Summary
Despite the discovery of many dinosaur eggs and nests over the past 100 years, articulated in-ovo embryos are remarkably rare. Here we report an exceptionally preserved, articulated oviraptorid embryo inside an elongatoolithid egg, from the Late Cretaceous Hekou Formation of southern China. The head lies ventral to the body, with the feet on either side, and the back curled along the blunt pole of the egg, in a posture previously unrecognized in a non-avian dinosaur, but reminiscent of a late-stage modern bird embryo. Comparison to other late-stage oviraptorid embryos suggests that prehatch oviraptorids developed avian-like postures late in incubation, which in modern birds are related to coordinated embryonic movements associated with tucking — a behavior controlled by the central nervous system, critical for hatching success. We propose that such pre-hatching behavior, previously considered unique to birds, may have originated among non-avian theropods, which can be further investigated with additional discoveries of embryo fossils.
Subject areas: Biological sciences, Evolutionary biology, Evolutionary processes, Phylogenetics
Graphical abstract
Highlights
-
•
A Late Cretaceous oviraptorid theropod dinosaur embryo is preserved in-ovo
-
•
Its head lies ventral to the body, and the back curled along the egg's blunt pole
-
•
Its posture is similar to that of a late-stage modern bird embryo
-
•
Avian tucking behavior possibly originated among non-avian theropods
Biological sciences; Evolutionary biology; Evolutionary processes; Phylogenetics
Introduction
Birds (Avialae) evolved from theropod dinosaurs during the Mesozoic, and many supposed ‘avian’ features characteristic of modern birds originated in their dinosaurian ancestors (Xu et al., 2014; Brusatte et al., 2015; Pittman et al., 2020), including aspects of reproductive biology (Zelenitsky and Therrien, 2008; Varricchio and Jackson, 2016; Norell et al., 1995; Varricchio et al., 2002). Non-avian theropods possess a mixture of reproductive features, including some that are avian-like and seen in today's birds, but others that are inherited from early reptilian ancestors or are unique features restricted to particular extinct dinosaur lineages (Horner, 2000; Zelenitsky, 2006; Yang et al., 2019b). Much of our knowledge on dinosaur reproduction has been developed from the study of eggs and embryos (if preserved), providing us with information on egg morphology and color, nest structure, nesting behavior, and early ontogeny/growth (e.g., Mikhailov, 2014; Tanaka et al., 2015; Wiemann et al., 2018; Yang et al., 2019b; Bi et al., 2021; Reisz et al., 2005; Norell et al., 2001).
Skeletons of dinosaurs are rarely preserved in ovo, but these embryonic fossils are important records of dinosaur reproduction as an embryo documents stages of early growth and development up until hatching. As for preserved eggshells of dinosaurs (e.g., Varricchio et al., 2002; Zelenitsky et al., 2002), aspects of their embryos are logically comparable in an evolutionary framework with those of their closest living relatives crocodiles and birds, the three clades of which belong to a group of diapsids called Archosauria.
With respect to in ovo embryonic posture, extant birds are known to have a degree of mobility, which allows them to develop different postures at different stages of development, and ultimately a characteristic tucking posture critical to successful hatching (Hamburger and Oppenheim, 1967; Hamburger, 1973; Oppenheim, 1973; Brooks, 1978; Rideout, 2012). The back of the embryo is against the air cell in the blunt pole of the egg and the head is tucked under the right wing with the beak pointing toward the lower slope of air cell (Oppenheim, 1973; Rideout, 2012). Such a posture or degree of mobility has not been observed in other extant archosaurs, the crocodilians (Ferguson, 1985; Casey and Sleigh, 2001), which instead have a sitting posture with the head bending upon the chest up to hatching (Deraniyagala, 1939), but it is not clear if deviations from this posture would increase mortality.
Owing to the scarcity of articulated dinosaur embryo fossils, it is unclear whether non-avian dinosaurs attained a bird-like posture before hatching, or were more similar to the ancestral archosaurian condition (i.e., as in living crocodiles, which we here presume to be plesiomorphic for Archosauria, although further evidence is needed from a range of tetrapods to determine the ancestral conditions of Archosauria and other clades). Although fossilized embryos of enantiornithine birds indicate that tucking behavior existed in the Cretaceous in early-diverging birds (Elżanowski, 1981; Zhou and Zhang, 2004). The prehatching posture of non-avian dinosaurs has rarely been commented on. A partial embryo of an oviraptorid—a non-avian theropod closely related to birds—was described to have the same posture as crocodilians (Norell et al., 1994, 2001), but the reasoning was not explained in detail. More in-depth comparative study from more recent discoveries is needed to illuminate the largely unexplored prehatching behavior of non-avian dinosaurs.
In this study, we describe a new non-avian theropod dinosaur embryo exquisitely preserved inside an egg, from the Late Cretaceous Hekou Formation of southern China. This articulated specimen is one of the most complete non-avian dinosaur embryos yet discovered, permitting detailed anatomical study and an unprecedented glimpse at the osteology of an in-ovo bird-like, but non-avian, theropod dinosaur. A comparison with the embryos of closely related taxa and extant archosaurs allows us to hypothesize aspects of prehatching behavior. Most importantly, we observe that oviraptorid embryos appear to take on aspects of avian-like tucking postures, and we propose that this prehatching behavior evolved prior to the origin of birds.
Results
Anatomical description
The embryo (YLSNHM01266) can be identified as an oviraptorid oviraptorosaur based on the following combination of characters: crenulated ventral margin of premaxilla; subantorbital portion of maxilla insets medially; external naris extends posteriorly, with posterior end situating above antorbital fenestra; parietal anteroposteriorly longer than frontal; edentulous skull; U-shaped mandibular symphysis; highly arched dentary. The oviraptorid affinity of YLSNHM01266 is also supported by phylogenetic analysis (see STAR Methods).
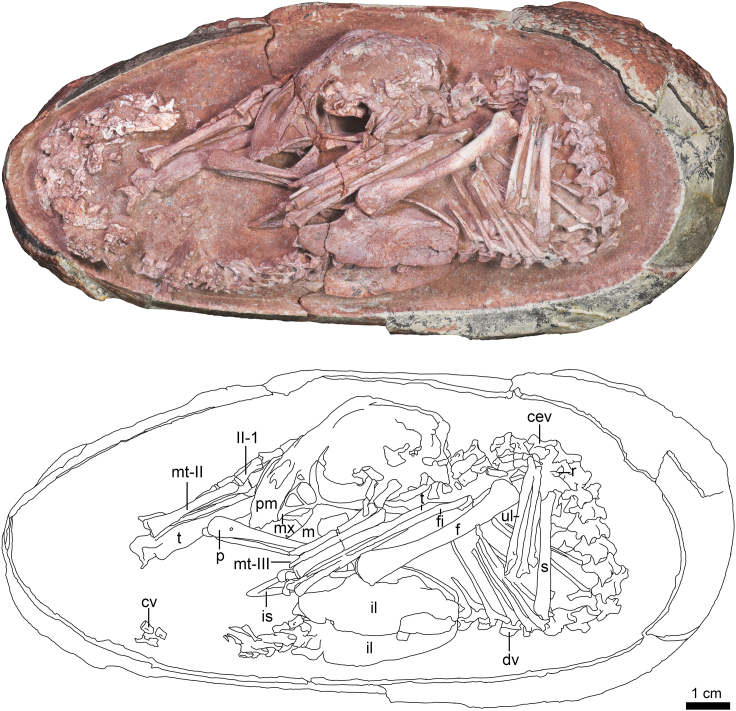
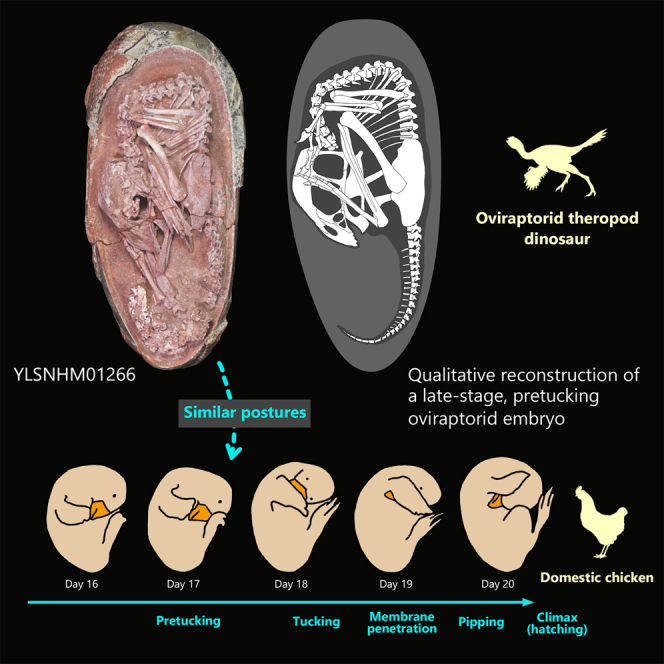
The articulated embryonic skeleton is preserved curled inside its egg (YLSNHM01266), with the skull positioned ventral to the body (Figure 1). The egg is elongate ovoid in shape with dimensions of 16.7 cm long by 7.6 cm wide, and has characteristics typical of the egg family Elongatoolithidae (see STAR Methods for eggshell analysis). The skeleton is almost complete, without much apparent postmortem disruption. The anterior surface of the skull faces toward the pointed pole and is situated about egg mid-length at the level of the ilium in-between the flexed hindlimbs, with a pes on either side. The anterior cervical vertebrae are in line with the long axis of the skull. The presacral vertebral column is strongly bent in an angular manner, so that the upper back of the embryo faces the blunt pole of the egg (similar flexion of the vertebral column is found in modern in ovo skeletons, e.g. Balanoff and Rowe, 2007: Figure 4, Day 18, and is not likely to be a taphonomic artifact). The skeleton is ∼23.5 cm in total length, measured from the anterior tip of the skull to the last preserved caudal vertebra, and occupies nearly the entire width of the egg and most of the length, with the exception of a ∼1.9 cm space between the dorsal vertebrae and the blunt pole of the egg. This space may represent the air cell, a space usually found between the back of the embryo and the blunt pole of bird eggs (e.g., Rahn et al., 1979). However, this inference is tentative and awaits further evidence. The posterodorsal, sacral and caudal vertebrae almost form a straight line along the long axis of the egg. Although the precise developmental stage of the embryo is unclear, it is likely to represent a late-stage embryo because the skeleton is well ossified and is large in size relative to the space inside the egg, as inferred in MPC 100/971 (Norell et al., 2001).
Figure 1.
Oviraptorid embryo inside an elongatoolithid egg (YLSNHM01266)
Abbreviations: cev, cervical vertebra; cv, caudal vertebra; dv, dorsal vertebra; f, femur; fi, fibula; II-1, pedal phalanx II-1; il, ilium; is, ischium; m, mandible; mt-I, metatarsal I; mt-III, metatarsal III; mx, maxilla; p, pubis; pm, premaxilla; r, radius; s, scapula; t, tibia; ul, ulna. Scale is 1 cm.
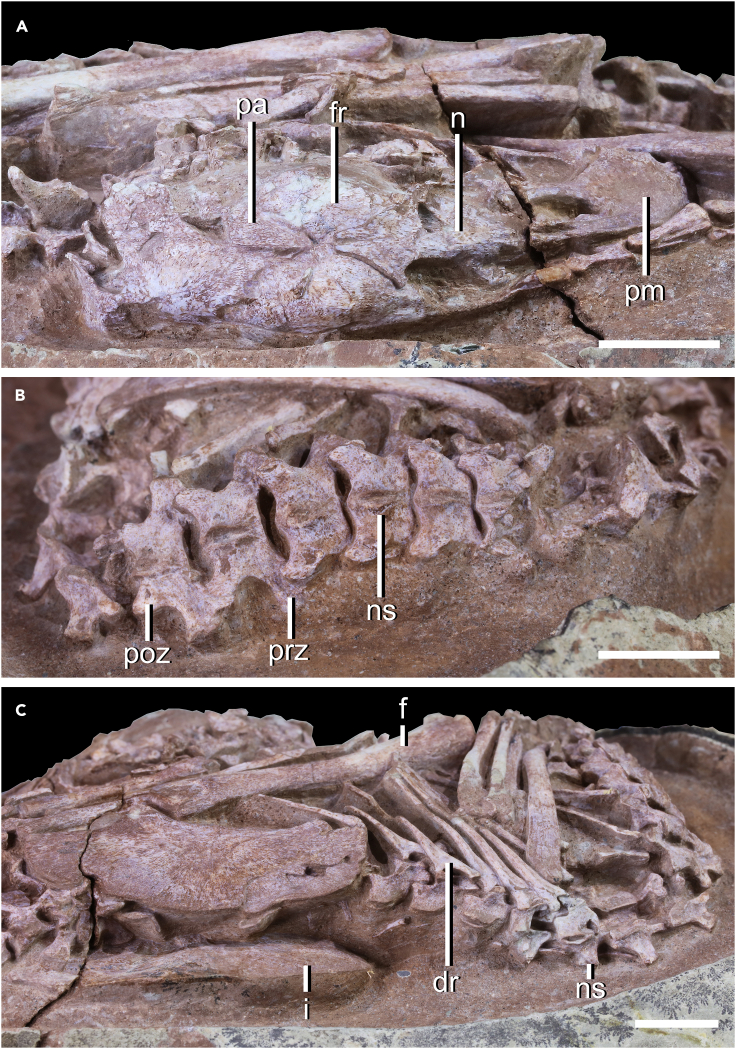
Both the cranium and the mandible of YLSNHM01266 are preserved and articulated (Figure 1). The skull is edentulous, as in caenagnathids and oviraptorids (Osmólska et al., 2004). The right and left premaxillae are not fused. No opening is observed in the premaxilla. The nasals are fused, as in all oviraptorids (including Yulong and IVPP V20182) and the caenagnathid perinate Beibeilong (Lü et al., 2013; Wang et al., 2016; Pu et al., 2017). In the dorsal view, the nasal contacts the frontal and forms a V-shaped margin (Figure 2A). The mandible of YLSNHM01266 lacks the lingual triturating shelf and lingual ridge, as in other oviraptorids except Gobiraptor (Lee et al., 2019). The dentary is anteroposteriorly short and highly arched, a typical feature of oviraptorid mandibles (Longrich et al., 2013; Ma et al., 2017, 2020).
Figure 2.
Skeleton of oviraptorid embryo (YLSNHM01266)
In (A) dorsal view of skull; (B) dorsal view of cervical and dorsal vertebrae; (C) dorsal view of posterodorsal vertebrae and pelvic region
Abbreviations: f, femur; fr, frontal; i, ilium; n, nasal; ns, neural spine; pa, parietal; pm, premaxilla; poz, postzygapophysis; prz, prezygapophysis. Scale is 1 cm.
With the vertebral column, YLSNHM01266 is estimated to have 22 presacral vertebrae (Figures 2B and 2C). The anterior dorsals have a low neural spine comparable to those of the cervicals, whereas those of the succeeding dorsals are slightly taller and more robust, and become lower again near the end of the dorsal series (Figures 2B and 2C). In general, the dorsal neural spines have a curved dorsal margin in lateral view, unlike those of some oviraptorosaurs, which are rectangular in some parts of the series (Balanoff and Norell, 2012). The prezygapophyses of the dorsals extend anterolaterally in the dorsal view (Figure 2B). The lateral extension of the prezygapophysis is more prominent in the dorsals than in the cervicals. However, the posterolateral extension of the postzygapophysis does not show obvious change along the presacral vertebrae series. The dorsal ribs are bicapitate. The capitula are well-developed with minor expansions at the articular ends. No uncinate process is observed on any of the dorsal ribs. The sacral vertebrae are not clearly observable but the proximal caudal vertebrae are exposed. One of the anterocaudal centra is visible laterally and bears a pleurocoel on its lateral surface. The more posterior caudals situated near the pointed pole of the egg are not visible as they are covered by secondary recrystallization.
With the forelimb, part of the left shoulder girdle and arm are visible in YLSNHM01266 (Figure 1). The coracoid and humerus may be hidden beneath the articulated ulna and radius, whereas the hand is likely missing. In the pelvic girdle, the ilium, ischium and pubis are all visible to varying extents. The ilium has a relatively straight to gently convex dorsal margin in lateral view (Figure 1). Only a small portion of the distal end of the ischium is visible, and its dorsal margin appears to be straight. The distal end of the pubis is expanded relative to the pubic shaft but there is no sign of pubic boot development. This suggests that pubic boot development mostly occurs post-hatching in oviraptorosaurs. It is noteworthy that the pubis points posteroventrally, similar to that of modern birds but unlike other oviraptorosaurs where it points downward or forward (Zhou et al., 2000; Osmólska et al., 2004; Makovicky and Zanno, 2011; Balanoff and Norell, 2012). The pubis has been subjected to some degree of postmortem disruption, as it is not articulated to the ilium, so it is unclear how much of its orientation is genuine. If the pubis was backward-pointing in life position, this may suggest a change in pubis orientation through ontogeny in oviraptorosaurs, implying that the retroverted pubis of birds could be a paedomorphic dinosaurian feature. However, this remains highly tentative until further evidence emerges. Both hindlimbs are strongly flexed. The left femur is articulated with the pelvic girdle and exposed in lateral view (Figure 1). The distal part of the femur lies near the left forearm elements on the anteroventral part of the rib cage; this bone may have been shifted slightly with the ilium postmortem. The femur is slightly longer than the ilium in YLSNHM01266, as in other oviraptorids where the lengths of the femur and the ilium are similar (Osmólska et al., 2004). The right hindlimb of YLSNHM01266 is also positioned such that the two flexed pedes are on each side of the skull (see Supplemental anatomical description).
Phylogenetic analysis
A phylogenetic analysis was conducted to test the relationships of YLSNHM01266 among oviraptorosaurs, by adding it to the dataset of Pu et al. (2017) which includes other perinate specimens (see STAR Methods). The analysis recovered a similar topology with other phylogenies (Pu et al., 2017; Funston and Currie, 2016) (Figure S1), except for placing Avimimus at the base of Oviraptorosauria and Nankangia within Caenagnathidae (which was previously recovered as an early-diverging oviraptorid). This is possibly due to the incorporation of specimens of immature individuals in the analysis, such that the interrelationships are influenced by ontogenetically-variable characters. Regardless, the phylogenetic analysis places YLSNHM01266 in Oviraptoridae (Figure S1), supporting our assignment based on comparative anatomy.
Discussion
Posture in non-avian dinosaur embryo fossils
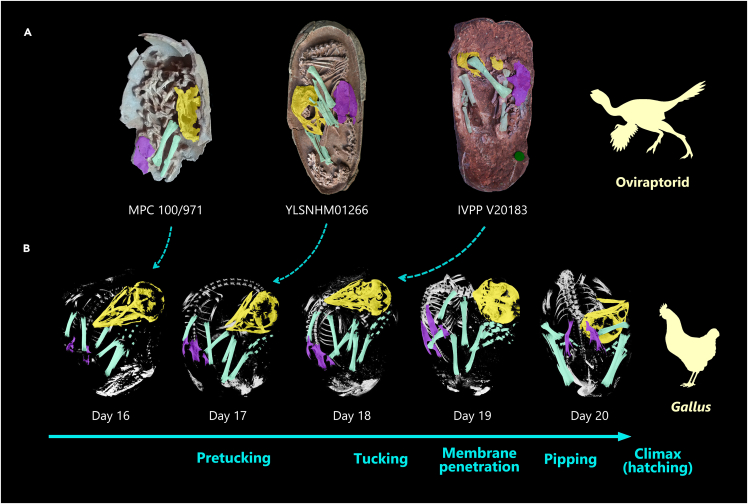
The immaculate articulated skeleton of YLSNHM01266 preserves a late-stage non-avian oviraptorid theropod dinosaur positioned within its egg. Among all Mesozoic dinosaurs, oviraptorids currently have the best record of embryonic fossils, although still only a handful of specimens are known. The new embryo is a keystone fossil that reveals postural differences among the known embryonic oviraptorids curled in ovo (Figure 3A). The skeleton is positioned so that the skull lies ventral to the body extending to the level of the ilium (and posteriorly), with the flexed hindlimbs positioned on either side. The anterior surface of the skull faces toward the pointed pole and is essentially parallel to the body, with the spine bent angularly so the upper back faces the blunt pole. Another oviraptorid egg, from Ganzhou, China (IVPP V20183 (Wang et al., 2016)), contains an embryo curled such the skull is also at the level of the ilium and the hindlimbs closely associated as in YLSNHM01266, but the skull points more inward toward the body rather than toward the pole (Figure 3A). Although the neck is not visible, the preserved body parts are in articulation so that the preserved position likely represents life position. In another highly-ossified, curled oviraptorid embryo from Mongolia (MPC 100/971 (Norell et al., 2001)), the skull faces toward the pole as in YLSNHM01266, but it resides at a level mostly anterior to the ilium and the hindlimbs are not very closely associated unlike YLSNHM01266 and IVPP V20183 (Figure 3A). Because these three oviraptorids are in similar sized eggs (Norell et al., 2001; Wang et al., 2016), their noted differences may represent different degrees of the curled posture (MPC 100/971 appears less curled than the other two embryos) or intervals of postural changes during incubation.
Figure 3.
In-ovo late-stage embryos of non-avian and avian dinosaurs
(A) Oviraptorid specimens (MPC 100/971, YLSNHM01266 & IVPP V20183), which potentially correspond to various tucking stages.
(B) Domestic fowl Gallus ontogenetic series (day 16-20) (modified from Rowe (2003)). Not to scale. Silhouettes modified from PhyloPic.
The postures in the known embryonic oviraptorids are difficult to assess relative to those of other non-avian dinosaurs, for two reasons. First, there is a scarcity of articulated specimens, particularly of non-oviraptorids. Second, meaningful comparisons may also be limited because likely influences on embryonic posture, such as egg shape, egg type, and embryo morphology, can differ significantly among non-avian dinosaurs. The perinate skeleton of a related oviraptorosaur Beibeilong (Caenagnathidae) is semi-articulated and associated with elongate eggs similar to those of oviraptorids. Unfortunately, the embryo was not preserved inside an egg (Pu et al., 2017), and thus the preserved posture is unlikely to represent the in-ovo life condition. Articulated embryonic remains within elongate eggs are also known for another pennaraptoran relative, Troodon formosus. In one specimen, the skull is closely associated with the flexed hindlimbs and faces posteriorly toward the pointed pole of the egg (Varricchio et al., 2002). This overall posture appears most similar to YLSNHM01266 among the articulated in-ovo oviraptorids. Although distantly related to oviraptorids, an early-diverging sauropodomorph Massospondylus is known from a late-stage embryo in a subspherical egg (Reisz et al., 2005). The posture appears to be distinct from that of oviraptorids as the skull is located near the pole, and the neck curls dorsally rather than ventrally (Reisz et al., 2010). This is an unusual embryonic head posture, which may be due to the presence of soft-shelled eggs in these dinosaurs (Norell et al., 2020) that lacked the rigidity to maintain the original posture after death. This atypical head posture alternatively could have been due to movements associated with the hatching process. This short roster of specimens is all of the currently available comparative data on articulated dinosaur skeletons in-ovo. This is why pre-hatching postures and behaviors for dinosaurs have been so unclear.
Prehatching behavior in non-avian dinosaurs and birds
Prehatching embryonic behaviors are poorly understood for non-avian dinosaurs and extinct early-diverging birds, but extant bird embryos and specimens like YLSNHM01266 described here provide insights. Extant bird embryos, via a unique tucking behavior, make a series of coordinated movements to change positions/postures late in incubation in order to prepare for hatching (Bekoff, 1994; Hamburger and Oppenheim, 1967) (Figure 3B). The tucking process consists of three phases, pre-tucking, tucking and post-tucking, described in detail by Hamburger and Oppenheim (1967). In the pre-tucking phase (on ∼17 day of domestic fowl Gallus), the embryo's beak is pointing toward the pointed pole early on and is positioned between the hindlimbs and feet, with the back curling at the blunt pole where the air cell resides (Rahn et al., 1979) (Figure 3B). The oviraptorid embryo of YLSNHM01266 appears to have this avian-like pre-tuck posture and a space possibly representing the air cell (Figure 3A). Other dinosaur embryos surveyed here indicate that a similar posture is also seen in a Troodon embryo, potentially, and in enantiornithine bird embryos from the Cretaceous of China (Zhou and Zhang, 2004) and Mongolia (Elżanowski, 1981). The oviraptorid embryo posture of MPC 100/971 may be preserved before the avian pre-tuck phase as the legs and feet are not as closely associated with the skull, which is perhaps the reason it was described initially as having a crocodilian-like posture (Norell et al., 2001) (Figure 3A).
Latter phases of pre-tucking or tucking, present in birds, may also be evident in the fossil embryos surveyed. During the first stage of tucking in birds (on ∼18 days of Gallus), the head eventually moves to a position where the beak and skull point inward and toward the right wing (Hamburger and Oppenheim, 1967; Oppenheim, 1973) (Figure 3B). In the subsequent tucking and post-tucking phases, the beak and head become tucked under the right wing then move toward the torso, where the beak is ultimately positioned behind the right shoulder (Hamburger and Oppenheim, 1967). In the oviraptorid embryo of IVPP V20183, it is unknown how the skull is positioned relative to the right forelimb as it is missing (Figure 3A). Because the skull appears more tucked into the body (45° from the egg long axis) than YLSNHM01266 (parallel to the egg long axis), IVPP V20183 is probably later in the tucking process. This position may represent late pre-tucking phase or later. In enantiornithine birds, advanced tucking is visible in one specimen where the beak is tucked under its right wing and points anteriorly as in extant birds (Elżanowski, 1981), presumably in a tucking phase or later.
Avian-like pre-hatching positions have been reported in embryonic specimens of early-diverging birds (Elżanowski, 1981; Zhou and Zhang, 2004) and here we also propose to observe them in non-avian pennaraptoran dinosaurs (Norell et al., 1994; Varricchio et al., 2002; Wang et al., 2016), including the newly described YLSNHM01266. We interpret that the late-stage oviraptorid embryos we surveyed demonstrate different positions associated with tucking behavior similar to modern birds, with MPC 100/971 (Norell et al., 1994) potentially representing a posture prior to tucking, YLSNHM01266 in the pre-tucking phase, and IVPP V20183 (Wang et al., 2016) corresponding to late pre-tucking or later phase (Figure 3). However, because of a lack of articulated forelimbs in these oviraptorid embryos, the precise placement of the tucked head and beak compared to that of birds is uncertain. The ultimate position in living birds, with the head tucked under the right wing is functionally related to hatching, which has been suggested to stabilize and direct the head/beak to be more effective and efficient during pipping and climax (Brooks, 1978; Hutt, 1930). Improper tucked posture is shown to significantly increase embryo mortality and reduce hatching success (Hutt, 1930; Herring et al., 2010).
Although the relationships between tucking and hatching are uncertain for extinct theropods, we hypothesize that the similarities in preserved positions of embryonic oviraptorids and early-diverging birds compared to prehatching positions in extant birds indicates at least some degree of tucking behavior among non-avian theropods, distinct from their more distant cousins like sauropodomorph dinosaurs and extant crocodilians. This now must be further tested with discoveries of additional embryo fossils—not only of theropod dinosaurs, but of sauropodomorphs and ornithischians more distantly related to birds—and more in-depth comparative studies of extant archosaurs, particularly crocodilians. Regardless, this new exceptional fossil embryo hints that some early developmental behaviors (tucking) often considered as uniquely avian may be rooted more deeply in the theropod lineage, as has already been observed with other features of reproduction (e.g., parent dinosaurs incubating nests of eggs).
Limitations of the study
More in-depth comparative study on the embryonic posture of non-avian dinosaurs and extant archosaurs is hindered by the scarcity of available in-ovo images and scans. Attempts at imaging YLSNHM01266 have been made using computed tomography (CT) and micro CT, but because of high density minerals and lack of contrast between bone and matrix, they did not provide useful anatomical data for bones still within the matrix. This is not unexpected, as CT imaging bones preserved in iron rich sediments is known to be difficult, as previously reported in other eggs from red beds in southern China (e.g., Wang et al. (2016)). As for extant archosaurs, literature on prehatching behavior is very limited except for domestic fowl Gallus. Thus, it is difficult to compare the postures of non-avian dinosaurs with those of other archosaurs (e.g., crocodilians) precisely and quantitatively (e.g., with geometric morphometrics). The primary purpose of this paper is to announce the exquisite specimen YLSNHM01266 and to use it as an anchor to propose hypotheses about pre-hatching posture in non-avian dinosaurs. It is thus beyond the time frame and scope of this paper to obtain firsthand scan data of a large sample of extant archosaur embryos. Future research on the prehatching behavior of non-avian dinosaurs will benefit from discoveries of additional embryo fossils (for comparison and evaluation of taphonomic factors), more precise determination of developmental stage, as well as studies on a broader sample of in-ovo, extant archosaur embryos.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Phylogenetic data matrix | This paper | https://doi.org/10.5281/zenodo.5708399 |
| Supplemental anatomical description | This paper | https://doi.org/10.5281/zenodo.5708399 |
| Software and algorithms | ||
| TNT 1.5 | Goloboff et al. (2008) | http://www.lillo.org.ar/phylogeny/tnt/ |
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Waisum Ma (w.ma.1@pgr.bham.ac.uk).
Materials availability
The specimen reported in this paper (YLSNHM01266) is accessioned at the public institute Yingliang Stone Nature History Museum (Nan’an, China). The specimen is available for research.
Experimental model and subject details
This fossil specimen reported (YLSNHM01266) is an embryo preserved in an egg. It was recovered from the Hekou Formation at Shahe Industrial Park, Ganzhou City, Jiangxi Province.
Institutional abbreviations
BP, Bernard Price Institute for Palaeontological Research, University of Witwatersrand, Johannesburg, South Africa; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; MPC, Paleontological Center, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia; NMNS, National Museum of Natural Science, Taichung, Taiwan, China; YLSNHM, Yingliang Stone Nature History Museum, Nan’an, China
Geological setting
There is no consensus on whether the strata of the Nanxiong Basin and the Ganzhou area are part of a generalized Nanxiong Group/Nanxiong Formation, or if it should be divided into Upper Cretaceous Yuanfu, Zhutian and Zhenshui formations of the Nanxiong Basin (Fang et al., 2009) or the Upper Cretaceous Ganzhou Group (Maodian and Zhoutian formations), or the Cretaceous-Paleogene Guifeng Group (Upper Cretaceous Hekou and Tangbian formations and Cretaceous-Paleogene Lianhe Formation) (Wen et al., 2016).
The embryo specimen YLSNHM01266, documented here, was recovered from the Hekou Formation at Shahe Industrial Park, Ganzhou City, Jiangxi Province. The Hekou Formation is now redefined as a red, fluvial, coarse clastic deposit (He et al., 2017), and mainly consists of a red conglomerate and glutenite, interbedded with a small amount of sandstone, siltstone, and locally interspersed tuff. The formation preserves dinosaur bones and eggs, and fossil plants (Department of Geology and Mineral Resources of Jiangxi Province, 1997). It is stratigraphically equivalent to the Dafeng Formation/Yuanfu Formation in the lower section of the Nanxiong Formation in Nanxiong, Guangdong Province (Ling, 1996).
Some scholars suggested these three formations from the Guifeng Group (Hekou, Tangbian, Lianhe) were likely formed around the same time, i.e., they are coeval and overlapping and deposited in different environments - comprising a deposystem composite of fluvial and alluvial deposits (Xie, 2001). At least K–Ar dating and paleomagnetic studies seem to provide some age constraints for these three formations. Paleomagnetic studies conducted in the 1990s have dated the Guifeng Group as late Campanian to Maastrichtian, ranging from 71.4–65.0 Ma (Gu, 1991; Zuo et al., 1999). More recent research that considers lithostratigraphy and sedimentary facies, by Chen et al. (2017) and Xi et al. (2019), considered the age of Guifeng Group as Coniacian–Maastrichtian, with the Hekou Formation as Coniacian–Santonian, Tangbian Formation as Campanian, and Lianhe Formation as Maastrichtian.
From the perspective of regional geological maps (He et al., 2017) (not only Shahe Industrial Park), Hekou Formation is also found in Nankang area, Longling Town, Gan County (=Ganxian) and other areas. In many studies, these areas are simply classified as Nanxiong Formation/Nanxiong Group (Maastrichtian /Campanian to Maastrichtian), such as Lü et al. (2014), Lü et al. (2015). Although the exact location of some fossils is unclear, the geological records of vertebrates in the Guifeng Group definitively need more detailed research.
Method details
Egg shell analysis
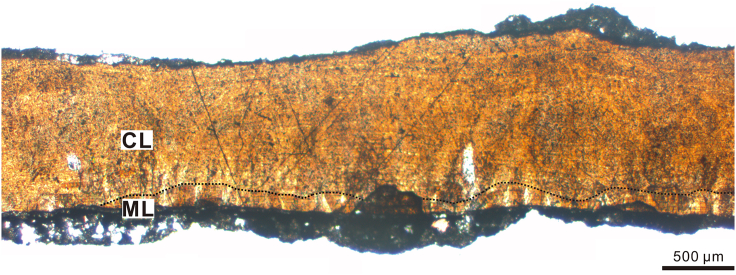
The egg containing an embryo, YLSNHM01266, measures 16.7 cm long, with an equatorial width of 7.6 cm. The surface ornamentation in the equatorial region is linearituberculate, consisting of closely spaced ridges. The blunt end shows nodose ornamentation, whereas the acute end is smooth. A piece of eggshell was removed from the egg and prepared as a petrographical thin section for histological studies. The shell thickness, including ornamentation, varies from 1.0 to 1.2 mm. Two calcitic ultrastructural layers, a mammillary (ML) and a continuous layer (CL), separated by an undulating boundary, were identified. The ML is severely eroded, likely due to calcium resorption by the developing embryo, which is described for other eggs containing embryos (e.g., Yang et al., 2019a). The thickness ratio of the mamillary layer to continuous layer would be roughly 1:4 in the presence of a complete ML. Characteristics of the aforementioned egg and eggshell, are indicative of the Elongatoolithidae (Zhao, 1975). This oofamily is common throughout the Cretaceous of Asia, and previous discoveries of embryos or adults associated with elongatoolithid eggs confirm their identity as oviraptorosaur (e.g., Norell et al., 1994; Norell et al., 1995; Dong and Currie, 1996; Weishampel et al., 2008; Wang et al., 2016; Pu et al., 2017; Norell et al., 2018).
Eggshell microstructures of YLSNHM01266, showing characteristics indicative of Elongatoolithidae
The undulating boundary between the continuous (CL) and mammillary (ML) layers is marked as a dotted black line. Note that the ML is severely eroded and thus only the upper parts of the mammillae are visible. Scale is 500 μm.
Phylogenetic analysis
The phylogenetic position of YLSNHM01266 was assessed by incorporating it into a dataset of Oviraptorosauria, which includes other oviraptorosaur perinates including the caenagnathid Beibeilong and oviraptorid Yulong (Pu et al., 2017). The data matrix includes a total of 35 oviraptorosaur taxa and three outgroups (Herrerasaurus, Velociraptor, Archaeopteryx), scored for 250 morphological characters (Pu et al., 2017). The data was analyzed in TNT (version 1.5) (Goloboff et al., 2008) using ‘New Technology’ search with default parameters for sectorial search, ratchet, tree drift and tree fusing, with minimum length tree found in 10 replicates. The recovered 25 most parsimonious trees were further subjected to ‘Traditional’ search using ‘tree bisection reconnection’, recovering 47 additional trees. A strict consensus tree was constructed from the 72 most parsimonious trees (each with a consistency index = 0.492; retention index = 0.661) (Figure S1A).
An additional set of phylogenetic analysis was conducted with the putative ontogenetically-variable characters for YLSNHM01266 and Beibeilong coded as ‘?’, following the implementation in Pu et al. (2017). This aims to minimize the effect of these characters on the phylogeny. Excluded characters were: 82, 84, 162, 165, 166, 184, 185, 187, 222, 224 and 248. All of the other settings were identical to the analysis of the original matrix. A strict consensus tree was calculated based on 432 most parsimonious trees (39 trees from ‘New Technology’ search; 393 trees from ‘Traditional’ search) (each with a consistency index = 0.495; retention index = 0.663) (Figure S1B).
Both analyses recovered YLSNHM01266 as an oviraptorid (Figure S1), corroborating our assignment based on comparative anatomy.
Quantification and statistical analysis
Phylogenetic analyses were performed in TNT (version 1.5).
Additional resources
Supplemental anatomical description: https://doi.org/10.5281/zenodo.5708399.
Acknowledgments
We thank Anthony Romilio (The University of Queensland) for digitizing the specimen with photogrammetry. We thank Willi Hennig Society for making TNT freely available and PhyloPic for providing silhouettes. L.X. is supported by the National Natural Science Foundation of China (No. 41888101, 41790455, 41772008), 111 Project (B20011) and Fundamental Research Funds for the Central Universities (265QZ201903). Research of D.K.Z is funded by Natural Sciences and Engineering Research Council of Canada with a project ID of 327513-2009. T.-R.Y. is supported by the research grant of Ministry of Science and Technology, Taiwan (MOST 108-2116-M-178-003-MY2).
Author contributions
L.X. and K.N. conceived the project. L.X. provided the information and interpretation on the geological setting. W.M. performed the comparative anatomical research and phylogenetic analysis. T.-R.Y. performed the eggshell analysis. All authors provided the interpretation on embryonic behavior. W.M. led the writing of the first draft of the manuscript. L.X., W.M., D.K.Z., T.-R.Y. and S.L.B. wrote and revised the final manuscript. All authors approved the manuscript for publication.
Declaration of interests
The authors declare no competing interests.
Published: December 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103516.
Supplemental information
Data and code availability
-
•
Dataset used in this study has been deposited at Zenodo. DOI is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information is available from the lead contact upon request.
References
- Balanoff A.M., Norell M.A. Osteology of Khaan mckennai (Oviraptorosauria: Theropoda) Bull. Am. Mus. Nat. Hist. 2012;2012:1–76. [Google Scholar]
- Balanoff A.M., Rowe T. Osteological description of an embryonic skeleton of the extinct elephant bird, Aepyornis (Palaeognathae: Ratitae) J. Vertebr. Paleontol. 2007;27:1–53. [Google Scholar]
- Bekoff A. Springer; 1994. Neuroembryology of Motor Behaviour in Birds. Perception and Motor Control in Birds. [Google Scholar]
- Bi S., Amiot R., De Fabrègues C.P., Pittman M., Lamanna M.C., Yu Y., Yu C., Yang T., Zhang S., Zhao Q. An oviraptorid preserved atop an embryo-bearing egg clutch sheds light on the reproductive biology of non-avialan theropod dinosaurs. Sci. Bull. 2021;66:947–954. doi: 10.1016/j.scib.2020.12.018. [DOI] [PubMed] [Google Scholar]
- Brooks W.S. Avian prehatching behavior: functional aspects of the tucking pattern. Condor. 1978;80:442–444. [Google Scholar]
- Brusatte S.L., O’connor J.K., Jarvis E.D. The origin and diversification of birds. Curr. Biol. 2015;25:R888–R898. doi: 10.1016/j.cub.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Casey M.B., Sleigh M.J. Cross-species investigations of prenatal experience, hatching behavior, and postnatal behavioral laterality. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2001;39:84–91. doi: 10.1002/dev.1032. [DOI] [PubMed] [Google Scholar]
- Chen L., Steel R.J., Guo F., Olariu C., Gong C. Alluvial fan facies of the Yongchong Basin: implications for tectonic and paleoclimatic changes during Late Cretaceous in SE China. J. Asian Earth Sci. 2017;134:37–54. [Google Scholar]
- Department of Geology and Mineral Resources of Jiangxi Province . China University of Geosciences Press; 1997. Stratigraphy (Lithostratic) of Jiangxi Province, Wuhan. [Google Scholar]
- Deraniyagala P.E.P. The tetrapod reptiles of Ceylon.–Vol. I. Testudinates and crocodilians. Colombo Museum Natural history series. Ceylon J. Sci. 1939;1:307–391. [Google Scholar]
- Dong Z.-M., Currie P.J. On the discovery of an oviraptorid skeleton on a nest of eggs at Bayan Mandahu, Inner Mongolia, People's Republic of China. Can. J. Earth Sci. 1996;33:631–636. [Google Scholar]
- Elżanowski A. Embryonic bird skeletons from the late Cretaceous of Mongolia. Palaeontol. Pol. 1981;42:147–176. [Google Scholar]
- Fang X.-S., Li P.-X., Zhang Z.-J., Zhang X.-Q., Lin Y.-L., Guo S.-B., Cheng Y.-M., Li Z.-Y., Zhang X.-J., Cheng Z.-W. Cretaceous strata in Nanxiong Basin of Guangdong and the evolution from the dinosaur egg to the bird egg. Acta Geosci. Sin. 2009;30:167–186. [Google Scholar]
- Ferguson M. In: Gans C., Billett F., Maderson P.F.A., editors. Vol. 14. John Wiley & Sons; 1985. Reproductive biology and embryology of the crocodilians. (Biology of the Reptilia). [Google Scholar]
- Funston G.F., Currie P.J. A new caenagnathid (Dinosauria: Oviraptorosauria) from the Horseshoe Canyon Formation of Alberta, Canada, and a reevaluation of the relationships of Caenagnathidae. J. Vertebr. Paleontol. 2016;36:e1160910. [Google Scholar]
- Goloboff P.A., Farris J.S., Nixon K.C. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. [Google Scholar]
- Gu X. The magnetic stratum of late Cretaceous red beds in Jitai basin, Jiangxi province. Geol. Sci. Technol. Jiangxi. 1991;18:185–188. [Google Scholar]
- Hamburger V. Springer; 1973. Anatomical and Physiological Basis of Embryonic Motility in Birds and Mammals. Neuroembryology. [Google Scholar]
- Hamburger V., Oppenheim R. Prehatching motility and hatching behavior in the chick. J. Exp. Zool. 1967;166:171–203. doi: 10.1002/jez.1401660203. [DOI] [PubMed] [Google Scholar]
- He F., Huang X., Li X. Occurrence rule and buried characteristics of dinosaur fossils in the Ganzhou Basin, Jiangxi Province. East China Geol. 2017;38:250–254. [Google Scholar]
- Herring G., Ackerman J.T., Eagles-Smith C.A. Embryo malposition as a potential mechanism for mercury-induced hatching failure in bird eggs. Environ. Toxicol. Chem. 2010;29:1788–1794. doi: 10.1002/etc.208. [DOI] [PubMed] [Google Scholar]
- Horner J.R. Dinosaur reproduction and parenting. Annu. Rev. Earth Planet. Sci. 2000;28:19–45. [Google Scholar]
- Hutt F. X.—studies in embryonic mortality in the fowl. I. The frequencies of various Malpositions of the chick embryo and their significance. Proc. R. Soc. Edinb. 1930;49:118–130. [Google Scholar]
- Lee S., Lee Y.-N., Chinsamy A., Lü J., Barsbold R., Tsogtbaatar K. A new baby oviraptorid dinosaur (Dinosauria: Theropoda) from the upper Cretaceous Nemegt Formation of Mongolia. PLoS One. 2019;14:e0210867. doi: 10.1371/journal.pone.0210867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L. The establishment of the Cretaceous Maodian formation, Hekou Formation and Tangbian formation in Jiangxi province. Geol. Sci. Technol. Jiangxi. 1996;23:55–59. [Google Scholar]
- Longrich N.R., Barnes K., Clark S., Millar L. Caenagnathidae from the upper Campanian Aguja Formation of west Texas, and a revision of the Caenagnathinae. Bull. Peabody Mus. Nat. Hist. 2013;54:23–49. [Google Scholar]
- Lü J., Currie P.J., Xu L., Zhang X., Pu H., Jia S. Chicken-sized oviraptorid dinosaurs from central China and their ontogenetic implications. Naturwissenschaften. 2013;100:165–175. doi: 10.1007/s00114-012-1007-0. [DOI] [PubMed] [Google Scholar]
- Lü J., Yi L., Brusatte S.L., Yang L., Li H., Chen L. A new clade of Asian Late Cretaceous long-snouted tyrannosaurids. Nat. Commun. 2014;5:1–10. doi: 10.1038/ncomms4788. [DOI] [PubMed] [Google Scholar]
- Lü J., Pu H., Kobayashi Y., Xu L., Chang H., Shang Y., Liu D., Lee Y.-N., Kundrat M., Shen C. A new oviraptorid dinosaur (Dinosauria: Oviraptorosauria) from the late Cretaceous of southern China and its paleobiogeographical implications. Sci. Rep. 2015;5:1–15. doi: 10.1038/srep11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Wang J., Pittman M., Tan Q., Tan L., Guo B., Xu X. Functional anatomy of a giant toothless mandible from a bird-like dinosaur: Gigantoraptor and the evolution of the oviraptorosaurian jaw. Sci. Rep. 2017;7:16247. doi: 10.1038/s41598-017-15709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Brusatte S.L., Lü J., Sakamoto M. The skull evolution of oviraptorosaurian dinosaurs: the role of niche partitioning in diversification. J. Evol. Biol. 2020;33:178–188. doi: 10.1111/jeb.13557. [DOI] [PubMed] [Google Scholar]
- Makovicky P.J., Zanno L.E. John Wiley & Sons; Oxford, UK: 2011. Theropod diversity and the refinement of avian characteristics. Living dinosaurs: The Evolutionary History of Modern Birds; pp. 9–29. [Google Scholar]
- Mikhailov K.E. Eggshell structure, parataxonomy and phylogenetic analysis: some notes on articles published from 2002 to 2011. Hist. Biol. 2014;26:144–154. [Google Scholar]
- Norell M.A., Clark J.M., Demberelyin D., Rhinchen B., Chiappe L.M., Davidson A.R., Mckenna M.C., Altangerel P., Novacek M.J. A theropod dinosaur embryo and the affinities of the Flaming Cliffs dinosaur eggs. Science. 1994;266:779–782. doi: 10.1126/science.266.5186.779. [DOI] [PubMed] [Google Scholar]
- Norell M.A., Clark J.M., Chiappe L.M., Dashzeveg D. A nesting dinosaur. Nature. 1995;378:774–776. [Google Scholar]
- Norell M.A., Clark J.M., Chiappe L.M. An embryonic oviraptorid (Dinosauria: Theropoda) from the upper Cretaceous of Mongolia. Am. Mus. Novit. 2001;2001:1–20. [Google Scholar]
- Norell M.A., Balanoff A.M., Barta D.E., Erickson G.M. A second specimen of Citipati osmolskae associated with a nest of eggs from Ukhaa Tolgod, Omnogov Aimag, Mongolia. Am. Mus. Novit. 2018;2018:1–44. [Google Scholar]
- Norell M.A., Wiemann J., Fabbri M., Yu C., Marsicano C.A., Moore-Nall A., Varricchio D.J., Pol D., Zelenitsky D.K. The first dinosaur egg was soft. Nature. 2020;583:406–410. doi: 10.1038/s41586-020-2412-8. [DOI] [PubMed] [Google Scholar]
- Oppenheim R.W. Elsevier; 1973. Prehatching and Hatching Behavior: A Comparative and Physiological Consideration. Studies on the Development of Behavior and the Nervous System. [Google Scholar]
- Osmólska H., Currie P.J., Barsbold R. In: The Dinosauria. Second Edition. Weishampel D.B., Dodson P., Osmolska H., editors. University of California Press; 2004. Oviraptorosauria. [Google Scholar]
- Pittman M., O'connor J., Field D.J., Turner A.H., Ma W., Makovicky P., Xu X. In: Pennaraptoran Theropod Dinosaurs: Past Progress and New Frontiers. Pittman M., Xu X., editors. Bulletin of the American Museum of Natural History; 2020. Pennaraptoran systematics. [Google Scholar]
- Pu H., Zelenitsky D.K., Lü J., Currie P.J., Carpenter K., Xu L., Koppelhus E.B., Jia S., Xiao L., Chuang H. Perinate and eggs of a giant caenagnathid dinosaur from the Late Cretaceous of central China. Nat. Commun. 2017;8:14952. doi: 10.1038/ncomms14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn H., Ar A., Paganelli C.V. How bird eggs breathe. Sci. Am. 1979;240:46–55. [Google Scholar]
- Reisz R.R., Scott D., Sues H.-D., Evans D.C., Raath M.A. Embryos of an Early Jurassic prosauropod dinosaur and their evolutionary significance. Science. 2005;309:761–764. doi: 10.1126/science.1114942. [DOI] [PubMed] [Google Scholar]
- Reisz R.R., Evans D.C., Sues H.-D., Scott D. Embryonic skeletal anatomy of the sauropodomorph dinosaur Massospondylus from the Lower Jurassic of South Africa. J. Vertebr. Paleontol. 2010;30:1653–1665. [Google Scholar]
- Rideout B.A. Investigating embryo deaths and hatching failure. Vet. Clin. Exot. Anim. Pract. 2012;15:155–162. doi: 10.1016/j.cvex.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Rowe T. 2003. Gallus gallus (Online). Digital Morphology. [Google Scholar]
- Tanaka K., Zelenitsky D.K., Therrien F. Eggshell porosity provides insight on evolution of nesting in dinosaurs. PLoS One. 2015;10:e0142829. doi: 10.1371/journal.pone.0142829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio D.J., Jackson F.D. Reproduction in Mesozoic birds and evolution of the modern avian reproductive mode. Auk. 2016;133:654–684. [Google Scholar]
- Varricchio D.J., Horner J.R., Jackson F.D. Embryos and eggs for the Cretaceous theropod dinosaur Troodon formosus. J. Vertebr. Paleontol. 2002;22:564–576. [Google Scholar]
- Wang S., Zhang S.K., Sullivan C., Xu X. Elongatoolithid eggs containing oviraptorid (Theropoda, Oviraptorosauria) embryos from the upper Cretaceous of southern China. BMC Evol. Biol. 2016;16:67. doi: 10.1186/s12862-016-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishampel D.B., Fastovsky D.E., Watabe M., Varricchio D., Jackson F., Tsogtbaatar K., Barsbold R. New oviraptorid embryos from Bugin-Tsav, Nemegt Formation (Upper Cretaceous), Mongolia, with insights into their habitat and growth. J. Vertebr. Paleontol. 2008;28:1110–1119. [Google Scholar]
- Wen C.H., Liu X.H., Lü B., Mao X.G., Chen J.S., Hou S.M., Zhou Z.B., Hou J.L., Wu H.B. The Cretaceous redbeds in Shicheng Basin, Jiangxi province: pedogenic and paleoenvironmental characteristics. Quat. Sci. 2016;36:1403–1416. [Google Scholar]
- Wiemann J., Yang T.-R., Norell M.A. Dinosaur egg colour had a single evolutionary origin. Nature. 2018;563:555–558. doi: 10.1038/s41586-018-0646-5. [DOI] [PubMed] [Google Scholar]
- Xi D., Wan X., Li G., Li G. Cretaceous integrative stratigraphy and timescale of China. Sci. China Earth Sci. 2019;62:256–286. [Google Scholar]
- Xie A.Z. Discussion about subdivide of Guifeng group and sedimentary system and character of facical model in Late Cretaceous in Xinjiang Basin. J. East China Geol. Inst. 2001;24:5–10. [Google Scholar]
- Xu X., Zhou Z., Dudley R., Mackem S., Chuong C.-M., Erickson G.M., Varricchio D.J. An integrative approach to understanding bird origins. Science. 2014;346:1253293. doi: 10.1126/science.1253293. [DOI] [PubMed] [Google Scholar]
- Yang T.-R., Engler T., Lallensack J.N., Samathi A., Makowska M., Schillinger B. Hatching asynchrony in oviraptorid dinosaurs sheds light on their unique nesting biology. Integr. Organismal. Biol. 2019;1:obz030. doi: 10.1093/iob/obz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.-R., Wiemann J., Xu L., Cheng Y.-N., Wu X.-C., Sander P.M. Reconstruction of oviraptorid clutches illuminates their unique nesting biology. Acta Palaeontol. Pol. 2019;64:581–596. [Google Scholar]
- Zelenitsky D.K. Reproductive traits of non-avian theropods. J. Paleontol. Soc. Korea. 2006;22:209. [Google Scholar]
- Zelenitsky D.K., Therrien F. Phylogenetic analysis of reproductive traits of maniraptoran theropods and its implications for egg parataxonomy. Palaeontology. 2008;51:807–816. [Google Scholar]
- Zelenitsky D.K., Modesto S.P., Currie P.J. Bird-like characteristics of troodontid theropod eggshell. Cretac. Res. 2002;23:297–305. [Google Scholar]
- Zhao Z. Microstructure of the dinosaurian eggshells of Nanxiong, Guangdong, and the problems in dinosaur egg classification. Vertebr. Palasiat. 1975;13:105–117. [Google Scholar]
- Zhou Z., Zhang F. A precocial avian embryo from the Lower Cretaceous of China. Science. 2004;306:653. doi: 10.1126/science.1100000. [DOI] [PubMed] [Google Scholar]
- Zhou Z.-H., Wang X.-L., Zhang F.-C., Xu X. Important features of Caudipteryx - evidence from two nearly complete new specimens. Vertebr. Palasiat. 2000;38:241–254. [Google Scholar]
- Zuo Y., Wu J., Zhou W. Lithostratigraphic division of volcanic terrain in late Mesozoic in Jiangxi. Geol. Rev. 1999;45:742–750. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Dataset used in this study has been deposited at Zenodo. DOI is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information is available from the lead contact upon request.