Abstract
Background
Several studies have recently suggested that liver disease and cirrhosis were risk factors for poor outcomes in patients with coronavirus disease 2019 (COVID-19) infections. However, no large data study has reported the clinical course of COVID-19 patients with chronic hepatitis B virus (HBV) infections. This study investigated whether HBV infection had negative impacts on the clinical outcomes of COVID-19 patients.
Methods
We performed a nationwide population-based cohort study with 19,160 COVID-19-infected patients in 2020 from the Korean Health Insurance Review and Assessment database. The clinical outcomes of COVID-19 patients with chronic HBV infections were assessed and compared to those of non-HBV-infected patients.
Results
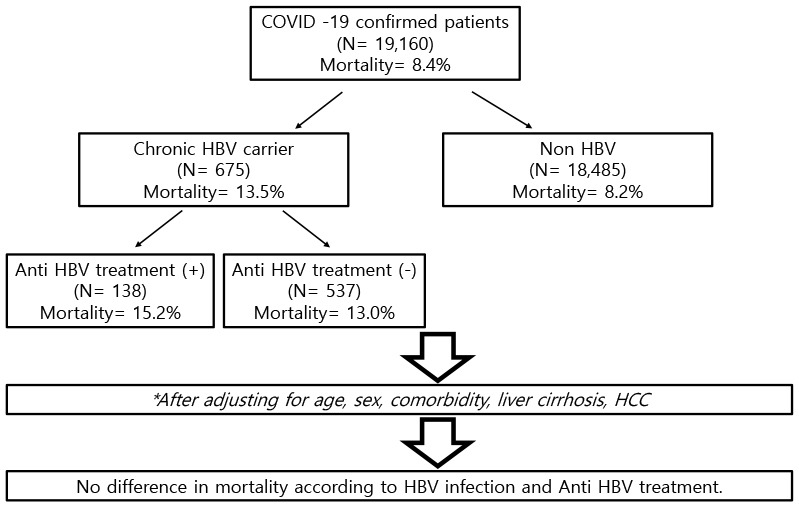
Of the 19,160 patients diagnosed with COVID-19, 675 (3.5%) patients had chronic HBV infections. The HBV-infected patients were older and had more commodities than the non-HBV infected COVID-19 patients. During the observation period, COVID-19-related mortality was seen in 1,524 (8.2%) of the non-HBV-infected 18,485 patients, whereas 91 (13.5%) in HBV-infected 675 patients died of COVID-19 infection. Compared to patients without HBV infections, a higher proportion of patients with chronic HBV infections required intensive care unit (ICU) admission and had organ failures. However, odds ratios for mortality, ICU admission, and organ failure were comparable between the two groups after adjusting for age, sex, and comorbid diseases including liver cirrhosis and hepatocellular carcinoma.
Conclusion
COVID-19-infected patients with HBV infections showed worse clinical courses than non-HBV-infected COVID-19 patients. However, after adjustment, chronic HBV infection itself does not seem to affect the clinical outcomes in COVID-19 patients.
Keywords: Coronavirus Disease 2019, Chronic Hepatitis B, Risk Factor, SARS-CoV-2, Coronavirus, COVID-19, Antiviral
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which is an RNA virus first reported in humans in December 2019 that rapidly spread worldwide and has resulted in a pandemic crisis. It was already well known that COVID-19 patients with comorbidities including diabetes, hypertension, chronic obstructive pulmonary disease, and cardiovascular disease had poor prognoses.1,2 Relatively, data on the effect of COVID-19 in patients with underlying liver diseases have been scarce. However, several studies on COVID-19 and chronic liver disease were published recently.3,4,5 Most studies showed that patient prognosis was poor in the presence of chronic liver disease.6,7 The clinical course of COVID-19 was influenced by the causes of chronic liver disease including viral hepatitis, nonalcoholic steatohepatitis, alcoholic fatty liver, the use of immunosuppressive agents in liver transplant recipients, and autoimmune hepatitis.8,9 As for viral hepatitis, most studies have focused on patients with hepatitis C virus.10 However, there are very few reports on the prognosis of COVID-19 in hepatitis B virus (HBV) carriers.9,11 In addition, there are no reports on whether there is a difference in the prognosis of patients undergoing treatment for HBV. Although many studies are underway to develop a treatment for COVID-19, no treatment has been proven effective yet. Recently, several experimental studies reported that nucleos(t)ide reverse transcriptase inhibitors (NRTIs) for HBV treatment were effective against COVID-19 through potential antiviral treatment mechanisms.11 Unlike Western countries, the prevalence of chronic HBV patients in Korea is high (4%), and there are many chronic HBV patients taking antiviral drugs for HBV. Therefore, the relatively greater number of HBV patients in Korea made it a favorable situation in which to confirm whether HBV infection status and antiviral therapy for HBV infections affected the prognosis of patients with COVID-19.
In this study, we aimed to report the clinical course of COVID-19 patients with chronic HBV infections, and also to determine whether the use of antiviral agents for HBV infections had an effect on the clinical course of COVID-19.
METHODS
Data source and collection
In this COVID-19 pandemic, studies using real-world clinical data are not easy because the patients have a variety of clinical outcomes, health conditions, and accessibility to healthcare institutions. Therefore, we decided to use a nationwide database of de-identified COVID-19 patient data from the Health Insurance Review and Assessment (HIRA) service database. The database contains claims records for all individuals who were tested for COVID-19 and hospitals that issued claims to the HIRA through October 31, 2020. The database includes detailed information regarding demographic characteristics, diagnoses, prescriptions, procedures, patient clinical outcomes, and survival. The diagnostic codes are assigned based on the Korean Classification of Diseases, seventh revision (KCD-7), which is a modified version of the tenth revision of the International Classification of Diseases (ICD-10). The use of drugs was identified using Anatomical Therapeutic Chemical codes and HIRA general name codes.
Patient definition and clinical outcomes
We identified COVID-19 patients using the following KCD-7/ICD-10 codes: B34.2 (coronavirus infection, unspecified site), B97.2 (coronavirus as the cause of diseases classified to other chapters), U18 (provisional assignment of new diseases of uncertain etiology or emergency use), and U07.1 (COVID-19). These diagnostic codes were given only if a definitive diagnosis of COVID-19 was made based on positive nasopharyngeal swab specimens tested with real-time reverse transcription-polymerase chain reaction assays.12 This definition was used in other similar studies, and its usefulness was confirmed.13 The index date was defined as the date of the COVID-19 diagnosis. All patients were followed until December 31, 2020.
Comorbidities were defined based on claims codes within one year before the index date and evaluated based on codes revised using the Charlson comorbidity index (Supplementary Table 1).14 Procedure codes were also used to identify cases that involved mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and continuous renal replacement therapy (CRRT) (Supplementary Table 2). Survival and the in-hospital stay length were defined as the primary study outcome. The secondary outcomes included the use of ventilation, ECMO, renal replacement therapy, and liver failure.
Anti-viral agents for HBV
This study identified all anti-viral agents for HBV patients including approved available drugs in Korea that were prescribed within one month before the index date. For the present study, anti-viral agent users were defined as patients with anti-viral agent use one month before the index date with estimated drug compliance of over 70%, whereas non-users were defined as patients who had never received antiviral agents or had received them three months before the index date. The detailed types and codes for antiviral agents are described in Supplementary Table 3.
Statistical analysis
Categorical and continuous variables are presented as counts, percentages, and mean ± SDs. The clinical characteristics were compared by the t-test for continuous variables and the χ2-Fisher’s exact test for categorical variables. Multivariate logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs). All tests were two-sided, and P values of < 0.05 were considered statistically significant. The analyses were performed with program R.
Ethics statement
The study protocol for the analysis of de-identified patient data was exempted from review by the Institutional Review Board (IRB) of Korea University Ansan Hospital (IRB No. 2021AS0062).
RESULTS
Baseline characteristics
By the end of October 2020, a total of 19,160 COVID-19 patients were enrolled, of which 675 patients were HBV-infected patients. The mean age of all patients was 52.8 years, of which males comprised 47.3%. The mean age of the HBV-infected patients was 59.3 years, which was higher than that of the non-HBV-infected patients at 52.6 years (P < 0.005). Overall, the HBV-infected patients had more comorbidities compared to the non-HBV-infected patients. Diabetes mellitus was a co-morbidity in 58.5% of the HBV-infected patients vs. 34.7% of the non-HBV-infected patients, and 49.8% vs. 37.3% had hypertension, 15.7% vs. 15.5% had cerebrovascular disease, 40.7% vs. 31.6% had chronic pulmonary disease, 9.5% vs. 3.9% had chronic renal disease, 19.1% vs. 1.3% had liver cirrhosis, 23.0% vs. 1.9% had liver cancer, and 46.1% vs. 14.3% had any other malignancies except liver cancer, respectively (Table 1).
Table 1. Baseline characteristics of the enrolled COVID-19-infected patients according to HBV infection with or without antiviral agent treatment.
| Characteristics | All patients (n = 19,160) | HBV-infected (n = 675) | Not HBV-infected (n = 18,485) | P value | Anti-viral agents user (n = 138) | Anti-viral agents non-user (n = 537) | P value | |
|---|---|---|---|---|---|---|---|---|
| Age, yr | 52.8 ± 12.5 | 59.3 ± 15.7 | 52.6 ± 21.7 | < 0.001 | 58.5 ± 12.0 | 59.5 ± 16.5 | 0.170 | |
| Range | < 0.001 | 0.009 | ||||||
| ≤ 39 | 5,604 (29.2) | 80 (11.9) | 5,524 (29.9) | 10 (7.2) | 70 (13.0) | |||
| 40–65 | 7,695 (40.2) | 355 (52.6) | 7,340 (39.7) | 88 (63.8) | 267 (49.7) | |||
| ≥ 65 | 5,861 (30.6) | 240 (35.6) | 5,621 (30.4) | 40 (29.0) | 200 (37.3) | |||
| Sex | 0.290 | 0.017 | ||||||
| Male | 9,065 (47.3) | 333 (49.3) | 8,732 (47.2) | 81 (58.7) | 252 (46.9) | |||
| Female | 10,095 (52.7) | 342 (50.7) | 9,753 (52.8) | 57 (41.3) | 285 (53.1) | |||
| Comorbidity | ||||||||
| Diabetes mellitus | 6,804 (35.5) | 395 (58.5) | 6,409 (34.7) | < 0.001 | 71 (51.5) | 324 (60.3) | 0.066 | |
| Hypertension | 7,239 (37.8) | 336 (49.8) | 6.903 (37.3) | < 0.001 | 62 (44.9) | 274 (51.0) | 0.215 | |
| Cerebrovascular disease | 3,001 (15.7) | 141 (20.9) | 2,860 (15.5) | < 0.001 | 24 (17.4) | 117 (21.8) | 0.291 | |
| Chronic pulmonary disease | 6,120 (31.9) | 275 (40.7) | 5,845 (31.6) | < 0.001 | 41 (29.7) | 234 (43.6) | 0.003 | |
| Chronic renal disease | 781 (4.1) | 64 (9.5) | 717 (3.9) | < 0.001 | 8 (5.8) | 56 (10.4) | 0.105 | |
| Liver cirrhosis | 377 (2.0) | 129 (19.1)) | 248 (1.3) | < 0.001 | 64 (46.4) | 65 (12.1) | < 0.001 | |
| Cancer | 2,947 (15.4) | 311 (46.1) | 2,636 (14.3) | < 0.001 | 92 (66.7) | 219 (40.8) | < 0.001 | |
| Hepatocellular carcinoma | 498 (2.6) | 155 (23.0) | 343 (1.9) | < 0.001 | 67 (48.6) | 88 (16.4) | < 0.001 | |
Data are shown as number (%) or mean ± SD. P values were calculated using the student’s t-test or the χ2 or Fischer exact test, as appropriate.
COVID-19 = coronavirus disease 2019, HBV = hepatitis B virus.
Clinical outcomes of COVID-19-infected patients according to HBV infection
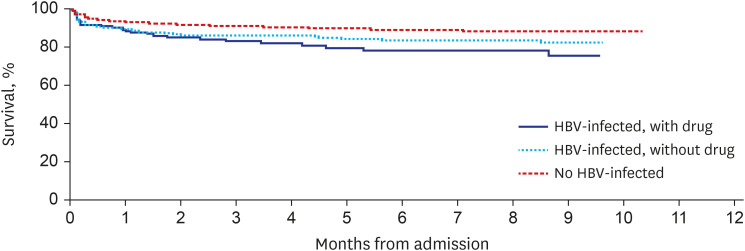
Among the total patients, 91 out of the 675 HBV patients died (13.5%) while, compared with 1524 out of the 18,486 non-HBV patients (8.2%) were dead. In addition, the medical history at admission to the intensive care unit (ICU) and the treatment for organ failure was significantly higher (Table 2). Fig. 1 shows the Kaplan-Meyer curve for overall survival compared according to HBV infection status. During an approximate 10-month follow-up period, the survival rate of the COVID-19 patients was 91.6%, of which the HBV patients comprised 86.5%, which was statistically significantly lower than that of the non-HBV-infected patients.
Table 2. Clinical outcomes and complications of the enrolled COVID-19-infected patients according to HBV infection with or without antiviral agent treatment.
| Clinical outcomes | Overall patients (n = 19,160) | HBV-infected patients (n = 675) | Non HBV-infected patients (n = 18,845) | P value | Anti-viral agents user (n = 138) | Anti-viral agents non-user (n = 537) | P value | |
|---|---|---|---|---|---|---|---|---|
| Death | 1,615 (8.4) | 91 (13.5) | 1,524 (8.2) | < 0.001 | 21 (15.2) | 70 (13.0) | 0.487 | |
| In-hospital stay length, days | 20.5 ± 12.9 | 19.9 ± 12.7 | 20.5 ± 12.9 | 0.259 | 19.8 ± 13.0 | 19.9 ± 12.7 | 0.914 | |
| ICU admission | 1,483 (7.7) | 85 (12.6) | 1,398 (7.6) | < 0.001 | 14 (10.1) | 71 (13.2) | 0.389 | |
| Complications | ||||||||
| Liver failure | 68 (0.4) | 13 (1.9) | 55 (0.3) | < 0.001 | 6 (4.3) | 7 (1.3) | 0.032 | |
| MV and ECMO | 971 (5.1) | 54 (8.0) | 917 (5.0) | 0.001 | 4 (2.9) | 50 (9.3) | 0.013 | |
| AKI | 635 (3.3) | 34 (5.0) | 601 (3.3) | 0.015 | 3 (2.2) | 31 (5.8) | 0.123 | |
| CRRT | 190 (1.0) | 19 (2.8) | 171 (0.9) | < 0.001 | 3 (2.2) | 16 (3.0) | 0.778 | |
Data are shown as number (%) or mean ± SD. P values were calculated using the student’s t-test or the χ2 or Fischer exact test, as appropriate.
COVID-19 = coronavirus disease 2019, HBV = hepatitis B virus, ICU = intensive care unit, MV = mechanical ventilation, ECMO = extracorporeal membrane oxygenation, AKI = acute kidney injury, CRRT = continuous renal-replacement therapy.
Fig. 1. Kaplan-Meier curve of overall survival in COVID-19-infected patients compared according to HBV infection status. The HBV-infected patients were divided into those treated with or without anti-viral agents.
COVID-19 = coronavirus disease 2019, HBV = hepatitis B virus.
In the analysis of overall mortality, HBV patients had a high mortality rate, and many patients were hospitalized in the ICU or received intensive treatment. However, after adjusting for age, sex, cirrhosis, and comorbid diseases, no statistical differences between the two groups were observed in all cause mortality, long in-hospital stay length (≥ 30 days), the ICU admission rate, progression to liver failure, the requirement for mechanical ventilator or ECMO, and CRRT for acute kidney injury (Table 3).
Table 3. Relative risk of overall clinical outcomes of COVID-19 according to HBV infection and antiviral agent treatment.
| Clinical outcomes | No. of patients | No. of events | Events rate | Crude OR (95% CI) | P value | Adjusted ORa (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | ||||||||
| Non HBV-infected | 18,485 | 1,524 | 8.2 | 1 | 1 | |||
| HBV-infected, with antiviral agents | 537 | 70 | 13.0 | 1.67 (1.28–2.14) | < 0.001 | 0.91 (0.68–1.21) | 0.545 | |
| HBV-infected, without antiviral agents | 138 | 21 | 15.2 | 2.00 (1.22–3.12) | 0.004 | 0.95 (0.54–1.61) | 0.867 | |
| Composite (mortality, ICU admission, liver failure, MV/ECMO, AKI/CRRT) | ||||||||
| Non HBV-infected | 18,485 | 2,552 | 13.8 | 1 | 1 | |||
| HBV-infected, with antiviral agents | 537 | 116 | 21.6 | 1.72 (1.39–2.11) | < 0.001 | 0.97 (0.76–1.23) | 0.823 | |
| HBV-infected, without antiviral agents | 138 | 33 | 23.9 | 1.83 (1.22–2.67) | 0.002 | 0.92 (0.58–1.44) | 0.720 | |
| ICU admission | ||||||||
| Non HBV-infected | 18,485 | 1,398 | 7.6 | 1 | 1 | |||
| HBV-infected, with antiviral agents | 537 | 71 | 13.2 | 1.86 (1.43–2.39) | < 0.001 | 1.22 (0.92–1.60) | 0.160 | |
| HBV-infected, without antiviral agents | 138 | 14 | 10.1 | 1.38 (0.76–2.32) | 0.256 | 0.96 (0.50–1.70) | 0.896 | |
| Long in-hospital stay length (≥ 30 days) | ||||||||
| Non HBV-infected | 18,485 | 3,508 | 19.0 | 1 | 1 | |||
| HBV-infected, with antiviral agents | 537 | 99 | 18.4 | 0.96 (0.77–1.20) | 0.752 | 1.03 (0.82–1.29) | 0.791 | |
| HBV-infected, without antiviral agents | 138 | 24 | 17.4 | 0.90 (0.56–1.37) | 0.636 | 1.08 (0.67–1.69) | 0.730 | |
COVID-19 = coronavirus disease 2019, HBV = hepatitis B virus, ICU = intensive care unit, MV = mechanical ventilation, ECMO = extracorporeal membrane oxygenation, AKI = acute kidney injury, CRRT = continuous renal-replacement therapy, OR = odds ratio, CI = confidence interval.
aAdjusted for age, sex, diabetes mellitus, hypertension, cerebrovascular disease, chronic respiratory disease, chronic renal failure, liver cirrhosis, malignancies.
Clinical outcomes of COVID-19-infected patients according to current antiviral treatment for HBV
Among the HBV patients, antiviral treatment did not reduce mortality or the length of in-hospital stays. However, the proportion of patients who progressed to liver failure was significantly higher in the antiviral treatment group. The number of patients with critical respiratory tract illnesses was lower in the antiviral-treated group with statistical significance (Table 2).
DISCUSSION
This study investigated the clinical outcomes of patients with chronic HBV infections using data from the HIRA Service. In COVID-19-infected patients, those with HBV infections had worse clinical prognoses than the HBV non-infected patients. However, HBV infection itself did not seem to affect the clinical outcomes of patients with COVID-19. In addition, antiviral drugs for HBV did not affect the clinical prognosis of patients with COVID-19.
Recently, SARS-CoV-2 infections were identified by the spike protein of SARS-CoV-2 in the cytosol of the huh-7 cell of the hepatoma cell line, the observation of ACE2 in cholangiocytes and hepatocytes by single-cell sequencing, and the detection of traces of SARS-CoV-2 in the liver of patients who died of COVID-19.15,16,17 The findings could indicate that the human liver can be used as a habitat for SARS-CoV-2 replication and activation. The histological examination of livers from deceased COVID-19 patients showed widespread vascular abnormalities, steatosis, and mitochondrial abnormalities, which were thought to be caused by SARS-CoV-2.18 Several studies reported that the prognosis of patients with COVID-19 infections was worse in patients with accompanying chronic liver disease, which lead to reduced liver function.3,18,19 This study also showed that the mortality rate of COVID-19 infections in HBV carriers was significantly higher than that of non-HBV-infected patients during the observation period. Compared to the non-HBV group, more patients in the HBV group were admitted to the ICU, progressed to liver failure, and required CCRT, mechanical ventilation, and ECMO.
However, the inflammation associated with chronic HBV infections causes liver cirrhosis and liver cancer, and patients with decreased liver function due to cirrhosis and liver cancer have poor prognoses. Therefore, the study data were analyzed by adjusting for HBV-related complications and other comorbidities. The results confirmed that HBV infection itself did not affect the prognosis of patients infected with COVID-19. This was also confirmed by the fact that there was no difference in the prognosis of COVID-19 patients receiving and not receiving current HBV treatment. Thus, it is thought that there is no direct relationship between HBV and the COVID-19 virus. This is consistent with the other studies with a relatively small number of patients that HBV itself did not affect the clinical outcomes of the COVID-19 after adjusting for comorbidities.9,11,18
Through this study, additional analysis was performed on whether antiviral drugs for HBV affected the clinical course of COVID-19. Several COVID-19 treatment attempts have recently been made, one of which is drug repositioning.20 This is used to check the feasibility “of treating patients by recycling a drug that is expected to have a therapeutic effect on the COVID-19 virus by considering the mechanisms of drugs used in the treatment of other viruses. Antiviral drugs for HBV could be a drug repositioned for COVID-19 virus treatment. HBV treatment drugs are based on the mechanism of inhibiting viral RNA synthesis. Many clinical studies have shown that the mechanism is effective in the treatment of the COVID-19 virus, and related research is ongoing.21,22 However, in this study, there was no difference in the clinical course of patients taking hepatitis B antiviral drugs compared to patients not taking antiviral drugs. Although there was no statistical significance, the patient group taking antiviral drugs for HBV had a worse mortality rate. This result seems to be because expense of antiviral drugs are covered only in chronic HBV infection patients with abnormal liver function tests, cirrhosis, or hepatocellular carcinoma by national health insurance system in Korea. These patients are more likely to progress to liver failure.
It was noteworthy that the proportion of patients with severe respiratory distress was significantly lower in the antiviral treatment group compared to the non-treated group. Many studies have suggested that anti-RNA-dependent RNA polymerase (RdRp) drugs can be used for COVID-19 virus treatment. NRTIs, such as tenofovir, abacavir, and lamivudine used for HBV treatment are potent inhibitors of RdRp. The drugs can tightly bind to the RdRp of the COVID-19 virus strain and thus, may prevent severe acute respiratory distress. Therefore, as in our study, patients taking antiviral drugs for HBV seemed to have fewer respiratory complications. However, further research is needed to confirm the causal relationship.
There were some limitations in this study. It was difficult to provide detailed clinical information on the patients because of the use of claims data. Performing detailed analysis was not allowed because sensitive personal information such as detailed clinical information, history, lifestyle, and the exact death cause of the patient was omitted. Since the direct cause of death cannot be confirmed in the data through HIRA, it is not possible to accurately confirm COVID-19 related mortality. And there may be diagnostic errors or claims code errors in this kind of data. For these reasons, it seems that the mortality rate in our study was rather higher than the mortality rate known so far. Data on HBV medication compliance and HBV DNA titers were also not provided. So, since we do not know about the activity of HBV, it seems that it cannot be seen as showing the exact relationship between HBV infection status and COVID-19. In addition, we did not fully take into account other various liver disease, such as chronic hepatitis C virus infection, alcoholic liver disease, and nonalcoholic fatty liver disease, which may affect liver-related adverse outcomes of COVID 19. Future studies should investigate the effect of HBV on clinical outcome of COVID-19 after adjusting chronic liver diseases caused by other causes.
Nevertheless, it is important to note that this study showed the clinical course of COVID-19 in a relatively large number of patients with chronic HBV, and in patients with anti-viral HBV treatment.
In conclusion, this study found a relatively high mortality rate, longer in-hospital stays, and a higher rate of intensive treatment in the ICU in chronic HBV patients infected with COVID-19. However, after adjustment, HBV infection itself seems not to affect clinical outcomes in patients with COVID-19. In addition, antiviral therapy for HBV did not reduce the mortality.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung YK.
- Data curation: Seo GH.
- Formal analysis: Seo GH.
- Methodology: Jung YK, Seo GH.
- Writing - original draft: Jung YK, Choe JW.
- Writing - review & editing: Yim HJ.
SUPPLEMENTARY MATERIALS
Charlson Comorbidity Index and associated KCD-7/ICD-10 codes
National Procedure Codes
Definition and claims codes for HBV infections and anti-viral agents
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YR, Kang MK, Song JE, Kim HJ, Kweon YO, Tak WY, et al. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: a multicenter study in South Korea. Clin Mol Hepatol. 2020;26(4):562–576. doi: 10.3350/cmh.2020.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73(3):705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Y, Pan H, She Q, Wang F, Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5(6):528–529. doi: 10.1016/S2468-1253(20)30080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, et al. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40(10):2394–2406. doi: 10.1111/liv.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS, 4th, Armstrong MJ, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74(6):1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2021;19(7):1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt AA, Yan P, Chotani RA, Shaikh OS. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021;41(8):1824–1831. doi: 10.1111/liv.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SH, Cho DH, Choi J, Baik SK, Gwon JG, Kim MY. Association between chronic hepatitis B infection and COVID-19 outcomes: a Korean nationwide cohort study. PLoS One. 2021;16(10):e0258229. doi: 10.1371/journal.pone.0258229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with coronavirus disease 2019 (COVID-19)- related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. 2020;71(16):2121–2128. doi: 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40(8):2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40(9):2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14(5):690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 21.Malik S, Gupta A, Zhong X, Rasmussen TP, Manautou JE, Bahal R. Emerging therapeutic modalities against COVID-19. Pharmaceuticals (Basel) 2020;13(8):188. doi: 10.3390/ph13080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang JH, Kim JW, Jeong SH, Myung HJ, Kim HS, Park YS, et al. Clevudine for chronic hepatitis B: antiviral response, predictors of response, and development of myopathy. J Viral Hepat. 2011;18(2):84–90. doi: 10.1111/j.1365-2893.2010.01281.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Charlson Comorbidity Index and associated KCD-7/ICD-10 codes
National Procedure Codes
Definition and claims codes for HBV infections and anti-viral agents