Abstract
Analgesic tolerance to opioids contributes to the opioid crisis by increasing the quantity of opioids prescribed and consumed. Thus, there is a need to develop non-opioid-based pain-relieving regimens as well as strategies to circumvent opioid tolerance. Previously, we revealed a non-opioid analgesic mechanism induced by median nerve electrostimulation at the overlaying PC6 (Neiguan) acupoint (MNS-PC6). Here, we further examined the efficacy of MNS-PC6 in morphine-tolerant mice with neuropathic pain induced by chronic constriction injury (CCI) of the sciatic nerve. Daily treatments of MNS-PC6 (2 Hz, 2 mA), but not electrostimulation at a non-median nerve-innervated location, for a week post-CCI induction significantly suppressed established mechanical allodynia in CCI-mice in an orexin-1 (OX1) and cannabinoid-1 (CB1) receptor-dependent fashion. This anti-allodynic effect induced by repeated MNS-PC6 was comparable to that induced by repeated gabapentin (50 mg/kg, i.p.) or single morphine (10 mg/kg, i.p.) treatments, but without tolerance, unlike repeated morphine-induced analgesia. Furthermore, single and repeated MNS-PC6 treatments remained fully effective in morphine-tolerant CCI-mice, also in an OX1 and CB1 receptor-dependent fashion. In CCI-mice receiving escalating doses of morphine for 21 days (10 mg/kg, 20 mg/kg and 50 mg/kg), single and repeated MNS-PC6 treatments remained fully effective. Therefore, repeated MNS-PC6 treatments induce analgesia without tolerance, and retain efficacy in opioid-tolerant mice via a mechanism that involves OX1 and CB1 receptors. This study suggests that MNS-PC6 is an alternative pain management strategy that maybe useful for combatting the opioid epidemic, and opioid-tolerant patients receiving palliative care.
Perspectives:
Median nerve stimulation relieves neuropathic pain in mice without tolerance and retains efficacy even in mice with analgesic tolerance to escalating doses of morphine, via an opioid-independent, orexin-endocannabinoid-mediated mechanism. This study provides a proof of concept for utilizing peripheral nerve stimulating devices for pain management in opioid-tolerant patients.
Keywords: Median nerve stimulation, neuropathic pain, morphine tolerance, orexins, endocannabinoids
INTRODUCTION
Opioids are commonly prescribed for postoperative pain77 or severe neuropathic pain.75 However, many patients continue to consume opioids well after surgery21 due to continued pain18 or postsurgical opioid over-prescription.5, 40 Analgesic tolerance developing after repeated opioid consumption7 further increases the risk of developing opioid dependence, withdrawal and/or overdose.38, 68 Therefore, unresolved severe chronic pain or opioid over-prescription may lead to opioid use disorder,22, 23 contributing to the ongoing opioid crisis.51, 64 Opioids are also used for relieving cancer pain where intolerable side effects induced by escalating doses of opioids due to analgesic tolerance in palliative care remain an unsolved clinical issue.9, 70 Therefore, it is an urgent need to develop alternative non-opioid pain management strategies as well as to develop strategies to reduce opioid tolerance.
Cannabinoids, having analgesic activity by acting at CB1 (CB1Rs) and/or CB2 cannabinoid receptors48 have been proposed as opioid-sparing agents to reduce opioid use.41 However, the efficacy of phyto- or synthetic cannabinoids for pain relief in humans remains a contentious issue due to modest efficacy and moderate adverse effects.11, 25, 27, 47, 54 An alternative strategy is to increase the levels of endocannabinoids (eCBs), such as 2-arachidonoylglycerol (2-AG) and anandamide (AEA), via inhibiting their respective degrading enzymes, monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH).76 However, a clinical trial with an FAAH inhibitor for chronic pain management was unsuccessful28 in spite of promising results in preclinical studies.26, 49 MAGL inhibitors also effectively reduced neuropathic pain and opioid tolerance in mice72, 76 but their effectiveness remains unknown in humans. Alternatively, a non-pharmacological method that can increase eCBs, which are synthesized on demand, may be an immediate solution to address the urgent issues associated with the opioid crisis.
We have previously revealed a non-opioid analgesic mechanism that can be induced by median nerve electro-stimulation (MNS) in mice.6 The PC6 (Neiguan) acupoint, located overlying the median nerve on the anterior forearm,69 was chosen as a standardized location to insert the acupuncture needle for MNS. We have proven that this procedure induced an analgesic effect equivalent to that induced by direct MNS in mice, and thus referred to the procedure as MNS-PC6 and MNS-PC6-induced analgesia as MNS-PC6-IA.6 We found that MNS-PC6 can activate orexin neurons in the lateral hypothalamus (LH), releasing orexins into the ventrolateral periaqueductal gray (vlPAG), the midbrain region that initiates descending pain inhibition. Orexins then activate the postsynaptic OX1 receptor (OX1R), a Gq protein-coupled receptor, in the vlPAG, resulting in 2-AG synthesis. 2-AG then produces retrograde inhibition of GABA release by activating presynaptic CB1Rs, leading to disinhibition of the vlPAG and ultimately inducing analgesia.6, 24 This analgesic effect, mediated by the OX1R-initiated-eCB-CB1R signaling pathway,24 is opioid-independent since it was resistant to naloxone or naltrexone at doses that completely abolished opioid analgesia.6 Thus, MNS-PC6 may provide an alternative analgesic strategy in opioid-tolerant patients.
Pioneered by Wall and Sweet,67 electrical stimulation at peripheral nerves by implanting electrodes has been reported to provide satisfactory long-term pain relief in patients with chronic pain.14, 36 Very recently, non-invasive stimulating devices via peripheral neuromodulation have been cleared by U.S. Food and Drug Administration (FDA) for the treatment chronic pain.58, 60, 61 However, no studies have been conducted to examine the effect of peripheral neuromodulation in opioid-tolerant individuals. Thus, we aim to provide a proof-of-concept preclinical animal study on the potential application of peripheral neuromodulation, via MNS-PC6, for chronic pain management in opioid-tolerant patients.
Previously, we have demonstrated that an opioid-independent, but orexin- and eCB-dependent, analgesic effect can be induced by a single administration of MNS-PC6 in both acute nociceptive and chronic neuropathic pain models.6 Here, using the same MNS-PC6 protocol and the same chronic pain model, we first examined whether repeated administration of MNS-PC6 could attenuate chronic constriction injury (CCI)-induced neuropathic pain in mice and whether repeated MNS-PC6-IA was devoid of tolerance. Subsequently, MNS-PC6 was administered to morphine-tolerant CCI-mice. For mechanistic studies, mice were pretreated with OX1R and CB1R antagonists, respectively, to ascertain the involvement of the OX1R-initiated eCB-CB1R signaling cascade6, 24 in repeated MNS-PC6-IA in morphine-tolerant CCI-mice. Lastly, to model morphine-tolerant patients more closely, MNS-PC6-IA was investigated in CCI-mice made highly tolerant to morphine by receiving escalating doses of the opioid.
METHODS
Animals
Male adult C57BL/6 mice aged 8–10 weeks (25–35 g) purchased from National Laboratory Animal Center, Taiwan, were used for all experiments. Female mice of a similar age were also used in a pilot study to examine possible sex difference of MNS-PC6-IA. All experiments adhered to the guidelines approved by the Institutional Animal Care and Use Committees in the College of Medicine, National Taiwan University, in accordance to the ARRIVE guidelines. Mice were housed in groups of 5 in plastic cages and maintained in a holding room with a 12 h light-dark cycle with free access to food and water ad libitum. On the experimental day, mice were moved in their home cages to the behaviour room and acclimated there for at least 1 h before testing. All experiments were conduction from 9 am to 6 pm.
Chronic constriction injury (CCI) surgery in mice
Mice were randomly divided into CCI and non-CCI groups. The CCI group received the CCI surgery as reported previously.6, 34 Briefly, the right sciatic nerve of the mouse, under anesthesia with intraperitoneal (i.p.) injection of sodium pentobarbital (80 mg/kg), was exposed and loosely ligated with a 4.0 silk suture. The incision was then closed, and the mouse was returned to its home cage. The non-CCI control group received the same surgical procedures, except sciatic nerve ligation.
Assessment of neuropathic pain
Neuropathic pain was well-established 7 days after the CCI surgery, as evidenced by mechanical allodynia in the hind paw of the injured side.43 The mechanical allodynic response was evaluated as reported before.6, 19 We measured the percentage of positive responses (withdrawal, flinching or licking) in a total of ten repetitive stimulations with a von Frey filament exerting 0.16 g of force onto the plantar surface of the paw on the injured side, in triplicates.
The MNS-PC6 procedure
For conducting the MNS-PC6 experiment in CCI mice, in addition to the MNS-PC6 group, three control groups (sham-MNS, non-MNS, and anesthesia-control) of mice were included, as reported previously.6 The MNS-PC6 group, under anesthesia with 2% isoflurane, received low-frequency electrical stimulation (2 Hz, 2 mA, 0.15 ms) for 20 min by an electrical stimulator (Trio 300, ITO, Co., Tokyo, Japan) through acupuncture needles inserted at PC6 acupoints bilaterally. The proportional location of the PC6 acupoint in a mouse was determined following the anatomical description in the WHO guidelines for human acupoints,69 a location at the anterior aspect of the forearm, between the palmaris longus and flexor carpi radialis tendons, proximal to the wrist crease, 1/6 of the distance between the wrist and cubital creases6 (Supplementary Information, Fig. S1). The sham-MNS group received needle insertions at bilateral PC6 acupoints but no electrical stimulation. The non-MNS group received the same electrical stimulation bilaterally, via the acupuncture needle, at the middle of the lateral deltoid muscle, a non-MNS region. Acupuncture needles (32-gauge, Yu Kuang, New Taipei City, Taiwan) were inserted perpendicularly to a depth of 1–3 mm at the PC6 or non-MNS location bilaterally. Successful stimulation of the PC6 acupoint through the needle was confirmed by the presence of paw twitches during MNS-PC6.15, 57 After MNS-PC6, mice were allowed to completely recover from anesthesia for 10 min and further acclimatized for 20 min before being subjected to the von Frey test. The anesthesia-control group received no treatment except anesthesia and vehicle injection, if stated. The sham-MNS group, non-MNS group and anesthesia-control group underwent the same anesthesia and recovery procedures as did the MNS-PC6 group.
To compare the analgesic effects induced by repeated administration of MNS-PC6 with that by analgesic agents, morphine (10 mg/kg, i.p.) and gabapentin (50 mg/kg, i.p.), were used. All treatments were given to CCI-mice once daily from Day 8 after CCI. For morphine-tolerant experiments, i.p. morphine injection was given to anesthetized mice at the commencement of the MNS-PC6 procedure. For the mechanistic studies, receptor antagonists were given by i.p. injection 15 min prior to MNS-PC6. For each treatment group (n = 6), the data was compiled from three different cohorts with two mice per condition in each cohort.
Drugs
AM251, 1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide, and naloxone were purchased from Sigma-Aldrich (St. Louis, MO, USA). SB-334867, N-(2-Methyl-6-benzoxazolyl)-N’−1,5-naphthyridin- 4-yl urea, and gabapentin were purchased from Tocris Bioscience (Bristol, UK). For i.p. injections, SB-334867 and AM251 were dissolved in 0.9% saline solution containing 10% (w/v) encapsin and 2% (v/v) dimethylsulfoxide (DMSO). Morphine and naloxone were dissolved in 0.9% saline. All drugs were prepared as the working concentrations for i.p. injections.
Statistical analysis
The experiments were designed to generate groups of equal size, using randomization and blinded analysis. Data are expressed as the mean ± S.E.M and “n” indicates the number of mice tested. All group data have been validated to be normally distributed by the Shapiro–Wilk test (Supplementary Information, Table S1), and thus parametric statistics were employed. No outliers were identified in the study therefore none were excluded from the statistical analysis. For the time course figures depicting anti-allodynic effect, differences among groups were analyzed by the two-way ANOVA with repeated measures over Day. Differences among groups at each time point or treatment groups were post hoc analyzed by the Bonferroni’s test. One-way ANOVA followed by Sidak’s post hoc test was used to analyze the difference in allodynic responses among groups. P values of <0.05 were considered statistically significant.
RESULTS
Repeated MNS-PC6 treatments decreased mechanical allodynia in CCI-mice
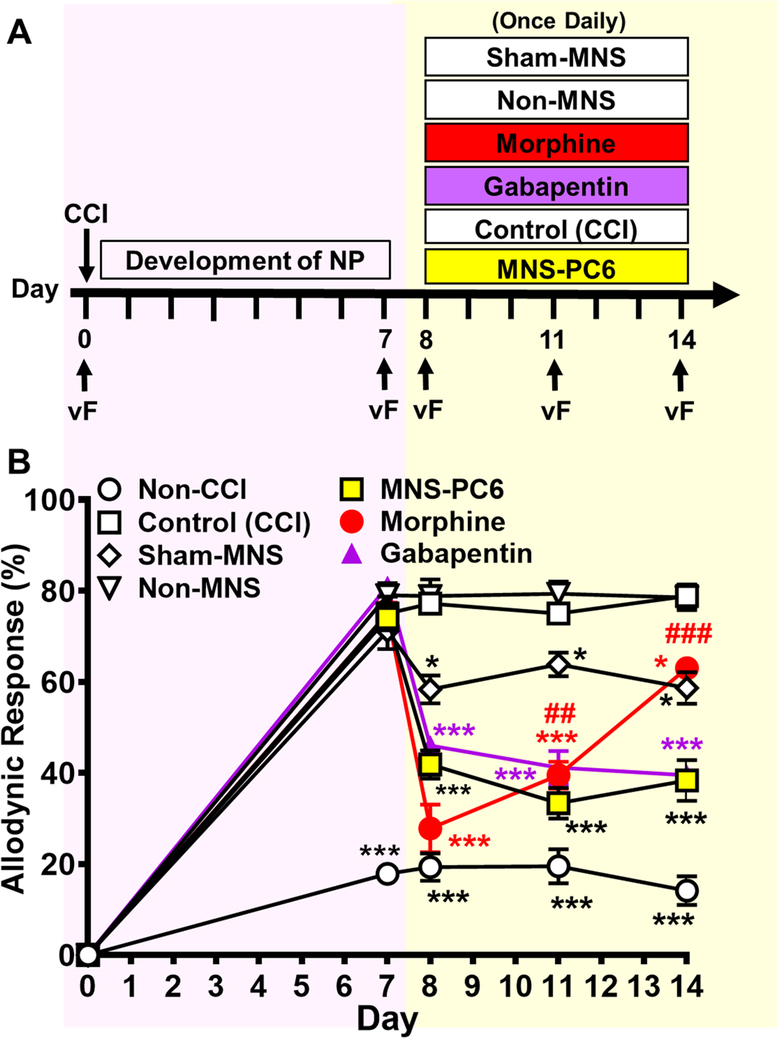
All mice received the CCI surgery, except the non-CCI group that received the same surgical procedures as in the CCI group but did not have the nerve ligated (Fig. 1A). As shown in Fig. 1B, a two-way ANOVA with repeated measures over Day showed main effects of Day [F(4,140) = 782, p < 0.001] and Treatment [F(6,35) = 107.2, p < 0.001], and a significant interaction between Day and Treatment [F(24,140) = 30.13, p < 0.001]. Mice receiving CCI surgery developed sustained neuropathic pain, as indicated by the increased mechanical allodynic response in the paw innervated by the lesioned nerve 7 days after surgery, lasting for more than 14 days (empty squares, Fig. 1B). In contrast, the non-CCI group did not develop long-term allodynia (empty circles, Fig. 1B).
Fig. 1. The anti-allodynic effect of repeated MNS-PC6 in CCI-mice, compared to repeated morphine or gabapentin.
(A) Mice received the CCI surgery on Day 0 and the mechanical sensitivity of the injured hindpaw to a 0.16 g von Frey filament (VF) was measured on Day 0 before CCI, and on Days 7, 8, 11 and 14 after CCI. The allodynic response was expressed by the percentage of nociceptive responses (withdrawal, flinching or licking) during 10 trials of VF stimulation. On Days 8–14, MNS-PC6, Sham-MNS or non-MNS (see Methods) was applied daily to CCI-mice. Gabapentin (50 mg/kg, i.p.) or morphine (10 mg/kg, i.p.) was also given to CCI-mice daily on Days 8–14. (B) Allodynic responses of CCI-mice receiving various treatments as described in Fig. 1A. Note that CCI-mice (empty squares), as compared to the Non-CCI group (empty circles) that received same surgical procedure without sciatic nerve ligation, developed mechanical allodynia that stabilized 7 days after CCI surgery. Furthermore, repeated MNS-PC6 (yellow squares), morphine (red circles) and gabapentin (purple triangles) all significantly reduced mechanical allodynia. Comparatively, sham-MNS also induced significant anti-allodynic effect, but in a lower magnitude. However, mice developed analgesic tolerance to morphine, but not to MNS-PC6 or gabapentin. *P<0.05, ***P<0.001 vs. the Control (CCI) group, ##P<0.05, ###P<0.001 vs. the allodynic response on Day 8, two-way ANOVA with repeated measures over time/ Bonferroni’s post hoc test. N=6 in each group.
On Days 8–14, CCI-mice were divided into 6 groups. As appropriate, each group received daily treatments of 1) MNS-PC6, 2) needle insertion at PC6 acupoints (Sham-MNS), 3) electro-stimulation at the non-median nerve-innervated location (Non-MNS), 4) anesthesia only [Control (CCI)], 5) morphine (10 mg/kg, i.p.) or 6) gabapentin (50 mg/kg, i.p.) (yellow shading, Fig. 1A). As shown in Figure 1B, allodynic responses measured on Days 8, 11 and 14 in repeated MNS-PC6-treated CCI-mice (yellow squares) were significantly reduced (P <0.05, two-way ANOVA followed by Bonferroni post hoc test, as compared with the Control (CCI) group (empty squares). Interestingly, repeated sham-MNS treatments (empty diamonds) also induced a significant anti-allodynic effect (P=0.024, two-way ANOVA followed by Bonferroni post hoc test). However, its anti-allodynic effect was much less prominent, as compared with the MNS-PC6 group (P<0.001, two-way ANOVA followed by Bonferroni post hoc test).
Two-way ANOVA followed by Bonferroni post hoc test showed that the antiallodynic effects in the repeated MNS-PC6 group did not decline over time (yellow squares, P=0.449, Day 11 vs. Day 8; P>0.999, Day 14 vs. Day 8), suggesting tolerance did not develop. Similarly, anti-allodynic effects induced by repeated treatments with gabapentin (50 mg/kg, i.p.) were also sustained on Days 8, 11 and 14 (purple triangles, P=0.649, Day 11 vs. Day 8; P=0.244, Day 14 vs. Day 8). In contrast, the anti-allodynic effects induced by repeated treatments with morphine (10 mg/kg, i.p.) declined quickly (red circles). On the first day (Day 8) of administration, morphine did induce a marked anti-allodynic effect, which was even greater than that induced by MNS-PC6 (red circles vs. yellow squares, P<0.05) or gabapentin (red circles vs. purple triangles, P<0.05). However, the morphine-induced anti-allodynic effect declined by Day 11 (P=0.004, Day 11 vs. Day 8). On Day 14, it declined even further (P<0.001, Day 14 vs. Day 8) to a level that was significantly smaller than the effect in the MNS-PC6 group (red circles vs. yellow squares,Day 14, P<0.001) or the gabapentin group (red circles vs. purple triangles, Day 14, P<0.001). This suggests analgesic tolerance develops within 1 week in mice treated daily with morphine (10 mg/kg, i.p.). Nonetheless, a small, but significant anti-allodynic response was still observed on Day 14 in this group (red circles vs. empty squares, Day 14, P=0.03).
Repeated MNS-PC6-IA was antagonized by an OX1R or CB1R antagonist, but not by an opioid receptor antagonist.
We further employed pharmacological approaches to examine whether the OX1R- and CB1R-mediated analgesic mechanism of MNS-PC6 6 is retained in repeated MNS-PC6-IA. A separate cohort of CCI-mice was daily treated with MNS-PC6 from Day 8 to Day 14 post CCI. On Day 15, in addition to the MNS-PC6 group, three antagonists-pretreated groups of mice were included. They were given i.p. injection of antagonists of OX1Rs (SB-334867), CB1Rs (AM251) and opioid receptors (naloxone), respectively, 15 min before receiving MNS-PC6 (Fig. 2A, green shading). As shown in Fig. 2B, one-way ANOVA showed a significant difference among treatment groups [F(7,40) = 15.27, p < 0.001]. Sidak’s post hoc analyses showed a significant anti-allodynic effect in the MNS-PC6 group on Day 15, as compared with the Control (CCI) group (yellow bar vs. open bar, P < 0.001). This MNS-PC6-IA effect was markedly reduced by SB-334867 (15 mg/kg, P=0.029) and AM251 (1.1 mg/kg, P=0.021), but not by naloxone (1.0 mg/kg, P=0.309). These antagonists at doses employed here have been reported to completely block the effects induced by their respective agonists at OX1Rs (orexin-A), CB1Rs (WIN55,212–2) and opioid receptors (morphine) 6, 29. Nonetheless, these antagonists per se did not affect allodynic responses in the control group of CCI-mice (Fig. 2B), suggesting they lack non-specific motor-impairing activity. These results suggest that repeated treatments with MNS-PC6 induce a sustained, opioid-independent analgesic effect through a mechanism mediated by OX1Rs and CB1Rs, likely through endogenous orexins and eCBs, respectively.
Fig. 2. The interaction of repeated MNS-PC6 with an OX1, CB1 or opioid receptor antagonist in CCI-mice.
(A) The protocol of CCI induction and allodynic response assessment is similar to that shown in Fig 1A. The treatment protocols for Control (CCI) and MNS-PC6 groups were extended for another day, Day 15, for a subsequent mechanistic study (green shading). Allodynic responses were measured on Day 15 in Control (CCI) and MNS-PC6 groups pretreated, respectively, with OX1R (SB-334867, 15 mg/kg, i.p.), CB1R (AM251, 1.1 mg/kg, i.p.) and opioid receptor (naloxone, 1 mg/kg, i.p.), antagonists 15 min before the MNS procedure. (B) Allodynic responses of CCI-mice receiving various treatments on Day 15 as described in Fig. 2A. Note that the OX1R or CB1R, but not opioid receptor, antagonist reversed MNS-PC6-IA. ***P<0.001 vs. the Control (CCI) group (open bar); #P<0.05, ##P<0.01, ###P<0.001 vs. the MNS-PC6 group (yellow bar), one-way ANOVA with Sidak’s post hoc test. N=6 in each group.
MNS-PC6 induced analgesia in morphine-tolerant mice via OX1Rs and CB1Rs.
Given that MNS-PC6-IA is opioid-independent (Fig. 2B),6 we subsequently examined whether MNS-PC6 could elicit analgesia in CCI-mice that had developed morphine tolerance. Mice daily treated with morphine (10 mg/kg, i.p.) from Day 8 to Day 15 (horizontal red bar, Fig. 3A) developed analgesic tolerance, as indicated by the diminished anti-allodynic effect of morphine on Day 15 (red bar, Fig. 3B), unlike the group daily treated with MNS-PC6 (yellow bar, Fig. 3B). As depicted in Fig 3B, one-way ANOVA showed a significant difference among treatment groups [F(7,40) = 15.27, p < 0.001]. Post hoc analyses by the Sidak’s test showed that on Day 15, MNS-PC6 administered immediately after morphine injection elicited significant analgesia, indicated by the decreased allodynic responses, in those morphine-tolerant CCI-mice (blue bar vs. red bar, Fig. 3B, P=0.002). This analgesic effect induced by single administration of MNS-PC6 in morphine-tolerant CCI-mice was antagonized by SB-334867 (15 mg/kg, i.p., left-slashed blue bar vs. blue bar, P=0.049) or AM251 (1.1 mg/kg, i.p., right-slashed blue bar vs. blue bar, P=0.022), pre-administered 15 min before MNS-PC6. These findings suggest that MNS-PC6-IA, via sequential activation of OX1Rs and CB1Rs by endogenous orexins and eCBs, respectively, remains functional in morphine-tolerant mice.
Fig. 3. Effects of single or repeated treatments of MNS-PC6 on allodynic responses of morphine-tolerant CCI-mice, and their interactions with OX1R and CB1R antagonists.
(A) The protocol of CCI induction and allodynic response assessment is similar to that shown in Fig. 1A. CCI-mice received morphine (10 mg/kg, i.p.) daily on Days 8–15. MNS-PC6 was either co-administered with the final dose of morphine on Day 15 (morphine/single-MNS group, horizontal red bar), or co-administered daily with morphine on Days 8–15 (morphine/repeated-MNS group, horizontal yellow-red bar). OX1R (SB-334867, 15 mg/kg, i.p.) or CB1R (AM251, 1.1 mg/kg, i.p.) antagonist was given 15 min before the MNS-PC6 procedure on Day 15. (B) Allodynic responses on Day 15 in Control (CCI), repeated MNS-PC6 and repeated morphine groups, respectively, with a MNS-PC6-co-treatment on Day 15 (blue bar), or MNS-PC6-co-treatments daily on Days 8–15 (green bar). OX1R (SB-334867, 15 mg/kg, i.p.) or CB1R (AM251, 1.1 mg/kg, i.p.) antagonist was given 15 min before the MNS-PC6 procedure on Day 15. Note that that pretreatment of OX1R or CB1R antagonist significantly reversed MNS-PC6-IA induced by either single or repeated MNS-PC6 in morphine-tolerant CCI-mice. *P<0.05, ***P<0.001 vs. the Control (CCI) group (open bar); ##P<0.01, ###P<0.001 vs. the repeated-morphine-treated control group (red bar); πP<0.05 vs. the repeated-morphine/single-MNS group (blue bar); δP<0.05, δδδP<0.001 vs. repeated-morphine/repeated-MNS group (green bar), one-way ANOVA with Sidak’s post hoc test. N=6 in each group.
Repeated MNS-PC6-IA efficacy is maintained in morphine-tolerant CCI-mice.
As repeated MNS-PC6-IA is devoid of tolerance (Fig. 1B), we subsequently tested whether the OX1R-CB1R cascade-mediated repeated MNS-PC6-IA remains tolerance-free in morphine-tolerant CCI-mice. From Day 8 to 15, MNS-PC6 was co-administered daily with morphine (10 mg/kg, i.p., horizontal yellow-red bar, Fig. 3A). As shown in Fig. 3B, on Day 15, the allodynic response in the co-treatment group (green bar) was significant lower (P=0.0006) than in the repeated-morphine treated group (red bar). This suggests that repeated co-administration of MNS-PC6 with morphine produces an analgesic effect that does not develop tolerance. This analgesic effect was significantly antagonized by SB-334867 (15 mg/kg, left-slashed green bar, P=0.027) or AM251 (1.1 mg/kg, right-slashed green bar, P=0.0001) given on Day 15 before co-administration of MNS-PC6 and morphine (Fig. 3B). These results suggest that repeated MNS-PC6-IA is also mediated by a mechanism involving OX1Rs, CB1Rs and 2-AG in the vlPAG, which is possibly the OX1R-PLC-DAGL-2AG-CB1R cascade in the vlPAG we reported previously 6, 24. Morphine does not produce cross tolerance with MNS-PC6-IA and hence MNS-PC6 can induce an analgesic effect comparable to the initial morphine response in morphine-tolerant CCI-mice.
MNS-PC6-IA is maintained in CCI-mice made profoundly opioid tolerant by escalating doses of morphine.
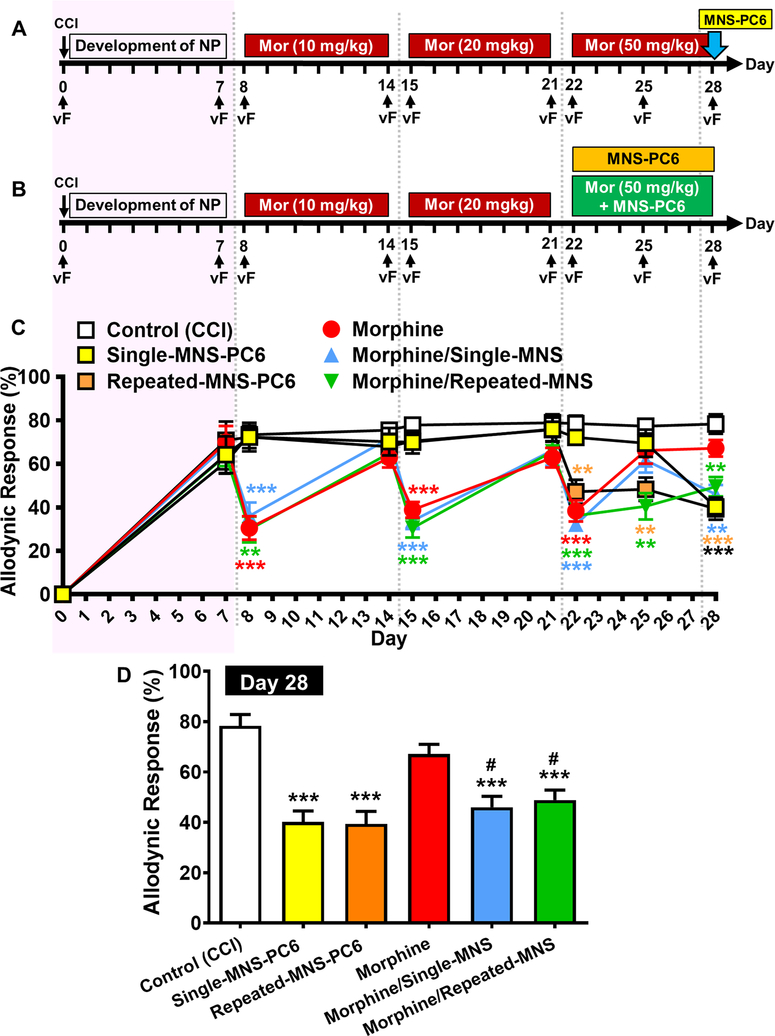
In the clinical setting, analgesic tolerance to escalating doses of morphine is a serious drawback in pain management, especially in palliative care.33 We therefore further examined effects of single and repeated doses of MNS-PC6 in CCI-mice that had developed analgesic tolerance induced by escalating doses of morphine. CCI-mice were randomly divided into 6 groups and received a treatment protocol as indicated in Fig. 4A and Fig. 4B. As shown in Fig. 4C, CCI-mice treated with morphine (i.p.) daily developed tolerance by 1 week at either an initial dose of 10 mg/kg on Days 8–14 after CCI (pink shading), a subsequent doubling dose (20 mg/kg) on Days 15–21, or a further escalating dose of 50 mg/kg on Days 22–28. On Day 28, in these highly morphine-tolerant CCI-mice, MNS-PC6 administered alone (yellow squares) or in combination with morphine (50 mg/kg) (blue triangles) induced a significant anti-allodynic effect (Fig. 4C). As compared with the morphine-tolerant group without MNS-PC6 treatment, MNS-PC6 elicited significant analgesia in mice tolerant to escalating doses of morphine (blue vs. red bars, Fig. 4D, P=0.012, one-way ANOVA followed by Sidak’s post hoc test). This suggests that MNS-PC6 can still effectively reduce pain even in mice that had developed profound analgesic tolerance to escalating doses of morphine.
Fig. 4. Effects of single or repeated MNS-PC6 on allodynic responses of CCI-mice with analgesic tolerance to escalating doses of morphine.
(A) The protocol of CCI induction and allodynic response assessment is similar to that shown in Fig. 1A. CCI-mice received morphine (i.p.) daily at escalating doses from 10 mg/kg on Days 8–14, then 20 mg/kg on Days 15–21, and finally 50 mg/kg on Days 22–28. MNS-PC6 was co-administered with the final dose of morphine on Day 28. Allodynic responses were measured on Days 0, 7, 8, 14, 15, 21, 22, 25 and 28. (B) Similar protocols of escalating morphine dosing treatments and MNS-PC6 as in (A) were performed in CCI-mice except MNS-PC6 was co-administered with morphine daily on Days 22–28. (C) Time courses of allodynic responses in CCI-mice receiving various treatments as described in protocol (A) and (B). Note that the morphine tolerance developed by 1 week after repeated injections at each dose, and analgesia re-emerged after injecting a higher dose of morphine. Co-administration of MNS-PC6 with morphine on Day 28 induced a significant antiallodynic effect in CCI-mice rendered morphine-tolerant by escalating doses (blue triangles). Repeated MNS-PC6-IA efficacy is retained in morphine-tolerant CCI-mice from Day 22–28 (green triangles). **P<0.01, ***P<0.001 vs. the Control (CCI) group, two-way ANOVA with repeat measures over time/ Bonferroni’s post hoc test. (D) Allodynic responses on Day 28 in the 6 treatment groups of CCI mice as described in (C), in addition to the control CCI group and the morphine daily-treated CCI group, CCI mice were treated with single or repeated MNS-PC6, or morphine-tolerant CCI-mice treated with single (morphine/single-MNS) or repeated MNS-PC6 (morphine/repeated-MNS). Note that a single administration of MNS-PC6 or repeated administration of MNS-PC6 both significantly suppressed allodynic responses in morphine-tolerant CCI-mice receiving escalating doses of morphine. ***P<0.001 vs. the Control (CCI) group (open bar); #P<0.05 vs. the morphine group (red bar), one-way ANOVA with Sidak’s post hoc test.
In another group of CCI-mice that had developed analgesic tolerance to 20 mg/kg morphine, MNS-PC6 was administered alone (horizontal orange bar, Fig. 4B) or in combination with 50 mg/kg morphine daily from Day 22 to Day 28 (horizontal green bar, Fig. 4B). In these morphine (10+20 mg/kg)-tolerant mice, daily treatments with MNS-PC6 alone for 7 days maintained a significant anti-allodynic effect without tolerance (orange symbols, Fig. 4C). In the group where MNS-PC6 was co-administered with 50 mg/kg morphine from Day 22 to Day 28 (green diamonds, Fig. 4C), a significant anti-allodynic effect was still observed on Day 25 and Day28. One-way ANOVA followed by Sidak’s post hoc test in Fig. 4D showed that repeated MNS-PC6 administered alone (orange bar, P<0.001) or co-administered with morphine (green bar, P<0.001) induced significant anti-allodynic effect, which is in contrast to the morphine-tolerant group (red bar, P=0.039, Fig. 4D). This suggests that repeated MNS-PC6 continues to maintain substantial analgesic efficacy even in CCI-mice with analgesic tolerance after rapidly escalating doses of morphine.
DISCUSSION
Repeated MNS-PC6 induces a tolerance-free analgesia in via OX1Rs and CB1Rs in mice with neuropathic pain.
Our previous study demonstrated that a single administration of MNS-PC6 on Day 8 significantly suppressed established mechanical allodynia in CCI-mice.6 Here, MNS-PC6 administered daily starting on Day 8 for 7 consecutive days induced a stable anti-allodynic effect with a similar efficacy throughout the 7-day treatment period (Fig. 1). The similar efficacy observed in single and repeated MNS-PC6 treated CCI-mice suggests that MNS-PC6-induced anti-allodynic effect in CCI-mice is not cumulative. Indeed, our previous study, using the hot plate test, has demonstrated that 20 min of MNS-PC6 induced an antinociceptive effect lasting for about one hour.6 The stable anti-allodynic effect of MNS-PC6 during the treatment period suggests that tolerance does not develop. This is in-line with previous clinical reports that electrical stimulation at the median nerve, either by a surgically-implanted electrode35, 67 or by percutaneous stimulation,66 effectively relieved chronic pain without tolerance.65 In certain cases, MNS even provided pain relief for years3, 36, 39 or decades.14, 62 Although their analgesic mechanism(s) were not identified, the opioid system is unlikely involved since pain relief was naloxone-insensitive.65 The current findings that repeated MNS-PC6-IA was prevented by OX1R and CB1R antagonists (Fig. 2), suggests it is mediated by endogenous orexins and eCBs. Our previous studies support orexins are probably released from the LH6 and MNS-PC6-IA is likely mediated by a disinhibition mechanism in the vlPAG through the OX1R-PLC-DAGL-2-AG-CB1R signaling cascade, which we first revealed using electrophysiological approaches.24 In a pilot study to examine the possible sex difference in MNS-PC6-IA, age-matched female mice were subjected to the same CCI surgery and pharmacological challenges on Day 8. It was found that MNS-PC6 exerted a significant anti-allodynic effect in female CCI-mice. Similarly, this anti-allodynic effect was blocked by SB 334867 and AM 251, but not by naloxone (Supplementary information Fig. S2). This suggests that MNS-PC6 can also induce an opioid-independent anti-allodynic effect mediated by OX1Rs and CB1Rs in female neuropathic mice. The present results also demonstrated that repeated gabapentin treatments significantly suppressed CCI-induced allodynia without developing analgesic tolerance (Fig. 1B). The clinical use of gabapentin for chronic pain is well recognized, 12 especially the off-label use for neuropathic pain.71 However, a significant portion of patients with chronic pain are refractory to gabapentin, and serious adverse effects such as drowsiness, ataxia, mental confusion, dependence, teratogenicity and respiratory depression have been reported.46, 53 Therefore, MNS-PC6 with the benefit of being tolerance-free should be explored as an alternative therapy for chronic pain.
It is noteworthy that in the sham-MNS group of CCI-mice that received needle insertion at PC6 acupoints, but did not receive electrical stimulation, a slight but statistically significant anti-allodynic response was observed. This effect may be attributed to mechanical stimulation of the median nerve from respiratory movement during needle insertion for 20 mins. A related observation in our previous study is that placing the stimulating electrode adjacent to the surgically exposed median nerve without electrostimulation induced a slight but statistically significant increase in orexin-A levels in the ventrolateral periaqueductal gray.6 However, since the sham-MNS-induced analgesia was much less efficacious than MNS-PC-IA, this treatment group was excluded from subsequent mechanistic studies in morphine-tolerant models in order to minimize the number of animals used.
Analgesic tolerance to cannabinoids.
Cannabinoids are analgesic and thus have been proposed to replace or supplement opioids for pain relief, especially in palliative care.13 However, analgesic tolerance to exogenous CB1R agonists after repeated administration, due to CB1R desensitization is commonly encountered in preclinical models,10, 20, 37 as also seen for opioids. Analgesic tolerance may contribute to the variable efficacy seen in meta-analyses of the use of cannabinoids for relieving chronic non-cancer pain.27, 47, 54 An alternative solution is to increase eCB levels by pharmacological approaches. However, body-wide pharmacological enhancements of AEA or/and 2-AG levels via inhibiting their respective major degrading enzymes, FAAH and MAGL, were also found to cause analgesic tolerance, possibly due to CB1R desensitization by eCB overload.8, 42
In contrast to previous studies employing exogenous cannabinoids or eCB degradation inhibitors to induce analgesia, the 2-AG-mediated MNS-PC6-IA here did not develop tolerance. This may be attributed to the pulsatile release of 2-AG that is synthesized on-demand during MNS-PC6-IA, like during eCB-mediated stress-induced analgesia (SIA),55 is at an optimal duration, concentration and location to avoid CB1R desensitization and thus does not induce analgesic tolerance.
The orexin-2-AG-mediated MNS-PC6-IA is retained in morphine-tolerant mice.
It has been reported that opioid-dependent SIA31 and PAG stimulation-induced analgesia32 exhibit cross tolerance with morphine. Here, we found the efficacy of MNS-PC6-IA elicited by either single or repeated administration is retained in morphine-tolerant mice (Fig. 3), suggesting no cross tolerance between MNS-PC6-IA and morphine analgesia. This further supports that MNS-PC6-IA is not opioid-mediated. Instead, it is eCB-mediated.
Altered eCB levels have been reported in mice with neuropathic pain or chronic morphine treatment, two conditions employed in this study, thus they may be confounding factors for the observed MNS-PC6-IA. The findings in mice, however, are inconsistent. Either elevated45 or unchanged72 2-AG levels have been reported in the brains of mice with neuropathic pain. In rats treated with morphine chronically, 2-AG, but not AEA, levels were decreased.63 However, the present results in mice suggest no change in the basal eCB tone of analgesic circuits since AM-251, a CB1R antagonist, per se did not alter the allodynic response in morphine-tolerant CCI-mice (Supplementary Fig. S3), Thus, in these mice, 2-AG, which is synthesized on-demand upon OX1R activation, is functional and sufficient to elicit analgesia.
Conversely, up-regulation of eCB and orexin systems has been reported in animal treated repeatedly with morphine. An elevation in eCB-mediated presynaptic inhibition of GABAergic synaptic transmission73 but no change in CB1R expression74 were found in vlPAG slices of morphine-tolerant rats. This would enhance eCB-mediated disinhibition in the vlPAG. In addition, repeated morphine treatments can increase the number of activated orexin neurons in the LH of rats.17 It was also found that one month of morphine chronic treatment significantly increased the number of orexin neurons in the LH of mice, and noted an ~50% increase in orexin neurons in postmortem brains of heroin addicts.56 Therefore, it is likely that the orexin-eCB signaling is upregulated in neuropathic, morphine-tolerant mice, perhaps to provide compensatory antinociceptive tone, although this upregulation is insufficient to counter the declining opioid system. The finding that the function of orexin-eCB signaling is upregulated in morphine-tolerant animals further suggests MNS-PC6 to be an efficient analgesic procedure via this signaling pathway in opioid-tolerant patients.
Applications of MNS-PC6-IA
As the US NIH is promoting non-pharmacological approaches for pain management as part of a strategic approach to address the urgent opioid crisis,1, 30 the utilization of non-opioid-based peripheral neuromodulation approaches may provide a faster option, as compared with novel pharmacotherapy, in terms of regulatory approval procedures. Advancements in biomedical engineering have led to the introduction of several non-invasive miniaturized electrical stimulators providing minimal current stimulation at peripheral nerves.2, 4, 16 This contrasts to the surgically-implanted invasive electrodes previously used for peripheral nerve stimulation.52 Recently, several peripheral nerve stimulators have been approved by the US FDA for the treatment of chronic pain.61 These devices can easily target the median nerve and may be able to achieve MNS-PC6-IA. Thus, the present study provides a preclinical proof of concept for using these devices for pain relief in morphine-tolerant patients, especially for terminal patients who have developed analgesic tolerance to escalating doses of morphine. These devices may also contribute to reducing the overuse of opioids, a leading contributor to the opioid crisis, by decreasing the amount of opioids prescribed. Very recently, a wearable wristband-like device, specifically designed to stimulate the median nerve,44 was cleared by the US FDA for the treatment of essential tremor.59 Although it has not been tested for the treatment of chronic pain, this device may provide an alternative option for chronic pain relief, especially in morphine tolerant patients. Although novel opioid-replacing analgesics are under development, peripheral neuromodulation may provide a faster solution to address the urgent opioid crisis.
Conclusions and Perspectives.
The present study provides a preclinical proof-of-concept for the application of median nerve stimulation as a pain-relieving therapy in morphine-tolerant patients. Adequate treatment of chronic pain remains an unmet medical need due to the significant adverse effects and limitations of current analgesics, particularly opioids, as well as the potential risk of opioid misuse.50
Supplementary Material
Fig. S1. Representative diagram of PC6 location in mice. The proportional location of the PC6 acupoint (red dot) in a mouse was determined following the anatomical description in the WHO guidelines for human acupoints [69], a location at the anterior aspect of the forearm, between the palmaris longus and flexor carpi radialis tendons, proximal to the wrist crease, 1/6 of the distance between the wrist and cubital creases. The image of mouse ventral side is adapted from Illustration Toolkit Neuroscience by Motifolio.
Fig. S2. The pilot study of MNS-PC6 on allodynic response in female mice with neuropathic pain. Female CCI-mice were induced and their allodynic responses were evaluated on Day 8 post CCI surgery via the mechanical sensitivity of the injured hindpaw to a 0.07 g von Frey filament. Female CCI-mice received MNS-PC6 with pre-treatment of OX1R antagonist (SB-334867, 15 mg/kg, i.p.), CB1R antagonist (AM251, 1.1 mg/kg, i.p.), opioid receptor antagonist (naloxone, 1 mg/kg, i.p.) or vehicle 15 mins prior. Similar to their male counterpart, MNS-PC6 signficantly suppressed allodynic responses in female CCI-mice, reversible by the OX1R or CB1R, but not opioid receptor, antagonist. One-way ANOVA followed by Sidak’s post hoc test was performed. N=7 in each group.
Fig. S3. The effects of OX1R, CB1R antagonists alone on allodynic responses in mice with neuropathic pain and morphine tolerance. CCI-mice were induced and their allodynic responses were evaluated as described in Figure 1. CCI-mice received morphine (10 mg/kg, i.p.) daily on Days 8–15. On Day 15, CCI-mice received pre-treatment of OX1R antagonist (SB-334867, 15 mg/kg, i.p.) or CB1R antagonist (AM251, 1.1 mg/kg, i.p.) 15 mins before morphine injection. Allodynic responses on Day 15 show that administration of OX1R antagonist or CB1R antagonist does not affect the allodynic response in CCI-mice. One-way ANOVA followed by Sidak’s post hoc test was performed. N=6 in each group.
Table S1. The Shapiro-Wilk analysis parameters showing all data in each group are normally distributed.
Research funding:
This study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST104-2745-B-002-004, MOST106-2321-B-002-019, MOST107-2321-B-002-010, MOST 108-2321-B-002-005, MOST 108-2320-B-002-029-MY3 and 109-2320-B-002-042-MY3 to L.-C.C.), National Health Research Institutes, Taiwan (NHRI-EX109-10733NI to L.-C.C.), Ministry of Education, Taiwan (107M4022-3 to L.-C.C.) and National Institute of Health, USA (DA041229 and DA047858 to K.M.).
Footnotes
Disclosures:
Conflicts of interest statement: The authors declare no conflicts of interest.
REFERENCES
- 1.Abbasi J. Robert Kerns, PhD: Researching Nondrug Approaches to Pain Management. JAMA. 319:1535–1537, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Banks GP, Winfree CJ. Evolving Techniques and Indications in Peripheral Nerve Stimulation for Pain. Neurosurg Clin N Am. 30:265–273, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 45:692–699, 1976 [DOI] [PubMed] [Google Scholar]
- 4.Charkhkar H, Christie BP, Pinault GJ, Tyler DJ, Triolo RJ. A translational framework for peripheral nerve stimulating electrodes: Reviewing the journey from concept to clinic. J Neurosci Methods. 328:108414, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Chen EY, Marcantonio A, Tornetta P 3rd. Correlation Between 24-Hour Predischarge Opioid Use and Amount of Opioids Prescribed at Hospital Discharge. JAMA Surg. 153:e174859, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YH, Lee HJ, Lee MT, Wu YT, Lee YH, Hwang LL, Hung MS, Zimmer A, Mackie K, Chiou LC. Median nerve stimulation induces analgesia via orexin-initiated endocannabinoid disinhibition in the periaqueductal gray. Proc Natl Acad Sci U S A. 115:E10720–E10729, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 393:1558–1568, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Crowe MS, Wilson CD, Leishman E, Prather PL, Bradshaw HB, Banks ML, Kinsey SG. The monoacylglycerol lipase inhibitor KML29 with gabapentin synergistically produces analgesia in mice. Br J Pharmacol. 174:4523–4539, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalal S, Bruera E. Pain Management for Patients With Advanced Cancer in the Opioid Epidemic Era. Am Soc Clin Oncol Educ Book. 39:24–35, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 77:475–487, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marzo V New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 17:623–639, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Dosenovic S, Jelicic Kadic A, Miljanovic M, Biocic M, Boric K, Cavar M, Markovina N, Vucic K, Puljak L. Interventions for Neuropathic Pain: An Overview of Systematic Reviews. Anesth Analg. 125:643–652, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Dzierzanowski T. Prospects for the Use of Cannabinoids in Oncology and Palliative Care Practice: A Review of the Evidence. Cancers (Basel). 11:129, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg E, Waisbrod H, Gerbershagen HU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin J Pain. 20:143–146, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Fu LW, Longhurst JC. Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptors. J Appl Physiol (1985). 106:1800–1809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabriel RA, Swisher MW, Ilfeld BM. Percutaneous peripheral nerve stimulation for acute postoperative pain. Pain Manag. 9:347–354, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 23:3106–3111, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 393:1537–1546, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 13:883–888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 81:300–318, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth Analg. 125:1733–1740, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hah JM, Sturgeon JA, Zocca J, Sharifzadeh Y, Mackey SC. Factors associated with prescription opioid misuse in a cross-sectional cohort of patients with chronic non-cancer pain. J Pain Res. 10:979–987, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth. 120:1335–1344, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, Liao HT, Mackie K, Chiou LC. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 31:14600–14610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphreys K, Saitz R. Should Physicians Recommend Replacing Opioids With Cannabis? JAMA. 321:639–640, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 147:281–288, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johal H, Devji T, Chang Y, Simone J, Vannabouathong C, Bhandari M. Cannabinoids in Chronic Non-Cancer Pain: A Systematic Review and Meta-Analysis. Clin Med Insights Arthritis Musculoskelet Disord. 13:1179544120906461, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur R, Sidhu P, Singh S. What failed BIA 10–2474 Phase I clinical trial? Global speculations and recommendations for future Phase I trials. J Pharmacol Pharmacother. 7:120–126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HJ, Chang LY, Ho YC, Teng SF, Hwang LL, Mackie K, Chiou LC. Stress induces analgesia via orexin 1 receptor-initiated endocannabinoid/CB1 signaling in the mouse periaqueductal gray. Neuropharmacology. 105:577–586, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy RM. Neuromodulation: The “Not-so-Hidden” Cure for the Opioid Crisis. Neuromodulation. 20:519–524, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Lewis JW, Sherman JE, Liebeskind JC. Opioid and non-opioid stress analgesia: assessment of tolerance and cross-tolerance with morphine. J Neurosci. 1:358–363, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer DJ, Hayes RL. Stimulation-produced analgesia: development of tolerance and cross-tolerance to morphine. Science. 188:941–943, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Mercadante S, Arcuri E, Santoni A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs. 2019 [DOI] [PubMed] [Google Scholar]
- 34.Ming-Tatt L, Khalivulla SI, Akhtar MN, Lajis N, Perimal EK, Akira A, Ali DI, Sulaiman MR. Anti-hyperalgesic effect of a benzilidine-cyclohexanone analogue on a mouse model of chronic constriction injury-induced neuropathic pain: Participation of the kappa-opioid receptor and KATP. Pharmacol Biochem Behav. 114–115:58–63, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Mirone G, Natale M, Rotondo M. Peripheral median nerve stimulation for the treatment of iatrogenic complex regional pain syndrome (CRPS) type II after carpal tunnel surgery. J Clin Neurosci. 16:825–827, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 14:216–221; discussion 222–213, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Morgan DJ, Davis BJ, Kearn CS, Marcus D, Cook AJ, Wager-Miller J, Straiker A, Myoga MH, Karduck J, Leishman E, Sim-Selley LJ, Czyzyk TA, Bradshaw HB, Selley DE, Mackie K. Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J Neurosci. 34:5152–5163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 164:1322–1334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nashold BS Jr., Goldner JL, Mullen JB, Bright DS. Long-term pain control by direct peripheral-nerve stimulation. J Bone Joint Surg Am. 64:1–10, 1982 [PubMed] [Google Scholar]
- 40.Neuman MD, Bateman BT, Wunsch H. Inappropriate opioid prescription after surgery. Lancet. 393:1547–1557, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, Lintzeris N, Khor KE, Farrell M, Smith A, Le Foll B. Opioid-Sparing Effect of Cannabinoids: A Systematic Review and Meta-Analysis. Neuropsychopharmacology. 42:1752–1765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okine BN, Norris LM, Woodhams S, Burston J, Patel A, Alexander SP, Barrett DA, Kendall DA, Bennett AJ, Chapman V. Lack of effect of chronic pre-treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: evidence for plastic changes in the endocannabinoid system. Br J Pharmacol. 167:627–640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 139:117–126, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Pahwa R, Dhall R, Ostrem J, Gwinn R, Lyons K, Ro S, Dietiker C, Luthra N, Chidester P, Hamner S, Ross E, Delp S. An Acute Randomized Controlled Trial of Noninvasive Peripheral Nerve Stimulation in Essential Tremor. Neuromodulation. 22:537–545, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, Di Marzo V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 52:415–422, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol. 9:13–21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabgay K, Waranuch N, Chaiyakunapruk N, Sawangjit R, Ingkaninan K, Dilokthornsakul P. The effects of cannabis, cannabinoids, and their administration routes on pain control efficacy and safety: A systematic review and network meta-analysis. J Am Pharm Assoc (2003). 60:225–234 e226, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 4:245–259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, Piomelli D. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3’-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 322:236–242, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Schneiderhan J, Clauw D, Schwenk TL. Primary Care of Patients With Chronic Pain. JAMA. 317:2367–2368, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Skolnick P The Opioid Epidemic: Crisis and Solutions. Annu Rev Pharmacol Toxicol. 58:143–159, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Slavin KV. Technical aspects of peripheral nerve stimulation: hardware and complications. Prog Neurol Surg. 24:189–202, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 111:1160–1174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 159:1932–1954, 2018 [DOI] [PubMed] [Google Scholar]
- 55.Suplita RL 2nd, Eisenstein SA, Neely MH, Moise AM, Hohmann AG. Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology. 54:161–171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thannickal TC, John J, Shan L, Swaab DF, Wu MF, Ramanathan L, McGregor R, Chew KT, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, Siegel JM. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med. 10, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tjen ALSC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: roles of endocannabinoids and GABA. J Appl Physiol (1985). 106:1793–1799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.US-FDA: StimQ Peripheral Nerve Stimulator (PNS) System 510(k) Premarket Notification.(Administration U.S.F.a.D., Ed.), 2016. [Google Scholar]
- 59.US-FDA: External Upper Limb Tremor Stimulator 510(k) Premarket Notification.(Administration U.S.F.a.D., Ed.), Silver Spring, MD, 2018. [Google Scholar]
- 60.US-FDA: SPRINT Peripheral Nerve Stimulation System, Extensa, SPRINT Peripheral Nerve Stimulation System, Endura, SPRINT Clinical Programmer Kit 510(k) Premarket Notification.(Administration U.S.F.a.D., Ed.), 2018. [Google Scholar]
- 61.US-FDA: FDA permits marketing of first medical device for relief of pain associated with irritable bowel syndrome in patients 11–18 years of age, 2019. [Google Scholar]
- 62.Van Calenbergh F, Gybels J, Van Laere K, Dupont P, Plaghki L, Depreitere B, Kupers R. Long term clinical outcome of peripheral nerve stimulation in patients with chronic peripheral neuropathic pain. Surg Neurol. 72:330–335; discussion 335, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Vigano D, Grazia Cascio M, Rubino T, Fezza F, Vaccani A, Di Marzo V, Parolaro D. Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoyl glycerol, in rat brain. Neuropsychopharmacology. 28:1160–1167, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Walker G The opioid crisis: a 21st century pain. Drugs Today (Barc). 54:283–286, 2018 [DOI] [PubMed] [Google Scholar]
- 65.Walker JB, Katz RL. Non-opioid pathways suppress pain in humans. Pain. 11:347–354, 1981 [DOI] [PubMed] [Google Scholar]
- 66.Walker JB, Katz RL. Peripheral nerve stimulation in the management of dysmenorrhea. Pain. 11:355–361, 1981 [DOI] [PubMed] [Google Scholar]
- 67.Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 155:108–109, 1967 [DOI] [PubMed] [Google Scholar]
- 68.Webster LR. Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg. 125:1741–1748, 2017 [DOI] [PubMed] [Google Scholar]
- 69.WHO: WHO Standard Acupuncture Point Locations in the Western Pacific Region. Organization WH (Ed.), World Health Organization Western Pacific Region, Manila, Philippines, 2008. [Google Scholar]
- 70.WHO. In: WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. WHO Guidelines Approved by the Guidelines Review Committee, Geneva, 2018. [PubMed] [Google Scholar]
- 71.Wiffen PJ, Derry S, Bell RF, Rice AS, Tolle TR, Phillips T, Moore RA. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 6:CD007938, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkerson JL, Niphakis MJ, Grim TW, Mustafa MA, Abdullah RA, Poklis JL, Dewey WL, Akbarali H, Banks ML, Wise LE, Cravatt BF, Lichtman AH. The Selective Monoacylglycerol Lipase Inhibitor MJN110 Produces Opioid-Sparing Effects in a Mouse Neuropathic Pain Model. J Pharmacol Exp Ther. 357:145–156, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson-Poe AR, Lau BK, Vaughan CW. Repeated morphine treatment alters cannabinoid modulation of GABAergic synaptic transmission within the rat periaqueductal grey. Br J Pharmacol. 172:681–690, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson-Poe AR, Morgan MM, Aicher SA, Hegarty DM. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience. 213:191–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong CS, Hui GK, Chung EK, Wong SH. Diagnosis and management of neuropathic pain. Pain Manag. 4:221–231, 2014 [DOI] [PubMed] [Google Scholar]
- 76.Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharmacology. 124:105–120, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004–2012. JAMA. 315:1654–1657, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative diagram of PC6 location in mice. The proportional location of the PC6 acupoint (red dot) in a mouse was determined following the anatomical description in the WHO guidelines for human acupoints [69], a location at the anterior aspect of the forearm, between the palmaris longus and flexor carpi radialis tendons, proximal to the wrist crease, 1/6 of the distance between the wrist and cubital creases. The image of mouse ventral side is adapted from Illustration Toolkit Neuroscience by Motifolio.
Fig. S2. The pilot study of MNS-PC6 on allodynic response in female mice with neuropathic pain. Female CCI-mice were induced and their allodynic responses were evaluated on Day 8 post CCI surgery via the mechanical sensitivity of the injured hindpaw to a 0.07 g von Frey filament. Female CCI-mice received MNS-PC6 with pre-treatment of OX1R antagonist (SB-334867, 15 mg/kg, i.p.), CB1R antagonist (AM251, 1.1 mg/kg, i.p.), opioid receptor antagonist (naloxone, 1 mg/kg, i.p.) or vehicle 15 mins prior. Similar to their male counterpart, MNS-PC6 signficantly suppressed allodynic responses in female CCI-mice, reversible by the OX1R or CB1R, but not opioid receptor, antagonist. One-way ANOVA followed by Sidak’s post hoc test was performed. N=7 in each group.
Fig. S3. The effects of OX1R, CB1R antagonists alone on allodynic responses in mice with neuropathic pain and morphine tolerance. CCI-mice were induced and their allodynic responses were evaluated as described in Figure 1. CCI-mice received morphine (10 mg/kg, i.p.) daily on Days 8–15. On Day 15, CCI-mice received pre-treatment of OX1R antagonist (SB-334867, 15 mg/kg, i.p.) or CB1R antagonist (AM251, 1.1 mg/kg, i.p.) 15 mins before morphine injection. Allodynic responses on Day 15 show that administration of OX1R antagonist or CB1R antagonist does not affect the allodynic response in CCI-mice. One-way ANOVA followed by Sidak’s post hoc test was performed. N=6 in each group.
Table S1. The Shapiro-Wilk analysis parameters showing all data in each group are normally distributed.