ABSTRACT
Atherosclerosis, a multifactorial vascular disease resulting from lipid metabolism disorders, features chronic inflammatory damage resulting from endothelial dysfunction, which usually affects multiple arteries. The carotid artery is a common site for clinical atherosclerosis evaluation. The aortic root is the standard site for quantifying atherosclerosis in mice. Due to the adverse reactions of first-line drugs, it is necessary to discover new drugs to prevent and treat atherosclerosis. Berberine (BBR) is one of the most promising natural products derived from herbal medicine Coptidis Rhizoma (Huanglian) that features significant anti-atherosclerosis properties. However, overall BBR mechanism against carotid atherosclerosis has not been clearly discovered. Our work aimed to investigate potential BBR mechanism in improving carotid atherosclerosis in ApoE knockout mice. Here, we proved that in ApoE −/− mice receiving high-fat diet for 12 weeks, BBR can reduce serum lipid levels, improve intimal hyperplasia, and antagonize carotid lipid accumulation, which may be achieved through regulating the PI3K/AKT/mTOR signaling pathway, regulating autophagy, promoting cell proliferation and inhibiting cell apoptosis. In summary, these data indicate that BBR can ameliorate carotid atherosclerosis. Therefore, it could be a promisingly therapeutic alternative for atherosclerosis.
KEYWORDS: Carotid atherosclerosis, berberine, endothelial dysfunction, signaling pathway
1. Introduction
Atherosclerosis, a progressive chronic inflammatory and metabolic disease, features lipid deposition, focal intimal thickening, smooth muscle cell proliferation, and plaque formation [1]. It usually impacts various large and medium arteries, especially branches of the aorta, as well as coronary, femoral and carotid artery, and is the main reason for heart disease and stroke [2,3]. With the development of society, atherosclerosis and its complications have caused an increase in morbidity and mortality worldwide, accounting for only one third of the death toll in the world [4,5]. Although atherosclerosis pathogenesis is still not fully understood, it is found to participate in lipid metabolism disorders, endothelial dysfunction, inflammation along with oxidative stress [6]. It has been reported that endothelial dysfunction and the formation of neointima are the early key steps in atherosclerosis development, which are conducive to vasospasm, thrombosis, macrophage penetration, cell growth, and inflammation that lead to atherosclerosis [7,8]. Therefore, it is vital to study the underlying mechanism of carotid atherosclerosis.
With complementary and alternative medicines increasingly popular with patients with atherosclerosis, natural derived compounds are increasingly used in Asian and Western countries. Berberine (BBR), an isoquinoline alkaloid and the main active ingredient in Chinese herbal medicine Coptidis Rhizoma (Huanglian), has been revealed to have a wide range of beneficial properties against antibiotics, anti-inflammatory, anti-apoptotic, anti-oxidant, and neuroprotective effects [9–14]. Recently, based on more and more in vitro and in vivo researches, BBR has protective influence in cardiovascular disease (CVD), like lowering serum lipid levels [15], and blood sugar [16], along with protecting vascular endothelium [17], with anti-inflammatory [18] and antioxidant [19] impacts. It is worth noting that BBR has presented beneficial impacts against atherosclerosis. BBR improves atherosclerosis in mice with hyperhomocysteinemia, which is relative to peroxisome proliferator-activated receptor γ (PPARγ) activation as well as subsequent inhibition on endothelial cell oxidative stress [20]. The anti-atherosclerotic properties of BBR can also prevent the formation of foam cells through inhibiting cholesterol accumulating in macrophages [21]. However, the underlying mechanism by which BBR protects aorta and improves atherosclerosis is still not clear.
Here, we use ApoE knockout (ApoE−/−) mice for experiments, which will develop hypercholesterolemia and spontaneously form complex plaques under a normal diet, and a high-fat diet can promote hyperlipidemia, which can form atherosclerotic lesions similar to humans [22]. It has been widely used in atherosclerosis research. We placed the perivascular collar around the carotid artery of ApoE−/− mice receiving a high-fat diet to establish an animal model of carotid atherosclerosis, and investigated the regulation of BBR on serum lipid levels, carotid artery histology, endothelial function, cell proliferation and apoptosis levels and PI3K/AKT/mTOR signaling pathway, aming to explore the protective impact of BBR on carotid atherosclerosis and its underlying mechanism [23,24]. This work provides an experimental basis for further research on the mechanism of BBR in preventing and treating carotid atherosclerosis, indicating that BBR may become a potential drug for the treatment of carotid atherosclerosis.
2. Materials & methods
2.1. Animals & treatment
Male 8-week-old wild-type (WT) C57BL/6 and ApoE−/− mice on C57BL/6 genetic background from Beijing Vital River Laboratory Animal Technology Co., Ltd., which our team housed under specific-pathogen-free environment (20–25°C, 55–65% humidity, 12 h light/dark cycle), and had free access to water and food, all procedures in strict reference to the Guidelines for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology, China, as well as approved by the Laboratory Animal Ethics Committee, Affiliated Hospital of Shandong University of Traditional Chinese Medicine.
Our team anesthetized ApoE−/− mice via intraperitoneally injecting pentobarbital sodium (50 mg/kg), later performed carotid artery surgery under dissecting microscopes, gently separated the right common carotid artery, where placed narrow collars (length 5 mm; inner diameter 0.3 mm; outer diameter 0.6 mm) around, and fixed the collar with three surgical threads. In C57BL/6 J mice, our team isolated the right common carotid artery without placing the right neck [25]. One week after operation, our team allocated by random ApoE−/− mice into 5 groups (n = 12) based on body weight: M group (ApoE −/− model mice), ATO+PIO group (ApoE−/− mice+ATO+PIO-4.6 mg/kg) (ATO and PIO, positive drug, Dalian, China), BBRL (ApoE−/− mice + BBR-78 mg/kg) (BBR, A0151, Chengdu, China), BBRM (ApoE−/− mice + BBR-117 mg/kg) and BBRH (ApoE−/− mice + BBR-156 mg/kg), fed a high-fat diet (with fat content of 21% and cholesterol content of 0.15%). WT C57BL/6 mice served as the control group (C group, n = 12), receiving normal diet. Our team treated mice in C and M group with physiological saline, and others with doses. Our team intervened all mice via oral gavage for 12 weeks, measured their weight at 0, 1, 3, 6, and 12 weeks after experiment.
2.2. Sample collection and treatment
After experiment, our team anesthetized the mice with isoflurane, took the blood from abdominal aorta, later sacrificed the mice via decapitation, separated and collected the right carotid artery under operating microscopes for further analysis.
2.3. ELISA analysis
After centrifuging the blood sample at 1300 × g for 10 min at 4°C to collect serum, our team added corresponding reagents in reference to biochemical kits (Nanjing Jiancheng Bioengineering Institute) instructions and procedures for serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) content determination [25].
2.4. Morphological analysis
After fixing the right carotid artery in 4% paraformaldehyde, embedding it in OCT, and cutting it into frozen sections (6 μm), our team applied HE staining (Wuhan Servicebio Biological Technology Co., Ltd.) to observe carotid plaque area and neointima formation to assess carotid atherosclerotic lesions, stained oil red O working solution (Sigma-Aldrich, St. Louis, U.S.) for 20 min, later decolorized it with 60% isopropanol for 10 min, washed thoroughly in phosphate buffered saline (PBS) and later acquired images under common optical microscopes to evaluate lipid droplet accumulation [26].
2.5. Immunofluorescence staining
After blocking the frozen sections with 5% FBS for 30 min to incubate with Ki67 antibody (Cell Signaling Technology, U.S.) and TUNEL antibody (Cell Signaling Technology) at 4°C overnight, our team marked the target protein with red or green fluorescence for visualization, stained the nuclei with DAPI (blue fluorescence) (Cell Signaling Technology), and later performed image acquisition via confocal microscopes (Leica, Germany) to evaluate the proportion of positive cells [27].
2.6. Western blot analysis
After fully lysing mice carotid artery tissue in RIPA lysis buffer (Cell Signaling Technology) for 30 min, our team centrifuged them at 12,000 r/min at 4°C for 15 min, took the supernate as the total protein extract to be tested, applied BCA kit (Beyotime) to measure the total protein concentration, loaded each well with 50 μg protein and separated them via 8–15% SDS-PAGE. Our team transferred the protein to PVDF film (Millipore, U.S.), blocking the film in 5% skim milk, via following primary antibodies overnight at 4°C: p-PI3K p85, PI3K p85, p-AKT, AKT, p-mTOR, mTOR, Beclin-1, P62, and GAPDH antibodies (1:1000 dilution). Our team incubated the films with anti-mouse and -rabbit IgG peroxidase-conjugated secondary antibodies, visualized bands with ECL kits (Millipore, U.S.) and quantified them via Quantity One Software (Bio-Rad, U.S.) [26].
2.7. Statistical analysis
With data being expressed as means ± standard deviation (SD) from at least three independent experiments, our team proceeded statistical analysis between two groups with t test as well as among multiple groups via one-way analysis of variance (ANOVA), P < 0.05 regarded as statistically significant.
3. Results
In this study, we hypothesized that BBR plays a role in improving carotid atherosclerosis, which may be mediated by regulating the PI3K/AKT/mTOR signaling pathway. First, we evaluated the effect of BBR on serum lipid levels, carotid artery lipid accumulation and intimal hyperplasia in carotid atherosclerotic ApoE−/− mice. To study the molecular mechanism, we tested the expression of cell proliferation and apoptosis, PI3K/AKT/mTOR and autophagy-related proteins.
3.1. BBR reduced serum lipid levels in ApoE−/− mice
In order to confirm the blood lipid-lowering impact of BBR, our team measured blood lipid levels in each group. Compared with C group, serum TC, TG and LDL-C levels in M group increased signally (Figure 1(a–c), while HDL-C levels were signally reduced (P < 0.0001) (Figure 1d). However, compared with M group, low-, medium- and high-dose BBR reduced serum TC, TG and LDL-C levels (P < 0.0001, P < 0.05, P < 0.001), of which BBRH group had a significant effect (Figure 1(a–c)). Only high-dose BBR increased HDL-C levels (P < 0.05) (Figure 1(d)). Throughout our experiment, the body weight of mice was recorded. All mice weight elevated in comparison with that before experiment, but there was no difference among the groups at each time point in ApoE −/− mice (Figure 1e).
Figure 1.
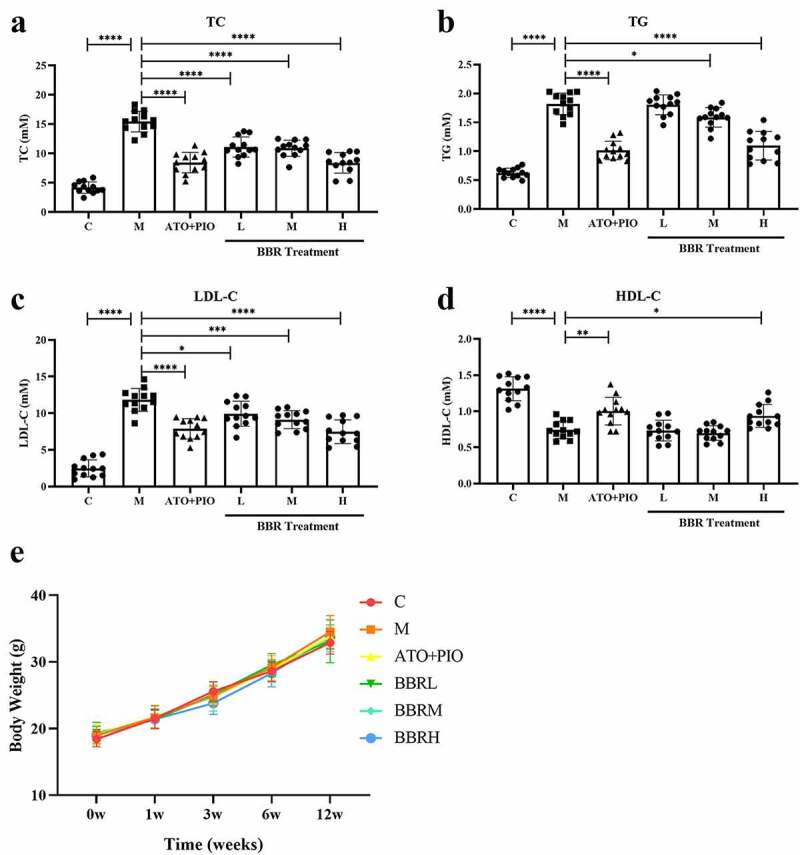
BBR reduced blood lipid levels in ApoE−/− mice. (a) TC level; (b) TG level; (c) LDL-C level; (d) HDL-C level; (e) Body weight; the findings were presented as mean ± SD; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
3.2. BBR reduced plaque area in carotid arteries in ApoE−/− mice
The pathological diagnosis of carotid artery vascular tissue was significantly different between the groups. In C group, vascular intima, media, and adventitia were neat and intact, with no obvious structural damage being observed (Figure 2); while in M group, obvious thickening of the vascular intima was observed near the intima, the smooth muscle in the vascular medium became less, and the atherosclerotic plaque increased (P < 0.0001) (Figure 2). Compared with the M group, low-, medium- and high-dose BBR showed a significant and dose-dependent inhibitory effect on these changes, the intima was visually thickened and relatively intact, atherosclerotic plaques were reduced, and atherosclerotic plaques were reduced (Figure 2). The ratio of hardened plaque area to lumen area was visually reduced (P < 0.01, P < 0.001, P < 0.0001) (Figure 2(b)).
Figure 2.
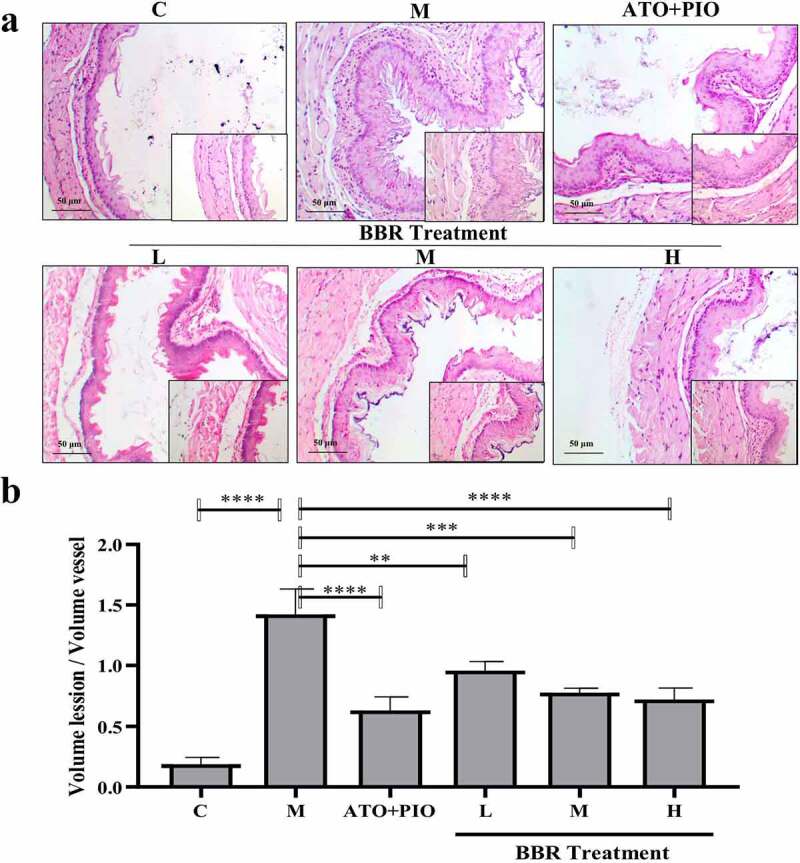
BBR reduced the plaque area in ApoE−/− mice carotid arteries. (a) H&E staining representative image of the carotid artery (large image was magnified 40; small image was magnified 200); (b) the ratio of carotid artery atherosclerotic plaque area to lumen area; the findings were presented as mean ± SD; ** P < 0.01, *** P < 0.001, **** P < 0.0001
3.3. BBR inhibited neointima formation in carotid arteries of ApoE−/− mice
Next, the impact of BBR on neointima formation was observed. Compared with C group, intimal hyperplasia was increased in M group (P < 0.0001) (Figure 3); while compared with M group, middle- and high-dose BBR could inhibit carotid artery neointimal hyperplasia in ApoE−/− mice (P < 0.05, P < 0.01), among which high-dose group had a significant impact (Figure 3).
Figure 3.

BBR inhibited neointima formation in ApoE−/− mice carotid arteries. (a) H&E staining representative image of carotid artery (magnification 200); (b) carotid artery intima/media thickness ratio; the findings were presented as mean ± SD; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
3.4. BBR inhibited lipid accumulation in carotid arteries of ApoE−/− mice
Oil red O staining results showed that compared with C group, carotid lipid droplets in M group elevated signally (P < 0.0001) (Figure 4); whereas in comparison with M group, medium and high-dose BBR could inhibit lipid droplets accumulating in ApoE−/− mice (P < 0.01, P < 0.001), and high-dose group had a significant effect (Figure 4).
Figure 4.
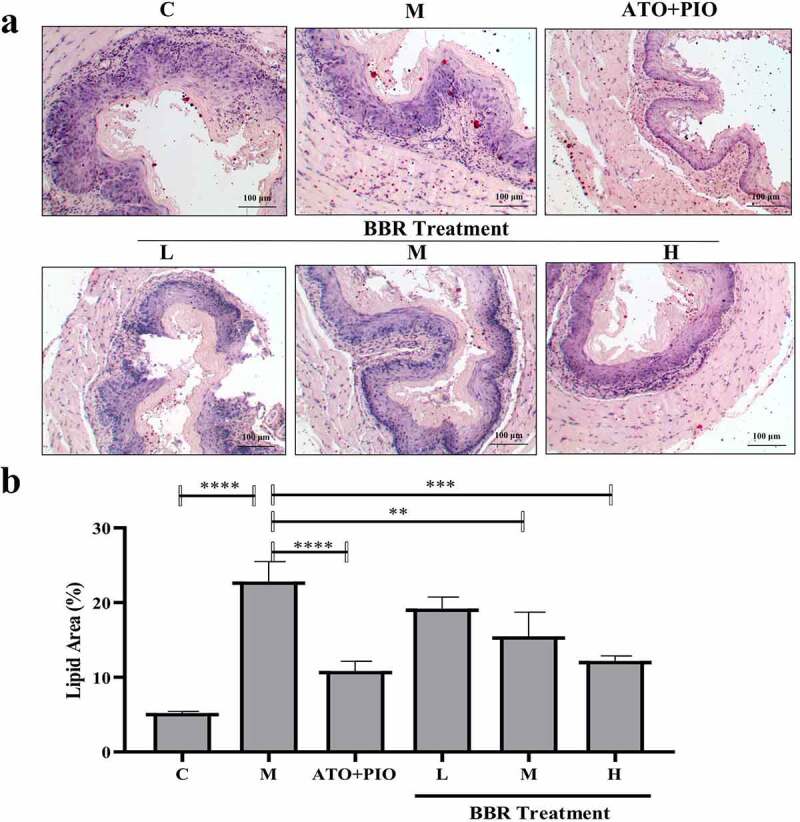
BBR inhibited lipid accumulation in ApoE−/− mice carotid arteries. (a) oil red O staining representative image of carotid artery (magnification 40); (b) Carotid artery lipid droplet area; the findings were presented as mean ± SD; ** P < 0.01, *** P < 0.001, **** P < 0.0001
3.5. BBR promoted cell proliferation and inhibited apoptosis in carotid arteries of ApoE−/− mice
Immunofluorescence results showed that compared with C group, the proportion of Ki67+ cells in the carotid artery of M group decreased (P < 0.0001) (Figure 5), and the proportion of TUNEL+ cells increased (P < 0.0001) (Figure 6), indicating that the cell proliferation rate reduced and the apoptosis rate was increased. Compared with M group, medium- and high-dose BBR promoted cell proliferation in a dose-dependent manner (P < 0.001, P < 0.0001) (Figure 5), medium- and high-dose BBR could inhibit cell apoptosis (P < 0.05, P < 0.001) (Figure 6), and high-dose group had a significant effect.
Figure 5.
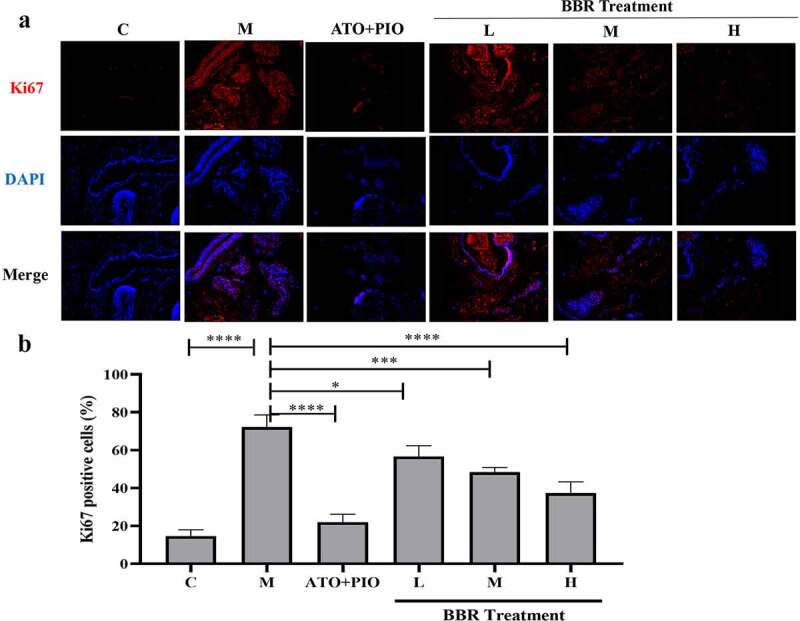
BBR promoted cell proliferation in ApoE−/− mice carotid arteries. (a) Ki67 immunofluorescence staining representative image of carotid artery (magnification 100), the first row represented Ki67 staining of proliferating cells (red), the second row represented nuclear staining by DAPI (blue), and the third row represented the merged image; (b) The proportion of Ki67+ cells in carotid arteries; the findings were presented as mean ± SD; ** P < 0.01, **** P < 0.0001
Figure 6.
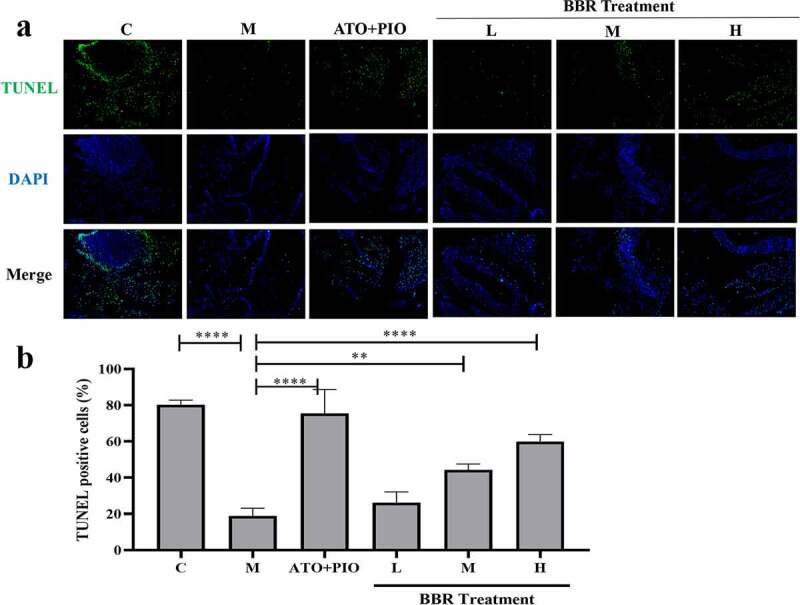
BBR inhibited cell apoptosis in ApoE−/− mice carotid arteries. (a) TUNEL immunofluorescence staining representative image of carotid artery (magnification 100), the first row represented TUNEL staining of apoptotic cells (green), the second represented nuclear staining via DAPI (blue), and the third represented the merged images; (b) the proportion of TUNEL+ cells in the carotid arteries; the findings were presented as mean ± SD; * P < 0.05, **** P < 0.0001
3.6. BBR regulated PI3K/AKT/mTOR signaling pathway in ApoE−/− mice carotid arteries
Aiming to confirm whether BBR regulatory mechanism was relative to PI3K/AKT/mTOR signaling pathway, our team measured relative protein expression via western blot. The findings revealed that compared with C group, p-PI3K and p-mTOR expressions elevated in M group (P < 0.0001), and p-AKT expression decreased (P < 0.0001); in comparison with M group, medium- and high-dose BBR reversed the changes in protein expression (P < 0.0001, P < 0.001, P < 0.01, P < 0.05) (Figure 7). Additionally, compared with C group, M group showed increased Beclin-1 and P62 protein expressions (P < 0.0001), while low-, medium-, and high-dose BBR reduced Beclin-1 and P62 levels in a dose-dependent manner of ApoE−/− mice (P < 0.05, P < 0.0001, P < 0.01) (Figure 7). These findings indicated that BBR modulated PI3K/AKT/mTOR signaling pathway in a dose-dependent manner to improve carotid atherosclerosis. The impact of BBR high-dose group is significant.
Figure 7.
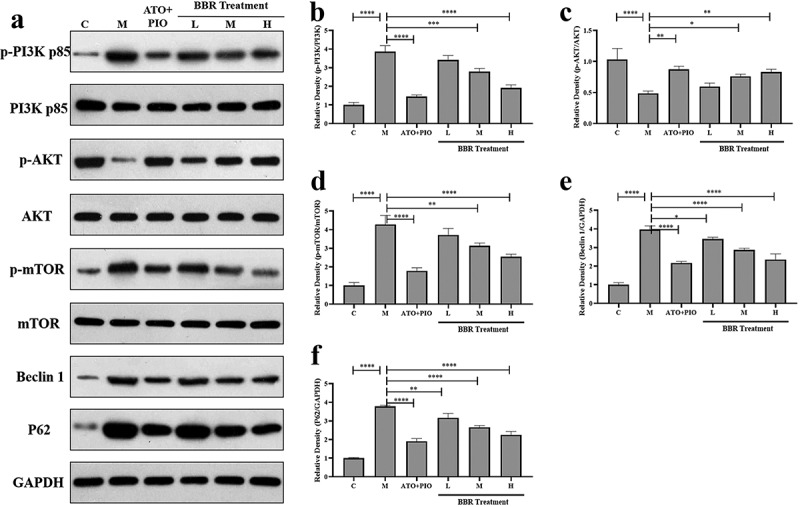
BBR regulated PI3K/AKT/mTOR signaling pathway in ApoE−/− mice carotid arteries. (a) representative image of protein expression relative to PI3K/AKT/mTOR signaling pathway in carotid arteries; (b-f) p-PI3K/PI3K, (b) p-AKT/AKT, (c) p-mTOR/mTOR, (d) Beclin-1, (e-f) P62 protein expression levels in carotid arteries; the findings were presented as mean ± SD; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
4. Discussion
It has been fully realized that carotid atherosclerosis is a complex pathophysiological process, which is related to hyperlipidemia, endothelial dysfunction, cell proliferation and autophagy [27,28]. In this study, ApoE −/− mice were placed in a carotid artery neck ring combined with a high-fat diet to establish an animal model of carotid atherosclerosis (survival rate after surgery was 100%) to explore the mechanism of BBR in improving the disease. Studies have shown that high-fat diet feeding increases the blood viscosity of mice, while the placement of the collar affects local blood circulation, which is based on local changes in the hemodynamic state of the carotid artery, which leads to carotid atherosclerosis [25]. Therefore, this animal model is similar to the pathophysiological conditions of human atherosclerosis and is considered a suitable research object [29–31].
For hundreds of years, Chinese herbal medicine has been widely applied to prevent and treat various diseases. Among them, BBR is a natural alkaloid isolated from the traditional Chinese herbal medicine Coptidis Rhizoma (Huanglian) [32]. Based on previous studies, BBR has various pharmacological properties, including microbial action, anti-inflammatory activity, and regulation of insulin secretion [33–35]. In recent years, BBR has a broader potential clinical significance in preventing and treating different human diseases [36–40]. Our study found that BBR can improve carotid atherosclerosis in a dose-dependent manner, manifested by lowering serum lipid levels, improving intimal hyperplasia, and antagonizing carotid lipid accumulation. In addition, this study clearly shows that the improvement effect of BBR on carotid atherosclerosis is achieved by mediating PI3K/AKT/mTOR signaling pathway, regulating autophagy, promoting cell proliferation and inhibiting cell apoptosis. Obviously, our research confirms and expands the potential mechanism of BBR in improving carotid atherosclerosis.
It has been previously reported that hyperlipidemia acts importantly in carotid atherosclerosis development [41,42]. Recent studies have shown that LDL-C promotes plaque formation, stimulates the proliferation of vascular smooth muscle, and is related to inflammation and lipid accumulation. In our study, the histological observation of the carotid artery showed that BBR reduced the formation and growth of carotid artery plaque and the proliferation of neointima in ApoE −/− mice, and inhibited lipid accumulation. In addition, cell proliferation and apoptosis have been proven to be one of the key steps in a variety of human diseases, including carotid atherosclerosis. Therefore, promoting cell proliferation and inhibiting cell apoptosis may be an effective strategy to prevent and treat carotid atherosclerosis. Our study found that BBR promoted carotid artery cell proliferation and inhibited cell apoptosis, which may be the key role of BBR in anti-carotid atherosclerosis.
PI3K/AKT/mTOR signaling pathway is relative to various cell functions, including cell survival, differentiation, invasion, proliferation, apoptosis and autophagy [43–45]. Studies have confirmed that PI3K/AKT/mTOR is an important signaling pathway of growth factors or cytokines involved in the proliferation and migration of vascular smooth muscle cells, and attenuates lipid accumulation, inflammation and apoptosis in ApoE −/− mice by enhancing autophagy in atherosclerosis. While selective inhibition of mTOR plays a beneficial role in reducing the development of plaque lesions in ApoE -/- mice [46–48]. PI3K is a substrate of tyrosine kinase receptor, and its activation leads to phosphorylation of AKT, which then promotes mTOR phosphorylation and activation to regulate cell function [49]. We found that BBR up-regulated p-PI3K and p-mTOR expressions but down-regulated p-AKT expression in a dose-dependent manner, confirming that BBR activates PI3K/AKT/mTOR signaling to improve atherosclerotic function. In addition, BBR also reduced autophagy activity markers Beclin-1 and P62 protein expression, confirming the effective effect of the mTOR-mediated negative control axis of autophagy [50].
5. Conclusions
The results of this study indicate that in ApoE −/− mice receiving high-fat diet for 12 weeks, BBR can reduce serum lipid levels, antagonize carotid lipid accumulation, and improve intimal hyperplasia, which may be through regulating PI3K/AKT The/mTOR signaling pathway, regulating autophagy, promoting cell proliferation and inhibiting cell apoptosis. In general, BBR is a promising anti-carotid atherosclerosis treatment strategy. In our future work, we can further study the treatment mechanism in order to provide a basis for clinical application and resource development and utilization.
Funding Statement
This work was supported by the Jinan Science and Technology Development Plan Project (NO. 202019151) and the Special Technical Project of TCM’s Prevention and Treatment of “ Carotid Atherosclerostic Plaque” Based on Heart Governing Blood and Vessels Theory (Luweihan [2021] No. 45).
Research highlights
1. BBR improves carotid atherosclerosis in ApoE −/− mice.
2. BBR regulates autophagy, promotes cell proliferation and inhibits cell apoptosis.
3. BBR regulates the PI3K/AKT/mTOR signaling pathway.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. 2016;109:708–715. [DOI] [PubMed] [Google Scholar]
- [2].Libby P, Bornfeldt KE, Tall AR.. Atherosclerosis: successes, surprises, and future challenges. Circ Res. 2016;118:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- [4].Lee GY, Kim JH, Oh GT, et al. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J Control Release. 2011;155:211–217. [DOI] [PubMed] [Google Scholar]
- [5].Barrett TJ. Macrophages in atherosclerosis regression. Arterioscler Thromb Vasc Biol. 2020;40:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lu H, Daugherty A. Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009;73:595–601. [DOI] [PubMed] [Google Scholar]
- [8].Tian DY, Jin XR, Zeng X, et al. Notch signaling in endothelial cells: is it the therapeutic target for vascular neointimal hyperplasia? Int J Mol Sci. 2017;18(8):1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng F, Wang Y, Li J, et al. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int J Cardiol. 2013;167:936–942. [DOI] [PubMed] [Google Scholar]
- [10].Li W, Hua B, Saud SM, et al. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol Carcinog. 2015;54:1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu S, Yu L, Zhu J, et al. Berberine promotes glucagon-like peptide-1 (7-36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol. 2009;200:159–165. [DOI] [PubMed] [Google Scholar]
- [12].Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20:574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang X, Zhao Y, Xu J, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pozsgay J, Szekanecz Z, Sarmay G. Antigen-specific immunotherapies in rheumatic diseases. Nat Rev Rheumatol. 2017;13:525–537. [DOI] [PubMed] [Google Scholar]
- [15].Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. [DOI] [PubMed] [Google Scholar]
- [16].Cok A, Plaisier C, Salie MJ, et al. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie. 2011;93:1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu L, Liu J, Huang Z, et al. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun. 2015;458:796–801. [DOI] [PubMed] [Google Scholar]
- [18].Bae A, Cheon G. Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages. Korean J Physiol Pharmacol. 2016;20:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sun Y, Yuan X, Zhang F, et al. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci Rep. 2017;7:11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li H, He C, Wang J, et al. Berberine activates peroxisome proliferator-activated receptor gamma to increase atherosclerotic plaque stability in Apoe(-/-) mice with hyperhomocysteinemia. J Diabetes Investig. 2016;7:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zimetti F, Adorni MP, Ronda N, et al. The natural compound berberine positively affects macrophage functions involved in atherogenesis. Nutr Metab Cardiovasc Dis. 2015;25:195–201. [DOI] [PubMed] [Google Scholar]
- [22].De Saint-Hubert M, Bauwens M, Deckers N, et al. In vivo molecular imaging of apoptosis and necrosis in atherosclerotic plaques using microSPECT-CT and microPET-CT imaging. Mol Imaging Biol. 2014;16:246–254. [DOI] [PubMed] [Google Scholar]
- [23].Li C, Wang Z, Wang C, et al. Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One. 2015;10:e124031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cai X, Li X, Li L, et al. Adiponectin reduces carotid atherosclerotic plaque formation in ApoE-/- mice: roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol Med Rep. 2015;11:1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xie Z, Yang Y, He Y, et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics. 2020;10(10):4694–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jing Y, Gao B, Han Z, et al. The protective effect of HOXA5 on carotid atherosclerosis occurs by modulating the vascular smooth muscle cell phenotype. Mol Cell Endocrinol. 2021;534:111366. [DOI] [PubMed] [Google Scholar]
- [27].Kou JY, Li Y, Zhong ZY, et al. Berberine-sonodynamic therapy induces autophagy and lipid unloading in macrophage. Cell Death Dis. 2017;8:e2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li W, Li Y, Zhao Y, et al. The protective effects of aloperine against ox-LDL-induced endothelial dysfunction and inflammation in HUVECs. Artif Cells Nanomed Biotechnol. 2020;48:107–115. [DOI] [PubMed] [Google Scholar]
- [29].Lu J, Sun M, Wu X, et al. Urate-lowering therapy alleviates atherosclerosis inflammatory response factors and neointimal lesions in a mouse model of induced carotid atherosclerosis. Febs J. 2019;286:1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pan X, Hou R, Ma A, et al. Atorvastatin upregulates the expression of miR-126 in apolipoprotein E-knockout mice with carotid atherosclerotic plaque. Cell Mol Neurobiol. 2017;37:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ma J, Yang S, Ma A, et al. Expression of miRNA-155 in carotid atherosclerotic plaques of apolipoprotein E knockout (ApoE(-/-)) mice and the interventional effect of rapamycin. Int Immunopharmacol. 2017;46:70–74. [DOI] [PubMed] [Google Scholar]
- [32].Kuo P, Chuang C, Yeh H, et al. Growth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the HER2/PI3K/Akt signaling pathway. J Agric Food Chem. 2017;59:8216–8224. [DOI] [PubMed] [Google Scholar]
- [33].Huang H, Pan P, Gong R, et al. Anti-inflammatory effects of three kinds of traditional Mongolian medicine monomer and its combination on LPS-stimulated RAW264.7 macrophages. Eur Rev Med Pharmacol Sci. 2016;20:950–958. [PubMed] [Google Scholar]
- [34].Pang B, Zhao H, Zhou Q, et al. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015(3):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: from in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449–461. [DOI] [PubMed] [Google Scholar]
- [36].Chen C, Yu Z, Li Y, et al. Effects of berberine in the gastrointestinal tract - a review of actions and therapeutic implications. Am J Chin Med. 2014;42:1053–1070. [DOI] [PubMed] [Google Scholar]
- [37].Li DX, Zhang J, Zhang Y, et al. Inhibitory effect of berberine on human skin squamous cell carcinoma A431 cells. Genet Mol Res. 2015;14:10553–10568. [DOI] [PubMed] [Google Scholar]
- [38].Ren K, Zhang W, Wu G, et al. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed Pharmacother. 2016;84:1748–1759. [DOI] [PubMed] [Google Scholar]
- [39].Tang F, Yang L. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;495:1482–1489. [DOI] [PubMed] [Google Scholar]
- [40].Xu X, Sun C, Ma Y, et al. Impacts of berberine on oxidized LDL-induced proliferation of human umbilical vein endothelial cells. Am J Transl Res. 2017;9:4375–4389. [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang Z, Zhang M, Li Y, et al. Simvastatin inhibits the additive activation of ERK1/2 and proliferation of rat vascular smooth muscle cells induced by combined mechanical stress and oxLDL through LOX-1 pathway. Cell Signal. 2013;25:332–340. [DOI] [PubMed] [Google Scholar]
- [42].Zhang L, Jia YH, Zhao XS, et al. Trichosanatine alleviates oxidized low-density lipoprotein induced endothelial cells injury via inhibiting the LOX-1/p38 MAPK pathway. Am J Transl Res. 2016;8:5455–5464. [PMC free article] [PubMed] [Google Scholar]
- [43].Jiang Y, Kou J, Han X, et al. ROS-dependent activation of autophagy through the PI3K/Akt/mTOR pathway is induced by hydroxysafflor yellow A-sonodynamic therapy in THP-1 macrophages. Oxid Med Cell Longev. 2017(8):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pompura SL, Dominguez-Villar M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J Leukoc Biol. 2018;6:1065–1076. DOI: 10.1002/JLB.2MIR0817-349R [DOI] [PubMed] [Google Scholar]
- [46].Zhai C, Cheng J, Mujahid H, et al. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS One. 2014;9:e90563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peng J, He X, Zhang L, et al. MicroRNA‑26a protects vascular smooth muscle cells against H2O2‑induced injury through activation of the PTEN/AKT/mTOR pathway. Int J Mol Med. 2018;42(3):1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li Z, Xu C, Sun D. MicroRNA-488 serves as a diagnostic marker for atherosclerosis and regulates the biological behavior of vascular smooth muscle cells. Bioengineered. 2021;12(1):4092–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saha A, Connelly S, Jiang J, et al. Akt phosphorylation and regulation of transketolase is a nodal point for amino acid control of purine synthesis. Mol Cell. 2014;55:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wan Q, Yang M, Liu Z, et al. Atmospheric fine particulate matter exposure exacerbates atherosclerosis in apolipoprotein E knockout mice by inhibiting autophagy in macrophages via the PI3K/Akt/mTOR signaling pathway. Ecotoxicol Environ Saf. 2021;208:111440. [DOI] [PubMed] [Google Scholar]


