Abstract
Klotho is an aging-suppressor gene. The purpose of this study was to investigate whether Klotho deficiency affects arterial structure. We found that Klotho-deficient (kl/kl) mice developed severe arterial calcification and elastin fragmentation. Klotho-deficient mice demonstrated higher levels of bone morphogenetic proteins (BMP2, BMP4) and runt-related transcription factor 2 (RUNX2) in aortas, indicating that Klotho deficiency upreglates expression of BMP2 and RUNX2 (a key transcription factor in osteoblasts). To exclude the potential involvement of hyperphosphatemia in arterial calcification, Klotho-deficient mice were given low phosphate diet (0.2%). Low phosphate diet normalized blood phosphate levels and abolished calcification in the lungs and kidneys, but it did not prevent calcification in the aortas in Klotho-deficient mice. Thus, Klotho deficiency per se might play a causal role in the pathogenesis of arterial calcification which is independent of hyperphosphatemia. In cultured mouse aortic smooth muscle cells (ASMCs), Klotho-deficient serum induced transition of ASMCs to osteoblasts. Klotho-deficient serum promoted BMP2/vitamin D3-induced protein expression of PIT2 and RUNX2, phosphorylation of SMAD1/5/8 and SMAD2/3, and extracellular matrix calcification. Interestingly, treatments with recombinant Klotho protein abolished BMP2/vitamin D3-induced osteoblastic transition and morphogenesis and calcification. Therefore, Klotho is a critical regulator in the maintenance of normal arterial homeostasis. Klotho deficiency-induced arterial calcification is an active process which involves osteoblastic transition of SMCs and activation of the BMP2-RUNX2 signaling.
Keywords: arterial calcification, osteoblast, BMP2, RUNX2, aortic smooth muscle cells
Introduction
Arterial calcification is largely seen in individuals older than 60 years and in patients with diabetes, atherosclerosis, hypertension, and chronic kidney disease (CKD) (Zhu, Mackenzie, Farquharson, & Macrae, 2012). The frequency of arterial calcification is linked to mortality and morbidity of cardiovascular diseases (Zhu et al., 2012). Arterial calcification is characterized by the deposition of calcium minerals in arteries, likely resulting in arterial stiffening. Arterial calcification is an important risk factor for systolic hypertension, ischemic heart disease and stroke. However, the pathogenesis of arterial calcification is largely unknown.
Klotho has been considered as anti-aging gene, which encodes a single-pass transmembrane protein of 1014 amino acids (130 kDa) (Kuro-o et al., 1997; Shiraki-Iida et al., 1998). Klotho gene is primarily expressed in kidneys and brain choroid plexus (Kuro-o et al., 1997). Notably, short-form Klotho protein with an apparent molecular weight of 65 kDa was found in the blood as a result of alternative RNA splicing or proteolytic cleavage (C. D. Chen, Podvin, Gillespie, Leeman, & Abraham, 2007; J. Chen, Fan, Wang, & Sun, 2018; K. Chen et al., 2020; Huang, 2010; Shiraki-Iida et al., 1998). Klotho deficiency reduced lifespan (mouse dies at 8 to 10 weeks of age) whereas overexpression of Klotho extended lifespan by 20-30% (Kuro-o et al., 1997). Although Klotho is detected in the blood (Hu, Kuro-o, & Moe; Huang; Xu & Sun, 2015), the physiological function of the circulating Klotho in vessels is poorly understood.
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) family that regulate bone formation (Lowery & de Caestecker, 2010). BMP2 and BMP4 have been reported to have osteogenic actions as mediators of arterial calcification (Hruska, Mathew, & Saab, 2005; Lowery & de Caestecker, 2010). BMP2 activates type I and type II serine/threonine kinase receptors which subsequently regulates intracellular Smad transcription factors. Activation of Smad signaling promotes osteoblastic transition through increasing transcription factors such as RUNX2, osterix (Osx), distal-less homeobox 5 (Dlx5), and Msh homeobox 2 (Msx2). RUN2 has been considered as an essential regulator of arterial calcification.
The purpose of this study was to investigate a hypothesis that Klotho deficiency leads to arterial calcification via promoting osteoblastic transition of arterial smooth muscle cells and upregulating BMP2 signaling.
Materials and methods
A detailed method section is available in the Online Supplemental Methods and Data.
Animal studies.
Heterozygous Klotho mutant (kl/+) mice with more than 9 generations in 129/Sv background were kindly provided by Dr. Kuro-o (Kuro-o et al., 1997). For dietary phosphate restriction, mice were fed with a low phosphate (Pi) diet containing 0.2% (wt/wt) inorganic phosphate (TD-09073, Harlan Teklad, Madison, WI) from weaning at 3 weeks of age.
Measurements of pulse wave velocity (PWV).
PWV was measured as we described recently (K. Chen, Zhou, & Sun, 2015; Lin, Chen, & Sun, 2016).
Plasma phosphate and calcium measurements.
Plasma samples were sent to the Yale University Mouse Metabolic Phenotyping Centers for measuring plasma inorganic phosphorous and calcium as described recently (Lin et al., 2016).
Immunohistochemistry (IHC).
IHC was performed as described previously (Lin & Sun, 2015a, 2015b). A detailed procedure is available in the Online Supplemental Methods and Data.
Cell cultures.
For details, refer to Online Supplemental Methods and Data. Klotho-deficient serum was generated as we described recently (Fan & Sun, 2016).
Western blotting.
Western blot was performed as described in our previous studies (K. Chen et al., 2015; Crosswhite, Chen, & Sun, 2014; Zhou, Chen, Lei, & Sun, 2015; Zhou, Chen, Wang, et al., 2015). A detailed procedure is available in the Online Supplemental Methods and Data.
RNA Isolation and RT-PCR.
The RT-PCR procedure was described previously (Lin & Sun, 2012). A detailed procedure is available in the Online Supplemental Methods and Data.
Statistical Analysis.
Data were analyzed using a one-way ANOVA. The Newman-Keuls procedure was used to assess differences between means. To compare data between two groups, student t test was used. Data were expressed as mean ± SEM. A probability value with p<0.05 were considered significant.
Results
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Klotho deficiency caused severe arterial calcification and increased the protein levels of BMP2, BMP4, and RUNX2 in aortas
Klotho deficient mice (kl/kl) lived for only 8 to 9 weeks. There was a loss of aortic tissue in 8 week-old kl/kl mice as revealed by H&E staining (Fig. 1A). Alizarin red staining showed severe arterial calcification in Klotho deficient mice (Fig 1A&B), suggesting that Klotho deficiency caused severe arterial calcification in mice. To investigate the potential mechanism of arterial calcification in Klotho deficient animals, we measured several key proteins in the regulation of osteogenesis using western blot. Klotho deficient mice at the earlier ages (4-6 weeks) had significantly higher levels in BMP2, BMP4, and RUNX2 in aortas (Fig. 1C-D), suggesting that Klotho deficiency upregulates these proteins. Upregulation of these factors may contribute to the development of klotho deficiency-induced vascular calcification. However, the levels of BMP2 and BMP4 in aortas of Klotho deficient mice decreased to the normal levels at ages of 8 weeks (Fig. 1C-D). Thus, this result suggests that BMP2 and BMP4 may not be involved in the maintenance of vascular calcification. The level of RUNX2 in aortas from Klotho deficient mice remained elevated at the age of 8 weeks. IHC analysis confirmed upregulation of matrix gla protein (MGP), alkaline phosphatase (ALP), and RUNX2 in Klotho-deficient mice (Fig. S1A-D). These data suggest that Klotho deficiency promotes osteogenesis in aortas.
Figure 1. Klotho deficiency caused severe arterial calcification and increased the protein levels of BMP2, BMP4, and RUNX2 in aortas.
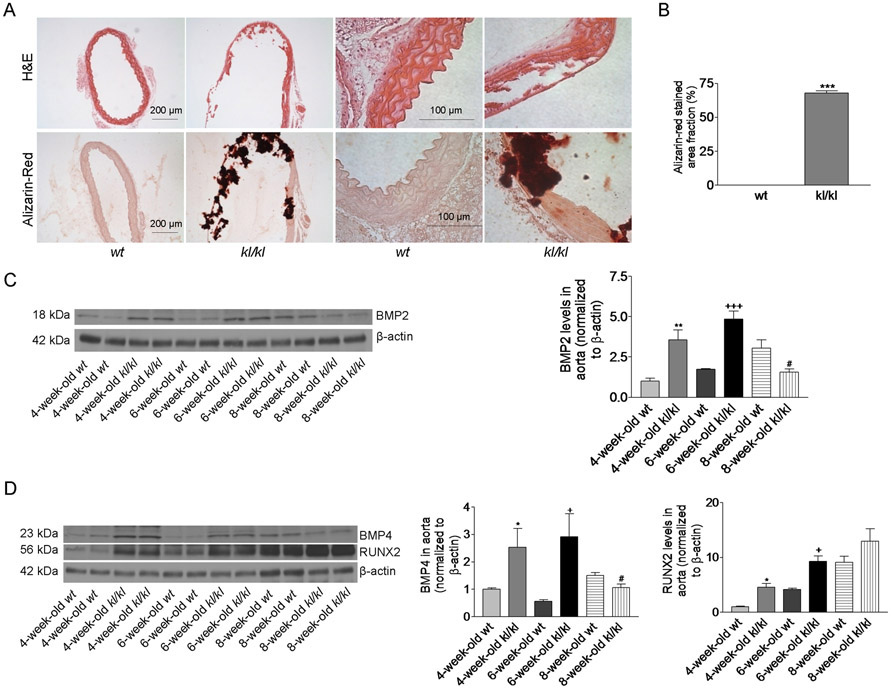
(A), H&E and Alizarin-Red staining of thoracic aorta tissue sections (original magnifications: ×200 and x100) from 8-week-old wt and kl/kl deficient mice. (B), The area fraction of Alizarin-Red positive-staining in aorta section (n=5). ***p<0.001, compared with wt mice. (C), Western blot analysis of BMP2 in aortas. (D), Western blot analysis of BMP4 and RUNX2 in aortas. n=5; *p<0.05, **p<0.01 vs 4-week-old wt; +p<0.05, +++p<0.001 vs 6-week-old wt; #p<0.05 vs 8-week-old wt. Data=means±SEM.
Low phosphate diet did not prevent arterial stiffening in Klotho-deficient mice
It has been reported that Klotho deficient mice developed hyperphosphatemia (Kuro-o et al., 1997). Hyperphosphatemia has been considered as an important regulator of arterial calcification in patients with chronic kidney disease (Kendrick & Chonchol, 2011). To exclude the possible involvement of hyperphosphatemia in arterial calcification, we fed Klotho-deficient mice with low phosphate diet (0.2%) immediately after weaning at the age of 3 weeks. Low phosphate diet prevented premature death in Klotho-deficient mice and largely improved the health condition as evidenced by increased body weights (Fig. 2A&B). Pulse wave velocity (PWV), an index of arterial stiffening, was increased significantly in Klotho-deficient mice on normal diet starting from 6 weeks of age (Fig. 2C). These data indicate that Klotho-deficient mice developed arterial stiffening at their early age. Low phosphate diet did not prevent arterial stiffening in Klotho-deficient mice (Fig. 2C).
Figure 2. Low phosphate did not prevent arterial stiffening in Klotho deficient mice.
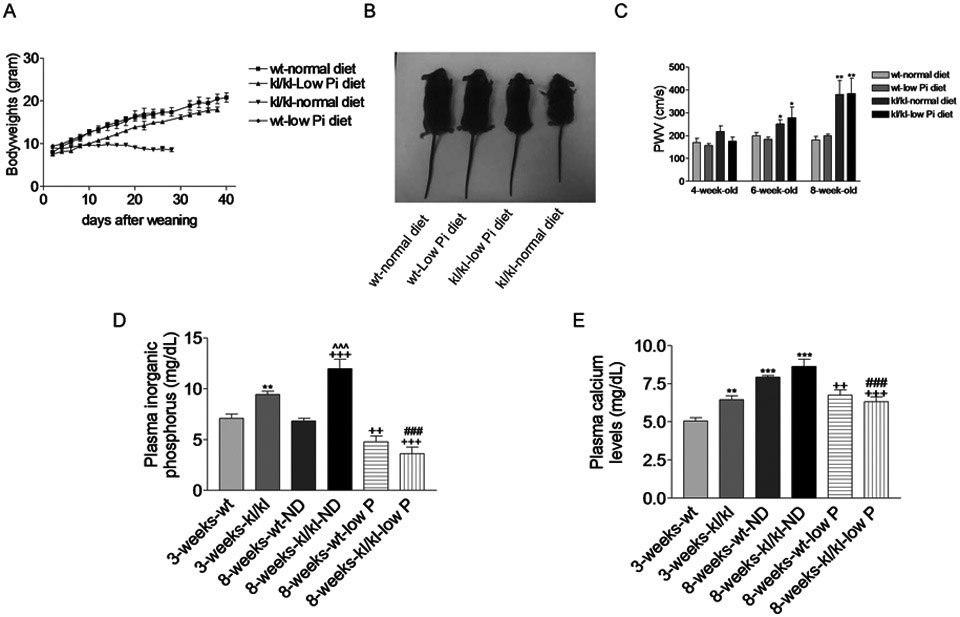
(A), Body weights. (B), Images of animals at 8 weeks old. (C), PWV in mice at different ages. n=5; * p<0.05, ** p<0.01 vs wt-normal diet. (D). Blood phosphorus levels. (E), Blood calcium levels. n=6; * *p<0.01, ***p<0.001 vs 3-weeks-wt; ++p<0.01, +++p<0.001 vs 8-weeks-wt-ND; ^^^p<0.001 vs 3-weeks-kl/kl; ###p<0.001 vs 8-weeks-kl/kl-ND. ND, normal diet. Data=means±SEM.
We found that Klotho-deficient mice displayed significantly higher levels of inorganic phosphate (hyperphosphatemia) and calcium levels (hypercalcemia) (Fig. 2D&E). Low phosphate diet abolished hyperphosphatemia and attenuated plasma calcium levels in Klotho-deficient mice (8 weeks) (Fig. 2D&E). These results indicated that Klotho deficiency-induced arterial stiffening is independent of hyperphosphatemia.
Low phosphate diet abolished lung and kidney calcification but did not prevent aortic calcification in Klotho-deficient mice
To determine organ specificity of calcification, we performed Alizarin red staining in aortas, lungs, and kidneys. Klotho deficient mice at the age of 3 weeks old did not develop obvious calcification in aortas (Fig. 3A), lungs (Fig. 3B), and kidneys (Fig. S2A). At 8 weeks old, Klotho-deficient mice fed with normal diet (ND) showed severe arterial calcification in aortas (Fig. 3C), lungs (Fig. 3D), and kidneys (Fig. S2B). With low phosphate diet, Klotho-deficient mice still developed aortic calcification albeit at a less severe degree (Fig. 3C), suggesting that Klotho deficiency-induced aortic calcification is largely independent of hyperphosphatemia. By contrast, low phosphate diet completely abolished calcification in the lung (Fig. 3D) and the kidney (Fig. S2B), suggesting that the development of calcification in lungs and kidneys is a passive calcium deposition process due to hyperphosphatemia. However, Klotho deficiency might directly promote aortic calcification in the normal blood levels of phosphate.
Figure 3. Low phosphate diet abolished lung calcification but did not prevent aortic calcification in Klotho-deficient mice.
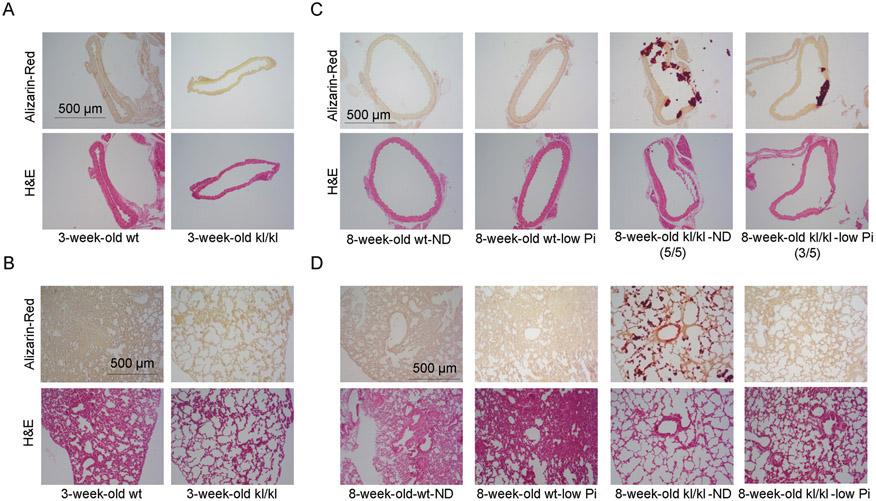
Alizarin-Red and H&E staining of aorta from 3-week-old kl/kl deficient mice (A) and 8-week-old kl/kl deficient mice (C). Alizarin-Red and H&E staining of lung from 3-week-old kl/kl deficient mice (B) and 8-week-old kl/kl deficient mice (D).
In addition, Klotho-deficient mice with normal diet displayed diminished elastin, smooth muscle α-actin, and collagen I in aortas (Fig. S2C-D), indicating arterial remodeling.
Serum levels of Klotho were dropped dramatically in kl/kl mice (Fig. S2E), confirming Klotho deficiency.
Klotho deficiency upregulated protein levels of BMP2, BMP4, RUNX2, and BMP signaling in aortas
To further study the potential molecular basis of aortic calcification in Klotho deficient animals fed on low phosphate diet, several key proteins in the regulation of osteoblastic transition were measured using western blot. Interestingly, at the age of 8 weeks, Klotho deficient mice fed on low phosphate diet displayed higher levels of BMP2, BMP4, and RUNX2 in aortas, compared to those of wt mice fed on low phosphate diet (Fig. 4A&B). The levels of pSMAD(1/5/8) and pSMAD(2/3) were also increased in aortas of Klotho deficient mice compared to those of wt mice (Fig. 4A&B). These results suggest that arterial calcification observed in Klotho deficient mice might be associated with the increases in arterial BMPs and/or BMP signaling.
Figure 4. Klotho deficiency upregulated protein levels of BMP2, BMP4, RUNX2, and BMP signaling in aortas.
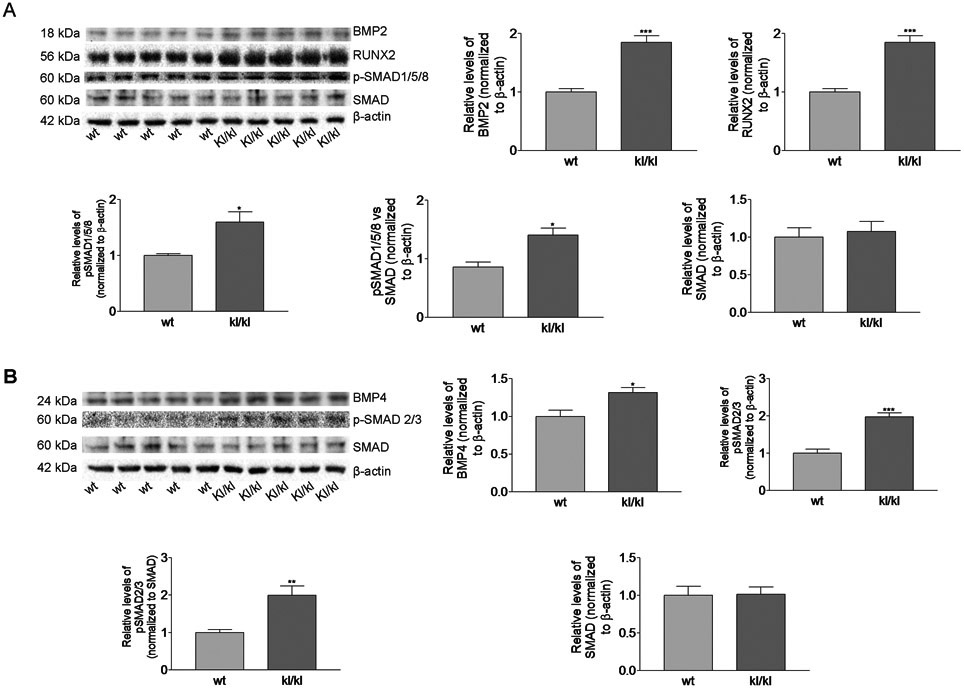
(A), Western blot analysis of BMP2, RUNX2, p-SMAD(1/5/8), and total SMAD in aorta lysates from mice. (B), Western blot analysis of BMP4, pSMAD(2/3), and total SMAD in aortic lysates. n=5; *p<0.05, **p<0.01, ***p<0.001 vs 8-week-old wt mice fed on low phosphate diet. Data=means±SEM.
Klotho-deficient serum (FBS) increased BMP2, BMP4, and RUNX2 protein levels in primary cultures of mouse aortic smooth muscle cells (ASMCs)
To study the molecular mechanism of arterial calcification in Klotho-deficient mice, we used primary culture of ASMCs isolated from wt mouse aortas. Short-form Klotho protein was detected in mouse aortic SMCs, but Klotho mRNA was not detectable (Fig. 5A&B), suggesting that short-form Klotho protein might come from fetal bovine serum in culture medium. Similar results were found in a cell line of mouse aortic smooth muscle cells (MOVAS) (Data not shown).
Figure 5. Klotho-deficient serum (FBS) increased BMP2, BMP4, and RUNX2 protein levels in primary cultures of mouse aortic smooth muscle cells (SMCs).
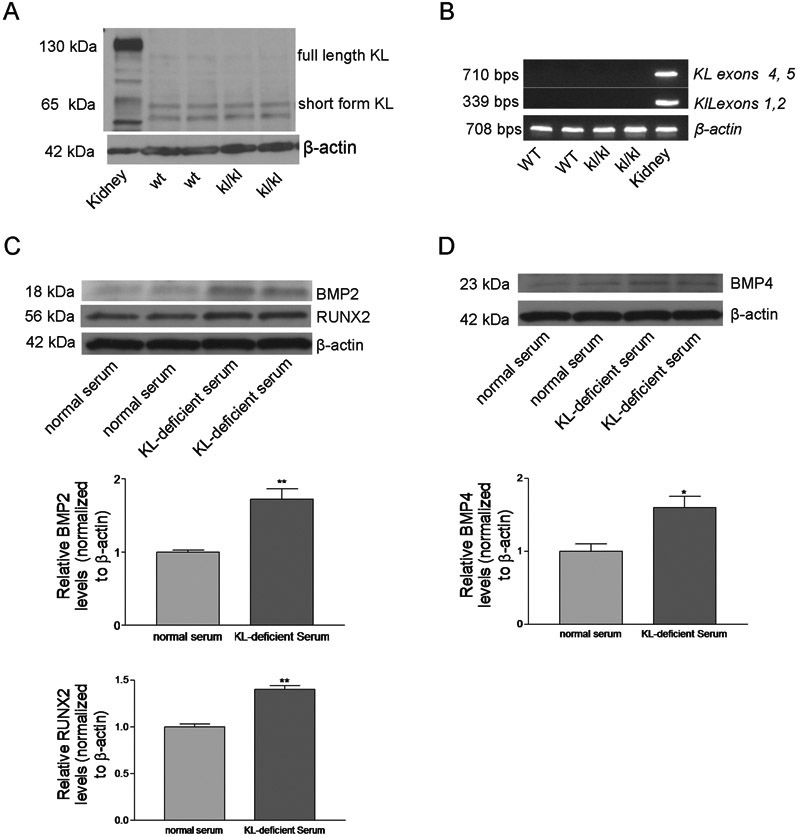
Aortic smooth muscle cells (SMCs) were isolated from 6-week-old kl/kl and wt mice. (A), Short form Klotho protein in primary mouse aortic SMCs was assessed by Western blot. (B), Klotho mRNA in primary mouse ASMCs were detected using RT-PCR. (C), BMP2 and RUNX2 protein expression were measured in cells incubated with Klotho-deficient serum (10%) or normal serum (10%) for 5 days. (D), BMP4 protein was measured after incubation with Klotho-deficient serum (10%) or normal serum (10%) for 5 days. n=5; *p<0.05, **p<0.01 vs normal serum. Data=means±SEM.
To determine the effect of Klotho deficiency on the regulators on calcification, we generated Klotho-deficient FBS (50% reduction in Klotho) as we described recently (Fan & Sun, 2016). Klotho-deficient FBS promoted BMP2, BMP4, and RUNX2 protein expression levels in cultured primary mouse ASMCs (Fig. 5C&D). Thus, Klotho may be a negative regulator for BMP2, BMP4, and RUNX2 expression in ASMCs.
Klotho-deficient serum promoted BMP2-VitD3-induced calcification in cultured mouse aortic smooth muscle cells (MOVAS)
Since in vitro calcification experiment requires long-term culturing of primary ASMCs which leads to cell detachment and death, we switched to a cell line of mouse aortic smooth muscle cells (MOVAS)(Afroze et al., 2003; Mackenzie et al., 2011) for studying calcification. As shown in Fig. 6A, BMP2 induced calcification of MOVAS in the presence of vitamin D3. Interestingly, Klotho-deficient serum promoted BMP2-VitD3-induced cell calcification and calcium deposition (Fig. 6A&B). Importantly, treatments with exogenous Klotho protein completely abolished BMP2-VitD3-induced calcification (Fig. 6A&B). These data suggest that Klotho is not only necessary but also sufficient to curb BMP2-VitD3-induced extracellular matrix calcification of cultured MOVAS.
Figure 6. Klotho-deficient serum promoted BMP2-VitD3-induced calcification in cultured mouse aortic smooth muscle cells (MOVAS).
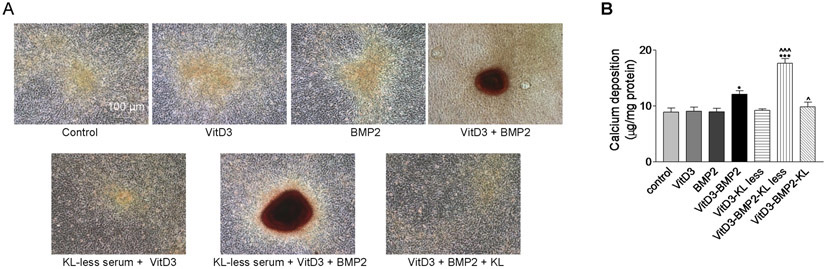
Confluent cells were incubated with 2.5% normal FBS or 2.5% KL-deficient FBS in the presence or absence of 1 nM of vitamin D3 or 200 ng/mL of BMP2 for two weeks. Culture medium was changed every 3 days. Representative photographs of Alizarin Red staining at the magnification of 100 (A). Quantification of calcium contents in extracellular matrix (B). n=4 independent experiments. *p<0.05, ***p<0.001 vs the control; ^p<0.05, ^^^p<0.001 vs VitD3-BMP2. Data=means±SEM.
Klotho-deficient serum promoted BMP2-induced phosphorylations of SMAD1/5/8 and SMAD 2/3 and increased BMP2-induced RUNX2 and PIT2 expression in cultured MOVAS
To further study the regulation of calcification in cultured MOVAS by Klotho, we assessed phosphorylations of SMAD and several key proteins in the regulation of calcification. BMP2 increased phosphorylations of SMAD in the presence of vitamin D3 (Fig. S3A). Importantly, Klotho-deficient serum promoted BMP2-induced phosphorylations of SMAD (Fig. S3A). Klotho deficient serum also potentiated BMP2-induced RUNX2 and PIT2 protein expressions (Fig. S3B). It is noteworthy that exogenous Klotho protein treatments completely abolished BMP2-induced phosphorylations of SMAD and protein expressions of RUNX2 and PIT2 (Fig. S3A&B). These results suggest that Klotho is both necessary and sufficient to inhibit BMP2-induced SMAD signaling and its down-stream protein expressions such as RUNX2 and PIT2 in cultured MOVAS.
Discussion
Arterial calcification is characterized by deposition of hydroxyapatite on elastic lamella of arteries. Hydroxyapatite is composed of calcium and phosphate. It was believed that aortic calcification is a passive process due to hyperphosphatemia in Klotho-deficient (kl/kl) mice(Kuro-o et al., 1997; Xu & Sun, 2015). Here, we report a surprising finding that restoring blood phosphate to normal levels by low phosphate diet did not prevent arterial calcification in Klotho-deficient mice (Fig. 3B). Thus, this finding provides the first evidence that Klotho deficiency might play a direct and active role in the pathogenesis of arterial calcification. Although the short-form Klotho protein (65 kDa) was detected in primary culture of mouse ASMCs, Klotho mRNA was not detectable (Fig. 5). Therefore, ASMCs do not express Klotho and the detected short-form Klotho protein comes from the circulation. This finding is supported by the published data that mouse arteries do not express Klotho protein (Lim et al., 2012; Lindberg et al., 2013). The short-form Klotho protein was detected in serum of kl/kl mouse (Fig. S2F). Therefore, these data suggest that the circulating Klotho acts directly on ASMCs as a hormone. The major source of the circulating Klotho protein is kidneys (Xu & Sun, 2015). It is interesting that a kidney-derived protein plays an important role in the maintenance of normal arterial homeostasis.
It is noticed that Klotho deficiency led to upregulation of BMP2 and BMP4 in aortas (Figs. 4A&B, S2C&D). Bone morphogenetic proteins (BMPs) are also members of the transforming growth factor-β family (Cai, Pardali, Sanchez-Duffhues, & ten Dijke). BMPs can trigger the differentiation of multipotential cells into the osteogenic lineage (Bostrom et al., 1993; Cai et al.). The BMP2 signaling pathway may promote the differentiation of myofibroblasts into the osteogenic lineage (Cheng, Shao, Charlton-Kachigian, Loewy, & Towler, 2003). Recent research showed that BMPs are involved in vascular calcification in low-density lipoprotein receptor-deficient (LDLR−/−) mice (Derwall et al., 2012). BMP2 accelerated atherosclerotic intimal calcification in BMP2 transgenic/apoE knockout mice (Nakagawa et al., 2010). Therefore, it is expected that the increases in BMP2 and BMP4 in aortas might contribute to arterial calcification in Klotho-deficient mice.
Another interesting finding is that Klotho protein suppressed BMP2/Vitamin D3-induced calcification in cultured mouse aortic smooth muscle cells (MOVAS) (Fig. 6). It is well documented that high level of phosphate or phosphate glycerol is required to generate extracellular matrix calcification in cell culture (Mackenzie et al., 2011). Our data suggest that both BMP2 and Vitamin D3 are required for the development of cell calcification in the absence of high level of phosphate. In our experimental condition, recombinant BMP2 protein itself did not directly induce calcification in cultured MOVAS. On the other hand, vitamin D3 has been shown to promote calcification in various vascular SMCs in the presence of 10 mM β-glycerophosphate (Jono, Nishizawa, Shioi, & Morii, 1998; Mantadakis et al., 2007). Indeed, high concentration of vitamin D was found both in Klotho deficient mice and Klotho knockout mice (Tsujikawa, Kurotaki, Fujimori, Fukuda, & Nabeshima, 2003; Yoshida, Fujimori, & Nabeshima, 2002). In this study, we found that Klotho deficiency promoted BMP2/vitamin D3-induced calcification in cultured MOVAS (Fig. 6). Importantly, treatment with recombinant Klotho protein completely abolished this Klotho-less serum-BMP2/vitamin D3-induced calcification. Therefore, Klotho is an important regulator in BMP2/vitamin D3-induced smooth muscle cell calcification.
Several lines of evidence showed that the BMP2–canonical Smad1/5/8 signaling pathway is linked to the bone remodeling processes including osteoblast differentiation and osteoclastogenesis and to the transition from vascular SMCs to osteoblast-like cells (Broege et al., 2013; Kee et al., 2014). Klotho deficiency upregulated phosphorylation of SMAD1/5/8 in aortas (Fig. 4). Interestingly, Klotho-deficient serum promoted BMP2-induced phosphorylations of SMAD1/5/8 and SMAD2/3 in cultured mouse ASMCs whereas Klotho protein treatments abolished BMP2-induced phosphorylation of SMAD (Fig. S3). Therefore, these data suggest that Klotho is a negative regulator of the BMP signaling. A further study is required to investigate how Klotho interacts with BMP2 and/or BMP2 receptors. Given that some effects of Klotho are mediated by FGF23 signaling (Kurosu et al., 2006; Razzaque, 2009; Urakawa et al., 2006), it would be interesting to investigate if the regulation of the BMP signaling by Klotho is FGF23-depedent.
RUNX2 (runt-related transcription factor) is a critical regulator for osteoblast differentiation and chondrocyte maturation (Komori et al., 1997; Otto et al., 1997). RUNX2 is a marker of osteoblasts and has been shown to play an important role in VSMC calcification in vitro and in vivo (Byon et al., 2008; N. X. Chen, Duan, O'Neill, & Moe, 2006; Mori, Shioi, Jono, Nishizawa, & Morii, 1999; Speer, Li, Hiremath, & Giachelli, 2010; Sun et al., 2012). Here, we showed that Klotho deficiency increased arterial RUNX2 levels associated with arterial calcification in mice (Fig. 4). Klotho-less serum directly upregulated RUNX2 levels in cultured mouse ASMCs (Fig. 5). In addition, Klotho-less serum enhanced BMP2-induced RUNX2 expression and matrix calcification in MOVAS in the presence of vitamin D3 (Fig. 6 & S3). These findings provide the first evidence that Klotho deficiency promoted osteoblastic transition. Interestingly, Klotho treatments abolished the upregulation of RUNX2 and matrix calcification in cultured MOVAS (Fig. 6 & S3). Taken together, these results suggest that RUNX2 might be a down-stream of Klotho-BMP pathway in the regulation of arterial calcification (Fig. S4). Most recent data indicated that functional cooperation between vitamin D receptor and RUNX2 is required for arterial calcification in response to vitamin D3 (Han et al., 2013).
Our results also suggest that Klotho might regulate PIT2 protein levels in matrix calcification in cultured MOVAS. PIT-1 and PIT-2 are sodium-dependent phosphate (NaPi) cotransporters, which have been identified as the predominant phosphate transporters in rat and human VSMCs (Li, Yang, & Giachelli, 2006; Villa-Bellosta, Bogaert, Levi, & Sorribas, 2007). PIT1 and PIT2 have been shown to regulate phosphate uptake and phosphate-induced matrix calcification in cultured vascular smooth muscle cells redundantly (Crouthamel et al., 2013). Currently, there is no antibody against mouse PIT1. Here, we showed that Klotho-deficient serum promoted BMP2-induced PIT2 protein levels in cultured MOVAS which was reversed by Klotho treatments (Fig. S3B). A further study is warranted to determine how Klotho regulates BMP2-induced PIT2 protein level in mouse aortic smooth muscle cells.
Supplementary Material
Perspective.
Low phosphate diet normalized blood phosphate levels and abolished calcification in lungs and kidneys but did not prevent the development of arterial calcification in Klotho-deficient mice. Klotho deficiency upregulated BMP2, BMP4 and RUNX2 expressions in aortas leading to aortic calcification in the absence of hyperphosphatemia. Klotho-deficient serum directly induced osteoblastic transition in cultured SMCs as evidenced by upregulation of BMP2 and RUNX2. Klotho-deficient serum promoted BMP2/VitD3-induced calcification, protein expressions of PIT2 and RUNX2, and phosphorylations of SMAD1/5/8 and SMAD2/3 in SMCs. Interestingly, treatments with Klotho protein abolished BMP2/VitD3-induced morphogenetic effects. Thus, Klotho deficiency-induced arterial calcification is an active process which requires activation of the BMP2-RUNX2 signaling pathway. Klotho may be a therapeutic target for vascular calcification.
Source of Funding
This work was supported by NIH R01 HL154147, HL118558, AG062375 and AG049780 grants.
Footnotes
Disclosure: No
Conflict of interest: No
References
- Afroze T, Yang LL, Wang C, Gros R, Kalair W, Hoque AN, … Husain M (2003). Calcineurin-independent regulation of plasma membrane Ca2+ ATPase-4 in the vascular smooth muscle cell cycle. Am J Physiol Cell Physiol, 285(1), C88–95. [DOI] [PubMed] [Google Scholar]
- Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, & Demer LL (1993). Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest, 91(4), 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broege A, Pham L, Jensen ED, Emery A, Huang TH, Stemig M, … Gopalakrishnan R (2013). Bone morphogenetic proteins signal via SMAD and mitogen-activated protein (MAP) kinase pathways at distinct times during osteoclastogenesis. J Biol Chem, 288(52), 37230–37240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, … Chen Y (2008). Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem, 283(22), 15319–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Pardali E, Sanchez-Duffhues G, & ten Dijke P (2012). BMP signaling in vascular diseases. FEBS Lett, 586(14), 1993–2002. [DOI] [PubMed] [Google Scholar]
- Chen CD, Podvin S, Gillespie E, Leeman SE, & Abraham CR (2007). Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A, 104(50), 19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fan J, Wang S, & Sun Z (2018). Secreted Klotho Attenuates Inflammation-Associated Aortic Valve Fibrosis in Senescence-Accelerated Mice P1. Hypertension, 71(5), 877–885. doi: 10.1161/hypertensionaha.117.10560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Wang S, Sun QW, Zhang B, Ullah MF, & Sun Z (2020). Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circulation Research. doi: 10.1161/circresaha.120.317348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Zhou X, & Sun Z (2015). Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension, 66(5), 1006–1013. doi: 10.1161/hypertensionaha.115.06033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NX, Duan D, O'Neill KD, & Moe SM (2006). High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant, 21(12), 3435–3442. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, & Towler DA (2003). MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem, 278(46), 45969–45977. [DOI] [PubMed] [Google Scholar]
- Crosswhite P, Chen K, & Sun Z (2014). AAV delivery of tumor necrosis factor-alpha short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension, 64(5), 1141–1150. doi:HYPERTENSIONAHA.114.03791 [pii] 10.1161/HYPERTENSIONAHA.114.03791 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouthamel MH, Lau WL, Leaf EM, Chavkin NW, Wallingford MC, Peterson DF, … Giachelli CM (2013). Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: redundant roles for PiT-1 and PiT-2. Arterioscler Thromb Vasc Biol, 33(11), 2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, … Yu PB (2012). Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol, 32(3), 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, & Sun Z (2016). The Anti-aging Gene Klotho Regulates Proliferation and Differentiation of Adipose-derived Stem Cells. Stem Cells. doi: 10.1002/stem.2305 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MS, Che X, Cho GH, Park HR, Lim KE, Park NR, … Choi JY (2013). Functional cooperation between vitamin D receptor and Runx2 in vitamin D-induced vascular calcification. PLoS One, 8(12), e83584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska KA, Mathew S, & Saab G (2005). Bone morphogenetic proteins in vascular calcification. Circ Res, 97(2), 105–114. [DOI] [PubMed] [Google Scholar]
- Hu MC, Kuro-o M, & Moe OW (2012). Secreted klotho and chronic kidney disease. Adv Exp Med Biol, 728, 126–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL (2010). Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int, 77(10), 855–860. [DOI] [PubMed] [Google Scholar]
- Jono S, Nishizawa Y, Shioi A, & Morii H (1998). 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation, 98(13), 1302–1306. [DOI] [PubMed] [Google Scholar]
- Kee HJ, Cho SN, Kim GR, Choi SY, Ryu Y, Kim IK, … Jeong MH (2014). Gallic acid inhibits vascular calcification through the blockade of BMP2-Smad1/5/8 signaling pathway. Vascul Pharmacol, 63(2), 71–78. [DOI] [PubMed] [Google Scholar]
- Kendrick J, & Chonchol M (2011). The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis, 58(5), 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, … Kishimoto T (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 89(5), 755–764. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, … Nabeshima YI (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390(6655), 45–51. doi: 10.1038/36285 [doi] [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, … Kuro-o M (2006). Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem, 281(10), 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang HY, & Giachelli CM (2006). Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res, 98(7), 905–912. [DOI] [PubMed] [Google Scholar]
- Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, & Hsiao LL (2012). Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation, 125(18), 2243–2255. doi:CIRCULATIONAHA.111.053405 [pii] 10.1161/CIRCULATIONAHA.111.053405 [doi] [DOI] [PubMed] [Google Scholar]
- Lin Y, Chen J, & Sun Z (2016). Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension, 67(3), 564–573. doi: 10.1161/hypertensionaha.115.06825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, & Sun Z (2012). Antiaging gene Klotho enhances glucose-induced insulin secretion by upregulating plasma membrane levels of TRPV2 in MIN6 beta-cells. Endocrinology, 153(7), 3029–3039. doi: 10.1210/en.2012-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, & Sun Z (2015a). Antiaging Gene Klotho Attenuates Pancreatic beta-Cell Apoptosis in Type 1 Diabetes. Diabetes, 64(12), 4298–4311. doi: 10.2337/db15-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, & Sun Z (2015b). In vivo pancreatic beta-cell-specific expression of antiaging gene Klotho: a novel approach for preserving beta-cells in type 2 diabetes. Diabetes, 64(4), 1444–1458. doi: 10.2337/db14-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg K, Olauson H, Amin R, Ponnusamy A, Goetz R, Taylor RF, … Larsson TE (2013). Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS One, 8(4), e60658. doi: 10.1371/journal.pone.0060658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery JW, & de Caestecker MP (2010). BMP signaling in vascular development and disease. Cytokine Growth Factor Rev, 21(4), 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Zhu D, Longley L, Patterson CS, Kommareddy S, & MacRae VE (2011). MOVAS-1 cell line: a new in vitro model of vascular calcification. Int J Mol Med, 27(5), 663–668. [DOI] [PubMed] [Google Scholar]
- Mantadakis E, Spanaki AM, Psaroulaki A, Fitrolaki D, Minadakis G, Michaeloudi E, … Briassoulis G (2007). Encephalopathy complicated by Guillain-Barre syndrome and hydrocephalus and associated with acute Bartonella quintana infection. Pediatr Infect Dis J, 26(9), 860–862. [DOI] [PubMed] [Google Scholar]
- Mori K, Shioi A, Jono S, Nishizawa Y, & Morii H (1999). Dexamethasone enhances In vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol, 19(9), 2112–2118. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Ikeda K, Akakabe Y, Koide M, Uraoka M, Yutaka KT, … Matsubara H (2010). Paracrine osteogenic signals via bone morphogenetic protein-2 accelerate the atherosclerotic intimal calcification in vivo. Arterioscler Thromb Vasc Biol, 30(10), 1908–1915. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, … Owen MJ (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 89(5), 765–771. [DOI] [PubMed] [Google Scholar]
- Razzaque MS (2009). The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol, 5(11), 611–619. doi:nrendo.2009.196 [pii] 10.1038/nrendo.2009.196 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, … Nabeshima Y (1998). Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett, 424(1-2), 6–10. [DOI] [PubMed] [Google Scholar]
- Speer MY, Li X, Hiremath PG, & Giachelli CM (2010). Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem, 110(4), 935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, … Chen Y (2012). Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res, 111(5), 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, & Nabeshima Y (2003). Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol, 17(12), 2393–2403. [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, … Yamashita T (2006). Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature, 444(7120), 770–774. doi:nature05315 [pii] 10.1038/nature05315 [doi] [DOI] [PubMed] [Google Scholar]
- Villa-Bellosta R, Bogaert YE, Levi M, & Sorribas V (2007). Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol, 27(5), 1030–1036. [DOI] [PubMed] [Google Scholar]
- Xu Y, & Sun Z (2015). Molecular basis of klotho: from gene to function in aging. Endocrine Reviews, 36(2), 174–193. doi: 10.1210/er.2013-1079 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujimori T, & Nabeshima Y (2002). Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology, 143(2), 683–689. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen K, Lei H, & Sun Z (2015). Klotho Gene Deficiency Causes Salt-Sensitive Hypertension via Monocyte Chemotactic Protein-1/CC Chemokine Receptor 2-Mediated Inflammation. Journal of the American Society of Nephrology, 26(1), 121–132. doi:ASN.2013101033 [pii] 10.1681/ASN.2013101033 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chen K, Wang Y, Schuman M, Lei H, & Sun Z (2015). Antiaging Gene Klotho Regulates Adrenal CYP11B2 Expression and Aldosterone Synthesis. Journal of the American Society of Nephrology. doi:ASN.2015010093 [pii] 10.1681/ASN.2015010093 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Mackenzie NC, Farquharson C, & Macrae VE (2012). Mechanisms and clinical consequences of vascular calcification. Front Endocrinol (Lausanne), 3, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


