Abstract
Although antibodies have attracted attention as next-generation biopharmaceuticals, the costs of purifying the products and of arranging the environment for cell cultivation are high. Therefore, there is a need to increase antibody efficacy and improve product quality as much as possible. Since antibodies are glycoproteins, their glycan structures have been found to affect the function of antibodies. Especially, afucosylation of the N-linked glycan in the Fc region is known to significantly increase antibody-dependent cellular cytotoxicity. In this study, we established a double-mutant ΔGMDΔGFT in which GDP-mannose 4,6-dehydratase and GDP-fucose transporter were knocked out in Chinese hamster ovary cells, a platform for biopharmaceutical protein production. By adapting ΔGMDΔGFT cells to serum-free medium and constructing suspension-cultured cells, we established host CHO cells with no detected fucosylated glycans and succeeded in production of afucosylated antibodies. We also demonstrated that, in culture in the presence of serum, fucosylation occurs due to contamination from serum components. Furthermore, we found that afucosylation of glycans does not affect cell growth after adaptation to serum-free medium as compared to wild-type CHO cells growth and does not significantly affect the expression levels of other endogenous fucose metabolism-related enzyme genes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10616-021-00501-3.
Keywords: Chinese hamster ovary cell, N-glycan, Fucosylation, GDP-fucose, Serum-free medium adaptation
Introduction
Protein-based pharmaceuticals are representative of the biopharmaceutical industry, and the market for them, with billions of dollars in annual sales, is growing (Aggarwal 2014; Walsh 2018). These products include various proteins such as antibodies, enzymes, hormones, cytokines, growth factors, and coagulation factors (Dumont et al. 2016; Walsh 2018). In addition, biopharmaceuticals to treat diseases such as cancer, immune diseases, infectious diseases, genetic diseases, Alzheimer's disease, and Parkinson's disease are being widely developed. In particular, antibody drugs occupy a large place in the current pharmaceutical market. In 2016, antibody drug sales accounted for approximately 66% of all biopharmaceuticals excluding vaccines, with 8 of the top 10 sales being antibody-related (Kesik-Brodacka 2018).
Chinese hamster ovary (CHO) cells are the main platform for producing these recombinant biopharmaceuticals. However, antibody drugs are much more expensive than conventional chemotherapeutic drugs. This is because culturing animal cells requires expensive culture media and culture facilities, and purifying the produced protein is expensive. In addition, antibody drugs are administered in higher dose than conventional protein drugs, resulting in higher treatment costs. Therefore, it is necessary to enhance the efficacy of antibody drugs as much as possible.
Many protein-based biopharmaceuticals, including antibodies, are glycoproteins. Added to these glycoproteins are oligosaccharides and sugar chains that contribute to the quality of the product by post-translational modification. Especially, it is important to add N-linked glycans to many recombinant biopharmaceutical proteins. The properties of glycosylation play major roles in altering protein stability, including factors such as half-life, folding, targeting, transporting, immunogenicity, and biological activity (Runkel et al. 1998; Walsh and Jefferis 2006; Jefferis 2016; Kuriakose et al. 2016). Glycosylation results in broad changes in the function of a single protein. Therefore, modification of the glycan structure can be a means to improve the quality of CHO-derived recombinant biopharmaceuticals.
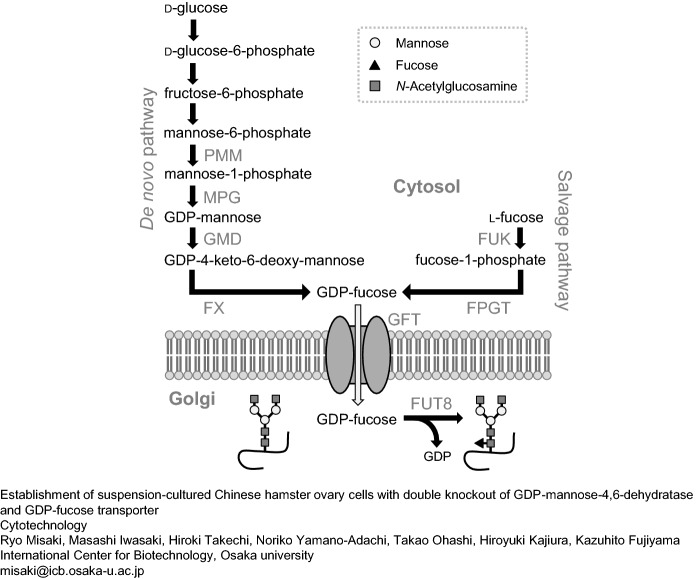
Afucosylated N-glycan of the human IgG1 Fc region was reported to increase the ability of natural killer cells to bind to the FcγRIIIa receptor (Shields et al. 2002; Shinkawa et al. 2003; Niwa et al. 2004; Iida et al. 2006). This property has been used to further improve the cancer therapeutic performance of monoclonal antibodies via antibody-dependent cellular cytotoxicity (ADCC) (Chung et al. 2012). α1,6-Fucosyltransferase (FUT8) adds fucose (core fucose) with α1,6-linkage to the reducing terminal N-acetylglucosamine (GlcNAc) of the N-linked glycan. The production of the donor substrate GDP-fucose of FUT8 takes place via the de novo synthetic or salvage pathway (Fig. 1). The de novo synthetic pathway is the main synthetic route of GDP-fucose catalyzed by GDP-mannose-4,6-dehydratase (GMD) and GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase. In contrast, the salvage pathway is catalyzed by l-fucose kinase (FUK) and fucose-1-phosphate guanylyltransferase (FPGT) using fucose as a substrate. The produced GDP-fucose is then transported by the GDP-fucose transporter (GFT) into the Golgi apparatus to be catalyzed by FUT8. At present, research to produce low-fucosylated or nonfucosylated N-glycans of monoclonal antibodies by targeting these genes is intensifying (Mori et al. 2004; Imai-Nishiya et al. 2007; Kanda et al. 2007; Malphettes et al. 2010; Haryadi et al. 2013; Louie et al. 2017).
Fig. 1.
Synthetic pathway of fucosylated N-glycans and GDP-fucose via the de novo and the salvage pathway. PMM phosphomannomutase, MPG mannose-1-phosphate guanylyltransferase (GDP-mannose pyrophosphorylase), FX GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase (GDP-L-fucose synthase), FUK fucose kinase, FPGT fucose-1-phophate guanylyltransferase
Transfer of fucose residue to the N-glycans was still remaining in CHO-DG44 cells under single GMD-knockdown (Imai-Nishiya et al., 2007). In contrast, serum-free fed-batch culture made no fucosylation of antibodies produced in GMD-knockout CHO cells (Kanda et al. 2007). However, even if only the expression of GMD is suppressed, there is a strong possibility that core fucosylation to the glycans may occur in the presence of extracellular GDP-Fuc contamination present in the culture medium containing serum. Thus, to solve this problem, we further knocked out GFT that transport GDP-Fuc into the Golgi lumen, which is the site of glycosylation. According from a previous report, knocking down or knocking out GFT expression markedly suppressed the fucosylation of endogenous and recombinant proteins (Omasa et al. 2008; Zhang et al. 2012; Chan et al. 2016). However, there have been few reports of double knockout of fucosylation-related enzyme genes. Although more potent inhibition of fucosylation to total intracellular glycans is expected, effects of the multi-mutations on cell growth, the adaptation to serum-free medium, the expression levels of other glycosylation-related enzyme genes, and N-linked glycan structure of a recombinant biopharmaceutical proteins still remain unclear. Hence, we examined these effects using CHO-K1 cell lines producing recombinant antibodies.
Mutation of FUT8 is a simple and attractive approach for inhibiting core fucosylation of N-linked glycans. In contrast, the FUT8 remains active in the mutant cells established in this study. This means that extracellular supplementation of monosaccharides or sugar nucleotides can exchange the α1,6-fucosylation to other core-glycosylation. A previous study showed that, using sugar nucleotides synthesized by adding an excess of L-fucose structural-like sugars such as L-arabinose as a donor substrate, FUT8 transfers those L-fucose-like sugars to the N-linked glycans of antibodies in competition with GDP-Fuc, resulting in an altered ADCC activity (Hossler et al. 2017). The mutant cell line established in this study could be used in the future to efficiently transfer an L-fucose-like sugar in place of the core fucose residue in N-linked glycans of the recombinant antibodies and confer high ADCC activity.
Materials and methods
Cells
Adherent CHO-K1 cells from ATCC were cultured in 5% CO2 at 37 °C in Ham’s F12 Nutrient Mixture (Nacalai tesque, Kyoto, Japan) containing 10% fetal bovine serum (FBS) or ProCHO™ AT serum-free medium (Lonza, Basel, Switzerland). Suspension-cultured cells were cultured in EX-CELL® CD CHO serum-free medium (Sigma-Aldrich, St. Louis, MO, USA). The number of viable cells was measured using cells cultured in 10 mL medium on 10-cm dishes. Each initial cell number was 1.4 × 105 cells and the sampling was conducted at every 48 h from 1-day culture. Immunoglobulin G (IgG) was produced in a 350-mL culture bag A-350NL (NIPRO, Osaka, Japan) without shaking. Viable cells of each cell line on a 10 cm-dish were stained by trypan blue and the number of cells were then counted using hemocytometers.
sgRNA design, plasmid construction, and screening of single cell lines
The target sequences of single guide RNA (sgRNA) for genome editing were designed as follows: 5′-CACCGTCGACCTGTATTAAATGAAC-3′ (sense 1 for GMD), 5′-CAGCTGGACATAATTTACTTGCAAA-3′ (anti-sense 1 for GMD), 5′-CACCGACATTTATATAAGAATCCAC-3′ (sense 2 for GMD), 5′-CTGTAAATATATTCTTAGGTGCAAA-3′ (anti-sense 2 for GMD), 5′-CACCGTGCAGCGCCCGCAGCAGAAA-3′ (sense 1 for GFT), 5′-CACGTCGCGGGCGTCGTCTTTCAAA-3′ (anti-sense 1 for GFT), 5′-CACCGCGCTGGTCGTCTCTCTCTAC-3′ (sense 2 for GFT), 5′-CGCGACCAGCAGAGAGAGATGCAAA-3′ (anti-sense 2 for GFT). These sense oligonucleotides were annealed to their pair antisense oligonucleotides, respectively. The annealed double strand DNAs were then introduced to pSpCas9n(BB)-2A-Puro (PX462) (Addgene, Cambridge, MA). Transfection to cells grown on 6-well plates was performed using Lipofectamine™ 2000 transfection reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. The transfected cells were sorted to 24-well plates as single cell clones.
Sequencing analysis
Genomic DNA of the transfectants was extracted by Kaneka Easy DNA Extraction Kit version 2 (Kaneka, Osaka, Japan) according to the manufacturer’s instructions. Mutated regions were amplified by PCR using the primer sets shown in Supplementary Table 1. The PCR products were subcloned to pGEM®-T Easy vector and sequenced using M13 forward or reverse primers.
Lectin blotting
Proteins were extracted from cultured CHO cells using CytoBuster™ Protein Extraction Reagent (Merck Millipore, Billerica, MA), separated in 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing condition, and transferred to a polyvinylidene difluoride membrane. The membrane was then washed with lectin buffer (10 mM Tris–HCl, 150 mM NaCl, and 0.05% Tween 20) and incubated with biotin-conjugated Aleuria aurantia lectin, AAL (J-Oil Mills, Tokyo, Japan). Fucosylated glycans were visualized by peroxidase-conjugated streptavidin (Merck Millipore) and Luminata™ Forte Western HRP Substrate (Merck Millipore).
Adaptation of cells to serum-free medium and preparation of suspension-cultured cells
Serum concentration in medium was reduced stepwise. Cells were cultured in Ham’s F12 Nutrient Mixture containing 2.5% or 1.0% FBS and ProCHO™ AT containing 0.5% or 0.25% or 0.1% FBS, respectively. Detached cells under the 0.1% FBS were harvested and adapted to EX-CELL® CD CHO serum-free medium.
Construction of expression vectors of IgG and establishment of cells stably producing the recombinant IgG
Genes encoding variable region of the heavy (H)- and light (L)-chains of human anti-dengue virus monoclonal antibody, D23-4F5E1, were cloned as previously reported (Sasaki et al. 2013). The coding region of H- and L-chains were introduced to pQCXIP (TaKaRa Bio Inc., Shiga, Japan) and pQCXIH (TaKaRa Bio Inc.) with a human H-chain and L-chain constant regions, respectively, as previously performed (Misaki et al. 2016). Transfection of the expression vectors to suspension-cultured GMD/GFT double-knockout CHO-K1 cells was conducted using Neon™ Transfection System (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells stably expressing both H- and L-chains were selected in EX-CELL® CD CHO serum-free medium containing 2 μg/mL puromycin dihydrochloride and 200 μg/ mL hygromycin B.
Purification of IgG
The 2-week cultured medium in a batch culture was centrifuged at 1500×g for 5 min and the supernatant was applied to a Protein G Sepharose™ 4 Fast Flow column (GE Healthcare UK Ltd., Buckinghamshire, UK) equilibrated with a binding buffer, 20 mM sodium phosphate buffer, pH 7.0. The IgG was eluted by 100 mM glycine buffer, pH 2.7 after washing with the binding buffer, and a neutralization buffer, 1 M Tris–HCl buffer, pH 9.0 was rapidly added and mixed with the eluted fractions.
Preparation of N-glycans
1.0 × 108 cells were washed with PBS(−) twice and lysed in cold-Radio-Immunoprecipitation Assay buffer. The cells lysate was then incubated on ice for 30 min and centrifuged at 4 °C at 1000×g for 5 min. The supernatant containing the total soluble proteins was dialyzed against ultrapure water and lyophilized. Purified IgGs were also dialyzed against ultrapure water and lyophilized. N-Glycans were released from the total soluble proteins or the purified IgGs by Glycopeptidase F (Takara Bio Inc.) according to the manufacturer’s instructions. The obtained N-glycans were lyophilized and labeled with 2-pyridylamine (PA) as described (Kondo et al. 1990). The PA-labeled N-glycans were separated from unreacted PA by phenol/chloroform extraction.
High-performance liquid chromatography (HPLC)
PA-labeled N-glycans were monitored on a HPLC apparatus (Hitachi 7000 HPLC system, Hitachi, Tokyo, Japan) at excitation and emission wavelengths of 310 nm and 380 nm, respectively. The PA-labeled N-glycans were fractionated using a 4.6 × 250 mm column (Cosmosil 5C18-P column, Nacalai Tesque, Kyoto, Japan) and eluted by linearly increasing the acetonitrile concentration in 0.02% trifluoroacetic acid from 0 to 7% for 35 min at a flow rate of 1.2 mL/min in a reversed-phase HPLC.
LC–MS/MS analysis of N-glycans
The molecular mass and composition of the PA-labeled N-glycans were monitored by using an LC–MS/MS system (1200 series, Agilent Technologies, Santa Clara, CA) equipped with HCT plus (Bruker Daltonics, Bremen, Germany). The mobile phase in the LC consisted of a mixture of solvent A (acetonitrile:acetic acid = 98:2, v/v) and solvent B (water:acetic acid:triethylamine = 92:5:3, v/v/v). The PA-labeled N-glycans were separated using a 2.0 × 150 mm column (Shodex Asahipak NH2P-50, Showa Denko, Tokyo, Japan) by linearly increasing the solvent B concentration from 20 to 55% for 35 min at a flow rate of 0.2 mL/min. The parameter of the MS/MS analysis was as follows: scan 350–5000 m/z, 5.0 psi nebulizer flow, 3.0 L/min dry gas flow rate, 300 °C dry temperature, 200,000 target count, and the MS/MS Frag. Ampl. of 1.0 V in the positive-ion mode. The relative amount of detected PA-labeled N-glycans was calculated on the basis of the peak area of the LC.
Estimation of intracellular GDP-fucose amount
Cultured 5.0 × 107 cells were homogenized in phosphate buffered saline (PBS) and centrifuged. The obtained supernatant was lyophilized as the donor substrate including GDP-fucose for the next enzymatic reaction. Recombinant mouse FUT8 was prepared using a baculovirus expression system as previously described (Ohashi et al. 2017). The mixture of cell extracts and FUT8 was incubated in PBS, 0.02 mg/mL GlcNAc2Man3GlcNAc2-Asn-Fmoc at 37 °C for 16 h. The mixture was then incubated at 100 °C for 3 min and centrifuged. The supernatant was subjected to HPLC, and the HPLC analysis was performed as described (Ohashi et al. 2017). The amount of GDP-fucose was calculated from the peak area of synthesized GlcNAc2Man3FucGlcNAc2-Asn-Fmoc.
Reverse transcription-PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from 3-day cultured cells using ISOGEN II (NIPPON GENE, Tokyo, Japan) according to the manufacturer’s instructions. The cDNA was synthesized using SuperScript™ VILO™ MasterMix (Thermo Fisher Scientific) according to the manufacturer’s instructions. The expression of GMD and GFT genes was confirmed by RT-PCR. β-Actin (ACTB) and FUT8 genes were also amplified as internal controls. The expression levels of enzymes related to the synthesis of fucosylated glycans, α-L-fucosidase 1 (FUC1), α-L-fucosidase 2 (FUC2), fucosyltransferase 4 (FUT4), FUT8, fucosyltransferase 9 (FUT9), and fucose-1-phosphate guanylyltransferase (FPGT), were evaluated by qRT-PCR. All primer sequences used in these PCR are shown in Supplementary Table 1.
Results
Establishment of GMD single-mutated and GMD/GFT double-mutated CHO cell lines
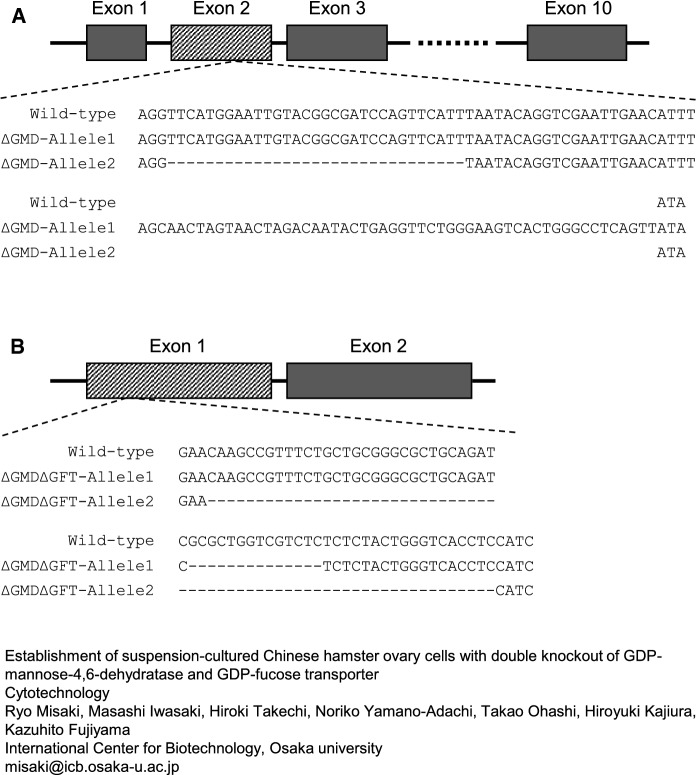
Sequencing analysis of each single cell line resulted in the establishment of GMD-knockout CHO-K1 (ΔGMD) with insertion and deletion of nucleotides on its alleles in exon 2 as shown in Fig. 2A. GMD/GFT double-knockout CHO-K1 (ΔGMDΔGFT) was succeeded by the deletion of nucleotides in exon 1 of the GFT gene in ΔGMD cells (Fig. 2B). The GMD and GFT gene expression in wild-type CHO-K1 and ΔGMDΔGFT cells was confirmed by RT-PCR (Supplementary Fig. 1). The GMD gene was amplified using cDNA synthesized from ΔGMDΔGFT-mRNA. The gene was longer compared to wild-type cells because of an insertion of nucleotide on one allele in ΔGMDΔGFT described above. The GFT gene was correctly amplified from wild-type- but not ΔGMDΔGFT-cDNA because the ΔGMDΔGFT-cDNA lacks nucleotide regions on its allele 1 and 2 which the PCR antisense primer anneals to (Fig. 2B and Supplementary Table 1). The internal control ACTB and FUT8 genes were amplified using both wild-type- and ΔGMDΔGFT-cDNAs.
Fig. 2.
Sequence of genomic DNA around the mutated region. Exon 2 of GMD in wild-type CHO-K1 cells, DNA-inserted allele 1 and -deleted allele 2 of ΔGMD cells (a); Exon 1 of GFT in wild-type CHO-K1 cells and both DNA-deleted alleles of ΔGMDΔGFT cells (b)
We then examined the reduction of fucosylation in total soluble proteins from ΔGMD and ΔGMDΔGFT cells by lectin blotting using AAL. Although total soluble proteins from ΔGMD cells showed a slight reduction of AAL-positive bands compared with the wild type, the ΔGMDΔGFT double mutation significantly decreased the reactivity of cell extracts to the AAL (Supplementary Fig. 2). The adherent wild-type CHO-K1 and both mutant cells were cultured in serum-reduced medium for 6 months, and finally their suspension cells adapting to serum-free medium were prepared.
N-glycan analysis of ΔGMD and ΔGMDΔGFT cells
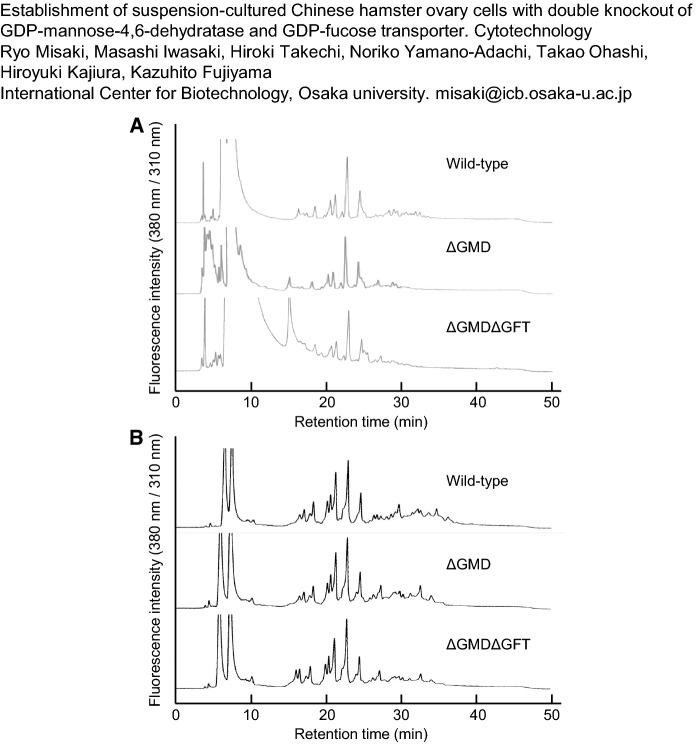
PA-labeled N-glycans from adherent cells were fractionated on RP-HPLCs (Fig. 3A). In these chromatograms, all PA-labeled N-glycans were eluted from 20 to 35 min on HPLC. The HPLC profiles after 25 min, which contains large amounts of fucosylated N-glycans, different between the wild type and the mutants. The detailed structures and ratios of the N-glycans are shown in Table 1. GN, M, Gal, NA, NG, and GL show N-acetylglucosamine, mannose, galactose, N-acetylneuraminic acid, N-glycolylneuraminic acid, and glucose, respectively. The italicized structures show fucosylated glycans. GMD knockout decreased the proportion of fucosylated N-glycans in wild-type CHO-K1 cells from 18.7 to 10.1% of the total amount of N-glycans. The N-glycan in ΔGMDΔGFT cells still contained 0.9% fucosylation even if the double knockout of GMD and GFT produced a significant reduction. The amounts of high-mannose-type and complex-type N-glycans in both mutants were almost the same as those in the wild-type cells. The occupancy of galactosylated N-glycans, which contribute to the increase in ADCC activity, also showed no difference among the wild-type, ΔGMD, and ΔGMDΔGFT cells. The RP-HPLC profiles of PA-labeled N-glycans from suspension cells that were adapted to serum-free medium are also shown in Fig. 3B. All PA-labeled N-glycans were eluted from 20 to 35 min in the RP-HPLC as those from adherent cells, and the HPLC profiles after 25 min from the mutants differed from those of the wild type. The distribution of the N-glycan structures from suspension cells is summarized in Table 2. Fucosylated N-glycans in wild-type cells made up 25.1% of the total amount of N-glycans, and the GMD single knockout reduced the proportion significantly, to 0.2%. Moreover, no fucosylated glycans were detected in ΔGMDΔGFT cells. Compared with the adherent cells, the suspension-cultured cells had almost the same amounts of high-mannose-type structures. On the other hand, complex-type structures increased by approximately 1.2- to 1.7-fold and, accordingly, the amounts of galactosylated N-glycans increased in the suspension cells.
Fig. 3.
RP-HPLC profiles of PA-labeled N-glycans from total soluble proteins of adherent wild-type CHO-K1 cells, ΔGMD cells, and ΔGMDΔGFT cells (a); of suspension-cultured wild-type CHO-K1 cells, ΔGMD cells, and ΔGMDΔGFT cells (b)
Table 1.
N-glycan structures in adherent cells
| Structure | Wild-type | ΔGMD | ΔGMDΔGFT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | |
| GLM9GN2 | 0.1 | 0.7 | – | – | – | ||||
| M5–9GN2 | 63.3 | 63.4 | 68.8 | 61.7 | 61.9 | 53.8 | 60.8 | 68.6 | 55.7 |
| M5FGN2 | – | 0.1 | – | – | – | – | – | – | – |
| M4GN2 | 2.3 | 3.8 | 4.3 | 3.5 | 4.0 | 5.1 | 3.3 | 3.4 | 3.5 |
| M4FGN2 | – | 0.5 | – | – | – | – | – | – | 0.2 |
| M3GN2 | 0.9 | 1.6 | 3.5 | 3.8 | 4.9 | 5.1 | 7.5 | 5.2 | 7.0 |
| M3FGN2 | 3.0 | 1.8 | 2.3 | 0.7 | 0.8 | 1.5 | 0.2 | – | – |
| M2GN2 | 0.7 | 4.9 | 2.7 | 4.3 | 4.2 | 6.2 | 10.5 | 7.1 | 4.9 |
| M2FGN2 | 6.1 | 5.2 | 5.3 | 1.5 | 2.6 | 4.1 | – | – | – |
| GNM3GN2 | – | 0.1 | – | 0.2 | 0.4 | 0.4 | 0.8 | 0.6 | 3.1 |
| GNM3FGN2 | 0.9 | 0.7 | 1.5 | 0.3 | 0.7 | 1.0 | – | – | 0.6 |
| GNM5GN2 | 2.4 | 0.4 | 1.6 | 1.3 | 0.8 | 1.6 | 1.2 | 0.5 | 0.2 |
| GN2M3GN2 | – | 0.1 | 0.1 | 0.9 | 0.7 | 0.8 | 1.5 | 1.8 | 2.5 |
| GN2M3FGN2 | 4.1 | 1.8 | 1.8 | 1.6 | 1.1 | 1.9 | 0.9 | 0.6 | – |
| GN3M3GN2 | – | – | – | 0.4 | 0.3 | 0.4 | 1.3 | 0.9 | 0.4 |
| GN3M3FGN2 | 1.1 | 1.1 | 0.7 | 0.6 | 0.8 | 0.7 | – | 0.1 | – |
| GN4M3GN2 | – | – | – | – | – | – | 0.1 | 0.1 | – |
| GalGNM3GN2 | 1.8 | 0.2 | 0.5 | 0.5 | 0.2 | 0.9 | 1.1 | 0.5 | 2.4 |
| GalGNM3FGN2 | 0.6 | 0.3 | 0.9 | 0.2 | 0.4 | 0.4 | – | – | 0.2 |
| GalGNM5GN2 | – | – | 0.1 | – | – | – | – | – | – |
| GalGN2M3GN2 | – | 0.2 | 0.1 | 0.4 | 0.3 | 0.4 | 0.7 | 0.8 | 1.6 |
| GalGN2M3FGN2 | 0.5 | – | 0.5 | 0.4 | 0.2 | 0.5 | – | – | – |
| GalGN3M3GN2 | 1.8 | 0.4 | 0.9 | 0.6 | – | 0.9 | 1.1 | 1.0 | 0.6 |
| GalGNM6GN2 | – | – | – | – | – | 0.5 | – | – | – |
| Gal2GN2M3GN2 | 2.4 | 2.4 | 0.4 | 5.7 | 5.6 | 2.2 | 6.1 | 5.1 | 10.4 |
| Gal2GN2M3FGN2 | 5.9 | 3.7 | 2.4 | 2.3 | 1.7 | 1.1 | – | – | – |
| Gal2GN3M3GN2 | – | 0.1 | – | 0.1 | – | 0.2 | – | 0.1 | 2.9 |
| Gal2GN3M3FGN2 | – | – | 0.1 | – | 0.2 | 0.1 | – | – | – |
| Gal3GN3M3GN2 | – | 5.9 | 0.1 | 2.6 | 3.8 | 1.7 | 0.3 | 1.8 | – |
| Gal3GN3M3FGN2 | 1.6 | 0.5 | 0.3 | 0.8 | 0.1 | 0.4 | – | – | – |
| Gal4GN4M3GN2 | – | – | – | 0.9 | – | – | – | 0.8 | 1.6 |
| Gal4GN4M3FGN2 | – | 0.2 | – | 0.3 | – | 0.5 | – | – | – |
| NAGalGNM3GN2 | – | – | – | – | – | – | – | 0.1 | – |
| NAGalGN2M3GN2 | – | – | 0.1 | 0.5 | – | 0.2 | – | – | 0.8 |
| NAGalGN2M3FGN2 | – | – | 0.1 | – | – | – | – | – | – |
| NAGalGN4M3GN2 | – | – | – | – | – | – | – | – | 0.2 |
| NAGal2GN2M3GN2 | – | – | 0.4 | 1.0 | 0.8 | 0.8 | 1.1 | 0.7 | – |
| NAGal2GN2M3FGN2 | 0.5 | – | – | 0.2 | 0.2 | 0.1 | – | – | – |
| NAGal2GN3M3GN2 | – | – | 0.1 | – | – | 0.4 | – | – | – |
| NAGal3GN3M3GN2 | – | 0.3 | 0.1 | 1.2 | 3.1 | 3.1 | 0.8 | 0.3 | 0.2 |
| NGGal2GN2M3GN2 | – | – | – | 0.1 | 0.2 | 0.1 | 0.3 | – | – |
| NGGal2GN2M3FGN2 | – | – | – | 0.2 | – | 0.2 | – | – | – |
| NGGal2GN3M3GN2 | – | – | – | – | – | – | – | – | 0.2 |
| NA2Gal2GN2M3GN2 | – | – | – | 0.2 | – | 0.6 | – | – | – |
| NA2Gal3GN3M3GN2 | – | – | – | 0.8 | – | 1.8 | 0.3 | – | 0.6 |
Analysis was conducted three times for each cell line (1st, 2nd, 3rd)
The italicized area shows the data of fucosylated glycans
Table 2.
N-glycan structures in suspension cells
| Structure | Wild-type | ΔGMD | ΔGMDΔGFT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | |
| GLM9GN2 | 0.1 | 0.8 | 0.4 | 1.5 | 0.8 | 0.9 | 0.2 | 0.7 | 0.6 |
| M5-9GN2 | 73.4 | 57.7 | 61.8 | 78.0 | 53.1 | 63.8 | 75.4 | 71.6 | 50.9 |
| M6FGN2 | 0.1 | – | – | – | – | – | – | – | – |
| M5FGN2 | 0.4 | – | 0.2 | – | – | – | – | – | – |
| M4GN2 | 0.4 | 1.1 | 1.4 | 0.5 | 2.0 | 0.8 | 0.8 | 1.1 | 1.1 |
| M4FGN2 | – | – | 0.2 | – | – | – | – | – | – |
| M3GN2 | 0.3 | 1.2 | 1.2 | 1.1 | 9.1 | 5.1 | 1.6 | 4.9 | 5.3 |
| M3FGN2 | 0.7 | 4.7 | 3.4 | – | 0.1 | – | – | – | – |
| M2GN2 | – | – | – | – | 2.8 | 0.7 | – | – | 1.1 |
| M2FGN2 | – | 0.4 | 0.5 | – | – | – | – | – | – |
| GNM3GN2 | 0.1 | 0.9 | 0.1 | 0.6 | 7.1 | 5.4 | 0.5 | 3.0 | 5.0 |
| GNM3FGN2 | 0.4 | 3.6 | 3.9 | – | – | – | – | – | – |
| GNM5GN2 | 0.3 | 0.6 | 0.5 | 0.3 | 0.3 | 0.5 | 0.2 | 0.7 | 0.9 |
| GN2M3GN2 | – | 0.7 | 0.4 | 0.8 | 4.4 | 5.7 | 1.6 | 3.9 | 6.0 |
| GN2M3FGN2 | 0.4 | 2.3 | 3.3 | – | – | 0.1 | – | – | – |
| GN3M3GN2 | 0.1 | 0.3 | 0.1 | 0.3 | 1.5 | 2.7 | 0.5 | 0.9 | 2.3 |
| GN3M3FGN2 | 0.2 | 1.5 | 1.4 | – | – | – | – | – | – |
| GN4M3GN2 | – | – | – | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| GN4M3FGN2 | – | 0.1 | 0.1 | – | – | – | – | – | – |
| GN5M3GN2 | – | – | – | – | 0.1 | – | – | – | – |
| GalGNM3GN2 | – | 0.6 | 0.7 | 0.4 | 2.7 | 1.4 | 0.4 | 2.0 | 1.1 |
| GalGNM3FGN2 | 0.3 | 1.1 | 1.0 | – | – | – | – | – | – |
| GalGNM5GN2 | – | 0.4 | 0.1 | 0.4 | 0.2 | 0.1 | 0.3 | – | 0.3 |
| GalGN2M3GN2 | – | 0.1 | 0.1 | 1.3 | 2.1 | 0.7 | 1.0 | 3.5 | 1.5 |
| GalGN2M3FGN2 | 0.5 | 0.7 | 1.2 | – | – | – | – | – | – |
| GalGN3M3GN2 | 1.4 | 1.7 | 1.9 | 0.5 | 1.3 | 1.8 | 0.8 | 1.1 | 1.0 |
| GalGN3M3FGN2 | – | – | – | 0.1 | – | – | – | – | - |
| GalGN4M3GN2 | – | – | 0.1 | – | 0.1 | 0.1 | – | – | 0.4 |
| GalGNM6GN2 | – | – | – | – | – | 0.1 | – | – | – |
| Gal2GN2M3GN2 | 0.9 | 1.6 | 2.7 | 9.3 | 4.9 | 3.3 | 8.6 | 3.4 | 10.1 |
| Gal2GN2M3FGN2 | 3.7 | 7.5 | 2.8 | 0.2 | – | – | – | – | – |
| Gal2GN3M3GN2 | – | 0.1 | 0.3 | 0.3 | 0.9 | 0.6 | 0.4 | 0.8 | 0.6 |
| Gal2GN3M3FGN2 | 0.1 | – | 0.1 | – | – | – | – | – | – |
| Gal2GN4M3GN2 | – | – | – | – | 0.1 | – | – | – | – |
| Gal3GN3M3GN2 | 0.1 | 0.9 | 0.9 | 2.7 | 2.9 | 1.3 | 2.0 | 1.2 | 3.2 |
| Gal3GN3M3FGN2 | 1.4 | 1.6 | 1.4 | – | – | – | – | – | – |
| Gal3GN4M3GN2 | 0.3 | – | – | – | – | – | 0.2 | – | – |
| Gal3GN4M3FGN2 | 0.3 | – | – | – | – | – | – | – | – |
| Gal4GN4M3GN2 | – | – | – | 0.8 | 1.3 | 1.4 | 1.9 | 0.5 | 2.3 |
| Gal4GN4M3FGN2 | 2.0 | 1.7 | 1.3 | – | – | – | – | – | – |
| NAGalGNM3GN2 | 0.1 | – | 0.2 | – | – | – | 0.3 | 0.1 | 0.1 |
| NAGalGNM4GN2 | – | – | 0.2 | – | – | – | 0.2 | – | – |
| NAGalGNM5GN2 | 0.1 | 0.1 | – | – | – | – | 0.2 | – | 0.2 |
| NAGalGN2M3GN2 | 0.1 | 0.1 | – | 0.1 | – | – | – | – | 0.2 |
| NAGalGN2M3FGN2 | 0.3 | – | – | – | – | – | – | – | – |
| NAGalGN4M3GN2 | – | 0.1 | – | – | – | – | – | – | – |
| NAGal2GN2M3GN2 | 0.5 | 1.0 | 1.7 | 0.7 | 0.4 | 1.4 | 2.2 | 0.3 | 1.8 |
| NAGal2GN2M3FGN2 | 5.6 | 2.1 | 1.7 | – | – | – | – | – | – |
| NAGal2GN3M3GN2 | – | 0.4 | – | – | 0.1 | – | – | 0.1 | 0.1 |
| NAGal3GN3M3GN2 | – | - | - | 0.1 | 0.6 | 0.6 | 0.6 | 0.1 | 1.9 |
| NAGal3GN3M3FGN2 | 1.4 | 1.2 | 1.4 | – | – | – | – | – | – |
| NAGal4GN4M3GN2 | – | – | – | – | 0.2 | 0.3 | – | – | 0.9 |
| NGGalGN2M3GN2 | – | – | – | – | 0.3 | 0.2 | 0.1 | – | 0.1 |
| NGGal2GN2M3GN2 | – | – | – | – | 0.3 | 0.2 | 0.3 | – | 0.1 |
| NGGal2GN2M3FGN2 | – | 0.2 | – | – | – | – | – | – | – |
| NGGal3GN3M3GN2 | – | – | – | – | 0.1 | – | – | – | –– |
| NA2Gal2GN2M3GN2 | 1.1 | 0.2 | – | – | 0.3 | 0.6 | 1.1 | – | 0.9 |
| NA2Gal2GN2M3FGN2 | 2.9 | 0.8 | 1.1 | – | – | – | – | – | – |
Analysis was conducted three times for each cell line (1st, 2nd, 3rd)
The italicized area shows the data of fucosylated glycans
Evaluation of cell growth
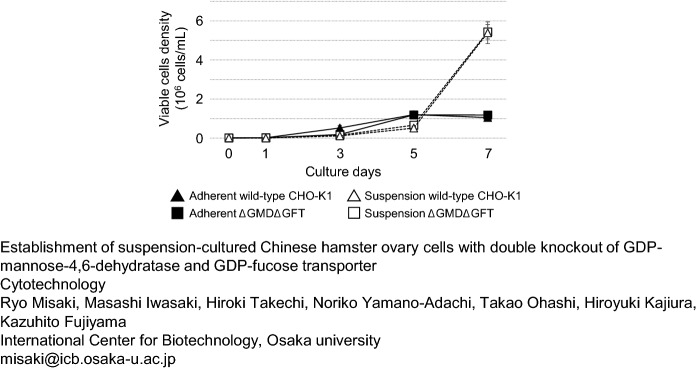
Figure 4 shows the number of viable wild-type CHO-K1, and ΔGMDΔGFT cells. During a 7-day cultivation, adherent ΔGMDΔGFT cells grew at a rate similar to that of adherent wild-type CHO-K1 cells. The total number of adherent cells increased from 1.4 × 105 to approximately 1.2 × 107 until day 5, and the growth rate decreased during the last 2 days. The growth rate of suspension-cultured ΔGMDΔGFT cells was also similar to that of the wild type. Unlike the adherent cells, however, their growth rate significantly increased after day 5.
Fig. 4.
Growth curve of wild-type CHO-K1 cells and ΔGMDΔGFT cells. Closed triangle: adherent wild-type CHO-K1 cells, closed square: Adherent ΔGMDΔGFT cells, opened triangle: wild-type CHO-K1 suspension cells, opened square: ΔGMDΔGFT suspension cells. Vertical and horizontal axis shows the cells density and culture days, respectively. Initial number of cultured cells was 1.4 × 105 cells. Mean (SE), n = 3
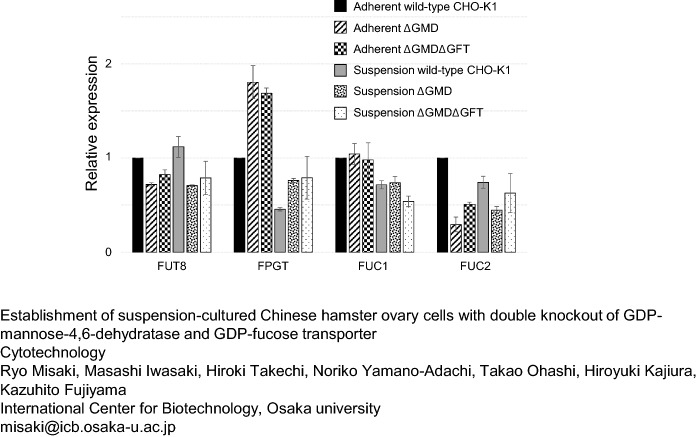
Expression levels of enzyme genes relating to fucosylated glycan synthesis
The gene expression levels of FUT8, FPGT, FUC1, and FUC2 in the wild-type and both mutant cell lines were estimated by qRT-PCR (Fig. 5). All gene expression levels in adherent wild-type CHO-K1 cells were normalized to 1. Expression of FUT4 and FUT9 was not found in CHO-K1 cells (data not shown). In both adherent and suspension cells, expression of FUT8 was reduced by GMD knockout but not by GFT knockout. GMD knockout significantly increased FPGT expression in adherent and suspension cells compared with the wild-type cells, though the expression levels decreased by approximately 50% by the adaptation to serum-free medium. Expression of FUC1 was not affected by the knockout of GMD or GFT, though the adaptation to serum-free medium decreased it in the suspension cells. GMD knockout also decreased FUC2 expression, but GFT knockout improved FUC2 expression in adherent and suspension-cultured mutant cells.
Fig. 5.
Relative gene expression of endogenous enzymes relating to the fucose metabolism. Bars mean adherent wild-type CHO-K1 cells, adherent ΔGMD cells, adherent ΔGMDΔGFT cells, suspension-cultured wild-type CHO-K1 cells, suspension-cultured ΔGMD cells, suspension-cultured ΔGMDΔGFT cells from the left, respectively. The expression amount of each gene from the wild-type CHO-K1 cells was normalized to 1. Mean (SE), n = 3
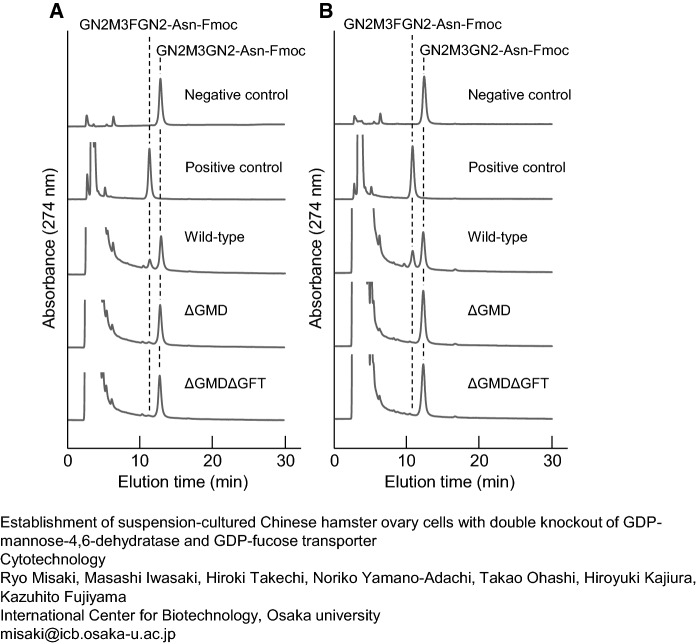
Analysis of amounts of intracellular GDP-fucose
The amounts of GDP-fucose in adherent wild-type CHO-K1, ΔGMD, and ΔGMDΔGFT cells cultivated under serum were estimated (Fig. 6A). An enzyme reaction using extracts of wild-type CHO-K1 cells resulted in the appearance of a new peak whose retention time corresponded to that of fucosylated GN2M3FGN2-Asn-Fmoc on RP-HPLC. The amount of GDP-fucose in 1.0 × 107 cells worked out to 87.9 pmol from the peak area of newly synthesized GN2M3FGN2-Asn-Fmoc. Although the amount of enzyme reaction product was significantly reduced by GMD following GFT knockout, 15.1 pmol and 8.5 pmol of GDP-fucose were still observed in 1.0 × 107 cells of ΔGMD and ΔGMDΔGFT, respectively. The results of the enzyme reaction using suspension cells are shown in Fig. 6B. As estimated from the peak area of GN2M3FGN2-Asn-Fmoc on RP-HPLC, 1.0 × 107 wild-type cells contained 130.5 pmol of GDP-fucose. In contrast, GN2M3FGN2-Asn-Fmoc was not detected by enzyme reaction using extracts of mutant, ΔGMD, or ΔGMDΔGFT cells.
Fig. 6.
Estimation of intracellular GDP-fucose in adherent cells (a); in suspension cells (b). Enzymatically produced GN2M3FGN2-Asn-Fmocs were eluted by HPLCs. Negative control: Fut8+GN2M3GN2-Asn-Fmoc, positive control: GDP-Fuc+Fut8+GN2M3GN2-Asn-Fmoc, wild-type: CHO-K1 extracts+Fut8+GN2M3GN2-Asn-Fmoc, ΔGMD: ΔGMD extracts+Fut8+GN2M3GN2-Asn-Fmoc, ΔGMDΔGFT: ΔGMDΔGFT extracts+Fut8+GN2M3GN2-Asn-Fmoc
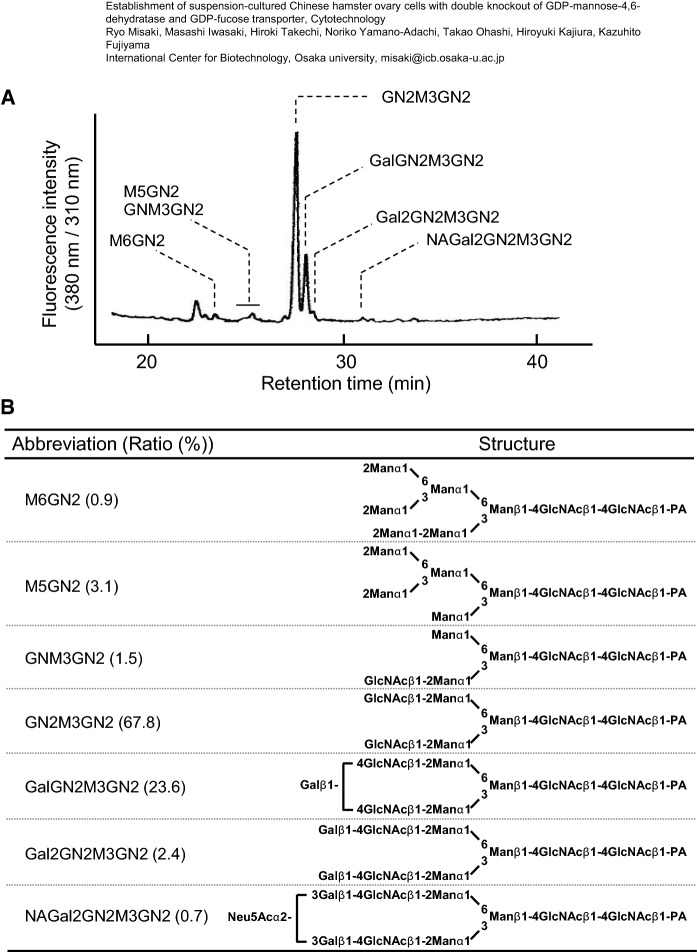
N-glycan analysis of recombinant human IgG produced in ΔGMDΔGFT cells
PA-labeled N-glycans prepared from recombinant human IgG produced in ΔGMDΔGFT suspension-cultured cells were fractionated on an RP-HPLC (Fig. 7A). Peaks containing PA-glycans are indicated with their abbreviations. A glycan structural analysis using an LC–MS/MS resulted in no detection of any fucosylated N-glycans (Fig. 7B). High-mannose-type, hybrid-type, asialo-type, and sialo-type N-glycans accounted for 4.0%, 69.3%, 26.0%, and 0.7% of the total N-glycan content, respectively.
Fig. 7.
N-Glycan analysis of recombinant human IgGs produced in ΔGMDΔGFT cells. RP-HPLC profiles of PA-labeled N-glycans of recombinant human IgGs produced in suspension-cultured ΔGMDΔGFT cells (a); N-glycan structure determined by HPLC and LC–MS analysis (b)
Discussion
In this study, we succeeded in establishing CHO-K1 cells that could synthesize afucosylated glycans by knockout of GMD and GFT, and examined the effects of afucosylation on serum-free adaptation of cells and the gene expression levels of other endogenous enzymes related to the synthesis of fucosylated glycans.
First, we destroyed the de novo synthetic pathway of GDP-fucose, which is a donor substrate of fucosyltransferases, by GMD knockout in CHO-K1 cells. The adherent ΔGMD cells showed a 54% reduction of fucosylated N-glycans compared with adherent wild-type cells, but 10% of fucosylated glycans were still found in ΔGMD. Galactosylation, which contributes to ADCC activity, was not affected by the GMD knockout. The knockout affected the amounts of high-mannose-type and complex-type N-glycans only slightly. Almost all fucosylation of N-glycans was lost by the double mutation of GMD and GFT, as 0.9% of fucosylation was detected. These results suggest that both the inactivation of the GDP-fucose de novo synthetic pathway and the loss of transport of the GDP-fucose from cytosol to the Golgi apparatus specifically suppressed the fucoylation of N-glycans but not the distribution patterns of other N-glycans. However, ΔGMD cells cultured in FBS contain approximately 10% fucosylated N-glycans, though a previous work mentioned that depletion of GMD produced no fucosylated glycans in CHO cells cultured in dialyzed FBS (Kanda et al. 2007). GDP-fucose should be synthesized only through the salvage pathway in ΔGMD cells because of the lack of the de novo synthetic function. In the salvage pathway, L-fucose kinase (FUK) and FPGT synthesize GDP-fucose from L-fucose via L-fucose-1-phosphate. ΔGMD cells cannot produce fucose. Therefore, L-fucose and GDP-fucose are thought to come from extracellular source, such as medium and FBS. Then, the internalized or FUK/FPGT-synthesized GDP-fucose was transported to the Golgi lumen by GFT and used to synthesize fucosylated glycans. Internalized fucosylated glycoproteins are degraded in lysosomes (Becker and Lowe 2003), and released free fucose residues may then be reused to synthesize GDP-fucose in ΔGMD cells. Similarly, fucosylated glycans of exogenous proteins from extracellular sources may have been detected in small amounts because GDP-fucose is not transported into the Golgi apparatus by GFT knockout. This means that at least the remove of serum from culture medium is required for the construction of cells without any fucosylated glycans. FBS contains many glycosylated enzymes and hormones, such as alkaline phosphatase, thyroid-stimulating hormone, and so on (Endo et al. 1988; Ikegami et al. 2014). Next, we developed wild-type CHO-K1, ΔGMD, and ΔGMDΔGFT cells adapted to serum-free medium, to which the current host cells should be adapted from the standpoints of both the effectiveness and safe industrial production of biopharmaceutical proteins. These cell lines were then established as suspended cells in the process of serum-free adaptation. The N-glycosylation pattern except for fucosylation among these cell lines were not affected by the serum-free adaptation. The adaptation of wild-type CHO-K1 cells to serum-free medium also produced no differences in the amounts of fucosylated N-glycans. However, knockout of GMD produced only 0.2% fucosylation in ΔGMD suspension cells, which is less than that in adherent ΔGMDΔGFT cells. Moreover, fucosylated N-glycans were not detected in ΔGMDΔGFT suspension cells. These results suggest that inactivation of GFT and adaptation to serum-free medium are required for the complete removal of fucosylated glycans from CHO cells. On the other hand, even suspension ΔGMD cells under serum-free were able to greatly suppress glycan fucosylation (0.7% relative to the suspension wild-type cells) compared to adherent ΔGMDΔGFT cells cultured in the presence of serum (5% relative to the adherent wild-type cells). Although the ΔGMD cells were not completely defucosylated in serum-free medium as previously reported (Kanda et al. 2007), a single mutation of GMD can be a strong way to effective production of antibodies with high ADCC activity under serum-free.
It was thought that the amount of fucosylated glycans depended on that of intracellular GDP-fucose. As shown in Fig. 6A, the amounts of GDP-fucose in 1.0 × 107 adherent wild-type and ΔGMD cells were 87.9 and 15.1 pmol, respectively. It was thought that the GDP-fucose contained in ΔGMD cells was internalized from medium with serum or synthesized using internalized L-fucose by FUK and FPGT, and the GDP-fucose was then used as a substrate for the production of approximately 10% fucosylated N-glycans. No remaining GDP-fucose was found in extracts of ΔGMD suspension cells, as shown in Fig. 6B. This means that these exogenous substrates were removed by the adaptation of ΔGMD cells to serum-free medium. Actually, we detected GDP-fucose from medium containing 10% FBS without any cells (data not shown). Similarly, 1.0 × 107 of adherent ΔGMDΔGFT cells contained 8.5 pmol of GDP-fucose, and the adaptation of ΔGMDΔGFT cells to serum-free medium resulted in the total absence of fucosylated N-glycans. In contrast, adaptation of wild-type CHO-K1 cells to serum-free medium increased the amount of intracellular GDP-fucose. Probably, suspension-cultured cells need supplementation with L-fucose or GDP-fucose and positively produce GDP-fucose using active GMD, FUK, and FPGT because serum-free medium contains no L-fucose. We further examined a potential of the suspension ΔGMDΔGFT cells for industrial biopharmaceutical protein production by expression of a recombinant human IgG under serum-free cultivation. Fucosylation was not detected in the purified IgGs as shown in Fig. 7 and the distribution of detected N-glycan structures was quite similar without the abnormal structures compared to trastuzumab produced in GFT-mutated CHO-DG44 cells reported by Chan et al. (2016).
Although Kanda et al. (2007) and Chan et al. (2016) demonstrated that GMD- and GFT-knockout did not affect cell growth of CHO-DG44 cells, respectively, there are no information about the effect of the GMD-GFT-double knockout on cell characteristics. First, the effects of the knockout of GMD and GFT on the expression of genes encoding other enzymes relating to fucosylation was analyzed. Overall, the expression level of each target gene except for FUT8 in suspension cells was lower than that in adherent cells. As shown in Fig. 4, the suppression of fucosylated N-glycans did not affect cell growth among the wild type or the mutants. Although increase of the number of both adherent ΔGMDΔGFT and wild-type cells was suppressed by the high-confluency, the number of suspension ΔGMDΔGFT and wild-type cells increased dramatically as well after the 5-day culture.
In this study, we succeeded in using GMD and GFT knockout to establish a novel CHO cell line, ΔGMDΔGFT, in which fucosylated N-glycans are undetectable and production of fucose-free human IgGs using the ΔGMDΔGFT cells. Moreover, as demonstrated in previous studies with GMD or GFT single knockout cell line (Kanda et al. 2007; Chan et al. 2016), we demonstrated that adaptation to serum-free medium did not affect the growth of ΔGMDΔGFT cells compared to that of wild-type CHO-K1 cells. This means that ΔGMDΔGFT cells can be used for the industrial production of recombinant biopharmaceutical proteins as a good host cell line. Especially, the production of the next generation of recombinant monoclonal antibodies has received a lot of attention, and the market is growing significantly every year. Not only improved productivity but also highly value-added antibody production are required. ΔGMDΔGFT cell with afucosylated N-glycans can be a useful host for IgGs because the removal of the core-fucosylation of IgG brings a huge increase of ADCC activity described above. On the other hand, Ohashi et al. and Hosser et al. reported that FUT8 can add D-arabinose and L-galactose instead of L-fucose as the core glycosylation (Ohashi et al. 2017; Hossler et al. 2017). Intriguingly, the IgG with arabinosylated N-glycan showed a high affinity to FcγRIII similar to or slightly higher than that of the IgG with afucosylated N-glycans. A previous study suggested that fucose residues are internalized from extracellular compartment via a specific plasma membrane transporter system (Wiese et al. 1994). Accumulation of fucose in HepG2 cells was facilitated by existence of other monosaccharides including D-arabinose except for glucose (Wiese et al. 1997). Although there is no information about uptake of D-arabinose via the same transporter, Hossler et al. 2017 showed that addition of high D-arabinose into cultured medium achieved its uptake to CHO cells. One research group demonstrated that combination of FUK and FPGT can produce GDP-D-arabinose from the substrate D-arabinose (Park et al. 1997). Substrate specificity of GFT is still unclear. For instance, Leishmania LPG2 is known to transport not only GDP-mannose but also GDP-fucose (Ma et al. 1997). However, endogenous GFT may have transported D-arabinose from cytosol to the Golgi lumen in the case of Hossler et al. 2017. Therefore, ΔGMDΔGFT cells may have a reduced capacity for D-arabinose uptake because of lack of GFT function. On the other hand, D-arabinose is efficiently transferred to N-glycans without competing with fucose under depletion of GMD. Low concentration of D-arabinose in cell cultured medium may economically achieve the production of arabinosylated N-glycans. ΔGMD cells in this study or previously established GMD-knockout cells (Kanda et al. 2007) are promising host cells for effective production of antibodies with arabinosylated N-glycans.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the research project of Manufacturing Technology Association of Biologics and Japan Agency for Medical Research and Development (AMED; 17ae0101003h0005 and 19ae0101066h0002).
Abbreviations
- GMD
GDP-mannose 4,6-dehydratase
- GFT
GDP-fucose transporter
- Man
Mannose
- Fuc
Fucose
- GlcNAc
N-Acetylglucosamine
- Fmoc
9-Fluorenylmethyloxycarbonyl
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aggarwal RS. What’s fueling the biotech engine-2012 to 2013. Nat Biotechnol. 2014;32:32–39. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41–53. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- Chan KF, Shahreel W, Wan C, et al. Inactivation of GDP-fucose transporter gene (Slc35c1) in CHO cells by ZFNs, TALENs and CRISPR-Cas9 for production of fucose-free antibodies. Biotechnol J. 2016;11:399–414. doi: 10.1002/biot.201500331. [DOI] [PubMed] [Google Scholar]
- Chung S, Quarmby V, Gao X, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. Mabs. 2012;4:326–340. doi: 10.4161/mabs.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J, Euwart D, Mei B, et al. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol. 2016;36:1110–1122. doi: 10.3109/07388551.2015.1084266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Ohbayashi H, Hayashi Y, et al. Structural study on the carbohydrate moiety of human placental alkaline phosphatase. J Biochem. 1988;103:182–187. doi: 10.1093/oxfordjournals.jbchem.a122228. [DOI] [PubMed] [Google Scholar]
- Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. 2012;28:147–176. doi: 10.5661/bger-28-147. [DOI] [PubMed] [Google Scholar]
- Haryadi R, Zhang P, Chan KF, Song Z. CHO-gmt5, a novel CHO glycosylation mutant for producing afucosylated and asialylated recombinant antibodies. Bioengineered. 2013;4:90–94. doi: 10.4161/bioe.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossler P, Chumsae C, Racicot C, et al. Arabinosylation of recombinant human immunoglobulin-based therapeutics. Mabs. 2017;9:715–734. doi: 10.1080/19420862.2017.1294295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S, Misaka H, Inoue M, et al. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcγRIIIa. Clin Cancer Res. 2006;12:2879–2887. doi: 10.1158/1078-0432. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Liao XH, Hoshino Y, et al. Tissue-specific posttranslational modification allows functional targeting of thyrotropin. Cell Rep. 2014;9(3):801–809. doi: 10.1016/j.celrep.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai-Nishiya H, Mori K, Inoue M, et al. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 2007;7:84. doi: 10.1186/1472-6750-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R. Posttranslational modifications and the immunogenicity of biotherapeutics. J Immunol Res. 2016;2016:5358272. doi: 10.1155/2016/5358272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y, Imai-Nishiya H, Kuni-Kamochi R, et al. Establishment of a GDP-mannose 4,6-dehydratase (GMD) knockout host cell line: a new strategy for generating completely non-fucosylated recombinant therapeutics. J Biotechnol. 2007;130:300–310. doi: 10.1016/j.jbiotec.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Kesik-Brodacka M. Progress in biopharmaceutical development. Biotechnol Appl Biochem. 2018;65:306–322. doi: 10.1002/bab.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A, Suzuki J, Kuraya N, et al. Improved method for fluorescence labeling of sugar chains with sialic acid residues. Agric Biol Chem. 1990;54:2169–2170. doi: 10.1271/bbb1961.54.2169. [DOI] [PubMed] [Google Scholar]
- Kuriakose A, Chirmule N, Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. J Immunol Res. 2016;2016:1298473. doi: 10.1155/2016/1298473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie S, Haley B, Marshall B, et al. FX knockout CHO hosts can express desired ratios of fucosylated or afucosylated antibodies with high titers and comparable product quality. Biotechnol Bioeng. 2017;114:632–644. doi: 10.1002/bit.26188. [DOI] [PubMed] [Google Scholar]
- Ma D, Russell DG, Beverley SM, et al. Golgi GDP-mannose uptake requires Leichmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J Biol Chem. 1997;272:3799–3805. doi: 10.1074/jbc.272.6.3799. [DOI] [PubMed] [Google Scholar]
- Malphettes L, Freyvert Y, Chang J, et al. Highly efficient deletion of FUT8 in CHO cell lines using zinc-finger nucleases yields cells that produce completely nonfucosylated antibodies. Biotechnol Bioeng. 2010;106:774–783. doi: 10.1002/bit.22751. [DOI] [PubMed] [Google Scholar]
- Misaki R, Fukura N, Kajiura H, et al. Recombinant production and characterization of human anti-influenza virus monoclonal antibodies identified from hybridomas fused with human lymphocytes. Biologicals. 2016;44(5):394–402. doi: 10.1016/j.biologicals.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, et al. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng. 2004;88:901–908. doi: 10.1002/bit.20326. [DOI] [PubMed] [Google Scholar]
- Niwa R, Shoji-Hosaka E, Sakurada M, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64:2127–2133. doi: 10.1158/0008-5472. [DOI] [PubMed] [Google Scholar]
- Ohashi H, Ohashi T, Kajiura H, et al. Fucosyltransferases produce N-glycans containing core L-galactose. Biochem Biophys Res Commun. 2017;483:658–663. doi: 10.1016/j.bbrc.2016.12.087. [DOI] [PubMed] [Google Scholar]
- Omasa T, Tanaka R, Doi T, et al. Decrease in antithrombin III fucosylation by expressing GDP-fucose transporter siRNA in Chinese hamster ovary cells. J Biosci Bioeng. 2008;106:168–173. doi: 10.1263/jbb.106.168. [DOI] [PubMed] [Google Scholar]
- Park S, Pastuszak I, Mengeling BJ, et al. Synthesis and utilization of GDP-D-arabinopyranoside. Anal Biochem. 1997;244:321–327. doi: 10.1006/abio.1996.9906. [DOI] [PubMed] [Google Scholar]
- Runkel L, Meier W, Pepinsky RB, et al. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta) Pharm Res. 1998;15:641–649. doi: 10.1023/A:1011974512425. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Setthapramote C, Kurosu T, et al. Dengue virus neutralization and antibody-dependent enhancement activities of human monoclonal antibodies derived from dengue patients at acute phase of secondary infection. Antivir Res. 2013;98(3):423–431. doi: 10.1016/j.antiviral.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/ibc.M210665200. [DOI] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks. Nat Biotechnol. 2018;36:1136–1145. doi: 10.1038/nbt.4305. [DOI] [PubMed] [Google Scholar]
- Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol. 2006;24:1241–1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- Wiese TJ, Dunlap JA, Yorek MA. L-fucose is accumulated via a specific transport system in eukaryotic cells. J Biol Chem. 1994;269:22705–22711. doi: 10.1016/S0021-9258(17)31703-9. [DOI] [PubMed] [Google Scholar]
- Wiese TJ, Dunlap JA, Yorek MA. Effect of L-fucose and D-glucose concentration on L-fucoprotein metabolism in human Hep G2 cells and changes in fucosyltransferase and alpha-L-fucosidase activity in liver of diabetic rats. Biochim Biophys Acta. 1997;1335:61–72. doi: 10.1016/S0304-4165(96)00123-7. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- Zhang P, Haryadi R, Chan KF, et al. Identification of functional elements of the GDP-fucose transporter SLC35C1 using a novel Chinese hamster ovary mutant. Glycobiology. 2012;22:897–911. doi: 10.1093/glycob/cws064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.