Abstract
Syncytial isopotentiality, resulting from a strong electrical coupling, emerges as a physiological mechanism that coordinates individual astrocytes to function as a highly efficient system in brain homeostasis. However, whether syncytial isopotentiality occurs selectively to certain brain regions or is universal to astrocytic networks remains unknown. Here, we have explored the correlation of syncytial isopotentiality with different astrocyte subtypes in various brain regions. By using a non-physiological K+-free/Na+ electrode solution to depolarize a recorded astrocyte in situ, the existence of syncytial isopotentiality can be revealed: the recorded astrocyte’s membrane potential remains at a quasi-physiological level due to strong electrical coupling with neighboring astrocytes. Syncytial isopotentiality appears in layer I of the motor, sensory and visual cortical regions, where astrocytes are organized with comparable cell densities, interastrocytic distances, and the quantity of directly coupled neighbors. Second, though astrocytes vary in their cytoarchitecture in association with neuronal circuits from layer I to VI, the established syncytial isopotentiality remains comparable among different layers in the visual cortex. Third, neurons and astrocytes are uniquely organized as barrels in layer IV somatosensory cortex; interestingly, astrocytes both inside and outside of the barrels do electrically communicate with each other and also share syncytial isopotentiality. Fourth, syncytial isopotentiality appears in radial-shaped Bergmann glia and velate astrocytes in the cerebellar cortex. Fifth, although fibrous astrocytes in white matter exhibit a distinct morphology, their network syncytial isopotentiality is comparable with protoplasmic astrocytes. Altogether, syncytial isopotentiality appears as a system-wide electrical feature of astrocytic networks in the brain.
Keywords: Astrocytes, gap junctions, electrical coupling, syncytial isopotentiality
Introduction
Astrocytes establish the largest syncytial networks through gap junction coupling in the brain (D’Ambrosio et al. 1998; Rouach et al. 2008; Wallraff et al. 2006; Xu et al. 2010). Although astrocytes have long been thought of as integral units of neural circuits, how astrocytes act as a system in brain function emerges as a current focus of research (Nimmerjahn and Bergles 2015). One mechanism revealed in our recent study shows that a strong electrical coupling enables hippocampal astrocytes to constantly equalize their membrane potentials so that a syncytial isopotentiality can be achieved (Ma et al. 2016).
While syncytial isopotentiality occurs in hippocampal astrocytes, it remains unknown whether this physiological mechanism operates in other parts of the brain. The answer to this question is important because the heterogeneity of astrocytes has been recognized ever since this star-shaped glial subtype was discovered, leading to the initial classification of protoplasmic astrocytes in grey matters and fibrous astrocytes in white matters (Kimelberg 2010; Verkhratsky and Nedergaard 2018). More recent studies showed that astrocytes in different brain subregions vary in their cell morphology, spatial organization, association with different neuronal circuitries, and gene expression (Houades et al. 2008; Houades et al. 2006; Morel et al. 2017; Nadarajah et al. 1996; Roux et al. 2011; Zhang and Barres 2010). Other than permitting ion flux across cells, another important property of gap junction coupling is to mediate biochemical coupling, such as propagation of Ca2+ transients and redistribution of metabolites (Giaume et al. 2010; Volterra et al. 2014). Interestingly, the levels of spontaneous calcium transients vary between cortical layers (Takata and Hirase 2008). This suggests that astrocytes may adopt unique layer specific function similar to cortical neuronal networks (Petersen 2007). Nevertheless, it is completely unknown how such a multifaceted heterogeneity is associated with regional astrocyte coupling strength and syncytial isopotentiality.
In mouse hippocampus, a strong interastrocytic electrical coupling and a minimum of 7–9 directly coupled nearest neighbors are two critical factors underpinning syncytial isopotentiality (Ma et al. 2016). However, it is yet unknown whether a combination of these structural and functional features would serve as a generic pattern of “astrocyte connectome” to underpin syncytial isopotentiality across the brain.
To answer the extent to which syncytial isopotentiality exists in the brain, we have chosen several well-recognized subtypes of astrocytes to correlate their morphology, spatial organization pattern, and syncytial isopotentiality expression. In doing so, CUBIC tissue clearing method was used to examine the morphology of different subtypes of astrocytes and their spatial organizations in Aldh1l1-eGFP mice with high-resolution (Susaki et al. 2014; Susaki et al. 2015). The existence of syncytial isopotentiality was examined by our newly developed methodology, which involves the use of K+-free/Na+-containing recording pipette solution (Ma et al. 2016).
We show that although the strength of syncytial isopotentiality varies among different astrocyte subtypes, the syncytial isopotentiality represents a general feature of astrocytic networks throughout the brain.
Methods
Animals.
All experimental procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of The Ohio State University. All experiments were performed with the use of BAC Aldh1l1-eGFP transgenic mice of both sexes at postnatal day (P) 21–28 (Yang et al. 2011; Zhong et al. 2016). Mice were housed in a 12 hour (hr) light/dark cycle and temperature controlled (22 ± 2°C) environment with ad libitum access to food and water.
Preparation of acute brain slices and freshly disassociated astrocytes.
Mice were anesthetized with 8% chloral hydrate in 0.9% NaCl saline followed by decapitation (Wang et al. 2013). Brains were removed quickly from skulls and placed into ice-cold oxygenated (95%O2/5%CO2) aCSF with reduced Ca2+ and increased Mg2+ (in mM: 125 NaCl, 3.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 0.1 CaCl2, 3 MgCl2 and 10 Glucose). Coronal or sagittal brain slices (250 μm) were cut at 4°C with a Vibratome (Pelco 1500) and transferred to the oxygenated standard aCSF (in mM: 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3.5 KCl, 2 CaCl2, 1 MgCl2 and 10 Glucose, osmolality, 295 ± 5 mOsm; pH 7.3–7.4). Brain slices were allowed to recover from preparation damage for at least 1 hr at the room temperature (RT) before electrophysiological recording.
To prepare freshly disassociated astrocytes, hippocampal brain slices were first incubated for 30 min in oxygenated aCSF supplemented with 10 μM SR101 at 34°C. Then, the CA1 region was dissected out and cut into ~1 mm2 pieces and transferred into a 1.5 mL Eppendorf tube containing oxygenated aCSF supplemented with 24U/mL papain and 0.8 mg/mL L-cysteine for 7 min at RT. After papain digestion, the tissues were gently triturated five to seven times into a cell suspension, which is then transferred into the recording chamber (Du et al. 2015; Du et al. 2018).
Optical tissue clearing and imaging.
Tissue clearing was performed as described (Susaki et al. 2014; Susaki et al. 2015; Tainaka et al. 2014). Anesthetized mice were cardially-perfused with 4% paraformaldehyde (PFA) in 0.1M phosphate-buffered saline (PBS). 1 mm mid-sagittal brain slices were sectioned and post-fixed in 4%PFA/0.1M PBS at 4°C overnight, followed by in ScaleCUBIC reagent-1 immersion for 3 days at RT. Brain slices were incubated in a blocking solution consisting of 5% normal donkey serum (NDS) and 0.1% Triton X-100 in PBS for 24 hrs at RT. Slices were incubated for 72 hrs at RT in a primary antibody solution consisting of antibodies diluted in a 10% NDS and 0.05% Triton X-100 in PBS solution. Brain slices were then washed with PBS 3 times for 3 hrs each wash, followed by incubation of the secondary antibody solution diluted in a 10% NDS and 0.05% Triton X-100 in PBS solution for 72 hrs at RT. Brain slices were washed in PBS 3 times for 3 hrs each wash and then mounted in ScaleCUBIC reagent-1. Primary antibody used was rabbit anti-NeuN (1:500, ABN78, Millipore) and secondary antibody was a Cy3-conjugated donkey anti-rabbit antibody (1:500, Jackson ImmunoResearch). Images were acquired by confocal microscopy (SP8, Leica).
Intracellular loading of 0.1% biocytin in [K+]P and subsequent treatment with 1:1,600 Streptavidin-Cy3 (Jackson ImmunoResearch) has been described in our previous report in detail (Xu et al. 2014).
Imaging acquisition for astrocyte identification in situ.
Polychrome V fluorescent imaging system (Till Photonics, Germany) was used to identify Aldh1l1-eGFP-positive astrocytes. An infrared differential interference contrast (IR-DIC) video camera was used to visualize astrocyte cell bodies for electrode placement in single and dual whole-cell patch clamp recording.
Electrophysiology.
Individual brain slices were transferred to the recording chamber mounted on an Olympus BX51WI microscope with constant oxygenated aCSF (2.0 mL/min) bath perfusion. Whole-cell patch clamp recordings were performed using a MultiClamp 700A amplifier and pClamp 9.2 software (Molecular Devices, Sunnyvale, CA). Borosilicate glass pipettes (Warner Instrument) were pulled from a Micropipette Puller (Model P-87, Sutter Instrument). The recording electrodes had a resistance of 3–5 MΩ when filled with the electrode solution containing (in mM) 140 KCl (or NaCl), 1.0 MgCl2, 0.5 CaCl2, 10 HEPES, 5 EGTA, 3 Mg-ATP, and 0.3 Na-GTP (pH=7.25–7.30, 280 ± 5 mOsm). The physiological 140 KCl-containing pipette solution is referred as [K+]P, whereas K+-free/140 mM NaCl-containing pipette solution is referred as [Na+]P in the following description. The membrane potential (VM) was recorded under current clamp mode in PClamp 9.2 program. The liquid junction potential was compensated prior to all recordings. In current clamp recording, the input resistance (Rin) was measured by “Resistance test” (a 63 pA/600 ms pulse) during recording. Recordings with initial Rin greater than 50 MΩ, or Rin varied greater than 10% during recording were discarded. All the experiments were conducted at RT. The procedure for transjunctional current measurement has been described in details in our previous reports (Ma et al. 2016; Ma et al. 2014; Xu et al. 2010; Xu et al. 2014). Specifically, dual patch whole-cell recording was made from pairs of neighboring astrocytes with [K+]P electrodes. Both astrocytes were held at −80 mV at resting, and a pair of command voltages (VCOM) of ±120 mV/25 ms was alternately delivered to one cell, and transjunctional currents were recorded in another cell in a pair.
Chemical reagents.
All chemicals were purchased from Sigma-Aldrich.
Data analyses.
The patch clamp recording data were analyzed by Clampfit 9.0 (Molecular Devices, Sunnyvale, CA). Statistical analysis was performed using Origin 8.0 (OriginLab, Northampton, MA) or IBM SPSS Statistics 25.0 (IBM, Armonk NY). Results are given as means ± SEM. Unpaired or paired Student’s t-test was performed for comparisons between two groups. One-way ANOVA followed by Tukey’s post-hoc multiple comparisons (denoted by ♦) or Dunnett’s post-hoc planned comparisons (denoted by ‡) tests were performed for multiple groups as indicated. Differences were considered statistically significant when p < 0.05.
The electrical coupling strength (S) is calculated from S = GGap / GK, where GGap and GK are gap junction and membrane K+ conductances, respectively (Ma et al. 2016).
Results
Syncytial isopotentiality in gap junction-coupled astrocytes.
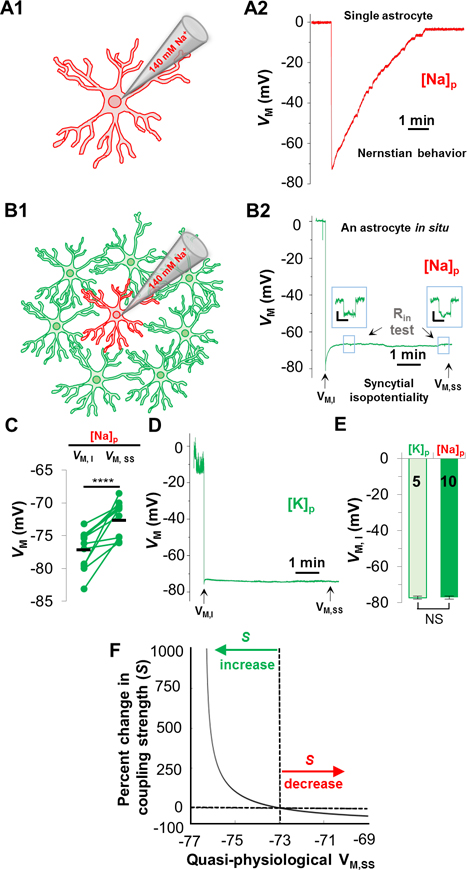
Astrocytes are known to behave as K+ electrodes due to predominant expression of K+ conductance (Kuffler et al. 1966; Ransom and Goldring 1973). Accordingly, in single freshly disassociated astrocytes, K+-free/Na+ pipette solution ([Na+]P depolarizes the membrane potential (VM) towards 0 mV, reflecting an anticipated Nernstian VM behavior of astrocytes (Figure 1A). However, this Nernstian VM behavior does not occur in gap junction coupled astrocyte in situ. Instead, a quasi-physiological VM remains throughout the [Na+]P recording (Figure 1B), resulting from a strong electrical coupling that equalizes the recorded astrocyte to have a VM comparable to its coupled neighbors. In the [Na+]P recording, the initial VM (VM,I) immediately following membrane break-in (−77.17 ± 0.89 mV) was ~5 mV more negative than the quasi-physiological VM at the steady-state (VM,SS) level (−72.71 ± 0.81 mV, n=10 recordings, p < 0.001, Figure 1C).
Figure 1. Astrocyte syncytial isopotentiality.
(A) In a single freshly disassociated astrocyte, K+-free/Na+ pipette solution [Na+]P progressively substituted the endogenous K+ content in whole-cell recording, leading to a VM depolarization towards 0 mV as predicted by Nernstian equation. (B) The VM recorded from an astrocyte in situ with [Na+]P disobeys the Nernstian prediction. Instead, the VM maintains at a quasi-physiological level. (B2) In [Na+]P recording, the initial VM (VM,I), and the quasi-physiological VM at the steady-state (VM,SS). Input resistance (RIn) was periodically monitored during recording. (C) VM,SS was significantly more depolarized than VM,I (p < 0.001, pairwise t-test). (D) VM,I and VM,SS was measured in whole-cell recording using 140 mM KCl recording pipette ([K+]P). (E) VM,I in [K+]P and [Na+]P recordings was not significantly different (p > 0.05, unpaired t-test). (F) The relationship between VM,SS and coupling strength (S) predicted by computational modeling. The VM,SS from hippocampal CA1 syncytium, −73 mV, is used as the baseline in the model to predict the relative difference in coupling strength (S%) between CA1 syncytium and a syncytium of another brain region.
As we have previously demonstrated, the VM,I is recorded under a condition where the intracellular K+ concentration ([K+]i) remains undisturbed and, therefore, corresponds to resting astrocyte VM (Ma et al. 2016). This notion was further confirmed by astrocyte recordings made with physiological [K+]P in hippocampal slices (Figure 1D). Specifically, in [K+]P recording, the resting astrocyte VM remained nearly constant from the initial VM,I (−77.22 ± 0.75 mV) to the steady-state VM,SS (−78.35 ± 0.74 mV, n=5 recordings, p > 0.05), and these values are comparable to the VM,I in [Na+]P recording (p > 0.05, Figure 1E). Therefore the VM,I in [Na+]P recording is a reliable readout of the resting astrocyte VM.
The amplitude of quasi-physiological VM,SS in [Na+]P recording is a functional readout of coupling strength of a syncytium. Specifically, weakening of electrical coupling shits the VM,SS of [Na+]P-recorded astrocyte towards the Nernstian predicted 0 mV, whereas strengthening of coupling shifts the VM,SS towards the physiological VM of neighboring astrocytes (Ma et al. 2016). Therefore, the coupling strength (S) of an unexplored syncytium can be quantitatively compared to hippocampal CA1 syncytium based on the difference in their VM,SS values (Figure 1F, Supp. Info). To ensure the consistency in the quality of the whole-cell recording, the input resistance (Rin) was periodically monitored in all the recordings (Figure 1B).
Syncytial isopotentiality occurs in different cortical regions
To answer whether syncytial isopotentiality also occurs in evolutionarily more recent parts of the cerebrum, such as neocortex, we selected three neocortical regions involved with higher-order behavioral functions: primary motor (MC), somatosensory (SC), and visual cortices (VC). We first examined layer 1 astrocytes in these regions because they are similarly associated with inhibitory neurons, and tuft dendrites ascending from layer 2/3 (L2/3) and layer 5 (L5) pyramidal neurons (Bear et al. 2016; Cauller et al. 1998; Hestrin and Armstrong 1996; Palmer et al. 2012; Prieto et al. 1994).
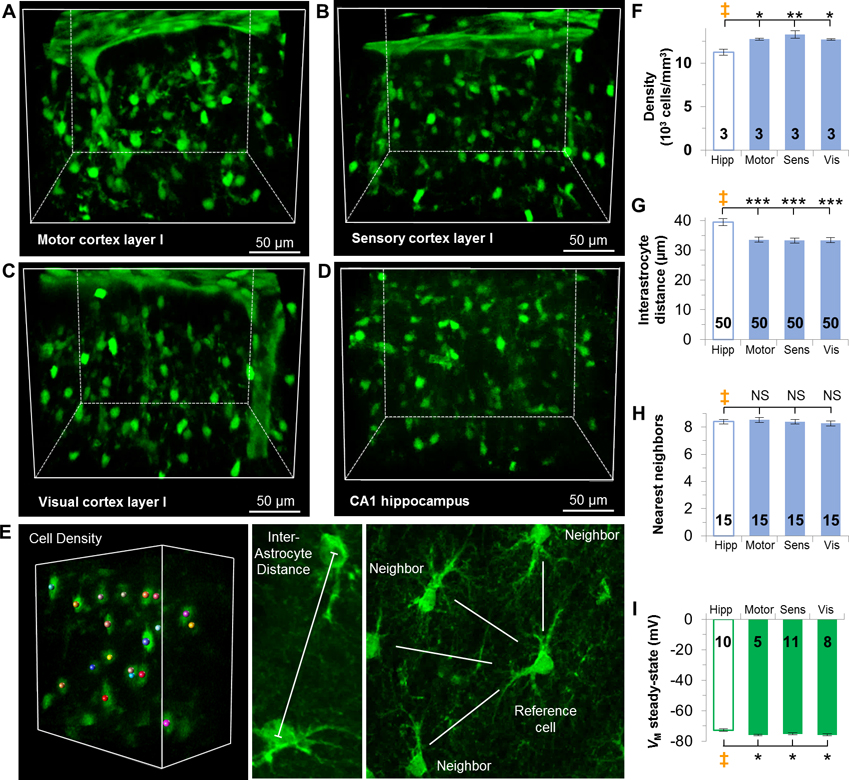
First, we used CUBIC tissue clearing method to visualize astrocytes in Aldh1l1-eGFP transgenic mice in these layer I regions and compared them with astrocytes in hippocampal CA1 stratum radiatum (Figure 2A–D). We quantitatively analyzed and compared the astrocyte cell density, interastrocytic distance, and the number of the nearest neighbors among these regions. For cell density analysis, individual astrocytes with full domains in a defined volume were quantified, whereas partial astrocytes at the volume edges were excluded. The interastrocytic distance was measured between nearest neighbors at the central points of the soma. In the nearest neighbor analysis, a randomly chosen astrocyte is set as the reference cell and astrocytes were considered as nearest neighbors if there was no intermediate astrocyte or a blood vessel between them (Figure 2E) (Xu et al. 2010).
Figure 2. Syncytial isopotentiality occurs in layer I cortical astrocytes across three brain regions.
(A-D) CUBIC optical clearing of brain tissue slices reveals the syncytial organization of astrocytes in layer I of the motor (A), sensory (B), and visual (C) cortices, and CA1 stratum radiatum hippocampal astrocytes (D). E. Representations of astrocyte syncytium anatomical parameters. (F) Astrocyte density is higher in layer I cortices than astrocytes in the hippocampus. (G) The interastrocytic distance was shorter in layer I cortices than the hippocampus. (H) The number of nearest neighbors was comparable between hippocampus and layer I cortices. (I) VM,SS in each layer I cortical area is significantly hyperpolarized than the hippocampus. F, G, H, I: one-way ANOVA, Dunnett’s post hoc (‡). *: p < 0.05, **: p < 0.01, ***: p < 0.005, NS: p > 0.05.
In comparison to the density of astrocytes in hippocampal CA1 stratum radiatum (11238 ± 331 cells/mm3), the layer I of all three cortical regions are populated with more astrocytes (motor cortex 12745 ± 102 cells/mm3, p < 0.05; sensory cortex, 13281 ± 331 cells/mm3, p < 0.01; visual cortex, 12732 ± 84 cells/mm3, p < 0.05, n=3 mice in each region, Figure 2F, Supp. Info. Table 2). Interastrocyte distance for hippocampal astrocytes averaged 39.45 ± 1.14 μm. Layer I astrocytes in each region were significantly shorter by ~6 μm than hippocampal astrocytes (motor cortex, 33.59 ± 0.9 μm, p < 0.001; sensory cortex, 33.3 ± 0.81 μm, p < 0.001; visual cortex, 33.4 ± 0.86 μm, p < 0.001, n=50 measurements in each region from 3 mice, Figure 2G, Supp. Info. Table 3). In contrast, hippocampal astrocytes and layer I cortical astrocytes are directly coupled with a comparable number of eight nearest neighbors (hippocampus, 8.4 ± 0.16 nearest neighbors; motor cortex, 8.53 ± 0.19 neighbors; sensory cortex, 8.4 ± 0.16 neighbors; visual cortex, 8.27 ± 0.18 neighbors, n=15 measurements in each region from 3 mice, p > 0.05, Figure 2H, Supp. Info. Table 4).
We next asked whether these anatomical differences could give rise to a strength of syncytial isopotentiality in layer I astrocyte networks differing from hippocampal CA1 syncytium. In [Na+]P recording, the quasi-physiological VM,SS appeared in all recorded layer I astrocytes at comparable levels of ~76 mV (motor cortex, −75.94 ± 0.58 mV, n=5 recordings; sensory cortex, −75.73 ± 0.69 mV, n=11 recordings; visual cortex, −76.01 ± 0.77 mV, n=8 recordings, p > 0.05). This is significantly closer to the physiological VM,I than hippocampal astrocytes (−72.74 ± 0.81 mV, n=10 recordings, p < 0.05, Figure 2I, Supp. Info. Table 5), indicating that layer I astrocyte networks have a stronger strength of syncytial isopotentiality. According to our model prediction, astrocyte networks in layer I motor, sensory and visual cortex are electrically coupled 615%, 263%, and 510% stronger, respectively, over the hippocampal astrocytes (Figure 1F, Supp. Info). While a combination of a higher astrocyte density and shorter interastrocytic distance apparently underlie a stronger strength of syncytial isopotentiality in cortical layer I regions, this view is contradicted by the results in the following sections.
Syncytial isopotentiality is a general feature across the cortical layers
The neocortex is composed of distinct laminar layers containing different populations of neurons. Such a distinctive laminar pattern is in part due to more densely packed neurons in the lower layers compared to the sparse number of neurons in layer I (Markram et al. 2015; Schuz and Palm 1989). It is conceivable that the disparity in neuronal density and circuits may be associated with different organizational patterns of astrocytes due to spatial constraints. Thus, we next selected the visual cortex, a region involved with visual processing, to examine if the strength of syncytial isopotentiality would differ in a laminar layer-specific manner.
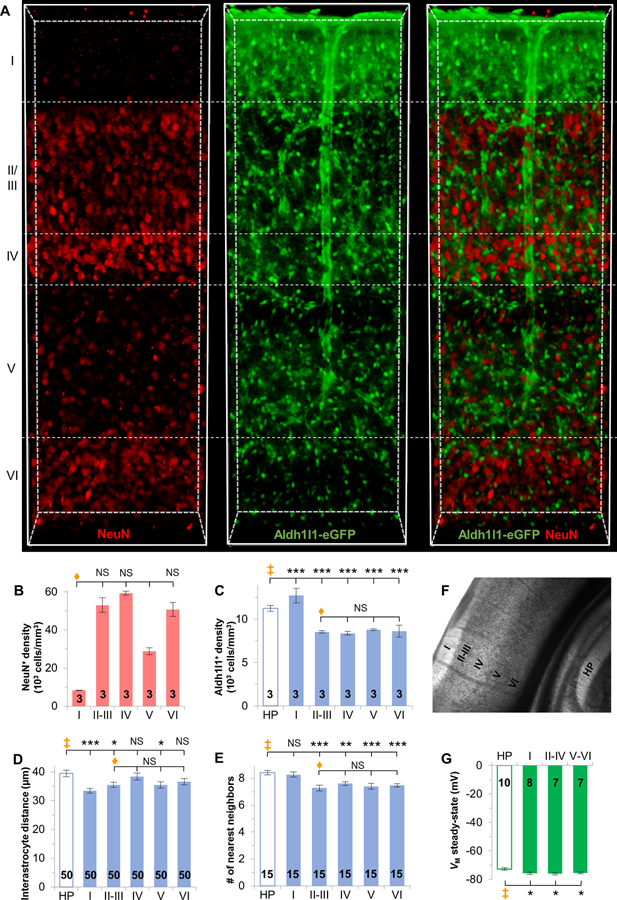
First, in visual cortex tissue that was made optically transparent, our NeuN staining results confirmed that the NeuN+ cells were indeed higher in layers II-III, IV, and VI as previously reported (Markram et al. 2015; Schuz and Palm 1989) (Figure 3B).
Figure 3. Syncytial isopotentiality is present in all cortical layers of the visual cortex.
(A) CUBIC optically-clear visual cortex with NeuN immunostaining in Aldh1l1-eGFP transgenic mice. (B) NeuN+ cell density varies across layers. (C) Aldh1l1-eGFP+ astrocytes are denser in layer I, but less dense in layers II-VI when compared to CA1 hippocampus. (D) Interastrocytic distance varies across cortical layers. (E) The number of nearest neighbors is significantly less in layers II-VI. (F) DIC imaging of the visual cortex layers as indicated. (G) VM,SS in the cortical layers was significantly more negative. B, C, D, E: one-way ANOVA, Tukey’s post hoc (♦); C, D, E, F: one-way ANOVA, Dunnett’s post hoc (‡). *: p < 0.05, **: p < 0.01, ***: p < 0.005, NS: p > 0.05.
In contrast to a higher density of astrocytes in layer I visual cortex, layer II-III, IV, V, and VI are populated with significantly fewer astrocytes compared to hippocampal CA1 syncytium (hippocampus, 11238 ± 331 cells/mm3; layer I, 12732 ± 84 cells/mm3; layer II-III, 8535 ± 146 cells/mm3; layer IV 8379 ± 205 cells/mm3; layer V, 8776 ± 106 cells/mm3; layer VI, 8629 ± 70 cells/mm3, n=3 mice, p < 0.001, Figure 3C, Supp. Info. Table 2). In terms of interastrocytic distance, layer IV (38.33 ± 0.86 μm) and VI (36.58 ± 1.11 μm) astrocytes are spaced with a distance comparable to that of the hippocampus (39.45 ± 1.14 μm), in the range of ~37–38 μm (n=50 measurements from 3 mice, p > 0.05). In contrast, layer I (33.45 ± 0.86 μm), II/III (35.5 ± 0.86 μm) and V (35.44 ± 0.86 μm) astrocytes reside more closely in the range of ~33–36 μm, and is significantly shorter than hippocampal astrocytes (n=50 measurements from 3 mice, p < 0.001 layer 1, p < 0.05 layer II/III and layer V, Figure 3D, Supp. Info. Table 3). As for the quantity of the nearest neighbors, each astrocyte in layer I visual cortex (8.27 ± 0.18 neighbors) and hippocampal CA1 stratum radiatum (8.4 ± 0.16 neighbors) is similarly adjacent to ~8 nearest neighbors (n=15 measurements from 3 mice, p > 0.05), while each astrocyte in layer II/III (7.27 ± 0.21 neighbors), IV (7.6 ± 0.13 neighbors), V (7.4 ± 0.21 neighbors) and VI (7.47 ± 0.13 neighbors) is surrounded by ~7 nearest neighbors (n=15 measurements from 3 mice, layer II/III and V: p < 0.001; layer IV: p < 0.01; layer VI; p < 0.01, Figure 3E, Supp. Info. Table 4). Overall, across the layers, each astrocyte has the requisite number of nearest neighbors, i.e. 7–9, to establish syncytial isopotentiality (Ma et al. 2016).
The anatomical differences between astrocytes in visual cortex across the layers suggest a possibility that the strength of syncytial isopotentiality may also vary. Since astrocyte density, interastrocytic distance and the number of nearest neighbors do not differ across layers II through VI (p > 0.05, Figure 3C–E), for the following functional study, we grouped layers II through IV as the upper cortical layers, and layers V through VI as the lower cortical layers. The cortical layers were visualized under DIC (Figure 3F), and astrocytes were randomly selected from these groups for electrophysiological detection of syncytial isopotentiality.
In [Na+]P recording, the VM,SS of astrocytes in layer II-IV (−76.09 ± 0.76 mV, n=7 recordings) and V-VI (−75.48 ± 0.69 mV, n=7 recordings) are comparable to layer I (−75.80 ± 0.79 mV, n=8 recordings), indicating that they have similar coupling strengths in these layer groups (p > 0.05). Similarly to layer I, the VM,SS of layers II-VI are significantly closer to the physiological VM,I than hippocampal astrocytes (−72.74 ± 0.81 mV, n=10 recordings) (p < 0.05). These VM,SS values corresponded to a 764% and 347% stronger syncytial coupling strength (S) in layer II-IV and V-VI, respectively, compared to astrocytes in CA1 stratum radiatum (Figure 3G, Supp. Info).
In summary, the strength of syncytial isopotentiality across layers II-VI appears to be comparable to layer I. Interestingly, this is achieved at a lower astrocyte density and fewer nearest neighbors than that of layer I syncytia. This suggests that there is no correlation between syncytial anatomy and the strength of syncytial isopotentiality.
Astrocyte networks in the barrel cortex communicate between compartments
Astrocytes in layer IV barrel fields of the somatosensory cortex have been reported to be organized into gap junctional-restricted compartments (Houades et al. 2008). Specifically, the Cx43 expression was found to be enriched within the barrels, but largely absent in the inter-barrel space (septa). This Cx43 expression pattern coincided with the observation of a restricted dye coupling of astrocytes occurred mainly inside the barrels, very weak in barrel septa, and totally absent between the barrels. While intercellular dye transfer signifies directional and spatial coupling of astrocytes in a syncytium, the lack of dye coupling is often associated with a concomitantly detectable electrical coupling of astrocytes in situ and cultured conditions due to a higher sensitivity of transjunctional current measurement (Ransom and Kettenmann 1990; Sontheimer et al. 1990; Xu et al. 2010).
Since Cx43 antibody immunostaining could not reliably be used to delineate the characteristic barrels in layer IV somatosensory cortex in our hands, we could not further characterize and compare the anatomy of astrocyte syncytia inside and outside of the barrels in this study.
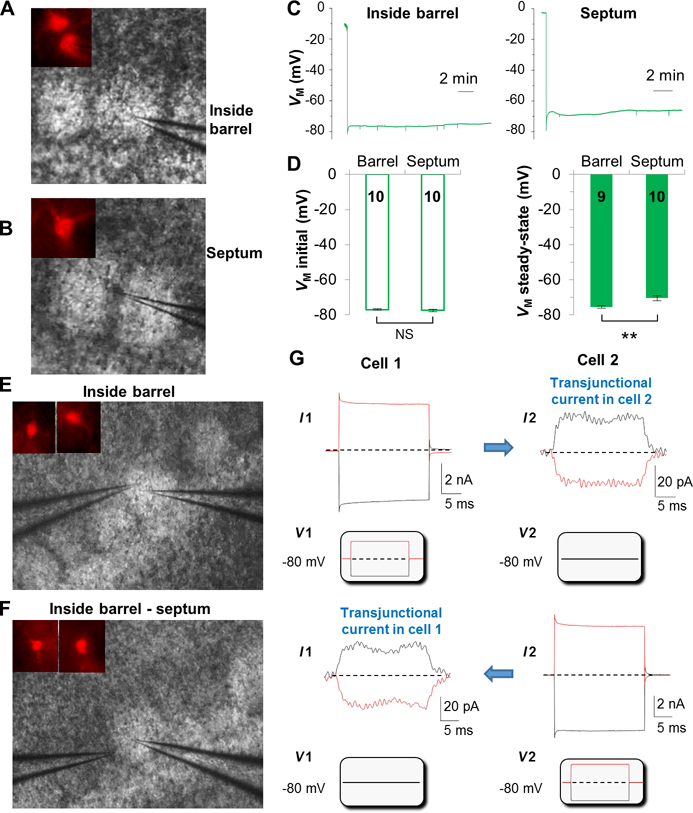
To answer whether gap junction coupling gives rise to syncytial isopotentiality inside and outside of the barrels, astrocytes in both regions were recorded with [Na+]P (Figure 4A,B). Astrocytes in both areas shared a comparable VM,I (barrel: −76.96 ± 0.35 mV, septum: −77.36 ± 0.72 mV, n=10 recordings for each group, p > 0.05), indicating a comparable resting astrocyte VM across the barrel fields (Figure 4D). Interestingly, a weaker syncytial coupling, i.e., a more depolarized VM,SS, was evident in astrocytes outside (−70.47 ± 1.39 mV, n=10 recordings) than inside the barrels (−75.79 ± 0.8 mV, n=9 recordings, p < 0.01, Figure 4C–D). Based on these VM,SS values, the strength of syncytial coupling inside and outside of the barrels is 505% stronger and 58% weaker, respectively, than that of hippocampal syncytium (Figure 1F). Despite the differences in coupling strengths, astrocytes appear to be strongly coupled throughout the barrel fields.
Figure 4. Astrocytes form an electrically uniform syncytium in somatosensory barrel cortex.
VM recordings with [Na+]P from an astrocyte inside the barrel (A), and an astrocyte in the septum (B). Astrocytes were identified by positive SR101 staining (insets in A and B). (C) [Na+]P VM recording from astrocyte inside the barrel and within the septum as indicated. (D) Astrocytes inside the barrel and in septum show comparable VM,I, but significantly different VM,SS. Dual patch recording from a pair of neighboring astrocytes inside the barrel (E), and another pair with one astrocyte inside the barrel and another in the adjacent septum (F). (G) The command voltages (Vcom), ±100 mV/50 ms, were alternately delivered to Cell1 and Cell2 as indicated below the recording traces, and the induced transjunctional current in the adjacent astrocyte is also indicated. Note that the transjunctional current is bidirectional.
The strong coupling strength of astrocytes in the space-restrained septum region opens the possibility that the observed quasi-physiological VM,SS could be in part due to sharing of syncytial coupling with barrel astrocytes. Therefore we used the more sensitive transjunctional currents measurement to examine this question (Xu et al. 2010; Zhong et al. 2016). In the first set of study, the double patch recordings were made from two neighboring astrocytes in the barrel (n=6 pairs) (Figure 4E), and the second set of recordings were made from one astrocyte inside and another in the adjacent septum (n=7 pairs) (Figure 4F). In both experimental groups, bidirectional transjunctional currents could be readily induced by command voltages that were alternately delivered to the two astrocytes in dual patch recording (Figure 4G); this was consistent with the electrical properties of gap junction channels from hippocampal astrocytes (Xu et al. 2010; Xu et al. 2014; Zhong et al. 2016). Importantly, these results unequivocally show that astrocytes inside and outside of the barrels do electrically communicate so that syncytial isopotentiality remains a shared electrical feature of astrocyte syncytium across the barrel fields. Due to a large variation of transjunctional currents from pair to pair and the low membrane resistance-caused voltage errors (Du et al. 2015; Ma et al. 2014; Xu et al. 2010), the transjunctional currents were not quantitatively comparable between the two experimental groups.
Syncytial isopotentiality exists in cerebellar Bergmann and velate glial networks
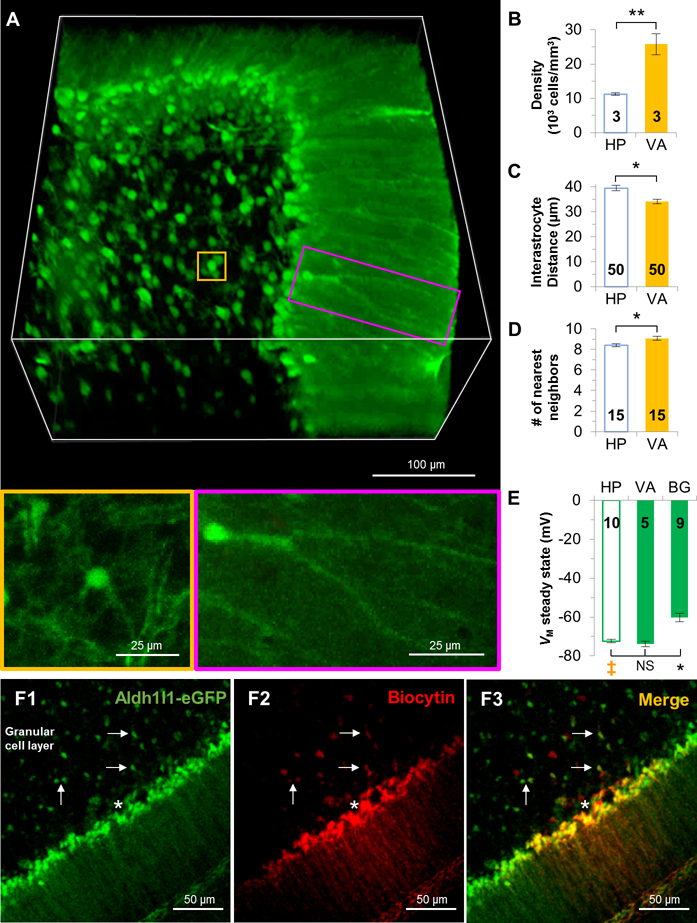
Although Bergman glia and velate astrocytes are derived from the same progenitor pool (Kita et al. 2013), they strikingly differ in their morphology. Bergmann glia are characterized by having their cell bodies arranged in rows alongside with the somata of Purkinje neurons, and extending of their processes along the Purkinje cell layer towards the pia in the cerebellum. Additionally, gap junction couplings are mainly formed at their distal processes (Muller et al. 1996) (Figure 5A). Velate astrocytes are cerebellar protoplasmic astrocytes that exhibit characteristic velate sheath processes and are more dispersed in arrangement (Chan-Palay and Palay 1972) (Figure 5A).
Figure 5. Syncytia established by Bergmann glia and velate astrocytes vary in coupling strength.
(A) CUBIC optically-clear cerebellum reveals the morphological and spatial organizational pattern heterogeneity of velate astrocytes (gold) and Bergmann glia (magenta). Velate astrocytes are higher in density (B), shorter in the interastrocytic distance (C), and adjacent with one more nearest neighbors (D) than hippocampal CA1 astrocytes. (E) The quasi-physiological VM,SS in Bergmann glia was significantly more depolarized than velate and hippocampal astrocytes. (F) Diffusion of pipette-loaded biocytin among Bergmann glia and to adjacent velate astrocytes. Recorded cell is marked by white asterisk. Arrows point to biocytin-positive velate astrocytes in the granular cell layer. B, C, D: unpaired t-test; E: one-way ANOVA, Dunnett’s post hoc (‡). *: p < 0.05, **: p < 0.01, NS: p > 0.05.
Bergmann glia was not included in this anatomical analysis due to the difficulty in discriminating individual somas. The syncytial arrangement of velate astrocytes was compared to CA1 hippocampal astrocytes. Interestingly, the density of velate astrocytes in granule cell layer is 2.5-folds higher than those in the hippocampus (hippocampus, 11238 ± 331 cells/mm3; velate astrocytes, 25811 ± 331 cells/mm3, n=3 mice, p < 0.01, Figure 5B, Supp. Info. Table 2). Consistent with a higher cell density, the interastrocytic distance of velate astrocytes is ~5 μm shorter than hippocampal astrocytes (hippocampus, 39.45 ± 1.14 μm; velate astrocytes, 34.07 ± 0.93 μm, n=50 measurements from 3 mice, p < 0.05, Figure 5C, Supp. Info. Table 3), and each velate astrocyte is accompanied by 9 nearest neighbors, which is one more than the 8 nearest neighbors in hippocampal syncytium (hippocampus, 8.4 ± 0.16; velate astrocytes, 9.07 ± 0.18 μm, n=15 measurements from 3 mice, p < 0.05, Figure 5D, Supp. Info. Table 4).
Next, [Na+]P recording was performed to answer whether the striking difference in cell morphology and syncytial arrangement correspond to different coupling strengths in velate astrocytes and Bergmann glia. The VM,I of Bergmann glia (−76.17 ± 0.36 mV, n=17 recordings) and velate astrocytes (−76.74 ± 0.77 mV, n=11 recordings) were comparable with each other and also with hippocampal astrocytes (−77.17 ± 0.89 mV, n=10 recordings) (p > 0.05), indicating that, despite diversity in morphology, they share a similar resting VM. The VM,SS of velate astrocytes (−73.82 ± 1.49 mV, n=5 recordings) was comparable with hippocampal astrocytes (−72.74±0.81 mV, n=10 recordings, p > 0.05, Figure 5E). However, the VM,SS appeared to be more depolarized in Bergmann glia (−60.16 ± 2.19 mV, n=9 recordings) compared with hippocampal astrocytes (p < 0.005, Figure 5E), which corresponds to a 91% weaker coupling strength than that of the hippocampal syncytium.
Another outstanding question to be determined is whether Bergmann glia and velate astrocytes form gap junction coupling, therefore, establishing a Bergmann glia-velate astrocyte hybrid syncytium. To answer this question, biocytin (0.1%) was included in the pipette solution. Similar to a previous report, biocytin diffused laterally into neighboring Bergmann glia (Muller et al. 1996). Additionally, biocytin also diffused into the adjacent velate astrocytes in the granular cell layer (Figure 5F). Therefore, Bergmann glia and velate astrocytes do couple into a shared syncytium. Since the coupling can be detected by the less-sensitive dye coupling, we did not proceed further to examine the electrical coupling between these two glial subtypes.
In summary, syncytial isopotentiality exists in the syncytial networks established by both Bergmann glia and velate astrocytes, and these two morphologically distinct astrocyte subtypes are coupled into a shared syncytium. However, a weak coupling strength was indicated in Bergmann glia soma recording with [Na+]P. Despite the anatomical heterogeneity in the cerebellum, there appears to be no correlation between syncytial anatomy and the strength of syncytial isopotentiality.
Fibrous astrocytes share syncytial isopotentiality in white matter
Fibrous astrocytes are a distinct class of astrocytes that extend long processes to make gap junction-coupling with neighboring astrocytes (Yamamoto et al. 1990a; Yamamoto et al. 1990b). Using biocytin as an intercellular tracer, a weaker coupling among fibrous astrocytes has been reported (Haas et al. 2006). This observation suggests that gap junction coupling in white matter may differ from protoplasmic astrocytes in grey matter.
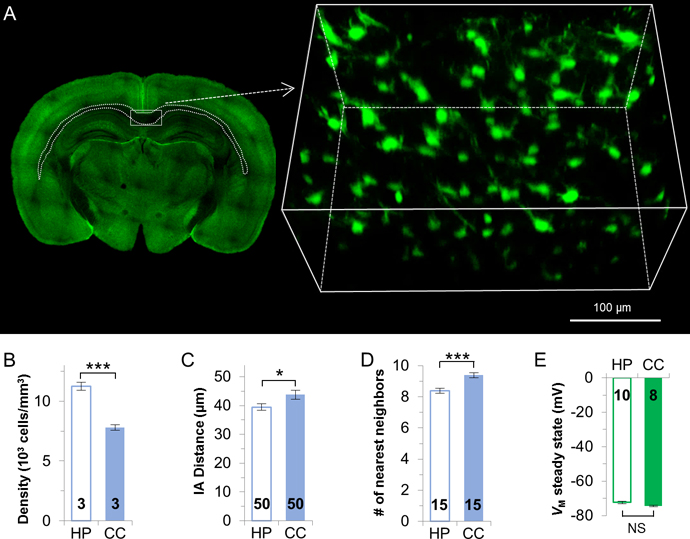
To examine how the syncytial anatomy in white matter differs from the grey matter, CUBIC tissue clearing was used to visualize fibrous astrocyte syncytium in the corpus callosum (Figure 6A). The density of fibrous astrocytes is ~31% less than protoplasmic astrocytes in the hippocampus (protoplasmic, 11238 ± 331 cells/mm3; fibrous, 7800±226 cells/mm3; n=3 mice, p < 0.005, Figure 6B, Supp. Info. Table 2). The interastrocytic distance of corpus callosum astrocytes is significantly longer than hippocampal astrocytes (protoplasmic, 39.45 ± 1.14 μm; fibrous, 43.76 ± 1.58 μm; n=50 measurements from 3 mice, p < 0.05, Figure 6C, Supp. Info. Table 3). Also, each fibrous astrocyte is surrounded by 9 nearest neighbors, which is significantly more than hippocampal astrocytes (protoplasmic, 8.4 ± 0.16 neighbors; fibrous, 9.4 ± 0.16 neighbors; n=15 measurements from 3 mice, p < 0.005, Figure 6D, Supp. Info. Table 4).
Figure 6. Syncytial isopotentiality appears in syncytium established by fibrous astrocytes in the corpus callosum.
(A) Representative image of the select region of the CUBIC optically-cleared corpus callosum. Fibrous astrocytes show a significantly lower density (B), a longer interastrocytic distance (C), and one more nearest neighbors (D) than hippocampal astrocytes. (E) Fibrous and hippocampal protoplasmic astrocytes share comparable VM,SS. B, C, D, E: unpaired t-test. *: p < 0.05, ***: p < 0.005, NS: p > 0.05.
A constrained dye coupling has been previously reported in corpus callosum fibrous astrocytes (Haas et al. 2006). However, in [Na+]P recording, the VM,I of fibrous astrocytes was comparable to protoplasmic astrocytes (fibrous, −75.39 ± 0.43 mV, n=8 recordings; protoplasmic, −77.17 ± 0.89 mV, n=10 recordings, p > 0.05), and the VM,SS in fibrous astrocytes (−74.51 ± 0.4 mV, n=8) was also comparable with hippocampal astrocytes (fibrous, −74.51 ± 0.4 mV, n=8 recordings; protoplasmic, −72.74 ± 0.81 mV, n=10 recordings p > 0.05, Figure 6E, Supp. Info. Table 5).
Together, the lack of correlation between the syncytial anatomy and the strength of syncytial isopotentiality was also observed in white and grey matters. These data indicate that syncytial isopotentiality is also a shared feature of fibrous astrocytes in the corpus callosum.
Discussion
While syncytial isopotentiality has been identified as a physiological mechanism in hippocampal astrocytes, to what extent does syncytial isopotentiality exist in astrocyte networks remains unknown. By examining morphologically heterogeneous populations of astrocytes in various brain regions, our results indicate that syncytial isopotentiality most likely operates as a system-wide physiological mechanism in the brain.
Anatomical heterogeneity of astrocytes and the astrocyte syncytium
Our understanding of astrocyte heterogeneity expands continuously from cytoarchitecture to embryonic origins, gene/protein expression, and physiological functions (Bayraktar et al. 2014; Ben Haim and Rowitch 2017). This calls into question of the extent to which syncytial isopotentiality exists in other parts of astrocyte networks. Additionally, while a strong electrical coupling is a prerequisite to achieving syncytial isopotentiality (Ma et al. 2016), Cx43 and 30 appear to be differentially expressed across the brain (Nadarajah et al. 1996; Nagy et al. 1999; Yamamoto et al. 1990b), and this is echoed by heterogeneity in astrocyte dye coupling in both grey and white matters (Claus et al. 2018; Haas et al. 2006; Houades et al. 2006). Thus the architecture and connectivity of astrocyte networks indeed differ across and within brain regions. Consequently, increasingly study now focus on the molecular programs dictating the wiring of neuron-astrocyte networks in developing and adult brain (Farmer et al. 2016; Martin et al. 2015; Stogsdill et al. 2017; Stork et al. 2014). However, little is known about how such a multifaceted heterogeneity is associated with the function of astrocytes at syncytial system levels and syncytial isopotentiality in particular.
Using CUBIC tissue clearing method to examine the morphology and spatial organization of astrocytes in Aldh1l1-eGFP transgenic mice with high resolution, our results show that the syncytial organization, in terms of density, interastrocytic distance, and nearest neighbors, vary across brain regions, and within a local region as described in the visual cortex (Figure 3).
In freshly dissociated hippocampal tissue preparation, a “miniature syncytium” with a minimum of 7–9 gap junction-coupled astrocytes is required to achieve syncytial isopotentiality (Ma et al. 2016). Here, our morphometrical analysis shows syncytial anatomy appears to have no correlation with the variable strength of syncytial isopotentiality between and within the same brain regions. These observations suggest that the “fine-tuning” of the syncytial coupling strength may depend on the number of process-process contacts and gap junction channels between neighboring astrocytes. In addition, the syncytial coupling strength may be dynamically regulated by local neural circuitry through mechanisms yet to be determined. Thus, this issue needs to be resolved at the ultrastructural and functional levels in future studies.
Syncytial isopotentiality - a universal electrical feature of astrocytic networks
Our results favor the notion that syncytial isopotentiality is a general feature of astrocyte networks. First, syncytial isopotentiality appears in protoplasmic astrocytes in different cortical regions, across cortical layers of the visual cortex, in the barrel fields of layer IV somatosensory cortex, and velate astrocytes in the cerebellum. Second, syncytial isopotentiality also exists in astrocyte networks established by morphologically distinct Bergmann glia. Third, syncytial networks established by fibrous astrocytes are strikingly different from those of protoplasmic astrocytes, but they share comparable strengths of syncytial isopotentiality.
An interesting observation from astrocytes in the layer IV barrel field of the somatosensory cortex suggested that astrocytes are organized as compartmentalized networks (Houades et al. 2008). In this study, the dye coupling analysis showed a strong gap junctional coupling of astrocytes within a barrel, but absent of coupling between barrels. This would create a preferential segregation of astrocyte communication directed within the barrel and not across barrels. In our transjunctional current measurement, septum astrocytes remain strongly electrically coupled to each other and also to astrocytes inside the barrels. From an electrical standpoint, astrocytes across the barrel fields still behave as a single cytoplasmic network, or syncytium. Given the different sensitivity between dye coupling and transjunctional current measurements (Ransom and Kettenmann 1990; Xu et al. 2010), these observations together imply that astrocyte syncytium can be organized in a way that is metabolically compartmentalized to segregate cell signaling and metabolites between barrels, but remain electrically coupled as an isopotential network. Different astrocyte subtypes establish a shared syncytium is also evident in the cerebellum, where Bergmann glia and velate astrocytes are coupled into a continuum syncytium.
Another unique compartmentalized domain in the thalamus, termed barreloids, have been described by Claus and colleagues (Claus et al. 2018). In a similar manner to the cortical barrel fields, dye coupling is confined within these thalamic barreloids, as the barreloid boundaries are formed by weakly coupled oligodendrocytes. This will be another interesting area to use our established methodology to examine if syncytial isopotentiality exists in this region and the strength of its electrical coupling. In addition, it remains unknown if astrocytes in the thalamic barreloids can electrically communicate across compartments despite the restricted dye coupling, as we’ve demonstrated in the cortical barrel fields (Figure 4G). The answer to these questions can provide insight into the open question of whether astrocytes form multiple syncytia, or a single syncytium throughout the brain.
Nevertheless, while our findings favor the view that syncytial isopotentiality is system-wide, exceptions may exist in unexplored brain regions. Thus an extensive examination of this issue to other brain regions is needed in the future.
Syncytial isopotentiality vary in strength from brain region to region
In the present study, we have also observed a heterogeneity in the strength of syncytial isopotentiality across the brain. The strength of syncytial isopotentiality is significantly stronger in all examined cortical regions compared with hippocampal CA1 syncytium. This immediately brings into the question of the purpose to have a more strongly coupled astrocyte syncytium in these particular brain regions. It can be postulated that a higher density of synapses would be better matched with a more strongly coupled syncytium so that the extracellular K+ and neurotransmitter concentrations can be maintained at the same physiological levels as other parts of the brain. Interestingly, a trend towards lower synaptic density in deeper mouse cortical layers has been observed (Schuz and Palm 1989), and this is consistent with an ordered decrease in the strength of syncytial isopotentiality from layer II-IV (764%) to layer V-VI (347%) compared to hippocampal CA1 syncytium. Nevertheless, synapse density should not be the only regulatory factor for the strength of network coupling. Evidently, networks established by fibrous astrocytes in the corpus callosum are in an environment composed of myelinated axons and devoid of synapses, but exhibit a coupling strength comparable to protoplasmic astrocytes.
In contrast, the strength of syncytial isopotentiality is weaker in Bergmann glia compared with hippocampal as well as velate astrocytes. Bergmann glia are distinct cells with gap junctions located within the unipolar processes. Although these cells are packed densely along the Purkinje cell layer, Bergmann glia coupling strength could not completely counteract the depolarization induced by Na+-loading in the recorded Bergmann glia. We have previously demonstrated that the number of direct neighbors is positively correlated with the coupling strength (Ma et al. 2016). Thus an insufficient amount of direct neighbors may underlie a weaker coupling strength measured from the soma, but the actual strength of syncytial isopotentiality in Bergmann glial network could be strong.
The strength of coupling of septum astrocytes in the layer IV somatosensory cortex barrel fields also appeared to be weaker than astrocytes inside the barrels. This is consistent with the reported weak Cx43 immunostaining and weak dye-coupling in the septum (Houades et al. 2008).
What is the relative contribution of different connexin subunits to syncytial isopotentiality emerges to be an interesting question from our study. The fibrous astrocytes in the corpus callosum are weak or absent in Cx30 expression (Nagy et al. 1999), but exhibit a similarly strong syncytial isopotentiality (Figure 6E). Inferring from this observation, Cx43 alone is sufficient to establish a network isopotential. Interestingly, the Cx30 expression appears to be dominant in the thalamus (Griemsmann et al. 2015), whether Cx30 expression is also sufficient to establish syncytial isopotentiality requires future study.
Is there an “astrocyte connectome” underpinning syncytial isopotentiality?
The term “connectome” refers to the structural and functional mapping of neural connectivity in the brain, ranging from large-scale brain structures to the nanoscale details of synapses. Although astrocytes are wired together into a syncytium, the current effort has been mainly focused on creating wiring diagrams of a variety of neurons in different brain areas.
In this study, we demonstrated that syncytial isopotentiality is likely a general feature of astrocytes in the brain. The astrocyte syncytium is able to accomplish this through low resistance pathways between cells that is estimated at 4.3 MΩ between neighboring hippocampal astrocytes (Ma et al. 2016). Interestingly, protoplasmic astrocytes interdigitate with fine processes with minimal overlap at the domain edges (Bushong et al. 2004; Bushong et al. 2002; Xu et al. 2014), whereas fibrous astrocytes, on the contrary, do not occupy individual domains with their processes overlapping extensively (Sun and Jakobs 2012). In either case, the fine processes should conceivably be high resistance structures. How astrocyte structural connectivity achieves low resistance pathways is unknown. Furthermore, it also isn’t known if there is a general connectivity pattern in establishing a syncytium, or if connectivity is heterogeneous in different astrocyte subtypes and in different brain regions.
The low resistance pathways between astrocytes is a question that has remained unanswered for over 50 years (Kuffler et al. 1966). The role of these pathways, connectivity, and the syncytium in brain function continues to be a mystery. Astrocyte connectomes at an ultrastructural resolution may yield insight into this fundamentally important neurobiological question.
Pathological implications affecting astrocyte syncytial isopotentiality
Astrocytes respond to neurological injuries and diseases by becoming “reactive”, exhibiting changes in cell morphology, genetic and molecular expression (Liddelow and Barres 2017; Sofroniew 2014). Our conclusion that syncytial isopotentiality is most likely system-wide immediately brings into question if this feature is impaired under pathological conditions.
As to gap junction coupling, Cx43 expression and dye coupling are reported to be affected in various disease models (Giaume et al. 2010). For instance, in a mouse model of tuberous sclerosis complex, the Cx43 expression is downregulated and astrocyte dye-coupling is substantially reduced (Xu et al. 2009). Conversely, in Alzheimer’s disease human samples and mouse models, the Cx43 expression and dye-coupling all have shown to increase (Nagy et al. 1996; Peters et al. 2009). These studies suggest that either a loss or gain of syncytial network connectivity may both occur in a context-dependent manner.
In addition, the syncytial architecture as a whole can be altered. Evidently, reactive astrocytes lose domain organization in epileptic conditions (Oberheim et al. 2008), but continue to maintain nearly exclusive domains under lesion-induced axonal degeneration (Wilhelmsson et al. 2006). Altogether, these observations suggest that pathological conditions may alter the spatial organization of astrocyte syncytium that in turn may disrupt the physiological syncytial isopotentiality. Nevertheless, no study has yet been carried out to determine a causal relationship between syncytial isopotentiality and the pathogenesis of various kinds of neurological disorders.
Supplementary Material
Main Points.
Syncytial isopotentiality is a universal feature of astrocytes in the brain.
Syncytial Isopotentiality varies in strength in different brain regions.
Acknowledgments
The authors thank Dr. Andrea Tedeschi for advice on CUBIC tissue clearing and confocal image techniques. This work is sponsored by grants from the National Institute of Neurological Disorders and Stroke RO1NS062784, R56NS097972 (MZ), P30NS104177 (Dr. Askwith), and National Science Foundation DMS1410935 (DT). SZ was a recipient of scholarships from Chinese Scholarship Council (CSC) (21406260143), WW was sponsored by a CSC fellowship (201606165056) and a grant from Natural Science Foundation of China (81400973). The authors declare no competing financial interest.
References
- Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. 2014. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 7:a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Connors BW, Paradiso MA. 2016. Neuroscience : exploring the brain.
- Ben Haim L, Rowitch DH. 2017. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18:31–41. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. 2004. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci 22:73–86. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW. 1998. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol 390:297–310. [PubMed] [Google Scholar]
- Chan-Palay V, Palay SL. 1972. The form of velate astrocytes in the cerebellar cortex of monkey and rat: high voltage electron microscopy of rapid Golgi preparations. Z Anat Entwicklungsgesch 138:1–19. [DOI] [PubMed] [Google Scholar]
- Claus L, Philippot C, Griemsmann S, Timmermann A, Jabs R, Henneberger C, Kettenmann H, Steinhauser C. 2018. Barreloid Borders and Neuronal Activity Shape Panglial Gap Junction-Coupled Networks in the Mouse Thalamus. Cereb Cortex 28:213–222. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM 2nd, Janigro D. 1998. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci 18:4425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma B, Kiyoshi CM, Alford CC, Wang W, Zhou M. 2015. Freshly dissociated mature hippocampal astrocytes exhibit passive membrane conductance and low membrane resistance similarly to syncytial coupled astrocytes. J Neurophysiol 113:3744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Wang W, Lutton AD, Kiyoshi CM, Ma B, Taylor AT, Olesik JW, McTigue DM, Askwith CC, Zhou M. 2018. Dissipation of transmembrane potassium gradient is the main cause of cerebral ischemia-induced depolarization in astrocytes and neurons. Exp Neurol 303:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Theroux JF, Peng J and others. 2016. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351:849–54. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. 2010. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11:87–99. [DOI] [PubMed] [Google Scholar]
- Griemsmann S, Hoft SP, Bedner P, Zhang J, von Staden E, Beinhauer A, Degen J, Dublin P, Cope DW, Richter N and others. 2015. Characterization of Panglial Gap Junction Networks in the Thalamus, Neocortex, and Hippocampus Reveals a Unique Population of Glial Cells. Cereb Cortex 25:3420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H. 2006. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex 16:237–46. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Armstrong WE. 1996. Morphology and physiology of cortical neurons in layer I. J Neurosci 16:5290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. 2008. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci 28:5207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houades V, Rouach N, Ezan P, Kirchhoff F, Koulakoff A, Giaume C. 2006. Shapes of astrocyte networks in the juvenile brain. Neuron Glia Biol 2:3–14. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. 2010. Functions of mature mammalian astrocytes: a current view. Neuroscientist 16:79–106. [DOI] [PubMed] [Google Scholar]
- Kita Y, Kawakami K, Takahashi Y, Murakami F. 2013. Development of cerebellar neurons and glias revealed by in utero electroporation: Golgi-like labeling of cerebellar neurons and glias. PLoS One 8:e70091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. 1966. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol 29:768–87. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA. 2017. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46:957–967. [DOI] [PubMed] [Google Scholar]
- Ma B, Buckalew R, Du Y, Kiyoshi CM, Alford CC, Wang W, McTigue DM, Enyeart JJ, Terman D, Zhou M. 2016. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 64:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Xu G, Wang W, Enyeart JJ, Zhou M. 2014. Dual patch voltage clamp study of low membrane resistance astrocytes in situ. Mol Brain 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S and others. 2015. Reconstruction and Simulation of Neocortical Microcircuitry. Cell 163:456–92. [DOI] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, Araque A. 2015. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:730–4. [DOI] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, Yang Y. 2017. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci 37:8706–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Moller T, Neuhaus J, Kettenmann H. 1996. Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia 17:274–84. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Thomaidou D, Evans WH, Parnavelas JG. 1996. Gap junctions in the adult cerebral cortex: regional differences in their distribution and cellular expression of connexins. J Comp Neurol 376:326–42. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Li W, Hertzberg EL, Marotta CA. 1996. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res 717:173–8. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. 1999. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88:447–68. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Bergles DE. 2015. Large-scale recording of astrocyte activity. Curr Opin Neurobiol 32:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. 2008. Loss of astrocytic domain organization in the epileptic brain. J Neurosci 28:3264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L, Murayama M, Larkum M. 2012. Inhibitory Regulation of Dendritic Activity in vivo. Front Neural Circuits 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters O, Schipke CG, Philipps A, Haas B, Pannasch U, Wang LP, Benedetti B, Kingston AE, Kettenmann H. 2009. Astrocyte function is modified by Alzheimer’s disease-like pathology in aged mice. J Alzheimers Dis 18:177–89. [DOI] [PubMed] [Google Scholar]
- Petersen CC. 2007. The functional organization of the barrel cortex. Neuron 56:339–55. [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Peterson BA, Winer JA. 1994. Morphology and spatial distribution of GABAergic neurons in cat primary auditory cortex (AI). J Comp Neurol 344:349–82. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Goldring S. 1973. Ionic determinants of membrane potential of cells presumed to be glia in cerebral cortex of cat. J Neurophysiol 36:855–68. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Kettenmann H. 1990. Electrical coupling, without dye coupling, between mammalian astrocytes and oligodendrocytes in cell culture. Glia 3:258–66. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. 2008. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322:1551–5. [DOI] [PubMed] [Google Scholar]
- Roux L, Benchenane K, Rothstein JD, Bonvento G, Giaume C. 2011. Plasticity of astroglial networks in olfactory glomeruli. Proc Natl Acad Sci U S A 108:18442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuz A, Palm G. 1989. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol 286:442–55. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. 2014. Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Minturn JE, Black JA, Waxman SG, Ransom BR. 1990. Specificity of cell-cell coupling in rat optic nerve astrocytes in vitro. Proc Natl Acad Sci U S A 87:9833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C. 2017. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. 2014. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron 83:388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Jakobs TC. 2012. Structural remodeling of astrocytes in the injured CNS. Neuroscientist 18:567–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S and others. 2014. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157:726–39. [DOI] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR. 2015. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc 10:1709–27. [DOI] [PubMed] [Google Scholar]
- Tainaka K, Kubota SI, Suyama TQ, Susaki EA, Perrin D, Ukai-Tadenuma M, Ukai H, Ueda HR. 2014. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159:911–24. [DOI] [PubMed] [Google Scholar]
- Takata N, Hirase H. 2008. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One 3:e2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M. 2018. Physiology of Astroglia. Physiol Rev 98:239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Liaudet N, Savtchouk I. 2014. Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nat Rev Neurosci 15:327–35. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. 2006. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26:5438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Putra A, Schools GP, Ma B, Chen H, Kaczmarek LK, Barhanin J, Lesage F, Zhou M. 2013. The contribution of TWIK-1 channels to astrocyte K(+) current is limited by retention in intracellular compartments. Front Cell Neurosci 7:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. 2006. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A 103:17513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Wang W, Kimelberg HK, Zhou M. 2010. Electrical coupling of astrocytes in rat hippocampal slices under physiological and simulated ischemic conditions. Glia 58:481–93. [DOI] [PubMed] [Google Scholar]
- Xu G, Wang W, Zhou M. 2014. Spatial organization of NG2 glial cells and astrocytes in rat hippocampal CA1 region. Hippocampus 24:383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zeng LH, Wong M. 2009. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol Dis 34:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. 1990a. LM and EM immunolocalization of the gap junctional protein connexin 43 in rat brain. Brain Res 508:313–9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. 1990b. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J Comp Neurol 302:853–83. [DOI] [PubMed] [Google Scholar]
- Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD. 2011. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 59:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. 2010. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20:588–94. [DOI] [PubMed] [Google Scholar]
- Zhong S, Du Y, Kiyoshi CM, Ma B, Alford CC, Wang Q, Yang Y, Liu X, Zhou M. 2016. Electrophysiological behavior of neonatal astrocytes in hippocampal stratum radiatum. Mol Brain 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.