Abstract
Background:
Our objective was to determine the association between racialized economic segregation and the hazard of breast cancer (BC) mortality in Maryland.
Methods:
Among 35,066 women (24,540 White; 10,526 Black) diagnosed with incident invasive BC in Maryland during 2007–2017, exposure to racialized economic segregation was measured at the census tract level using Index of Concentration at the Extremes metrics. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox proportional hazards regression for the association between racialized economic segregation and the hazard of BC mortality, accounting for clustering at the census tract level. Models were adjusted for age and stratified by race, median age (<60 years, ≥60 years), and clinical characteristics.
Results:
Overall, the hazard of BC mortality was 1.84 times as high (95% CI: 1.64, 2.06) for the least privileged quintile of racialized economic segregation compared to the most privileged quintile. This association differed significantly (p-interaction< 0.05) by race and age, with 1.20 (95% CI: 0.90, 1.60) times the hazard of BC mortality for Black women versus 1.66 (95% CI: 1.41, 1.95) times the hazard for White women, and with greater hazards for younger women (HR: 2.17, 95% CI: 1.83, 2.57) than older women (HR: 1.62, 95% CI: 1.40, 1.88).
Conclusions:
Our results suggest that BC survival disparities exist in Maryland among women residing in the least privileged census tracts with lower income households and higher proportions of Black residents.
Impact:
Our findings provide new insights into the BC mortality disparities observed among women in Maryland.
Keywords: Breast cancer, Mortality, Index of Concentration at the Extremes (ICE), Racial disparities, Residential segregation, Socioeconomic inequality
Introduction
Breast cancer is the second leading cause of cancer deaths among Black/African American (AA) women (1). While breast cancer mortality rates are decreasing, Black-White racial disparities in breast cancer mortality rates are increasing (1). Data suggest that racial mortality disparities are due to the higher prevalence of aggressive breast cancer subtypes, late stage of presentation (2, 3), and the high prevalence of comorbidities among Black/AA women (4–6). These mortality disparities are also due to racially patterned differences in access to resources in part due to race-based segregation (7–11), which is compounded by persistent socioeconomic inequalities by race in the United States.
Residential racial and economic segregation remain upstream factors that influence access to equitable healthcare, independent of individual-level risk factors (12), including for breast cancer care (13, 14). For example, unequal access to opportunities and resources such as employment, wealth, income, housing, and education can significantly influence access to high-quality healthcare services, such as cancer prevention/screening, early detection, and treatment services (1, 15). Additionally, access to goods and services and limitations of the built environment (e.g., green spaces, inaccessible or nonexistent sidewalks) can directly affect cancer health outcomes and exposure to cancer risk factors across the cancer continuum (16). While several studies have examined the contribution of residential racial segregation to the observed disparities in breast cancer mortality (7–11, 17), these studies did not explicitly consider how racial composition intersected with the economic conditions of these communities.
The Index of Concentration at the Extremes (ICE) is a segregation metric that reflects the extent to which an area’s population is concentrated into extremes of deprivation and privilege. The ICE was initially introduced in the field of social sciences as a measure of economic residential segregation and was expanded to racial and racialized economic segregation, to capture the extremes of high-income White communities and low-income Black communities (18–20). Therefore, ICE captures both racial and economic elements of residential segregation at the same time.
Utilizing data sources from the Maryland Cancer Registry (MCR) and statewide census-level data, we examined the relationships between residential segregation (racial and economic), Black/White race, prognostic/initial treatment factors, and risk of breast cancer mortality among breast cancer patients. Maryland is an ideal setting to explore these relationships, given its large Black/AA population (30% of residents) that is nearly double the national average (14%) (21). Of the ~4,500 women each year that are diagnosed with invasive breast cancer in the state of Maryland, one-third identify as Black/AA (22). The population in Maryland also has varying levels of economic status, as Prince George’s County, Maryland has one of the highest median household incomes in the US according to 2019 data ($84,920 compared to Baltimore City’s $50,379) (23). We hypothesized that breast cancer patients diagnosed in Maryland living in less vs. more privileged census tracts across social dimensions of race and income would have higher risk of breast cancer mortality.
Materials and Methods
Study population
The MCR collects data for all cancer diagnoses among residents of the state, including patient demographics, clinical, and diagnostic information, and treatment. The MCR routinely links to additional data sources to capture missed cancers and obtain additional information on previously reported cases. Each year the MCR identifies potentially unreported cancer cases by matching the MCR master database with Maryland death certificates that indicate that a patient had cancer. Facilities and physicians are then followed up for information. The registry also links to the Maryland Vital Statistics, Social Security Death Index (SSDI), and the National Death Index (NDI) for information on vital statistics including cause of death. For the present study, a total of 59,230 women diagnosed with breast cancer in Maryland during 2007–2017 were identified from the MCR. The study was approved by the Institutional Review Board (IRB) at the Maryland Department of Health and was deemed exempt by the IRB at the Johns Hopkins Bloomberg School of Public Health.
Analytic Population
To be included in the study analysis women age ≥18 years had to be diagnosed with primary breast cancer as their first cancer between 2007–2017 (excluded n=10,525). The analysis was then restricted to non-Hispanic Black (NHB) and non-Hispanic White (NHW) women for the study’s research question (excluded n=3,788). Women diagnosed with in situ breast cancer (n=7,532) or missing age at diagnosis (n=2) or follow-up time (n=1,295) were excluded. Additionally,1,022 women were excluded due to missing data associated with variables needed to create the socioeconomic index (i.e., zip code, census tract). The final analytical population included 35,066 women (24,540 NHW; 10,526 NHB).
Exposures
The primary exposures were the census tract level ICE measure which was estimated using equations from Scally and colleagues (18–20, 24) and data from the US Census American Community Survey 2011–2015, which had the greatest overlap with the year of diagnosis among this study population. The description of these equations for the ICE measures are found in Supplemental Table 1. Economic segregation was defined as the difference in the number of persons living in households earning ≤ $25,000 compared to the number of persons living in households earning ≥ $100,000 as a proportion of the total population with household income information. Racial segregation was defined as the difference in the number of Black persons compared to the number of White persons as a proportion of the total persons with race/ethnicity information. Racialized economic segregation was defined as difference in the number of Black persons in households earning ≤ $25,000 compared to White persons in households earning ≥ $100,000 as a proportion of the total persons with income and race/ethnicity information. The segregation indices were calculated in reverse of previous use and range from −1 to 1, such that a value of −1 indicates concentrated privilege (i.e., advantage) and a value of 1 indicates concentrated deprivation (i.e., disadvantage). Quintiles for segregation were constructed based on total number of cases to create equally sized groups and the quintile cut-offs for the segregation indices are presented in Supplemental Table 2. For consistently, Quintile 5 is interpreted as the least privileged whereas Quintile 1 is interpreted as the most privileged group (reference).
The MCR collects self-reported race from the demographics or diagnostic parts of a patient’s record. Race is entered by the facilities at time of diagnosis, and the MCR uses motor vehicle records to enter race when this data is missing. Race was categorized as NHB/AA and NHW (referent), hereto referred to as Black and White, as a representation of differing sociopolitical experiences. The following variables were also collected from the demographic and diagnostic parts of the patients’ records and were evaluated for this current analysis: age at diagnosis, type of initial cancer treatment (surgery only; surgery + radiation; surgery + chemotherapy; surgery + chemotherapy and radiation; no surgery; and treatment unknown), and tumor characteristics including estrogen receptor (ER)/progesterone receptor (PR) status, HER2 status, breast cancer SEER Summary stage (localized, regional, distant, unknown), and tumor grade (I, II, III-IV, unknown). HER2 receptor status was also available, although only for 71% of cases, so triple negative breast cancer was also evaluated (ER negative (ER−), PR negative (PR−), and HER2 negative (HER2−)).
Ascertainment of outcome: breast cancer-specific mortality
Vital statistics information was ascertained by the MCR through linkage to the Maryland Vital Statistics, SSDI and NDI. Data for date and cause of death were most complete through November 5, 2019 from Vital Statistics. The primary cause of death was coded using the ICD-10 codes for breast cancer deaths (C50).
Statistical Analysis
Demographic, clinical and tumor characteristics of the study population were compared by race as counts and proportions. The census tract level segregation indices were summarized by county (Supplemental Table 3) and mapped by census tract using percentile ranking in Maryland. Percentile ranking maps the segregation indices by their ranking in comparison to other census tracts as opposed to the absolute value of the segregation index. Survival time was calculated as time since diagnosis to mortality or date of last linkage to the death registries. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox proportional hazards regression, accounting for clustering at the census tract level with robust standard error specification. We evaluated the proportional hazards assumption by visualizing the cumulative hazards over time and testing an interaction with time in the Cox model. While the cumulative hazards generally appeared proportional over time, there was evidence of non-proportional hazards using an interaction with time. Thus, the HRs are interpreted as average effect over time. As a sensitivity analysis, we also reported the overall HRs by ≤ 5 years and > 5 years.
Age was considered a potential confounder and was adjusted for using natural cubic splines with knots at the 5th, 35th, 65th and 95th percentiles. Subgroups were also evaluated by overall median age at diagnosis (<60 years, ≥60 years), adjusting for age continuously within each analysis. Tumor stage, hormone receptor status, tumor grade, and treatment were considered potential mediators and not confounders and were therefore not adjusted for in models; however, these variables are also prognostic subgroups of interest, so supplemental analyses were additionally reported stratified by these variables. To evaluate the effects of hormone/endocrine therapy, we restricted data to women with ER+ breast cancer. HER2 receptor status was missing for 29% of women, so stratified analyses were not conducted by this subtype. To test for interactions across subgroups, models with and without interactions terms between the strata variable and segregation index were compared using the likelihood ratio test. Since stratified models essentially allow each variable within the model to have a different relationship with the outcome in each stratum, the models used to test the interactions also included interaction terms between age and the strata variable for age-adjusted models as well as between race and the strata variable for age- and race-adjusted models. Since the likelihood ratio test does not allow for clustered variance, the likelihood ratio tests were run on models without accounting for clustering as the standard errors were similar with and without clustering. All analyses were conducted using Stata Version 14.1. All tests were two-sided and p-values < 0.05 were considered statistically significant.
Data Availability:
The data analyzed in this study are available from the Maryland Cancer Registry. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request with the permission of Maryland Cancer Registry.
Results
Among the 35,066 women in the study, there were over 216,295 person-years of follow-up and 3,739 deaths due to breast cancer. Table 1 describes demographics, clinical and diagnostic factors, and tumor characteristics of the women diagnosed with breast cancer, overall and stratified by race. The median age at breast cancer diagnosis overall was 61 years (interquartile range [IQR] = 52, 71). Black women were younger at diagnosis (median= 58 years, IQR= 49, 67) compared to White women (mean=61 years, IQR= 52, 71). The median follow-up time since diagnosis was 5.9 years (IQR = 3.3, 8.8).
Table 1.
Characteristics of Black and White women diagnosed with invasive breast cancer in the Maryland Cancer Registry, 2007–2017
| Race/Ethnicity | Overall N=35066 | |||||
|---|---|---|---|---|---|---|
| White N=24540 | Black N=10526 | |||||
| No. | % | No. | % | No. | % | |
| SEER Stage | ||||||
| Local | 12212 | 49.8 | 4309 | 40.9 | 16521 | 47.1 |
| Regional | 5296 | 21.6 | 2829 | 26.9 | 8125 | 23.2 |
| Distant | 973 | 4.0 | 561 | 5.3 | 1534 | 4.4 |
| Unknown | 6059 | 24.7 | 2827 | 26.9 | 8886 | 25.3 |
| Grade | ||||||
| 1 | 4460 | 18.2 | 1260 | 12 | 5720 | 16.3 |
| 2 | 11510 | 46.9 | 3965 | 37.7 | 15475 | 44.1 |
| 3 | 7206 | 29.4 | 4659 | 44.3 | 11865 | 33.8 |
| Unknown | 1364 | 5.6 | 642 | 6.1 | 2006 | 5.7 |
| Tumor Phenotype | ||||||
| ER+/PR+ | 17502 | 71.3 | 6031 | 57.3 | 23533 | 67.1 |
| ER+/PR− | 2432 | 9.9 | 1107 | 10.5 | 3539 | 10.1 |
| ER−/PR+ | 233 | 0.9 | 172 | 1.6 | 405 | 1.2 |
| ER−/PR− | 3577 | 14.6 | 2775 | 26.4 | 6352 | 18.1 |
| Unknown | 796 | 3.2 | 441 | 4.2 | 1237 | 3.5 |
| Treatment | ||||||
| Surgery only | 5461 | 22.3 | 1927 | 18.3 | 7388 | 21.1 |
| Surgery + Radiation | 7342 | 29.9 | 1973 | 18.7 | 9315 | 26.6 |
| Surgery + Chemotherapy | 3438 | 14.0 | 1684 | 16 | 5122 | 14.6 |
| Surgery + Radiation + Chemo | 3882 | 15.8 | 2417 | 23 | 6299 | 18.0 |
| No surgery | 1964 | 8.0 | 1129 | 10.7 | 3093 | 8.8 |
| Unknown | 2453 | 10.0 | 1396 | 13.3 | 3849 | 11.0 |
| Hormone Therapy * | ||||||
| No | 4452 | 22.3 | 2184 | 30.5 | 6636 | 24.5 |
| Yes | 12093 | 60.5 | 3662 | 51.2 | 15755 | 58.1 |
| Unknown | 3427 | 17.2 | 1305 | 18.2 | 4732 | 17.4 |
| Triple Negative Breast Cancer | ||||||
| No | 15107 | 61.6 | 5813 | 55.2 | 20920 | 60.0 |
| Yes | 1677 | 6.8 | 1587 | 15.1 | 3264 | 9.3 |
| Unknown | 7756 | 31.6 | 3126 | 29.7 | 10882 | 31.0 |
| Med. | IQR | Med. | IQR | Med. | IQR | |
| Age at Diagnosis | 61 | 52, 71 | 58 | 49, 67 | 60 | 51, 70 |
| Years Since Diagnosis | 6.0 | 3.5, 9.0 | 5.4 | 3.1, 8.5 | 5.9 | 3.3, 8.8 |
| Year of Diagnosis | 2012 | 2009, 2015 | 2013 | 2010, 2015 | 2012 | 2010, 2015 |
| ICE Segregation Index | ||||||
| Economic | −0.30 | −0.46, −0.09 | −0.11 | −0.35, 0.10 | −0.26 | −0.44, −0.04 |
| Racial | −0.72 | −0.87,−0.46 | 0.46 | −0.21, 0.80 | −0.57 | −0.82, 0.03 |
| Racialized economic | −0.31 | −0.45, −0.17 | 0.01 | −0.15, 0.16 | −0.23 | −0.41, −0.05 |
Abbreviations: No. = number, med. = median, IQR = interquartile range (25th percentile, 75th percentile), ER = estrogen receptor, PR = progesterone receptor, ICE = index of concentration at the extremes
Hormone therapy among ER positive only
Tumor characteristics and breast cancer treatments also differed by race. Black women were diagnosed with later stage and higher grade breast cancers compared to White women. Black women had higher prevalence of ER−/PR− tumors compared to White women (26.4% vs. 14.6%). Among women with ER+ breast cancer, Black women were less likely to receive hormone therapy (51.2%) than White women (60.5%). HER2 status combined with ER and PR status was only available for 69% of women, among which 21.4% of Black women had triple negative breast cancer (ER−/PR−/HER2−) in comparison to 10% of White women. Black women were also more likely to receive the combination of surgery, chemotherapy, and radiation (23%) compared to White women (15.8%) and less likely (18.7%) to receive the combination of surgery and radiation compared to White women (29.9%).
Distribution of segregation indices in Maryland
The census tract segregation indices are summarized with boxplots by Maryland county in Figure 1. The overall median census-tract economic, racial, and racialized economic segregation across Maryland was −0.20, −0.47, and −0.18, respectively, all leaning towards greater privilege (below zero). Baltimore County was made up of the largest number of census tracts (N=211) while Garrett County and Somerset County had the least number of census tracts (N=7). Howard County (−0.54) had the highest economic privilege, Garrett County (−0.97) had the highest racial privilege, and Calvert County (−0.41) had the highest racialized economic privilege. Baltimore city had the lowest economic (0.23), racial (0.62), and racialized economic (0.24) privilege. Figure 2 describes each index visually by census-tract across Maryland, with greater economic segregation (decreasing privilege) centered around census-tracts near Baltimore city, northwest Maryland, southeast Maryland, and smaller pockets around the Washington DC area. Greater racial segregation was observed near the Baltimore city and Washington DC areas. Lastly, racialized economic segregation was greatest again near the Baltimore city and Washington DC areas but also in northwest and southeast Maryland.
Figure 1.
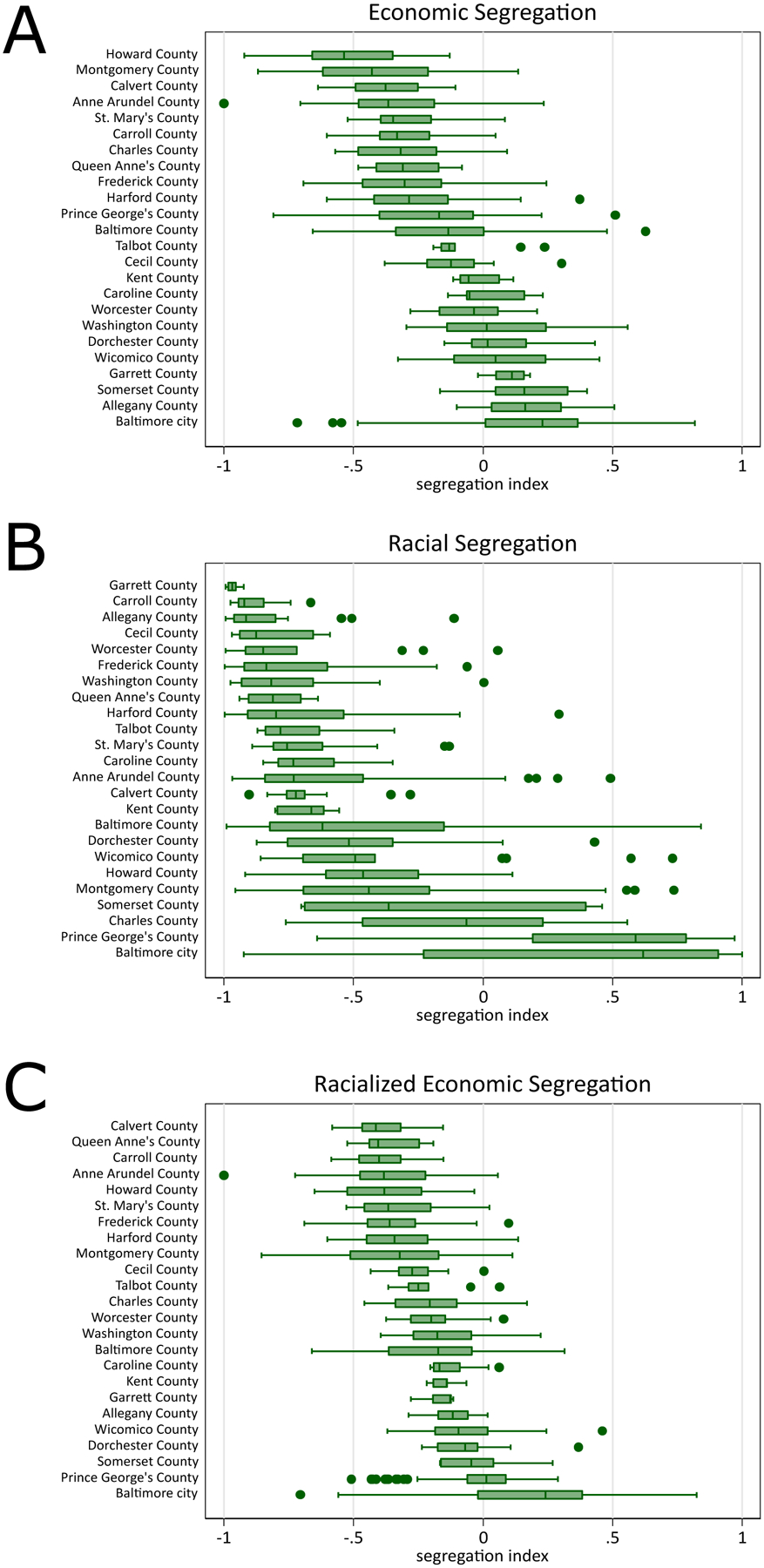
Distribution of economic and Black-White racial segregation indices by county in Maryland based on the American Community Survey, 2011–2015
Panel A: For economic segregation, a value towards −1 indicates higher concentration of high-income households (i.e., higher economic privilege) and a value towards 1 indicates higher concentration of low-income households (i.e., lower economic privilege.
Panel B: For racial segregation, a value of towards −1 indicates higher concentration of White households (i.e., higher racial privilege) and a value towards 1 indicates higher concentration of Black households (i.e., lower racial privilege).
Panel C: For racialized economic segregation, a value of towards −1 indicates higher concentration of high-income White households (i.e., higher racialized economic privilege) and a value towards 1 indicates higher concentration of low-income Black households (i.e., lower racialized economic privilege).
Figure 2.
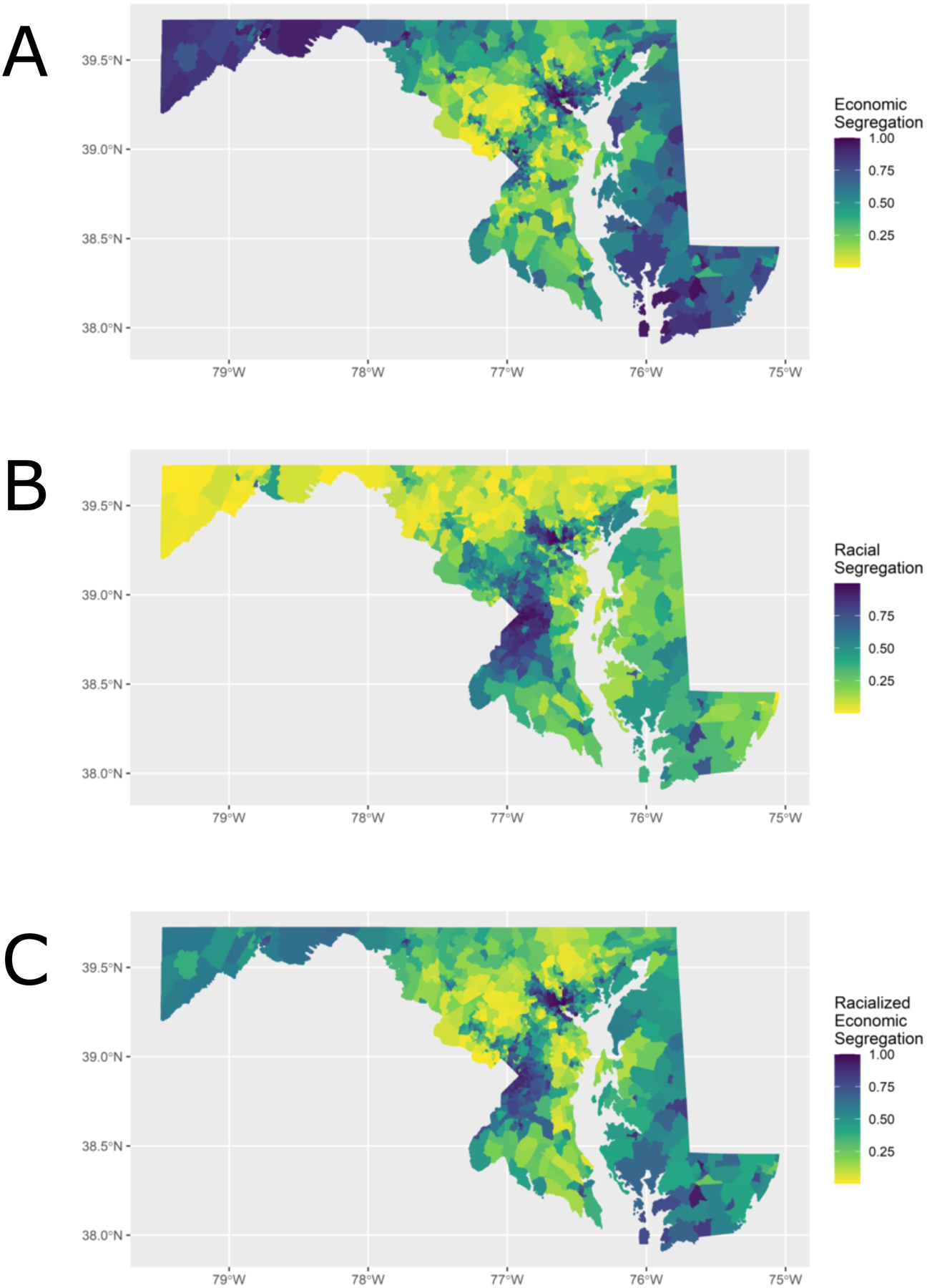
Economic and Black-White racial segregation indices by census tract (ranked by percentiles) in Maryland, 2011–2015
The segregation indices are percentile ranked from 0 to 1 so that gradient in color is distributed evenly based on rank rather than absolute value.
Panel A: For economic segregation, a percentile towards 0 indicates higher concentration of high-income households (i.e., higher economic privilege) and a percentile towards 1 indicates higher concentration of low-income households (i.e., lower economic privilege.
Panel B: For racial segregation, a percentile of towards 0 indicates higher concentration of White households (i.e., higher racial privilege) and a percentile towards 1 indicates higher concentration of Black households (i.e., lower racial privilege).
Panel C: For racialized economic segregation, a percentile towards 0 indicates higher concentration of high-income White households (i.e., higher racialized economic privilege) and a percentile towards 1 indicates higher concentration of low-income Black households (i.e., lower racialized economic privilege).
Economic segregation and breast cancer mortality
The age-adjusted HR and 95% CI for the association between each segregation index and breast cancer mortality, overall and by race and age are shown in Table 2. Additional supplemental analyses stratified by prognostic factors (Supplemental Figure 1 and Supplemental Tables 4–5) and time since (≤5 years, >5 years) (Supplemental Table 6) were similar across strata. For each segregation index, the HRs are in comparison to the most privileged (Quintile 1).
Table 2.
Age-adjusted hazard ratios and 95% confidence intervals for breast cancer mortality associated with economic and Black-White racial segregation indices among women diagnosed with breast cancer, overall and by race and age, Maryland 2007–2017
| Race | Age | ||||
|---|---|---|---|---|---|
| Overall | Black | White | <60 | 60+ | |
| Deaths | 3739 | 1447 | 2292 | 1665 | 2074 |
| Person-Years | 216295 | 61727 | 154568 | 111699 | 104596 |
| Economic Segregation | |||||
| Quintile 1 | (reference; most privileged/least deprived) | ||||
| Quintile 2 | 1.18*** (1.05, 1.33) | 1.17 (0.94, 1.46) | 1.16** (1.02, 1.31) | 1.27*** (1.06, 1.53) | 1.10 (0.94, 1.29) |
| Quintile 3 | 1.21*** (1.08, 1.35) | 1.23* (1.00, 1.51) | 1.12* (0.98, 1.28) | 1.36*** (1.15, 1.61) | 1.08 (0.93, 1.26) |
| Quintile 4 | 1.41*** (1.25, 1.58) | 1.30*** (1.07, 1.59) | 1.31*** (1.15, 1.49) | 1.54*** (1.29, 1.85) | 1.30*** (1.12, 1.49) |
| Quintile 5 | 1.67*** (1.50, 1.86) | 1.43*** (1.19, 1.72) | 1.45*** (1.26, 1.66) | 2.01*** (1.71, 2.36) | 1.44*** (1.25, 1.66) |
| P-interaction | 0.87 | 0.04 | |||
| Racial Segregation | |||||
| Quintile 1 | 1.00 (reference; most privileged/least deprived) | ||||
| Quintile 2 | 1.06 (0.95, 1.19) | 1.29 (0.77, 2.14) | 1.03 (0.92, 1.15) | 1.09 (0.92, 1.30) | 1.05 (0.91, 1.20) |
| Quintile 3 | 1.12** | 1.32 | 1.02 | 1.15 | 1.11 |
| (1.00, 1.25) | (0.82, 2.13) | (0.91, 1.15) | (0.96, 1.36) | (0.97, 1.28) | |
| Quintile 4 | 1.42*** (1.28, 1.58) | 1.44 (0.91, 2.28) | 1.16** (1.03, 1.31) | 1.62*** (1.38, 1.90) | 1.29*** (1.13, 1.49) |
| Quintile 5 | 1.57*** (1.42, 1.74) | 1.27 (0.80, 2.00) | 1.17 (0.96, 1.44) | 1.83*** (1.56, 2.14) | 1.41*** (1.23, 1.62) |
| P-interaction | 0.61 | 0.03 | |||
| Racialized Economic Segregation | |||||
| Quintile 1 | 1.00 (reference; most privileged/least deprived) | ||||
| Quintile 2 | 1.14** (1.01, 1.30) | 1.17 (0.83, 1.64) | 1.11 (0.98, 1.25) | 1.20* (1.00, 1.45) | 1.10 (0.93, 1.29) |
| Quintile 3 | 1.34*** (1.19, 1.51) | 1.17 (0.85, 1.59) | 1.26*** (1.12, 1.25) | 1.53*** (1.28, 1.84) | 1.21** (1.05, 1.41) |
| Quintile 4 | 1.42*** (1.26, 1.60) | 1.13 (0.84, 1.2) | 1.23*** (1.09, 1.40) | 1.77*** (1.48, 2.11) | 1.20** (1.03, 1.40) |
| Quintile 5 | 1.84*** (1.64, 2.06) | 1.20 (0.90, 1.60) | 1.66*** (1.41, 1.95) | 2.17*** (1.83, 2.57) | 1.62*** (1.40, 1.88) |
| P-interaction | 0.04 | 0.004 | |||
p-value<0.1,
p-value<0.05,
p-value<0.01
For economic segregation, a quintile towards 1 indicates higher concentration of high-income households (i.e., higher economic privilege) and a quintile towards 5 indicates higher concentration of low-income households (i.e., lower economic privilege). For racial segregation, a quintile towards 1 indicates higher concentration of White households (i.e., higher racial privilege) and a quintile towards 5 indicates higher concentration of Black households (i.e., lower racial privilege). For racialized economic segregation, a quintile of towards 1 indicates higher concentration of high-income White households (i.e., higher racialized economic privilege) and quintile towards 5 indicates higher concentration of low-income Black household (i.e., lower racialized economic privilege).
Overall, women in the least privileged quintile of economic segregation had 1.67 times the hazard (95% CI: 1.50, 1.86) of breast cancer mortality than women in the most privileged quintile. The associations for breast cancer mortality comparing the least to the most economically privileged were similar by race with a HR of 1.43 (95% CI: 1.19, 1.72) and 1.45 (95% CI: 1.26, 1.66) for Black and White women, respectively.
The increased hazard of breast cancer mortality associated with living in the least economically privileged areas compared to the most economically privileged areas was stronger for women aged < 60 years (HR: 2.01, 95% CI: 1.71, 2.36) versus women aged ≥ 60 years (HR 1.44, 95% CI: 1.25, 1.66) (p-interaction=0.04) (Table 2). In supplemental analyses, models were adjusted for race and results were slightly attenuated although still statistically significant, but the interaction by age was no longer significant (Supplemental Table 4).
Racial segregation and breast cancer mortality
Overall, the hazard of breast cancer mortality was 1.57 times as high (95% CI: 1.42, 1.74) for women in the least privileged quintile of racial segregation compared to the most privileged quintile (Table 2). The association was similar by race, although among Black women the association was not statistically significant and with wide confidence intervals, while the association was only statistically significant for quintile 4 among White women (HR: 1.16, 95% CI: 1.03, 1.31).
The association between racial segregation and breast cancer mortality differed by age and stage. The HR comparing the least to the most racially privileged was 1.83 (95% CI: 1.56, 2.14) among women aged < 60 years and 1.41 (95% CI: 1.23, 1.62) among women aged ≥ 60 years (p-interaction=0.03) (Table 2). In supplemental results presented for prognostic factors adjusted for age, the association between racial segregation and breast cancer mortality only differed by stage (p-interaction= 0.02) (Supplemental Table 4). In supplemental models additionally adjusted for race, the results were strongly attenuated, and interactions were no longer significant (Supplemental Table 5). Only Quintile 4 (the second to least privileged) overall and across age were still significantly associated with increased breast cancer mortality.
Racialized economic segregation and breast cancer mortality
Overall, the hazard of breast cancer mortality was 1.84 times as high (95% CI: 1.64, 2.06) for the least privileged quintile of racialized economic segregation compared to the most privileged quintile (Table 2). The associations for racialized economic segregation were similar and increased across quintiles among Black women, but not statistically significant and with wide confidence intervals. The association differed by race, such that the least privileged quintile of racialized economic segregation compared to the most privileged quintile was associated with 1.20 (95% CI: 0.90, 1.60) times the hazard of breast cancer mortality for Black women as opposed to 1.66 (95% CI: 1.41, 1.95) times as high for White women (p-interaction=0.04).
The association for racialized economic segregation and breast cancer mortality also differed by age (Table 2). Comparing the least racially and economically privileged to the most racially and economic privileged, the association was stronger for women aged <60 years (HR: 2.17, 95% CI: 1.83, 2.57) versus women aged ≥ 60 years (HR: 1.62, 95% CI: 1.40, 1.88) (p-interaction=0.004). In supplemental analyses additionally adjusting for race, results were attenuated; however, the associations still suggested increased breast cancer mortality for decreasing racialized economic privilege (Supplemental Table 5).
Discussion
Our study examined associations between racialized economic segregation and breast cancer mortality in a cohort of Black and White women diagnosed with invasive breast cancer in Maryland. This is the first study, to our knowledge, to evaluate the associations between ICE metrics and breast cancer mortality by subgroups of race and age. Overall, women in living in neighborhoods with higher concentrations of lower incomes and/or with more Black residents had nearly twice as high the hazard of breast cancer mortality in comparison to women living in neighborhoods with higher concentrations of higher incomes and/or more White residents. In associations stratified by race, only the association between economic segregation and breast cancer mortality was statistically significant among Black women. Age was a significant effect modifier for associations between economic, racial, and racialized economic segregation and breast cancer mortality, with stronger hazard ratios ranging between 1.83–2.17 among younger women (< 60 years at diagnosis).
While other studies have examined the impact of residential segregation on breast cancer mortality using different metrics (i.e., isolation index (7), measures of socioeconomic status and urbanization (25), location quotient of racial residential segregation (9), racial index of dissimilarity (8), Information Theory Index (10) and “hyper-segregation” (11)), we chose to use the ICE. To date, ICE metrics have only been utilized in a few breast cancer studies: one study comparing ER status in breast cancer cases reported to SEER (20), another study measuring breast cancer survival in Milwaukee, Wisconsin (26), and a study of cancer incidence, including breast cancer in Massachusetts (27). The single ICE metric which combines race/ethnicity and income as a measure of racialized economic segregation is a validated measure of place-based health disparities (18–20, 24, 28).
ICE encapsulates an aspect of social inequity that would be missed if we only considered inequities by the percentage of the population among certain incomes or race/ethnicity and highlights unequal group relationships (24). These otherwise hidden inequities in Maryland can be graphically depicted, as illustrated in Figure 2. Unsurprisingly, areas of Maryland where racialized economic segregation were greatest (Figure 2) have the highest age-adjusted female breast cancer mortality rates as described by the 2019 Maryland Department of Health Cancer Data report (2012–2016): Baltimore City (27 per 100,000); Prince George’s Co. near Washington DC (25.1 per 100,000); and Worcester County in southeast Maryland (30.7 per 100,000) (29).
We stratified results by race to explore racial differences in the associations between racialized economic segregation and breast cancer mortality. Black communities generally experience the highest degree of residential segregation of any US group (30, 31). Blacks are more likely to live in segregated areas for numerous reasons, including factors associated with racial discrimination, immigrant settling patterns, and economic disparity (11, 30, 32). In turn, residential segregation tends to limit social, economic and educational opportunities and resources and is linked to increased poverty in these minority communities (33). Health outcomes can also be negatively impacted by segregation through exposure to substandard housing, lack of access to medical services, and social isolation (12, 30, 34). The only statistically significant association observed among Black women was for the association between economic segregation and breast cancer mortality and results were similar among White women. We did find a significant interaction with individual race and racialized economic segregation with increasing breast cancer mortality among White women living in less privileged areas and no association among Black women. We found no interaction between individual race and racial segregation. Russell and colleagues also explored interactions with individual race and did not find race to be a significant modifier in the association between residential racial composition and breast cancer mortality among women diagnosed with breast cancer in Georgia (22). In additional models, we adjusted for race and found that race attenuated point estimates, most strongly for racial segregation. These results suggest individual race and residential racial composition may be interrelated in the Maryland context.
We observed significant effect modification by age, with a steeper ICE gradient among women under age 60 as compared to women aged 60 and older. This finding may suggest that access to healthcare and insurance status, specifically Medicare that is available to adults as of age ≥ 65 years, reduces disparities in breast cancer mortality arising from racialized economic segregation. In line with this hypothesis, in a study among seniors in the Surveillance, Epidemiology, and End Results–Medicare database (66 to 85 years of age), Haas and colleagues did not find a significant association between Black segregation and mortality (HR, 1.03; 95% CI, 0.87–1.21). However, this study was also unable to account for residential socioeconomic status (7). To our knowledge, our findings among younger women have not been explored in other studies of breast cancer mortality and residential segregation and they indicate that age should be considered as a significant factor in this association.
This study had several strengths and limitations. We examined the impact of racialized economic segregation on breast cancer mortality among a large cohort of invasive breast cancer patients of which 30% were Black. We had sufficient power to evaluate associations among subgroups by race, prognostic factors (i.e., age, hormone receptor status, stage), and treatment modalities. We used ICE metrics that more accurately represent the extremes of economic and racialized segregation in America than other metrics of social inequality. We utilized census tracts for these ICE metrics which might capture the social experience more accurately than county-level metrics. We did not adjust for covariates as we hypothesized that these factors would function as mediators in our analyses, and therefore confounding beyond age could be present if these factors were not actual mediators. We also lacked behavioral risk factor data for risk factors such as physical activity and obesity which could be impacted by racial and economic segregation (35, 36). Evaluation of these factors could be important for further identification of at-risk groups.
In conclusion, our results suggest that breast cancer survival disparities exist in Maryland among women residing in the least privileged census tracts with lower income households and higher proportions of Black residents. We observed significantly stronger associations between racialized economic segregation and breast cancer mortality among younger women. Our findings for ICE were more extreme than those of racial segregation. We believe that these studies that only looked at segregation by race may have underestimated the effects of segregation on cancer outcomes and the intersection between race and socioeconomics should be considered as a critical driver of cancer health disparities. Our study findings provide new insights into the racial disparities in breast cancer mortality observed among women diagnosed with invasive breast cancer in Maryland.
Supplementary Material
Acknowledgements:
This work was supported by the American Cancer Society (MRSG-19-010-01-CPHPS awarded to A Connor). We would also like to acknowledge support by the NCI Cancer Center Support Grant (P30 CA006973). Cancer data was provided by the Maryland Cancer Registry, Center for Cancer Prevention and Control, Department of Health, with funding from the State of Maryland and the Maryland Cigarette Restitution Fund. The collection and availability of cancer registry data is also supported by the Cooperative Agreement NU58DP006333, funded by the Centers for Disease Control and Prevention. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Footnotes
Conflict of Interest:
Conflicts of interest/Competing interests: Drs. Connor, Dibble, Visvanathan, and Dean and Ms. Hayes do not have any conflicts of interest to disclose. All contributions by Dr. Maneet Kaur were during her affiliation with Johns Hopkins Bloomberg School of Public Health; however, at the time of manuscript submission, Dr. Kaur was employed by Flatiron Health, an independent subsidiary of Roche. Dr. Kaur reported stock ownership in Roche.
References
- 1.American Cancer Society. Cancer Facts & Figures for African Americans 2019–2021. Atlanta: American Cancer Society, 2019. [Google Scholar]
- 2.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. Am J Epidemiol. 2002;155:534–45. [DOI] [PubMed] [Google Scholar]
- 4.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–72. [DOI] [PubMed] [Google Scholar]
- 5.Ashing K, Rosales M, Lai L, Hurria A. Occurrence of comorbidities among African-American and Latina breast cancer survivors. J Cancer Surviv. 2014;8:312–8. [DOI] [PubMed] [Google Scholar]
- 6.Satia JA. Diet-related disparities: understanding the problem and accelerating solutions. J Am Diet Assoc. 2009;109:610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas JS, Earle CC, Orav JE, Brawarsky P, Keohane M, Neville BA, et al. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008;113:2166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulson MR, Beaulieu-Jones BR, Kenzik KM, Dechert TA, Ko NY, Sachs TE, et al. Residential Racial Segregation and Disparities in Breast Cancer Presentation, Treatment, and Survival. Ann Surg. 2021;273:3–9. [DOI] [PubMed] [Google Scholar]
- 9.Pruitt SL, Lee SJ, Tiro JA, Xuan L, Ruiz JM, Inrig S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer. 2015;121:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell EF, Kramer MR, Cooper HL, Gabram-Mendola S, Senior-Crosby D, Jacob Arriola KR. Metropolitan area racial residential segregation, neighborhood racial composition, and breast cancer mortality. Cancer Causes Control. 2012;23:1519–27. [DOI] [PubMed] [Google Scholar]
- 11.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. 2010;35:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White K, Haas JS, Williams DR. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res. 2012;47:1278–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean LT, Gehlert S, Neuhouser ML, Oh A, Zanetti K, Goodman M, et al. Social factors matter in cancer risk and survivorship. Cancer Causes Control. 2018;29:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean L, Subramanian SV, Williams DR, Armstrong K, Charles CZ, Kawachi I. The role of social capital in African-American women’s use of mammography. Soc Sci Med. 2014;104:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumanyika S Obesity Treatment in Minorities. In: Wadden T, Stunkard A, editors. Handbook of Obesity Treatment. New York: Guilford Press; 2002. p. 416–46. [Google Scholar]
- 16.Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121:2314–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin LJ, Gaglioti AH, Beyer KM, Zhou Y, Moore MA, Nash R, et al. Neighborhood-Level Redlining and Lending Bias Are Associated with Breast Cancer Mortality in a Large and Diverse Metropolitan Area. Cancer Epidemiol Biomarkers Prev. 2021;30:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G. Public Health Monitoring of Privilege and Deprivation With the Index of Concentration at the Extremes. Am J Public Health. 2016;106:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger N, Waterman PD, Gryparis A, Coull BA. Black carbon exposure, socioeconomic and racial/ethnic spatial polarization, and the Index of Concentration at the Extremes (ICE). Health Place. 2015;34:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N, Singh N, Waterman PD. Metrics for monitoring cancer inequities: residential segregation, the Index of Concentration at the Extremes (ICE), and breast cancer estrogen receptor status (USA, 1992–2012). Cancer Causes Control. 2016;27:1139–51. [DOI] [PubMed] [Google Scholar]
- 21.“Profile of General Population and Housing Characteristics: 2010 Demographic Profile Data”. U.S. Census Bureau. Archived from the original on 2014-03-05. Retrieved 08/09/2018. [Google Scholar]
- 22.Russell E, Kramer MR, Cooper HL, Thompson WW, Arriola KR. Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health. 2011;88:1117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Census Bureau. “Quick Facts” Retrieved 05/04/2021 (https://www.census.gov/quickfacts/fact/table/princegeorgescountymaryland/INC110219#INC110219).
- 24.Scally BJ, Krieger N, Chen JT. Racialized economic segregation and stage at diagnosis of colorectal cancer in the United States. Cancer Causes Control. 2018;29:527–37. [DOI] [PubMed] [Google Scholar]
- 25.Parise CA, Caggiano V. Regional Variation in Disparities in Breast Cancer Specific Mortality Due to Race/Ethnicity, Socioeconomic Status, and Urbanization. J Racial Ethn Health Disparities. 2017;4:706–17. [DOI] [PubMed] [Google Scholar]
- 26.Bemanian A, Beyer KM. Measures Matter: The Local Exposure/Isolation (LEx/Is) Metrics and Relationships between Local-Level Segregation and Breast Cancer Survival. Cancer Epidemiol Biomarkers Prev. 2017;26:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger N, Feldman JM, Kim R, Waterman PD. Cancer Incidence and Multilevel Measures of Residential Economic and Racial Segregation for Cancer Registries. JNCI Cancer Spectr. 2018;2:pky009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieger N, Waterman PD, Batra N, Murphy JS, Dooley DP, Shah SN. Measures of Local Segregation for Monitoring Health Inequities by Local Health Departments. Am J Public Health. 2017;107:903–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maryland Department of Health. 2019. Cancer Data. Cigarette Restitution Fund Program. Cancer Prevention, Education, Screening and Treatment Program. https://health.maryland.gov/phpa/cancer/SiteAssets/Pages/surv_data-reports/2019%20CRF%20Cancer%20Report.pdf.
- 30.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polednak AP. Segregation, discrimination and mortality in U.S. blacks. Ethn Dis. 1996;6:99–108. [PubMed] [Google Scholar]
- 32.Jones C The impact of racism on health. Ethn Dis. 2002;12:S2–10–3. [PubMed] [Google Scholar]
- 33.Acevedo-Garcia D Residential segregation and the epidemiology of infectious diseases. Soc Sci Med. 2000;51:1143–61. [DOI] [PubMed] [Google Scholar]
- 34.Duncan DT, Aldstadt J, Whalen J, White K, Castro MC, Williams DR. Space, race, and poverty: Spatial inequalities in walkable neighborhood amenities? Demogr Res. 2012;26:409–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu CY, Woo A, Hawkins C, Iman S. The Impacts of Residential Segregation on Obesity. J Phys Act Health. 2018;15:834–9. [DOI] [PubMed] [Google Scholar]
- 36.Lopez R Black-white residential segregation and physical activity. Ethn Dis. 2006;16:495–502. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study are available from the Maryland Cancer Registry. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request with the permission of Maryland Cancer Registry.


