Abstract
Objective:
Studies demonstrate rapid antidepressant and anti-suicidal ideation effects of subanesthetic ketamine. The specific subcomponents of depression that are most closely tied to reduction of suicidal ideation with ketamine treatment are less explored.
Methods:
Exploratory, post hoc analysis of data from a randomized clinical trial of ketamine vs midazolam in patients with major depressive disorder (MDD) and clinically significant suicidal ideation examined changes in factor analysis-derived symptom clusters from standard measures of depression (Hamilton Depression Rating Scale, HDRS; Beck Depression Inventory, BDI) and mood disturbance (Profile of Mood States, POMS), and their relationship to severity of suicidal ideation (Beck Scale for Suicidal Ideation; SSI). Ratings obtained before and one day after blinded intravenous infusion were decomposed into component factors or published subscales. Treatment effects on factors/subscales were compared between drugs, correlations with changes in suicidal ideation were tested, and stepwise regression was used to derive predictors of change in SSI.
Results:
Factor scores for HDRS Psychic Depression, HDRS Anxiety, BDI Subjective Depression, POMS Depression and POMS Fatigue improved more with ketamine than midazolam. Stepwise regression showed across both drugs that improvement in HDRS Psychic Depression, POMS Depression, and HDRS Anxiety predicted 51.6% of the variance in reduction of suicidal ideation.
Limitations:
Secondary analysis of clinical trial data.
Conclusions:
Ketamine’s rapid effects on suicidal ideation appear to be mostly a function of its effects on core mood and anxiety symptoms of MDD, with comparatively little contribution from neurovegetative symptoms with the potential exception of vigor/fatigue.
Trial Registration:
Data used in this secondary analysis came from ClinicalTrials.gov identifier: NCT01700829.
Introduction
U.S. age-adjusted suicide rates have increased 33% in the past twenty years (Hedegaard et al., 2020) — a period of declining deaths due to other causes including ischemic heart disease, neoplasms and motor vehicle accidents (Woolf and Schoomaker, 2019). Declining US life expectancy has been linked partly to increased suicide rates (Case and Deaton, 2020). Ninety percent of deaths by suicide are associated with psychiatric illness with depressive disorders implicated in 60% of these (Mann, 2003; Mann et al., 2005), suggesting that effective treatment of depression may reduce suicide risk.
Suicidal behavior is typically preceded by suicidal thoughts, and treatment with traditional, monoaminergic antidepressants reduces suicidal ideation in part via reduction in overall depression severity (Gibbons et al., 2012; Grunebaum et al., 2013). Suicidal crises require urgent action (Fu et al., 2020; Ionescu et al., 2019; Ionescu et al., 2016; Mallick and McCullumsmith, 2016) but standard antidepressant agents typically require several weeks of treatment before significantly relieving symptoms (Schatzberg and DeBattista, 2015). Ketamine is a rapidly acting antidepressant and anti-suicidal ideation agent capable of relieving suicidal ideation within hours rather than weeks (Al Jurdi et al., 2015; Ballard et al., 2017; Bartoli et al., 2017; Grunebaum et al., 2018; Zarate et al., 2012). As such, it has the potential to transform treatment of patients presenting with acute suicidal ideation (Bartoli et al., 2017; Reinstatler and Youssef, 2015; Sanacora et al., 2017).
In our previous trial (Grunebaum et al., 2018), ketamine’s effects on suicidal ideation were stronger than the midazolam comparator. Ketamine was superior to midazolam in reduction of Profile of Mood States (POMS) Depression score but fell short of significance on the Hamilton Depression Rating Scale (HDRS-17). This raised the question of which depressive symptom components ketamine affected most and how these changes might be related to its rapid reduction of suicidal thoughts.
Depression is a heterogenous entity, and we had previously found that mood components of depression–and not neurovegetative symptoms–are related to suicidal thinking cross-sectionally (Keilp et al., 2012). We further observed improvement in mood components of depression are most strongly related to reductions in suicidal ideation during pharmacologic treatment (Keilp et al, 2018a), and that the effectiveness of a particular treatment is related to its efficacy in reducing these core mood symptoms (Grunebaum et al., 2013). We sought to investigate whether ketamine may act similarly, and more rapidly.
In this post-hoc analysis: we decomposed depression severity ratings from the HDRS and BDI into factor analysis-derived symptom clusters (Grunebaum et al., 2005); generated subscores from an additional mood rating (POMS); and examined changes in these more specific symptom measures one day following a single blinded infusion with either ketamine or midazolam. Our goal was to determine which symptom factor scores or subscales were most strongly affected by ketamine, and to determine if changes in these symptoms were related to reductions in suicidal ideation at day one. Data from our published, midazolam-controlled clinical trial of ketamine for MDD with clinically significant suicidal ideation (Grunebaum et al., 2018) was used for this analysis. We hypothesized, as in our prior work (Grunebaum et al., 2013, Keilp et al, 2018a), that reduction in the core mood dimensions of these rating scales would be most associated with ketamine effects, and that reductions in these would most strongly correlate with reduction in suicidal ideation.
Methods
Participants
Trial methods and primary results have been reported (Grunebaum et al., 2018). Briefly, we conducted a randomized clinical trial (N=80) testing adjunctive sub-anesthetic intravenous ketamine (0.5 mg/kg) versus midazolam (0.02 mg/kg) in patients with MDD and clinically-significant suicidal ideation, defined as a score of ≥4 on the clinician-rated SSI (Brown et al., 2000; Price et al., 2014). Primary outcome was change in SSI score at 24-hours post-infusion. Main eligibility criteria were: age 18-64 years old; DSM-IV MDD (First, 1994) with score ≥16 on the 17-item HDRS (Hamilton, 1960); SSI ≥4; and voluntary admission to an inpatient research unit. Exclusions included: unstable medical or neurological illness; significant EKG abnormality; pregnancy or lactation; current psychosis; history of ketamine abuse or dependence; drug or alcohol dependence within six months of study enrollment; suicidal ideation due to binge substance abuse or withdrawal; previous inadequate response to ketamine; prior adverse reaction to ketamine or midazolam; daily opiate medication (equivalent to >20mg oxycodone) over the three days prior to infusion; a score of <25 on the Mini Mental State Exam for those over 60 years old (Folstein et al., 1975); lack of capacity or inadequate English comprehension for informed consent. The study was approved by the New York State Psychiatric Institute’s Institutional Review Board. Written informed consent was obtained from all participants, and the study was registered at ClinicalTrials.gov (identifier: NCT01700829).
Patients were randomized to ketamine or midazolam infused in 100mL normal saline over 40 minutes. Participants and study staff were blind to treatment. Baseline characteristics between treatment groups differed only in frequency of borderline personality disorder, which was not found to affect the primary outcome — summarized in Table 1 (for additional information see Grunebaum et al., 2018).
Table 1:
Baseline demographic and clinical characteristics by treatment group (N=80)
| Midazolam (N=40), mean (SD) |
Ketamine (N=40), mean (SD) |
|
|---|---|---|
| Age | 40.7 (13.1) | 38.4 (13.2) |
| Female, no. (%) | 26 (65%) | 22 (55%) |
| SSI | 15.7 (6.9) | 14.3 (6.3) |
| No of Past MDD ep, median (range) | 3 (1 to "too many to count") | 4.5 (1 to "too many to count") |
| Hx of Past Suicide Attempt at Randomization, no. (%) | 22 (55) | 17 (42.5) |
| Baseline HDRS-24 | 30.25 (6) | 29.35 (6.2) |
| Baseline BDI | 33.9 (8.1) | 31.8 (8.1) |
| Baseline POMS | 101.7 (35.2) | 95.92 (37.4) |
SSI = Beck Scale for Suicidal Ideation; MDD = Major Depressive Disorder; HDRS-24 = 24 item Hamilton Depression Rating Scale; BDI = Beck Depression inventory; POMS = Profile of Mood States
Outcome Measures
SI was assessed via the SSI, one day prior and one day after infusion. Depressive symptoms were assessed using the clinician-rated HDRS (Hamilton, 1960), the self-rated BDI (Beck et al., 1961) and self-rated POMS (McNair, 1981). Factor analyses of the HDRS and BDI have been described previously (Grunebaum et al., 2005), and derived factors have been studied in prior publications (Grunebaum et al., 2005; Grunebaum et al., 2013; Keilp et al., 2018a; Keilp et al., 2012; Keilp et al., 2018b; Milak et al., 2010; Milak et al., 2005). The HDRS was decomposed into five factors assessing: Psychic Depression; Loss of Motivation; Disturbed Thinking; Anxiety; and, Sleep Disturbance. For example, the Psychic Depression factor includes items such as depressed mood, worthlessness and hopelessness. The BDI was decomposed into three factors: Subjective Depression; Self-Blame; and Somatic Complaints. For instance, the Subjective Depression factor comprises items including sadness and pessimism. The POMS is comprised of six standard subscales assessing: Depression; Tension/Anxiety; Anger; Confusion; Fatigue; and, Vigor. Further details regarding scale items comprising each factor or subscale score are presented in Supplemental Tables s1 - s3. The suicidal ideation item on the HDRS, which loads on the Psychic Depression factor (Grunebaum et al., 2005), was removed in the calculation of this factor score for analyses, given its redundancy with items on the SSI (see Keilp et al., 2012) as in our previous analyses of treatment effects with this scale (Keilp et al., 2018). The suicidal ideation item of the BDI does not load on any one factor for the BDI (Keilp et al., 2012), and thus is not included in factor score computations for the BDI. There are no items on the POMS that ask directly about suicidal ideation.
Statistical Analyses
Total scores on the SSI, HDRS, BDI and POMS were compared across treatment and time using repeated measures Analysis of Variance (ANOVA), with Drug (2 levels, ketamine vs. midazolam) and Time (2 levels, baseline one day prior to infusion and one day after) as factors, to illustrate global treatment effects.
Depression factors and POMS subscales were compared hierarchically as follows: for each of the three rating scales, a repeated-measures General Linear Model was applied with Factor Score (5 levels for HDRS, 3 for BDI, and 6 for POMS), Drug (2 levels), and Time (2 levels, baseline and 24-hours post-infusion) as factors. A significant Drug x Time, or Factor Score x Drug x Time effect led to examination of specific factor scores in a repeated measures Analysis of Variance with Drug and Time as factors.
Pearson correlations were computed between changes in each factor/subscale and change in SSI, between baseline and one day post-infusion. These change scores were examined in all participants, and in those treated with ketamine alone.
A stepwise linear regression was then run in all participants with factor/subscales showing any significant effect of time or drug x time) as potential predictors of reduction in SSI. This regression was then repeated in the ketamine treated participants alone, with factor/subscales that showed a differential effect for ketamine. In our initial approach to this analysis in ketamine-treated participants, we included marginal effects (p<.055) in order to insure including potentially relevant predictors. We then repeated these analyses including only those measures that clearly met significance criteria (p<.05) in hierarchical analyses, which allow for exploration of omnibus effects without adjustment for multiple comparisons (versus if these analyses had been strictly univariate). Analyses were run in SPSS version 26 (IBM Corp, Armonk, New York).
Results
Total Score Analyses
Compared with midazolam, ketamine was associated with greater reduction in SSI score (Drug x Time interaction F[1, 78]=7.41, p=0.008). The Drug x Time interaction on the HDRS total score, however, was not significant (Drug x Time interaction F[1, 78]=2.53, p=0.116). This effect was marginal for BDI and POMS total scores (Drug x Time interaction for BDI: F[1, 72]=3.86, p=.053; Drug x Time interaction for POMS: F[1, 78]=3.86, p=0.053).
Depression Factor Score Analyses
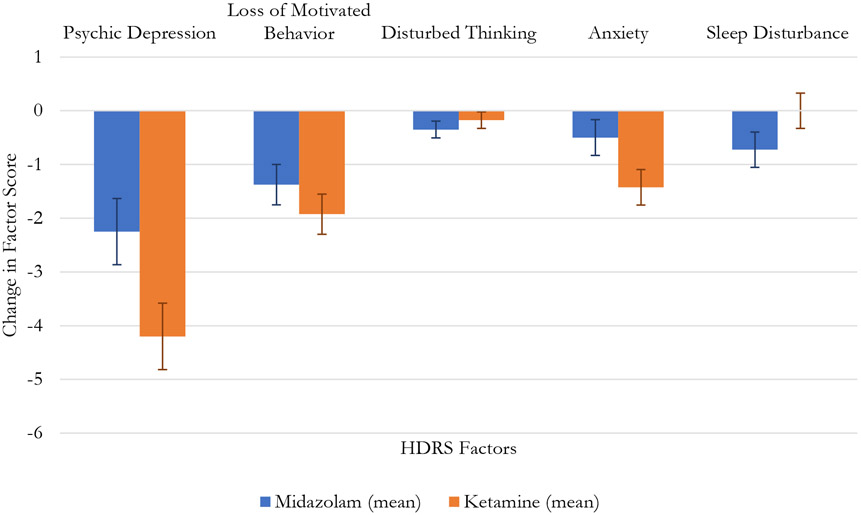
In the omnibus analysis of the five HDRS factors, there was a significant Factor Score x Drug x Time interaction (F[2, 144]=3.89, p=.023). Changes across treatment are illustrated in Figure 1. In univariate comparisons, the Drug x Time effect, illustrating a superiority for ketamine, was significant for the HDRS Psychic Depression factor score (F[1, 78]=4.99, p=.028), and marginal for the HDRS Anxiety factor score (F[1, 78]=3.88, p=.052), but none of the other three factor scores were statistically significant (Table 2.; Figure 1).
Fig. 1.
Change in Hamilton Depression Rating Scale Factor Scores
Table 2:
Factor Scores Across Treatment Conditions and Assessment Time Points
| Pre Midazolam (SD) |
Post Midazolam (SD) |
Pre Ketamine (SD) |
Post Ketamine (SD) |
Significance: Time Effect |
Significance: Drug Effect |
Significance: Time Effect x Drug Effect |
|
|---|---|---|---|---|---|---|---|
| Total Scores | |||||||
| SSI | 16.9 (5.8) | 13.3 (8.4) | 15.55 (6.2) | 7.2 (6.4) | <0.001 | 0.004 | 0.008 |
| HDRS-24 | 30.3 (6.0) | 24.0 (8.8) | 29.4 (6.2) | 20.0 (9.7) | <0.001 | 0.091 | 0.116 |
| BDI | 33.1 (9.2) | 25.0 (12.2) | 31.7 (8.0) | 18.8 (10.7) | <0.001 | 0.065 | 0.053 |
| POMS | 101.7 (35.2) | 85.5 (46.2) | 95.9 (37.4) | 61.3 (45.5) | <0.001 | 0.064 | 0.053 |
| HDRS Factors | |||||||
| HDRS: Psychic Depression | 11.1 (2.7) | 8.8 (4.0) | 11.5 (2.7) | 7.3 (3.9) | <0.001 | 0.0378 | 0.028 |
| HDRS: Loss of Motivated Behavior | 6.1 (1.5) | 4.7 (2.1) | 5.8 (1.9) | 3.8 (2.7) | <0.001 | 0.100 | 0.303 |
| HDRS: Disturbed Thinking | 0. 8 (1.3) | 0.4 (0.7) | 0.5 (1.2) | 0.4 (0.8) | 0.019 | 0.504 | 0.427 |
| HDRS: Anxiety | 4.3 (2.4) | 3.8 (2.0) | 4.1 (1.8) | 2.7 (1.7) | <0.001 | 0.119 | 0.052 |
| HDRS: Sleep Disturbance | 3.1 (1.8) | 2.3 (1.8) | 2.5 (1.8) | 2.5 (1.7) | 0.122 | 0.617 | 0.122 |
| BDI Factors | |||||||
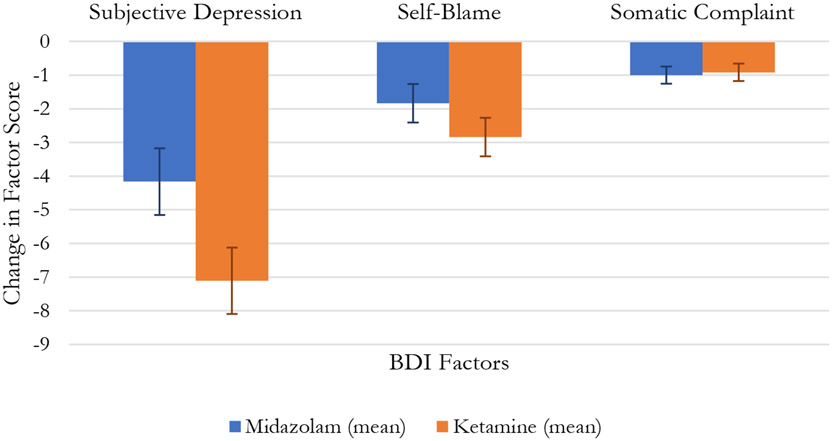
| BDI: Subjective Depression | 17.3 (4.9) | 13.1 (6.1) | 17.5 (4.2) | 10.4 (6.8) | <0.001 | 0.246 | 0.039 |
| BDI: Self-Blame | 8.4 (3.5) | 6.5 (4.0) | 7.6 (3.2) | 4.7 (3.2) | <0.001 | 0.073 | 0.220 |
| BDI: Somatic Complaint | 2.7 (2.1) | 1.7 (1.9) | 2.4 (1.8) | 1.5 (1.6) | <0.001 | 0.517 | 0.825 |
| POMS Subscales | |||||||
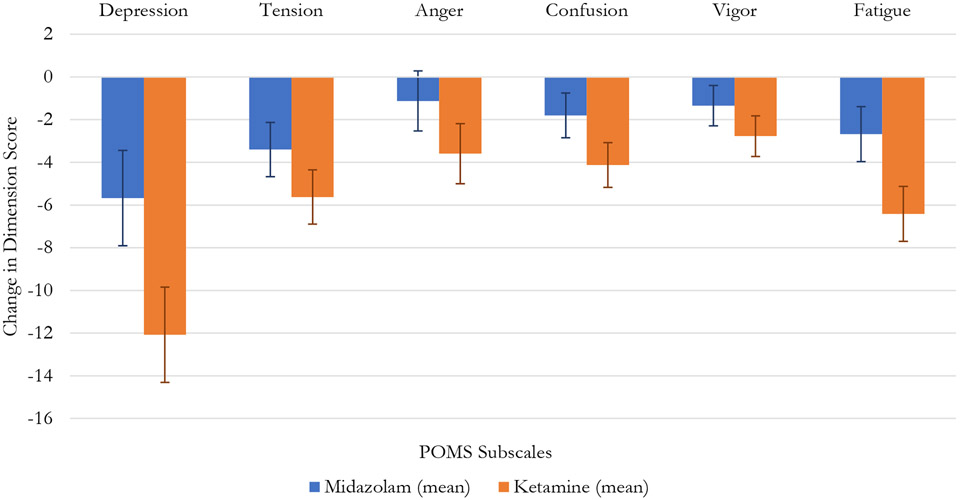
| POMS: Depression | 39.5 (12.8) | 33.9 (17.7) | 35.6 (14.6) | 23.6 (16.1) | <0.001 | 0.023 | 0.046 |
| POMS: Tension | 20.2 (9.4) | 16.8 (10.5) | 19.2 (8.7) | 13.6 (9.4) | <0.001 | 0.273 | 0.219 |
| POMS: Anger | 10.3 (9.9) | 9.1 (11.5) | 10.1 (9.7) | 6.5 (8.6) | 0.020 | 0.482 | 0.219 |
| POMS: Confusion | 15.7 (5.5) | 13.9 (6.4) | 15.4 (5.3) | 11.3 (7.2) | <0.001 | 0.216 | 0.122 |
| POMS: Vigor | 3.5 (3.4) | 27.2 (5.6) | 2.9 (2.5) | 26.3 (6.0) | 0.003 | 0.836 | 0.290 |
| POMS: Fatigue | 19.4 (6.3) | 16.7 (8.2) | 18.5 (6.5) | 12.1 (7.6) | <0.001 | 0.048 | 0.043 |
SSI = Beck Scale for Suicidal Ideation; MDD = Major Depressive Disorder; HDRS-24 = 24 item Hamilton Depression Rating Scale; BDI = Beck Depression inventory; POMS = Profile of Mood Stat
In the omnibus analysis of the three BDI factor scores, there was a significant Factor Score x Drug x Time interaction (F[4, 312]=4.42, p=.002). Changes in these scores across treatment are illustrated in Figure 2. In univariate comparisons, the Drug x Time effect, showing superiority for ketamine, was significant for the BDI Subjective Depression factor score (F[1, 72]=4.44, p=.039), but not the other factors (Table 2.; Figure 2.).
Fig. 2.
Change in Beck Depression Index Factors
In the omnibus analysis of the six POMS subscales, there was a marginally significant Drug x Time interaction across all subscale scores ((F[1, 78]=3.51, p=.051), with a non-significant Factor Score x Drug x Time interaction (F[5, 390]=1.62, p=.153), indicating a trend toward a global effect across all subscales. Changes in these subscales across treatment are illustrated in Figure 3. In univariate comparisons, a Drug x Time effect, illustrating a superiority for ketamine, was significant for the POMS Depression subscale (F[1, 78]=4.10 p=.046) and the POMS Fatigue subscale (F[1, 78]=4.24 p=.043) (Table 2.; Figure 3).
Fig. 3.
Change in Profile of Mood States Subscale Scores.
Correlations with Change in Suicidal Ideation
Across all participants, irrespective of drug group, change in SSI was significantly correlated with change in all depression factor/subscales except for HDRS Disturbed thinking (p = .90). Effect sizes were largest for HDRS Psychic Depression (r = .62, p < .001), BDI Subjective Depression (r = .61, p < .001), and POMS Depression (r = .61, p < .001).
Within the ketamine-treated group, the strongest correlations of change in SSI were with (in descending order of effect size): POMS Vigor (r = −.69, p < .001; scale is reverse scored from others), POMS Confusion (r = .63, p < .001) and POMS Depression (r = .62, p < .001), BDI Subjective Depression (r = .61, p < .001), HDRS Loss of Motivation (r = .55, p < .001) and HDRS Psychic Depression (r = .54, p < .001). Correlations were significant with all factor/subscales except HDRS Disturbed Thinking (p =.31) and Sleep Disturbance (p = .09).
We explored two other possible contributions to the decline in suicidal ideation: concurrent medications and neuropsychological changes. Neither the presence/absence of concomitant medications, their number, nor specific class of coadministered medication (antidepressants, lithium, benzodiazepines, or antipsychotics) contributed additional variance to our prediction of SSI decline. Two neuropsychological tasks, Choice Reaction Time and Stroop Interference, (in the same sample, reported in (Keilp et al., 2021)) showed relative improvement in ketamine vs. midazolam treated participants. However, as additional factors in stepwise regression, neither contributed to prediction of SSI decline.
Stepwise Regression of Change in Suicidal Ideation
A stepwise regression model with all participants, including all significant factor/subscale scores and drug assignment, found three predictors accounting for 51.6% of the variance in reduction of SSI score after blinded infusion (final model: F[3,76] = 26.99, p < .001). These were change in HDRS Psychic Depression (β = .34, t = 3.34, p = .001), change in POMS Depression (β = .41, t = 4.17, p < .001) and change in HDRS Anxiety (β = .20, t = 2.39, p = .019).
In the ketamine group, a similar model was extracted (final model: F[2,37] = 15.35, p < .001) accounting for 45.4% of the variance in the reduction in suicidal ideation. This model included two variables, change in HDRS Psychic Depression (β = .30, t = 2.10, p = .002) and POMS Depression (β = .47, t = 3.33, p = .002). Change in HDRS Anxiety fell short of significance in this model (t = 1.76, p = .087).
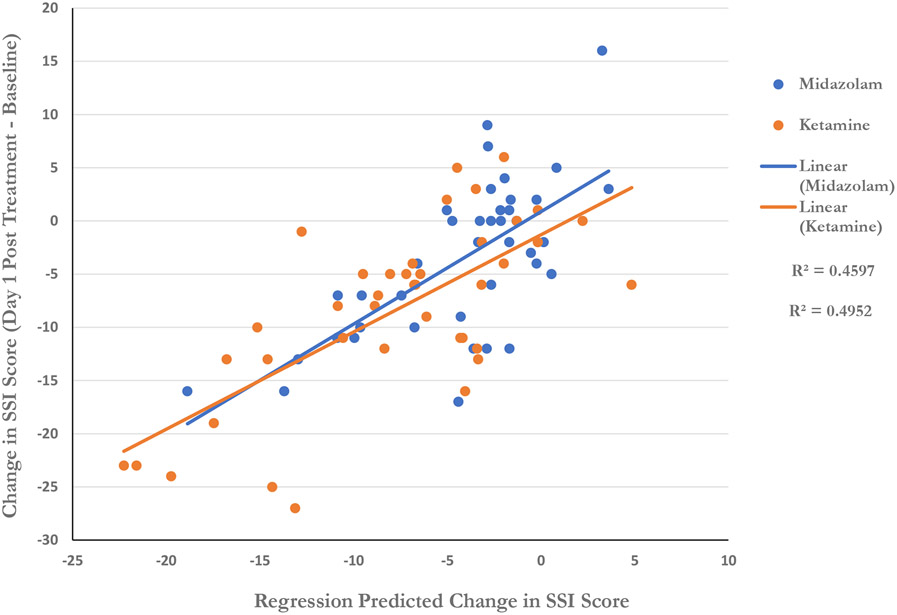
Using the final model derived in both drug groups, including all participants in the study, predicted scores versus actual change in SSI was calculated and plotted in Figure 4. Separate regression lines were plotted to depict the relationship for each drug. Regression lines overlap significantly, suggesting a common mechanism for reducing suicidal thinking across both drugs.
Fig. 4.
Regression Prediction of Change in Suicidal Ideation
Regression prediction based on linear combination of change in HDRS Psychic Depression, HDRS Anxiety, and POMS Depression scores (see text for details).
We repeated the regression analysis in the ketamine group using only those change scores that were clearly significant hierarchically i.e. HDRS Psychic Depression and BDI Subjective Depression (POMS scales would not be included because for the omnibus effect across all subscales p = .051). An equation including change in HDRS Psychic Depression (β = .35, t = 2.49, p = .018) and BDI Subjective Depression (β = .41, t = 2.88, p = .007 ) was significant (final model: F[2,39] = 13.38, p < .001) and predicted 42.0% of variance in the reduction of SSI score in the ketamine group.
Discussion
One day after treatment, ketamine exhibited superiority to midazolam for reduction of suicidal ideation primarily by its stronger impact on core mood disturbance (i.e. HDRS Psychic Depression, BDI Subjective Depression, and POMS Depression) with little apparent contribution of change in somatic symptoms except possibly for fatigue/vigor. Changes in core mood ratings across both drugs exhibited the strongest correlations to changes in suicidal thinking. A stepwise linear regression revealed that a linear combination of changes in HDRS Psychic Depression, POMS Depression, and HDRS Anxiety accounted for 51.6% of the variance in SSI change with treatment, regardless of drug assignment. A comparable result was observed in the ketamine group alone.
Consistent with our prior studies of other antidepressants, changes in mood and anxiety components of standard, multi-dimensional depression scales were most strongly related to decline in suicidal ideation. It is noteworthy that after considering these robust associations, there is an additional 50% of decline in suicidal thinking that is unexplained. This may partly be related to error variance within each scale (i.e. none is a “perfect” measure) or to idiosyncratic factors at the individual level that are difficult to capture in any set of rating scales.
Another domain which may help further elucidate the anti-SI effects of ketamine is its relation to anhedonia (Ballard et al., 2017). Nascent research suggests that reduction in SI following ketamine treatment may be independently associated with lessened anhedonia as reflected in specialized measures (Lally et al, 2015). Future work may capture additional symptomatology related to suicidal thinking by probing domains that standard depression scales do not target very well, such as: psychic pain; feelings of burdensomeness, entrapment; or rumination (Shneidman, 1993; De Beurs et al., 2019).
Our earlier work indicates that suicidal ideation is most strongly correlated with the core mood components of depression (Keilp et al., 2012), and suicidal ideation is reduced in concert with clinical improvement produced by oral antidepressant medications in these symptoms (Grunebaum et al., 2005; Keilp et al., 2018a). Results here extend this association to ketamine treatment and reinforce the importance of improving mood as a means of reducing suicidal ideation, in the context of any antidepressant treatment. The speed with which ketamine acts to improve mood symptoms appears to be key to its rapid effects on suicidal ideation. This contrasts with the actions of many antidepressant treatments which initially improve neurovegetative symptoms such as sleep, appetite, and energy (Machado-Vieira et al., 2010) while improving mood more slowly. Mood effects may only emerge weeks into treatment, even after the initiation of multiple treatments (Belmaker and Agam, 2008). In addition, whereas midazolam’s mood effects appeared to wane after Day 1 post-infusion, ketamine’s effects appeared to last longer (Grunebaum et al., 2018).
It is noteworthy that reduction in SSI score in the ketamine group was most strongly correlated with increase in POMS Vigor score, although change in this score did not differentiate between ketamine and midazolam. However, reduction in the POMS Fatigue score did differentiate between drugs, with ketamine superior. We have recently reported that Choice Reaction Time task performance improved to a greater degree in response to ketamine relative to midazolam (Keilp et al., 2021). Taken together, these findings suggest that improved energy is another feature of the ketamine response – a characteristic that should be studied further, particularly given the fatigue-inducing effects of antidepressant drugs for many patients.
Current antidepressant medications are thought to relieve depression via modulation of brain monoamines, but this mechanism of action may be less optimal for addressing acute suicidal states. Ketamine’s rapid effects on glutamatergic and GABAergic systems may be more effective for this clinical need (Abdallah et al., 2015; Duman, 2014; Milak et al., 2020; Manji et al., 2001). Research into specific effects of ketamine and its metabolites including preferential localization to certain neural circuits is in its nascency (Abdallah et al., 2017; Mann et al., 2013). Investigation into regional neuroanatomical action of ketamine and induction of down-stream neurotransmission is a promising area for future research (Milak et al., 2016; Milak et al., 2020).
Our group has published other analyses using data from this trial relevant to in vivo ketamine metabolites and potential interactions of ketamine with opioid receptors (Grunebaum et al., 2019; Grunebaum et al., 2020), and a limitation of this study is its post-hoc approach. A medication washout was not required prior to the study intervention, which may have led to interactions with assigned treatments that may have altered treatment effects – however, there was no difference in frequencies of major classes of concomitant medications at baseline between the two groups (Grunebaum et al., 2018).
These results reinforce the connection between core mood disturbance and suicidal thinking, and the necessity to rapidly improve mood as a means of treating acutely suicidal patients. The findings suggest that ketamine’s relative effectiveness in the treatment of acute suicidal states is due to its rapid effects on these mood-related components of depression, and perhaps should be considered a promising treatment for acute suicide risk. A new generation of mood and vigor enhancing antidepressant agents capitalizing on elucidation of ketamine’s mechanism and improving on its roughly one-week duration of effect, would truly be a sea-change for depression patients.
Highlights.
Ketamine reduces SI in concert with MDD symptoms; primarily mood symptoms (73/85)
Improvements in mood correlate with improvements in SI in ketamine and midazolam (81/85)
Reduction in fatigue and improvement in vigor may be related to lessened SI (76/85)
*SI: Suicidal Ideation; MDD: Major Depressive Disorder
Acknowledgements
The authors thank all participants who volunteered their time and trust; the New York State Psychiatric Institute 5-South unit staff; the Institutional Review Board; the Data and Safety Monitoring Board; the Biological Studies Unit; and, Manny de la Nuez for essential contributions.
Funding/ Support
Secondary analysis of trial data funded by NIMH grant R01 MH-096784 to Dr. Grunebaum.
Footnotes
Conflict of Interest
Drs. Mann and Burke receive royalties for the commercial use of the Columbia Suicide Severity Rating Scale which was not part of this analysis. The other authors report no financial or other relationships pertinent to the subject of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Annabella Hochschild, Sackler School of Medicine, Tel Aviv University.
John G. Keilp, Molecular Imaging and Neuropathology Division, New York State Psychiatric Institute, and Columbia University Irving Medical Center, New York, NY.
Sean P. Madden, Molecular Imaging and Neuropathology Division, New York State Psychiatric Institute, and Columbia University Irving Medical Center, New York, NY.
Ainsley K. Burke, Molecular Imaging and Neuropathology Division, New York State Psychiatric Institute, and Columbia University Irving Medical Center, New York, NY.
J. John Mann, Molecular Imaging and Neuropathology Division, New York State Psychiatric Institute, and Columbia University Irving Medical Center, New York, NY..
Michael F. Grunebaum, Molecular Imaging and Neuropathology Division, New York State Psychiatric Institute, and Columbia University Irving Medical Center, New York, NY.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH, 2015. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66:509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, et al. , 2017. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology 42:1210–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Jurdi RK, Swann A, Mathew SJ, 2015. Psychopharmacological Agents and Suicide Risk Reduction: Ketamine and Other Approaches. Curr Psychiatry Rep 17:81. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, Ameli R, Niciu MJ, Brutsche NE, et al. , 2017. Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord 218:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Yarrington JS, Farmer CA, Richards E, Machado-Vieira R, Kadriu B, Niciu MJ, Yuan P, Park L, et al. , 2018. Characterizing the course of suicidal ideation response to ketamine. J Affect Disord 241:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G, 2017. Ketamine as a rapid-acting agent for suicidal ideation: A meta-analysis. Neurosci Biobehav Rev 77:232–36. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 47:343–52. [DOI] [PubMed] [Google Scholar]
- Beck AT, Lester D, Albert N, 1973. Suicidal wishes and symptoms of depression. Psychol Rep 33:770. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J, 1961. An inventory for measuring depression. Arch Gen Psychiatry 4:561–71. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G, 2008. Major depressive disorder. N Engl J Med 358:55–68. [DOI] [PubMed] [Google Scholar]
- De Beurs D, Fried EI, Wetherall K, Cleare S, DB OC, Ferguson E, O'Carroll RE, RC OC, 2019. Exploring the psychology of suicidal ideation: A theory driven network analysis. Behav Res Ther 120:103419. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM, 1998. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11:125–36. [DOI] [PubMed] [Google Scholar]
- Brown GK, Beck AT, Steer RA, Grisham JR, 2000. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol 68:371–7. [PubMed] [Google Scholar]
- Case A, Deaton A, 2020. Deaths of despair and the future of capitalism. Princeton University Press, Princeton. [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr., 2010. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, 2014. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety 31:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, 2018. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL., Gibbon M, Williams JBW., 1994. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York State Psychiatric Institute, Biometrics Research, New York. [Google Scholar]
- First MB, 1997. User's guide for the structured clinical interview for DSM-IV axis II personality disorders : SCID-II. American Psychiatric Press, Washington, DC. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–98. [DOI] [PubMed] [Google Scholar]
- Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, Hough D, Manji H, Drevets WC, et al. , 2020. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation With Intent: Double-Blind, Randomized Study (ASPIRE I). J Clin Psychiatry 81. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Brown CH, Hur K, Davis J, Mann JJ, 2012. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry 69:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glue P, Menkes DB, 2017. Ketamine and Suicidal Ideation: Direct Effect or Epiphenomenon? J Clin Psychopharmacol 37:282–83. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, et al. , 2018. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am J Psychiatry 175:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Parris MS, Burke AK, Suckow RF, Cooper TB, Mann JJ, 2019. Ketamine metabolite pilot study in a suicidal depression trial. J Psychiatr Res 117:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Liu J, Huang YY, Marcott S, Burke AK, Mann JJ, 2020. Opioid Receptor μ-1 and Ketamine Effects in a Suicidal Depression Trial: A Post Hoc Exploration. J Clin Psychopharmacol 40:420–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp J, Li S, Ellis SP, Burke AK, Oquendo MA, Mann JJ, 2005. Symptom components of standard depression scales and past suicidal behavior. J Affect Disord 87:73–82. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp JG, Ellis SP, Sudol K, Bauer N, Burke AK, Oquendo MA, Mann JJ, 2013. SSRI versus bupropion effects on symptom clusters in suicidal depression: post hoc analysis of a randomized clinical trial. J Clin Psychiatry 74:872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Curtin SC, Warner M, 2020. Increase in Suicide Mortality in the United States, 1999–2018. NCHS Data Brief:1–8. [PubMed] [Google Scholar]
- Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, Pavone KJ, Petrie SR, Dording C, et al. , 2019. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. J Affect Disord 243:516–24. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, Nyer M, Cassano P, Mischoulon D, et al. , 2016. Rapid and Sustained Reductions in Current Suicidal Ideation Following Repeated Doses of Intravenous Ketamine: Secondary Analysis of an Open-Label Study. J Clin Psychiatry 77:e719–25. [DOI] [PubMed] [Google Scholar]
- Joiner TEP, Jeremy W; Rudd M. David 2004. Is There a Window of Heightened Suicide Risk If Patients Gain Energy in the Context of Continued Depressive Symptoms? Prof Psychol Res PR 35:84–89. [Google Scholar]
- Keilp JG, Ellis SP, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, Grunebaum MF, 2018a. Suicidal ideation declines with improvement in the subjective symptoms of major depression. J Affect Disord 227:65–70. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Grunebaum MF, Gorlyn M, LeBlanc S, Burke AK, Galfalvy H, Oquendo MA, Mann JJ, 2012. Suicidal ideation and the subjective aspects of depression. J Affect Disord 140:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Madden SP, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, 2018b. The lack of meaningful association between depression severity measures and neurocognitive performance. J Affect Disord 241:164–72. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Madden SP, Marver JE, Frawley A, Burke AK, Herzallah MM, Gluck M, Mann JJ, Grunebaum MF, 2021. Effects of Ketamine Versus Midazolam on Neurocognition at 24 Hours in Depressed Patients With Suicidal Ideation. J Clin Psychiatry 82. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA Jr., 2015. Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol 29:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA, 2010. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals (Basel) 3:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick F, McCullumsmith CB, 2016. Ketamine for Treatment of Suicidal Ideation and Reduction of Risk for Suicidal Behavior. Curr Psychiatry Rep 18:61. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS, 2001. The cellular neurobiology of depression. Nat Med 7:541–7. [DOI] [PubMed] [Google Scholar]
- Mann JJ, 2003. Neurobiology of suicidal behaviour. Nat Rev Neurosci 4:819–28. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, Hegerl U, Lonnqvist J, Malone K, et al. , 2005. Suicide prevention strategies: a systematic review. JAMA 294:2064–74. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Roose SP, McGrath PJ, 2013. Clinical handbook for the management of mood disorders. Cambridge University Press, Cambridge. [Google Scholar]
- McNair D, Lorr M, Droppleman LF., 1981. Manual for the Profile of Mood States. Educational and Industrial Testing Service, San Diego. [Google Scholar]
- Milak MS, Keilp J, Parsey RV, Oquendo MA, Malone KM, Mann JJ, 2010. Regional brain metabolic correlates of self-reported depression severity contrasted with clinician ratings. J Affect Disord 126:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ, 2005. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry 62:397–408. [DOI] [PubMed] [Google Scholar]
- Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, Mao X, Rodriguez CI, Oquendo MA, et al. , 2016. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry 21:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Rashid R, Dong Z, Kegeles LS, Grunebaum MF, Ogden RT, Lin X, Mulhern ST, Suckow RF, et al. , 2020. Assessment of Relationship of Ketamine Dose With Magnetic Resonance Spectroscopy of Glx and GABA Responses in Adults With Major Depression: A Randomized Clinical Trial. JAMA Netw Open 3:e2013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, et al. , 2013. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak W, Grendas LN, Sanmarco LM, Estecho IG, Arena Á R, Eberhardt N, Rodante DE, Aoki MP, Daray FM, et al. , 2019. Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR. EBioMedicine 50:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J, Gorman DR., 1962. The brief psychiatric rating scale. Psychological Reports:779–812. [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, Soleimani L, Charney DS, Foulkes AL, et al. , 2014. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety 31:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar R, Fam J, Yeo EY, Dawe GS, 2015. Ketamine and suicidal ideation in depression: Jumping the gun? Pharmacol Res 99:23–35. [DOI] [PubMed] [Google Scholar]
- Reinstatler L, Youssef NA, 2015. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D 15:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association Council of Research Task Force on Novel, B., et al. , 2017. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry 74:399–405. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, DeBattista C, 2015. Manual of clinical psychopharmacology, Eighth edition. ed. American Psychiatric Publishing, a division of American Psychiatric Association, Washington, DC. [Google Scholar]
- Shneidman ES, 1993. Suicide as psychache. J Nerv Ment Dis 181:145–7. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, 2018. Antisuicidal Effects of Ketamine: A Promising First Step. Am J Psychiatry 175:97–98. [DOI] [PubMed] [Google Scholar]
- Woolf SH, Schoomaker H, 2019. Life Expectancy and Mortality Rates in the United States, 1959–2017. JAMA 322:1996–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, et al. , 2012. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]