Abstract
Behavioral interventions to improve cognitive function in older adults are widespread and can vary from theater classes to cognitive training programs. However, the effectiveness in maintaining different cognitive domains varies greatly both across and within intervention types. To date, no systematic reviews have synthesized findings across more than a few types of interventions (e.g., cognitive vs. exercise). This systematic review examined 11 types of behavioral interventions and the respective transfer to 19 cognitive domains, as well as transfer to everyday function. Study inclusion criteria were: peer-reviewed articles in English, samples of healthy adults aged 65 and older, and randomized controlled trials of behavioral interventions with reported cognitive outcomes. The 2017 search yielded 75 eligible articles comprising cognitive training, exercise training, combination interventions, cognitively-stimulating activities, and action video games. In general, process- (n = 26) and strategy-based (n = 16) cognitive training improved the trained domains but had weak transfer to non-trained domains. Aerobic training (n = 13) most consistently improved executive function, and strength/resistance (n = 8) and aerobic/resistance combination training (n = 6) most consistently improved cognitive inhibition and visual working memory. Combination interventions (n = 15 nonfactorial, n = 3 factorial) showed promise in improving verbal delayed recall and executive function. Few studies examined cognitively-stimulating activities or action video games, leaving inconclusive results about their effect on cognitive function. Few studies examined everyday function (n = 9), however, process- and strategy-based training demonstrated notable long-term transfer. Recommendations for future research and practice are highlighted.
Keywords: Randomized controlled trial, Healthy older adults, Cognition, Cognitive training, Exercise training, Systematic review
1. Introduction
Increased life expectancy and decreased mortality rates have resulted in a substantial rise in the number of adults aged 65+ (Blazer et al., 2015; Ortman et al., 2014). Coupled with an increased proportion of older adults relative to working-age adults (Ortman et al., 2014), population aging contributes to an imbalance between demand for resources such as health and caregiving services, and the available supply of financial, workforce, and social supports to provide these resources. Cognitive impairment is a leading cause of disability in older adults, with estimated costs of over $226 billion in 2015 (Andrews et al., 2017). As impairments in fluid cognitive abilities, or abilities involving adaptation or integration of novel information (Cattell, 1963), are associated with future cognitive dysfunction (Pandya et al., 2017) and loss of independence (Blazer et al., 2015; Hsu et al., 2017; Lau et al., 2015; Tomaszewski Farias et al., 2018), preserving fluid abilities may reduce cognitive impairment-related disability and the burdens associated with disability. This systematic review examines the efficacy of a breadth of behavioral interventions on cognitive function in healthy older adults.
Currently, there are myriad programs promoting activities to help cognition with varying levels of empirical support (Laditka et al., 2012). Accumulating evidence suggests older adults and physicians alike consistently identify three major activities as important for maintaining cognitive function: social, cognitive, and physical activity (Friedman et al., 2013, 2015; Kim et al., 2015). For this review, we refined and classified these activities into five broad areas: (1) cognitively-stimulating activities, (2) cognitive training, (3) action video games, (4) exercise training, and (5) combination interventions.
1.1. Cognitively-stimulating activities, cognitive training, and action video games
Cognitively-stimulating activities capitalize on existing cognitive processes and target general intellectual stimulation most commonly through engagement in leisure activities (Park et al., 2007; Stine-Morrow et al., 2014). Activities range broadly and include photography (Park et al., 2014), crossword puzzles or other games (Verghese et al., 2003), reading (Verghese et al., 2003), fine and performing art-based activities such as playing musical instruments and acting (Verghese et al., 2003), activities thought to increase cognitive reserve such as education (Stern, 2002, 2003, 2013), and stress-reduction activities (e.g., mindfulness) that incorporate cognitive elements such as attention regulation (Malinowski and Shalamanova, 2017). A large body of observational retrospective work demonstrates that more engagement in cognitively-stimulating activities is associated with better cognitive function in later life (Barnes et al., 2006; Fountain-Zaragoza and Prakash, 2017; Gard et al., 2014; Mitchell et al., 2012; Verghese et al., 2003; Wayne et al., 2014; Wilson et al., 2003). The observational link between engagement in cognitively-stimulating activities and better cognitive function has implications for interventions incorporating these activities (Stern, 2013). Although there is a scarcity of experimental studies involving cognitively-stimulating activities, emerging evidence suggests that some leisure activities such as volunteering or photography that incorporate social, physical, and cognitive elements can improve memory and reasoning (Carlson et al., 2008; Park et al., 2014; Stine-Morrow et al., 2014).
Cognitive training is a specific type of cognitive intervention designed to improve a targeted cognitive domain, usually through repeated practice of cognitive tasks (Lampit et al., 2015b; Mowszowski et al., 2016; Reijnders et al., 2013; Shah et al., 2017). There are two major cognitive training types: process-based, which train cognitive domains through adaptive practice of specific fluid tasks, and strategy-based, which focus on teaching and practicing strategies, frequently mnemonic devices for memory (Lustig et al., 2009). Generally, process-based and strategy-based cognitive training improve the targeted ability (e.g., processing speed, attention, semantic memory, and reasoning; Ball et al., 2002; Lustig et al., 2009), as well as everyday function (Edwards et al., 2018; Lustig et al., 2009; Mowszowski et al., 2016; Ross et al., 2016).
After the success of cognitive training interventions on improving and maintaining cognitive function (e.g., Rebok et al., 2014; Willis et al., 2006), there has been a surge in both commercializing empirically-valid cognitive training programs (e.g., Brain HQ, Posit Science; Shah et al., 2017) and examining cognitive benefits of already-available commercial games not explicitly designed to improve cognitive function (e.g., 3D immersive virtual reality games; García-Betances et al., 2018; Shah et al., 2017). Such commercially-available games, called action video games or serious games, may impact cognition through improving discrimination, attention, and speed of processing skills (Dye et al., 2009; Toril et al., 2014; Zelinski and Reyes, 2009). Observational research demonstrates a relationship between action video game engagement and better speed of processing and reasoning (Bediou et al., 2018), and randomized controlled trials (RCTs) suggest such games may improve cognitive function (Bediou et al., 2018).
1.2. Exercise and exergames
Exercise is related to better cognitive function in healthy older adults (Barnes and Yaffe, 2011; Bherer et al., 2013; Colcombe and Kramer, 2003; Kirk-Sanchez and McGough, 2014) and, along with cognitive training and blood pressure management, is believed to be one of the most promising avenues for dementia prevention (National Academies of Sciences, Engineering, & Medicine, 2017). Observational studies found associations between better cognition and both lifetime (Gill et al., 2015) and late-life exercise engagement (Hamer et al., 2013), suggesting that previously-inactive older adults may benefit from exercise adoption. However, causality cannot be assumed in this observational work as higher-functioning adults may be more likely to adopt exercise behaviors due to ability or health literacy. Numerous experimental studies have demonstrated that exercise interventions improve cognitive abilities (Kelly et al., 2014b), but benefits have been more consistent for aerobic training, or combined cardiovascular fitness and resistance training (Colcombe and Kramer, 2003). Exercise most consistently improves executive function (Colcombe and Kramer, 2003; Kelly et al., 2014b); transfer effects to other cognitive domains have been inconsistent (Kelly et al., 2014b).
Despite widespread knowledge about the benefits of exercise, few older adults report engaging in such activities (Kruger et al., 2007). To address this, research has shifted to identifying enjoyable, accessible activities that benefit older adults. To that end, there is increasing interest in exergames, or video games that include movement such as the Xbox and Wii that incorporate cognitively-demanding activities but are not explicitly designed to improve cognitive performance (Bediou et al., 2018). A bout of exercise during exergaming can achieve similar energy expenditure as other aerobic activities (Sween et al., 2014), suggesting that exergames may confer benefits that are distinct from action video games. A small but growing literature suggests older adults may receive cognitive benefit from exergame engagement (Monteiro-Junior et al., 2016).
1.3. Combination interventions
In an effort to save time and resources or determine which combination of activities is most beneficial for cognition, combination interventions are becoming increasingly popular. The rationale for combination interventions varies, but the underlying assumption, supported by retrospective observational work (Wang et al., 2013), is that engagement in a variety of healthful behaviors has additive benefits. Typically, combination interventions combine cognitive and exercise training because they have the strongest evidence of improving or maintaining cognitive function in older adults (National Academies of Sciences, 2017). Interventions combining cognitive and exercise training suggest transfer to cognitive function in older adults (He et al., 2018; Rahe et al., 2015), but others caution that the effects are not greater compared to pure cognitive training (Zhu et al., 2016). In this review, two combination interventions will be examined (adapted from Collins, 2018): nonfactorial, or interventions that incorporate multiple activities without evaluating each activity separately (e.g., dual-task training, exergames), and factorial, or interventions that include both combination and single-activity training (e.g., a combination physical activity-cognitive training with separate physical activity and cognitive training arms as well).
1.4. Transfer to everyday function
Another major issue in behavioral interventions for older adults is the question of whether intervention gains transfer to everyday function. Everyday function is commonly assessed through self-report or direct observation of simulated activities individuals do in their everyday life. Several authors have called for research on the transfer of behavioral interventions to everyday function (Greenwood and Parasuraman, 2016; Kelly et al., 2014a), but systematic reviews typically overlook intervention transfer to everyday function. To our knowledge, only one meta-analysis examining Useful Field of View training included everyday function outcomes (UFOV; Edwards et al., 2018).
1.5. Aims
To date, several systematic reviews and meta-analyses examined the effect of specific behavioral interventions on cognitive function in older adults (e.g., Kelly et al., 2014a, 2014b; Noice et al., 2013). However, to our knowledge, there are no studies that synthesize findings across a wide breadth of behavioral interventions. We address this gap through an examination of cognitively-stimulating activities, cognitive training, action video games, exercise training, and combination interventions. Importantly, this review will emphasize comparisons between interventions, but will also consider within-intervention comparisons, addressing a limitation in the literature. Within- and between-intervention comparisons are critical as there may be some intervention types with similar strengths across trials of the same type (e.g., appropriate active control groups), or with systematic weaknesses across trials (e.g., inadequate power). Additionally, identifying which interventions are best at improving particular domains would better inform which combinations of activities may be most potent for maintaining cognitive function in older adults with specific impairments. Intervention details hypothesized to affect transfer, such as duration, adaptation (i.e., increased difficulty to adjust for training gains), and follow-up period (Kelly et al., 2014a) will be considered.
This systematic review critically evaluated the transfer of 11 types of behavioral interventions on cognitive outcomes in healthy older adults. Specifically, the aims of this systematic review were to (1) summarize similarities and differences between cognitive intervention types and their outcomes, (2) identify the behavioral intervention type (s) that confer benefits across the most cognitive domains, and (3) highlight cross-intervention limitations that should be addressed in future behavioral RCTs. An exploratory aim was to examine how many studies included everyday function and which interventions, if any, conferred such benefits. In order to narrow the scope of this review, the following behavioral interventions were examined: cognitively-stimulating activities (education, art, and stress-reduction interventions), cognitive (process- and strategy-based) training, action video games, exercise (aerobic, strength/resistance, or aerobic/resistance combination), and combination (nonfactorial and factorial) interventions.
2. Methods
2.1. Search strategy and selection criteria
PubMed, PsycINFO, Cochrane Central Register of Controlled Trials, International Clinical Trials Registry, and United States National Institutes of Health databases were used to identify relevant articles published before May 15, 2017. Searches were conducted from May 15 to June 2, 2017. For each search, three search terms were included to represent (1) type of cognitive intervention, (2) cognitive outcomes, and (3) healthy older population. Wildcard searches (denoted by *) were used when appropriate. Search terms were compiled by all co-authors based on search terms from previous systematic reviews and meta-analyses and keywords from relevant empirical articles. The search term for behavioral interventions included two components: either cognit*, brain, exercise, activity, fitness, educ, exergam*, video gam*, computer, mental stimulat*, cognit* stimulat*, social, mindful*, yoga, relaxation, meditation, memory, executive function, processing, attention, dual task, Wii, Nintendo, Xbox, Kinect, serious (i.e., serious games), computer, aerobic, strength, flexibility, resistance, balance, or dance; and a second component of either “train*” or “intervention.” Each intervention term was combined with an outcome term, “cognit*,” which was expected to include objective and subjective cognitive outcomes. Finally, the population term, “healthy older adults,” was used to include community-dwelling healthy older adults without a dementia diagnosis. In total, 68 sets of search terms were used. Search results were filtered by human subjects, written in English, and participants 65 and older. Additionally, meta-analysis and systematic review papers (n = 43 articles) were cross-referenced to manually search relevant empirical articles not recovered with the search engines. See Appendix A for the full list of reviews used for cross-reference. Articles included in the systematic review are denoted by an asterisk in the References list.
The following inclusion criteria were used to identify eligible studies: (1) peer-reviewed, (2) an RCT with reported posttest results (i.e., not a protocol paper), (3) included at least one cognitive outcome, (4) behavioral (i.e., not pharmaceutical), and (5) healthy and community-dwelling older adults 65 years of age and older (i.e., no clinical diagnosis of mild cognitive impairment or other clinical-based population). Multiple papers based on the same study sample were included. For example, if several manuscripts using data from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial came up in the search results, they were all included for full evaluation (Ball et al., 2002; Rebok et al., 2014; Willis et al., 2006). Four reviewers (BNS, SAF, CEW, and JH) independently reviewed articles in-depth and extracted data independently based on the inclusion criteria above. Each article was cross-checked by at least one additional reviewer, and any discrepancies between the two were discussed by additional reviewers (CBP, LAR) to achieve consensus.
2.2. Quality assessment
Methodological quality was assessed using a 10-item checklist modified from the 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group (Furlan et al., 2009) and PEDro scale (Maher et al., 2003). The final items assessed: adequate randomization, concealed treatment allocation, sufficient intervention details, tester blinding to randomization, similar dropout rates across training groups, intention-to-treat analysis, acceptable compliance, similar assessment timing across groups, and freedom from reporting bias (Appendix B). If information was omitted from the article, reviewers referred to protocol papers and ClinicalTrials.gov. Each item was scored either 1 (No), 2 (Unclear), or 3 (Yes), with a possible quality control range of 10–30.
2.3. Operationalization of terms and domain organization
We a priori identified four types of control groups: no-contact controls, social controls, active controls, and active + social controls. No-contact controls were controls in which there was no contact between researchers and participants between assessments and included waitlist controls. Social controls involved the same amount of interaction between participants and study staff but no reasonable expectation of the activity improving cognitive function. For example, if the intervention was a group-based education class of five participants that met once per week for six weeks (60 min/session), the social control would also need to have the same amount of participant-to-participant and participant-to-staff interaction quality conducted in groups of five participants once per week for six weeks (60 min/session). That is, the duration and quality of social interaction is equivalent; however, there is uncertainty or no (reasonable) expectancy of cognitive or outcome benefits. Active controls were defined as conditions in which all components but the critical “active” component were present; that is, there was a reasonable belief among the control group that activities would confer cognitive benefits (Boot et al., 2013; Rebok, 2016; Wu et al., 2017). For example, if the intervention was a group-based, trainer-led cognitive training that lasted for six 1-h sessions across six weeks, an active control could be 12 h of at-home DVD lectures. That is, there is a reasonable expectation that education lectures could confer cognitive benefits, but the social interactions with staff are not equivalent. Active + social controls match both social interactions with staff and had reasonable expectations that the activities would confer benefits. For example, if participants in the intervention were assigned to 10 h of group-based, trainer-led aerobic training, the active + social controls would be assigned to 10 h of group-based, trainer-led toning and stretching.
Since studies operationalized cognitive domains differently (e.g., Trailmaking Task B as executive function, working memory, or divided attention), we organized all cognitive tasks into the following domains: (1) cognitive speed, (2) (psycho)motor function, (3) verbal attention, (4) visual attention, (5) multimodal (i.e., both verbal and visual) attention, (6) cognitive inhibition, (7) verbal immediate recall (or short-term memory), (8) visual immediate recall (or short-term memory) (9) multimodal immediate recall, (10) verbal working memory, (11) visual working memory, (12) verbal delayed recall, (13) visual delayed recall, (14) multimodal delayed recall, or (15) executive function. The domains were chosen based on a modified version of the structure presented by Angevaren et al. (2008). If a cognitive task or domain did not fit into the domains presented in that review, the authors (BNS, SAF, CEW, and JH) reviewed additional sources (Lezak et al., 2004) and met with additional reviewers (CBP, LAR) to reach a consensus on the most appropriate domain. Furthermore, results were organized from simple to more complex domains (i.e., from lowest to highest level of cognitive function). All cognitive measures organized by domain, as well as domain definitions, are in Appendix C. Definitions were modified from existing neuropsychological references (Lezak et al., 2004). Additional cognitive domains that did not fit the above hierarchy included (16) subjective cognition (17) dementia status, (18) verbal perception, and (19) visual perception. Unless otherwise stated, all cognitive measures were objective assessments. In our exploratory analysis, everyday function was defined as self-reported or observation-based tasks simulating activities completed in everyday life such as grocery shopping, transportation, or bathing.
3. Results
3.1. Search strategies and study selection
An initial data search yielded 6397 articles from five search engines: Pubmed (n = 1457 articles), PsycINFO (n = 3070 articles), Cochrane Central Register of Controlled Trials (n = 1862 articles), the International Clinical Trials Registry Platform (n = 8 articles), and the US National Institutes of Health database (n = 0 articles). Cross-referencing from systematic reviews or meta-analyses (n = 43 articles) identified an additional 86 articles. Across both search strategies, a total of 6483 articles were screened, and 3756 articles were excluded based on title and abstract. The remaining 2727 articles were screened in full. Of those, 2652 were excluded. Excluded studies were: not peer-reviewed (n = 1), a protocol paper with no reported results (n = 16), not an RCT (n = 44), not reporting cognitive outcome measures (n = 26), not behavioral interventions with posttest cognitive measures (n = 9), not age-eligible or did not include community-dwelling older adults (n = 50), or a systematic review or meta-analysis and pulled for cross-check purposes only (n = 58). Duplicated articles were also excluded (n = 2448), leaving 75 unique articles for inclusion in the current review (Fig. 1).
Fig. 1.

Selection process of the systematic review.
Of the 75 articles, several had multiple intervention groups for different categories of intervention; those articles were included under multiple intervention categories. For example, if a study had a cognitive intervention group and a separate exercise intervention group, the study would be reported twice: once in the cognitive category and once in the corresponding exercise (i.e., aerobic, strength/resistance, or aerobic/resistance combination) category. The total number of interventions for each category was: education (n = 3), fine and performing arts (henceforth “theatre” since only theatre interventions emerged from the search; n = 2), stress-reduction training (n = 2), process-based cognitive training (n = 26), strategy-based cognitive training (n = 16), action video games (n = 3), aerobic exercise (n = 13), strength/resistance (n = 8), combination aerobic/strength (n = 6), and combination (nonfactorial n = 15; factorial n = 3).
3.2. Study characteristics
Of the 75 studies, 16 included multiple intervention groups (Ball et al., 2002; Barnes et al., 2013; Belchior et al., 2013; Cheng et al., 2012; Gajewski and Falkenstein, 2012; Goghari and Lawlor-Savage, 2017; Klusmann et al., 2010, 2011; Legault et al., 2011; Moul et al., 1995; Panton et al., 1990; Rebok et al., 2014; Shatil, 2013; Stigsdotter and Backman, 1989; Stigsdotter-Neely and Backman, 1993; Willis et al., 2006). Cognitive (43%, 42/97) and exercise (28%, 27/97) training comprised most of the interventions.
Generally, there were only a few notable between-intervention differences. First, exercise training interventions were generally of longer duration compared to other interventions (see Table 1). For example, aerobic training lasted an average of 21.61 weeks compared to approximately eight weeks for process-based cognitive training. Similarly, the total number of intervention minutes was substantially longer for exercise vs. other intervention types, with the exception of education and factorial interventions; factorial interventions likely lasted longer because they tended to include exercise components. For example, aerobic and strength combination training lasted an average of over 77 h (4668 min) compared to over 16 h (968 min) for process-based cognitive training. In general, most interventions did not adequately describe their adaptive training approach. In strategy-based cognitive, action video game, and nonfactorial training, fewer than half of the studies adequately described their adaptation strategy. Critically, no education, aerobic and strength combination, mindfulness, or theatre interventions adequately described their adaptation procedure, if any, over the intervention period (Table 1). Although several studies acknowledged adaptation over the intervention period, aspects such as when or at what threshold difficulty was increased were insufficiently described.
Table 1.
Intervention Characteristics.
| Study | Intervention description | Dosage | Duration (Tot. Min.) | Adapted | Setting (G = Group; I = Ind.) | Delivery (T = Trainer-Led, I = Ind.-Led) | Control group | QCS |
|---|---|---|---|---|---|---|---|---|
| Cognitively-stimulating activities: education interventions | ||||||||
| de Medeiros et al. (2011) | Structured autobiographical writing course | 1×/week (90 min each) | 8 weeks (720 min) | ? | G | T | NC; AS | 26 |
| Klusmann et al. (2010) | Computer course taught how to operate computer, writing, playing, calculating, surfing internet, email, drawing, etc. | 3×/week (90 min each; 75 sessions total) | 26 weeks (6750 min) | N | G | T | NC | 26 |
| Klusmann et al. (2011) | Computer course taught how to operate computer, writing, playing, calculating, surfing internet, email, drawing, etc. | 3×/week (90 min each; 75 sessions total) | 26 weeks (6750 min) | N | G | T | NC | 26 |
| Cognitively-stimulating activities: theatre interventions | ||||||||
| Noice and Noice (2009) | Theatre | 2×/week (60 min each) | 4 weeks (480 min) | YN | G | T | NC; AS | 23 |
| Noice and Noice (2013) | Theatre; 2 experiments presented in paper | 2×/week (60 min each) | 4 weeks (480 min) | YN | G | T | NC | 22 |
| Cognitively-stimulating activities: stress-reduction training | ||||||||
| Galvin et al. (2006) | Relaxation response (meditation, imagery and relaxation techniques) | 1 90 min session + at-home practice: 7×/week (20 min each) | 5 weeks (450–1150 min) | ? | ? | T | NC | 22 |
| Oken et al. (2006) | Iyengar yoga (progressive relaxation, visualization, meditation) | 1×/week (90 min each) | 26 weeks (2340 min) | N | I | T | NC; AS | 26 |
| Process-based cognitive training | ||||||||
| Ball et al. (2002) | Speed of processing | 2×/week (60–75 min each; 10 sessions total; up to 4 booster sessions) | 5–6 weeks (600–750 min; up to 1050 min with booster) | YA | G | T | NC | 30 |
| Barnes et al. (2013) | Mental activity (Posit Science) | 3×/week (60 min each) | 12 weeks (2160 min) | YA | I | I | AS | 30 |
| Belchior et al. (2013) | UFOV | 6 sessions (90 min each) | 2–3 weeks (540 min) | YA | I | T | NC; AS | 24 |
| Borella et al. (2010) | Verbal working memory | 3 sessions (60 min each) | 2 weeks (180 min) | YA | I | T | AS | 24 |
| Borella et al. (2013) | Verbal working memory | 3 sessions (60 min each) | 2 weeks (180 min) | YA | I | T | AS | 25 |
| Carretti et al. (2013) | Verbal working memory | 3 sessions (50–70 min each) | 2 weeks (150–210 min) | YA | I | T | AS | 25 |
| Dahlin et al. (2008) | Updating task | 3×/week (45 min each) | 5 weeks (675 min) | YA | G | T | NC | 24 |
| Gajewski and Falkenstein (2012) | Mental activation | 2×/week (90 min each) | 16 weeks (2880 min) | YN | G | T | NC | 19 |
| Goghari and Lawlor-Savage, 2017 | Logic and planning | 5×/week (30 min each) | 8 weeks (1200 min) | YA | I | I | NC | 23 |
| Goghari and Lawlor-Savage, 2017 | Working memory | 5×/week (30 min each) | 8 weeks (1200 min) | YA | I | I | NC | 23 |
| Lampit et al. (2014) | COGPACK | 3×/week (30–45 min each) | 12 weeks (1080–1620 min) | YN | G | T | AS | 28 |
| Lee et al. (2012) | RehaCom program | 3×/week (30 min each) | 6 weeks (540 min) | YN | I | I | AS | 23 |
| Legault et al. (2011) | Computer-based memory intervention (word lists and recall) | 2×/week (40–48 min each for 2 months), then 1×/week (40–48 min each for 2 months) | 16 weeks (960–1152 min) | YA | G | I | AS | 23 |
| Millán-Calenti et al. (2015) | TeleCognitio | 2×/week, (20 min each) | 12 weeks (480 min) | YN | I | I | NC | 24 |
| Mozolic et al. (2011) | Modality-specific attention | 1×/week (60 min each) | 8 weeks (480 min) | YA | I | T | AS | 24 |
| Nouchi et al. (2012) | Brain Age game (Nintendo) | 5×/week (15 min each) | 4 weeks (300 min) | ? | I | I | AS | 25 |
| O’Brien et al. (2013) | Speed of processing | 2×/week (70 min each) | 10 weeks (1400 min) | YA | G | I | NC | 27 |
| Rebok et al. (2014) | Speed of processing | 2×/week (60–75 min each; 10 sessions total; up to 4 booster sessions) | 5–6 weeks (600–750 min; up to 1050 min with booster) | YA | G | T | NC | 30 |
| Shatil (2013) | CogniFit | 3×/week (40 min each) | 16 weeks (1920 min) | YN | G | I | AS | 23 |
| Smith et al. (2009) | Posit Science | 5×/week (60 min each) | 8 weeks (2400 min) | YA | I | I | AS | 30 |
| Stepankova et al. (2014) | Computerized verbal n-back task | low frequency group: 2×/week (20–30 min each); high frequency group: 4×/week (20–30 min each) |
5 weeks (low: 200–300 min; high: 400–600 min) | YA | I | I | NC | 25 |
| Stigsdotter and Backman (1989) | Problem solving, logical thinking, and visuospatial skills | 8 sessions (90 min each) | 8 weeks (720 min) | N | G | T | NC | 23 |
| Stigsdotter-Neely and Backman (1993) | Problem solving, logical thinking, and visuospatial skills | 8 sessions (90 min each) | 8 weeks (720 min) | N | G | T | NC | 20 |
| Willis et al. (2006) | Speed of processing | 2×/week (60–75 min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min; up to 1050 with booster) | YA | G | T | NC | 30 |
| Wolinsky et al. (2013) | Speed of processing | ?×/week (10 h total) | 6–8 weeks (600 min.) | YA | I | I | AS | 28 |
| Zelinski et al. (2011) | Computerized cognitive program (Brain Fitness Program, Posit Science) | 5×/week (60 min each) | 8 weeks (2400 min.) | YA | I | I | AS | 30 |
| Strategy-based cognitive training | ||||||||
| Ball et al. (2002) | Instruction and practice on memory strategies | 2×/week (60–75min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min; up to 1050 min with booster) | YN | G | T | NC | 30 |
| Ball et al. (2002) | Reasoning | 2×/week (60–75min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min; up to 1050 min with booster) | YN | G | T | NC | 30 |
| Best et al. (1992) | Practical instruction on use of external memory cues, with practice encouraged between sessions | 2×/week (45–60 min each) | 2 weeks (180–240 min) | N | G | T | NC; AS | 22 |
| Cao et al. (2016) | Presentation followed by practice; reasoning only | 2×/week (60 min each; 24 sessions total) | 12 weeks (1440 min) | N | G | T | AS | 28 |
| Cavallini et al. (2015) | Mnemonic devices | ?×/week; (? min each; 5 sessions total) | 3 weeks (?) | ? | I | I | AS | 24 |
| Cheng et al. (2012) | Presentation followed by practice; reasoning only | 2×/week (60 min each; 24 sessions total; up to 3 booster sessions) | 12 weeks (1440 min; up to 1620 with booster) | N | G | T | NC | 30 |
| Craik et al. (2007) | Learning and practice of memory strategies/ techniques, with homework | ?×/week (? min each) | 12 weeks (? min) | N | G | T | NC | 23 |
| Dawson et al. (2014) | Instruction period followed by practice of meta-cognitive strategy | x?/week (60 min each; 3 group sessions and 9 individual sessions) | 8 weeks (1020 min) | N | G;I | T | AS | 26 |
| McDougall et al. (2010) | Progressive muscle relaxation followed by a discussion of homework; lecture about memory component followed by practice of memory strategies | ?×/week (60 min each; 8 sessions total; up to 4 90 min booster sessions) | 8 weeks (480 min; up to 840 min with booster) | N | G | T | AS | 26 |
| Miller et al. (2013) | Brain Fitness (Dakim Inc.) | 5×/week (20–25 min each; 40 sessions total) | 8 weeks (800–1000 min) | YN | I | I | NC | 25 |
| Nouchi et al. (2016) | Arithmetic and language problem solving | 1×/week (15min each) | 23 weeks (345 min) | N | G | T | NC | 27 |
| Rebok et al. (2014) | Instruction and practice on memory strategies | 2×/week (60–75min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min; up to 1050 min with booster) | YN | G | T | NC | 30 |
| Rebok et al. (2014) | Reasoning | 2×/week (60–75min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min; up to 1050 min with booster) | YN | G | T | NC | 30 |
| Requena et al. (2016) | Cognitive and emotional intervention (memory and cognitive stimulation) | 1×/week (? min each; 192 sessions total) | 192 weeks (?) | ? | G | T | AS | 23 |
| Willis et al. (2006) | Instruction and practice on memory strategies | 2×/week (60–75min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min; up to 1050 min with booster) | YN | G | T | NC | 30 |
| Willis et al. (2006) | Reasoning | 2×/week (60–75min each; 10 sessions total; up to 4 booster sessions | 5–6 weeks (600–750 min.; up to 1050 min with booster) | YN | G | T | NC | 30 |
| Action video game interventions | ||||||||
| Belchior et al. (2013) | Medal of Honor (Sony PlayStation 2) | ?×/week (90 min each; 6 sessions total) | 2–3 weeks (540 min) | YA | I | T | NC; AS | 24 |
| Goldstein et al. (1997) | Video games (Super Tetris) | 1×/week (60 min each) | 5 weeks (300 min) | N | I | I | NC | 24 |
| Torres (2008) | 7 casual video games (QBeez, Supper Granny 3, ZooKeeper, PenguinPush, Bricks, Pingyn and memory games) | 1×/week (? min each) | 8 weeks (?) | N | ? | ? | AS | 20 |
| Aerobic training | ||||||||
| Albinet et al. (2010) | Walking/running and circuit training | 3×/week (60 min each) | 12 weeks (2160 min) | YA | ? | T | AS | 27 |
| Bakken et al. (2001) | Calisthenics, stationary biking, walking | 3×/week (60 min each) | 8 weeks (1440 min) | YN | G | T | NC | 20 |
| Barnes et al. (2013) | Dance | 3×/week (60 min each) | 12 weeks (2160 min) | YA | G | T | AS | 30 |
| Kimura and Hozumi (2012) | Combination style dance workout | ?×/week (40 min each; 4 sessions total) | 8 weeks (160 min) | YA | G | T | AS | 27 |
| Maclean et al. (2014) | Rhythmic musical intervention | 1 session | 1 week (?) | N | ? | T | AS; S | 27 |
| Maki et al. (2012) | Walking | 1×/week (90 min each) | 12 weeks (1080 min) | N | G | T | AS | 27 |
| Moul et al. (1995) | Walking | 5×/week (30–40 min each) | 16 weeks (2400–3200 min) | YN | G | T | NC | 24 |
| Muscari et al. (2010) | Cycle ergometer, treadmill, and free-body activity | 3×/week (60 min each) | 52 weeks (9360 min) | YA | G | T | AS | 28 |
| Oken et al. (2006) | Walking | 1×/week (60 min each) | 26 weeks (1560 min) | YA | G | T | NC | 26 |
| Panton et al. (1990) | W alking/j ogging | 3×/week (30 min each) | 26 weeks (2340 min) | YA | G | T | NC | 21 |
| Shatil (2013) | Fitness Forever (TM) senior exercise video | 3×/week (45 min each) | 16 weeks (2160 min) | N | G | T | AS | 23 |
| Smiley-Oyen et al. (2008) | aerobic exercise equipment | 3×/week (60 min each) | 40 weeks (7200 min) | YA | G | T | AS | 28 |
| van Uffelen et al. (2008) | Walking | ?×/week (? min each) | 52 weeks (?) | YN | ? | T | AS | 29 |
| Strength/resistance training | ||||||||
| Cassilhas et al. (2007) | Sets of 8 repetitions using equipment (chest press, leg press, vertical traction, abdominal crunch, leg curl, lower back) | 3×/week (60 min each) | 24 weeks (4320 min) | YA | G | T | AS | 27 |
| Kimura et al. (2010) | Three phases: conditioning, strengthening/balancing, functional training | 2×/week (90 min each) | 12 weeks (2160 min) | YA | G | T | AS | 26 |
| Liu-Ambrose et al. (2008) | Strengthening and balance (Otago) | 3×/week (30 min each) | 26 weeks (2160 min) | YA | I | T;I | NC | 28 |
| Liu-Ambrose et al. (2010) | Bicep curls, tricep extensions, seated rowing, lat pulldown exercises, leg presses, hamstring curls, and calf raises; also included minisquats, minilunges, lunge walks | 1 or 2×/week (60 min each) | 52 weeks (3120–6240 min) | YA | G | T | AS | 30 |
| Liu-Ambrose et al. (2012) | Bicep curls, tricep extensions, seated rowing, lat pulldown exercises, leg presses, hamstring curls, and calf raises; also included minisquats, minilunges, lunge walks | 1 or 2×/week (60 min each) | 52 weeks (3120–6240 min) | YA | G | T | AS | 28 |
| Moul et al. (1995) | Alternate days of upper or lower body exercise | 5×/week (30–40 min each) | 16 weeks (2400–3200 min) | YN | G | T | NC | 24 |
| Panton et al. (1990) | Nautilus exercise machines (leg extension, side leg curl, super pullover, duo-decline press, 10 degree chest, rotary torso, low back, lateral raise, over-head press, multi-biceps | 3×/week (30 min each) | 26 weeks (2340 min) | YN | G | T | NC | 21 |
| Perrig-Chiello et al. (1998) | Machine exercises (leg press, bench press, leg curls, seated row exercise, leg extension exercise, preacher curls, trunk curls, back extension) | 1×/week (? min each) | 8 weeks (?) | ? | ? | ? | AS | 25 |
| Aerobic/resistance combination training | ||||||||
| Gajewski and Falkenstein (2012) | Aerobic (step, floor movement exercises), cardio (machines) and strength exercises (8 sets, repeated in 3 × 15 series) | 2×/week (90 min each) | 16 weeks (2880 min) | YN | G | T | NC | 19 |
| Klusmann et al. (2010) | Endurance, strength, flexibility balance/coordination | 3×/week (90 min each; 75 sessions total) | 26 weeks (6750 min) | ? | G | T | NC | 26 |
| Klusmann et al. (2011) | Endurance, strength, flexibility balance/coordination | 3×/week (90 min each; 75 sessions total) | 26 weeks (6750 min) | ? | G | T | NC | 26 |
| Legault et al. (2011) | Walking, flexibility | 2×/week (60 min each) + at-home walking (up to 150min/week) | 16 weeks (1920–4320 min) | YN | G;I | T;I | AS | 23 |
| Okumiya et al. (1996) | Light aerobics (e.g., walking), calisthenics (e.g., stretching), and muscle strengthening (e.g., weightbearing exercises) | 2×/week (60 min each) | 24 weeks (2880 min) | ? | G | T | NC | 26 |
| Williamson et al. (2009) | Aerobic, strength, balance, and flexibility | adaptation phase: 3×/week (40–60 min each), Transition phase: 2×/week (40–60 min each), Maintenance phase: 1×/week (40–60 min each) | 52 weeks (6290–7410 min) | YN | G;I | T | AS | 26 |
| Combination (nonfactorial) interventions | ||||||||
| Chambon et al. (2014) | Participants read information and instructions on a particular task, performed the task, then feedback on performance | 2×/week (60 min each) | 12 weeks (1440 min) | YA | I | T | NC; AS | 23 |
| Cheng et al. (2012) | Presentation on strategies followed by practice of strategies and real-world problem solving; memory, reasoning, problem solving, map reading, handcraft making, health and physical exercise | 2×/week (60 min each; 24 sessions total; up to 3 booster sessions) | 12 weeks (1440 min; up to 1620 min with booster) | ? | G | T | NC | 30 |
| Falbo et al. (2016) | Physical-cognitive dual-task | 2×/week (60 min each) | 12 weeks (1440 min) | ? | G | T | AS | 21 |
| Hiyamizu et al. (2011) | Strength and balance + cognitive dual-task | 2×/week (60 min each; 24 sessions total) | 12 weeks (1440 min) | ? | I | T | AS | 28 |
| Kitazawa et al. (2015) | Dual task net-step exercise | 1×/week (30 min each) | 8 weeks (240 min) | YA | G | T | NC | 27 |
| Lampit et al. (2015a,b) | Multi-domain cognitive intervention based on COGPACK | 3×/week (60 min each; 36 sessions total) | 12 weeks (2160 min) | YA | G | T | AS | 26 |
| Li et al. (2010) | Dual-task | 1×/week (60 min each) | 5 weeks (300 min) | YN | G | T | NC | 23 |
| Luo et al. (2016) | Presentation on strategies followed by practice of strategies and real-world problem solving; memory, reasoning, map reading, handcraft making, health, physical exercise | 2×/week (60 min each; 24 sessions total) | 12 weeks (1440 min) | ? | G | T | NC | 28 |
| Maillot et al. (2012) | Wii games | 2×/week (60 min each) | 12 weeks (1440 min) | YN | G | T | NC | 25 |
| Sato et al. (2015) | Water-based exercise + cognitive intervention | 1×/week (60 min each) | 10 weeks (600 min) | YN | G | T | AS | 25 |
| Schattin et al. (2016) | Exergame from dividat | 3×/week (30 min each; 24 sessions total) | 8 weeks (720 min) | YA | I | T | AS | 26 |
| Stigsdotter and Backman (1989) | Training of recoding operations, attention, relaxation | ?×/week (90 min each; 8 sessions) | 8 weeks (720 min) | N | G | T | NC | 23 |
| Stigsdotter-Neely and Backman (1993) | training of recoding operations, attention, relaxation | ?×/week (90 min each; 8 sessions) | 8 weeks (720 min) | N | G | T | NC | 20 |
| Uemura et al. (2012) | Dual-task | 1×/week (35 min each) | 24 weeks (840 min) | YA | G | T | AS | 23 |
| Westlake and Culham (2007) | Balance + reading or distraction task (dual-task) | 3×/week (60 min each) | 8 weeks (1440 min) | YA | G | T | AS | 22 |
| Combination (factorial) interventions | ||||||||
| Barnes et al. (2013) | Group exercise (dance-based aerobic exercise) + mental activity (Posit Science) | 3×/week (60 min each) for cognitive; 3×/week (60 min each) for exercise |
12 weeks (4320 min) | YA | G | T | AS | 30 |
| Legault et al. (2011) | Combined cognitive (computer-based memory) and exercises (walking, flexibility exercises) | 56 sessions (2440–48 min sessions for cognitive, 3260 min sessions for exercise training) | 16 weeks (2880–3072 min) | YA | G | T;I | AS | 23 |
| Shatil (2013) | CogniFit + Fitness Forever exercise video | 3×/week (40 min each) for cognitive; 3×/week (45 min each) for exercise |
16 weeks (4080 min) | YN | G | T;I | AS | 23 |
Note. QCS = Quality Control Score; N = no adaptation; YA = yes adaptation and adequately described; YN = yes adaptation and inadequately described;? = insufficient detail; NC = no-contact control; S = social control; AS = active + social control; see Section 3.2 for modified definition of AS.
While many interventions were implemented in group settings, 58% of process-based cognitive training and 67% of video game interventions were in individual settings. Additionally, most cognitive interventions were exclusively trainer-led with the exception of process-based training (50% individual-led; 13 of 26) and video game (33% individual-led; 1 of 3) interventions. Across interventions, there was a similar breakdown of no-contact vs. active and social control groups. Generally, no-contact controls comprised half to two-thirds of control group types except for process-based cognitive training (46%), education (75%), aerobic training (31%), strength/resistance training (43%), and factorial (0%) interventions. Few studies included multiple control groups (Table 1). Interestingly, the type of control group has changed over time (See Table 1). Of the eight studies published before 2000, most (75%) used a no-contact control group, one used an active + social control, and one used both no-contact and active + social control groups (see Table 1). In comparison, of the 52 interventions published since 2010, 16 (30%) included only a no-contact control group, 31 (60%) included only an active + social control group, and three included both no-contact and active + social controls. Alternative control groups are becoming more common in publications (see Table 1).
The mean quality assessment score across all studies was 25.36 (SD = 2.75) and ranged from 19 to 30 (Table 1). Study scores were similar across intervention types, with the exception of video game and theatre interventions, which had mean quality scores lower by at least 1 point compared to studies of other intervention types. For example, the mean quality control score of papers reporting on theatre interventions was 22.5, and the mean quality control score of aerobic interventions was 25.92. Across all interventions, the most common problem was failure to complete intention-to-treat (ITT) analysis. Although ITT is not always appropriately described or applied (Hollis and Campbell, 1999), including only those who adhere to intervention protocols in analyses may overestimate intervention effectiveness in real-world dissemination from a public health perspective.
Notably, the a priori definitions of the social, active, and active + social control groups were not representative of the control groups in the included studies. Most interventions incorporated some (but not all) aspects of both social and active control groups. For example, educational lectures that occurred with less intensity (i.e., duration or frequency) than the intervention group would have both (1) some social interaction with the research staff over that of no-contact controls and (2) a reasonable expectation by the participants that there would be cognitive benefits. As such, we modified our definition of active + social controls to be any condition where at least some elements of both social and active controls were present.
3.3. Cognitively-stimulating activities: education interventions, n = 3
Participants in education interventions were either taught computer skills or completed an autobiographical writing course. Neither intervention explicitly described adaptation, though participants did receive feedback from group members in the writing class. The average intervention duration was 79 h (4740 min). All interventions were group-based and trainer-led. No-contact control groups were used in all studies. The average quality control score was 26 (Table 1).
3.3.1. Transfer effects
There was most consistent immediate posttest transfer to subjective cognition; verbal delayed recall also improved, but this was less consistent (Fig. 2; Appendix D). Lastly, one study evaluated long-term (i.e., assessed any time after immediate posttest) transfer and found no evidence of transfer to verbal delayed recall (Appendix D).
Fig. 2.
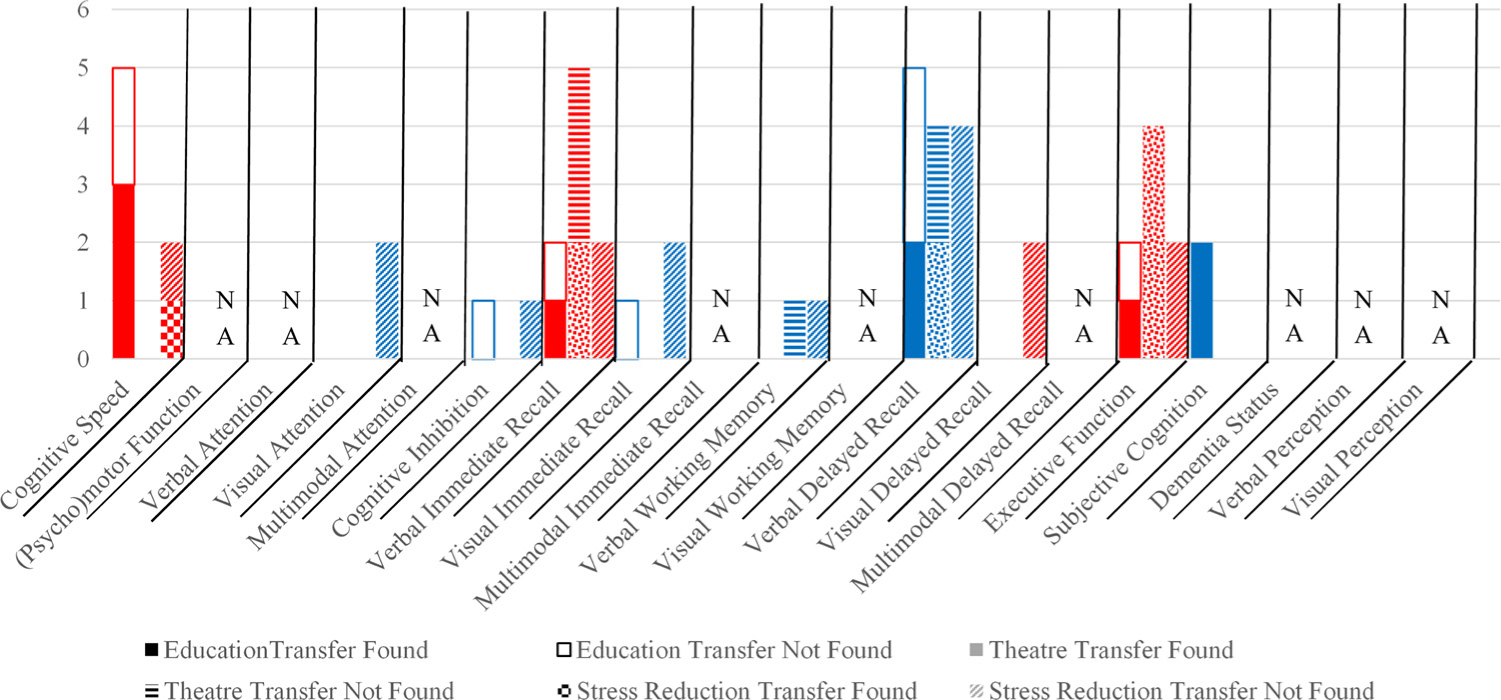
Summary results for cognitively-stimulating activities, n = 7.
Note. Cognitively-stimulating activities included education (n = 3), theatre (n = 2), and stress-reduction (n = 2) interventions. Red and blue colors provided to aid in readability of chart. Red = cognitive speed, verbal immediate recall, visual delayed recall, or executive function. Blue = visual attention, cognitive inhibition, visual immediate recall, verbal working memory, verbal delayed recall, or subjective cognition. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Cognitively-stimulating activities: theatre interventions, n = 2
In the theatre courses, participants were asked to participate in increasingly demanding theatre experiences similar to college-level theatre courses. Although adaptation was described as increasing in difficulty over the intervention period, a specific description of how the course difficulty was modified was not provided. The average intervention duration was 8 h (480 min). All interventions were group-based and trainer-led. No-contact control groups were used in all studies. The average quality control score was 21.5 (Table 1).
3.4.1. Transfer effects
There was most consistent transfer to executive function and inconsistent improvements on verbal immediate and delayed recall (Fig. 2; Appendix D). Notably, two measures of executive function, verbal fluency and the Means-End Problem-Solving Procedure, consistently improved after theatre interventions. No studies measured long-term transfer (Appendix D).
3.5. Cognitively-stimulating activities: stress-reduction training, n = 2
Both stress-reduction training interventions incorporated relaxation, meditation, and visualization activities aimed at relaxing participants. Adaptation throughout the intervention period was not described in either study. The average intervention duration was 24 h (1395 min). Of those with adequate description, the study modality was individual-based. Both studies were trainer-led, and both were compared against a no-contact control. The average quality control score was 24 (Table 1).
3.5.1. Transfer effects
Stress-reduction training did not confer benefits to any cognitive domain except inconsistently to cognitive speed (Fig. 2; Appendix D). No studies evaluated long-term transfer.
3.6. Cognitive training: process-based cognitive training, n = 26
Process-based interventions targeted a range of cognitive domains, including speed of processing, attention, working memory and general memory, logic and planning, problem solving, and included a variety of commercialized cognitive training programs (e.g., Posit Science, COGPACK, CogniFit). Sample process-based activities included decreasing the stimulus display speed while adding distractor tasks, playing working memory card games, and practicing pattern recognition (no strategies taught). Adaptation was frequently defined as increasing difficulty of the cognitive task as a measure of performance accuracy on the task (e.g., maintaining 75% accuracy before increasing difficulty of the task). Manipulation of difficulty varied based on the task but generally involved decreasing time allowed to respond on a task, increasing complexity of the task, or increasing the amount of information to monitor (e.g., memory load; Table 1). The average intervention duration was 17 h (968 min). A majority (58%, 15/26) of the interventions were individual-based, and half were trainer-led (13/26). No-contact controls were used in half of the interventions (13/26). The average quality control score was 25.38 (Table 1).
3.6.1. Transfer effects
In general, the most promising cognitive domains demonstrating immediate transfer were cognitive speed, verbal working memory, and visual working memory. Visual attention and executive function were commonly assessed but did not show consistent transfer (Fig. 3; Appendix D). Notably, the Useful Field of View (UFOV), verbal learning tests, the Cattell Culture Fair Test, and the Trailmaking Tasks were commonly used but did not show general intervention-related improvements with the exception of UFOV composites or subtest 4 (Appendix D). Ten studies evaluated long-term transfer; the trained domains most frequently maintained better performance compared to the control group (Appendix D).
Fig. 3.

Summary results for cognitive training, n = 42.
Note. Cognitive training consisted of process-based (n = 26) and strategy-based (n = 16) cognitive training interventions. Red and blue colors provided to aid in readability of chart. Red = cognitive speed, verbal attention, multimodal attention, verbal immediate recall, multimodal immediate recall, visual working memory, visual delayed recall, executive function, dementia status, or visual perception. Blue = visual attention, cognitive inhibition, visual immediate recall, verbal working memory, verbal delayed recall, multimodal delayed recall, or subjective cognition. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. Cognitive training: strategy-based cognitive training, n = 16
Strategy-based training primarily involved instruction and practice in memory or reasoning strategies, such as mnemonics and method of loci. For example, participants were taught how to pair target words with locations on the body such as eye-cake (ACTIVE; Ball et al., 2002; Rebok et al., 2014; Willis et al., 2006). Only one strategy-based training intervention was computerized; participants played more than 400 computerized exercises incorporating memory training techniques and practice of techniques (Brain Fitness; Miller et al., 2013). None of the strategy-based interventions reviewed included a clear description of adaptation (i.e., interventions were described as adaptive, but specific thresholds for increasing difficulty were not discussed); however, most interventions adjusted difficulty based on participant performance. The average intervention duration was 12 h (713 min). A majority of the interventions were group-based (88%, 14/16) and trainer-led (88%, 14/16). No-contact controls were used in a majority (69%, 11/16) of the studies. The average quality control score was 27.12 (Table 1).
3.7.1. Transfer effects
Verbal immediate recall was the most commonly improved domain, followed by verbal delayed recall. Executive function was frequently assessed; however, transfer was not consistent (Appendix D, Fig. 3). Notably, verbal learning tests, verbal fluency, and Trailmaking Task variants were commonly administered; verbal learning tests frequently improved after training. Thirteen studies included long-term transfer, and trained domains most frequently maintained gains across the follow-up period (Appendix D).
3.8. Action video game interventions, n = 3
Participants played games ranging from Tetris to Medal of Honor, a first-person shooter video game. Although all action video games are presumably adaptive, only one was explicitly adaptive, in which participants advanced to more challenging levels upon completion of a level. The average intervention duration was 7 h (420 min). Of those with adequate detail, two interventions were individual-based; one was trainer-led. No-contact and active + social controls were equally used across all studies. The average quality control score was 22.67 (Table 1).
3.8.1. Transfer effects
There was weak evidence of immediate transfer to cognitive speed, visual attention, and dementia status (Fig. 4; Appendix D). No studies measured long-term transfer (Appendix D).
Fig. 4.

Summary results for action video game interventions, n = 3.
Note. Red and blue colors provided to aid in readability of chart. Red = cognitive speed or dementia status. Blue = visual attention or cognitive inhibition. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.9. Exercise: aerobic training, n = 13
Intervention activities included walking/running, aerobic activities on machines (e.g., stationary bicycles and treadmills), or dancing. Adaptation was frequently defined as increasing difficultly as a percentage of one’s age- and sex-specific heart rate. The average intervention duration was 49 h (2911 min). All interventions with adequate detail were group-based and trainer-led. A majority of the interventions (69%, 9/13) had an active + social control group. The average quality control score was 25.92 (Table 1).
3.9.1. Transfer effects
Executive function was the cognitive domain most likely to improve after aerobic training. Additionally, there was weak evidence of transfer to cognitive speed and verbal perception (Table 5; Appendix D). There was strong evidence of no transfer to visual attention or cognitive inhibition. Notably, the Stroop task, verbal fluency, and the Trailmaking Task were common measures assessed across studies; none of these measures demonstrated consistent immediate transfer. No studies assessed long-term transfer (Appendix D).
3.10. Exercise: strength/resistance training, n = 8
All interventions included machine-based exercises aimed to increase strength, and balance components. Adaptation was defined as increasing weights after the ability to complete a series of weight repetitions comfortably. The average intervention duration was 47 h (2803 min). A majority of the interventions were group-based (75%, 6/8) and trainer-led (88%, 7/8). Half of the interventions were compared against a no-contact control group (4/8). The average quality control score was 26.12 (Table 1).
3.10.1. Transfer effects
Cognitive inhibition and visual working memory were the domains most frequently improved after training. Additionally, there was strong evidence that (psycho)motor function and executive function were the least frequently improved cognitive domains (Fig. 5; Appendix D). No studies assessed long-term transfer (Appendix D).
Fig. 5.

Summary results for exercise training, n = 27.
Note. Exercise interventions consisted of aerobic (n = 13), strength/resistance (n = 8), and aerobic/resistance combination training (n = 6). Red and blue colors provided to aid in readability of chart. Red = cognitive speed, verbal immediate recall, visual working memory, visual delayed recall, executive function, or dementia status. Blue = (psycho) motor function, visual attention, cognitive inhibition, visual immediate recall, verbal working memory, verbal delayed recall, subjective cognition, or verbal perception. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.11. Exercise: aerobic/resistance combination training, n = 6
All interventions included a combination of light-to-moderate aerobic activities, such as walking, and strength or flexibility/balance exercises. Adaptation was generally poorly-described but was defined as increasing aerobic intensity and resistance through the intervention period. The average intervention duration was 74 h (4523 min). All interventions were group-based and trainer-led. No-contact control groups were used in a majority of the studies (67%, 4/6). The average quality control score was 24.33 (Table 1).
3.11.1. Transfer effects
Aerobic/resistance combination training most frequently improved verbal delayed recall (Appendix D). Additionally, there was strong evidence that cognitive speed, verbal immediate recall, and executive function were not likely to improve after training (Fig. 5; Appendix D). Notably, the MMSE was commonly administered and did not show transfer. No studies assessed long-term transfer (Appendix D).
3.12. Combination (nonfactorial) interventions, n = 15
Intervention activities included multicomponent cognitive training, combination cognitive and exercise activities including exergaming, and dual-task interventions where participants were asked to complete cognitive tasks such as counting backwards while performing a walking or balance task. Adaptation of the interventions was generally inadequately described, although dual-task interventions more frequently described either the cognitive or balance task adaptation as broadly increasing in difficulty over the intervention period. The average intervention duration was 19 h (1092 min). A majority of the interventions were group-based (80%, 12/15), and all were trainer-led. The average quality control score was 24.67 (Table 1).
3.12.1. Transfer effects
There was most consistent transfer to verbal immediate and delayed recall. There were less consistent benefits to cognitive speed, verbal or visual working memory, executive function, or dementia status (Fig. 6; Appendix D). Four studies included a long-term follow-up; verbal delayed recall was the most frequent domain that maintained training gains (Appendix D). Notably, the Trailmaking Task B and MMSE were commonly administered measures and consistently did not show transfer.
Fig. 6.
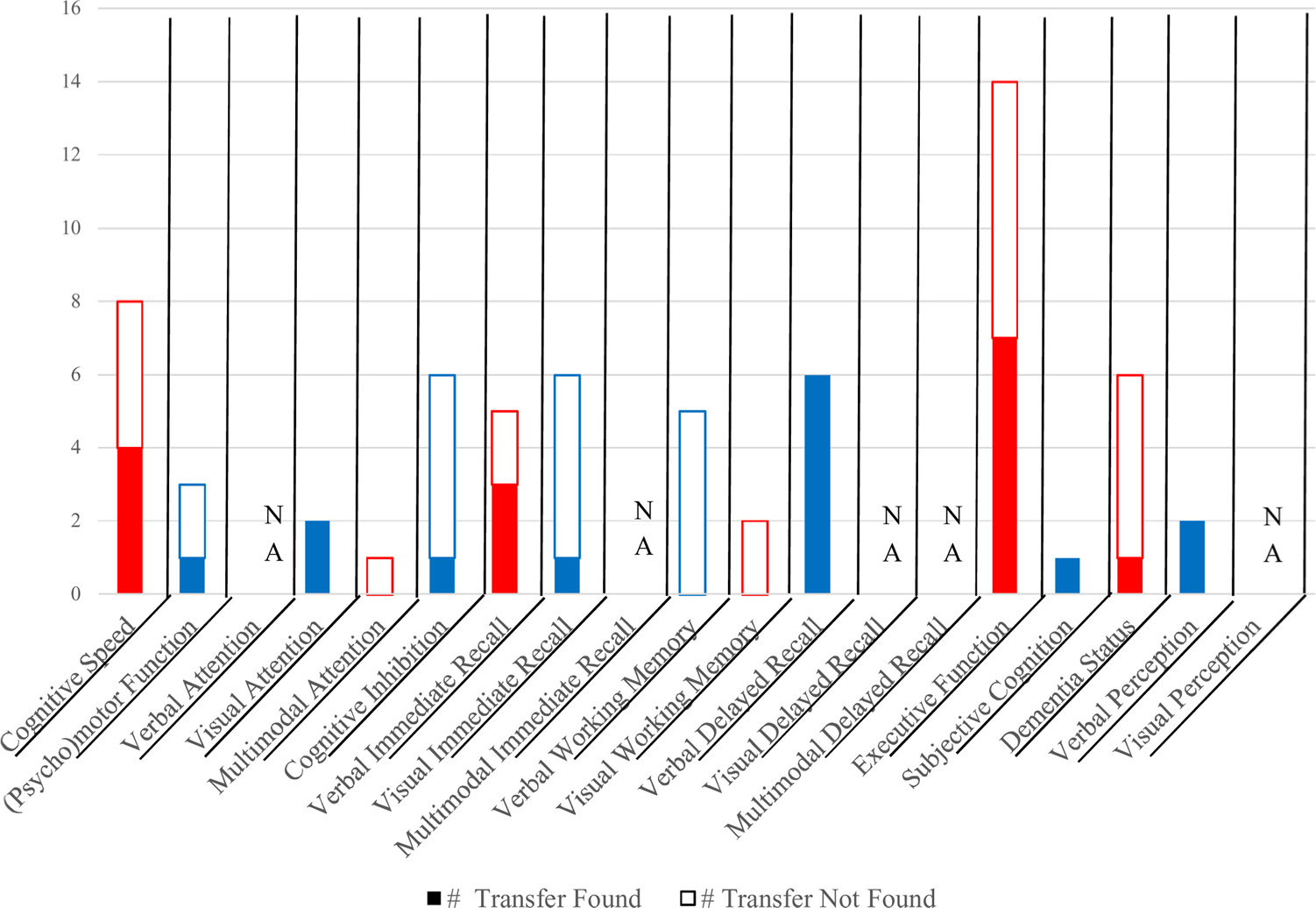
Summary results for combination (nonfactorial) interventions, n = 15.
Note. Red and blue colors provided to aid in readability of chart. Red = cognitive speed, multimodal attention, verbal immediate recall, visual working memory, executive function, or dementia status. Blue = (psycho)motor function, visual attention, cognitive inhibition, visual immediate recall, verbal working memory, verbal delayed recall, subjective cognition, or verbal perception. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.13. Combination (factorial) interventions, n = 3
Factorial designs included interventions with multiple intervention components, as well as separate intervention groups for separate components. For example, a possible factorial design would include a cognitive-only intervention group, an aerobic-only intervention group, and a cognitive + aerobic exercise intervention group. All three studies included four intervention groups: a computerized cognitive intervention (all of which were process-based), an aerobic exercise intervention, a combination process-based cognitive and exercise intervention, and an active + social control group. The combination group received more intervention time than the other groups, with the exception of one study, where active control components were added to equalize intervention time across groups. The cognitive components were all adaptive based on participant performance, and only one exercise component was adaptive based on increasing difficultly as a percentage of one’s age- and sex-specific heart rate. The average intervention duration was 63 h (3760 min). All interventions were group-based and trainer-led. All interventions used active + social control groups. The average quality control score was 25.33 (Table 1).
3.13.1. Transfer effects
There was most consistent transfer to cognitive speed and visual attention. In addition, there was strong evidence of no transfer to executive function (Fig. 7; Appendix D). Notably, the Trailmaking Task was commonly administered and consistently did not show transfer. No studies assessed long-term transfer (Appendix D).
Fig. 7.

Summary results for combination (factorial) interventions, n = 3.
Note. Red and blue colors provided to aid in readability of chart. Red = cognitive speed, verbal immediate recall, visual working memory, visual delayed recall, or executive function. Blue = visual attention, cognitive inhibition, verbal working memory, or verbal delayed recall. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.14. Everyday function
Nine studies evaluated transfer to everyday function (Ball et al., 2002; Cavallini et al., 2015; Lampit et al., 2014; Maki et al., 2012; McDougall et al., 2010; Mozolic et al., 2011; Noice and Noice, 2013; Rebok et al., 2014; Willis et al., 2006). Cognitive training interventions, specifically those from the ACTIVE trial (Ball et al., 2002; Jobe et al., 2001; Rebok et al., 2014; Willis et al., 2006), most consistently included everyday functional outcomes, and provided the largest breadth of everyday function assessments. Measures of everyday function included everyday speed of processing, everyday problem solving, self-reported driving or life space, self-reported (instrumental) activities of daily living (ADL/IADL) or difficulties completing everyday tasks, and performance-based IADL (Observed Tasks of Daily Living-Revised [OTLD-R] and Timed Instrumental Activities of Daily Living [TIADL]). There was significant transfer to observed everyday function in one theatre intervention (OTDL-R; Noice and Noice, 2013). Additionally, there was significant immediate (Bayer Activities of Daily Living, Lampit et al., 2014) and long-term transfer to self-reported IADL function across five and ten years in both process- and strategy-based training (MDS-Health Care, Rebok et al., 2014; Willis et al., 2006). Interestingly, there was no short-term transfer in self-reported (Ball et al., 2002) or observed (Direct Assessment of Functional Activities, McDougall et al., 2010) IADL function; however, all of the cognitive training interventions in the ACTIVE trial demonstrated transfer to self-reported IADL across ten years (Rebok et al., 2014). Of the studies included in this review, there was no consistent transfer from cognitive training to everyday speed of processing, everyday problem solving, or driving space. However, other literature using the ACTIVE trial and other studies excluded from this review have repeatedly demonstrated such transfer over time (see Section 4.2.1). Aerobic training transferred to self-reported IADL difficulties but not lifespace (Maki et al., 2012). See Fig. 8 for more detail.
Fig. 8.
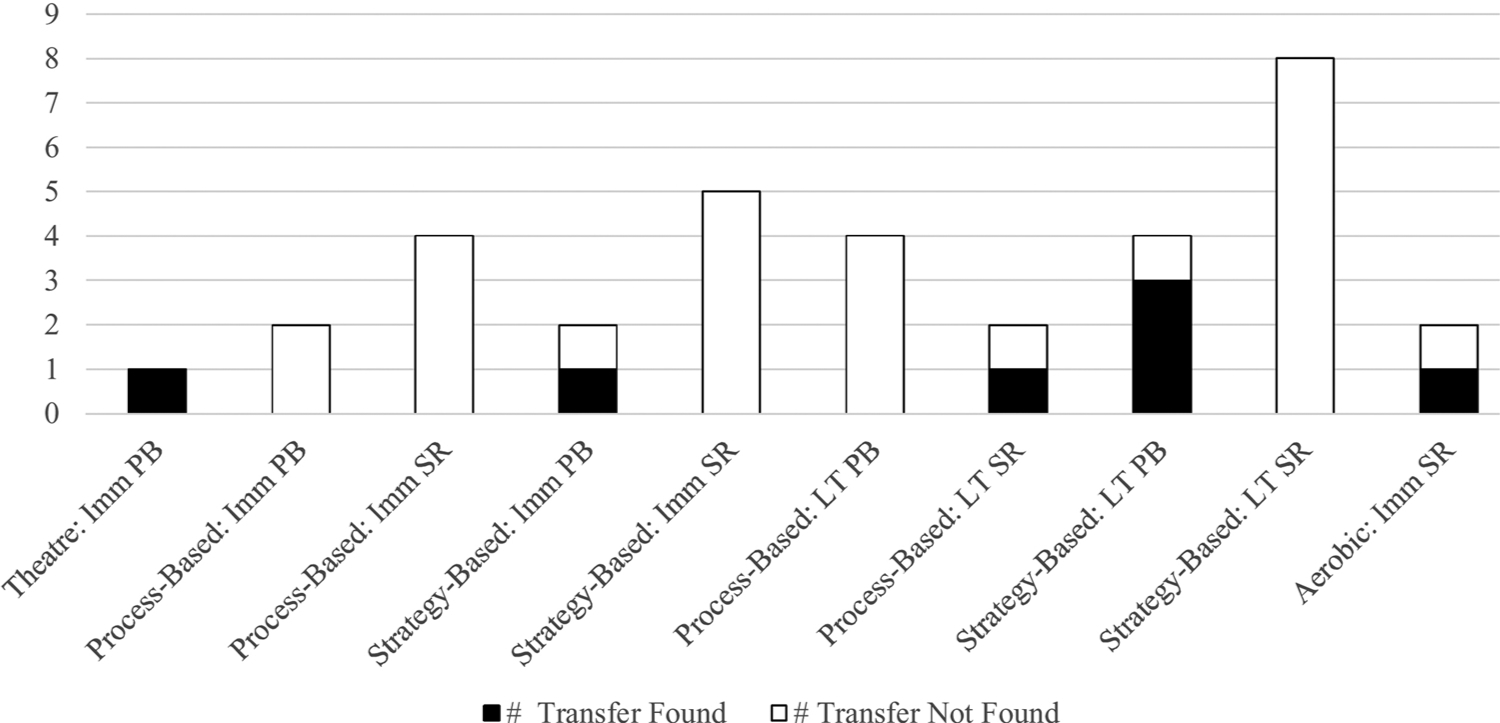
Transfer of behavioral interventions to everyday function, n = 9.
Note. Imm = Immediate Outcome, LT = Long-Term Outcome, PB = Performance-Based Outcome, SR = Self-Report Outcome.
4. Discussion
This systematic review of 75 peer-reviewed publications examined the effects of randomized controlled trial behavioral interventions on cognition in healthy older adults. To date, this is the first systematic review to examine cognitive outcomes across a breadth of intervention types. Generally, cognitively-stimulating activity interventions had inconsistent evidence of transfer but showed promise in improving verbal delayed recall (education, n = 3), executive function (theatre, n = 2), and cognitive speed (stress-reduction training, n = 2). Process-based cognitive training (n = 26) most consistently transferred to cognitive speed, verbal, and visual working memory. Strategy-based cognitive training (n = 16) most consistently transferred to verbal immediate recall. Similar to other reviews (e.g., Edwards et al., 2018; Kelly et al., 2014b), there was consistent evidence of near transfer (i.e., transfer to the same or similarly-trained tasks) and weak-to-moderately consistent evidence for far cognitive transfer (i.e., transfer to untrained tasks) in cognitive training. Similar to cognitively-stimulating activities, action video games (n = 3) showed promise in transferring to dementia status via the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), but strong conclusions should not be drawn due to the few number of studies. Aerobic training (n = 13) most consistently transferred to executive function, while strength/resistance training (n = 8) most consistently transferred to cognitive inhibition and visual working memory. Similarly, aerobic/resistance combination training (n = 6) showed most consistent improvements in visual working memory. Lastly, combination nonfactorial interventions (n = 15) most consistently transferred to verbal delayed recall, and factorial interventions (n = 3) most consistently transferred to cognitive speed and visual attention. While factorial interventions show promise, strong conclusions should not be drawn given the few studies.
4.1. Limitations of existing behavioral interventions
This review highlighted several limitations of the existing literature. First, the best way to group interventions was unclear, especially when interventions were inadequately described or included a wide breadth of activities. When possible, more nuanced intervention categories (e.g., process- or strategy-based cognition) were created, but several studies were vague in documenting specific tasks. Relatedly, terminology surrounding the cognitive training literature was inconsistent. For example, cognitive training trials in our review ranged from training memory mnemonics (Ball et al., 2002), to cognitive and emotional training (Requena et al., 2016), to Sudoku (Gajewski and Falkenstein, 2012). Complicating this is the commercialization of these terms in order to lend credence to other cognitively-stimulating activities that lack the scientific rigor of RCTs (Shah et al., 2017). These challenges exemplify why it is vital to include adequate descriptions of intervention activities, either in text, supplementary materials, or on a website in order to understand which activities led to which outcomes (Edwards et al., 2018; Kelly et al., 2014a). Additionally, it is critical to standardize terminology, particularly the terms cognitive intervention, cognitive training, and cognitively-stimulating activities (National Academies of Sciences, 2017). This systematic review has attempted to operationalize these terms to assist with terminology standardized in the field of behavioral interventions.
Control group descriptions and inconsistencies were another limitation of the literature. We attempted to divide control groups into active- or social-only control groups; however, studies consistently provided inadequate information to ascertain expectancy, equivalent interactions between staff, or other information critical to evaluate placebo effectiveness. This finding is consistent with previous work (Boot et al., 2013; Simons et al., 2016) and highlights a continued limitation in the field to adequately describe active control groups both in terms of what specific activities were done, as well as explicitly measuring and controlling for differential expectancy effects. Although attempts to describe components of an adequate control group were made, few have actually designed and implemented such controls. As a result, it was difficult to assess the appropriateness of the control groups or determine meaningful trends based on control group type. Despite our a priori definitions of the types of control groups, most control groups (other than no-contact or wait-list controls) were a combination of active and social that did not fit a clear group. For example, a control group that attended group lectures for less duration than a cognitive intervention group includes both active (e.g., learning new material at a lecture) and social (e.g., engagement with other attendees and staff at end of lecture) components, but it would not be a true active + social control by the a priori definition because of the non-equivalency of the duration. Further complicating this was the “reasonable expectancy of effects.” Because older adults cite numerous activities, ranging from crossword puzzles to exercise to social engagement, as beneficial for cognitive health (Friedman et al., 2011, 2015; Friedman et al., 2013; Wilcox et al., 2009), we argue that placebo effects would be present in most control groups other than no-contact controls. We recommend that researchers use guidelines, such those from the Cochrane Back Review Group, in order to ensure that sufficient detail is included (Furlan et al., 2009). Additionally, we recommend that researchers consider which control group(s) are most appropriate for their research questions. Some researchers argue that no-contact control groups are never appropriate (e.g., Simons et al., 2016), whereas others suggest they may be appropriate depending on the aims of the study (e.g., Rebok, 2016). Interestingly, our review noted a large decrease on the reliance of no-contact control groups (75% prior to 2000 to 30% since 2010). This indicates a positive change in the field; however, when possible, including multiple control groups may be the best way to examine the relative effects of behavioral interventions on cognitive and everyday function in older adults (Ross et al., 2016,in press).
Relatedly, several studies failed to provide adequate information on other intervention details such as the number of sessions per week, number of minutes per session, or intervention modality (e.g., group setting or trainer-led). If details were not provided in the manuscript, we cross-referenced protocol or other cited manuscripts, as well as reviewed information from sites such as ClinicalTrials.gov. The rationale for this was to demonstrate the level of information available to possible community stakeholders. Since the ultimate goal of behavioral intervention research is dissemination, researchers should not be the gatekeepers of information that would be necessary for the public to implement these interventions. As the use of database repositories and publishing protocols becomes more ubiquitous, we anticipate this limitation will be less pervasive. In the meantime, researchers should ensure enough information about their intervention is available to the public to ensure intervention fidelity can be maintained when translated into practice.
Another challenge in the review process was inadequate breadth of cognitive assessments across several domains, particularly (psycho) motor function, subjective cognition, and dementia status. Relatedly, our definitions and categorization of the cognitive domains assume some degree of orthogonality, when this is not the case in reality (i.e., we do not have “pure” measures of cognitive speed, executive function, or other domains that do not incorporate elements of each other). This limits our confidence to generalize the presence or absence of transfer to these domains. In particular, intervention-related changes in subjective cognition may elucidate the extant limitation of understanding psychosocial mechanisms of transfer. Changes in subjective cognition may capture changes in proposed psychological mechanisms like cognitive self-efficacy (Simons et al., 2016). Testing mechanisms requires at least three assessments (Gelfand et al., 2009), thus reiterating the importance of more than pre-posttest-only assessments.
Relatedly, everyday function was only examined in nine articles. Despite repeated calls to include everyday functional outcomes, few behavioral interventions in older adults include it as a primary or secondary outcome. Moreover, there is a lack of consensus on the operationalization of everyday function. For instance, everyday function assessments in this review included basic ADLs, IADLs, and life or driving space assessments. Some studies combined simplistic physical manipulation of everyday stimuli (e.g., coins, phonebook) with complex high-level cognitive tasks (e.g., Everyday Problems Test) in one composite score, making it difficult to ascertain specifically which everyday function(s) were likeliest to benefit from training (Ball et al., 2002; Rebok et al., 2014; Willis et al., 2006). Additionally, everyday function assessments include tasks that may not be as relevant for current or future cohorts (e.g., sorting change, using a phonebook). While there was transfer to everyday function in this review, it is not recommended to use these measures without considering their ecological validity.
Further complicating the operationalization of “everyday function” is the emergence of ecological cognitive assessments, or everyday cognition, (e.g., Gamaldo and Allaire, 2016; Scott et al., 2015), which are traditional neuropsychological assessments modified to be taken on portable technologies in everyday life. While this is vital for evaluating the ecological validity of neuropsychological assessments, it does not fall under the purview of everyday function. We propose using the term everyday function for self-reported or direct observation of activities, or performance on simulated activities, that reflect tasks encountered regularly in life. While this is a broad definition, it differentiates the ability to perform real-world tasks from completing traditional neuropsychological tests in daily life. Our definition focuses on the stimuli (i.e., traditional neuropsychological test versus real-world tasks); however, it is also important to consider the frequency (Gamaldo and Allaire, 2016) and location of assessment. Researchers are increasingly implementing burst designs of repeated assessments within a day or across months. We propose that such temporal-focused measurement include a demarcation so that they can be better differentiated in the literature. For example, self-reported or simulated tasks mimic real-world behaviors assessed infrequently (e.g., across years) are everyday function whereas such tasks assessed in burst designs are monthly (or quicker timescale, as appropriate) everyday cognition or monthly everyday function.
A last limitation of existing behavioral interventions is inconsistent assessments of long-term transfer effects. Few studies (Appendix D) provided long-term follow-up assessments, here defined as any assessment that occurred after the immediate posttest. Follow-up periods ranged from months to multiple years post-intervention; there was no consistent post-intervention benchmark to evaluate long-term transfer across studies. Failure to measure long-term outcomes limits our ability to evaluate the presence of “sleeper effects,” or downstream benefits of an intervention (Edwards et al., 2017; Wolinsky et al., 2015). This is especially true for everyday function, where there was consistent evidence of gradual training-related changes at five and ten years posttest compared to more proximal follow-ups (Ball et al., 2002; Edwards et al., 2018; Rebok et al., 2014; Ross et al., 2017; Willis et al., 2006). Failure to include long-term assessments also reduces the ability to measure post-study adherence. For example, it remains unseen whether one must continually engage in aerobic training to receive executive function benefits or whether engagement in a short time-span is sufficient to offset age-related declines (National Academies of Sciences, 2017).
4.2. Strengths, limitations, and recommendations
This systematic review includes several strengths that improve the understanding of behavioral interventions in older adulthood. The wide breadth of behavioral interventions reviewed allows researchers and practitioners the opportunity to incorporate components of other interventions into new (ideally) factorial intervention designs. It also provides an overview of the differential cognitive benefits across a variety of behavioral interventions. This allows for comparisons across interventions and may facilitate creating more effective individualized multi-domain interventions. For example, if one wishes to enhance a wider breadth of cognitive domains, combining process-based cognitive and aerobic training would be a more effective approach than combining aerobic training with a mindfulness intervention.
An additional strength of this systematic review is that only peer-reviewed RCTs were included. By only including RCTs, the gold standard of intervention evaluation, conclusions made about the effectiveness of behavioral interventions are strengthened. Lastly, several critical aspects of interventions, such as control group type, adaptation, and delivery methods, were documented. Previous reviews have considered a few of these possible intervention factors (Edwards et al., 2018; Kelly et al., 2014a), but it is not standard practice across systematic reviews due to varied reporting in studies. Quantifying these reported intervention components can be incorporated into future meta-analyses to examine how study-specific variables, such as control group type or duration of intervention, impact transfer (Edwards et al., 2018).
Another strength of this systematic review is the organization of cognitive tests into cognitive domains. Empirical studies do not consistently identify cognitive assessments as measuring the same cognitive domain or function (e.g., MMSE as a measure of global cognitive function vs. dementia status or Flanker as attention vs. executive function), and few systematic reviews provide the detail necessary to evaluate patterns of transfer relevant to specific measures. For example, the current review was able to identify Trailmaking Task B as a frequently-used measure of executive function. Although it is commonly used, it generally did not improve after either process- or strategy-based cognitive training. This information is important because it suggests that either cognitive training is not associated with improved executive function performance, or cognitive training is not associated with improvements in Trailmaking Task B and other executive function measures should be considered. Furthermore, the current review provides a structure for future researchers to use when organizing results into various cognitive domains.
Despite the strengths, there are limitations worth noting. The inclusion criteria of this systematic review, while necessary, limited the types of interventions and populations included. For example, certain cognitively-stimulating social interventions such as Senior Odyssey (Stine-Morrow et al., 2008), Experience Corps (Carlson et al., 2008), and the Synapse Project (Park et al., 2014) were excluded from our review primarily due to pseudo-randomization or the sample age range. Additionally, studies including adults under 65 were excluded because of search filters and conventional older adult age thresholds. Older adults with diagnosed cognitive impairment or probable dementia were also excluded. Although this is not necessarily a limitation, it reduces external validity to only older adults without apparent cognitive dysfunction. Additionally, intervention inclusion criteria in healthy older adult samples generally require a degree of functional independence, so transfer to less physically healthy samples cannot be inferred from this review. Future interventions should explore alternative administration methods to increase access to at-risk, mobility-limited older adults to address this limitation (Payne and Stine-Morrow, 2017). A last limitation of our review is our categorization of the novel behavioral interventions. For example, “arts” interventions were originally conceptualized as including any fine or performing arts-based activities. However, there are differences within this category that suggest the underlying process by which they impact cognition is not similar. Although music therapy interventions did not make it through the screening process, such interventions would have been classified as “arts.” However, music therapy works in part through dopaminergic stimulation (Stegemöller, 2014); other arts interventions like theatre do not have evidence of this mechanistic process. Future reviews should consider proposed mechanisms of transfer when categorizing interventions in reviews and meta-analyses.
4.2.1. Implications and recommendations for research
Future research to examine the most effective combination of behavioral activities to improve cognitive and everyday function in older adults is warranted. There is a trend in behavioral interventions to include multiple intervention components (e.g., cognitive training targeting multiple cognitive domains). For example, two behavioral interventions included over ten different activities into the same intervention (Gajewski and Falkenstein, 2012; Shatil, 2013). This is also reflected in some commercial products that offer over 30 cognitive games. When designing an intervention, researchers may be tempted to incorporate multiple games in hopes of improving multiple cognitive domains through one intervention or sustain participant engagement and enjoyment. However, including too many intervention components makes it difficult to disentangle which aspects of the intervention are (in)effective (Collins et al., 2014; Ross et al., 2017, 2018). For example, if one intervention included games targeting a variety of cognitive domains and improved memory, it is impossible to know which game(s) drove the transfer effect. Behavioral interventions can be costly to researchers and burdensome on participants, so it is ideal to only include components that are most influential on cognition. To address this limitation, we recommend implementing the multiphase optimization strategy (MOST) to test each component (e.g., cognitive domain trained). MOST is an approach that can help researchers refine interventions into their most effective components while maintaining resource constraints (Collins et al., 2014). Briefly, researchers design screening experiments that include different combinations of intervention components. The results are used to make decisions about which components to keep or remove. MOST was successful in designing smoking cessation interventions (Collins et al., 2011) but has not been utilized in behavioral interventions in older adults. For example, a researcher may design an experiment that includes ten computer games and use MOST to systematically test and refine the most effective combination(s) of these games.
Furthermore, psychological mechanisms of transfer should be emphasized in future intervention research. Such mechanisms are frequently post hoc speculations but are not incorporated into interventions. As a result, they remain theoretical (Simons et al., 2016) or are examined using methodologically subpar standards (Alsubaie et al., 2017 for review). Addressing this limitation requires large sample sizes, statistically-sophisticated techniques (Preacher et al., 2010), and multiple waves of data above traditional pre-posttest intervention designs (Gelfand et al., 2009). Hypothesized psychological mechanisms of transfer include increased motivation (Boot et al., 2013; Motter et al., 2016; Rabipour and Raz, 2012; Simons et al., 2016), expectancy of intervention gains (Boot et al., 2013; Motter et al., 2016), exposure to novelty (Motter et al., 2016), social contact with trainers and/or other participants (Boot et al., 2013; Motter et al., 2016; Simons et al., 2016), and experimenter demand (Simons et al., 2016). To our knowledge, three studies directly tested moderators or mediators of speed of processing training (Edwards et al., 2013; Kaur et al., 2014; Sharpe et al., 2014). Contrary to assumptions (e.g., Simons et al., 2016), evidence suggests psychosocial moderators or mediators play little effect in speed of processing training gains. Psychosocial factors such as greater self-efficacy are either unrelated to cognitive training gains (Sharpe et al., 2014) or are related to smaller training gains (Kaur et al., 2014), opposite of what some researchers propose (Simons et al., 2016). What does appear to drive the transfer of cognitive training to cognitive and everyday function is proximal training gains (Edwards et al., 2013) or baseline moderators (Ball et al., 2013; Rebok et al., 2013; Willis and Caskie, 2013). To directly examine mechanisms underlying intervention effects, the use of active control groups would address psychosocial aspects of training such as increased motivation, expectancy of training gains, exposure to novelty, social contact, and experimenter demand. Researchers should also include psychosocial measures before and after an intervention, including cognitive self-efficacy and depression, in order to determine whether these are truly mechanisms of transfer.
Some argue that there is an opportunity cost associated with activities that may confer “less” cognitive benefit compared to other activities. Because time is a finite resource, it is believed that engaging in certain activities takes time away from engagement in other activities. Specifically, Simons et al. (2016) propose that cognitive training may replace exercise time, and older adults could engage in exercise were they not participating in cognitive training. However, there is no empirical evidence for this (Lampit et al., 2015b), and it should be explored before researchers make recommendations to not engage in evidence-based healthful behaviors. Ways to empirically evaluate this include (1) assessing how participants would have spent their time if they were not engaging in the intervention or (2) performing an extensive pre-intervention assessment to evaluate participants’ daily activities. Relatedly, multiple health behavior changes, or the transfer of health behaviors from one domain to another (e.g., engaging in healthful eating after beginning exercise; Noar et al., 2008), has been proposed as a mechanism for cognitive intervention transfer (Simons et al., 2016). Multiple behavior changes have been demonstrated in near transfer activities (Fleig et al., 2015), but it is unclear whether far transfer occurs. To test this, future work should incorporate repeated assessments before, during, and after the intervention period to assess behavioral changes as a result of engaging in an intervention study.
In addition to cognitive transfer, future work should examine everyday function and consider such outcomes at least as, if not more, important. Although some cite limited cognitive transfer as evidence of failure to establish transfer to everyday function (Simons et al., 2016), few studies a priori identified everyday function (e.g., IADL) as an outcome of interest (but see Ball et al., 2002; Cavallini et al., 2015; Lampit et al., 2014; Maki et al., 2012; McDougall et al., 2010; Mozolic et al., 2011; Noice and Noice, 2013; Rebok et al., 2014; Willis et al., 2006). Both within the current systematic review and other literature, notable exceptions to this are the ACTIVE (Jobe et al., 2001) and Iowa Healthy and Active Minds Study (IHAMS; Wolinsky et al., 2015) trials that found reductions in IADL difficulties (Wolinsky et al., 2015) and driving mobility (Ross et al., 2016, 2017) and safety (Ball et al., 2010) among other everyday functions (see Edwards et al., 2018 for review). The argument that behavioral interventions, namely cognitive interventions, are not worth pursuing falsely equates absence of evidence as evidence of absence. That is, there is an (unfounded) assumption that the failure to find cognitive training transfer to everyday function is due to true lack of transfer rather than a lack of including everyday functional outcomes in extant studies. There is an acknowledged dearth of literature of behavioral interventions on everyday function that researchers have said is necessary to address (Greenwood and Parasuraman, 2016; Kelly et al., 2014a), but emerging evidence suggests there are additional beneficial effects on IADLs (Edwards et al., 2013, 2005; Willis et al., 2006), driving (Ball et al., 2010; Ross et al., 2016, 2017), physical function (Ross et al., 2018; Smith-Ray et al., 2013, 2014), expected medical expenditures (Wolinsky et al., 2009), and dementia risk (Edwards et al., 2017). For more detail, see the recent review by Edwards et al. (2018). Future work should more seriously consider transfer effects to clinically and practically-meaningful outcomes, especially because the ultimate goal of behavioral interventions is to improve everyday function.
Furthermore, long-term follow-up of intervention outcomes should be examined. To ensure the ability to compare across studies, the timing of long-term follow-up assessments should be standardized. Booster effects could also be incorporated to inform whether additional sessions are needed, and if so, how often. Because older adults participating in behavioral interventions are likely healthy and functionally independent, the inclusion of long-term assessments would inform if intervention engagement can offset or delay meaningful impairment in older adulthood. Capturing the true nature of any phenomenon requires measuring variables at twice the rate of the expected timescale (Shannon, 1949; Shiyko and Ram, 2011), so post-intervention measures should be at least twice as frequently as the expected rate of change in the outcome(s). Including long-term assessments will also better capture long-term processes. For example, everyday function more consistently transferred years after intervention administration (e.g., Ball et al., 2010; Rebok et al., 2014; Ross et al., 2016, 2017, 2018). Failure to include long-term assessments would have missed this slower time-scale change. Particularly with healthy samples who perform at or near ceiling at baseline (e.g., no reported impairments), long-term assessments inform whether the intervention successfully altered the transition to suboptimal everyday function.
Lastly, researchers should use database repositories or publish extensive protocols with detailed information about their interventions. This is critical to aid in both replication, intervention dissemination, and open science. While the interventions themselves may be proprietary, details such as how many sessions over what length of time are informative for both researchers and the public alike. Making this information publicly available will ultimately democratize intervention research and hold researchers responsible for information dissemination and transparency.
Based on our review, we recommend the following: (1) explicitly test each intervention component via MOST and factorial intervention designs, (2) recruit large samples with at least three waves of data to statistically test mechanisms of transfer, such as expectancy effects and multiple health behavior changes (Motter et al., 2016), (3) examine meaningful everyday functional outcomes, (4) include long-term follow-up assessments at least twice as frequently as the expected timescale to capture slow-scale changes in outcomes, and (5) make all study information publicly available.
4.2.2. Implications and recommendations for practice and policy
A majority of the behavioral interventions included trained staff in charge of intervention administration. As a result, two major practical limitations arise: (1) there is a high cost to train and maintain staff, and (2) participation is limited to functionally independent older adults with resources to make frequent visits to training facilities. To reduce costs and increase access to older adults with suboptimal functioning, researchers should collaborate with community partners to provide their interventions across a variety of delivery methods, including at-home settings, churches or other social groups older adults are likely to frequent, and self-guided interventions. If the ultimate goal of behavioral interventions is to promote healthy aging, creative collaborations that move away from traditional laboratory-based intervention delivery are necessary to meet older adults at their current level of functioning.
A final recommendation is to increase access and knowledge of beneficial activities to older adults and healthcare professionals. Older adults frequency cite mental, physical, social, and leisure activity engagement as ways to maintain cognitive function (Friedman et al., 2011, 2013, 2015; Kim et al., 2015; Wilcox et al., 2009). Unfortunately, activities researchers know are not evidence-based, such as crossword puzzles, are among the most-frequently mentioned activities believed to maintain mental acuity (Kim et al., 2015). In fact, few older adults report receiving information about preventing cognitive decline from primary care providers and instead receive information from television, magazines, and newspapers (Friedman et al., 2013). When primary care providers do advise patients on ways to delay cognitive impairment, they most commonly advise engagement in exercise and intellectual stimulation (Friedman et al., 2013). While general and lifetime engagement in physical or cognitive activity may help prevent cognitive impairment, the usefulness of this advice to older adult consumers is questionable. Instead, geriatric care providers and researchers should work together to dispel myths about activities that are not evidence-based in addition to providing older adults with accurate information about effective activities and interventions. Moving forward, we recommend the following for practitioners and policy makers: (1) collaborate with researchers and existing social networks older adults utilize to provide multiple venues for intervention access, and (2) increase access and knowledge to geriatric care practitioners (and individuals) to reduce spreading incorrect information about cognitive health.
5. Conclusion
There is inconsistent evidence of transfer from behavioral interventions to cognitive outcomes in healthy older adults. There is consistent evidence of transfer from cognitive training to the trained cognitive domains, and evidence that aerobic training confers benefits to executive function. Preliminary RCTs utilizing novel behavioral interventions like mindfulness and theatre show promise in conferring cognitive benefits, but strong conclusions should not be drawn until such findings are replicated in larger samples. In general, the reviewed studies included poor intervention descriptions, lack of consistency in control group design, and little examination of long-term effects. In the future, research should include both cognitive and everyday functional outcomes, as well as adapt existing interventions to reach a broader, more inclusive and diverse sample of older adults.
Supplementary Material
Acknowledgements
Special thanks to Daniel Goldschmidt for his thoughtful feedback.
Funding
Briana N. Sprague received support by the Joseph and Jean Britton Distinguished Graduate Fellowship and the Kligman Fellowship through The College of Health and Human Development and The Center for Healthy Aging, The Pennsylvania State University. Christina E. Webb and Jinshil Hyun were partially supported by the National Institute on Aging Grant T32 AG049676, The Pennsylvania State University.
Footnotes
Conflict of interest
All authors have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arr.2019.04.002.
Note: Articles with asterisks denote articles included in the systematic review.
References2
- *Albinet CT, Boucard G, Bouquet CA, Audiffren M, 2010. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur. J. Appl. Physiol. 109 (4), 617–624. 10.1007/s00421-010-1393-y. [DOI] [PubMed] [Google Scholar]
- Alsubaie M, Abbott R, Dunn B, Dickens C, Keil TF, Henley W, Kuyken W, 2017. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: a systematic review. Clin. Psychol. Rev. 55, 74–91. 10.1016/j.cpr.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Desai U, Kirson NY, Enloe CJ, Ristovska L, King S, ... Kahle-Wrobleski K, 2017. Functional limitations and health care resource utilization for individuals with cognitive impairment without dementia: findings from a United States population-based survey. Alzheimer’s Demen. Diagn. Assess. Dis. Monitor. 6, 65–74. 10.1016/j.dadm.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L, 2008. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochr. Database Syst. Rev. 10.1002/14651858.CD005381.pub3. Issue 3. Art. No.: CD005381. [DOI] [PubMed] [Google Scholar]
- *Bakken RC, Carey JR, Di Fabio RP, Erlandson TJ, Hake JL, Intihar TW, 2001. Effect of aerobic exercise on tracking performance in elderly people: a pilot study. Phys. Ther. 81 (12), 1870–1879. [PubMed] [Google Scholar]
- *Ball KK, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, ... Advanced Cognitive Training for Independent an Vital Elderly Study Group, 2002. Effects of cognitive training interventions with older adults: a randomized controlled trial. J. Am. Med. Assoc. 288 (18), 2271–2281. 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Edwards JD, Ross LA, McGwin G Jr., 2010. Cognitive training decreases motor vehicole collision involvement of older drivers. J. Am. Geriatr. Soc. 58 (11), 2107–2113. 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Ross LA, Roth DL, Edwards JD, 2013. Speed of processing training in the ACTIVE study: how much is needed and who benefits? J. Aging Health 25 (8S), 65S–84S. 10.1177/0898264312470167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K, 2013. The Mental Activity and eXercise (MAX) Trial: a randomized controlled trial to enhance cognitive function in older adults. J. Am. Med. Assoc. Intern. Med 173 (9), 797–804. 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, 2011. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10 (9), 819–828. 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, de Leon CF, Bennett DA, 2006. The relation of lifetime cognitive activity and lifetime access to resources to late-life cognitive function in older African Americans. Aging Neuropsychol. Cogn. 13 (3–4), 516–528. 10.1080/138255890969519. [DOI] [PubMed] [Google Scholar]
- Bediou B, Adams DM, Mayer RE, Tipton E, Green CS, Bavelier D, 2018. Meta-analysis of action video game impact on perceptual, attentional, and cognitive skills. Psychol. Bull. 144 (1), 77–110. 10.1037/bul0000130. [DOI] [PubMed] [Google Scholar]
- *Belchior P, Marsiske M, Sisco SM, Yam A, Bavelier D, Ball K, Mann WC, 2013. Video game training to improve selective visual attention in older adults. CHB 29 (4), 1318–1324. 10.1016/j.chb.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Best DL, Hamlett KW, Davis SW, 1992. Memory complaint and memory performance in the elderly: the effects of memory-skills training and expectancy change. Appl. Cogn. Psychol. 6 (5), 405–416. 10.1002/acp.2350060505. [DOI] [Google Scholar]
- Bherer L, Erickson KI, Liu-Ambrose T, 2013. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 1–8. 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG, Yaffe K, Liverman CT, 2015. Cognitive aging: Progress in Understanding and Opportunities for Action. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Boot WR, Simons DJ, Stothart C, Stutts C, 2013. The pervasive problem with placebos in psychology: why active control groups are not sufficient to rule out placebo effects. Perspect. Psychol. Sci. 8 (4), 445–454. 10.1177/1745691613491271. [DOI] [PubMed] [Google Scholar]
- *Borella E, Carretti B, Riboldi F, de Beni R, 2010. Working memory training in older adults: evidence of transfer and maintenance effects. Psychol. Aging 25 (4), 767–778. 10.1037/a0020683. [DOI] [PubMed] [Google Scholar]
- *Borella E, Carretti B, Zanoni G, Zavagnin M, de Beni R, 2013. Working memory training in old age: an examination of transfer and maintenance effects. Arch. Clin. Neuropsychol. 28 (4), 331–347. 10.1093/arclin/act020. [DOI] [PubMed] [Google Scholar]
- *Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, ... Yao D, 2016. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front. Aging Neurosc. 8, 70. 10.3389/fnagi.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Saczynski JS, Rebok GW, Seeman T, Glass TA, McGill S, ... Fried LP, 2008. Exploring the effects of an “everyday” activity program on executive function and memory in older adults: Experience Corps. Gerontologist 48 (6), 793–801. 10.1093/geront/48.6.793. [DOI] [PubMed] [Google Scholar]
- *Carretti B, Borella E, Zavagnin M, de Beni R, 2013. Gains in language comprehension related to working memory training in healthy older adults. Int. J. Geriatr. Psychiatry 28 (5), 539–546. 10.1002/gps.3859. [DOI] [PubMed] [Google Scholar]
- *Cassilhas RC, Viana VAR, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT, 2007. The impact of resistance exercise on the cognitive function of the elderly. Med. Sci. Sports Exerc. 39 (8), 1401–1407. 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- Cattell RB, 1963. Theory of fluid and crystallized intelligence: a critical experiment. J. Educ. Psychol. 54 (1), 1–22. 10.1037/h0046743. [DOI] [PubMed] [Google Scholar]
- *Cavallini E, Bottiroli S, Capotosto E, De Beni R, Pavan G, Vecchi T, Borella E, 2015. Self-help memory training for healthy older adults in a residential care center: specific and transfer effects on performance and beliefs. Int. J. Geriatr. Psychiatry 30 (8), 870–880. 10.1002/gps.4230. [DOI] [PubMed] [Google Scholar]
- *Chambon C, Herrera C, Romaiquere P, Paban V, Alescio-Lautier B, 2014. Benefits of computer-based memory and attention training in healthy older adults. Psychol. Aging 29 (3), 731–743. 10.1037/a0037477. [DOI] [PubMed] [Google Scholar]
- *Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, ... Li C, 2012. The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Med. 10 (30), 1–13. 10.1186/1741-7015-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, 2003. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 14 (2), 125–130. 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Collins LM, 2018. Optimization of Behavioral, Behavioral, and Biomedical Interventions: the Multiphase Optimization Strategy (MOST). Springer. [Google Scholar]
- Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, ... Fiore MC, 2011. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann. Behav. Med. 41 (2), 208–226. 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Trail JB, Kugler KC, Baker TB, Piper ME, Mermelstein RJ, 2014. Evaluating individual intervention components: making decisions based on the results of a factorial screening experiment. Transl. Behav. Med. 4 (3), 238–251. 10.1007/s13142-013-0239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Craik FIM, Winocur G, Palmer H, Binns MA, Edwards M, Bridges K, ... Stuss DT, 2007. Cognitive rehabilitation in the elderly: effects on memory. J. Int. Neuropsychol. Soc. 13 (1), 132–142. 10.1017/S1355617707070166. [DOI] [PubMed] [Google Scholar]
- *Dahlin E, Stigsdotter Neely A, Larsson A, Bäckman L, Nyberg L, 2008. Transfer of learning after updating training mediated by the striatum. Science 320 (5882), 1510–1512. 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- *Dawson D, Richardson J, Troyer A, Binns M, Clark A, Polatajko H, ... Bar Y, 2014. An occupation-based strategy training approach to managing age-related executive changes: a pilot randomized controlled trial. Clin. Rehabil. 28 (2), 118–127. 10.1177/0269215513492541. [DOI] [PubMed] [Google Scholar]
- *de Medeiros K, Mosby A, Hanley KB, Pedraza MS, Brandt J, 2011. A randomized clinical trial of a writing workshop intervention to improve autobiographical memory and well-being in older adults. Int. J. Geriatr. Psychiatry 26 (8), 803–811. 10.1002/gps.2605. [DOI] [PubMed] [Google Scholar]
- Dye MWG, Green CS, Bavelier D, 2009. Increasing speed of processing with action video games. Curr. Direct. Psychol. Sci. 18 (6), 321–326. 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Fausto BA, Tetlow AM, Corona RT, Valdés EG, 2018. Systematic review and meta-analyses of useful field of view cognitive training. Neurosci. Biobehav. Rev. 84, 72–91. 10.1016/j.neubiorev.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Ruva CL, O’Brien JL, Haley CB, Lister JJ, 2013. An examination of mediators of the transfer of cognitive speed of processing training to everyday functional performance. Psychol. Aging 28 (2), 314–321. 10.1037/a0030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK, 2005. The impact of speed of processing training on cognitive and everyday performance. Aging Mental Health 9 (3), 262–271. 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt F, 2017. Speed of processing training results in lower risk of dementia. Alzheimer’s Dement. Transl. Res. Clin. Interv. 3 (4), 603–611. 10.1016/j.trci.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Falbo S, Condello G, Capranica L, Forte R, Pesce C, 2016. Effects of physical-cognitive dual task training on executive function and gait performance in older adults: a randomized controlled trial. BioMed Res. Int. 2016, 5812092. 10.1155/2016/5812092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig L, Küper C, Lippke S, Schwarzer R, Wiedemann AU, 2015. Cross-behavior associations and multiple health behavior change: a longitudinal study on physical activity and fruit and vegetable intake. J. Health Psychol. 20 (5), 525–534. 10.1177/1359105315574951. [DOI] [PubMed] [Google Scholar]
- Fountain-Zaragoza S, Prakash RS, 2017. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front. Aging Neurosci. 9 (11), 1–15. 10.3389/fnagi.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Becofsky K, Anderson LA, Bryant LL, Hunter RH, Ivey SL, ... Lin SY, 2015. Public perceptions about risk and protective factors for cognitive health and impairment: a review of the literature. Int. Psychogeriatr. 27 (8), 1263–1275. 10.1017/S1041610214002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Laditka SB, Laditka JN, Wu B, Liu R, Price AE, ... Sharkey JR, 2011. Ethnically diverse older adults’ beliefs about staying mentally sharp. Int. J. Aging Hum. Dev. 73 (1), 27–52. 10.2190/AG.73.1.b. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Rose ID, Anderson LA, Hunter R, Bryant LL, Wu B, ... Tseng W, 2013. Beliefs and communication practices regarding cognitive functioning among consumers and primary care providers in the United States, 2009. Prevent. Chron. Dis. 10, 1–13. 10.5888/pcd10.120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan AD, Pennick V, Bombardier C, van Tulder M, 2009. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine J. 34 (18), 1929–1941. 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- *Gajewski PD, Falkenstein M, 2012. Training-induced improvement of response selection and error detection in aging assessed by task switching: effects of cognitive, physical, and relaxation training. Front. Hum. Neurosci. 6, 130. 10.3389/fnhum.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Galvin JA, Benson H, Deckro GR, Fricchione GL, Dussek JA, 2006. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement. Therap. Clin. Pract. 12 (3), 186–191. 10.1016/j.ctcp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Gamaldo AA, Allaire JC, 2016. Daily fluctuations in everyday cognition: is it meaningful? J. Aging Health 38 (5), 834–849. 10.1177/0898264315611669. [DOI] [PubMed] [Google Scholar]
- García-Betances RI, Cabrera-Umpiérrez MF, Arredondo MT, 2018. Computerized neurocognitive interventions in the context of the brain training controversy. Rev. Neurosci. 29 (1), 55–69. 10.1515/revneuro-2017-0031. [DOI] [PubMed] [Google Scholar]
- Gard T, Holzel BK, Lazar SW, 2014. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann. N. Y. Acad. Sci. 1307, 89–103. 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand LA, Mensinger JL, Tenhave T, 2009. Mediation analysis: a retrospective snapshot of practice and more recent directions. J. Gen. Psychol. 136 (2), 153–176. 10.3200/GENP.136.2.153-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SJ, Friedenreich CM, Sajobi TT, Longman RS, Drogos LL, Davenport MH, ... Poulin MJ, 2015. Association between lifetime physical activity and cognitive functioning in middle-aged and older community dwelling adults: results from the brain in motion study. J. Int. Neuropsychol. Soc. 21 (10), 816–830. 10.1017/S1355617715000880. [DOI] [PubMed] [Google Scholar]
- *Goghari VM, Lawlor-Savage L, 2017. Comparison of cognitive change after working memory training and logic and planning training in healthy older adults. Front. Aging Neurosci. 9, 39. 10.3389/fnagi.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Goldstein JH, Cajko L, Oosterbroek M, Michielsen M, Van Houten O, Salverda F, 1997. Video games and the elderly. Soc. Behav. Personal. 25 (4), 345–352. 10.2224/sbp.1997.25.4.345. [DOI] [Google Scholar]
- Greenwood PM, Parasuraman R, 2016. The mechanisms of far transfer from cognitive training: review and hypothesis. Neuropsychology 30 (6), 742–755. 10.1037/neu0000235. [DOI] [PubMed] [Google Scholar]
- Hamer M, Lavoie KL, Bacon SL, 2013. Taking up physical activity later in life and healthy ageing: the English longitudinal study of ageing. Br. J. Sports Med. 0, 1–6. 10.1136/bjsports-2013-092993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yang L, Zhou J, Yao L, Pang MCY, 2018. Dual-task training effects on motor and cognitive functional abilities in individuals with stroke: a systematic review. Clin. Rehabilit. 10.1177/0269215518758482. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- *Hiyamizu M, Morioka S, Shomoto K, Shimada T, 2011. Effects of dual task balance training on dual task performance in elderly people: a randomized controlled trial. Clin. Rehabil. 26 (1), 58–67. 10.1177/0269215510394222. [DOI] [PubMed] [Google Scholar]
- Hollis S, Campbell F, 1999. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 319 (7211), 670–674. 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-L, Hsu W-C, Chang C-C, Lin K-J, Hsiao I-T, Fan Y-C, Bai C-H, 2017. Everyday cognition scales are related to cognitive function in the early stage of probable Alzheimer’s diesase and FDG-PET findings. Scient. Rep. 7 (1), 1719. 10.1038/s41598-017-01193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball KK, Tennstedt SL, Marsiske M, Willis SL, ... Kleinman K, 2001. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control. Clin. Trials 22 (4), 453–479. 10.1016/S0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Dodson JE, Steadman L, Vance DE, 2014. Predictors of improvement following speed of processing training in middle-aged and older adults with HIV: a pilot study. Am. Assoc. Neurosci. Nurs. 46 (1), 23–33. 10.1097/JNN.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S, 2014a. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res. Rev. 15, 28–43. 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S, 2014b. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res. Rev. 16, 12–31. 10.1016/j.aar.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kim S, Sargent-Cox KA, Anstey KJ, 2015. A qualitative study of older and middle-aged adults’ perception and attitudes towards dementia and dementia risk reduction. J. Adv. Nurs. 71 (7), 1694–1703. 10.1111/jan.12641. [DOI] [PubMed] [Google Scholar]
- *Kimura K, Hozumi N, 2012. Investigating the acute effect of an aerobic dance exercise program on neuro-cognitive function in the elderly. Psychol. Sport Exer. 13 (5), 623–629. 10.1016/j.psychsport.2012.04.001. [DOI] [Google Scholar]
- *Kimura K, Obuchi S, Arai T, Nagasawa H, Shiba Y, Watanabe S, Kojima M, 2010. The influence of short-term strength training on health-related quality of life and executive cognitive function. J. Physiol. Anthropol. 29 (3), 95–101. 10.2114/jpa2.29.95. [DOI] [PubMed] [Google Scholar]
- Kirk-Sanchez NJ, McGough EL, 2014. Physical exercise and cognitive performance in the elderly: current perspectives. Clin. Interv. Aging 9, 51–62. 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kitazawa K, Showa S, Hiraoka A, Fushiki Y, Sakauchi H, Mori M, 2015. Effect of a dual-task net-step exercise on cognitive and gait function in older adults. J. Geriatr. Phys. Therapy 38 (3), 133–140. 10.1519/JPT.0000000000000029. [DOI] [PubMed] [Google Scholar]
- *Klusmann V, Evers A, Schwarzer R, Heuser I, 2011. Activity experiences shape perceived fitness trajectories: results from a 6-month randomized controlled trial in older women. Aging Neuropsychol. Cogn. 18 (3), 328–339. 10.1080/13825585.2011.553272. [DOI] [PubMed] [Google Scholar]
- *Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, Dimeo FC, 2010. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial . J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 65 (6), 680–688. 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- Kruger J, Carlson SA, Buchner D, 2007. How active are older Americans? Prevent. Chron. Dis. 4 (3), A53. [PMC free article] [PubMed] [Google Scholar]
- Laditka JN, Laditka SB, Lowe KB, 2012. Promoting cognitive health: a web site review of health systems, public health departments, and senior centers . Am. J. Alzheimers Dis. Other Demen. 27 (8), 600–608. 10.1177/1533317512460564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Lampit A, Hallock H, Moss R, Kwok S, Rosser M, Lukjanenko M, ... Valenzuela M, 2014. The timecourse of global cognitive gains from supervised computer-assisted cognitive training: a randomised, active-controlled trial in elderly with multiple dementia risk factors. J. Prevent. Alzheimer’s Dis. 1 (1), 33–39. 10.14283/jpad.2014.18. [DOI] [PubMed] [Google Scholar]
- *Lampit A, Hallock H, Suo C, Naismith SL, Valenzuela M, 2015a. Cognitive training-induced short-term functional and long-term structural plastic change is related to gains in global cognition in healthy older adults: a pilot study. Front. Aging Neurosci. 7, 14. 10.3389/fnagi.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampit A, Valenzuela M, Gates NJ, 2015b. Computerized cognitive training is beneficial for older adults. J. Am. Geriatr. Soc. 63 (12), 2610–2615. 10.1111/jgs.13825. [DOI] [PubMed] [Google Scholar]
- Lau KM, Parikh M, Harvey DJ, Huang C-J, Tomaszewski Farias S, 2015. Early cognitively-based functional limitations predict loss of independence in instrumental activities of daily living in older adults. Neuropsychol. Soc. 21 (9), 688–698. 10.1017/S1355617715000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Lee Y, Lee C-R, Hwang B, 2012. Effects of computer-aided cognitive rehabilitation training and balance exercise on cognitive and visual perception ability of the elderly. J. Phys. Therapy Sci. 24 (9), 885–887. 10.1589/jpts.24.885. [DOI] [Google Scholar]
- SHARP-P Study Group,*Legault C, Jennings JM, Katula JA, Dagenbach D, Gaussoin SA, Sink KM, ... 2011. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the Seniors Health and Activity Research Program Pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatr. 11 (27). 10.1186/1471-2318-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, 2004. Neuropsychological Assessment, 4th ed. Oxford University Press, New York. [Google Scholar]
- *Li KZH, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley PA, 2010. Benefits of cognitive dual-task training on balance performance in healthy older adults. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 65 (12), 1344–1352. 10.1093/gerona/glq151. [DOI] [PubMed] [Google Scholar]
- *Liu-Ambrose T, Doanldson MG, Ahamed Y, Graf P, Cook WL, Close J, ... Khan KM, 2008. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J. Am. Geriatr. Soc. 56 (10), 1821–1830. 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- *Liu-Ambrose T, Nagamatsu LS, Graf P, Beatte BL, Ashe MC, Handy TC, 2010. Resistance training and executive function: a 12-month randomized controlled trial. Arch. Intern. Med. 170 (2), 170–178. 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC, 2012. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol. Aging 33 (8), 1690–1698. 10.1016/j.,neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- *Luo C, Zhang X, Cao X, Gan Y, Li T, Cheng Y, ... Li C, 2016. The lateralization of intrinsic networks in the aging brain implicates the effects of cognitive training. Front. Aging Neurosci. 8, 32. 10.3389/fnagi.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidler R, Reuter-Lorenz PA, 2009. Aging, training, and the brain: a review and future directions. Neuropsychol. Rev. 19 (4), 504–522. 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Maclean LM, Brown LJE, Astell AJ, 2014. The effect of rhythmic musical training on healthy older adults’ gait and cognitive function. Gerontologist 54 (4), 624–633. 10.1093/geront/gnt050. [DOI] [PubMed] [Google Scholar]
- Maher CG, Sherrington C, Hebert RD, Moseley AM, Elkins M, 2003. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 83 (8), 713–721. [PubMed] [Google Scholar]
- *Maillot P, Perrot A, Hartley A, 2012. Effects of interactive physical-activity videogame training on physical and cognitive function in older adults. Psychol. Aging 27 (3), 589–600. 10.1037/a0026268. [DOI] [PubMed] [Google Scholar]
- Maki Y, Ura C, Yamaguchi T, Murai T, Isahai M, Kaiho A, ... Yamaguchi H, 2012. Effects of intervention using a community-based walking program for prevention of mental decline: a randomized controlled trial. J. Am. Geriatr. Soc. 60 (3), 505–510. 10.1111/j.1532-5415.2011.03838.x. [DOI] [PubMed] [Google Scholar]
- Malinowski P, Shalamanova L, 2017. Meditation and cognitive ageing: the role of mindfulness meditation in building cognitive reserve. J. Cogn. Enhan. 1 (2), 96–106. 10.1007/s41465-017-0029-0. [DOI] [Google Scholar]
- *McDougall GJ Jr., Becker H, Pituch K, Acee TW, Vaughan PW, Delville CL, 2010. The SeniorWISE study: improving everyday memory in older adults. Arch. Psychiatr. Nurs. 24 (5), 291–306. 10.1016/j.apnu.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Millán-Calenti JC, Lorenzo T, Núñez-Naveira L, Buján A, Rodríguez-Villamil JL, Maseda A, 2015. Efficacy of a computerized cognitive training application on cognition and depressive symptomatology in a group of healthy older adults: a randomized controlled trial. Achr. Gerontol. Geriatr. 61 (3), 337–343. 10.1016/j.archger.2015.08.015. [DOI] [PubMed] [Google Scholar]
- *Miller KJ, Dye RV, Kim J, Jennings JL, O’Toole E, Wong J, Siddarth P, 2013. Effect of a computerized brain exercise program on cognitive performance in older adults. Am. J. Geriatr. Psychiatry 21 (7), 655–663. 10.1016/j.jagp.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Mitchell MB, Cimino CR, Benitez A, Brown CL, Gibbons LE, Kennison RF, ... Piccinin AM, 2012. Cognitively stimulating activities: effects on cognition across four studies with up to 21 years of longitudinal data. J. Aging Res. 2012, 1–12. 10.1155/2012/461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Junior RS, Otero Vaghetti CA, Nascimento OJM, Laks J, Camaz Deslandes A, 2016. Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons. Neur. Regen. Res. 11 (2), 201–204. 10.4103/1673-5374.177709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter JN, Devanand DP, Doraiswamy PM, Sneed JR, 2016. Clinical trials to gain FDA approval for computerized cognitive training: what is the ideal control condition? Front. Aging Neurosci. 8 (249), 1–6. 10.3389/fnagi.2016.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Moul JL, Goldman B, Warren B, 1995. Physical activity and cognitive performance in the older population. J. Aging Phys. Act. 3 (2), 135–145. [Google Scholar]
- Mowszowski L, Lampit A, Walton CC, Naismith SL, 2016. Strategy-based cognitive training for improving executive functions in older adults: a systematic review. Neuropsychol. Rev. 26 (3), 252–270. 10.1007/s11065-016-9329-x. [DOI] [PubMed] [Google Scholar]
- *Mozolic JL, Long AB, Morgan AR, Rawley-Payne M, Laurienti PJ, 2011. Neurobiol. Aging 32 (4), 655–668. 10.1016/j.neurobiolaging.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Muscari A, Giannoni C, Pierpaoli L, Berzigotti A, Maietta P, Foschi E, ... Zoli M, 2010. Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: a randomized controlled trial. Int. J. Geriatr. Psychiatry 25 (10), 1055–1064. 10.1002/gps.2462. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, & Medicine, 2017. Preventing Cognitive Decline and Dementia: A Way Forward. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Noar SM, Chabot M, Zimmerman RS, 2008. Applying health behavior theory to multiple behavior change: considerations and approaches. Prev. Med. 46 (3), 275–280. 10.1016/j.ypmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- *Noice H, Noice T, 2009. An arts intervention for older adults living in subsidized retirement homes. Neuropsychol. Dev. Cogn. 16 (1), 56–79. 10.1080/13825580802233400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Noice H, Noice T, 2013. Extending the reach of an evidence based theatrical intervention. Exp. Aging Res. 39 (4), 398–418. 10.1080/0361073X.2013.808116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noice T, Noice H, Kramer AF, 2013. Participatory arts for older adults: a review of benefits and challenges. Gerontologist 54 (5), 741–753. 10.1093/geront/gnt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, ... Kawashima R, 2012. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PLoS ONE 7 (1), e29676. 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nouchi R, Taki Y, Takeuchi H, Nozawa T, Sekiguchi A, Kawashima R, 2016. Reading aloud and solving simple arithmetic calculation intervention (learning therapy) improves inhibition, verbal episodic memory, focus attention and processing speed in healthy elderly people: evidence from a randomized controlled trial. Front. Hum. Neurosci. 10, 217. 10.3389/fnhum.2016.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *O’Brien JL, Edwards JD, Maxfield ND, Peronto CL, Williams VA, Lister JJ, 2013. Cognitive training and selective attention in the aging brain: an electrophysiological study. Clin. Neurophysiol. 124 (11), 2198–2208. 10.1016/j.clinph.2013.05.012. [DOI] [PubMed] [Google Scholar]
- *Oken BS, Zajdel D, Kishiyama S, Flegal K, Dehen C, Haas M, … Leyva J, 2006. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern. Ther. Health Med. 12 (1), 40–47. [PMC free article] [PubMed] [Google Scholar]
- *Okumiya K, Matsubayashi K, Wada T, Kimura S, Doi Y, Ozawa T, 1996. Effects of exercise on neurobehavioral function in community-dwelling older people more than 75 years of age. J. Am. Geriatr. Soc. 44 (5), 569–572. 10.1111/j.1532-5415.1996.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H, 2014. An Aging Nation: The Older Population in the United States (P25–1140). Retrieved from http://www.census.gov/library/publications/2014/demo/p25-1140.html.
- Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL, 2017. Predictors of reversion from mild cognitive impairment to normal cognition. Dement. Geriatr. Cogn. Disord. 43 (3–4), 204–214. 10.1159/000456070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Panton LB, Graves JE, Pollock ML, Hagberg JM, Chen W, 1990. Effect of aerobic and resistance training on fractionated reaction time and speed of movement. J. Gerontol. Ser. A: Biol. Sci. Med. Sci 45 (1), M26–M31. 10.1093/geronj/45.1.M26. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH, Meade ML, Stine-Morrow E, 2007. Improving cognitive function on older adults: nontraditional approaches. J. Gerontol. Ser. B: Psychol. Sci. Soc. Sci. 62B (Special Issue 1), 45–52. 10.1093/geronb/62.special_issue_1.45. [DOI] [PubMed] [Google Scholar]
- Park DC, Lodi-Smith J, Drew L, Haber S, Hebrank A, Bischof GN, Aamodt W, 2014. The impact of sustained engagement on cognitive function in older adults: the Synapse Project. Psychol. Sci. 25 (1), 103–112. 10.1177/0956797613499592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Stine-Morrow EAL, 2017. The effects of home-based cognitive training on verbal working memory and language comprehension in older adulthood. Front. Aging Neurosci. 9 (256), 1–20. 10.3389/fnagi.2017.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Perrig-Chiello P, Perrig WJ, Ehrsam R, Staehelin HB, Krings F, 1998. The effects of resistance training on well-being and memory in elderly volunteers. Age Ageing 27 (4), 469–475. 10.1093/ageing/27.4.469. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Zyphur MJ, Zhang Z, 2010. A general multilevel SEM framework for assessing multilevel mediation. Psychol. Methods 15 (3), 209–233. 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- Rabipour S, Raz A, 2012. Training the brain: fact and fad in cognitive and behavioral remediation. Brain Cogn. 79, 159–179. 10.1016/j.bandc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Rahe J, Petrelli A, Kaesberg S, Fink GR, Kessler J, Kalbe E, 2015. Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clin. Interv. Aging 2015 (10), 297–310. 10.2147/CIA.S74071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, 2016. Selecting control groups: to what should we compare behavioral interventions? In: Gitlin LN, Czaja SJ (Eds.), Behavioral Intervention Research: Designing, Evaluating, and Implementing. Springer Publishing Company, New York, NY. [Google Scholar]
- *Rebok GW, Ball KK, Guey LT, Jones RN, Kim H-Y, King JW, ... Willis SL, 2014. Ten year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 62, 16–24. 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Langbaum JBS, Jones RN, Gross AL, Parisi JM, Spira AP, Kueider AM, Petras H, Brandt J, 2013. Memory training in the ACTIVE study: how much is needed and who benefits? J. Aging Health 25 (8S), 21S–42S. 10.1177/0898264312461937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders J, van Heugten C, van Boxtel M, 2013. Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Ageing Res. Rev. 12, 263–275. 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- *Requena C, Turrero A, Ortiz T, 2016. Six-year training improves everyday memory in healthy older people. Randomized controlled trial. Front. Aging Neurosci. 8, 135. 10.3389/fnagi.2016.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Edwards JD, O’Connor ML, Ball KK, Wadley VG, Vance DE, 2016. The transfer of cognitive speed of processing training to older adults’ driving mobility across 5 years. J. Gerontol. Ser. B: Psychol. Sci. Soc. Sci. 71 (1), 87–97. 10.1093/geronb/gbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Freed SA, Edwards JD, Phillips CB, Ball KK, 2017. The impact of three cognitive training programs on driving cessation across 10 years: a randomized controlled trial. Gerontologist 57 (5), 838–846. 10.1093/geront/gnw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Sprague BN, Phillips CB, O’Connor ML, Dodson JE, 2018. The impact of three cognitive training interventions on older adults’ physical functioning across 5 years. J. Aging Health 30 (3), 475–498. 10.1177/0898264316682916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, Webb CE, Whitaker C, Hicks JM, Schmidt EL, Samimy S, ... Visscher KM, 2019. The effects of Useful Field of View training on brain activity and connectivity. J. Gerontol. Ser. B: Psychol. Sci. 10.1093/geronb/gby041. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Sato D, Seko C, Hashitomi T, Sengoku Y, Nomura T, 2015. Differential effects of water-based exercise on the cognitive function in independent elderly adults. Aging Clin. Exp. Res. 27 (2), 149–159. 10.1007/s40520-014-0252-9. [DOI] [PubMed] [Google Scholar]
- *Schättin A, Arner R, Gennaro F, de Bruin ED, 2016. Adaptations of prefrontal brain activity, executive functions, and gait in healthy elderly following exergame and balance training: a randomized-controlled study. Front. Aging Neurosci. 8, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SB, Graham-Engeland JE, Engeland CG, Smyth JM, Almeida DM, Katz MJ, ... Sliwinksi MJ, 2015. The Effects of Stress on Cognitive Aging, Physiology, and Emotion (ESCAPE) project. BMC Psychiatry 15, 146. 10.1186/s12888-015-0497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah TM, Weinborn M, Verdile G, Sohrabi HR, Martins RN, 2017. Enhancing cognitive functioning in healthy older adults: a systematic review of the clinical significance of commercially available computerized cognitive training in preventing cognitive decline. Neuropsychol. Rev. 27, 62–80. 10.1007/s11065-016-9338-9. [DOI] [PubMed] [Google Scholar]
- Shannon CE, 1949. Communication in the presence of noise. Proc. IRE 37 (1), 10–21. 10.1109/JRPROC.1949.232969. [DOI] [Google Scholar]
- Sharpe C, Holup AA, Hansen KE, Edwards JD, 2014. Does self-efficacy affect responsiveness to cognitive speed of processing training? J. Aging Health 26 (5), 789–806. 10.1177/0898264314531615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Shatil E, 2013. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone?. A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 5 (8), 1–12. 10.3389/fnagi.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyko MP, Ram N, 2011. Conceptualizing and estimating process speed in studies employing ecological momentary assessment designs: a multilevel variance decomposition approach. Multivar. Behav. Res. 46 (6), 875–899. 10.1080/00273171.2011.625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, Stine-Morrow EAL, 2016. Do “brain-training” programs work? Psychol. Sci. Publ. Interest 17 (3), 103–186. 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- *Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P, 2008. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascularfitness” hypotheses. Ann. Behav. Med. 36 (3), 280–291. 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM, 2009. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J. Am. Geriatr. Soc 57 (4), 594–603. 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ray RL, Hughes SL, Prohaska TR, Little DM, Jurivich DA, Hedeker D, 2013. Impact of cognitive training on balance and gait in older adults. J. Gerontol. Ser. B: Psychol. Sci. Soc. Sci 70, 357–366. 10.1093/geronb/gbt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ray RL, Makowski-Woidan B, Hughes SL, 2014. A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Educ. Behav. 41 (Suppl. 1), 62S–69S. 10.1177/1090198114537068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemöller EL, 2014. Exploring a neuroplacticity model of music therapy. J. Music Ther. 51 (3), 211–227. 10.1093/jmt/thu023. [DOI] [PubMed] [Google Scholar]
- *Stepankova H, Lukavsky J, Buschkuehl M, Kopecek M, Ripova D, Jaeggi SM, 2014. The malleability of working memory and visuospatial skills: a randomized controlled study in older adults. Dev. Psychol. 50 (4), 1049–1059. 10.1037/a0034913. [DOI] [PubMed] [Google Scholar]
- Stern Y, 2002. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. [PubMed] [Google Scholar]
- Stern Y, 2003. The concept of cognitive reserve: a catalyst for research. J. Clin. Exp. Neuropsychol. 25 (5), 589–593. [DOI] [PubMed] [Google Scholar]
- Stern Y, 2013. Cognitive reserve: implications for assessment and intervention. Folia Phoniatr. Logopaed. 65 (2), 49–54. 10.1159/000353443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Stigsdotter A, Backman L, 1989. Multifactorial memory training with older adults: how to foster maintenance of improved performance. Gerontology 35 (5–6), 260–267. 10.1159/000213035. [DOI] [PubMed] [Google Scholar]
- *Stigsdotter-Neely A, Backman L, 1993. Long-term maintenance of gains from memory training in older adults: two 3 1/2-year follow-up studies. J. Gerontol.: Psychol. Sci. 45 (5), 233–237. 10.1093/geronj/48.5.P233. [DOI] [PubMed] [Google Scholar]
- Stine-Morrow EAL, Parisi JM, Morrow DG, Park DC, 2008. The effects of an engaged lifestyle on cognitive vitality: a field experiment. Psychol. Aging 23 (4), 778–786. 10.1037/a0014341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow EAL, Payne BR, Roberts BW, Kramer AF, Morrow DG, Payne L, ... Janke MC, 2014. Training versus engagement as paths to cognitive enrichment with aging. Psychol. Aging 29 (4), 891–906. 10.1037/a0038244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sween J, Flynnt Wallington S, Sheppard V, Taylor T, Llanos AA, Adams-Campbell LL, 2014. The role of exergaming in improving physical activity: a review. J. Phys. Act. Health 11 (4), 864–870. 10.1123/jpah.2011-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski Farias S, Giovannetti T, Payne BR, Marsiske M, Rebok GW, Schaie KW, ... Gross AL, 2018. Self-perceived difficulties in everyday function precede cognitive decline among older adults in the ACTIVE study. J. Int. Neuropsychol. Soc 24 (1). 10.1017/S1355617717000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toril P, Reales JM, Ballesteros S, 2014. Video game training enhances cognition of older adults: a meta-analytic study. Psychol. Aging 29 (3), 706–716. 10.1037/a0037507. [DOI] [PubMed] [Google Scholar]
- *Torres A, 2008. Cognitive effects of videogames on older people. Int. J. Disabil. Hum. Dev. 10 (1), 55–58. 10.1515/ijdhd.2011.003. [DOI] [Google Scholar]
- *Uemura K, Yamada M, Nagai K, Tateuchi H, Mori S, Tanaka B, Ichihashi N, 2012. Effects of dual-task switch exercise on gait and gait initiation performance in older adults: preliminary results of a randomized controlled trial. Arch. Gerontol. Geriatr. 54 (2), e167–e171. 10.1016/j.archger.2012.01.002. [DOI] [PubMed] [Google Scholar]
- *van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M, 2008. Walking or vitamin B for cognition in older adults with mild cognitive impairment?. A randomised controlled trial. Br. J. Sports Med. 42 (5), 344–351. 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, ... Buschke H, 2003. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 348 (25), 2508–2516. 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Wang H-X, Jin Y, Hendrie HC, Liang C, Yang L, Cheng Y, ... Gao S, 2013. Late life leisure activities and risk of cognitive decline. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 68 (2), 205–213. 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne PM, Walsh JN, Taylor-Piliae RE, Wells RE, Papp KV, Donovan NJ, Yeh GY, 2014. Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. J. Am. Geriatr. Soc. 62 (1), 25–39. 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Westlake KP, Culham EG, 2007. Sensory-specific balance training in older adults: effect on proprioceptive reintegration and cognitive demands. Phys. Ther. 87 (10), 1274–1283. 10.2522/ptj.20060263. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Sharkey JR, Mathews AE, Laditka JN, Laditka SB, Logsdon RG, ... Liu R, 2009. Perceptions and beliefs about the role of physical activity and nutrition on brain health in older adults. Gerontologist 49 (S1), S61–S71. 10.1093/geront/gnp078. [DOI] [PubMed] [Google Scholar]
- Williamson JD, Espeland M, Kritchevsky SB, Newman AB, King AC, Pahor M, ... Miller ME, 2009. Changes in cognitive function in a randomized trial of physical activity: results of the Lifestyle Interventions and Independence for Elders Pilot Study. J. Gerontol. Ser. A: Med. Sci 64 (6), 688–694. 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Caskie GIL, 2013. Reasoning training in the ACTIVE study: how much is needed and who benefits? J. Aging Health 25 (8S), 43S–64S. 10.1177/0898264313503987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Willis SL, Tennstedt S, Marsiske M, Ball KK, Elias J, Koepke KM, ... Wright E, 2006. Long-term effects of cognitive training on everyday functional outcomes in older adults. J. Am. Med. Assoc. 296 (23), 2805–2814. 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, 2003. Assessment of lifetime participation in cognitively stimulating activities. J. Clin. Exp. Neuropsychol. 25 (5), 634–642. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke HW, Kosinski M, Unverzagt FW, Smith DM, Jones RN, ... Tennstedt SL, 2009. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv. Res. 9 (1), 109. 10.1186/1472-6963-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM, 2013. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS ONE 8 (5), e61624. 10.1371/journal.pone.0061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM, 2015. The effect of cognitive speed of processing training on the development of additional IADL difficulties and the reduction of depressive symptoms: results from the IHAMS randomized controlled trial. J. Aging Health 27 (2), 335–354. 10.1177/0898264314550715. [DOI] [PubMed] [Google Scholar]
- Wu R, Rebok GW, Lin FV, 2017. A novel theoretical life course framework for triggering cognitive development across the lifespan. Hum. Dev. 59, 342–365. 10.1159/000458720. [DOI] [Google Scholar]
- Zelinski EM, Reyes R, 2009. Cognitive benefits of computer games for older adults. Gerontechnology 8 (4), 220–235. 10.4017/gt.2009.08.04.004.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Zelinski EM, Spina LM, Yaffe K, Ruff R, Kennison RF, Hahncke HW, Smith GE, 2011. Improvement in memory with plasticity-based adaptive cognitive training: results of the 3-month follow-up. J. Am. Geriatr. Soc. 59 (2), 258–265. 10.1111/j.1532-5415.2010.03277.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Yin S, Lang M, He R, Li J, 2016. The more the better?. A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 31, 67–79. 10.1016/j.arr.2016.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


