Abstract
Resolution is an active and highly coordinated process that occurs in response to inflammation in order to limit tissue damage and promote repair. When the resolution program fails, inflammation persists. It is now understood that failed resolution is a major underlying cause of many chronic inflammatory diseases. Here, we will review the major failures of resolution in atherosclerosis, including the imbalance of pro-inflammatory to pro-resolving mediator production, impaired clearance of dead cells, and functional changes in immune cells that favor ongoing inflammation. In addition, we will briefly discuss new concepts that are emerging as possible regulators of resolution and highlight the translational significance for the field.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disability worldwide.1 The rising prevalence of obesity and diabetes, two major risk factors for ASCVD, have exacerbated this further in the past decade, setting off a race to develop new therapies. Recent years have seen major advances in both the scientific understanding and clinical treatment of atherosclerosis. Conventional therapy focuses on lowering low-density lipoprotein (LDL) levels, yet many patients suffer recurrent clinical events despite having achieved “goal” LDL levels.2, 3 This continued cardiovascular risk after optimal pharmacotherapy is referred to as “residual risk”. Clinical studies have now confirmed decades of pre-clinical research demonstrating that this enhanced risk is largely due to inflammation. The landmark CANTOS trial showed that in patients with already well-controlled LDL levels, blocking interleukin (IL)-1β led to reduced cardiovascular events independent of cholesterol lowering.3 Subsequently, the COLCOT and LoDoCo 2 trials showed that treatment with colchicine, an anti-inflammatory medication that targets multiple inflammatory pathways, also leads to fewer cardiac events in patients with known ASCVD.4, 5 While these studies mark a turning point in the treatment of ASCVD, they have simultaneously highlighted new challenges. Inhibition of the immune response compromises host defense, leaving patients susceptible to infectious complications. This was confirmed by both COLCOT, which reported a higher rate of pneumonia in patients receiving colchicine, and CANTOS, which observed a higher rate of fatal infection in patients receiving anti-IL-1β therapy.4, 6 Therefore, while targeting chronic inflammation in ASCVD is a priority, it is crucial to identify a way to do so without impairing the ability to mount an acute inflammatory response. A growing body of recent work suggests that the chronic inflammation associated with atherosclerosis results not only from excessive inflammation, but also from the failure of inflammation to resolve. Inflammation resolution is an active process that involves production of specific pro-resolving proteins and lipids that terminate the acute inflammatory response and guide tissue repair. This review will discuss recent advances in the study of inflammation resolution and how these concepts can be applied to our understanding of atherosclerosis in order to develop novel therapeutics.
Atherosclerosis is an Inflammatory Disease.
Atherogenesis arises in medium and large-sized arteries and typically begins at sites of perturbed or non-laminar flow. At these sites, endothelial cells are dysfunctional and permissive to the passage of circulating lipoproteins into the intimal space. Once there, lipoproteins become trapped in the subendothelial layer where they undergo enzymatic and oxidative modifications. Both native LDL and oxidized LDL (oxLDL) play an important role in atherogenesis. Oligopeptides derived from the apoplipoprotein B of native LDL drive T cell responses, while oxLDL elicits a robust innate inflammatory response.7 In addition, trapped lipoproteins activate endothelial cells and vascular smooth muscle cells (VSMCs) to increase their expression of adhesion molecules and production of chemokines. In turn, this leads to recruitment of leukocytes into the vessel wall. Monocytes, in particular inflammatory Ly6chigh monocytes in mice, make up the majority of the infiltrating cells and differentiate into macrophages (Mϕs). Mϕs take up lipoproteins, producing the cholesterol ester-laden “foam cells” that are characteristic of early lesions.8 The interaction of Mϕs with modified lipoproteins triggers pro-inflammatory cascades that augment leukocyte recruitment. As plaques become more advanced, lymphocytes, dendritic cells, and neutrophils accumulate alongside Mϕs. The pro-inflammatory milieu within the growing lesion, together with excess intracellular cholesterol, activates ER stress pathways leading to apoptotic cell death. Apoptotic cells that are not cleared in a timely fashion progress to necrosis by activating self-hydrolytic enzymes, which leads to swelling of the cell, irreparable damage to the cytoplasmic membrane, and cell disruption in a process known as “secondary necrosis”.9 These necrotic cells coalesce to form a nidus of debris and lipid that becomes the necrotic core within the plaque. VSMCs migrate from the media to the intima where they elaborate extracellular matrix that forms the collagen cap overlying the lesion. This matrix also contributes to further entrapment of lipoproteins. Growing evidence suggests that VSMCs within the intima also undergo metaplasia to become Mϕs, contributing to foam cell formation. The fate of the plaque is ultimately determined by its growth and stability. Some plaques will suffer from continued leukocyte recruitment, ongoing proliferation of Mϕs, and excessive apoptosis. This then leads to heightened inflammation, expanding necrotic cores, thinning fibrous caps, and endothelial erosion, rendering the lesion more prone to cause clinical events. While the pro-inflammatory mechanisms associated with atherosclerosis have been long appreciated (and recently elegantly reviewed8, 10-13), it is now clear that failures of resolution are an equally important contributor to chronic inflammation.
Atherosclerosis Results from Failures of Resolution.
Inflammation is a physiologic process aimed at protecting the host, however the protective nature of the process relies on an adequate resolution phase. If resolution fails, the inflammatory phase persists, leading to tissue damage and development of chronic disease. In recent years, it has become evident that several aspects of the resolution program fail in advanced atherosclerosis.14-16
Specialized Pro-Resolving Mediators.
The specialized pro-resolving mediators (SPMs) comprise a family of ligands that function in a receptor-dependent fashion on a wide range of target cells. They are derived from arachidonic acid (AA), docosahexaenoic acid (DHA), or eicosapentaenoic acid (EPA) by the action of cyclooxygenases (COX) or lipoxygenases (LOX). This generates multiple classes of SPMs including the lipoxins (LX), resolvins (Rv), protectins, and maresins (Figure 1). SPMs can be produced by a wide variety of cell types including macrophages, neutrophils, and lymphocytes. In addition, transcellular synthesis has been observed between leukocytes, platelets, and epithelial cells, with intermediates being formed in one cell type and then synthesized to SPMs by the other.17
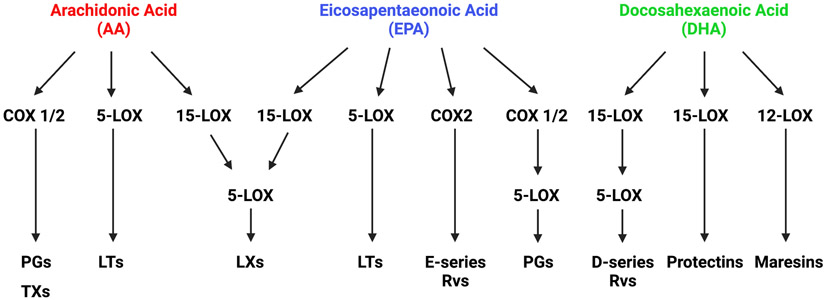
Figure 1. Simplified scheme of lipid mediator production.
The fatty acids, including arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), are precursors for the generation of pro-inflammatory and pro-resolving lipid mediators. Through the action of enzymes including 5-lipoxygenase (5-LOX), 15-lipoxygenase (15-LOX), and cyclooxygenase (COX) 1 and 2, they give rise to multiple families. These include prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), lipoxins (LXs), resolvins (Rvs), protectins, and maresins.
The ability of SPMs to influence plaque progression in mice has been demonstrated by a variety of studies in which the administration of exogenous SPMs to mice with established atherosclerosis led to reduced overall lesion area, smaller necrotic cores, reduced immune cell infiltration, enhanced lesional efferocytosis, and thicker collagen caps (Table 1). In humans, low levels of circulating SPMs correlate with the presence of atherosclerosis.18 Further, plasma from symptomatic patients suffering from stroke showed significantly lower RvD1 levels compared with asymptomatic patients.19 Recent work has identified an imbalance of pro-inflammatory and pro-resolving lipid mediators within lesions as a key feature of advanced atherosclerosis. In human carotid arteries, regions of advanced disease were shown to have a high LTB4:SPM ratio compared with early plaque regions.20 Apoe−/− mice fed a Western diet for 4, 8, or 16 weeks showed a steady increase in prostaglandin and LTB4 content within the aortas. Simultaneously, there was a decrease in RvD2 and maresin 1.21 Similarly, atheroprone Ldlr−/− mice fed a Western diet for 17 weeks had higher LTB4:SPM ratios within the aorta compared to Ldlr−/− mice fed for only 8 weeks. Administration of RvD1 to Ldlr−/− mice with established plaque led to smaller necrotic cores, increased lesional efferocytosis, and thicker collagen caps during progression of disease. RvD1 treatment also led to an increase in overall SPM content, suggesting that RvD1 can trigger a local feed-forward mechanism within the vessel wall.20 Together, these data suggest that the local balance of pro-resolving to pro-inflammatory mediators plays a pathogenic role in disease.
Table 1.
The effects of manipulating pro-resolving mediator pathways in animal models of atherosclerosis.
| Animal Model | Diet | Outcome | References | |
|---|---|---|---|---|
| Lipoxygenases | ||||
| 5-LOX knockout | Alox5−/−/Ldlr−/− | Western | ↓ aortic plaque size | 146 |
| 12/15 LOX overexpression | 12/15-LOX-transgenic Apoe−/− mice | Chow | ↓ aortic plaque size ↑ LXA4 |
147, 148 |
| 12/15 LOX overexpression | 12/15-LOX transgenic Apoe−/− mice | Western | ↔ aortic plaque size | 148 |
| 15-LOX Mφ overexpression | Human 15-LOX transgenic rabbit | Western | ↓ aortic plaque size | 149 |
| SPMs | ||||
| AT-LXA4 (osmotic pump) | Apoe−/− mice | Western | ↓ aortic plaque size | 29 |
| RvD1 (i.p.) | Ldlr−/− mice | Western | ↓ aortic plaque necrosis, ROS ↑ fibrous cap thickness, efferocytosis |
20 |
| RvD2 and MaR1 (i.p.) | Apoe−/− mice | Western | ↓ aortic plaque necrosis, Mϕ content ↑ fibrous cap thickness |
21 |
| RvE1 (p.o. gavage) | ApoE*3 Leiden transgenic mice | Western | ↓ aortic plaque size, expression of pro-atherogenic genes | 150 |
| RvE1 (p.o. topical) | Rabbit | Western | ↓ aortic plaque size, inflammatory cell infiltration | 151 |
| Receptors | ||||
| ALX/FPR2 knockout | Fpr2−/− Apoe−/− mice | Chow | ↓ aortic plaque size (16 weeks diet) | 29 |
| Western | ↔ aortic plaque size (4 weeks diet) | 29 | ||
| Fpr2−/− Ldlr−/− mice | Western | ↓ aortic plaque size (12 weeks diet) | 27 | |
| ↓Mϕ content, collagen content | ||||
| Western | ↔ aortic plaque size (16-20 weeks diet) | 27 | ||
| Fpr2−/− marrow into Ldlr−/− mice | Western | ↓ aortic plaque size (17-20 weeks diet) | 27, 30 | |
| BLT1 knockout | Blt1−/− Apoe−/− mice | Western | ↓ aortic plaque size (4-12 weeks diet) | 32, 33 |
| ↔ aortic plaque size (19 weeks diet) | 32 | |||
| ChemR23 knockout | Chemr23−/− Apoe−/− mice | Western | ↑ aortic arch plaque area, ↑ aortic root plaque size, ↑ Mϕ content (8 weeks diet) | 34 |
| Western | ↑ aortic arch plaque area, ↔ aortic root plaque size, ↑ Mϕ content (12 weeks diet) | 34 | ||
| Chemr23GFP/GFP Apoe−/− mice | Western | ↓ aortic root plaque size, ↓ Mϕ content (4 and 12 weeks diet) | 35 | |
| Chemr23−/− marrow into Ldlr−/− mice | Western | ↑ aortic plaque size, plaque necrosis, Mϕ content (12 weeks diet) | 34 | |
| Chemr23GFP/GFP marrow into Apoe−/− mice | Western | ↓ aortic plaque size (6 weeks diet) | 35 | |
| GPR32 overexpression | hGPR32mycTg Fpr2−/− Apoe−/− | ↓ aortic plaque size, plaque necrosis | 36 | |
| Pro-resolving Proteins | ||||
| Annexin A1 knockout | Anxa1−/− Apoe−/− mice | Western | ↑ aortic plaque size | 46 |
| Ac2-26 (i.v.) | Apoe−/− mice | Western | ↓ aortic plaque size and Mϕ content | 46 |
| Ac2-26 (nanoparticles i.v.) | Ldlr−/− mice | Western | ↓ aortic plaque size, plaque necrosis, ROS ↑ fibrous cap thickness |
30 |
| IL-10 knockout | Il10−/− Apoe−/− mice | Western | ↑ aortic plaque size | 41 |
| IL-10 overexpression in Mϕs | Ldlr−/− mice | Western | ↓ aortic plaque size | 42, 43 |
| IL-10 (nanoparticles i.v.) | Ldlr−− mice | Western | ↓ aortic plaque necrosis, ROS | 44 |
| ↑ fibrous cap thickness | ||||
Modified from Kasikara, Doran, et al.15
Several studies have shown that SPM production is dependent on the cellular milieu. Under inflammatory conditions, 5-LOX in Mϕs is phosphorylated, leading to its translocation to the nuclear membrane where it interacts with 5-LOX activating protein (FLAP) and promotes the synthesis of LTB4. Under non-inflammatory conditions or in response to RvD1, 5-LOX remains unphosphorylated and instead is localized to the cytoplasm. There, in sequence with 12/15-LOX, it favors production of LXA4.22 Signaling through the MER proto-oncogene tyrosine kinase (MerTK) receptor, an efferocytosis receptor, limits phosphorylation of 5-LOX and increases LXA4 production.22, 23 Examination of human carotid atherosclerotic lesions showed that in regions of advanced plaque, 5-LOX had a predominantly nuclear localization compared to healthy regions of arteries.24 In addition, high-mobility group box 1 (HMGB1) signals through the receptor for advanced glycation end products (RAGE) to phosphorylate 5-LOX, increase 5-LOX nuclear localization, and increase LTB4 production. Alternatively, when C1q is present, HMGB1 and C1q form a tetramolecular complex that links RAGE and the leukocyte-associated Ig-like receptor-1 (LAIR-1). This prevents phosphorylation of 5-LOX and increases production of LXA4, RvD1, and RvD2 (Figure 2).25
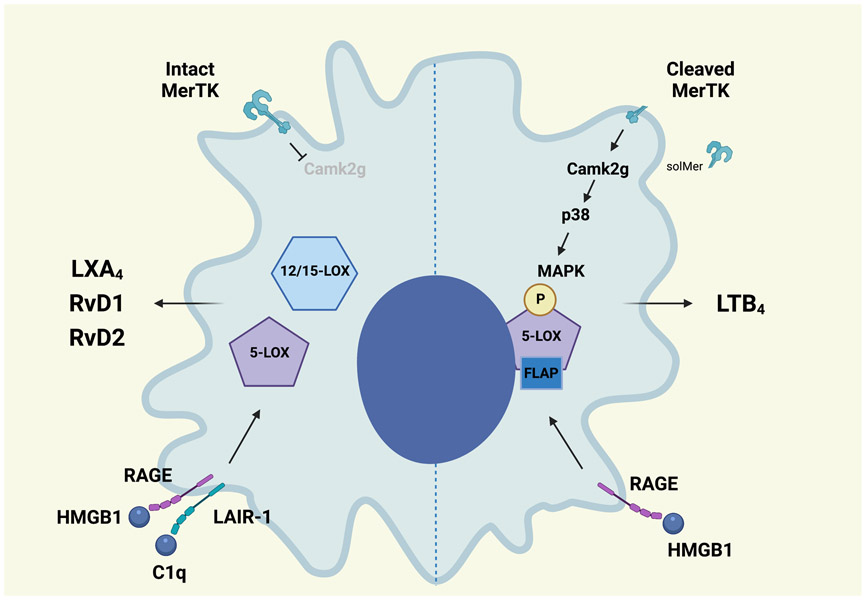
Figure 2. Nuclear localization of 5-lipoxygenase specifies lipid mediator production in response to receptor signaling.
Signaling by MerTK inhibits Calcium/calmodulin-dependent kinase 2γ (CaMK2g) activity and turns off a p38MAPK-MK2 cascade. This favors 5-LOX translocation to the cytoplasm where it is brought into proximity with 12/15-LOX and promotes lipoxin A4 (LXA4) production. Pro-inflammatory stimuli lead to MerTK cleavage and increased CaMK2g/p38MAPK-MK2 signaling that phosphorylates 5-LOX, enhancing its interaction with 5-LOX activating protein (FLAP). This promotes nuclear localization and leukotriene B4 (LTB4) production. The interaction of high-mobility group box 1 (HMGB1) with the receptor for advanced glycation end products (RAGE) also promotes phosphorylation of 5-LOX and increased LTB4 production. When complement 1q (C1q) is present, HMGB1 and C1q form a complex with RAGE and the leukocyte-associated Ig-like receptor-1 (LAIR-1). This tetramer favors cytoplasmic 5-LOX and increases SPM production. (Illustration credit: Ben Smith)
SPM Receptors.
The SPMs effect their cellular functions through their actions on G-protein coupled receptors in mice and humans, including N-formyl peptide receptor 2 (ALX/FPR2), LTB4 receptor-1 (BLT1), Chemerin receptor 23 (ChemR23), and G protein-coupled receptor 18 (GPR18). In addition, humans express GPR32 for which there is currently no known ortholog in mice. In vitro and in vivo, a variety of athero-relevant functions have been attributed to SPM receptor signaling, including modulation of efferocytosis, leukocyte recruitment, and cytokine production (Table 2).
Table 2.
Resolving mediators, receptors, and their athero-relevant downstream effects.
| Receptor | Ligands | Athero-relevant downstream effects | References |
|---|---|---|---|
| ALX/FPR2 | Annexin A1 (Ac2-26) | ↑ efferocytosis, neutrophil apoptosis | 30, 46, 152-156 |
| ↓ PMN infiltration to tissue | |||
| AT-LXA4 | ↑ efferocytosis | 157 | |
| LXA4 | ↑ efferocytosis, anti-inflammatory cytokine production | 158-161 | |
| ↓ PMN infiltration to tissue, pro-inflammatory cytokine production | |||
| RvD1 | ↑ efferocytosis, anti-inflammatory cytokine production | 28, 162-165 | |
| ↓ PMN infiltration to tissue, pro-inflammatory cytokine production | |||
| RvD3 | ↓ PMN infiltration to tissue, pro-inflammatory cytokine production | 166 | |
| SAA | ↑ leukocyte chemotaxis, pro-inflammatory cytokine production | 26, 167 | |
| BLT1 | LTB4 (agonist) | ↑ leukocyte chemotaxis, foam cell formation | 32 |
| RvE1 (antagonist) | ↓ PMN infiltration to tissue, NF-κB activation, platelet aggregation, LTB4-induced calcium mobilization | 168-170 | |
| RvE2 (antagonist) | ↑ phagocytosis | 168, 171 | |
| ↓ PMN chemotaxis/infiltration to tissue | |||
| ChemR23/ERV | RvE1 | ↑ phagocytosis | 168, 169, 172, 173 |
| ↓ PMN chemotaxis/infiltration to tissue, pro-inflammatory cytokine production | |||
| RvE2 (partial agonist) | ↑ phagocytosis | 170, 171 | |
| ↓ PMN chemotaxis/infiltration to tissue | |||
| Chemerin | ↑ adhesion and chemotaxis of leukocytes | 174, 35 | |
| ↓ pro-inflammatory cytokines | |||
| ↑ apoptosis | |||
| FPR1 | Annexin A1 (Ac2-26) | ↓ PMN chemotaxis/infiltration to tissue, leukocyte:endothelial cell interaction | 154, 175, 176 |
| GPR18/DRV2 | RvD2 | ↑ efferocytosis | 165, 177-179 |
| ↓ PMN infiltration to tissue, pro-inflammatory cytokine production, leukocyte:endothelial cell interaction, leukocyte activation | |||
| GPR32/DRV1 | AT-RvD1 | ↑ phagocytosis, 12/15-LOX expression | 36 |
| ↓ oxLDL uptake | |||
| LXA4 | ↑ efferocytosis, anti-inflammatory cytokine production | 158-161 | |
| ↓ PMN infiltration to tissue, pro-inflammatory cytokine production | |||
| RvD1 | ↑ efferocytosis, anti-inflammatory cytokine production | 28, 162-164 | |
| ↓ PMN infiltration to tissue, pro-inflammatory cytokine production | |||
| RvD3 | ↑ efferocytosis | 166 | |
| RvD5 | ↑ phagocytosis, ↓ pro-inflammatory cytokine production | 180 |
Modified from Kasikara, Doran, et al.15
ALX/FPR2
ALX/FPR2 can promote either pro-inflammatory or pro-resolving signaling, dependent on the ligand. When activated by serum amyloid A (SAA), ALX/FPR2 promotes inflammatory cytokine signaling.26, 27 In contrast, when activated by LXA4 or RvD1, ALX/FPR2 promotes Mϕ skewing toward a pro-reparative phenotype and increases efferocytosis.28 This suggests that in advanced plaques, where levels of pro-resolving ligands are low, pro-inflammatory ALX/FPR2 signaling predominates. Genetic deletion of Fpr2 in Ldlr−/− or Apoe−/− mice led to reduced atherosclerotic lesion area compared with control mice early in the course of plaque development.27, 29 As lesions progressed with longer lengths of feeding, the difference between the Fpr+/+ and Fpr−/− groups was lost.27, 29 In other studies, the authors utilized a bone marrow transplantation model to generate chimeric Fpr2−/− → Ldlr−/− mice. Interestingly, in this model, the mice receiving Fpr2−/− marrow developed significantly less plaque than those receiving wild type marrow despite long lengths of Western diet feeding.27, 30 This suggests that there may be a role for FPR2 on non-hematopoietic cells in advanced atherosclerosis. Recently, a series of novel ALX/FPR2 agonists were developed that specifically target the pro-resolving properties of the receptor and decreased the number of adherent human neutrophils in an in vitro assay. While not yet used in vivo, this study suggests that it is possible to target the SPM receptors with small molecule agonists.31
BLT1
The role of BLT1 in atherosclerosis also appears to be dependent on stage of lesion development. Similar to ALX/FPR2, the BLT1 receptor has multiple ligands with opposing functions. These include LTB4 which acts an agonist, as well as RvE1 and RvE2 that act as antagonists. In Blt1−/− Apoe−/− mice fed a Western diet, there was a decrease in aortic lesion size after 4 or 8 weeks of feeding compared to Apoe−/− mice. However, after 19 weeks of Western diet, there was no difference in lesion size.32 A subsequent study confirmed these findings, showing that Blt1−/− Apoe−/− mice fed a Western diet for 6 or 12 weeks had a reduction in lesion area.33 Interestingly, these authors also found a markedly diminished number of SMCs, Mϕs, and T cells in the vessel wall of Blt1−/− Apoe−/− mice. They found that BLT1 is upregulated in human atherosclerotic lesions, and frequently co-localized with SMCs and Mϕs, suggesting that SPMs may also regulate vascular cells in ways that are not yet fully understood.32
ChemR23
Deletion of the ChemR23 receptor led to increased atherosclerotic lesion area in Apoe−/− or Ldlr−/− mice versus controls.34 This increase in atherosclerosis was also associated with increased lesional Mϕ content, heightened pro-atherogenic signaling in Mϕs, enhanced foam cell formation, and reduced efferocytosis in vitro as well as increased necrotic core size in vivo. This stands in contrast to another study that utilized Apoe−/− mice in which the ChemR23 gene was replaced with eGFP (ChemR23eGFP/eGFP Apoe−/−). In this study, ChemR23eGFP/eGFP Apoe−/− mice fed a western diet for either 4 or 12 weeks had smaller plaques and less lesional Mϕ accumulation than control mice.35 While the explanation for this is not known, it is possible that the stage of lesion development influences the availability of ligands in the local microenvironment. Since ChemR23 can be stimulated by the inflammatory ligand, chemerin, as well as the resolving ligands, RvE1 and RvE2, it is interesting to hypothesize that the balance of pro-inflammatory:pro-resolving ligands may influence receptor function in a paradigm similar to that described for LTB4:SPMs above. In human atherosclerotic lesions from coronary and carotid arteries, ChemR23 expression was found primarily in Mφs surrounding the necrotic core. Interestingly, ChemR23 expression was increased in plaques from patients on lipid-lowering therapy with statins, suggesting that receptor expression in addition to mediator abundance is dynamic. 34
GPR32
Recently, the role of the GPR32 receptor was investigated in vivo. Because there is no murine homolog of GPR32, the authors created a transgenic mouse model expressing human GPR32 on an Fpr2−/− Apoe−/− background (hGPR32mycTg Fpr2−/− Apoe−/− mice). After 12 weeks of Western diet, these mice had reduced lesion area and smaller necrotic cores compared to Fpr2−/− Apoe−/− mice. Using zymosan-elicited Mϕs in a peritonitis model of sterile inflammation, the authors showed that expression of hGPR32 led to enhanced efferocytosis and increased expression of 12/15-LOX. Administration of the GPR32 agonist, AT-RvD1, further enhanced these phenotypes.36
Non-Lipid Pro-Resolving Mediators.
Pro-resolving proteins
The pro-resolving cytokine, IL-10, has been detected in human and murine atherosclerotic plaques, where it is produced primarily by Mϕs.37 Through inhibition of nuclear factor (NF)-κB, IL-10 impedes the release of multiple pro-inflammatory mediators including intracellular adhesion molecule 1 (ICAM-1), IL-1β, monocyte chemotactic protein 1 (MCP-1), and tumor necrosis factor-α (TNF-α). IL-10 upregulates cholesterol efflux receptors and downregulates scavenger receptors, which limits foam cell formation.38 Upregulation of IL-10 expression in Mϕs leads to increased Rac activity, which promotes efferocytosis.39 In addition, it can inhibit the secretion of matrix metalloproteinase-9 (MMP-9) to prevent degradation of the fibrous caps in advanced atherosclerosis.40 Deletion of Il10 from Apoe−/− mice led to dramatically increased lesion area and heightened MMP activity.41 Overexpression of IL-10 in Mϕs of Ldlr−/− mice led to reduced atherosclerotic lesion area compared to control mice.42, 43 Delivery of IL-10 to established atherosclerotic plaques using lesion-targeting nanoparticles led to decreased necrotic core area, increased collagen cap thickness, and a trend toward increased lesional efferocytosis (Table 1).44 Notably, many of the SPMs, including LXA4, RvD1 and RvE1, lead to increased expression of IL-10 in Mϕs, linking these two important arms of the resolution program.17
Annexin A1 (AnxA1), is also important in atherosclerosis and has been associated with a variety of pro-resolving functions, including inhibiting leukocyte recruitment, boosting efferocytosis, and reprogramming Mϕs toward a pro-reparative phenotype.45 Deletion of AnxA1 in Apoe−/− mice led to increased atherosclerotic lesion area.46 Administration of recombinant AnxA1 to Ldlr−/− mice with established lesions led to a reduction in overall lesion area and decreased necrotic core size.47 Similarly, treatment of Apoe−/− mice with a bioactive peptide derived from AnxA1, Ac2-26, led to a reduction in lesion area and macrophage content by blocking chemokine-dependent integrin activation.46 When delivered directly to plaques of Ldlr−/−mice using a lesion-targeting nanoparticle, Ac2-26 decreased plaque necrosis, reduced lesional oxidative stress, thickened collagen caps, and increased intraplaque IL-10 expression (Table 1).30
Pro-resolving gases
Nitric oxide (NO) is known to have both pro-apoptotic and anti-apoptotic properties depending on the local concentration. In atherosclerosis, high levels of inducible nitric oxide synthase (iNOS) lead to high concentrations of NO, which favor apoptosis and clearance of leukocytes.48 In addition, NO can nitrosylate the NLRP3 inflammasome in order to prevent its assembly in Mϕs.49 Hydrogen sulfide (H2S) is also a potent inhibitor of the inflammasome50. In vitro and in an in vivo murine peritonitis model, H2S led to enhanced efferocytosis and reduced pro-inflammatory cytokine expression.51 Enhancing H2S activity in Ldlr−/− or Apoe−/− mice led to reduced plaque formation, lower expression of ICAM-1, and reduced aortic expression of pro-inflammatory cytokines.52, 53 Conversely, genetic targeting of the H2S synthetic enzyme, cystathionine γ-lyase led to exacerbated atherosclerosis in Apoe−/− mice.54 Carbon monoxide (CO) may also play a role in resolution in atherosclerosis. Heme oxygenase (HO-1) is an enzyme induced by oxidative stress, inflammation, and oxidized lipoproteins that produces CO as a byproduct of heme degradation.55 It is expressed at low levels in endothelial cells and VSMCs but is highly upregulated in pro-resolving Mϕs.56 CO and HO-1 are known to suppress pro-inflammatory cytokine production and block leukocyte infiltration into tissue. In a murine peritonitis model, inhaled CO led to lower levels of leukocyte infiltration to the peritoneum and increased efferocytosis by Mϕs.57 Induction of HO-1 expression and activity in Ldlr−/− mice inhibited the formation of atherosclerotic lesions.58 Importantly, CO has been shown to increase levels of the SPMs, again indicating that these arms of the resolution program are closely linked.57
Tissue hypoxia is a contributor to the development of atherosclerosis. Recently, it was shown that ventilating atheroprone mice with carbogen gas (95% O2 and 5% CO2) led to reduced plaque hypoxia. This was associated with increased lesional efferocytosis, and smaller necrotic cores. In vitro, hypoxia led to decreased expression of MerTK by Mϕs, which may be the mechanism by which this occurs.59
Efferocytosis and the Clearance of Dead Cells.
Cell death is a quintessential part of the resolution process and a crucial mechanism by which the immune cell burden within developing lesions is reduced. Cells within plaques undergo cell death in a variety of ways including necroptosis, pyroptosis, ferroptosis, and apoptosis.60-63 The engulfment of dead cells by professional phagocytes is called efferocytosis. This process is mechanistically distinct from classical phagocytosis in that it requires phagocyte expression of receptors that recognize ligands on dead cells. It is a highly regulated and efficient process that rapidly allows the phagocyte to reorganize its cytoskeleton and upregulate metabolism to process the large amount of nutritional cargo represented by the ingested apoptotic cell (AC). When efferocytosis is properly executed, it leads to the downregulation of pro-inflammatory signaling and upregulation of pro-resolving pathways, including SPM production. It is now well appreciated that efferocytosis is impaired in chronic inflammatory diseases, including atherosclerosis.62, 64 Emerging data from GWAS studies have identified SNPs in genes related to efferocytosis pathways. One example is phosphatase and actin regulator 1 (PHACTR1), in which rs9349379 was associated with early-onset myocardial infarction.65 Subsequent studies showed that the risk allele (rs9349379-G) was associated with lower levels of PHACTR1 expression in Mϕs and impaired efferocytosis in human monocyte-derived Mϕs as well as human lesional Mϕs compared to the common allele.66 In vitro, the loss of PHACTR1 from human or murine Mϕs led reduced ability to engulf ACs and therefore inhibited efferocytosis. Mice lacking hematopoietic expression of PHACTR1 (Phactr1−/− marrow transplanted to Ldlr−/− or Apoe−/− mice) had increased atherosclerotic lesion area, larger necrotic cores, and thinning collagen caps.66, 67
Compromise of efferocytosis receptors
Impaired efferocytosis occurs during atherogenesis for several reasons (Figure 3). Efferocytosis receptors, including LDL Receptor Related Protein 1 (LRP1) and MerTK, can be proteolytically cleaved or degraded during advanced atherosclerosis.68, 69 In response to oxLDL, LRP1 is ubiquitinated and then bound by epsins 1 and 2, leading to its internalization and degradation. Mϕs in which epsins are genetically deleted had increased total and surface levels of LRP1. Apoe/− mice in which epsins 1 and 2 were deleted from myeloid cells had smaller atherosclerotic lesions, smaller necrotic cores, and diminished inflammatory cell content within the aortas after Western feeding.70 Deletion of LRP1 from hematopoietic cells or macrophages in atheroprone mice also led to increased overall plaque area and necrotic core size.71-73 MerTK and LRP1 are targets of a disintegrin and metalloproteinase 17 (ADAM17), which can cleave the extracellular domain from the receptors, rendering the remaining receptor incapable of signaling.69 In the case of MerTK, it also generates a soluble fragment, known as soluble Mer (solMer), which can compete for binding to the bridging molecule Gas6, decreasing its availability and further impairing efferocytosis.74 Levels of MerTK decrease with progression of atherosclerosis while ADAM17 and solMer accumulate within plaques from both mice and humans.75-77 Loss of MerTK in atheroprone mice (Mertk−/− Ldlr−/−) leads to increased lesion size, larger necrotic core area, and reduced lesional SPMs.22, 69, 75, 76 In a mouse model in which MerTK was replaced by a cleavage-resistant mutant (MertkCR Ldlr−/− mice), there were significantly smaller necrotic cores, enhanced lesional efferocytosis, and higher levels of SPMs compared with control mice.75 This suggests that cleavage of the MerTK receptor plays a causative role in atheroprogression.
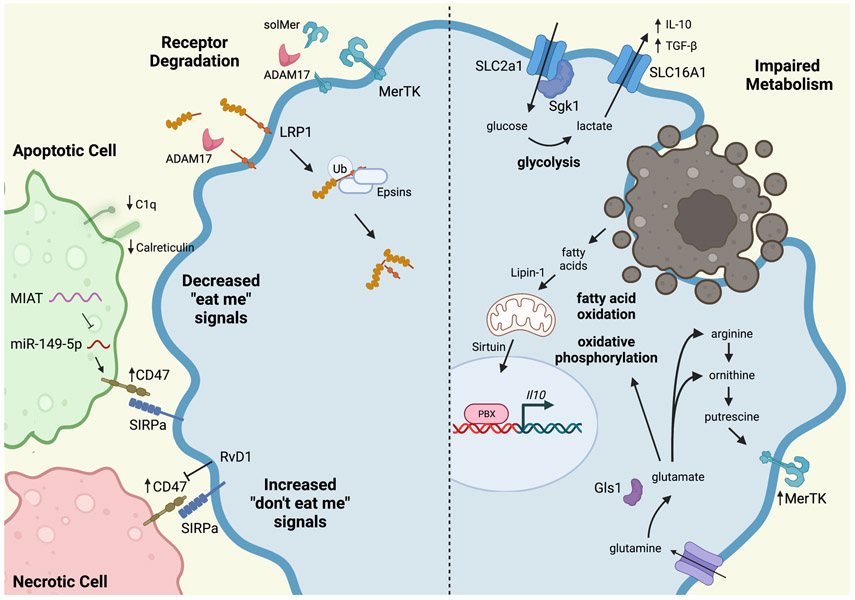
Figure 3. Mechanisms of Defective Efferocytosis in Atherosclerosis.
A variety of impairments contribute to defective efferocytosis in atherosclerosis. Efferocytosis receptors, including MerTK and LRP1, can be cleaved by ADAM17. LRP can also be ubiquitinated and degraded by Epsins. Apoptotic cells express decreased levels of “eat me” signals, such as C1q and calreticulin. Apoptotic and necrotic cells inappropriately express the “don’t eat me” signal, CD47, that prevents their efficient uptake by macrophages. This can be overcome by treatment with resolvin D1 (RvD1). Metabolism also plays an important role in efficient efferocytosis, particular in continual efferocytosis where successive apoptotic cells must be consumed. Glycolysis produces lactate, which increases IL-10 production and enhances subsequent efferocytosis. Digestion of apoptotic cells provides fatty acids as a substrate for fatty acid oxidation and oxidative phosphorylation, which also increase production of IL-10. Apoptotic cell-derived arginine and ornithine are converted to putrescine, which leads to enhanced, persistent MerTK expression on the macrophage, further enhancing efferocytosis. Glutamine is converted to glutamate, which contributes to oxidative phosphorylation, but also produces arginine and ornithine to feed the polyamine synthesis pathway. (Illustration Credit: Ben Smith).
It is worth noting that the role of ADAM17 is not completely straightforward. Despite the atherogenic role of ADAM17 suggested by the above studies, low levels of ADAM17 in Ldlr−/− mice (hypomorphic Adam17ex/ex mice that express low residual ADAM17 expression) led to larger atherosclerotic lesions, with more lesional macrophages compared to littermate controls.78 In a subsequent study, myeloid-specific Adam17−/− mice (Adam17flox/flox Lyz2Cre Apoe−/−) fed a western diet for 12 weeks developed significantly more atherosclerotic lesion area but reduced lesional macrophage area and increased collagen content while the endothelial-specific loss of Adam17 (Adam17flox/flox BmxCre Apoe−/−) led to significantly smaller lesions. This made clear that the effects of ADAM17 on atherosclerosis are cell type specific and will require further investigation.79
Several pathways have been elucidated that play into the regulation of MerTK in atherosclerosis. For example, calcium/calmodulin-dependent kinase 2 gamma (CaMK2γ) activity is heightened in Mϕs within advanced atherosclerotic plaques of humans and mice.80 In vitro, this was shown to prevent signaling of an ATF6/LXRα pathway that drives MerTK expression. Genetic deletion of CaMK2γ (Camk2g−/− Ldlr−/− mice) or delivery of nanoparticles carrying CaMK2γ siRNA to Ldlr−/− mice restored signaling of this pathway, leading to increased MerTK expression, reduced necrotic core size, and enhanced lesional efferocytosis.80, 81
Loss of bridging moleculesx
In addition to efferocytosis receptors, bridging molecules that allow for the indirect binding of ACs can be compromised in advanced atherosclerosis. For example, milk fat globule-EGF factor 8 (MFGE8) is a bridging molecule that interacts with integrin αvβ3 whose lesional expression declines during atheroprogression in mice.82 Ldlr−/− mice lacking MFGE8 (bone marrow transfer of Mfge8−/− marrow into Ldlr−/− mice) have increased atherosclerotic lesion area and increased plaque necrosis.82 Similarly, Transglutaminase 2 (TG2) acts as a bridging molecule for integrin β3.83 When marrow from Tg2−/− mice is transplanted into Ldlr−/− mice, the resulting lesions are larger, contain more Mϕs, and have larger necrotic cores.84 The complement component, C1q, is another bridging molecule whose loss in Ldlr−/− mice leads to increased atherosclerotic lesion area and heightened accumulation of lesional ACs.85 While C1q serves multiple functions in inflammation, it is known to promote resolution and efferocytosis through its ability to interact with HMBG1 to influence 5-LOX localization and enhance SPM production in Mϕs (Figure 2).25
Inappropriate signaling from apoptotic cells
In advanced atherosclerosis, ACs themselves can contribute to defective efferocytosis (Figure 3). For example, dead cells in lesions express lower amounts of the ‘eat me’ signal, calreticulin. GWAS showed that human carriers of the cardiovascular risk allele at chromosome 9p21 have lower intraplaque expression of CDKN2B and reduced lesional expression of calreticulin.65, 86 Similarly, Apoe−/− mice lacking Cdkn2b have decreased levels of calreticulin and ACs from these animals are resistant to engulfment. Fed a Western diet, Cdknb−/− Apoe−/− mice have increased plaque area and larger necrotic cores.86 There can also be inappropriate expression of ‘don’t-eat-me’ signals. One such signal is CD47, whose expression renders ACs inedible to efferocytes.87 CD47 expression is significantly upregulated in human atherosclerotic plaques compared to healthy arteries.87 Treating Apoe−/− mice with an anti-CD47 antibody led to smaller necrotic cores and enhanced lesional efferocytosis. It is not yet known what leads to increased CD47 on the ACs in advanced plaques, however, in vitro studies have suggested that signaling through the TNF receptor, TNFR1, and NF-κB may lead to its upregulation.87 Another potential mechanism of CD47 regulation is via the microRNA, miR-149-5, which acts as a negative regulator of CD47. Recently, the long non-coding RNA, myocardial infarction-associated transcript (MIAT) was found to act as a ‘sponge’ for miR-149-5, thereby increasing CD47 expression. Of note, MIAT is highly expressed in symptomatic human plaques and advanced murine atherosclerosis, suggesting that in advanced disease, MIAT leads to upregulation of CD47.88, 89 This was supported by studies showing that Miat−/− Apoe−/− mice had decreased CD47 expression, increased efferocytosis, and decreased plaque necrosis.89 Interestingly, MIAT was also found to promote transdifferentiation of SMCs into inflammatory macrophage-like cells, suggesting another mechanism by which MIAT promotes atherosclerosis.88
Metabolic reprogramming by efferocytosis
When accomplished effectively, the outcome of efferocytosis is not only clearance of dead cells, but also a shift toward a pro-reparative Mϕ phenotype, which includes metabolic reprogramming. RNA sequencing of efferocytes actively engulfing ACs revealed a genetic signature associated with phagocytosis.90 Subsequent studies have shown important roles for glycolysis, oxidative phosphorylation (OXPHOS), and their related metabolic pathways in efficient efferocytosis. Binding of ACs to Mϕs leads to upregulation of the glucose transporter SLC2A1 (GLUT1) and serum/glucocorticoid-regulated kinase 1 (SGK1) that enhance glucose uptake and activate aerobic glycolysis.90 This pathway was shown to be critical for the successive clearance of ACs, known as ‘continual efferocytosis’, since blocking glucose uptake or glycolytic flux led to failures in actin polymerization that prevented further AC engulfment.90 Deletion of Slc2a1 from myeloid cells in atheroprone mice (Slc2a1flox/flox Lyz2 Cre marrow transplanted into Ldlr−/− mice) led to increased necrotic core size and decreased lesional collagen content.91 Another solute transporter, SLC16A1, was found to be upregulated by Mϕs in response to AC internalization. SLC16A1 is responsible for the release of lactate, the end-product of glycolysis, from the cell. Interestingly, lactate acts in a paracrine fashion on neighboring cells to enhance their production of IL-10 and TGF-β, further linking glycolysis to the resolution program.90
OXPHOS also appears to promote resolution. Strategies that promote OXPHOS enhance the expression of resolution genes and inhibiting aspects of the OXPHOS pathways leads to impaired efferocytosis.92 Fatty acid oxidation (FAO or β-oxidation) provides reducing equivalents to OXPHOS. Therefore, changes in FAO may contribute to OXPHOS phenotypes in Mϕs. One study showed that loss of Lipin-1, a phosphatase with transcriptional coregulator activity, is required for FAO and OXPHOS in pro-reparative macrophages.93 While lipin-1 deficient Mϕs were able to ingest a single AC, they had a specific impairment in their ability to take up subsequent cells.94 Interestingly, these functions were attributable to the transcriptional role and not the enzymatic activity of lipin-1. The loss of lipin-1 from myeloid cells (Lpin1flox/flox Lyz2Cre) in hypercholesterolemic mice led to increased lesion area and necrotic core size.95 Another study showed that activation of FAO in response to efferocytosis stimulated the mitochondrial respiratory chain, leading to sirtuin-dependent activation of transcription factor PBX1 and IL-10 expression. Disruption of the electron transport chain led to decreased IL-10 and impaired resolution.96 Recently, it was shown that glutaminolysis, which also feeds substrates into the OXPHOS pathway, promotes efficient efferocytosis. In response to ingestion of an AC, glutaminase-1 (GLS1) is upregulated to produce glutamate that is then metabolized via a non-canonical transaminase-dependent OXPHOS pathway. Loss of GLS1 from Mϕs impaired efferocytosis in vitro and genetic deletion of GLS1 from atheroprone mice fed a Western diet (Gls1−/− Lyz2Cre Apoe−/− mice) led to augmented plaque size and an increased number of lesional ACs.97 These authors also showed that Gls1 expression is reduced in atheroprogression, suggesting that loss of GLS-1 during plaque development may contribute to failed efferocytosis in vivo. Glutamate can be further metabolized into ornithine, arginine, or polyamines. When ACs are degraded, Mϕs take up a load of arginine and ornithine that are further metabolized by arginase (Arg1) and ornithine decarboxylase (ODC) into putrescine. Putrescine promotes Rac1 activation to facilitate actin rearrangement and engulfment of ACs. Interestingly, the loss of Arg1 or ODC did not affect the ability of Mϕs to take up a single AC but led to a dramatic reduction in the ability of the Mϕ to carry out successive rounds of efferocytosis. This underscores that the upregulation of this pathway occurs in response to AC-derived arginine/ornithine rather than endogenously generated polyamines. Interestingly, genetic deletion of Arg1 or nanoparticle-mediated silencing of lesional ODC in Ldlr−/− mice did not reduce overall atherosclerotic burden in the progression of disease. However, in a model of atherosclerotic regression, loss of either Arg1 or ODC impaired plaque remodeling and led to persistently large necrotic cores and thinner collagen caps than observed in control mice.98 The authors went on to show that ODC-dependent putrescine synthesis sustains MerTK expression in vitro and in vivo, leading to enhanced efferocytosis and increased IL-10 expression.99
Efferocytosis triggers a pro-resolving macrophage phenotype
As alluded to in prior sections, efficient efferocytosis increases the production of pro-resolving mediators. After ingesting ACs, Mϕs quickly upregulate lipoxins and the D-series resolvins.100 The reciprocal relationship between efferocytosis and pro-resolving mediator production creates a positive feedback loop, in which treatment of Mϕs with IL-10 or SPMs enhances efferocytosis and increased efferocytosis then leads to heightened production of these pro-resolving mediators. This could be particularly important in diseases such as atherosclerosis where the burden of ACs is high and continual efferocytosis is important for maintaining homeostasis. Further, this may also be another mechanism by which the lower content of lesional SPMs in late-stage lesions contributes to impaired efferocytosis.
A recent paper identified a novel mechanism by which efferocytosis shifts the pool of murine Mϕs or human monocyte-derived Mϕs toward a pro-resolving phenotype. The authors found that nucleotides derived from the digestion of AC-derived DNA activate an mTorc2-dependent pathway that upregulates the transcription factor, Myc. This promotes proliferation of the efferocytosing Mϕs, thereby expanding the pool of pro-resolving Mϕs. Silencing DNase2a, which is responsible for the degradation of AC-derived DNA, or Rictor, which is a key component of the mTorc2 pathway, blocked proliferation. Further, the authors found that in murine models of atherosclerotic regression, efferocytosis and Mϕ proliferation are necessary for the resolution of necrotic cores and when DNase2a or Rictor are silenced within lesional Mϕs of Ldlr−/− mice with established plaque, regression is impaired.101
Necroptosis
Until recently, it was thought that the necrotic cores of atherosclerotic plaques were solely the result of secondary necrosis due to failed efferocytosis, however, it is now appreciated that necroptosis contributes to this. Like apoptosis, necroptosis is a specific cell death program. When a death receptor is triggered, receptor-interacting protein kinase 1 (RIP1) and caspase-8 are activated to promote apoptosis. In situations where caspase-8 is inactive or overwhelmed, RIP1 instead interacts with RIP kinase 3 (RIP3) to form the ‘necrosome’. The necrosome can then recruit mixed lineage-kinase domain-like (MLKL) and assemble the necrotic pore.63 This leads to active disruption of the mitochondrial, lysosomal, and plasma membranes and results in leakage of cell contents that can act as DAMPs and augment inflammation.102 Necroptotic cells (NCs) release high levels of the pro-inflammatory lipid mediators thromboxane B2 and PGE2, which likely contribute to their inflammatory nature in tissue.103 NCs as well RIP1, RIP3, and MLK have been identified in advanced and symptomatic, but not early, human plaques, suggesting that necroptosis may contribute to the progression of disease.104, 105 The mechanisms by which NCs are cleared from tissue are not yet well understood. A recent study showed that while Mϕs tend to wholly engulf ACs, they instead ‘nibble’ NCs. The authors found that NCs express high levels of the ‘don’t eat me’ signal, CD47, that contributes to their inefficient uptake. When co-cultures of Mϕs and NCs were treated with an anti-CD47 antibody, there was a significant increase in their ingestion (Figure 3).106 Whereas ACs activated CDC42 and provoked a wide ‘mouth-like’ phagocytic cup, NCs instead activated RhoA that led to a thin ‘arm-like’ structure that failed to encompass the dead cell. Anti-CD47 treatment produced the wider ‘mouth-like’ structure even in interacting with NCs. Similarly, treatment of Apoe−/− mice with anti-CD47 during atheroprogression led to fewer lesional NCs and less plaque necrosis.106 Anti-CD47 treatment also led to reduced pro-inflammatory mediator production and upregulation of RvD1 production by NCs.103, 106 Treatment of mice with RvD1 led to enhanced NC clearance through shifting the balance of RhoA:CDC42 and facilitating release of the ‘eat me’ signal, calreticulin.103 Deletion of Mlkl−/− from hypercholesterolemic mice led to decreased Mϕ death, smaller necrotic cores, and increased collagen cap thickness compared to control mice.103 In addition, the Mlkl−/− mice were found to have increased total plaque SPMs and an improved SPM:pro-inflammatory lipid mediator balance.103 Together, this suggests that increased necroptosis in advanced plaques is another mechanism by which inflammation resolution is impaired in atherosclerosis.
Macrophage Egress vs Retention in Plaque
At the conclusion of an inflammatory response, it is crucial that immune cells be able to leave the site of tissue injury in order to return to homeostasis. In atherosclerosis, there is accumulating evidence that Mϕs are inappropriately retained within the vessel wall, adding to the growth of the plaque. This is due to both the increased expression of molecules that promote retention and impairment of those that enhance movement. Cholesterol loading of Mϕs leads to upregulation of the Mϕ chemostasis factors, semaphorin 3E and netrin-1, which both promote Mϕ survival and decrease Mϕ emigration from tissue.107-109 The loss of hematopoietic netrin-1 in Ldlr−/− mice led to reduced atherosclerotic plaque size and lower lesional Mϕ content.107 New work has now shown that temporally silencing netrin-1 can also promote plaque regression. Using a model of tamoxifen-induced netrin-1 deletion in hyperlipidemic mice (Ntn1flox/floxCx3cr1creERT2+), the authors showed that deletion of myeloid netrin-1 led to a dramatic reduction of plaque size and complexity. Netrin-1−/− Mϕs had a pro-resolving phenotype in plaque that favored increased IL-10 expression and enhanced lesional efferocytosis. Further, utilizing bead-labeling to track plaque Mϕs, the authors showed that loss of netrin-1 changed macrophage dynamics to favor egress from plaque. Through transcriptional profiling, they found that netrin-deleted Mϕs expressed higher levels of CCR7 which they hypothesized is the mechanism by which netrin-1 modulated Mϕ trafficking.110 Studies have previously shown that CCR7 is expressed at low levels in plaque Mϕs and is upregulated in emigrating Mϕs during regression.111, 112 Blocking CCR7 leads to retention of Mϕs within plaque.112 Interestingly, a netrin-1 mutation has recently been identified in a family with premature atherosclerosis. In vitro, the product of this variant had a diminished ability to promote chemotaxis of Mϕs, suggesting that the role of netrin-1 is clinically relevant.113
T regulatory cells
In addition to Mϕs, Tregs have emerged as potent regulators of the immune response and important cellular mediators of resolution. These cells have been shown to be atheroprotective through their ability to elaborate IL-10 and TGF-β, limit excessive inflammation, foster skewing of Mϕs toward a pro-resolving phenotype, and to directly promote resolution themselves.114-117 In humans, low levels of circulating Tregs are associated with an increased risk of acute coronary syndromes.118 In addition, advanced or symptomatic atherosclerotic plaques contain fewer Tregs than early or asymptomatic plaques.119, 120 In mouse models of atherosclerosis, Treg numbers decline in the circulation as well as in aortic plaques and their function is impaired as atherosclerosis progresses.121-123 Depletion of Tregs in hypercholesterolemic mouse models leads to increased atherosclerosis.39, 117, 124 Either the adoptive transfer of Tregs or antibody-mediated expansion of Tregs also leads to decreased plaque progression.39, 117, 125, 126 Together, these suggest that Tregs play an important role in the regulation of atherosclerosis.
We have previously shown that Tregs are able to specifically regulate resolution processes, including efferocytosis. Co-culture of Mϕs with Tregs led to enhanced AC uptake in vitro. Conversely, Mϕs isolated from mice in which Tregs had been depleted (either by an anti-CD25 antibody depleting strategy or using a Foxp3-diptheria toxin receptor mouse model) had reduced capacity for efferocytosis.39 This was due to the ability of activated Tregs to elaborate IL-13 that led to upregulation of IL-10 in Mϕs in order to promote a STAT3/Vav1/Rac1 pathway that promoted actin rearrangement and facilitated AC engulfment.39 In a recent study, co-culture of Mϕs and Tregs in vitro led to upregulation of SPMs including 15R-LXA4, RvD1 and RvD6 as well as of the SPM receptors, Gpr18, ALX/FPR2, and ChemR23, which may also explain the positive effect of Tregs on efferocytosis.127 In a positive feedback look, efferocytosis also increases Treg numbers in vivo, as infusion of ACs to mice led to a TGF-β-dependent expansion of Tregs.128
A fascinating study has recently identified a role for Tregs in conditioning lymphatic endothelial cells in order to regulate leukocyte trafficking in the resolution phase. In this study, the authors found that activation of Tregs led to their upregulation of lymphotoxin α1β2 (LTα1β2) that promoted their interaction with lymphatic endothelial cells and upregulated adhesion molecules on the endothelial cells. The net effect was movement of leukocytes out of inflamed tissue and into the lymphatic system, which promoted interaction of Tregs with effector T cells and dendritic cells within the draining lymph nodes in order to terminate immune responses.129 Endothelial cells also secrete developmental endothelial locus-1 (DEL-1) during the resolution phase. DEL-1 has previously been shown to regulate leukocyte recruitment, promote efferocytosis, and increase SPM production.130 Recently, it was found that DEL-1 can also promote induction of de novo Tregs in the periphery. Together, this may explain the observation that DEL-1 overexpression attenuated atherogenesis in mice fed a high-fat diet.131, 132 In addition, this provides another example of endothelial cross talk with Tregs that promotes resolution and homeostasis.
Tregs have now also been shown to induce regression of atherosclerosis. In a murine model of regression, mice were rendered hypercholesterolemic by injection of a gain-of-function PCSK9 variant accompanied by Western diet-feeding to establish plaques and then subsequently treated with an apolipoprotein B antisense oligonucleotide (apoB-ASO) and a chow diet to rapidly lower cholesterol. The authors found that regressing plaques have significantly higher number of Tregs and that these were predominantly induced Tregs generated in the periphery.127 In a subset of mice undergoing regression, they depleted Tregs using an anti-CD25 antibody. Strikingly, they found that the depletion of Tregs blocked regression overall. Whereas Mϕs in regressing lesions upregulated pro-reparative Mϕ markers such as Arg1 and CD206, those with Treg depletion did not. Treg-depleted plaques also showed evidence of Mϕ retention and blocked egress from the lesion and Mϕs in these lesions showed decreased efferocytosis and lower IL-10.127 Together, these studies suggest that Tregs play an important role in atherosclerosis and that the loss of Tregs during atheroprogression may contribute to failed resolution and impede plaque regression.
Other Processes Contributing to Failed Resolution.
Aging and Senescence.
Aging contributes to a variety of diseases including neurodegenerative disease and ASCVD. The persistent inflammation associated with aging is becoming increasingly well characterized as a failure of resolution. Aging has been shown to impair efferocytosis in vitro and in vivo.133 Intriguingly, the transfer of young peritoneal Mϕs to aged mice reproduced the poor efferocytosis observed in older mice, suggesting that there is an “aging milieu” that impairs Mϕ function.134-137 Aging in mice also leads to an imbalance in pro-inflammatory and pro-resolving mediators, with an increase in LTs and prostaglandins but a decrease in D-series resolvins and lipoxins.136, 138 Administration of RvD1 and RvD3 led to enhanced resolution and improved efferocytosis.136 This is now supported by human data that shows elderly patients have an imbalance of lipoxins:leukotrienes.138 Cellular senescence, which is a process in which cells cease to divide yet survive and undergo distinctive phenotypic shifts, is also common in aging.139 The senescence ‘phenotype’ exacerbates inflammation and also impairs clearance of dead cells.138, 140 Senescent cells release factors that promote cleavage of MerTK, contributing to impaired efferocytosis. This was rescued by exogenous administration of RvD1.140 In addition, Gas6, which facilitates binding of ACs to MerTK, was found to be downregulated in aged mice.137 Together, these suggest a mechanism by which efferocytosis and inflammation resolution are impaired with advanced age.
Clonal Hematopoiesis.
A novel link between aging and inflammation, known as clonal hematopoiesis of indeterminate potential (CHIP) has recently been uncovered. This occurs when somatic mutations arise in a subset of genes in hematopoietic progenitor cells, conferring a proliferative advantage to them. To date, mutations in JAK2, DNTM3A, TET2, and ASXL1 have been associated with CHIP. These are rare in young people but are found in >10% of those ≥ 70 years old.141 Those who have these clones are at increased risk of hematological cancers as well as ASCVD. The mechanisms by which CHIP increases risk of these diseases is an active area of investigation, but data thus far suggests that the mutations associated with CHIP lead to pro-inflammatory changes in myeloid cells.141 Recently, the potential role for the resolving program has begun to be explored. When marrow from mice expressing a gain of function mutation in the Jak2 gene was transplanted to atheroprone mice (Jak2V617F marrow into Ldlr−/− mice), the authors observed increased lesion size with larger necrotic cores and defective lesional efferocytosis compared to controls. Mechanistically, this was attributed to lower surface levels of MerTK on Mϕs and increased MerTK receptor cleavage. Interestingly, lesions from the Jak2V617F → Ldlr−/− mice also demonstrated enhanced phagocytosis of erythrocytes due to lower levels of the ‘don’t eat me’ signal CD47 on their surface. In vitro, the enhanced ingestion of erythrocytes led to competition for ingestion of ACs by Mϕs, suggesting that this may further contribute to impaired efferocytosis. In similar models, where JakV617F is specifically expressed in Mϕs or in chimeric mice that model CHIP by transplanting a 1:4 ratio of JakV617F:WT marrow to Ldlr−/− mice, the authors found similarly increased lesion area with larger necrotic cores compared to controls. Further, they found that deletion of essential inflammasome components including caspase 1, caspase 11, and Aim2 reversed these findings. In particular, Aim2 was found to be overexpressed in JakV617F Mϕs and in lesions of JakV617F mutant mice. This suggests that CHIP mutations are linked to important resolving functions, including efferocytosis. This will be an exciting and important area of study in the coming years.
Therapeutic Possibilities and Future Directions
From the bench to the bedside, it is now clear that the immune system is a major driver of ASCVD and targeting inflammation in atherosclerosis can prevent major adverse cardiac events.3-6 This strategy is unfortunately hampered by infectious complications that arise as a result of suppressing host immune defense. Approaches that instead promote resolution may therefore offer an attractive alternative. Administration of exogenous SPMs is currently being studied as a therapeutic in several clinical trials for other inflammatory diseases, however, the short half-life of these mediators complicates their use in the treatment of chronic disease. While clinical trials that administered both EPA and DHA as a means of boosting production of endogenous production have yielded mixed results, the recent Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) treated patients with EPA alone and found a 25% reduction in cardiovascular events in patients with established ASCVD or major ASCVD risk factors.142, 143. While the trial was aimed at lowering triglyceride levels as an independent risk factor for ASCVD, pre-clinical studies suggest that treatment with EPA would boost production of the E-series resolvins and it is exciting to hypothesize that this could have contributed to the observed risk reduction.144 While this has not yet been assessed in this patient population, this data is likely forthcoming, and would certainly lend support to the concept of pro-resolving therapy for ASCVD. Already, RvE1 analogs are actively being studied in clinical trials for the treatment of other inflammatory diseases (Thetis Pharmaceuticals). Further, the synthesis of small molecule agonists of the SPM receptor, FPR2, brings resolution-targeting therapeutics one step closer.31
Targeting efferocytosis in human ASCVD is also emerging as a promising therapeutic strategy. In a study of cancer patients being treated with a humanized anti-CD47 antibody (magrolimab), those receiving anti-CD47 therapy had a significant reduction in vascular inflammation as measured by 18F-fluorodeoxyglucose positron-emission tomography and computed tomography (18F-FDG PET CT).145 Further studies will be necessary to determine whether this is sufficient to reduce cardiovascular events. Other recent studies have harnessed advances in nanotechnology to target atherosclerotic plaques. A number of studies have used atherosclerotic plaque-targeting nanoparticles to deliver pro-resolving proteins such as Annexin A1 or to silence components of pathways that negatively regulate pro-resolving pathways.30, 81, 99, 101 While these have not yet been used in clinical trials, there is a precedent for the use of nanotechnology in treating human disease and the hope is that these pre-clinical studies will provide the base on which to build the next clinical trials.
Sources of Funding
This work was supported by grants from the National Institutes of Health (NIH; R01HL159487) and the American Heart Association (17FTF33660643).
NONSTANDARD ABBREVIATIONS AND ACRONYMS:
- AC
apoptotic cell
- ADAM17
a disintegrin and metalloproteinase 17
- AnxA1
annexin A1
- apoB
apolipoprotein B
- Arg1
arginase 1
- ASCVD
atherosclerotic cardiovascular disease
- BLT1
LTB4 receptor-1
- CaMK2γ
calcium/calmodulin-dependent kinase 2 gamma
- ChemR23
chemerin receptor 23
- CHIP
clonal hematopoiesis of indeterminate potential
- CO
carbon monoxide
- DEL-1
developmental endothelial locus-1
- FLAP
5-LOX activating protein
- FPR2
N-formyl peptide receptor 2
- GLS1
glutaminase-1
- GLUT1
glucose transporter SLC2A1
- GPR18
G-protein–coupled receptor 18
- GPR32
G-protein–coupled receptor 32
- H2S
hydrogen sulfide
- HMGB1
high-mobility group box 1
- HO-1
heme oxygenase-1
- ICAM-1
intracellular adhesion molecule 1
- IL
interleukin
- iNOS
inducible NO synthase
- LAIR-1
leukocyte-associated Ig-like receptor-1
- LDL
low-density lipoprotein
- LOX
lipoxygenase
- LRP1
low-density lipoprotein receptor-related protein 1
- LTα1β2
lymphotoxin α1β2
- LXA4
lipoxin A4
- Mϕ
macrophage
- MCP-1
monocyte chemotactic protein 1
- MerTK
MER proto-oncogene tyrosine kinase
- MFGE8
milk fat globule-EGF factor 8
- MIAT
myocardial infarction–associated transcript
- MLKL
mixed lineage-kinase domain-like protein
- MMP-9
matrix metalloproteinase-9
- NC
necroptotic cell
- NF-κB
nuclear factor kappa B
- ODC
ornithine decarboxylase
- oxLDL
oxidized low-density lipoprotein
- OXPHOS
oxidative phosphorylation
- PHACTR1
phosphatase and actin regulator 1
- RAGE
receptor for advanced glycation end products
- REDUCE-IT
Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial
- RIP1
receptor-interacting protein kinase 1
- RIP3
receptor-interacting protein kinase 3
- RvD1
resolvin D1
- SAA
serum amyloid A
- SGK1
serum/glucocorticoid-regulated kinase 1
- SPM
specialized pro-resolving mediator
- TG2
transglutaminase 2
- TNF-α
tumor necrosis factor-α
Footnotes
Disclosures
None.
REFERENCES
- 1.Roth GA, Mensah GA and Fuster V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J Am Coll Cardiol. 2020;76:2980–2981. [DOI] [PubMed] [Google Scholar]
- 2.Aday AW and Ridker PM. Targeting Residual Inflammatory Risk: A Shifting Paradigm for Atherosclerotic Disease. Front Cardiovasc Med. 2019;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradhan AD, Aday AW, Rose LM and Ridker PM. Residual Inflammatory Risk on Treatment With PCSK9 Inhibition and Statin Therapy. Circulation. 2018;138:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, Lopez-Sendon J, Ostadal P, Koenig W, Angoulvant D, Gregoire JC, Lavoie MA, Dube MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC and Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 5.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL and LoDoCo2 Trial I. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838–1847. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ and Group CT. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 7.Gistera A and Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13:368–380. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgozoglu L and Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. [DOI] [PubMed] [Google Scholar]
- 9.Silva MT, do Vale A and dos Santos NM. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P The changing landscape of atherosclerosis. Nature. 2021;592:524–533. [DOI] [PubMed] [Google Scholar]
- 11.Roy P, Orecchioni M and Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf D and Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soehnlein O and Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredman G and MacNamara KC. Atherosclerosis is a major human killer and non-resolving inflammation is a prime suspect. Cardiovascular Research. 2021;117:2563–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasikara C, Doran AC, Cai B and Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128:2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore KJ, Koplev S, Fisher EA, Tabas I, Bjorkegren JLM, Doran AC and Kovacic JC. Macrophage Trafficking, Inflammatory Resolution, and Genomics in Atherosclerosis: JACC Macrophage in CVD Series (Part 2). J Am Coll Cardiol. 2018;72:2181–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basil MC and Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN and Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30:2792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazan HA, Lu Y, Jun B, Fang Z, Woods TC and Hong S. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot Essent Fatty Acids. 2017;125:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M and Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N and Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res. 2016;119:1030–1038. [DOI] [PubMed] [Google Scholar]
- 22.Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G and Tabas I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016;113:6526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai B, Kasikara C, Doran AC, Ramakrishnan R, Birge RB and Tabas I. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci Signal. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu H, Gabrielsen A, Agardh HE, Wan M, Wetterholm A, Wong CH, Hedin U, Swedenborg J, Hansson GK, Samuelsson B, Paulsson-Berne G and Haeggstrom JZ. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci U S A. 2006;103:8161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Xiang A, Peng T, Doran AC, Tracey KJ, Barnes BJ, Tabas I, Son M and Diamond B. HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc Natl Acad Sci U S A. 2019;116:23254–23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T and Rovati GE. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br J Pharmacol. 2014;171:3551–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petri MH, Laguna-Fernández A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK and Bäck M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovascular Research. 2015;105:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA and Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–34. [DOI] [PubMed] [Google Scholar]
- 29.Petri MH, Laguna-Fernandez A, Arnardottir H, Wheelock CE, Perretti M, Hansson GK and Back M. Aspirin-triggered lipoxin A4 inhibits atherosclerosis progression in apolipoprotein E(−/−) mice. Br J Pharmacol. 2017;174:4043–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokzhad O and Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maciuszek M, Ortega-Gomez A, Maas SL, Perretti M, Merritt A, Soehnlein O and Chapman TM. Synthesis and evaluation of novel cyclopentane urea FPR2 agonists and their potential application in the treatment of cardiovascular inflammation. Eur J Med Chem. 2021;214:113194. [DOI] [PubMed] [Google Scholar]
- 32.Subbarao K, Jala VR, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT and Haribabu B. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:369–75. [DOI] [PubMed] [Google Scholar]
- 33.Heller EA, Liu E, Tager AM, Sinha S, Roberts JD, Koehn SL, Libby P, Aikawa ER, Chen JQ, Huang P, Freeman MW, Moore KJ, Luster AD and Gerszten RE. Inhibition of atherogenesis in BLT1-deficient mice reveals a role for LTB4 and BLT1 in smooth muscle cell recruitment. Circulation. 2005;112:578–86. [DOI] [PubMed] [Google Scholar]
- 34.Laguna-Fernandez A, Checa A, Carracedo M, Artiach G, Petri MH, Baumgartner R, Forteza MJ, Jiang X, Andonova T, Walker ME, Dalli J, Arnardottir H, Gistera A, Thul S, Wheelock CE, Paulsson-Berne G, Ketelhuth DFJ, Hansson GK and Back M. ERV1/ChemR23 Signaling Protects Against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages. Circulation. 2018;138:1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Vorst EPC, Mandl M, Muller M, Neideck C, Jansen Y, Hristov M, Gencer S, Peters LJF, Meiler S, Feld M, Geiselhoringer AL, de Jong RJ, Ohnmacht C, Noels H, Soehnlein O, Drechsler M, Weber C and Doring Y. Hematopoietic ChemR23 (Chemerin Receptor 23) Fuels Atherosclerosis by Sustaining an M1 Macrophage-Phenotype and Guidance of Plasmacytoid Dendritic Cells to Murine Lesions-Brief Report. Arterioscler Thromb Vasc Biol. 2019;39:685–693. [DOI] [PubMed] [Google Scholar]
- 36.Arnardottir H, Thul S, Pawelzik SC, Karadimou G, Artiach G, Gallina AL, Mysdotter V, Carracedo M, Tarnawski L, Caravaca AS, Baumgartner R, Ketelhuth DF, Olofsson PS, Paulsson-Berne G, Hansson GK and Back M. The resolvin D1 receptor GPR32 transduces inflammation-resolution and atheroprotection. J Clin Invest. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallat Z, Heymes C, Ohan J, Faggin E, Leseche G and Tedgui A. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 1999;19:611–6. [DOI] [PubMed] [Google Scholar]
- 38.Han X, Kitamoto S, Lian Q and Boisvert WA. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J Biol Chem. 2009;284:32950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proto JD, Doran AC, Gusarova G, Yurdagul A Jr., , Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, Kuriakose G, Bhattacharya J and Tabas I. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity. 2018;49:666–677 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waehre T, Halvorsen B, Damas JK, Yndestad A, Brosstad F, Gullestad L, Kjekshus J, Froland SS and Aukrust P. Inflammatory imbalance between IL-10 and TNFalpha in unstable angina potential plaque stabilizing effects of IL-10. Eur J Clin Invest. 2002;32:803–10. [DOI] [PubMed] [Google Scholar]
- 41.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK and Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–7. [PMC free article] [PubMed] [Google Scholar]
- 42.Han X, Kitamoto S, Wang H and Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 2010;24:2869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han X and Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost. 2015;113:505–12. [DOI] [PubMed] [Google Scholar]
- 44.Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, Milton J, Carvalho Leitao R, Rosa Fernandes L, Hasan M, Gao H, Nguyen V, Harris J, Tabas I and Farokhzad OC. Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS Nano. 2016;10:5280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugimoto MA, Vago JP, Teixeira MM and Sousa LP. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J Immunol Res. 2016;2016:8239258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, Doring Y, Zarbock A and Soehnlein O. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kusters DH, Chatrou ML, Willems BA, De Saint-Hubert M, Bauwens M, van der Vorst E, Bena S, Biessen EA, Perretti M, Schurgers LJ and Reutelingsperger CP. Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR−/− Mice on Western Type Diet. PLoS One. 2015;10:e0130484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace JL, Ianaro A, Flannigan KL and Cirino G. Gaseous mediators in resolution of inflammation. Semin Immunol. 2015;27:227–33. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez-Cuellar E, Tsuchiya K, Hara H, Fang R, Sakai S, Kawamura I, Akira S and Mitsuyama M. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J Immunol. 2012;189:5113–7. [DOI] [PubMed] [Google Scholar]
- 50.Castelblanco M, Lugrin J, Ehirchiou D, Nasi S, Ishii I, So A, Martinon F and Busso N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J Biol Chem. 2018;293:2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dufton N, Natividad J, Verdu EF and Wallace JL. Hydrogen sulfide and resolution of acute inflammation: A comparative study utilizing a novel fluorescent probe. Sci Rep. 2012;2:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Q, Pan L and Ji Y. H 2S protects against diabetes-accelerated atherosclerosis by preventing the activation of NLRP3 inflammasome. J Biomed Res. 2019;34:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C and Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:173–9. [DOI] [PubMed] [Google Scholar]
- 54.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhotak S, Meng QH and Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–34. [DOI] [PubMed] [Google Scholar]
- 55.Siow RC, Sato H and Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovascular Research. 1999;41:385–94. [DOI] [PubMed] [Google Scholar]
- 56.Back M, Yurdagul A Jr., , Tabas I, Oorni K and Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16:389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM and Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190:6378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y and Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–12. [DOI] [PubMed] [Google Scholar]
- 59.Marsch E, Theelen TL, Demandt JA, Jeurissen M, van Gink M, Verjans R, Janssen A, Cleutjens JP, Meex SJ, Donners MM, Haenen GR, Schalkwijk CG, Dubois LJ, Lambin P, Mallat Z, Gijbels MJ, Heemskerk JW, Fisher EA, Biessen EA, Janssen BJ, Daemen MJ and Sluimer JC. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–53. [DOI] [PubMed] [Google Scholar]
- 60.Ouyang S, You J, Zhi C, Li P, Lin X, Tan X, Ma W, Li L and Xie W. Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis. 2021;12:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian Z, Zhao Y, Wan C, Deng Y, Zhuang Y, Xu Y, Zhu Y, Lu S and Bao Z. Pyroptosis in the Initiation and Progression of Atherosclerosis. Front Pharmacol. 2021;12:652963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doran AC, Yurdagul A Jr., and Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rayner KJ. Cell Death in the Vessel Wall: The Good, the Bad, the Ugly. Arterioscler Thromb Vasc Biol. 2017;37:e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yurdagul A Jr., , Doran AC, Cai B, Fredman G and Tabas IA. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med. 2017;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myocardial Infarction Genetics C, Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Ardissino D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Mannucci PM, Schwartz SM, Siscovick DS, Yee J, Friedlander Y, Elosua R, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Kathiresan S, Meigs JB, Williams G, Nathan DM, MacRae CA, O'Donnell CJ, Salomaa V, Havulinna AS, Peltonen L, Melander O, Berglund G, Voight BF, Kathiresan S, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Musunuru K, Daly MJ, Purcell S, Voight BF, Purcell S, Nemesh J, Korn JM, McCarroll SA, Schwartz SM, Yee J, Kathiresan S, Lucas G, Subirana I, Elosua R, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Samani NJ, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall A, Wellcome Trust Case Control C, Schunkert H, Erdmann J, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Schunkert H, Samani NJ, Erdmann J, Ouwehand W, Hengstenberg C, Deloukas P, Scholz M, Cambien F, Reilly MP, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE, Rader DJ, Scheffold T, Berger K, Stoll M, Huge A, Girelli D, Martinelli N, Olivieri O, Corrocher R, Morgan T, Spertus JA, McKeown P, Patterson CC, Schunkert H, Erdmann E, Linsel-Nitschke P, Lieb W, Ziegler A, Konig IR, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Engert JC, Do R, Xie C, Anand S, Kathiresan S, Ardissino D, Mannucci PM, Siscovick D, O'Donnell CJ, Samani NJ, Melander O, Elosua R, Peltonen L, Salomaa V, Schwartz SM and Altshuler D. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasikara C, Schilperoort M, Gerlach B, Xue C, Wang X, Zheng Z, Kuriakose G, Dorweiler B, Zhang H, Fredman G, Saleheen D, Reilly MP and Tabas I. Deficiency of macrophage PHACTR1 impairs efferocytosis and promotes atherosclerotic plaque necrosis. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T, Ding L, Wang Y, Yang O, Wang S and Kong J. Genetic deficiency of Phactr1 promotes atherosclerosis development via facilitating M1 macrophage polarization and foam cell formation. Clin Sci (Lond). 2020;134:2353–2368. [DOI] [PubMed] [Google Scholar]
- 68.Gorovoy M, Gaultier A, Campana WM, Firestein GS and Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol. 2010;88:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C and Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem. 2011;286:33335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brophy ML, Dong Y, Tao H, Yancey PG, Song K, Zhang K, Wen A, Wu H, Lee Y, Malovichko MV, Sithu SD, Wong S, Yu L, Kocher O, Bischoff J, Srivastava S, Linton MF, Ley K and Chen H. Myeloid-Specific Deletion of Epsins 1 and 2 Reduces Atherosclerosis by Preventing LRP-1 Downregulation. Circ Res. 2019;124:e6–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF and Fazio S. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yancey PG, Ding Y, Fan D, Blakemore JL, Zhang Y, Ding L, Zhang J, Linton MF and Fazio S. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation. 2011;124:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Overton CD, Yancey PG, Major AS, Linton MF and Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–7. [DOI] [PubMed] [Google Scholar]
- 74.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM and Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, Spite M, Fredman G and Tabas I. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017;127:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen PL, Tedgui A and Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–31. [DOI] [PubMed] [Google Scholar]
- 77.Garbin U, Baggio E, Stranieri C, Pasini A, Manfro S, Mozzini C, Vallerio P, Lipari G, Merigo F, Guidi G, Cominacini L and Fratta Pasini A. Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovascular Research. 2013;97:125–33. [DOI] [PubMed] [Google Scholar]
- 78.Nicolaou A, Zhao Z, Northoff BH, Sass K, Herbst A, Kohlmaier A, Chalaris A, Wolfrum C, Weber C, Steffens S, Rose-John S, Teupser D and Holdt LM. Adam17 Deficiency Promotes Atherosclerosis by Enhanced TNFR2 Signaling in Mice. Arterioscler Thromb Vasc Biol. 2017;37:247–257. [DOI] [PubMed] [Google Scholar]
- 79.van der Vorst EP, Zhao Z, Rami M, Holdt LM, Teupser D, Steffens S and Weber C. Contrasting effects of myeloid and endothelial ADAM17 on atherosclerosis development. Thromb Haemost. 2017;117:644–646. [DOI] [PubMed] [Google Scholar]
- 80.Doran AC, Ozcan L, Cai B, Zheng Z, Fredman G, Rymond CC, Dorweiler B, Sluimer JC, Hsieh J, Kuriakose G, Tall AR and Tabas I. CAMKIIgamma suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J Clin Invest. 2017;127:4075–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tao W, Yurdagul A Jr., , Kong N, Li W, Wang X, Doran AC, Feng C, Wang J, Islam MA, Farokhzad OC, Tabas I and Shi J. siRNA nanoparticles targeting CaMKIIgamma in lesional macrophages improve atherosclerotic plaque stability in mice. Sci Transl Med. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A and Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–77. [DOI] [PubMed] [Google Scholar]
- 83.Toth B, Garabuczi E, Sarang Z, Vereb G, Vamosi G, Aeschlimann D, Blasko B, Becsi B, Erdodi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fesus L and Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–92. [DOI] [PubMed] [Google Scholar]
- 84.Boisvert WA, Rose DM, Boullier A, Quehenberger O, Sydlaske A, Johnson KA, Curtiss LK and Terkeltaub R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26:563–9. [DOI] [PubMed] [Google Scholar]
- 85.Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ and Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T and Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL and Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fasolo F, Jin H, Winski G, Chernogubova E, Pauli J, Winter H, Li DY, Glukha N, Bauer S, Metschl S, Wu Z, Koschinsky ML, Reilly M, Pelisek J, Kempf W, Eckstein HH, Soehnlein O, Matic L, Hedin U, Backlund A, Bergmark C, Paloschi V and Maegdefessel L. Long Noncoding RNA MIAT Controls Advanced Atherosclerotic Lesion Formation and Plaque Destabilization. Circulation. 2021;144:1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ye ZM, Yang S, Xia YP, Hu RT, Chen S, Li BW, Chen SL, Luo XY, Mao L, Li Y, Jin H, Qin C and Hu B. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N, Kucenas S, Rathmell JC, Makowski L and Ravichandran KS. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563:714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freemerman AJ, Zhao L, Pingili AK, Teng B, Cozzo AJ, Fuller AM, Johnson AR, Milner JJ, Lim MF, Galanko JA, Beck MA, Bear JE, Rotty JD, Bezavada L, Smallwood HS, Puchowicz MA, Liu J, Locasale JW, Lee DP, Bennett BJ, Abel ED, Rathmell JC and Makowski L. Myeloid Slc2a1-Deficient Murine Model Revealed Macrophage Activation and Metabolic Phenotype Are Fueled by GLUT1. J Immunol. 2019;202:1265–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batista-Gonzalez A, Vidal R, Criollo A and Carreno LJ. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front Immunol. 2019;10:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schilke RM, Blackburn CMR, Rao S, Krzywanski DM, Finck BN and Woolard MD. Macrophage-Associated Lipin-1 Promotes beta-Oxidation in Response to Proresolving Stimuli. Immunohorizons. 2020;4:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chandran S, Schilke RM, Blackburn CMR, Yurochko A, Mirza R, Scott RS, Finck BN and Woolard MD. Lipin-1 Contributes to IL-4 Mediated Macrophage Polarization. Front Immunol. 2020;11:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blackburn CMR, Schilke RM, Vozenilek AE, Chandran S, Bamgbose TT, Finck BN and Woolard MD. Myeloid-associated lipin-1 transcriptional co-regulatory activity is atheroprotective. Atherosclerosis. 2021;330:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, Ben-Sahra I, Gius DR, Yvan-Charvet L, Chandel NS, Schumacker PT and Thorp EB. Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab. 2019;29:443–456 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merlin J, Ivanov S, Dumont A, Sergushichev A, Gall J, Stunault M, Ayrault M, Vaillant N, Castiglione A, Swain A, Orange F, Gallerand A, Berton T, Martin JC, Carobbio S, Masson J, Gaisler-Salomon I, Maechler P, Rayport S, Sluimer JC, Biessen EAL, Guinamard RR, Gautier EL, Thorp EB, Artyomov MN and Yvan-Charvet L. Non-canonical glutamine transamination sustains efferocytosis by coupling redox buffering to oxidative phosphorylation. Nat Metab. 2021;3:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yurdagul A Jr., , Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, Kolluru GK, Rymond CC, Gerlach BD, Zheng Z, Kuriakose G, Kevil CG, Koomen JM, Cleveland JL, Muoio DM and Tabas I. Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell Metab. 2020;31:518–533 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yurdagul A Jr., , Kong N, Gerlach BD, Wang X, Ampomah P, Kuriakose G, Tao W, Shi J and Tabas I. ODC (Ornithine Decarboxylase)-Dependent Putrescine Synthesis Maintains MerTK (MER Tyrosine-Protein Kinase) Expression to Drive Resolution. Arterioscler Thromb Vasc Biol. 2021;41:e144–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dalli J and Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gerlach BD, Ampomah PB, Yurdagul A Jr., , Liu C, Lauring MC, Wang X, Kasikara C, Kong N, Shi J, Tao W and Tabas I. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vandenabeele P, Galluzzi L, Vanden Berghe T and Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. [DOI] [PubMed] [Google Scholar]
- 103.Hosseini Z, Marinello M, Decker C, Sansbury BE, Sadhu S, Gerlach BD, Bossardi Ramos R, Adam AP, Spite M and Fredman G. Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation. Arteriosclerosis, thrombosis, and vascular biology. 2021;41:1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, Hedin U, Sad S, Guo L, Kolodgie FD, Virmani R, Ruddy T and Rayner KJ. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016;2:e1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karunakaran D, Nguyen MA, Geoffrion M, Vreeken D, Lister Z, Cheng HS, Otte N, Essebier P, Wyatt H, Kandiah JW, Jung R, Alenghat FJ, Mompeon A, Lee R, Pan C, Gordon E, Rasheed A, Lusis AJ, Liu P, Matic LP, Hedin U, Fish JE, Guo L, Kolodgie F, Virmani R, van Gils JM and Rayner KJ. RIPK1 Expression Associates With Inflammation in Early Atherosclerosis in Humans and Can Be Therapeutically Silenced to Reduce NF-kappaB Activation and Atherogenesis in Mice. Circulation. 2021;143:163–177. [DOI] [PubMed] [Google Scholar]
- 106.Gerlach BD, Marinello M, Heinz J, Rymut N, Sansbury BE, Riley CO, Sadhu S, Hosseini Z, Kojima Y, Tang DD, Leeper NJ, Spite M, Barroso M, Rayner KJ and Fredman G. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27:525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A and Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wanschel A, Seibert T, Hewing B, Ramkhelawon B, Ray TD, van Gils JM, Rayner KJ, Feig JE, O'Brien ER, Fisher EA and Moore KJ. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol. 2013;33:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA and Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schlegel M, Sharma M, Brown EJ, Newman AAC, Cyr Y, Afonso MS, Corr EM, Koelwyn GJ, van Solingen C, Guzman J, Farhat R, Nikain CA, Shanley LC, Peled D, Schmidt AM, Fisher EA and Moore KJ. Silencing Myeloid Netrin-1 Induces Inflammation Resolution and Plaque Regression. Circ Res. 2021;129:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA and Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL and Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruikman CS, Vreeken D, Zhang H, van Gils MJ, Peter J, van Zonneveld AJ, Hovingh GK and van Gils JM. The identification and function of a Netrin-1 mutation in a pedigree with premature atherosclerosis. Atherosclerosis. 2020;301:84–92. [DOI] [PubMed] [Google Scholar]
- 114.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S and Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T and Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. [DOI] [PubMed] [Google Scholar]
- 116.Josefowicz SZ, Lu LF and Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A and Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. [DOI] [PubMed] [Google Scholar]
- 118.Mor A, Luboshits G, Planer D, Keren G and George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–7. [DOI] [PubMed] [Google Scholar]
- 119.Dietel B, Cicha I, Voskens CJ, Verhoeven E, Achenbach S and Garlichs CD. Decreased numbers of regulatory T cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis. 2013;230:92–9. [DOI] [PubMed] [Google Scholar]
- 120.George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A and Shamiss A. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis. 2012;222:519–23. [DOI] [PubMed] [Google Scholar]
- 121.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G and George J. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. [DOI] [PubMed] [Google Scholar]
- 122.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX and Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tabas I and Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity. 2017;47:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T and Hansson GK. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Foks AC, Frodermann V, ter Borg M, Habets KL, Bot I, Zhao Y, van Eck M, van Berkel TJ, Kuiper J and van Puijvelde GH. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. [DOI] [PubMed] [Google Scholar]
- 126.Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, Bobik A and Agrotis A. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. 2012;126:1256–66. [DOI] [PubMed] [Google Scholar]
- 127.Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, Sansbury BE, Corr EM, van Solingen C, Koelwyn GJ, Shanley LC, Beckett L, Peled D, Lafaille JJ, Spite M, Loke P, Fisher EA and Moore KJ. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ Res. 2020;127:335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kleinclauss F, Perruche S, Masson E, de Carvalho Bittencourt M, Biichle S, Remy-Martin JP, Ferrand C, Martin M, Bittard H, Chalopin JM, Seilles E, Tiberghien P and Saas P. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Piao W, Xiong Y, Li L, Saxena V, Smith KD, Hippen KL, Paluskievicz C, Willsonshirkey M, Blazar BR, Abdi R and Bromberg JS. Regulatory T Cells Condition Lymphatic Endothelia for Enhanced Transendothelial Migration. Cell Rep. 2020;30:1052–1062 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kourtzelis I, Li X, Mitroulis I, Grosser D, Kajikawa T, Wang B, Grzybek M, von Renesse J, Czogalla A, Troullinaki M, Ferreira A, Doreth C, Ruppova K, Chen LS, Hosur K, Lim JH, Chung KJ, Grossklaus S, Tausche AK, Joosten LAB, Moutsopoulos NM, Wielockx B, Castrillo A, Korostoff JM, Coskun U, Hajishengallis G and Chavakis T. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol. 2019;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kakino A, Fujita Y, Nakano A, Horiuchi S and Sawamura T. Developmental Endothelial Locus-1 (Del-1) Inhibits Oxidized Low-Density Lipoprotein Activity by Direct Binding, and Its Overexpression Attenuates Atherogenesis in Mice. Circ J. 2016;80:2541–2549. [DOI] [PubMed] [Google Scholar]
- 132.Li X, Colamatteo A, Kalafati L, Kajikawa T, Wang H, Lim JH, Bdeir K, Chung KJ, Yu X, Fusco C, Porcellini A, De Simone S, Matarese G, Chavakis T, De Rosa V and Hajishengallis G. The DEL-1/beta3 integrin axis promotes regulatory T cell responses during inflammation resolution. J Clin Invest. 2020;130:6261–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Linehan E and Fitzgerald DC. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol (Bp). 2015;5:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ Jr., and Walsh K. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aprahamian T, Takemura Y, Goukassian D and Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arnardottir HH, Dalli J, Colas RA, Shinohara M and Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol. 2014;193:4235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frisch BJ, Hoffman CM, Latchney SE, LaMere MW, Myers J, Ashton J, Li AJ, Saunders J 2nd, , Palis J, Perkins AS, McCabe A, Smith JN, McGrath KE, Rivera-Escalera F, McDavid A, Liesveld JL, Korshunov VA, Elliott MR, MacNamara KC, Becker MW and Calvi LM. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via Interleukin1B. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gangemi S, Pescara L, D'Urbano E, Basile G, Nicita-Mauro V, Davi G and Romano M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp Gerontol. 2005;40:612–4. [DOI] [PubMed] [Google Scholar]
- 139.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rymut N, Heinz J, Sadhu S, Hosseini Z, Riley CO, Marinello M, Maloney J, MacNamara KC, Spite M and Fredman G. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. FASEB J. 2020;34:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaiswal S and Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. 2020;17:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., , Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM and Investigators R-I. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 143.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., , Juliano RA, Jiao L, Granowitz C, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM and Investigators R-I. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. [DOI] [PubMed] [Google Scholar]
- 144.Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, Patel M, Collier DJ and Dalli J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ Res. 2020;126:75–90. [DOI] [PubMed] [Google Scholar]
- 145.Jarr KU, Nakamoto R, Doan BH, Kojima Y, Weissman IL, Advani RH, Iagaru A and Leeper NJ. Effect of CD47 Blockade on Vascular Inflammation. N Engl J Med. 2021;384:382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, Funk CD and Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–6. [DOI] [PubMed] [Google Scholar]
- 147.Merched AJ, Ko K, Gotlinger KH, Serhan CN and Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Merched AJ, Serhan CN and Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics. 2011;4:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV and Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salic K, Morrison MC, Verschuren L, Wielinga PY, Wu L, Kleemann R, Gjorstrup P and Kooistra T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis. 2016;250:158–65. [DOI] [PubMed] [Google Scholar]
- 151.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J and Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C and Maderna P. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–605. [DOI] [PubMed] [Google Scholar]
- 153.Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ and Perretti M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol. 2005;78:639–46. [DOI] [PubMed] [Google Scholar]
- 154.Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D and Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–30. [DOI] [PubMed] [Google Scholar]
- 155.Watson RW, Rotstein OD, Nathens AB, Parodo J and Marshall JC. Neutrophil apoptosis is modulated by endothelial transmigration and adhesion molecule engagement. J Immunol. 1997;158:945–53. [PubMed] [Google Scholar]
- 156.Zouki C, Ouellet S and Filep JG. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J. 2000;14:572–80. [DOI] [PubMed] [Google Scholar]
- 157.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR and Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. Journal of the American Society of Nephrology : JASN. 2002;13:2497–507. [DOI] [PubMed] [Google Scholar]
- 158.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N and Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–7. [DOI] [PubMed] [Google Scholar]
- 159.Ariel A, Chiang N, Arita M, Petasis NA and Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–72. [DOI] [PubMed] [Google Scholar]
- 160.Hachicha M, Pouliot M, Petasis NA and Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chiang N, Fierro IM, Gronert K and Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA and Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Norling LV, Dalli J, Flower RJ, Serhan CN and Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G and Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lopategi A, Flores-Costa R, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Titos E and Claria J. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J Leukoc Biol. 2019;105:25–36. [DOI] [PubMed] [Google Scholar]
- 166.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA and Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liang TS, Wang JM, Murphy PM and Gao JL. Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem Biophys Res Commun. 2000;270:331–5. [DOI] [PubMed] [Google Scholar]
- 168.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N and Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–7. [DOI] [PubMed] [Google Scholar]
- 169.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA and Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. The Journal of Experimental Medicine. 2005;201:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oh SF, Pillai PS, Recchiuti A, Yang R and Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D and Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE and Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fredman G, Van Dyke TE and Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mariani F and Roncucci L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm Res. 2015;64:85–95. [DOI] [PubMed] [Google Scholar]
- 175.Perretti M and D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nature Reviews Immunology. 2009;9:62. [DOI] [PubMed] [Google Scholar]
- 176.Walther A, Riehemann K and Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5:831–40. [DOI] [PubMed] [Google Scholar]
- 177.Chiang N, Dalli J, Colas RA and Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Colas RA, Souza PR, Walker ME, Burton M, Marques RM, Zaslona Z, Curtis AM and Dalli J. Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increases Systemic Inflammation and Cardiovascular Disease. Circ Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M and Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA and Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]