1. Background
In August 2021, two Japanese men, aged 38 and 30, died within days after receiving their second dose of the Moderna COVID-19 vaccine. Both had been vaccinated with the same lot of the vaccine [1]. Further investigation revealed that some black substances were spotted in syringes and a vial, with pink substances found in another syringe. A good understanding of vaccine manufacturing, quality control, incident investigation and pharmacovigilance is helpful in determining if vaccine contamination was the cause of death in these cases.
2. Vaccine manufacturing and quality control
The manufacture of a vaccine begins from the research process, followed by rigorous clinical testing and finally approval from the relevant authorities for its use with the general public. Vaccine formulations are intended to optimise vaccine stability and efficacy. The final vaccine product may include adjuvants to boost immune response, stabilisers to extend shelf life, and preservatives to eliminate microorganisms for multi-dose vial formulations, and it may be delivered parenterally via multi-dose vials, via single-dose pre-filled syringes, or by other methods of administration, e.g., orally or by inhalation.
Good manufacturing practice and quality assurance both play a key role in avoiding contamination and product defects. All operations in the manufacturing process should be performed with stringent quality control, which includes safety, potency, purity, sterility, and other product-specific tests. Manufacturers also provide product documentation such as the recommended storage conditions, dosage, shelf life, expiry date extension, and supporting distribution circumstances.
Vaccines may be produced in stages in different facilities. For example, a vaccine can be produced by the manufacturer in one location, but packaged in another facility elsewhere. It is important that strict quality control is maintained at all sites. Finally, suitable conditions of transport and storage, as specified by the manufacturer, should be maintained at all stages from vaccine production to final administration to the patient.
3. Incident investigation
Following the two post-vaccination deaths in Japan in August, another vaccine centre in Gunma prefecture also reported tiny, black substances in a vial from another vaccine lot. The Japanese authorities conducted an urgent investigation and found foreign material contained in 39 unused vials [2]. The material found was a few millimetres in size, though its elements are yet to be determined.
In the case of foreign materials discovered in several unopened vials, the most probable cause was thought to be related to friction between two pieces of metal in the machinery that put stoppers on the vials, and the material was confirmed to be stainless steel [1].
The vaccine vials were reportedly produced in Spain [3]. Moderna Inc, Takeda Pharmaceutical Co Ltd and the Japanese authorities recalled three vaccine batches, totalling 1.63 million doses, on September 1st, 2021. By then, the vaccines had been distributed to 863 immunisation centres around Japan, with approximately 500,000 people given doses from the three suspended batches [2]. In September 2021, the Ministry of Health of Japan again reported that another 49-year-old man died after receiving a dose of Moderna COVID-19 vaccine that was among batches later recalled from use by its distributor in the country.
In these cases, the official statement from the authority stated that a manufacturing error caused the contamination, with contamination by foreign substances, and also from incorrectly inserted needles – vial coring [1].
4. Coring
When a syringe is pushed into a medicine vial's stopper, fragmentation of the plug may occur. The fragment may be “cored” inside the needle bore, and then injected into the body [4]. Coring can occur in up to 40.8 per cent of cases even with a fine needle of gauge size 24 [5].
Unless a patient is allergic to latex, the immediate effects of injecting a piece of rubber into them would not be seen clinically. Still, this may potentially result in embolism and adverse reactions varying from autopsy-detected clinically occult pulmonary granulomas to local tissue infarction, pulmonary infarction and death [6].
Other inert particle contaminants may not represent a substantial concern in healthy tissue. However, they may significantly influence tissue perfusion in individuals with a compromised vital organ microvascular structure. Additionally, if such debris enters the left side of the circulation and obstructs a cerebral vessel, neurologic deficits can occur [7].
5. Biovigilance
Biovigilance is a surveillance system that comprises the detection, gathering, and analysis of information for tracking adverse events (AEs) related to biological products. These products include cells, tissues, organs and vaccines [8]. The World Health Organization (WHO) has published a guideline for national authorities on quality assurance for biological products that lists procedures for approval of manufacturers' biological products and the post-licensing monitoring of products [9].
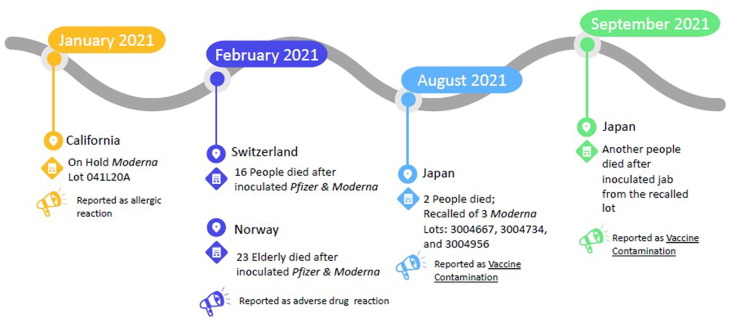
Prior to the COVID-19 vaccine recall in Japan, the U.S. state of California also paused and then resumed the administration of Moderna COVID-19 vaccine as a result of its biovigilance in January 2021, when less than 0.002% of vaccinees had some form of allergic reaction [10]. Switzerland and Norway also released interim biovigilance news of suspected death in adults over 80 years old. The vaccine's biovigilance events are depicted in Fig. 1 in chronological order.
Fig. 1.
COVID-19 vaccine biovigilance events in chronological order.
To detect and assess the vaccination risk–benefit profile, the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA) conduct post-licensure vaccine safety monitoring using the Vaccine Adverse Event Reporting System (VAERS).
6. Vaccine manufacturing and quality control
The WHO guideline stipulates that for vaccine production, a specific master formula for each batch must be recorded and supported by validation of the consistency of the process and the product. This should be done at both extremes of production volumes. The product stability should be similarly validated. Besides these, the manufacturer has to prepare a clear policy for handling the starting material, packaging materials, bulk and finished products, including sampling, quarantine, release and storage [11].
The FDA guidelines for industry development and licensure of COVID-19 vaccines require quality control in the manufacture of each drug substance and product. Facilities for manufacture, toxicity studies, characterization of the immune response in animal models, details of clinical trials including of the trial population, design, efficacy considerations and safety considerations have to be audited before the vaccine can be mass-produced. Measures for post-licensure safety evaluation, post-marketing safety studies and assessment of long-term vaccine effectiveness are also part of the FDA's considerations before the vaccine can be licensed [12].
7. Potential solutions
Transitioning from a vial to a pre-filled syringe would prevent incidents of “coring.” Every time a needle is pushed into a pharmaceutical vial to extract a vaccine, it introduces another possibility of admixture-related contamination. To eliminate the chance of medication contamination, single-use syringes should be considered [13]. Furthermore, pre-filled syringes ensure that healthcare professionals deliver each dose precisely since it is pre-filled with the right amount.
The vials rely on stoppers and seals which serve to protect the contents and ensure closure integrity. For example, for their COVID-19 vaccine vials, Pfizer-BioNTech and Moderna use synthetic bromobutyl and chlorobutyl rubber stoppers, respectively, along with plastic flip-off caps. In contrast, contamination of the Moderna COVID-19 vaccine at Japan appears to have resulted from incorrectly inserted needles causing bits of the vials' rubber stopper to break off. Thus, a coating barrier layer applied to the rubber stopper that could act as a barrier to the coring or transfer of elastomer components to the vaccine is crucial [14].
8. Concluding remarks
Vaccine contamination is an important concern which may aggravate vaccine hesitancy. It can be minimized or prevented by good manufacturing, transport and storage practices, and other measures such as the utilization of pre-filled syringes. In addition, robust surveillance measures should be put in place, with swift investigation of reported incidents and decisive action taken to reassure the public.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Blair G. The Guardian. Guardian Media Group; United Kingdom: 2021. Japan’s Moderna Covid vaccine rollout hit by recall and contamination scares. [Google Scholar]
- 2.Swift R., O'Donnell C. Reuters; New York: 2021. Moderna to recall COVID-19 doses in Japan after stainless steel contaminants found. [Google Scholar]
- 3.Novak M. Japan Halts Moderna Vaccinations After Foreign Substances Found in 39 Vials. Available online: https://gizmodo.com/japan-halts-moderna-vaccinations-after-foreign-substanc-1847561489. Accessed November 15 2021. Gizmodo. United States: G/O Media; 2021.
- 4.Eskander J., Cotte J., Glenn E., Friedman S., Rosinia F. The incidence of coring and fragmentation of medication vial rubber stoppers. J Clin Anesth. 2015;27(5):442–444. doi: 10.1016/j.jclinane.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Wani T., Wadhwa A., Tobias J.D. The incidence of coring with blunt versus sharp needles. J Clin Anesth. 2014;26(2):152–154. doi: 10.1016/j.jclinane.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Roth J.V. How to enter a medication vial without coring. Anesth Analg. 2007;104:1615. doi: 10.1213/01.ane.0000260552.76585.53. [DOI] [PubMed] [Google Scholar]
- 7.Lehr HANS.-ANTON., Brunner JOACHIM, Rangoonwala RAMZAN, James kirkpatrick C. Particulate Matter Contamination of Intravenous Antibiotics Aggravates Loss of Functional Capillary Density in Postischemic Striated Muscle. Am J Respir Crit Care Med. 2002;165(4):514–520. doi: 10.1164/ajrccm.165.4.2108033. [DOI] [PubMed] [Google Scholar]
- 8.Singh S., Chandel S., Sarma P., Reddy DibbantiHarikrishna, Mishra A., Kumar S., et al. Biovigilance: A Global Perspective. Perspect Clin Res. 2019;10(4):155. doi: 10.4103/picr.PICR_89_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Vaccine Adverse Event Reporting System (VAERS). Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html. Accessed November 14 2021. 2021.
- 10.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Expert Committee on Biological Standardization. WHO good manufacturing practices for biological products; Annex 2. Available online https://www.who.int/biologicals/areas/vaccines/Annex_2_WHO_Good_manufacturing_practices_for_biological_products.pdf?ua=1. Accessed 15 Nov 2021. 2015.
- 12.FDA. 2021 Biologics Recalls. 2020.
- 13.Yoneda S., Torisu T., Uchiyama S. Development of syringes and vials for delivery of biologics: current challenges and innovative solutions. Expert Opin Drug Deliv. 2021;18(4):459–470. doi: 10.1080/17425247.2021.1853699. [DOI] [PubMed] [Google Scholar]
- 14.Ramakanth D., Singh S., Maji P.K., Lee Y.S., Gaikwad K.K. Advanced packaging for distribution and storage of COVID-19 vaccines: a review. Environ Chem Lett. 2021;19(5):3597–3608. doi: 10.1007/s10311-021-01256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]