Abstract
Background: L-triiodothyronine (LT3) has been increasingly used in combination with levothyroxine in the treatment of hypothyroidism. A metal coordinated form of LT3, known as poly-zinc-liothyronine (PZL), avoided in rats the typical triiodothyronine (T3) peak seen after oral administration of LT3.
Objectives: To evaluate in healthy volunteers (i) the pharmacokinetics (PK) of PZL-derived T3 after a single dose, (ii) the pharmacodynamics of PZL-derived T3, (iii) incidence of adverse events, and (iv) exploratory analysis of the sleep patterns after LT3, PZL, or placebo (PB) administration.
Methods: Twelve healthy volunteers 18–50 years of age were recruited for a Phase 1, double-blind, randomized, single-dose PB-controlled, crossover study to compare PZL against LT3 or PB. Subjects were admitted three separate times to receive a randomly assigned capsule containing PB, 50 μg LT3, or 50 μg PZL, and were observed for 48 hours. A 2-week washout period separated each admission.
Results: LT3-derived serum T3 levels exhibited the expected profile, with a Tmax at 2 hours and return to basal levels by 24–36 hours. PZL-derived serum T3 levels exhibited ∼30% lower Cmax that was 1 hour delayed and extended into a plateau that lasted up to 6 hours. This was followed by a lower but much longer plateau; by 24 hours serum T3 levels still exceeded ½ of Cmax. Thyrotropin levels were similarly reduced in both groups.
Conclusion: PZL possesses the necessary properties to achieve a much improved T3 PK. PZL is on track to provide hypothyroid patients with stable levels of serum T3.
Keywords: liothyronine, slow release, metal coordination, hypothyroidism, thyroid, thyroxine
Introduction
For decades, hypothyroidism was treated with desiccated extracts of porcine thyroid glands, which contain thyroxine (T4) and triiodothyronine (T3) (1,2). With the development of thyrotropin (TSH) radioimmunoassay and the discovery that humans activate T4 to T3 (3), treatment with levothyroxine (LT4) became the standard of care (4,5). Nonetheless, deiodination of T4 to T3 may not be sufficient to account for the normal thyroidal secretion of T3. First noticed around 1974 (6), subsequent studies revealed that LT4-treated patients maintain ∼10% lower serum T3 levels compared with euthyroid individuals with similar TSH levels (7,8).
Some LT4-treated patients with normal TSH levels complain of residual symptoms of hypothyroidism (9–11). Compared with the general population, LT4-treated patients weigh ∼10 pounds more (7), have a slower rate of energy expenditure (12), show slightly higher serum cholesterol levels (13), and are more likely to be on therapy to lower cholesterol levels (14). The extent to which lower T3 levels contribute to the residual symptoms is unknown. In LT4-treated thyroidectomized rats, tissue euthyroidism only occurs after normalization of serum T3 (15). Such clear-cut evidence is not available for LT4-treated patients.
L-triiodothyronine (LT3) has been commercially available since 1956, but the pharmacokinetics (PK) of current LT3 products raise potential concerns (16–18). A tablet of LT3 causes a T3 peak 2–3 hours after dosing, which sometimes is associated with palpitations, depending on the dose and duration of treatment. Notwithstanding, little evidence exists of adverse reactions (AR) to appropriate doses of LT3 and a growing number of endocrinologists prescribe LT4 plus LT3 to treat hypothyroidism. The combined analysis of 20 clinical trials that included ∼1000 hypothyroid patients on combination therapy for up to ∼1 year did not reveal an increased frequency of AR when compared with LT4-treated patients (2).
A retrospective analysis of 400 patients on LT3 for several years also revealed no concerning cardiovascular or fracture trends, but did reveal an increased risk of being prescribed antipsychotic medication and a possible concern about an increased risk of breast cancer and use of antidepressants (19). Thus, professional medical societies have become more accepting of combination therapy and have recommended the development of sustained-release T3 preparations (4,20).
Poly-zinc-liothyronine (PZL) is a prodrug that uses metal coordination chemistry to control and sustain the absorption of T3. It is a polymeric coordination complex of zinc and T3 designed to adhere to the intestinal mucosal lining, where it creates a depot from which T3 is slowly released and absorbed over time. Studies in rats validated these concepts using oral administration of PZL (21).
As reported for the chemical reactions between the dianion of tyrosine, which has similar functional groups to T3, and zinc (22), T3 also acts as a tridentate ligand when binding to zinc through the participation of the amino acid group and the phenol group. When the phenol group is deprotonated, it serves as an additional zinc-binding site, thereby expanding coordination mode and favoring the formation of supramolecular structures, such as PZL. The inherent mucoadhesive properties of supramolecular metal coordinated (MC) complexes extend their intestinal transit time and allow for the slow release of T3 from the MC complexes through ligand exchange (e.g., hydrolysis). The released T3 is absorbed into the bloodstream as T3 would normally (23). MC molecules adhere to the mucosa by mechanisms that include coordinate covalent bonding, hydrogen bonding, halogen bonding, metal-halogen bonding, and electrostatic interactions (24).
Here we report the first study of PZL in volunteers, a double-blinded placebo (PB)-controlled crossover investigation of the PK and pharmacodynamics (PD) of PZL-derived T3 in humans.
Materials and Methods
This clinical trial was conducted under the Food and Drug Administration (FDA) exploratory investigational new drug (IND) application (IND-137796), and approved by the University of Chicago IRB and Clinical Research Center (CRC) committees (IRB20-1341).
Objectives
To evaluate:
-
(i)
PK of PZL-derived T3 after a single dose of PZL by repetitive measurements of serum T3 levels.
-
(ii)
PD of PZL-derived T3 through monitoring of serum TSH and free T4 (fT4) levels, as well as heart rate and blood pressure.
-
(iii)
Occurrence of AR or adverse events (AE) to a single dose of PZL through clinical interview and examination, metabolic panels, electrocardiogram (ECG).
-
(iv)
Exploratory analysis of the sleep patterns.
Investigational plan
This is a Phase 1, double-blind, randomized, single-dose PB-controlled, crossover study to test PZL in healthy volunteers (Fig. 1).
FIG. 1.
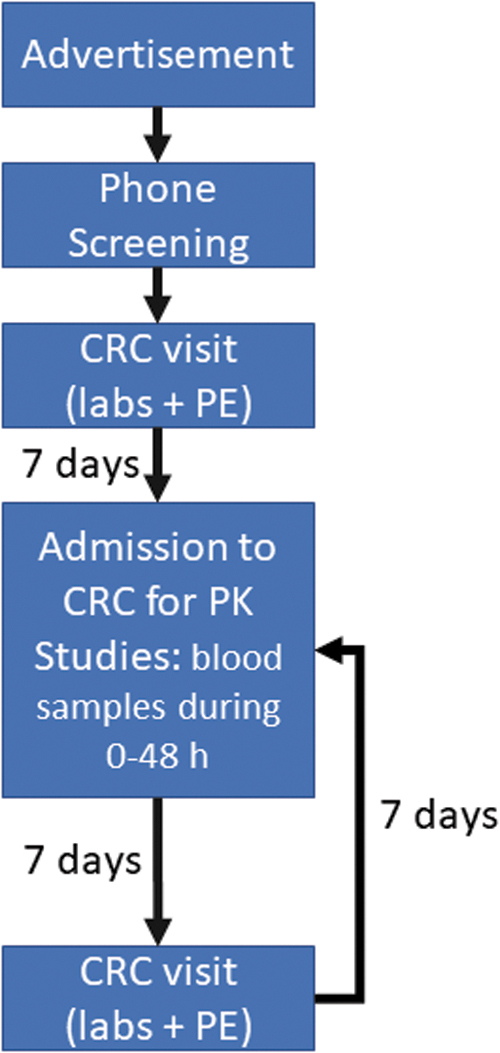
Flow chart of the study design, including timing between steps. Color images are available online.
Recruitment and screening
Healthy male and female volunteers 18–50 years of age, sleeping at least 7 hours/night but no more than 9 hours/night, between 22:00 and 08:00 hours, were recruited from the community through advertisement. BMI <30 was an inclusion criterion, to ensure sufficient female representation in the study women with BMI 33 was recruited.
Initial screening involved a telephone questionnaire. Exclusion criteria included the following: use of steroids, oral contraceptives, or any medications that affect thyroid hormone absorption or metabolism; ingestion of kelp, soy, biotin; pregnancy, lactation; diagnosis of sleep disorders (including obstructive sleep apnea), prediabetes/diabetes, endocrine dysfunction, psychiatric, eating disorders, gastrointestinal disease that affects T3 absorption, drug or nicotine use, habitual alcohol use of >2 drinks/day, caffeine intake of >500 mg/day, bariatric surgery, weight >100 kg, dietary restrictions, night shifts.
After screening, subjects visited the CRC, where they consented to the study. Qualifying individuals were in good health based on the medical history, physical examination. They underwent a 12-lead ECG, complete blood count (CBC), complete metabolic panel (CMP), serum TSH, fT4, total T3, and thyroid antibodies to thyroperoxidase and thyroglobulin; women underwent a pregnancy test. Participants were eligible if ECG, CBC, CMP, and TFTs were normal.
Investigational product, dosage, and delivery system
Identical, off-white, size-0 capsules (Capsugel® Vcaps®; Lonza CHI) coated for duodenal delivery of contents were prepared by Catalent, Inc. (San Diego, CA). They contained current good manufacturing practice (CGMP) grade PB (sterile excipient powder mixed with 5 μg CGMP grade zinc chloride), PZL (56 μg) prepared by Synthonics, Inc. (Blacksburg, VA), or Na-T3 (53 μg) prepared by Peptido, GmbH. The amounts of PZL and Na-T3 were calculated to contain 50 μg LT3, based on previous clinical experience with LT3 (16) and recommended dietary allowance of zinc (10 mg/day). Fifty-microgram LT3 is a pharmacologic dose, which was used because of the baseline T3 levels in these normal volunteers. Capsules were labeled using three different alpha-numeric codes and delivered to the Investigational Drug Service Pharmacy at the University of Chicago (kept at 4°C until use).
Admission to the CRC
Each volunteer was assigned (blindly and randomly) to a predefined sequence of treatment arms. In the morning of the trial (overnight fasting), subjects were admitted to the CRC, received their treatment, and were observed for 48 hours in the CRC. Subjects had timed blood draws and were monitored three times a day for AR through vital signs and physical examination (including neurological screening); an ECG was obtained before discharge. After discharge, these individuals returned to the CRC for a 7-day follow-up visit, for physical examination, ECG, CBC, and CMP. After a 2-week washout period, volunteers were readmitted to one of the remaining treatment arms and the cycle was repeated. Thus, all individuals completed the three treatment arms, separated by 2-week washout periods.
Pharmacokinetics
An intravenous catheter was inserted into a forearm vein and left in place for serial blood sampling collected at specified times post-treatment and sent to the central laboratory for T3 levels using ECLIA—electrochemiluminescence immunoassay—on Cobas, Roche (interassay variability 1.3%; reference range: 80–200 ng/dL). A total of 13 blood samples were drawn: −30 minutes, −15 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 6 hours, 8 hours, 12 hours, 18 hours, 24 hours, 36 hours, and 46 hours.
Pharmacodynamics
The same blood samples obtained in the PK studies were also processed for determination of fT4 and TSH levels using ECLIA—electrochemiluminescence immunoassay—on Cobas, Roche (interassay variability and reference range: fT4: 1.8%, 0.93–1.7 ng/dL; TSH: 1.5%, 0.27–4.2 μIU/mL). Systolic and diastolic arterial blood pressure, as well as pulse, were measured at 30-minute intervals during the first inpatient day from the nondominant arm using ambulatory monitoring equipment (Oscar II, SunTech Medical Instruments).
Sleep timing, duration, and fragmentation were assessed using wrist actigraphy monitors (Actiwatch Spectrum; Philips Respironics, Bend, OR) (25). Actiwatches were worn during the 2-day inpatient sessions and for the subsequent four outpatient days for a total of 6 days. Our primary measure of habitual sleep timing was at the midpoint of sleep, but we also calculated nocturnal sleep duration and indicators of sleep quality (sleep efficiency and sleep fragmentation). For screening, the Pittsburgh Sleep Quality Index was administered to assess sleep quality (26) and the Horne–Ostberg questionnaire to assess chronotype (27).
During the admissions, participants completed five validated questionnaires regarding vigor, mood, affect, sleepiness, hunger, and appetite. All questionnaires were completed at regular intervals up to 11 times during each admission; day 1 at 13:30, 15:30, 18:30, and 20:30 hours; day 2 at 9:00, 11:00, 13:30, 15:30, 18:30, and 20:30 hours; and day 3 at 9:00 hours. Subjective alertness was assessed using the Visual Analog Scale (VAS) for Global Vigor and Mood (28). Subjective mood of participants was measured with the Positive and Negative Affect Scales (PANAS) (29). Visual Analog Scales (30) to assess hunger and appetite were completed as described (31,32). Sleepiness was assessed with the Stanford Sleepiness Scale (SSS) (33).
ARs and AEs
An independent safety monitor (Dr. Richard Abrams, Rush University Medical Center, Chicago, IL) accessed all data and knew the treatment assignment of each volunteer. Based on clinical experience with the use of LT3 (34–37), he conferenced with Dr. Dumitrescu after each study arm was completed and, in all cases, recommended the continuation of the studies.
Statistical methods
For the sample size calculation, we used summary statistics (mean, standard deviation [SD], and interquartile range) for Cmax and Tmax from a previous study for LT3 (16). Based on preclinical data, we expect PZL-derived T3 to exhibit a decrease of 30% in Cmax (mean = 242) and an increase in Tmax of ∼6 hours (mean = 8.5). Assuming an SD = 100 for Cmax, we found that a sample of 12 individuals can detect a 30% decrease or higher in the mean Cmax with a power of 90%. We further found that this sample of 12 individuals can detect an increase of 1 hour or more in the mean Tmax with a power of 90%. Data analysis was conducted using statistical software R, and mixed-effects regression models were fit using the R package lme4 (38).
Results
Thirteen individuals were screened and 12 ultimately consented and were enrolled in the trial (Table 1). They were 31.6 ± 9.9 years old; four were women. Eleven individuals completed the trial as planned, one individual missed a study arm and was excluded. The baseline characteristics of this individual were indistinguishable from the rest of the group.
Table 1.
Demographics of All Enrolled Volunteers
| ID no. | Sex | Age, years |
|---|---|---|
| A | F | 20–24 |
| B | M | 20–24 |
| C | M | 20–24 |
| D | M | 25–29 |
| E | M | 35–39 |
| F | F | 20–24 |
| G | M | 35–39 |
| H | M | 40–44 |
| I | F | 30–34 |
| J | F | 45–49 |
| K | M | 20–24 |
| L | M | 30–34 |
Age ranges are provided to protect patient privacy.
T3 PK
Descriptive statistics and two-way analysis of variance
In the LT3-arm, T3 serum levels increased from a baseline of ∼110 ng/dL to a Cmax of ∼300 ng/dL between 2 and 3 hours (2.7 ± 0.53) after dose delivery (Table 2; Fig. 2). T3 levels decreased from ∼300 to 150 ng/dL by 12 hours, and then much less (dropping to 130 ng/dL) for the next 12 hours (Table 2; Fig. 2). It took ∼8 hours for the serum T3 levels to decrease to ½ of Cmax (Table 2; Fig. 2). These two distinct phases of elimination are compatible with a two-compartment model for this experimental system (16,18), but we cannot exclude that the drop in serum TSH might have decreased thyroidal T3 secretion as well. T3 levels returned to baseline between 36 and 46 hours (Table 2). Two individuals exhibited atypical T3 profiles (Cmax delayed by 6–24 hours) for no identifiable reasons and were excluded from further analyses.
Table 2.
Serum Triiodothyronine in Volunteers During 48 Hours After Taking a Capsule of Poly-Zinc-Liothyronine, L-triiodothyronine, or Placebo
| Time (hours) | PZL (ng/dL) |
LT3 (ng/dL) |
PB (ng/dL) |
n | |||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | ||
| −0.5 | 109 | 5.1 | 115 | 7.1 | 114 | 4.6 | 9 |
| −0.25 | 108 | 5.2 | 110 | 6.2 | 111 | 4.9 | 9 |
| 1 | 122 | 11.6 | 140 | 9.5 | 108 | 4.5 | 9 |
| 2 | 208 | 40.2 | 298 | 37.5** | 105 | 3.8 | 9 |
| 3 | 230 | 35.9 | 304 | 29.4* | 102 | 3.4 | 9 |
| 4 | 221 | 26.3 | 265 | 25.3 | 101 | 3.5 | 9 |
| 6 | 221 | 22.0 | 205 | 16.1 | 100 | 4.4 | 9 |
| 8 | 203 | 18.9 | 178 | 13.1 | 101 | 5.5 | 9 |
| 12 | 165 | 14.6 | 141 | 8.5 | 100 | 5.6 | 9 |
| 18 | 147 | 12.1 | 129 | 7.3 | 102 | 5.2 | 9 |
| 24 | 143 | 12.4 | 122 | 5.2 | 99 | 4.6 | 9 |
| 36 | 110 | 7.0 | 102 | 6.0 | 97 | 6.5 | 9 |
| 46 | 102 | 6.3 | 100 | 4.8 | 97 | 6.2 | 9 |
p < 0.05 versus PZL; **p < 0.01 versus PZL by two-way ANOVA; normal reference range is 80–200 ng/dL.
ANOVA, analysis of variance; LT3, L-triiodothyronine; PZL, poly-zinc-liothyronine; SEM, standard error of mean.
FIG. 2.
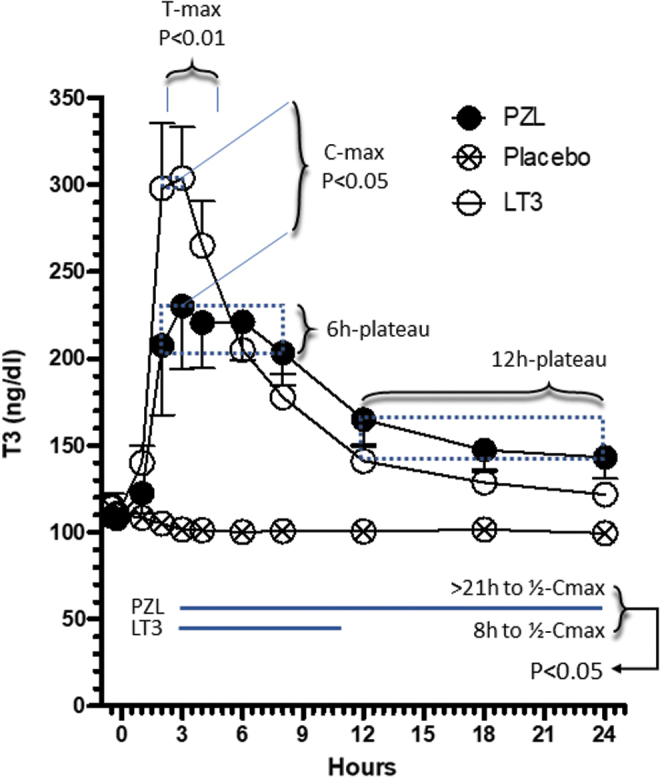
T3 serum kinetics in volunteers after taking a capsule of PZL, LT3, or PB. Serum T3 values during the first 24 hours after treatment; T3 levels are stable between 2 and 3 hours after LT3 (p > 0.05) but after PZL, T3 levels are stable for a longer time, between 2 and 8 hours (p > 0.05); T3 concentrations at 2 and 3 hours are significantly different (two-way ANOVA followed by Bonferroni post-test). ANOVA, analysis of variance; LT3, L-triiodothyronine; PB, placebo; PZL, poly-zinc-liothyronine; T3, triiodothyronine. Color images are available online.
In the PZL arm, T3 serum levels increased from a baseline of ∼110 ng/dL to a Cmax of ∼220 ng/dL between 2 and 8 hours (4.7 ± 2.3) after dose delivery (Table 2; Fig. 2). Tmax was delayed in the PZL arm by 1 hour (Table 2; Fig. 2). T3 levels decreased slowly from ∼220 to 170 ng/dL up until 12 hours and then slowed down further, dropping to 150 ng/dL for the next 12 hours (Table 2; Fig. 2). By 24 hours serum T3 levels still exceeded ½ of Cmax. T3 levels returned to baseline by 46 hours (Table 2).
In the PB arm, T3 levels ranged from a baseline of ∼115 to 100 ng/dL by end of the study (Table 2; Fig. 2).
Mixed-effects models
We next fitted mixed-effects models for T3 levels under each time point considering patients as random effects to incorporate the repeated measures into the model. This model is suitable for individually randomized trials with longitudinal continuous outcomes. The analysis confirms that the T3 levels in the LT3 arm have a steeper slope and reach higher levels during the first 3 hours followed by a faster decrease compared with the PZL arm. In addition, starting at the 12 hours time-point, T3 levels were modeled at higher levels in the PZL arm (Table 3).
Table 3.
Mathematical Modeling of L-triiodothyronine- and Poly-Zinc-Liothyronine-Derived Triiodothyronine
| Time (hours) | Mixed effects |
PW mixed effects |
AUC |
|||||
|---|---|---|---|---|---|---|---|---|
| LT3 | PZL | p | LT3-PZL | p | LT3 | PZL | p | |
| 1 | 140 | 122 | 0.15 | |||||
| 2 | 298 | 208 | 0.05 | |||||
| 3 | 304 | 230 | 0.07 | |||||
| 4 | 265 | 221 | 0.12 | |||||
| 6 | 205 | 221 | 0.33 | |||||
| 8 | 178 | 203 | 0.10 | |||||
| 12 | 141 | 165 | 0.04 | |||||
| 18 | 129 | 147 | 0.06 | |||||
| 24 | 122 | 143 | 0.02 | |||||
| 36 | 102 | 110 | 0.08 | |||||
| 46 | 100 | 102 | 0.50 | |||||
| 1–2 | 73 | 0.12 | ||||||
| 2–3 | −17 | 0.55 | ||||||
| 3–4 | −55 | 0.04 | ||||||
| 4–12 | −6.35 | 0.06 | ||||||
| 12–46 | 0.9 | 0.03 | ||||||
| 0–3 | 704 | 554 | 0.14 | |||||
| 3–12 | 1775 | 1828 | 0.66 | |||||
| 12–24 | 1559 | 1808 | 0.05 | |||||
| 24–48 | 2349 | 2574 | 0.05 | |||||
| 0–48 | 6387 | 6765 | 0.24 | |||||
Data shown for T3 levels in ng/dL; AUC is ng·hour/dL; LT3-PZL is the difference between the fixed effect of time under LT3 and PZL; for the PW mixed-effects model the effect of baseline response was 0.6 (SE = 0.07); the linear fixed effect of time under the PZL between 1 and 2 hours was 85.1 (SE = 26.4), between 2 and 3 hours was 22.6 (SE = 14.1), between 3 and 4 hours was −0.59 (SE = 20.1), between 4 and 12 hours was −8.1 (SE = 2.3), and between 12 and 46 hours was −2.0 (SE = 0.4).
AUC, area under the curve; PB, placebo; PW, piecewise; SE, standard error; T3, triiodothyronine.
To study the integrated T3 levels, we calculated the area under each curve (AUC) for multiple time segments. Between 0 and 3 hours, the integrated T3 levels are slightly higher in the LT3 arm, whereas from 12 to 24 hours and 24–48 hours they are higher in the PZL arm (Table 3). Note that the overall 0–48 hours AUC is similar for both LT3 and PZL (Table 3).
We next used the piecewise mixed-effects model with change points at 1, 2, 3, 4, and 12 hours, with average levels at −30 and −15 minutes as a covariate and with random intercept and random slopes across the patients within each group. This model is useful when analyzing longitudinal data sets to model segmented change over time. It predicted a clear difference between the LT3 and PZL curves between 3 and 46 hours (Table 3).
T3 PD
TSH and fT4 levels
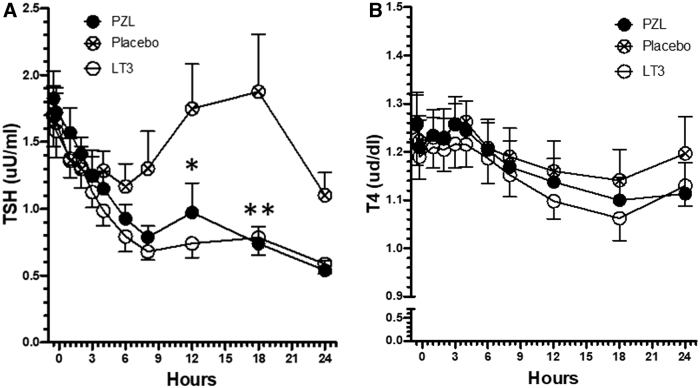
In the PB arm, serum TSH and fT4 exhibited a reciprocal variation that reflects their normal circadian rhythmicity (Fig. 3A, B). Serum TSH dropped by ∼30% by 6 hours into the study (3:00 PM) only to return to baseline values by 18 hours (3:00 AM), which was then followed by another ∼30% drop by 24 hours (9:00 AM; Fig. 3A). At the same time, serum fT4 levels varied much less, ∼10%, with reciprocal peaks and valleys (Fig. 3B). The elevation in serum T3 levels observed in the LT3 arm and PZL arm provoked a similar reduction in serum TSH levels in both groups that reached ∼40% by 9 hours, and ∼70% by 24 hours, with disruption of the circadian rhythmicity (Fig. 3A). No significant changes were observed in fT4 serum levels (Fig. 3B).
FIG. 3.
T4 and TSH serum kinetics in volunteers after taking a capsule of PZL, LT3, or PB. (A) Serum TSH values; *p < 0.05 for both PZL and LT3 versus PB; **p < 0.001 for both PZL and LT3 versus PB; all statistics by two-way ANOVA followed by Bonferroni post-test; (B) serum T4 levels. TSH, thyrotropin; T4, thyroxine.
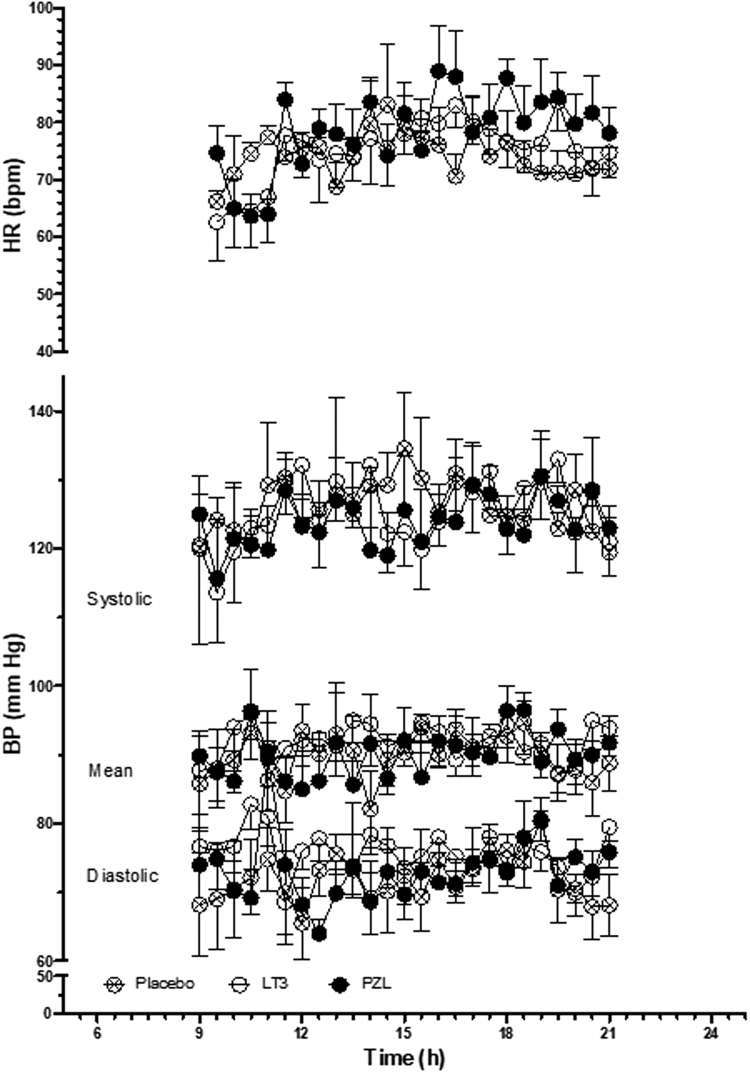
Heart rate
Heart rate tended to increase during the day to a maximum around 4:00 PM, and then slowly return to baseline (Fig. 4), with no differences observed among treatment arms (Table 4).
FIG. 4.
Heart rate, systolic, diastolic, and mean blood pressures in volunteers after taking a capsule of PZL, LT3, or PB.
Table 4.
Heart Rate in Volunteers During the First 12 Hours After Taking a Capsule of Poly-Zinc-Liothyronine, L-triiodothyronine, or Placebo
| Heart rate (bpm) |
|||
|---|---|---|---|
| PZL | LT3 | PB | |
| Number of values | 24 | 24 | 24 |
| Minimum | 64 | 63 | 64 |
| 25% percentile | 75 | 74 | 75 |
| Median | 79 | 76 | 79 |
| 75% percentile | 84 | 80 | 84 |
| Maximum | 89 | 84 | 89 |
| Mean | 79 | 75 | 79 |
| SD | 7.0 | 5.6 | 7.0 |
| SE | 1.4 | 1.1 | 1.4 |
| Lower CI of mean | 76 | 73 | 76 |
| Upper CI of mean | 81 | 78 | 81 |
No statistically significant differences were observed by ANOVA.
BP, blood pressure; CI, 95% confidence interval; SD, standard deviation.
Blood pressure
Systolic and diastolic blood pressures, as well as mean arterial pressure, remained stable throughout the day (Fig. 4), with no differences observed among treatment arms (Table 5).
Table 5.
Systolic, Diastolic, and Mean Blood Pressure in Volunteers During the First 12 Hours After Taking a Capsule of Poly-Zinc-Liothyronine, L-triiodothyronine, or Placebo
| Systolic BP (mmHg) |
Diastolic BP (mmHg) |
Mean BP |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PZL | LT3 | PB | PZL | LT3 | PB | PZL | LT3 | PB | |
| Number of values | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Minimum | 115.7 | 113.6 | 119.5 | 64.00 | 65.50 | 68.60 | 85.00 | 82.14 | 86.33 |
| 25% percentile | 121.3 | 122.3 | 123.1 | 70.00 | 69.27 | 73.47 | 86.71 | 88.13 | 89.89 |
| Median | 123.5 | 124.0 | 125.3 | 73.00 | 70.80 | 76.00 | 90.00 | 90.14 | 91.60 |
| 75% percentile | 127.0 | 129.6 | 129.3 | 74.72 | 74.55 | 77.92 | 91.90 | 92.86 | 94.17 |
| Maximum | 130.6 | 133.0 | 134.6 | 89.50 | 80.57 | 82.80 | 96.50 | 94.63 | 96.00 |
| Mean | 123.9 | 125.6 | 126.2 | 73.18 | 71.83 | 75.77 | 90.09 | 90.01 | 91.66 |
| SD | 3.659 | 4.919 | 3.886 | 4.786 | 3.346 | 3.180 | 3.372 | 3.163 | 2.746 |
| SE | 0.732 | 0.984 | 0.777 | 0.957 | 0.670 | 0.636 | 0.675 | 0.633 | 0.549 |
| Lower CI of mean | 122.4 | 123.5 | 124.6 | 71.20 | 70.45 | 74.46 | 88.70 | 88.70 | 90.52 |
| Upper CI of mean | 125.4 | 127.6 | 127.8 | 75.15 | 73.21 | 77.08 | 91.49 | 91.31 | 92.79 |
No statistically significant differences were observed by ANOVA.
ARs or AEs
There were no deaths, no AR, or serious AE. All AE were mild and resolved without complications; none were definitively linked to LT3 or PZL treatment, as they were evenly distributed among arms (seven AE per arm). In the PB arm: anxiety, tiredness, faint episode, dizziness, back pain, knee pain, neck stiffness; in the LT3 arm: cold upper extremity, headache, blurry vision, nausea, nosebleed, low-grade fever, dizziness; and in the PZL arm: dizziness, tiredness, low-grade fever, loose stools, sluggishness, headache (2 × ), sore throat. One woman had a delayed period during the trial with subsequent periods being on time, and another woman reported a heavier period in the month after the completion of the trial. No pregnancy occurred for any of the volunteers for the duration of the trial or during the 1 month afterward.
All CBCs, CMPs, and ECGs obtained before, during, and 1 week after each admission arm were unremarkable (exceptions hereunder). One volunteer had positive thyroid autoantibodies in the presence of normal TFTs. One volunteer had slight elevation in total bilirubin of 1.2 mg/dL (0.1–1.0) at screening and ranged from 0.9 to 2.5 mg/dL during the trial, with the higher level of 2.5 obtained during the PB arm. Three women had low normal red blood cell (RBC) at screening, 4.49 × 106/μL, 4.82 × 106/μL, and 4.84 × 106/μL (normal: 4.47–5.91 × 106) and developed lower RBC during the trial, 3.98 × 106/μL, 4.09 × 106/μL, and 4.38 × 106/μL, respectively. A fourth woman and one man had low RBC at screening 4.23 × 106/μL and 4.41 × 106/μL, respectively, both reaching a lower level of 3.97 × 106/μL during the trial. Although the four women maintained a hemoglobin level in the normal range, the man with low RBC reached a lower hemoglobin level of 12.6 g/dL (normal range >13.5 g/dL).
Exploratory analysis of the sleep patterns
For all sleep outcomes, mean data were calculated for each participant from all days of collection, with a maximum of six contributing days within an arm. Ten participants completed actigraphy during the PZL arm, seven had scorable data for all 6 days, two had 5 days, and one had 4 days contribution to the calculated means. Nine individuals completed actigraphy and had scorable data over all 6 days during the LT3 arm. For the PB arm, 10 individuals completed actigraphy of which 9 had 6 days of data and 1 had 3 days. No differences were observed in mean sleep outcomes including sleep onset, sleep offset, time in bed, sleep efficiency, and fragmentation among the groups (Table 6).
Table 6.
Sleep Parameters in Volunteers During the First Night After Taking a Capsule of Poly-Zinc-Liothyronine, L-triiodothyronine, or Placebo
| PZL | LT3 | PB | |
|---|---|---|---|
| Bedtime (minutes from midnight) | 11 ± 72 | −2.9 ± 57 | 12 ± 62 |
| Wake time (minutes from midnight) | 503 ± 73 | 509 ± 47 | 520 ± 71 |
| Time in bed (minutes) | 492 ± 37 | 512 ± 49 | 509 ± 40 |
| Assumed sleep (minutes) | 436 ± 46 | 442 ± 48 | 448 ± 32 |
| Actual sleep (minutes) | 394 ± 48 | 401 ± 44 | 408 ± 30 |
| Percent sleep (%) | 90 ± 3.1 | 91 ± 2.5 | 91 ± 1.9 |
| WASO (minutes) | 41 ± 11 | 40 ± 12 | 39 ± 10 |
| Sleep efficiency (%) | 80 ± 5.9 | 78 ± 4.2 | 80 ± 4.0 |
| Sleep fragmentation (%+%) | 17 ± 5.6 | 19 ± 7.5 | 17 ± 5.2 |
Numbers are the mean ± SD; for the time data, those are in minutes from midnight.
Bedtime, the beginning of the rest interval; waketime, the end of the rest interval; time in bed, total minutes from bedtime to waketime; actual sleep, the total number of epochs within the entire rest interval scored as sleep multiplied by the epoch length in minutes, percent sleep is the percentage of scored total sleep over the sleep interval multiplied by 100; WASO, the total number of epochs scored as wake within the sleep interval multiplied by the epoch length in minutes; sleep efficiency, the percentage of scored total sleep over the time in bed thus including time in bed before initial sleep onset and final awakening; sleep fragmentation, the sum of the percent mobile and percent immobile bouts as defined by actiware software for the entire rest interval.
WASO, wake after sleep onset.
For the analysis of the vigor, mood, affect, sleepiness, hunger, and appetite questionnaires, all ratings taken from 13:30 on day 1 to 9:00 on day 3 were collapsed to derive one mean value for each individual during each of the three laboratory sessions. Repeated-measures analysis of variance (ANOVA) revealed there was no difference between treatment arms for VAS global vigor and mood (Supplementary Figs. S1 and S2), positive and negative affect (PANAS; Supplementary Figs. S3 and S4), hunger (Supplementary Figs. S5–S10), appetite (Supplementary Figs. S11–S15), or sleepiness (SSS; Supplementary Fig. S16).
Discussion
Professional medical associations have called for trials to be performed with slow-release formulations of LT3 (4,20), as past studies on LT3 were not comprehensive, and in most cases were not designed to detect long-term ARs. Multiple strategies have been developed to answer those calls (39), with different degrees of success, including the oral administration of T3 sulfate (40,41), and specially formulated T3 tablets designed to delay its absorption (42,43).
Here we report the results of a phase 1 clinical trial in which PZL was tested as a slow-release T3 formulation. Whereas the serum T3 profile observed after the LT3 dose was consistent with previous studies, the serum T3 profile after the PZL dose was substantially different. There was a 6-hour plateau in T3 levels around the Cmax that was ∼30% lower compared with LT3, which reflects the delayed but continued intestinal absorption of T3. At the same time, T3 levels in the circulation remained above ½ Cmax for more than 24 hours, which was in contrast with the swing in serum T3 levels observed after LT3 administration. Mixed-effect and piecewise mixed-effects mathematical models, as well as sectional AUCs, confirmed these findings. The 6-hour plateau combined with the relative stability of T3 levels during the first 24 hours, set up an optimistic scenario for achieving stable T3 levels during administrations of PZL every 24 hours.
Despite the differences in PK, the reduction in serum TSH was similar in both LT3 and PZL-treatment arms. This indicates that the prolonged and constrained elevation in serum T3 levels associated with PZL administration (at the dose of 50 μg T3) caused similar cumulative PD effects, despite the absence of the marked T3 peak in the circulation. Accordingly, the total AUC for serum T3 was similar for LT3 and PZL. In addition, no ARs were reported. Only mild AEs were observed, similar in frequency in all study arms.
This study shows that a copolymer of T3 and zinc possesses the necessary properties to achieve a much improved T3 PK. This was a proof-of-principle study required by the FDA regulatory process. It is conceivable that more consistent delivery of T3 could be achieved in the final steps of the drug product development, which includes refinement of the formulation with appropriate excipients and delivery method (i.e., coated tablets instead of enteric capsules). The successful development of PZL adds to the arsenal of new strategies and molecules that are being developed to provide stable replacement levels of T3 for patients that suffer from hypothyroidism.
Supplementary Material
Acknowledgments
The authors thank the director of the CRC at the University of Chicago Medical Center, Dr. Arlene Chapman, clinical research nurses Kathy Reilly, Imani Wilson, Sally Pirowski, Leeon Jones and bionutritional research manager Jennifer Kilkus for their dedicated help with the execution of the trial. The authors also thank the Kovler Diabetes Center executive director Peggy Hasenauer, clinical research coordinators Cristy Miles, Gail Gannon, Colleen Bender, Triniece Pearson and Rabia Ali, Melanie Norstrom (IRB, Department of Medicine), and pharmacist Judy Pi (Investigational Drug Service Pharmacy Manager) for coordination of different aspects of the trial. The authors thank Dr. Richard Abrams from Rush University Medical Center for his valuable oversight as independent safety monitor.
Consent
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' Contributions
A.M.D. directed all studies; interpreted data and prepared article; E.C.H. coordinated screening and recruitment, performed all sleep studies, interpreted the data and edited article; M.A., O.D., and M.E. conducted clinical trial; M.G., conducted statistical analyses; A.C.B. created the clinical protocol, interpreted data, and prepared article.
Author Disclosure Statement
A.C.B. is a consultant for Synthonics, Allergan, Abbvie and BLA Technology. The other authors have nothing to disclose.
Funding Information
The National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health provided funding for the clinical trial under Award Number R44DK116396.
Supplementary Material
References
- 1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. 2017. Hypothyroidism. Lancet 390:1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Idrees T, Palmer S, Maciel RMB, Bianco AC 2020 Liothyronine and desiccated thyroid extract in the treatment of hypothyroidism. Thyroid 30:1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAninch EA, Bianco AC. 2016. The history and future of treatment of hypothyroidism. Ann Intern Med 164:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. . 2014. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 24:1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89. [DOI] [PubMed] [Google Scholar]
- 6. Stock JM, Surks MI, Oppenheimer JH. 1974. Replacement dosage of L-thyroxine in hypothyroidism. A re-evaluation. N Engl J Med 290:529–533. [DOI] [PubMed] [Google Scholar]
- 7. Peterson SJ, McAninch EA, Bianco AC. 2016. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab 101:4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. 2011. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 6:e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. 2002. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol 57:577–585. [DOI] [PubMed] [Google Scholar]
- 10. Wekking EM, Appelhof BC, Fliers E, Schene AH, Huyser J, Tijssen JG, et al. . 2005. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol 153:747–753. [DOI] [PubMed] [Google Scholar]
- 11. Roberts ND 1996 British Thyroid Foundation Newsletter.
- 12. Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. 2016. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid 26:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAninch EA, Rajan KB, Miller CH, Bianco AC. 2018. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab 103:4533–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Idrees T, Prieto WH, Casula S, Ajith A, Ettelson M, Andreotti Narchi FA, et al. . 2021. Use of statins among patients taking levothyroxine: an observational drug utilization study across sites. J Endocr Soc 5:bvab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G, et al. . 2015. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest 125:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonklaas J, Burman KD, Wang H, Latham KR. 2015. Single-dose T3 administration: kinetics and effects on biochemical and physiological parameters. Ther Drug Monit 37:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. 2007. Twenty-four hour hormone profiles of TSH, Free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes 115:261–267. [DOI] [PubMed] [Google Scholar]
- 18. Van Tassell B, Wohlford GFt, Linderman JD, Smith S, Yavuz S, Pucino F, et al. . 2019. Pharmacokinetics of L-triiodothyronine in patients undergoing thyroid hormone therapy withdrawal. Thyroid 29:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leese GP, Soto-Pedre E, Donnelly LA. 2016. Liothyronine use in a 17 year observational population-based study—the tears study. Clin Endocrinol 85:918–925. [DOI] [PubMed] [Google Scholar]
- 20. Jonklaas J, Bianco AC, Cappola AR, Celi FS, Fliers E, Heuer H, et al. . 2021. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid 31:156–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Da Conceicao RR, Fernandes GW, Fonseca TL, Bocco B, Bianco AC. 2018. Metal coordinated poly-zinc-liothyronine provides stable circulating triiodothyronine levels in hypothyroid rats. Thyroid 28:1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li DQ, Zhou J, Liu X. 2007. The one-dimensional chain polymer of aqua(tyrosinato)zinc(II). Acta Crystallogr C 63(Pt 8):m371–m373. [DOI] [PubMed] [Google Scholar]
- 23. Price JD, Piccariello T, Palmer S. 2014. Metal-coordinated pharmaceuticals. Drug Dev Deliv 14:34–39. [Google Scholar]
- 24. Smart JD 2005. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev 57:1556–1568. [DOI] [PubMed] [Google Scholar]
- 25. Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. 1997. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 85:207–216. [DOI] [PubMed] [Google Scholar]
- 26. Knutson KL, Ryden AM, Mander BA, Van Cauter E. 2006. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med 166:1768–1774. [DOI] [PubMed] [Google Scholar]
- 27. Roepke SE, Duffy JF. 2010. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat Sci Sleep 2010:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monk TH 1989. A Visual Analogue Scale technique to measure global vigor and affect. Psychiatry Res 27:89–99. [DOI] [PubMed] [Google Scholar]
- 29. Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 30. Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, et al. . 2000. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 84:405–415. [DOI] [PubMed] [Google Scholar]
- 31. Fernstrom MH, Krowinski RL, Kupfer DJ. 1987. Appetite and food preference in depression: effects of imipramine treatment. Biol Psychiatry 22:529–539. [DOI] [PubMed] [Google Scholar]
- 32. Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, et al. . 2016. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 39:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. 1973. Quantification of sleepiness: a new approach. Psychophysiology 10:431–436. [DOI] [PubMed] [Google Scholar]
- 34. Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, et al. . 2011. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab 96:3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Celi FS, Zemskova M, Linderman JD, Babar NI, Skarulis MC, Csako G, et al. . 2010. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol 72:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamai H, Fujino R, Shizume K, Kuma K, Suematsu H. 1975. [Changes in responsivity to TRH test and T3-suppression test after surgical treatment of hyperthyroidism (author's transl)]. Nihon Naibunpi Gakkai Zasshi 51:985–996. [DOI] [PubMed] [Google Scholar]
- 37. Stamp TC, Doar JW, Wynn V. 1969. Observations on some effects of L-triiodothyronine on carbohydrate and lipid metabolism in man. J Clin Pathol 22:132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bates D, Maechler M, Bolker B, Walker W. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- 39. Idrees T, Price JD, Piccariello T, Bianco AC. 2019. Sustained release T3 therapy: animal models and translational applications. Front Endocrinol (Lausanne) 10:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santini F, Giannetti M, Ricco I, Querci G, Saponati G, Bokor D, et al. . 2014. Steady state serum T3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3'-triiodothyronine sulfate (T3S). Endocr Pract 20:680–689. [DOI] [PubMed] [Google Scholar]
- 41. Santini F, Ceccarini G, Pelosini C, Giannetti M, Ricco I, Querci G, et al. . 2019. Treatment of hypothyroid patients with L-thyroxine (L-T4) plus triiodothyronine sulfate (T3S). A phase II, open-label, single center, parallel groups study on therapeutic efficacy and tolerability. Front Endocrinol (Lausanne) 10:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jonklaas J, Burman KD. 2016. Daily administration of short-acting liothyronine is associated with significant triiodothyronine excursions and fails to alter thyroid-responsive parameters. Thyroid 26:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hennemann G, Docter R, Visser TJ, Postema PT, Krenning EP. 2004. Thyroxine plus low-dose, slow-release triiodothyronine replacement in hypothyroidism: proof of principle. Thyroid 14:271–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.