Abstract
Introduction: We analyzed the association of age with ventilation practice and outcomes in critically ill COVID–19 patients requiring invasive ventilation.
Methods: Posthoc analysis of the PRoVENT–COVID study, an observational study performed in 22 ICUs in the first 3 months of the national outbreak in the Netherlands. The coprimary endpoint was a set of ventilator parameters, including tidal volume normalized for predicted bodyweight, positive end–expiratory pressure, driving pressure, and respiratory system compliance in the first 4 days of invasive ventilation. Secondary endpoints were other ventilation parameters, the use of rescue therapies, pulmonary and extrapulmonary complications in the first 28 days in the ICU, hospital– and ICU stay, and mortality.
Results: 1122 patients were divided into four groups based on age quartiles. No meaningful differences were found in ventilation parameters and in the use of rescue therapies for refractory hypoxemia in the first 4 days of invasive ventilation. Older patients received more often a tracheostomy, developed more frequently acute kidney injury and myocardial infarction, stayed longer in hospital and ICU, and had a higher mortality.
Conclusions: In this cohort of invasively ventilated critically ill COVID–19 patients, age had no effect on ventilator management. Higher age was associated with more complications, longer length of stay in ICU and hospital and a higher mortality.
Keywords: age, coronavirus disease 2019, COVID-19, critical care, invasive ventilation, mortality
INTRODUCTION
The coronavirus disease 2019 (COVID–19) pandemic has resulted in worldwide recurrent surges of patients in need for urgent and intense medical care [1], and as of early–November 2021 5 million patients have died from this new disease [2]. Many hospitalized COVID–19 patients need admission to an intensive care unit (ICU), most often for escalation of respiratory support that includes invasive ventilation [3].
Aging is associated with various changes in lung physiology [4]. Due to changes in the structure of the thoracic cage, aging is known to reduce chest wall compliance. However, lung compliance increases with age because of a decrease in elastic recoil. Second, aging is associated with so–called ‘senile emphysema’ [5]. Due to a decrease in the supporting structures of lung parenchyma, the risk for early closure of small airways increases which could result in air trapping. The increased incidence of comorbidities in elderly may also mandate a different ventilation approach. For example, the combination of a reduced respiratory system reserve and an increased incidence of pulmonary disease in elderly patients may require a higher FiO2, while the higher incidence of cardiovascular disease in the elderly may actually reduce the possibility of, for example, ventilation with higher pressures. Indeed, one small prospective cohort study showed that elderly patients with acute respiratory failure received ventilation with lower pressures compared to younger patients [6]. However, this was not confirmed by a more recently published study, showing no age dependent variations in ventilator settings in such patients [7].
Several risk factors for contracting severe COVID–19 have been identified and described. Elderly patients, but also patients with underlying cardiovascular or respiratory conditions are most vulnerable to develop a complicated SARS–CoV–2 infection [8–10], and are at a higher risk for mortality of this disease [11–13]. Aging itself, however, is linked to the development of comorbidities and functional disabilities. Indeed, patients aged > 65 years are three times more often diagnosed with multiple chronic diseases [14], including comorbidities like cancer, cardiovascular diseases, and diabetes mellitus. All these are well–known predictors for mortality [15–17]. Older age is also associated with immunological alterations and inflammation, which may also translate into a higher risk of dying from an infectious disease [16].
It is unknown whether age–related differences exist in ventilator settings in critically ill COVID–19 patients. It also remains uncertain to which extent the association of age with mortality in COVID–19 patients requiring invasive ventilation is mediated by the increased prevalence of comorbidities in elderly patients. In the context of these uncertainties, we assessed the database of a large national observational study [18, 19]. We hypothesized that age has an independent effect on ventilator management and has an association with outcome in critically ill invasively ventilated COVID–19 patients.
MATERIALS AND METHODS
Design, study sites, and participants
This is a posthoc analysis of a national multicenter observational study, named ‘Practice of VENTilation in COVID–19 patients’ (PRoVENT–COVID) [18]. This study included more than 40% of all critically ill COVID–19 patients admitted to a Dutch ICU in the first 3 months of the national outbreak. The study protocol was approved by the Institutional Review Board of the Amsterdam UMC, location AMC, Amsterdam, the Netherlands on 7 April 2020 (W20_157 # 20.171), and hence at the other 21 hospital that eventually participated in the study. The need for written informed consent was waived because of the observational nature. The study was registered at clinicaltrials.gov (study identifier NCT04346342).
Adult patients were eligible if admitted to the ICU of a participating hospital, and receiving invasive ventilation for respiratory failure related to COVID–19, confirmed by RT–PCR. For the current analysis, we excluded patients that were transferred to an ICU in a non–participating hospital within the first hour of invasive ventilation.
Data collection
Multiple in–person and virtual meetings were organized at the Amsterdam University Medical Centers, location ‘AMC’, to train data collectors, that were all doctors in training or medical residents. During these meetings, data entry instructions were given, the database structure was explained, and data entry was trained. Each data collector was supervised by an experienced researcher in the domain of critical care. If inaccuracies, outliers and errors were found after data review, queries were sent and resolved by local investigators. Patient characteristics, anthropometric data, medical history, and available severity scores as recorded in the electronic patient records, severity of acute respiratory distress syndrome (ARDS) according to the current Berlin definition for this syndrome [20], and the extent of lung involvement on chest computed tomography or chest radiographs was collected for all patients at baseline. Different disease severity scores, e.g., the Acute Physiology and Chronic Health Evaluation (APACHE) II or IV score, the Simplified Acute Physiology Score (SAPS) II and the Sequential Organ Failure Assessment (SOFA) score, were used in the participating hospitals. The disease severity score documented in each hospital was collected at baseline, i.e., in the first 24 hours in the ICU. Laboratory test results, hemodynamic parameters, kidney function, fluid balance, and use and dose of continuous sedation, muscle paralysis, and vasopressors were captured daily up to calendar day 4.
Ventilator settings and key ventilation variables and parameters, and the use of adjunctive rescue therapies for refractory hypoxemia, including alveolar recruitment maneuvers, prone positioning, use of neuromuscular blocking agents (NMBAs), and extracorporeal membrane oxygenation (ECMO) was collected at fixed time points 3 times per day (08:00, 16:00 and 24:00) up to calendar day 4 or until death or ICU discharge, if that occurred first. From these three measurement points, the daily mean was calculated for each respiratory variable.
Pulmonary and extrapulmonary events were recorded until ICU day 28, ICU discharge or date of death, whichever came first.
Patients’ location and life status were collected up to day 90.
Study endpoints
The coprimary endpoint of this current analysis was a set of 4 key ventilator settings and ventilation parameters: tidal volume normalized for predicted bodyweight (VT PBW), positive end–expiratory pressure (PEEP), driving pressure (ΔP), and respiratory system compliance (Crs) during the first 4 calendar days.
Secondary endpoints were other ventilation parameters and use of rescue therapies for hypoxemia, pulmonary and extrapulmonary complications, ICU and hospital discharge, the number of days alive and free from invasive ventilation at day 28, and mortality at ICU and hospital discharge and at day 28 and 90.
Definitions
Pulmonary and extrapulmonary events were defined as pneumothorax, tracheostomy, reintubation, acute kidney injury and need for renal replacement therapy, and thromboembolic events, including pulmonary embolism, deep venous thrombosis, ischemic stroke, myocardial infarction, and systemic arterial thrombosis.
VT per predicted bodyweight (PBW) was calculated as follows:
| [eq. 1a]; |
| [eq. 1b]; and |
| [eq. 2]. |
ΔP and mechanical power (MP) were calculated using the following equations:
| [eq. 3]; and |
| [eq. 4] |
Crs was calculated as follows:
| [eq. 5] |
Power calculation
We did not perform a formal power calculation––instead, the number of patients available in the database was used as the sample size.
Statistical analysis plan
Patients were categorized into 4 age groups using the age quartiles. The day of the start of ventilation was merged with the first full calendar and named ‘day 1’. The following days were named ‘day 2’ and ‘day 3’. No assumptions for missing data were made.
Categorical variables are presented as numbers and proportions, continuous variables are reported with median and interquartile ranges. Age groups were compared using the Kruskal–Wallis test for continuous variables and Fisher exact tests for categorical variables. If differences were found, a posthoc Dunn test was used for pairwise comparison.
Distribution plots were constructed to show the key ventilator parameters for the four age groups. Time-to-event outcomes are presented in Kaplan–Meier curves, and age groups are compared with the Log–rank test.
To adjust for the unequal distribution of effect modifiers between the 4 age groups, multivariable models were made for ICU and hospital mortality, and 28– and 90-day mortality. The following variables were considered for adjustment in these models: (i.) gender; (ii.) body mass index (BMI); (iii.) history of hypertension, heart failure, diabetes mellitus, chronic kidney disease, chronic obstructive pulmonary disease, active hematological or solid cancer; (iv.) use of angiotensin-converting enzyme inhibitors, use of angiotensin II receptor blockers, and use of vasopressor or inotropic medication; (v.) PaO2 to FiO2 ratio; and (vi.) mean arterial blood pressure, heart rate, plasma creatinine, fluid balance, and arterial pH. These baseline covariates were selected according to clinical relevance and as used in previous studies [18, 21].
All analyses were conducted in R, version 4.0.5. A P < 0.05 was considered statistically significant.
RESULTS
Participants
Patient flow is shown in Supplementary Figure 1. A total of 1340 patients in 22 ICUs were screened for eligibility; major reasons for exclusions were that patients had an alternate diagnosis or did not receive invasive ventilation. Of the remaining 1122 patients, the median age was 65 [57 to 72] years. Baseline demographics of the 4 age groups are presented in Table 1. Older patients were shorter, weighed less, had a lower BMI and were more often diagnosed with a medical history of arterial hypertension, heart failure, diabetes mellitus, or COPD. Home medication like angiotensin–converting enzyme inhibitors and blockers, beta–blockers, statins, and calcium channel blockers were more often used at home in the higher age groups. At the first day of invasive ventilation, older patients were more often in need of vasopressors and inotropic drugs, and older patients had a higher cumulative fluid balance and a lower urine output.
Table 1. Patient characteristics according to age category at baseline.
| Age 22 to 57 years (n = 287) | Age 58 to 65 years (n = 286) | Age 66 to 72 years (n = 283) | Age 73 to 85 years (n = 266) | P value | |
| Age, years | 52.0 [47.0 to 55.0] | 62.0 [60.0 to 64.0] | 69.0 [67.0 to 71.0] | 75.0 [74.0 to 77.0] | <0.001 |
| Male | 200 (69.7) | 217 (75.9) | 203 (71.7) | 197 (74.1) | 0.370 |
| Height, cm | 178.0 [170.0 to 185.0] | 178 [170.0 to 184.0] | 175.0 [170.0 to 180.0] | 174.0 [168.5 to 180.0] | <0.001 |
| Weight, kg | 90.0 [80.8 to 105.0] | 89.0 [78.2 to 98.0] | 85.0 [75.6 to 92.2] | 82.0 [75.0 to 90.0] | <0.001 |
| Body Mass Index, kg/m2 | 28.9 [26.2 to 32.7] | 27.7 [25.4 to 30.6] | 27.2 [24.8 to 29.7] | 27.0 [24.9 to 29.4] | <0.001 |
| Severity of illness* | |||||
| SAPS II, % (no) | 35.7 (99/277) | 34.3 (92/268) | 33.6 (91/271) | 30.8 (77/250) | |
| *Modified SAPS II | 24.0 [19.0 to 29.0] | 24.0 [19.0 to 31.0] | 24.5 [19.0 to 32.0] | 26.0 [20.0 to 34.0] | 0.361 |
| APACHE II, no (%) | 26.0 (72/277) | 25.4 (68/268) | 17.7 (48/271) | 22.4 (56/250) | |
| *Modified APACHE II | 12.0 [10.0 to 15.0] | 12.0 [9.0 to 15.0] | 15.0 [9.0 to 19.0] | 15.0 [10.0 to 20.0] | 0.026 |
| APACHE IV, no (%) | 45.5 (126/277) | 40.7 (109/268) | 41.7 (113/271) | 36.8 (92/250) | |
| *Modified APACHE IV | 44.0 [37.2 to 55.0] | 44.0 [35.0 to 56.5] | 49.0 [36.8 to 59.2] | 49.0 [34.8 to 62.0] | 0.469 |
| SOFA, no (%) | 53.4 (148/227) | 54.1 (145/268) | 46.5 (126/271) | 44.4 (111/250) | |
| SOFA | 7.0 [5.0 to 8.0] | 7.0 [6.0 to 10.0] | 7.0 [6.0 to 10.0] | 8.0 [7.0 to 12.5] | <0.001 |
| Comorbidities | |||||
| Arterial hypertension | 53 (18.5) | 105 (36.7) | 108 (38.2) | 114 (42.9) | <0.001 |
| Heart failure | 3 (1.0) | 10 (3.5) | 16 (5.7) | 20 (7.5) | <0.001 |
| Diabetes mellitus | 44 (15.3) | 62 (21.7) | 80 (28.3) | 64 (24.1) | 0.002 |
| Chronic kidney disease | 8 (2.8) | 14 (4.9) | 9 (3.2) | 16 (6.0) | 0.204 |
| Baseline creatinine | 71.0 [60.0 to 87.0] | 77.0 [64.0 to 98.0] | 78.0 [63.0 to 98.0] | 84.0 [66.8 to 111.2] | <0.001 |
| Liver cirrhosis | 2 (0.7) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0.329 |
| Chronic obstructive pulmonary disease | 8 (2.8) | 25 (8.7) | 34 (12.0) | 21 (7.9) | <0.001 |
| Active hematological neoplasia | 3 (1.0) | 5 (1.7) | 4 (1.4) | 4 (1.5) | 0.911 |
| Active solid neoplasia | 3 (1.0) | 7 (2.4) | 8 (2.8) | 10 (3.8) | 0.193 |
| Neuromuscular disease | 4 (1.4) | 0 (0.0) | 2 (0.7) | 2 (0.8) | 0.258 |
| Immunosuppression | 7 (2.4) | 8 (2.8) | 5 (1.8) | 4 (1.5) | 0.710 |
| Previous medication | |||||
| Systemic steroids | 6 (2.1) | 8 (2.8) | 10 (3.5) | 14 (5.3) | 0.216 |
| Inhalation steroids | 34 (11.8) | 37 (12.9) | 33 (11.7) | 21 (7.9) | 0.244 |
| Angiotensin-converting enzyme inhibitor | 25 (8.7) | 45 (15.7) | 62 (21.9) | 57 (21.4) | <0.001 |
| Angiotensin II receptor blocker | 18 (6.3) | 35 (12.2) | 30 (10.6) | 44 (16.5) | 0.002 |
| Beta-blockers | 28 (9.8) | 52 (18.2) | 63 (22.3) | 68 (25.6) | <0.001 |
| Insulin | 16 (5.6) | 22 (7.7) | 21 (7.4) | 19 (7.1) | 0.744 |
| Metformin | 29 (10.1) | 47 (16.4) | 52 (18.4) | 47 (17.7) | 0.020 |
| Statins | 35 (12.2) | 76 (26.6) | 110 (38.9) | 109 (41.0) | <0.001 |
| Calcium channel blockers | 29 (10.1) | 45 (15.7) | 59 (20.8) | 64 (24.1) | <0.001 |
| Transferred under invasive ventilation from another hospital | 59 (20.6) | 53 (18.5) | 48 (17.0) | 41 (15.4) | 0.436 |
| Days between admission and start of invasive ventilation | 0.0 [0.0 to 0.0] | 0.0 [0.0 to 0.0] | 0.0 [0.0 to 0.0] | 0.0 [0.0 to 0.0] | 0.508 |
| Use of non-invasive mechanical ventilation before intubation | 28/259 (10.8) | 14/256 (5.5) | 24/258 (9.3) | 19/236 (8.1) | 0.152 |
| Duration of non-invasive ventilation, hours | 7.0 [2.0 to 23.0] | 7.0 [3.5 to 19.0] | 8.0 [2.8 to 9.5] | 8.0 [1.0 to 17.0] | 1.000 |
| Chest CT-scan performed at baseline | 111/276 (40.2) | 93/270 (34.4) | 78/269 (29.0) | 81/257 (31.5) | 0.023 |
| Percentage lung parenchyma affected | 0.561 | ||||
| 0% | 7/111 (6.3) | 3/93 (3.2) | 3/78 (3.8) | 1/81 (1.2) | |
| 25% | 29/111 (26.1) | 27/93 (29.0) | 29/78 (37.2) | 31/81 (38.3) | |
| 50% | 38/111 (34.2) | 26/93 (28.0) | 21/78 (26.9) | 22/81 (27.2) | |
| 75% | 30/111 (27.0) | 33/93 (35.5) | 19/78 (24.4) | 22/81 (27.2) | |
| 100% | 7/111 (6.3) | 4/93 (4.3) | 6/78 (7.7) | 5/81 (6.2) | |
| Chest x-ray performed at baseline | 136/162 (84.0) | 152/176 (86.4) | 157/185 (84.9) | 157/176 (89.2) | 0.506 |
| Quadrants affected | 0.810 | ||||
| 1 | 13 (9.8) | 12 (7.8) | 8 (5.0) | 9 (5.8) | |
| 2 | 32 (24.1) | 37 (24.0) | 38 (23.8) | 32 (20.8) | |
| 3 | 34 (25.6) | 39 (25.3) | 45 (28.1) | 50 (32.5) | |
| 4 | 54 (40.6) | 66 (42.9) | 69 (43.1) | 63 (40.9) | |
| Laboratory tests | |||||
| pH | 7.4 [7.3 to 7.4] | 7.4 [7.3 to 7.4] | 7.4 [7.3 to 7.4] | 7.3 [7.3 to 7.4] | <0.001 |
| PaO2 | 10.7 [9.2 to 14.2] | 10.3 [8.8 to 12.6] | 10.9 [9.5 to 13.3] | 11.2 [9.7 to 13.3] | 0.008 |
| SaO2 | 95.0 [93.0 to 97.4] | 94.2 [92.0 to 96.8] | 95.0 [93.0 to 97.0] | 95.0 [93.0 to 97.0] | 0.030 |
| PaCO2 | 5.6 [4.9 to 6.5] | 5.9 [5.0 to 6.9] | 6.1 [5.3 to 7.1] | 5.9 [5.0 to 6.9] | 0.003 |
| Lactate | 1.1 [0.9 to 1.4] | 1.1 [0.9 to 1.4] | 1.2 [0.9 to 1.5] | 1.2 [1.0 to 1.6] | 0.002 |
| Worst PaO2/FiO2 ratio, mm Hg | 126.6 [94.7 to 164.5] | 117.9 [91.8 to 160.3] | 120.2 [96.1 to 157.3] | 126.2 [97.4 to 161.6] | 0.401 |
| Need for advanced support | |||||
| Continuous sedation | 277/287 (96.5) | 276/286 (96.5) | 267/277 (95.0) | 253/263 (95.1) | 0.691 |
| Need for vasopressor use | 198/287 (69.0) | 223/286 (78.0) | 225/281 (80.1) | 217/266 (81.6) | 0.002 |
| Need for inotropic use | 6/287 (2.1) | 6/286 (2.1) | 16/281 (5.7) | 17/266 (6.4) | 0.009 |
| Fluid balance, mL | 418.0 [-126.0 to 1206.0] | 513.0 [-26.3 to 1209.0] | 456.1 [-25.5 to 1252.8] | 780.0 [144.0 to 1557.0] | 0.001 |
| Urine output, mL | 875.0 [511.2 to 1377.5] | 657.0 [350.0 to 1120.0] | 720.0 [370.0 to 1165.0] | 505.0 [255.0 to 877.5] | <0.001 |
Data presented as median with interquartile range [25th to 75th quartile] or n (%). *Age component is removed from the APACHE and SAPS Score. *Total numbers are different because different scores were used in the participating hospitals.
SAPS, Simplified Acute Physiology Score; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CT, Computed Tomography.
Ventilation characteristics
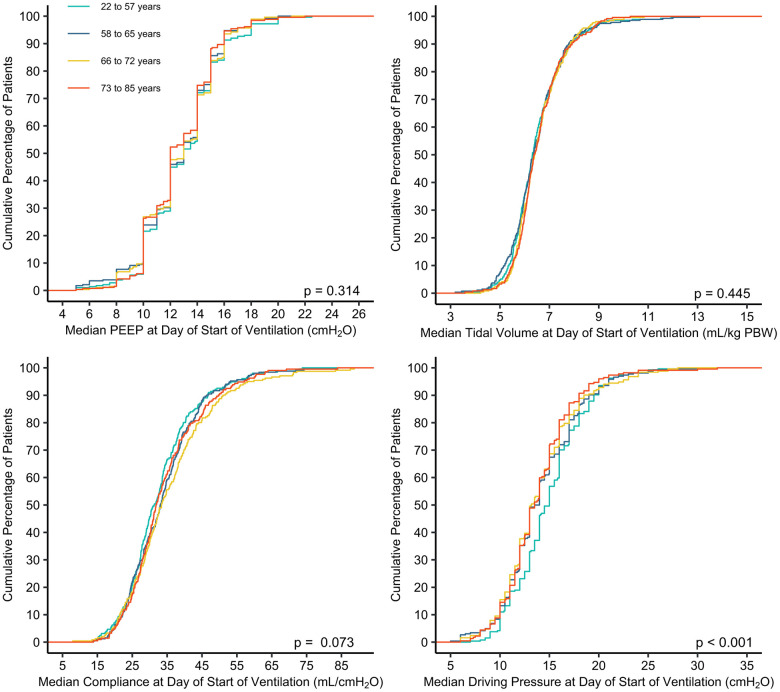
Key ventilator settings are shown in Table 2, Figure 1, and Supplementary Figures 2–5. On the first day of ventilation, median VT PBW, PEEP, ΔP and Crs were largely similar between the 4 age groups. Some differences reached statistical significance, but differences were too small to have a clinical meaning.
Table 2. Characteristics of mechanical ventilation and laboratory results in the first day of ventilation.
| Age 22 to 57 years (n = 287) | Age 58 to 65 years (n = 286) | Age 66 to 72 years (n = 283) | Age 73 to 85 years (n = 266) | P value | |
| Mode of mechanical ventilation | |||||
| Volume control | 32/271 (11.8) | 35/267 (13.1) | 33/267 (12.4) | 41/248 (16.5) | 0.398 |
| Pressure control | 163/271 (60.1) | 153/267 (57.3) | 149/267 (55.8) | 123/248 (49.6) | 0.103 |
| Pressure support | 12/271 (4.4) | 20/267 (7.5) | 13/267 (4.9) | 12/248 (4.8) | 0.380 |
| Synchronized Intermitted Mandatory Ventilation | 19/271 (7.0) | 12/267 (4.5) | 25/267 (9.4) | 22/248 (8.9) | 0.131 |
| Airway Pressure Release Ventilation | 9/271 (3.3) | 10/267 (3.7) | 10/267 (3.7) | 5/248 (2.0) | 0.652 |
| INTELLIVENT-Adaptive Support Ventilation | 11/271 (4.1) | 10/267 (3.7) | 12/267 (4.5) | 11/248 (4.4) | 0.971 |
| Other | 25/271 (9.2) | 27/267 (10.1) | 25/267 (9.4) | 34/248 (13.7) | 0.310 |
| Use of assisted ventilation | 76/287 (26.5) | 78/282 (27.7) | 88/283 (31.1) | 88/265 (33.2) | 0.285 |
| Tidal volume (n/N), mL/kg PBW* | (274/287) 6.4 [5.8 to 7.0] | (274/286) 6.4 [5.9 to 7.1] | (263/283) 6.5 [5.9 to 7.1] | (243/266) 6.5 [6.0 to 7.1] | 0.445 |
| PEEP, (n/N) cmH2O* | (287/287) 13.0 [11.0 to 15.0] | (286/286) 12.7 [11.0 to 14.6] | (279/283) 13.0 [10.7 to 14.8] | (262/266) 12.2 [10.8 to 14.2] | 0.314 |
| Driving pressure (n/N), cmH2O* | (264/287) 14.7 [12.5 to 17.0] | (265/286) 13.8 [11.7 to 16.3] | (252/283) 13.2 [11.3 to 15.7] | (227/266) 13.5 [11.6 to 15.7] | <0.001 |
| Compliance (n/N), mL/cmH2O* | (256/287) 32.4 [25.9 to 38.3] | (258/286) 33.8 [27.1 to 41.7] | (241/283) 34.7 [27.7 to 43.3] | (215/266) 32.6 [27.3 to 40.7] | 0.073 |
| Mechanical power (n/N), J/min* | (256/287) 19.2 [16.0 to 23.7] | (257/286) 19.3 [15.9 to 23.1] | (241/283) 17.9 [14.7 to 22.3] | (214/266) 17.2 [14.6 to 20.9] | <0.001 |
| Peak pressure (n/N), cmH2O* | (264/287) 27.7 [25.0 to 30.8] | (267/286) 26.7 [23.3 to 30.0] | (257/283) 26.0 [23.3 to 29.2] | (227/266) 26.2 [23.6 to 29.0] | <0.001 |
| Total respiratory rate (n/N), breaths per minute* | (287/287) 22.0 [20.0 to 24.3] | (286/286) 22.0 [19.5 to 24.5] | (282/283) 21.3 [19.3 to 24.0] | (258/266) 21.3 [19.1 to 23.7] | 0.053 |
| Minute ventilation (n/N), L/min* | (275/287) 9.8 [8.6 to 11.4] | (277/286) 10.0 [8.5 to 11.6] | (269/283) 9.6 [8.2 to 11.3] | (245/266) 9.3 [8.2 to 10.6] | 0.005 |
| Minute volume corrected (n/N), mL/kg/min PBW* | (274/287) 139.1 [121.9 to 158.3] | (274/286) 139.9 [124.8 to 162.9] | (263/283) 137.7 [123.7 to 159.6] | (243/266) 137.2 [122.8 to 155.0] | 0.782 |
| FiO2 (n/N)* | (286/287) 0.6 [0.5 to 0.7] | (286/286) 0.6 [0.5 to 0.7] | (281/283) 0.6 [0.5 to 0.7] | (258/266) 0.6 [0.5 to 0.7] | 0.283 |
| PaO2 (n/N), mmHg* | (284/287) 81.0 [71.5 to 99.3] | (286/286) 78.7 [71.3 to 93.4] | (280/283) 82.4 [72.7 to 95.4] | (264/266) 83.3 [75.0 to 96.0] | 0.018 |
| PaCO2 (n/N), mmHg* | (284/287) 42.9 [38.3 to 48.4] | (286/286) 44.6 [39.8 to 49.5] | (280/283) 46.1 [39.9 to 52.0] | (264/266) 45.0 [39.1 to 50.9] | 0.002 |
| EtCO2 (n/N), mmHg* | (264/287) 38.0 [33.8 to 43.8] | (257/286) 37.7 [33.3 to 42.8] | (261/283) 36.3 [31.9 to 42.0] | (231/266) 35.3 [31.6 to 39.9] | <0.001 |
Data presented as median with interquartile range [25th to 75th quartile] or n (%). *Mean of all values available at the first day of ventilation. Total numbers are different because of missing or unmeasured values. EtCO2, End-Tidal Carbon Dioxide; FiO2, inspired fraction of oxygen; ICU, Intensive Care.
Figure 1.
Cumulative frequency distribution of median PEEP, tidal volume, compliance and driving pressure at start day of invasive ventilation. Mean values were calculated from three or four measurements available on the first day of ventilation. The Kruskal-Wallis test was used to calculate p-values.
Mechanical power and peak pressure decreased from the younger to the older age groups at the first day of ventilation (Table 2). The difference in mechanical power and peak pressure disappeared in subsequent days (Supplementary Table 1). EtCO2 was lower but PaCO2 was higher in older age groups, and PaO2 was lower in the second age quartile (Table 2); only the difference in EtCO2 persisted in subsequent days (Supplementary Table 1) Use of adjunctive therapies for refractory hypoxemia was not affected by age, except for the use of NMBAs, which was less used with higher age (Table 3).
Table 3. Clinical outcome according to age group.
| Age 22 to 57 years (n = 287) | Age 58 to 65 years (n = 286) | Age 66 to 72 years (n = 283) | Age 73 to 85 years (n = 266) | P value | |
| 28-day mortality | 36/281 (12.8) | 59/279 (21.1) | 100/279 (35.8) | 123/263 (46.8) | <0.001 |
| 90-day mortality | 46/255 (18.0) | 72/251 (28.7) | 120/267 (44.9) | 145/242 (59.9) | <0.001 |
| In hospital mortality | 43/259 (16.6) | 71/256 (27.7) | 113/255 (44.3) | 140/252 (55.6) | <0.001 |
| ICU mortality | 42/277 (15.2) | 71/278 (25.5) | 110/274 (40.1) | 133/262 (50.8) | <0.001 |
| Length of hospital stay, days | 24.0 [17.0 to 33.0] | 26.0 [16.0 to 41.0] | 22.0 [14.0 to 39.0] | 21.5 [10.0 to 36.0] | 0.008 |
| Length of hospital stay in survivors, days | 25.0 [18.5 to 35.5] | 30.0 [20.0 to 46.5] | 32.5 [20.3 to 49.8] | 33.0 [25.8 to 52.0] | <0.001 |
| Length of ICU stay, days | 15.0 [10.0 to 23.0] | 17.0 [10.0 to 30.0] | 16.0 [8.3 to 26.0] | 14.0 [7.0 to 25.0] | 0.037 |
| Length of ICU stay in survivors, days | 15.0 [10.0 to 22.8] | 20.0 [12.0 to 31.0] | 18.0 [10.0 to 34.0] | 20.0 [13.0 to 38.0] | <0.001 |
| Ventilator-free days at day 28 | 13.0 [0.0 to 19.0] | 4.0 [0.0 to 17.0] | 0.0 [0.0 to 14.2] | 0.0 [0.0 to 9.7] | <0.001 |
| Duration of ventilation, days | 13.0 [9.0 to 21.0] | 15.0 [9.0 to 26.0] | 15.0 [8.0 to 24.0] | 13.0 [6.0 to 22.0] | 0.023 |
| Duration of ventilation in survivors, days* | 13.0 [8.0 to 21.2] | 17.0 [10.0 to 28.3] | 17.0 [10.0 to 31.0] | 19.0 [12.0 to 34.0] | <0.001 |
| Tracheostomy* | 35/283 (12.4) | 62/284 (21.8) | 48/280 (17.1) | 45/265 (17.0) | 0.029 |
| Reintubation* | 32/282 (11.3) | 42/284 (14.8) | 33/278 (11.9) | 33/264 (12.5) | 0.631 |
| Pneumothorax* | 2/283 (0.7) | 3/275 (1.1) | 2/267 (0.7) | 2/259 (0.8) | 0.970 |
| Thrombotic complications*& | 72/287 (25.1) | 95/286 (33.2) | 74/283 (26.1) | 78/266 (29.3) | 0.135 |
| Pulmonary embolism | 55/287 (19.2) | 75/286 (26.2) | 61/283 (21.6) | 58/266 (21.8) | 0.236 |
| Deep vein thrombosis | 17/287 (5.9) | 20/286 (7.0) | 9/283 (3.2) | 11/266 (4.1) | 0.156 |
| Ischemic stroke | 3/287 (1.0) | 10/286 (3.5) | 8/283 (2.8) | 10/266 (3.8) | 0.148 |
| Myocardial infarction | 2/287 (0.7) | 0/286 (0.0) | 7/283 (2.5) | 7/266 (2.6) | 0.007 |
| Systemic arterial thrombosis | 1/287 (0.3) | 1/286 (0.3) | 2/283 (0.7) | 0/266 (0.0) | 0.805 |
| Acute kidney injury* | 89/287 (31.0) | 140/285 (49.1) | 126/281 (44.8) | 141/265 (53.2) | <0.001 |
| Need for renal replacement* | 35/287 (12.2) | 62/286 (21.7) | 57/283 (20.1) | 51/266 (19.2) | 0.013 |
| Adjunctive therapies refractory hypoxemia** | 162/284 (57.0) | 174/282 (61.7) | 159/282 (56.4) | 152/265 (57.4) | 0.563 |
| Prone positioning | 156/284 (54.9) | 169/282 (59.9) | 155/282 (55.0) | 145/265 (54.7) | 0.533 |
| Alveolar recruitment maneuver | 15/242 (6.2) | 16/239 (6.7) | 18/239 (7.5) | 15/214 (7.0) | 0.946 |
| Other adjunctive therapies** | 156/287 (54.4) | 134/286 (46.9) | 143/283 (50.5) | 104/266 (39.1) | 0.003 |
| Neuromuscular blocking agents | 156/287 (54.4) | 133/286 (46.5) | 141/283 (49.8) | 104/266 (39.1) | 0.003 |
| Extracorporeal membrane oxygenation | 7/285 (2.5) | 2/282 (0.7) | 2/278 (0.7) | 1/262 (0.4) | 0.142 |
Data presented as median with interquartile range [25th to 75th quartile] or n (%). Totals are different due to missing data. *Assessed at day 28. **Assessed in the first four days of ventilation. &One could have more than one thrombotic complication. Total numbers are different because of missing or unmeasured values.
ICU, Intensive Care Unit.
Pulmonary and extrapulmonary events
Pulmonary and extrapulmonary complications are presented in Table 3 and Supplementary Table 2. Tracheostomy was more often used in the older compared to the youngest patients. No differences in other pulmonary events were found. There was no effect of age on thrombotic complications, only the incidence of myocardial infarction was higher in the older age groups compared to the younger age groups. Acute kidney injury (AKI) occurred less often in the youngest age group compared to the older age groups, as was the need for renal replacement therapy.
Outcomes
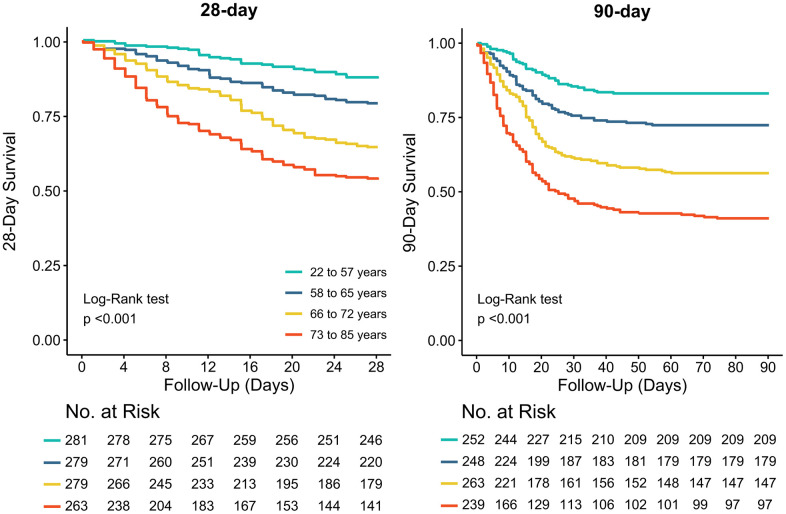
Patient outcomes are shown in Table 3, Supplementary Table 2 and Figure 2. In survivors, length of hospital and ICU stay increased while number of ventilator–free days decreased from the younger to the older age groups. Mortality rates increased from the lowest to the higher age group. After adjustment from effect modifiers, ICU– and hospital mortality, and 28– and 90–day were all higher in older patients (Supplementary Tables 3, 4).
Figure 2.
Kaplan-Meier curves for 28-day and 90-day mortality per age group. The Log-Rank test was used to calculate P values.
DISCUSSION
The results of this posthoc analysis of the PRoVENT–COVID study can be summarized as follows: (i.) there were no clinically meaningful differences in the key ventilator parameters between the 4 age groups; (ii.) on the first calendar day, mechanical power and peak pressure were lower in older patients but this effect disappeared in the succeeding days; (iii.) on the first four calendar days, EtCO2 was lower while PaCO2 was slightly higher in older patients; (iv) use of NMBAs was lower in older patients; (v) tracheostomy was more often used in older patients; (vi.) the incidence of AKI and the need for renal replacement therapy, and myocardial infarction was higher in older patients; (vii.) older patients stayed longer in the ICU and hospital; and (viii.) had higher mortality rates.
Our study has several strengths. The study included a large number of centers, both academic and non–academic, increasing the generalizability of the findings. Data were collected in a short time interval of 3 months, which minimizes the risk of changes in care over time. Data were collected by trained data collectors, which improved the quality of the data. Patients were followed until day 90, enabling for reporting on outcomes after stay in ICU. Of note, median age and other baseline characteristics are comparable to that in other studies [22, 23]. Also, in line with previous studies, the second and third age group had an evidently smaller range than the first and last age group, suggesting that middle–aged patients were the most prominent group admitted to the ICU.
Our findings suggest that ventilator management is not affected by age. Indeed, we found only minor, clinical meaningless, differences in key ventilator variables. The younger age groups had a higher BMI that could, at least in part, explain the higher median ΔP and Ppeak, and the higher mechanical power. Indeed, with a higher BMI higher thoracic pressures may be needed due to an increased stiffness of the chest wall [24]. Previous studies have shown higher EtCO2 values in older patients [25–27], but this was not seen in our cohort. Actually, the opposite relation between EtCO2 and age could be explained by the higher BMI in the younger age group, as an higher BMI may be associated with an increased production of carbon dioxide [28]. Of note, on the first day of mechanical ventilation, we did find a slightly higher PaCO2 but lower EtCO2 in older patients than in younger patients, but this difference disappeared in the following days. The age dependent reduction in body mass could also explain the lower use of NMBAs in older patients [29]. An association of higher age with lower use of NMBAs has been described before [30]. Other explanations for these differences include age–related differences in clearance of NMBAs, and maybe also the higher incidence of acute kidney injury (AKI) in older patients [29]. As AKI also affects clearance of opioids [31], the higher effective dose of opioids may have prevented use of NMBAs as well. Furthermore, physicians might be reluctance to use NMBAs in elderly patients because of the increased risk of prolonged immobility and thus ICU–acquired weakness [32].
Age is known to be a risk factor for complications like AKI, need for renal replacement therapy, and myocardial infarction [33–36]. Therefore, the increased incidence of these complications in older age groups was expected.
We found a strong association of age with mortality. This is, at least in part, in line with previous studies showing that age is a risk factor for mortality in invasively ventilated ICU patients in general [37–40], and in COVID–19 in particular [13, 41–43]. After adjusting for comorbidities and other effect modifiers, mortality rates remained significantly higher in the older patients. The 28–day mortality rate in our oldest age group was higher than that reported in a prospective study performed in elderly COVID–19 patients [44]. Interestingly, in that study it was shown that when patients were classified according to their frailty scale, mortality increased in vulnerable and frail patients. The level of frailty defines how vulnerable patients are for both physical and psychosocial factors. Frailty can be considered as a marker of biological age and, in addition to calendar age, can provide important prognostic information about clinical outcomes of ICU patients [44, 45]. Unfortunately, frailty was not, or incomplete reported in the medical records in the hospitals that participated in our study, but taken together the differences in mortality between our study and the previous study [44] suggest that patients in our cohort could have been frail more often.
In survivors, older patients stayed longer in the ICU and in the hospital, had a higher incidence of tracheostomy, and received ventilation for more days than younger patients. This may suggest that treatment discontinuation was not more common in elderly patients, but this could also be explained by the fact that older patients may have had already further disease progression or were in a higher need for supportive care. As data on treatment discontinuation were not collected in this analysis, this remains uncertain.
The findings of our study expand the current knowledge about the effects of age on ventilator management and outcomes in critically ill invasively ventilated COVID–19 patients. Lung–protective ventilation was well applied during the first COVID–19 outbreak, also in older patients. The higher mortality rates in older patients could help in decision–making about preventive measures. For example, these findings support guidelines to prioritize the elderly in vaccination programs. These insights may also further support a patient in deciding whether, and to what extent, ICU admission is still desirable.
Our analysis has several limitations. First, the question arises whether ‘door selection’ for ICU admission may has occurred. Particularly in the elderly, there is a possibility that ICU admission may no longer be considered beneficial if there is a relatively severe disease or premorbid functioning. Unfortunately, we could not collect data on ‘Do Not Resuscitate’ (DNR) codes or treatment discontinuation, e.g., withholding or withdrawal medical care in a reliable way. This cohort represents the first months of the pandemic in the Netherlands, during which an understandable emphasis was put on patient care rather than on reporting DNR codes in the patient records. However, since mortality is strongly influenced by the decision to discontinue treatment, this may have interfered with our findings [46]. Second, there is an intercountry difference in the willingness of patients to consider ICU admission. Compared to other countries, doctors as well as patients seem to be more reluctant to proceed with ICU admission when the situation worsens [47]. This could result in a selection bias and should be considered when extrapolating these results to other countries with a more liberal ICU admission policy. In fact, we expect the association of age with mortality to be even stronger in those countries. As mentioned above, we could also not collect data on the frailty, which is another important limitation. In addition, the PRoVENT–COVID trial was conducted in the first three months of the national outbreak in the Netherlands. Due to the introduction of e.g., dexamethasone and improved prophylaxis against venous thromboembolic events, and also the vaccination program, current ICU cohorts might be different.
CONCLUSIONS
In this cohort of critically ill invasively ventilated COVID–19 patients, there were no meaningful differences in ventilator management between groups based on age quartiles. The use of adjunctive therapies for refractory hypoxemia was not affected by age, except for use of NMBAs that decreased with higher age. Older patients developed complications more often, had a longer duration of ventilation and higher mortality rates.
Supplementary Material
ACKNOWLEDGMENTS
The PRoVENT-COVID investigators (see supplement).
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- ARDS
Acute Respiratory Distress Syndrome
- BMI
Body Mass Index
- EtCO2
End tidal Carbon dioxide
- COPD
Chronic Obstructive Pulmonary Disease
- COVID-19
coronavirus disease 2019
- Crs
Respiratory system compliance
- ECMO
Extracorporeal membrane oxygenation
- FiO2
Inspired Oxygen Fraction
- PaO2
Partial Pressure of Oxygen
- PEEP
Positive End-Expiratory Pressure
- PBW
Predicted Body Weight
- SAPS
The Simplified Acute Physiology Score
- SOFA
Sequential Organ Failure
- VT
Tidal Volume
- ΔP
Driving Pressure
Footnotes
AUTHOR CONTRIBUTIONS: LH, ASN, FP and MJS designed the study and wrote the protocol. LH and ASN analyzed the data. LH and MJS drafted the manuscript. All authors made a substantial contribution to data interpretation. All authors read and approved the manuscript.
CONFLICTS OF INTEREST: Ary Serpa Neto reports personal fees from Dräger, outside of the submitted work. Marcus Schultz reports personal fees from Hamilton and Xenios/Novalung, outside of the submitted work. The other authors declare no conflicts of interest.
FUNDING: PRoVENT–COVID was funded by Amsterdam UMC, location AMC, Amsterdam, The Netherlands.
REFERENCES
- 1.Medicine JHUa. COVID-19 Dashboard by the Center for Systems Science and Engineering. 2021. https://coronavirus.jhu.edu/map.html
- 2.WHO. World Health Organization Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/
- 3.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020; 324:782–93. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 4.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006; 1:253–60. 10.2147/ciia.2006.1.3.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992; 101:793–9. 10.1378/chest.101.3.793 [DOI] [PubMed] [Google Scholar]
- 6.Gee MH, Gottlieb JE, Albertine KH, Kubis JM, Peters SP, Fish JE. Physiology of aging related to outcome in the adult respiratory distress syndrome. J Appl Physiol (1985). 1990; 69:822–9. 10.1152/jappl.1990.69.3.822 [DOI] [PubMed] [Google Scholar]
- 7.Antonia Koutsoukou MK, Orfanos S, Rovina N, Dimitrakopoulou C, Kotanidou A, Koutsoukou A. ARDS in Aged Patients: Respiratory System Mechanics and Outcome. Health Sci J, 2017; 11:498 10.21767/1791-809X.1000498 [DOI] [Google Scholar]
- 8.Salzberger B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Hanses F. Epidemiologie von SARS-CoV-2-Infektion und COVID-19 [Epidemiology of SARS-CoV-2 infection and COVID-19]. Internist (Berl). 2020; 61:782–8. 10.1007/s00108-020-00834-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020; 94:91–5. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z, Li Y, Cheng L, Li W, Jia H, Ma X. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY). 2020; 12:12493–503. 10.18632/aging.103579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020; 368:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 13.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, et al. , and COVID-19 Lombardy ICU Network. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020; 180:1345–55. 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition--multimorbidity. JAMA. 2012; 307:2493–4. 10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirani V, Naganathan V, Blyth F, Le Couteur DG, Gnjidic D, Stanaway FF, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Multiple, but not traditional risk factors predict mortality in older people: the Concord Health and Ageing in Men Project. Age (Dordr). 2014; 36:9732. 10.1007/s11357-014-9732-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age (Dordr). 2013; 35:563–72. 10.1007/s11357-012-9381-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019; 394:1365–75. 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 18.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LD, Dongelmans DA, Hollmann MW, Horn J, Vlaar AP, Schultz MJ, Neto AS, Paulus F, and PRoVENT-COVID Collaborative Group. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021; 9:139–48. 10.1016/S2213-2600(20)30459-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boers NS, Botta M, Tsonas AM, Algera AG, Pillay J, Dongelmans DA, Horn J, Vlaar AP, Hollmann MW, Bos LD, Paulus F, Neto AS, Schultz MJ, and PRoVENT-COVID investigators†. PRactice of VENTilation in Patients with Novel Coronavirus Disease (PRoVENT-COVID): rationale and protocol for a national multicenter observational study in The Netherlands. Ann Transl Med. 2020; 8:1251. 10.21037/atm-20-5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, and ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 21.Schavemaker R, Schultz MJ, Lagrand WK, van Slobbe-Bijlsma ER, Serpa Neto A, Paulus F, and The PRoVENT-Covid Collaborative Group. Associations of Body Mass Index with Ventilation Management and Clinical Outcomes in Invasively Ventilated Patients with ARDS Related to COVID-19-Insights from the PRoVENT-COVID Study. J Clin Med. 2021; 10:1176. 10.3390/jcm10061176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021; 47:60–73. 10.1007/s00134-020-06294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards-Belle A, Orzechowska I, Gould DW, Thomas K, Doidge JC, Mouncey PR, Christian MD, Shankar-Hari M, Harrison DA, Rowan KM, and ICNARC COVID-19 Team. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020; 46:2035–47. 10.1007/s00134-020-06267-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013; 369:2126–36. 10.1056/NEJMra1208707 [DOI] [PubMed] [Google Scholar]
- 25.Nunn JF, Hill DW. Respiratory dead space and arterial to end-tidal carbon dioxide tension difference in anesthetized man. J Appl Physiol. 1960; 15:383–9. 10.1152/jappl.1960.15.3.383 [DOI] [PubMed] [Google Scholar]
- 26.St Croix CM, Cunningham DA, Kowalchuk JM, McConnell AK, Kirby AS, Scheuermann BW, Petrella RJ, Paterson DH. Estimation of arterial PCO2 in the elderly. J Appl Physiol (1985). 1995; 79:2086–93. 10.1152/jappl.1995.79.6.2086 [DOI] [PubMed] [Google Scholar]
- 27.Satoh K, Ohashi A, Kumagai M, Sato M, Kuji A, Joh S. Evaluation of Differences between PaCO2 and ETCO2 by Age as Measured during General Anesthesia with Patients in a Supine Position. Journal of Anesthesiology. 2015; 2015:710537. 10.1155/2015/710537 [DOI] [Google Scholar]
- 28.De Jong A, Chanques G, Jaber S. Mechanical ventilation in obese ICU patients: from intubation to extubation. Crit Care. 2017; 21:63. 10.1186/s13054-017-1641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee LA, Athanassoglou V, Pandit JJ. Neuromuscular blockade in the elderly patient. J Pain Res. 2016; 9:437–44. 10.2147/JPR.S85183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arroliga AC, Thompson BT, Ancukiewicz M, Gonzales JP, Guntupalli KK, Park PK, Wiedemann HP, Anzueto A, and Acute Respiratory Distress Syndrome Network. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2008; 36:1083–8. 10.1097/CCM.0B013E3181653895 [DOI] [PubMed] [Google Scholar]
- 31.Ball M, Moore RA, Fisher A, McQuay HJ, Allen MC, Sear J. Renal failure and the use of morphine in intensive care. The Lancet. 1985; 325:784–6. 10.1016/S0140-6736(85)91448-5 [DOI] [PubMed] [Google Scholar]
- 32.deBacker J, Hart N, Fan E. Neuromuscular Blockade in the 21st Century Management of the Critically Ill Patient. Chest. 2017; 151:697–706. 10.1016/j.chest.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 33.Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am. 2012; 96:87–91. 10.1016/j.mcna.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK. Cardiovascular Risks Associated with Gender and Aging. J Cardiovasc Dev Dis. 2019; 6:19. 10.3390/jcdd6020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao CT, Wang J, Wu HY, Huang JW, Chien KL. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: a population-based study. Geroscience. 2018; 40:201–17. 10.1007/s11357-018-0013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008; 52:262–71. 10.1053/j.ajkd.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 37.Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002; 136:25–36. 10.7326/0003-4819-136-1-200201010-00007 [DOI] [PubMed] [Google Scholar]
- 38.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F, and ALIVE Study Group. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004; 30:51–61. 10.1007/s00134-003-2022-6 [DOI] [PubMed] [Google Scholar]
- 39.Quality of Life After Mechanized Ventilation in the Elderly Study Investigators. 2-month mortality and functional status of critically ill adult patients receiving prolonged mechanical ventilation. Chest. 2002; 121:549–58. 10.1378/chest.121.2.549 [DOI] [PubMed] [Google Scholar]
- 40.Santa Cruz R, Villarejo F, Figueroa A, Cortés-Jofré M, Gagliardi J, Navarrete M. Mortality in Critically Ill Elderly Individuals Receiving Mechanical Ventilation. Respir Care. 2019; 64:473–83. 10.4187/respcare.06586 [DOI] [PubMed] [Google Scholar]
- 41.Jung C, Fjølner J, Bruno RR, Wernly B, Artigas A, Bollen Pinto B, Schefold JC, Wolff G, Kelm M, Beil M, Sviri S, van Heerden PV, Szczeklik W, et al. , and COVIP Study Group. Differences in mortality in critically ill elderly patients during the second COVID-19 surge in Europe. Crit Care. 2021; 25:344. 10.1186/s13054-021-03739-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leoni ML, Lombardelli L, Colombi D, Bignami EG, Pergolotti B, Repetti F, Villani M, Bellini V, Rossi T, Halasz G, Caprioli S, Micheli F, Nolli M. Prediction of 28-day mortality in critically ill patients with COVID-19: Development and internal validation of a clinical prediction model. PLoS One. 2021; 16:e0254550. 10.1371/journal.pone.0254550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillon A, Laurent E, Godillon L, Kimmoun A, Grammatico-Guillon L. Long-term mortality of elderly patients after intensive care unit admission for COVID-19. Intensive Care Med. 2021; 47:710–2. 10.1007/s00134-021-06399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung C, Flaatten H, Fjølner J, Bruno RR, Wernly B, Artigas A, Bollen Pinto B, Schefold JC, Wolff G, Kelm M, Beil M, Sviri S, van Heerden PV, et al. , and COVIP study group. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021; 25:149. 10.1186/s13054-021-03551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flaatten H, de Lange DW, Artigas A, Bin D, Moreno R, Christensen S, Joynt GM, Bagshaw SM, Sprung CL, Benoit D, Soares M, Guidet B. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 2017; 43:1319–28. 10.1007/s00134-017-4718-z [DOI] [PubMed] [Google Scholar]
- 46.Flaatten H, Guidet B, de Lange DW, Beil M, Leaver SK, Fjølner J, van Heerden PV, Sigal S, Szczeklik W, Jung C. The importance of revealing data on limitation of life sustaining therapy in critical ill elderly Covid-19 patients. J Crit Care. 2022; 67:147–8. 10.1016/j.jcrc.2021.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas LE, Karakus A, Holman R, Cihangir S, Reidinga AC, de Keizer NF. Trends in hospital and intensive care admissions in the Netherlands attributable to the very elderly in an ageing population. Crit Care. 2015; 19:353. 10.1186/s13054-015-1061-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.