Abstract
Pleckstrin homology (PH) domain binding to D3-phosphorylated phosphatidylinositides (PI) provides a reversible means of recruiting proteins to the plasma membrane, with the resultant change in subcellular localization playing a key role in the activation of multiple intracellular signaling pathways. Previously we found that the T-cell-specific PH domain-containing kinase Itk is constitutively membrane associated in Jurkat T cells. This distribution was unexpected given that the closely related B-cell kinase, Btk, is almost exclusively cytosolic. In addition to constitutive membrane association of Itk, unstimulated JTAg T cells also exhibited constitutive phosphorylation of Akt on Ser-473, an indication of elevated basal levels of the phosphatidylinositol 3-kinase (PI3K) products PI-3,4-P2 and PI-3,4,5-P3 in the plasma membrane. Here we describe a defect in expression of the D3 phosphoinositide phosphatase, PTEN, in Jurkat and JTAg T cells that leads to unregulated PH domain interactions with the plasma membrane. Inhibition of D3 phosphorylation by PI3K inhibitors, or by expression of PTEN, blocked constitutive phosphorylation of Akt on Ser-473 and caused Itk to redistribute to the cytosol. The PTEN-deficient cells were also hyperresponsive to T-cell receptor (TCR) stimulation, as measured by Itk kinase activity, tyrosine phosphorylation of phospholipase C-γ1, and activation of Erk compared to those in PTEN-replete cells. These data support the idea that PH domain-mediated association with the plasma membrane is required for Itk activation, provide evidence for a negative regulatory role of PTEN in TCR stimulation, and suggest that signaling models based on results from Jurkat T-cell lines may underestimate the role of PI3K in TCR signaling.
A major advance in our understanding of signal transduction pathways has come from the realization that many signaling proteins possess one or more self-contained domains that mediate important regulatory interactions with other cellular structures. Many of these domains, such as Src homology domains 2 and 3 (SH2 and SH3 domains), were initially identified as mediators of protein-protein interactions, but it is now apparent that some domains are also involved in high-affinity binding to certain modified phospholipids. Just as SH2 domains have been demonstrated to mediate a regulatable, reversible association between two proteins, depending on the presence or absence of a phosphate group on key tyrosine residues, so it has recently become clear that pleckstrin homology (PH) domains can mediate a similar association with the plasma membrane, depending on the presence or absence of phosphate on the D3 position of the myo-inositol ring of phosphatidylinositides (PI) (6, 12, 30, 44, 63). This property of PH domains forms the basis for a regulatable, reversible association of PH domain-containing proteins with the phosphoinositide-rich regions of the plasma membrane, an event that plays an important role in regulating the activities of several enzymes important in signaling pathways.
The importance of PH domain-mediated interactions with the plasma membrane is well illustrated by the mechanisms of activation of the ubiquitous serine/threonine kinase Akt (also known as protein kinase B) and the B-cell and mast cell protein tyrosine kinase (PTK) Btk. Both kinases possess PH domains within their amino termini. Akt plays an important role in growth control and protection from apoptosis, and is activated only when sufficient levels of both PI-3,4-P2 and PI-3,4,5-P3 are present in the plasma membrane (10, 12, 16, 18). The mechanism of activation involves PH domain-dependent corecruitment of Akt and the Akt kinase PDK1 (which also contains a PH domain) to the plasma membrane by high-affinity interactions with PI-3,4-P2 and PI-3,4,5-P3, respectively. Colocalized PDK1 phosphorylates Akt on Thr-308, catalyzing autophosphorylation on Ser-473, and activating the kinase (2, 71). The Btk kinase plays a vital role in B-cell development and is required for signaling Ca2+ influx following antigen receptor engagement. Btk activation requires phosphorylation by membrane-resident Src family kinases of the activation loop within the kinase domain of Btk, a process that is facilitated when Btk translocates to the plasma membrane via interaction of its PH domain with D3-phosphorylated phosphoinositides. Btk bearing a PH domain mutation that prevents the binding of D3-phosphorylated phosphoinositides causes the disease states X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (xid) in mice (40, 54, 61, 67).
Whether or not D3-phosphorylated phosphoinositides accumulate in the plasma membrane is determined largely by the balance between the enzymatic activities that catalyze the addition and removal of phosphate from the D3 position of this molecule. The multiple isoenzymes of phosphatidylinositol 3-kinase (PI3K) comprise the principal enzymatic activity that catalyzes the addition of phosphate onto the D3 position of PI, generating PI-3-P, PI-3,4-P2, and PI-3,4,5-P3 from PI, PI-4-P, and PI-4,5-P2, respectively (10, 12, 29). Opposing this activity is the dual-specificity phosphatase PTEN (also known as MMAC1 and TEP1), which possesses activity against D3-phosphorylated phosphoinositides as well as against phosphotyrosine residues in some proteins (5, 10, 53).
PTEN has been shown to be a tumor suppressor gene that is either deleted or mutated in a high percentage of human glioblastomas and endometrial, prostate, breast, and hematopoietic cancers (5, 10, 53, 64). Similarly, a spontaneous mutation in PI3K that causes constitutive activation leads to cellular transformation (39). These findings underscore the importance of PI3K, PTEN, and the ability to appropriately regulate D3-phosphoinositide metabolism in regulating growth control and sensitivity to apoptosis. Consistent with the hypothesis that the lipid phosphatase activity of PTEN is responsible for its growth suppression function, introduction of wild-type PTEN, but not of lipid phosphatase-inactive PTEN, into tumor cell lines causes G1 arrest of the cell cycle in glioblastoma cells (32, 60) and apoptosis in carcinomas (46, 78). In addition, tumor cells lacking PTEN activity have elevated levels of PI-3,4-P2 and PI-3,4,5-P3, and high levels of activated Akt, consistent with the idea that the basal level of D3-phosphorylated phosphoinositides plays a key role in the regulation of cell growth and sensitivity to apoptotic stimuli (19, 34, 57, 70).
PI3K and PTEN are also important for T-cell growth and function. Overexpression of PTEN in Jurkat T cells results in apoptosis, which can be rescued by coexpression of constitutively active Akt (75). Additionally, T cells from PTEN+/− mice exhibit reduced activation-induced cell death and increased proliferation upon activation compared to those of their PTEN+/+ littermates (21). Likewise, increased PI3K expression protects T cells from CD95-mediated apoptosis (35). PI3K has also been implicated as being an important mediator of T-cell receptor (TCR) signaling and T-cell activation. PI3K is rapidly activated following TCR stimulation (11, 20, 26, 76). Furthermore, pharmacologic inhibitors of PI3K have been found to inhibit TCR-stimulated Erk2 activation, interleukin 2 production, and T-cell proliferation (23, 25, 69, 74), and PTEN overexpression in Jurkat T cells inhibits Erk activation (75).
In addition to Akt, there are a number of proteins that have been implicated in TCR signaling and that either themselves possess D3-phosphorylated PI-binding PH domains—phospholipase C (PLC)-γ1, Vav, PKD, Itk, and Tec—or are regulated by proteins that do—Ras (regulated by SOS and Ras-GAP) and protein kinase C (PKC; regulated by PDK1) (15, 22, 44, 45). Itk (also known as Emt or Tsk) and Tec are members of the Tec family of nonreceptor PTKs, which also includes Btk, Bmx, and Txk (also known as Rlk). All Tec PTKs, with the exception of Txk/Rlk, have an amino-terminal PH domain as part of their domain structure (54, 62). However, with the exception of Btk, the role of the PH domain in regulating the activities of these kinases has not been well established. In resting cells Btk is found almost exclusively in the cytosol, and it is targeted to the plasma membrane only transiently by the high-affinity interaction between its PH domain and PI-3,4,5-P3 upon antigen receptor stimulation (41, 47, 48, 73). The Itk tyrosine kinase is expressed predominantly in T cells. Itk is involved in T-cell development, as well as TCR and CD28 signaling, and appears to be required for sustaining the rise in intracellular Ca2+ levels that follows T-cell activation (49, 50, 61). It has been suggested that Itk activation should follow a regulatory mechanism largely similar to that of Btk (3, 36, 66). Surprisingly, however, we previously found that more than 50% of Itk is localized to the membrane fraction of unstimulated Jurkat T cells (7, 68). Membrane localization of Itk was not sufficient to induce its tyrosine phosphorylation and activation. However, TCR stimulation of these cells did result in tyrosine phosphorylation and activation of Itk. This activation required ZAP-70 and was associated with no detectable net change in the fraction of membrane-bound Itk (7, 68).
In trying to understand the mechanism for the unexpected constitutive targeting of Itk to the plasma membrane in Jurkat T cells, we considered a membrane-targeting model for Itk similar to that of Btk, meaning a PH domain-mediated interaction with the plasma membrane. Under such a model, the constitutive localization of Itk to the plasma membrane would indicate either that the Itk PH domain has a different binding specificity than the Btk PH domain or that D3-phosphoinositide metabolism is abnormal in Jurkat T cells, permitting the basal accumulation of D3-phosphorylated phosphoinositides. Given that PTEN is frequently mutated in hematopoietic cell lines, we hypothesized that defects in PTEN function in the Jurkat leukemic T-cell line could result in accumulation of PI-3,4,5-P3 in the plasma membrane and that this could be responsible for the constitutive membrane localization of Itk. In the present study, we report defective expression of the PTEN protein in Jurkat leukemic T-cell lines. Although the PTEN gene was transcribed in JTAg T cells, it harbors mutations in exon 7 of both alleles. These mutations introduce premature termination codons, resulting in truncation of PTEN within the C-terminal C2 domain and the rapid degradation of the truncated protein (33). The PTEN deficiency in JTAg T cells results in constitutive phosphorylation of Akt on Ser-473 and constitutive membrane localization of Itk. Treatments that would be expected to reduce the levels of PI-3,4,5-P3 and PI-3,4-P2, such as pharmacologic inhibition of PI3K or reintroduction of wild-type PTEN, reduced Akt phosphorylation and caused redistribution of Itk from the plasma membrane to the cytosol. The importance of basally elevated PI-3,4,5-P3 levels in targeting Itk to the plasma membrane was further supported by the demonstration that the intact PH domain of Itk is required for this interaction. Examining the effect of PTEN reexpression upon signaling events downstream of TCR engagement, we found that vector-transfected Jurkat T cells are hyperresponsive compared to PTEN-transfected cells in terms of Itk kinase activity, PLC-γ1 tyrosine phosphorylation, and Erk activation, suggesting that the PTEN deficiency in Jurkat T cells causes constitutive activation and premature priming of TCR signaling pathways.
MATERIALS AND METHODS
Cells and antibodies.
Simian virus 40 T antigen-transfected human leukemic Jurkat T cells (JTAg) were maintained in RPMI 1640 (Life Technologies, Inc., Gaithersburg, Md.) supplemented with 7.5% fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, and 10 μg of ciprofloxacin (Bayer, Kankakee, Ill.)/ml. Cells were cultured in 2.5% fetal bovine serum overnight, prior to use. Normal T cells were purified from peripheral blood of healthy donors from the National Institutes of Health blood bank (Bethesda, Md.) by negative depletion with a monoclonal antibody cocktail against CD11b, CD14, CD16, CD19, and major histocompatibility complex (MHC) class II purchased from PharMingen (San Diego, Calif.). The purified T cells were allowed to rest overnight before being subjected to membrane preparation and immunoprecipitation. The OKT3 monoclonal antibody to human CD3 and polyclonal rabbit antisera specific for human ZAP-70 and Lck have been described elsewhere (8, 43). The anti-phosphotyrosine monoclonal antibody, 4G10, was from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Polyclonal rabbit antisera specific for human Itk were kindly provided by G. Mills (University of Texas M.D. Anderson Cancer Center, Houston) and were used to immunoprecipitate Itk. A mouse monoclonal antibody, 2F12, directed to the N-terminal 26 amino acids of Itk was the gift of L. Berg (University of Massachusetts, Worcester) and was used in immunoblotting. The 3023 rabbit antisera to LAT were a kind gift of L. E. Samelson (National Cancer Institute, Bethesda, Md.). Antibodies to Akt and phospho-Akt (Ser-473) were from New England Biolabs (Beverly, Mass.). PTEN immunoblotting was performed with a cocktail of anti-PTEN antibodies, including N-19 (goat polyclonal, directed to the N terminus; Santa Cruz Biotechnology, Santa Cruz, Calif.), A2B1 (mouse monoclonal, to amino acids 388 to 400 at the C terminus; Santa Cruz), A2B1 (mouse monoclonal, to the C-terminal 10 amino acids; Chemicon), and Ab-2 (mouse monoclonal, to amino acids 345 to 390; Oncogene). Antibodies to the myc and Flag epitope tags are the mouse monoclonal antibody 9E10 and anti-Flag from Santa Cruz Biotechnology and IBI Kodak (New Haven, Conn.), respectively.
Cell stimulation and lysis.
Cells were harvested by centrifugation, washed once, and resuspended in cold RPMI 1640 medium at a density of 108/ml. After equilibration to 37°C for 10 min, the cells were stimulated with OKT3 (1:50 ascites) for the indicated duration. Stimulation was terminated by addition of 5 volumes of 4°C lysis buffer [20 mM HEPES (pH 7.4), 1% Triton X-100, 50 mM β-glycerophosphate, 2 mM EGTA, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 10% glycerol, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, 100 μg of 4-(2-aminoethyl)benzenesulfonyl fluoride/ml]. After a 30-min incubation on ice, postnuclear lysates were prepared by a 10-min centrifugation at 4°C and 21,000 × g. The lysates were either directly analyzed by Western blotting or subjected to immunoprecipitation followed by immunoblotting or a kinase assay.
Immunoprecipitation and Western blot analysis.
Postnuclear whole-cell lysates were incubated with the rabbit polyclonal antibody to human Itk and goat anti-rabbit immunoglobulin G agarose (Sigma, St. Louis, Mo.) for 2 to 16 h at 4°C. Immunoprecipitates that were to be analyzed by immunoblotting were washed three times with the above lysis buffer supplemented with 150 mM NaCl. Whole-cell lysates and immunoprecipitates to be analyzed by Western blotting were denatured by heating to 95°C in Nu-PAGE sample buffer, subjected to electrophoresis on either a 4 to 12% Nu-PAGE gradient or 6% or 10% Tris-glycine gels, and transferred to a nitrocellulose membrane according to the manufacturer's instructions (NOVEX, San Diego, Calif.). Concentrations for blotting antibodies varied according to the manufacturer's recommendations. In particular, the anti-PTEN blotting was performed using a cocktail of four anti-PTEN antibodies (see above) at a 1:100 dilution. The blots were developed with the ECL system of Amersham Pharmacia Biotech (Piscataway, N.J.) and autoradiographed on BMR film (Eastman Kodak Co., Rochester, N.Y.).
Transient transfection of JTAg T cells.
JTAg T cells in the logarithmic-growth phase were transfected by electroporation. Cells were resuspended in complete growth medium at a density of 4 × 107/ml, and 300 μl of the cell suspension was mixed with 0.6 to 30 μg of plasmid DNA in a 4-mm gap electroporation cuvette for 15 min before being subjected to a single pulse from a BMX ECM 830 square-wave electroporator at 300 V for 10 ms. The cells were transferred to culture dishes and incubated overnight in complete medium. Transfection efficiencies were typically between 85 and 90% among the live cells as assessed by transfection with pCMV-GFP. The transfected cells were then left unstimulated or stimulated with an anti-CD3 monoclonal antibody (OKT3; 1:100 ascites) 14 to 20 h posttransfection. Cell viability was assessed by trypan blue; it was typically 80% in the overnight cultures, and cell equivalents used in the experiments were based on live cells.
Construction of expression plasmids.
The construct pSRα-Flag-PTEN, encoding PTEN carrying a triplicated Flag epitope tag, was generated by a two-step procedure. The sequence encoding the hemagglutinin (HA) epitope tags was removed from pSRα-HA-Srf I (pSRα-JNK1, a gift of M. Karin [University of California, San Diego], was modified by removal of the Jnk1 open reading frame and creation, by site-directed mutagenesis, of an SrfI site 3′ of the HA coding sequence to generate pSRα-HA-Srf I) and replaced with a DNA sequence encoding three Flag epitope tags by using the ExSite and QuickChange kits (Stratagene, La Jolla, Calif.) to generate pSRα-Flag-Srf I. The open reading frame of wild-type human PTEN was then amplified from the Est clone AA187786 (Genome Systems Inc., St. Louis, Mo.) by PCR with the primer pair 5′-ATGACAGCCATCATCAAAGAGATCG-3′ and 5′-TTTATTTTCATGGTGTTTTATCCCTCT-3′. The resulting fragment was inserted into the SrfI site of pSRα-Flag-Srf I to create pSRα-Flag-PTEN (PTEN-WT). The pSRα-Flag-PTEN C124S (PTEN-C/S) mutant was prepared by QuickChange mutagenesis (Stratagene) using the primer 5′-CATGTTGCAGCAATTCACTCTAAAGCTGGAAAGGGACG-3′ and its complement, and using wild-type pSRα-Flag-PTEN as the template. For the construction of pEGFP-Itk, pcDNA3-Itk was digested with XbaI, blunted with Klenow fragment, and then digested with Asp718 to liberate the Itk coding sequence, which was ligated into the pEGFP N1 vector between the Asp718 and SmaI sites. The cloning of C-terminally myc-tagged wild-type and R29C (xid) Itk into the mammalian expression vector pEF-BOS has been described previously (7).
In vitro Itk kinase assay.
Itk-associated tyrosine kinase activity was assessed by an immune complex kinase assay. Anti-Itk immunoprecipitates from lysates were washed twice with lysis buffer plus 150 mM NaCl, twice with 4°C LiCl wash buffer (100 mM Tris-HCl [pH 7.5]–0.5 M LiCl), and twice with 4°C distilled water. To each sample of washed beads, 30 μl of kinase reaction mixture (10 mM MgCl2, 10 mM HEPES [pH 7.0], 2 mM sodium orthovanadate, 5 μCi of [γ-32P]ATP [Amersham Pharmacia Biotech]) and 5 μg of RR-SRC substrate peptide (Sigma) were added. The reaction was performed at room temperature for 15 min with frequent mixing, then terminated by addition of acetic acid to 30% of the total volume. The products were centrifuged briefly, and supernatants were spotted onto p81 phosphocellulose disks (Life Technologies, Inc.). After four to six washes with 75 mM phosphoric acid, 32P incorporation was measured by liquid scintillation counting. In some assays (where indicated), the kinase activity was normalized to the relative amount of Itk recovered in the anti-Itk immunoprecipitates. The relative amount of Itk was measured by densitometric analysis of X-ray films using the public-domain NIH Image program (developed at the National Institutes of Health).
Preparation of cytosolic and membrane fractions.
Cells (2.5 × 107) were centrifuged quickly in cold phosphate-buffered saline after OKT3 stimulation and resuspended in 1.5 ml of cold hypotonic lysis solution [20 mM HEPES (pH 7.6), 5 mM sodium pyrophosphate, 5 mM EGTA, 1 mM MgCl2, 10 μg of aprotinin/ml, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 1 mM sodium orthovanadate]. The cell suspension was incubated on ice for 30 min, followed by cellular disruption with 10 passes of a Dounce homogenizer. After centrifugation at 100,000 × g at 4°C for 1 h, the supernatant was collected as the cytosolic fraction. The pellet was solubilized in 1.5 ml of the membrane solubilization buffer [1% Triton X-100, 20 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM MgCl2, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride), 1 mM sodium orthovanadate] on ice for 30 min, followed by centrifugation at 100,000 × g at 4°C for 1 h. This supernatant was taken as the membrane fraction. The quality of the membrane and cytosolic fractions was routinely assessed by Western blotting for LAT (membrane) and ZAP-70 (cytosol).
Confocal fluorescence microscopy.
JTAg cells were suspended at 2 × 107 cells/ml in normal growth medium, and 500-μl aliquots were electroporated with 25 μg of pEGFP-Itk or 25 μg of pEGFP-Itk plus 25 μg of one of the following: pSRα-Flag, pSRα-Flag-PTEN, or pSRα-Flag-PTEN-C/S. Electroporation was carried out in a Bio-Rad Gene Pulser II using 250 V at 960 μF. The cells were cultured overnight in normal growth medium with a final serum concentration of 20%. Live cells were visualized for green fluorescent protein (GFP) and Hoechst 33342-stained nuclei by confocal microscopy on a Zeiss LSM510 microscope fit with a warmed stage.
Luciferase reporter assay.
The NF-AT luciferase reporter gene (10 μg) was cotransfected with either empty vector (pSRα-Flag-Srf I) or wild-type PTEN (pSRα-Flag-PTEN) (30 μg) by electroporation. Cells were cultured at 37°C for 16 h. Cells were harvested and assayed using the Luciferase Assay System (Promega, Madison, Wis.) and a model LB 953 Autolumat (Perkin-Elmer, Gaithersburg, Md.). A β-galactosidase reporter gene was also included in the cotransfections, and the β-galactosidase activity (Tropix, Bedford, Mass.) was used to normalize the luciferase reporter gene data.
Northern blot analysis.
Total RNA was isolated from normal CD4+ T cells and JTAg T cells using RNA STAT-60 (TEL-TEST). RNA (25 to 40 μg) was resolved on a formaldehyde denaturing, 0.85% agarose gel, transferred onto a GeneScreen Plus Hybridization Transfer membrane (NEN Life Science Products, Inc.), and hybridized at 60°C overnight with a 32P-labeled (Boehringer Mannheim) PTEN cDNA probe corresponding to the full-length coding region.
Cloning and sequencing of PTEN exon 7-containing genomic fragment.
Genomic fragments containing the PTEN exon 7 sequence were amplified from JTAg T-cell genomic DNA (ReadyAmp Genomic System; Promega) by PCR using two primer pairs, PTENex7f (5′-TGACAGTTTGACAGTTAAAGG-3′)–PTENex7r (5′-GGATATTTCTCCCAATGAAAG-3′) and PTEN7-F (5′-ACCATGCAGATCCTCAGTTTGTG-3′)–PTEN7-R (5′-CTCATGTTACAATGCCATAAGGC-3′). PCR was performed with 200 ng of genomic DNA, 200 nmol of each primer, 200 nmol of deoxynucleoside triphosphates/liter, and 5 U of Pfu DNA polymerase (Promega) in a final volume of 50 μl. After an initial denaturing at 95°C for 3 min, 30 cycles of denaturing (94°C) for 30 s, annealing (at 55°C for the PTENex7f–PTENex7r primer pair and at 60°C for the PTEN7-F–PTEN7-R primer pair) for 1 min, and extension (72°C) for 1 min were performed on a DNA thermal cycler (HYBAID). The final extension was performed for 10 min. The PCR products were purified from the gel by Geneclean (Bio 101) and cloned into the pCR 4Blunt-TOPO vector (Invitrogen). Sequences from 11 clones containing PTEN exon 7 were determined by using both T3 and T7 primers to read both strands.
RESULTS
Itk is distributed to the cytosol of normal human T cells and the membrane of JTAg cells.
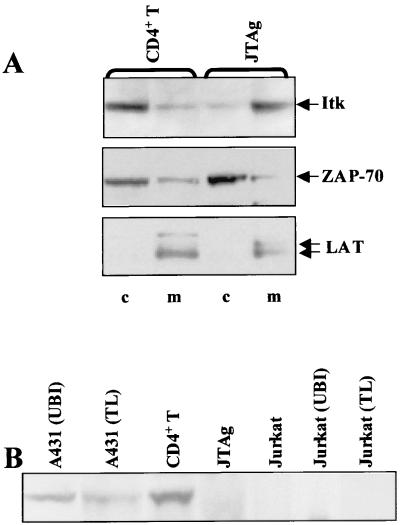
We previously found that Itk is constitutively localized to the membrane fraction in Jurkat T cells (68). This finding was unexpected, since the related Tec family kinase Btk demonstrates a predominantly cytosolic distribution pattern in resting B cells and mast cells (41, 47, 48, 73). In considering the possibility that this difference may stem more from a difference between normal T cells and Jurkat T cells, rather than a difference between Itk and Btk, we assessed the subcellular distribution of Itk in normal CD4+ T cells compared to that in JTAg cells (Fig. 1A). In sharp contrast to the predominant membrane distribution of Itk in the JTAg cells, Itk in the resting normal human CD4+ T cells was found to be localized predominantly within the cytosolic fraction. The distribution pattern in the normal CD4+ T cells is similar to that of Btk in mast cells and B cells (41, 47, 48, 73). The predominant distribution of ZAP-70 and LAT into the cytosolic and membrane fractions, respectively, is consistent with minimal cross-contamination between the two fractions.
FIG. 1.
Constitutive localization of Itk to the membrane is correlated with absence of PTEN expression. (A) The cytosolic (c) and membrane (m) fractions prepared from normal human CD4+ T cells and JTAg T cells were immunoblotted with a monoclonal antibody to Itk (2F12). The membrane was stripped and reblotted with rabbit antisera to the cytosolic protein ZAP-70 (1213) and the transmembrane protein LAT (3023). (B) Whole-cell lysates (total protein, approximately 20 μg for JTAg and Jurkat E6 cells, and 10 μg for CD4+ T cells, A431 cells, and Jurkat [Upstate Biotechnology Inc. {UBI}] and Jurkat [Transduction Laboratories {TL}] cells) were immunoblotted with an antibody cocktail of four anti-PTEN antibodies (see Materials and Methods). The two A431 lysates (from UBI and TL) were included as positive controls.
Expression of PTEN is deficient in Jurkat cells.
Membrane localization of Tec family kinases has been shown to be mediated by high-affinity interaction of the PH domain of the kinase with certain phosphoinositides, principally PI-3,4,5-P3 (47, 48, 66, 67, 73). This suggested to us that the enzymatic processes that control the levels of PI-3,4,5-P3 might be misregulated in Jurkat T cells, leading to constitutive Itk localization at the plasma membrane (7, 68). Given the importance of PTEN in regulating PI-3,4,5-P3 levels and the fact that PTEN activity is deficient in many transformed cell lines, we examined the levels of PTEN protein expression in Jurkat, JTAg, and normal human CD4+ T cells and in the A431 cell line (positive control) (Fig. 1B). PTEN expression could be readily detected in the A431 cell line and in the CD4+ T cells, but not in Jurkat cell lines obtained from three different sources, nor in JTAg cells, even when Jurkat and JTAg lysates were overloaded. PTEN was also readily detectable in total T cells and peripheral blood mononuclear cells (data not shown).
The PTEN gene is transcribed and mutated in exon 7 in JTAg T cells.
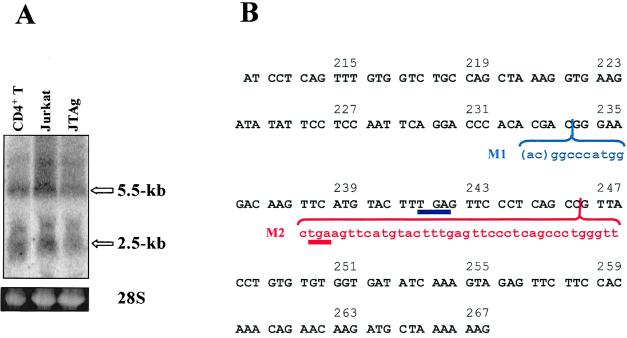
To find the mechanisms responsible for the PTEN deficiency, we first examined the expression of PTEN mRNA by Northern blot analysis. Using a full-length PTEN cDNA probe, we could detect in both normal human CD4+ T cells and JTAg T cells comparable amounts of multiple PTEN transcripts with the expected major species of 2.5 and 5.5 kb (31), suggesting that the PTEN gene is present and transcribed in JTAg T cells (Fig. 2A). To assess if the PTEN gene in JTAg T cells contains mutations that could generate unstable message or protein, we cloned and sequenced PTEN exon 7, since mutations in this exon have previously been reported in a Jurkat subline (64). Sequences from 11 exon 7 clones were determined (Fig. 2B). No wild-type sequence was observed, and all clones contained one of two mutations. The first mutation contains a 2-bp deletion (AC) followed by a 9-bp insertion (GGCCCATGG) at codon 234, which resulted in a frameshift and a downstream stop codon at codon 241. The second mutation is a 39-bp insertion (CTGAAGTTCATGTACTTTGAGTTCCCTCAGCCCTGGGTT) at codon 246, which generated a new stop codon at codon 247.
FIG. 2.
Transcriptional expression and truncated translation of PTEN in JTAg T cells. (A) Northern blot analysis. Two major PTEN RNA species (5.5 and 2.5 kb) are indicated by open arrows. Relative levels of total RNA loading are shown as ethidium bromide staining of 28S rRNA (lower panel). (B) DNA sequencing analysis. The PTEN exon 7 sequence is shown in boldface uppercase letters. Mutation 1 (M1), containing a 2-bp deletion (in parentheses) and a 9-bp insertion, is shown in lowercase blue letters. Mutation 2 (M2), the 39-bp insertion mutation, is shown in lowercase red letters. The stop codons introduced by these mutations are underlined in blue (M1) or red (M2). Numbers refer to the codon number.
Basal levels of phosphoinositide lipids are high in PTEN-deficient Jurkat cells.
Since Akt phosphorylation and activation are critically dependent on the presence of both PI-3,4,5-P3 and PI-3,4-P2 in the plasma membrane, and the production of these lipids represents the rate-limiting step in Akt activation, the phosphorylation status of Akt has been proven to provide an accurate measure of the levels of these phospholipids within the cell. In these studies we have used Ser-473 phosphorylation of Akt as a measure of the levels of PI-3,4,5-P3 and PI-3,4-P2 in the plasma membrane. Under resting conditions (2.5% fetal calf serum overnight), the levels of Akt phosphorylation were high in JTAg T cells (Fig. 3A). Indeed, Akt was maximally phosphorylated under these resting conditions, as demonstrated by the fact that its phosphorylation could not be further increased by cross-linking of CD3, which strongly activates PI3K (11, 76), suggesting that the basal levels of PI-3,4,5-P3 and PI-3,4-P2 are high in these cells. The hyperphosphorylation of Akt corresponds to increased kinase activity of this enzyme (1).
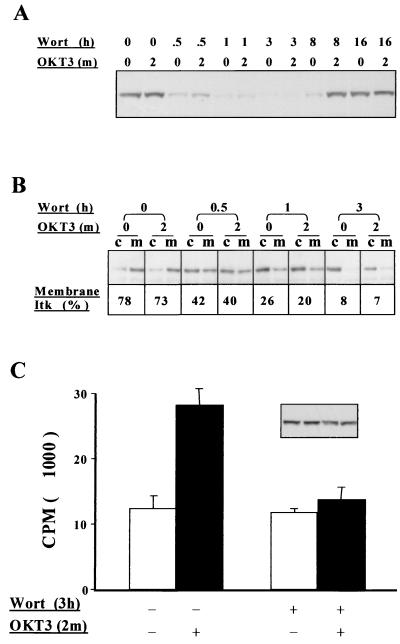
FIG. 3.
Effects of wortmannin on Akt phosphorylation and membrane distribution and kinase activity of Itk. JTAg T cells were treated with wortmannin (100 nM) for the indicated times prior to stimulation by cross-linking CD3 (OKT3 ascites). (A) Whole-cell lysates were immunoblotted with an anti-phospho-Akt antibody which recognizes the Ser-473-phosphorylated, active form of Akt. (B) The cytosolic (c) and membrane (m) fractions were prepared from the same samples and immunoblotted with an anti-Itk monoclonal antibody (2F12). The percentage of total Itk localized to the membrane fraction was determined by densitometric analysis of the X-ray films. (C) Itk was immunoprecipitated with rabbit polyclonal anti-Itk antisera from 107 cells treated with or without wortmannin (100 nM) and OKT3 as indicated and was subjected to an in vitro kinase assay. The inset shows an Itk blot of the Itk immunoprecipitate, indicating that equal amounts of Itk were used in the kinase assay.
Inhibition of PI3K activity results in reduced Akt phosphorylation and a redistribution of Itk from the plasma membrane to the cytosol.
To further study the effect of unopposed PI3K activity in PTEN-deficient Jurkat cells upon the activation of Akt and membrane localization of Itk, we pretreated JTAg cells with the PI3K inhibitors wortmannin and LY294002 (Calbiochem). Cells pretreated with 100 nM wortmannin for the indicated times exhibited a reduction in Akt Ser-473 phosphorylation that could be seen within 30 min and reached a peak (no detectable phospho-Akt) at 3 h (Fig. 3A). It is noteworthy that after 8 h of wortmannin pretreatment Akt phosphorylation on Ser-473 could once again be detected, but only upon CD3 cross-linking. By 16 h, basal Akt phosphorylation on Ser-473 had returned to the levels observed in untreated JTAg cells. The total amount of Akt did not change during the time course of wortmannin treatment (data not shown). Similar results were obtained when 100 μM LY294002 was used in place of 100 nM wortmannin (data not shown).
In parallel, we prepared cytosolic and membrane fractions from the same cells and examined the subcellular localization of Itk. Initially, about 75% of Itk distributed to the membrane fraction. Prolonged wortmannin pretreatment resulted in a redistribution of Itk from the membrane to the cytosolic fraction (Fig. 3B). The degree of redistribution to the cytosol was directly correlated with the loss of Ser-473 phosphorylation on Akt, with more than 90% of Itk appearing in the cytosol of JTAg cells after 3 h of wortmannin treatment.
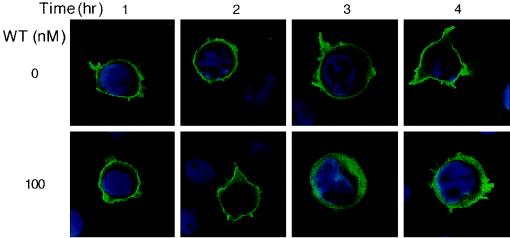
To further examine the effect of wortmannin treatment upon the subcellular localization of Itk in Jurkat cells, and to confirm that the distribution that we observed in the crude membrane fractions is also reflective of what is occurring in the plasma membrane, we transiently transfected JTAg cells with Itk-GFP and examined its subcellular distribution using fluorescence confocal microscopy. In the absence of treatment, Itk-GFP distributed to the plasma membranes of the cells. After treatment of the cells with 100 nM wortmannin for 3 to 4 h, the Itk-GFP fusion protein clearly showed a diffuse cytoplasmic pattern (Fig. 4). An intermediate staining pattern was discerned after 2 h of wortmannin treatment. This time course is consistent with that measured by subcellular fractionation (Fig. 3B) and suggests that the crude membrane fraction is reliably reflecting events occurring at the plasma membrane.
FIG. 4.
Inhibition of PI3K with wortmannin results in a shift of plasma membrane-associated Itk into the cytoplasm. JTAg cells expressing GFP-tagged Itk were treated with vehicle (0.1% dimethyl sulfoxide) or 100 nM wortmannin and examined by fluorescence confocal microscopy at 1, 2, 3, and 4 h after the beginning of treatment. To better visualize the cytoplasmic compartment, the nuclei were stained with Hoechst 33342.
The effect of prolonged wortmannin treatment, and the consequent loss of Itk from the plasma membrane, upon Itk kinase activity was also examined. Immune complex kinase assays were performed on Itk immunoprecipitates from JTAg whole-cell lysates of cells that were treated with either wortmannin or vehicle for 3 h and then either left unstimulated or stimulated with an antibody to CD3 (Fig. 3C). While wortmannin pretreatment had no effect upon unstimulated Itk kinase activity, it completely blocked the TCR-stimulaed increase in activity, suggesting that, while membrane recruitment is not sufficient for Itk activation, it is required. An aliquot of the Itk immunoprecipitates was blotted for Itk and showed that comparable amounts of Itk were used in each kinase reaction (Fig. 3C, inset).
Reintroduction of PTEN into JTAg T cells restores the normal Itk distribution pattern and reduces Akt phosphorylation.
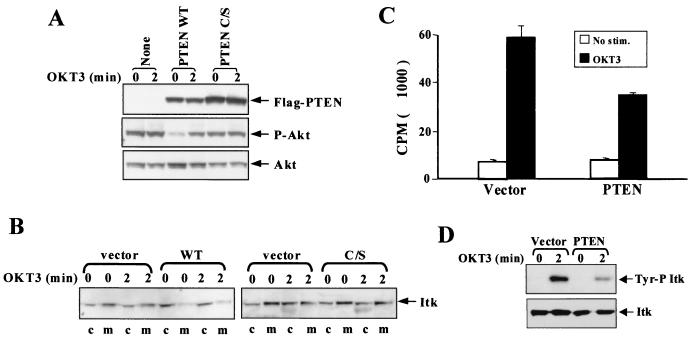
Having found that prolonged inhibition of JTAg with PI3K inhibitors could cause Itk to assume the cytosolic distribution pattern typical of normal, nontransformed T cells, we tested to see whether restoration of PTEN could similarly reverse the constitutive membrane localization of Itk. JTAg T cells were transiently transfected with vector alone or with PTEN expression vectors encoding wild-type PTEN-Flag (PTEN-WT) or PTEN-Flag bearing an inactivating C124S mutation (PTEN-C/S). Expression of both PTEN proteins could be detected 16 h posttransfection as indicated by anti-Flag immunoblotting (Fig. 5A, top panel). Despite transfection of the cells with equal amounts of plasmid, the levels of wild-type PTEN expression were consistently found to be lower than those of the inactive PTEN mutant, suggesting that high-level expression of active PTEN is deleterious to these cells. While significantly less PTEN-WT than PTEN-C/S was expressed, only PTEN-WT expression decreased basal Akt phosphorylation (Fig. 5A, center panel). It is interesting that while wild-type PTEN expression does block basal Akt Ser-473 phosphorylation, it also unmasks the capacity of CD3 cross-linking to stimulate Ser-473 phosphorylation, which can be seen neither in vector control-transfected cells nor in cells expressing phosphatase-dead PTEN (Fig. 5B).
FIG. 5.
Reexpression of wild-type PTEN reduces Akt phosphorylation and restores the predominant cytosolic localization of Itk in JTAg T cells. Flag-tagged PTEN was expressed in JTAg T cells 18 h postelectroporation. (A) Each whole-cell lysate of 2 × 105 cell equivalents was immunoblotted for the level of PTEN expression with an anti-Flag antibody (top). The same membrane was stripped and reblotted for phosphorylated Akt (center) and Akt (bottom). (B) Cytosolic (c) and membrane (m) fractions were prepared from the same samples, and the Itk distribution pattern was examined by immunoblotting. (C) Itk was immunoprecipitated with 2F12 from 2 × 107 JTAg T cells transfected with the vector or PTEN-WT and then subjected to an in vitro kinase assay. (D) The amounts of Itk used in the kinase assay and their degree of tyrosine phosphorylation were measured by Western blot analysis.
Once again the ability of Itk to distribute to the membrane fraction correlated with the degree of basal Ser-473 phosphorylation of Akt. Expression of phosphatase-active PTEN, but not the inactive C/S mutant of PTEN, resulted in a marked redistribution of Itk from the membrane to the cytosolic fraction (Fig. 5B). Similar results were obtained when Itk-GFP distribution was examined by confocal fluorescence microscopy of JTAg cells transfected with the vector control, wild-type PTEN, and phosphatase-inactive PTEN (Fig. 6). In both the vector- and PTEN-C/S-transfected cells, Itk-GFP is localized to the plasma membrane, while PTEN-WT-transfected cells exhibit cytosolic distribution of Itk-GFP.
FIG. 6.
PTEN expression in JTAg cells prevents plasma membrane association of Itk. GFP-tagged Itk was coexpressed in JTAg cells with vector (pSRα-Flag-Srf I), PTEN-WT, or the inactive mutant, PTEN-C/S, and examined by fluorescence confocal microscopy. To better visualize the cytoplasmic compartment, the nuclei were stained with Hoechst 33342.
The effect of restored PTEN expression upon Itk kinase activity was also examined (Fig. 5C). As with inhibition of PI3K activity, expression of PTEN had no effect upon basal Itk kinase activity. However, unlike wortmannin treatment, which completely blocked TCR-mediated Itk activation, Itk could still be activated upon CD3 cross-linking in the PTEN-expressing JTAg cells, although activation was typically only 50 to 60% of that observed in control cells. The lower kinase activity of Itk in PTEN-expressing JTAg T cells was also correlated with decreased Itk tyrosine phosphorylation (Fig. 5D).
The PH domain of Itk is required for its membrane localization.
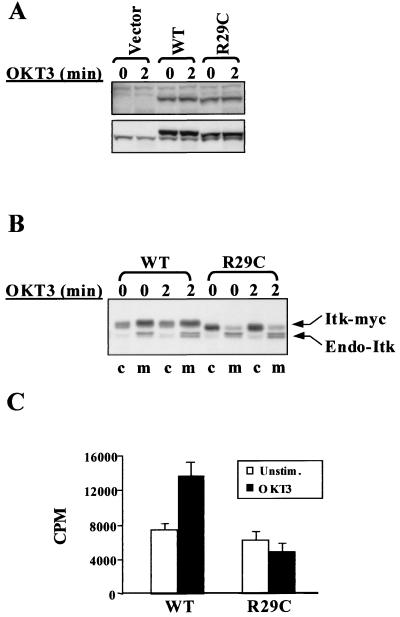
Together, the above data argue that Itk is localized to the plasma membrane due to an accumulation of D3-phosphorylated phosphoinositides as the result of unopposed basal PI3K activity in the absence of counterbalancing PTEN activity. Since the ability of Tec family kinases to bind to phosphoinositides depends upon the function of their PH domains, this model would predict that disruption of Itk's PH domain would lead to a predominantly cytosolic distribution of JTAg T cells. Several mutations found in the PH domain of Btk dramatically reduce the affinity of Btk for PI-3,4,5-P3 and induce XLA in humans and xid in mice. myc-tagged Itk bearing a mutation comparable to that found in the PH domain of Btk from xid mice (ItkR29Cmyc) was expressed in JTAg cells and compared with myc-tagged wild-type Itk (ItkWTmyc) with respect to partitioning between the membrane and cytosolic fractions. When expressed at a level roughly comparable to that of endogenous Itk (Fig. 7A), ItkR29Cmyc showed drastically reduced membrane localization, with most of the kinase appearing in the cytosol (Fig. 7B). In contrast, both ItkWTmyc and endogenous Itk from the same cells were localized primarily to the membrane fraction (Fig. 7B). Consistent with the results from the wortmannin pretreatment experiments, the capacity of Itk to bind to the membrane fraction is required in order for TCR engagement to activate Itk kinase activity. Immune complex kinase assays measured no increase in the kinase activity of ItkR29Cmyc isolated from OKT3-stimulated JTAg T cells, while ItkWTmyc could be activated normally in response to OKT3 stimulation (Fig. 7C).
FIG. 7.
Targeting of Itk to the membrane and sensitivity of Itk to CD3 stimulation requires an intact PH domain. Six micrograms of each pSRα-Itk-myc construct or vector was electroporated into 1.2 × 107 JTAg T cells to get about two- to threefold overexpression of the recombinant Itk over endogenous Itk. (A) The levels of Itk expression were monitored by Western blot analysis of the whole-cell lysates with an anti-myc antibody (9E10) (top) or an anti-Itk antibody (2F12) (bottom). (B) The cytosol (c) and membrane (m) fractions from pSR-Itk(WT) and pSR-Itk(R29C) transfectants were electrophoresed on a 6% Tris-glycine gel to resolve the endogenous and transfected Itk's and then immunoblotted with an anti-Itk antibody (2F12). (C) The recombinant Itk was immunoprecipitated with an anti-myc antibody (9E10) from 2 × 107 JTAg T cells transfected with either pSR-Itk(WT) or pSR-Itk(R29C) and was analyzed in an in vitro kinase assay.
Overexpression of PTEN in Jurkat T cells attenuates CD3-stimulated Itk kinase activity, phosphorylation of PLC-γ1, and Erk activation.
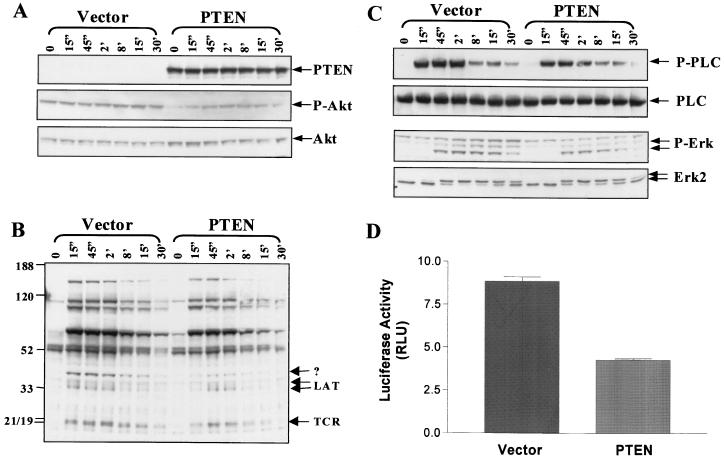
It is clear from the data presented in Fig. 5 and 6 that the PTEN expression status of Jurkat T cells affects both basal biochemical events in these cells (the phosphorylation status of Akt and membrane localization of Itk) and biochemical changes initiated by TCR signaling. This point is illustrated by the facts that CD3 cross-linking leads to increased Akt phosphorylation only in PTEN-replete or wortmannin-treated JTAg T cells and that PTEN expression reduces by about 50% the capacity of Itk to become activated upon CD3 cross-linking. In light of these observations, we examined whether other early, TCR-initiated signaling events are sensitive to the PTEN status of JTAg T cells. JTAg T cells were transiently transfected with either vector or the PTEN-WT plasmid (Fig. 8A). As was seen earlier (Fig. 5A), expression of PTEN caused markedly reduced Ser-473 phosphorylation of Akt and rendered Akt sensitive to increased Ser-473 phosphorylation in response to CD3 cross-linking (Fig. 8A). We first examined the effect of PTEN expression upon OKT3-stimulated tyrosine phosphorylation of proteins recovered from JTAg whole-cell lysates. Anti-phosphotyrosine immunoblotting showed that many of the proteins that become tyrosine phosphorylated in response to CD3 cross-linking exhibited either minor reductions in the overall level of tyrosine phosphorylation or a shorter duration of phosphorylation (Fig. 8B). A few proteins exhibited a more pronounced sensitivity to PTEN expression. Of particular note are bands of ∼21 kDa and of ∼36 and 38 kDa, which are likely to represent TCRζ and LAT, respectively, and a band of ∼40 kDa, which remains unidentified. These three proteins all displayed CD3-stimulated tyrosine phosphorylation that was delayed, transient, and reduced in the presence of PTEN expression. The effect of PTEN overexpression on tyrosine phosphorylation of these proteins does not seem to be the consequence of decreased cell viability, since these cells responded as well as vector-transfected cells to CD3 stimulation in terms of tyrosine phosphorylation of other proteins such as ZAP-70, pp90, and pp95. Better understanding of the mechanism and significance of these events awaits further investigation.
FIG. 8.
Effect of PTEN expression on signaling pathways downstream of the TCR. JTAg T cells were transiently transfected with PTEN as described for Fig. 5 and stimulated with OKT3 for the indicated times (prime, minutes; double prime, seconds). (A) The expression level of PTEN and degree of Akt Ser-473 phosphorylation were determined by immunoblotting of whole-cell lysates. (B) The extent of protein tyrosine phosphorylation initiated upon CD3 cross-linking (OKT3) was determined by antiphosphotyrosine (4G10) Western blotting of whole-cell lysates. (C) PLC-γ1 was immunoprecipitated and blotted for phosphotyrosine (4G10) (top). The membrane was stripped and reblotted for PLC-γ1 (second panel). Erk activation was assessed either by immunoblotting of the whole-cell lysates with anti-active Erk (P-Erk) (third panel) or by detection of the electrophoretic shift of phosphorylated Erk-2 on a 10% Tris-glycine gel (bottom panel). (D) A total of 1.2 × 107 JTAg T cells were cotransfected with 10 μg of pNF-AT-Luc and 5 μg of the pβ-Gal control plasmid. Cells also received 30 μg of either the pSRα-PTEN-Flag or the pSRα-Flag (empty vector) plasmid. Lysates were prepared 16 h after transfection and were analyzed for luciferase and β-galactosidase activities. Relative light units (RLU) of the luciferase activity normalized for β-galactosidase activity are shown.
Because Tec family kinases have been implicated as regulators of PLC (54, 61, 67), we examined more closely the effect of PTEN expression upon TCR-stimulated PLC-γ1 tyrosine phosphorylation. The ability of CD3 cross-linking to induce PLC-γ1 tyrosine phosphorylation was partially attenuated in JTAg T cells expressing PTEN, compared to control-transfected cells (Fig. 8C). This was especially true of the earliest time points analyzed (15 s, 45 s, and 2 min), while the signals were more comparable at 8 and 15 min. This pattern is reminiscent of what has been reported for TCR-stimulated T cells from Rlk-Itk double-knockout mice (65), suggesting that the reduced anti-CD3-stimulated Itk kinase activity observed in PTEN-expressing JTAg T cells may be responsible for the decreased PLC-γ1 tyrosine phosphorylation. Another signaling pathway that has been shown to be sensitive to the status of Tec kinases is that of Erk activation, so the effect of PTEN expression on the ability of CD3 cross-linking to activate Erk was assessed using antisera specific for activated Erk and by detection of the electrophoretic shift induced upon Erk phosphorylation. PTEN expression had a subtle but highly reproducible effect on Erk activation, as is shown in Fig. 8C, leading to weaker and more transient Erk activation. We also examined the effect of PTEN expression upon NF-AT activity, as measured by an NF-AT luciferase reporter assay. PTEN expression caused a 50% reduction in basal NF-AT activity (Fig. 8D). Taken in aggregate, these results indicate that PTEN not only plays a role in maintaining the basal signaling tone of T cells but also plays a role in terminating signals initiated by TCR engagement.
DISCUSSION
The PH domain is a modular protein domain that affects membrane localization of proteins based on modulation of phosphoinositide lipids in the plasma membrane. Btk, the most extensively studied of the Tec family kinases, translocates to the plasma membrane via a PH domain–PI-3,4,5-P3 interaction, which is required for efficient tyrosine phosphorylation and activation, in response to antigen receptor stimulation (40, 54, 61, 67). Whether the T-cell-specific Tec family kinase Itk is regulated by a similar mechanism has only recently begun to be addressed (7, 14, 68). Our previously published observation of constitutive membrane localization of Itk in unstimulated Jurkat T cells (68), an observation that has subsequently been confirmed by two other labs (7, 14), is in disaccord with Itk following the model of Btk activation. The obvious moiety mediating Itk's aberrant membrane localization is its PH domain. Like Btk, the other Tec-family PH domains have the highest binding affinity for PI-3,4,5-P3 (54). Therefore, it seemed reasonable to consider the possibility that the metabolism of D3-phosphorylated phosphoinositides is inappropriately regulated in Jurkat T cells, leading to a basal accumulation of PI-3,4,5-P3 and inappropriate recruitment of Itk to the plasma membrane. The simplest scenario for the creation of such an imbalance in D3-phosphoinositide metabolism requires either that PI3K be hyperactive or that PTEN be hypoactive.
Given that PTEN is functionally absent in a number of human cell lines and that mutations within PTEN have been noted previously in a Jurkat subline (64), we reasoned that Jurkat T cells might lack a functional PTEN allele. This could potentially explain both the transformed phenotype of these cells and the constitutive targeting of Itk to the membrane. Indeed, we found that Jurkat leukemic T-cell lines do not express detectable levels of PTEN protein, even though PTEN could be readily detected in normal human T cells isolated from peripheral blood. The fact that the antibody cocktail that we used to detect PTEN recognizes epitopes at both the N and C termini of PTEN suggested that either no PTEN mRNA was being made or the translated protein was unstable. A recent report showing that appropriately sized PCR products could be amplified from a Jurkat cDNA library with PTEN exonic primers argued for the latter possibility (75). However, the demonstrated existence of an intronless PTEN pseudogene (ψPTEN) on chromosome 9p21 that is highly homologous to PTEN, with more than 98% identity (13, 17, 31, 42), made us concerned that this approach might be subject to false positives due to the almost unavoidable contamination of cDNA libraries with small amounts of genomic DNA. To avoid potential ψPTEN-related ambiguities, we used Northern blot analysis to directly detect PTEN transcripts in normal and JTAg T cells. This analysis revealed comparable levels of PTEN message in both JTAg and normal T cells, suggesting a posttranslational mechanism in the PTEN deficiency in Jurkat T cells.
We therefore considered the possibility that the JTAg PTEN gene contains mutations that result in an unstable translation product. Previous work by Sakai et al. indicating, by PCR–single-strand conformation polymorphism analysis, the presence of mutations in exon 7 of PTEN in a Jurkat subline caused us to focus on this region of the gene (64). In our analysis of PTEN exon 7 we found no wild-type sequences. All sequences contained one or the other of the described mutations (Fig. 2B) (64), suggesting that both PTEN alleles of JTAg are affected. Both mutations cause premature termination of PTEN translation. PTEN with a truncation within this exon is expected to have minimal phosphatase activity and to be highly unstable (33). Interestingly, PTEN mutations in this region are associated with many cases of Cowden disease and Bannayan-Zonana syndrome—human disorders associated with loss of PTEN function (55). Our results thus support the notion that mutations in both alleles of the PTEN gene in Jurkat T-cell lines encode the production of truncated PTEN that has low phosphatase activity and is subject to rapid degradation. This leads to a functional deficiency of PTEN in these cells, resulting in basal accumulation of D3-phosphorylated phosphoinositides.
It is likely that defective PTEN expression, and the resultant accumulation of PI-3,4-P2 and PI-3,4,5-P3, contributes to the transformed phenotype of Jurkat T cells. This supposition is supported by the fact that restoration of expression of PTEN ultimately leads to loss of growth and increased cell death beginning approximately 24 h after transfection (reference 75 and our unpublished observations). This effect is likely to be mediated largely by activated Akt. PI-3,4-P2 recruits Akt, via its PH domain, to the plasma membrane, where Akt becomes phosphorylated on Thr-308 by PDK1, which is juxtaposed to Akt at the membrane by virtue of high-affinity binding of the PH domain of PDK1 with PI-3,4,5-P3. Akt then autophosphorylates on Ser-473 and becomes fully active (71). Active Akt provides a strong antiapoptotic signal (10, 16, 18) and has been found to protect T cells from Fas-mediated and activation-induced cell death (21, 35, 56). It is unclear why CD3 cross-linking, which does activate PI3K in Jurkat T cells (11, 20, 26, 76), did not cause increased Akt phosphorylation. Possibly, most of the available Akt was already phosphorylated due to high levels of PI-3,4-P2 and PI-3,4,5-P3. Alternatively, this may indicate TCR-stimulated activation of other phosphatases, such as SHIP, which may have held the levels of PI-3,4-P2 and PI-3,4,5-P3 fairly constant (24).
The degree of Itk partitioning to the membrane fraction was directly correlated with the amount of Ser-473 phospho-Akt recovered from the cells. Given the close correlation between the cellular levels of PI-3,4-P2 and PI-3,4,5-P3 and the ability of Akt to become phosphorylated at Ser-473 (16, 18), this would suggest that the ability of Itk to bind to the plasma membrane is directly dependent upon the concentration of D3-phosphorylated PI in the plasma membrane. This hypothesis is supported by the observation that membrane targeting of Itk required an intact PH domain (7, 14). Both pharmacologic inhibition of PI3K and exogenous expression of PTEN could release Itk from the membrane and relocalize it to the cytosol, which is consistent with the hypothesis that the constitutive targeting of Itk to the plasma membrane was due the accumulation of PI-3,4,5-P3 in the plasma membrane as a result of the absence of PTEN expression in these cells.
The time course that we observe for the loss of Akt phosphorylation and loss of Itk from the cell membrane using inhibitors of PI3K predicts a gradual decline in PI-3,4-P2 and PI-3,4,5-P3 levels due to inefficient catabolism of basally accumulated D3-phosphorylated phosphoinositides in the absence of PTEN. This would explain why Mills and colleagues were not able to block activation of Itk by 30 min of Jurkat cell treatment with pharmacologic inhibitors of PI3K (51). The first time point at which Akt Ser-473 phosphorylation became completely undetectable was 3 h after the addition of wortmannin. By 8 h after wortmannin treatment, basal Akt phosphorylation was still undetectable; however, by this time, CD3 cross-linking could stimulate Akt phosphorylation. An explanation for these observations is perhaps provided by the fact that wortmannin is an irreversible inhibitor of PI3K that is unstable in aqueous solution (59). This being the case, it is likely that by 8 h all of the wortmannin has degraded and sufficient translation of new PI3K has occurred to support Akt phosphorylation when the PI3K was activated by TCR stimulation. Sixteen hours following wortmannin addition, sufficient PI3K would be resynthesized to support Akt activation in the absence of TCR stimulation. The ectopic expression of wild-type PTEN in JTAg T cells was also able to reduce basal Akt phosphorylation. As in the cells incubated with wortmannin for 8 h, the Akt in the PTEN-expressing cells showed increased phosphorylation in response to TCR stimulation. This seems reasonable, since PTEN and Akt should have equal access to the D3-phosphorylated phosphoinositides, allowing for Akt phosphorylation prior to catabolism of the D3-phosphorylated phosphoinositides by PTEN. Together these results provide strong evidence for the basal accumulation of D3-phosphorylated phosphoinositides in JTAg T cells as a result of the absence of PTEN activity. In addition, these data support the idea that TCR engagement itself, without a contribution from CD28, can activate the antiapoptotic pathway mediated by activated Akt in Jurkat T cells.
It has remained an open question whether or not Itk is regulated by the same general mechanism as has been demonstrated for Btk. The Btk activation model holds that efficient activation requires the activation of PI3K to create high-affinity membrane binding sites for the PH domain of Btk. This then results in the recruitment of Btk to the plasma membrane, whereupon it becomes phosphorylated by activated membrane-resident Src family PTKs (61, 67). The fact that Itk requires an intact PH domain and the presence of PI-3,4,5-P3 in the plasma membrane in order to be activated in response to CD3 cross-linking strongly supports the notion that Itk must be able to translocate to the plasma membrane as a function of the interaction between its PH domain and PI-3,4,5-P3 in order to be activated in response to TCR engagement, and is consistent with other recently published results (7, 14). However, it should be noted that membrane recruitment is not sufficient for Itk activation; additional TCR-initiated events requiring the kinase activity of ZAP-70 and expression of the membrane linker protein LAT (68) and the cytoplasmic adapter protein SLP-76 (R. L. Wange and X. Shan, unpublished data) also are required in order for Itk activation by TCR engagement to be observed.
One caveat against full conformity to the Btk model is the fact that we have been unable to detect translocation of Itk to the plasma membrane in JTAg T cells that express PTEN ectopically and are stimulated by CD3 cross-linking. This is unlikely to be a result of the overexpression of PTEN, since, in the same cells, OKT3-stimulated phosphorylation of Akt on Ser-473 could be observed. A more likely explanation is that the translocation is rapid and transient and occurs earlier than the 2-min-poststimulation time point that was analyzed in these studies. This supposition is based upon the fact that full activation of Itk kinase activity under our stimulation conditions occurs after only 45 s of stimulation (68) and that most of the active kinase is recovered in the cytosolic fraction (R. L. Wange and X. Shan, unpublished data). Nonetheless, our results are in good general agreement with the Btk-derived model of Tec family kinase activation in response to antigen receptor stimulation, and they clearly demonstrate the importance of PI3K and PTEN in regulating the subcellular redistribution and activation of Tec family kinases.
Over the past 5 years there has been substantial controversy in the literature regarding the importance of PI3K in the process of T-cell activation, especially as to whether PI3K is a necessary signaling component of the CD28 coreceptor (9, 23, 37, 38, 52, 58, 72, 77). An analysis of the literature reveals that most of the studies conducted with normal T cells have supported a role for PI3K in CD28 function (23, 58, 77), while studies carried out with Jurkat T-cell lines have consistently found PI3K to be dispensable for CD28 function in these cells (38, 52, 72). The finding that Jurkat T-cell lines lack PTEN expression, and therefore have high basal levels of D3-phosphorylated phosphoinositides, may provide an explanation for the discrepant results reported for these two model systems.
In addition to uncovering an explanation for the unexpected constitutive membrane localization of Itk to the plasma membrane, we also provide evidence that PTEN deficiency may contribute to maintaining an elevated basal signaling state in Jurkat cells. This is suggested by both the elimination of basal Akt Ser-473 phosphorylation and the 50% reduction in basal NF-AT reporter activity in cells expressing PTEN compared to those that do not. Akt and NF-AT activity are linked by GSK3, which phosphorylates NF-AT, causing nuclear expulsion of the transcription factor (4). In its active state, Akt phosphorylates and inactivates GSK3, potentially promoting enhanced NF-AT activity due to nuclear accumulation. In addition, Itk has also been more directly implicated in the activation of NF-AT (28).
Our results also indicate that PTEN may play a role in limiting TCR-initiated signals. This is suggested by the approximately 50% reduction in both Itk kinase activity and PLC-γ1 tyrosine phosphorylation in response to CD3 cross-linking, and by the more-transient Erk activation kinetics that were observed in the PTEN-expressing cells. An inhibitory effect of PTEN expression in Jurkat T cells upon Erk activation has also been reported recently (75), confirming this observation. Since Tec family kinases are thought to play a role in PLC-γ1 tyrosine phosphorylation and activation, it is intriguing that the greatest effect of PTEN expression upon TCR-stimulated signaling events, other than reduced Akt phosphorylation and Itk kinase activity, was seen upon PLC-γ1 tyrosine phosphorylation. We are currently in the process of developing JTAg cell lines that will inducibly express PTEN, and we hope that this reagent will allow us to look at the effect of PTEN expression on more-distal TCR-initiated signaling events, such as transcriptional activation and interleukin 2 production.
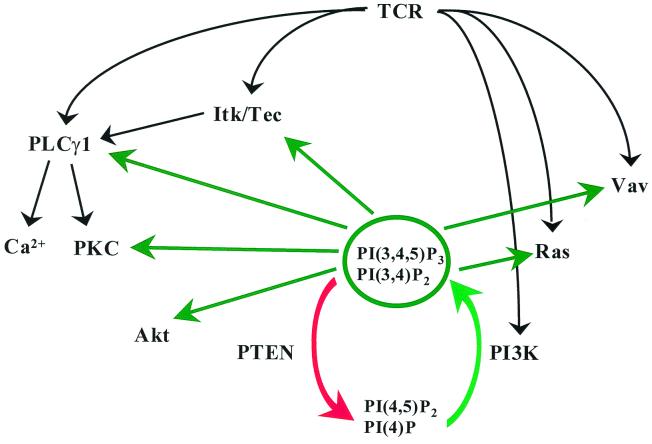
There are a host of other signaling molecules, involved in TCR signaling, that either possesses PH domains themselves or are affected by PH domain-containing proteins. Further investigation will be required in order to determine which additional signaling pathways, other than those mediated by Itk and Akt, are affected by the PTEN-deficient status of Jurkat T cells. A number of important signaling molecules that could potentially be affected by the PTEN status are depicted in Fig. 9. One would predict that Tec, the other PH-domain-containing Tec-family kinase that is expressed in T cells, would behave similarly to Itk and would also show constitutive plasma membrane association in Jurkat cells. As for Itk, the degree of Tec activation that could be achieved following TCR stimulation would be dependent upon the PTEN status of the cell. Also PLC-γ1 and Vav, which are both key effectors of TCR signaling, both possess PH domains, and PLC-γ1 has been shown to be regulated by PI3K in a PH domain-dependent manner (27). Ras pathways might be altered through the PH domain-containing upstream effectors Ras-GAP, which stimulates the GTPase activity of Ras and is thought to be a Ras effector, and SOS, which is a guanine nucleotide exchange factor for Ras. Both molecules possess PH domains with preferences for D3-phosphorylated phosphoinositides (63). All PKC isozymes have been found to be substrates for PDK1, the same PH domain-containing kinase that phosphorylates Akt (15, 22, 45). PDK1 phosphorylates a site within the activation loop of PKC; phosphorylation of this site is associated with increased kinase activity. Consequently, there is the potential that multiple TCR-initiated signaling pathways are affected by the PTEN status of the cell. However, given that TCR stimulation itself leads to PI3K activation and the accumulation of D3-phosphorylated phosphoinositides, in some ways the PTEN deficiency can be thought of as providing a partial TCR signal. In the absence of any compensatory down-modulatory effects (yet to be determined), one might expect that the biggest difference between PTEN-replete and -deficient cells would be related to the rate of onset and termination of signals. Also, with the exception of the activation of Akt, most signaling events that are regulated by PI3K also require a secondary signal of some sort, such as activation of ZAP-70 in the case of Itk and PLC-γ1 activation, or the production of diacylglycerol, in the case of PKC activation. Therefore, it seems likely that only a subset of PI3K-dependent pathways will be significantly perturbed in Jurkat T cells as a consequence of the absence of PTEN.
FIG. 9.
Multiple TCR-stimulated signaling pathways are sensitive to the level of D3-phosphorylated phosphoinositides. Opposing activities of PI3K (heavy green arrow) and PTEN (red arrow) are shown regulating the pool of D3-phosphorylated phosphoinositides. The potential for D3-phosphorylated phosphoinositides to regulate signaling molecules activated in response to TCR activation is depicted by light green arrows. Of the signaling proteins indicated, Itk, Tec, PLC-γ1, Akt, and Vav all contain PH domains that preferentially bind to PI-3,4,5-P3. Ras does not contain a PH domain, but it is subject to regulation by SOS and Ras-GAP, which both contain PI-3,4,5-P3-binding PH domains. Likewise, the various isozymes of PKC do not possess PH domains but are all phosphorylated upon their activation loop by PDK1, which has a PH domain that binds PI-3,4,5-P3 with high affinity.
It is interesting that of the more than 100 different proteins that have been found to have PH domains, only the Tec family kinases possess tyrosine kinase activity, and as we and others have shown, an intact PH domain is required for antigen receptor-mediated activation of this class of kinases. To our knowledge, no other tyrosine kinase has yet been shown to be subject to negative regulation by PTEN. It is tempting to speculate, therefore, that the ability of PTEN expression in JTAg T cells to reduce or eliminate tyrosine phosphorylation of PLC-γ1, as well as the proteins at 21 (TCRζ), 36 and 38 (LAT), and 40 kDa in response to CD3 cross-linking, indicates that these proteins are substrates of either Itk or Tec. Such a finding would be significant, given how little is known about the substrate repertoire of Tec family kinases, and would be the first indication of a role for these kinases in the phosphorylation of LAT and TCR subunits. An alternative hypothesis would be that PTEN may directly dephosphorylate these proteins via its phosphotyrosine phosphatase activity. We are currently working to differentiate between these two hypotheses.
Much of the credit for the rapid advances achieved over the last decade in our understanding of the signaling events that are initiated in T cells upon TCR stimulation has to go to the Jurkat T-cell line, which has demonstrated tremendous predictive power for the signaling events that occur in normal T cells. The advantages of this system are many and include the substantial body of data that has already been collected with this system, the ready availability of standardized reagents, and the relative ease and cost-effectiveness of growing large numbers of cells for biochemical manipulation. But what has made the Jurkat system indispensable as a means for dissecting T-cell signaling pathways has been the amenability of these cells to molecular biological manipulation, ranging from transient expression of exogenous cDNAs to the relative ease with which somatic mutations can be induced in these cells. A great many mutant Jurkat T-cell lines that lack important signaling molecules have consequently been generated. These have proven to be invaluable tools for the performance of structure-function studies of these molecules and have aided tremendously in our understanding of the roles of these molecules in TCR signaling. However, the discovery that Jurkat T cells lack a key component responsible for regulating the level of D3-phosphorylated phosphoinositides points up the need for caution in using this model system to study signaling events that are under the control of these phospholipids.
ACKNOWLEDGMENTS
We thank L. Berg, G. Crabtree, M. Karin, G. Mills, and L. E. Samelson for their kind gifts of reagents, N.-P. Weng and K. Liu for assistance in purifying normal T cells from whole blood, M.-C. Seminario for preparation of JTAg genomic DNA, and J. Wood and T. Herndon for critical review of the manuscript.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Andjelkovic M, Maira S M, Cron P, Parker P J, Hemmings B A. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19:5061–5072. doi: 10.1128/mcb.19.7.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beals C R, Sheridan C M, Turck C W, Gardner P, Crabtree G R. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 5.Besson A, Robbins S M, Yong V W. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605–611. doi: 10.1046/j.1432-1327.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 6.Blomberg N, Baraldi E, Nilges M, Saraste M. The PH superfold: a structural scaffold for multiple functions. Trends Biochem Sci. 1999;24:441–445. doi: 10.1016/s0968-0004(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 7.Bunnell S C, Diehn M, Yaffe M B, Findell P R, Cantley L C, Berg L J. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 8.Burkhardt A L, Stealey B, Rowley R B, Mahajan S, Prendergast M, Fargnoli J, Bolen J B. Temporal regulation of non-transmembrane protein tyrosine kinase enzyme activity following T cell antigen receptor engagement. J Biol Chem. 1994;269:23642–23647. [PubMed] [Google Scholar]
- 9.Cai Y C, Cefai D, Schneider H, Raab M, Nabavi N, Rudd C E. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 10.Cantley L C, Neel B G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrera A C, Rodriguez-Borlado L, Martinez-Alonso C, Merida I. T cell receptor-associated alpha-phosphatidylinositol 3-kinase becomes activated by T cell receptor cross-linking and requires pp56lck. J Biol Chem. 1994;269:19435–19440. [PubMed] [Google Scholar]
- 12.Chan T O, Rittenhouse S E, Tsichlis P N. Akt/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 13.Chiariello E, Roz L, Albarosa R, Magnani I, Finocchiaro G. Identification of PTEN-related sequences in glioma cells and in non-neoplastic cell lines. Cancer Lett. 1999;138:1–4. doi: 10.1016/s0304-3835(98)00357-7. [DOI] [PubMed] [Google Scholar]
- 14.Ching K A, Kawakami Y, Kawakami T, Tsoukas C D. Emt/Itk associates with activated TCR complexes: role of the pleckstrin homology domain. J Immunol. 1999;163:6006–6013. [PubMed] [Google Scholar]
- 15.Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C S, Newton A C, Schaffhausen B S, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 16.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahia P L, FitzGerald M G, Zhang X, Marsh D J, Zheng Z, Pietsch T, von Deimling A, Haluska F G, Haber D A, Eng C. A highly conserved processed PTEN pseudogene is located on chromosome band 9p21. Oncogene. 1998;16:2403–2406. doi: 10.1038/sj.onc.1201762. [DOI] [PubMed] [Google Scholar]
- 18.Datta S R, Brunet A, Greenberg M E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 19.Davies M A, Koul D, Dhesi H, Berman R, McDonnell T J, McConkey D, Yung W K, Steck P A. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- 20.de Aos I, Metzger M H, Exley M, Dahl C E, Misra S, Zheng D, Varticovski L, Terhorst C, Sancho J. Tyrosine phosphorylation of the CD3-epsilon subunit of the T cell antigen receptor mediates enhanced association with phosphatidylinositol 3-kinase in Jurkat T cells. J Biol Chem. 1997;272:25310–25318. doi: 10.1074/jbc.272.40.25310. [DOI] [PubMed] [Google Scholar]
- 21.Di Cristofano A, Kotsi P, Peng Y F, Cordon-Cardo C, Elkon K B, Pandolfi P P. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 22.Dutil E M, Toker A, Newton A C. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1) Curr Biol. 1998;8:1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 23.Eder A M, Dominguez L, Franke T F, Ashwell J D. Phosphoinositide 3-kinase regulation of T cell receptor-mediated interleukin-2 gene expression in normal T cells. J Biol Chem. 1998;273:28025–28031. doi: 10.1074/jbc.273.43.28025. [DOI] [PubMed] [Google Scholar]
- 24.Edmunds C, Parry R V, Burgess S J, Reaves B, Ward S G. CD28 stimulates tyrosine phosphorylation, cellular redistribution and catalytic activity of the inositol lipid 5-phosphatase SHIP. Eur J Immunol. 1999;29:3507–3515. doi: 10.1002/(SICI)1521-4141(199911)29:11<3507::AID-IMMU3507>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Exley M, Varticovski L. Evidence for phosphatidylinositol 3-kinase-dependent T cell antigen receptor (TCR) signal transduction. Mol Immunol. 1997;34:221–226. doi: 10.1016/s0161-5890(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 26.Exley M, Varticovski L, Peter M, Sancho J, Terhorst C. Association of phosphatidylinositol 3-kinase with a specific sequence of the T cell receptor zeta chain is dependent on T cell activation. J Biol Chem. 1994;269:15140–15146. [PubMed] [Google Scholar]
- 27.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowell D J, Shinkai K, Liao X C, Beebe A M, Coffman R L, Littman D R, Locksley R M. Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 29.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 30.Fruman D A, Rameh L E, Cantley L C. Phosphoinositide binding domains: embracing 3-phosphate. Cell. 1999;97:817–820. doi: 10.1016/s0092-8674(00)80792-8. [DOI] [PubMed] [Google Scholar]
- 31.Fujii G H, Morimoto A M, Berson A E, Bolen J B. Transcriptional analysis of the PTEN/MMAC1 pseudogene, psiPTEN. Oncogene. 1999;18:1765–1769. doi: 10.1038/sj.onc.1202492. [DOI] [PubMed] [Google Scholar]
- 32.Furnari F B, Huang H J, Cavenee W K. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–5008. [PubMed] [Google Scholar]
- 33.Georgescu M M, Kirsch K H, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 35.Hausler P, Papoff G, Eramo A, Reif K, Cantrell D A, Ruberti G. Protection of CD95-mediated apoptosis by activation of phosphatidylinositide 3-kinase and protein kinase B. Eur J Immunol. 1998;28:57–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<57::AID-IMMU57>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Heyeck S D, Wilcox H M, Bunnell S C, Berg L J. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J Biol Chem. 1997;272:25401–25408. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 37.Hutchcroft J E, Bierer B E. Signaling through CD28/CTLA-4 family receptors: puzzling participation of phosphatidylinositol-3 kinase. J Immunol. 1996;156:4071–4074. [PubMed] [Google Scholar]
- 38.Hutchcroft J E, Franklin D P, Tsai B, Harrison-Findik D, Varticovski L, Bierer B E. Phorbol ester treatment inhibits phosphatidylinositol 3-kinase activation by, and association with, CD28, a T-lymphocyte surface receptor. Proc Natl Acad Sci USA. 1995;92:8808–8812. doi: 10.1073/pnas.92.19.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez C, Jones D R, Rodriguez-Viciana P, Gonzalez-Garcia A, Leonardo E, Wennstrom S, von Kobbe C, Toran J L, Borlado L, Calvo V, Copin S G, Albar J P, Gaspar M L, Diez E, Marcos M A, Downward J, Martinez A, Merida I, Carrera A C. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawakami Y, Kitaura J, Hata D, Yao L, Kawakami T. Functions of Bruton's tyrosine kinase in mast and B cells. J Leukoc Biol. 1999;65:286–290. doi: 10.1002/jlb.65.3.286. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami Y, Yao L, Miura T, Tsukada S, Witte O N, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S K, Su L K, Oh Y, Kemp B L, Hong W K, Mao L. Alterations of PTEN/MMAC1, a candidate tumor suppressor gene, and its homologue, PTH2, in small cell lung cancer cell lines. Oncogene. 1998;16:89–93. doi: 10.1038/sj.onc.1201512. [DOI] [PubMed] [Google Scholar]
- 43.Kung P, Goldstein G, Reinherz E L, Schlossman S F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 44.Leevers S J, Vanhaesebroeck B, Waterfield M D. Signaling through phosphoinositide 3-kinases: the lipids take center stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 45.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 46.Li D M, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA. 1998;95:15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Rawlings D J, Park H, Kato R M, Witte O N, Satterthwaite A B. Constitutive membrane association potentiates activation of Bruton tyrosine kinase. Oncogene. 1997;15:1375–1383. doi: 10.1038/sj.onc.1201308. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Wahl M I, Eguinoa A, Stephens L R, Hawkins P T, Witte O N. Phosphatidylinositol 3-kinase-gamma activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci USA. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao X C, Littman D R. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 50.Liu K Q, Bunnell S C, Gurniak C B, Berg L J. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Cuevas B, Gibson S, Khan H, LaPushin R, Imboden J, Mills G B. Phosphatidylinositol 3-kinase is required for CD28 but not CD3 regulation of the TEC family tyrosine kinase EMT/ITK/TSK: functional and physical interaction of EMT with phosphatidylinositol 3-kinase. J Immunol. 1998;161:5404–5412. [PubMed] [Google Scholar]
- 52.Lu Y, Phillips C A, Trevillyan J M. Phosphatidylinositol 3-kinase activity is not essential for CD28 costimulatory activity in Jurkat T cells: studies with a selective inhibitor, wortmannin. Eur J Immunol. 1995;25:533–537. doi: 10.1002/eji.1830250234. [DOI] [PubMed] [Google Scholar]
- 53.Maehama T, Dixon J E. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 54.Mano H. Tec family of protein-tyrosine kinases: an overview of their structure and function. Cytokine Growth Factor Rev. 1999;10:267–280. doi: 10.1016/s1359-6101(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 55.Marsh D J, Coulon V, Lunetta K L, Rocca-Serra P, Dahia P L, Zheng Z, Liaw D, Caron S, Duboue B, Lin A Y, Richardson A L, Bonnetblanc J M, Bressieux J M, Cabarrot-Moreau A, Chompret A, Demange L, Eeles R A, Yahanda A M, Fearon E R, Fricker J P, Gorlin R J, Hodgson S V, Huson S, Lacombe D, Eng C. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998;7:507–515. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- 56.Mok C L, Gil-Gomez G, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady H J. Bad can act as a key regulator of T cell apoptosis and T cell development. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myers M P, Pass I, Batty I H, Van der Kaay J, Stolarov J P, Hemmings B A, Wigler M H, Downes C P, Tonks N K. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 59.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 60.Ramaswamy S, Nakamura N, Vazquez F, Batt D B, Perera S, Roberts T M, Sellers W R. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rawlings D J. Bruton's tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin Immunol. 1999;91:243–253. doi: 10.1006/clim.1999.4732. [DOI] [PubMed] [Google Scholar]
- 62.Rawlings D J, Witte O N. The Btk subfamily of cytoplasmic tyrosine kinases: structure, regulation and function. Semin Immunol. 1995;7:237–246. doi: 10.1006/smim.1995.0028. [DOI] [PubMed] [Google Scholar]
- 63.Rebecchi M J, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Annu Rev Biophys Biomol Struct. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- 64.Sakai A, Thieblemont C, Wellmann A, Jaffe E S, Raffeld M. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–3415. [PubMed] [Google Scholar]
- 65.Schaeffer E M, Debnath J, Yap G, McVicar D, Liao X C, Littman D R, Sher A, Varmus H E, Lenardo M J, Schwartzberg P L. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 66.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawlings D J, Kinet J P. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scharenberg A M, Kinet J P. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell. 1998;94:5–8. doi: 10.1016/s0092-8674(00)81214-3. [DOI] [PubMed] [Google Scholar]
- 68.Shan X, Wange R L. Itk/Emt/Tsk activation in response to CD3 cross-linking in Jurkat T cells requires ZAP-70 and Lat and is independent of membrane recruitment. J Biol Chem. 1999;274:29323–29330. doi: 10.1074/jbc.274.41.29323. [DOI] [PubMed] [Google Scholar]
- 69.Shi J, Cinek T, Truitt K E, Imboden J B. Wortmannin, a phosphatidylinositol 3-kinase inhibitor, blocks antigen-mediated, but not CD3 monoclonal antibody-induced, activation of murine CD4+ T cells. J Immunol. 1997;158:4688–4695. [PubMed] [Google Scholar]
- 70.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 71.Toker A, Newton A C. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 72.Truitt K E, Shi J, Gibson S, Segal L G, Mills G B, Imboden J B. CD28 delivers costimulatory signals independently of its association with phosphatidylinositol 3-kinase. J Immunol. 1995;155:4702–4710. [PubMed] [Google Scholar]
- 73.Varnai P, Rother K I, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- 74.Von Willebrand M, Jascur T, Bonnefoy-Berard N, Yano H, Altman A, Matsuda Y, Mustelin T. Inhibition of phosphatidylinositol 3-kinase blocks T cell antigen receptor/CD3-induced activation of the mitogen-activated kinase Erk2. Eur J Biochem. 1996;235:828–835. doi: 10.1111/j.1432-1033.1996.00828.x. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Gjorloff-Wingren A, Saxena M, Pathan N, Reed J C, Mustelin T. The tumor suppressor PTEN regulates T cell survival and antigen receptor signaling by acting as a phosphatidylinositol 3-phosphatase. J Immunol. 2000;164:1934–1939. doi: 10.4049/jimmunol.164.4.1934. [DOI] [PubMed] [Google Scholar]
- 76.Ward S G, Ley S C, MacPhee C, Cantrell D A. Regulation of D-3 phosphoinositides during T cell activation via the T cell antigen receptor/CD3 complex and CD2 antigens. Eur J Immunol. 1992;22:45–49. doi: 10.1002/eji.1830220108. [DOI] [PubMed] [Google Scholar]
- 77.Ward S G, Wilson A, Turner L, Westwick J, Sansom D M. Inhibition of CD28-mediated T cell costimulation by the phosphoinositide 3-kinase inhibitor wortmannin. Eur J Immunol. 1995;25:526–532. doi: 10.1002/eji.1830250233. [DOI] [PubMed] [Google Scholar]
- 78.Wick W, Furnari F B, Naumann U, Cavenee W K, Weller M. PTEN gene transfer in human malignant glioma: sensitization to irradiation and CD95L-induced apoptosis. Oncogene. 1999;18:3936–3943. doi: 10.1038/sj.onc.1202774. [DOI] [PubMed] [Google Scholar]