Abstract
Genome integrity is essential for life and, as a result, DNA repair systems evolved to remove unavoidable DNA lesions from cellular DNA. Many forms of life possess the capacity to remove interstrand DNA cross-links (ICLs) from their genome but the identity of the naturally-occurring, endogenous substrates that drove the evolution and retention of these DNA repair systems across a wide range of life forms remains uncertain. In this review, we describe more than a dozen chemical processes by which endogenous ICLs plausibly can be introduced into cellular DNA. The majority involve DNA degradation processes that introduce aldehyde residues into the double helix or reactions of DNA with endogenous low molecular weight aldehyde metabolites. A smaller number of the cross-linking processes involve reactions of DNA radicals generated by oxidation.
Keywords: DNA damage, interstrand cross-links, ICL, DNA repair, endogenous DNA damage
Unavoidable DNA damage and its repair
DNA provides the blueprint and operating instructions for every living organism on earth [1]. The genetic information encoded in the sequence of DNA nucleobases must be maintained for the entire lifetime of a cell, many years in some cases [2]. Some elements of DNA structure are exceptionally stable. For example, the half-life for spontaneous hydrolysis of the phosphodiester linkages in the DNA backbone is 3 × 107 y [3]. However, there are a number of chemical “weak spots” that render the DNA in cells susceptible to reactions that alter its structure and function. As a result, cellular DNA continually sustains low levels of unavoidable damage induced by reactions with water, reactive oxygen species, and aldehyde metabolites [4–6].
For example, cytosine and 5-methylcytosine residues undergo spontaneous and enzyme-catalyzed hydrolytic deamination to yield mispaired uracil and thymine residues in duplex DNA [7–12], a process that leaves a common mutational signature in the genetic code of living organisms [13]. The generation of reactive oxygen species in cells[14, 15] leads to DNA strand breaks via reactions with the sugar-phosphate backbone[16, 17] and oxidatively-damaged nucleobases such as 8-oxoguanine and formamidopyrimidines (Fapy) [18–20]. It is important to recognize that, given the large number of nucleotides in a genome (e.g. 12.8 billion in a diploid human cell) [21] even very inefficient reactions can produce significant numbers of DNA lesions. For example, the spontaneous hydrolytic loss of purine residues from DNA (depurination) occurs with a half-life of 730 y, yet this process generates more than 10,000 apurinic (AP) sites per day in each human cell [5, 22, 23]. As an interesting aside, a number of unavoidable DNA degradation processes (possibly including some that generate interstrand cross-links) [24] continue after cell death and limit the ability to recover sequence information from ancient DNA samples [25–27].
Error-prone replication of damaged DNA can introduce mutations in the genetic code [13, 28, 29]. Mutations contribute to the evolution of species [12, 30–33] but, generally, are either silent or detrimental to individual organisms [34–36]. For example, in mammals, mutations and genomic instability arising from DNA damage may contribute to cancer, aging, and neurodegeneration [37–43].
The relentless nature of endogenous DNA damage combined with requirements for genetic stability in living organisms, creates a selection pressure that has driven the evolution and retention of DNA repair systems across all forms of life [36, 44–52]. The human genome contains at least 130 genes encoding proteins involved in DNA repair [53]. DNA is the only molecule in the cell that benefits from such an extensive repair network. Logically, repair systems evolved to handle the common types of unavoidable DNA damage that block replication or cause genetic instability (e.g. mutations) [44–46, 48, 52]. In other words, endogenous DNA damage and replication errors [54] generate the substrates that explain the existence of most known DNA-repair systems. For example, the base excision repair enzyme uracil DNA glycosylase (UDG) excises uracil residues that arise from the unavoidable hydrolytic deamination of cytosine residues or the misincorporation of 2’-deoxyuridine in cellular DNA [12, 45, 55–58].
Cross-link repair pathways exist – but what are the endogenous substrates?
Interestingly, organisms ranging from bacteria to plants to humans possess the capacity to repair interstrand DNA-DNA cross-links (ICLs), in which the two complementary strands of the double helix are covalently bonded to one another [59–61]. The removal of ICLs from cellular DNA was first studied more than 50 years ago in the context of xenobiotics such as sulfur mustards, nitrogen mustards, mitomycin C, psoralens, and cisplatin [62–65]. However, it seems unlikely that ICL repair pathways evolved to protect cells from such external threats, given that agents of this type are not ubiquitous in the environment. Rather, the existence of ICL repair pathways across many diverse forms of life suggests that the generation of ICL(s) in genomic DNA is an unavoidable consequence of cellular life. However, the identity of these naturally-occurring ICLs remains uncertain. In this review, we consider endogenous chemical processes with the potential to generate ICLs in the DNA of living organisms.
Repair of Interstrand Cross-links in Cellular DNA.
Before considering the formation and structure of potential endogenous ICLs, it may be useful to briefly review the repair of ICLs in vertebrates. ICLs are exceptionally toxic [66] because they block the strand separation required for read-out and replication of genetic information embedded in the nucleotide sequence of DNA [67–69]. Cells really have “no good options” for dealing with cross-links in their DNA. Unrepaired cross-links may lead to cell senescence, cell death, and tissue dysfunction [37, 70–72]. On the other hand, the repair of cross-links can be error-prone, with the potential to generate genetic mutations and deletions [73–77]. In humans, endogenous ICLs may contribute to aging, neurodegeneration, and cancer [37–43].
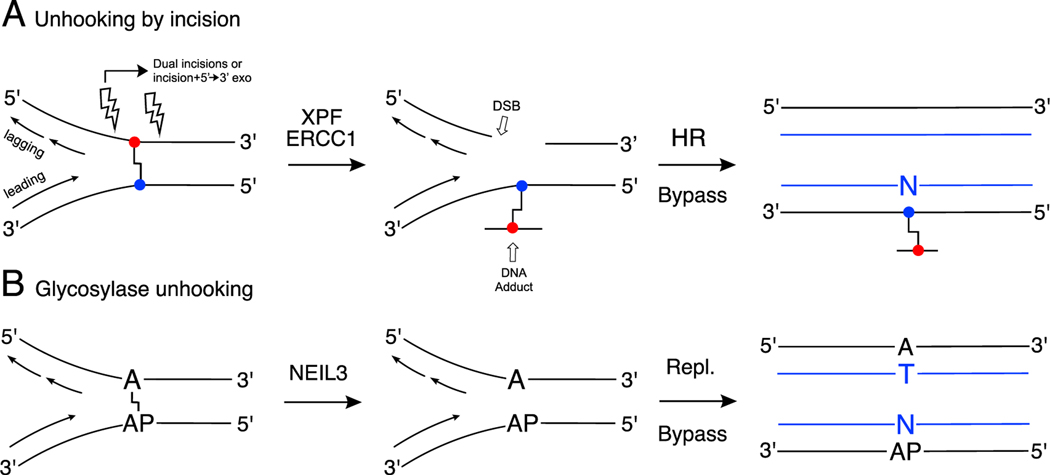
The majority of ICL repair occurs during DNA replication [78–80]. When replication forks collide with an ICL, complex DNA damage response pathways are activated [67, 80, 81]. The Fanconi anemia pathway is critical for vertebrate cross-link repair [40, 42, 82, 83]. Association of the FANCI-FANCD2 proteins with a stalled replication fork is followed by ubiquitylation of this protein complex [67, 84–86]. This, in turn, leads to recruitment of nucleases that make incisions to “unhook” the cross-link (these incisions are depicted by lightning bolts in Scheme 1A) [67, 87, 88]. The endonuclease XPF-ERCC1 and the scaffolding protein SLX4 are critical in the incision process [42, 89, 90]. A number of other endonucleases that may be involved in cross-link unhooking have been identified, including MUS81-EME1, SLX1, FAN1, and SNM1A, although the circumstances under which they become involved remains uncertain [42, 89, 91–93]. There is evidence that the 5’→3’ exonuclease activity of SNM1A may digest through the ICL after the initial incision, thereby alleviating the need for a second incision [93– 96]. After unhooking, the resulting ICL remnant – now simply a large DNA adduct – is bypassed by error-prone translesion synthesis (TLS) polymerases such as pols η, κ, ι, and ν (eta, kappa, iota, and nu).[97–100] Subsequent extension after bypass often involves Rev1 and pol ζ (zeta) [67, 101–103]. The double-strand break induced by the incision(s) is repaired by RAD51-dependent homologous recombination [67, 73, 77, 104–106].
Scheme 1.
Simplified views of ICL repair pathways. Panel A: Dual incision, Fanconi- Anemia pathway (DSB = double strand break, HR = homologous recombination, Bypass involves translesion synthesis polymerases). Panel B: NEIL3-dependent unhooking of the dA-AP ICL (Repl. indicates normal error-free replication). Newly synthesized strands are shown in blue (right side).
Fanconi anemia is a clinically heterogeneous disease caused by mutations in one of twenty two proteins involved in replication-coupled ICL repair [107, 108]. Patients display symptoms that can include bone marrow failure, congenital skeletal anomalies, and increased risk of acute myeloid leukemia and other cancers [107–110]. A defining feature of the disease is that patient’s cells display genomic instability and hypersensitivity to interstrand cross-linking agents [111]. Thus, the symptoms of Fanconi anemia are thought to arise from failure to resolve endogenous ICLs during replication; however, as noted above, the nature of the unavoidable ICLs in cellular DNA that drive disease progression is not known [40, 112, 113]. The molecular events underlying the symptoms experienced by individuals with Fanconi anemia may extend beyond DNA repair deficits [114].
Several other ICL repair pathways have been described. Semlow et al. recently discovered an unprecedented repair pathway in which the base excision repair enzyme NEIL3 unhooks ICLs derived from AP sites in DNA and from psoralen (Scheme 1B) [115–117]. This pathway is discussed below in the context of AP- and acetaldehyde-derived ICLs. Seidman’s group discovered a FANCM-dependent ICL traverse that defers repair until after replication [118, 119]. Finally, replication-independent repair is important in post-mitotic and rarely dividing cells [120–23].
The three-dimensional structure of an ICL in duplex DNA may be important in replication-independent recognition and repair, with more distorted ICLs recognized and repaired more readily than those that retain normal B-DNA-like structures [120–123]. On the other hand, the three-dimensional structure adopted by cross-linked DNA may not be directly relevant to replication-dependent repair in which stalling of advancing replication forks generates strand-separated, Y-shaped, or X-shaped structures at the cross-link [89]. The capacity of the BER glycosylase NEIL3 to unhook various ICLs likely depends on the chemical stability of glycosidic bonds or imine linkages at the cross-linking site [117].
The role of aldehydes in the formation of endogenous ICLs is rooted in their unique chemical properties.
Subsequent sections of this review describe reactions of DNA with a variety of endogenous, low molecular weight aldehydes. As a result, it may be useful to examine some general properties of the aldehyde functional group that are relevant to its DNA-modifying properties. Aldehyde functional groups are electrophiles that can covalently modify both proteins and nucleic acids [124–128]. The reactivity of aldehydes (and their corresponding iminium ions) is most commonly associated with toxicity in biological systems [124–133], but has been exploited in the development of covalent enzyme inhibitors [134–137], probes for the identification of protein kinase substrates [138], preparation of protein conjugates,[139–141] characterization of macromolecular interactions in cells [142–144], ribosome-targeting antibiotics [145], and methods for fixing biological samples [143, 146].
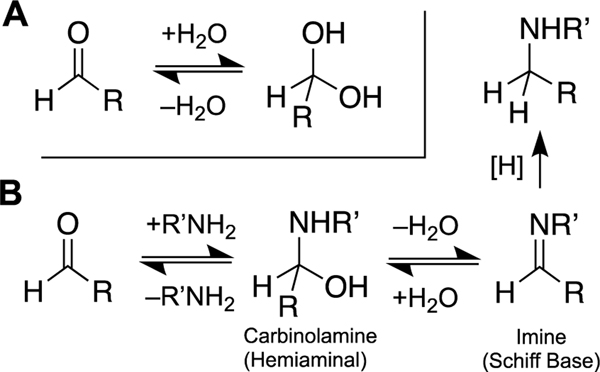
Nucleophiles readily attack the electrophilic carbon atom of the aldehyde carbonyl group (Scheme 2) [147, 148]. However, unlike most classical DNA-modifying electrophiles, such as epoxides or alkylsulfonates, the reactivity of aldehydes is not irreversibly quenched by reaction with nucleophiles such as water. Rather, water and many other nucleophiles including thiols add rapidly, but reversibly, to the aldehyde (Scheme 2A) [147, 149–152]. The presence of thiols does not prevent the reaction of an aldehyde with nitrogen nucleophiles, though thiols may depress the rates of these reactions (see Figure 4 in ref [153]). Thus, aldehydes can diffuse through biological systems, attaching reversibly to water, thiols, and other biomolecules, until they come to rest at thermodynamically-favored locations.
Scheme 2.
Reversible addition of nucleophiles to aldehydes. Panel A: Reversible hydration of an aldehyde. Panel B: The reaction of an amine with an aldehyde reversibly generates an imine. When the reaction is conducted in the presence of a water-compatible hydride donor such as NaBH3CN, the equilibria are drawn irreversibly forward to generate good yields of the corresponding alkylamine. This type of reaction is known as a “reductive amination of an aldehyde”.
Nucleobases react readily with aldehyde functional groups to generate DNA adducts [6, 129, 131, 154–162]. Indeed, most of the processes considered as candidates for the generation of endogenous ICLs in this review involve reversible reactions of aldehydes with nucleophilic nitrogen atoms in the DNA bases. Aldehydes react reversibly with both endocyclic and exocyclic nitrogen atoms in the nucleobases [154, 155]. Generally, adducts on the exocyclic NH2 groups are formed more slowly, but display greater kinetic stability and are more readily characterized [154]. Attack of an amine group on an aldehyde residue generates a carbinolamine (also sometimes referred to as a hemiaminal, Scheme 2B) [154, 163, 164]. This can be followed by dehydration to produce the corresponding imine (also known as a Schiff base, Scheme 2B) [163, 164]. While the mechanism and rate of imine formation have been studied in detail [163, 164], relatively little is known about the factors that control the equilibrium yields of imine resulting from the reactions of various amines and aldehydes [165].
Imines can be irreversibly converted to the corresponding alkylamine adduct by hydride (H:–) reducing agents, such as sodium cyanoborohydride (NaBH3CN), sodium borohydride, or sodium triacetoxyborane (Scheme 2B) [157, 166–173]. Biological agents such as glutathione, NAD(P)H, and ascorbate also have the potential to reduce some nucleobase-imine adducts [174–176]. Imines derived from enolizable aldehydes also have the potential to generate enamines.[177]
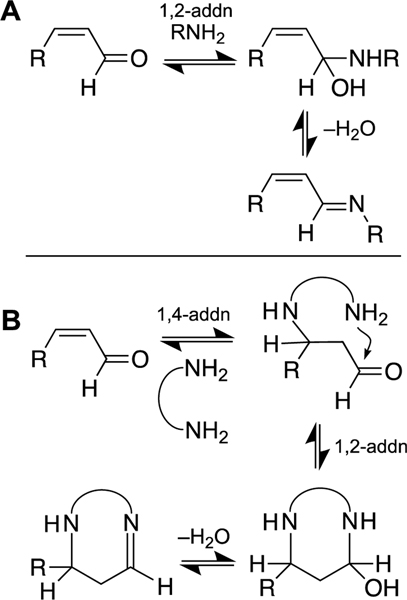
The nucleobases of DNA also react readily with α,β-unsaturated aldehydes [156, 160, 178–182]. The addition of nucleophiles to α,β-unsaturated aldehydes can occur either at the aldehyde (1,2-addition) or the double bond (conjugate, 1,4-addition, Scheme 3) [156, 160, 178–182]. Cyclic adducts and cross-links can result from a sequence of reactions involving initial 1,4-addition of a nucleophile, followed by 1,2-addition of a second nucleophilic center to the aldehyde unit (Scheme 3B) [130, 156, 160, 178–182].
Scheme 3.
Addition of amines to α,β-unsaturated aldehydes. Panel A: Reversible 1,2-addition to the aldehyde group. Panel B: Sequential 1,4-addition and 1,2-addition reactions can generate cyclic DNA adducts or DNA ICLs in which two nitrogen atoms are bridged by a 3-carbon, “propano” linker. There are a number of stereochemical considerations in these reactions: the configuration of the alkene in the α,β-unsaturated aldehyde can be E or Z (cis or trans), 1,4- addition has the potential to generate a new stereocenter, carbinolamine formation generates a new stereocenter, and imines can exist in E or Z configurations.
ICLs derived from abasic sites in cellular DNA.
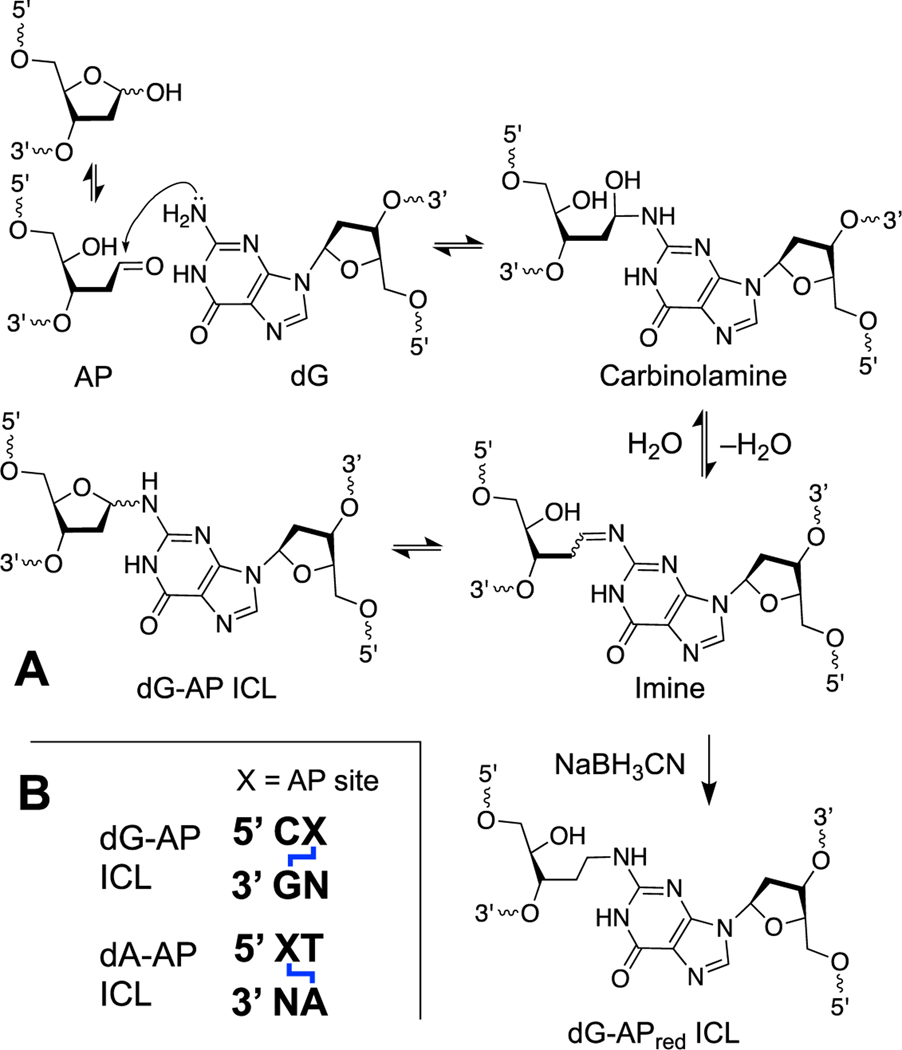
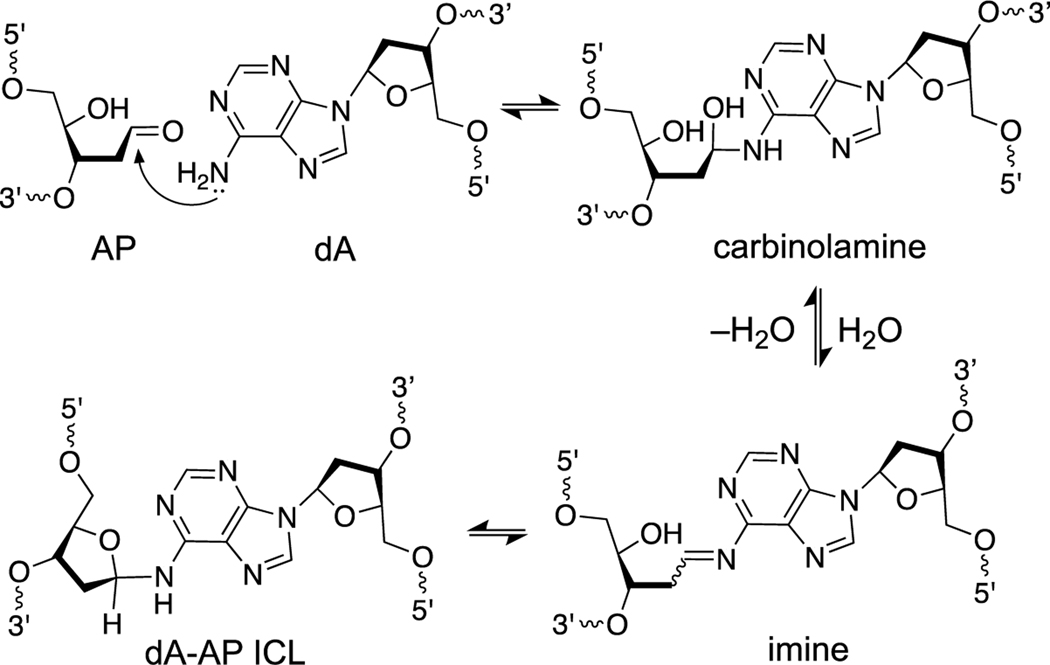
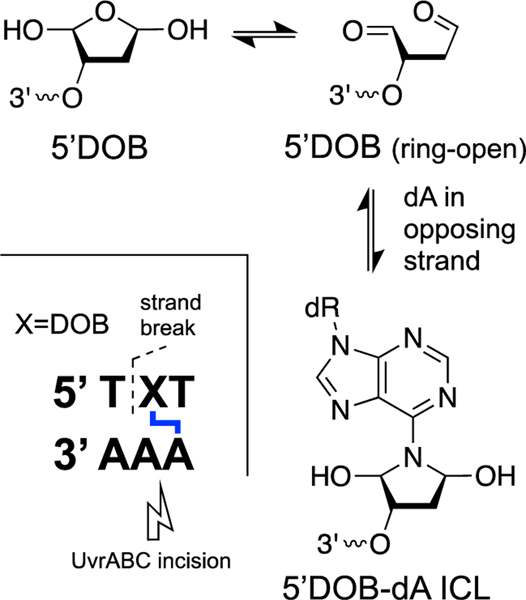
Abasic (apurinic, AP) sites are generated by the spontaneous [5, 183, 184] and enzymatic [10, 55, 56, 185, 186] release of nucleobases from the DNA backbone and are among the most common unavoidable lesions present in cellular DNA [23]. The AP sugar residue in DNA exists as an equilibrating mixture of the ring-closed hemiacetal (99%) and the ring-opened aldehyde (1%, Scheme 4) [187]. Thus, an AP site in DNA positions an electrophilic aldehyde group on the interior of the double helix. Reactions of the AP aldehyde with adenine or guanine residues on the opposing strand of duplex DNA generate ICLs [188–191].
Scheme 4.
Panel A: Formation mechanism and chemical structure of native and reduced dG-AP ICLs. Panel B: DNA sequence motifs that support formation of dG-AP and dA-AP ICLs.
The dG-AP ICL has been observed in sequences where a guanine residue is offset one nucleotide to the 5’-side of the AP site on the opposing strand (Scheme 4) [188, 189]. Typical equilibrium yields of this ICL formed in duplex DNA are in the range of 2–3% [189]. Significantly higher (approximately 20%) yields of the corresponding chemically stable alkylamine ICL (dG-APred) can be generated when the reaction is carried out in the presence of the water-compatible hydride reagent, NaBH3CN (Scheme 4) [188, 189, 192–194]. This reductive amination reaction draws equilibria forward via irreversible reduction of the imine and has been exploited for the preparation of DNA duplexes containing structurally-defined, chemically-stable, site-specific ICLs [193, 194]. Significantly higher yields of dG-APred have been observed in sequences containing mispaired bases near the AP site [195]. It remains to be seen whether biological agents such as glutathione, ascorbate, or NAD(P)H can stabilize the dG-AP ICL by reduction.
The dA-AP ICL has been observed in sequences where an adenine residue is offset one nucleotide to the 3’-side of the AP site on the opposing strand (Schemes 4B and 5) [190, 196]. Typical equilibrium yields of the dA-AP ICL in duplex DNA range from 15–70% depending upon the flanking sequences [190]. Reduction of the dA-AP ICL by chemical or biological reagents has not been described. In some cases, ICL yields are dramatically increased by the presence of mispaired bases near the AP site [195, 196].
Scheme 5.
Formation mechanism and chemical structure of the dA-AP ICL.
The formation of AP-derived ICLs can be detected by gel electrophoretic analysis within minutes of AP site generation in duplex DNA though the reactions require approximately 48–100 h to reach final equilibrium yields [189–191]. Once formed, the AP-derived ICLs are quite stable. The dissociation of the isolated dG-AP ICL occurs with a half-life of about 160 h and dissociation of the dA-AP ICL occurs with a half-life of 60–90 h, depending upon the sequence context (37 ˚C, pH 7) [192, 197].
Importantly, the dA-AP ICL is sufficiently stable to block DNA replication by a highly processive, strand-displacing polymerase [198]. Similarly, in single molecule experiments, the dA-AP ICL is stable against dissociation when the flanking DNA on one side is unzipped in a protein nanopore [196]. The kinetic stability of the AP-derived ICLs may stem from the fact that they exist as cyclic aminoglycosides, rather than as the hydrolytically-labile imine form [192, 197, 199]. Literature precedents indicate that cyclic nucleobase adducts of this type, including N6-glycosyladenine, N2-tetrahydropyranoyl-dG, N2-tetrahydrofuranosyl-dG, N2-glucopyranosyl-dG and N2-ribosyl-dG derivatives, possess substantial stability [200–204].
The preferred sequence motifs for the dA-AP and dG-AP ICLs reflect an interplay of the right-handed helical twist of DNA and the groove location of the exocyclic NH2 groups involved in the cross-links [194, 205]. These structural issues are illustrated by the recently solved NMR structure (pdb code 6xah) of the dA-AP ICL located in a 5’CXT/5’AAG sequence (where X=AP and the underlined A is the site of ICL attachment) [206]. The NMR structure shows that the dA-AP cross-link in duplex DNA exists in the ring-closed (aminoglycoside) form and, at least in this sequence context, is exclusively in the β configuration. Juxtapositioning of the N6-dA amino group and C1 of the AP site within bonding distance, while maintaining the flanking unpaired adenine and thymine bases stacked within the duplex is achieved by a modest unwinding of the DNA at the site of the ICL [206]. Otherwise, the duplex retains a normal B-DNA structure. The relatively undistorted nature of the dA-AP ICL may limit recognition and removal of this lesion by replication-independent repair [120, 121, 123].
Recent studies by Walters’ group identified an unprecedented pathway for repair of the dA-AP ICL. They showed that the dA-AP ICL blocks DNA replication in Xenopus egg extracts and is unhooked at the stalled replication fork by the DNA glycosylase NEIL3 (Scheme 1B) [116, 117]. Unhooking of the dA-AP ICL by NEIL3 regenerates the native adenine residue and an AP site. Translesion synthesis across the AP site is error-prone [207], but the NEIL3 unhooking pathway evades double-strand breaks, homologous recombination, and bypass of a bulky adduct that can introduce point mutations and deletions. NEIL3 was shown to be the “front line” mechanism for unhooking of the dA-AP ICL – engaging before the Fanconi anemia pathway is activated by the stalled replication fork. When the NEIL3 unhooking pathway was inhibited by various means, the classical Fanconi anemia pathway engaged to resolve the dA-AP ICL [116].
In biochemical experiments, the glycosylase domain of NEIL3 is competent to unhook the dA-AP ICL selectively in splayed-arm substrates that mimic the strand-separated DNA at the leading edge of a replication fork [116, 208]. In Xenopus extracts, replication-dependent unhooking of dA-AP by NEIL3 requires both the glycosylase domain and the C-terminal domains of the enzyme [115]. NEIL3 is recruited to the dA-AP ICL by association of the tandem GRF1 and GRF2 zinc finger domains with regions of single-stranded DNA at the leading edge of the stalled replication fork[209] and binding of the NPL4-type zinc-finger (NZF) domain to ubiquitin chains that are added to the stalled CMG helicase by the E2-ubiquitin ligase TRAIP [115]. The GRF domains also regulate NEIL3 activity on splayed substrates such as the dA-AP ICL.[209] The discovery of this specialized NEIL3 repair pathway for unhooking of the dA-AP ICL may provide circumstantial evidence that this ICL is important in living systems [115, 116, 208].
Complex ICLs arising from strand breaks at abasic sites in duplex DNA.
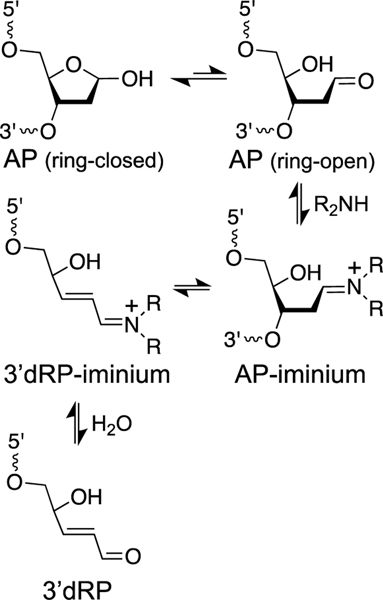
As noted above, AP sites are common, unavoidable lesions in cellular DNA [5, 22, 23]. AP sites can be converted to DNA strand breaks by β-elimination of the 3’-phosphate group (Scheme 6) [183]. This reaction can be catalyzed by biogenic polyamines such as spermine, spermidine, and putrescine [168, 183, 210, 211] and by amine residues on proteins and peptides [171, 212]. The amine-catalyzed elimination reaction proceeds via iminium ion intermediates to produce an α,β-unsaturated sugar remnant (referred to here at the 3’dRP group) on the 3’-terminus of the resulting strand break [213]. Mass spectroscopic studies have detected the 3’dRP lesion in cellular DNA [214, 215].
Scheme 6.
Elimination of the 3’-phosphoryl group from an abasic (AP) site generates electrophilic α,β-unsaturated iminium ion (3’dRP-iminium) and α,β-unsaturated aldehyde (3’dRP) sugar remnants in duplex DNA.
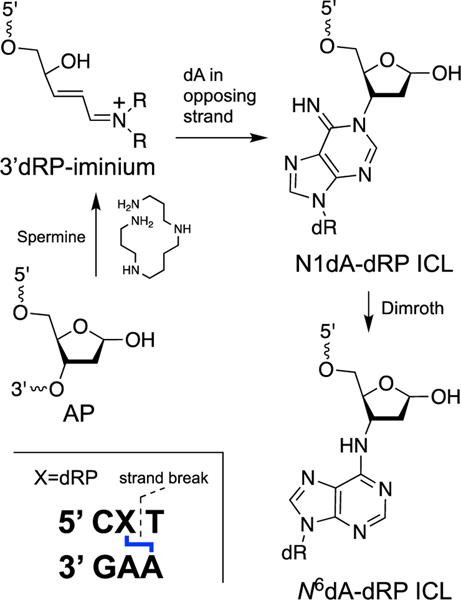
Recent work has shown that the electrophilic α,β-unsaturated iminium ion or aldehyde generated by cleavage of an AP site in duplex DNA can lead to formation of an ICL at the strand break. Specifically, spermine-mediated strand cleavage at an abasic site located in a 5’CXT/5’AAG sequence (where X = AP site and the ICL attachment is to the underlined A) leads to cross-link formation by reaction with an adenine residue on the opposing strand (Scheme 7) [117]. The ICL is chemically-stable and is a complete block to DNA replication by the strand-displacing ϕ29 DNA polymerase [117]. Chemical structure(s) of the ICL consistent with the data are shown in Scheme 7. Chemical precedents gleaned from reactions of the low molecular weight α,β-unsaturated aldehyde acrolein with 2’-deoxyadenosine and polydeoxyadenylic acid suggest that ICL formation in this case proceeds via conjugate addition of N1-dA to the 3’dRP end group [182, 216]. An alternative N6-dA attachment (indistinguishable on the basis of mass spectrometric data) could arise either via direct N6-attack or Dimroth rearrangement of an initial N1-adduct [182].
Scheme 7.
Spermine-catalyzed generation of a strand break in duplex DNA, followed by attack of an adenine residue from the opposing strand on the 3’dRP sugar remnant, produces a complex (clustered) lesion composed of an ICL adjacent to a DNA strand break.
In light of the propensity for various nucleobases to form adducts with α,β-unsaturated aldehydes [126, 180–182, 216–220], it seems likely that strand cleavage at abasic sites can give rise to a family of ICLs derived from reactions of various nucleobases with the 3’-α,β-unsaturated sugar remnant. The general architecture of the dA-dRP lesion is analogous to that of the ICLs generated by 5’-dioxobutane lesions and C4’-oxidized abasic sites described below.
Acetaldehyde-derived ICLs.
Acetaldehyde is mutagenic and carcinogenic in cellular and animal experiments [221, 222]. Exposure to acetaldehyde comes from a variety of sources including various combustion processes, bacterial metabolism of glucose, and its presence in foods [126, 223, 224]. Most human exposure to acetaldehyde is, strictly speaking, not from endogenous sources. Rather, acetaldehyde arises primarily from the metabolism of ethanol to acetaldehyde [221, 222]. The acetaldehyde generated by ethanol oxidation is further metabolized to acetate by the enzyme ALDH2 [221, 222]. Individuals with genetic variations that compromise the function of ALDH2 have an increased risk of alcohol-related cancers, presumably due to elevated levels of acetaldehyde-derived DNA damage experienced following ethanol consumption [221, 222].
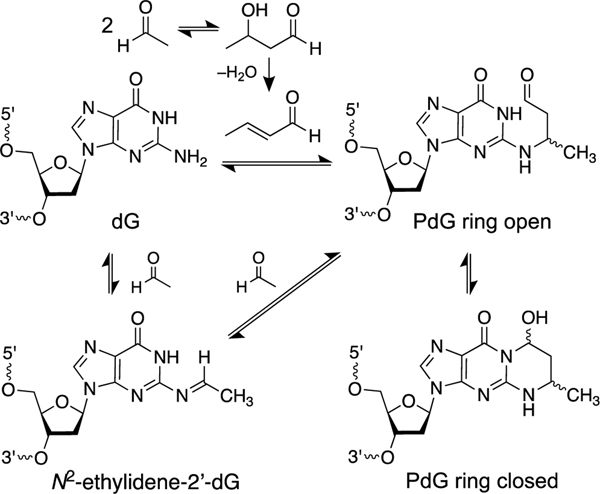
Acetaldehyde generates a variety of DNA adducts [157, 175, 221, 222, 225–227]. Most important in the context of this review, early work suggested that acetaldehyde generates ICLs in cellular DNA [228, 229]. Although a single equivalent of acetaldehyde can generate an intrastrand cross-link between two guanine residues in DNA (Figure 1) [226, 230], there is no evidence for the generation of interstrand DNA cross-links involving reaction of a single acetaldehyde unit with nucleobases on the opposing strands of the double helix, despite the fact that such an ICL is structurally reasonable, given the existence of the analogous formaldehyde-derived ICLs (see below). Rather, the acetaldehyde-derived ICLs that have been characterized arise from initial generation of an 1,N2-α-methyl-γ-hydroxypropano-2’-deoxyguanosine adduct (αMeγOHPdG, abbreviated as PdG in Scheme 8) in duplex DNA (Scheme 8) [157, 231, 232]. This guanine adduct is formed either via an aldol condensation of two equivalents of acetaldehyde in solution to generate crotonaldehyde [175, 233] or via reaction of a second equivalent of acetaldehyde with the N2-ethylidene-2’-deoxyguanosine adduct on DNA (Scheme 8) [157]. This ICL-precursor adduct has been detected in the DNA of cultured cells treated with acetaldehyde[234] and in DNA isolated from the blood of ALDH2-deficient alcoholics [235]. Biogenic amines and histones may catalyze the formation of the αMeγOHPdG adduct [236–239].
Figure 1.
Acetaldehyde generates intrastrand G-G cross-links.
Scheme 8.
Condensation of two equivalents of acetaldehyde gives rise to a propano-2’- deoxyguanosine (PdG) adduct in cellular DNA. The propano-2’-deoxyguanosine lesions can generate ICLs (Scheme 9).
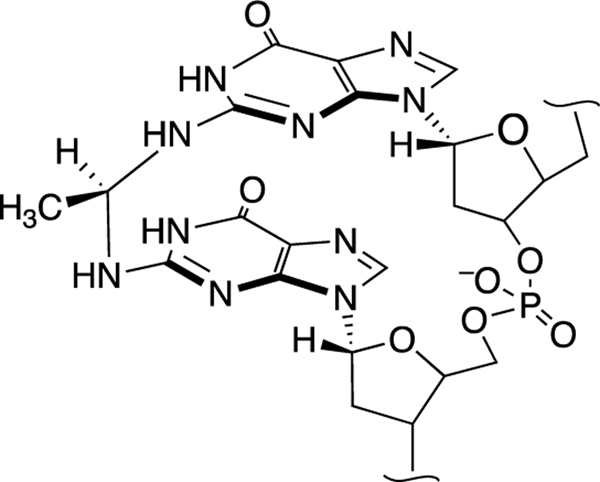
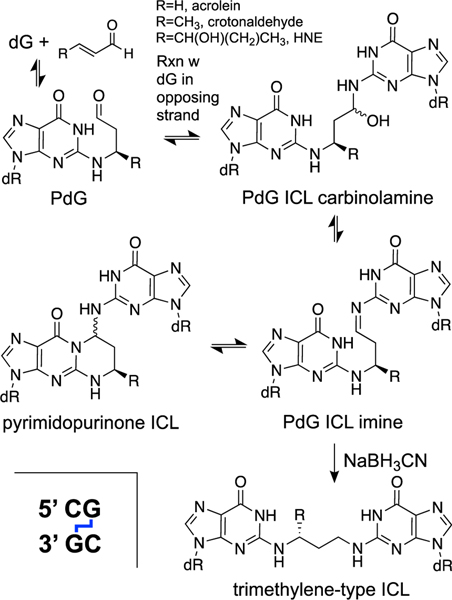
The αMeγOHPdG adducts are stable and, in nucleosides and in single-stranded DNA, exist predominantly in the ring-closed form (Scheme 8) [218, 232]. On the other hand, in duplex DNA, when the dG adduct is paired with a cytosine residue, the adduct undergoes partial ring-opening that enables the aldehyde group at the γ-position to react with the exocyclic NH2-group of a guanine residue on the opposing strand to generate ICLs at 5’CG sequences (Scheme 9). Starting from the α-R-isomer of the αMeγOHPdG adduct installed in a 5’CG sequence in duplex DNA, ICL formation reaches an equilibrium yield of approximately 40% over 21 days at pH 7 and 37 ˚C [157, 231, 232, 240]. In duplex DNA this ICL does not disrupt base pairing (PDB code: 2hmd) [241]. The nucleosidic ICL remnant obtained by digestion of duplexes containing this cross-link exists in the cyclic pyrimidopurinone form and is quite stable (Scheme 9) [157, 231, 232, 240]. In duplex DNA, however, the ICL exists as the ring-opened carbinolamine (Scheme 9) [160, 232, 240]. This cross-linked DNA duplex is stable enough to be analyzed by HPLC, isolated, and subjected to ligation reactions [231, 240, 242], but the ICL is completely dissociated by thermal denaturation of the duplex [240]. Treatment of duplex DNA containing the αMeγOHPdG ICL with NaBH3CN for 24 h in pH 7 buffer generated the corresponding, chemically-stable trimethylene-type ICL (Scheme 9) [240]. It is not known whether biological agents such as NAD(P)H, ascorbate, or glutathione have the capacity to reduce this ICL.
Scheme 9.
Reversible generation of imine and carbinolamine ICLs from propano-2’- deoxyguanosine (PdG) adducts at 5’CG sequences in duplex DNA and conversion to chemically-stable trimethylene-type ICLs by the reducing agent sodium cyanoborohydride (NaBH3CN).
Replication-dependent repair of the αMeγOHPdG ICL has been examined in a Xenopus egg extract [242]. The evidence indicates involvement of two distinct unhooking pathways for this ICL. The dual-incision, Fanconi anemia pathway is activated and contributes to unhooking and bypass of the ICL [242]. Interestingly, a second, faster, Fanconi-independent unhooking pathway also was observed. Both unhooking pathways are dependent upon the convergence of two replication forks on the ICL [242]. The corresponding reduced (chemically stable) αMeγOHPdGred ICL was unhooked exclusively by the Fanconi pathway, suggesting that the Fanconi-independent pathway exploits the reversible nature of this imine/carbinolamine cross-link [242]. The Fanconi-independent pathway did not involve the glycosylase NEIL3 or generation of an AP site [242]. Overall, the evidence is consistent with a Fanconi-independent unhooking reaction that regenerates native dG on one strand and the αMeγOHPdG adduct on the opposing strand [242]. Bypass of the adduct generated by the Fanconi-independent unhooking process is error-prone[243, 244], requires Rev1 and pol ζ [242, 244], and shows a mutation signature identical to that of the αMeγOHPdG adduct [242]. The Fanconi-independent unhooking reaction could be catalyzed by some as-yet-unidentified protein at the replication fork. However, it seems equally possible that the faster, Fanconi-independent unhooking process observed for the αMeγOHPdG-ICL results from spontaneous dissociation of the ICL induced by strand separation of the duplex at the stalled replication forks. The idea that this PdG ICL could dissociate spontaneously in the strand-separated DNA at a stalled replication fork on the timescale of the replication-coupled repair assay is supported by earlier experiments showing that the structurally-related ICL derived from the 1,N2-γ-hydroxypropano-2’-deoxyguanosine adduct readily dissociated (within 1 h) under conditions that disrupt the duplex structure (unbuffered water) [160, 240].
The observed engagement of the Fanconi anemia pathway in the repair of acetaldehyde-induced ICLs in a Xenopus egg extract is consistent with several previous observations: 1. cells deficient in Fanconi anemia proteins are hypersensitive to acetaldehyde [245, 246], 2. acetaldehyde activates the Fanconi anemia response in cells, and 3. acetaldehyde induces replication-dependent double-strand breaks in mammalian cells [246–248]. Recent work showed that Aldh2–/–Fancd2–/– knockout mice show profound health deficits including susceptibility to lymphoblastic leukemia, dramatic decreases in their hematopoietic stem cell pool, and ethanol-induced bone marrow failure [249–251]. This meshes with results showing that genetic deficiencies in ALDH2 activity in Fanconi anemia patients correlate with more rapid progression toward bone marrow failure [252]. Curiously, Aldh2–/–Rev–/–mice do not display the same phenotype as Aldh2–/–Fanca–/– animals despite apparent involvement of Rev1 repair of the acetaldehyde-derived ICLs via the Fanconi anemia pathway.
Overall, these results suggest acetaldehyde (and perhaps other endogenous ALDH2 substrates) generates ICLs that require the Fanconi pathway for repair [249–251]. It is important to note that a number of different biogenic aldehydes are substrates for ALDH2 including acetaldehyde, 4-hydroxynonenal, and acrolein [253–255]. While the ICL precursor lesion αMeγOHPdG has been found in cellular DNA [235], the actual ICLs derived from this precursor have not been detected in cellular DNA. It seems possible that acetaldehyde-derived intrastrand cross-links[226, 230] could be relevant to some of the observations surrounding Fancd2–/–Aldh2–/– cell lines and animals, considering evidence that FANCD2 is involved in cellular tolerance of intrastrand cross-links induced by UV-light [256, 257].
ICLs derived from α,β-unsaturated aldehydes, acrolein, crotonaldehyde, and 4-hydroxy-2-nonenal.
A number of α,β-unsaturated aldehydes including acrolein, crotonaldehyde, and 4-hydroxy-2-nonenal (HNE) are generated by endogenous metabolism of amino acids and polyamines, and via lipid peroxidation [125, 126, 156, 217, 258–261]. These compounds are also ubiquitous in foods and the environment [126, 156, 217, 258–261].
These α,β-unsaturated aldehydes have the potential to react with all of the DNA nucleobases, but the best characterized adducts are those with 2’-deoxyguanosine [126, 180–182, 216–220]. As described above in the context of the crotonaldehyde adducts derived from the condensation of acetaldehyde, α,β-unsaturated aldehydes generate PdG-type adducts on guanine residues in cellular DNA (Schemes 8 and 9) [218, 259, 262]. PdG adducts generated by α,β-unsaturated aldehydes are removed by nucleotide excision repair[263] and potentially by ALKB [264], but these lesions have been detected in cellular DNA [217, 244, 258, 265]. The PdG adducts derived from acrolein, crotonaldehyde, and HNE serve as precursors for the generation of ICLs in duplex DNA [160, 232, 240, 266–268]. The properties of these ICLs are similar to the crotonaldehyde-derived ICLs described above in the context of acetaldehyde. Starting from the corresponding R-isomers of the PdG adducts in DNA, the equilibrium yields of ICLs (pH 7, 37 ˚C) are approximately 50% after 7 days for acrolein, 40% after 21 days for crotonaldehyde, and 85% after 2 months for HNE [160]. To date, ICLs derived from α,β-unsaturated aldehydes have not been detected in cellular DNA.
Acrolein, crotonaldehyde, and HNE are detoxified in cells by ALDH enzymes including ALDH2, ALDH1A1 and ALDH7A1 [253–255, 269, 270]. Thus, the cellular levels of these α,β-unsaturated aldehydes are expected to increase in ALDH2-deficient individuals and in Aldh2–/– cell lines [235]. As a result, ICLs derived from acrolein, crotonaldehyde, and HNE could contribute to the genetic toxicity induced by mutations in, or knockout of, the Aldh2 gene [112, 249, 250]. It is noteworthy, however, that chicken DT-40 cells deficient in FANCD2 are not hypersensitive to acrolein or crotonaldehyde (in contrast, these cell lines are hypersensitive acetaldehyde or formaldehyde) [246]. These results suggest that acrolein and crotonaldehyde do not generate significant levels of ICLs in DT-40 cells, or that the ICLs derived from these chemicals are unhooked by a Fanconi-independent pathway, or that the ICLs dissociate spontaneously during DNA replication.
Malondialdehyde-derived ICL.
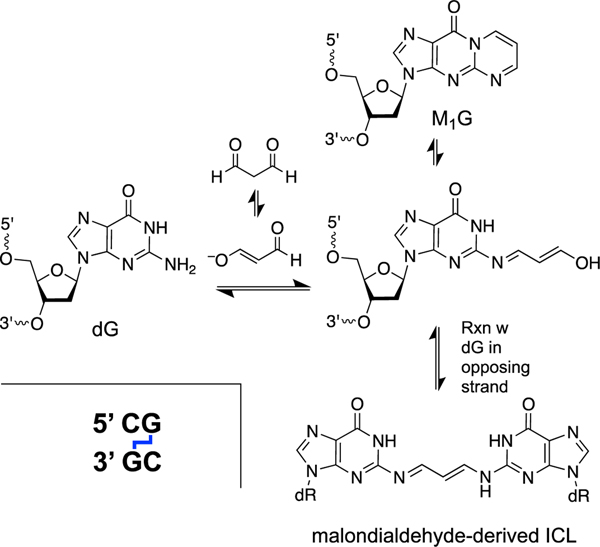
Malondialdehyde is an endogenous product of thromboxane biosynthesis and lipid peroxidation[271–273] that exists as a mixture of the dialdehyde, the enol, and (predominantly) β-alkoxyacrolein at physiological pH (Scheme 10). This compound is a bifunctional electrophile that forms cyclic adducts with nucleobases [274–279].
Scheme 10.
Formation an ICL from the malondialdehyde-2’-deoxyguanosine adduct (M1G) in duplex DNA.
The major lesion formed by the reaction of malondialdehyde with DNA is the 2’-deoxyguanosine adduct, commonly referred to as M1G [274, 276]. The M1G adduct has been detected in the DNA of healthy humans [276]. Interestingly, base propenal residues generated by oxidative DNA strand cleavage serve as malondialdehyde equivalents, capable of generating the M1G lesion [280, 281].
Similar to the PdG adducts described above, the M1G adduct paired with a complementary cytosine residue in duplex DNA exists in the ring-opened form that has the potential to generate cross-links [282]. Indeed, gel electrophoretic analysis provided evidence that malondialdehyde generates an ICL at 5’CG sequences [283]. This ICL has not been well characterized because it is generated in low yields and is unstable [283, 284], but the proposed[283] structure shown in Scheme 10 is consistent with the chemical expectations and the data [283]. Interestingly, it was not possible to generate the malondialdehyde ICL from the M1G lesion using the same approaches employed with great success to characterize the PdG-type ICLs [285]. The three-dimensional structure of the malondialdehyde ICL in duplex DNA has been modeled by a dG-dG trimethylene cross-link (lower structure, Scheme 9) [286].
Malondialdehyde is a substrate for ALDH2[255] and, as a result, has the potential to contribute to the genotoxicity observed in Aldh2–/– cell lines and individuals with ALDH2 deficiencies. Malondialdehyde also can generate DNA-protein cross-links (DPCs) [287]. No direct evidence has been reported supporting the presence of malondialdehyde-derived ICLs in cellular DNA.
Formaldehyde-derived ICLs.
Formaldehyde is ubiquitous in the environment and in foods [288]. In addition, formaldehyde is unavoidably generated by endogenous metabolic processes including demethylation of histones in the cell nucleus [125, 126]. Formaldehyde concentrations in the range of 30–300 μM have been reported in mammalian tissue and blood [289–291]. Formaldehyde has been classified as a human carcinogen [292–294].
Formaldehyde forms reversible covalent adducts with both DNA and proteins [154, 155, 295]. These chemical properties underlie the use of formaldehyde as an embalming agent and a fixative in molecular and cell biology [146]. Formaldehyde is also used to cross-link proteins to their DNA-binding sites in chromatin immunoprecipitation (ChIP) assays [144, 296]. Formaldehyde can condense with other aldehydes to generate complex mixtures of DNA adducts [157, 297].
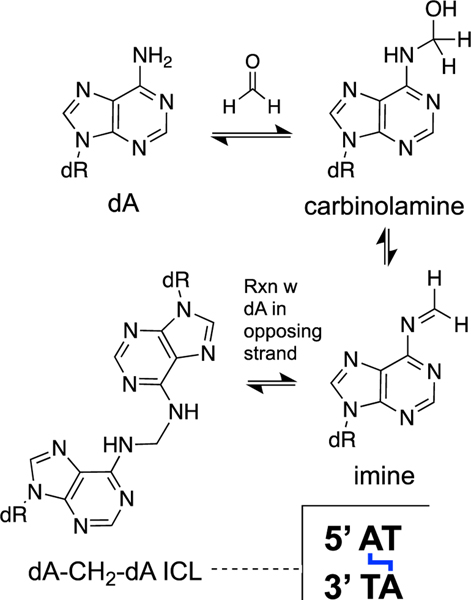
Formaldehyde reversibly forms adducts with all of the DNA bases [154, 155]. Adducts on the endocyclic nitrogens of the nucleobases typically form faster but are less stable, while adducts with the exocyclic nitrogens (–NH2) form more slowly but are more stable [154, 155]. The hydroxymethyl (carbinolamine) adducts generated by the reaction of the exocyclic amino groups of dGMP and dAMP with formaldehyde decompose with half-lives in the range of 2–6 h at neutral pH and 23 ˚C [146, 298].
Formaldehyde generates cross-links between nucleobases in duplex DNA [159, 299, 300]. Analysis of hydrolysates of DNA treated with formaldehyde provided evidence for dG-CH2-dG, dA-CH2-dA, dG-CH2-dA, dC-CH2-dG and dC-CH2-dA cross-links [159, 299, 300]. The dA-CH2-dA cross-link has been detected in the hepatic DNA of rats treated with formaldehyde-releasing chemicals such as N-nitrosodimethylamine [301]. These studies also detected background levels of the cross-link in untreated animals, presumably arising from endogenous formaldehyde [301]. In general, it is not possible to know whether cross-link “remnants” obtained by the digestion of DNA arise from interstrand or intrastrand cross-links. However, in this case, there is good in vitro evidence that formaldehyde generates ICLs in duplex DNA [299, 300, 302–305]. Hopkins’ group rigorously characterized the structure and properties of the dA-CH2-dA ICL formed at 5’AT sequences in duplex DNA (Scheme 11) [300, 302]. This ICL is formed slowly and in low yields in vitro, with <1% yield generated over the course of 9 days when a oligomeric DNA duplex was treated with formaldehyde (25 mM) in pH 6 phosphate buffer (50 mM) containing NaCl (25 mM) at 25 ˚C [300, 302]. Once formed, the dA-CH2-dA ICL is quite stable both in duplex DNA and as the dinucleoside ICL remnant, a feature that enables detection and characterization of the lesion [146, 299, 300, 302].
Scheme 11.
Formaldehyde generates an ICL at 5’AT sequences in duplex DNA.
There is indirect evidence suggesting that formaldehyde has the capacity to generate intrastrand G-G and G-A cross-links in DNA.[306] This process is analogous to the better-characterized generation of intrastrand cross-links by acetaldehyde (Figure 1) [226, 230]. In addition, formaldehyde-mediated DNA-protein cross-links have been detected in cells [128, 307].
In humans, formaldehyde is detoxified by the glutathione-dependent formaldehyde dehydrogenase activity of the enzyme alcohol dehydrogenase 5 (ADH5) [308–310]. Other enzymes involved in the removal of formaldehyde include ADH1, ALDH2, and cytochrome P450 2E1 (CYP2E1) [308, 309]. Formate resulting from oxidation of formaldehyde may be shunted into the one-carbon metabolic cycle for use in the biosynthesis of nucleosides [310, 311]. Vertebrate cell lines deficient in Fanconi anemia proteins such as FANCD2 or FANCB are hypersensitive to formaldehyde [245, 246]. Furthermore, simultaneous knockout of ALD5 and FANCL or FANCD2 is lethal to vertebrate cells [312]. Adh5–/–Fancd2–/– mice suffer from bone marrow failure, liver and kidney dysfunction, and fatal malignancies [72]. Together, the results suggest that aldehyde detoxification and cross-link repair pathways work together to protect the mammalian genome from formaldehyde-derived damage.
Glyoxal-derived ICLs.
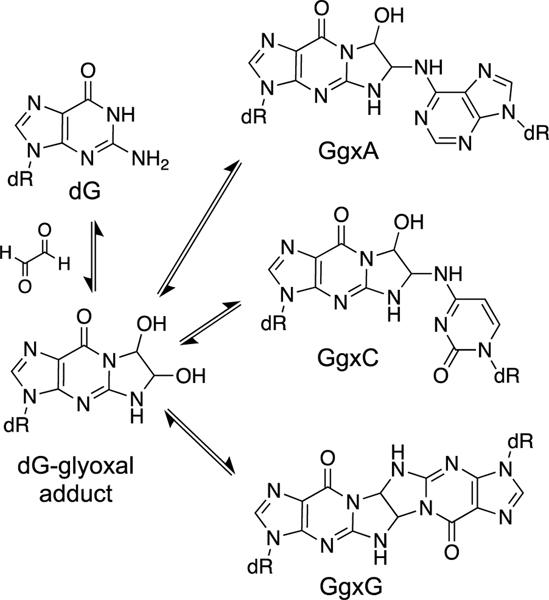
Glyoxal is generated in cells by a variety of processes including carbohydrate metabolism, amino acid metabolism, lipid oxidation, and DNA oxidation [126, 223]. This dialdehyde generates DNA adducts with dA, dC, and dG [313]. Similar to acrolein and MDA, glyoxal forms a cyclic adduct with dG [314, 315]. The cyclic dG adduct is stable, decomposing with half-life of 595 h in duplex DNA (pH 7.4, 37 ˚C) [316]. Glyoxal-DNA adducts may be removed by the enzyme DJ-1/Park7 although this issue remains controversial [317, 318].
Reactions in the context of nucleosides indicate that glyoxal has the potential to generate DNA cross-links involving dG/dG, dC/dC, dG/dC, and dG/dA attachments [313, 319]. For example, reaction of the glyoxal-2’-deoxyguanosine nucleoside adduct with 2’-deoxyguanosine (300 mM) in pH 7 buffer at 60 ˚C for 6 days produced a 4–5% yield of the GgxG linkage (Scheme 12) [319]. Higher yields (71%) of the GgxC dinucleoside were obtained in DMSO (60 ˚C, 6 d) [319]. While these dinucleoside cross-link attachments are formed slowly, they are chemically stable, showing no dissociation over 15 h at pH 7.4, 37 ˚C [316]. The GgxC, GgxA, and GgxG cross-link remnants have been detected in human placental DNA hydrolysates at levels of 3.50, 2.49, and 1.26 per 108 nucleotides, respectively [320]. Similarly, the GgxC and GgxA adducts were detected in leukocyte DNA hydrolysates at levels of 2.10 and 1.94 per 108 nucleotides [321, 322]. It is uncertain whether the cross-linked nucleobases detected after DNA digestion[316, 320–322] arise from intrastrand cross-links, interstrand cross-links, or both. In general, we are not aware of any biochemical studies (e.g. gel electrophoretic or mass spectrometric analyses of intact duplexes) characterizing glyoxal-derived ICLs in duplex DNA. Experiments in chicken DT-40 cells suggest that the Fanconi anemia ICL repair pathway does not play a significant role in mitigating the cytotoxicity of glyoxal [246].
Scheme 12.
Studies with nucleosides suggest that the glyoxal-dG adduct has the potential to generate ICLs.
Other endogenous 1,2-diketo compounds such as methylglyoxal and 4,5-dioxovaleric acid form DNA adducts and have the potential to generate cross-links [131, 323–329].
ICLs derived from nitric oxide and nitrous acid.
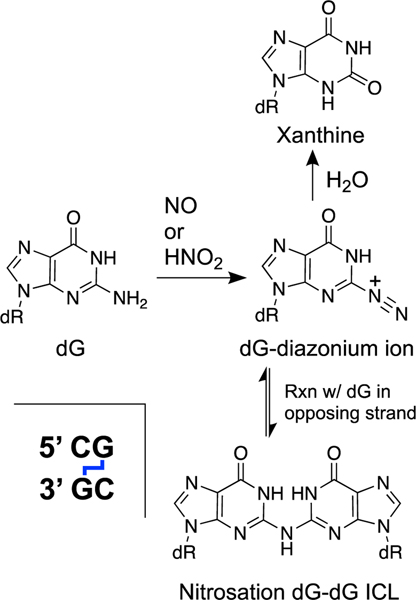
The endogenous signaling agent nitric oxide decomposes under cellular conditions to generate reactive oxygen and reactive nitrogen species including the nitrosating agent N2O3 [330, 331]. Nitrosating agents generate nucleobase diazonium ions that can be attacked by water to generate deaminated bases [332]. Alternatively, nucleobase diazonium ions can react with the exocyclic amino group of a purine residue on the opposing strand of DNA to generate an ICL (Scheme 13) [332–335]. The dG-dG ICL predominates over the dG-dA ICL (formally, these cross-links might be more accurately described as inosine-guanine, inosine-adenine, or guanine-nebularine ICLs). Treatment of duplex DNA with nitrous acid (500 mM, pH 4.15, 100 min) generates the dG-dG ICL (2% yield) selectively at 5’CG sequences[333] in DNA and the resulting connection of the two purine residues by a single nitrogen atom at these sites in duplex DNA is accommodated by extrusion of the two cytosine base pairing partners from the double helix [336]. Deamination products predominate over the dG-dG ICL when DNA is treated with nitric oxide under physiologically-relevant conditions (a 6:94 ratio of dG-dG to 2’-deoxyxanthosine) [337, 338]. Nitrosative DNA damage has been characterized in a mouse model of inflammation, but nitrosation-derived ICLs were not among the lesions analyzed in these in vivo experiments [331].
Scheme 13.
The endogenous signaling agent nitric oxide (NO) can decompose inside cells to yield nitrosating agents that generate nucleobase diazonium ions that, in turn, produce ICLs in duplex DNA.
An ICL derived from the 5-(2’-deoxyuridinyl)methyl radical.
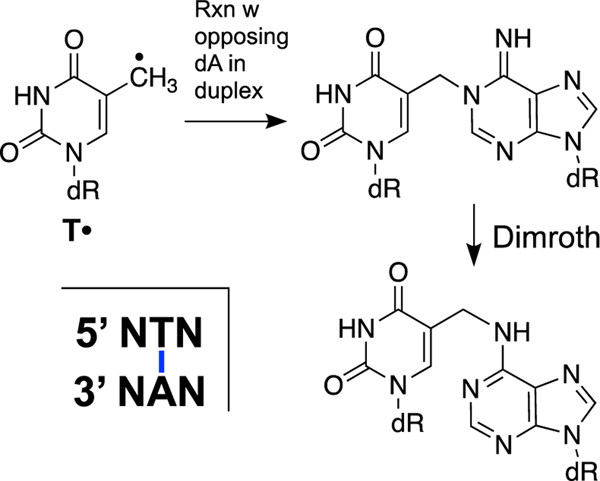
The generation of reactive oxygen and nitrogen species is an unavoidable consequence of aerobic life [14, 15]. These include superoxide radical (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (HO•), nitric oxide (NO), and peroxynitrite (ONOO–) [14, 15, 330, 331]. Ionizing radiation is also unavoidable in the environment and generates oxygen radicals in cells [339]. Hydroxyl radicals derived from oxidative stress or ionizing radiation inflict a wide variety of damages on the nucleobases and deoxyribose backbone of DNA [16, 17, 20, 340, 341]. The 5-(2’-deoxyuridinyl)methyl radical (T•, Scheme 14) can arise by hydrogen atom abstraction from the methyl group of thymine residues or by one-electron oxidation of the nucleobase [342, 343]. Greenberg’s group showed that the 5-(2’-deoxyuridinyl)methyl radical can generate an ICL via reaction with the directly opposing adenine residue [344, 345]. The cross-linking reaction can take place in aerobic solution (unlike some radical processes that are inhibited by O2) and involves rate-limiting rotation of the thymidine radical to the syn-glycosidic conformation that positions the radical for addition to the N1 position of the opposing adenine residue [346]. The initial C5-N1 ICL subsequently isomerizes to the C5-N6 ICL via a Dimroth rearrangement [346]. This ICL is chemically stable and would be expected to block replication and transcription [344– 346].
Scheme 14.
ICL formation involving reaction of the 5-(2’-deoxyuridinyl)methyl radical (T•) with the directly-opposing adenine residue in duplex DNA.
It is worth noting that the 5-(2’-deoxyuridinyl)methyl radical and the analogous radical derived from 5-methylcytosine can generate intrastrand cross-links via addition to the C8-position of an adjacent guanine residue [347, 348].
ICL derived from 2’-deoxyguanosine radical cation.
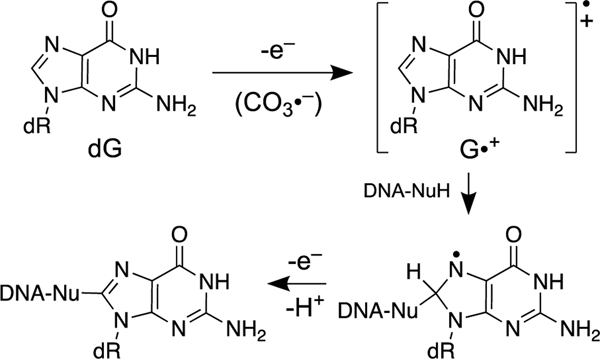
Under conditions of inflammation, reactions of superoxide radical (O2•−), nitric oxide (NO•), and CO2 can give rise to the carbonate radical anion (CO3•−) via nitrosoperoxycarbonate [349]. In addition, carbonate radical anion is generated under Fenton reaction conditions in bicarbonate buffer [350]. In duplex DNA, one-electron oxidation of guanine residues by CO3•− produces the guanine radical cation (G•+) [343]. Ionizing radiation and high intensity UVC light also can generate G•+ in DNA [343]. Nucleophilic attack of water on G•+ can lead to either 8-oxoguanine or formamidopyridine (Fapy) lesions [343]. Attack of the lysine amine residue on the G•+ intermediate generates DNA- protein cross-links [343, 351]. An analogous reaction of G•+ with an amine residue of a nucleobase on the opposing strand of duplex DNA has the potential to generate an ICL (Scheme 15). Indeed, exposure of duplex DNA to UVC light leads to products that migrate slowly in denaturing gels, consistent with the generation of an ICL(s) derived from the G•+ intermediate [352]. These slowly-migrating products display partial lability to piperidine workup [352]. It is proposed that this ICL is generated by nucleophilic attack of the exocyclic N4-amino group in the opposing cytosine residue on G•+ [343]; however, the formation, structure, and properties of these putative ICLs remains largely uncharacterized. It is worth noting that the G•+ intermediate also can give rise to intrastrand G-T cross-links [343, 353].
Scheme 15.
Generation of the guanine radical cation in duplex DNA may lead to ICLs.
A complex ICL generated by the oxidative DNA strand-cleavage product, 5’-dioxobutane.
Hydrogen atom abstraction from the C5’ position of deoxyribose sugars in DNA by agents such as hydroxyl radical can generate a strand break with a 5’-dioxobutane end group (DOB) [17, 354]. The DOB lesion exists as an equilibrating mixture of the ring-closed and the electrophilic dialdehyde forms. The DOB end group generates ICLs in sequences where an adenine residue is offset one nucleotide to the 3’-side of the lesion on the opposing strand (Scheme 16) [355]. The cross-linking reaction generates 30–40% yields of the ICL over the course of 15–20 h (k1 = 2.6 × 10−5 s−1, pH 7.2, 37 ˚C) [355]. The reaction is reversible and the isolated ICL dissociates with a half-life of approximately 11 h (pH 7.2, 37 ˚C). A slower reaction eliminates the 5’DOB sugar remnant from the duplex altogether (k2 = 5.6 × 10−6 s−1) [355]. Treatment with NaBH3CN does not stabilize the ICL against dissociation [355], a feature that may hinder detection and quantitation of this lesion in cellular DNA. Nonetheless, the ICL was stable enough for mass spectrometric analyses results of which the ICL structure shown in Scheme 16 [355].
Scheme 16.
Oxidative strand cleavage induced by abstraction of a C5’-hydrogen atom can generate a strand break with a 5’-dioxobutane (5’DOB) terminus on one side of the break. Reaction of the 5’DOB group with an adenine residue on the opposing strand of duplex DNA can generate a complex lesion composed of an ICL adjacent to a strand break.
Studies employing a chemically-stable synthetic analog of the DOB-derived ICL[356] provided evidence that the bacterial NER complex UvrABC generates a deleterious double-strand break via incision on the 3’-side of the cross-linked adenine residue (Scheme 16) [357]. The results of these studies highlight the challenges presented to cellular repair systems by this type of complex (clustered) lesion consisting of an ICL adjacent to a single-strand break.
ICLs derived from the C4’-oxidized abasic site in duplex DNA.
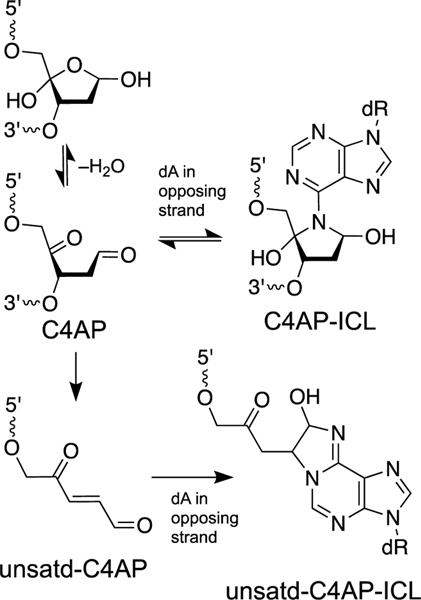
The C4’-oxidized abasic site (C4AP) can arise from abstraction of the C4’-hydrogen atom from the sugar-phosphate backbone of DNA by agents such as hydroxyl radical [17]. This lesion is generated by exposure of DNA to ionizing radiation or antibiotics such as bleomycin [17]. Greenberg’s group provided rigorous characterization of two different types of ICL that can arise from this lesion [358, 359]. The C4AP lesion, similar to the 5’DOB lesion described above, exists as an equilibrium mixture of the ring-closed form and an electrophilic, ring-opened keto-aldehyde form (Scheme 17). The C4AP lesion can generate an ICL via reaction with an adenine residue located one base to the 3’side of the lesion on the opposing strand (Scheme 17) [358, 359]. The reaction generates 15– 40% yield of the ICL (depending on local sequence) over the course of 10–12 h (k = 5.5 × 10-5 s−1, pH 7.2, 37 ˚C) [358, 359]. The isolated ICL is unstable, dissociating with a half-life of approximately 3 h [358, 359]. Treatment with NaBH3CN does not stabilize the ICL [358, 359]. The proposed structure of the C4AP ICL is shown in Scheme 17. As an aside, Tris buffer has the potential to quench the reactivity of C4AP by formation of a tridentate adduct [360].
Scheme 17.
Oxidative strand cleavage arising from abstraction of the C4’-hydrogen atom can generate the C4’-oxidized abasic site (C4AP). The C4AP lesion can generate an ICL by reaction with an adenine residue on the opposing strand. Elimination of the 3’-phosphoryl group from the C4AP lesion produces the unsaturated C4AP lesion that can react with adenine or cytosine residues on the opposing strand to generate a complex lesion consisting of an ICL adjacent to a DNA strand break.
Elimination of the 3’-phosphoryl group from the C4AP lesion generates a strand break containing the α,β-unsaturated keto-aldehyde sugar remnant on the 3’-terminus of the break (unsatd-C4AP, Scheme 17). Interestingly, this strand cleavage reaction is catalyzed by an adenine residue directly opposing the C4AP lesion [358, 359]. The α,β-unsaturated sugar remnant derived from C4AP generates ICLs in 1–30% yields (depending upon local sequence) via reactions with cytosine or adenine residues on the opposing strand (unsatd-C4AP ICL, Scheme 17). The elimination reaction that generates unsatd-C4AP is rather slow and the nicked ICLs grow in over the course of 40–50 h (pH 7.2, 37 ˚C) [359]. The C4AP ICLs are the fast-forming (kinetic) products and the unsaturated-C4AP ICLs are the late-forming (thermodynamic) products.
The ICLs generated by the unsaturated C4AP are stable and the chemical structure of the C4AP-dA cross-linkage was rigorously determined by independent chemical synthesis combined with mass spectrometric analyses [359]. ICL formation by the unsaturated C4AP residue is inhibited by the presence of the dithiothreitol (1 mM) in the reaction mixture, presumably due to conjugate addition of the thiol to the α,β-unsaturated keto-aldehyde functional group. When this nicked ICL lesion is processed by the bacterial NER complex UvrABC, 15% of the incisions involved a dysfunctional repair pathway that generates a double-strand break [361].
A cross-link remnant arising from the reaction of 2’-deoxycytidine with the unsatd-C4AP residue has been detected in DNA hydrolysates of cultured human lymphocytes treated with either bleomycin or ionizing radiation [362]. Following exposure to these DNA-damaging agents, this lesion was removed from cellular DNA more slowly than the repair of typical oxidative nucleobase lesions, with a half-life of 10 h [362]. It remains uncertain whether the dC-unsaturated-C4AP cross-link remnant detected in the DNA hydrolysates arises from an interstrand cross-link, intrastrand cross-link, or both. Chemical synthesis of a duplex containing the unsaturated C4AP-dA ICL at a defined location has been reported and may prove useful in future DNA repair studies [363].
ICL generated by furan-modified adenine residues in DNA.
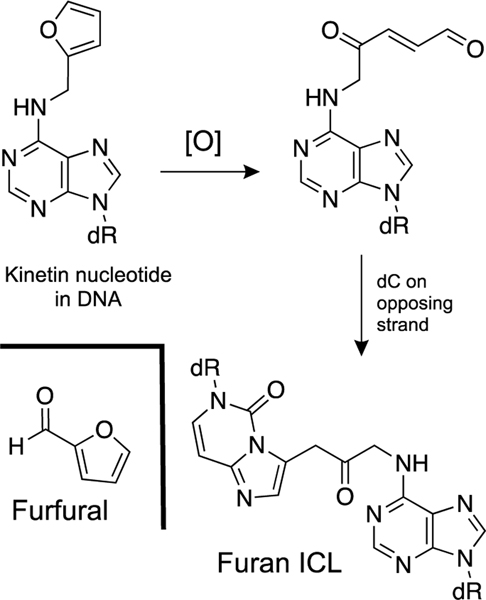
Furfural is generated by the hydroxylation of the C5’-position of deoxyribose residues in DNA [364]. Furfural has the potential to generate DNA adducts. Specifically, there is some evidence for the presence of N6-furfuryladenine residues in cellular DNA [365]. The formation of this lesion would involve condensation of furfural with the exocyclic amino group on adenine followed by reduction of the N6-furfuryl imine adduct. To our knowledge the reduction of N6-imine adduct in DNA is unprecedented.
Madder’s group provided gel electrophoretic evidence that oxidation of N6-furfuryladenine residue by N-bromosuccinimide in DNA can generate a low yield of an unstable ICL in a duplex where the furan lesion is opposed by cytosine residues [366]. The proposed structure for the resulting ICL is shown in Scheme 18. This reaction is analogous to the oxidation of furan to a 1,4-dialdehyde product that can modify DNA [367].
Scheme 18.
Possible mechanism for generation of an ICL from an N6-furfuryladenine residue in duplex DNA.
ICLs generated by ultraviolet light.
For many organisms, UV-light presents an unavoidable threat to their genetic material. Indeed, DNA damage associated with increased levels of UV-B flux resulting from depletion of the ozone layer has been proposed as the mechanism of a terrestrial mass extinction event on earth 359 million years ago [368]. UV-light generates a number of DNA lesions including, most famously, intrastrand DNA cross-links involving cyclobutane dimer formation [369]. Several early studies provided evidence that UV-irradiation also generates ICLs in duplex DNA [370–372]. The idea that ICLs can be generated by ultraviolet irradiation of DNA was further supported by more recent work [373, 374]. Together, the studies suggest that non-B-DNA conformations or increased duplex dynamics associated with local melting favor UV-induced ICL formation [370–374]. Otherwise, nothing is known regarding the yields of these ICLs, the sequence(s) in which they form, their chemical structure(s), or their biological significance.
ICLs generated by psoralen and other natural products.
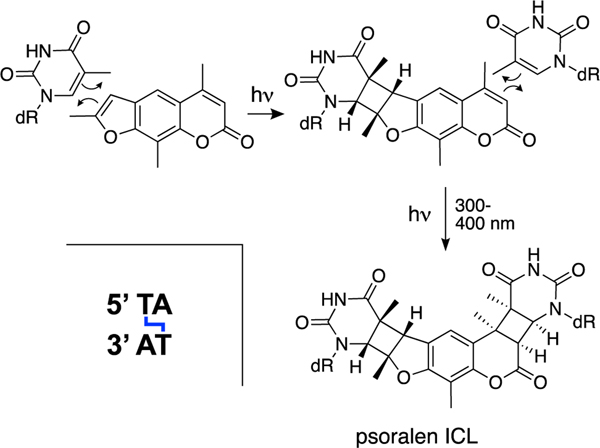
The psoralens are a group of furocoumarin natural products derived from plants such as celery, limes, lemons, and cow parsley [375]. These compounds form DNA photoadducts including ICLs [375]. Psoralen-derived ICLs are not endogenous lesions but have been widely employed in studies of ICL repair. Psoralens bind weakly to DNA by intercalation[376, 377] and irradiation of psoralen-DNA complexes with 300–400 nm light induces reactions between the pi-bonds in the natural product and the 5,6-double bonds of thymine and cytosine residues (Scheme 19) [375, 378–381]. Psoralen ICLs are generated preferentially at 5’-TA sequences[382] via sequential photoinduced [2+2] cycloadditions of thymine residues with the 4’,5’-double bond in the furan ring of the psoralen, followed by reaction of the 3,4-double bond of the pyrone system in the natural product (Scheme 19) [375, 380]. Duplexes containing a psoralen ICL display some local unwinding at the cross-link site but, overall, retain a largely B-form structure [378–384].
Scheme 19.
Psoralens are plant-derived (not endogenous) natural products that generate ICLs when the DNA-bound complex is exposed to UV-irradiation.
There are a number of other plant-derived and microbial natural products that can induce ICLs. These include colibactin [385–387], azinomycin B [388–391], the mitomycins [392], and pyrrolizidine alkaloids such as FR900482 and dehydromonocrotaline [205, 393–395]. It is not clear that environmental cross-linking agents could represent a universal genomic threat of the type required to explain the evolution of ICL repair pathways.
Conclusions.
DNA repair pathways evolved to handle unavoidable damage to cellular DNA. In most cases, the endogenous lesions that drove the evolution of known DNA repair pathways can be inferred. On the other hand, the identity of the endogenous lesion(s) underlying the evolution of ICL repair pathways found in most life forms remains uncertain. In this review, we described more than a dozen processes by which ICLs might be unavoidably introduced into the DNA of living cells. Given the large number of processes by which endogenous ICLs plausibly can be generated, it seems likely that multiple reaction pathways contribute to the total load of ICLs in cellular DNA. The relative importance of different DNA cross-linking pathways may vary depending upon many factors including species, growth conditions, tissue-type, and pathophysiological conditions.Much work remains to define the formation, properties, and cellular occurrence of ICLs. In humans, endogenous ICLs may contribute to cancer, neurodegeneration, and aging [37–43]. Molecular understanding of the formation, consequences, and repair of endogenous ICLs – including how these features are affected by individual variations in the human genome – may enable preventive, predictive, personalized medical approaches that mitigate disease and increase healthspan.
Acknowledgements
We are grateful to the National Institutes of Health for support of this work (R01ES021007 and R01GM131071)
Funding
National Institutes of Health
Footnotes
Conflict of Interest
None of the authors have competing or conflicting interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Crick FHC, Central dogma of molecular biology, Nature, 227 (1970) 561–563. [DOI] [PubMed] [Google Scholar]
- [2].Arrojo e Drigo R, Lev-Ram V, Tyagi S, Ramachandra R, Deerinck T, Bushong E, Phan S, Orphan V, Lechene C, Ellisman MH, Hetzer MW, Cell Metab, 30 (2019) 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fekry M,I, Gates KS, DNA-catalyzed hydrolysis of DNA phosphodiesters, Nat. Chem. Biol, 5 (2009) 710–711. [DOI] [PubMed] [Google Scholar]
- [4].Lindahl T, Instability and decay of the primary structure of DNA, Nature, 362 (1993) 709–715. [DOI] [PubMed] [Google Scholar]
- [5].Gates KS, An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals, Chem. Res. Toxicol, 22 (2009) 1747–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].De Bont R, van Larebeke N, Endogenous DNA damage in humans: a review of quantitative data, Mutagenesis, 19 (2004) 169–185. [DOI] [PubMed] [Google Scholar]
- [7].Frederico LA, Kunkel TA, Shaw BR, A sensitive genetic asssay for detection of cytosine deamination: determination of rate constants and the activation energy, Biochemistry, 29 (1990) 2532–2537. [DOI] [PubMed] [Google Scholar]
- [8].Lindahl T, Nyberg B, Heat-induced deamination of cytosine residues in deoxyribonucleic acid, Biochemistry, 13 (1974) 3405–3410. [DOI] [PubMed] [Google Scholar]
- [9].Shapiro R, Danzig M, Acidic hydrolysis of deoxycytidine and deoxyuridine derivatives. The general mechanism of deoxyribonucleoside hydrolysis, Biochemistry, 11 (1972) 23–29. [DOI] [PubMed] [Google Scholar]
- [10].Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL, Saksena G, Harris S, Shah RR, Resnick MA, Getz G, Gordenin DA, An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers, Nature Genet, 45 (2013) 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pham P, Bransteitter R, Goodman MF, Reward versus risk: DNA cytidine deaminases triggering immunity and disease, Biochemistry, 44 (2005) 2703–2715. [DOI] [PubMed] [Google Scholar]
- [12].Lewis CAJ, Crayle J, Zhou S, Swanstrom R, Wolfenden R, Cytosine deamination and the precipitous decline ofspontaneous mutation during Earth’s history, Proc. Nat. Acad. Sci. USA, (2016) 8194–8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Helleday T, Eshtad S, Nik-Zainal S, Mechanisms underlying mutational signatures in human cancers, Nat. Rev. Genet, 15 (2014) 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM, Biological and physiological role of reactive oxygen species -- the good, the bad and the ugly, Acta Physiol, 214 (2015) 329–348. [DOI] [PubMed] [Google Scholar]
- [15].Halliwell B, Gutteridge JMC, Role of free radicals and catalytic metal ions in human disease: an overview, Methods Enzymol, 186 (1990) 1–85. [DOI] [PubMed] [Google Scholar]
- [16].Greenberg MM, Elucidating DNA damage and repair processes by independently generating reactive and metastable intermediates, Org. Biomol. Chem, 5 (2007) 18–30. [DOI] [PubMed] [Google Scholar]
- [17].Pratviel G, Bernadou J, Meunier B, Carbon-hydrogen bonds of DNA sugar units as targets for chemical nucleases and drugs, Angew. Chem. Int. Ed. Eng, 34 (1995) 746–769. [Google Scholar]
- [18].Fleming AM, Burrows CJ, 8-Oxo-7,8-dihydroguanine, Friend and Foe: Epigenetic-like Regulator Versus Initiator of Mutagenesis, DNA Repair, 56 (2017) 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Greenberg MM, The formamidopyrimidines: Purine lesions formed in competition with 8-oxopurines from oxidative stress, Acc. Chem. Res, 45 (2012) 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cadet J, Wagner JR, DNA OA Base Damage by Reactive Oxygen Species, and UV Radiation, a.J.R.W. Jean Cadet1, DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation, Cold Spring Harb. Perspect. Biol, 5 (2013) a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lander ES, Initial impact of the sequencing of the human genome, Nature, 470 (2011) 187–197. [DOI] [PubMed] [Google Scholar]
- [22].Lindahl T, Nyberg B, Rate of depurination of native deoxyribonucleic acid, Biochemistry, 11 (1972) 3610–3618. [DOI] [PubMed] [Google Scholar]
- [23].Chen H, Yao L, Brown C, Rizzo CJ, Turesky RJ, Quantitation of Apurinic/Apyrimidinic Sites in Isolated DNA and in Mammalian Tissue with a Reduced Level of Artifacts, Anal. Chem, (2019) 7403–7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hansen AJ, Mitchell DL, Wiuf C, Paniker L, Brand TB, Binladen J, Gilichinsky DA, Rønn R, Willerslev E, Cross-links rather than strand breaks determine access to ancient DNA sequences from frozen sediments, Genetics, 173 (2006) 1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dabney J, Meyer M, Pääbo S, Ancient DNA damage, Cold Spring Harb. Perspect. Biol, 5:a012567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Allentoft ME, Collins M, Harker D, Haile J, Oskam CL, Hale ML, Campos PF, Samaniego JA, Gilbert MTP, Willerslev E, Zhang G, Scofield RP, Holdaway RN, Bunce M, The half-life of DNA in bone: measuringdecay kinetics in 158 dated fossils, Proc. R. Soc. B, 279 (2012) 4724–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Orlando L, Gilbert MTP, Willerslev E, Reconstructing ancient genomes and epigenomes, Nat. Rev. Genetic, 16 (2015) 395–408. [DOI] [PubMed] [Google Scholar]
- [28].Friedberg EC, McDaniel LD, Schultz RA, The role of endogenous and exogenous DNA damage and mutagenesis, Curr. Opin. Genetics Devel, 14 (2004) 5–10. [DOI] [PubMed] [Google Scholar]
- [29].Kucab JE, Zou X, Morganella S, Joel M, Nanda AS, Nagy E, Gomez C, Degasperi A, Harris R, Jackson SP, Arlt VM, Phillips DH, Nik-Zainal S, A compendium of mutational signatures of environmental agents, Cell, 177 (2019) 821–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Francioli LC, Polak PP, Koren A, Menelaou A, Chun S, Renkens I, Genome of the Netherlands Consortium, van Dujin CM, Swertz M, Wijmenga C, van Ommen G, Slagboom PE, Boomsma DI, Ye K, Guryev V, Arndt PF, Kloosterman WP, de Bakker PIW, Sunyaev SR, Genome-wide patterns and properties of de novo mutations in humans, Nature Genet, 47 (2015) 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schroeder JW, Yeesin P, Simmons LA, Wang JD, Sources of spontaneous mutagenesis in bacteria, Crit. Rev. Biochem. Mol. Biol, 53 (2018) 29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lynch M, Ackerman MS, Gout J-F, Long H, Sung W, Thomas WK, Foster PL, Genetic drift, selection and the evolution of the mutation rate, Nat. Rev. Genet, 17 (2016) 704–714. [DOI] [PubMed] [Google Scholar]
- [33].Hershberg R, Mutation—The Engine of Evolution: Studying Mutation and Its Role in the Evolution of Bacteria, Cold Spring Harb. Perspect. Biol, 7 (2015) a018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tomasetti C, Li L, Vogelstein B, Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention, Science, 355 (2017) 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lodato MA, Rodin RE, Bohrson CL, Coulter ME, Barton AR, Kwon M, Sherman MA, Vitzthum CA, Luquette LJ, Yandava CN, Yang P, Chittenden TW, Hatem NE, Ryu SC, Woodworth MB, Park PJ, Walsh CA, Aging and neurodegeneration are associated with increased mutations in single human neurons, Science, 359 (2018) 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nik-Zainal S, Hall BA, Cellular survival over genomic perfection, Science, 366 (2019) 802–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Niedernhofer LJ, Lalai AS, Hoeijmakers JHJ, Fanconi anemia (cross)linked to DNA repair, Cell, 123 (2005) 1191–1198. [DOI] [PubMed] [Google Scholar]
- [38].Tiwari V, Wilson III DM, DNA damage and associated DNA repair defects in disease and premature aging, Am. J. Hum. Genet, 105 (2019) 237–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Driscoll M, Diseases Associated with Defective Responses to DNA Damage, Cold Spring Harb. Perspect. Biol, 4 (2012) a012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Duxin JP, Walter JC, What is the DNA repair defect underlying Fanconi anemia, Curr. Opin. Cell. Biol, 37 (2015) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vermeij WP, Hoeijmakers JHJ, Pothof J, Aging: not all DNA damage is equal, Curr. Opin. Gen. Devel, 26 (2014) 124–130. [DOI] [PubMed] [Google Scholar]
- [42].Kottemann MC, Smogorzewska A, Fanconi anaemia and the repair of Watson and Crick DNA crosslinks, Nature, 493 (2013) 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Garinis GA, van der Horst GTJ, Vijg J, Hoeijmakers JHJ, DNA damage and aging: new-age ideas for an age-old problem, Nat. Cell Biol, 10 (2008) 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tubbs A, Nussenszweig A, Endogenous DNA Damage as a Source of Genomic Instability in Cancer, Cell, 168 (2017) 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lindahl T, Barnes DE, Repair of endogenous DNA damage, Cold Spring Harb. Perspect. Biol, 65 (2000) 127–134. [DOI] [PubMed] [Google Scholar]
- [46].Lindahl T, Endogenous damage to DNA, Phil. Trans. R. Soc. Lond. B, 351 (1996) 1529–1538. [DOI] [PubMed] [Google Scholar]
- [47].DiRuggiero J, Robb FT, Early Evolution of DNA Repair Mechanisms, in: The Genetic Code and the Origin of Life, Springer, Boston, MA, 2004. [Google Scholar]
- [48].Barnes DE, Lindahl T, Repair and genetic consequences of endogenous DNA base damage in mammalian cells, Annu. Rev. Genet, 38 (2004) 445–476. [DOI] [PubMed] [Google Scholar]
- [49].Strauss BS, Why Is DNA Double Stranded? The Discovery of DNA Excision Repair Mechanisms, Genetics, 209 (2018) 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brunette GJ, Jamalruddin MA, Baldock RA, Clark NL, Bernstein KA, Evolution-based screening enables genome-wide prioritization and discovery of DNA repair genes, Proc. Nat. Acad. Sci. USA, 116 (2019) 19593–19599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eisen JA, Hanawalt PC, A phylogenomic study of DNA repair genes, proteins, and processes, Mutat. Res, 435 (1999) 171–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lindahl T, The Intrinsic Fragility of DNA (Nobel Lecture), Angew. Chem. Int. Ed. Eng, 55 (2016) 8528–8534. [DOI] [PubMed] [Google Scholar]
- [53].Wood RD, Mitchell M, Sgouros J, Lindahl T, Human DNA repair genes, Science, 291 (2001) 1284–1289. [DOI] [PubMed] [Google Scholar]
- [54].Ganai RA, Johansson E, DNA Replication—A Matter of Fidelity, Mol. Cell, 62 (2016) 745–755. [DOI] [PubMed] [Google Scholar]
- [55].Krokan HE, Drabløs F, Slupphaug G, Uracil in DNA – occurrence, consequences and repair, Oncogene, 21 (2002) 8935–8948. [DOI] [PubMed] [Google Scholar]
- [56].Guillet M, Bioteux S, Origin of endogenous DNA abasic sites in Saccaromyces cerevisiae, Mol. Cell Biol, 23 (2003) 8386–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lindahl T, Ljunquist S, Siegert W, Nyberg B, Sperens B, DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. , J. Biol. Chem, 252 (1977) 3286–3294. [PubMed] [Google Scholar]
- [58].Shu X, Liu M, Lu Z, Zhu C, Meng H, Huang S, Zhang X, Yi C, Genome-wide mapping reveals that deoxyuridine is enriched in the human centromeric DNA, Nat. Chem. Biol, 14 (2018) 680–687. [DOI] [PubMed] [Google Scholar]
- [59].McVey M, Strategies for DNA interstrand cross-link repair: Insights from worms, flies, frogs, and slime molds, Env. Mol. Mutagenesis, 51 (2010) 646–658. [DOI] [PubMed] [Google Scholar]
- [60].Enderle J, Dorn A, Puchta H, DNA-and DNA-Protein-Crosslink Repair in Plants, Int. J. Mol. Sci, 20 (2019) 4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McHugh PJ, Ward TA, Chovanec M, A Prototypical Fanconi Anemia Pathway in Lower Eukaryotes?, Cell Cycle, 11 (2012) 3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lawley PD, Brookes P, Molecular Mechanism of the Cytotoxic Action of Difunctional Alkylating Agents and of Resistance to this Action, Nature, 206 (1965) 480–483. [DOI] [PubMed] [Google Scholar]
- [63].Cole RS, Repair of DNA Containing Interstrand Crosslinks in Escherichia coli: Sequential Excision and Recombination, Proc. Nat. Acad. Sci. USA, 70 (1973) 1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kohn KW, Steigbigel NH, Spears CL, Cross-linking and Repair of DNA in Sensitive and Resistant Strains of E. Coli Treated With Nitrogen Mustard, Proc. Nat. Acad. Sci. USA, 53 (1965) 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zwelling LA, Kohn KW, Ross WE, Wwig RA, Anderson T, Kinetics of formation and disappearance of a DNA cross-linking effect in mouse leukemia L1210 cells treated with cis- and trans-diamminedichloroplatinum(II), Cancer Res, 39 (1978) 1762–1768. [PubMed] [Google Scholar]
- [66].Lawley PD, Phillips DH, DNA adducts from chemotherapeutic agents, Mutation Res, 355 (1996) 13–40. [DOI] [PubMed] [Google Scholar]
- [67].Clauson C, Schärer OD, Niedernhofer LJ, Advances in understanding the complex mechanisms of DNA interstrand cross-link repair, Cold Spring Harbor Perspectives in Biology, 5 (2013) a012732/012731-a012732/012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schärer OD, DNA interstrand crosslinks: natural and drug-induced DNA adducts that induce unique cellular responses, ChemBioChem, 6 (2005) 27–32. [DOI] [PubMed] [Google Scholar]
- [69].Brookes P, Lawley PD, The reaction of mono and di-functional alkylating agents with nucleic acids, Biochem. J, 80 (1961) 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GTJ, Meinecke P, Kleijer WJ, Vijg J, Jaspers NGJ, Hoeijmakers JHJ, A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis, Nature, 444 (2006) 1038–1043. [DOI] [PubMed] [Google Scholar]
- [71].Bergstrahl DT, Sekelsky J, Interstrand crosslink repair: can XPF-ERCC1 be let off the hook?, Trends in Genetics, 24 (2007) 70–76. [DOI] [PubMed] [Google Scholar]
- [72].Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu L, Swenberg JA, Crossan GP, Patel KJ, Endogenous formaldehyde is a hemapoietic stem cell genotoxin and metabolic carcinogen, Mol. Cell, 60 (2015) 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Greenberg RB, Alberti M, Hearst JE, Chua MA, Saffran WA, Recombinational and mutagenic repair of psoralen interstrand cross-links in Saccharomyces cerevisiae, J. Biol. Chem, 276 (2001) 31551–31560. [DOI] [PubMed] [Google Scholar]
- [74].Shen X, Li L, Mutagenic repair of interstrand crosslinks, Env. Mol. Mutagenesis, 51 (2010) 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zheng H, Wang X, Warren AJ, Legerski RJ, Nairn RS, Hamilton JW, Li L, Nucleotide excision repair- and polymerase eta-mediated error-prone removal of mitomycin C interstrand cross-links, Mol. Cell Biol, 23 (2003) 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vogel EW, Barbin A, Nivard MJ, Stack HF, Waters MD, Lohman PH, Heritable and cancer risks of exposures to anticancer drugs: inter-species comparisons of covalent deoxyribonucleic acid- binding agents, Mutat. Res, 400 (1998) 509–540. [DOI] [PubMed] [Google Scholar]
- [77].Jonnalagadda VS, Matsuguchi T, Engelward BP, Interstrand crosslink-induced homologous recombination carries an increased risk of deletions and insertions, DNA Repair, 4 (2005) 594–605. [DOI] [PubMed] [Google Scholar]
- [78].Akkari YM, Bateman RL, Reifsteck CA, DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links, Mol. Cell Biol, 20 (2000) 8283–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D’Andrea AD, S- phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51, Blood, 100 (2002) 2414–2420. [DOI] [PubMed] [Google Scholar]
- [80].Räschle M, Knipscheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, Walter JC, Mechanism of replication-coupled DNA interstrand cross-link repair, Cell, 134 (2008) 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhang J, Dewar JM, Budzowska M, Motnenko A, Cohn MA, Walter JC, DNA interstrand cross-link repair requires replication-fork convergence, Nat. Struct. Mol. Biol, 22 (2015) 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ceccaldi R, Sarangi P, D’Andrea AD, The Fanconi anaemia pathway: new players and new functions, Nat. Rev. Mol. Cell Biol, 17 (2016) 337–349. [DOI] [PubMed] [Google Scholar]
- [83].Datta A, Brosh RMJ, Holding All the Cards—How Fanconi Anemia Proteins Deal with Replication Stress and Preserve Genomic Stability, Genes, 10 (2019) 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Knipscheer P, Räschle M, Smogorzewska A, Enolu M, Ho TV, Schärer OD, Elledge SJ, Walter JC, The Fanconi Anemia Pathway Promotes Replication-Dependent DNA Interstrand Cross-Link Repair, Science, 326 (2009) 1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Longerich S, Kwon Y, Tasi M-S, Hiaing AS, Kupfer GM, Sung P, Regulation of FANCD2 and FANCI monoubiquitination by their interaction and by DNA, Nucleic Acids Res, 42 (2014) 5657–5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liang C-C, Li Z, Lopez-Martinez D, Nicholson WV, Vénien-Bryan C, Cohn MA, The FANCD2–FANCI complex is recruited to DNA interstrand crosslinks before monoubiquitination of FANCD2, Nat. Commun, 7 (2016) doi: 10.1038/ncomms12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Klein D, Boonen RA, Long DT, Szypowska AA, Räschle M, Walter JC, Knipsheer P, XPF- ERCC1 acts in Unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4, Mol. Cell, 54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Klein DD, Hoogenboom WS, Boonen RA, Knipsheer P, Recruitment and positioning determine the specific role of the XPF-ERCC1 endonuclease in interstrand crosslink repair, EMBO J, 36 (2017) 2034–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang J, Walter JC, Mechanism and regulation of incisions during DNA interstrand cross-link repair, DNA Repair, 19 (2014) 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hodskinson MRG, Silhan J, Crossan GP, Garayoechea JI, Mukherjee S, Johnson CM, Schärer OD, Patel KJ, Mouse SLX4 Is a Tumor Suppressor that Stimulates the Activity of the Nuclease XPF-ERCC1 in DNA Crosslink Repair, Mol. Cell, 54 (2014) 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].McHugh PJ, XPF–ERCC1: Linchpin of DNA crosslink repair, PLoS Genet, 16 (2020) e1008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thongthip S, Bellani MA, Gregg SQ, Sridhar S, Conti BA, C Y, Seidman M, Smogorzewska A, Fan1 deficiency results in DNA interstrand cross-link repair defects, enhanced tissue karyomegaly, and organ dysfunction, Genes Dev, 30 (2016) 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Buzon B, Grainger R, Huang S, Rzadki C, Junop MS, Structure-specific endonuclease activity of SNM1A enables processing of a DNA interstrand crosslink, Nucleic Acids Res, 46 (2018) 9057–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Abdullah UB, McGouran JF, Brolih S, Ptchelkine D, El-Sagheer AH, Brown T, McHugh PJ, RPA activates the XPF-ERCC1 endonuclease to initiate processing of DNA interstrand crosslinks, EMBO J, 36 (2017) 2047–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang AT, Sengerová B, Cattell E, Inagawa T, Hartley JM, Kiakos K, Burgess -Brown NA, Swift LP, Enzlink JH, Schofield CJ, Gilead O, Hartley JA, McHugh PJ, Human SNM1A and XPF–ERCC1 collaborate to initiate DNA interstrand cross-link repair, Genes Dev, 25 (2011) 1859–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schärer OD, ERCC1-XPF endonuclease—positioned to cut, EMBO J, 36 (2017) 1993–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Roy U, Schärer OD, Involvement of Translesion Synthesis DNA Polymerases in DNA Interstrand Crosslink Repair, DNA Repair (Amst), 44 (2016) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ho TV, Schärer OD, Translesion DNA synthesis polymerases in DNA interstrand crosslink repair, Env. Mol. Mutagenesis, 51 (2010) 552–566. [DOI] [PubMed] [Google Scholar]
- [99].Klug AR, Harbut MB, Lloyd RS, Minko IG, Replication Bypass of N2-deoxyguanosine Interstrand Cross-Links by Human DNA Polymerases η and ι, Chem. Res. Toxicol, 25 (2012) 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Price NE, Li L, Gates KS, Wang Y, Replication and repair of a reduced 2’-deoxyguanosine- abasic site cross-link in human cells, Nucleic Acids Res, 45 (2017) 6486–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Budzowska M, Graham TGW, Sobeck A, Waga S, Walter JC, Regulation of the Rev1-pol zeta complex during bypass of a DNA interstrand cross-link, EMBO J, 34 (2015) 1971–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sarkar S, Davies AA, Ulrich HD, McHugh PJ, DNA interstrand crosslink repair during G1 involves nucleotide excision repair and DNA polymerase ζ, EMBO J, 25 (2006) 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sharma S, Canman CE, REV1 and DNA polymerase zeta in DNA interstrand cross-link repair, Env. Mol. Mutagenesis, 53 (2010) 725–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hinz JM, Role of homologous recombination in DNA interstrand cross-link repair, Env. Mol. Mutagenesis, 51 (2010) 582–603. [DOI] [PubMed] [Google Scholar]
- [105].Michl J, Zimmer J, Tarsounas M, Interplay between Fanconi anemia and homologous recombination pathways in genome integrity, EMBO J, 35 (2016) 909–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Long DT, Räschle M, Joukov V, Walter JC, Mechanism of RAD51-dependent DNA interstrand cross-link repair, Science, 333 (2011) 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Niraj J, Färkkilä A, D’Andrea AD, The Fanconi Anemia Pathway in Cancer, Annu. Rev. Cancer Biol, 3 (2019) 457–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rageul J, Kim H, Fanconi Anemia and the Underlying Causes of Genomic Instability, Env. Mol. Mutagenesis, 61 (2020) 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Neveling K, Endt D, Hoehn H, Schindler D, Genotype-phenotype correlations in Fanconi anemia, Mutation Res, 668 (2009) 73–91. [DOI] [PubMed] [Google Scholar]
- [110].Tischkowitz MD, Hodgson SV, Fanconi anaemia J. Med. Genet, 40 (2003) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Auerbach AD, Wolman SR, Susceptibility of Fanconi’s anaemia fibroblasts to chromosome damage by carcinogens, Nature, 261 (1976) 494–496. [DOI] [PubMed] [Google Scholar]
- [112].Garaycoechea JI, Patel KJ, Why does the bone marrow fail in Fanconi anemia?, Blood, 123 (2014) 26–34. [DOI] [PubMed] [Google Scholar]
- [113].Sakai W, Sugasawa K, Importance of finding the bona fide target of the Fanconi anemia pathway, Genes Environ., 41 (2019) 10.1186/s41021-41019-40122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Brosh RMJ, Bellani M, Liu Y, Seidman MM, Fanconi Anemia: a DNA Repair Disorder Characterized by Accelerated Decline of the Hematopoietic Stem Cell Compartment and Other Features of Aging, Ageing Res. Rev, 33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wu RA, Semlow DR, Kamimae-Lanning AN, Kochenova OV, Chistol G, Hodskinson MRG, Amunugama R, Sparks JL, Wang M, Deng L, Mimoso CA, Low E, Patel KJ, Walter JC, TRAIP is a master regulator of DNA interstrand crosslink repair, Nature, 567–272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Semlow DR, Zhang J, Budzowska M, Drohat AC, Walter JC, Replication-dependent unhooking of DNA interstrand cross-links by the NEIL3 glycosylase, Cell, 167 (2016) 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yang Z, Nejad MI, Gamboa Varela J, Price NE, Wang Y, Gates KS, A role for the base excision repair enzyme NEIL3 in replication-dependent repair of interstrand cross-links derived from psoralen and abasic sites, DNA Repair, 52 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Huang J-C, Liu S, Bellani MA, Thazhathveetil AK, Ling C, de Winter JP, Wang Y, Wang W, Seidman MM, The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand cross-links, Mol. Cell, 52 (2013) 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rohleder F, Kuper J, Kisker C, Huang J-C, Seidman M, Xue Y, Wang W, Round A, FANCM interacts with PCNA to promote replication traverse of DNA interstrand crosslinks, Nucleic Acids Res, 44 (2016) 3219–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kato N, Kawasoe Y, Williams HL, Coates E, Roy U, Shi Y, Beese LS, Schärer OD, Yan H, Gottesman ME, Takahashi TS, Gautier J, Sensing and processing of DNA interstrand crosslinks by the mismatch repair pathway, Cell Rep., 21 (2017) 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Williams HL, Gottesman ME, Gautier J, The differences between ICL repair during and outside of S phase, Trends Biochem. Sci, 38 (2013) 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Williams HL, Gottesman ME, Gautier J, Replication-Independent Repair of DNA Interstrand Crosslinks, Mol. Cell, 47 (2012) 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Oh S, Bae W, Alfhili MA, Lee MH, Nucleotide Excision Repair XPA -1, and the Translesion Synthesis Complex, POLZ‐ 1 and REV-1, Are Critical for Interstrand Cross-Link Repair in Caenorhabditis elegans Germ Cells, Biochemistry, 59 (2020) 3554–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Fritz KS, Petersen DR, An overview of the chemistry and biology of reactive aldehydes, Free Rad. Biol. Med, 59 (2013) 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].O’Brien PJ, Siraki AG, Shangari N, Aldehyde sources, metabolism, molecular toxicity, and possible effects on human health, Crit. Rev. Toxicol, 35 (2005) 609–662. [DOI] [PubMed] [Google Scholar]
- [126].Voulgaridou G-P, Anestopoulos I, Franco R, Panayiotidis MI, Pappa A, DNA damage induced by endogenous aldehydes: current state of knowledge, Mutation Res., 711 (2011) 13–27. [DOI] [PubMed] [Google Scholar]
- [127].LoPachin RM, Gavin T, Molecular mechanisms of aldehyde toxicity: a chemical perspective, Chem. Res. Toxicol, 27 (2014) 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liu C-W, Tian X, Hartwell HJ, Leng J, Chi L, Lu K, Swenberg JA, Accurate Measurement of Formaldehyde-Induced DNA-Protein Cross-Links by High-Resolution Orbitrap Mass Spectrometry, Chem. Res. Toxicol, 31 (2018) 350–357. [DOI] [PubMed] [Google Scholar]
- [129].Weng M.-w., Lee H-W, Park S-H, Hu Y, Wang H-T, Chen L-C, Rom WN, Huang WC, Lepor H, Wu X-R, Yang CS, Tang M.-s., Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobaccosmoke carcinogenesis, Proc. Nat. Acad. Sci. USA, 115 (2018) E6152–E6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lin D, Li W, Tian X, Peng Y, Zheng J, In Vitro DNA Adduction Resulting from Metabolic Activation of Diosbulbin B and 8- Epidiosbulbin E Acetate, Chem. Res. Toxicol, 32 (2019) 38–48. [DOI] [PubMed] [Google Scholar]
- [131].Shuck SC, Wuenschell GE, Termini JS, Product Studies and Mechanistic Analysis of the Reaction of Methylglyoxal with Deoxyguanosine, Chem. Res. Toxicol, 31 (2008) 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lim H-K, Chen J, Sensenhauser C, Cook K, Preston R, Thomas T, Shook B, Jackson PF, Rassnick S, Rhodes K, Gopaul V, Salter R, Silva J, Evans DC, Overcoming genotoxicity of a pyrrolidine substituted arylindenopyrimidine as a potent dual adenosine A2A/A1 antagonist by minimizing bioactivation to an iminium ion reactive intermediate, Chem. Res. Toxicol, 24 (2011) 1012–1030. [DOI] [PubMed] [Google Scholar]
- [133].Otero A, Chapela M-J, Atanassova M, Vieites JM, Cabado AG, Cyclic imines: chemistry and mechanism of action: a review, Chem. Res. Toxicol, 24 (2011) 1817–1829. [DOI] [PubMed] [Google Scholar]
- [134].Gates KS, Silverman RB, 5-(Aminomethyl)-3-aryl-2-oxazolidinones. A novel class of mechanism-based inactivators of monoamine oxidase B, J. Am. Chem. Soc, 112 (1990) 9364–9372. [Google Scholar]
- [135].Luo M, Gates KS, Henzl MT, Tanner JJ, Diethylaminobenzaldehyde Is a Covalent, Irreversible Inactivator of ALDH7A1, ACS Chem. Biol, 10 (2015) 693–697. [DOI] [PubMed] [Google Scholar]
- [136].Perni RB, Britt SD, Court JJ, Courtney LF, Deininger DD, Farmer LJ, Gates CA, Harbeson SL, Kim JL, Landro JA, Levin RB, Luong Y-P, O’Malley ET, Pitlik J, Rao BG, Thomson JA, Tung RD, Van Drie JH, Wei Y, Inhibitors of Hepatitis C Virus NS3.4A Protease 1. Non- charged Tetrapeptide Variants, Bioorg. Med. Chem. Lett, 13 (2003) 4059–4063. [DOI] [PubMed] [Google Scholar]
- [137].Wyatt JW, Korasick DA, Qureshi IA, Campbell AC, Gates KS, Tanner JJ, Inhibition, crystal structures, and in-solution oligomeric structure of aldehyde dehydrogenase 9A1, Arch. Biochem. Biophys, 691 (2020) 108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Maly DJ, Allen JA, Shokat KM, A mechanism-based cross-linker for the identification of kinase-substrate pairs, J. Am. Chem. Soc, 126 (2004) 9160–9161. [DOI] [PubMed] [Google Scholar]
- [139].Liang SI, McFarland JM, Rabuka D, Gartner ZJ, A Modular Approach for Assembling Aldehyde-Tagged Proteins on DNA Scaffolds, J. Am. Chem. Soc, 136 (2014) 10850–10853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Spears RJ, Fascione MA, Site-Selective Incorporation and Ligation of Protein Aldehydes, Org. Biomol. Chem, 14 (2016) 7622–7638. [DOI] [PubMed] [Google Scholar]
- [141].Casi G, Huguenin-Dezot N, Zuberbühler K, Scheuermann J, Neri D, Site-Specific Traceless Coupling of Potent Cytotoxic Drugs to Recombinant Antibodies for Pharmacodelivery, J. Am. Chem. Soc, 134 (2012) 5887–5892. [DOI] [PubMed] [Google Scholar]
- [142].Engreitz JM, Sirokman K, McDonel P, Shishkin AA, Surka C, Russell P, Grossman SR, Chow AY, Guttman M, Lander ES, RNA-RNA interactions enable specific targeting of noncoding RNAs to nascent pre-mRNAs and chromatin sites, Cell, 159 (2014) 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC, Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking, BioTechniques, 37 (2004) 790–802. [DOI] [PubMed] [Google Scholar]
- [144].Hoffman EA, Frey BL, Smith LM, Auble DT, Formaldehyde cross-linking: a tool for the study of chromatin complexes, J. Biol. Chem, 290 (2015) 26404–26411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hansen JL, Ippolito JA, Ban N, Nissen P, Moore PB, Steitz TA, The structures of four macrolide antibiotics bound to the large ribosomal subunit, Mol. Cell, 10 (2002) 117–128. [DOI] [PubMed] [Google Scholar]
- [146].Karmakar S, Harcourt EM, Hewings DS, Scherer F, Lovejoy AF, Kurtz DM, Ehrenschwender T, Barandun LJ, Roost C, Alizadeh AA, Kool ET, Organocatalytic Removal of Formaldehyde Adducts From RNA and DNA Bases, 2015, 7 (2015) 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kanchuger MS, Byers LD, Acyl Substituent Effects on Thiohemiacetal Equilibria, J. Am. Chem. Soc, 101 (1979) 3005–3010. [Google Scholar]
- [148].Appel R, Mayr H, Quantification I. of the Electrophilic Reactivities of Aldehydes, and Enones, R.A.a.H. Mayr, Quantification of the Electrophilic Reactivities of Aldehydes, Imines, and Enones, J. Am. Chem. Soc, 133 (2011) 8240–8251. [DOI] [PubMed] [Google Scholar]
- [149].Lienhard GE, Jencks WP, Thiol Addition to the Carbonyl Group. Equilibria and Kinetics, J. Am. Chem. Soc, 88 (1966) 3982–3995. [DOI] [PubMed] [Google Scholar]
- [150].Sander EG, Jencks WP, Jencks E.G.S.a.W.P, Equilibria for Additions to the Carbonyl Group, J. Am. Chem. Soc, 90 (1968) 6154–6162. [Google Scholar]
- [151].Rendina AR, Hermes JD, Cleland WW, A novel method for determining rate constants for dehydration of aldehyde hydrates, Biochemistry, 23 (1984) 5148–5156. [DOI] [PubMed] [Google Scholar]
- [152].Greenzaid P, Luz Z, Samuel D, A nuclear magnetic resonance study of the reversible hydration of aliphatic aldehydes and ketones. II. The acid-catalyzed oxygen exchange of acetaldehyde, J. Am. Chem. Soc, 89 (1967) 756–759. [Google Scholar]
- [153].Kallen RG, Jencks WP, The mechanism of the condensation of formaldehyde with tetrahydrofolic acid, J. Biol. Chem, 241 (1966) 5851–5863. [PubMed] [Google Scholar]
- [154].McGhee JD, von Hippel PH, Formaldehyde as a probe of DNA structure. I. Reaction with exocyclic groups of DNA bases, Biochemistry, 14 (1975) 1281–1296. [DOI] [PubMed] [Google Scholar]
- [155].McGhee JD, von Hippel PH, Formaldehyde as a probe of DNA structure. II. Reaction with endocyclic imino groups of DNA bases, Biochemistry, 14 (1975) 1297–1303. [DOI] [PubMed] [Google Scholar]
- [156].Zhang S, Villalta PW, Wang M, Hecht SS, Analysis of Crotonaldehyde- and Acetaldehyde- Derived 1,N2-Propanodeoxyguanosine Adducts in DNA from Human Tissues Using Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry, Chem. Res. Toxicol, 19 (2006) 1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS, Identification of DNA Adducts of Acetaldehyde, Chem. Res. Toxicol, 13 (2000) 1149–1157. [DOI] [PubMed] [Google Scholar]
- [158].Medeiros MHG, DNA Damage by Endogenous and Exogenous Aldehydes, J. Braz. Chem. Soc., 30 (2019) 2000–2009. [Google Scholar]
- [159].Chaw YFM, Crane LE, Lange P, Shapiro R, isolation and identification of cross-links from formaldehyde-treated nucleic acids, Biochemistry, 19 (1980) 5525–5531. [DOI] [PubMed] [Google Scholar]
- [160].Stone MP, Cho YJ, Huang H, Kim HY, Kozekov ID, Kozekova A, Wang H, Minko IG, Lloyd RS, Harris TM, Rizzo CJ, Interstrand cross-links induced by alpha, beta-unsaturated aldehydes derived from lipid peroxidation and environmental sources, Acc. Chem. Res, 41 (2008) 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Gates KS, Covalent Modification of DNA by Natural Products, in: Kool ET (Ed.) Comprehensive Natural Products Chemistry, Pergamon, New York, 1999, pp. 491–552. [Google Scholar]
- [162].Byrns MC, Predecki DP, Peterson LA, Characterization of nucleoside adducts of cis −2-butene- 1,4-dial, a reactive metabolite of furan, Chem. Res. Toxicol, 15 (2002) 373–379. [DOI] [PubMed] [Google Scholar]
- [163].Cordes EH, Jencks WP, On the mechanism of Schiff base formation and hydrolysis, J. Am. Chem. Soc, 84 (1962) 832–837. [Google Scholar]
- [164].Cordes EH, Jencks WP, The mechanism of hydrolysis of Schiff bases derived from aliphatic amines, J. Am. Chem. Soc, 85 (1963) 2843–2848. [Google Scholar]
- [165].Saggiomo V, Lüning U, On the formation of imines in water - a comparison, Tet. Lett., 50 (2009) 4663–4665. [Google Scholar]
- [166].Borch RF, Bernstein MD, Durst HD, The cyanohydridoborate anion as a selective reducing agent, J. Am. Chem. Soc, 93 (1971) 2897–2904. [Google Scholar]
- [167].Borch RF, Hassid AI, A new method for the methylation of amines, J. Org. Chem, 37 (1972) 1673–1674. [Google Scholar]
- [168].Yang Z, Price NE, Johnson KM, Wang Y, Gates KS, Interstrand cross-links arising from strand breaks at true abasic sites in duplex DNA, Nucleic Acids Res, 45 (2017) 6275–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Minko IG, Jacobs AC, de Leon AR, Gruppi F, Donley N, Harris TM, Rizzo CJ, McCullough AK, Lloyd RS, Catalysts of DNA strand cleavage at apurinic/apyrmidinic sites, Sci. Rep, 6 (2016) DOI: 10.1038/srep28894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Marchand C, Krajewski K, Lee H-F, Antony S, Johnson AA, Amin R, Roller PP, Kvaratskhelia M, Pommier Y, Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites, Nucleic Acids Res, 34 (2006) 5157–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Kurtz AJ, Dodson ML, Lloyd RS, Evidence for multiple imino intermediates and identification of reactive nucleophiles in peptide-catalyzed beta-elimination at abasic sites, Biochemistry, 41 (2002) 7054–7064. [DOI] [PubMed] [Google Scholar]
- [172].Sun B, Latham KA, Dodson ML, Lloyd RS, Studies on the catalytic mechanism of five DNA glycosylases, J. Biol. Chem, 270 (1995) 19501–19508. [DOI] [PubMed] [Google Scholar]
- [173].Fromme JC, Verdine GL, Structure of a trapped endonuclease III-DNA covalent intermediate, EMBO J, 22 (2003) 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Koissi N, Fishbein JC, Trapping of a cross-link formed by a major purine adduct of a metabolite of the carcinogen N-nitrosomorpholine by inorganic and biological reductants, Chem. Res. Toxicol, 26 (2013) 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Vaca CE, Fangay J-L, Schweda EKH, Studies of the action of acetaldehyde with deoxynucleosides, Chem. Biol. Interact, 98 (1995) 51–67. [DOI] [PubMed] [Google Scholar]
- [176].Wang M, Yu N, Chen L, Villalta PW, Hochalter JB, Hecht SM, Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine, Chem. Res. Toxicol, 19 (2006) 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Sanchez D, Bastida D, Burés J, Isart C, Pineda O, Vilarrasa J, Relative Tendency of Carbonyl Compounds To Form Enamines, Org. Lett, 14 (2012) 536–539. [DOI] [PubMed] [Google Scholar]
- [178].Eder E, Hoffman C, Identification and Characterization of Deoxyguanosine Adducts of Mutagenic beta-Alkyl-Substituted Acrolein Congeners, Chem. Res. Toxicol, 6 (1993) 486–494. [DOI] [PubMed] [Google Scholar]
- [179].Calow ADJ, Carbó JJ, Cid J, Fernández E, Whiting A, Understanding α,β-Unsaturated Imine Formation from Amine Additions to α,β-Unsaturated Aldehydes and Ketones: An Analytical and Theoretical Investigation, J. Org. Chem, 79 (2014) 5163–5172. [DOI] [PubMed] [Google Scholar]
- [180].Pawlowicz AJ, Klika KD, Kronberg L, The structural identification and conformational analysis of the products from the reaction of acrolein with 2’-deoxycytidine, 1-methylcytosine and calf thymus DNA, Eur. J. Org. Chem, 2007 (2007) 1429–1437. [Google Scholar]
- [181].Pawlowicz AJ, Kronberg L, Characterization of adducts formed in the reactions of acrolein with thymidine and calf thymus DNA, Chem. Biodiversity, 5 (2008) 177–188. [DOI] [PubMed] [Google Scholar]
- [182].Pawlowicz AJ, Munter T, Zhao Y, Kronberg L, Formation of acrolein adducts with 2’- deoxyadenosine in calf thymus DNA, Chem. Res. Toxicol, 19 (2006) 571–576. [DOI] [PubMed] [Google Scholar]
- [183].Lindahl T, Andersson A, Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid, Biochemistry, 11 (1972) 3618–3623. [DOI] [PubMed] [Google Scholar]
- [184].Nooner T, Dutta S, Gates KS, Chemical properties of the leinamycin 2’-deoxyguanosine adduct, Chem. Res. Toxicol, 17 (2004) 942–949. [DOI] [PubMed] [Google Scholar]
- [185].Krokan HE, Bjørås M, Base Excision Repair, Cold Spring Harb. Perspect. Biol, 5 (2013) a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Brooks SC, Adhikary S, Rubinson EH, Eichman BF, Recent advances in the structural mechanisms of DNA glycosylases, Biochim. Biophys. Acta, 1834 (2013) 247–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Wilde JA, Bolton PH, Mazumdar A, Manoharan M, Gerlt JA, Characterization of the equilibrating forms of the abasic site in duplex DNA using 17O-NMR, J. Am. Chem. Soc, 111 (1989) 1894–1896. [Google Scholar]
- [188].Dutta S, Chowdhury G, Gates KS, Interstrand crosslinks generated by abasic sites in duplex DNA, J. Am. Chem. Soc, 129 (2007) 1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Johnson KM, Price NE, Wang J, Fekry MI, Dutta S, Seiner DR, Wang Y, Gates KS, On the Formation and Properties of Interstrand DNA-DNA Cross-links Forged by Reaction of an Abasic Site With the Opposing Guanine Residue of 5’-CAp Sequences in Duplex DNA, J. Am. Chem. Soc, 135 (2013) 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Price NE, Johnson KM, Wang J, Fekry M, I., Wang Y, Gates KS, Interstrand DNA−DNA Cross-Link Formation Between Adenine Residues and Abasic Sites in Duplex DNA, J. Am. Chem. Soc, 136 (2014) 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Catalano MJ, Price NE, Gates KS, Effective molarity in a nucleic acid controlled reaction, Bioorg. Med. Chem. Lett, 26 (2016) 2627–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Catalano MJ, Liu S, Andersen N, Yang Z, Johnson KM, Price NA, Wang Y, Gates KS, Chemical structure and properties of the interstrand cross-link formed by the reaction of guanine residues with abasic sites in duplex DNA, J. Am. Chem. Soc, 137 (2015) 3933–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Imani-Nejad M, Guo X, Housh K, Nel C, Yang Z, Price NE, Wang Y, Gates KS, Preparation and purification of oligodeoxynucleotide duplexes containing a site-specific, reduced, chemically stable covalent interstrand cross-link between a guanine residue and an abasic site, Methods Mol. Biol, 1973 (2019) 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Imani-Nejad M, Price NE, Haldar T, Lewis C, Wang Y, Gates KS, Interstrand DNA Cross- Links Derived from Reaction of a 2- Aminopurine Residue with an Abasic Site, ACS Chem. Biol, 14 (2019) 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Guo X, Imani-Nejad M, Gu L-Q, Gates KS, Selective covalent capture of a DNA sequence corresponding to a cancer-driving C>G mutation in the KRAS gene by a chemically reactive probe: optimizing a cross-linking reaction with non- canonical duplex structures, RSC Adv, 9 (2019) 32804–32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Imani-Nejad M, Shi R, Zhang X, Gu L-Q, Gates KS, Sequence-specific covalent capture coupled with high-contrast nanopore detection of a disease-derived nucleic acid sequence, ChemBioChem, 18 (2017) 1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Price NE, Catalano MJ, Liu S, Wang Y, Gates KS, Chemical and structural characterization of interstrand cross-links formed between abasic sites and adenine residue in duplex DNA, Nucleic Acids Res, 43 (2015) 3434–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Yang Z, Johnson KM, Price NE, Gates KS, Characterization of interstrand DNA-DNA cross- links derived from abasic sites using bacteriophage φ29 DNA polymerase, Biochemistry, 54 (2015) 4259–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Catalano MJ, Ruddraraju KV, Gates KS, Crystal structure of a nucleoside model of the interstrand cross-link formed by the reaction of 2’-deoxyguanosine and an abasic site in duplex DNA, Acta Cryst. E, E72 (2016) 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Mons S, Fleet GWJ, An approach to the generation of simple analogues of the antitumor agent spicamycin, Org. Biomol. Chem, 1 (2003) 3685–3691. [DOI] [PubMed] [Google Scholar]
- [201].Takamura-Enya T, Watanabe M, Totsuka Y, Kanazawa T, Matsushima-Hibya Y, Koyama K, Sugimura T, Wakabayashi K, Mono(ADP-ribosyl)ation of 2’-deoxyguanosine residue in DNA by an apoptosis-inducing protein, pierisin-1, from cabbage butterfly, Proc. Nat. Acad. Sci. USA, 98 (2001) 12414–12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Fuller WD, Sanchez RA, Orgel LE, Studies in prebiotic synthesis VI. Synthesis of purine nucleosides, J. Mol. Biol, 67 (1972) 25–33. [DOI] [PubMed] [Google Scholar]
- [203].Young-Sciame R, Wang M, Chung F-L, Hecht SM, Reactions of alpha-acetoxy-N- nitrosopyrrolidine and alpha-acetoxy-N-nitrosopiperidine with deoxyguanosine: formation of N2- tetrahydrofuranyl and N2-tetrahydropyranyl adducts, Chem. Res. Toxicol, 8 (1995) 607–616. [DOI] [PubMed] [Google Scholar]
- [204].Ochs S, Severin T, Reaction of 2’-deoxyguanosine with glucose, Carbohydrate Res, 266 (1995) 87–94. [Google Scholar]
- [205].Hopkins PB, Millard JT, Woo J, Weidner MF, Kirchner JJ, Sigurdsson ST, Raucher S, Sequence preferences of DNA interstrand cross-linking agents: Importance of minimal DNA structural reorganization in the cross-linking reactions of mechlorethamine, cisplatin, and mitomycin C, Tetrahedron, 47 (1991) 2475–2489. [Google Scholar]
- [206].Kellum AH, Qiu DY, Voehler MW, Martin W, Gates KS, Stone MP, Structure of a Stable Interstrand DNA Cross-Link Involving a β- N- Glycosyl Linkage Between an N6- dA Amino Group and an Abasic Site, Biochemistry, (2020) doi: 10.1021/acs.biochem.1020c00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Choi J-Y, Lim S, Kim EJ, Jo A, Guengerich FP, Translesion synthesis across abasic lesions by human B-family and Y-family DNA polymerases alpha, delta, eta, iota, kappa, and REV 1, J. Mol. Biol, 404 (2010) 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Imani-Nejad M, Housh K, Rodriguez AA, Haldar T, Kathe S, Wallace SS, Eichman BF, Gates KS, Unhooking of an interstrand cross-link at DNA fork structures by the DNA glycosylase NEIL3, DNA Repair, 86 (2020) 102752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Rodriguez AA, Wojtaszek JL, Greer BH, Haldar T, Gates KS, Williams RS, Eichman BF, An autoinhibitory role for the GRF zinc finger domain of DNA glycosylase NEIL3, J. Biol. Chem, (2020) doi: 10.1074/jbc.RA1120.015541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Male R, Fosse VM, Kleppe K, Polyamine-induced hydrolysis of apurinic sites in DNA and nucleosomes, Nucleic Acid Res, 10 (1982) 6305–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].McHugh PJ, Knowland J, Novel reagents for chemical cleavage at abasic sites and UV photoproducts in DNA, Nucleic Acids Res, 23 (1995) 1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].McCullough AK, Sanchez A, Dodson ML, Marapaka P, Taylor JS, Lloyd RS, The reaction mechanism of DNA glycosylase/AP lyases at abasic sites, Biochemistry, 40 (2001) 561–568. [DOI] [PubMed] [Google Scholar]
- [213].Mazumder A, Gerlt JA, Absalon MJ, Stubbe J, Cunningham RP, Withka J, Bolton PH, Stereochemical studies of the b-elimination reactions at aldehydic abasic sites in DNA: endonuclease III from Escherichia coli, sodium hydroxide, and Lys-Trp-Lys, Biochemistry, 30 (1991) 1119–1126. [DOI] [PubMed] [Google Scholar]
- [214].Rahimoff R, Kosmatchev O, Kirchner A, j. Pfaffeneder, F. Spada, V. Brantl, M. Müller, T. Carell, 5-Formyl- and 5-carboxydeoxycytidines do not cause accumulation of harmful repair intermediates in stem cells, J. Am. Chem. Soc, 139 (2017) 10359–10364. [DOI] [PubMed] [Google Scholar]
- [215].Luke AM, Chastain PD, Pachkowski BF, Afonin V, Takeda S, Kaufman DG, Swenberg JA, Nakamura J, Accumulation of true single strand breaks and AP sites in base excision repair deficient cells, Mutation Res, 694 (2010) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Smith RA, Williamson DS, Cerny RL, Cohen SM, Detection of 1,N6-propanodeoxyadenosine in acrolein-modified polydeoxyadenylic acid and DNA by 32P-postlabeleing, Cancer Res, 50 (1990) 3005–3012. [PubMed] [Google Scholar]
- [217].Burcham PC, Genotoxic lipid peroxidation products: Their DNA damaging properties and role in formation of endogenous DNA adducts, Mutagenesis 13 (1998) 287–305. [DOI] [PubMed] [Google Scholar]
- [218].Chung F-L, Young R, Hecht SM, Formation of Cyclic 1,N2-Propanodeoxyguanosine Adducts in DNA upon Reaction with Acrolein or Crotonaldehyde, Cancer Res, 44 (1984) 990–995. [PubMed] [Google Scholar]
- [219].Chenna A, Iden CR, Characterization of 2’-deoxycytidine and 2’-deoxyuridine adducts formed in reactions with acrolein and 2-bromoacrolein, Chem. Res. Toxicol, 6 (1993) 261–268. [DOI] [PubMed] [Google Scholar]
- [220].Chenna A, Reiger RA, Iden CR, Characterization of Thymidine Adducts Formed by Acrolein and 2-bromoacrolein, Carcinogenesis, 13 (1992) 2361–2365. [DOI] [PubMed] [Google Scholar]
- [221].Seitz HK, Stickel F, Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism, Genes, Nutr., 5 (2010) 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Brooks PJ, Enoch M-A, Goldman D, Li T-K, Yokohama A, The Alcohol Flushing Response: An Unrecognized Risk Factor for Esophageal Cancer from Alcohol Consumption, PLoS Med., 6 (2009) e1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].O’Brien PJ, Siraki AG, Shangari N, Aldehyde Sources, Metabolism, Molecular Toxicity Mechanisms, and Possible Effects on Human Health, Crit. Rev. Toxicol, 35 (2005) 609–662. [DOI] [PubMed] [Google Scholar]
- [224].Tagaino R, Washio J, Abiko Y, Tanda N, Sasaki K, Takahashi N, Metabolic property of acetaldehyde production from ethanol and glucose by oral Streptococcus and Neisseria, Sci. Rep, 9 (2019) 10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Fraenkel-Conrat H, Singer B, Nucleoside adducts are formed by cooperative reaction of acetaldehyde and alcohols: Possible mechanism for the roleofethanolincarcinogenesis, Proc. Nat. Acad. Sci. USA, 85 (1988) 3758–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Sonohara Y, Yamamoto J, Tohashi K, Takasuka R, Matsuda T, Iwai S, Kuraoka I, Acetaldehyde forms covalent GG intrastrand crosslinks in DNA, Sci. Rep, 9 (2019) 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Brooks PJ, Theruvathu JA, DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis, Alcohol, 35 (2005) 187–193. [DOI] [PubMed] [Google Scholar]
- [228].Ristow H, Obe G, Acetaldehyde induces cross-links in DNA and causes sister-chromatid exchanges in human cells, Mutat. Res, 58 (1978) 115–119. [DOI] [PubMed] [Google Scholar]
- [229].Lambert B, Chen Y, He S-M, Sten M, DNA crosslinks in human leucocyctes treated with vinyl acetate and acetaldehyde in vitro, Mutat. Res, 146 (1985) 301–303. [DOI] [PubMed] [Google Scholar]
- [230].Matsuda T, Kawanishi M, Yagi T, Matsui S, Takebe H, Specific tandem GG to TT base substitutions induced by acetaldehyde are due to intra-strand crosslinks between adjacent guanine bases, Nucleic Acids Res, 26 (1998) 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Lao Y, Hecht SS, Synthesis and Properties of an Acetaldehyde-Derived Oligonucleotide Interstrand Cross-Link, Chem. Res. Toxicol, 18 (2005) 711–721. [DOI] [PubMed] [Google Scholar]
- [232].Cho Y-J, Wang H, Kozekov ID, Kurtz AJ, Jacob J, Voehler M, Smith J, Harris TM, Lloyd RS, Rizzo CJ, Stone MP, Stereospecific formation of interstrand carbinolamine DNA cross-links by crotonaldehyde- and acetaldehyde-derived alpha-CH3, gamma-OH, N2-propano-2’-deoxyguanosine adducts in the 5’-CpG-3’ sequence, Chem. Res. Toxicol, 19 (2006) 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Guthrie JP, The Aldol Condensation of Acetaldehyde: the Equilibrium Constant for the I Reaction and the Rate Constant for the Hydroxide Catalyzed RetroAldol Reaction, Can. J. Chem, 52 (1974) 2037–2040. [Google Scholar]
- [234].Carrião C, Garcia M, Angeli JPF, Freitas FP, Gomes OF, de Oliveira TF, Loureiro APM, Di Mascio P, Medeiros MHG, [13C2]-Acetaldehyde Promotes Unequivocal Formation of 1,N2- Propano-2’-deoxyguanosine in Human Cells, J. Am. Chem. Soc, 133 (2011) 9140–9143. [DOI] [PubMed] [Google Scholar]
- [235].Matsuda T, Yabushita H, Kanaly RA, Shibutani S, Yokoyama A, Increased DNA Damage in ALDH2-Deficient Alcoholics, Chem. Res. Toxicol, 19 (2006) 1374–1378. [DOI] [PubMed] [Google Scholar]
- [236].Nozière B, Córdova A, A Kinetic and Mechanistic Study of the Amino Acid Catalyzed Aldol Condensation of Acetaldehyde in Aqueous and Salt Solutions, J. Phys. Chem. A, 112 (2008) 2827–2837. [DOI] [PubMed] [Google Scholar]
- [237].Theruvathu JA, Jaruga P, Nath RG, Dizdaroglu M, Brooks PJ, Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde, Nucleic Acids Res, 33 (2005) 3513–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Sako M, Inagaki S, Esaka Y, Deyashiki Y, Histones accelerate the cyclic 1,N2-propanoguanine adduct-formation of DNA by the primary metabolite of alcohol and carcinogenic crotonaldehyde, Bioorg. Med. Chem. Lett, 13 (2003) 3497–3498. [DOI] [PubMed] [Google Scholar]
- [239].Sako M, Yaekura I, Deyashiki Y, Chemo- and regio-selective modifications of nucleic acids by acetaldehyde and crotonaldehyde, Nucleic Acids Res. Suppl, 2 (2002) 21–22. [DOI] [PubMed] [Google Scholar]
- [240].Kozekov ID, Nechev LV, Moseley MS, Harris CM, Rizzo CJ, Stone MP, Harris TM, DNA Interchain Cross-Links Formed by Acrolein and Crotonaldehyde, J. Am. Chem. Soc, 125 (2003) 50–61. [DOI] [PubMed] [Google Scholar]
- [241].Dooley PA, Zhang M, Korbel GA, Nechev LV, Harris CM, Stone MP, Harris TM, NMR determination of the conformation of a trimethylene interstrand cross-link in an oligodeoxynucleotide duplex containing a 5′-d(GpC) motif, J. Am. Chem. Soc, 125 (2003) 62–72. [DOI] [PubMed] [Google Scholar]
- [242].Hodskinson MR, Bolner A, Sato K, Kamimae-Lanning AN, Rooijers K, Witte M, Mahesh M, Silhan J, Petek M, Williams DM, Kind J, Chin JW, Patel KJ, Knipsheer P, Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms, Nature, 579 (2020) 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Liu X, Lao Y, Yang I-Y, Hecht SS, Moriya M, Replication-Coupled Repair of Crotonaldehyde/Acetaldehyde-Induced Guanine-Guanine Interstrand Cross-Links and Their Mutagenicity, Biochemistry, 45 (2006) 12898–12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP, Chemistry and Biology of DNA Containing 1,N2-Deoxyguanosine Adducts of the α,β-Unsaturated Aldehydes Acrolein, Crotonaldehyde, and 4-Hydroxynonenal, Chem. Res. Toxicol, 22 (2009) 759–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Ghosh S, Sur S, Yerram SR, Rago C, Bhunia AK, Hossain MZ, Paun BC, Ren YR, Iacobuzio-Donahue CA, Azad NA, Kern SE, Hypersensitivities for Acetaldehyde and Other Agents among Cancer Cells Null for Clinically Relevant Fanconi Anemia Genes, Am. J. Pathol, 184 (2014) 26–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Ridpath JR, Nakamura A, Tano K, Luke AM, Sonoda E, Arakawa H, Buerstedde J-M, Gillespie DAF, Sale JE, Yamazoe M, Bishop DK, Takata M, Takeda S, Watanabe JA, Swenberg JA, Nakamura J, Cells Deficient in the FANC/BRCA Pathway Are Hypersensitive to Plasma Levels of Formaldehyde, Cancer Res, 67 (2007) 11117–11122. [DOI] [PubMed] [Google Scholar]
- [247].Abraham J, Balbo S, Crabb D, Brooks PJ, Alcohol Metabolism in Human Cells Causes DNA Damage and Activates the Fanconi Anemia–Breast Cancer Susceptibility (FA-BRCA) DNA Damage Response Network, Alcohol Clin. Exp. Res, 35 (2011) 2113–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Kotova N, Vare D, Schultz N, Meesters DG, Stępnik M, Grawé J, Helleday T, Jenssen D, Genotoxicity of alcohol is linked to DNA replication-associated damage and homologous recombination repair, Carcinogenesis, 34 (2013) 325–330. [DOI] [PubMed] [Google Scholar]
- [249].Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ, The Fanconi Anaemia DNA repair pathway counteracts the toxic effects of naturally produced aldehydes, Nature, 375 (2011) 53–58. [DOI] [PubMed] [Google Scholar]
- [250].Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ, Genotoxic Consequences of Endogenous Aldehydes on Mouse Haematopoietic Stem Cell Function, Nature, 489 (2012) 571–575. [DOI] [PubMed] [Google Scholar]
- [251].Oberbeck N, Lengevin F, King G, de Wind N, Crossan GP, Patel KJ, Maternal aldehyde elimination during pregnacy preserves the fetal genome, Mol. Cell, 55 (2014) 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Hira A, Yabe H, Yoshida K, Okuno Y, Shiraishi Y, Chiba K, Tanaka HM, S. , J. Nakamura, S. Kojima, a. et, Variant ALDH2 is associated with accelerated progression of bone marrow failure in Japanese Fanconi anemia patients, Blood, 122 (2013) 3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Klyosov AA, Kinetics and Specificity of Human Liver Aldehyde Dehydrogenases Toward Aliphatic, Aromatic, and Fused Polycyclic Aldehydes, Biochemistry 35 (1996) 4457–4467. [DOI] [PubMed] [Google Scholar]
- [254].Yoval-Sánchez B, Rodríguez-Zavala JS, Differences in susceptibility to inactivation of human aldehyde dehydrogenases by lipid peroxidation byproducts, Chem. Res. Toxicol, 25 (2012) 722–729. [DOI] [PubMed] [Google Scholar]
- [255].Chen C-H, Ferreira JCB, Gross ER, Mochly-Rosen D, Targeting Aldehyde Dehydrogenase 2: New Therapeutic Opportunities, Physiol. Rev, 94 (2014) 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Federico MB, Vallerga MB, Radl A, Paviolo NS, Bocco JL, Di Giorgio M, Soria G, Gottifredi V, Chromosomal Integrity after UV Irradiation Requires FANCD2-Mediated Repair of Double Strand Breaks, PLoS Genet., 12 (2016) e1005792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Fu D, Dudimah F, Zhang J, Pickering A, Paneerselvam J, Palrasu M, Wang H, Fei P, Recruitment of DNA polymerase eta by FANCD2 in the early response to DNA damage, Cell Cycle, 12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Nath RG, Ocando JE, Chung F-L, Detection of 1,N-propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues, Cancer Res, 56 (1996) 452–456. [PubMed] [Google Scholar]
- [259].Chung F-L, Nath RG, Ocando J, Nishikawa A, Zhang L, Deoxyguanosine adducts of t-4 hydroxy-2-nonenal are endogenous DNA lesions in rodents and humans: detection and potential sources, Cancer Res, 60 (2000) 1507–1511. [PubMed] [Google Scholar]
- [260].Stevens JF, Maier CS, Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease, Mol. Nutr. Food Res, 52 (2008) 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Esterbauer H, Schaur RJ, Zollner H, Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes, Free Rad. Biol. Med., 11 (1991) 81–128. [DOI] [PubMed] [Google Scholar]
- [262].Winter CK, Segall HJ, Haddon WF, Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro, Cancer Res, 46 (1986) 5682–5686. [PubMed] [Google Scholar]
- [263].Choudhury S, Dyba M, Pan J, Roy R, Chung F-L, Repair kinetics of acrolein- and (E)-4-hydroxy-2-nonenal-derived DNA adducts in human colon cell extracts, Mutat. Res, 751-752 (2013) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Singh V, Fedeles BI, Li D, Delaney JC, Kozekov ID, Kozekov K, Marnett LJ, Rizzo CJ, Essigmann JM, Mechanism of Repair of Acrolein- and Malondialdehyde-Derived Exocyclic Guanine Adducts by the α- Ketoglutarate/Fe(II) Dioxygenase AlkB, Chem. Res. Toxicol, 27 (2014) 1619–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Yang J, Balbo S, Villalta PW, Hecht SS, Analysis of Acrolein-Derived 1,N2- Propanodeoxyguanosine Adductsin Human Lung DNA from Smokers and Nonsmokers, Chem. Res. Toxicol, 32 (2019) 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Wang H, Kozekov ID, Harris TM, Rizzo CJ, Site-specific Synthesis and Reactivity of Oligonucleotides Containing Stereochemically Defined 1,N2-deoxyguanosine Adducts of the Lipid Peroxidation Product trans-4-hydroxynonenal, J. Am. Chem. Soc, 125 (2003) 5687–5700. [DOI] [PubMed] [Google Scholar]
- [267].Huang H, Kozekov ID, Kozekova A, Wang H, Lloyd RS, Rizzo CJ, Stone MP, DNA Cross- Link Induced by trans-4-hydroxynonenal, Environ. Mol. Mutagen, 51 (2010) 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Kawanishi M, Matsuda T, Nakayama A, Takebe H, Matsui S, Yagi T, Molecular analysis of mutations induced by acrolein in human fibroblast cells using supF shuttle vector plasmids, Mutat. Res, 417 (1998) 65–73. [DOI] [PubMed] [Google Scholar]
- [269].Brocker C, Cantore M, Failli P, Vasiliou V, Aldehydel dehydrogenase 7A1 (ALDH7A1) attenuates reactive aldehyde and oxidative stress induced cytotoxicity, Chem. Biol. Interact, 191 (2011) 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Makia NL, Bojang P, Falkner KC, Conklin DJ, Prough RA, Murine hepatic aldehyde dehydrogenase 1a1 is a major contributor to oxidation of aldehydes formed by lipid peroxidation, Chem. Biol. Interact, 191 (2011) 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].del Rio D, Stewart AJ, Pellegrin N, A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress, Nutr. Metab. Cardiovas. Dis, 15 (2005) 316–328. [DOI] [PubMed] [Google Scholar]
- [272].Marnett LJ, Lipid peroxidation-DNA damage by malondialdehyde, Mutat. Res, 424 (1999) 83–95. [DOI] [PubMed] [Google Scholar]
- [273].Dennis KJ, Shibamoto T, Gas chromatographic determination of malonaldehyde formed by lipid peroxidation, Free Rad. Biol. Med, 7 (1989) 187–192. [DOI] [PubMed] [Google Scholar]
- [274].Chaudhary AK, Reddy GR, Blair IA, Marnett LJ, Characterization of an N6-oxopropenyl-2’- deoxyadenosine adduct in malondialdehyde-modified DNA using liquid chromatography/electrospray ionization tandem mass spectrometry, Carcinogenesis, 17 (1996) 1167–1170. [DOI] [PubMed] [Google Scholar]
- [275].Stone K, Uzieblo A, Marnett LJ, Studies of the Reaction of Malondialdehyde with Cytosine Nucleosides, Chem. Res. Toxicol, 3 (1990) 467–472. [DOI] [PubMed] [Google Scholar]
- [276].Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ, Detection of endogenous malondialdehyde-deoxyguanine adducts in human liver, Science, 265 (1994) 1580–1582. [DOI] [PubMed] [Google Scholar]
- [277].Basu AK, O’Hara SM, Valladier P, Stone K, Mols O, Marnett LJ, Identification of adducts formed by reaction of guanine nucleosides with malondialdehyde and structurally related aldehydes, Chem. Res. Toxicol, 1 (1988) 53–59. [DOI] [PubMed] [Google Scholar]
- [278].Basu AK, Marnett LJ, Unequivocal demonstration that malondialdehyde is a mutagen, Carcinogenesis, 4 (1983) 331–333. [DOI] [PubMed] [Google Scholar]
- [279].Moschel RC, Leonard NJ, Fluorescent Modification of Guanine. Reaction With Substituted Malondialdehydes, J. Org. Chem, 41 (1976) 294–300. [DOI] [PubMed] [Google Scholar]
- [280].Plastaras JP, Riggins JN, Otteneder M, Marnett LJ, Reactivity and mutagenicity of endogenous DNA oxopropenylating agents: base propenals, malondialdehyde, and N-oxopropenyllysine, Chem. Res. Toxicol, 13 (2000) 1235–1242. [DOI] [PubMed] [Google Scholar]
- [281].Dedon PC, Plastaras JP, Rouzer CA, Marnett LJ, Indirect mutagenesis by oxidative DNA damage: formation of the pyrimidopurinone adduct of deoxyguanosine by base propenal, Proc. Nat. Acad. Sci. USA, 95 (1998) 11113–11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Mao H, Schnetz-Boutaud NC, Weisenseel JP, Marnett LJ, Stone MP, Duplex DNA catalyzes the chemical rearrangement of a malondialdehyde deoxyguanosine adduct, Proc. Nat. Acad. Sci. USA, 96 (1999) 6615–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ, Malondialdehyde, a Product of Lipid Peroxidation, Is Mutagenic in Human Cells, J. Biol. Chem, 278 (2003) 31426–31433. [DOI] [PubMed] [Google Scholar]
- [284].Basu AK, Marnett LJ, Romano LJ, Dissociation of malondialdehyde mutagenicity in Salmonella typhimurium from its ability to induce interstrand DNA cross-links, Mutat. Res, 129 (1984) 39–46. [DOI] [PubMed] [Google Scholar]
- [285].Wang H, Kozekov ID, Kozekova A, Tamura P, Marnett LJ, Harris TM, Rizzo CJ, Site- specific synthesis of oligonucleotides containing malondialdehyde adducts of deoxyguanosine and deoxyadenosine via a post-synthetic modification strategy, Chem. Res. Toxicol, 19 (2006) 1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Dooley PA, Tsarouhtsis D, Korbel GA, Nechev LV, Shearer J, Zegar IS, Harris CM, Stone MP, Harris TM, Structural Studies of an Oligodeoxynucleotide Containing a Trimethylene Interstrand Cross-Link in a 5′-(CpG) Motif: Model of a Malondialdehyde Cross-Link, J. Am. Chem. Soc, 123 (2001) 1730–1739. [DOI] [PubMed] [Google Scholar]
- [287].Voitkun V, Zhitkovich A, Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro, Mutat. Res, 424 (1999) 97–106. [DOI] [PubMed] [Google Scholar]
- [288].IARC, Formaldehyde, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 100F (2006) 401–435. [Google Scholar]
- [289].Tong ZQ, Han CS, Luo WH, Wang XH, Li H, Luo HJ, Zhou JN, Qi JS, He RQ, Accumulated hippocampal formaldehyde induces age-dependent memory decline, Age, 35 (2013) 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Heck HD, White E, Casanova-Schmitz M, Determination of formaldehyde in biological tissues by gas chromatography/mass spectrometry, Biomed. Mass Spectrom, 9 (1982) 347–353. [DOI] [PubMed] [Google Scholar]
- [291].Luo W, Li H, Zhang Y, Ang CY, Determination of formaldehyde in blood plasma by high- performance liquid chromatography with fluorescence detection, J. Chromatogr. B Biomed Sci. Appl., 753 (2001) 253–257. [DOI] [PubMed] [Google Scholar]
- [292].Zhang L, Freeman LEB, Nakamura J, Hecht SS, Vandenberg JJ, Smith MT, Sonawane BR, Formaldehyde and leukemia: epidemiology, potential mechanisms, and implications for risk assessment, Env. Mol. Mutagenesis, 51 (2010) 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Kennedy-Darling J, Smith LM, Measuring the formaldehyde protein-DNA cross-link reversal rate, Anal. Chem, 86 (2014) 5678–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Swenberg JA, Moeller BC, Lu K, Rager JE, Fry R, Starr TB, Formaldehyde Carcinogenicity Research: 30 Years and Counting for Mode of Action, Epidemiology, and Cancer Risk Assessment, Toxicol. Pathol, 41 (2013) 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Metz B, Kersten GFA, Hoogerhout P, Brugghe HF, Timmermans HAM, de Jong A, Meiring H, ten Hove J, Hennink WE, Crommelin DJA, Jiskoot W, Identification of formaldehyde-induced modifications in proteins: reactions with model peptides, J. Biol. Chem, 279 (2004) 6235–6243. [DOI] [PubMed] [Google Scholar]
- [296].Orlando V, Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation, Trends Biochem. Sci, 25 (2000) 99–104. [DOI] [PubMed] [Google Scholar]
- [297].Pluskota-Karwatka D, Le Curieux F, Munter T, Sjöholm R, Kronberg L, Formation of conjugate adducts in the reactions of malondialdehyde-acetaldehyde and malondialdehyde-formaldehyde with guanosine, Chem. Res. Toxicol, 18 (2005) 300–307. [DOI] [PubMed] [Google Scholar]
- [298].Shishodia S, Zhang D, El-Sagheer AH, Brown T, Claridge TDW, Schofield CJ, Hopkinson RJ, NMR analyses on N-hydroxymethylated nucleobases - implications for formaldehyde toxicity and nucleic acid demethylases, Org. Biomol. Chem, 16 (2018) 4021–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Cheng G, Shi Y, Sturla SJ, Jalas JR, McIntee EJ, Villalta PW, Wang M, Hecht SS, Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: formation of cyclic deoxyguanosine adducts and formaldehyde cross-links, Chem. Res. Toxicol, 16 (2003) 145–152. [DOI] [PubMed] [Google Scholar]
- [300].Huang H, Hopkins PB, DNA interstrand cross-linking by formaldehyde: nucleotide sequence preference and covalent structure of the predominant cross-link formed in synthetic oligonucleotides, J. Am. Chem. Soc, 115 (1993) 9402–9408. [Google Scholar]
- [301].Wang M, Cheng G, Villalta PW, Hecht SS, Development of liquid chromatography electrospray ionization tandem mass spectrometry methods for analysis of DNA adducts of formaldehyde and their application to rats treated with N-nitrosodimethylamine or 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, Chem. Res. Toxicol, 20 (2007) 1141–1148. [DOI] [PubMed] [Google Scholar]
- [302].Huang H, Solomon MS, Hopkins PB, Formaldehyde preferentially crosslinks duplex DNA through deoxyadenosine residues at the sequence 5’-d(AT), J. Am. Chem. Soc, 114 (1992) 9240–9241. [Google Scholar]
- [303].Haselkorn R, Doty P, Reaction of formaldehyde with polynucleotides, J. Biol. Chem, 236 (1961) 2837–2745. [PubMed] [Google Scholar]
- [304].Freifelder D, DAvidson PF, Physicochemical studies on the reaction of formaldehyde with DNA, Biochem. J, 3 (1963) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].van der Eb AJ, van Kesteren LW, van Bruggen EFJ, Structural properties of adenovirus DNA’s, Biochem. Biophys. Acta, 182 (1969) 530–541. [DOI] [PubMed] [Google Scholar]
- [306].Kawanishi M, Matsuda T, Yagi T, Genotoxicity of formaldehyde: molecular basis of DNA damage and mutation, Front. Environ. Sci, 2 (2014) 1–8. [Google Scholar]
- [307].Nakamura J, Nakamura M, DNA-protein crosslink formation by endogenous aldehydes and AP sites, DNA Repair, 88 (2020) 102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, O’Brien PJ, The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes, Chem. Biol. Interact, 130-132 (2001) 285–296. [DOI] [PubMed] [Google Scholar]
- [309].Nakamura J, Holley DW, Kawamoto T, Bultman SJ, The failure of two major formaldehyde catabolism enzymes (ADH5 and ALDH2) leads to partial synthetic lethality in C57BL/ 6 mice, Genes Environ, 42 (2020) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Hopkinson RJ, Schofield CJ, Decipering functions of intracellular formaldehyde: linking cancer and aldehyde metabolism, Biochemistry, 57 (2018) 904–906. [DOI] [PubMed] [Google Scholar]
- [311].Burgos-Barragan G, Wit N, Meiser J, Dingler FA, Pietzke M, Mulderring L, Pontel LB, Rosado IV, Brewer TF, Cordell RL, Monks PS, Chang CJ, Vazquez A, Patel KJ, Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism, Nature, 548 (2017) 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Rosado IV, Langevin F, Crossan GP, Takata M, Patel KJ, Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway, Nat. Struct. Mol. Biol, 18 (2011) 1432–1434. [DOI] [PubMed] [Google Scholar]
- [313].Olsen R, Molander P, Øvrebø S, Ellingsen DG, Thorud S, Thomassen Y, Lundanes E, Greibrokk T, Backman J, Sjöholm R, Kronberg L, Reaction of glyoxal with 2’-deoxyguanosine, 2’- deoxyadenosine, 2’-deoxycytidine, cytidine, thymidine, and calf thymus DNA: Identification of DNA adducts, Chem. Res. Toxicol, 18 (2005) 730–739. [DOI] [PubMed] [Google Scholar]
- [314].Shapiro R, Hachmann J, Reaction of guanine derivatives with 1,2-dicarbonyl compounds, Biochemistry, 5 (1966) 2799–2807. [DOI] [PubMed] [Google Scholar]
- [315].Loeppky RN, Cui W, Goelzer P, Park M, Y. Q., Glyoxal-guanine DNA adducts: Detection, stability, and formation in vivo from nitrosamines, IARC Sci. Publ, 150 (1999) 155–168. [PubMed] [Google Scholar]
- [316].Kasai H, Iwamoto-Tanaka N, Fukada S, DNA modifications by the mutagen glyoxal: adduction to G and C, deamination of C and GC and GA cross-linking, Carcinogenesis, 19 (1998) 1459–1465. [DOI] [PubMed] [Google Scholar]
- [317].Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart J-C, Jurkunas UV, Nadal M, Bouloc P, Dairou J, Lamouri A, Guanine glycation repair by DJ-1/Park7 and its bacterial homologs, Science, 357 (2017) 208–211. [DOI] [PubMed] [Google Scholar]
- [318].Jun YW, Kool ET, Small substrate or large? Debate of the mechanism of glycation repair by DJ- 1, Cell Chem. Biol, 27 (2020) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Brock AK, Kozekov ID, Rizzo CJ, Harris CM, Coupling products of nucleosides with the glyoxal adduct of deoxyguanosine, Chem. Res. Toxicol, 17 (2004) 1047–1056. [DOI] [PubMed] [Google Scholar]
- [320].Chen H-JC, Chen Y-C, Analysis of glyoxal-induced DNA cross-links by capillary liquid chromatography nanospray ionization tandem mass spectrometry, Chem. Res. Toxicol, 22 (2009) 1334–1341. [DOI] [PubMed] [Google Scholar]
- [321].Chen H-JC, Chang Y-L, Teng Y-C, Hsiao C-F, Lin T-S, A Stable Isotope Dilution Nanoflow Liquid Chromatography Tandem Mass Spectrometry Assay for the Simultaneous Detection and Quantification of Glyoxal-Induced DNA Cross-Linked Adducts in Leukocytes from Diabetic Patients, Anal. Chem, 89 (2017) 13082–13088. [DOI] [PubMed] [Google Scholar]
- [322].Chen H-JC, Liu C-T, Li Y-J, Correlation between glyoxal-induced DNA cross-links and hemoglobin modifications in human blood measured by mass spectrometry, Chem. Res. Toxicol, 32 (2019) 179–189. [DOI] [PubMed] [Google Scholar]
- [323].Vaca CE, Fang J-L, Conradi M, Hou SM, Development of a 32P-postlabelling method for the analysis of 2’-deoxyguanosine-3’-monophosphate and DNA adducts of methylglyoxal, Carcinogenesis, 15 (1994) 1887–1894. [DOI] [PubMed] [Google Scholar]
- [324].Murata-Kamiya N, Kamiya H, Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA, Nucleic Acids Res, 29 (2001) 3433–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Rahman A, Shahabuddin SM Hadi, Formation of strand breaks and interstrand cross-links in DNA by methylglyoxal, J. Biochem. Toxicol, 5 (1990) 161–166. [DOI] [PubMed] [Google Scholar]
- [326].Petrova KV, Millsap AD, Stec DF, Rizzo CJ, Characterization of the Deoxyguanosine−Lysine Cross-Link of Methylglyoxal, Chem. Res. Toxicol, 27 (2014) 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Douki T, Onuki J, Medeiros MHG, Bechara EJH, Cadet J, Di Mascio P, DNA alkylation by 4,5-dioxovaleric acid, the final oxidation product of 5-aminolevulinic acid, Chem. Res. Toxicol, 11 (1998) 150–157. [DOI] [PubMed] [Google Scholar]
- [328].Frischmann M, Bidmon C, Angerer J, Pischetsrieder M, Identification of DNA Adducts of Methylglyoxal, Chem. Res. Toxicol, 18 (2005) 1586–1592. [DOI] [PubMed] [Google Scholar]
- [329].Shapiro R, Cohen BI, Shiuey S-J, Maurer H, On the reaction of guanine with glyoxal, pyruvaldehyde, and kethoxal, and the structure of acylguanines. A new synthesis of N2-alykylguanines, Biochemistry, 8 (1969) 238–245. [DOI] [PubMed] [Google Scholar]
- [330].Radi R, Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine, Proc. Nat. Acad. Sci. USA, 115 (2018) 5839–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Mangerich A, Knutson CG, Parry NM, Sureshkumar M, Yea W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z, Taghizadeh K, Wishnok JS, Wogan GN, Fox JG, Tannenbaum SR, Dedon PC, Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer, Proc. Nat. Acad. Sci. USA, 109 (2012) 10753–10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Shapiro R, Pohl SH, Reaction of ribonucleosides with nitrous acid. Side products and kinetics, Biochemistry, 7 (1968) 448–455. [DOI] [PubMed] [Google Scholar]
- [333].Kirchner JJ, Sigurdsson ST, Hopkins PB, Interstrand Cross-Linking of Duplex DNA by Nitrous Acid: Covalent Structure of the dG-to-dG Cross-Link at the Sequence 5’-CG, J. Am. Chem. Soc, 114 (1992) 4021–4027. [Google Scholar]
- [334].Dubelman S, Shapiro R, A method for the isolation of cross-linked nucleosides from DNA: application to cross-links induced by nitrous acid, Nucleic Acids Res, 4 (1977) 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Geiduschek EP, “Reversible” DNA, Proc. Nat. Acad. Sci. USA, 47 (1961) 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Edfeldt NBF, Harwood EA, Sigurdsson ST, Hopkins PB, Reid BR, Solution structure of a nitrous acid induced DNA interstrand cross-link, Nucleic Acids Res, 32 (2004) 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Dong M, Wang C, Deen WM, Dedon PC, Absence of 2’-deoxyoxanosine and presence of abasic sites in DNA exposed to nitric oxide at controlled physiological concentrations, Chem. Res. Toxicol, 16 (2003) 1044–1055. [DOI] [PubMed] [Google Scholar]
- [338].Caulfield JL, Wishnok JS, Tannenbaum SR, Nitric oxide-induced interstrand cross-links in DNA, Chem. Res. Toxicol, 16 (2003) 571–574. [DOI] [PubMed] [Google Scholar]
- [339].Yamaguchi H, Uchihori Y, Yasuda N, Takada M, Kitamura H, Estimation of yields of OH radicals in water irradiated by ionizing radiation, J. Radiat. Res, 46 (2005) 333–341. [DOI] [PubMed] [Google Scholar]
- [340].Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H, Free radical-induced damage to DNA: mechanisms and measurement, Free Rad. Biol. Med, 32 (2002) 1102–1115. [DOI] [PubMed] [Google Scholar]
- [341].Cadet J, Douki T, Gasparutto D, Ravanat J-L, Oxidative damage to DNA: formation measurement and biochemical features, Mutat. Res, 531 (2003) 5–23. [DOI] [PubMed] [Google Scholar]
- [342].Ding H, Greenberg MM, gamma-Radiolysis and hydroxyl radical produce interstrand cross-links in DNA involving thymidine, Chem. Res. Toxicol, 20 (2007) 1623–1628. [DOI] [PubMed] [Google Scholar]
- [343].Cadet J, Wagner JR, Shafirovich V, Geacintov NE, One-electron oxidation reactions of purine and pyrimidine bases in cellular DNA, Int. J. Radiat. Biol, 90 (2014) 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Hong IS, Greenberg MM, Efficient DNA interstrand cross-link formation from a nucleotide radical, J. Am. Chem. Soc, 127 (2005) 3692–3693. [DOI] [PubMed] [Google Scholar]
- [345].Hong IS, Ding H, Greenberg MM, Oxygen independent DNA interstrand cross-link formation by a nucleotide radical, J. Am. Chem. Soc, 128 (2006) 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Ding H, Majumdar A, Tolman JR, Greenberg MM, Multinuclear NMR and kinetic analysis of DNA interstrand cross-link formation, J. Am. Chem. Soc, 130 (2008) 17981–17987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Zhang Q, Wang Y, Independent Generation of 5-(2′-Deoxycytidinyl)methyl Radical and the Formation of a Novel Cross-Link Lesion between 5-Methylcytosine and Guanine, J. Am. Chem. Soc, 125 (2003) 12795–12802. [DOI] [PubMed] [Google Scholar]
- [348].Hong H, Cao H, Wang Y, Wang Y, Identification and Quantification of a Guanine-Thymine Intrastrand Cross-Link Lesion Induced by Cu(II)/H2O2/Ascorbate, Chem. Res. Toxicol, 19 (2006) 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Lonkar P, Dedon PC, Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates, Int. J. Cancer, 128 (2011) 1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Illés E, Mizrahi A, Marks V, Meyerstein D, Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate, Free Rad. Biol. Med, 131 (2019) 1–6. [DOI] [PubMed] [Google Scholar]
- [351].Perrier S, Hau J, Gasparutto D, Cadet J, Favier A, Ravanat J-L, Characterization of Lysine- Guanine Cross-Links upon One-Electron Oxidation of a Guanine-Containing Oligonucleotide in the Presence of a Trilysine Peptide, J. Am. Chem. Soc, 128 (2006) 5703–5710. [DOI] [PubMed] [Google Scholar]
- [352].Cuesta-López S, Menoni H, Angelov D, Peyrard M, Guanine radical chemistry reveals the effect of thermal fluctuations in gene promoter regions, Nucleic Acids Res, 39 (2011) 5276–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Yun BH, Geacintov NE, Shafirovich V, Generation of GuanineThymidine Cross-Links in DNA by Peroxynitrite/Carbon Dioxide, Chem. Res. Toxicol, 24 (2011) 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Chen B, Bohnert T, Zhou X, Dedon PC, 5’-(2-Phosphoryl-1,4-dioxobutane) as a product of 5’- oxidation of deoxyribose in DNA: elimination as trans-1,4-dioxo-2-butene and approaches to analysis, Chem. Res. Toxicol, 17 (2004) 1406–1413. [DOI] [PubMed] [Google Scholar]
- [355].Guan L, Greenberg MM, DNA interstand cross-link formation by the 1,4-dioxobutane abasic site, J. Am. Chem. Soc, 131 (2009) 15225–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Ghosh S, Greenberg MM, Synthesis, of cross-linked DNA containing oxidized abasic site analogues, J. Org. Chem, 79 (2014) 5948–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Ghosh S, Greenberg MM, Nucleotide excision repair of chemically stabilized analogues of DNA interstrand cross-links produced from oxidized abasic sites, Biochemistry, 53 (2014) 5958–5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Sczepanski JT, Jacobs AC, Greenberg MM, Self-promoted DNA interstrand cross-link formation by an abasic site, J. Am. Chem. Soc, 130 (2008) 9646–9647. [DOI] [PubMed] [Google Scholar]
- [359].Sczepanski JT, Jacobs AC, Majumdar A, Greenberg MM, Scope and mechanism of interstrand crosslink formation by the C4’-oxidized abasic site, J. Am. Chem. Soc, 131 (2009) 11132–11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Dhar S, Kodama T, Greenberg MM, Selective detection and quantitation of oxidized abasic sites in DNA, J. Am. Chem. Soc, 129 (2007) 8702–8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Sczepanski JT, Jacobs AC, Van Houten B, Greenberg MM, Double strand break formation during nucleotide excision repair of a DNA interstrand cross-link, Biochemistry, 48 (2009) 7565–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Regulus P, Duroux B, Bayle P-A, Favier A, Cadet J, Ravanat J-L, Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion, Proc. Nat. Acad. Sci. USA, 104 (2007) 14032–14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Taverna Porro ML, Saint-Pierre C, Gasparutto D, Ravanat J-L, Solid-phase synthesis of branched oligonucleotides containing a biologically relevant dCyd341 interstrand crosslink DNA lesion, Org. Biomol. Chem, 18 (2020) 1892–1899. [DOI] [PubMed] [Google Scholar]
- [364].Pratviel G, Pitié M, Bernadou J, Meunier B, Furfural as a marker of DNA cleavage by hydroxylation at the 5’-carbon of deoxyribose, Angew. Chem. Int. Ed. Eng, 30 (1991) 702–704. [Google Scholar]
- [365].Barciszewski J, Siboska GE, Pedersen BO, Clark BFC, Rattan SIS, Evidence for the presence of kinetin in DNA and cell extracts, FEBS Lett, 393 (1996) 197–200. [DOI] [PubMed] [Google Scholar]
- [366].Carrette LLG, Madder A, A synthetic oligonucleotide model for evaluating the oxidation and crosslinking properties of natural furan-modified DNA, ChemBioChem, 15 (2014) 103–107. [DOI] [PubMed] [Google Scholar]
- [367].Byrns MC, Vu CC, Neidgh JW, Abad J-L, Jones RA, Peterson LA, Detection of DNA Adducts Derived from the Reactive Metabolite of Furan, cis-2-Butene-1,4-dial, Chem. Res. Toxicol, 19 (2006) 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Marshall JEA, Lakin J, Troth I, Wallace-Johnson SM, UV-B radiation was the Devonian- Carboniferous boundary terrestrial extinction kill mechanism, Sci. Adv, 6 (2020) eaba0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Taylor JS, Unraveling the Molecular Pathway from Sunlight to Skin Cancer Acc. Chem. Res, 27 (1994) 76–82. [Google Scholar]
- [370].Marmur J, Grossman L, Ultraviolet light induced linking of deoxyribonucleic acid strands and its reversal by photoreactivating enzyme, Proc. Nat. Acad. Sci. USA, 47 (1961) 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Gliˆsin VR, Doty P, The effect of temperature on the cross-linking of deoxyribonucleic acid strands by ultraviolet radiation, Biochim. Biophys. Acta, 61 (1962) 458–460. [Google Scholar]
- [372].Freifelder D, Davidson PF, Letter to the editor, Biochem. J, 3 (1963) 97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Nejedlý K, Kittner R, Kypr J, Genomic DNA regions whose complementary strands are prone to UV light-induced cross-linking, Arch. Biochem. Biophys, 388 (2001) 216–224. [DOI] [PubMed] [Google Scholar]
- [374].Lowe JD, Nguyen HT, Or A, Attri AK, Minton KW, UV-induced interstrand cross-linking of d(GT)n•d(CA)n is facilitated by a structural transition, J. Biol. Chem, 261 (1986) 10051–10057. [PubMed] [Google Scholar]
- [375].Hearst JE, Photochemistry of the psoralens, Chem. Res. Toxicol, 2 (1989) 69–75. [DOI] [PubMed] [Google Scholar]
- [376].Wiesenhahn G, Hearst JE, DNA unwinding induced by photoaddition of psoralen derivatives and determination of dark-binding equilibrium constants by gel electrophoresis, Proc. Nat. Acad. Sci. USA, 75 (1978) 2703–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Hearst JE, Photochemistry and nucleic acid structure, J. Investig. Dermatol, 77 (1981) 39–44. [DOI] [PubMed] [Google Scholar]
- [378].Haran TE, Crothers DM, Phased psoralen cross-links do not bend the DNA double-helix, Biochemistry, 27 (1988) 6967–6971. [DOI] [PubMed] [Google Scholar]
- [379].Spielmann HP, Dwyer TJ, Hearst JE, Wemmer DE, Solution structures of psoralen monoadducted and cross-linked DNA oligomers by NMR spectroscopy and restrained molecular dynamics, Biochemistry, 34 (1995) 12937–12953. [PubMed] [Google Scholar]
- [380].Spielmann HP, Dwyer TJ, Sastry SS, Hearst JE, Wemmer DE, DNA structural reorganization upon conversion of a psoralen furan-side monoadduct to an interstrand cross-link: implications for DNA repair, Proc. Nat. Acad. Sci. USA, 92 (1995) 2345–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Hwang G-S, Kim J-K, Choi B-S, The solution structure of a psoralen cross-linked DNA duplex by NMR and relaxation matrix refinement, Biochem. Biophys. Res. Commun, 219 (1996) 191–197. [DOI] [PubMed] [Google Scholar]
- [382].Sinden RR, Hagerman PJ, Interstrand psoralen cross-links do not introduce appreciable bends in DNA, Biochemistry, 23 (1984) 6299–6303. [DOI] [PubMed] [Google Scholar]
- [383].Lukin M, de los Santos C, NMR Structures of Damaged DNA, Chem. Rev, 106 (2006) 607–686. [DOI] [PubMed] [Google Scholar]
- [384].Tomic MT, Wemmer DE, KIm S-H, Structure of a psoralen cross-linked DNA in solution by nuclear magnetic resonance, Science, 238 (1987) 1722–1725. [DOI] [PubMed] [Google Scholar]
- [385].Xue M, Kim CS, Healy AR, Wernke KM, Wang Z, Frischling C, Shine EE, Wang W, Herzon SB, Crawford JM, Structure elucidation of colibactin and its DNA cross-links, Science, 365 (2019) eaax2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, Balskus EP, The human gut bacterial genotoxin colibactin alkylates DNA, Science, 363 (2019) DOI: 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Bossuet-Greif N, Vignard J, Taieb F, Mirey G, Dubois D, Petit C, Oswald E, Nougayrèdea J-P, Bossuet-Greif a.V. Nadège, Taieb bFrédéric,Mirey aGladys,Dubois bDamien,Petit aClaude,Oswald aEric,Nougayrèdea aJean-Philippe, The Colibactin Genotoxin Generates DNA Interstrand Cross-Links in Infected Cells, mBio, 9 (2018) e02393–02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Zang H, Gates KS, DNA binding and alkylation by the “left half” of azinomycin B, Biochemistry, 39 (2001) 14968–14975. [DOI] [PubMed] [Google Scholar]
- [389].Armstrong RW, Salvati ME, Nguyen M, Novel interstrand crosslinks induced by the antitumor antibiotic carzinophilin/azinomycin B, J. Am. Chem. Soc, 114 (1992) 3144–3145. [Google Scholar]
- [390].Mullins EA, Warren GM, Bradley NP, Eichman BF, Structure of a DNA glycosylase that unhooks interstrand cross-links, Proc. Nat. Acad. Sci. USA, 114 (2017) 4400–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Coleman RS, Perez RJ, Burk CH, Navarro A, Studies on the Mechanism of Action of Azinomycin B: Definition of Regioselectivity and Sequence Selectivity of DNA Cross-Link Formation and Clarification of the Role of the Naphthoate, 124, 44 (2002) 13008–13017. [DOI] [PubMed] [Google Scholar]
- [392].Tomasz M, Mitomycin C: small, fast and deadly (but very selective), Chem. Biol, 2 (1995) 575–579. [DOI] [PubMed] [Google Scholar]
- [393].Rieben WK, Coulombe RAJ, DNA Cross-Linking by Dehydromonocrotaline Lacks Apparent Base Sequence Preference, Toxicol. Sci, 82 (2004) 497–503. [DOI] [PubMed] [Google Scholar]
- [394].Huang H, Pratnum TK, Hopkins PB, Covalent structure of the DNA-DNA interstrand crosslink formed by reductively activated FR66979 in synthetic DNA duplexes, J. Am. Chem. Soc, 116 (1994) 2703–2709. [Google Scholar]
- [395].Woo J, Sigurdsson ST, Hopkins PB, DNA interstrand cross-linking reactions of pyrrole-derived, bifunctional electrophiles: evidence for a common target site in DNA, J. Am. Chem. Soc, 115 (1993) 3407–3415. [Google Scholar]