Abstract
Background:
Neuroimaging studies reveal structural and functional including neurochemical brain abnormalities in individuals with substance use disorders compared to healthy controls. However, whether and to what extent such dysfunction is reversible with abstinence remains unclear, and a review of studies with longitudinal within-subject designs is lacking. We performed a systematic review of longitudinal neuroimaging studies to explore putative brain changes associated with abstinence in treatment-seeking individuals with substance use disorders.
Methods:
Following PRISMA guidelines, we examined articles published up to May 2021 that employed a neuroimaging technique and assessed neurobiological recovery in treatment-seeking participants at a minimum of two time-points separated by a period of abstinence (longer than 24 hours apart) or significant reduction in drug use.
Results:
Forty-five studies met inclusion criteria. Encouragingly, in this limited but growing literature, the majority of studies demonstrated at least partial neurobiological recovery with abstinence. Structural recovery appeared to occur predominantly in frontal cortical regions, the insula, hippocampus, and cerebellum. Functional and neurochemical recovery was similarly observed in prefrontal cortical regions but also in subcortical structures. The onset of structural recovery appears to precede neurochemical recovery, which begins soon after cessation (particularly for alcohol); functional recovery may require longer periods of abstinence.
Conclusions:
The literature is still growing and more studies are warranted to better understand abstinence-mediated neural recovery in individuals with substance use disorders. Elucidating the temporal dynamics between neuronal recovery and abstinence will enable evidence-based planning for more effective and targeted treatment of substance use disorders, potentially preempting relapse.
Keywords: substance use disorders, addiction, recovery, abstinence, neuroimaging, longitudinal, alcohol, cocaine
1. INTRODUCTION
Substance use disorders (SUD) are chronically relapsing disorders. They are characterized by compulsive drug-seeking and drug-taking behaviors despite a decrease in the pleasure derived from the drug and harmful or even catastrophic consequences. Impairments in response inhibition and salience attribution, functions of the prefrontal cortex, are hypothesized to contribute to the cycle of addiction (Goldstein and Volkow, 2002, 2011). Indeed, neuroimaging studies provide reliable evidence for structural and functional including neurochemical abnormalities in the prefrontal cortex and numerous other cortical and subcortical brain regions with chronic exposure to substances of abuse, irrespective of the specific drug consumed (Chang et al., 2007; Ende et al., 2013; Ersche et al., 2013; Fritz et al., 2014; Luijten et al., 2017; Moselhy et al., 2001; Sullivan and Pfefferbaum, 2005). Importantly, these alterations often parallel changes in cognitive functioning (Chanraud et al., 2007; Moreno-Lopez et al., 2012), affective symptoms (London et al., 2004), and treatment outcomes (Rando et al., 2011) implying a clinically significant impact.
However, whether these neurobiological changes associated with long-term substance use are permanent or recover with abstinence has been a matter of debate. Commonly, studies investigating the potential for human brain recovery with abstinence compare data from a group of current substance users with those from abstainers (i.e., former users) as well as to those from healthy volunteers. Although necessary (to explore such between-group effects), these cross-sectional studies are often confounded by between-subject variability (e.g., comorbid drug use or mood symptoms) and preclude the possibility of teasing apart pre-existing vulnerabilities (e.g., traits and/or environmental insults associated with greater likelihood of drug consumption) from the effects of chronic drug use. Moreover, cross-sectional studies have a propensity for both Type I (reporting false positives) and Type II errors (driven by lack of power/statistical sensitivity to detect subtle changes) due to small sample sizes. In contrast, designs that use longitudinal data can control for within-subject variability (each subject serves as their own control while their brain outcomes are measured repeatedly over time) and provide greater statistical power. These designs allow for a more direct examination of change while accounting for (at least some of) the extraneous variables that may contribute to results.
Although changes in brain structure and function including neurochemistry during abstinence have previously been reviewed (see Charlet et al., 2018; Moeller and Paulus, 2018 for recent reviews), the current manuscript uniquely focuses on longitudinal neuroimaging studies that examined within-subject brain changes between early and protracted abstinence in treatment-seeking humans with SUDs. Appropriate within-subject modeling of brain changes with abstinence is critical for a better understanding of the neurobiological trajectory that may help identify mechanisms associated with sustained long-term abstinence or with the propensity for relapse. Importantly, these results may also help to identify vital individualized predictors of, and targets for, timely prevention and treatment to enhance recovery and reduce the chronically relapsing nature of drug addiction.
2. METHODS
We performed a systematic literature search to identify neuroimaging studies investigating the effects of alcohol/drug abstinence on brain structure, function, and neurochemistry. First, we searched Pubmed using a search term that included (“magnetic resonance imaging” OR “MRI” OR “functional magnetic resonance imaging” OR “fMRI” OR “diffusion tensor imaging” OR “DTI” OR “positron emission tomography” OR “PET” OR “electroencephalography” OR “EEG” OR “magnetic resonance spectroscopy” OR “MRS” OR “single photon emission computed tomography” OR “SPECT”). These terms were combined with a term related to SUDs (“substance use disorder” OR “addiction” OR “dependence” OR “drug abuse” OR “alcohol” OR “cocaine” OR “crack” OR “speed” OR “methamphetamine” OR “amphetamine” OR “opioids” OR “heroin” OR “hallucinogens” OR “MDMA” OR “ecstasy” OR “mushrooms” OR “ketamine” OR “sedative” OR “tobacco” OR “nicotine” OR “cannabis” OR “marijuana”) as well as with a term referring to abstinence or treatment (“abstinence” OR “cessation” OR “treatment” OR “recovery”; note we did not use the term “relapse” because our focus was on the effects of recovery based on abstinence or significant reduction in drug use). The initial search was limited to full text articles and studies published in English, in a peer-reviewed journal in any year and were assessed using Endnote X9 following the PRISMA guidelines.
This initial database search yielded 7,749 records, and included studies published up to May 2021. Titles and abstracts from all articles identified through the search were screened. Articles were excluded for being non-relevant (i.e., not related to SUD), case studies, reviews (i.e., literature or systematic), clinical trials (since they examine the impact of a treatment intervention and not of abstinence-mediated recovery, which is the focus of this review), meta-analyses and/or non-human research. A total of 106 articles remained and were assessed more closely for eligibility. Full text articles and studies adhering to the following criteria were included; (1) employed a neuroimaging technique; (2) assessed participants at a minimum of two time-points with an inter-scan duration of greater than 24 hours, separated by a period of abstinence or significant reduction in drug use; (3) SUD sample at the first follow-up was at least n=10; and (4) participants were defined as seeking treatment for SUD. Forty-five studies met eligibility criteria (Figure 1). We summarized the imaging modalities, brain regions, abstinence period prior to any of the scans, statistical analysis thresholds, and within-subject changes in structural, functional, and neurochemical outcomes (emerging during the defined abstinence period) in Table 1.
Figure 1.
PRISMA flowchart detailing the procedure used to for article inclusion.
Table 1:
Summary of Brain Recovery in in Human Substance-Using Samples. Studies are sorted by imaging measure, drug type and by the increasing duration of abstinence (at the final neuroimaging scan).
| (A) STRUCTURAL STUDIES | |||||||
| Study | Sample (at each time point) | Imaging Modality/ Whole brain analysis or a priori ROI assessed | Abstinence Length (at each scan) | Statistical Threshold for Longitudinal Changes | Crosssectional Results (SUD vs. HC) | Within-Group Longitudinal Changes | |
| Alcohol | |||||||
| GM and WM | |||||||
| van Eijk et al. (2013) |
T1 HC: n=55 SUD: n=49 T2 HC: n=20 SUD: n=49 |
3T MRI: T1- weighted MPRAGE ROI (anterior frontal gyrus, cingulate gyrus, insula, parietal and temporal lobes, cerebellum, and hippocampus); total WM | T1: 1 day T2: 2 weeks |
p<0.05; whole-brain FWE-corrected |
SUD at T1 vs. HC at T1 ↓ GM volume: middle and inferior frontal gyrus, cingulate and precentral gyrus, insula, precuneus, superior parietal lobule, middle temporal gurus, and cerebellum SUD at T2 vs. HC at T2 Not reported |
HC NS SUD ↑ GM volume: cingulate gyrus, insula, temporal gyrus, precuneus, parietal lobule, and cerebellum |
|
| Kuhn et al. (2014) |
T1 HC: n=32 SUD: n=42 T2 HC: n=32 SUD: n=42 |
3T MRI: T1- weighted MPRAGE ROI (hippocampal subfields) | T1: ~ 9 days T2: 2 weeks |
p<0.017; Bonferroni corrected for 3 ROIs |
SUD at T1 vs. HC at T1 ↓ GM volume: hippocampus (subfield CA2+3) SUD at T2 vs. HC at T2 NS |
HC NS SUD ↑ GM volume: hippocampus (subfield xCA2+3) |
|
| Wang et al. (2016) |
T1 HC: n=20 SUD: n=49 T2 HC: n=20 SUD: n=49 |
3T MRI: T1- weighted MPRAGE ROI in subcortical regions (volume: hippocampus, amygdala, caudate, putamen, pallidum, nucleus accumbens); cortical thickness; surface area in cortical areas | T1: 1 day T2: 2 weeks |
Subcortical regions: p<0.05 Cortical thickness and surface area: cluster-wise correction with Monte Carlo simulations (vertex-wise threshold p< 0.05, 5,000 iterations) |
SUD at T1 vs. HC at T1 ↓ GM volume: hippocampu s, amygdala, putamen, and nucleus accumbens ↓ Cortical thickness: medial OFC, middle frontal, superior frontal, and inferior frontal lobe, rostral ACC, inferior parietal lobe, fusiform, inferior temporal and lateral occipital lobe Surface area: NS SUD at T2 vs. HC at T2 ↓ GM volume: hippocampus, amygdala, putamen, and nucleus accumbens ↓ Cortical thickness: middle frontal, superior frontal, and inferior frontal lobe, inferior parietal lobe, fusiform, inferior temporal and lateral occipital lobe Surface area: NS |
HC NS SUD ↑ Cortical thickness: medial OFC, middle frontal, superior frontal, rostral ACC, cuneus, and inferior parietal and lateral occipital regions; ↑in global sulci > gyri |
|
| Bach et al. (2020) |
T1 HC: n=74 SUD: n=62 T2 HC: N/A SUD: n=62 |
3T MRI: T1- weighted MPRAGE Whole-brain volume and cortical thickness | T1: ~12 days T2: 27 days |
p<0.05; FWE-corrected |
SUD at T1 vs. HC at T1 ↓ GM volume: superior, inferior and middle frontal gyrus, posterior cingulate gyrus, insula, fusiform gyrus, hippocampus, amygdala, thalamus, putamen, precuneus, and cuneus ↓ cortical thickness: superior frontal cortex, rostral middle frontal cortex, caudal middle frontal cortex and medial and lateral OFC, superior parietal cortex, occipital cortex, precuneus, cuneus, lingual and fusiform cortex SUD at T2 vs. HC at T1 ↓ GM volume: posterior cingulate gyrus, insula, fusiform gyrus, hippocampus, thalamus, and precuneus ↓ cortical thickness: superior parietal cortex, occipital cortex, and precuneus |
HC N/A SUD ↑ GM volume: middle and inferior frontal gyri, middle cingulate gyrus, insula, supramarginal gyrus, and precuneus ↑ cortical thickness: superior frontal cortex, rostral middle frontal cortices, lateral OFC, insula, and lateral occipital cortex |
|
| Durazzo et al. (2017) |
T1 HC: n=33 SUD: n=82 T2 HC: N/A SUD: n=42 |
1.5T MRI: T1- weighted MPRAGE ROI (frontal, parietal, temporal, occipital lobes GM and WM) |
T1: 1 week T2: 4 weeks |
p < .024 |
SUD at T1 vs. HC at T1 ↓ GM volume: frontal, parietal, temporal, and occipital SUD at T2 vs. HC at T1 ↓ GM volume: frontal, parietal, and occipital |
HC N/A SUD ↑ GM volume: frontal |
|
| Durazzo and Meyerhoff (2020) |
T1 HC: n=33 SUD: n=78 T2 HC: N/A SUD: 24 |
1.5T MRI: T1- weighted MPRAGE ROI (DLPFC, OFC, ACC, precentral gyri, and insula) | T1: 1 week T2: 4 weeks |
p<0.05; corrected |
SUD at T1 vs. HC at T1 ↓ GM volume: rostral ACC, medial OFC, insula and total frontal cortex SUD at T2 vs. HC at T1 ↓ GM volume: rostral ACC and insula |
HC N/A SUD NS |
|
| Gazdzinski et al. (2008) |
T1 HC: 14 SUD: n=24 (13smokers, 11 non-smokers) T2 HC: N/A SUD: n=24 |
1.5T MRI: T1- weighted MPRAGE ROI (hippocampus) |
T1: 1 week T2: 1 month |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 NS SUD at T2 vs. HC at T1 NS |
HC N/A SUD ↑ GM volume: hippocampus (in both smokers and non-smokers) |
|
| Mon et al. (2013) |
T1 HC: n=17 (not genotyped) SUD: n=41 (26 Val/Val BDNF, 15 Val/Met BDNF) T2 HC: n=17 SUD: n=41 |
1.5T MRI: T1- weighted MPRAGE ROI (GM major lobes and subcortical regions: thalamus, lenticular nuclei, caudate, brainstem, and cerebellum; WM major lobes) | T1: 1 week T2: 5 weeks |
p≤0.027 and p≤0.017 respectively for cortical and subcortical regions |
Not reported |
HC NS SUD Val/Val BDNF carriers ↑ GM volume: frontal, parietal, and temporal lobes, and thalamus ↓ GM volume: caudate Val/Met BDNF carriers ↑ GM volume: cerebellum, temporal lobe ↑ WM volume: frontal lobe parietal, and temporal lobes (at trend level) |
|
| Demirakca et al. (2011) |
T1 HC: n=66 SUD: n=50 T2 HC: N/A SUD: n=14 |
1.5T MRI: T1- weighted MPRAGE Global GM, WM, and ROI (cingulate gyrus, OFC, insula, thalamus, amygdala, and hippocampus) | T1: 5 weeks T2: 4 months |
p<0.05 (FWE) |
SUD at T1 vs. HC at T1 ↓ GM volume: anterior and middle cingulate gyrus, insula, superior temporal gyrus, parahippoca mpal gyrus, amygdala, SUD at T2 vs. HC at T1 N/A |
HC N/A SUD ↑ total GM and WM volume ↑ GM volume: OFC, cingulate gyrus, and insula |
|
| Shear et al. (1994) |
T1 HC: n=N/A SUD: n=24 T2 HC: N/A SUD: n=15 |
1.5T MRI: Protondensity and T2weighted scans (Total GM and WM) | T1: 1 month T2: 4 months |
p<0.05 | N/A |
HC N/A SUD ↑ total WM volume |
|
| Hoefer et al. (2014) |
T1 HC: n=35 (not genotyped)sUD: n=84 (subset: 26 Val/Val BDNF, 15 Val/Met BDNF) T2 HC: n=N/A SUD: n=121 T3 HC: 16 SUD: n=37 |
1.5 T MRI: T1- weighted MPRAGE ROI: hippocampus | T1: 1 week T2: 1 month T3: 7 months |
p<0.05 (uncorrected) |
SUD at T1 vs. HC at T1 ↓ GM volume: hippocampus SUD at T2 vs. HC at T3 ↓ GM volume: hippocampus SUD at T2 vs. HC at T3 ↓ GM volume: hippocampus |
HC NS SUD ↑ GM at trend level: hippocampus (between T1 and T3) in Val/Val carriers No effect of smoking |
|
| Cardenas et al. (2007) |
T1 HC: n=18 SUD: n=47 T2 HC: n=8 SUD: n=17 |
1.5T MRI: T1- weighted MPRAGE Whole-brain (GM; WM) |
T1: ~1 week T2: 7 months |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 ↓ GM and WM volume: DLPFC ↓ GM: temporal region SUD at T2 vs. HC at T2 Not reported |
HC NS SUD ↑ GM volume: OFC and parietal lobes |
|
| Durazzo et al. (2015) |
T1 HC: n=32 SUD: n=82 (46smokers, 36 non-smokers) T2 HC: N/A SUD: n=82 T3 HC: n=15 SUD: n=36 |
1.5T MRI: T1- weighted MPRAGE ROI (GM: frontal, parietal, temporal, occipital lobes, thalamus, caudate, lenticular nucleus, and cerebellum; WM: frontal, parietal, temporal, occipital lobes) | T1: 1 week T2: 5 weeks T3: 7.5 months |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 ↓ GM volume: frontal and parietal lobes, thalamus, and lenticular nucleus (in smokers + non-smokers) SUD at T2 vs. HC at T1 ↓ GM volume: frontal and parietal lobes, thalamus, and lenticular nucleus (in smokers + non-smokers) SUD at T3 vs. HC at T1 ↓ GM volume: frontal and parietal lobes, thalamus, and lenticular nucleus (in smokers + non-smokers) |
HC NS SUD ↑ GM volume: frontal and parietal lobes and thalamus, and cerebellum (T2>T1)>(T3>T1) frontal, parietal, occipital lobes, thalamus, caudate, and cerebellum (T3>T2) frontal and parietal lobes, thalamus, and cerebellum (T2-T3>T1-T2) ↑ WM volume: parietal, temporal, and occipital lobes (T2-T3) |
|
| Zou et al. (2018) |
T1 HC: n=17 SUD: n=65 T2 HC: N/A SUD: n=82 T3 HC: n=17 SUD: n=36 |
1.5T MRI: T1- weighted MPRAGE ROI (DLPFC, OFC, ACC, insula, amygdala, and hippocampus) | T1: 1 week T2: 1 month T3: 7.5 months |
p<0.023; modified Bonferroni correction |
SUD at T1 vs. HC at T1 ↓ GM volume: DLPFC, ACC, insula (at trend level), and hippocampus SUD at T2 vs. HC at T1 ↓ GM volume: DLPFC, ACC, insula (at trend level), and hippocampus SUD at T3 vs. HC at T3 ↓ GM volume: hippocampus |
HC NS SUD ↑ GM volume: DLPFC, OFC, insula, and hippocampus (T2>T1) DLPFC, OFC, ACC, insula, and hippocampus (T3>T2) DLPFC, OFC, and insula (T3>T2>>T1) Hippocampus (T3>T2>T1) |
|
| Gazdzinski et al. (2005) |
T1 HC: n=17 SUD: n=18 T2 HC: N/A SUD: n=23 T3 HC: n=17 SUD: n=7 |
1.5T MRI: T1- weighted MPRAGE Whole-brain |
T1: 1 week T2: 1 month T3: 6–12 months for SUD; 2 years for HC |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 Not reported SUD at T2 vs. HC at T3 Not reported |
HC NS SUD ↑ total GM volume (T1-T2<<T2<T3) |
|
| Pfefferbaum et al. (1995) |
T1 HC: n=58 SUD: n=58 T2 HC: n=58 SUD: n=58 T3 HC: n=11 SUD: n=19 |
1.5T MRI: T1- weighted Spin Echo ROI (anterior and posterior cortical GM and WM volume, basal ganglia, thalamus, and subcortical WM) |
T1: ~12 days T2: 1 month T3: up to 12 months (~130 days) |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 ↓ GM and WM volume: anterior and posterior cortical regions ↓ GM volume: thalamus SUD AT T2 VS. HC AT T2 ↓ GM and WM volume: anterior and posterior cortical regions ↓ GM volume: thalamus SUD at T3 vs. HC at T1 ↓ WM volume: anterior and posterior cortical regions |
HC NS SUD ↑ GM volume: anterior cortical regions (at trend level) (T2>T1) |
|
| DTI | |||||||
| Gazdzinski et al. (2010) |
T1 HC: n=22 SUD: n=36 (20smokers, 16 non-smokers) T2 HC: 22 SUD: n=21 (11smokers, 10 non-smokers) |
1.5T MRI: DTI single-shot doublerefocused spinecho echo-planar imaging sequence ROI (frontal, temporal, parietal and occipital white matter) | T1: ~4.5 days T2: ~1 month (SUD) T2: 1 year (HC) |
p<0.05; uncorrected |
SUD AT T1 VS. HC at T1 ↑ MD: frontal, temporal and parietal white matter in non-smokers ↑ MD: frontal white matter insmokers SUD at T2 vs. HC at T2 NS |
HC NS SUD ↑ FA: temporal white matter ↓ MD: mean diffusivity in frontal, temporal, parietal, and occipital in non-smokers and ↑ WM volume in frontal and temporal lobes in smokers |
|
| Alhassoon et al. (2012) |
T1 HC: n=15 SUD: n=15 T2 HC: n=15 SUD: n=15 |
1.5T MRI: DTI single-shot, stimulated-echo sequence with spiral acquisition ROI (genu and body of corpus collosum) | T1: 2 weeks T2: 1 year |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 ↓ FA & ↑RD SUD at T2 vs. HC at T2 NS |
HC NS SUD ↑ FA, ↓ RD: Genu and body of the corpus collosum |
|
| Pfefferbaum et al (2014) |
T1 HC: n=56 SUD: n= 47 T2 HC: n=56 SUD: n=27 |
1.5T MRI: DTI single shot spinecho, echo-planar diffusion-weighted sequence ROI: 27 ROIs (21 bilateral and 6 midline regions) were examined |
T1: 47 weeks (mean) T2: 1–8 years (325.5 ± 391.9 weeks) |
p<0.001; tfce |
SUD at T1 vs. HC at T1 Not reported SUD at T2 vs. HC at T2 ↓ FA |
HC ↓ FA SUD ↑ FA: 20 out of 27 ROIs including corpus collosum, the fornix, corona radiata |
|
| Cocaine | |||||||
| GM | |||||||
| Parvaz et al. (2016) |
T1 HC: n=12 SUD: n=19 T2 HC: n=12 SUD: n=19 |
4T MRI: T1- weighted 3DMDEFT Whole-brain | T1: ~3 weeks T2: 6 months |
p<0.005; uncorrected >50 contiguous voxels |
SUD at T1 vs. HC at T1 Not reported SUD at T2 vs. HC at T2 Not reported |
HC NS SUD ↑ GM volume: IFG, vmPFC, OFC |
|
| Methamphetamine | |||||||
| GM | |||||||
| Ruan et al. (2018) |
T1 HC: n=27 SUD: n=19 T2 HC: N/A SUD: n=29 |
3T MRI: T1- weighted scan (sequence not reported) Whole-brain | T1: 6 months T2: 12 months |
p<0.05 (FWE); >500 contiguous voxels |
SUD at T1 vs. HC at T1 ↓GM volume: precentral gyrus, fusiform gyrus, caudate, and cerebellum SUD at T2 vs. HC at T2 Not reported |
HC N/A SUD ↑ GM volume: cerebellum ↓ GM volume: cingulate gyrus |
|
| DTI | |||||||
| Zhuang et al. (2016) |
T1 HC: n=19 SUD: n=20 T2 HC: N/A SUD: n=19 |
3T MRI: single-shot EPI DWI Whole-brain + ROI (based on group differences) | T1: 6 months T2: 13 months |
p<0.05; uncorrected |
SUD at T1 vs. HC at T1 ↓FA and ↑RD : internal capsule and superior corona radiata ↑MD: internal capsule SUD at T2 vs. HC at T1 Not reported |
HC N/A SUD ↓FA: internal capsule and superior corona radiata |
|
| Heroin | |||||||
| GM | |||||||
| Wang et al (2012b) |
T1 HC: n=19 SUD: n=20 T2 HC: N/A SUD: n=20 |
1.5T MRI: T1- weighted scan (sequence not reported) Whole-brain | T1: 3 days T2: 1 month |
p< 0.001; uncorrected, >100 clusters |
SUD at T1 vs. HC at T1 ↓ GM volume: superior and middle frontal gyrus, posterior cingulate, and inferior occipital gyrus SUD at T2 vs. HC at T1 ↓ GM volume: middle frontal gyrus, posterior cingulate, and inferior occipital gyrus |
HC N/A SUD NS |
|
| DTI | |||||||
| Wang et al (2013) |
T1 HC: n=20 SUD: n=20 T2 HC: N/A SUD: n=20 |
1.5T MRI: DTI (sequence not reported) Whole-brain | T1: 3 days T2: 1 month |
< 0.001; uncorrected, >15 voxels |
SUD at T1 vs. HC at T1 ↓ FA: frontal gyrus and cingulate gyrus SUD at T2 vs. HC at T1 NS |
HC N/A SUD NS |
|
| (B) FUNCTIONAL STUDIES | |||||||
| Study | Sample (at each time point) | Imaging Modality / Whole brain analysis or a priori ROI assessed | Task or Stim uli | Abstinence Length (at each scan) | Statistical Threshold for Longitudinal Changes | Crosssectional Results (SUD vs. HC) | Within-Group Longitudinal Changes |
| Alcohol | |||||||
| Mon et al (2009) |
T1 HC: n=28 SUD: n=58 T2 HC: n=13 SUD: n=41 (22 cigarett esmokers, 19 non-smokers) |
Perfusion MRI (ASL): Frontal and Parietal GM | Rest: Eyes closed | T1: 6 days T2: 34 days |
p<0.05 |
SUD at T1 vs. HC at T1 ↓ Cerebral blood flow: Frontal and parietal GM SUD at T2 vs. HC at T1 ↓ Cerebral blood flow: Parietal GM Non-smokers NS Smokers ↓ Cerebral blood flow: Frontal and parietal GM |
HC NS SUD NS Non-smokers ↑ Cerebral blood flow: Frontal and parietal GM Smokers NS |
| Durazzo et al., (2010) |
T1 HC: 28 SUD: n=57 T2 HC: N/A SUD: n=41 SUD labeled as Abs or Rel based on a 12 months clinical follow-up |
Perfusion MRI (ASL): Frontal and parietal GM | N/A | T1: 7 ± 3 days T2: 35 ± 11 days |
p<0.05 |
SUD-Abs at T1 vs. SUDRel at T1 vs. HC at T1 ↓ Cerebral blood flow in Rel compared to both Abs and HC: Frontal and parietal GM SUD-Abs at T2 vs. SUDRel at T2 vs. HC at T1 ↓ Cerebral blood flow in Rel compared to both Abs and HC: Frontal GM |
HC N/A SUD NS |
| Colrain et al (2012) |
T1 HC: N/A SUD: n=15 T2 HC: N/A SUD: n=15 |
EEG (Sleep evoked potentials): FP1, FP2, Fz, FCz, Cz, CPz, Pz | Auditory stimuli | T1: 165 days T2: 394 days |
p<0.05 | N/A |
HC N/A SUD ↑ N550 and ↑ P900 amplitudes: Frontal electrodes |
| Nicotine | |||||||
| Janes et al., (2009) |
T1 HC: N/A SUD: n=13 T2 HC: N/A SUD: n=13 |
Task fMRI: Whole-brain | Cue reactivity (smokingrelated versus neutral pictures) | T1: prequit T2: 52 ± 11 days |
P<0.005 | N/A |
HC N/A SUD ↑ BOLD activation (smoking vs. neutral pictures): Frontal cortex (BA 6, 9, 44, 46), ACC (BA 24, 32), PCC (BA 31), temporal cortex (BA 22, 41, 42), parietal cortex (BA 1, 2, 4, 7, 40), and caudate. ↓ BOLD activation (smoking vs. neutral pictures): hippocampus |
| Cocaine | |||||||
| Balodis et al. (2016) |
T1 HC: n=28 SUD: n=29 T2 HC: n=28 SUD: n=29 |
Task fMRI: Whole-brain and ROI (VS) | MID task (reward and loss anticipatio n, reward and loss outcome) | T1: 2 months T2: 5 months |
p< 0.05 (FWE) |
N/A |
HC ↓ BOLD activation (reward outcome): Ventromedial PFC extending to ACC SUD ↑ BOLD activation (reward anticipation): Precuneus, posterior cingulate extending to culmen, midbrain, thalamus extending to striatal regions |
| Moeller et al (2012) |
T1 HC: n=13 SUD: n=15 T2 HC: N/A SUD: n=15 |
Task fMRI: Whole brain | Drug Stroop task (drug and neutral words) | T1: ~3 weeks T2: 6 months |
p<0.05 (cluster-corrected); >15 contiguous voxels |
SUD at T1 vs. HC at T1 ↓ BOLD activation: Midbrain SUD at T2 vs. HC at T1 NS |
HC N/A SUD ↑ BOLD activation: Midbrain and thalamus |
| Alper et al. (1998) |
T1 HC: N/A SUD: n=17 T2 HC: N/A SUD: n=17 T3 HC: N/A SUD: n=17 |
Quantitative EEG: frontal absolute and relative power, asymmetry and coherence: delta frequency band | Rest: Eyes closed | T1: 5–10 days (3.2±2.4 days) T2: 1 month T3: 6 months |
p<0.05 | N/A |
HC N/A SUD NS |
| Parvaz et al (2017) |
T1 HC: n=18 SUD: n=19 T2 HC: N/A SUD: n=19 |
Task EEG: C1, Cz, C2, CP1, CPz and CP2 | Passive Picture Viewing (pleasant, unpleasant, drug, and neutral pictures) | T1: ~3 weeks T2: 6 months |
p<0.05 |
SUD at T1 vs. HC at T1 ↓ LPP amplitude: Pleasant versus neutral pictures ↑ LPP amplitude: Drug versus neutral pictures SUD at T2 vs. HC at T1 ↓ LPP amplitude: Pleasant versus neutral pictures ↑ LPP amplitude: Drug versus neutral pictures |
HC N/A SUD ↑ LPP amplitude: Pleasant versus neutral pictures NS: Drug versus neutral pictures |
| Heroin | |||||||
| Wang et al., (2011) |
T1 HC: n=16 SUD: n=15 T2 HC: N/A SUD: n=15 |
Resting fMRI: Whole-brain | Rest: Eyes closed | T1: 3 days T2: 1 month |
p<0.005 |
SUD at T1 vs. HC at T1 ↑ BOLD activation: bilateral OFC ↓ BOLD activation: Cerebellar tonsil SUD at T2 vs. HC at T1 ↓ BOLD activation: Cerebellar tonsil |
HC N/A SUD ↓ BOLD activation: Cerebellar tonsil, frontopolar PFC (BA 10), subgenual ACC (BA 25) |
| Other SUD | |||||||
| Forster et al (2016)sUD: alcohol or polysubst ance |
T1 HC: N/A SUD: n=26 T2 HC: N/A SUD: n=21 (of whom 12 had AUD) |
Task fMRI: Whole brain | BART (Contrasts : Decision events; Success feedback; Explode feedback) | T1: ~1–4 weeks T2: 3 months |
p<0.05 (cluster-corrected); >30 contiguous voxels | N/A |
HC N/A SUD ↑ BOLD activation: dorsal premotor cortex (Decision events) caudal ACC and PCC (Success feedback) ↓ BOLD activation: IFG and caudal ACC, extending into the cingulum (Explode feedback) |
| (C) NEUROCHEMICAL STUDIES | |||||||
| Study | Sample (at each time point) | Imaging Modality /Brain Region | Task or Stimuli | Abstine nce (at each scan) | Statistical Threshold for Longitudinal Changes | Crosssectional Results (SUD vs. HC) | Within-Group Longitudinal Changes with Abstinence |
| Alcohol | |||||||
| Ceccarini et al (2014) |
T1 HC: n=17 SUD: n=26 T2 HC: N/A SUD: n=19 |
[18F] PET Whole brain | Alcohol administra tion | T1: >1 week (6±2 days) T2: 1 month |
p<0.05 (FWE); >200 contigluous voxels |
SUD at T1 vs. HC at T1 ↓ CB1R availability: Frontal, temporal, mesotempor al, parietal and occipital lobes, and striatum SUD at T2 vs. HC at T1 N/R |
HC N/A SUD NS |
| Ceccarini et al (2020) |
T1 HC: n=32 SUD: n=16 T2 HC: N/A SUD: n=10 T3 HC: N/A SUD: n=8 |
[18F] PET Whole brain and ROI | Not specified | T1: <2 week T2: 2 month T3: 6 months |
pcluster<0.05 (cluster le/ ; vel) |
SUD at T1 vs. HC at T1 ↓mGluR5 availability: Corticolimbic regions SUD at T2 vs. HC at T1 ↓mGluR5 availability: Corticolimbic regions (esp. in the thalamus and hippocampus) SUD at T3 vs. HC at T1 NS in most regions ↓mGluR5 availability: the thalamus and hippocampus, and the NAcc |
HC N/A SUD at T2 vs. T1 ↑ mGluR5 availability: bilateral cortical (middle frontal gyrus, superior orbitofrontal gyrus, anterior cingulate cortex, insula, and left inferior temporal lobe) and subcortical (encompassing the hippocampus, parahippoca mpal gyrus, and putamen) clusters SUD at T3 vs. T1same as T2 vs. T1 |
| Gazdzinski et al. (2008) |
T1 HC: 14 SUD: n=24 T2 HC: N/A SUD: n=24 (13 cigarett esmokers, 11 non-smokers) |
1.5T 1H MRS ROI (medial temporal lobe) | Rest | T1: ~6 days T2: ~32 days |
p<0.05 with a Bonferroni correction |
SUD at T1 vs. HC at T1 ↓ NAA, ↓ Cho: Medial temporallobe SUD at T2 vs. HC at T1 ↓ Cho, ↓ NAA: Medial temporallobe |
HC N/A SUD NS Non-smokers ↑ NAA: Medial temporallobe (trend) ↑ Cho: Medial temporallobe Smokers NS |
| Mon et al (2012) |
T1 HC: n=16 SUD: n=20 T2 HC: N/A SUD: n=36 |
5T 1H single voxel MRS ROI (DLPFC, ACC, the parieto-occipital cortex) | Rest | T1: ~9 days T2: ~34 days |
p<0.05 |
SUD at T1 vs. HC at T1 ↓ Glu, ↓Glx, ↓NAA, Cr: ACC SUD at T2 vs. HC at T1 NS |
HC N/A SUD ↑ Glu, ↑ Glx, ↑NAA, ↑Cho ratios to water: ACC |
| Durazzo et al. (2006) |
T1 HC: n= 29 (20 cigarett esmokers, 9 non-smokers) SUD: n=36 (19 cigarett esmokers, 17 non-smokers) T2 HC: N/A SUD: n=25 (14 cigarett esmokers, 11 non-smokers) |
1.5T 1H MRS ROI (major cerebral lobes, thalamus, cerebellar vermis) | Rest | T1: ~7 days T2: ~34 days |
p<0.02 (FWE; Metabolite concentration in GM); p<0.01 (FWE; Metabolite concentration in WM) |
SUD at T1 vs. HC at T1 N/R SUD at T2 vs. HC at T1 ↓ NAA, ↓ Cho: Parietal WM |
HC N/A SUD ↑ NAA: Frontal GM and WM ↑ Cho: Frontal and parietal GM & WM, and occipital WM ↑ mI and ↑ Cr: Frontal WM Non-smokers ↑ NAA: Frontal WM ↑ Cho: Frontal temporal and parietal GM & WM, and occipital WM Smokers ↑ NAA: Frontal GM ↓ NAA: parietal and occipital WM ↑ Cho: Frontal GM |
| Bartsch et al. (2007) |
T1 HC: n=10 SUD: n=24 T2 HC: n=10 SUD: n=15 |
1.5T 1H MRS ROI (mesial frontal lobe and cerebellum |
Rest | T1: <1week (3 ± 1 days) T2: 6–7 weeks (38 ± 3 days) |
p<0.05 (FWE) at voxel level |
SUD at T1 vs. HC at T1 NS SUD at T2 vs. HC at T2 NS |
HC NS SUD ↑ NAA: Mesial frontal lobe ↑ Cho: Cerebellum |
| Parks et al. (2002) |
T1 HC: n=12 SUD: n=31 T2 HC: n=7 SUD: n=23 T3 HC: n=7 SUD: n=11 |
1.5T 1H MRS ROI (anterior frontal lobe and anterior cerebellar vermis) | Rest | T1: ~5 days T2: 3 weeks T3: 3 months |
p<0.05 |
SUD at T1 vs. HC at T1 ↓ NAA and ↓ Cho: Cerebellum SUD at T2 vs. HC at T1 N/R SUD at T3 vs. HC at T1 N/R |
HC NS SUD ↑ NAA: Cerebellum (T3>T2) |
| Ende et al.(2005) |
T1 HC: n=30 SUD: n=33 T2 HC: n=12 SUD: n=33 (14 abstine nt) T3 HC: N/A SUD: n=26 (11 abstinent) |
1.5T 1H MRS ROI [frontal lobe, superior fontal gyrus, ACC, pons and cerebellum (cortex and vermis)] | Rest | T1: <5 weeks T2: 3 months T3: 6 months |
p< 0.008 (crosssectional analysis: Bonferronicorrected for six subregions) p< 0.025 [longitudinal analysis: Bonferronicorrected for two (NAA and Cho) metabolites] |
SUD at T1 vs. HC at T1 ↓ NAA, ↓ Cho, and ↓ Cr: Frontal WM, cerebellar cortex and cerebellar vermis SUD at T2 vs. HC at T1 N/R SUD at T3 vs. HC at T1 NS |
HC N/R SUD N/R Abstainers ↑ Cho: all ROIs (T2>T1) Non-Abstainers N/R |
| Nicotine | |||||||
| Rademacher et al (2016) |
T1 HC: n=15 SUD: n=30 T2 HC: N/A SUD: n=15 |
18F-FDOPA PET: ROIs [Cerebellum, VS, caudate (dorsal and ventral) and Putamen (dorsal and ventral)] | Not specified | T1: 1 day T2: 3 months |
p< 0.05 (Dubey/Armit age-Parmar correction) |
SUD at T1 vs. HC at T1 ↓ DA synthesis: Dorsal and ventral caudate SUD at T2 vs. HC at T1 N/R |
HC N/A SUD ↑ DA synthesis: Dorsal and ventral caudate |
| Methamphetamine | |||||||
| Burger et al. (2018) |
T1 HC: n=22 SUD: n=31 T2 HC: N/A SUD: n=22 |
3T 1H MRS ROI (ACC, DLPFC, and frontal WM) | Rest | T1: ~ 1.5 weeks T2: ~ 5 weeks |
p<0.05 |
SUD at T1 vs. HC at T1 ↓ NAA and ↓ NAA+NAAG: DLPFC ↓Cho: Frontal WM SUD at T2 vs. HC at T1 ↓ NAA and ↑ mI: ACC ↓ NAA and ↓ NAA+NAAG : DLPFC ↓Cho: Frontal WM |
HC N/A SUD ↓ NAA, ↓ NAA+NAAG and ↑ mI: ACC ↓ NAA, ↓ NAA+NAAG and ↓ mI: Frontal WM |
| Heroin | |||||||
| Xu et al (2015) |
T1 HC: n=20 SUD: n=55 (assigned to either Placebo or Jitai groups) T2 HC: N/A SUD: n=49 T3 HC: N/A SUD: n=43 T4 HC: N/A SUD: n=37 |
[99mTc]TRO DAT-1sPECT ROIs (striatum) | Rest: Placebo versus Jitai (a traditional Chinese medicine) | T1: 20 days T2: 3 months T3: 6 months T4: 12 months |
p<0.05 |
SUD at T1 vs. HC at T1 ↓ DAT (32%):Striatum SUD at T2 vs. HC at T1 N/R SUD at T3 vs. HC at T1 N/R SUD at T4 vs. HC at T1 ↓ DAT:Striatum (left) |
HC N/A SUD ↑ DAT (22%):Striatum (T4>T1) Jitai ↑ DAT (15%):Striatum (T3>T1) ↑ DAT (18%):Striatum (T4>T1) Placebo ↑ DAT (25%):Striatum (T4>T1) |
ACC, anterior cingulate cortex; AD, axial diffusivity; ASL, arterial spin labeling; BART, balloon analog risk task; BOLD, Blood-oxygen dependent Level; BDNF, brain-derived neurotrophic factor; CB1R, cannabinoid 1 receptor; Cho, choline; CSF, cerebral spinal fluid; DA, dopamine; DAT, dopamine transporter; DG, dentate gyrus; DLPFC, dorsolateral; prefrontal cortex; DTI, diffusion tensor imaging; DWI, diffusion weighted imaging; EEG, electroencephalography; FA, functional anisotropy; FDG, 18Ffluorodeoxyglucose; FDOPA, 6-[18F]fluoro-L-DOPA (FDOPA); fMRI, functional magnetic resonance imaging; FWE, family wise error; FDR, false discovery rate; Glx, glutamate+glutamine concentration; GM, gray matter; GRASS, gradient-recalled acquisition in the steady state; HC, healthy controls; IFG, inferior frontal gyrus; LPP, late positive potential; 3D-MDEFT, three-dimensional-modified driven equilibrium Fourier transform; MD, mean diffusivity; mI, myo-inositol; MID, monetary incentive delay; MPH, methylphenidate; MPRAGE, magnetization-prepared gradient-echo sequence; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAA, N-acetylaspartate; NAcc, nucleus accumbens; NAAG, N-acetylaspartate-glutamate; N/A, not applicable; N/R, not reported; NS, not significant; OFC, orbitofrontal cortex; PET, positron emission tomography; PFC, prefrontal cortex; RD, radial diffusivity; ROI, region of interest; SPECT, single-photon emission computed tomography; SUD, substance use disorder; T1–4, time points 1 through 4; tfce, threshold-free cluster enhancement; VS, ventral striatum; WM, white matter; vmPFC, ventromedial prefrontal cortex; ↓, decreased; ↑, increased; =, no change. The table is arranged by imaging modalities (structural, functional and then neurochemical studies), within each modalities the studies are group by the substance (alcohol, cocaine, methamphetamine, heroin, nicotine, and other SUD) and within each substance the studies are arranged by the increasing duration of abstinence at the final scan. Sample sizes are reported for only those individuals who were abstinent on the study day. Default longitudinal comparison is between the first and the last scan, unless specified otherwise.
3. STRUCTURAL STUDIES
Abnormalities in brain structure are well documented in individuals with SUD (Mackey et al., 2019). Magnetic resonance imaging (MRI) allows for the detection of subtle variations in the volume and shape of cortical and subcortical regions as well as cortical thickness, area and folding patterns (Ashburner and Friston, 2000). Voxel-based morphometry is a technique that segments brain images into gray matter (GM) volume, white matter (WM) volume, and cerebrospinal fluid to index neuroanatomical abnormalities (Ashburner and Friston, 2000). Although both GM and WM can be assessed using this method, changes in WM integrity are evaluated more accurately using imaging techniques, such as diffusion tensor imaging (DTI), which provides more subtle information about tissue microstructure and organization (Jones et al., 2013; Whitwell, 2009). Common measures computed from DTI are fractional anisotropy, an indicator of WM track myelination (Nucifora et al., 2007), radial diffusivity, a marker of myelin integrity (Harsan et al., 2006), mean diffusivity, a marker of the magnitude of (isotropic) water diffusivity, and axial diffusivity, a marker of the magnitude of diffusion parallel to the fiber tracts.
3.1. Alcohol
3.1.1. Gray Matter
Structural changes have reliably been reported in individuals with alcohol use disorders (AUD) who have achieved abstinence. Using a regions of interest (ROI) approach in 49 alcohol-dependent patients, van Eijk et al., (2013) revealed GM volume increases in several brain regions including the cingulate gyrus, insula, temporal gyrus, precuneus, parietal lobule, and cerebellum following 2 weeks of abstinence (van Eijk et al., 2013). These results were partially supported by a more recent study that used a whole-brain approach to investigate GM volume recovery in 62 individuals with AUD (Bach et al., 2020). Here, GM volume increased in the middle and inferior frontal gyri, middle cingulate gyrus, insula, supramarginal gyrus, and precuneus from approximately 12 days of abstinence to 27 days. Cortical thickness measures show a similar pattern (Wang et al., 2016; Bach et al., 2020). Specifically, in the first 2 weeks of abstinence in 49 alcohol-dependent individuals increased cortical thickness was observed in the medial orbitofrontal cortex (OFC), middle frontal, superior frontal, rostral anterior cingulate cortex (ACC), cuneus, and inferior parietal and lateral occipital regions; greater cortical thickness recovery in sulci compared to gyri, particularly for frontal regions, suggested that sulci may be more sensitive than gyri to excessive alcohol consumption and abstinence-induced recovery (Wang et al., 2016). The Bach et al., (2020) study similarly reported increases primarily in frontal regions encompassing the superior frontal cortices, lateral OFC, and rostral middle frontal cortex, and in the insula and lateral occipital cortex in 62 alcohol-dependent individuals. In general, in these studies, GM (volume and/or thickness) increased in brain regions that showed reductions when individuals with AUD were compared to healthy controls.
When studies investigated abstinence durations of a least 4 weeks, increases in frontal regions became more apparent. For example, increased frontal GM volume (but not parietal, temporal or occipital) was found in 42 individuals with AUD following 4 weeks of abstinence (Durazzo et al., 2017). A study from the same group showed that differences between individuals with AUD and controls in GM volume in frontal regions, such as the OFC (but not the rostral anterior cingulate cortex or insula) observed at one week of abstinence dissipated following 4 weeks of abstinence; yet within-subject frontal changes did not achieve significance when examined longitudinally (Durazzo and Meyerhoff, 2020). Interestingly, following 5 weeks of abstinence, Mon and colleagues (2013) reported that in 41 alcohol-dependent individuals, GM volume increases in the frontal lobe were genotype-specific for a polymorphism (Val66Met) in the neurotrophin, brain-derived neurotrophic factor (BDNF) (Mon et al., 2013). Given that BDNF supports the survival of neurons and promotes neurogenesis in the brain (Lu and Chow, 1999), it has clear relevance to GM volume recovery. Specifically, relative to Val/Val carriers, Met carriers have a significant reduction (~30%) in BDNF secretion, which may compromise their potential for GM volume recovery (Chen et al., 2008). Indeed, while some GM changes were observed with abstinence in alcohol-dependent individuals who were Val/Met carriers (n=15) (increased GM volume in the cerebellum exclusively in this group and increases in the temporal lobe in both genotype groups), only those who were Val/Val carriers (n=26) had increased frontal GM volume following abstinence (Mon et al., 2013). There were also GM volume increases in the parietal lobe and the thalamus, and decreased GM volume in the caudate, again exclusive to Val/Val carriers. Of note, collapsed across genotype group, increases in frontal and parietal GM volume were related to improvement in working memory, with the latter also associated with improvement in processing speed, and increases in all cortical and subcortical GM were related to improvement in visuospatial learning (Mon et al., 2013).
Significant recovery in frontal GM volume, including in the OFC, was observed in investigations following 4 and 7 months of abstinence. Demirakca et al., (2011) showed increases in GM volume in the OFC, as well as the cingulate gyrus, and insula after 4 months of abstinence in 14 alcohol-dependent individuals (Demirakca et al., 2011), while Cardenas et al., (2007) demonstrated increased GM volume in the OFC and parietal lobes following 7 months of abstinence in 17 alcohol-dependent individuals (Cardenas et al., 2007).
Many studies have adopted an ROI approach to specifically examine the hippocampus, a structure that is highly susceptible to neuronal injury (Geddes et al., 2003) but also possesses high potential for neuronal plasticity and regeneration (Leuner and Gould, 2010). Studies have reported increased hippocampal GM volume following 2 weeks (Kuhn et al., 2014), 4 weeks (Gazdzinski et al., 2008), and 7.5 months of abstinence (Zou et al., 2018). Interestingly, similarly to Mon et al., (2013), Hoefer et al., (2014) reported a trend towards increased hippocampal volume following 7 months of abstinence in Val/Val but not Met/Val carriers or controls (Hoefer et al., 2014). Differences from healthy control participants even after this abstinence duration (reduced hippocampal volumes in the AUD) suggest only partial recovery of the hippocampus (Hoefer et al., 2014; Zou et al., 2018). Encouragingly, better visuospatial processing (Hoefer et al., 2014) and visual short- and long-term memory (Gazdzinski et al., 2008) were observed even with only partial hippocampal recovery. In contrast, other studies have reported no change in hippocampal volume early in abstinence [2 weeks (van Eijk et al., 2013; Wang et al., 2016)] or with longer periods of abstinence [4 months (Demirakca et al., 2011)]. Similarly no significant changes in GM volume were found in several other subcortical regions examined as ROIs, such as the amygdala (Demirakca et al., 2011; Wang et al., 2016; Zou et al., 2018) and putamen/lenticular nucleus (Durazzo et al., 2015; Mon et al., 2013; Wang et al., 2016) following abstinence; results observed in the caudate were mixed (Durazzo et al., 2015; Mon et al., 2013; Wang et al., 2016).
Investigations that include more than two time-points provide a window into the trajectory/slope of brain volume recovery over abstinence. Overall, results in individuals with AUD suggest that over protracted abstinence (~ 1 year), the majority of GM volume recovery occurs within the first month of abstinence (Durazzo et al., 2015; Gazdzinski et al., 2005; Pfefferbaum et al., 1995). A large cohort scanned over three time-points (n=82 at 1 week and 1 month; and n=36 at 7.5 months) demonstrated increased GM volumes in the frontal, parietal and occipital lobes, thalamus, caudate, and cerebellum over 7.5 months of abstinence with greater increases occurring between 1 week and 1 month of abstinence compared to 1 month and 7.5 months of abstinence (specifically for frontal and parietal lobes, thalamus, and cerebellum) (Durazzo et al., 2015). With the exception of GM volume in the thalamus, these changes over the 7.5 month of abstinence were associated with better processing speed, a relationship that was only evident in non-smoking individuals (n=18) and did not extend to smokers with AUD (n=18) (Durazzo et al., 2015). Interestingly, a follow-up study in a similar cohort and similar time points (n=65 at 1 week; n=82 at 1 month; and n=36 at 7.5 months; 23 participants had no/unusable data from 1 week of abstinence and thus were first assessed at 1 month of abstinence) revealed that the rate of recovery may be region-specific (Zou et al., 2018). Specifically, whereas the dorsolateral prefrontal cortex (DLPFC), OFC, and insula showed greater increases in GM volume within the first month of abstinence relative to 1 week and 7.5 months, the ACC increased only between 1 month and 7.5 months of abstinence. Together, these studies indicate that GM volume recovery follows a nonlinear trajectory (i.e., steeper slope earlier in recovery) that may vary region by region (Durazzo et al., 2015; Zou et al., 2018).
3.1.2. White Matter
Studies using voxel-based morphometry to index morphological abnormalities in WM have reported mixed results, perhaps because of this technique’s limited sensitivity to quantifying WM volume. Mon et al., (2013) observed increased WM volume in the frontal lobes (and trends for the parietal and temporal lobes) in Val/Met carriers of the BDNF gene following 5 weeks of abstinence. Given the GM findings and BDNF’s contributions as described above, this unexpected finding remains to be replicated in larger sample sizes. Following 4 months of abstinence, two studies reported increased total WM volume (Demirakca et al., 2011; Shear et al., 1994), while following 7.5 months of abstinence, another study demonstrated increased WM volume specifically in the parietal, temporal, and occipital lobes, with the predominance of recovery occurring between 1 and 7.5 months (Durazzo et al., 2015). In contrast, other studies have reported no changes in regional WM volume following 7 months of abstinence (Cardenas et al., 2007) or in total WM volume following 2 weeks (van Eijk et al., 2013), 4 weeks (Durazzo et al., 2017) and 12 months of abstinence (Pfefferbaum et al., 1995).
Using diffusion tensor imaging, the pattern of results is more consistent. Following one month of abstinence, Gazdzinski et al., (2010) observed increased fractional anisotropy in the frontal, temporal, parietal, and occipital lobes and a decrease in mean diffusivity in the temporal lobe in non-smoking in alcohol-dependent individuals (n=10), an effect not present in alcohol-dependent smokers (n=11) (Gazdzinski et al., 2010), suggesting that smoking may selectively affect WM microstructure and recovery in alcohol-dependent individuals. Increased fractional anisotropy and reduced radial diffusivity were seen in the genu and body of the corpus callosum during the first year of abstinence in 15 alcohol-dependent individuals (Alhassoon et al., 2012). Although not directly correlated with the diffusion metrics, significant improvements in working memory at follow-up were noted in these subjects. Increased fractional anisotropy in multiple brain areas (20 out of 27 ROIs assessed, including the corpus collosum, the fornix, and corona radiata) was also observed over a follow-up period of up to 8 years in individuals with alcohol dependence (Pfefferbaum et al., 2014).
3.2. Stimulants and Opioids
A study from our group investigated regional GM volume recovery in 19 abstinent individuals with cocaine use disorder who either achieved abstinence or significantly reduced their cocaine use from baseline (≥ 3 weeks after last drug use) to 6 month follow-up (Parvaz et al., 2016a). Using a whole-brain approach, we demonstrated that GM volume increased in the ventromedial PFC, OFC and inferior frontal gyrus with the latter increases associated with improvements in cognitive flexibility and decision-making measured by the Wisconsin Card Sorting Test and Iowa Gambling Task, respectively (Parvaz et al., 2016a). In another whole-brain study examining individuals with methamphetamine use (n=29), cerebellar GM volume increased, but the cingulate gyrus GM volumes decreased, from 6 months to 12 months of abstinence (Ruan et al., 2018). Over a similar timeframe, Zhuang et al., (2016) showed that compared with baseline (at a 6 months abstinence), individuals with methamphetamine use had continued fractional anisotropy reductions in the internal capsule and superior corona radiata at 13 months of abstinence (Zhuang et al., 2016).
To date only two studies used a longitudinal design to investigate structural recovery in individuals with an opioid use disorder. Employing a whole-brain approach, Wang et al., (2012b) examined the effects of one month of abstinence (compared to a 3 day abstinent scan) in 20 treatment-seeking males with heroin use disorder. While there were no significant longitudinal improvements, the superior frontal gyrus GM abnormalities (as compared to healthy controls) that were documented after 3 days of abstinence were not detectable following one month of abstinence (abnormalities in the other cortical regions, including the middle frontal gyrus, persisted) (Wang, X. et al., 2012). Similarly, although white matter showed no within-subject longitudinal changes with abstinence, abnormalities in fractional anisotropy in the frontal gyrus and cingulate gyrus that were evident after 3 days of abstinence were no longer detectable following one month of abstinence (Wang, X. et al., 2013).
3.3. Interim Summary
Among individuals with AUD, GM volume recovery following abstinence was predominantly assessed via an ROI approach with findings generally indicating increased GM volume in cortical regions spanning the frontal (Cardenas et al., 2007; Demirakca et al., 2011; Durazzo et al., 2017; Durazzo et al., 2015; Gazdzinski et al., 2005; Mon et al., 2013; Pfefferbaum et al., 1995; van Eijk et al., 2013; Zou et al., 2018) and temporal, parietal, and occipital lobes (Cardenas et al., 2007; Durazzo et al., 2015; Mon et al., 2013; van Eijk et al., 2013) as well as the insula (Bach et al., 2020; Demirakca et al., 2011; van Eijk et al., 2013; Zou et al., 2018). Increases were also noted in the hippocampus (Gazdzinski et al., 2005; Gazdzinski et al., 2008; Hoefer et al., 2014; Kuhn et al., 2014; Zou et al., 2018), thalamus (Durazzo et al., 2015; Mon et al., 2013), and cerebellum (Durazzo et al., 2015; Gazdzinski et al., 2005; Mon et al., 2013; van Eijk et al., 2013) but not in the caudate [where mixed results were reported (Durazzo et al., 2015; Mon et al., 2013; Wang et al., 2016)] and lentiform nucleus/putamen (Durazzo et al., 2015; Mon et al., 2013; Wang et al., 2016). Encouragingly, GM recovery occurred as early as 2 weeks post-cessation in select regions (Kuhn et al., 2014; van Eijk et al., 2013) and multi-time point studies suggest that the majority of GM recovery occurs within the first month of abstinence (Durazzo et al., 2015; Gazdzinski et al., 2005; Pfefferbaum et al., 1995; Zou et al., 2018). These changes are associated with improved cognitive function and may be more discernable in certain subgroups of individuals (e.g., with select genes and/or non-smokers).
Given the paucity of studies investigating WM, the general pattern of its recovery remains unclear. While studies using non-DTI methods report both regional and global increases (Demirakca et al., 2011; Durazzo et al., 2015; Mon et al., 2013; Shear et al., 1994), as well as some mixed findings, DTI studies more consistently point to increased WM integrity (as measured by fractional anisotropy) of the corpus collosum following abstinence (Alhassoon et al., 2012; Pfefferbaum et al., 2014).
The majority of the above reviewed studies have been conducted in individuals with AUD following abstinence. Only a handful of studies have examined structural recovery in individuals with substance use disorders other than alcohol including stimulant and opioid use disorder, and no study has investigated structural recovery in treatment-seeking marijuana users. Future research that addresses structural changes associated with these substances following abstinence is clearly warranted.
4. FUNCTIONAL STUDIES
Human brain function is commonly assessed using imaging modalities such as functional MRI (fMRI) and psychophysiological tools such as electroencephalography (EEG). Functional MRI measures local changes in cerebral blood flow and brain metabolism using the blood oxygen level-dependent (BOLD) signal, which is an indirect measure of neural activity that relies on a cascade of physiological events linking neural activity in specific brain regions to the MRI signal. Electroencephalography assesses electrical signals with high temporal resolution, allowing to track human brain function in almost real time, although it is limited by poor spatial resolution. These techniques provide an evaluation of how the brain works dynamically, its physiology, regional connectivity and functional architecture either during rest or in response to specific stimuli. Accordingly, these tools can index neural changes and reorganization that are associated with cessation of or reduction in substance use in individuals with SUD.
4.1. Alcohol
In contrast to the numerous structural neuroimaging studies, to date there have only been a few neuroimaging studies that examined longitudinal changes in brain function during abstinence in AUD. Mon et al. (2009) used arterial spin labeling to examine longitudinal cerebral blood flow changes after 1 month of sobriety relative to baseline (one week of abstinence) in individuals with AUD (n=41) as compared to light social drinkers (n=13). Similarly to the GM volume results reported above, at baseline and compared to the light drinkers, individuals with AUD showed lower cerebral blood flow in frontal and parietal GM. Longitudinally, although there were no significant changes with abstinence in the entire sample of individuals with AUD, recovery (i.e., increase to the level of light social drinkers) in frontal and parietal GM cerebral blood flow was observed only in non-smoking AUD (n=19) but not in the smokers (n=22) (Mon et al., 2009). A subsequent study from the same group divided the 41 individuals with AUD to those who remained abstinent (n=19) vs. those who relapsed (n=22) at 12-months follow-up; here again, although there were no longitudinal changes across the entire group, recovery in cerebral blood flow was observed in those who maintained abstinence but not in those who relapsed (Durazzo et al., 2010). Taken together, these two studies suggest that longitudinal recovery between 1 week and 1 month of abstinence in cerebral blood flow can be observed in non-smoking AUD and/or those who can maintain abstinence.
Further evidence of functional recovery in abstaining alcohol users (n=15) is provided by an EEG study that reported recovery of sleep evoked potentials, recorded from frontal electrodes, after a longer-term (>12 months) abstinence (Colrain et al., 2012). These sleep evoked potentials (mainly the K-complex comprised of the N550 and P900 amplitudes), previously reported to be reduced in individuals with AUD as compared to healthy controls by the same group (Colrain et al., 2009), reflect the functional integrity of the underlying cortex (Colrain, 2005; Tononi and Cirelli, 2006), also representing memory consolidation (Poe et al., 2010).
4.2. Nicotine and Cocaine
In a longitudinal fMRI cue-reactivity study, Janes et al, (2009) reported an increase in fMRI BOLD activity in prefrontal, temporal and parietal regions in response to smoking-related relative to neutral pictures in 13 tobacco-dependent individuals from a pre-cessation baseline to about 1–2 months of abstinence (52 ± 11 days). These results suggest that reactivity to substance-related cues increased during the early phase of abstinence, which is consistent with the trajectory of incubation of cue-reactivity (or craving) as has previously been shown in individuals with other types of SUD using self-reported (Bedi et al., 2011; Li et al., 2015; Wang, G. et al., 2013; Wang, G.B. et al., 2012) and EEG correlates of (Parvaz et al., 2016b) cue-induced craving.
Two fMRI studies investigated longitudinal changes as a function of abstinence duration in individuals with cocaine use disorders, both reporting improved activation in the midbrain and the thalamus. In the first study we used a monetarily rewarded drug Stroop task and showed decreased fMRI BOLD activation (overall task versus baseline) in the midbrain of 15 treatment-seeking cocaine-addicted individuals compared to 13 non-addicted healthy controls at baseline (after detoxification; ≥ 3 weeks after last drug use). After about 6 months of mostly abstinence/substantially reduced drug use, the fMRI BOLD signal in cocaine-addicted individuals was comparable to that in non-addicted healthy controls at baseline. We interpreted these results to suggest a restoration of dopaminergic activity, supported by correlations with reduced drug-seeking behavior in these subjects (Moeller et al., 2012). The later study by Balodis et al. (2016) used the Monetary Incentive Delay task (Knutson et al., 2000) in a larger sample of cocaine-addicted individuals (n=29) and non-addicted healthy controls (n=28), to show similar increases from approximately 2 to 5 months of abstinence in the midbrain and the thalamus, and in the posterior cingulate cortex and the precuneus. Notably, the increase in midbrain activity correlated positively with abstinence duration at follow-up (Balodis et al., 2016). Taken together these results suggest that recovery in the midbrain and thalamus in response to salient reward-relevant tasks is associated with better clinical outcomes (i.e., reduced drug seeking and longer abstinence duration).
Using EEG, our group focused on the late positive potential (an event-related potential observed typically at centroparietal recording sites and indicative of bottom-up attentional change) to report that motivated attention to pleasant cues, which was lower at baseline (after detoxification; ≥ 3 weeks after last drug use) in 19 treatment-seeking cocaine-addicted individuals compared to healthy controls, increased with six months of significantly reduced cocaine use (Parvaz et al., 2017). This increase in reactivity to pleasant cues correlated with longer duration of abstinence at baseline and with decreased craving at follow-up. Nevertheless, reactivity to pleasant cues in the cocaine-addicted individuals at follow-up was still lower than that in healthy controls at baseline, suggesting only a partial recovery with 6 months of significant reduction of cocaine use. Notably, motivated attention to drug-related cues, which was increased in the cocaine-addicted individuals at baseline as compared to the healthy controls, did not change at follow-up, highlighting the protracted nature of the disproportionate attention attributed to drug-related cues in drug addiction (Parvaz et al., 2017). Similarly, a previous study investigating the EEG power in delta band frequency [that reflects frontal cortical regulation of behavioral impulses or concentration/attention allocation to extraneous cues (Fernandez et al., 1995)] also did not show abstinence-related recovery in 17 cocaine addicted individuals from 5 – 10 days to 1 or 6 months of abstinence (Alper et al., 1998). Taken together, these results suggest that while motivated attention to non-drug-related reinforcers may partially recover with 6 months of abstinence or significant reduction in cocaine use, the processes that underlie heightened attention allocation to drug-related cues and maladaptive impulse control may persist. The plasticity in reactivity to salient (including drug) reinforcers could therefore serve as an important target for long-term interventions.
4.3. Heroin and Other SUD
In a resting-state fMRI study, Wang et. al., (2011) showed higher BOLD fMRI signal in the OFC and lower activity in the cerebellar tonsil in 15 individuals with heroin use disorder at 3 days of abstinence compared to 16 non-addicted healthy controls; the activity in the cerebellar tonsil continued to decline as assessed after 1 month of abstinence when activity of frontopolar and subgenual ACC regions was also decreased (Wang et al., 2011). Although, no longitudinal changes were observed in the OFC, the absence of significant cross-sectional differences with the non-addicted healthy controls at 1-month suggests that the OFC activity may have recovered during the first month of abstinence.
In an fMRI study that employed the Balloon Analogue Risk task, 21 treatment-seeking individuals with SUD (12 AUD, four polysubstance dependence, two opioid dependence and one each sedative/hypnotic/anxiolytic dependence, cannabis dependence, and amphetamine dependence) were scanned first at 1 – 4 weeks (baseline) and then at 3 months of abstinence (follow-up) (Forster et al., 2016). Compared to baseline, at follow-up there were increased activations in the dorsal premotor cortex to decision events (when participants are deciding whether or not to inflate the balloon) and in the caudal anterior and posterior cingulate cortices for the success feedback (when participants are informed whether they succeeded and the balloon did not explode on that trial), while the inferior frontal gyrus and the caudal anterior cingulate cortex showed decreased activations to the failure feedback (when the balloon exploded) (Forster et al., 2016). Together, findings were interpreted to reflect an increased surprise signal to unexpected outcomes (i.e., high risk successes and low risk balloon explosions) in recovery, suggestive of the formation of stronger expectancies (Alexander and Brown, 2010, 2014). This study is unique in combining treatment-seeking individuals across different alcohol/drug classes potentially enhancing generalizability of results if replicated in additional samples of individuals with SUDs.
4.4. Interim Summary
There is a paucity of neuroimaging studies examining changes in brain function with abstinence in individuals with SUD. Studies in AUD point to a general recovery in frontal brain regions, showing increased cerebral blood flow in non-smoking and/or abstinent AUD (Durazzo et al., 2010; Mon et al., 2009) and increased amplitude of the EEG-derived auditory sleep evoked potentials (Colrain et al., 2012). In nicotine users, increased reactivity to smoking-related cues during the first 2 months of abstinence (Janes et al., 2009) supports the notion of incubation of cue-reactivity/craving during earlier phases of abstinence (Li et al., 2016). Reports of abstinence-mediated recovery in cocaine use disorder paint a more complex picture with a pattern of recovery that may depend on context. Within the first six months of abstinence, midbrain and thalamic responses to salient stimuli (including money) recover (Balodis et al., 2016; Moeller et al., 2012) with a similar, albeit partial, recovery in reactivity to pleasant cues as documented using an EEG-derived marker of bottom-up/automatic processing (Parvaz et al., 2017). In contrast, heightened reactivity to drug-related cues (Parvaz et al., 2017) or dysregulated attention allocation to extraneous cues (Alper et al., 1998) may be more protracted. In heroin users, however, some evidence for recovery in the OFC resting activity within the first month of abstinence (Wang et al., 2011) warrants replication and further validation. Overall, these studies point to both cortical and subcortical functional recovery during the first year of abstinence in alcohol and cocaine use disorders. More studies are needed to explore functional (cognitive and emotional) recovery in drug addiction (across all drugs of abuse and alcohol) and the effects of context and time in this non-linear multi-layered process. A drug related context may be a crucial variable predisposing addicted individuals to relapse especially at specific times in abstinence as potentially amenable for timely interception.
5. NEUROCHEMICAL STUDIES
In humans, chemical and molecular integrity of brain cells and tissue are quantified using either nuclear imaging techniques such as Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT) or Magnetic Resonance Spectroscopy (MRS). The two nuclear imaging modalities provide a quantitative assessment of regional distribution and kinetics of chemical compounds labeled with short-lived positron (in PET) or gamma (in SPECT) emitting isotopes in the living body. The molecules labeled with these isotopes bind to specific proteins (i.e., receptors and transporters) and can be measured in the tissues of interest as a function over time; PET is also used to assess the cerebral metabolic rate of glucose utilization as well as regional cerebral blood flow. Magnetic Resonance Spectroscopy is also a molecular neuroimaging technique, but does not use ionizing radiation of PET and SPECT, instead leveraging magnetic fields to localize a specific volume of tissue for spectral analysis. Each frequency in the spectrum corresponds to a metabolite nucleus, and the amplitude represents its concentration within the volume. This technique is typically used to study neuronal integrity, most reliably quantifiable via N-acetylaspartate [NAA; a surrogate marker for neuronal density and integrity (Licata and Renshaw, 2010)], choline-containing compounds [Cho; a marker of cell membrane integrity, increased in diseases with increased membrane turnover or axonal injury (Lin et al., 2012)], Creatine [Cr; a metabolite that provides a measure of cellular energy storage (Rackayova et al., 2017)], and myo-inositol [mI; a marker of glial cell density and therefore a measure of inflammation (Brand et al., 1993)], together providing a snapshot of the chemical environment of the selected brain region.
5.1. Alcohol
Most longitudinal studies employed MRS in AUD, showing partial recovery within the first three months of abstinence (Bartsch et al., 2007; Durazzo et al., 2006; Mon et al., 2012; Parks et al., 2002) of the deficits documented in this population within one month of abstinence as compared to light drinkers, encompassing reduced NAA in the ACC (Mon et al., 2012), medial temporal lobe (Gazdzinski et al., 2008), the cerebellum (Ende et al., 2005; Parks et al., 2002) and parietal (Durazzo et al., 2006) and frontal WM (Ende et al., 2005), as well as reduced Cr in the cerebellum and frontal WM (Ende et al., 2005). The partial nature of the recovery is evident in both a differential pattern of results for the different neurochemicals within different ROIs and their different recovery trajectories whereby, for example, cerebellar NAA levels increased from 3 weeks to 3 months of abstinence (n=11), whereas cerebellar Cho levels as well as frontal NAA remained below that of controls during this time frame (Parks et al., 2002). Adding to the evidence for partial neurochemical recovery, Ende et al. (2005) showed increased Cho levels in frontal WM, cerebellar cortex and vermis in AUD individuals following 3-months of complete abstinence (n=14), but reported no change in NAA levels over 3 (n=14) or 6 months (n=11) of abstinence (Ende et al., 2005). These measures of neurochemical recovery correlated with both cognitive function and brain structure. For example, increased levels of fronto-mesial NAA and cerebellar Cho from baseline (<1 week abstinence) to 6 – 7 weeks of abstinence in 15 individuals with AUD were associated with global volumetric brain gain as well as improved attention (Bartsch et al., 2007). In another study, Mon et al (2012) observed lower concentrations of glutamate and glutamate + glutamine in the ACC in treatment-seekers with AUD (n=20) at approximately nine days of abstinence, which normalized (compared to healthy controls, n=16) over four weeks of sustained abstinence but were not associated with cognitive improvement (Mon et al., 2012).
Similarly to the above reviewed structural and functional studies, the lack of consistency of results between these studies may reflect diverse subject characteristics including comorbid cigarette smoking. For example, Durazzo et al. (2006) reported significant increases in NAA (frontal GM and WM), Cho (frontal and parietal GM & WM, and occipital WM), and mI and Cr (frontal WM) concentrations in 25 individuals with AUD following approximately one-month of abstinence. However, when further stratified based on cigarette smoking status, the results diverged between non-smokers (n=11) and smokers (n=14) such that the former drove most of these changes with the latter showing increased NAA and Cho only in frontal GM and a decrease in NAA in parietal and occipital WM, suggesting that cigarette smoking may adversely affect metabolite recovery in AUD (Durazzo et al., 2006). A later study from the same group reported a similar trend for a partial recovery in Cho and NAA in non-smokers but not in smokers (Gazdzinski et al., 2008). Taken together, studies using MRS present an encouraging, albeit not entirely consistent, pattern of results with respect to changes in Cho and NAA recovery with abstinence in AUD. Low sample sizes may be another source contributing to this discrepancy.
In a PET study Ceccarini et al (2014) reported a global deficiency (−16.1%) of the endocannabinoid signaling pathways, especially in the availability of the type 1 cannabinoid receptor (CB1R), in individuals with AUD (n=26) as compared to healthy controls (n=17). Such blunted CB1R availability in the cerebellum and parieto-occipital cortex, the ventral striatum and the mesotemporal lobe did not recover after one month of abstinence (−17.0%), highlighting persistent deficits in the endocannabinoid signaling pathways, at least within the first month of abstinence in AUD (Ceccarini et al., 2014). In a recent PET study, the same group showed reduced corticolimbic metabotropic glutamate receptor 5 (mGluR5) availability in AUD at baseline (<2 weeks post-detoxification), which recovered up to the levels observed in healthy controls across 2 and 6 months of abstinence in most cortical and subcortical regions, except for the hippocampus, nucleus accumbens and thalamus. Interestingly, lower striatopallidal mGluR5 availability at baseline was associated with higher propensity to relapse at 6 months and its longitudinal normalization was associated with lower craving. Together, these results suggest that, unlike deficits in CB1R, those in mGluR5 availability normalize with abstinence in AUD (Ceccarini et al., 2014; Ceccarini et al., 2020), and such normalization, especially in the mGluR5 availability, is associated with decreased craving in this population (Ceccarini et al., 2020).
5.2. Nicotine, Methamphetamine and Heroin
Using 6-[18F]fluoro-L-DOPA (FDOPA)-PET, Rademacher et al (2016) compared presynaptic dopamine function between 15 nonsmokers and 30 nicotine-dependent smokers studied before and after 3 months of abstinence. Results revealed a 15% to 20% lower capacity of dopamine synthesis in the dorsal and ventral regions of the caudate nuclei of sated smokers as compared to non-smokers, which normalized during three months of abstinence to the level of non-smoking controls. Interestingly, this time course is consistent with earlier research suggesting that the cholinergic system takes approximately three months to normalize in abstinent tobacco smokers (Cosgrove et al., 2009).
Using MRS, Burger et al. (2018) showed lower NAA and NAA with n-acetyl-aspartyl-glutamate concentration in the DLPFC and lower Cho concentration in frontal WM in 31 individuals with a methamphetamine use disorder compared to 22 non-addicted controls. In contrast to the results of partial NAA recovery with abstinence in the AUD population, a longitudinal examination (n=22) from acute (up to 2 weeks) to short-term (up to 6 weeks) abstinence revealed further reduction in NAA and NAA with n-acetyl-aspartyl-glutamate concentrations in the ACC and frontal WM. Over time, there were also decreased levels of myo-inositol in the left frontal WM, while an increase in myo-inositol was seen in the ACC (Burger et al., 2018).
Treatment-seeking heroin users (n=55), randomly assigned to receive either Placebo or Jitai (a traditional Chinese medicine that has been approved by the China Food and Drug Administration for treatment of opioid addiction), were scanned with SPECT using [99mTc]TRODAT-1 to examine longitudinal changes in dopamine transporter concentration from baseline (almost 20 days abstinent), to three, six, and 12 months of abstinence (Xu et al., 2015). At baseline, compared to healthy controls (n=20; scanned once), the heroin addicted individuals showed lower dopamine transporter concentrations in the striatum (by 30%). Longitudinal analyses showed that the individuals assigned to the Jitai group had a steady increase in the dopamine transporter concentrations, while in the placebo group results were mixed, such that there was an increase from baseline to three months, a slight decrease from three to six months and then an increase from six to 12 months follow-up. Importantly, both groups showed a longitudinal increase in dopamine transporter concentrations from baseline to 12 months follow-up (by 20%) (Xu et al., 2015).
5.3. Interim Summary
Neurochemical techniques, especially MRS in alcohol use, have been predominantly used to quantify molecular recovery as a function of abstinence in individuals with SUD. Most studies in AUD have shown consistent increases in NAA concentration within the first 3 months of abstinence (Bartsch et al., 2007; Durazzo et al., 2006; Parks et al., 2002). The frontal cortex and the cerebellum have been the most studied ROIs, whereas some studies have also examined the parietal cortex and the medial temporal lobe. Early recovery (within one month of abstinence) shows increased NAA in the frontal cortex and the medial temporal lobe and increased Cho in frontal, temporal, parietal and occipital lobes, driven by non-smoking individuals with AUD (Durazzo et al., 2006; Gazdzinski et al., 2008). During more protracted abstinence (2 to 6 months) studies show conflicting results. For example, whereas some show increase in the cerebellar Cho (Bartsch et al., 2007; Ende et al., 2005), others do not (Parks et al., 2002), and whereas some show no change in frontal NAA (Ende et al., 2005; Parks et al., 2002), others do (Bartsch et al., 2007). Thus, in AUD, variability of results was observed based on length and status of abstinence, the metabolite and ROI under investigation, and cigarette smoking. In methamphetamine use disorder there was no recovery as a function of abstinence, with results instead suggesting continued reduction in NAA in the ACC and frontal WM up to 5 weeks of abstinence (Burger et al., 2018).
Nuclear imaging results suggest a dopaminergic recovery with abstinence. For example, a PET study in nicotine users showed increased dopamine synthesis in the dorsal and ventral caudate with over 5 weeks of abstinence (Rademacher et al., 2016). A study in heroin users showed recovery in dopamine transporter concentration in the striatum after 6 – 12 months of abstinence (Xu et al., 2015). In AUD, although mGluR5 availability showed overall recovery in both cortical and subcortical regions during the first 6 months of abstinence (Ceccarini et al., 2020), CB1R availability did not recover, at least within the first month of abstinence (Ceccarini et al., 2014).
6. DISCUSSION
Longitudinal studies that assess within-subject changes in brain morphology and function including neurochemistry following sustained abstinence are optimal for identifying the potential for and the trajectory of neural recovery in individuals with SUD. Overall, the reviewed research suggests that neural deficits dissipate following a period of sustained abstinence from substance use in individuals with SUD. In each of the three reviewed sections, sustained abstinence was predominantly associated with (at least partial) recovery, such that over time deficits in select regions appeared to normalize, implying that these abnormalities are likely consequences of substance consumption rather than premorbid or risk factors for SUD. Importantly, these neural substrates may serve as potential biomarkers that can be targeted for treatment of SUDs.
Structural recovery occurred predominantly in frontal cortical regions, the insula, hippocampus, and cerebellum. In addition to prefrontal cortical regions, functional recovery was also observed in subcortical structures (midbrain, striatum, thalamus). While reversal of neural damage was evident across studies and modalities used, numerous instances of regional specificity and variability/inconsistencies in time-course and pattern of these changes were noted. These discrepancies may reflect between-study differences in the use of ROI versus whole-brain approaches (with the former increasing the susceptibility to selection biases), inter-scan intervals and clinical characteristics (e.g., concurrent substance use, severity of SUD), calling for more and larger studies of this type. A question to explore is whether specific brain regions may be faster or more amenable to recovery, particularly the frontal cortex, while other regions may show a slower trajectory or be more impervious to change.
For structural studies, where recovery was primarily indexed as GM volume increases in individuals with AUD, greater changes occurred relatively early in the course of abstinence (i.e., within the first month of cessation), while relatively less change occurred with longer abstinence [i.e., post 6 months (Durazzo et al., 2015; Pfefferbaum et al., 1995; Zou et al., 2018)] indicating that GM structural recovery may follow a nonlinear trajectory (Gazdzinski et al., 2005). More longitudinal multi-interval studies that assess WM integrity are needed to determine the trajectory of WM recovery, particularly studies that employ DTI, rather than voxel-based morphometry, to more sensitively index changes in WM microstructure and organization following abstinence.
Similarly, early recovery was observed in neurometabolite levels in individuals with AUD (Ende et al., 2005; Parks et al., 2002). However, such early recovery was not observed in CB1R availability (Ceccarini et al., 2014) and the recovery in mGluR5 availability was most evident several months post-cessation (Ceccarini et al., 2020), suggesting a heterogeneous functional molecular recovery profile. An exception was noted for individuals with a methamphetamine use disorder, where studies generally demonstrated that neural abnormalities may worsen with abstinence, even up to a year (Burger et al., 2018; Ruan et al., 2018; Zhuang et al., 2016), raising the possibility that neurotoxic effects are not only associated with current methamphetamine use, but also with withdrawal from the drug, and/or with premorbid factors. Similar maladaptive longitudinal change was seen in chronic cigarette smokers who showed increased reactivity to smoking related cues during the course of early abstinence (Janes et al., 2009). Such an increase in (or incubation of) drug cue-reactivity during the initial phase of abstinence has been consistently seen in animal models of addiction, and is now being observed in human studies as well (Li et al., 2016). Overall, evidence suggests that recovery is not a uniform process but instead may occur along a non-linear trajectory (i.e., different phases of recovery). This non-linear trajectory mirrors findings from a mega-analysis that demonstrated the absence of substance-specific linear effects on brain volume where both the impact of the drug and GM recovery may be more complex than can be elucidated by a simple linear analysis (Mackey et al., 2019). While speculative, it is plausible that functional recovery may be dependent, to some degree, on initial structural recovery, which may explain its delayed onset. More specifically, because GM volume reductions in specific regions may be related to alteration in functional response (Fu et al., 2008; Yuan et al., 2010), improvement in brain function may be subsequent to neural repair of structural networks (Crews et al., 2005). However, it is also possible that structural imaging is simply more sensitive and hence can detect effects earlier compared to functional imaging (Johansen-Berg, 2012).
It is important to note that the dynamic brain changes observed within the first few days following cessation may be associated, in part, with withdrawal and compensatory actions in response to drug removal [e.g. (Wang, X. et al., 2012)]. Thus, any damage associated with excessive drug use needs to be teased apart from that associated with the sub-acute, residual, effects of the drug (Fernandez-Serrano et al., 2011). It has been suggested that a minimum of two weeks of abstinence must be maintained to make this distinction (Schulte et al., 2014), which indeed has been undertaken by all but three studies reviewed here [(Kuhn et al., 2014; van Eijk et al., 2013; Wang et al., 2016)]. Considering the other end of the time spectrum, only one study (Pfefferbaum et al., 2014) assessed an abstinence period greater than 13 months, prohibiting the examination of the long-term trajectory of recovery, which clearly awaits future studies. Studies examining relationships between protracted abstinence length and neural outcomes suggest that recovery is ongoing and not the result of a single neural process (He et al., 2018; O’Neill et al., 2001). Future studies employing multiple assessments at different time-points and combining different imaging modalities over extended abstinence periods (>13-months), are warranted to accurately capture the precise recovery time-course associated with protracted abstinence in individuals with SUD.
A goal of this body of neuroimaging research is to leverage these data to improve prognostic outcomes in individual with SUDs, thus linking neural recovery to clinical improvements is fundamental. Findings from the reviewed studies demonstrate that structural (Durazzo et al., 2006; Gazdzinski et al., 2008; Hoefer et al., 2014; Mon et al., 2013; Parvaz et al., 2016a) and functional (DeVito et al., 2012; Forster et al., 2016) including neurochemical (Bartsch et al., 2007; Durazzo et al., 2006) improvements correlate with enhanced cognitive performance, indicating putative neurobiological substrates for cognitive recovery following abstinence in individuals with SUD. While not assessed in every study or across every substance, these findings are encouraging given that cognitive dysfunction is a vulnerability factor for relapse (Stevens et al., 2014) and better cognitive function is associated with achieving more favorable treatment outcomes in recovering individuals (Sofuoglu et al., 2013). Further, while not assessed in any of the reviewed studies, predictors of relapse beyond cognition, such as craving, have been associated with changes in GM volume (Makris et al., 2004). Thus, the full breadth of improved clinical outcomes and their relation to neural regeneration over abstinence remains to be discerned. Lastly, demonstrating that better brain health is achievable with abstinence may serve as a powerful motivational tool to encourage cessation and inspire treatment engagement among individuals with SUD and treatment providers.
The prevalence of cigarette smoking among individuals with SUD is high (John et al., 2003; Weinberger et al., 2018; Weinberger and Sofuoglu, 2009; Zale et al., 2015) and evidence suggests that cigarette smoking negatively affects neural recovery. Compared to non-smokers, heavy cigarette smokers have lower GM volume (Brody et al., 2004; Gallinat et al., 2006), lower global cerebral blood flow (Domino et al., 2004), and altered neurochemistry (Ashok et al., 2019; Moffett et al., 2007). Functional MRI studies suggest that nicotine may modulate task-induced BOLD responses and have performance enhancing properties (Hahn et al., 2009). Collectively, this evidence underscores the importance to control for the confounding effects of cigarette smoking on results. While some of the studies in individuals with AUD prospectively parsed smokers from non-smokers to determine if recovery differed as a function of cigarette smoking [e.g., (Durazzo et al., 2006; Durazzo et al., 2015; Gazdzinski et al., 2008; Mon et al., 2009)], this was not done for any other SUD. Some studies did, however, include baseline cigarette smoking as a covariate in longitudinal analyses when differences between the control and SUD group emerged (Mon et al., 2013). Given that it is common for individuals in treatment for SUD to also quit smoking cigarettes (Orleans and Hutchinson, 1993), future studies may consider modeling the trajectory of cigarette consumption over SUD abstinence to appropriately disassociate the effects of the primary substance from cigarette use on the changes in neural outcomes.
Several limitations of this review are worth noting. First, this review included studies that employed behavioral and/or other interventions [e.g., cognitive behavioral therapy (Balodis et al., 2016; DeVito et al., 2012) and traditional Chinese medicine (Xu et al., 2015)] to facilitate abstinence. These treatments may have induced positive neural or neurochemical changes over abstinence (DeVito et al., 2017; Seminowicz et al., 2013). Disassociating the intervention effects from those related directly to abstinence is yet to be achieved. Second, a caveat of this research is that treatment-seekers in general, or those who successfully achieve abstinence, might possess less neurobiological vulnerability and be more apt to neural recovery compared to those who are unable to quit or maintain abstinence (e.g., non-treatment-seekers and/or relapsers) (Martinez et al., 2011; Wang, G.J. et al., 2012). Third, methodologies varied across studies (e.g., sample characteristics, abstinence length (see Figure 3 for the distribution of inter-scan interval in structural, functional, and neurochemical studies), treatment administered, imaging techniques, specific ROIs examined, statistical thresholds) making direct between-study comparisons difficult. In addition, while within-study paradigms offer benefits over cross-sectional designs, these studies must still ensure that adequate control groups are enrolled and assessed at equivalent time-points to the SUD group and the influence of potential confounders are controlled for in the analyses (e.g., age, comorbid substance use, baseline SUD severity). Fourth, our inferences are based on a limited number of studies, and most studies had limited sample sizes, primarily due to high attrition rates. High attrition rate has been a major limitation in conducting longitudinal studies in human drug addiction, partially because of the highly mobile, unstable, and transient lifestyles of many study participants (BootsMiller et al., 1998). Lastly, there is a predominance of studies examining recovery in treatment-seeking individuals with AUD, while recovery in other SUD (e.g., opiates and cannabis) has been generally overlooked, representing a critical gap in this area of research as seen in Figure 2. Nevertheless, the literature is growing, and we anticipate that future studies, with larger sample sizes, and longer follow-up periods will help clarify these issues.
Figure 3: Distribution of inter-scan interval in structural, functional, and neurochemical studies.

The violin plot shows the distribution of the interval between scans (in days) grouped by structural, functional and neurochemical studies. Each violin plot shows the distribution of the duration of abstinence in substance using samples for each drug represented by the black dots, as well as the interquartile range (IQR) as a white box, the median as the black line within the white box and 95% confidence interval as a thin black line.
Figure 2: Summary of the number of longitudinal comparisons (for participants with Substance Use Disorder) in the collected studies for each imaging modality and substance.
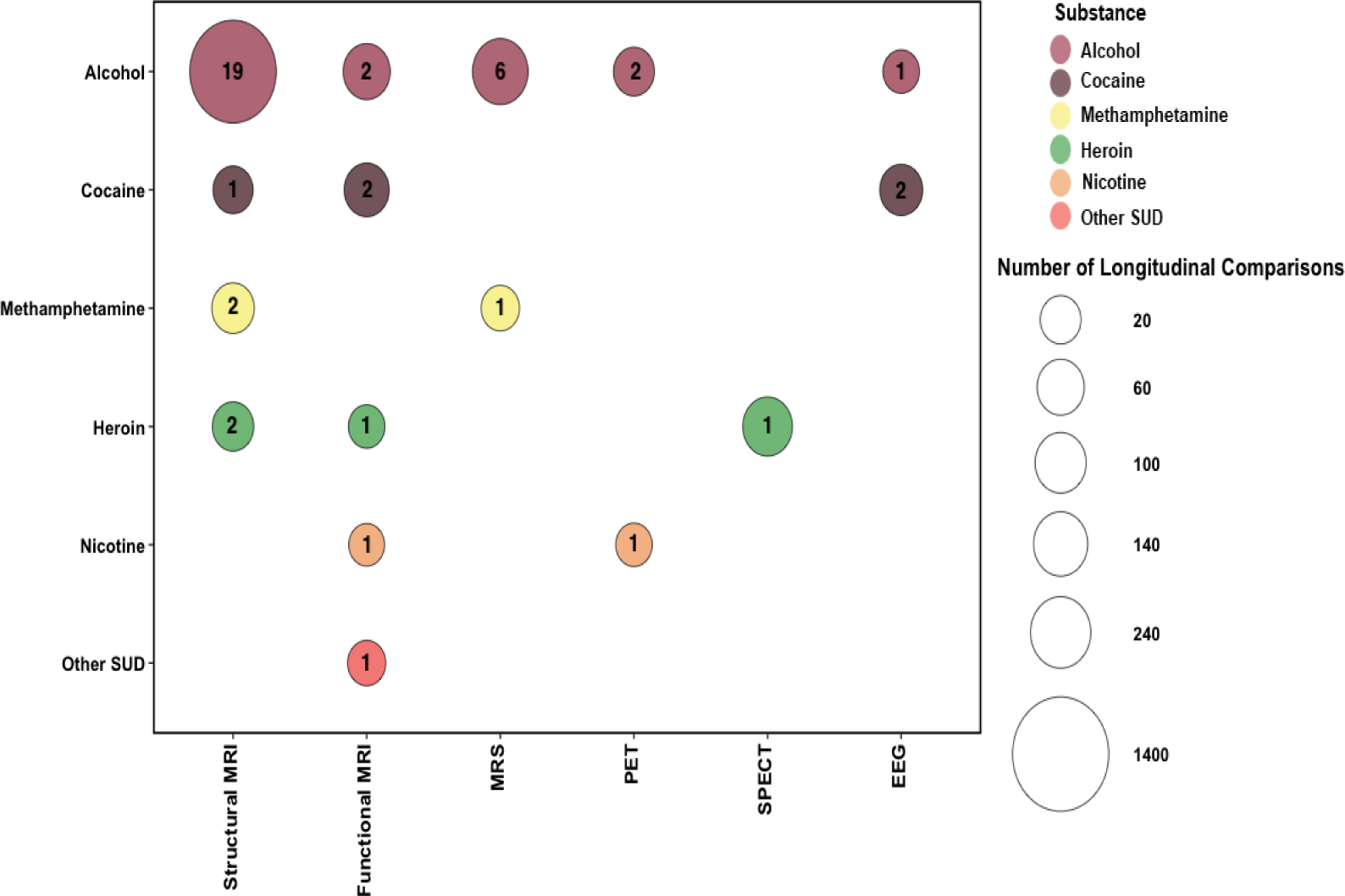
The bubble plot summarizes the number of substance using participants in the collected studies for the individual imaging modalities (structural MRI, functional MRI, MRS, PET, SPECT, and EEG) and is grouped by the substance (alcohol, cocaine, methamphetamine, heroin, nicotine, and other SUD). Each bubble represents the intersection between the imaging modality and the substance, the bubble size corresponds with the number of longitudinal comparisons, and the number of collected studies is represented by the number within each bubble.
The use of imaging techniques in addiction research has increased substantially in the last decade and many of these studies have been instrumental in providing evidence that structural and functional including neurochemical deficits may recover with even short periods of abstinence. Beyond providing hope for individuals with SUD and encouraging them to seek treatment, and providing evidence-based treatment, characterizing these neurobiological processes may help to identify novel biomarkers that can be targeted for SUD timely intervention. Capturing the trajectory of neural changes over abstinence (or even using a harm-reduction approach) may help establish a neuroscience-informed framework for developing pharmacological, psychotherapeutic, and/or neuromodulatory interventions that can mimic and/or enhance the brain’s ability to repair itself, restoring cognitive function, and contributing to positive long-term treatment outcomes in individuals with SUD.
Highlights.
Structural recovery occurred predominantly in frontal cortical regions, the insula, hippocampus, and cerebellum.
In addition to prefrontal cortical regions, functional recovery was also observed in subcortical structures (midbrain, striatum, thalamus).
A question to explore is whether specific brain regions may be more amenable to recovery, particularly the frontal cortex, while other regions may be more impervious to change.
Characterizing these neurobiological processes may help to identify novel biomarkers that can be targeted for timely intervention for substance use disorders.
Results provide hope for treatment-seeking individuals with substance use disorders and encourage them to seek treatment.
Role of funding sources
This work was supported by grants from the National Institute on Drug Abuse (K01DA043615 to MAP; T32007135 and Fonds de la Recherche en Santé du Québec and the Canada First Research Excellence Fund awarded to McGill University for the Healthy Brains for Healthy Lives to RAR; T32MH087004 that supports FA; and R01DA041528, R01AT010627, R01DA048301, and R01DA047851 to RZG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of interest
Authors do not have any conflict of interest with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander WH, Brown JW, 2010. Computational models of performance monitoring and cognitive control. Top Cogn Sci 2(4), 658–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW, 2014. A general role for medial prefrontal cortex in event prediction. Front Comput Neurosci 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I, 2012. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res 36(11), 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper KR, Prichep LS, Kowalik S, Rosenthal MS, John ER, 1998. Persistent QEEG abnormality in crack cocaine users at 6 months of drug abstinence. Neuropsychopharmacology 19(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2000. Voxel-based morphometry--the methods. Neuroimage 11(6 Pt 1), 805–821. [DOI] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Howes OD, 2019. Tobacco smoking and dopaminergic function in humans: a meta-analysis of molecular imaging studies. Psychopharmacology (Berl) 236(4), 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Koopmann A, Bumb JM, Vollstadt-Klein S, Reinhard I, Rietschel M, Witt SH, Wiedemann K, Kiefer F, 2020. Leptin predicts cortical and subcortical gray matter volume recovery in alcohol dependent patients: A longitudinal structural magnetic resonance imaging study. Horm Behav 124, 104749. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, Potenza MN, 2016. Neurofunctional Reward Processing Changes in Cocaine Dependence During Recovery. Neuropsychopharmacology 41(8), 2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M, 2007. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130(Pt 1), 36–47. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H, 2011. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69(7), 708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BootsMiller BJ, Ribisl KM, Mowbray CT, Davidson WS, Walton MA, Herman SE, 1998. Methods of ensuring high follow-up rates: lessons from a longitudinal study of dual diagnosed participants. Subst Use Misuse 33(13), 2665–2685. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D, 1993. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15(3–5), 289–298. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED, 2004. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55(1), 77–84. [DOI] [PubMed] [Google Scholar]
- Burger A, Brooks SJ, Stein DJ, Howells FM, 2018. The impact of acute and short-term methamphetamine abstinence on brain metabolites: A proton magnetic resonance spectroscopy chemical shift imaging study. Drug Alcohol Depend 185, 226–237. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ, 2007. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34(3), 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K, 2014. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J Neurosci 34(8), 2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Leurquin-Sterk G, Crunelle CL, de Laat B, Bormans G, Peuskens H, Van Laere K, 2020. Recovery of Decreased Metabotropic Glutamate Receptor 5 Availability in Abstinent Alcohol-Dependent Patients. J Nucl Med 61(2), 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N, 2007. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102 Suppl 1, 16–32. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL, 2007. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32(2), 429–438. [DOI] [PubMed] [Google Scholar]
- Charlet K, Rosenthal A, Lohoff FW, Heinz A, Beck A, 2018. Imaging resilience and recovery in alcohol dependence. Addiction 113(10), 1933–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Bath K, McEwen B, Hempstead B, Lee F, 2008. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp 289, 180–188; discussion 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, 2005. The K-complex: a 7-decade history. Sleep 28(2), 255–273. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Padilla M, Baker FC, 2009. The impact of alcoholism on sleep evoked Delta frequency responses. Biol Psychiatry 66(2), 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Padilla ML, Baker FC, 2012. Partial recovery of alcohol dependence-related deficits in sleep evoked potentials following 12 months of abstinence. Front Neurol 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK, 2009. beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 66(6), 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, Zou J, Sullivan EV, 2005. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res 29(8), 1504–1513. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K, 2011. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res 35(9), 1678–1685. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Dong G, Kober H, Xu J, Carroll KM, Potenza MN, 2017. Functional neural changes following behavioral therapies and disulfiram for cocaine dependence. Psychol Addict Behav 31(5), 534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN, 2012. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend 122(3), 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK, 2004. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry 28(2), 319–327. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Mon A, Meyerhoff DJ, 2010. Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol 44(3), 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, Meyerhoff DJ, 2006. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res 30(3), 539–551. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, 2020. Changes of frontal cortical subregion volumes in alcohol dependent individuals during early abstinence: associations with treatment outcome. Brain Imaging Behav 14(5), 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ, 2017. Regional brain volume changes in alcohol-dependent individuals during early abstinence: associations with relapse following treatment. Addict Biol 22(5), 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Yeh PH, Meyerhoff DJ, 2015. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict Biol 20(5), 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, Wichert S, Rabinstein J, Frischknecht U, Mann K, Vollstadt-Klein S, 2013. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res 37(10), 1643–1649. [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K, 2005. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry 58(12), 974–980. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, Bullmore ET, 2013. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol 23(4), 615–624. [DOI] [PubMed] [Google Scholar]
- Fernandez T, Harmony T, Rodriguez M, Bernal J, Silva J, Reyes A, Marosi E, 1995. EEG activation patterns during the performance of tasks involving different components of mental calculation. Electroencephalogr Clin Neurophysiol 94(3), 175–182. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A, 2011. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev 35(3), 377–406. [DOI] [PubMed] [Google Scholar]
- Forster SE, Finn PR, Brown JW, 2016. A preliminary study of longitudinal neuroadaptation associated with recovery from addiction. Drug Alcohol Depend 168, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N, Lotze M, 2014. Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology 39(11), 2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, Ming F, Yang Z, 2008. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett 438(3), 322–326. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M, 2006. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci 24(6), 1744–1750. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ, 2005. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend 78(3), 263–273. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ, 2010. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain 133(Pt 4), 1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ, 2008. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res 162(2), 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes DM, LaPlaca MC, Cargill RS, 2nd, 2003. Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol 184(1), 420–427. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159(10), 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12(11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA, 2009. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cereb Cortex 19(9), 1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Boehm N, Grucker D, Ghandour MS, 2006. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 83(3), 392–402. [DOI] [PubMed] [Google Scholar]
- He Q, Huang X, Turel O, Schulte M, Huang D, Thames A, Bechara A, Hser YI, 2018. Presumed structural and functional neural recovery after long-term abstinence from cocaine in male military veterans. Prog Neuropsychopharmacol Biol Psychiatry 84(Pt A), 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefer ME, Pennington DL, Durazzo TC, Mon A, Abe C, Truran D, Hutchison KE, Meyerhoff DJ, 2014. Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol 48(7), 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Frederick B, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ, 2009. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol 17(6), 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, 2012. The future of functionally-related structural change assessment. Neuroimage 62(2), 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U, 2003. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol 38(6), 606–612. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R, 2013. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73, 239–254. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D, 2000. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Charlet K, Schubert F, Kiefer F, Zimmermann P, Heinz A, Gallinat J, 2014. Plasticity of hippocampal subfield volume cornu ammonis 2+3 over the course of withdrawal in patients with alcohol dependence. JAMA Psychiatry 71(7), 806–811. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, 2010. Structural plasticity and hippocampal function. Annu Rev Psychol 61, 111–140, C111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, Bao YP, Shi J, Sun HQ, Lu L, 2015. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol 20(3), 513–522. [DOI] [PubMed] [Google Scholar]
- Li X, Venniro M, Shaham Y, 2016. Translational Research on Incubation of Cocaine Craving. JAMA Psychiatry 73(11), 1115–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF, 2010. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci 1187, 148–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Liao HJ, Merugumala SK, Prabhu SP, Meehan WP 3rd, Ross BD, 2012. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav 6(2), 208–223. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W, 2004. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61(1), 73–84. [DOI] [PubMed] [Google Scholar]
- Lu B, Chow A, 1999. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res 58(1), 76–87. [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kuhn S, Machielse MW, Sescousse G, 2017. Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 74(4), 387–398. [DOI] [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, Brooks S, Caparelli E, Chye YY, Cousijn J, Dagher A, Desrivieres S, Feldstein-Ewing S, Foxe JJ, Goldstein RZ, Goudriaan AE, Heitzeg MM, Hester R, Hutchison K, Korucuoglu O, Li CR, London E, Lorenzetti V, Luijten M, Martin-Santos R, May A, Momenan R, Morales A, Paulus MP, Pearlson G, Rousseau ME, Salmeron BJ, Schluter R, Schmaal L, Schumann G, Sjoerds Z, Stein DJ, Stein EA, Sinha R, Solowij N, Tapert S, Uhlmann A, Veltman D, van Holst R, Whittle S, Wright MJ, Yucel M, Zhang S, Yurgelun-Todd D, Hibar DP, Jahanshad N, Evans A, Thompson PM, Glahn DC, Conrod P, Garavan H, Group EAW, 2019. Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. Am J Psychiatry 176(2), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS Jr., Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC, 2004. Decreased absolute amygdala volume in cocaine addicts. Neuron 44(4), 729–740. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E, 2011. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168(6), 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Paulus MP, 2018. Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Prog Neuropsychopharmacol Biol Psychiatry 80(Pt B), 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Woicik PA, Maloney T, Alia-Klein N, Honorio J, Telang F, Wang GJ, Wang R, Sinha R, Carise D, Astone-Twerell J, Bolger J, Volkow ND, Goldstein RZ, 2012. Enhanced midbrain response at 6-month follow-up in cocaine addiction, association with reduced drug-related choice. Addict Biol 17(6), 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM, 2007. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81(2), 89–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, Meyerhoff DJ, 2013. Brain-derived neurotrophic factor genotype is associated with brain gray and white matter tissue volumes recovery in abstinent alcohol-dependent individuals. Genes Brain Behav 12(1), 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ, 2009. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res 33(8), 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ, 2012. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend 125(1–2), 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Stamatakis EA, Fernandez-Serrano MJ, Gomez-Rio M, Rodriguez-Fernandez A, Perez-Garcia M, Verdejo-Garcia A, 2012. Neural correlates of hot and cold executive functions in polysubstance addiction: association between neuropsychological performance and resting brain metabolism as measured by positron emission tomography. Psychiatry Res 203(2–3), 214–221. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A, 2001. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36(5), 357–368. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Lee SK, Melhem ER, 2007. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 245(2), 367–384. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ, 2001. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res 25(11), 1673–1682. [PubMed] [Google Scholar]
- Orleans CT, Hutchinson D, 1993. Tailoring nicotine addiction treatments for chemical dependency patients. J Subst Abuse Treat 10(2), 197–208. [DOI] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR, 2002. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res 26(9), 1368–1380. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, Goldstein RZ, 2016a. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ, 2016b. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry 73(11), 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Malaker P, Sinha R, Alia-Klein N, Goldstein RZ, 2017. Abstinence reverses EEG-indexed attention bias between drug-related and pleasant stimuli in cocaine-addicted individuals. J Psychiatry Neurosci 42(2), 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, Zahr NM, Sullivan EV, 2014. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry 1(3), 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO, 1995. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 19(5), 1177–1191. [DOI] [PubMed] [Google Scholar]
- Poe GR, Walsh CM, Bjorness TE, 2010. Cognitive neuroscience of sleep. Prog Brain Res 185, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackayova V, Cudalbu C, Pouwels PJW, Braissant O, 2017. Creatine in the central nervous system: From magnetic resonance spectroscopy to creatine deficiencies. Anal Biochem 529, 144–157. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Prinz S, Winz O, Henkel K, Dietrich CA, Schmaljohann J, Mohammadkhani Shali S, Schabram I, Stoppe C, Cumming P, Hilgers RD, Kumakura Y, Coburn M, Mottaghy FM, Grunder G, Vernaleken I, 2016. Effects of Smoking Cessation on Presynaptic Dopamine Function of Addicted Male Smokers. Biol Psychiatry 80(3), 198–206. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R, 2011. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry 168(2), 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X, Zhong N, Yang Z, Fan X, Zhuang W, Du J, Jiang H, Zhao M, 2018. Gray matter volume showed dynamic alterations in methamphetamine users at 6 and 12months abstinence: A longitudinal voxel-based morphometry study. Prog Neuropsychopharmacol Biol Psychiatry 81, 350–355. [DOI] [PubMed] [Google Scholar]
- Schulte MH, Cousijn J, den Uyl TE, Goudriaan AE, van den Brink W, Veltman DJ, Schilt T, Wiers RW, 2014. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev 34(7), 531–550. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR, 2013. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 14(12), 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N, 1994. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res 18(1), 172–176. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM, 2013. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Verdejo-Garcia A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W, 2014. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat 47(1), 58–72. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, 2005. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 180(4), 583–594. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2006. Sleep function and synaptic homeostasis. Sleep Med Rev 10(1), 49–62. [DOI] [PubMed] [Google Scholar]
- van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G, 2013. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res 37(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L, 2013. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PloS one 8(7), e68791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, Shi J, 2012. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (Berl) 221(4), 701–708. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS, 2012. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17(9), 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Demirakca T, van Eijk J, Frischknecht U, Ruf M, Ucar S, Hermann D, Mann K, Kiefer F, Ende G, 2016. Longitudinal Mapping of Gyral and Sulcal Patterns of Cortical Thickness and Brain Volume Regain during Early Alcohol Abstinence. Eur Addict Res 22(2), 80–89. [DOI] [PubMed] [Google Scholar]
- Wang X, Li B, Zhou X, Liao Y, Tang J, Liu T, Hu D, Hao W, 2012. Changes in brain gray matter in abstinent heroin addicts. Drug Alcohol Depend 126(3), 304–308. [DOI] [PubMed] [Google Scholar]
- Wang X, Yu R, Zhou X, Liao Y, Tang J, Liu T, Shan B, Hao W, 2013. Reversible brain white matter microstructure changes in heroin addicts: a longitudinal study. Addict Biol 18(4), 727–728. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou X, Liao Y, Tang J, Liu T, Hao W, 2011. Brain function of heroin addicts after withdrawal. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36(8), 733–738. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Gbedemah M, Wall MM, Hasin DS, Zvolensky MJ, Goodwin RD, 2018. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002–14: emerging disparities in vulnerable populations. Addiction 113(4), 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M, 2009. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse 35(1), 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, 2009. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J Neurosci 29(31), 9661–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu Y, Li Y, Deng Y, Huang Y, Yuan J, Lv R, Wang Y, Zhang G, Guo Z, Han M, Liu X, Fu D, 2015. Longitudinal changes of dopamine transporters in heroin users during abstinence. Psychopharmacology (Berl) 232(18), 3391–3401. [DOI] [PubMed] [Google Scholar]
- Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, von Deneen KM, Liu Y, Gold MS, Tian J, 2010. Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neurosci Lett 482(2), 101–105. [DOI] [PubMed] [Google Scholar]
- Zale EL, Dorfman ML, Hooten WM, Warner DO, Zvolensky MJ, Ditre JW, 2015. Tobacco Smoking, Nicotine Dependence, and Patterns of Prescription Opioid Misuse: Results From a Nationally Representative Sample. Nicotine Tob Res 17(9), 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang W, Tang Y, Zhong N, Jiang H, Du J, Wang J, Zhao M, 2016. Persistent Microstructural Deficits of Internal Capsule in One-Year Abstinent Male Methamphetamine Users: a Longitudinal Diffusion Tensor Imaging Study. J Neuroimmune Pharmacol 11(3), 523–530. [DOI] [PubMed] [Google Scholar]
- Zou X, Durazzo TC, Meyerhoff DJ, 2018. Regional Brain Volume Changes in Alcohol-Dependent Individuals During Short-Term and Long-Term Abstinence. Alcohol Clin Exp Res 42(6), 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]


