Abstract
Due to the complex pathophysiological mechanism, spinal cord injury (SCI) has become one of the most intractable central nervous system (CNS) diseases to therapy. Stem cell transplantation, mesenchymal stem cells (MSCs) particularly, appeals to more and more attention along with the encouraging therapeutic results for the functional regeneration of SCI. However, traditional cell transplantation strategies have some limitations, including the unsatisfying survival rate of MSCs and their random diffusion from the injection site to ambient tissues. The application of biomaterials in tissue engineering provides a new horizon. Biomaterials can not only confine MSCs in the injured lesions with higher cell viability, but also promote their therapeutic efficacy. This review summarizes the strategies and advantages of biomaterials reinforced MSCs transplantation to treat SCI in recent years, which are clarified in the light of various therapeutic effects in pathophysiological aspects of SCI.
Keywords: MSCs transplantation, Spinal cord injury, Biomaterials, Functional regeneration
1. Introduction
Spinal cord injury (SCI) is a devasting central nervous system (CNS) disease accompanying motor and sensory dysfunction, which severely hinders the patients’ quality of life. Falls, traffic accidents, violence, and X-sports are primary reasons resulting in traumatic SCI [1]. Pharmacological and surgical intervention are both adopted for the SCI treatment in clinic. Pharmacological therapy represented by methylprednisolone (MP) administration can scavenge free radicals and reduce inflammation response. Nevertheless, it faces some controversies about complicated side effects and unconvincing neurological recovery [2]. Besides, the surgery is suggested to implement when canal integrity is seriously damaged. The removal of cracked bone, disk, and ligament fragments can decompress the spinal cord and limit secondary injury, which is helpful for the improvement of motor function and nerve recovery. However, the therapeutic outcomes of current pharmacological intervention or surgical intervention still cannot meet the requirement of nerve regeneration and functional recovery. Scientists believe that intricate pathophysiological mechanisms, such as cells loss and death, the lack of neurotrophins or neuroprotective/neuroregenerative factors, axonal breakage, demyelination, glial scar (axon regeneration inhibitory structure) formation, inflammation, and other inhibitory factors, are underlying reasons impeding the recovery of neural reconnection after spinal cord injury [3,4]. Consequently, therapeutic strategies targeting to single pathophysiological mechanism is likely to fail to cure the multifactorial disease.
During the past decades, stem cell transplantation has been proposed as a potential strategy for SCI treatment and has extensively attracted the attention of researchers [5,6]. Mesenchymal stem cells (MSCs) [7,8], neural stem and progenitor cells (NSPCs) [9], [10], [11], and embryonic stem cells (ESCs) [12,13] are typical types of stem cells being investigated for SCI treatment. Among them, MSCs own distinct therapeutic mechanisms from other stem cells. Apart from the capability of multipotential differentiation [14,15], paracrine effects [16], [17], [18] and the mitochondrial transfer [19] are typically emphasized mechanisms for the comprehensive therapeutic effects of MSCs in the SCI treatment. However, the failure of MSCs transplantation in the clinical trial implies that the inherent therapeutic characteristics of MSCs administered to humans are not as vigorous as demonstrated in preclinical studies [20]. Variable biodistribution rather than retention in the injured site after administration might be one of reasons. In addition, when exposed to the nasty microenvironment upon SCI without nutrient extracellular matrix (ECM), MSCs are not able to survive for an extended period or retain their physiological functions either.
With the development of regenerative medicine and the application of biomaterials in tissue engineering, MSCs transplantation combined with biomaterials attracts amounts of attention and brings unexplored insight for remedy after severe injury of the spinal cord [21,22]. Some biomaterials can be applied as scaffolds to support the growth of MSCs and confine their distribution [23,24] while a few biomaterials are capable of promoting their therapeutic efficacy via inducing differentiation [25], [26], [27] or provoking functional secretion [28], [29], [30]. Especially, biomaterials-based scaffolds can serve as delivery platforms for MSCs, the varied factors derived from MSCs, and even the anti-inflammation chemical drugs. Due to the flexibility in the modification of biomaterials, controlled release of the harbored therapeutic components can be achieved as well. Benefiting from the synergistic functions, biomaterials reinforced MSCs transplantation has come to the front stage in therapy for SCI.
Herein, this review attempts to provide a comprehensive overview of the recent development of biomaterials reinforced MSCs transplantation regarding the various therapeutic effects in pathophysiological aspects of SCI. Moreover, some existing limitations and future perspectives of this field were also discussed.
2. Pathophysiology
Over the past decades, the pathophysiological researches of SCI have been developed rapidly following with novel proposal of management principles and intervention strategies. Herein, we will primarily discuss the pathophysiological process of traumatic injury in the spinal cord, which can be briefly divided into primary and secondary injury. Traumatic events, such as vehicle accident, fall and sports, produce immediate mechanical damage and dislocation of the vertebral column. The fractured and displaced bone fragments, disk material as well as ligaments result in the compression or transection of the spinal cord. The subsequent events, including blood vessel damage and blood-spinal cord barrier (BSCB) disruption, evoke severe hemorrhage, exposing the spinal cord to an influx of inflammatory cells, cytokines, and vasoactive peptides [4]. Within a few minutes, the spread of the spinal cord swelling is accelerated and aggravates the compression of the spinal cord. Furthermore, the swelling exacerbates secondary ischemia by autoregulation of blood flow ceases [31,32].
Nevertheless, the secondary injury cascade initiated by the above series of events is more fatal and which substantially obstructs the progress in functional repairment. Increased cell permeability, pro-apoptotic signals, ischemia, and BSCB disruption further exacerbates damage to the injured spinal cord [33]. When apoptosis or necrosis happens to neurons and astrocytes, the over-released excitotoxic neurotransmitter glutamate together with ischemia microenvironment can induce the imbalance of ionic homeostasis [34,35]. It has been demonstrated that high intracellular calcium concentration activates calpains which intervene the functional regulation of mitochondria, causing both neurons and glia death [36], [37], [38]. The dead neural cells release substantive ATP, DNA, and potassium to activate microglial cells propagating inflammation response and ongoing neuron apoptosis or necrosis [39]. Besides, overexpressed glutamate causes overactivation of N-methyl-d-aspartate (NMDA) [40], α-amino-3‑hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) [41], and kainate receptor, inducing oligodendrocyte death and axonal injury. As a result, unscathed axons become demyelinated and thus unable to conduct impulses after SCI. In the meantime, phagocytic inflammatory cells can not only clear myelin debris, but also release cytotoxic by-products such as free radicals (e.g., O2−, hydrogen peroxide [42,43], and peroxynitrite [44], [45], [46]), which can directly oxidize lipids, DNA and proteins [4,43,47,48], and further conduce neurons and astrocytes necrosis or apoptosis.
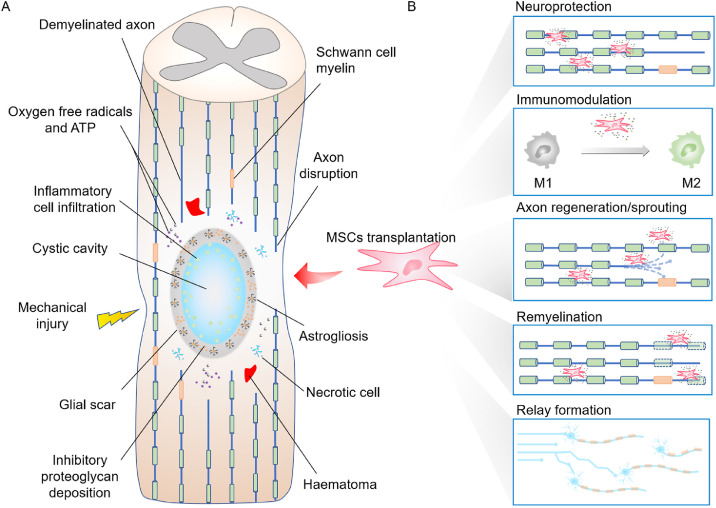
The severe traumatic primary injury rapidly induces dramatic secondary cascade injury and finally generates cystic cavities and glial scar (Fig. 1A). A wide range of cells and cytokines are involved in the pathological process of SCI and forms an inconducive microenvironment to nerve regeneration in the end. Therefore, the combination of multiple strategies is more facile to implement meaningful neural circuit connectivity than a single strategy.
Fig. 1.
Illustration of the therapeutic effects of MSCs transplantation for the treatment of SCI. (A) The pathological mechanisms of SCI. (B) The five commonly recognized mechanisms of MSCs transplantation.
3. MSCs and repair of SCI
Stem cell transplantation has been broadly researched for the treatment of diverse diseases, and some proposed strategies have successfully entered the clinical trial stage in recent years [5,6,49]. Apart from the potential of differentiation, different stem cells have their own unique merits. Among them, MSCs have attracted amounts of attention due to their ample sources (e.g., bone marrow, umbilical cord, adipose tissue, and amniotic fetus), easy isolation/expansion, convenient preservation, less ethical concerns, low immunogenicity as well as inflammation tropism [7,14,[50], [51], [52], [53]. For SCI treatment, MSCs exhibit specific superiority (Fig. 1B), and amounts of preclinical researches were conducted on animal models using rats, dogs or macaco rhesus. MSCs could exert positive influences on nerve regeneration and functional recovery through its intrinsic functions or by loading different therapeutic agents including various neuroprotective/neuroregenerative factors and drugs. There are also some projects aimed at upregulating the expression of recovery-related cytokines in MSCs via gene transfection to enhance the therapeutic effects of MSCs-based strategies. Traditionally, like other stem cells, MSCs are expected to trans-differentiate to neural lineage cells [54,55], rebuild the injured neuronal circuit [15] or form perineurium-like sheath to protect host myelin and axons [56] after SCI. However, MSCs prefer to differentiate toward the mesenchymal rather than neural lineage. Additionally, it has been demonstrated that MSCs are inferior to neural stem cell regarding to the differentiation capability to neuron [57].
More and more evidences indicated that the paracrine activity instead of MSC-derived neuron-like cells maybe the primary contributor [16], [17], [18],58,59]. MSCs can secret various factors such as immunomodulatory, anti-inflammatory, neurotrophic/neuroprotective, and angiogenetic factors so that they are able to present synergistic therapeutic effect on the SCI treatment via regulating the rigorous host microenvironment. For instance, many neurotrophic factors, such as vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), glial cell derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophin 1 factor (NT-1), NT-3, and basic fibroblast growth factor (bFGF) [17,50,60], can prevent nerve degeneration and apoptosis, as well as support neurogenesis, remyelination, and axonal growth. Meanwhile, the secretion of anti-inflammatory cytokines including tumor necrosis factor (TNF) β1, interleukin (IL)-13, IL-18 binding protein (IL-18 BP), ciliary neurotrophic factor (CNTF) and IL-10, can modulate neuroinflammation [50,61,62], which are beneficial to reduce the glial scar and subsequently promote the functional recovery.
To this end, several researches applied the extracellular vesicles (EVs) which are secreted by MSCs harboring diverse active molecules for the SCI treatment [55,[63], [64], [65], [66], [67]. As an advanced cell-free therapy strategy, MSCs-derived exosomes could efficaciously mitigate the disorder of spinal cord through anti-inflammation, promotion of microglial/macrophage polarization, reduction in A1 astrocytes, and protection for BSCB [68]. Specifically, Romanelli et al. [69] administrated exosomes generated by human umbilical cord-derived MSCs (hUC-MSCs) to the rats of spinal cord trauma. Their data showed that exosomes directly interacted with activated microglia in vitro and inhibited the expression of pro-inflammatory cytokines during secondary injury. The expression of IL-1β and IL-6 was downregulated, and the expansion of glia scar was inhibited. Thus, the recovery of motor function was further confirmed through Basso, Beattie and Bresnahan (BBB) score eventually. In consideration of hypoxic microenvironment around the lesion, Liu et al. [70] explored the functional difference and imperceptible mechanisms attributed to exosomes derived from MSCs under hypoxia (HExos) and those under normoxia (Exos). They found that HExos could promote behavioral reappear by shifting microglial polarization from M1 to M2 phenotype in vivo and in vitro. A further mRNA array helped them to confirm that miR-216a-5p was potentially involved in HExos-mediated microglial polarization. The same team also demonstrated the role of MSCs-derived exosomes in suppressing the activation of A1 neurotoxic reactive astrocytes following SCI in their previous study [71]. Since the destruction of the BSCB is usually the inevitable result of SCI, Lu et al. [72] identified that MSCs derived exosome-conducted treatment could inhibit the migration of pericytes and improve the integrity of BSCB through nuclear factor κB (NF-κB) p65 signaling in pericytes. The effective recovery of motor functions induced by EVs instead of MSCs accelerates the development of cell-free stem cell therapies.
Most recently, mitochondrial transfer from MSCs to damaged cells has gradually emerged as a promising therapeutic cue and is proposed as a novel mechanism for MSCs to treat CNS diseases [73], [74], [75], [76]. Reduced oxygen delivery in the pathological environment of SCI can lead to damage to mitochondria, causing insufficient energy supply and a series of additional injury events such as oxidative stress and calcium overload [77,78]. Mitochondrial transfer from MSCs to ambient damaged cells can effectively replace dysfunctional mitochondria and replenish energy for nerve regeneration. Li et al. [74] depicted that bone marrow derived MSCs could donate the mitochondria to injured motor neurons via gap junction. Additionally, internalization of mitochondria inhibited cellular apoptosis and promoted cell survival in motor neurons post deprivation of oxygen and glucose. Specifically, mitochondria transplantation presented comparable therapeutic effect with MSCs transplantation on SCI model rats.
4. MSCs combined with biomaterials in the repair of SCI
Although there is certain evidence from various SCI animal models in which testified improvement following MSCs transplantation [7,14,54,79,80], a small part of projects can enter upon clinical trials [81], [82], [83]. The reasons for this status quo can be divided into two aspects. One is existent gap between the animal models of SCI and clinical cases. Most preclinical data are based on mild to moderately severe chest contusion models. However, thoracic contusion is rare in humans, and more than 60% of SCI occurs in the cervical spine [84]. The other is the differences in immunity. Although many studies have been conducted in immunodeficient animals, they may not be able to accurately simulate the immune situation of patients with SCI. Up to now, only one product, Stemirac, MSCs extracted from the patients’ bone marrow and multiplied in the lab, was approved by Japan government for the SCI treatment in 2018. However, the approval of Stemirac also struck fierce debate [85]. Some rigorous challenges, including the unsatisfying survival rate of transplanted MSCs as well as limited accumulation in the injured site [83,86,87], need to be overcome urgently for the clinical transformation. Moreover, the nasty microenvironment in the lesion largely hinders the therapeutic functions of MSCs. With the development of biomaterials and regenerative medicine, their applications in the remedy of the injured spinal cord have exhibited much promising potential [22,[88], [89], [90]. The biomaterials discussed here are materials that are employed for contacting and interacting with the biological components [91], playing a pivotal role in the artificial manipulation of cellular biological activities for regenerative medicine [92], [93], [94], [95].
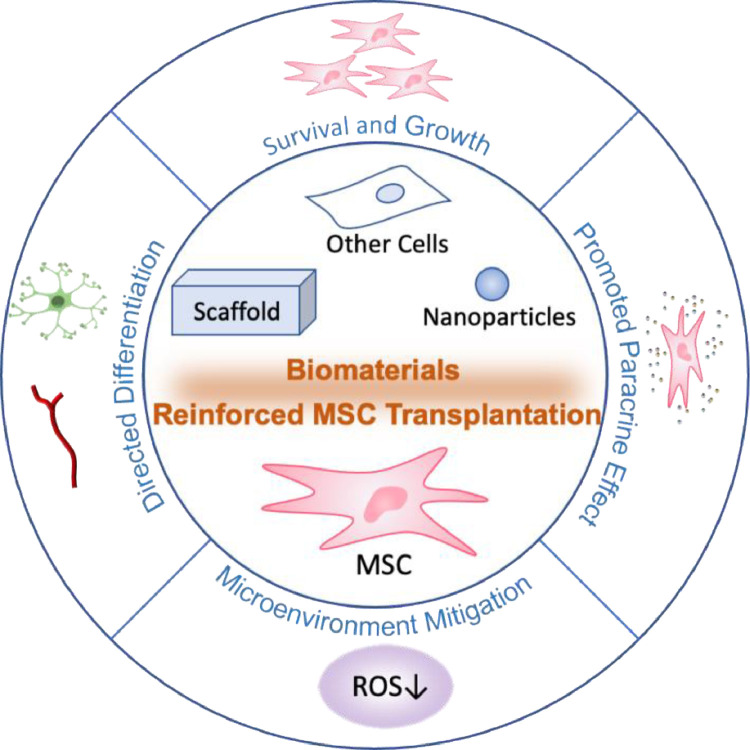
Generally, hydrogels which have similar physico chemical properties to the natural extracellular matrix such as collagen and hyaluronic acid (HA), are broadly utilized to combine with MSCs to synergistically mitigate the SCI damage. Meanwhile, appropriate adhesive molecules (e.g., laminin, fibronectin, fibrinogen, adhesive peptide) and growth factors (e.g., BDNF) are co-delivered into biomaterial scaffolds with cells to further promote the therapeutic effect of MSCs. Some synthetic materials can also be developed as functional scaffold to support MSCs. Apart from the bare scaffold, some inorganic nanoparticles combined with MSCs as well as multicellular co-transplantation system are also investigated in the preclinical studies for their therapeutic capacity in SCI model. In this section, we will discuss about the recent researches focusing on biomaterials reinforced MSCs transplantation in the repair of SCI (Fig. 2).
Fig. 2.
Biomaterials are able to promote the therapeutic effects of MSCs for the repair of SCI.
4.1. Biomaterial scaffolds
Biocompatible scaffolds constructed by diverse natural or synthetical biomaterials to support the transplanted MSCs have been extensively explored during the past decades [24,96,97]. ECM is demonstrated as a crucial element to provide structural and trophic support for cellular adhesion and survival. Moreover, ECM plays an essential part in maintaining and preserving the stemness of stem cells, which is essential for their regeneration activity. However, the ECM is absent in the injured spinal lesion, resulting in the limited survival rate of transplanted MSCs. Consequently, the biomaterial scaffolds can remedy the situation by facilitating the replenish and transports of oxygen and nutrients to sustain cell activity and avoid detachment-induced programmed cell death [98]. Natural biomaterials derived from the components of ECM, such as collagen, HA, gelatin, fibrin, fibronectin and arginine-glycine-aspartic acid (RGD) peptide, have been proposed and characterized as the scaffolds or modifiers. For instance, Oliveira et al. [99] compared and researched three natural ECM-like hydrogels, gellan gum (GG, functionalized with a fibronectin peptide), collagen, and a hydrogel rich in laminin epitopes (NVR-gel), combined with MSCs in promoting axonal regeneration. They found all the hydrogel-based systems supported MSCs survival. Synthetic polymers have unique strengths in the stability and controllability of physicochemical properties of the scaffolds, including porosity, stiffness, and degradability. Reasonably, synthetic biomaterial-constructed scaffolds represented by poly(lactic-co-glycolic acid) (PLGA) scaffolds, have become an integral element of cell scaffolds. Briefly, the biomaterial scaffolds are able to optimize the cell niche to maintain maximum cell viability and function, and thus induce efficient improvement through complicated mechanisms (Table 1).
Table 1.
Mesenchymal stem cells (MSCs) combined with biomaterial scaffolds in SCI.
| Mechanisms | Scaffolds | SCI types | Phase | Animal model | Ref. |
|---|---|---|---|---|---|
| Neuroprotection | GS | complete | acute | SD rat | [56] |
| hydroxyapatite-collagen | complete | acute | SD rat | [153] | |
| CS-HEC—Col/GP | incomplete | subacute | C57 mice | [154] | |
| Immunomodulation | collagen | incomplete | acute | SD rat | [103] |
| fibrin hydrogel | complete | acute | SD rat | [123] | |
| RGD ECM hydrogel | incomplete | acute/chronic | B6.129P-Cx3cr1tm1Litt/J mice | [124] | |
| HG RGD + ECM | incomplete | – | C57 mice | [125] | |
| Axonal sprouting/regeneration | PLGA | complete | acute | SD rat | [15] |
| collagen | complete | chronic | SD rat | [101] | |
| collagen | complete | chronic | canine | [102] | |
| Pep-HA | complete | acute | SD rat | [112] | |
| HA-PH-RGD/F | incomplete | subacute | Wistar rat | [116] | |
| fibrin matrix | incomplete | subacute | rat | [121] | |
| alginate | intermedia | intermedia | Wistar albino rat | [122] | |
| HA hydrogel | complete | acute | SD rat | [130] | |
| GS | complete | acute | SD rat | [139] | |
| chitosan | complete | acute | C57BL/6 J mice | [155] | |
| p(HEMA-AEMA) | complete | subacute | Wistar rat | [126] | |
| Neuronal relay formation | GS | complete | acute | Beagle canine | [80] |
| Myelin regeneration | PLGA | incomplete | acute | SD rat | [8] |
| GS | complete | acute | SD rat | [141] |
4.1.1. Collagen-based scaffolds
Type Ⅰ collagen attracts extensive attentions via superior advantages containing proper mechanical strength, simple fabrication process and the capacity to promote cellular adhesion. Xiao et al. [100] transplanted the collagen scaffolds named NeuroRegen scaffolds (NRS) with MSCs. NRS could promote cell retention, integration, and differentiation efficiently at the injury site and exert positive immunomodulatory and neurotrophic influences. Additionally, the functional collagen scaffolds could suppress scar formation and induce newborn neuron production. The sensory and motor functional improvement was observed while the sense function in bowel and bladder was regained as well. In another research, Wang et al. [101] applied NRS combined with MSCs to promote functional recovery after scar resection in rats with chronic SCI. After surgical resection of scar and implantation of NRS combined with MSCs, they observed neurofilament regeneration and β-tubulin-Ⅲ positive neural regeneration in the lesion site. Meanwhile, the beneficial outcome accompanied with no negative effects on locomotor function. Li et al. [102] demonstrated that MSCs seeded NRS was also beneficial to the lager animal. The grafts could minimize the area of glial scar in injured spinal cord at 1-year post-implantation in canines with chronic SCI. A mechanism study of repair capacity of collagen scaffolds delivered MSCs in SCI treatment was carried out by Peng et al. [103]. They assessed the transformation of classically activated macrophage/alternatively activated macrophage polarization in a hemisected SCI rat model. They also observed more M2-type of macrophages in the co-transplantation group compared to the control group, suggesting that the co-transplantation system could protect MSCs through conducing polarization from M1 to M2 phenotype, which was able to form an anti-inflammatory microenvironment to inhibit the formation of chronic glia scar and provide alignment guidance for nerve regeneration. Interestingly, the implantation of collagen scaffolds or MSCs alone was failed to repair the nerve function [104]. As promising carriers to deliver MSCs, the inner structure of collagen scaffolds attributed to different crosslinking and fabrication process is of significance to nerve regeneration. Porous collagen-based scaffolds can promote neuron-like differentiation and reduce astrogliosis [105]. Declared by Zou et al. [106], aligned collagen sponge scaffold performed better capabilities to encourage cell migration and guide axonal elongation compared to random collagen scaffolds.
Attempts to modify and optimize the collagen scaffold are also underway. Cholas et al. [107] studied dehydrothermally (DHT) or 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) mediated crosslinked collagen scaffolds incorporating different therapeutic agents including MSCs to boost reparative response in the rat spinal cord. They found that physical crosslinking (mediated by DHT) and chemical crosslinking (mediated by EDAC) exhibited different effects on the collagen scaffolds in diverse aspects, including return of bladder function, hindlimb function, axon regeneration, angiogenesis, inflammatory response, and astrocyte response. Donor MSC-derived bioactive molecules might promote nerve preservation and regeneration [103]. Taking the high affinity between fibronectin (FN) and collagen, they modified the hydroxyapatite-collagen (HC) scaffold with FN. More deposition of FN on the HC scaffolds could ameliorate the adhesion and proliferation of MSCs.
Apart from the cell proliferation, cell communications or interactions are essential for the secretion of paracrine factors. Nevertheless, cell encapsulation in 3D scaffolds may prevent cells establishing intercellular contacts. Spheroid cell culture is a promising technique improving cell survival and function by preserving cell-to-cell interactions [108]. He et al. [109] found that MSCs spheroids-laden collagen hydrogels could promote neuronal differentiation and inhibit inflammatory response attributed to the activation of PI3K-Akt signaling pathway caused by elevated intrinsic cell-cell communication and cell-extracellular matrix interactions.
4.1.2. HA-based scaffolds
HA is another important component of the ECM [110] and has also been investigated as the biomaterial scaffold to support transplanted MSCs for SCI treatment. Especially, CD44, a cell-surface receptor which also exists on the MSC surface, can specifically interact with HA [111]. Nevertheless, functional modification is usually conducted to further promote the function of HA scaffolds. Li et al. [112] tethered HA hydrogel scaffold with an adhesive peptide PPFLMLLKGSTR, and synergized with MSCs (Pep-Sca-MSCs) to promote recovery from spinal cord transection. The applied peptide is a motif sequence obtained from laminin-5 α3 and is verified as the major binding site for α3β1 integrin. Thus, the MSCs could adhere on the scaffold tightly with promoted survival. Observation results of neurofilaments (NF) and Luxol fast blue staining suggested that Pep-Sca-MSC-treated group showed a greater number of NF-200 positive neural fibers, indicating the effective axonal regeneration. Furthermore, combining the results of locomotor function recovery, the cooperation of MSCs and the scaffold rather than single implantation of either MSCs or the scaffold was necessary for effective functional regeneration of neural tissue. Despite of more and more exosome-mediated therapeutic outcome in SCI repair, this strategy has the same problems of easy loss from injured spinal cord as MSCs. Therefore, biomaterials scaffold with adhesivity can be applied to encapsulate exosomes for tissue regeneration [113], [114], [115]. The adhesive HA scaffold developed by Li et al. could also assist the transplantation of the human MSCs derived exosomes to the injured lesion for the treatment of SCI. The results in vivo indicated that the transplanted exosome could induce comprehensive mitigation of the microenvironment after SCI [65].
In another example, Zaviskova et al. [116] developed an injectable scaffold based on hydroxyphenyl derivative of HA hydrogel with modification of RGD peptide. RGD was applied for the cellular adhesion in the scaffold. Additionally, fibrinogen was also utilized to enhance the proliferation of MSCs. Bare hydrogel (HA-PH-RGD), fibrinogen tethered hydrogel (HA-PH-RGD/F), or HA-PH-RGD/F combined with MSCs was injected into the lesion. All of them could promote axonal growth toward the lesion site, but HA-PH-RGD/F integrated with MSCs exhibited synergistically enhanced recovery of SCI.
4.1.3. Gelatin sponge scaffolds
Gelatin is another common component of ECM which is designed as scaffolds loading MSCs. The certain functions of three-dimensional gelatin sponge (3D GS) scaffolds, decreasing the glia scar, cystic cavity and inflammation around the lesion, have been demonstrated [117], implying the possible cue for the improvement of neurons survival and nerve regeneration. The attractive performance of GS scaffolds in modulating deleterious microenvironment is helpful to maintain the viability of MSCs. Ma et al. [56] delivered MSCs with 3D GS scaffolds to the injured spinal cord and MSCs could survive up to 8 weeks with the scaffold supporting. Moreover, 3D GS could direct the formation of MSC-derived perineurium-like sheath, serving as a physical and chemical barrier for the enwrapped host myelin and axons. Li et al. [118] developed a NT-3/fibrin coated GS scaffold (NF-GS) for SCI repair. MSCs integrated to the NF-GS possessed high cellular viability with excellent cell distribution and phenotype in vitro. Release profile indicated that NF-GS was able to sustainably release NT-3 up to 28 day Ameliorative paralysis hindlimb locomotion with increased amplitude and shortened latency of cortical motor evoked potentials in the hemisected spinal cord of canine were observed in later research [119].
Proper physical electrical stimulation promoting neuron-like cell differentiation of MSCs is demonstrated by Zhang's group [120]. NT-3 and retinoic acid stimulated MSCs were loaded into 3D GS scaffold, and then the system was grafted into the transected rat spinal cord. Governor Vessel electro-acupuncture (GV-EA) treatment was applied after 24 h. Higher survival rate and more neural differentiation were found at 30 days after GV-EA treatment in comparison with no electro-acupuncture treatment. Furthermore, Qazi et al. [30] demonstrated the control of gelatin-based scaffolds pore size was another effective method to enhance cell communications and modulate MSCs angiogenic paracrine effects. MSCs were seeded into gelatin-based scaffolds with varied pore size (∼200, ∼302 and ∼382 μm). The results indicated that medium pore caused effectively cellular aggregation, but smaller pores prevented cell infiltration and larger ones leaded to cell flow through the scaffolds. These differences are underlying regulators dominating intercellular and cell-matrix interactions.
4.1.4. Other ECM-like scaffolds
Apart from collagen, HA, and gelatin, there are many other biomaterials with great compatibility have been developed as the scaffold for the cell therapy to the post-traumatic spinal cord, such as fibrin [121], alginate [122], etc. For instance, 3D MSC-laden microfibers to mimic nerve fibers were fabricated via electrospinning by Yao et al. [123]. The special architecture of the fibrin scaffold enabled the MSC-aligned adhesion to form microscale cell fibers and enhanced their neural differentiation. After transplanting the 3D MSC-laden microfibers in the lesion, host neurons could translocate to the injury gap, further promoting the regeneration of nerve fiber.
Although researches using the component of ECM to mimic the cell niche can promote the therapeutic effect of MSCs to some extent, ECM actually consists of various components and single content may not mimic the actual situation very well. To this end, Veglianese's group implanted MSCs onto a carbomer/agarose scaffold modified with PEG and RGD (HG RGD). After incubation for 14 d, the ECM secreted by MSCs was deposited in the scaffold. Thus, the HG RGD+ECM could be obtained after lyophilization. Subsequently, MSCs were loaded for SCI treatment. The fabricated biomimetic scaffold, HG RGD+ECM, could not only optimize the viability and density of MSCs, but also delivery the paracrine neuroprotective factors (e.g., chemokine (C—C motif) ligand 2 (CCL2)) effectively. After implantation in the injured site, MSCs combined with HG RGD+ECM could modulate inflammation and increase M2 macrophages, leading to a promoted functional recovery in a mouse SCI model [124,125].
4.1.5. Synthetic polymer-based scaffolds
Despite of the quantitative advantages dominated by natural biomaterial-based scaffolds in biomaterials reinforced MSCs for the treatment of SCI, some appropriate chemical-synthetic biomaterials were also successfully designed as scaffolds to delivery MSCs.
Ropper et al. [8] fabricated a tailored PLGA scaffold with 350–500 μm pore diameter, for human derived MSCs (hMSCs) implantation in the hemisection model of SD rats. They demonstrated that the tailored PLGA scaffolds could realize maximize stemness maintenance benefitted from fine-tuned softness, smoothness, and pore size ranges. MSCs embedded in the PLGA scaffolds exerted neurotropism, anti-inflammation, angiogenesis and so on, achieved robust motor and sensory improvement in the end. Yang et al. [15] designed a PLGA scaffold possessed 50 micro-channels in the 3-mm-diameter conduit. The as-fabricated PLGA scaffolds could impede cyst and scar formation, and MSCs encapsulated in the scaffolds were able to differentiate into neuron-like cells in the existence of activated or preconditioned Schwann cells (SCs). The therapeutic system exhibited significant potential for the rehabilitation of SCI. In addition to PLGA scaffolds, a SIKVAV-modified highly superporous poly(2-hydroxyethyl methacrylate) (HEMA) hydrogel with oriented pores was constructed by Aleš Hejčl's team [126]. Their results indicated that connective tissue and blood vessels quickly infiltrated the scaffold within the first week after therapeutic intervention, and axons gradually infiltrated from the first month.
Other synthetic polymers, such as PCL (polycaprolactone) and PLA (poly-L-lactic acid), were also designed as porous PCL scaffolds with high-volume PCL microtubes [127] and aligned microfiber-based conduit [128] for SCI repair, respectively. Both of two different scaffolds could promote axon regeneration, implying that they are prospective polymers combined with MSCs transplantation for functional restoration following spinal cord trauma.
4.2. Inorganic nanoparticles
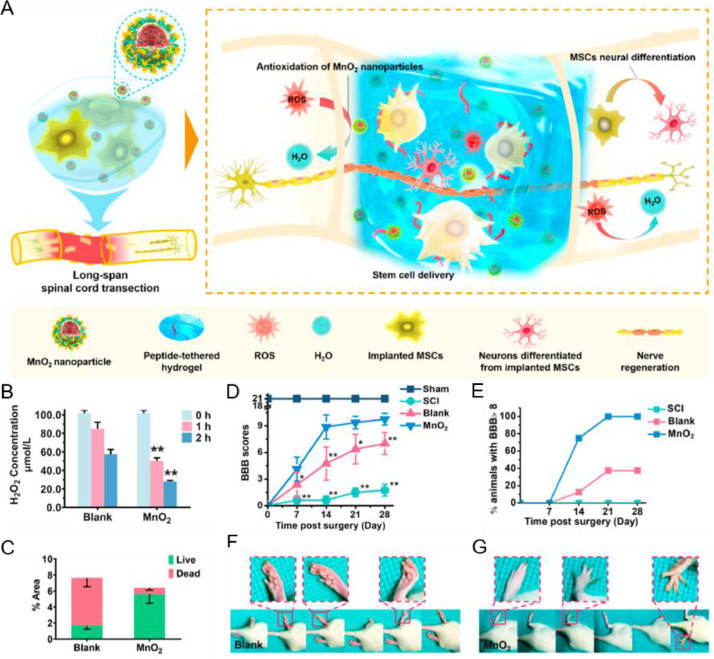
Apart from biomaterial scaffolds, some inorganic nanoparticles, such as silica, iron oxide, ceria, MnO2, and selenium are applied to treat SCI. On the one hand, inorganic nanoparticles with reactive oxygen species (ROS)-scavenging ability can be utilized as protectors to enhance the proliferation of the implanted MSCs and promote the regeneration of the host neurons [129]. The inflammation response after SCI is accompanied by excessive production of ROS, which contributes to the formation of inhibitory microenvironment hindering the functional recovery. In this regard, our group [130] developed a MnO2 nanoparticle-dotted HA hydrogel loading with MSCs to promote spinal cord recovery through mitigating the ROS microenvironment. MnO2 nanoparticle could scavenge the H2O2 effectively in vitro and in vivo, and thus protect the MSCs from the damage caused by ROS. Synergized with MnO2, the therapeutic efficacy of MSCs was significantly promoted (Fig. 3). Apart from MnO2 nanoparticles, inorganic nanoparticles capable of scavenging ROS can be adopted for the SCI treatment as well. For example, Cho et al. [131] developed PEG-decorated silica nanoparticles (PSiNPs) to evaluate membrane sealing ability and neuroprotective effect of the nanoformulation after spinal cord trauma. The results revealed that PSiNPs effectively sealed membrane-injured neurons, significantly reduced the formation of ROS, and obviously inhibited the process of lipid peroxidation of the membrane. Kim et al. [132] prepared ceria nanoparticles with an average size of 19.5 nm to regulate overexpressed ROS in the microenvironment of SCI. Upon the oxidative stress induced by H2O2, CeO2 could directly regulate ROS under the subcellular level and the neuronal viabilities obviously increased. Selenium nanoparticles (SeNPs) own similar capacity of ROS scavenging. Rao's group modified SeNPs with the soluble polysaccharide–protein complex and PG-6 peptide for SCI treatment [133]. With the loading of therapeutic agents monosialotetrahexosylganglioside (GM1) and tetramethylpyrazine (TMP), the as-constructed SeNPs@GM1/TMP could mitigate the ROS level, prevent mitochondrial dysfunction of neurons, and notably enhance the functional repair of rats after SCI. However, there are no researches combined CeO2 nanoparticles or SeNPs with MSCs to resolve obstacles in the road of SCI repair up to date. According to their function, we believe related investigations will be carried out in the near future.
Fig. 3.
A MnO2 nanoparticle-dotted hydrogel synergized with MSCs could promot spinal cord repair through reducing the ROS level in the microenvironment. (A) Schematic illustration of the fabrication of MnO2 nanoparticle-dotted hydrogel synergizing with MSCs for the promotion of spinal cord repair. (B) Evaluation of the concentration of H2O2 after incubation with blank or MnO2 nanoparticle-dotted hydrogels for 1 h or 2 h. (C) Analysis of the cytoviability of the MSCs after incubation with blank or MnO2 nanoparticle-dotted hydrogels for 24 h. (D) Monitoring of the BBB scores during 28 d post surgery. (E) Analysis of the percentage of rats with a BBB score > 8. (F-G) Typical photographs of animal walking gaits at the end of treatment (Day 28) in the Blank group (F) and the MnO2 group (G). (Reproduced from [130], Copyright 2019 American Chemical Society).
On the other hand, some inorganic nanoparticles can directly alter the biological functions of MSCs. For instance, Kim et al. [134] employed iron oxide nanoparticle (IONP) to stimulate MSCs. IONP could be partially ionized and the released iron ions could activate the JNK and c-Jun signaling cascades in MSCs resulting in enhanced expression of therapeutic growth factors. In addition, due to the inherent magnetic property of IONP [135], the exosome-mimetic nanovesicles derived from the INOP-incorporated MSCs (NV-IONP) could effectively accumulate in lesion via the guidance of an external magnetic field after intravenous administration. Consequently, NV-IONP harboring numerous therapeutic growth factors delivered in the lesion was capable for anti-apoptosis, angiogenesis, and inflammation attenuation, leading to alleviated damage and improved function of spinal cord.
4.3. Multicellular co-transplantation system
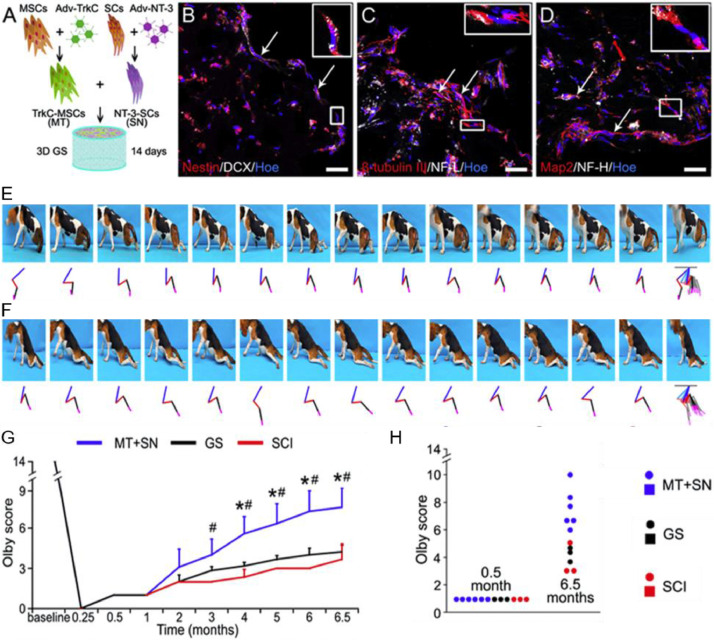
As mentioned above, cell therapy has been widely investigated for SCI treatment, and various cells exhibit promising potential in functional regeneration. Since different cell lineage owns unique merits, multicellular co-transplantation system may perform a synergistic therapeutic effect via multiple mechanisms, including the intrinsic function of each cell and the intercellular interaction [136]. SCs, playing a crucial role in the endogenous repair process of SCI [137], are usually co-transplanted with MSCs [138]. For example, Zeng et al. [139] cocultured SCs and MSCs in a 3D GS scaffolds for the treatment of SCI. SCs would induce the differentiation of MSCs toward neuron-like cells. Meanwhile, fibronectin secreted by MSCs in the early stage would accumulate on GS scaffold to support the cellular adhesion and survival. Interestingly, fibronectin could further promote the neurite elongation of MSC-differentiated neurons. Consequently, the as-fabricated co-transplantation system resulted in a synergistic therapeutic effect in spinal cord transection model. In another work, a multichannel PLGA scaffold was designed and fabricated for the co-transplantation of MSCs and activated SCs, which could further improve the axonal regeneration and promote the functional recovery [15]. The successful application of multicellular co-transplantation system requires not only the well-designed scaffold but also suitable types of cells with appropriate modification. It has been reported that NT-3 could protect neurons, promote axon regeneration, and promote neuronal differentiation of NSCs [140] or MSCs [141]. Therefore, Wu et al. [75] genetically modified SCs and MSCs with NT-3 gene or TrKC (receptor of NT-3) gene, respectively. Subsequently, NT-3-SCs and TrKC-MSCs were co-transplanted with a 3D gelatin sponge scaffold in the gap of spinal cord of Beagle canines. A variable number of MSC-derived neural tissue was integrated with regenerating corticospinal tract (CST) nerve fibers and 5-HT nerve fibers, which might be attributed to the formation of synapse-like structure derived from the implanted MSCs. Thus, the gradual restoration of paralyzed limb motor function in the SCI canines with the treatment of the upgraded co-transplantation system (Fig. 4).
Fig. 4.
Cotranplantation of NT-3-SCs with TrkC-MSCs could promote the recovery of paralyzed limb motor function in canine with complete SCI. (A) Schematic illustration of the construction method of MSC-derived neural network tissue in the 3D gelatin sponge scaffold. (B-D) TrkC-MSCs co-culturing with NT-3-SCs for 14 d enabled the neural lineage differentiation in vitro. (E-H) Exploration of the pelvic limb motor functional recovery from the 1st month after SCI. (Reproduced from [80], Copyright 2018 Elsevier B.V.).
4.4. How do biomaterials help?
As mentioned above, multiple kinds of biomaterials have been utilized in MSCs transplantation-based combinatorial strategies for SCI treatment. The roles of biomaterials in this promising remedial intervention can be overall concluded from two sides (Table 2). One aspect is that biomaterials dominant the fates of grafted MSCs via complex physical properties. Fundamentally, the topography (inner structure, pore size etc.) and mechanical properties (stiffness, viscoelasticity, and rheological properties) of biomaterials exert their influences on the survival, proliferation, and adhesion of MSCs through different signaling pathways. Besides, the introduction of biomaterials can help to manipulate the rigorous microenvironment and thus prevent the grafts from deleterious attacks around injured spinal cord. In addition, appropriate modification can engineer MSCs secretome directly [142]. Viewing on the cure of spinal cord trauma and regeneration of injured tissue, some biomaterials themselves also have the intrinsic therapeutic capability, including inhibiting glial scar formation, subsiding inflammation, stimulating angiogenesis and promoting neurogenesis [143,144]. For instance, HA has immanent favorable functions in CNS tissue repair and regeneration. As confirmed by Wang's team [145], low molecular weight-hyaluronan tetrasaccharide (LMW-HA4), the degradation product of HA, can upregulate the expression of NF-κB and c-IAP2 to inhibit caspase-3 expression and induce astrocytes to increase the expression of BDNF and VEGF, leading to self-protective effects on spinal cord compression injuries. Kushchayev's team conducted the investigation of neuroprotective role of HA scaffold in hemisection SCI [146]. Compared to the control group, the area of lesion, fibrous scar, and inflammatory cells were all decreased in HA scaffold-treated rats, although there were no differences in behavioral assessment scores.
Table 2.
The contributions of biomaterials to MSCs in the combinatorial strategies for SCI repair.
| Types | Biomaterials | Mechanisms/Functions | Ref. |
|---|---|---|---|
| Scaffolds | collagen | Regulating adhesion, proliferation, and differentiation through focal adhesion kinase-Src (FAK-Src) and RhoA/ROCK signaling pathway | [101], [102], [103],153] |
| HA | Promoting cellular adhesive growth through reacting with CD44; Restoring protective microenvironment via anti-inflammation and reducing glia scar |
[112,130] | |
| RGD/fibrinogen modified HA-PH | Enhancing adhesion and proliferation | [116] | |
| gelatin | Mitigating inflammation; Inhibiting necrosis and apoptosis; Promoting neuron-like differentiation |
[56,80,139,141] | |
| RGD ECM | Integrin binding | [124] | |
| fibrin | Inducing oriented adhesion, promoting neuron-like differentiation and axonal sprouting/regeneration | [121,123] | |
| alginate | Axonal sprouting/regeneration | [122] | |
| chitosan | Rheological properties similar to nerve tissue contributed to MSCs viability; Synergistically modulating inflammation with MSCs |
[155] | |
| PLGA | Maintaining stemness | [8,15] | |
| p(HEMA-AEMA) | Promoting integration to host tissue | [126] | |
| RGD modified agarose/carbomer | RGD motif increased MSCs adhesion; ECM deposition maintained MSCs viability | [125] | |
| Inorganic nanoparticles | MnO2 | Scavenging ROS | [130] |
| iron oxide | Targeting MSCs to injured spinal cord and stimulating MSCs secretion | [135] | |
| Multicellular co-transplantation system | gelatin | Supporting co-cultured cell growth to realize neuronal relay formation and promote axonal sprouting/regeneration | [80,139] |
| PLGA | Immunomodulation | [15] |
In consideration of the mechanism summarized above, biomaterials with superior biocompatibility, nourishment, appropriate mechanical parameters, microenvironment manipulation or neuroprotection capability, are all of potential to reinforce the MSCs transplantation for the SCI treatment.
5. Limitations and outlook
Stem cell transplantation in spinal cord injury repair has captured extensive attention of scientists during the past few years. The therapeutic strategy combined with biomaterial scaffolds at least successfully remedy some drawbacks up to a point, represented by disappointing survival rate and limited retention in the lesioned site, which are the reasons conducing the failure of clinical transformation. Nonetheless, there are amounts of roadblocks waiting to be cleared.
Firstly, despite a crowd of beneficial findings from animal models with injured spinal cord in the preclinical experiments, the source of MSCs, culture conditions, transplantation parameters (e.g., cell numbers, implanted site, timing of treatment), and route of delivery, are all need to be clarified to create a safe therapy [147,148]. Secondly, it is urgent to propose and adopt a clinical-grade protocol in non-clinical trials to achieve reproducibility of the procedure. Thirdly, in-depth research about the therapeutic mechanism of MSCs still needs to be continuously carried out. To date, some researchers believe that neural differentiation at the injury/graft site after MSCs transplantation is the major cause of nerve tissue regeneration and even motor-sensory function recovery [148]. As amounts of researches continue to practice, the finding of the false immuno-positive phenomenon caused by cell fusion has brought the theory of neuron-like differentiation of MSCs into controversy [149]. On the other hand, taking the paracrine effect of MSCs as the starting point, people gradually explore a new avenue to intervene in SCI [150]. This hypothesis supposes that biomolecules, such as neurotrophic factors including insulin-like growth factor (IGF)-1, BDNF, VEGF, granulocyte macrophage-colony stimulating factor (GM-CSF), fibroblast growth factor (FGF)-2, and transforming growth factor (TGF)-β, secreted by transplanted MSCs at the lesion site, can activate or modulate the endogenous neuro-restoration processes. The secretome of MSCs contains a variety of factors as mentioned above. However, the current potential mechanism for the beneficial influence of the MSC secretome on SCI intervention is too superficial. For instance, what are the respective roles of every biomolecule secreted by MSCs? Which of them play a pivotal part? Once these problems are well figured out, it will undoubtedly promote the emergence of more novel SCI treatment methods and contribute to reaching more controlled effects of transplanted MSCs even the application of identified biomolecules for cell-free stem cell therapy [18]. Of course, the development of biodegradable scaffold aimed at facilitating and optimizing bioactive molecule delivery, and realizing effective intervention in situ, are equally important.
Low efficiency of expansion represents another defect of MSCs seeded with biomaterial scaffolds. Microcarriers, small particles with diameters ranging between 100 and 400 mm, are novel tackles developed for MSCs expansion and induction of MSCs into different cell lineages attributed to their ability to modulate cell shape and cell organization like aggregate size [151]. Microcarrier culture system could enhance MSCs proliferation in-fold compared to traditional monolayer cultures. And the advanced cell culture system could mimic a three-dimensional environment, inducing cell differentiation. In addition, microcarriers could be directly applied in delivering MSCs to targeted areas for repair and regeneration of tissue [152]. Nevertheless, few pieces of research are focusing on combining biomaterial microcarriers with MSCs transplantation, though it has been researched in bone, cartilage, skin, vascular, CNS, adipose tissue, and liver repair.
In addition to accurate mechanism explorations and more innovative treatment researches, there should be extra efforts to promote the clinical application of the MSCs transplantation therapy for SCI. There is an obvious gap between the animal models of SCI and clinical cases, such as injury site, immunity and so on, which determined corresponding changes on the aspect of transplantation/administration route, detailed dosing paraments, and recovery level even prognosis. Therefore, it is urgent to align preclinical models more closely with the clinical patients. And developing SCI animal models using large animals (e.g., canine and rhesus) is encouraged for the preclinical trials.
Conflicts of interest
We declare that we have no financial and personal relationship with other people or organizations that can inappropriately influence our work.
Acknowledgements
This work was supported by National Key Research and Development Project of Stem Cell and Transformation Research (2019YFA0112100 , 2019YFA0112102) and National Natural Science Foundation of China (81973252, 81620108028).
References
- 1.GBD. Traumatic brain injury spinal cord injury. global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(1):56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva N.A., Sousa N., Reis R.L., Salgado A.J. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Ren H., Chen X., Tian M., Zhou J., Ouyang H., Zhang Z. Regulation of inflammatory cytokines for spinal cord injury repair through local delivery of therapeutic agents. Adv Sci. 2018;5(11) doi: 10.1002/advs.201800529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3(1):1–21. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 5.Assinck P., Duncan G.J., Hilton B.J., Plemel J.R., Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra H.S., Sarda K. Clinical translation of stem cell based interventions for spinal cord injury - Are we there yet? Adv Drug Deliv Rev. 2017;120(1):41–49. doi: 10.1016/j.addr.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Cofano F., Boido M., Monticelli M., Zenga F., Ducati A., Vercelli A., et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. doi: 10.3390/ijms20112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ropper A.E., Thakor D.K., Han I., Yu D., Zeng X., Anderson J.E., et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proc Natl Acad Sci U S A. 2017;114(5):E820–E829. doi: 10.1073/pnas.1616340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefifard M., Rahimi-Movaghar V., Nasirinezhad F., Baikpour M., Safari S., Saadat S., et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment: a systematic review and meta-analysis. Neuroscience. 2016;322(13):377–397. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Ma W., Zhan Y., Zhang Y., Xie X., Mao C., Lin Y. Enhanced neural regeneration with a concomitant treatment of framework nucleic acid and stem cells in spinal cord injury. ACS Appl Mater Interfaces. 2020;12(2):2095–2106. doi: 10.1021/acsami.9b19079. [DOI] [PubMed] [Google Scholar]
- 11.Fischer I., Dulin J.N., Lane M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat Rev Neurosci. 2020;21(7):366–383. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin M.C., Medress Z.A., Azad T.D., Doulames V.M., Veeravagu A. Stem cell therapies for acute spinal cord injury in humans: a review. Neurosurg Focus. 2019;46(3):E10. doi: 10.3171/2018.12.FOCUS18602. [DOI] [PubMed] [Google Scholar]
- 13.Fandel T.M., Trivedi A., Nicholas C.R., Zhang H., Chen J., Martinez A.F., et al. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell. 2016;19(4):544–557. doi: 10.1016/j.stem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Qu J., Zhang H. Roles of mesenchymal stem cells in spinal cord injury. Stem Cells Int. 2017;2017 doi: 10.1155/2017/5251313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang E.Z., Zhang G.W., Xu J.G., Chen S., Wang H., Cao L.L., et al. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol Sin. 2017;38(5):623–637. doi: 10.1038/aps.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead B., Logan A., Berry M., Leadbeater W., Scheven B.A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veneruso V., Rossi F., Villella A., Bena A., Forloni G., Veglianese P. Stem cell paracrine effect and delivery strategies for spinal cord injury regeneration. J Control Release. 2019;300:141–153. doi: 10.1016/j.jconrel.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Han D., Zheng X., Wang X., Jin T., Cui L., Chen Z. Mesenchymal stem/stromal cell-mediated mitochondrial transfer and the therapeutic potential in treatment of neurological diseases. Stem Cells Int. 2020;2020 doi: 10.1155/2020/8838046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh H., Yokota K., Fehlings M.G. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front Cell Neurosci. 2019;13:248. doi: 10.3389/fncel.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuhrmann T., Anandakumaran P.N., Shoichet M.S. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv Healthc Mater. 2017;6(10) doi: 10.1002/adhm.201601130. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K., Shi Z., Zhou J., Xing Q., Ma S., Li Q., et al. Potential application of an injectable hydrogel scaffold loaded with mesenchymal stem cells for treating traumatic brain injury. J Mater Chem B. 2018;6(19):2982–2992. doi: 10.1039/c7tb03213g. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q., Shi B., Ding J., Yan L., Thawani J.P., Fu C., et al. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019;88(1):57–77. doi: 10.1016/j.actbio.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 25.Hong K.H., Song S.C. 3D hydrogel stem cell niche controlled by host-guest interaction affects stem cell fate and survival rate. Biomaterials. 2019;218 doi: 10.1016/j.biomaterials.2019.119338. [DOI] [PubMed] [Google Scholar]
- 26.Li N., Xue F., Zhang H., Sanyour H.J., Rickel A.P., Uttecht A., et al. Fabrication and characterization of pectin hydrogel nanofiber scaffolds for differentiation of mesenchymal stem cells into vascular cells. ACS Biomater Sci Eng. 2019;5(12):6511–6519. doi: 10.1021/acsbiomaterials.9b01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafiee A., Kehtari M., Zarei Z., Soleimani M., Varshochian R., Ahmadi A., et al. An in situ hydrogel-forming scaffold loaded by PLGA microspheres containing carbon nanotube as a suitable niche for neural differentiation. Mater Sci Eng C. 2020;120 doi: 10.1016/j.msec.2020.111739. [DOI] [PubMed] [Google Scholar]
- 28.Ogle M.E., Doron G., Levy M.J., Temenoff J.S. Hydrogel culture surface stiffness modulates mesenchymal stromal cell secretome and slters senescence. Tissue Eng Part A. 2020 doi: 10.1089/ten.tea.2020.0030. [DOI] [PubMed] [Google Scholar]
- 29.Qazi T.H., Mooney D.J., Duda G.N., Geissler S. Niche-mimicking interactions in peptide-functionalized 3D hydrogels amplify mesenchymal stromal cell paracrine effects. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119639. [DOI] [PubMed] [Google Scholar]
- 30.Qazi T.H., Tytgat L., Dubruel P., Duda G.N., Vlierberghe S., Geissler S. Extrusion printed scaffolds with varying pore size as modulators of MSC angiogenic paracrine effects. ACS Biomater Sci Eng. 2019;5(10):5348–5358. doi: 10.1021/acsbiomaterials.9b00843. [DOI] [PubMed] [Google Scholar]
- 31.Martirosyan N.L., Feuerstein J.S., Theodore N., Cavalcanti D.D., Spetzler R.F., Preul M.C. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J Neurosurg Spine. 2011;15(3):238–251. doi: 10.3171/2011.4.SPINE10543. [DOI] [PubMed] [Google Scholar]
- 32.Werndle M.C., Saadoun S., Phang I., Czosnyka M., Varsos G.V., Czosnyka Z.H., et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med. 2014;42(3):646–655. doi: 10.1097/CCM.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 33.Alizadeh A., Dyck S.M., Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arundine M., Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34(4–5):325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 35.Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflug Arch Eur J Phy. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 36.Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., Vaccaro A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4(4):451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Lemasters J.J., Theruvath T.P., Zhong Z., Nieminen A.L. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787(11):1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasola A., Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50(3):222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Li S., Stys P.K. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20(3):1190–1198. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossman S.D., Wolfe B.B., Yasuda R.P., Wrathall J.R. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem. 2000;75(1):174–184. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- 41.Sánchez-Gómez M.V., Alberdi E., Pérez-Navarro E., Alberch J., Matute C. Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J Neurosci. 2011;31(8):2996–3006. doi: 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X., Kim H.K., Chung J.M., Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131(3):262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall E.D. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8(2):152–167. doi: 10.1007/s13311-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall E.D. In: Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Kobeissy FH, editor. CRC Press/Taylor & Francis; Boca Raton (FL): 2015. The contributing role of lipid peroxidation and protein oxidation in the course of CNS injury neurodegeneration and neuroprotection: an overview; pp. 49–61. [Google Scholar]
- 45.Nakamura T., Lipton S.A. Nitric oxide-dependent protein post-translational modifications impair mitochondrial function and metabolism to contribute to neurodegenerative diseases. Antioxid Redox Signal. 2020;32(12):817–833. doi: 10.1089/ars.2019.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipton S.A., Choi Y.B., Pan Z.H., Lei S.Z., Chen H.S., Sucher N.J., et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 47.Bao F., Chen Y., Dekaban G.A., Weaver L.C. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88(6):1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- 48.Hausmann O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41(7):369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y., Tang F., Xiao Z., Han G., Wang N., Yin N., et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26(5):891–900. doi: 10.3727/096368917X695038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boido M., Piras A., Valsecchi V., Spigolon G., Mareschi K., Ferrero I., et al. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2014;16(8):1059–1072. doi: 10.1016/j.jcyt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Dasari V.R., Veeravalli K.K., Dinh D.H. Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells. 2014;6(2):120–133. doi: 10.4252/wjsc.v6.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelagalli A., Nardelli A., Lucarelli E., Zannetti A., Brunetti A. Autocrine signals increase ovine mesenchymal stem cells migration through Aquaporin-1 and CXCR4 overexpression. J Cell Physiol. 2018;233(8):6241–6249. doi: 10.1002/jcp.26493. [DOI] [PubMed] [Google Scholar]
- 53.Zachar L., Bacenkova D., Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho S.R., Kim Y.R., Kang H.S., Yim S.H., Park C.I., Min Y.H., et al. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone marrow in a rat model of spinal cord injury. Cell Transplant. 2016;25(7):1423. doi: 10.3727/096368916X692078. [DOI] [PubMed] [Google Scholar]
- 55.Luo H., Xu C., Liu Z., Yang L., Hong Y., Liu G., et al. Neural differentiation of bone marrow mesenchymal stem cells with human brain-derived neurotrophic factor gene-modified in functionalized self-assembling peptide hydrogel in vitro. J Cell Biochem. 2019;120(3):2828–2835. doi: 10.1002/jcb.26408. [DOI] [PubMed] [Google Scholar]
- 56.Ma Y.H., Zeng X., Qiu X.C., Wei Q.S., Che M.T., Ding Y., et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials. 2018;160:37–55. doi: 10.1016/j.biomaterials.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 57.Zou Y.L., Zhao Y.N., Xiao Z.F., Chen B., Ma D.Z., Shen H., et al. Comparison of regenerative effects of transplanting three-dimensional longitudinal scaffold loaded-human mesenchymal stem cells and human neural stem cells on spinal cord completely transected rats. ACS Biomater Sci Eng. 2020;6(3):1671–1680. doi: 10.1021/acsbiomaterials.9b01790. [DOI] [PubMed] [Google Scholar]
- 58.Baez-Jurado E., Hidalgo-Lanussa O., Barrera-Bailon B., Sahebkar A., Ashraf G.M., Echeverria V., et al. Secretome of mesenchymal stem cells and its potential protective effects on brain pathologies. Mol Neurobiol. 2019;56(10):6902–6927. doi: 10.1007/s12035-019-1570-x. [DOI] [PubMed] [Google Scholar]
- 59.Sobacchi C., Palagano E., Villa A., Menale C. Soluble factors on stage to direct mesenchymal stem cells fate. Front Bioeng Biotechnol. 2017;5:32. doi: 10.3389/fbioe.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolar M.K., Itte V.N., Kingham P.J., Novikov L.N., Wiberg M., Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7(1):12605. doi: 10.1038/s41598-017-12969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gnecchi M., Danieli P., Malpasso G., Ciuffreda M.C. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol. 2016;1416:123–146. doi: 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- 62.Guillen M.I., Platas J., Perez Del Caz M.D., Mirabet V., Alcaraz M.J. Paracrine anti-inflammatory effects of adipose tissue-derived mesenchymal stem cells in human monocytes. Front Physiol. 2018;9:661. doi: 10.3389/fphys.2018.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruppert K.A., Nguyen T.T., Prabhakara K.S., Toledano Furman N.E., Srivastava A.K., Harting M.T., et al. Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci Rep. 2018;8(1):480. doi: 10.1038/s41598-017-18867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo Y., Kim H.S., Hong I.S. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019;2019 doi: 10.1155/2019/5126156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20(6):4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 66.Guo S.W., Perets N., Betzer O., Ben-Shaul S., Sheinin A., Michaelevski I., et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13(9):10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 67.Huang J.H., Xu Y., Yin X.M., Lin F.Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2020;424(1):133–145. doi: 10.1016/j.neuroscience.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 68.Liu W.Z., Ma Z.J., Li J.R., Kang X.W. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12(1):102. doi: 10.1186/s13287-021-02153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romanelli P., Bieler L., Scharler C., Pachler K., Kreutzer C., Zaunmair P., et al. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front Neurol. 2019;10:1225. doi: 10.3389/fneur.2019.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu W., Rong Y.L., Wang J.X., Zhou Z., Ge X.H., Ji C.Y., et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflamm. 2020;17:47. doi: 10.1186/s12974-020-1726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu W., Wang Y.X., Gong F.Y., Rong Y.L., Luo Y.J., Tang P.Y., et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotraum. 2019;36(3):469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 72.Lu Y.H., Zhou Y., Zhang R.Y., Wen L.L., Wu K.M., Li Y.F., et al. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci-Switz. 2019;13:209. doi: 10.3389/fnins.2019.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gollihue J.L., Patel S.P., Eldahan K.C., Cox D.H., Donahue R.R., Taylor B.K., et al. Effects of mitochondrial transplantation on bioenergetics, cellular incorporation, and functional recovery after spinal cord injury. J Neurotrauma. 2018;35(15):1800–1818. doi: 10.1089/neu.2017.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H., Wang C., He T., Zhao T., Chen Y.Y., Shen Y.L., et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9(7):2017–2035. doi: 10.7150/thno.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakamura Y., Park J.H., Hayakawa K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp Neurol. 2020;324 doi: 10.1016/j.expneurol.2019.113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun T., Qiao H., Pan P.Y., Chen Y., Sheng Z.H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4(3):413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Q., Xie Y., Ordaz J.D., Huh A.J., Huang N., Wu W., et al. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 2020;31(3):623–641. doi: 10.1016/j.cmet.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71(2):281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 79.Morita T., Sasaki M., Kataoka-Sasaki Y., Nakazaki M., Nagahama H., Oka S., et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221–231. doi: 10.1016/j.neuroscience.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 80.Wu G.H., Shi H.J., Che M.T., Huang M.Y., Wei Q.S., Feng B., et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials. 2018;181:15–34. doi: 10.1016/j.biomaterials.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 81.Silvestro S., Bramanti P., Trubiani O., Mazzon E. Stem cells therapy for spinal cord injury: an overview of clinical trials. Int J Mol Sci. 2020;21(2):659. doi: 10.3390/ijms21020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soria-Zavala Karla, García-Sánchez Julián, Rodríguez-Barrera Roxana. Mesenchymal stem cells for clinical use after spinal cord injury. IntechOpen. 2020 doi: 10.5772/intechopen.91839. [DOI] [Google Scholar]
- 83.Cheng H., Liu X., Hua R., Dai G., Wang X., Gao J., et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dvorak M.F., Noonan V.K., Fallah N., Fisher C.G., Rivers C.S., Ahn H., et al. Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J Neurotraum. 2014;31(18):1540–1547. doi: 10.1089/neu.2013.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cyranoski D. Japan's approval of stem-cell treatment for spinal-cord injury concerns scientists. Nature. 2019;565(7741):544–545. doi: 10.1038/d41586-019-00178-x. [DOI] [PubMed] [Google Scholar]
- 86.Fan X., Wang J.Z., Lin X.M., Zhang L. Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety. Neural Regen Res. 2017;12(5):815–825. doi: 10.4103/1673-5374.206653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Griffin J.M., Bradke F. Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem. EMBO Mol Med. 2020;12(3):e11505. doi: 10.15252/emmm.201911505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y., Li L.M., Mu J.F., Chen J.C., Feng S.Q., Gao J.Q. Implantation of a functional TEMPO-hydrogel induces recovery from rat spinal cord transection through promoting nerve regeneration and protecting bladder tissue. Biomater Sci-Uk. 2020;8(6):1695–1701. doi: 10.1039/c9bm01530b. [DOI] [PubMed] [Google Scholar]
- 89.Higuchi A., Kumar S.S., Benelli G., Ling Q.D., Li H.F., Alarfaj A.A., et al. Biomaterials used in stem cell therapy for spinal cord injury. Prog Mater Sci. 2019;103:374–424. [Google Scholar]
- 90.Papa S., Pizzetti F., Perale G., Veglianese P., Rossi F. Regenerative medicine for spinal cord injury: focus on stem cells and biomaterials. Expert Opin Biol Th. 2020;20(10):1203–1213. doi: 10.1080/14712598.2020.1770725. [DOI] [PubMed] [Google Scholar]
- 91.Jo J.I., Gao J.Q., Tabata Y. Biomaterial-based delivery systems of nucleic acid for regenerative research and regenerative therapy. Regen Ther. 2019;11:123–130. doi: 10.1016/j.reth.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanson S., D'Souza R.N., Hematti P. Biomaterial-mesenchymal stem cell constructs for immunomodulation in composite tissue engineering. Tissue Eng Part A. 2014;20(15–16):2162–2168. doi: 10.1089/ten.tea.2013.0359. [DOI] [PubMed] [Google Scholar]
- 93.Inoo K., Yamamoto M., Tabata Y. Preparation of cell aggregates incorporating gelatin hydrogel microspheres of sugar-responsive water solubilization. J Tissue Eng Regen M. 2020;14(8):1050–1062. doi: 10.1002/term.3076. [DOI] [PubMed] [Google Scholar]
- 94.Lee W., Choi J.H., Lee S., Song J.E., Khang G. Fabrication and characterization of silk fibroin microfiber-incorporated bone marrow stem cell spheroids to promote cell-cell interaction and osteogenesis. ACS Omega. 2020;5(29):18021–18027. doi: 10.1021/acsomega.0c01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park A., Choi J.H., Lee S., Been S., Song J.E., Khang G. Application of double network of gellan gum and pullulan for bone marrow stem cells differentiation towards chondrogenesis by controlling viscous substrates. J Tissue Eng Regen Med. 2020;14(11):1592–1603. doi: 10.1002/term.3116. [DOI] [PubMed] [Google Scholar]
- 96.Ishihara M., Kishimoto S., Nakamura S., Fukuda K., Sato Y., Hattori H. Biomaterials as cell carriers for augmentation of adipose tissue-derived stromal cell transplantation. Biomed Mater Eng. 2018;29(5):567–585. doi: 10.3233/BME-181009. [DOI] [PubMed] [Google Scholar]
- 97.Wang X., Rivera-Bolanos N., Jiang B., Ameer G.A. Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine. Adv Funct Mater. 2019;29(23) [Google Scholar]
- 98.Iwashita M., Ohta H., Fujisawa T., Cho M., Ikeya M., Kidoaki S., et al. Brain-stiffness-mimicking tilapia collagen gel promotes the induction of dorsal cortical neurons from human pluripotent stem cells. Sci Rep. 2019;9(1):3068. doi: 10.1038/s41598-018-38395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oliveira E., Assuncao-Silva R.C., Ziv-Polat O., Gomes E.D., Teixeira F.G., Silva N.A., et al. Influence of different ECM-like hydrogels on neurite outgrowth induced by adipose tissue-derived stem cells. Stem Cells Int. 2017;2017 doi: 10.1155/2017/6319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao Z., Tang F., Zhao Y., Han G., Yin N., Li X., et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with NeuroRegen scaffolds and mesenchymal stem cells. Cell Transplant. 2018;27(6):907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang N., Xiao Z., Zhao Y., Wang B., Li X., Li J., et al. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J Tissue Eng Regen Med. 2018;12(2):e1154–e1163. doi: 10.1002/term.2450. [DOI] [PubMed] [Google Scholar]
- 102.Li X., Tan J., Xiao Z., Zhao Y., Han S., Liu D., et al. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci Rep. 2017;7:43559. doi: 10.1038/srep43559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peng Z., Gao W., Yue B., Jiang J., Gu Y., Dai J., et al. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J Tissue Eng Regen Med. 2018;12(3):e1725–e1736. doi: 10.1002/term.2358. [DOI] [PubMed] [Google Scholar]
- 104.Han Q., Jin W., Xiao Z., Ni H., Wang J., Kong J., et al. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31(35):9212–9220. doi: 10.1016/j.biomaterials.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 105.Kourgiantaki A., Tzeranis D.S., Karali K., Georgelou K., Bampoula E., Psilodimitrakopoulos S., et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen Med. 2020;5(1):12. doi: 10.1038/s41536-020-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zou Y.L., Ma D.Z., Shen H., Zhao Y.N., Xu B., Fan Y.H., et al. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater Sci-UK. 2020;8(18):5145–5156. doi: 10.1039/d0bm00431f. [DOI] [PubMed] [Google Scholar]
- 107.Cholas R., Hsu H.P., Spector M. Collagen scaffolds incorporating select therapeutic agents to facilitate a reparative response in a standardized hemiresection defect in the rat spinal cord. Tissue Eng Part A. 2012;18(19–20):2158–2172. doi: 10.1089/ten.TEA.2011.0577. [DOI] [PubMed] [Google Scholar]
- 108.Sart S., Tsai A.C., Li Y., Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2014;20(5):365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He J., Zhang N., Zhu Y., Jin R., Wu F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials. 2021;265 doi: 10.1016/j.biomaterials.2020.120448. [DOI] [PubMed] [Google Scholar]
- 110.Trujillo S., Vega S.L., Song K.H., San Felix A., Dalby M.J., Burdick J.A., et al. Engineered full-length fibronectin-hyaluronic acid hydrogels for stem cell engineering. Adv Healthc Mater. 2020;9(21) doi: 10.1002/adhm.202000989. [DOI] [PubMed] [Google Scholar]
- 111.Kwon M.Y., Wang C., Galarraga J.H., Pure E., Han L., Burdick J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials. 2019;222 doi: 10.1016/j.biomaterials.2019.119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L.M., Han M., Jiang X.C., Yin X.Z., Chen F., Zhang T.Y., et al. Peptide-tethered hydrogel scaffold promotes recovery from spinal cord transection via synergism with mesenchymal stem cells. ACS Appl Mater Inter. 2017;9:3330–3342. doi: 10.1021/acsami.6b12829. [DOI] [PubMed] [Google Scholar]
- 113.Han C.S., Zhou J., Liang C., Liu B., Pan X.B., Zhang Y., et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci-UK. 2019;7(7):2920–2933. doi: 10.1039/c9bm00101h. [DOI] [PubMed] [Google Scholar]
- 114.Wang C.G., Wang M., Xu T.Z., Zhang X.X., Lin C., Gao W.Y., et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L.L., Fan C.X., Hao W.P., Zhuang Y., Liu X.R., Zhao Y.N., et al. NSCs migration promoted and drug delivered exosomes-collagen scaffold via a bio-specific peptide for one-step spinal cord injury repair. Adv Healthc Mater. 2021 doi: 10.1002/adhm.202001896. [DOI] [PubMed] [Google Scholar]
- 116.Zaviskova K., Tukmachev D., Dubisova J., Vackova I., Hejcl A., Bystronova J., et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J Biomed Mater Res A. 2018;106(4):1129–1140. doi: 10.1002/jbm.a.36311. [DOI] [PubMed] [Google Scholar]
- 117.Du B.L., Zeng C.G., Zhang W., Quan D.P., Ling E.A., Zeng Y.S. A comparative study of gelatin sponge scaffolds and PLGA scaffolds transplanted to completely transected spinal cord of rat. J Biomed Mater Res A. 2014;102(6):1715–1725. doi: 10.1002/jbm.a.34835. [DOI] [PubMed] [Google Scholar]
- 118.Li G., Che M.T., Zhang K., Qin L.N., Zhang Y.T., Chen R.Q., et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–248. doi: 10.1016/j.biomaterials.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 119.Li G., Che M.T., Zeng X., Qiu X.C., Feng B., Lai B.Q., et al. Neurotrophin-3 released from implant of tissue-engineered fibroin scaffolds inhibits inflammation, enhances nerve fiber regeneration, and improves motor function in canine spinal cord injury. J Biomed Mater Res A. 2018;106(8):2158–2170. doi: 10.1002/jbm.a.36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang K., Liu Z., Li G., Lai B.Q., Qin L.N., Ding Y., et al. Electro-acupuncture promotes the survival and differentiation of transplanted bone marrow mesenchymal stem cells pre-induced with neurotrophin-3 and retinoic acid in gelatin sponge scaffold after rat spinal cord transection. Stem Cell Rev Rep. 2014;10(4):612–625. doi: 10.1007/s12015-014-9513-4. [DOI] [PubMed] [Google Scholar]
- 121.Mukhamedshina Y.O., Akhmetzyanova E.R., Kostennikov A.A., Zakirova E.Y., Galieva L.R., Garanina E.E., et al. Adipose-derived mesenchymal stem cell application combined with fibrin matrix promotes structural and functional recovery following spinal cord injury in rats. Front Pharmacol. 2018;9:343. doi: 10.3389/fphar.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blasko J., Szekiova E., Slovinska L., Kafka J., Cizkova D. Axonal outgrowth stimulation after alginate/mesenchymal stem cell therapy in injured rat spinal cord. Acta Neurobiol Exp (Wars) 2017;77(4):337–350. [PubMed] [Google Scholar]
- 123.Yao S., He F., Cao Z., Sun Z., Chen Y., Zhao H., et al. Mesenchymal stem cell-laden hydrogel microfibers for promoting nerve fiber regeneration in long-distance spinal cord transection injury. ACS Biomater Sci Eng. 2020;6(2):1165–1175. doi: 10.1021/acsbiomaterials.9b01557. [DOI] [PubMed] [Google Scholar]
- 124.Papa S., Vismara I., Mariani A., Barilani M., Rimondo S., De Paola M., et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J Control Release. 2018;278:49–56. doi: 10.1016/j.jconrel.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 125.Caron I., Rossi F., Papa S., Aloe R., Sculco M., Mauri E., et al. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135–147. doi: 10.1016/j.biomaterials.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 126.Hejcl A., Ruzicka J., Proks V., Mackova H., Kubinova S., Tukmachev D., et al. Dynamics of tissue ingrowth in SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores after bridging a spinal cord transection. J Mater Sci Mater Med. 2018;29(7):89. doi: 10.1007/s10856-018-6100-2. [DOI] [PubMed] [Google Scholar]
- 127.Shahriari D., Koffler J.Y., Tuszynski M.H., Campana W.M., Sakamoto J.S. Hierarchically ordered porous and high-volume polycaprolactone microchannel scaffolds enhanced axon growth in transected spinal cords. Tissue Eng Pt A. 2017;23(9–10):415–425. doi: 10.1089/ten.tea.2016.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hurtado A., Cregg J.M., Wang H.B., Wendell D.F., Oudega M., Gilbert R.J., et al. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials. 2011;32(26):6068–6079. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.An Z., Yan J., Zhang Y., Pei R. Applications of nanomaterials for scavenging reactive oxygen species in the treatment of central nervous system diseases. J Mater Chem B. 2020;8(38):8748–8767. doi: 10.1039/d0tb01380c. [DOI] [PubMed] [Google Scholar]
- 130.Li L., Xiao B., Mu J., Zhang Y., Zhang C., Cao H., et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano. 2019;13(12):14283–14293. doi: 10.1021/acsnano.9b07598. [DOI] [PubMed] [Google Scholar]
- 131.Cho Y., Shi R.Y., Ivanisevic A. Ben Borgens R. Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury. J Neurosci Res. 2010;88(7):1433–1444. doi: 10.1002/jnr.22309. [DOI] [PubMed] [Google Scholar]
- 132.Kim J.W., Mahapatra C., Hong J.Y., Kim M.S., Leong K.W., Kim H.W., et al. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Adv Sci. 2017;4(10) doi: 10.1002/advs.201700034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rao S.Y., Lin Y.P., Du Y.X., He L.Z., Huang G.N., Chen B.L., et al. Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J Mater Chem B. 2019;7(16):2648–2656. doi: 10.1039/c8tb02520g. [DOI] [PubMed] [Google Scholar]
- 134.Kim H.Y., Kumar H., Jo M.J., Kim J., Yoon J.K., Lee J.R., et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18(8):4965–4975. doi: 10.1021/acs.nanolett.8b01816. [DOI] [PubMed] [Google Scholar]
- 135.Niu X.Q., Chen J.J., Gao J.Q. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci. 2019;14(5):480–496. doi: 10.1016/j.ajps.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baudequin T., Tabrizian M. Multilineage constructs for scaffold-based tissue engineering: a review of tissue-specific challenges. Adv Healthc Mater. 2018;7(3) doi: 10.1002/adhm.201700734. [DOI] [PubMed] [Google Scholar]
- 137.Oudega M., Xu X.M. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23(3–4):453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- 138.Ban D.X., Ning G.Z., Feng S.Q., Wang Y., Zhou X.H., Liu Y., et al. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6(6):707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- 139.Zeng X., Ma Y.H., Chen Y.F., Qiu X.C., Wu J.L., Ling E.A., et al. Autocrine fibronectin from differentiating mesenchymal stem cells induces the neurite elongation in vitro and promotes nerve fiber regeneration in transected spinal cord injury. J Biomed Mater Res A. 2016;104(8):1902–1911. doi: 10.1002/jbm.a.35720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lai B.Q., Che M.T., Du B.L., Zeng X., Ma Y.H., Feng B., et al. Transplantation of tissue engineering neural network and formation of neuronal relay into the transected rat spinal cord. Biomaterials. 2016;109:40–54. doi: 10.1016/j.biomaterials.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Zeng X., Qiu X.C., Ma Y.H., Duan J.J., Chen Y.F., Gu H.Y., et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184–201. doi: 10.1016/j.biomaterials.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 142.Wechsler M.E., Rao V.V., Borelli A.N., Anseth K.S. Engineering the MSC secretome: a hydrogel focused approach. Adv Healthc Mater. 2021 doi: 10.1002/adhm.202001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han S., Lee J.Y., Heo E.Y., Kwon I.K., Yune T.Y., Youn I. Implantation of a Matrigel-loaded agarose scaffold promotes functional regeneration of axons after spinal cord injury in rat. Biochem Biophys Res Commun. 2018;496(3):785–791. doi: 10.1016/j.bbrc.2018.01.157. [DOI] [PubMed] [Google Scholar]
- 144.Li X., Zhang C., Haggerty A.E., Yan J., Lan M., Seu M., et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials. 2020;245 doi: 10.1016/j.biomaterials.2020.119978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J., Rong W., Hu X., Liu X., Jiang L., Ma Y., et al. Hyaluronan tetrasaccharide in the cerebrospinal fluid is associated with self-repair of rats after chronic spinal cord compression. Neuroscience. 2012;210:467–480. doi: 10.1016/j.neuroscience.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 146.Kushchayev S.V., Giers M.B., Eng D.H., Martirosyan N.L., Eschbacher J.M., Mortazavi M.M., et al. Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. J Neurosurg-Spine. 2016;25(1):114–124. doi: 10.3171/2015.9.SPINE15628. [DOI] [PubMed] [Google Scholar]
- 147.Sart S., Agathos S.N., Li Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol Prog. 2013;29(6):1354–1366. doi: 10.1002/btpr.1825. [DOI] [PubMed] [Google Scholar]
- 148.Teixeira F.G., Carvalho M.M., Sousa N., Salgado A.J. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70(20):3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maltman D.J., Hardy S.A., Przyborski S.A. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59(3):347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 150.Hawryluk G.W., Mothe A., Wang J., Wang S., Tator C., Fehlings M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21(12):2222–2238. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martin Y., Eldardiri M., Lawrence-Watt D.J., Sharpe J.R. Microcarriers and their potential in tissue regeneration. Tissue Eng Part B-Re. 2011;17(1):71–80. doi: 10.1089/ten.TEB.2010.0559. [DOI] [PubMed] [Google Scholar]
- 152.Koh B., Sulaiman N., Fauzi M.B., Law J.X., Ng M.H., Idrus R.B.H., et al. Three dimensional microcarrier system in mesenchymal stem cell culture: a systematic review. Cell Biosci. 2020;10:75. doi: 10.1186/s13578-020-00438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yao Z.P., Liu W.G., Song C.Y. Effect of mesenchymal stem cell-incorporated hydroxyapatite-collagen scaffold on tissue repair in acute spinal cord injury, and the mechanism involved. Trop J Pharm Res. 2020;19(5):1099–1103. [Google Scholar]
- 154.Zhang J.N., Cheng T., Chen Y.H., Gao F., Guan F.X., Yao M.H. A chitosan-based thermosensitive scaffold loaded with bone marrow-derived mesenchymal stem cells promotes motor function recovery in spinal cord injured mice. Biomed Mater. 2020;15(3) doi: 10.1088/1748-605X/ab785f. [DOI] [PubMed] [Google Scholar]
- 155.Boido M., Ghibaudi M., Gentile P., Favaro E., Fusaro R., Tonda-Turo C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci Rep. 2019;9(1):6402. doi: 10.1038/s41598-019-42848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]