Abstract
Background
As common progenitor cells of osteoblasts and adipocytes, bone marrow mesenchymal (stromal) stem cells (BMSCs) play key roles in bone homeostasis, tissue regeneration, and global energy homeostasis; however, the intrinsic mechanism of BMSC differentiation is not well understood. Plasticity in energy metabolism allows BMSCs to match the divergent demands of osteo-adipogenic differentiation. Targeting BMSC metabolic pathways may provide a novel therapeutic perspective for BMSC differentiation unbalance related diseases.
Scope of review
This review covers the recent studies of glucose, fatty acids, and amino acids metabolism fuel the BMSC differentiation. We also discuss recent findings about energy metabolism in BMSC differentiation.
Major conclusions
Glucose, fatty acids, and amino acids metabolism provide energy to fuel BMSC differentiation. Moreover, some well-known regulators including environmental stress, hormone drugs, and biological and pathological factors may also influence BMSC differentiation by altering metabolism. This offers insight to the significance of metabolism on BMSC fate determination and provides the possibility of treating diseases related to BMSC differentiation, such as obesity and osteoporosis, from a metabolic perspective.
Keywords: Bone marrow mesenchymal (stromal) stem cells differentiation, Energy metabolism, Environmental factors, Hormone drugs, Biological factors, Pathological factors
Abbreviations
- AdipoR1
adiponectin receptor 1
- KDM4B
lysine-specific demethylase 4B
- AGEs
advanced glycation end products
- αKG
ketoglutaric acid
- ALP
alkaline phosphatase
- LDL
low-density lipoprotein
- AN
anorexia nervosa
- MSCs
mesenchymal stem cells
- BMA
bone marrow adiposity
- NAD+
nicotinamide adenine dinucleotide
- BMAT
bone marrow adipose tissue
- OXPHOS
oxidative phosphorylation
- BMSCs
bone marrow mesenchymal (stromal) stem cells
- ON
osteonectin
- Dex
dexamethasone
- OADs
oral antidiabetic drugs
- DPP4
dipeptidyl peptidase-4
- OVX
ovariectomized
- ECM
extracellular matrix
- PDH
pyruvate dehydrogenase
- FAS
fatty acid synthase
- PGC1α
peroxlsome proliferator activated receptor-γ coactivator-1α
- Glut1
glucose transporter 1
- PPARγ
peroxisome proliferator-activated receptor γ
- GLS
glutaminase
- PTH
parathyroid hormone
- hESCs
human embryonic stem cells
- ROS
reactive oxygen species
- hMSCs
human bone marrow mesenchymal stem cells
- Runx2
Runx family transcription factor 2
- HIF1α
hypoxia-inducible factor 1α
- SBA
separation-based anorexia model
- hiPSCs
human induced pluripotent stem cells
- SMG
simulated microgravity
- HPAA
high molecular weight polyacrylic acid Sirt sirtuin
- IGF-1
insulin-like growth factor 1
- SSC
skeletal stem cells
- KDM6B
lysine-specific demethylase 6B
- T2DM
type 2 diabetes mellitus
1. Introduction
Roughly 50 years ago, Friedenstein et al. first discovered mesenchymal stem cells (MSCs) in bone marrow [1], and these non-hematopoietic stem cell populations showed stem-like characteristics [2]. Since then, extensive research has been conducted using in vitro and in vivo models to better understand the character of MSCs. Because it is easier to isolate bone marrow mesenchymal stem cells (BMSCs) and because they exhibit more powerful self-renewal ability and multi-lineage differentiation potential, the regulators participating in BMSC differentiation process have attracted attention. Previous studies focus mainly on transcriptional factors such as Runx2, PPARγ [[3], [4], [5], [6], [7], [8]], hormone drugs [[9], [10], [11]], environmental stress such as extracellular matrix (ECM) [[12], [13], [14], [15]], and some biological/pathological factors [16].
In recent years, an increase has been noted in studies on the energy metabolism that occurs during BMSC differentiation [17,18]. Because BMSCs demand substantial energy to maintain bone homeostasis [17], both genetic and functional studies have demonstrated that energy metabolism, including glucose, fatty acid, and amino acid metabolism, all function as critical regulators in BMSC differentiation [18,19]. Dysregulation of energy metabolism in BMSCs consequently disturbs the balance between bone formation and bone resorption [18]. Furthermore, restoring the energy metabolism of BMSCs can improve diseases such as obesity and osteoporosis by balancing osteo-adipogenic differentiation [[20], [21], [22]]. Nevertheless, there hurdles remain in achieving clearer understanding about fuel choices and cellular metabolism in BMSCs. This review addresses our understanding of glucose, lipid, and amino acid metabolism during BMSC differentiation (Figure 1, Table 1). We also discuss the role of some well-known regulators (e.g., environmental stress, hormone drugs, biological and pathological factors) in influencing BMSC differentiation via metabolism (Figure 1, Table 2). Targeting BMSC metabolic pathways provide a novel therapeutic perspective for BMSC differentiation unbalances related diseases.
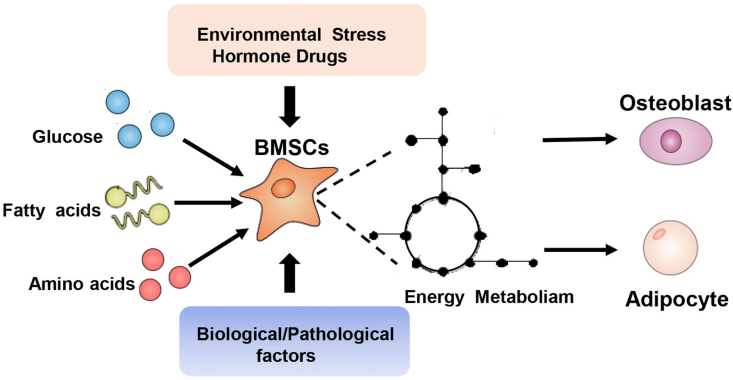
Figure 1.
The role of energy metabolism during BMSC differentiation. Glucose, fatty acids, and amino acid participate in BMSC differentiation. Moreover, environmental stress, hormone drugs, biological factors, and pathological factors influence differentiation by altering metabolism.
Table 1.
Functions and mechanism of glucose, fatty acids and amino acids metabolism during BMSC differentiation.
| Metabolites/Metaboilc pathway | Functions | Mechanism | References |
|---|---|---|---|
| Glucose and metabolic pathway | |||
| Glucose uptake | Promotes osteogenic differentiation of hMSCs | Blocks ubiquitination of Runx2 | [26,27] |
| Glycolysis | Promotes osteogenic differentiation of primary BMSCs and ST2 cells | Activates the RhoA/ROCK pathway | [[29], [30], [31]] |
| OXPHOS | Activates osteogenic differentiation of C3H10T1/2 cells | Promotes intracellular β-catenin signaling or downregulates HIF1α level | [34,35] |
| No change during osteogenesis of hMSCs and ST2 cells Contributes to adipogenesis of hMSCs | No Increases supramolecular organization of cytochrome c oxidation |
[36,37] [38] |
|
| Fatty acids and metabolic pathway | |||
| Palmitate | Inhibits osteogenic differentiation of hMSCs | Inhibits the expression of Bmp2 and glucose metabolism | [41,42] |
| Oleate | Promotes osteogenic differentiation of hMSCs | Prevents attenuation of the insulin signaling pathway | [[42], [43], [44]] |
| Arachidonic acid | Promotes adipogenic and inhibits osteogenic differentiation of hMSCs | No | [44] |
| Fatty acids oxidation | Promotes osteogenic differentiation of skeletal progenitor cells | As the energy source | [45] |
| Amino Acids and metabolic pathway | |||
| Glutamine | Promotes BMSCs differentiated toward osteoblasts | Provides ATP through the TCA cycle to meet energetic and synthetic demands | [49,50] |
| Glutaminase | Promotes BMSCs differentiated toward osteoblasts | Increases α-ketoglutarate production | [49] |
| Ketoglutaric acid | Promotes osteogenic potential of BMSCs | Decreases histone methylations accumulations | [52] |
| Kynurenine | Inhibits osteogenic differentiation in BMSCs and hMSCs | Upregulates miR-493-5b while downregulates miR-210-3b to elevate oxidative stress | [[53], [54], [55]] |
Table 2.
Environmental stress, hormone drugs and biological/pathological factors regulate BMSCs differentiation via altering energy metabolism.
| Other factors | Fuctions | Metabolic mechanism | References |
|---|---|---|---|
| Environmental stress | |||
| ECM stiffness | High ECM stiffness promotes osteogenic differentiation of hMSCs and BMSCs | Enhances ATP levels and AMPK activation; promotes mitochondriogenesis and declines mitochondria fission | [[57], [58], [59]] |
| Hypoxia | Suppresses adipogenesis and enhances osteogenesis of hMSCs | Promotes pyruvate dehydrogenase (PDH) by PDH kinase (PDK) and inhibits glycolytic enzymes | [63] |
| Inhibits both osteogenic and adipogenic differentiation of MSCs | Inhibits oxidative and enhances glycolysis | [[64], [65], [66]] | |
| Microgravity | Suppresses osteogenic differentiation of MSCs; promotes adipogenic differentiation of BMSCs | Inhibits OXPHOS and decreases the expression of important energy sensor Sirt1; upregulates leptin expression | [73,74] |
| Hormone drugs | |||
| PTH | Inhibits adipogenesis in murine cell lines of adipocyte progenitors BMSCsadipo, enhances osteogenesis of BMSCs | Impaires insulin signaling, enhances glycolysis and ATP production rate; promotes BMAT lipolysis and provides more fatty acids to fuel osteogenic differentiation | [76,77] |
| Dexamethasone | Inhibits osteogenic differentiation of BMSCs | Impaires mitochondrial function and downregulates mitochondrial metabolism AMPK/PGC-1 α/Sirt3 axis to induce ROS overproduction | [79,80] |
| Biological factors | |||
| MicroRNAs | MiR-34a overexpression impaires osteogenic differentiation of hMSCs; miR-181a/b can promote osteogenesis; | Inhibits lactate dehydrogenase-A (LDHA), hexokinase 2 (HK2), and Glut1-mediated glycolysis; enhances mitochondrial metabolism | [26,86,87] |
| MicroRNA-200a-3p suppresses osteogenic differentiation of MSCs | Inhibits GLS | [88] | |
| Adiponectin | Promotes osteoblastogenesis in C3H10T1/2 cells | Induces ALP, osteopontin expression | [92,96,97] |
| Inhibits the adipogenic commitment of BMSCs | Increases AdipoR1, KDM6B and KDM4B to inhibit PPARγ | [92,98,99] | |
| Agin | Inhibits osteogenic differentiation and promtes adipogenic differentiation of BMSCs | Downregulates mitochondria metabolism related NAD+, PGC-1α levels as well as citrate contents in bone matrix | [[101], [102], [103], [104], [105]] |
| Pathological factors | |||
| Obesity/diabetes mellitus | Inhibits osteogenic differentiation and promotes adipogenic differentiation of BMSCs | Increases DPP4 to inhibiting glucose metabolism and lipolysis | [[110], [111], [112]] |
| Osteoporosis | Impaires osteogenic differentiation of MSCs, SSCs and BMSCs | Inhibits fatty acid synthase expression; decreases mitochondrial biogenesis, PGC1α level and Sirt 3 expression | [104,[114], [115], [116], [117]] |
| Anorexia nervosa | Increases adipogenic differentiation of BMSCs while blunts osteogenic differentiation | Downregulates Sirt1 expression; blocks BMPs signaling and activates the inflammatory NF-κB signaling via adipokines derived from bone marrow adipose in pathological state | [[121], [122], [123]] |
2. Glucose, fatty acids, and amino acids metabolism during BMSC differentiation
2.1. Glucose metabolism in BMSC differentiation
2.1.1. Glucose uptake
(Table 1) Glucose has been long known as an important nutrient for bone cells [23]. Studies from the early 1960s demonstrated that bone explants, as well as primary cultures of calvarial osteoblasts, demand high levels of glucose to proliferate [[23], [24], [25]]. More recent evidence shows that altered glucose uptake has been implicated in affecting BMSC differentiation. For example, in differentiating human bone marrow mesenchymal stem cells (hMSCs), inhibiting the expression of osteogenic differentiation markers like osteopontin, alkaline phosphatase (ALP), and osteonectin (ON) is accompanied by lower glucose uptake and glucose transporter 1 (Glut1) expression [26]. Moreover, Glut1 modulates the posttranslational modification of osteogenic differentiation marker Runx2 and blocks ubiquitination of Runx2 [27]. Finally, selective deletion of Glut1 in osteoblast precursors suppresses osteogenesis in vitro and in vivo [27]. Zhou et al. have reported that using low glucose medium can change human-induced pluripotent stem cells (hiPSCs) and H9 human embryonic stem cells (hESCs) successfully into functional hMSCs with the capability of tri-lineage differentiation in vitro (adipogenesis, osteogenesis, and chondrogenesis) [28]. These findings indicate that Glut1 mediates glucose uptake which promotes osteogenic differentiation of BMSCs isolated from both mice and humans, and lower glucose levels may contribute to the maintenance of stemness of functional hMSCs.
2.1.2. Glycolysis
Glycolysis is thought to preserve the “stemness” of the proliferating BMSCs [18]. However, some recent findings highlight that aerobic glycolysis is the predominant source of energy that promotes the differentiation of BMSCs [[29], [30], [31]]. The inhibition of glycolysis suppresses osteogenesis of primary bone marrow mesenchymal progenitors [29], while blunting glycolysis pathways in BMSC-like ST2 cells also leads to decreased osteogenesis and mineralization [30]. Moreover, glycolytic agonist promotes osteogenic differentiation of BMSCs via activation of RhoA/ROCK [31]. These findings indicate the critical role of glycolysis in future therapies using BMSCs [31]. Conversely, there is another study that implied that osteogenic differentiation of BMSCs requires a metabolic switch from glycolysis to increased mitochondrial oxidative phosphorylation (OXPHOS) to ensure a sufficient energy supply [32]. Next we discuss the role of OXPHOS in BMSC differentiation.
2.1.3. Oxidative phosphorylation (OXPHOS)
OXPHOS is crucial to regulating BMSC differentiation [32]. Studies have shown that OXPHOS activates in BMSCs during osteogenic differentiation [33], maybe via downregulating hypoxia-inducible factor 1 (HIF-1) expression [34] or promoting β-catenin acetylation in C3H10T1/2 cells [35], while inhibiting OXPHOS reduces the osteogenic potential of C3H10T1/2 cells [35]. However, Pattappa et al. found no change in OXPHOS during hMSCs osteogenesis [36], and a subsequent study by Esen et al. found similar results in ST2 cells [37]. Conversely, Hofmann et al. confirmed that OXPHOS supercomplexes can serve as a hallmark of adipogenic but not osteogenic differentiation of hMSCs [38]. Taken together, OXPHOS is critical in regulating osteogenic differentiation of C3H10T1/2 cells through promoting the intracellular β-catenin signaling pathways or downregulating the HIF1α level. However, OXPHOS is not necessary for osteogenic differentiation of hMSCs and ST2 cells, while contributing to adipogenic differentiation in hMSCs.
2.2. Fatty acids metabolism in BMSC differentiation
It was not until 1987 that Fleisch and colleagues identified fatty acids as a substrate capable of supplying energy to bone tissue and bone cells [39]. Subsequent studies revealed that fatty acids are second only to glucose as a main nutritional determinant for skeletal progenitor cells [40]. In vitro study has demonstrated that long-chain saturated fatty acids such as palmitate can inhibit osteogenic differentiation of hMSCs [41,42] which can be mitigated by oleate [[42], [43], [44]]. Similarly, arachidonic acid (omega-6 fatty acid derived from linoleic acid) favored adipogenic differentiation and inhibited osteogenic differentiation in vitro of hMSCs [44]. Fatty acid metabolism can also promote osteogenic differentiation of skeletal progenitor cells [45]. Nick et al. recently found that enhancing fatty acid oxidation promotes osteogenic differentiation of skeletal progenitor cells, while suppressing fatty acids oxidation of skeletal progenitor cells through serum lipid deprivation can promote chondrogenic differentiation over osteogenesis [45]. Furthermore, fatty acids-specific receptor GPR120 transcripts are present in primary BMSCs, whose expression levels gradually increase during osteogenic induction [46]; thus, fatty acids function as an energy source to facilitate bone formation, and depriving fatty acids suppresses osteogenic differentiation possibly by inhibiting fatty acid oxidation and expression of specific receptors. Additional studies with a new model are needed to fully understand how the MSCs utilize fatty acids in health and metabolic bone diseases states.
2.3. Amino acids metabolism in MSC differentiation
2.3.1. Glutamine
Glutamine is the most abundant amino acid in circulation [47]. In addition to its direct contribution to protein synthesis, glutamine functions as an important energy source and an essential carbon and nitrogen donor for the synthesis of amino acids, nucleotides, glutathione, and hexosamine [47]. It has been 30 years since Biltz et al. first reported that isolated calvaria and long bones exhibited active glutamine consumption and metabolism [48]; subsequently, the role of glutamine in bone has drawn increasing attention. More recent studies have indicated that human and mouse BMSCs consume glutamine during differentiation [49]. In line with these roles, recent research shows that during the process of BMSCs differentiated toward osteoblasts, glutamine metabolism provides ATP through the TCA cycle to meet energetic and synthetic demands [49,50]. In addition to glutamine, glutaminase (GLS) also promotes osteogenic differentiation of BMSCs [51]. Deletion of GLS in BMSCs resulted in a reduction of overall osteoblast numbers and capability of bone formation [49]. Furthermore, Wang et al. found that glutamine metabolite ketoglutaric acid (αKG) promotes the osteogenic potential of BMSCs by decreasing the accumulations of histone methylations [52]. This evidence suggests that glutamine itself, glutaminase, and glutamine metabolites αKG can promote osteogenic differentiation of BMSCs. Targeting the glutamine metabolism process in BMSCs may provide a new clue for bone loss therapy.
2.3.1. Kynurenine
Kynurenine, a metabolite of tryptophan, has been thought to inhibit osteogenic differentiation of BMSCs [53]. With age, kynurenine accumulates in BMSCs and suppresses osteogenic differentiation by impairing autophagy, promoting early senescence, and altering cellular bioenergetics [53]. In vivo experiments using adult (6–8 months) mice injected intraperitoneally with kynurenine (10 mg/kg) for four weeks showed a reduction of osteogenic differentiation in BMSCs accompanied by restricted osteoblastic bioenergetics and energy production [54]. In vitro study also reported that hMSCs treated by kynurenine showed elevating oxidative stress and decreasing osteogenic differentiation [55]. Regarding the mechanism, kynurenine blunts BMSC differentiation by altering miRNAs levels [55]. For example, kynurenine-treated hMSCs upregulated miR-493-5b to block osteogenic potential and downregulated miR-210-3b to increase ROS level [53]. Taken together, kynurenine inhibits osteogenic differentiation of mouse BMSCs and hMSCs perhaps through altering miRNA levels (upregulated miR-493-5b, downregulated miR-210-3b) to increase ROS level.
3. Environmental stress, hormone drugs, and biological/pathological factors regulate BMSCs differentiation by altering energy metabolism
Emerging evidence has indicated that environmental stress, hormone drugs, and biological/pathological factors all impact BMSC differentiation by altering intercellular metabolism [18]. Here we focus on environmental stress such as extracellular matrix (ECM) stiffness, hypoxia, and microgravity and discuss hormone drugs like parathyroid hormone (PTH) and dexamethasone (Dex); in addition, biological factors including microRNAs, adiponectin, aging and pathological factors like obesity, diabetes mellitus, osteoporosis, and anorexia nervosa are introduced in this section (Table 2).
3.1. Environmental stress
3.1.1. ECM stiffness
ECM is involved in cellular metabolism via regulating glucose, lipid, and amino acid metabolism [56]. Recent in-depth studies have shown that extracellular matrix stiffness has been implicated in BMSC differentiation via regulating energy metabolism [57,58]. In vitro study has confirmed that cultured hMSCs on stiff (20 kPa) substrate showed enhanced osteogenesis accompanied by higher intracellular ATP levels and AMPK activation than on soft (1 kPa) substrate [57]. AMPK inhibitor treatment decreased ATP levels and osteogenesis marker ALP expression in hMSCs while AMPK activators can reverse ATP and ALP levels [57]. Other recent work has also proved that the HPAA (high-molecular-weight polyacrylic acid)-crosslinked collagen membranes (HCM) can self-mineralize and simulate extracellular matrix (ECM) stiffness, which expedites in situ bone regeneration to guide osteogenic differentiation of BMSCs [58,59]. Regarding the mechanism, HCM promotes mitochondriogenesis and declines mitochondria fission to respond to high energy demand during osteogenic differentiation of BMSCs [59]. This in vitro evidence indicates that high ECM stiffness may promote mitochondriogenesis, increase ATP, and decline mitochondria fission to enhance osteogenic differentiation of BMSCs. Whether another metabolic pathway contributes to this process is unknown, so additional in vitro and in vivo studies are needed.
3.1.2. Hypoxia
Studies from recent decades have shown that hypoxia, an important feature of the BMSC niche, plays an important role in maintaining BMSC differentiation [60]. Previous studies indicated that hypoxia suppressed adipogenesis and associated HIF1α and PPARγ expression in hMSCs and enhanced osteogenesis associated Runx2 expression [61,62]. A recent study found that shRNA-mediated knockdown of HIF1α in hMSCs suppressed osteogenesis through inhibition of pyruvate dehydrogenase (PDH) by PDH kinase (PDK) and activation of glycolytic enzymes [63]. Other studies have suggested that hypoxia (1%) signals shifts in metabolism from oxidative to glycolysis, while the change of metabolism inhibits both osteogenic [64] and adipogenic [65] differentiation of MSCs [16,66]. These results imply that hypoxia can regulate differentiation of hMSCs and mice MSCs by altering energy metabolism, but criteria should be put forward to standardize the experiment system, and reasonable care should be taken when performing a direct extrapolation of in vitro findings to in vivo situations.
3.1.3. Microgravity
Studies have shown that the culture of MSCs under microgravity (μg) affects their differentiation [67,68]. Exposure to simulated microgravity decreases the differentiation of BMSCs to osteoblasts which can pose negative effects on bone formation and bone volume [69]. Previous findings have reported that space flight also affects mitochondrial function and whole-body energy homeostasis; it also attenuates the expression of insulin-like growth factor 1 (IGF-1) [[70], [71], [72]] which alters osteoblast differentiation. In vitro evidence has shown that simulated microgravity (SMG) suppresses osteogenic differentiation of MSCs by inhibiting OXPHOS accompanied by decreased the expression of important energy sensor sirtuin 1 (Sirt1) [73]. Moreover, upregulating Sirt1 using resveratrol, an activator of Sirt1, recovered SMG-inhibited OXPHOS and osteogenic differentiation of MSCs [73]. Conducting in vivo studies, Dai et al. found that BMSCs derived from C57BL/6 mice after 28 days of hindlimb suspension exhibited downregulated Runx2 and upregulated PPARγ accompanied by upregulated lipid metabolism regulator leptin expression [74]. In sum, microgravity suppresses MSCs’ osteogenic differentiation by inhibiting OXPHOS and promoting adipogenic differentiation by upregulating leptin expression. Insights regarding the relationship between energy metabolism and MSC differentiation under microgravity could provide a novel therapeutic target for disuse-related bone loss.
3.2. Hormone drugs
3.2.1. Parathyroid hormone (PTH)
Parathyroid hormone (PTH) has proved an effective bone anabolic drug for over a decade. Previous evidence suggests that PTH promotes osteoblast lineage cell differentiation by stimulating aerobic glycolysis via IGF signaling [75]. A more recent study implied that PTH also promoted BMSC differentiation by regulating cellular metabolic status as an anabolic stimulus [76]. Tencerova et al. found PTH-treated murine cell lines of adipocyte progenitors BMSCsadipo exhibited reducing adipogenesis accompanied by impaired insulin signaling while enhancing glycolysis and the ATP production rate [77]. In addition, oligomycin (an inhibitor of oxidative phosphorylation) treatment significantly changed cellular bioenergetics of BMSCsadipo which were associated with decreased adipocytic differentiation [76]. Maridas et al. reported that PTH enhances effects on BMAT lipolysis and provides more fatty acids to fuel osteogenic differentiation of BMSCs [77]. Altogether, PTH inhibits adipogenic differentiation of BMSCs by impairing insulin signaling and enhancing glycolytic and ATP production, and PTH also enhances BMAT lipolysis and produces more fatty acids to fuel BMSCs osteogenesis.
3.2.2. Dexamethasone (Dex)
Dexamethasone (Dex) is a potent glucocorticoid drug. Frequently, Dex is used to enhance osteogenic, chondrogenic, and adipogenic differentiation of BMSCs [78]. Recent studies have shown how Dex-treated BMSCs exhibit compromised osteogenic differentiation and mitochondrial dysregulation [79,80]. Regarding the mechanism, Dex can impair mitochondrial function to induce ROS overproduction and disturb BMSC metabolism [79], thus causing compromised osteogenic differentiation of BMSCs [79]. In alignment with these findings is another study that demonstrated how Dex treatment down-regulated mitochondrial metabolism-related Sirt3 expression, induced high ROS levels and eventually impaired BMSC osteogenic differentiation in vitro [80]. Resveratrol treatment could ameliorate mitochondrial impairment and restore osteogenic capacity in Dex-exposed BMSCs by triggering the AMPK/PGC-1 α/Sirt 3 axis [80]. This evidence indicates that Dex inhibits mitochondrial metabolism-related Sirt3 and blunts the osteogenic differentiation of BMSCs.
3.3. Biological factors
3.3.1. microRNAs
Although there have many studies on microRNAs regulating the differentiation of BMSCs [[81], [82], [83], [84], [85]], the intrinsic mechanism still needs to be further explored. Recently, Hong et al. reported that miR-34a overexpression impaired osteogenic differentiation of hMSCs in vitro by inhibiting lactate dehydrogenase-A (LDHA), hexokinase 2 (HK2), and Glut1-mediated glycolysis [26]. Zheng et al. found that miR-181a/b can promote osteogenesis by enhancing mitochondrial metabolism [86]. Hundreds of microRNAs were also identified in MSCs mitochondria during osteogenic differentiation, and these microRNAs may impact MSC differentiation through gathering in mitochondria of MSCs to control mitochondrial respiration [87]. In addition to regulating glycolysis and mitochondrial function, microRNA-200a-3p suppresses osteogenic differentiation of MSCs by inhibiting GLS [88], and GLS overexpression reversed the inhibitory effects of overexpressed microRNA-200a-3p on osteogenic differentiation of MSCs [88]. These new findings reveal that microRNA can influence glycolysis, mitochondrial function, and glutamine metabolism to regulate osteogenic differentiation of MSCs isolated from mice and humans.
3.3.2. Adiponectin
Adiponectin is the most abundant circulating adipokine and is primarily involved in glucose metabolism and insulin resistance [89]. Within the bone, BMSC-derived bone marrow adipocytes continuously release adiponectin into the bone niche which soaks all cells, including osteoblast and osteoclast progenitors [90,91]. Moreover, both mRNA and protein expression of adiponectin receptors 1 and 2 (AdipoR1 and R2) are detectable in BMSCs [92], indicating adiponectin signaling participates in BMSC function regulation. Recently, adiponectin has been proven a critical regulator in BMSC differentiation [89]. Some in vitro studies have indicated that within the bone marrow niche, adiponectin is apt to promoteosteogenic differentiation [[92], [93], [94], [95], [96]]. Supplementation of full-length [92,96] or globular [97] adiponectin can increase the expression of the osteogenic-related genes ALP [92,96,97] and osteopontin [92,97] in C3H10T1/2 cells [92]. Silencing AdipoR1 by siRNA in C3H10T1/2 cells significantly reduced adiponectin-induced osteogenic differentiation in vitro [92]. In an in vivo study, 5-week-old adiponectin knockout mice exhibited decreased trabecular structure and mineralization and increased bone marrow adiposity [98]. Importantly, adiponectin inhibits the adipogenic differentiation of BMSCs via downregulated lysine-specific demethylases (lysine-specific demethylase 6B (KDM6B) and lysine-specific demethylase 4B (KDM4B)) [98]. Earlier studies confirmed that KDM4B and KDM6B increase osteogenic and reduce adipogenic differentiation [99] by removing methylated histone lysine residues and enabling dynamic and reversible regulation of transcription [99]. The absence of KDM4B and KDM6B reportedly increased the expression of PPARγ in hMSCs and thus switched the differentiation fate from osteogenic to adipogenic in vitro [99]. Indeed, the presence of fatty bone marrow in adiponectin knockout mice was attributed to reduced KDM4B and KDM6B expression in BMSCs, triggering adipogenesis and ultimately causing a reduction in osteoblasts and an increase in adipocytes on the trabecular surfaces [98]. In sum, these findings indicate that adiponectin perhaps promotes osteogenic differentiation of BMSCs by increasing AdipoR1 or lysine-specific demethylases (KDM6B and KDM4B) to inhibit PPARγ expression.
3.3.3. Aging
It has long been known that bone loss during aging is accompanied by increased bone marrow adiposity due to the shift of BMSC differentiation from osteoblasts to adipocytes [100]. Aged BMSCs exhibit diminished content and abnormal ultrastructure in mitochondrial, thus resulting in decreased oxygen consumption, ATP synthesis, NAD+ level, and increased ROS generation during osteogenic differentiation [101]. Therefore, replenishing these decreased metabolites can enhance BMSC osteogenesis and prevent skeletal degeneration, or benefit regenerative strategies. For example, diminishing levels of the key mitochondrial metabolite NAD+ is often seen in aged BMSCs [102], and decreasing NAD+ led to decreased activity of sirtuins (NAD+-dependent deacetylases), disrupted mitochondrial metabolism, and oxidative stress [102]. Accordingly, administration of nicotinamide mononucleotide, a key NAD+ intermediate, promoted BMSC expansion, enhanced osteogenesis, decreased adipogenesis and protected bone against aging in mice [103]. In addition to NAD+, PGC1α is another master regulator of mitochondrial biogenesis and oxidative metabolism to influence skeletal stem cells (SSCs) differentiation during aging [104]. PGC-1α decreased with aging in Prx1+ SSCs, and loss of PGC-1α promoted adipogenic differentiation at the expense of decreased osteogenic differentiation [104]. Mice with overexpression of PGC-1α in SSCs reversed the unbalance of SSC osteo-adipo differentiation during aging [104]. Moreover, Guan Min's group also found that citrate in bone microstructure is derived from the tricarboxylic acid cycle, which can increase the energy of biomolecular synthesis such as nucleic acid and protein required for BMSC differentiation [105]. They also discovered that citrate in bone matrix is lost during aging and that increasing citrate in aging BMSCs can improve the osteogenic differentiation ability [105]. Taken together, current evidence indicates that aging downregulate mitochondria metabolism related NAD+, PGC-1α level as well as citrate contents in bone matrix and then lead to inhibited osteogenic differentiation of BMSCs. Increasing NAD+, the PGC-1α level, and citrate can enhance BMSC osteogenesis and improve aged-related phenotype.
3.4. Pathological factors
3.4.1. Obesity and diabetes mellitus
MSCs are purported to play a vital role in obesity and diabetes mellitus and have received increasing attention as a new target for therapy. Rubin et al. reported that BMSC adipogenesis is one of the main reasons for obesity [106]. Recent data has confirmed that improving metabolic status may promote BMSC osteogenesis and ultimately alleviate obesity [18]. Dipeptidyl peptidase-4 (DPP4) was correlated with lipid accumulation [107] and can degrade circulating glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) to maintain glucose homeostasis [108,109]. Recently, Ambrosi et al. determined that DPP4 increased in multipotent CD45−CD31-Sca1+CD24+ cells in obesity mice, and inhibition DPP4 enhanced BMSC osteogenesis and improved obesity [110]. Additionally, over a decade, DPP4 inhibitors (DPP4is), commonly called gliptins, work as valuable oral antidiabetic drugs (OADs) for the treatment of type 2 diabetes mellitus (T2DM) by extending the half-life of native incretins [111,112]. Taken together, obesity and diabetes mellitus lead to abnormal glucose metabolism and lipid accumulation and ultimately trigger BMSCs adipogenic differentiation. Inhibiting DPP4 can accelerate glucose metabolism and lipolysis and promote BMSC osteogenic differentiation, which can be used in treating obesity and diabetes mellitus.
3.4.2. Osteoporosis
Impaired osteogenic differentiation capability of MSCs plays an important role during the pathogenesis of osteoporosis [113]. The hMSCs from osteoporotic patients exhibited lower osteogenic capacity and higher levels of palmitic acid in the plasma membrane [41]. Can we target MSCs metabolism to reverse their differentiation and treat osteoporosis? Glenske et al. have reported that testosterone inhibits hMSCs’ adipogenic differentiation of postmenopausal women by reducing the occurrence of lipid droplets thus improving osteoporosis [114]. Similarly, Bermeo et al. found that inhibition fatty acid synthase (FAS) [115] significantly increased bone formation and decreased marrow adipose in ovariectomized (OVX) treated C57BL/6 mice [116], suggesting FAS inhibition enhanced osteogenic and impaired adipogenic differentiation of BMSCs. Beyond fatty acid, Yu et al. have confirmed that compromised mitochondrial biogenesis and declined PGC1α level are related to OVA-induced osteoporosis [104]. Importantly, decreasing mitochondrial biogenesis and PGC1α levels impair Prx1+ SSC osteogenesis, and induction PGC1α expression in SSCs can improve OVA-induced osteoporosis [104]. Moreover, Guo et al. have reported that advanced glycation end products (AGEs) induce BMSCs adipogenesis and osteoporosis, which is accompanied by compromised mitochondrial dysfunction and decreased mitochondria metabolic regulatory enzyme Sirt3 expression [22]. Overexpression of Sirt3 significantly inhibited BMSC adipogenesis and osteoporosis in the senescent phenotype mouse model [22,117]. These in vivo and in vitro studies suggest that inhibition of FAS and increase of mitochondria Sirt3 in BMSCs can alleviate aberrant differentiation-related osteoporosis. However, more animal models are needed to explore the intrinsic mechanisms.
3.4.3. Anorexia nervosa
Anorexia nervosa (AN) patients are always accompanied by high bone marrow adiposity (BMA) and osteoporosis [[118], [119], [120]]. More recently, Louvet et al. have reported BMSCs from caloric-restricted mice (separation-based anorexia model (SBA)) present a down-regulation of the important energy sensor Sirt1, which is accompanied by an increase in adipogenesis at the expense of osteogenesis, and overexpression of Sirt1 by resveratrol leads to a decrease in adipogenesis and an increase in osteogenesis of BMSCs [121]. They also found that tibias from SBA mice displayed low levels of Sirt1 mRNA which are restored by resveratrol treatment. Similarly, this recovery of Sirt1 levels also returned the BMA, BV/TV, and Tb.Th in cultured tibias from SBA mice [121]. Mechanismly, chronic energy deficiency in female mice causes a decrease of Sirt1 expression in BMSCs, resulting in critical changes of Runx2 and Foxo1 acetylation levels and thus blunting BMSC differentiation capacity [121]. Additionally, Abdallah et al. have revealed that bone marrow adipocytes significantly inhibit the commitment of murine BMSCs into osteoblastic cell lineage by blocking BMPs signaling and activating inflammatory NF-κB signaling [122]. Mechanismly, bone marrow adipocytes may secrete adipokines to activate NF-κB pathway [122]. Previous evidence highlighted that adipokines like adiponectin, leptin, and adipose-derived estrogen were important for energy metabolism regulation in BMSC differentiation [123]. Taken together, this evidence suggests that increasing Sirt1 levels in BMSCs and inhibiting the excessive secretion of adipokines can recover BMSC osteogenic differentiation to ameliorate AN.
4. Unresolved questions, challenges, and potential opportunities
In recent years, a significant expansion in scope and investigation have been witnessed in the metabolic regulation of MSCs, driven by technological advances and a renewed interest in cellular metabolism. To date, the intrinsic mechanism about how MSCs use glucose, fatty acids, and amino acids during differentiation remains unclear, especially lacking in vivo validation. In addition, studies are exploring how environmental stress like ECM stiffness, hypoxia, microgravity, and other physical factors impact cellular energy metabolic status to MSC differentiation. This issue indeed needs further in vivo evidence for confirmation. In subsequent studies, genetic mice with conditional modification of encoding metabolism genes will be available and will be key tools in this effort. Moreover, new technological developments will facilitate important discoveries in BMSC metabolism during differentiation, while unraveling the metabolic difference among quiescent, proliferating, and differentiating BMSCs—as well as among BMSCs found in different locations—will be of interest to improve cell expansion for tissue engineering applications.
In exploring how energy metabolism influences MSC differentiation, we also met some certain challenges. First, it is difficult to mimic the BMSCs native niche in vitro; in fact, we observed that specific metabolic changes in vitro are not always recapitulated in vivo. Thus, studying BMSCs in their native microenvironment will be essential. Developing new animal models with intravital labeling BMSCs by specific metabolic markers may provide new insight for direct visual analysis of BMSC' metabolic changes. Another methodology that prove helpful to understanding of BMSC metabolism in vivo is the development of a device for the isolation of BMSCs while preserving their metabolic profiles, thereby permitting further analysis. Additionally, using material like hydrogels to develop new culture systems in vitro that mimic the BMSC’ native microenvironment, with physiological extracellular matrix stiffness, oxygen concentration, and suitable culture media, will prove more useful to retaining the metabolic characteristics of primary BMSCs.
Second, MSCs are heterogeneous. More advanced technologies need to be developed to mimic the complicated bone marrow niche environment and analyze dynamics change at single cells level. Particularly in the study of heterogeneity of BMSCs, single-cell sequencing provides new insights to advance understanding of the functional heterogeneity of BMSC subpopulations from RNA and protein levels. Recent evidence has revealed the heterogeneity of BMSCs under development and stress conditions: Sivaraj et al. have characterized the heterogeneity of BMSCs during skeletal development [124] and identified a subpopulation of metaphyseal MSCs (mpMSCs) that can generate bone cells and LepR+ marrow stromal cells subpopulations [124]. He et al. have revealed distinctive heterogeneity within human embryonic limb bud mesenchyme and epithelium and determined that perichondrial embryonic skeletal stem/progenitor cell (eSSPC) are marked by the adhesion molecule CADM1 and highly express FOXP1/2 transcriptional factors [125]. They also observed that among human embryonic limb bud mesenchyme subpopulations, only the subpopulation 2 exhibited increased ATP metabolic genes expression [125]. Sheng et al. have reported that peri-arteriolar LepR+ osteolectin+ BMSCs for osteogenesis and lymphopoiesis in the bone marrow are maintained by mechanical stimulation and depleted during aging [126]. CXCL12+ cell populations were sensitive to mechanical loading and upregulated BMP4 expression and osteogenesis [127]. Qin'group has found that peri-arteriolar marrow adipogenic lineage precursors (MALPs) express high myofibroblast genes (such as Myl9, Col9a1, Col10a1 et al.) to participate in bone marrow repair after radiation damage [128]. They also observed some metabolic genes related to fatty acid oxidation and fatty acid metabolic process were significantly upregulated in MALPs after radiation [128]. They did not discuss the metabolic change of the MALP subpopulation.
Studies about metabolic heterogeneity among BMSC subpopulations remain limited, but a recent paper from Joffin et al. [129] highlights the importance of mitochondrial metabolism in PDGFRβ+ adipocyte progenitor cell fate, especially in adipogenic progenitor cells (APC) and fibro-inflammatory precursors (FIP), which are two subpopulations of PDGFRβ+ adipocyte progenitor cells with distinctive metabolism in white adipose tissue [129]. They also highlighted that inhibiting mitochondrial activity blocked APCs adipogenesis and promoted expression of higher inflammatory factors in FIPs, which were restored by enhancing mitochondrial activity [129]. In addition to Joffin et al., Shao et al. have identified a HIF1α-dependent regulatory mechanism controlling adipocyte MSCs subpopulations (APCs and FIPs) function in mice and demonstrated the ability of the anti-cancer drug Imatinib to promote metabolically beneficial adipogenesis in obesity [130]. The findings of Joffin and Shao highlighted the importance of mitochondrial metabolism for maintaining function of adipose-tissue-resident PDGFRβ+ MSCs and how such processes were be disturbed during obesity [131]. In the future, identifying metabolic heterogeneity among BMSC subpopulations may be an effective approach to restoring BMSC aberrant differentiation and contribute to tissue engineering applications.
Finally, increasing evidence suggests important roles of the skeletal system as an endocrine organ. Nevertheless, the interplay between metabolism during BMSC differentiation and whole-body metabolism remains largely unexplored. Better understanding about how metabolic heterogeneity among BMSC subpopulations affects health, including conditions like obesity and diabetes, and whether pharmacological targeting of the dysregulated metabolic pathways in specific BMSC subpopulations can restore their function will be of considerable interest.
Funding
This research was sponsored by the National Natural Science Foundation of China (81772409), the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (CX2021030), and the Space Medical Experiment Project of China Manned Space Program (HYZHXM01024). Special thanks to Yi Lyu and Zhouqi Yang (NPU) for their assistance.
Conflict of interest
The authors do not disclose any conflicts of interest with respect to this manuscript.
References
- 1.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell & Tissue Kinetics. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein A.J., Chailakhyan R.K., Latsinik N.V., Panasyuk A.F., Keiliss-Borok I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida C.A., Furuichi T., Fujita T., Fukuyama R., Kanatani N., Kobayashi S., et al. Core-binding factor β interacts with Runx2 and is required for skeletal development. Nature Genetics. 2002;32:633–638. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- 4.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., et al. The novel Zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang H., Zhang X., Zhu C., Tang X., Yu F., Shang G.W., et al. Molecular mechanisms of PPAR- γ governing MSC osteogenic and adipogenic differentiation. Current Stem Cell Research and Therapy. 2016;11:255–264. doi: 10.2174/1574888x10666150531173309. [DOI] [PubMed] [Google Scholar]
- 7.Okitsu Y., Takahashi S., Minegishi N., Kameoka J., Kaku M., Yamamoto M., et al. Regulation of adipocyte differentiation of bone marrow stromal cells by transcription factor GATA-2. Biochemical and Biophysical Research Communications. 2007;364:383–387. doi: 10.1016/j.bbrc.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Fujimori K., Amano F. Forkhead transcription factor Foxa1 is a novel target gene of C/EBP β and suppresses the early phase of adipogenesis. Gene. 2011;473:150–156. doi: 10.1016/j.gene.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Ren G.W., Zhang L.Y., Zhao X., Xu G.W., Zhang Y.Y., Roberts A.I., et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. 2006. [DOI] [PubMed] [Google Scholar]
- 12.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Ge C., Yang Q., Zhao G., Yu H., Kirkwood K.L., Franceschi R.T. Interactions between extracellular signal-regulated kinase 1/2 and p38 MAP kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity. Journal of Bone and Mineral Research. 2012;27:538–551. doi: 10.1002/jbmr.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taleb S., Cancello R., Clement K., Lacasa D. Cathepsin S promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147:4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 15.Sul H.S. Minireview:Pref-1:role in adipogenesis and mesenchymal cell fate. Molecular Endocrinology. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q., Shou P., Zheng C., Jiang M., Cao G., Yang Q., et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death & Differentiation. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Confavreux C.B., Levine R.L., Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Molecular and Cellular Endocrinology. 2009;30(1–2):21–29. doi: 10.1016/j.mce.2009.04.004. 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gastel N., Carmeliet G. Metabolic regulation of skeletal cell fate and function in physiology and disease. Nature Metabolism. 2021;3(1):11–20. doi: 10.1038/s42255-020-00321-3. [DOI] [PubMed] [Google Scholar]
- 19.Rendina-Ruedy E., Rosen C.J. Lipids in the bone marrow: an evolving perspective. Cell Metabolism. 2020;31(2):219–231. doi: 10.1016/j.cmet.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita K., Dzau V.J. Mesenchymal stem cells in obesity: insights for translational applications. Laboratory Investigation. 2017;97(10):1158–1166. doi: 10.1038/labinvest.2017.42. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Ma T. Metabolic regulation of mesenchymal stem cell in expansion and therapeutic application. Biotechnology Progress. 2015;31(2):468–481. doi: 10.1002/btpr.2034. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y., Jia X., Cui Y., Song Y., Wang S., Geng Y., et al. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biology. 2021;41:101915. doi: 10.1016/j.redox.2021.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borle A.B., Nichols N., Nichols G., Jr. Metabolic studies of bone in vitro. I. Normal bone. Journal of Biological Chemistry. 1960;235:1206–1210. [PubMed] [Google Scholar]
- 24.Cohn D.V., Forscher B.K. Aerobic metabolism of glucose by bone. Journal of Biological Chemistry. 1962;237:615–618. [PubMed] [Google Scholar]
- 25.Peck W.A., Birge S.J., Jr., Fedak S.A. Bone cells: biochemical and biological studies after enzymatic isolation. Science. 1964;146(3650):1476–1477. doi: 10.1126/science.146.3650.1476. [DOI] [PubMed] [Google Scholar]
- 26.Hong M., Zhang X.B., Xiang F., Fei X., Ouyang X.L., Peng X.C. MiR-34a suppresses osteoblast differentiation through glycolysis inhibition by targeting lactate dehydrogenase-A (LDHA). In Vitro Cellular Developmental Biology. Animal. 2020;56(6):480–487. doi: 10.1007/s11626-020-00467-0. [DOI] [PubMed] [Google Scholar]
- 27.Wei J., Shimazu J., Makinistoglu M.P., Maurizi A., Kajimura D., Zong H., et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;18(7):1576–1591. doi: 10.1016/j.cell.2015.05.029. 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Liao J., Fang C., Mo C., Zhou G., Luo Y. One-step derivation of functional mesenchymal stem cells from human pluripotent stem cells. Bio-Protocol. 2018;20(22) doi: 10.21769/BioProtoc.3080. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.Y., Long F. Notch signaling suppresses glucose metabolism in mesenchymal progenitors to restrict osteoblast differentiation. Journal of Clinical Investigation. 2018;128(12):5573–5586. doi: 10.1172/JCI96221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Z., Kho J., Dawson B., Jiang M.M., Chen Y., Ali S., et al. Nitric oxide modulates bone anabolism through regulation of osteoblast glycolysis and differentiation. Journal of Clinical Investigation. 2021;131(5) doi: 10.1172/JCI138935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Jiang G., Zhao X., Gong Y. Dimethyloxalylglycine promotes bone marrow mesenchymal stem cell osteogenesis via Rho/ROCK signaling. Cellular Physiology and Biochemistry. 2016;39(4):1391–1403. doi: 10.1159/000447843. [DOI] [PubMed] [Google Scholar]
- 32.Li Q., Gao Z., Chen Y., Guan M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein & Cell. 2017;8(6):439–445. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C.T., Shih Y.R., Kuo T.K., Lee O.K., Wei Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 34.Shum L.C., White N.S., Mills B.N., Bentley K.L., Eliseev R.A. Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells and Development. 2016;25(2):114–122. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shares B.H., Busch M., White N., Shum L., Eliseev R.A. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. Journal of Biological Chemistry. 2018;293(41):16019–16027. doi: 10.1074/jbc.RA118.004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pattappa G., Heywood H.K., de Bruijn J.D., Lee D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. Journal of Cellular Physiology. 2011;226(10):2562–2570. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- 37.Esen E., Chen J., Karner C.M., Okunade A.L., Patterson B.W., Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metabolism. 2013;17(5):745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofmann A.D., Beyer M., Krause-Buchholz U., Wobus M., Bornhäuser M., Rödel G. OXPHOS supercomplexes as a hallmark of the mitochondrial phenotype of adipogenic differentiated human MSCs. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamek G., Felix R., Guenther H.L., Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. Biochemical Journal. 1987;248(1):129–137. doi: 10.1042/bj2480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Liu X., Zuo B., Zhang L. The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging and Disease. 2015;7(4):514–525. doi: 10.14336/AD.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaepe K., Werner J., Glenske K., Bartges T., Henss A., Rohnke M., et al. ToF-SIMS study of differentiation of human bone-derived stromal cells: new insights into osteoporosis. Analytical and Bioanalytical Chemistry. 2017;409(18):4425–4435. doi: 10.1007/s00216-017-0386-7. [DOI] [PubMed] [Google Scholar]
- 42.Glenske K., Schäpe K., Wieck A., Failing K., Werner J., Rohnke M., et al. Effect of long term palmitate treatment on osteogenic differentiation of human mesenchymal stromal cells - impact of albumin. BoneKEy Reports. 2020;13:100707. doi: 10.1016/j.bonr.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palomer X., Pizarro-Delgado J., Barroso E., Vázquez-Carrera M. Palmitic and oleic acid: the Yin and Yang of fatty acids in type 2 diabetes mellitus. Trends in Endocrinology and Metabolism. 2018;29(3):178–190. doi: 10.1016/j.tem.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Casado-Díaz A., Dorado G., Quesada-Gómez J.M. Influence of olive oil and its components on mesenchymal stem cell biology. World Journal of Stem Cells. 2019;11(12):1045–1064. doi: 10.4252/wjsc.v11.i12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gastel N., Stegen S., Eelen G., Schoors S., Carlier A., Daniëls V.W., et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020;579(7797):111–117. doi: 10.1038/s41586-020-2050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao B., Huang Q., Jie Q., Lu W.G., Wang L., Li X.J., et al. GPR120: a bi-potential mediator to modulate the osteogenic and adipogenic differentiation of BMMSCs. Scientific Reports. 2015;14(5):14080. doi: 10.1038/srep14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10(11):1564. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biltz R.M., Letteri J.M., Pellegrino E.D., Palekar A., Pinkus L.M. Glutamine metabolism in bone. Mineral and Electrolyte Metabolism. 1983;9(3):125–131. [PubMed] [Google Scholar]
- 49.Yu Y., Newman H., Shen L., Sharma D., Hu G., Mirando A.J., et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metabolism. 2019;29(4):966–978.e4. doi: 10.1016/j.cmet.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou T., Yang Y., Chen Q., Xie L. Glutamine metabolism is essential for stemness of bone marrow mesenchymal stem cells and bone homeostasis. Stem Cells International. 2019;2019:8928934. doi: 10.1155/2019/8928934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skerry T.M. The role of glutamate in the regulation of bone mass and architecture. Journal of Musculoskeletal and Neuronal Interactions. 2008;8(2):166–173. [PubMed] [Google Scholar]
- 52.Wang Y., Deng P., Liu Y., Wu Y., Chen Y., Guo Y., et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nature Communications. 2020;11(1):5596. doi: 10.1038/s41467-020-19360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel D., Potter M., Anaya J.M., McGee-Lawrence M.E., Hamrick M.W., Hill W.D., et al. Kynurenine induces an age-related phenotype in bone marrow stromal cells. Mechanism of Ageing and Development. 2021;195:111464. doi: 10.1016/j.mad.2021.111464. [DOI] [PubMed] [Google Scholar]
- 54.Refaey M.E., McGee-Lawrence M.E., Fulzele S., Kennedy E.J., Bollag W.B., Elsalanty M., et al. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. Journal of Bone and Mineral Research. 2017;32(11):2182–2193. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalton S., Smith K., Singh K., Kaiser H., Kolhe R., Mondal A.K., et al. Accumulation of kynurenine elevates oxidative stress and alters microRNA profile in human bone marrow stromal cells. Experimental Gerontology. 2020;130:110800. doi: 10.1016/j.exger.2019.110800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge H., Tian M., Pei Q., Tan F., Pei H. Extracellular matrix stiffness: new areas affecting cell metabolism. Frontiers in Oncology. 2021;11:631991. doi: 10.3389/fonc.2021.631991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie J., Bao M., Hu X., Koopman W.J.H., Huck W.T.S. Energy expenditure during cell spreading influences the cellular response to matrix stiffness. Biomaterials. 2021;267:120494. doi: 10.1016/j.biomaterials.2020.120494. [DOI] [PubMed] [Google Scholar]
- 58.Li J., Yan J.F., Wan Q.Q., Shen M.J., Ma Y.X., Gu J.T., et al. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomaterialia. 2021;125:112–125. doi: 10.1016/j.actbio.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Wan M.C., Tang X.Y., Li J., Gao P., Wang F., Shen M.J., et al. Upregulation of mitochondrial dynamics is responsible for osteogenic differentiation of mesenchymal stem cells cultured on self-mineralized collagen membranes. Acta Biomaterialia. 2021;136:137–146. doi: 10.1016/j.actbio.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 60.Chen W., Zhuo Y., Duan D., Lu M. Effects of hypoxia on differentiation of mesenchymal stem cells. Current Stem Cell Research and Therapy. 2020;15(4):332–339. doi: 10.2174/1574888X14666190823144928. [DOI] [PubMed] [Google Scholar]
- 61.Wagegg M., Gaber T., Lohanatha F.L., Hahne M., Strehl C., Fangradt M., et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Marsboom G., Toth P.T., Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beegle J., Lakatos K., Kalomoiris S., Stewart H., Isseroff R.R., Nolta J.A., et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33(6):1818–1828. doi: 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu S.H., Chen C.T., Wei Y.H. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2013;31(12):2779–2788. doi: 10.1002/stem.1441. [DOI] [PubMed] [Google Scholar]
- 65.Jorgensen C., Khoury M. Musculoskeletal progenitor/stromal cell-derived mitochondria modulate cell differentiation and therapeutical function. Frontiers in Immunology. 2021;12:606781. doi: 10.3389/fimmu.2021.606781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papandreou I., Cairns R.A., Fontana L., Lim A.L., Denko N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metabolism. 2006;3(3):187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 67.Grimm D., Wehland M., Corydon T.J., Richter P., Prasad B., Bauer J., et al. The effects of microgravity on differentiation and cell growth in stem cells and cancer stem cells. Stem Cells Translational Medicine. 2020;9(8):882–894. doi: 10.1002/sctm.20-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue L., Li Y., Chen J. Duration of simulated microgravity affects the differentiation of mesenchymal stem cells. Molecular Medicine Reports. 2017;15(5):3011–3018. doi: 10.3892/mmr.2017.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basso N., Jia Y., Bellows C.G., Heersche J.N. The effect of reloading on bone volume, osteoblast number, and osteoprogenitor characteristics: studies in hind limb unloaded rats. Bone. 2005;37(3):370–378. doi: 10.1016/j.bone.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 70.Dai Z.Q., Wang R., Ling S.K., Wan Y.M., Li Y.H. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Proliferation. 2007;40(5):671–684. doi: 10.1111/j.1365-2184.2007.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gershovich J.G., Buravkova L.B. Morphofunctional status and osteogenic differentiation potential of human mesenchymal stromal precursor cells during in vitro modeling of microgravity effects. Bulletin of Experimental Biology and Medicine. 2007;144(4):608–613. doi: 10.1007/s10517-007-0387-1. [DOI] [PubMed] [Google Scholar]
- 72.Merzlikina N.V., Buravkova L.B., Romanov Y.A. The primary effects of clinorotation on cultured human mesenchymal stem cells. Journal of Gravitational Physiology. 2004;11(2):P193–P194. [PubMed] [Google Scholar]
- 73.Liu L., Cheng Y., Wang J., Ding Z., Halim A., Luo Q., et al. Simulated microgravity suppresses osteogenic differentiation of mesenchymal stem cells by inhibiting oxidative phosphorylation. International Journal of Molecular Sciences. 2020;21(24):9747. doi: 10.3390/ijms21249747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai S., Kong F., Liu C., Xiao F., Dong X., Zhang Y., et al. Effect of simulated microgravity conditions of hindlimb unloading on mice hematopoietic and mesenchymal stromal cells. Cell Biology International. 2020;44(11):2243–2252. doi: 10.1002/cbin.11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Esen E., Lee S.Y., Wice B.M., Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. Journal of Bone and Mineral Research. 2015;30(11):1959–1968. doi: 10.1002/jbmr.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tencerova M., Rendina-Ruedy E., Neess D., Færgeman N., Figeac F., Ali D., et al. Metabolic programming determines the lineage-differentiation fate of murine bone marrow stromal progenitor cells. Bone Research. 2019;14(7):35. doi: 10.1038/s41413-019-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maridas D.E., Rendina-Ruedy E., Helderman R.C., DeMambro V.E., Brooks D., Guntur A.R., et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. Federation of American Societies for Experimental Biology Journal. 2019;33(2):2885–2898. doi: 10.1096/fj.201800948RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H., Pang B., Li Y., Zhu D., Pang T., Liu Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy. 2012;14(4):423–430. doi: 10.3109/14653249.2011.652735. [DOI] [PubMed] [Google Scholar]
- 79.Lv Y.J., Yang Y., Sui B.D., Hu C.H., Zhao P., Liao L., et al. Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics. 2018;8(9):2387–2406. doi: 10.7150/thno.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L., Wang B.Z., Xie J., Zhang R.Y., Jin C., Chen W.K., et al. Therapeutic effect of SIRT3 on glucocorticoid-induced osteonecrosis of the femoral head via intracellular oxidative suppression. Free Radical Biology and Medicine. 2021;176:228–240. doi: 10.1016/j.freeradbiomed.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Jeong B.C., Kang I.H., Hwang Y.C., Kim S.H., Koh J.T. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death & Disease. 2014;5 doi: 10.1038/cddis.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J., Guan X., Guo F., Zhou J., Chang A., Sun B., et al. miR-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death & Disease. 2013;4:e845. doi: 10.1038/cddis.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamam D., Ali D., Kassem M., Aldahmash A., Alajez N.M. microRNAs as regulators of adipogenic differentiation of mesenchymal stem cells. Stem Cells and Development. 2015;24:417–425. doi: 10.1089/scd.2014.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su X., Liao L., Shuai Y., Jing H., Liu S., Zhou H., et al. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death & Disease. 2015;6 doi: 10.1038/cddis.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding W., Li J., Singh J., Alif R., Vazquez-Padron R.I., Gomes S.A., et al. miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE-/- mice. Cardiovascular Research. 2015;106:131–142. doi: 10.1093/cvr/cvv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng H., Liu J., Tycksen E., Nunley R., McAlinden A. MicroRNA-181a/b-1 over-expression enhances osteogenesis by modulating PTEN/PI3K/AKT signaling and mitochondrial metabolism. Bone. 2019;123:92–102. doi: 10.1016/j.bone.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng H., Liu J., Yu J., McAlinden A. Expression profiling of mitochondria-associated microRNAs during osteogenic differentiation of human MSCs. Bone. 2021;151:116058. doi: 10.1016/j.bone.2021.116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lv R., Pan X., Song L., Sun Q., Guo C., Zou S., et al. MicroRNA-200a-3p accelerates the progression of osteoporosis by targeting glutaminase to inhibit osteogenic differentiation of bone marrow mesenchymal stem cells. Biomedicine & Pharmacotherapy. 2019;116:108960. doi: 10.1016/j.biopha.2019.108960. [DOI] [PubMed] [Google Scholar]
- 89.Lewis J.W., Edwards J.R., Naylor A.J., McGettrick H.M. Adiponectin signalling in bone homeostasis, with age and in disease. Bone Research. 2021;9(1):1. doi: 10.1038/s41413-020-00122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fazeli P.K., Horowitz M.C., MacDougald O.A., Scheller E.L., Rodeheffer M.S., Rosen C.J., et al. Marrow fat and bone--new perspectives. Journal of Clinical Endocrinology & Metabolism. 2013;98(3):935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DiMascio L., Voermans C., Uqoezwa M., Duncan A., Lu D., Wu J., et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. The Journal of Immunology. 2007;178(6):3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- 92.Lee H.W., Kim S.Y., Kim A.Y., Lee E.J., Choi J.Y., Kim J.B. Adiponectin stimulates osteoblast differentiation through induction of COX2 in mesenchymal progenitor cells. Stem Cells. 2009;27(9):2254–2262. doi: 10.1002/stem.144. [DOI] [PubMed] [Google Scholar]
- 93.Wu X., Huang L., Liu J. Effects of adiponectin on osteoclastogenesis from mouse bone marrow-derived monocytes. Experimental and Therapeutic Medicine. 2019;17(2):1228–1233. doi: 10.3892/etm.2018.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Y.Y., Chen C.Y., Chuang T.Y., Lin Y., Liu H.Y., Mersmann H.J., et al. Adiponectin receptor 1 regulates bone formation and osteoblast differentiation by GSK-3β/β-catenin signaling in mice. Bone. 2014;64:147–154. doi: 10.1016/j.bone.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 95.Kajimura D., Lee H.W., Riley K.J., Arteaga-Solis E., Ferron M., Zhou B., et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metabolism. 2013;17(6):901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oshima K., Nampei A., Matsuda M., Iwaki M., Fukuhara A., Hashimoto J., et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochemical and Biophysical Research Communications. 2005;331(2):520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 97.Chen T., Wu Y.W., Lu H., Guo Y., Tang Z.H. Adiponectin enhances osteogenic differentiation in human adipose-derived stem cells by activating the APPL1-AMPK signaling pathway. Biochemical and Biophysical Research Communications. 2015;461(2):237–242. doi: 10.1016/j.bbrc.2015.03.168. [DOI] [PubMed] [Google Scholar]
- 98.Wu Y., Tu Q., Valverde P., Zhang J., Murray D., Dong L.Q., et al. Central adiponectin administration reveals new regulatory mechanisms of bone metabolism in mice. American Journal of Physiology. Endocrinology and Metabolism. 2014;306(12):E1418–E1430. doi: 10.1152/ajpendo.00048.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ye L., Fan Z., Yu B., Chang J., Al Hezaimi K., Zhou X., et al. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11(1):50–61. doi: 10.1016/j.stem.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grote C., Reinhardt D., Zhang M., Wang J. Regulatory mechanisms and clinical manifestations of musculoskeletal aging. Journal of Orthopaedic Research. 2019;37(7):1475–1488. doi: 10.1002/jor.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bellantuono I., Aldahmash A., Kassem M. Aging of marrow stromal (skeletal) stem cells and their contribution to age-related bone loss. Biochimica et Biophysica Acta. 2009;1792(4):364–370. doi: 10.1016/j.bbadis.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Neri S., Borzì R.M. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules. 2020;10(2):340. doi: 10.3390/biom10020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song J., Li J., Yang F., Ning G., Zhen L., Wu L., et al. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death & Disease. 2019;10(5):336. doi: 10.1038/s41419-019-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.[Yu B., Huo L., Liu Y., Deng P., Szymanski J., Li J., et al. PGC-1α controls skeletal stem cell fate and bone-fat balance in osteoporosis and skeletal aging by inducing TAZ. Cell Stem Cell. 2018;23(2):193–209.e5. doi: 10.1016/j.stem.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fu X., Li Y., Huang T., Yu Z., Ma K., Yang M., et al. Runx2/Osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Advanced Science. 2018;5(4):1700755. doi: 10.1002/advs.201700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubin C.T., Capilla E., Luu Y.K., Busa B., Crawford H., Nolan D.J., et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rufinatscha K., Radlinger B., Dobner J., Folie S., Bon C., Profanter E., et al. Dipeptidyl peptidase-4 impairs insulin signaling and promotes lipid accumulation in hepatocytes. Biochemical and Biophysical Research Communications. 2017;485(2):366–371. doi: 10.1016/j.bbrc.2017.02.071. [DOI] [PubMed] [Google Scholar]
- 108.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 109.Ahrén B., Hughes T.E. Inhibition of dipeptidyl peptidase-4 augments insulin secretion in response to exogenously administered glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pituitary adenylate cyclase-activating polypeptide, and gastrin-releasing peptide in mice. Endocrinology. 2005;146(4):2055–2059. doi: 10.1210/en.2004-1174. [DOI] [PubMed] [Google Scholar]
- 110.Ambrosi T.H., Scialdone A., Graja A., Gohlke S., Jank A.M., Bocian C., et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–784.e6. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marguet D., Baggio L., Kobayashi T., Bernard A.M., Pierres M., Nielsen P.F., et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mari A., Sallas W.M., He Y.L., Watson C., Ligueros-Saylan M., Dunning B.E., et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2005;90(8):4888–4894. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- 113.Jiang Y., Zhang P., Zhang X., Lv L., Zhou Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Proliferation. 2021;54(1) doi: 10.1111/cpr.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Glenske K., Schuler G., Arnhold S., Elashry M.I., Wagner A.S., Barbeck M., et al. Effects of testosterone and 17β-estradiol on osteogenic and adipogenic differentiation capacity of human bone-derived mesenchymal stromal cells of postmenopausal women. BoneKEy Reports. 2019;11:100226. doi: 10.1016/j.bonr.2019.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriological Reviews. 1976;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bermeo S., Al Saedi A., Vidal C., Khalil M., Pang M., Troen B.R., et al. Treatment with an inhibitor of fatty acid synthase attenuates bone loss in ovariectomized mice. Bone. 2019;122:114–122. doi: 10.1016/j.bone.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 117.Shimizu M., Higuchi K., Bennett B., Xia C., Tsuboyama T., Kasai S., et al. Identification of peak bone mass QTL in a spontaneously osteoporotic mouse strain. Mammalian Genome. 1999;10(2):81–87. doi: 10.1007/s003359900949. [DOI] [PubMed] [Google Scholar]
- 118.Bredella M.A., Fazeli P.K., Miller K.K., Misra M., Torriani M., Thomas B.J., et al. Increased bone marrow fat in anorexia nervosa. Journal of Clinical Endocrinology & Metabolism. 2009;94(6):2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ecklund K., Vajapeyam S., Feldman H.A., Buzney C.D., Mulkern R.V., Kleinman P.K., et al. Bone marrow changes in adolescent girls with anorexia nervosa. Journal of Bone and Mineral Research. 2010;25(2):298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Badr S., Legroux-Gérot I., Vignau J., Chauveau C., Ruschke S., Karampinos D.C., et al. Comparison of regional bone marrow adiposity characteristics at the hip of underweight and weight-recovered women with anorexia nervosa using magnetic resonance spectroscopy. Bone. 2019;127:135–145. doi: 10.1016/j.bone.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 121.Louvet L., Leterme D., Delplace S., Miellot F., Marchandise P., Gauthier V., et al. Sirtuin 1 deficiency decreases bone mass and increases bone marrow adiposity in a mouse model of chronic energy deficiency. Bone. 2020;136:115361. doi: 10.1016/j.bone.2020.115361. [DOI] [PubMed] [Google Scholar]
- 122.Abdallah B.M. Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling. Journal of Biomedical Science. 2017;24(1):11. doi: 10.1186/s12929-017-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sadie-Van Gijsen H., Crowther N.J., Hough F.S., Ferris W.F. The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cellular and Molecular Life Sciences. 2013;70(13):2331–2349. doi: 10.1007/s00018-012-1211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sivaraj K.K., Jeong H.W., Dharmalingam B., Zeuschner D., Adams S., Potente M., et al. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Reports. 2021;36(2):109352. doi: 10.1016/j.celrep.2021.109352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He J., Yan J., Wang J., Zhao L., Xin Q., Zeng Y., et al. Dissecting human embryonic skeletal stem cell ontogeny by single-cell transcriptomic and functional analyses. Cell Research. 2021;31(7):742–757. doi: 10.1038/s41422-021-00467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen B., Tasdogan A., Ubellacker J.M., Zhang J., Nosyreva E.D., Du L., et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591(7850):438–444. doi: 10.1038/s41586-021-03298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takada I., Kouzmenko A.P., Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nature Reviews Rheumatology. 2009;5(8):442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 128.Zhong L., Yao L., Tower R.J., Wei Y., Miao Z., Park J., et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife. 2020;9 doi: 10.7554/eLife.54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Joffin N., Paschoal V.A., Gliniak C.M., Crewe C., Elnwasany A., Szweda L.I., et al. Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell. 2021;28(4):702–717.e8. doi: 10.1016/j.stem.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shao M., Hepler C., Zhang Q., Shan B., Vishvanath L., Henry G.H., et al. Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell. 2021;28(4):685–701.e7. doi: 10.1016/j.stem.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kajimura S. Bioenergetics matter to metabolic health-from a fat progenitor view. Cell Stem Cell. 2021;28(4):589–591. doi: 10.1016/j.stem.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]