Abstract
Before 2016, human papillomavirus (HPV) vaccination was recommended on a 3-dose schedule. However, many vaccine-eligible US females received fewer than 3 doses, which provided an opportunity to evaluate the real-world vaccine effectiveness (VE) of 1, 2, and 3 doses. We analyzed data on cervical intraepithelial neoplasia (CIN) grades 2–3 and adenocarcinoma in situ (designated CIN2+) from the HPV Vaccine Impact Monitoring Project (HPV-IMPACT; 2008–2014). Archived tissue from CIN2+ lesions was tested for 37 types of HPV. Women were classified by number of doses received ≥24 months before CIN2+ detection. Using a test-negative design, VE was estimated as 1 minus the adjusted odds ratio from a logistic regression model that compared vaccination history for women whose lesions tested positive for HPV-16/18 (vaccine-type cases) with that for women who had all other CIN2+ lesions (controls). Among 3,300 women with available data on CIN2+, typing results, and vaccine history, 1,561 (47%) were HPV-16/18–positive, 136 (4%) received 1 dose of HPV vaccine, 108 (3%) received 2 doses, and 325 (10%) received 3 doses. Adjusted odds ratios for vaccination with 1, 2, and 3 doses were 0.53 (95% confidence interval (CI): 0.37, 0.76; VE = 47%), 0.45 (95% CI: 0.30, 0.69; VE = 55%), and 0.26 (95% CI: 0.20, 0.35; VE = 74%), respectively. We found significant VE against vaccine-type CIN2+ after 3 doses of HPV vaccine and lower but significant VE with 1 or 2 doses.
Keywords: case-control studies, cervical intraepithelial neoplasia, early detection of cancer, human papillomavirus 16, human papillomavirus 18, papillomavirus infections, uterine cervical neoplasms, vaccines
Oncogenic types of human papillomavirus (HPV) cause most cervical cancers, with HPV-16 and −18 being implicated in approximately 70% (1, 2). Clinical trials have demonstrated that vaccines targeting HPV-16 and −18 have high efficacy in preventing precancerous cervical lesions associated with HPV-16 or −18 among women with no evidence of infection with those types of HPV (3). Three vaccines targeting HPV-16 and −18 have been licensed in the United States. Since 2006, HPV vaccination has been recommended for females aged 11–12 years, and through age 26 years for those not previously vaccinated (4). Through 2015, nearly all doses of HPV vaccine administered in the United States were quadrivalent vaccine (5).
Administration of HPV vaccine was initially recommended on a 3-dose schedule: an initial dose followed by additional doses at 1–2 months and 6 months (4). HPV vaccine coverage in the United States has gradually increased, but it has lagged behind that of other vaccines recommended for the same age groups; in 2017, 69% of adolescent females aged 13–17 years received at least 1 dose and 49% received all recommended doses (6). Interest in reduced dose schedules, which would simplify vaccination programs worldwide and would likely increase series completion, prompted additional clinical trials. In 2016, the US Advisory Committee for Immunization Practices recommended a 2-dose schedule, with the initial dose followed by a second at 6–12 months for persons who initiate the series before their 15th birthday. This recommendation was informed by noninferior immunogenicity data from trials comparing a 2-dose schedule in adolescents with a 3-dose schedule in females aged 16–26 years in whom clinical efficacy was previously established (7). Accumulating data suggest there might be high effectiveness against vaccine-type infection with a single dose; post hoc analyses of data from clinical trials suggest that efficacy against infection is similar for 1 dose and 3 doses (8–10). Several trials designed to evaluate 1-dose vaccination schedules have begun, including trials being carried out in Costa Rica and Tanzania (8, 11).
Evaluating outcomes in women who received fewer than the recommended number of doses of HPV vaccine can provide evidence on the real-world effectiveness of 1 and 2 doses. Several studies of vaccine effectiveness (VE) have shown effectiveness against prevalent HPV infections (i.e., detection of HPV DNA in cervical or vaginal swab specimens), anogenital warts, abnormal cervical cytology, and cervical lesions; in a 2018 review, Markowitz et al. (9) highlighted methodological challenges, including systematic differences between women according to number of doses received, and the inability to control for prevalent infections at baseline. Ten studies have evaluated the effectiveness of 1, 2, and 3 doses of HPV vaccine against cervical disease without consideration of HPV type, but no studies have reported on the VE of 1 or 2 doses against vaccine-type high-grade cervical lesions (12–21).
Active population-based surveillance for cervical intraepithelial neoplasia (CIN) grades 2 and 3 and adenocarcinoma in situ, collectively referred to as CIN2+, has been conducted in 5 communities in the United States since 2008. As part of this surveillance, archived diagnostic blocks of CIN2+ are used for HPV detection and typing. These surveillance data have been used to estimate VE against vaccine-type cervical lesions (22). Our aim in this analysis was to estimate VE against vaccine-type CIN2+ by number of vaccine doses.
METHODS
The Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT), a US cervical precancer surveillance effort led by the Centers for Disease Control and Prevention, has been described previously (22–24). Ongoing population-based surveillance has been conducted for histologically confirmed CIN2+ among female residents aged ≥18 years of 5 catchment areas in 5 states since 2008, with a combined adult female population of approximately 1.5 million. Surveillance sites include Monroe County, New York; New Haven County, Connecticut; Davidson County, Tennessee; and portions of Alameda County, California, and Multnomah and Washington counties, Oregon. This public health surveillance activity was exempted from institutional review board review at the Centers for Disease Control and Prevention and most surveillance sites. Institutional review board approval was obtained from 1 surveillance site as required (24). Briefly, histopathology laboratories reported CIN2+ diagnoses occurring among female residents of the catchment areas to project staff at 5 surveillance sites. For each case, the incidence date was defined as the date of the earliest CIN2+ diagnosis. The final diagnosis for each case was defined as the highest grade of CIN2+ identified within 6 months after the incidence date.
Archived diagnostic tissue from the subset of women aged 18–39 years was submitted to the Centers for Disease Control and Prevention’s HPV laboratory for HPV DNA typing, as previously described (22). Briefly, serial sections were obtained from 1 specimen, and after confirmation of the fact that tissue representative of a high-grade lesion was present, DNA was extracted and tested using the Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, Indiana), which detects 37 types of HPV. Specimens with inadequate or HPV-negative Linear Array results were retested with the INNO-LiPA HPV Genotyping Extra Assay (Innogenetics N.V., Ghent, Belgium) (25). Specimens negative for the genomic control probe and HPV in Linear Array and INNO-LiPA were considered inadequate.
Project staff collected additional information on demographic factors, screening, and vaccination histories for women aged 18–39 years through reviews of medical records, including paper charts and electronic medical records, obtained from the screening provider and other health-care providers. Investigators at each surveillance site sought information on history of HPV vaccination through at least 2 of the following 3 methods: 1) review of medical records from the screening provider and other providers who might have documented vaccination, 2) review of the state vaccine registry, and 3) patient interview. Women were classified as vaccinated if the medical record or vaccine registry contained documentation of HPV vaccination or if a medical record noted a vaccination history; self-report alone was not sufficient to document vaccination. Women were classified as unvaccinated if they reported that they had not been vaccinated, if their medical record noted a lack of HPV vaccination or refusal of vaccination, or if they had maintained continuous enrollment in a health insurance plan while vaccine-eligible but did not make a claim for HPV vaccine. All other women were classified as having unknown vaccination status.
For this analysis, data on cases reported for the years 2008–2014 as of May 2018 were included. We included women with CIN2+ who were age-eligible for vaccination (aged ≤26 years in 2006 when the first HPV vaccine was approved for use in the United States) and had a valid HPV DNA typing result (Figure 1). Women with unknown vaccination status were excluded.
Figure 1.
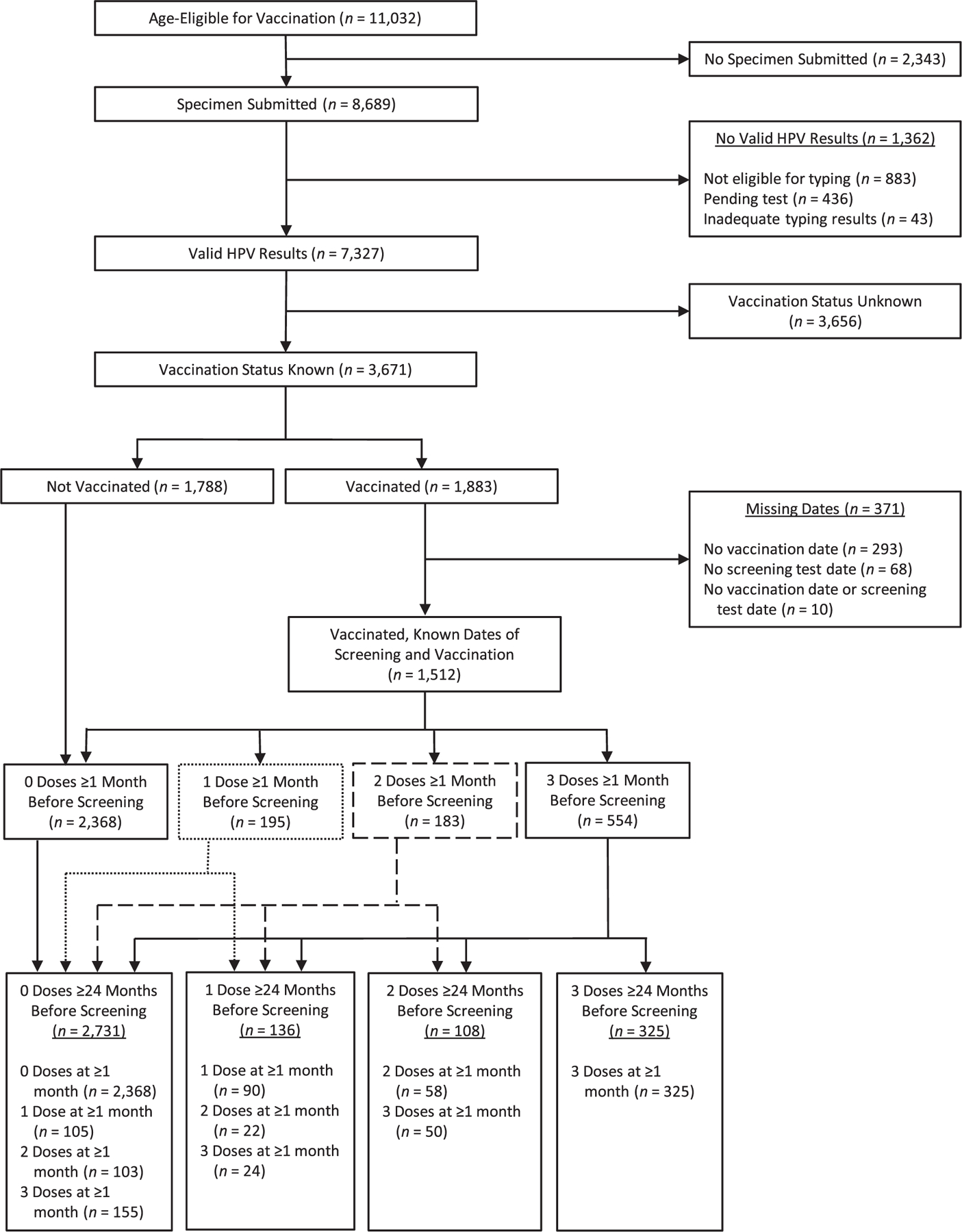
Inclusion of women with cervical intraepithelial neoplasia grades 2–3 and adenocarcinoma in situ in an analysis of human papillomavirus (HPV) vaccine effectiveness, HPV-IMPACT, United States, 2008–2014. The numbers adjacent to solid or broken lines show the number of women reassigned from dose groups ≥1 month before screening. For example, of the 183 women who received 2 doses of HPV vaccine ≥1 month before screening, 58 received 2 doses ≥24 months before screening and were assigned to the 2-dose group, 22 received 1 dose ≥24 months before screening and were reassigned to the 1-dose group, and 103 received both doses less than 24 months before screening and were reassigned to the 0-dose group. HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
Because HPV vaccines work by preventing infection and it may take years after infection for CIN2+ lesions to develop, we defined a buffer period between vaccination and CIN2+ diagnosis to decrease the likelihood of including lesions caused by HPV types acquired before vaccination. In a prior analysis, Hariri et al. (22) demonstrated statistically significant VE after a 24-month buffer period and higher VE with longer buffer periods. Based on this earlier analysis, our understanding of natural history, and observations from intention-to-treat analyses from HPV vaccine trials (3), we defined the number of vaccine doses as those received ≥24 months prior to the screening test for the primary analysis (Figure 1).
HPV-16/18 positivity was defined as detection of HPV-16 or HPV-18, regardless of whether other types of HPV were also detected, using hierarchical categorization. A previous analysis of data from this surveillance system found no difference in HPV type attribution by hierarchical or proportional categorization (26). We compared demographic and clinical characteristics between HPV-16/18–positive CIN2+ women and women with all other CIN2+, as well as by number of vaccine doses received ≥24 months prior to diagnosis. We evaluated statistical significance using the χ2 test or Fisher’s exact test. We implemented a test-negative design, a type of case-control study which uses the adjusted odds ratio (aOR) for vaccination to compare persons who have similar conditions but different results when tested for a vaccine-type disease in order to estimate VE (VE = (1 – aOR) × 100) (27). Specifically, we used logistic regression to evaluate the association between number of vaccine doses among women with HPV-16/18–positive CIN2+ (cases) and women whose CIN2+ did not have detectable HPV-16/18 (referred to as HPV-16/18–negative CIN2+ (controls)). Controls included women who had lesions in which other HPV types were detected and lesions testing negative for HPV. We developed models comparing 1, 2, and 3 doses of vaccine with 0 doses; comparing 2 and 3 doses of vaccine with 1 dose; and comparing 3 doses of vaccine with 2 doses. Variables considered for adjustment included race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic/other race), surveillance site, type of health insurance (public, private, or other), birth cohort (1979–1986 or 1987–1994), age group at diagnosis (18–20, 21–24, 25–29, or 30–34 years), diagnosis period (2008–2010 or 2011–2014), and age at vaccination (12–18, 19–22, or 23–26 years; evaluated in the vaccinated subset only because age at vaccination was not relevant for the unvaccinated reference group in the main analysis). Variables were selected using backwards elimination and change-in-estimate criteria (≥10% change in the odds ratio estimate for vaccine dose). We tested for statistical interactions between number of doses, birth cohort, age group at diagnosis, and diagnosis period.
Assumptions made in the main VE analysis were tested using several sensitivity analyses. To evaluate use of the 24-month buffer period, we repeated the analysis with 1-month, 12-month, and 36-month buffers. To evaluate the influence of doses given during the 24 months before the screening test, we excluded all women who had received any doses during this interval. To evaluate the potential influence of excluding the large number of cases with unknown vaccination status, we included them as unvaccinated cases. To evaluate the potential influence of non-HPV-16/18 types in lesions with multiple types, we excluded all women with more than 1 HPV type detected. To evaluate the influence of lesions without HPV detected, we excluded HPV-negative women from the control group.
All analyses were conducted in SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
From 2008 to 2014, a total of 11,032 women with high-grade cervical lesions (CIN2+) reported to HPV-IMPACT were age-eligible for vaccination (Figure 1). Of these, 8,689 women (78.8%) had specimens submitted for HPV typing, and 7,327 submitted specimens (84.3%) had valid HPV results; 1,513 specimens (20.6%) with valid results had more than 1 HPV type detected, and 248 (3.4%) had 0 types detected. Among specimens with typing results, 3,671 (50.1%) were from women with known vaccination status, including 1,788 not vaccinated and 1,883 vaccinated. Among vaccinated women, 371 were excluded because missing dates of vaccination and/or screening prevented determination of the interval between vaccination and the screening test. The primary analysis included 3,300 vaccine-eligible women, of whom 932 (28.2%) had received doses of vaccine ≥1 month before the screening test (554 with 3 doses, 183 with 2 doses, and 195 with 1 dose). Counting only doses received ≥24 months before the screening test, 325 women (9.8%) had 3 doses, 108 (3.3%) had 2 doses, and 136 (4.1%) had 1 dose. Women who received all doses less than 24 months before the screening test and those who were never vaccinated comprised the 0-dose group (2,731 women (82.7%)). No woman received 2 doses of vaccine with an interval of ≥6 months between doses and the first dose before age 15 years, as currently recommended (not shown).
Compared with women with HPV-16/18–negative CIN2+, those with HPV-16/18–positive CIN2+ were more frequently diagnosed before 2011, born in the earlier cohort, not vaccinated or vaccinated at an older age, diagnosed with CIN grade 3/adenocarcinoma in situ, and non-Hispanic white (Table 1). Differences in proportion HPV-16/18–positive were also noted by surveillance site and health insurance status. Comparing women who were unvaccinated (0 doses) with women who were vaccinated ≥24 months prior to diagnosis (any doses), many characteristics differed between the 2 groups (Table 2). Comparing women who received 3 doses ≥24 months prior to diagnosis with women who received 1 or 2 doses, differences were noted in year of diagnosis, age at vaccination, race/ethnicity, site, and insurance status.
Table 1.
Characteristics of Women With CIN2+ According to Detection of Human Papillomavirus Type 16 or 18, HPV-IMPACT, United States, 2008–2014
| Characteristic | HPV-16/18–Positive Cases (n = 1,561) |
HPV-16/18–Negative Casesa (n = 1,739) |
P Valueb | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Year of diagnosis | <0.001 | ||||
| 2008–2010 | 897 | 57.5 | 813 | 46.8 | |
| 2011–2014 | 664 | 42.5 | 926 | 53.2 | |
| Age at diagnosis, years | 0.079 | ||||
| 18–20 | 99 | 6.3 | 124 | 7.1 | |
| 21–24 | 587 | 37.6 | 718 | 41.3 | |
| 25–29 | 692 | 44.3 | 716 | 41.2 | |
| 30–34 | 183 | 11.7 | 181 | 10.4 | |
| Birth cohort | <0.001 | ||||
| 1979–1986 | 1,080 | 69.2 | 1,071 | 61.6 | |
| 1987–1995 | 481 | 30.8 | 668 | 38.4 | |
| Age at vaccinationc, years | <0.001 | ||||
| 12–18 | 33 | 21.3 | 169 | 40.8 | |
| 19–22 | 59 | 38.1 | 141 | 34.1 | |
| 23–26 | 63 | 40.6 | 104 | 25.1 | |
| Diagnosis | <0.001 | ||||
| CIN grade 2 | 631 | 40.4 | 1,190 | 68.4 | |
| CIN grade 2/3 | 253 | 16.2 | 222 | 12.8 | |
| CIN grade 3/AIS | 677 | 43.4 | 327 | 18.8 | |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic white | 974 | 62.4 | 917 | 52.7 | |
| Non-Hispanic black | 197 | 12.6 | 353 | 20.3 | |
| Hispanic | 148 | 9.5 | 195 | 11.2 | |
| Other | 140 | 9.0 | 148 | 8.5 | |
| Missing data | 102 | 6.5 | 126 | 7.2 | |
| Surveillance site | <0.001 | ||||
| California | 152 | 9.7 | 174 | 10.0 | |
| Connecticut | 414 | 26.5 | 592 | 34.0 | |
| New York | 519 | 33.2 | 505 | 29.0 | |
| Oregon | 254 | 16.3 | 263 | 15.1 | |
| Tennessee | 222 | 14.2 | 205 | 11.8 | |
| Type of health insurance | 0.003 | ||||
| Private | 877 | 56.2 | 950 | 54.6 | |
| Public | 347 | 22.2 | 466 | 26.8 | |
| Other/none | 97 | 6.2 | 113 | 6.5 | |
| Missing data | 240 | 15.4 | 210 | 12.1 | |
| No. of HPV doses received ≥24 months before screening | <0.001 | ||||
| 0 | 1,406 | 90.1 | 1,325 | 76.2 | 0 |
| 1 | 47 | 3.0 | 89 | 5.1 | 1 |
| 2 | 35 | 2.2 | 73 | 4.2 | 2 |
| 3 | 73 | 4.7 | 252 | 14.5 | 3 |
Abbreviations: AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2, 2/3, or 3 and AIS; HPV, human papillomavirus; HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
The HPV-16/18–negative group included women with lesions of other HPV types or no types detected.
χ2 test.
Among vaccinated women only (155 HPV-16/18–positive cases and 414 HPV-16/18–negative cases).
Table 2.
Characteristics of Women With CIN2+ According to Number of Doses of Human Papillomavirus Vaccine Received ≥24 Months Before the Screening That Led to Diagnosis, HPV-IMPACT, United States, 2008–2014
| Characteristic | 0 Doses (n = 2,731) |
Any Doses (n = 569) |
P Valuea | 1 Dose (n = 136) |
2 Doses (n = 108) |
3 Doses (n = 325) |
P Valueb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Year of diagnosis | <0.001 | <0.001 | ||||||||||
| 2008–2010 | 1,604 | 58.7 | 106 | 18.6 | 34 | 25.0 | 35 | 32.4 | 37 | 11.4 | ||
| 2011–2014 | 1,127 | 41.3 | 463 | 81.4 | 102 | 75.0 | 73 | 67.6 | 288 | 88.6 | ||
| Age at diagnosis, years | <0.001 | 0.806 | ||||||||||
| 18–20 | 193 | 7.1 | 30 | 5.3 | 7 | 5.1 | 7 | 6.5 | 16 | 4.9 | ||
| 21–24 | 1,040 | 38.1 | 265 | 46.6 | 63 | 46.3 | 56 | 51.9 | 146 | 44.9 | ||
| 25–29 | 1,170 | 42.8 | 238 | 41.8 | 59 | 43.4 | 38 | 35.2 | 141 | 43.4 | ||
| 30–34 | 328 | 12.0 | 36 | 6.3 | 7 | 5.1 | 7 | 6.5 | 22 | 6.8 | ||
| Birth cohort | <0.001 | 0.719 | ||||||||||
| 1979–1986 | 1,896 | 69.4 | 255 | 44.8 | 65 | 47.8 | 48 | 44.4 | 142 | 43.7 | ||
| 1987–1995 | 835 | 30.6 | 314 | 55.2 | 71 | 52.2 | 60 | 55.6 | 183 | 56.3 | ||
| Age at vaccination, years | 0.065 | |||||||||||
| 12–18 | 202 | 35.5 | 37 | 27.2 | 34 | 31.5 | 131 | 40.3 | ||||
| 19–22 | 200 | 35.1 | 55 | 40.4 | 43 | 39.8 | 102 | 31.4 | ||||
| 23–26 | 167 | 29.3 | 44 | 32.4 | 31 | 28.7 | 92 | 28.3 | ||||
| Diagnosis | 0.043 | 0.903 | ||||||||||
| CIN grade 2 | 1,483 | 54.3 | 338 | 59.4 | 84 | 61.8 | 61 | 56.5 | 193 | 59.4 | ||
| CIN grade 2/3 | 393 | 14.4 | 82 | 14.4 | 19 | 14.0 | 15 | 13.9 | 48 | 14.8 | ||
| CIN grade 3/AIS | 855 | 31.3 | 149 | 26.2 | 33 | 24.3 | 32 | 29.6 | 84 | 25.8 | ||
| Race/ethnicity | 0.002 | 0.002 | ||||||||||
| Non-Hispanic white | 1,553 | 56.9 | 338 | 59.4 | 63 | 46.3 | 61 | 56.5 | 214 | 65.8 | ||
| Non-Hispanic black | 438 | 16.0 | 112 | 19.7 | 39 | 28.7 | 24 | 22.2 | 49 | 15.1 | ||
| Hispanic | 302 | 11.1 | 41 | 7.2 | 17 | 12.5 | 5 | 4.6 | 19 | 5.8 | ||
| Other | 235 | 8.6 | 53 | 9.3 | 11 | 8.1 | 12 | 11.1 | 30 | 9.2 | ||
| Missing data | 203 | 7.4 | 25 | 4.4 | 6 | 4.4 | 6 | 5.6 | 13 | 4.0 | ||
| Surveillance site | 0.003 | <0.001 | ||||||||||
| California | 271 | 9.9 | 55 | 9.7 | 19 | 14.0 | 9 | 8.3 | 27 | 8.3 | ||
| Connecticut | 803 | 29.4 | 203 | 35.7 | 28 | 20.6 | 37 | 34.3 | 138 | 42.5 | ||
| New York | 843 | 30.9 | 181 | 31.8 | 40 | 29.4 | 37 | 34.3 | 104 | 32.0 | ||
| Oregon | 453 | 16.6 | 64 | 11.2 | 32 | 23.5 | 10 | 9.3 | 22 | 6.8 | ||
| Tennessee | 361 | 13.2 | 66 | 11.6 | 17 | 12.5 | 15 | 13.9 | 34 | 10.5 | ||
| Type of health insurance | <0.001 | <0.001 | ||||||||||
| Private | 1,466 | 53.7 | 361 | 63.4 | 66 | 48.5 | 63 | 58.3 | 232 | 71.4 | ||
| Public | 704 | 25.8 | 109 | 19.2 | 39 | 28.7 | 23 | 21.3 | 47 | 14.5 | ||
| Other/none | 177 | 6.5 | 33 | 5.8 | 10 | 7.4 | 6 | 5.6 | 17 | 5.2 | ||
| Missing data | 384 | 14.1 | 66 | 11.6 | 21 | 15.4 | 16 | 14.8 | 29 | 8.9 | ||
Abbreviations: AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2, 2/3, or 3 and AIS; HPV, human papillomavirus; HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
χ2 test comparing 0 doses with any doses.
χ2 test comparing 1, 2, and 3 doses.
Comparing HPV-16/18–positive CIN2+ cases and HPV-16/18–negative CIN2+ controls, adjusted odds ratios for vaccination with 1, 2, and 3 doses ≥24 months before screening were 0.53 (95% confidence interval (CI): 0.37, 0.76), 0.45 (95% CI: 0.30, 0.69), and 0.26 (95% CI: 0.20, 0.35), respectively (Figure 2; unadjusted odds ratios are shown in Web Table 1, available at https://academic.oup.com/aje). The corresponding VE estimates for 1, 2, and 3 doses compared with 0 doses were 47%, 55%, and 74%. An interaction was identified between number of vaccine doses and birth cohort, so results are also presented stratified by birth cohort (Figure 2). In the later cohort (born in 1987–1994), VE estimates ranged from 59% for 1 dose to 83% for 3 doses. In the earlier cohort (born in 1979–1986), VE estimates were lower, and confidence intervals for 1 and 2 doses included 1. Direct comparisons by number of doses among vaccinated women indicated little difference between 1 and 2 doses and nearly 40% greater effectiveness for 3 doses than for 1 or 2 doses (Table 3).
Figure 2.
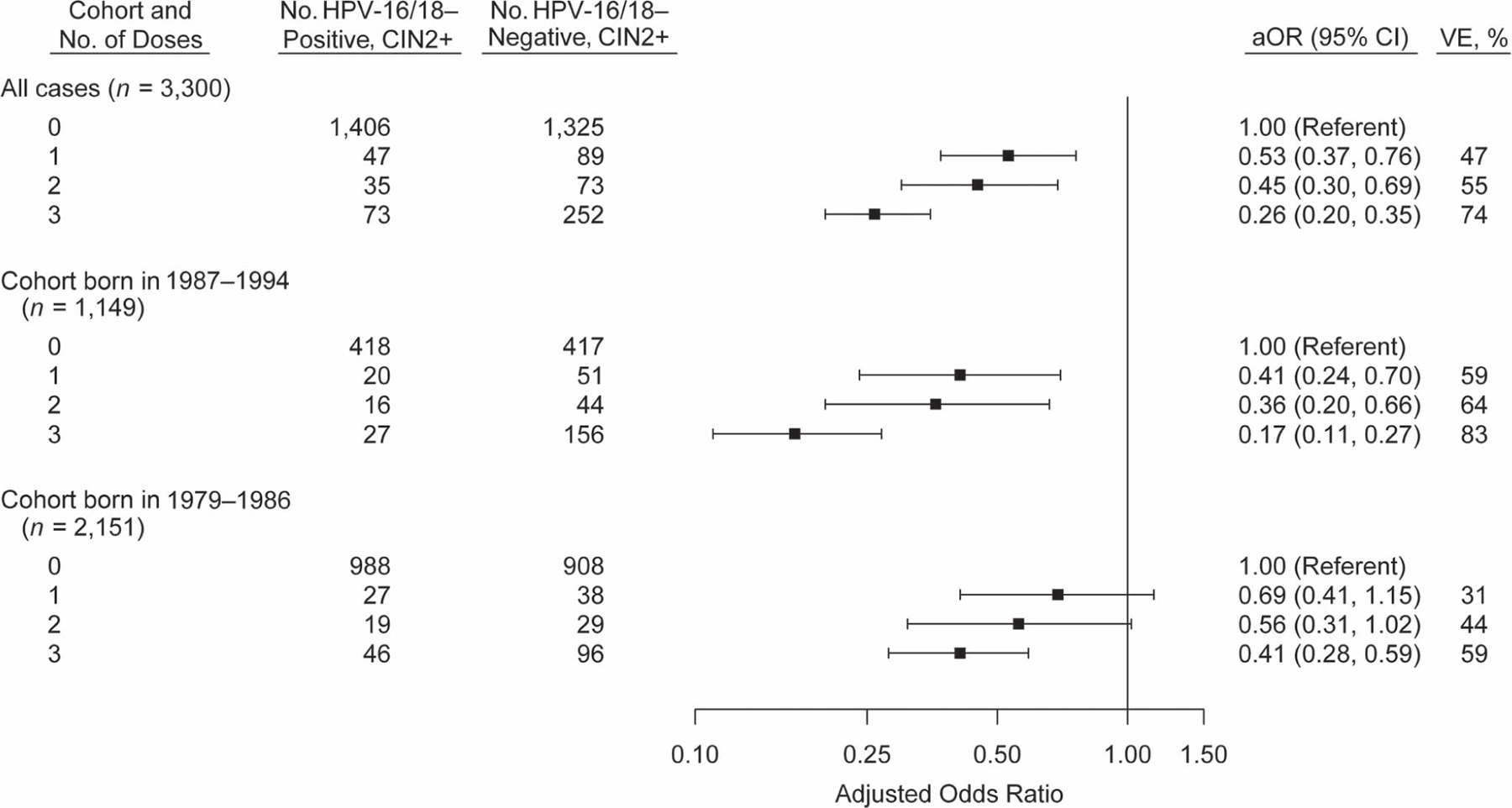
Vaccination history of women with cervical intraepithelial neoplasia grades 2–3 and adenocarcinoma in situ (CIN2+) with and without human papillomavirus (HPV) type 16 or 18 (HPV-16/18), adjusted odds ratios (aORs), and vaccine effectiveness (VE), overall and by birth cohort, HPV-IMPACT, United States, 2008–2014. Odds ratios were adjusted for surveillance site, race/ethnicity, and health insurance status. P for interaction (cohort × number of doses) < 0.01. Bars, 95% confidence intervals (CIs). HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
Table 3.
Unadjusted and Adjusted Odds Ratios Comparing Numbers of Human Papillomavirus Vaccine Doses Received in CIN2+ Cases With and Without Human Papillomavirus Type 16/18 Among Vaccinated Women With CIN2+, HPV-IMPACT, United States, 2008–2014
| No. of HPV Vaccine Doses Given ≥24 Months Before Screening | OR | 95% CI | Adjusted ORa | 95% CI |
|---|---|---|---|---|
| Versus 1 dose | ||||
| 1 | 1.00 | Referent | 1.00 | Referent |
| 2 | 0.91 | 0.53, 1.55 | 0.96 | 0.55, 1.68 |
| 3 | 0.55 | 0.35, 0.85 | 0.61 | 0.38, 0.99 |
| Versus 2 doses | ||||
| 2 | 1.00 | Referent | 1.00 | Referent |
| 3 | 0.60 | 0.37, 0.98 | 0.64 | 0.39, 1.05 |
Abbreviations: CI, confidence interval; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2, 2/3, or 3 and adenocarcinoma in situ; HPV, human papillomavirus; HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project; OR, odds ratio.
Adjusted for surveillance site, race/ethnicity, health insurance status, and age at vaccination.
Results of sensitivity analyses are shown in Figure 3. The VE estimates for 1 and 3 doses, but not 2 doses, generally increased with the length of the buffer period. Excluding women given any doses during the 24-month buffer period led to a higher VE estimate for 1 dose (65%) but similar results for 2 and 3 doses, compared with the primary analysis with the 24-month buffer; this may have been due to longer time between vaccination and CIN2+ diagnosis for women with exactly 1 dose (median, 47 months) than for those with more than 1 dose but only 1 eligible dose (median, 32 months). In an analysis categorizing women with unknown vaccination status as unvaccinated, VE estimates were similar to those of the primary analysis. Exclusion of all women for whom multiple HPV types were detected yielded a similar VE estimate for 3 doses and lower VE estimates for 2 doses or 1 dose (Web Table 1). Exclusion of the small proportion (3%) of controls with no HPV detected had no meaningful impact on results (not shown).
Figure 3.
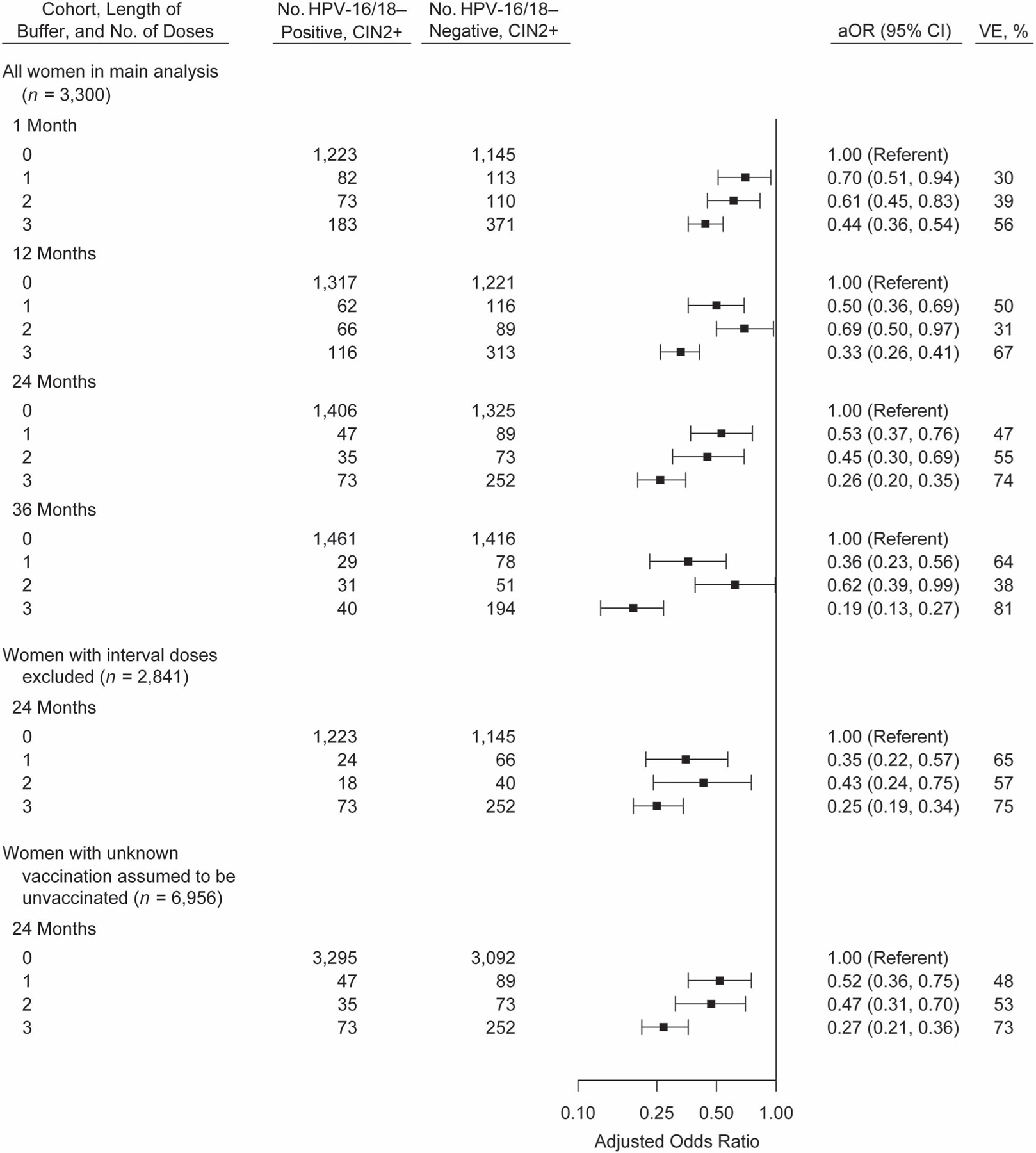
Vaccination history of women with cervical intraepithelial neoplasia grades 2–3 and adenocarcinoma in situ (CIN2+) with and without human papillomavirus (HPV) type 16 or 18 (HPV-16/18), adjusted odds ratios (aORs), and vaccine effectiveness (VE) in sensitivity analyses with varying classification of vaccine doses, HPV-IMPACT, United States, 2008–2014. Odds ratios were adjusted for surveillance site, race/ethnicity, and health insurance status. The buffer was defined as the time between the date of the first vaccine dose and the date of the screening test that led to CIN2+ diagnosis. Bars, 95% confidence intervals (CIs). HPV-IMPACT, Human Papillomavirus Vaccine Impact Monitoring Project.
DISCUSSION
In this analysis, estimates of HPV VE against vaccine-type CIN2+ disease increased with the number of documented vaccine doses among women in 5 US communities during 2008–2014. VE was 47%, 55%, and 74% for 1, 2, and 3 doses of HPV vaccine, respectively. Estimated VE was higher for the cohort born in 1987–1994 (59%–83%) than for that born in 1979–1986 (31%–59%). While the 3-dose VE estimates are lower than those in per protocol analyses from randomized controlled trials, they align well with intention-to-treat or total-vaccinated-cohort analyses of vaccine-type high-grade cervical lesions from those trials (45%–70%, depending on trial and outcome) (3). To our knowledge, this is the first published analysis to have included HPV-16/18 typing data in an analysis of the effectiveness of fewer than 3 HPV vaccine doses for prevention of cervical lesions, and it extends previous findings from this project on VE for ≥1 dose administered at least 24 months prior to diagnosis (22, 28).
Previously, at least 10 studies have reported on VE against cervical cytological or histological outcomes by number of doses (9, 12–21). None of those studies evaluated VE against vaccine-type disease; rather, they used all-cause CIN2+ histology or cytological abnormalities, which would include many cases associated with HPV types not targeted by vaccine. A major limitation of most studies published to date is that they have been conducted in settings of catch-up vaccination programs; among these, most have found higher VE estimates for 3 doses than for 1 or 2, and little to no evidence for VE with fewer than 3 doses (e.g., adjusted effect estimates near or exceeding 1) (12–17). In our analysis, like other studies that included adult catch-up vaccinees, we found the highest VE with 3 doses; however, in contrast to those studies, we found evidence of some (albeit lower) protection with fewer than 3 doses. A few studies have evaluated VE in women who were mainly vaccinated at younger ages; these studies were conducted in settings without catch-up vaccination extending into adulthood, by restricting the analysis to women who had been vaccinated as adolescents, or by only including cohorts who were vaccine-eligible as adolescents (18–21). In all 4 of these studies, similar VEs were estimated for 1 and 3 doses of HPV vaccine. This may suggest that women who are vaccinated at younger ages need fewer doses for equal protection or that there is confounding in analyses that include women who received catch-up vaccination. Women who received fewer than 3 doses might have been more likely to initiate vaccination after exposure to HPV infection than those who received 3 doses.
We observed higher VE in later birth cohorts. Women in the earlier birth cohort (1979–1986), diagnosed with CIN2+ at ages 22–35 years, were ≥20 years of age when the vaccine was first recommended; their median age at vaccination was 23 years. In contrast, women born in the later cohort (1987–1995) were diagnosed with CIN2+ at ages 18–27 years and were as young as 11 years of age when the vaccine was first recommended; their median age at vaccination was 19 years. In both cohorts, most women were vaccinated as part of catch-up programs, and many women were likely vaccinated after they became sexually active (29). The higher VE observed in the later cohort is probably due to the higher likelihood that they were vaccinated before exposure to HPV-16/18. Because of the small numbers of women vaccinated as adolescents in our data, it was not feasible to stratify all vaccine dose groups by age at vaccination.
During the surveillance period, a 3-dose HPV vaccination schedule was recommended in the United States (4). No women in this analysis received 2 doses according to the currently recommended 2-dose schedule; therefore, this analysis was not a direct evaluation of the current recommendation (7). In our main analysis, we found that the estimate for 2 doses was intermediate between the estimates for 1 and 3 doses, suggestive of a dose response. In some sensitivity analyses, the VE estimate for 2 doses was lower than the estimate for 1 dose, suggesting that estimates were sensitive to the buffer period or simply imprecise.
Notably, previous CIN2+ VE studies have included outcomes associated with nonvaccine HPV types; we incorporated HPV typing data and implemented analytical methods that assume a specific impact of individual vaccination on vaccine-type cases. Half of the CIN2+ outcomes in this analysis were not HPV-16/18–positive and could not have been prevented by the quadrivalent HPV vaccine, the pre-dominant vaccine available during the surveillance period—assuming that the vaccine confers no cross-protection for other HPV types. In the United States, national monitoring of HPV DNA prevalence through self-collected vaginal swabs has not identified a significant decrease in any individual nonvaccine HPV type (30); however, cross-protection against nonvaccine types has been reported in other countries, particularly for bivalent vaccine (31, 32). If the US quadrivalent vaccination program reduced the prevalence of non–vaccine-type CIN2+, it would reduce the observed VE in this analysis. On the other hand, our VE estimates could have been biased if there is type replacement from HPV vaccination. Pneumococcal vaccine, another multivalent vaccine, has been shown to lead to considerable type replacement (33). However, little consistent evidence for type replacement exists for HPV vaccine (30, 34); therefore, we did not implement analytical approaches that have been developed to counter associated bias (33).
Our analysis had some of the same inherent methodological challenges and potential for bias as other observational VE studies, mainly because persons who receive fewer than 3 doses in the setting of a 3-dose recommendation differ from those who follow recommendations. We observed that women who received 1 or 2 doses differed from women who received 3 doses with regard to several demographic characteristics, including race, insurance coverage, and site of residence. While we adjusted for measured confounders, the presence of unmeasured confounders, including behaviors, is likely. Demographic characteristics differed between women who were HPV-16/18–positive and women who were HPV-16/18–negative, and many of these differences were related to age and likelihood of vaccination; however, compared with a traditional case-control study, the cases and controls used in this test-negative analysis were probably more comparable. For example, cases and controls all had risk factors for CIN2+ and participated in cervical cancer screening; nearly all had HPV detected in their diagnostic specimens, and exclusion of women with no HPV detected yielded very similar results (27). Although data were collected through population surveillance, selection bias created by excluding women with incomplete data is possible (35). For example, we did not know the vaccination status of 50% of women. A previous analysis showed that women with unknown vaccination status had similar HPV-16/18 prevalence as women known to be unvaccinated (22). In the sensitivity analysis including these women as unvaccinated, results were similar to those of the primary analysis. Specimens for typing were not available for all women; however, specimen availability was unlikely to be affected by vaccination status and should not have biased VE results.
In conclusion, this analysis of CIN2+ cases identified through population-based surveillance supports significant VE with 1, 2, and 3 doses of HPV vaccine, although 3-dose effectiveness was higher than 1-dose effectiveness. Our findings should be interpreted with the understanding of biases that might affect VE estimates by number of doses. The higher VE in the younger cohort underscores the importance of vaccination at younger ages, before exposure to HPV through sexual activity. At the same time, these data provide encouraging evidence that some women who were vaccinated at ages older than the recommended age of 11–12 years can still receive protection against HPV-16/18–associated CIN2+. Continued surveillance will provide data for further evaluation of the effectiveness of 1 dose of HPV vaccine, for which there is increasing international interest. Additionally, as adolescents who were vaccinated following the 2016 recommendation reach the age for cervical cancer screening, future analyses can better evaluate the VE of 2 doses administered consistent with current recommendations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a cooperative agreement with the Centers for Disease Control and Prevention’s Emerging Infections Program (grants U50CK000482 (California), U50CK000488 (Connecticut), U50CK000486 (New York), U50CK000484 (Oregon), and U50CK000491 (Tennessee)).
We thank Rayleen Lewis for development of graphical presentations.
Additional members of the HPV-IMPACT Working Group include Manideepthi Pemmaraju, Sheelah Blankenship, Stephanie Allen, and Dr. Tiffanie Markus (Department of Health Policy, Vanderbilt University Medical Center); Dr. Martin Whiteside (Director, Tennessee Comprehensive Cancer Control Program, Tennessee Department of Health); Monica Brackney, James Meek, Kyle Higgins, and Dr. James Hadler (Connecticut Emerging Infections Program, Yale School of Public Health); Dr. Lynn Sosa (Connecticut Department of Public Health); Erin Whitney, Kayla Saadeh, and Deanna Fink (California Emerging Infections Program); Dr. Michael Silverberg (Division of Research, Kaiser Permanente Northern California); Dr. Nasreen Abdullah, Shannon Allain, and Sara Ehlers (Oregon Health Authority); Mary Scahill, Marina Oktapodas, and Christina Felsen (University of Rochester Medical Center); Angela Cleveland (Centers for Disease Control and Prevention); and Rebecca Dahl (Maximus Federal, contractor to the Centers for Disease Control and Prevention).
This work was presented at the 32nd International Papillomavirus Conference, Sydney, New South Wales, Australia, October 2–6, 2018.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations:
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- CIN2+
cervical intraepithelial neoplasia grades 2–3 and adenocarcinoma in situ
- HPV
human papillomavirus
- VE
vaccine effectiveness
Footnotes
L.M.N. has received personal fees from Merck & Company, Inc. (Kenilworth, New Jersey) outside the range of the current work. The other authors report no potential conflicts of interest.
Contributor Information
Michelle L. Johnson Jones, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia
Julia Warner Gargano, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Melissa Powell, Oregon Department of Human Services, Portland, Oregon.
Ina U. Park, Department of Family and Community Medicine, School of Medicine, University of California, San Francisco, San Francisco, California
Linda M. Niccolai, Department of Epidemiology of Microbial Diseases and Connecticut Emerging Infections Program, School of Public Health, Yale University, New Haven, Connecticut
Nancy M. Bennett, Center for Community Health and Prevention, University of Rochester Medical Center, Rochester, New York; Department of Medicine, School of Medicine and Dentistry, University of Rochester, Rochester, New York
Marie R. Griffin, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee
Troy Querec, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Elizabeth R. Unger, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia
Lauri E. Markowitz, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia
REFERENCES
- 1.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol 2009;10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J, Quint W, Banks L, et al. The biology and life-cycle of human papillomaviruses. Vaccine 2012; 30(suppl 5):F55–F70. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012;30(suppl 5):F123–F138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014;63(RR-5):1–30. [PubMed] [Google Scholar]
- 5.Markowitz LE, Gee J, Chesson H, et al. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr 2018;18(2 suppl):S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morbid Mortal Wkly Rep 2018;67(33): 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65(49):1405–1408. [DOI] [PubMed] [Google Scholar]
- 8.Kreimer AR, Herrero R, Sampson JN, et al. Evidence for single-dose protection by the bivalent HPV vaccine—review of the Costa Rica HPV vaccine trial and future research studies. Vaccine 2018;36(32):4774–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markowitz LE, Drolet M, Perez N, et al. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine 2018;36(32):4806–4815. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Joshi S, Muwonge R, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018;36(32):4783–4791. [DOI] [PubMed] [Google Scholar]
- 11.National Library of Medicine, National Institutes of Health. A Dose Reduction Immunobridging and Safety Study of Two HPV Vaccines in Tanzanian Girls (DoRIS) Bethesda, MD: National Library of Medicine; 2016. https://clinicaltrials.gov/ct2/show/NCT02834637 Accessed December 4, 2018. [Google Scholar]
- 12.Brotherton JML, Malloy M, Budd AC, et al. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: observational cohort of young women in Australia. Papillomavirus Res 2015;1:59–73. [Google Scholar]
- 13.Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014;348(7948):g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertig DM, Brotherton JM, Budd AC, et al. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med 2013;11: Article 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofstetter AM, Ompad DC, Stockwell MS, et al. Human papillomavirus vaccination and cervical cytology outcomes among urban low-income minority females. JAMA Pediatr 2016;170(5):445–452. [DOI] [PubMed] [Google Scholar]
- 16.Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014;111(9):1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverberg MJ, Leyden WA, Lam JO, et al. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: a population-based case-control study. Lancet Child Adolesc Health 2018;2(10):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Bell C, Sun M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ 2016;188(12):E281–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dehlendorff C, Sparén P, Baldur-Felskov B, et al. Effectiveness of varying number of doses and timing between doses of quadrivalent HPV vaccine against severe cervical lesions. Vaccine 2018;36(43):6373–6378. [DOI] [PubMed] [Google Scholar]
- 20.Verdoodt F, Dehlendorff C, Kjaer SK. Dose-related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: a Danish nationwide cohort study [published online ahead of print March 20, 2019]. Clin Infect Dis doi: 10.1093/cid/ciz239. [DOI] [PubMed] [Google Scholar]
- 21.Brotherton JM, Budd A, Rompotis C, et al. Is one dose of human papillomavirus vaccine as effective as three?: a national cohort analysis [published online ahead of print July 15, 2019]. Papillomavirus Res 10.1016/j.pvr.2019.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariri S, Bennett NM, Niccolai LM, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States—2008–2012. Vaccine 2015;33(13):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariri S, Johnson ML, Bennett NM, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer 2015;121(16):2775–2781. [DOI] [PubMed] [Google Scholar]
- 24.Hariri S, Markowitz LE, Bennett NM, et al. Monitoring effect of human papillomavirus vaccines in US population, Emerging Infections Program, 2008–2012. Emerg Infect Dis 2015;21(9):1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri S, Steinau M, Rinas A, et al. HPV genotypes in high grade cervical lesions and invasive cervical carcinoma as detected by two commercial DNA assays, North Carolina, 2001–2006. PloS One 2012;7(3):e34044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hariri S, Unger ER, Schafer S, et al. HPV type attribution in high-grade cervical lesions: assessing the potential benefits of vaccines in a population-based evaluation in the United States. Cancer Epidemiol Biomarkers Prev 2015;24(2): 393–399. [DOI] [PubMed] [Google Scholar]
- 27.Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med 1980;303(10):549–552. [DOI] [PubMed] [Google Scholar]
- 28.Powell SE, Hariri S, Steinau M, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine 2012;31(1):109–113. [DOI] [PubMed] [Google Scholar]
- 29.Abma JC, Martinez GM. Sexual activity and contraceptive use among teenagers in the United States, 2011–2015. Natl Health Stat Report 2017;(104):1–23. [PubMed] [Google Scholar]
- 30.Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017;216(5):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017;17(12):1293–1302. [DOI] [PubMed] [Google Scholar]
- 32.Woestenberg PJ, King AJ, van Benthem BHB, et al. Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217(2):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews N, Waight PA, Borrow R, et al. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS One 2011;6(12):e28435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesher D, Soldan K, Lehtinen M, et al. Population-level effects of human papillomavirus vaccination programs on infections with nonvaccine genotypes. Emerg Infect Dis 2016;22(10):1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verani JR, Baqui AH, Broome CV, et al. Case-control vaccine effectiveness studies: preparation, design, and enrollment of cases and controls. Vaccine 2017;35(25): 3295–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


