Keywords: brain clearance, brain extracellular matrix, brain fluid transport, cerebrospinal fluid, glymphatic system
Abstract
The brain harbors a unique ability to, figuratively speaking, shift its gears. During wakefulness, the brain is geared fully toward processing information and behaving, while homeostatic functions predominate during sleep. The blood-brain barrier establishes a stable environment that is optimal for neuronal function, yet the barrier imposes a physiological problem; transcapillary filtration that forms extracellular fluid in other organs is reduced to a minimum in brain. Consequently, the brain depends on a special fluid [the cerebrospinal fluid (CSF)] that is flushed into brain along the unique perivascular spaces created by astrocytic vascular endfeet. We describe this pathway, coined the term glymphatic system, based on its dependency on astrocytic vascular endfeet and their adluminal expression of aquaporin-4 water channels facing toward CSF-filled perivascular spaces. Glymphatic clearance of potentially harmful metabolic or protein waste products, such as amyloid-β, is primarily active during sleep, when its physiological drivers, the cardiac cycle, respiration, and slow vasomotion, together efficiently propel CSF inflow along periarterial spaces. The brain’s extracellular space contains an abundance of proteoglycans and hyaluronan, which provide a low-resistance hydraulic conduit that rapidly can expand and shrink during the sleep-wake cycle. We describe this unique fluid system of the brain, which meets the brain’s requisites to maintain homeostasis similar to peripheral organs, considering the blood-brain-barrier and the paths for formation and egress of the CSF.
CLINICAL HIGHLIGHTS
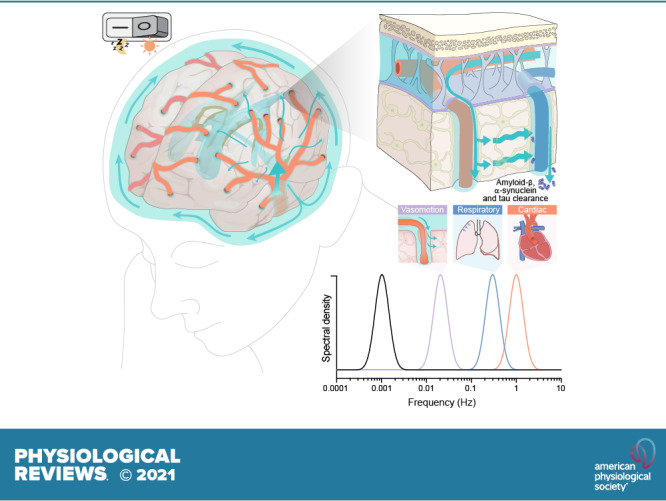
The recent discovery of the glymphatic pathway and the rediscovery of dural lymphatic vessels have reshaped our understanding of brain fluid dynamics and how the brain parenchyma rids itself from harmful proteins such as amyloid-β, α-synuclein, and tau especially during sleep. Along with this understanding has come a novel insight into the pathology behind common neurodegenerative disorders such as Alzheimer’s disease and traumatic brain injury, as well as the edema formation after ischemic stroke. Clinical imaging tools for studying glymphatic function are already in use and undergoing rapid development and show great promise to become established as prognostic and diagnostic tools in the near future.
1. INTRODUCTION
The brain is an exceptional organ that processes sensory input, computes decisions, and coordinates behavioral output. The demands on the brain are more complex and multifaceted than for any other organ. Typically, the brain shifts between functional states to fine tune itself for performing optimally its various tasks, such as cognition and problem solving, responding to dangers or stress, relaxing, thinking, wondering, exercising, meditating, reminiscing, resting, sleeping, dreaming, or healing. In its most demanding states, when a threatening situation requires absolute alertness, the brain focuses on processing and execution of the tasks at hand, putting aside the dull aspects of brain housekeeping. Conversely, in situations that are less demanding, and especially when we fall asleep, restorative homeostatic processes become a dominant activity of the brain (1). The process of reprioritizing between housekeeping and attentional or creative processes is unique to brain and is intimately linked to the sleep-wake cycle. The sleeping brain is focused on the restorative task of cleaning up the mess of metabolic waste products generated during active wakefulness (2). During sleep, the glymphatic system pumps large amounts of cerebrospinal fluid (CSF) into the neuropil in a process facilitated by expansion of the extracellular space volume that occurs during nonrapid eye movement (NREM) sleep (3). The fresh CSF supply mixes with extracellular fluid and flushes out metabolic waste products, notably amyloid-β (4). The resetting of homeostasis and the rejuvenation derived from healthy sleep prepare the organism to embark on the new day of challenges. Indeed, obtaining sufficient sleep is a necessary requirement for maintaining normal cognitive function (5), and prolonged sleep deprivation inevitably brings serious psychiatric and neurological consequences in previously healthy individuals (6).
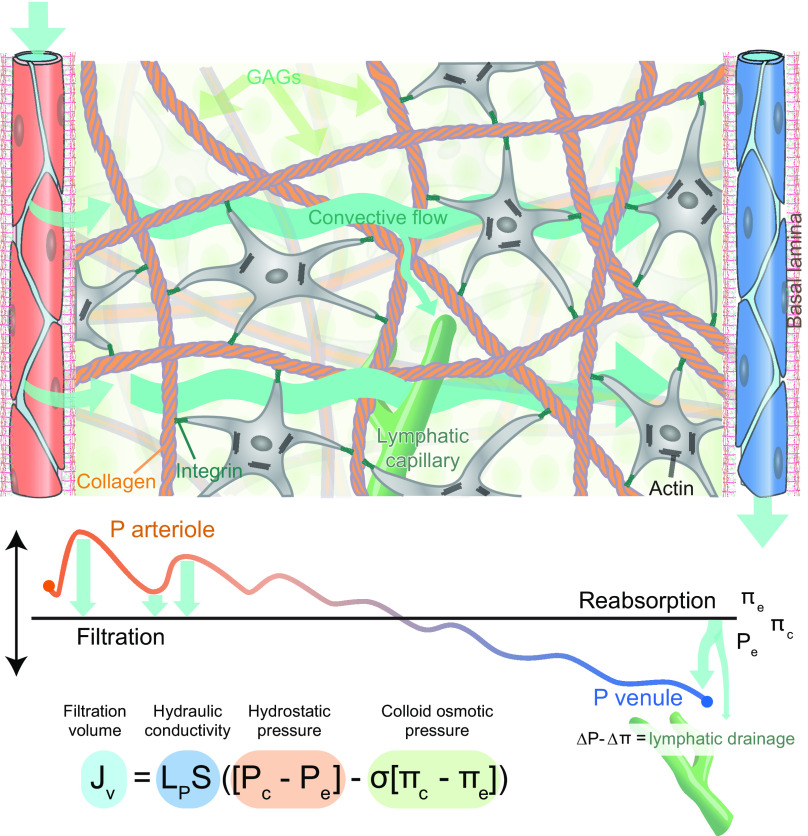
How, then, do organs other than the brain manage to remove their metabolic waste? In peripheral tissues, proteins in the extracellular space are removed more or less continuously by the lymphatic system, propelled by a constant influx to the tissue of an ultrafiltrate from porous blood capillaries (7, 8). According to the Starling equation, inflow of fluid and proteins from plasma is driven by the hydrostatic pressure at the arterial end of the capillary bed, while reuptake of fluid is powered by the osmotic gradient in the venous end of the capillaries. However, accumulating evidence questions the fitness of the Starling principle by demonstrating that excess fluid and extracellular proteins are primarily transported out of peripheral tissue via the intricate network of blind-ended lymphatic capillaries dispersed within the tissue (9).
The brain also differs from other organs with respect to fluid dynamics. In particular, the brain is isolated from peripheral fluid exchange by the presence of tight endothelial and epithelial junctions that forms several distinct barriers sealing the brain and CSF from direct communication with the blood stream and peripheral extracellular fluid. The endothelial cell tight junctions are the key structural component of the blood-brain barrier (BBB), which is an evolutionarily conserved hallmark of the vertebrate central nervous system (CNS) (10, 11). A tightly regulated internal milieu likely improves the processing power of neural networks and otherwise stabilizes the CNS in the face of a changing external milieu, thus imparting an evolutionary benefit (12, 13). However, the BBB also presents a physiological problem, since the tight junctions dramatically reduce influx of an ultrafiltrate of plasma into the neuropil compared with peripheral tissues. The lack of fluid influx brings a risk of stasis and accumulation of protein waste. In fact, brain tissue is uniquely vulnerable to aggregation of toxic proteins, which would be rapidly removed from peripheral tissues by lymphatic drainage. Age-related accumulation of toxic proteins such as amyloid-β, tau, and α-synuclein represents the core problem in many neurodegenerative disorders (14), thus underscoring the importance of efficient brain clearance in healthy aging (15). The mammalian brain has developed an alternate source of fluid and a unique drainage mechanism to ensure constant extracellular fluid flow, much as seen in peripheral organs via lymphatic flow. The primary source of brain extracellular fluid is the CSF, which is produced by the choroid plexus at rate of at least 430–1,000 mL per day in humans (16–19). CSF transport into the neuropil is a functional substitute for the lack of transcapillary fluid influx. The unique construction of the cerebral vasculature with its thousands of penetrating arteries piercing the brain parenchyma, each surrounded by vascular endfeet of astrocytes, creates a low-resistance periarterial fluid flow pathway that enable fast transport of “clean” CSF deep into the brain. The vascular endfeet of astrocytes plaster along all segments of the cerebral vasculature to create the perivascular space that enables fast low-resistance transport of CSF into brain (20). The astrocytic endfeet are loosely interconnected by gap junctions and are covered by glycocalyx similar to that in peripheral capillaries, thus allowing easy fluid exchange with the neuropil. Hydrostatic pressure generated by arterial pulsatility propels CSF into the tissue, much as arterial pulsatility drives an ultrafiltrate of plasma into peripheral tissues (21). The directional flow through the brain parenchyma is facilitated by aquaporin-4 (AQP4) water channels that are strategically positioned on the astrocytic endfeet lining the perivascular spaces (22). Thus the overall organization of tissue fluid flow in CNS resembles that of peripheral tissue (FIGURE 1). To accommodate for the lack of lymphatic vessels in brain, the perivenous channels functionally act as the efferent lymphatic vessels of the brain and thus transport excess fluid and proteins out of the neuropil (2). While the brain is devoid of lymphatics per se, there is a network of classical lymphatic vessels in the dura mater (the fibrous membrane surrounding the brain), and these dural vessels are, along with cranial and spinal nerves, the main channel for returning CSF to the general circulation (23, 24).
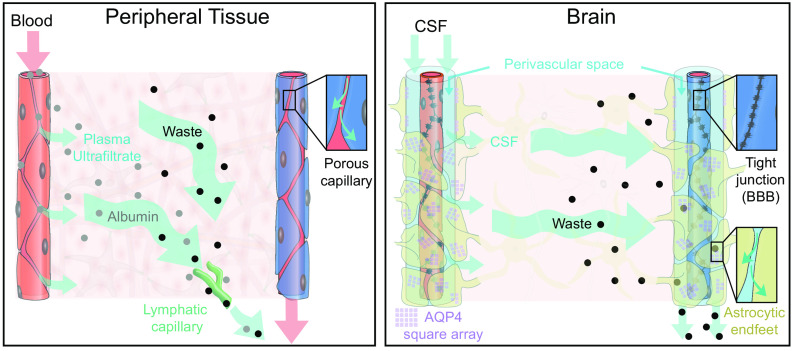
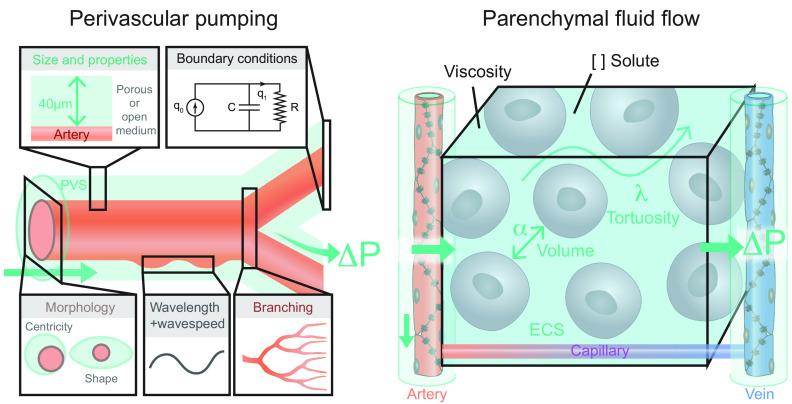
FIGURE 1.
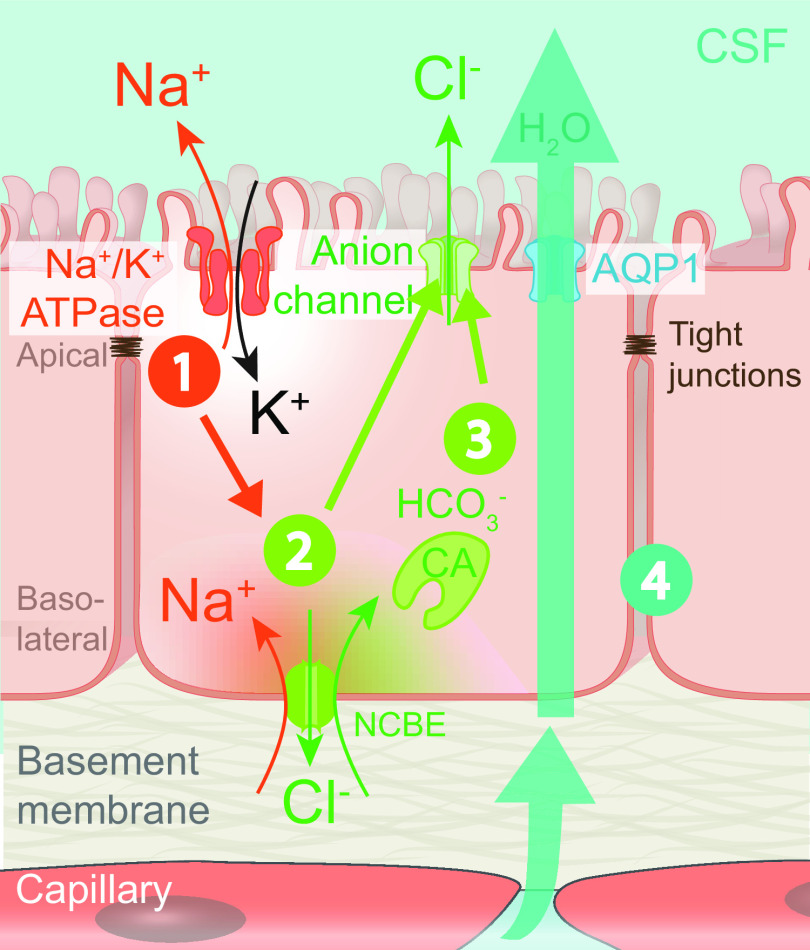
Comparison of fluid and solute flow in peripheral tissues and brain. The source of fluid in peripheral tissue is the porous capillaries, which are permeable to plasma and globular proteins like albumin (gray circles). In addition, endothelial cells express aquaporin-1 (AQP1) water channels. A hydrostatic pressure gradient drives an ultrafiltrate of plasma and globular proteins into the tissue at the arterial end of the capillary bed, while most of the fluid and extracellular proteins, including waste products (black circles) are transported out of peripheral tissues by lymphatic vessels. In the brain, the blood brain barrier (BBB) formed by capillary endothelial cell tight junctions largely prevents influx of vascular fluid and proteins. Instead, the brain produces its own fluid, cerebrospinal fluid (CSF). CSF is transported into deep parts of the brain along the periarterial spaces. The unique perivascular spaces of the central nervous system are annular CSF-filled tunnels with low resistance to fluid flow, which are created by a convoluted surface of loosely interconnected astrocytic endfeet plastering the entire cerebral vasculature, including arteries, capillaries and veins. The arterial wall pulsatility drives CSF influx into the neuropil, facilitated by the aquaporin-4 (AQP4) water channels on the astrocytic endfeet. AQP4 channels form square arrays that occupy up to 60% of the surface of astrocytic endfeet facing the perivascular space (John Rash, personal communication). In contrast, brain endothelial cells express few or no water channels. The extracellular fluid and protein waste produced during neural activity are transported to and leave the brain along perivenous spaces for ultimate export by meningeal lymphatic vessels and along cranial and spinal nerve sheaths.
How does the brain turn fluid flow on and off depending on the state of brain activity? Data on this subject are scarce, but it is notable that the extracellular space is almost devoid of collagen and other incompressible fibrous materials. The extracellular matrix in brain is composed mostly of proteoglycans and hyaluronan (25), which can absorb and release water molecules quickly, thus dynamically fine-tuning the volume of the extracellular space and thereby adjusting its hydraulic conductivity (8). In fact, direct measurements of brain extracellular volume have shown that state-dependent changes in brain fluid transport are controlled by arousal-controlled shrinkage and sleep-induced expansion of the extracellular space volume (1), possibly supported by similar control mechanisms that up- and downregulate CSF influx in the periarterial space (26).
The aim of this review is to provide a detailed description of the current understanding of brain fluid dynamics, which in the past years has undergone a major overhaul. Diffusion was long believed to be the predominate transport mechanism within the brain, even though early work had already shown simple diffusion to be insufficient as a clearance mechanism for brain, one of the most metabolically active tissues in the body. The discovery of the glymphatic pathway provided not only an explanation to the decades old mystery but also opened up a new biological field of study, which is helping us to understand how brain clearance is maintained in the healthy brain, why we need to sleep, and the root cause of neurodegenerative diseases (27). The biological constraints of state-dependent higher brain functions have rarely been considered, but emerging evidence points to a deterioration of homeostatic glial function in the genesis of most, if not all, neurological and psychiatric diseases (28, 29).
We shall describe the organization and physiological drivers of the glymphatic system, as well as the molecular components of brain extracellular matrix and their role in parenchymal flow. We shall also describe the drivers of convective CSF flow in perivascular spaces and how glymphatic flow is modulated depending on brain state. The core thesis underlying this review is that a proper understanding of fluid transport in peripheral tissues provides insight into the less understood fluid flows in the CNS. Therefore, we shall review the current knowledge of fluid transport and the lymphatic system in peripheral tissues and then draw parallels to brain. Also, the glymphatic system is at multiple sites linked to extra-axial CSF flow, which include CSF production sites, CSF flow in the ventricles and subarachnoid space, and CSF egress sites. Therefore, we must describe the dynamics of fluid transport in these compartments outside the brain tissue proper to explain their interplay with the glymphatic system. Next, we shall describe the pathophysiological changes of fluid flow in various brain disorders and how novel imaging tools and biomarkers are rapidly revolutionizing our understanding of brain physiology. We contend that new insights into brain fluid dynamics will lead to the discovery of novel pathophysiological mechanisms, diagnostics tools, and therapeutics avenues.
2. THE DELINEATION OF BRAIN FLUID TRANSPORT THROUGH TIME
2.1. Introduction
The study of brain fluid transport is as old as the study of the brain itself, and the earliest notions of structures involved in brain fluid transport are found in the first known biological academic works stemming from ancient Greece. Whereas some of the earliest works were descriptive and often contained fanciful postulates, the revival of science during the renaissance led to a revolution of the understanding of brain fluid systems. The discoveries of key components of the nervous system cellular components and long-distance signaling mechanisms occurred simultaneously with the recognition of brain fluid transport, reflecting that both processes are integral to CNS function. Production of CSF by the choroid plexus, the CSF flow pathways, and the description of perivascular spaces as being conduits of CNS to the lymphatic drainage were all discovered in the same century as nerve fibers, action potentials, and synapses (30).
The field of brain fluid transport encompasses CSF formation and flow pathways, ventricular morphology, brain extracellular space constitution, brain clearance, CSF drainage sites, and many other factors. We present a run-through of the findings that over the centuries have helped researchers piece together an in-depth understanding of the brain’s fluid environment. This historical account is also a tour de force recalling major scientific personalities whose names will surely ring a bell for biologists and clinicians alike, due to the scattering of their eponymous discoveries across neuroanatomy and physiology. In this section, we go through the earliest discoveries such as the identification of the brain ventricles and the CSF that laid the groundwork for understanding brain fluid transport and link these early discoveries with modern day findings such as ultrastructural studies of fluid flow pathways in brain, physiological systems like the glymphatic system, and CSF and brain clearance pathways such as dural lymphatics. For a more detailed description of the history of the discovery of CSF we refer the reader to excellent reviews (31, 32), and for a more in-depth understanding of the early literature of CSF, perivascular spaces, choroid plexus, and the BBB, we refer to these classic reviews (33–35).
2.2. Early Identification of the Ventricles and Their Contents
The historical medical literature contains reports of colorless fluid leaking from skull fractures and other neurotrauma. The oldest description of this fluid, which we now know as CSF, is recorded in the Edwin Smith papyrus scroll written in Egyptian hieroglyphs around 1500 BC, which is perhaps derived from an even older lost text dating from 3000 BC. The papyrus describes fluid leakage from a comminuted skull fracture (31, 36). However, for many centuries the content of the brain ventricles and subarachnoid space remained unknown, and the discovery of CSF is thus relatively new. The first written description of the brain ventricles in an academic text is found in Aristotle’s Historia Animalium from the fourth century B.C. (31, 37). A later Greek philosopher, Galen of Pergamon (129-c. 200 A.D.), believed that the ventricles harbored the spiritus animalis: a life-giving vapor providing vigor to the body and mind (38). This hypothesis prevailed for more than a millennium, and it was not until the 16th century that the development of a more empirical science led to the recognition that the ventricles are not air-filled cavities. One of the first anatomically correct drawings of the ventricular system was created by Leonardo Da Vinci in the 15th century, who cleverly created a cast by injecting hot wax into the ventricular system of an ox brain and thereby produced the first three-dimensional morphological depiction of the cerebral ventricles (FIGURE 2) (32). In the Renaissance, other anatomists started to question the theories of Galen. The renowned anatomist Andreas Vesalius came up with the argument that, since the ventricular system is conserved among species, it could hardly be the abode of spiritus animalis, which was supposedly a quality unique to man (38, 40). Eventually, Galen’s theories about the ventricles were rejected, and several academics came to mention the presence of fluid in the CNS, among them the eponymously famous English doctor Thomas Willis. In 1664, he described the fluid in the cerebral ventricles and had the insight that the choroid plexus might possess a glandular activity as the source of said fluid (32). The Bolognese doctor Antonio Maria Valsalva, of the eponymous maneuver, reported in 1692 on the presence of fluid surrounding the spinal cord of a dog undergoing dissection (31, 32, 41). The formal discovery of CSF as a fluid extending from the ventricles to the subarachnoid space and the first estimation of its total volume are attributed to another Italian doctor, Domenico Cotugno, of the University of Naples. His 1764 work on sciatic nerve pain includes a detailed description of CSF based on his dissections of 20 human cadavers (41). Cotugno explained why something so seemingly obvious as the presence of CSF in CNS had been largely overlooked by other anatomists, noting important methodological factors such as the customary dissection of previously decapitated heads. In Cotugno’s approach, the heads were severed from the fresh cadavers in his laboratory, which inevitably resulted in spillage of a clear fluid (3 Neapolitan ounces in his estimation, where 1 Neapolitan ounce = 26.72 mL) from the foramen magnum (32, 41). In the subsequent century, research into CSF and its spaces accelerated, and the focus shifted from merely anatomical and descriptive to include investigations of the physiology of the CSF system and speculations about its function.
FIGURE 2.

Timeline of significant events and discoveries pertaining to brain fluid transport. The black arrow superimposed over the cerebral artery illustrates the passage of time. The timeline spans over millenniums and includes the first clinical description of cerebral spinal fluid (CSF) in ancient Egypt found in the Edwin Smith papyrus scroll from 1500 BC, the first morphologically correct drawing of the ventricular system by Leonardo da Vinci in 15th century, and the description of CSF flow as the third circulation by Harvey Cushing in the 20th century. Many other substantial discoveries extending beyond those illustrated in the figure established the foundation to our modern understanding of brain fluid transport but due to space limitations cannot be included in the figure (see FIGURE 3). Images are courtesy of The New York Academy of Medicine Library (Edwin Smith Papyrus scroll) and from Ref. 31 (Da Vinci drawings; with permission from Springer International), Ref. 39 (schematic by Harvey Cushing; with permission from The Archives of Surgery), and Ref. 49 (Arachnoid granulations drawing by Key and Retzius).
2.3. Outlining of CSF Pathways, Production, and Egress Sites and Investigations Into Its Function
Perhaps because Cotugno mentioned CSF as an incidental finding in his work on sciatic pain, it was not known to the wider medical audience until discussed by the 19th century French physiologist Francois Magendie (32, 42). Magendie discovered the median aperture that connects the fourth ventricle to the cisterna magna, thus revealing the ventricular system to be in open contact with the subarachnoid space, which provides a pathway for flow of CSF (42). He also coined the term liquide céphalo-spinal (in English cerebrospinal fluid), as it is now universally known (31, 32). Despite the intuition of Thomas Willis, the site of CSF production remained a matter of discussion in the scientific community late into the 20th century. Magendie proposed that CSF was produced in the leptomeninges, but his contemporary colleagues Ernest Faivre and Hubert von Lushcka (who also discovered the lateral apertures, a second communication between the fourth ventricle and the subarachnoid space) had proposed that the choroid plexus was its source, due to their histological findings of cellular inclusions in the choroid plexus epithelium suggestive of secretory activity (33, 42). This view was supported by subsequent histological and pharmacological studies showing increased cytoplasmic volume of choroid plexus cells from animals treated with the muscarinic agonist pilocarpine, which evokes an increase in CSF pressure (42, 43). Definitive proof of CSF secretion by the choroid plexus did not come until 1960, when Rougemont in elegant experiments managed to collect the choroid plexus fluid secreted in vivo and showed by analysis that its electrolyte composition was similar to ventricular CSF and distinct from that of a plasma transudate (33, 44). Later, George B. Hassin of the University of Illinois discovered that a complete surgical removal of choroid plexus did not completely ablate CSF production (45), whereupon it was generally conceded that there must also be extrachoroidal CSF production (33).
A detailed understanding of the flow pathways of CSF arose early in the 20th century. Whereas Magendie had originally proposed an ebb and flow movement of CSF, it became apparent that it was largely unidirectional flow and was thus designated the third circulation by American neurosurgeon Harvey Cushing in 1926 (46). The third circulation was proposed as a circulation parallel to the bloodstream, whereby CSF moved by bulk flow driven by the active production of CSF in choroid plexus, thus draining from the ventricular system and into the subarachnoid space, and then flowing along the cerebral hemispheres eventually to find egress to the bloodstream through arachnoid granulations in the dural sinuses (FIGURE 2). Cushing’s view was largely supported by work of his former student Lewis H. Weed, who in a series of papers claimed that CSF drains directly into the bloodstream rather than via the lymphatics (47, 48). Weed’s experimental studies were supported by human cadaver studies of the preceding century, in which Ernst Axel Henrik Key and Magnus Gustav Retzius of Karolinska University injected blue-colored gelatin into the CSF compartment and showed its subsequent accumulation in the arachnoid granulations (FIGURE 2) (49). Although contemporary scientists had reported CSF drainage along perineural sheaths and via the nasal mucosa to the lymphatics, which were also labeled in the dye experiments of Key and Retzius, these sites were considered by Weed and Cushing as minor accessory drainage paths (43, 47, 49). Another matter of considerable debate concerned the role of brain perivascular spaces in the circulation of CSF. From their earliest identification in the mid-18th century up until today, the histological composition and functional significance of the brain perivascular space have been discussed at length (35, 50). Today the spaces are known as Virchow-Robin spaces, due to Rudolf Virchow’s account of them in 1851 and that of Charles-Philippe Robin in 1859 (35). In the late 19th century, the first hypotheses arose proposing perivascular spaces to be a specialized brain lymphatic system (51). That view was furthered when Sir Frederick Walker Mott published in the beginning of the 20th century what was considered compelling histological evidence of a canalicular system, where the subarachnoid space was held to be in direct communication with the perivascular space, which in turn communicated with perineuronal spaces, thus constituting a direct fluid pathway from the neuron to the subarachnoid space (35, 43). Cushing and Weed were convinced of the existence of this canalicular system, though they disagreed with Mott about the directionality of the fluid flow in the perivascular spaces (35, 43, 47). Mott believed that fluid from the subarachnoid space was driven inward into the perivascular spaces toward neurons, finding ultimate drainage via cerebral capillaries, while Weed and Cushing argued that under physiological conditions the flow should proceed from the neuron toward the subarachnoid space. That view fit with their third circulation model (FIGURE 3), and Weed showed experimentally that perivascular inflow of his CSF tracer, potassium ferrocyanide (Prussian blue), only occurred under pathological conditions such as after injecting hypertonic saline into the bloodstream (43, 47). Eventually, some of the findings of Mott were identified as artifacts caused by histological procedures, and the perineuronal spaces were attributed to neuronal shrinkage postmortem, thus not representing an actual open communication with the perivascular spaces (35).
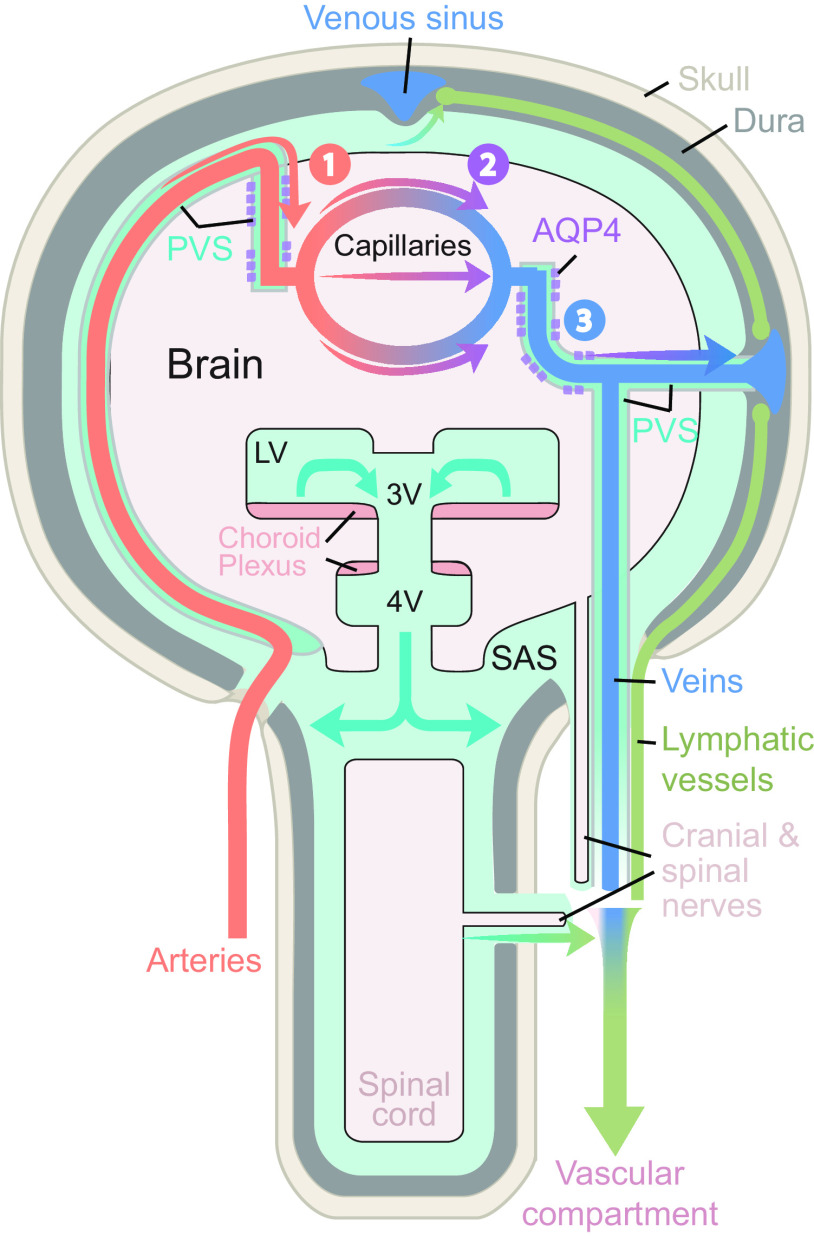
FIGURE 3.
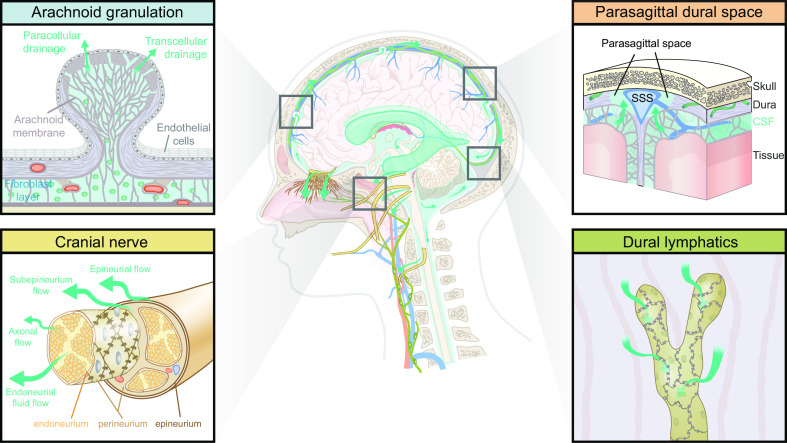
Schematic of brain fluid transport. Cerebrospinal fluid (CSF) is produced primarily in the choroid plexus which sits in the lateral, 3rd, and 4th ventricles, but it has been suggested that a substantial contribution from extrachoroidal sources contributes to the overall production of CSF. CSF produced within the ventricles flows into the subarachnoid space through the lateral and median apertures and into the basal cisterns (see sect. 5) (33, 52). The subarachnoid space contains around 80% of the total CSF volume, compared with 20% within the ventricles (53). Subarachnoid CSF extends all over the brain and spinal cord and keeps the nervous system buoyant and thus allows it to maintain its shape, despite the lack of a fibrous extracellular matrix. From the subarachnoid space, CSF can either flow into the glymphatic system or egress from the intracranial or intraspinal space directly into the peripheral circulation (see sect. 6 for intra-axial fluid dynamics and sect. 12 for CSF egress). The CSF that enters the periarterial spaces is pumped forward in an anterograde manner along the major cerebral arteries and from there it enters the brain through the perivascular spaces of the thousands of penetrating arteries that perforate the brain tissue (1) (see sect. 9 for physiological drivers) (4, 21, 54). From the periarterial and periarteriolar spaces, CSF is driven into the brain extracellular space over the glia limitans perivascularis in a process that is supported by the polarized expression of aquaporin 4 (AQP4) water channels on the abluminal membrane of the astrocytic endfeet (2) (see sect. 7 for details on fluid flow in brain extracellular space and sect. 8 for roles of AQP4 in brain fluid transport) (4, 22, 55). The flow of fluid through the tortuous brain extracellular space is probably driven by a combination of diffusion and convection. Extracellular fluid with metabolites and waste products is drained into perivenous spaces and finds efflux from the brain following the large cerebral veins (3) (4, 56, 57). CSF from the subarachnoid space and extracellular fluid finds egress from the intracranial compartment to peripheral circulation through several different egress pathways, including dural lymphatics, perineuronal pathways, parasagittal spaces, arachnoid villi, and granulations and adventitia of large cerebral vessels (58). PVS, perivascular space; SAS, subarachnoid space.
Another unique property of the brain vasculature and extracellular fluid became evident in the 19th century with the first reports indicating the existence of the BBB (34). In 1885, Paul Ehrlich injected various dyes subcutaneously in living animals and studied their spread in different organs, observing very limited penetration of most dyes into the brain parenchyma (59). Ehrlich firmly believed that this phenomenon was not due to confinement of the dye within the blood vessels but instead due to a special property of the nervous system in excluding the dye uptake (59). In 1900, German neurologist Max Lewandowsky (60) showed that the respiratory toxin sodium ferrocyanide caused toxic effects when injected into the subarachnoid space, whereas the same dose injected intravenously had only minimal effects. Lewandowsky concluded from this simple experiment that there must be a barrier between the blood and the brain that is circumvented upon injection into the subarachnoid space (34, 43, 61). This discovery led to speculations regarding the physiological function of such a barrier. Publishing in 1909, Edwin Ellen Goldmann reported similarities between the way intravenously administered dye accumulated in the placenta and in the choroid plexus, which led him to believe that the choroid plexus extracted nutrients from the bloodstream and delivered them to brain via the CSF, thus proposing a nutritive role of CSF flow (34). Although this concept was initially accepted by many, it seemed at odds with the enormously rich capillary vascularization of the brain tissue (34). The Latvian/Soviet physiologist and biochemist, Lina Solomonovna Stern, was likely the first to suggest a twofold path for entry of compounds in the blood into the brain, consisting of transport through the choroid plexus and across the endothelial cells of the brain vasculature (61). Stern also proposed that the function of the BBB was to help maintain a constant chemical composition of the CSF and thus a stable environment for the milieu intérieur of brain parenchyma relative to other organs (61). With the technological advances of modern medicine and science from the last half of the 20th century, many concepts theorized in the previous century were not only proven to be true but also elaborated upon in great detail.
2.4. Modern CSF History: Outlining the Microanatomy, Detailing Flow Pathways with In Vivo Imaging, and Specifying Functions in Physiology and Pathophysiology
Factors that drive fluid flow and regional variations in flow properties within different intracranial compartments have been outlined in great detail in the last 50 yr. The bulk flow of CSF from the ventricles to the subarachnoid space as first proposed by Weed has since been quantified by a variety of approaches. The rate of CSF production was measured in humans by following the washout of a radiolabeled tracer using a ventriculo-cisternal perfusion system, giving a rate of 0.3 mL/min (62), which suggests a total CSF volume turnover three to five times per day, given the estimated total CSF volume of 130–150 mL (see sect. 5 regarding CSF production rates) (33, 34). The observed high rate of CSF production led Hugh Davson to formulate the sink hypothesis, where the rapid export of CSF toward the bloodstream functioned as a sink for the brain parenchyma, with transfer of extracellular compounds to the CSF by simple diffusion. The rapid turnover of CSF thus prevents build-up of metabolic waste products in the brain, driven by a concentration gradient between the extracellular fluid and the CSF (33, 34). The diffusional component of Davson’s sink hypothesis was considered inadequate by Helen FitzGerald Cserr, who in a range of studies reported that 10–20% of total CSF production arose in the brain parenchyma and was thus of extrachoroidal origin. Furthermore, she noted that when tracer compounds of different molecular weights were injected into the brain parenchyma, they had similar rates of travel away from the injection site, thus indicative of extracellular fluid convective flow rather than simple diffusion, which would have been dependent on molecular weight (see sect. 11.4) (33, 52, 63, 64). For some time, researchers paid scant attention to the role of perivascular spaces in brain extracellular fluid clearance, due to an abiding belief that 1) these spaces were not in open contact with the brain capillaries and thus could not possibly function as sources or egress sites of CSF, and 2) due to the pial-glial barrier membrane, the spaces were not in open contact with brain extracellular space (33, 35, 64). Electron microscopy studies conducted in the 1960s showed that certain aspects of these assumptions were incorrect and that leptomeningeal cells and astrocytic endfeet forming sheaths around the outer layer of the perivascular space were permeable, such that compounds injected into the CSF freely pass from the perivascular spaces into the brain extracellular space (65). Cserr eventually reported that tracers injected into the parenchyma indeed accumulated in perivascular spaces and near the ventricles, which led her to conclude that extracellular fluid must flow from brain through perivascular spaces and into the subarachnoid space, as well as flowing along white matter fiber tracts (64). This scenario recalls the concept of perivascular flow as originally proposed by Weed in 1910 (42, 63). In 1985, Patricia A. Grady published a novel finding indicating that flow of CSF in perivascular spaces actually constituted a kind of circulation, where CSF from the subarachnoid space flows into the neuropil via perivascular spaces around penetrating arterioles and then through the basal lamina of capillaries to egress the neuropil along perivascular spaces surrounding veins (66). Grady drew these conclusions based on tracer studies using the infusion of horseradish peroxidase into the lateral ventricle or cisterna magna of cats and dogs, followed by observations of the time-dependent spread of the tracer in histologically stained brain sections. She saw that after as little as 10 min after infusion, the perivascular system was outlined by tracer around arteries and also veins, thus suggesting a flow velocity much faster than could possibly be obtained by simple diffusion (66).
In 2012 the Maiken Nedergaard group (4) made key observations regarding the unique properties of extracellular fluid flow in the brain parenchyma, which they designated as the glymphatic system, a unique extracellular space clearance system in the brain. Their model showed how extracellular fluid movement is dependent on fluid inflow from CSF along periarterial spaces (the Virchow-Robin spaces). The astrocytic endfeet that plaster and fully enwrap the vasculature creates the unique perivascular space. The perivascular spaces are open and provide little resistance for CSF influx driven by arterial pulsatility (21, 54). Furthermore, the polarized expression of AQP4 water channels on the vascular side of astrocytic endfeet facilitate CSF movement into and out of the neuropil (4). Their work showed that deletion of AQP4 (AQP4 knockout mice) caused a 55% reduction in the clearance of exogenous amyloid-β (4). Remarkably, they found that the glymphatic system was primarily active during sleep, thus presenting a novel restorative function of sleep as a means of clearing the brain of metabolic waste products that accumulated during wakefulness (1).
The term glymphatic system was coined due its dependence on AQP4 expression in glial endfeet and due to its role in clearing the extracellular space of metabolites, thus in analogy to the lymphatic system that serves this role in all organs other than the brain. The glymphatic system concept provides a completely new understanding of CSF flow in perivascular spaces and the important role of AQP4 expression in vascular astrocytic endfeet for brain extracellular fluid exchange. The brain-cleansing function of the glymphatic system effectively removes proteins involved in several neurodegenerative diseases, i.e., amyloid-β, tau, and α-synuclein in Alzheimer’s disease and Parkinson’s disease, from the brain by bulk flow (27, 67). The understanding of CSF egress has also gone through a complete revision from the earlier thinking that arachnoid granulations in superior sagittal sinus were the only exit sites. Jonathan Kipnis and Kari Alitalo reported in 2015 on the existence of lymphatic vessels in dorsal dura mater, which were later shown to drain solutes in the CSF (23, 68). Even though the dural lymphatic vessels had been described in the 18th century by the Italian anatomist Paolo Mascagni, their presence was forgotten or ignored for more than 200 yr and they were until recently never considered important for brain fluid clearance (69). However, the work by Kipnis, Alitalo, and others shows dural lymphatics to be important egress routes for metabolic wastes from brain, and important mediators of inflammatory processes in the CNS, making this brain extracellular fluid egress path highly relevant for the understanding of many neurological disorders (70, 71).
In summary, studies of CSF and its physiological role are as old as the discipline of biology itself. As presented in this section, many scientists over the ages have labored to understand CSF and brain fluid clearance; many of the names mentioned in this section are known even to casual students of medical history or neuroanatomy. Many aspects of this field of study have sparked controversy, and even to this day some discussions initiated a century ago continue to be debated enthusiastically, such as the types of fluid flow existing in brain parenchyma or the directionality of flow in the perivascular space. More often than not, this ignites fruitful scientific discussion, leading to new important experiments, and thus facilitates progress in the field. However, too often have lingering misconceptions and incorrect description of past literature hampered progress, both in the past and in present time (18, 72). Our understanding of brain clearance pathways and their involvement in various neurological disorders is currently in a state of rapid flux. It is thus imperative that in the future, rigorous and creative work recalls the history. Avoiding misconceptions and improving the technical approaches will both aid in deciphering CSF clearance and brain fluid dynamics.
2.5. Outstanding Questions
Several times through history, hypotheses and models of brain fluid transport have been taken as fact, and this static thinking has delayed new discoveries and prevented important scientific questions from being asked and investigated. How do we prevent our current understanding of brain fluid transport from petrifying, and how can we ensure that scientists approach the field with an open mind, making a critical examination of old literature and creatively testing new and old hypotheses by designing original experiments?
Artifacts caused by death or chemical handling of tissue samples for histological examination have often led to misconceptions about brain fluid transport. For example, fluid-filled spaces disappear and flow tracers in the postmortem brain are translocated into surrounding tissue. How do we prevent artifacts of currently used techniques from blurring our understanding and misleading the field in the future?
2.6. Interim Summary Section
The earliest description of CSF stems from physician notes from ancient Egypt written down around 1500 BC. The first descriptions of the brain’s ventricular system come from ancient Greece, where Aristotle wrote about brain ventricles in the fourth century BC. A more precise description of CSF in terms of volume and location was first provided by Domenico Cotugno in the late 18th century.
From the late 19th to mid-20th century, the macroscopic brain fluid pathways were laid out in great detail. Francois Magendie described the open communication between the intraventricular CSF and that of the subarachnoid space. The choroid plexus was identified as the most important source of CSF, and CSF drainage pathways were identified at the arachnoid granulations and in the nasal submucosa. Harvey Cushing conceptually described the flow of CSF from production site to drainage sites as the third circulation.
Perivascular spaces were first identified in the mid-19th century by Rudolf Virchow and Charles-Philippe Robin, and it was then suggested that they might function as brain lymphatic channels. In the late 20th century, these perivascular spaces within the brain were shown to be flow pathways of compounds injected both into the CSF or brain parenchyma. In the new millennium, Maiken Nedergaard presented the mechanisms and physiological importance of perivascular CSF flow for brain clearance in her description of the glymphatic pathway, where fluid flow in the brain parenchyma is established primarily by glial cells and their expression of the water channel AQP4. Drainage of CSF and brain clearance was furthermore shown to be dependent on dural lymphatics by Jonathan Kipnis and Kari Alitalo in the 2010s.
The modern view of the third circulation includes CSF production in the choroid plexus and at extrachoroidal sites, flow of CSF through the ventricular system and into the subarachnoid space, CSF flow into the glymphatic system (which is crucial for brain clearance and homeostasis), and lastly, drainage of CSF and brain extracellular fluid through dural lymphatics, perineuronal spaces, parasagittal spaces and arachnoid granulations.
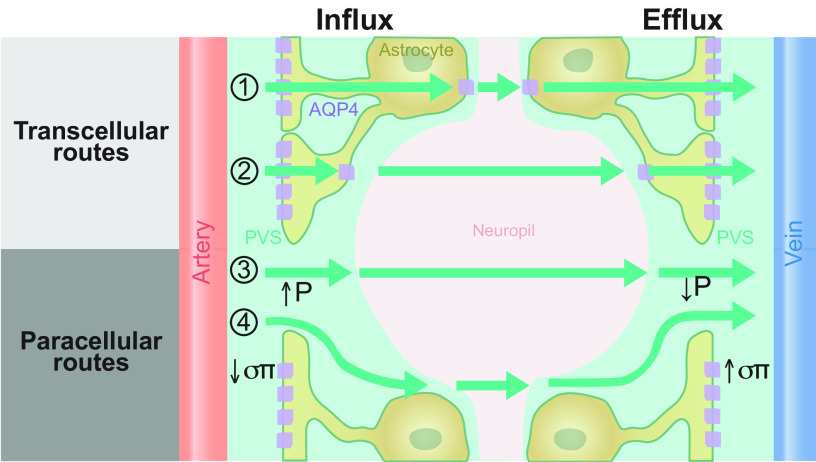
The glymphatic system conceptualizes brain fluid transport into three segmental fluid transport pathways: 1) periarterial CSF influx, 2) AQP4-supported influx and dispersion of CSF in the extracellular space, and 3) perivenous efflux. Meningeal lymphatic vessels surround the large venous sinuses and export, at least in part, perivenous fluid, thereby adding a fourth segment to brain fluid transport. Segments 1, 3, and 4 can be viewed as a plumbing network, while extracellular fluid dispersion in segment 2 represents the functionally important part of the glymphatic system that delivers metabolites and removes waste.
3. DEVELOPMENT OF BRAIN FLUID SYSTEMS
3.1. Introduction
The brain fluid system consists of several compartments, cellular and vascular components, and multiple barriers that successively mature during embryonic and postnatal development. The functional properties of the brain barriers (see sect. 5 for adult function of BBB), the composition of the CSF, and the shape of the cerebral ventricles in developmental and adult states of the organism differ. These key components of the brain fluid systems serve distinct functions in cell proliferation and morphogenesis during CNS development. In this section we shall describe the development of the structures involved in the adult brain fluid systems, giving an account of their properties and function across development, and emphasize the point at which they have adopted their typical functions in adult CNS. For the sake of objectivity, when describing the time course of development, we mainly refer to the developmental day of the animal in which the experiments were conducted. For readers wishing to translate the experimental animal developmental days to corresponding human landmarks, we refer to a translational tool (73). We also refer to prior excellent reviews for a more detailed description of the development of the choroid plexus (74), BBB (75), and brain vascularization (76).
3.2 Development of the Glymphatic System
The rodent glymphatic system does not develop and mature until after birth, suggesting that the need of brain clearance is lesser during development or is perhaps maintained in different ways. The earliest sign of perivascular CSF transport begins at embryonic day 17.5 (E17.5) of the 20-day gestation in mice, with the onset of perivascular CSF tracer transport at proximal arterial branches emanating from the Circle of Willis (FIGURE 4) (77). The first indications of intra-axial glymphatic fluid flow are seen around postnatal day 1 (P1) in the hippocampus. Subsequently, CSF influx initiates in neocortical regions, proceeding from the ventral to dorsal aspect of the brain, reaching maturity at P14 (77). The development of the glymphatic system depends on the formation of a cover of astrocytic endfeet lining the perivascular spaces, along with polarization of AQP4 expression toward the vascular side of astrocytic endfeet (77). The development and maturation of the glymphatic system relies heavily on the signaling molecule platelet-derived growth factor B (PDGF-B), for which receptors are expressed on pericytes throughout development (77). Knockout of PDGF-B in mice eliminates astrocytic AQP4 polarization, probably due to reduced vascular recruitment of pericytes. Pericytes are important for the development of AQP4 polarization at the astrocytic endfeet (see sect. 8 on AQP4) (77). Pericytes are also essential components of the neurovascular unit, with particular involvement in the development and maintenance of the BBB. PDGF-B signaling leading to pericyte recruitment is mediated during angiogenesis by secretion from endothelial cells at the tip of the endothelial sprout and from PDGF-B derived within the extracellular matrix (75, 78). Recruited pericytes contribute to several intercellular signaling pathways engaging endothelial cells and astrocytes in the formation and maintenance of the BBB (75). Thus the postnatal development of the glymphatic system goes hand in hand with the maturation of the BBB.
FIGURE 4.
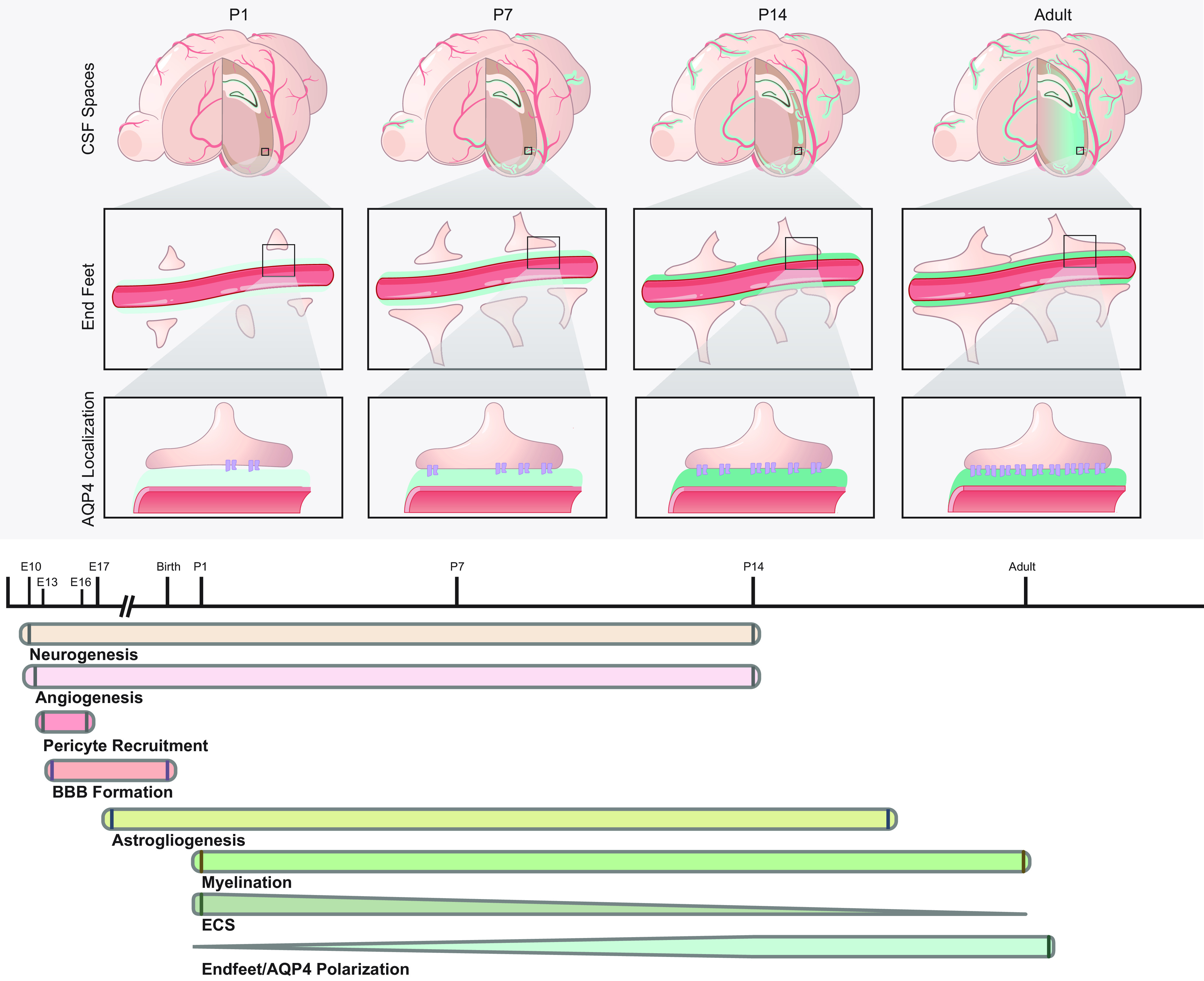
Development of the glymphatic system. The glymphatic system develops postnatally. The earliest signs of perivascular cerebral spinal fluid (CSF) flow appear proximal to Circle of Willis at embryonic day 17.5 (E17.5) in mice. Actual parenchymal CSF inflow is first evident in the hippocampus at postnatal day 1 (P1). From P7 to P14 the neocortex starts to show widespread CSF tracer inflow, proceeding from the basal to the dorsal aspect of the brain. By P14, the development of the murine glymphatic system is functionally and morphologically complete. The onset of glymphatic flow requires the development of astrocytic endfeet with polarized expression of aquaporin-4 (AQP4). BBB, blood-brain barrier; ECS, extracellular space.
3.3. Development of CSF Egress Sites
Egress of cranial CSF in adult mammals takes place through meningeal lymphatics and perineuronal sheaths to the cervical lymphatic system or through arachnoid villi/granulations into the bloodstream (see sect. 12). CSF drainage from the subarachnoid space and the flow of CSF from the ventricles to the subarachnoid space seem to emerge relatively late in brain development, at around 14–24 wk gestational age for humans and E18 in rats (79, 80). In mouse, meningeal lymphatics develop just before birth, but for the most part undergoes its maturation during the first postnatal month (24). The formation of lymphatic vessels begins at the base of the skull in association with the larger vessels and cranial nerves, and progresses to involve latero-ventral parts of the dura, such that they make their first appearance around the foramen magnum and jugular foramen, then at the pterygopalatine artery and middle meningeal artery, and at later developmental stages come to involve the superior sagittal sinus and the confluence of sinuses (24). Lymphangiogenesis in dura mater depends on the release of vascular endothelial growth factor-C (VEGF-C) by vascular smooth muscle cells and from the pituitary and pineal gland (24). The current thinking on the development of the arachnoid villi and the larger arachnoid granulations remains somewhat a mattery of controversy. Some reports indicate the appearance of a primitive arachnoid villi at embryonic week 26 in the human, with anatomically mature arachnoid villi evident from week 35, which provides histological, but not functional, evidence for their capacity to support CSF egress to the bloodstream during prenatal development (81). However, the villi are not abundant in human infancy, and studies in late gestation fetal and neonatal sheep (aged 2–6 days) show that the majority of CSF egress is via perineuronal and lymphatic drainage (82, 83). Furthermore, the lateral lacunae parallel to the superior sagittal sinus have been detected in histological examination of human fetal brain. MRI tracer studies following intrathecal gadobutrol administration reveal that these structures drain CSF from the subarachnoid space to dura mater in the adulthood (81, 84). It remains to be elucidated if these and other pathways are functional in the human fetus.
3.4. Ventricular Development and Morphology
At the earliest stages of brain development, the ventricles and ventricular CSF play an essential role. In the human embryo, the process of neurulation occurs during the fourth week of gestation, whereupon the neural plate folds longitudinally and forms the neural tube (FIGURE 5) (85). Initially, the neural tube is in open communication with the amniotic fluid through the anterior and posterior neuropore, but these openings subsequently close to form an enclosed fluid cavity filled with amniotic fluid, which can be considered the first CSF (86). Concomitant with the formation of the neural tube, a series of coordinated events involving cell proliferation, migration, and apoptosis lead to accelerated growth in the anterior portion of the neural tube as well as formation of a number of flexures, which give rise to three brain vesicles that are precursors of the forebrain, midbrain, and hindbrain (85, 87). The lumen of these three brain vesicles is destined to develop into the ventricular system, whereas the lumen in the posterior part of the neural tube gives rise to the central canal in the spinal cord (85, 87). An occlusion emerging between the brain ventricles and spinal canal arguably facilitates the rapid growth of the brain ventricles by increasing intraluminal pressure specifically in that region, thus resulting in a massive expansion of the ventricular volume (85, 88). In human embryos, this occlusion occurs around gestational week 4 (89). The developmental occlusion seems to be initiated by processes occurring in the tissue lateral to the neuroectoderm, which are also important in promoting the formation of the neural folds. The maintenance of the occlusion is dependent on extracellular Ca2+ and intracellular signaling-mediated by calmodulin and cAMP (90). As the CNS develops further, the ventricles eventually attain their adult morphology, whereby the anterior, dorsal, and posterior proliferation zones of the telencephalon define the elongated shape of the lateral ventricles. The embryonic forebrain ventricle develops into the adult lateral ventricles and the third ventricle, the midbrain ventricle gives rise to the Sylvian aqueduct, and the hindbrain ventricle eventually forms the fourth ventricle (85). Around the ninth fetal month, the ventricular system has an appearance resembling that of the adult (91). The communication between the ventricular system and the subarachnoid space is established relatively late in fetal development; the foramen of Magendie reportedly appears at 4 mo and foramina of Luschka at 6 mo or later in the human fetus (80).
FIGURE 5.
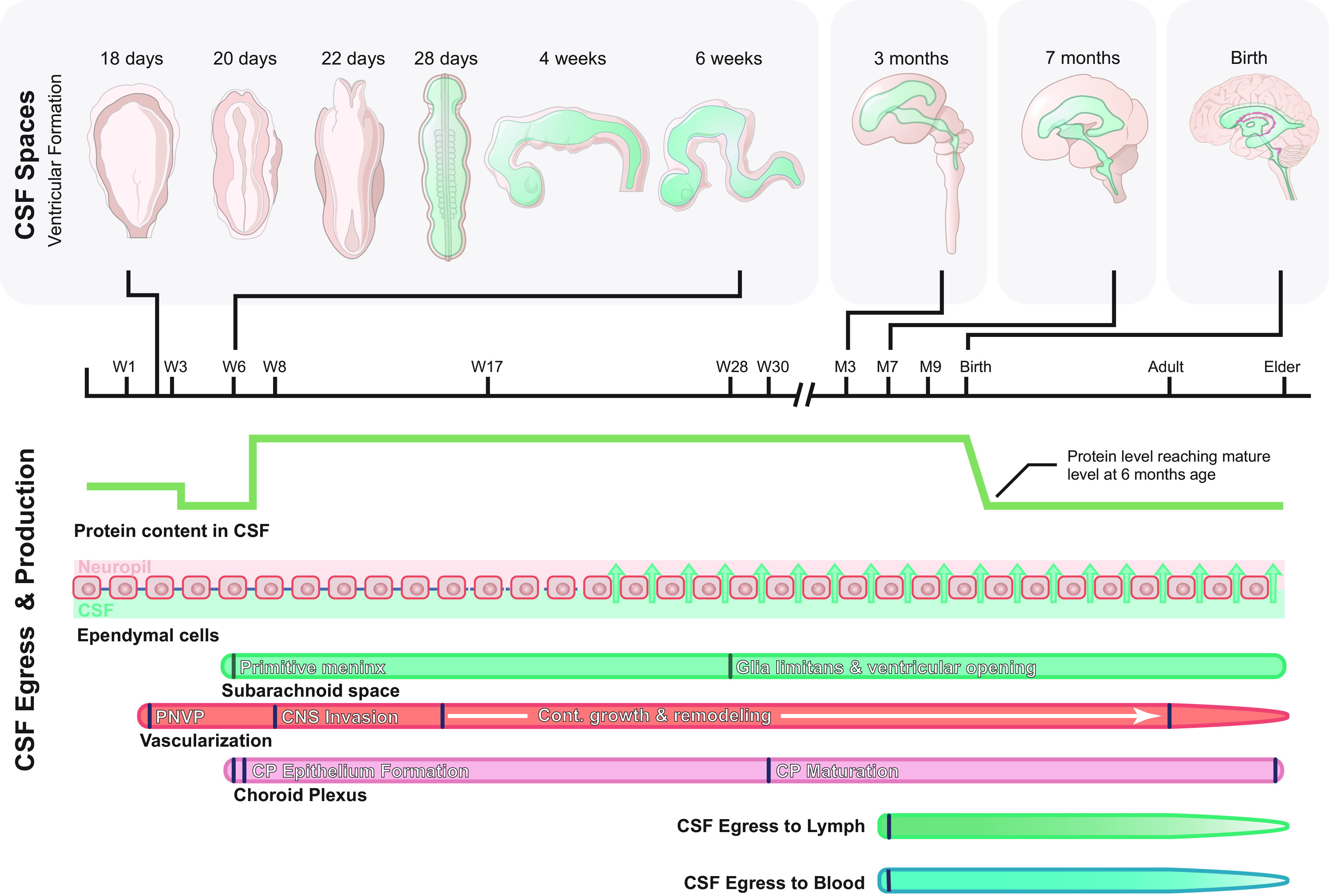
Development of ventricles, cerebral spinal fluid (CSF) spaces, and egress sites. The initial development of the cerebral ventricles occurs during neurulation, when the neural plate infolds to form the neural tube, which is initially in open contact with the amniotic fluid. Around day 28 in human gestation the anterior and posterior neuropores close, and the first CSF is formed from the captive remnant of amniotic fluid trapped in the neural tube lumen. During the coming weeks and months, infolding of the neural tube results in the formation of the primitive ventricular system consisting of the three brain vesicles. With further growth of the central nervous system (CNS), these vesicles eventually inflate under CSF pressure to form the ventricles of the adult CNS. Strap junctions between ependymal cells create a tight barrier between the CSF compartment and the brain extracellular space, which likely allows for this inflation. In humans, the ventricular compartment and subarachnoid space become communicated between embryonic months 4 and 6, with the opening of the foramina of Luschka and Magendie. Vascularization and formation of the choroid plexus occurs very early in brain development, with onset at embryonic day 10 (E10) in mice and around embryonic week 6 in human brain. CSF egress systems develop only after birth, although there must be some mechanism supporting egress in utero. PNVP, perineural vascular plexus; W, gestational week; M, gestational month.
3.5 CSF Composition in the Embryo and Fetus
The composition of CSF is tightly regulated during development. Unlike the adult CSF, the embryonic and fetal CSF has a high protein content, which begins to decline after birth, only falling to adult levels at ∼6 mo of age (10). The high protein content and the ionic composition of the embryonic CSF results from the balance of tightly regulated influx and efflux mechanisms, which are distinct from those at the adult blood-CSF barrier (10). The composition of CSF during development is tuned to meet the changing requirements of the fetal brain (10). After the closure of the neuropore, CSF composition begins to deviate from that of amniotic fluid (86). Initially, the total protein content declines both in CSF and amniotic fluid, but after the formation of the choroid plexus around week 7, protein content begins to increase in CSF, whereas it continues declining in the amniotic fluid (86). Specific signaling molecules involved in neural stem cell differentiation (e.g., sonic hedgehog) peak in embryonic CSF at very specific timepoints, even preceding the development of the choroid plexus (86). Hence, some components in embryonic CSF are likely derived either from the neuroepithelium lining the ventricles or from the blood after the developing brain initiates vascularization (10, 87). Before choroid plexus development in chick embryos, growth factors such as fibroblast growth factor 2 and other blood-born peptides can freely enter the embryonic CSF from the bloodstream by a transcellular route through vascular endothelial cells in the ventral mesencephalon and the anteroventral part of the prosencephalon (87, 92, 93). Furthermore, a wide range of specific transporters as well as ion channels are already expressed in the embryonic endothelial cells, e.g., the glucose transporter 1 (GLUT-1), aquaporin-1 (AQP1) water channels, and the inwardly rectifying potassium channel Kir4.1 (10, 87). The CSF is of cardinal importance in brain development for two main reasons: first, due to the high content of protein and proteoglycans in the CSF compartment, a critical colloid osmotic pressure gradient between the CSF space and amniotic fluid is established, which probably drives the massive fluid influx that contributes to developmental ventricular inflation (94, 95). This inflation is facilitated by strap junctions (see sect. 3.8) between cells of the neuroepithelium, and these embryo-specific junctions then prevent fluid flow into the neural extracellular space (10). Ventricular inflation is critical for normal proliferation of neural cells and brain expansion, as exemplified by the attenuated brain growth after experimental deflation of the CSF space or in the anencephaly occurring in embryos with failed closure of the anterior neuropore (85, 94). The second important factor of the CSF in development is that the enclosed CSF compartment is an important medium for distributing growth factors with tight temporal and spatial restriction when the choroid plexus forms and thus is critical for CNS morphogenesis (74, 87).
3.6. Development of Brain Vasculature
The earliest blood supply to the embryonic nervous system entails diffusion of gases and solutes from a perineural vascular plexus covering the surface of the neural tube, which in human embryos appears at weeks 3 and 4 (FIGURE 5) (96, 97). Around the eighth gestational week in humans, the plexus sends out branches that penetrate the brain parenchyma and start to form an intrinsic vascular plexus (98, 99). This penetration starts with a fusion of the basement membranes of the capillary and glia limitans externa, which is the outer glial membrane of the brain composed of glial endfeet. The nascent vessels penetrate the glia limitans and grow into the nervous tissue in a funnel defined by basement membrane and external glial endfeet layer, which remains in open contact with the subarachnoid space, thus forming the future perivascular spaces (98). The surface plexus will eventually develop into the leptomeningeal surface vasculature (76). The molecular machinery driving the formation of intrinsic brain vasculature has been characterized in considerable detail in animal models. The neuroectoderm secretes vascular endothelial growth factor (VEGF)-A, which activates VEGFR2 receptors on the angioblasts of the perineuronal vascular plexus to initiate the formation of endothelial sprouts. These penetrate the neural tissue and form the intrinsic vascular plexus via a process called sprouting angiogenesis (76). Several other factors are crucial for the development of the intrinsic brain vasculature including, e.g., Wnt ligands (see review for detailed description of CNS angiogenesis; Ref. 76). Neurons are the primary source of VEGF-A during embryonic development, but after the completion of vascular remodeling, glia take over the role of paracrine VEGF-A secretion (76). The coordinated interaction between the endothelial cells, pericytes, neurons, and astrocytes (which together form the neurovascular unit) contributes to angiogenesis and the maintenance of brain vasculature during embryonic development and in postnatal life (76).
3.7. Development of Choroid Plexus
In humans, the anlage to the choroid plexus can be seen from around embryonic week 6, and an actual choroid plexus from week 7 (FIGURE 5) (95). The first steps in the development of choroid plexus takes place around E8.5–E9.5 in mouse, when repression of the neuronal fate of certain neuroepithelial cells is mediated by increased expression of antagonistic Hes transcription factors, which by downregulation of neurogenin-2 promotes the choroid plexus fate (for details of involved transcription factors, see review, Ref. 74). The choroid plexus develops within each primitive ventricle starting with the hindbrain, followed by development in the forebrain and midbrain. In humans, maturation of the choroid plexus epithelium goes through four morphological stages: 1) pseudostratified, 2) high columnar, 3) cuboidal, and 4) cubiform cells, in which the nuclei move to the basal part of the cell (74). Simultaneously, microvilli develop and mature at the apical side of the choroid plexus epithelium, which substantially increases the surface area of choroid plexus cells, thus increasing the surface area for CSF production. Tight junctions between the choroid plexus epithelium cells form the blood-CSF barrier, in which apicobasal polarity of the choroid plexus epithelium appears at a very early stage of choroid plexus development (74, 95). Vascularization of the choroid plexus likewise occurs early in development, in a process where signaling between the roof plate neuroepithelium, choroid plexus epithelium, and pericytes instigates formation of fenestrated capillaries in the choroid plexus (74).
3.8. Brain Barriers During Development
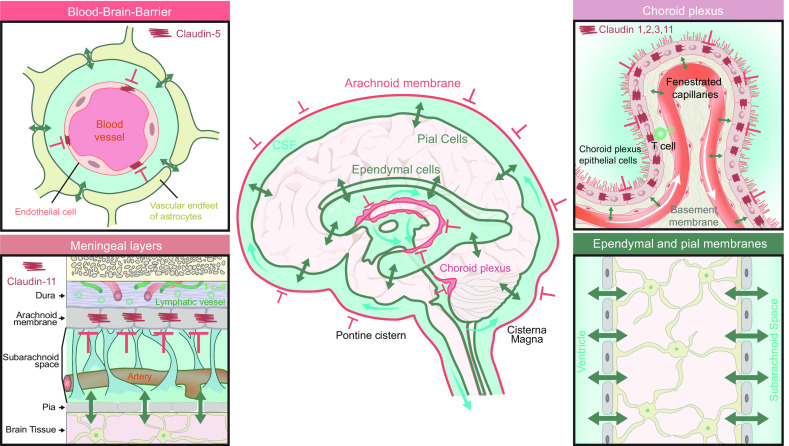
The developing CNS is endowed with at least four barriers, some of which are transiently present, whereas others persist throughout adulthood. The four barriers are classified as the inner-CSF barrier, the BBB, blood-CSF barrier, and outer-CSF barrier. The inner-CSF barrier is a transient barrier during development maintained by “strap” junctions between the neuroepithelial cells and later by the radial glia that line the ventricular walls (100). Strap junctions are morphologically different than tight junctions upon freeze fracture electron microscopy, and the molecular scaffold differs somewhat from that of tight junctions by, e.g., not expressing genes of zonula occludens proteins (100, 101). The inner-CSF barrier is critical for normal CNS development since it allows for ventricular inflation by supporting the growth of colloid osmotic pressure in the ventricles and limits the passage of bioactive compounds into the developing brain parenchyma. The strap junctions are lost in the course of development of the ependymal lining of the ventricles and are replaced in the adult brain by gap junctions that result in a much more permeable barrier allowing for passage of relatively large molecules across the ependyma (101). In sheep, strap junctions start declining in presence at midgestation and are replaced by gap junctions at around day 125 of the 147-day gestation (101).
The BBB separates the contents of the blood from the brain extracellular space, while simultaneously allowing facilitated transport of certain substances. Tight junctions between endothelial cells limits paracellular passage of compounds between blood and brain, while supporting very limited transcytotic activity across brain endothelial cells that declines in healthy aging (102, 103). The earliest penetrating blood vessels sprouting from the perineural plexus appear around E10 in mouse and are thought to be leaky (104). Subsequently, the primitive BBB is formed in part under control by the canonical Wnt/beta-catenin signaling pathway, whereby Wnts specifically released by neural cells (Wnt7a/b) induces expression of the tight junction protein claudin-3 as well as GLUT-1 in brain endothelial cells (102, 105). Around E15 in mice, the primitive BBB is fully established, although the exact timing varies between species and different brain regions (104). In the adult BBB, the endothelial specific claudin-5 is crucial for sealing the BBB, and lack of claudin-5 results in death soon after birth in mice (106). Claudin-3 is not present in the adult BBB, and whether it holds an important role in BBB development has recently been questioned (107). The outer-CSF barrier in the adult consists of the component barriers: the arachnoid barrier layer that separates the subarachnoid space CSF from the dura mater and its fenestrated capillaries, a blood-CSF barrier where tight junctions between endothelial cells in blood vessels traversing subarachnoid space restrict passage of solutes from blood to CSF, as well as a permeable barrier between brain and CSF comprised of gap junctions between astrocytic endfeet in the glia limitans (59, 108). In the developing human embryo and fetus, the outer-CSF barriers differ anatomically from the adult barriers. The subarachnoid space is formed around week 7, and communication with the intraventricular CSF is not established until embryonic weeks 7–10, though a physiological communication seems to develop before the formation of the macroscopic median and lateral foramina (108, 109). The tight junctions between arachnoid epithelial cells in the barrier layer seem to be present at the formation of the subarachnoid space (108). Electron microscopy studies in rat indicate that at E12 capillaries in the pia mater are fenestrated and the neuroepithelium and radial glia cells form an incomplete barrier between the brain tissue and CSF (59). Also in the rat, from E14 onward, the pial capillaries become nonfenestrated and junctional structures develop between radial glial endfeet; by E16–E18 these have become fully developed to form a subarachnoid space where CSF is in limited contact with the brain parenchyma (59, 108). In human gestational weeks 25–28, the radial glial endfeet layer has transitioned into the external astrocytic endfeet layer forming the glia limitans externa, where gap junctions between astrocytic endfeet in glia limitans superficialis provide a permeable barrier between CSF and brain extracellular space (108).
3.9. Outstanding Questions
Is brain clearance during early development and before maturation of the glymphatic system maintained by simple diffusion or by other active transport systems? Alternately, is the capacity for local protein degradation in embryonic and fetal brain so high that protein export is not necessary?
What other molecular cues and cells are necessary for development of the glymphatic pathway besides pericytes and PDGF-B signaling?
Does the glymphatic pathway play a role in postnatal brain development, e.g., in synapse formation and pruning, and in myelination?
3.10. Interim Summary Section
The primitive ventricles and earliest CSF are formed during neurulation, when occlusion of the anterior and posterior neuropore of the neural tube entraps amniotic fluid that becomes the nascent CSF. The composition of CSF changes from that of amniotic fluid and, unlike CSF of the adult, is high in protein content. The colloid osmotic pressure drives a net influx of fluid into the primitive ventricles inflating the anterior part of the neural tube, which has proven to be critical for cortical development. The choroid plexus develops later, and signaling molecules important for development are produced either by the neuroepithelium and secreted into the CSF or delivered by the early brain vasculature.
The subarachnoid space develops around embryonic week 7 in humans and is sealed exteriorly by tight junctions between arachnoid epithelial cells since its formation. In the earliest embryonic stage, the pial capillaries are fenestrated, but during embryonic development they become nonfenestrated and the BBB is established very early during development.
During early CNS development, exchange between CSF and the developing brain is restricted. Ventricular CSF is first contained by strap junctions linking the neuroepithelial and later by radial glia cells lining the ventricles, both of which form an inner CSF barrier. CSF within the subarachnoid space is also restricted from freely exchanging with the CNS by junctions between radial glial endfeet that form an outer CSF barrier. The inner CSF barrier becomes permeable when the ependymal cell layer develops and the outer CSF barrier with the development of glia limitans externa.
The glymphatic system develops postnatally. The earliest signs of glymphatic system function are observed at P1 in the mouse hippocampus. The glymphatic system then progressively develops in a ventral to dorsal manner and reaches maturity at P14. The glymphatic system relies on the development of astrocytic endfeet and polarized AQP4 expression in a process that is dependent on PDGF-B. Dural lymphatics also develop late and mature postnatally in a process dependent on VEGF-C.
4. EVOLUTION OF THE BRAIN’S FLUID PATHWAYS
4.1. Introduction
Among living organisms there is an enormous range in the complexity and size of the nervous system. While the nematode Caenorhabditis elegans has exactly 302 neurons, the human brain contains around 86 billion neurons, and larger animals like whales and elephants have even bigger brains (110–113). Sufficiently small brains may not need a specialized fluid clearance system or indeed any specialized vasculature to deliver nutrients or clear waste products. In certain evolutionary lineages, brains grew so large that diffusion was no longer sufficient to deliver the necessary oxygen and nutrients throughout the brain, and intrinsic brain vasculature consequently developed. Similarly, we can reasonably assume that when brains acquire a certain size, some brain clearance mechanism other than diffusion also becomes necessary. The glymphatic system has been found to mediate fluid clearance in brain of rodents and other mammals, but relatively little is known about its corresponding importance in nonmammalian species. The minimal requirements for the existence of a functioning glymphatic fluid transport system are that CSF is produced and that an intrinsic vascular network ensheathed by glial cells forms a perivascular fluid conduit, wherein exchange of fluid with the brain extracellular space is facilitated by AQP4 in astrocytic endfeet. In this section we review the comparative neuroanatomy of multiple species, aiming to arrive at some understanding of how and when the components of the glymphatic system first appeared in evolution, and if alternate solutions for brain clearance ever arose.
4.2. Vasculature Developed in Primitive Chordates When Brains Grew Large
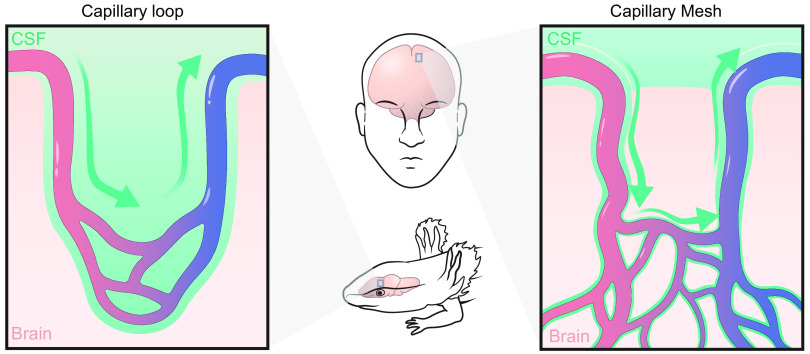
The vascular tree provides not only a scaffold for the glymphatic system, but the cardiorespiratory forces that act upon it are important drivers of glymphatic fluxes (see sect. 9). The CSF-filled perivascular spaces piercing the brain shorten diffusion paths for metabolic waste products in the brain extracellular space, and the flow in these spaces speeds up clearance substantially. Therefore, the presence of an intrinsic brain vasculature, where blood vessels irrigate nervous tissue, seems to be a necessary requirement for a functional glymphatic system. In evolution, the penetration of blood vessels into the brain parenchyma and the formation of intrinsic vasculature of the brain seem to have arisen in early chordates (FIGURE 6) (114). Almost none of the Protostomia (e. g., insects) nor early Deuterostomia are endowed with intrinsic vasculature, and brains or cephalic ganglia of such organisms are typically nourished by diffusion from an open circulation as seen in insects or via a superficial, nonpenetrating vasculature such as that in the Cephalocordate lancelet (114, 115). The brain of the lamprey, a member of Cyclostomata (jawless fish), contains capillary loops piercing the brain surface, whereas arteries or veins are confined to the surface, and the spinal cord is nourished from a superficial vascular plexus. The hagfish, another member of Cyclostomata, has an intrinsic capillary mesh that is supplied by penetrating arteries (114). Intrinsic brain vasculature is an established feature among all vertebrates (116, 117), although structure of the intrinsic vasculature differs between different classes and subclasses of vertebrates. There are two main forms of microvasculature architectures, namely 1) a rich anastomosing capillary meshwork fed and drained by singly piercing arterioles and veins, and 2) the capillary loop structure, where capillaries form nonanastomosing hairpin-like loops with few or no branch points, supplied by a single artery and vein that enter the brain together in pairs (FIGURE 7) (118). The capillary anastomosing meshwork is a feature of the human brain and the majority of other mammals, with the notable exception of marsupials, which harbor capillary loops (119, 120). Capillary loops are also seen in many nonmammalian vertebrates, including amphibians and reptiles, and even in some invertebrates, such as the earthworm (114, 118). Originally, it was suggested that the capillary loop represents a more primitive microvascular organization compared with the anastomosing network, since it was most often found in more primitive classes of vertebrates (114, 118, 119). However, there are species with a capillary loop structure in almost every class of vertebrates, including mammals, which raises the question what are the physiological differences and advantages of the two confirmations (114, 118, 119, 121). It is difficult to resolve this evolutionary question given the present evidence, but we suppose that the capillary loop represents a more basal conformation of the intrinsic brain vasculature, which can recur as an atavism across many orders (114, 121). The physiological difference between the two capillary patterns is not fully understood. Surprisingly, the anastomosing network does not perform better than the capillary loops in rescuing from cerebral ischemia following an experimentally induced arterial embolism (118). It has been hypothesized that, by shortening diffusion distances, the capillary meshwork is superior to capillary loops for facilitating gas exchange. On the other hand, capillary loops could be beneficial with respect to counter-current exchange, which would tend to stabilize the blood composition across all intrinsic brain segments, while boosting oxygen delivery by increasing CO2 levels in the capillaries (the Bohr effect) (119).
FIGURE 6.
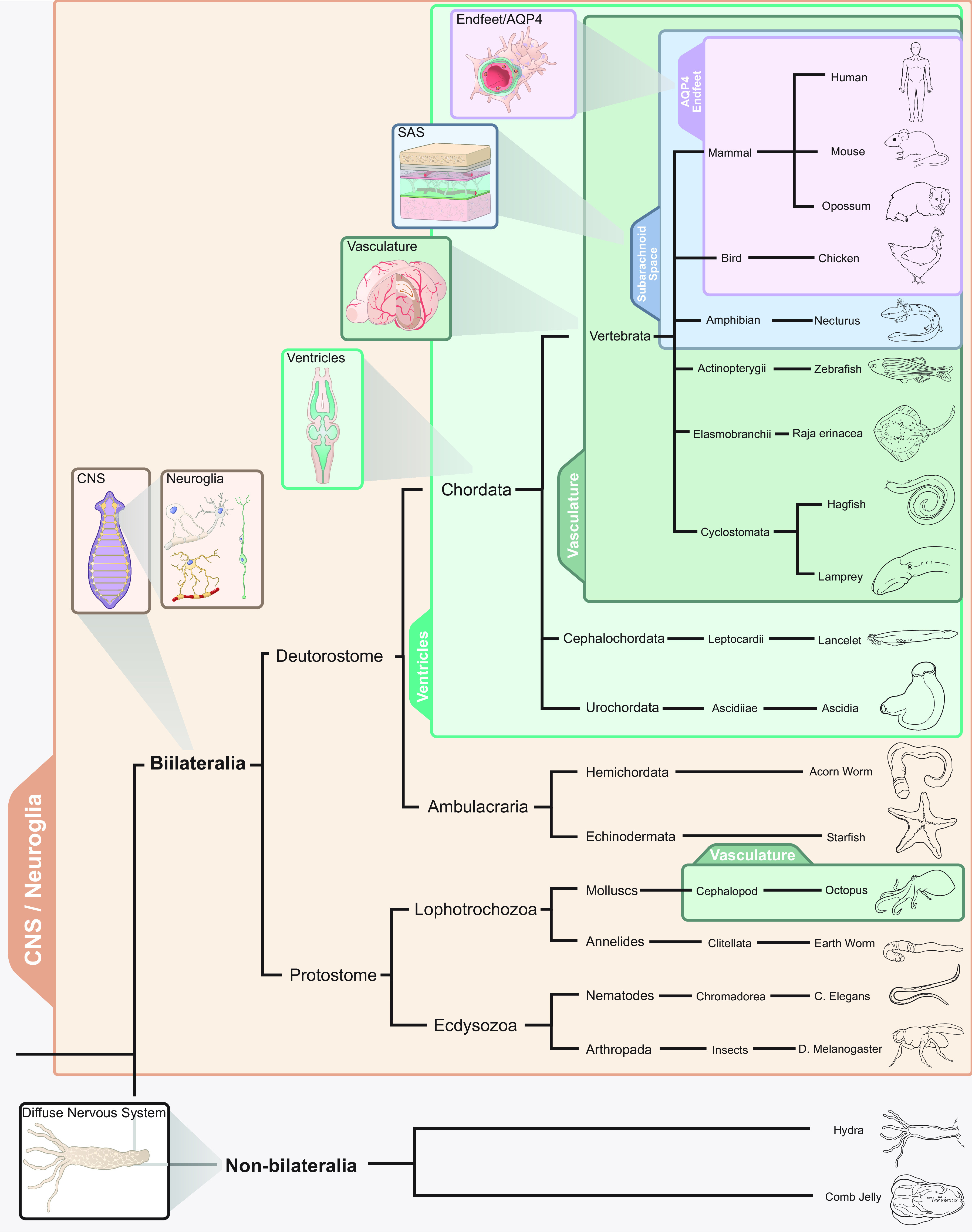
Phylogenetic development of the glymphatic system. When the nervous system evolved from diffuse neural networks to form centralized ganglia, the first supporting neuroglial cells also appeared. Thus, all bilateria have neuroglia. Ventricles developed at a phylogenetic stage when the nervous system progressed from being a plate-like structure to form a neural tube with a lumen containing cerebral spinal fluid (CSF), which is a feature of all chordates. In vertebrate evolution, brains might have grown so large that the tissue could not be nourished by diffusion from a vascular surface plexus, thus requiring the development of intrinsic brain vascularization. The subarachnoid space covering the external surface of the brain with CSF is seemingly present in all terrestrial vertebrates, such that extensions of the subarachnoid space into the brain parenchyma (i.e., the perivascular spaces) are likely also to be present in amphibians, reptiles, birds, and mammals. The evolutionary innovation of a perivascular endfeet layer of protoplasmic astrocytes with expression of aquaporin 4 (AQP4) polarized toward the blood vessel seems to be a feature of mammalian and avian brains. Thus, since the glymphatic pathway appears fully developed in mammals and birds, we surmise that it must also have been present in their common ancestor, before the divergence of sauropsids (ancestors of reptiles and avians) and synapsids (ancestors of mammals). CNS, central nervous system; SAS, subarachnoid space.
FIGURE 7.
Capillary conformations in evolution. Intrinsic brain vasculature takes two forms in vertebrates. A capillary loop structure (left) consists of a single artery and single vein that feed into a capillary with no or very few branch points and without anastomoses with other capillaries. A capillary meshwork (right) consists of arteries and veins that penetrate singly into the brain and feed into a rich anastomosing network of capillaries. These 2 conformations of vasculature also result in 2 different types of perivascular spaces. In capillary loops, a single perivascular space contains artery, vein, and capillary. In capillary meshworks, the artery and vein run in their own perivascular spaces. It is not yet established whether the hypothetical pericapillary space actually exists, but certain data supports that proposition. CSF, cerebral spinal fluid.
In summary, intrinsic brain vasculature is a feature of all vertebrates and some invertebrates. Intrinsic vasculature consists either of a capillary meshwork with arteries and veins entering the brain alone or a capillary loop structure, where arteries and veins enter in pairs and capillaries do not anastomose. The glymphatic system has been described mainly in rodents, which harbor capillary meshworks and show that a single artery traversing its perivascular space can act as an important pump for driving the anterograde flow of CSF (21). Whether the capillary loop perivascular space with bidirectional blood flow, as seen in amphibians and marsupials, is less efficient than the typical mammalian perivascular space for driving CSF influx is an interesting topic for future research.
4.3. Evolution of CSF, Ventricles, and Subarachnoid Space
CSF in the ventricles and the subarachnoid space provides the fluid that flows into the glymphatic system. CSF is found only in the deuterostomes; even the most specialized protostomes such as cephalopods do not possess cerebral ventricles or CSF (122). Among deuterostomes, an enclosed ventricular cavity containing CSF appears in the most primitive chordates such as the urochordate ascidia and the Cephalochordate lancelet (FIGURE 5) (122). The nascent CSF in these animals is composed of seawater, since in their larval stage these animals have a CNS composed of a neural tube with a patent anterior neuropore, which is open to the surrounding water (122). When these organisms reach adulthood, the anterior neuropore closes and the lumen of their open ventricular system becomes an enclosed fluid system (122). Other than the earliest chordates, intraventricular CSF is present in all chordates, including teleosts (bony fish), amphibians, birds, and mammals (122).
In all mammalian brains there is a subarachnoid space in open contact with the intraventricular CSF, with communication through macroscopic openings in the fourth ventricle through the lateral apertures (foramina of Luschka). Humans and some other mammals also have an open communication between the subarachnoid space and the ventricles through the median aperture (123). Birds, reptiles, and amphibians possesses subarachnoid space CSF but macroscopic communication between the intraventricular CSF and the external subarachnoid space CSF is absent (123, 124). The compositions of the internal and external CSF in these animals are similar, and it seems likely that a microscopic communication between the two compartments exists (116, 124–126). This putative communication is probably obtained through the thin tela choroidea of the fourth ventricle, which in certain Orders contains fenestrations (see sect. 5.7.3 for a description of tela choroidea) (124).
In all terrestrial vertebrates, the external CSF space (or subarachnoid space) is sealed exteriorly by the arachnoid barrier membrane, from the blood by the BBB, and interiorly by glia limitans superficialis and perivascularis, which are permeable membranes allowing the mixing of fluid between the external CSF and the brain extracellular space (127). In aquatic vertebrates (jawless fishes, cartilaginous fishes, and perhaps bony fishes), there is no external CSF. Instead, the outer brain surface is bordered either by connective tissue or sometimes by the so-called perimeningeal fluid (123, 124). When present, the perimeningeal fluid differs in composition from the ventricular CSF, and CSF solutes do not freely move into the perimeningeal fluid. The ionic composition of the perimeningeal fluid resembles that of plasma, and its protein content is higher than that of the CSF, although it is not simply a blood plasma ultrafiltrate (127). The perimeningeal fluid is also sealed from the periphery by an arachnoid barrier membrane as seen in elasmobranches and in the teleosts zebrafish and goldfish (127–129). Elasmobranches like sharks and rays have an inner barrier composed of tight junctions between astrocytic endfeet in the glia limitans externa and perivascularis, which shields the brain parenchyma from the perimeningeal fluid and blood (130). It is not well characterized whether teleosts and aquatic vertebrates other than elasmobranches have an impermeable glia limitans externa, but the BBB of the teleost zebrafish is formed by tight junctions between endothelial cells, and its perivascular astrocytic endfeet most likely form a permeable membrane, which implies that the glia limitans externa would also be permeable (129).
In summary, terrestrial vertebrates possess ventricular and subarachnoid space CSF compartments and lack tight junctions between ependymal cells lining the ventricles and astrocytic endfeet in the glia limitans superficialis and perivascularis. As such, the CSF is in free communication with the brain extracellular fluid. In aquatic vertebrates, there is no external CSF compartment, but rather a perimeningeal fluid with ionic composition resembling that of plasma. In elasmobranchs, this fluid covers the brain surface and is delimited externally by an arachnoid barrier membrane and internally by an impermeable glia limitans superficialis and perivascularis. Teleosts possess a BBB, and their glia limitans perivascularis and superficialis are most likely permeable. No animals in the protostome clade, which includes insects and cephalopods, possess CSF.
4.4. Evolution of Perivascular Spaces
Perivascular spaces are protrusions of the subarachnoid space around penetrating arteries and veins, which disappear at the capillary level (131). These spaces are effective conduits for CSF flow into the glymphatic system and shorten the distance between CSF and the extracellular space in all brain regions, including deep subcortical structures (4, 21). In mammals, extensions of the subarachnoid space follow the penetrating arterioles and ascending veins into the brain parenchyma (50). At the basal parts of the mammalian brain, penetrating arterioles enter the parenchyma from the subarachnoid space. The arterioles are surrounded by a protrusion of leptomeningeal cells from the arachnoidea, which form the inner wall of the perivascular space, as well as by a protrusion of pia mater cells that contact the astrocytic endfeet, thus forming the external wall of the perivascular space (50). Cortical arterioles entering from the convexity of the cortex, and all venules are surrounded by only one layer of leptomeninges (50). Both the basal and convexity cortex arterioles are thus in open communication with the subarachnoid space (50). The leptomeningeal membrane disappears at the capillary level, where it is functionally replaced by the astrocytic endfeet enwrapping the capillary endothelium, which are separated only by the basal lamina (50). Perivascular spaces naturally require that there be an intrinsic brain vasculature, which is found in all adult vertebrates. However, perivascular spaces also exist in protostome cephalopods such as the octopus, which do not possess CSF (132, 133). The perivascular channels of octopus brain contain collagen, which is assumed to structurally support the vessels, and aid in keeping them open under various pressure conditions. It has also been proposed they function as lymph ducts to remove extracellular fluid from the nervous system (132, 133). The perivascular spaces in cephalopods are lined by glial endfeet, which morphologically appear to form an impermeable barrier. Indeed, transcriptome analysis revealed that octopus brain contains claudins and many other proteins involved in BBB formation in vertebrates (134, 135). In vertebrates, as described above, the intrinsic brain vascularization consists of either capillary loops or a capillary meshwork. These two conformations result in different perivascular space structures. In the amphibian Necturus maculosus (the mud puppy), capillary loops pierce the parenchyma, and its perivascular spaces contain the entire loop such that the inflow and outflow of blood occurs in the same perivascular space. This is opposite to the circumstance in mammals, where only one penetrating arteriole or venule is found in each perivascular space (116). Also, the pial-glial endfeet membrane lines the perivascular space through the entire length of the mud puppy brain capillary loop, and the perivascular space is open around the entire capillary loop (FIGURE 7) (116). The ultrastructure of the perivascular spaces and their communication with the subarachnoid space also varies between mammals and other vertebrates. In elasmobranches like the skate and shark, the BBB is maintained by tight junctions between glial endfeet; in these species the perivascular space is in open communication with the bloodstream, allowing blood-borne tracers to freely enter perivascular spaces (136). Thus the perivascular space in elasmobranches does not seem to serve as a source of fresh fluid. There are reports of perivascular spaces in species of carp (teleost) and lizard (reptile) that traverse the entire brain parenchyma and would thus link the internal and external CSF compartment, at least in reptiles (124–126). In the zebrafish, another teleost, the dorsal meninges contain nonlumenized lymphatic endothelial cells, which are capable of taking up intracerebrally delivered tracer molecules, thus strongly indicating the presence of a drainage path from the brain parenchyma to meninges, despite its lack of an external CSF space (124–126, 137, 138).
In summary, perivascular spaces are found in all animals with intrinsic brain vasculature, which includes vertebrates and cephalopods. CSF-filled perivascular spaces are a feature of terrestrial vertebrates, which all possess subarachnoid spaces. Perivascular spaces in cephalopods do not contain CSF but are supported by large amounts of collagen, whereas perivascular spaces in elasmobranches are filled with perimeningeal fluid, which is barred from mixing with brain extracellular fluid by a perivascular glial barrier membrane. However, it has been suggested both for cephalopods and elasmobranches that these spaces act much as lymph ducts aiding in the removal of waste from brain (127, 133). Thus these spaces might represent an alternate brain clearance system to the glymphatic system seen in mammals, although experimental evidence supporting that hypothesis is lacking.
4.5. Astrocytes and Astrocytic Endfeet
Astrocytes and their endfeet processes terminate around the perivascular spaces to establish a permeable barrier that facilitates the mixing of CSF with brain extracellular fluid in the glymphatic system (4). Neuroglia is the collective term in mammals for astrocytes, oligodendrocytes, ependymal cells, microglia, and other cell types, which are chiefly responsible for myelination, structural support, metabolic and ionic homeostasis, and immunological functions among several other roles (13, 139, 140). In addition, all the subtypes of neuroglial cells have been implicated in information processing, including short- and long-term memory, learning, and synaptic formation. All mammals have protoplasmic astrocytes, although their morphology and function vary somewhat between species. Human protoplasmic astrocytes are larger and have more complex arborization than do those of nonhuman primates; this difference is even more notable when compared with astrocytes of rodents (140, 141). Particular properties of human astrocytes are arguably related to our unique cognitive abilities. For example, grafting human glial progenitor cells into mice increases their performance in certain cognitive tasks (142). Most of the protoplasmic astrocytes in the mammalian brain send processes to contact with microvessels, and their processes terminate with endfeet covering the brain’s vasculature (141). The endfeet of human protoplasmic astrocytes completely enwrap the vessels, forming a cobblestone-like appearance with multiple apposed, round endfeet. In contrast, the rodent endfeet form rosettes, resulting in a more porous perivascular endfeet layer (141). Neuroglia seem to have arisen in the phylogenetic stage when the nervous system became centralized in ganglia rather than being a diffuse net (FIGURE 6) (140, 143). Diffuse nervous systems in animals such as hydra and comb jelly do not contain clusters of neurons, whereas the first central nervous systems are seen in primitive Bilateralia like the flatworm, earthworm, and roundworm and are composed of several ganglionic neuronal masses, usually with a segmental organization (139). Glial-like cells first appear in what is considered some of the earliest Bilateralia, being present in Acoela worms, in which nonneuronal cells extend lamellar processes into the neuropil to surround neurites, thus forming the phylogenetically earliest form of supportive cells of the nervous system (139). The neuroglia of different phyla, while performing similar functions, differ in their structure and gene expression, suggesting that neuroglia probably developed independently several times in evolutionary history (139). In the brain of the common fruit fly, Drosophila melanogaster, a hemolymph-brain barrier is formed by glial cells connected by septate junctions, thus forming one of the earliest complete compartmentalization of the brain (139). At this stage in evolution, glial cells have also become critical for neuronal development and survival of the organism (139). Many Deuterostomes, the superphylum encompassing the chordates, echinoderms, and hemichordates, possess only radial glia cells (139). In Echinodermata like the sea urchin, radial glial cells are the only type of glia in the CNS (139). In some primitive vertebrates like the elasmobranchs (sharks, rays, and skates), radial glia cells predominate, but in species where the brain tissue has made a transition from a “laminar” organization with thin brain parenchyma and large ventricles to an “elaborate” organization with thick brain parenchyma and more compact ventricles, a larger proportion of the neuroglia develop into parenchymal glia resembling astrocytes. The increase in parenchymal astrocytes populations with increasing brain thickness implies greater demand for neuroglia in the maintenance of brain homeostasis that cannot be accommodated by radial glia alone. In most advanced vertebrates like mammals and birds, astrocytes and oligodendrocytes are the predominant neuroglia, and radial glial cells mainly exist during development, although certain radial glia cells persist during adulthood, such as the Muller glia in retina and Bergman glia, e.g., during gliosis and in specific brain regions (139, 140). The presence of protoplasmic astrocytes is a requirement for developing glymphatic function, which requires that finger-like extensions from the astrocytes form perivascular endfeet expressing AQP4 channels. The ensheathment of brain vasculature by astrocytic endfeet is seen in mammals, birds, reptiles, and elasmobranches, whereas amphibians and teleost fish have less complete coverage (144, 145). Radial glial can also make contact with the vasculature and can even form endfeet, for example in the frog Rana pipiens and several other species (145), suggesting that the primitive radial glia may have been progenitors for the diversification of specialized astrocytes.
4.6. Aquaporin-4 Water Channels
The proper function of the glymphatic system is highly dependent on the polarized expression of AQP4 on astrocytic endfeet (4). Loss of AQP4 polarization results in diminished CSF influx and reduced clearance (15, 55). A complete absence of AQP4, as seen in AQP4 knockout mice, although not fatal, substantially reduces glymphatic function (4, 22). Therefore, the polarized expression of AQP4 water channels is a critical aspect of the functioning of the glymphatic system.
AQP4 is a member of the diverse family of aquaporin water channels, which comprise more than 850 transmembrane proteins expressed in bacteria, plants, fungi, and animals (146–153). Cells contain 60–95% water, in which ions and most biomolecules are dissolved, and cells in multicellular organisms are bathed in an extracellular fluid, which is also composed almost entirely of water (148). Particular aquaporin subtypes are differentially expressed between kingdoms, seemingly having evolved for specialized functions. These functions are as varied as pore-closing mechanisms of plant aquaporins that preserve internal water during drought and aquaporins controlling glycerol permeability of certain insect cells, which are part of protective responses to desiccation or freezing temperatures (154, 155).
The polarized expression of AQP4 toward astrocytic endfeet is a feature of mammalian brain (FIGURE 6) (156). Mammals differ somewhat with respect to the cellular distribution of AQP4; whereas mice have 5–10 times higher AQP4 expression localized to the endfoot membrane, humans astrocytes show a distinctly lower level of polarization (157). Expression patterns of AQP4 in nonmammalian vertebrates are less well documented. In the zebrafish, AQP4 is expressed in the radial glia but is not polarized (137, 156, 158). There is little information regarding AQP4 expression in neuroglia of amphibia and reptiles (156). AQP4 is present in the brain of the elasmobranch dogfish, but its cellular distribution is unknown (159). In birds, AQP4 is polarized toward astrocytic perivascular endfeet, suggesting that they may have a glymphatic system similar to that in mammals (160). In summary, polarized AQP4 expression toward astrocytic endfeet is found in mammals and birds, but zebrafish do not show polarized AQP4 expression. AQP4 expression patterns in amphibia and reptiles are not well described.
4.7. Evolution of the Glymphatic System
The glymphatic system is a feature of all mammalian brains studied so far, including mouse, rat, pig, nonhuman primates, and human (4, 57, 161–167). Thus the different components of the glymphatic system first described in mouse, which include perivascular flow of CSF, polarized AQP4 expression on the astrocytic endfeet, perivenous efflux of CSF tracers, and a global brain influx and clearance rate exceeding that possible for simple diffusion, have all been identified in individual studies of nonhuman primates and humans (84, 157, 162, 164–170). Also, there are several physiological and pathological similarities across species, e.g., the occurrence of increased brain clearance during sleep, altered expression and polarization of AQP4 with aging, and declining brain clearance with aging (164, 169–171). MRI studies have provided the main part of insights concerning human glymphatics, albeit without providing the temporal and spatial resolutions afforded by certain techniques used in basic research (see BOX 3). This technical limitation makes it difficult to directly compare the glymphatic system in rodents and humans. Certain anatomical and molecular expression patterns differ between rodents and human, as outlined in the sections above, namely with respect to the tighter and denser astrocytic endfeet layer forming the glia limitans perivascularis in humans, the lower polarization index of AQP4 in humans compared with rodents, and the higher level of AQP4 on the human parenchymal astrocytic membrane (141, 157). The functional significance of these differences is not known. There are differences between rodents and humans regarding CSF flow on the brain surface. Rodents like mouse and rat, in which the glymphatic system was first described, have lissencaphalic brains, whereas humans and most other large mammals have gyrencephalic brains, characterized by folded cortexes with gyri and sulci (172). In gyrencephalic brains, sulci are CSF filled spaces where the subarachnoid space expands, such that CSF flow in the sulci exceeds that in the gyri, where the subarachnoid space is relatively thin. In line with this anatomic distinction, MRI tracers delivered to the CSF compartment in humans have been found to flow primarily within the brain sulci, where the major cerebral leptomeningeal arteries run, and are not evenly spread in the subarachnoid space over the entire brain convexities (165). This pattern of brain surface CSF flow of the gyrencephalic brain is nonetheless similar to that seen in the rodent lissencephalic brain, where the flow of CSF tracers also follows the courses of the major leptomeningeal arteries, i.e., the posterior, middle and anterior cerebral artery (4, 57). However, there are some key differences between the CSF flow over the dorsal convexities of lissencephalic versus gyrencephalic brains. In the human brain, the periarterial flow of MRI contrast agent delivered into the CSF compartment seems to flow in a space around the leptomeningeal surface arteries of the dorsal cortex, which forms a space much larger than that described for rodents, in which the surface flow of CSF tracers is largely confined to a narrow periarterial space (4, 131, 165). One possible explanation for this species difference is that CSF reservoirs within the sulci and fissures in the generally large dorsal cortex of gyrencephalic brains in effect shorten the transport distance from the CSF reservoir to the perivascular space and into the neuropil. The corresponding flow distances are shorter in the smaller lissencephalic brains of rat and mouse, in which the preponderance of CSF inflow to the perivascular spaces arises in the basal cisterns around the Circle of Willis. This view is supported by studies of the glymphatic system in the gyrencephalic brain of pigs, in which CSF tracer influx from the sulci to the neuropil substantially exceeded that from the neighboring gyri (167). Thus, from the perspective of brain fluid clearance, gyrencephalic brains can support relatively greater CSF flow in the subarachnoid space over the dorsal cortexes through the network of sulci and fissures, thus resulting in more efficient fluid exchange between these CSF reservoirs and the brain extracellular space. The anatomic substrate for CSF uptake from the subarachnoid space into the perivascular spaces is not well understood.
BOX 3. Imaging the Glymphatic System
Two-Photon MicroscopyThe discovery of the glymphatic pathway was largely enabled by the use of two-photon laser microscopy, which constitutes a unique tool to image in vivo processes in the cortex in a relatively noninvasive rodent preparation (FIGURE 27). Here, optical access to cortex is provided through a craniotomy or thinned skull window, which, when correctly undertaken, causes minimal stress in the underlying cortex (4). Two-photon laser microscopy allows for superfast multichannel imaging (30 Hz and more) and, with the use of fluorescent tracers/particles injected into the fluid compartments of the brain (bloodstream, CSF, and/or extracellular fluid), enables the monitoring of fluid dynamics with high temporal and spatial resolution under various physiological and pathological conditions (21). When combined with transgenic mouse lines in which fluorescent proteins are expressed under the control of specific genes to label different cell lines, two-photon laser microscopy also works as a tool to define the anatomical pathways of fluid dynamics. When combined with the calcium ion sensitive protein GCaMP, cellular activity can be related to the fluid flow patterns (4, 469). Shortcomings of two-photon microscopy include the small field of view, limited penetrance into the parenchyma to a maximum depth <1,000 µm, and the somewhat invasive procedure.
Dorsal Cortex Macroscopic Transcranial Epifluorescence Imaging Transcranial macroscopic imaging is an increasingly used technique to study fluid flow in the brain. This technique entails epifluorescence imaging through a low magnification objective with a wide field of view, which affords imaging of a large part of the dorsal cortex at once, through an intact cranium (469, 576). The benefits of this technique include the wide field of view, which allows for quantification and comparison of flow in different brain regions, between hemispheres, and in different vascular compartments. This technique can also be used with transgenic mice expressing GCaMP, such that regional cortical activity can be related to CSF flow patterns. Limitations of mesoscopic epifluorescence imaging include the inability to separate emittance stemming from cortex from that of the subarachnoid space and the restricted optical penetrance of epifluorescence light, such that the deeper cortical layers and subcortical structures evade imaging.
MRI ImagingMRI provides brain-wide imaging of the living brain that is applicable in animal models and clinical studies. Multiple imaging sequences can provide information about vascular conformation (angiography), diffusion (diffusion-weighted MRI), and anatomy in one session. MRI imaging of the CSF compartment can be contrast-enhanced or contrast free. In contrast-enhanced MRI, a paramagnetic tracer is delivered intrathecally to the CSF compartment, usually through the cisterna magna in rodents or by lumbar puncture in humans (161, 162, 164, 165, 405, 469, 758). Alternately, a BBB-permeable agent can be delivered to the CSF or brain compartment by intravascular administration. With contrast-enhanced MRI, whole brain CSF flow can be imaged with high specificity, thus providing good options for quantification and comparison of tracer distribution between subjects and conditions. The contrast agent can also be injected before imaging, and its distribution in brain and lymphatic system visualized later (759). In humans, where the process of CSF inflow to the neuropil and tracer clearance occurs over at least 24 h, contrast enhanced MRI is also applicable as a tool to monitor CSF clearance, without requiring repeated injections of contrast agent (164, 165). Contrast-free MRI techniques have been developed for rodent studies, which can visualize CSF compartments including the ventricles, subarachnoid space and basal cisterns, and perivascular spaces without requiring a contrast agent (407). Although having lower specificity and less fitness for quantitation than contrast-enhanced MRI, the contrast-free MRI methods provide the option of imaging at multiple timepoints separated by days or weeks and as such is ideal for monitoring CSF compartment dynamics in the same individual over time without requiring multiple invasive procedures. Overall limitations of MRI are the low temporal and spatial resolution. However, new techniques are being developed that improve the sampling rate by reducing spatial resolution, such as ultra-fast magnetic resonance encephalography. That procedure can image the entire brain at 1 Hz and thus provide information about effects of pulsatory waves in the brain parenchyma that drive fluid flow in the glymphatic pathway (495).
PET and SPECT ImagingPositron emission tomography (PET) and single photon emission computer tomography (SPECT) allow for whole body dynamic imaging of intrathecally delivered radioisotopes. Quantitation of radioisotope concentration in PET and SPECT imaging is superior to the contrast quantification in contrast-enhanced MRI, thus providing better information on regional tracer concentration differences and the clearance rate of CSF from the neuropil. Furthermore, whole body PET or SPECT imaging provides simultaneous information about radiotracer concentrations at various CSF egress pathways (277, 637). Limitations of these methods include their enormous expense, as well poor spatial resolution (2–4 mm) and short half-life of PET radionuclides, which often require access to a nearby cyclotron/radiopharmaceutical laboratory.
CT ScanningCT scanning provides whole brain and body imaging at very high (100 µm) resolution in vivo. Temporal resolution is relatively low, and available CT tracers have poor penetration into neuropil. Radiation dosimetry is an issue in human experimentation.
Histological MethodsHistological analysis of brain sections from animals infused with fluorescent, fixable tracers can reveal uptake in brain fluid compartments, which can be combined with histochemical and immunohistochemical staining and imaging with epifluorescence or confocal microscopy. These techniques allow for brain-wide imaging of tracer distribution, as well as provide high magnification of cells and extracellular matrix components involved in fluid dynamics in brain. Electron microscopy provide even higher magnification of subcellular components but is particularly prone to fixation artifacts (4, 56). Novel whole skull clearing techniques such as uDISCO allow for immunohistochemical staining of skull and brain in toto, and can thus be used, in combination with light sheet microscopy, to image connections between the CSF compartment, dural lymphatics, and other canals and compartments, although with some loss of resolution compared with ordinary microscopic techniques such as confocal imaging of brain sections (760). Histological methods have the major limitation presented by postmortem and fixation artifacts, which can result in nonphysiological distribution of CSF tracers and the spurious formation of tissue pseudocompartments that do not exist in vivo.
In summary, the glymphatic system is a feature of all mammalian brains studied so far, including rodents, pigs, nonhuman, and human primates. Species differences in astrocytic endfeet morphology and AQP4 expression patterns could indicate some divergence of glymphatic functioning between species, but present technological limitations have made difficult a direct comparison. CSF flow over the dorsal cortexes seem to differ between lissencephalic and gyrencephalic brains. It appears that the CSF-filled sulci of gyrencephalic brains facilitate glymphatic influx to the underlying brain, whereas there is less inflow into the adjacent gyri. While it seems likely that birds thus have a functional glymphatic system, there have been no functional studies in Aves. However, based on the comparative neuroanatomy presented above, it is plausible that birds harbor the vascular, cellular, and molecular components required to constitute a glymphatic system. From this, we can also infer that the glymphatic system most likely arose before the divergence of sauropsids (reptiles and avians) and synapsids (mammals), but further histological and in vivo studies are needed to pinpoint the stages of evolutionary development of the glymphatic system.
4.8. Comparison of the Evolution of the Glymphatic System With That of the Peripheral Lymphatics
At what stage in evolution did the peripheral lymphatic system first develop and are stages in the evolution of the cardiovascular system related to the formation of the lymphatic system? What can the evolution of the peripheral lymphatic and cardiovascular system tell us about the evolution of the glymphatic system?
The peripheral blood vessels found in mammals are formed by endothelial cells connected by intercellular junctions and linked in their basal aspect to the vascular basal lamina (see sect. 7) (173, 174). Blood vessel formation by endothelial cells is a characteristic of all vertebrates (173, 174). The lymphatic system, which is also a vascular system based on endothelial cells, is similarly found only in vertebrates, having been described in teleosts, amphibia, reptiles, and birds (175). It thus seems possible that the evolution of the lymphatic system could have been inseparable from the transition to a cardiovascular system composed of a network of endothelial channels, rather than by apposed basement membranes of the tissues that they irrigate, as is found in many nonvertebrate chordates (173, 174). Invertebrates do not have any lymphatic vasculature, and all excess extracellular fluid, together with cellular waste and recycled proteins, flows directly back into the blood circulation (176). The transition from blood vessels forming a passive barrier composed of extracellular matrix to a structural barrier formed by endothelium thus mirrors the evolutionary advent of lymphatic vasculature in vertebrates (173–177). Transgenic mice lacking proteins critical for lymphatic vascular development, i.e., PROX1 or podoplanin knockout mice, suffer perinatal death, showing that the lymphatic system is crucial for mammalian survival (178, 179). Thus, at some evolutionary stage when the blood vasculature no longer served unassisted to remove excess extracellular fluid, the lymphatic vasculature emerged out of a vital necessity to ensure extracellular fluid homeostasis.
How, then, might the evolution of the glymphatic system have temporally related to the evolution of intrinsic brain vasculature? As outlined in the sections above, our best guess is that the glymphatic system first appeared before the divergence between synapsids (mammals) and sauropsids (reptiles and avians). The glymphatic system is only present in those vertebrates, which generally harbor a larger and more complex CNS. On the other hand, intrinsic brain vasculature is present in all vertebrates and in some invertebrates, suggesting a primacy over glymphatics. Indeed, various components of the glymphatic system are lacking in lower order vertebrate brains, including absence of AQP4 expression or endfeet polarization and the lack of protoplasmic astrocytes forming a glia limitans perivascularis. AQP4 knockout animals, which are functionally glymphatic knockouts, are viable, whereas lymphatic vasculature knockout is lethal perinatally. Furthermore, AQP4 knockout mice show no developmental, behavioral, or gross neuroanatomical deficits (180, 181). Does it follow that vertebrates lacking a glymphatic system have no alternate pathway for brain fluid clearance? Considering the evolution of the peripheral lymphatic system, it seems clear that its necessity emerged with the formation of blood vessels from endothelial cells (173, 174, 176, 182). Brain vasculature in vertebrates is similarly composed of endothelial cells, forming an even tighter physical barrier than that in the periphery, due to the BBB (75). It is estimated that 20% of CSF in mammalian brain is produced by extrachoroidal tissues, and transcapillary fluid influx across the BBB is likely one of the main sources of extrachoroidal CSF (33, 52, 183). Thus there is necessarily fluid influx into the brain extracellular space, and the brains of vertebrates with no glymphatic system must have some alternate pathway to drain away excess fluid and avoid catastrophic brain edema. It seems most likely that periarterial and perivenous spaces, perhaps together with pericapillary spaces, constitute a sufficient drainage route for brain extracellular fluid. Perivascular spaces are highly conserved among all animals with intrinsic brain vasculature, and these spaces, as proposed by the Austrian neurologist Heinrich Obersteiner more than a 100 yr ago, probably constitute the most primitive influx and efflux pathways for extracellular fluid, though the anatomy and location of barrier membranes differ between species (35). Understanding better the development of perivascular spaces both in phylogeny and ontogeny could provide information about the properties of intrinsic brain clearance mechanisms and the advantages conferred by the development of a glymphatic system.
4.9. Outstanding Questions
The avian brain harbors the anatomical structures and molecular machinery that drive the glymphatic system in mammals. Does this mean that the avian brain has a functional glymphatic system, and if so, does it share all the main characteristics with mammals, i.e., perivascular transport, AQP4 dependence, and sleep-wake regulation?
Comparative neuroanatomy and molecular biology suggest that the glymphatic pathway developed before the split of sauropsids (reptiles and avians) and synapsids (mammals). Do reptiles possess a glymphatic system and at what point during evolution did the glymphatic system first develop?
The brain of zebrafish, a teleost (bony fish), does not have external CSF and contains radial glial cells with unpolarized expression of AQP4. Nonetheless, they effectively transport intracerebral macromolecules to lymphatic endothelial cells in the dorsal meninges. Does the zebrafish brain have a more primitive glymphatic system, e.g., without AQP4, or does it clear the brain with alternate mechanistic elements?
The complex and large brain of the octopus has perivascular channels that are proposed to function as lymphatic-like channels much akin to the perivascular spaces of the mammalian glymphatic system. However, cephalopods, like other protostomes, do not have ventricles or CSF. Thus a different intra-axial fluid flow must exist in brain of these animals. What type of brain fluid transport takes place in the complex CNS of the octopus?
Elasmobranches (sharks and rays) have a glial BBB, and their perivascular spaces are in open communication with blood. How does this arrangement affect brain clearance in sharks compared with vertebrates with endothelial BBB?
4.10. Interim Summary Section
The earliest phylogenetic need for an intrinsic brain vasculature arose in jawless fishes similar to modern lamprey and hagfish. The presence of an intrinsic vasculature is an established feature of all jawed vertebrates. The brain vasculature arose from the need to sustain metabolism when simple diffusion from a surface vascular plexus no longer sufficed for the metabolic demands of the tissue.
Intraventricular CSF compartments are found in all chordates. External CSF compartments equivalent to the mammalian subarachnoid space are found in terrestrial vertebrates like amphibians, birds, and reptiles. In these latter species, there is microscopic communication channels between the internal and external CSF spaces. In nonterrestrial vertebrates, an external fluid pool sometimes surrounds the brain, e.g., in teleosts and elasmobranches. In elasmobranchs, this fluid freely exchanges with the bloodstream, and a barrier composed of tight junctions between endfeet in the glia limitans prevents mixing of this fluid with brain extracellular fluid. Thus open exchange of subarachnoid space CSF and its solutes with the brain extracellular space seems to be a feature of terrestrial vertebrates.
Perivascular spaces, which are important fluid conduits for the inflow of CSF and outflow of extracellular fluid from the brain in the mammalian glymphatic system, occur in various conformation across different species. Perivascular spaces exist in animals with no CSF at all such as the octopus and in animals with no external CSF such as shark and zebrafish. In these species, perivascular spaces can be composed of collagen bundles, or simply a fluid freely exchanged with the blood. Despite this lack of compartmentalization, intrathecally administered compounds clear dorsally toward meningeal lymphatic endothelial cells in the zebrafish brain, thus suggesting that an alternative brain clearance pathway indeed exists.
Astrocytic endfeet covering the brain vasculature, and the polarized expression of AQP4 toward the abluminal side of the endfeet, are necessary conditions for a functional glymphatic system, as outlined in rodents. Mammals, reptiles, birds, and cartilaginous fish have all been shown to harbor a nearly complete coverage of brain microvasculature by astrocytic endfeet. Humans have a less complete endfoot coverage than rodents, but it is unknown if this morphologic distinction imparts any differences in glymphatic function. Animals such as amphibians and teleosts do harbor some endfoot processes, which are formed by radial glia cells.
AQP4 expression is documented in brains of several nonmammalian orders such as teleosts and elasmobranches, but detailed information about their cellular and subcellular distributions is lacking. Zebrafish express AQP4 in radial glial cells, but the expression is not polarized. Birds have polarized expression of AQP4 toward astrocytic endfeet, similar to that in mammals.
The glymphatic system is present in rodents with lisencephalic brains and in gyrencephalic mammals like pigs, cats, nonhuman, and primates and humans. All components that constitute the glymphatic system in mammals are also found in birds, although the presence of a functional glymphatic system is not yet confirmed in avian brain. Arguments based on comparative neuroanatomy led us to speculate that the glymphatic system developed before the divergence of sauropsids (reptiles and avians) and synapsids (mammals).
5. MACROSCOPIC ORGANIZATION OF INTRAVENTRICULAR AND EXTRA-AXIAL FLUID TRANSPORT IN BRAIN
5.1. Introduction
Unlike any other organs, the brain is completely immersed in a fluid, CSF, and is confined within a bony vault, which is a closed and rigid cavity. The density of CSF (1.007 g/cm3) is similar to that of brain tissue (1.040 g/cm3), such that the buoyant forces of the surrounding fluid reduce the brain weight (∼1,400 g) to ∼46 g. One advantage of this anatomic arrangement is that it eliminates the need for the fibrous elements that maintain the shape of many other organs like the kidney or uterus. Indeed, brain tissue is practically devoid of connective tissue except around large vessels and meninges (see sect. 7). The maintenance of the brain’s structural integrity requires that it be suspended, floating like a yolk in an egg. If it were not for its buoyancy in CSF, the brain mass would compress the arteries at the Circle of Willis and disrupt its own blood flow.
A unique feature of fluid flow in the CNS is its adaptation to the brain’s lack of porous capillaries. The tight capillary junctions of the BBB effectively restrict influx of vascular fluid, which is an essential aspect of peripheral tissue homeostasis. Instead, the choroid plexus secretes CSF, which then circulates throughout the brain by glymphatic flow in a network of annular perivascular tunnels formed by the vascular endfeet of astrocytes. Thus the CNS uses the glymphatic system as an alternate strategy to maintain its tissue fluid perfusion. Although not structurally identical, the brain’s glymphatic system is best viewed as a functional analogue of the fluid flow mechanisms supported by the porous capillaries and lymphatic vessels in peripheral tissues (FIGURE 1). The glymphatic system is properly viewed as sophisticated biological engineering to compensate for the physiological constraints imposed by the BBB and the confined space of the cranium.
In this section, we shall first describe the unique features of fluid production and transport in the CNS. We shall also describe the multiple functions of the meningeal membranes, including their shielding of the brain tissue, their barrier function, and their role as an immune interface separating CNS from peripheral tissues. For detailed reviews on the immune function of the CNS membranes and barriers, we refer to several excellent reviews on the topic (184–186).
5.2. Choroid Plexus and CSF Production
The choroid plexus is a highly vascularized secretory epithelium that resides in all four cerebral ventricles. The choroid plexus is a folded structure with papillary-like extensions that project into the lumen of the CSF-filled ventricles (FIGURE 8). The epithelium of the choroid plexus is a specialization of the ependymal cell layer that lines all ventricular surfaces (17). Both the choroid plexus epithelium and the associated ependymal cells are endowed with cilia protruding into the ventricles. Cilia in the ventricles may play multiple important roles during the development of the CNS (189), migration of neural progenitors along the subventricular spaces (190), and CSF transport (191), as covered in prior reviews (192, 193). Ependymal cells are of neuroepithelial origin; lineage studies show that the choroid plexus epithelial cells originate from the embryonic roof plate that occupies the dorsal midline of the vertebrate neural tube. These epithelial cells commit to their lineage at an early stage of brain development, even before the onset of neurogenesis in mice (194, 195). The cells of the choroid plexus epithelium are cuboidal in shape, covered by microvilli at the apical membrane, and rest on a basement membrane. They form a continuous plexus, which is densely coupled by tight junctions just below the apical surface. These tight junctions in the choroid plexus form the selectively permeable blood-CSF barrier, which together with the BBB and the arachnoid barrier, maintain the milieu intérieur of the CNS (108). A dense reticular network of fenestrated capillaries located below the choroid plexus basement membrane allows an ultrafiltrate of plasma to come in direct contact with the choroidal epithelial cells. For details of the choroid plexus functional anatomy, we refer the reader to Refs. 196, 197.
FIGURE 8.
Barriers separate the central nervous system (CNS) from peripheral tissues, but fluid can exchange with relative ease between all the CNS compartments due to the lack of tight barriers within the CNS. Middle: the tight barriers of the adult human brain in red and porous membranes in green. CNS is separated from peripheral tissues by several tight barriers (red lines), which include the blood brain barrier (BBB), the blood-choroid barrier, and the arachnoid membrane. Each of these barriers is created by expression of tight junctions containing claudins and other junctional proteins. The brain surfaces, i.e., the pial membrane and the ependymal cells layers covering the ventricles, are permeable membranes (green lines). Both the cells covering the ventricular surface (ependymal cells) and the cortical surfaces (pial cells) are connected by gap junctions, but gaps allow exchange of cerebral spinal fluid (CSF) with the extracellular fluid (green lines). Of note, astrocytes rather than endothelial cells form a vascular barrier in the external and intermediate zones of the median eminence and the adjacent ventral part of the arcuate nucleus, but not in other regions of the hypothalamus (187, 188). Also shown is the localization of the choroid plexus in the lateral, 3rd, and 4th ventricles, the meningeal layer, and the subarachnoid space. Top left inset: the BBB is a tight barrier that restricts fluid and solute exchange with the vascular compartment while the perivascular endfeet of astrocytes are loosely connected by gap junctions. Bottom left inset: The meningeal membranes consist of dura mater, arachnoid mater, and pia mater. The arachnoid membrane is a tight barrier expressing claudin-11 that separates the peripheral tissue and dura mater from the subarachnoid space. Pia mater covers all surfaces of the brain and spinal cord, but does not represent a barrier for fluid exchange. Together, these 2 membranes are known as the leptomeninges. Top right inset: choroid plexus is a simple structure consisting of a single layer of tight junction-coupled choroidal epithelial cells resting on a basement membrane containing a complex network of fenestrated capillaries. Bottom right inset: The brain surface is covered by loosely connected pial cells, whereas the ependymal lining of the ventricular surfaces also lacks tight junctions. Astrocytic processes contact both the pial and the ependymal layers, thereby creating a second semipermeable layer that possibly acts as a filter. Thus CSF can exchange with brain extracellular fluid at all of the brain surface.
There is a general concordance in the literature that the choroid plexus is the primary site of CSF production. The concurring studies have used an array of approaches, including surgical removal of choroidal tissue or chemical and pharmacological manipulations of CSF production. Most of these classical studies suggested that the choroid plexus produces ∼80% of the total CSF volume, whereas the remaining 20% is actively secreted by endothelial cells across the BBB, which has an enormous capillary surface area estimated at 100–200 cm2·g tissue−1 (198). However, despite this commonly accepted view, other lines of evidence suggest that the choroid plexus produces little or no CSF: Compelling arguments against the choroid plexus being involved in CSF secretion are reviewed in Ref. 199. Our view is that the choroid plexus and the capillaries likely both contribute to CSF production and that their relative importance for CSF production may change depending on the state of brain activity, autonomic drive, age, disease states, position of the head, blood pressure, hydration state, and other factors. The emerging field of CSF secretion benefits from the rapid development of new tools and methodologies that are expected to provide critical new insights (200–202).
Despite decades of research, the precise mechanisms by which the choroid plexus produces CSF remains a matter of debate (FIGURE 9). This debate is focused on the established fact that the expression pattern of membrane transporters in choroid plexus differs radically from that of any other secretory epithelia. Most notably, the key players in fluid secretion, Na+/K+-ATPase (NKA) and the electroneutral Na+, K+, Cl− cotransporter (NKCC1), are present in the apical membrane of the choroid plexus rather than in the basolateral membrane, as in other organs (203–205). In classical secretory glands, such as the salivary glands and the exocrine pancreas, NKA and NKCC1 are located at the basolateral membrane, such that NKCC1 drives Cl− into the cells against an electrochemical gradient (206). In turn, the high intracellular Cl− concentration combined with an abundant polarized expression of Cl− channels supports Cl− secretion at the apical membrane. Meanwhile, extracellular Na+ enters the extracellular gaps between neighboring cells and is propelled into the secreted fluid by the electrostatic forces generated by the intracellular Cl− transport. An interesting aspect of this model is that Na+ can permeate the tight junctions connecting epithelial secretory cells (204). Thus secretory cells can secrete Cl− thanks to an appropriate polarized arrangement of transporters and channels, while Na+ is exported by passive electrostatic forces into the extracellular space.
FIGURE 9.
The classical model of cerebral spinal fluid (CSF) production. 1: In this model of CSF secretion, the Na+-K+-ATPase is the primary driver of CSF production, and secretes Na+ directly into CSF. 2: The activity of the Na+-K+-ATPase lowers intracellular Na+, which in turn promotes Na+ influx across the basolateral membrane, which depends on Na+//Cl− cotransporters to produce an increase in cytosolic anions (Cl- and ) concentrations. 3: In turn, the high density of anion channels at the apical membrane facilitates efflux of Cl- and . 4: Water is drawn into CSF by the increase in osmolarity supported by aquaporin 1 (AQP1) water channels. AQP1 at the basolateral membrane likely also contributes to water influx to the cell. NCBE, Na+-driven Cl−/ exchangers.
The location of NKCC1 and NKA in the apical membrane of choroid epithelial cells suggests that their secretion of fluid (i.e., CSF) follows a fundamentally different principle than for typical secretory membranes. According to the classical model of CSF production, NKA rather than NKCC1 is the central player (207, 208). Because NKA is present in the apical membrane, its activity will result in direct secretion of Na+ into the CSF. This process will naturally reduce the intracellular Na+ concentration, which in turn drives the import of from blood by the Na+-driven Cl−/ exchangers (NCBE, NBCn2) in the basolateral membrane. In conjunction with local production of from CO2 via carbonic anhydrase, the rising intracellular concentrations of Cl− and together create an electrochemical gradient that drives the apical export by the Cl−/ exchanger (AE2). This is predicated upon the high permeability of apically located anion channels to Cl− and ions. The net result is that choroidal epithelial cells propel Na+ together with /Cl− from the cytosol directly into CSF. The resultant osmotic gradient drives in turn an influx of water from plasma into the ventricles, which is supported by the AQP1 water channels present at high density in the apical membranes and at more moderate levels on the basolateral membrane of choroidal epithelial cells (209, 210). In fact, analysis of samples from rabbits and dogs indicates that the osmolarity of CSF is 6 mosmol/kgH2O higher than that of plasma (211, 212), although others have found contrary results (204). We also note that genetic deletion of AQP1 in mice reduced CSF production by only 20%, which does not support the idea that osmosis is a major driver of CSF production (209, 210).
Thus the classical model of CSF secretion ignores the high NKCC1 expression in the apical membrane of choroidal epithelial cells (FIGURE 9). This omission is based on the notion that NKCC1 supports the inward movement of Na+, K+, and Cl− in other cell types (213). The unusual apical location of NKCC1 in choroidal epithelial cells would thus predict that choroid plexus should absorb rather than secrete CSF. It has been argued that NKCC1 acts as a macroscopic K+ buffering mechanism to avoid excessive accumulation of this depolarizing cation (214, 215). Maintaining extracellular K+ within the physiological range is a high priority for neural tissue, since elevations of brain extracellular K+ concentration ([K+]) are associated with potentially catastrophic increases in neuronal excitability, resulting in seizures (216). The equilibrium potential of NKCC1 is close to the neuronal resting membrane potential, and the transporter is therefore largely inactive under physiological conditions. However, NKCC1 will become activated when [K+] in CSF exceeds normal limits, such as during ischemia. Thus NKCC1 in the choroid plexus may function as a potent macroscopic K+ buffer ready to export excess K+ quickly out of the brain, possibly via the K+-Cl− (KCC3) cotransporter located in the basolateral membrane of choroidal epithelial cells (204). More recent studies have suggested that NKCC1 in the choroid plexus has instead a reversed directionality, thus driving Na+, K+, and Cl− out of the choroidal epithelial cells to support CSF production (217). This alternative model awaits independent confirmation and is not supported by the findings from cryo-EM showing that NKCC1 is not symmetric and that reversing ion transport thereby is unlikely (213, 218). Resolving this conundrum calls for additional research using better methodologies than are presently in use (217, 219). We anticipate that the classical concepts of CSF production are likely to be revised upon development of more advanced molecular and imaging techniques.
5.3. Extrachoroidal CSF Production
As noted above, the choroid plexus is usually considered the primary source of CSF. However, several lines of evidence support the contrary notion that CSF is, in whole or in part, a product of extrachoroidal sources. For detailed discussion of this concept, please refer to Ref. 199. One of the strongest arguments in favor of alternate production sites is that surgical removal of the choroid plexus does not completely eliminate CSF production (220). Most authors have favored the idea that extrachoroidal CSF production reflects the influx of vascular fluid across the BBB (199). This model holds that ion and solute transporters located in the BBB mediate water influx via cotransport with solutes such as glucose and amino acids (221, 222). Influx of vascular water is believed to occur primarily at the microvascular bed, given the vast total surface area of brain capillaries. Conversely, it has been argued that the surface area of the choroid plexus is insufficient to support the high production rate of CSF, and consequently CSF must be supplemented by fluid transport across the larger surface area of brain capillaries, see discussions in Refs. 18, 198. The extracellular fluid generated by influx of water across the BBB will flow, at least in part, into the ventricles and thereby contribute to the total CSF production. Thus it is not possible to account for the capillary contribution to CSF production, since existing techniques cannot distinguish the CSF pools produced by the choroid plexus versus capillary influx. Recent work shows that arterioles in the subarachnoid space express AQP1 water channels and the NKKC1 cotransporter, suggesting that CSF is also produced in the subarachnoid space (223). The spinal cord may also contribute to CSF production, but its relatively small volume (35 g, corresponding to only 2% of human brain weight) has made it difficult to collect reliable data on how much CSF is produced from the spinal cord and its rich surrounding vascular network (18). MRI measurements of CSF flow show that the flow volume in the foramen magnum is severalfold larger than that in the cerebral aqueduct connecting the third and fourth ventricles, thus supporting the proposition that the choroid plexus is not the only source of CSF (224).
5.4. CSF Production Rate in Humans and Experimental Animals
Although the production of CSF has been studied for several decades, surprisingly little is known about the regulation of its secretion rate. In this section, we discuss the existing quantitative literature on CSF production and draw attention to unresolved key questions and gaps in knowledge.
Pappenheimer and colleagues (225) pioneered the measurement of CSF production in experimental animals. His indirect tracer dilution method based on ventriculo-cisternal perfusion was first tested in awake goats with chronically implanted cannulas. The Pappenheimer method entails infusion of a tracer into a lateral ventricle while collecting CSF from the cisterna magna. CSF production is then calculated based on the dilution of the tracer in the downstream CSF sample. The indirect tracer dilution method suffers from several limitations. For example, a large volume of fluid containing the tracer, typically comprising close to one third of the estimated rate of CSF production, must be injected into the lateral ventricle, which brings a risk of activating mechanosensitive receptors, lowering the brain temperature, and changing CSF osmolarity, all of which could perturb the measurements. Also, any diffusion of CSF tracers into the periventricular tissue will bias the calculation of the CSF production rate by this method, as reviewed in Ref. 199. In fact, it has been reported that the apparent CSF production rate increases as a function of the infusion rate used during the Pappenheimer technique (226). It is therefore important to take into consideration that essentially the entire existing quantitative literature on CSF production is based on the Pappenheimer indirect dilution technique and should as such be considered with caution.
A recently introduced method for direct measurement of CSF production has several distinct advantages over the Pappenheimer method. In this new approach, a cannula is inserted into a lateral ventricle while CSF efflux via the aqueduct is blocked by injection of mineral oil in the fourth ventricle through a cannula inserted in the cisterna magna. Thus newly produced CSF is directly collected from the lateral ventricle without the need for tracer administration in rats (227, 228) and mice (202). The technique does suffer from the limitation that it misses CSF produced by the choroid plexus in the fourth ventricle or arising outside the ventricles. This may pose a problem, since 40–60% of total CSF production in rabbits is said to occur in the fourth ventricle (229). In fact, while the indirect tracer dilution technique indicated CSF production at a rate of 3.38 µL/min in rats (230), direct CSF measurements in the lateral ventricles gave CSF production rates in the range of only 0.39–1.40 µL/min (227, 228). The discrepancy may well reflect the contribution of CSF produced in the fourth ventricle, but one must also consider the possibility that additional CSF is produced by extrachoroidal sources outside the ventricles, which would be included in the rate estimated based on the indirect tracer dilution technique, but not the direct CSF measurements in the ventricles. To our knowledge, this possibility has not been directly addressed. Given present technical limitations, it is simply impossible to conclude if the discrepancy between indirect and direct CSF measurements represents an artifact or a real difference. Clearly, new approaches are needed to establish the relative contributions of choroidal and extrachoroidal CSF production.
For obvious reasons, direct measurements of CSF production are not possible in healthy human brain. However, this has been attempted intraoperatively in patients undergoing invasive treatment for glioma or other CNS tumors. Older studies in patients with ventricular catheters to deliver antineoplastic drugs reported an average CSF production rate of 0.37 mL/min (62, 231). In recent years, CSF production in healthy individuals has mostly been studied using noninvasive MRI measurements of CSF outflow through the cerebral aqueduct. Analysis of fluid flow in phantom models of the aqueduct indicates that this approach is generally accurate but prone to error in cases where the aqueduct is narrow or when CSF flow velocity is high (232). MRI-based measurements of CSF production rate in adult human brain are between 0.3 and 0.7 mL/min, which corresponds to 430-1,000 mL/day (18, 19). Measurements in awake volunteers show a nightly peak of 0.7 mL/min occurring at 2:00 AM, versus an evening nadir of only 0.2 mL/min at 6:00 PM, suggesting some relationship with the sleep-wake cycle and/or circadian rhythm. In that study, total CSF production was estimated to average 650 mL/day (233), that is to say, within the range of other reports. It is important to note that measurements of CSF flow at the aqueduct do not include CSF production in the fourth ventricle or the extrachoroidal CSF production outside the ventricles. In fact, a recent study reported the CSF flow in the aqueduct amounted to 0.6 mL/min (0.86 L/day), while the CSF flow through the foramen magnum in the same patients was more than threefold higher (2.7 mL/min or 3.9 L/day) (234). The negative value and high standard error reported in this publication may reflect that respiration initiates rhythmic rostral-caudal movement of CSF, which complicates the calculations (235). Taken together, MRI studies suggest that CSF production in humans is under circadian control and that extrachoroidal CSF production may be a more significant contributor to the total rate of CSF production than is currently recognized. However, these observations await independent replication and confirmation.
5.5. Physiological and Pharmacological Manipulations of CSF Production
Numerous studies have used pharmacological manipulations to study the molecular mechanisms involved in CSF production. The most effective inhibitor of CSF production is the NKA inhibitor, ouabain, which suppresses CSF production by 40–100% (236–238). However, ouabain has high systemic toxicity due to its nonspecific elimination of ionic gradients across the plasma membranes of all cell types. Thus ouabain may likely perturb CSF production by exerting a variety of unintended toxic effects. The carbonic anhydrase inhibitor acetazolamide is also highly effective at reducing CSF production (204), as might be predicted from the contribution of the Na+-driven Cl−/ electrochemical gradient in the choroid plexus mentioned above. In contrast, the NKCC1 inhibitor furosemide only inhibited CSF secretion when applied at high and nonspecific concentrations, while the more selective NKCC1 inhibitor bumetanide had no effect on CSF formation when delivered intravenously (239). Intraventricular delivery of bumetanide has been reported to reduce CSF production, a finding consistent with the apical localization of NKCC1 in choroidal epithelial cells. Unfortunately, high and thus nonspecific concentrations of bumetanide (10–100 µM) were utilized in most of the previous studies. To ensure pharmacological specificity, it is imperative to employ low concentrations of bumetanide in the order of the Ki (∼0.1 µM) for specific inhibition of NKCC1 to avoid the unintended inhibition of other anion and cation transporters that takes place at concentrations in the range of 2–4 µM (239, 240) Amiloride, which blocks the epithelial sodium channel (ENaC), the Na+/H+ exchangers (NHE-1), and the pan-acid sensing ion channels (ASIC), does not suppress CSF production when delivered in blood or in the ventricle (237). The Cl− transport inhibitor DIDS reduces CSF production when delivered via blood, which is consistent with the high expression of Cl−/ transporters at the basolateral membrane of choroid epithelial cells (204). In the clinic, several of these agents, most notably acetazolamide and furosemide, are used to reduce CSF production and thereby lower intracranial pressure (ICP) in patients suffering from idiopathic intracranial hypertension (241). Topiramate is increasingly prescribed for idiopathic intracranial hypertension and is popular for this indication because it also acts as an antimigraine prophylactic agent. The antimigraine mechanism of topiramate is via weak inhibition of carbonic anhydrase activity along with blockade of voltage-dependent sodium and calcium channels and direct effects both on glutamatergic and GABAergic transmission (241). In a rat study, topiramate was very effective in blocking CSF production, lowering ICP by 22%, while acetazolamide, amiloride, and furosemide had no such effects when administered at clinically relevant doses (242).
What is known about the physiological regulation of CSF production in humans? A study using MRI to estimate flow in the aqueduct reported 50% lower CSF production in healthy aged individuals compared with young subjects (243). Also, patients suffering from Alzheimer’s disease exhibited lower CSF production compared with aged-matched controls (244). Only a few studies have addressed the effect of age or Alzheimer’s pathology on CSF production in rodents, despite its obvious clinical importance. One study reported that CSF production increased threefold in rats as they matured from 8 to 12 wk of age (227). This observation was confirmed by the finding that CSF production increased by 20% in rats from 3 to 12 mo of age, while production declined by 55% as the rats aged from 12 to 30 mo, which is near the lifespan of rats (245). In mice, aging suppressed CSF production most significantly at later stages of life and, overall, CSF production in mice aged 22 mo was 50% lower compared with that in mice aged 2–3 mo (202). Interestingly, the same study found that young female mice produced 30% more CSF than littermate males based on measurements using the direct method (202), yet glymphatic flow in mice is comparable and decreases as a function of aging in both sexes (246). The choroid plexus expresses multiple sex hormone receptors, including estrogen receptors (247–250), which might mediate gender differences in CSF production. A clinical study reported that premenstrual women exhibit a greater total CSF volume than men, but it is uncertain how this observation may relate to CSF production (251). The choroid plexus vasculature is densely innervated by sympathetic adrenergic and parasympathetic cholinergic nerve fibers (248, 252–254), which predicts for autonomic regulation of blood flow and possibly of CSF production. Indeed, surgical denervation or pharmacological suppression of adrenergic sympathetic signaling increased CSF production in rodents (255), whereas cholinergic pharmacological stimulation increased CSF production (256). Wakefulness is associated with lower CSF production than during anesthesia in mice (202). However, the impact of anesthesia does not appear to be simply a function of suppression of sympathetic tone. Isoflurane anesthesia increases CSF production more potently than the α2 agonist xylazine, albeit the latter treatment more efficiently inhibits noradrenaline release (202). The choroid plexus epithelium also expresses serotonergic receptors, the activation of which increases blood flow in the choroid plexus, while not affecting cerebral blood flow (257). Serotonin and other neuromodulators regulating perfusion of the choroid plexus may indirectly modulate CSF production, but this remains to be established experimentally.
5.6. Ventricular System
The ventricular system consists of the lateral, third, and fourth ventricles. The internal lumens of the ventricles are interconnected, and each ventricle contains choroid plexus that contributes to total CSF production (FIGURE 8). The ventricles in healthy young adults have a volume of ∼25 mL, representing <20% of total CSF volume (130–150 mL). CSF leaves the fourth ventricle via the median (foramen of Magendie) and the bilateral lateral (foramina of Luschka) apertures, subsequently draining into the cisterna magna and cerebellopontine angle cistern, respectively. Of note, there is also no foramen of Magendie in rodents; instead, CSF leaves the rodent fourth ventricles via the two laterally positioned foramina of Luschka in fetal and neonate rats (258, 259) and in adult mice (202). A minor proportion of CSF from the fourth ventricle will then flow down the central canal, which terminates in the conus medullaris of the spinal cord, also known as the ventriculus terminalis or the fifth ventricle. A histological analysis found that the central canal closes in almost all human subjects aged more than 20 yr (260). The central canal may however play a role in CSF transport: Syringomyelia is caused by closed CSF-filled cysts, which slowly expand to block the central canal and are often associated with pain and also atrophy of the spinal tissue including the dorsal white matter tracts, causing weakness and loss of spinal reflexes (261). All surfaces of the ventricles and the central canal are lined with a layer of loosely joined ependymal cells linked by gap junctions that presents no barrier for the exchange of fluid and solutes between CSF and the brain or spinal cord tissue (10, 262). This arrangement is critical for the pathway of CSF/extracellular glymphatic fluid exchange, as shall be discussed below.
5.7. Meninges
The meninges consist of three distinct membranes surrounding the brain and spinal cord (FIGURE 8). The three membranes are the dura mater, which directly faces the cranial vault and the underlying arachnoid mater, and the arachnoid mater, which separates the subarachnoid space from the dura mater. The innermost membrane, the very thin pia mater also faces the subarachnoid space and intimately covers the brain. Like the BBB and choroidal epithelial cells, the arachnoid mater expresses an abundance of tight junctions (claudin-11) that effectively contain the CSF fluid within the brain compartment by restricting the passage of fluid and solutes between CNS and peripheral tissues (108).
5.7.1. Dura mater.
Dura mater is a thick, vascularized membrane plastering the inner surface of skull and the intervertebral space surrounding the spinal cord, where the epidural space contains a rich network of blood vessels and fat that separates the dura mater from the bony structure of the vertebrae. Dura mater consists mostly of collagen fibers and forms a continuous sheath enveloping the dural venous sinuses, the middle meningeal arteries, and the meningeal lymphatic vessels. Dura mater also forms the falx cerebri and tentorium cerebelli at critical positions supporting the soft brain tissue. Blood vessels in the dura mater do not present a barrier for leakage of solutes from plasma. CSF tracers enter the dura mater and are drained by meningeal lymphatic vessels, suggesting that the dura mater represents an immunological interface for CNS, consistent with its high concentration of resident immune cells (23). Meningeal lymphatic vessels expressing all the classical markers of the lymphatic vasculature reside in the dura mater (23, 68). In the setting of multiple sclerosis and other neuroimmune diseases, leukocytes can form aggregates in the dura mater, also designated as a tertiary lymphoid tissue (263).
5.7.2. Arachnoid mater.
The arachnoid membrane is attached to the inward surface of the dura mater, facing the subarachnoid space. The blood-arachnoid-cerebrospinal fluid interface has been called the outer CSF barrier because of its functional resemblance to the BBB and the blood-CSF barrier that separate the CNS from peripheral tissues. The arachnoid membrane is composed of leptomeningeal fibroblasts densely connected by tight junctions formed by claudin-11 (108). The arachnoid membrane extends arachnoid trabeculae or villi that traverse the underlying CSF-filled subarachnoid space and merge with the underlying pia mater. The arachnoid trabeculae consist of collagen fibers incompletely covered by leptomeningeal cells.
5.7.3. Pia mater.
The pia mater is the membrane formed by a monolayer of cells that loosely adheres to the brain and spinal cord surfaces. Pial cells are in direct contact with the glia limitans superficialis – a dense network of astrocytic processes facing the outer surfaces of the CNS. The pial cells are not connected by tight junctions and therefore do not present a barrier to the influx of CSF solutes into the underlying neuropil, granted that the glia limitans likely slows the exchange of larger solutes. The pia mater and the ependymal cells of the ventricles meet without any interposed neuronal tissue, adhering to each other to form a connective tissue membrane called the tela choroidea. This connective tissue membrane is the attachment site of the rather free-floating choroid plexus in the fourth ventricle, and it contains a rich network of vessels supplying the choroid plexus. The tela choroidea forms partly at the roof and the lateral recesses of the fourth ventricle.
5.8. The Subarachnoid Space
The subarachnoid space is positioned between the arachnoid barrier membrane and the pia mater. It is filled with CSF, thus accounting for the part of CSF volume not contained in the ventricles. The arachnoid trabeculae provide support for the leptomeningeal vasculature, while also serving to reduce the movement of the brain within the skull (FIGURE 8). The subarachnoid space forms several cisterns or CSF-filled cavities, of which the cisterna magna positioned between the cerebellum and medulla oblongata is the largest. The pontine cistern lying on the ventral surface of the pons contains the basilar artery and the anteroinferior cerebellar artery, which are both important entry points for glymphatic CSF transport. The interpeduncular cistern lying at the base of the skull is continuous with the chiasmatic cistern under the diencephalon. Several other minor cisterns surround the brain. The main cistern in the spinal cord is the lumbar cistern, which is located at the base of the spinal cord, wrapping the filum terminale and cauda equina. The human spinal cord terminates around the first and second lumbar vertebrae and the lumbar cistern below that point is therefore the preferred site for injecting agents into CSF or drawing CSF via lumbar puncture.
5.9. Outstanding Questions
What controls CSF production during the different states of brain activity? Do neuromodulators play a role? Does blood flow regulation to the choroid plexus play an unappreciated role?
Is CSF produced in part by extrachoroidal sources and not only by the choroid plexus? If so, what are the relative contributions from each of the separate sources (e.g., BBB) and do the relative contributions change as a function of the state of brain activity, blood pressure, or age or in neurodegenerative diseases or neuroinflammation?
Why does CSF production decrease with healthy aging? Does the lowering of CSF production contribute to Alzheimer’s disease?
Why is CSF production not suppressed by increases in intracranial pressure? Does the abnormal pattern of CSF flow with reflux of CSF back into the fourth ventricle contribute to neurodegeneration in hydrocephalus?
5.10. Interim Summary Section
CNS is separated from peripheral tissues by the BBB, the arachnoid barrier, the blood-CSF barrier, and the barriers formed by astrocytes in select regions of the hypothalamus. These barriers contain tight junctions and allow only limited exchange of fluid and solutes. Within CNS, glial limitans at the brain surfaces or in between select regions forms semipermeable borders, thus slowing, but not preventing, exchange of fluid and solutes between the different CNS compartments. It has yet to be established whether glia limitans is an active and selective filter allowing some but not all solutes to pass.
The molecular machinery of CSF production in the choroid plexus has not been established, although several models have been proposed. Little is also known about how CSF secretion is regulated. Animal and human studies have shown that CSF production declines in aging. Emerging evidence points toward extrachoroidal CSF secretion contributing significantly to total CSF production. If so, parenchymal production of CSF would complement the arterial influx of CSF and thus contribute to the parenchymal flow that drives glymphatic brain clearance.
The meninges and in particular dura mater have in recent years been shown to be an active site for immune interactions between CNS and peripheral tissues. The meningeal lymphatic vessels are important drainage routes for CSF and merge with the cervical lymphatic vasculature. The meninges house a large number of immune cells and can, under certain conditions like multiple sclerosis, be viewed as a tertiary lymphoid tissue.
6. MACROSCOPIC ORGANIZATION OF INTRA-AXIAL FLUID TRANSPORT: THE GLYMPHATIC SYSTEM
6.1. Introduction
Billions of neurons are continuously running an assortment of ion pumps and transporters required for the maintenance of membrane potentials and the generation of action potentials in human brain, while forming and remodeling trillions of synapses at any given moment. It is not surprising that the brain requires a large supply of energy to maintain its normal activity (264). The human brain also synthesizes over 3 g of protein every day to carry out its various functions, which is a rate three- to fourfold higher than that for skeletal muscle (265, 266). Brain proteins are continuously produced, and are likewise broken down, with a mean half-life ranging between 3 and 13 days (267). While there is some recycling of amino acids in situ, there is a need for a robust mechanism for removing such large amounts of protein destined for degradation. In the periphery, this task is performed by the uninterrupted clearance of extracellular fluid by the lymphatic vasculature, which export their contents into the venous drainage, whereupon the proteins are recycled in the liver. In the brain, the glymphatic system fulfills the task of clearance, thereby compensating for the existence of the BBB and the lack of proper lymphatic vessels (50). The perivascular spaces are fluid-filled compartments surrounding most intracerebral vessels that function as connecting pathways for passage of CSF into and out of the brain. The organization and structure of the perivascular space network therefore run parallel to the cerebral vascular network, enabling them to function somewhat analogously to the lymphatic vasculature in the periphery. This section will present the unique architecture of the cerebral vasculature that provides the structural foundation for the glymphatic system. Due to this unique organization, CSF closely follows the arterial vascular network as it flows into the brain (CSF entry), primarily along the three main cerebral arteries (anterior, middle, and posterior cerebral arteries), and subsequently follows the venous vascular network as it flows out of the brain (extracellular fluid exit), chiefly along the internal cerebral vein of the deep venous system and along the caudal rhinal vein (the inferior anastomotic vein of Labbé in humans) of the superficial venous system in rodents. We shall also describe the macroscopic transport route that CSF follows from the subarachnoid space, traveling centripetally toward the deeper subcortical brain regions through arterial perivascular spaces. The section also highlights what is known about the macroscopic extracellular fluid exit routes that closely follow the perivascular spaces surrounding veins but also occur along white matter tracts and subependymal routes neighboring the ventricular system. The macroscopic anatomy of the glymphatic pathway will be described here, while sect. 7 provides further insight into the microscopic anatomy of the glymphatic pathway. For a comprehensive review of perivascular spaces, we refer the reader to a recent in-depth review (50). Subsequent sections will introduce important drivers (sect. 9) and functions (sect. 10) of glymphatic physiology.
6.2. Vascular Organization of the Brain
The arterial system of the brain is divided into anterior and posterior systems. The anterior system is derived from the internal carotid arteries, which branch off the common carotid arteries on each side of the cranium. The posterior system is fed from the two vertebral arteries, which merge at the level of the pons to give rise to the basilar artery. The anterior and posterior systems meet to form the Circle of Willis, which gives rise to the main trunks of the anterior, middle, and posterior cerebral arteries, which together irrigate the majority of the brain parenchyma (FIGURE 10). The Circle of Willis provides a built-in redundancy that helps to ensure continuous blood supply by forming an anastomosis of the anterior and posterior circulations. Cerebral venous drainage is achieved by two pathways, namely the deep and superficial venous systems. The deep venous system arises from the deep medullary and subependymal veins draining into the internal cerebral vein and the basal vein, which join to form the great vein of Galen. The inferior sagittal sinus joins the great vein of Galen to form the straight sinus, which ultimately joins the superficial venous system at the confluence of the sinuses. The superficial venous drainage system is formed by pial veins that feed into the superior sagittal sinus, the occipital sinus, the transverse sinus, the cavernous sinus, and the sigmoid sinus, all of which ultimately drain into the internal jugular veins. The blood supply to the brain is unlike that of other typical solid organs such as the kidney, pancreas, and spleen. In the periphery, the arterial system travels alongside the venous drainage, together entering the organ at roughly the same location and then proceeding to bifurcate outwards with an arterial and venous branch at each bifurcation point. However, the brain arterial system enters at a different foramen (foramen lacerum) than does the venous system (jugular foramen), and the two sides of the brain vasculature take completely separate trajectories. The main cerebral arteries pass along sulci and other surface features of the brain underneath the arachnoid mater, and pierce the pia mater and the parenchyma to form a perivascular space, traveling deep into the tissue in a centripetal fashion (50). In the periphery, arterial blood supply is primarily centrifugal, as in the hepatic, renal, and splenic arteries. While superficial cerebral veins take opposite trajectories to arteries, they are also partially covered by a pial layer and form a relatively incomplete perivascular space. Brain veins also have several distinctive properties compared with those in the periphery, since they do not harbor valves to force directional flow. Instead, they rely mostly on external forces (e.g., arterial pressure and gravity) to drive directional venous outflow, and furthermore drain the brain in a centrifugal pattern, as distinct from the centripetal pattern seen in extra-CNS tissues. This peculiar branching network of the brain vasculature (and its accompanying perivascular spaces) defies an established principle known as Murray’s law. A basic physical principle for transfer networks that is observed by vascular and respiratory structures and plant xylem, Murray’s law holds that flow is maximized when the sum of the cubes of the radii of two branches equals the cube of the radius of the prebifurcation channel. This law governs the vascular branching pattern in almost all mammalian organs, thus ensuring a minimal physiological cost of delivering and removing vital substances. In fact, the microvascular organization of the cerebral cortex, for example, eludes an adequate explanation and its number of bifurcations and changes in vessel diameter deviate significantly from that predicted by Murray’s law (268). In the kidney, lung, and liver, the morphology of the vascular network is determined by the functional unit of the organ such as the renal pyramid for urine production, the alveoli for gas exchange, and the hepatic sinusoid for the production of bile, respectively. In neocortex, no such relationship has been observed across several species for the homologous functional unit, which is the cortical column (269, 270). At first glance, the brain microvascular organization seems largely random, but most evidence suggests that the capillary density is determined by the diffusion distance of O2 in the face of avid consumption in neurons (271). Every brain cell is in close proximity (10–20 µm) to a capillary, and the mean diffusion distance for O2 to diffuse is quite uniform across the parenchyma (with some important variations between gray and white matter, which are consistent with the differing energy demands of the two tissues) (272). Importantly, this spatial relationship is conserved across development, such that that distance between penetrating arteries is conserved despite the rapid expansion of the embryonic brain tissue, suggesting that this vascular structure has a crucial function throughout life (98). Seemingly, the vascular geometry is optimized for the global delivery of blood and removal of substances rather than serving some specific function of the organ in question. Since the BBB restricts the movement of blood-borne fluid and its solutes (other than gases) into the brain parenchymal, the perivascular spaces circumscribing the cerebral vascular network are ideally positioned to carry out a role analogous to that of the lymphatic vasculature in the periphery.
FIGURE 10.
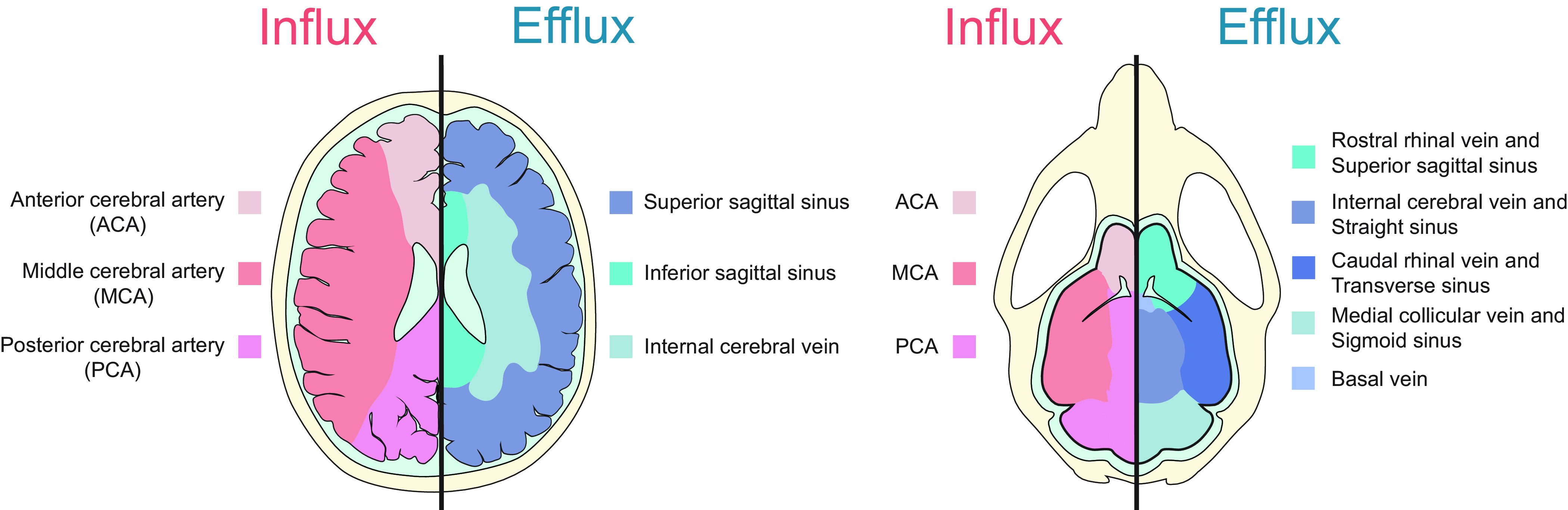
Influx and efflux of intra-axial fluid follows the arterial and venous vascular territories in the human and rodent brain. Cerebrospinal fluid (CSF) enters the brain along the arterial vascular system of the brain that consists primarily of the 3 large cerebral arteries: the anterior cerebral artery (ACA), the middle cerebral artery (MCA), and the posterior cerebral artery (PCA). Intra-axial fluid exits along the perivascular spaces surrounding the draining venous system, which consists of a superficial and deep system.
6.3. Pathways of CSF Entry to the Brain
The mechanism and directionality by which CSF exchanges with extracellular fluid within perivascular spaces, while understood in general terms, remain in need of further clarification. However, the preponderance of evidence favors the centripetal entry of CSF (from the brain surface downwards to deep regions) along arterial perivascular spaces, in conjunction with a centrifugal exit of extracellular fluid (arising in the deep venous system to the superficial venous system) along venous perivascular spaces. Notwithstanding, there is some evidence for the centrifugal exit of extracellular fluid exclusively along arterial perivascular spaces and the absence of CSF entry to the parenchyma (273). The most important evidence for mainly centripetal CSF transport into the parenchyma is currently furnished by MRI studies of paramagnetic tracers delivered into the intrathecal space in humans. Although this approach lacks sufficient spatial resolution to determine whether or not fluid exchange occurs within intraparenchymal perivascular spaces, it does enable the real-time visualization of macroscopic intra-axial fluid transport. Most such studies have used gadobutrol (605 Da), a gadolinium-based macrocyclic contrast agent that is highly hydrophilic and is therefore easily distributed both in CSF and extracellular fluid (165). Intrathecal delivery of gadobutrol in the spinal canal of humans is followed by entry of the tracer in the cisterna magna as soon as 20 min after infusion at the lumbar spinal cord (164, 165). The tracer then distributes within the subarachnoid cisterns and sulci following the perivascular space surrounding the main cerebral arteries (anterior, middle, and posterior) as they traverse across the cortical surface in an anterograde direction (161, 164, 165, 274). The now diluted tracer comes to cover the entire intracranial subarachnoid space by ∼8 h after infusion, with concentration peaking between 10 and 15 h (275). Once the tracer makes an appearance in the overlying subarachnoid space, it then distributes into the adjacent deeper regions of the brain in a centripetal fashion (164, 165, 275, 276). Cortical signal enhancement can be seen as early as 4 h after tracer injection and reaches its maximum by ∼10–24 h (275). MRI studies using a linear contrast agent (Gd-DTPA, 938 Da) (276) or single photon emission computed tomography (SPECT)-CT studies using 99mTc-DTPA (277) found similar temporospatial distribution patterns after intrathecal delivery in healthy adults. The latter procedure revealed that increasing the volume of the injectate did not alter the degree of tracer penetration in the brain, suggesting that transport does not depend exactly on how the tracer is delivered (277). It is worth noting that certain brain regions demonstrate much slower tracer distribution kinetics than others. Thus, while tracer eventually reaches all brain regions, the first sign of contrast enhancement occurred much earlier in cerebral and cerebellar cortices, white matter tracts, and limbic structures and was relatively delayed in striatum, thalamus, and deep cerebellar nuclei (161, 164, 165, 274–277). This may likely reflect the differing distances of the perivascular space paths. There might also be kinetic differences between cortical areas, such that the parietal lobe had slower uptake compared with frontal, temporal, and occipital cortexes (276). If so, this could be due to the relatively low tracer concentration found in the cerebral convexity in some studies, or might alternately indicate that certain brain regions have inherently slower CSF-extracellular fluid exchange, a property that might confer greater vulnerability to pathology (164, 165). In all studies in healthy humans with intrathecal administration, CSF tracers seem to be excluded from accessing the ventricular system, suggesting that the outflow of CSF being produced by the choroid plexus prevented its entry, or alternately that the tracer became too diluted in the large fluid volume of the lateral ventricles (164, 165, 275, 276). Taken together, these studies definitively show that tracers delivered into the intrathecal space in humans are distributed across the entire brain, suggesting the CSF freely exchanges with the extracellular fluid and that centripetal CSF transport predominates. The transport mechanism of centripetal CSF movement has long been held to be primarily explicable by diffusion, a process that consists of the movement of solutes by Brownian motion down a concentration gradient, such that their flux declines with increasing molecular weight, and occurs in an isotropic direction from a point source (see sect. 11). Diffusion works well at short distances, as is amply demonstrated for the transport of O2 from hemoglobin in red blood cells within capillaries to the nearest neuron. However, this process is rather slow and inefficient in the context of fluid in the human CNS, where diffusion paths can be up to a meter (even more in giraffes). Advection, on the other hand, refers to a fast and directional flow driven by a pressure gradient that can efficiently cover large distances in a brief time. The quintessential example of this is presented by the vascular system, which uses advection to ensure that life-sustaining molecules reach their specific target tissue (see sect. 11.4 for further discussion on the contributions of advection and diffusion). Final distribution to the target by simple diffusion supports a fast and continuous arrival of nutrient molecules across the blood vessel wall to recipient cells only a few microns away. Recent evidence has shown that CSF entry into brain does not diverge from this model and that its transport is likewise best explained by a combination of diffusion and advection (also called bulk flow) (278). All tracer studies performed in humans have indicated that the observed transport exceeds that explicable by diffusion alone, which calculations and modeling predict would suffice for only a few millimeters per day, far less than the observations of several centimeters per day (164). Recent work in rats used an innovative framework of optimal mass transport to calculate the relative contribution of bulk flow (i.e., advection) and diffusion on the transport of Gd-DOTA after injection into the cisterna magna, which is not generally accessible in human studies (279–281). This body of work found that CSF transport in the subarachnoid space was predominately explicable by bulk flow, while the centripetal entry of tracer to the brain from CSF was due both to diffusion and advection (279). The overall consensus is that the perivascular spaces likely contribute to the advective component (21, 282), while diffusion predominates in the extracellular space (283–285). This scenario is analogous to the vascular system and thus implicitly requires a pump-like mechanism to generate a pressure gradient sufficient to drive directional flow within perivascular spaces. The directional macroscopic intra-axial fluid flow in brain is now understood to be driven by the glymphatic system, as will be covered in detail in subsequent sections.
6.4. Pathways of Extracellular Fluid Exit From the Brain
As described below, the majority (∼80%) of extracellular fluid leaves the brain parenchyma through an intra-axial pathway that does not require mixing with CSF in the subarachnoid compartment. Most studies have identified perivascular spaces as the pathway for the egress of extracellular fluid from the neuropil en route to its extracranial destinations. However, if CSF indeed enters the brain in a predominantly centripetal fashion, then it makes little sense that extracellular fluid would be using these same paths as an exit route. This argument implies that there must be an anatomic compartmentalization of the inflowing and outflowing fluids. This condition is analogous to that in the peripheral lymphatics, where the vascular system is the source of lymph, but the downstream lymphatic vasculature provides the drainage route. How exactly are these putative pathways in brain compartmentalized to ensure that the entering fresh fluid is continuously removed without being mixed with the extracellular metabolites and waste protein to be cleared? MRI in human subjects shows that intrathecally administered tracers are initially widely distributed throughout the brain, but begin to be cleared as early as 24 h after application, and are substantially eliminated by 80 h (∼3 days) (275). At 4 wk after the tracer injection, there was no detectable tracer signal in any brain region, suggesting complete clearance of the contrast (164). The anatomical route for this clearance is uncertain due to the spatial resolution limitations of MRI and the logistic difficulty of repeatedly scanning patients over several days. However, tracer enhancement in the tissue occurs several hours (24 h) after the maximal signal in subarachnoid space CSF, and the CSF signal declines continuously after this zenith, supporting the notion that tracers are not cleared back into the subarachnoid space (161, 164, 165, 286). Likewise, simultaneously with brain signal enhancement, the first MR signal can be detected in the cervical lymph nodes, which are an important extracranial exit site (see sect. 12 on CSF egress sites). The concurrent enhancement between these compartments, and not with CSF, indicates that extracellular fluid most likely bypasses the CSF to clear directly toward peripheral lymphatics via intra-axial efflux. How exactly these flow pathways interconnect and to what extent each route contributes to global CSF efflux from the brain await experimental demonstration. The majority of available evidence suggests that perivascular spaces are the primary outflow routes; however, intrathecal tracers penetrate deep into subcortical white matter, which extends further than the known anatomy of cortical perivascular spaces (164). Work in animals has identified that the compartmentalization of inflowing CSF is restricted to arterial perivascular space, while the removal of extracellular fluid is directed primarily toward venous perivascular spaces. However, several additional pathways cooperate to drive clearance, most notably the white matter tracts as mentioned above, along with transependymal and subependymal routes. Early work showed that horseradish peroxidase delivered into the subarachnoid CSF rapidly enters the brain along arterial perivascular space in cats and dogs, and the same was later shown in mouse studies with fluorescent dyes in conjunction with two-photon microscopy (4, 66). At later time points after application, the fluorescent tracers were observed surrounding capillaries and subsequently made an appearance at venules and veins, which supports the concept of directional flow along the perivascular spaces (4, 66, 287).
Although tracing CSF is a powerful method to evaluate how fluid circulates through the parenchyma, such approaches are subject to the caveat that movement of tracer molecules is hindered by the tissue through charge interactions or size restrictions. An attractive alternative approach is to deliver the tracers directly into the extracellular space via an intraparenchymal injection. When delivered into cortex, thalamus, or striatum, the tracer later appears at perivenous spaces that do not strictly follow the vascular network but rather define an anatomical pathway moving posteromedial toward the internal cerebral vein of the deep venous system and posterolateral and ventrally along the external capsule toward the caudal rhinal vein (the inferior anastomotic vein of Labbé in humans) of the superficial venous system (4). Prior studies have already shown that tracers injected into the caudate nucleus of rat brain could be found traveling outward and inward, away from the site of delivery (63, 64, 288, 289). That distribution pattern suggested that extracellular fluid flows along a system of extracellular spaces connecting to perivascular spaces but also along white matter tracts and within the subependymal layer of the ventricular ependyma (63). The observation that extracellular fluid in the neighboring parenchyma flows toward the ventricles had also been shown as early as 1865 by Wilhelm His, Sr. and replicated several times since then (290, 291). The basement membranes under the ependyma and between ependymal cells form labyrinthine structures that connect the basement membranes of the subependymal vessels, thus serving as convenient conduits for flow (63, 292). It is possible that this pathway has not yet been observed in humans due to the dilution of intraparenchymal tracers in the large CSF volume within the ventricles. White matter tracts also appear to be important flow routes out of the brain, especially when intraparenchymal tracers are delivered into the frontal lobe at the gray-white matter boundary in anesthetized rats. Three-dimensional (3-D)-serial reconstructions from such experiments showed that the tracers diffused medially and posteriorly along these tracts, suggesting that fiber tract organization can serve as a guide for flow along their trajectory (293). These regional divisions in extracellular fluid flow appear to be present also in deep brain structures such as the thalamus and caudate nucleus in rats (294). Although these are neighboring structures, extracellular fluid traced by MR imaging of Gd-DTPA does not cross from one structure to another. Furthermore, efflux from the caudate nucleus drained toward the ventral and lateral cortex (approximating the paths of the internal cerebral and caudal rhinal veins) along myelinated fiber tracts (4, 295). However, extracellular fluid flow from caudate into thalamus is restricted by compact myelin fascicles that act as a barrier structure (296). Interestingly, when the myelin was destroyed in rat model of multiple sclerosis, the barrier was interrupted and abnormal flow into the thalamus was observed (296). This observation has interesting implications for how white matter injury could bring about abnormal flow patterns and reduce CSF clearance. In experiments using the demyelination model, these two deep brain structures exhibited very different half-lives for the tracer, with faster clearance from thalamus than from caudate. This difference may reflect the relatively high expression of AQP4 water channels and the high astrocyte-to-neuron ratio in thalamus relative to caudate, as is further suggested by findings that AQP knockout significantly reduced the clearance of the Gd-DTPA from thalamus but not caudate nucleus (294, 297). Moreover, stimulating neuronal activity in the thalamus with a painful sensory stimulus reduced the efflux rate for several hours, suggesting that extracellular clearance might be slowed down during a period of neuronal excitation (1, 298). Regional propensity for slow extracellular clearance in brain regions that are clinically relevant for Alzheimer’s disease was also seen in healthy humans, with the entorhinal cortex and the entorhinal subcortical white matter being among the last regions to clear the tracer (286). A large degree of intersubject variability in efflux was seen after intrathecal tracer delivery in healthy humans, both in permeation to the brain and clearance rates between brain regions (165). This variability needs to be further studied, as it might hold the key to explaining the sporadic nature of several of the neurodegenerative disorders that are characterized by the formation of abnormal protein deposits.
In summary, we have explained in detail how the glymphatic system compartmentalizes the inflow of CSF along arterial perivascular space and the outflow of extracellular fluid along venous perivascular space, white matter tracts, and subependymal spaces. Although the intra-axial outflow system might seem unorganized compared with the highly structured organization of the peripheral lymphatic vasculature, it is quite the opposite; the availability of a multitude of outflow routes brings built-in redundancy that assures several fail-safes in the event that one pathway is compromised. All outflow pathways have the same goal, to deliver CNS waste to the periphery.
6.5. Outstanding Questions
What are the anatomical boundaries that separate extracellular fluid flow pathways within the brain? Do functionally separate flow compartments overlap with the anatomic distribution of degeneration seen in age-related neurodegenerative disorders?
What is the exact efflux route of extracellular fluid from brain? What path does extracellular fluid follow to reach the large caliber veins that drain the brain parenchyma?
To what extent do the exit routes of extracellular fluid change across aging and disease? Do these outflow sites serve as bottlenecks that, if disturbed, promote the abnormal accumulation of intra-axial solutes?
How variable is perivascular space morphology across brain regions and individuals and to what degree does this variability predict tracer transport?
6.6. Interim Summary Section
The blood supply of the brain is unique compared with other organs. Cerebral arteries and veins take separate trajectories, and the entire vasculature is surrounded by perivascular spaces. Its peculiar branching pattern defies established geometric principles of Murray’s law, suggesting rather that it is optimized for supporting extracellular fluid flow. The vascular organization of the central nervous system ideally positions the perivascular network to carry out a role analogous to the lymphatic vasculature in the periphery.
The glymphatic system compartmentalizes the inflow of CSF along arterial perivascular spaces. MRI studies definitively show that tracers delivered into the intrathecal space in humans are distributed throughout the entire brain, supporting the notions that CSF freely exchanges with the extracellular fluid and that CSF is transported into the brain in a centripetal fashion. The fluid transport kinetics are best explained by a combination of diffusion and advection, in a process more appropriately called dispersion.
The majority of extracellular fluid leaves the brain parenchyma along venous perivascular spaces, white matter tracts, and/or subependymal spaces. Preferential pathways for flow seem to be highly organized, and the presence of variability between individuals needs to be further studied, as this might provide an additional imaging biomarker to explain the sporadic nature of age-related neurodegenerative disorders.
7. MICROSCOPIC FLOW IN PERIPHERAL AND NERVOUS TISSUE
7.1. Introduction
Multicellular tissues need constant delivery of nutrients and removal of metabolic waste products. In most multicellular organisms, blood circulation is a conduit for fast and efficient transfer of nutrients and metabolites, but not all cells are in direct contact with the vascular network. Instead, the extracellular fluid serves as a local distributor of solutes and as a medium for the removal of waste from the metabolically active parenchymal cells. Excess fluid and proteins are transported out of peripheral tissue by lymphatic vessels and ultimately recycled or inactivated in the liver. We here review the fundamentals of fluid transport in peripheral tissues to convey a better understanding of how the basic features of fluid transport are replicated in the CNS by the glymphatic system. Similar to other tissues, neural cells require prompt delivery of energy metabolites and removal of waste substances to maintain their homeostatic control. Yet, unlike most peripheral tissues, the brain is physiologically separated from the blood compartment by tight barriers. In the absence of large transvascular contributions to tissue fluid flow in the brain, the intra-axial glymphatic system serves as a conduit for fast and efficient delivery of nutrients and metabolites, which acts across an expansive network of perivascular spaces that are characteristic of cerebrovascular architecture. This intramedullary perivascular space system also acts as a stand-in for the absent lymphatic vasculature in the CNS, by transporting excess fluid, metabolites and waste proteins out of the parenchyma and into the peripheral circulation. Despite certain key differences, many of the features governing fluid transport in peripheral systems are also applicable in the CNS, as shall be illustrated in the following sections. We shall also compare the composition and properties of the extracellular matrix in peripheral tissues and brain. The chief difference here is that neural tissue lacks the fibrous matrix proteins that provide structural support to peripheral tissues. For example, collagen is only present along the larger vessels and in the meningeal membranes. The extracellular matrix in brain is mostly composed of water-binding hyaluronan and proteoglycans. This specialized composition of the brain extracellular matrix might be a prerequisite for the rapid changes in the extracellular space volume that occur during physiological state transitions. Hyaluronan and proteoglycans can quickly expand by binding a large number of water molecules, thereby increasing the hydraulic conductivity of the brain’s extracellular space. Such a mechanism may contribute to CSF influx into the neuropil during sleep. The very large water-binding capacity of hyaluronan and proteoglycans might also be important for the understanding of the rapidly developing edema in the setting of stroke and traumatic brain injury. For an in-depth review of the extracellular matrix and fluid transport in peripheral tissues, we refer to Ref. 8. This section provides a description of the principles of the glymphatic system, while the drivers and functions of the system are discussed in sects. 9 and 10.
7.2. Extracellular Matrix Components and Membrane Receptors in Peripheral Tissues
A general feature of all tissues is that parenchymal cells are embedded in the extracellular matrix along with nerves, blood vessels, and lymphatic vessels. The three main components of the extracellular matrix are collagen, proteoglycans, and hyaluronan (FIGURE 11) (8). Collagen constitutes the major building block of the scaffold of the extracellular matrix in peripheral tissues. Indeed, collagen is the most abundant protein in mammals and is present in especially high densities in skin, tendons, muscle, cartilage, outer surfaces of organs, and bone. In contrast, there is little collagen in the brain, other than that at structures of the basal lamina surrounding the larger cerebral vessels and certain parts of the meninges.
FIGURE 11.

The extracellular matrix components and their specialization in the basal lamina. A: the extracellular matrix of peripheral tissue contains the fibrous proteins collagen, laminin, and fibronectin, which are embedded in proteoglycans and hyaluronan. Proteoglycans and hyaluronan both contain glycosaminoglycans (GAGs), which have a large capacity for binding water and Na+. Insert: proteoglycans contain a core protein, which is the binding target of numerous repeating disaccharide chains. Hyaluronan is also composed of a repeating disaccharide chain but differs with respect to its lack of core proteins. Hyaluronan interacts with proteoglycans but not with collagen, laminin, and fibronectin. Most of the extracellular matrix proteins interact with multiple classes of membrane receptors, including integrins and CD44. These receptors are coupled via linker proteins on the cytosolic site with the actin cytoskeleton. B: the basal lamina consists of a repetitive, highly organized interweaving of collagen IV, laminin, fibronectin, and proteoglycans. The basal lamina is permeable to soluble proteins such as albumin, and the presence of gaps between the endothelial cells allows free passage of an ultrafiltrate of plasma into the tissue, driven by hydrostatic pressure (blue arrows). The basal lamina induces polarized expression of channels, transporters, and ion pumps in the basolateral membrane (e.g., exocrine glands).
Collagen glycoproteins form a family with at least 28 members, all of which share a basic structural unit consisting of three α-chains assembled into a right-handed triple helix (299). Thousands of the triple helix molecules are packed together in clusters held in place by lateral interactions to form collagen fibrils, which have a diameter of 50–200 nm. These fibrils in turn interact with other extracellular matrix components to form supramolecular networks of high tensile strength. Type I collagen is the most abundant conformation, while type IV collagen forms the specialized two-dimensional sheets of the basal lamina. Collagen fibrils bind to at least four different classes of membrane receptors, including integrin receptors, receptor tyrosine kinases (also called discoidin domain receptors), immunoglobulin-like receptors (involved in the coagulation cascade), and mannose receptors; together these receptors regulate many basic cellular functions including proliferation and differentiation (300).
Multiadhesive proteins (i.e., laminin and fibronectin) interact both with collagen and membrane receptor binding sites and often have several functions (301). Fibronectin is a high molecular weight extracellular protein that participates in collagen fiber formation and links collagen to various membrane receptors, including integrins (302–304). Laminin is a heterotrimeric protein that is abundantly present in the basal lamina (see sect.7.3) (305). Elastin is primarily present in tissues that require high elasticity, such as large arteries, ligaments, and cartilage, and also in skin and the bladder wall (301). Other extracellular matrix components include tenascins and thrombospondins, both of which are glycoproteins having additional roles extending beyond adhesion to include processes such as proliferation, antiangiogenesis, apoptosis, immune regulation, and differentiation (301).
7.2.1. Proteoglycans.
These are glycosylated proteins composed of a core protein to which glycosaminoglycan (GAG) sidechains are attached. GAGs are polysaccharides composed of repeating units of a disaccharide (FIGURE 11A, inset). The most common types of GAGs, chondroitin sulfate and heparan sulfate, are mainly composed of polymers of either N-acetylgalactosamine or N-acetylglucosamine, respectively, and glucuronic acid. The core protein of proteoglycans is glycosylated in the Golgi apparatus and then enzymatically sulfonated before its export to the extracellular space by vesicular release. Proteoglycans interact strongly with collagen and may direct the assembly of collagen monomers into their characteristic beaded filament conformation (306, 307).
Hyaluronan is a unique GAG since its constituent hyaluronan polysaccharide does not bind to a protein core. Furthermore, hyaluronan is not sulfated and exhibits a wide range of molecular sizes spanning from 5 to 20,000 kDa, even exceeding 34,000 kDa in the umbilical cord (308). Hyaluronan does not interact with collagen, fibronectin, and laminin, but it does bind to proteoglycans via linker proteins, often forming very large macromolecular structures in the extracellular matrix. Despite their large size, these structures may be transportable to some extent, since the content of GAG and hyaluronan can rapidly decrease when hydraulic conductivity increases (309, 310). Hyaluronan is produced by three types of hyaluronan synthase (HAS1-3) in the plasma membrane, whereupon it is exported to the extracellular space by ATP-binding cassette transporters (311). The rate of production of hyaluronan may be autoregulated by its own concentration in the extracellular space (312, 313).
Hyaluronan also interacts with multiple receptors on the plasma membrane, most notably CD44, hyaluronan-mediated motility receptor, and intercellular adhesion molecule-1. CD44 is present in most tissues, where it participates in many basic cellular functions, including migration, proliferation and differentiation, as well as the cellular activation in inflammation and cancer (314). In general, these hyaluronan receptors have multiple roles, with effects on MAPK and other downstream signaling pathways and participation in inflammatory processes and wound repair (315–319).
Thus the extracellular matrix in peripheral tissue presents a complex biochemical landscape in which its diverse components interact with each other and with a diverse variety of membrane receptors. The relevant membrane receptors are connected on the cytosolic side via linker proteins with the cellular cytoskeleton, thus creating a tissue-wide network of interconnected structures, a scenario that may call for some revisions of the traditional conception of cells as autonomous components of the organism. In general, the adhesion receptors are multifunctional, with participation in cell signaling and a gamut of fundamental cellular processes. Adhesion receptors are activated in the setting of inflammation and changes in the cellular adhesive forces are a central aspect of tissue edema formation.
7.3. The Vascular Basal Lamina in the Periphery
The basal lamina, also known as the basement membrane, is a specialization of the extracellular matrix, which typically functions as an anchoring layer for epithelial cells in peripheral tissues (301, 305) (FIGURE 11B). The epithelial cells become functionally polarized around their basolateral attachment to the basal lamina, which in turn creates a boundary between the vascular wall and the tissue. As mentioned above, the basal lamina differs from other parts of the extracellular matrix due to its greater structural complexity and denser packing. The extracellular matrix components are organized in a repetitive manner, distinctively different from the typically loose composition of the main part of the extracellular matrix. The matrix proteins within the basal lamina also differ somewhat from those of the extracellular space. The basal lamina is composed of collagen type IV, laminin, nidogen (or entactin: a glycoprotein that forms complexes with laminin), fibronectin, and heparan sulfate (or perlecan) (299, 301). The main proteoglycans of the basal lamina are heparan sulfate chains, which also interact extensively with other matrix components, while acting as a “filler” due to their extensive hydration and Na+ binding (320). Proteoglycans and glycoproteins in the basal lamina are anchored to the plasma membrane by syndecans and glypican, which are best described as transmembrane proteoglycans (321–323). Thus the basal lamina creates a rigid yet porous structure, which acts as a sieve allowing the entry of a plasma ultrafiltrate containing albumin and other plasma proteins into the extracellular space, while preventing extravasation of the larger complement proteins and cellular components such as erythrocytes and leukocytes. Although the term glycocalyx is used often to describe the basal lamina, it is not entirely synonymous: glycocalyx denotes in more general terms the interwoven meshwork of extracellular matrix glycoproteins, i.e., proteoglycans, syndecans, proteins, and lipids that covers the membrane of many cells, including gram-positive bacteria and endothelial cells (319). The endothelial cell glycocalyx is present at the basolateral (tissue) side and luminal (blood) side, and both sides contribute to the ultrafiltration of plasma entering the tissue. In brain, astrocytic vascular endfeet adhere to the basal lamina to create the perivascular tunnels surrounding all segments of the vascular tree (see sect. 7.9.). In the brain, the perivascular spaces represent a critical element of glymphatic fluid transport.
7.4. Structure and Organization of the Peripheral Lymphatic System
The drawback of having the extracellular fluid serving as a distributor of signaling compounds and metabolic substrates in peripheral tissue is that this setup requires an active removal mechanism to avoid accumulation of fluid in the extravascular compartment, i.e., lymphedema. The lymphatic system plays a pivotal role in tissue homeostasis by clearing excess fluid and metabolic waste from peripheral tissues. In addition, the lymphatic system participates in many aspects of normal and pathological immune responses including tolerance, autoimmunity, and inflammation (324). In-depth reviews on the peripheral lymphatic system include Refs. 9, 325, 326. The lymphatic system is organized into five principle segments (FIGURE 12) (327) as follows: a network of blind lymphatic capillaries formed by lymphatic endothelial cells, which are surrounded by an incomplete basal lamina (I) (328). These capillaries contain flaps extending from the borders of the lymphatic endothelial cell, which lack junctions at their tip but are anchored on the sides by button (cell-cell junction composed of cadherin and several tight junction subclass proteins). The flaps act as one-way valves allowing the unidirectional uptake of fluid, soluble proteins, and immune cells (329). The initial lymphatic capillaries merge into larger lymphatic collectors bordered by smooth muscle cells aligned along their length (II). These collectors are also surrounded by a more organized basal lamina occasionally containing fibroblasts. The collecting lymphatic vessels are equipped with bicuspid valves, which are macroscopic structures encouraging drainage away from distal sites. Each segment of the collector vessel situated between two valves is known as a lymphangion. Rhythmic muscular contractions push open the valves, thus driving the forward flow of lymph to the downstream lymphangion, while discouraging backflow. Lymph in multiple larger prenodal collecting lymph vessels will eventually be transported to a lymph node (III), which it will exit via a single postnodal collecting lymphatic channel before finally reaching either the thoracic or right lymphatic ducts (IV), which dump the lymph into venous circulation via the subclavian veins (49).
FIGURE 12.
Schematic overview of the lymphatic system in peripheral tissues. The lymphatic vascular tree is composed of several distinct segments. I: blind lymphatic capillaries, for which the primary function is the uptake of excess extracellular fluid. The capillaries have no smooth muscle cells, and their fluid uptake is believed to rely on the unique valve-like microstructural organization of endothelial cells’ flaps and button. A magnified illustration of flaps extending from the borders of the lymphatic endothelial cell. The flaps lack cell adhesions at their tips and are only anchored on the sides by buttons (red), which are cell-cell junctions created by cadherin and tight junction proteins. Uptake of fluid and solutes is unidirectional, and efflux cannot occur because any increase in the intraluminal back-pressure will close the flaps. Also, the basal lamina surrounding lymphatic capillaries is incomplete, facilitating uptake of larger structures, such as immune cells. II: lymphatic collectors are surrounded by smooth muscle cells that are in part oriented along the lymphatic vessel wall rather than encircling the vessel as in blood vessels. They are also endowed with valves that open in response to an increase in pressure created by contraction of the smooth muscle cells. The segment between two valves is called a lymphangion. Intrinsic properties of the muscle cells, the fluid load, and the autonomic nervous system regulate lymph vessel contractility. III: lymph nodes receive lymph from large prenodal collecting lymph vessels. Lymph within the node is transported from the afferent to the efferent lymphatics via a peripheral path (blue arrows pointing to flow from the subcapsular to the medullary sinus) or a central path (flow from the subcapsular sinus to the centrally placed B-cell follicles and T-cell cortex, not shown). IV: postnodal collecting lymphatic channel is shown. Approximately 50% of lymph fluid is transferred to the venous compartment within the node, whereas little exchange of proteins take place at this site. Thus most proteins entering the node, and ∼50% of the total lymph production, exit via the postnodal collecting lymphatic channels and are returned to the venous circulation by the thoracic or right lymphatic ducts (not shown) (49).
About 3–5 liters of lymph are returned to the general circulation each day at the thoracic duct. However, it is estimated that at least 50% of the total lymph volume is absorbed by the 500–600 lymph nodes scattered around the body, such that adult humans must produce closer to 6–10 liters of lymph daily (330, 331). Similar to plasma, albumin and serum globulins are the main protein constituents of lymph. The protein content in lymph is reportedly in the range of 1.5–3.4 g/dl, which is somewhat less than that of plasma. However, lymph also contains tissue-specific proteins, such as extracellular matrix proteins and intracellular proteins derived from the surrounding tissue (331). For additional details, see reviews discussing the composition of the lymphatic fluid (8, 332). Most existing data suggest that the total protein concentration in lymph does not change along the prenodal sections of the lymphatic system, which suggests that the initial segments of the lymphatic vessels, including the lymphatic capillaries and prenodal lymphangia, are simple conduits for fluid transport that do not substantially modify its composition (8).
7.5. Formation and Transport of Lymph in the Periphery
According to the traditional view, the formation of the extracellular fluid follows Starling’s principle (BOX 1 and FIGURE 13). In this model, capillary fluid exchange reflects the balance between hydrostatic and colloid osmotic (oncotic) pressure gradients. Thus the pressure gradient between the arterial side of the microcirculation and the extracellular tissue space would drive into the tissue an ultrafiltrate of plasma, which is ultimately transported out of the tissue by the lymphatic system. The hydrostatic gradient is always higher than the oncotic gradient, resulting in a net gain of fluid and solutes, which are removed by the lymphatic vasculature and transported back to the general circulation. Recent work has called for a revision of the original Starling’s principle by proposing that tissue fluid homeostasis depends more on lymphatic function than on venous reabsorption due to the high colloid osmotic forces in the venous end of the capillary network (337, 338). Also, experimental measurements of tissue hydrostatic and colloid osmotic pressure yield values much lower than those required for the principle to be strictly correct. Due to these discrepancies, the revised Starling principle posits that the endothelial glycocalyx determines the colloid osmotic pressure of the tissue (62). Thus the emerging concept is that it is the endothelial cell glycocalyx and the lymphatic vasculature that together regulate tissue fluid flow and thereby are the major contributing factors in fluid homeostasis failure and edema development.
BOX 1. Starling’s law and convective tissue flow
Starling’s law predicts that net fluid movement between capillaries and the extracellular medium should depend on the balance between two opposing forces: the hydrostatic and the colloid osmotic (oncotic) pressure gradients (FIGURE 13). These two fundamental driving forces are represented in Starling’s equation, which states that the tissue fluid flow is equal to (Pc − Pe) − σ (πc − πe), where Pc is the hydrostatic pressure in the capillary, Pe is the hydrostatic pressure in the extracellular space, πc the colloid osmotic pressure in the capillary, πe is the colloid osmotic pressure in the extracellular space, and σ is the reflection coefficient that adjusts for the permeability of the capillary to soluble proteins. The relatively higher hydrostatic pressure in capillaries (Pc > Pe) lying close to the arterioles will drive an influx of fluid and plasma proteins, most notably albumin, through pores and into the extracellular space (312). As the hydrostatic pressure falls and equalizes with the extracellular space (Pc ≈ Pe) in the capillaries more distal to the arteriole, the higher colloid osmotic pressure in these capillaries (πc > πe) will partially drive back the excess fluid in the extracellular space into capillaries at the venous side of the capillary bed (333, 334). The remaining fluid is transported out of the tissue by lymphatic vessels.
The presence of a porous endothelium enables an ultrafiltrate of plasma to flow into the extracellular space in peripheral tissues (335). The constant influx of the plasma ultrafiltrate and the ongoing drainage by the venous and lymphatic systems sets up a directional convective flow with a velocity in the range of 6–120 µm/min within the tissue (323, 336). The fenestrations in the capillaries are created by gaps that normally separate endothelial cells, covered by the basal lamina also called glycocalyx (see below). The basal lamina or glycocalyx forms a 100- to 500-nm thick, continuous coating of the blood vessel endothelial cells, which can be permeated by solutes of diameter as large as ∼5 nm (321). Recent evidence suggests that the filter properties of the pore of the glycocalyx (πg) play a much greater role in determining the tissue colloid osmotic pressure (πe) than the tissue itself (58, 73). The albumin concentration is ∼40–60% of that in plasma and the ion concentrations in the extracellular space are fairly similar to those in plasma (8).
FIGURE 13.
Starling’s principle of hydrostatic pressure driving convective fluid flow in peripheral tissue. Capillaries in peripheral tissues are fenestrated and covered by a basal lamina. This arrangement allows an ultrafiltrate of plasma containing relatively low molecular weight solutes such as glucose and globular proteins to flow into the tissue, driven by hydrostatic pressure (Pc). Pc oscillates with the cardiac cycle, exhibiting higher pressures during systole and lower pressures during diastole, which drive temporally changing filtration volumes rather than continuous inflow. The directional flow of fluid and its solutes away from the arterial end of the capillary bed is supported by the colloid osmotic (oncotic) pressure (πc) absorbing fluid in the venous end of the capillary bed. The remaining fluid and solutes (ΔP − Δπ) are transported out of the tissue by lymphatic capillaries (green). The ultrafiltrate of plasma do not experience unrestricted flow within the tissue, because of interference from cells and the fibrous elements of the extracellular matrix proteins (mostly collagen). Fluid flows primarily within the gelatinous pockets of the proteoglycans/hyaluronan containing glycosaminoglycan (GAGs.) When the hydration of GAGs is high, the hydraulic resistance is low, resulting in faster and more substantial fluid transport. On the other hand, dehydration of proteoglycans/hyaluronan will increase the hydraulic resistance and thereby reduce the inflow of the plasma ultrafiltrate. The relative contribution of the endothelial cell glycocalyx and hydration of GAG in regulating the rate of plasma ultrafiltration has not been determined. The tension within the tissue is created by the fibrillary network of collagen connected to the cytoskeleton by integrin receptors and serves to limit the extent of GAG hydration.
The uptake of excess fluid into the lymphatic capillaries could theoretically result from several distinct mechanisms, which include hydrostatic and colloid osmotic pressure gradients, active transport, and vesicular transport (8). The latter two mechanisms have been largely rejected in recent years, and it is now generally accepted that hydrostatic pressure and active transport are the predominant mechanisms of fluid transport. These forces entrap lymph in the blind-ended lymphatic capillaries, which are endowed with valve-like flaps that support fluid influx but discourage efflux (7). In principle, the hydrostatic pressure of the tissue and the phasic increases in pressure evoked by the composite of arterial pulsation, postural changes, tissue compression, and smooth muscle cell and skeletal muscle contractions together drive excess extracellular fluid into the blind-ended lymph capillaries. Theoretical considerations predict that a pressure gradient of just 0.09 mmHg/mm should suffice to drive a large fluid volume into the lymphatic capillaries (339). A pressure gradient in the range of 0.08 to 2.7 mmHg/mm has been confirmed by measurement between the lymphatic capillary lumen and more distal tissue, using a micropipette technique, suggesting that the hydrostatic pressure within tissue is more than sufficient to drive extracellular fluid into lymph capillaries (340, 341). Pressure recordings have also shown that cardiac and respiratory activities both drive rhythmic changes of transmural lymphatic pressure, thus pointing to a functional role of these dynamic forces in driving lymphatic flux (342, 343). The occurrence of active transendothelial transport of solutes is best documented for the case of lipids in the lymphatic vessels of the intestine (344). There are few reports of active transport of fluid and solutes in other tissues, but it is known that overhydration of mice drives increased fluid transport across lymphatic endothelial membranes, perhaps suggestive of an active process (345). The lymph is actively transported out of the tissue by contraction of the lymphangioma and the other indirect forces noted above (8).
7.6. The Properties of the Extracellular Matrix, Hydraulic Conductivity, and Mechanical Stress in Peripheral Extracellular Space
The relative volume fraction of the extracellular space ranges from 10% in skeletal muscle tissue to almost 50% in skin (8). The exact composition of the extracellular matrix also differs across organs, but one shared characteristic is the high abundance of glycosaminoglycans. GAGs are negatively charged at physiological pH; this negative charge, in conjunction with a large hydration capacity, is responsible for the gel-like nature of the extracellular matrix (312). Inorganic cations are also entrapped in large quantities by the GAGs. In particular, Na+ can reach a concentration of 250 mM in cartilage, thus exceeding almost twofold the free Na+ concentration in plasma (89). An important feature of the extracellular matrix is that GAGs are underhydrated under physiological conditions, such that they can readily incorporate more water (312). Under physiological conditions, the extracellular matrix fibrillary network composed of collagen, laminin, fibronectin, elastin, and linker glycoproteins limits the water-binding capacity of GAGs (346, 347). In addition, the extracellular matrix fibrillary network interacts with the integrin receptor and other membrane receptors such as CD44. The plasma membrane integrin receptors are connected with cytosolic actin filaments via linker proteins such as α-actinin, filamin, and tensin, whereby the cytoskeleton forms another fibrillary network that contributes to tissue tension (336). Thus a complex network of extra- and intracellular fibrillary structures keeps GAG hydration within certain limits, while active lymphatic drainage maintains tissue fluid homeostasis. Pathological conditions, such as injury or inflammation, degrade the fibrillary network and its connections with cell adhesion receptors, resulting in a decrease in tissue tension that leads to excessive hydration of GAGs and tissue swelling (312).
The hydraulic conductivity of the extracellular space is a measure of the permeability of the tissue to fluid, which is the inverse of its hydraulic resistance (8, 348). Hydraulic conductivity increases nonlinearly with increasing hydration of the GAGs, which in turn increases the velocity of tissue fluid flow. The best way of explaining the consequences of increased tissue hydration is in the setting of inflammation, which is associated with considerable increases in fluid flow. Conversely, fibrosis will lead to an increased local concentration of fibrous matrix glycoproteins, which consequently decreases the hydraulic conductivity and attenuates fluid flow. Interestingly, hydraulic conductivity increases in the setting of lymphedema, but this increase is counteracted by a compensatory increase in fibrous extracellular matrix components in a strain of mice with constitutively defective lymphatic vessels (349).
Not all domains of the extracellular space are available for solute dispersion. Collagen and proteoglycans physically fill part of the volume, thereby presenting an impediment to flow, which is quantified experimentally by measuring the volume of tissue accessible to a large molecular weight tracer such as labelled albumin (350, 351). These volume fractions are not constant but vary with the hydration state of the tissue. By experimentally manipulating the hydration of rat skin, one can establish the linear relationship between hydration and volume fraction available for albumin (312, 352–354). Thus the hydration of the GAGs controls the extracellular space available for tracer dispersion and thereby the hydraulic conductivity.
Given these properties, the extracellular space is best characterized in materials science terminology as a poroelastic medium, comparable to a wet sponge filled with gel-like fluid. While hydration increases the pore size of the sponge, the elastic components of the tissue are set in motion by external forces such as arterial pulsatility, which exerts mechanical stress on the cells by two principal mechanisms: first, the hydrostatic pulse pressure propels movement of extracellular fluid downstream from the pressure source (355). Second, the concurrent stretching of the collagen fibers is directly transmitted to the plasma membrane via integrin receptors (348). These forces are in turn transmitted to the cytosol by the binding of integrin or CD44 to the cytoskeleton. Unsurprisingly, given the abundance of signal transduction pathways, shear stress can alter gene expression (355). For example, a nonphysiological increase in extracellular fluid flow induced expression of smooth muscle actin and other components of contractile tissue in cultured fibroblasts (356).
7.7. Edema Formation in the Setting of Acute Injury or Inflammation in Peripheral Tissue
Since the gel-like GAGs are not fully hydrated under physiological conditions, the tension exerted by the extracellular matrix fibrillary network (collagen, laminin, and fibronectin) and its binding via plasma membrane receptors (integrin, CD44) prevents the unrestricted swelling that would otherwise occur in the absence of solid tissue elements that prevent additional water binding. Loss of the tension stored in the extracellular matrix fibrils and/or intracellular cytoskeleton results in uncontrolled absorption of water by GAGs, while parallel osmotic forces drive influx of Na+ into the GAG-containing proteoglycan/hyaluronan matrix (312, 357, 358) (FIGURE 14). This process is strikingly expressed when the structure of collagen is altered by acute burn injury. Within minutes of the injury, the transcapillary rates of protein and fluid influx increase by several hundredfold (359). Reed and Rubin (312) calculated that an impossibly high 250–300 mmHg increase in the transcapillary pressure would be needed to explain the rapid fluid influx observed in burn injury. This calculation argues that the rapidly evolving edema must not be simply a consequence of increased permeability of capillary endothelial cells but rather due to loss of control of GAG hydration when collagen is denatured in the burn injury. A similar mechanism may underlie the slower edema formation occurring in inflammation (204, 358, 361, 362) or traumatic injury (212, 321). In these conditions, matrix metalloproteinase enzymes (MMPs) are activated (363, 364). The activated MMPs degrade extracellular matrix fibrils, including collagen IV in the basement membrane (365, 366) and detach the integrin binding to extracellular matrix fibrils (237). As in burn injury, the enzymatic degradation of extracellular matrix fibrils reduces the tension on GAGs, resulting in greater absorption of water. Another important mechanism to take into consideration is the interactions between extracellular matrix with integrin and other plasma membrane receptors (347, 367). Integrins are bound to the cellular cytoskeleton via linker proteins, contributing to the overall tension exerted on GAGs (368–370). Injury and inflammation increase intracellular Ca2+ signaling, which is associated with degradation of the actin cytoskeleton (301, 360, 371) and activation of adhesion receptors (357, 372). Thus abnormal cytosolic Ca2+ will lead to actin degradation and diminished linkage to the extracellular matrix proteins, resulting in a reduction in tissue tension and thereby increased GAG hydration. In support of this model, disruption of actin by the fungal toxin cytochalasin reduced tissue hydrostatic pressure and induced edema (373). Conversely, phalloidin (Amanita phalloides toxin) stabilizes actin and attenuates transcapillary albumin influx following anaphylaxis (374). At later stages of edema, exposure of intracellular osmolytes (mostly degraded proteins) from dying cells may increase the oncotic pressure gradient, possibly driving uptake of additional fluid uptake and tissue edema.
FIGURE 14.
Glycosaminoglycans (GAGs) swell in the setting of injury and increases tissue fluid flow. During normal physiological conditions, solid elements in the extracellular space, including collagen, as well as the cellular tension, together limit GAG hydration in peripheral tissue (355). After a burn injury, the degradation and conformational changes of collagen and cellular elements lead to rapid influx of vascular fluid resulting in massive swelling and an enormous increase in GAG hydration (204, 344, 358, 359). Tissue swelling is slower in onset and less severe in the setting of inflammation or tissue injury. In these conditions, matrix metalloproteinases (MMPs) are activated, resulting in a breakdown of the fibrillar extracellular matrix proteins and interruption of cellular adhesion complexes, mainly integrins. The resultant decrease in tissue tension leads to an increase in GAG hydration and tissue swelling. Ca2+ signaling in the setting of tissue injury can also be associated with a breakdown of the actin cytoskeleton and thus result in a more modest increase in GAG hydration (360). In fibrotic scar tissue, the relative amounts of the fibrillar extracellular matrix structures and the actin cytoskeleton increase, thus placing GAGs under additional pressure and reducing their hydration. Indeed, hydraulic resistance has an inverse relationship with GAG hydration. The more water that GAGs bind, the lower their resistance to flow, resulting in an increase in convective flow. On the other hand, fibrosis reduces the tissue fluid flow rate.
7.8. Microscopic Flow in Brain: Extracellular Matrix Components and Membrane Receptors in CNS Tissues
We shall now consider how to apply observations in peripheral tissues for obtaining a better understanding of fluid flow in the neuropil. The first question that we pose is as follows: how does the CNS extracellular matrix differ from that of peripheral tissues? Remarkably, the adult brain is practically devoid of collagen, laminin, and fibronectin. The brain has four primary extracellular matrix structures: the neural extracellular matrix, perineuronal nets, the meningeal membranes, and the basal lamina surrounding the large vasculature (FIGURE 15) (375). The most abundant proteoglycan in CNS is chondroitin sulfate proteoglycan (CSPG), which consists of chondroitin sulfate surrounding one of at least 16 different core proteins (25, 376). CSPG is more highly sulfated in the adult CNS than in peripheral tissues, which confers additional negative charge (377). The greater diversity of CSPGs in CNS has called for a specific nomenclature to distinguish among the various subtypes. Lecticans (hyalectans) are a subgroup of CSPGs that contain a lectin domain in the core protein of the hyaluronic acid-binding domain. The lectican category includes neurocan, brevican, versican, and aggrecan (25, 377). Whereas neurocan and brevican are primarily, if not exclusively, present in CNS, versican is expressed both in brain and peripheral tissues, while aggrecan is typically found in brain and cartilage (25, 378). The main sites of versican and brevican production is in astroglial cells (379–381), while neurocan appears to be secreted by neurons (382). In general, lecticans act as a hub for linking multiple matrix molecules (including hyaluronan and tenascin R) to form very large macromolecular extracellular aggregates. Furthermore, lecticans bind to the CD44 receptor (383). The C terminus also allows lectican to form linkages with selectins and other carbohydrates present on the outer plasma membrane of target cells (384). In addition to CSPG, the brain extracellular matrix contains high concentrations of the GAG hyaluronan (also known as a mucopolysaccharide), as well as the brain-specific matrix protein tenascin-R and multiple other linker proteins. Hyaluronan is synthesized by hyaluronan synthases embedded in the cell membrane. It is highly enriched in the brain at all times, and its steady-state concentration is defined by the balance between the activities of hyaluronan synthases and hyaluronidase. The three known human hyaluronan synthase genes (Has1-3) are all constitutively expressed at low levels in brain (385).
FIGURE 15.
Brain extracellular matrix. A: the extracellular matrix (ECM) in the brain is distinct from that in the periphery with respect to its near absence of protein components, being practically devoid of collagen, except collagen IV in the basement membranes, fibronectin, elastin, and laminins. The brain extracellular matrix is primarily composed of glycosaminoglycans (GAGs), mostly hyaluronan and chondroitin sulfate proteoglycans (CSPGs). CSPGs have a wide array of isoforms termed lecticans, including aggrecan, neurocan, brevican, and versican, which are linked together in a matrix via tenascin R. The actin cytoskeleton of cells in the neuropil bind to the CSPGs in the extracellular matrix via CD44 and other non-CD44 adhesion receptors. B: the vascular basement membrane is one of the few locations in the CNS where the extracellular matrix is fibrous, being composed of structural proteins similar to those found in peripheral basement membranes. In particular, the vascular basement membrane is composed of a 3-dimensional matrix of collagen IV and laminins. The isoform of laminin varies between different segments of the vascular network. The protein-rich vascular basement membrane matrix is stabilized by cross-linked nidogen and perlecan. Brain endothelial cells are interconnected via cadherin and the zonula occludens tight junctions, which together form the blood-brain barrier (BBB). The BBB inhibits the free entry of plasma water and many of its solutes from the vasculature. Endothelial cells attach onto the basement membrane via CD44 and integrin receptors in an interaction resulting in the upregulation of tight junction proteins, thus conferring to them their barrier properties. Astrocyte endfeet connected by gap junctions line the opposite side of the basement membrane and attach to the basement membrane via dystroglycan and integrin receptors. The dystroglycan receptor is composed of α- and β-subunits. The attachment of the α-dystroglycan to agrin in the basement membrane, together with the dystrophin-associated protein complex (i.e., dystrophin, dystrobrevin, and α-syntrophin), stabilize the localization of orthogonal arrays of intramembranous proteins composed primarily of aquaporin-4 (AQP4) water channels.
Tenascin-R is a trimer linker glycoprotein, which is exclusively present in the CNS. Tenascin-R characteristically binds to the COOH-terminal domain of lecticans, with highest affinity for brevican, and thereby consolidates the extracellular matrix structure (320, 386, 387). A second unique feature of the CNS extracellular matrix is the presence of perineuronal nets that form a macromolecular structure on the surface primarily of interneurons, which debut toward the end of the critical developmental period for neuroplasticity (388, 389). Perineuronal nets have received considerable attention in relation to their inhibition of synaptic plasticity in learning and in the aftermath of CNS injury (388, 390, 391). Perineuronal nets are important for the normal organization of the brain, but its committed nature acts as an impediment to reorganization after an insult. Perineuronal nets are primarily composed of extracellular matrix molecules, including CSPGs, hyaluronan, and tenascin-R (392). The extent to which the perineuronal nets limit diffusion and flow of solutes around interneurons is presently unknown, but steric hindrance from perineuronal nets against the passage of large molecules seems likely to be a factor influencing the microenvironment around interneurons. Perineuronal nets are primarily found in cortex, hippocampus, thalamus, brainstem, and spinal cord, which also highlights the regional variation in extracellular matrix organization that is characteristic of the CNS (393).
Our second question about the extracellular matrix is as follows: which peripheral tissue has an extracellular matrix most resembling that of brain? Posing this question relates to the deeper issue of how the particulars of extracellular matrix architecture conforms to tissue-specific requirements. In fact, and perhaps surprisingly, the structure of the brain extracellular matrix is most similar to the proteoglycan component of cartilage, as both are rich in aggrecan and hyaluronan, but with the obvious distinction that collagen is largely absent from brain (25). Cartilage contains an abundance of aggrecan-hyaluronan complexes, which appear to serve as a cushion at load-bearing joints (25). These complexes are known to bind large a quantity of water that is extruded when the cartilage is compressed, and returns upon release of the pressure (25). These aggrecan-hyaluronan complexes in the brain might conceivably serve a similar sponge-like function as GAGs in the cartilage. The capacity to buffer against pressure spikes is critical for the brain since it is confined within an incompressible skull and is subject to cardiac- and respiration-induced deformations that may cause cyclic extrusion and hydration of water molecules resident in the extracellular matrix (394).
Why then is the brain extracellular matrix so distinct from that of most peripheral tissues? More specifically, why does the brain possess a lectican-assembled matrix, as opposed to one consisting primarily of a protein scaffold, as is typical of other tissues? One possibility is that this design is required to constrain cell migration by providing insufficient traction to cells (25). CSPGs are upregulated in the glial scar after brain injury and prevent the migration of axons across the lesion (320). This feature also seems to be coopted by glioblastoma cells, which upregulate their expression of several extracellular matrix molecules and receptors, thus conferring a pathological ability to migrate through tissue. Intriguingly, brain metastases of peripheral tumors are usually unable to spread deeply but rather are seeded close to their vascular entry point, and some regions of CNS with a particularly rigid architecture (i.e., white matter) appear resistant to peripheral metastases (395). In contrast, glioblastoma cells aggressively infiltrate throughout the parenchyma, suggesting that the nature of their interaction with the extracellular matrix imparts a special proclivity to migrate within the brain, thus evading the surgeon’s scalpel. The absence in the brain of a protein-filled extracellular matrix is also thought to account for the pattern of liquefactive necrosis seen after cerebral ischemia. Cell necrosis after a stroke leaves a cavity filled with CSF, whereas a comparable lesion in peripheral tissues would result in coagulative necrosis, with a large intact extracellular matrix of normal structure (396).
In summary, the composition of the brain extracellular matrix differs from that in other tissues principally with respect to its almost complete lack of fibrous structures and low matrix protein content. The brain extracellular matrix is also characterized by its high negative charge derived from GAGs, which can support more extensive binding of Na+ and water of hydration than is typical for other tissues. Although much remains to be elucidated, the brain extracellular matrix seems positioned to play a key role in fluid and solute transport.
7.9. The Basal Laminae (Basement Membranes) of the Brain
The basement membrane forms a 3-D network of proteins composed of laminins, collagen type IV, nidogen-1 (and/or -2), and heparan sulfate proteoglycans (perlecan or agrin) (FIGURE 15B). However, the CNS contains three distinct forms of basement membranes, namely the vascular (endothelial and smooth muscle), the pial, and glial basement membranes. To appreciate best the complexity of these different membranes, they should be considered in the context of development. The developing vasculature, which is of mesodermal origin, penetrates into the embryonic brain, which consists of a covering of pia mater of neural crest origin and the underlying parenchyma, a neuroectodermal tissue. The three cell layers are of distinct lineage, each expressing their own characteristic basement membrane. Consequently, the three layers are in direct contact with each other throughout the branching vasculature network, traveling first from pial arteries, to penetrating arterioles, then to capillaries, and finally the postcapillary venules, which converge to form the downstream venous system. Although, the molecular composition of the three brain basement membranes does not differ fundamentally from that of the peripheral basement membrane, its constituent cells form a three-layered structure that confers regional specialization and provides a physical substrate for the subsequent formation of the perivascular space.
Brain endothelial cells (EC), smooth muscle cells (SMC), pericytes, and astrocytes (also perhaps microglia) adhere to the different basement membrane layers via transmembrane proteins in the integrin and dystroglycan receptor families (397). Dystroglycan receptors, which are expressed in extracellular space and astrocyte endfeet, consist of an extracellular form of α-dystroglycan that binds to basement membrane proteins, whereas the membrane-bound β-dystroglycan ties the α-dystroglycan to the actin cytoskeleton of astrocytes (398, 399). Of the several integrin isoforms expressed in the brain basement membrane, some play a specific role in intracellular signaling. For example, binding of EC β1-integrin with collagen IV in the basement membrane induces the expression of interendothelial claudin-5, which is responsible for forming the tight junctions that give the BBB its barrier function (400). Collagen and laminin are the two major components of the basement membrane and thus provide the main part of its structural stability, while nidogen and perlecan aid in cross linking between matrix components (401). Laminin, however, is also responsible for most of the biological activity of the basement membrane and likely accounts for the highly regional structural variation between the different basement membrane layers. Laminins are multidomain glycoproteins composed of three polypeptides (α, β, γ) that form an asymmetric cruciform structure through disulfide bonds (385). There are several types of the laminin subunits (five α, four β, three γ), thus allowing for upwards of 18 isoforms (401). The laminin type varies significantly across the cerebrovascular network. At the large pial arteries in the subarachnoid space, the vascular basement membrane [laminin 411 and 511 (α4,5; β1; γ1)] is physically separate from the pial basement membrane (laminin 111). As the penetrating arteriole enters parenchyma, the pial basement membrane and the glial basement membrane (laminin 211) merge into a single structure called the parenchymal basement membrane (397). Astrocyte endfeet following along the vessels are firmly attached to the parenchymal basement membrane via dystroglycan receptors to form the glia limitans perivascularis, and on the brain surface to form the glia limitans superficialis (402). At this vascular segment, the vascular basement membrane and parenchymal basement membrane are still separate, but the picture is quite different in the capillaries, where the vascular and parenchymal basement membranes fuse together to form a single basement membrane composed of a combination of laminins from endothelial (laminins 411 and 511) and astrocytic (laminin 211) sources (397). Therefore, at the level of the capillary, the space between the vascular and parenchymal basement membrane seemingly disappears. On the other hand, recent studies suggest that the basement membranes may remain separate, therefore implying the possibility of a perivascular space exists around capillaries (131). Be that as it may, the space clearly reappears at the postcapillary venule, but with expression of endothelial laminin consisting mainly of laminin 411 along with “patchy” expression of laminin 511 (401). The spaces and layers created by the various basement membranes are the boundaries for the formation of perivascular space in the brain and are therefore of vital importance in the organization of intra-axial fluid flow.
7.10. Structure and Organization of the Brain’s Glymphatic System
For centuries, the extracellular fluid of the brain parenchyma was considered to be stagnant and functionally separate from the CSF (18). The removal of waste products from the brain was believed to rely exclusively on proteolytic degradation and/or transport of selected solutes across the BBB (403). Yet, certain neurotoxic peptides such as amyloid-β form pathological extracellular deposits despite the presence of several transporters capable of mediating their clearance across the BBB (404). However, clearance at the BBB is restricted to molecules with substrate-specific transporters. Also, the lack of lymphatics in brain calls for an alternate pathway for bulk clearance of excess extracellular matrix constituents and protein. This role is served by the glymphatic system, which consists of 1) an afferent path for entry of fresh CSF into the parenchyma along arterial perivascular space, 2) mixing and exchange of the new CSF with the extracellular fluid, and 3) an efferent exit path of the mixed CSF-extracellular fluid along venous perivascular space or other intra-axial outflow routes (FIGURE 16).
FIGURE 16.
Schematic representation of the organization of the glymphatic system. Cerebrospinal fluid (CSF) flow into the glymphatic system initiates at the basal cisterns of the subarachnoid space, flowing toward perivascular spaces of large cerebral arteries (see text for details). 1: From here, CSF flows into the perivascular spaces of penetrating arterioles that enter the brain parenchyma (4). In a process facilitated by aquaporin 4 (AQP4) channels expressed on the adluminal cell membrane of perivascular astrocytic endfeet, inward flow occurs over the perivascular glia limitans and into the brain extracellular space (22). 2: A directional flow of extracellular fluid is established through the narrow extracellular space toward the perivenous spaces (56). This flow rids the extracellular space of metabolic products and accumulated fluid, and provides a source of fresh extracellular fluid (1). 3: From the perivenous spaces, extracellular fluid exits the brain parenchyma. The glymphatic system has been shown to form a closed connection with dural lymphatics, so it seems likely that perivenous extracellular fluid finds egress through these channels draining into the venous system (70). Other egress pathways are perineuronal paths, perivenous spaces along dural sinuses and major cerebral vessels exiting the skull, and arachnoid granulations. See sect. 12 for details on egress of fluid from the intracranial compartment.
7.10.1. CSF entry.
The entry of CSF into the brain has been demonstrated experimentally in rodents (4), nonhuman primates (166), and humans (164) to begin at the basal cistern of the subarachnoid space and proceed along the perivascular space of the large cerebral arteries. Detailed knowledge of the anatomical structure of this compartment is lacking, and its nature is thus a matter of controversy. Some research groups suggest that the perivascular space lies between the pial basement membrane and the vascular basement membrane, while others regard it as being in the subarachnoid space proper. Imaging experiments suggest that the perivascular space along leptomeningeal arteries is most likely identical with the subarachnoid space, as intracisternally delivered tracers in rodents can be seen filling up a space on either side of the artery that exceeds in diameter that of the central vessel (21, 279, 405–407) and is broadly distributed between the sulci in humans (164, 165). To distinguish this space from other fluid compartments in the brain, it was designated as the paravascular space (4, 408). However, if this space were in fact synonymous with the subarachnoid space, then the anatomic terms would have overlapping meaning. Therefore, we have chosen to expand the meaning of the term perivascular space to encompass all fluid compartments that surround vessels. Irrespective of the particular compartment in which transport is occurring, CSF entry takes place along the distribution of arterial tributaries. The geometry of the perivascular space appears to be optimized to reduce hydraulic resistance and maximize flow (409). As CSF reaches the initial segment of a penetrating arteriole, it enters the space formed by the pial basement membrane (adjacent to the vascular basement membrane) and the glial basement membrane, which remain distinct and unfused. This space is directly contiguous with the subarachnoid space, which explains why centripetal CSF transport begins as soon as the tracer directly approaches the arteriole (164, 165). CSF enters along the perivascular space until it reaches the vascular segment, where the pial basement membrane disappears and the vascular basement membrane fuses with the glial basement membrane. At this point, it is uncertain if the fluid traverses into the space between the vascular basement membrane and any remaining pial basement membrane or if it instead continues to flow through the basement membrane of the vessel. There is also conflicting evidence about the exact number of pial layers accompanying the artery (131, 406), and it seems that this number might also vary based on anatomical location (50). However, calculations suggest that the hydraulic resistance within basement membranes may be too high to allow much flow along this path (410, 411). On the other hand, the pial basement membrane does not present a barrier to flow, and tracers are seen readily redistributing through it under certain conditions (21, 412). Recent work suggests that the vascular and glial basement membrane might not merge completely at this level, which would explain the presence of CSF tracers at the capillary level, suggesting that a patent perivascular space may exist throughout the brain vascular network (4, 131). The interior of the spaces also contains perivascular macrophages, perivascular fibroblasts, and pericytes, which may have an as yet unknown role in modulating CSF flow (413). Throughout the descending pathway for CSF into cerebral cortex, the outer wall of the perivascular space is formed by the parenchymal basement membrane (including the pial and glial basement membranes), which provides the structural support for the glia limitans perivascularis (402). In gray matter, protoplasmic astrocytes tile the parenchyma and spread complex lamellar processes outwards, forming domains with minimal overlap with their neighboring cells (414). In all species that have been examined, at least one of these cell processes extends one or several perivascular endfeet, such that virtually the entire intraparenchymal vasculature is ensheathed by the glia limitans perivascularis (415). The astrocyte endfeet are said to cover between 63 and 99% of the capillary cross section by attaching to laminins in the glial basement membrane through dystroglycan receptors (402, 415, 416). The presence of agrin in the glial basement membrane orchestrates the localization of an orthogonal array of intramembranous proteins (OAPs) to the perivascular endfeet by binding to α-dystroglycan (402, 417). OAPs consist primarily of AQP4 water channel tetramers and inwardly rectifying K+ channels (Kir4.1), which play a role in facilitating water entry into the parenchyma via AQP4 (402, 418). The zones of incomplete coverage of the perivascular space by astrocytic endfeet were initially estimated to form 20–50 nm clefts (415), but studies using cryofixation show that astrocytic coverage can be much sparser than previously thought, generating clefts ∼1-µm wide at the level of the capillary (416). Endeet attachment and OAP polarity are lost or severely reduced under pathological conditions but have some capacity for recovery after injury, further suggesting that this boundary could be highly dynamic (419, 420). It remains to be demonstrated at what level of the vascular tree CSF enters the parenchyma and if it indeed enters across AQP4 water channels into astrocytes or flows between the endfeet clefts (see sect. 8 on the role of AQP4). In all plausible scenarios, CSF must cross at least one basement membrane in the brain, as is likewise seen in the periphery.
7.10.2. CSF-extracellular fluid mixing.
Passing beyond the glial basement membrane, CSF next enters the extracellular space of the brain, which contains the extracellular fluid (FIGURE 16). The biophysics of precisely how CSF mixes or drives extracellular fluid flow downstream awaits further clarification. However, most evidence suggests that CSF and extracellular fluid come into direct contact, thus forming a single, continuous fluid compartment with roughly similar compositions at either end (421). The extracellular fluid bathes all cells in the tissue and contains inorganic ions, neurotransmitters, metabolites, peptides, neurohormones, and other macromolecules of the extracellular matrix. The geometry of the extracellular space is critical for determining the concentration of these solutes and also limits the rate at which they can arrive and leave the compartment. The volume fraction of the brain extracellular space is between 14 and 24%, as measured with ion-selective microelectrodes, which indicates that the total extracellular fluid exceeds that of CSF (285, 421). Extracellular fluid moves in the extracellular spaces between cells that have been described as sheets and tunnels of widths predicted to measure between 38 and 64 nm (422–424). The tortuosity of the extracellular space has been calculated to be 40% of that expected for diffusion in an unrestricted medium (285, 423). This difference is likely due to the geometry of the extracellular space, the presence of dead space microdomains, and additional factors hindering fluid transport such as the network of extracellular matrix molecules (423). In the new technique of super-resolution shadow imaging (SUSHI), loading the extracellular fluid with a cell-impermeable dye reveals that the extracellular space volume fraction can range between 5 and 36% (mean ∼20%) in hippocampus, which is at the upper range of estimates by ion-selective electrodes. These broad spatial and regional variations of extracellular space volume fraction suggest heterogeneity for fluid transport rates in brain tissue (425, 426). Additional studies have also shown that extracellular space volume fraction is larger in cortical layers V and VI compared with III and IV, suggesting that CSF-extracellular fluid exchange might vary depending on the cortical depth and local cellular architectonics (427). The novel SUSHI imaging approach has also revealed the extracellular space to have a width up to 100–300 nm. This is much larger than previous predictions (425, 428), so it remains to be clarified if extracellular space size is indeed greater in living brain, such as is seen in other tissues (429). Nevertheless, extracellular space volume fraction is not a static parameter but changes dynamically as a function of neural activity, cell volume, postnatal development, and disease (285). One important aspect of this dynamic is evident during transition from sleep to awake states, whereupon the extracellular space decreases from 23 to 14% (1). We have proposed this to be due to changes in intracellular volume that are mediated by activation of norepinephrine receptors during the awake state, but other regulatory mechanisms are certainly possible. The decline in brain extracellular volume fraction upon waking closely matches the decrease in extracellular space volume observed after repetitive electrical stimulation of rat spinal cord (24 to 17%), thus demonstrating that neuronal activity has a strong influence on the extracellular space geometry (430). The extracellular matrix also plays a role in maintaining extracellular space volume by attracting Na+ and water into the matrix via the negatively charged side chains of extracellular matrix macromolecules (431). During aging, decreases in the concentrations of CSPGs and fibronectin coincide with a declining extracellular space volume (431). The microenvironment therefore provides a high (albeit variable) hydraulic resistance to flow throughout the glymphatic pathway, yet CSF tracers are abundantly present within brain parenchyma and even at the level of the capillaries (4). Additional work shall be required to determine if advection occurs to a significant extent within the extracellular space. Although physiological models suggest this to be unlikely, available models do not incorporate the highly dynamic nature of the extracellular space volume fraction; for further discussion, see sect. 11 on modeling of brain fluid transport in the extracellular space (432–434). The finding that the perivascular space continues through to the venous side of the vascular tree also raises the notion that parenchymal advection might not be necessary to drive bulk flow, due to the short cell-to-capillary distances in brain (10–20 µm) (131, 272). A portion of CSF bulk flow might continue through the capillary perivascular space, with diffusion of solutes toward or away from the perivascular space (depending on the direction of the concentration gradient), which could explain why dispersion is the predominant mechanism of CSF tracer entry to brain (278). Future work should aim to construct a comprehensive model of the brain-wide extracellular space to identify if there is any underlying organization in the extracellular space that would be conducive to directional flow; this hypothesized structure might be termed a glymphatic connectome.
7.10.3. Removal of extracellular fluid.
Many experiments carried out in our laboratory and elsewhere concur in showing that solutes in the extracellular fluid are cleared at a rate faster than is explicable by diffusion alone (FIGURE 16) (435). Tracers spanning a wide range of molecular masses (900–69,000 Da) are cleared from the brain at similar rates, despite a fivefold difference in their diffusion coefficients, which is indirect evidence in support of bulk flow (64). Experimental measurements of the rate of extracellular fluid outflow in rat brain are in the range 0.1–0.3 µl/min (435). While the concept of bulk flow clearance of extracellular fluid is generally accepted, the driving force for this outflowing of fluid is still a matter of debate. Initially, clearance was considered to result from extrachoroidal production of extracellular fluid at the BBB, since its magnitude was well within the range of known influx rates for Na+ and Cl− from plasma, and given the enormous surface area of brain capillaries (435). Although this model might well be valid, the true magnitude of extrachoroidal flow awaits experimental confirmation. The majority of evidence points to inflow of CSF mainly via arterial perivascular spaces. Like any fluid, CSF water is nearly incompressible, such that its entry to the brain must be matched by the exit of an equal volume at some downstream location (conservation of mass). Therefore, the volumetric flow rate at which CSF enters parenchyma should closely match the rate of extracellular fluid efflux, in the absence of any changes to the geometry or some unknown mechanism for fluid storage within the tissue (e.g., intracellular uptake). Evidence garnered as early as the 1990s had already shown that tracers injected into the CSF enter along arterial perivascular space and subsequently accumulate in the venous perivascular space and white matter tracts (66, 287, 435). This classical view was recently confirmed using two-photon imaging in anesthetized mice, showing the entry of CSF tracers along the arterial perivascular space of cortex and its subsequent appearance in the venous perivascular space (4, 54, 436). There is some conflicting evidence in rodents, whereby tracers injected directly into the parenchyma find their way to arterioles, suggesting an outflow of extracellular fluid along the arterial perivascular space (273). This proposed efflux pathway between smooth muscle cells and through the vascular basement membrane of arteries was named the intramural periarterial drainage model (IPAD) (437). The IPAD is primarily based on histological evidence that is vulnerable to death and perfusion artifacts, which could displace flow tracers and create fluid spaces in locations where they do not exist in vivo (412). Two in vivo studies support the IPAD model but are confounded by their methodology whereby high intracerebral injection volume and BBB breakdown likely altered the physiological flow pathways (438, 439). Furthermore, high resistance in the proposed flow pathway of the IPAD model makes it inefficient as a fluid conduit, and several physiological disadvantages of such a design makes it unlikely to be an important efflux pathway, as described in the following sections.
The observation that CSF enters along arterial perivascular space and subsequently shows up at the venous perivascular space indicates that CSF influx might be a direct driver for extracellular fluid efflux. There are several possible scenarios for such a mechanism: 1) the flow of CSF into arterial perivascular space continues down along the capillary, either through an existing perivascular space or along channels created by basement membranes, eventually connecting with the venous side perivascular space; 2) the entirety of the flow continues into the extracellular space and re-enters the venous perivascular space at some downstream location; and 3) a combination of these processes and/or some unknown pathway constitute the drivers of extracellular fluid efflux. The caveat in answering this question concerns whether all venous perivascular spaces (or some subset thereof) drain exclusively into subarachnoid space, or rather find another intra- or extra-axial efflux route. Measurements in rats suggest that between 10 and 20% of extracellular fluid drains back into the subarachnoid space CSF (64), which would imply that the majority of venous perivascular spaces follow an intramedullary route to exit the skull. If flow is to continue along the perivascular space, there must be a pressure gradient from the vicinity of the artery toward the venous side (278). Such a pressure gradient along the arterial/venous perivascular space has not yet been measured, but there are several models for how it might occur. The best developed theory is that CSF flow is driven by perivascular (peristaltic) pumping caused by the arterial wall pulsation of the cardiac cycle (21, 54, 66, 282, 440). If CSF flow does indeed occur, either through the capillary perivascular space or basement membrane, this would have important implications for the diffusion of O2, glucose, and other nutrients across the BBB. Would such soluble nutrients merge with the perivascular space flow and be carried downstream, thus potentially missing their target, or is the capillary flow so slow that diffusion predominates, thus having a negligible effect on the delivery of substances? For scenario 2, where the entire volume of afferent fluid from the arterial perivascular space enters the extracellular space to drive fluid efflux, the only conclusions are presently drawn from modeling rather than experimental approaches (432–434). The existing models generally concur in proposing that the hydraulic resistance is too high to allow advection to occur within the parenchyma (see sect. 11, for a discussion about diffusion versus advection in brain extracellular space). However, such conclusions are mostly drawn either from assumptions of an idealized Voronoi geometry (432) (that is to say the partitioning of a space into volumes equidistant from some set of objects) or from serial reconstructions of a block of tissue (424, 434). The measured permeabilities reported for extracellular space range from 10 to 4,000 nm2, suggesting high variability or high uncertainty (434, 441, 442) and seem to be much lower when estimated from models of fixed tissue. Another potential reason for the large discrepancy in permeability estimates is that the extracellular space could be conducive to bulk flow in certain brain regions (i.e., white matter tracts) and less so in other regions (i.e., gray matter). The highly organized bundles of axonal tracts in white matter allow for the anisotropic diffusivity of water, which is the principal behind tractography in MR diffusion tensor imaging (443). This unique organization allows for extracellular fluid bulk flow speed up to 10.5 µm/min in the white matter of cat brain compared with <2 µm/min in the gray matter (183). The lower hydraulic resistance in white matter tracts might even divert fluid away from venous perivascular space, as alluded to in scenario 3, above. It is also possible that arterial and venous perivascular space display zonation, similar to that seen in the underlying vasculature, that would drive directional flow (444). Higher expression of AQP4 is seen in perivenous and pericapillary astrocytic endfeet relative to those in the periarterial perivascular space (4), yet more endfeet are seen surrounding capillaries than at arterioles and venules (445). To our knowledge, it remains unknown if the glial basement membrane or the extracellular matrix display any such arterial-venous zonation of the perivascular space.
7.11. Formation and Transport of Glymphatic Fluid
The fluid entering the glymphatic pathway is composed of water and inorganic ions derived from blood plasma. This is either due to the production of CSF at the choroid plexus or the extrachoroidal secretion of extracellular fluid by the BBB (see sect. 5). The relative contribution of extracellular fluid secretion to flow, and its point of entry in the glymphatic pathway, both require further clarification. This process would not follow Starling’s principle, contrary to findings in the periphery, due to the presence of the BBB making the brain capillary much less “porous.” A small volume of glymphatic fluid could derive from water of metabolism in living cells, but for the present we ignore this contribution.
In all situations the fluid entering the glymphatic system is nearly isotonic to plasma, but in contrast to the periphery, it consists of CSF, which is not simply an ultrafiltrate of plasma (TABLE 1) (446): the CSF is acellular and has higher concentrations of Na+, Cl−, and Mg2+, but lower concentrations of K+, , Ca2+, PO43-, glucose, and proteins than are found in plasma. Especially, the deficit in protein and glucose content requires increased Na+ and Cl− to maintain tonicity and charge. Likewise, the higher Mg2+ concentration in CSF partially offsets the lower Ca2+. Clearly, CSF composition is regulated by CNS cells, rather than being an ultrafiltrate with an ionic composition identical to that of its source, the blood plasma. There is no clear consensus whether CSF and extracellular fluid are truly distinct fluid compartments with somewhat differing compositions. The present evidence suggests that they indeed differ in composition (421) due to the extracellular fluid’s greater proximity to neurons and astrocytes that take up Na+, K+, and , and due to the relative concentrating of Cl− in the spatially confined extracellular space. Whether the composition differences arise out of physiological necessity, or due to effects of the anatomic site of sampling, remains unknown. The [K+] is lower at its point of secretion by the choroid plexus compared with the point of sampling in the subarachnoid space (447), indicating a gradient in its composition across different segments of the glymphatic pathway.
Table 1.
Concentrations of solutes in plasma and lumbar CSF in human
| Solute | Plasma | CSF |
|---|---|---|
| Sodium, mM | 150 | 147 |
| Sodium,* mEq/L | 140 | 144 |
| Potassium, mM | 4.63 | 2.86 |
| Magnesium, mM | 0.81 | 1.12 |
| Calcium, mM | 2.35 | 1.14 |
| Chloride, mM | 99 | 113 |
| Bicarbonate, mM | 26.8 | 23.3 |
| Inorganic phosphate, mg/dL | 4.7 | 3.4 |
| Protein, mg/dL | 6,800 | 28 |
| Glucose, mg/dL | 100 | 50–80 |
| Osmolality, mosmol/kgH2O | 289 | 289 |
| pH | 7.397 | 7.307 |
| pco2, mmHg | 41.1 | 50.5 |
See Ref. 446. CSF, cerebral spinal fluid. *mEq/liter data for sodium employ actual volumes of respective fluids.
In the periphery, lymphatic fluid is formed secondary to the high hydrostatic pressure pulses inside the arteriole, which are generated by the cardiac cycle. The relatively lower pressure in the extracellular fluid pulls more fluid out of the blood vessels to create lymph (448). In CNS, the CSF is driven into parenchyma through a related mechanism termed perivascular pumping, which is also generated by the cardiac cycle (21). The fluid that enters the neuropil functions in the manner of lymph by providing the cells with water, ions, and other vital substances present in CSF. This inflow is facilitated by the expression of AQP4 water channels on astrocyte endfeet (4, 22), which functions similarly as does AQP1 on the luminal membrane of the endothelial cells in the peripheral capillaries (449) and likewise AQP2 on the lymphatic vasculature (8, 345). CSF entry might play an additional role in CNS to that of lymph by replenishing or buffering (e.g., K+) of the extracellular fluid to preserve optimal ionic composition for neuronal function. The entry into brain of a fluid source of tightly controlled solute composition would also promote the clearance function of the glymphatic pathway, since its sparse concentration of proteins will favor the removal of extracellular waste proteins via a concentration gradient between CSF and extracellular fluid. If the rate of CSF turnover diminishes (e.g., in aging), the concentration of the harmful solutes would rise in the afferent fluid entering the glymphatic system, such that the concentration gradient between the CSF and the extracellular fluid would be reduced, thus slowing down the rate and magnitude of its clearance.
Also, in analogy to lymphatics, the glymphatic efflux of extracellular fluid helps maintain the degree of tissue hydration and aids in the removal of excess protein and harmful metabolites (4). Inhibiting glymphatic function by knocking out AQP4 water channels in mice is not lethal but results in increased brain water content and enhanced accumulation of toxic proteins such as amyloid-β in the brain (155, 450). Since the volumes of CSF influx and extracellular fluid efflux must match, any net accumulation of fluid will result in cerebral edema. Several studies from one research group show that mechanically obstructing cervical lymphatics can cause a state of lymphogenic encephalopathy resembling the lymphedema seen in the peripheral tissues (451–453). Recent experimental approaches using transgenic mice that lack lymphatic vasculature did not find any alterations in brain water content (68), suggesting that adaptive mechanisms or alternative pathways can compensate for the blocked outflow. Due to the lower driving pressure, glymphatic fluid efflux is expected to be much smaller than is typical for lymphatic fluid. Flow rates of 14 µl/h were measured in a single rat lymphatic vessel, which matches the entire global outflow of extracellular fluid (6–18 µl/h) (435). Therefore, reductions in glymphatic efflux might lead to the slow, insidious accumulation of proteins such as occurs in Alzheimer’s disease, in contrast to the rapid onset of peripheral lymphedema that develops in days to weeks after lymphatic vessel resection.
7.12. The Properties of the Extracellular Matrix, Hydraulic Conductivity, and Mechanical Stress
The forces that are driving extracellular fluid flow in the periphery (hydrostatic and colloid osmotic pressure) should likewise apply in the glymphatic system. Frameworks such as Starling’s principle might roughly translate to the CNS, but their applicability will depend on the degree of coverage by the astrocytic endfeet on the perivascular space, and will have less reliance on the glycocalyx. Hydrostatic pressure in the arterial perivascular space (PVS) must exceed the pressure in the extracellular space (PPVS > Pextracellular) to drive CSF flow into the tissue. The converse (Pextracellular > PPVS) could potentially hold true on the venous side, which would drive efflux of extracellular fluid into the venous perivascular space. However, the existence of spatial pressure gradients within the intracranial compartment awaits experimental confirmation. If the colloid osmotic pressure of the extracellular fluid exceeds that of CSF (σπextracellular> σπPVS), CSF should also move into the tissue. Opposite flow toward the venous perivascular space could occur in the case that colloid osmotic pressure of the exiting fluid is sufficiently high due to accumulation of solutes to drive water movement from the extracellular fluid into the perivascular space (σπPVS> σπextracellular). The net entry of any osmotically active particle would be expected to serve a buffering function comparable to that of albumin in the periphery, since the protein concentration of CSF is so much lower than in plasma. Permeability of the basement membranes and the astrocyte endfeet will regulate the ability of CSF to cross into brain parenchyma, much in the manner of the porous endothelial cell layer in the periphery. The permeability will also determine the magnitude of the reflection coefficient, σ. The enormously abundant AQP4 channels (primarily the M23 isoform), which account for up to 60% of the endfeet surface area (J. Rash, personal communication), are estimated to impart a water permeability of the endfeet as high as 0.2–0.6 cm/s (432), greatly exceeding the permeability of the entire astrocyte soma, which is only 0.05 cm/s (454). The AQP4 channels confer a high permeability to water since cells deficient in these channels exhibit an approximately sevenfold reduction in permeability (to 0.007 cm/s) (454). The permeability of the glial basement membrane has not been quantified, but this barrier is known to be permissive to the entry of a broad variety of tracers both through the glia limitans superficialis and perivascularis (4, 455). Permeability measurements of the basement membrane in the eye suggest that this type of structure is highly permeable to fluid but that its hydraulic conductivity can decrease as much as 66% upon application of pressure. This implies by extension that ICP may be a factor in the contribution of the glial basement membrane in regulating flow rate into brain (456).
Once fluid leaves the perivascular space, the brain extracellular space can be considered a porous medium, much more resembling that in peripheral tissues. Flow through a porous medium typically follows Darcy’s law, which states that flow rate is dependent on the permeability of the medium (e.g., pore size), the viscosity of the fluid, and the pressure drop over a given distance (278). As explained in the sections above, pore size is partly determined by extracellular space volume fraction, which is dynamically changing in the living brain. However, much as in the periphery, the extracellular matrix can also determine the hydraulic resistance of the tissue. Knocking out expression of the linker protein tenascin-R in mice alters the extracellular matrix structure in cortex sufficiently to reduce extracellular space volume fraction from 20 to 15% (457). A permanent reduction in extracellular space could potentiate the accumulation of ions such as K+ after neuronal firing, which might well result in a state of hyperexcitability. Interestingly, mice lacking the enzyme that produces hyaluronan [hyaluronan synthetase 3 (Has3)] had an extracellular volume fraction of only 7%, compared with 12% in wild-type mice, which occurred in association with onset of spontaneous seizures (458, 459).
Hyaluronan, one of the principal GAGs in the brain, possesses several interesting properties that might modulate parenchymal fluid flow. Increased concentration and mean molecular weight of hyaluronan increases the viscosity and elasticity of an aqueous solution (460). This property makes hyaluronan an excellent mechanical lubricant at the junction of tissues like cartilage and muscle. The osmotic pressure of hyaluronan in solution is not ideal, i.e., does not follow the Van’t Hoff equation relating osmotic pressure to molality, but rather increases exponentially much like in an albumin solution (461). This osmotic buffering property makes hyaluronan an excellent regulator of extracellular matrix water content. However, increasing the viscosity of extracellular fluid could potentially lead to slower extracellular fluid clearance. Interestingly, the concentration of hyaluronan in brain and CSF increases with healthy aging and even more so in certain brain diseases (462–464), which may be a factor in the age-related decline in glymphatic clearance (15, 27). There is limited evidence regarding the viscosity in the extracellular space of the living brain. One technical approach using super-resolution imaging of self-assembling single carbon nanotubes indicated that the viscosity of extracellular fluid in neonatal mouse ranges between 1 and 50 mPa·s (428). This range of values overlaps but can also exceed the range for CSF (0.7-1 mPa·s) (465), suggesting that extracellular fluid solutes and the composition of extracellular matrix must play an important role in determining its value. The local viscosity of extracellular space is spatially inhomogeneous and does not seem to vary with the size of the fluid space in question (428). The large range in the measured viscosities in extracellular fluid also suggests that the flow rate within the tissue can vary considerably, as is indeed the case (∼0.1–10 µm/s for a 35-nm quantum dot). Intriguingly, the local application of hyaluronidase enzyme caused an expansion of the extracellular space and increased the diffusion coefficient, indicating a global reduction in viscosity (428). In muscle fascia, this experimental treatment dramatically increased the flow rate through the tissue (466). The brain and the peripheral extracellular matrix share the feature that they are in constant flux, with continuous production and breakdown of matrix components. These dynamics could contribute to diurnal or age-associated changes in flow. Integrins, for example, can switch between an active and inactive conformation, by a mechanism determined by the extracellular concentrations of Ca2+ and Mg2+ (467). Concentrations of these bivalent cations, which are known to maintain integrins in an inactive state, are higher during sleep than while awake (468), raising the possibility that cellular attachments to the extracellular matrix and basement membrane might change across the state of behavioral arousal. In summary, the fluid clearance in brain shares several similarities but important differences compared with corresponding phenomena in peripheral tissues.
7.13. Edema Formation in Brain
Cerebral ischemia activates MMP-2 and -9 and several other extracellular proteases. The consequences of MMP-2 and -9 activation on brain edema are currently ascribed to degradation of the neurovascular unit and opening of the BBB, rather than a decrease in extracellular fluid pressure (36, 41, 106). However, it seems plausible that protease activation in brain might degrade matrix proteins and disrupt their connections with cell receptors, which, in conjunction with a breakdown of the actin cytoskeleton, would result in reduced tissue tension. This could permit a catastrophic increase in GAG hydration, as alluded to above. Certainly, injury of the BBB will facilitate fluid and solute influx, but this pathway cannot by itself explain the onset of edema in the setting of injury, since the prevailing pressure gradients between the capillary lumen and the tissue are too small. Recent evidence has further shown that rapid changes in arterial diameters can cause the perivascular space to increase in volume, thus driving more CSF flow into the ischemic tissue (469). The entry of Na+ and fluid from CSF was the primary contributor to the swelling of ischemic brain tissue (469). Thus a decrease in extracellular fluid pressure or an increase in extracellular fluid colloid osmotic pressure in brain may contribute to edema in the setting of acute injury or inflammation, much as in peripheral tissues.
7.14. Outstanding Questions
Do mural cells (i.e., perivascular macrophages, perivascular fibroblasts, and pericytes), which have an established role in scavenger functions in the perivascular space, also have a role in fluid transport?
At what branch of the vascular tree does CSF enter the parenchyma? Does the undefined astrocytic glycocalyx play a role in filtering the inflowing CSF?
Is there an underlying organization to the extracellular space that is conducive to directional flow, in the manner of a glymphatic connectome?
Do the molecular constituents of the extracellular matrix in the CNS play a substantial role in the dynamic regulation of the extracellular space volume? How do regional differences in the extracellular matrix contribute to modulating flow?
The extracellular matrix suffers significant changes during aging and disease. How do differences in extracellular matrix composition (e.g., reactive astrocytosis and glial scar) affect flow?
7.15. Interim Summary Section
Similar to peripheral tissues, neural tissue has a basic need for fluid-based delivery of energy metabolites and removal of solutes and waste products. The “in-house” production of CSF by the choroid plexus along with the existence of the unique perivascular spaces created by AQP4-enriched astrocytic endfeet can be viewed as an evolutionary adaption that is a prerequisite for tissue fluid transport within the confines of the BBB in the specialized CNS.
The lymphatic system represents a highly efficient path for removal of excess fluid and protein waste in peripheral tissues. Emerging evidence suggests that the Starling principle of fluid homeostasis should be reevaluated: it is likely the lymphatic system that transport the greater part of extracellular fluid back to the venous system, rather than local osmotic forces driving fluid back into the postcapillary venules.
The lack of fibrous structures in the brain extracellular space, combined with its high content of hyaluronan and proteoglycans, grants the extracellular matrix the ability to rapidly expand or shrink depending on its water content. The hydraulic conductivity of the extracellular space is a function of the hydration state of the extracellular matrix. The unique viscoelastic properties of the brain’s extracellular matrix enable the brain to change its hydraulic conductivity across the sleep-wake cycle, thereby ensuring that pressure waves generated by the cardiac or respiratory cycles drive parenchymal fluid transport more efficiently during sleep than in the awake state.
8. THE ROLE OF AQUAPORIN-4 IN FLUID TRANSPORT
8.1. Introduction
Aquaporins are a class of water channels present on the plasma membrane of cells, where they facilitate water exchange between the intracellular milieu and the surrounding environment. Among many aquaporin isoforms, AQP4 is expressed in the basolateral membrane of principal cells of the kidney collecting duct, where it is believed to provide an efflux route for fluid after water reabsorption (470). Similarly, AQP4 is the most important isoform for brain fluid transport. In the brain, AQP4 is exclusively expressed by astrocytes, with a typical highly polarized and dense expression in the perivascular endfeet surrounding the brain vasculature. The unique distribution of AQP4 perivascular endfeet abutting the vascular wall subserves several vital functions in CNS physiology. Their proximity to the perivascular space enables AQP4 to dictate the degree of fluid influx and efflux, thus playing a critical role in regulating intra-axial fluid flow. Various tracer studies using global knockout mice have shown a dependence of intra-axial flow on AQP4 expression (22), and intra-axial flow is also decreased in mouse lines with specific disruption of the perivascular polarization of AQP4 (22). Therefore, we know that polarized AQP4 expression is fundamental for fluid transport, but it remains uncertain whether fluid enters directly through AQP4 or if the water channels facilitate an indirect process that enables water influx to occur. AQP4 expression exhibits a zonation pattern with higher polarization to pericapillary and perivenous spaces compared with periarterial spaces (4), but the degree to which this spatial distribution contributes to directional fluid transport has yet to be established. Nonetheless, we do know that fluid flow is significantly reduced in circumstances where there is absence of AQP4 polarization and is highest when AQP4 polarization is maximal. Evidence suggests that AQP4 expression and its polarization are dynamically regulated and that both conditions need to be met for effective fluid transport. Further work will aim to elucidate how exactly astrocytes and perivascular AQP4 expression facilitate fluid influx and whether pharmacologically blocking or enhancing transport through these water channels can serve for treating human disease. This section discusses in detail the role that AQP4 water channels play in intra-axial fluid transport, but we refer the reader to a review presenting a more general coverage of AQP4 in CNS physiology (155).
8.2. Polarization of AQP4 on Astrocytic Endfeet
AQP4 water channels coat almost the entire cerebral vasculature and CSF barriers in the murine brain and can also be found on some ependymal cells lining the ventricles (471). Clustering of AQP4 occurs within orthogonal arrays of intramembranous particles (OAPs), sometimes called square arrays (see sect. 7.10 for a description of OAPs). The endfoot membrane adjacent to the capillary has up to 60% coverage by arrays, but this coverage declines virtually to nil proceeding toward the astrocytic soma (471). Endfeet often form multiple layers around blood vessels, but the presence of OAPs drops drastically after the first layer, which is indicative of their importance to the perivascular compartment (471). The degree of polarization is sometimes quantified as a polarization index, which is calculated as the proportion of perivascular AQP4 expression relative to the background expression at the soma (15, 55, 77). While this strict polarization is characteristic of the murine brain, polarization is less pronounced in human cortical tissue (157). The relatively lower polarization in human brain reflects the higher AQP4 expression in the astrocytic soma within the parenchyma. However, it is hard to determine if this is a true feature of healthy human brain or reflects the sampling conditions of patients undergoing neurosurgical intervention for diagnoses of epilepsy, intracranial neoplasms, or treatment for aneurysms, any of which pathologies might result in abnormal AQP4 expression. Thus further evidence is required to evaluate if humans normally possess the same degree of vascular polarization of AQP4 as do rodents (see sect 4 for the evolution of the glymphatic pathway). Polarization seems to specifically face toward the cerebral vasculature since AQP4 arrays are formed by the dystrophin-associated protein complex (DAPC), which depends on binding to laminins and agrin proteins that are found almost exclusively in the vascular basement membrane (BM). When components of the DAPC are knocked out, AQP4 polarization is lost. However, certain mutant mouse lines (e.g., α-syntrophin knockouts) retain some degree of polarization, suggesting a degree of redundancy in the polarization mechanism, perhaps involving other components of the DAPC such as β-syntrophin and β1-integrin (155). However, knockout lines that target the polarizing scaffolding for AQP4 display deficits in glymphatic function and K+ clearance and are resistant to edema formation, which is a phenotype remarkably similar to that seen in global AQP4 knockouts (472, 473).
8.3. Putative Functions of Polarized AQP4 Expression to the Endfoot
There are several hypotheses about the function of the highly organized AQP4 arrays. For example, polarized AQP4 expression has been proposed to play a role in astrocyte migration during development, K+ homeostasis, neuronal excitability, and extracellular space dynamics (474). Additionally, there is evidence that the arrays have a role in intracranial fluid homeostasis (155). As a member of the water-selective subfamily of aquaporins (155), the positioning of AQP4 on the adluminal surface of the perivascular filopodia of astrocytes confers to them an ability to regulate the transport of water from the blood and the perivascular space into the neuropil. Indeed, global knockout of AQP4 in mice results in decreased influx of labeled water from plasma into brain (475, 476), which is further supported by the observation that these mice are protected from cerebral edema (474). However, the endfeet and their high density of square AQP4 arrays also form the outer wall of the perivascular space and are therefore poised to support CSF entry to the neuropil.
8.4. Role of Endfoot AQP4 Square Arrays on Intra-Axial Fluid Transport
Global AQP4 knockout mice have reduced influx of CSF tracers compared with wild-type animals (4). However, the absolute dependence of CSF influx on AQP4 was recently challenged by a single study in which there was no discernible difference between the knockout line and wild types (283, 412). Nonetheless, a comprehensive replication study using four different global knockout lines confirmed the initial findings (22). Meta-analysis of over a dozen studies with a total of 144 animals showed a consistent dependence of CSF influx on AQP4 expression (22). Further evaluation suggested that results of the negative study could have been compromised by use of an unconventional anesthetic that suppresses glymphatic function (3), the wide age range of the experimental animals (12–24 wk), and a nonstandard tracer injection protocol (22).
AQP4 has also been shown to play an important role in the efflux of extracellular fluid (4), and, indeed, global AQP4 knockout animals exhibit higher brain water content compared with wild-type controls, which might contribute to their slightly elevated extracellular space volume (284, 477). However, transgenic mice lacking perivascular or ependymal AQP4 expression have normal brain water content, suggesting that it is specifically the subpial (glia limitans) AQP4 that determines extracellular fluid clearance (478). Infusion of artificial CSF into Aqp4-/- mice results in a supranormal increase in ICP compared with wild-type mice, supporting a role for AQP4 in the removal of extracellular fluid (479). Furthermore, the clearance of CSF tracers such as amyloid-β and mannitol injected into the brain parenchyma of Aqp4-/- mice is reduced by ∼70% compared with wild-type mice (4). That finding was contested by one study (283) but has been confirmed by eight separate studies consisting of 87 Aqp4-/- rodents (22, 412). Again, the discordant findings in the one study might have resulted from variant methodologies (22).
AQP4 might also play a role in the transport of oxygen into brain tissue (155). AQP4 knockout animals have slower O2 diffusion into brain (480), which might be a factor in the higher capillary density seen in these animals (476). However, the particular pathway followed by fluid en route to the neuropil, and whether it flows directly through AQP4 channels or follows an indirect route, remain unknown. In the following sections, we aim to explore the implications of the either transcellular (through astrocyte endfeet) or paracellular transport routes (between astrocyte endfeet) for intra-axial fluid flow.
8.5 Transcellular Transport Routes
CSF water could enter directly into the endfeet via AQP4 water channels (transcellular routes, FIGURE 17). For such a direct pathway to operate, certain physiological conditions would need to be met. Driving fluid into a cell under hydrostatic pressure (e.g., perivascular pumping) would encounter a high degree of hydraulic resistance, making it unlikely to be a primary driving mechanism for CSF influx (481). In addition, AQP4 is highly selective for solute-free water, so fluid entry would have to be accompanied by the cotransport of osmoles (e.g., Na+, K+, and Cl−) into the astrocyte. In astrocytes, this ionic transport could be primarily driven by the Na+/K+-ATPase, voltage-gated chloride channels (CIC-2), or volume-regulated anion channels (482). However, such a process would lead to the accumulation of intracellular osmoles, which would have to be removed from the astrocyte or through the syncytium out toward the extracellular space at a distal location, where egress of the excess intracellular fluid would also occur. Astrocytes possess the required transporters to support directional movement of intracellular Na+ and Cl−, but whether this mechanism actually suffices to drive intracellular fluid transport remains unknown (483). In the kidney, AQP4 is thought to play a role in water efflux from the cell, and OAPs have a high density of Kir4.1 channels, which are primarily involved in K+ extrusion. Therefore, it is conceivable that AQP4 may play a dominant role in water outflow rather than inflow. It might be that water is transported at specific zones of the astrocyte (i.e., the endfeet), rather than transporting ions and fluid across the entire cell body. While plausible, such a scenario would require the expression of AQP4 square arrays on the parenchymal side of the endfeet membranes, along with complementary ion channels to drive osmole efflux, a model that has not been systematically evaluated (484). In either scenario, intracellular fluid transport would be decoupled from solute transport, which is a distinct process.
FIGURE 17.
Plausible fluid transport routes and their dependence on the polarized expression of aquaporin 4 (AQP4) at perivascular astrocytic endfeet. Directional fluid transport from arterial to venous vasculature would require differential conditions at the site of influx (left) and efflux (right ) of fluid. Possible flow pathways can be largely divided into transcellular or paracellular transport routes. Transcellular routes (top row) would consist of direct entry of fluid through AQP4 channels, and could be subdivided into the following: 1: trans-astrocytic fluid transport, where fluid enters the astrocyte at the endfoot and continues through the soma and the greater glial syncytium following a largely intracellular pathway, or 2: trans-endfoot fluid transport, where fluid enters into the endfoot and rapidly exits into the extracellular space (ECS) without entering the soma, thus following a largely extracellular pathway interspersed with short segments of intracellular transport. Alternatively, fluid may also follow paracellular routes (bottom row), which would consist of fluid transport through the clefts formed by neighboring endfeet. This primarily extracellular transport route would rely indirectly on AQP4 water channel expression. 3: Hydrostatic pressure gradients, between the arterial and venous sides of the extracellular space could drive directional fluid flow toward the region of lower pressure (Partery > PECS > Pvein). 4: osmotic pressure gradients could be generated by astrocytic ionic fluxes (e.g., K+ spatial buffering) that in part depend on AQP4 polarization. Directional transport of extracellular osmoles could potentially drive fluid entry between endfeet toward efflux sites (σπvein > σπECS > σπartery). PVS, perivascular space.
8.6. Paracellular Transport Routes
Alternately, AQP4 may play an indirect role in fluid transport allowing fluid flux across the glial basement membrane to enter the extracellular space through clefts formed between the endfeet (paracellular routes). This process would mirror fluid transport in peripheral tissues and would be governed by principles much as those specified in the Starling hypothesis. One possibility is that CSF inflow would be driven by a hydrostatic pressure gradient between the arterial perivascular space and the lower pressure venous perivascular space. Although this scenario seems plausible, given the presence of the arterio-venous pressure gradient, technical limitations have limited our ability to measure corresponding pressure gradients at the smaller scale between these parenchymal regions within the intracranial compartment. In support of this model, recent work used immunohistochemical labeling of the protein megalencephalic leukoencephalopathy with subcortical cysts‐1 (MLC-1) to outline the endfeet; results demonstrated that the size of endfeet is highly heterogenous and is positively correlated with the diameter of proximal vessels (485). Results of modeling suggested that the decreasing size of the endfeet at microvascular branches and the declining pressure within the perivascular space ensures net CSF influx from the perivascular space into the parenchyma. In such a scenario, the decreasing endfoot-endfoot junction density would predict that fluid flow may in fact take place within clefts. The second possible explanation is that a directional osmotic gradient due to specialized ionic fluxes in astrocytes (e.g., K+ spatial buffering) might generate osmotic gradients in the extracellular space, which could potentially drive flow through endfeet clefts and into the neuropil. Modeling studies have had some success in showing that this could indeed be a plausible mechanism (486). In this last scenario, flow into the parenchyma would only occur when the driving ionic fluxes are present, suggesting that transport would be highly dependent on tissue-level neuronal activity and functional state.
8.7. Dynamic Regulation of AQP4 Polarization
The manner in which AQP4 facilitates the directional transport of fluid has not yet been elucidated. Zonation between the arterial and venous neurovascular units would necessarily be a fundamental aspect of such a system. However, polarization of AQP4 expression could be under dynamic regulation from various factors. Recent work showed that AQP4 polarization is under circadian regulation and appears to be particularly important for fluid transport during intervals of sleep and inactivity (55). Perivascular polarization of AQP4 is highest during the resting phase, and declining polarization during the active phase coincides with a loss in the day-night difference in glymphatic influx (55). AQP4 expression also changes rapidly after acute ischemic stroke, being strongly downregulated in the infarct and overexpressed in the penumbra (487, 488). We suppose that additional regulatory mechanisms for AQP4 polarization may emerge. In the principal cells of the collecting duct in the kidney, AQP2 serves to increase water permeability of the plasma membrane to allow for the reabsorption of free water (470). This process depends on intracellular signaling whereby antidiuretic hormone (vasopressin) binds to vasopressin-1 receptors, which causes the translocation of cytosolic AQP2 to the membrane, where it can aid in water reabsorption (470). It is unknown if an analogous process operates in the brain, perhaps under hormonal regulation. A recent paper showed a similar phenomenon for AQP4 in spinal cord vasculature and that blocking translocation to the membrane decreased edema formation after injury (489). AQP4 clustering has also been shown to be regulated by vasopressin (470), but there may be a broader spectrum of peptide hormones or signaling pathways involved in the regulation of polarization.
8.8. Outstanding Questions
Does fluid flow follow a primarily transcellular or paracellular route?
Is fluid flow coupled to synchronous physiological processes within the neuropil that could enable directional fluid transport (e.g., neuronal activity or K+ spatial buffering)?
If fluid follows a transcellular route entering directly through AQP4, then do inward and outward flowing solutes follow a separate route? To what extent is fluid transport similar or different to solute transport?
Are any of the other aquaporin family isoforms important for intra-axial fluid transport in brain?
Does spatial zonation of AQP4 expression play a role in driving directional fluid transport? What factors (e.g., developmental, hormonal, circadian, etc.) regulate AQP4 polarization?
8.9. Interim Section Summary
Aquaporin-4 is expressed throughout the body, but in the brain, it is especially enriched in astrocytes, where its expression is notably polarized toward the perivascular endfeet surrounding the brain vasculature. AQP4 clusters at the endfeet within orthogonal array of proteins (OAPs) or square arrays.
AQP4 expression plays a critical role in regulating intra-axial fluid flow. Global AQP4 knockout mice have reduced influx of CSF tracers compared with wild-type animals. Likewise, CSF tracers injected into the brain parenchyma of Aqp4-/- mice have slower clearance than in wild-type mice. Adequate polarization to the endfeet seems to be critical for facilitating fluid transport, since knockout animals that have preserved AQP4 expression but lack polarization have reduced tracer transport.
AQP4 might regulate flow through either transcellular or paracellular transport routes. Transcellular routes would consist of direct entry of fluid through AQP4 channels. Paracellular routes would consist of fluid transport through the clefts formed by neighboring endfeet and would rely indirectly on AQP4 water channel expression. Future work and new techniques are necessary to elucidate the exact pathway whereby AQP4 facilitates intra-axial fluid transport in the brain.
9. PHYSIOLOGICAL DRIVERS OF THE GLYMPHATIC SYSTEM
9.1. Introduction
How is fluid movement in the glymphatic system established? Thus far, multiple factors have been shown to drive fluid movement in the glymphatic system. Lying in close apposition to the vascular tree, the glymphatic system is driven by many of the same processes that ensure cerebral blood perfusion, namely pressure waves generated by the cardiac cycle, the arterial/venous pressure gradient, and autoregulation of blood vessel diameters. Blood flow in brain is furthermore affected by the respiratory cycle, whereby changes in intrathoracic pressure propagate to effects on the cardiac cycle, venous pressure, and intracranial pressure (ICP). These forces likely also act on the glymphatic pathway. Whereas cardiorespiratory factors are probably very important for flow in perivascular spaces, other factors in the brain extracellular space also contribute to directing fluid flow, e.g., the polarized expression of AQP4 expression on the vascular side of astrocytic endfeet and the extracellular space volume fraction. In this section, we consider in detail the different physiological drivers that enable and drive flow in the glymphatic system.
9.2. Intracranial Oscillations
The brain, unlike other organs, is entirely encased by a protective bony structure, the skull. The rigid walls of the cranium form the outermost barrier of the intracranial compartment, meaning that maximal intracranial volume cannot exceed a fixed quantity. The intracranial vault is filled with the brain, which occupies 80% of the space (intracellular compartment: 66% and extracellular compartment: 14%), blood occupies 10% (arterial, capillary, venous compartment), and CSF occupies 10% of the volume (20% of which in the ventricles and the rest in the cranial and spinal subarachnoid space) (421). Seminal work by Monro and Kellie (490), and extended by Burrows, determined that, due to the intracranial volume being fixed, any increase in extracellular volume would require an accompanying and proportional decrease in an adjacent compartment to prevent a rise in ICP. Therefore, derangements in intracranial volume are measured clinically by using ICP as a surrogate of the effective equilibrium between compartments. The most dynamic of these compartments is the CSF space. In cases where the brain swells or blood volume increases, CSF exits the intracranial compartment so as to maintain total volume. In disorders such as hydrocephalus, where this accommodation cannot be achieved, ICP rises to dangerous levels. The Monro-Kellie doctrine can also be extended to posit that even small changes in the volume of a compartment can cause CSF shifts. This turns out to be a quite important concept for in understanding fluid transport in the brain, since this process is a primary physiological driver of CSF flow.
CSF volume is a passive contributor to intracranial volume, as can be seen in the circumstance of cortical atrophy due to aging or the formation of hydrocephalus ex vacuo. Therefore, the compartments that can exhibit fluctuations in volume driving CSF flow are the brain tissue itself and the cerebral blood volume. Under physiological conditions, it is believed that the brain tissue has limited potential to significantly change its volume within a short timescale (seconds to minutes). However, this volume constraint might not hold at longer timescales such as across the day, arousal and brain states, or periods of neurodevelopment (months to years). Since both compartments are primarily composed of water, it follows that any large volume change would most likely be driven by fluid shifts, since the macromolecular content (i.e., metabolites, amino acids, lipids, and nucleic acids) of the brain tissue is largely stable. If volume changes were to occur, these would have to be the net result of changes in either intracellular or extracellular space volume. Intracellular volume could increase or decrease by altering the total number of cells or by swelling/shrinking of the cellular compartment. On the other hand, extracellular volume might vary secondarily to intracellular volume shifts or is perhaps independently regulated (e.g., by the extracellular matrix hydration state, see sect. 7). Yet, the most dynamic compartment at short timescales is definitely the cerebral blood volume (CBV), which consists of arterial, capillary, and venous vascular volumes. Thus arterial and venous CBV are the volume fractions with the most physiological variation. Arterial CBV constitutes 20–30% of the total vascular volume, while venous CBV accounts for 70–80% (491). Much as with brain tissue, the length of the vascular network is unlikely to change within short time scales, but this parameter does vary significantly across development and disease states. However, the diameters of both the arteries and veins are highly dynamic, as is consequently their volume. This is particularly relevant since the volume fraction of perivascular spaces in certain brain regions have been shown with 7 T magnetic resonance imaging to range from 0.22% in the basal ganglia to 0.74% in thalamus (492). This suggests that even a small change in CBV has enormous effects on the volume of fluid within perivascular spaces.
A fluctuation in CBV can occur acutely, as when bending over to tie your shoelace, which evokes a sensation of pressure building up inside your head, or it can occur rhythmically, as when you feel your heart beating inside your head as you jog. The intracranial environment is continuously subjected to the cumulative effect of all the mechanical pressure waves introduced into the skull. These rhythmic oscillations are sometimes organized by their frequency range, into very high (0.6-2 Hz), high (0.15–0.6 Hz), low (0.05–0.15 Hz), very low (0.02–0.05 Hz), and still slower ranges, which we denominate ultralow (0.01–0.02 Hz) and superlow (0.005–0.01 Hz) for the purpose of the present description (FIGURE 18) (496). The nomenclature and frequencies encompassed by each frequency band vary broadly within the literature and definitely require harmonization in the years ahead (495, 499, 503). Several oscillations reported in the literature may actually refer to the same process, but having been measured in different compartments using different techniques, they cannot be cross validated between studies. Moreover, the exact physiological underpinnings behind each oscillation band are still being worked out. For the purpose of this review, we shall organize the various oscillations into two categories: extracranial oscillators and intracranial oscillators. Extracranial oscillators like the cardiac and respiratory cycles introduce mechanical pressure waves into the skull and are primarily in the high- and very high-frequency ranges. However, an important caveat is that these higher frequencies have faster or slower cardiac and respiratory rates in different species. For example, a resting heart rate in humans is close to 1 Hz, while mice can have heart rates of up to 12 Hz, suggesting that the physiological process underlying these oscillations must have species-specific frequency bands (504). On the other hand, intracranial oscillators are more numerous and much more complex. Intracranial oscillators can be further divided into neural and vascular oscillators. Neural oscillations, which are the most studied, are primarily driven by phasic activity within and between neural circuits. These oscillations are generally measured used scalp electroencephalogram (EEG) or local field potential recordings and have frequency bands ranging from ultrafast (200–600 Hz), fast (80–200 Hz), gamma (30–80 Hz), beta (10–30 Hz), theta (4–10 Hz), delta (1.5-4 Hz), slow 1 (0.5–1.5 Hz), slow 2 (0.2–0.5 Hz), slow 3 (0.07–0.2 Hz), and slow 4 (0.025–0.07 Hz) (493). These frequency ranges reported are not universally accepted nomenclature and there is significant variability in the criteria used. We recommend that readers check the frequency ranges used in individual studies and also take into account species-specific considerations. The mechanism by which neural oscillators modify intracranial volume is not precisely known, but has recently become a significant research topic due to the role of CBV in the blood oxygenation level-dependent (BOLD) response recorded in functional (f)MRI (500). Neural activity has been shown to cause cells to swell and shrink, and the resultant changes in extracellular volume could cause flow changes (505). An alternative mechanism is that neural activity is coupled to hemodynamics via neurovascular coupling in a process known as functional hyperemia (506). Examples of neural oscillators that have been shown to change intracranial volume and drive CSF are described below. The second type of intracranial oscillators are vascular oscillators, which are driven intrinsically by the vasculature. Vascular oscillators are divided into three separate processes: myogenic, neurogenic, and endothelial (nitric oxide dependent and independent) oscillations (496). Myogenic (low frequency) oscillations are generated by a process primarily known as vasomotion (507). This process is believed to be the cause of fluctuations in blood flow and mean arterial pressure, which are known as Traube-Hering-Mayer or Mayer waves or simply M waves (507). Neurogenic (very low frequency) oscillations are primarily the result of innervation of the autonomic nervous system on the microvasculature. These neurogenic oscillators might play a role in entraining the various rhythms and are found in the same frequency band as the Lundberg B waves seen in ICP recordings (499, 508). Endothelial oscillations in the brain are less well known but have in the periphery been shown to be either nitric oxide (NO)-dependent (ultralow) or NO-independent (superlow) (496, 497). It is important to note that the CSF compartment will be exposed to the net effect of all these coinciding oscillations, such that CSF flow is best described by a system of coupled oscillators. The following sections will discuss several of the oscillatory rhythms that are known to drive CSF flow.
FIGURE 18.
Intracranial oscillations as drivers for brain fluid transport. A: oscillations can be measured by different techniques in separate compartments, such as neural oscillations measured using EEG or local field potential (493). They display important state-dependent changes such as the predominance of delta waves, K complexes, and sleep spindles during nonrapid eye movement sleep (494). Vascular oscillations are divided into extracranial and intracranial oscillations. Extracranial oscillations are the cardiac (very high frequency) and respiratory (high frequency) cycles, while intracranial oscillations are divided into myogenic (low), neurogenic (very low), and endothelial components [Kiviniemi et al. (495) and Stefanovska et al (496)]. Endothelial oscillations consist of nitric oxide (NO)-dependent (ultralow) and NO-independent (superlow) mechanisms (497). Several neural and vascular oscillations have been measured in CSF. Arterial pulsations from the very high frequency cardiac cycle drive perivascular flow [Mestre et al. (21), Iliff et al. (54), and Harrison et al. (407)]. Flow changes are also driven by the respiratory cycle (498) (Strik et al. (499). Myogenic oscillations, also known as vasomotion, have been measured in cerebral spinal fluid (CSF) [van Veluw et al. (439)]. These myogenic oscillations are sometimes described as ultraslow, Mayer (M) waves, or Lundberg C waves (499) [Drew et al. (500), Lundberg (769), Lang et al. (770)]. B: diagram of coupled oscillators that drive CSF flow. Extracranial oscillators such as the cardiac and respiratory (Resp.) cycles transmit pressure waves into the intracranial compartment by modulating the amount of blood entering the vascular compartment, which is measured as cerebral blood flow (CBF) and cerebral blood volume (CBV). Neural processes like functional hyperemia in turn also modulate CBF and CBV [Buszaki et al. (493)]. Changes in CBF and CBV can be evaluated from functional MRI blood oxygen level-dependent (BOLD) contrast signals [Fultz et al. (501)]. The direction of coupling between the various oscillators is an exciting and growing field to determine the multiple processes underlying CSF flow (502).
9.3. Cardiac Cycle
9.3.1. Background: blood flow in the brain.
Cerebral perfusion is higher than in most other organs, such that the brain receives 12–20% of cardiac output, despite accounting for only 2% of total human body weight (509–511). Cardiac output, the volume of blood pumped by the heart in a minute, is defined as the product of the stroke volume (volume of blood ejected with each beat) and the heart rate (the number of times the heart beats in a minute). As such, cardiac output depends on body size and changes in response to demand, increasing from perhaps 4 L/min at rest up to 35 L/min in an elite athlete during exercise (512). Cerebral blood flow (CBF; ml·100 g−1·min−1) is the volume of blood that passes through the brain with each minute, which is defined by the cerebral perfusion pressure (CPP) and is inversely related to the cerebrovascular resistance (CVR) of the brain vasculature. CPP is determined as the pressure difference between mean arterial blood pressure (MAP) and intracranial pressure (CPP = MAP − ICP), or rather the brain venous pressure, should that exceed the ICP. The CBF is then defined as the CPP divided by the cerebrovascular resistance (CBF = CPP/CVR). In other words, CBF theoretically increases with rising mean arterial pressure and decreases in response to lower MAP, with elevations of ICP or venous pressure, or following increases in cerebrovascular resistance. Normally, cerebral autoregulation maintains CBF within a narrow range during physiological conditions, despite the huge variations in cardiac output across daily activity (509). The magnitude of CBF is generally kept within a twofold range, which is maintained by the effectors of cerebral autoregulation that alter the resistance of the brain vasculature (509). The autoregulated change in resistance mainly occurs in arteries and arterioles, where modulation of vascular smooth muscle cell tone can cause either vessel dilation or constriction (509, 510). The role of cerebrovascular autoregulation is explained in further detail in sect. 9.5.
The total volume of blood within the brain is furthermore a dynamic quantity. Thus CBV is defined as the product of CBF and mean transit time (MTT), which indicates the time taken for blood to pass through the brain (CBV = CBF × MTT). In healthy adults, the CBF is ∼50 mL 100 g·tissue−1·min−1 and the MTT ∼12 s, such that the CBV is ∼5 mL/100 cm3 tissue (5% vol/vol) (513). As mentioned above, under normal conditions, CBF is relatively stable; however, there are certain exceptions, such as during hypercapnia, where CBF can increase substantially. CBV is a direct function of CBF and is sometimes represented using Grubb’s exponent, which states that CBV = 0.8 × CBF0.38 in nonhuman primates (514). An interesting feature of this relationship is that at lower CBF values, the CBV changes are of much greater magnitude than those occurring during higher CBF states. The majority of the CBV is found on the venous side of the vascular tree. The total intracranial volume of an average young male person is ∼1,400 mL, of which blood represents 100–130 mL and CSF 75 mL (490). There is a profound difference between the total cerebral perfusion and the CSF production rate. While a brain may receive blood at a rate of ∼700 mL/min, the CSF production rate is a relatively minuscule 0.3–0.7 mL/min (see sect. 5.4 regarding CSF production rates) (490). In other words, blood flow exceeds CSF production by a factor of 1,000–2,000. Given that the entire vascular tree in human brain encompasses >600 km of densely packed arteries, arterioles, capillaries, venules, and veins, it is evident that flow through the cerebral blood vessels must exert substantial forces on the surrounding intracranial structures (510). Indeed, cardiovascular dynamics pump CSF in the perivascular spaces of leptomeningeal surface vessels, which thus ensures the supply of fresh CSF to the glymphatic system (21).
9.3.2. Glymphatic flow and the cardiac cycle.
Ultrafast MR encephalography reveals that cardiovascular pulse waves in phase with the R component of the electrocardiogram travel along the major cerebral arteries, descend downwards to the ventricles, and proceed from there toward the brainstem and cerebellum (see TABLE 2) (495). In that study, cardiac cycle-induced pulse waves were also observed in the CSF, and the authors hypothesized that propagating pulse waves traversing the brain parenchyma could cyclically accelerate and deaccelerate CSF flow in the perivascular spaces of penetrating arterioles. The anterograde flow of CSF in perivascular spaces of leptomeningeal surface vessels has been studied microscopically in various animal models (FIGURE 19). The anterograde flow velocity in the perivascular spaces of the middle cerebral artery was ∼18 µm/s and was faster in the center of the space and slower near the perivascular space wall, as well as around vessel bifurcations (21, 282). The blood flow in a neighboring artery was ∼1,000 times faster than the local CSF flow (516). The dynamics of CSF flow speed in the perivascular space is similar to blood flow, meaning that its velocity periodically increases and decreases in synchrony with the cardiac cycle (21). The velocity peak matches that of the heartbeat, indicating that the cardiac pulse wave propagating along the artery is a main driving force of CSF inflow (21). In fact, the displacement of the artery wall during the cardiac pulse wave matches the dynamic movement of CSF both in velocity and timing, which strongly suggests CSF is pumped forward by the distension of the artery during systole (21). The importance of the cardiac cycle in pumping CSF forward into the periarterial spaces is also demonstrated by observations that pharmacological agents altering cardiovascular dynamics can impact CSF flow. Thus treatment of rats with the β1-adrenoreceptor agonist dobutamine increases heart rate and cardiac output and also stimulates CSF flow in the perivascular space proximal to the Circle of Willis, whereas angiotensin-II treatment induces hypertension and reduces the velocity of CSF flow (21, 407). In line with this, dobutamine treatment increases the inflow of CSF tracer to the brain parenchyma, thus confirming that raising the CSF flow rate in periarterial spaces leads to increased glymphatic influx (54). However, the relationship is more complicated than it might seem at first glance. The pulse wave pumping CSF forward is dependent not just on heart rate but also on its various component features, including pulse pressure, blood pressure, and also ICP. Pharmacological studies of the relationship between pressure waves and CSF inflow have generally suffered from a lack of specificity for these various component factors, so there is still no clear understanding of how they separately determine the rate of CSF flow (438, 440, 517). This reflects the difficulties in dissecting out the different cardiac and vascular components affecting perivascular flow and glymphatic function in separate entities, which not only relate to drugs but also various physiological states. Thus dynamic cardiac forces as well as arterial caliber changes affect influx rate by altering pumping efficacy and periarterial space resistance, and in similar ways, venous pulsations and venous blood volume changes likely affect efflux rate. It should also be noted that a reduction in CSF influx may be an indirect consequence of excess fluid accumulation and failure of fluid drainage out of the CNS compartments. Most of the efflux paths are difficult to access experimentally due to their deep location. Future studies must aim to disentangle the interconnection between the drivers of glymphatic influx and efflux. According to the terminology used in fluid mechanics, CSF inflow in the perivascular spaces is described by the Reynolds number (the ratio of inertial to viscous forces within a fluid), which describes the extent of turbulent flow, and the Peclet number, which is the ratio of advective to diffusive transport. For CSF flow in the perivascular space around the middle cerebral artery, the Reynolds number is typically 0.001, suggesting a strictly laminar flow, and the Peclet number is typically 1,000, implying that advective transport dominates over diffusion (21).
Table 2.
Cyclic changes in neural activity and cardiorespiratory forces that drives CSF flow and glymphatic activity during sleep and wakefulness
| Frequency | Brain State | Cause | Physiological Driver on CSF dynamics | Effect on Ventricular CSF | Effect on Glymphatics | References |
|---|---|---|---|---|---|---|
| Very high: 0.8–1.2 Hz or every ∼1 s | Wakefulness and sleep | Cardiovascular cycle | Arterial pulsations | Minor low amplitude contribution compared with respiratory forces (Human) | Forward pumping of CSF in periarterial surface vessels (Mice) | (21, 54, 495, 498, 515) |
| High: 0.4-0.25 Hz or every ∼4 s | Wakefulness and sleep | Respiratory cycle | Venous pulsations, drop in venous blood volume and changes in ICP | Inspiration causes largest amplitude changes during wakefulness in CSF flow leading to increased inward flow from 3rd to 4th ventricle (Human) | Venous pulsations are believed to drive extracellular fluid efflux from brain* | (495, 498, 515) |
| Very low: 0.05 Hz or every ∼20 s | NREM stage 2 sleep | Sleep spindles or K complexes oscillations trigger vasodilation followed by vasoconstriction | Change in BOLD signal reflecting dynamic changes in vascular tone | Largest changes in intraventricular CSF flow, also relative to wakefulness Leads to increased inward flow from 3rd to 4th ventricle (Human) | A drop in arterial blood volume could lower perivascular space resistance to CSF flow and large oscillations in arterial blood volume could act as a pump* | (469, 501) |
| Low: 0.1 Hz or every ∼10 s | Wakefulness | Increased neural activity in the γ-band leading to vasodilation followed by vasoconstriction | Arterial constriction that follows the neural activity driven dilation | Little or no effect (Human) | Increased brain extracellular space clearance. Likely occurring by the same mechanisms as above, albeit with lower amplitude changes (Mice) | (439, 501) |
ICP, intracranial pressure, CSF, cerebrospinal fluid; NREM, nonrapid eye movement; BOLD, blood oxygenation level-dependent. *Hypothesis, not experimentally proven.
FIGURE 19.

Physiological drivers of the glymphatic system. Flow in the glymphatic system is established by different physiological processes driving intracerebral pressure gradients. Top left inset: anterograde cerebral spinal fluid (CSF) flow in perivascular spaces of leptomeningeal surface arteries is driven by perivascular pumping. The distension of the arterial wall caused by the cardiac impulse wave pumps CSF forward in a pulsatile fashion, where increased CSF flow velocity occurs in phase with the R component of the ECG (systole) (21). Bottom left inset: the respiratory cycle changes intrathoracic pressure, which affects central venous pressure and also the cardiac cycle (respiratory bradycardia). This in turn affects venous pressure in brain as well as intracranial pressure (ICP). Intraventricular CSF flow is thus highly dependent on the respiratory cycle, where inspiration leads to a drop in venous blood volume in brain, thus causing increased flow of CSF into the ventricular system. Forced inspiration can increase this CSF flow rate (498, 515). In the glymphatic system, the venous pressure changes caused by the respiratory cycle are believed to have earlier and greater effects on perivenous spaces than periarterial spaces (495). This might impose a pressure gradient facilitating extracellular fluid flow toward the venous compartment during each inspiration. Top right inset: increased brain extracellular volume is associated with increased glymphatic function that occurs during sleep. The 60% increase in extracellular space volume that typically occurs during nonrapid eye movement (NREM) sleep causes a drop in hydraulic resistance that promotes fluid flow through the parenchyma (1). ECS, extracellular space. Bottom right inset: changes in arterial and arteriolar diameter caused by autoregulation of smooth muscle cell tone have been associated both with intraventricular CSF flow and brain clearance. In the 4th ventricle, increased CSF inflow deeper into the ventricular system has an anticorrelation with the blood oxygen level-dependent (BOLD) signal on functional MRI. This suggests that loss of cerebral blood volume due to vasoconstriction following the hyperemia induced by global increases in neural activity during NREM sleep increases CSF flow (501). Brain fluid clearance in mice has also been associated with vasomotion, both in association with 0.1-Hz neuronal oscillations occurring during wakefulness and during evoked functional hyperemia (439). PVS, perivascular space.
9.4. Respiratory Cycle
9.4.1. Background: respiratory cycle and changes in ICP and venous pressure.
Intracranial pressure has a close relationship with venous pressure. Due to the lack of physical valves in the brain and cranial veins, venous pressure in brain is quite sensitive to central venous pressure, which in turn tracks the changes in intrathoracic pressure across the respiratory cycle (490). Thus the respiratory cycle evokes venous pressure pulsations in cerebral veins, which in turn provoke cyclic changes in ICP that reflect expiration and inspiration (518). Besides this respiratory component, the ICP pressure waveform has two other components, namely the cardiac cycle and slow wave vasomotion (M waves and B waves, see FIGURE 18) (518). The amplitude of the respiratory component is the largest among the three ICP pressure wave components (490, 518). In other words, under physiological conditions the venous pulsations caused by the respiratory cycle provoke the largest changes in ICP. Furthermore, the respiratory cycle can affect CSF flow (495, 498), but exactly how cyclic ICP changes and respiration-induced changes in venous volume may each affect CSF is not known in detail.
9.4.2. Glymphatic flow and respiration.
Intraventricular CSF flow is driven by inspiration-related intrathoracic pressure changes and can thus be increased by forced inspiration and decreased with breath holding (498). The intraventricular CSF flow during inspiration is in an inward direction, thus causing CSF to move from the fourth ventricle to the cerebral aqueduct and onwards to the third and lateral ventricles (FIGURE 19 and TABLE 2) (515). The CSF flow during inspiration is driven by the loss of venous volume, such that the drop in intrathoracic pressure during inspiration causes increased venous blood outflow and consequently increased CSF inflow to the ventricles (515). The respiratory-driven venous pulsations travel through the brain parenchyma in a centripetal manner, initiating in the perivenous area in cortical regions and propagating toward the center of the brain (495). The role of the respiratory cycle on microscale CSF and extracellular fluid flow is poorly understood (50). Histological evidence shows that CSF tracers entering the brain along the glymphatic pathway, or delivered intrathecally, find their efflux from the brain through white matter tracts and along perivenous spaces around large caliber draining veins including the transverse sinus (that drain subcortical white matter tracts), straight sinus, and inferior sagittal sinus (4, 56, 436). Venous pulsations and consequently increased pressure difference between arterial and venous compartments are believed to drive fluid efflux from the brain parenchyma by a mechanism resembling the process driving fluid influx in periarterial spaces on the brain surface during the cardiac cycle (21, 495). Compared with the wall distensions on the arterial side caused by the cardiac cycle, venous wall pulsations are likely of a much larger amplitude due to the larger caliber of the veins and show greater changes in blood volume relative to arterial blood volume. The more distensible venous wall compared with the rigid, smooth muscle cell-covered arterial walls likely permit bigger volume changes in the venous compartment than in the arterial compartment during changes in CPP, ICP, and respiratory rate. Unfortunately, in vivo studies investigating the nature of perivenous efflux of extracellular fluid are lacking, likely due to present technical limitations as well as a traditional focus placed on studying dynamics of the periarterial CSF spaces (50). In fact, the respiratory cycle has a modulatory effect on the arterial perivascular spaces, which has some consequences on the influx of CSF to the glymphatic system (21). This modulation is probably caused by a drop in MAP due to respiratory bradycardia, which in turn affects the CPP, and then modulates the amplitude of the arterial wall distension, a phenomenon that is responsible for pumping CSF forward (21).
9.5. Vasodynamics
9.5.1. Background: slow vasomotion and functional hyperemia in brain.
Dynamic changes in vessel diameter arise not only from the cardiac cycle but also due to autoregulation of vessel tone. Thus vasoconstriction and dilation result from changes in the tonus of vascular smooth muscle cells in the walls of brain arteries and arterioles (519). Slow vasomotion in the absence of external stimuli falls within a broad range of frequencies centered around 0.1 Hz in awake human, mouse, and rat (520). It is debated whether these low-frequency fluctuations in vasomotion are caused by periodic changes in neuronal activity and smooth muscle contractility, but the rhythmic increases in arteriole diameter are phase locked with increased electrical activity in the γ-band, which is the so-called ultra-slow electroencephalographic oscillation (520). The vasomotion occurs synchronously over multiple transhemispheric brain regions and is dependent in part on the cortical projections of the corpus callosum (520). Functional hyperemia represents another kind of slow vasomotion but unlike the vasodilations driven by ultra-slow fluctuations in neuronal activity, functional hyperemia is dependent on external stimuli, and is not only temporally locked to the stimuli, but also spatially confined to just those cortical areas involved in processing the given stimulus (516, 519). Our understanding of vasomotion on intra-axial CSF flow is still limited, although there are reports of increased brain clearance associated with the cyclic ultra-slow oscillations of neuronal activity and also with functional hyperemia. However, the linking causal mechanisms have yet to be identified. At a macroscopic scale, vasomotion has been shown to produce pulsations that propagate over large parts of the brain; the associated intraventricular CSF flow seems to depend on changes in CBV probably caused by caliber changes in cerebral blood vessels that are coupled to oscillations in neuronal activity (439, 501). Thus there is a strong association between intra-axial CSF flow and vasomotion, but finer details such as driving forces, directionality, and physiological function remain to be elucidated.
9.5.2. Glymphatic flow and vasomotion.
During NREM sleep, 0.05-Hz oscillations in neuronal activity are followed by increased inflow of CSF through the ventricular system in humans (501). fMRI studies of the BOLD signal showed anticorrelation with CSF inflow through the fourth ventricle (501). Thus, whenever the intracerebral blood volume decreases, CSF flows into the ventricles (see TABLE 2). Sleep spindles or K complexes oscillations during NREM stage 2 preceded both the drop in BOLD signal and the increase in ventricular CSF inflow. This increasing effect on intraventricular CSF flow indicates that slow waves in NREM sleep could be a driver of the increased glymphatic activity during sleep, conceivably by a mechanism whereby the arterial constriction following upon neurogenic vasodilation causes a drop in resistance for CSF flow in the perivascular space. In contrast, during wakefulness only small amplitude CSF oscillations were seen in the ventricular system, and these oscillations tracked the respiratory cycle at 0.25 Hz as mentioned above. New ultrafast MR encephalography methods applied in humans have revealed parenchyma vasomotor waves propagating in a rhythmic fashion, also during wakefulness (439, 495). These waves were more temporally complex and spatially widespread than are cardiorespiratory waves, and a power spectrum analysis showed various frequency components centered around two main peaks. Changes in signal stemming from vascular changes were associated with oscillating neuronal activity occurring at 0.023–0.73 Hz, whereas signal changes at 0.001–0.023 Hz are believed to result from changes in vascular tone driven by sympathetic and parasympathetic activity from the autonomic innervations of the cerebral vasculature (495). Studies in mice support the notion that vasomotion associated with oscillations in neuronal activity during wakefulness is a driver of intra-axial fluid flow. Indeed, vasomotion during wakefulness driven by oscillating neural activity centered around 0.1 Hz has been associated with increased clearance of brain parenchymal tracers, as revealed by mouse studies with intravital two-photon microscopy (439). That increased power of vasomotion can boost brain parenchymal clearance rates was furthermore corroborated in that study, which showed that evoking functional hyperemia in mice by visual stimulation increased the clearance rate by about one third, compared with nonstimulated controls (FIGURE 19 and TABLE 2) (439). However, that study was confounded by artifactual BBB breakdown, resulting in leakage of blood components into the perivascular spaces. As such, further studies are needed to elucidate fully the mechanism of functional hyperemia and vasomotion in regulating brain clearance. The glymphatic inhibition that occurs during isoflurane anesthesia, when cerebral vessels are fully dilated, is thought to be due in part to loss of vasomotion during the full dilation (3, 439). A similar mechanism is likely responsible for the glymphatic inhibition seen during hypercapnia, although in both instances a reduction in perivascular space cross-sectional area might also have been responsible for the attenuated glymphatic function (521). The difference in glymphatic activity that is seen between the awake and sleeping states can partly be explained by differences in hemodynamics between these two brain states. During deep sleep, oscillations in cerebral blood volume are of much larger amplitude than during wakefulness, with the largest amplitude occurring during REM sleep compared with NREM sleep (522). Somewhat surprisingly, neurovascular coupling is also stronger during sleep than in the awake state, meaning that sensory stimulations during sleep drives larger fluctuations in arterial diameters (522). Thus the large displacements of blood that are seen during sleep, both as a response to cyclic electrophysiological changes in neural activity, as well as by sensory evoked functional hyperemia, would in turn cyclically allow greater volumes of CSF to enter the brain during sleep than during wakefulness.
9.6. Hydraulic Resistance
As fluid flows through the glymphatic system, the greatest resistance and thus the slowest flow rate occurs through the tortuous brain extracellular space (see sect. 7 on extracellular matrix). The volume fraction of the brain extracellular space changes dynamically, typically varying between 14 and 24% of the total brain volume, but can fall to 5% during ischemia (285). The extracellular space is geometrically complex and harbors lengthy and tortuous paths that circumvent cells, along with other paths that terminate in a cul-de-sac. Furthermore, the extracellular space contains an extracellular matrix that increases the viscosity of the extracellular space fluid and also harbors receptors and negatively charged structures that can interact with and temporarily bind certain constituents of the extracellular space fluid, thus slowing the flow of fluid and its solutes through the extracellular space (285). The extracellular matrix of the brain differs from that of other organs with respect to its almost complete lack of fibrous components like collagen and for its abundance of negatively charged chondroitin sulfate proteoglycans as well as aggrecan-hyaluronan complexes, which likely facilitate rapid water uptake of the brain extracellular space (25, 377); see sect. 7 on extracellular matrix for more information on this subject. Perhaps due to this complexity, the extracellular space volume in brain can change dynamically to an extent greater than previously supposed, such that the resistance to extracellular fluid flow can be tuned up or down (1). Consequently, the fluid movement into and through the neuropil encounters several switching sites, where parenchymal glymphatic flow can increase or decrease depending on brain state (3).
9.6.1. Brain state-dependent regulation.
The glymphatic pathway is primarily active during NREM sleep, when periarterial and parenchymal CSF inflow is severalfold higher than during wakefulness (see FIGURE 20) (1). The increased glymphatic function during sleep is associated with a 60% increase in extracellular volume, and the resultant drop in resistance to flow likely facilitates the increased glymphatic influx observed during sleep (1). Noradrenergic signaling from the locus coeruleus is a critical factor in arousal and waking, and pharmacological blockade of this signaling in awake mice attenuated the loss in extracellular space volume compared with the sleep state, in association with increased glymphatic function, attaining an activity similar to that during normal sleep (1). Also in humans, sleep is crucial to brain clearance and one night of sleep deprivation leads to reduced clearance of intrathecally delivered MRI contrast agent from the brain parenchyma across multiple brain regions (524). In humans, extracellular space volume cannot easily be measured, but instead an indirect estimate can be obtained using diffusion weighted MRI. Sleep studies have shown that changes in the extracellular space volume in human brain are complex and show a regional pattern, with increases in diffusivity in cerebellum and temporal pole and decreases in thalamus, insula, striatum, and parahippocampus (525). Diffusivity in human brain decreases from morning to evening, suggesting that prolonged wakefulness shrinks the extracellular space volume (526). In one study, a night of sleep deprivation led to decreased diffusivity measured in the morning, compared with that after a night with normal sleep (771). However, a similar study did not find any such effect of sleep deprivation (525, 527). Overall, the expansion of the extracellular space that enables increased glymphatic activity in rodents likely also occurs in human brain, but the dynamics might be more complex and show regional variability. The enhancement of glymphatic function during sleep is associated with higher delta power on EEG recordings, which is typically observed during NREM sleep, and also occurs upon treatment with noradrenergic antagonists, as with certain anesthetic regiments (1, 3) (FIGURE 20). Thus mice anesthetized with ketamine/xylazine or isoflurane/dexmedetomidine show increases in glymphatic function that are comparable to those in natural sleep, whereas isoflurane anesthesia alone is less efficient in increasing glymphatic influx (1, 3). Besides correlating positively with increased delta power in the EEG band, glymphatic influx is also inversely correlated with heart rate during anesthesia, such that anesthetic agents that increase delta power and decrease heart rate are optimal for preserving glymphatic function (3, 528). The mechanism by which the extracellular space expands during sleep and anesthesia is currently unknown but likely involves norepinephrine-regulated ion channels and transporters (529, 530). One potential mechanism that could increase extracellular space volume is the declining blood volume that follows upon sleep spindles or K complexes oscillations during NREM stage 2, which drives CSF influx into the ventricular system (501). Such a loss of CBV would likely also provoke increased cross-sectional area of periarteriolar spaces, thus causing a drop in perivascular space resistance and increased inflow of CSF to the periarterial spaces of penetrating arterioles. This increased perivascular inflow could in turn result in increased inward flow of CSF to the neuropil, where negatively charged extracellular matrix proteins might readily absorb the additional water and thus expand the extracellular space. In a similar manner, the stimulation of glymphatic function by heart rate reductions during anesthesia could be explained by a reduction in CBF leading to reduced CBV, causing in turn an increased CSF inflow by lowering the resistance in periarterial and periarteriolar spaces. However, this is a complex matter since different anesthetic drugs have distinct effects on heart rate, stroke volume, and blood vessel autoregulation depending on their mechanisms of action. This means that effects of anesthesia on CBF and CBV are difficult to predict. This also holds true for their effects on glymphatic function, and further studies are needed to pin down the relationship between brain state, cardiovascular components, and their impacts on glymphatic flow.
FIGURE 20.
Glymphatic activity and cerebrospinal fluid (CSF) egress during sleep and wakefulness. The glymphatic system is primarily active during deep sleep, when brain clearance more than doubles compared with wakefulness. This is likely due to an increase in extracellular volume fraction, which in rats is ∼14% during wakefulness but increases to 23% in a process dependent on the loss of noradrenergic signaling from the locus coeruleus (1). This could occur by a mechanism whereby perivascular space access is shut off during periods of high noradrenergic tonus, but then opens up for fluid influx during the low noradrenergic tonus of sleep. The rate of CSF egress from the subarachnoid space shows an inverse relationship with glymphatic activity, thus increasing during wakefulness and decreasing during sleep (523). Top row: the relationship between sleep and glymphatic activity. It is important to note that many details of the interconnection between the various stages of sleep and glymphatic flow remain to be determined. For example, the activity of the glymphatic system during rapid eye movement (REM) sleep is unknown. Bottom row: the critical importance of the extracellular volume fraction on glymphatic flow in the active state (left) and during inactivity [non-REM (NREM) sleep, right]. See sect. 12.10 for details on CSF egress. ISF, interstitial fluid.
9.6.2. Permeability.
The fluid flow in the glymphatic system from periarterial spaces toward the neuropil and onwards into perivenous spaces depends on the polarized expression of AQP4 water channels in the plasma membrane of astrocytic endfeet facing the perivascular space (4). AQP4 knockout animals, animals treated with AQP4 inhibitors, and animals treated with AQP4 antisense oligonucleotides all show attenuated CSF influx to the brain parenchyma and decreased clearance rates of extracellular tracer molecules (4, 22, 531, 532). The polarized expression of AQP4 to the astrocytic endfeet lining the perivascular space, as opposed to the soma and nonvascular processes, is critical for glymphatic function (15). With knockout of α-syntrophin, normal AQP4 polarization is lost, and AQP4 expression becomes diffusely spread throughout the glial cell. In mice with α-syntrophin knockout, inflow of CSF to the brain parenchyma is dramatically attenuated (22). Natural variations in the level of AQP4 polarization are found to be entrained by the circadian rhythm (55). Thus expression of the AQP4 gene and several other genes involved in the dystrophin-associated complex, which anchors AQP4 toward the adluminal site of the astrocytic endfeet, increases during the day, when mice are normally sleeping (55). The increased polarization of AQP4 during the day was associated with increased CSF influx and higher brain clearance rate, thus highlighting the importance of the polarized AQP4 expression for facilitating glymphatic flow. Loss of perivascular localization of AQP4 water channels is seen during reactive astrogliosis and has been associated with decreased glymphatic function in healthy aging and several disease models and across species (15, 56, 533). The mechanism by which AQP4 drives water into the parenchyma remains to be elucidated (see sect. 8).
9.7. Outstanding Questions
Arterial pulsations drive CSF influx, but modeling studies have concluded that these are not sufficient drivers. What additional pressure gradients generated during the cardiac cycle might contribute to driving directional CSF flow along periarterial spaces?
How do changes in intracranial pressure and venous pressure affect glymphatic influx and efflux?
Shrinkage of the extracellular space contributes in significant ways to the brain state control of glymphatic flow. However, it is likely that multiple other mechanisms suppress CSF influx in the awake state. CSF influx into the peri-arterial spaces surrounding the large caliber arteries in the basal cisterns are likely another site of regulation. This is plausible because the drivers of glymphatic flow, i.e., heart rate, respiration, and vasomotion, do not exhibit sufficiently pronounced diurnal changes to explain readily the dramatic increase in glymphatic flow during sleep, nor do diurnal changes in the rate of CSF production explain this difference.
9.8. Interim Summary Section
With their close apposition to the vascular system of brain, cardiovascular pulse waves drive glymphatic pathway influx and potentially also efflux. Cardiac pulse waves generate anterograde flow in the periarterial spaces of surface arteries by a pumping mechanism. Changes in vessel diameter likely also contribute to changes in CSF flow, possibly by altering the resistance to flow in the perivascular space.
Respiratory cyclic changes in intrathoracic pressure drive intraventricular CSF flow, probably by inducing a transient loss of intracranial venous blood volume. Respiratory venous pulsations travelling in a centripetal fashion from cortical to deep brain regions are similarly believed to drive efflux of extracellular fluid from brain along the perivenous spaces of the glymphatic system, but this has yet to be experimentally proven.
Vasomotion driven by slow oscillations in neuronal activity as occurs during sleep drive flow of intraventricular CSF, which is likely due to changes in blood volume that affect CSF flow. Cyclic changes in vessel caliber occurs also during wakefulness and propagate over the brain parenchyma with very low frequency. These cyclic changes in arterial diameter likely also affect the CSF flow in periarterial spaces by adjusting the resistance of the periarterial channels.
Flow in the extracellular space is facilitated by polarized astrocytic AQP4 expression and by dynamic changes in extracellular volume that may change across the sleep-wake cycle. Extracellular matrix proteins in the brain such as hyaluronan and proteoglycans can rapidly expand or shrink depending on their hydration state, which likely aids in rapid adjustment of extracellular space resistance.
10. PHYSIOLOGICAL FUNCTIONS OF GLYMPHATIC SYSTEM
10.1. Introduction
The glymphatic system transports CSF into the brain and in equal measure removes extracellular fluid from the brain, carrying along with it dissolved metabolic waste products. As far as we know, the glymphatic system extends to every part of the brain, spinal cord, and retina. While mainly viewed as an efferent pathway to drive solutes and fluid out of the brain, the glymphatic systems also functions as an afferent pathway that can deliver molecules and fluid to the brain. The glymphatic pathway thus exists as a parallel circuit to the intrinsic brain vasculature and can perform functions similar to those of the blood and lymphatic vasculature in nonneuronal organs, while remedying the obstacles to brain fluid clearance due the presence of the BBB and lack of lymphatic vessels. In this section, we shall summarize the roles of the glymphatic system in brain fluid and metabolite clearance, delivery of nutrients, and finally in volume neurotransmission.
10.2. Clearance
Brain clearance is the designation for the brain’s ability to rid itself of excess extracellular fluid, metabolites, and waste products derived from the breakdown of intra- and extracellular proteins. In simple terms, brain clearance can take place by three different mechanisms, namely glymphatic efflux, local degradation, and export across the BBB (27, 75, 404). Local degradation can occur via the intracellular processes of ubiquitination and by autophagy, which are discussed in detail elsewhere (14, 534–536). Different brain proteins are apt to have their own preferential route of clearance/degradation, which would depend on molecular size and the likelihood of their cellular release. These processes are modulated by global factors such as age, arousal state, brain region, cellular location, and cardiovascular health (FIGURE 21). Furthermore, a given protein is not cleared exclusively by one solitary mechanism but rather by the contributions of various possible clearance paths. As an example, immunoglobulins in the CSF are cleared by transport over the blood-CSF barrier (see sect. 5) and by bulk flow of CSF (539, 540). The several clearance mechanisms can work in concert, for example, when glymphatic transport carries a protein toward its efflux transporter expressed at the BBB. As such, dissecting out the precise contributions of distinct clearance mechanisms is difficult. However, the glymphatic pathway has been shown to be a substantial mediator of brain clearance, not only of solutes lacking a BBB transport mechanism, but also for clearance of molecules that are substrates for BBB transporters (4, 450).
FIGURE 21.
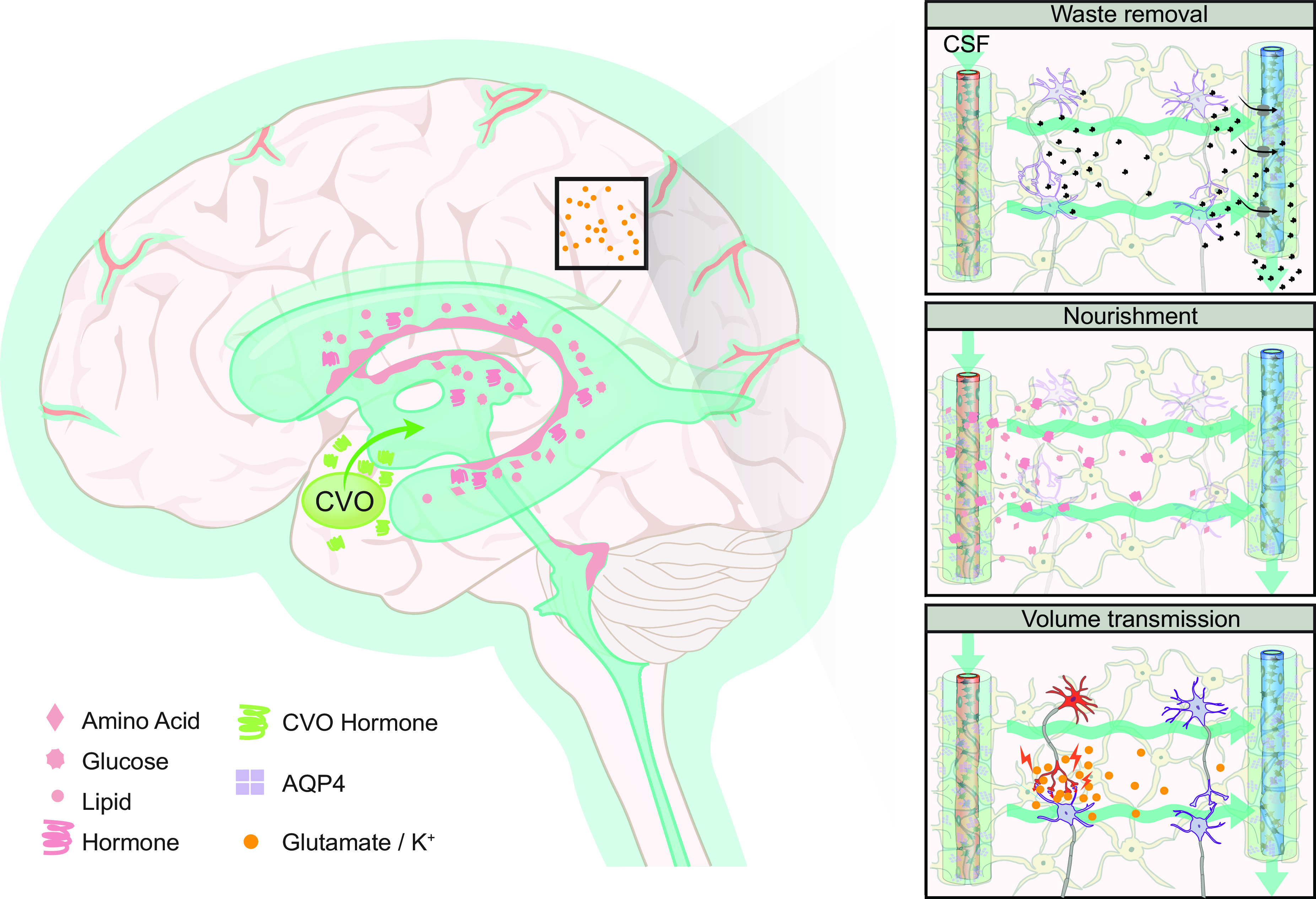
Glymphatic system and its role in clearance, nourishment, and volume transmission. The glymphatic system serves various physiological functions. By establishing extracellular fluid efflux, the glymphatic system clears the brain parenchyma of extracellular metabolites and waste products (1, 4). Furthermore, the influx of cerebrospinal fluid (CSF) to the brain along periarterial spaces also provides nourishment to the brain, whereby the glymphatic system distributes glucose to neurons and astrocytes, and delivers lipids and apolipoprotein E arising in the choroid plexus throughout the brain (436, 537, 538). Certain vitamins like folate and ascorbate are exclusively delivered to the brain parenchyma by CSF. Finally, the glymphatic system may play a role in local volume transmission, which refers to the transport of “spill-over” neurotransmitter from a synapse to neighboring non-postsynaptic cells. Glymphatic flow in the extracellular space would theoretically increase local volume transmission, as may occur during nonrapid eye movement (NREM) sleep, when large volumes of CSF wash through the neuropil. Interesting, NREM sleep is characterized physiologically by large scale synchrony of neuronal activity, which fits with the notion of widespread humoral action supported volume transmission, as opposed to the tight spatiotemporal coupling of neurotransmission during wakefulness. Global volume transmission refers to the brain-wide delivery of neuropeptides or hormones with CSF, arising in circumventricular organs or from the choroid plexus. CVO, circumventricular organs; AQP4, aquaporin 4.
On the other hand, the clearance of proteins that are neither enzymatically degraded nor exported by specialized BBB transporters in the manner of amyloid-β might rely entirely on glymphatic efflux. Inert tracers such as inulin and mannitol, which have no putative receptors or BBB transporters, can thus be used to investigate the purely glymphatic clearance rates (420). In mice with impaired glymphatic efflux due to AQP4 knockout, the clearance rate of radiolabeled inulin injected into frontal cortex declined by ∼25% after 30 min and that of radiolabeled mannitol injected into striatum declined by ∼70% after 2 h compared with animals with normal AQP4 expression (4, 541). In awake mice, in which glymphatic transport is minimal, the clearance rate over 4 h of inulin injected into the frontal cortex was reduced by half relative to that in sleeping mice (1). The importance of the glymphatic system in brain clearance has also been shown using antisense oligonucleotides for mRNA interference. Thus intraventricular injection of antisense oligonucleotides that specifically target AQP4 mRNA provoked a 30% reduction in brain AQP4 protein expression over a 28-day period (531). In concert with this reduction, there was a 23% reduction in the washout of mutant huntingtin protein into the CSF and a 49% reduction of its washout into blood plasma in a mouse model of Huntington’s disease. This confirms that a subacute inhibition of the glymphatic system can have dramatic effects on the brain clearance of a neurotoxic protein. An even stronger effect was found after acute pharmacological glymphatic inhibition, which can be provoked with the AQP4 antagonist TGN-020 (542). In one study, wild-type mice were treated with an intraperitoneal injection of TGN-020 and 15 min later received an intrastriatal injection of tau protein (532). Subsequent measurements of tau and phosphorylated tau in CSF indicated a 90% reduction in their clearance from brain after the pharmacological blockade of AQP4.
Amyloid-β is a product of neuronal metabolism, which has been heavily implicated in the pathology of Alzheimer’s disease and is a protein with more complex clearance pathways than most other proteins (see pathology in sect. 13) (14). Amyloid-β clearance is unique in that it occurs partly by specialized BBB transport, which heavily depends on glymphatic transport. This has been demonstrated in studies crossing APP/PS1 transgenic Alzheimer’s disease model mice, which have increased amyloid-β accumulation, with the glymphatic-deficient AQP4 knockout mouse (see sect. 13 for description of APP/PS1 mouse model). Offspring with increased amyloid-β deposition and glymphatic inhibition showed potentiation of amyloid-β deposition accumulation in hippocampus and cortical areas, as well as around leptomeningeal and cortical vessels compared with the APP/PS1 mice with an intact glymphatic system (450). The increased amyloid-β deposition was not due to augmented amyloid-β production or impaired amyloid-β degradation but rather to a reduction in amyloid-β clearance by glymphatic efflux. Similar observations were made in APP/PS1 mice with an genetically intact glymphatic system, but with ligation of the cervical lymphatics (543). Thus, while mice normally have clearance of amyloid-β via a specific BBB transporter, this pathway greatly relies on the patency of glymphatic efflux to the downstream cervical lymphatic system. By extension, the glymphatic system is an important brain clearance mechanism for various substances in the brain extracellular space, potentially via coupling to BBB transport. Amyloid-β, along with tau and α-synuclein, is involved in the pathogenesis of neurodegenerative disorders and is normally cleared at least in part by the glymphatic pathway (see sect. 8 for pathological processes in the glymphatic pathway) (56, 544). Furthermore, substrate transporter proteins such as apolipoprotein E that are abundantly secreted by the choroid plexus are ultimately transported from the brain parenchyma by glymphatic efflux, while small molecular weight metabolites are also cleared from the brain in part by glymphatic transport (537, 541).
10.3. Nourishment
While CSF is driven into the brain along perivascular spaces of penetrating arterioles, it carries solutes typical of CSF along with any further solutes that were transported into the perivascular spaces across the BBB. Many essential nutrients (like glucose, lactate, amino acids, ions, certain vitamins, and certain hormones, see review for details, Ref. 75) are transported by facilitated diffusion across the BBB and are thus directly available to CNS cells via the capillary network. The glymphatic system might aid in transporting these nutrients by tissue fluid dispersion from the capillary to cells that are distal to a capillary (see sect. 7). However, CSF influx represents an alternative source of some of these nutrients and indeed is the only source for substances lacking a specific transport mechanism in the BBB (FIGURE 21) (196). The glucose concentration in CSF is about one-third of the plasma concentration, and this CSF glucose pool is available for transport into astrocytes and neurons (see TABLE 1 for CSF solute concentrations) (538). In addition, lipids and several isoforms of their transporter apolipoprotein E, which is produced by the choroid plexus and secreted into the CSF, obtain brain-wide distribution by the glymphatic system (FIGURE 21) (436, 537). Certain vitamins (e.g., folate) are provided to the brain almost exclusively by way of secretion from the choroid plexus into the CSF (545). The importance of CSF as a medium for brain nourishing is exemplified by the phenotype of a loss of function mutation of the folate receptor-α. Children with this mutation undergo neurodegeneration due to central folate deficiency resulting directly from impaired transcytosis of folate over the choroid plexus epithelium and into the CSF (545). Glymphatic transport might also be critical for other vitamins, insulin-like growth factor I and II, transthyretin, and many other peptides and proteins that are transported from blood into CSF or produced and secreted from choroid plexus (196), although details remain to be elucidated. However, the brain-wide glymphatic transport of sugars and lipid compounds by convection through the glymphatic system suggests an essential role in brain nourishment and that all solutes within the CSF are likely dispersed throughout the brain by way of the glymphatic system.
10.4. Volume Transmission
Volume transmission represents an elusive concept of uncertain physiological importance, despite its pervasive acceptance in the literature (285). Volume transmission can be strictly local in its scope, e.g., due to synaptic spillover of ions or neurotransmitters into the adjacent extracellular space, where they can reach neighboring neurons to exert parasynaptic effects. The concept of volume transmission is popular in the field of dopamine research, since the range and duration of action of synaptically released dopamine depend on the local patency of the reuptake process. Disturbances in striatal dopamine reuptake due to disease may shift dopamine signaling toward a larger, less selective sphere of influence, resulting in symptoms of movement disorder. In the absence of any significant dopamine reuptake sites in cerebral cortex, volume transmission may be essential for proper encoding of dopaminergic signals (546). Volume transmission is also believed to function at a brain-wide scale, whereby neurotransmitters or neurohormones contained in the CSF can affect the brain globally (285). The glymphatic system is poised to play an important role both in local and global volume transmission scenarios (FIGURE 21). In local volume transmission, the proposed advection in the extracellular space will increase the distance traveled by a neurotransmitter molecular form its point of release and thus amplify its sphere of influence and duration of action. Thus we hypothesize that the glymphatic system would tend to expand the local volume transmission, albeit in the face of increasing dilution in the CSF. The glymphatic system could maintain global volume transmission by ensuring brain-wide distribution of neuroactive peptides or hormones. Circumventricular organs can release neuroactive hormones into the CSF with global effects. Also, peptides and hormones that are synthesized in the choroid plexus (e.g., BDNF) (196) and are then released into CSF or transported via the bloodstream into CSF (e.g., leptin) (196) could obtain widespread access to the brain parenchyma via the glymphatic system. Although not qualifying as volume transmission per se, hippocampal neurogenesis has been associated with signaling from CSF-born factors and their effects on the secretion of type I interferon from the choroid plexus (547). A more classical example of volume transmission is provided by the transport of tumor necrosis factor to distal sites after repetitive stimulation of peripheral C fibers. The distal effects of the tumor necrosis factor on susceptible synapses causes widespread hypersensitivity to painful stimuli (548). In the case of the pineal gland, its release of melatonin into the bloodstream follows a circadian pattern, which could act globally to harmonize a brain-wide physiological state. Like some other circumventricular organs, the pituitary gland circumvents the need for brain-wide distribution of bioactive molecules by the glymphatic system by releasing hormones directly into the bloodstream (549). This is enabled by its unique anatomical position in the sella turcica, which is an indentation in the sphenoid bone at the base of the skull. Sitting within this indentation, the pituitary gland is sealed from the subarachnoid space by the diaphragma sellae, which is a specialized part of basal dura mater endowed with a slit-like aperture for the pituitary gland (549). This intricate design has likely evolved to avoid direct distribution of pituitary hormones to CNS regions.
10.5. Outstanding Questions
How much does cytosolic degradation (i.e., ubiquitination and autophagy) versus glymphatic efflux contribute to protein clearance? Is all protein waste eliminated both by cytosolic degradation and glymphatic efflux? Do the elimination characteristics differ with regard to cell types and brain regions? Do they change with age?
Does the glymphatic system actively contribute to delivery of nutrients, hormones, and signaling molecules to the CNS? Would impaired glymphatic function interfere with brain metabolism and signaling by imposing a lack of nutrients and attenuated distribution of signaling molecules by volume transmission?
Are there regional variances in clearing efficacy of the glymphatic system?
10.6. Interim Summary Section
The most important physiological function of the glymphatic system is the clearance of brain metabolic waste. Brain clearance occurs mainly during sleep and is of crucial importance even for the clearance of compounds that are also degraded locally or transported across the BBB, e.g., lactate and amyloid-β.
The glymphatic influx of CSF delivers glucose and other metabolites to neurons and glial cells within the tissue, and also conveys a multitude of signaling molecules, nutrients, and vitamins within CSF. The importance of these glymphatic functions largely remains unexplored.
Volume transmission is a nonsynaptic signaling mechanism that is believed to be important for brain function, but remains hypothetical. The architecture of the glymphatic pathway makes it a likely pathway for volume transmission of neuroactive substances distal to their source, both local and global.
11. MODELING INTRA-AXIAL FLUID TRANSPORT
11.1. Introduction
Measuring fluid transport in the perivascular spaces that course deep into the neuropil or within the sheets and tunnels of the brain’s extracellular space is hindered by several technical limitations. Available imaging methods such as MRI lack the necessary spatial resolution to visualize flow in these microscopic compartments, while optical techniques rely on the invasive use of fluorescent tracers to label the fluid. The various technical limitations are further described in BOX 3. A potential solution to these problems would be to develop physiologically realistic models to evaluate fluid transport in the brain. Computational models of intra-axial fluid transport incorporate realistic geometries and known biomechanical properties of the tissue to inform us further on the processes that govern fluid flow. The models developed to date possess a high degree of variability in terms of their constituent parameters and the underlying hypotheses to be tested. The modeling attempts that have proven most relevant to understanding intra-axial (glymphatic) fluid transport can largely be divided into two types: 1) perivascular pumping, and 2) parenchymal transport models (FIGURE 22). The first type of model attempts to evaluate if arterial pulsations can generate a pumping mechanism in the perivascular space that can contribute to directional flow. The latter type of model seeks to determine what conditions may be necessary to support fluid flow within the microscopic and tortuous environment of the extracellular space. This section provides a bird’s eye view of the existing modeling studies and offers insight into how these models inform physiological experimentation and serve the goal of building more sophisticated models that would benefit from interdisciplinary input. However, for in-depth coverage of the models themselves, we refer the reader to superb reviews (278, 557). Additionally, not all of the comprehensive models of various aspects of ventricular and spinal CSF flow can be covered in this section; for further reading, please refer to TABLE 3 and Refs. 558, 559.
FIGURE 22.
Overview of existing models evaluating intra-axial fluid transport. The two main types of models are perivascular pumping (left) and parenchymal fluid flow (right). Perivascular pumping models consist of an artery with a surrounding perivascular space (PVS). Most such models implement a concentric anulus in which the artery lies central to the larger PVS, but modifying the centricity and the shape of the PVS cross-section to better represent in vivo measurements greatly reduces the calculated hydraulic resistance (409). The size and porosity (or lack thereof) of the PVS also affect the hydraulic conductivity (21, 409) The velocity of the arterial pulsation (wave speed) divided by the frequency of the pulsation (heart rate) indicates the wavelength. Models have used wide ranges of wavelengths due to the difference in resting heart rates between mice and humans, and due to inconsistent use of pulse wave velocity measurements collected from different blood vessels and species (278). Including outflowing boundary conditions such as intracranial compliance (C) and cranial cerebral spinal fluid outflow resistance (R) modifies the volume flow rate through the system (554). Few studies have evaluated how vascular branching and bifurcations affect wave speed transmission and arterial rigid motions (411, 555). The majority of perivascular pumping models have found that a static pressure gradient (ΔP) across the PVS is required for arterial pulsations to drive net flow at a rate comparable to those measured in vivo (21, 282, 555, 556). Parenchymal fluid flow models primarily consist of a segment of brain tissue interposed between an artery and a vein. The majority of such models to date have not included the interposed capillary network, which could but present an interesting extension. The extracellular space (ECS) geometry can be idealized or reconstructed from serial electron microscopic images. The volume and tortuosity of the ECS is approximated using the alpha (α) and lambda (λ) terms measured from real time iontophoresis experiments. The viscosity and concentration of solutes in the extracellular fluid can also be defined in these models. Static pressure gradients from the arterial side to the venous side have been tested as possible drivers of parenchymal fluid flow.
Table 3.
Glossary of common terms used in fliud dynamics
| Term | Definition |
|---|---|
| Advection | The transport of solute by bulk motion of fluid driven by a pressure gradient. The Peclet number, a dimensionless ratio, is greater than 1 when advection transports solute more quickly than diffusion. Since molecules of all sizes travel at the same rate and direction as the carrier fluid, this transport process is independent of the molecular size of the solute. This term is sometimes used synonymously with convection. |
| Convection | The transport of fluid by bulk motion, sometimes referred to as bulk flow. The term advection refers to the transport of a solute within the convective fluid. Thereby, the transport of the solute together with its fluid carrier also occurs by convection, but only the solute is advected by the fluid. Sometimes this term is erroneously used to refer to the combined effects of advection and diffusion. |
| Diffusion | The motion of solute or fluid from an area of high solute concentration to an area of low concentration. The rate at which diffusion occurs depends on the temperature, medium viscosity, and size of the molecules. The gradual movement of the solute is achieved by random walks (i.e., Brownian motion) and therefore occurs at the same rate in all directions, which is sometimes referred to as isotropic diffusion. The Peclet number is <1 when diffusion transports solute more quickly than advection. |
| Dispersion | The transport of a fluid that includes both advective and diffusive properties. There are specific types of dispersive transport, such as Taylor dispersion, where small concentration gradients are formed between the rapidly flowing fluid in the center of the perivascular space and the slower flow at the walls (shearing), causing diffusive transport to spread out the solute further and enhance its distribution. In some modeling studies, Taylor dispersion has been referred to as a primarily diffusive transport that is improved by the shearing forces caused by the arterial pulsations. |
| Volume fraction (alpha, α) | The proportion of tissue volume occupied by the extracellular space relative to the total tissue volume. The magnitude of α is calculated by fitting a function to data obtained from real time iontophoresis experiments and is reported as a fraction (α ≈ 0.2) that can be converted to a percentage (∼20%). |
| Tortuosity (lamba, λ) | The relative hindrance encountered by solute when moving through the extracellular space, compared with transport in a free and open environment. The magnitude of λ is computed by comparing the diffusion coefficients of a substance in brain (D*) and in a freely diffusible medium (D) such as agarose gel. This parameter is also estimated by fitting data from iontophoresis experiments, and is reported as the square root of the ratio between D and D* (λ ≈ 1.6). |
| Peristalsis | A radially symmetric contraction and dilation that travels along the length of a tube (e.g., gastrointestinal tract). To drive perivascular flow, the wave speed of the arterial pulsation needs to be sufficiently slow that fluid in the proximal segments is propelled prior to fluid in the distal segments. |
| Wavelength | The quotient between the wave speed and the frequency of the wave. When referring to arterial pulsations, the wave speed is the rate at which the systolic pressure wave travels from the heart to the perivascular space (1–5 m/s) and the frequency is the heart rate (1 Hz in humans compared with 5–10 Hz in mice). The wavelength relative to the length of the perivascular space is critical for peristaltic pumping to be possible. If the wavelength is much longer than the length of the space, then the radial displacement of the wall occurs almost simultaneously along the entire length of the vessel and would then propel the fluid in either direction, thus being unable to generate net flow. |
11.2. Perivascular Pumping
The observation that the brain pulsates in synchrony with the heart beat was first recorded in the Edwin Smith papyrus from 1500 BC, which is believed to be a reproduction of an even older papyrus scroll authored by Egyptian physician Imhotep around 3000 BC (36). The author stated that he felt the brain surface pulsate against his finger in a patient who had sustained open skull trauma, a common injury in those warlike times (560). Yet, the idea that arterial pulsations drive CSF flow inside the closed skull was more formally proposed by John O’Connell as recently as 1943 (561). That concept was naturally extended by Bradbury et al. (288) to posit that arterial pulsations facilitate fluid transport within perivascular spaces, allowing for the exchange between extracellular fluid and the surrounding CSF. However, their contention was that pulsations contributed to back and forth mixing of CSF and extracellular fluid, rather than driving any directional flow (562). The concept of perivascular pumping gained purchase when Marshall Rennels and Patricia Grady experimentally confirmed that arterial pulsations drove directional flow along perivascular spaces into the brain (66). Since then, compelling experimental evidence has consistently shown that arterial pulsations drive CSF flow within perivascular spaces around arteries in the anterograde direction of blood flow from large to smaller vessels (21, 282). Nonetheless, some studies have shown that fluid transport occurs in the direction opposite to blood flow, proceeding from small to large vessels (retrograde), and that this flow occurs in the basement membrane of the arterial wall as opposed to the perivascular space proper (438, 439). These reports sparked a lively debate about the anatomical path and direction of fluid flow that is actually a continuation of a debate beginning in the late 1800s. For instance, Rudolf Virchow’s definition differed from Charles Philippe Robin’s concept of what constituted the boundaries of the perivascular space; while Virchow’s space was “subadventitial” and in direct contact with the subarachnoid CSF compartment, Robin’s space was “intradventitial” and closed off from the subarachnoid space (35). This varying terminology and seemingly conflicting definitions make their composite eponym (Virchow-Robin spaces) conceptually imperfect as a descriptor of these important spaces. The disagreement on the flow routes was clear, but there was also some dispute about the general direction of the flow. For example, Harvey Cushing criticized the experiments of Frederick Mott, considering his observations of anterograde flow of CSF toward capillaries to be an artifact of the high-pressure injections of the dye (35). We can suppose that these luminaries would be gratified to know that we continue their tradition by debating the nature of CSF flow to this day (408, 412, 563).
A primary reason for the conflicting experimental conclusions was, and remains, attributed to differences in methodological approaches. One potential solution to test theories about CSF flow without interference from confounding experimental variables is to use modeling techniques. Here, the system can be reduced to its simplest elements to establish if pulsations of the arterial wall are plausibly sufficient to drive directional flow. Two main ideologies about perivascular transport predominate today, and the prevalent models reflect these two ideas. The first model evaluates anterograde flow into the brain along perivascular spaces, while the second model would explain retrograde flow within the confines of the basement membrane. The overarching conclusion derived from most anterograde flow models suggests that arterial pulsations would not be sufficient to drive bulk flow without supplement from other drivers (557). However, recent work has shown that a departure from the traditional concentric annulus structure of the perivascular space and adopting a geometry informed by experiments significantly reduces hydraulic resistance (409). Perivascular spaces at the brain surface of live mice have an oval cross section, which is bisected by the leptomeningeal artery to define separate flow pathways on either side of the artery (409, 412, 564, 565), while penetrating arteries lie eccentrically to the surrounding perivascular space (409, 412); both of these structural features seem to optimize the spaces to facilitate flow (409). This highlights the vital importance of undertaking additional work to map out the shape and dimensions of the entire perivascular space network to support a complete model of flow. Recent endeavors have used geometries extracted from published experimental data, which is a very promising future direction (555, 556). Even so, almost all models consider a section of perivascular space in isolation and do not consider the behavior when it is connected to the rest of the system. Therefore, defining the boundary conditions calls for special attention, since including in the model experimental values for intracranial compliance and CSF outflow resistance drastically changes the results from a scenario where pulsations alone cannot drive flow, to one more accurately reflecting the experimental data, through the use of realistic geometries (554, 556). Bradbury and Cserr’s initial conjecture that there is no net flow in perivascular spaces has also been supported by recent models stating that dispersion via a primarily diffusive mechanism aided by back-and-forth movement generated by arterial pulsations would suffice to support solute transport, without the need for net anterograde flow (433, 566). A recent revision of this concept suggests that an oscillatory dispersive clearance mechanism would be ineffective in the absence of a slow anterograde net flow (567). For retrograde transport models, the overall conclusion is that, despite the high hydraulic resistance presented by basement membrane, reverse transport remains possible, albeit at a much slower rate than what has been measured experimentally (411, 438, 557, 568). However, if arterial pulsations are the sole driving mechanism, the functional equivalent of a valve-like structure or mechanism (e.g., impedance pumping) or wave reflections are needed to explain transport in the opposing direction of the arterial pressure wave (569–572). A proposed directional permeability of the basement membrane created by the extracellular matrix microarchitecture has not been experimentally confirmed (569). Such an arrangement seems prima facia to be unlikely, which probably led to authors of that study to speculate that arterial pulsations would be a weak driver of retrograde fluid transport. Recently, that hypothesis was revised to propose that the cyclic contraction and relaxation of smooth muscle cells as in vasomotion (see sect. 9 on physiological drivers of intra-axial fluid flow) could contribute to retrograde fluid flow within basement membranes (573), a concept that has received some experimental validation (439). The high variability seen in the results obtained from existing models emphasizes their most notable pitfall, namely that models are necessarily bound by the assumptions made during their formulation. There remains a need to develop physiologically more complex and accurate models that could incorporate more of the relevant parameters. Until this is accomplished, the inferences to be drawn from existing models must remain of a provisional nature.
Nevertheless, existing models can still provide the means to test simple hypotheses about CSF flow or in some cases even aid in generating hypotheses. For example, one recurring result from perivascular pumping models is that a small pressure gradient of only ∼0.02 mmHg/cm suffices to explain the perivascular bulk flow seen in experimental studies (555, 556). While some have attributed this gradient to the transient increase in intracranial pressure (ICP) during tracer injection (even for very small volumes), this rise is several orders of magnitude higher (2–3 mmHg) and relatively transient (5 min) compared with the tonic pressure gradient needed to drive flow (412). Moreover, recent work has shown that the tracer injection does not in fact drive net flow of perivascular fluid and so seems unlikely to be the explanatory factor (574). A separate line of thinking holds that it is CSF production that sets up the pressure gradient (282), but this effect seems too large (555) and does not fit with experimental findings that CSF production rate can be high in states where perivascular flow is at its lowest (3, 202). Specifically, CSF production under isoflurane anesthesia is 33% higher than under ketamine-xylazine anesthesia (202), but tracer influx is nonetheless severely restricted under isoflurane, suggesting that perivascular flow is not simply the servant of CSF production (3, 575). Therefore, some authors have concluded that the source of this minute pressure gradient remains to be discovered (555). Most experimental studies evaluating perivascular fluid transport have been performed in anesthetized rodents, so it crucial to take into consideration brain state, given that arterial pulsations are also present in awake conditions, when a profound glymphatic inhibition prevails (1, 576). Therefore, as yet unknown features of state changes (e.g., perivascular space morphology) may need to be considered. For example, mean ICP in rats anesthetized with fentanyl/fluanisone and midazolam shows a slow decrease of ∼0.04–0.05 mmHg/min up to 75 min after the induction of anesthesia, which is in the range of pressures needed to drive flow (577). This declining ICP could be an effect of fluctuations in CBF, heart/respiratory rate, delta power, norepinephrine levels, or any combination of these factors. At 45 min after induction, a spectral component in the 8–6 Hz appears in the ICP waveform, which is consistent with the heart rate, despite being absent before anesthesia (577). Therefore, it is entirely plausible that arterial pulsations only contribute to flow during certain brain states and that presently unknown pressure gradients could enable state-dependent bulk flow.
The clear conclusion to be drawn from the above consideration is that more experimental data are necessary to develop more robust models. For instance, it remains to be established whether the outer boundary of the perivascular space is rigid or deformable, which would determine if the brain tissue also pulsates along with the artery. Two-photon imaging in mice showed that the outer wall of the perivascular space deforms during arterial diameter changes, suggesting that pressure increases in the space can displace brain tissue, which is therefore likely to pulsate in synchrony with the artery (578). This deformable property of brain tissue can be studied using lubrication theory to evaluate if arterial pulsations might indeed induce steady streaming of CSF from the perivascular space and into the brain (579). This modeling approach shows that the peristaltic wave could potentially drive flow through the glial outer wall of the perivascular space and that this process would be highly dependent on the permeability and rigidity of the brain tissue (579). Further modeling work has investigated how increased permeability from polarized expression of AQP4 on the astrocyte endfeet might facilitate the entry of CSF into the parenchyma (432). Imaging studies have also shown that the biomechanical properties of brain tissue can shape the degree of arterial diameter changes, suggesting that variable brain tissue rigidity along the vascular network can also influence the arterial vasodynamics (580). All these results beg the question of how changes in extracellular matrix properties, loss of AQP4 polarization, and astrogliosis as occur during aging and pathology might affect this exchange (see sect. 8 on pathology).
However, these considerations also raise additional questions about how the arterial pulsation propagates through the vasculature and whether it can indeed drive directional flow. One of the main critiques of perivascular pumping models follows from observations that the wavelength (=wave speed/heart rate) of the arterial pulsation greatly exceeds the length of the perivascular space; in other words, the arterial pressure wave travels so fast (1–5 m/s) that the entire arterial wall essentially moves synchronously, rather than as a pulsation traveling along the vasculature like a peristaltic wave in the intestine (278, 556). Clinically and experimentally, the wave speed can be measured as the pulse wave velocity using ultrasound imaging. Here, the wave speed is computed as the temporal delay in the peak flow velocity between two locations separated by a known distance (e.g., pulse wave velocity between the brachial artery and the arteries in the ankle). This metric is useful for determining the degree of stiffening in the large elastic arteries (i.e., aorta) seen in aging and diseases like atherosclerosis or arterial hypertension. This is mainly because pressure waves travel faster through stiffer tissues, so findings of an increased pulse wave velocity generally correlate with cardiovascular risk factors. The converse also holds in healthy, compliant arteries, where the incoming wave is partly transmitted and partly reflected, resulting in a dampening of the pressure wave and a slowing of the pulse wave velocity (581). It is critical to note that these measurements are primarily derived from blood flow measurements (flow motion) and do not necessarily measure vessel diameter changes (vasomotion) (507). As such, the exact relationship between intraluminal flow changes and the external vessel diameter changes, which is the relevant metric for perivascular flow, needs further consideration, since these effects are not linearly related and might differ at certain segments of the vascular network, depending on the local biomechanical properties of the vessel wall (54, 581). While most modeling studies have used point estimates of pulse wave velocity from the aorta or carotid artery in mice or humans (556, 582, 583), wave speed varies broadly throughout the vascular network (584). Therefore, while it is appealing to consider the pressure wave as a uniform, nondecaying traveling wave, this could hardly be further from the truth. The wave speeds reported in the common carotid artery (∼2,500 mm/s) differs by two orders of magnitude from the pulse wave velocity calculated in the cortical capillary network of live mice (∼25 mm/s) (583, 585). Therefore, the use of 1-D and 2-D models to simulate arterial pulsations might yield useful insight for the present but await further integration of more realistic wave speeds. Nonetheless, computational fluid dynamical models that incorporate accurate 3-D depictions of angioarchitecture are most likely needed to correctly evaluate how the pulse wave travels through the finely branching vasculature and how rigid arterial motions modify perivascular flow (555).
An additional outstanding question is whether perivascular spaces are open (278) or are porous (406, 586). This factor significantly alters how flows are modeled, since flow in open spaces is best described by the Navier-Stokes equation, while flow in a porous medium is best described by the Darcy-Brinkman equation (278). Prior work concluded that perivascular spaces at the brain surface and in the neuropil contain fibroblasts, collagen fibers, and structural elements of the extracellular matrix, which implies a porous medium (406, 586). Therefore, some early models included porous perivascular spaces and still predicted anterograde flow (587). However, the presence of a porous medium drastically reduces hydraulic conductivity (588). Particle tracking velocimetry experiments in surface perivascular spaces showed parabolic velocity profiles and predominantly smooth particle tracks, suggesting that these spaces might be empty (21, 278). Further analysis of the particle tracks showed that they were more consistent with ballistic transport suggestive of smooth motion within an open space, rather than the diffusive transport that might be expected within a porous medium (588). These conflicting findings might relate to histological processing artifacts, since surface perivascular spaces were much larger in live mice and shrink to the point of disappearance during tissue fixation (21, 282, 408, 412, 564, 589). The spatial dimensions of the perivascular space are most likely determined by the accompanying artery, and the loss of intravascular pressure during fixation causes the arteries to collapse, while fixation alone causes capillaries to shrink in diameter by 13% and penetrating vessels by 34% (590). Tissue shrinkage and chemical fixation plausibly causes cross linking of the sparse collagen fibrils and other elements of the extracellular matrix, resulting in the appearance of a porous and small perivascular space. Indeed, the fixation artifact of perivascular spaces has remained an active research topic since the late 19th and early 20th century (35). Consequently, 3-D models of the perivascular network should probably be informed by in vivo model systems and caution should be taken in interpreting modeling results from the architecture of fixed specimens.
11.3. Parenchymal Transport
The parenchymal transport models are perhaps the most complex to organize and discuss. This is primarily due to the varying interpretations of where and how transparenchymal fluid transport occurs. In 1865, the famous anatomist Wilhelm His proposed that perivascular spaces play the role of lymphatic vasculature in the brain (35). Advection-dominated clearance of brain metabolites from the brain parenchyma has long been suspected and has been reliably demonstrated over decades of research using different techniques, thus withstanding the test of time (4, 15, 63, 64, 591, 592). This kind of bulk flow of extracellular fluid was measured in white matter (183) and emerged as an established feature of parenchymal fluid transport in 2004 (435). Nonetheless, the nature of the driver behind this flow was never conclusively settled; some proposed that it was due to hydrostatic and osmotic pressure gradients (183), and others held that it resulted from secretion of extracellular fluid at the BBB (435). In contrast, seminal work by Charles Nicholson and others showed that fluid transport in gray matter was largely explicable by diffusion alone (183, 285). One of the main techniques used to evaluate this was real time iontophoresis, which entails measuring the recovery of a cell-impermeable cation [tetramethylammonium (TMA+)] upon its delivery into the extracellular space, and its subsequent transport toward an ion-selective microelectrode placed perhaps 100 µm away (285). The elapsed time for the TMA+ to arrive at the microelectrode and the amount that is recovered are used to compute the extracellular volume fraction [also known as alpha (α)] and the tortuosity [also known as lamba (λ)] (285). This technique formed the basis for modern understanding of solute transport in the parenchyma and was subsequently supplemented by imaging modalities like integrative optical imaging (285) and fluorescence recovery after photo-bleaching (283, 284). Although these techniques provide useful information on tracer transport in the parenchyma, their spatial resolution is restricted to only a few micrometer, thus limiting their interpretations regarding brain-wide transport.
In 2012, our group used in vivo imaging to show that CSF enters the brain via bulk flow along arterial perivascular spaces, thus replicating results from Rennels and Grady (4, 66). Moreover, we also showed that this process is regulated by expression of AQP4 water channels on astroglial cells (4, 22), thus presenting a previously unknown function for the highly polarized expression of these channels. Bulk flow clearance of solutes from the brain primarily occurred along perivascular spaces of large draining veins and was also dependent on AQP4 (4). Furthermore, we later found that this process is only active during sleep and anesthesia with certain compounds, which has been a common feature of all prior work evaluating bulk flow clearance (1, 591). Our analysis showed that the transport of amyloid-β was more prominent during sleep compared with wakefulness and was dependent on AQP4 (1, 4). These experimental findings provided a unifying explanation to processes that had previously seemed separate and unrelated, while placing astrocytes at center stage in the role of regulating solute clearance and parenchymal fluid transport in the brain. Although supporting a broad range of initial conclusions, little knowledge was garnered from these studies about the exact pathway followed by CSF from its entry in the periarterial spaces to its exit via the perivenous spaces (see sect. 8). This gap in knowledge has unfortunately led various research groups to adopt multiple arbitrary definitions of what exactly comprises parenchymal glymphatic transport. Modeling studies implement realistic geometries of the extracellular space but have not yet incorporated the wide regional heterogeneity of that geometry or the known differences between fluid transport in gray and white matter (424, 432–434, 593) (for further discussion on the specific geometries and their potential pitfalls, see sect. 7).
The “ground-truth” for the magnitudes of the extracellular space volume (α) and tortuosity (λ) is in all cases derived from real-time iontophoresis data (285). Although this technique is widely reproducible and yields results comparable to those obtained using radioisotopes, using fluorescence methods, and from apparent diffusion coefficients of water measured by diffusion-weighted MRI, there are important assumptions made in their computation (285). Essentially, the transport of TMA+ is assumed to abide by Fick’s laws, such that its transport should be mediated exclusively by diffusion that occurs in a homogenous and isotropic manner. However, if bulk flow was present in the extracellular space, the transport of TMA+ would rather be governed by Darcy’s law, which calculates the superficial velocity of the fluid by accounting for the pressure gradient driving the flow and the hydraulic conductivity of the pore size. Ray and colleagues (593) noticed that individual iontophoresis measurements vary widely in the range of 70–90% in a given experiment. Rather than concluding that this was due exclusively to inherent variability of the experimental technique, they reasoned that if a convective flow field were present between the two microelectrodes, the TMA+ concentration would vary depending on the direction of the flow. In such a scenario, if the TMA+ were diffusing in the same direction as the convective flow field, less of it would be available at the microelectrode, resulting in an artifactual overestimation of the volume (α). In contrast, if the TMA+ were diffusing against the direction of the convective flow field, the escaping solute would be returned to the microelectrode, thus resulting in greater accumulation and consequently a lower α measurement. Since it would be technically impossible to align the microelectrodes with the orientation of the flow field, the authors decided to test if a random sampling of flow directions could explain the high variability in the experimental measurements (593). Surprisingly, their findings fit exceptionally well with the observed spread of the iontophoresis data from Cserr et al. (594) and Kress et al. (15) and were compatible with a diffusive transport mechanism with an underlying parenchymal bulk flow velocity of 50 µm/min (593). This also suggests that the flow direction in individual segments of the parenchyma need not always be in the same direction. This novel approach highlights that greater efforts are needed to define the geometry of the extracellular space using complementary techniques. See, for example, MRI analysis of diffusion parameters in Refs. 525, 527.
The artery-to-vein segment is most commonly chosen for modeling of parenchymal transport (557). This segment is preferred for evaluating transport from periarterial spaces through the parenchyma and toward perivenous spaces. Jin and colleagues (269, 432, 595) used the cortical vascular geometry from nonhuman primates, which consists of a quasihexagonal configuration with a ∼2:1 ratio of arterioles to venules. This microvascular unit was further reduced into sixths and a computational domain consisting of two arterioles and one venule, which was justified by the symmetric nature of the domains and the need to lower computational cost (432). However, we note that there may be substantial interspecies variation in this organization. For example, the cortical microvascular unit is also quasihexagonal in mice, but the arterioles to venules ratio is reversed, with a value of 1:2 in a computational domain similar to that of Jin et al. (432). Mice have three times more ascending veins than penetrating arterioles, while in rats the ratio is 1.8–2.6:1. While capillary networks have not been incorporated into these models, it seems that rodents and humans have topologically equivalent capillaries, which suggests that this component can be scaled interchangeably when these ratios are included in the model (596). The overwhelming majority of models have employed a simple 1:1 arteriole to venule ratio, with the intervening tissue’s width being of a spacing similar to the mean diameter of the vessels (410, 434, 566). However, the distance between the vessels in these models is 200–300 µm (434, 566), which is supported by measurements in nonhuman primates, and exceeds the <200 µm average distances seen in mouse cortex, while the rest of the parameters governing the arterial pulsation are from mice (270). This highlights the importance of using literature values from the same species (ideally the same strain) in modeling studies if one is to retain the most realistic relationships possible.
Most parenchymal transport models have predicted that diffusion alone is most likely a sufficient driver for fluid transport in the extracellular space (557). There is also a general consensus that the hydrostatic and hydrodynamic pressure gradients between arteries and veins required to drive parenchymal bulk flow are implausibly high (>1 mmHg/mm) even at inflated extracellular space volumes (∼32%) (432, 434). The main problem arising from most models is that these spatial pressure gradients have never been experimentally measured. This is due in part to the technical limitations imposed by most commercial pressure transducer systems, which have a resolution of 1 mmHg that is inadequate to measure such small pressure gradients. Certainly, future technical development should aim to enable better measurements, but for the present this limitation presents an important caveat for claims derived from these models. While parenchymal bulk flow appears to be improbable in models, the majority of human MRI studies evaluating the transport of intrathecal gadolinium-based contrast agent have surmised that tracer data do not fit a purely diffusive transport mechanism (164, 597, 598). Computational models predict that the estimated apparent diffusion coefficient for intrathecal tracer exceeds by 5–26% that obtained from diffusion tensor imaging alone, suggesting a greater potential for solute transport in the gray matter than was previously expected (597). A separate study showed that a stochastic glymphatic flow model considering the arterial-to-venous transport of fluid between randomly placed vessels did not suffice to explain the observed tracer distribution (598). However, upon addition to the model of a “glymphatic velocity field” derived from the macroscopic traveling wave of the arterial pulsation through the skull, it matched the experimental data remarkably well (495, 598, 599). This latter point suggests that there might be superimposed hydrodynamic pressure gradients at the macroscopic scale in addition to the arteriovenous gradients at the microscopic scale.
In conclusion, while models can attempt to address important gaps in knowledge arising from the present lack of data or technical limitations, it is still crucial to appreciate their pitfalls. Rather than changing or removing parameters, efforts should be directed toward developing more comprehensive models with the ultimate goal of increasing the degree of complexity to mirror more closely the in vivo condition. Although reduced and simplified models certainly have their use, the field as a whole should move toward multiscale modeling aiming to encompass more of what is known of microscopic flows into the realm of macroscopic flow modeling (TABLE 4).
Table 4.
The good, the bad, and the ugly of modeling studies
| “Good” Modeling Studies | “Bad” Modeling Studies |
|---|---|
| • Use realistic geometries and dimensions collected from experimental datasets. • Account for interspecies and condition differences in physiological parameters and biomechanical properties of tissues. • Appreciate that their conclusions are limited by the assumptions included in the model.Titles state that they are modeling studies when they do not include experimental evidence. • Are primarily hypothesis testing, but acknowledge that they are unable to fully confirm or reject a hypothesis pertaining to a complex biological process and are therefore mainly supportive evidence.Generate hypotheses and provide constructive and insightful conclusions that can be experimentally tested. • Work toward building more complex and realistic models.Include sensitivity testing. |
• Use simplified geometries and dimensions not derived from experimental datasets. • Use parameter values from different species, ages, conditions (e.g., awake, sleep, anesthesia, etc.), and live/fixed tissue interchangeably. • Overstate the level of confidence about their conclusions and consider them equal to results from experimental approaches. • Include confirmatory biological conclusions in their study titles, without stating that they are modeling studies despite the absence of experimental evidence. • Draw conclusions about biological phenomena without acknowledging the inherent assumptions of the model. • Are believed sufficient to confirm or reject hypotheses about complex biological phenomena. • Do not include sensitivity testing. |
11.4. Mechanisms of Fluid Transport within the CNS
Evolution has optimized the transport of fluid and solute within living organisms over the course of billions of years. As life evolved from single cells to multicellular organisms these transport mechanisms had to similarly scale in complexity to assure the timely and low-cost delivery of nutrients from the environment to all organismal cells. Larger species evolved cardiovascular systems consisting of an intricate network of branching pipes to achieve this goal. In biological systems, fluid transport can largely be driven by diffusion, advection (bulk flow), or a combination of these, termed dispersion. The nomenclature for these processes varies in the literature so when referring to fluid flow, the term used here is advection. Convection is synonymous with advection and is sometimes misused in the literature to denote both advective and diffusive transport.
The vascular system has harnessed the benefits of both advection and diffusion to deliver its contents. The flow of blood within arteries, capillaries, and veins driven by the heartbeat is predominantly advective in nature. This process aids the delivery of several liters of blood across meter-long distances over the course of a few minutes. As species evolved and became larger, so do the length and caliber of the flow channels and the size of the pump (i.e., heart). Employing advective transport has significantly reduced the time needed to distribute blood throughout the body and ensures a steady supply of life-sustaining compounds. However, once the vasculature reaches the capillary network, it has branched several times, causing the flow to be reduced several orders of magnitude from meters per second in the ascending aorta to millimeters per second in the smallest capillaries. This unique branching pattern serves two large advantages: 1) it forms dense capillary networks that ensure that any cell is not too far from the nearest blood vessel reducing the time needed to deliver nutrients broadly throughout the body; and 2) the reduced flow speed increases the transit time of blood allowing for enough time to exchange molecules between the blood and the tissue. An example of this is the loading and unloading of O2 and CO2 to and from the red blood cell to the neighboring tissue outside the capillary (278). Therefore, while transport of hemoglobin inside the blood vessel is predominantly advective, transport across the capillary wall to the target cell is predominantly diffusive; highlighting that, at short distances, diffusion can be equally effective to advective transport.
The nervous and lymphatic systems mimic several strategies of the vascular system. In the periphery, blood vessels and nerves travel together in neurovascular bundles to reach their corresponding downstream targets, branching at exactly the same points and following parallel routes (600). While this peculiar organization might not hold true in the CNS, it seems logical to assume that CNS fluid flow might also share features with the other fluid systems in the body. The glymphatic pathway similarly is composed of large bore flow channels, i.e., perivascular spaces, that contain advective CSF transport that possess flow speeds between 9.4 and 28 µm/s (21). As flow within these spaces branches, the volumetric flow rate (Qin, FIGURE 23A) is divided between each daughter branch, typically slowing down at each bifurcation due to attenuation of the systolic pressure wave. At the level of the smallest daughter branch, the flow is greatly reduced down to speeds on the order of nm/s (434). However, if all the daughter branches reconnect downstream, the slow flows begin to combine together resulting in a final flow rate (Qout) that would roughly equal Qin. Assuming this is a closed system without leaks, the net transport through the system is primarily explained by the process that predominates over the majority of the pathway (i.e., advection). However, if one were to measure flow speeds at one segment at the level of the large arteries, we would obtain flow speeds compatible with bulk fluid flow (µm/s). Conversely, if one measures flow speeds at the level of the capillary these would be so slow (nm/s) that diffusive, isotropic transport might dominate over the flow. In neither situation, would the experiment provide true insight about net transport through the entirety of the system (Qin or Qout). Transport kinetics within 0.12 µm3 of neuropil (434) can be primarily diffusive in nature, while on a global scale, clearance from the parenchyma is largely compatible with advection (Qout). This feature supports why experiments consisting of injecting labeled tracers into the parenchyma and measuring their elimination rate from the brain (large length scales) arrive at conclusions compatible with advective transport, while studies that evaluate local spatial distribution (small length scales) infer a primarily diffusive transport. The first method most likely provides a much better estimate of the biologically relevant process of brain clearance. However, technical advances in MR imaging and optimal mass transport modeling have improved evaluations of the contributions of advection-diffusion within different brain regions (279, 601, 602). Future work will continue to determine to what extent the branching network of intra-axial flow is a closed system.
FIGURE 23.

Reframing the contributions of advection and diffusion to glymphatic clearance. A: 2-dimensional representation of the glymphatic pathway, composed of a brain-wide network of perivascular spaces that branch along the cerebral vasculature. Cerebrospinal fluid (CSF) flows into the system (Qin) through the large surface perivascular spaces of the cerebral arteries flowing at several microns per second (µm/s) and transports solutes like oxygen and carbon dioxide by a primarily advective process. As the flow pathways bifurcate and divide into daughter branches, glymphatic fluid flow slows down perhaps up to several nanometers per second (nm/s) near the capillaries in the tissue where diffusion would dominate transport of most solutes. The flow continues along the closed system toward the outflow routes and coalesces increasing in speed. The total outflow rate (Qout) might not be exclusively composed of fluid coming from perivenous spaces but might also include white matter tracts and subependymal pathways. The sum of all these outflow pathways should theoretically equal the inflow rate (Qin = Qout). B: ions and small molecules (e.g., H2O: 18 Da; Na: 23 Da; O2: 32 Da; and K: 39 Da) can be transported quickly and efficiently across short distances via diffusion, while larger molecules (e.g., lactate: 90 Da, and glucose: 180 Da), peptides and protein aggregates (e.g., Aβ1-40: 4.3 kDa; Aβ1-42: 4.5 kDa; albumin: 66.5 kDa, Aβ oligomers: 100-200 kDa; and tau aggregates: 242 kDa) might rely more so on the advective modes of transport for clearance. C: glymphatic function is dependent on the interplay between effective advective transport via perivascular spaces and adequate access to the extracellular space. Dysfunction in glymphatic clearance could be due to variations in either or both of these processes. RBCs, red blood cells; AQP4, aquaporin 4.
In recent years, a lively debate in the field has focused on determining if solute clearance from the brain is dependent on advection or diffusion (412). While understanding the fluid dynamics is fruitful, it is important to note that practically no physiological fluid system relies exclusively on one or the other but rather a finely tuned balance between both mechanisms. Therefore, perturbations to either advective or diffusive transport will impact the effectiveness of solute clearance. All available studies evaluating the relative contribution of advection-diffusion have consisted of a point estimate limited to a particular point in time and space and used different measurement techniques that possess unique methodological limitations. This inferential approach most likely results in an oversimplification of global intra-axial fluid flow properties across brain regions and brain states. Evidence from our group and others has shown that perivascular flow is highly dependent on arterial pulsations (21), blood pressure (21, 405), and arterial diameter (469), among others, while the extracellular space (ECS) is a highly dynamic environment across brain regions (285), levels of arousal (1), age (285), and disease (285, 422). Therefore, it would seem counterintuitive to make definitive extrapolations about fluid transport from evaluating only one of these conditions. Rather we propose reframing the debate toward embracing and understanding this added complexity and heterogeneity.
Global reductions in solute clearance from the brain are believed to be an important cause of neurological diseases. However, if a lesson is taken from renal physiology, reductions in the glomerular filtration rate in the kidney can be caused by several things including decreases in renal blood flow or changes to Bowman’s capsule that lower the filtration fraction. Glymphatic solute clearance can also be reduced by similar mechanisms, mainly the slowing of perivascular fluid flow and restrictions in the exchange between the incoming CSF and the extracellular fluid. Therefore, fluctuations in CSF inflow speeds, which are an advective mode of transport or restricted access to the extracellular space that can be governed by diffusive transport, can both significantly affect clearance, either separately or synergistically. In a similar sense, the solute of interest will also determine which mechanism of transport is likely to be the most important for its clearance (FIGURE 23B). Small molecules and ions (e.g., H2O, Na, O2, and K) can be transported quickly and efficiently across short distances via diffusion, while larger molecules (e.g., lactate and glucose), peptides, and protein aggregates (e.g., Aβ, albumin, Aβ oligomers, and tau aggregates) might rely more on the advective modes of transport (FIGURE 23B). It is therefore helpful to use a similar framework to think about glymphatic dysfunction, while contributions from both modes of transport are required for clearance, modulation in one could potentially counteract a subtle deficit in the other (FIGURE 23C). Glymphatic disruption might be the result of a pathological decrease in one or the other, or a combination of both. For example, Aqp4-/- animals have larger ECS volumes with increased diffusivity in the extracellular space compared with wild-type animals (284). A similar effect can be seen in wild-type animals that are treated with an AQP4-specific inhibitor, TGN-020 (603, 604). This should cause an expected increase in flow through the glymphatic pathway, as is seen in states such as sleep (1). Counterintuitively, glymphatic function is drastically reduced in both the global AQP4 knockout animals (4) and wild-type mice treated with TGN-020 (532). Indicating that perhaps both effective perivascular flow and access to the extracellular space is needed for optimal function. Potential disruptors of perivascular flow are arteriosclerosis, arterial hypertension, and occlusion of the space by red blood cells, fibrin/fibronectin deposits, or other aggregates. Pathological changes that might restrict access to the extracellular space are loss of AQP4 polarization, reactive astrogliosis, β-amyloid deposition, changes in the ECM properties, or a loss in capillary density. Together, variations in these two separate processes could contribute to disrupting glymphatic function.
More importantly, the relative contributions of both advection and diffusion are likely to be highly dynamic across time of day, brain state, or development. This latter point further supports that inferring global transport from one cross-sectional measurement in time across a condition is less productive than understanding how both mechanisms cooperate to drive effective brain clearance (see TABLE 3).
11.5. Outstanding Questions
In vivo experiments have shown that perivascular space morphology changes significantly during tissue processing and that these spatial distortions strongly influence the modeling results. Therefore, we pose the question; what is the geometry of the perivascular space network (in vivo) in its entirety, spanning from leptomeningeal arteries to draining veins, and how does it connect to parenchymal transport routes?
Much is known about pulse wave propagation in the arterial tree, but how can this knowledge be further implemented into perivascular flow models? Are pulsations of the arterial wall the only contributor to flow or are pressure waves transmitted into the intracranial space from the Circle of Willis responsible for rigid motions of the arterial tree that could drive flow?
Attempts to generate 3-D models of the extracellular space by reconstructing tissue sections have been restricted to micrometer-scaled volumes. Despite being a significant technical and computational undertaking, will larger volume reconstructions shed further insight into macroscale extracellular fluid transport (i.e., gray-white matter transition zones)?
Are there unknown or unrecognized pressure gradients within the perivascular space and the intracranial compartment that could drive flow? Will the development of more precise measurement techniques be able to detect gradients on the order of 0.02 mmHg?
What experimental evidence is needed for microscale models to be incorporated into the much more establish macroscale models of CSF flow?
11.6. Interim Section Summary
There are two main models of perivascular transport: those consisting of anterograde flow into the brain within the perivascular space and those evaluating retrograde flow out of the brain within basement membranes. In both cases, the general consensus is that arterial pulsations alone are not sufficient to drive net flow in any direction, thus contradicting experimental results that show bulk flow is in the direction of blood flow. Studies have proposed that an as yet unknown pressure gradient or the presence of a valve-like mechanism could explain these opposing results.
Models evaluating parenchymal fluid transport have relied on idealized geometries of the extracellular space (e.g., Voronoi diagrams) or have endeavored to simulate flow through a reduced subvolume reconstructed from only 180 µm3 of rat CA1 hippocampus using electron microscopy of fixed tissue. Such models have concluded that diffusive transport predominates in the extracellular space and that the pressure gradients required to drive bulk flow are implausibly high. This caveat holds even in the context of the inflated extracellular space volume fraction (∼32%) that is believed to underlie the enhanced solute clearance observed during the sleep state.
Moreover, there is large heterogeneity in the parameters and geometries used in all existing models, which is likely responsible for certain disagreements between experimental and model results. Careful attention should be given to the morphology of the perivascular space, the characteristics of the arterial pulsation (shape, wave speed, and wavelength), and the outflow boundary conditions (intracranial compliance and CSF outflow resistance).
There is a dire need for better modeling to further our understanding processes that are currently limited by our inability to measure certain physiological parameters. However, it is critical here to remain cognizant of the potential pitfalls of insufficiently supported models. Future models should aim to adhere better to the true anatomy and physiology of the system to emulate properly the biological circumstances. Researchers should be aware that models can only present supportive evidence but are unable to decisively accept or reject a hypothesis without accompanying experimental validation.
12. EGRESS OF CSF AND INTRACRANIAL SOLUTES TO THE PERIPHERAL CIRCULATION
12.1. Introduction
The CSF surrounding the human brain is replenished at a rate of ∼600 mL/day (see sect. 5 for CSF production). Normally, the efflux of CSF from the CNS closely matches the formation of new CSF; any excess accumulation of CSF or other fluids can significantly increase intracranial pressure, since the inflexible skull surrounding the brain leaves little room for expansion. It is therefore unsurprising that there are abundant pathways for draining intracranial fluid from the CNS to the periphery, although elucidating their precise nature has proven difficult. Several studies have shown that blockage of intracranial fluid drainage pathways impairs fluid transport along the glymphatic system. Thus net fluid clearance from the brain entails a functional glymphatic system to clear the parenchyma, in conjunction with effective drainage pathways that remove intracranial fluid and metabolic waste products to the peripheral circulatory systems. In this section, we shall provide anatomical details about the known egress paths draining CSF and extracellular fluid to the bloodstream and cervical lymphatics and shed some light on the volumetric distribution of CSF egress along these different egress paths. Finally, we shall discuss the dynamic changes in CSF egress that occur within the normal range of physiology, brain state, and circadian rhythms. For more details on CSF egress and its history, we refer to an excellent review (58), and for further details on dural lymphatics including their important role in the brain’s immune response we refer the reader to other valuable reviews (185, 605).
12.2. The Drainage of Cerebrospinal Fluid through Blood or Lymph
When evaluating the fluid efflux pathways from CNS, it is important to consider the different anatomical pathways that extracellular fluid and its dissolved contents traverse as they travel from deep within the CNS parenchyma to reach peripheral lymphatics or the bloodstream. In gross terms, we divide the path into two components: 1) intra-axial efflux and 2) extra-axial CSF/extracellular fluid egress (FIGURE 24 and BOX 1). The intra-axial path of extracellular fluid efflux includes perivenous efflux, efflux in the spaces between fiber tracts in white matter, and subependymal flow beneath the ependymal membrane lining the cerebral ventricles (see sect. 6 for intra-axial efflux) (4, 64, 288, 289, 606). These efflux pathways together constitute the routes whereby brain extracellular fluid exits the brain. The extra-axial CSF/extracellular fluid egress sites, which we shall describe in this section, include perineuronal sheets along cranial and spinal nerves (607, 608), dural lymphatics (23, 68), arachnoid villi/granulations (609), dural spaces along sinuses (610), and the adventitia of major cerebral vessels entering or leaving the skull through the cranial foramina (611). These drainage pathways, whereby intracranial fluid finds its way to the extracranial circulation, are thus important for draining the subarachnoid space CSF and likewise critical for removing from the intracranial compartment solutes that have been cleared from the neuropil by the glymphatic system. Certain solutes within the extracellular space, notably amyloid-β, are cleared in part by transport across the BBB of CNS capillaries and venules or by choroid plexus epithelium cells (75, 612, 613).
FIGURE 24.
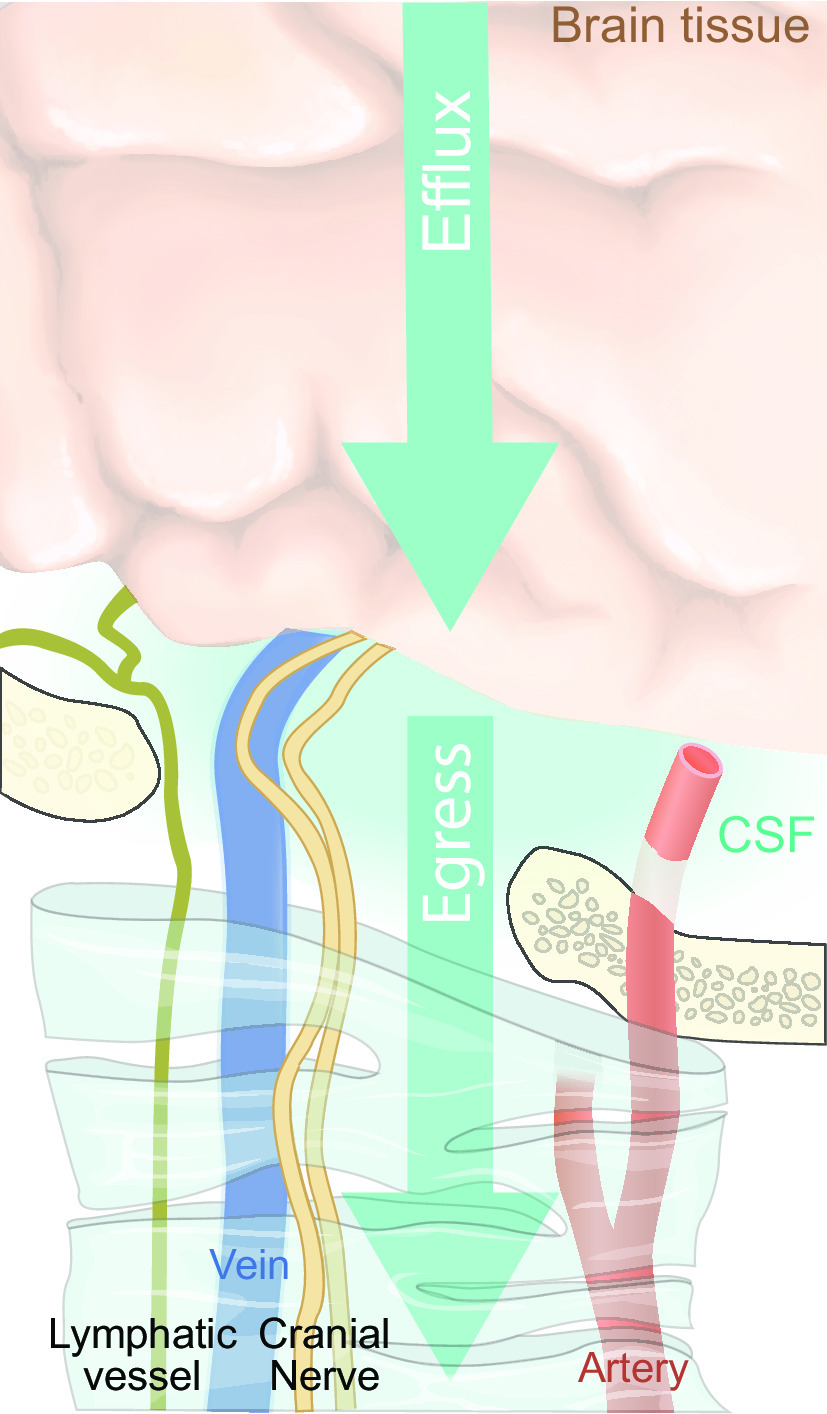
Extracellular fluid efflux and intracranial fluid egress. To distinguish between outflow of fluid from the brain parenchyma and outflow from the intracranial and spinal compartments to the peripheral circulatory systems, we have defined the following terms that we shall use categorically to distinguish between these two serially connected fluid outflow systems. Efflux: glymphatic efflux, also known as brain clearance, is the process by which extracellular fluid and its solutes in intra-axial compartments of the brain parenchyma transfers to cerebrospinal fluid (CSF) compartments or directly to egress sites. For example, glymphatic efflux includes the outflow of waste proteins in the brain extracellular space along major cerebral draining veins, possibly connecting with dural lymphatics and dural parasagittal spaces. Egress: this refers to the transport of CSF, extracellular fluid, solutes and cells from the central nervous system compartment to the periphery, e.g., the transport of CSF tracers from the subarachnoid space through dural lymphatics to the cervical lymphatic system.
As can be inferred from the above summary, the outflow of intracranial fluid and its constituent solutes from the CNS are a complex and multifaceted process. Historically, the arachnoid granulations and the bloodstream of the superior sagittal sinus have been considered the main egress site for CSF, while cervical lymphatics were thought to be an accessory pathway (see sect. 2). However, quantitative data suggest that equal proportions of CSF egress via the bloodstream and the cervical lymphatics (614). Moreover, there is a lack of observations in vivo showing that arachnoid granulations in fact drain CSF into blood, which calls into question their importance in this function (58). In fact, arachnoid granulations may play little to no role in CSF egress under physiological conditions, as suggested by observations that rodents and human children have patent CSF egress, despite their absence of arachnoid granulations and the sparseness of their arachnoid villi (58, 615, 616). Nonetheless, it is too early to discount completely the arachnoid granulations as an egress path, since some fraction of CSF seems to egress directly to the bloodstream (614, 617, 618). Other, still unidentified egress sites may support egress of CSF to the bloodstream. Furthermore, the absolute volume of fluid leaving the CNS through any particular outflow path seems not to be static but instead depends on dynamic physiological parameters including brain state, ICP, and body position, as well as pathological changes (e.g., lymphatic vessel degeneration) and aging (55, 185, 523, 618, 619).
12.3. Perineuronal Egress
To evaluate properly the communication between subarachnoid space and peri-neuronal egress paths, it is important to consider first the potential pathways created by the organization of the tissue (BOX 2). Peripheral nerves are bundles of axon fascicles ensheathed by the epineurium (622). Under the epineurium, a membrane known as the perineurium enwraps individual fascicles of axons (FIGURE 25). Finally, each axon is supported within the fascicles by a connective tissue called the endoneurium, which defines a space filled with endoneurial fluid bathing the axons. Egress of tracers delivered to the subarachnoid space has been found in each of these different compartments of peripheral nerves. However, the volume fractions of egress along the epineurium, subepineurial space, and within the endoneurial fluid or intra-axonally is currently unknown (FIGURE 25) (616, 629, 632–634). The accumulation of subarachnoid space tracers around cranial nerves is similar across many different species, indicating this to be a major outflow route for CSF entering the cervical lymphatics (616, 626, 627, 629, 632, 634, 635). Indeed, perineuronal drainage of a tracer injected into the subarachnoid space has been described for cranial nerves I, II, V, VII, IX, X, and XII (616, 626, 635, 636). Outflow of CSF along the olfactory nerves running through the cribriform plate seems to be volumetrically the largest perineuronal outflow path in man and in other species (618, 637–639). However, egress of CSF along other cranial nerves also contributes to the net outflow to the cervical lymphatics (635). Furthermore, specialized sensory organs such as the eye and inner ear are directly connected to perineuronal fluid pathways, and CSF might serve as a source for perilymph and endolymph in the inner ear, although the connection might be primarily through the cochlear aqueduct and not perineuronal (626, 633, 640). In the case of the optic nerve, there is a net flux of fluid from the retina and corpus vitreum into the subarachnoid space, thus suggesting that perineuronal fluid flow along cranial nerves is not necessarily unidirectional throughout the entire course of the nerve and that drainage of fluid from the perineuronal space toward the cervical lymphatics can occur at specific sites along the cranial nerve (626, 633, 634). The ultrastructure of these sites of communication between the perineuronal outflow pathways and the cervical lymphatics is currently unknown.
BOX 2. Cranial nerves and their fluid spaces
The epineurial membrane is a connective tissue membrane of mesodermal origin that is considered the functional equivalent of the dura mater of the brain and spinal cord, and, as such, does not possess a barrier function (FIGURE 25, bottom left inset) (620, 621). The perineurium forms a membrane of squamous epithelium of ectodermal origin, which tracks the nerve roots as they exit the spinal cord, appearing to be a fusion of the pia mater and arachnoid mater that enwraps the entire bundle of fascicles in each spinal nerve, as well as each individual fascicle. The perineurial membrane is a barrier membrane composed of tight junctions between epithelial cells, such that peripheral nerves are protected by a blood-nerve barrier functionally similar to the BBB, endowed with specialized transporters for ions, glucose, and amino acids (622, 623). Within the endoneurium compartment, the axons are bathed in an endoneurial fluid with a composition likely similar to that of CSF, thus with a low protein content but an ionic composition similar to that of plasma, though hypertonic concentrations have also been reported (624, 625). There is considerable evidence for the existence of a direct and open communication between the subarachnoid space and the endoneurial fluid compartment of peripheral nerves, and CSF is likely to be a source of endoneurial fluid (624, 626–628). Thus CSF flow from the subarachnoid space (and probably likewise from extracellular fluid in the CNS) into the endoneurium of peripheral nerves is a potential egress pathway. Conversely, tracers injected into the subarachnoid space have also been seen to occupy a space lying between the perineurium and epineurium (subepineurial space) of the extracranial portion of the olfactory nerves (629, 630). This subepineurial space has been reported to communicate openly with the subarachnoid space, although the ultrastructural details of such a connection have not been described (629). As the perineuronal membrane is a selectively permeable continuation of the leptomeninges (which contains the arachnoid barrier layer), it is clear that some sort of communication between these compartments must exist if CSF is to reach the subepineurial space of the peripheral nerves. However, the anatomical location and histological and molecular organization of this communication remain unknown. Lymphatic vessels are embedded into the epineurium of the proximal parts of cranial nerves both intra- and extracranially, sometimes in very close proximity to the perineuronal membrane (68, 631). Therefore, the available evidence indicates that the epineurium is a potentially important route for egress of CSF that is drained along the perineuronal pathway.
FIGURE 25.
Cerebrospinal fluid (CSF) egress sites in the cranium. CSF can drain from the intracranial subarachnoid space via many different routes. In general, CSF egress proceeds either to the cervical lymphatic system or directly into the bloodstream. Drainage to the cervical lymphatic system can occur through several different structures. Perineuronal drainage, especially along the olfactory nerves, seems to be one of the most important egress pathways. CSF egress along the perineuronal routes can occur within the nerve axons, in the endoneurial fluid or in a subepineurial space, or even in the epineurial connective tissue. The perineurium is an epithelial barrier membrane sealed by tight junctions. Lymphatics associated with the cranial nerves can also direct CSF out of the cranium, as can the dural lymphatics. Present in dorsal and basal dura mater, the dural lymphatics take up macromolecules from the CSF. Morphological characteristics of the lymphatics in the basal dura mater hint that these vessels might be better conduits for lymphatic drainage than are the dorsal dural lymphatics. Nonvessel like structures designated as the parasagittal dura spaces that drain CSF have also been identified in dura mater. These spaces can convey CSF to the lymphatics in dura mater, and also to the arachnoid granulations. The only structures known to drain CSF directly to blood are the arachnoid villi/granulations, which are protrusions of the arachnoidea through slits in the dura mater. CSF egress along this pathway may occur by transcellular and paracellular routes.
12.3.1. Olfactory nerve fluid egress.
The magnitude of egress of CSF along the olfactory nerve exceeds that of the other cranial nerves (608, 618, 637). The pathway by which the olfactory nerves leave the cranium is unique; whereas the other cranial nerves form bundles leaving the skull through a single macroscopic foramen, the olfactory nerves transverse the skull through ∼20 bilateral foramina in the human cribriform plate, and, as such, do not form an actual nerve trunk but instead an array of olfactory filaments (641). The cribriform plate is highly perforated, with foramina comprising some 20% of its total surface area in the mouse (639). CSF from the subarachnoid space drains through the foramina of the cribriform plate and into the nasal cavity, eventually passing into the cervical lymphatics. This pathway was first identified more than 150 yr ago by Quincke (1872), Schwalbe (1869), and Key and Retzius (1876) (607, 618, 629). The pathway by which CSF drains along the olfactory nerve to the lymphatic system includes at least three serially connected fluid conduits, namely the subarachnoid space, the perineuronal space, and the nasal lymphatic vessels. The flow pathway linking these three compartments is still not fully understood at an ultrastructural level. Movement of CSF from the subarachnoid space around the olfactory bulb into the perineuronal spaces of the olfactory nerves seems to occur in cul-de-sacs of the subarachnoid space surrounding the olfactory fila in the cribriform plate (630, 642). In the cribriform plate, the dura mater of the cranium fuses with the bone periosteum to create a dense connective tissue that binds the nerves to the bone. Intrathecally delivered CSF tracers crop up in this connective tissue, suggesting that CSF can egress through the arachnoid barrier membrane in the vicinity of the subarachnoid space evagination (630, 642). In fact, the tight junction protein claudin-11, which is normally present in the arachnoid barrier membrane, is absent near the cribriform plate on the basal side of the olfactory bulb (643). There are some reports of the presence of subarachnoid space prolongations continuing along the path of the olfactory nerve through the cribriform plate (618, 644). However, neither light nor electron microscopy have revealed an actual continuous and open space between the subarachnoid space and perineuronal fluid compartments of the olfactory nerve in the nasal cavity (630). The nature of the communication between perineuronal fluid of the olfactory nerve and the nasal lymphatics also remains somewhat controversial, with evidence supporting either a direct flow into lymphatic vessels fused to the epineurium of the olfactory nerve, or CSF flow into the nasal submucosa, from whence it is taken up by lymphatics (629, 630, 634, 636, 638, 642). Many independent studies have shown that tracers injected into CSF compartments can be detected in the nasal submucosa, giving compelling evidence that CSF can leak from the perineuronal spaces into the mucosa (629, 630, 642). This claim is also supported by the histological finding that the connective tissue membrane of the epineurium has few or no tight junctions (642). Other tracer studies, however, have shown that the lymphatics of the nasal submucosa might not drain to the deep cervical lymph nodes, thus not supporting that CSF egress occurs through nasal submucosa (23). Instead, there may be a direct flow pathway between the perineuronal space and cervical lymphatic, thus permitting more efficient CSF egress. Indeed, lymphatic vessels have been observed to be fused to the olfactory nerve epineurium, thus presenting a channel for draining CSF tracers directly from the perineuronal spaces, bypassing the nasal submucosa (634). The existence of this direct flow pathway is also corroborated by recent findings in humans, where intrathecally delivered MRI tracer was absent from the nasal mucosa, but in approximately one half of patients was detected in tubular structures around the proximal extracranial portion of the olfactory nerves (645). Finally, dural lymphatics present another potential outflow path into the cervical lymphatics through the cribriform plate. Lymphatics do exist in the dura mater around the olfactory bulb, and they traverse the cribriform plate (68, 639). However, there is only minor uptake of CSF tracer into the dural lymphatic vessels compared with the main pathway along the perineuronal spaces (616). The existence of the perineuronal CSF drainage route along the olfactory nerve in human has been shown 1) in clinical studies finding egress of an intravenously delivered, BBB-permeable positron emission tomography (PET) tracer into the nasal turbinates; 2) by histological postmortem studies revealing congested blood in perineuronal spaces around the olfactory nerves within the nasal cavity, as well as in the nasal mucosa; and 3) by postmortem tracer studies (637, 638, 646). In summary, the olfactory nerve constitutes a substantial CSF drainage pathway that is conserved across species. The exact ultrastructural details of the flow pathway remain controversial, and detailed examinations of the egress path have hitherto been performed ex vivo and are thus likely to be confounded by various artifacts. Nonetheless, the olfactory nerve CSF egress pathway is certainly the largest contributor to CSF drainage among the cervical lymphatics.
12.3.2. Fluid egress along other cranial nerves.
Although the olfactory nerve supports quantitatively the largest CSF egress among the cranial nerves, almost every cranial nerve likely makes some contribution. Thus perineuronal drainage of tracer injected into the subarachnoid space has also been described for cranial nerves II, V, VII, VIII, IX, X, and XII (616, 626, 635, 636, 647). In the case of the optic nerve (CNII), intracisternally injected CSF tracer was detected close to the junction where the nerve enters the orbit, both in the subarachnoid space around the optic nerve, as well as in the surrounding dura. This corroborates the observations from the olfactory nerve that tracers from the subarachnoid space enter a dural space in close proximity to the foramina of the skull, suggesting that the arachnoid barrier layer is locally leaky, thus permitting movement of fluid between the subarachnoid space and dura mater (636). In sheep injected intracisternally with CSF tracer, there was no evidence for communication between the optic nerve and lymphatic vessels in the orbit (636). In fact, a recent study suggests that the site of egress along the optic nerve lies intracranially, anterior to the optic chiasm, and that the optic nerve primarily drains fluid retrogradely from the retina rather than CSF from the subarachnoid space (633). The fluid transport within the optic nerve has been seen to follow perivascular routes, as well as along the axons themselves and within the endoneurium (632, 633). The egress of tracer from the optic nerve to the peripheral circulation is most likely obtained through lymphatic vessels in the overlying dura mater anterior to the optic chiasm (68, 633, 635). However, horseradish peroxidase injected intrathecally in rabbits has also been noted to pass into veins within the subarachnoid space of the optic nerve, both by intercellular and intracellular mechanisms (632).
Drainage along the vestibulocochlear nerve (CNVIII) follows a similar pattern as for the olfactory and optic nerves, such that CSF tracers flow in a space between the epineurium and perineurium, as well as beneath the perineurium surrounding individual axons (i.e., in the endoneurial fluid) (626). The outflow of fluid along the vestibulocochlear nerve seems to be in direct contact with the perilymph of the scala tympani (616, 626). In one study where horseradish peroxidase was injected intrathecally in rabbits, the tracer was found to drain to lymphatics in the modiolus (the bony core of cochlea) (626). Another study with intrathecal injection of India ink did not indicate any flow to lymphatics of the inner ear (616).
The specifics of CSF egress along other cranial nerves are known in considerably less detail. However, a general description of the path seems to be that the nerves are tracked to a certain extent by an extension of the subarachnoid space along its intracranial course, which ends in a cul-de-sac. Dural lymphatics are present in the surrounding dura mater of most cranial nerves, extending along their intracranial course and thus providing a potential intracranial egress site (68, 631). However, it has also been observed that, despite abundant perineuronal localization of CSF tracers in the intracranial course of a cranial nerve, little or no CSF tracer is seen inside the lymphatic vessels of the surrounding dura mater (616, 635). Thus it remains an open question whether egress sites along cranial nerves other than the olfactory and optic nerves are generally connected to intracranial dural lymphatics or only after the nerves exit the skull.
12.4. Dural Lymphatics
The presence of lymphatics of dura mater has been known for centuries but has only recently been described in detail through the use of the lymphatic-specific antibodies LYVE1 and PROX1 to map out the anatomical distribution of lymphatics in the cranial dura mater, which importantly demonstrates a role in brain CSF clearance (23, 68, 69, 611, 616, 631, 648, 649). The dural lymphatic vessels are located in close proximity to the large vessels of the dura mater, namely those around the superior sagittal sinus, sinus of confluences, and the transverse sinuses, as well as along the branches of the middle meningeal artery in the dorsal dura mater and along the petrosquamosal and sigmoid sinus in the basal dura mater (FIGURE 25) (23, 68, 648). Morphologically, the lymphatic vessels in the dorsal dura mater are of smaller diameter and often discontinuous compared with the lymphatic vessels in the basal dura mater along the petrosquamosal and sigmoid sinus (648). These are of generally larger caliber and harbor valves and show other morphological features making them more likely to serve as efficient drainage pathways (648). Thus there is an ongoing discussion of whether dural lymphatics are important egress sites relative to other egress pathways, and whether lymphatic vessels of basal dura mater are more important CSF drainage pathways than those in the dorsal dura mater. Concerning the latter controversy, independent studies have reported that both dorsal and basal dural lymphatic vessels are able to take up intrathecally delivered tracers (23, 68, 648). However, several research groups have reported uptake exclusively in the basal lymphatics (68, 635, 648). Thus morphological cues and evidence from the preponderance of tracer studies indicate a more important role for the basal dural lymphatics in CSF drainage. It has been postulated that the dorsal dural lymphatics could merely be draining the dural extracellular space, and studies in nonhuman primates and humans have shown that dorsal dural lymphatics running along the superior sagittal sinus take up extravasated MRI tracer delivered intravenously, thus indicating this to be at least in part a role of the dorsal lymphatic vasculature (650). However, other compelling lines of evidence indicate an important role of the dorsal dural lymphatics in brain clearance (70). Following injection of Visudyne into the CSF and its subsequent photoconversion into a compound with specific toxicity for the lymphatic endothelium, there occurs an ablation of the dorsal dural lymphatics. Extirpating the lymphatics of the dorsal dura mater by this procedure leads to decreased drainage of CSF to cervical lymph nodes (70). Furthermore, the ablation also leads to decreased CSF inflow into the brain parenchyma, suggesting that the glymphatic system is inhibited when dural lymphatics are ablated (70). That study thus shows an important role of dorsal dural lymphatics relative to basal lymphatics and also reveals that dural lymphatics are a substantial contributor to CSF egress and brain clearance overall. Whether the dorsal and basal dural lymphatics actually represent a continuation of the same lymphatic vascular system remains to be elucidated. Also, the precise site and ultrastructure of the drainage pathway that leads CSF and brain extracellular fluid from the CNS, through the impermeable arachnoid barrier, and into the dural lymphatics have not yet been identified. Studies with intrathecally delivered tracer have shown the flow to first enter dural lymphatic vessels lying over the olfactory bulb and sinus transversus in dorsal dura mater, and in the basal dura mater in close proximity to skull foramina, indicating that the arachnoid barrier layer might be permeable in these areas (68, 71, 648). Another important question for the future is why there is glymphatic inhibition after dural lymphatics ablation. Glymphatic efflux has been found to occur around the transverse sinus, straight sinus, and inferior sagittal sinus, and glymphatic perivenous efflux pathway might be directly linked to dural lymphatic vessels, which are present at some of these sites (4, 23, 56, 68, 436, 648). Fluid spaces within the dura (see sect. 12.6), which have been shown in humans to drain CSF borne tracers, could be the reservoirs or sinks for glymphatic efflux, from whence dural lymphatics drain CSF and brain extracellular fluid, although this conjecture has yet to be experimentally proven (84). Lastly, dural lymphatics are also seen in the dura mater lining the intracranial portions of many cranial nerves, which could thus also contribute to what is collectively designated as perineuronal CSF drainage (68, 631, 635).
12.5. Arachnoid Villi/Granulations
The arachnoid villi and granulations are invaginations of the arachnoid membrane passing through slits in the dura mater connective tissue, which protrude into the venous sinuses or the lateral lacunae of the dura mater (651). The distinction between arachnoid villi and granulations depends on their size; the arachnoid granulations are larger and can sometimes be seen by the naked eye. The arachnoid villi and granulations are especially prominent at the superior sagittal sinus but are also seen at the transverse sinuses, confluence of sinuses, and cavernous sinus (609). The ultrastructure of the arachnoid villi/granulations has been outlined in many species, including humans (FIGURE 25). These structures consist of a central core in open communication with the subarachnoid space, in which there is a network of arachnoid cells, fibroblasts, and connective tissue (610). Around the core, the arachnoid barrier membrane lies as a continuation of the arachnoid barrier membrane of the subarachnoid space. In contact with this membrane lies a fibrous capsule formed by dural fibroblasts and connective tissue as well as endothelial cells from the venous sinus wall (610). In humans, the apical part of the granulation forms a cap cell layer, which is devoid of the fibrous capsule and endothelial lining, whereby the arachnoid barrier membrane is in direct contact both with CSF and blood (652). In primates and canines, the arachnoid villi/granulations are covered by endothelium in their entirety. The endothelial and arachnoid layers of the villi and granulations are both linked by tight junctions (610). Drainage through the arachnoid villi/granulations has been suggested to occur along two distinct paths. The first path consists of transcellular CSF transport across the membrane by transcytosis. Morphological features consistent with transcytosis have been observed in this structure, as indicated by the presence of numerous vacuoles in the endothelial cells (52, 653). The other drainage path is paracellular and is believed to egress through extracellular cisternal spaces (654). Tracers injected into the CSF can accumulate within the arachnoid villi (616), but since any tracers proceeding to enter the bloodstream would not be revealed by histological methods, a quantitative measure of egress through arachnoid villi/granulations is still lacking. Ex vivo and in vitro studies have revealed that the arachnoid granulations favor flow in the physiological direction proceeding from subarachnoid space to the bloodstream, indicating that the histological structure and/or the molecular machinery responsible for egress must harbor a valve-like function that prevents retrograde flow from the bloodstream into CSF (654, 655). That rodents and human children possess only sparse arachnoid granulations indirectly hints toward this egress pathway being less substantial than other egress paths, which are conserved among most mammals (e.g., olfactory nerve drainage or dural lymphatics) (615, 616). It has been postulated that the relative importance of arachnoid granulation egress might increase during pathological states, e.g., during raised ICP (58, 609).
12.6. Dural Spaces Along Venous Sinuses
A recent MRI study in humans used gadobutrol (605 Da) to monitor the egress pathways of CSF in man at 3, 6, 24, and 48 h after tracer administration. Gadobutrol is a nonionic hydrophilic molecule, which does not cross the BBB and can therefore be used to trace efflux pathways (84). Gadobutrol was found to accumulate in a 2-mm-wide parasagittal space in the dorsal dura mater parallel to the superior sagittal sinus and posterior to middle segment, thus at the very location where cortical veins empty into the superior sagittal sinus (FIGURE 25) (84). Labeling in this space was observed in all study participants, with peak intensity seen at the 24-h scan. A human cadaver study has described the parasagittal space as consisting of endothelial-lined channels within the dura (656). In the MRI study, the extent of gadobutrol influx to the parasagittal space exceeded what might have been expected from the anatomical descriptions of arachnoid granulations, suggesting that it may have arrived by alternate routes (84). One possible mechanism for this could be perivenous efflux of CSF along cortical veins, which has been described in rodents (4). The identity of the final egress pathway taken by fluid passing through the dural parasagittal space remains an open question. Among the potential routes are the dural lymphatics, fenestrated postcapillary venules, dural venous sinuses through arachnoid granulations and villi, or efflux along the adventitia of the jugular vein. The dural parasagittal spaces also take up intravenously delivered MRI contrast agent, thus indicating the presence of an open communication with the blood circulation (657). This space might be an important interface between CSF antigens and immune cells (658).
12.7. Adventitia of Major Cerebral Vessels
Intracisternally administered tracers have been found to accumulate in the connective tissue sheets of larger veins and arteries exiting the skull, from whence they drain into nearby lymphatic vessels (4, 56, 611, 659, 660). Tracers injected into the brain parenchyma or subarachnoid space have been found to permeate the dura mater at the base of the skull in close proximity to the carotid artery and also to infiltrate the adventitia of the internal carotid artery outside the cranial vault (611, 659). Other studies have found drainage of intracisternally injected carbon particles to the adventitia of the cerebellar, basilar, and vertebral arteries (660). Thus close to the foramina where major vessels traverse from the intracranial to extracranial compartment, CSF and brain extracellular fluid together exit the skull through spaces in the vascular adventitia. These adventitial egress spaces for CSF have not yet been studied in the same detail as other egress paths, and their relative importance is thus unknown.
12.8. Spinal Cord Egress Sites
Cranial CSF also finds egress through outflow pathways associated with the spinal cord, especially around nerve roots. This pathway was first identified by Brierley and Field (607) in rabbits injected with India ink in the lateral ventricles. The ink accumulated around the dorsal and anterior nerve roots of the spinal cord, as well as in the paravertebral lymph nodes and lymph nodes in the thorax, abdomen, and pelvis (607). Intrathecally delivered gadobutrol delivered at the lumbar subarachnoid space in human patients peak in blood levels much before they peak at cranial egress sites, showing that spinal CSF primarily leaves the intrathecal compartment at the spinal level and not cranial level (661). Human PET studies with intrathecal administration of tracers by lumbar puncture also show signs of CSF egress along spinal cord nerve roots (277). The draining around the nerve roots could well be conducted via dural lymphatics, which richly cover the nerve roots (662, 663). Arachnoid granulations have also been observed in close proximity to the nerve roots, thus presenting an alternate clearance route (664). Others have reported relatively higher outflow to the lymphatic system at intervertebral segments of the sacral spine (665). Overall, spinal cord CSF egress seems to occur primarily via invaginations of the subarachnoid space around the nerve roots through structures resembling those draining cranial CSF.
12.9. Volumetric Distribution Along Various Egress Paths
12.9.1. Distribution of CSF egress to blood and cervical lymphatics.
Much research on the distribution of fluid egress from the subarachnoid space has focused on defining the relative proportion of CSF tracers that is cleared by the cervical lymphatics relative to clearance into the blood. Many such studies have employed injection of radiolabeled tracers into various CSF compartments and then followed the recovery rates of the injected tracer in lymph and blood plasma (609). Other techniques used are fluorescent CSF tracers, with dynamic monitoring of fluorescence intensity at various extracranial sites (635). As described above, several different egress pathways exist, and their anatomical location and miniscule scale makes a precise measurement of CSF egress difficult. The most precise and accurate measurements such as radiotracer experiments are inherently invasive, whereas the less invasive fluorescent tracer assessments tend to be biased by differences in compartment volume, flow rate, and fractional CSF content between cervical lymphatic vessels and cranial blood vessels. Historically, the most precise measurements of the egress ratios to cranial lymphatics and to bloodstream have been done in awake sheep using a dual tracer method. Here, injections of different radiotracers into the CSF compartment and into the bloodstream are followed by dual-channel monitoring of radioactivity in the blood and cervical lymph, while adjusting for the amount of tracer lost by plasma filtration and for the amount of tracer that recirculates into the lymphatics (614). Results of that complex study procedure indicated 48% recovery of tracer in lymph, thus suggesting that approximately half of tracer clearance occurred through cervical lymphatics (614). This is most likely an underestimate of the true lymphatic contribution, since ligation of lymphatic vessels seems apt to increase resistance in this pathway and thus decrease flow rate, and furthermore, were any lymphatic vessel by error improperly cannulated or ligated, the resultant excess drainage would be misattributed as blood activity. Similar studies in rat have estimated that 30% of CSF tracer is drained through cervical lymphatics in rats, and 10–15% in the cat, and that the drainage toward blood was two to three times higher in both species (666). Conversely, measurements of fluorescent CSF tracer egress in lymph and blood of mice have shown arrival of the tracer at the mandibular lymph nodes within 11 min of administration, whereas appearance of tracer in the posterior facial veins (which drain the venous sinuses in mice) was delayed until 25 min, thus indicating that lymphatic drainage might be the only substantial egress site for CSF in mouse brain (635). The ratio between CSF egress to blood and cervical lymphatics in human is not known; however, MRI studies showed that tracer accumulation in cervical lymph nodes occurs rather slowly, peaking at 24–48 h after lumbar injection of gadobutrol (162). The discrepant findings of relative efflux of CSF drainage to cervical lymphatics and to blood likely depend on several factors. First, tracer studies of CSF flow are actually monitoring the path taken by the tracer molecule, which might show a predilection for either lymphatic or blood egress. Indeed, sorting of flow of tracers along different paths might depend on their molecular weight and polarity. Second, species differences such as the lack of arachnoid granulations in rodents may be relevant. Finally, the available experimental methodologies do not afford absolute quantitation of dynamic tracer concentrations across all tissue compartments. Whole body PET/SPECT-CT may suffice for this purpose in animal models, and by extension in human studies. Overall, the plethora of possible pathways makes it technically very challenging to attain absolute measurements of the various volumes CSF egress by all different channels.
12.9.2. Distribution of CSF egress along paths leading to cervical lymphatics.
The perineuronal drainage of CSF along the olfactory nerve is a major egress site (618). The egress along the olfactory nerve pathway can be blocked by focal injection of fibrosis-inducing agent kaolin, by surgical removal of olfactory nerves, or by sealing the cribriform plate with glue (618, 639). Such experiments involving obstruction of egress along olfactory nerve pathways suggest that the pathway could normally account for ∼87% of total CSF drainage to the cervical lymphatics in rabbit (618). In humans, the highest concentration of an intravenously delivered and BBB permeable PET tracer detected in any extracerebral site was in the nasal turbinates, thus suggesting a major contribution of the olfactory nerve to the cervical lymphatics clearance pathway also in humans (637). Quantitative measurements of other egress paths are lacking.
12.10. Physiological Factors Impacting Intracranial Fluid Egress
The egress of fluid from the cranial vault is a dynamic process. The diversity of parallel egress paths has likely developed to complement each other, thus ensuring continuous outflow of fluid during various conditions. Some egress paths might serve an accessory role and thus may be recruited when the outflow requirement exceeds limits of the normally predominant egress pathway. Dynamic changes in egress volumes can be seen both in physiological and pathological conditions. Thus normal postural changes (e.g., moving from a standing to a sitting position) provoke changes in ICP, which cause a compensatory increase in fluid egress rate (667, 668). The capacity of the egress system to cope with rapid ICP changes would likely be more important in bipedal animals than in quadrupeds, which have a horizontal body position. Another physiological variable impacting CSF egress is the production rate for CSF, as reviewed in sect. 5. Interestingly, peak CSF production occurs in the middle of the night in humans, perhaps due to circadian rhythms in the activity of the choroid plexus epithelium (233, 669). The lymphatic system has higher flow rates during wakefulness (likely due to movement), whereas glymphatic pathway activity increases dramatically during sleep (1, 523). The relative proportions of drainage into the cervical lymphatics and into blood may thus depend on various dynamically changing factors.
12.10.1 Physiological modulators of CSF egress.
CSF outflow via cervical lymphatics and through arachnoid granulations increases during episodes of elevated ICP. Thus experimentally increased ICP provoked by a ventriculo-cisternal perfusion system heightened the pressure recorded in cervical lymphatic vessels and stimulated the flow rate of lymph (670). This perfusion procedure increased lymphatic outflow 2.7-fold, while elevating clearance toward blood 3.9-fold. This suggests a ceiling effect for the lymphatic pathway, or alternately that the arachnoid villi/granulations have a somewhat higher capacity for CSF outflow in response to transiently increased ICP (617). Another factor impacting fluid egress is head position. Upon tilting the head downwards by 20°, there is increased tracer accumulation in the nasal submucosa, whereas tilting the head upwards by 20° more than doubles the amount of CSF tracer reaching the spinal cord (618). Such differences could be driven by pressure differences between CSF in the cranium and spinal cord that vary with head position (671). The glymphatic system is also affected by body position in mice, being more efficient in the supine position (619). The interplay between up and downregulation of CSF egress and glymphatic activity has yet to be elucidated, although it is known that glymphatic activity is inversely related with CSF egress to the cervical lymphatics (55, 523). Furthermore, anesthetics such as ketamine-xylazine that favor glymphatic activity increase CSF egress by the olfactory route, whereas isoflurane anesthesia, which inhibits glymphatic function, leads to increased CSF egress through the basal part of the skull around the vagus nerve (647, 672).
Increased outflow of CSF tracers through cervical lymphatics has furthermore been observed during wakefulness relative to an anesthetized state in mice (523). Similarly, increased clearance of CSF tracer from the spinal cord in humans was seen in the aftermath of physical activity as compared with rest, again perhaps in relation to effects of bipedal posture (673). Such flow changes could arise either from increased CSF flow rate in the subarachnoid space or in lymphatic vessels or from both in combination. CSF flow in the subarachnoid space and ventricles is largely driven by respiration and to a lesser extent by hemodynamics, such that the increased amplitude and rate of respiratory and cardiac activity during wakefulness and physical activity are likely to be contributing factors (see sect. 9 for driving factors of brain fluid transport) (498). Flow in lymphatic vessels depends on the pumping actions of intrinsic smooth muscle cell, changes in body position, and the activity of surrounding skeletal muscle. However, hemodynamic variables such as cardiac output and central venous pressure are certainly contributing factors. Both will affect the local pressure in the tissues they drain, as well as the pressure gradient between lymphatic vessels and the venous structures into which they drain (see review for details, Ref. 674). Certain anesthetics reduce the contractility of lymphatic vessels (675), and the reduced skeletal muscle activity and hemodynamic changes during sleep likely also contribute to the nocturnally decreased CSF egress. Finally, there is an established link between activity brain state and intraventricular CSF flow in the cerebral aqueduct, thus suggesting that neuronal activity could play an intrinsic role in the rate of CSF egress (55, 501). On the other hand, one night of sleep deprivation in human patients did not affect the clearance rate of the subarachnoid space of intrathecally delivered MRI contrast agent gadobutrol, suggesting that circadian rhythm and behavioral factors might be more important than brain state in governing egress of CSF (524).
12.11. Outstanding Questions
How is the intersection of the subarachnoid space and the egress paths structurally organized? Elucidating the ultrastructure of the meningeal membranes and the molecular organization of junction proteins in these areas represents an exciting topic with implications for the pathogenesis of various diseases. Is there a functional equivalent of directional valves, and do potential bottleneck for egress arise in the aged or diseased brain that present targets for therapeutic interventions to alleviate glymphatic dysfunction?
Perineuronal egress represents the one of the most important egress pathways, having been described in all compartments along the cranial nerves (intraaxonal, endoneurial, perineurial, subepineurial, and epineurial). Are all of these paths real, or do some represent postmortem/histological artifacts?
CSF is also transported directly into venous blood, but the only known egress site is the arachnoid villi/granulations, which are not well characterized in all species and develop in human only during childhood. Thus there might be other unknown structures draining CSF to the blood compartment. Could the rich network of postcapillary venules in the dura mater take up CSF from parasagittal dural spaces?
Besides changes in CSF production rate, are egress rates altered by changes in glymphatic activity and/or by other gating mechanisms within the egress pathway itself?
12.12. Interim Summary Section
CSF egress is a complex and dynamic outflow process with multiple pathways, some of which serve as main egress sites and others are seemingly accessorial and operate only under certain physiological conditions. Such a multifaceted egress system is likely to have developed to ensure continued drainage of fluid from the cranial vault under diverse physiological conditions. The alternating drainage pathways do not likely function to maintain a constant ICP. Even under physiological conditions the ICP is highly dynamic and can fluctuate within a range of (−2 mmHg to 12 mmHg) in mice under physiological challenges such as Valsalva and postural changes.
CSF and extracellular fluid egress occur by perineuronal drainage, through dural lymphatics, through arachnoid granulations, through parasagittal spaces in dura, and within the adventitia of major cerebral blood vessels.
CSF and extracellular fluid are drained into the bloodstream and also the cervical lymphatic system. The exact branching ratio is debated. Older studies using radiolabeled tracers suggest an approximately a 50:50 partitioning, but those studies were invasive and most likely underestimated the contribution of cervical lymphatic drainage. Newer studies based on fluorescence microscopy suggest that the cervical lymphatics play a dominant role in draining CSF. Yet, the experimental approaches in these studies also harbor their own confounding factors, and it remains an important task to quantify the drainage and how it is regulated along the different egress pathways.
Drainage of CSF along the olfactory nerve has been reported as a major drainage pathway across several species including human. Yet other studies have questioned the importance of CSF drainage along the olfactory nerve and suggest a greater role for dural lymphatics or parasagittal dural spaces. The ultrastructure of the flow pathway within the peripheral nerve compartments and their communication with the cervical lymphatics remains to be elucidated. The relative importance of other drainage pathways remains less clear, and evidence for an important role of arachnoid granulations is weak.
13. PATHOLOGY IN BRAIN FLUID TRANSPORT SYSTEMS
13.1. Introduction
Dysfunction of the glymphatic system and brain fluid transport in general has been implicated in the pathology of many neurological disorders. The intricate organization of the glymphatic system and the CSF egress pathways harbor multiple sites where pathological processes can impair the fluid flow, which in turn reduces the brain’s capability for clearance and homeostasis. In this section, we review the pathological processes that have been found to occur in the glymphatic system with healthy aging, as well as in conditions such as Alzheimer’s disease, traumatic brain injury (TBI), and ischemic stroke, among others. The pathophysiological processes in which the glymphatic system has been implicated can be roughly grouped into acute and chronic glymphatic pathologies. Acute glymphatic pathology is mainly seen during ischemic stroke, subarachnoid hemorrhage, and acute traumatic brain injury, any of which events can either up- or downregulate perivascular CSF flow to a harmful extent. Chronic glymphatic pathologies are chronic changes that attenuate glymphatic system drivers such as cardiovascular pumping or AQP4 polarization, wherein long-term impairments in brain clearance can lead to the harmful buildup of proteinaceous waste. We shall describe the pathophysiology within the various disorders that instigate glymphatic failure and hypothesize how these perturbations can explain clinical phenotypes, while also providing promising targets for future clinical prognostic, diagnostic, and therapeutic tools. We refer to reader to reviews for detailed information regarding glymphatic system pathology (27, 67, 676–678) but emphasize that the rapid development in our understanding of pathological processes in the glymphatic system makes it important to stay abreast of the newest original literature.
13.2. Acute Pathological Processes in the Glymphatic Pathway
13.2.1. Stroke.
Early studies of the glymphatic system in stroke focused on the hemorrhagic variants such as subarachnoid hemorrhage (SAH), which was shown to significantly reduce glymphatic influx of CSF in rats and nonhuman primates (163, 166, 679). The glymphatic defect in SAH was caused by occlusion of the perivascular space due to extracellular deposition of fibrin and fibrinogen derived from the extravasated blood plasma (163, 166). Treatment by delivering tissue plasminogen activator (tPA) through the glymphatic pathway was able to rescue CSF clearance and glymphatic influx in a preclinical model in nonhuman primates (163, 166). tPA is a specific serine protease that breaks down components of the extracellular matrix such as fibronectin and laminins, so it is possible that intracisternal tPA may also alter the geometry of CSF inflow pathways, independently of its role in fibrinolysis. However, it remains unknown if this strategy could be used to enhance glymphatic function in settings other than in SAH (680). Glymphatic inhibition after SAH may also be a consequence of the rapid activation of astrocytes and a loss of their AQP4 polarization, ultimately resulting in accumulation of tau protein and helper and cytotoxic T cells in the parenchyma (681). Loss of capillary pulsatility could also play a role in the glymphatic inhibition after SAH (682). The transfer of blood breakdown products into the arterial perivascular space via glymphatic flow may contribute to the vasospasm arising from perivasculitis (681, 683). Accumulating evidence shows that erythrocytes in subarachnoid space are cleared via cervical and meningeal lymphatics, suggesting that these pathways play a role in resolving the bleed, but conversely it is also plausible that perturbation of these same pathways can cause faulty CSF drainage and thus be a driver for post-SAH hydrocephalus (681, 684).
The role of glymphatic function in the pathophysiology of intracerebral hemorrhage is less clear. In a rodent model of intracerebral hemorrhage induced by the injection of collagenase into striatum there was no effect on glymphatic clearance compared with sham treatment (163). However, other data suggest that cranial burr holes and intraparenchymal injections (e.g., for delivery of collagenase) can by themselves affect glymphatic inflow of CSF tracers (420). Therefore, it is still unknown if the sham and intracerebral hemorrhage groups in (28) both exhibited global unnoted reductions in glymphatic function, perhaps due to artifactual reactive gliosis that obscured the intrinsic effect of hematoma formation on glymphatic function. Future studies should focus on isolating the glymphatic clearance of the hematoma and the role that this process plays in resolving intracerebral hemorrhage.
The continuous inflow of CSF into the parenchyma also drives edema formation after acute ischemic stroke (469). Within minutes after a middle cerebral artery occlusion (MCAO) in mice, there is a rapid entry of CSF into the ischemic hemisphere. This inrush of fluid is the first contributor to tissue swelling, rather than the previously emphasized vascular sources (469). The normal polarized expression of astrocytic AQP4 facilitated the formation of edema, whereas AQP4 knockout animals were protected from edema after MCAO (469). In a model of ischemic edema in spinal cord, the primary driver of swelling seemed to be the translocation of AQP4 to the astrocyte endfeet, which was blocked by the typical antipsychotic trifluoperazine, which has antidopaminergic and antiadrenergic actions (489). In later stages of stroke (3 h after infarct), glymphatic influx is reduced; it is unclear what causes this early inhibition of influx before BBB opening, but it could be a consequence of the onset of edema formation (163). The later stages of edema (i.e., vasogenic edema) beings to form several hours after the insult. Vasogenic edema also seems to interfere with CSF inflow (685), but more work is required to evaluate if a reduction in extracellular fluid volume contributes to the progression of this delayed type of edema. The previously described bulk flow of edematous fluid along white matter tracts may prove to be an important factor in edema resolution. CSF egress along cervical and meningeal lymphatics might also contribute to the maturation of the infarct core by aiding in the presentation of CNS antigens, but the data are conflicting in this regard. Mice lacking lymphatic vasculature due to genetic interference in VEGF-C signaling have shown both increased and decreased infarct volumes after MCAO (686, 687). There are several methodological differences between the latter two studies that could contribute to the discrepant results, so more work is needed to elucidate the role of impaired CSF egress in driving stroke severity and recovery.
13.3. Chronic Pathological Changes in the Glymphatic Pathway
Proteostasis is the dynamic balance between production and degradation of proteins within the cell. The degradation process includes both intracellular (i.e., proteasomal degradation and autophagy) and extracellular protein clearance mechanisms. Disordered proteostasis promotes the abnormal deposition of proteins such as occurs during healthy aging and in neurodegenerative diseases. Proteopathic disorders (a.k.a., proteinopathies, protein conformational disorders, and protein misfolding diseases) are a group of diseases caused by structurally abnormal proteins that accumulate and deposit in cells and their surrounding extracellular matrix, which disrupts proteostasis and leads to cellular degeneration.
While the glymphatic system aids in the extracellular clearance of toxic proteins, it also plays a crucial role in the removal of other macromolecules and excess fluid. Dysfunction in these clearance processes contributes to the pathogenesis of a broad array of diseases, extending from edema to pure proteopathic disorders like Huntington’s disease. Therefore, it is imperative to determine the manner whereby glymphatic pathology (glymphopathy) might contribute to abnormal proteostasis and promote the progression of proteopathies. However, proteopathies are a subset of the broader class of glymphopathies, which are characterized by the abnormal accumulation of fluid and its various solutes within the CNS.
13.4. Variation in Glymphatic System Efficacy and Their Potential Role as Risk Factors for Developing Neurodegenerative Disease
13.4.1. Aging.
Glymphatic function initiates in infancy, reaches a peak in young adulthood, and declines throughout the remaining lifespan (15). In humans, this is experimentally demonstrated in MRI-contrast studies, where the intrathecally administered agent is cleared from the brain parenchyma at a much slower rate in older patients than in the young. The same age-dependent decline was seen for clearance of CSF within the ventricular system (170). In aging rodents, there is likewise a reduction in the inflow of CSF in perivascular spaces and reduced trans-pial CSF flow in middle-aged and aged animals compared with the young, occurring in association with a 40% reduction in the amyloid-β clearance rate from the aged brain (15). Among the various factors resulting in the age-related decline in glymphatic function, the two primary instigators are 1) AQP4 mislocalization in the course of astrogliosis, and 2) reduced arterial wall compliance due to arteriosclerosis (FIGURE 26) (15). These two processes independently lead to decreased transport of fluid across the astrocytic endfeet and into the brain extracellular space and reduce the efficacy of periarterial space CSF pumping due to attenuation of vasomotion and arterial wall pulsatility (15, 21, 54). Altered astroglial AQP4 expression has also been identified in the aging human brain (169). Increased total AQP4 expression but not AQP4 mislocalization was found to be a feature of the cognitively intact aging human brain (169). Drainage pathways are also affected in aging human brain, which is accompanied by an increase in CSF outflow resistance (694). Consequently, elderly individuals show greater ICP increases after constant infusion of artificial CSF into the subarachnoid space by lumbar puncture. This age-dependent effect on ICP reflects, at least in part, the declining patency of the CSF egress routes, such that the infusion cannot be accommodated (694). In mice, aging has been associated with slowed outflow of CSF solutes to blood and cervical lymphatics, as well as with the reduced accumulation of CSF solutes in cervical lymph nodes (70, 635). This is likely due to decreased coverage and reduced vessel diameter of meningeal lymphatics in the aged mouse (70). These morphological changes were associated with differential expression patterns of 607 genes compared with young mice, which included genes involved in immune and inflammatory responses, extracellular matrix organization, and endothelial tube morphogenesis, among many other functions (70). The altered lymphatic vessel distribution associated with aging occurs in concert with decreased glymphatic function as well as cognitive decline. Treatment of aged mice with vascular endothelial growth factor-C (VEGF-C) reversed the decreases in lymphatic vessel diameter, which was associated with improved drainage of CSF tracers to the deep cervical lymph nodes, improved glymphatic function, and rescue of cognitive function (70). It is difficult to assess the causal relationship between the factors associated with declining brain fluid transport during aging such as decreased CSF production, glymphatic inhibition, and dural lymphatics degeneration. Furthermore, other clearance mechanisms are also affected by aging and thus contribute to the net drop in clearance efficacy. For example, the abundance of perivascular macrophages doubles in aging rodent brain, but their phagocytic ability declines, suggesting decreased clearance efficacy by this pathway (695, 696).
FIGURE 26.

A reduction in glymphatic fluid transport has been demonstrated to occur in many neurological disorders as well as healthy aging. In healthy aging of mice, impaired brain clearance arises to due to loss of aquaporin 4 (AQP4) polarization, which abrogates the removal of extracellular amyloid-β (15). In neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease, reduced glymphatic function has been linked to build-up of amyloid-β (450, 688) and α-synuclein, respectively (544, 689). In an ischemic stroke model in mice, spreading ischemic depolarizations cause massive vasoconstriction as well as an ionic shift that leads to increased cerebral spinal fluid (CSF) influx to brain, resulting in edema (469). In mouse and nonhuman primate models of subarachnoid hemorrhage, blood components including fibrin and fibrinogen occlude periarterial spaces and impair CSF influx (163). Both mild and moderate traumatic brain injury (TBI) have been associated with reduced glymphatic function in rodents. In human TBI, there is a pathological buildup of hyperphosphorylated tau, typically seen in perivascular areas (56, 690, 691). Potential biomarkers for TBI (S100-B, for example) are washed out of the brain parenchyma by the glymphatic system predominantly during sleep. As such, sleep disruption due to the standard clinical practice of administering repeated neurological exams could thus be impairing glymphatic function and interfering with the detection of blood-based biomarkers in TBI (692). A rodent model of noncommunicating hydrocephalus and human cases of normal pressure hydrocephalus have been associated with impaired glymphatic clearance of brain parenchyma (162, 164,165, 533, 693). RBC, red blood cells. SAH, subarachnoid hemorrhage; PVS, perivascular space; GFAP, glial fibrillary acidic protein; NSE, neuron-specific enolase.
13.4.2. Sleep.
Clearance of the brain extracellular space primarily occurs during sleep, when upregulation of glymphatic drivers such as increased extracellular space volume, larger fluctuations in blood volume, and larger amplitudes in vasomotion-driven vessel caliber changes result in increased glymphatic activity and enhanced CSF flow into the ventricular system (see sect. 9 for physiological drivers) (1, 501, 522). Sleep disturbances are associated with diverse chronic diseases including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and TBI (697–700). The causal relationship between sleep disturbance and many of these disorders is not fully understood. Although it is likely in many cases that neurodegenerative disease debut predates the occurrence of sleep problems, poor sleep is nonetheless a risk factor for developing Alzheimer’s disease (700). Reduced duration and quality of sleep are seen with aging and could also contribute to the glymphatic inhibition seen in the elderly (15, 701). Thus glymphatic inhibition due to poor sleep quality is a potential risk factor for certain neurodegenerative diseases and could also be implicated in the causality chain whereby aging is a risk factor for many neurological disorders. We propose that glymphatic inhibition as a result of sleep disturbances could be a common pathway leading to cognitive impairment in the elderly and promoting the progression of the aforementioned neurodegenerative disorders.
One night of sleep deprivation in human patients leads to decreased clearance of the brain parenchyma of the MRI tracer gadobutrol, and even after one night of normal sleep, sleep-deprived patients still show higher levels of gadobutrol in brain compared with patients not exposed to sleep deprivation (524). An episode of sleep reduces the levels of amyloid-β and tau protein in extracellular fluid and in CSF, both in humans and in preclinical studies in mice (702–704). During wakefulness in mice, the tau concentration in extracellular fluid doubles relative to that in the sleeping state, and amyloid-β levels increase by about a third, probably due to reduced clearance and high norepinephrine levels (412, 702, 703). The cleansing effect of sleep has been shown in mice, where the clearance rate of intracortically injected amyloid-β more than doubled during sleep, whereas in humans, a single night of sleep deprivation leads to a 30% increase in amyloid-β and a 50% increase in tau concentration in the CSF in healthy young volunteers (1, 702, 704). Preclinical studies furthermore showed that sleep deprivation leads to a reduced washout of metabolites from the brain parenchyma (541). Single nucleotide polymorphisms (SNPs) of the AQP4 gene have been associated with altered NREM sleep in humans and with the ability of coping with prolonged sleep deprivation, thus suggesting a possible link between glymphatic function during sleep and the preservation of cognitive function (705). In conclusion, sleep enhances glymphatic function and sleep disruption is associated with reduced brain and CSF clearance of potentially harmful proteins. Genetic variation in the efficiency of sleep and reduced sleep quality with aging are potential risk factors for sporadic neurodegenerative diseases and for increased progression of these disorders in the elderly. Finally, sleep disturbances arising after disease onset could contribute to a facilitation of the cognitive decline in neurodegenerative diseases.
13.4.3. Small vessel disease and hypertension.
As described in sect. 9, glymphatic flow relies on pulsatile changes in arterial diameter to pump CSF forward along the periarterial spaces of leptomeningeal arteries. Furthermore, intra-axial clearance has been associated with vasomotion (21, 439). Thus the occurrence of anterograde flow in the glymphatic system depends on arterial distensions that pump fluid into the brain. In several animal models of cardiovascular disease, glymphatic dysfunction has been associated with the reduced vessel pulsatility, that is to say a reduced deflection of the arterial wall in response to systolic pulses in the arterial blood pressure (54, 706, 707). Diseases that impair compliance and reactivity of the arterial wall will likely reduce vessel pulsatility and thus decrease CSF pumping in the periarterial spaces. Small vessel disease and hypertension are associated with thickening of the vascular wall of small vessels, along with fibrinoid necrosis and lipohyalinosis, inflammation, white matter hyperintensities, and microinfarcts (420, 708). Although the underlying mechanisms remain to be fully elucidated, decreased glymphatic function occurs in several animal models of small vessel disease including diabetes (709). Such findings support the prediction that vascular pathologies reduce vessel pulsatility and impair CSF pumping into the glymphatic system (50, 420). Acute induction of hypertension in mice upon intravenous injection of angiotensin II altered the waveform and propagation velocity of pulsatile waves in cerebral arterial walls, probably triggered by contraction of smooth muscle cells secondary to the raised intraluminal pressure (21). The acute hypertension also provoked a 40% reduction in perivascular CSF flow velocity in the same mice. In rats with chronic hypertension [spontaneously hypertensive rats (SHR)], dynamic contrast-enhanced MRI showed a global decrease in glymphatic function at age 7–9 wk (just before the onset of the hypertension), which persisted in the now hypertensive rats aged 19–21 wk (405). In the immunohistochemical arm of that study, there were no observed differences in glial fibrillary acidic protein (GFAP) and AQP4 expression between the hyper- and normotensive rats, suggesting that vascular pathology was the primary factor responsible for suppression of CSF inflow in that model. A recent study showed that SHRs aged 8–9 mo also exhibited a significant reduction in glymphatic function, now associated with loss of AQP4 polarity and widening of the perivascular space. Together, the two studies suggest that vascular pathology alone suffices to reduce glymphatic function in young SHR but that this early pathology sets the stage for later progression of astroglial changes in the neuropil that contribute to further glymphatic dysfunction (710). Glymphatic dysfunction or AQP4 mislocalization has also been reported in several conditions and diseases that overlap with aspects of small vessel disease, including healthy aging, cerebral amyloid angiopathy, microinfarcts, chronic kidney disease, and stroke (15, 163, 711–714). Another aspect of vascular status with relevance for glymphatic function is the BBB. Although experimental details are lacking, it seems plausible that BBB defects could be harmful to perivascular flow, since even small changes in fluid influx over the vascular endothelium likely exert substantial changes in flow dynamics and perhaps directionality.
13.5. Neurodegenerative Disorders
13.5.1. Alzheimer’s disease.
Alzheimer’s disease is the most common neurodegenerative disorder, and the ongoing demographic shift is placing an ever-greater socioeconomic burden from this illness on many countries (712). Although the pathologic processes leading to the development of Alzheimer’s disease are complex, the hallmark neuropathological features of Alzheimer’s disease are altered turnover and increased deposition of amyloid-β, tau hyperphosphorylation, oxidative stress, microglial proliferation, and reactive gliosis (712).
In the healthy brain, the glymphatic system efficiently clears amyloid-β from the extracellular space (see sect. 10) (4). Any prolonged attenuation of glymphatic function increases amyloid-β deposition rates. This has been shown in the amyloid precursor protein (APP)/presenilin 1 (PS1) mouse model of Alzheimer’s disease, where cortical amyloid-β deposition increases with age due to expression of chimeric mouse-human mutant APP and human mutant PS1. In one study of APP/PS1 mice, knockout of AQP4 led to a 25–50% increase in the brain levels of soluble and insoluble amyloid-β at 12 mo of age and accelerated the formation of amyloid plaques compared with animals with an intact glymphatic system. In another study in animals aged 3 mo, AQP4 knockout increased the levels of intraneuronal amyloid-β aggregates but did not alter the extracellular concentrations (FIGURE 26) (450, 715). Thus glymphatic efflux is important in clearing the neuropil of amyloid-β in these models. The clearance mechanism is likely due to the concerted effects of direct glymphatic efflux to the cervical lymphatics, transport of amyloid-β to efflux transporters at the BBB, and delivery and efflux of ApoE (see sect. 10 on glymphatic functions) (537, 715). Similar observations have been made for the pathogenic tau protein, such that the levels of total and phosphorylated tau cleared to the CSF compartment were almost completely abolished in AQP4 knockout animals and in wild-type mice treated with the AQP4 inhibitor TGN-020 (533). Where these animal models of glymphatic inhibition represent an all or none condition, glymphatic system dysfunction in humans shows more graded variations. Indeed, intrathecally delivered gadobutrol to human patients manifests in substantial interindividual variability in terms of glymphatic delivery of the tracer to different brain regions (165). It is an important question for the future whether individual differences in glymphatic function could be a risk factor for developing Alzheimer’s disease, and whether the natural decrease in glymphatic function described in studies of aging could explain why Alzheimer’s disease usually debuts in the aged population. In APP/PS1 mice with no genetic modification of glymphatic function, the glymphatic attenuation associated with astrogliosis caused by natural aging precedes the onset of amyloid-β deposition (688). The loss of AQP4 polarization associated with normal aging, by setting the stage of glymphatic failure, may thus be a risk factor for developing Alzheimer’s disease (169, 532, 714, 716). Furthermore, interindividual genetic variation in the AQP4 gene could potentially impose either loss or gain of function in the glymphatic pathway, although direct evidence linking SNPs in the AQP4 gene with glymphatic function is lacking. However, two SNPs in the AQP4 gene have been shown to correlate with levels of amyloid-β in the temporal lobe of patients in the Alzheimer’s disease spectrum: rs72878794 was associated with decreased levels, while rs151244 was associated with greater amyloid-β burden (717). SNPs within the AQP4 gene have also been associated both with increased and reduced rates of cognitive decline in patients with Alzheimer’s disease (717, 718). Obtaining a better understanding of the functional implications of these SNPs on the AQP4 channel and its expression pattern, as well as their effect on glymphatic function, could aid our understanding of the pathology of sporadic Alzheimer’s disease and potentially lead to new biomarkers and therapeutic targets. While interindividual variation in glymphatic function may pose a risk for developing sporadic Alzheimer’s disease, it is also evident from rodent studies that, after onset of the disease, glymphatic function deteriorates quickly. Thus, Alzheimer’s disease pathologies such as deposition of amyloid-β plaques and hyperphosphorylated tau are inherently harmful to glymphatic function, implying a feed-forward mechanism in disease progression. Reactive astrogliosis and associated AQP4 mislocalization are observed in several mouse models of Alzheimer’s disease and are also evident in autopsy samples from human Alzheimer’s disease patients. AQP4 mislocalization proved to be a predictor of the extent of amyloid-β burden and Braak staging at time of death (169, 532, 714). Other factors may also contribute to the glymphatic inhibition after disease onset. For example, Alzheimer’s disease patients had a weakened negative coupling between the BOLD signal on fMRI and CSF influx in the fourth ventricle, which was associated with increasing cortical amyloid-β levels asserted with PET scans, suggesting that cardiovascular pumping of CSF might be impaired in these patients (719). A general impairment in the clearing of solutes from CSF seems to be a feature of the Alzheimer’s disease brain. In a PET tracer study, there was a 33% reduction in the CSF clearance rate of tracer from the ventricles of patients compared with healthy controls (637). The ventricular clearance rate was inversely correlated with the level of amyloid-β deposition in cortex (637). Also, a recent MRI study in human brain based on ultrafast 10-Hz scanning found pulse propagation generated by the drivers of glymphatic flow (cardiac, respiration, and slow vasomotion) differed with regard to arrival latency and propagation speed in Alzheimer’s disease patients (720, 721). Thus human clinical studies are in agreement with the preclinical findings of reduced glymphatic function in Alzheimer’s disease models. Overall, several of the physiological drivers that ensure flow through the glymphatic system seem to be affected in Alzheimer’s disease after clinical onset (see sect. 9).
CSF drainage pathways are also affected in Alzheimer’s disease (see sect. 12.3 for CSF egress). In humans, the drainage of an intravenously administered PET tracer that is able to cross the BBB toward the nasal cavity was reduced by 66% in patients with Alzheimer’s disease relative to age-matched controls (637). This is an indication that reduced CSF drainage along the olfactory nerves could play a pathophysiological role in the reduced CSF turnover in patients with Alzheimer’s disease, a proposition that is supported by autopsy studies of patients showing degeneration of the olfactory epithelium and abnormal olfactory nerve morphology (722). Furthermore, development of anosmia in the elderly is a risk factor for the development of Alzheimer’s disease and other neurodegenerative disorders (723). Rodent studies have confirmed that degeneration of olfactory nerves diminishes CSF outflow into the nasal cavity, thus supporting the idea that attenuated outflow along this pathway could promote Alzheimer’s disease pathology (639, 723).
Dural lymphatics is another important CSF egress pathway that has been implicated in Alzheimer’s disease pathology. In rodents, increased lymphatic coverage improves brain clearance, whereas knockout of meningeal lymphatics impairs the clearance of amyloid-β injected into the brain (70). In two transgenic mouse models with increased rates of amyloid-β deposition, the coverage and morphology of lymphatic vessels in dorsal dura mater are unaffected, and drainage to the cervical lymph nodes appeared normal (70). Thus amyloidosis does not seem itself to promote pathological changes to dural lymphatics. However, ablation of dorsal dural lymphatics or ligation of deep cervical lymph nodes in Alzheimer’s disease mouse models leads to increased amyloid-β and tau burden both in brain and the meninges (70, 724). Conversely, increased coverage and size of lymphatic vasculature by VEGF-C treatment did not decrease levels of amyloid-β in mouse models of Alzheimer’s disease (70). As such, dural lymphatics and CSF egress toward cervical lymphatics are important for normal amyloid-β clearance, and the interconnected impairment of these egress pathways could act as a risk factor for amyloid-β accumulation in brain, perhaps leading to an earlier onset or accelerated course of the disease.
13.5.2. Parkinson’s disease.
Parkinson’s disease, the second most common neurodegenerative disease, is characterized pathologically by intracellular and intra-axonal inclusion bodies consisting of aggregated α-synuclein protein (Lewy bodies) and degeneration of the nigrostriatal dopamine neurons (725). Striatal dopamine depletion accounts for the cardinal motor symptoms of Parkinson’s disease, but damage to multiple other neuronal populations contributes to the complex clinical presentation, which includes cognitive impairment, sleep disturbances, anosmia, depression, autonomic failure, and constipation (726, 727).
Under physiological conditions, the glymphatic pathway aids in clearing α-synuclein from brain parenchyma (544). As such, AQP4 knockout mice show reduced ability to clear human recombinant α-synuclein after intrastriatal injection (544, 728). The α-synuclein accumulation seen in Parkinson’s disease is directly harmful to glymphatic function. In the A53T transgenic mouse, which bears a mutant human α-synuclein variant associated with Parkinson’s disease, overexpression of the protein leads to intraneuronal accumulation of α-synuclein. The glymphatic system is inhibited via loss of AQP4 polarization occurring in this model of Parkinson’s disease (544). AQP4 seems to accumulate on the astrocytic membranes facing α-synuclein-positive neurons, which, together with increased levels of AQP4 and neuroinflammation, is likely driving the loss of AQP4 polarization (544). Glymphatic system impairment seems to aggravate the disease course, since AQP4+/− mice receiving intrastriatal injections of α-synuclein show increased loss of dopaminergic neurons in substantia nigra and more severe loss of motor function (728). The preclinical findings of glymphatic impairment in Parkinson’s disease have been corroborated by diffusion tensor imaging of perivascular spaces in human patients. Here it was found that lower diffusivity in the perivascular spaces was a feature of Parkinson’s disease patients compared with healthy controls (729). The drainage of CSF and extracellular fluid from brain toward dural lymphatics and into the cervical lymphatic system is also perturbed in Parkinson’s disease. In a clinical study where participants received intravenous injections of the MRI tracer gadobutrol, which subsequently leaks into the dural extracellular space, the flow of tracer through dural lymphatics was slower in Parkinson’s disease patients compared with healthy controls (730). There was no sign of gross morphological differences in the cross-sectional area estimated by MRI in lymphatic vasculature either around superior sagittal sinus or the sigmoid sinus (730). Rather, the reduction in dural lymphatic flow may result from changes in the structural integrity of the lymphatic endothelium, since a mouse model of Parkinson’s disease showed a reduction in the expression of the tight junction proteins occludin and zonula ocludens-1 in the dural lymphatic vasculature (730). Reduced flow through dural lymphatics and into cervical lymphatics seems to play a role in Parkinson’s disease pathology; cervical lymphatic vessel ligation exacerbated α-synuclein aggregation in mouse models of Parkinson’s disease (544, 730). In A53T mice with ligated cervical lymph nodes, the attenuation of glymphatic function was further reduced compared with that in A53T mice with intact lymphatics. In addition, there was increased astrogliosis and further loss of polarization of AQP4 expression toward the astrocytic endfeet in the ligated animals (544). These findings suggest that perturbed drainage toward the cervical lymphatic system leads to increased brain levels of α-synuclein aggregates, occurring at least in part via glymphatic inhibition. Other factors at play in reducing flow in dural lymphatics might include inhibition of autophagy of α-synuclein in brain and the occurrence of meningeal inflammation (544, 730). Postmortem samples of temporal lobe from patients who had died with Parkinson’s disease showed increased AQP4 immunoreactivity, with an inverse relationship between local AQP4 expression and α-synuclein levels in cortical layers V-VI (i.e., the location of corticofugal projection neurons) (689). These observations suggest a brain-wide disturbance in glymphatic function in sporadic Parkinson’s disease, as distinct from localized effects in the nigrostriatal pathway. Such global effects could play a role in the cognitive impairment often seen in these patients.
13.5.3. Traumatic brain injury.
TBI is a risk factor for developing dementia later in life, despite a seemingly uneventful recovery from the initial injury (731). TBI is categorized in terms of its severity as mild, moderate, and severe (732). Repetitive events of mild TBI have been associated with chronic traumatic encephalopathy (CTE), especially in players of certain contact sports and in military veterans exposed to explosive shockwaves. Symptoms of CTE include emotional lability, aggression, depression as well as memory and cognitive impairment (731). The chronic neuropathological changes associated with single TBI of moderate or severe intensity or repetitive mild TBI include brain atrophy and accumulation of neurofibrillary tangles of hyperphosphorylated tau, as well as amyloid-β plaques (731, 732), although in a spatial pattern distinct from the distributions typically seen in Alzheimer’s disease. Abnormal tau deposition can be seen up to five decades after battle-related trauma in nondemented Vietnam war veterans (733).
The accumulation of hyperphosphorylated tau in CTE patients is especially evident in perivascular areas of neocortex and also in the depths of cortical sulci and in superficial cortical layers (691, 734). These preferred locations for tauopathy overlap with the distribution of leptomeningeal surface vessels, and components of the neurovascular unit (i.e., capillary endothelial cells, smooth muscle cells, basal lamina, and astroglial endfeet), which constitute key elements of the glymphatic system. Normally, intracortical tau is washed out of the brain parenchyma by glymphatic efflux along a subset of large caliber veins, including the caudal rhinal veins, internal cerebral veins, and inferior sagittal sinus, but this process seems to be impaired after TBI (56, 690, 735). In a model of repetitive mild TBI in rats, injury led to perturbation of glymphatic flow, with increased influx and decreased efflux of intrathecally administered CSF tracer in amygdala and olfactory bulb after three mild impacts to the left temporal lobe (690). In a model of moderate TBI in mice, there was evidence of glymphatic pathway failure due to AQP4 mislocalization in astrocytes of the ipsilateral hemisphere 1 day after the injury, which spread to both hemispheres at seven days postinjury, and persisted for at least 28 days (56). The importance of glymphatic clearance of excess tau protein after TBI is highlighted by the finding that a moderate injury in AQP4 knockout mice provoked higher phosphorylated tau concentration in brain and exacerbated the delayed axonal degeneration and neuroinflammation compared with animals with normal AQP4 expression (56).
CSF egress pathways are also involved in the pathological changes following TBI. Reduced drainage of intracisternally delivered microscopic beads to the deep cervical lymph nodes was seen as soon as 2 h after a single mild closed-skull TBI to the right inferior temporal lobe (736). This reduction in drainage seems to be due at least in part to inhibition of dural lymphatics. Thus reduced uptake of intrathecally delivered LYVE-1 antibodies was found in the dural lymphatics around the transverse sinus after a TBI injury (736). Morphologic changes of dural lymphatics consistent with active lymphangiogenesis ensued from the first week after TBI, which could plausibly contribute to the normalization of drainage toward the deep cervical lymph nodes that was seen 2 mo postinjury. The same study showed that increases in ICP, which are often seen after TBI, lead to reduced transport of CSF tracers to the deep cervical lymph nodes after 3 h and reduced uptake to the dorsal dural lymphatics at 3 and 24 h after the injury (736). Thus increases in ICP seem to diminish the egress of CSF to the cervical lymphatics in the hours after the TBI event. Interestingly, preexisting dural lymphatic dysfunction provoked by Visudyne injection and subsequent photoablation of dural lymphatic vessels resulted in aggravation of the TBI, with increased neuroinflammation and more pronounced deficits in memory and motor learning at 2 wk after the injury (736). Attenuated functioning of dural lymphatic vessels is seen in natural aging, and rescuing lymphatic vessel degeneration with VEGF-C treatment reduced the level of gliosis occurring post-TBI to that seen in younger animals (736).
The transport of conventional biomarkers of TBI such as GFAP and S100B in the bloodstream is mediated by the glymphatic pathway, at least in rodents (FIGURE 26). In a mouse model of closed-head TBI, four different measures that inhibit glymphatic function (i.e., sleep deprivation, CSF drainage via cisternotomy, AQP4 knockout, and acetazolamide) were all shown to decrease the efflux of the trauma biomarkers S100B, GFAP, and neuron-specific enolase to serum (692). We contend that aspects of common clinical practice in the treatment of TBI patients, which include sleep deprivation due to frequent neurological testing, along with ventriculotomy after severe injury traumatic brain, may well interfere in the egress of biomarkers to the blood (692, 737). Sleep or rest immediately after TBI is emerging as a critical factor for removal of cellular debris by the glymphatic system, thereby improving long-term recovery.
13.5.4. Other neurodegenerative disorders.
Brain fluid transport is also involved in or affected by other neurodegenerative disorders. In Huntington’s disease, a CAG trinucleotide repeat expansion in the gene encoding the protein huntingtin leads to a mutant variant of the protein, which causes neuronal dysfunction and ultimately leads to apoptosis (14, 738). Aggregates of mutant huntingtin protein are typically found inside the affected cell nuclei but can also be detected extracellularly in the CSF compartment (14, 739). The CSF level of mutant huntingtin has a correlation with the disease state both in clinical and preclinical studies, and reduction of mutant huntingtin production with antisense therapy leads to reduced CSF protein levels (738, 739). The transport of the normally intracellular mutant huntingtin protein to CSF was facilitated by leakage from dying cells but also via active secretion from nonapoptotic cells, followed by spreading through the brain extracellular space via glymphatic system efflux (531). It remains to be studied whether glymphatic inhibition leads to an aggravated phenotype of Huntington’s disease.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder affecting motor neurons in the spinal cord, brainstem, and motor cortex, leading to progressive paralysis (14). The disease can result from mutations in various genes, including TAR DNA-binding protein-43, superoxide dismutase-1, and fused-in-sarcoma, and is recognized by cytoplasmic inclusion bodies composed of proteins encoded by the mutated gene (740). In preclinical models of ALS, the glymphatic system aids in promoting efflux of disease-related proteins, and inhibition of the glymphatic system by knocking out AQP4 results in increased extracellular levels of toxic variants of superoxide dismutase-1 in cervical and lumbar spinal cord, without an increase in total tissue levels (741). Evidence points toward abnormal glymphatic function in mouse models of the disease, thus suggesting that impaired CNS clearance could play a role in its pathophysiology. As such, increased glymphatic influx was found at the cervical level of the spinal cord, whereas the clearance rates of tracer injected into lumbar spinal cord was reduced in an animal model of the disease (741). In several mouse models of ALS, there is loss of astrocyte AQP4 polarization, together with an overall increase in total AQP4 and GFAP concentrations, with these changes seemingly being more prominent in the ventral horn of the spinal cord compared with the dorsal horn (742). There was no loss of AQP4 polarization in postmortem samples from a small group of human patients with ALS, but several patients showed signs of abnormally high levels of AQP4 expression (742). Knocking out AQP4 in an animal model of ALS led to earlier and more rapid disease onset, reduced life span, and greater loss of motor strength (742). Thus preclinical evidence points toward glymphatic inhibition being part of the disease pathophysiology and suggests that the degree of glymphatic inhibition could affect the overall course of ALS.
13.6. Hydrocephalus and Intracranial Expansive Processes
13.6.1. Neonatal hydrocephalus.
A rat model of neonatal hydrocephalus is caused by a genetic mutation in the Ccdc39 gene, which impairs cilia function in choroid plexus and ependymal cells, thus diminishing intraventricular CSF flow and associated glymphatic function (693). In these mice, diminished inflow of CSF to the perivascular spaces of leptomeningeal surface arteries was seen from postnatal day 5, coinciding with the first signs of ventricular enlargement. At later developmental stages, when hydrocephalus becomes more prominent, there is a complete absence of CSF inflow to the brain. Interestingly, the hydrocephalus that can develop after germinal matrix hemorrhage is preventable by inhibiting astrogliosis and AQP4 mislocalization, which also rescued glymphatic function (FIGURE 26) (743).
13.6.2. Idiopathic normal pressure hydrocephalus.
Idiopathic normal pressure hydrocephalus (iNPH) affects 1–3% of all patients aged over 65 yr, and is recognized by progressive ventriculomegaly and a triad of neurological symptoms including gait ataxia, urinary incontinence, and dementia (744). Such patients suffer from impaired glymphatic influx and efflux, as indicated by monitoring the dynamics of signal enhancement and clearance of the MRI contrast agent gadobutrol delivered by lumbar puncture (164, 165). CSF flow is also affected in iNPH patients and shows reflux of CSF from the subarachnoid space into the ventricular system (165). Brain biopsies of iNPH patients have revealed AQP4 delocalization away from astrocytic endfeet, thus presenting a likely mechanism for the glymphatic dysfunction observed in this group of patients (533). Timely diversion of CSF via a surgical shunting procedure can arrest or alleviate the progression of symptoms, making iNPH one of the few treatable forms of dementia.
13.6.3. Glioma.
The outflow of CSF tracer to the blood was dramatically reduced in a mouse model of glioma, which is indicative of reduced CSF turnover (745). Similarly, efflux along cranial nerves was severely diminished, with an aberrant flow of CSF from cisterna magna toward the spinal cord rather than through the more proximal glymphatic pathway and cranial egress sites. Expanding intracranial masses like gliomas are likely to cause alterations in tissue organization and also elevate ICP, which can prevent CSF flow into otherwise healthy regions of the brain parenchyma. Although it remains to be studied in animal models, we speculate that similar symptoms and manifestation should be seen in nonneoplastic conditions with localized tissue expansion, e.g., noncommunicating hydrocephalus. VEGF-C-driven increase in dural lymphatic drainage has been shown to improve the immune response against glioblastoma (746).
13.7. Depression and Other Psychiatric Disorders
Major depressive disorder (MDD) has a yearly prevalence of 6% and likely affects 20% of all people at some point during their lifetime, thus creating an enormous socioeconomic burden and loss of well-being (747). The chief symptoms of MDD include persistently depressed mood, weight loss or gain, insomnia or hypersomnia, anhedonia, extreme guilt, and cognitive and memory difficulties (747). Patients with MDD tend to have episodes of relapse and recurrence, and especially late-life depression is further associated with increased risk of developing cognitive impairment and Alzheimer’s disease, as well as many other disorders. The pathophysiology behind MDD remains elusive, but hyperactivity in the hypothalamic-pituitary-adrenal axis and an aggravated stress response are consistent findings. The chronic unavoidable stress model in mice leads to depression-like symptoms including weight loss, immobility, and higher scores in behavioral tests of anxiety and anhedonia. In studies of glymphatic function in this mouse model, there was globally reduced glymphatic influx to the brain, with the largest decline relative to controls in the anterior part of the brain (748, 749). A reduced glymphatic efflux rate was also observed, manifesting as delayed clearance of exogenous CSF solutes such as amyloid-β from the brain parenchyma, with notably increased levels of endogenous amyloid-β1–42 found in the hippocampus and neocortex (748, 749). Glymphatic dysfunction was linked to reduced astrocytic expression of AQP4 and loss of its polarization in MDD mice. Furthermore, there were decreased levels of the AQP4-anchoring protein dystrophin-associated complex and lower expression of extracellular matrix proteins such as laminin and agrin, probably contributing to the loss of AQP4 polarization. Interestingly, similar patterns of abnormal AQP4 expression have been found in human autopsy samples of patients with a history of MDD, namely decreased endfeet AQP4 expression in orbitofrontal cortex, and lower expression levels of AQP4 mRNA in hippocampus and the noradrenergic locus coeruleus (750–752).
The typical hyperactivity in the hypothalamic-pituitary-adrenal axis occurring in MDD entails overproduction of glucocorticoid stress hormones, which may contribute to the cerebral atrophy reported in imaging studies. Produced in the adrenal glands, glucocorticoids are suspected of contributing to aspects of MDD, notably the cognitive symptoms (753). Treatment of naïve mice with the glucocorticoid receptor agonist dexamethasone produced glymphatic inhibition and AQP4 mislocalization similar to that seen in the mouse model of depression, whereas treatment with the glucocorticoid receptor antagonist mifepristone rectified the AQP4 mislocalization and glymphatic dysfunction (748). Moreover, treatment with the antidepressant drug fluoxetine, a serotonin-selective reuptake inhibitor, attenuated the glymphatic dysfunction in this model of depression (749).
13.8. Multiple Sclerosis and Inflammation
Multiple sclerosis is a disorder with focal demyelination in gray matter and white matter tracts throughout the nervous system caused by autoimmune neuroinflammation. In a mouse model of multiple sclerosis [experimental autoimmune encephalomyelitis (EAE)], there was a loss of AQP4 localization and glymphatic dysfunction in the spinal cord but not in brain (754). This atypical presentation was attributed to the greater susceptibility of the mouse spinal cord to the demyelination occurring after immunization with myelin basic protein. It seems plausible that involvement of the brain in EAE would likewise cause widespread AQP4 delocalization and impaired glymphatic function. The dural lymphatics, which have a close functional linkage with the glymphatic system, have been implicated in the same mouse model of multiple sclerosis (71). Lymphangiogenesis is not seen in the dorsal dural lymphatics in EAE, although they are considered important for trafficking immune cells to the brain parenchyma (71, 755). Indeed, ablation of dorsal dural lymphatics and inhibition of lymphangiogenesis were both associated with an attenuated inflammatory response, suggesting an important role of these vessels in neuroinflammation (71, 755). The dural sinuses are important immune interfaces between CNS derived antigens transported with the CSF and T cells circulating in the bloodstream (658). In the EAE model, increased numbers of T cells reactive to myelin oligodendrocyte glycoprotein are found in the dura mater (658). In the special case of inflammation associated with insertion of foreign bodies into the brain (e.g., EEG electrodes), the dorsal dura mater shows some lymphangiogenesis, which was functionally associated with increased inflow to the brain through the glymphatic system (756). Systemic inflammation caused by intraperitoneal injections of bacterial lipopolysaccharide (LPS) causes reduced glymphatic influx at 3 h after the injection (757). The increased heart rate occurring at 3 h post-LPS injection could contribute to the reduction in glymphatic function, whereas cerebral blood flow measured with laser Doppler, AQP4 polarization, astrocytic GFAP, BBB integrity, and brain levels of inflammatory cytokines were all normal in this model of systemic inflammation (3, 757). Overall, inflammatory conditions affect both intra- and extra-axial fluid transport, but the mechanisms are not fully understood. The effect of neuroinfectious disease on brain fluid transport represent and exciting topic for future studies.
13.9. Environmental Factors
13.9.1. Substance use disorders.
Excessive alcohol use places a major burden on public health and is a risk factor for developing several chronic diseases including dementia (761). Acute alcohol consumption affects glymphatic fluid influx and efflux, as does chronic heavy use. However, the mechanisms leading to impaired glymphatic function differ between the acute and chronic alcohol settings (762). In a mouse model of acute moderate alcohol intake, reduced influx and efflux in the glymphatic pathway was likely caused by reduced pulsatility of surface and penetrating arteries, as well as of ascending veins (762). The effect was likely a consequence of ethanol-induced release of β-endorphin, and, as such, the transient glymphatic inhibition could largely be prevented by coadministration of the opioid antagonist naloxone (762). In that study, the acute administration of ethanol did not affect AQP4 expression or polarization, and glymphatic inhibition recovered within 24 h. Conversely, chronic moderate or high alcohol administration in rats led to irreversible inhibition of glymphatic influx and efflux, which was likely mediated by astrogliosis and AQP4 delocalization (762, 763). Surprisingly, acute and chronic low doses of alcohol both served to boost glymphatic influx (763, 764). This potential beneficial effect of low-dose ethanol was associated with decreased expression of the astroglial marker GFAP and increased vessel pulsatility due to induction of endothelial nitric oxide synthase by ethanol. Similar to chronic moderate alcohol use, repeated cocaine administration for 5 days led to inhibition of glymphatic influx and efflux (706). The impairment was associated with reduced pulsatility of surface and penetrating arteries as well as in ascending veins, along with AQP4 delocalization away from the astrocytic endfeet (706).
13.10. Iatrogenic Conditions
13.10.1. Craniotomy.
Unilateral craniotomy acutely inhibits glymphatic influx bilaterally and is associated with decreasing pulsatility in penetrating arteries in mice (707). The inhibition of pulsatility lasts at least 2 wk after craniotomy but spontaneously improves to control levels after 28 days in all brain regions except the subcortical white matter. Fifty-six days after the craniotomy, glymphatic function in white matter was fully restored to baseline levels (707). The persistent glymphatic inhibition after craniotomy was associated with astrogliosis and AQP4 delocalization. Surprisingly, cranioplasty did not significantly accelerate the restoration of glymphatic function, although it did reduce the level of astrogliosis and attenuated the locomotor and cognitive impairment otherwise seen postcraniotomy. These latter observations may reflect that mice are capable of quickly generating a rather rigid membrane on top of the dura that may in part take over the function of the skull to e.g., restore ICP (707).
13.10.2. Anesthesia.
The activity in the glymphatic pathway is state dependent, being highest during deep sleep or under ketamine-xylazine anesthesia and lowest during wakefulness (1) and is further reduced by active running, as has been shown in mice (765). However, the activity of the glymphatic pathway during anesthesia is highly dependent on the type of anesthetic used, possibly related to differing pharmacological effects on noradrenergic signaling in brain (3); use of noradrenergic agonists in anesthetic regimens is apt to suppress glymphatic function. Electroencephalography showed increased delta band power in association with increased glymphatic influx, such that anesthetics with specific effects on the delta band would alter glymphatic function in predictable ways. There is a report that certain anesthetics can improve glymphatic function by increasing the polarized expression of AQP4 (766). For further details on what increases or decreases glymphatic function during different anesthetic agents, see sect. 9.
13.11. Therapeutic Options
13.11.1. Intrathecal therapy by way of glymphatic system.
The glymphatic system’s role in driving the brain wide distribution of CSF and its solutes has the potential to be used for administration of drugs and gene therapy. Several types of drugs are administered intrathecally, including anesthetics, antineoplastics, and antibiotics, while intrathecal antisense RNA therapy is also in rapid development. Manipulating the glymphatic system with drugs or intravenous infusion of hypertonic saline can boost delivery to brain of intrathecally delivered drugs like immunoglobulins, oxycodone, and naloxone (576, 767).
13.11.2. Vagus nerve stimulation.
Electrical stimulation of the vagus nerve is a Food and Drug Administration-approved treatment for several neurological disorders including migraine and refractory epilepsy, as well as MDD (768). Stimulation of the vagal nerve in mice led to substantial increase in glymphatic influx globally and an especially pronounced increase in the more rostral parts of the brain (768). Vagal stimulation is naturally associated with declining heart and respiratory rates, suggesting that the parasympathetic cardiorespiratory response could play a role in the increased glymphatic influx (768).
13.12. Outstanding Questions
How do openings in the BBB affect glymphatic flow?
Does reactive gliosis always impair glymphatic clearance due to loss of AQP4 polarization? Do other pathologic changes of astrocytes contribute to the impairment in glymphatic flow that is a hallmark of most, if not all, neurodegenerative disorders?
How does microglial cell activation affect glymphatic flow?
Does vascular amyloidosis suppress or even reverse the direction of CSF influx along the periarterial spaces?
How do chronic derangements in glymphatic function (glymphopathies) contribute to the development of neurodegenerative diseases of aging (e.g., Alzheimer’s and Parkinson’s disease) that are characterized by abnormal proteostasis and deposition of neurotoxic proteins?
13.13. Interim Summary Section
Healthy aging leads to a decline in function of the glymphatic pathway. This attenuation is a likely multifactorial in nature. Astrogliosis is linked to a loss of AQP4 polarization toward astrocytic endfeet, and reduced arterial wall compliance leads to less efficient CSF pumping in the perivascular space. Many other factors, including impaired sleep and a lower CSF production rate might also be in play.
Amyloid-β accumulates in brain as a function of natural aging, lack of sleep, obstruction of CSF egress pathways, and glymphatic inhibition. Amyloid-β itself suppresses glymphatic fluid transport, which may be a feed-forward process in Alzheimer’s disease progression.
Astrogliosis and loss of AQP4 polarization toward astrocytic endfeet are common denominators of all neurological disorders studied so far that inflict perturbations in the glymphatic pathway. Loss of glymphatic pathway function as a result of astrogliosis or loss of AQP4 polarity has been documented in experimental models of Alzheimer’s disease, Parkinson’s disease, TBI, subarachnoid hemorrhage, iNPH, and small vessel disease.
Edema formation after acute ischemic stroke is the result of a rapid increase in influx of CSF through the glymphatic system facilitated by AQP4. Thus early tissue swelling after stroke is caused by fluid influx from the CSF compartment rather than the vascular compartment, questioning the traditional models of edema formation.
14. SUMMARY
This review addresses the functional roles of brain fluid transport and its underlying structural organization. Our main thesis is that brain tissue, much like peripheral tissues, has a basic homeostatic requirement for fluid flow to disperse metabolites and export waste products. Due to the specialized properties of nervous tissue, fluid transport has accommodated the physiological requirements of an electrically active organ that is suspended in fluid within a closed cavity. Other complicating factors for brain fluid transport include the existence of a tight BBB that largely eliminates capillary filtration and the lack of a parenchymal lymphatic system. The restrictions placed on fluid transport within neural tissue have demanded the evolution of the sophisticated network of extra-axial and intra-axial fluid transport systems described in this review. The specific adaptions of this network include the elaborate structure of the choroid plexus, the perivascular spaces, and the polarized expression of AQP4 water channels on astrocytes aligned with the capillary bed. Together this arrangement enables unidirectional fluid transport and recapitulates the simple organization of capillary filtration and extracellular tissue flow in peripheral tissues (FIGURE 1). Following this logic, the ongoing explorations of brain fluid flow have a firm basis in the established knowledge about blood perfusion and lymphatic flow in peripheral tissue. For excellent reviews, see Refs. 8, 335, 338, 674.
Yet, the most important difference from peripheral tissue is that the brain possesses the unique ability to modulate tissue fluid transport dynamically. During wakefulness, extracellular fluid flow falls almost to zero, likely reflecting the requirement of higher brain function for absolute precision of neural transmission that precludes the broad dispersion of neuroactive substances by active fluid transport. For example, synaptic spillover of glutamate reduces the temporal and spatial specificity of glutamatergic signaling. The expansion of the extracellular space that supports glymphatic flow may preclude the neurocomputional demands of the waking state. Sleep, on the other hand, is a state in which the brain is uncoupled from its surroundings and local synapses fire in synchrony, thus presenting the brain an opportunity to clean up the metabolic mess that accumulated during wakefulness. In fact, the biological necessity for sleep may reflect the brain’s needs to reestablish homeostasis and clear certain toxic proteins such as amyloid-β as well as small molecules like adenosine and lactate that had accumulated in the awake state.
We have in each section of this review highlighted some of the most pertinent and interesting questions to be addressed in the near future, but our list is by no means comprehensive. We also expect that equally novel and interesting aspects of brain fluid transport will be revealed and explored over the next few years. Of particular interest is how fluid transport interacts with neural circuits and whether a dysregulation of fluid transport and ion homeostasis can contribute to abnormal brain states such as psychosis or sun-downing, the late-day confusion often plaguing patients with Alzheimer’s disease.
We give an account of the history of thinking about the fluid content in brain, beginning with the description in the Edwin Smith papyrus from ancient Egypt. The ventricular system came to be described in the renaissance times, and details of its anatomy were established in the 19th century. We stress that many aspects of brain fluid transport remain to be discovered, and this exploration is driven by methodological innovations in physiology, microscopy, and brain imaging techniques. The glymphatic/lymphatic systems represent a new biological concept that likely participates in most, if not all, physiological and pathological processes in the CNS. As such, the field of research about the glymphatic system is rapidly evolving and presents an enormous opportunity for young researchers viewing a fresh topic with a fresh mind. Novel discoveries about the glymphatic system run the full gamut of neurobiology, spanning from evolutionary and developmental biology, the sleep-wake cycle, glia and vascular research, normal brain aging, and acute and chronic neurological disorders.
GRANTS
This work is supported by the Novo Nordisk and Lundbeck Foundations, the European Research Council (ERC) under the European Union’s (EU) Horizon 2020 Research and Innovation Program Grant Agreement 742112, EU Horizon 2020 Research and Innovation Program Grant 666881 (SVDs@target), National Institute of Neurological Disorders and Stroke Grant R01-NS-100366, National Institute on Aging Grant RF1-AG-057575, US Army Research Office Grant Multidisciplinary University Research Initiative W911NF1910280 (to M.N.), Foundation Leducq Transatlantic Networks of Excellence Program, and the Adelson and the Simon Foundations.
DISCLAIMERS
The views and conclusions contained in this opinion article are solely those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the National Institutes of Health, Army Research Office, or the US government. The US government is authorized to reproduce and distribute reprints for government purposes notwithstanding any copyright notation herein
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.N. conceived and designed the review; M.K.R., H.M. and M.N. prepared figures; M.K.R., H.M. and M.N. drafted manuscript; M.K.R., H.M. and M.N. edited and revised manuscript; M.K.R., H.M. and M.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dan Xue and Austin C. Fisher for creating all graphic content included in this review. Dan Xue designed the graphical abstract and FIGURES 1, 3, and 8–27 and edited all figures. Austin C. Fisher designed FIGURES 2, 4, 5, 6, and 7. We also thank Kjeld Møllgård, Peter Bork, and Henrik Clausen, Copenhagen University, Denmark; Helge Wiig, University of Bergen, Norway; Doug Kelley and Jack Thomas, University of Rochester; Jeff Iliff, Washington University, Seattle, WA; John Rash, Colorado State University; Alex Verkhratsky, Manchester University, UK; Lynn Bilston, University of New South Wales, Australia; and Paul Cumming, Bern University, Switzerland for valuable discussions and comments on the manuscript and the principles underlying brain fluid transport.
FIGURE 27.
Technological approaches to imaging of glymphatic system. Different techniques used to image the glymphatic system plotted in a 3-dimensional plot depicting the typical qualities of each imaging modality in 3 different measures, namely temporal resolution, spatial resolution, and volume imaging. It should be noted that any of these imaging modalities can be adjusted in a manner changing their parameters. For example, increasing temporal resolution of functional MRI brings a loss in spatial resolution, as occurs in ultrafast magnetic encephalography, or is at the expense of limiting the brain volume imaged (407, 495). Histology provides the highest spatial resolution and affords brain wide imaging, albeit with a loss of precise temporal control, and at a cost of considerable workload. Furthermore, histological preparations all suffer from postmortem changes such as loss of extracellular volume, as well as preparation artifacts (shrinkage) that might confound the results. Tracers are traditionally delivered into cisterna magna or intrathecally (550–553), but noninvasive approaches to imaging fluid transport are under fast development. CT, computed topography; Macro, macroscopic transcranial imaging; PET, positron emission tomography; MRI, magnetic resonance imaging; 2p, two-photon microscopy.
REFERENCES
- 1.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolic clearance from the adult brain. Science 342: 373–377, 2013. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nedergaard M. Neuroscience. Garbage truck of the brain. Science 340: 1529–1530, 2013. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 5: eaav5447, 2019. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat 3: 553–567, 2007. [PMC free article] [PubMed] [Google Scholar]
- 6.Waters F, Chiu V, Atkinson A, Blom JD. Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry 9: 303–303, 2018. doi: 10.3389/fpsyt.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73: 1–78, 1993. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92: 1005–1060, 2012. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 9.Alitalo K. The lymphatic vasculature in disease. Nat Med 17: 1371–1380, 2011. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 10.Saunders NR, Dziegielewska KM, Mollgard K, Habgood MD. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J Physiol 596: 5723–5756, 2018. doi: 10.1113/JP275376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16: 1–13, 2004. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25: 5–23, 2005. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkhratsky A, Ho MS, Parpura V. Evolution of neuroglia. Adv Exp Med Biol 1175: 15–44, 2019. doi: 10.1007/978-981-13-9913-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4: 49–60, 2003. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 15.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845–861, 2014. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johanson CE. J Choroid Plexus–Cerebrospinal Fluid Circulatory Dynamics: Impact on Brain Growth, Metabolism, and Repair. Totowa, NJ: Humana Press, 2008. [Google Scholar]
- 17.Lehtinen MK, Bjornsson CS, Dymecki SM, Gilbertson RJ, Holtzman DM, Monuki ES. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci 33: 17553–17559, 2013. doi: 10.1523/JNEUROSCI.3258-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11: 10, 2014. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang TY, Chung HW, Chen MY, Giiang LH, Chin SC, Lee CS, Chen CY, Liu YJ. Supratentorial cerebrospinal fluid production rate in healthy adults: quantification with two-dimensional cine phase-contrast MR imaging with high temporal and spatial resolution. Radiology 233: 603–608, 2004. doi: 10.1148/radiol.2332030884. [DOI] [PubMed] [Google Scholar]
- 20.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci 23: 9254–9262, 2003. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9: 4878, 2018. doi: 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Pla V, Du T, Kress BT, Wang X, Plog BA, Thrane AS, Lundgaard I, Abe Y, Yasui M, Thomas JH, Xiao M, Hirase H, Asokan A, Iliff JJ, Nedergaard M. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 7: e40070, 2018. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K. Development and plasticity of meningeal lymphatic vessels. J Exp Med 214: 3645–3667, 2017. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruoslahti E. Brain extracellular matrix. Glycobiology 6: 489–492, 1996. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- 26.Hauglund NL, Pavan C, Nedergaard M. Cleaning the sleeping brain–the potential restorative function of the glymphatic system. Curr Opin Physiol 15: 1–6, 2020. doi: 10.1016/j.cophys.2019.10.020. [DOI] [Google Scholar]
- 27.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 17: 1016–1024, 2018. doi: 10.1016/S1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4: AN20120010, 2012. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry 7: 272–281, 2020. doi: 10.1016/S2215-0366(19)30302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glickstein M. Golgi and Cajal: the neuron doctrine and the 100th anniversary of the 1906 Nobel Prize. Curr Biol 16: R147–151, 2006. doi: 10.1016/j.cub.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 31.Deisenhammer F, Sellebjerg F, Teunissen CE, Tumani H. Cerebrospinal Fluid Clinical Neurology. Cham, Switzerland: Springer International, 2015, p. 1–441. doi: 10.1007/978-3-319-01225-4. [DOI] [Google Scholar]
- 32.Herbowski L. The maze of the cerebrospinal fluid discovery. Anat Res Int 2013: 596027, 2013., doi: 10.1155/2013/596027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cserr HF. Physiology of the choroid plexus. Physiol Rev 51: 273–311, 1971. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- 34.Davson H. Review lecture. The blood-brain barrier. J Physiol 255: 1–28, 1976. doi: 10.1113/jphysiol.1976.sp011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woollam DH, Millen JW. Perivascular spaces of the mammalian central nervous system. Biol Rev 29: 251–283, 1954. doi: 10.1111/j.1469-185X.1954.tb00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Middendorp JJ, Sanchez GM, Burridge AL. The Edwin Smith papyrus: a clinical reappraisal of the oldest known document on spinal injuries. Eur Spine J 19: 1815–1823, 2010. doi: 10.1007/s00586-010-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aristotle, Thompson DA. Historia Animalium. Oxford, UK: The Clarendon Press, 1829. [Google Scholar]
- 38.Milhorat TH. The third circulation revisited. J Neurosurg 42: 628–645, 1975. doi: 10.3171/jns.1975.42.6.0628. [DOI] [PubMed] [Google Scholar]
- 39.Prolo D. Harvey Cushing selected papers on neurosurgery. Arch Surg 99: 676, 1969. doi: 10.1001/archsurg.1969.01340170128031. [DOI] [Google Scholar]
- 40.Catani M, Sandrone S. Brain Renaissance: from Vesalius to Modern Neuroscience. Oxford, UK: Oxford University Press, 2015. [Google Scholar]
- 41.Di Ieva A, Yaşargil MG. Liquor cotunnii: the history of cerebrospinal fluid in Domenico Cotugno’s work. Neurosurgery 63: 352–358, 2008. doi: 10.1227/01.NEU.0000320438.99843.9F. [DOI] [PubMed] [Google Scholar]
- 42.Weed LH. Meninges and cerebrospinal fluid. J Anat 72: 181–215, 1938. [PMC free article] [PubMed] [Google Scholar]
- 43.Mott FW. The Oliver-Sharpey Lectures on the cerebro-spinal fluid. Lancet 176: 1–8, 1910. doi: 10.1016/S0140-6736(00)52276-4. [DOI] [Google Scholar]
- 44.de Rougemont J, Ames A 3rd, Nesbett FB, Hofmann HF. Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J Neurophysiol 23: 485–495, 1960. doi: 10.1152/jn.1960.23.5.485. [DOI] [PubMed] [Google Scholar]
- 45.Hassin GB. Changes in the brain in plexectomized dogs. Arch Neur Psych 38: 1224–1239, 1937. doi: 10.1001/archneurpsyc.1937.02260240104008. [DOI] [Google Scholar]
- 46.Cushing H. The third circulation and its channels. Lancet 2: 851–857, 1925. [Google Scholar]
- 47.Weed LH. The absorption of cerebrospinal fluid into the venous system. Am J Anat 31: 191–221, 1923. doi: 10.1002/aja.1000310302. [DOI] [Google Scholar]
- 48.Casper ST. Atlantic conjunctures in Anglo-American Neurology: Lewis H. Weed and Johns Hopkins Neurology, 1917-1942. Bull History Med 82: 646–671, 2008. doi: 10.1353/bhm.0.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Key R. Studien in der Anatomie des Nervensystems und des Bindesgewebe. Stockholm, Sweden: Samson and Wallin, 1876. [Google Scholar]
- 50.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE. and colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel D. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 16: 137–153, 2020. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 51.Obersteiner H, Hill A. The Anatomy of the Central Nervous Organs in Health and Disease. Lancaster, PA: Blakiston, 1890. [Google Scholar]
- 52.McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg 59: 369–383, 1983. doi: 10.3171/jns.1983.59.3.0369. [DOI] [PubMed] [Google Scholar]
- 53.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128: 309–316, 2011. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33: 18190–18199, 2013. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 11: 4411, 2020. doi: 10.1038/s41467-020-18115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34: 16180–16193, 2014. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: 1299–1309, 2013. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proulx ST. Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci 78: 2429–2457, 2021. doi: 10.1007/s00018-020-03706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balslev Y, Saunders NR, Mollgard K. Ontogenetic development of diffusional restriction to protein at the pial surface of the rat brain: an electron microscopical study. J Neurocytol 26: 133–148, 1997. [ doi: 10.1023/A:1018527928760. [DOI] [PubMed] [Google Scholar]
- 60.Lewandowsky M. Zur lehre der cerebrospinalflussigkeit. Z Klin Med 40: 480–494, 1900. [Google Scholar]
- 61.Saunders NR, Dreifuss JJ, Dziegielewska KM, Johansson PA, Habgood MD, Mollgard K, Bauer HC. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci 8: 404, 2014. doi: 10.3389/fnins.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cutler RW, Page L, Galicich J, Watters GV. Formation and absorption of cerebrospinal fluid in man. Brain 91: 707–720, 1968. doi: 10.1093/brain/91.4.707. [DOI] [PubMed] [Google Scholar]
- 63.Cserr HF, Cooper DN, Milhorat TH. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res 25: 461–473, 1977. doi: 10.1016/S0014-4835(77)80041-9. [DOI] [PubMed] [Google Scholar]
- 64.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol Renal Physiol 240: F319–F328, 1981. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 65.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40: 648–677, 1969. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘Paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: 47–63, 1985. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 67.Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13: 379–394, 2018. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2121: 991–999, 2015. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandrone S, Moreno-Zambrano D, Kipnis J, van Gijn J. A (delayed) history of the brain lymphatic system. Nat Med 25: 538–540, 2019. doi: 10.1038/s41591-019-0417-3. [DOI] [PubMed] [Google Scholar]
- 70.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560: 185–191, 2018. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21: 1380–1391, 2018. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferris CF. Rethinking the conditions and mechanism for glymphatic clearance. Front Neurosci 15: 1–8, 2021. doi: 10.3389/fnins.2021.624690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 105: 7–17, 2001. doi: 10.1016/S0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 74.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci 16: 445–457, 2015. doi: 10.1038/nrn3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev 99: 21–78, 2019. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walchli T, Wacker A, Frei K, Regli L, Schwab ME, Hoerstrup SP, Gerhardt H, Engelhardt B. Wiring the vascular network with neural cues: a CNS perspective. Neuron 87: 271–296, 2015. doi: 10.1016/j.neuron.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 77.Munk AS, Wang W, Bechet NB, Eltanahy AM, Cheng AX, Sigurdsson B, Benraiss A, Mae MA, Kress BT, Kelley DH, Betsholtz C, Mollgard K, Meissner A, Nedergaard M, Lundgaard I. PDGF-B is required for development of the glymphatic system. Cell Rep 26: 2955–2969, 2019. doi: 10.1016/j.celrep.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468: 562–566, 2010. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mashayekhi F, Draper CE, Bannister CM, Pourghasem M, Owen-Lynch PJ, Miyan JA. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain 125: 1859–1874, 2002. doi: 10.1093/brain/awf182. [DOI] [PubMed] [Google Scholar]
- 80.Johal J, Paulk PB, Oakes PC, Oskouian RJ, Loukas M, Tubbs RS. A comprehensive review of the foramina of Luschka: history, anatomy, embryology, and surgery. Childs Nerv Syst 33: 1459–1462, 2017. doi: 10.1007/s00381-017-3480-4. [DOI] [PubMed] [Google Scholar]
- 81.Gomez DG, DiBenedetto AT, Pavese AM, Firpo A, Hershan DB, Potts DG. Development of arachnoid villi and granulations in man. Acta Anat (Basel) 111: 247–258, 1982. doi: 10.1159/000145473. [DOI] [PubMed] [Google Scholar]
- 82.Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M. Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol 283: R869–R876, 2002. doi: 10.1152/ajpregu.00173.2002. [DOI] [PubMed] [Google Scholar]
- 83.Mollanji R, Papaiconomou C, Boulton M, Midha R, Johnston M. Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 281: R1215–R1223, 2001. doi: 10.1152/ajpregu.2001.281.4.R1215. [DOI] [PubMed] [Google Scholar]
- 84.Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun 11: 354, 2020. doi: 10.1038/s41467-019-14195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowery LA, Sive H. Totally tubular: the mystery behind function and origin of the brain ventricular system. Bioessays 31: 446–458, 2009. doi: 10.1002/bies.200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chau KF, Springel MW, Broadbelt KG, Park HY, Topal S, Lun MP, Mullan H, Maynard T, Steen H, LaMantia AS, Lehtinen MK. Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev Cell 35: 789–802, 2015. doi: 10.1016/j.devcel.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bueno D, Parvas M, Nabiuni M, Miyan J. Embryonic cerebrospinal fluid formation and regulation. Semin Cell Dev Biol 102: 3–12, 2020. doi: 10.1016/j.semcdb.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Schoenwolf GC, Desmond ME. Neural tube occlusion precedes rapid brain enlargement. J Exp Zool 230: 405–407, 1984. doi: 10.1002/jez.1402300309. [DOI] [PubMed] [Google Scholar]
- 89.Desmond ME. Description of the occlusion of the spinal cord lumen in early human embryos. Anat Rec 204: 89–93, 1982. doi: 10.1002/ar.1092040112. [DOI] [PubMed] [Google Scholar]
- 90.Desmond ME, Levitan ML. Brain expansion in the chick embryo initiated by experimentally produced occlusion of the spinal neurocoel. Anat Rec 268: 147–159, 2002. doi: 10.1002/ar.10146. [DOI] [PubMed] [Google Scholar]
- 91.Bjarkam CR. Neuroanatomi. Copenhagen, Denmark: Munksgaard, 2015, p. 399. [Google Scholar]
- 92.Martin C, Bueno D, Alonso MI, Moro JA, Callejo S, Parada C, Martin P, Carnicero E, Gato A. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev Biol 297: 402–416, 2006. doi: 10.1016/j.ydbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 93.Parvas M, Parada C, Bueno D. A blood-CSF barrier function controls embryonic CSF protein composition and homeostasis during early CNS development. Dev Biol 321: 51–63, 2008. doi: 10.1016/j.ydbio.2008.05.552. [DOI] [PubMed] [Google Scholar]
- 94.Desmond ME, Jacobson AG. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev Biol 57: 188–198, 1977. doi: 10.1016/0012-1606(77)90364-5. [DOI] [PubMed] [Google Scholar]
- 95.Dziegielewska KM, Ek J, Habgood MD, Saunders NR. Development of the choroid plexus. Microsc Res Tech 52: 5–20, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 96.Ruhrberg C, Bautch VL. Neurovascular development and links to disease. Cell Mol Life Sci 70: 1675–1684, 2013. doi: 10.1007/s00018-013-1277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Rahilly R, Muller F. The meninges in human development. J Neuropathol Exp Neurol 45: 588–608, 1986. doi: 10.1097/00005072-198609000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Marin-Padilla M. The human brain intracerebral microvascular system: development and structure. Front Neuroanat 6: 38, 2012. doi: 10.3389/fnana.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tata M, Ruhrberg C, Fantin A. Vascularisation of the central nervous system. Mech Dev 138: 26–36, 2015. doi: 10.1016/j.mod.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Møllgård K, Balslev Y, Lauritzen B, Saunders NR. Cell junctions and membrane specializations in the ventricular zone (germinal matrix) of the developing sheep brain: a CSF-brain barrier. J Neurocytol 16: 433–444, 1987. doi: 10.1007/BF01668498. [DOI] [PubMed] [Google Scholar]
- 101.Whish S, Dziegielewska KM, Mollgard K, Noor NM, Liddelow SA, Habgood MD, Richardson SJ, Saunders NR. The inner CSF-brain barrier: developmentally controlled access to the brain via intercellular junctions. Front Neurosci 9: 16, 2015. doi: 10.3389/fnins.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 7: a020412, 2015. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang AC, Stevens MY, Chen MB, Lee DP, Stahli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, Vest RT, Chaney A, Lehallier B, Olsson N, Du Bois H, Hsieh R, Cropper HC, Berdnik D, Li L, Wang EY, Traber GM, Bertozzi CR, Luo J, Snyder MP, Elias JE, Quake SR, James ML, Wyss-Coray T. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583: 425–430, 2020. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell 163: 1064–1078, 2015. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, Von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/β-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183: 409–417, 2008. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161: 653–660, 2003. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Castro Dias M, Coisne C, Lazarevic I, Baden P, Hata M, Iwamoto N, Francisco DM, Vanlandewijck M, He L, Baier FA, Stroka D, Bruggmann R, Lyck R, Enzmann G, Deutsch U, Betsholtz C, Furuse M, Tsukita S, Engelhardt B. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci Rep 9: 1–16, 2019. doi: 10.1038/s41598-018-36731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brochner CB, Holst CB, Mollgard K. Outer brain barriers in rat and human development. Front Neurosci 9: 75, 2015. doi: 10.3389/fnins.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brocklehurst G. The development of the human cerebrospinal fluid pathway with particular reference to the roof of the fourth ventricle. J Anat 105: 467–475, 1969. [PMC free article] [PubMed] [Google Scholar]
- 110.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513: 532–541, 2009. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 111.Burger JR, George MA, Leadbetter C, Shaikh F. The allometry of brain size in mammals. J Mammal 100: 276–283, 2019. doi: 10.1093/jmammal/gyz043. [DOI] [Google Scholar]
- 112.Armstrong E. A look at relative brain size in mammals. Neurosci Lett 34: 101–104, 1982. doi: 10.1016/0304-3940(82)90159-8. [DOI] [PubMed] [Google Scholar]
- 113.Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, Nguyen KC, Tang LT, Bayer EA, Duerr JS, Bulow HE, Hobert O, Hall DH, Emmons SW. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 571: 63–71, 2019. doi: 10.1038/s41586-019-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Craigie EH. The architecture of the cerebral capillary bed. Biol Rev Camb Philos Soc 20: 133–146, 1945. doi: 10.1111/j.1469-185x.1945.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 115.Schirmeier S, Klambt C. The Drosophila blood-brain barrier as interface between neurons and hemolymph. Mech Dev 138: 50–55, 2015. doi: 10.1016/j.mod.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Brightman MW. Perivascular spaces in the brains of Necturus maculosus Rafinesque and Mus norwegicus albinus. Anat Rec 117: 427–447, 1953. doi: 10.1002/ar.1091170307. [DOI] [PubMed] [Google Scholar]
- 117.Gillilan LA. A comparative study of the extrinsic and intrinsic arterial blood supply to brains of submammalian vertebrates. J Comp Neurol 130: 175–196, 1967. doi: 10.1002/cne.901300302. 7251938 [DOI] [Google Scholar]
- 118.Ernst S. Brain Function and the Evolution of Cerebral Vascularization. New York: The American Museum of Natural History New York, 1962. [Google Scholar]
- 119.Snyder GK, Gannon B, Baudinette RV, Nelson J. Functional organization of cerebral microvasculature in a marsupial, the northern quoll (Dasyurus hallucatus). J Exp Zool 251: 349–354, 1989. doi: 10.1002/jez.1402510311. [DOI] [PubMed] [Google Scholar]
- 120.Wislocki GB, Campbell AC. The unusual manner of vascularization of the brain of the opossum (Didelphys virginiana). Anat Rec 67: 177–191, 1937. doi: 10.1002/ar.1090670205. [DOI] [Google Scholar]
- 121.Lazzari M, Franceschini V. Structural and spatial organisation of brain parenchymal vessels in the lizard, Podarcis sicula: a light, transmission and scanning electron microscopy study. J Anat 197: 167–175, 2000. doi: 10.1046/j.1469-7580.2000.19720167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bueno D, Garcia-Fernandez J. Evolutionary development of embryonic cerebrospinal fluid composition and regulation: an open research field with implications for brain development and function. Fluids Barriers CNS 13: 5, 2016. doi: 10.1186/s12987-016-0029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cserr HF, Bundgaard M. Blood-brain interfaces in vertebrates: a comparative approach. Am J Physiol Regul Integr Comp Physiol 246: R277–R288, 1984. [DOI] [PubMed] [Google Scholar]
- 124.Jones HC. Comparative aspects of the cerebrospinal fluid systems in vertebrates. Sci Prog 66: 171–190, 1979. [PubMed] [Google Scholar]
- 125.Bakay L. Phylogenesis of the perivascular spaces of the brain. Nature 160: 789–790, 1947. doi: 10.1038/160789a0. [DOI] [PubMed] [Google Scholar]
- 126.Van Rijssel RG. Circulation of cerebrospinal fluid in Carassius gibelio. Arch Neurol Psychiatry 56: 522–543, 1946. doi: 10.1001/archneurpsyc.1946.02300220035003. [DOI] [PubMed] [Google Scholar]
- 127.Bundgaard M, Cserr HF. Barrier membranes at the outer surface of the brain of an elasmobranch, Raja erinacea. Cell Tissue Res 265: 113–120, 1991. doi: 10.1007/BF00318145. [DOI] [PubMed] [Google Scholar]
- 128.Momose Y, Kohno K, Ito R. Ultrastructural study on the meninx of the goldfish brain. J Comp Neurol 270: 327–336, 1988. doi: 10.1002/cne.902700303. [DOI] [PubMed] [Google Scholar]
- 129.Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, Kim KW. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull 75: 619–628, 2008. doi: 10.1016/j.brainresbull.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 130.Gotow T, Hashimoto PH. Plasma membrane organization of astrocytes in elasmobranchs with special reference to the brain barrier system. J Neurocytol 13: 727–742, 1984. doi: 10.1007/BF01148491. [DOI] [PubMed] [Google Scholar]
- 131.Hannocks MJ, Pizzo ME, Huppert J, Deshpande T, Abbott NJ, Thorne RG, Sorokin L. Molecular characterization of perivascular drainage pathways in the murine brain. J Cereb Blood Flow Metab 38: 669–686, 2018. doi: 10.1177/0271678X17749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gray EG. Electron microscopy of the glio-vascular organization of the brain of octopus. Phil Trans Royal Soc London Series B Biol Sci 255: 13–32, 1997. [Google Scholar]
- 133.Stephens PR, Young JZ. The glio-vascular system of cephalopods. Phil Trans Royal Soc London Series B Biol Sci 255: 1–12, 1997. [Google Scholar]
- 134.Bundgaard M, Abbott NJ. Fine structure of the blood-brain interface in the cuttlefish Sepia officinalis (Mollusca, Cephalopoda). J Neurocytol 21: 260–275, 1992. doi: 10.1007/BF01224760. [DOI] [PubMed] [Google Scholar]
- 135.Zhang X, Mao Y, Huang Z, Qu M, Chen J, Ding S, Hong J, Sun T. Transcriptome analysis of the Octopus vulgaris central nervous system. PLoS One 7: e40320–11, 2012. doi: 10.1371/journal.pone.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brightman MW, Reese TS, Olsson Y, Klatzo I. Morphologic aspects of the blood-brain barrier to peroxidase in elasmobranchs. Prog Neuropathol 1: 146–161, 1971. [Google Scholar]
- 137.Quinonez-Silvero C, Hubner K, Herzog W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev Biol 457: 181–190, 2020. doi: 10.1016/j.ydbio.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 138.van Lessen M, Shibata-Germanos S, van Impel A, Hawkins TA, Rihel J, Schulte-Merker S. Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. eLife 6: 1–24, 2017. doi: 10.7554/eLife.25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Verkhratsky A, Ho MS, Zorec R, Parpura V. Neuroglia in Neurodegenerative Diseases. New York: Springer, 2019, p. 129–148. [Google Scholar]
- 140.Verkhratsky A, Nedergaard M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc Lond B Biol Sci 371: 20150428, 2016. doi: 10.1098/rstb.2015.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci 29: 3276–3287, 2009. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12: 342–353, 2013. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hartline DK. The evolutionary origins of glia. Glia 59: 1215–1236, 2011. doi: 10.1002/glia.21149. [DOI] [PubMed] [Google Scholar]
- 144.Bodega G, Suarez I, Rubio M, Fernandez B. Distribution and characteristics of the different astroglial cell types in the adult lizard (Lacerta lepida) spinal cord. Anat Embryol (Berl) 181: 567–575, 1990. doi: 10.1007/BF00174628. [DOI] [PubMed] [Google Scholar]
- 145.King JS. A comparative investigation of neuroglia in representative vertebrates: a silver carbonate study. J Morphol 119: 435–465, 1966. doi: 10.1002/jmor.1051190405. [DOI] [PubMed] [Google Scholar]
- 146.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels–from atomic structure to clinical medicine. J Physiol 542: 3–16, 2002. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Finn RN, Chauvigne F, Hlidberg JB, Cutler CP, Cerda J. The lineage-specific evolution of aquaporin gene clusters facilitated tetrapod terrestrial adaptation. PLoS One 9: e113686, 2014. doi: 10.1371/journal.pone.0113686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Benga G. Birth of water channel proteins-the aquaporins. Cell Biol Int 27: 701–709, 2003. doi: 10.1016/S1065-6995(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 149.Hohmann I, Bill RM, Kayingo I, Prior BA. Microbial MIP channels. Trends Microbiol 8: 33–38, 2000. doi: 10.1016/s0966-842x(99)01645-5. [DOI] [PubMed] [Google Scholar]
- 150.Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P. The role of aquaporins in cellular and whole plant water balance. Biochim Biophys Acta 1465: 324–342, 2000. doi: 10.1016/s0005-2736(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 151.Magni F, Sarto C, Ticozzi D, Soldi M, Bosso N, Mocarelli P, Kienle MG. Proteomic knowledge of human aquaporins. Proteomics 6: 5637–5649, 2006. doi: 10.1002/pmic.200600212. [DOI] [PubMed] [Google Scholar]
- 152.Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624, 2008. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 153.Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S. Aquaporins in yeasts and filamentous fungi. Biol Cell 97: 487–500, 2005. doi: 10.1042/BC20040144. [DOI] [PubMed] [Google Scholar]
- 154.Finn RN, Chauvigne F, Stavang JA, Belles X, Cerda J. Insect glycerol transporters evolved by functional co-option and gene replacement. Nat Commun 6: 7814, 2015. doi: 10.1038/ncomms8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev 93: 1543–1562, 2013. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gleiser C, Wagner A, Fallier-Becker P, Wolburg H, Hirt B, Mack AF. Aquaporin-4 in astroglial cells in the CNS and supporting cells of sensory organs-a comparative perspective. Int J Mol Sci 17, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eidsvaag VA, Enger R, Hansson HA, Eide PK, Nagelhus EA. Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels. Glia 65: 964–973, 2017. doi: 10.1002/glia.23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grupp L, Wolburg H, Mack AF. Astroglial structures in the zebrafish brain. J Comp Neurol 518: 4277–4287, 2010. doi: 10.1002/cne.22481. [DOI] [PubMed] [Google Scholar]
- 159.Cutler CP, Maciver B, Cramb G, Zeidel M. Aquaporin 4 is a ubiquitously expressed isoform in the dogfish (Squalus acanthias) shark. Front Physiol 2: 107, 2011. doi: 10.3389/fphys.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoshimura K, Sugiura K, Ohmori Y, Aste N, Saito N. Immunolocalization of aquaporin-4 in the brain, kidney, skeletal muscle, and gastro-intestinal tract of chicken. Cell Tissue Res 344: 51–61, 2011. doi: 10.1007/s00441-011-1134-5. [DOI] [PubMed] [Google Scholar]
- 161.Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open 4: 2058460115609635, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eide PK, Vatnehol SA, Emblem KE, Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep 8: 7194, 2018. doi: 10.1038/s41598-018-25666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 45: 3092–3096, 2014. doi: 10.1161/STROKEAHA.114.006617. [DOI] [PubMed] [Google Scholar]
- 164.Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, Mardal KA, Eide PK. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3: e121537, 2018. doi: 10.1172/jci.insight.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ringstad G, Vatnehol SA, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140: 2691–2705, 2017. doi: 10.1093/brain/awx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, Gakuba C, Emery E, Hantraye P, Vivien D, Aron-Badin R, Gaberel T. Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke 48: 2301–2305, 2017. doi: 10.1161/STROKEAHA.117.017014. [DOI] [PubMed] [Google Scholar]
- 167.Bèchet NB, Shanbhag NC, Lundgaard I. Glymphatic pathways in the gyrencephalic brain. J Cereb Blood Flow Metab 271678X21996175, 2021. doi: 10.1177/0271678x21996175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meng Y, Abrahao A, Heyn CC, Bethune AJ, Huang Y, Pople CB, Aubert I, Hamani C, Zinman L, Hynynen K, Black SE, Lipsman N. Glymphatics visualization after focused ultrasound-induced blood–brain barrier opening in humans. Ann Neurol 86: 975–980, 2019. doi: 10.1002/ana.25604. [DOI] [PubMed] [Google Scholar]
- 169.Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, Iliff JJ. Association of perivascular localization of aquaporin-4 with cognition and alzheimer disease in aging brains. JAMA Neurol 74: 91–99, 2017. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 170.Zhou Y, Cai J, Zhang W, Gong X, Yan S, Zhang K, Luo Z, Sun J, Jiang Q, Lou M. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol 87: 357–369, 2020. doi: 10.1002/ana.25670. [DOI] [PubMed] [Google Scholar]
- 171.Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 115: 4483–4488, 2018. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kelava I, Lewitus E, Huttner WB. The secondary loss of gyrencephaly as an example of evolutionary phenotypical reversal. Front Neuroanat 7: 1–9, 2013. doi: 10.3389/fnana.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Monahan-Earley R, Dvorak AM, Aird WC. Evolutionary origins of the blood vascular system and endothelium. J Thromb Haemost 11: 46–66, 2013. doi: 10.1111/jth.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Muñoz-Chápuli R, Pérez-Pomares JA. Origin of the Vertebrate Endothelial Cell Lineage. Ontogeny and Phylogeny. Amsterdam, The Netherlands: Elsevier Inc., 2010, p. 465–486. [Google Scholar]
- 175.Oliver G, Alitalo K. The lymphatic vasculature: Recent progress and paradigms. Annu Rev Cell Dev Biol 21: 457–483, 2005. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- 176.Lee BB, Suami H. Ontogeny and Phylogeny of Lymphatics: Embryological Aspect. Amsterdam, The Netherlands: Elsevier Inc., 2019, p. 5–17. [Google Scholar]
- 177.Choi I, Lee S, Hong YK. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med 2: a00644, 2012. doi: 10.1101/cshperspect.a006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J 22: 3546–3556, 2003. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999. doi: 10.1016/S0092-8674(00)81511-1. doi:. [DOI] [PubMed] [Google Scholar]
- 180.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest 100: 957–962, 1997. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6: 159–163, 2000. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 182.Hong YK, Shin JW, Detmar M. Development of the lymphatic vascular system: a mystery unravels. Dev Dyn 231: 462–473, 2004. doi: 10.1002/dvdy.20179. [DOI] [PubMed] [Google Scholar]
- 183.Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol Fluid Physiol 238: F42–F49, 1980. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- 184.Lempriere S. Meningeal lymphatic flow slows after mild traumatic brain injury. Nat Rev Neurol 16: 600, 2020. doi: 10.1038/s41582-020-00423-2. [DOI] [PubMed] [Google Scholar]
- 185.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 100: 375–388, 2018. [ doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127: 3210–3219, 2017. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia 52: 228–233, 2005. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- 188.Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res 132: 10–26, 2000. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 189.Ringers C, Olstad EW, Jurisch-Yaksi N. The role of motile cilia in the development and physiology of the nervous system. Philos Trans R Soc Lond B Biol Sci 375: 20190156, 2020. doi: 10.1098/rstb.2019.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311: 629–632, 2006. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 191.Faubel R, Westendorf C, Bodenschatz E, Eichele G. Cilia-based flow network in the brain ventricles. Science 353: 176–178, 2016. doi: 10.1126/science.aae0450. [DOI] [PubMed] [Google Scholar]
- 192.Narita K, Takeda S. Cilia in the choroid plexus: their roles in hydrocephalus and beyond. Front Cell Neurosci 9: 39–39, 2015. doi: 10.3389/fncel.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jiménez AJ, Domínguez-Pinos MD, Guerra MM, Fernández-Llebrez P, Pérez-Fígares JM. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2: e28426, 2014. doi: 10.4161/tisb.28426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thomas T, Dziadek M. Capacity to form choroid plexus-like cells in vitro is restricted to specific regions of the mouse neural ectoderm. Development 117: 253–262, 1993. doi: 10.1242/dev.117.1.253. [DOI] [PubMed] [Google Scholar]
- 195.Watanabe M, Kang YJ, Davies LM, Meghpara S, Lau K, Chung CY, Kathiriya J, Hadjantonakis AK, Monuki ES. BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J Neurosci 32: 15934–15945, 2012. doi: 10.1523/JNEUROSCI.3227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol 273: 57–68, 2015. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 197.Spector R, Johanson CE. The mammalian choroid plexus. Sci Am 261: 68–74, 1989. doi: 10.1038/scientificamerican1189-68. [DOI] [PubMed] [Google Scholar]
- 198.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11: 26, 2014. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Oreskovic D, Rados M, Klarica M. Cerebrospinal fluid secretion by the choroid plexus? Physiol Rev 96: 1661–1662, 2016. doi: 10.1152/physrev.00021.2016. [DOI] [PubMed] [Google Scholar]
- 200.Evans PG, Sokolska M, Alves A, Harrison IF, Ohene Y, Nahavandi P, Ismail O, Miranda E, Lythgoe MF, Thomas DL, Wells JA. Non-invasive MRI of blood-cerebrospinal fluid barrier function. Nat Commun 11: 2081, 2020. doi: 10.1038/s41467-020-16002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shipley FB, Dani N, Xu H, Deister C, Cui J, Head JP, Sadegh C, Fame RM, Shannon ML, Flores VI, Kishkovich T, Jang E, Klein EM, Goldey GJ, He K, Zhang Y, Holtzman MJ, Kirchhausen T, Wyart C, Moore CI, Andermann ML, Lehtinen MK. Tracking calcium dynamics and immune surveillance at the choroid plexus blood-cerebrospinal fluid interface. Neuron 108: 623–639, 2020. doi: 10.1016/j.neuron.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu G, Mestre H, Sweeney AM, Sun Q, Weikop P, Du T, Nedergaard M. direct measurement of cerebrospinal fluid production in mice. Cell Rep 33: 108524, 2020. doi: 10.1016/j.celrep.2020.108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129: 957–970, 2004. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93: 1847–1892, 2013. doi: 10.1152/physrev.00004.2013. [DOI] [PubMed] [Google Scholar]
- 205.Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res 7: 15, 2010. doi: 10.1186/1743-8454-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Proctor GB. The physiology of salivary secretion. Periodontol 2000 70: 11–25, 2016. doi: 10.1111/prd.12116. [DOI] [PubMed] [Google Scholar]
- 207.Wright EM. Transport processes in the formation of the cerebrospinal fluid. Rev Physiol Biochem Pharmacol 83: 3–34, 1978. [PubMed] [Google Scholar]
- 208.Wright EM, Saito Y. The choroid plexus as a route from blood to brain. Ann N Y Acad Sci 481: 214–220, 1986. doi: 10.1111/j.1749-6632.1986.tb27152.x. [DOI] [PubMed] [Google Scholar]
- 209.Oshio K, Song Y, Verkman AS, Manley GT. Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir Suppl 86: 525–528, 2003. doi: 10.1007/978-3-7091-0651-8_107. [DOI] [PubMed] [Google Scholar]
- 210.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J 19: 76–78, 2005. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 211.Davson H. A comparative study of the aqueous humour and cerebrospinal fluid in the rabbit. J Physiol 129: 111–133, 1955. doi: 10.1113/jphysiol.1955.sp005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Davson H, Segal M. Physiology of the CSF and Blood-Brain Barriers. Boca Raton, FL: 1996, p. 1832. [Google Scholar]
- 213.Yang X, Wang Q, Cao E. Structure of the human cation-chloride cotransporter NKCC1 determined by single-particle electron cryo-microscopy. Nat Commun 11: 1016, 2020. doi: 10.1038/s41467-020-14790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wu Q, Delpire E, Hebert SC, Strange K. Functional demonstration of Na+-K+-2Cl− cotransporter activity in isolated, polarized choroid plexus cells. Am J Physiol Cell Physiol 275: C1565–C1572, 1998. doi: 10.1152/ajpcell.1998.275.6.C1565. [DOI] [PubMed] [Google Scholar]
- 215.Gregoriades JM, Madaris A, Alvarez FJ, Alvarez-Leefmans FJ. Genetic and pharmacological inactivation of apical Na+-K+-2Cl− cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am J Physiol Cell Physiol 316: C525–C544, 2019. doi: 10.1152/ajpcell.00026.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Frohlich F, Bazhenov M, Iragui-Madoz V, Sejnowski TJ. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist 14: 422–433, 2008. doi: 10.1177/1073858408317955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Steffensen AB, Oernbo EK, Stoica A, Gerkau NJ, Barbuskaite D, Tritsaris K, Rose CR, MacAulay N. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat Commun 9: 2167, 2018. doi: 10.1038/s41467-018-04677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chew TA, Orlando BJ, Zhang J, Latorraca NR, Wang A, Hollingsworth SA, Chen DH, Dror RO, Liao M, Feng L. Structure and mechanism of the cation-chloride cotransporter NKCC1. Nature 572: 488–492, 2019. doi: 10.1038/s41586-019-1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.MacAulay N, Rose CR. CrossTalk opposing view: NKCC1 in the luminal membrane of choroid plexus is outwardly directed under basal conditions and contributes directly to cerebrospinal fluid secretion. J Physiol 598: 4737–4739, 2020. doi: 10.1113/JP279868. [DOI] [PubMed] [Google Scholar]
- 220.Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA. Cerebrospinal fluid production by the choroid plexus and brain. Science 173: 330–332, 1971. doi: 10.1126/science.173.3994.330. [DOI] [PubMed] [Google Scholar]
- 221.Loo DD, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci U S A 93: 13367–13370, 1996. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.MacAulay N, Hamann S, Zeuthen T. Water transport in the brain: role of cotransporters. Neuroscience 129: 1031–1044, 2004. doi: 10.1016/j.neuroscience.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 223.Li Q, Aalling NN, Forstera B, Erturk A, Nedergaard M, Mollgard K, Xavier AL. Aquaporin 1 and the Na(+)/K(+)/2Cl(-) cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system. Fluids Barriers CNS 17: 15, 2020. doi: 10.1186/s12987-020-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lindstrom EK, Ringstad G, Mardal KA, Eide PK. Cerebrospinal fluid volumetric net flow rate and direction in idiopathic normal pressure hydrocephalus. NeuroImage Clin 20: 731–741, 2018. doi: 10.1016/j.nicl.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Heisey SR, Held D, Pappenheimer JR. Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am J Physiol 203: 775–781, 1962. doi: 10.1152/ajplegacy.1962.203.5.775. [DOI] [PubMed] [Google Scholar]
- 226.Oreskovic D, Klarica M, Vukic M, Marakovic J. Evaluation of ventriculo-cisternal perfusion model as a method to study cerebrospinal fluid formation. Croat Med J 44: 161–164, 2003. [PubMed] [Google Scholar]
- 227.Karimy JK, Kahle KT, Kurland DB, Yu E, Gerzanich V, Simard JM. A novel method to study cerebrospinal fluid dynamics in rats. J Neurosci Methods 241: 78–84, 2015. doi: 10.1016/j.jneumeth.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, Furey CG, Zhou X, Mansuri MS, Montejo J, Vera A, DiLuna ML, Delpire E, Alper SL, Gunel M, Gerzanich V, Medzhitov R, Simard JM, Kahle KT. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med 23: 997–1003, 2017. doi: 10.1038/nm.4361. [DOI] [PubMed] [Google Scholar]
- 229.Welch K. Secretion of cerebrospinal fluid by choroid plexus of the rabbit. Am J Physiol 205: 617–624, 1963. doi: 10.1152/ajplegacy.1963.205.3.617. [DOI] [PubMed] [Google Scholar]
- 230.Chodobski A, Szmydynger-Chodobska J, Johanson CE. Vasopressin mediates the inhibitory effect of central angiotensin II on cerebrospinal fluid formation. Eur J Pharmacol 347: 205–209, 1998. doi: 10.1016/S0014-2999(98)00229-5. [DOI] [PubMed] [Google Scholar]
- 231.Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg 25: 430–436, 1966. doi: 10.3171/jns.1966.25.4.0430. [DOI] [PubMed] [Google Scholar]
- 232.Brinkmann G, Harlandt O, Muhle C, Brossmann J, Heller M. Quantification of fluid flow in magnetic resonance tomography: an experimental study of a flow model and liquid flow measurements in the cerebral aqueduct in volunteers. Rofo 172: 1043–1051, 2000. doi: 10.1055/s-2000-9217. [DOI] [PubMed] [Google Scholar]
- 233.Nilsson C, Stahlberg F, Thomsen C, Henriksen O, Herning M, Owman C. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol Regul Integr Comp Physiol 262: R20–R24, 1992. doi: 10.1152/ajpregu.1992.262.1.R20. [DOI] [PubMed] [Google Scholar]
- 234.Piechnik SK, Summers PE, Jezzard P, Byrne JV. Magnetic resonance measurement of blood and CSF flow rates with phase contrast–normal values, repeatability and CO2 reactivity. Acta Neurochir Suppl 102: 263–270, 2008. doi: 10.1007/978-3-211-85578-2_50. [DOI] [PubMed] [Google Scholar]
- 235.Chen L, Beckett A, Verma A, Feinberg DA. Dynamics of respiratory and cardiac CSF motion revealed with real-time simultaneous multi-slice EPI velocity phase contrast imaging. NeuroImage 122: 281–287, 2015. doi: 10.1016/j.neuroimage.2015.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ames A 3rd, Higashi K, Nesbett FB. Effects of Pco2 acetazolamide and ouabain on volume and composition of choroid-plexus fluid. J Physiol 181: 516–524, 1965. doi: 10.1113/jphysiol.1965.sp007780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Davson H, Segal MB. The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol 209: 131–153, 1970. doi: 10.1113/jphysiol.1970.sp009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Segal MB, Burgess AM. A combined physiological and morphological study of the secretory process in the rabbit choroid plexus. J Cell Sci 14: 339–350, 1974. [DOI] [PubMed] [Google Scholar]
- 239.McCarthy KD, Reed DJ. The effect of acetazolamide and furosemide on cerebrospinal fluid production and choroid plexus carbonic anhydrase activity. J Pharmacol Exp Ther 189: 194–201, 1974. [PubMed] [Google Scholar]
- 240.Rangroo Thrane V, Thrane AS, Wang F, Cotrina ML, Smith NA, Chen M, Xu Q, Kang N, Fujita T, Nagelhus EA, Nedergaard M. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med 19: 1643–1648, 2013. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 15: 78–91, 2016. doi: 10.1016/S1474-4422(15)00298-7. [DOI] [PubMed] [Google Scholar]
- 242.Scotton WJ, Botfield HF, Westgate CS, Mitchell JL, Yiangou A, Uldall MS, Jensen RH, Sinclair AJ. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia 39: 209–218, 2019. doi: 10.1177/0333102418776455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.May C, Kaye JA, Atack JR, Schapiro MB, Friedland RP, Rapoport SI. Cerebrospinal fluid production is reduced in healthy aging. Neurology 40: 500–503, 1990. doi: 10.1212/wnl.40.3_part_1.500. [DOI] [PubMed] [Google Scholar]
- 244.Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, Saul TA. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57: 1763–1766, 2001. doi: 10.1212/WNL.57.10.1763. doi:. [DOI] [PubMed] [Google Scholar]
- 245.Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, Johanson CE, Silverberg GD. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS 9: 3, 2012. doi: 10.1186/2045-8118-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Giannetto M, Xia M, Stæger FF, Metcalfe T, Vinitsky HS, Dang JA, Xavier AL, Kress BT, Nedergaard M, Hablitz LM. Biological sex does not predict glymphatic influx in healthy young, middle aged or old mice. Sci Rep 10: 16073, 2020. doi: 10.1038/s41598-020-72621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lindvall-Axelsson M, Owman C. Actions of sex steroids and corticosteroids on rabbit choroid plexus as shown by changes in transport capacity and rate of cerebrospinal fluid formation. Neurol Res 12: 181–186, 1990. doi: 10.1080/01616412.1990.11739940. [DOI] [PubMed] [Google Scholar]
- 248.Nilsson C, Lindvall-Axelsson M, Owman C. Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res Brain Res Rev 17: 109–138, 1992. doi: 10.1016/0165-0173(92)90011-a. [DOI] [PubMed] [Google Scholar]
- 249.Hong-Goka BC, Chang FL. Estrogen receptors alpha and beta in choroid plexus epithelial cells in Alzheimer’s disease. Neurosci Lett 360: 113–116, 2004. doi: 10.1016/j.neulet.2004.01.075. [DOI] [PubMed] [Google Scholar]
- 250.Skipor J, Thiery JC. The choroid plexus–cerebrospinal fluid system: undervaluated pathway of neuroendocrine signaling into the brain. Acta Neurobiol Exp (Wars) 68: 414–428, 2008. [DOI] [PubMed] [Google Scholar]
- 251.Teasdale GM, Grant R, Condon B, Patterson J, Lawrence A, Hadley DM, Wyper D. Intracranial CSF volumes: natural variations and physiological changes measured by MRI. Acta Neurochir Suppl (Wien) 42: 230–235, 1988. doi: 10.1007/978-3-7091-8975-7_45. [DOI] [PubMed] [Google Scholar]
- 252.Edvinsson L, Hakanson R, Lindvall M, Owman C, Svensson KG. Ultrastructural and biochemical evidence for a sympathetic neural influence on the choroid plexus. Exp Neurol 48: 241–251, 1975. doi: 10.1016/0014-4886(75)90154-5. [DOI] [PubMed] [Google Scholar]
- 253.Edvinsson L, Lindvall M. Autonomic vascular innervation and vasomotor reactivity in the choroid plexus. Exp Neurol 62: 394–404, 1978. doi: 10.1016/0014-4886(78)90063-8. [DOI] [PubMed] [Google Scholar]
- 254.Lindvall M, Edvinsson L, Owman C. Sympathetic nervous control of cerebrospinal fluid production from the choroid plexus. Science 201: 176–178, 1978. doi: 10.1126/science.663649. [DOI] [PubMed] [Google Scholar]
- 255.Lindvall M, Edvinsson L, Owman C. Effect of sympathomimetic drugs and corresponding receptor antagonists on the rate of cerebrospinal fluid production. Exp Neurol 64: 132–145, 1979. doi: 10.1016/0014-4886(79)90010-4. [DOI] [PubMed] [Google Scholar]
- 256.Lindvall M, Edvinsson L, Owman C. Reduced cerebrospinal fluid formation through cholinergic mechanisms. Neurosci Lett 10: 311–316, 1978. doi: 10.1016/0304-3940(78)90245-8. [DOI] [PubMed] [Google Scholar]
- 257.Faraci FM, Mayhan WG, Heistad DD. Effect of serotonin on blood flow to the choroid plexus. Brain Res 478: 121–126, 1989. doi: 10.1016/0006-8993(89)91483-2. [DOI] [PubMed] [Google Scholar]
- 258.Brocklehurst G. The significance of the evolution of the cerebrospinal fluid system. Ann R Coll Surg Engl 61: 349–356, 1979. [PMC free article] [PubMed] [Google Scholar]
- 259.Jones H, Sellars R. The movement of fluid out of the ventricles in fetal and neonatal rats. Eur J Pediatr Surg 37: 130–133, 1982. doi: 10.1055/s-2008-1059831. [DOI] [Google Scholar]
- 260.Kasantikul V, Netsky MG, James AE Jr.. Relation of age and cerebral ventricle size to central canal in man. Morphological analysis. J Neurosurg 51: 85–93, 1979. doi: 10.3171/jns.1979.51.1.0085. [DOI] [PubMed] [Google Scholar]
- 261.Bertram CD, Brodbelt AR, Stoodley MA. The origins of syringomyelia: numerical models of fluid/structure interactions in the spinal cord. J Biomech Eng 127: 1099–1109, 2005. doi: 10.1115/1.2073607. [DOI] [PubMed] [Google Scholar]
- 262.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res 40: 2583–2599, 2015. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pikor NB, Prat A, Bar-Or A, Gommerman JL. Meningeal tertiary lymphoid tissues and multiple sclerosis: a gathering place for diverse types of immune cells during CNS autoimmunity. Front Immunol 6: 657, 2015. doi: 10.3389/fimmu.2015.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.DiNuzzo M, Nedergaard M. Brain energetics during the sleep-wake cycle. Curr Opin Neurobiol 47: 65–72, 2017. doi: 10.1016/j.conb.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Smeets JS, Horstman AM, Schijns O, Dings JT, Hoogland G, Gijsen AP, Goessens JP, Bouwman FG, Wodzig W, Mariman EC, van Loon LJ. Brain tissue plasticity: protein synthesis rates of the human brain. Brain 141: 1122–1129, 2018. doi: 10.1093/brain/awy015. [DOI] [PubMed] [Google Scholar]
- 266.Sundaram SK, Muzik O, Chugani DC, Mu F, Mangner TJ, Chugani HT. Quantification of protein synthesis in the human brain using L-[1-11C]-leucine PET: incorporation of factors for large neutral amino acids in plasma and for amino acids recycled from tissue. J Nucl Med 47: 1787–1795, 2006. [PubMed] [Google Scholar]
- 267.Fornasiero EF, Mandad S, Wildhagen H, Alevra M, Rammner B, Keihani S, Opazo F, Urban I, Ischebeck T, Sakib MS, Fard MK, Kirli K, Centeno TP, Vidal RO, Rahman RU, Benito E, Fischer A, Dennerlein S, Rehling P, Feussner I, Bonn S, Simons M, Urlaub H, Rizzoli SO. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat Commun 9: 4230, 2018. doi: 10.1038/s41467-018-06519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cassot F, Lauwers F, Lorthois S, Puwanarajah P, Duvernoy H. Scaling laws for branching vessels of human cerebral cortex. Microcirculation 16: 331–344, 2009. doi: 10.1080/10739680802662607. [DOI] [PubMed] [Google Scholar]
- 269.Adams DL, Piserchia V, Economides JR, Horton JC. Vascular supply of the cerebral cortex is specialized for cell layers but not columns. Cereb Cortex 25: 3673–3681, 2015. doi: 10.1093/cercor/bhu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci 16: 889–897, 2013. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hadjistassou C, Bejan A, Ventikos Y. Cerebral oxygenation and optimal vascular brain organization. J R Soc Interface 12: 20150245, 2015. doi: 10.1098/rsif.2015.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res 58: 312–328, 1999. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 273.Morris AW, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol 131: 725–736, 2016. doi: 10.1007/s00401-016-1555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Edeklev CS, Halvorsen M, Lovland G, Vatnehol SA, Gjertsen O, Nedregaard B, Sletteberg R, Ringstad G, Eide PK. Intrathecal use of gadobutrol for glymphatic MR imaging: prospective safety study of 100 patients. AJNR Am J Neuroradiol 40: 1257–1264, 2019. doi: 10.3174/ajnr.A6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Watts R, Steinklein JM, Waldman L, Zhou X, Filippi CG. Measuring glymphatic flow in man using quantitative contrast-enhanced MRI. AJNR Am J Neuroradiol 40: 648–651, 2019. doi: 10.3174/ajnr.A5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dyke JP, Xu HS, Verma A, Voss HU, Chazen JL. MRI characterization of early CNS transport kinetics post intrathecal gadolinium injection: Trends of subarachnoid and parenchymal distribution in healthy volunteers. Clin Imaging 68: 1–6, 2020. doi: 10.1016/j.clinimag.2020.04.043. [DOI] [PubMed] [Google Scholar]
- 277.Verma A, Hesterman JY, Chazen JL, Holt R, Connolly P, Horky L, Vallabhajosula S, Mozley PD. Intrathecal (99m)Tc-DTPA imaging of molecular passage from lumbar cerebrospinal fluid to brain and periphery in humans. Alzheimers Dement (Amst) 12: e12030, 2020. doi: 10.1002/dad2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Thomas JH. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J R Soc Interface 16: 20190572, 2019. doi: 10.1098/rsif.2019.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Koundal S, Elkin R, Nadeem S, Xue Y, Constantinou S, Sanggaard S, Liu X, Monte B, Xu F, Van Nostrand W, Nedergaard M, Lee H, Wardlaw J, Benveniste H, Tannenbaum A. Optimal mass transport with lagrangian workflow reveals advective and diffusion driven solute transport in the glymphatic system. Sci Rep 10: 1990, 2020. doi: 10.1038/s41598-020-59045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ratner V, Zhu L, Kolesov I, Nedergaard M, Benveniste H, Tannenbaum A. Optimal-mass-transfer-based estimation of glymphatic transport in living brain. Proc SPIE Int Soc Opt Eng 94131J, 2015. doi: 10.1117/12.2076289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, Tannenbaum A. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. NeuroImage 152: 530–537, 2017. doi: 10.1016/j.neuroimage.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bedussi B, Almasian M, de Vos J, VanBavel E, Bakker EN. Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J Cereb Blood Flow Metab 38: 719–726, 2018. doi: 10.1177/0271678X17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6: 1–16, 2017. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci 24: 8049–8056, 2004. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev 88: 1277–1340, 2008. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab 39: 1355–1368, 2019. doi: 10.1177/0271678X18760974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhang ET, Richards HK, Kida S, Weller RO. Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol 83: 233–239, 1992. doi: 10.1007/BF00296784. [DOI] [PubMed] [Google Scholar]
- 288.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol Fluid Physiol 240: F329–F336, 1981. doi: 10.1152/ajprenal.1981.240.4.F329. [DOI] [PubMed] [Google Scholar]
- 289.Cserr HF. Relationship between cerebrospinal fluid and interstitial fluid of brain. Fed Proc 33: 2075–2078, 1974. [PubMed] [Google Scholar]
- 290.Bertrand C. Diffusion and absorption within the brain; an experimental study with Prussian blue and India ink in rabbits and cats. J Neuropathol Exp Neurol 11: 53–61, 1952. doi: 10.1097/00005072-195201000-00006. [DOI] [PubMed] [Google Scholar]
- 291.Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, Bakker EN. Clearance from the mouse brain by convection of interstitial fluid toward the ventricular system. Fluids Barriers CNS 12: 23, 2015. doi: 10.1186/s12987-015-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Leonhardt H, Desaga U. Recent observations on ependyma and subependymal basement membranes. Acta Neurochir (Wien) 31: 153–159, 1975. doi: 10.1007/BF01406287. [DOI] [PubMed] [Google Scholar]
- 293.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol Regul Integr Comp Physiol 266: R292–R305, 1994. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 294.Lei Y, Han H, Yuan F, Javeed A, Zhao Y. The brain interstitial system: anatomy, modeling, in vivo measurement, and applications. Prog Neurobiol 157: 230–246, 2017. doi: 10.1016/j.pneurobio.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 295.Han H, Li K, Yan J, Zhu K, Fu Y. An in vivo study with an MRI tracer method reveals the biophysical properties of interstitial fluid in the rat brain. Sci China Life Sci 55: 782–787, 2012. doi: 10.1007/s11427-012-4361-4. [DOI] [PubMed] [Google Scholar]
- 296.Wang A, Wang R, Cui D, Huang X, Yuan L, Liu H, Fu Y, Liang L, Wang W, He Q, Shi C, Guan X, Teng Z, Zhao G, Li Y, Gao Y, Han H. The drainage of interstitial fluid in the deep brain is controlled by the integrity of myelination. Aging Dis 10: 937–948, 2019. doi: 10.14336/AD.2018.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Teng Z, Wang A, Wang P, Wang R, Wang W, Han H. The effect of aquaporin-4 knockout on interstitial fluid flow and the structure of the extracellular space in the deep brain. Aging Dis 9: 808–816, 2018. doi: 10.14336/AD.2017.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shi C, Lei Y, Han H, Zuo L, Yan J, He Q, Yuan L, Liu H, Xu G, Xu W. Transportation in the interstitial space of the brain can be regulated by neuronal excitation. Sci Rep 5: 17673, 2015. doi: 10.1038/srep17673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Exposito JY, Valcourt U, Cluzel C, Lethias C. The fibrillar collagen family. Int J Mol Sci 11: 407–426, 2010. doi: 10.3390/ijms11020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Plant AL, Bhadriraju K, Spurlin TA, Elliott JT. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta 1793: 893–902, 2009. doi: 10.1016/j.bbamcr.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 301.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802: 31–47, 2014. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 302.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20: 495–501, 2008. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schwarzbauer JE, Sechler JL. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr Opin Cell Biol 11: 622–627, 1999. doi: 10.1016/s0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 304.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26: 397–419, 2010. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yurchenco PD, O’Rear JJ. Basal lamina assembly. Curr Opin Cell Biol 6: 674–681, 1994. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 306.Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem 277: 49120–49126, 2002. doi: 10.1074/jbc.M206891200. [DOI] [PubMed] [Google Scholar]
- 307.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem 278: 37698–37704, 2003. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 308.Saari H, Konttinen YT, Friman C, Sorsa T. Differential effects of reactive oxygen species on native synovial fluid and purified human umbilical cord hyaluronate. Inflammation 17: 403–415, 1993. doi: 10.1007/BF00916581. [DOI] [PubMed] [Google Scholar]
- 309.Ostgaard G, Reed RK. Increased lymphatic hyaluronan output and preserved hyaluronan content of the rat small intestine in prolonged hypoproteinaemia. Acta Physiol Scand 152: 51–56, 1994. doi: 10.1111/j.1748-1716.1994.tb09783.x. [DOI] [PubMed] [Google Scholar]
- 310.Price FM, Levick JR, Mason RM. Changes in glycosaminoglycan concentration and synovial permeability at raised intra-articular pressure in rabbit knees. J Physiol 495: 821–833, 1996. doi: 10.1113/jphysiol.1996.sp021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Schulz T, Schumacher U, Prehm P. Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J Biol Chem 282: 20999–21004, 2007. doi: 10.1074/jbc.M700915200. [DOI] [PubMed] [Google Scholar]
- 312.Reed RK, Rubin K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res 87: 211–217, 2010. doi: 10.1093/cvr/cvq143. [DOI] [PubMed] [Google Scholar]
- 313.Rugheimer L, Johnsson C, Maric C, Hansell P. Hormonal regulation of renomedullary hyaluronan. Acta Physiol (Oxf) 193: 191–198, 2008. doi: 10.1111/j.1748-1716.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 314.Misra S, Hascall VC, Markwald RR, Ghatak S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front Immunol 6: 201, 2015. doi: 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol 117: 1343–1350, 1992. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Turley EA. The role of a cell-associated hyaluronan-binding protein in fibroblast behaviour. Ciba Found Symp 143: 121–133, 1989. doi: 10.1002/9780470513774.ch8. [DOI] [PubMed] [Google Scholar]
- 317.Turley EA, Austen L, Vandeligt K, Clary C. Hyaluronan and a cell-associated hyaluronan binding protein regulate the locomotion of ras-transformed cells. J Cell Biol 112: 1041–1047, 1991. doi: 10.1083/jcb.112.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Laurent C, Johnson-Wells G, Hellstrom S, Engstrom-Laurent A, Wells AF. Localization of hyaluronan in various muscular tissues. A morphological study in the rat. Cell Tissue Res 263: 201–205, 1991. doi: 10.1007/BF00318761. [DOI] [PubMed] [Google Scholar]
- 319.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 7: 79–89, 1999. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 320.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 14: 722–729, 2013. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 321.Dane MJ, van den Berg BM, Lee DH, Boels MG, Tiemeier GL, Avramut MC, van Zonneveld AJ, van der Vlag J, Vink H, Rabelink TJ. A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol 308: F956–F366, 2015. doi: 10.1152/ajprenal.00532.2014. [DOI] [PubMed] [Google Scholar]
- 322.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, Oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A 86: 5385–5389, 1989. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hansen KC, D’Alessandro A, Clement CC, Santambrogio L. Lymph formation, composition and circulation: a proteomics perspective. Intl Immunol 27: 219–227, 2015. doi: 10.1093/intimm/dxv012. [DOI] [PubMed] [Google Scholar]
- 325.Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science 369: eaax4063, 2020. doi: 10.1126/science.aax4063. [DOI] [PubMed] [Google Scholar]
- 326.Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21(st) century: novel functional roles in homeostasis and disease. Cell 182: 270–296, 2020. doi: 10.1016/j.cell.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Curran RE, Mosher MB, Owens ES, Fenstermacher JD. Cerebrospinal fluid production rates determined by simultaneous albumin and inulin perfusion. Exp Neurol 29: 546–553, 1970. doi: 10.1016/0014-4886(70)90079-8. [DOI] [PubMed] [Google Scholar]
- 328.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 438: 946–953, 2005. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 329.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204: 2349–2362, 2007. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kramer GC, Sibley L, Aukland K, Renkin EM. Wick sampling of interstitial fluid in rat skin: further analysis and modifications of the method. Microvasc Res 32: 39–49, 1986. doi: 10.1016/0026-2862(86)90042-7. [DOI] [PubMed] [Google Scholar]
- 331.Santambrogio L. The lymphatic fluid. Int Rev Cell Mol Biol 337: 111–133, 2018. doi: 10.1016/bs.ircmb.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 332.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 333.Agostoni E, Zocchi L. Starling forces and lymphatic drainage in pleural liquid and protein exchanges. Respir Physiol 86: 271–281, 1991. doi: 10.1016/0034-5687(91)90086-x. [DOI] [PubMed] [Google Scholar]
- 334.Hu X, Adamson RH, Liu B, Curry FE, Weinbaum S. Starling forces that oppose filtration after tissue oncotic pressure is increased. Am J Physiol Heart Circ Physiol 279: H1724–H1736, 2000. doi: 10.1152/ajpheart.2000.279.4.H1724. [DOI] [PubMed] [Google Scholar]
- 335.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng 9: 229–256, 2007. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 336.Dafni H, Israely T, Bhujwalla ZM, Benjamin LE, Neeman M. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res 62: 6731–6739, 2002. [PubMed] [Google Scholar]
- 337.Levick JR. Capillary filtration-absorption balance reconsidered in light of dynamic extravascular factors. Exp Physiol 76: 825–857, 1991. doi: 10.1113/expphysiol.1991.sp003549. [DOI] [PubMed] [Google Scholar]
- 338.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87: 198–210, 2010. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 339.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev 70: 987–1028, 1990. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 340.Hogan R. Lymph formation in the bat wing. In: Progress in Microcirculation Research First Australasian Symposium on the Microcirculation Sydney 1980. Kensington, Australia: Univ. of New South Wales, 1981, p. 261–282. [Google Scholar]
- 341.Zhang WB, Aukland K, Lund T, Wiig H. Distribution of interstitial fluid pressure and fluid volumes in hind-limb skin of rats: relation to meridians? Clin Physiol 20: 242–249, 2000. doi: 10.1046/j.1365-2281.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- 342.Moriondo A, Mukenge S, Negrini D. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol 289: H263–H269, 2005. doi: 10.1152/ajpheart.00060.2005. [DOI] [PubMed] [Google Scholar]
- 343.Negrini D, Moriondo A, Mukenge S. Transmural pressure during cardiogenic oscillations in rodent diaphragmatic lymphatic vessels. Lymphat Res Biol 2: 69–81, 2004. doi: 10.1089/lrb.2004.2.69. [DOI] [PubMed] [Google Scholar]
- 344.Dixon JB. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab 21: 480–487, 2010. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res 106: 920–931, 2010. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Adair TH, Guyton AC. Modification of lymph by lymph nodes. III. Effect of increased lymph hydrostatic pressure. Am J Physiol Heart Circ Physiol 249: H777–H782, 1985. doi: 10.1152/ajpheart.1985.249.4.H777. [DOI] [PubMed] [Google Scholar]
- 347.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J 22: 2324–2333, 2003. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Adair TH, Guyton AC. Modification of lymph by lymph nodes. II. Effect of increased lymph node venous blood pressure. Am J Physiol Heart Circ Physiol 245: H616–H622, 1983. doi: 10.1152/ajpheart.1983.245.4.H616. [DOI] [PubMed] [Google Scholar]
- 349.Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol 176: 1122–1129, 2010. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Blumberg BS, Ogston AG. Further evidence on the protein complexes of some hyaluronic acids. Biochem J 68: 183–188, 1958. doi: 10.1042/bj0680183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ogston AG, Sherman TF. Effects of hyaluronic acid upon diffusion of solutes and flow of solvent. J Physiol 156: 67–74, 1961. doi: 10.1113/jphysiol.1961.sp006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Reed RK, Lepsoe S, Wiig H. Interstitial exclusion of albumin in rat dermis and subcutis in over- and dehydration. Am J Physiol Heart Circ Physiol 257: H1819–H1827, 1989. doi: 10.1152/ajpheart.1989.257.6.H1819. [DOI] [PubMed] [Google Scholar]
- 353.Wiig H, Tenstad O, Bert JL. Effect of hydration on interstitial distribution of charged albumin in rat dermis in vitro. J Physiol 569: 631–641, 2005. doi: 10.1113/jphysiol.2005.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Negrini D, Tenstad O, Wiig H. Interstitial exclusion of albumin in rabbit lung measured with the continuous infusion method in combination with the wick technique. Microcirculation 10: 153–165, 2003. doi: 10.1080/713773611. [DOI] [PubMed] [Google Scholar]
- 355.Adair TH. Studies of lymph modification by lymph nodes. Microcirc Endothelium Lymphatics 2: 251–269, 1985. [PubMed] [Google Scholar]
- 356.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118: 4731–4739, 2005. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 357.Demling RH. The burn edema process: current concepts. J Burn Care Rehabil 26: 207–227, 2005. [PubMed] [Google Scholar]
- 358.Lund T, Wiig H, Reed RK. Acute postburn edema: role of strongly negative interstitial fluid pressure. Am J Physiol Heart Circ Physiol 255: H1069–H1074, 1988. doi: 10.1152/ajpheart.1988.255.5.H1069. [DOI] [PubMed] [Google Scholar]
- 359.Lund T, Onarheim H, Wiig H, Reed RK. Mechanisms behind increased dermal imbibition pressure in acute burn edema. Am J Physiol Heart Circ Physiol 256: H940–H948, 1989. doi: 10.1152/ajpheart.1989.256.4.H940. [DOI] [PubMed] [Google Scholar]
- 360.Goldman N, Chandler-Militello D, Langevin HM, Nedergaard M, Takano T. Purine receptor mediated actin cytoskeleton remodeling of human fibroblasts. Cell Calcium 53: 297–301, 2013. doi: 10.1016/j.ceca.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nedrebo T, Berg A, Reed RK. Effect of tumor necrosis factor-α, IL-1β, and IL-6 on interstitial fluid pressure in rat skin. Am J Physiol Heart Circ Physiol 277: H1857–H1862, 1999. doi: 10.1152/ajpheart.1999.277.5.H1857. [DOI] [PubMed] [Google Scholar]
- 362.Nedrebo T, Rk R. Different serotypes of endotoxin (lipopolysaccharide) cause different increases in albumin extravasation in rats. Shock 18: 138–141, 2002. doi: 10.1097/00024382-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 363.Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol 40: 1317–1333, 2008. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol 114: 997–1008, 2004. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 365.Paul R, Heil P, Spatz JP, Schwarz US. Propagation of mechanical stress through the actin cytoskeleton toward focal adhesions: model and experiment. Biophys J 94: 1470–1482, 2008. doi: 10.1529/biophysj.107.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Roach DM, Fitridge RA, Laws PE, Millard SH, Varelias A, Cowled PA. Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur J Vasc Endovasc Surg 23: 260–269, 2002. doi: 10.1053/ejvs.2002.1598. [DOI] [PubMed] [Google Scholar]
- 367.Hayakawa K, Tatsumi H, Sokabe M. Mechano-sensing by actin filaments and focal adhesion proteins. Commun Integr Biol 5: 572–577, 2012. doi: 10.4161/cib.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Chabadel A, Banon-Rodriguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, Jurdic P, Anton IM, Saltel F. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell 18: 4899–4910, 2007. doi: 10.1091/mbc.e07-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ciobanasu C, Faivre B, Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol 92: 339–348, 2013. doi: 10.1016/j.ejcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 370.Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci 62: 1081–1099, 2005. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Langevin HM, Nedergaard M, Howe AK. Cellular control of connective tissue matrix tension. J Cell Biochem 114: 1714–1719, 2013. doi: 10.1002/jcb.24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 38: 646–651, 2007. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 373.Berg A, Rubin K, Reed RK. Cytochalasin D induces edema formation and lowering of interstitial fluid pressure in rat dermis. Am J Physiol Heart Circ Physiol 281: H7–H13, 2001. doi: 10.1152/ajpheart.2001.281.1.H7. [DOI] [PubMed] [Google Scholar]
- 374.Bronstad A, Reith A, Berg A, Reed RK. Effect of the cytoskeletal fixation agent phalloidin on transcapillary albumin transport and interstitial fluid pressure in anaphylaxis in the wistar rat. Microcirculation 9: 197–205, 2002. doi: 10.1080/mic.9.3.197.205. [DOI] [PubMed] [Google Scholar]
- 375.Yamaguchi Y. Isolation and characterization of nervous tissue proteoglycans. Methods Mol Biol 171: 35–39, 2001. doi: 10.1385/1-59259-209-0:035. [DOI] [PubMed] [Google Scholar]
- 376.Margolis RK, Margolis RU. Nervous tissue proteoglycans. Experientia 49: 429–446, 1993. doi: 10.1007/BF01923587. [DOI] [PubMed] [Google Scholar]
- 377.Herndon ME, Lander AD. A diverse set of developmentally regulated proteoglycans is expressed in the rat central nervous system. Neuron 4: 949–961, 1990. doi: 10.1016/0896-6273(90)90148-9. [DOI] [PubMed] [Google Scholar]
- 378.Morawski M, Bruckner G, Arendt T, Matthews RT. Aggrecan: beyond cartilage and into the brain. Int J Biochem Cell Biol 44: 690–693, 2012. doi: 10.1016/j.biocel.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 379.Bignami A, Perides G, Rahemtulla F. Versican, a hyaluronate-binding proteoglycan of embryonal precartilaginous mesenchyma, is mainly expressed postnatally in rat brain. J Neurosci Res 34: 97–106, 1993. doi: 10.1002/jnr.490340110. [DOI] [PubMed] [Google Scholar]
- 380.Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem 269: 10119–10126, 1994. doi: 10.1016/S0021-9258(17)36998-3. [DOI] [PubMed] [Google Scholar]
- 381.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem 267: 10003–10010, 1992. doi: 10.1016/S0021-9258(19)50191-0. [DOI] [PubMed] [Google Scholar]
- 382.Margolis RU, Margolis RK. Aggrecan-versican-neurocan family proteoglycans. Methods Enzymol 245: 105–126, 1994. doi: 10.1016/0076-6879(94)45008-0. [DOI] [PubMed] [Google Scholar]
- 383.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev 80: 1267–1290, 2000. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 384.Varki A. Selectin ligands. Proc Natl Acad Sci U S A 91: 7390–7397, 1994. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Novak U, Kaye AH. Extracellular matrix and the brain: components and function. J Clin Neurosci 7: 280–290, 2000. doi: 10.1054/jocn.1999.0212. [DOI] [PubMed] [Google Scholar]
- 386.Bruckner G, Morawski M, Arendt T. Aggrecan-based extracellular matrix is an integral part of the human basal ganglia circuit. Neuroscience 151: 489–504, 2008. doi: 10.1016/j.neuroscience.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 387.Perides G, Erickson HP, Rahemtulla F, Bignami A. Colocalization of tenascin with versican, a hyaluronate-binding chondroitin sulfate proteoglycan. Anat Embryol (Berl) 188: 467–479, 1993. doi: 10.1007/BF00190141. [DOI] [PubMed] [Google Scholar]
- 388.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133: 2331–2347, 2010. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 389.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71: 1073–1089, 2011. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 390.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248–1251, 2002. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 391.Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31: 9332–9344, 2011. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.van ‘t Spijker HM, Kwok JC. A sweet talk: the molecular systems of perineuronal nets in controlling neuronal communication. Front Integr Neurosci 11: 33, 2017. doi: 10.3389/fnint.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Krishnaswamy VR, Benbenishty A, Blinder P, Sagi I. Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights. Cell Mol Life Sci 76: 3229–3248, 2019. doi: 10.1007/s00018-019-03182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sloots JJ, Biessels GJ, Zwanenburg JJ. Cardiac and respiration-induced brain deformations in humans quantified with high-field MRI. NeuroImage 210: 116581, 2020. doi: 10.1016/j.neuroimage.2020.116581. [DOI] [PubMed] [Google Scholar]
- 395.Paganetti PA, Caroni P, Schwab ME. Glioblastoma infiltration into central nervous system tissue in vitro: involvement of a metalloprotease. J Cell Biol 107: 2281–2291, 1988. doi: 10.1083/jcb.107.6.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Adigun R, Basit H, Murray J. Necrosis, Cell (Liquefactive, Coagulative, Caseous, Fat, Fibrinoid, And Gangrenous). Treasure Island, FL: StatPearls, 2020. [Google Scholar]
- 397.Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 37: 3300–3317, 2017. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Winder SJ. The complexities of dystroglycan. Trends Biochem Sci 26: 118–124, 2001. doi: 10.1016/S0968-0004(00)01731-X. [DOI] [PubMed] [Google Scholar]
- 399.Zaccaria ML, Di Tommaso F, Brancaccio A, Paggi P, Petrucci TC. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience 104: 311–324, 2001. doi: 10.1016/S0306-4522(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 400.Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, del Zoppo GJ. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab 31: 1972–1985, 2011. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Yousif LF, Di Russo J, Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr 7: 101–110, 2013. doi: 10.4161/cam.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wolburg H, Noell S, Wolburg-Buchholz K, Mack A, Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist 15: 180–193, 2009. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- 403.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443: 780–786, 2006. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 404.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain–implications for Alzheimer diseaser. Nat Rev Neurol 12: 248, 2016. doi: 10.1038/nrneurol.2016.36. [DOI] [PubMed] [Google Scholar]
- 405.Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier AL, Gjedde A, Benveniste H, Nedergaard M. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci 39: 6365–6377, 2019. doi: 10.1523/JNEUROSCI.1974-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, Abbott NJ, Meyerand ME, Sorokin L, Stanimirovic DB, Thorne RG. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol 596: 445–475, 2018. doi: 10.1113/JP275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, Nahavandi P, Thomas DL, Lythgoe MF, Wells JA. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. eLife 7: 1–14, 2018. doi: 10.7554/eLife.34028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Bakker EN, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AW, Weller RO, Carare RO. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 36: 181–194, 2016. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Tithof J, Kelley DH, Mestre H, Nedergaard M, Thomas JH. Hydraulic resistance of periarterial spaces in the brain. Fluids Barriers CNS 16: 19, 2019. doi: 10.1186/s12987-019-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Asgari M, de Zelicourt D, Kurtcuoglu V. How astrocyte networks may contribute to cerebral metabolite clearance. Sci Rep 5: 15024, 2015. doi: 10.1038/srep15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Faghih MM, Sharp MK. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS 15: 17, 2018. doi: 10.1186/s12987-018-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Mestre H, Mori Y, Nedergaard M. The brain’s glymphatic system: current controversies. Trends Neurosci 43: 458–466, 2020. [ doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Faraco G, Park L, Anrather J, Iadecola C. Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med (Berl) 95: 1143–1152, 2017. doi: 10.1007/s00109-017-1573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26: 523–530, 2003. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 415.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58: 1094–1103, 2010. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 416.Korogod N, Petersen CC, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. eLife 4: e05793, 2015. doi: 10.7554/eLife.05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Noell S, Wolburg-Buchholz K, Mack AF, Beedle AM, Satz JS, Campbell KP, Wolburg H, Fallier-Becker P. Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur J Neurosci 33: 2179–2186, 2011. doi: 10.1111/j.1460-9568.2011.07688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, Kurachi Y, Ottersen OP. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 26: 47–54, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 419.Kubotera H, Ikeshima-Kataoka H, Hatashita Y, Allegra Mascaro AL, Pavone FS, Inoue T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci Rep 9: 1263, 2019. doi: 10.1038/s41598-018-37419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 131: 2257–2274, 2017. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci 37: 620–628, 2014. doi: 10.1016/j.tins.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A 103: 5567–5572, 2006. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Nicholson C, Hrabětová S. Brain extracellular space: the final frontier of neuroscience. Biophys J 113: 2133–2142, 2017. doi: 10.1016/j.bpj.2017.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Kinney JP, Spacek J, Bartol TM, Bajaj CL, Harris KM, Sejnowski TJ. Extracellular sheets and tunnels modulate glutamate diffusion in hippocampal neuropil. J Comp Neurol 521: 448–464, 2013. doi: 10.1002/cne.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Tonnesen J, Inavalli V, Nagerl UV. Super-resolution imaging of the extracellular space in living brain tissue. Cell 172: 1108–1121, 2018. doi: 10.1016/j.cell.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 426.Hrabetova S, Cognet L, Rusakov DA, Nagerl UV. Unveiling the extracellular space of the brain: from super-resolved microstructure to in vivo function. J Neurosci 38: 9355–9363, 2018. doi: 10.1523/JNEUROSCI.1664-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Lehmenkuhler A, Sykova E, Svoboda J, Zilles K, Nicholson C. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience 55: 339–351, 1993. doi: 10.1016/0306-4522(93)90503-8. [DOI] [PubMed] [Google Scholar]
- 428.Godin AG, Varela JA, Gao Z, Danne N, Dupuis JP, Lounis B, Groc L, Cognet L. Single-nanotube tracking reveals the nanoscale organization of the extracellular space in the live brain. Nat Nanotechnol 12: 238–243, 2017. doi: 10.1038/nnano.2016.248. [DOI] [PubMed] [Google Scholar]
- 429.Benias PC, Wells RG, Sackey-Aboagye B, Klavan H, Reidy J, Buonocore D, Miranda M, Kornacki S, Wayne M, Carr-Locke DL, Theise ND. Structure and distribution of an unrecognized interstitium in human tissues. Sci Rep 8: 4947, 2018. doi: 10.1038/s41598-018-23062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Sykova E, Vargova L, Kubinova S, Jendelova P, Chvatal A. The relationship between changes in intrinsic optical signals and cell swelling in rat spinal cord slices. NeuroImage 18: 214–230, 2003. doi: 10.1016/S1053-8119(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 431.Sykova E, Mazel T, Hasenohrl RU, Harvey AR, Simonova Z, Mulders WH, Huston JP. Learning deficits in aged rats related to decrease in extracellular volume and loss of diffusion anisotropy in hippocampus. Hippocampus 12: 269–279, 2002. doi: 10.1002/hipo.1101. [DOI] [PubMed] [Google Scholar]
- 432.Jin BJ, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148: 489–501, 2016. doi: 10.1085/jgp.201611684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Asgari M, de Zelicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep 6: 38635, 2016. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, Ottersen OP, Nagelhus EA, Mardal KA, Pettersen KH. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A 114: 9894–9899, 2017. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45: 545–552, 2004. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 436.Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, Nagelhus EA, Nedergaard M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 3: 2582, 2013. doi: 10.1038/srep02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Albargothy NJ, Johnston DA, Macgregor M, Roy S, Ajay OW, Hawkes CA, Carare RO. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 136: 139–152, 2018. doi: 10.1007/s00401-018-1862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Arbel-Ornath M, Hudry E, Eikermann-Haerter K, Hou S, Gregory JL, Zhao L, Betensky RA, Frosch MP, Greenberg SM, Bacskai BJ. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta Neuropathol 126: 353–364, 2013. doi: 10.1007/s00401-013-1145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP, Greenberg SM, Bacskai BJ. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105: 549–561, 2020. doi: 10.1016/j.neuron.2019.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, Park JW, Bankiewicz K. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 14: 69–78, 2006. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Smith JH, Humphrey JA. Interstitial transport and transvascular fluid exchange during infusion into brain and tumor tissue. Microvasc Res 73: 58–73, 2007. doi: 10.1016/j.mvr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 442.Basser PJ. Interstitial pressure, volume, and flow during infusion into brain tissue. Microvasc Res 44: 143–165, 1992. doi: 10.1016/0026-2862(92)90077-3. [DOI] [PubMed] [Google Scholar]
- 443.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 4: 316–329, 2007. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Lavina B, Gouveia L, Sun Y, Raschperger E, Rasanen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554: 475–480, 2018. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 445.McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab 31: 795–806, 2011. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wood JH. Physiology, Pharmacology, and Dynamics of Cerebrospinal Fluid. Boston, MA: Springer, 1980. doi: 10.1007/978-1-4684-1039-6_1. [DOI] [Google Scholar]
- 447.Bito LZ, Davson H. Local variations in cerebrospinal fluid composition and its relationship to the composition of the extracellular fluid of the cortex. Exp Neurol 14: 264–280, 1966. doi: 10.1016/0014-4886(66)90114-2. [DOI] [PubMed] [Google Scholar]
- 448.Levick JR. Revision of the Starling principle: new views of tissue fluid balance. J Physiol 557: 704, 2004. doi: 10.1113/jphysiol.2004.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A 90: 7275–7279, 1993. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener 10: 58, 2015. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Foldi M, Zoltan OT, Sonkodi S, Sebok S, Domonkos H, Fekete E, Deak I. Lymphogenic encephalopathy and brain edema. Angiologica 5: 372–376, 1968. [PubMed] [Google Scholar]
- 452.Foldi M, Csillik B, Joo F, Zoltan OT. Electron microscopic alterations in the central nervous system in experimental lymphogenic encephalopathy. I. Angiologica 4: 50–56, 1967. [PubMed] [Google Scholar]
- 453.Csanda E, Foldi M, Obal F, Zoltan OT. Cerebral oedema as a consequence of experimental cervical lymphatic blockage. Angiologica 5: 55–63, 1968. doi: 10.1159/000157734. [DOI] [PubMed] [Google Scholar]
- 454.Solenov E, Watanabe H, Manley GT, Verkman AS. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol 286: C426–C432, 2004. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 455.Wagner HJ, Barthel J, Pilgrim C. Permeability of the external glial limiting membrane of rat parietal cortex. Anat Embryol (Berl) 166: 427–437, 1983. doi: 10.1007/BF00305928. [DOI] [PubMed] [Google Scholar]
- 456.Fisher RF. The water permeability of basement membrane under increasing pressure: evidence for a new theory of permeability. Proc R Soc Lond B Biol Sci 216: 475–496, 1982. doi: 10.1098/rspb.1982.0087. [DOI] [PubMed] [Google Scholar]
- 457.Sykova E, Vorisek I, Mazel T, Antonova T, Schachner M. Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur J Neurosci 22: 1873–1880, 2005. doi: 10.1111/j.1460-9568.2005.04375.x. [DOI] [PubMed] [Google Scholar]
- 458.Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y. Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci 34: 6164–6176, 2014. doi: 10.1523/JNEUROSCI.3458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Perkins KL, Arranz AM, Yamaguchi Y, Hrabetova S. Brain extracellular space, hyaluronan, and the prevention of epileptic seizures. Rev Neurosci 28: 869–892, 2017. doi: 10.1515/revneuro-2017-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Laurent TC, Laurent UB, Fraser JR. The structure and function of hyaluronan: an overview. Immunol Cell Biol 74: A1–7, 1996. [ doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 461.Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev 58: 255–315, 1978. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- 462.Laurent UB, Laurent TC, Hellsing LK, Persson L, Hartman M, Lilja K. Hyaluronan in human cerebrospinal fluid. Acta Neurol Scand 94: 194–206, 1996. doi: 10.1111/j.1600-0404.1996.tb07052.x. [DOI] [PubMed] [Google Scholar]
- 463.Jenkins HG, Bachelard HS. Developmental and age-related changes in rat brain glycosaminoglycans. J Neurochem 51: 1634–1640, 1988. doi: 10.1111/j.1471-4159.1988.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 464.Cargill R, Kohama SG, Struve J, Su W, Banine F, Witkowski E, Back SA, Sherman SLS. Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol Aging 33: 830, 2012. doi: 10.1016/j.neurobiolaging.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Bloomfield IG, Johnston IH, Bilston LE. Effects of proteins, blood cells and glucose on the viscosity of cerebrospinal fluid. Pediatr Neurosurg 28: 246–251, 1998. doi: 10.1159/000028659. [DOI] [PubMed] [Google Scholar]
- 466.Day TD. Connective tissue permeability and the mode of action of hyaluronidase. Nature 166: 785–786, 1950. doi: 10.1038/166785b0. [DOI] [PubMed] [Google Scholar]
- 467.Zhang K, Chen J. The regulation of integrin function by divalent cations. Cell Adh Migr 6: 20–29, 2012. doi: 10.4161/cam.18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352: 550–555, 2016. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, Mortensen KN, Staeger FF, Bork PA, Bashford L, Toro ER, Tithof J, Kelley DH, Thomas JH, Hjorth PG, Martens EA, Mehta RI, Solis O, Blinder P, Kleinfeld D, Hirase H, Mori Y, Nedergaard M. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367: eaax7171, 2020. doi: 10.1126/science.aax7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Noda Y, Sohara E, Ohta E, Sasaki S. Aquaporins in kidney pathophysiology. Nat Rev Nephrol 6: 168–178, 2010. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 471.Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A 95: 11981–11986, 1998. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A 100: 13615–13620, 2003. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J 18: 542–544, 2004. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- 474.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta 1758: 1085–1093, 2006. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 475.Ohene Y, Harrison IF, Nahavandi P, Ismail O, Bird EV, Ottersen OP, Nagelhus EA, Thomas DL, Lythgoe MF, Wells JA. Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study. NeuroImage 188: 515–523, 2019. doi: 10.1016/j.neuroimage.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Zhang Y, Xu K, Liu Y, Erokwu BO, Zhao P, Flask CA, Ramos-Estebanez C, Farr GW, LaManna JC, Boron WF, Yu X. Increased cerebral vascularization and decreased water exchange across the blood-brain barrier in aquaporin-4 knockout mice. PLoS One 14: e0218415, 2019. doi: 10.1371/journal.pone.0218415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare O, Laake P, Klungland A, Thoren AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A 108: 17815–17820, 2011. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Vindedal GF, Thoren AE, Jensen V, Klungland A, Zhang Y, Holtzman MJ, Ottersen OP, Nagelhus EA. Removal of aquaporin-4 from glial and ependymal membranes causes brain water accumulation. Mol Cell Neurosci 77: 47–52, 2016. doi: 10.1016/j.mcn.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18: 1291–1293, 2004. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 480.Thrane AS, Takano T, Rangroo Thrane V, Wang F, Peng W, Ottersen OP, Nedergaard M, Nagelhus EA. In vivo NADH fluorescence imaging indicates effect of aquaporin-4 deletion on oxygen microdistribution in cortical spreading depression. J Cereb Blood Flow Metab 33: 996–999, 2013. doi: 10.1038/jcbfm.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Smith AJ, Verkman AS. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J 32: 543–551, 2018. doi: 10.1096/fj.201700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Elorza-Vidal X, Gaitan-Penas H, Estevez R. Chloride channels in astrocytes: structure, roles in brain homeostasis and implications in disease. Int J Mol Sci 20: 1034, 2019. doi: 10.3390/ijms20051034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Felix L, Delekate A, Petzold GC, Rose CR. Sodium fluctuations in astroglia and their potential impact on astrocyte function. Front Physiol 11: 871, 2020. doi: 10.3389/fpls.2020.00871, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Zeuthen T. Water-transporting proteins. J Membr Biol 234: 57–73, 2010. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- 485.Wang MX, Ray L, Tanaka KF, Iliff JJ, Heys J. Varying perivascular astroglial endfoot dimensions along the vascular tree maintain perivascular-interstitial flux through the cortical mantle. Glia 69: 715–728, 2020. doi: 10.1002/glia.23923. [DOI] [PubMed] [Google Scholar]
- 486.Halnes G, Pettersen KH, Øyehaug L, Rognes ME, Einevoll GT. Astrocytic ion dynamics: implications for potassium buffering and liquid flow. In: Computational Glioscience, edited by De Pittà M, Berry H.. Cham, Switzerland: Springer International Publishing, 2019, p. 1. [Google Scholar]
- 487.Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A 100: 2106–2111, 2003. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Frydenlund DS, Bhardwaj A, Otsuka T, Mylonakou MN, Yasumura T, Davidson KG, Zeynalov E, Skare O, Laake P, Haug FM, Rash JE, Agre P, Ottersen OP, Amiry-Moghaddam M. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci U S A 103: 13532–13536, 2006. doi: 10.1073/pnas.0605796103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Kitchen P, Salman MM, Halsey AM, Clarke-Bland C, MacDonald JA, Ishida H, Vogel HJ, Almutiri S, Logan A, Kreida S, Al-Jubair T, Winkel Missel J, Gourdon P, Tornroth-Horsefield S, Conner MT, Ahmed Z, Conner AC, Bill RM. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 181: 784–799, 2020. doi: 10.1016/j.cell.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Wilson MH. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab 36: 1338–1350, 2016. doi: 10.1177/0271678X16648711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Hua J, Liu P, Kim T, Donahue M, Rane S, Chen JJ, Qin Q, Kim SG. MRI techniques to measure arterial and venous cerebral blood volume. NeuroImage 187: 17–31, 2019. doi: 10.1016/j.neuroimage.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Zong X, Lian C, Jimenez J, Yamashita K, Shen D, Lin W. Morphology of perivascular spaces and enclosed blood vessels in young to middle-aged healthy adults at 7T: dependences on age, brain region, and breathing gas. NeuroImage 218: 116978, 2020. doi: 10.1016/j.neuroimage.2020.116978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science 304: 1926–1929, 2004. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 494.McCormick L, Nielsen T, Nicolas A, Ptito M, Montplaisir J. Topographical distribution of spindles and K-complexes in normal subjects. Sleep 20: 939–941, 1997. doi: 10.1093/sleep/20.11.939. [DOI] [PubMed] [Google Scholar]
- 495.Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M. Ultra-fast magnetic resonance encephalography of physiological brain activity–glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36: 1033–1045, 2016. doi: 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Stefanovska A. Coupled oscillators: complex but not complicated cardiovascular and brain interactions. Conf Proc IEEE Eng Med Biol Soc 2006: 437–440, 2006. doi: 10.1109/iembs.2006.259557. [DOI] [PubMed] [Google Scholar]
- 497.Kvernmo HD, Stefanovska A, Kirkeboen KA, Kvernebo K. Oscillations in the human cutaneous blood perfusion signal modified by endothelium-dependent and endothelium-independent vasodilators. Microvasc Res 57: 298–309, 1999. doi: 10.1006/mvre.1998.2139. [DOI] [PubMed] [Google Scholar]
- 498.Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci 35: 2485–2491, 2015. doi: 10.1523/JNEUROSCI.3246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Strik C, Klose U, Erb M, Strik H, Grodd W. Intracranial oscillations of cerebrospinal fluid and blood flows: analysis with magnetic resonance imaging. J Magn Reson Imaging 15: 251–258, 2002. doi: 10.1002/jmri.10084. [DOI] [PubMed] [Google Scholar]
- 500.Drew PJ, Mateo C, Turner KL, Yu X, Kleinfeld D. Ultra-slow oscillations in fMRI and resting-state connectivity: neuronal and vascular contributions and technical confounds. Neuron 107: 782–804, 2020. doi: 10.1016/j.neuron.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366: 628–631, 2019. doi: 10.1126/science.aax5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Musizza B, Stefanovska A, McClintock PV, Palus M, Petrovcic J, Ribaric S, Bajrovic FF. Interactions between cardiac, respiratory and EEG-delta oscillations in rats during anaesthesia. J Physiol 580: 315–326, 2007. doi: 10.1113/jphysiol.2006.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Kiviniemi V. Endogenous brain fluctuations and diagnostic imaging. Hum Brain Mapp 29: 810–817, 2008. doi: 10.1002/hbm.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Ho D, Zhao X, Gao S, Hong C, Vatner DE, Vatner SF. Heart rate and electrocardiography monitoring in mice. Curr Protoc Mouse Biol 1: 123–139, 2011. doi: 10.1002/9780470942390.mo100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Lipton P. Effects of membrane depolarization on light scattering by cerebral cortical slices. J Physiol 231: 365–383, 1973. doi: 10.1113/jphysiol.1973.sp010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 37: 161–181, 2014. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Aalkjær C, Boedtkjer D, Matchkov V. Vasomotion–what is currently thought? Acta Physiol (Oxf) 202: 253–269, 2011. doi: 10.1111/j.1748-1716.2011.02320.x. [DOI] [PubMed] [Google Scholar]
- 508.Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J Neurosurg 22: 581–590, 1965. doi: 10.3171/jns.1965.22.6.0581. [DOI] [PubMed] [Google Scholar]
- 509.Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology 123: 1198–1208, 2015. doi: 10.1097/ALN.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 510.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18: 419–434, 2017. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Xing CY, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan LJ, Zhang R. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 37: 2848–2856, 2017. doi: 10.1177/0271678X16676826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.King J, Lowery DR. Physiology, Cardiac Output. Treasure Island, FL: StatPearls, 2021. [NBK470455/?report=classic] [PubMed] [Google Scholar]
- 513.Hua J, Qin Q, Pekar JJ, van Zijl PC. Measurement of absolute arterial cerebral blood volume in human brain without using a contrast agent. NMR Biomed 24: 1313–1325, 2011. doi: 10.1002/nbm.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Grubb RL Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 5: 630–639, 1974. doi: 10.1161/01.STR.5.5.630. doi:. [DOI] [PubMed] [Google Scholar]
- 515.Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Identification of the upward movement of human CSF in vivo and its relation to the brain venous system. J Neurosci 37: 2395–2402, 2017. doi: 10.1523/JNEUROSCI.2754-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Rungta RL, Chaigneau E, Osmanski BF, Charpak S. Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron 99: 362–375, 2018. doi: 10.1016/j.neuron.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Bilston LE, Fletcher DF, Brodbelt AR, Stoodley MA. Arterial pulsation-driven cerebrospinal fluid flow in the perivascular space: a computational model. Comput Methods Biomech Biomed Engin 6: 235–241, 2003. doi: 10.1080/10255840310001606116. [DOI] [PubMed] [Google Scholar]
- 518.Hall A, O’Kane R. The best marker for guiding the clinical management of patients with raised intracranial pressure-the RAP index or the mean pulse amplitude? Acta Neurochir (Wien) 158: 1997–2009, 2016. doi: 10.1007/s00701-016-2932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96: 17–42, 2017. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Mateo C, Knutsen PM, Tsai PS, Shih AY, Kleinfeld D. Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent “resting-state”. Connectivity. Neuron 96: 936–948, 2017. doi: 10.1016/j.neuron.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Goodman JR, Iliff JJ. Vasomotor influences on glymphatic-lymphatic coupling and solute trafficking in the central nervous system. J Cereb Blood Flow Metab 40: 1724–1734, 2020. doi: 10.1177/0271678X19874134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Turner KL, Gheres KW, Proctor EA, Drew PJ. Neurovascular coupling and bilateral connectivity during NREM and REM sleep. eLife 9: 1–1, 2020. doi: 10.7554/eLife.62071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Ma Q, Ries M, Decker Y, Muller A, Riner C, Bucker A, Fassbender K, Detmar M, Proulx ST. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137: 151–165, 2019. doi: 10.1007/s00401-018-1916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Eide PK, Vinje V, Pripp AH, Mardal KA, Ringstad G. Sleep deprivation impairs molecular clearance from the human brain. Brain 144: 863–874, 2021. doi: 10.1093/brain/awaa443. [DOI] [PubMed] [Google Scholar]
- 525.Demiral ŞB, Tomasi D, Sarlls J, Lee H, Wiers CE, Zehra A, Srivastava T, Ke K, Shokri-Kojori E, Freeman CR, Lindgren E, Ramirez V, Miller G, Bandettini P, Horovitz S, Wang GJ, Benveniste H, Volkow ND. Apparent diffusion coefficient changes in human brain during sleep– does it inform on the existence of a glymphatic system? NeuroImage 185: 263–273, 2019. doi: 10.1016/j.neuroimage.2018.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Voldsbekk I, Maximov II, Zak N, Roelfs D, Geier O, Due-Tønnessen P, Elvsåshagen T, Strømstad M, Bjørnerud A, Groote I. Evidence for wakefulness-related changes to extracellular space in human brain white matter from diffusion-weighted MRI. NeuroImage 212: 116682, 2020. doi: 10.1016/j.neuroimage.2020.116682. [DOI] [PubMed] [Google Scholar]
- 527.Voldsbekk I, Groote I, Zak N, Roelfs D, Geier O, Due-Tønnessen P, Løkken LL, Strømstad M, Blakstvedt TY, Kuiper YS, Elvsåshagen T, Westlye LT, Bjørnerud A, Maximov II. Sleep and sleep deprivation differentially alter white matter microstructure: a mixed model design utilising advanced diffusion modelling. NeuroImage 226: 117540, 2021. doi: 10.1016/j.neuroimage.2020.117540. [DOI] [PubMed] [Google Scholar]
- 528.Ozturk BO, Monte B, Koundal S, Dai F, Benveniste H, Lee H. Disparate volumetric fluid shifts across cerebral tissue compartments with two different anesthetics. Fluids Barriers CNS 18: 1, 2021. doi: 10.1186/s12987-020-00236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res 37: 2496–2512, 2012. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Rasmussen R, O’Donnell J, Ding F, Nedergaard M. Interstitial ions: A key regulator of state-dependent neural activity? Prog Neurobiol 193: 101802, 2020. doi: 10.1016/j.pneurobio.2020.101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Caron NS, Banos R, Yanick C, Aly AE, Byrne LM, Smith ED, Xie Y, Smith SE, Potluri N, Findlay Black H, Casal L, Ko S, Cheung D, Kim H, Seong IS, Wild EJ, Song JJ, Hayden MR, Southwell AL. Mutant huntingtin is cleared from the brain via active mechanisms in Huntington disease. J Neurosci 41: 780–796, 2021. doi: 10.1523/JNEUROSCI.1865-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, Ahmed Z, Fisher A, Meftah S, Murray TK, Ottersen OP, Nagelhus EA, O’Neill MJ, Wells JA, Lythgoe MF. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 143: 2576–2593, 2020. doi: 10.1093/brain/awaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Eide PK, Hansson HA. Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol Appl Neurobiol 44: 474–490, 2018. doi: 10.1111/nan.12420. [DOI] [PubMed] [Google Scholar]
- 534.Hjerpe R, Bett JS, Keuss MJ, Solovyova A, McWilliams TG, Johnson C, Sahu I, Varghese J, Wood N, Wightman M, Osborne G, Bates GP, Glickman MH, Trost M, Knebel A, Marchesi F, Kurz T. UBQLN2 mediates autophagy-independent protein aggregate clearance by the proteasome. Cell 166: 935–949, 2016. doi: 10.1016/j.cell.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem 84: 435–464, 2015. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Thibaudeau TA, Anderson RT, Smith DM. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun 9: 1097, 2018. doi: 10.1038/s41467-018-03509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 11: 74, 2016. doi: 10.1186/s13024-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, Sun W, Goldman S, Blekot S, Nielsen M, Takano T, Deane R, Nedergaard M. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun 6: 6807, 2015. doi: 10.1038/ncomms7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Muraszko K, Sung C, Walbridge S, Greenfield L, Dedrick RL, Oldfield EH, Youle RJ. Pharmacokinetics and toxicology of immunotoxins administered into the subarachnoid space in nonhuman primates and rodents. Cancer Res 53: 3752–3757, 1993. [PubMed] [Google Scholar]
- 540.Strazielle N, Ghersi-Egea JF. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm 10: 1473–1491, 2013. doi: 10.1021/mp300518e. [DOI] [PubMed] [Google Scholar]
- 541.Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, Nedergaard M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37: 2112–2124, 2017. doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Huber VJ, Tsujita M, Nakada T. Identification of aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg Med Chem 17: 411–417, 2009. doi: 10.1016/j.bmc.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 543.Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol 29: 176–192, 2019. doi: 10.1111/bpa.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R, Xiao M, Hu G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl Neurodegener 8: 7, 2019. doi: 10.1186/s40035-019-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Grapp M, Wrede A, Schweizer M, Huwel S, Galla HJ, Snaidero N, Simons M, Buckers J, Low PS, Urlaub H, Gartner J, Steinfeld R. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4: 2123, 2013. doi: 10.1038/ncomms3123. [DOI] [PubMed] [Google Scholar]
- 546.Fuxe K, Agnati LF, Marcoli M, Borroto-Escuela DO. Volume transmission in central dopamine and noradrenaline neurons and its astroglial targets. Neurochem Res 40: 2600–2614, 2015. doi: 10.1007/s11064-015-1574-5. [DOI] [PubMed] [Google Scholar]
- 547.Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346: 89–93, 2014. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Kronschlager MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkuhler J. Gliogenic LTP spreads widely in nociceptive pathways. Science 354: 1144–1148, 2016. doi: 10.1126/science.aah5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Ganapathy MK, Tadi P. Anatomy, Head and Neck, Pituitary Gland. Treasure Island, FL: StatPearls, 2021. [NBK551529] [PubMed] [Google Scholar]
- 550.Xavier AL, Hauglund NL, von Holstein-Rathlou S, Li Q, Sanggaard S, Lou N, Lundgaard I, Nedergaard M. Cannula implantation into the cisterna magna of rodents. J Vis Exp 135: 57378, 2018. doi: 10.3791/57378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Sweeney AM, Pla V, Du T, Liu G, Sun Q, Peng S, Plog BA, Kress BT, Wang X, Mestre H, Nedergaard M. In vivo imaging of cerebrospinal fluid transport through the intact mouse skull using fluorescence macroscopy. J Vis Exp 149: e59774, 2019. doi: 10.3791/59774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Ramos M, Burdon Bechet N, Battistella R, Pavan C, Xavier AL, Nedergaard M, Lundgaard I. Cisterna magna injection in rats to study glymphatic function. Methods Mol Biol 1938: 97–104, 2019. doi: 10.1007/978-1-4939-9068-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 11: 107, 2013. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ladron-de-Guevara A, Shang JK, Nedergaard M, Dh K. Perivascular pumping in the mouse brain: Realistic boundary conditions reconcile theory, simulation, and experiment. bioRxiv 2020.07.02.183608, 2020. doi: 10.1101/2020.07.02.183608. [DOI] [PMC free article] [PubMed]
- 555.Daversin-Catty C, Vinje V, Mardal KA, Rognes ME. The mechanisms behind perivascular fluid flow. PLoS One 15: e0244442, 2020. doi: 10.1371/journal.pone.0244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Kedarasetti RT, Drew PJ, Costanzo F. Arterial pulsations drive oscillatory flow of CSF but not directional pumping. Sci Rep 10: 10102, 2020. doi: 10.1038/s41598-020-66887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Martinac AD, Bilston LE. Computational modelling of fluid and solute transport in the brain. Biomech Model Mechanobiol 19: 781–800, 2020. doi: 10.1007/s10237-019-01253-y. [DOI] [PubMed] [Google Scholar]
- 558.Kurtcuoglu V, Jain K, Martin BA. Modelling of cerebrospinal fluid flow by computational fluid dynamics. In: Biomechanics of the Brain, edited by Miller K. Cham, Switzerland: Springer, 2019, p. 215–241. [Google Scholar]
- 559.Linninger AA, Tangen K, Hsu CY, Frim D. Cerebrospinal fluid mechanics and its coupling to cerebrovascular dynamics. Annu Rev Fluid Mech 48: 219–257, 2016. doi: 10.1146/annurev-fluid-122414-034321. [DOI] [Google Scholar]
- 560.Breasted JH, New York Historical Society Library. The Edwin Smith Surgical Papyrus. Pub. in Facsimile and Hieroglyphic Transliteration, with Translation and Commentary. Chicago, IL: University of Chicago Press, 1930. [Google Scholar]
- 561.O’Connell JE. The vascular factor in intracranial pressure and the maintenance of the cerebrospinal fluid circulation. Brain 66: 204–228, 1943. doi: 10.1093/brain/66.3.204. [DOI] [Google Scholar]
- 562.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res 545: 103–113, 1991. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 563.Bakker E, Naessens DM, VanBavel E. Paravascular spaces: entry to or exit from the brain? Exp Physiol 104: 1013–1017, 2019. doi: 10.1113/EP087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Coles JA, Myburgh E, Brewer JM, McMenamin PG. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog Neurobiol 156: 107–148, 2017. doi: 10.1016/j.pneurobio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 565.Coles JA, Stewart-Hutchinson PJ, Myburgh E, Brewer JM. The mouse cortical meninges are the site of immune responses to many different pathogens, and are accessible to intravital imaging. Methods 127: 53–61, 2017. doi: 10.1016/j.ymeth.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Rey J, Sarntinoranont M. Pulsatile flow drivers in brain parenchyma and perivascular spaces: a resistance network model study. Fluids Barriers CNS 15: 20, 2018. doi: 10.1186/s12987-018-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Troyetsky DE, Tithof J, Thomas JH, Kelley DH. Dispersion as a waste-clearance mechanism in flow through penetrating perivascular spaces in the brain. Sci Rep 11: 4595, 2021. doi: 10.1038/s41598-021-83951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Diem AK, Tan M, Bressloff NW, Hawkes C, Morris AW, Weller RO, Carare RO. A simulation model of periarterial clearance of amyloid-beta from the brain. Front Aging Neurosci 8: 18, 2016. doi: 10.3389/fnagi.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Diem AK, MacGregor Sharp M, Gatherer M, Bressloff NW, Carare RO, Richardson G. Arterial pulsations cannot drive intramural periarterial drainage: significance for abeta drainage. Front Neurosci 11: 475, 2017. doi: 10.3389/fnins.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Sharp MK, Diem AK, Weller RO, Carare RO. Peristalsis with oscillating flow resistance: a mechanism for periarterial clearance of amyloid beta from the brain. Ann Biomed Eng 44: 1553–1565, 2016. doi: 10.1007/s10439-015-1457-6. [DOI] [PubMed] [Google Scholar]
- 571.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 238: 962–974, 2006. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 572.Coloma M, Schaffer JD, Carare RO, Chiarot PR, Huang P. Pulsations with reflected boundary waves: a hydrodynamic reverse transport mechanism for perivascular drainage in the brain. J Math Biol 73: 469–490, 2016. doi: 10.1007/s00285-015-0960-6. [DOI] [PubMed] [Google Scholar]
- 573.Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front Aging Neurosci 11: 1, 2019. doi: 10.3389/fnagi.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Raghunandan A, Ladron-de-Guevara A, Tithof J, Mestre H, Du T, Nedergaard M, Thomas JH, Kelley DH. Bulk flow of cerebrospinal fluid observed in periarterial spaces is not an artifact of injection. eLife 10: e65958, 2021. doi: 10.7554/eLife.65958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, Lu H. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology 127: 976–988, 2017. doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Plog BA, Mestre H, Olveda GE, Sweeney AM, Kenney HM, Cove A, Dholakia KY, Tithof J, Nevins TD, Lundgaard I, Du T, Kelley DH, Nedergaard M. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight 3: e120922, 2018. doi: 10.1172/jci.insight.120922. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Eftekhari S, Westgate CS, Johansen KP, Bruun SR, Jensen RH. Long-term monitoring of intracranial pressure in freely-moving rats; impact of different physiological states. Fluids Barriers CNS 17: 39, 2020. doi: 10.1186/s12987-020-00199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Kedarasetti RT, Turner KL, Echagarruga C, Gluckman BJ, Drew PJ, Costanzo F. Functional hyperemia drives fluid exchange in the paravascular space. Fluids Barriers CNS 17: 52, 2020. doi: 10.1186/s12987-020-00214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Romano F, Suresh V, Galie PA, Grotberg JB. Peristaltic flow in the glymphatic system. Sci Rep 10: 21065, 2020. doi: 10.1038/s41598-020-77787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Gao YR, Greene SE, Drew PJ. Mechanical restriction of intracortical vessel dilation by brain tissue sculpts the hemodynamic response. NeuroImage 115: 162–176, 2015. doi: 10.1016/j.neuroimage.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.van de Vosse FN, Stergiopulos N. Pulse wave propagation in the arterial tree. Annu Rev Fluid Mech 43: 467–499, 2011. doi: 10.1146/annurev-fluid-122109-160730. [DOI] [Google Scholar]
- 582.Herold V, Parczyk M, Morchel P, Ziener CH, Klug G, Bauer WR, Rommel E, Jakob PM. In vivo measurement of local aortic pulse-wave velocity in mice with MR microscopy at 17.6 Tesla. Magn Reson Med 61: 1293–1299, 2009. doi: 10.1002/mrm.21957. [DOI] [PubMed] [Google Scholar]
- 583.Williams R, Needles A, Cherin E, Zhou YQ, Henkelman RM, Adamson SL, Foster FS. Noninvasive ultrasonic measurement of regional and local pulse-wave velocity in mice. Ultrasound Med Biol 33: 1368–1375, 2007. doi: 10.1016/j.ultrasmedbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 584.Reymond P, Merenda F, Perren F, Rufenacht D, Stergiopulos N. Validation of a one-dimensional model of the systemic arterial tree. Am J Physiol Heart Circ Physiol 297: H208–H222, 2009. doi: 10.1152/ajpheart.00037.2009. [DOI] [PubMed] [Google Scholar]
- 585.Santisakultarm TP, Cornelius NR, Nishimura N, Schafer AI, Silver RT, Doerschuk PC, Olbricht WL, Schaffer CB. In vivo two-photon excited fluorescence microscopy reveals cardiac- and respiration-dependent pulsatile blood flow in cortical blood vessels in mice. Am J Physiol Heart Circ Physiol 302: H1367–H1377, 2012. doi: 10.1152/ajpheart.00417.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Lam MA, Hemley SJ, Najafi E, Vella NG, Bilston LE, Stoodley MA. The ultrastructure of spinal cord perivascular spaces: implications for the circulation of cerebrospinal fluid. Sci Rep 7: 12924, 2017. doi: 10.1038/s41598-017-13455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Wang P, Olbricht WL. Fluid mechanics in the perivascular space. J Theor Biol 274: 52–57, 2011. doi: 10.1016/j.jtbi.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 588.Min Rivas F, Liu J, Martell BC, Du T, Mestre H, Nedergaard M, Tithof J, Thomas JH, Kelley DH. Surface periarterial spaces of the mouse brain are open, not porous. J R Soc Interface 17: 20200593, 2020. doi: 10.1098/rsif.2020.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci 37: 2904–2915, 2017. [ doi: 10.1523/JNEUROSCI.3390-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Steinman J, Koletar MM, Stefanovic B, Sled JG. 3D morphological analysis of the mouse cerebral vasculature: comparison of in vivo and ex vivo methods. PLoS One 12: e0186676, 2017. doi: 10.1371/journal.pone.0186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Groothuis DR, Vavra MW, Schlageter KE, Kang EW, Itskovich AC, Hertzler S, Allen CV, Lipton HL. Efflux of drugs and solutes from brain: the interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab 27: 43–56, 2007. doi: 10.1038/sj.jcbfm.9600315. [DOI] [PubMed] [Google Scholar]
- 592.Hladky SB, Barrand MA. Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 15: 30, 2018. doi: 10.1186/s12987-018-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Ray L, Iliff JJ, Heys JJ. Analysis of convective and diffusive transport in the brain interstitium. Fluids Barriers CNS 16: 6, 2019. doi: 10.1186/s12987-019-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Cserr HF, DePasquale M, Nicholson C, Patlak CS, Pettigrew KD, Rice ME. Extracellular volume decreases while cell volume is maintained by ion uptake in rat brain during acute hypernatremia. J Physiol 442: 277–295, 1991. doi: 10.1113/jphysiol.1991.sp018793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Hirsch S, Reichold J, Schneider M, Szekely G, Weber B. Topology and hemodynamics of the cortical cerebrovascular system. J Cereb Blood Flow Metab 32: 952–967, 2012. doi: 10.1038/jcbfm.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Smith AF, Doyeux V, Berg M, Peyrounette M, Haft-Javaherian M, Larue AE, Slater JH, Lauwers F, Blinder P, Tsai P, Kleinfeld D, Schaffer CB, Nishimura N, Davit Y, Lorthois S. Brain capillary networks across species: a few simple organizational requirements are sufficient to reproduce both structure and function. Front Physiol 10: 233, 2019. doi: 10.3389/fphys.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Valnes LM, Mitusch SK, Ringstad G, Eide PK, Funke SW, Mardal KA. Apparent diffusion coefficient estimates based on 24 hours tracer movement support glymphatic transport in human cerebral cortex. Sci Rep 10: 9176, 2020. doi: 10.1038/s41598-020-66042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Croci M, Vinje V, Rognes ME. Uncertainty quantification of parenchymal tracer distribution using random diffusion and convective velocity fields. Fluids Barriers CNS 16: 32, 2019. doi: 10.1186/s12987-019-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Rajna Z, Raitamaa L, Tuovinen T, Heikkila J, Kiviniemi V, Seppanen T. 3D multi-resolution optical flow analysis of cardiovascular pulse propagation in human brain. IEEE Trans Med Imaging 38: 2028–2036, 2019. doi: 10.1109/TMI.2019.2904762. [DOI] [PubMed] [Google Scholar]
- 600.Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res 104: 428–441, 2009. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- 601.Elkin R, Nadeem S, Haber E, Steklova K, Lee H, Benveniste H, Tannenbaum A. GlymphVIS: visualizing glymphatic transport pathways using regularized optimal transport. Med Image Comput Glyphic Assist Interv 11070: 844–852, 2018. doi: 10.1007/978-3-030-00928-1_95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Elkin R, Nadeem S, Lee H, Benveniste H, Tannenbaum A. Fisher-rao regularized transport analysis of the glymphatic system and waste drainage. Med Image Comput Rao Assist Interv 12267: 573–582, 2020. doi: 10.1007/978-3-030-59728-3_56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Debacker C, Djemai B, Ciobanu L, Tsurugizawa T, Le Bihan D. Diffusion MRI reveals in vivo and non-invasively changes in astrocyte function induced by an aquaporin-4 inhibitor. PLoS One 15: e0229702, 2020. doi: 10.1371/journal.pone.0229702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Alshuhri MS, Gallagher L, Work LM, Holmes WM. Direct imaging of glymphatic transport using H217O MRI. JCI Insight 6: 141159, 2021. doi: 10.1172/jci.insight.141159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Alves De Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol 38: 597–620, 2020. doi: 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
- 606.Cserr HF, Ostrach LH. Bulk flow of interstitial fluid after intracranial injection of Blue Dextran 2000. Exp Neurol 45: 50–60, 1974. doi: 10.1016/0014-4886(74)90099-5. [DOI] [PubMed] [Google Scholar]
- 607.Brierley JB, Field EJ. The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat 82: 153–166, 1948. [PubMed] [Google Scholar]
- 608.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal fluid Res 2: 6, 2005. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal fluid Res 7: 9, 2010. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Kida S, Yamashima T, Kubota T, Ito H, Yamamoto S. A light and electron microscopic and immunohistochemical study of human arachnoid villi. J Neurosurg 69: 429–435, 1988. doi: 10.3171/jns.1988.69.3.0429. [DOI] [PubMed] [Google Scholar]
- 611.Johnston M, Armstrong D, Koh L. Possible role of the cavernous sinus veins in cerebrospinal fluid absorption. Cerebrospinal Fluid Res 4: 3, 2007. doi: 10.1186/1743-8454-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Benveniste H, Elkin R, Heerdt PM, Koundal S, Xue Y, Lee H, Wardlaw J, Tannenbaum A. The glymphatic system and its role in cerebral homeostasis. J Appl Physiol (1985) 129: 1330–1340, 2020. doi: 10.1152/japplphysiol.00852.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Lee H, Mortensen K, Sanggaard S, Koch P, Brunner H, Quistorff B, Nedergaard M, Benveniste H. Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med 79: 1568–1578, 2018. doi: 10.1002/mrm.26779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Boulton M, Flessner M, Armstrong D, Hay J, Johnston M. Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol Regul Intgr Comp Physiol 274: R88–R96, 1998. doi: 10.1152/ajpregu.1998.274.1.R88. [DOI] [PubMed] [Google Scholar]
- 615.Jayatilaka P. Arachnoid granulations and arachnoid villi in mammals by ceylon. J Med Sci 18: 25–30, 1969. [Google Scholar]
- 616.Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19: 480–488, 1993. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 617.Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol Regul Intgr Comp Physiol 275: R889–R896, 1998. doi: 10.1152/ajpregu.1998.275.3.R889. [DOI] [PubMed] [Google Scholar]
- 618.Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol 339: 519–534, 1983. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The effect of body posture on brain glymphatic transport. J Neurosci 35: 11034–11044, 2015. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Shanthaveerappa TR, Bourne GH. The ‘perineural epithelium’, a metabolically active, continuous, protoplasmic cell barrier surrounding peripheral nerve fasciculi. J Anat 96: 527–537, 1962. [PMC free article] [PubMed] [Google Scholar]
- 621.Thomas PK. The connective tissue of peripheral nerve: an electron microscope study. J Anat 97: 35–44, 1963. [PMC free article] [PubMed] [Google Scholar]
- 622.Peltonen S, Alanne M, Peltonen J. Barriers of the peripheral nerve. Tissue Barriers 1: e24956, 2013. doi: 10.4161/tisb.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Froehner SC, Davies A, Baldwin SA, Lienhard GE. The blood-nerve barrier is rich in glucose transporter. J Neurocytol 17: 173–178, 1988. doi: 10.1007/BF01674204. [DOI] [PubMed] [Google Scholar]
- 624.Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol 121: 291–312, 2011. doi: 10.1007/s00401-010-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Wadhwani KC, Rapoport SI. Transport properties of vertebrate blood-nerve barrier: Comparison with blood-brain barrier. Prog Neurobiol 43: 235–279, 1994. doi: 10.1016/0301-0082(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 626.Manzo RP, Gomez DG, Potts DG. Cerebrospinal fluid absorption in the rabbit. Inner ear pathways. Acta Otolaryngol 109: 389–396, 1990. doi: 10.3109/00016489009125160. [DOI] [PubMed] [Google Scholar]
- 627.Nakao Y, Tabuchi T, Sakihama N, Nakajima S. Extracellular fluid pathway inside the facial nerve fascicles. Ann Otol Rhinol Laryngol 106: 503–505, 1997. doi: 10.1177/000348949710600611. [DOI] [PubMed] [Google Scholar]
- 628.Takeda T, Takeuchi S, Kishimoto S, Saito H. Interstitial fluid pressure in the facial nerve: Relationship between facial nerve pressure and cerebrospinal fluid pressure. Am J Otolaryngol 10: 345–350, 1989. [DOI] [PubMed] [Google Scholar]
- 629.Gomez DG, Fenstermacher JD, Manzo RP, Johnson D, Potts DG. Cerebrospinal fluid absorption in the rabbit: olfactory pathways. Acta Otolaryngol 100: 429–436, 1985. doi: 10.3109/00016488509126567. [DOI] [PubMed] [Google Scholar]
- 630.Walter BA, Valera VA, Takahashi S, Ushiki T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol Appl Neurobiol 32: 388–396, 2006. doi: 10.1111/j.1365-2990.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 631.Furukawa M, Shimoda H, Kajiwara T, Kato S, Yanagisawa S. Topographic study on nerve-associated lymphatic vessels in the murine craniofacial region by immunohistochemistry and electron microscopy. Biomed Res 29: 289–296, 2008. doi: 10.2220/biomedres.29.289. [DOI] [PubMed] [Google Scholar]
- 632.Gomez DG, Manzo RP, Fenstermacher JD, Potts DG. Cerebrospinal fluid absorption in the rabbit. Optic pathways. Graefes Arch Clin Exp Ophthalmol 226: 1–7, 1988. doi: 10.1007/BF02172707. [DOI] [PubMed] [Google Scholar]
- 633.Wang X, Lou N, Eberhardt A, Yang Y, Kusk P, Xu Q, Forstera B, Peng S, Shi M, Ladron-de-Guevara A, Delle C, Sigurdsson B, Xavier AL, Erturk A, Libby RT, Chen L, Thrane AS, Nedergaard M. An ocular glymphatic clearance system removes beta-amyloid from the rodent eye. Sci Transl Med 12: eaaw3210, 2020. doi: 10.1126/scitranslmed.aaw3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Zakharov A, Papaiconomou C, Johnston M. Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat Res Biol 2: 139–146, 2004. doi: 10.1089/lrb.2004.2.139. [DOI] [PubMed] [Google Scholar]
- 635.Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8: 1434, 2017. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Zakharov A, Papaiconomou C, Djenic J, Midha R, Johnston M. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol Appl Neurobiol 29: 563–573, 2003. doi: 10.1046/j.0305-1846.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- 637.de Leon MJ, Li Y, Okamura N, Tsui WH, Saint-Louis LA, Glodzik L, Osorio RS, Fortea J, Butler T, Pirraglia E, Fossati S, Kim HJ, Carare RO, Nedergaard M, Benveniste H, Rusinek H. Cerebrospinal fluid clearance in alzheimer disease measured with dynamic PET. J Nucl Med 58: 1471–1476, 2017. doi: 10.2967/jnumed.116.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1: 2, 2004. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Norwood JN, Zhang Q, Card D, Craine A, Ryan TM, Drew PJ. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife 8: e44278, 2019. doi: 10.7554/eLife.44278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res 362: 25–37, 2018. doi: 10.1016/j.heares.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Leboucq N, Menjot de Champfleur N, Menjot de Champfleur S, Bonafe A. The olfactory system. Diagn Interv Imaging 94: 985–991, 2013. doi: 10.1016/j.diii.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 642.Erlich SS, McComb JG, Hyman S, Weiss MH. Ultrastructural morphology of the olfactory pathway for cerebrospinal fluid drainage in the rabbit. J Neurosurg 64: 466–473, 1986. doi: 10.3171/jns.1986.64.3.0466. [DOI] [PubMed] [Google Scholar]
- 643.Weller RO, Sharp MM, Christodoulides M, Carare RO, Mollgard K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol 135: 363–385, 2018. doi: 10.1007/s00401-018-1809-z. [DOI] [PubMed] [Google Scholar]
- 644.Jackson RT, Tigges J, Arnold W. Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol 105: 180–184, 1979. doi: 10.1001/archotol.1979.00790160014003. [DOI] [PubMed] [Google Scholar]
- 645.Melin E, Eide PK, Ringstad G. In vivo assessment of cerebrospinal fluid efflux to nasal mucosa in humans. Sci Rep 10: 1–10, 2020. doi: 10.1038/s41598-020-72031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Lowhagen P, Johansson BB, Nordborg C. The nasal route of cerebrospinal fluid drainage in man. A light-microscope study. Neuropathol Appl Neurobiol 20: 543–550, 1994. doi: 10.1111/j.1365-2990.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 647.Stanton EH, Persson ND, Gomolka RS, Lilius T, Sigurðsson B, Lee H, Xavier AL, Benveniste H, Nedergaard M, Mori Y. Mapping of CSF transport using high spatiotemporal resolution dynamic contrast-enhanced MRI in mice: effect of anesthesia. Magn Reson Med 85: 3326–3317, 2021. doi: 10.1002/mrm.28645. [DOI] [PubMed] [Google Scholar]
- 648.Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572: 62–66, 2019. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- 649.Foldi M, Gellert A, Kozma M, Poberai M, Zoltan OT, Csanda E. New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat (Basel) 64: 498–505, 1966. doi: 10.1159/000142849. [DOI] [PubMed] [Google Scholar]
- 650.Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 6: e29738, 2017. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.von During M, Andres KH. Sensory nerve fiber terminals in the arachnoid granulations of non-human primates. Neurosci Lett 127: 121–124, 1991. doi: 10.1016/0304-3940(91)90909-d. [DOI] [PubMed] [Google Scholar]
- 652.Ohta K, Inokuchi T, Hayashida Y, Mizukami T, Yoshida T, Kawahara T. Regional diminution of von Willebrand factor expression on the endothelial covering arachnoid granulations of human, monkey and dog brain. Kurume Med J 49: 177–183, 2002. doi: 10.2739/kurumemedj.49.177. [DOI] [PubMed] [Google Scholar]
- 653.Tripathi R. Tracing the bulk outflow route of cerebrospinal fluid by transmission and scanning electron microscopy. Brain Res 80: 503–506, 1974. doi: 10.1016/0006-8993(74)91033-6. [DOI] [PubMed] [Google Scholar]
- 654.Glimcher SA, Holman DW, Lubow M, Grzybowski DM. Ex vivo model of cerebrospinal fluid outflow across human arachnoid granulations. Invest Ophthalmol Vis Sci 49: 4721–4728, 2008. doi: 10.1167/iovs.08-2238. [DOI] [PubMed] [Google Scholar]
- 655.Grzybowski DM, Holman DW, Katz SE, Lubow M. In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Invest Ophthalmol Vis Sci 47: 3664–3672, 2006. doi: 10.1167/iovs.05-0929. [DOI] [PubMed] [Google Scholar]
- 656.Fox RJ, Walji AH, Mielke B, Petruk KC, Aronyk KE. Anatomic details of intradural channels in the parasagittal dura: a possible pathway for flow of cerebrospinal fluid. Neurosurgery 39: 84–90, 1996. PMC] doi: 10.1097/00006123-199607000-00017. [DOI] [PubMed] [Google Scholar]
- 657.Wu CH, Lirng JF, Ling YH, Wang YF, Wu HM, Fuh JL, Lin PC, Wang SJ, Chen SP. Noninvasive characterization of human glymphatics and meningeal lymphatics in an in vivo model of blood–brain barrier leakage. Ann Neurol 89: 111–114, 2021. doi: 10.1002/ana.25928. [DOI] [PubMed] [Google Scholar]
- 658.Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, Papadopoulos Z, Kanamori M, Salvador AF, Baker W, Lemieux M, Da Mesquita S, Cugurra A, Fitzpatrick J, Sviben S, Kossina R, Bayguinov P, Townsend RR, Zhang Q, Erdmann-Gilmore P, Smirnov I, Lopes MB, Herz J, Kipnis J. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184: 1000–1017, 2021. doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Casley Smith JR, Foldi Borcsok E, Foldi M. The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. Brit J Exp Pathol 57: 179–188, 1976. [PMC free article] [PubMed] [Google Scholar]
- 660.Wang HJ, Casley-Smith JR. Drainage of the prelymphatics of the brain via the adventitia of the vertebral artery. Acta Anat (Basel) 134: 67–71, 1989. doi: 10.1159/000146736. [DOI] [PubMed] [Google Scholar]
- 661.Eide PK, Mariussen E, Uggerud H, Pripp AH, Lashkarivand A, Hassel B, Christensen H, Hovd MH, Ringstad G. Clinical application of intrathecal gadobutrol for assessment of cerebrospinal fluid tracer clearance to blood. JCI Insight 6: 147063, 2021. doi: 10.1172/jci.insight.147063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Jacob L, Boisserand LS, Geraldo LH, de Brito Neto J, Mathivet T, Antila S, Barka B, Xu Y, Thomas JM, Pestel J, Aigrot MS, Song E, Nurmi H, Lee S, Alitalo K, Renier N, Eichmann A, Thomas JL. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10: 4594, 2019. doi: 10.1038/s41467-019-12568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Miura M, Kato S, von Ludinghausen M. Lymphatic drainage of the cerebrospinal fluid from monkey spinal meninges with special reference to the distribution of the epidural lymphatics. Arch Histol Cytol 61: 277–286, 1998. doi: 10.1679/aohc.61.277. [DOI] [PubMed] [Google Scholar]
- 664.Fricke B, Andres KH, Von During M. Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech 53: 96–105, 2001. doi: 10.1002/jemt.1074. [DOI] [PubMed] [Google Scholar]
- 665.Ma Q, Decker Y, Muller A, Ineichen BV, Proulx ST. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J Exp Med 216: 2492–2502, 2019. doi: 10.1084/jem.20190351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Bradbury MW, Cole DF. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol 299: 353–365, 1980. doi: 10.1113/jphysiol.1980.sp013129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Andresen M, Hadi A, Petersen LG, Juhler M. Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochir 157: 109–113, 2015. doi: 10.1007/s00701-014-2250-2. [DOI] [PubMed] [Google Scholar]
- 668.Brimioulle S, Moraine JJ, Norrenberg D, Kahn RJ. Effects of positioning and exercise on intracranial pressure in a neurosurgical intensive care unit. Phys Ther 77: 1682–1689, 1997. doi: 10.1093/ptj/77.12.1682. [DOI] [PubMed] [Google Scholar]
- 669.Myung J, Schmal C, Hong S, Tsukizawa Y, Rose P, Zhang Y, Holtzman MJ, De Schutter E, Herzel H, Bordyugov G, Takumi T. The choroid plexus is an important circadian clock component. Nat Commun 9: 1062, 2018. doi: 10.1038/s41467-018-03507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Silver I, Li B, Szalai J, Johnston M. Relationship between intracranial pressure and cervical lymphatic pressure and flow rates in sheep. Am J Physiol Regul Integr Comp Physiol 277: R1712–R1717, 1999. doi: 10.1152/ajpregu.1999.277.6.R1712. [DOI] [PubMed] [Google Scholar]
- 671.Klarica M, Rados M, Draganic P, Erceg G, Oreskovic D, Marakovic J, Bulat M. Effect of head position on cerebrospinal fluid pressure in cats: comparison with artificial model. Croatian Med J 47: 233–238, 2006. [PMC free article] [PubMed] [Google Scholar]
- 672.Benveniste H, Heerdt PM, Fontes M, Rothman DL, Volkow ND. Glymphatic system function in relation to anesthesia and sleep states. Anesth Analg 128: 747–758, 2019. doi: 10.1213/ANE.0000000000004069. [DOI] [PubMed] [Google Scholar]
- 673.Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol 287: R1450–R1455, 2004. doi: 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- 674.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594: 5749–5768, 2016. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Bachmann SB, Proulx ST, He Y, Ries M, Detmar M. Differential effects of anaesthesia on the contractility of lymphatic vessels in vivo. J Physiol 597: 2841–2852, 2019. doi: 10.1113/JP277254. [DOI] [PubMed] [Google Scholar]
- 676.Benveniste H, Lee H, Volkow ND. The glymphatic pathway: waste removal from the CNS via cerebrospinal fluid transport. Neuroscientist 23: 454–465, 2017. doi: 10.1177/1073858417691030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology 65: 106–119, 2019. doi: 10.1159/000490349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science 370: 50–56, 2020. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Golanov EV, Bovshik EI, Wong KK, Pautler RG, Foster CH, Federley RG, Zhang JY, Mancuso J, Wong ST, Britz GW. Subarachnoid hemorrhage-induced block of cerebrospinal fluid flow: role of brain coagulation factor III (tissue factor). J Cereb Blood Flow Metab 38: 793–808, 2018. doi: 10.1177/0271678X17701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Mayer M. Biochemical and biological aspects of the plasminogen activation system. Clin Biochem 23: 197–211, 1990. doi: 10.1016/0009-9120(90)90601-p. [DOI] [PubMed] [Google Scholar]
- 681.Pu T, Zou W, Feng W, Zhang Y, Wang L, Wang H, Xiao M. Persistent malfunction of glymphatic and meningeal lymphatic drainage in a mouse model of subarachnoid hemorrhage. Exp Neurobiol 28: 104–118, 2019. doi: 10.5607/en.2019.28.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.McConnell ED, Wei HS, Reitz KM, Kang H, Takano T, Vates GE, Nedergaard M. Cerebral microcirculatory failure after subarachnoid hemorrhage is reversed by hyaluronidase. J Cereb Blood Flow Metab 36: 1537–1552. 2016. doi: 10.1177/0271678X15608389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Luo C, Yao X, Li J, He B, Liu Q, Ren H, Liang F, Li M, Lin H, Peng J, Yuan TF, Pei Z, Su H. Paravascular pathways contribute to vasculitis and neuroinflammation after subarachnoid hemorrhage independently of glymphatic control. Cell Death Dis 7: e2160, 2016. doi: 10.1038/cddis.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Chen J, Wang L, Xu H, Xing L, Zhuang Z, Zheng Y, Li X, Wang C, Chen S, Guo Z, Liang Q, Wang Y. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun 11: 3159, 2020. doi: 10.1038/s41467-020-16851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Blaumanis OR, Rennels ML, Grady PA. Focal cerebral edema impedes convective fluid/tracer movement through paravascular pathways in cat brain. Adv Neurol 52: 385–389, 1990. [PubMed] [Google Scholar]
- 686.Esposito E, Ahn BJ, Shi J, Nakamura Y, Park JH, Mandeville ET, Yu Z, Chan SJ, Desai R, Hayakawa A, Ji X, Lo EH, Hayakawa K. Brain-to-cervical lymph node signaling after stroke. Nat Commun 10: 5306, 2019. doi: 10.1038/s41467-019-13324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Yanev P, Poinsatte K, Hominick D, Khurana N, Zuurbier KR, Berndt M, Plautz EJ, Dellinger MT, Stowe AM. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab 40: 263–275, 2020. doi: 10.1177/0271678X18822921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93: 215–225, 2016. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Hoshi A, Tsunoda A, Tada M, Nishizawa M, Ugawa Y, Kakita A. Expression of aquaporin 1 and aquaporin 4 in the temporal neocortex of patients with Parkinson’s disease. Brain Pathol 27: 160–168, 2017. doi: 10.1111/bpa.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Christensen J, Wright DK, Yamakawa GR, Shultz SR, Mychasiuk R. Repetitive mild traumatic brain injury alters glymphatic clearance rates in limbic structures of adolescent female rats. Sci Rep 10: 6254, 2020. doi: 10.1038/s41598-020-63022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4: 134ra60, 2012. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 35: 518–526, 2015. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Emmert AS, Iwasawa E, Shula C, Schultz P, Lindquist D, Dunn RS, Fugate EM, Hu YC, Mangano FT, Goto J. Impaired neural differentiation and glymphatic CSF flow in the Ccdc39 rat model of neonatal hydrocephalus: genetic interaction with L1cam. Dis Model Mech 12: dmm040972, 2019. doi: 10.1242/dmm.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Albeck MJ, Skak C, Nielsen PR, Olsen KS, Borgesen SE, Gjerris F. Age dependency of resistance to cerebrospinal fluid outflow. J Neurosurg 89: 275–278, 1998. doi: 10.3171/jns.1998.89.2.0275. [DOI] [PubMed] [Google Scholar]
- 695.Koellhoffer EC, McCullough LD, Ritzel RM. Old maids: aging and its impact on microglia function. Int J Mol Sci 18: 769–725, 2017. doi: 10.3390/ijms18040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Liu Y, Jacobowitz DM, Barone F, McCarron R, Spatz M, Feuerstein G, Hallenbeck JM, Sirén AL. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab 14: 348–352, 1994. doi: 10.1038/jcbfm.1994.43. [DOI] [PubMed] [Google Scholar]
- 697.Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury: a review. CNS Drugs 25: 175–185, 2011. doi: 10.2165/11584870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 698.Stefani A, Högl B. Sleep in Parkinson’s disease. Neuropsychopharmacology 45: 121–128, 2020. doi: 10.1038/s41386-019-0448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Veauthier C. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Rep 15: 1–10, 2015. doi: 10.1007/s11910-014-0514-0. [DOI] [PubMed] [Google Scholar]
- 700.Wen Y, Schwartz S, Borenstein AR, Wu Y, Morgan D, Anderson WM. Sleep, cognitive impairment, and alzheimer’s disease: a systematic review and meta-analysis. Sleep 40: 1–18, 2017. doi: 10.1016/j.sleep.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 701.Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron 94: 19–36, 2017. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363: 880–884, 2019. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326: 1005–1007, 2009. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, Patterson BW, Baty J, Morris JC, Ovod V, Mawuenyega KG, Bateman RJ. Effect of sleep on overnight CSF amyloid-β kinetics running. Ann Neurol 83: 197–1017, 2018. doi: 10.1002/ana.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Ulv Larsen SM, Landolt HP, Berger W, Nedergaard M, Knudsen GM, Holst SC. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol 18: e3000623, 2020. doi: 10.1371/journal.pbio.3000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Chen W, Huang P, Zeng H, Lin J, Shi Z, Yao X. Cocaine-induced structural and functional impairments of the glymphatic pathway in mice. Brain Behav Immun 20: 1–8, 2020. doi: 10.1016/j.bbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 707.Plog BA, Lou N, Pierre CA, Cove A, Kenney HM, Hitomi E, Kang H, Iliff JJ, Zeppenfeld DM, Nedergaard M, Vates GE. When the air hits your brain: decreased arterial pulsatility after craniectomy leading to impaired glymphatic flow. J Neurosurg 133: 210–223, 2019. doi: 10.3171/2019.2.jns182675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 114: 1462–1473, 2018. doi: 10.1093/cvr/cvy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 37: 1326–1337, 2017. doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Xue Y, Liu N, Zhang M, Ren X, Tang J, Fu J. Concomitant enlargement of perivascular spaces and decrease in glymphatic transport in an animal model of cerebral small vessel disease. Brain Res Bull 161: 78–83, 2020. doi: 10.1016/j.brainresbull.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 711.Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, Lu M, Zhang T, Liu A, Chen J. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging 50: 96–106, 2017. doi: 10.1016/j.neurobiolaging.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease–insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol 13: 612–623, 2017. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 713.Viggiano D, Wagner CA, Martino G, Nedergaard M, Zoccali C, Unwin R, Capasso G. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol 16: 452–469, 2020. doi: 10.1038/s41581-020-0266-9. [DOI] [PubMed] [Google Scholar]
- 714.Yang J, Lunde LK, Nuntagij P, Oguchi T, Camassa LM, Nilsson LN, Lannfelt L, Xu Y, Amiry-Moghaddam M, Ottersen OP, Torp R. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis 27: 711–722, 2011. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- 715.Feng W, Zhang Y, Wang Z, Xu H, Wu T, Marshall C, Gao J, Xiao M. Microglia prevent beta-amyloid plaque formation in the early stage of an Alzheimer’s disease mouse model with suppression of glymphatic clearance. Alzheimers Res Ther 12: 125–125, 2020. doi: 10.1186/s13195-020-00688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Steward CE, Venkatraman VK, Lui E, Malpas CB, Ellis KA, Cyarto EV, Vivash L, O’Brien TJ, Velakoulis D, Ames D, Masters CL, Lautenschlager NT, Bammer R, Desmond PM. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J Neuroimaging 31: 569–510, 2021. doi: 10.1111/jon.12837. [DOI] [PubMed] [Google Scholar]
- 717.Chandra A, Farrell C, Wilson H, Dervenoulas G, De Natale ER, Politis M, Alzheimer’s Disease Neuroimaging Initiative. Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer’s disease spectrum. Neurobiol Aging 97: 1–9, 2021. doi: 10.1016/j.neurobiolaging.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 718.Burfeind KG, Murchison CF, Westaway SK, Simon MJ, Erten-Lyons D, Kaye JA, Quinn JF, Iliff JJ. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement (NY) 3: 348–359, 2017. doi: 10.1016/j.trci.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Han F, Chen J, Belkin-Rosen A, Gu Y, Luo L, Buxton O, Liu X. The coupling of global brain activity and cerebrospinal fluid inflow is correlated with Alzheimer’s disease related pathology. BioRxiv 2020.06.04.134726, 2020. doi: 10.1101/2020.06.04.134726. [DOI]
- 720.Rajna Z, Mattila H, Huotari N, Tuovinen T, Kruger J, Holst SC, Korhonen V, Remes AM, Seppanen T, Hennig J, Nedergaard M, Kiviniemi V. Cardiovascular brain impulses in Alzheimer’s disease. Brain awab144, 2021. doi: 10.1093/brain/awab144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Tuovinen T, Kananen J, Rajna Z, Lieslehto J, Korhonen V, Rytty R, Mattila H, Huotari N, Raitamaa L, Helakari H, Elseoud AA, Kruger J, LeVan P, Tervonen O, Hennig J, Remes AM, Nedergaard M, Kiviniemi V. The variability of functional MRI brain signal increases in Alzheimer’s disease at cardiorespiratory frequencies. Sci Rep 10: 21559, 2020. doi: 10.1038/s41598-020-77984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Talamo BR, Feng WH, Perez-Cruet M, Adelman L, Kosik K, Lee MY, Cork LC, Kauer JS. Pathologic changes in olfactory neurons in Alzheimer’s disease. Ann N Y Acad Sci 640: 1–7, 1991. doi: 10.1111/j.1749-6632.1991.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 723.Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 73: 93–101, 2016. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Cao X, Xu H, Feng W, Su D, Xiao M. Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res Bull 143: 83–96, 2018. doi: 10.1016/j.brainresbull.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 725.Tan EK, Chao YX, West A, Chan LL, Poewe W, Jankovic J. Parkinson disease and the immune system–associations, mechanisms and therapeutics. Nat Rev Neurol 16: 303–318, 2020. doi: 10.1038/s41582-020-0344-4. [DOI] [PubMed] [Google Scholar]
- 726.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers 3: 17013, 2017. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 727.Cumming P, Borghammer P. Molecular imaging and the neuropathologies of Parkinson’s disease. Curr Top Behav Neurosci 11: 117–148, 2012. doi: 10.1007/7854_2011_165. [DOI] [PubMed] [Google Scholar]
- 728.Cui H, Wang W, Zheng X, Xia D, Liu H, Qin C, Tian H, Teng J. Decreased AQP4 expression aggravates ɑ-synuclein pathology in parkinson’s disease mice, possibly via impaired glymphatic clearance. J Mol Neurosci. In press. doi: 10.1007/s12031-021-01836-4. [DOI] [PubMed] [Google Scholar]
- 729.Chen HL, Chen PC, Lu CH, Tsai NW, Yu CC, Chou KH, Lai YR, Taoka T, Lin WC. Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson’s disease. Oxidative Med Cell Longevity 2021: 1–10, 2021. doi: 10.1155/2021/4034509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Ding XB, Wang XX, Xia DH, Liu H, Tian HY, Fu Y, Chen YK, Qin C, Wang JQ, Xiang Z, Zhang ZX, Cao QC, Wang W, Li JY, Wu E, Tang BS, Ma MM, Teng JF, Wang XJ. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat Med 27: 411–418, 2021. doi: 10.1038/s41591-020-01198-1. [DOI] [PubMed] [Google Scholar]
- 731.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9: 211–221, 2013. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nat Rev Dis Primers 2: 16084, 2016. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- 733.Mohamed AZ, Cumming P, Gotz J, Nasrallah F, Department of Defense Alzheimer’s Disease Neuroimaging Initiative. Tauopathy in veterans with long-term posttraumatic stress disorder and traumatic brain injury. Eur J Nucl Med Mol Imaging 46: 1139–1151, 2019. doi: 10.1007/s00259-018-4241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Iverson GL, Gardner AJ, Shultz SR, Solomon GS, McCrory P, Zafonte R, Perry G, Hazrati LN, Keene CD, Castellani RJ. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 142: 3672–3693, 2019. doi: 10.1093/brain/awz286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Li L, Chopp M, Ding G, Davoodi-Bojd E, Zhang L, Li Q, Zhang Y, Xiong Y, Jiang Q. MRI detection of impairment of glymphatic function in rat after mild traumatic brain injury. Brain Res 1747: 147062–147062, 2020. doi: 10.1016/j.brainres.2020.147062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, Ennerfelt HE, Shapiro D, Nguyen BH, Frost EL, Lammert CR, Kipnis J, Lukens JR. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun 11: 1–18, 2020. doi: 10.1038/s41467-020-18113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Plog BA, Nedergaard M. Why have we not yet developed a simple blood test for TBI? Expert Rev Neurother 15: 465–468, 2015. doi: 10.1586/14737175.2015.1031112. [DOI] [PubMed] [Google Scholar]
- 738.Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, Blair NF, Craufurd D, Priller J, Rickards H, Rosser A, Kordasiewicz HB, Czech C, Swayze EE, Norris DA, Baumann T, Gerlach I, Schobel SA, Paz E, Smith AV, Bennett CF, Lane RM, Phase 1–2a IONIS-HTTRx Study Site Teams. Targeting Huntingtin expression in patients with Huntington’s disease. N Engl J Med 380: 2307–2316, 2019. doi: 10.1056/NEJMoa1900907. [DOI] [PubMed] [Google Scholar]
- 739.Southwell AL, Smith SE, Davis TR, Caron NS, Villanueva EB, Xie Y, Collins JA, Li Ye M, Sturrock A, Leavitt BR, Schrum AG, Hayden MR. Ultrasensitive measurement of huntingtin protein in cerebrospinal fluid demonstrates increase with Huntington disease stage and decrease following brain huntingtin suppression. Sci Rep 5: 1–11, 2015. doi: 10.1038/srep12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Ng Kee Kwong KC, Mehta AR, Nedergaard M, Chandran S. Defining novel functions for cerebrospinal fluid in ALS pathophysiology. Acta Neuropathol Commun 8: 1–18, 2020. doi: 10.1186/s40478-020-01018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Hirose M, Asano M, Watanabe-Matsumoto S, Yamanaka K, Abe Y, Yasui M, Tokuda E, Furukawa Y, Misawa H. Stagnation of glymphatic interstitial fluid flow and delay in waste clearance in the SOD1-G93A mouse model of ALS. Neurosci Res 4: S0168-0102(20)30489-2, 2020. doi: 10.1016/j.neures.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 742.Watanabe-Matsumoto S, Moriwaki Y, Okuda T, Ohara S, Yamanaka K, Abe Y, Yasui M, Misawa H. Dissociation of blood-brain barrier disruption and disease manifestation in an aquaporin-4-deficient mouse model of amyotrophic lateral sclerosis. Neurosci Res 133: 48–57, 2018. doi: 10.1016/j.neures.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 743.Ding Y, Zhang T, Wu G, McBride DW, Xu N, Klebe DW, Zhang Y, Li Q, Tang J, Zhang JH. Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp Neurol 320: 113003, 2019. doi: 10.1016/j.expneurol.2019.113003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, Alper SL, Lundgaard I, Nedergaard M, Kahle KT. Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 26: 285–295, 2020. doi: 10.1016/j.molmed.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Ma Q, Schlegel F, Bachmann SB, Schneider H, Decker Y, Rudin M, Weller M, Proulx ST, Detmar M. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci Rep 9: 14815, 2019. doi: 10.1038/s41598-019-51373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Song E, Mao T, Dong H, Boisserand LS, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577: 689–694, 2020. doi: 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers 2: 16065, 2016. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 748.Wei F, Song J, Zhang C, Lin J, Xue R, Shan LD, Gong S, Zhang GX, Qin ZH, Xu GY, Wang LH. Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology (Berl) 236: 1367–1384, 2019. doi: 10.1007/s00213-018-5147-6. [DOI] [PubMed] [Google Scholar]
- 749.Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl) 234: 365–379, 2017. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- 750.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16: 634–646, 2011. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Medina A, Watson SJ, Bunney W Jr, Myers RM, Schatzberg A, Barchas J, Akil H, Thompson RC. Evidence for alterations of the glial syncytial function in major depressive disorder. J Psychiatr Res 72: 15–21, 2016. doi: 10.1016/j.jpsychires.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry 73: 613–621, 2013. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Tae WS, Kim SS, Lee KU, Nam EC, Choi JW, Park JI. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol 32: 671–676, 2011. doi: 10.3174/ajnr.A2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Fournier AP, Gauberti M, Quenault A, Vivien D, Macrez R, Docagne F. Reduced spinal cord parenchymal cerebrospinal fluid circulation in experimental autoimmune encephalomyelitis. J Cereb Blood Flow Metab 39: 1258–1265, 2019. doi: 10.1177/0271678X18754732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, Karpus WJ, Sandor M, Fabry Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun 10: 229, 2019. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Hauglund NL, Kusk P, Kornum BR, Nedergaard M. Meningeal lymphangiogenesis and enhanced glymphatic activity in mice with chronically implanted EEG electrodes. J Neurosci 40: 2371–2380, 2020. doi: 10.1523/JNEUROSCI.2223-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Manouchehrian O, Ramos M, Bachiller S, Lundgaard I, Deierborg T. Acute systemic LPS-exposure impairs perivascular CSF distribution in mice. J Neuroinflammation 18: 1–13, 2021. doi: 10.1186/s12974-021-02082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Cai X, Qiao J, Kulkarni P, Harding IC, Ebong E, Ferris CF. Imaging the effect of the circadian light-dark cycle on the glymphatic system in awake rats. Proc Natl Acad Sci U S A, 117: 668–669, 2020. doi: 10.1073/pnas.1914017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Xue Y, Liu X, Koundal S, Constantinou S, Dai F, Santambrogio L, Lee H, Benveniste H. In vivo T1 mapping for quantifying glymphatic system transport and cervical lymph node drainage. Sci Rep 10: 14592, 2020. doi: 10.1038/s41598-020-71582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Förstera B, Zhao S, Bhatia HS, Parra-Damas A, Mrowka L, Theodorou D, Rempfler M, Xavier AL, Kress BT, Benakis C, Steinke H, Liebscher S, Bechmann I, Liesz A, Menze B, Kerschensteiner M, Nedergaard M, Ertürk A. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat Neurosci 22: 317–327, 2019. doi: 10.1038/s41593-018-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392: 1015–1035, 2018. doi: 10.1016/s0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Liu Q, Yan L, Huang M, Zeng H, Satyanarayanan SK, Shi Z, Chen D, Lu JH, Pei Z, Yao X, Su H. Experimental alcoholism primes structural and functional impairment of the glymphatic pathway. Brain Behav Immun 85: 106–119, 2020. doi: 10.1016/j.bbi.2019.06.029. [DOI] [PubMed] [Google Scholar]
- 763.Lundgaard I, Wang W, Eberhardt A, Vinitsky HS, Reeves BC, Peng S, Lou N, Hussain R, Nedergaard M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep 8: 2246, 2018. doi: 10.1038/s41598-018-20424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Cheng Y, Liu X, Ma X, Garcia R, Belfield K, Haorah J. Alcohol promotes waste clearance in the CNS via brain vascular reactivity. Free Radic Biol Med 143: 115–126, 2019. doi: 10.1016/j.freeradbiomed.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 765.von Holstein-Rathlou S, Petersen NC, Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett 662: 253–258, 2018. doi: 10.1016/j.neulet.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Gao X, Ming J, Liu S, Lai B, Fang F, Cang J. Sevoflurane enhanced the clearance of Abeta1-40 in hippocampus under surgery via up-regulating AQP-4 expression in astrocyte. Life Sci 221: 143–151, 2019. doi: 10.1016/j.lfs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 767.Lilius TO, Blomqvist K, Hauglund NL, Liu G, Stæger FF, Bærentzen S, Du T, Ahlström F, Backman JT, Kalso EA, Rauhala PV, Nedergaard M. Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release 304: 29–38, 2019. doi: 10.1016/j.jconrel.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 768.Cheng KP, Brodnick SK, Blanz SL, Zeng W, Kegel J, Pisaniello JA, Ness JP, Ross E, Nicolai EN, Settell ML, Trevathan JK, Poore SO, Suminski AJ, Williams JC, Ludwig KA. Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul 13: 1024–1030, 2020. doi: 10.1016/j.brs.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 769.Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scan Suppl 36: 1–193, 1960. [PubMed] [Google Scholar]
- 770.Lang EW, Diehl RR, Timmermann L, Baron R, Deuschl G, Mehdorn HM, Zunker P. Spontaneous oscillations of arterial blood pressure, cerebral and peripheral blood flow in healthy and comatose subjects. Neurol Res 21: 665–669, 1999. doi: 10.1080/01616412.1999.11740995. [DOI] [PubMed] [Google Scholar]
- 771.Elvsåshagen T, Norbom LB, Pedersen PO, Quraishi SH, Bjørnerud A, Malt UF, Groote IR, Westlye LT. Widespread changes in white matter microstructure after a day of waking and sleep deprivation. PLoS One 10: e0127351, 2015. doi: 10.1371/journal.pone.0127351. [DOI] [PMC free article] [PubMed] [Google Scholar]