Abstract
Vitamin D is best known for its role in maintaining bone health and calcium homeostasis. However, it also exerts a broad range of extra-skeletal effects on cellular physiology and on the immune system. Vitamins D2 and D3 share a high degree of structural similarity. Functional equivalence in their vitamin D-dependent effects on human physiology is usually assumed but has in fact not been well defined experimentally. In this study we seek to redress the gap in knowledge by undertaking an in-depth examination of changes in the human blood transcriptome following supplementation with physiological doses of vitamin D2 and D3. Our work extends a previously published randomized placebo-controlled trial that recruited healthy white European and South Asian women who were given 15 µg of vitamin D2 or D3 daily over 12 weeks in wintertime in the UK (Nov-Mar) by additionally determining changes in the blood transcriptome over the intervention period using microarrays. An integrated comparison of the results defines both the effect of vitamin D3 or D2 on gene expression, and any influence of ethnic background. An important aspect of this analysis was the focus on the changes in expression from baseline to the 12-week endpoint of treatment within each individual, harnessing the longitudinal design of the study. Whilst overlap in the repertoire of differentially expressed genes was present in the D2 or D3-dependent effects identified, most changes were specific to either one vitamin or the other. The data also pointed to the possibility of ethnic differences in the responses. Notably, following vitamin D3 supplementation, the majority of changes in gene expression reflected a down-regulation in the activity of genes, many encoding pathways of the innate and adaptive immune systems, potentially shifting the immune system to a more tolerogenic status. Surprisingly, gene expression associated with type I and type II interferon activity, critical to the innate response to bacterial and viral infections, differed following supplementation with either vitamin D2 or vitamin D3, with only vitamin D3 having a stimulatory effect. This study suggests that further investigation of the respective physiological roles of vitamin D2 and vitamin D3 is warranted.
Keywords: adaptive immunity, ethnicity, immunomodulation, innate immunity, human transcriptome, vitamin D supplementation, vitamin D3 , vitamin D2
Introduction
Vitamin D is a pro-hormone that is essential for human health. While vitamin D is best known for its role in maintaining bone health and calcium homeostasis, it also exerts a broad range of extra-skeletal effects on cellular physiology and on the immune system (1–6) and multiple studies have linked poor vitamin D status with increased risk of osteoporotic and stress fractures, increased risk of developing cardiovascular diseases and some cancers, poor modulation of the immune system, higher mortality including death from cancer, and the pathogenesis of immune mediated inflammatory diseases (7–10). It is recommended that individuals maintain serum 25-hydroxyvitamin D (25(OH)D) concentrations (the accepted biomarker of systemic vitamin D status) of at least 25 nmol/L throughout the year and throughout the life course (11). However, vitamin D deficiency and inadequacy (defined as serum 25(OH)D concentrations below 25 nmol/L and 50 nmol/L, respectively) is considered a global pandemic and a public health issue of great importance in the human population, especially in older people, individuals not exposed to sufficient sunlight and, importantly, ethnic groups with darker skin tone (12–14).
Vitamin D is the overarching term used to describe both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Both the plant/fungus-derived vitamin D2 and the animal-derived vitamin D3 forms can be found in some (albeit limited) human foods or are available as food supplements. However, vitamin D3 is also produced in the skin by the action of ultraviolet B radiation from the sun. Following two successive hydroxylation steps, in the liver and kidney, respectively, the active form of the vitamin, 1,25-dihydroxyvitamin D3 (1,25(OH)D3) binds to the intracellular vitamin D receptor (VDR) and, in complex with the retinoid X receptor (RXR), the heterodimer regulates expression of hundreds of genes through the vitamin D response element (VDRE) (15); other tissues also express the 25(OH)D 1-α-hydroxylase although kidney is considered the most important for production of circulating 1,25(OH)D (16). Both forms of vitamin D can be double hydroxylated into their active metabolites and they bind to the VDR with similar affinity (17), but there remains some controversy around whether or not they elicit identical biological responses (17–19). Moreover, there are differences in their catabolism and in their binding affinity to vitamin D binding protein (DBP), the major vitamin D transport protein in blood. Vitamin D2 binds DBP with lower affinity and is catabolised faster (20, 21).
The functional equivalence, or otherwise, of vitamins D2 and D3 for human health has been a subject of much debate in recent years, with some authors suggesting that the two compounds have equal efficacy while others provide evidence that vitamin D3 increases circulating serum 25(OH)D concentration more efficiently than vitamin D2 (22–26). In acute studies comparing the efficacy of vitamins D2 and D3 in raising serum 25(OH)D concentration, vitamin D2 is less effective than D3 when given as single bolus (22). However, the findings from studies following the daily administration of D2 or D3 over longer time-periods are more equivocal, with clinical trials showing higher efficacy of D3 (27, 28) or equal efficacy (29, 30). Furthermore, a recent meta-analysis of vitamin D supplementation trials found that reduced cancer mortality was seen only with vitamin D3 supplementation, not with vitamin D2 supplementation, and indicated that all-cause mortality was significantly lower in trials with vitamin D3 supplementation than in trials with vitamin D2 (9). Moreover, a recent study of US women makes the intriguing observation that the two independent metabolites, 25(OH)D3 and 25(OH)D2 appear to have totally different associations with depression (31). Supplementation with one form of vitamin D can reduce circulating concentrations of the other form. For example, our recent comparative trial of vitamin D2 versus D3 revealed that serum 25(OH)D3 concentration was reduced over the 12-week intervention in participants given vitamin D2, compared with the average 12-week concentration in participants given placebo [(28), see Figure 1C , for example]. The implications of such reciprocal depletion remain to be explored.
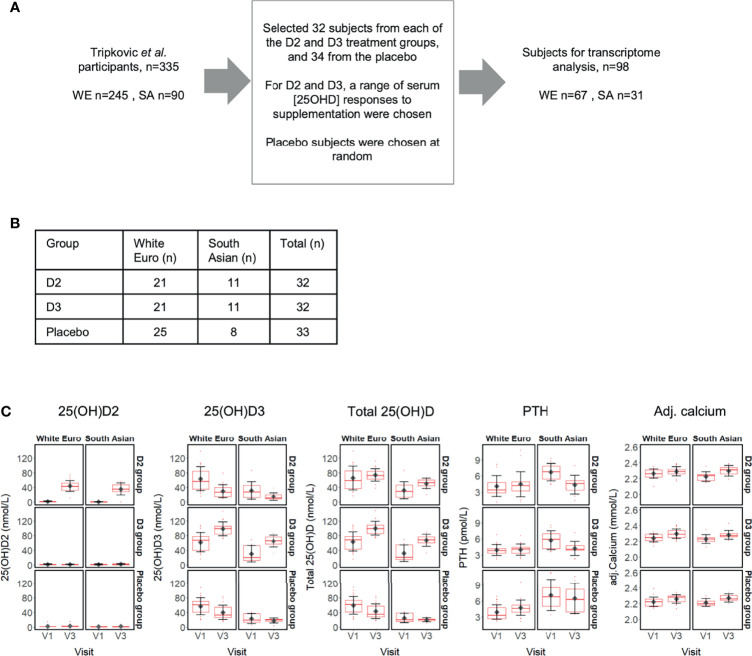
Figure 1.
(A) Selection of 98 subject participants from the Tripkovic et al. (2017) study (28) for transcriptomic analysis of their V1 (baseline) and V3 (12-week) samples in the present study; (B) Ethnicity and treatment group membership for the 97 subjects for which transcriptomic data was obtained (data for one South Asian subject from the placebo group did not pass quality control); (C) Metadata on serum concentrations of 25(OH)D2, 25(OH)D3, total 25(OH)D, PTH and calcium (albumin-adjusted) for the 97 subjects (see Supplementary Data File 1 for details). Participants were selected to provide comparable numbers between the placebo and the two vitamin D treatment groups, covering the full range of serum responses to supplementation within the D2 and D3 treatment groups, as judged from the measured changes in serum 25(OH)D2 or 25(OH)D3 concentrations between V1 and V3.
There is limited understanding of the effects of vitamin D supplementation on gene expression in vivo in humans because of the diversity of experimental designs used. This includes: (i) diverse sampling intervals, ranging from hours to years following vitamin D supplementation (32, 33); (ii) use of substantially different doses ranging from physiological (moderate) to supra-physiological doses; and (iii) relatively small participant numbers so most studies were considerably underpowered (33, 34). Currently, there is no robust evidence, from in vivo human genome-wide expression analysis, about which specific cellular pathways are influenced by vitamin D supplementation. Moreover, the influence of vitamin D2, as distinct from vitamin D3, on gene expression in humans has not yet been evaluated, even though vitamin D2 is also used widely as a supplement and food fortificant.
We have addressed this gap in knowledge by investigating gene expression in a relatively large cohort of healthy white European and South Asian women who participated in a randomised double-blind placebo-controlled trial – the D2-D3 Study - that compared the relative efficacy of vitamins D2 and D3 in raising serum 25(OH)D concentration. The study concluded that vitamin D3 was superior to vitamin D2 in raising serum 25(OH)D concentration (28). As an integral part of the original study design, we also investigated gene expression in the participants over the 12-week trial period. This allowed us to examine the effects of vitamin D supplementation on the transcriptome and to determine whether these effects might differ following supplementation with vitamin D2 compared with vitamin D3. We provide evidence in support of the hypothesis that the biological effects of vitamin D2 and D3 differ in humans. Consequently, a more comprehensive analysis of the biological effects of the two forms of vitamin D on human physiology is warranted.
Materials and Methods
The recruitment of individuals as part of the ‘D2-D3 Study’ was described previously (28) and the clinical trial has been registered (ISRCTN23421591). This study received ethical approval from the South-East Coast (Surrey) National Health Service Research Ethics Committee (11/LO/0708) and the University of Surrey Ethics Committee (EC/2011/97/FHMS). All participants gave written informed consent in agreement with the Helsinki Declaration before commencing study activities. Briefly, 335 women of both South Asian (SA) and white European (WE) descent were randomised to one of three intervention groups for 12-weeks and provided with daily doses of vitamin D within fortified foods (both orange juice and biscuits) (28). Participants were randomised to placebo, 15 μg/d vitamin D2 or 15 μg/d vitamin D3. Of the 335 participants reported previously by Tripkovic et al (28), 97 were selected for transcriptome analysis, representing 67 WE and 30 SA participants; allocations to each treatment group are reported in Figure 1 and Supplementary Data File 1 . Participants were selected at random from both ethnic groups to form a balanced placebo group. We undertook transcriptome analysis at baseline (V1) and at 12 weeks after treatment commenced (V3).
Blood Transcriptome Analysis
Whole peripheral blood (2.5 ml) was collected using PAXgene Blood RNA tubes (BD Biosciences and Diagnostics). The tubes were inverted ten times immediately after drawing blood, stored upright at 15-25°C for 24 hours, followed by a -20°C freezer for 24 hours and then into a -80°C freezer for long-term storage. Transcriptomic analysis was conducted essentially as described in previous studies (35, 36). Total RNA was isolated using the PAXgene Blood RNA Kit (Qiagen) following the manufacturer’s recommendations. cRNA was synthesised and fluorescently labelled with Cy3-CTP from 200 ng of total RNA using the Low Input QuickAmp Labelling Kit, One Color (Agilent Technology). Labelled cRNA was hybridised on a Sure Print G3 Human Gene Expression 8 x 60K v2 microarray slide (Agilent Technologies). Standard manufacturer’s instructions for one colour gene-expression analysis were followed for labelling, hybridisation and washing steps. Extracted RNA was quantified using NanoDrop ND2000 spectrophotometer (Thermo Scientific). RNA quality and integrity was evaluated using either the Bioanalyzer 2100 or the TapeStation 4200 (Agilent Technologies). Only RNA samples with an RNA Integrity Number (RIN) of >7.0 were subjected to DNA microarray analysis. Microarrays were hybridised at 65°C for 17 hours in an Agilent Hybridization Oven on a rotisserie at 10 rpm. The washed microarrays were scanned using an Agilent Microarray Scanner with a resolution of 2 μm.
Transcriptome Data Processing and Differential Expression Analysis
Raw scanned microarray images were processed using Agilent Feature Extraction software (v11.5.1.1) with the Agilent 039494_D_F_20140326_human_8x60K_v2 grid, and then imported into R for normalization and analysis using the LIMMA package (37). Normalised data for all participants (V3 – V1) was assessed by principal component analysis to screen for any batch effects ( Supplementary Figure 3 ). Microarray data were background-corrected using the ‘normexp’ method (with an offset of 50) and quantile normalised, producing expression values in the log base 2 scale. The processed data were then filtered to remove probes exhibiting low signals across the arrays, retaining non-control probes that are at least 10% brighter than negative control probe signals on at least 41 arrays (~20% of the arrays in the analysis). Data from identical replicate probes were then averaged to produce expression values at the unique probe level. Initial data exploration identified one sample (participant 0017, V3 time point) with array data that was a notable outlier from the group and therefore both the V1 and V3 microarrays for this subject were excluded from all subsequent analysis, reprocessing the data as above in their absence before proceeding.
Tests for differential expression were performed using LIMMA, applying appropriate linear model designs to identify: (i) significant differences in the transcriptional responses occurring across the 12-week V1 to V3 period of the study between the treatment and placebo groups for each ethnic cohort (example contrasts tested, in the format ethnicity_treatment_time: (WE_D2_V3 - WE_D2_V1) - (WE_P_V3-WE_P_V1) = 0, and (WE_D3_V3 - WE_D3_V1) - (WE_P_V3 - WE_P_V1) = 0); and ii) to determine significant changes for each ethnic cohort within each treatment group between the V3 and V1 sampling points, blocking on subject identity (example contrasts tested: WE_D2_V3 - WE_D2_V1 = 0, WE_D3_V3 - WE_D3_V1 = 0). Blocking on subject identity was used to control for inter-subject variability. Significance p-values were corrected for multiplicity using the Benjamini and Hochberg method, obtaining adjusted p-values (adj.P.Val).
Functional Enrichment and Network Analysis
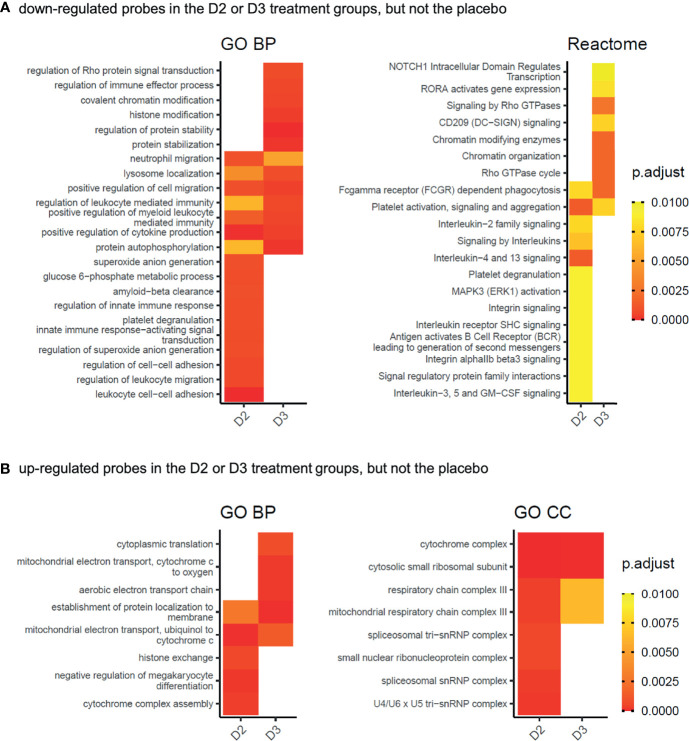
Functional enrichment analysis of lists of genes of interest possessing valid ENTREZ gene identifiers was performed using the R package clusterProfiler (38). The software produces adjusted p-values (p.adjust) using the Benjamini and Hochberg correction method. Construction and analysis of protein-protein interaction networks from sets of genes was undertaken in Cytoscape (39) using the STRING plugin (40). Cytoscape was also used to construct and visualise commonality in the functional categories identified as being significantly enriched in the genes responding to the experimental treatments. To visualise categories identified from the D2 or D3 treatment groups but not from the placebo (e.g. Figure 4 ) significantly enriched categories (p.adjust < 0.01) from all groups were imported such that treatment group nodes (D2, D3 and placebo (P)) are linked to functional category nodes by edges assigned the corresponding p.adjust values. All nodes with an edge connection to the placebo treatment node were then removed and the resulting networks further filtered to retain only those nodes with at least one edge connection with p.adjust<=0.001 (p.adjust<=0.01 for Reactome pathways). The results were finally summarised as heatmap plots ( Figure 4 ).
Figure 4.
Gene Ontology biological process (GO BP), cellular compartment (GO CC), or Reactome pathway functional categories significantly enriched in the gene products represented by the probes (A) significantly down-regulated (adj.P.Val ≤ 0.05) in the D2 or D3 treatment groups of the WE cohort, but not in the placebo group, and (B) significantly up-regulated (adj.P.Val ≤ 0.05) in the D2 or D3 treatment groups of the WE cohort, but not in the placebo group. Gene products represented by the significantly down-regulated probes in the comparisons WE D2 V3 v V1, WE D3 V3 v V1 and WE P V3 v V1 from Figure 2A , and possessing ENTREZ identifiers, were subjected separately to functional enrichment analysis using compareCluster (38). The details for each group are given in Supplementary Data File 4 . Significantly enriched categories (p.adjust ≤ 0.01) from all groups were processed as described in the Methods section to visualise categories identified from the D2 or D3 treatment groups but not by the placebo. Heatmap tiles that are blank correspond to categories that did not meet the significance criteria applied during the processing. The complete networks for each differentially expressed group of genes are shown in Supplementary Data File 5 .
Weighted gene co-expression network analysis was performed in R using the WGCNA (41) and CoExpNets (42) packages. A normalised expression data matrix generated by filtering to retain probes with signals more markedly above background (30% brighter than negative control probe signals on at least 41 arrays) was used as input, consisting of 12,169 unique probes. Signed scale-free networks were constructed separately for the data for the SA and WE ethnic groups using 50 iterations of the k-means clustering option in the CoExpNets ‘getDownstreamNetwork’ function to refine the clustering process ( Supplementary Data File 6 ). Pearson correlations between cluster module eigengenes and metadata for serum 25(OH)D2, 25(OH)D3, total 25(OH)D and PTH concentrations were calculated using pairwise complete observations.
Gene Set Enrichment Analysis (GSEA)
GSEA was performed using the R package clusterProfiler (38), applying the default parameters which implement the fgsea algorithm and correct for multiple testing using the Benjamini and Hochberg method. Gene sets were obtained from MSigDB via the msigdbr package (43). Ranked gene lists for interrogation were derived from LIMMA analysis of the unfiltered microarray data, pre-ranking genes according to their t-statistic.
Results
The study volunteers were South Asian (SA) and white European (WE) women based in the United Kingdom, aged between 20 and 64 years [n=335; (28)] and participation was for 12 weeks over the winter months (October to March, in Surrey, UK; latitude, 51°14’ N). Serum measurements, including concentrations of total 25(OH)D, 25(OH)D2 and 25(OH)D3, were determined from fasting blood samples taken at the start [baseline defined as Visit 1 (V1)] and at weeks 6 (V2) and 12 (V3). To determine whether changes in serum 25(OH)D concentration as a result of vitamin D supplementation are associated with physiological changes at the level of gene expression, we investigated the whole blood transcriptome from a representative subset of the study participants (n=98) using total RNA isolated from V1 and V3 blood samples and Agilent Human Whole Genome 8 × 60K v2 DNA microarrays ( Figure 1 ). Participants for transcriptome analysis were selected to provide comparable numbers between the placebo and the two vitamin D treatment groups, covering the full range of serum responses to supplementation within the D2 and D3 treatment groups, as judged from the measured changes in serum 25(OH)D2 or 25(OH)D3 concentration between V1 and V3 ( Supplementary Data File 1 ).
Metadata for the Transcriptome Analysis Cohort
Following microarray data quality control, transcriptome data were available for both the V1 and V3 samples of 97 study participants ( Figure 1B ); the RNA from one participant failed quality control and was excluded. Similar to data reported previously for the entire study cohort (28), the sub-set of participants in the present study showed increased concentrations of 25(OH)D2 and 25(OH)D3 only after supplementation with vitamin D2 and vitamin D3, respectively, and higher total 25(OH)D in both treatment groups ( Figure 1C ). In the vitamin D3 treatment groups the mean 25(OH)D3 serum levels rose by 59% (WE) and 116% (SA) over the 12-week intervention. Conversely, mean serum 25(OH)D3 concentrations fell across the 12 weeks of the study in the placebo groups who did not receive additional vitamin D; 25(OH)D3 baseline vitamin D concentration in the SA ethnic group tended to be lower than for the WE group and dropped, respectively, by 23% and 29% relative to baseline V1 ( Figure 1C and Supplementary Data File 1 ). It is notable that, in the vitamin D2 treatment group, serum 25(OH)D3 concentration decreased to a greater extent over the 12-week period, by 52% (SA) and 53% (WE). Serum 25(OH)D2 was low in the absence of specific supplementation, typically less than 5 nmol/L (152/162 samples analysed). Serum calcium concentration, appropriately adjusted for serum albumin concentration, was maintained within a normal clinical range across all sample groups, while the concentration of parathyroid hormone (PTH) was stable between V1 and V3 only within the SA placebo group, the WE vitamin D2 and WE vitamin D3 intervention groups. PTH concentrations increased at V3 compared with V1 in the WE placebo group, but decreased in both the vitamin D2 and D3 intervention groups in the SA ethnic cohort.
Effects of Supplementation With Either Vitamin D2 or D3 on Global Gene Expression
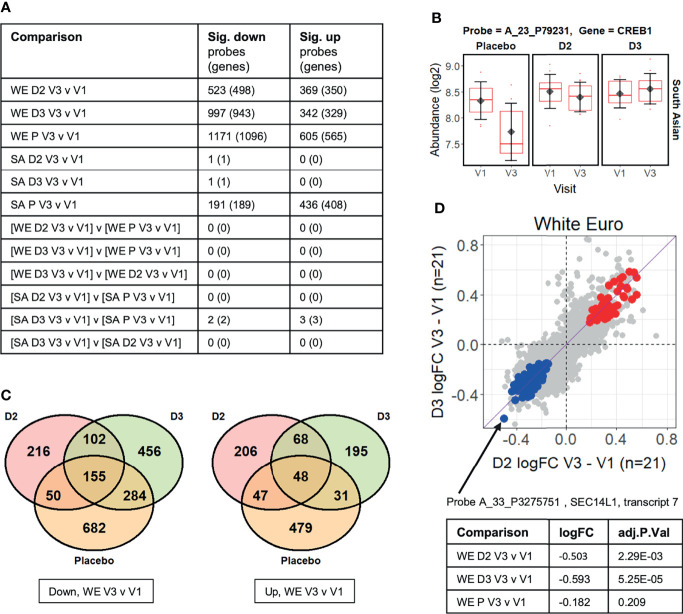
Filtering of the normalised microarray data to select for probes showing signals at least 10% above background in at least 41 (~20%) of the arrays yielded transcript abundance data from 20,662 probes corresponding to 17,588 different genomic features (12,436 of which were annotated with an ENTREZ gene identifier). Using the filtered data, we identified significant differences in the transcriptional responses occurring across the 12-week V1 to V3 period of the study between the vitamin D2, vitamin D3 and placebo treatment groups for each ethnic cohort. In a separate analysis, we determined significant changes within each group between the V3 and V1 sampling points ( Figure 2 and Supplementary Data File 2 ). The former was tested in a ‘difference-in-difference’ analysis according to the generalised null hypothesis [treatment1.V3 – treatment1.V1] - [treatment2.V3 – treatment2.V1] = 0 ( Figure 2A , rows 7-12), and the latter using treatment.V3 – treatment.V1 = 0 ( Figure 2A , rows 1-6).
Figure 2.
(A) Summary of limma differential expression test results identifying significant changes (adj.P.Val ≤ 0.05) in transcript probe abundance within and between the experimental groups (see Supplementary Data File 2 for full details). Both the number of unique microarray probes, and the number of unique genome loci they represent, are indicated. WE = “white European”; SA = “South Asian”; D2 = vitamin D2 treatment group; D3 = vitamin D3 treatment group; P = placebo group. (B) The CREB1 gene probe signal is significantly different (adj.P.Val=0.021) between the vitamin D3-treated group and the placebo group over the course of the V1 to V3 study period in the South Asian cohort (i.e. [SA D3 V3 v V1] v [SA P V3 v V1] comparison from Figure 2A ). (C) Venn diagrams showing the numbers of probes in the white European (WE) cohort that are specifically significantly (adj.P.Val ≤ 0.05) down- or up-regulated in V3 compared to V1 in each treatment group. (D) Comparison of the log2 fold-changes (FC) in abundance occurring from V1 to V3 in the WE cohort in the D2 and D3 treatment groups. Probes significantly up-regulated in both D2 and D3 groups but not the placebo are coloured red, while those similarly down-regulated are shown in blue (see Supplementary Data File 3 ). A probe detecting SEC14L1 shows the largest decreases in abundance in those down-regulated by D2 and D3 but not placebo.
No significant changes in probe signals (adj.P.Val<=0.05) were identified in the ‘difference-in-difference’ analysis for the data for the WE ethnic group, but five were found in the corresponding analysis of the SA cohort ( Figure 2A ). Furthermore, difference-in-difference analysis of the entire study data, ignoring ethnicity, did not yield statistically significant changes between the D2 or D3 treatment groups and the placebo control (data not shown). We note that another ‘difference-in-difference’ study on the influence of vitamin D on gene expression (the BEST-D trial) failed to identify any significant changes in gene expression following long-term vitamin D treatment in a Caucasian cohort (32). The observed log2 fold-change (log2FC) in signal abundances between V3 and V1 in the study typically fell well within +/- 1, and, in such a context, it is likely that the sample sizes used within the study restrict our ability to reliably identify individual gene expression responses using this highly conservative approach.
We consider that the strong interpersonal differences in gene expression across individuals in the study population are likely to hinder detection of small statistically significant gene expression changes across treatment groups from microarray-derived data. It is known that expression of any particular gene and its magnitude of change over time can vary widely across individuals in a human population, and the differences in response across time within an individual is considered more physiologically meaningful. This issue is considered further in the Discussion. In this study we observe that many statistically significant differences are detected when evaluating gene expression changes within an individual across time (as described below where we examine paired observations with two time points per subject, V1 and V3).
The five significant changes that were identified as occurring in response to treatment of the SA ethnic group with vitamin D3, relative to the placebo, are summarised in Supplementary Figure 1 . These are driven primarily by the marked changes in the placebo group between the V1 and V3 sampling points, and may be associated with the sustained low serum 25(OH)D3, or high PTH, concentrations observed in this group of subjects. One of these differentially expressed genes encodes the cAMP response element binding protein CREB1 ( Figure 2B ) which is part of the cAMP-PKA-CREB signaling pathway in bone cells which contributes to the regulation of skeletal metabolism in response to PTH concentrations (44).
In contrast to the difference-in-difference analysis, the direct within-group comparisons of transcript abundance measurements at V3 versus V1 per subject identified large numbers of significant changes in gene expression, most notably in the WE D2, WE D3 and WE placebo groups ( Figures 2A, C and Supplementary Data File 2 ). These statistically significant gene expression changes in the WE ethnic cohort arising in the vitamin D treatment groups are considered in more detail below. While there is some overlap in the groups of differentially expressed genes, only 13% of down-regulated differentially expressed genes (102 of 774) were common between the two vitamin D treatment groups. Conversely, 28% (216 of 774) and 59% (456 of 774) were uniquely down-regulated by D2 and D3, respectively (excluding those additionally down-regulated in the placebo group over the equivalent 12-week intervention)( Figure 2C ). As noted above, serum 25(OH)D3 concentration fell over the 12-week intervention period in the vitamin D2-treated group; thus, changes in gene expression observed in this group could a priori either be due to the influence of vitamin D2 itself, or could be attributable to the depletion of the endogenous 25(OH)D3 reserves. It was therefore important to have included comparative transcriptomic analysis of the placebo group in this study to distinguish gene expression changes resulting from vitamin D2 per se from those changes arising due to the depletion of 25(OH)D3 in the vitamin D2 treatment group.
It is notable that expression of a large number of genes was altered between the first and last visits (V1 to V3) in the non-treated placebo group. While some of these changes will be attributable to the reduced synthesis of vitamin D that is seen in northern latitudes over the winter months, there is also significant over-representation of genes known to exhibit seasonal differences in gene expression (45) ( Supplementary Figure 2 ). There is however no unique association of seasonally expressed genes with the data for the WE placebo group; a similar significant enrichment in ‘seasonal genes’ is also observed for the significant changes in gene expression identified in the WE D2 and WE D3 treatment groups ( Supplementary Figure 2 ) and therefore it is unlikely that this apparent seasonal effect exclusively reflects vitamin D-specific effects. There was no overlap between genes significantly down-regulated exclusively in the placebo group and those significantly up-regulated exclusively in the vitamin D treatment groups (or vice versa). Consistent with the 12-week period of the study, seasonal changes in gene expression are therefore a background feature of all the data collected, and is therefore a factor that needs to be considered when undertaking such vitamin D supplementation studies.
Probe A_33_P3275751, detecting SEC14L1 gene transcription, showed the largest decrease in response to both forms of vitamin D, but not placebo participants, in the group of probe signals with significantly reduced abundance in the V3 samples compared with V1 ( Figure 2D ). This probe was originally designed to detect ‘transcript variant 7’ and hybridizes to a location at the 3’ end of the SEC14L1 locus, detecting several splice variants. High expression of SEC14L1 is significantly associated with lymphovascular invasion in breast cancer patients, where transcript abundance correlates positively with higher grade lymph node metastasis, and poor prognostic outcome (46). Its overexpression is also frequent in prostate cancer where SEC14L1 has been identified as a potential biomarker of aggressive progression of the disease (47, 48).
Genes Significantly Down-Regulated by Both Vitamins D2 and D3, But Not Placebo, Are Enriched for Functions Associated With Immune Responses
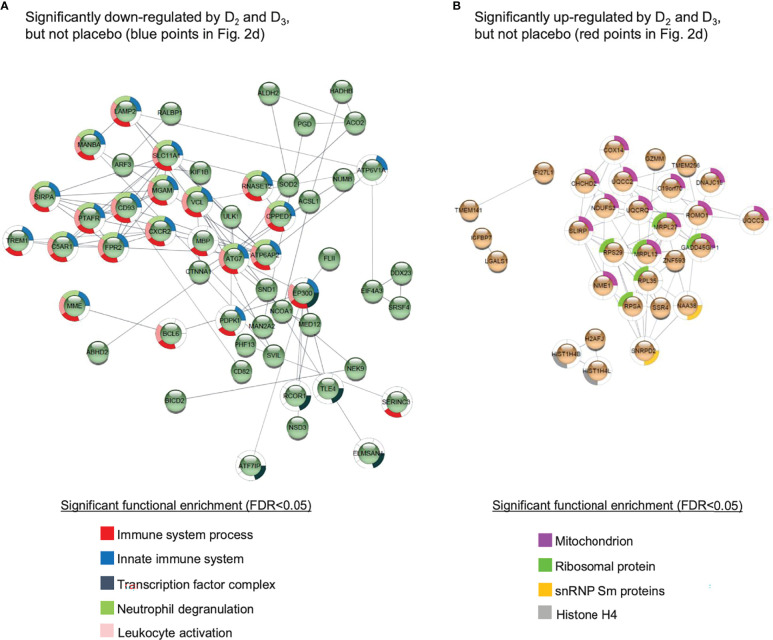
Given the relatively small sample sizes used in this study, it is considered more appropriate to examine enrichment of cellular pathways among differentially expressed genes rather than focusing on changes in individual genes. Functional enrichment analysis of the genes represented by the probes specifically repressed by vitamins D2 and D3 (blue data points in Figure 2D ) suggests that both supplements have suppressive effects on the immune response in the WE group ( Figure 3 and Supplementary Data File 3 ). Indeed, a significant (p = 1.98 × 10−6) protein-protein interaction network derived from the Homo sapiens medium confidence interactions curated in the STRING database (49) is centred on a highly connected group of proteins associated with the innate immune response, neutrophil degranulation and leukocyte activation ( Figure 3A ; all protein groups are detailed in Supplementary Data File 3 ). This includes the histone acetyltransferase, EP300, known to function as a transcriptional coactivator with VDR, the vitamin D receptor protein (50). Collectively, these findings are consistent with the emerging view that vitamin D exposure leads to a shift from a pro-inflammatory to a more tolerogenic immune status (1, 2).
Figure 3.
Protein-protein interaction networks for gene products corresponding to the probes (A) significantly down-regulated or (B) significantly up-regulated in both the D2 and D3 treatment groups of the WE cohort, but not the placebo group. Details given in Supplementary Data File 3 . The networks were generated using the STRING database of Homo sapiens medium confidence (0.4) interactions, and only connected nodes are shown. Networks for both (A, B) are significantly enriched for interactions compared to randomised sets, yielding p-values of 1.98 × 10-6 and 4.99 ×10-9, respectively.
Genes Significantly Up-Regulated by Both Vitamins D2 and D3, But Not Placebo, Include Components of Histone H4 and the Spliceosome
A similar analysis of the probes specifically induced by D2 and D3 (red points in Figure 2D ) produced a significant (p = 4.99 × 10−9) protein-protein interaction network enriched primarily for mitochondrial and ribosomal proteins, but also involving two subunits of histone H4 and SNRPD2, a core component of the SMN-Sm complex that mediates spliceosomal snRNP assembly ( Figure 3B ).
Comparative Functional Enrichment Analysis Supports Roles for Vitamins D2 and D3 in the Suppression of Immunity, and in Chromatin Modification and Spliceosome Function
As a complementary approach to the functional enrichment analysis of specific subsets of genes whose expression is altered by supplementation with vitamin D2 and D3 but not by placebo treatment, a comparative functional enrichment analysis of all significant changes in each treatment group was performed (see Methods). This aimed to identify functional categories that are more extensively affected by vitamin D2, or by vitamin D3, or by both vitamins D2 and D3, than by the placebo, and took into consideration the changes occurring in the placebo treatment group across the 12 weeks of the study ( Figure 4 and Supplementary Data File 4 ). Consistent with the earlier analysis, functional categories associated with immunity and immune response pathways are prominent among those genes repressed by vitamin D supplementation ( Figure 4A ), while mitochondrial, ribosomal and spliceosomal functions are prominent in the induced genes ( Figure 4B ). Although the two vitamin treatments share many common categories identified from this analysis, the results also highlight some differences between the respective effects of D2 or D3 supplementation. For example, ‘histone exchange’ is significant only in the vitamin D2 up-regulated genes, and ‘chromatin modifying enzymes’ are significant only in the D3 down-regulated genes.
Overall, the observed differences in gene expression from the blood transcriptome presented in this study suggest that the physiological effects of vitamin D3 and D2 may be dissimilar.
Weighted Gene Correlation Network Analysis (WGCNA) Identifies Modules of Co-Expressed Genes That Significantly Correlate With Serum Markers of Vitamin D Supplementation in the WE and SA Ethnic Groups
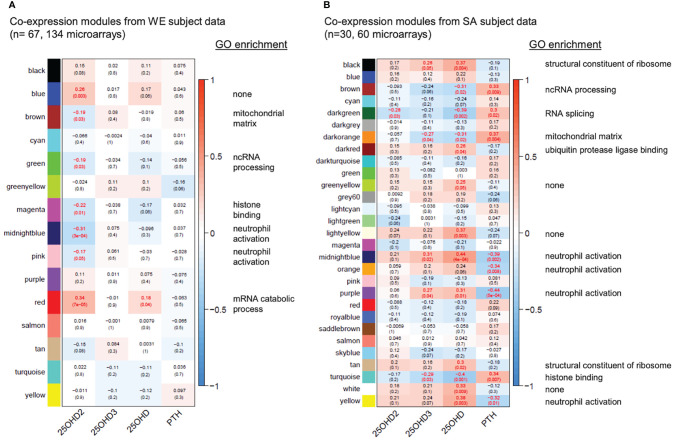
WGCNA quantifies both the correlations between individual pairs of genes or probes across a data set, and also the extent to which these probes share the same neighbours (41). The WGCNA process creates a dendrogram that clusters similarly abundant probes into discrete branches, and subsequent cutting of the dendrogram yields separate co-expression modules, representing putatively co-regulated sets of genes. The first principal component of the expression matrix of each module defines the expression profile of the eigengene for the module, and this can then be correlated with experimental metadata. By allowing phenotypic traits to be associated with relatively small numbers (tens) of module eigengenes, instead of thousands of individual variables (i.e. gene probes), WGCNA both alleviates the multiple testing problem associated with standard differential expression analysis and also directly relates experimental traits to gene expression data in an unsupervised way that is agnostic of the experimental design.
WGCNA was used to construct separate signed co-expression networks for the WE and SA ethnic groups as described in the Methods, and Pearson correlations between expression of the module eigengenes and serum 25(OH)D2, 25(OH)D3, total 25(OH)D and parathyroid hormone (PTH) concentrations were calculated ( Figure 5 and Supplementary data files 6 – 8 ). These results therefore ignore whether the data originates from the vitamins D2, D3 or placebo treatment groups and focus solely on the relationship between the serum metabolite concentrations and gene expression. A significant negative correlation was observed between 25(OH)D2 concentration and expression modules in the WE co-expression network that are enriched for immune-associated functions (midnight blue and pink modules in Figure 5A ). This is consistent with the results from the different analytical approaches (presented in Figures 3 , 4 ). Similarly, the only module exhibiting a significant positive correlation with 25(OH)D2 (and total 25(OH)D concentration), the red expression module, is enriched for GO categories associated with the ribosome and mRNA processing ( Supplementary Data File 6 ). No significant correlations with serum 25(OH)D3 concentrations were detected in the WE cohort.
Figure 5.
Gene co-expression networks for the WE (A) and SA (B) ethnic groups. For each group of subjects, signed scale-free networks were constructed from the expression data using WGCNA to cluster probes with similar expression characteristics across all samples into discrete modules (colours on the y-axis: colours in (A, B) are independent). Module membership for the modules of genes identified in the WGCNA analysis of the data from the South Asian and white European cohorts are detailed in Supplementary Data File 6 . The colour scale to the right of each panel represents the Pearson correlation (from -1 to +1) between the expression profile for each module’s eigengene and the respective serum 25(OH)D2, 25(OH)D3, total 25(OH)D and PTH concentrations (x-axis). The Pearson correlation coefficients are also provided in each box, followed in brackets by an adjusted p-value, testing for the significance of each correlation. Statistically significant correlations (adjusted p-value ≤ 0.05) are indicated in red. A headline significant GO functional enrichment category (p.adjust ≤ 0.01) for each significant module is shown to the right; GO test results for each module are summarised in Supplementary Data Files 7 , 8 ).
Interestingly, stronger and more numerous correlations were observed in the co-expression network for the SA cohort ( Figure 5B ). In agreement with the WE network, an expression module significantly associated with the ribosome (black module in Figure 5B ) showed a general positive correlation with total 25(OH)D concentration whereas a module enriched for histone binding (turquoise) had a significant negative correlation with 25(OH)D concentration. Strikingly however, modules enriched for immune response functions (midnight blue, orange, purple, yellow in Figure 5B ) were consistently positively correlated with total 25(OH)D and usually 25(OH)D3, but not 25(OH)D2 concentrations, in the SA network, and negatively correlated with PTH concentrations. This raises the possibility that vitamin D supplementation may exert different effects on the immune system depending on ethnicity of the individual, may indicate that PTH status has an influence on the outcome and may reflect physiological differences resulting from the low baseline vitamin D status in SA women. In this context we observed that PTH concentration at the V1 sampling point in the SA cohort was elevated compared with that in the WE cohort ( Figure 1C ).
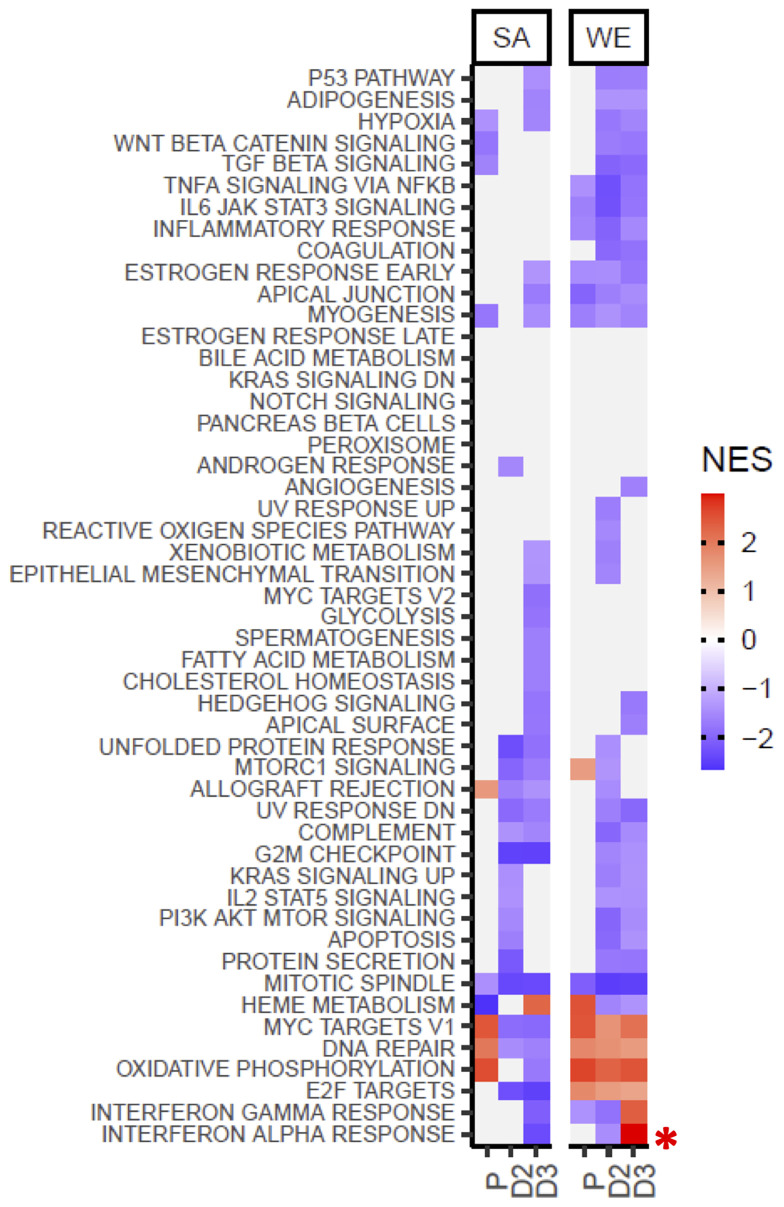
Gene Sets That Respond to Interferon Alpha and Gamma Show Divergent Behaviour in the Vitamin D2 and D3 Treatment Groups of the WE Cohort
The molecular signatures database (MSigDB) hallmark gene sets represent coherent gene expression signatures related to well-defined biological states or processes (https://www.gsea-msigdb.org/gsea/msigdb/). Gene set enrichment analysis (GSEA) was performed to identify statistically significant (padjust<=0.05), concordant changes in expression of these hallmark sets between the V3 and V1 sampling times for the placebo, vitamin D2, and vitamin D3 treatment groups ( Figure 6 and Supplementary Data File 9 ). Interestingly, in the WE cohort, the gene sets defining the signature responses to interferon alpha and gamma exhibited divergent behaviour in the vitamin D2 and D3 treatment groups. This is evident as significant up-regulation over the course of the intervention study in the D3 group but significant down-regulation in the D2 group (see also Supplementary Figures 4 , 5 illustrating the core genes accounting for the enrichment signals). This differed from the SA cohort where significant down-regulation was observed in the D3 group (see also Supplementary Figure 6 ). It is acknowledged that some of the observed differences between the WE and SA groups could reflect the differences in the respective sample sizes. However, in cases where statistically significant differences are found, such as the reciprocal trend in the respective interferon responses these are not likely to be attributable to sample size differences.
Figure 6.
Gene Set Enrichment Analysis using the MSigDB hallmark gene sets indicates divergent behaviour for the interferon alpha and gamma response gene sets following supplementation with vitamin D2 and D3 in the WE cohort. Coloured tiles in the heatmap correspond to gene sets exhibiting a statistically significant (padjust≤ 0.05), concordant change between the V3 and V1 sampling times for the placebo (P), vitamin D2 (D2) or vitamin D3 (D3) treatment groups in the SA or WE cohorts. Grey tiles indicate non-significance. A positive normalised enrichment score (NES) indicates up-regulation of a gene set in V3 relative to V1, and conversely down-regulation is indicated by a negative NES score. Full results are provided in Supplementary Data File 9 , and the behavior of the leading edge, core genes accounting for the significant enrichment signals in the interferon alpha and gamma response sets are illustrated in Supplementary Figures 4 – 6 .
Discussion
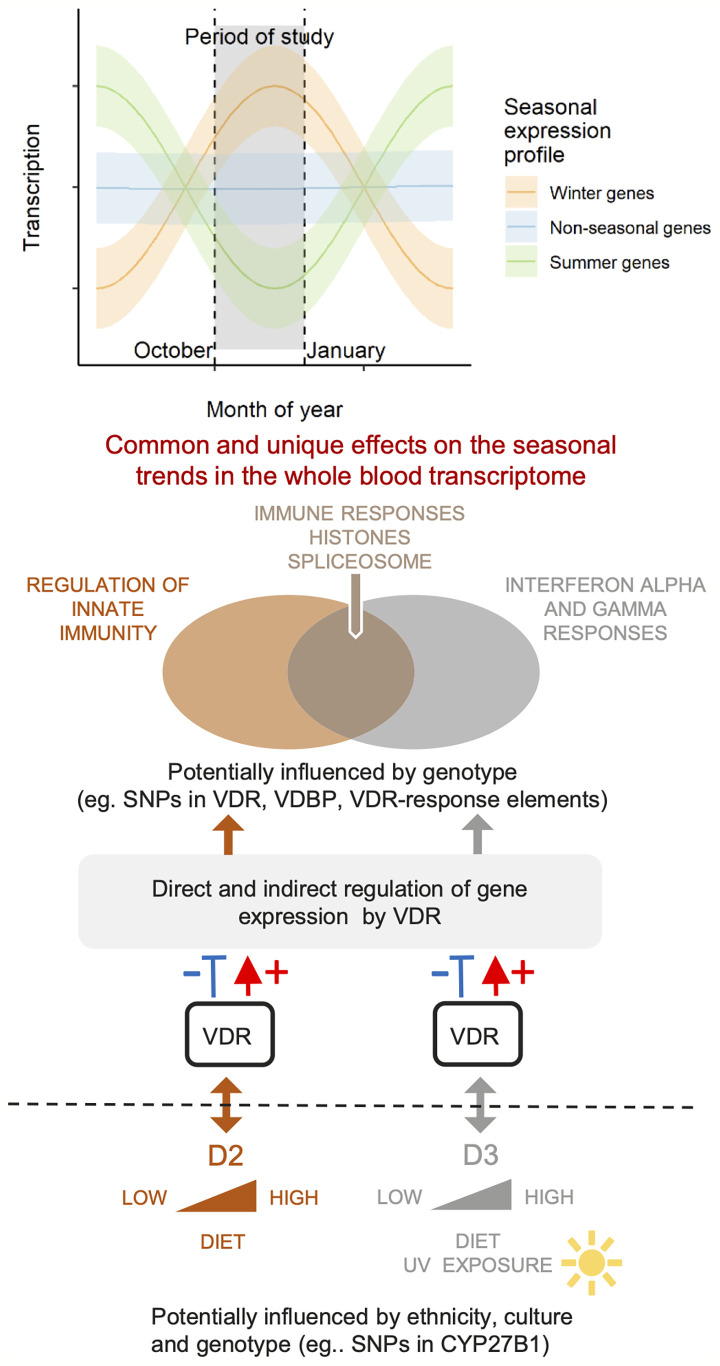
In the present study, we conducted a longitudinal blood transcriptome analysis in 97 of the original cohort of 335 women from two ethnic backgrounds, South Asian (SA) and white European (WE), reported by Tripkovic at al (28). Extensive changes in gene expression in all three treatment groups were observed, some of which were unique to the vitamin D2-treated or D3-treated groups, with the majority exhibiting downregulation of transcription over the 12-week intervention period. Gene expression changes in the placebo group are likely to be attributable, at least in part, to seasonal drops in vitamin D status ( Figure 2 ) (45, 51). The effects of vitamin D supplementation on gene expression take place superimposed on the natural seasonal changes that would have taken place in the absence of intervention. Figure 7 presents a schematic diagram to help visualize the possible interplay between genetic factors, seasonal changes, vitamin D status and whole blood transcriptome expression.
Figure 7.
A schematic diagram of this study in the context of genetic and seasonal factors that can influence physiological vitamin D status and the whole blood transcriptome. Dietary supplementation with vitamin D2 or D3 boosts the native levels of the active forms of these vitamins in the blood, and generates overlapping but different effects on the seasonal trends in gene expression. Non-supplemented placebo subjects remain on their natural trajectories for levels of active D2 and D3, and for their seasonal expression profile. Selected differentially expressed pathways from this study are indicated on the Venn diagram.
Statistically significant gene expression changes were mainly detected as differences within each treatment group (i.e. differences at baseline (V1) versus 12 weeks after the intervention (V3) within each individual participant). In this respect, our longitudinal study design partly circumvents the problems that are often encountered with heterogeneity in inter-individual responses in omic studies of human populations, which make it difficult to detect robust changes between different groups of individuals. Recent longitudinal multi-omics studies are revealing strong interpersonal differences among individuals that can hinder statistical comparisons across different treatment populations or across disease cases and controls [e.g (52, 53).; M.P. Snyder, personal communication]. Our strategy to examine the changes in gene expression within each individual, from baseline to the treatment endpoint has enabled us to detect differences in the trajectory and magnitude of gene expression and, in combination with pathway analysis, has allowed us to extract physiologically meaningful information from the transcriptome data; this could not have been achieved by examining differences across the different treatment groups. This in vivo human transcriptome study illustrates long-term effects on gene expression. The majority of differentially expressed genes identified in this study were down-regulated by vitamin D supplementation, with many of these encoded pathways involved in immunity. Observed gene expression changes are consistent with vitamin D exerting a modulating effect on the immune system, leading towards a more tolerogenic state, a concept reviewed by (1).
Vitamins D2 and D3 Do Not Influence Expression of the Same Genes in Whole Blood
Excluding genes that were also differentially expressed over the 12-week intervention in the placebo group, only 13% of down-regulated differentially expressed genes were common between the two treatment groups while 28% and 59% were uniquely down-regulated by vitamins D2 and D3, respectively ( Figure 2C ). For example, some biological processes such as histone modification and covalent chromatin modification are downregulated following vitamin D3 supplementation only, while spliceosomal function are upregulated by vitamin D2 only. Functional categories of genes enriched among the upregulated genes, following supplementation with either vitamin D2 or D3, include translation, mitochondrial and spliceosome function ( Figure 3B and Supplementary Data File 4 ); statistically enriched biological cellular component terms in these functional categories are ribosomal proteins, components of the mitochondrial respiratory chain, two subunits of the histone H4 and snRNP Sm protein components of the spliceosome assembly. It is known that, in addition to influencing transcription, vitamin D can also influence post-transcriptional events by recruiting co-regulators (54). In this context it is relevant that components of the spliceosome, such as snRNP Sm proteins that mediate both transcriptional control and splicing decisions, leading to alternatively spliced transcripts (55), were upregulated by vitamin D supplementation in this study.
In light of the finding that vitamins D2 and D3 influence expression of different genes in the human blood transcriptome, recently we undertook a parallel in vitro study with a model rat cell line (56). We examined the respective influences of the two physiologically active forms of vitamin D, 1,25(OH)D3 and 1,25(OH)D2 on differentiation and global gene expression in differentiating rat CG4 oligodendrocyte precursor cells and revealed considerable differences in the influence of the two types of vitamin D on gene expression at 24 h and after 72 h following onset of differentiation. We demonstrated that 1,25(OH)D3 and 1,25(OH)D2 respectively influenced expression of 1,272 and 574 genes at 24 h following addition of the vitamin to the culture, where many of the changes in expression were specific to one or the other form of the vitamin (56). This study provides evidence of the different direct effects of the two active vitamin D metabolites on gene expression in vitro in cultured cells and provides some evidence that the changes we observed in the in vivo study may reflect the influence of the physiologically active forms of the vitamin.
Influence of Vitamin D on Expression of Genes Encoding Immune Pathways
In common to both the D2 and D3 treatment groups, but not the placebo group, we found that many different pathways of the immune system are differentially expressed (largely down-regulated) by vitamin D, consistent with the notion that one physiological role of vitamin D is to restrain, or balance, the activity of the immune system (1, 2) ( Figures 3A , 4 and Supplementary Data Files 3 – 5 ). The immunomodulatory effects of vitamin D on both innate and adaptive immunity are well documented (57–59). In this regard, our observation that vitamin D2 and D3 supplementation in the WE cohort was associated with divergent patterns of expression for interferon alpha (type I) and interferon gamma (type II) signature gene sets stands out as particularly interesting ( Figure 6 ). Vitamin D appears to modulate type I interferon activity. For example, it enhances the effects of type I interferon treatment on mononuclear cells from patients with multiple sclerosis (60), which parallels evidence for modest benefits of vitamin D as an adjunct treatment with type II interferon in multiple sclerosis patients (61, 62). Vitamin D may also help suppress symptoms in autoimmune diseases such as systemic lupus erythematosus (63), which are associated with chronic over-activity of interferon signalling and tentatively designated interferonopathies. Moreover, type I interferons play a critical role in defence against viral infections. Basal expression of type I interferon-stimulated genes, in the absence of infection, is key to priming a rapid and effective response to viral infection (64). There is currently intense interest in vitamin D as both a potential prophylactic and a therapeutic agent for treatment of SARS-CoV-2 infection. One of the proposed modes of action of vitamin D is modulation of interferon activity (65); in this context our observation that vitamin D3 (but not vitamin D2) enhances the expression of genes involved in the interferon alpha response, is highly relevant to susceptibility to viral infection. Indeed, a recent genome-wide association study (GWAS) in 2,244 critically ill Covid-19 patients identified genetic variants leading to reduced interferon type I signalling that are associated with severe Covid-19 disease (66).
Gene Expression Changes in Response to Vitamin D Are Partially Attributable to Ethnicity
We have also found differences in gene expression response to vitamin D according to ethnicity (with the caveat that the sample size of the SA group was smaller than the WE group and the average baseline vitamin D status was significantly lower relative to the WE group). Unlike the white European group, differences in gene expression across treatment groups were found in the South Asian group ( Figures 2A, B ) who received supplementation with vitamin D3, where three genes were significantly upregulated and two down-regulated after the 12-week intervention relative to the placebo group ( Figure 2 and Supplementary Figure 1 ). cAMP-responsive element binding protein 1 (CREB1), one of the three upregulated genes, encodes a transcription factor that binds as a homodimer to the cAMP-responsive element. CREB1 protein is phosphorylated by several protein kinases and induces transcription of genes in response to hormonal stimulation of the cAMP pathway. The protein kinase A (PKA) pathways are activated by the parathyroid hormone PTH in response to low serum calcium levels to maintain serum calcium homeostasis, primarily by promoting vitamin D 1α-hydroxylation in the kidney. Both PTH and 1,25 (OH)2 vitamin D have similar effects in promoting the maturation of osteocytes and opposing the differentiation of osteoblasts into osteocytes (67, 68). It is relevant to note that those in the SA cohort had elevated serum PTH at baseline relative to the WE cohort.
Ethnic differences in response are also suggested from the Weighted Gene Correlation Network Analysis (WGCNA) and the Gene Set Enrichment Analysis (GSEA), where the response to vitamin D3 intake appeared to have the opposite effect on the type I and II interferon pathways in the SA group compared with the WE group ( Figure 6 and Supplementary Figures 4 , 6 ). However, direct correlation between the stimulation of immune responses, other than the interferon pathways, with an increase in serum 25(OH)D3 concentration was evident in the SA group only. This is the opposite of that observed in the WE group where the effect of vitamin D supplementation was to suppress several immune pathways.
The transcriptome results differ considerably between the two different ethnic groups. While some of the differences may be attributable to differences in sample sizes studied between the two groups it is also possible that some of the differences represent genuine differences in the respective physiological responses of the two ethnic groups. Alternatively, the differences may reflect the starting physiological status of the SA participant group, who had considerably lower baseline vitamin D concentrations (and higher PTH concentrations) than the WE group. The importance of clarifying these different responses is given particular impetus by the emerging evidence for the interplay between ethnicity, skin tone, vitamin D status and susceptibility to viral infection, Covid-19 being of particular current relevance here (12, 13, 69, 70). The transcriptomic data presented in this study might provide a useful context for further studies aimed at understanding the role of vitamin D in influencing the immune response to SARS-CoV-2 infection, particularly in relation to severe Covid-19. It is notable that the recent GenOMICC study of severe Covid-19 disease highlighted a link between severe Covid-19 and reduced type I interferon signalling (66). Our finding that vitamin D3 appears to stimulate type I interferon signalling could be relevant in the context of its use as a prophylactic treatment. It should be noted here that in order to have a beneficial effect, a vitamin D replete status would be required prior to exposure to the virus, contributing innate immunity; administration of vitamin D following admission to hospital, even at high bolus doses, would not provide any clinical benefit because the virus would have already established itself. Recent clinical trials have borne out this latter point.
Concluding Remarks
Our ability to detect differences in the effects of vitamin D2 and vitamin D3 may have been negatively impacted by the relatively low statistical power and by the inclusion of two different ethnic groups among the 97 participants, since the transcriptome results from the two ethnic groups are clearly different; the number of participants represents a weakness of the present study. It will be important to replicate this study using a larger cohort in order to verify, or otherwise, the key findings from this study. From power calculations it is considered that for a whole human microarray-based gene expression study of this nature, and with gene expression changes of the magnitude we observe, at least 400 participants of each ethnic group should be recruited for each treatment, giving a total cohort size of 2,400. In this context, the biological interpretation of our findings should be considered as preliminary, requiring independent verification. A second limitation of this study was our failure to take account of the contribution of potential changes in blood cell composition across the seasons and across ethnicity; it is known that blood cell composition can vary significantly throughout the year (51, 71).
It is difficult to compare the findings of the present study with other reported in vivo studies because of the considerable differences in experimental design, including the use of supra-physiological vitamin D doses (up to 2,000 μg single bolus doses), different human population types and small sample sizes, which make statistical analysis not feasible [e.g. (33, 34)]. Furthermore, our study is unique in that it compared gene expression in participants given either of the two commonly used forms of vitamin D, vitamin D2 and vitamin D3. The present study evaluated overall expression changes in a complex blood cell population, which is also known to change in cellular composition across seasons (51). Furthermore, this study has focussed on identification of statistically significant pathway, or gene set, enrichment following sustained vitamin D supplementation, rather than a gene-focussed analysis; the latter approach is limited given the effect sizes and sample size of this study.
This study suggests that a more detailed consideration of the system-wide effects of these two forms vitamin D is warranted. This is perhaps of particular importance to at-risk ethnic groups, including black and South Asian populations who reside in northern latitudes. The studies would need to be time course-based (temporal) to track gene expression changes within individuals from baseline to defined sampling times and would need in-built control to account for seasonal gene expression changes. They would need to be designed specifically to answer whether different ethnicity or different vitamin D baseline concentrations give rise to different responses to vitamin D supplementation.
Since some pathways appear to be regulated specifically by vitamin D3, or in some cases, in opposing directions by vitamin D3 and D2, future studies should investigate whether vitamin D2 supplementation might counteract some of the benefits of vitamin D3 on human health. This possibility is prompted by the findings from this cohort that the circulating concentration of 25(OH)D3 within vitamin D2-treated participants was significantly lower after the 12-week intervention than in the placebo group, who received no vitamin D supplements – suggesting that the former might be depleted by the latter. The results from this study suggest that guidelines on food fortification and supplementation with specific forms of vitamin D may need revisiting.
Data Availability Statement
All microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-8600.
Ethics Statement
This study received ethical approval from the South-East Coast (Surrey) National Health Service Research Ethics Committee (11/LO/0708) and the University of Surrey Ethics Committee (EC/2011/97/FHMS). All participants gave written informed consent in agreement with the Helsinki Declaration before commencing study activities. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CS and SL-N conceived of the study. CS, GB, and SL-N supervised the project. LD, GB, and LT carried out the experiments, AH, GB, CS, LD, CM-L, HW, and RE analyzed and interpreted the transcriptome data. CS, AH, and GB wrote the first draft of the manuscript. AH generated the figures and all authors contributed to the final manuscript.
Funding
This work was funded by BBSRC (UK) DRINC award BB/I006192/1 to SL-N and CS, and by a University of Brighton Innovation Seed Fund Award (to CS).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to particularly thank Michael Chowen CBE DL and Maureen Chowen for their generous philanthropic donation (to CS).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.790444/full#supplementary-material
Metadata on serum concentrations of 25(OH)D2, 25(OH)D3, total 25(OH)D, PTH and calcium (albumin-adjusted) for the 97 subjects subjected to transcriptomic analysis [selected from the cohort described by Tripkovic et al. (28)].
[Note: data file is 9.1 MB.] Significant differences in the transcriptional responses occurring across the 12-week V1 to V3 period of the study between the vitamin D2, vitamin D3 and placebo treatment groups for each ethnic cohort, and significant changes within each group between the V3 and V1 sampling points (see Figure 2 ).
Functional analysis of the genes represented by the probes specifically repressed by D2 and D3 in the white European cohort (blue data points in ) or induced by D2 and D3 in the white European cohort (red data points in Figure 2D ).
Comparative functional enrichment analysis of all significant changes in each treatment group in the white European cohort ( Figure 2C ; and see Figure 4 ).
Networks illustrating all the functional categories significantly enriched (p.adjust < 0.01) in analysis of the gene products represented by the probes significantly up- or down-regulated (adj.P.Val < 0.05) in the white European cohort treatment groups (from data presented in Supplementary Data File 4 ).
Module membership for the modules of genes identified in the WGCNA analysis of the data from the South Asian and white European cohorts, as presented in Figure 5 .
Gene ontology functional enrichment analysis results for the modules of genes identified in the WGCNA analysis of the data from the white European cohort.
Gene ontology functional enrichment analysis results for the modules of genes identified in the WGCNA analysis of the data from the South Asian cohort.
Gene Set Enrichment Analysis results for the analysis reported in Figure 6 .
References
- 1. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and Immune Function. Nutrients (2013) 5(7):2502–21. doi: 10.3390/nu5072502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s Effect on Immune Function. Nutrients (2020) 12(5):1248. doi: 10.3390/nu12051248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pilz S, Zittermann A, Trummer C, Theiler-Schwetz V, Lerchbaum E, Keppel MH, et al. Vitamin D Testing and Treatment: A Narrative Review of Current Evidence. Endocr Connect (2019) 8(2):R27–43. doi: 10.1530/EC-18-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwalfenberg GK. A Review of the Critical Role of Vitamin D in the Functioning of the Immune System and the Clinical Implications of Vitamin D Deficiency. Mol Nutr Food Res (2011) 55(1):96–108. doi: 10.1002/mnfr.201000174 [DOI] [PubMed] [Google Scholar]
- 5. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev (2016) 96(1):365–408. doi: 10.1152/physrev.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The Nonskeletal Effects of Vitamin D: An Endocrine Society Scientific Statement. Endocr Rev (2012) 33(3):456–92. doi: 10.1210/er.2012-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holick MF. Vitamin D Deficiency. N Engl J Med (2007) 357(3):266–81. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 8. Bouillon R. Extra-Skeletal Effects of Vitamin D. Front Horm Res (2018) 50:72–88. doi: 10.1159/000486072 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Fanf F, Tang J, Jia L, Feng Y, Xu P, et al. Association Between Vitamin D Supplementation and Mortality: Systematic Review and Meta-Analysis. Br Med J (2019) 366:l4673. doi: 10.1136/bmj.l4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damoiseaux J, Smolders J. The Engagement Between Vitamin D and the Immune System: Is Consolidation by a Marriage to Be Expected? EBioMedicine (2018) 31:9–10. doi: 10.1016/j.ebiom.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. SACN . SACN (Scientific Advisory Committee on Nutrition) Vitamin D and Health Report. (2016) London: TSO. [Google Scholar]
- 12. Kohlmeier M. Avoidance of Vitamin D Deficiency to Slow the COVID-19 Pandemic. BMJ Nutr Prev Health (2020) 3(1):67–73. doi: 10.1136/bmjnph-2020-000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lanham-New SA, Webb AR, Cashman KD, Cashman D, et al. Al E. Vitamin D and SARS-CoV-2 Virus/COVID-19 Disease. BMJ Nutr Prev Health (2020) 3(1):106–10. doi: 10.1136/bmjnph-2020-000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buttriss JL, Lanham-New SA, Steenson S, Levy L, Swan GE, Darling AL, et al. Implementation Strategies for Improving Vitamin D Status and Increasing Vitamin D Intake in the UK: Current Controversies and Future Perspectives: Proceedings of the 2nd Rank Prize Funds Forum on Vitamin D. Br J Nutr (2021) 1–21. doi: 10.1017/S0007114521002555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long MD, Sucheston-Campbell LE, Campbell MJ. Vitamin D Receptor and RXR in the Post-Genomic Era. J Cell Physiol (2015) 230(4):758–66. doi: 10.1002/jcp.24847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeLuca HF. Overview of General Physiologic Features and Functions of Vitamin D. Am J Clin Nutr (2004) 80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S [DOI] [PubMed] [Google Scholar]
- 17. Tsugawa N, Nakagawa K, Kawamoto Y, Tachibana Y, Hayashi T, Ozono K, et al. Biological Activity Profiles of 1alpha,25-Dihydroxyvitamin D2, D3, D4, D7, and 24-Epi-1alpha,25-Dihydroxyvitamin D2. Biol Pharm Bull (1999) 22(4):371–7. doi: 10.1248/bpb.22.371 [DOI] [PubMed] [Google Scholar]
- 18. Zarei A, Hulley PA, Sabokbar A, Javaid MK, Morovat A. 25-Hydroxy- and 1alpha,25-Dihydroxycholecalciferol Have Greater Potencies Than 25-Hydroxy- and 1alpha,25-Dihydroxyergocalciferol in Modulating Cultured Human and Mouse Osteoblast Activities. PloS One (2016) 11(11):e0165462. doi: 10.1371/journal.pone.0165462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chun RF, Hernandez I, Pereira R, Swinkles L, Huijs T, Zhou R, et al. Differential Responses to Vitamin D2 and Vitamin D3 Are Associated With Variations in Free 25-Hydroxyvitamin D. Endocrinology (2016) 157(9):3420–30. doi: 10.1210/en.2016-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hollis BW. Comparison of Equilibrium and Disequilibrium Assay Conditions for Ergocalciferol, Cholecalciferol and Their Major Metabolites. J Steroid Biochem (1984) 21(1):81–6. doi: 10.1016/0022-4731(84)90063-3 [DOI] [PubMed] [Google Scholar]
- 21. Houghton LA, Vieth R. The Case Against Ergocalciferol (Vitamin D2) as a Vitamin Supplement. Am J Clin Nutr (2006) 84(4):694–7. doi: 10.1093/ajcn/84.4.694 [DOI] [PubMed] [Google Scholar]
- 22. Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of Vitamin D2 and Vitamin D3 Supplementation in Raising Serum 25-Hydroxyvitamin D Status: A Systematic Review and Meta-Analysis. Am J Clin Nutr (2012) 95(6):1357–64. doi: 10.3945/ajcn.111.031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D Deficiency as a Public Health Issue: Using Vitamin D2 or Vitamin D3 in Future Fortification Strategies. Proc Nutr Soc (2017) 76(3):392–9. doi: 10.1017/S0029665117000349 [DOI] [PubMed] [Google Scholar]
- 24. Biancuzzo RM, Holick MF, Winter MR. Reply to S Lanham-New Et al. Am J Clin Nutr (2010) 92(4):999–1000. doi: 10.3945/ajcn.110.000240 [DOI] [PubMed] [Google Scholar]
- 25. Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, et al. Fortification of Orange Juice With Vitamin D(2) or Vitamin D(3) is as Effective as an Oral Supplement in Maintaining Vitamin D Status in Adults. Am J Clin Nutr (2010) 91(6):1621–6. doi: 10.3945/ajcn.2009.27972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lanham-New S, Vieth R, Heaney R. Vitamin D2 and Vitamin D3 Comparisons: Fundamentally Flawed Study Methodology. Am J Clin Nutr (2010) 92(4):999. doi: 10.3945/ajcn.2010.30099 [DOI] [PubMed] [Google Scholar]
- 27. Zittermann A, Ernst JB, Gummert JF, Borgermann J. Vitamin D Supplementation, Body Weight and Human Serum 25-Hydroxyvitamin D Response: A Systematic Review. Eur J Nutr (2014) 53(2):367–74. doi: 10.1007/s00394-013-0634-3 [DOI] [PubMed] [Google Scholar]
- 28. Tripkovic L, Wilson LR, Hart K, Johnsen S, de Lusignan S, Smith CP, et al. Daily Supplementation With 15 Mug Vitamin D2 Compared With Vitamin D3 to Increase Wintertime 25-Hydroxyvitamin D Status in Healthy South Asian and White European Women: A 12-Wk Randomized, Placebo-Controlled Food-Fortification Trial. Am J Clin Nutr (2017) 106(2):481–90. doi: 10.3945/ajcn.116.138693 [DOI] [PubMed] [Google Scholar]
- 29. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as Effective as Vitamin D3 in Maintaining Circulating Concentrations of 25-Hydroxyvitamin D. J Clin Endocrinol Metab (2008) 93(3):677–81. doi: 10.1210/jc.2007-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zittermann A, Pilz S, Berthold HK. Serum 25-Hydroxyvitamin D Response to Vitamin D Supplementation in Infants: A Systematic Review and Meta-Analysis of Clinical Intervention Trials. Eur J Nutr (2020) 59(1):359–69. doi: 10.1007/s00394-019-01912-x [DOI] [PubMed] [Google Scholar]
- 31. Li P, Zhao Y, Fan X, Wang J, Lu W, Zheng X. Independent Associations of Serum 25-Hydroxyvitamin D3 and D2 With Depressive Symptoms in Females. J Affect Disord (2021) 296:342–9. doi: 10.1016/j.jad.2021.09.102 [DOI] [PubMed] [Google Scholar]
- 32. Berlanga-Taylor AJ, Plant K, Dahl A, Lau E, Hill M, Sims D, et al. Genomic Response to Vitamin D Supplementation in the Setting of a Randomized, Placebo-Controlled Trial. EBioMedicine (2018) 31:133–42. doi: 10.1016/j.ebiom.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neme A, Seuter S, Malinen M, Nurmi T, Tuomainen TP, Virtanen JK, et al. In Vivo Transcriptome Changes of Human White Blood Cells in Response to Vitamin D. J Steroid Biochem Mol Biol (2019) 188:71–6. doi: 10.1016/j.jsbmb.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 34. Hossein-nezhad A, Spira A, Holick MF. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PloS One (2013) 8(3):e58725. doi: 10.1371/journal.pone.0058725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, et al. Mistimed Sleep Disrupts Circadian Regulation of the Human Transcriptome. Proc Natl Acad Sci USA (2014) 111(6):E682–91. doi: 10.1073/pnas.1316335111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, et al. Effects of Insufficient Sleep on Circadian Rhythmicity and Expression Amplitude of the Human Blood Transcriptome. Proc Natl Acad Sci United States America (2013) 110(12):E1132–41. doi: 10.1073/pnas.1217154110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res (2015) 43(7):e47. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu G, Wang LG, Han Y, He QY. Clusterprofiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics: J Integr Biol (2012) 16(5):284–7. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res (2003) 13(11):2498–504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res (2017) 45(D1):D362–D8. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Langfelder P, Horvath S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinf (2008) 9:559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Botia JA. CoExpNets: Co-Expression Network Management Based on WGCNA + K-Means. R Package Version 0.1.0. 0.1.0. (2019), R package. [Google Scholar]
- 43. Dolgalev I. Msigdbr: MSigDB Gene Sets for Multiple Organisms in a Tidy Data Format. R Package Version 7.2.1. (2020). [Google Scholar]
- 44. Zhang R, Edwards JR, Ko SY, Dong S, Liu H, Oyajobi BO, et al. Transcriptional Regulation of BMP2 Expression by the PTH-CREB Signaling Pathway in Osteoblasts. PloS One (2011) 6(6):e20780. doi: 10.1371/journal.pone.0020780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ, et al. Widespread Seasonal Gene Expression Reveals Annual Differences in Human Immunity and Physiology. Nat Commun (2015) 6:7000. doi: 10.1038/ncomms8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sonbul SN, Aleskandarany MA, Kurozumi S, Joseph C, Toss MS, Diez-Rodriguez M, et al. Saccharomyces Cerevisiae-Like 1 (SEC14L1) is a Prognostic Factor in Breast Cancer Associated With Lymphovascular Invasion. Mod Pathol (2018) 31(11):1675–82. doi: 10.1038/s41379-018-0092-9 [DOI] [PubMed] [Google Scholar]
- 47. Agell L, Hernandez S, Nonell L, Lorenzo M, Puigdecanet E, de Muga S, et al. A 12-Gene Expression Signature is Associated With Aggressive Histological in Prostate Cancer: SEC14L1 and TCEB1 Genes are Potential Markers of Progression. Am J Pathol (2012) 181(5):1585–94. doi: 10.1016/j.ajpath.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 48. Burdelski C, Barreau Y, Simon R, Hube-Magg C, Minner S, Koop C, et al. Saccharomyces Cerevisiae-Like 1 Overexpression is Frequent in Prostate Cancer and has Markedly Different Effects in Ets-Related Gene Fusion-Positive and Fusion-Negative Cancers. Hum Pathol (2015) 46(4):514–23. doi: 10.1016/j.humpath.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 49. Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING Database in 2017: Quality-Controlled Protein–Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res (2017) 45(Database issue):D362–D8. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fetahu IS, Hobaus J, Kallay E. Vitamin D and the Epigenome. Front Physiol (2014) 5:164. doi: 10.3389/fphys.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sailani MR, Metwally AA, Zhou W, Rose SMS, Ahadi S, Contrepois K, et al. Deep Longitudinal Multiomics Profiling Reveals Two Biological Seasonal Patterns in California. Nat Commun (2020) 11(1):4933. doi: 10.1038/s41467-020-18758-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou W, Sailani MR, Contrepois K, Zhou Y, Ahadi S, Leopold SR, et al. Longitudinal Multi-Omics of Host-Microbe Dynamics in Prediabetes. Nature (2019) 569(7758):663–71. doi: 10.1038/s41586-019-1236-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Contrepois K, Wu S, Moneghetti KJ, Hornburg D, Ahadi S, Tsai MS, et al. Molecular Choreography of Acute Exercise. Cell (2020) 181(5):1112–30 e16. doi: 10.1016/j.cell.2020.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanchez-Martinez R, Zambrano A, Castillo AI, Aranda A. Vitamin D-Dependent Recruitment of Corepressors to Vitamin D/retinoid X Receptor Heterodimers. Mol Cell Biol (2008) 28(11):3817–29. doi: 10.1128/MCB.01909-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou R, Chun RF, Lisse TS, Garcia AJ, Xu J, Adams JS, et al. Vitamin D and Alternative Splicing of RNA. J Steroid Biochem Mol Biol (2015) 148:310–7. doi: 10.1016/j.jsbmb.2014.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mengozzi M, Hesketh A, Bucca G, Ghezzi P, Smith CP. Vitamins D3 and D2 Have Marked But Different Global Effects on Gene Expression in a Rat Oligodendrocyte Precursor Cell Line. Mol Med (Cambridge Mass) (2020) 26(1):32. doi: 10.1186/s10020-020-00153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science (2006) 311(5768):1770–3. doi: 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 58. Hayes CE, Nashold FE. Vitamin D and Multiple Sclerosis Vol. 2. Feldman D, editor. San Diego (CA, US): Elsevier Inc; (2018) p. 989–1024. [Google Scholar]
- 59. Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I Interferon Suppresses Type II Interferon-Triggered Human Anti-Mycobacterial Responses. Science (2013) 339(6126):1448–53. doi: 10.1126/science.1233665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feng X, Wang Z, Howlett-Prieto Q, Einhorn N, Causevic S, Reder AT. Vitamin D Enhances Responses to Interferon-Beta in MS. Neurol Neuroimmunol Neuroinflamm (2019) 6(6):e622. doi: 10.1212/NXI.0000000000000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hupperts R, Smolders J, Vieth R, Holmoy T, Marhardt K, Schluep M, et al. Randomized Trial of Daily High-Dose Vitamin D3 in Patients With RRMS Receiving Subcutaneous Interferon Beta-1a. Neurology (2019) 93(20):e1906–e16. doi: 10.1212/WNL.0000000000008445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Camu W, Lehert P, Pierrot-Deseilligny C, Hautecoeur P, Besserve A, Jean Deleglise AS, et al. Cholecalciferol in Relapsing-Remitting MS: A Randomized Clinical Trial (CHOLINE). Neurol Neuroimmunol Neuroinflamm (2019) 6(5):e597. doi: 10.1212/NXI.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zheng R, Gonzalez A, Yue J, Wu X, Qiu M, Gui L, et al. Efficacy and Safety of Vitamin D Supplementation in Patients With Systemic Lupus Erythematosus: A Meta-Analysis of Randomized Controlled Trials. Am J Med Sci (2019) 358(2):104–14. doi: 10.1016/j.amjms.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 64. Mostafavi S, Yoshida H, Moodley D, LeBoite H, Rothamel K, Raj T, et al. Parsing the Interferon Transcriptional Network and Its Disease Associations. Cell (2016) 164(3):564–78. doi: 10.1016/j.cell.2015.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gauzzi MC, Fantuzzi L. Reply to Jakovac: COVID-19, Vitamin D, and Type I Interferon. Am J Physiol Endocrinol Metab (2020) 319(2):E245–6. doi: 10.1152/ajpendo.00315.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic Mechanisms of Critical Illness in Covid-19. Nature (2020) 591(7848):92–8. doi: 10.1038/s41586-020-03065-y [DOI] [PubMed] [Google Scholar]
- 67. St John HC, Meyer MB, Benkusky NA, Carlson AH, Prideaux M, Bonewald LF, et al. The Parathyroid Hormone-Regulated Transcriptome in Osteocytes: Parallel Actions With 1,25-Dihydroxyvitamin D3 to Oppose Gene Expression Changes During Differentiation and to Promote Mature Cell Function. Bone (2015) 72:81–91. doi: 10.1016/j.bone.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. St John HC, Bishop KA, Meyer MB, Benkusky NA, Leng N, Kendziorski C, et al. The Osteoblast to Osteocyte Transition: Epigenetic Changes and Response to the Vitamin D3 Hormone. Mol Endocrinol (2014) 28(7):1150–65. doi: 10.1210/me.2014-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kmietowicz Z. Sixty Seconds on … Vitamin D. BMJ (2020) 371:m3872. doi: 10.1136/bmj.m3872 [DOI] [PubMed] [Google Scholar]
- 70. Weir EK, Thenappan T, Bhargava M, Chen Y. Does Vitamin D Deficiency Increase the Severity of COVID-19? Clin Med (Lond) (2020) 20(4):e107–e8. doi: 10.7861/clinmed.2020-0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu B, Taioli E. Seasonal Variations of Complete Blood Count and Inflammatory Biomarkers in the US Population - Analysis of NHANES Data. PloS One (2015) 10(11):e0142382. doi: 10.1371/journal.pone.0142382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metadata on serum concentrations of 25(OH)D2, 25(OH)D3, total 25(OH)D, PTH and calcium (albumin-adjusted) for the 97 subjects subjected to transcriptomic analysis [selected from the cohort described by Tripkovic et al. (28)].
[Note: data file is 9.1 MB.] Significant differences in the transcriptional responses occurring across the 12-week V1 to V3 period of the study between the vitamin D2, vitamin D3 and placebo treatment groups for each ethnic cohort, and significant changes within each group between the V3 and V1 sampling points (see Figure 2 ).
Functional analysis of the genes represented by the probes specifically repressed by D2 and D3 in the white European cohort (blue data points in ) or induced by D2 and D3 in the white European cohort (red data points in Figure 2D ).
Comparative functional enrichment analysis of all significant changes in each treatment group in the white European cohort ( Figure 2C ; and see Figure 4 ).
Networks illustrating all the functional categories significantly enriched (p.adjust < 0.01) in analysis of the gene products represented by the probes significantly up- or down-regulated (adj.P.Val < 0.05) in the white European cohort treatment groups (from data presented in Supplementary Data File 4 ).
Module membership for the modules of genes identified in the WGCNA analysis of the data from the South Asian and white European cohorts, as presented in Figure 5 .
Gene ontology functional enrichment analysis results for the modules of genes identified in the WGCNA analysis of the data from the white European cohort.
Gene ontology functional enrichment analysis results for the modules of genes identified in the WGCNA analysis of the data from the South Asian cohort.
Gene Set Enrichment Analysis results for the analysis reported in Figure 6 .
Data Availability Statement
All microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-8600.