Abstract
The probability that fruit ingestion may protect human health is an intriguing vision and has been studied around the world. Therefore, fruits are universally promoted as healthy. Over the past few decades, the number of studies proposing a relationship between fruit intake and reduced risk of major chronic diseases has continued to grow. Fruits supply dietary fiber, and fiber intake is linked to a lower incidence of cardiovascular disease and obesity. Fruits also supply vitamins and minerals to the diet and are sources of phytochemicals that function as phytoestrogens, antioxidant and anti-inflammatory agents, and other protective mechanisms. So, this review aims to summarize recent knowledge and describe the most recent research regarding the health benefits of some selected red fruits.
Keywords: antioxidant activity, phenolic compounds, volatile compounds, vitamins, minerals, fatty acids, fibers, consumer perception, health benefits
1. Introduction
Nowadays, people are concerned about having a varied and healthy diet. Therefore, the acceptability and consumption of red fruits, especially the species of several families, such as Rosaceae (strawberry, raspberry, blackberry, and sweet cherry), and Ericaceae (blueberry, cranberry) has undergone significant increases not only due to their high nutritive value, characteristic taste, flavor and nutraceutical characteristics, but also due to their known health-promoting properties (as dietary sources of bioactive compounds) [1,2,3,4,5]. There is the recognition that red fruits are a good source of many bioactive ingredients and nutrients, including vitamins (vitamins A, C, and E), minerals (calcium, phosphorus, iron, magnesium, potassium, sodium, manganese, and copper), dietary fiber, and antioxidants [1,3]. They are also a rich source of bioactive compounds, and studies have shown that they have essential positive effects on the human diet and health, which could be mainly ascribed to the presence of several health-related compounds such as organic acids, phenolics, and sugars (glucose, fructose) [6,7,8]. Intake of fresh fruits enhanced both mental and physical health and facilitated the prevention of various non-communicable diseases as well, such as neurological disease, cardiovascular disease, diabetes mellitus, obesity, osteoarthritis, and some cancers [8]. For example, Huang et al. [9] showed that strawberries are a rich source of anti-inflammatory polyphenols such as anthocyanins, which have been shown to decrease the postprandial meal-induced increases in inflammation and oxidative stress in overweight healthy adults, mainly if the strawberry drink was consumed before the meal. In addition, the ease of transport due to their size makes them even more recommendable to be consumed in all situations. The qualitative and quantitative composition, the nutritional value, and consumer’s acceptability of the fruits in general and the red fruits, in particular, will vary with the species, cultivar, genotype, maturity stage, agricultural practice, environmental conditions, plant nutrition, soil conditions as well as subsequent storage conditions [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. This review aims to summarize and update knowledge concerning the composition of red fruits and to describe the most recent research regarding the health benefits of some selected red fruits.
2. Red Fruits Composition
2.1. Vitamins and Minerals
Red fruit berries are the best dietary sources of bioactive compounds, namely vitamins and minerals with antioxidant properties [1,2]. Since red fruits do not usually undergo any processing to be consumed, their antioxidant properties are not reduced. According to Nile and Park [17], 100 g of the edible portion of raspberries, blackberries, or blueberries could provide more than 50% of Recommended Dietary Allowance for manganese, vitamin C (ascorbic acid), and vitamin B9 (folic acid).
2.1.1. Vitamins
Vitamins are organic substances with strong antioxidant potential that our bodies cannot synthesize in sufficient quantities yet are essential for their good development, even in trace amounts. According to Rodriguez-Amaya [18], there are 14 known vitamins grouped into two large groups of molecules, fat-soluble (A, D, E, and K) and water-soluble (B group and C).
Under the name of red fruits is a set of fruits of black or red color, mostly arranged in berries, like strawberry, cherry, red raspberry, black raspberry, blackberry, cranberry, blue-berry, blackcurrants, and grapes. Vitamin C or ascorbic acid is the most quantified in red fruits and is one of the main antioxidant compounds present in this type of fruit. It is a water-soluble carbohydrate-derived compound, known for its high antioxidant activity due to the neutralization of free radicals and other reactive oxygen species, and acidic properties due to the presence of a 2,3-enediol moiety (Figure 1) [3].
Figure 1.
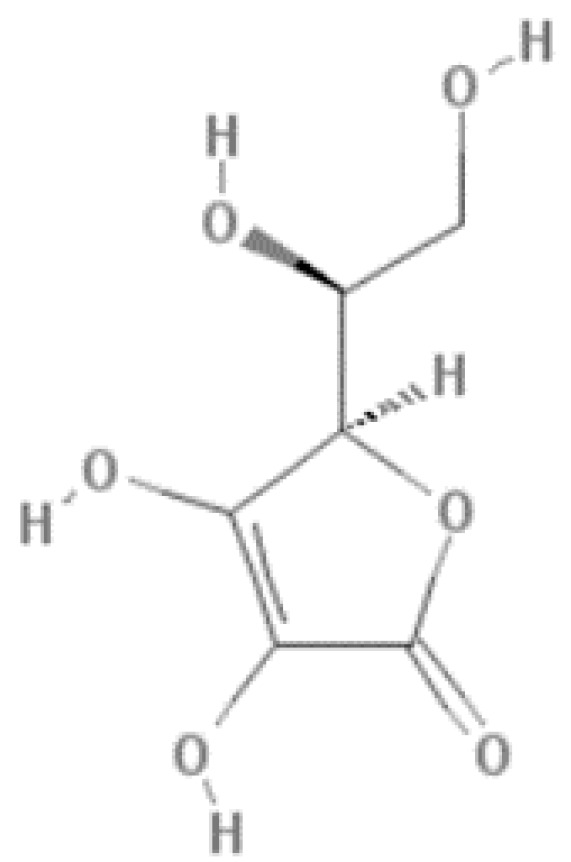
Structure of ascorbic acid.
Contrary to vitamin C, vitamin A is not found in fruits, at least in large quantity, with some exceptions such as the mango, the cantaloupe, and even the watermelon. A total of 1–2 mg per day is the human requirement for vitamin B2 (riboflavin). The largest source of this vitamin is the green vegetable, unlike fruits that are relatively poor in riboflavin [3] Vitamin B6 (riboflavin) is not present in large quantities in red fruits, being present in appreciable quantities in grapes, prunes, avocados, and bananas [3].
Table 1 presents the composition in vitamins of some red fruits found by several authors, and wide variations in the contents of vitamins can be observed. For instance, the level of vitamin C ranged from 5 to 100 g/100 g fresh weight (FW) among cultivars of cherry, cranberry, blackberry, blueberry, red raspberry, and strawberry. Blueberries are the richest fruits in ascorbic acid, while the content of raspberries is similar to that of strawberries, and blackberries have a lower value of about 34 mg/100 g FW [19]. However, the ascorbic acid content in blackberry is about 2–3 times the content in red currants and about 8–9 times less than in blackcurrants [20]. Cranberry is rich in vitamins, such as vitamin C. As with strawberries, and depending on the storage conditions, cranberries also have significant losses of vitamin C after storage [21]. Cranberry is a fruit that has lately been recognized as new functional food and nutraceutical. For instance, cranberries are known to have a unique function for maintaining urinary tract health [22]. According to Dorofejeva et al. [23], in cultivated cranberries, this bioactive compound is about 10 mg/100 g dry weight (DW).
Table 1.
The composition in Vitamin C (ascorbic acid) and other vitamins of some red fruits (units at the Table footnote).
| Red Fruits | |||||||
|---|---|---|---|---|---|---|---|
| Sweet Cherry | Cranberry | Blackberry | Blueberry | Raspberry | Strawberry | References | |

|

|

|

|

|

|
||
| Vitamin C (Ascorbic acid) |
62.4 a | 10 b | 34–52 a | 10–100 a | 5–92.2 a | 5–90 a | [2,19,23,24,25,26,27] |
| Vitamin B6 (Pyridoxine) |
790 c | 606 c | 1999 c | 1744 c | [28] | ||
| Vitamin B2 (Riboflavin) |
247 c | 69 c | 216 c | 93 c | [28] | ||
Units: a mg/100 g Fresh weight (FW); b mg/100 g Dry weight (DW); c μg/g.
2.1.2. Minerals
Usually, fruits are not recognized as primary sources of mineral intake. Even so, according to the Dietary Approaches to Stop Hypertension (DASH) fruits contribute an average of 5.8%, 17.3%, 33.0%, and 6.6% to the intakes of calcium, magnesium, potassium, and zinc, respectively [29]. Red fruits are not only a source of vitamins [1], but are also a rich source of minerals (phosphorus, calcium, iron, potassium, magnesium, manganese, sodium, and copper) [2]. Table 2 shows the mineral composition of sweet cherry, blackberry, blueberry, raspberry, and strawberry.
Table 2.
Mineral content (mg/100 g FW) of some red fruits.
| Sweet Cherry | Blackberry | Blueberry | Raspberry | Strawberry | References | |
|---|---|---|---|---|---|---|
| Phosphorus (P) |
12.2 | 7–29 | 8.6 | 5.7 | 6.6 | [2,30,31] |
| Potassium (K) |
90.9 | 77–349 | 70.1 | 71.8 | 51.2 | [2,30,31] |
| Calcium (Ca) |
- | 6–29 | - | 1.14 | 2.20 | [2,30] |
| Magnesium (Mg) | 12.2 | 6–44.8 | 4.9 | 15.9 | 8.78 | [2,30,31] |
| Zinc (Zn) |
0.69 | 0.07–0.44 | 0.13 | 0.37 | 0.13 | [2,30,31] |
| Iron (Fe) |
1.16 | 0.28–1.28 | 1.24 | 1.06 | 1.0 | [2,30,31] |
Among the fruits analyzed, blackberries and cherry are the fruits with the highest concentration of minerals. There are important differences between the different researchers regarding the concentration of minerals [2,19,23,30]. This is explained, as previously mentioned, since in most cases comparisons are made between fruits of different cultivars, subject to different soil and climatic conditions, and post-harvest handling techniques [2]. The effects of cultivars and cultivation conditions on the composition of strawberries are demonstrated by Hakala et al. [31].
2.2. Sugars and Organic Acids
Generally, fructose and glucose are the main sugars present in red fruits, while citric and malic acids are the main organic acids present in this type of fruit. Mikulic-Petkovsek et al. [32] studied the sugars and organic acids content in fruit of 25 wild and cultivated berry species and found that glucose and fructose were the most abundant sugars in berry fruits and the main organic acids were citric and malic acid. Instead, Viljakainen et al. [33] also showed significant variations in sugars and organic acids between different berry cultivars.
Table 3 presents the composition of some of the most common red fruits in sugars and organic acids addressed by different authors and it is consensual that different factors such as genotype, cultural practices, climate conditions, altitude, and season can affect the variation and content of such compounds. The data present in Table 3 and from other studies showed the occurrence and the variation in different sugars and organic acids. Glucose was the main sugar found in the strawberry, up to 80% [34], and citric acid the main organic acid in strawberry and blueberry juice, contributing with 62–84% and 73–90% of the total acid content, respectively [34,35]. In blueberries, a high content of fructose and citric acid as a main sugar and acid were also found, respectively [36]. In addition, Zhang et al. [36] also found high content of citric acid (1.862–13.424 mg/g) and quinic acid (0.147 to 5.445 mg/g) as the major organic acids present in blueberry fruits.
Table 3.
The average content of main sugars and organic acids in different types of red fruits.
| Red Fruits | Main Sugars | Main Organic Acids | References |
|---|---|---|---|
| Berries | Fructose (18.0–57.2 g/L) Glucose (22.2–50.0 g/L) Sucrose (0.2–5.1 g/L) |
Citric acid (2.9–16.2 g/L) Malic acid (3.3–24.7 g/L) |
[33] |
| Raspberry (Rubus idaeus) |
Fructose 35–45% of total sugars Glucose 30–35% of total sugars Sucrose 30–35% of total sugars |
[42] | |
| Strawberry (Fragaria × ananassa) |
Frutose (1.07–3.079 g/100 g) Glucose (2.236–4.802 g/100 g) Sucrose (0.352–7.571 g/100 g) |
Citric acid (643.32 mg/100 mL) Malic acid (203.98 mg/100 mL) |
[43,44] |
| Blueberry (Vaccinium corymbosum) |
Fructose (70.40–304.52 mg/g DW) Glucose (13.86–57.36 mg/g DW) Sucrose (0.56–7.90 mg/g DW) |
Citric acid (13.34–75.11 mg/g DW) Quinic acid (2.86–11.56 mg/g DW) Malic acid (1.02–7.21 mg/g DW) |
[45] |
| Sweet cherry (Prunus avium) |
Malic acid Oxalic acid Shikimic acid |
[37] |
In cherries, the most abundant soluble sugars observed were glucose and fructose, together with the sugar alcohol sorbitol, while malic, oxalic, and shikimic acids were the most abundant organic acids [37].
In strawberry, Urün et al. [38] found high content of citric (522.4–711.5 mg/g FW) and malic acid (159.8–266.7 mg/g FW) and fructose was the sugar present in the highest concentration (2.17–4.43% of total sugars). Also, Morais et al. [39] showed enhancement in the total soluble solids content in strawberry plants inoculated with the PGPB strain Pedobacter sp. CC1.
In white and red grapes, tartaric, malic, and citric acids are the most preponderant organic acids. In a study of 24 red grape varieties [40], the titratable acidity, expressed as equivalent of tartaric acid, varied from 3.9 (‘Moreto Boal’) to 13.5 g/L (‘Tinta Miúda’ and ‘Jean’), these results are following the tartaric acid concentration that ranged from 2.49 to 7.70 g/L of tartaric acid. In another study with red grapes from the Douro and Dão regions, Portugal, it was found that red grapes from the Douro Region had a higher content of tartaric acid (average values 6.21 g/L) compared to red grapes from Dão (4.96 g/L) [41].
2.3. Dietary Fibers
There are several definitions of dietary fibers [46], according to the Institute of Medicine, [47], the term dietary fiber consists of non-digestible carbohydrates and lignin that are intrinsic and intact in plants. The term includes cellulose, hemicellulose, lignin, pectins, gums, mucilages, and a non-carbohydrate component [1]. Anita and Abraham [48] classified the dietary fiber into two categories: cellulose, hemicellulose, and lignin, which are water-insoluble/less fermented, being the pectins, gums, and mucilages water-soluble/well-fermented fibers.
The benefits of a high fiber diet have long been known [49], and we are always incited to eat fiber-rich foods [50]. Thus, in addition to the direct consumption of fiber-rich products and ingredients, the food industry has been searching for new sources of dietary fiber and developing new products with fiber supplementation [51]. Diets rich in fruits with high content of fibers have been related to the decreased incidence of several types of diseases [52]. Indeed, the modulation of function of the intestinal tract [50], lower risk of colorectal cancer [52], reduction in the total and LDL cholesterol [52] and cardiovascular disease, reduction onset risk or symptoms of metabolic syndrome and type 2 diabetes, are benefits of a fiber-rich diet. In addition, these types of diet are usually relatively low in calories compared to meals rich in other food types [53]. It is recommended that adults should ingest about 20 to 35 g of dietary fiber per day [54]. The fiber content of several red fruits is present in Table 4. Among red fruits, the cranberry presents a higher content in fiber followed by the raspberries and blackberries. Strawberries and cherries appear to be the least rich in fiber, with values between 1.3 and 2.2 mg/100 g FW. According to Akimov et al. [55], insoluble nutritional fiber is dominant in raspberry (68.6%). The necessary daily norm for the consumption of nutritional fiber is 20 g [54]. One hundred grams of raspberry fruit provides the human organism with 11.7% of such components.
Table 4.
Dietary fiber of some red fruits.
| Red Fruits | |||||||
|---|---|---|---|---|---|---|---|
| Sweet Cherry | Cranberry | Blackberry | Blueberry | Raspberry | Strawberry | References | |
| Dietary fiber (mg/100 g FW) |
2.1 | 35.7 c | 4.5–5.3 | 1.9–2.4 | 5.8–6.5 | 1.3–2.2 | [2,30,31] |
| Estimated Fiber Components a | |||||||
| Serving Size a | 1 cup(138 g) | 1 cup(144 g) | 1 cup(148 g) | 1 cup(123 g) | 1 cup(152 g) | ||
| Total (100 g) | 2.2 | 5.3 | 2.8 | 6.5 | 2.0 | [56,57,58] | |
| Insoluble (100 g) | 1.6 | 4.7 | 2.4 | 5.3 | 1.5 | ||
| Soluble (100 g) | 0.6 | 0.6 | 0.3 | 1.2 | 0.5 | ||
| Pectin b (100 g) | 0.7 | 1.4 | 0.8 | 1.6 | 0.7 | ||
2.4. Lipids and Fatty Acids
The increased consumption of red fruits is associated with their nutritional value, which offers many health benefits, particularly in the prevention of cardiovascular diseases and reduction in cancer risks. Red fruits, when consumed in the raw form, present reduced levels of fat, generally below 1% (Table 5). Pacifico et al. [59] studying two sweet cherry cultivars (‘Del Monte’ and ‘Della Recca’) found differences in their lipid composition. Similar findings were described by Kafkas et al. [60] in nine strawberry cultivars (‘Call-Giant4′, ‘Camarosa’, ‘Fern’, ‘Festival’, ‘Kabarla’, ‘Redlans Hope’, ‘Sweet Charlie’, ‘Whitney’, and ‘Gianna’) of Turkey. Kafkas et al. [61] in seven raspberry cultivars (‘Heritage’, ‘Canby’, ‘Willamette’, ‘Hollanda Boduru’, ‘Newburgh’, ‘Tulameen’, and ‘Meeker’) and Fadavi et al. [62] in 25 pomegranates varieties grown in Iran also found differences in the lipid percentage and fatty acid composition. According to Al Juhaimi et al. [63], the fatty composition of grapes is dependent on the local origin. The same conclusion was achieved by Melgarejo and Artés [64] when comparing the oil content and fatty acid composition of the oilseed of seven sweet Spanish varieties with some Oriental pomegranate varieties. Al-Maiman and Ahmad [65] also observed a slight modification in lipid and fatty acid content during different stages of pomegranate maturation (unripe fruits, half-ripe fruits, and full-ripe fruits). Wang and Wang [66] analyzing the effect of storage conditions on several cranberries’ varieties and concluded that storage temperature greatly affected their fatty acid profile. The types of product processed, as well as its residues, also affect the lipid content and the fatty acid composition. Recently, Zafra-Rojas et al. [67] found that blackberry residues comprised of peel, seeds, and pulp exhibited lower content of fatty acids when compared with the commercial product.
Table 5.
Total fat and fatty acids (g/100 g FW) composition of different red fruits in the raw form. Adapted from [68].
| Red Fruits | Total Fat | Fatty Acids | ||
|---|---|---|---|---|
| Saturated | Monounsaturated | Polyunsaturated | ||
| Raspberry (Rubus idaeus) |
0.65 | 0.019 | 0.064 | 0.375 |
| Sweet cherry (Prunus avium) |
0.20 | 0.038 | 0.049 | 0.052 |
| Strawberry (Fragaria × ananassa) |
0.30 | 0.015 | 0.043 | 0.155 |
| Grapefruit, pink and red (Citrus × paradisi) |
0.14 | 0.021 | 0.020 | 0.036 |
| Cranberry (Vaccinium oxycoccos) |
0.13 | 0.008 | 0.018 | 0.055 |
| Pomegranate (Punica granatum) |
1.17 | 0.120 | 0.093 | 0.079 |
| Blackberry (Rubus fruticosus) |
0.49 | 0.014 | 0.047 | 0.280 |
| Blueberry (Vaccinium corymbosum) |
0.33 | 0.028 | 0.047 | 0.146 |
The lipid fraction of red fruits is rich in unsaturated fatty acids, particularly polyunsaturated fats (Table 6). The berry fruits (raspberry, strawberry, blackberry, and blueberry) are a remarkably good source of polyunsaturated fats (Table 6), with a significant amount of linoleic (C18:2, n-6), and linolenic (C18:3, n-3) fatty acids. Red fruits also contain considerable amounts of oleic acid (C18:1, n-9), palmitic acid (C16:0), α-linolenic acid (C18:3, n-3), stearic acid (C18:0), and myristic acid (C14:0). Contrary to all red fruits considered in this review, lipid fraction in pomegranate seeds consist mainly of punicic acid (C18:3, n-5), a conjugated isomer of α-linolenic acid [69].
Table 6.
Predominant fatty acids in the seed oil of several red fruits.
| Red Fruits | Main Fatty Acids | References |
|---|---|---|
| Sour cherry (Prunus cerasus) |
Linoleic acid, oleic acid, palmitic acid, α-linolenic acid and stearic acid | [70] |
| Sweet cherry (Prunus avium) |
Linoleic acid, oleic acid, palmitic acid, α-linolenic acid, and myristic acid | [71] |
| Strawberry (Fragaria × ananassa) |
Linoleic acid, linolenic acid, oleic acid, palmitic acid and stearic acid | [61,72] |
| Grapefruit (Citrus × paradisi) |
Linoleic acid, oleic acid, palmitic acid, stearic acid, and linolenic acid | [73] |
| Cranberry (Vaccinium oxycoccos) |
Linoleic acid, oleic acid, linolenic acid, palmitic acid, and stearic acid | [74] |
| Pomegranate (Punica granatum) |
Punicic acid, linoleic acid, oleic acid and palmitic acid | [75] |
| Raspberry (Rubus idaeus) |
Linoleic acid, linolenic acid, oleic acid, palmitic acid and stearic acid | [72,76] |
| Blackberry (Rubus fruticosus) |
Linoleic acid, α-linolenic acid, oleic acid, palmitic acid, and stearic acid | [77] |
| Blueberry (Vaccinium corymbosum) |
Linoleic acid, α-linolenic acid, oleic acid, palmitic acid, and stearic acid | [77] |
Lipids present in fruits are crucial in the metabolism since they are included in cell membranes, increase resistance to viral infections and catarrhal diseases [78]. In red fruits, the low lipid content associated with low cholesterol levels makes these fruits highly appreciated by consumers. Nowadays, consumers have increasingly been more concerned with the nutritional and caloric value of the food they consume, looking for healthier, innovative, safe, and easy-to-use products. Therefore, the consumption of red fruits has increased, in part due to an array of health benefits. Additionally, they present some type of fatty acids with numerous positive effects on human health, as an anticancer and neuroprotective agent, is also associated with cardiovascular diseases protection [79]. Fatty acids in red fruits are mainly in the form of polyunsaturated acids, which are necessary to build cell membranes and the covering of nerves as well as for proper blood clotting, muscle movement, and protection against inflammation [80]. Polyunsaturated fatty acids are essential for normal body functions and are associated with significant beneficial cardiovascular effects [81].
According to the Food and Agriculture Organization/World Health Organization, about 2–4% of daily energy should come in the form of essential fatty acids with an additional 3% energy for pregnant or breastfeeding mothers [82]. The dominant fatty acids found in red fruits are omega-6 linoleic acid, omega-9 oleic acid, and omega-3 linolenic acid, which are linked to an array of health benefits. Omega-3 and omega-6 are crucial for the prevention and treatment of cardiovascular disease [81]. Their incorporation in a diet can result in a decrease in mortality from coronary artery and cardiovascular diseases [81]. Still linked to the reduction in cardiovascular disorders is omega-9, the most common monounsaturated fatty acid, which has also been linked to beneficial effects for diabetes [83].
2.5. Polyphenols
2.5.1. Phenolic Acids and Flavonoids
Phenolic compounds are ubiquitous in plants, and as in other types of fruits they are present in high amounts in red fruits. They are one of the most studied secondary metabolites due to their bioactive functions such as anti-proliferative, anti-diabetic, anticancer, anti-microbial, anti-inflammatory, and antiviral, along with their high antioxidant capacity [84]. Phenolics are a group of hydroxylated molecules gathered in different types of structures with a common aromatic ring (Figure 2), and currently, about 8000 different structures of plant phenolics are known [85].
Figure 2.
The basic carbon skeleton of phenolics.
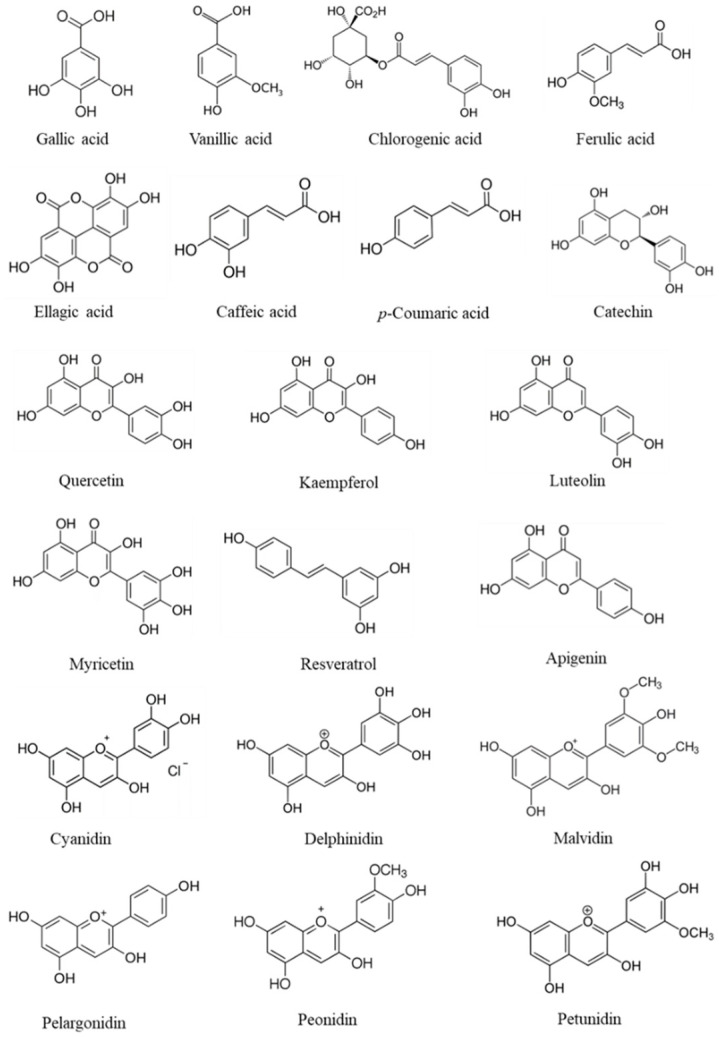
There are many criteria to classify or distinguish phenolics, however, the most commonly used is the division of phenolics into flavonoids and non-flavonoids. According to Działo et al. [85], flavonoids are based on two aromatic rings connected by a bridge consisting of three carbons (C6-C3-C6), which are divided into six main sub-classes: flavonols, flavones, flavanones, flavan-3-ols, isoflavones, and anthocyanidins. In nature, flavonoids occur usually in association with sugar as glycosides. The second class of phenolics is the non-flavonoid molecules [85], which can be divided into 3 subgroups: phenolic acids, lignans (C6-C3)2, and stilbenes C6-C2-C6 [86]. Phenolic acids can be divided into two categories: hydroxybenzoic (C6-C1) acid derivatives and hydroxycinnamic (C6-C3) acid derivatives. The first group includes molecules such as hydroxybenzoic, gallic, vanillic, and ellagic acid, and in the second group p-coumaric, caffeic, ferulic, and chlorogenic acids are the most representative [87]. Flavonoids include catechin, quercetin, kaempferol, luteolin, and myricetin, as the most relevant compounds [85,87,88]. Anthocyanidins include cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin as the most representative molecules [87,89]. Figure 3, illustrates the chemical structures of the most common phenolics found in fruits.
Figure 3.
Chemical structures of phenolics that are commonly found in fruits.
According to the literature, in red fruits diverse and high amounts of phenolics can be found; however, each type of fruit contains a typical phenolic profile. Table 7 summarizes the most common type of red fruits and the main type of phenolics often associated with them. Certain similarities in the phenolic profile can be observed within the same plant genera.
Table 7.
Some examples of main polyphenols are found in different types of red fruits.
| Red Fruits | Polyphenols | References |
|---|---|---|
| Bilberry (Vaccinium mytillus) |
Delphinidin, cyanidin, petunidin, peonidin, malvidin, gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, sinapic acid | [90,91] |
| Blueberry (Vaccinium corymbosum) |
Cyanidin 3-O-glucoside, cyanidin 3-O-glucuronide, cyanidin 3-O-arabinoside, malvidin 3-acetylglucoside, malvidin 3-O-glucoside, peonidin-3-O-glucoside, petunidin 3-acetylglucoside, petunidin-3-O-glucoside, quercetin-3-O-rutinoside, quercetin-3-O-galactoside, quercetin-3-O-glucoside, kaempferol-3-O-glucoside, quercetin-3-O-diglycoside, quercetin 3-O-arabinoside, trans-5-caffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid | [92,93] |
| Cranberry (Vaccinium oxycoccos) |
Cyanidin-3-O-galactoside, cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, peonidin-3-O-galactoside, peonidin-3-O-arabinoside, gallic acid, catechin, epicatechin, procyanidin A2 and p-coumaric acid, rutin, benzoic acid, caffeic acid | [94,95] |
| Lingonberry (Vaccinium vitis-idaea) |
Cyanidin-3-O-galactoside, cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, proanthocyanidin A, proanthocyanidin B, ferulic acid, quercetin-3-O-glactoside, quercetin-3-O-glucoside, quercetin-3-O-arabinoside, quercetin-3-O-rhamnoside, kaempferol-pentoside, kaempferol-rhamnoside | [96] |
| Gooseberry (Ribes uva-crispa) |
Cyanidin3-O-glucoside, petunidin-3-O-glucoside, pelargonidin chloride, caffeic acid, epigallocatechin gallate, p-coumaric acid, rutin, kaempferol, resveratrol | [93,97] |
| Black currant (Ribes nigrum) |
Delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, petunidin-3-O-rutinoside, pelargonidin-3-O-rutinoside, peonidin-3-O-rutinoside, epigallocatechin, catechin, epicatechin, neochlorogenic acid, chlorogenic acid, myricetin-malonylglucoside; quercetin-3-O-galactoside, quercetin-3-O-glucoside, quercetin-3-O-rutinoside, quercetin-3-6-malonylglucoside, kaempferol-3-O-glucoside, isorhamnetin-3-O-glucoside, kaempferol-malonylglucoside | [98,99,100] |
| Red currant (Ribes pallidum) |
Cyanidin-3-O-glucoside, cyanidin-3-O-sophoroside, cyanidin-3-O-rutinoside, cyanidin-3-O-xylosylrutinoside, gallic acid, catechin, syringic acid, cinnamic acid, chlorogenic acid, ferulic acid | [100,101] |
| Red raspberry (Rubus idaeus) |
Cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, petunidin-3-O-glucoside, gallic acid, syringic acid, ferulic acid, quercetin | [93,101] |
| Strawberry (Fragaria × ananassa) |
Cyanidin-3-O-glucoside, cyanidin-3-O-glucoside, cyanidin-3-O-malonylglucoside, pelargonidin-3-O-glucoisde, pelargonidin-3-O-rutinoside, pelargonidin-3-O-acetylglucoside, procyaniin dímers and pentamers, gallic acid, catechin, epicatechin, ferulic acid, p-coumaric acid, cinnamic acid, ellagic acid, quercetin-3-malonylglucoside, kaempferol-3-O-glucuronide, kaempferol-3-O-malonylglucoside | [102] |
| Crowberry (Empetrum nigrum) |
Cyanidin-3-galactoside, chlorogenic acid, protocatechuic acid, batatasin-II, epicatechin, quercetin, kaempferol | [103] |
In general, the majority of phenolics present in red fruit belong to the main classes: phenolic acids and anthocyanins; nonetheless, it is possible to find other types even if they are at lower concentrations. Table 8 and Table 9, shows the average levels of different types of phenolic acids and flavonoids often found in red fruits.
Table 8.
Example of phenolic acid contents found in the most common red fruits.
| Red Fruits | Phenolic Acids (mg/kg FW) | References | |||||
|---|---|---|---|---|---|---|---|
| p-Coumaric | Caffeic | Chlorogenic | Ferulic | Gallic | Ellagic | ||
| Bilberry (Vaccinium mytillus) |
2.0–3.0 | 1.0–5.0 | 210.0–297.0 | 2.0–8.0 | 52.0–85.0 | 8.0–14.0 | [104] |
| Blueberry (Vaccinium corymbosum) |
n.d.-55.29 | 2.0–27.35 | n.d.-700.0 | 9.60–22.0 | n.d.-18.0 | n.d.-1.0 | [104,105] |
| Cranberry (Vaccinium oxycoccos) |
n.d. | 20.7–25.3 | n.d. | 60.5 | n.d. | n.d. | [106] |
| Lingonberry (Vaccinium vitis-idaea) |
37.6–251.1 | 20.1–48.5 | n.d. | 16.2–221.7 | n.d.-47.5 | n.d. | [106,107] |
| Gooseberry (Ribes uva-crispa) |
43.0–49.0 | 1.67–3.53 | n.d. | 6.0–6.4 | n.d. | n.d. | [106] |
| Black currant (Ribes nigrum) |
31.66–31.72 | n.d | 21.30–21.32 | 17.47–17.49 | n.d. | 3.14–3.16 | [105] |
| Red currant (Ribes xpallidum) |
8.24–8.28 | 12.73–12.79 | n.d. | n.d. | n.d | n.d. | [105] |
| Red raspberry (Rubus idaeus) |
1.0–18.0 | 3.22–10.8 | n.d. | 0.6–9.4 | 210.0–220.0 | n.d.-41.42 | [105,106,108] |
| Strawberry (Fragaria × ananassa) |
20.0–49.0 | 1.71–4.2 | n.d. | n.d.-3.2 | 21.0–41.0 | n.d.-68.4 | [105,106,109,110] |
n.d.: not detected.
Table 9.
Example of flavonoid contents found in the most common red fruits.
| Red Fruits | Flavonoids (mg/kg FW) | References | |||
|---|---|---|---|---|---|
| Kaempferol | Myricetin | Quercetin | Luteolin | ||
| Bilberry (Vaccinium mytillus) |
n.d. | n.d.-21.0 | n.d.-41.2 | n.d. | [104,114] |
| Blueberry (Vaccinium corymbosum) |
18.0 | n.d.-34.0 | 31.0–83.0 | n.d.-8.0 | [104,115] |
| Cranberry (Vaccinium oxycoccos) |
n.d.-6.1 | 43.0–230.0 | 73.0–250.0 | n.d. | [114,115] |
| Lingonberry (Vaccinium vitis-idaea) |
n.d.-10.3 | n.d. | n.d.-34.7 | n.d. | [114] |
| Gooseberry (Ribes uva-crispa) |
n.d.-19.0 | n.d. | n.d.-22.0 | n.d. | [114] |
| Black currant (Ribes nigrum) |
n.d.-23.0 | n.d.-245.0 | 22.7–122.0 | n.d. | [114] |
| Red currant (Ribes xpallidum) |
n.d.-8.8 | n.d.-42.9 | n.d.-29.0 | n.d. | [114,116] |
| Red raspberry (Rubus idaeus) |
n.d.-1.0 | n.d. | 6.5–90.0 | n.d. | [108,115] |
| Strawberry (Fragaria × ananassa) |
n.d.-5.0 | n.d. | 6.0–19.0 | n.d. | [108,115] |
| Crowberry (Empetrum nigrum) |
n.d. | 44.0–49.0 | 53.0–56.0 | n.d. | [114] |
n.d.: not detected.
Certain similarities in phenolic content can be observed within the same plant family and genus. For example, flavonols and hydroxycinnamic acids are typical of the Ericaceae family, genus Vaccinium (bilberry, blueberry, cranberry, and lingonberry) [90,92,94,95,96], while flavonols dominate in gooseberry, black and red currant (Grossulariaceae family, genus Ribes) [98,101]. Instead, ellagic acid is the main phenolic compound in fruits from the Rosaceae family, genus Fragaria and Rubus (strawberry and red raspberry) [93,101,102].
Despite the similarity between red fruits, each species has its typical profile. For example, Häkkinen et al. [111] reported that blueberry is rich in quercetin and caffeic acid, while bilberry and lingonberry have residual concentrations of quercetin. Hydroxycinnamates dominated in all cherry samples and represented 60–74% by weight of the phenols in the fresh and stored samples of the varieties ‘Saco’, ‘Summit’, and ‘Van’, and 45% by weight of the phenols in the cv. Burlat samples, which were richer in anthocyanins. The relative and total levels of hydroxycinnamates, anthocyanins, flavonols, and flavan-3-ols varied among sweet cherry cultivars and during storage. Moreover, cold storage induced decreased total phenol levels in the varieties ‘Summit’ and ‘Van´, however increased total phenol levels in the varieties ‘Burlat’ and ‘Saco’ [112,113].
2.5.2. Anthocyanins
Anthocyanins are generally accepted as the largest and most important group of phenolics in red fruits. Anthocyanins are water-soluble compounds responsible for the blue, purple, red, or black color of many fruits, including red fruits. Until now, there are about 17 anthocyanidins properly identified in nature, however only six of them, cyanidin, delphinidin, petunidin, peonidin, pelargonidin, and malvidin, are found in most foods and plants [89]. When anthocyanidins are coupled with sugars, anthocyanins are formed [87]. Table 10 shows some average levels of the main anthocyanins most common in red fruits.
Table 10.
Example of anthocyanin contents found in the most common red fruits.
| Red Fruits | Anthocyanin (mg/kg FW) | References | |||||
|---|---|---|---|---|---|---|---|
| Delphinidin | Cyanidin | Petunidin | Pelargonidin | Peonidin | Malvidin | ||
| Bilberry (Vaccinium mytillus) |
562.0–2913.0 | 488.0–955.0 | 437.0–705.0 | n.d. | 33.0–560.0 | 492.0–937.0 | [123,124,125] |
| Blueberry (Vaccinium corymbosum) |
405.0–768.0 | 82.8–379.0 | 294.8–319.0 | n.d. | 20.6–50.0 | 524.0–669.0 | [110,123,125,126,127] |
| Cranberry (Vaccinium oxycoccos) |
n.d.-10.8 | 13.2–313.0 | n.d.-10.0 | n.d.-185.3 | n.d.-310.0 | n.d.-25.0 | [110,123,126,127] |
| Lingonberry (Vaccinium vitis-idaea) |
n.d. | 19–769.0 | n.d. | n.d. | n.d.-6.0 | n.d | [124,126] |
| Gooseberry (Ribes uva-crispa) |
72.6–84.6 | 43.5–323.0 | n.d. | n.d. | n.d. | n.d | [126,128] |
| Black currant (Ribes nigrum) |
270.0–2940 | 166.5–1100.0 | n.d.-2.0 | n.d. | 8.0–110 | n.d.-180 | [123,125,128] |
| Red currant (Ribes xpallidum) |
n.d. | 360.0–217.0 | n.d. | n.d. | n.d. | n.d. | [123,126] |
| Red raspberry (Rubus idaeus) |
n.d. | 385.0–980.0 | n.d. | 9.0–660.0 | n.d. | n.d.-44.9 | [110,123,126] |
| Strawberry (Fragaria × ananassa) |
n.d. | 10.0–66.0 | n.d. | 162.0–336.4 | n.d. | n.d.-8.5 | [110,123,126,129] |
| Crowberry (Empetrum nigrum) |
430.0–1183.0 | 550.0–775.0 | 240.0–421.0 | n.d. | 220–1037.0 | 997.0–1550.0 | [123,126] |
n.d.: not detected.
Cyanidin-3-O-rutinoside and cyanidin-3-O-xylosylrutinoside were the main anthocyanins found in black raspberries [117], cyanidin-3-O-rutinoside and cyanidin-3-O-glucoside were the main anthocyanins found in sweet cherry [112,118], and delphinidin-3-O-galactoside was the main anthocyanin in blueberries [119]. The application of gibberellic acid, abscisic acid, and glycine-betaine at pre-harvest increased anthocyanin content in cherries [120]. In red raspberry cyanidin-3-O-sophoroside and cyanidin-3-O-glucoside were the main phenolics found [16,110,121]. Guiné et al. [93] found that red raspberry was also rich in ferulic acid, vanillic acid, and delphinidin-3-O-glucoside, while gooseberry was rich in chlorogenic acid and cyanidin-3-O-glucoside. Strawberry was shown to be rich in pelargonidin and ellagic acid [110,122].
Grapes are the main dietary source of anthocyanins. They are considered to have diverse biological properties and therefore are regarded as secondary metabolites with potential nutritional value. Anthocyanins are mainly localized in berry skin. Grape anthocyanins are the 3-O-monoglucosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin. Glucosylated derivatives of these anthocyanins, esterified at the C6 position of glucose with acetyl or coumaroyl groups have also been found, albeit at low concentrations. The monomeric anthocyanins in grape skin extracts were mainly malvidin (1.40–7.09 mg/g of skin), in particular, malvidin-3-glucoside (0.62–6.09 mg/g of skin) [40].
In cherry, extracts from stems of cv. Lapins and kernels of cv. Early Bigi presented high levels of total phenolics, flavonoids, and ortho-diphenols. In general, major phenolic compounds identified in stems and kernels were sakuranetin and catechin, respectively. Moreover, antioxidant activities showed a positive correlation with the increments in phenolic compounds [130].
During production, the correct combination of scion × rootstock can produce fruits with higher firmness, weight, sugars, vitamins, and phenolic compounds that boost the fruit’s antioxidant activity. Orchard management, such as applying drip irrigation and summer pruning, can also increase the total phenolic content, while application of growth regulators can result in improved storability, increased red coloring, increased fruit size, and reduced cracking. Salicylic acid, oxalic acid, acetylsalicylic acid, and methyl salicylate are promising growth regulators as they also increase total phenolics, anthocyanins, and induce the higher activity of antioxidant enzymes [131]. The application of biostimulants, such as glycine-betaine and Ascophyllum nodosum extracts increased polyphenols content and antioxidant capacity in cherries [132,133]. In grape, it was also observed that the application of chitosan on the whole vine before and after veraison led to the increased concentration of total phenolic compounds, anthocyanins, and tannins [134]. In this study, it was also observed that chitosan application not only induced the synthesis of phenolic compounds but also acted as a facilitator of phenolic transfer from leaves towards grape berries. Furthermore, the application of other foliar mitigation treatments (kaolin (5%) and potassium silicates (0.1 and 0.05%)) influenced the grape berry quality, namely the concentration of total anthocyanins and monomeric anthocyanins [135].
The tannin profiles of five grapes (Vitis vinifera L.) varieties were studied by Cosme et al. [136]. Depending on the grape variety, the polymeric fractions in skins represented 91–99% and the distribution of the mean degree of polymerization (mDP) of the skin proanthocyanidins ranged from 3.8 to 81.0.
According to data presented in Table 7, Table 8, Table 9 and Table 10, and many others reported in the literature, anthocyanins are the most important group of phenolics in red fruits, while flavonoids and phenolic acids are present in lower amounts. The most abundant anthocyanins are cyanidin and delphinidin, and in flavonoids are quercetin, kaempferol, and myricetin. Caffeic acid, p-coumaric acid, and ferulic acid are the most common phenolic acids found in red fruits.
Although it is common to find this profile in red fruits, the differences in their levels often found within the same genus and species are due to genotype, cultural practices, environmental conditions, fruit ripeness, and postharvest and storage conditions. Howard et al. [137] found that total phenolics, hydroxycinnamic acids, and anthocyanidins in blueberries can vary significantly between genotypes and between growing seasons, and from their point of view different genotypes should be always screened over multiple seasons to identify phenolic-rich germplasm. Similar results were achieved by Nour et al. [99] when they studied the variation in anthocyanins profile and content along with different genotypes of blackcurrant, and they report that genotype is one factor that significantly influences the content of phenolics. In addition, Vagiri et al. [98] found a significant variation in phenolics in blackcurrant due to genotypes, ontogenic stage, and location. In 2012, Aaby et al. [102] found a significant variation in phenolic profile and content in 27 cultivars of strawberries during ripening, which is a crucial stage of fruit development and has a crucial role in phenolic accumulation. Zhang et al. [138] studying the effect of maturation in the accumulation of anthocyanins in strawberry (Fragaria × ananassa Duch.), found that the major groups of compounds such as anthocyanins, amino acids, and sugars alter during growth and maturation. The same authors concluded that each stage of strawberry development has its unique metabolic profile, with the most drastic changes occurring at the transition toward the red-ripened stage. Beekwilder et al. [139] reported an increment in the level of anthocyanins during raspberry fruit ripening, while the levels of ellagitannins and proanthocyanidins decreased in the same period, probably due to differences in enzymatic activity of glycosyltransferases, which interfere in biosynthetic pathways of anthocyanins. Atkinson et al. [140] also found that cultural practices could interfere significantly with the content of phenolics. They found that using mulches in strawberry cultivation can increase the concentration of ellagic acid and ascorbic acid due to significant increments in light reflection. Vyas et al. [141] showed that environmental conditions had significant effects on the concentration of anthocyanins and proanthocyanidins in lingonberry, and the levels of these two types of antioxidant compounds were positively correlated with latitude, altitude, reduced temperature, and increased precipitation of the collection sites.
Another important set of factors that affects the content and availability of phenolics in red fruits are the post-harvest factors, which include the storage conditions and processing. Kozos et al. [142] found that storing blueberries in a controlled atmosphere for six weeks decreases the loss of anthocyanins compared to a normal atmosphere. The same authors found that to preserve the high quality of blueberries, the fruits must be cooled quickly after harvest and stored in a cold room with a controlled atmosphere. Also, Srivastava et al. [143] observed retention higher than 40% in ellagic acid and quercetin, two important antioxidant phenolics, when blueberries were stored at 1 °C for 35 days. Mullen et al. [144] observed an increment in ellagitannins of red raspberries when they were stored at 4 °C for 3 days. In addition, Ayala-Zavala [145] found that temperatures of 0 °C retained higher contents of antioxidant compounds in strawberries for longer periods than those stored at 5 °C or 10 °C.
The loss of phenolic content in fruits are expected when they are converted into juices or jams, due to the complexity of processing steps involved. In general, when fruits are converted into juices, a frozen or refrigeration step is followed by blanching, milling, depectinization, pressing, pasteurization, and if necessary clarification and concentration. All these steps can play a major role in phenolic degradation. For example, anthocyanin retention in cranberry juice is generally lower than 50% due to losses during various stages of processing [146]. Buchert et al. [147], comparing the effects of the usage of different enzyme preparations on anthocyanin composition of bilberry and blackcurrant juices during the juice preparation, observed that the enzyme activities of β-galactosidase, α-arabinosidase, and β-glucosidase varied significantly, and the presence of β-galactosidase resulted in complete loss of delphinidin, cyanidin, petunidin and malvidin galactosides in the juice. They also observed that the pressing step resulted in marked losses of anthocyanins due to physical removal of the anthocyanin-rich skins as well as the anthocyanins binding to cell wall polysaccharides. Furthermore, another study reported that frozen highbush blueberries (Vaccinium corymbosum) [148] showed a pronounced deterioration of phenolic compounds when frozen blueberries were processed into juice and concentrate. Nonetheless, Lee et al. [149] observed an increment of malvidin-glycosides and total anthocyanins increased by 51% and 60%, respectively, in pasteurized juices and concentrates compared to the fresh fruit, probably caused by the step of concentration. However, they noted that delphinidin-glycosides with pasteurization decreased by 26%. Pasteurization was found to exert a significantly destructive effect on the anthocyanin content of strawberry juice in 60–70% [150] due to the high temperatures used in this processing step. In jams, another way to process fruits the tendencies are similar to those observed in juices. Kim and Padilla-Zakour [151], studying the processing effect on phenolics and antioxidant capacity in anthocyanin-rich cherry, plum, and raspberry, found that the processing steps and heating during jam making decreased the contents of total phenolics by 48% for cherry, 57% for plum and 36% for raspberry. The content of total anthocyanins decreased 86% in cherry, 84% in plum, and 45% in raspberry [151]. Jam making generally involves fruit tissues disruption followed by heating under a high acidic environment to avoid any bacterial contaminations, resulting in considerable losses of anthocyanins [152]. However, some phenolics such as ellagic acid can increase between 4.6 fold in black raspberry [152] and 1.5 to 2.5 fold during the processing of raspberry [153] and strawberry [154] jams. This increase may be correlated to an easier extractability of ellagic acid in this heat and high acid environment [151].
Despite these variations, numerous studies demonstrated that various phytochemical constituents of red fruits exhibit a wide range of biological effects [84,155], including antioxidant activities, which will be addressed in point three of this work.
2.6. Aroma and Flavor Compounds
Due to the increased interest in the health benefits of small berry fruits, research in berries’ flavor quality has also increased. Volatiles found in small berries are diverse and give unique flavors to different fruits. Fruit flavor also depends upon taste (sweetness and sourness, and low or no astringency) and aroma (concentration of volatile organic compounds). Nevertheless, while the environment may alter the flavor quality of small fruits, genetic factors seem to determine the flavor profile quantitatively and qualitatively [156].
Strawberries (Fragaria × ananassa) are among the most appreciated fruits worldwide. The modern garden-strawberries varieties present largeness, beautiful red color, and extended shelf life. However, the sensory quality is often censured by consumers, as they seem deficient in flavor and fragrance [157].
Numerous volatiles, of several chemical natures, have been identified in ripe strawberries including alcohols, aldehydes, esters, furanones, ketones, and terpenes [158]. Wild strawberries, opposite to garden varieties, present intense flavor, and fragrance [159]. These varieties provide an appreciated source of volatile compounds for breeding new commercial strawberries with improved aroma [160]. One example is Fragaria moschata or musk strawberries, which are recognized for their well-known aroma.
At present, few musk strawberries survive in farm plantings. One example is the Italian clone ‘Profumata di Tortona’, considered one of the most fragrant strawberries [161]. The berries of this variety are characterized by an intense red color, a pale flesh, a delightful sour sweet taste, and astringent flavor, presenting aromas of caramel, mango, and tropical-fruits scent [158,162].
Ulrich and Olbricht [163,164] identified significant differences in the presence of individual esters, ketones, and terpenes between Fragaria × vesca samples and Fragaria × ananassa cultivars. Mainly in esters (ethyl hexanoate, methyl butanoate, and methyl hexanoate) that were found in higher concentrations in Fragaria × ananassa compared with wild samples. Contrariwise, the ester methyl anthranilate (2-aminobenzoic acid methyl ester), the aroma compound associated with strawberry aroma/flavor [165], presents the characteristic fragrance of wild strawberries with a fruity, concord grape, musty with a floral powdery nuance, was more abundant in Fragaria × vesca. Likewise, ketones (2-pentanone, 2-heptanone, and 2-nonanone) and terpenes (myrtenal, myrtenil acetate, α-terpineol) are present in higher levels in F. vesca, excepting the monoterpene linalool, more abundant in cultivated strawberries.
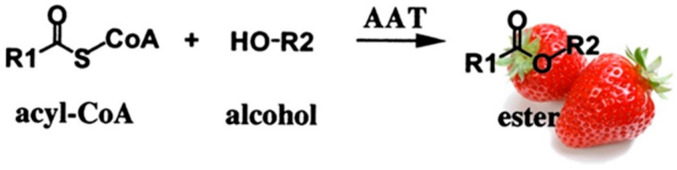
As it was mentioned before, esters are important volatile compounds in fruit flavor, and in strawberry, several esters have been identified [166]. The reaction of transacylation from acyl-CoA into alcohol is called esterification (Figure 4). The enzyme that catalyses the reaction is alcohol acyltransferase (AAT). Ueda et al. [167] concluded that, in strawberry, the alcohol moieties of the esters produced revealed the alcohols mainly synthesized in the fruit, and the acid moieties reflected the acyl-CoA specificity of the AAT enzyme. The strawberry AAT enzyme had a high activity with hexanol and with acetyl- or butyl-CoAs [168,169].
Figure 4.
Schematic representation of the esterification reaction catalyzed by the alcohol acyltransferase (ATT).
Recently, Negri et al. [159] identified 131 volatile compounds in ripe berries of ‘Profumata di Tortona’ and F. vesca cv. ‘Regina delle Valli’, a number exceeding the aroma compounds usually found in commercial strawberries. Moreover, 80 volatile compounds have been already identified by Schwieterman et al. [170] in 35 strawberry-garden varieties. In total, and according to Ulrich et al. [171], 979 volatile compounds were identified, 590 of which were found since 1997.
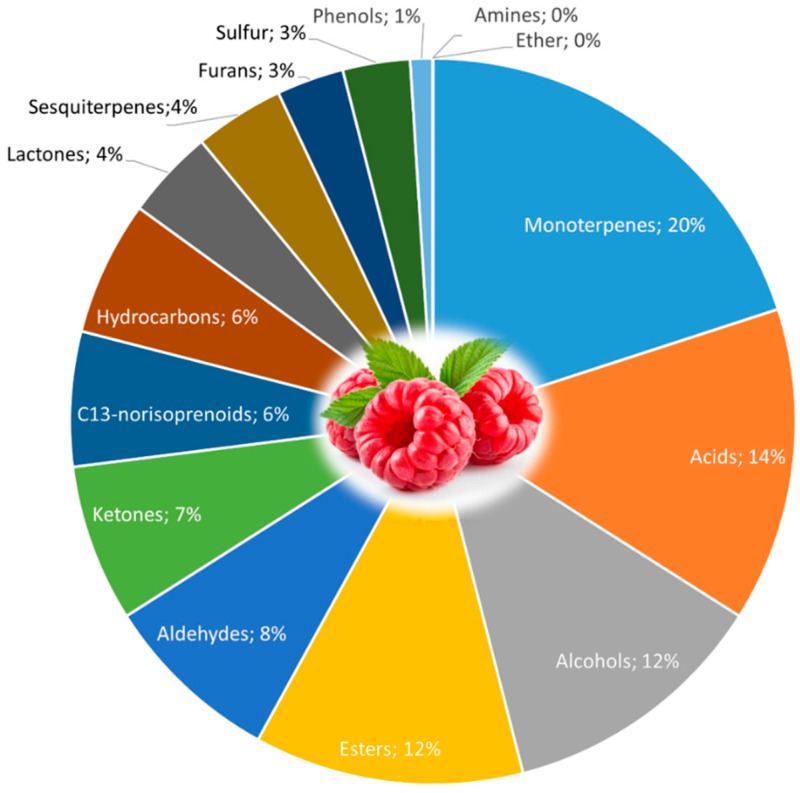
Raspberry volatiles are important for the perception of their sensory quality and mold resistance [172], Figure 5. As to other fruits, volatile compounds are influenced by numerous factors including ripeness, climate, soil, cultivar variation, among others [173].
Figure 5.
Volatile compounds in raspberry fruit (Rubus idaeus L.) and their chemical class. Adapted from [172].
The volatile compounds in raspberry are mostly free forms of different metabolites, most of them present in the form of glycoside bound to sugars, able to release free volatile compounds by enzymatic or chemical cleavage, the process that can occur during plant maturation, industrial treatments, and fruits processing [174].
Honkanen et al., in 1980 [175], identified a total of 75 volatile components in raspberry oil in the pressed juice. More than 40 were not reported previously. These comprised 2,5-dimethyl-4-methoxy-3(2H) furanone, 5-methyl-4-hydroxy-3(2H) furanone, 2,5-dimethyl-4-hydroxy-3(2H) furanone, terpenes, and a few esters including ethyl 5-hydroxyoctanoate and ethyl 5-hydroxydecanoate, that had not been identified in natural products [175].
The aroma of blackberry fruits affects the quality of either fresh or processed fruits. The volatile compounds responsible for the fruit flavor are biosynthesized through metabolic pathways during ripening, harvest, post-harvest, and storage periods. According to Georgilopoulos and Gallois [176], it seems that the aroma of blackberry juice is mainly due to the presence of furfural, 3-methyl-butanal, 3-methyl-1-butanol, phenylacetaldehyde, and trans-furan linalool oxide. However, Du and Qian [156] state that the most potentially important odor-active compounds in ‘Black Diamond’, a blackberry variety, are ethyl hexanoate, ethyl butanoate, 1-octen-3-one, 2-heptanol, cis-3-hexenol, linalool, nonanal, trans-2-hexenol, methional, ethyl 3-hydroxyhexanoate, α-ionone, β-ionone, furaneol, and 5-isoprenyl-2-dimethyl-divinyltetrahydrofuran.
Blueberry aroma depends on the interaction of dozens of volatile compounds [177]. Most research has been conducted, in recent years, with the aim of understanding the full complexity of blueberry flavor. For instance, Beaulieu et al., in 2014 [178], when studying five Louisiana-grown RAB cultivars (‘Brightwell’, ‘Climax’, ‘Premier’, ‘Powder Blue’, and ‘Tifblue’) found compounds such as linalool, methyl 3-methylbutanoate, 1,8-cineole, (E)-2-hexanal, (Z)-3-hexenal, (Z)-3-hexenyl acetate, limonene, hexyl acetate, hexanal, and α-terpineol. More recently, Farneti et al. [179] studied the intricacy of blueberry aroma by untargeted volatile organic compounds analysis, performed by solid-phase microextraction- gas chromatography-mass spectrometry (SPME-GC-MS) and proton transfer reaction—time of flight—mass spectrometry (PTR-ToF-MS). They found that the compounds usually considered responsible for blueberry aroma are synthesized by the fruit in the ripe stage. They are monoterpenes (such as linalool), (Z)-2-hexen-1-ol, and hexanal. Several compounds can be detected in fruits at the pink stage of ripening like the (E)-2-hexenal.
Hirvi et al., in 1981 [180], found a huge number of volatile compounds in cranberry samples. A total of 70 components were identified in cranberries from two continents, Europe and America. The aroma of cranberries is characterized by the presence of several aromatic metabolites together with α-terpineol (terpinolene, p-mentha-1,8-diene, thujene, p-mentha-1,8-diol, carvenone, 1-phenylethanol, 3-phenylpropan-1-ol, trans-cinnamyl alcohol, 2-(4-hydroxyphenyl)-ethanol, 2-(4-methoxyphenyl) ethanol, salicylaldehyde, 4-methoxybenzaldehyde, 3-phenylpropanal, trans-cinnamaldehyde, vanillin, 4-hydroxyacetophenone, 4-methoxyacetophenone, 4-(4-hydroxphenyl) butan-2-one, 4-(4-hydroxy-3-methoxyphenyl) butan-2-one, methyl cinnamate, 2-phenylethyl formate, and some γ-and α-lactones. Nevertheless, not all these compounds are aromatic in the sense that they can be perceived by the human nose.
When considering the potential contribution of a volatile compound to the aroma of fruit, the determination of the odor-activity value (OAV) and odor detection threshold is of extreme importance. An OAV is used to estimate odor potency in terms of the ratio of the concentration of a volatile compound to its odor detection threshold. In 2016, Zhu et al. [181] identified several compounds and also studied their OAVs. They found that the hexanal, pentanal, (E)-2-heptenal, (E)-2-hexenal, (E)-2-octenal, (E)-2-nonenal, ethyl 2-methylbutyrate, β-ionone, 2-methylbutyric acid, and octanal contributed greatly to the cranberry aroma. So, we can say that the list proposed by Zhu et al. [181], presents the true form of cranberry aroma/flavor.
3. Red Fruits: Consumers’ Perception of Health Benefits
The consumer’s quality perception can be influenced by the fruit’s intrinsic attributes, but also by extrinsic indicators provided by the seller of the fruit; like brands, product origin, or quality labels [182]. According to Grunert [183], consumers are interested in the benefits of fruit consumption and how it can help them attain their values. This means that consumers, besides judging the fruit quality, also ponder if the specific fruit properties are desirable and likely to fulfill their preferences and needs by linking the fruit characteristics to self-relevant consequences and personal values [184]. Recent studies have mentioned pleasure-seeking as the primary motivation for fruit consumption [185]. People seem to enjoy looking at, handling and selecting the fruits they want to eat. Freshness, firmness, size, color, and flavor are important attributes for fruit selection, and visually attractive fruits are likely to act as a promoter of purchase and consumption intentions [186]. Although overall fruit quality is the key factor behind purchase decisions [187], the price cannot be discarded as part of the decision process by consumers [188,189]. Availability, packaging, convenience, and brand are other search determinants often cited in the literature to affect consumer food choices [190,191,192,193]. Furthermore, consumers still see a link between healthier food and less-than-optimal sensory attributes [185,194], which can lead to a lower intake of healthier foods, although some consumer segments characterized by older people and women are willing to trade taste for health benefits [195].
Plant science research has been primarily focused on the increase in production, with health benefits being a minor concern. However, consumers’ awareness of the health effect of fruit intake against chronic diseases makes this a major topic for discussion and research [196]. The food industry is currently adapting its market trends to accommodate sustainability values, especially those related to health benefits, as they are increasingly searched by the consumers [197], based, for fruits, on the phytonutrients present in these foods [196].
The current use of phytonutrients by food producers and the knowledge of their effect on the prevention of chronic disease points out the need for a careful look at crop production strategies (fertilization, season, soil fertility, and irrigation) affecting the quantitative and qualitative profiles of these compounds, but also to post-harvest techniques (processing or packaging) that can modify phytonutrients [196]. There is mounting evidence of the potential health benefits of a fruit-rich diet. The ingestion of phytochemicals from fruits and their positive influence on several diseases (cancer, heart disease, stroke, hypertension, birth defects, cataracts, diabetes, diverticulosis, and obesity) were established [197,198,199]. There are many phytochemicals present in fruits that can be responsible for their health-promoting activities. Vitamins, carotenoids, phenolic acids, trihydroxystilbenes, or flavonoids, are often associated with the prevention of certain cancers and cardiovascular diseases, but also phytoestrogens, organosulfur compounds, fiber, or isothiocyanates (reviewed by [200,201]). The majority of small fruits and grapes have a high antioxidant activity, and present in their composition several compounds of interest [202,203].
In cherry, phenolic extracts inhibited low-density lipoprotein oxidation in vitro in a dose-dependent manner. Extracts of freshly harvested cherries exhibited significantly higher antioxidant activities than extracts of stored samples at cold temperature. The cv. Summit samples had the highest antioxidant activity. Differences in the antioxidant effects of the cherry samples were positively correlated with their levels of p-coumaroylquinic acid (p < 0.1) but negatively correlated with their cyanidin-3-O-rutinoside levels (p < 0.05) [113].
There are still many studies to be conducted for the advancement of functional foods into the diet routines of consumers. Although there has been a strong emphasis on showing how fruits can prevent diseases and increase health, rather than just providing nutrients, further research has to focus on how to effectively inform consumers of nutrition and health properties. For instance, although it appears that 87% of Americans believe that certain foods have health benefits, with fruits recognized as the top food category with health benefits [204], other authors point out the lack of awareness of consumers to such facts [205]. An increase in consumers’ knowledge of the health benefits of fruit consumption will, most likely, increase the intake of fruits, allowing their long-term marketplace success [206], with clear benefits, not only for the consumer’s health but also for producers and sellers. Some works point out that an increase in the public awareness of the link between diet and health can be responsible for higher per capita consumption of fruits [201] (see Figure 6).
Figure 6.
Health effects of red fruits.
In the past few years, many studies have shown that phenolics and other molecules present in red fruits have different bioactive properties, including antioxidant activity. The antioxidant activity of red fruits and their bioactive molecules, including phenolics have been assessed using in vitro assays, measuring its ability to reduce and trap free radicals and reactive oxidant species (ROS) produced from a wide range of physiological processes. Different methods such as oxygen radical absorbance capacity (ORAC), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) or ferric reducing antioxidant power assay (FRAP) [155,207], among others, have been used to assess this capacity. The results from many authors have shown that red fruits have high levels of antioxidant activity due to their high content of phenolics and vitamin C, among other compounds. Some examples of the antioxidant capacity of red fruits are shown in Table 11.
Table 11.
Some examples of antioxidant activity values of red fruits by ORAC method.
| Red Fruits | Antioxidant Activity | References |
|---|---|---|
| ORAC (µmol Trolox/g FW) | ||
| Bilberry (Vaccinium myrtillus) | 14.4–122.7 | [90,208] |
| Blueberry (Vaccinium corymbosum) | 10.3–51.9 | [108,209,210] |
| Cranberry (Vaccinium oxycoccos) | 18.5–96.8 | [211,212,213] |
| Lingonberry (Vaccinium vitis-idaea) | 38.1 | [211] |
| Gooseberry (Ribes uva-crispa) | 17.0–41.5 | [214] |
| Black currant (Ribes nigrum) | 36.9–93.1 | [214] |
| Red currant (Ribes xpallidum) | 1.27–32.6 | [213] |
| Red raspberry (Rubus idaeus) | 7.8–45.2 | [108,214,215] |
| Strawberry (Fragaria × ananassa) | 20.2–22.1 | [108,216] |
These and other results from antioxidant activity assays performed by many authors have shown that red fruits can serve as a good natural source of bioactive and antioxidant molecules in the human diet, due to the high contents of anthocyanins, other flavonoids, and phenolic acids. For example, Tulipani et al. [217] found that eating strawberries (500 g every day) for 16 consecutive days, increased the plasma total antioxidant capacity (TAC) and the antihaemolytic defenses of human erythrocytes due to its high content of anthocyanins and vitamin C. Vasileiou et al. [218], reported that regular consumption of cranberries could prevent bacterial adherence to uroepithelial cells, which reduces the development of urinary tract infections, which could be related to its richness of phenolics, mainly anthocyanins. Riso et al. [219] found that the consumption of blueberries-based drinks with high content of anthocyanins for six weeks, reduced significantly the levels of oxidized DNA bases and increased the resistance to oxidatively induced DNA damage. In a study with 12 colored berries including black currants, blueberries, and red and black raspberries [220], it was found a strong correlation between the phenolic composition and the high levels of antioxidant activity. The same authors have concluded that phenolic components may be used directly to evaluate the potential health benefits of berries and other types of fruits.
Also, several diseases are related to a diet low in certain minerals. Low levels of potassium intake are reflected in higher blood pressure [221], higher risk of developing kidney stones since the acid-base metabolism is positively affected by potassium [222]. The heartbeat and muscle contraction are also affected by the level of potassium in the body [223]. Calcium is important for bones and tooth formation and decreases the risk of osteoporosis [224]. The magnesium levels in the body affect protein synthesis, muscle contraction, body temperature regulation, and the activation of moreover 100 enzymes [6]. Skeletal mineralization and some cellular functions such as phospholipid synthesis and intracellular regulatory, glycolysis, gluconeogenesis, DNA and RNA synthesis, cellular protein phosphorylation, depend on the presence of the inorganic phosphate [28]. On the other hand, the biosynthesis of amino acids is related to the presence of nitrogen. Even so, this mineral is also an essential component of nucleic acids, cofactors, and other metabolites [6].
Indeed, food choice is one of the most frequent human decisions and is determined by a complex set of factors and interrelated determinants [225]. Although several models attempting to explain that process have been proposed, one of the most accepted is the proposed Total Food Quality Model [226]. This model can be divided into three parameters: ‘search’, ‘experience’ and ‘credence’ attributes. The first two (search attributes, like appearance or price, and experience characteristics, like flavor or taste) are those more easily observed by consumers and can be straightforwardly experienced by them. For credence properties, like health and nutritional benefits, the consumer cannot validate those claims [227]. This must be where most effort should be employed; the increase in the consumers’ awareness of the health benefits of the intake of fruits, always considering the latest scientific progress and keeping in mind that these credence properties are a matter of credibility and trust that cannot be broken.
Hence, all encouraging actions that increase the intake in those fruits, and, therefore, of health-promoting compounds, are of great importance. This is even more important in our current society, where the fast food supply is large, widely available, and more easily responds to the fast-paced life of consumers, with nutritionally poor foods taking the place of a healthier diet.
4. Final Remarks
Fruits, especially small fruits, are rich in bioactive compounds such as vitamins and phenolic compounds, with well-demonstrated potential health benefits. However, we are at the beginning of acquiring knowledge to understand the variation in these compounds throughout the fruit development and the postharvest period. How climate change and innovative crop production technology affect plant performance, yield, and fruit quality is at the moment largely unknown. Further research should be aimed to develop species-specific strategies that improve both fruit quality and nutritional properties, without significantly affecting the yield. New fruit species and cultivars with improved traits are needed to produce fruits with excellent quality, and a high consumer appeal, that is well adapted to the different growing regions and future climate change.
Acknowledgments
The authors would like to thank the Chemistry Research Centre-Vila Real (CQ-VR) and CITAB/Inov4Agro Center for the Research and Technology of Agro-Environmental and Biological Sciences/Institute for Innovation, Capacity Building, and Sustainability of Agri-Food Production for their financial support.
Author Contributions
Conceptualization, F.C. and B.G.; writing—original draft preparation, F.C., T.P., A.A., M.C.M., I.O., A.V., B.G. Writing—review and editing, F.C., T.P., A.A., M.C.M., E.B., R.A., J.F.-C., I.O., A.V., B.G.; Visualization, A.V., B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds FCT Portuguese Foundation for Science and Technology-Portugal and COMPETE under the projects UIDB/00616/2020, UIDP/00616/2020, and UIDB/04033/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skrovankova S., Sumczynski D., Mlcek J., Jurikova T., Sochor J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015;16:24673–24706. doi: 10.3390/ijms161024673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Souza V.R., Pereira P.A., Da Silva T.L., Lima L.C.O., Pio R., Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- 3.Gomes-Rochette N.F., Da Silveira Vasconcelos M., Nabavi S.M., Mota E.F., Nunes-Pinheiro D.C., Daglia M., De Melo D.F. Fruit as potent natural antioxidants and their biological effects. Curr. Pharm. Biotechnol. 2016;17:986–993. doi: 10.2174/1389201017666160425115401. [DOI] [PubMed] [Google Scholar]
- 4.Olas B. Berry phenolic antioxidants—Implications for human health? Front. Pharmacol. 2018;9:78. doi: 10.3389/fphar.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Ahuja J.K.C., Burton-Freeman B.M. Characterization of the nutrient profile of processed red raspberries for use in nutrition labeling and promoting healthy food choices. Nutr. Healthy Aging. 2019;5:225–236. doi: 10.3233/NHA-190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicente A.R., Manganaris G.A., Sozzi G.O., Crisosto C.H. Nutritional quality of fruits and vegetables. In: Florkowski W.J., Shewfelt R.L., Brueckner B., Prussia S.E., editors. Postharvest Handling: A Systems Approach. 2nd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2009. pp. 57–106. [DOI] [Google Scholar]
- 7.Badjakov I., Nikolova M., Gevrenova R., Kondakova V., Todorovska E., Atanassov A. Bioactive compounds in small fruits and their influence on human health. Biotechnol. Biotechnol. Equip. 2008;22:1581–1587. doi: 10.1080/13102818.2008.10817517. [DOI] [Google Scholar]
- 8.Jaglan P., Buttar H.S., Al-bawareed O.A., Chibisov S. Potential health benefits of selected fruits: Apples, blueberries, grapes, guavas, mangos, pomegranates, and tomatoes. In: Singh R.B., Watanabe S., Isaza A.A., editors. Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases. Academic Press; San Diego, CA, USA: 2022. pp. 359–370. Chapter 24. [DOI] [Google Scholar]
- 9.Huang Y., Park E., Edirisinghe I., Burton-Freeman B.M. Maximizing the health effects of strawberry anthocyanins: Understanding the influence of the consumption timing variable. Food Funct. 2016;7:4745–4752. doi: 10.1039/C6FO00995F. [DOI] [PubMed] [Google Scholar]
- 10.Serrano M., Guillen F., Martinez-Romero D., Castillo S., Valero D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005;53:2741–2745. doi: 10.1021/jf0479160. [DOI] [PubMed] [Google Scholar]
- 11.Pelayo-Zaldivar C., Ebeler S., Kader A. Cultivar and harvest date effects on flavor and other quality attributes of California strawberries. J. Food Qual. 2005;28:78–97. doi: 10.1111/j.1745-4557.2005.00005.x. [DOI] [Google Scholar]
- 12.Keutgen A.J., Pawelzik E. Quality and nutritional value of strawberry fruit under long term salt stress. J. Agric. Food Chem. 2008;107:1413–1420. doi: 10.1016/j.foodchem.2007.09.071. [DOI] [Google Scholar]
- 13.Usenik V., Fabcic J., Stampar F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.) Food Chem. 2008;107:185–192. doi: 10.1016/j.foodchem.2007.08.004. [DOI] [Google Scholar]
- 14.Faniadis D., Drogoudi P.D., Vasilakakis M. Effects of cultivar, orchard elevation, and storage on fruit quality characters of sweet cherry (Prunus avium L.) Sci. Hortic. 2010;125:301–304. doi: 10.1016/j.scienta.2010.04.013. [DOI] [Google Scholar]
- 15.Pinto T., Vilela A., Pinto A., Nunes F.M., Cosme F., Anjos R. Influence of cultivar and of conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus) J. Sci. Food Agric. 2018;98:4616–4624. doi: 10.1002/jsfa.8990. [DOI] [PubMed] [Google Scholar]
- 16.Anjos R., Cosme F., Gonçalves A., Nunes F.M., Vilela A., Pinto T. Effect of agricultural practices, conventional vs organic, on the phytochemical composition of ‘Kweli’ and ‘Tulameen’ raspberries (Rubus idaeus L.) Food Chem. 2020;2020:126833. doi: 10.1016/j.foodchem.2020.126833. [DOI] [PubMed] [Google Scholar]
- 17.Nile S.H., Park S.W. Edible berries: Bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Amaya D.B. A Guide to Carotenoid Analysis in Foods. ILSI Press; Washington, DC, USA: 2001. [Google Scholar]
- 19.Jabłońska-Ryś E., Zalewska-Korona M., Kalbarczyk J. Antioxidant Capacity, Ascorbic Acid and Phenolics Content in Wild Edible Fruits. J. Fruit Ornam. Plant Res. 2009;17:115–120. [Google Scholar]
- 20.Stewart D., McDougall G.J., Sungurtas J., Verrall S., Graham J., Martinussen I. Metabolomic approach to identifying bioactive compounds in berries: Advances toward fruit nutritional enhancement. Mol. Nutr. Food Res. 2007;51:645–651. doi: 10.1002/mnfr.200700056. [DOI] [PubMed] [Google Scholar]
- 21.Rudy S., Dziki D., Krzykowski A., Gawlik-Dziki U., Polak R., Róžiło R., Kulig R. Influence of pre-treatments and freeze-drying temperature on the process kinetics and selected physico-chemical properties of cranberries (Vaccinium macrocarpon Ait.) LWT-Food Sci. Technol. 2015;63:497–503. doi: 10.1016/j.lwt.2015.03.067. [DOI] [Google Scholar]
- 22.Maki K.C., Kaspar K.L., Khoo C., Derrig L.H., Scild A.L., Gupta K. Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am. J. Clin. Nutr. 2016;103:1434–1442. doi: 10.3945/ajcn.116.130542. [DOI] [PubMed] [Google Scholar]
- 23.Dorofejeva K., Rakcejeva T., Galoburda R., Dukalska L., Kviesis J. Vitamin C content in Latvian cranberries dried in convective and microwave vacuum driers. Procedia Food Sci. 2011;1:433–440. doi: 10.1016/j.profoo.2011.09.067. [DOI] [Google Scholar]
- 24.Sinelli N., Spinardi A., di Egidio V., Mignani I., Casiraghi E. Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy. Postharvest Biol. Technol. 2008;50:31–36. doi: 10.1016/j.postharvbio.2008.03.013. [DOI] [Google Scholar]
- 25.Harb J., Khraiwesh B., Streif J., Reski R., Frank W. Characterization of blueberry monodehydroascorbate reductase gene and changes in levels of ascorbic acid and the antioxidative capacity of water soluble antioxidants upon storage of fruits under various conditions. Sci. Hortic. 2010;125:390–395. doi: 10.1016/j.scienta.2010.04.031. [DOI] [Google Scholar]
- 26.Paes J., Dotta R., Barbero G.F., Martínez J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids. 2014;95:8–16. doi: 10.1016/j.supflu.2014.07.025. [DOI] [Google Scholar]
- 27.Gündüz K., Serçe S., Hancock J.F. Variation among highbush and rabbit eye cultivars of blueberry for fruit quality and phytochemical characteristics. J. Food Compos. Anal. 2015;38:69–79. doi: 10.1016/j.jfca.2014.09.007. [DOI] [Google Scholar]
- 28.Nemzer B., Vargas L., Xia X., Sintara M., Feng H. Phytochemical and physical properties of blueberries, tart cherries, strawberries, and cranberries as affected by different drying methods. Food Chem. 2018;262:242–250. doi: 10.1016/j.foodchem.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 29.Lin P.-H., Aickin M., Champagne C., Craddick S., Sacks F.M., McCarron P., Most-Windhauser M.M., Rukenbrod F., Haworth L. Food group sources of nutrients in the dietary patterns of the DASH-Sodium trial. J. Am. Diet. Assoc. 2003;103:488–496. doi: 10.1053/jada.2003.50065. [DOI] [PubMed] [Google Scholar]
- 30.USDA-ARS (US Department of Agriculture, Agricultural Research Service) USDA Nutrient Database for Standard Reference, Release 25, Software 1.2.2, from the Nutrient Data Laboratory. [(accessed on 28 December 2021)]; Available online: http://www.nal.usda.gov/fnic/foodcomp.
- 31.Hakala M., Lapvetelainen A., Houpalahti Kallio H., Tahvonen R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compost. Anal. 2003;16:67–80. doi: 10.1016/S0889-1575(02)00165-5. [DOI] [Google Scholar]
- 32.Mikulic-Petkovsek M., Schmitzer V., Slatnar A., Stampar F., Veberic R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012;77:10. doi: 10.1111/j.1750-3841.2012.02896.x. [DOI] [PubMed] [Google Scholar]
- 33.Viljakainen S., Visti A., Laakso S. Concentrations of organic acids and soluble sugars in juices from Nordic berries. Acta Agric. Scand. B Soil Plant Sci. 2010;52:101–109. doi: 10.1080/090647102321089846. [DOI] [Google Scholar]
- 34.Forney C.F., Kalt W., Jordan J.A., Vinqvist-Tymchuk M.R., Fillmore S.A.E. Compositional changes in blueberry and cranberry fruit during ripening. Acta Hortic. 2012;926:331–337. doi: 10.17660/ActaHortic.2012.926.46. [DOI] [Google Scholar]
- 35.Cekic C., Ozgen M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.) J. Food Compost. Anal. 2010;23:540–544. doi: 10.1016/j.jfca.2009.07.002. [DOI] [Google Scholar]
- 36.Zhang J., Nie J.-Y., Li J., Zhang H., Li Y., Farooq S., Bacha S.A.S., Wang J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020;19:2352–2361. doi: 10.1016/S2095-3119(20)63236-1. [DOI] [Google Scholar]
- 37.Correia S., Queirós F., Ribeiro C., Vilela A., Aires A., Barros A.I., Schouten R., Silva A.P., Gonçalves B. Effects of calcium and growth regulators on sweet cherry (Prunus avium L.) quality and sensory attributes at harvest. Sci. Horti. 2019;248:231–240. doi: 10.1016/j.scienta.2019.01.024. [DOI] [Google Scholar]
- 38.Urün I., Attar S.H., Sönmez D.A., Gündesli M.A., Ercişli S., Kafkas N.E., Bandić L.M., Duralija B. Comparison of polyphenol, sugar, organic acid, volatile vompounds, and antioxidant capacity of commercially grown strawberry cultivars in Turkey. Plants. 2021;10:1654. doi: 10.3390/plants10081654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morais M.C., Mucha Â., Ferreira H., Gonçalves B., Bacelar E., Marques G. Comparative study of plant growth-promoting bacteria on the physiology, growth and fruit quality of strawberry. J. Sci. Food Agric. 2019;99:5341–5349. doi: 10.1002/jsfa.9773. [DOI] [PubMed] [Google Scholar]
- 40.Costa E., Cosme F., Jordão A.M., Mendes-Faia A. Anthocyanin profile and antioxidant activity from 24 grape varieties cultivated in two Portuguese wine regions. OENO One. 2014;48:51–62. doi: 10.20870/oeno-one.2014.48.1.1661. [DOI] [Google Scholar]
- 41.Costa E., Cosme F., Rivero-Pérez M.D., Jordão A.M., González-SanJosé M.L. Influence of wine region provenance on phenolic composition, antioxidant capacity and radical scavenger activity of traditional Portuguese red grape varieties. Eur. Food Res. Technol. 2015;241:61–73. doi: 10.1007/s00217-015-2434-x. [DOI] [Google Scholar]
- 42.Milivojevic J.M., Nikolic M.D., Maksimovic J.J.D., Radivojevic D.D. Generative and fruit quality characteristics of primocane fruiting red raspberry cultivars. Turk. J. Agric. For. 2011;35:289–296. doi: 10.3906/tar-1001-617. [DOI] [Google Scholar]
- 43.Li J., Zhang C., Liu H., Liu J., Jiao Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020;2020:7236534. doi: 10.1155/2020/7236534. [DOI] [Google Scholar]
- 44.Lee J., Kim H.-B., Noh Y.H., Min S.R., Lee H.S., Jung J., Park K.H., Kim D.S., Nam M.H., Kim T.I., et al. Sugar content and expression of sugar metabolism-related gene in strawberry fruits from various cultivars. J. Plant Biotechnol. 2018;45:90–101. doi: 10.5010/JPB.2018.45.2.090. [DOI] [Google Scholar]
- 45.Correia S., Gonçalves B., Aires A., Silva A., Ferreira L., Carvalho R., Fernandes H., Freitas C., Carnide V., Silva A.P. Effect of harvest year and altitude on nutritional and biometric characteristics of blueberry cultivars. J. Chem. 2016;2016:8648609. doi: 10.1155/2016/8648609. [DOI] [Google Scholar]
- 46.Ha M.A., Jarvis M., Mann J. A definition for dietary fibre. Eur. J. Clin. Nutr. 2000;54:861–864. doi: 10.1038/sj.ejcn.1601109. [DOI] [PubMed] [Google Scholar]
- 47.Institute of Medicine . Dietary Reference Intakes Proposed Definition of Dietary Fiber. A Report of the Panel on the Definition of Dietary Fiber and The Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board, National Academy Press; Washington, DC, USA: 2001. [DOI] [Google Scholar]
- 48.Anita F.P., Abraham P. Clinical Dietetics and Nutrition. Delhi Oxford University Press; Calcutta, India: 1997. pp. 73–77. [DOI] [Google Scholar]
- 49.Fayet-Moore F., Cassettari T., Tuck K., McConnell A., Petocz P. Dietary fibre intake in Australia. Paper II: Comparative examination of food sources of fibre among high and low fibre consumers. Nutrients. 2018;10:1223. doi: 10.3390/nu10091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson J.W., Baird P., Davis R.H., Jr., Ferreri S., Knudtson M., Koraym A., Waters V., Williams C.L. Health benefits of dietary fibre. Nutr. Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 51.Chau C.F., Huang Y.L. Comparison of the chemical composition and physicochemical properties of different fibres prepared from peel of the Citrus sinensis L. Cv. Liucheng. J. Agric. Food Chem. 2003;51:2615–2618. doi: 10.1021/jf025919b. [DOI] [PubMed] [Google Scholar]
- 52.Terry P., Giovannucci E., Michels K.B., Bergkvist L., Hansen H., Holmberg L., Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Nat. Canc. Instit. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 53.Marlett J.A., McBurney M.I., Slavin J.L. Position of the American Dietetic Association health implications of dietary fiber. J. Am. Diet. Assoc. 2002;102:993–1000. doi: 10.1016/S0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 54.Tutelyan V.A. Norms of physiological needs in energy and nutrients for different groups of the population of the Russian Federation. Vopr. Pitan. 2009;78:4–15. [PubMed] [Google Scholar]
- 55.Akimov M.Y., Koltsov V.A., Zhbanova E.V., Akimova O.M. Nutritional value of promising raspberry varieties. IOP Conf. Ser. Earth Environ. Sci. 2021;640:022078. doi: 10.1088/1755-1315/640/2/022078. [DOI] [Google Scholar]
- 56.U.S. Department of Health and Human Services and U.S. Department of Agriculture Dietary Guidelines for Americans 2015–2020. [(accessed on 31 August 2018)]; Available online: http://health.gov/dietaryguidelines/2015/guidelines/
- 57.Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Padayachee A., Day L., Howell K., Gidley M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017;57:59–81. doi: 10.1080/10408398.2013.850652. [DOI] [PubMed] [Google Scholar]
- 59.Pacifico S., Di Maro A., Petriccione M., Galasso S., Piccolella S., Di Giuseppe A.M.A., Scortichini M., Monaco P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014;64:188–199. doi: 10.1016/j.foodres.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 60.Kafkas E., Gunaydin S., Ercisli S., Ozogu Y., Unlu M.A. Fat and fatty acid composition of strawberry cultivars. Chem. Nat. Compd. 2009;45:861–863. doi: 10.1007/s10600-010-9489-5. [DOI] [Google Scholar]
- 61.Kafkas E., Ozgen M., Ozogul Y., Turemis N. Phytochemical and fatty acid profile of selected red raspberry cultivars: A comparative study. J. Food Qual. 2008;31:67–78. doi: 10.1111/j.1745-4557.2007.00184.x. [DOI] [Google Scholar]
- 62.Fadavi A., Barzegar M., Azizi M.H. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J. Food Compos. Anal. 2006;19:676–680. doi: 10.1016/j.jfca.2004.09.002. [DOI] [Google Scholar]
- 63.Al Juhaimi F., Geçgel Ü., Gülcü M., Hamurcu M., Özcan M.M. Bioactive properties, fatty acid composition and mineral contents of grape seed and oils. S. Afr. J. Enol. Vitic. 2017;38:103–108. doi: 10.21548/38-1-1042. [DOI] [Google Scholar]
- 64.Melgarejo P., Artés F. Total lipid content and fatty acid composition of oilseed from lesser known sweet pomegranate clones. J. Sci. Food Agric. 2000;80:1452–1454. doi: 10.1002/1097-0010(200008)80:10<1452::AID-JSFA665>3.0.CO;2-L. [DOI] [Google Scholar]
- 65.Al-Maiman S.A., Ahmad D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 2002;76:437–441. doi: 10.1016/S0308-8146(01)00301-6. [DOI] [Google Scholar]
- 66.Wang C.Y., Wang S.Y. Effect of storage temperatures on fruit quality of various cranberry cultivars. Acta Hortic. 2009;810:853–862. doi: 10.17660/ActaHortic.2009.810.114. [DOI] [Google Scholar]
- 67.Zafra-Rojas Q., Cruz-Cansino N., Delgadillo-Ramírez A., Alanis E., Añorve-Morga J., Quintero-Lira A., Castañeda A., Ramírez-Moreno E. Organic acids, antioxidants, and dietary fiber of Mexican blackberry (Rubus fruticosus) residues cv. Tupy. J. Food Qual. 2018;1:5950761. doi: 10.1155/2018/5950761. [DOI] [Google Scholar]
- 68.US Department of Agriculture USDA National Nutrient Database for Standard Reference, Release April 2018. [(accessed on 26 November 2018)]; Available online: https://ndb.nal.usda.gov/ndb/search/list.
- 69.Pereira de Melo I., Carvalho E., Filho J. Pomegranate seed oil (Punica granatum L.): A source of punicic acid (conjugated α-linolenic acid) J. Hum. Nutr. Food Sci. 2014;2:1024. [Google Scholar]
- 70.Dziadek K., Kopeć A., Czaplicki S. The petioles and leaves of sweet cherry (Prunus avium L.) as a potential source of natural bioactive compounds. Eur. Food Res. Technol. 2018;244:1415–1426. doi: 10.1007/s00217-018-3055-y. [DOI] [Google Scholar]
- 71.Bastos C., Barros L., Dueñas M., Calhelha R.C., Queiroz M.J.R.P., Santos-Buelga C., Ferreira I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015;173:1045–1053. doi: 10.1016/j.foodchem.2014.10.145. [DOI] [PubMed] [Google Scholar]
- 72.Pieszka M., Migdał W., Gąsior R., Rudzińka M., Bederska-Łojewska D., Pieszka M., Szczurek M. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015;2015:659541. doi: 10.1155/2015/659541. [DOI] [Google Scholar]
- 73.Shinagawa F.B., Santana F.C., Araujo E., Purgatto E., Mancini-Filho J. Chemical composition of cold pressed Brazilian grape seed oil. Food Sci. Technol. 2018;38:164–171. doi: 10.1590/1678-457x.08317. [DOI] [Google Scholar]
- 74.Parker T.D., Adams D.A., Zhou K., Harris M., Yu L. Fatty acid composition and oxidative stability of cold-pressed edible seed Oils. J. Food Sci. 2003;68:1240–1243. doi: 10.1111/j.1365-2621.2003.tb09632.x. [DOI] [Google Scholar]
- 75.Fernandes L., Pereira J.A., Lopéz-Cortés I., Salazar Domingo M., Ramalhosa E., Casal S. Lipid composition of seed oils of different pomegranate (Punica granatum L.) cultivars from Spain. Int. J. Food Stud. 2015;4:95–103. doi: 10.7455/ijfs/4.1.2015.a8. [DOI] [Google Scholar]
- 76.Celik F., Ercisli S. Lipid and fatty acid composition of wild and cultivated red raspberry fruits (Rubus idaeus L.) J. Med. Plants Res. 2009;3:583–585. [Google Scholar]
- 77.Li Q., Wang J., Shahidi F. Chemical characteristics of cold-pressed blackberry, black raspberry, and blueberry seed oils and the role of the minor components in their oxidative stability. J. Agric. Food Chem. 2016;64:5410–5416. doi: 10.1021/acs.jafc.6b01821. [DOI] [PubMed] [Google Scholar]
- 78.Luginina E.A., Egoshina T.L. Biochemical composition of fruits of wild growing berry plants. In: Weisfeld L., Opalko A.I., Bekuzarova S.A., editors. Temperate Horticulture for Sustainable Development and Environment: Ecological Aspects. Apple Academic Press Inc.; New York, NY, USA: 2018. 20p [Google Scholar]
- 79.Nagy K., Tiuca I.-D. Importance of fatty acids in physiopathology of human body. [(accessed on 28 August 2018)];IntechOpen. 2017 doi: 10.5772/67407. Available online: https://www.intechopen.com/books/fatty-acids/importance-of-fatty-acids-in-physiopathology-of-human-body. [DOI] [Google Scholar]
- 80.Dominguez L.J., Barbagallo M. Not All Fats Are Unhealthy. In: Sánchez-Villegas A., Sánchez-Tainta A., editors. The Prevention of Cardiovascular Disease through the Mediterranean Diet. Academic Press; London, UK: 2018. pp. 35–58. [Google Scholar]
- 81.Ander B.P., Dupasquier C.M., Prociuk M.A., Pierce G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003;8:164–172. [PMC free article] [PubMed] [Google Scholar]
- 82.Sanjeev P., Chaudhary D.P., Sreevastava P., Saha S., Rajenderan A., Sekhar J.C., Chikkappa G.K. Comparison of fatty acid profile of specialty maize to normal maize. J. Am. Oil Chem. Soc. 2014;91:1001–1005. doi: 10.1007/s11746-014-2429-y. [DOI] [Google Scholar]
- 83.Johnson M., Bradford C. Omega-3, Omega-6 and Omega-9 fatty acids: Implications for cardiovascular and other diseases. J. Glycom. Lipidom. 2014;4:123. doi: 10.4172/2153-0637.1000123. [DOI] [Google Scholar]
- 84.Johansson E., Hussain A., Kuktaite R., Andersson S.C., Olsson M.E. Contribution of organically grown crops to human health. Int. J. Environ. Res. Public Health. 2014;11:3870–3893. doi: 10.3390/ijerph110403870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Działo M., Mierziak J., Korzun U., Preisner M., Szopa J., Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016;17:160. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balasundrama N., Sundramb K., Sammana S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 87.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 88.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colak N., Primetta A.K., Riihinen K.R., Jaakola L., Grúz J., Strnad M., Torun H., Ayaz F.A. Phenolic compounds and antioxidant capacity in different-colored and non-pigmented berries of bilberry (Vaccinium myrtillus L.) Food Biosci. 2017;20:67–78. doi: 10.1016/j.fbio.2017.06.004. [DOI] [Google Scholar]
- 91.Lätti A.K., Riihinen K.R., Kainulainen P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008;56:190–196. doi: 10.1021/jf072857m. [DOI] [PubMed] [Google Scholar]
- 92.Wang L.J., Wu J., Wang H.-X., Li S.-S., Zheng X.-C., Du H., Xu Y.-J., Wang L.-S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods. 2015;16:295–304. doi: 10.1016/j.jff.2015.04.027. [DOI] [Google Scholar]
- 93.Guiné R.P.F., Soutinho S.M.A., Gonçalves F.J. Phenolic compounds and antioxidant activity in red fruits produced in organic farming. [(accessed on 20 August 2020)];Croat. J. Food Sci. Technol. 2014 6:15–26. Available online: http://hdl.handle.net/10400.19/2233. [Google Scholar]
- 94.Brown P.N., Shipley P.R. Determination of anthocyanins in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2011;94:459–466. doi: 10.1093/jaoac/94.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biswas N., Balac P., Narlakanti S.K., Enamul Haque M.D., Mehedi Hassan M.D. Identification of phenolic compounds in processed cranberries by HPLC method. J. Nutr. Food Sci. 2013;3:181. doi: 10.4172/2155-9600.1000181. [DOI] [Google Scholar]
- 96.Ek S., Kartimo H., Mattila S., Tolonen A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea) J. Agric. Food Chem. 2006;54:9834–9842. doi: 10.1021/jf0623687. [DOI] [PubMed] [Google Scholar]
- 97.Chiang C.-J., Kadouh H., Zhou K. Phenolic compounds and antioxidant properties of gooseberry as affected by in vitro digestion. LWT Food Sci. Technol. 2013;51:417–422. doi: 10.1016/j.lwt.2012.11.014. [DOI] [Google Scholar]
- 98.Vagiri M., Ekholm A., Johansson E., Andersson S.C., Rumpunen K. Major phenolic compounds in black currant (Ribes nigrum L.) buds: Variation due to genotype, ontogenetic stage and location. Food Chem. 2015;63:1274–1280. doi: 10.1016/j.lwt.2015.04.006. [DOI] [Google Scholar]
- 99.Nour V., Stampar F., Veberic R., Jakopic J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013;141:961–966. doi: 10.1016/j.foodchem.2013.03.105. [DOI] [PubMed] [Google Scholar]
- 100.Aneta W., Jan O., Magdalena M., Joanna W. Phenolic profile, antioxidant and antiproliferative activity of black and red currants (Ribes spp.) from organic and conventional cultivation. Int. J. Food Sci.Techn. 2013;48:715–726. doi: 10.1111/ijfs.12019. [DOI] [Google Scholar]
- 101.Adina F., Cecilia G., Felicia G., Carmen D., Ovidiu T. Identification and quantification of phenolic compounds from red currant (Ribes rubrum L.) and raspberries (Rubus idaeus L.) Int. J. Pharm. Phytoch. Ethnomed. 2017;6:30–37. doi: 10.18052/www.scipress.com/IJPPE.6.30. [DOI] [Google Scholar]
- 102.Aaby K., Mazur S., Nes A., Skrede G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012;132:86–97. doi: 10.1016/j.foodchem.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 103.Väisänen M., Martz F., Kaarlejärvi E., Julkunen-Tiitto R., Stark S. Phenolic responses of mountain crowberry (Empetrum nigrum ssp. hermaphroditum) to global climate change are compound specific and depend on grazing by reindeer (Rangifer tarandus) J. Chem. Ecol. 2013;39:1390–1399. doi: 10.1007/s10886-013-0367-z. [DOI] [PubMed] [Google Scholar]
- 104.Može Š., Polak T., Gašperlin L., Koron D., Vanzo A., Ulrih N.P., Abram V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.) J. Agric. Food Chem. 2011;59:6998–7004. doi: 10.1021/jf200765n. [DOI] [PubMed] [Google Scholar]
- 105.Jakobek L., Seruga M., Novak I., Medvidović-Kosanović M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Deulsche Lebensm. Rundsch. 2007;103:369–378. [Google Scholar]
- 106.Mattila P., Hellström J., Törrönen R. Phenolic acids in berries, fruits and beverages. J. Agric. Food Chem. 2006;54:7193–7199. doi: 10.1021/jf0615247. [DOI] [PubMed] [Google Scholar]
- 107.Pilat B., Zadernowski R., Czaplicki S., Jeż M. Cold storage, freezing and lyophilisation and its effect on transformations of phenolic compounds in lingonberry (Vaccinium vitis-idaea L.) Pol. J. Natur. Sc. 2018;33:101–113. [Google Scholar]
- 108.Jakobek L., Seruga M. Influence of anthocyanins, flavonols and phenolic acids on the antiradical activity of berries and small fruits. Int. J. Food Prop. 2012;15:122–133. doi: 10.1080/10942911003754684. [DOI] [Google Scholar]
- 109.Williner M.R., Pirovani M.E., Guemes D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003;83:842–845. doi: 10.1002/jsfa.1422. [DOI] [Google Scholar]
- 110.Pinto M.S., Lajolo F.M., Genovese M.I. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria x ananassa Duch.) Food Chem. 2008;107:1629–1635. doi: 10.1016/j.foodchem.2007.10.038. [DOI] [Google Scholar]
- 111.Häkkinen S., Heinonen M., Kärenlampi S., Mykkänen H., Ruuskanen J., Törrönen R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999;32:345–353. doi: 10.1016/S0963-9969(99)00095-2. [DOI] [Google Scholar]
- 112.Gonçalves B., Landbo A.-K., Knudsen D., Silva A.P., Moutinho-Pereira J., Rosa E., Meyer A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.) J. Agric. Food Chem. 2004;52:523–530. doi: 10.1021/jf030595s. [DOI] [PubMed] [Google Scholar]
- 113.Gonçalves B., Landbo A.-K., Let M., Silva A.P., Rosa E., Meyer A.S. Storage affects the phenolic profiles and antioxidant activities of cherries (Prunus avium L.) on human low-density lipoproteins. J. Sci. Food Agric. 2004;84:1013–1020. doi: 10.1002/jsfa.1752. [DOI] [Google Scholar]
- 114.Bhagwat S., Haytowitz D.B., Holden J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. [(accessed on 18 November 2018)];2013 Available online: http://www.ars.usda.gov/nutrientdata.
- 115.Harnly J.M., Doherty R.F., Beecher G.R., Holden J.M., Haytowitz D.B., Bhagwat S., Gebhardt S. Flavonoid content of U.S. fruits, vegetables and nuts. J. Agric. Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 116.Mattila P.H., Hellström J., Karhu S., Pihlava J.-M., Veteläinen M. High variability in flavonoid contents and composition between different North-European currant (Ribes spp.) varieties. Food Chem. 2016;204:14–20. doi: 10.1016/j.foodchem.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 117.Tulio A.Z., Jr., Reese R.N., Wyzgoski F.J., Rinaldi P.L., Fu R., Scheerens J.C., Miller A.R. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J. Agric. Food Chem. 2008;56:1880–1888. doi: 10.1021/jf072313k. [DOI] [PubMed] [Google Scholar]
- 118.Gonçalves B., Silva A.P., Moutinho-Pereira J., Bacelar E., Rosa E., Meyer A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.) Food Chem. 2007;103:976–984. doi: 10.1016/j.foodchem.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 119.Aires A., Carvalho R., Matos M., Carnide V., Silva A.P., Gonçalves B. Variation of chemical constituents, antioxidant activity, and endogenous plant hormones throughout different ripening stages of highbush blueberry (Vaccinium corymbosum L.) cultivars produced in centre of Portugal. J. Food Biochem. 2017;41:e12414. doi: 10.1111/jfbc.12414. [DOI] [Google Scholar]
- 120.Correia S., Aires A., Queirós F., Carvalho R., Schouten R., Silva A.P., Gonçalves B. Climate conditions and spray treatments induce shifts in health promoting compounds in cherry (Prunus avium L.) fruits. Sci. Hortic. 2020;263:109147. doi: 10.1016/j.scienta.2019.109147. [DOI] [Google Scholar]
- 121.Kassim A., Poette J., Paterson A., Zait D., McCallum S., Woodhead M., Smith K., Hackett C., Graham J. Environmental and seasonal influences on red raspberry anthocyanin antioxidant contents and identification of quantitative traitsloci (QTL) Mol. Nutr. Food Res. 2009;53:625–634. doi: 10.1002/mnfr.200800174. [DOI] [PubMed] [Google Scholar]
- 122.Corona G., Tang F., Vauzour D., Rodriguez-Mateos A., Spencer J.P.E. Assessment of the anthocyanidin content of common fruits and development of a test diet rich in a range of anthocyanins. J. Berry Res. 2011;1:209–216. doi: 10.3233/JBR-2011-022. [DOI] [Google Scholar]
- 123.Ogawa K., Sakakibara H., Iwata R., Ishii T., Sato T., Goda T., Shimoi K., Kumazawa S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008;56:4457–4462. doi: 10.1021/jf800406v. [DOI] [PubMed] [Google Scholar]
- 124.Lee J., Finn C.E. Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: Anthocyanin and free amino acid composition. J. Funct. Foods. 2012;4:213–218. doi: 10.1016/j.jff.2011.10.007. [DOI] [Google Scholar]
- 125.Zoratti L., Jaakola L., Häggman H., Giongo L. Anthocyanin profile in berries of wild and cultivated Vaccinium spp. along altitudinal gradients in the Alps. J. Agric. Food Chem. 2015;63:8641–8650. doi: 10.1021/acs.jafc.5b02833. [DOI] [PubMed] [Google Scholar]
- 126.Koponen J.M., Happonen A.M., Mattila P.H., Törrönen A.R. Contents in anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007;55:1612–1619. doi: 10.1021/jf062897a. [DOI] [PubMed] [Google Scholar]
- 127.Karaaslan N.M., Mehmet Y. Determination of anthocyanins in cherry and cranberry by high performance liquid chromatography–electrospray ionization–mass spectrometry. Eur. Food Res. Technol. 2016;242:127–135. doi: 10.1007/s00217-015-2524-9. [DOI] [Google Scholar]
- 128.Köhkönen M.P., Heinämäki J., Ollilainen V., Heinonen M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003;83:1403–1411. doi: 10.1002/jsfa.1511. [DOI] [Google Scholar]
- 129.da Silva F.L., Escribano-Bailón M.T., Alonso J.J.P., Rivas-Gonzalo J.C., Santos-Buelga C. Anthocyanin pigments in strawberry. LWT-Food Sci.Technol. 2007;40:374–382. doi: 10.1016/j.lwt.2005.09.018. [DOI] [Google Scholar]
- 130.Afonso S., Oliveira I.V., Meyer A.S., Aires A., Saavedra M.J., Gonçalves B. Phenolic profile and bioactive potential of stems and seed kernels of sweet cherry fruit. Antioxidants. 2020;9:1295. doi: 10.3390/antiox9121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Correia S., Schouten R., Silva A.P., Gonçalves B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.) Front. Plant Sci. 2017;8:2166. doi: 10.3389/fpls.2017.02166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gonçalves B., Moutinho-Pereira J., Santos A., Silva A.P., Bacelar E., Correia C., Rosa E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006;26:93–104. doi: 10.1093/treephys/26.1.93. [DOI] [PubMed] [Google Scholar]
- 133.Gonçalves B., Morais M.C., Sequeira A., Ribeiro C., Guedes F., Silva A.P., Aires A. Quality preservation of sweet cherry cv. ‘Staccato’ by using glycine-betaine or Ascophyllum nodosum. Food Chem. 2020;322:126713. doi: 10.1016/j.foodchem.2020.126713. [DOI] [PubMed] [Google Scholar]
- 134.Singh R.K., Soares B., Goufo P., Castro I., Cosme F., Pinto-Sintra A.L., Inês A., Oliveira A.A., Falco V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of Grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) tissues. Antioxidants. 2019;8:525. doi: 10.3390/antiox8110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singh R.K., Afonso J., Nogueira M., Oliveira A.A., Cosme F., Falco V. Silicates of potassium and aluminium (kaolin); comparative foliar mitigation treatments and biochemical insight on grape berry quality in Vitis vinifera L. (cv. Touriga National and Touriga Franca) Biology. 2020;9:58. doi: 10.3390/biology9030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cosme F., Ricardo-da-Silva J.M., Laureano O. Tannin profiles of Vitis vinifera L. cv. red grapes growing in Lisbon and from their monovarietal wines. Food Chem. 2009;112:197–204. doi: 10.1016/j.foodchem.2008.05.058. [DOI] [Google Scholar]
- 137.Howard L.R., Clark J.R., Brownmiller C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003;83:1238–1247. doi: 10.1002/jsfa.1532. [DOI] [Google Scholar]
- 138.Zhang J., Wang X., Yu O., Tang J., Gu X., Wan X., Fang C. Metabolic profiling of strawberry (Fragaria × ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011;62:1103–1118. doi: 10.1093/jxb/erq343. [DOI] [PubMed] [Google Scholar]
- 139.Beekwilder J., Jonker H., Meesters P., Hall R.D., van der Meer I.M., Ric de Vos C.H. Antioxidants in raspberry: on-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005;53:3313–3320. doi: 10.1021/jf047880b. [DOI] [PubMed] [Google Scholar]
- 140.Atkinson C.J., Dodds P.A.A., Ford Y.Y., Le Mière J., Taylor J.M., Blake P.S., Paul N. Effects of cultivar, fruit number and reflected photosynthetically active radiation on Fragaria x ananassa productivity and fruit ellagic acid and ascorbic acid concentrations. Ann. Bot. 2006;97:429–441. doi: 10.1093/aob/mcj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vyas P., Curran N.H., Igamberdiev A.U., Debnath S.C. Antioxidant properties of lingonberry (Vaccinium vitis-idaea L.) leaves within a set of wild clones and cultivars. Can. J. Plant Sci. 2015;95:663–669. doi: 10.4141/cjps-2014-400. [DOI] [Google Scholar]
- 142.Kozos K., Ochmian I., Chełpiński P. The effects of rapid chilling and storage conditions on the quality of Brigitta Blue cultivar highbush blueberries. Folia Hortic. 2014;26:147–153. doi: 10.1515/fhort-2015-0006. [DOI] [Google Scholar]
- 143.Srivastava A., Akoh C.C., Yi W., Fischer J., Krewer G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007;55:2705–2713. doi: 10.1021/jf062914w. [DOI] [PubMed] [Google Scholar]
- 144.Mullen W., Stewart A.J., Lean M.E.J., Gardner P., Duthie G.G., Crozier A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J. Agric. Food Chem. 2002;50:5197–5201. doi: 10.1021/jf020141f. [DOI] [PubMed] [Google Scholar]
- 145.Ayala-Zavala J.F., Wang S.Y., Wang C.Y., González-Aguilar A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci. Technol. 2004;37:687–695. doi: 10.1016/j.lwt.2004.03.002. [DOI] [Google Scholar]
- 146.Pappas E., Schaich K. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009;49:741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- 147.Buchert J., Koponen J.M., Suutarinen M., Mustranta A., Lille M., Törrönen R., Poutanen K. Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J. Sci. Food Agric. 2005;85:2548–2556. doi: 10.1002/jsfa.2284. [DOI] [Google Scholar]
- 148.Skrede G., Wrolstad R.E., Durst R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.) J. Food Sci. 2000;65:357–364. doi: 10.1111/j.1365-2621.2000.tb16007.x. [DOI] [Google Scholar]
- 149.Lee J., Durst R.W., Wrolstad R.E. Impact of juice processing on blueberry anthocyanins and polyphenolics: Comparison of two pretreatments. J. Food Sci. 2002;67:1660–1667. doi: 10.1111/j.1365-2621.2002.tb08701.x. [DOI] [Google Scholar]
- 150.Narwojsz A., Borowska E.J. Cranberry and strawberry juices-influence of method production on antioxidants content and antioxidative capacity. Pol. J. Nat. Sci. 2010;25:209–214. doi: 10.2478/v10020-010-0018-6. [DOI] [Google Scholar]
- 151.Kim D.-O., Padilla-Zakour O.I. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: Cherry, plum, and raspberry. J. Food Sci. 2004;69:S395–S400. doi: 10.1111/j.1365-2621.2004.tb09956.x. [DOI] [Google Scholar]
- 152.Savikin K., Zdunić G., Janković T., Tasić S., Menković N., Stević T., Dordević B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum. Nutr. 2009;64:212–217. doi: 10.1007/s11130-009-0123-2. [DOI] [PubMed] [Google Scholar]
- 153.Amakura Y., Umino Y., Tsuji S., Tonogai Y. Influence of jam processing on the radical scavenging activity and phenolic content in berries. J. Agric. Food Chem. 2000;48:6292–6297. doi: 10.1021/jf000849z. [DOI] [PubMed] [Google Scholar]
- 154.Pinto M.S., Lajolo F.M., Genovese M.I. Bioactive compounds and antioxidant capacity of strawberry jams. Plant Foods Hum. Nutr. 2007;62:127–131. doi: 10.1007/s11130-007-0052-x. [DOI] [PubMed] [Google Scholar]
- 155.Paredes-López O., Cervantes-Ceja M.L., Vigna-Pérez M., Hernández-Pérez T. Berries: Improving human health and healthy aging, and promoting quality life-A Review. Plant Foods Hum. Nutr. 2010;65:299–308. doi: 10.1007/s11130-010-0177-1. [DOI] [PubMed] [Google Scholar]
- 156.Du X., Qian M. In: Flavor Chemistry of Small Fruits: Blackberry, Raspberry, and Blueberry. Flavor and Health Benefits of Small Fruits, In. Flavor and Health Benefits of Small Fruits. Du X., Qian M., editors. American Chemical Society; Washington, DC, USA: 2010. pp. 27–43. (ACS Symposium Series). [DOI] [Google Scholar]
- 157.Colquhon T., Levin L., Mosckowitz H., Whiteker V., Clark D., Folta K. Framing the perfect strawberry: An exercise in a consumer-assisted selection of fruit crops. J. Berry Res. 2012;2:45–61. doi: 10.3233/JBR-2011-027. [DOI] [Google Scholar]
- 158.Jetti R.R., Yang E., Kurnianta A., Finn C., Qian M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007;72:S487–S496. doi: 10.1111/j.1750-3841.2007.00445.x. [DOI] [PubMed] [Google Scholar]
- 159.Negri A.S., Allegra D., Simoni L., Rusconi F., Tonelli C., Espen L., Galbiati M. Comparative analysis of fruit aroma patterns in the domesticated wild strawberries “Profumata di Tortona” (F. moschata) and “Regina delle Valli” (F. vesca) Front. Plant Sci. 2015;6:56. doi: 10.3389/fpls.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ulrich D., Hoberg E. Flavour analysis in plant breeding research on strawberries. In: Schieberle P., Engel K.-H., editors. Frontiers of Flavour Sciences. Deutsche Forschungsanstalt Lebensmittelchemie; Garching, Germany: 2000. pp. 161–163. [Google Scholar]
- 161.Urruty L., Giraudel J.L., Lek S., Roudeillac P., Montury M. Assessment of strawberry aroma through SPME/GC and ANN methods. Classification and discrimination of varieties. J. Agric. Food Chem. 2002;50:3129–3136. doi: 10.1021/jf0116799. [DOI] [PubMed] [Google Scholar]
- 162.Pet’ka J., Leitner E., Parameswaran B. Musk strawberries: The flavor of a formerly famous fruit reassessed. Flavour Fragr. J. 2002;27:273–279. doi: 10.1002/ffj.3095. [DOI] [Google Scholar]
- 163.Ulrich D., Olbricht K. Diversity of volatile patterns in sixteen Fragaria vesca L. accessions in comparison to cultivars of Fragaria × ananassa. J. App. Bot. Food Qual. 2013;86:37–46. doi: 10.5073/JABFQ.2013.086.006. [DOI] [Google Scholar]
- 164.Ulrich D., Olbricht K. Diversity of metabolite patterns and sensory characters in wild and cultivated strawberries. J. Berry Res. 2014;4:11–17. doi: 10.3233/JBR-140067. [DOI] [Google Scholar]
- 165.Barbey C.R., Hogshead M.H., Harrison B., Schwartz A.E., Verma S., Oh Y., Lee S., Folta K.M., Whitaker V.M. Genetic Analysis of Methyl Anthranilate, Mesifurane, Linalool, and Other Flavor Compounds in Cultivated Strawberry (Fragaria × ananassa) Front Plant Sci. 2021;12:615749. doi: 10.3389/fpls.2021.615749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fan Z., Hasing T., Johnson T., Garner D.M., Schwieterman M.L., Barbey C.R., Colquhoun T.A., Sims C.A., Resende M.F.R., Whitaker V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021;8:66. doi: 10.1038/s41438-021-00502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ueda Y., Tsuda A., Bai J.H., Fujishita N., Chachin K. Characteristic pattern of aroma ester formation from banana, melon, and strawberry with reference to the substrate specificity of ester synthetase and alcohol contents in pulp. J. Jpn. Soc. Food Sci. Technol. 1992;39:183–187. doi: 10.3136/nskkk1962.39.183. [DOI] [Google Scholar]
- 168.Olias J.M., Sanz C., Rios J.J., Perez A.G. Substrate specificity of alcohol acyltransferase from strawberry and banana fruits. In: Rouseff R.L., Leahy M.M., editors. Fruit Flavors: Biogenesis, Characterization and Authentication. American Chemical Society; Washington, DC, USA: 1995. pp. 134–141. [Google Scholar]
- 169.Aharoni A., Keizer L.C., Bouwmeester H.J., Sun Z., Alvarez-Huerta M., Verhoeven H.A., Blaas J., van Houwelingen A.M., De Vos R.C., van der Voet H., et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell. 2000;12:647–662. doi: 10.1105/tpc.12.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schwieterman M.L., Colquhoun T.A., Jaworski E.A., Bartoshuk L.M., Gilbert J.L., Tieman D.M., Odabasi A.Z., Moskowitz H.R., Folta K.M., Klee H.J., et al. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE. 2014;9:e88446. doi: 10.1371/journal.pone.0088446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ulrich D., Kecke S., Olbricht K. What do we know about the chemistry of strawberry aroma? J. Agric. Food Chem. 2018;66:3291–3301. doi: 10.1021/acs.jafc.8b01115. [DOI] [PubMed] [Google Scholar]
- 172.Aprea E., Carlin S., Giongo L., Grisenti M., Gasperi F. Characterization of 14 raspberry cultivars by solid-phase microextraction and relationship with Gray Mold susceptibility. J. Agric. Food Chem. 2010;58:1100–1105. doi: 10.1021/jf902603f. [DOI] [PubMed] [Google Scholar]
- 173.Forney C.F., Kalt W., Jordan M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience. 2000;35:1022–1026. doi: 10.21273/HORTSCI.35.6.1022. [DOI] [Google Scholar]
- 174.Ikan R. Naturally Occurring Glycosides. John Wiley; Chichester, UK: New York, NY, USA: 1999. [Google Scholar]
- 175.Honkanen E., Pyysalo T., Hirvi T. The aroma of finnish wild raspberries, Rubus idaeus, L. Z. Lebensm. Unters. Forsch. 1980;171:180–182. doi: 10.1007/BF01042646. [DOI] [Google Scholar]
- 176.Georgilopoulos D.N., Gallois A.N. Flavour compounds of a commercially concentrated blackberry juice. Food Chem. 1988;28:141–148. doi: 10.1016/0308-8146(88)90143-4. [DOI] [Google Scholar]
- 177.Gilbert J.L., Guthart M.J., Gezan S.A., De Carvalho M.P., Schwieterman M.L., Colquhoun T.A., Bartoshuk L.M., Sims C.A., Clark D.G., Olmstead J.W. Identifying breeding priorities for blueberry flavor using biochemical, sensory, and genotype by environment analyses. PLoS ONE. 2015;10:e0138494. doi: 10.1371/journal.pone.0138494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beaulieu J.C., Stein-Chisholm R.E., Boykin D.L. Qualitative analysis of volatiles in Rabbiteye blueberry cultivars at various maturities using Rapid Solid-phase Microextraction. J. Am. Soc. Hortic. Sci. 2014;139:167–177. doi: 10.21273/JASHS.139.2.167. [DOI] [Google Scholar]
- 179.Farneti B., Khomenko I., Grisenti M., Ajelli M., Betta E., Algarra A.A., Giongo L. Exploring blueberry aroma complexity by chromatographic and Direct-Injection Spectrometric Techniques. Front. Plant Sci. 2017;8:617. doi: 10.3389/fpls.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hirvi T., Honkanen E., Pyysalo T. The aroma of cranberries. Z. Lebensm. Unters. Forsch. 1981;172:365–367. doi: 10.1007/BF01127665. [DOI] [Google Scholar]
- 181.Zhu J.C., Chen F., Wang L.Y., Niu Y.W., Chen H., Wang W., Xiao Z. Characterization of the key aroma volatile compounds in cranberry (Vaccinium macrocarpon Ait.) using Gas Chromatography-Olfactometry (GC-O) and Odor Activity Value (OAV) J. Agric. Food Chem. 2016;64:4990–4999. doi: 10.1021/acs.jafc.6b01150. [DOI] [PubMed] [Google Scholar]
- 182.Caswell J.A., Noelke C.M., Mojduszka E.M. Unifying Two Frameworks for Analyzing Quality and Quality Assurance for Food Products. In: Krissoff B., Bohman M., Caswell J.A., editors. Global Food Trade and Consumer Demand for Quality. Kluwer Academic/Plenum Publishers; New York, NY, USA: 2002. pp. 43–61. [Google Scholar]
- 183.Grunert K.G. Food quality and safety: Consumer perception and demand. Eur. Rev. Agric. Econ. 2005;32:369–391. doi: 10.1093/eurrag/jbi011. [DOI] [Google Scholar]
- 184.Poole N.D., Martínez L.M.C., Jiménez F.V. Quality perceptions under evolving information conditions: Implications for diet, health and consumer satisfaction. Food Policy. 2007;32:175–188. doi: 10.1016/j.foodpol.2006.05.004. [DOI] [Google Scholar]
- 185.Mai R., Hoffmann S. How to combat the unhealthy = tasty intuition: The influencing role of health consciousness. J. Public Policy Mark. 2015;34:63–83. doi: 10.1509/jppm.14.006. [DOI] [Google Scholar]
- 186.Dixon H., Mullins R., Wakefield M., Hill D. Encouraging the consumption of fruit and vegetables by older Australians: An experimental study. J. Nutr. Educ. Behav. 2004;36:245–249. doi: 10.1016/S1499-4046(06)60387-4. [DOI] [PubMed] [Google Scholar]
- 187.Batt P.J. Consumer sovereignty: Exploring consumer needs. In: Johnson G.I., Hofman P.J., editors. Agri-Product Supply-Chain Management in Developing Countries. ACIAR; Canberra, Australia: 2004. pp. 77–87. [Google Scholar]
- 188.Riediger N.D., Shooshtari S., Moghadasian M.H. The influence of sociodemographic factors on patterns of fruit and vegetable consumption in Canadian adolescents. J. Am. Diet. Assoc. 2007;107:1511–1518. doi: 10.1016/j.jada.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 189.Yeh M.C., Ickes S.B., Lowenstein L.M., Shuval K., Ammerman A.S., Farris R., Katz D.L. Understanding barriers and facilitators of fruit and vegetable consumption among a divers multi-ethnic population in the USA. Health Promot. Int. 2008;23:42–51. doi: 10.1093/heapro/dam044. [DOI] [PubMed] [Google Scholar]
- 190.Irmak C., Vallen B., Robinson S. The impact of product name on dieters’ and nondieters’ food evaluations and consumption. J. Consum. Res. 2011;38:390–405. doi: 10.1086/660044. [DOI] [Google Scholar]
- 191.Mai R., Symmank C., Seeberg-Elverfeldt B. Light and pale colors in food packaging: When does this package cue signal superior healthiness or inferior tastiness? J. Retail. 2016;92:426–444. doi: 10.1016/j.jretai.2016.08.002. [DOI] [Google Scholar]
- 192.Vila-López N., Küster-Boluda I. Commercial versus technical cues to position a new product: Do hedonic and functional/healthy packages differ? Soc. Sci. Med. 2018;198:85–94. doi: 10.1016/j.socscimed.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 193.Shaikh A.R., Yaroch A.L., Nebeling L., Yeh M.-C., Resnicow K. Psychosocial predictors of fruit and vegetable consumption in adults. Am. J. Prev. Med. 2008;34:535–543. doi: 10.1016/j.amepre.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 194.Werle C., Trendel O., Ardito G. Unhealthy food is not tastier for everybody: The “healthy = tasty” French intuition. Food Qual Prefer. 2013;28:116–121. doi: 10.1016/j.foodqual.2012.07.007. [DOI] [Google Scholar]
- 195.Verbeke W. Functional foods: Consumer willingness to compromise on taste for health? Food Qual Prefer. 2006;17:126–131. doi: 10.1016/j.foodqual.2005.03.003. [DOI] [Google Scholar]
- 196.Patil B.S., Uckoo R.M., Jayaprakasha G.K., Palma M.A. Consumers’ changing perceptions of quality: Revisiting the science of fruit and vegetable cultivation for improved health benefits; Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): International Symposia on Postharvest Knowledge for the Future and Consumer and Sensory Driven Improvements to Fruits and Nuts; Brisbane, Australia. 17 August 2014; pp. 459–468. [Google Scholar]
- 197.Samoggia A., Nicolodi S. Consumer’s Perception of Fruit Innovation. J. Int. Food Agribus. Mark. 2017;9:92–108. doi: 10.1080/08974438.2016.1266567. [DOI] [Google Scholar]
- 198.Van Duyn M.A.S. Year 2000 Dietary Guidelines: The Case for Fruits and Vegetables First. Produce for Better Health Foundation; Wilmington, DE, USA: 1999. [Google Scholar]
- 199.Kader A. Importance of fruits, nuts and vegetables in human nutrition and health. Perish. Handl. Q. 2001;106:6. [Google Scholar]
- 200.Ströhle A. Vegetables and fruits in prevention: The German Nutrition Society (DGE) opinion confirms: High consumption of vegetables and fruits reduces risk of contracting diseases. Dtsch. Apoth. Ztg. 2012;152:75–77. [Google Scholar]
- 201.Scheerens J.C. Phytochemicals and the consumer: Factors affecting fruit and vegetable consumption and the potential for increasing small fruit in the diet. Hort Technol. 2001;11:547–556. doi: 10.21273/HORTTECH.11.4.547. [DOI] [Google Scholar]
- 202.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Etherton T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;113:71–88. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 203.Yahia E.M. The contribution of fruit and vegetable consumption to human health. In: De La Rosa L.A., Alvarez-Parrilla E., González-Aguilar G.A., editors. Fruit and Vegetable Phytochemicals. John Wiley & Sons; Hoboken, NJ, USA: 2017. pp. 3–51. [Google Scholar]
- 204.Association A.D. A Place on the Plate for Functional Foods. 2011. [(accessed on 15 November 2018)]. Available online: http://www.foodinsight.org/Content/3842/REVISED%20ADA%20Functional%20Foods%20Webcast%20Deck%207.26.2011%20V2.pdf.
- 205.Rekhy R., Khan A., Eason J., Mactavish-West H., Lister C., Mcconchie R. Australian consumer awareness of health benefits associated with vegetable consumption. Nutr. Diet. 2017;74:175–184. doi: 10.1111/1747-0080.12328. [DOI] [PubMed] [Google Scholar]
- 206.Verbeke W. Consumer acceptance of functional foods: Sociodemographic, cognitive and attitudinal determinants. Food Qual. Prefer. 2005;16:45–57. doi: 10.1016/j.foodqual.2004.01.001. [DOI] [Google Scholar]
- 207.Hidalgo G.-I., Almajano M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants. 2017;6:7. doi: 10.3390/antiox6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bunea A., Rugina D.O., Pintea A.M., Sconta Z., Bunea C.I., Socaciu C. Comparative polyphenolic content and antioxidant activities of some wild and cultivated blueberries from Romania. Not. Bot. Horti Agrobo. 2011;39:70–76. doi: 10.15835/nbha3926265. [DOI] [Google Scholar]
- 209.Connor A.M., Luby J.J., Tong C.B.S. Variability in antioxidant activity in blueberry and correlations among different antioxidant activity assays. J. Am. Soc. Hortic. Sci. 2002;127:238–244. doi: 10.21273/JASHS.127.2.238. [DOI] [Google Scholar]
- 210.Rodrigues E., Poerner N., Rockenbach I.I., Gonzaga L.V., Mendes C.R., Fett R. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Ciência Tecnol. Aliment. 2011;31:911–917. doi: 10.1590/S0101-20612011000400013. [DOI] [Google Scholar]
- 211.Zheng W., Wang Y.S. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries and lingonberries. J. Agric. Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 212.Yu L.L., Zhou K.K., Parry J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005;91:723–729. doi: 10.1016/j.foodchem.2004.06.044. [DOI] [Google Scholar]
- 213.Haytowitz D.B., Bhagwat S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. [(accessed on 18 November 2018)];2010 Available online: http://www.ars.usda.gov/nutrientdata.
- 214.Moyer A.R., Hummer E.K., Finn E.C., Frei B., Wrolstad E.R. Anthocyanins, phenolics and antioxidant capacity in diverse small fruits: Vaccinium, Rubus and Ribes. J. Agric. Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- 215.Wang S., Lin H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varieties with cultivar and developmental stage. J. Agric. Food Chem. 2000;18:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- 216.Kevers C., Falkowski M., Tabart J., Defraigne J.-O., Dommes J., Pincemail J. evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food Chem. 2007;55:8596–8603. doi: 10.1021/jf071736j. [DOI] [PubMed] [Google Scholar]
- 217.Tulipani S., Alvarez-Suarez J.M., Busco F., Bompadre S., Quiles J.L., Mezzetti B., Battino M. Strawberry consumption improves plasma antioxidant status and erythrocyte resistance to oxidative haemolysis in humans. Food Chem. 2011;128:180–186. doi: 10.1016/j.foodchem.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 218.Vasileiou I., Katsargyris A., Theocharis S., Giaginis C. Current clinical status on the preventive effects of cranberry consumption against urinary tract infections. Nutr. Res. 2013;33:595–607. doi: 10.1016/j.nutres.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 219.Riso P., Klimis-Zacas D., Del Bo’ C., Martini D., Campolo J., Vendrame S., Møller P., Loft S., De Maria R., Marisa Porrini M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013;52:949–961. doi: 10.1007/s00394-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 220.Chen L., Xin X., Yuan Q., Su D., Liu W. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agric. 2014;94:180–188. doi: 10.1002/jsfa.6216. [DOI] [PubMed] [Google Scholar]
- 221.McCarron D.A., Reusser M.E. Are low intakes of calcium and potassium important causes of cardiovascular disease? Am. J. Hypertens. 2001;14:S206–S212. doi: 10.1016/S0895-7061(01)02090-8. [DOI] [PubMed] [Google Scholar]
- 222.Zerwekh J.E., Odvina C.V., Wuermser L.-A., Pak C.Y.C. Reduction of renal stone risk by potassium-magnesium citrate during 5 weeks of bed rest. J. Urol. 2007;177:2179–2184. doi: 10.1016/j.juro.2007.01.156. [DOI] [PubMed] [Google Scholar]
- 223.Ignarro L.J., Balestrieri M.L., Napoli C. Nutrition, physical activity, and cardiovascular disease: An update. Cardiovasc. Res. 2007;73:326–340. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 224.Cohen A.J., Roe F.J.C. Review of risk factors for osteoporosis with particular reference to a possible aetiological role of dietary salt. Food Chem. Toxicol. 2000;38:237–253. doi: 10.1016/S0278-6915(99)00145-3. [DOI] [PubMed] [Google Scholar]
- 225.Köster E.P. Diversity in the determinants of food choice: A psychological perspective. Food Qual. Prefer. 2009;20:70–82. doi: 10.1016/j.foodqual.2007.11.002. [DOI] [Google Scholar]
- 226.Grunert K.G., Larsen H.H., Madsen T.K., Baadsgaard A. Market Orientation in Food and Agriculture. Kluwer Academic; Dordrecht, The Netherlands: 1996. [Google Scholar]
- 227.Brunsø K., Birch D., Memery J., Temesi Á., Lakner Z., Lang M., Dean D., Grunert K.G. Core dimensions of food-related lifestyle: A new instrument for measuring food involvement, innovativeness and responsibility. Food Qual. Prefer. 2021;91:104192. doi: 10.1016/j.foodqual.2021.104192. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.