Abstract
Simple Summary
Glioblastoma multiforme (GBM) is the most aggressive tumor type in the central nervous system. Hypoxia, defined as a lack of sufficient oxygen in tissues, is the most detrimental factor for the survival of GBM patients, promoting drug resistance, and invasion and inhibition of immune responses. Traditionally, tumor hypoxia has been studied from a narrow viewpoint, excluding the immune system and focusing primarily on the effect of hypoxia on blood vessels and tumor cells. More recently, however, evidence highlighting the important role of immunosurveillance has been uncovered for multiple tumors, including GBM. Thus, connecting the knowledge gained from traditional hypoxia studies with findings from recent immunological studies is urgently needed to better understand the role of hypoxia in cancer.
Abstract
Hypoxia is a hallmark of glioblastoma multiforme (GBM), the most aggressive cancer of the central nervous system, and is associated with multiple aspects of tumor pathogenesis. For example, hypoxia induces resistance to conventional cancer therapies and inhibits antitumor immune responses. Thus, targeting hypoxia is an attractive strategy for GBM therapy. However, traditional studies on hypoxia have largely excluded the immune system. Recently, the critical role of the immune system in the defense against multiple tumors has become apparent, leading to the development of effective immunotherapies targeting numerous cancer types. Critically, however, GBM is classified as a “cold tumor” due to poor immune responses. Thus, to improve GBM responsiveness against immunotherapies, an improved understanding of both immune function in GBM and the role of hypoxia in mediating immune responses within the GBM microenvironment is needed. In this review, we discuss the role of hypoxia in GBM from a clinical, pathological, and immunological perspective.
Keywords: glioblastoma multiforme (GBM), hypoxia, antitumor immunity, immunotherapy
1. Introduction
Tumor cells have distinct metabolic features compared to normal cells. For example, although normal cells usually suppress glycolysis under normoxic conditions (i.e., the Pasteur effect), tumor cells preferentially use glycolysis despite the presence of oxygen, a phenomenon known as the Warburg effect [1]. Lactic acid accumulation resulting from the Warburg effect is metabolic hallmark of the tumor microenvironment (TME), leading to low pH. Critically, these unique metabolic characteristics can inhibit antitumor immune responses, making the TME more favorable for tumor progression [2]. Thus, a precise understanding of metabolic programming within the TME is essential for the development of effective antitumor therapy.
Oxygen is the most basic and important component of cellular metabolism. Many enzymes, such as oxygenase, require oxygen for their function [3], and large amounts of energy are generated by oxidative phosphorylation compared to glycolysis [4]. A lack of oxygen leads to hypoxia—a hallmark of many cancers that is linked to tumor progression and worse clinical outcomes for patients [5]. Hypoxia in cancer can result from the fast proliferation of tumor cells; this causes some tumor cells to be located far from oxygen-supplying blood vessels (>180 μm) [6], leading to limited oxygen diffusion. In addition, the TME often promotes angiogenesis, which can result in the formation of abnormal, closed blood vessels, further inducing hypoxia [7]. Critically, the presence of hypoxia reprograms tumor cells through multiple proteins, such as hypoxia-inducible factor (HIF)-1α. Such hypoxia-adapted tumor cells are more invasive and resistant to therapies, and they can also evade immunosurveillance.
Beyond tumor cells themselves, tumor-infiltrating immune cells are also under hypoxic conditions in the TME. Normoxic ambient air contains 21% O2, whereas O2 concentrations of 2–9% (14.4–64.8 mmHg) are present in tissue under physiological normoxia. In contrast, some parts of normal organs, such as the bone marrow and thymus, as well as the TME, are hypoxic, containing approximately 1% O2 (7.2 mmHg) [8]. A number of studies have investigated immune cell function in the context of hypoxia; however, these have reported contradictory results regarding whether hypoxia promotes beneficial or adverse effects on immune cells. For example, CD8+ T cells activated under hypoxia show stronger cytotoxic effects against tumor cells than those activated under normoxia [9]. Similarly, natural killer (NK) cells cultured under hypoxic conditions following normoxia are more highly activated than normoxic NK cells [10]. Conversely, hypoxia downregulates interferon (IFN)-γ production by CD8+ T cells under T cell receptor (TCR) stimulation and attenuates NK cell-mediated cytotoxicity [11,12]. Intriguingly, NK cell activity is enhanced by short-term, but not long-term, hypoxia [13,14], and continuous activation of CD8+ T cells under hypoxia promotes characteristics distinct from those present in acutely activated CD8+ T cells [15]. This suggests that while a HIF-1α-mediated glycolytic burst enhances the activity of cytotoxic cells, mitochondrial dysfunction in response to long-term hypoxia attenuates cytotoxic and inflammatory functions. Overall, these complex hypoxia-associated phenotypes are dependent on numerous different factors and experimental conditions, suggesting that understanding the role of hypoxia in antitumor immunity is likely to be more complicated than expected.
Of all the organs in the body, the brain requires the highest amount of oxygen. Thus, although the brain comprises only 2% of total body weight, it consumes 20% of the body’s oxygen. Oxygen levels in the brain differ depending on the region. For example, the oxygen level in the midbrain is approximately 0.5%, whereas in the pia, it is 8% [16]. Brain tumors have been shown to contain 1.25% O2, with the peritumoral area containing 2.5% O2 [17]. Thus, brain tumor regions are mostly hypoxic compared to normal brain tissue. Glioblastoma multiforme (GBM), the most aggressive brain tumor type, can be classified as a hypoxic tumor. Irradiation is the most frequently used antitumor therapy for brain tumors; however, hypoxia-mediated stemness promotes cellular resistance to irradiation. Thus, studies aimed at understanding the effects of hypoxia within brain tumors are urgently needed. In this review, we discuss recent findings on the role of hypoxia in tumor biology and in antitumor immunity against brain tumors.
2. Glioblastoma Multiforme (GBM)
2.1. GBM
Tumors of the central nervous system (CNS) are relatively rare compared to other peripheral tumors, with CNS tumors showing an estimated frequency of about 1% amongst those detected in all tumor sites [18]. Most CNS tumors, about 70% of cases, are non-malignant, half of which are meningiomas. In 30% of cases, however, CNS tumors are malignant, with glioblastomas accounting for about 50% of all CNS malignancies [19]. Despite its low overall incidence, glioblastoma is an important tumor type due to the high average years of life lost from this cancer, which amounts to 20.1 years [20]. In addition, according to a report from the Australian Institute of Health and Welfare (AIHW), between 1988–1992 and 2013–2017, the estimated percentage change in the 5-year survival rate for brain tumor was only about 2.3%, whereas all cancers combined showed an increase of 18.3% [21].
Most GBMs are primary tumors, although a small portion develop from low-grade astrocytoma and thus are known as secondary GBM [22]. A study by Verhaak et al. [23] further reported that GBM can be divided into four subtypes based on gene expression patterns: classical, proneural, neural, and mesenchymal. The classical type includes amplifications of chromosome 7 and epidermal growth factor receptor (EGFR), as well as a homozygous deletion spanning the Ink4a/ARF locus. The mesenchymal type shows high expression of chitinase-3-like protein 1 (CHI3L1) and tyrosine-protein kinase Met (c-Met), with neurofibromatosis type 1 (NF1) mutation/deletion or low expression of NF1. Mesenchymal type GBM also displays a higher percentage of necrosis and inflammation than other types. The proneural type has platelet-derived growth factor receptor A (PDGFRA) abnormalities and mutations on both tumor protein p53 (TP53) and isocitrate dehydrogenase 1 (IDH1), similar to secondary GBM. In contrast, the neural type is similar to normal brain tissue in terms of the gene expression pattern. Recently, however, the Verhaak group suggested that the neural GBM subtype might, in fact, be contamination from normal brain tissue [24]. Because IDH mutation is associated with better prognosis, proneural subtype GBM is thought to be associated with better patients outcomes, whereas mesenchymal subtype GBM is associated with worse outcomes [25].
2.2. Clinical Approaches for GBM
To improve the survival of GBM patients, maximal resection of tumor tissues is recommended. This reduces mass effect and enhances efficacy of adjuvant therapies, leading to increased survival rates [26]. However, despite the rapid development of improved detection methods, complete resection is often difficult due to the presence of complex vasculature, location of the tumor, and fear of damage to intact brain tissues. Thus, in many cases, resection is ineffective, and recurrence after surgery is common [27].
Current standard care for GBM is Stupp’s regimen, which involves radiotherapy (2 Gy per day, 5 days per week, up to a total of 60 Gy), with concomitant temozolomide (TMZ) treatment (Figure 1a) [28]. Similar to other chemotherapies, TMZ induces DNA damage via methylation of O6 and N7 positions on guanine and the N3 position on adenine, which promotes cell death. Sensitivity to TMZ treatment is largely dependent on methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter [29]. MGMT repairs DNA, and consequently, patients with MGMT methylation, which inhibits expression of this gene, are sensitive to TMZ treatment [30]. In 2015, a device delivering alternating electric fields, also known as tumor-treating fields (TTFs), was approved by the US Food and Drug Administration for GBM patients [31]. In phase 3 trials, this device promoted a significant increase in overall survival (OS) and progression-free survival (PFS) compared to TMZ alone [32].
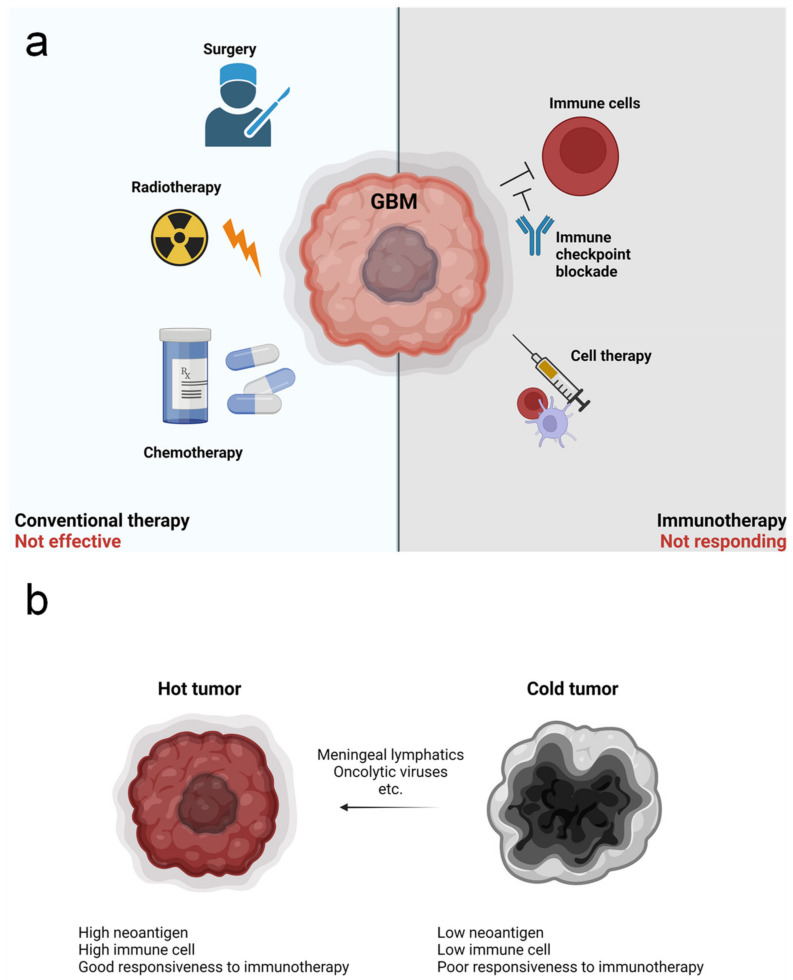
Figure 1.
Therapeutic approaches for glioblastoma multiforme (GBM) and hurdles to treatment. (a) There are multiple strategies to care for GBM patients. Conventional GBM therapy involves surgery, followed by radiotherapy and concomitant chemotherapy. However, this is not fully effective. Recently, immunotherapies have been developed and shown promising results for other tumors. Immune checkpoint blockade approaches for inhibiting immunosuppression, and cell therapies, such as dendritic cell (DC) vaccines, are now being tested for GBM. However, responsiveness to these therapies is poor. (b) Tumors can be classified as “hot” or “cold”. Hot tumor shows high levels of neoantigens, increased infiltration of immune cells, and better responsiveness to therapies relative to cold tumors. Thus, several approaches for converting cold tumors into hot tumors, such as by manipulating lymphatics or through the use of oncolytic viruses, are being studied.
Complex vasculature is also highly associated with GBM progression, and therefore, anti-angiogenesis therapies, including bevacizumab, have been tested. In phase 3 trials, bevacizumab treatment improved PFS; however, disappointingly, a significant improvement in OS was not observed [33,34]. Furthermore, long-term treatment with bevacizumab is associated with increased hypoxia and invasiveness [35]. Alternatively, vessel normalization via TIE2 activation and angiopoietin-2 (ANG2) inhibition showed promising results in rodent models, leading to less hypoxia and invasion, although this strategy has not been tested in humans [36].
In recent years, the emergence of cancer immunotherapy was expected to be a game changer for treatment of various tumor types, including GBM. Toward this goal, many strategies have been suggested, such as dendritic cell (DC) vaccines, immune checkpoint inhibitors, chimeric antigen receptor (CAR) T cell therapy, and adoptive cell therapy. Although none of these have shown promising results for GBM patients, a massive number of studies remain ongoing [37]. Currently, the most popular strategy for GBM immunotherapy is immune checkpoint blockade, such as with anti-programmed cell death 1 (PD-1) therapy. However, recent phase 3 clinical trials using nivolumab in unmethylated-MGMT GBM with radiotherapy and methylated-MGMT GBM with chemoradiotherapy did not show any improvement in OS and PFS [38]. Although it is too early to judge, many concerns regarding the use of immunotherapy for GBM exist due to the fact that GBM is classified as a “cold tumor” with poor immune cell infiltration [39]. Thus, various strategies for converting GBM into “hot” tumor have been suggested, including the use of oncolytic viruses and/or manipulating meningeal lymphatics (Figure 1b) [40,41].
3. GBM and Hypoxia
3.1. Histological Characteristics of GBM
GBM tumors display unique features, such as necrotic foci, pseudopalisades, and microvascular hyperplasia (Figure 2a), which are thought to be important for the fast growth and invasiveness of GBM cells [42]. Pseudopalisades may result from the migration of tumor cells escaping from a hypoxic region to form the invasive front edge. Pseudopalisading cells shape microvascular hyperplasia, forming tuft microaggregates around the edge of blood vessels and leading to the formation of glomeruloid bodies [43]. These features are largely mediated by angiogenesis-induced hypoxia. For example, excessive expression of vascular endothelial growth factor (VEGF) induces the hyper-proliferation of endothelial cells, resulting in defective and permeable blood vessels that can be easily disrupted [44]. This abnormal vasculature in the GBM microenvironment inhibits the delivery of oxygen, as well as drugs and immune cells [36]. In addition, hypoxia resulting from abnormal vessels promotes the invasion of tumor cells, a main hurdle for therapies against GBM [42].
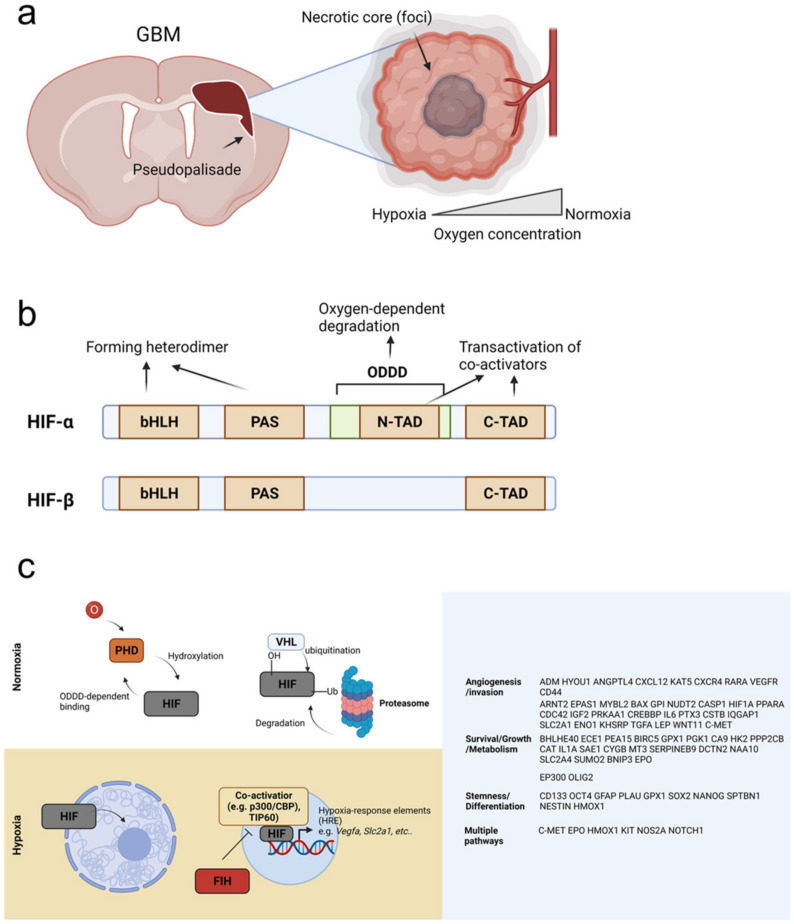
Figure 2.
Responses to hypoxia in GBM tumors. (a) GBM tissue shows aggressive invasiveness and pseudopalisades. Oxygen is supplied by blood vessels, and thus, tumor cells located far from vessels become hypoxic due to poor oxygen diffusion, forming necrotic core (foci). Tumor cells that escape from hypoxia form pseudopalisades. (b) Hypoxia-inducible factor (HIF)-α and HIF-β have basic helix–loop–helix (bHLH), Per–Arnt–Sim (PAS), and C-terminal (C-TAD) domains. The bHLH and PAS domain are responsible for forming the heterodimer, and the C-TAD domain promotes transactivation of co-activators. HIFα also has an N-terminal (N-TAD) domain and an oxygen-dependent degradation domain (ODDD), which mediate its oxygen-dependent degradation via the ubiquitin–proteasome pathway. The N-TAD also participates in transactivation of co-activators. (c) Under normoxia, oxygen-dependent prolyl hydroxylase (PHD) enzyme is active and binds to HIF in an ODDD-dependent manner. PHD hydroxylates HIF, which allows von Hippel–Lindau (VHL) to bind and recruit E3 ubiquitinase. This enzyme ubiquitinates HIF, targeting it for binding and degradation by the proteasome. In contrast, under hypoxia, HIF is stable and translocates into the nucleus, where it binds to co-activators, such as p300/CBP or TIP60, and turns on expression of hypoxia-response element (HRE) genes. HIF can regulate multiple cellular processes via activation of these HRE genes. HIF-suppressors, including factor-inhibiting HIF (FIH), function to inhibit binding between HIF and its co-activators and block HRE activation.
3.2. Cellular Sensing of Hypoxia
Cells can sense the surrounding oxygen level through multiple molecular mechanisms. Of these, the most well-studied is the highly conserved HIF pathway [42], which acts as the major oxygen-sensing pathway in metazoan species [45]. The transcription factor HIF is a heterodimer formed from two distinct subunits, HIFα and HIFβ. In humans, HIFα has three isoforms. HIF-1α is ubiquitously expressed and overexpressed by tumor cells [46,47]. In contrast, HIF-2α is expressed in distinct cell populations, such as in subsets of tumor-associated macrophages (TAMs) [48]. HIF-3α is also selectively expressed, although its expression in immune cells is not clear [49,50]. Target genes for HIF-1α and HIF-2α show some degree of overlap; however, a subset of genes is distinctly regulated by each transcription factor [51]. HIF-3 can also function as a transcriptional activator for a unique set of genes [52], although it is most commonly known to be a dominant-negative regulator of HIF-1, due to its lack of a C-terminal (C-TAD) domain [53]. In contrast, the aryl hydrocarbon receptor nuclear translocator (ARNT), HIF-1β is expressed ubiquitously [42].
HIF-1α, HIF-2α, and HIF-1β all contain a basic helix–loop–helix (bHLH) domain, a Per–Arnt–Sim (PAS) domain, and a C-TAD domain. HIF-α also has additional oxygen-dependent degradation domain (ODDD) and an N-terminal (N-TAD) domain. The bHLH and PAS domains form the heterodimer and bind to hypoxia-response elements (HREs) [54], whereas the C-TAD and N-TAD domains are involved in transactivation of coactivators, such as p300/CBP (Figure 2b) [55].
In normoxia, HIFα is bound to prolyl hydroxylase 1–3 (PHD1–3) via the ODDD, and PHD hydroxylates two prolyl residues of HIFα. PHD is regulated by O2 levels due to its 2-oxoglutarate-dependent and iron-dependent dioxygenase domains [56]. Hydroxylation of HIFα allows it to bind von Hippel–Lindau (VHL), which recruits E3 ubiquitin ligases. These promote the ubiquitination of HIFα and its subsequent degradation by the proteasome [57]. Conversely, under hypoxic conditions, PHD activity is lost, and stable HIFα translocates into the nucleus, where it binds HIFβ and coactivators, such as p300/CBP (Figure 2c) [58]. Factor-inhibiting HIF (FIH), an O2-dependent hydroxylase, also functions in HIFα regulation by blocking HIFα binding to coactivators [59]. HIF target genes are reviewed in detail elsewhere (e.g., [60]) (Figure 2c).
3.3. HIF, HRE Genes, and GBM
As noted above, oxygen-dependent gene expression is mainly mediated by HIF and downstream HRE genes. The HIF-dependent hypoxic response regulates multiple cellular activities, including metabolism, migration, angiogenesis, and differentiation [61], and this pathway promotes invasiveness in hypoxic GBM cells via multiple mechanisms. For example, carbonic anhydrase 9 (CA9), a zinc-dependent enzyme that catalyzes the conversion of CO2 into bicarbonate, is known to be affected by hypoxia and is highly expressed in GBM cells [62]. Hypoxia also stabilizes the EGFRvIII protein by promoting interaction with integrin β3 in GBM cells [63] and further induces recruitment of the integrins αvβ3 and αvβ5 to the surface of GBM cells, leading to activation of focal adhesion kinase (FAK) [64]. Procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 (PLOD2), an enzyme that regulates collagen cross-linking, is also controlled by hypoxia in a HIF-1α-dependent manner [65]. Collectively, these protein interactions promote invasion of GBM cells. In addition, epithelial-to-mesenchymal transition (EMT)- and metastasis-related genes, such as recombination signal binding protein for immunoglobulin kappa J (RBPJ) [66], zinc finger E-box-binding homeobox 1 (ZEB1) [67], and Twist-related protein 1 (TWIST1), are known to be regulated by hypoxia via the HIF-1α pathway [68]. Hypoxia has also been reported to induce expression of C-X-C chemokine receptor type 4 (CXCR4) in GBM cells and of CXCL12 in endothelial cells, and both C-C motif chemokine receptor type 5 (CCR5) and C-C motif chemokine ligand 4 (CCL4) are positively regulated by hypoxia [69,70,71].
Several key pathways are involved in cellular adaptation to hypoxia. For example, hypoxia suppresses cap-dependent protein translation at the level of translation initiation [72]. This suppression is primarily regulated by protein kinase R (PKR)-like endoplasmic reticulum (ER) kinase (PERK) and the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) [73]. However, some genes are continuously translated under hypoxia, such as those associated with stress response. These commonly include genes related to antioxidant response, amino acid transport, metabolism, and autophagy. Further, when activated PERK phosphorylates eIF2α to induce translational suppression [74], the remaining ribosomes are able to translate mRNAs encoding proteins for unfolded protein response (UPR), such as ATF4 [75]. In addition, activation of inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α) in response to hypoxia promotes activation of functional X-box binding protein 1 (XBP1), which regulates multiple metabolic pathways [76,77]. Mitochondrial functions are also regulated by hypoxia, as 0.3% O2 is the rate-limiting threshold for electron transport complex (ETC) activity [78]. Likewise, the tricarboxylic acid (TCA) cycle is reduced, mitochondria translocate to the perinuclear site, and mitochondrial fission and mitophagy are induced in both a HIF-dependent and HIF-independent manner [79,80,81,82]. The most prominent aspect of adaptation to hypoxia is upregulation of glucose uptake and glycolysis. This is mediated by HIF-1α, which directly regulates expression of glucose transporter 1 (GLUT1), GLUT3, hexokinase 1 and 2, enolase 1, phosphoglycerate kinase 1 (PGK1), pyruvate kinase M2 (PKM2), lactate dehydrogenase A (LDHA), and phosphoinositide-dependent kinase 1 (PDK1) [83,84]. PDK1 inhibits conversion of pyruvate to acetyl CoA, thereby promoting lactate production [85]. This glycolysis-mediated enrichment of lactate and H+ ions lowers the surrounding pH, and critically, both the presence of lactate and low pH are harmful for antitumor immunity [86]. Hypoxia adaptation mechanisms are also involved in cell death pathways. For example, hypoxia induces autophagic cell death in apoptosis-competent cells via BCL2-interacting protein 3 (BNIP3) [87] and promotes necrosis of neuronal cells [88]. However, alarmin release by necrotic cells further promotes progression of glioblastoma stem-like cells [89].
Critically, hypoxia is also associated with increased radioresistance in GBM. Although the underlying mechanism is not clear, several molecular pathways have been implicated in this phenomenon. In one instance, it was shown that the mitogen-activated protein kinase (MAPKK; MEK)/Extracellular signal-regulated kinase (EKR) pathway promotes hypoxia-mediated radioresistance via the activity of DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) and HIF-1α [90]. Another study found that phospholipase C gamma (PLCγ) binding to fibroblast growth factor receptor 1 (FGFR1) induces protein kinase C (PKC) activation in response to HIF-1α regulation, and this also induces radioresistance [91]. In addition, hypoxia promotes glioma stem cell (GSC) formation by inducing stem cell marker genes, including octamer-binding transcription factor 4 (OCT4), NANOG, SRY-Box transcription factor 2 (SOX2), Kruppel-like factor 4 (KLF4), and cMYC, while downregulating expression of glial fibrillary acidic protein (GFAP) [92,93], and this was further shown to be critical for inducing radioresistance.
HIF proteins and HRE genes also regulate a number of angiogenesis-related molecules, such as VEGFs, placenta growth factors (PGFs), angiopoietin (ANGPT), CXCL12, and platelet-derived growth factor B (PDGF-B) [94]. In response to hypoxia, VEGFs and PGFs bind to VEGF receptor (VEGFR)-1 and -2 on endothelial cells and induce proliferation and survival via the ERK/PI3K/AKT pathways [95]. Rho GTPase-mediated migration and membrane type matrix metalloproteinase (MMP)-mediated extracellular matrix (ECM) degradation are also induced by VEGF/PGF binding [96,97]. Among the angiogenesis-related molecules induced by hypoxia, the most well-studied protein is VEGF-A. Notably, although this protein is critical for homeostatic vasculature, hypoxia-mediated VEGF-A overexpression induces vascular permeability, which in turn, inhibits the delivery of drugs and immune cells, limits perfusion, and even further promotes hypoxia [94].
4. Hypoxia and Antitumor Immunity
4.1. Hypoxia in an Immunological Niche
Like other cells, immune cells need proper oxygen levels for survival and function. However, despite mechanisms to maintain homeostasis and normoxia in most instances, some niches can become hypoxic due to anatomic characteristics of organs or burst of cellular expansion. This is referred to as “physiological hypoxia” [50], and in some cases, it is necessary for proper organ function. The bone marrow is one of the most well-characterized physiologically hypoxic organs [98]. Here, hypoxia is critical for maintaining hematopoietic stem cell (HSC) homeostasis [99]. Although it remains controversial, HIF-1β was shown to be required for quiescence, survival, and development of HSCs, whereas HIF-2α is dispensable for HSC function [100,101]. Germinal centers (GCs) are another example of a physiologically hypoxic environment. During maturation of B cells, the oxygen gradient decreases within the GC [102], possibly as a result of increased oxygen consumption by expanding B cells. Notably, GC hypoxia was found to affect phenotype, proliferation, and class switching of B cells [102,103]. The reproductive organs also show physiological hypoxia. For example, although exact O2 tension values in seminiferous tubules remain controversial, the testicular interstitium is hypoxic, showing O2 tension values of about 12 to 15 mmHg [104]. Likewise, the vagina is hypoxic under normal conditions [105], and physiological hypoxia in the placenta modulates immune function by protecting the fetus from the maternal immune system; accordingly, HIF dysfunction is associated with placental defects [106]. HIF-1α-mediated gene expression in trophoblasts regulates non-classical class I histocompatibility antigen to prevent attack from natural killer (NK) cells [107], and HIF-1α-mediated programmed cell death 1 ligand 1 (PD-L1) was further found to inhibit T cell responses [108]. The intestinal mucosa is also hypoxic [109], and here, it was shown that physiological hypoxia regulates epithelial barrier function and resident immune cells [110].
4.2. Antitumor Immunity
In general, antitumor immunity is similar to persistent antiviral immunity [111]. Although many different immune cells can participate in antitumor responses, T cells are thought to be the most important antitumor immune cells. When tumor antigen is released, antigen-presenting cells (APCs), such as DCs, take-up antigen and migrate into the lymph nodes (LN), where they present their antigen to T cells. Antigen-specific T cells then undergo priming and clonal expansion, and these activated T cells migrate into the tumor area. CD4+ T cells orchestrate antitumor immunity and CD8+ T cells recognize and directly kill tumor cells [112]. However, tumor cells often poorly express major histocompatibility complex (MHC) class I molecule to escape from CD8+ T cell-mediated immunosurveillance [113]. In addition, most tumors have antigens that resemble our self-antigens, thereby inducing tolerance [114]. This limits antitumor T cell activity, and as a result of persistent activation, T cells become exhausted and lose their function. Exhausted CD8+ T cells, for example, will express PD-1 on the surface, which binds to PD-L1 on tumor cells or myeloid cells and inhibits T cell function [115,116].
Another class of T cells known as γδ T cells also participate in antitumor immunity and are highly correlated with favorable patient outcomes [117]. These cells can recognize tumor cells via γδTCR or NK-like receptors [118], the upregulation of their ligands of which are induced by transformation and cellular stress [119]. For example, tumor cells highly express NK group 2 member D (NKG2D)-ligands, such as MHC class I polypeptide-related sequence A/B (MICA/B) in humans and retinoic acid early transcript 1 (RAE-1) for mice [120].
Beyond T cells, myeloid cells, including macrophages, monocytes, and neutrophils, can also act as antitumor cells via phagocytosis or the production of inflammatory cytokines [121,122]. However, most tumor-infiltrating myeloid cells are immunosuppressive; these are known as myeloid-derived suppressive cells (MDSCs). MDSCs can suppress antitumor immunity through multiple mechanisms, including interleukin (IL)-10 secretion [123]. Likewise, regulatory T cells (Tregs) also suppress antitumor immune responses [124], and various other immune cells, such as innate lymphoid cells (ILCs) [125], B cells [126], and eosinophils [127], can promote antitumor or protumor responses, depending on the context and environment.
4.3. Antitumor Immunity in the GBM
Unlike peripheral tumors, antitumor immunity against brain tumors has been poorly described. Because of the strong blood–brain barrier (BBB), infiltration of lymphocytes is limited, and thus, the brain is considered to be an immune-privileged organ [128]. Microglia are the predominant immune cells in the brain, although a limited number of other immune cells, such as T cells and mast cells, are also present [129,130]. Likewise, the brain tumor microenvironment is also primarily enriched with microglia and bone marrow-derived macrophages [131]. Due to these unique characteristics, as well as low frequency of neoantigen, antitumor immunity in the brain and responsiveness to immunotherapies is quite poor (Figure 3) [132]. Thus, brain tumors such as GBM are often referred to as “cold tumors” [133].
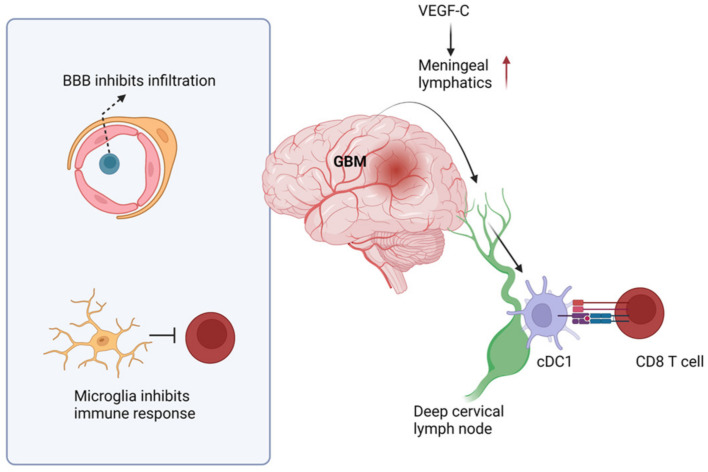
Figure 3.
Immune responses in the GBM. GBM antigens are drained by meningeal lymphatics, and classical DC-1s (cDC1s) present antigen to CD8+ T cells in the deep cervical lymph node. However, due to the strong blood–brain barrier (BBB), immune cell infiltration into the parenchyma is limited. In addition, the predominant microglia suppress immune responses. However, enhancing lymphatics via VEGF-C treatment has shown promising results to improve survival in animal models.
However, a recent study found that that classical DC-1s (cDC1s) can infiltrate into the GBM area and present antigen to T cells in deep cervical LNs (dcLNs) [134]. This study further suggested that CD141+ cDC1s can present antigen in human GBM patients. Regardless, the role of cDC1s and CD8+ T cells in GBM is negligible without immunotherapies, such as anti-PD-L1 treatment [135]. Further, our group showed that CD4+ and CD8+ T cells are dispensable for OS of GBM patients and animals [136]. These studies suggest that although immune responses do occur in the GBM microenvironment, they are too weak to protect host. Consistent with these observations, Song et al. showed that meningeal lymphatics are dampened by GBM progression, suggesting this is one reason for poor anti-GBM immunity [41]. Notably, they further showed that if lymphatics are improved by VEGF-C application, most GBM-bearing animals can survive (Figure 3) [41]. However, extracranial antigen presentation was found to be unable to promote tumor eradication without immunotherapies in a melanoma brain metastasis model [137]. This suggests that antigen presentation in the periphery is unlikely to be sufficient for inducing anti-brain tumor immunity, and further study is needed.
Macrophages and microglia are also important components of anti-GBM immunity. Past in vitro studies have suggested that macrophages can be divided by two groups: M1 and M2. M1 macrophages are related to Th1 responses, whereas M2 macrophages regulate wound healing and Th2 responses [138]. However, recent studies have shown that subsets of macrophages are more complex than previously thought. Tumor-associated macrophages (TAMs), for example, are neither M1- nor M2-like, and rather show mixed phenotypes [139]. Within the tumor microenvironment, most TAMs are M2-like cells; however, proinflammatory TAMs that can phagocytose tumor cells also exist [140]. One well-known mechanism by which this occurs is via the SIRPα–CD47 axis. CD47 is a “do not eat me” signal that inhibits phagocytosis of SIRPα-expressing TAMs. Thus, we can improve phagocytosis by TAMs using CD47 blockade [141]. In addition, the depletion of M2-like TAMs by blocking colony stimulating factor 1 receptor (CSF1R) is also considered to be a promising therapeutic strategy [142]. Conversely, although they are dominant type of immune cells in the brain, the role of microglia in GBM is still unclear. Microglia are usually located at the surrounding edge of the tumor mass rather than inner area [143], but the reason for this is not known, and more studies on microglia in GBM are needed.
NK cells and γδ T cells are also able to kill GBM cells [144,145]. Notably, although in vivo blocking of NK1.1 does not affect OS of GBM-bearing mice [136], another study showed that NK1.1-blockade increases GBM size [146], suggesting an antitumor role for NK cells. Intriguingly, anti-GBM NK cell activity was found to be dependent on the gut microbiota [146]. In addition, NK cells display more potent activity against stem-like GBM cells [144], although direct contact between NK cells and GBM stem cells (GSCs) via αv integrin induces TGF-β-mediated NK cell suppression [147]. Thus, more studies on the role of NK cells in GBM are needed. Other groups have focused on the role of γδ T cells; one murine study showed that Vγ1, Vγ4, and Vγ7 T cells are present in the brain tumor area [148], with Vγ7 comprising the most dominant γ chain. This study further showed that Vδ1, Vδ4, and Vδ6.3 T cells are able to infiltrate into GBM tissue, and in this case, Vδ6.3 is the most prevalent δ chain. In addition, findings from this investigation suggested that γδ T cells are diminished at the terminal stage of tumor progression due to apoptosis, with Vδ6.3+ T cells showing the most vulnerability to cell death. Preferential infiltration of Vγ9Vδ2 T cells was also observed in human brain tumor tissue [149], and interestingly, these cells can preferentially kill mesenchymal GBM cells via NKG2D [150]. Further, despite limited investigation, one study suggested that B cells are immunosuppressive within the GBM [151].
4.4. The Role of Hypoxia in Anti-GBM Immune Responses
Hypoxia has been shown to affect multiple functions of anti-cancer immune cells. For example, in vitro culture of CD8+ T cells under hypoxic conditions promotes reduced levels of proliferation, cytokine production, and cytotoxicity. Melanoma-infiltrating CD8+ T cells are also highly exhausted and malfunctional due to severe hypoxia [11]. Similarly, immune cells in GBM core tissue, which primarily comprise M2 macrophages and regulatory T cells, are highly hypoxic (Figure 4a). CD8+ T cells within the hypoxic core are also exhausted, and peripheral CD8+ T cells cultured in vitro under hypoxic conditions phenocopy CD8+ T cells from the GBM core [152].
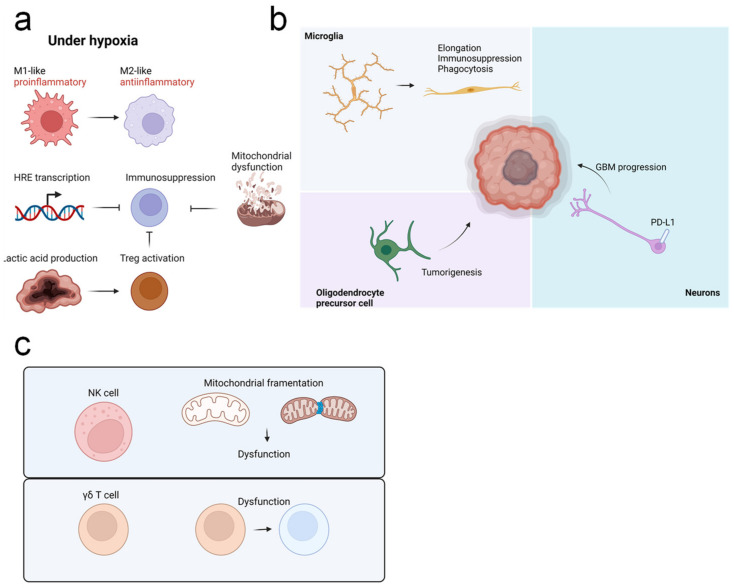
Figure 4.
The effect of hypoxia on anti-GBM immune responses. (a) Under hypoxia, macrophages preferentially show an immunosuppressive M2-like phenotype, rather than an inflammatory M1-like phenotype. Tumor-infiltrating lymphocytes are suppressed in response to activation of HRE genes and mitochondrial dysfunction, and accumulation of lactic acid supports stability and function of regulatory T cells (Tregs), to further suppress inhibit responses. (b) Microglia in the tumor area show an elongated phenotype; these cells are immunosuppressive, with enhanced phagocytosis ability. Oligodendrocyte precursor cells are thought to be a precursor for GBM cells. Connection with neurons also supports GBM progression, whereas PD-L1 expression from neurons is associated with improved prognosis of GBM patients. (c) Hypoxia inhibits functions of natural killer (NK) cells and γδ T cells and promotes dysfunction of NK cell mitochondria.
Expression of HRE genes, including PD-L1, is induced by hypoxia in tumor cells and immune cells [153]. However, in melanoma, hypoxia-induced metabolic stress also inhibits mitochondrial biogenesis in tumor-infiltrating lymphocytes (TILs). Competition between tumor cells and TILs further suppresses the metabolic activity of TILs and inhibits reactivity against immune checkpoint blockade [154], and these immunosuppressive effects are enhanced if hypoxia is chronically persistent [15]. Notably, although PD-1 blockade alone is unable to rescue mitochondrial dysfunction, metabolic reprogramming is sufficient to reverse the exhaustion of TILs [155]. However, TILs in the GBM may be different from those present in subcutaneous tumor models. One study found that modulation of hypoxia using metformin is not sufficient to reinvigorate CD8+ T cell responses in a GBM model [136], and further study is needed.
CD4+ T cells include various subsets; T helper 1 (Th1) cells resemble CD8+ T cells, whereas Tregs show weaker glycolysis and are more oxidative than effector T cells [156]. One study using a B16 melanoma model reported that glucose uptake is closely related to Treg stability, as these cells utilize lactic acid to stabilize their suppressive identity [157]. In the GBM microenvironment, oxidative phosphorylation promotes immunosuppression of Tregs, whereas glycolysis enhances migration (Figure 4a). Further, in a hypoxic microenvironment, Tregs in the GBM use fatty acids for immunosuppression [158], and this is tightly regulated by HIF-1α.
Because hypoxia transiently disrupts the BBB, it may be related to inflammation and inflammation-associated features of the microglia [159]. In Alzheimer’s disease (AD), acute hypoxia induces the M1 transition of microglia [160]. However, hypoxia also inhibits mitochondrial metabolism and promotes the cell cycle arrest of microglia in AD [161]. In GBM tissue, microglia are highly distributed near pseudopalisades and do not escape from hypoxia. In addition, microglia under hypoxia show elongated morphology and increased phagocytosis capability (Figure 4b) [162]. However, the precise role of microglia and hypoxic microglia in GBM is unknown and requires further investigation. Intriguingly, recent studies have shown that other brain-resident cells can participate in GBM progression. For example, oligodendrocyte precursor cells (OPCs) may be associated with GBM progression, and these cells have been proposed as a possible origin of GBM cells [163]. Synaptic and electric communication between GBM cells and neurons also promotes GBM progression [164]. In addition, it was shown that astrocytes suppress immune response in the GBM microenvironment [165]. Because these cell types are also affected by hypoxia, this should be further studied in the context of GBM. Intriguingly, one study reported that neuronal expression of PD-L1, which is known to be regulated by hypoxia, is related to better prognosis of GBM patients [166], thus suggesting a possible role for hypoxic brain cells in GBM progression (Figure 4b).
Hypoxia is also related to dysfunction of NK cells (Figure 4c) [167], as hypoxia-associated mitochondrial fragmentation disrupts NK cell-mediated antitumor immunity [168]. However, the precise effect of hypoxia on NK cell function within the GBM microenvironment remains unclear. Similarly, cytotoxicity of γδ T cells, which are similar to, but distinct from, NK cells, and can kill tumor cells via both γδTCR and NKG2D, is also dampened by hypoxia (Figure 4c) [169]. Further, in the GBM microenvironment, γδ T cells are apoptotic and malfunctional [148], due to the fact that GBM patients receive radiochemotherapy, which also kills γδ T cells. Thus, MGMT-modified γδ T cell therapy might represent an alternative treatment strategy [170]. However, in a murine model, γδ T cells were found to be apoptotic without any treatment [148]. Thus, we expect that the tumor microenvironment suppresses γδ T cell function. Results from our previous study suggest that hypoxia is the critical mechanism mediating suppression of γδ T cells in the GBM [136]. Specifically, we found that when metformin is given to GBM-bearing mice, tumor cell respiration is inhibited, and the remaining oxygen can be utilized by γδ T cells. In addition, adoptive γδ T cell therapy with metformin or HIF-1α is able to prolong overall survival of GBM-bearing mice. Thus, rescuing hypoxia of γδ T cells could be beneficial for γδ T cell-mediated anti-GBM immunity.
5. Clinical Perspectives
GBM is one of the most immunologically poor tumor types. Although a number of mechanisms, such as the BBB, participate in immunosuppression, hypoxia is a critical immunosuppressive factor in the GBM microenvironment. Thus, even when immune cells are able to infiltrate into the GBM microenvironment, this hypoxic niche suppresses their antitumor functions. As discussed above, hypoxia inhibits multiple immune cells that are important for antitumor immunity, including CD8+ T cells and γδ T cells. Conversely, functions of immunosuppressive cells, such as Tregs and M2 macrophages, are enhanced by hypoxia. As a consequence of this strong immunosuppression, clinical trials assessing the use of immunotherapy for the treatment of GBM have been unsuccessful. An improved understanding of the unique features of anti-GBM immunity is therefore urgently needed to overcome these hurdles and develop effective treatment options. In particular, studies aimed at further investigating the effects of hypoxia on the multiple types of GBM-infiltrating immune cells will help to elucidate the mechanisms by which hypoxia suppresses immune function and determine how this could be overcome. One possible approach is through the use of immunotherapy combined with anti-hypoxic strategies, such as vessel normalization. Alternatively, cell therapy using engineered hypoxia-resistant immune cells may be another option for next-generation immunotherapy against hypoxic tumors.
6. Conclusions
Hypoxia is a classic hallmark of tumors [5], and GBM is one of the most hypoxic tumors. Hypoxia affects multiple aspects of GBM biology and pathology, including vasculature, invasiveness, resistance to drugs, and antitumor immune responses [42,171]. Critically, hypoxia-driven invasion and angiogenesis are highly associated with poor prognosis, and hypoxia-mediated resistance to conventional therapies, including chemotherapy and radiation, is a significant hurdle when caring for GBM patients. Furthermore, recently developed immunotherapies are also not effective against GBM, as it is considered to be a “cold tumor”, with low neoantigen levels and poor immune cell infiltration [132]. There are many reasons why GBM is a “cold tumor”, and hypoxia is one critical factor that promotes immunosuppression within the GBM microenvironment [61]. Although the detrimental role of hypoxia in immune cell function has been well-studied, the precise impact of hypoxia on anti-GBM immunity is unclear. In particular, CD8+ T cells are known to be suppressed by hypoxia, but unlike in other tumors, a re-oxygenation strategy was not effective for restoring the CD8+ T cell function in GBM. In contrast, improving the oxygen metabolism of γδ T cells was sufficient to increase the survival period of GBM-bearing animals, suggesting a critical role for these cells [136]. Thus, the effect of hypoxia on various immune cell types within the GBM is a critical area of investigation, as targeting hypoxia may be beneficial for improving the efficacy of conventional therapy and immune responses against this deadly tumor.
Acknowledgments
The authors thank the members of the Laboratory of Host Defenses for helpful discussions. Figures were created with BioRender.com.
Author Contributions
Conceptualization, J.H.P. and H.K.L.; writing—original draft preparation, J.H.P. and H.K.L.; writing—review and editing, J.H.P. and H.K.L.; visualization, J.H.P. and H.K.L.; supervision, H.K.L.; project administration, J.H.P. and H.K.L.; funding acquisition, J.H.P. and H.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of Korea (NRF-2021M3A9D3026428 and NRF-2021M3A9H3015688) funded by the Ministry of Science and ICT of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vadlakonda L., Dash A., Pasupuleti M., Anil Kumar K., Reddanna P. Did we get pasteur, warburg, and crabtree on a right note? Front. Oncol. 2013;3:186. doi: 10.3389/fonc.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boedtkjer E., Pedersen S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020;82:103–126. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- 3.Giaccia A.J., Simon M.C., Johnson R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–2194. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein T., Gatenby R.A., Brown J.S. The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS ONE. 2017;12:e0185085. doi: 10.1371/journal.pone.0185085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 6.Thomlinson R.H., Gray L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown J.M., Giaccia A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 8.Simon M.C., Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gropper Y., Feferman T., Shalit T., Salame T.M., Porat Z., Shakhar G. Culturing CTLs under Hypoxic Conditions Enhances Their Cytolysis and Improves Their Anti-tumor Function. Cell Rep. 2017;20:2547–2555. doi: 10.1016/j.celrep.2017.08.071. [DOI] [PubMed] [Google Scholar]
- 10.Lim S.A., Moon Y., Shin M.H., Kim T.J., Chae S., Yee C., Hwang D., Park H., Lee K.M. Hypoxia-Driven HIF-1alpha Activation Reprograms Pre-Activated NK Cells towards Highly Potent Effector Phenotypes via ERK/STAT3 Pathways. Cancers. 2021;13:1904. doi: 10.3390/cancers13081904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharping N.E., Menk A.V., Whetstone R.D., Zeng X., Delgoffe G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017;5:9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar S., Germeraad W.T., Rouschop K.M., Steeghs E.M., van Gelder M., Bos G.M., Wieten L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS ONE. 2013;8:e64835. doi: 10.1371/journal.pone.0064835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balsamo M., Manzini C., Pietra G., Raggi F., Blengio F., Mingari M.C., Varesio L., Moretta L., Bosco M.C., Vitale M. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur. J. Immunol. 2013;43:2756–2764. doi: 10.1002/eji.201343448. [DOI] [PubMed] [Google Scholar]
- 14.Velasquez S.Y., Killian D., Schulte J., Sticht C., Thiel M., Lindner H.A. Short Term Hypoxia Synergizes with Interleukin 15 Priming in Driving Glycolytic Gene Transcription and Supports Human Natural Killer Cell Activities. J. Biol. Chem. 2016;291:12960–12977. doi: 10.1074/jbc.M116.721753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharping N.E., Rivadeneira D.B., Menk A.V., Vignali P.D.A., Ford B.R., Rittenhouse N.L., Peralta R., Wang Y., Wang Y., DePeaux K., et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 2021;22:205–215. doi: 10.1038/s41590-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erecinska M., Silver I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001;128:263–276. doi: 10.1016/S0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 17.Beppu T., Kamada K., Yoshida Y., Arai H., Ogasawara K., Ogawa A. Change of oxygen pressure in glioblastoma tissue under various conditions. J. Neurooncol. 2002;58:47–52. doi: 10.1023/A:1015832726054. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 19.Miller K.D., Ostrom Q.T., Kruchko C., Patil N., Tihan T., Cioffi G., Fuchs H.E., Waite K.A., Jemal A., Siegel R.L., et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021;71:381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- 20.Burnet N.G., Jefferies S.J., Benson R.J., Hunt D.P., Treasure F.P. Years of life lost (YLL) from cancer is an important measure of population burden--and should be considered when allocating research funds. Br. J. Cancer. 2005;92:241–245. doi: 10.1038/sj.bjc.6602321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Australian Institute of Health and Welfare . Cancer in Australia 2021. AIHW; Canberra, Australia: 2021. [Google Scholar]
- 22.Ohgaki H., Kleihues P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 23.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q., Hu B., Hu X., Kim H., Squatrito M., Scarpace L., deCarvalho A.C., Lyu S., Li P., Li Y., et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell. 2017;32:42–56.e46. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huse J.T., Phillips H.S., Brennan C.W. Molecular subclassification of diffuse gliomas: Seeing order in the chaos. Glia. 2011;59:1190–1199. doi: 10.1002/glia.21165. [DOI] [PubMed] [Google Scholar]
- 26.Stummer W., van den Bent M.J., Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: New arguments in an old discussion. Acta Neurochir. 2011;153:1211–1218. doi: 10.1007/s00701-011-1001-x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson T.A., Karajannis M.A., Harter D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014;5:64. doi: 10.4103/2152-7806.132138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakomy R., Kazda T., Selingerova I., Poprach A., Pospisil P., Belanova R., Fadrus P., Vybihal V., Smrcka M., Jancalek R., et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front. Oncol. 2020;10:840. doi: 10.3389/fonc.2020.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3:198–210. doi: 10.1016/j.gendis.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitange G.J., Carlson B.L., Schroeder M.A., Grogan P.T., Lamont J.D., Decker P.A., Wu W., James C.D., Sarkaria J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro. Oncol. 2009;11:281–291. doi: 10.1215/15228517-2008-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassman A.B., Joanta-Gomez A.E., Pan P.C., Wick W. Current usage of tumor treating fields for glioblastoma. Neurooncol. Adv. 2020;2:vdaa069. doi: 10.1093/noajnl/vdaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stupp R., Taillibert S., Kanner A.A., Kesari S., Steinberg D.M., Toms S.A., Taylor L.P., Lieberman F., Silvani A., Fink K.L., et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 33.Chinot O.L., Wick W., Mason W., Henriksson R., Saran F., Nishikawa R., Carpentier A.F., Hoang-Xuan K., Kavan P., Cernea D., et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert M.R., Dignam J.J., Armstrong T.S., Wefel J.S., Blumenthal D.T., Vogelbaum M.A., Colman H., Chakravarti A., Pugh S., Won M., et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Vinals F., Inoue M., Bergers G., Hanahan D., Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J.S., Kim I.K., Han S., Park I., Kim C., Bae J., Oh S.J., Lee S., Kim J.H., Woo D.C., et al. Normalization of Tumor Vessels by Tie2 Activation and Ang2 Inhibition Enhances Drug Delivery and Produces a Favorable Tumor Microenvironment. Cancer Cell. 2016;30:953–967. doi: 10.1016/j.ccell.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Dapash M., Castro B., Hou D., Lee-Chang C. Current Immunotherapeutic Strategies for the Treatment of Glioblastoma. Cancers. 2021;13:4548. doi: 10.3390/cancers13184548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oronsky B., Reid T.R., Oronsky A., Sandhu N., Knox S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2020;10:574012. doi: 10.3389/fonc.2020.574012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buerki R.A., Chheda Z.S., Okada H. Immunotherapy of Primary Brain Tumors: Facts and Hopes. Clin. Cancer Res. 2018;24:5198–5205. doi: 10.1158/1078-0432.CCR-17-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forsyth P.A., Abate-Daga D. Oncolytic Virotherapy for Malignant Gliomas. J. Clin. Oncol. 2018;36:1440–1442. doi: 10.1200/JCO.2017.77.3192. [DOI] [PubMed] [Google Scholar]
- 41.Song E., Mao T., Dong H., Boisserand L.S.B., Antila S., Bosenberg M., Alitalo K., Thomas J.L., Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689–694. doi: 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteiro A.R., Hill R., Pilkington G.J., Madureira P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells. 2017;6:45. doi: 10.3390/cells6040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brat D.J., Van Meir E.G. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: A new world of angiogenesis research. Am. J. Pathol. 2001;158:789–796. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bates D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain S., Maltepe E., Lu M.M., Simon C., Bradfield C.A. Expression of ARNT, ARNT2, HIF1 alpha, HIF2 alpha and Ah receptor mRNAs in the developing mouse. Mech. Dev. 1998;73:117–123. doi: 10.1016/S0925-4773(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 47.Zhong H., De Marzo A.M., Laughner E., Lim M., Hilton D.A., Zagzag D., Buechler P., Isaacs W.B., Semenza G.L., Simons J.W. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 48.Talks K.L., Turley H., Gatter K.C., Maxwell P.H., Pugh C.W., Ratcliffe P.J., Harris A.L. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 2000;157:411–421. doi: 10.1016/S0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan C. Hypoxia-inducible factor 3 biology: Complexities and emerging themes. Am. J. Physiol. Cell Physiol. 2016;310:C260–C269. doi: 10.1152/ajpcell.00315.2015. [DOI] [PubMed] [Google Scholar]
- 50.Taylor C.T., Colgan S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keith B., Johnson R.S., Simon M.C. HIF1alpha and HIF2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang P., Yao Q., Lu L., Li Y., Chen P.J., Duan C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014;6:1110–1121. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Makino Y., Cao R., Svensson K., Bertilsson G., Asman M., Tanaka H., Cao Y., Berkenstam A., Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 54.Erbel P.J., Card P.B., Karakuzu O., Bruick R.K., Gardner K.H. Structural basis for PAS domain heterodimerization in the basic helix--loop--helix-PAS transcription factor hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA. 2003;100:15504–15509. doi: 10.1073/pnas.2533374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freedman S.J., Sun Z.Y., Poy F., Kung A.L., Livingston D.M., Wagner G., Eck M.J. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc. Natl. Acad. Sci. USA. 2002;99:5367–5372. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fong G.H., Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008;15:635–641. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- 57.Cockman M.E., Masson N., Mole D.R., Jaakkola P., Chang G.W., Clifford S.C., Maher E.R., Pugh C.W., Ratcliffe P.J., Maxwell P.H. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 58.Kallio P.J., Pongratz I., Gradin K., McGuire J., Poellinger L. Activation of hypoxia-inducible factor 1alpha: Posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc. Natl. Acad. Sci. USA. 1997;94:5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lando D., Peet D.J., Gorman J.J., Whelan D.A., Whitelaw M.L., Bruick R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyd N.H., Tran A.N., Bernstock J.D., Etminan T., Jones A.B., Gillespie G.Y., Friedman G.K., Hjelmeland A.B. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics. 2021;11:665–683. doi: 10.7150/thno.41692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vito A., El-Sayes N., Mossman K. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells. 2020;9:992. doi: 10.3390/cells9040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marotta D., Karar J., Jenkins W.T., Kumanova M., Jenkins K.W., Tobias J.W., Baldwin D., Hatzigeorgiou A., Alexiou P., Evans S.M., et al. In vivo profiling of hypoxic gene expression in gliomas using the hypoxia marker EF5 and laser-capture microdissection. Cancer Res. 2011;71:779–789. doi: 10.1158/0008-5472.CAN-10-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Z., Han L., Dong Y., Tan Y., Li Y., Zhao M., Xie H., Ju H., Wang H., Zhao Y., et al. EGFRvIII/integrin beta3 interaction in hypoxic and vitronectinenriching microenvironment promote GBM progression and metastasis. Oncotarget. 2016;7:4680–4694. doi: 10.18632/oncotarget.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skuli N., Monferran S., Delmas C., Favre G., Bonnet J., Toulas C., Cohen-Jonathan Moyal E. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: A novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308–3316. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 65.Xu Y., Zhang L., Wei Y., Zhang X., Xu R., Han M., Huang B., Chen A., Li W., Zhang Q., et al. Procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 promotes hypoxia-induced glioma migration and invasion. Oncotarget. 2017;8:23401–23413. doi: 10.18632/oncotarget.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maciaczyk D., Picard D., Zhao L., Koch K., Herrera-Rios D., Li G., Marquardt V., Pauck D., Hoerbelt T., Zhang W., et al. CBF1 is clinically prognostic and serves as a target to block cellular invasion and chemoresistance of EMT-like glioblastoma cells. Br. J. Cancer. 2017;117:102–112. doi: 10.1038/bjc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joseph J.V., Conroy S., Pavlov K., Sontakke P., Tomar T., Eggens-Meijer E., Balasubramaniyan V., Wagemakers M., den Dunnen W.F., Kruyt F.A. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1alpha-ZEB1 axis. Cancer Lett. 2015;359:107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Yang M.H., Wu M.Z., Chiou S.H., Chen P.M., Chang S.Y., Liu C.J., Teng S.C., Wu K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 69.Zagzag D., Lukyanov Y., Lan L., Ali M.A., Esencay M., Mendez O., Yee H., Voura E.B., Newcomb E.W. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: Implications for angiogenesis and glioma cell invasion. Lab. Investig. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 70.Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E., Capla J.M., Galiano R.D., Levine J.P., Gurtner G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y., Liu T., Yang N., Xu S., Li X., Wang D. Hypoxia and macrophages promote glioblastoma invasion by the CCL4-CCR5 axis. Oncol. Rep. 2016;36:3522–3528. doi: 10.3892/or.2016.5171. [DOI] [PubMed] [Google Scholar]
- 72.Braunstein S., Karpisheva K., Pola C., Goldberg J., Hochman T., Yee H., Cangiarella J., Arju R., Formenti S.C., Schneider R.J. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Van den Beucken T., Magagnin M.G., Jutten B., Seigneuric R., Lambin P., Koritzinsky M., Wouters B.G. Translational control is a major contributor to hypoxia induced gene expression. Radiother. Oncol. 2011;99:379–384. doi: 10.1016/j.radonc.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 74.Brewer J.W., Diehl J.A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 76.Bouchecareilh M., Higa A., Fribourg S., Moenner M., Chevet E. Peptides derived from the bifunctional kinase/RNase enzyme IRE1alpha modulate IRE1alpha activity and protect cells from endoplasmic reticulum stress. FASEB J. 2011;25:3115–3129. doi: 10.1096/fj.11-182931. [DOI] [PubMed] [Google Scholar]
- 77.Liu C.Y., Schroder M., Kaufman R.J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 78.Wilson D.F., Rumsey W.L., Green T.J., Vanderkooi J.M. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J. Biol. Chem. 1988;263:2712–2718. doi: 10.1016/S0021-9258(18)69126-4. [DOI] [PubMed] [Google Scholar]
- 79.Hayek I., Fischer F., Schulze-Luehrmann J., Dettmer K., Sobotta K., Schatz V., Kohl L., Boden K., Lang R., Oefner P.J., et al. Limitation of TCA Cycle Intermediates Represents an Oxygen-Independent Nutritional Antibacterial Effector Mechanism of Macrophages. Cell Rep. 2019;26:3502–3510. doi: 10.1016/j.celrep.2019.02.103. [DOI] [PubMed] [Google Scholar]
- 80.Thomas L.W., Staples O., Turmaine M., Ashcroft M. CHCHD4 Regulates Intracellular Oxygenation and Perinuclear Distribution of Mitochondria. Front. Oncol. 2017;7:71. doi: 10.3389/fonc.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuhrmann D.C., Brune B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017;12:208–215. doi: 10.1016/j.redox.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W., et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 83.Nakazawa M.S., Keith B., Simon M.C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer. 2016;16:663–673. doi: 10.1038/nrc.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atas E., Oberhuber M., Kenner L. The Implications of PDK1-4 on Tumor Energy Metabolism, Aggressiveness and Therapy Resistance. Front. Oncol. 2020;10:583217. doi: 10.3389/fonc.2020.583217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J.X., Choi S.Y.C., Niu X., Kang N., Xue H., Killam J., Wang Y. Lactic Acid and an Acidic Tumor Microenvironment suppress Anticancer Immunity. Int. J. Mol. Sci. 2020;21:8363. doi: 10.3390/ijms21218363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azad M.B., Chen Y., Henson E.S., Cizeau J., McMillan-Ward E., Israels S.J., Gibson S.B. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niquet J., Baldwin R.A., Allen S.G., Fujikawa D.G., Wasterlain C.G. Hypoxic neuronal necrosis: Protein synthesis-independent activation of a cell death program. Proc. Natl. Acad. Sci. USA. 2003;100:2825–2830. doi: 10.1073/pnas.0530113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papale M., Buccarelli M., Mollinari C., Russo M.A., Pallini R., Ricci-Vitiani L., Tafani M. Hypoxia, Inflammation and Necrosis as Determinants of Glioblastoma Cancer Stem Cells Progression. Int. J. Mol. Sci. 2020;21:2660. doi: 10.3390/ijms21082660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marampon F., Gravina G.L., Zani B.M., Popov V.M., Fratticci A., Cerasani M., Di Genova D., Mancini M., Ciccarelli C., Ficorella C., et al. Hypoxia sustains glioblastoma radioresistance through ERKs/DNA-PKcs/HIF-1alpha functional interplay. Int. J. Oncol. 2014;44:2121–2131. doi: 10.3892/ijo.2014.2358. [DOI] [PubMed] [Google Scholar]
- 91.Gouaze-Andersson V., Delmas C., Taurand M., Martinez-Gala J., Evrard S., Mazoyer S., Toulas C., Cohen-Jonathan-Moyal E. FGFR1 Induces Glioblastoma Radioresistance through the PLCgamma/Hif1alpha Pathway. Cancer Res. 2016;76:3036–3044. doi: 10.1158/0008-5472.CAN-15-2058. [DOI] [PubMed] [Google Scholar]
- 92.Mathieu J., Zhang Z., Zhou W., Wang A.J., Heddleston J.M., Pinna C.M., Hubaud A., Stadler B., Choi M., Bar M., et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kolenda J., Jensen S.S., Aaberg-Jessen C., Christensen K., Andersen C., Brunner N., Kristensen B.W. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J. Neurooncol. 2011;103:43–58. doi: 10.1007/s11060-010-0357-8. [DOI] [PubMed] [Google Scholar]
- 94.Abou Khouzam R., Brodaczewska K., Filipiak A., Zeinelabdin N.A., Buart S., Szczylik C., Kieda C., Chouaib S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2020;11:613114. doi: 10.3389/fimmu.2020.613114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karar J., Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zahra F.T., Sajib M.S., Ichiyama Y., Akwii R.G., Tullar P.E., Cobos C., Minchew S.A., Doci C.L., Zheng Y., Kubota Y., et al. Endothelial RhoA GTPase is essential for in vitro endothelial functions but dispensable for physiological in vivo angiogenesis. Sci. Rep. 2019;9:11666. doi: 10.1038/s41598-019-48053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H., Keiser J.A. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: Role of flt-1. Circ. Res. 1998;83:832–840. doi: 10.1161/01.RES.83.8.832. [DOI] [PubMed] [Google Scholar]
- 98.Parmar K., Mauch P., Vergilio J.A., Sackstein R., Down J.D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takubo K., Goda N., Yamada W., Iriuchishima H., Ikeda E., Kubota Y., Shima H., Johnson R.S., Hirao A., Suematsu M., et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 100.Guitart A.V., Subramani C., Armesilla-Diaz A., Smith G., Sepulveda C., Gezer D., Vukovic M., Dunn K., Pollard P., Holyoake T.L., et al. Hif-2alpha is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122:1741–1745. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- 101.Krock B.L., Eisinger-Mathason T.S., Giannoukos D.N., Shay J.E., Gohil M., Lee D.S., Nakazawa M.S., Sesen J., Skuli N., Simon M.C. The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood. 2015;125:3263–3272. doi: 10.1182/blood-2014-10-607267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho S.H., Raybuck A.L., Stengel K., Wei M., Beck T.C., Volanakis E., Thomas J.W., Hiebert S., Haase V.H., Boothby M.R. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abbott R.K., Thayer M., Labuda J., Silva M., Philbrook P., Cain D.W., Kojima H., Hatfield S., Sethumadhavan S., Ohta A., et al. Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination. J. Immunol. 2016;197:4014–4020. doi: 10.4049/jimmunol.1601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reyes J.G., Farias J.G., Henriquez-Olavarrieta S., Madrid E., Parraga M., Zepeda A.B., Moreno R.D. The hypoxic testicle: Physiology and pathophysiology. Oxid. Med. Cell Longev. 2012;2012:929285. doi: 10.1155/2012/929285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wagner G., Levin R. Oxygen tension of the vaginal surface during sexual stimulation in the human. Fertil. Steril. 1978;30:50–53. doi: 10.1016/S0015-0282(16)43395-9. [DOI] [PubMed] [Google Scholar]
- 106.Kozak K.R., Abbott B., Hankinson O. ARNT-deficient mice and placental differentiation. Dev. Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 107.Wakeland A.K., Soncin F., Moretto-Zita M., Chang C.W., Horii M., Pizzo D., Nelson K.K., Laurent L.C., Parast M.M. Hypoxia Directs Human Extravillous Trophoblast Differentiation in a Hypoxia-Inducible Factor-Dependent Manner. Am. J. Pathol. 2017;187:767–780. doi: 10.1016/j.ajpath.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barsoum I.B., Smallwood C.A., Siemens D.R., Graham C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 109.He G., Shankar R.A., Chzhan M., Samouilov A., Kuppusamy P., Zweier J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. USA. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Colgan S.P., Taylor C.T. Hypoxia: An alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim P.S., Ahmed R. Features of responding T cells in cancer and chronic infection. Curr. Opin. Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 113.Cornel A.M., Mimpen I.L., Nierkens S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers. 2020;12:1760. doi: 10.3390/cancers12071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nelson C.E., Thompson E.A., Quarnstrom C.F., Fraser K.A., Seelig D.M., Bhela S., Burbach B.J., Masopust D., Vezys V. Robust Iterative Stimulation with Self-Antigens Overcomes CD8(+) T Cell Tolerance to Self- and Tumor Antigens. Cell Rep. 2019;28:3092–3104. doi: 10.1016/j.celrep.2019.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 116.Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., Nair V.S., Xu Y., Khuong A., Hoang C.D., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park J.H., Lee H.K. Function of gammadelta T cells in tumor immunology and their application to cancer therapy. Exp. Mol. Med. 2021;53:318–327. doi: 10.1038/s12276-021-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hayday A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 120.Huang M., Sun R., Wei H., Tian Z. Simultaneous knockdown of multiple ligands of innate receptor NKG2D prevents natural killer cell-mediated fulminant hepatitis in mice. Hepatology. 2013;57:277–288. doi: 10.1002/hep.25959. [DOI] [PubMed] [Google Scholar]
- 121.Gul N., van Egmond M. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015;75:5008–5013. doi: 10.1158/0008-5472.CAN-15-1330. [DOI] [PubMed] [Google Scholar]
- 122.Anfray C., Ummarino A., Andon F.T., Allavena P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells. 2019;9:46. doi: 10.3390/cells9010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Veglia F., Sanseviero E., Gabrilovich D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021;21:485–498. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shimizu J., Yamazaki S., Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J. Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 125.Jacquelot N., Seillet C., Wang M., Pizzolla A., Liao Y., Hediyeh-Zadeh S., Grisaru-Tal S., Louis C., Huang Q., Schreuder J., et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 2021;22:851–864. doi: 10.1038/s41590-021-00943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsou P., Katayama H., Ostrin E.J., Hanash S.M. The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 2016;76:5597–5601. doi: 10.1158/0008-5472.CAN-16-0431. [DOI] [PubMed] [Google Scholar]
- 127.Davis B.P., Rothenberg M.E. Eosinophils and cancer. Cancer Immunol. Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 128.Pachter J.S., de Vries H.E., Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 2003;62:593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- 129.Herz J., Filiano A.J., Smith A., Yogev N., Kipnis J. Myeloid Cells in the Central Nervous System. Immunity. 2017;46:943–956. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pasciuto E., Burton O.T., Roca C.P., Lagou V., Rajan W.D., Theys T., Mancuso R., Tito R.Y., Kouser L., Callaerts-Vegh Z., et al. Microglia Require CD4 T Cells to Complete the Fetal-to-Adult Transition. Cell. 2020;182:625–640. doi: 10.1016/j.cell.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lim M., Xia Y., Bettegowda C., Weller M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018;15:422–442. doi: 10.1038/s41571-018-0003-5. [DOI] [PubMed] [Google Scholar]
- 133.Sampson J.H., Gunn M.D., Fecci P.E., Ashley D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer. 2020;20:12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Johanns T.M., Ward J.P., Miller C.A., Wilson C., Kobayashi D.K., Bender D., Fu Y., Alexandrov A., Mardis E.R., Artyomov M.N., et al. Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer Immunol. Res. 2016;4:1007–1015. doi: 10.1158/2326-6066.CIR-16-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bowman-Kirigin J.A., Saunders B.T., Desai R., Wang A.Z., Schaettler M.O., Liu C.J., Livingstone A.J., Kobayashi D.K., Durai V., Kretzer N.M., et al. The conventional dendritic cell 1 subset primes CD8+ T cells and traffics tumor antigen to drive anti-tumor immunity in the brain. bioRxiv. 2021 doi: 10.1101/2021.09.13.460088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park J.H., Kim H.J., Kim C.W., Kim H.C., Jung Y., Lee H.S., Lee Y., Ju Y.S., Oh J.E., Park S.H., et al. Tumor hypoxia represses gammadelta T cell-mediated antitumor immunity against brain tumors. Nat. Immunol. 2021 doi: 10.1038/s41590-020-00860-7. [DOI] [PubMed] [Google Scholar]
- 137.Taggart D., Andreou T., Scott K.J., Williams J., Rippaus N., Brownlie R.J., Ilett E.J., Salmond R.J., Melcher A., Lorger M. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8(+) T cell trafficking. Proc. Natl. Acad. Sci. USA. 2018;115:E1540–E1549. doi: 10.1073/pnas.1714089115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bohlson S.S., O’Conner S.D., Hulsebus H.J., Ho M.M., Fraser D.A. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front. Immunol. 2014;5:402. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hambardzumyan D., Gutmann D.H., Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016;19:20–27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Quail D.F., Joyce J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell. 2017;31:326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., Olson O.C., Quick M.L., Huse J.T., Teijeiro V., et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Darmanis S., Sloan S.A., Croote D., Mignardi M., Chernikova S., Samghababi P., Zhang Y., Neff N., Kowarsky M., Caneda C., et al. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep. 2017;21:1399–1410. doi: 10.1016/j.celrep.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Castriconi R., Daga A., Dondero A., Zona G., Poliani P.L., Melotti A., Griffero F., Marubbi D., Spaziante R., Bellora F., et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J. Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 145.Bryant N.L., Suarez-Cuervo C., Gillespie G.Y., Markert J.M., Nabors L.B., Meleth S., Lopez R.D., Lamb L.S., Jr. Characterization and immunotherapeutic potential of gammadelta T-cells in patients with glioblastoma. Neuro Oncol. 2009;11:357–367. doi: 10.1215/15228517-2008-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D’Alessandro G., Antonangeli F., Marrocco F., Porzia A., Lauro C., Santoni A., Limatola C. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 2020;50:705–711. doi: 10.1002/eji.201948354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaim H., Shanley M., Basar R., Daher M., Gumin J., Zamler D.B., Uprety N., Wang F., Huang Y., Gabrusiewicz K., et al. Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021;131 doi: 10.1172/JCI142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beck B.H., Kim H., O’Brien R., Jadus M.R., Gillespie G.Y., Cloud G.A., Hoa N.T., Langford C.P., Lopez R.D., Harkins L.E., et al. Dynamics of Circulating gammadelta T Cell Activity in an Immunocompetent Mouse Model of High-Grade Glioma. PLoS ONE. 2015;10:e0122387. doi: 10.1371/journal.pone.0122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee M., Park C., Woo J., Kim J., Kho I., Nam D.H., Park W.Y., Kim Y.S., Kong D.S., Lee H.W., et al. Preferential Infiltration of Unique Vgamma9Jgamma2-Vdelta2 T Cells Into Glioblastoma Multiforme. Front. Immunol. 2019;10:555. doi: 10.3389/fimmu.2019.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chauvin C., Joalland N., Perroteau J., Jarry U., Lafrance L., Willem C., Retiere C., Oliver L., Gratas C., Gautreau-Rolland L., et al. NKG2D Controls Natural Reactivity of Vgamma9Vdelta2 T Lymphocytes against Mesenchymal Glioblastoma Cells. Clin. Cancer Res. 2019;25:7218–7228. doi: 10.1158/1078-0432.CCR-19-0375. [DOI] [PubMed] [Google Scholar]
- 151.Lee-Chang C., Rashidi A., Miska J., Zhang P., Pituch K.C., Hou D., Xiao T., Fischietti M., Kang S.J., Appin C.L., et al. Myeloid-Derived Suppressive Cells Promote B cell-Mediated Immunosuppression via Transfer of PD-L1 in Glioblastoma. Cancer Immunol. Res. 2019;7:1928–1943. doi: 10.1158/2326-6066.CIR-19-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim A.R., Choi S.J., Park J., Kwon M., Chowdhury T., Yu H.J., Kim S., Kang H., Kim K.M., Park S.H., et al. Spatial immune heterogeneity of hypoxia-induced exhausted features in high-grade glioma. Oncoimmunology. 2022;11:2026019. doi: 10.1080/2162402X.2022.2026019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noman M.Z., Desantis G., Janji B., Hasmim M., Karray S., Dessen P., Bronte V., Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Najjar Y.G., Menk A.V., Sander C., Rao U., Karunamurthy A., Bhatia R., Zhai S., Kirkwood J.M., Delgoffe G.M. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight. 2019;4:e124989. doi: 10.1172/jci.insight.124989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scharping N.E., Menk A.V., Moreci R.S., Whetstone R.D., Dadey R.E., Watkins S.C., Ferris R.L., Delgoffe G.M. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F., Sullivan S.A., Nichols A.G., Rathmell J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watson M.J., Vignali P.D.A., Mullett S.J., Overacre-Delgoffe A.E., Peralta R.M., Grebinoski S., Menk A.V., Rittenhouse N.L., DePeaux K., Whetstone R.D., et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591:645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miska J., Lee-Chang C., Rashidi A., Muroski M.E., Chang A.L., Lopez-Rosas A., Zhang P., Panek W.K., Cordero A., Han Y., et al. HIF-1alpha Is a Metabolic Switch between Glycolytic-Driven Migration and Oxidative Phosphorylation-Driven Immunosuppression of Tregs in Glioblastoma. Cell Rep. 2019;27:226–237. doi: 10.1016/j.celrep.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Halder S.K., Milner R. Mild hypoxia triggers transient blood-brain barrier disruption: A fundamental protective role for microglia. Acta Neuropathol. Commun. 2020;8:175. doi: 10.1186/s40478-020-01051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang F., Zhong R., Li S., Fu Z., Cheng C., Cai H., Le W. Acute Hypoxia Induced an Imbalanced M1/M2 Activation of Microglia through NF-kappaB Signaling in Alzheimer’s Disease Mice and Wild-Type Littermates. Front. Aging Neurosci. 2017;9:282. doi: 10.3389/fnagi.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.March-Diaz R., Lara-Ureña N., Romero-Molina C., Heras-Garvin A., Ortega-de San Luis C., Alvarez-Vergara M.I., Sanchez-Garcia M.A., Sanchez-Mejias E., Davila J.C., Rosales-Nieves A.E., et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat. Aging. 2021;1:385–399. doi: 10.1038/s43587-021-00054-2. [DOI] [PubMed] [Google Scholar]
- 162.Saavedra-Lopez E., Roig-Martinez M., Cribaro G.P., Casanova P.V., Gallego J.M., Perez-Valles A., Barcia C. Phagocytic glioblastoma-associated microglia and macrophages populate invading pseudopalisades. Brain Commun. 2020;2:fcz043. doi: 10.1093/braincomms/fcz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dufour A., Gontran E., Deroulers C., Varlet P., Pallud J., Grammaticos B., Badoual M. Modeling the dynamics of oligodendrocyte precursor cells and the genesis of gliomas. PLoS Comput Biol. 2018;14:e1005977. doi: 10.1371/journal.pcbi.1005977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Venkatesh H.S., Morishita W., Geraghty A.C., Silverbush D., Gillespie S.M., Arzt M., Tam L.T., Espenel C., Ponnuswami A., Ni L., et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573:539–545. doi: 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henrik Heiland D., Ravi V.M., Behringer S.P., Frenking J.H., Wurm J., Joseph K., Garrelfs N.W.C., Strahle J., Heynckes S., Grauvogel J., et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 2019;10:2541. doi: 10.1038/s41467-019-10493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu Y., Carlsson R., Ambjorn M., Hasan M., Badn W., Darabi A., Siesjo P., Issazadeh-Navikas S. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J. Neurosci. 2013;33:14231–14245. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Teng R., Wang Y., Lv N., Zhang D., Williamson R.A., Lei L., Chen P., Lei L., Wang B., Fu J., et al. Hypoxia Impairs NK Cell Cytotoxicity through SHP-1-Mediated Attenuation of STAT3 and ERK Signaling Pathways. J. Immunol. Res. 2020;2020:4598476. doi: 10.1155/2020/4598476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zheng X., Qian Y., Fu B., Jiao D., Jiang Y., Chen P., Shen Y., Zhang H., Sun R., Tian Z., et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019;20:1656–1667. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 169.Sureshbabu S.K., Chaukar D., Chiplunkar S.V. Hypoxia regulates the differentiation and anti-tumor effector functions of gammadeltaT cells in oral cancer. Clin. Exp. Immunol. 2020;201:40–57. doi: 10.1111/cei.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lamb L.S., Pereboeva L., Youngblood S., Gillespie G.Y., Nabors L.B., Markert J.M., Dasgupta A., Langford C., Spencer H.T. A combined treatment regimen of MGMT-modified gammadelta T cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci. Rep. 2021;11:21133. doi: 10.1038/s41598-021-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chedeville A.L., Madureira P.A. The Role of Hypoxia in Glioblastoma Radiotherapy Resistance. Cancers. 2021;13:542. doi: 10.3390/cancers13030542. [DOI] [PMC free article] [PubMed] [Google Scholar]