Summary
Background
Neuroinflammatory diseases such as encephalitis, meningitis, multiple sclerosis and other neurological diseases with inflammatory components, have demonstrated a need for diagnostic biomarkers to define treatable and reversible neuroinflammation. The development and clinical validation of a targeted translational inflammation panel using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) could provide early diagnosis, rapid treatment and insights into neuroinflammatory mechanisms.
Methods
An inflammation panel of 13 metabolites (neopterin, tryptophan, kynurenine, kynurenic acid, 3-hydroxykynurenine, xanthurenic acid, anthranilic acid, 3-hydroxyanthranilic acid, quinolinic acid, picolinic acid, arginine, citrulline and methylhistamine) was measured based on a simple precipitation and filtration method using minimal CSF volume. The chromatographic separation was achieved using the Acquity UPLC BEH C18 column in combination with a gradient elution within a 12-min time frame. Acute encephalitis (n=10; myelin oligodendrocyte glycoprotein encephalitis n=3, anti-N-methyl-D-aspartate encephalitis n=2, acute disseminated encephalomyelitis n=2, herpes simplex encephalitis n=1, enteroviral encephalitis n=1) and frequency-matched non-inflammatory neurological disease controls (n=10) were examined.
Findings
The method exhibited good sensitivity as the limits of quantification ranged between 0.75 and 3.00 ng mL−1, good linearity (r2 > 0.99), acceptable matrix effects (<± 19.4%) and high recoveries (89.8-109.1 %). There were no interferences observed from common endogenous CSF metabolites, no carryover and concordance with well-established clinical methods. The accuracy and precision for all analytes were within tolerances, at <± 15 mean relative error and < 15 % coefficient of variation respectively. All analytes in matrix-matched pooled human CSF calibrators and human CSF extracts were stable for 24 h after extraction and two freeze-thaw cycles.
Interpretation
The method was successfully applied to a pilot study investigating acute brain inflammation case-control groups. Statistical discrimination between encephalitis (n=10) and control groups (n=10) was achieved using orthogonal partial least squares discriminant analysis and heatmap cluster analysis. Statistical analysis of the measured metabolites identified significant alterations of seven metabolites in the tryptophan-kynurenine pathway (tryptophan, kynurenine, kynurenic acid, 3-hydroxykynurenine, anthranilic acid, 3-hydroxyanthranilic acid, quinolinic acid), arginine and neopterin in presence of acute neuroinflammation. Furthermore, elevated ratios of CSF kynurenine/tryptophan ratio, quinolinic acid/kynurenic acid and anthranilic acid/3-hydroxyanthranilic acid provided strong discriminative power for neuroinflammatory conditions. Studies of large groups of neurological diseases are required to explore the sensitivity and specificity of the inflammation panel.
Funding
Financial support for the study was granted by Dale NHMRC Investigator grant APP1193648, Petre Foundation, Cerebral Palsy Alliance and Department of Biochemistry at the Children's Hospital at Westmead.
Keywords: clinical validation, cerebrospinal fluid, neuroinflammation, neopterin, tryptophan-kynurenine pathway, nitric oxide pathway
Research in context.
Evidence before this study
The significant morbidity and mortality rates of neuroinflammatory diseases has resulted in efforts to measure metabolites contributing to the pathophysiological processes. Several studies in neuroinflammatory diseases have reported alterations in the pterin, tryptophan-kynurenine and nitric oxide pathways. Moreover, our previous untargeted CSF metabolomics study identified statistical significance of nine tryptophan-kynurenine metabolites, four nitric oxide pathway metabolites and neopterin in acute encephalitis.
Added value of this study
Diagnostic biomarkers are important to define treatable and reversible neuroinflammation. In this study we developed and clinically validated an inflammation panel measuring thirteen cerebrospinal fluid metabolites from pterin, tryptophan-kynurenine and nitric oxide pathways. The inflammatory panel shows promising robustness and reproducibility in this pilot study.
Implications of all available evidence
Early detection of inflammation is crucial to ensure efficient treatments and improved clinical outcomes. We present a time and cost efficient LC-MS/MS method for routine applicability to translational clinical care.
Alt-text: Unlabelled box
Introduction
Inflammation of the brain is increasingly recognised in acute neuroinflammatory diseases (such as encephalitis and multiple sclerosis), but also neurodevelopmental, neuropsychiatric and neurodegenerative processes.1, 2, 3, 4, 5 The immune response upon inflammation induces the initiation and alteration of a wide range of neuroactive metabolites. The analysis of cerebrospinal fluid (CSF) specimens holds promise in the diagnosis of neurological pathologies.6
Inflammatory responses are coordinated through cellular and molecular interactions mediated by chemokines, cytokines or reactive oxygen species.7 The production of inflammatory mediators is formed from peripherally derived immune cells, CNS glia and endothelial cells. For example, C-reactive protein (CRP) is a clinical biomarker used to measure systemic inflammation.8, 9, 10 Pro-inflammatory cytokine and chemokines (such as interleukin-6 (IL-6), IL-8, interferon-γ (IFN-γ), IFN-inducible protein-10 (IP-10), chemokine [C-X-C motif] ligand 13, B-cell activating factor) are associated with acute and chronic neuroinflammatory diseases,11, 12, 13, 14, 15 however these cytokines/chemokines have limitations in clinical translation due to limited data on normative ranges and prolonged turnaround time involved in batch testing.16,17 Although peripheral blood biomarkers are less invasive, cost efficient and easy to access, they fail to discriminate neuroinflammation from systemic inflammation. By contrast, cerebrospinal fluid (CSF) is the closest biofluid to the brain and directly reflects the pathophysiological alterations of the CNS. The importance of simultaneous CSF and plasma analyses has been demonstrated in several studies.18, 19, 20
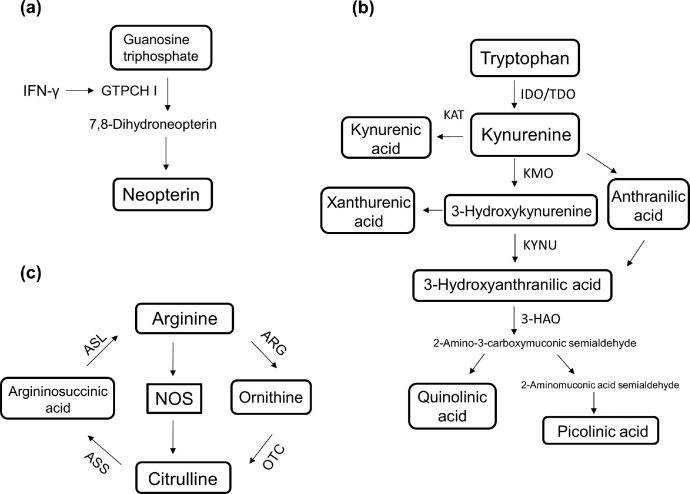
Metabolites are crucial for cellular functions, and small molecule metabolites such as amines, amino acids, neurotransmitters, fatty acids and products of cellular activities hold great potential bridging the knowledge of human diseases.21 Our previous untargeted metabolomics study demonstrated CSF neopterin, nine tryptophan-kynurenine metabolites and four nitric oxide pathway metabolites differentiated acute encephalitis from non-inflammatory controls.22 Neopterin is metabolised from the guanosine triphosphate pathway and is a direct product generated by the stimulation of γ-interferon (Figure 1). Neopterin is a well-established and valuable acute clinical biomarker of inflammation and infection in the central nervous system.23,24 The kynurenine pathway is a major route for the metabolism of tryptophan and plays a key role in neuroinflammation-mediated kynurenine dysregulation.25,26 Under basal conditions, indoleamine 2,3-dioxygenase plays a minor role in the metabolism of tryptophan.27 Inflammation-induced activation of indoleamine 2,3-dioxygenase stimulates the accelerated breakdown of tryptophan and imbalance formation of neuroprotective (e.g. kynurenic acid) and neurotoxic metabolites (e.g. 3-hydroxykynurenine, quinolinic acid) (Figure 1). Nitric oxide is a critical gasotransmitter involved in the regulation of physiological processes, neurotransmission, defence mechanisms, acute and chronic inflammation.28,29 The short half-life, rapid diffusion rate and gaseous nature poses an analytical challenge in the quantification of nitric oxide, therefore the measurement of more stable metabolites in the nitric oxide pathway is more practical. Nitric oxide is synthesised by nitric oxide synthases from arginine and citrulline (Figure 1). Arginine and citrulline play an important role in the immune responses and their availability during inflammation may serve as potential neuroinflammatory biomarkers.30
Figure 1.
Summary of targeted pathways associated with neuroinflammation (a) neopterin pathway, (b) tryptophan-kynurenine pathway and (c) nitric oxide pathway. Modifications from Yan et al.21
Preliminary studies predominantly employ discovery-based untargeted approaches, however, to successfully translate research data there is a growing demand for quantitative-driven methods. Metabolites in the tryptophan-kynurenine pathway, nitric oxide pathway and neopterin have been measured in CSF using gas chromatography-mass spectrometry, high performance liquid chromatography coupled to ultra-violet or fluorescence detection, LC-MS/MS and enzyme immunoassay.31, 32, 33, 34, 35, 36, 37 However, to date there has been no method reported to simultaneously quantify and validate neopterin and metabolites from the tryptophan-kynurenine pathway and nitric oxide pathway in human CSF for diagnostic purposes and clinical translation.
This study aimed to develop and clinically validate a translational LC-MS/MS method capable of detecting and quantifying neopterin and metabolites from the tryptophan-kynurenine pathway and nitric oxide pathway. We intend to deliver a simple and cost-effective method capable of processing samples individually with a rapid turnaround time for translational clinical care.
Methods
Chemicals and reagents
3-hydroxyanthranilic acid, 3-hydroxykynurenine, anthranilic acid, kynurenic acid, quinolinic acid, l-tryptophan, l-kynurenine, picolinic acid, xanthurenic acid, arginine and citrulline were purchased as powders from Sigma Aldrich (Sydney, Australia). Neopterin and methylhistamine were purchased from Novachem (Victoria, Australia). The thirteen metabolites are associated with neuroinflammation21 and reported as statistically significant in an untargeted CSF metabolomics study22 and hence selected as targeted metabolites of interest for the neuroinflammation panel.
D3-kynurenic acid and 15N-neopterin were supplied by CDN Isotopes (Quebec, Canada). D3-tryptophan, D4-kynurenine, 13C2-3-hydroxykynurenine, D3-3-hydroxyanthranilic acid, 13C6-arginine and D7-citrulline were from Toronto Research Chemicals (Toronto, Canada). HPLC grade acetonitrile, Ammonium formate, activated charcoal, metaphosphoric acid (MPA), ethylenediaminetetraacetic acid (EDTA) and Whatman filter paper were purchased from Sigma Aldrich (Sydney, Australia). Fresh Type I Water from a Millipore Elix Essential and Synergy-UV were used. Formic acid was supplied by Fisher Chemical (Fair Lawn, New Jersey). The Nanosep 0.2 μM and Nanosep with 3K omega centrifugal devices were supplied by Pall Australia Pty Ltd (Victoria, Australia). The catalogue numbers of the reagents and chemicals are provided in Supplemental Table 1.
Study design and participants
The study was conducted across two groups of human CSF patient samples that have not been used in any previous studies, obtained from the Department of Biochemistry at the Children's Hospital at Westmead, Sydney, Australia (Supplemental Table 2). The encephalitis group (n=10, eight females, two males; mean [median] age 6y 4mo [5y], range 1–16y) all fulfilled encephalitis criteria11 (myelin oligodendrocyte glycoprotein encephalitis n=3, anti-N-methyl-D-aspartate encephalitis n=2, acute disseminated encephalomyelitis n=2, herpes simplex encephalitis n=1, enteroviral encephalitis n=1). CSF was frozen within 1 hour of sampling and stored at –80°C until the time of processing. The CSF tube analysed in the pilot study was not used for routine testing and thawed only once after sampling.
The controls were frequency-matched based on age and gender (n=10; six females, four males; mean [median] age 7y 3mo [7y 6mo], range 3mo–14y) had non-inflammatory neurological diseases.
Ethics
The Sydney Children's Hospitals Network Ethics Committee approved this study (LNR/14/SCHN/275; 2019/ETH06182), including informed consent from parents and/or guardians.
Preparation of standard solutions
Matrix matched in-house calibrators and tri level quality controls were prepared by appropriately spiking charcoal stripped pooled human CSF. Charcoal stripped pooled human CSF was prepared by adding 3 g activated charcoal (Sigma Aldrich, Australia) to 50 mL of pooled human CSF and mixed using a shaker for 4 hours at 5 °C, followed by centrifugation at 3000 g for 20 minutes and filtration of the supernatant using a Whatman filter paper (Sigma Aldrich, Australia).
A 5 μg mL−1 mixed standard solution containing all thirteen metabolites was prepared and stored in -40°C. The mixed standard was added to charcoal stripped pooled human CSF to prepare the calibrators. A 2 μg mL−1 mixed internal standard solution was prepared in water and stored in aliquots of 100 μL and stored at -40°C.
CSF sample preparation
Two different sample preparation protocols were employed. Two aliquots of 80 μL were prepared from the calibrators and human CSF patient and control samples for LC-MS/MS analyses.
The analysis of tryptophan, kynurenine, kynurenic acid, xanthurenic acid, 3-hydroxyanthranilic acid and quinolinic acid was prepared by deproteinising 80 μL of human CSF with 20 μL of MPA/EDTA solution in Nanosep 0.2 μM centrifugal devices. The mixed IS solution was added to the precipitation reagent MPA/EDTA solution resulting in an overall ten-fold dilution. The CSF samples were vortexed for 60 seconds and precipitated in ice at 4°C for 20 minutes. This was followed by centrifugation for 15 minutes at a temperature of 5°C and velocity of 4000 g. The supernatant was collected and injected into the LC-MS/MS.
The analysis of neopterin, 3-hydroxykynurenine, anthranilic acid, picolinic acid, arginine, citrulline and methylhistamine were prepared by adding 20 μL of the diluted mixed IS solution to 80 μL of human CSF in Nanosep 0.2 μM centrifugal devices. The CSF samples were vortexed for 60 seconds, left in ice at 4°C for 20 minutes and subsequently centrifuged for 15 minutes at a temperature of 5°C and velocity of 4000 g. The supernatant was collected and injected into the LC-MS/MS.
Seven calibration points were prepared similarly by spiking charcoal stripped pooled human CSF with the mixed standard solution at concentrations of 5, 20, 50, 100, 250, 500 and 1000 ng mL−1. Three quality control (QC) samples were prepared by spiking charcoal stripped pooled human CSF with another mixed standard solution at 10 (low QC), 300 (medium QC) and 2000 ng/mL (high QC).
Liquid Chromatography coupled to tandem mass spectrometry
Ultra performance liquid chromatography coupled to tandem mass spectrometry analyses were performed using a Waters ACQUITY UPLC I-Class System UPLC system coupled to a Xevo TQ-XS triple quadrupole mass spectrometer (Waters, Manchester, UK). The same chromatographic programme and mass spectrometric parameters was applied to both sample preparation methods.
The chromatographic separation of metabolites was achieved using the Acquity UPLC BEH C18 column (2.1 mm × 150 mm 1.7 μm particle size) at a flow rate of 0.25 mL min−1 and an injection volume of 5 μL. The mobile phases consisted of 10 mM ammonium formate and 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile (B). The gradient programme was: 0 to 2.5 minutes (0 % B), 2.5 to 4.5 minutes (0-10 % B), 4.5 to 6.5 minutes (10-30 % B), 6.5 to 8 minutes (30-35 % B), 8 to 8.5 minutes (35- 100 % B), 8.5 to 8.6 minutes (100-0 % B) and 8.6 to 12 minutes (0 % B).
The mass spectrometer was operated in the multiple reaction monitoring mode using positive electrospray ionisation. The precursor ion, most abundant product ions, optimal cone voltage and collision energy were identified for each analyte using the MassLynx 4.1 Software. The source conditions were as follows: source temperature of 150 °C, desolvation temperature was 450 °C, nitrogen gas flow rate of 900 L hr−1, cone voltage 20 V and capillary voltage of 2.50 kV.
Method Validation
Method Validation was performed in accordance with the International Organization for Standardization technical standard for medical laboratories38,39 and National Association of Testing Authorities Australia General Accreditation Guidance for validation and verification of quantitative and qualitative test methods.40
Linearity of the calibration standards 5, 20, 50, 100, 250, 500 and 1000 ng mL−1 in matrix-matched CSF were run in triplicate and assessed based on its ability to establish an analyte: internal standard ratio response proportional (r2 > 0.99) to the concentration of an analyte. The working ranges for 3- hydroxykynurenine, 3-hydroxyanthranilic acid, anthranilic acid, picolinic acid and methylhistamine (0- 250 ng mL−1); neopterin, quinolinic acid, kynurenine, kynurenic acid and xanthurenic acid (0- 500 ng mL− 1); tryptophan, arginine and citrulline (0-1000 ng mL−1) were assessed.
The limit of detection (LOD) and limit of quantification (LOQ) was determined by spiking decreasing concentrations of the mixed stock solution into blank stripped human CSF. The LOD of an analyte exhibits a signal-to-noise ratio (S/N) is equal to 3 and the LOQ a S/N equal to 10 whereby the instrument can reliably quantify the analyte and fulfil the requirement of <15 % coefficient of variation (CV) and ± 15 % mean relative error (MRE). Selectivity was investigated using a mixed aqueous standard solution containing 40 compounds closely related to the inflammation panel including amino acids, biogenic amines and pterins were spiked into the matrix-matched blank calibrator and human CSF extract.
Accuracy and precision were determined using the different concentrations of QC samples performed in replicates (n = 6) on five independent days. Accuracy was measured using % MRE and deemed accurate when % MRE values were within the acceptable range of ± 15 % MRE. Intra- and inter-day precision was measured using % CV and regarded as precise when values fulfilled the criteria of not exceeding 15 % CV.
Matrix effect and recovery studies were evaluated using the standard addition method. For matrix effect human CSF and haemolysed CSF samples were spiked with 80 or 160 ng mL−1 of the mixed standard solution and measured using a seven point neat standard calibration curve. Haemolysed CSF experiments were conducted on haemoglobin concentrations of 12, 6 and 3 g L−1 using Nanosep 0.2 μM centrifugal devices and Nanosep with 3K omega centrifugal devices. The differences in concentration between the spiked and blank CSF samples represent the measured concentration of the spiked analytes. Neat standard calibrators were used to determine the concentration of the spiked analytes. Matrix effect is reported as a percentage whereby > 0 implies presence of ion enhancement and < 0 means ion suppression. An acceptable range for ion enhancement or suppression is within ± 20 %. The recovery of the analytes was determined by comparing the detector response of the spiked samples before and after extraction.
A series of stability experiments were performed to assess the stability of the analytes under different experimental conditions (post-preparative, freeze-thaw and long-term). The comparison of concentrations of freshly prepared matrix-matched calibrators, matrix-matched quality controls and CSF samples stored under different conditions were examined. For post-preparative stability, matrix matched calibrators and QC's and CSF sample extracts were left in the autosampler at a maintained constant temperature of 8 °C and re-analysed at 24 h and 48 h. The freeze-thaw stability cycles were assessed using matrix-matched QC samples over a total of three cycles. Low and medium QCs were frozen at -40 °C and thawed in an ice-cold water bath at room temperature. Long-term stability was conducted by testing low and medium QCs stored in -40 °C at three different time intervals; initial (t0), followed by once every two months for 6 months (t1, t2 and t3). The concentrations of analytes must not deviate greater than 15 % to the initial concentration to be regarded as stable.
For the robustness study, minor changes in the column temperatures, composition of mobile phases and injection volumes were conducted to understand the appropriate limits to the parameters of the method. Carryover was assessed by the injection of blank solutions following a high concentration calibrator (2000 ng ml−1), performed in replicates. A method is deemed free from carryover when the blank injections do not yield a peak area greater than the LOD of the analyte and a signal to noise (S/N) ratio higher than three.
A correlation study of this novel LC-MS/MS method was conducted with other collaborating laboratories using existing protocols41, 42, 43 on ten CSF samples for the assessment of tryptophan, kynurenine, kynurenic acid, 3-hydroxykynurenine, anthranilic acid, 3-hydroxyanthranilic acid, quinolinic acid, arginine, citrulline and neopterin. Bland-Altman analysis was used to assess the agreement between the quantitative methods.
Statistical methods
Data analyses were performed using Metaboanalyst 5.0 and SPSS version 25 (IBM Corp. Armonk, NY, USA) and graphs were generated by GraphPad Prism 8 (GraphPad, San Diego, USA). Supervised orthogonal partial least squares-discriminant analysis (OPLS-DA) was used to identify intrinsic variation in the targeted metabolites between the encephalitis and control groups. A heatmap of the metabolites and samples using Pearson's correlation provided visualisation of the metabolite data. The Mann-Whitney U test was conducted and identified statistical significance in nine metabolite concentrations between encephalitis and controls. P-values of <0.05 was considered statistically significant.
Role of funding source
Financial support for the study was granted by Dale NHMRC Investigator grant APP1193648, Petre Foundation and Cerebral Palsy Alliance. Funding sources had no role in the design of this study, and did not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Results
Method Optimisation
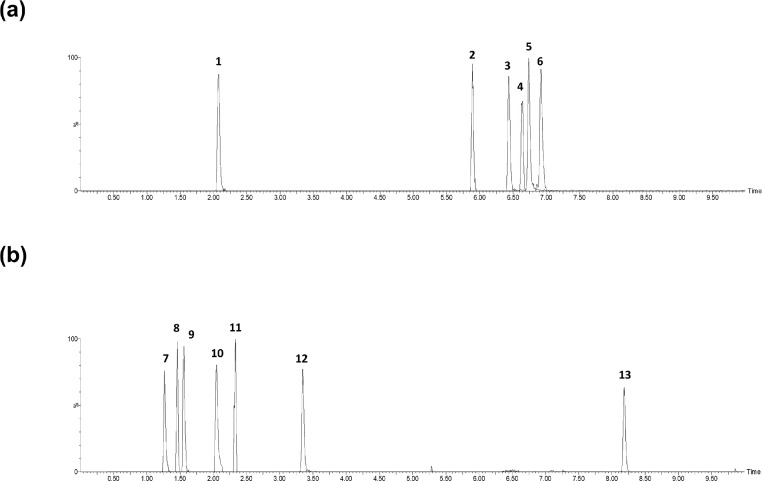
The qualitative and quantitative analysis of the targeted analytes was achieved through the determination of retention time, the monoisotopic mass [M + H]+ of the ionised precursor ion and a minimum of two product fragment ions for confirmatory purposes (Table 1). The ion transitions were operated using the in-built software MassLynx-Intellistart (v.4.1, Waters) to optimise collision energy, cone voltage and dwell time. All analytes eluted within a twelve min window with arginine as the first to elute at 1.41 min and anthranilic acid eluting last at 8.25 min. The extracted ion chromatograms of the metabolites in the neuroinflammation panel are presented in Figure 2.
Table 1.
Retention times, precursor ion and product ions of the targeted analytes.
| Analyte | Retention time (min) | Precursor ion[M + H]+ | 1st product ion |
2nd product ion |
Cone Voltage | ||
|---|---|---|---|---|---|---|---|
| m/z | Collision energy (eV) | m/z | Collision energy (eV) | ||||
| Neopterin | 2.34 | 254 | 206 | 16 | 190 | 20 | 25 |
| Tryptophan | 6.74 | 205 | 188 | 10 | 118 | 24 | 24 |
| Kynurenine | 5.91 | 209 | 146 | 18 | 192 | 8 | 18 |
| Kynurenic acid | 6.85 | 190 | 144 | 20 | 116 | 28 | 40 |
| 3-hydroxykynurenine | 3.21 | 225 | 162 | 18 | 110 | 8 | 22 |
| Xanthurenic acid | 6.46 | 206 | 160 | 18 | 188 | 28 | 34 |
| Anthranilic acid | 8.25 | 138 | 120 | 10 | 92 | 22 | 20 |
| 3-hydroxyanthranilic acid | 6.66 | 154 | 136 | 12 | 108 | 20 | 26 |
| Quinolinic acid | 2.10 | 168 | 106 | 14 | 150 | 8 | 24 |
| Picolinic acid | 2.17 | 124 | 106 | 10 | 96 | 16 | 22 |
| Arginine | 1.41 | 175 | 116 | 12 | 158 | 10 | 26 |
| Citrulline | 1.47 | 176 | 159 | 8 | 113 | 16 | 22 |
| Methylhistamine | 1.44 | 126 | 109 | 35 | 97 | 35 | 13 |
| 15N-Neopterin | 2.34 | 255 | 207 | 18 | 191 | 20 | 28 |
| D3-Tryptophan | 6.74 | 209 | 192 | 10 | 148 | 16 | 30 |
| D4-Kynurenine | 5.91 | 213 | 196 | 8 | 150 | 20 | 22 |
| D3-Kynurenic acid | 6.86 | 195 | 149 | 10 | 121 | 28 | 30 |
| 13C2-3-hydroxykynurenine | 3.20 | 229 | 161 | 6 | 211 | 8 | 44 |
| D3-3-hydroxyanthranilic acid | 6.66 | 157 | 139 | 10 | 111 | 18 | 10 |
| 13C6-Arginine | 1.41 | 181 | 121 | 14 | 164 | 10 | 30 |
| D7-Citrulline | 1.47 | 183 | 120 | 16 | 166 | 8 | 22 |
Figure 2.
Representative extracted ion chromatograms of the targeted metabolites in human cerebrospinal fluid. (a) Metabolites prepared using the MPA/EDTA solution sample preparation method:1 quinolinic acid,2 kynurenine,3 xanthurenic acid4 3-hydroxyanthranilic acid5 tryptophan and6 kynurenic acid. (b) Metabolites prepared by adding 20 μL of diluted mixed IS solution sample preparation method:7 methylhistamine,8 arginine,9 citrulline,10 picolinic acid,11 neopterin (c)12 3-hydroxykynurenine and13 anthranilic acid.
Validation Experiments
The calibration curves for the analytes were linear over the range from the LOQ to 1000 ng mL−1, with correlation coefficients (r2) higher than 0.99 (Table 2). The LOD and LOQ concentrations of the analytes were in the range of 0.20-0.75 and 0.75-3.00 ng mL−1 (Table 2). The method was highly selective to the targeted analytes with no interferences from other endogenous components in human CSF and matrix-matched charcoal stripped pooled human CSF.
Table 2.
Linearity, LOD and LOQ of the validated method.
| Analyte | Linearity (r2) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|
| Neopterin | 0.998 | 0.20 | 1.00 |
| Tryptophan | 0.996 | 0.30 | 1.00 |
| Kynurenine | 0.995 | 0.20 | 1.00 |
| Kynurenic acid | 0.994 | 0.20 | 0.75 |
| 3-hydroxykynurenine | 0.996 | 0.30 | 2.00 |
| Xanthurenic acid | 0.988 | 0.30 | 1.00 |
| Anthranilic acid | 0.990 | 0.25 | 1.00 |
| 3-hydroxyanthranilic acid | 0.993 | 0.50 | 2.00 |
| Quinolinic acid | 0.992 | 0.20 | 0.75 |
| Picolinic acid | 0.997 | 0.30 | 2.00 |
| Arginine | 0.990 | 0.30 | 1.50 |
| Citrulline | 0.992 | 0.20 | 1.00 |
| Methylhistamine | 0.993 | 0.75 | 3.00 |
The results for accuracy, intra- and inter-run precision of the analytes are summarised in Table 3. The accuracy ranged from -13.5 to 15.6 % MRE. The intra- and inter-day precision values of the analytes were ≤ 15 % for all QC concentrations. The data met the acceptable method validation criteria, indicating the performance of the analytical method is accurate and precise.
Table 3.
Accuracy and precision data of the method for the targeted analytes at different QC concentration levels (n = 6). For metabolites with a lower working range (neopterin, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, anthranilic acid, quinolinic acid, picolinic acid and methylhistamine) only two QC concentrations were assessed.
| Analyte | Accuracy (% MRE) |
Intra-run precision(% CV) |
Inter-run precision(% CV) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 ng/mL | 300ng/mL | 2000 ng/mL | 10 ng/mL | 300ng/mL | 2000 ng/mL | 10 ng/mL | 300ng/mL | 2000 ng/mL | |
| Neopterin | -6.60 | -2.96 | 5.56 | 4.05 | 9.52 | 4.55 | |||
| Tryptophan | 12.3 | 6.69 | 7.97 | 11.0 | 10.6 | 7.62 | 6.59 | 8.20 | 8.53 |
| Kynurenine | -12.2 | 1.05 | 11.9 | 6.29 | 3.78 | 7.41 | 5.78 | 7.48 | 13.1 |
| Kynurenic acid | 2.29 | -12.9 | 7.09 | 4.98 | 4.02 | 3.82 | 14.8 | 12.4 | 8.42 |
| 3-hydroxykynurenine | 2.27 | 8.87 | 7.44 | 9.49 | 13.8 | 8.34 | |||
| Xanthurenic acid | -2.84 | -3.31 | 1.34 | 7.24 | 10.8 | 4.23 | 14.5 | 12.2 | 11.5 |
| Anthranilic acid | 6.76 | -4.02 | 6.08 | 8.36 | 4.08 | 2.75 | |||
| 3-hydroxyanthranilic acid | -3.96 | 10.7 | 4.90 | 6.16 | 12.3 | 8.51 | |||
| Quinolinic acid | 11.09 | 9.3 | 11.8 | 9.48 | 12.6 | 8.45 | |||
| Picolinic acid | 5.53 | 2.32 | 5.19 | 4.24 | 8.49 | 3.62 | |||
| Arginine | 15.6 | 0.10 | -6.81 | 14.9 | 8.79 | 5.24 | 8.88 | 11.3 | 12.2 |
| Citrulline | 11.8 | 12.1 | 12.3 | 5.74 | 4.26 | 8.16 | 9.89 | 8.67 | 9.95 |
| Methylhistamine | -13.5 | -10.9 | 11.6 | 12.3 | 14.1 | 6.95 | |||
The recoveries for all analytes ranged from 89.8 to 109.1 % demonstrating the suitability of the sample preparation method for the extraction of the targeted metabolites in human CSF. The standard addition method was used to assess the matrix effects present in human CSF (Table 4). In human CSF, the matrix effects of the targeted analytes were within tolerances (± 20%), ranging from -19.4 to 14.9 %. This indicates the method is not affected by ionisation suppression or enhancement. Furthermore, CSF blood contamination can cause misinterpretations of data analysis and metabolic profiles.44 The results for matrix effects investigated at three haemoglobin concentration levels (12, 6 and 3 g L−1) and using two different centrifugal devices (Nanosep 0.2 μM and Nanosep with 3K omega centrifugal devices). The Nanosep 0.2 μM centrifugal devices showed 3-hydroxyanthranilic acid and kynurenic acid were not affected at a haemoglobin concentrations of 12 and 6 g L−1 respectively. The Nanosep with 3K omega centrifugal devices showed acceptable matrix effects for tryptophan, kynurenic acid and 3-hydroxyanthranilic acid at 12 g L−1 and neopterin and kynurenine at 3 g L−1. 3-hydroxykynurenine, xanthurenic acid, anthranilic acid, arginine, citrulline and methylhistamine showed significant ion suppression in haemolysed CSF whilst quinolinic acid and picolinic acid were enhanced.
Table 4.
Matrix effect and recovery results of all analytes (n = 5).
| Analyte | Matrix effect of human CSF (%) | Recovery of human CSF (%) |
|---|---|---|
| Neopterin | -19.4 | 97.0 |
| Tryptophan | -12.2 | 95.6 |
| Kynurenine | -4.3 | 94.0 |
| Kynurenic acid | -10.4 | 93.1 |
| 3-hydroxykynurenine | 1.04 | 89.8 |
| Xanthurenic acid | 7.76 | 109.1 |
| Anthranilic acid | -16.6 | 91.7 |
| 3-hydroxyanthranilic acid | 4.15 | 92.2 |
| Quinolinic acid | 3.94 | 103.3 |
| Picolinic acid | 14.9 | 107.8 |
| Arginine | -9.69 | 95.2 |
| Citrulline | -16.8 | 93.9 |
| Methylhistamine | -17.5 | 109.0 |
For post-preparative stability, the analytes in medium and high QC concentrations were able to maintain stability for 48 hours in the autosampler at a constant temperature of 4 °C. At the low QC concentration tryptophan, kynurenic acid, 3-hydroxykynurenine, xanthurenic acid, arginine, neopterin and methylhistamine were unstable. Most analytes in human CSF were stable for 48 hours in the autosampler at a constant temperature of 4 °C except for arginine, neopterin and methylhistamine. In a clinical setting, stock solutions are used over an extended period, hence, freeze-thaw and long-term stability experiments were subjected to extensive analysis (Table 5). A majority of the metabolites at all concentration were stable for at least two freeze-thaw cycles with the exception of xanthurenic acid, 3-hydroxyanthranilic acid, arginine and methylhistamine. The long-term storage of matrix-matched working solutions showed the metabolites were stable for at least 180 days.
Table 5.
Long term stability and freeze-thaw stability of matrix-matched quality control samples (n = 5)
| Analyte | Long- term stability (% difference) after 180 days |
Freeze-thaw stability (cycles) |
||
|---|---|---|---|---|
| 10 ng/mL | 300 ng/mL | 10 ng/mL | 300 ng/mL | |
| Neopterin | -7.0 | -8.7 | 2 | 2 |
| Tryptophan | 3.0 | -6.6 | 2 | 3 |
| Kynurenine | -9.6 | -1.4 | 3 | 3 |
| Kynurenic acid | -6.02 | 8.78 | 2 | 2 |
| 3-hydroxykynurenine | 4.3 | -11.6 | 2 | 2 |
| Xanthurenic acid | 1.7 | 5.6 | 1 | 1 |
| Anthranilic acid | 10.1 | 7.9 | 2 | 2 |
| 3-hydroxyanthranilic acid | 1.0 | 2.9 | 1 | 2 |
| Quinolinic acid | -6.7 | 3.7 | 3 | 3 |
| Picolinic acid | 10.1 | 7.9 | 2 | 2 |
| Arginine | 6.9 | -3.6 | 1 | 2 |
| Citrulline | 0.4 | -10.7 | 2 | 3 |
| Methylhistamine | 9.9 | 13.3 | 1 | 1 |
Following the injection of a high concentration calibrator the responses of analytes in the blank samples were zero or significantly less than the LOD peak areas. This supported the method is free from carryover and the gradient programme is efficient in flushing out any remaining analytes between runs.
The rapid increase and demand for diagnostic tests in medical laboratories requires a quantitative method to demonstrate the clinical measurement of a potential biomarker is reproducible and reliable. Bland-Altman analysis method is commonly used in a diagnostic setting to evaluate tests conducted in clinical laboratories.45 The correlation study evaluated the estimated bias and the limits of agreement for the targeted analytes with published methods (Table 6). The estimated biases are within clinical requirements and the developed method is in agreement with existing standards, although their estimated standard deviations are large.
Table 6.
Summary of estimated bias and standard deviation of estimated bias data using Bland-Altman analysis.
| Analyte | Estimated Bias | Standard deviation of estimated bias |
|---|---|---|
| Tryptophan | -13.9 | 59.5 |
| Kynurenine | -5.91 | 63.6 |
| Kynurenic acid | -11.8 | 51.0 |
| 3-hydroxykynurenine | -7.54 | 52.8 |
| Anthranilic acid | 8.17 | 28.4 |
| 3-hydroxyanthranilic acid | -3.12 | 80.4 |
| Quinolinic acid | -2.63 | 31.3 |
| Arginine | -1.31 | 2.14 |
| Citrulline | -1.50 | 0.83 |
| Neopterin | 13.3 | 15.2 |
Application to patients with neuroinflammation
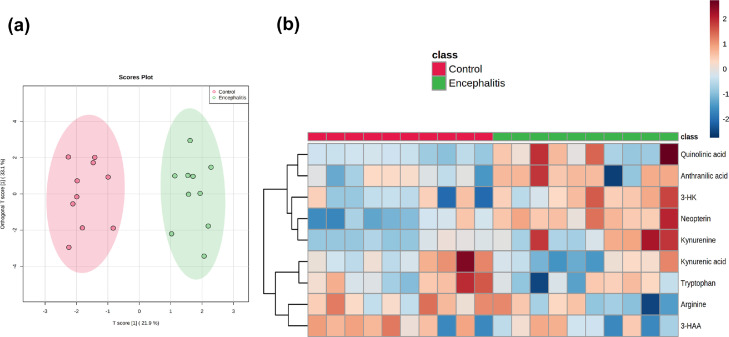
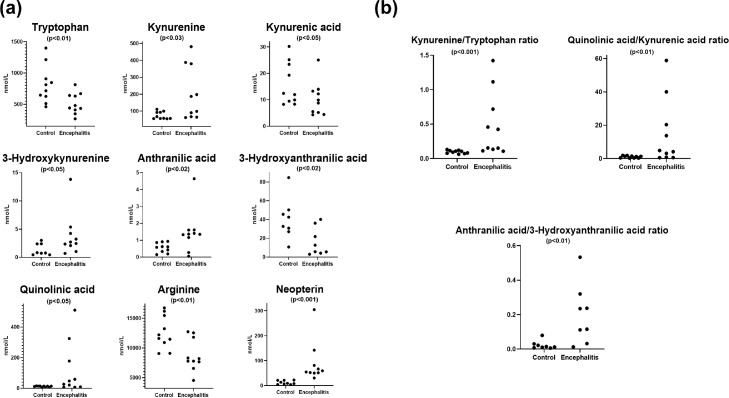
In the case-control studies, seven metabolites (neopterin, tryptophan, kynurenine, kynurenic acid, quinolinic acid, arginine and citrulline) had detectable concentrations above the lower limit in all patients and controls. There were six metabolites (3-hydroxykynurenine, xanthurenic acid, anthranilic acid, 3-hydroxyanthranilic acid, picolinic acid and methylhistamine) that had a small percentage of samples below the lower detection limit (Supplemental Table 3). Targeted metabolomics of the CSF samples was applied to identify metabolites whose distributions differed statistically significantly between the groups. The OPLS-DA score plot showed discrimination between encephalitis and controls (Figure 3a). A heatmap was constructed to illustrate the distinct metabolic differences between encephalitis and controls, which identified nine metabolites were different in the encephalitis patients (Figure 3b). An effect study using Cohen's criteria46 showed a large difference (Supplemental Table 4) in nine metabolites between CSF samples of encephalitis patients and controls. The effect sizes were supported by the Mann-Whitney U test. The levels of kynurenine, 3-hydroxykynurenine, anthranilic acid, quinolinic acid and neopterin were significantly higher in patients compared to controls. Tryptophan, kynurenic acid, 3-hydroxyanthranilic acid and arginine were significantly lower in patients compared to controls (Figure 4a). The quantification of metabolites in tryptophan-kynurenine pathway further supported the findings in our previous untargeted CSF metabolomics study and the discriminative power of elevated kynurenine/tryptophan (p<0.001) and anthranilic acid/3-hydroxyanthranilic acid ratio (p<0.01) at identifying brain inflammation (Figure 4b). In our analysis a marked increase in quinolinic acid and decrease in kynurenic acid resulted in an elevation of the quinolinic acid/kynurenic ratio (p<0.01) (Figure 4b).
Figure 3.
Statistical analysis of the CSF acute neuroinflammation patients and non-inflammatory disease comparison group. (a) Orthogonal partial least squares discriminant analysis score plot showing a distinct separation of 10 control patients (red dots) from 10 patients with encephalitis (green dots). (b) Heatmap of the measured metabolites showed nine differentially expressed metabolites between encephalitis and control groups using Pearson's correlation. The changes in metabolites showed five metabolites were elevated in encephalitis (kynurenine, 3-hydroxykynurenine, anthranilic acid, quinolinic acid, neopterin) and four metabolites decreased (tryptophan, kynurenic acid, 3-hydroxyanthranilic acid, arginine). The encephalitis and control groups were labelled with green and red bars respectively. The blue coloured cells represent downregulation and red coloured cells represent upregulation.
Figure 4.
Quantitative data in nmol L−1 of individual CSF patient samples for tryptophan-kynurenine metabolites, arginine, neopterin and ratios in encephalitis patients (n = 10) in comparison with controls (n = 10). Comparison of the encephalitis and control groups were analysed by Mann-Whitney U test. (a) An increase was observed in kynurenine, 3-hydroxykynurenine, anthranilic acid, quinolinic acid and neopterin, whereas the levels of tryptophan, kynurenic acid, 3-hydroxyanthranilic acid and arginine decreased in encephalitis compared to controls (b) An elevation of tryptophan/kynurenine, quinolinic acid/kynurenine acid and anthranilic acid/3-hydroxyanthranilic acid ratios was observed in acute encephalitis.
Discussion
To our knowledge, this is the first multi-analyte CSF inflammation assay clinically validated in an accredited pathology laboratory. We focus on three major metabolic pathways (neopterin, tryptophan-kynurenine and nitric oxide) that have been extensively reported in human studies associated with CNS inflammation. The analysis of multiple biochemical pathways implicated in inflammation may improve diagnostic sensitivity and specificity and the potential to address the knowledge gaps for differential diagnosis. The simple protein precipitation and filtration sample preparation procedure in combination with the Acquity UPLC BEH C18 column and tandem mass spectrometry demonstrated the ability of the method to successfully distinguish a panel of targeted metabolites with diverse physicochemical properties. The simplicity of the complementary sample preparation procedures allows minimal volumes of CSF to be used, rapid preparation duration, and a rapid patient sample turn-around time of 4 h can be achieved. These are extremely beneficial for running the quantification method in a fast-paced clinical laboratory setting.
The enzyme-linked immunosorbent assay (ELISA) is a widely used application with commercially available kits for several metabolites in the inflammation panel (neopterin, tryptophan, kynurenine, kynurenic acid, quinolinic acid, arginine and citrulline).47,48 However, the commercial ELISA kits are mainly restricted for research use only and require batch testing. The strength of the LC-MS/MS approach is the capability of performing individualised testing and providing rapid turnaround times for patient samples with clinical urgency.
An advantage of this method is the transitioning of potential biomarkers towards clinical application in acute care management. The principal focus for many studies is the development of accurate methods for measuring metabolite levels to generate and interpret data. A challenge in CSF assays is the lack of access to matrix-matched calibrators and QCs. We believe our translational LC-MS/MS method offers high throughput and time efficient routine applicability.
This method was successfully applied to analyse thirteen metabolite concentrations in human CSF samples from encephalitis patients and non-inflammatory neurological disease controls. The present study shows elevated and decreased concentrations of metabolites in the pterin, tryptophan-kynurenine and nitric oxide pathways. Whilst some of these metabolites can define the presence of neuroinflammation, they are unable to discriminate inflammation in different diseases. To-date, there are limited studies that compare different diseases using the same experimental methodology. Future larger groups using this translational assay can determine whether the inflammation panel may help separate different neuroinflammatory conditions, and aid in the differentiation of disease states. The purpose of including our pilot encephalitis study is to show validation on the robustness of the biomarkers, and reproducibility. There has been extensive evidence demonstrating correlations between neuroinflammation and neopterin, tryptophan-kynurenine and nitic oxide pathways, without evaluating clinical reference ranges. The establishment of normative ranges for different age groups is needed to facilitate the translational impact of this inflammation panel.
A limitation of this study is the heterogeneity of acute encephalitis diseases used in our pilot study, and the small sample size (n=10). Further studies with more homogenous and larger groups with other human central nervous system diseases will need to be conducted to demonstrate the sensitivity, specificity and reproducibility of the inflammation panel. Furthermore, the inflammation panel is derived from our published untargeted metabolomics study which determined the metabolite changes in acute encephalitis compared to controls,22 rather than a broad range of different neuroinflammatory diseases. Future untargeted studies in different neuroinflammatory groups may discover novel biomarkers. The simultaneous analysis of CSF and blood samples will also be useful in addressing whether the origins of observed metabolite changes are purely from CSF, or secondary to blood brain barrier disruptions.
Conclusion
The alterations in metabolite profiles of human diseases often occur well before the signs of clinical symptoms. A rapid, reproducible and cost-effective method has been developed and validated for an inflammation panel using LC-MS/MS and holds great promise to be implemented into routine screening in a diagnostic laboratory. The simple sample preparation procedure in combination with a short chromatographic run time allows a patient sample turn-around time of 4 hours. The method was applied to encephalitis groups which successfully measured the CSF metabolites showing discriminative trends in the tryptophan-kynurenine pathway, arginine and neopterin. Our primary objective was to demonstrate the sensitivity and specificity of the panel in a diagnostic laboratory to ensure the applicability of the assay in a day-to-day routine setting. Further confirmation and validation in larger groups of human central nervous system diseases with neuroinflammatory and non-inflammatory aetiology and simultaneous peripheral analysis is required in future research. More importantly, extensive studies are needed to define normative ranges of these metabolites in different age groups.
Declaration of interests
The authors have declared that no conflict of interest exists.
Acknowledgments
Contributors
J. Yan: Conceptualization, Investigation, Methodology, Formal Analysis, Validation, Writing - original draft. V.X. Han: Validation, Writing – reviewing & editing. B. Heng: Validation, Writing – reviewing & editing. G.J. Guillemin: Validation, Writing – reviewing & editing. S. Bandodkar: Conceptualization, Investigation, Validation, Verification of underlying data, Supervision, Funding acquisition, Writing – reviewing & editing. R.C. Dale: Conceptualization, Investigation, Validation, Verification of underlying data, Supervision, Funding acquisition, Writing – reviewing & editing.
Data sharing statement
The data supporting the findings of this study and de-identified individual participant data are available in the article and/or supplementary material. Readers are welcome to contact the corresponding author for the raw data used in this work.
Acknowledgements
The authors would like to thank the hospital scientists at the Children's Hospital at Westmead, Dr Beena Devanapalli, Dr Lihua Zeng and Shirley Alvarez and Dr Sharron Chow from Macquarie University for their contribution to this research. We would also like to thank the Biochemistry team at the Children's Hospital at Westmead for their support and encouragement. Financial support for the study was granted by Dale NHMRC Investigator grant APP1193648, Petre Foundation, Cerebral Palsy Alliance and Department of Biochemistry at the Children's Hospital at Westmead.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103917.
Appendix. Supplementary materials
References
- 1.Jiang N.M., Cowan M., Moonah S.N., Petri W.A., Jr. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol Med. 2018;24(9):794–804. doi: 10.1016/j.molmed.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kothur K., Bandodkar S., Wienholt L., et al. Etiology is the key determinant of neuroinflammation in epilepsy: Elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia. 2019;60(8):1678–1688. doi: 10.1111/epi.16275. [DOI] [PubMed] [Google Scholar]
- 3.Dahm T., Rudolph H., Schwerk C., Schroten H., Tenenbaum T. Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shives K.D., Tyler K.L., Beckham J.D. Molecular mechanisms of neuroinflammation and injury during acute viral encephalitis. Journal of Neuroimmunology. 2017;308:102–111. doi: 10.1016/j.jneuroim.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson J., Nutma E., van der Valk P., Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wishart D.S., Lewis M.J., Morrissey J.A., et al. The human cerebrospinal fluid metabolome. J Chromatogr B. 2008;871(2):164–173. doi: 10.1016/j.jchromb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felger J.C., Haroon E., Patel T.A., et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25(6):1301–1311. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sproston N.R., Ashworth J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Clos T.W., Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30(3):261–277. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 11.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Quist-Paulsen E., Aukrust P., Kran A.B., et al. High neopterin and IP-10 levels in cerebrospinal fluid are associated with neurotoxic tryptophan metabolites in acute central nervous system infections. J Neuroinflammation. 2018;15(1):327. doi: 10.1186/s12974-018-1366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepennetier G., Hracsko Z., Unger M., et al. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. Journal of Neuroinflammation. 2019;16(1):219. doi: 10.1186/s12974-019-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kothur K., Wienholt L., Brilot F., Dale R.C. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine. 2016;77:227–237. doi: 10.1016/j.cyto.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ramesh G., MacLean A.G., Philipp M.T. Cytokines and Chemokines at the Crossroads of Neuroinflammation, Neurodegeneration, and Neuropathic Pain. Mediators of Inflammation. 2013;2013 doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monastero R.N., Pentyala S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int J Inflam. 2017;2017 doi: 10.1155/2017/4309485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Academies of Sciences E . In: Medicine. Biomarkers of Neuroinflammation: Proceedings of a Workshop. Bain L, Keren NI, Norris SMP, editors. The National Academies Press; Washington, DC: 2018. p. 90. [Google Scholar]
- 18.Bettcher B.M., Johnson S.C., Fitch R., et al. Cerebrospinal Fluid and Plasma Levels of Inflammation Differentially Relate to CNS Markers of Alzheimer's Disease Pathology and Neuronal Damage. J Alzheimers Dis. 2018;62(1):385–397. doi: 10.3233/JAD-170602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J., Khademi M., Fugger L., et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proceedings of the National Academy of Sciences. 2020;117(23):12952–12960. doi: 10.1073/pnas.1912839117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch J., Vacas S., Terrando N., et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. Journal of Neuroinflammation. 2016;13(1):211. doi: 10.1186/s12974-016-0681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J., Kuzhiumparambil U., Bandodkar S., Dale R.C., Fu S. Cerebrospinal fluid metabolomics: detection of neuroinflammation in human central nervous system disease. Clin Transl Immunol. 2021;10(8):e1318. doi: 10.1002/cti2.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan J., Kuzhiumparambil U., Bandodkar A., Bandodkar S., Dale R.C., Fu S. Cerebrospinal fluid metabolites in tryptophan-kynurenine and nitric oxide pathways: biomarkers for acute neuroinflammation. Dev Med Child Neurol. 2021;63(5):552–559. doi: 10.1111/dmcn.14774. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann G., Wirleitner B., Fuchs D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res. 2003;52(8):313–321. doi: 10.1007/s00011-003-1181-9. [DOI] [PubMed] [Google Scholar]
- 24.Dale R.C., Brilot F., Fagan E., Earl J. Cerebrospinal fluid neopterin in paediatric neurology: a marker of active central nervous system inflammation. Dev Med Child Neurol. 2009;51(4):317–323. doi: 10.1111/j.1469-8749.2008.03225.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell B., Charych E., Lee A., Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8(12) doi: 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mithaiwala M.N., Santana-Coelho D., Porter G.A., O'Connor J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells. 2021;10(6) doi: 10.3390/cells10061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung A.W., Terentis A.C., King N.J., Thomas S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond) 2015;129(7):601–672. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 28.Wijnands K.A.P., Castermans T.M.R., Hommen M.P.J., Meesters D.M., Poeze M. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7(3):1426–1463. doi: 10.3390/nu7031426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy G., Clark J.M., Buzás E.I., Gorman C.L., Cope A.P. Nitric oxide, chronic inflammation and autoimmunity. Immunology Letters. 2007;111(1):1–5. doi: 10.1016/j.imlet.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Luiking Y.C., Have G.A.M.T., Wolfe R.R., Deutz N.E.P. Arginine de novo and nitric oxide production in disease states. American Journal of Physiology-Endocrinology and Metabolism. 2012;303(10):E1177–E1E89. doi: 10.1152/ajpendo.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan E.B., Frugier T., Lim C.K., et al. Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J Neuroinflammation. 2015;12:110. doi: 10.1186/s12974-015-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson A.M., Croteau D., Ellis R.J., et al. HIV, prospective memory, and cerebrospinal fluid concentrations of quinolinic acid and phosphorylated Tau. J Neuroimmunol. 2018;319:13–18. doi: 10.1016/j.jneuroim.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs K.R., Lim C.K., Blennow K., et al. Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer's disease and relationship to amyloid-β and tau. Neurobiol Aging. 2019;80:11–20. doi: 10.1016/j.neurobiolaging.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Coutinho L.G., Christen S., Bellac C.L., et al. The kynurenine pathway is involved in bacterial meningitis. J Neuroinflammation. 2014;11(1):169. doi: 10.1186/s12974-014-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez-Neri I., Castro E., Montes S., et al. Arginine, citrulline and nitrate concentrations in the cerebrospinal fluid from patients with acute hydrocephalus. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851(1-2):250–256. doi: 10.1016/j.jchromb.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 36.Martens-Lobenhoffer J., Sulyok E., Czeiter E., et al. Determination of cerebrospinal fluid concentrations of arginine and dimethylarginines in patients with subarachnoid haemorrhage. J Neurosci Methods. 2007;164(1):155–160. doi: 10.1016/j.jneumeth.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Fominykh V., Brylev L., Gaskin V., et al. Neuronal damage and neuroinflammation markers in patients with autoimmune encephalitis and multiple sclerosis. Metab Brain Dis. 2019;34(5):1473–1485. doi: 10.1007/s11011-019-00452-x. [DOI] [PubMed] [Google Scholar]
- 38.ISO . 3 ed. International Organization for Standardization; Geneva, Switzerland: 2012. ISO 15189 Medical laboratories - Requirements for quality and competence. [Google Scholar]
- 39.ISO . International Organization for Standardization; Geneva, Switzerland: 2019. ISO/TS 20914 Medical laboratories – Practical guidance for the estimation of measurement uncertainty. [Google Scholar]
- 40.NATA . National Association of Testing Authorities; Rhodes, Australia: 2018. General Accreditation Guidance – Validation and verification of quantitative and qualitative test methods. [Google Scholar]
- 41.Jones S.P., Franco N.F., Varney B., et al. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PloS one. 2015;10(6) doi: 10.1371/journal.pone.0131389. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guillemin G.J., Cullen K.M., Lim C.K., et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27(47):12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsubara Y., Heininger J.A., Lin Y.Y. Improved diagnosis of classical vs atypical phenylketonuria by liquid chromatography. Clin Chem. 1984;30(2):278–280. [PubMed] [Google Scholar]
- 44.Batllori M., Casado M., Sierra C., et al. Effect of blood contamination of cerebrospinal fluid on amino acids, biogenic amines, pterins and vitamins. Fluids Barriers CNS. 2019;16(1):34. doi: 10.1186/s12987-019-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misyura M., Sukhai M.A., Kulasignam V., Zhang T., Kamel-Reid S., Stockley T.L. Improving validation methods for molecular diagnostics: application of Bland-Altman, Deming and simple linear regression analyses in assay comparison and evaluation for next-generation sequencing. J Clin Pathol. 2018;71(2):117–124. doi: 10.1136/jclinpath-2017-204520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J. Academic Press; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 47.Edén A., Marcotte T.D., Heaton R.K., et al. Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.González-Sánchez M., Jiménez J., Narváez A., et al. Kynurenic Acid Levels are Increased in the CSF of Alzheimer's Disease Patients. Biomolecules. 2020;10(4) doi: 10.3390/biom10040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.