Abstract
Background
FOLFOX plus bevacizumab is a standard of care (SOC) for first-line treatment of microsatellite-stable metastatic colorectal cancer (MSS mCRC). This study randomized patients to SOC or SOC plus avelumab (anti-PD-L1) plus CEA-targeted vaccine.
Methods
Patients with untreated MSS mCRC enrolled to a lead-in arm assessing safety of SOC + immuno-oncology agents (IO). Next, patients were randomized to SOC or SOC + IO. The primary endpoint was progression-free survival (PFS). Multiple immune parameters were analyzed.
Results
Six patients enrolled to safety lead-in, 10 randomized to SOC, and 10 to SOC + IO. There was no difference in median PFS comparing SOC versus SOC + IO (8.8 months (95% CI: 3.3-17.0 months) versus 10.1 months (95% CI: 3.6-16.1 months), respectively; hazard ratio 1.061 [P = .91; 95% CI: 0.380-2.966]). The objective response rate was 50% in both arms. Of patients analyzed, most (8/11) who received SOC + IO developed multifunctional CD4+/CD8+ T-cell responses to cascade antigens MUC1 and/or brachyury, compared to 1/8 who received SOC alone (P = .020). We detected post-treatment changes in immune parameters that were distinct to the SOC and SOC + IO treatment arms. Accrual closed after an unplanned analysis predicted a low likelihood of meeting the primary endpoint.
Conclusions
SOC + IO generated multifunctional MUC1- and brachyury-specific CD4+/CD8+ T cells despite concurrent chemotherapy. Although a tumor-directed immune response is necessary for T-cell–mediated antitumor activity, it was not sufficient to improve PFS. Adding agents that increase the number and function of effector cells may be required for clinical benefit.
Keywords: colorectal cancer, Avelumab, therapeutic vaccine, combination immunotherapy, FOLFOX, immune response
This article presents the findings of a randomized phase II clinical trial comparing FOLFOX-based standard of care to FOLFOX-based standard of care plus avelumab plus AdCEA vaccine. Prospective studies of effects of FOLFOX plus bevacizumab on circulating cascade antigen-specific T cells, peripheral blood immune cell subset frequencies, serum cytokines, and soluble factors are also reported.
Implications for Practice.
This randomized, phase II trial investigated the clinical benefit and effects on peripheral immune measures of standard of care FOLFOX + bevacizumab (SOC) versus SOC + avelumab (anti-PD-L1) + a CEA-targeted adenoviral vaccine (AdCEA) as first-line treatment for microsatellite stable, metastatic colorectal cancer. Despite a lack of improvement in clinical outcomes in the experimental arm, the addition of avelumab + AdCEA to SOC was biologically active and produced multifunctional T-cell responses to cascade antigens MUC1 and brachyury. The addition of agents that can increase the number and function of these cells may be needed to translate biologic activity (ie, cascade antigen-specific responses) into clinical activity in prospective studies.
Introduction
An estimated 32,000 patients are diagnosed with metastatic colorectal cancer (mCRC) annually in the US and the 5-year overall survival (OS) is 14.3%.1 FOLFOX-based chemotherapy plus the anti-VEGF monoclonal antibody bevacizumab as first-line therapy for mCRC is associated with a median progression-free survival (PFS) of approximately 10 months and a median OS of 25-29 months.2,3
Patients with mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) mCRC were identified as a subgroup that can experience durable responses and survival benefit with immune checkpoint inhibition (ICI) such as programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) blockade with or without CTLA-4 blockade in first-line4 and subsequent settings.5-7 Pembrolizumab is approved for first-line and subsequent treatment of patients with dMMR mCRC. Nivolumab, with or without ipilimumab, has an indication for patients who have received fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy.
Immune checkpoint inhibition, however, is minimally active in mCRC patients with mismatch repair proficient/microsatellite stable (pMMR/MSS) disease.5,8-12 Although immune-stimulatory features of various chemotherapies have been reported,13-16 it is unknown whether combining immuno-oncology (IO) agents with SOC regimens for pMMR/MSS mCRC can improve outcomes.
Therapeutic vaccines targeting tumor-associated antigens (TAAs) are of interest in MSS mCRC in light studies showing that vaccines can lead to increased T-cell infiltration into tumors (eg, prostate cancer) that typically lack a robust immune infiltrate.17,18 However, once in the tumor microenvironment, effector cell activity can drive interferon (IFN)-γ production and upregulation of checkpoint molecules,19 thus hampering antitumor activity. In this situation, PD-1/PD-L1 blockade may negate this mechanism of immune escape and enable a vaccine-derived antitumor response while providing additional synergy with chemotherapy, since cancer cells undergo immunogenic cell death when exposed to certain types of chemotherapy, including oxaliplatin.14,15 Furthermore, blocking PD-1/PD-L1 interactions during antigen presentation in tumor-draining lymph nodes may enhance tumor-specific immune responses.20
Avelumab is a fully human IgG1 anti-PD-L1 monoclonal antibody approved by the US Food and Drug Administration for multiple oncologic indications.21-24 Adenovirus (Ad)5 [E1-, E2b-]-CEA (AdCEA) is an adenovirus serotype-5-based vector platform capable of inducing CD4+/CD8+ T-cell responses to CEA. In phase I/II trials, AdCEA was well-tolerated, with the common side effects of mild injection-site reactions and constitutional symptoms.25,26
Here, we present the findings of a randomized phase II clinical trial comparing FOLFOX-based standard of care (SOC) to FOLFOX-based SOC plus avelumab plus AdCEA vaccine. We also report prospective studies of the effects of FOLFOX plus bevacizumab on circulating cascade antigen-specific T cells, peripheral blood immune cell subset frequencies, serum cytokines, and soluble factors.
Methods
Study Design
This investigator-initiated, randomized, open-label, phase II study evaluated the safety and clinical activity of modified (m)FOLFOX627 plus bevacizumab SOC alone or in combination with AdCEA vaccine plus avelumab (SOC + IO) in patients with previously untreated mCRC. Patients received treatment at the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health in Bethesda, Maryland, or at the Lombardi Comprehensive Cancer Center, Georgetown University Medical Center (GUMC) in Washington, DC.
Statistical Methods
The primary objective was to determine if there was a difference in PFS between patients treated with SOC versus SOC + IO. Median PFS with SOC alone was estimated to be 10 months. The goal of this study was to determine whether SOC + IO would increase median PFS to 18 months. Assuming exponential PFS curves, the hazard ratio (HR) for SOC is 0.0693, or an approximately 7% probability of progressing each month when the median PFS is 10 months. An assumed median PFS of 18 months for the combination arm would correspond to an HR of 0.0385. The resulting HR for comparison of the 2 overall actuarial curves would be 1.80. Following the principles of a phase 2.5 design, for sample size purposes, to compare these curves and detect a difference with a 0.10 one-tailed log-rank test, 35 evaluable patients per arm (70 total) were planned for randomization. A total of 52 progression events would confer 80% power to compare the curves.
Kaplan-Meier curves and a 2-tailed log-rank test were the primary methods of analysis. The hazard ratios were calculated using a Cox proportional hazards model. Binary measures were compared between 2 groups using Fisher’s exact test. Changes in laboratory parameters between 2 points in time were assessed for statistical significance using a Wilcoxon signed-rank test. Parameters compared between patients who responded to treatment or not were assessed for the significance of the difference using a Mann-Whitney test. All P-values are 2-tailed and reported without adjustment for multiple comparisons.
Patients
Eligible patients were ≥18 years of age with previously untreated pMMR/MSS mCRC, with measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST v.1.1). Patients who received adjuvant therapy for resectable colon cancer (including oligometastatic disease) were eligible if treatment was > 6 months prior to enrollment. Eligible patients had Eastern Cooperative Oncology Group performance status of 0-2, adequate vital organ function, and absence of severe comorbid illness. Full eligibility criteria are listed in the Supplementary Material.
Enrollment
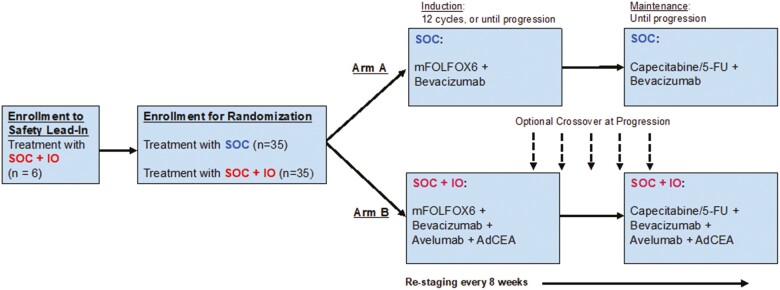
Patients were first enrolled on a lead-in arm assessing the safety of SOC + IO (avelumab and AdCEA plus mFOLFOX6 plus bevacizumab) (Fig. 1). After the safety of the SOC + IO regimen was established, patients were randomized 1:1 to receive SOC (Arm A) or SOC + IO (Arm B). Randomization occurred at a central location, which maintained the confidentiality of the randomization process from the study investigators. The central office used randomization assignment sheets created by software that randomly assigned participants to treatment, with instructions created by the study statistician. This study was randomized with variable block sizes of either 2 or 4 participants per block, and without stratification.
Figure 1.
Enrollment schema: Patients were first enrolled on a safety lead-in arm. After the safety of the SOC + IO combination was established, patients were randomized to SOC (Arm A) or SOC + IO (Arm B). After completion of induction mFOLFOX-based treatment, patients received maintenance capecitabine or 5-fluorouracil plus bevacizumab. Patients were treated until progression. Patients randomized to SOC were given the option to cross over to the SOC + IO regimen on progression if clinically appropriate. SOC, standard of care; SOC + IO, standard of care plus immuno-oncology agents.
Protocol Treatment
Treatment continued until disease progression, unacceptable toxicity, withdrawal from the study, or study completion. Restaging CT scans of the chest, abdomen, and pelvis were performed every 8 weeks.
Arm A: SOC
Patients randomized to the control Arm A received bevacizumab 5 mg/kg intravenously (IV), oxaliplatin 85 mg/m2 IV over 2 hours, leucovorin 400 mg/m2 IV over 2 hours (concurrently with oxaliplatin), then 5-FU 400 mg/m2 by IV push, then 5-FU 2,400 mg/m2 continuous infusion over 46 hours; each cycle repeated every 2 weeks. After 12 cycles, patients without disease progression transitioned to a maintenance regimen consisting of continuous oral capecitabine 625 mg/m2 twice daily28 or 5-FU 2,400 mg/m2 continuous infusion over 46 hours every 2 weeks plus bevacizumab 5 mg/kg IV every 2 weeks.
Arm B: SOC + Immuno-oncology Agents (SOC + IO)
The first 6 patients treated in the safety lead-in, and subsequently all patients randomized to SOC + IO, received avelumab and AdCEA. In response to toxicity observed in the first patient treated on the safety lead-in, the 5-FU bolus was removed from the experimental SOC + IO regimen (discussed below).
AdCEA 5 × 1011 viral particles were administered subcutaneously with cycles 1, 2, and 3, cycles 5, 7, and 9, and every 6 cycles thereafter. The remainder of the treatment regimen was avelumab 10 mg/kg IV over 60 min every 2 weeks, bevacizumab 5 mg/kg IV, oxaliplatin 85 mg/m2 IV over 2 hours, leucovorin 400 mg/m2 IV over 2 hours concurrently with oxaliplatin, then 5-FU 2,400 mg/m2 continuous infusion over 46 hours; each cycle was repeated every 2 weeks. After 12 cycles, patients who did not experience disease progression transitioned to a maintenance regimen consisting of oral capecitabine 625 mg/m2 twice daily plus bevacizumab 5 mg/kg IV every 2 weeks plus avelumab 10 mg/kg IV every 2 weeks plus AdCEA every 6 cycles.
Crossover
At radiographic disease progression, patients receiving SOC had the option to cross over and receive SOC + IO if clinically appropriate and after counseling regarding standard treatment options. After crossover, patients continued treatment until progression or unacceptable toxicity.
SARS-CoV-2 Amendment for Bevacizumab Dosing
At the outset of the SARS-CoV-2 pandemic, the clinical protocol was amended to allow all patients to receive bevacizumab every 4 weeks at the every 3-week dose (7.5 mg/kg IV every 4 weeks), as opposed to bevacizumab 5 mg/kg IV every 2 weeks, to minimize exposure to the healthcare setting. An every-4-weeks regimen was selected for its ease of integration into the every-2-weeks cycle design of this study. At the time of amendment, all affected patients had completed induction and were in the maintenance phase of treatment.
Safety and Dose-Limiting Toxicity
Adverse events were graded according to the NCI’s Common Terminology Criteria for Adverse Events v.5. Per protocol, recording of grade 1 adverse events was not required. The evaluation period for dose-limiting toxicity (DLT) ran from the first dose of avelumab to 28 days later and was assessed only in the 6 patients enrolled in the safety lead-in. A DLT was defined as any captured adverse event outside the known safety profile of the standard and investigational agents.
Correlative Analyses
Immune assays
Blood samples collected at baseline and around days 29, 56, 111, and 167 were analyzed for immune measures. For analysis of peripheral blood mononuclear cells (PBMCs), blood was collected in sodium heparin tubes and PBMCs were separated by Ficoll-Hypaque density gradient separation. Cells were cryopreserved in 90% heat-inactivated human AB serum and 10% dimethyl sulfoxide at a concentration of 1 × 107 cells/mL prior to analysis. For serum assays, blood was collected in serum separator tubes, centrifuged, and stored at –80 °C prior to analysis.
PBMC analyses
Cryopreserved PBMCs collected from patients before and during therapy with SOC or SOC + IO were examined by multicolor flow cytometry to identify 138 peripheral immune cell subsets (Supplementary Table S1), using methodology previously described.29,30 Subsets evaluated included 10 parental cell types (CD4+ and CD8+ T cells, regulatory T cells (Tregs), natural killer (NK) cells, NKT cells, conventional dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), B cells, myeloid-derived suppressor cells (MDSCs), and monocytes). In addition to these standard parental PBMC subsets, 128 refined subsets characterized by expression of maturation and/or functional markers of the parental cell types were also examined. The 128 refined subsets are described elsewhere.30 Flow cytometry files were analyzed using FlowJo v.9.7 for Macintosh, with nonviable cells excluded and negative gates based on fluorescence-minus-one controls. The frequency of all subsets was calculated as a percentage of PBMCs to eliminate bias that might occur in smaller populations with fluctuations in leukocyte subpopulations. Immune subsets with a potentially biologically relevant change following therapy were defined as those with P < .05, most patients having a >25% change, and difference in medians >0.05% of PBMCs. P values are reported without adjustment for multiple comparisons; given the large number of PBMC subsets assessed as well as the small number of patients evaluated, P < .05 should be considered trends. In addition, excluding subsets with median differences between groups of <0.05% of PBMCs eliminates very rare subsets with minor differences that may not be of biological significance.
To determine if SOC therapy influences major peripheral immune subsets at time points earlier than were evaluated in the current study, research bloods were collected from 6 patients with newly diagnosed metastatic CRC, who were enrolled on a blood collection protocol to evaluate the immunologic status of colorectal cancer patients. PBMCs collected from these patients before and after 1 and 2 weeks of FOLFOX + bevacizumab therapy were assessed by flow cytometry for changes in the frequency of the classic cell types indicated above (excluding cDC and pDC), as well as for PD-1 and PD-L1 expression within these cell types.
TAA-specific T cells were analyzed, using methods previously described,31 in cryopreserved PBMCs isolated from patients before and during SOC or SOC + IO therapy. PBMCs were stimulated in vitro with overlapping 15-mer peptide pools encoding CEA, brachyury, and MUC1, and analyzed by intracellular cytokine staining. Peptide pools encoding human leukocyte antigen (HLA) and CEFT (a mixture of peptides of cytomegalovirus, Epstein-Barr virus, influenza, and tetanus toxin) served as negative and positive controls, respectively. The absolute number of viable CD4+ or CD8+ T lymphocytes that produced the cytokines IFN-γ, tumor necrosis factor (TNF)-α, or interleukin (IL)-2, or were positive for a degranulation marker (CD107a) at the end of expansion was calculated per 1 × 106 cells plated at the start of the stimulation assay. This calculation accounts not only for the percentage but also the total number of viable antigen-specific T cells expanded in the stimulation assay. The background signal obtained with the HLA peptide pool was subtracted. Multifunctional TAA responses, defined as CD4+ or CD8+ T cells expressing ≥2 of IFN-γ, TNF-α, IL-2, or CD107a, were quantified before and after vaccination. The frequency of patients developing a >3-fold and >10-fold increase in multifunctional TAAs after versus before vaccination was determined.
Serum Analyses
Anti-Ad5 neutralizing antibodies were measured as previously described25,32-34 in serum obtained from patients before and during SOC + IO treatment. Serum levels of cytokine/soluble factors were determined before and during SOC or SOC + IO treatment using commercially available kits per the manufacturer’s instruction. IL-8 was measured by AlphaLISA (PerkinElmer), soluble CD27 (sCD27) and soluble CD40 ligand (sCD40L) were measured using Instant ELISA kits (Life Technologies), and soluble PD-L1 (sPD-L1), IFN-γ, and transforming growth factor (TGF)-β1 were measured using ELISA kits from Abcam, ThermoFisher, and R&D, respectively.
Results
Patient Demographics
Between April 2017 and October 2019, 26 patients were enrolled in this study. Twenty-four patients were treated at the NCI and 2 at GUMC. Baseline demographic and tumor data are summarized in Table 1. While 20% (2/10) of patients randomized to SOC + IO had tumors with BRAF V600E mutations versus 0/10 (0%) of those randomized to SOC, this difference was not statistically meaningful (P = .47). In addition, 7/10 (70%) of patients randomized to SOC had RAS mutations versus a lower fraction, 2/9 (22.2%) of patients randomized to SOC + IO (P = .067).
Table 1.
Baseline characteristics of patients randomized to SOC or SOC plus IO, and patients assigned to SOC plus IO on the safety lead-in arm.
| Characteristic | Number (%) | |||
|---|---|---|---|---|
| Standard of care (n = 10) | Standard of care + IO (n = 10) | Lead-In (n = 6) | ||
| Gender | Female | 7 (70%) | 4 (40%) | 1 (16.7%) |
| Male | 3 (30%) | 6 (60%) | 5 (83.3%) | |
| Primary tumor location | Left | 6 (60%) | 8 (80%) | 3 (50%) |
| Right | 4 (40%) | 2 (20%) | 3 (50%) | |
| Prior adjuvant therapy | Yes | 4 (40%) | 1 (10%) | 0 |
| No | 6 (60%) | 9 (90%) | 6 (100%) | |
| RAS Status | Wild Type | 3 (30%) | 7 (70%) | 2 (33.3%) |
| Mutant | 7 (70%) | 2 (20%) | 2 (33.3%) | |
| Unknown | 0 | 1 (10%) | 2 (33.3%) | |
| BRAF V600E Status | Wild Type | 10 (100%) | 8 (80%) | 3 (50%) |
| Mutant | 0 | 2 (20%) | 1 (16.7%) | |
| Unknown | 0 | 0 | 2 (33.3%) | |
Abbreviations: IO, immuno-oncology.
Safety
No treatment-emergent adverse events (TEAE) were observed outside the expected safety profile of any individual agent. Grade ≥ 2 TEAEs are summarized in Table 2; one grade 5 adverse event is described below. Safety data for the 6 patients treated in the safety lead-in are included in the SOC + IO column of Table 2.
Table 2.
Treatment-related adverse events.
| Adverse eventa | Standard of care (n = 10) | Standard of care + IO (n = 16) | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Any | 10 (100%) | 7 (70%) | 2 (20%) | 14 (87.5%) | 11 (68.8%) | 3 (18.8%) | 1 (6.3%) | |
| Abdominal pain | 1 (6.3%) | |||||||
| Acute kidney injury | 1 (10%)b | |||||||
| Alanine aminotransferase increased | 1 (10%) | 3 (18.8%) | 2 (12.5%) | |||||
| Alkaline phosphatase increased | 2 (20%) | 3 (18.8%) | ||||||
| Anemia | 2 (20%) | 4 (40%) | 3 (18.8%) | 2 (12.5%) | ||||
| Anorexia | 1 (6.3%) | |||||||
| Anxiety | 1 (6.3%) | |||||||
| Arthralgia | 1 (6.3%) | |||||||
| Aspartate aminotransferase increased | 2 (20%) | 1 (10%) | 1 (6.3%) | 1 (6.3%) | ||||
| Cardiac disorders—Other: Coronary vasospasm | 1 (6.3%) | |||||||
| Cough | 2 (20%) | 3 (18.8%) | ||||||
| Dehydration | 1 (10%) | 2 (12.5%) | ||||||
| Diarrhea | 2 (20%) | 1 (6.3%) | ||||||
| Dizziness | 1 (6.3%) | |||||||
| Duodenal ulcer | 1 (10%) | |||||||
| Dysgeusia | 3 (18.8%) | |||||||
| Dysphagia | 1 (10%) | |||||||
| Dyspepsia | 1 (6.3%) | |||||||
| Eczema | 1 (6.3%) | |||||||
| Epistaxis | 1 (6.3%) | |||||||
| Eye infection | 1 (10%) | |||||||
| Fatigue | 4 (40%) | 5 (31.3%) | 3 (18.8%) | |||||
| Febrile neutropenia | 1 (10%) | |||||||
| Flu-like symptoms | 2 (20%) | 2 (12.5%) | ||||||
| Folliculitis | 1 (10%) | |||||||
| Gastroesophageal reflux | 1 (10%) | 2 (12.5%) | ||||||
| Headache | 1 (10%) | 1 (6.3%) | ||||||
| Hyperglycemia | 2 (12.5%) | |||||||
| Hypertension | 1 (10%) | 1 (6.3%) | ||||||
| Hypoalbuminemia | 1 (10%) | |||||||
| Hypocalcemia | 1 (10%) | |||||||
| Hypothyroidism | 2 (12.5%) | |||||||
| Hypokalemia | 1 (10%) | 2 (20%) | 1 (6.3%) | |||||
| Hypophosphatemia | 3 (30%) | 1 (10%) | 2 (12.5%) | 3 (18.8%) | ||||
| Infusion-related reaction | 1 (10%) | 4 (25%) | 2 (12.5%) | |||||
| Injection-site reaction | 3 (18.8%) | |||||||
| Insomnia | 1 (6.3%) | |||||||
| Jaw pain | 1 (6.3%) | |||||||
| Lipase increased | 1 (6.3%) | 1 (6.3%) | ||||||
| Lymphocyte count decreased | 2 (20%) | 2 (20%) | 1 (10%) | 6 (37.5%) | 3 (18.8%) | 2 (12.5%) | ||
| Mucositis oral | 1 (10%) | 1 (6.3%) | ||||||
| Nasal congestion | 1 (6.3%) | |||||||
| Nausea | 2 (20%) | 5 (31.3%) | ||||||
| Neutropenic typhlitis | 1 (6.3%) | |||||||
| Neutrophil count decreased | 2 (20%) | 2 (20%) | 1 (10%) | 2 (12.5%) | 2 (12.5%) | 1 (6.25%) | ||
| Pain | 1 (10%) | |||||||
| Palmar-plantar erythrodysesthesia syndrome | 3 (30%) | 4 (25%) | ||||||
| Pelvic pain | 1 (6.3%) | |||||||
| Peripheral motor neuropathy | 2 (12.5%) | |||||||
| Peripheral sensory neuropathy | 2 (20%) | 3 (18.8%) | ||||||
| Platelet count decreased | 1 (10%) | 1 (10%) | 3 (18.8%) | |||||
| Proteinuria | 4 (40%) | 1 (6.3%) | ||||||
| Serum amylase increased | 2 (20%) | |||||||
| Sinus tachycardia | 1 (6. 3%) | |||||||
| Skin infection | 1 (6.3%) | |||||||
| Tenosynovitis | 1 (6. 3%) | |||||||
| Thromboembolic event | 1 (6. 3%) | |||||||
| Upper respiratory infection | 1 (10%) | |||||||
| Urinary tract infection | 1 (6. 3%) | |||||||
| Vaginal infection | 1 (6. 3%) | |||||||
| Vomiting | 1 (6. 3%) | |||||||
| Weight loss | 1 (10%) | 1 (6.3%) | ||||||
| White blood cell count decreased | 4 (40%) | 1 (10%) | 4 (25%) | 2 (12.5%) | ||||
One patient who received SOC + IO on the safety lead-in arm developed grade 5 neutropenic typhlitis.
When a patient had multiple events identified by a particular term, only the worst grade is represented.
One patient randomized to SOC developed acute kidney injury requiring hemodialysis. This was attributed to oxaliplatin, and renal function recovered.
Abbreviations: IO, immuno-oncology; SOC + IO, AdCEA vaccine plus avelumab.
The first patient who enrolled in the safety lead-in died after developing neutropenic typhlitis complicated by bowel perforation. This occurred after completing the DLT period. In light of a report describing increased incidence of grade 3/4 febrile neutropenia when combining mFOLFOX6 with pembrolizumab,35 the 5-fluorouracil (5-FU) bolus was removed from the SOC + IO regimen subsequent to the grade 5 event, and the amended regimen was well-tolerated thereafter. Two patients who received avelumab developed grade 2 steroid-responsive joint disorders (one arthralgia; one tenosynovitis).
Efficacy
All patients who received at least one cycle of therapy were evaluable for PFS and response. Due to slow accrual, a preliminary, unplanned futility analysis was conducted with 8 patients randomized to SOC and 7 to SOC + IO. The PFS curves overlapped. Using conditional power, given the results to date and the original goal of identifying a difference between a median of 10 and 18 months PFS, there was approximately 40% probability of identifying a statistically meaningful difference in PFS between arms if the study accrued to the planned goal. As a result of the early lack of difference, the limited probability of finding a successful result if the hypothesized difference were true, and the slow accrual, the trial closed to accrual in December 2019 (n = 10 patients randomized to each arm).
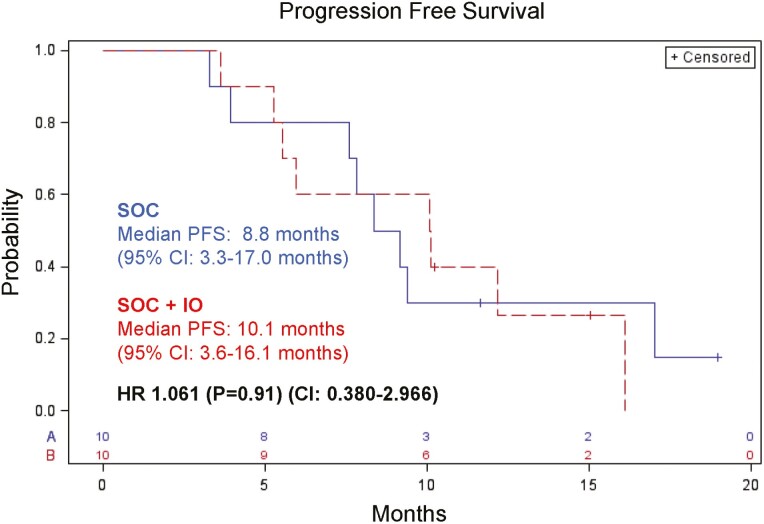
At the time of final survival analysis in August 2020, there was no difference in PFS between patients randomized and treated with SOC versus those randomized and treated with SOC + IO. Median PFS was 8.8 months for SOC (95% CI: 3.3-17.0 months) and 10.1 months for SOC + IO (95% CI: 3.6-16.1 months; HR: 1.061; P = .91; CI: 0.380-2.966) (Fig. 2). Median OS was 18.6 months (95% CI: 8.8-22.7 months) and 15.1 months (95% CI: 5.4 months–not estimable) for SOC and SOC + IO, respectively; HR 0.777; P = .67; CI: 0.243-2.484) (Supplementary Fig. S1).
Figure 2.
Progression-free survival in months since enrollment for patients treated with SOC or SOC plus immunotherapy (SOC + IO). SOC, standard of care; SOC + IO, SOC plus immuno-oncology agents.
There were no complete responses. The objective response rate (ORR) was 50% (5/10) for both the SOC and SOC + IO arms. Including patients treated with SOC + IO on the safety lead-in, the ORR for SOC + IO was 56.3% (9/16). Best overall response (BOR) for SOC was partial response (PR = 5) and stable disease (SD = 5). BOR for all patients who received SOC + IO was PR = 9, SD = 7. All PRs were confirmed.
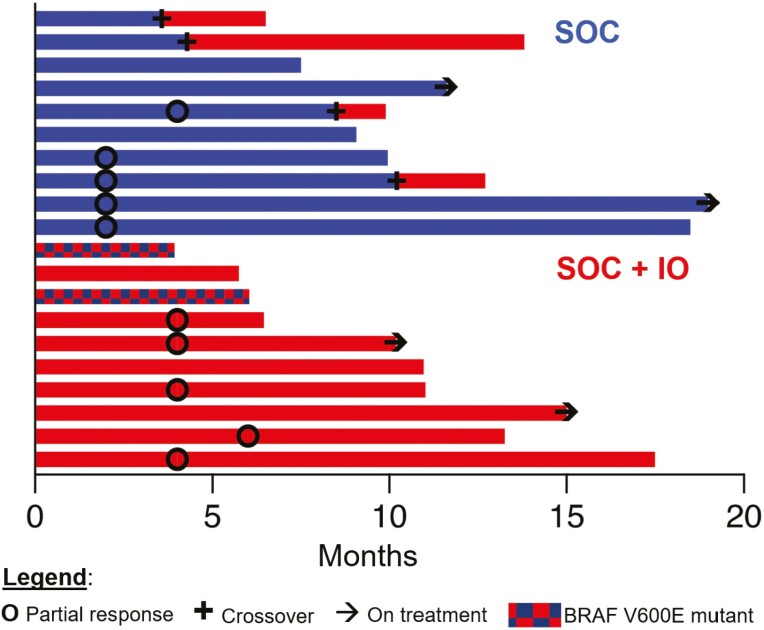
Four patients who progressed on SOC crossed over to receive SOC + IO. No patients subsequently had objective responses. One patient who crossed over had a prolonged period of SD (8.8 months). Swimmer plots in Fig. 3 depict individual PFS, time to response, duration of response, and time of crossover, if applicable.
Figure 3.
Outcomes: Swimmer plot depicts the time of partial response, crossover (if applicable), and ongoing responses at the time of analysis in relation to months on treatment. Treatment regimen and BRAF V600E mutational status are indicated by color.
Maintenance Therapy
Nine of 10 (90%) and 7/10 (70%) patients randomized to SOC and SOC + IO, respectively, went on to receive maintenance therapy. With the exception of one patient per arm, all patients received capecitabine (as opposed to 5-FU + leucovorin) maintenance therapy.
Peripheral Blood Immune Cell Subsets Exploratory Analyses
We evaluated the effect of the SOC and SOC + IO regimens on the immune cell subsets in peripheral blood, comparing baseline to 1 month on treatment. For patients treated with either SOC or SOC + IO, analyses of PBMC subsets showed no statistical changes in the 10 classic PBMC subsets evaluated. These included CD4+ and CD8+ T cells, Tregs, NK and NKT cells, B cells, cDCs and pDCs, MDSCs, and monocytes (Supplementary Table S2). However, trends of changes in refined PBMC subsets were observed in each treatment group. In patients treated with SOC alone, there were increases in CD4+ T cells expressing the proliferative marker Ki67, central memory CD4+ T cells expressing Ki67, NK cells expressing Ki67, and NK cells expressing the activating receptor NKp30 (Supplementary Table S2). In patients who received SOC + IO, there were increases in CD8+ T cells expressing Ki67, effector memory CD8+ T cells expressing Ki67, NK cells expressing Ki67, and intermediate monocytes (Supplementary Table S2).
Changes in major immune subsets were also interrogated before and after 1 and 2 weeks of FOLFOX + bevacizumab in 6 patients with mCRC enrolled on a blood collection protocol. In these patients, no significant changes in the frequencies of classic immune cell types, including CD4 and CD8 T cells, Tregs, B cells, NK cells, NK-T cells, and MDSCs, as well as PD-1 or PD-L1 expressing classic immune subsets, were observed after 1 or 2 weeks of SOC therapy.
Serum Cytokines and Soluble Factors Exploratory Analyses
Comparison of baseline versus on-treatment serum cytokines and soluble factors showed no statistical changes in sCD27, sPD-L1, IL-8, or IFN-γ in SOC-treated patients. However, there were reductions in the immunosuppressive cytokine TGF-ß and sCD40L after SOC therapy (Supplementary Table S3A). Platelets, which are a potential source of these analytes, as well as neutrophils and the neutrophil:lymphocyte ratio were also reduced after 1 month of SOC therapy (Supplementary Table S3A). Similar changes were observed in patients treated with SOC + IO (Supplementary Table S3B).
Tumor-associated Antigen-specific T Cells Exploratory Analyses
We assessed the development of multifunctional TAA-specific T cells, defined as CD4+ or CD8+ T cells expressing ≥2 of IFN-γ, TNF-α, IL-2, or CD107a, against cascade antigens not encoded by the vaccine. Patients were scored for the development of multifunctional TAA-specific T cells using a 3-fold and 10-fold cutoff post-therapy versus pre-therapy. Using a 10-fold change in post versus pre, a significantly greater frequency of patients in the SOC + IO arm (compared to SOC arm) developed multifunctional T cells against the cascade antigen brachyury (0/8 vs. 5/11, P = .045) and either the cascade antigen brachyury or MUC1 (1/8 vs. 8/11, P = .020), respectively (Table 3). This increase in multifunctional antigen-specific T cells in patients receiving SOC + IO was observed despite most patients in this arm developing anti-Ad5 antibodies after receiving multiple vaccinations as part of the IO regimen (Supplementary Table S4).
Table 3.
Frequency of patients developing multifunctional T-cell responses against the TAAs CEA, brachyury, and MUC1 during therapy.
| Multifunctional T-cell responses | ||||||
|---|---|---|---|---|---|---|
| Response at any time point post (vs pre) | Arm | CEA | Brachyury | MUC1 | Brachyury or MUC1 | Any TAA |
| >3-fold increase | SOC | 5/8 (63%) | 6/8 (75%) | 6/8 (75%) | 7/8 (88%) | 7/8 (88%) |
| SOC + IO | 7/10 (70%) | 9/11 (82%) | 8/11 (73%) | 11/11 (100%) | 11/11 (100%) | |
| >10-fold increase | SOC | 3/8 (38%) | 0/8 (0%) | 1/8 (13%) | 1/8 (13%) | 3/8 (38%) |
| SOC + IO | 2/10 (20%) | 5/11 (45%) | 4/11 (36%) | 8/11 (73%) | 8/11 (73%) | |
Immune responses were calculated by comparing the absolute number of CD4+ or CD8+ T cells producing cytokine (IFN-γ, TNF-α, IL-2) or positive for CD107a per 1 × 106 PBMCs plated at the start of the in vitro stimulation assay. Multifunctional T-cell responses, or CD4+ and CD8+ T cells expressing 2 or more of IFN-γ, TNF-α, IL-2, or CD107a were quantified. Any background signal obtained with the negative control peptide pool, HLA, was subtracted. The frequency of patients developing a >3-fold (A) and >10-fold (B) increase in multifunctional T cells tested at any time during therapy are shown.
Abbreviations: HLA, human leukocyte antigen; IO, immuno-oncology; SOC, standard of care; SOC + IO, AdCEA vaccine plus avelumab; TAA, tumor-associated antigen.
We investigated whether changes in any other immune parameters differed between patients treated with SOC and SOC + IO. Compared to patients treated with SOC, individuals treated with SOC + IO had trends of greater increases in PD-1+ CD8+ T cells, and greater decreases in PD-L1+ CD4+ T cells, B cells, NKG2D+ NK, and CD56br CD16+ NK (double-positive NK) (Supplementary Fig. S2).
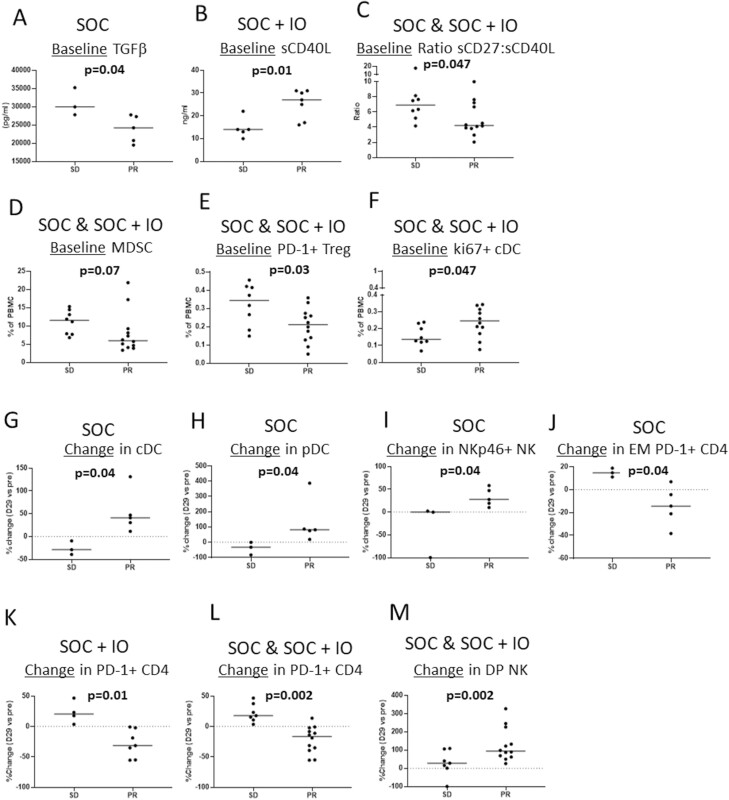
To investigate the association with clinical response, immune parameters were compared in each arm separately, as well as in both arms combined, between those patients developing a BOR of SD versus a BOR of PR. In patients receiving SOC alone, there were lower baseline levels of TGF-β in those who developed a PR compared to those with SD (Fig. 4A). In the SOC + IO arm, patients who developed a BOR of PR had higher baseline levels of sCD40L than patients with SD (Fig. 4B). When both arms were combined (SOC and SOC + IO), baseline samples from patients who experienced a PR had lower ratios of sCD27:sCD40L, lower levels of MDSCs, lower levels of PD-1+ Tregs, and higher levels of cDCs expressing Ki67 compared to patients with SD (Fig. 4C-F). There were also notable changes after 1 month of therapy that was associated with clinical response. In patients receiving SOC alone, greater increases in cDCs, pDCs, and NK cells expressing the activating receptor NKp46 and greater decreases in effector memory CD4+ T cells expressing PD-1 were noted in patients who had a PR compared to those who had SD (Fig. 4G-J). In the SOC + IO arm, early decreases in CD4+ T cells expressing PD-1 were noted in patients who developed a PR compared to an early increase in patients who had SD (Fig. 4K). This differential effect on PD-1+ CD4+ T cells in patients developing PR versus SD was also noted when both arms were combined (Fig. 4L). In addition, greater early increases in CD56br CD16+ NK (double-positive NK) were noted in patients who developed a PR compared to those with SD when both treatment arms were combined (Fig. 4M).
Figure 4.
Immune parameters associated with clinical response. Immune parameters at baseline (A-F) and early changes after 1 month of therapy (G-M) were evaluated in each arm (A-B, G-K) and in a pool of patients from both arms (C-F, L-M). Immune parameters that correlate with the likelihood of BOR of SD vs. BOR of PR are shown. BOR, best overall response; DP, double-positive; EM, effector memory; PR, partial response; SD, stable disease.
Discussion
In this randomized phase II trial, the SOC + IO regimen was well tolerated and the frequency and severity of TEAEs did not differ from each agent’s expected safety profile. The trial closed to accrual after an unplanned futility analysis predicted a low likelihood of the trial meeting its primary endpoint. Median PFS was similar between arms, and the objective radiographic response rate was the same (50%) in both arms. This study was not powered to detect a statistically meaningful difference in OS.
This trial did not stratify by BRAF V600E or RAS mutational status. Two patients in the SOC + IO arm had BRAF V600E mutant disease, an aggressive feature with a poorer prognosis compared to wild type.36 Conversely, most patients randomized to the SOC arm had tumors harboring RAS mutations (Table 1). These differences were not statistically meaningful. Therefore, it is unclear if this heterogeneity skewed outcomes.
Tumor-associated cascade antigens, in this case, brachyury and MUC1, were analyzed for potential indication of epitope spreading due to antitumor effects. Notwithstanding the lack of improvement in median PFS, the SOC + IO regimen yielded multifunctional CD4+ and/or CD8+ T-cell responses to cascade TAAs (MUC1 or brachyury) > 10-fold higher than baseline in 8/11 (73%) patients analyzed. In contrast, only 1/8 (13%) patients analyzed developed responses >10-fold higher than baseline after SOC treatment. Both MUC1 and brachyury are therapeutic targets of interest.37-42 In contrast to the findings with cascade antigens, there was not a difference in the development of T cells targeting CEA, the antigen encoded by the vaccine, in patients receiving SOC versus SOC + IO. This is a phenomenon that has been observed in preclinical models, in which a vaccine targeting CEA produced CEA-specific T-cell responses, but led to an even greater level of T-cell responses against cascade antigens.43
Reports from trials in prostate cancer, a malignancy similar to MSS mCRC in that it lacks a robust immune infiltrate, suggest that vaccine-derived T cells do migrate to tumor.17,18 Therefore, providing immunologic enhancement to tumor-infiltrating T cells derived from the SOC + IO regimen could be useful in future clinical trials. While these multifunctional T cells may be necessary for immune-mediated clinical activity, they are clearly not sufficient. Generating enough cells and facilitating their activity may be necessary components needed to achieve improved PFS that were lacking in this study.
When designing novel experimental regimens, the fact that vaccine and/or PD-1/PD-L1 blockade alone are not sufficient to produce meaningful clinical activity in pMMR/MSS mCRC5,8-11 should not overshadow the importance of ICI’s ability to counteract immune escape driven by treatments such as FOLFOX or therapeutic vaccine. For example, genes encoding PD-1 and other inhibitory receptors were upregulated in tumor-infiltrating lymphocytes after FOLFOX treatment in preclinical studies.44 Analysis of PD-L1 expression on pre- and post-treatment samples from 9 mCRC patients confirmed that FOLFOX upregulates tumoral PD-L1 expression.44 In addition to addressing PD-L1 expression from chemotherapy, PD-L1 expression in tumor that results from increased T-cell activity (eg, from vaccine) and subsequent IFN-γ production has been well characterized,19 supporting the inclusion of PD-1/PD-L1 blockade in regimens that include vaccine.
Our prospective analysis of classic and refined immune cell subset frequencies detected statistically meaningful changes in refined subsets that were distinct to each treatment arm 1 month on treatment. The significance of these changes is unclear. In contrast, the lack of change in Tregs and MDSCs seen in the classic subset analyses is notable since some studies have reported that chemotherapy can cause fluctuations in these populations.45-47 Additionally, there were no post-treatment changes in total CD4+ and CD8+ T cells, NK cells, NKT cells, B cells, cDCs, pDCs, or monocytes.
We identified associations between baseline data and changes in refined subsets with response to treatment (partial responses vs. stable disease). Patients who experienced a PR when treated with SOC or SOC + IO had lower baseline frequencies of MDSCs and PD-1+ Tregs, but higher levels of cDCs expressing Ki67 (Fig. 4D-F). We also associated high baseline sCD40L and low sCD27:sCD40L ratios with objective response to the SOC + IO regimen (sCD40L) and either regimen (sCD27:sCD40L), respectively (Fig. 4B, C). There was not a difference in the degree of decline in sCD40L after therapy between patients with PR versus those with SD. Soluble CD40L is known to have immunosuppressive functions and the source of this soluble factor is activated T lymphocytes and platelets.48 The higher baseline level of sCD40L seen in patients with PR may indicate that these patients at some point had a higher level of T-cell activation prior to therapy. These findings warrant further investigation of these refined subsets and circulating factors as biomarkers for response to these treatment regimens.
In conclusion, the addition of avelumab and the AdCEA vaccine to FOLFOX-based SOC did not improve median PFS or ORR compared to SOC alone in this small, randomized trial. However, the SOC + IO regimen yielded biological activity in the form of substantial increases in multifunctional CD4+ and CD8+ T cells specific for the cascade antigens MUC1 and brachyury. Identifying therapeutic regimens that can harness these cells and lead to improved clinical outcomes is key, as is further investigation of the potential biomarkers of response identified in this study.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Mohammad Bagheri, MD, and Christopher Heery, MD for their contributions to this clinical trial, as well as Bonnie L. Casey and Debra Weingarten for their editorial assistance in the preparation of this manuscript.
This research was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, and via Cooperative Research and Development Agreements (CRADAs) between the National Cancer Institute and EMD Serono Research & Development Institute, Inc., USA, an affiliate of Merck KGaA, Darmstadt, Germany, and between the National Cancer Institute and ImmunityBio. Preliminary results were presented as a short oral presentation at the virtual ESMO World GI Congress in 2021; see Redman, J. et al. Annals of Oncology, Volume 31, S227.
Contributor Information
Jason M Redman, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Yo-Ting Tsai, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Benjamin A Weinberg, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Renee N Donahue, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Shruti Gandhy, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Margaret E Gatti-Mays, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Houssein Abdul Sater, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Marijo Bilusic, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Lisa M Cordes, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Seth M Steinberg, Biostatistics and Data Management Section, Office of the Clinical Director, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Jennifer L Marte, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Caroline Jochems, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Sunnie S Kim, University of Colorado Cancer Center, Aurora, CO, USA.
John L Marshall, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Sheri McMahon, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Erica Redmond, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Jeffrey Schlom, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
James L Gulley, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Julius Strauss, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Patient Consent for Publication
No personally identifiable patient data were discussed in this article.
Ethics Approval
All patients gave written informed consent for participation. This study was approved by the National Cancer Institute’s Institutional Review Board. The trial registration number is NCT03050814.
Conflict of Interest
Benjamin A. Weinberg: Taiho, Lilly, Sirtex, HalioDx, Bayer, and Diiachi Sankyo/AstraZeneca (Other—Personal fees); Bayer, HalioDx, and Diiachi Sankyo/AstraZeneca (C/A); Ipsen (Other—Research support). Sunnie S. Kim: Daiichi-Sankyo and Merck (C/A); GlaxoSmithKline and Merck (RF unrelated to the work here). John L. Marshall: Bayer, Amgen, Taiho, Merck, Pfizer, Daiichi, Caris and Indivumed (Other—Personal fees); Bayer, Caris, Indivumed (Other—research support). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: J.M.R., Y-T.T., R.N.D., B.A.W., S.G., M.E.G-M., H.A.S., M.B., L.M.C., S.S.K., J.L.M., S.M., E.R., J.S., J.L.G. and J.S. Provision of study material or patients: J.M.R., B.A.W., S. ., M.E.G-M., H.A.S., M.B., S.S.K., J.L.M., S.M., E.R., J.L.G. and J.S. Collection and/or assembly of data: J.M.R., B.A.W., Sh.G., M.E.G-M., H.A.S., M.B., S.S.K., J.L.M., S.M., E.R., J.L.G., J.S., Y-T.T, and R.N.D. Data analysis and interpretation: J.M.R., Y-T.T. and R.N.D. Manuscript writing: J.M.R., Y-T.T. and R.N.D.
Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. National Cancer Institute Surveillance, Epiemiology, and End Results Program. National Institutes of Health. Available at https://seer.cancer.gov. Accessed 20 April 2021.
- 2. Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326-5334. 10.1200/JCO.2008.16.3212 [DOI] [PubMed] [Google Scholar]
- 3. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. 10.1001/jama.2017.7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andre T, Shiu K, Kim T, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J Clin Oncol. 2020;38(suppl 18): Abstract LBA4. [Google Scholar]
- 5. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andre T, Lonardi S, Wong M, et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. J Clin Oncol. 2018;36(suppl 4): Abstract 553. [Google Scholar]
- 7. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 8. Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(21):3485-3490. 10.1200/JCO.2010.28.3994 [DOI] [PubMed] [Google Scholar]
- 9. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Overman MJ, Kopetz S, McDermott R, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol 2016;34(suppl 15): Abstract 3501. [Google Scholar]
- 12. O’Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLOS ONE. 2017;12(12):e0189848. 10.1371/journal.pone.0189848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59-73. 10.1038/nri2216 [DOI] [PubMed] [Google Scholar]
- 14. Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482-491. 10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 15. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51-72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 16. Rebe C, Demontoux L, Pilot T, et al. Platinum derivatives effects on anticancer immune response. Biomolecules 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gulley JL, Heery CR, Madan RA, et al. Phase I study of intraprostatic vaccine administration in men with locally recurrent or progressive prostate cancer. Cancer Immunol Immunother. 2013;62(9):1521-1531. 10.1007/s00262-013-1448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benci JL, Xu B, Qiu Y, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540-1554.e12. 10.1016/j.cell.2016.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dammeijer F, van Gulijk M, Mulder EE, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 2020;38(5):685-700.e8. 10.1016/j.ccell.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 21. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64. 10.1016/S1470-2045(17)30900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. 10.1056/NEJMoa2002788 [DOI] [PubMed] [Google Scholar]
- 24. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morse MA, Chaudhry A, Gabitzsch ES, et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother. 2013;62(8):1293-1301. 10.1007/s00262-013-1400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balint JP, Gabitzsch ES, Rice A, et al. Extended evaluation of a phase 1/2 trial on dosing, safety, immunogenicity, and overall survival after immunizations with an advanced-generation Ad5 [E1-, E2b-]-CEA(6D) vaccine in late-stage colorectal cancer. Cancer Immunol Immunother. 2015;64(8):977-987. 10.1007/s00262-015-1706-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheeseman SL, Joel SP, Chester JD, et al. A ‘modified de Gramont’ regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer. 2002;87(4):393-399. 10.1038/sj.bjc.6600467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843-1852. 10.1016/S0140-6736(14)62004-3 [DOI] [PubMed] [Google Scholar]
- 29. Lepone LM, Donahue RN, Grenga I, et al. Analyses of 123 peripheral human immune cell subsets: defining differences with age and between healthy donors and cancer patients not detected in analysis of standard immune cell types. J Circ Biomark. 2016;5:5. 10.5772/62322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donahue RN, Lepone LM, Grenga I, et al. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J Immunother Cancer. 2017;5:20. 10.1186/s40425-017-0220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gatti-Mays ME, Strauss J, Donahue RN, et al. A phase I dose-escalation trial of BN-CV301, a recombinant poxviral vaccine targeting MUC1 and CEA with costimulatory molecules. Clin Cancer Res. 2019;25(16):4933-4944. 10.1158/1078-0432.CCR-19-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gabitzsch ES, Xu Y, Balint JP Jr, Hartman ZC, Lyerly HK, Jones FR. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother. 2010;59(7):1131-1135. 10.1007/s00262-010-0847-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gabitzsch ES, Xu Y, Balcaitis S, Balint JP Jr, Jones FR. An Ad5[E1-, E2b-]-HER2/neu vector induces immune responses and inhibits HER2/neu expressing tumor progression in Ad5 immune mice. Cancer Gene Ther. 2011;18(5):326-335. 10.1038/cgt.2010.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gabitzsch ES, Xu Y, Balint JP Jr, Balcaitis S, Sanders-Beer B, Jones FR. Induction and comparison of SIV immunity in Ad5 naïve and Ad5 immune non-human primates using an Ad5 [E1-, E2b-] based vaccine. Vaccine. 2011;29(45):8101-8107. 10.1016/j.vaccine.2011.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shahda S, Noonan A, Bekaii-Saab T, et al. A phase II study of pembrolizumab in combination with mFOLFOX6 for patients with advanced colorectal cancer. J Clin Oncol 2017;35(suppl 15):Abstract 3541. [Google Scholar]
- 36. Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148(1):88-99. 10.1053/j.gastro.2014.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45-60. 10.1038/nrc1251 [DOI] [PubMed] [Google Scholar]
- 38. Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265(25):15286-15293. [PubMed] [Google Scholar]
- 39. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428. 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927-939. 10.1016/j.cell.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 41. Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533-544. 10.1172/JCI38379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471-2478. 10.1158/1078-0432.CCR-06-2353 [DOI] [PubMed] [Google Scholar]
- 43. Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328-4337. 10.1158/0008-5472.CAN-04-0073 [DOI] [PubMed] [Google Scholar]
- 44. Dosset M, Vargas TR, Lagrange A, et al. PD-1/PD-L1 pathway: an adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology. 2018;7(6):e1433981. 10.1080/2162402X.2018.1433981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maeda K, Hazama S, Tokuno K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011;31(12):4569-4574. [PubMed] [Google Scholar]
- 46. Kanterman J, Sade-Feldman M, Biton M, et al. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74(21):6022-6035. 10.1158/0008-5472.CAN-14-0657 [DOI] [PubMed] [Google Scholar]
- 47. Limagne E, Euvrard R, Thibaudin M, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016;76(18):5241-5252. 10.1158/0008-5472.CAN-15-3164 [DOI] [PubMed] [Google Scholar]
- 48. Schlom J, Jochems C, Gulley JL, Huang J. The role of soluble CD40L in immunosuppression. Oncoimmunology. 2013;2(1):e22546. 10.4161/onci.22546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.