ABSTRACT
Heart rate (HR) targets are commonly used to administer exercise intensity in sport and clinical practice. However, as exercise protracts, a time-dependent dissociation between HR and metabolism can lead to a misprescription of the intensity ingredient of the exercise dose.
Purpose
We tested the hypothesis that a slow component of HR (i.e., scHR) occurs in all intensity domains, greater than the slow component of oxygen uptake (scV˙O2), and we developed an equation to predict it across exercise intensities.
Method
Eighteen healthy, postmenopausal women (54 ± 4 yr) performed on a cycle ergometer: i) a ramp incremental test for thresholds and V˙O2max detection; ii) 30-min constant work exercise at 40%, 50%, 60%, 70%, and 80% V˙O2max for the measurement of scHR, scV˙O2, stroke volume, and body temperature (T°). scHR and scV˙O2 were compared by two-way repeated-measures ANOVA (intensity and variable). Pearson correlation was calculated between the slow component of all variables, relative intensity, and domain. scHR (in beats per minute) was predicted with a linear model based on exercise intensity relative to the respiratory compensation point (RCP).
Results
A positive scHR was present in all domains, twice the size of scV̇O2 (P < 0.001), and significantly correlated with the slow components of V̇O2 (r2 = 0.46), T° (r2 = 0.52), and relative intensity (r2 = 0.66). A linear equation accurately predicts scHR based on %RCP (r2 = 0.66, SEE = 0.15).
Conclusions
A mismatch exists between the slow components of HR and metabolic intensity. Whenever exercise is prescribed based on HR, target values should be adjusted over time to grant that the desired metabolic stimulus is maintained throughout the exercise session.
Key Words: KINETICS, SLOW COMPONENT, EXERCISE THERAPY, TRAINING, AEROBIC METABOLISM
“Exercise is medicine” and induces benefits in both performance and health in a dose–response manner (1,2). The exercise dose can be quantified through the elements of frequency, intensity, time, and type, as described in the frequency, intensity, time, and type scheme (3). Among these, intensity is the most elusive term of the “exercise prescription” and can be classified as “absolute” or “relative” (4). Absolute intensity is typically quantified through the direct measurement of speed/power or its oxygen consumption (V˙O2) equivalent and defines, for example, the energy cost of a given activity (5). Relative intensity is commonly quantified based on fixed fractions of maximal or “reserve” oxygen consumption (%V˙O2max or %V˙O2R) or, more accurately, based on individually determined exercise intensity boundaries (5), and dictates the extent of the disturbance of the body’s homeostasis (5–9). The actual implementation of a given absolute or relative exercise intensity target requires the appropriate translation of the desired metabolic intensity into a load or speed (10). Whenever the quantification or implementation of workload is impossible or impractical (e.g., unavailable individual V˙O2/workload relationship, workload resulting from combinations of speed and inclination, or a complex movement task in a real-life context), heart rate (HR) remains one of the most commonly used methods to prescribe intensity in sport and clinical practice. The prescription of exercise intensity using HR targets requires the continuous monitoring of HR and relies on the existence and constancy over time of a linear relationship with V˙O2 (absolute, relative to max/reserve and metabolic equivalents) (11–13). However, the validity of HR as an index of metabolic intensity over time has been recently questioned; an increase of HR over time, totally or partially dissociated from the slow component of V˙O2 (scV˙O2) has been observed during constant work exercise in healthy adults (14) and individuals with obesity (15).
In particular, HR seems to display a positive slope as a function of time (i.e., an HR slow component (scHR)) at all exercise intensities (i.e., below the gas exchange threshold (GET), moderate exercise domain), whereas V˙O2 shows this behavior only at intensities exceeding GET (i.e., heavy and severe exercise intensity domains) (14). A slow increase in HR over time during exercises lasting more than 10 min has been classically described under the name of “cardiovascular drift” (16–19), in association with a parallel increase in body temperature (T°) due to dehydration and hyperthermia (16–19) and with a decrease (16–19) in stroke volume (SV). More recently, it was suggested that an increase in HR over time (i.e., a “true slow component”) occurs even before 10 min of exercise, unrelated to the dehydration, hyperthermia, or a decrease in SV that characterize the traditionally described “cardiovascular drift” (14). However, the physiological underpinnings of this phenomenon remain to be fully elucidated (14). Moreover, the relationship between the slow component of HR and intensity domain has received very little attention. Whatever the physiological cause of the slow component of HR may be, the practical implication of this phenomenon is that prescribing exercise intensity based on HR targets leads to an unanticipated, undesired, and domain-specific reduction in work rate/metabolic intensity during a prolonged exercise session that is intended to provide a constant training load (14,15). The results of the aforementioned studies performed on adult males, healthy and with obesity, have raised the awareness of the scientific community on this perhaps overlooked problem in exercise implementation. In this context, menopausal women represent a large and increasing portion of the population in which accurate exercise prescription is crucial to promote and maintain health (20–22). As women entering this status may experience increments in resting HR and systolic and diastolic blood pressure (23,24), the development of a research-informed framework for optimal exercise prescription requires the collection of population-specific data (25,26). Such information is essential to grant the desired stimulus is maintained throughout the exercise sessions (27). Therefore, in the present study, we analyzed the HR, SV, V˙O2, and T° kinetics during constant work exercise performed at different relative intensities and domains in a group of postmenopausal women with the aim to i) verify the existence of an increase over time of HR across domains, ii) quantify the amplitude of this phenomenon, iii) establish the relationship between the slow component of HR and that of V˙O2 across domains, and iv) quantify the relationship between the slow component of HR and other physiological variables (i.e., kinetics of V̇O2, SV, T°, and relative exercise intensity) toward a possible prediction model.
METHODS
Participants
Eighteen recreationally active postmenopausal women (Table 1) were recruited by advertisement within the local community and agreed to participate in this study. Inclusion criteria were female sex and age between 45 and 65 yr, and menopausal status (i.e., absence of menstrual cycles for a minimum of 12 months); exclusion criteria were smoking and any medical condition or therapy that could influence the physiological responses during testing. The subjects were fully informed of any risk and discomfort associated with the experiments before giving their written consent to participate in the study. All procedures were approved by the Committee for Approval of Human Research–CARU of the University of Verona (no. 16-2019).
TABLE 1.
Overview of anagraphic, anthropometrics, and functional characteristics.
| No. | Age (yr) | T-MP (yr) | Height (cm) | Weight (kg) | BMI (kg·m−2) | V˙O2max (ml·min−1·kg−1) | GET (%V˙O2max) | RCP (%V˙O2max) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 18 | 54.0 ± 3.6 | 4.2 ± 2.7 | 163.9 ± 5.5 | 59.0 ± 8.1 | 22.0 ± 3.0 | 36.4 ± 5.3 | 57.0% ± 8.9% | 81.2% ± 4.7% |
Values are expressed as mean ± SD: age, T-MP, height, weight, BMI, V˙O2max, GET, and RCP.
T-MP, time from menopause.
Protocol
After medical clearance and anthropometric measurements (body mass (digital scale, Seca877; Seca, Leicester, United Kingdom), height (vertical stadiometer; Seca), and skinfold thickness (Holtain T/W skinfold caliper; Holtain Limited, Crymych, United Kingdom) (28)), subjects visited the laboratory on six occasions within a maximum of 1 month. On the first visit, they performed a ramp incremental test to exhaustion on an electromagnetically braked cycle ergometer (Sport Excalibur; Lode, Groningen, the Netherlands). On the successive appointments, each separated by a minimum of 2 d, subjects performed, in a randomized order, five constant work exercises on the same cycle ergometer, respectively, at 40%, 50%, 60%, 70%, and 80% of their V˙O2max, as determined from the ramp incremental test, each lasting 30 min or until exhaustion.
Participants were instructed to avoid caffeine consumption and physical activity, respectively, for at least 8 and 24 h before each testing session. Tests were conducted at the same time of the day in an environmentally controlled laboratory (22°C–25°C, 55%–65% relative humidity). Ergometer position was chosen during the first ramp incremental test and recorded for the successive appointments. Moreover, to minimize the variability of glycogen oxidation, participants consumed the following standardized meal 2 h before all the testing sessions: 500 cc of water and 2 g·kg−1 of low glycemic index carbohydrates (11).
Ramp Incremental Protocol
The ramp incremental protocol consisted of a 3-min baseline cycling at 30 W, followed by a 10–15 W·min−1 increase in power output (PO) until volitional exhaustion. Participants were asked to pick a self-selected cadence in the range of 70–90 rpm and to maintain it throughout all tests. Breath-by-breath pulmonary gas exchange, ventilation, and HR were continuously measured using a metabolic cart (Quark B2; Cosmed, Firenze, Italy) (29). Capillary blood samples (20 μL) were drawn from the ear lobe before and at the first, third, fifth, and seventh minutes after exhaustion. Samples were immediately analyzed using an electroenzymatic technique (Biosen C-Line; EKF Diagnostics, Barleben, Germany), and the highest value was considered as the peak of blood lactate accumulation ([La−]max) for the incremental test.
Constant Work Protocol
The constant work exercise consisted of a 3-min baseline cycling at 30 W, followed by an instantaneous increase in PO that was maintained for 30 min or until exhaustion. The absolute PO for each of the five trials was chosen so that it would elicit a V˙O2 equal to 40%, 50%, 60%, 70%, and 80% of the previously identified V˙O2max. To this aim, the individual V˙O2/PO relationship derived from the incremental exercise was corrected for the V˙O2 mean response time and slow component, by applying the mathematical model recently proposed by Caen et al. (10).
Breath-by-breath pulmonary gas exchange, ventilation, and HR were continuously measured with the same method described for the ramp incremental.
SV was determined continuously by electrical bioimpedance meter (PhysioFlow®; Manatec type PF05L1, Paris, France) that uses resting blood pressure (ERKA; Perfect Aneroid Clinica 48, Hamburg, Germany) and changes in transthoracic impedance during cardiac ejection to calculate SV (30). After skin preparation, six electrodes were used as per the manufacturer’s instructions: two at the base of the neck, two on the back at the same level as the xiphoid process, and two on the chest.
Tympanic temperature was taken as a proxy of core body temperature and was measured by an infrared thermometer (Braun®; ThermoScan, Lausanne, Switzerland) (31) from the inner ear at rest, during the last minute of baseline cycling, and at the 3rd, 5th, 10th, 15th, 20th, 25th, and 30th minutes of exercise.
Data-Analysis
For both the incremental and constant work protocols, gas exchange variables were sampled breath-by-breath, whereas HR, SV, and cardiac output were sampled beat by beat. Aberrant data points (that lay 3 SD from the local mean) were removed, and thereafter, data were linearly interpolated at 1-s and then mediated at 5-s intervals. For the incremental test, GET and respiratory compensation point (RCP) were determined with the standard technique by three experts independently (32). Respiratory exchange ratio (R) was calculated as carbon dioxide production/V˙O2. V˙O2max and peak PO were determined, respectively, as the average V˙O2 of the last 30 s of exercise and the as highest mechanical PO achieved upon exhaustion during the ramp incremental exercise (33).
For each constant work exercise, we calculated 1) a 1-min mean of V˙O2, HR, and SV for the last minute of baseline and the for the 1st, 3rd, 5th, and then for every 5th additional minute until the end of the exercise (i.e., 30th minute or exhaustion); 2) the individual slow components of the V˙O2, HR, SV, quantified as the slope of the linear fitting of the 1-s interpolated data, from the 5th minute to the end of the exercise and named scV˙O2, scHR, and slow component of SV (scSV), respectively; and 3) the individual slow components of T° quantified as the slope of the linear fitting of the 5-min data, from the 5th minute to the end of the exercise and named slow component of body temperature (scT°). For each intensity, all the slow components were expressed in absolute units; scHR and scV˙O2 were also expressed relative to the individual reserve calculated as the difference between maximal and resting values observed during the ramp incremental protocol and were named %scHR and %scV˙O2, respectively.
Finally, each trial was further classified as belonging to the moderate, heavy, and severe domain based on the mean V˙O2 at the 5th minute of the exercise with the following rule:
if V˙O2 at the 5th min < V˙O2 at GET → MODERATE domain → score 1
if V˙O2 at the 5th min > V˙O2 at GET and < V˙O2 at RCP → HEAVY domain → score 2
if V˙O2 at the 5th min > V˙O2 at RCP → SEVERE domain → score 3
Statistical Analysis
Data description
All data are presented as mean ± SD. After assumption verification (i.e., normality, homogeneity of variance), a one-way repeated-measures ANOVA was performed to compare the value at the fifth minute and the slow component of V˙O2, PO, HR, SV, and T° across the different intensities relative to %V˙O2max.
To verify the presence and amplitude of scHR in exercise domains and its relationship with scV˙O2
%scV˙O2 and %scHR were compared with a control value equal to 0 and to each other across the three exercise intensity domains by two-way repeated-measures ANOVA (variable and domain). Post hoc analyses were performed using the Holm–Sidak method.
To test the possible interaction among the aforementioned variables and scHR, we propose a regression including scV˙O2, scHR, scSV, scT, intensity domains, %V˙O2max, and %RCP followed by multiple linear regression
A Pearson correlation coefficient was calculated between the slow component of V˙O2, HR, SV, T°, exercise domain, %V˙O2max, and %RCP.
To develop a multiple linear model for the prediction of the individual increments over time of scHR, we proceed as follows: i) the parameters were ordered by correlation coefficient with scHR, and ii) a forward multiple regression was initially run including these variables: scV˙O2, scSV, scT°, intensity domains, %V˙O2max, and %RCP; this analysis identified nonsignificant (P ≥ 0.05) and cross-correlated predictors (i.e., correlation coefficient >±0.70) that were discarded from the model. Then a forward multiple regression was run again until significant and not cross-correlated predictors and the best model were identified (34). Power analysis was conducted a priori, based on the expected SD of HR seen during constant load exercise in previous articles (14,15) as the main variable. To identify significant differences, with an α error of 0.05 and a statistical power (1 − β) of 0.95, a n value of 8 subjects was necessary (G*Power 3.1). All statistical analyses were performed using SigmaPlot version 14.0, and α was set in advance at the 0.05 level. Statistical significance was accepted when P < α.
RESULTS
Subjects’ characteristics are reported in Table 1. The average time from menopause was 4 ± 3 yr (from 1 to 8 yr); although the average body mass index (BMI) was indicative of a normal-weight population, the rather high average V˙O2max per kilogram (36.4 ± 5.3 mL·min−1·kg−1) and the results of the International Physical Activity Questionnaire (IPAQ- Short Form) (2250 ± 1340 MET·wk−1) were indicative of a moderately active to active lifestyle.
Subjects’ mean ± SD of V˙O2max, peak PO, and HRmax, measured at the end of the ramp incremental, were 2.12 ± 0.26 L·min−1, 172 ± 22 W, and 171 ± 9 bpm, respectively. A plateau (i.e., an increase in V˙O2 <50% of the expected based on the increase in PO) was present in 16 of the 18 subjects. Furthermore, the values of %HRmax, Rmax, and [La−]max upon exhaustion (101% ± 5%, 1.14 ± 0.24, and 8.6 ± 1.1 mmol·L−1, respectively) indicate that a maximal effort was reached. Subjects’ GET and RCP were detected at V˙O2 of 1.20 ± 0.18 L·min−1 (57% ± 9% V˙O2max) and 1.72 ± 0.18 L·min−1 (81% ± 5% V˙O2max), respectively.
For constant work exercises, an overview of the subjects’ mean ± SD response at the fifth minute of exercise intensity is reported in Table 2; the profiles of the variables are displayed as a function of time in Figure 1. A one-way repeated-measures ANOVA on values at fifth minute showed, as expected, a significant effect of relative exercise intensity (i.e., %V˙O2max) on PO, V˙O2, and HR (for all variables, P < 0.001), whereas no main effect of intensity was found on either SV (P = 0.28) or T° (P = 0.55). Post hoc analysis revealed significant differences between all the intensities for PO, V˙O2, and HR (for all intensities, P < 0.001).
TABLE 2.
Variables at fifth minute of constant work exercises.
| V˙O2max (%) | PO (W) | V˙O2 (L·min−1) | HR (bpm) | SV (mL) | T° (°C) |
|---|---|---|---|---|---|
| 40 | 35 ± 9 | 0.92 ± 0.11 | 100 ± 13 | 91.1 ± 12.5 | 37 ± 0.2 |
| 50 | 51 ± 14a | 1.11 ± 0.17a | 109 ± 12a | 98.6 ± 15.2 | 36.9 ± 03 |
| 60 | 74 ± 14a,b | 1.31 ± 0.20a,b | 121 ± 12a,b | 101.0 ± 12.7 | 36.9 ± 0.3 |
| 70 | 96 ± 16a,b,c | 1.53 ± 0.22a,b,c | 136 ± 12a,b,c | 98.4 ± 10.3 | 37.0 ± 0.3 |
| 80 | 120 ± 17a,b,c,d | 1.75 ± 0.26a,b,c,d | 153 ± 10a,b,c,d | 100.4 ± 9.8 | 37.0 ± 0.2 |
| Main effect | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.28 | P = 0.55 |
Mean ± SD of PO, V˙O2, HR, SV, and T° at the 5th minute of different exercise intensities relative to the V˙O2max. Main effects for the relative exercise intensity are shown in the bottom line of the tables. Data in boldface are significant (P < 0.05).
aSignificant difference from 40%.
bSignificant difference from 50%.
cSignificant difference from 60%.
dSignificant difference from 70%.
FIGURE 1.
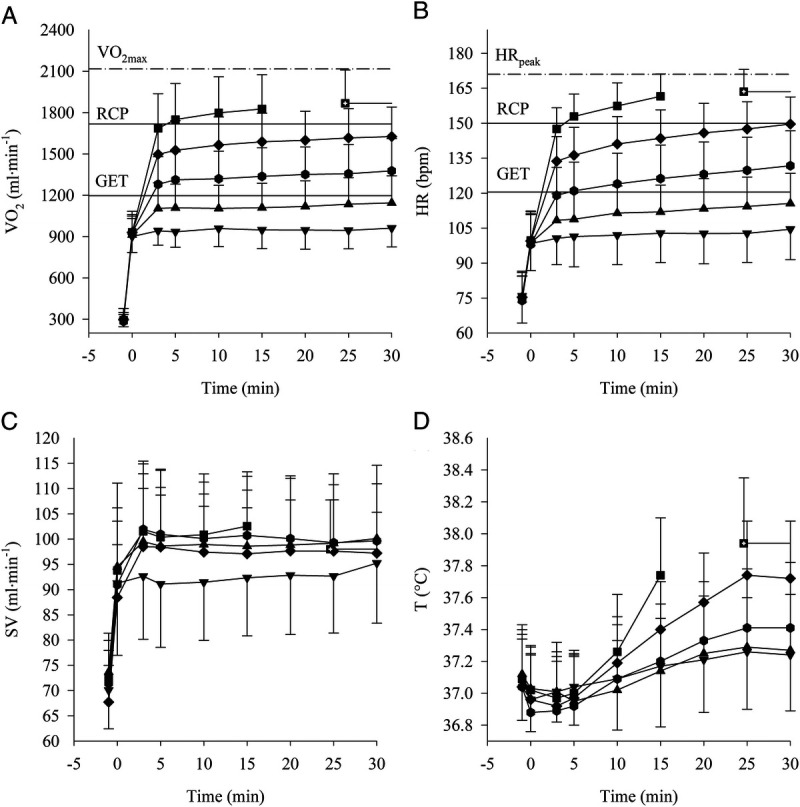
Data collected during CWR at 40% (▼), 50% (▲), 60% (●), 70% (♦), and 80% (■) of V̇O2max are plotted as a function of time. Mean ± SD of HR (A), oxygen uptake (B), SV (C), and body temperature (D). The three horizontal line plots in panels A and B indicate from the lowest to the highest: GET, RCP, and peak value as identify from the ramp test.
Subjects’ mean ± SD of scV˙O2, scHR, scSV, and scT° are reported in Table 3 as increments in absolute units per minute across exercise intensity (i.e., %V˙O2max). A one-way repeated-measures ANOVA revealed a main effect of relative exercise intensity on scV˙O2, scHR, and scT° (P < 0.001) and no effect on scSV (P = 0.12). On scV˙O2, the post hoc analysis revealed a significantly lower value for the 40% when compared with all the intensities greater than 60% V˙O2max (P < 0.001, for all the conditions), whereas 50% was significantly lower compared with all the values greater than 70% V˙O2max (P < 0.001, for all the conditions). When comparing the scHR, all the values increased from 40% to 80% V˙O2max and were significantly different from each other (P < 0.05 for all the conditions). Lastly, on the scT°, the post hoc analysis revealed a significantly greater value at 80% compared with all the intensities (P < 0.001, for all the conditions); likewise, the value at 70% was greater compared with the intensities at 40% and 50% V˙O2max (P < 0.001, for both conditions).
TABLE 3.
Variables’ slow component at different exercise intensities.
| V˙O2max (%) | scV˙O2 (mL·min−2) | scHR (bpm·min−2) | scSV (mL·min−1) | scT° (°C·min−1) |
|---|---|---|---|---|
| 40 | 0.31 ± 0.83 | 0.11 ± 0.10 | 0.11 ± 0.19 | 0.01 ± 0.01 |
| 50 | 1.13 ± 1.32 | 0.25 ± 0.13a | −0.05 ± 0.16 | 0.01 ± 0.01 |
| 60 | 2.38 ± 1.57a | 0.42 ± 0.15a,b | −0.08 ± 0.32 | 0.02 ± 0.01 |
| 70 | 3.18 ± 2.02a,b | 0.57 ± 0.18a,b,c | −0.13 ± 0.25 | 0.03 ± 0.01a,b |
| 80 | 4.01 ± 2.21a,b | 0.70 ± 0.16a,b,c,d | −0.03 ± 0.33 | 0.06 ± 0.02a,b,c,d |
| Main effect | P < 0.001 | P < 0.001 | P = 0.08 | P < 0.001 |
Means ± SD absolute increments per minute of scV˙O2, scHR, scSV, and scT° at different intensities relative to V˙O2max. Main effects for the relative exercise intensity are shown in the bottom line of the tables. Data in boldface are significant (P < 0.05).
aSignificant difference from 40%.
bSignificant difference from 50%.
cSignificant difference from 60%.
dSignificant difference from 70%.
Subjects’ mean ± SD of %scV˙O2 and %scHR were, respectively, 0.05 ± 0.07 and 0.22 ± 0.15 %min−1 for the moderate domain, 0.15 ± 0.12 and 0.49 ± 0.22 %min−1 for the heavy domain, and 0.21 ± 0.11 and 0.80 ± 0.17 %min−1 for the severe domain. %scV˙O2 and %scHR are plotted as a function of %V˙O2max in Figure 2. A two-way repeat-measures ANOVA (domain and variable) on the %scV˙O2 and %scHR in the moderate, heavy, and severe domains showed a main effect of the domain and the variable (for both factors, P < 0.001). A post hoc analysis showed that both variables, in all domains, were significantly different from a control column equal to zero. Moreover, %scHR was significantly greater compared with %scV˙O2 in all domains (P < 0.001). Regarding the %scV˙O2, heavy and severe domains were not different from each other, but both were significantly greater compared with the moderate domain. Finally, the %scHR significantly increased from moderate to heavy, to severe intensity domain (P < 0.001 for all post hoc comparisons across domains). The results of the Pearson correlation analysis between scHR and scV˙O2, scSV, scT°, exercise intensity (expressed both as %V˙O2max and %RCP), and domain (i.e., 1, 2, 3) are presented in Table 4 as coefficients of determination (r2). All the previously listed variables, except for scSV, had a significant moderate to high correlation with scHR.
FIGURE 2.
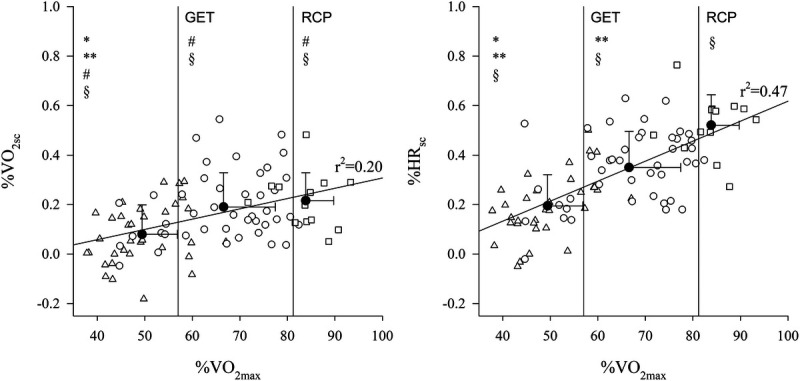
Individual data (white symbols) of %slow component per minute of oxygen uptake (V˙O2; left panel) and HR (right panel) are plotted as a function of %V˙O2max. Data points falling in the moderate domain are indicated by (Δ) in the heavy domain by (○) and in the severe domain by (□). Regression lines with the coefficient of determination are shown. The two vertical lines in each panel indicate the group-average value of GET and RCP, expressed as %V̇O2max. The three black dots (●) in each graph indicates mean ± SD values of %scV̇O2 and %HR grouped by intensity domain. Relative to each domain group, *indicates a significant difference from heavy and **from the severe domains; #indicates a significant difference from %scHR within the same domain; §indicates a significant difference from “0.”
TABLE 4.
Correlation matrix between the slow components and exercise intensities.
| scSV (mL·min−1) | scT° (°C·min−1) | scV˙O2 (mL·min−2) | Intensity (domain) | Intensity (%V˙O2max) | Intensity (% of RCP) | |
|---|---|---|---|---|---|---|
| scHR (bpm·min−2) | −0.07* | 0.52** | 0.46** | 0.51** | 0.63** | 0.66** |
| scSV (mL·min−1) | 0.01 | 0.00 | 0.02 | 0.07* | 0.06* | |
| scT° (°C·min−1) | 0.33** | 0.34** | 0.61** | 0.47** | ||
| scV˙O2 (mL·min−2) | 0.21** | 0.33** | 0.29** | |||
| Intensity (domain) | 0.51** | 0.62** | ||||
| Intensity (%V˙O2max) | 0.91** |
Correlation matrix with coefficient of determinations (r2) between slow components of HR, SV, T°, V˙O2, relative exercise intensity as domains, %V˙O2max, and %RCP.
*Significant effect: P < 0.05.
**Significant effect: P < 0.001.
Moreover, the iterative application of forward multiple linear regression identified the following significant, not cross-correlated predictors of scHR: scV˙O2, scT°, and %RCP and the following predicting equations for the individual scHR:
Relative exercise intensity, as indicated by %RCP, was the single most relevant predictor of scHR. The use of this single predictor as opposed to the above more complex equation yielded the following equation and performance:
DISCUSSION
The purposes of this study were i) to confirm the presence, ii) to describe the amplitude iii) and the relationship with V˙O2 of the scHR in all exercise domains in postmenopausal women, and iv) to verify the possible relationship between the scHR and the slow component of V˙O2, SV, and body temperature across domains/exercise relative intensities toward the development of a possible prediction model. The study confirmed, in the specific postmenopausal population, the existence of an scHR that increases in proportion to the intensity and across domains. In all domains, the scHR over time is significantly larger than the scV˙O2. The scHR over time can be accurately predicted from a simple equation based on %RCP only.
The individual and anthropometric characteristics (BMI and percent body fat) of the subjects enrolled in the study were in line with what we expected from the existing literature for a healthy postmenopausal population (35). However, the average relative high value of V˙O2max of 36.4 ± 5.3 mL·min−1·kg−1 places these mean between the 75th and 80th percentiles of the age-specific American College of Sport Medicine fitness distribution. The phenomenon of a delayed increase in HR over time during constant work exercises of prolonged duration has been previously described in association with changes in SV and T° in male individuals under the name of “cardiovascular drift” (18,19). Two recent studies with an overall sample size of 33 male-only individuals (17 healthy adults and 16 individuals with obesity) specifically investigated the existence of the scHR in relation to intensity domains and V˙O2 kinetics; however, the aforementioned studies did not simultaneously record changes in SV and T° possibly associated with the cardiovascular drift phenomenon (14,15). By contextually analyzing the time course of body temperature, and cardiovascular and metabolic variables during constant work exercise, the current study contributes to fill a lack of knowledge on the existence and the relationship with other cardiometabolic variables of the scHR, across relative intensities and domains, in adult women after menopause. In addition, this is the first study to examine more than one intensity in a single domain. In agreement with previous findings in adult males, our data confirm the presence of an scHR in all domains of exercise, including moderate. Although it had been previously reported that the amplitude of the scHR increases from the moderate to the heavy to the severe domain (14,15), our study is the first to demonstrate that the dynamics of the scHR is a linear function of exercise intensity. Interestingly, the absolute and relative amplitude of the scHR in our study were markedly smaller (i.e., ~0.21 bpm and 0.22% per minute) than the values reported in young males at a comparable intensity of exercise (i.e., ~1 bpm and 1.5% per minute for moderate-intensity exercise) (14). The difference may be partially explained by the fact that our determination of the scHR was calculated from the fifth minute of exercise (i.e., when a temporary steady state in HR is typically reached) (29) as opposed to the third minute of exercise, when HR may still be raising in the least fit individuals (36). In fact, a smaller dynamic of HR (i.e., ~0.18 bpm and 0.12% per minute, more similar to the values in our current study) had been previously documented in endurance-trained athletes between the 10th and 60th minutes of constant, moderate-intensity sessions (18,37). Moreover, a smaller potential for HR excursion typically characterizes older individuals with a smaller HR reserve compared with younger individuals (25); therefore, the age difference of ~25 yr in the populations of the studies could at least partially explain the observed smaller HR dynamic over time in our older sample compared with younger adults (14). The aforementioned findings and considerations suggest that future studies aimed at quantifying the dynamics of HR over time during constant work exercise be mindful of the time window in which the phenomenon is measured and make sure that different ages and both sexes are evaluated.
Regarding the relationship with other physiological variables, in agreement with previous work (14,15), our data confirm a greater amplitude of the scHR compared with the scV˙O2 in all domains of exercise, with a ratio of 2:1 roughly for all domains. Moreover, our study demonstrates a significant yet mild correlation between scHR and scV˙O2, which suggests that scHR is only partially related to metabolism (i.e., <50% of the variability in scHR is explained by changes in scV˙O2). The slow component of the V˙O2 kinetics is a very well-described phenomenon that has been discussed in detail in several reviews (38–41). Although the physiological underpinnings of this phenomenon remain to be fully elucidated, it is generally agreed that i) the scV˙O2 is absent in the moderate-intensity domain of exercise, and ii) it is present in the form of a delayed exponential (and increased gain) in the heavy domain and iii) in the form of a linear projection to V˙O2max in the severe domain of exercise (37,41). We would like to specify that our study was focused on the quantification of the amplitude of the scV˙O2 rather than on accurate determination of its kinetics that would have been impossible with only one exercise repetition. The choice of using a linear function aimed at reducing the impact of the variability of the V˙O2 signal on the identification of the amplitude of the change over time, rather than endorsing a linear over an exponential fitting of the response in the heavy domain of exercise.
The data from the current study agree with the literature in that we find an increase in the scV˙O2 with increasing intensity. However, the scV˙O2 over time different from 0 in the moderate domain seems in contrast with previous findings in healthy young males (14) and adults affected by obesity (15). In the majority of studies, the scV˙O2 in the moderate-intensity is quantified as the difference between the V˙O2 at 3rd–6th minute to the 10th minute of exercise. As such, small differences in breath by breath V˙O2, in a small group of individuals, may be difficult to confirm. In our study, the large sample size, the long exercise duration (i.e., 30 min), and the comparison with 0 may have emphasized a difference that is statistically significant, yet practically very small (total amplitude of ~20 mL·min−1 that is well below the minimum detectable difference for this variable, i.e., ~100 mL·min−1) (42). In agreement with this view, a drift in V˙O2 comparable to that observed in our study (i.e., ~1 mL·min−2) had been described by Trinity et al. (19) in a group of young individuals exercising for 60 min in the moderate domain. Alternatively, it is also plausible that in our sample of postmenopausal women, a delayed “metabolic shift” between aerobic and anaerobic metabolisms (43) may characterize the moderate domain of exercise.
The notion that V˙O2 slowly projects to its max, when a constant-load exercise above the heavy to severe boundary is performed, is supported by several studies on V˙O2 kinetics (38–40). Therefore, the fact that, in our study, V˙O2 did not reach its peak at the higher intensities (i.e., 80% V˙O2max) may be somewhat surprising. However, a V˙O2 upon exhaustion about 6% to 7% lower compared with V˙O2max has been previously described in individuals exercising up to 10% above the heavy to severe boundary for 30 min (44–46). We hypothesize that, although a slow component of V˙O2 is always present above critical intensity, which would theoretically project to V˙O2max (47), this projection may be very slow in the lower portion of the severe domain (45,46), to the extent that the subject may not reach V˙O2max within the window of observation or before exhaustion.
The physiological basis of the slow increase in HR over time has received less attention than the scV˙O2. Its origin has been attributed to either an indirect consequence of the exercise/temperature-related reduction of SV or to the direct chronotropic effect of hyperthermia/catecholamines/signaling from exercising muscles (19,48,49). A progressive decrease in SV and mean arterial pressures has been described as part of the cardiovascular drift phenomenon that occurs when an exercise between 50% and 75% V˙O2max is prolonged above 10th minute (19); in this context, larger tendencies to a decrease have been described for the more prolonged exercises (18,19,50). The peripheral displacement of blood volume in conjunction with thermoregulatory increases in skin blood flow has been reported to reduce venous return, in turn lowering SV and causing a secondary increase in HR to maintain a constant cardiac output (19). Alternatively, recent literature suggests that the observed reduction of SV over time is in fact secondary to the reduction of ventricular filling time that is caused by the increase in HR (19). The continuous increase in HR observed overtime after the 10th minute of a constant work exercise >45% V˙O2max has been attributed to the direct effect of either temperature or circulating catecholamine on the sinoatrial node or via muscle thermoreflexes (19,51). In our study, we did not observe significant SV decreases at all exercise intensities; this confirms previous work on exercises of a comparable or shorter duration (14,19). Moreover, in line with previous studies, we found no correlation between the scHR and changes of SV over time (14,15). On the contrary, T° was significantly increased after 30 min of exercise (~0.2°C in the moderate exercise, ~0.5°C in the heavy domain, and ~0.8°C during the severe domain), with values similar to those reported in the literature for exercises of a comparable duration (15,19). Moreover, a significant mild-to-good correlation was found between increments of T° and HR (r2 = 0.52). The aforementioned finding confirms in postmenopausal women and in a larger range of intensities the findings of previous studies in adult males (50,52,53) and supports a role of heat accumulation in determining the HR increases over time. Larger changes in T° (i.e., ~1°C vs 0.2°C–0.5°C of our study) and stronger correlations with scHR (i.e., r2 = 0.95) had been reported in young males for exercises of double the duration conducted in the upper end of the moderate-intensity domain (50). The higher relative intensity compared with our study (57% vs 49% V˙O2max in our study) is the likely cause for the larger heat accumulation in Coyle’s young men compared with our postmenopausal women. In fact, a linear relationship was previously described between heat accumulation and relative exercise intensities between 25% and 75% V˙O2max (53). Our data, enclosed between 40% and 80% V˙O2max, are in line with these observations and show significant scT° increments associated with exercise intensity expressed both as %RCP and as %V˙O2max (r2 = 0.66 and r2 = 0.63, respectively). In summary, our data support the hypothesis that the scHR is at least in part attributable to the increases in T° as previously described in adult men (15,19).
Whatever the causes of scHR may be, this phenomenon has practical implications in the field of exercise prescription, yet this phenomenon is often ignored in both healthy adults (14) and clinical populations (15). When exercise is anchored to HR targets and HR demonstrates an increase over time that is dissociated from V˙O2, a time-dependent reduction workload (approximately 14% in 17 min) will be observed along with a decrease in the metabolic load of the training session (14,36). Our findings align with the aforementioned reports. To obtain a gross estimate of the effect of ignoring the scHR on the overall training load of a constant HR-target session, we proceeded as follows: we first calculated the POs that correspond to a given target HR based on the HR/PO relationship either of the 5th (PO@5) or the 30th minute of exercise (PO@30). Then we calculated the V˙O2 at the 5th (CL5) and 30th minute (CL30) of a constant load trial performed at PO@5, by using the V˙O2/PO relationship of the 5th and 30th minutes; we also calculated the V˙O2 at the 30th minute (CT30) for PO@30 by using the V˙O2/PO relationship of the 30th minute. Finally, we calculated the total session V˙O2 as the area of the triangles defined by the aforementioned points on a V˙O2/time relationship for either a constant load or a constant HR target training session and translated these values into kilocalories using the energy equivalent of O2. Based on the aforementioned procedure and on the embedded assumptions, our data, the practical impact of ignoring the scHR while prescribing exercise anchored to HR targets is a reduction in PO from ≃6% to ≃15% and in V˙O2 from ≃1% to ≃12% over a 30-min session. Importantly, the metabolic load reduction would have a marginal impact on the energy expenditure (≃5% reduction in kilocalories per 30-min session); however, an undesired switch in the domain from heavy to moderate and severe to heavy could occur at the intensities closer to the respective boundaries. In this context, to be able to predict the scHR would be very useful to avoid an undesired reduction in both absolute and relative training load. The prediction of scHR derived from exercise intensity relative to RCP (equation 2) can be used to dynamically correct HR targets based on the anticipated HR drift. This strategy allows maintaining the target metabolic intensity/training load throughout prolonged exercise in postmenopausal women.
Interestingly, relative exercise intensity, as indicated by %RCP, was the single most relevant predictor of scHR. Recent data have raised the attention on the limitations of using %V˙O2max for the definition and the implementation of exercise intensity domains (27,54). In fact, the %V˙O2max that corresponds to the metabolic intensities that separate moderate from heavy and heavy from severe exercise has been found to be more variable than expected between subjects. Our finding that percentage of the intensity at the heavy to severe boundary (as measured by RCP) is the single most relevant predictor of the scHR, stronger than %V˙O2max, corroborates the importance of the direct measurement of these boundaries at the individual level rather than the use of an average value to grant that a desired and homogeneous stimulus is administered through exercise. In our study, we used RCP as a marker of the heavy to severe boundary (42), yet the correspondence/equivalence among different indexes of this critical intensity has been the object of an unsettled discussion (55,56). Although entering in this debate is beyond this article, it is our contempt that different indexes of the heavy to severe boundary (e.g., RCP, critical power, maximum lactate steady state, deoxyhemoglobin deflection point) all occur at an identical metabolic intensity (42,55), and provided that they are determined with the appropriate protocols and that they are correctly “translated” in homogeneous units (10,57), they should be considered equivalent. Importantly, the submaximal nature of the heavy to severe boundary and the possibility to measure or estimate it with different methodological approaches (42,55,58–60) make the implementation of this measure feasible on a large scale.
Limitations
The developed predictive equation does not take into account factors that may potentially affect HR kinetics such as age, sex, altitude, fatigue, overtraining, nutrition, and hydration. Further studies are needed to verify the accuracy of the proposed predictive equation outside of the specific population and to take into account sex and age and other factors that could play a role as predictors of scHR and better understand the mechanistic bases of this phenomenon.
CONCLUSIONS
This investigation demonstrated that an scHR is present in all domains of exercise, with its amplitude being larger with increasing intensity and about twice as large as the scV˙O2. Whenever the implementation of the workload is impossible or impractical and exercise is prescribed on HR targets, we need to be mindful of the mismatch between the slow components of HR and metabolic load/V˙O2. An adjusted HR target over time would grant that the desired stimulus is maintained throughout the exercise session in a given individual.
Acknowledgments
Funding from the Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Italy, supported this study. We would like to thank the participants in this study.
Results of the present study do not constitute an endorsement by the American College of Sport Medicine and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. No conflicts of interest, financial or otherwise, are declared by the authors.
Contributor Information
MASSIMO TESO, Email: massimo.teso@univr.it.
ALESSANDRO L. COLOSIO, Email: alessandro.colosio@ugent.be.
REFERENCES
- 1.McLaughlin M, Jacobs I. Exercise is medicine, but does it interfere with medicine? Exerc Sport Sci Rev. 2017;45(3):127–35. [DOI] [PubMed] [Google Scholar]
- 2.Borde R, Hortobágyi T, Granacher U. Dose–response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45(12):1693–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garber CE Blissmer B Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. [DOI] [PubMed] [Google Scholar]
- 4.Herold F, Müller P, Gronwald T, Müller NG. Dose–response matters!—a perspective on the exercise prescription in exercise-cognition research. Front Psychol. 2019;10:2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamnick NA, Pettitt RW, Granata C, Pyne DB, Bishop DJ. An examination and critique of current methods to determine exercise intensity. Sports Med. 2020;50(10):1729–56. [DOI] [PubMed] [Google Scholar]
- 6.Vanhatalo A Black MI DiMenna FJ, et al. The mechanistic bases of the power–time relationship: muscle metabolic responses and relationships to muscle fibre type. J Physiol. 2016;594(15):4407–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flück M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol. 2006;209(Pt 12):2239–48. [DOI] [PubMed] [Google Scholar]
- 8.Jones AM, Wilkerson DP, Fulford J. Muscle [phosphocreatine] dynamics following the onset of exercise in humans: the influence of baseline work-rate. J Physiol. 2008;586(3):889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(23):4795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caen K, Boone J, Bourgois JG, Colosio AL, Pogliaghi S. Translating ramp V˙O2 into constant power output: a novel strategy that minds the gap. Med Sci Sports Exerc. 2020;52(9):2020–8. [DOI] [PubMed] [Google Scholar]
- 11.Colosio AL, Lievens M, Pogliaghi S, Bourgois JG, Boone J. Heart rate–index estimates aerobic metabolism in professional soccer players. J Sci Med Sport. 2020;23(12):1208–14. [DOI] [PubMed] [Google Scholar]
- 12.Colosio AL, Pedrinolla A, Da Lozzo G, Pogliaghi S. Heart rate–index estimates oxygen uptake, energy expenditure and aerobic fitness in rugby players. J Sports Sci Med. 2018;17(4):633–9. [PMC free article] [PubMed] [Google Scholar]
- 13.McArdle W, Katch F, Katch V. Exercise Physiology: Nutrition, Energy, and Human Performance. 7th ed. Baltimore (MD): Lippincott Williams & Wilkins; 2010. 1104 p. [Google Scholar]
- 14.Zuccarelli L, Porcelli S, Rasica L, Marzorati M, Grassi B. Comparison between slow components of HR and V˙O2 kinetics: functional significance. Med Sci Sports Exerc. 2018;50(8):1649–57. [DOI] [PubMed] [Google Scholar]
- 15.Zuccarelli L, Sartorio A, De Micheli R, Tringali G, Grassi B. Obese patients decrease work rate in order to keep a constant target heart rate. Med Sci Sports Exerc. 2021;53(5):986–93. [DOI] [PubMed] [Google Scholar]
- 16.Mostardi R, Kubica R, Veicsteinas A, Margaria R. The effect of increased body temperature due to exercise on the heart rate and on the maximal aerobic power. Eur J Appl Physiol Occup Physiol. 1974;33(3):237–45. [DOI] [PubMed] [Google Scholar]
- 17.Watso JC, Farquhar WB. Hydration status and cardiovascular function. Nutrients. 2019;11(8):1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyle EF, González-Alonso J. Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev. 2001;29(2):88–92. [DOI] [PubMed] [Google Scholar]
- 19.Trinity JD, Pahnke MD, Lee JF, Coyle EF. Interaction of hyperthermia and heart rate on stroke volume during prolonged exercise. J Appl Physiol. 2010;109(3):745–51. [DOI] [PubMed] [Google Scholar]
- 20.Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW. Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther. 2019;23(2):170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dąbrowska-Galas M, Dąbrowska J, Ptaszkowski K, Plinta R. High physical activity level may reduce menopausal symptoms. Medicina (Kaunas). 2019;55(8):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dugan SA, Gabriel KP, Lange-Maia BS, Karvonen-Gutierrez C. Physical activity and physical function: moving and aging. Obstet Gynecol Clin North Am. 2018;45(4):723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nio AQX, Stöhr EJ, Shave R. The female human heart at rest and during exercise: a review. Eur J Sport Sci. 2015;15(4):286–95. [DOI] [PubMed] [Google Scholar]
- 24.Rael B Barba-Moreno L Romero-Parra N, et al. Cardiorespiratory response to exercise in endurance-trained premenopausal and postmenopausal females. Eur J Appl Physiol. 2021;121(3):903–13. [DOI] [PubMed] [Google Scholar]
- 25.Riebe D, Ehrman J, Liguori G, Magal M. ACSM’s Guidelines for Exercise Testing and Prescription—Tenth Edition. Philadelphia (PA): Wolters Kluwer; 2018. 651 p. [Google Scholar]
- 26.Bull FC Al-Ansari SS Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iannetta D Inglis EC Mattu AT, et al. A critical evaluation of current methods for exercise prescription in women and men. Med Sci Sports Exerc. 2020;52(2):466–73. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari L, Teso M, Colosio AL, Pogliaghi S. Performance and anthropometrics of classic powerlifters: which characteristics matter? J Strength Cond Res. 2020; Mar 12. [DOI] [PubMed] [Google Scholar]
- 29.de Roia G, Pogliaghi S, Adami A, Papadopoulou C, Capelli C. Effects of priming exercise on the speed of adjustment of muscle oxidative metabolism at the onset of moderate-intensity step transitions in older adults. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1158–66. [DOI] [PubMed] [Google Scholar]
- 30.Charloux A Lonsdorfer-Wolf E Richard R, et al. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol. 2000;82(4):313–20. [DOI] [PubMed] [Google Scholar]
- 31.Morán-Navarro R Courel-Ibáñez J Martínez-Cava A, et al. Validity of skin, oral and tympanic temperatures during exercise in the heat: effects of wind and sweat. Ann Biomed Eng. 2019;47(1):317–31. [DOI] [PubMed] [Google Scholar]
- 32.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). 1986;121(6):2020–7. [DOI] [PubMed] [Google Scholar]
- 33.Colosio AL, Baldessari E, Basso E, Pogliaghi S. Respiratory and muscular response to acute non-metabolic fatigue during ramp incremental cycling. Respir Physiol Neurobiol. 2019;270:103281. [DOI] [PubMed] [Google Scholar]
- 34.Field A. Discovering Statistics Using SPSS. 2nd ed. Thousand Oaks (CA): Sage Publications, Inc; 2005. xxxiv, 779. [Google Scholar]
- 35.Moreira H, Passos B, Rocha J, Reis V, Carneiro A, Gabriel R. Cardiorespiratory fitness and body composition in postmenopausal women. J Hum Kinet. 2014;43(1):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adami A, Pogliaghi S, De Roia G, Capelli C. Oxygen uptake, cardiac output and muscle deoxygenation at the onset of moderate and supramaximal exercise in humans. Eur J Appl Physiol. 2011;111(7):1517–27. [DOI] [PubMed] [Google Scholar]
- 37.González-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107(6):824–30. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012;2(2):933–96. [DOI] [PubMed] [Google Scholar]
- 39.Rossiter HB. Exercise: kinetic considerations for gas exchange. Compr Physiol. 2011;1(1):203–44. [DOI] [PubMed] [Google Scholar]
- 40.Grassi B, Rossiter HB, Zoladz JA. Skeletal muscle fatigue and decreased efficiency: two sides of the same coin? Exerc Sport Sci Rev. 2015;43(2):75–83. [DOI] [PubMed] [Google Scholar]
- 41.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc. 2016;48(11):2320–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keir DA Fontana FY Robertson TC, et al. Exercise intensity thresholds: identifying the boundaries of sustainable performance. Med Sci Sports Exerc. 2015;47(9):1932–40. [DOI] [PubMed] [Google Scholar]
- 43.Colosio AL, Caen K, Bourgois JG, Boone J, Pogliaghi S. Bioenergetics of the VO2 slow component between exercise intensity domains. Pflugers Arch Eur J Physiol. 2020;472(10):1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iannetta D, Inglis EC, Fullerton C, Passfield L, Murias JM. Metabolic and performance-related consequences of exercising at and slightly above MLSS. Scand J Med Sci Sports. 2018;28(12):2481–93. [DOI] [PubMed] [Google Scholar]
- 45.Hill DW, McFarlin BK, Vingren JL. Exercise above the maximal lactate steady state does not elicit a VO2 slow component that leads to attainment of VO2max. Appl Physiol Nutr Metab. 2021;46(2):133–40. [DOI] [PubMed] [Google Scholar]
- 46.Lucia A, Hoyos J, Chicharro JL. The slow component of V˙O2 in professional cyclists. Br J Sports Med. 2000;34(5):367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murias JM, Pogliaghi S, Paterson DH. Measurement of a true V˙O2max during a ramp incremental test is not confirmed by a verification phase. Front Physiol. 2018;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escourrou P, Freund PR, Rowell LB, Johnson DG. Splanchnic vasoconstriction in heat-stressed men: role of renin–angiotensin system. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(6):1438–43. [DOI] [PubMed] [Google Scholar]
- 49.Jose AD, Stitt F, Collison D. The effects of exercise and changes in body temperature on the intrinsic heart rate in man. Am Heart J. 1970;79(4):488–98. [DOI] [PubMed] [Google Scholar]
- 50.Fritzsche RG, Switzer TW, Hodgkinson BJ, Coyle EF. Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J Appl Physiol. 1999;86(3):799–805. [DOI] [PubMed] [Google Scholar]
- 51.Orizio C, Perini R, Comandè A, Castellano M, Beschi M, Veicsteinas A. Plasma catecholamines and heart rate at the beginning of muscular exercise in man. Eur J Appl Physiol Occup Physiol. 1988;57(5):644–51. [DOI] [PubMed] [Google Scholar]
- 52.Davies CT, Brotherhood JR, Zeidifard E. Temperature regulation during severe exercise with some observations on effects of skin wetting. J Appl Physiol. 1976;41(5 Pt 1):772–6. [DOI] [PubMed] [Google Scholar]
- 53.Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966;21(6):1757–62. [DOI] [PubMed] [Google Scholar]
- 54.Iannetta D Keir DA Fontana FY, et al. Evaluating the accuracy of using fixed ranges of METs to categorize exertional intensity in a heterogeneous group of healthy individuals: implications for cardiorespiratory fitness and health outcomes. Sports Med. 2021;51:2411–21. [DOI] [PubMed] [Google Scholar]
- 55.Keir DA, Pogliaghi S, Murias JM. The respiratory compensation point and the deoxygenation break point are valid surrogates for critical power and maximum lactate steady state. Med Sci Sports Exerc. 2018;50(11):2375–8. [DOI] [PubMed] [Google Scholar]
- 56.Broxterman RM, Craig JC, Richardson RS. The respiratory compensation point and the deoxygenation break point are not valid surrogates for critical power and maximum lactate steady state. Med Sci Sports Exerc. 2018;50(11):2379–82. [DOI] [PubMed] [Google Scholar]
- 57.Iannetta D, Inglis EC, Pogliaghi S, Murias JM, Keir DA. A “step-ramp-step” protocol to identify the maximal metabolic steady state. Med Sci Sports Exerc. 2020;52(9):2011–9. [DOI] [PubMed] [Google Scholar]
- 58.Iannetta D Fontana FY Maturana FM, et al. An equation to predict the maximal lactate steady state from ramp-incremental exercise test data in cycling. J Sci Med Sport. 2018;21(12):1274–80. [DOI] [PubMed] [Google Scholar]
- 59.Fontana FY, Colosio AL, Keir DA, Murias JM, Pogliaghi S. Identification of critical intensity from a single lactate measure during a 3-min, submaximal cycle-ergometer test. J Sports Sci. 2017;35(22):2191–7. [DOI] [PubMed] [Google Scholar]
- 60.Fontana FY, Keir DA, Bellotti C, De Roia GF, Murias JM, Pogliaghi S. Determination of respiratory point compensation in healthy adults: can non-invasive near-infrared spectroscopy help? J Sci Med Sport. 2015;18(5):590–5. [DOI] [PubMed] [Google Scholar]


