Abstract
Fetal pain perception has important implications for fetal surgery, as well as for abortion. Current neuroscientific evidence indicates the possibility of fetal pain perception during the first trimester (<14 weeks gestation). Evidence for this conclusion is based on the following findings: (1) the neural pathways for pain perception via the cortical subplate are present as early as 12 weeks gestation, and via the thalamus as early as 7–8 weeks gestation; (2) the cortex is not necessary for pain to be experienced; (3) consciousness is mediated by subcortical structures, such as the thalamus and brainstem, which begin to develop during the first trimester; (4) the neurochemicals in utero do not cause fetal unconsciousness; and (5) the use of fetal analgesia suppresses the hormonal, physiologic, and behavioral responses to pain, avoiding the potential for both short- and long-term sequelae. As the medical evidence has shifted in acknowledging fetal pain perception prior to viability, there has been a gradual change in the fetal pain debate, from disputing the existence of fetal pain to debating the significance of fetal pain. The presence of fetal pain creates tension in the practice of medicine with respect to beneficence and nonmaleficence.
Keywords: fetal pain, fetal analgesia, fetal anesthesia, fetal nociception, fetal awareness
Introduction
The controversy regarding fetal pain is complicated. Relevant factors include neuroanatomical development, consciousness, and physiologic, behavioral, and hormonal responses, as well as medical, ethical, and legal considerations. The onset of fetal pain capacity plays a vital role in identifying ethical issues surrounding painful procedures involving the fetus, such as fetal surgery, abortion, and feticide. Defining the time frame during which fetal pain capacity develops must first be addressed. Current neuroscientific evidence indicates that the onset of fetal pain perception is possible during the first trimester.
At the center of the controversy regarding fetal pain is a decades-long debate concerning when the human fetus begins to perceive pain and what areas of the brain are necessary for pain perception. Estimates for the onset of fetal pain perception over the past 20 years range from the first trimester (AAPLOG 2018; Derbyshire and Bockmann 2020; Pierucci 2020) until some point after birth (RCOG 2010).
The brainstem, thalamus, cortical subplate, and cortex have been implicated in fetal pain capacity. The predominant position has been that the potential for fetal pain perception emerges mid-gestation. This position is mirrored at the legislative level, by laws in 13 states which recognize fetal pain capacity at 20–22 weeks gestation (Guttmacher 2021) and by the American Academy of Pediatrics (AAP 2016), which supports proactive pain management, particularly in extremely preterm infants, born as early as 21–22 weeks (Ahmad et al. 2017). In the field of fetal medicine, however, fetal surgeons and anesthesiologists, routinely administer fetal analgesia at increasingly earlier gestations in the second trimester (>14 weeks gestation) to ameliorate pain and improve outcome. Consideration of fetal pain capacity and negative long-term neuroadapative phenomena have prompted anesthesiologists to recommend fetal analgesia from the second trimester onwards (Gupta, Wimalasundera, and Moore 2021). In 2021, the American Society of Anesthesiologists’ and the North American Fetal Therapy Network’s (NAFTNet) Consensus Statement provided evidence-based protocols for the administration of analgesia and anesthesia to the fetus and recommended the administration of fetal analgesia for all invasive maternal-fetal procedures (Chatterjee et al.). Some prominent researchers, likewise, propose fetal pain capacity beginning as early as 12 weeks gestation via the cortical subplate (Derbyshire and Bockmann 2020; Pierucci 2020), while other medical professionals raise the possibility of pain perception earlier in the first trimester (AAPLOG 2018; ACP 2021), based on neuroanatomical development of the thalamus and brainstem once the minimal necessary anatomy for pain processing is present at 7–8 weeks gestation (Derbyshire 2006, 2008).
Other organizations, however, dispute fetal pain capability prior to the presence of a developed cortex, based on the hypothesis of cortical necessity. In the U.K., the Royal College of Obstetricians and Gynaecologists’ (RCOG) most recent 2010 report on fetal awareness states that fetal pain is not structurally possible until 24 weeks gestation, and is unlikely to be functionally possible until after birth. In the U.S., the American College of Obstetricians and Gynecologists (ACOG 2020) and the Society for Maternal Fetal Medicine (SMFM 2021) state that fetal pain is not structurally possible until at least 24–25 weeks gestation, that the fetus cannot be conscious of pain “until the third trimester at the earliest,” (>28 weeks gestation), and cannot perceive pain as such until “late in the third trimester” (ACOG 2020). These organizations cite evidence of cortical necessity for pain perception based on a 2005 systematic review study (Lee et al. 2005) and the 2010 RCOG report. The SMFM additionally relies on correlation with case studies of adult post-lobotomy patients dating from the 1950s, some of whom experienced indifference to pain (Terrier, Lévêque, and Amelot 2019). The SMFM notes, however, that correlation with neonatal pain research “cannot be extrapolated to the fetus” because “these reported findings are specific to the neonate” (2021).
Conversely, the American Academy of Pediatrics uses validated neonatal pain assessment tools for extremely preterm infants born less than 28 weeks gestation (and as early as 23 weeks gestation), stating that pain scales “are essential for the rating and management of neonatal pain, and their use has been strongly recommended by the AAP and by international researchers” (AAP 2016, 2). These pain scales, which utilize behavioral and physiological indicators of pain, including facial expressions and limb movements, are utilized for assessing and treating acute pain in low gestational age infants prior to the cortical developmental milestones indicated by RCOG, ACOG, and SMFM, calling into question the need for cortical maturity for pain perception. Numerous researchers have also challenged the hypothesis of cortical necessity, noting that pain perception occurs prior to 24 weeks gestation without reaching the cortex (Derbyshire and Bockmann 2020; Pierucci 2020; Sekulic et al. 2016; Van de Velde and De Buck 2012). This growing debate highlights the lack of medical, scientific, and international consensus regarding the recognition and treatment of pain at earlier gestational ages, prior to cortical development.
The fetal pain debate has medical and ethical implications regarding painful procedures involving the fetus. Since 1981, the field of fetal surgery has grown exponentially, with successful treatment of life-threatening and devastating conditions of the fetus, such as myelomeningocele, congenital diaphragmatic hernia, hypoplastic left heart syndrome, and twin-twin transfusion syndrome (Adzick 2020). As the field of fetal medicine continues to expand, the rate at which fetuses are exposed to noxious stimuli continues to rise. Fetal interventions increasingly include fetal analgesia (pain relief) and anesthesia (loss of physical sensation with or without loss of consciousness) at earlier gestational ages in an effort to alleviate acute pain and to prevent the long-term neuroadaptive consequences of fetal exposure to early or repetitive noxious stimuli (Chatterjee et al. 2021; Gupta, Wimalasundera, and Moore 2021). Additionally, research since 2018 indicates that transplacental transfer of maternal anesthesia is likely insufficient for fetal pain management; therefore, direct fetal analgesia is recommended, administered intramuscularly or via the umbilical vein (Bellieni 2020; Chatterjee et al. 2021).
The fetal pain debate is also complicated by political and legal issues regarding abortion and feticide. Recognition of fetal pain capability at 12 weeks gestation, for example, has the potential to impact second- and third-trimester abortions (Guttmacher 2019), as well as to affect feticide procedures, which may not be reflected in abortion statistics. Feticide procedures are typically used in selective reductions or as adjuncts in second- and third-trimester abortions. Consideration of fetal pain also raises ethical and legal concerns regarding fetal suffering, informed consent, patient autonomy, the patient–physician relationship, and the role of the government.
Pain is distinct from nociception. Nociception is defined as the “neural process of encoding noxious stimuli” (IASP 2017), while pain is defined as an unpleasant experience “associated with, or resembling that associated with, actual or potential tissue damage” (Raja et al. 2020, 1977), and suffering as the state of being in pain. The controversy over the definition of pain and distinctions relevant to the ethical impact of these definitions will be discussed further below. The consequences of nociception may include pain perception as well as autonomic and behavioral responses (IASP 2017). As noted by prominent anesthesiologists, pain perception typically occurs concomitantly with autonomic and behavioral responses (Van de Velde and De Buck 2012); therefore, these responses in the context of a noxious stimulus provide surrogate markers for pain perception as is the accepted practice in neonatal medicine.
Nociception may be likened to the transmission of electrical impulses through a power cord to an appliance. When the electrical impulse reaches the appliance, the appliance can function. Similarly, neural transmission of noxious stimuli must travel to the brainstem, the subplate, or the cortex, before pain perception can occur. Debate exists as to which level these impulses must reach in order to trigger perception of pain. While nociception and pain perception typically occur concurrently, dissociation is possible. For example, when general anesthesia is used during surgery, neural transmission of noxious stimuli may occur, but perception of pain may be prevented by the effects of anesthesia.
Awareness or consciousness of pain has been categorized as internal and external. External awareness (awareness of one’s environment) involves awareness of external stimuli and has been linked to brainstem function, while internal awareness (awareness of one’s thoughts and memories) has been associated with cortical function (Stanojevic et al. 2021). External awareness of pain represents an unreflected experience of “being in pain” (Derbyshire and Bockmann 2020, 5), such as a neonate who experiences, but will not remember, pain from a heel lance. In contrast, internal awareness, represents a self-reflective experience of knowing that one is in pain, such as a 4-year-old who remembers and fears the pain of an immunization. Neurodevelopmentally, the self-reflective experience of pain does not emerge until cortical synapses develop during the first 2 years of postnatal life (Kadić and Kurjak 2018). It is the standard of care, per the American Academy of Pediatrics, however, to address unreflected pain in infants and toddlers (2016). This raises the important issue of addressing unreflected pain in the developing fetus.
The ability to perceive pain requires intact and functioning neural pathways for the transmission of painful stimuli from the periphery to the brain. The development of these pathways begins during the first trimester and continues into the postnatal period and beyond. There has been disagreement as to which structures in the brain are necessary for pain perception in the fetus and at what stage these pathways become functional. In the past 15 years, new research and clinical practice, particularly in the field of fetal medicine and anesthesiology, have demonstrated an evolving understanding of fetal pain perception, which challenge existing policies and raise ethical issues regarding proper pain management during fetal procedures.
Background
This paper explores the topic of fetal pain and reviews literature on ‘fetal pain,’ ‘fetal analgesia,’ and ‘fetal anesthesia.’ Sources for this literature review were obtained via PubMed searches from January 2005 through August 2021, English language only, where full papers were accessible, as well as from an internal examination and analysis of selected references cited by these sources. Dissertations, opinion editorials, and abstract-only pieces were excluded. The following topics are important background for the discussion of fetal pain: the history of neonatal pain management, procedural memory, and the definition of pain.
A Brief History of Neonatal Pain Management
The history of neonatal pain management is foundational to the topic of fetal pain. Many of the same issues in the neonatal pain debate have resurfaced in relation to fetal pain perception. Until the late 1980s, neonatal pain was largely unrecognized and untreated. Preterm and term infants were considered insensitive to pain due to limited cortical development. Surgeries and other invasive procedures were routinely performed on neonates without analgesia (Anand 2019). Medical skepticism of neonatal pain resulted from decades of research which showed variable observable responses to noxious stimuli in neonates compared to older infants and children (Rodkey and Riddell 2013). Neonatal responses to repeated pin pricks, electric shocks, and pinching, for example, showed subtle responses in preterm, critically ill, or neurologically compromised infants, which were often dismissed. In healthy, term infants, reactions to these noxious stimuli included withdrawal and crying. Due to the immaturity of the cerebral cortex, however, these responses in both term and preterm infants were regarded as inconsistent, nonspecific, and reflexive, occurring at subcortical levels, and therefore, not indicative of pain perception.
The assumption that newborns were insensitive to pain due to an undeveloped cortex was widely adopted by the scientific and medical communities until the late 1980s, despite dissenting views. Anesthesia, if administered to infants, was utilized for the benefit of the surgeon in order to suppress movement during surgical procedures, by means of paralytic medications. Analgesia was considered unnecessary during surgery due to lack of higher cortical function and the infant’s inability to remember the pain. Additionally, the risks and challenges of administering anesthesia to neonates, especially to critically ill infants, at a time when no standard medical practice had been established, contributed to hesitancy in anesthesia usage during invasive procedures. Alleviation of pain, if addressed, was limited to nonpharmacologic interventions such as swaddling or feeding. The scientific consensus was a presumption against neonatal pain.
In 1987, Anand and colleagues conducted a small randomized controlled trial of preterm infants undergoing cardiac surgery which revolutionized the understanding and management of neonatal pain (Anand, Sippell, and Green 1987). In this study, 16 preterm infants were intubated, paralyzed, and given nitrous oxide prior to cardiac surgery (patent ductus arteriosus ligation). Eight of the infants received intraoperative pain management with the opioid analgesic, fentanyl, while eight infants did not (non-fentanyl group). Compared to those who received fentanyl, the non-fentanyl group mounted immediate and substantial stress responses intra- and post-operatively, including elevated adrenaline and noradrenaline levels, and sustained significant intra- and post-operative morbidity, including intraventricular hemorrhage. This study, coupled with humanitarian concerns and parental activism, challenged the accepted scientific, medical, and international consensus which had held that it was impossible for the neonate to experience pain due to an undeveloped cortex. Slowly, the consensus changed, and a rapid growth in neonatal pain research and management resulted, which improved intraoperative and post-operative outcomes (Anand 2019).
Recognition of neonatal pain became a springboard for the use of physiological, hormonal, and behavioral indicators of pain in preterm, term, cognitively impaired, and sedated neonates. Further research demonstrated that preterm and critically ill infants were not insensitive to pain, as previously thought, but may have subtle or blunted behavioral reactions to painful stimuli due to limited energy reserves (Anand, Sippell, and Green 1987; Maxwell, Fraga, and Malavolta 2019). One prominent example of change in clinical practice was male infant circumcision. In the 1970s to 1990s, when infant circumcisions were performed without analgesia, several studies demonstrated indicators of pain via behavioral, physiologic, and hormonal responses (crying, changes in heart rate, blood pressure, oxygen saturation, and cortisol levels) (AAP 1999). Consequently, the standard of care changed to provide pain management for invasive and noxious procedures for neonates (AAP 2016, 2012, 1999), including extremely preterm infants born as early as 21–22 weeks gestation (Ahmad et al. 2017; Rysavy et al. 2015). In 2016, the American Academy of Pediatrics noted that the use of the Neonatal Pain and Sedation Scale (NPASS) is a validated pain assessment tool for preterm infants beginning at 23 weeks gestation (AAP 2016, 3). The NPASS utilizes facial expressions and physiologic and behavioral responses as accepted indicators of pain in extremely low gestational age infants, prior to cortical development. Neonatal pain research also resulted in the discovery of the long-term effects of pain experienced early in life.
Procedural Memory and Sensitization
Though infants lack the recall to remember painful procedures experienced early in life, research has demonstrated the existence of procedural memory of pain, which causes long-lasting negative neurophysiological effects that may endure for years following painful experiences (Van de Velde and De Buck 2012; Walker 2019). Exposure to early or repetitive noxious stimuli can lead to prolonged neuroadaptive effects on the developing nervous system due to greater neuronal and synaptic plasticity in early development. For example, preterm infants exposed to repeated, medically indicated heel lances for 4 weeks demonstrated significantly increased physiologic changes (heart rate and oxygen saturation), compared to age-matched preterm infants receiving a heel lance for the first time (Johnston and Stevens 1996). Likewise, male infants who were circumcised without analgesia demonstrated stronger reactions to immunizations months later, raising concerns that exposure to a single painful procedure early in development can lead to long-lasting effects (AAP 2012). Exposure to noxious stimuli not only adversely affects subsequent neurophysiological development, but can also result in sensitization. For example, preterm infants exposed to repeated painful procedures, such as heel lances, can become sensitized to respond to non-painful stimuli in the same manner (AAP 2016).
In 2016, the American Academy of Pediatrics (AAP) issued a policy statement regarding the prevention and management of neonatal pain, which addresses procedural memory as follows:
The prevention and alleviation of pain in neonates, particularly preterm infants, is important not only because it is ethical but also because exposure to repeated painful stimuli early in life is known to have short- and long-term adverse sequelae. These sequelae include physiologic instability, altered brain development, and abnormal neurodevelopment, somatosensory, and stress response systems which can persist into childhood. (2)
These findings are important considerations in the field of fetal medicine and fetal anesthesia, as exposure of the fetus to early or repetitive noxious interventions may also result in long-term neurodevelopmental consequences (Gupta, Wimalasundera, and Moore 2021; Van de Velde and De Buck 2012).
Defining Pain
In 1979, the International Association for the Study of Pain (IASP) defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” The IASP further noted that “Pain is always subjective” (IASP, 1979, 250). In 2020, an expanded definition added that the “inability to communicate does not negate the possibility that a human or nonhuman animal experiences pain” (Raja et al. 2020, 1977).
The IASP definition of pain holds significant authority in the international medical and scientific communities. It is frequently cited as evidence against the possibility of fetal pain when cortical development and self-reflection are viewed as necessary for pain perception. (Cohen and Sayeed 2011; Derbyshire 2008; Lee et al. 2005). The two-part IASP definition requires both a sensory and an emotional experience of pain. While the sensory component is not in question, the requirement for pain to have a self-reflective emotional component has been disputed, as it excludes the possibility of pain not only in the fetus, but also in the infant, the toddler, and the cognitively impaired (ACP 2021; Bellieni 2019; Raja et al. 2020). The IASP definition has been criticized particularly in its inapplicability to those who are unable to express emotional and self-reflective elements of pain, such as the fetus:
...such a demanding definition of pain restricts pain almost exclusively to fairly mature human beings. To ease that restriction it might be worthwhile to consider a less sophisticated definition, which focuses less on subjective reflection (knowing that I am in pain) and more on the immediate and unreflective feel of pain (being in pain) (Derbyshire and Bockmann 2020, 5).
In clinical practice, neonatal pain assessment tools address the immediate, unreflective nature of pain in preterm neonates born as early as 23 weeks gestation (AAP 2016). These assessments include the Neonatal Facial Coding System (NFCS), the Premature Infant Pain Profile-Revised (PIPP-R), the Neonatal Pain and Sedation Scale (NPASS), and the Behavioral Infant Pain Profile (Maxwell, Fraga, and Malavolta 2019). These protocols provide multidimensional pain assessment tools, relying on behavioral and physiologic indicators of pain. In summary, the IASP definition of pain is useful for healthy older children and adults; however, specialized pain assessment tools are needed in earlier stages of human development, when overt expression or articulation of the pain experience may not be possible. These concerns are likewise present in a parallel way when considering fetal pain.
The Evidence for Fetal Pain
The subjective experience of pain during early human development cannot be directly known by others, and signs of pain perception must be used to determine onset of fetal pain capacity. Fetal pain perception may be inferred from indirect, objective evidence, such as milestones of neuroanatomical development, from physiologic, hormonal, and behavioral responses, and from relevant clinical practice in fetal medicine and fetal anesthesiology. As in the realm of neonatal pain research, there are gaps in medical knowledge that currently hinder comprehensive understanding of fetal pain perception. Notwithstanding, what follows is objective evidence in support of the subjective experience of fetal pain.
Neuroanatomical Evidence
Fetal pain perception requires that the pathways for pain signal transmission are present and functioning, at least at an immature level. The sensory systems for both tactile and nociceptive stimuli develop early in embryologic development, preceding the development of the olfactory, vestibular, auditory, and visual systems (Borsani et al. 2019). These nociceptive pathways arise along a continuum of maturation rather than as a single discrete event or endpoint (Benatar and Benatar 2001; Sekulic et al. 2016) beginning in utero and continuing postnatally.
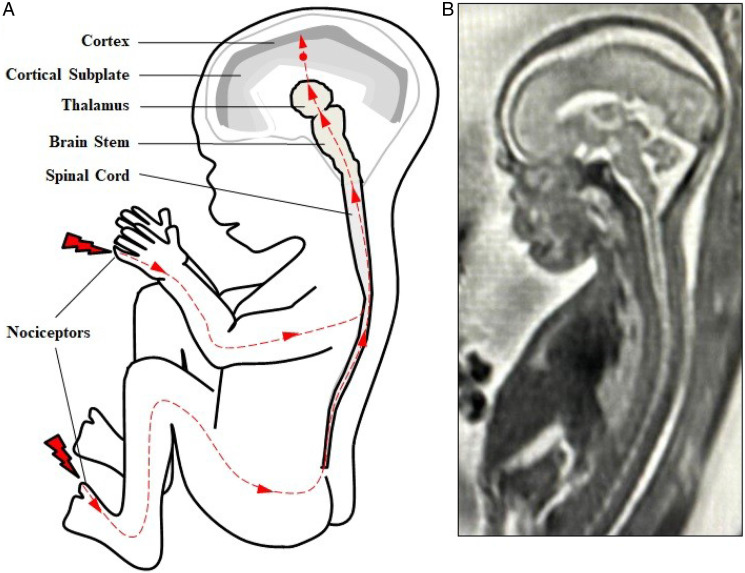
Nociception involves the transmission of impulses through the sensory nervous system from peripheral free nerve endings to the spinal cord to the brainstem and thalamus via the spinothalamic tract and then to cortical structures, including the cortical subplate and cortex (see Figure 1 and Table 1). Noxious stimuli are first sensed by peripheral nociceptors in the perioral area at 7.5 weeks gestation, the hands (10 weeks), and most areas of the body by 14 weeks gestation (Humphrey 1964). Nerve fibers from these peripheral receptors reach the spinal cord beginning at 7–8 weeks gestation (Okado and Kojima 1984). Projections from the spinal cord reach the brainstem and thalamus beginning at 7 weeks gestation (Derbyshire 2006, 2008). Nerve fibers from the thalamus then project to the cortical subplate, a structure in the fetus, discovered in 1974, which is a waiting compartment for neurons which later migrate to the fetal cortex (Judaš, Sedmak, and Pletikos 2010). The first thalamocortical nerve fibers from the thalamus project to the cortical subplate beginning at 12–15 weeks gestation (Bystron et al. 2008; Kostović and Judaš 2002; RCOG 2010), earlier than the 20–22 weeks that has been reported in previous studies (Hevner 2000; Kostović and Rakic 1990; Lee et al. 2005). Thalamocortical fibers are then noted to “massively invade the subplate zone” between 15–26 weeks gestation (Kostović and Judaš 2002, 146). Finally, thalamocortical fibers project from the thalamus to the cortex beginning at 23–24 weeks gestation (Kostović 2020; Lee et al. 2005).
Figure 1.
(Fetal nociceptive pathways. (A) Nociception involves neural pathways extending from peripheral nociceptors to the spinal cord to the brainsteam and thalamus then to cortical structures. Projections from the thalamus (thalamocortical fibers) first invade the cortical subplate beginning at 12 weeks gestation (red dot). Thalamocortical projections to the cortex begin at approximately 24 weeks gestation. (B) T2-weighted magnetic resonance imaging (MRI) of a fetus at 21 weeks gestation.
Table 1.
Development of Fetal Nociceptive Pathways
| Fetal Nociceptive Pathways | Description | Gestational Age, wk | Source |
| Peripheral nociceptors | Present in perioral area (7.5 weeks), hands (10 weeks), abdomen (15 weeks). Responsiveness present in most areas of body by 14–15 weeks. | 7.5–15 | Humphrey 1964 |
| Spinal cord | Peripheral afferents reach spinal cord | 7–8 | Okado and Kojima 1984 |
| Brainstem/thalamus | Spinothalamic tract fibers reach thalamus | 7–8 | Derbyshire 2006, 2008 |
| Cortical subplate | Thalamocortical fibers reach subplate | 12–15 | Bystron et al. 2008 Kostović and Judaš 2002 RCOG 2010 |
| Cortex | Thalamocortical fibers reach cortex | 23–24 | Kostović 2020 Lee et al. 2005 |
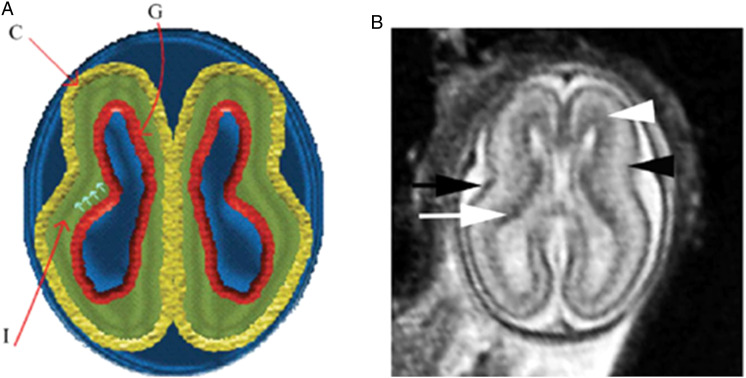
In the second trimester and third trimesters, the subplate neurons migrate to their final location in the cerebral cortex, and the subplate largely disintegrates by 32–34 weeks gestation. In animal studies, areas of the subplate have shown topographic similarity to innervation of the future cortex. That is to say, sensory nerves are arranged in predictable anatomic locations within the subplate that mirror their future locations in the cortex (Bellieni 2020; Wess et al. 2017). The somatosensory region of the subplate has been noted to develop earlier than other regions of the subplate and is four times thicker than the developing cortex at mid-gestation (Judaš, Sedmak, and Kostović 2013; Lowery et al. 2007). The subplate is noted to subserve “important sensory functions” (Lowery et al. 2007, 277). Researchers highlight that “the structures and mechanisms used for pain processing during fetal or neonatal life are unique and completely different from those used by adults,” which notably includes the subplate during early human development (Lowery et al. 2007, 276). MRI of the cortical subplate at 19 weeks gestation is shown in Figure 2, indicated by the black arrowhead (Saleem 2013, 895).
Figure 2.
Normal multi-layered magnetic resonance imaging (MRI) appearance of fetal brain early in gestation. (A) A diagram representing the fetal brain at 19 weeks of gestation shows surface and multi-layered appearance of the parenchyma with an inner germinal matrix (G), intermediate layer (l), and a developing cortex. (C). The small arrows point to the direction of the migrating neurons from germinal matrix to the developing cortex. (B) Axial balanced fast field echo MR image of a normal brain at 19 weeks of gestation shows a smooth surface and multi-layered parenchyma with an inner hypointense germinal matrix (white arrow), an intermediate layer, and an outer hypointense developing cortex (black arrow). Two additional sublayers can be identified: subventricular zone (white arrowhead) and subplate (black arrowhead). Subventricular zone is thick in the frontal region and shows slightly hypointense signal as it contains germinal matrix with increased cell pro-duction. The subplate zone appears slightly hyperintense as it has high water content, because of extracellular matrix. Reproduced by permission from SAGE Publications (Saleem 2013).
Although nociception, or the transmission of pain signals, can lead to the sensation of pain, this is not always the case. For example, if the spinal cord is severed, (Benatar and Benatar 2001; Lee et al. 2005) nociception still can occur below the level of the injury, but no sensation of pain results because signal transmission to the brain is prevented. Likewise, in unconscious, anesthetized patients, nociception may occur, but pain may not be perceived due to the effects of anesthesia. The question arises as to when consciousness develops in utero, resulting in external, and later, internal awareness. In the fetus, awareness and perception are thought to progress gradually along a continuum, rather than as a binary switch activated by cortical development at 24 weeks gestation, as will be discussed below (Benatar and Benatar 2001).
Four hypotheses have been proposed regarding structures or functions necessary for pain perception: 1) cortical necessity after 24 weeks gestation (RCOG 2010; SMFM et al. 2021; Stanojevic et al. 2021); 2) cortical subplate beginning at 12 weeks gestation; (Derbyshire and Bockmann 2020); 3) brainstem and thalamus (Brusseau 2008; Merker 2007; Sekulic et al. 2016), which are present after 7–8 weeks gestation (Derbyshire 2006, 2008); and 4) the onset of fetal consciousness, estimated at varying gestational ages (Lee et al. 2005).
Hypothesis 1
Cortical necessity (>24 weeks)
The first hypothesis, adopted by ACOG, RCOG, and SMFM, states that the cortex is the essential endpoint necessary for pain perception. Functional cortical connections, which are not present until after 24 weeks gestation, are viewed as the minimum necessary neuroanatomy for conscious pain perception. This hypothesis regards pain perception as akin to lighting a fuse on a firecracker, with the fuse representing the pathway from nociceptor to cortex, and the firecracker representing the cortex. The painful stimulus lights the fuse, and travels in a linear manner along the fuse until it reaches the firecracker resulting in a conscious pain experience. According to the ‘firecracker’ hypothesis, pain perception is not structurally possible until the stimulus reaches the cortex at 24 weeks gestation, and is likely not functionally possible until 29–30 weeks when EEG and somatosensory evoked potentials (SEP) demonstrate cortical response to noxious stimuli (Lee et al. 2005). Functional studies of fetal pain perception have relied largely on measurements of cortical activity in the second half of gestation. Sensory evoked potentials which measure electrical activity in the cortex in response to sensory stimulation, can be recorded in the fetus at 29 weeks gestation, while EEG measurements have recorded cortical activity in the fetus at 20 weeks (Lee et al. 2005; Lowery et al. 2007). However, SMFM notes that both EEG and MRI have failed to provide “valid, objective measures” of fetal pain perception (2021). Therefore, the interpretation and meaning of fetal EEGs, remains uncertain. Furthermore, the brain patterns of fetal (and neonatal) EEGs, during sleep and awake cycles, for example, are challenging to interpret in regard to fetal awareness. The gold standard for neonatal EEG interpretation of sleep-wake patterns, for example, relies on EEG in combination with observable behavioral characteristics, highlighting the need for correlation of imaging studies with clinical behavior (Dereymaeker et al. 2017).
This highlights the need to correlate functional imaging or neurophysiologic studies with fetal behavioral cues, pain responses, and context. Additionally, reliance on measurements of mature nociceptive or cortical function has been questioned. As noted by researchers in 2019, “It is now recognised that the developing motor and sensory systems are able to function long before they have completed their neural maturation” (Borsani et al. 2019, 225). This has raised growing concern that the necessity of the cortex for pain perception may have been overstated, and that fetal pain perception is possible even with an immature cortex (Bellieni 2020; Bernardes et al. 2021a; Derbyshire and Bockmann 2020; Merker 2007).
Proponents of the cortical necessity hypothesis view all fetal responses to noxious stimuli prior to 24 weeks gestation as unconscious, reflexive, subcortical reactions, not indicative of a pain experience (Lee et al. 2005; RCOG 2010; SMFM et al. 2021). This is in contrast to comparable responses to noxious stimuli in extremely preterm infants and neonates, in which these same physiologic and behavioral reactions are viewed as signs of pain (AAP 2016). Additionally, there is no identified difference in pain responses in the fetus or neonate after 24 weeks gestation that demonstrates impact from cortical connections (Gibbins et al. 2008). Instead, the same indicators of pain that are present after 24 weeks, are already present, developing, and maturing prior to 24 weeks gestation. Fetal pain responses prior to 24 weeks gestation, similar to those found in newborns, have been demonstrated in research as well as in clinical practice in neonatology and in fetal medicine. For example, in extremely preterm infants (>23 weeks gestation), “facial actions are the most prominent pain indicators” (Gibbins et al. 2008, 452). These accepted indicators of pain in the preterm neonate have also been demonstrated in the fetus at 23 weeks gestation. In 2021, research by Bernardes et al. demonstrated, for the first time, acute pain facial responses following anesthetic puncture of the fetal thigh during fetal surgery at 23 weeks gestation, noting the following:
It has been suggested that the experience of pain is not possible until the cortex becomes functional and the connection among periphery, spinal cord and thalamus are completely developed, which does not occur before 24 weeks pregnancy. However, we demonstrated here, for the first time, the existence of fetal pain response before this time-point…supporting fetal pain as a neuroadaptive phenomenon that emerges even with an immature cortex. (2021a)
Pain perception can occur without reaching the cortical level in extremely preterm infants at similar gestational ages. As noted by renowned neuroscientists Kostović and Jovanov-Milošević in 2006, “The connection between the thalamus and the cortex was considered as an anatomical substrate for the prospective pain sensation in the preterm infant, although there is recent evidence that pain sensation in preterms does not reach the cortical level” (419).
The necessity of an intact cortical system has also been questioned by studies which describe the occurrence of pain perception in children and adults with severe damage to or absence of the somatosensory cortex (Aleman and Merker 2014; Merker 2007). Research of infants and children born without a cortex (anencephaly and hydranencephaly), in which the highest functioning level of brain tissue is the brainstem (anencephaly) or diencephalon (hydranencephaly), have demonstrated intact awareness of pain, accompanied by crying, avoidance, and withdrawal, as well as the ability to be soothed (Aleman and Merker 2014; Sekulic et al. 2016). Other evidence includes research in which the somatosensory cortex has been stimulated or ablated (destroyed). It would be expected that electrical stimulation of the somatosensory cortex would cause pain and that ablation of this region of the cortex would eliminate pain perception; however, this did not occur (Lowery et al. 2007). Instead, ablation of the thalamus blocked the pain response (Brusseau 2008; Lowery et al. 2007; Marchant 2014). Similar findings are reported in studies of patients with Déjerine–Roussy syndrome. In this thalamic pain syndrome, isolated lesions in the thalamus due to stroke or metastases result in debilitating pain triggered by minor stimuli, such as light touch (Klit, Finnerup, and Jensen 2009). Ablation of these thalamic lesions alleviates pain (Patel et al. 2017).
In 2012, a study by Mazzola et al. analyzed pain response to electrical stimulation of the insula and operculum in adult patients with drug-resistant epilepsy. A minority of patients (10.4–12.8%) reported painful sensations in response to stimulation. A 2016 study by Feinstein et al., however, noted that extensive damage to the operculum and absence of the insular cortex did not prevent the experience of “excessive” pain, thus providing “direct evidence that the insula, anterior cingulate, and amygdala are not necessary for feeling the suffering inherent to pain (1499)." The authors conclude that cortical structures may be necessary for “regulation of pain rather than providing the decisive substrate for pain’s conscious experience” (1499). Due to significant gaps in medical knowledge, it is unclear how adult pain perception translates to the unique “structures and mechanisms used for pain processing during fetal or neonatal life” (Lowery et al. 2007, 276) These studies, however, challenge the hypothesis of cortical necessity and support the hypotheses of pain perception via the cortical subplate or subcortical structures prior to 24 weeks gestation.
Hypothesis 2
Cortical subplate (>12 weeks)
The second hypothesis holds that the cortical subplate is the minimum structure necessary for the fetus to feel pain as early as 12 weeks gestation. In 2020, 10 years after the RCOG report was released, a review on fetal pain by Derbyshire and Bockmann stated that, based on existing research, the potential for fetal pain exists once thalamic projections reach the subplate beginning at 12 weeks gestation. This study is of particular significance as Derbyshire, a principal author of the 2010 RCOG report, previously held that fetal pain was not structurally possible until 24 weeks gestation, and was unlikely to be functionally possible until birth. Subplate innervation beginning at 12 weeks raises the possibility of fetal pain perception in the first trimester resulting in what RCOG states could represent a “rawer, more primitive, form of pain or suffering” (2010, 6) before full maturation of the cortex which does not occur until postnatal life (Kadić and Kurjak 2018).
Technology is still evolving to ethically measure functional connectivity in the human subplate and thalamus in response to noxious stimuli (Lowery et al. 2007). In animal models, however, researchers have found that the subplate is functional and active in responding to sensory stimuli before cortical function and maturation are possible, and that sensory responses are not “dependent on the onset of thalamocortical transmission to layer 4” of the cortex, as previously believed (Wess et al. 2017, 12602). According to renowned neuroscientists, “Subplate neurons and afferent synaptic connections are the most significant functional network during the preterm age when cortical synaptic arrangements are very immature or absent…The functional activity of SP [subplate] may also be crucial for early response to painful stimulation” (Kostović and Jovanov-Milošević 2008, 6–7). This underscores the role of the subplate in fetal pain perception, not just as a waiting compartment, but as a functionally active and responsive precursor of the fetal cortex, beginning as early as 12 weeks gestation.
Hypothesis 3
Brainstem and thalamus (>7–8 weeks)
The third hypothesis postulates that subcortical structures including the brainstem and thalamus, located below the level of the cortex and subplate, are sufficient for pain perception (Merker 2007; Sekulic et al. 2016) via the spinothalamic tract which relays nociceptive information from the spinal cord to the thalamus beginning between 7–15 weeks gestation. Early thalamic development is evident by 5–6 weeks gestation (Mojsilović and Zečević 1991). The first nociceptor axons from the periphery connect with the spinal cord beginning at 7–8 weeks gestation (RCOG 2010), while the spinothalamic nerve fibers begin reaching the thalamus at 7 weeks gestation (Derbyshire 2006, 2008). This raises the possibility of fetal pain perception in the first trimester, after 7–8 weeks gestation, once the minimum neuroanatomy is present. This is uncertain, however, due to the structural and functional immaturity of the thalamus at this stage in development (RCOG 2010). Technology is still evolving to assess the function of the thalamus. As noted by prominent researchers, “Fetal development of the thalamus occurs much earlier than the sensory cortex but functional evidence for thalamic sensory processing will require novel neuroimaging techniques or the recording of thalamic field potentials from fetuses” (Lowery et al. 2007, 276). The minimum necessary neuroanatomy for fetal pain perception, however, has developed in the first trimester via the cortical subplate, via the thalamus, or both.
Evidence also suggests the fetus has a heightened sensitivity to pain due to the lack of descending inhibitory pathways which appear in the postnatal period (Andrews and Fitzgerald 1994; Fisk et al. 2001; Sekulic et al. 2016; Van de Velde and De Buck 2012). This hypersensitivity leads to what has been described as “hyperexcitability and a generalised movement of all limbs” in response to tactile stimulation (Derbyshire 2008, 119) and a fetus that is “extremely sensitive to painful stimuli” (Sekulic et al. 2016, 1031). More coordinated responses become possible during the third trimester, and hypersensitivity diminishes as the descending pathways mature in postnatal development (Hatfield 2014).
Hypothesis 4
Consciousness
The fourth hypothesis holds that the perception of pain depends not just on the structure and function of the nociceptive pathways, but also on the state of consciousness of the fetus. Few would dispute this assertion. Considerable uncertainty remains, however, regarding what level of consciousness may be required for pain perception and at what timepoint this consciousness begins to develop in the fetus. The level of consciousness is important to consider, given that pain typically cannot be sensed during states of unconsciousness, such as situations of general anesthesia. Two factors have been proposed to affect the onset of fetal consciousness: the intrauterine environment and the level of fetal development.
Neurochemicals in the intrauterine environment have been proposed to induce a constant unconscious state in the fetus throughout gestation (Mellor et al. 2005). This frequently cited hypothesis, however, (Lee et al. 2005; RCOG 2010) has been discredited by recent research. In the warm, buoyant intrauterine environment, the fetus is exposed to endocrine neuroinhibitors (ENIn) such as adenosine, allopregnanolone, pregnanolone, and prostaglandin D2 which provide a sedative effect and create the optimal environment for neurodevelopment. Research in 2018, however, concluded that the concentration of these neurochemicals in the fetus is not sufficient to block pain perception which would require concentrations 10–20 times greater than naturally found in utero (Bellieni, Vannuccini, and Petraglia). Additionally, maternal levels of these neurochemicals, which can be higher than fetal levels, likewise, do not confer anesthetic or analgesic effects. The sedative effects of naturally occurring levels of ENIn, therefore, do not functionally provide analgesia or anesthesia to the fetus or prevent arousability from noxious or other stimuli (Bellieni, Vannuccini, and Petraglia 2018; Platt 2011; Sekulic et al. 2016).
Controversy also surrounds the level of fetal development required for the fetus to be aware or conscious of pain. Cortical development in the third trimester has been proposed as a determinant of fetal consciousness (ACOG 2021; Lee et al. 2005; RCOG 2010); however, consciousness is also mediated by subcortical structures such as the thalamus and brainstem (Brusseau 2008; Merker 2007). Evidence for consciousness at the level of the brainstem and thalamus has been demonstrated in infants and children with hydranencephaly and anencephaly who lack a developed cortex, as will be discussed below. Evidence for consciousness also includes fetal action planning and learning.
Researchers note that fetal awareness may be deduced once the fetus is capable of action planning and learning (Stanojevic et al. 2021), noting that “pain perception/behavior requires the subjective ability to evaluate the environment and form coordinated responses” (Apkarian 2018, 1253). Such evidence of action planning begins by 13 weeks gestation with purposeful, targeted hand movements, and “a surprisingly advanced level of motor planning” is established by 22 weeks gestation (Kadić and Kurjak 2018, 185). Studies by Castiello et al. (2010) and Zoia et al. (2007) have likewise demonstrated purposeful movements requiring action planning and learning in the fetus prior to 24 weeks gestation, highlighting fetal awareness prior to cortical connectivity. Research in 2010 analyzed action planning versus reflexive behavior in the fetus (Castiello et al.). Fetal movements in twin gestations were studied, utilizing 4-D ultrasound from 14 weeks gestation. The study noted action planning via purposeful movements of the fetuses toward the co-twin, as well as self-directed movements. The study also noted differentiation in the velocity of targeted fetal movements. Slower velocity of fetal movements was noted toward the co-twin as well as with self-directed movements toward the sensitive eye regions, when compared to non-targeted movements toward the uterine wall, for example. The presence of such action planning, learning, and social behavior raises consideration of fetal consciousness and awareness by 14 weeks gestation.
Other research of human infants and children born without a cortex have demonstrated intact awareness of pain (Aleman and Merker 2014; Sekulic et al. 2016). Research with decerebrate and decorticate experimental animals (cortex surgically removed) has likewise demonstrated the ability to perceive and respond to pain, with crying, fear, and avoidance (Sekulic et al. 2016). These studies suggest an emerging level of consciousness once the brainstem, including the thalamus (diencephalon), is present during fetal development (Brusseau 2008), not as an all-or-none event occurring at a distinct gestational age (Derbyshire 1999). A useful analogy to consider is the post-operative patient emerging from general anesthesia who transitions through a spectrum of unconsciousness to increasing levels of consciousness until becoming fully awake, alert, aware, and responsive. Throughout the transition from the anesthetized state to full consciousness, increasing degrees of awareness to stimuli have been noted (Bonhomme et al. 2019; Sanders et al. 2017). This line of analysis raises the possibility that fetal pain perception and awareness, mediated at the level of the thalamus and brainstem, may be possible after 7–8 weeks gestation, or at the level of the thalamus and subplate, from 12 weeks gestation.
Pain perception is a complex, multi-layered experience, and gaps in medical knowledge remain significant. Overreliance on a single parameter, such as neuroanatomical development or functional imaging, is inadvisable in light of evolving research and technology, without correlation and context. Neurophysiologic data, from EEG and fMRI, for example, are also limited in utero due to technical and ethical considerations (Stanojevic et al. 2021). Instead, correlation with physiologic, hormonal, or behavioral evidence is necessary. In the 1980s, for example, based on then-current neuroscientific evidence, medical students were taught to inform patients that bone marrow biopsies performed with local anesthesia would result in a painless experience, due to the absence of nerve fibers deep within the bone (Platt 2011). In clinical practice, however, this hypothesis contrasted sharply with the clinical pain experienced by patients during these biopsies. Only later, with developing technology and further studies, were nerve fibers deep in the bone tissue identified (McCredie 2007). The history of medicine underscores the limitations of medical knowledge, the evolving expertise of the neurosciences, and the need to consider a multimodal, precautionary approach to pain assessment.
Physiologic and Hormonal Responses
The extremely preterm infant of 22–27 weeks gestation and the fetus of similar gestation share a predominantly fetal physiology (Pierucci 2020). Therefore, similar pain assessment tools have been used to study both groups. Responses to pain are generally grouped as physiologic, hormonal, and behavioral. As noted above, these markers are already recognized and accepted as signs of pain in extremely preterm infants and neonates (AAP 2016). As responses may be affected by a variety of factors, context and graduated pain scales are important considerations (Bellieni 2012).
Physiologic responses to pain in the fetus, measurable via ultrasound, include changes in heart rate and cerebral blood flow. Though pain typically results in increased heart rate, this may differ in the fetus, as bradycardia may result from fetal stress. Other factors may affect fetal heart rate including gestational age, cord compression, oxygen levels (hypoxemia), underlying disease processes, and fetal ability to sustain heart rate elevation. Decreased heart rate is a known complication of fetal surgery, for example, and medication (atropine) is routinely administered to support fetal heart rate (Brusseau and Mizrahi-Arnaud 2013). Due to the ambiguity associated with heart rate, cerebral blood flow measurements of the middle cerebral artery have also been used to measure fetal response to noxious stimuli. Increased blood flow to the brain, measured by middle cerebral artery pulsatility, occurs in response to painful stimuli based on fetal studies conducted as early as 16–18 weeks gestation (De Buck, Deprest, and Van de Velde 2008; Kadić and Predojević 2012). This pain response, also seen in children and adults, represents a brain-sparing reaction to protect essential organs during threatened or ongoing tissue injury (Brusseau and Mizrahi-Arnaud 2013; Teixeira et al. 1999).
Noxious stimuli also stimulate a stress hormonal response in the fetus via the hypothalamic-pituitary-adrenal (HPA) axis, which is functional early in the second trimester, based on studies of fetal response during needling through the abdomen during intrahepatic blood transfusions (Fisk et al. 2001; Smith et al. 2000; Van de Velde and De Buck 2012). Measurements of cortisol, adrenaline, and β-endorphins (endogenous opioids released by the pituitary gland) are elevated in response to noxious stimuli and have a deleterious effect on the hemodynamic stability of the fetus. Stress responses vary according to gestational age, with β-endorphins occurring earlier in gestation than cortisol (Anand, Sippell, and Green 1987; Gitau et al. 2001; Fisk et al. 2001). In studies by Giannakoulopoulos et al. (1994, 1999), needling through the fetal abdomen to access the intrahepatic vein resulted in rises in cortisol levels by 183%, noradrenaline levels by 196%, and β-endorphin by 590% compared to controls. As in neonatal pain research, the use of analgesics prior to painful procedures in the fetus has been shown to suppress these stress responses and the resultant disruption in fetal homeostasis (Fisk et al. 2001; Lowery et al. 2007; Rokyta 2008).
Behavioral Evidence
Fetal behavior is an indication of underlying neurologic function. Fetal response to noxious stimuli increases with gestational age and development. Such responses include withdrawal reflexes, whole body movements away from the noxious stimulus, and facial expressions. Ethically questionable research conducted in the 1930s to 1960s by University of Pittsburgh anatomists, Hooker and Humphrey, sought to determine fetal motor responses to tactile stimulation (Hooker 1936, 1942, 1952; Humphrey 1964). Their research utilized more than 150 intact, living fetuses obtained from surgical abortions via hysterotomy (or less commonly through spontaneous preterm delivery) which were immediately suspended in saline solution. Fetal responses to repeated stimulation of the skin and musculature with monofilaments or bead-tipped glass rods were recorded, until demise occurred 8–20 min after placental separation (Hooker 1942). Fetal withdrawal reflexes to tactile stimulation of the perioral area were noted, beginning at 7.5 weeks gestation, showing “generalized movement of the head and upper trunk away from the stimulus” (Wilson 2014, 144). Responsiveness to tactile stimulation rapidly extends to other areas of the face, hands, feet, trunk and limbs by 14 weeks, generating whole body movement away from potentially noxious stimuli (Hooker 1952; Myers et al. 2004). Limitations of these studies included the increasing effects of anoxia on the responsiveness of the fetuses following placental separation. At these early gestations, fetal anesthesiologists have noted that the fetus responds to sensory stimuli “in a comparable way to the neonate” (Myers et al. 2004, 241).
Withdrawal from noxious stimuli represents an appropriate protective response to prevent tissue injury (Van de Velde and De Buck 2012). As such, a potentially noxious stimulus may trigger a spinal cord-mediated reflex, while also activating the developing nociceptive pathways to the thalamus and subplate beginning in the first trimester (Derbyshire and Bockmann 2020; RCOG 2010). For example, touching a hot stove triggers both a reflexive response and nociceptive transmission to the thalamus (Benatar and Benatar 2001). Thus, withdrawal reactions to noxious stimuli cannot be disregarded prior to 24 weeks gestation (RCOG 2010, Lee et al. 2005), but indicate appropriate protective, nocifensive responses and raise the possibility of concomitant pain perception via the spinothalamic tract as early as 7–12 weeks gestation.
Other potential behavioral markers of fetal pain include the movement of facial musculature and facial expressions, which have been shown to occur in response to noxious stimuli by mid-gestation. Facial musculature develops by 16 weeks gestation, and facial movements are observable on 4-D ultrasound at 20 weeks (AboEllail and Hata 2017). Fetal facial expressions become more distinguishable and varied after 24 weeks, with increased neuromuscular development and the deposition of adipose tissue. While the Neonatal Facial Coding System (NFCS) assesses 10 different facial expressions in neonates, fetuses may only demonstrate a fraction of these due to developmental immaturity. For example, in extremely preterm infants of 24–28 weeks gestation, four facial pain responses were observed most frequently: brow lowering (or brow bulge), eyes squeezed shut (eye squeeze), deepening of the nasolabial furrow, and vertical mouth stretch (Gibbins et al. 2008). These indices reflect the earlier development of the upper facial muscles compared to the lower facial muscles. Fetal pain assessments have also been conducted using the NFCS during painful intramuscular injections prior to fetal surgery (Bernardes et al. 2021a, 2021b, 2018). Facial expressions (horizontal mouth stretch, open lips, vertical mouth stretch, and brow lowering) were elevated in those fetuses receiving injections compared to controls. As noted above, Bernardes et al. demonstrated pain-related facial expressions, comparable to those observed in neonates, during anesthetic puncture of the fetal thigh at 23 weeks gestation (2021a).
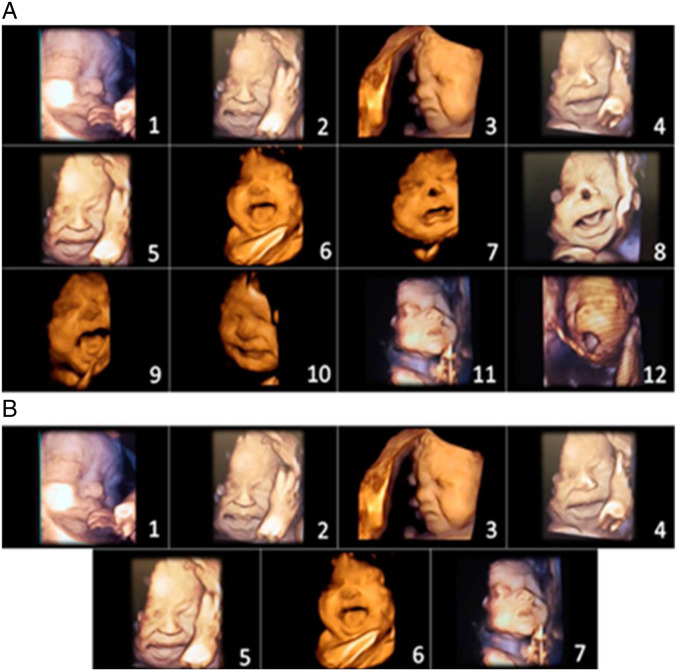
Figure 3 shows fetal facial expressions in response to anesthetic injection into the fetal thigh during third trimester fetal surgery (Bernardes et al. 2021b, 5). The 2021 Society of Maternal Fetal Medicine report, however, does not view such facial expressions in the late second and third trimesters as evidence of fetal pain, stating, “because the facial nucleus and circuitry required for facial expressions arise from the brainstem and not the cortex, these indirect measures do not reflect any experience of pain or suffering.” Such assertions, which conflict with accepted neonatal pain assessment tools at the same and earlier gestational ages, raise concerns about overreliance on neuroanatomical hypotheses, as was done in the era of untreated neonatal pain, rather than correlation with clinical behavior, context, and other markers of pain perception.
Figure 3.
(A) Initial items from neonatal facial coding system and 2 supplementary items. 1. Brow lowering. 2. Eyes squeezed shut. 3. Deepening of the nasolabial furrow. 4. Open lips. 5. Horizontal mouth stretch. 6. Vertical mouth stretch. 7. Lip purse. 8. Taut tongue. 9. Tongue protrusion. 10. Chin quiver. 11. Neck deflection. 12. Yawning. (B) Final items from the Fetal-5 Scale. 1. Brow lowering. 2. Eyes squeezed shut. 3. Deepening of the nasolabial furrow. 4. Open lips. 5. Horizontal mouth stretch. 6. Vertical mouth stretch. 7. Neck deflection. Reproduced by permission from IASP (Bernardes et al. 2021b).
Other behavioral responses include gross motor movements of the fetus in response to noxious stimuli. These responses are also apparent, based on studies done after 16–18 weeks gestation (RCOG 2010). In a 1994 study, needles passed through the fetal trunk (without analgesia) for medically indicated blood transfusions in second- and third-trimester fetuses (20–34 weeks gestation) resulted in “vigorous body and breathing movements” and significant stress hormone responses (Giannakoulopoulos et al. 1994, 77). The researchers concluded that, “the fetus mounts a similar hormonal response to that which would be mounted by older children and adults to stimuli which they would find painful” (80). Further studies have noted that administration of analgesia resulted in suppression of a fetal stress response (Fisk et al. 2001) as well as a fetus that is “still and appears quiescent and calm” (Derbyshire and Bockmann, 2020, 6) Based on such studies, fetal therapies such as percutaneous fetal cardiac interventions (FCI), which treat structural heart defects, now utilize fetal analgesia. In FCI, intramuscular opioids (for fetal analgesia) and paralytics (for fetal immobilization) are standardly administered to the fetus before needling of the fetal heart in order to prevent movement and pain, and to suppress hemodynamic instability and hormonal stress responses (Schidlow, Tworetzky, and Wilkins-Haug 2014).
Limited data exist regarding fetal responses to noxious stimuli prior to 15 weeks gestation as governmental regulations in the United States have precluded nontherapeutic research on living fetuses since the 1960s (Wilson 2014). Therapeutic fetal interventions, with potentially noxious stimuli, have historically taken place after 15–16 weeks gestation (Bellieni et al. 2013). Earlier diagnostic procedures, such as amniocentesis and chorionic villus sampling, may incidentally injure the fetus, but this complication is rare. In a small 1995 study, accidental fetal injury during amniocentesis demonstrated brisk fetal withdrawal responses at 15–18 weeks gestation (Petrikovsky and Kaplan 1995). Prior to 15 weeks, the fetus typically has limited exposure to noxious stimuli apart from abortion and feticide. Though feticide and dilation and evacuation (D&E) abortions may utilize ultrasound, there is a lack of published studies on observed fetal responses, likely due to ethical considerations. Physician testimony offered during state legislative hearings, however, has reported fetal withdrawal and flailing during feticide prior to 18 weeks gestation (Ohio Senate Bill 2019).
The Significance of Fetal Pain
In the past decade, there has been a gradual shift in the literature concerning fetal pain, from disputing the existence of fetal pain to debating the significance of fetal pain (Bellieni et al. 2018; Cohen and Sayeed 2011; Dadlez and Andrews 2018; Kluge 2015; Watson 2012). As the medical evidence has shifted in acknowledging fetal pain perception prior to viability (generally defined as 22–24 weeks gestation), this knowledge has important implications for therapeutic fetal procedures, abortion, and feticide.
Fetal Surgery
Fetal analgesia is routinely given before open or fetoscopic surgeries after mid-gestation to prevent pain and to avoid the deleterious short and long-term effects of exposure to painful stimuli. Ethical and medical concerns have resulted in a slow shift in the use of analgesia to earlier gestational ages. Anesthesia recommendations published in 2021 recommend the use of analgesia from the second trimester onwards (14+ weeks gestation) (Gupta, Wimalasundera, and Moore 2021) or whenever invasive procedures are performed on the fetus (Chatterjee et al. 2021), while researchers suggest consideration of pain management from 12 weeks gestation (Derbyshire and Bockmann, 2020).
Usage of fetal analgesia during all invasive procedures, however, is not universal. Some fetal needling procedures, for example, may not utilize fetal analgesia due to the brevity of the procedure and due to maternal and fetal risks of additional needling in administering direct fetal analgesia. Recent 2021 recommendations by the American Society for Anesthesiologists and the North American Fetal Therapy Network (NAFTNet), however, recommend adequate direct fetal anesthesia in all invasive procedures to “inhibit the humoral stress response, decrease fetal movement, and blunt any perception of pain” as well as to avoid “short- and long-term adverse effects on the developing central nervous system” (Chatterjee et al. 2021, 1167). A 10-year review (2001–2011) of studies involving fetal surgery evaluated the employment of fetal analgesia during invasive procedures ranging from 16–37 weeks gestation. The review found that a majority of these procedures did not administer direct fetal analgesia, but rather relied on maternal anesthesia. None of the studies described the fetal response to noxious stimulation (Bellieni et al. 2013). Recent research indicates that maternal anesthesia is insufficient for fetal pain management due to inadequate transplacental passage, and that administration of direct fetal analgesia is necessary (Bellieni 2020; Chatterjee et al. 2021). These studies, as well as consideration of acute fetal stress responses and long-term effects on neurodevelopment, continue to impact the standard of care for fetal analgesia. Fetal pain assessment, effective pain management, and balancing maternal and fetal safety pose unique challenges in fetal analgesia and anesthesia, as research and understanding of fetal pain continue to expand.
Abortion and Feticide
Fetal pain raises ethical issues not only for fetal surgery, but also for abortion and feticide. Feticide procedures, generally performed in the second and third trimesters, involve the injection of a lethal agent (such as potassium chloride, digoxin, lidocaine, etc.) into the fetal head, trunk, umbilical cord, or less commonly, the amniotic fluid, in order to induce cardiac asystole (Maurice et al. 2019). Alternatively, umbilical cord occlusion (UCO), radiofrequency ablation (RFA), and transection of the umbilical cord (procedures which may be used in fetal reduction in multifetal gestations or as an adjunct to second- or third-trimester surgical abortions) cause hypoxia-induced cardiac arrest (King et al. 2017). Concerns have been raised that such procedures may cause pain to the fetus from needling of the fetal head or trunk, from the painful effects of agents such as potassium chloride, and from cardiac ischemia (Blickstein and Oppenheimer 2016). Because the injection of potassium chloride is known to cause pain, its use in the death penalty (lethal injection) and in animal euthanasia in veterinary medicine must be preceded by administration of anesthesia and confirmed unresponsiveness (Dresser 2014; American Veterinary Medical Association 2020). Dilation and evacuation (D&E) abortions, generally performed in the second trimester (after 13 weeks gestation), have likewise raised concerns regarding fetal pain. In D&E abortions, the fetus is removed from the uterus in pieces via grasping forceps (Cunningham et al. 2018), resulting in repetitive noxious stimuli until demise occurs. ACOG’s latest 2013 practice guidelines for second-trimester abortions, however, do not include recommendations for fetal analgesia prior to abortion, noting “fetal perception of pain may not occur until the third trimester” (ACOG 2013, 1395).
Ethical and Legal Considerations
If the fetus is capable of experiencing pain and suffering, which research convincingly indicates is possible beginning in the first trimester, then arguably a moral obligation exists to prevent, mitigate, and treat fetal pain, whenever it can foreseeably be anticipated, according to the principles of justice, beneficence, and nonmaleficence. This concern arises in a notable way for cases of abortion or feticide. When these principles are applied unevenly in consideration of the pregnant woman rather than the fetus, the autonomy of the woman and the degree of maternal-fetal attachment may be framed as preeminent considerations that reduce the need to attend to or address the reality of fetal pain (Arora and Salazar 2014; Furedi 2001).
Organizations favoring abortion generally oppose fetal pain legislation, while organizations opposing abortion are generally in support of such legislation. State legislative hearings, in Ohio, for example, have included among opponents of fetal pain legislation various abortion rights groups, such as Planned Parenthood, National Abortion Rights Action League (NARAL), and the American Civil Liberties Union (ACLU), while proponents are generally affiliated with groups such as the American College of Pediatricians, Right to Life groups, and religiously affiliated organizations (Ohio Senate Bill 2019). Fetal pain laws in 13 states seek to mandate that abortion counseling includes information about fetal pain capability or to prohibit abortion once the fetus is pain-capable (Guttmacher 2021). Several state laws currently stipulate fetal pain capability at 20–22 weeks gestation, while ACOG and Planned Parenthood websites claim that fetal pain is not possible until after 24–30 weeks gestation (ACOG 2021; Planned Parenthood 2018). As of 2021, Senate Bill 61, the Pain-Capable Unborn Child Protection Act, is the latest federal bill to seek to ban abortion at 22 weeks gestation, citing protection from pain as a compelling government interest in limiting abortion (U.S. Congress 2021).
Abortion rights advocates assert that fetal pain laws are beyond the purview of the government and are unconstitutional, as Roe v. Wade (1973) and Planned Parenthood v. Casey (1992) established viability, not pain capacity, to be a compelling government interest, when considering possibilities for limiting abortion. Fetal pain legislation is construed as an effort to erode the autonomy of the pregnant woman and to intrude upon the patient–physician relationship. Abortion rights proponents also cite Roe in stating that the fetus is not a ‘person’ protected by the Fourteenth Amendment. Therefore, the ability to feel pain should not confer further consideration to the previable fetus (Dadlez and Andrews 2018; Watson 2012).
Some opponents of fetal pain laws acknowledge the conflict in recognizing the moral status of a pain-capable fetus and in upholding a woman’s autonomy (Dadlez and Andrews 2018). They maintain that decisions regarding the fetus and pain management should be addressed and resolved within the patient–physician relationship (Andaya and Campo-Engelstein 2021). Because unnecessary pain raises ethical concerns, it is noteworthy that abortion rights advocates will occasionally support procedures for rendering a pain-capable fetus insensate by the use of anesthesia prior to abortion (Dadlez and Andrews 2018; Watson 2012). This would allow abortions to follow the same ethical guidelines used internationally to prevent cruelty to animals:
“...abortion guidelines should be correspondingly restructured. They should follow the same pattern as the international guidelines for killing animals...and they should bring the death about as quickly and painlessly as possible” (Kluge 2015, 13)
Other abortion rights proponents, however, state that administering fetal anesthesia and analgesia introduces unnecessary procedures and risks to the pregnant woman which outweigh the temporary pain that may be experienced by the fetus during abortion or feticide procedures (Cohen and Sayeed 2011).
Abortion rights advocates view late term abortion for fetal anomalies as compassionate care to prevent psychological pain and suffering for the woman and her family. According to a survey of abortion providers, alleviating a woman’s psychological pain due to fatal fetal abnormalities outweighs fetal pain considerations during abortion:
“…the possibility of a brief period of physical pain [for the fetus] is less pressing for pregnant people and their families than the anticipated anguish of watching their terminally ill baby suffer and die” (Andaya and Campo-Englestein 2021, 5).
Proponents of fetal pain legislation argue that permitting pain that is remediable is unethical and that there is a moral obligation to prevent cruelty and suffering (Pierucci 2020; ACP 2021). In the case of fatal fetal anomalies, which are frequently cited as reasons for second- and third-trimester abortions, resources such as perinatal palliative and hospice care can be offered to provide compassionate options for the fetus, the woman, and her family (McCarthy et al. 2012). Between 40–85% of women will typically choose perinatal hospice or palliative care for a fatal fetal anomaly, if given the option (Flaig et al. 2019). A survey of abortion providers regarding life-limiting fetal diagnoses, however, did not mention this option as part of patient counseling (Andaya and Campo-Engelstein 2021).
Abortion opponents also express concern that inconsistent terminology and information affects informed consent, de-humanizes the fetus, and could be construed as manipulative (Dixon 2008; Kaczor 2011). For example, abortion rights advocates and abortion providers preferentially avoid terminology such as ‘baby’ and ‘fetus’ (Andaya and Campo-Engelstein 2021) or images of the fetus because of “not wanting to give the fetus human status” (Williams 2005, 2085). This dichotomy in terminology and the shielding of the pregnant woman from medical information may unduly influence the patient’s decision-making process, particularly in a time of crisis when reliance on medical counsel is high.
Reports over the past 20 years show that fetal pain is a concern of women considering abortion (Andaya and Campo-Engelstein 2021; Furedi 2001; RCOG 2010). Research, however, notes a reluctance among providers to discuss fetal pain due to concerns of causing emotional distress for the pregnant woman (Andaya and Campo-Engelstein 2021). This has raised the issue of medical paternalism potentially precluding appropriate patient counseling, education, and informed consent. In Gonzales v. Carhart (2007), abortion providers noted that pertinent medical information about the abortion procedures was not typically disclosed to patients. In the majority opinion, Justice Kennedy noted that the omission of information necessitates government involvement:
It is, however, precisely this lack of information concerning the way in which the fetus will be killed that is of legitimate concern to the State...The State has an interest in ensuring so grave a choice is well informed. It is self-evident that a mother who comes to regret her choice to abort must struggle with grief more anguished and sorrow more profound when she learns, only after the event, what she once did not know... (IV.A)
The integrity of the medical profession depends on informed consent (ACOG 2021), including a full and accurate disclosure regarding the fetus, fetal pain capacity, and the methodologies involved in abortion or feticide procedures. Medical ethics also imposes the requirement to avoid cruelty and unnecessary pain and suffering. Advocates of fetal pain legislation propose that fetal pain laws attempt to address both of these concerns, by informing women of fetal pain capability and in excluding abortion once fetal pain is possible based on a compelling government interest. In contrast, clinicians in fetal medicine are already voluntarily addressing these issues in developing standards of care for the use of analgesia and anesthesia during invasive fetal procedures (Chatterjee et al. 2021; Gupta, Wimalasundera, and Moore 2021; Wood et al. 2021).
A dichotomy exists in the practice of medicine. Disparate treatment of two indistinguishable human fetuses occurs, in which one fetus is accorded patient status and humanity, to whom beneficence and nonmaleficence are owed, while for the other fetus, this status is withheld. This cognitive dissonance disturbs and causes significant tension within the practice of medicine in view of objective medical evidence as well as in light of the fundamental mission of medical professionals as healers. There is an ethical obligation to prevent unnecessary pain and suffering, as well as an obligation for beneficence and nonmaleficence, which must be judiciously applied to both the pregnant woman and the fetus, while safety and health concerns are carefully balanced.
Conclusion
Until the late 1990s, fetal pain was largely unrecognized and untreated. Over the past 20 years, research in the fields of fetal pain and fetal medicine has changed this understanding. Denial of fetal pain capacity beginning in the first trimester, potentially as early as 8–12 weeks gestation, is no longer tenable. To paraphrase the 2016 American Academy of Pediatrics policy statement in terms of fetal, rather than neonatal, pain, “The prevention of pain in the fetus should be the goal of all health care professionals, not only because it is ethical, but also because repeated painful exposures have the potential for deleterious consequences” (1). Precise determination of fetal pain onset in the first trimester is challenging for several reasons, including the subjective elements related to pain and its perception, and due to gaps in medical knowledge. The development of fetal pain perception along a continuum of maturation rather than at a distinct gestational age, also impacts the discussion. Additionally, fetal research of responses to noxious stimuli in the first trimester is limited by technical and ethical considerations. In disputed or reasonably doubtful ethical situations of this kind, it is proper to yield to a precautionary principle, presuming pain when uncertainty exists. As summed up by the American Society of Anesthesiologists’ and the North American Fetal Therapy Network’s Consensus Statement in 2021, “Because it remains uncertain exactly when a fetus has the capacity to feel pain, it is best to administer adequate fetal anesthesia in all invasive maternal-fetal procedures” (Chatterjee et al. 2021, 1165).
Biographical Note
Bridget Thill, MD, MS is a former Air Force general practice physician who cared for military personnel in the U.S. and overseas before transitioning to medical bioethics. She received a master’s degree in bioethics from the University of Mary. She works as an independent researcher and writer in medical bioethics.
Footnotes
Declaration of Conflicting Interest: The author(s) declared that there is no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Bridget Thill, MD, MS https://orcid.org/0000-0002-7363-4560
References
- AboEllail M. A. M., Hata T. 2017. “Fetal face as important indicator of fetal brain function.” Journal of Perinatal Medicine 45 (6): 729‐736. doi: 10.1515/jpm-2016-0377. [DOI] [PubMed] [Google Scholar]
- Adzick N. S. 2020. “The IFMSS Tower Lecture: Fetal Surgery Past, Fetal Surgery Present, and Fetal Surgery Yet to Come!.” Fetal Diagnosis and Therapy 47 (12): 859‐864. doi: 10.1159/000507349. [DOI] [PubMed] [Google Scholar]
- Ahmad K. A., Frey C. S., Fierro M. A., Kenton A. B., Placencia F. X. 2017. “Two-Year Neurodevelopmental Outcome of an Infant Born at 21 Weeks’ 4 Days’ Gestation.” Pediatrics 140 (6): e20170103. doi: 10.1542/peds.2017-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman B., Merker B. 2014. “Consciousness Without Cortex: A Hydranencephaly Family Survey.” Acta Paediatrica 103 (10): 1057‐1065. doi: 10.1111/apa.12718. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Task Force on Circumcision . 2012. “Circumcision Policy Statement.” Pediatrics 130 (3): 585‐586. 10.1542/peds.2012-1989. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Task Force on Circumcision . 1999. “Circumcision policy statement. American Academy of Pediatrics. Task Force on Circumcision.” Pediatrics 103 (3): 686‐693. https://pubmed.ncbi.nlm.nih.gov/10049981/. [PubMed] [Google Scholar]
- American Academy of Pediatrics . 2016. “Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. “Prevention and management of procedural pain in the neonate: an update.” Pediatrics 137 (2): e20154271. doi: 10.1542/peds.2015-4271. [DOI] [PubMed] [Google Scholar]
- American Association of Pro-Life Obstetricians and. Gynecologists . 2018. “AAPLOG practice bulletin no. 2: Fetal pain.” Issues in Law & Medicine 33 (2): 237‐246. doi: 10.1097/01.AOG.0000431056.79334.cc. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists . 2020. Facts are Important: Fetal Pain. ACOG. https://www.acog.org/advocacy/facts-are-important/fetal-pain. [Google Scholar]
- American College of Obstetricians and Gynecologists . 2013. “ACOG Practice Bulletin No. 135: Second-trimester abortion.” Obstetrics and Gynecology 121 (6): 1394‐406. doi: 10.1097/01.AOG.0000431056.79334.cc., no. [DOI] [PubMed] [Google Scholar]
- American College of Pediatricians . 2021. “Fetal Pain: What is the Scientific Evidence?.” Issues Law Med 36 (1): 113‐122. https://acpeds.org/position-statements/fetal-pain. [PubMed] [Google Scholar]
- American Veterinary Medical Association . 2020. AVMA Guidelines for the Euthanasia of Animals. American Veterinary Medical Association. Accessed September 10, 2021. https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals. [Google Scholar]
- Anand K. J. S. 2019. “Discovering Pain In Newborn Infants.” Anesthesiology 131 (2): 392‐395. 10.1097/ALN.0000000000002810. [DOI] [PubMed] [Google Scholar]
- Anand K. J., Sippell W. G., Aynsley-Green A. 1987. “Randomised Trial Of Fentanyl Anaesthesia In Preterm Babies Undergoing Surgery: Effects On The Stress Response.” Lancet 329 (8527): 243‐8. doi: 10.1016/s0140-6736(87)90065-1. [DOI] [PubMed] [Google Scholar]
- Andaya E., Campo-Engelstein L. 2021. “Conceptualizing Pain and Personhood in the Periviable Period: Perspectives from Reproductive Health and Neonatal Intensive Care Unit Clinicians.” Social Science & Medicine 269 (2021): 113558. doi: 10.1016/j.socscimed.2020.113558. [DOI] [PubMed] [Google Scholar]
- Andrews K., Fitzgerald M. 1994. “The Cutaneous Withdrawal Reflex in Human Neonates: Sensitization, Receptive Fields, and the Effects of Contralateral Stimulation.” Pain 56 (1): 95‐101. doi: 10.1016/0304-3959(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Apkarian A. V. 2018. “Nociception, Pain, Consciousness, and Society: A Plea for Constrained Use of Pain-related Terminologies.” The Journal of Pain 19 (11): 1253‐1255. doi: 10.1016/j.jpain.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Arora Kavita Shah, Salazar Christina. 2014. “Fetal Pain Legislation.” AMA Journal of Ethics 16 (10): 818‐821. doi: 10.1001/virtualmentor.2014.16.10.pfor1-1410. [DOI] [PubMed] [Google Scholar]
- Bellieni C. V. 2020. “Analgesia for Fetal Pain During Prenatal Surgery: 10 Years of Progress.” Pediatric Research 89 (7): 1612‐1618. doi: 10.1038/s41390-020-01170-2. [DOI] [PubMed] [Google Scholar]
- Bellieni C. V., Vannuccini S., Petraglia F.. 2018. “Is Fetal Analgesia Necessary During Prenatal Surgery?” The Journal of Maternal-Fetal & Neonatal Medicine 31 (9): 1241‐1245. doi: 10.1080/14767058.2017.1311860. [DOI] [PubMed] [Google Scholar]
- Bellieni C. V. 2019. “New Insights into Fetal Pain.” Seminars in Fetal and Neonatal Medicine 24: 4101001. doi: 10.1016/j.siny.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Bellieni C. V. 2012. “Pain Assessment in Human Fetus and Infants.” The AAPS Journal 14 (3): 456‐461. doi: 10.1208/s12248-012-9354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellieni C. V., Tei M., Stazzoni G., Bertrando S., Cornacchione S., Buonocore G. 2013. “Use of Fetal Analgesia During Prenatal Surgery.” The Journal of Maternal-Fetal & Neonatal Medicine 26 (1): 90‐95. doi: 10.3109/14767058.2012.718392. [DOI] [PubMed] [Google Scholar]
- Benatar D., Benatar M. 2001. “A Pain in the Fetus: Toward Ending Confusion About Fetal Pain.” Bioethics 15 (1): 57‐76. doi: 10.1111/1467-8519.00212. [DOI] [PubMed] [Google Scholar]
- Bernardes L. S., Rosa A. S., Carvalho M. A., Ottolia J., Rubloski J. M., Castro D., Velloso A., Silva V. A., Andrade D. C. 2021. a. “Acute Pain Facial Expressions In 23‐Week Fetus.” Ultrasound in Obstetrics & Gynecology, doi: 10.1002/uog.23709. Published ahead of print, June 15, 2021a. [DOI] [PubMed] [Google Scholar]
- Bernardes LS, Carvalho MA, Harnik SB, Teixeira MJ, Ottolia J, Castro D, Velloso A, Francisco R, Listik C, Galhardoni R, Aparecida da Silva V, Moreira LI, de Amorim Filho AG, Fernandes AM, Ciampi de Andrade D. 2021. b. “Sorting Pain Out of Salience: Assessment of Pain Facial Expressions In The Human Fetus.” Pain Reports 6 (1): e882. relabeled as Figure 3. doi: 10.1097/PR9.0000000000000882. Figure 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes L. S., Ottolia J. F., Juliana Fontan Ottolia. Cecchini M., Filho A. G. d. A., Teixeira M. J., Francisco R. P. V., de Andrade D. C., Manoel Jacobsen Teixeira, Rossana Pulcineli Vieira Francisco, Daniel Ciampi de Andrade. Grupo de Estudo da Dor Fetal . 2018. “On the Feasibility of Accessing Acute Pain-Related Facial Expressions in the Human Fetus And Its Potential Implications: A Case Report.” Pain Reports 3 (5): e673. doi: 10.1097/PR9.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blickstein Isaac, Oppenheimer Inbar. 2016. Compassionate Treatment of Fetal Pain. Current Pediatric Research, 64‐68. http://www.currentpediatrics.com/articles/compassionate-treatment-of-fetal-pain.html. [Google Scholar]
- Bonhomme V., Staquet C., Montupil J., Defresne A., Kirsch M., Martial C., Vanhaudenhuyse A., Chatelle C., Larroque S. K., Raimondo F., Demertzi A., Bodart O., Laureys S., Gosseries O. 2019. “General Anesthesia: A Probe To Explore Consciousness.” Frontiers in Systems Neuroscience 13: 36. doi: 10.3389/fnsys.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani E., Della Vedova A. M., Rezzani R., Rodella L. F., Cristini C. 2019. “Correlation Between Human Nervous System Development And Acquisition of Fetal Skills: An Overview.” Brain and Development 41 (3): 225‐233. doi: 10.1016/j.braindev.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Brusseau R. 2008. “Developmental Perspectives: Is The Fetus Conscious?” International Anesthesiology Clinics 46 (3): 11‐23. doi: 10.1097/AIA.0b013e318181a88e. [DOI] [PubMed] [Google Scholar]
- Brusseau R., Mizrahi-Arnaud A. 2013. “Fetal Anesthesia and Pain Management for Intrauterine Therapy.” Clinics in Perinatology 40 (3): 429‐442. doi: 10.1016/j.clp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Bystron I., Blakemore C., Rakic P. 2008. “Development of the Human Cerebral Cortex: Boulder Committee Revisited.” Nature Reviews Neuroscience 9 (2): 110‐122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Castiello U., Becchio C., Zoia S., Nelini C., Sartori L., Blason L., D'Ottavio G., Bulgheroni M., Gallese V. 2010. “Wired to Be Social: The Ontogeny of Human Interaction.” PloS One 5 (10): e13199. doi: 10.1371/journal.pone.0013199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Arendt K. W., Moldenhauer J. S., Olutoye O. A., Jagroop Mavi Parikh. Parikh J. M., Tran K. M., Zaretsky M. V., Zhou J., Rollins M. D. 2021. “Anesthesia for Maternal-Fetal Interventions: A Consensus Statement From the American Society of Anesthesiologists Committees on Obstetric and Pediatric Anesthesiology and the North American Fetal Therapy Network.” Anesthesia & Analgesia 132 (4): 1164‐1173. doi: 10.1213/ANE.0000000000005177. [DOI] [PubMed] [Google Scholar]
- Cohen I. G., Sayeed S. 2011. “Fetal Pain, Abortion, Viability, and the Constitution.” Journal of Law, Medicine & Ethics 39 (2): 235‐242. doi: 10.1111/j.1748-720X.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- Cunningham F. Gary, Leveno Kenneth J., Bloom Steven L., Dashe Jodi S., Hoffman Barbara L., Casey Brian M., Spong Catherine Y., eds 2018. “Abortion” in Williams Obstetrics. 25th edition, 346‐370. New York: McGraw-Hill Education. [Google Scholar]
- Dadlez E. M., Andrews W. L. 2018. “Legislating Pain Capability: Sentience and the Abortion Debate.” In The Palgrave Handbook of Philosophy and Public Policy, 661‐675. Cham: Palgrave Macmillan, doi: 10.1007/978-3-319-93907-0_50. [DOI] [Google Scholar]
- De Buck F., Deprest J., Van de Velde M. 2008. “Anesthesia for fetal surgery.” Current Opinion in Anaesthesiology 21 (3): 293‐297. doi: 10.1097/ACO.0b013e3282fe6e70. [DOI] [PubMed] [Google Scholar]
- Derbyshire S. W. G. 1999. “Locating the beginnings of pain.” Bioethics 13 (1): 1‐31. doi: 10.1111/1467-8519.00129. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW. 2008. “Fetal Pain: Do We Know Enough to Do the Right Thing?” Reproductive Health Matters 16 (31): 117‐26. doi: 10.1016/S0968-8080(08)31370-6. [DOI] [PubMed] [Google Scholar]
- Derbyshire S. W., Bockmann J. C. 2020. “Reconsidering Fetal Pain.” Journal of Medical Ethics 46 (1): 3‐6. doi: 10.1136/medethics-2019-105701. [DOI] [PubMed] [Google Scholar]
- Derbyshire S. W. G. 2006. “Can Fetuses Feel Pain?” Bmj 332 (7546): 909‐912. doi: 10.1136/bmj.332.7546.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereymaeker A., Pillay K., Vervisch J., De Vos M., Van Huffel S., Jansen K., Naulaers G. 2017. “Review of sleep-EEG in preterm and term neonates.” Early Human Development 113: 87‐103. doi: 10.1016/j.earlhumdev.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon Darrin P. 2008. “Informed Consent or Institutionalized Eugenics-How the Medical Profession Encourages Abortion of Fetuses with Down Syndrome.” Issues L. & Med 24: 3. https://pubmed.ncbi.nlm.nih.gov/18771038/. [PubMed] [Google Scholar]
- Dresser R. 2014. “Drugs and the death penalty.” Hastings Center Report 44 (1): 9‐110. doi: 10.1002/hast.247. [DOI] [PubMed] [Google Scholar]
- Feinstein J. S., Khalsa S. S., Salomons T. V., Prkachin K. M., Frey-Law L. A., Lee J. E., Tranel D., Rudrauf D. 2016. “Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala.” Brain Structure and Function 221 (3): 1499‐1511. doi: 10.1007/s00429-014-0986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk N. M., Gitau R., Teixeira J. M., Giannakoulopoulos X., Cameron A. D., Glover V. A. 2001. “Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling.” Anesthesiology 95 (4): 828‐835. doi: 10.1097/00000542-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Flaig F., Lotz J. D., Knochel K., Borasio G. D., Führer M., Hein K. 2019. “Perinatal Palliative Care: A qualitative study evaluating the perspectives of pregnancy counselors.” Palliative Medicine 33 (6): 704‐711. doi: 10.1177/0269216319834225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furedi A. 2001. “Issues for service providers: a response to points raised.” Journal of Medical Ethics 27, suppl 2: ii28‐ii32. doi: 10.1136/jme.27.suppl_2.ii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakoulopoulos X., Sepulveda W., Kourtis P., Glover V, Fisk N. M. 1994. “Fetal plasma cortisol and beta-endorphin response to intrauterine needling.” Lancet 344 (8915): 77‐81. doi: 10.1016/s0140-6736(94)91279-3. [DOI] [PubMed] [Google Scholar]
- Giannakoulopoulos X., Teixeira J., Fisk N., Glover V. 1999. “Human fetal and maternal noradrenaline responses to invasive procedures.” Pediatric Research 45 (4): 494‐499. doi: 10.1203/00006450-199904010-00007. [DOI] [PubMed] [Google Scholar]
- Gibbins S., Stevens B., Beyene J., Chan P. C., Bagg M., Asztalos E. 2008. “Pain Behaviours in Extremely Low Gestational Age infants.” Early Human Development 84 (7): 451‐458. doi: 10.1016/j.earlhumdev.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Gitau R., Fisk N. M., Teixeira J. M. A., Cameron A., Glover V. 2001. “Fetal Hypothalamic-Pituitary-Adrenal Stress Responses to Invasive Procedures Are Independent of Maternal Responses1.” The Journal of Clinical Endocrinology & Metabolism 86 (1): 104‐109. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- Gupta Ritu, Wimalasundera Ruwan, Moore Phil. 2021. “Anaesthesia for Uncommon and Emerging Procedures.” In Anaesthesia for Uncommon and Emerging Procedures. Michael S. Green. 277-288, edited by Goudra Basavana G., ed. Cham, Switzerland: Springer, doi: 10.1007/978-3-030-64739-1. [DOI] [Google Scholar]
- Guttmacher Institute . 2019. Induced Abortion in the United States. Guttmacher Institute. Last modified September 2019. https://www.guttmacher.org/fact-sheet/induced-abortion-united-states#. [Google Scholar]
- Guttmacher Institute . 2021. Counseling and Waiting Periods for Abortion. Guttmacher Institute. Last modified September 1, 2021. https://www.guttmacher.org/state-policy/explore/counseling-and-waiting-periods-abortion. [Google Scholar]
- Hatfield L. 2014. “Neonatal Pain: What′s Age Got To Do With It?” Surgical Neurology International 5, Suppl 13: 479‐89. doi: 10.4103/2152-7806.144630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner R. F. 2000. “Development of Connections in the Human Visual System During Fetal Mid-Gestation: A Dil-Tracing Study.” Journal of Neuropathology & Experimental Neurology 59 (5): 385‐392. doi: 10.1093/jnen/59.5.385. [DOI] [PubMed] [Google Scholar]
- Hooker Davenport. 1936. “Early fetal activity in mammals.” Yale Journal of Biology and Medicine 8 (6): 579. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2601429/. [PMC free article] [PubMed] [Google Scholar]
- Hooker Davenport. 1952. “Early Human Fetal Activity (1952): Selected films from a physiological study under the direction of Davenport Hooker.” YouTube Video 16: 56. April 23, 2015. https://www.youtube.com/watch?v=KkK4d9pIneE. [Google Scholar]
- Hooker D. 1942. “Fetal reflexes and instinctual processes.” Psychosomatic Medicine 4 (2): 199‐205. https://journals.lww.com/psychosomaticmedicine/citation/1942/04000/fetal_reflexes_and_instinctual_processes.9.aspx. [Google Scholar]
- Humphrey T. 1964. Some correlations between the appearance of human fetal reflexes and the development of the nervous system. In Progress in Brain Research, Vol. 4, 93‐135. Elsevier, doi: 10.1016/S0079-6123(08)61273-X. [DOI] [Google Scholar]
- International Association for the Study of Pain . 1979. “Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy.” Pain 6: 249‐252. https://pubmed.ncbi.nlm.nih.gov/460932/. [PubMed] [Google Scholar]
- International Association for the Study of Pain . 2017. ““IASP Terminology.” International Association for the Study of Pain.” Last modified December 14, 2017. https://www.iasp-pain.org/resources/terminology/#nociception.
- Johnston CC, Stevens BJ. 1996. “Experience in a neonatal intensive care unit affects pain response.” Pediatrics 98 (5): 925‐30. https://pubmed.ncbi.nlm.nih.gov/8909487/. [PubMed] [Google Scholar]
- Judaš M., Sedmak G., Pletikos M. 2010. “Early history of subplate and interstitial neurons: from Theodor Meynert (1867) to the discovery of the subplate zone (1974).” Journal of Anatomy 217 (4): 344‐367. doi: 10.1111/j.1469-7580.2010.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judaš M., Sedmak G., Kostović I. 2013. “The significance of the subplate for evolution and developmental plasticity of the human brain.” Frontiers in Human Neuroscience 7: 423. doi: 10.3389/fnhum.2013.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor C. 2011. “Philosophy and Theology: Notes on Fetal Interventions.” The National Catholic Bioethics Quarterly 11 (4): 789‐794. Accessed September 10, 2021. https://digitalcommons.lmu.edu/phil_fac/89/. [Google Scholar]
- Kadić A.S., Kurjak A. 2018. “Cognitive functions of the fetus.” Ultraschall in der Medizin - European Journal of Ultrasound 39 (02): 181‐189. doi: 10.1055/s-0043-123469. [DOI] [PubMed] [Google Scholar]
- Kadić A.S., Predojević M. 2012. “Fetal neurophysiology according to gestational ageSeminars in Fetal and Neonatal Medicine.” Seminars in Fetal and Neonatal Medicine 17 (5): 256‐260. doi: 10.1016/j.siny.2012.05.007. [DOI] [PubMed] [Google Scholar]
- King J. R., Conturie C. L., Ouzounian J. G., Korst L. M., Llanes A., Chmait R. H. 2017. “Umbilical cord occlusion via laser coagulation in monochorionic multifetal gestations before and after 20 weeks of gestation.” Fetal Diagnosis and Therapy 42 (1): 9‐16. doi: 10.1159/000448948. [DOI] [PubMed] [Google Scholar]
- Klit H., Finnerup N. B., Jensen T. S. 2009. “Central post-stroke pain: clinical characteristics, pathophysiology, and management.” The Lancet Neurology 8 (9): 857‐868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- Kluge E.-H. W. 2015. “Ethical considerations on methods used in abortions.” Health Care Analysis 23 (1): 1‐18. doi: 10.1007/s10728-012-0232-1. [DOI] [PubMed] [Google Scholar]
- Kostovic I., Rakic P. 1990. “Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain.” The Journal of Comparative Neurology 297 (3): 441‐470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostović I., Jovanov-Milošević N. 2006. “The development of cerebral connections during the first 20-45 weeks' gestation.” Seminars in Fetal and Neonatal Medicine 11 (6): 415‐422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N. 2008. “Subplate zone of the human brain: historical perspective and new concepts.” Collegium Antropologicum 32 Suppl 1: 3‐18. https://www.researchgate.net/publication/5447730_Subplate_zone_of_the_human_brain_Historical_perspective_and_new_concepts. [PubMed] [Google Scholar]
- Kostović I. 2020. “The enigmatic fetal subplate compartment forms an early tangential cortical nexus and provides the framework for construction of cortical connectivity.” Progress in Neurobiology 194: 101883. doi: 10.1016/j.pneurobio.2020.101883 10.1016/j.pneurobio.2020.101883. [DOI] [PubMed] [Google Scholar]
- Kostović I., Judaš M.. 2002. “The role of the subplate zone in the structural plasticity of the developing human cerebral cortex.” Neuroembryology and Aging 1 (4): 145‐153. doi: 10.1159/000066270. [DOI] [Google Scholar]
- Lee S. J., Ralston H. J. P., Drey E. A.John Colin Partridge, Partridge J. C., Rosen M. A. 2005. “Fetal Pain.” JAMA 294 (8): 947‐954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- Lowery C. L., Hardman M. P., Manning N., Clancy B., Whit Hall R., Anand K. J. S. 2007. “Neurodevelopmental changes of fetal painSeminars in perinatology.” Seminars in Perinatology 31 (5): 275‐282. doi: 10.1053/j.semperi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Marchant A. 2014. “‘Neonates Do Not Feel Pain’: A Critical Review of the Evidence.” Bioscience Horizons: The International Journal of Student Research 7: hzu006. doi: 10.1093/biohorizons/hzu006. [DOI] [Google Scholar]
- Maurice P., Letourneau A., Benachi A., Jouannic J. M. 2019. “Feticide in Second‐ and Third‐Trimester Termination of Pregnancy For Fetal Anomalies: Results of A National Survey.” Prenatal Diagnosis 39(13): 1269‐1272. doi: 10.1002/pd.5594. [DOI] [PubMed] [Google Scholar]
- Maxwell L. G., Fraga M. V., Malavolta C. P. 2019. “Assessment of Pain in the Newborn.” Clinics in Perinatology 46 (4): 693‐707. doi: 10.1016/j.clp.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Mazzola L., Isnard J., Peyron R., Mauguière F. 2012. “Stimulation of the Human Cortex and The Experience of Pain: Wilder Penfield’s Observations Revisited.” Brain 135 (2): 631‐640. doi: 10.1093/brain/awr265. [DOI] [PubMed] [Google Scholar]
- McCarthy E. A., Watkins A., Shub A., Walker S. P. 2012. “Intrapartum Fetal Pain Management in Lethal Osteogenesis Imperfecta.” Prenatal Diagnosis 32 (10): 1013‐1015. doi: 10.1002/pd.3935. [DOI] [PubMed] [Google Scholar]
- McCredie J. 2007. “Nerves in Bone: The Silent Partners.” Skeletal Radiology 36 (6): 473‐475. doi: 10.1007/s00256-006-0253-7. [DOI] [PubMed] [Google Scholar]
- Mellor D. J., Diesch T. J., Gunn A. J., Bennet L. 2005. “The importance of ‘awareness’ for understanding fetal pain.” Brain Research Reviews 49 (3): 455‐471. doi: 10.1016/j.brainresrev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Merker B. 2007. “Consciousness Without a Cerebral Cortex: A Challenge For Neuroscience And Medicine.” Behavioral and Brain Sciences 30 (1): 63‐81. doi: 10.1017/S0140525X07000891. [DOI] [PubMed] [Google Scholar]
- Mojsilović J, Zečević N. 1991. “Early Development of The Human Thalamus: Golgi and Nissl study.” Early Human Development 27 (1-2): 119‐144. doi: 10.1016/0378-3782(91)90033-y. [DOI] [PubMed] [Google Scholar]
- Myers L. B., Bulich L. A., Hess P., Miller N. M. 2004. “Fetal Endoscopic Surgery: Indications And Anaesthetic Management.” Best Practice & Research Clinical Anaesthesiology 18 (2): 231‐258. doi: 10.1016/j.bpa.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Ohio Senate Bill 23 . 2019. Human Rights and Heart Beat Protection Act of 2019, 2nd Hearing, Before the Ohio Legislature Committee on Health, Human Services and Medicaid. March 19. 134th General Assembly. https://www.legislature.ohio.gov/legislation/legislation-committee-documents?id=GA133-SB-23. (Statement of Gary L. George, M.D., Southwest Ohio Guild of the Catholic Medical Association). [Google Scholar]
- Okado Nobuo, Kojima Tokuzo. 1984. “Ontogeny of the Central Nervous System: Neurogenesis, Fibre Connection, Synaptogenesis And Myelination In The Spinal Cord.” In Vol. 94 Clinics in Developmental Medicine: Continuity of Neural Functions from Prenatal to Postnatal Life, edited by Prechtl H. F., 31‐45. Philadelphia, PA: JB Lippincott Co. [Google Scholar]
- Patel R. A., Chandler J. P., Jain S., Gopalakrishnan M., Sachdev S. 2017. “Dejerine-Roussy Syndrome From Thalamic Metastasis Treated With Stereotactic Radiosurgery.” Journal of Clinical Neuroscience 44: 227‐228. doi: 10.1016/j.jocn.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Petrikovsky B. M., Kaplan G. P. 1995. “Fetal Responses to Inadvertent Contact With The Needle During Amniocentesis.” Fetal Diagnosis and Therapy 10 (2): 83‐85. doi: 10.1159/000264210. [DOI] [PubMed] [Google Scholar]
- Pierucci R. 2020. “Fetal Pain: The Science Behind Why It Is The Medical Standard Of Care.” The Linacre Quarterly 87 (3): 311‐316. doi: 10.1177/0024363920924877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planned Parenthood Great Plains Votes . 2018. “Why Politicians lie about Fetal Pain,” Planned Parenthood Great Plains Votes. Last modified April 18, 2018. https://www.plannedparenthoodaction.org/planned-parenthood-great-plains-votes/blog/why-politicians-lie-about-fetal-pain. [Google Scholar]
- Platt M. W. 2011. “Fetal Awareness And Fetal Pain: The Emperor’s New Clothes.” Archives of Disease in Childhood - Fetal and Neonatal Edition 96 (4): F236‐F237. doi: 10.1136/adc.2010.195966. [DOI] [PubMed] [Google Scholar]
- Raja S. N., Carr D. B., Cohen M., Finnerup N. B., Flor H., Gibson S., Keefe F. J., Mogil J. S., Ringkamp M., Sluka K. A., Song X.-J., Stevens B., Sullivan M. D., Tutelman P. R., Ushida T., Vader K. 2020. “The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises.” Pain 161 (9): 1976‐1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey E. N., Riddell R. Pillai. 2013. “The Infancy of Infant Pain Research: The Experimental Origins of Infant Pain Denial.” The Journal of Pain 14 (4): 338‐350. doi: 10.1016/j.jpain.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rokyta R. 2008. “Fetal Pain.” Neuro Endocrinology Letters 29 (6): 807‐814. https://pubmed.ncbi.nlm.nih.gov/19112406/. [PubMed] [Google Scholar]
- Royal College of Obstetricians and Gynaecologists . 2010. “Fetal Awareness: Review of Research and Recommendations for Practice.” Accessed September 10, 2021. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/fetal-awareness---review-of-research-and-recommendations-for-practice/.
- Rysavy M. A., Abhik Das. Bell E. F., DasStollVohr A.Betty R., Hintz S. R., Stoll B. J., Vohr B. R., Carlo W. A., Shankaran S., Walsh M. C., Tyson J. E., Cotten C. M., Smith P. B., Murray J. C., Colaizy T. T., Brumbaugh J. E., Higgins R. D. 2015. “Between-Hospital Variation in Treatment And Outcomes in Extremely Preterm Infants.” New England Journal of Medicine 372: 1801‐1811. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S. N. 2013. “Fetal Magnetic Resonance Imaging (MRI).” Journal of Child Neurology 28 (7): 890‐908. doi: 10.1177/0883073813486296. [DOI] [PubMed] [Google Scholar]
- Sanders R. D., Gaskell A., Raz A., Winders J., Stevanovic A., Rossaint R., Boncyk C., Defresne A., Tran G., Tasbihgou S., Meier S., Vlisides P. E., Fardous H., Hess A., Bauer R. M., Absalom A., Mashour G. A., Bonhomme V., Coburn M., Sleigh J. 2017. “Incidence of Connected Consciousness after Tracheal Intubation.” Anesthesiology 126 (2): 214‐222. doi: 10.1097/ALN.0000000000001479. [DOI] [PubMed] [Google Scholar]
- Tworetzky W., Wilkins-Haug L., Schidlow D. 2014. “Percutaneous Fetal Cardiac Interventions for Structural Heart Disease.” American Journal of Perinatology 31 (07): 629‐636. doi: 10.1055/s-0034-1383884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic S., Gebauer-Bukurov K., Cvijanovic M., Kopitovic A., Ilic D., Petrovic D., Capo I., Pericin-Starcevic I., Christ O., Topalidou A. 2016. “Appearance of Fetal Pain Could be Associated With Maturation of The Mesodiencephalic Structures.” Journal of Pain Research 9: 1031‐1038. doi: 10.2147/JPR.S117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. P., Gitau R., Glover V., Fisk N. M. 2000. “Pain and Stress in The Human Fetus.” European Journal of Obstetrics & Gynecology and Reproductive Biology 92 (1): 161‐165. doi: 10.1016/S0301-2115(00)00441-3. [DOI] [PubMed] [Google Scholar]
- Society for Maternal-Fetal Medicine (SMFM) Society of Family Planning. (SFP) Norton M. E., Cassidy A., Ralston S. J., Chatterjee D., Farmer D., Beasley A. D., Dragoman M. 2021. “Society for Maternal-Fetal Medicine Consult Series #59: The Use of Analgesia And Anesthesia For Maternal-Fetal Procedures.” American Journal of Obstetrics and Gynecology. Published ahead of print, August 27, 2021. doi: 10.1016/j.ajog.2021.08.031. [DOI] [PubMed] [Google Scholar]
- Stanojevic M., Kurjak A., Kadic A. S., Barisic L. S., Jakovljević M. 2021. “Fetal Awareness.” Donald School Journal of Ultrasound in Obstetrics and Gynecology 15 (2): 188‐194. doi: 10.5005/jp-journals-10009-1700. [DOI] [Google Scholar]
- Teixeira J. M. A., Glover V., Fisk N. M. 1999. “Acute cerebral redistribution in response to invasive procedures in the human fetus.” American Journal of Obstetrics and Gynecology 181 (4): 1018‐1025. doi: 10.1016/s0002-9378(99)70340-6. [DOI] [PubMed] [Google Scholar]
- Terrier L.-M., Lévêque M., Amelot A. 2019. “Brain Lobotomy: A Historical and Moral Dilemma with No Alternative?” World Neurosurgery 132: 211‐218. doi: 10.1016/j.wneu.2019.08.254. [DOI] [PubMed] [Google Scholar]
- U.S. Congress . 2021. Senate. Pain-Capable Unborn Child Protection Act. S.61. 117th Cong., 1st sess. Introduced in Senate January, Vol. 27. https://www.congress.gov/bill/117th-congress/senate-bill/61/titles?r=28&s=1. [Google Scholar]
- Van de Velde M., De Buck F. 2012. “Fetal and Maternal Analgesia/Anesthesia for Fetal Procedures.” Fetal Diagnosis and Therapy 31 (4): 201‐209. doi: 10.1159/000338146. [DOI] [PubMed] [Google Scholar]
- Walker S. M. 2019. “Long-Term Effects of Neonatal PainSeminars in Fetal and Neonatal Medicine.” Seminars in Fetal and Neonatal Medicine 24 (4): 101005. doi: 10.1016/j.siny.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Watson K. 2012. “Abortion Bans Premised on Fetal Pain Capacity.” Hastings Center Report 42 (5): 10‐11. doi: 10.1002/hast.78. [DOI] [PubMed] [Google Scholar]
- Wess J. M., Isaiah A., Watkins P. V., Kanold P. O. 2017. “Subplate Neurons Are The First Cortical Neurons To Respond To Sensory Stimuli.” Proceedings of the National Academy of Sciences 114 (47): 12602‐12607. doi: 10.1073/pnas.1710793114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. 2005. “Framing the Fetus in Medical Work: Rituals And Practices.” Social Science & Medicine 60 (9): 2085‐2095. doi: 10.1016/j.socscimed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Wilson E. K. 2014. “Ex utero: live human fetal research and the films of Davenport Hooker.” Bulletin of the History of Medicine 88 (1): 132‐160. doi: 10.1353/bhm.2014.0002. [DOI] [PubMed] [Google Scholar]
- Wood C. L., Zuk J., Rollins M. D., SilveiraSilveira L. J.John R. Feiner, Feiner J. R., Zaretsky M., Chatterjee D. 2021. “Anesthesia for Maternal-Fetal Interventions: A Survey of Fetal Therapy Centers in the North American Fetal Therapy Network.” Fetal Diagnosis and Therapy 48 (5): 1‐11. doi: 10.1159/000514897. [DOI] [PubMed] [Google Scholar]
- Zoia S., Blason L., D’Ottavio G., Bulgheroni M., Pezzetta E., Scabar A., Castiello U. 2007. “Evidence of Early Development of Action Planning in The Human Foetus: A Kinematic Study.” Experimental Brain Research 176 (2): 217‐226. doi: 10.1007/s00221-006-0607-3. [DOI] [PubMed] [Google Scholar]