Abstract
ATP1A1 encodes the α1 subunit of the sodium-potassium ATPase, an electrogenic cation pump highly expressed in the nervous system. Pathogenic variants in other subunits of the same ATPase, encoded by ATP1A2 or ATP1A3, are associated with syndromes such as hemiplegic migraine, dystonia, or cerebellar ataxia. Worldwide, only 16 families have been reported carrying pathogenic ATP1A1 variants to date. Associated phenotypes are axonal neuropathies, spastic paraplegia, and hypomagnesemia with seizures and intellectual disability. By whole exome or genome sequencing, we identified 5 heterozygous ATP1A1 variants, c.674A>G;p.Gln225Arg, c.1003G>T;p.Gly335Cys, c.1526G>A;p.Gly509Asp, c.2152G>A;p.Gly718Ser, and c.2768T>A;p.Phe923Tyr, in 5 unrelated children with intellectual disability, spasticity, and peripheral, motor predominant neuropathy. Additional features were sensory loss, sleep disturbances, and seizures. All variants occurred de novo and are absent from control populations (MAF GnomAD = 0). Affecting conserved amino acid residues and constrained regions, all variants have high pathogenicity in silico prediction scores. In HEK cells transfected with ouabain-insensitive ATP1A1 constructs, cell viability was significantly decreased in mutants after 72h treatment with the ATPase inhibitor ouabain, demonstrating loss of ATPase function. Replicating the haploinsufficiency mechanism of disease with a gene-specific assay provides pathogenicity information and increases certainty in variant interpretation. This study further expands the genotype-phenotype spectrum of ATP1A1.
ATP1A1 encodes the α1 unit of a sodium-potassium ATPase being highly expressed in the nervous system. With an autosomal-dominant inheritance, associated phenotypes comprise Charcot-Marie-Tooth disease (CMT)1,2, hereditary spastic paraplegia (HSP)3, and intellectual disability4, hypomagnesemia, hypokalemia, and seizures.5 Heterozygous missense mutations lead to a reduced ATPase function, indicating haploinsufficiency.1
We aimed at showing the pathogenicity of 5 newly identified heterozygous missense variants and broadening the known genotype-phenotype spectrum1-3,5 of ATP1A1.
Methods
All patients or legal representatives gave written informed consent. Clinical examinations were performed by experienced child neurologists. Using site-specific protocols, ATP1A1 variants were identified by whole exome or genome sequencing. In both children and parents, variants were validated by Sanger sequencing. Cosegregation analyses confirmed that all 5 variants occured de novo.
To assess variant pathogenicity, HEK cells were transfected with ouabain-insensitive ATP1A1 plasmids encoding either the wildtype (wt) or mutants in replicates of 8. As a negative control, we used wt-ATP1A1 plasmids that are not insensitive to ouabain. 24 hours after transfection, cells were treated with the ATPase inhibitor ouabain at a concentration of 0.5µM. After 72 hours, cell survival was measured by luciferase (CellTiter-Glo) assay. For normalization, we calculated the viability ratio of treated and untreated cells, each transfected with the same plasmid. For statistical analyses, we used a 1-way ANOVA to compare all mutants with wt, wt-ouabain, and untransfected cells, using the Tukey posttest for α-error correction.
Results
Phenotype Description
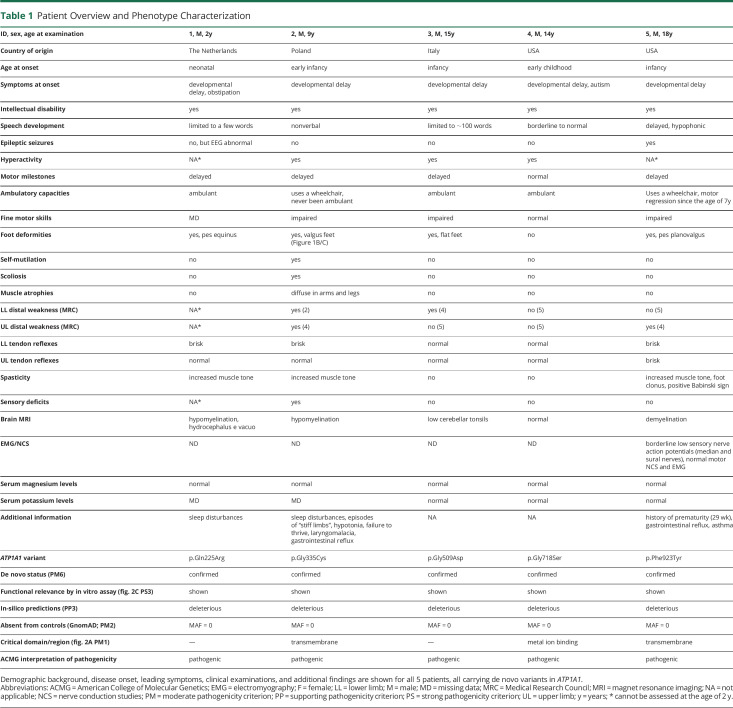
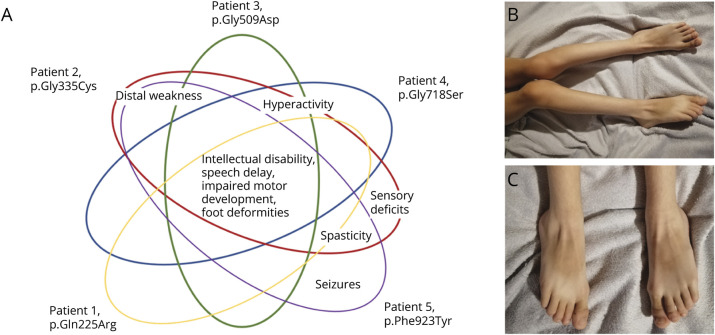
All 5 children were male and of Caucasian origin. First symptoms were observed in early childhood or even neonatally (Table 1). At the time of examination, the patients were 2–18 years old. A variable degree of intellectual disability was present in all patients (Figure 1A). Speech development ranged from absent to borderline normal. Motor milestones were delayed in 4 of 5 children. At current age, spasticity and distal muscle weakness were the leading motor symptoms (3/4) and foot deformities occurred in all examined patients (4/4). Deep tendon reflexes were brisk in 3 of 4 cases. Additional sensory deficits were reported in one patient (patient 2), who had a tendency to self-mutilation and distal muscle atrophies (Figure 1B, C) as well. This and one other patient (patient 5) were using a wheelchair by the time of examination. One patient (patient 5) had epileptic seizures. Brain MRIs had shown signs of hypomyelination or demyelination in 3 of 5 patients, and low cerebellar tonsils were reported in one patient (patient 3). Serum magnesium and potassium levels were normal in all examined patients. Additional findings were sleep disturbances and gastrointestinal reflux in 2 patients.
Table 1.
Patient Overview and Phenotype Characterization
Figure 1. Clinical Phenotypes Associated With Novel ATP1A1 Variants.
(A) Graphic depiction of distinct and overlapping phenotypes in 5 patients. (B) Diffuse muscle atrophies, increased muscle tone, and pes valgus in patient 2. (C) Pes planovalgus in patient 2, aggravated by neuropathy.
Molecular Genetics
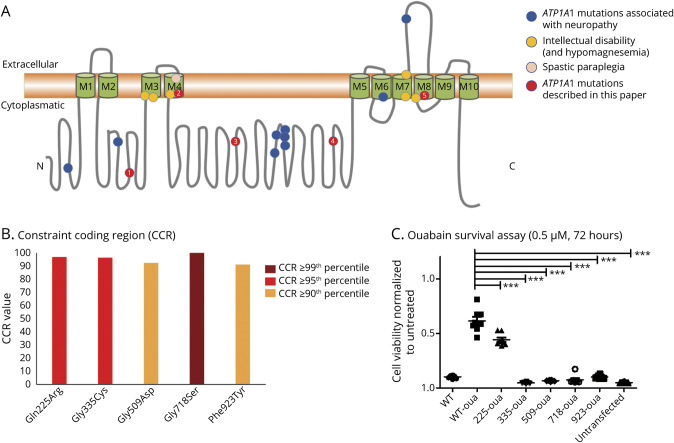
We identified 5 de novo missense variants in ATP1A1 (ENST00000295598.10; NM_000701.8; Figure 2A) that are all absent from control populations (GnomAD): c.674A>G;p.Gln225Arg, c.1003G>T;p.Gly335Cys, c.1526G>A;p.Gly509Asp, c.2152G>A;p.Gly718Ser, and c.2768T>A;p.Phe923Tyr (Table 1). All variants affect conserved (GERP scores: 4.82–5.33) and constrained amino acid positions (Figure 2B). The variants p.Gly335Cys and p.Phe923Tyr are located within transmembrane domains, whereas p.Gln225Arg, p.Gly509Asp, and p.Gly718Ser lie in intracellular loops, the latter affecting the metal binding site (Figure 2A). In silico pathogenicity predictions (CADD scores: 23.9–29.9, median: 29.0) were deleterious for all exchanges.
Figure 2. Functional Characteristics of Novel ATP1A1 Variants.
((A) Schematic depiction of the ATP1A1 protein showing the localization of previously reported and novel variants, sorted by phenotypes. Blue: ATP1A1 mutations associated with neuropathy: Leu48Arg, Ser207Phe, Ile592Thr, Ala597Thr, Pro600Ala, Pro600Thr, Asp601Phe, Asp811Ala, Gly877Ser. Yellow: ATP1A1 mutations associated with intellectual disability (and renal hypomagnesemia): Leu302Arg, Gly303Arg, Pro333Arg, Met859Arg, Gly864Arg, Asp933Asn. Rose: ATP1A1 mutation associated with spastic paraplegia: Leu337Pro. Red: Novel ATP1A1 mutations described in this article: Gln225Arg (patient 1), Gly335Cys (patient 2), Gly509Asp (patient 3), Gly718Ser (patient 4), Phe923Tyr (patient 5). (B) ATP1A1 variants are mapped to highly constrained coding regions (CCRs) of the gene.6 High constraint means that the disruption of this region would most likely cause haploinsufficiency. (C) Luminescence-based ouabain survival assay in HEK cells transfected with ouabain-insensitive ATP1A1 plasmids for all 5 mutants and wildtype (oua-wt), as well as wt-ATP1A1 (not ouabain-insensitive) in comparison with untransfected cells. After ouabain (0.5uM) treatment for 72h, cell viability is shown as a ratio with untreated cells (mean) transfected with the same plasmid. N, 8 Statistics: One-way ANOVA, α-error correction: Tukey posttest.
Functional Assessment
Compared with the ouabain-insensitive wt, cell viability was significantly reduced for all variants (Figure 2C). In the presence of the pathogenic mutant, the otherwise resistant plasmid led to cell death when treated with the unselective ATPase inhibitor ouabain, demonstrating the expected loss of function. To show that cell death was not an effect of transfection, we normalized each treated mutant to untreated cells transfected with the same plasmid.
Discussion
In this work, we describe a complex but consistent phenotype (Figure 1A) not adhering to distinct disease patterns such as neuropathy, spastic paraplegia, or intellectual disability that have all been described in association with pathogenic ATP1A1 variants before,1-5 (eTable 1, links.lww.com/WNL/B742) but combining those and other clinical features in a broader spectrum. In a linear protein model (Figure 2A), the variant positions do not correlate with the distinct subphenotypes. Why a certain variant causes one specific clinical syndrome and what determines disease severity is therefore not clear today. Although it is often challenging to determine pathogenicity of single variants in large proteins, the availability of a specific bioassay allowed us to show haploinsufficiency of each variant. All herein reported missense variants occurred de novo, and other genetic causes more likely to be causative were ruled out by whole exome or genome sequencing.
We conclude that the ATP1A1-associated disease spectrum is broader than previously thought. Our results show each variant's pathogenicity and expand the genotype–phenotype spectrum.
Acknowledgment
We thank the patients and their parents for participating in this study. We also acknowledge the iHope program that provided whole genome sequencing for patient 4.
Appendix. Authors
Study Funding
This project has been funded by NIH (R01NS105755). MD has received funding by the German Research Foundation German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, DO 2386/1-1).
Disclosure
A. Malhotra and R. J. Taft are full-time employees at Illumina, Inc. The remaining authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Lassuthova P, Rebelo AP, Ravenscroft G, et al. . Mutations in ATP1A1 cause dominant Charcot-Marie-Tooth Type 2. Am J Hum Genet. 2018;102(3):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Guo L, Lin S, et al. . ATP1A1 mutations cause intermediate Charcot-Marie-Tooth disease. Hum Mutat. 2019;40(12):2334-2343. [DOI] [PubMed] [Google Scholar]
- 3.Stregapede F, Travaglini L, Rebelo A, et al. . Hereditary spastic paraplegia is a novel phenotype for germline de novo ATP1A1 mutation. Clin Genet. 2020;97(3):521. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z, Li J, Ji T, Wu Y, Gao K, Jiang Y. ATP1A1 de novo mutation-related disorders: clinical and genetic features. Front Pediatr. 2021;9:657256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlingmann KP, Bandulik S, Mammen C, et al. . Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet. 2018;103(5):808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havrilla JM, Pedersen BS, Layer RM, Quinlan AR. A map of constrained coding regions in the human genome. Nat Genet. 2019;51(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]