Abstract
Background:
“Best Case/Worst Case” (BC/WC) is a communication tool to support shared decision making in older adults with surgical illness. We aimed to adapt and test BC/WC for use with critically ill older adult trauma patients.
Methods:
We conducted focus groups with 48 trauma clinicians in Wisconsin, Texas, and Oregon. We used qualitative content analysis to characterize feedback and adapted the tool to fit this setting. Using rapid sequence iterative design, we developed an implementation tool kit. We pilot tested this intervention at two trauma centers using a pre-post study design with older trauma patients in the ICU. Main outcome measures included study feasibility, intervention acceptability, quality of communication, and clinician moral distress.
Results:
BC/WC for trauma patients uses a graphic aid to document major events over time, illustrate plausible scenarios, and convey uncertainty. We enrolled 86 of 116 eligible patients and their surrogates (48 pre/38 post intervention). The median patient age was 72 (51-95) and mean Geriatric Trauma Outcome Score was 126.1 (±30.6). We trained 43 trauma attendings and trafellows to use the intervention. 94% could perform essential tool elements after training. The median end-of-life communication score (scale 0 – 10) improved from 4.5 to 6.6 (p = 0.006) after intervention as reported by family and from 4.1 to 6.0 (p = 0.03) as reported by nurses. Moral distress did not change. However, there was improvement (less distress) reported by physicians regarding “Witnessing providers giving false hope” from 7.34 to 5.03 (p = 0.022). Surgeons reported the tool put multiple clinicians on the same page and was useful for families, but tedious to incorporate into rounds.
Conclusion:
BC/WC trauma ICU is acceptable to clinicians and may support improved communication in the ICU. Future efficacy testing is threatened by enrollment challenges for severely injured older adults and their family members.
Level of Evidence:
Level III, therapeutic
Keywords: communication, older adults, prognosis
BACKGROUND
Each year nearly 500,000 older adults are admitted to the hospital with traumatic injury,1, 2 now the 5th leading cause of death for older Americans. For many severely injured older adults, traumatic injury is a terminal event leading to 20% in-hospital and 40% one-year mortality.3 The burdens of trauma care include invasive surgical procedures and intensive care unit (ICU) admission—interventions that older Americans typically prefer to avoid.4-6 Although trauma surgeons frequently communicate bad news and discuss prognosis with family members, they still encounter conflict among clinical team members and with patients and families during treatment discussions, particularly related to prognosis and end-of-life care.7, 8 These conflicts adversely affect patient safety, prolong ICU length of stay, and lead to moral distress for clinicians. Given the high treatment burdens and frequency of poor prognosis, severely injured older adults might benefit from communication interventions that clarify patients’ goals, reduce unwanted invasive procedures, and mitigate ICU conflict.
Surgeons are taught to communicate by quantifying discrete complications, mortality statistics and disclosing risk for specific procedures. This standard does not provide a framework for translating the complexity of traumatic injury into an accessible narrative about the consequences of treatment decisions—a narrative to which families and patients could associate their personal values and preferences.9 Scenario planning is a strategy to support decision making in the setting of uncertainty, which is used widely outside of healthcare by businesses and military planners. By appealing to deeply held concerns of decision makers, a well-constructed scenario can encourage people to comprehend a new, previously unimaginable reality and prepare for major shifts in a way simple forecasting, e.g., 20% mortality, cannot.10-12
Using feedback from older adults and surgeons, our team developed and tested a novel communication tool for acute care surgery called Best Case/Worst Case13 that uses scenario planning to illustrate treatment options, acknowledge uncertainty, and convey a clear message about the patient’s overall prognosis. Although we demonstrated improvements in patient engagement in our initial study,14 it was not possible to measure and compare values-aligned outcomes in this cohort because of broad prognostic uncertainty and heterogeneous care goals.
With the availability of robust prognostic calculators in geriatric trauma surgery,15 we could target a more clinically homogeneous group of patients with worse prognosis and greater need for concurrent palliative care.16-18 As the trauma setting differs from acute care surgery with multi-disciplinary teams caring for patients and conversations that occur in the ICU after the patient has received surgery and other treatments for stabilization, the objective of this study was to both adapt and test the original Best Case/Worst Case tool for older adults with traumatic injury and their families.
METHODS
We performed this study at the University of Wisconsin (UW), Oregon Health & Science University (OHSU) and the University of Texas, Southwestern (UT). The pre/post interventional study was performed at OHSU and Parkland Memorial (PMH) hospitals from July 2017 to March 2020. UW served as the IRB of record for all three study sites and approved this study, which was funded by the National Institutes for Aging, 1R21AG055876-01 and registered at ClinicalTrials.gov NCT03188055.
Intervention Development
We conducted six focus groups with 48 trauma clinicians (Supplemental Digital Content [SDC] 1). Via email, we invited all clinicians based on their clinical role in trauma care to participate and purposefully selected respondents to maximize group diversity based on gender, role in patient care, and years of experience. Each participant received $200 to compensate for their time. The first round (three focus groups, one per site) included attending trauma surgeons, neurosurgical consultants, and trauma fellows at each site. A non-clinical member (J.L.T.) of the research team with extensive training in both stakeholder engagement and clinician communication education moderated all six focus groups. She first showed a 10-minute video demonstrating how the Best Case/Worst Case tool could be used for a decision-making conversation between a trauma surgeon and family members of an older adult admitted to the ICU after a motor vehicle collision at highway speeds.19 Subsequent focus group questions focused on when to use the tool, how clinicians might integrate mortality estimates from the Geriatric Trauma Outcomes Score (GTOS)15 within the framework of the tool and how the tool might be used when an acute decision about operative intervention was not needed. (SDC 2)
Using qualitative content analysis of the focus group transcripts, we identified three primary differentiators between trauma ICU care and acute care surgery: 1) trauma care requires input and coordination of multiple specialists, 2) prognosis is likely to evolve over the patient’s hospital course, and 3) discrete decisions about surgical intervention are not needed for many patients. With this understanding, we conceptualized a communication tool that can be used daily during rounds, is not time consuming, and is available to multiple team members and consultants. (Figure 1) Three members of our team observed trauma ICU rounds and used a process of rapid cycle iteration and live testing to integrate scenario planning and completion of the novel Best Case/Worst Case graphic aid within the fast pace of ICU rounds.20
Figure 1. Graphic aid for the Best Case/Worst Case trauma ICU intervention.
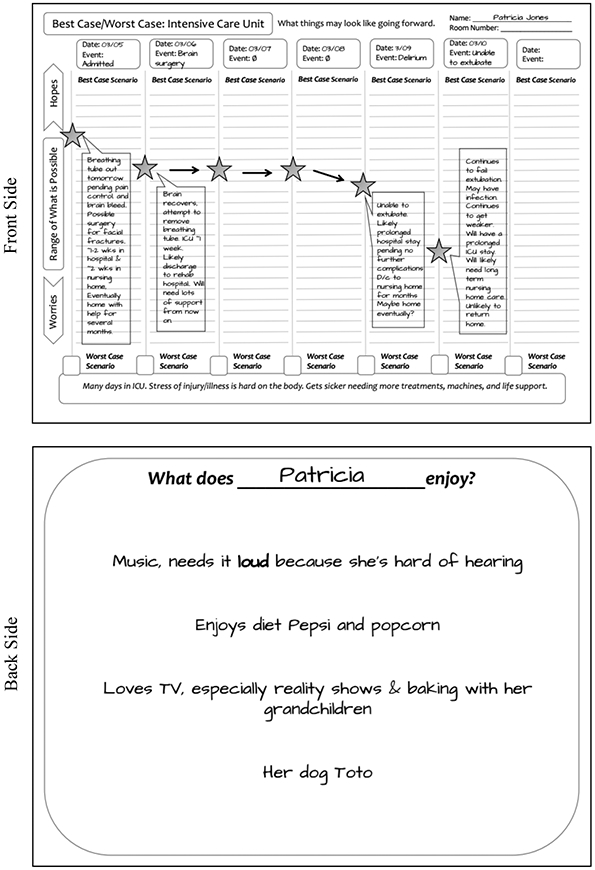
Patricia Jones is a 73-year-old who presents as a Level I trauma following a fall down a flight of stairs. Injuries notable for bilateral rib fractures, multiple facial fractures and an intracranial bleed. She was intubated in the trauma bay due to poor oxygenation and worsening GCS and admitted to the ICU. Overnight, Patricia’s neurological examination worsens, and a repeat head CT shows significant midline shift. She is taken to the OR with neurosurgery for craniotomy and hematoma evacuation.
We conducted a second round of focus groups and included trauma ICU nurses, advanced practice providers (APPs), nurse navigators, case managers, and chaplains to understand how the trauma team might integrate the tool within their practice. We showed a revised video demonstrating use of the tool on daily ICU rounds.21 Focus group questions sought feedback regarding this novel version of the Best Case/Worst Case tool and how different trauma team members might use the graphic aid to coordinate care and communicate with patients and families. (SDC 3)
Intervention Testing
We conducted a pre-post pilot study at OSHU and PMH to test the feasibility of study procedures, the acceptability of the intervention for clinicians, patients, and families and to understand how the intervention might impact outcomes for a future study, specifically quality of communication, clinician moral distress, intensity of treatment, and access to palliative care. We did not randomize the intervention at the patient or clinician level. Instead, we used a pre/post study design due to concerns about contamination of the control group after intervention training for trauma team members within the same institution.
Participants
We aimed to enroll 100 patients (50 per treatment group) age 65 and older with GTOS score of 110 or more and their family members. Due to significant enrollment challenges we expanded the criteria to include patients age 50 and older and admission to the ICU after traumatic injury. Patients who did not have decision-making capacity were eligible to participate if their family member serving as their formally or informally designated surrogate spoke English and did not have severe hearing or visual impairment. We obtained written informed consent for patient and family participants who received up to $35 for completion of study procedures.
We invited all trauma attendings, fellows and APPs at both sites to participate in intervention training and nurses practicing in the trauma ICU to complete study surveys. Clinicians who participated in intervention training received $245 and incentives worth $5 for survey completion.
Intervention delivery
We adapted the training program used successfully with acute care surgeons based on adult education theory.22 Training included expert demonstration followed by three increasingly complex exercises led by expert coaches, wherein learners use the tool in clinical scenarios with coaches playing team members and other study staff playing family members. To evaluate intervention fidelity, instructors completed a post-training evaluation scoring the trainee on essential tool elements, while trainees completed a similar self-assessment. (SDC 4) We distributed a brochure explaining the graphic aid and information about the communication tool, including QR codes linked to video demonstrations (SDC 5) for all other ICU clinicians, specifically, bedside nurses, social workers, and case managers.
Data collection
We administered the Quality of Communication (QOC)23 survey to family members and bedside nurses of study-enrolled patients within 48 – 96 hours of ICU admission. We administered the Family Inpatient Communication Survey (FICS), a measure of communication with surrogates about patient care, and the Goal Concordant Care (GCC) Survey to family members at 10 days after admission. We administered the Trauma Quality of Life (TQoL) survey to patients or to family members for patients without decision making capacity 30 days after injury. To reduce missing data, surveys were administered in person, via telephone, and electronically. We administered the After-Death Bereaved Family Member Interview (ADBFMI) survey to family members of decedents at 30 days. We administered the Moral Distress Survey in person, without collecting identifiers, at the start of the study and upon study completion to clinicians present on the unit at multiple time points. We reviewed electronic health records (EHR) to determine the patient’s GTOS, intensity of treatment received (intubation and mechanical ventilation, tracheostomy, feeding tube insertion, hemodialysis, enteral or parenteral nutrition, and cardiopulmonary resuscitation) and receipt of palliative care. On study completion, we solicited surgeons for feedback about the communication tool.
Data analysis
Based on prior studies, we estimated the standard deviation for the QOC measurement to be around 3.5.24, 25 We estimated 84% power to detect a medium-large effect of 2.00 points on the QOC scale, and 96% power to detect a large effect of 2.50 points. We performed intention-to-treat analysis with 2-sided tests for significance. For continuous outcome variables we used a two-sample t-test and for categorical outcomes we used Fisher’s Exact Test to compare groups. All analyses were conducted at the 0.05 significance level using the R statistical software, version 3.5.2 (R Foundation for Statistical Computing).
RESULTS
Over 28 months we screened 1,970 patients, identified 116 eligible patients and enrolled 86 patients, 38 in intervention and 48 in usual care control. (Figure 2) Demographic characteristics did not differ between groups. (Table 1) Family members were on average 10 years younger than patients, and 80% were female (SDC 6). We trained 38 trauma surgeons and fellows, and 7 APPs to use the Best Case/Worst Case trauma ICU tool. All achieved competency, although learners and instructors reported challenges using the GTOS to generate a plausible story about prognosis. (Figure 3)
Figure 2: Study CONSORT diagram.
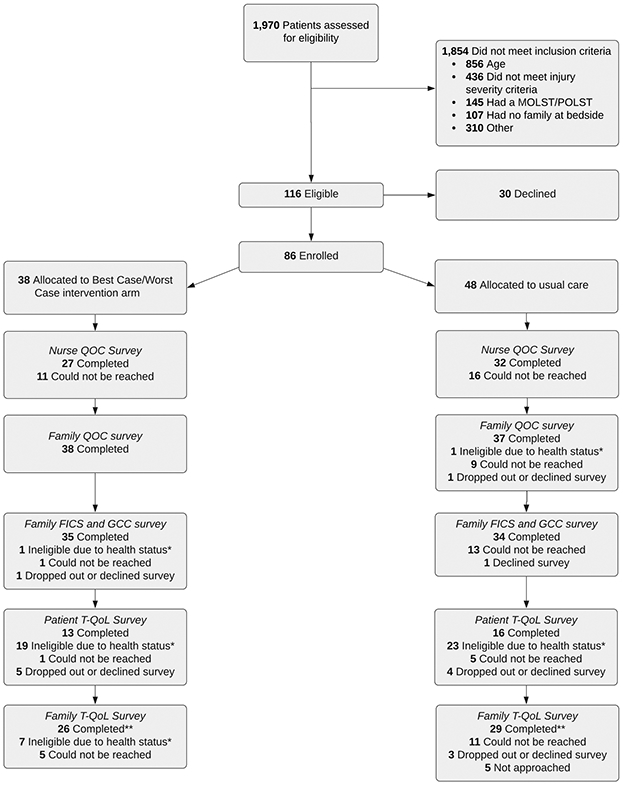
*Ineligible due to health status includes participants who did not have decision making capacity, had died or were incapacitated
**3 family members completed the After Death Bereaved Family Member Interview instead of the T-QoL survey
Table 1.
Patient Characteristics
| Intervention (n=38) |
Control (n=48) |
|
|---|---|---|
| Age in years: mean (sd) | 73 (11) | 74 (7) |
| Male gender: n (%) | 25 (66) | 32 (67) |
| Had decision making capacity at enrollment | 12 (32) | 22 (46) |
| Race/ethnicity (self-reported): n (%) | ||
| White or Caucasian | 34 (90) | 37 (77) |
| Black or African American | 1 (3) | 3 (6) |
| Asian | 1 (3) | 2 (4) |
| American Indian or Alaska Native | 0 (0) | 2 (4) |
| Unknown or No response | 2 (5) | 4 (8) |
| Hispanic, Latino, or Spanish origin: n (%) | 3 (8) | 3 (6) |
| Educational attainment: n (%) | ||
| Some High School or Less | 3 (8) | 3 (9) |
| High School Diploma or GED | 10 (27) | 9 (26) |
| Vocational Degree or Some College | 12 (32) | 8 (23) |
| College Degree | 7 (19) | 6 (17) |
| Graduate School Degree or Higher | 5 (14) | 9 (26) |
| Indication or reason for admission/mechanism of injury: n (%) | ||
| Motor vehicle or motorcycle collision | 12 (32) | 17 (35) |
| Gunshot would (GSW) | 0 (0) | 1 (2) |
| Fall from standing/sitting | 13 (34) | 19 (40) |
| Fall from height | 10 (26) | 7 (15) |
| Other | 3 (8) | 4 (8) |
| Comorbidities: n (%) | ||
| 0 | 4 (11) | 3 (6) |
| 1 | 12 (32) | 18 (38) |
| 2+ | 22 (58) | 27 (56) |
| GTOS: mean (sd) | ||
| GTOS Score | 129.6 (36) | 124 (28) |
| Probability of dying during index admission (%) | 17.8 (18) | 16.2 (16) |
| Probability of discharge to a nursing home, long-term acute care, or hospice (%) | 26.2 (16) | 23.3 (12) |
| Complete days spent in the ICU: mean (sd) | 4.9 (4) | 3.9 (3) |
| Patient death: n (%) | 6 (16) | 7 (15) |
Figure 3.
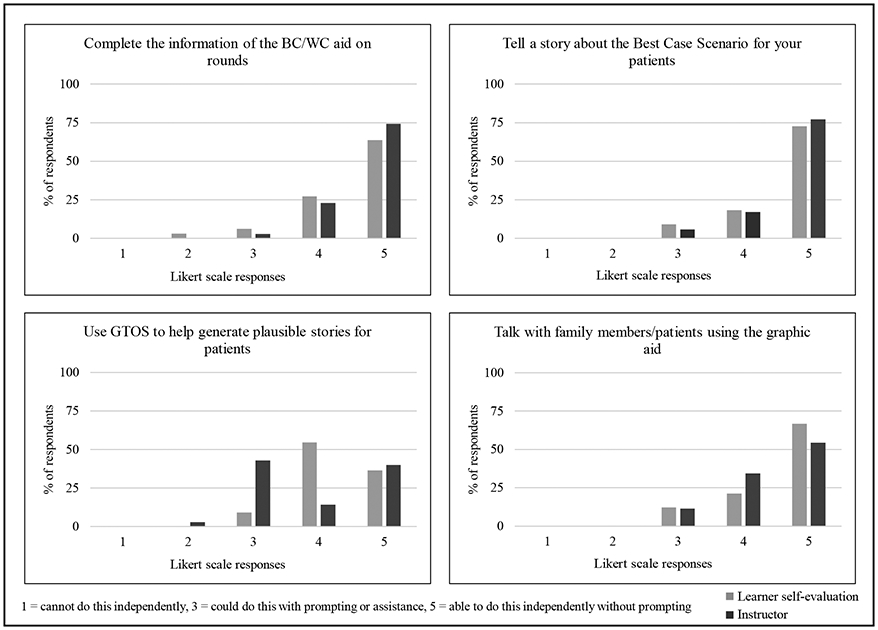
Selected post-training assessment questions as reported by instructors and learners
Quality of Communication
General communication scores were high (scale 0 – 10, 10 connotes better communication) and there was no significant difference between intervention and control for family or nurse reported general communication scores: family-reported mean 8.5 vs. 8.3, p = 0.709, nurse-reported mean 7.7 vs. 6.7, p = 0.063 (intervention vs. control). However, end-of-life communication scores were significantly higher as reported by family and nurses: family-reported mean 6.6 vs. 4.5, p = 0.006, nurse-reported mean 6 vs. 4.1, p = 0.03 (intervention vs. control). (Figure 4) This subscale includes questions such as, “How good is your loved one’s doctor at talking with you about when or how your family member might get sicker?” in contrast to general communication questions, which ask, “How good is your family member or friend’s doctor or nurse practitioner at using words you can understand?”
Figure 4.
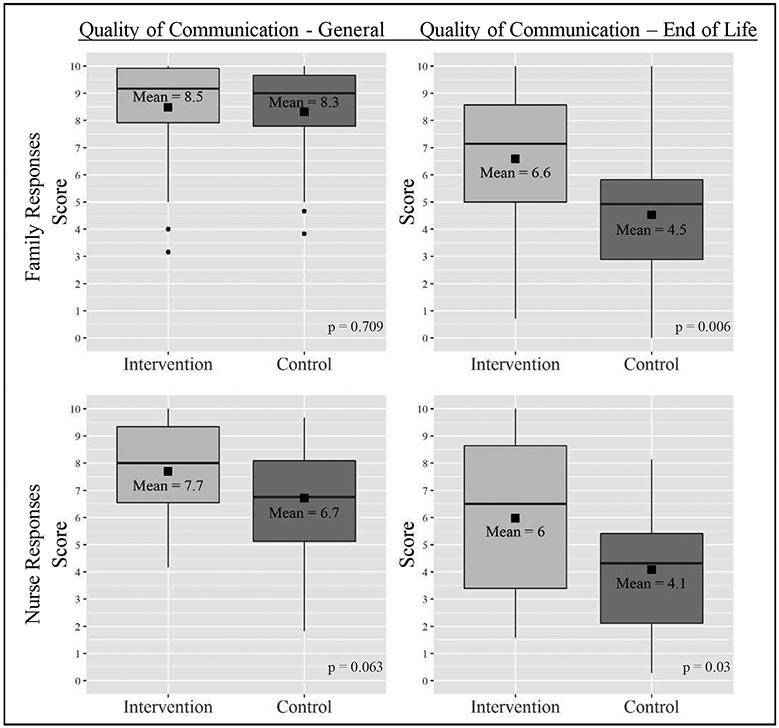
Quality of Communication as reported by family members and nurses caring for study patients (higher scores = better communication)
Moral distress
Ninety trauma ICU clinicians completed the MDS survey at the start of the study and 122 completed the survey at the end of the study with an estimated response rate for those present on the unit of 79% and 94% respectively. In this 21-item survey, each question measures both the level of distress (0 - 4) and the frequency of disturbance (0 −4) for each item, and the score is calculated by multiplying the response to both. Thus, each question will have a score from 0 – 16, a score of 4 or more for one item is considered moderately high.26, 27 For summary scores, moral distress was high with wide variation of scores and did not differ between study groups: mean score all respondents 73.4 ±39.3 upon study completion as compared to 71.7 ±42.5 at study commencement. Consistent with the moral distress literature, MDS was higher in nurses 82.6 ±40.9 than in physicians 51.4 ±24.0 upon study completion but was not different within groups based on intervention assignment. On exploratory analysis, evaluating questions specifically related to the intervention (questions 2, 3, 4, 7, 13 and 18), we found a statistically significant difference, consistent with less distress, in the physician responses to question 2 “Witness providers giving false hope” mean score 5.0 ±2.9 upon study completion as compared to 7.3 ±5.2 p = 0.022 at study commencement. (Table 2)
Table 2.
Moral Distress as Reported by Physicians and Nurses
| Post-intervention mean (sd) |
Pre-intervention mean (sd) |
p-value | |
|---|---|---|---|
| Physician Responses | (n=36) | (n=38) | |
| Composite score | 51 (24) | 61 (32) | 0.15 |
| Q2: Witness healthcare providers giving “false hope” to the patient or family | 5 (3) | 7.3 (5) | 0.022* |
| Q3: Follow the family’s wishes to continue life support even though I believe it is not in the best interest of the patient. | 5.8 (4) | 6 (5) | 0.889 |
| Q4: Initiate extensive life-saving actions when I think they only prolong death. | 4.5 (4) | 4.7 (4) | 0.845 |
| Q7: Continue to participate in care for a hopelessly ill person who is being sustained on a ventilator, when no one will make a decision to withdraw support. | 4.4 (3) | 5.6 (5) | 0.256 |
| Q13: Request nurses or others not to discuss the patient’s prognosis with the patient or family. | 0.6 (1) | 0.6 (1) | 0.984 |
| Q18: Witness diminished patient care quality due to poor team communication. | 5 (4) | 6 (4) | 0.188 |
| Nurse Responses | (n=86) | (n=52) | |
| Composite Score | 82.6 (40) | 79.5 (47) | 0.698 |
| Q2: Witness healthcare providers giving “false hope” to a patient or family. | 7 (5) | 6.6 (5) | 0.655 |
| Q3: Follow the family’s wishes to continue life support even though I believe it is not in the best interest of the patient. | 8.3 (5) | 7.1 (5) | 0.162 |
| Q4: Initiate extensive life-saving actions when I think they only prolong death. | 7.3 (5) | 6.3 (5) | 0.223 |
| Q7: Continue to participate in care for a hopelessly ill person who is being sustained on a ventilator, when no one will make a decision to withdraw support. | 7.3 (5) | 5.9 (5) | 0.11 |
| Q13: Follow the physician’s request not to discuss the patient’s prognosis with the patient or family. | 1.8 (3) | 2.5 (4) | 0.281 |
| Q18: Witness diminished patient care quality due to poor team communication. | 5 (4) | 5.6 (4) | 0.496 |
Q = question, higher scores signify more distress,
denotes statistical significance
Other measures and missingness
There was no difference in communication about care as reported by family members: mean FICS 114.5 ±23 vs. 113.8 ±20.9, intervention vs. control. There were high rates of missingness for measures of goal concordant care and TQoL making it difficult to evaluate the effect of the intervention on these outcomes. (SDC 7, 8, 9) We administered three ADBFMI surveys to family members of three decedents, which caused enough distress for research staff interviewing bereaved family members that we dropped this survey from this study. We found no evidence that patients had received palliative care in the EHR and there was no difference in the intensity of treatment received between groups. (SDC 10)
Surgeon reflections
We interviewed 12 of the 38 trauma surgeons, fellows and APPs trained to use the tool. Clinicians were generally supportive of the tool as a strategy to communicate with families. They noted it helped to keep team members and different clinical specialties on the same page, frame family meetings, and to support families who decided to pursue comfort-focused care. For example, “Right, yeah. Initially I found the whole thing really tedious, but I think you know, when I saw some of its benefit with patient’s family, then it was- made it easier to use it every day.” Some continued using it with non-study patients. Respondents reported families appreciated the tool, but also noted incorporating the tool into their routine on rounds could be tedious. As one surgeon noted, “It might not be the best tool to use for every patient for every provider, but I think that it was an important perspective to gain on how we can talk to families” Nearly all said they did not incorporate data about mortality or discharge disposition available with the GTOS calculator into their use of the tool.
DISCUSSION
With stakeholder input and a rapid iterative design strategy, we adapted the Best Case/Worst Case communication tool to the trauma setting. Instead of constructing a discrete treatment decision, this novel intervention uses scenario planning and a daily acknowledgement of the interplay between major events and prognosis to illustrate a range of outcomes and how patients might experience treatment along the way. By using a graphic aid to illustrate “what we are hoping for,” “what we are worried about,” and the evolution of the story over time, including setbacks and improvements, the tool aims to keep everyone on the same page and facilitate dialogue about treatments and outcomes with older adult trauma patients, their families, and the trauma ICU team.
Trauma surgeons and team members were generally supportive of this novel intervention, although they registered concerns about daily use. We also found sizable improvements in the quality of end-of-life communication as reported by family members and nurses and possibly a reduction in providers giving false hope. These outcomes should be interpreted with caution given the preliminary nature of this study and the simple pre/post study design. We also found particularly refractory challenges with enrollment, even after substantially expanding eligibility criteria. More than 25% of eligible participants declined, and we could not locate or reach family surrogates for nearly half of those who were otherwise eligible. After more than two (pre-pandemic) years attempting to fill this study, we stopped with 15% less than our planned enrollment. While these challenges are not uncommon for trauma-focused researchers, they pose a significant barrier for expanding the knowledge base in trauma care. This study has important implications for patients and families, surgeons, and investigators.
For patients and families, using a daily narrative about treatment and prognosis to describe plausible outcomes may provide context for critical downstream decisions. Studies of intensive care show that diagnoses and interventions build over time. With each new diagnosis corresponding treatments are added. Boss et al15 note that intensivists initially view each failing organ as a problem to be fixed, while families see their loved one as a whole person, not discrete organ systems. Over time, families and clinicians switch. As therapeutic options are exhausted the clinical team sees the patient as dying, yet families have adopted the fragmented view and see each new problem as treatable. This building clinical momentum in the ICU is well described.28-30 Multiple small treatment decisions are made without considering how each discrete intervention aligns with patient preferences until major events, like failure to liberate from a ventilator or imminent death, prompt discussion of goals of care.31, 32 Interventions that orient patients, families, and clinicians to the patient’s overall trajectory, rather than each isolated problem to be fixed, may help disrupt clinical momentum and support earlier identification of patients for whom such interventions are inconsistent with their goals.
For surgeons, trauma team members, and ICU clinicians, there is tension between needing to efficiently care for multiple critically ill people simultaneously and providing high quality communication. Although the pressure to manage multiple competing interests cannot be overstated, this is not a zero-sum game. Providing treatments that are inconsistent with patients’ goals, prolongation of the dying process, ICU conflict among clinicians about futility, and extended family meetings all contribute to increased workload and decreased wellbeing of the clinical workforce.33-38 Although our intervention is designed to take less than 3 minutes upon completion of the systems review on rounds, (by describing the major event of the previous day and the patient’s “outlook” in the best-case scenario,20 see: https://youtu.be/31pv2Rlp6R4 ) consistent daily use of the tool will be difficult to achieve unless clinicians understand how it helps them.
It is curious that trauma team members trained to use the Best Case/Worst Case tool did not incorporate the predictive information from the GTOS during training or in practice. The GTOS is easy to use and, during the study, the GTOS calculator was readily available online. We suspect this reflects a larger misconception about prognostic tools and clinician communication, as much of the surgical literature has focused on developing more accurate predictive algorithms and risk estimators, 39-42 yet there is little guidance about how they might be used in practice. While it is not surprising that surgeons focus on risk and mortality statistics, the interface between this type of clinician facing information and translation of these numbers clinically is remarkably unexplored and underdeveloped.43, 44
For researchers, difficulty enrolling patients in studies of trauma care is a serious problem for future patients when the evidence base is compromised by the context of providing care in this setting. Emergent and urgent treatment, underlying social conditions leading to trauma, and the prevalence of cognitive dysfunction in this patient population make interventional studies extremely difficult. Innovative strategies to support healthcare delivery research for trauma patients include waiver of consent, use of passive data collection from quality registries and voluntary participant assent for survey completion (in contrast to obtaining written informed consent), combined with infrastructure for collaboration like the Coalition for National Trauma Research (CNTR) will be essential to support future high-impact clinical trials.
This study has both strengths and limitations. While we are delighted that this intervention appears to improve end-of-life communication and possibly reduce some clinician moral distress, this is a preliminary study designed to test the feasibility of study procedures and acceptability of the intervention. As such, the suggestion of benefit needs further study. We are currently designing a larger effectiveness study for a multisite randomized clinical trial with a stepped-wedge design (randomization by study site) and supported by CNTR. In addition, the Best Case/Worst Case trauma ICU intervention was developed by a broad range of trauma care clinicians at multiple sites in the United States, but unique attention to local norms and clinical practices will be needed to implement it sustainably at other centers. Although we designed and tested this intervention for older adults with traumatic injury given the vulnerability of this cohort, the tool can also be used for younger patients. A stronger implementation strategy might be consistent use of scenario planning and the graphic aid for all patients in the trauma ICU regardless of age.
CONCLUSION
The Best Case/Worst Case trauma ICU intervention can be implemented on daily rounds to improve the quality of communication with patients and families and keep trauma team members on the same page about the patient’s overall healthcare trajectory. This preliminary study has promising results, but future study is needed to evaluate the effectiveness of this intervention on quality of communication, clinician moral distress and appropriate utilization of healthcare resources for patients with serious traumatic illness.
Supplementary Material
Table: Characteristics of Focus Group Respondents. pdf
Document: Interview Guide for Focus Groups Round 1. pdf
Document: Interview Guide for Focus Groups Round 2. pdf
Table: Assessment questions for instructors and learners for Best Case/Worst Case trauma ICU training. pdf
Figure: Brochure Describing the Best Case/Worst Case Trauma ICU Intervention, Provided to Trauma Team Members. pdf
Table: Family Participant Characteristics. pdf
Table: Receipt of Goal Concordant Care as Reported by Family Respondents. pdf
Table: Trauma Quality of Life Survey Missingness. pdf
Figure: Trauma Quality of Life Scores as Reported by Patients and Family Members. pdf
Table: Life Supporting Treatments Received Between 0- and 10-Days From Admission. pdf
Funding Statement:
This project was supported by a NIH Exploratory/Developmental Research Grant Award R21AG055876, Schwarze-PI. Christopher Zimmermann is supported by a NIH 2T32HL110853-06 Training Grant.
Footnotes
Conflict of Interest Statement:
The authors have no relevant financial conflicts of interest to declare.
References:
- 1.Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54(10):1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Injury Prevention and Control. 10 Leading Causes of Nonfatal Injuries, United States, 2014, All Races, Both Sexes, Disposition: All Cases, Ages: 65-85 Available from: webappa.cdc.gov/sasweb/ncipc/nfilead2001.html.
- 3.Fleischman RJ, Adams AL, Hedges JR, Ma OJ, Mullins RJ, Newgard CD. The optimum follow-up period for assessing mortality outcomes in injured older adults. J Am Geriatr Soc. 2010;58(10):1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–6. [DOI] [PubMed] [Google Scholar]
- 5.Barnato AE, Herndon MB, Anthony DL, Gallagher PM, Skinner JS, Bynum JP, Fisher ES. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007;45(5):386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Approaching Death: Improving Care at the End of Life. Field MJ, Cassel CK, editors. Washington, DC: The National Academies Press; 1997. 456 p. [PubMed] [Google Scholar]
- 7.Mosenthal AC, Murphy PA. Trauma care and palliative care: time to integrate the two? J Am Coll Surg. 2003;197(3):509–16. [DOI] [PubMed] [Google Scholar]
- 8.Cocanour CS. End-of-life care in trauma. J Trauma Acute Care Surg. 2015;79(6):891–6. [DOI] [PubMed] [Google Scholar]
- 9.King JS, Moulton BW. Rethinking informed consent: the case for shared medical decision-making. Am J Law Med. 2006;32(4):429–501. [DOI] [PubMed] [Google Scholar]
- 10.Wack P. Uncharted waters ahead. Harvard Business Review. 1985. [Google Scholar]
- 11.Wack P. Shooting the rapids. Harvard Business Review. 1985. [Google Scholar]
- 12.Schwartz P. The Art of the Long View: Planning for the Future in an Uncertain World: New York, New York: Doubleday/Currency; 1991. [Google Scholar]
- 13.Best Case/Worst Case (BC/WC) SURGEON Communication Tool - Whiteboard Video [Video]. YouTube: March 29, 2016. Available from: https://youtu.be/FnS3K44sbu0. [Google Scholar]
- 14.Taylor LJ, Nabozny MJ, Steffens NM, Tucholka JL, Brasel KJ, Johnson SK, Zelenski A, Rathouz PJ, Zhao Q, Kwekkeboom KL, et al. Best Case/Worst Case: A Framework to Improve Surgeon Communication in High-Stakes Surgical Decisions. JAMA Surg. 2017;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao FZ, Wolf SE, Nakonezny PA, Minhajuddin A, Rhodes RL, Paulk ME, Phelan HA. Estimating Geriatric Mortality after Injury Using Age, Injury Severity, and Performance of a Transfusion: The Geriatric Trauma Outcome Score. J Palliat Med. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosenthal AC, Weissman DE, Curtis JR, Hays RM, Lustbader DR, Mulkerin C, Puntillo KA, Ray DE, Bassett R, Boss RD, et al. Integrating palliative care in the surgical and trauma intensive care unit: a report from the Improving Palliative Care in the Intensive Care Unit (IPAL-ICU) Project Advisory Board and the Center to Advance Palliative Care. Crit Care Med. 2012;40(4):1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosenthal AC, Murphy PA, Barker LK, Lavery R, Retano A, Livingston DH. Changing the culture around end-of-life care in the trauma intensive care unit. J Trauma. 2008;64(6):1587–93. [DOI] [PubMed] [Google Scholar]
- 18.Mosenthal AC. Dying of Traumatic Brain Injury—Palliative Care too Soon, or too Late? JAMA Surg. 2020;155(8):731. [DOI] [PubMed] [Google Scholar]
- 19.Best Case/Worst Case (BC/WC) ICU Decision [Video]. YouTube: Oct 5, 2018. Available from: https://youtu.be/3Kz59dW0cak. [Google Scholar]
- 20.Best Case/Worst Case (BC/WC) INTENSIVE CARE Communication Tool - Whiteboard Video [Video]. YouTube: Oct 19, 2020. Available from: https://youtu.be/31pv2Rlp6R4. [Google Scholar]
- 21.Best Case/Worst Case (BC/WC) ICU [Video]. YouTube: Oct 5, 2018. Available from: https://youtu.be/93I18zvt4Xg. [Google Scholar]
- 22.Kolb DA. Experiential Learning: Experience as the Source of Learning and Development. 1st ed. Upper Saddle River, New Jersey Prentice-Hall; 1984. [Google Scholar]
- 23.Song MK, Sereika SM. An evaluation of the Decisional Conflict Scale for measuring the quality of end-of-life decision making. Patient Educ Couns. 2006;61(3):397–404. [DOI] [PubMed] [Google Scholar]
- 24.Engelberg R, Downey L, Curtis JR. Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care. J Palliat Med. 2006;9(5):1086–98. [DOI] [PubMed] [Google Scholar]
- 25.Curtis JR, Back AL, Ford DW, Downey L, Shannon SE, Doorenbos AZ, Kross EK, Reinke LF, Feemster LC, Edlund B. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: a randomized trial. JAMA. 2013;310(21):2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamric AB, Borchers CT, Epstein EG Development and Testing of an Instrument to Measure Moral Distress in Healthcare Professionals. AJOB Prim. Res 2012;3(2):8. [Google Scholar]
- 27.Corley MC, Elswick RK, Gorman M, Clor T. Development and evaluation of a moral distress scale. J Adv Nurs. 2001;33(2):250–6. [DOI] [PubMed] [Google Scholar]
- 28.Lynn J, Arkes HR, Stevens M, Cohn F, Koenig B, Fox E, Dawson NV, Phillips RS, Hamel MB, Tsevat J. Rethinking fundamental assumptions: SUPPORT's implications for future reform. Study to Understand Prognoses and Preferences and Risks of Treatment. J Am Geriatr Soc. 2000;48(5 Suppl):S214–21. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman SR, Shim JK, Russ AJ. Old age, life extension, and the character of medical choice. J Gerontol B Psychol Sci Soc Sci. 2006;61(4):S175–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman WA. Medical decisions: an appeal for reasonableness. Pediatrics. 1996;98(6 Pt 1):1182–4. [PubMed] [Google Scholar]
- 31.Kruser JM, Cox CE, Schwarze ML. Clinical Momentum in the Intensive Care Unit. A Latent Contributor to Unwanted Care. Ann Am Thorac Soc. 2017;14(3):426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruser JM, Benjamin BT, Gordon EJ, Michelson KN, Wunderink RG, Holl JL, Schwarze ML. Patient and Family Engagement During Treatment Decisions in an ICU: A Discourse Analysis of the Electronic Health Record. Crit Care Med. 2019;47(6):784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azoulay E, Timsit JF, Sprung CL, Soares M, Rusinova K, Lafabrie A, Abizanda R, Svantesson M, Rubulotta F, Ricou B, et al. Prevalence and factors of intensive care unit conflicts: the conflicus study. Am J Respir Crit Care Med. 2009;180(9):853–60. [DOI] [PubMed] [Google Scholar]
- 34.Studdert DM, Mello MM, Burns JP, Puopolo AL, Galper BZ, Truog RD, Brennan TA. Conflict in the care of patients with prolonged stay in the ICU: types, sources, and predictors. Intensive Care Med. 2003;29(9):1489–97. [DOI] [PubMed] [Google Scholar]
- 35.Danjoux Meth N, Lawless B, Hawryluck L. Conflicts in the ICU: perspectives of administrators and clinicians. Intensive Care Med. 2009;35(12):2068–77. [DOI] [PubMed] [Google Scholar]
- 36.Embriaco N, Papazian L, Kentish-Barnes N, Pochard F, Azoulay E. Burnout syndrome among critical care healthcare workers. Curr Opin Crit Care. 2007;13(5):482–8. [DOI] [PubMed] [Google Scholar]
- 37.Wujtewicz M, Wujtewicz MA, Owczuk R. Conflicts in the intensive care unit. Anaesthesiol Intensive Ther. 2015;47(4):360–2. [DOI] [PubMed] [Google Scholar]
- 38.Ervin JN, Kahn JM, Cohen TR, Weingart LR. Teamwork in the intensive care unit. Am Psychol. 2018;73(4):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesen BM, Bronsert MR, Aasen DM, Singh AB, Lambert-Kerzner A, Henderson WG, Hammermeister KE, Meguid RA. Use of Surgical Risk Preoperative Assessment System (SURPAS) and patient satisfaction during informed consent for surgery. J Am Coll Surg. 2020;230(6):1025–33. e1. [DOI] [PubMed] [Google Scholar]
- 40.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–42.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertsimas D, Dunn J, Velmahos GC, Kaafarani HM. Surgical risk is not linear: derivation and validation of a novel, user-friendly, and Machine-learning-based predictive optimal trees in emergency surgery risk (Potter) calculator. Ann Surg. 2018;268(4):574–83. [DOI] [PubMed] [Google Scholar]
- 42.Turner PL, Saager L, Dalton J, Abd-Elsayed A, Roberman D, Melara P, Kurz A, Turan A. A nomogram for predicting surgical complications in bariatric surgery patients. Obes Surg. 2011;21(5):655–62. [DOI] [PubMed] [Google Scholar]
- 43.Kopecky KE, Urbach D, Schwarze ML. Risk Calculators and Decision Aids Are Not Enough for Shared Decision-Making. JAMA Surg. 2018;In press. [DOI] [PubMed] [Google Scholar]
- 44.Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared decision making: the need for patient-clinician conversation, not just information. Health Affairs. 2016. Apr 1;35(4):627–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table: Characteristics of Focus Group Respondents. pdf
Document: Interview Guide for Focus Groups Round 1. pdf
Document: Interview Guide for Focus Groups Round 2. pdf
Table: Assessment questions for instructors and learners for Best Case/Worst Case trauma ICU training. pdf
Figure: Brochure Describing the Best Case/Worst Case Trauma ICU Intervention, Provided to Trauma Team Members. pdf
Table: Family Participant Characteristics. pdf
Table: Receipt of Goal Concordant Care as Reported by Family Respondents. pdf
Table: Trauma Quality of Life Survey Missingness. pdf
Figure: Trauma Quality of Life Scores as Reported by Patients and Family Members. pdf
Table: Life Supporting Treatments Received Between 0- and 10-Days From Admission. pdf


