Abstract
Legionella pneumophila (L. pneumophila) is one of the most threatening nosocomial pathogens. The implementation of novel and more effective surveillance and diagnostic strategies is mandatory to prevent the occurrence of legionellosis outbreaks in hospital environments. On these bases, the present review is aimed to describe the main clinical and molecular features of L. pneumophila focusing attention on the latest findings on drug resistance mechanisms. In addition, a detailed description of the current guidelines for the disinfection and surveillance of the water systems is also provided. Finally, the diagnostic strategies available for the detection of Legionella spp. were critically reviewed, paying the attention to the description of the culture, serological and molecular methods as well as on the novel high-sensitive nucleic acid amplification systems, such as droplet digital PCR.
Keywords: Legionella pneumophila, surveillance strategies, diagnostic strategies, antibiotic resistance, virulence factors
1. Introduction
Legionella species (spp.) are Gram-negative, aerobic, rod-shaped, non-spore-forming bacteria which are ubiquitous in water environments, such as lakes, rivers, hot springs, and ponds, as well as in composted materials and moist soil. The genus Legionella currently includes 66 species and more than 70 serotypes, half of them recognized as opportunistic pathogens for humans [1].
Legionella takes its name from an outbreak of pneumonia occurring in Philadelphia during a conference of U.S. military veterans in 1997. During this conference, 221 veterans of the American Legion contracted Legionella infection via water transmission. Of these, 34 veterans died highlighting for the first time the health problem represented by Legionella outbreaks [2]. A year later, Legionella pneumophilia (LP) was recognized as the etiologic agent of Legionnaires’ disease (LD) [3]. Currently, 15 different serogroups of LP were recognized of which serogroup 1 is the most clinically relevant accounting for 80–90% of Legionnaires’ disease cases, while other serogroups (2–15) are less common and occasionally cause legionellosis.
Other etiological agents of legionellosis are Legionella longbeachae, Legionella micdadei, Legionella bozemanii and Legionella dumoffii [4,5]. In particular, L. longbeachae is the causative agent of approximately 30% of community-acquired LD in New Zeeland and Australia [6], while L. micdadei represents the second most common cause of LD in the United States and Europe [7].
As widely known, Legionella spp. colonizes freshwater and moist soil environments as intracellular bacteria, infecting and replicating inside eukaryotic cells, such as free-living amoebae, monocytes and alveolar macrophages. In this way, Legionella is protected from different chemical and physical agents, thus developing several resistance mechanisms [8]. Frequent hosts of Legionella spp. are different amoebae including Acanthamoeba, Naegleria, Balmuthia, Dictyostelium and ciliate cells, such as Tetrahymena [9]. It has also been demonstrated that Legionella spp. Are naturally part of microbial ecosystems associated with complex biofilm communities, where they can survive in water systems [10].
Although Legionella spp. Are in low concentrations in natural environments, they can increase significantly in artificial water systems, where conditions are favorable for their proliferation due to the presence of biofilms and temperatures ranging between 25 °C and 45 °C, with a gold-standard temperature of 37 °C [10,11].
In the last few years, the incidence of Legionella infection has increased in both the United States and Europe. According to the reports from the European Centre for Disease Prevention and Control, in 2019, the overall notification rate in the EU/EEA was 2.2 cases per 100,000 inhabitants. Specifically, the annual rate from 2015 to 2019 has increased from 1.4 to 2.2 cases per 100,000 population, with the highest rate reported in Slovenia (9.4 cases per 100,000 population) [12].
Noteworthy, the incidence of legionellosis in Italy has increased over the years, reaching 52.9 cases per million inhabitants in 2019, which shows a slight increase compared to the previous year (48.9/1,000,000) [13].
During the last two years, some studies reported an opposite trend in the notification rate of Legionella infections compared with the above-mentioned data. Specifically, Fischer and colleagues evaluated the trends of legionellosis from 2000 to 2020 in Switzerland and other European countries. The highest notification rate was recorded in 2018 with 6.7/100,000 cases, while the trend after COVID-19 restriction measures showed a temporary decrease of 35%. A recent report by the Federal Office of Public Health (FOPH) showed also a reduction of LD cases of 32% compared with the expected case numbers observed from 2015 to 2019 [14]. As already mentioned, 52.9/100,000 cases of legionellosis were reported in Italy in 2019, while preliminary data for 2020 show a 35% decrease in the number of reported cases [15]. Such a decrement in the notification rate of legionellosis was surprising as the water tanks and pipes were not properly monitored due to the lock-down restrictions, therefore, an increment of Legionella exposure and infection was expected [16]. Recently, De Giglio and colleagues revealed that the water systems of some hospital wards showed a higher L. pneumophila contamination due to the 3-month closure during the COVID-19 emergency. In addition, Liang and colleagues and Chao and Lai described the possible increased risk of Legionella exposure after the end of lock-down [17,18]. Another possible explanation for the low notification rate observed during the COVID-19 pandemic could be related to similar symptoms existing between SARS-CoV-2 and LP pneumonia; therefore, an underestimation of LD pneumonia could have occurred during the pandemic [14,15].
The epidemiological data mentioned above suggest that the incidence of legionellosis and its associated health risks are expected to keep increasing due to global challenges, such as urbanization, climatic changes and new economic approaches. Therefore, the improvement of techniques and strategies for the environmental surveillance and management of Legionella in critical environments, such as health care structures and long care facilities is one of the most important challenges for the prevention of legionellosis.
On these bases, the present manuscript aims to provide a broad and updated description of past and current strategies implemented for the management of LP in healthcare environments. To the best of our knowledge, this review represents an advancement of current knowledge on LP diagnosis and treatment providing an updated description of novel treatments, water disinfection methods and molecular diagnostic techniques as widely discussed in the following chapters.
2. Legionella Clinical and Molecular Features
Among Legionella spp., Legionella pneumophila is the most implicated in human infections and responsible for different clinical manifestations: Legionnaires’ disease, severe pneumonia that can lead to permanent lung damage or death, Pontiac fever, a milder influenza-like disease, and rarer forms of extrapulmonary infection [19,20].
Patients’ risk factors associated with LD include older age, male sex, smoking, chronic lung diseases, cardiovascular diseases, or renal disorders [21].
The most common clinical symptoms of both LD and Pontiac fever are cough, muscle ache, headache, and shortness of breath. Fever and fatigue may precede the onset of cough. In LD, the symptoms usually appear after 2–14 days of incubation, while symptoms of Pontiac fever occur a few hours to three days after being exposed to the bacteria and typically last less than a week [22].
The main clinical feature of LD is pneumonia, which is clinically and radiographically characterized by irregular, unilobular infiltrates, which may progress to permanent lesions [23]. Signs and symptoms of lower respiratory tract infections are absent in Pontiac fever. Moreover, LD can also be associated with gastrointestinal symptoms, such as diarrhea, nausea, and vomiting [22].
Regarding LD, it was widely demonstrated that serious complications, including lung failure or death, may occur. In some cases, the presence of rare extrapulmonary complications, including cellulitis, skin abscesses, septic arthritis, endocarditis, meningitis, and peritonitis, was observed in immunocompromised patients [24,25,26].
It has been also described that LD mortality rate ranges between 1 to 10%, while Pontiac fever have usually a benign course without requiring any specific treatments [27,28].
From a clinical point of view, Legionella infections are divided into community-acquired and nosocomial infections. The second one can be acquired in health care settings during hospitalization. The nosocomial infections arise at least 48 h after admission, during the hospital stay, or after discharge, while community-acquired infections are already present at the time of admission [29].
After infection, LP grows in alveolar macrophages of human lungs using an escape mechanism resulting from the evolution of an opportunistic infection mechanism which LP uses in parasitized protozoa [30]. This mechanism consists of bypassing the canonical bactericidal endocytic pathway and forming an ER-associated replication-permissive compartment, called Legionella-containing vacuoles (LCV) [31,32].
Remarkably, many studies have shown that the LP genome contains several virulence genes involved in the entire infection cycle, which have been described as the most important factors affecting the ability of Legionella to grow and survive in alveolar macrophages and in free-living amoebae [33]. These virulence factors, termed pathogenicity island locus (PAIs) and encoded by specific DNA regions in the genome of pathogenic bacteria, are associated with Legionella pathogenicity while the same genes are not present in non-pathogenic strains [33,34]. Accordingly, an important role in the pathogenicity of Legionella is played by structures of the cell surface [35]. Specifically, adherence and subsequent invasion of the bacterium into the alveolar macrophages and protozoa are favored by the expression of some surface proteins.
The most abundant surface protein synthesized by LP is a 60-kDa heat shock protein (Hsp60) encoded by the high temperature protein B (htpB) gene. This protein modulates macrophage function via a mechanism that involves surface interactions, enhancing invasion and cytokine expression in macrophages in the pathogenesis of LD [36].
Of note, recombinant major outer membrane protein (MOMP), a protein encoded by the mompS gene, plays an important role during the attachment of Legionella to host cells. Specifically, this protein mediates the activation of alternative pathways of Complement Receptor 1 (CR1) and Complement Receptor 3 (CR3), leading to phagocytosis of LP by human monocytes [37].
Another protein associated with the virulence of LP is a 24 kDa protein encoded by the macrophage infectivity potentiator (Mip) gene which increases LP entrance in macrophages. Mip is exposed on Legionella’s surface and is involved in cell penetration. Interestingly, Mip is known to be one of the first genes associated with the ability of LP to replicate in eukaryotic cells [38].
Other important structures that play a crucial role in the infection process of Legionella are the secretion systems. It is widely known that many pathogenic bacteria use specialized protein secretion systems to introduce virulent effector proteins or other factors into host cells.
Specifically, Legionella is able to control the formation of LCVs and other pathogen-host interaction structures through the secretion of several proteins. Among the secretion systems, the putative type I Lss secretion machinery, type II PilD-dependent Lsp, type IVA lvh, and type IVB Icm/Dot secretion pathways are those involved in Legionella infectivity [39].
The LP type I Lss secretion system (T1SS) is responsible for the secretion of the repeats-in-toxin protein rtxA, which contributes to cellular entry and subsequent attachment to host cells (D’Auria G et al., 2008). rtxA is also involved in the intracellular survival and trafficking of the bacteria [34]. Particularly, the locus encoding for T1SS, named lssXYZABD, includes the typical components of a type I secretion system, including an ATP-binding cassette transporter (LssB) and a membrane fusion protein (LssD) [40]. Interestingly, all LP strains described in the study of Qin and colleagues contained the lssXYZABD locus. In contrast, the lssXYZABD locus was not found in non-L. pneumophila species, suggesting that the lssXYZABD secretion system plays an important role in LP biology [41].
LP also has a PilD-dependent type II secretion system (T2SS), termed Lsp, which is involved in the Legionella secretion pathway. This secretion system is required for Legionella virulence and environmental persistence. It has also been demonstrated that LP T2SS Lsp promotes intracellular infection of lung epithelial cells, attenuates cytokine secretion from infected macrophages and epithelia, and limits the number of cytokine transcripts in infected macrophages [42].
Type IV secretion systems (T4SSs) have been divided into two subclasses: type IVA (T4ASS), which resembled the Vir system of Agrobacterium tumefaciens, and type IVB (T4BSS), which is comparable to the Tra/Trb bacterial conjugation systems [39]. Specifically, the secretion systems type IVA lvh and type IVB Icm/Dot are present in LP.
In particular, the Legionella vir homolog (lvh) locus forms the protein for a second type IV secretion system that contributes to conjugation, virulence, and survival of the bacteria in the environment [43]. Previous studies have reported that the lvh and rtxA regions are more abundant in strains associated with human disease [44,45,46].
The gene cluster encoding T4ASS, referred to as lvh, is located in a region with high GC content, which allows the mobility of lvh region in the genus Legionella, contributing to the interspecies exchange of genetic information [47].
Once again, in the study of Qin and colleagues, the lvh genes were found in seven non-L. pneumophila and 40 LP strains, showing that the sequence of T4ASS lvh genes is highly conserved in these strains [41].
The type IVB secretion system (T4BSS) is termed Icm/Dot (Intracellular multiplication/defective organelle trafficking) and is a conjugation system used for the transport and injection of DNA or toxins into target cells [48,49,50].
In LP, the T4BSS is encoded by two separate pathogenicity regions on the chromosome. The first region contains 17 genes (icmTSRQ-PONMLKEGCDJBF), while the second region contains icmXWV and dotABCD [51].
Alveolar macrophages appear to be the primary cell type that is targeted by T4SS effectors and support intracellular bacterial replication [52]. Moreover, T4SS enables LP to manipulate a variety of cellular processes, including membrane trafficking, protein synthesis, ubiquitylation and autophagy [53,54,55,56].
Two-component systems (TCS), also known as two-component response regulators, are widespread signal transduction devices in bacteria that allow them to respond to environmental stimuli mainly via changes in gene expression. These systems are used by many pathogenic bacteria, including Legionella, to control the expression of their virulence genes [57]. In particular, the CpxRA TCS consists of the sensor histidine kinase (CpxA) and the cytoplasmic response regulator (CpxR). It recognizes various envelope stressors and promotes the transcription of genes that helps bacteria to overcome these stressors [57].
Of note, CpxRA is involved in the regulation of Dot/Icm system components and effectors. CpxR has been shown to directly activate the expression of the four Dot/Icm system components icmR, icmV, icmW and lvgA and to activate and repress 11 translocated effector proteins [58].
From these perspectives, the identification of particular virulence factors or drug resistance genes could be useful to clinicians to administer the most effective treatment and predict the prognosis of patients.
The most important virulence factors and their corresponding encoding genes are summarized in Table 1.
Table 1.
L. pneumophila virulence factors.
| Virulence Factors | Encoding Genes | Roles | |
|---|---|---|---|
| Surface Proteins | Hsp60 | htpB | Attachment in host cells, modulation of invasion and cytokine expression in macrophages [36] |
| MOMP | momps | Activation of an alternative pathway of complement CR1 and CR3, phagocytosis [37] | |
| Mip | Mip | Penetration and replication in host cells [38] | |
| Secretion systems | Type I Lss | lssXYZABD locus | Secretion of rtxA, attachment and penetration in host cells [34,59] |
| Type II Lsp | PilEL | Secretion of other virulence factors [42] | |
| Type IV | Lvh | Conjunction, secretion of virulence factors, Legionella survival [43,60] | |
| IcM/Dot | Conjunction, transport and injection of DNA [48,49] | ||
| Two component system | CpxRA | cpxA, cpxR, cpxRA | Transcription of anti-stressor genes, regulation of IcM/Dot system effectors [57,58] |
3. Pharmacological Treatment of Legionellosis and Management of LP Resistant Clones
The clinical manifestations of Legionella infections range from benign, mild disease to a more severe form with increased morbidity and mortality, especially in untreated patients. As the clinical and radiological symptoms of Legionella infection are nonspecific, empiric antibiotic treatment, effective towards a broad spectrum of common pneumonia pathogens, is recommended in the case of a suspected LP infection in order to reduce the morbidity and mortality associated with this disease [61,62].
Current American and European guidelines recommend macrolides and fluoroquinolones (azithromycin and levofloxacin, respectively), as first-line treatments for severe and moderate LD [63]. In detail, according to the British Thoracic Society (BTS) recommendations, the administration of 500 mg azithromycin or levofloxacin (500 mg IV/d) every 24 h is recommended in the case of mild LD pneumonia. In the presence of patients with severe LD pneumonia or in case of drug resistance, the second-line treatment is based on the combination of levofloxacin (500 mg IV/d) or another fluoroquinolone plus azithromycin (500 mg IV every 24 h) [64].
In a recent study by Miyashita and colleagues, it was observed that fluoroquinolones and macrolides have potent antimicrobial activity against both extracellular and intracellular Legionella spp., suggesting the good efficacy of these drugs in the treatment of Legionella infections [65].
Specifically, macrolides are bacteriostatic agents that bind reversibly to the 50S ribosomal subunit and inhibit protein synthesis [66]. They are effective against a wide range of bacteria, including intracellular pathogens. Macrolides, especially azithromycin, reach their peak concentration within 2–3 h and are rapidly absorbed and distributed throughout body tissues and cells [66].
In addition to macrolides, fluoroquinolones (levofloxacin, moxifloxacin, and ciprofloxacin) have increasingly become the standard treatment for Legionella infection, because they have a broad spectrum of activity against Gram-positive and Gram-negative organisms [67].
In particular, fluoroquinolones inhibit DNA gyrase subunit A blocking the transcription of bacterial DNA, resulting in bacterial cell death. Additionally, some studies recently reported that fluoroquinolones could be superior to macrolides due to their broad spectrum of activity and fewer adverse effects [68,69,70].
Despite the efficacy of macrolides and fluoroquinolones, treatment failures have been recently reported in the literature, indicating the possibility of developing resistance to traditional therapies [71]. In this context, Bakheit and colleagues have described some general mechanisms implicated in multiple bacterial resistance to macrolides. One mechanism is an active efflux pump, which ejects the drug from the bacteria cell. At least 16 different genes have been identified in connection with this mechanism. Another resistance mechanism involves some modifications in the ribosomal subunits, which reduce the binding of the antimicrobials to the ribosomal target site [71].
Regarding Legionella, mutations in genes encoding 23S rRNA or ribosomal proteins are known to be responsible for macrolide resistance in LP strains [71]. In particular, Descours and colleagues focused on mutations affecting key determinants of reduced susceptibility to macrolides, such as genes encoding 23S rRNA (rrl), L4 (rplD), and L22 (rplV) ribosomal proteins. The results highlighted that an initial mutation on ribosomal L4/L22 proteins causes moderately reduced sensitivity to macrolides and additional mutations in genes encoding 23S rRNA are responsible for an increased resistance [71].
In addition, some studies have demonstrated that the presence of the lpeAB genes and the efflux pump component lpeAB are associated with reduced susceptibility to azithromycin [72,73,74,75]. In particular, Massip and colleagues showed that lpeAB genes encode components of a trimeric efflux pump responsible for resistance to azithromycin among other macrolides while Vandewalle-Capo and colleagues demonstrated that the reduced sensitivity to azithromycin in ST1 strains was linked to the presence of lpeAB genes. More recently, Jia X and colleagues have tested 25 strains of Legionella, showing how the expression of lpeAB was responsible for reduced azithromycin susceptibility in all these strains. Similarly, Cocuzza and colleagues demonstrated the role of lpeAB efflux pump system in LP isolates which reduces susceptibility to azithromycin [73].
Additionally, fluoroquinolone-resistant Legionella strains have recently been identified in patients treated with these agents [76]. Notably, it has been described that bacterial resistance to quinolones is the result of chromosomal mutations of the DNA gyrase gene, leading to a decreased affinity of the drug for the enzyme. Similar to macrolides, alterations in drug efflux may also occur, resulting in a lower intracellular concentration of the drug [77].
Some researchers have also demonstrated that LP fluoroquinolone resistance in tested strains is associated with mutations affecting the type II topoisomerase-encoding genes (i.e., DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE)). Among these, gyrA is the primary target of fluoroquinolone in LP. When mutations affect gyrA, the susceptibility of DNA gyrase towards fluoroquinolones is reduced. Another target of fluoroquinolones is parC gene involved in the formation of topoisomerase IV. Consequently, the presence of additional mutations on parC is responsible for a stronger reduction of LP susceptibility to fluoroquinolones [77].
Recently, Hennbique and colleagues detected mutant gyrA sequences in mixtures of fluoroquinolone-resistant and susceptible LP strains by using digital PCR (dPCR) systems. These data suggest that dPCR allows rapid and accurate detection and quantification of these resistant mutants in respiratory samples [78].
To overcome these drug resistance mechanisms, recent studies focused their attention on the development of new therapies for the treatment of resistant LP. Among the most promising therapies, antisense therapy can eliminate or re-sensitize pathogens by targeting the LP vesicle trafficking pathway. Specifically, antisense therapies mediate the intracellular trafficking pathway to prevent the fusion of phagosomes and lysosomes in macrophages. In this way, bacteria in lysosomes are effectively killed by lysosomal enzymes [79]. Other current studies are evaluating the therapeutic potential of recombinant DNA vaccines against some virulence factors, such as peptidoglycan-associated lipoprotein (PAL) and PilE. In particular, an increased and stronger cellular and humoral immune response has been observed in mice after vaccine administration which resulted in a faster remission of the infection [80,81].
All these data suggest that it is important to analyze Legionella isolated, especially in hospital water systems, in order to early detect changes in antibiotic resistance patterns thus preventing potential outbreaks caused by antibiotic-resistant bacteria.
4. Monitoring of Legionella spp. in Hospital Environments and Water Disinfection Strategies
As stated by some recent reports, legionellosis represents a public health problem, which is escalating rapidly worldwide [82,83,84].
Of note, the main reservoirs for Legionella spp. are represented by water distribution systems, especially in large public buildings, households and industrial facilities [85]. However, the most dangerous type of colonization occurs in water distribution systems, cooling towers and hydric pipeline of hospitals, because in these environments LP can multiply causing severe infection in immunosuppressed hospitalized patients. Indeed, the most common route of exposure is represented by the inhalation of aerosol droplets containing Legionella, and the risk of transmission increases when the complexity of hospital water systems and patients’ susceptibility are considered [86]. Despite human-to-human transmission of legionellosis being very unlikely, a case was recently documented [87].
According to the latest available data from the European Centre for Disease Prevention and Control (ECDC), 10,672 confirmed cases of Legionellosis were reported in 2018, of which 6% were hospital-acquired. Specifically, in Italy, there were 3199 cases of legionellosis in 2019, of which 3.8% were hospital-acquired cases [88].
On these bases, environmental monitoring of LP in hospitals is a useful strategy to prevent nosocomial LD [89]. In order to prevent and reduce Legionella colonization and nosocomial cases of legionellosis, national and international guidelines started recommending environmental monitoring and remedial measures to control Legionella spp. contamination in water. In line with the World Health Organization (WHO), one of the best approaches to assess the health risks associated with Legionella colonization is the implementation of a water safety plan (WSP) [90].
According to the WHO recommendations, WSPs are based on the risk analysis methodology, a process for identifying hazardous events and determining the risks associated with the occurrence of Legionella spp. contamination. Specifically, WHO recommendations promote the assessment of risks and the development of measures for their control. Briefly, an effective water safety plan describes and monitors water systems, identifies potential hazards, assesses and prioritizes potential risks, identifies and implements control measures to mitigate the risk, defines corrective actions and verifies water system managing programs [91].
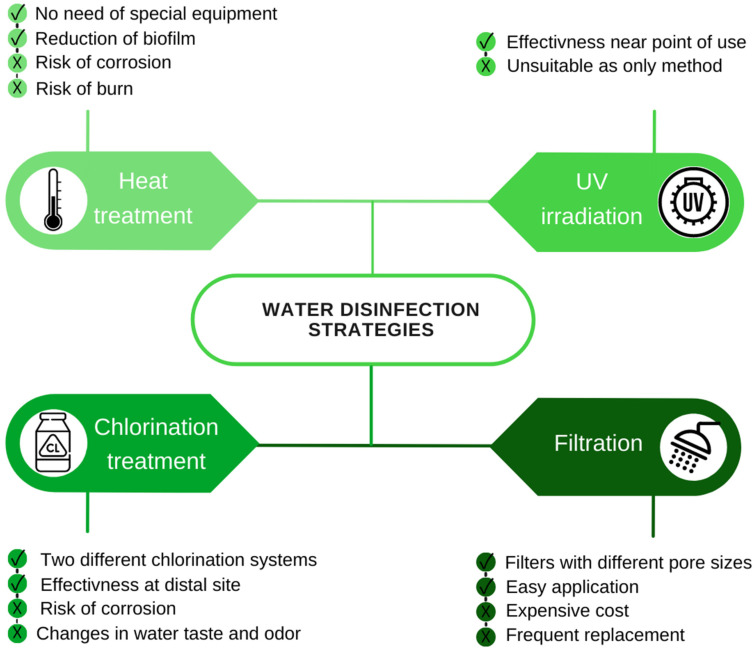
Different treatments are currently available for the disinfection of water. These treatments can be physical (heat treatment and ultraviolet irradiation) or chemical (use of metal ions and oxidizing agents) [12] (Figure 1).
Figure 1.
Pros (√) and cons (×) of the mainly adopted water disinfection strategies.
Heat treatment consists in raising the water temperature to at least 60 °C in order to inactivate Legionella. Currently, two main heat treatments are used. In the first method, the thermal shock is performed by maintaining the temperature of the water between 60 °C and 80 °C for three days. For effective disinfection, hot water at 60 °C must flow in all pipes for at least 30 min during the three-day treatment. In the second method, after a first heat-shock treatment at 70 °C, it is possible to keep the temperature at 55–60 °C at the distal points. Numerous public structures have adopted this technique because it does not require special equipment and it is also capable of reducing biofilms and eliminating Legionella colonies. However, heat treatments are not always applicable due to the high temperatures which lead to the corrosion of pipes, significant energy consumption and risk of burns [92].
Ultraviolet (UV) light irradiation has shown great efficacy in eradicating Legionella [93]. In particular, UV acts on bacterial DNA producing thymine dimers that inhibit DNA replication. Due to the lack of residual power, UV irradiation alone is not sufficient to control the presence of Legionella in the system. Other limits related to the use of UV are the presence of biofilms, the turbidity of the water and sediments which could act as a shield to the radiation. Therefore, this technique is not suitable as the only method for a whole building, because it does not have a residual effect, but is more effective near the point of consumption [93].
Filtration is a physical method based on the use of filters with pores of 0.22 µm placed at the points of use (POU), such as taps and showers. Filters are usually in hospitals to protect patients and medical staff in vulnerable departments. There are several different filtration systems that can remove contaminants: ultrafiltration, microfiltration, nanofiltration, and reverse osmosis processes. The disadvantage lies in the cost and frequent replacement, especially for hard water. Additionally, microbial filtration systems applied to POU require high water pressure to achieve the desired flow rate. Filters with small pore sizes need higher applied pressure than filters with large pore sizes [94].
Another physical method involves the use of ionization chambers with copper-silver electrodes installed on water pipes. Copper and silver have a bactericidal effect through the ionization of the cell wall of the microorganism which causes an alteration of cell permeability. In addition, both ions interfere with cellular respiration leading to cell lysis and death [95].
Interestingly, the work of Cloutman-Green and colleagues shows that it is possible to control LP incidence in a new hot water network at low temperatures (room temperature) using copper-silver ionization [96]. Therefore, ionization represents an easy-to-use method that is not affected by water temperature and its bactericidal effect can persist for several weeks thanks to the accumulation of copper in the biofilm. However, it has the disadvantage of being unsuitable for zinc pipes, as this metal causes the inactivation of silver ions. The use of ions requires constant maintenance of the electrodes and a careful evaluation of the doses depending on the characteristics of the system.
A similar method is based on the use of hydrogen peroxide and silver salts, which act synergistically with a complementary mechanism capable of radically destroying the protein material of the biofilm, penetrating deeply and inactivating microorganisms, including Legionella. It is a preventive measure that can be used as an alternative to heat or chlorine treatment [97].
Among the chemical methods, chlorination, chloramination and chlorine dioxide (CLO2) purifications are chemical processes that increase residual chlorine in water, resulting in the formation of toxic byproducts (chloroform, trihalomethanes bromoform, dibromochloromethane and bromodichloromethane) [94,98].
Chlorine is available in gaseous form and in the form of sodium or calcium hypochlorite. Although chlorination is the most used chemical method, it must be applied continuously in water tanks. In particular, there are two different methods of chlorination: intermittent and continuous.
The first method, also known as shock hyperchlorination, involves a single injection of chlorine into the water for 1–2 h until a high concentration (20–50 mg/L) of free residual chlorine is reached throughout the system, including distal sites. The other method involves a continuous injection of chlorine (0.5–1.0 mg/L) in the form of calcium or sodium hypochlorite. This method ensures a residual chlorine concentration throughout the water system, minimizing Legionella colonization at distal sites [98].
In this context, Paranjape and colleagues and Mouchtouri and colleagues showed that the effect of chlorination in cooling towers in Canada and Greece was crucial to minimizing the colonization and recolonization of Legionella spp. [99,100].
However, the significant disadvantage of hyperchlorination is the relative inability to eradicate the organism from the water distribution system, facilitating potential recontamination. In addition, strict and continuous monitoring of chlorine levels is required for the effectiveness of this method. Furthermore, chlorine has a significant corrosive effect on the water distribution pipes and can cause a change in the taste and odor of the water [101].
Similar to chlorination, chloramination is characterized by the formation of monochloramine, a weaker oxidant than chlorine but more effective and stable than chlorine dioxide [102]. Monochloramine has also a significant anti-Legionella activity and long-term efficacy, especially in complicated pipe networks [98]. The use of this chemical produces an excess of ammonia and a minor chloramine residue, due to the potential of monochloramine to react with organic substances in water creating byproducts. Additionally, rubber and plastic parts used in water systems have also been observed to be affected by chloramine [102].
Another chemical disinfection method is based on the use of chlorine dioxide (ClO2), an antioxidant that kills waterborne pathogens and associated biofilms. In addition, ClO2 is excellent in controlling the taste and color of drinking water. However, one disadvantage of using ClO2 in Legionella control is the potential corrosion of iron pipes [103].
Finally, ozone injected into water is another chemical method that has been used to control Legionella in building water systems [104]. It acts quickly by damaging bacterial DNA, and it is more effective than chlorine. On the other hand, it has no residual power and has limited effectiveness because it does not penetrate biofilms. In concentrated form, it can damage pipes. Its high cost and the need for specialized maintenance personnel make it an effective but not a commonly used method [98].
More recently, novel methods for the inactivation of LP in drinking water have been developed using LED emitting UV-C rays. In particular, LED UV-C irradiation of drinking water at 255 nm and 0.5 mJ/cm2 of fluence effectively reduced the log of all the tested LP serogroups [105]. These further data encourage the adoption of LED at POU due to its low cost of production and durability.
5. Detection of Legionella pneumophila and Diagnosis of Legionellosis
Specific detection methods have been developed for the diagnosis of LP infection using specimens obtained from the respiratory tract (e.g., sputum, bronchoalveolar lavage (BAL)) or liquid biopsy samples like serum or urine samples. The main diagnostic approaches include bacterial culture, serological and antibody-based assays, nucleic acid detection systems (e.g., polymerase chain reaction) and urine antigen tests [20].
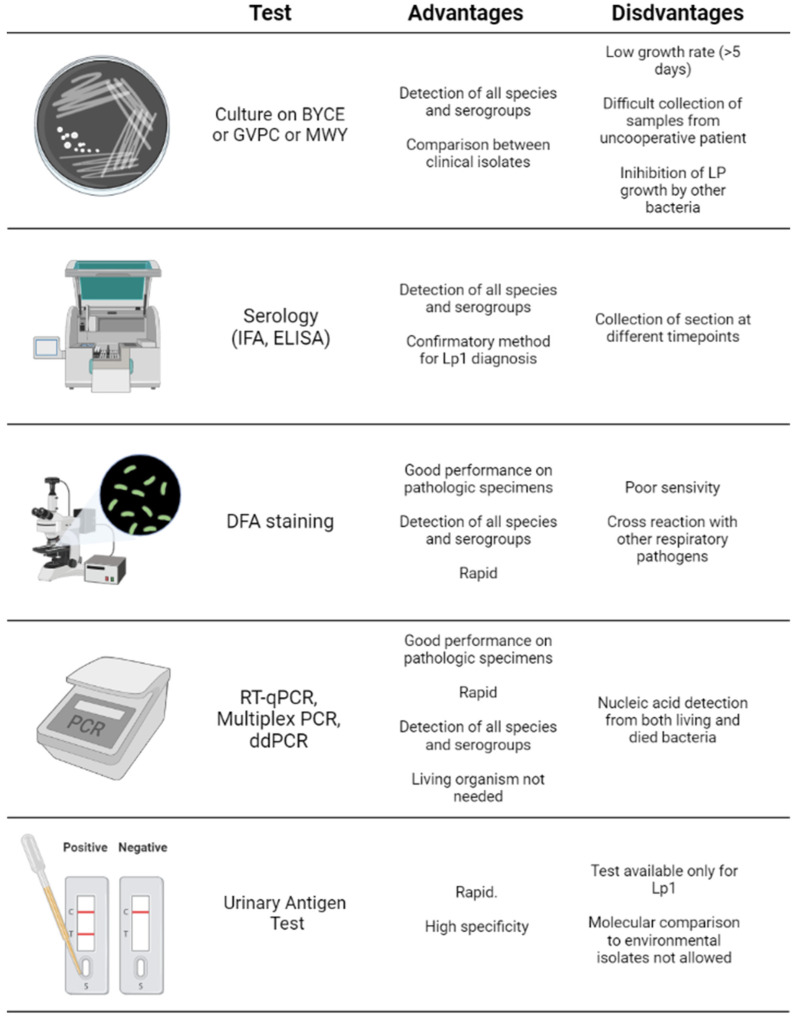
Isolation by culture methods is considered the gold standard for the diagnosis of Legionella infections. The most used medium for the growth of Legionella spp. is BCYE (Buffered Charcoal Yeast Extract), which consists of CYE agar base supplemented with cysteine, iron salts and α-ketoglutarate in ACES (n-(2-acetamido)-2-aminoethanesulfonic acid) buffer, which ensures an optimal pH (pH 6.9) for the growth of Legionella. In this medium, the yeast extract serves as a source of nutrients, while activated carbon is used to eliminate various toxic compounds produced in the soil, especially after exposure to light, such as reactive oxygen species [106].
In the presence of contaminating microorganisms, heat and/or acid pre-treatments of waters samples should be performed. Particularly, the water samples can be treated with HCl pH 2.2 for 5–20 min or heated up to 50 °C for 30 min or 60 °C for 1–3 min depending on the levels of contamination [107].
Additionally, other selective media are used to isolate Legionella spp. from potentially contaminated clinical samples. The most commonly used selective media is MWY (Wadowsky-Yee Medium) and Glycine Vancomycin Polymyxin Cycloheximide (GVPC) medium. Both media contain polymyxin B, which inhibits the growth of Gram-negative bacteria, vancomycin which targets Gram-positive bacteria while glycine impairs the bacterial wall facilitating the action of antibiotics [1].
The MWY medium also contains azinomycin which acts against yeasts, while dyes give a characteristic color to certain Legionella species. The GVPC medium, instead, is enriched with cycloheximide which suppresses the growth of fungi but is highly toxic by contact and inhalation. In this regard, there is also the possibility to use a different formulation, called Glycine Vancomycin Polymyxin Natamycin (GVPN), which contains natamycin, a non-toxic antifungal as effective as cycloheximide [1].
Interestingly, Di Tommaso and colleagues evaluated the diagnostic performance of BCYE and MWY in 951 Legionella-positive water samples from hospital environments. It was found that MWY allowed the detection of Legionella in 89.2% of the samples. In particular, MWY is essential to detect Legionella in samples contaminated by multiple organisms (52.6%, 349/663).
In samples contaminated by Legionella only, a higher frequency of positive samples was recorded using BCYE (94.8%, 273/288) compared to MWY (85.1%, 245/288). These findings confirm the appropriateness of the ISO 11,731:2017 update encouraging the use of selective media for correct detection of LP [108].
Regarding the growth of Legionella on culture media, it forms colonies under microaerophilic conditions (2.5% CO2) showing considerable pleomorphism. Initially, the colonies are small and punctiform, however, after several days of incubation they increase in diameter and reach up to 3–4 mm. They appear circular shape in shape, and it is possible to distinguish a white central part and shiny grey-white clear margins with a mucous consistency. It is also known that LP colonies emit yellow-green fluorescence when observed under UV lamps [109].
All Legionella spp. can be detected with culture methods; thus, these represent the gold standard for the diagnosis of legionellosis. Unfortunately, culture methods have some critical issues, mostly represented by the low growth rate of LP, which requires long waiting times (often taking 5 days or more to grow). Moreover, only half of patients with LD produce sputum. Furthermore, the culture method is impractical in some cases because the growth of Legionella can be inhibited by the presence of other bacteria [110].
Besides culture methods, serological tests can be used to assess Legionella infection. Among these methods, indirect immunofluorescent assays (IFA) and enzyme-linked immunosorbent assays (ELISA or EIA) are the most frequently performed tests [111]. However, the use of these techniques has declined significantly, due to the development of faster and user-friendly methods, such as the urinary antigen test. Indeed, both IFA and ELISA required the collection of two serum samples at different time points lengthening the time of diagnosis. Despite this limitation, serological tests remain relevant for retrospective epidemiological investigations and when the infectious agent cannot be isolated despite clear evidence of LD [112].
Another detection method is direct fluorescent-antibody (DFA) staining, a rapid method for the direct detection of Legionella spp. in respiratory secretions and tissue samples. The limitation of this method is his poor sensitivity due to cross-reactions with other respiratory pathogens [12].
More recently, nucleic acid amplification systems have gained the attention of researchers and clinicians for their high-sensitive diagnostic value for different diseases. The current COVID-19 pandemic has demonstrated the high diagnostic value of different molecular methods, including quantitative reverse transcription PCR (RT-qPCR), droplet digital PCR (ddPCR), biosensors and other point-of-care systems, able to detect the pathogen in different types of samples [113,114,115]. In line with these recent findings in COVID-19, the polymerase chain reaction (PCR) has been used for the detection of Legionella in respiratory secretions (e.g., sputum or BAL) as well as urine and serum samples [116,117,118]. PCR has numerous advantages compared to other diagnostic methods. Some studies have reported that PCR is more sensitive than culture methods, suggesting that it is a useful tool for identifying sources of infections [119,120,121]. One of the most important advantages of this method is the rapidity of the results as PCR results can be available approximately 4 h after sample collection and are easier to interpret than LP culture, which requires a longer time (approximately 5–8 days) [121].
Remarkably, some studies have described that multiplex PCR is capable of simultaneously detecting and discriminating different Legionella species or serogroups. This method is preferably used in outbreaks or for surveillance strategies. The amplification of nucleic acids has many advantages. Firstly, PCR does not require previous culture isolations. Notably, it does not need the presence of living organisms, which is important during the detection of Legionella, especially in patients under pharmacological treatments with antibiotics [122,123,124]. However, these methods also detect nucleic acids from dead or dying bacteria, organisms associated with amoeba and viable but nonculturable (VBNC) microorganisms overestimating the real bacterial load.
In order to overcome these critical points, some researchers have proposed the use of a droplet digital PCR (ddPCR) system as a novel and time-saving method for the rapid detection of Legionella spp. [125,126,127].
Falzone and colleagues demonstrated the high sensitivity and accuracy of ddPCR compared to RT-qPCR in detecting LP in water samples with a low bacterial load. Specifically, RT-qPCR detected a low concentration of L. pneumophila at a very late Ct value, while ddPCR precisely quantified the concentrations of L. pneumophila. Of note, it was described that ddPCR is not affected by the presence of fragmented DNA, suggesting that this method can be used to detect the pathogen in patients with suspected legionellosis under antibiotic treatments.
Similarly, Logan-Jackson and colleagues used ddPCR to characterize the abundance of Legionella spp. and five specific Legionella species from samples (groundwater) obtained from exposed sites. Using ddPCR, the authors were able to quantify low DNA concentration in water samples as well as the abundance of DNA in a host-pathogen interaction context identifying the co-occurrence of pathogenic Legionella spp. (L. pneumophila, L. anisa, L. longbeachae, L. bozemanii, and L. micdadei) and amoebae species.
Although these studies suggested that ddPCR should be used for the monitoring of water samples, Legionella molecular tests need improvements before commercialization [128,129,130].
Finally, when PCR is not available or when sputum cannot be obtained, urine antigen testing represents a good diagnostic alternative. The sensitivity of urine antigen tests ranges from 70% to 80% with a 100% specificity [130]. Legionella antigens can be detected in urine the day after the onset of symptoms and persist for a couple of weeks. Even if the urine antigen test is negative but Legionella infection is suspected, it is reasonable to perform PCR or culture on a lower respiratory tract specimen [131].
The main advantages of the urine antigen test are its rapid turnaround time and high specificity; however, this test can be used only for the detection of LP serotype 1 [132]. While LP serotype 1 causes over 80% of reported cases of LD in most regions of the world, L. longbeachae is widely distributed in some regions, such as Australia and New Zealand, limiting the utility of the urine antigen test [6]. However, antigen tests for the detection of L. longbeachae are under development [133] (Figure 2).
Figure 2.
Advantages and pitfalls of the culture, serological and molecular methods for the diagnosis of legionellosis.
6. Conclusions
Despite the development of more accurate diagnostic methods and increasingly accurate water monitoring and sanitation strategies, Legionella spp. infections in hospital environments still represent a significant public health problem. Before the COVID-19 pandemic, epidemiological data showed an increase in the number of infections caused by this pathogen suggesting the need for better preventive strategies. During the pandemic, the monitoring of water systems and the traceability of legionellosis cases were inadequate; therefore, a further increase in the number of Legionella infections is expected in the coming years. In this scenario, significant advancements have been obtained in the management and monitoring of Legionella. Overall, the data reported in the present manuscript can be summarized as follow:
-
-
The precise characterization of LP clinical and molecular features is essential to identify potential virulence or drug resistance factors useful for the selection of the most effective antibiotic treatment;
-
-
Disinfection strategies using chemicals, filters or UV lamps are essential in both water tanks, pipes and POU to reduce the risk of LP water contamination;
-
-
The use of molecular high-sensitive diagnostic methods, such as ddPCR, besides the standard culture methods, could be useful to correctly diagnose legionellosis in suspected LP pneumonia.
To further corroborate the clinical validity of these advancements, further studies are needed to validate the diagnostic accuracy of novel diagnostic techniques and the efficacy of new treatment and disinfection protocols.
Author Contributions
Conceptualization, M.S., M.L.M. and G.G.; methodology, G.G., R.R., A.L., V.S., D.C. and C.L.; formal analysis, G.G., R.R., G.P., A.C., D.C. and C.M.; investigation, G.G., R.R., A.L., A.C. and C.L.; data curation, M.S.; writing—original draft preparation, G.G. and R.R.; writing—review and editing, C.L. and M.S.; visualization, all authors.; supervision, M.L.M. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data described in the present manuscript are all available on PubMed NCBI.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ditommaso S., Giacomuzzi M., Memoli G., Garlasco J., Zotti C.M. Comparison of BCYEα+AB agar and MWY agar for detection and enumeration of Legionella spp. in hospital water samples. BMC Microbiol. 2021;21:48. doi: 10.1186/s12866-021-02109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser D.W., Tsai T.R., Orenstein W., Parkin W.E., Beecham H.J., Sharrar R.G., Harris J., Mallison G.F., Martin S.M., McDade J.E., et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N. Engl. J. Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 3.Brenner D.J., Steigerwalt A.G., McDade J.E. Classification of the Legionnaires’ disease bacterium: Legionella pneumophila, genus novum, species nova, of the family Legionellaceae, familia nova. Ann. Intern. Med. 1979;90:656–658. doi: 10.7326/0003-4819-90-4-656. [DOI] [PubMed] [Google Scholar]
- 4.Potts A., Donaghy M., Marley M., Othieno R., Stevenson J., Hyland J., Pollock K.G., Lindsay D., Edwards G., Hanson M.F., et al. Cluster of Legionnaires disease cases caused by Legionella longbeachae serogroup 1. Euro Surveill. 2013;18:20656. doi: 10.2807/1560-7917.es2013.18.50.20656. [DOI] [PubMed] [Google Scholar]
- 5.Waldron P.R., Martin B.A., Ho D.Y. Mistaken identity: Legionella micdadei appearing as acid-fast bacilli on lung biopsy of a hematopoietic stem cell transplant patient. Transpl. Infect. Dis. 2015;17:89–93. doi: 10.1111/tid.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu V.L., Plouffe J.F., Pastoris M.C., Stout J.E., Schousboe M., Widmer A., Summersgill J., File T., Heath C.M., Paterson D.L., et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: An international collaborative survey. J. Infect. Dis. 2002;186:127–128. doi: 10.1086/341087. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Environmental Protection Agency|US EPA. [(accessed on 6 January 2022)];2016 Available online: https://www.epa.gov/
- 8.Ryan K., Ray G. Sherris Medical Microbiology. 6th ed. The McGraw Hill Education Companies; New York, NY, USA: 2015. [Google Scholar]
- 9.Boamah D.K., Zhou G., Ensminger A.W., O’Connor T.J. From Many Hosts, One Accidental Pathogen: The Diverse Protozoan Hosts of Legionella. Front. Cell Infect. Microbiol. 2017;7:477. doi: 10.3389/fcimb.2017.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu Khweek A., Amer A.O. Factors Mediating Environmental Biofilm Formation by Legionella pneumophila. Front. Cell Infect. Microbiol. 2018;8:38. doi: 10.3389/fcimb.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhoads W.J., Garner E., Ji P., Zhu N., Parks J., Schwake D.O., Pruden A., Edwards M.A. Distribution System Operational Deficiencies Coincide with Reported Legionnaires’ Disease Clusters in Flint, Michigan. Environ. Sci. Technol. 2017;51:11986–11995. doi: 10.1021/acs.est.7b01589. [DOI] [PubMed] [Google Scholar]
- 12.ECDC, European Centre for Disease Prevention and Control. [(accessed on 14 January 2022)]. Available online: https://www.ecdc.europa.eu/en.
- 13.Rota M.C., Bella A., Caporali M.G., Nicolau A., Drasar V., Ricci M.L., Scaturro M., Gumá M., Crespi S. Travel-associated Legionnaires’ disease: Would changing cluster definition lead to the prevention of a larger number of cases? Epidemiol. Infect. 2018;147:62. doi: 10.1017/S0950268818003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer F.B., Mäusezahl D., Wymann N.M. Temporal trends in legionellosis national notification data and the effect of COVID-19, Switzerland, 2000–2020. medRxiv. 2022 doi: 10.1101/2022.01.19.22269395. [DOI] [PubMed] [Google Scholar]
- 15.Rota M.C., Caporali M.G., Bella A., Scaturro M., Giannitelli S., Ricci M.L. Il Sistema di Sorveglianza Della Legionellosi in Italia: I Risultati del 2019. [(accessed on 15 January 2022)]. Available online: https://www.epicentro.iss.it/ben/2020/4/sorveglianza-legionellosi-italia-2019.
- 16.Palazzolo C., Maffongelli G., D’Abramo A., Lepore L., Mariano A., Vulcano A., Bartoli T.A., Bevilacqua N., Giancola M.L., Di Rosa E., et al. Legionella pneumonia: Increased risk after COVID-19 lockdown? Italy, May to June 2020. Euro Surveill. 2020;25:2001372. doi: 10.2807/1560-7917.ES.2020.25.30.2001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J., Swanson C.S., Wang L., He Q. Impact of building closures during the COVID-19 pandemic on Legionella infection risks. Am. J. Infect. Control. 2021;49:1564–1566. doi: 10.1016/j.ajic.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao C.M., Lai C.C. Increasing legionella in Taiwan during COVID-19 pandemic. Am. J. Infect. Control. 2022;50:237–238. doi: 10.1016/j.ajic.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal S., Abell V., File T.M., Jr. Nosocomial (Health Care-Associated) Legionnaire’s Disease. Infect. Dis. Clin. N. Am. 2017;31:155–165. doi: 10.1016/j.idc.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Mondino S., Schmidt S., Rolando M., Escoll P., Gomez-Valero L., Buchrieser C. Legionnaires’ Disease: State of the Art Knowledge of Pathogenesis Mechanisms of Legionella. Annu. Rev. Pathol. Mech. Dis. 2020;15:439–466. doi: 10.1146/annurev-pathmechdis-012419-032742. [DOI] [PubMed] [Google Scholar]
- 21.Kenagy E., Priest P.C., Cameron C.M., Smith D., Scott P., Cho V., Mitchell P., Murdoch D.R. Risk Factors forLegionella longbeachaeLegionnaires’ Disease, New Zealand. Emerg. Infect. Dis. 2017;23:1148–1154. doi: 10.3201/eid2307.161429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunette G.W., Kozarsky P.E., Magill J.A., Shlim D.R., Whatley A.D. CDC Health Information for International Travel 2010, Chapter 5—Other Infectious Diseases Related to Travel. Elsevier; Amsterdam, The Netherlands: 2009. pp. 290–411. [Google Scholar]
- 23.Poirier R., Rodrigue J., Villeneuve J., Lacasse Y. Early Radiographic and Tomographic Manifestations of Legionnaires’ Disease. Can. Assoc. Radiol. J. 2017;68:328–333. doi: 10.1016/j.carj.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Franco-Garcia A., Varughese T.A., Lee Y.J., Papanicolaou G., Rosenblum M.K., Hollmann T.J., Koehne G., Boulad F., Babady N.E., Tang Y.-W., et al. Diagnosis of Extrapulmonary Legionellosis in Allogeneic Hematopoietic Cell Transplant Recipients by Direct 16S Ribosomal Ribonucleic Acid Sequencing and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Open Forum Infect. Dis. 2017;4:140. doi: 10.1093/ofid/ofx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banderet F., Blaich A., Soleman E., Gaia V., Osthoff M. Septic arthritis due to Legionella cincinnatiensis: Case report and review of the literature. Infection. 2017;45:551–555. doi: 10.1007/s15010-016-0964-1. [DOI] [PubMed] [Google Scholar]
- 26.Chitasombat M.N., Ratchatanawin N., Visessiri Y. Disseminated extrapulmonary Legionella pneumophila infection presenting with panniculitis: Case report and literature review. BMC Infect. Dis. 2018;18:467. doi: 10.1186/s12879-018-3378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dooling K.L., Toews K.-A., Hicks L.A., Garrison L.E., Bachaus B., Zansky S., Carpenter L.R., Schaffner B., Parker E., Petit S., et al. Active Bacterial Core Surveillance for Legionellosis—United States, 2011–2013. MMWR. Morb. Mortal. Wkly. Rep. 2015;64:1190–1193. doi: 10.15585/mmwr.mm6442a2. [DOI] [PubMed] [Google Scholar]
- 28.Isenman H.L., Chambers S.T., Pithie A.D., MacDonald S.L., Hegarty J.M., Fenwick J.L., Maze M.J., Metcalf S.C., Murdoch D.R. Legionnaires’ disease caused by Legionella longbeachae: Clinical features and outcomes of 107 cases from an endemic area. Respirology. 2016;21:1292–1299. doi: 10.1111/resp.12808. [DOI] [PubMed] [Google Scholar]
- 29.Sikora A., Zahra F. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2022. Nosocomial Infections. [Google Scholar]
- 30.Oliva G., Sahr T., Buchrieser C. The Life Cycle of L. pneumophila: Cellular Differentiation Is Linked to Virulence and Metabolism. Front. Cell. Infect. Microbiol. 2018;8:3. doi: 10.3389/fcimb.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiner B., Swart A.L., Welin A., Weber S., Personnic N., Kaech A., Freyre C., Ziegler U., Klemm R., Hilbi H. ER remodeling by the large GTPase atlastin promotes vacuolar growth of Legionella pneumophila. EMBO Rep. 2017;18:1817–1836. doi: 10.15252/embr.201743903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner B., Weber S., Hilbi H. Formation of the Legionella-containing vacuole: Phosphoinositide conversion, GTPase modulation and ER dynamics. Int. J. Med. Microbiol. 2018;308:49–57. doi: 10.1016/j.ijmm.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Cianciotto N.P. Pathogenicity of Legionella pneumophila. Int. J. Med. Microbiol. 2001;291:331–343. doi: 10.1078/1438-4221-00139. [DOI] [PubMed] [Google Scholar]
- 34.Dowling J.N., Saha A.K., Glew R.H. Virulence factors of the family Legionellaceae. Microbiol. Rev. 1992;56:32–60. doi: 10.1128/mr.56.1.32-60.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heuner K., Steinert M., Dietrich C., Fischer G., Kohler R.F., Hacker J. Legionella. John Wiley & Sons, Inc.; New York, NY, USA: 2014. Function and Expression of Legionella pneumophila Surface Factors; pp. 3–48. [Google Scholar]
- 36.Garduño R.A., Garduño E., Hoffman P.S. Surface-Associated Hsp60 Chaperonin of Legionella pneumophila Mediates Invasion in a HeLa Cell Model. Infect. Immun. 1998;66:4602–4610. doi: 10.1128/IAI.66.10.4602-4610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellinger-Kawahara C., Horwitz M.A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J. Exp. Med. 1990;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields B.S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842X(96)10041-X. [DOI] [PubMed] [Google Scholar]
- 39.De Buck E., Anné J., Lammertyn E. The role of protein secretion systems in the virulence of the intracellular pathogen Legionella pneumophila. Microbiology. 2007;153:3948–3953. doi: 10.1099/mic.0.2007/012039-0. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi S., Heuner K. Description of a putative type I secretion system in Legionella pneumophila. Int. J. Med. Microbiol. 2003;293:349–358. doi: 10.1078/1438-4221-00276. [DOI] [PubMed] [Google Scholar]
- 41.Qin T., Zhao D., Zhu L., Ren H., Li Y., Liu X., Li X., Li W., Zhao N., Lu J., et al. Legionella pneumophila Risk from Cooling Tower Systems in China. Appl. Environ. Microbiol. 2021;88:e0192121. doi: 10.1128/AEM.01921-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Zant A., Asare R., Graham J.E., Abu Kwaik Y. Role for RpoS but Not RelA of Legionella pneumophila in Modulation of Phagosome Biogenesis and Adaptation to the Phagosomal Microenvironment. Infect. Immun. 2006;74:3021–3026. doi: 10.1128/IAI.74.5.3021-3026.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandyopadhyay P., Liu S., Gabbai C.B., Venitelli Z., Steinman H.M. Environmental Mimics and the Lvh Type IVA Secretion System Contribute to Virulence-Related Phenotypes of Legionella pneumophila. Infect. Immun. 2007;75:723–735. doi: 10.1128/IAI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samrakandi M.M., Cirillo S.L.G., Ridenour D.A., Bermudez L.E., Cirillo J.D. Genetic and Phenotypic Differences between Legionella pneumophila Strains. J. Clin. Microbiol. 2002;40:1352–1362. doi: 10.1128/JCM.40.4.1352-1362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang B., Heron B.A., Gray B.R., Eglezos S., Bates J.R., Savill J. A Predominant and Virulent Legionella pneumophila Serogroup 1 Strain Detected in Isolates from Patients and Water in Queensland, Australia, by an Amplified Fragment Length Polymorphism Protocol and Virulence Gene-Based PCR Assays. J. Clin. Microbiol. 2004;42:4164–4168. doi: 10.1128/JCM.42.9.4164-4168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang B., Yuan Z., Heron B.A., Gray B.R., Eglezos S., Bates J.R., Savill J. Distribution of 19 major virulence genes in Legionella pneumophila serogroup 1 isolates from patients and water in Queensland, Australia. J. Med. Microbiol. 2006;55:993–997. doi: 10.1099/jmm.0.46310-0. [DOI] [PubMed] [Google Scholar]
- 47.Kozak N.A., Buss M., Lucas C.E., Frace M., Govil D., Travis T., Olsen-Rasmussen M., Benson R.F., Fields B.S. Virulence Factors Encoded by Legionella longbeachae Identified on the Basis of the Genome Sequence Analysis of Clinical Isolate D-4968. J. Bacteriol. 2010;192:1030–1044. doi: 10.1128/JB.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finsel I., Hilbi H. Formation of a pathogen vacuole according toLegionella pneumophila: How to kill one bird with many stones. Cell. Microbiol. 2015;17:935–950. doi: 10.1111/cmi.12450. [DOI] [PubMed] [Google Scholar]
- 49.Qiu J., Luo Z.-Q. Legionella and Coxiella effectors: Strength in diversity and activity. Nat. Rev. Genet. 2017;15:591–605. doi: 10.1038/nrmicro.2017.67. [DOI] [PubMed] [Google Scholar]
- 50.Kubori T., Nagai H. The Type IVB secretion system: An enigmatic chimera. Curr. Opin. Microbiol. 2015;29:22–29. doi: 10.1016/j.mib.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Nagai H., Kubori T. Type IVB Secretion Systems of Legionella and Other Gram-Negative Bacteria. Front. Microbiol. 2011;2:136. doi: 10.3389/fmicb.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Copenhaver A.M., Casson C.N., Nguyen H.T., Fung T.C., Duda M.M., Roy C.R., Shin S. Alveolar Macrophages and Neutrophils Are the Primary Reservoirs for Legionella pneumophila and Mediate Cytosolic Surveillance of Type IV Secretion. Infect. Immun. 2014;82:4325–4336. doi: 10.1128/IAI.01891-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong N., Niu M., Hu L., Yao Q., Zhou R., Shao F. Modulation of membrane phosphoinositide dynamics by the phosphatidylinositide 4-kinase activity of the Legionella LepB effector. Nat. Microbiol. 2016;2:16236. doi: 10.1038/nmicrobiol.2016.236. [DOI] [PubMed] [Google Scholar]
- 54.Moss S.M., Taylor I., Ruggero D., Gestwicki J.E., Shokat K.M., Mukherjee S. A Legionella pneumophila Kinase Phosphorylates the Hsp70 Chaperone Family to Inhibit Eukaryotic Protein Synthesis. Cell Host Microbe. 2019;25:454–462. doi: 10.1016/j.chom.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y.-H., Lucas M., Evans T.R., Abascal-Palacios G., Doms A.G., Beauchene N.A., Rojas A.L., Hierro A., Machner M.P. RavN is a member of a previously unrecognized group of Legionella pneumophila E3 ubiquitin ligases. PLoS Pathog. 2018;14:e1006897. doi: 10.1371/journal.ppat.1006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rolando M., Escoll P., Nora T., Botti J., Boitez V., Bedia C., Daniels C., Abraham G., Stogios P.J., Skarina T., et al. Legionella pneumophila S1P-lyase targets host sphingolipid metabolism and restrains autophagy. Proc. Natl. Acad. Sci. USA. 2016;113:1901–1906. doi: 10.1073/pnas.1522067113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt S., Raivio T.L. Just scratching the surface: An expanding view of the Cpx envelope stress response. FEMS Microbiol. Lett. 2011;326:2–11. doi: 10.1111/j.1574-6968.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 58.Altman E., Segal G. The Response Regulator CpxR Directly Regulates Expression of Several Legionella pneumophila icm/dot Components as Well as New Translocated Substrates. J. Bacteriol. 2008;190:1985–1996. doi: 10.1128/JB.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Auria G., Jiménez N., Peris-Bondia F., Pelaz C., Latorre A., Moya A. Virulence factor rtx in Legionella pneumophila, evidence suggesting it is a modular multifunctional protein. BMC Genom. 2008;9:14. doi: 10.1186/1471-2164-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stone B., Abu Kwaik Y. Expression of Multiple Pili by Legionella pneumophila: Identification and Characterization of a Type IV Pilin Gene and Its Role in Adherence to Mammalian and Protozoan Cells. Infect. Immun. 1998;66:1768–1775. doi: 10.1128/IAI.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma L., Losier A., Tolbert T., Cruz C.S.D., Marion C.R. Atypical Pneumonia: Updates on Legionella, Chlamydophila, and Mycoplasma Pneumonia. Clin. Chest Med. 2017;38:45–58. doi: 10.1016/j.ccm.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carratalà J., Garcia-Vidal C. An update on Legionella. Curr. Opin. Infect. Dis. 2010;23:152–157. doi: 10.1097/QCO.0b013e328336835b. [DOI] [PubMed] [Google Scholar]
- 63.Woodhead M., Blasi F., Ewig S., Garau J., Huchon G., Ieven M., Ortqvist A., Schaberg T., Torres A., van der Heijden G., et al. Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections—Full version. Clin. Microbiol. Infect. 2011;17((Suppl. 6)):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris M., Clark J., Coote N., Fletcher P., Harnden A., Mckean M., Thomson A., on behalf of the British Thoracic Society Standards of Care Committee British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax. 2011;66((Suppl. S2)):ii1–ii23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 65.Miyashita N., Kobayashi I., Higa F., Aoki Y., Kikuchi T., Seki M., Tateda K., Maki N., Uchino K., Ogasawara K., et al. In vitro activity of various antibiotics against clinical strains of Legionella species isolated in Japan. J. Infect. Chemother. 2018;24:325–329. doi: 10.1016/j.jiac.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Bakheit A.H., Al-Hadiya B.M., Abd-Elgalil A.A. Profiles Drug Substances Excipients and Related Methodology. In: Brittain H.G., editor. Volume 39. Elsevier; Amsterdam, The Netherlands: 2014. pp. 1–40. [DOI] [PubMed] [Google Scholar]
- 67.Wimer S.M., Schoonover L., Garrison M.W. Levofloxacin: A therapeutic review. Clin. Ther. 1998;20:1049–1070. doi: 10.1016/S0149-2918(98)80104-5. [DOI] [PubMed] [Google Scholar]
- 68.Erdogan H., Can F., Demirbilek M., Timurkaynak F., Arslan H. In vitro activity of antimicrobial agents against Legionella isolated from environmental water systems: First results from Turkey. Environ. Monit. Assess. 2010;171:689–691. doi: 10.1007/s10661-010-1356-0. [DOI] [PubMed] [Google Scholar]
- 69.Bonaldo G., Andriani L.A., D’Annibali O., Motola D., Vaccheri A. Cardiovascular safety of macrolide and fluoroquinolone antibiotics: An analysis of the WHO database of adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2019;28:1457–1463. doi: 10.1002/pds.4873. [DOI] [PubMed] [Google Scholar]
- 70.Postma D.F., Spitoni C., van Werkhoven C.H., van Elden L.J.R., Oosterheert J.J., Bonten M.J.M. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: Post-hoc analysis of a cluster-randomized trial. BMC Infect. Dis. 2019;19:17. doi: 10.1186/s12879-018-3630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Descours G., Ginevra C., Jacotin N., Forey F., Chastang J., Kay E., Etienne J., Lina G., Doublet P., Jarraud S. Ribosomal Mutations Conferring Macrolide Resistance in Legionella pneumophila. Antimicrob. Agents Chemother. 2017;61:e02188-16. doi: 10.1128/AAC.02188-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massip C., Descours G., Ginevra C., Doublet P., Jarraud S., Gilbert C. Macrolide resistance inLegionella pneumophila: The role of LpeAB efflux pump. J. Antimicrob. Chemother. 2017;72:1327–1333. doi: 10.1093/jac/dkw594. [DOI] [PubMed] [Google Scholar]
- 73.Jia X., Ren H., Nie X., Li Y., Li J., Qin T. Antibiotic Resistance and Azithromycin Resistance Mechanism of Legionella pneumophila Serogroup 1 in China. Antimicrob. Agents Chemother. 2019;63:e00768-19. doi: 10.1128/AAC.00768-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vandewalle-Capo M., Massip C., Descours G., Charavit J., Chastang J., Billy P.A., Boisset S., Lina G., Gilbert C., Maurin M., et al. Minimum inhibitory concentration (MIC) distribution among wild-type strains of Legionella pneumophila identifies a subpopulation with reduced susceptibility to macrolides owing to efflux pump genes. Int. J. Antimicrob. Agents. 2017;50:684–689. doi: 10.1016/j.ijantimicag.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Cocuzza C.E., Martinelli M., Perdoni F., Giubbi C., Vinetti M.E.A., Calaresu E., Frugoni S., Scaturro M., Ricci M.L., Musumeci R. Antibiotic Susceptibility of Environmental Legionella pneumophila Strains Isolated in Northern Italy. Int. J. Environ. Res. Public Health. 2021;18:9352. doi: 10.3390/ijerph18179352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shadoud L., Almahmoud I., Jarraud S., Etienne J., Larrat S., Schwebel C., Timsit J.-F., Schneider D., Maurin M. Hidden Selection of Bacterial Resistance to Fluoroquinolones In Vivo: The Case of Legionella pneumophila and Humans. EBioMedicine. 2015;2:1179–1185. doi: 10.1016/j.ebiom.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.AlMahmoud I., Kay E., Schneider M., Maurin M. Mutational paths towards increased fluoroquinolone resistance in Legionella pneumophila. J. Antimicrob. Chemother. 2009;64:284–293. doi: 10.1093/jac/dkp173. [DOI] [PubMed] [Google Scholar]
- 78.Hennebique A., Bidart M., Jarraud S., Beraud L., Schwebel C., Maurin M., Boisset S. Digital PCR for Detection and Quantification of Fluoroquinolone Resistance in Legionella pneumophila. Antimicrob. Agents Chemother. 2017;61:e00628-17. doi: 10.1128/AAC.00628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pashaei-Asl R., Khodadadi K., Pashaei-Asl F., Haqshenas G., Ahmadian N., Pashaiasl M., Baghdadabadi R.H. Legionella Pneumophila and Dendrimers-Mediated Antisense Therapy. Adv. Pharm. Bull. 2017;7:179–187. doi: 10.15171/apb.2017.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mobarez A.M., Ahmadrajabi R., Khoramabadi N., Salmanian A.H. Recombinant flagellin-PAL fusion protein of Legionella pneumophila induced cell-mediated and protective immunity against bacteremia in BALB/c mice. World J. Microbiol. Biotechnol. 2017;33:175. doi: 10.1007/s11274-017-2315-5. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y., Yang Z., Dong Y., Chen Y. Recombinant PAL/PilE/FlaA DNA vaccine provides protective immunity against Legionella pneumophila in BALB/c mice. BMC Biotechnol. 2020;20:28. doi: 10.1186/s12896-020-00620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benedict K.M., Reses H., Vigar M., Roth D.M., Roberts V.A., Mattioli M., Cooley L.A., Hilborn E.D., Wade T.J., Fullerton K.E., et al. Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2013–2014. MMWR. Morb. Mortal. Wkly. Rep. 2017;66:1216–1221. doi: 10.15585/mmwr.mm6644a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Granseth G., Bhattarai R., Sylvester T., Prasai S., Livar E. Notes from the Field: Two Cases of Legionnaires’ Disease in Newborns After Water Births—Arizona, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2017;66:590–591. doi: 10.15585/mmwr.mm6622a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiss D., Boyd C., Rakeman J.L., Greene S.K., Fitzhenry R., McProud T., Musser K., Huang L., Kornblum J., Nazarian E.J., et al. A Large Community Outbreak of Legionnaires’ Disease Associated With a Cooling Tower in New York City, 2015. Public Health Rep. 2017;132:241–250. doi: 10.1177/0033354916689620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaikwad U.N., Jinna S. Environmental surveillance of Legionella pneumophila in distal water supplies of a hospital for early identification & prevention of hospital-acquired legionellosis. Indian J. Med Res. 2018;147:611–614. doi: 10.4103/ijmr.IJMR_527_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sikora A., Wójtowicz-Bobin M., Kozioł-Montewka M., Magryś A., Gładysz I. Prevalence of Legionella pneumophila in water distribution systems in hospitals and public buildings of the Lublin region of eastern Poland. Ann. Agric. Environ. Med. 2015;22:195–201. doi: 10.5604/12321966.1152064. [DOI] [PubMed] [Google Scholar]
- 87.Correia A.M., Ferreira J.S., Borges V., Nunes A., Gomes B., Capucho R., Gonçalves J., Antunes D.M., Almeida S., Mendes A., et al. Probable Person-to-Person Transmission of Legionnaires’ Disease. N. Engl. J. Med. 2016;374:497–498. doi: 10.1056/NEJMc1505356. [DOI] [PubMed] [Google Scholar]
- 88.Rota M.C., Caporali M.G., Bella A., Scaturro M., Giannitelli S., Ricci M.L. I risultati del sistema di sorveglianza della legionellosi in Italia nel 2020 durante la pandemia di COVID-19. Boll. Epidemiol. Naz. 2021;2:9–16. doi: 10.53225/BEN_019. [DOI] [Google Scholar]
- 89.Lagana P., Soraci L., Gambuzza M.E., Mancuso G., Delia S.A. Innate Immune Surveillance in the Central Nervous System Following Legionella pneumophila Infection. CNS Neurol. Disord. Drug Targets. 2017;16:1080–1089. doi: 10.2174/1871527316666171123210420. [DOI] [PubMed] [Google Scholar]
- 90.WHO—World Health Organization—Legionella and the Prevention of Legionellosis. [(accessed on 7 January 2022)]. Available online: https://www.who.int/
- 91.Gamage S.D., Ambrose M., Kralovic S.M., Roselle G.A. Water Safety and Legionella in Health Care: Priorities, Policy, and Practice. Infect. Dis. Clin. N. Am. 2016;30:689–712. doi: 10.1016/j.idc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 92.Orlikowski J., Ryl J., Jazdzewska A., Krakowiak S. Effect of Thermal Shock During Legionella Bacteria Removal on the Corrosion Properties of Zinc-Coated Steel Pipes. J. Mater. Eng. Perform. 2015;25:2711–2719. doi: 10.1007/s11665-016-2103-7. [DOI] [Google Scholar]
- 93.Cates E.L., Torkzadeh H. Can incorporation of UVC LEDs into showerheads prevent opportunistic respiratory pathogens? Microbial behavior and device design considerations. Water Res. 2019;168:115163. doi: 10.1016/j.watres.2019.115163. [DOI] [PubMed] [Google Scholar]
- 94.Springston J.P., Yocavitch L. Existence and control of Legionella bacteria in building water systems: A review. J. Occup. Environ. Hyg. 2017;14:124–134. doi: 10.1080/15459624.2016.1229481. [DOI] [PubMed] [Google Scholar]
- 95.Zhou J., Wang T., Xie X. Rationally designed tubular coaxial-electrode copper ionization cells (CECICs) harnessing non-uniform electric field for efficient water disinfection. Environ. Int. 2019;128:30–36. doi: 10.1016/j.envint.2019.03.072. [DOI] [PubMed] [Google Scholar]
- 96.Cloutman-Green E., Barbosa V.L., Jimenez D., Wong D., Dunn H., Needham B., Ciric L., Hartley J.C. Controlling Legionella pneumophila in water systems at reduced hot water temperatures with copper and silver ionization. Am. J. Infect. Control. 2019;47:761–766. doi: 10.1016/j.ajic.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Casini B., Aquino F., Totaro M., Miccoli M., Galli I., Manfredini L., Giustarini C., Costa A.L., Tuvo B., Valentini P., et al. Application of Hydrogen Peroxide as an Innovative Method of Treatment for Legionella Control in a Hospital Water Network. Pathogens. 2017;6:15. doi: 10.3390/pathogens6020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sciuto E., Laganà P., Filice S., Scalese S., Libertino S., Corso D., Faro G., Coniglio M. Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms. 2021;9:577. doi: 10.3390/microorganisms9030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paranjape K., Bédard É., Whyte L.G., Ronholm J., Prévost M., Faucher S.P. Presence of Legionella spp. in cooling towers: The role of microbial diversity, Pseudomonas, and continuous chlorine application. Water Res. 2020;169:115252. doi: 10.1016/j.watres.2019.115252. [DOI] [PubMed] [Google Scholar]
- 100.Mouchtouri V.A., Goutziana G., Kremastinou J., Hadjichristodoulou C. Legionella species colonization in cooling towers: Risk factors and assessment of control measures. Am. J. Infect. Control. 2010;38:50–55. doi: 10.1016/j.ajic.2009.04.285. [DOI] [PubMed] [Google Scholar]
- 101.Digiano F.A., Zhang W. Pipe Section Reactor to Evaluate Chlorine-Wall Reaction. J. Am. Water Work. Assoc. 2005;97:74–85. doi: 10.1002/j.1551-8833.2005.tb10805.x. [DOI] [Google Scholar]
- 102.Kirmeyer G.J., LeChevallier M., Barbeau H., Martel K., Thompson G., Radder L., Klement W., Flores A. Optimizing Chloramine Treatment. 2nd ed. Prepared for the Water Research Foundation; Denver, CO, USA: 2004. [Google Scholar]
- 103.Vincenti S., de Waure C., Raponi M., Teleman A.A., Boninti F., Bruno S., Boccia S., Damiani G., Laurenti P. Environmental surveillance of Legionella spp. colonization in the water system of a large academic hospital: Analysis of the four–year results on the effectiveness of the chlorine dioxide disinfection method. Sci. Total Environ. 2019;657:248–253. doi: 10.1016/j.scitotenv.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 104.Muraca P.W., Goetz A., Yu V.L. Disinfection of Water Distribution Systems for Legionella: A Review of Application Procedures and Methodologies. Infect. Control Hosp. Epidemiol. 1990;11:79–88. doi: 10.2307/30144266. [DOI] [PubMed] [Google Scholar]
- 105.Buse H.Y., Hall J.S., Hunter G.L., Goodrich J.A. Differences in UV-C LED Inactivation of Legionella pneumophila Serogroups in Drinking Water. Microorganisms. 2022;10:352. doi: 10.3390/microorganisms10020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bertolino G., Marras L., Sanna C., Carrucciu G., Schintu M., Coroneo V. Ten-Year Retrospective Analysis of Legionella Diffusion in Hospital Water Systems and Its Serogroup Seasonal Variation. Adv. Exp. Med. Biol. 2020;1282:93–103. doi: 10.1007/5584_2020_484. [DOI] [PubMed] [Google Scholar]
- 107.Percival S.L., Williams D.W. In: Microbiology of Waterborne Diseases. 2nd ed. Percival S.L., Yates M.V., Williams W.D., Chalmers R.M., Gray N.F., editors. Academic Press; Cambridge, MA, USA: 2014. pp. 155–175. Chapter 8. [DOI] [Google Scholar]
- 108.Ditommaso S., Giacomuzzi M., Memoli G., Garlasco J., Zotti C.M. The use of BCYE medium for the detection of Legionella in environmental water samples: An appropriate update to ISO 11731:2017 standard? Diagn. Microbiol. Infect. Dis. 2022;102:115593. doi: 10.1016/j.diagmicrobio.2021.115593. [DOI] [PubMed] [Google Scholar]
- 109.Water Quality-Enumeration of Legionella. International Organization for Standardization; Geneva, Switzerland: 2017. [(accessed on 20 January 2022)]. Available online: https://www.iso.org/standard/61782.html. [Google Scholar]
- 110.Kimura S., Tateda K., Ishii Y., Horikawa M., Miyairi S., Gotoh N., Ishiguro M., Yamaguchi K. Pseudomonas aeruginosa Las quorum sensing autoinducer suppresses growth and biofilm production in Legionella species. Microbiology. 2009;155:1934–1939. doi: 10.1099/mic.0.026641-0. [DOI] [PubMed] [Google Scholar]
- 111.Chaudhry R., Sreenath K., Agrawal S.K., Valavane A. Legionella and Legionnaires’ Disease: Time to Explore in India. Indian J. Med. Microbiol. 2018;36:324–333. doi: 10.4103/ijmm.IJMM_18_298. [DOI] [PubMed] [Google Scholar]
- 112.Mercante J.W., Winchell J.M. Current and Emerging Legionella Diagnostics for Laboratory and Outbreak Investigations. Clin. Microbiol. Rev. 2015;28:95–133. doi: 10.1128/CMR.00029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Falzone L., Musso N., Gattuso G., Bongiorno D., Palermo C.I., Scalia G., Libra M., Stefani S. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int. J. Mol. Med. 2020;46:957–964. doi: 10.3892/ijmm.2020.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Falzone L., Gattuso G., Tsatsakis A., Spandidos D.A., Libra M. Current and innovative methods for the diagnosis of COVID-19 infection (Review) Int. J. Mol. Med. 2021;47:100. doi: 10.3892/ijmm.2021.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Musso N., Falzone L., Stracquadanio S., Bongiorno D., Salerno M., Esposito M., Sessa F., Libra M., Stefani S., Pomara C. Post-Mortem Detection of SARS-CoV-2 RNA in Long-Buried Lung Samples. Diagnostics. 2021;11:1158. doi: 10.3390/diagnostics11071158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stout J.E., Yu V.L. Legionellosis. N. Engl. J. Med. 1997;337:682–687. doi: 10.1056/NEJM199709043371006. [DOI] [PubMed] [Google Scholar]
- 117.Lindsay D.S.J., Abraham W.H., Findlay W., Christie P., Johnston F., Edwards G.F.S. Laboratory diagnosis of legionnaires’ disease due to Legionella pneumophila serogroup 1: Comparison of phenotypic and genotypic methods. J. Med. Microbiol. 2004;53:457. doi: 10.1099/0022-1317-53-5-457. [DOI] [PubMed] [Google Scholar]
- 118.Rantakokko-Jalava K., Jalava J. Development of Conventional and Real-Time PCR Assays for Detection of Legionella DNA in Respiratory Specimens. J. Clin. Microbiol. 2001;39:2904–2910. doi: 10.1128/JCM.39.8.2904-2910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bonetta S., Ferretti E., Balocco F., Carraro E. Evaluation ofLegionella pneumophilacontamination in Italian hotel water systems by quantitative real-time PCR and culture methods. J. Appl. Microbiol. 2010;108:1576–1583. doi: 10.1111/j.1365-2672.2009.04553.x. [DOI] [PubMed] [Google Scholar]
- 120.Touron-Bodilis A., Pougnard C., Frenkiel-Lebossé H., Hallier-Soulier S. Usefulness of real-time PCR as a complementary tool to the monitoring of Legionella spp. and Legionella pneumophila by culture in industrial cooling systems. J. Appl. Microbiol. 2011;111:499–510. doi: 10.1111/j.1365-2672.2011.05063.x. [DOI] [PubMed] [Google Scholar]
- 121.Collins S., Stevenson D., Walker J., Bennett A. Evaluation of Legionella real-time PCR against traditional culture for routine and public health testing of water samples. J. Appl. Microbiol. 2017;122:1692–1703. doi: 10.1111/jam.13461. [DOI] [PubMed] [Google Scholar]
- 122.Benitez A.J., Winchell J.M. Clinical application of a multiplex real-time PCR assay for simultaneous detection of Legionella species, Legionella pneumophila, and Legionella pneumophila serogroup 1. J. Clin. Microbiol. 2014;52:709. doi: 10.1128/JCM.03391-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benitez A.J., Winchell J.M. Rapid detection and typing of pathogenic nonpneumophila Legionella spp. isolates using a multiplex real-time PCR assay. Diagn. Microbiol. Infect. Dis. 2016;84:298–303. doi: 10.1016/j.diagmicrobio.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.El Basha N.R., Shaaban H.H., El Atroush H.A., Sherif M.M., El Kholy A.A. The use of multiplex PCR for the detection of atypical pathogens in Egyptian children with CAP: A high rate of Bordetella pertussis in early infancy. J. Egypt. Public Health Assoc. 2019;94:5. doi: 10.1186/s42506-018-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falzone L., Gattuso G., Lombardo C., Lupo G., Grillo C.M., Spandidos D.A., Libra M., Salmeri M. Droplet digital PCR for the detection and monitoring of Legionella pneumophila. Int. J. Mol. Med. 2020;46:1777–1782. doi: 10.3892/ijmm.2020.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Logan-Jackson A., Rose J.B. Cooccurrence of Five Pathogenic Legionella spp. and Two Free-Living Amoebae Species in a Complete Drinking Water System and Cooling Towers. Pathogens. 2021;10:1407. doi: 10.3390/pathogens10111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Logan-Jackson A.R., Rose J.B. Water Age Effects on the Occurrence and Concentration of Legionella Species in the Distribution System, Premise Plumbing, and the Cooling Towers. Microorganisms. 2021;10:81. doi: 10.3390/microorganisms10010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marinelli L., Cottarelli A., Solimini A.G., Del Cimmuto A., De Giusti M. Evaluation of timing of re-appearance of VBNC Legionella for risk assessment in hospital water distribution systems. Ann. Ig. 2017;29:431–439. doi: 10.7416/ai.2017.2175. [DOI] [PubMed] [Google Scholar]
- 129.Eble D., Gehrig V., Schubert-Ullrich P., Köppel R., Füchslin H. Comparison of the culture method with multiplex PCR for the confirmation of Legionella spp. and Legionella pneumophila. J. Appl. Microbiol. 2021;131:2600–2609. doi: 10.1111/jam.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peci A., Winter A.-L., Gubbay J.B. Evaluation and Comparison of Multiple Test Methods, Including Real-time PCR, for Legionella Detection in Clinical Specimens. Front. Public Health. 2016;4:175. doi: 10.3389/fpubh.2016.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ueda A., Oki M., Yanagi H., Ozawa H., Takagi A. Clinical Characteristics of Legionella Pneumonia Diagnosed with Legionella Urinary Antigen Test. Tokai J. Exp. Clin. Med. 2016;41:8–13. [PubMed] [Google Scholar]
- 132.Fields B.S., Benson R.F., Besser R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Badoux P., Kracht-Kosten L., Herpers B., Euser S. Method Comparison of the ImmuView L. pneumophila and L. longbeachae Urinary Antigen Test with the BinaxNOW Legionella Urinary Antigen Card for Detection of Legionella pneumophila Serogroup 1 Antigen in Urine. J. Clin. Microbiol. 2020;58:1429. doi: 10.1128/JCM.01429-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data described in the present manuscript are all available on PubMed NCBI.