Abstract
Pathologies of the vasculature including the microvasculature are often complex in nature, leading to loss of physiological homeostatic regulation of patency and adequate perfusion to match tissue metabolic demands. Microvascular dysfunction is a key underlying element in the majority of pathologies of failing organs and tissues. Contributing pathological factors to this dysfunction include oxidative stress, mitochondrial dysfunction, endoplasmic reticular (ER) stress, endothelial dysfunction, loss of angiogenic potential and vascular density, and greater senescence and apoptosis. In many clinical settings, current pharmacologic strategies use a single or narrow targeted approach to address symptoms of pathology rather than a comprehensive and multifaceted approach to address their root cause. To address this, efforts have been heavily focused on cellular therapies and cell-free therapies (e.g., exosomes) that can tackle the multifaceted etiology of vascular and microvascular dysfunction. In this review, we discuss 1) the state of the field in terms of common therapeutic cell population isolation techniques, their unique characteristics, and their advantages and disadvantages, 2) common molecular mechanisms of cell therapies to restore vascularization and/or vascular function, 3) arguments for and against allogeneic versus autologous applications of cell therapies, 4) emerging strategies to optimize and enhance cell therapies through priming and preconditioning, and, finally, 5) emerging strategies to bolster therapeutic effect. Relevant and recent clinical and animal studies using cellular therapies to restore vascular function or pathologic tissue health by way of improved vascularization are highlighted throughout these sections.
Keywords: angiogenesis, cell therapy, endothelial dysfunction, stem cells, vascularization
INTRODUCTION
Given the importance of the vasculature, especially the microvasculature in tissue health, it is not surprising that compromised or dysfunctional vessels contribute to many disease conditions. In numerous cases, microvascular repair or regeneration to restore microcirculatory health are necessary components to treat the disease. Instances of microvascular insufficiency, i.e., situations of reduced perfusion due to low vascular densities and/or compromised vasodynamics, contribute to or cause tissue ischemia, nonhealing ulcers, limited tissue metabolism and health, and even complications related to aging (1–8). Loss of microvessel integrity due to insult or disease results in microvascular collapse during tissue inflammation and repair, complicating healing (9, 10).
Microvascular dysfunction in the coronary circulation is often missed by angiography due to the small nature of the vessels but instead can be determined by the coronary flow reserve (CFR), a metric obtained via Doppler ultrasound (11–13). CFR is the ratio of hyperemic coronary flow velocity during an adenosine or dobutamine stress compared with resting coronary flow velocity, and a CFR ≤ 2.5 is considered coronary microvascular dysfunction (CMD) (11, 14). CMD can also be defined as endothelial dysfunction with constriction to acetylcholine, and <20% coronary dilation to nitroglycerin (14). CMD has been correlated to peripheral microvascular dysfunction as well as skin microvascular function with retinal microangiopathy and renal microvascular restrictive index, supporting the current discussion that skin microvascular function should be used as a screening indicator of retinal, renal, and possibly cardiac microvascular functions (15–17). Although skin microvascular function can be measured with a variety of non- to mildly invasive clinical assessments (18), more studies are needed to determine if skin microvascular function is an accurate indicator of target organ microvascular function.
Microvascular regeneration or repair is a multistage process involving progressive changes in microvessel phenotypes and microvascular networks. Importantly, repairs leading to effective perfusion involve not only deriving new microvessels and vessel segments, but the coordinated organization of these elements into a new or existing network that now meets the perfusion, metabolic, and functional demands of the tissue (19–21). Adaptation of the neovessels in the context of this network reorganization is a dynamic, multicell process involving neovessel pruning or remodeling into arterioles, capillaries, or venules (22). Critically important to vascularization outcomes is the establishment of vasoactive regulation of the new microvasculature to enable proper physiological responses to tissue function (1, 2, 23, 24).
Current pharmacological clinical approaches in microvascular pathologies are aimed at treating their symptomatic consequences. For example, in CMD, clinically indicated first line treatment includes β blockers to reduce inotropism to preserve oxygen demand and reduce chest pain as well as enhance vasorelaxation by blocking renin release (25). However, such an approach does not address the pathologic microvessels themselves or their regeneration. For pathologies such as myocardial infarction (MI) or peripheral artery disease, microvascular regeneration is still limited to the experimental realm (26–28). Recombinant vascular endothelial growth factor (VEGF) and other growth factor therapies for microvascular regeneration following MI, for instance, have completed the clinical trials, with the Vascular Endothelial Growth Factor in Ischemia for Vascular Angiogenesis, Euroinject One Trial, and Kuopio Angiogenesis trial reporting nonsignificant effects relative to placebo (19). Given the biological complexity intrinsic to microvascular regeneration, repair, and tissue vascularization, many cell-based approaches are showing greater promise in regenerating or improving microvascular form and function. Here, these cell therapies act mostly in a paracrine factor through secreted factors or exosomes [containing factors, microRNA (miRNA), etc.], or through direct cell interactions such as differentiation, or also through multiple cell interactions such as interactions of donor perivascular niche with endothelial cells (29–32).
There are numerous tissue sources for cells and cell systems capable of regenerating or repairing the microvasculature, each with distinct advantages and disadvantages. Equally varied are the molecular and physiological mechanisms by which these cells elicit a therapeutic benefit. Cellular therapies and cell-free therapies using exosomes or conditioned media have been known to promote angiogenesis, stabilize microvasculatures, and restore vasoactivity to improve vascular densities and perfusion (23, 24, 33). These effects can be accompanied by reduced oxidative stress, normalized mitochondrial and endoplasmic reticular (ER) function, and limited vascular cell senescence and apoptosis (34, 35). The significant potential of vascular and microvascular cell-based therapies are being realized in ongoing and recently completed clinical trials, building on current levels of understanding and highlighting future directions for investigation. The objective of this review is to integrate current progress along with the major issues that span stem cell and vascular research fields, with emphasis on microvascular regeneration where applicable.
STEM CELL SOURCES, UNIQUE CHARACTERISTICS, ADVANTAGES, AND DISADVANTAGES
Stem cells are defined by their ability to self-renew and differentiate. However, their potential to carry out those abilities is variable throughout development and through the lifespan (36). Since their discovery in the early 1960s (37), there has been a race for discovering sources of stem cells that can be used therapeutically, including for microvascular repair. In this section, we briefly detail each of the major sources of stem cells and provide a summary of their methods of isolation (also described in Fig. 1) as well as the unique characteristics, advantages, and disadvantages of each stem cell type (Table 1).
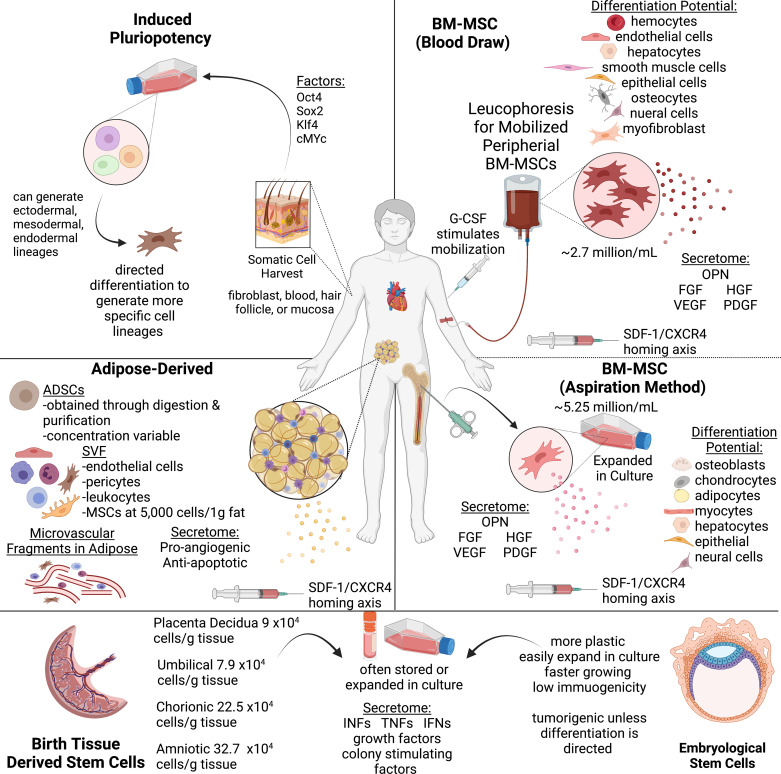
Figure 1.
Cell sources and isolation strategies. Locational sources and strategies for isolation and differentiation of stem cells including bone marrow-derived mesenchymal cells, adipose-derived cells and microvascular fragments, stem cells from birth derived tissues, and embryonic stem cells. Image created with BioRender.com. ADSCs, adipose derived stem cells; BM-MSCs, bone marrow mesenchymal stem cells; CXCR, C-X-C chemokine receptor; FGF, fibroblast growth factor; G-CSF, granulocyte colony stimulating factor; HGF, hepatocyte growth factor; MSCs, mesenchymal stem cells; OPN, osteopontin; SDF-1, stromal cell derived factor-1; SVF, stromal vascular fraction; VEGF, vascular endothelial growth factor.
Table 1.
Advantageous and disadvantageous qualities of stem cell sources
| Advantage | Disadvantage | |
|---|---|---|
| Bone marrow | Enhanced engraftment and retention of other stem cells Greater transcriptomic heterogeneity (vs. adipose MSC) |
Higher immunogenicity (vs. ADSC) Invasive harvesting procedure Lower cell yield (vs. ADSC)Enter senescence earlier (vs. ADSC) |
| Adipose | Ease of isolationHigher cell yield (vs. BM-MSC) Autologous potential Low immunogenicity (vs. BM-MSC) Higher immunosuppression capacity (vs. BM-MSC) Heterogeneous cell population |
Lower transcriptomic heterogeneity (vs. BM-MSC) Poor survival/engraftmentInconsistency among donors/samples |
| Human umbilical | High concentration of cells Limited senescence during passaging |
Genomic/chromosomal tests may be needed to rule out abnormalities |
| Embryonic | High plasticity Low immunogenicity |
Ethical concernsRisk of teratoma formation |
| Amniotic | High plasticity High concentration of cells |
Only available starting second trimester limiting autologous use. |
| Placental | Chorionic Villi MSC: Available first trimester Ideal for fetal therapeutic applications Limited senescence during passaging |
Chorionic Villi MSC: Relatively poor engraftment/retention |
| Induced pluripotent | High plasticity Higher immunosuppression Multiple locations for harvest/minimally invasive |
hiPSC-CM: Poor retention/engraftmentHigh costLong time to procure |
| Microvascular fragments | Quicker revascularization of target tissue Supplement to other stem cell sources |
Lower cell fraction than SVF (macrophages, stem cells, etc.) |
ADSCs, adipose derived stem cells; BM-MSCs, bone marrow mesenchymal stem cells; hiPSC-CM, human induced pluripotent stem cell; MSCs, mesenchymal stem cells; SVF, stromal vascular fraction.
Bone Marrow-Derived Stem Cells
The normal function of the bone marrow is to generate hemocytes for circulation in the blood. Beyond hemogenesis, bone marrow contains mesenchymal stem cells (BM-MSCs) that can differentiate into supporting cell types within the blood/bone niche, such as osteoblasts, chondrocytes, and myocytes (38). Once isolated, BM-MSCs can differentiate beyond their biological niche and follow lineages of endothelial cells, myocytes, hepatocytes, epithelial cells, or neural cells generated by coculturing with cytokines during in vitro expansion for cell therapy applications (39).
One of the first methods of isolation entails aspirating the bone marrow from the superior iliac crest under mild anesthesia (conscious sedation followed by local anesthetic) (40, 41). However, a more recent, less invasive method involves mobilizing the BM-MSCs pharmacologically with granulocyte colony-stimulating factor, then harvesting by a peripheral blood draw and isolation with fibrin microbead binding (42). Direct comparison of the concentration of mesenchymal stem cells (MSCs) between the two methods showed that bone aspirate yielded ∼5.25 million cells/mL versus a yield of ∼2.7 million cells/mL using the blood draw method (43). In addition, studies have shown that both methods are similar in terms of cell survival, development of graft versus host disease, and differentiation capacity in vitro, however, the peripheral blood draw method is favored for cell therapy/microvascular repair therapies due to a greater ease of harvest and less patient discomfort (44, 45).
Adipose-Derived Stem Cells
Like BM-MSCs, adipose-derived stem cells (ADSCs) can also serve as a source of multipotent MSCs to be used for cell therapy. Primary benefits relate to the ease in harvesting adipose tissue, usually via lipo-aspiration, a higher cell yield due to the presence of more stem cells in the fat than bone marrow, and less patient discomfort (46). ADSCs are constituents of the stromal vascular fraction (SVF)—a collection of mesenchymal and microvascular cells, including perivascular support cells (47). Enzymatic-based isolation methods to isolate the SVF use collagenase, centrifugation, and filtration, giving a much higher cell yield than mechanical disruption alone. This has led to the development of medical equipment that performs this automated process in the operating suite (48).
SVF contains a nonuniform mixture of cells that work together via directed differentiation and paracrine signaling pathways to enhance revascularization via angiogenesis (49, 50). The heterogeneous population of SVF is composed of MSCs (CD90), endothelial cells (CD31), pericytes (CD146), hematopoietic stem cells (CD45), macrophages (CD11b), B (CD19) and T (CD4) cells, dendritic cells (CD14), and endothelial progenitor cells (EPCs). SVF can be further refined to produce homogeneous proangiogenic cell populations such as adipose-derived mesenchymal stem cells (AD-MSCs) or EPCs (51, 52). Albeit, EPC therapy in-it-of-itself is understudied and is limited by ambiguous definition, overlapping markers, diverse culturing methods, and limited understanding of mechanisms of action (53). Alternatively, therapy with whole SVF is known to increase circulation of host regenerative cells such as EPCs (54, 55). Overall, SVF has been shown to contain a higher stromal cell population than bone marrow (56). In addition, the benefit of having multiple locations for harvesting and yielding more cell populations gives adipose tissue a greater autologous benefit (57). In summary, adipose tissue (specifically SVF) offers an arguably greater benefit for microvascular repair therapies because of its multiple locations available for harvesting, ease of harvest, and heterogeneous cell populations that can help facilitate physiologic vascular remodeling (57, 58).
Embryonic and Induced Pluripotent Stem Cells
Embryonic stem cells (ESCs) are derived from the inner mass of the blastocyst resulting in embryo destruction or from single blastomeres as an embryo-preserving technique that is similar to preimplantation genetic testing routinely used during assisted reproduction. Clinical grade human ESCs are typically derived from donated excess in vitro fertilization cryopreserved embryos and then stored long-term as cell lines. The use of ESCs has been a historically inflammatory subject, particularly in the United States where laws around ESC research vary widely by state. With the development of induced pluripotent stem cells (iPSCs), enthusiasm for ESC research has somewhat faded (59). iPSCs are adult somatic cells that have been induced to pluripotency using the Yamanaka transcription factors. Both ESCs and iPSCs are capable of self-renewal without differentiation or with differentiation into many different cell lineages and/or types (60, 61). Published protocols have demonstrated that iPSCs and ESCs can be coaxed into different lineages, including vascular endothelial cells (62), smooth muscle cells (63), and cardiomyocytes (64, 65).
One example of a protocol to differentiate iPSCs into endothelial cells (iPSC-ECs) was demonstrated by Ikuno et al. (66) using VEGF and cyclic adenosine monophosphate (cAMP) after the iPSCs were directed down a mesodermal germ lineage. iPSC-ECs have been shown to be capable of forming microvascular structures in an in vitro three-dimensional (3-D) culture (60) and in vivo (67). iPSC-ECs and human umbilical vein endothelial cells (HUVECs) were compared for angiogenic potential in 3-D culture and in vitro (mice) to determine the regenerative potential of iPSC-ECs in their current state of therapeutic development (60). At 2 wk in 3-D culture, capillary sprouting was much more abundant in the HUVEC samples whereas the iPSC-ECs had significantly decreased total network length, fewer vessel branch points, and fewer segments formed compared with the HUVEC samples. Inferior angiogenic potential of iPSC versus HUVEC was attributed to lower matrix metalloproteinase-9 (MMP-9) expression and activity, highlighting the necessity for standardization of iPSC production (60). In vitro, both iPSC-EC and HUVEC implants formed vessels containing lumens and were perfused by the host following implantation, as measured by the presence of erythrocytes, at days 7 and 14 (67). By day 7, both groups demonstrated comparable perfusion of the matrices and by day 14 vessel densities were comparable between the groups. The iPSC-ECs had significantly less α-smooth muscle actin from fibroblast-derived pericyte-coated vessels than HUVECs, indicating lower vascular maturity. Further research is needed to overcome the barriers to iPSC-EC translation potential such as improving the maturity of these vessel-like structures as well as investigating whether modifying iPSC-EC MMP-9 expression or activity can boost angiogenic efficiency of iPSCs similar to HUVECs.
A more robust differentiation protocol has been developed by Wang et al. in 2020. Delivery of modified messenger RNA (mRNA) for the erythroblast transformation-specific variant transcription factor 2 during the intermediate mesodermal stage of differentiation allowed vigorous and reproducible differentiation of 13 human iPSC lines into iPSC-ECs with high efficiency, forming perfused vascular networks in vivo that were lined primarily with the human iPSC-ECs. Six-week-old NOD-SCID mice were subdermally implanted with 1 × 106 iPSC-ECs in a collagen 1, fibrin, fibroblast growth factor (FGF), and erythropoietin matrix. After 7 days, the grafts were perfused and were phenotypically, transcriptionally, and functionally comparable with host endothelial cells (68). Effectiveness of iPSC-EC may be further enhanced by methods to facilitate maturation of cell function, as has been done in iPSC-derived cardiomyocytes by optimizing culture conditions such as extracellular matrix or 3-D culture conditions mimicking host environment. Indeed, growth of iPSC-ECs co-cultured with iPSC-derived spinal motor neurons in spinal cord organoid chips leads to increased maturation and transcription of vascular interaction pathways over growth in 96-well plates. Additional studies are necessary to determine appropriate maturation conditions. Overall, the continued development of iPSC-ECs is warranted, as it allows for opportunities to tailor treatment to individual patient-specific conditions, such as iPSC-EC microvascular grafts that produce factor VIII for hemophilia A (69).
One prevailing argument against the use of cellular therapeutics in a clinical setting is the risk of tumor or teratoma formation; however, the risk of tumorigenesis is not the same across all cell types (70, 71). Specifically, the high level of plasticity of ESCs and iPSCs makes them hard to control or predict the lineage after delivery (72, 73) and can result in the generation of teratomas following ESCs or iPSC administration (74). Nevertheless, studies have shown that even this can be combatted by directing ESC lineage in vitro before administration, which leads to reduced teratoma formation. For example, teratomas were not observed in over 200 animals transplanted with human ESC-derived cardiomyocytes when direct differentiation was used to establish a mesodermal or progenitor cardiomyocyte phenotype first (75). Moving forward, strict protocols such as this should be developed and followed to standardize a priming method for these cells that do not risk teratoma formation. Most obviously, the biggest ethical concern comes from the destruction of a viable human embryo in ESC research and clinical application (70, 76). In vitro fertilization produces multiple viable embryos that cannot all be implanted, and extras have been historically donated to research or stored in a biobank as an alternative to being discarded. Since the therapeutic use of ESC may not be viable due to ethical concerns, use of cell sources with similar properties to ESCs should be considered.
Umbilical, Amniotic, and Placental-Derived Stem Cells
Although inducing pluripotency poses a time and cost barrier and use of embryological stem cells is controversial, extra-fetal tissues present a multipotent-ready source of stem cells. These include tissues from the layers of the placental membrane after birth, amniotic fluid at different time points in utero, and the umbilical cord, making extra-fetal tissues relatively free of risks and ethical issues (77). From the umbilical cord, there are several MSC populations (umbilical cord blood-derived mesenchymal stem cell; UCB-MSCs) that can be isolated from the cord, cord lining, subamnion, or Whartons Jelly (78). Amniotic MSCs can be procured at any time during pregnancy, usually during diagnostic amniocentesis, or in some cases, therapeutic amnioreduction with the fluid undergoing centrifugation and culture (79). Placental MSCs are procured from whole organ during delivery or from diagnostic chorionic villus sampling specimens through digestion, centrifugation, and culture and have been shown to enhance angiogenesis in wound healing models after therapeutic delivery (79–81). The use of extra-fetal stem cells may be especially poignant for fetal interventions given their relation, however, their use is also applicable in the adult population considering some of their inherent advantages over adult stem cells (82).
Unique Regenerative Mechanisms, Advantages, and Disadvantages of Various Stem Cells
Although the proposed mechanisms of effect are similar across cell sources, there are a few nuances that distinguish the various stem cell sources. Bone marrow aspiration is an invasive procedure and it leads to a relatively lower cell yield compared with adipose. However, since BM-MSCs were one of the first cell therapy sources discovered they have been more heavily researched (83). On the contrary, ADSC yield is inconsistent among donors but provide a heterogeneous source of supporting cells and MSCs (84, 85). Despite inconsistencies in fat depot volumes and ADSC yields, MSCs from adipose tissue are ∼500 times more (∼5,000 cells/1 g fat) abundant than in BM-MSCs (57, 86). Furthermore, ADSCs enter senescence later than BM-MSCs, possess a greater proliferative capacity, and have superior protein secretion (FGF and insulin-like growth factor) and immunomodulatory effects (87). On the other hand, BM-MSCs show a preferential benefit in differentiating into osteogenic and chondrogenic lineages as well as benefits in proteins secreted [stromal cell derived factor-1 (SDF-1) and hepatocyte growth factor (HGF)] (88). This reveals the importance of selecting the appropriate source for a given clinical application.
To get a better understanding of the genomic landscape of adipose versus bone marrow MSCs, single-cell RNA sequencing data revealed these main differences: ADSCs showed higher immunosuppression capacity, lower transcriptomic heterogeneity, and lower immunogenicity than BM-MSCs, whereas BM-MSCs showed higher levels of metabolic activity, respiration, and oxygen consumption (89). Whether these differences impact effectiveness of microvascular regeneration should be investigated.
Despite the limited number of cells retrieved at the time of harvest, stem cells from bone marrow or adipose tissue can be expanded in culture but often undergo differentiation or enter senescence following 8 wk in culture (90). However, MSCs taken from birth-derived tissues, such as umbilical, placental, and Wharton’s Jelly-derived MSCs have a high proliferative capacity in culture and do not undergo senescence after prolonged passaging (86). Their collection is also noninvasive. One thing to note, though, is the health status of the fetus may not be known; therefore, genomic and chromosomal tests may need to be done to ensure no donor cell abnormalities (78).
Induced pluripotent stem cells have been shown to have similar plasticity to embryonic sources in terms of karyotype, phenotype, telomerase activity, and capacity for differentiation (70). However, they have also been shown to harbor epigenetic changes that can alter their differentiation capacity away from embryonic like cell types toward somatic cell types (91). In addition, iPSCs take a long time to expand and direct their differentiation, and thus are associated with a higher cost (92). Furthermore, iPSCs possess the ability to mobilize and engraft as well, but not along the same homing SDF-1/C-X-C motif chemokine receptor 4 (CXCR4) axis as BM- or AD-MSCs. iPSCs ability to engraft is mediated by integrin-β1 adhesion and requires lineage direction for targeting tissue in vascular therapeutics (93).
Angiogenic mechanisms have been shown to be different between cell sources and should be considered when choosing a source for cellular therapy. In fibrin-angiogenesis models, AD-MSCs use plasminogen activator-plasmin axis by endothelial cells for vessel invasion and elongation, with MMPs serving to regulate capillary diameter. Furthermore, they exhibit upregulation of angiogenic factors urokinase plasminogen activator, HGF, and tumor necrosis factor-α (TNFα) (94). This aspect of AD-MSC angiogenesis is more akin to fibroblast-mediated angiogenesis than BM-MSC-mediated angiogenesis. BM-MSCs execute angiogenesis in part through MMPs without involvement of the plasminogen activator-plasmin axis (95).
Paracrine angiogenic mechanism between cell sources is also known to vary and has been elegantly reviewed by Maacha et al. (96). To briefly summarize, MSC exosomes have been shown to carry proangiogenic miRNAs and differ between sources. MicroRNAs are small-size noncoding RNAs that negatively affect protein expression post-transcription and have recently garnered a lot of attention. Some proangiogenic miRNAs of AD-MSC exosomes include miRNA 148, 532-5p, 378, let-7f, 125a, 31, and 181b, with the latter three repressing antiangiogenic δ like canonical notch ligand 4, hypoxia inducing factor 1 (HIF-1), and transient receptor potential cation channel subfamily M member 7 expression (96). BM-MSC exosomes carry proangiogenic miRNAs 132, 494, 19a, 21a-5p, 210-3p, and 210 with the latter repressing antiangiogenic Efna3 expression in endothelial cells (96, 97). Finally, endometrial MSC exosomes have been shown to carry proangiogenic miRNA-21-5p. In terms of soluble factors, the total angiogenic potential is characterized as highest in Wharton’s Jelly, BM, and placental MSCs versus lower potential in AD- and UCB-MSCs (96). The AD-MSC secretome was shown to be high in VEGF/VEGF-D, insulin-like growth factor 1, interleukin (IL)-8, MMP-3, and MMP-9 and relatively lower in transforming growth factor-β (TGFβ-1), VEGF-A, HGF, bFGF, and angiopoietin-1. BM-MSCs were characterized as high in VEGF-D and lower relatively in VEGF, macrophage colony-stimulating factor, IL-1ra, SDF-1α, MCP-1, IGF-1, IL-8, MMP-3, and MMP-9. Amniotic MSC secretome was characterized as high in VEGF, TGFβ-1, VEGF-A, HGF, bFGF, and angiopoietin-1 (96). These differences may have functional impact for various therapeutic applications. When choosing sources of stem cells, attention should be paid to specific mechanisms of action pertinent to each cell type for the hypothesis in question, as well as the advantages and disadvantages of each cell type.
MECHANISMS OF ACTION FOR STEM CELL REGENERATION OF VASCULAR FUNCTION
Around the early 2000s, the use of stem cells as a cellular therapy for stimulating microvascular growth garnered excitement based on observations of apparent cell adoption of a vascular cell phenotype and mapping to vascular fate. Based on these initial findings, it was thought that stem cells mediated their therapeutic abilities through direct differentiation replacing the injured cells of the host tissue (98). Although direct cell interactions including differentiation are indeed supported (99), the relatively low rate of vascular integration and evidence of little to no incorporation (100) suggested that paracrine mechanism might play a more dominant role. Thus, the prevailing theory today is the paracrine hypothesis, which states that stem cells exert their regenerative effects through secretion of factors that enhance or regulate physiological and molecular processes. Evidence of this notion comes from the ability to use stem cell-conditioned media and yielding the same or similar therapeutic effects. This section explores the specific mechanisms, both direct and indirect (paracrine) of stem cell-mediated microvascular regeneration. Stem cell effects on angiogenesis and immunomodulatory effects will be described in each subsection as relevant and all information is summarized in Fig. 2. Effects of each stem cell source on microvascular regenerative parameters specifically are summarized in Table 2. In further support of expanded impacts, additional examples of stem cell effects on functional dilation, atherosclerosis, mitochondrial function, ER stress, DNA and lipid oxidation, and vascular aging are also provided. In these sections, the focus shifts to the specific stem cell types (e.g., mesenchymal) that dominate the literature. The lack of parallel studies across stem cell sources emphasizes the need for systematic comparisons of stem cell effects for optimizing therapy translation.
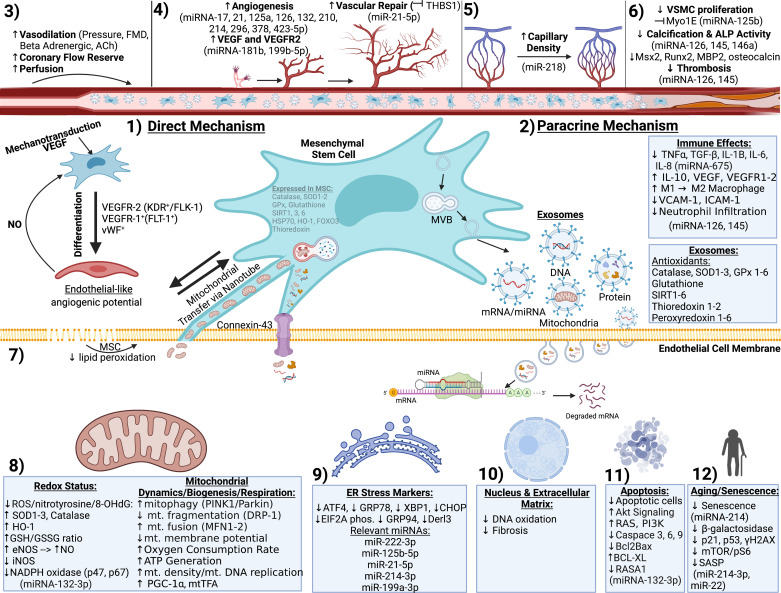
Figure 2.
Mechanisms of stem cell-mediated vascular functional regeneration. Mesenchymal stem cell therapies improve vascular health through direct (1) or paracrine (2) mechanisms to rejuvenate vasodilatory function (3), angiogenesis and vascular/capillary density (4 and 5), reduce calcification, smooth muscle proliferation, and thrombosis (6), reduce lipid peroxidation (7), attenuate oxidative stress and restore mitochondrial function (8), abrogate endoplasmic reticulum (ER) stress (9), DNA oxidation and fibrosis (10), apoptosis, and senescence (11). Image created with BioRender.com. eNOS, endothelial nitric oxide synthase; MSC, mesenchymal stem cell; MVB, multivesicular body; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
Table 2.
Microvascular regenerative effects of stem cell therapies
| Study Model | Administrative Route | Pathologic State | Microvascular End Points | Additional Results and Notes | Reference(s) |
|---|---|---|---|---|---|
| Bone-marrow MSCs | |||||
| Mice | Tail IV injection | Stroke |
|
|
(101,102) |
| Rats | Injection to ischemic areaTail IV injectionIntra-arterialIntracavernous injection | MIMICerebral ischemiaErectile dysfunction | Rats treated with MSCs displayed:
|
|
(103) (104) (105) (106) |
| Rabbit | Saline injection into ischemic thigh muscle | Hindlimb ischemia |
|
|
(107) |
| Human (phase I–II) | Trans endocardial injection | Nonischemic dilated cardiomyopathy |
|
(108) | |
| Human (phase I–II) (M/F 20–80 yr old) | Intra-arterial | Severe PAD |
|
|
(109) |
| Human(phase I–II) (M/F 18–80 yr old) | Myocardial injection | Myocardial ischemia/ coronary heart disease |
|
|
(110) |
| Adipose-derived MSCs | |||||
| Mice (exosome therapy) | Subcutaneous injection | Wound healing in diabetes |
|
↑upregulation of mmu_circ_0000250 miRNA in ADSC modified exosomes showed greater vascular density than ADSC exosomes alone | (111) |
| Rat | Intra-cardiac injection | MI | MSCs, relative to the no cell control, displayed:
|
Study compares BM-MSCs, AMSCs, and umbilical cord blood derived (UCBMSCs), as well as their exosomes. | (112) |
| Swine | Renal artery injection | Renal artery stenosis | MSC treated groups showed:
|
The study compared endothelial progenitor cells with adipose MSCs | (113) |
| iPSCs | |||||
| Rat (exosome therapy) | Intramuscular injection | Hindlimb ischemia | • ↑ micro-vessels per aortic ring (aortic ring assay) • ↑ CD31 expression (IHC) • ↑ % blood perfusion • ↑ IGFBP-3, PTX3, ALIX (angiogenesis antibody array) |
Study compared iVPCs exosome therapy with RAEC exosome therapy and showed greater vessel length in the iVPCs group. | (114) |
| Birth derived | |||||
| Mice (human placental MSCs) | Injection of hydrogel | Hindlimb ischemia | MSC treated animals had:
|
|
(115) |
| Rat (endothelial like umbilical cord cells) | Tail vein injection | Aging and diabetes |
|
|
(116) |
| Rat (human amnion-derived MSCs) | Injection into the myocardium | Myocardial infarction |
|
Heart function of MSC-treated groups were still significantly different than the sham model | (117) |
| Rat (Human placenta derived MSCs) | Implantation of PCL-MSC vascular graft | Hyperlipidemia |
|
|
(118) |
| Other | |||||
| Mice (Aortic MSCs compared with BM MSCs) | IV injection | Whole thorax irradiation in lung | MSCs treated animals had:
|
Limited immune cell infiltration (flow cytometry)superoxide dismutase mimetic treatment limits endothelial loss of radiation damage | (119) |
| Mice (MSCs derived from kidneys) | Subcutaneous | Ischemic kidney | MSCs transplanted into Kidney displayed: • ↑ Lectin and CM-DiI dye (IHC) |
• ↓ plasma creatinine • ↓TUNEL + nuclei • ↑Ki-67 nuclei |
(120) |
ACh, acetylcholine; EF, ejection fraction; F, female; IHC, immunohistochemistry; IV, intravenous, iVPCs, induced vascular progenitor cells, M, male; MI, myocardial infarction; MSC, mesenchymal stem cell; NOS, nitric oxide synthase; PAD, peripheral artery disease; PRU, renal artery resistance; PWV, pulse wave velocity; RAEC, rat aortic endothelial cells; SBP, systolic blood pressure; SMA, smooth muscle actin; SNP, sodium nitroprusside.
Direct Cell Interactions
There is evidence that MSCs are capable of direct differentiation into pericyte and endothelial-like cells (121, 122). Differentiation into the latter has been shown through mechanotransductive stimulation such as shear stress and MSC-endothelial extracellular matrix (ECM) protein interactions (99, 123–126). Differentiation also occurs in the presence of VEGF via Rho/ROCK and myocardin-related transcription factor A signaling and CYR61/CNN-1 gene upregulation (32). Nitric oxide released from nearby endothelial cells and S-nitrosoglutathione reductase also mediate differentiation into endothelial-like cells (127, 128). These differentiated cells are considered “like” endothelial cells in that they express a milieu of endothelial cell markers including VEGF receptors 1 and 2 and von Willebrand factor. Notably, differentiation is not complete. For example, as MSC-derived endothelial networks lack other endothelial markers such as platelet endothelial cell adhesion molecule 1 (129). It should also be noted that many of these studies are in vitro, so whether these conditions can cause endothelial-like differentiation in vivo should be determined.
Endothelial-like cells exhibit behavior akin to resident endothelial cells, namely, that they have angiogenic and vasculogenic potential to create new patent cell networks (116, 126, 130). Growth determination factor 11 and TGFβ receptor/ERK/EIF4E pathway further enhance endothelial-like differentiation and angiogenic potential (131). Autologous BM-MSC-derived endothelial-like cells have been used in 3-D bioprinting for the fabrication of carotid and femoral grafts that maintain patency with minimal inflammation when implanted in dogs (132).
These MSC differentiated endothelial-like cells can mediate recovery in the setting of vascular disorders (103, 115, 120). However, direct differentiation and engraftment does not always lead to recovery of organ function. For instance, amnion-derived MSCs differentiated to cardiomyocyte and endothelial-like cells in a rat model of heart failure, but this came without recovery of cardiac function or reduction in fibrosis (117). To note, functional vascular recovery is often achieved despite very low cell therapy engraftment and differentiation (107), leading to the era of the current paracrine hypothesis.
Stem cells can also associate directly with endothelial cells to mediate recovery through juxtacrine interactions (125, 133). Through gap junctions, such as connexin 43, miRNA, glucose, protein, and even mitochondria can be transferred between MSC and endothelial cells (134). In a mouse stroke model, the flux of glucose from endothelial cells to MSC allows for reduced VEGF uptake, angiogenic signaling, permeability, and inflammatory response as a protective recovery mechanism. This mechanism can be blocked by gap junction inhibition (101, 102). Following an ischemia/reperfusion-like injury in HUVECs, mitochondria from MSCs can be directly transferred from tunneling nanotubes rescuing oxygen consumption rate (135). Transfer of the aforementioned organelles is initiated upon MSC recognition of phosphatidylserines present on apoptotic-induced cultured endothelial cells. Other than direct transfer, stem cells can also release factors and mitochondria in a paracrine manner.
Indirect (Paracrine) Cell Interactions
The “paracrine hypothesis” suggests stem cells exert much of their beneficial effect on damaged tissue through the paracrine action of their secretome (i.e., exocytosed contents or vesicles) rather than engraftment of stem cells into damaged tissue (114). Using cell culture systems that prevent direct contact while allowing exchange of factors in the shared media, stem cells can exert regenerative, differentiation induction, and proangiogenic effects via paracrine mechanisms (136, 137). Much of the focus of the field has been on the potential of exosomes as a key therapeutic mechanism as well as an opportunity as a cell-free therapeutic alternative. To reflect this emphasis in the field, exosomes will be the focus in this section as well as highlighting nonexosome (e.g., conditioned media) paracrine effects when appropriate.
Exosomes are membrane-bound vesicles ranging from 30 to 150 nm released from cells that function in a paracrine manner transporting proteins, lipids, RNAs, microRNAs, mitochondria, and more between cells (30, 114, 138). Exosomes are formed through invaginations of the late endosome, where cytoplasmic contents are encapsulated in multivesicular bodies. Proteins from the ER and Golgi complex can be sorted into multivesicular bodies then released to the extracellular environment through fusion and exocytosis where exosomes often circulate to target cells via chemoattraction (139). Cellular recognition of exosomes occurs through either opsonized free floating exosomes, through adhesion, or via antigen recognition (140). Exosomes mediate their effects via intracellular signaling pathways that can be initiated after exosome soluble signaling or juxtacrine signaling. Genetic or protein content transfer can occur via fusion, macropinocytosis, or receptor-/raft-mediated endocytosis, albeit the latter two may also result in lysosomal degeneration as is the case with phagocytosis (139).
The utilization of MSC-derived exosomes circumvents the limited cell numbers as exosomes can be continuously harvested from media of cell cultures and have been shown to have efficacy as a vascular therapeutic. Antioxidant proteins can be released to have a direct restorative effect or miRNAs can indirectly influence host cell function through regulation of genetic expression (111). For example, in Yang et al.’s study, exosomal miRNA-181b-5p (181 b-Exos) increased protein expression of factors that promoted angiogenesis in brain microvascular endothelial cells after oxygen deprivation. Endothelial progenitor cells and AD-MSCs exosomes carry other proangiogenic miRNAs, such as miRNA-126, 296, 278, and 210 (141).
Exosomal therapy shows regenerative potential in vascular settings. Secrotomes isolated from human adipose mesenchymal stem cells increased vascularized granulation tissue and endothelial cell density of the dermis of in vivo mouse wounds compared with controls. Promisingly, injury sites in the group treated with secretomes expressed significantly higher levels of CD31 (a marker of vascular endothelial cells) (142). In vitro treatment with induced vascular progenitor cell (iVPC) exosomes resulted in increased vessel length and area of endothelial cells. In vivo, iVPC-Exo therapy significantly increased perfusion of an ischemic hindlimb model. The results suggest a similar angiogenic effect whether iVPCs or iVPC-Exo are administered, and that the effects of iVPCs are likely due to exosome secretion (114). A comparative study examined the effect of exosomes isolated from BM-MSCs, AD-MSCs, and UCB-MSCs for the treatment of MI in rats. All three types limited damage from induced MIs, stimulated angiogenesis via increased VEGF, bFGF, and HGF, and increased microvascular density, with AD-MSCs having the most significant effect (112). Signaling cascades initiated by the stem cell secretome increased the ability of fibroblasts, keratinocytes, and vascular endothelial cells to migrate. MSC exosomes also have applications for attenuating pathologies associated with vascular barrier dysfunction (143, 144).
Stem cell exosome-based therapy has many advantages compared with cellular therapy. Studies have shown that administration of stem cell-derived exosomes leads to similar outcomes compared with administration of the stem cells themselves (114). In some studies, exosomes even outperform their parent cells for microvascular regeneration (104). Without the stem cell, there is no evidence of tumorigenesis (114) and risk of immunogenicity (145) and cell emboli are greatly reduced. There are also practical advantages to noncell therapy including avoiding complex FDA guidance of cell therapy, simplified large-scale pharmaceutical production, and ease of storage compared with cells. Exosomes may also be used synthetically as drug/gene therapy delivery vehicles, enhancing bioavailability due to their size and have potential for homing to site of interest through targeting peptides on their membranes (146). The major disadvantages to exosomal therapy from a pharmaceutical standpoint is low yield of exosomal release from mammalian cells, cumbersome methodology for isolation, and ensuring batch to batch consistency in exosomal contents (146). Alternative methods that are potentially more amenable to large-scale exosome production are currently being investigated and need to be verified for clinical use in variety of stem cell sources and pathologies (147). Other methods such as changing culturing conditions are described by Phan et al. (138). Evaluation of surface markers is currently done for ensuring batch consistency (148), but this still does not fully ensure that inside contents are uniform throughout.
Stem Cell Remediation of ER Stress
The ER is an important site for protein, lipid and sterol synthesis, protein trafficking and folding, and calcium storage and release. ER stress occurs when the capacity of the ER to fold proteins is outpaced by synthesis of proteins, due to physiologic or pathologic strain (149). In a pig study of renal artery stenosis and in porcine kidney tubular cell culture with induced ER stress (via thapsigargin treatment), AD-MSCs and EPCs were able to both restore renal blood flow and glomerular filtration rate (albeit AD-MSC were more effective) as well as similarly improve microvascular density in the inner and middle cortex (113). EPCs reduced oxidative stress (NADPH oxidase p67and p47 subunits) whereas AD-MSCs reduced inflammation (decreased TNFα and IL-1β, increased IL-10), caspase-3-mediated apoptosis, and ER stress via reduced C/EBP homologous protein (CHOP), GRP94, and Derlin-3. EPCs were also able to increase VEGF whereas both EPCs and AD-MSC reduced p67 and p47 and both decreased fibrosis. Interestingly, this mechanism for MSC repair seems to be contact dependent via a direct cell effect, as culturing with an insert plate to only allow exchange of culture media alone did not replicate these regenerative effects. MSCs can also reduce ER stress via blocking palmitic acid-mediated HUVEC endothelial-to-mesenchymal transition dependent on MSC secretion of stanniocalcin-1 (150).
Stem Cell Rejuvenation of Mitochondrial Function
One major element of stem cell-mediated vascular rejuvenation is antioxidation of host tissue (151). MSCs are highly resistant to oxidative stress since they constitutively express catalase, superoxide dismutase 1–3 (SOD1–3), glutathione peroxidase (GPx), SIRT 1, 3, and 6, and thioredoxin, heme oxygenase-1, as well as having abundant antioxidant molecule glutathione (GSH) and redox sensitive forkhead box O3 signaling (151–156). These antioxidative elements have been shown to mediate recovery from vascular injury and disease in part by reducing oxidative stress and its markers [nitrotyrosine, and 8-hydroxydeoxyguanosine (8-OHdG)] and increasing host endothelial cell/vascular smooth muscle cell (VSMC) antioxidative protein content and/or activity (34, 119, 157). For example, BM-MSCs upregulate aortic heme oxygenase-1 (HO-1) and catalase to reduce oxidative stress in mouse radiation-induced aortic injury, accompanied by reduced fibrosis, aortic thickening, apoptosis, and inflammation (TNFα and intercellular adhesion molecule 1) (158).
Some of these antioxidative factors have been shown to be secreted or trafficked in exosomes to vascular targets, including catalase, SOD1-3, GPx1-7, GSH, SIRT1-6, peroxiredoxin 1–6, and thioredoxin 1–2 (34, 119, 151, 159). Under hypoxic conditions of pulmonary arterial hypertension, MSC exosomes reduced reactive oxygen species (ROS) by increasing GSH/GSSH balance and also positively affected the citric acid cycle by increasing pyruvate dehydrogenase and glutamate dehydrogenase 1, restoring energy balance and oxygen consumption rate in VSMC (160). Furthermore, miRNA-132-3p delivered by MSC exosomes to endothelial cells reduces ROS by downregulating Ras p21 protein activator 1 and increasing Ras and PI3K expression (161). MSC exosomal-mediated reduction in ROS can also be accompanied by increasing endothelial- but reducing inducible-nitric oxide synthase, thus restoring nitric oxide bioavailability (106). Reduced cellular oxidative stress from antioxidative MSCs ameliorates endothelial lipid peroxidation and DNA oxidative damage and maintains mitochondrial DNA replication and stability (162–165). More details about the emerging understanding of the antioxidative role of stem cells are summarized by Stavely and Nurgali (151).
Healthy mitochondria exist in a balance between fission, fusion, biogenesis, and mitophagy, and deviation from these leads to increased oxidative stress, reduced respiration, and vascular function (4). Stem cell therapies have also been shown to mediate shifts in the mitochondrial bioenergetics, dynamics, and respiration. Zhu et al. (166) found that in HUVECs and rat aortas, high glucose insult led to excessive dynamin-related protein-1-mediated mitochondrial fragmentation, ROS production, reduced membrane potential and ATP production, blunted mitophagy leading to increased caspase-3 and Bax-mediated apoptosis. All these observations were reversed when cocultured or infused with BM-MSCs, including a return in mitochondrial morphology to a filamentous fused network. Notably, the authors found an increase in mitophagy mediators Pink1 and Parkin with MSC therapy, small inhibiting RNA (siRNA) inhibition of which ameliorated the restorative effects MSCs. In another study, diabetic endothelial dysfunction was treated with MSC-conditioned media (167). MSC-conditioned media reduced ROS, prevented apoptosis (increased Bcl-2, decreased Bax), increased migration and tubulogenesis in HUVECs subjected to high glucose, and improved vasodilation in thoracic aorta. These effects were mediated by increased expression of SIRT1 and mitochondrial biogenesis activator peroxisome proliferator-activated receptor (PPAR) γ coactivator 1 (PGC-1α), specifically through the phosphoinositide 3-kinase/protein kinase-B (Akt)/SIRT1 and SIRT1/AMP-activated protein kinase/PGC-1α axes.
Mitochondrial functional recovery from MSC therapy can also occur via the direct and paracrine mechanisms through donation of whole mitochondria, mitochondrial membrane fragments, or mitochondrial DNA through tunneling nanotubes, connexin 43 gap junctions, cell fusion, and exosomes/extracellular vesicles (EVs) (105, 135, 165, 168, 169). Apoptotic endothelial cells (HUVECs) exposed to oxygen/glucose deprivation and reoxygenation express phosphatidylserines that serve as a stem cell recognition site for the formation of nanotubules and nearly unidirectional transfer of mitochondria from stem cell to injured endothelial cell (135). In a rat model of stroke (middle cerebral artery occlusion/reperfusion), BM-MSCs were found to increase angiogenesis, motor function, microvascular basal and maximal oxygen consumption rate, and extracellular acidification rate and reduce infarct size and lactic acid production (105). Interestingly, vessel density in the peri-infarct area correlated with host cells that have received BM-MSC mitochondria. Restorative effects were not seen with nanotube inhibition with annexin V or latrunculin-A. Since these inhibitors do not reduce paracrine signaling, the BM-MSCs direct therapeutic mechanism was postulated to be through engraftment and subsequent direct mitochondrial transfer. In coronary artery VSMCs, MSCs nanotube-mediated mitochondrial trafficking is bidirectional and a signal for MSC proliferation (170). With hydrogen peroxide (H2O2) exposure, HUVECs transfer their mitochondria to MSCs where they are degraded via autophagy (171). This induces heme oxygenase-1 production and subsequent mitochondrial biogenesis (PGC-1α and mitochondrial transcription factor A) and mitochondrial donation from MSCs to HUVECs. HUVECs then exhibit increased mitofusion 1–2 and reduced apoptosis. The restorative response was dependent on mitochondria being “damaged” (high superoxide) and intact fission and fusion mechanisms. In lipopolysaccharide-induced lung injury model (pulmonary microvascular endothelial cells and in vivo), there is increased ROS with reduced mitochondrial membrane potential, basal and maximal oxygen consumption rate and ATP turnover, and diminished vascular barrier function. This was reversed by MSC-exosomes but not exosomes devoid of mitochondria (165).
Stem Cell Effect on Vascular Dilative Function, Aging, Atherosclerosis, and Hypertension
These antioxidative effects on mitochondrial biogenesis/dynamics/mitophagy confer functional benefits in terms of vasodilative function including that of microvessels. Indeed, it has been shown that mitochondrial state 3 and 4 respiratory function correlates with both acetylcholine and flow-mediated (but not nitric oxide mediated) vasodilation (172). Intravenous SVF delivery reversed aging-associated loss of β-adrenergic agonist (norepinephrine) dilation and improved dobutamine-induced coronary flow reserve, an indicator of coronary microvascular function in a rodent model (1). Myogenic responsiveness to intraluminal pressure changes is improved in mesenteric arteries via an H2S-mediated mechanism after treatment with UCB-MSCs in mice with endothelial nitric oxide synthase (eNOS) knockout (173). There is improved endothelial-dependent (acetylcholine), but not -independent (nitric oxide) dilation with MSC therapy in rat thoracic aorta (high-glucose model) and during rabbit limb blood flow analysis (limb ischemia model) with improved perfusion (107). Interestingly, both acetylcholine and flow-mediated dilation operate via activation of eNOS and nitric oxide. In a rat model of brain death and ischemia reperfusion, BM-MSC conditioned media mediates a restoration of aortic vasodilatory sensitivity to acetylcholine, no change in maximal endothelium-independent response, and is less sensitive to constrictive phenylephrine (174). Furthermore, these aortas treated with conditioned media exhibited less neutrophilic infiltration and reduced caspase-3, 8, 9, 12, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 (CD54) expression, and nitrotyrosine (174). Interestingly, the regenerative effect of BM-MSCs on vasodilatory function to flow may be gender specific, as flow-mediated dilation was significantly restored in male but not female patients in the POSEIDEN-DCM study of dilated nonischemic cardiomyopathy (108).
Reduction of endothelium-dependent dilation in aging is associated with oxidative stress-induced senescence and short telomeres, reversed by antioxidant therapy (175). In HUVECs and in aged mice, gingival-derived MSC exosomes attenuate oxidative stress-induced senescence by reducing senescence-associated gene expression of B-galactosidase, p21, p53, phosphorylated γH2AX, and mTOR/pS6 signaling (176). Human iPSC exosomes reverse the effects of senescence (via high glucose insult) on HUVECs. Specifically, they reduce the proportion of senescent cells (senescence-associated B-galactosidase) to increase cell viability, and restore ability to form capillary-like structures (177). MSC exosomes reduce DNA damage, inflammation, fibrosis, and senescence-associated phenotype via ataxia-telangiectasia mutated/p53/p21 signaling in radiation-induced lung endothelial injury via miRNA-214-3p (178).
Aging-associated atherosclerosis and calcification represents another avenue in which stem cell therapy offers regenerative potential. In mouse aortic VSMCs with induced calcification by β-glycerophosphate, MSC-conditioned media reduces alkaline phosphatase activity, reduces intracellular calcium content, mRNA expression of Msx2, Runx2, osteocalcin, bone morphogenic protein-2, protein expression of bone morphogenic protein-2 and Runx2, reduced TNFα, IL-1β, IL-6, caspase-3, and increased Bcl-2/Bax ratio (179). In rat aortic VSMCs exposed to advanced glycation end products with induced calcification, BM-MSC exosomes calcification and ROS production are enhanced via miRNA-146a targeting of thioredoxin-interacting protein (180). In a rat model of hyperlipidemia, a synthetic vascular graft embedded with small EVs from human placenta-derived MSCs reduced calcification, thrombosis, and improved graft patency with M1–M2 macrophage phenotypic switch (118). These EVs contained VEGF, miRNA-126, and miRNA-145 (118). Rat aortic neointimal hyperplasia and vascular smooth muscle proliferation is inhibited by BM-MSC exosomal miRNA-125-b, which represses myo1e expression (181). These effects of stem cells on atherosclerosis and calcification are likely important for microvascular function as well, as coronary artery disease severity is correlated with microvascular dysfunction (182, 183), albeit microvascular disease may precede coronary artery disease (184).
MSC therapy can also mediate microvascular regeneration in the setting of increased hypertension and peripheral resistance. MSC therapy leads to a reduction in both circulating renin-angiotensin-aldosterone and the angiotensin type 1 receptor and increased angiotensin type 2 receptor expression in a chronic renal artery stenosis rat model (185). The reduction in sympathoexcitation reduces systolic blood pressure, albeit it does not lower systolic blood pressure to prestenotic levels. Most importantly, MSC therapy leads to recovery of the microvascular tree in the stenotic kidney (185) allowing for improved glomerular filtration rate and renal plasma flow (186). Human amniotic MSCs reduce portal pressure in portal hypertension alongside recovered microvascular function (187). Furthermore, there was reduced oxidative stress and inflammation and enhanced nitric oxide together culminating in enhanced liver function tests.
AUTOLOGOUS VERSUS ALLOGENEIC (SELF VS. SHELF)
There is current discussion about whether autologous or allogeneic sourcing is most appropriate for stem cell therapy and often referred to as “self or shelf.” Allogeneic cells can be more readily obtained, however, autologous cells better match host biologic and immunologic dynamics. If one were to imagine the “ideal stem cell donor candidate,” its’ properties would include a young person with no prior disease history, healthy lifestyle habits whose stem cells would be at no risk for causing rejection/immune response, maximum potential for engraftment, survival, proliferation, maximal, and consistent secretion of therapeutic factors. Likewise, the ideal stem cell recipient for most successful therapeutic benefit would be someone with minimal disease progression (preventative care) with limited comorbidities and healthy lifestyle habits. In reality, patients often have multiple comorbidities and progressive disease presentation. Similarly, donor stem cells, even from an ideal stem cell donor candidate, can still potentially elicit immune responses (188). How would one choose the universal stem cell donor that these allogeneic cellular therapeutics are based on? What would be the criteria? The dynamics of autologous versus allogeneic strategies are described in this section and compared in Fig. 3.
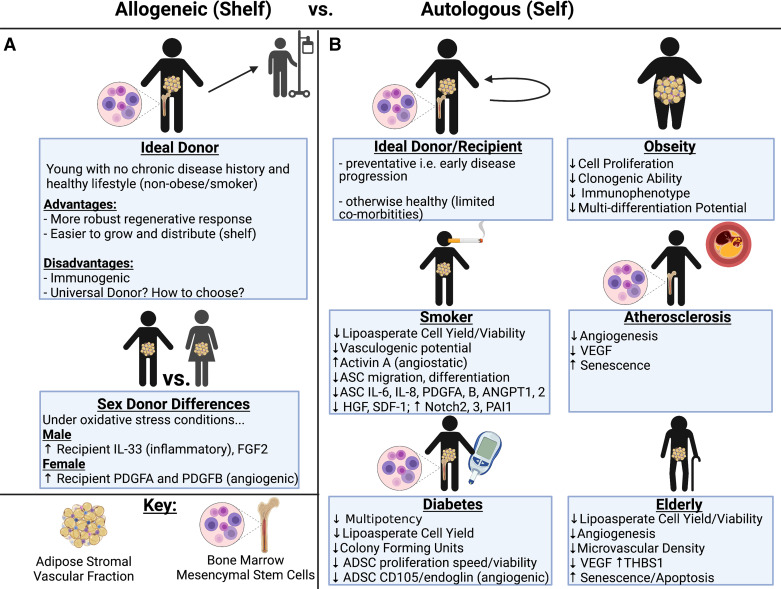
Figure 3.
Allogeneic vs. autologous stem cell therapeutic strategies in differing patient populations. The advantages and disadvantages of allogeneic (shelf) stem cell therapies are shown with attention paid to donor sex differences (A). For the autologous (self) strategy, effectiveness of stem cell therapies are shown in “ideal” recipients as well as various patient populations such as obesity, smoking, atherosclerosis, diabetes, and aging (B). Image created with BioRender.com. HGF, hepatocyte growth factor; SDF-1, stromal cell derived factor-1; VEGF, vascular endothelial growth factor.
Potentially, allogeneic stem cells could be produced in enormous quantities and readily available on a large pharmaceutical scale for clinical use, i.e., “off the shelf.” Issues of collection, aging, and disease states can be avoided with use of an ideal donor, who may be a young individual without chronic disease history and a healthy lifestyle (189). The challenges of allogeneic stem cell sources include immune reactions to the human leukocyte antigens (HLAs) on donor cells and infection transmission from donor to patient (189). On the other hand, MSCs are relatively hypoimmunogenic (190) and approximately half of international clinical trials from 2004–2018 use allogeneic MSCs as their source (191, 192). The mechanism behind why MSCs exhibit a hypoimmunogenic property is due to their capacity to be conditional antigen presenting cells in the early phase of an immune response (193, 194), then immunomodulate through secretion of soluble factors (195, 196). However, a newer area of research involves CRISPR-Cas9 gene editing to increase immune compatibility of allogeneic stem cells used for therapy (197, 198).
An additional consideration for allogeneic stem cells is sex as a biological variable (199, 200). SVF from male versus female mice exhibits slightly different cellular composition (201). Under oxidative stress conditions, SVF from male donors showcases some differences, including increased IL-33 (inflammatory) and FGF in SVF-HUVEC cocultures, whereas female SVF exhibited increased proangiogenic secretion of platelet-derived growth factor α and β and decreased IL-1β (202). Albeit, SVF antioxidant and VEGF gene and protein expression were similar between the sexes. These differences need to be further explored for any potential differences in therapeutic efficacy, in terms of cell efficacy as well as male to female or female to male stem cell delivery.
Pivoting to autologous stem cells, their use dismisses the immunological concerns of non-self-tissue. However, autologous stem cell use can be complicated by age and disease states that cause cellular dysfunction. Most logically, patients with gene-linked diseases may not receive any benefit via therapy with autologous cells. In addition, the vast number of cells necessary to isolate and expand ex vivo makes collection from autologous donors challenging, especially in states of disease or aging (189).
In a 2018 study by Redondo et al., BM-MSCs derived from patients with progressive multiple sclerosis (MS) were compared with MSCs from controls with the goal of determining whether the inflammatory conditions of MS compromise MSC function. The data showed that the MS-derived MSCs had decreased potential for expansion that was inversely related to the duration of progressive MS. In addition, the MS-derived MSC cells produced markers of early aging in vitro, including accelerated telomere shortening and senescence compared with control (203). In a 2021 study, MSCs obtained from the adipose of pigs with atherosclerotic renal artery stenosis displayed decreased levels of VEGF and capacity for angiogenesis, and increased rates of cellular senescence compared with control cells (204). Hyperglycemia can also influence MSC kinetics. MSCs isolated from abdominal fat of human diabetic donors between the age of 40 and 72 yr showed significantly fewer colony forming units, lower proliferation rates, and a lower number of cells displaying CD105+ (an angiogenic biomarker) compared with cells from nondiabetic age-matched controls (205). The results of these studies suggest that in patients with chronic disease states, autologous stem cells may not be the best option.
Age can also factor in, as adipose-derived SVF from old Fischer-344 rats (24 mo) has been compared with that of young rats (4 mo). In vivo, subcutaneous implants of age-dependent SVF-laden collagen constructs showed that old SVF resulted in decreased total and perfused vessels, and smaller, less mature vessels than those embedded with young SVF after 4 wk of implantation. They also found increased expression of thrombospondin-1, a protein correlated with decreased angiogenesis and migration in old SVF samples. Neutralization of thrombospondin-1 with antibodies before implantation ameliorated this effect (206). Another study revealed a negative correlation in yield of human SVF cells with age (19–71 yr old) in female donors (207).
Other donor characteristics that should be considered are lifestyle and modifiable factors. For example, viable SVF yield has a significant negative correlation to smoking level of the donor (208). ADSCs from smokers has exhibited reduced vasculogenic potential via increased angiostatic activin A expression, reduced ADSC migration and differentiation, reduced platelet-derived growth factor α and β, ANGPT1, ANGPT2, HGF, SDF-1, and increased Notch2, Notch3, and plasminogen activator inhibitor-1 (209). Increased physical activity of patients has also been associated with significantly lower viability of SVF without affecting the initial cell yield (208). Finally, ADSCs isolated from patients of a range of body mass index (BMI) have been compared. As BMI increases, there is an inverse correlation to differentiation potential, proliferation, and colony forming potential (199, 210). Further studies need to be done to ascertain more lifestyle, genetic, or naturally mitigating factors that can nullify a stem cell therapies beneficial affects to a vascular bed.
STRATEGIES TO IMPROVE STEM CELL ENGRAFTMENT AND FUNCTION
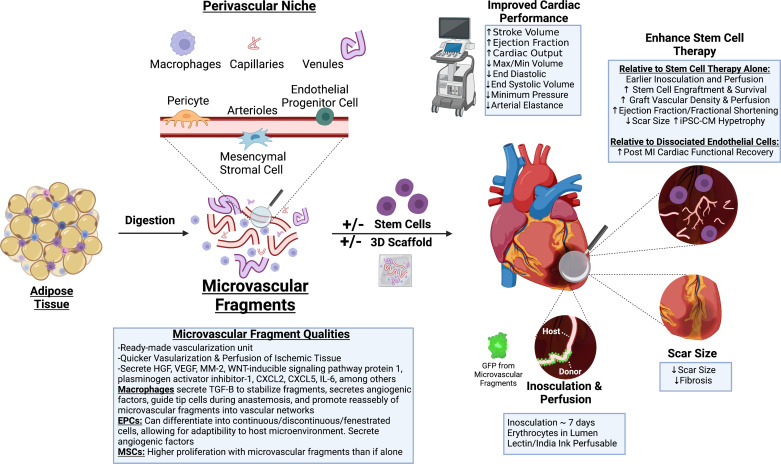
Stem cell therapies have been historically limited in their effectiveness by low engraftment and retention, and autologous potential is plagued by imperfections in stem cell function described previously. These described shortcomings of cell sources and therapies, either from autologous or allogeneic sources, give rise to the important discussion of whether these cells can be modified in ways to improve or exceed their initial potential. These limitations can potentially be ameliorated by priming or preconditioning the cells before therapeutic use with the goal of exposing exogenous cells with factors mimicking the potentially harsh environment in which they will be administered, thus improving cell engraftment and therapeutic robustness. Common strategies include hypoxic or ROS preconditioning, pharmacologic priming, gene editing, extracellular supportive methods, and co-therapies with microvascular fragments, all of which will be discussed in the following section and illustrated in Fig. 4.
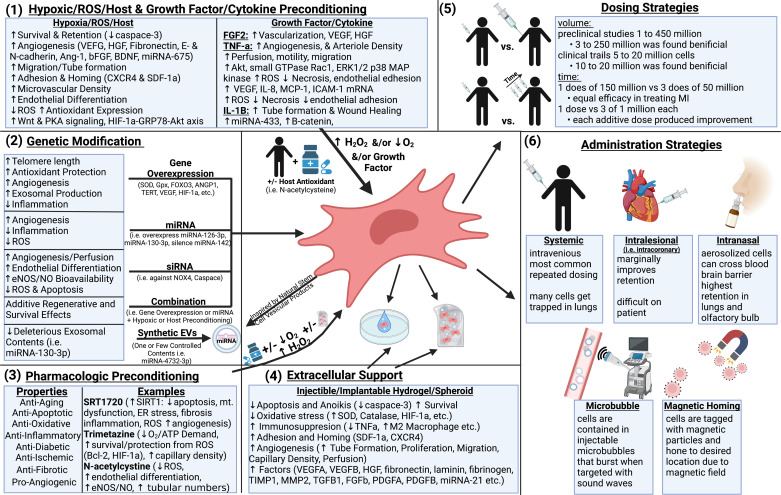
Figure 4.
Priming, preconditioning, dosing, and administrative strategies to enhance stem cell potency and effectiveness. Optimal therapeutic performance by stem cells can be elicited via hypoxic/ reactive oxygen species (ROS) preconditioning and preconditioning with growth factors and cytokines (1), with genetic modification (2), with pharmacologic intervention (3), extracellular support such as hydrogels (4), or by utilization of novel dosing (5) or administration (6) strategies. Image created with BioRender.com. CXCR, C-X-C chemokine recptor; eNOS, endothelial nitric oxide synthase; HIF-1, hypoxia inducing factor 1; SDF-1, stromal cell derived factor-1.
Preconditioning with Hydrogen Peroxide
Repeated replication of stem cells causes senescence and damage via oxidative stress, similar to the aging of in vivo somatic cells. Reactive oxygen species, and reactive nitrogen species (RNS), are produced during cell expansion primarily via mitochondrial complexes, peroxisomes, and other cell processes. Although low acute levels of ROS are important for normal cellular functions, high chronic levels are capable of causing cellular harm and dysfunction (211). In addition, oxidative stress in the host environment can decrease stem cell engraftment (212).
For these reasons, methods to enhance stem cell resistance to oxidative stress, or conditioning them to be “accustomed” to oxidative stress has been an exciting area of research. One priming technique is preconditioning of stem cells with H2O2. For example, H2O2-preconditioned ADSC exosomes yielded improved microvascular density in rat skin flap model of ischemia reperfusion with reduced inflammation and apoptosis, increased flap survival, tube length, and blood perfusion units compared with unconditioned ADSC exosomes (213). Another study found that H2O2-preconditioning improved survival, migration, proliferation, tissue engraftment, wound closure, and microvascular density in mice with full thickness excisional wounds compared with unconditioned MSCs (214). The mechanism for this was upregulation of cyclin D1, SDF-1, and CXCR4/7 receptors as well as decreased Bax/Bcl-2 ratio, cleaved caspase 3/9, and inhibited p16 and glycogen synthase kinase 3β expression (214). Preconditioning also enhanced oxidative stress resistance, as preconditioned MSCs were able to resist increases in superoxide fluorescence and mitochondrial membrane depolarization during oxidative challenge compared with unconditioned cells (214).
Hypoxic Preconditioning
Hypoxic preconditioning is a common method to induce stem cell resistance to oxidative stress in preparation for therapeutic utilization in ischemic tissue. Hao et al. (215, 216) found that chorionic villi-derived MSCs subjected to hypoxic conditioning exhibited better survival and angiogenic capacity, including reduced caspase-3, activation of Akt signaling, increased VEGFA, HGF, and culminating in increased endothelial cell proliferation, migration, and tube formation. Mouse peripheral blood mononuclear cells subjected to hypoxic preconditioning show increased (compared with nonpreconditioned cells) antioxidant gene expression, lower ROS levels, and higher survivability in oxidative stress conditions and in ischemic hindlimb muscle tissue. This corresponded with greatly improved microvascular density and perfusion 28 days after therapy (217). Antioxidant and cytoprotective genes upregulated included heme oxygenase-1, autocrine motility factor, hexokinase-2, IL-1β, VEGF, and induced nitric oxide synthase (217). Hypoxia preconditioning of MSCs also can upregulate proteins supportive of the angiogenic process, fibronectin 1, E-cadherin, N-cadherin, and many integrins (218, 219). Hypoxia can also upregulate proangiogenic and cytoprotective miRNA expression, such as miRNA-675 (decrease HIF-1α negative regulators to increase VEGF) (220). Hypoxia preconditioned adipose MSCs have also been shown to reverse rat erectile dysfunction by upregulating VEGF and its receptor, angiotensin 1, basic FGF, brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, SDF-1 and CXCR4, and neuronal nitric oxide synthase, culminating in increased angiogenesis (221). Furthermore, hypoxia preconditioned BM-MSCs release more VEGFA and SDF-1α than normoxic, becoming “endothelial-like” cells with angiogenic potential (222). VEGF is also produced more by hypoxia preconditioning of MSCs, enhancing angiogenesis when injected into rabbit molar pulp cavities (223).
Mechanisms behind hypoxia preconditioning increasing VEGF and angiogenic capacity could be wnt-dependent through protein kinase A signaling and HIF-1α-GRP78-Akt axis (31, 224, 225) or through PrPC-dependent JAK2/STAT3 activation and inactivation of caspase-3 (226). One reason for increased survival after hypoxia preconditioning may be due to enhanced autophagy. In EPCs engrafted in ischemic limb, which exhibited greater microtubule-associated protein 1A/1B light chain 3 stained autophagic structures, restoration of blood perfusion, and enhanced arteriogenesis and angiogenesis after EPC transplant in rat ischemic abdominal wall muscle (227).
Cell-free therapeutic strategies also benefit from hypoxia preconditioning (219, 228). Human AD-MSCs derived EVs cultivated under hypoxic conditions demonstrated improved tube formation of targeted HUVECs compared with normoxic cultivation or supernatant (229). In human UCB-MSC exosomes, angiogenesis, proliferation, and migration are further enhanced by hypoxic preconditioning than nonhypoxic preconditioning in HUVEC cell culture and mouse femur fracture models (230). Meta-analysis has been done comparing hypoxic to normoxic preconditioned exosomes for cardiac repair after MI, but the same analysis has not been done for vascular parameters and would be useful to the field (231).
Finally, hypoxia preconditioning has benefits for tissue engineering. In 3-D bioprinted SVF-vascularized bone grafts, short-term hypoxic preconditioning facilitates microvascular growth with increased VEGFA and HIF-1α expression in vitro and in vivo and inosculation with host (mouse) vasculature (232). Hypoxic preconditioning of primary rat myoblasts caused downregulation of miRNA-1, miRNA-206, and angiopoietin-1 with upregulation of VEGFA with greater percentage volume of blood vessels in a Matrigel construct (233).
Priming with Growth Factors and Cytokines
Use of growth factors and cytokines can also have therapeutic benefit for regenerative stem cell therapy. Dental pulp MSCs secretion of VEGF and HGF as well as vasculogenic ability is enhanced by preconditioning with FGF (223). Priming ADSCs with TNFα has shown a multitude of changes: improved proliferation, cell motility and migration, increased adhesion to monocytes (albeit decreased adhesion to endothelial cells), increased mRNA of VEGF, IL-8, monocyte chemoattractant-1, intercellular adhesion molecule-1, increased ROS generation, and increased positive regulators of angiogenesis (Akt, small GTPase Rac1, ERK1/2, and p38 MAPK) (234). In mouse hindlimb ischemia, TNFα-primed ADSCs increased blood flow recovery, arteriole density, and reduced necrosis (234). The angiogenic properties of human lung mesenchymal stromal cells in wound healing and tube formation assays are enhanced by stimulation with IL-1β through NF-κB dependent-miRNA-433 targeting of Dickkopf Wnt signaling pathway inhibitor 1 and subsequent β-catenin upregulation (235).
Genetically Modified Stem Cells
The capacity for induction of angiogenesis by stem cells can be greatly enhanced by genetic modification. For instance, BM-MSCs overexpressing VEGF-A165 leads to higher amounts of VEGF released, homing to hypoxic sites, enhanced angiogenesis, and perfusion over nonexpressed MSCs in limb ischemia models (236–238). Sometimes, a mixed strategy of preconditioning (i.e., hypoxic) and genetic overexpression is developed. Fierro and coworkers (239) explain in their MSC-VEGF formulation that their cells are thawed 48 h before injection to be preconditioned by hypoxia, allowing for 10% of cells to be retained one month after injection, as opposed to 1% without the hypoxic preconditioning.
Telomerase reverse transcriptase (TERT) and/or myocardin overexpressing adipose and bone-marrow MSCs not only increases viability and proliferation with reduced apoptosis, but also increases blood flow and arteriogenesis in mouse ischemic hind limb (240). Although the driving concept for TERT overexpression is to enhance stem cell function, TERT can be enhanced in recipient cells via exosomal mechanisms (241–243), and TERT itself is known to reduce oxidative stress and recover vascular function (244, 245). Therefore, whether stem cells are able to mediate vascular recovery via TERT donation or upregulation is warranted future inquiry.
Overexpression of HIF-1α via lentivirus in rat BM-MSCs leads to exosomes that transferred HIF-1α to hypoxic-injured HUVECs and improved their tube formation, increased VEGF, platelet-derived growth factor, and angiotensin 1 expression, preserved migration, and enhanced proliferation, all more efficiently than nonoverexpressed control exosomes (246). HIF-1α overexpression also leads to increased Jagged1 notch ligand and exosomal production carrying proangiogenic miRNA-15, miRNA-16, miRNA-17, miRNA-31, miRNA-126, miRNA-145, miRNA-320a, miRNA-424, with greater in vivo angiogenesis in mouse subcutaneous Matrigel plug than control nonoverexpressed exosomes (247).
Stem cells can also be made to overexpress miRNA to negatively regulate harmful processes that can overall enhance stem cell regenerative properties. Overexpressing miRNA-126-3p in human UCB-MSCs along with coculture with HUVECs (as well as exosomal treatment) resulted in increased tube formation, migration, and proliferation (248). In a rat tail vein arterialization model, human UCB-MSCs overexpressing miRNA-126-3p exhibited higher and accelerated re-endothelization and reduced inflammation (TNFα) and intimal hyperplasia, all showcasing the ability of miRNA overexpression for treatment of vein graft disease (248). As described in the study, microRNA-126-3p targeted HUVEC SPRED-1 and PIK3R2, negative regulators of VEGF, and then lowered their mRNA and protein concentration, alongside increased Akt and ERK1/2 phosphorylation, explaining their proangiogenic capabilities. On the contrary, some deleterious miRNAs could be targeted in stem cells to improve their function. For example, miRNA-142 is upregulated in aged mouse bone marrow MSC and increases ROS by disrupting pexophagy (autophagy of peroxisomes) via Epas1 (249).
Small interfering RNA (siRNA) can also be used for priming of stem cells. For instance, siRNA against caspase-3 in BM-MSCs allows for greater survival against H2O2-mediated apoptosis, showcasing the promise for such a strategy in ischemic and oxidative environments (250). In diabetic mice, BM-MSCs with siRNA targeted against NADPH oxidase 4 showed reduced oxidant levels, decreased adipogenic differentiation, decreased PPARγ, enhanced endothelial differentiation, greater tubular formation, and greater phosphorylated eNOS and nitric oxide bioavailability (251). Transplanted NADPH oxidase 4 siRNA diabetic MSCs into wild-type hindlimb ischemia showed blood flow recovery as well as increased collateral artery diameters and capillary density compared with non-siRNA diabetic MSCs (251).
Manipulation of stem cell EVs, exosomal content, or synthesis of simple synthetic EVs inspired by stem cell contents may be an advantageous strategy for the field, as major hurdles include heterogeneous composition of EVs with potentially deleterious miRNAs such as MSC-derived miR-130b-3p, which has been shown to promote lung cancer cell proliferation, migration, and invasion (252). Found naturally in dental pulp MSCs, microRNA-4732-3p has in vitro and in vivo antioxidant and angiogenic properties (i.e., improved tube formation, migration, and enhanced areal percentage of CD31). MicroRNA-4732-3p was incorporated into synthetic liposomes via electroporation and was delivered intramyocardially to infarcted rats, resulting in preserved cardiac function and reduced scar tissue (252). Therefore, a strategy where miRNAs are first studied and identified in natural exosomal therapeutics and then “mimicked” in synthetic liposomes has the advantages of homogeneity and upscaled copy number, potentially increasing dosage per delivery. Indeed, the synthetic miRNA-4732-3p liposomes outperformed control MSC EVs (252).
Genetically enhanced stem cells can exhibit antioxidative, antisenescent, and anti-inflammatory properties. PGC-1α overexpression enhances angiogenesis in diabetic conditions by protecting against oxidative conditions (253). Embryonic stem cells genetically edited to express active FOXO3 were derived into endothelial cells that led to vascular regeneration in a mouse ischemic injury (254). An intriguing strategy to increase stem cell therapeutic effectiveness relies not only on reducing donor stem cell ROS, but also ROS microenvironment of the host. Zhu et al. (255) used mouse BM-MSCs triple overexpressing SOD1 + glutathione peroxidase (intracellular antioxidants) + SOD3 (extracellular antioxidant), then transplanted into control, diabetic, or diabetic + N-acetylcysteine antioxidant treatment models of critical limb ischemia. Bone marrow MSC survival, proliferation, and migration was significantly enhanced by this treatment strategy, as was release of VEGF, FGF-2, HGF, and placenta growth factor-2, and these effects were mediated in part by loss of ROS (superoxide and hydrogen peroxide) induced advanced glycation end products (255). Survival of BM-MSCs was over 50% after 1–3 wk after injection versus 30%–35% with either overexpression or N-acetylcysteine alone versus 10% without overexpression/N-acetylcysteine treatment. This is significant as survival is typically around 1% for systemic injections and 10% for targeted injection (intracoronary or intramyocardial) (255). In addition, laser Doppler determined that this particular stem cell strategy enhanced blood flow recovery, increased capillary density, and reduced inflammatory infiltration in the ischemic limb (255). This study suggests that perhaps priming/preconditioning of the host themselves would be of benefit for stem cell therapeutic effectiveness.
Pharmacologic Preconditioning
Pharmacologic strategies can also be used to improve the effectiveness of stem cell treatment, often in combination with hypoxia/H2O2 preconditioning or with extracellular support. SIRT1 activation is often used in aging conditions due to its ability to enhance survival and respond to stress and inflammatory responses via targeting p53, FOXOs, and NF-κB. Liu et al. used SRT1720 SIRT1 activation combined with hypoxic and H2O2 strategy in aged rat and human BM-MSCs for therapeutic application in rat MI. Angiogenesis was greatly increased by this strategy as well as reduced stem cell apoptosis, fibrosis, scar size, and improved cardiac performance. The improved survival was due to upregulated Fas apoptosis inhibitory molecule, of which siRNA targeting ameliorated antiapoptosis (256). SIRT1 also has a role in diabetes, as diabetic rat BM early outgrowth cells exhibit reduced SIRT1 mRNA and reduced tube formation in vitro and neovascularization in vivo. There was also reduced secretion of proangiogenic ELR+ CXC chemokines CXCL1, CXCL3, and CXCL5 (257). SRT170 reversed these findings to improve angiogenesis in vitro (257).
ADSCs from aged equines with concomitant metabolic syndrome showcase enhanced ROS, senescence, ER stress, and mitochondrial dysfunction. Preconditioning with resveratrol (SIRT1 activator) and 5-azacytidine (inhibits DNA methylation) reduced ROS, enhanced SOD activity, promoted mitochondrial fusion over fission, restored mitochondrial membrane potential, restored elongated (vs. punctate) morphology, and restored mitophagy (increased PINK). Resveratrol and 5-azacytidine also reduced ER stress (reduced PERK), were anti-inflammatory (reduced TNFα, IL-6, and increased regulatory T-cells), decreased apoptosis, and increased proliferation of ADSCs, and microvesicles from these cells enhance vascularization in horses with suspensory ligament injury (258–260).
Other drugs to improve stem cell-mediated angiogenesis include 5-aza-dc, 2,4-dinitrophenol, and dimethyloxalylglycine (261). 5-Aza-2′-deoxycytidine increased transdifferentiation and endothelial marker expression and angiogenesis on Matrigel of BM stem cells (262). In BM-MSC, 2,4-dinitrophenol (electron transport chain blocker) and dimethyloxalylglycine (prolyl hydrolase inhibitor) increased atrial natriuretic peptide and VEGF, as well as HIF-1α, respectively promoting angiogenesis in rat MI models (263).
Pharmacologic preconditioning is also commonly used for antioxidative or anti-ischemic purposes. Trimetazine (anti-ischemic) lowers tissue demand for oxygen by lowering fatty acid oxidation and reducing ATP demand, making the host microenvironment more amenable for stem cell integration and therapeutic effect. Trimetazine preconditioned BM-MSCs were protected against ROS and had higher survival than nonpreconditioned, and exert enhanced neovascularization, capillary density, and cardiac function in ischemia reperfusion injury in part through enhanced HIF-1α and Bcl-2 (264, 265).
In an in vitro setting, BM-MSCs from diabetic rats preconditioned with antioxidant N-acetylcysteine showed reduced peroxisome proliferator-activated receptor γ, dichlorodihydrofluorescein and nitrotyrosine (reduced ROS), greater adiponectin, greater endothelial cell differentiation (percent CD31+), increased tubules, eNOS, and phosphorylated eNOS, and NO concentration compared with diabetic rat MSCs without preconditioning (251). Iron-chelator deferoxamine improved survival and total antioxidant capacity of human ADSCs secretome and increased VEGF receptor-α and angiopoietin 1 mRNA (266, 267). Mitotempol is a mitochondrial ROS scavenger that was used to precondition dysfunctional diabetic mouse ADSCs, which increased their survival, differentiation potential, migration, and proangiogenic qualities in diabetic mice with critical limb ischemia similar to control (nondiabetic) ADSCs (268). Preconditioning with antioxidant celastrol, in combination with an injectable hydrogel scaffold, led to increased rat and human BM-MSC survival, VEGFA and SDF-1α expression, and neovascular density postimplant into a scratched HUVEC wound closure culture model (269). This demonstrates the potential for a combination strategy with pharmacologic or other preconditioning strategies with extracellular support systems. For more pharmacologic strategies, refer to detailed reviews by Noronha et al. (261) and Miceli et al. (219).
Extracellular Support
It is known that the ability of endothelial cells to adapt to varied tissue environments and vascularize in an organotypic manner is lost after two-dimensional (2-D) culture (270). One strategy to increase engraftment is to administer the stem cells within an extracellular matrix designed to support the cells. For example, hydrogels are physiochemical mimics of the 3-D microenvironment that stem cells naturally inhabit, and therefore may be more encouraging of their natural development and therapeutic milieu. There are many types of hydrogel (natural: alginate, fibrin, collagen, chitosan, hyaluronic acid, etc. and synthetic: polyethylene glycol, methylcellulose etc.), each with advantages and limitations (271). Rat BM-MSCs encapsulated in n-isopropylacrylamide thermosensitive hydrogel further promotes angiogenesis and wound healing in diabetic foot ulcer (272). In a biomimetic pullulan-collagen hydrogel, murine BM-MSCs were seeded and produced greater angiogenic cytokines (VEGF) than in standard culture and showed enhanced viability, engraftment, and angiogenesis with accelerated healing faster than standard culture in murine excisional wound healing model (273). In rat critical limb ischemia, hydrogel with BM-MSCs greatly increased capillary density, number of blood vessels, and capillary to muscle fiber ratio compared with cell therapy or cell-free hydrogel alone (274). A detailed review of hydrogel applications, including advantages and disadvantages of hydrogel and cargo types for critical limb ischemia are provided by Xing et al. (271).
Exosomes can also be loaded into hydrogels. For example, Zhang et al. used UCB-MSC exosomes in hyaluronic acid hydrogel with nanohydroxyapatite poly-ε-caprolactone scaffolding to repair cranial bone defects in rat in part through enhanced vascular recovery. In vitro the exosome hydrogel contributed to enhanced proliferation, migration, and angiogeneic differentiation of endothelial progenitor cells, potentially via miRNA-21 upregulation of the NOTCH1/DLL4 pathway (275). Hydrogel microenvironments can also influence stem cell characteristics. A newly designed polyethylene glycol maleate citrate and β-tricalcium phosphate hydrogel (with rat BM-MSCs) showed enhanced osteogenic and angiogenic properties in spinal fusion surgery, and the enhanced angiogenic properties were due to effects of the biomaterial in inducing MSC-exosomal angiogenic function (276). One advantage of using an exosome-laden hydrogel is the stable release of exosomes over time. Injectable chitosan-graft-aniline tetramer with exosomes were able to promote angiogenesis with greater tube formation, migration, and proliferation and accelerated diabetic wound healing while also promoting M2 macrophage phenotype (277).
In a 2018 study by Jeong et al., an injectable decellularized matrix (IDM) was combined with human ADSCs. They found that the addition of IDM reduced rates of anoikis (cell death due to loss of extracellular matrix) and upregulated mRNA expression of angiogenic factors in vitro. Conversely, mouse hindlimb ischemic tissue was used to compare IDM + human ADSCs treatment to ADSCs alone and found that engraftment was enhanced in the IDM + ADSCs group. Angiogenic paracrine expression and microvessel density were increased in the IDM + ADSCs group as well (278). A more recent study in 2021 found that gelatin microspheres were embedded with thymosin b4 (Tb4), an actin-sequestering protein known to support cardiac cells and promote vasculogenesis and cell engraftment. Tb4 has also been shown to promote cell survival in hypoxic conditions. Treatment with human iPSC-CMs + Tb4 into induced MIs in porcine increased stem cell engraftment and induced vasculogenesis compared with either treatment alone (279).
Spheroids are another strategy to generate a 3-D environment that encourages aggregation and cell-cell interaction that may better mimic natural conditions and self-assembling. The most common method is the hanging drop method, where MSC are contained in droplets of determined volume on lids carefully placed on culture plates containing media that prevents drop evaporation. MSC in spheroids exhibit further enhanced antioxidative, anti-inflammatory, and antiapoptotic properties such as reduced cleaved caspase-3, TNFα and enhanced catalase, HIF-1α, and superoxide dismutase, compared with 2-D monolayer culture (280). Meta-analysis of 28 studies of MSC in spheroids shows enhanced angiogenic potential from bone marrow, adipose, and UCB-MSC sources across nine of those studies (7 in vitro and 4 in vivo) till April 2019. In vitro, MSC spheroids lead to higher expression of VEGFA, VEGFB, HGF, fibronectin, laminin, fibrinogen, tissue inhibitor of MMP-1, MMP-2, TGFβ1, FGFb, SDF-1α, HIF-1α, platelet-derived growth factor α and β, among others (280). However, in vivo models of wound healing (mice, AD-MSC), ischemic flap (mice, AD-MSC), renal ischemia-reperfusion (rat, AD-MSC), and hindlimb ischemia (mouse, UCB-MSC) demonstrate that MSC spheroids were associated with higher regenerative capacity for wound closure, cell engraftment, survival, differentiation into endothelial cells, secretion of VEGF, FGF2, intercellular adhesion molecule, vascular adhesion molecule, neuron-glial antigen 2, and HGF along with higher angiogenesis and vascularization versus 2-D culture (280). MSC spheroids can also be preconditioned with hypoxia or drugs such as DMOG, which further enhance oxidative resistance, survival, adhesion, CXC-chemokine receptor 4-homing to injury, and angiogenic potential (281). More comprehensive details on the role for extracellular support and biomaterials for enhanced stem cell contribution to angiogenesis are provided by Lee et al. (282) and Noronha et al. (261).
Microvessel Fragments and Heterogenous Cell Populations
Initial stem cell therapy studies used heterotypic cell populations versus homogeneous cell types. For example, experiments commonly used whole bone marrow versus specific MSC populations. Seemingly motivated by the cell therapy potential and a reductionist approach, the studies then detailed/focused on isolation and characterization of stem cell subtypes. The question remains whether a heterogeneous cell mixture is beneficial. The importance of this question is most provocatively highlighted by recent use of adipose-derived microvascular fragments and SVF. Both cell populations are derived from adipose tissue and SVF can be viewed as a more digested form of the same cell mixture. Importantly, both cell populations contain endothelial cells, smooth muscle cells, pericytes, immune cells, stem cells, and other stromal cells.
When it comes to improving engraftment to restore tissue and vascular function, one promising line of study is co-delivery of the cells of interest with native microvessel fragments (arterioles, venules, and capillaries) harvested from fat tissue. Transplanted microvessels have potential to improve engraftment and maturation of co-transplanted cells [e.g., human iPSC-cardiomyocytes (iPSC-CM)] by several mechanisms, including improved angiogenesis and secretion of proangiogenic factors, anastomoses with host vasculature, immunomodulation [promoting M1–M2 shift in macrophage phenotype (283)], and ability to adapt to varied host tissue environments. Of particular importance for microvessel fragments may be the “perivascular niche,” the idea that vasculature is made up of endothelial and smooth muscle cells, but also contains a microenvironment made of pericytes, immune cells, and regenerative cells (284, 285). These extravascular cells are necessary for native microvascular function, for example, pericytes secrete hepatocyte growth factor to promote vessel sprouting (286). This means that microvessels not only support cotransplanted stem cells; they may in fact contain their own regenerative milieu of cells that encourage angiogenesis and anastomoses, thereby increasing vascular density and perfusion. The emerging dynamics of microvascular fragment therapy is described in greater detail by Laschke et al. (287) and in Fig. 5.
Figure 5.
Microvascular fragment co-therapeutic strategies to enhance stem cell potency and effectiveness. The “perivascular niche” of microvascular fragments allows for effective therapeutic applications in myocardial infarction to reduce scar size, induce angiogenesis, inosculation, and perfusion, with improved cardiac performance. Furthermore, microvascular fragments optimize stem cell therapy when delivered as a co-therapeutic (287,288). Image created with BioRender.com. ESCs, embryonic stem cells; iPSC-CM, induced pluripotent stem cell cardiomyocyte like; MSCs, mesenchymal stem cells.
Stem and immune cells that are physically associated in the vascular wall or perivascular area of microvascular fragments such as endothelial progenitor cells and mesenchymal stromal cells are included when delivering microvascular fragments (287, 289, 290). Since these stem cells are delivered in their native microenvironment with microvascular fragments, they have higher proliferation rate and differentiation potential (291). Microvascular fragments are most easily isolated from adipose tissue via liposuction and limited enzymatic digestion (similar to adipose SVF albeit with a shorter digestion time).
Separately, stromal vascular fractions and microvascular fragments have historically been used for tissue engineered grafts. In a rat MI model, SVF on an implanted vicryl graft preserved microvascular perfusion and heart function due to SVF engraftment into coronary microvessels (23). Nunes et al. (292) found that SVF cells have angiogenic potential, incorporate into neovascular sites, and form perfused microvascular networks that anastomose with host microvascular fragments. These SVF-derived vascular networks also form a functional interface with parenchymal cells i.e., hepatocytes in their liver tissue mimic model. However, as mentioned earlier, isolation of SVF cells eliminates donor microvascular fragments and there is argument that utilization of microvascular fragments as a vascularization unit is more appropriate and efficacious.
Spater et al. (293) compared adipose SVF and microvascular fragments seeded on collagen-glycosaminoglycan scaffolds implanted into full thickness skin defect mouse dorsal skinfold chambers. Cellular composition (endothelial, perivascular, adipocytes, and stem cells) was comparable between SVF and microvascular fragments, albeit viability was higher in the latter with higher microvascular density, collagen count, and better incorporation into host tissue. Microvascular fragments/networks grafts themselves have been used in the setting of MI in mice to improve vascularization via inosculation and improved perfusion. These transplanted microvascular networks inosculate with host around day 7, complete with arterioles, venules, and capillaries containing erythrocytes culminating in reduced left ventricular infarct size and function (288). Considering the advantages of microvascular fragments, it is curious whether their supplementation of cell-based therapies would be of additive benefit.
There is emerging evidence that stem cell therapy can be enhanced by co-delivery with microvessel fragments that promote anastomosis with host vasculature, reducing ischemic conditions when administered alongside stem cell therapy (294). Whole microvessel inclusion is important because it allows quicker perfusion than waiting for them to assemble and mature. In a 2020 study by Sun et al., infarcts in rats were treated with microvessels plus human iPSC-CMs, iPSC-CMs alone, or iPSC-CMs cotransplanted with a suspension of dissociated endothelial cells via direct injection into the heart muscle. When compared with the iPSC-CMs only group, the microvessel plus iPSC-CMs group had significantly increased engraftment and stem cell survival as well as increased graft vascularity. The transplanted microvessels anastomosed with host vasculature and increased graft perfusion in the first week compared with iPSC-CM only group. The microvessels have been shown to secrete proangiogenic paracrine factors that may be the cause of earlier and more robust blood perfusion (294). In addition, cardiac function was improved only in the animals that received microvessels. Moreover, ready-made microvessel fragments allowed a sixfold increase in iPSC-CM survival, reduced scar size, reversed fractional shortening and ejection fraction loss, and twofold increase in vascular area and graft perfusion 5-days post-transplant (295).
In the context of stem cell transplantations in type 1 diabetes, Hiscox et al. (296) found that a engineered mouse islets with microvessel fragments increased islet stability. Very recent research by Aghazadeh et al. used microvessels cotransplanted with pancreatic progenitor cells into the subcutaneous space of the diabetic mice and results showed that a functional connection formed quickly (within 1 wk) leading to a significant decrease in pancreatic progenitor cell death. The progenitor cells developed into β cells and successfully reduced blood glucose levels ∼8 wk after transplantation into the subcutaneous space. This study also demonstrated that the implanted microvessels were better at connecting to host vasculature than single endothelial cells (29).
As adipose-derived microvessel fragments have been found to be rich in resident proangiogenic stromal and MSCs, a study more than a decade ago posed the intriguing question whether fragments from other tissue types would display similar angiogenesis and network formation (297). The investigators chose the microvessel fragments of a unique and highly differentiated tissue, brain cortex microvascular fragments (BMFs), to compare with rodent epididymal fat microvessel fragments (FMFs). They found that the BMFs, like the FMFs, could generate new microcirculation, indicating that the potential of microvessel fragments is not limited to FMFs and is probably independent of the tissue source. Notably, the BMFs formed vessel networks with higher vascular density sooner and with lower permeability (297).
These studies support that microvascular fragments provide an exciting new avenue for regenerative medicine. This is coupled with the ease of isolation and preservation advantages [can be cryopreserved without loss of function (298)]. Microvascular fragments themselves can also be primed, for example with erythropoietin or insulin-like growth factor 1 to increase angiogenic potential (299–301), or with other factors based on specific patient needs, such as hepatocyte growth factor for diabetic recipients (286). Just like stem cells, priming of microvascular fragments from autologous aged patients may be necessary, as vascularization function wanes with aging (302). More focus is necessary in the field on consistent microvascular fragment isolation protocols, specific cellular/fragment balance, advantages/disadvantages of autologous versus allogeneic microvascular fragment inclusion, priming conditions for microvascular fragment and stem cell co-therapy, and which combinations and in what proportions/doses are most efficient and complimentary with various stem cell therapies.
DOSING AND ADMINISTRATION STRATEGIES
What is the Ideal Dose?
Initially used to treat leukemia in the 1950s, there are currently 4,400 active clinical trials using cell-based therapeutics (303) with 900 focused on treating cardiovascular diseases (according to a search on February 2022 on grants.gov). One question being continually studied is the dosing strategy for cell-based therapies due to a deficit in the regulation and standardization of cell therapy doses. A few preclinical studies have begun testing varying doses of cells with studies ranging from administering 1 million to 450 million MSCs to improve cardiac function in a cardiovascular disease state in pigs and sheep (304). Three preclinical studies using a MI model in large animals treated with similar doses of mesenchymal cell therapies reported varying cardiac function results upon follow up. Hamamoto et al. (305) reported that 25 million allogeneic mesenchymal precursor cells directly injected into the epicardium during time of infarct reduced scar size, improved end diastolic, end systolic, and ejection fraction measures in sheep. However, Hashemi et al. (306) administered 24 million allogeneic MSCs via catheter to infarcted region 3 days post MI in pigs which improved only scar size. Both aforementioned results are inconsistent with a study done by Schuleri et al. (307) that showed administering 20 million autologous MSCs directly into the infarct resulted in no improvement in scar formation in a pig MI model. Although two of the studies demonstrated MSCs efficacy in improving cardiac function following MI at set end points, the therapeutic mechanism was not explored. A time-course (308) and 4-wk end point (309) in a rat MI model with intravenously (iv) injected 5 × 106 allogeneic BM-MSCs was explored to examine the mechanistic effects of MSCs in the heart. Both studies showed that intravenously administered MSCs home to the infarcted area and increase capillary density via angiogenesis that correlated to improved heart function (308, 309). The varying results of favorable cardiac outcomes could be due to the degree of dose and method of injection, as well as the source of the cells being used (304).
Unlike pharmaceuticals that can be repeatedly administered as well as at varying concentrations, the use of cell-based therapies in clinical trials is primarily and historically been a one-time administration, therefore the long-term therapeutic potential of cell therapies needs to be examined. A clinical trial using a one-time administration of autologous bone marrow mononuclear cells to treat peripheral artery disease showed significant improvement in the ankle-branchial index and increased skin ulcer healing by 6 mo (109). To further examine long-term efficacy on the vasculature, a clinical trial used autologous BM-MSCs in improving coronary artery disease (110). At a 3-year follow-up, patients receiving the cell therapy reported reduced angina and reduced need for revascularization interventions (110). This data suggests that cell-based therapies are not only safe to use in patients long-term but can promote healing in tissue through angiogenesis. However, it is naive to think that one administration of cells is enough to provide life-long therapeutic remedy. Despite clinical trials reporting improvements long-term, there is little evidence that a majority of administered cells persist past a few months (310). Hong et al. (311) examined this phenomenon in mice after administration of 100,000 cardiac progenitor cells into the coronaries or the myocardium. At 35-days post-administration there were ∼1,000 cells left in the entirety of the heart (311). To mitigate this precipitous decline in cell retention, recent preclinical studies have been examining the efficacy of repeated dosing strategies for cell-based therapies. Guo et al. (312) administered 0, 1, 2, or 3 intraventricular doses, 14 days apart, of 1 million cardiac mesenchymal cells to 3-wk-old MI regions in mice. Mice receiving the three doses of MSCs had improved left ventricular ejection fraction following each administration (312). However, what if a higher dose was administered? It is important to remember that the continual administration of autologous stem-cell therapies is restricted by the quantity available for harvest from a patient as well as if the patient donating or receiving the cell therapy has any pathologies/conditions that would affect the cellular therapeutic. Another important consideration is the patient’s current medication intake, and how these can affect stem cell therapeutic potency and whether dosing or administration strategies should be altered based on patient medication (313). Finally, there is considerable variation in how proper dosing should be defined for exosome-based therapies, such as numbers of exosomes versus total exosomal protein or RNA content (97). It is crucial that accuracy and reproducibility be the driving force alongside intended therapeutic effect in how dosing of cell and cell-based therapies are determined.
How Should Cells Be Administered?
Although the initial consensus that stem cell therapies are safe and several studies mentioned in this review demonstrate therapeutic gains, the results can be variable, possibly due to the utilization of different routes of administration. The most common routes are topical, intravenous, intra-arterial, intramuscular, and intralesional (190). Intravenous administration is the most broadly studied due to the low risk of adverse events, repeated dosing if necessary, and is easier on the patient (310). An early study using a coronary ligation MI model in mice found that intravenous administration of isogenic MSCs 3-h post-ischemia resulted in a reduced infarct size, improved left ventricular function, and increased capillary density 4 wk later (309). These results were corroborated in a 12-wk end point (314). Together, these studies demonstrate that intravenous administration of a cell therapy results in increased angiogenesis and functional improvement to an ischemic injury in as little as 28 days that is sustained 12-wk postadministration. To investigate this repeated dosing via intravenous administration route, Hong et al. used a large animal MI model given a single 150 million dose or repeated (50 million/day for 3 days) doses of human ADSCs 28 days following the ischemic injury. Both treatment groups had improved ejection fraction, coronary flow reserve, and angiogenesis in the peri-infarct area. However, the single dose resulted in a significant decrease in the area of the perfusion defect (315). Shortly after studies demonstrating that intravenous administration of MSCs improved cardiac parameters after injury, in 2007 it was reported that the MSCs homed to the infarcted region of the myocardium (316). In a time-course study using a rat model of chronic MI, it was shown that 24 h following intravenous administration of 5 × 106 MSCs ∼52% of injected cells was retained in the lungs while less than 1% were in the heart (317). However, no MSCs were detected in the lungs or heart at 30 days postinjection (317). Interestingly, despite the same method of administration, varying degrees of cell engraftment and biodistribution have been reported. For example, in an aged rat model, a tail vein injection of 6 million SVF cells resulted in ∼5% of SVF cells engrafting into the myocardium, with the largest portion being retained in the carotid artery (∼50% of total nucleated cells), ∼40% in the aorta, and ∼18% in the lung 28 days postadministration (24). Importantly, with the ever-growing evidence that intravenous administration of a cell therapy results in a majority of the cells getting trapped in the lungs, the possibility of adverse events need to be examined. However, it is important to note that extensive preclinical and clinical trials have shown the safety of stem cell therapies using varying sources (cell type and allogeneic vs. autologous), doses, and route of administration.
As discussed previously, the prevailing hypothesis for the therapeutic mechanism is the paracrine or secretory hypothesis due to repeated studies reporting low engraftment with a steady decline following administration. The biodistribution and the effect MSCs have on other organs leading to improvements in heart function and angiogenesis have been investigated. Luger et al. (318) examined intravenous delivery of human MSCs in immunocompetent mice with acute or chronic MI receiving the cell therapy 24 h or 4 wk postinjury, respectively. In the acute MI and chronic MI models, MSC retention in the heart was markedly low despite being measured shorty after administration. However, both groups showed improved left ventricular function as early as 1 wk following administration of the cell therapeutic. Interestingly, the acute MI model showed a reduction in the number of splenic natural killer cells. Normally following an MI, the natural killer cells residing in the spleen migrate to the site of injury producing a robust proinflammatory response with deleterious effects on healing and repair (319), but the supplementation of MSCs resulted in low splenic and cardiac natural killer cell numbers over the course of the study (10 wk) (318). MSCs also reduced the number of splenic and cardiac neutrophils (318) that can orchestrate a macrophage switch to a healing phenotype (320). This phenomenon was demonstrated by Dayan et al. (321) when left ventricular function was improved at 2 and 4 wk following an administration of human UC-MSCs 48 h after coronary artery ligation in MOD/SCID mice, which equated to a reduced number of macrophages in the myocardium with the remaining macrophages expressed as a pro-reparative and anti-inflammatory phenotype (M1 to M2). The following data have been corroborated using AD-MSCs (322). Mounting evidence suggests that despite a wide biodistribution from intravenous cell injection, there is a beneficial effect on organs with low engraftment (such as the heart), possibly due to cell therapy regulating the host’s immune cells via homing in lymphoid organs such as the spleen (310).
Many studies have been developing methods in conjunction with intravenous administration to increase cell engraftment/homing to the area of interest. A technique termed, “ultrasound targeted microbubble destruction” (UTMD), was developed in the early 2000s and uses a bioactive substance incorporated into microbubbles that can then be injected into a patient’s blood stream. Following injection, an ultrasound wand is placed over the area of interest where the sound waves burst the bubbles, thus promoting accumulation as a targeted therapeutic (323). Bone marrow mononuclear cells combined with UTMD in a rat model of ischemic hindlimb resulted in the mononuclear cells attaching to the endothelium and increased collateral vessel development (324). These results were mirrored in a rabbit MI model where UTMBD with BM-MSCs improved cardiac function in the infarcted heart, specifically ejection fraction and increased the collateral circulation (325). In more recent years, a modified version of UTMD has arisen using superparamagnetic nanoparticles (SPIONs) and external magnetic fields. The SPIONs have been added to AD-MSCs and intravenously injected to Wistar rats subjected to stroke (326). A strong magnet was placed next to the ischemic area for 30 min yielding a high initial recruitment of AD-MSCs to the brain, but researchers reported that the engrafted cells showed signs of death after 1 day (326). Contrary to these results, a study in a Wistar rat model of sciatic nerve damage demonstrated that intravenously injected AD-MSCs with SPIONs home to the injured nerve following a 24-h exposure to a magnetic field (327). At 7-days postinjection, 3.3% of administered cells still remained because cell viability and cell differentiation ability were unaffected by the SPIONs (327). The utilization of microbubbles or SPIONs represents a promising advancement in the application of cell therapies to increase homing while circumventing the need for direct injection/delivery. Albeit the intravenous administration of cell therapies has been widely discussed and used, in recent years researchers have been exploring other ways to deliver stem cells to patients.
The newest forms of cell administration, specifically for stromal cells isolated from adipose tissue, have been reported in solid, liquid, and gas phases. The solid phase of administration combines platelet poor plasma with adipose derived stromal cells to form gelatin seeded matrix that could be advantageous in dermal applications (328). As previously mentioned, both adipose-derived stem cells and adipose-derived stromal vascular fraction cells have been combined with collagen matrices and either injected (278) or implanted subdermally (293). In both cases, the utilization of a solid/semi-solid phase in conjunction with a cell therapy greatly increased the angiogenic potential, microvascular density, and cellular engraftment into the host tissue (278, 293). The stromal cells, typically in the liquid phase for most applications discussed and used in the clinic, are often injected into a vein or organ. More recently, stromal cells were used in combination with a micro-dermal roller aimed at increasing treatment penetration for improved restoration through the layers of the skin (328). In an animal model with a distal dermal flap developing necrosing, microneedeling with a dermal roller significantly increased the number of new vessels in the flap, improving viability (329). On human patients, microneedeling was combined with conditioned medium from human ESC cultures and resulted in improved skin pigmentation and decreased the look of wrinkles compared with microneedeling alone; however, the subdermal vascular effects of this therapy regime were not examined (330).
Finally, stromal cells in the liquid form have been combined with a nebulizer to deliver the therapeutic as an aerosolized treatment. The short- and long-term safety/efficacy of intranasal administration of MSCs was examined with human MSCs administered in repeating doses to juvenile immunodeficient mice, with no adverse events nor tumor formation reported. One hour postintranasal administration resulted in a majority of the cells accumulating in the lungs compared with the majority of the cells found in the stomach 1-day postadministration. Following up, cells could not be detected in the brain, lungs, liver, heart, spleen, kidney, or stomach 1-wk postadministration (331). The intranasal route of administration has the added benefit of bypassing the blood-brain barrier noninvasively, and for that reason it has been used to administer neural stem cells to the brain as a treatment for Parkinson’s disease (332, 333) and Alzheimer’s disease (334). The pace of scientific community in exploring the therapeutic mechanism behind cell and cell-based therapies combined with the novel developments in delivery and dosing strategies (Fig. 4) bring the use of stem cells, autologous or allogeneic, ever closer to a therapeutic for a wide range of disease and pathologic states.
FUTURE PERSPECTIVES
In consideration of designing an appropriate approach for the mediation of vascular and microvascular regeneration, this review highlighted critical questions necessary to move the field forward. What is the optimal therapeutic dose? How should cells be administered? What cells should be used? How should cells be conditioned, if at all? Is there an advantage to the use of homogenous or heterogeneous cell populations? Should cellular components (e.g., exosomes or secreted factors) be used in lieu of cells? How do various clinical situations and presentations effect how we answer these questions? These questions, while still completely unanswered, framed our exploration of the field’s current state and identification of future research directions. To provide quality evidence-based solutions to these questions, the field must embrace emerging trends in scientific rigor, reproducibility, and ethics. For instance, quality control standards are only beginning to be suggested in the field, and should be undertaken (335). This is especially true for cell-free studies using exosomes, as standardization is needed in terms of how we define dosage (based off of exosomal count vs. total protein content). Further comparative studies and discussion are needed to determine whether the advantages of exosomal therapy over stem cell therapies warrants such a refined approach (336). What potentially unique positive effects are we missing by removing the cellular component? Indeed, there is emerging discussion on the benefits of heterogeneity of using cell fractions or combined cell therapies versus one therapy with one isolated cell type, perhaps similar discussion is needed in the debate for cellular versus cell-free therapies (337–339). Finally, ensuring studies are representative of all facets of our population, including the inclusion of proper consideration of sex and gender as a biological variable (200) are necessary so that the answers we determine for these questions are translatable and equitable for all.
CONCLUSIONS
Cellular therapies represent a quickly evolving movement with exciting promise to overcome many limitations of current clinical standards. At the same time, the field is still young and concepts of optimal standardization in terms of which cells to use and in what situations, isolation practices, conditions for culture and expansion, ideal donor characteristics, dosing, and administration are not yet established. This review highlights the most recent trends in these areas as it relates to vascular regeneration in an effort to take stock of the current state of the field, in hopes that future approaches become more standardized. Isolation practices, unique characteristics, pros and cons of various cell types are provided in Fig. 1 and Table 1. Although the majority of the field has moved to accept the paracrine hypothesis as the prevailing theory for the majority of stem cell-mediated vascular regeneration, there is still relevance for the direct mechanism, such as differentiation and direct transfer of cellular contents which should not be wholly discounted. These mechanisms can improve vasodilative function, angiogenesis, vascular density, mitochondrial function while reducing calcification and smooth muscle proliferation, reduce lipid and DNA oxidation, ER stress fibrosis, senescence, and apoptosis according to Fig. 2, and microvascular recovery in particular is summarized in Table 2. There has been much debate about the merits of autologous versus allogeneic strategies, with advantages and disadvantages to both as highlighted in Fig. 3. The most feasible answer to the debate currently is that the use of autologous or allogeneic stem cells will likely depend on individual clinical characteristics and needs with each strategy having appropriate clinical situations. These situations should balance ease of using reproducible large-scale manufactured cell therapies versus cell therapies that can be more tailored to individual patient needs, all while keeping patient health status and goals of treatment in mind. The potential disadvantages of autologous therapy may be mitigated by advances in cell priming and preconditioning as well as alternative dosing and administration as highlighted in Figs. 4 and 5. In all, cellular therapeutics rejuvenate vascular dynamics in acute and chronic pathologies in animal and human models with wide opportunities for further optimization for individual pathologic states as well as donor and recipient health status.
GRANTS
This work was funded by Gheen’s Foundation (to A. J. LeBlanc), Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada Grant RGPIN 06621-2017 (to S. S. Nunes Vasconcelos), Canadian Institutes of Health Research Grant PJT153160 (to S. S. Nunes Vasconcelos), National Institute on Aging Grants AG053585 (to A. J. LeBlanc) and R01 AG049821 (to W. L. Murfee), Ministère du Développement économique, de la Création d'emplois et du Commerce, Ontario Ministry of Research and Innovation Grant ER17 13 420 149 (to S. S. Nunes Vasconcelos), and U.S. Department of Defense Grants W81XWH-19-RTRP-IDA and W81XWH-13-2-0057 (to A. J. LeBlanc).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.L. conceived and designed research; E.P.T. and G.R. analyzed data; E.P.T., V.S., G.R., D.B., and A.J.L. prepared figures; E.P.T., V.S., G.R., D.B., S.S.N., J.B.H., and W.L.M. drafted manuscript; E.P.T., V.S., G.R., D.B., S.S.N., J.B.H., W.L.M., and A.J.L. edited and revised manuscript; E.P.T., V.S., G.R., D.B., S.S.N., J.B.H., W.L.M., and A.J.L. approved final version of manuscript.
REFERENCES
- 1.Rowe G, Kelm NQ, Beare JE, Tracy E, Yuan F, LeBlanc AJ. Enhanced β-1 adrenergic receptor responsiveness in coronary arterioles following intravenous stromal vascular fraction therapy in aged rats. Aging (Albany NY) 11: 4561–4578, 2019. doi: 10.18632/aging.102069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe G, Tracy E, Beare JE, LeBlanc AJ. Cell therapy rescues aging-induced β-1 adrenergic receptor and GRK2 dysfunction in the coronary microcirculation. Geroscience 44: 329–348, 2022. doi: 10.1007/s11357-021-00455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracy E, Rowe G, LeBlanc AJ. Cardiac tissue remodeling in healthy aging: the road to pathology. Am J Physiol Cell Physiol 319: C166–C182, 2020. doi: 10.1152/ajpcell.00021.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracy EP, Hughes W, Beare JE, Rowe G, Beyer A, LeBlanc AJ. Aging induced impairment of vascular function—mitochondrial redox contributions and physiological/clinical implications. Antioxid Redox Signal 35: 974–1015, 2021. doi: 10.1089/ars.2021.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev 25: 604–614, 2009. doi: 10.1002/dmrr.1004. [DOI] [PubMed] [Google Scholar]
- 6.Roustit M, Loader J, Baltzis D, Zhao W, Veves A. Microvascular changes in the diabetic foot. In: The Diabetic Foot, edited by Veves A, Giurini J, Guzman R.. Cham: Humana, 2018, p. 173–188. [Google Scholar]
- 7.Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109: 1121–1126, 2004. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 8.Wei J, Cheng S, Bairey Merz CN. Coronary microvascular dysfunction causing cardiac ischemia in women. JAMA 322: 2334–2335, 2019. doi: 10.1001/jama.2019.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benton RL, Maddie MA, Gruenthal MJ, Hagg T, Whittemore SR. Neutralizing endogenous VEGF following traumatic spinal cord injury modulates microvascular plasticity but not tissue sparing or functional recovery. Curr Neurovasc Res 6: 124–131, 2009. doi: 10.2174/156720209788185678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fassbender JM, Whittemore SR, Hagg T. Targeting microvasculature for neuroprotection after SCI. Neurotherapeutics 8: 240–251, 2011. doi: 10.1007/s13311-011-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W, Lee JCY, Leung ST, Lai A, Lee T-F, Chiang JB, Cheng YW, Chan HL, Yiu KH, Goh VK, Pennell DJ, Ng MY. Long-term prognosis of patients with coronary microvascular disease using stress perfusion cardiac magnetic resonance. JACC Cardiovasc Imaging 14: 602–611, 2021. doi: 10.1016/j.jcmg.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Merz NB. Testing for coronary microvascular dysfunction. JAMA 322: 2358, 2019. doi: 10.1001/jama.2019.16625. [DOI] [PubMed] [Google Scholar]
- 13.Merz NB, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA) developing evidence-based therapies and research agenda for the next decade. Circulation 135: 1075–1092, 2017. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RD, Petersen JW, Mehta PK, Wei J, Johnson BD, Handberg EM, Kar S, Samuels B, Azarbal B, Kothawade K, Kelsey SF, Sharaf B, Shaw LJ, Sopko G, Bairey Merz CN, Pepine CJ. Prevalence of coronary endothelial and microvascular dysfunction in women with symptoms of ischemia and no obstructive coronary artery disease is confirmed by a new cohort: the NHLBI-sponsored women’s ischemia syndrome evaluation-coronary vascular dysfunction (WISE-CVD). J Interv Cardiol 2019: 7169275, 2019. doi: 10.1155/2019/7169275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 44: 2137–2141, 2004. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TT, Shaw JE, Robinson C, Kawasaki R, Wang JJ, Kreis AJ. Diabetic retinopathy is related to both endothelium-dependent and -independent responses of skin microvascular flow. Diabetes Care 34: 1389–1393, 2011. doi: 10.2337/dc10-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulon P, Constans J, Gosse P. Impairment of skin blood flow during post-occlusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens 26: 56–63, 2012. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- 18.Hellmann M, Roustit M, Cracowski JL. Skin microvascular endothelial function as a biomarker in cardiovascular diseases? Pharmacol Rep 67: 803–810, 2015. doi: 10.1016/j.pharep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc AJ, Krishnan L, Sullivan CJ, Williams SK, Hoying JB. Microvascular repair: post-angiogenesis vascular dynamics. Microcirculation 19: 676–695, 2012. doi: 10.1111/j.1549-8719.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secomb TW, Pries AR. The microcirculation: physiology at the mesoscale. J Physiol 589: 1047–1052, 2011. doi: 10.1113/jphysiol.2010.201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghazadeh Y, Khan ST, Nkennor B, Nunes SS. Cell-based therapies for vascular regeneration: past, present and future. Pharmacol Ther 231: 107976, 2022. doi: 10.1016/j.pharmthera.2021.107976. [DOI] [PubMed] [Google Scholar]
- 22.Pries AR, Secomb TW. Making microvascular networks work: angiogenesis, remodeling, and pruning. Physiology (Bethesda) 29: 446–455, 2014. doi: 10.1152/physiol.00012.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leblanc AJ, Touroo JS, Hoying JB, Williams SK. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 302: H973–H982, 2012. doi: 10.1152/ajpheart.00735.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelm NQ, Beare JE, Yuan F, George M, Shofner CM, Keller BB, Hoying JB, LeBlanc AJ. Adipose-derived cells improve left ventricular diastolic function and increase microvascular perfusion in advanced age. PLoS One 13: e0202934, 2018. doi: 10.1371/journal.pone.0202934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res 116: 856–870, 2020. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annex BH, Cooke JP. New directions in therapeutic angiogenesis and arteriogenesis in peripheral arterial disease. Circ Res 128: 1944–1957, 2021. doi: 10.1161/CIRCRESAHA.121.318266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng-Blichfeldt J-P, Gosens R, Dean C, Griffiths M, Hind M. Regenerative pharmacology for COPD: breathing new life into old lungs. Thorax 74: 890–897, 2019. doi: 10.1136/thoraxjnl-2018-212630. [DOI] [PubMed] [Google Scholar]
- 28.Fine LG. Restoring the function of a diseased kidney via its microvasculature. Nephron Exp Nephrol 126: 82, 2014. doi: 10.1159/000360672. [DOI] [PubMed] [Google Scholar]
- 29.Aghazadeh Y, Poon F, Sarangi F, Wong FTM, Khan ST, Sun X, Hatkar R, Cox BJ, Nunes SS, Nostro MC. Microvessels support engraftment and functionality of human islets and hESC-derived pancreatic progenitors in diabetes models. Cell Stem Cell 28: 1936–1949.e8, 2021. doi: 10.1016/j.stem.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 88: 487–514, 2019. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 31.Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, Chen Y, Ma B, Wei J, Han Q, Zhao RC. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev 27: 456–465, 2018. doi: 10.1089/scd.2017.0296. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Zhang R, Wang S-J, Zhang C-L, Mao L-B, Zhuang C-Y, Tang Y-Y, Luo X-G, Zhou H, Zhang T-C. Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor—a signaling pathway. Int J Biochem Cell Biol 45: 1447–1456, 2013. doi: 10.1016/j.biocel.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol 310: H455–H465, 2016. doi: 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eirin A, Zhu X-Y, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant 27: 1080–1095, 2018. doi: 10.1177/0963689718780942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buono L, Scalabrin S, De Iuliis M, Tanzi A, Grange C, Tapparo M, Nuzzi R, Bussolati B. Mesenchymal stem cell-derived extracellular vesicles protect human corneal endothelial cells from endoplasmic reticulum stress-mediated apoptosis. Int J Mol Sci 22: 4930, 2021. doi: 10.3390/ijms22094930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration 85: 3–10, 2013. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 37.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197: 452–454, 1963. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 38.Tan S, Barker N. Engineering the niche for stem cells. Growth Factors 31: 175–184, 2013. doi: 10.3109/08977194.2013.859683. [DOI] [PubMed] [Google Scholar]
- 39.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther 21: 1045–1056, 2010. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech 6: e441–e445, 2017. doi: 10.1016/j.eats.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowen JE. Technical issues in harvesting and concentrating stem cells (bone marrow and adipose). PM R 7: S8–S18, 2015. doi: 10.1016/j.pmrj.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Chan T-M, Harn H-J, Lin H-P, Chou P-W, Chen JY-R, Ho T-J, Chiou T-W, Chuang H-M, Chiu S-C, Chen Y-C, Yen S-Y, Huang M-H, Liang B-C, Lin S-Z. Improved human mesenchymal stem cell isolation. Cell Transplant 23: 399–406, 2014. doi: 10.3727/096368914X678292. [DOI] [PubMed] [Google Scholar]
- 43.Chong P-P, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 30: 634–642, 2012. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 44.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, Ehninger G, Johnston L, Maziarz RT, Pulsipher MA, Porter DL, Mineishi S, McCarty JM, Khan SP, Anderlini P, Bensinger WI, Leitman SF, Rowley SD, Bredeson C, Carter SL, Horowitz MM, Confer DL; Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 367: 1487–1496, 2012. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M, Huang B. The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res Ther 3: 48, 2012. doi: 10.1186/scrt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamat P, Frueh FS, McLuckie M, Sanchez-Macedo N, Wolint P, Lindenblatt N, Plock JA, Calcagni M, Buschmann J. Adipose tissue and the vascularization of biomaterials: stem cells, microvascular fragments and nanofat—a review. Cytotherapy 22: 400–411, 2020. doi: 10.1016/j.jcyt.2020.03.433. [DOI] [PubMed] [Google Scholar]
- 47.Stachura A, Paskal W, Pawlik W, Mazurek MJ, Jaworowski J. The use of adipose-derived stem cells (ADSCs) and stromal vascular fraction (SVF) in skin scar treatment—a systematic review of clinical studies. J Clin Med 10: 3637, 2021. doi: 10.3390/jcm10163637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8: 145, 2017. doi: 10.1186/s13287-017-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. J Plast Reconstr Aesthet Surg 69: 170–179, 2016. doi: 10.1016/j.bjps.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Jones VM, Suarez-Martinez AD, Hodges NA, Murfee WL, Llull R, Katz AJ. A clinical perspective on adipose-derived cell therapy for enhancing microvascular health and function: Implications and applications for reconstructive surgery. Microcirculation 28: e12672, 2021. doi: 10.1111/micc.12672. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, Xia J, Qiu X, Wang P, Jia R, Chen Y, Yang B, Dai Y. In vitro evaluation of endothelial progenitor cells from adipose tissue as potential angiogenic cell sources for bladder angiogenesis. PLoS One 10: e0117644, 2015. doi: 10.1371/journal.pone.0117644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue S, Zhang H-T, Zhang P, Luo J, Chen Z-Z, Jang X-D, Xu R-X. Functional endothelial progenitor cells derived from adipose tissue show beneficial effect on cell therapy of traumatic brain injury. Neurosci Lett 473: 186–191, 2010. doi: 10.1016/j.neulet.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 53.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637, 2012. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol 29: 61–66, 2009. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 55.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 287: C572–C579, 2004. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 56.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15: 641–648, 2013. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv 36: 1111–1126, 2018. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Madonna R, Caterina RD. In vitro neovasculogenic potential of resident adipose tissue precursors. Am J Physiol Cell Physiol 295: C1271–C1280, 2008. doi: 10.1152/ajpcell.00186.2008. [DOI] [PubMed] [Google Scholar]
- 59.Ilic D, Ogilvie C. Concise review: human embryonic stem cells—what have we done? What are we doing? Where are we going? Stem Cells 35: 17–25, 2017. doi: 10.1002/stem.2450. [DOI] [PubMed] [Google Scholar]
- 60.Bezenah JR, Kong YP, Putnam AJ. Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Sci Rep 8: 2671, 2018. doi: 10.1038/s41598-018-20966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karagiannis P, Takahashi K, Saito M, Yoshida Y, Okita K, Watanabe A, Inoue H, Yamashita JK, Todani M, Nakagawa M, Osawa M, Yashiro Y, Yamanaka S, Osafune K. Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev 99: 79–114, 2019. doi: 10.1152/physrev.00039.2017. [DOI] [PubMed] [Google Scholar]
- 62.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 99: 4391–4396, 2002. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Mounayri O, Mihic A, Shikatani EA, Gagliardi M, Steinbach SK, Dubois N, Dacosta R, Li RK, Keller G, Husain M. Serum-free differentiation of functional human coronary-like vascular smooth muscle cells from embryonic stem cells. Cardiovasc Res 98: 125–135, 2013. doi: 10.1093/cvr/cvs357. [DOI] [PubMed] [Google Scholar]
- 64.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8: 228–240, 2011. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Raikwar SP, Mueller T, Zavazava N. Strategies for developing therapeutic application of human embryonic stem cells. Physiology (Bethesda) 21: 19–28, 2006. doi: 10.1152/physiol.00034.2005. [DOI] [PubMed] [Google Scholar]
- 66.Ikuno T, Masumoto H, Yamamizu K, Yoshioka M, Minakata K, Ikeda T, Sakata R, Yamashita JK. Efficient and robust differentiation of endothelial cells from human induced pluripotent stem cells via lineage control with VEGF and cyclic AMP. PLoS One 12: e0173271, 2017. [Erratum in PLoS One 12: e0176238, 2017]. doi: 10.1371/journal.pone.0173271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bezenah JR, Rioja AY, Juliar B, Friend N, Putnam AJ. Assessing the ability of human endothelial cells derived from induced-pluripotent stem cells to form functional microvasculature in vivo. Biotechnol Bioeng 116: 415–426, 2019. doi: 10.1002/bit.26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang K, Lin R-Z, Hong X, Ng AH, Lee CN, Neumeyer J, Wang G, Wang X, Ma M, Pu WT, Church GM, Melero-Martin JM. Robust differentiation of human pluripotent stem cells into endothelial cells via temporal modulation of ETV2 with modified mRNA. Sci Adv 6: eaba7606, 2020. doi: 10.1126/sciadv.aba7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumeyer J, Lin R-Z, Wang K, Hong X, Hua T, Croteau SE, Neufeld EJ, Melero-Martin JM. Bioengineering hemophilia A-specific microvascular grafts for delivery of full-length factor VIII into the bloodstream. Blood Adv 3: 4166–4176, 2019. doi: 10.1182/bloodadvances.2019000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and safety issues of stem cell-based therapy. Int J Med Sci 15: 36–45, 2018. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki J, Shimizu Y, Tsuzuki K, Pu Z, Narita S, Yamaguchi S, Katagiri T, Iwata E, Masutomi T, Fujikawa Y, Shibata R, Murohara T. No influence on tumor growth by intramuscular injection of adipose-derived regenerative cells: safety evaluation of therapeutic angiogenesis with cell therapy. Am J Physiol Heart Circ Physiol 320: H447–H457, 2021. doi: 10.1152/ajpheart.00564.2020. [DOI] [PubMed] [Google Scholar]
- 72.Nussbaum J, Minami E, Laflamme MA, Virag JAI, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J 21: 1345–1357, 2007. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 73.Matsu-Ura T, Sasaki H, Okada M, Mikoshiba K, Ashraf M. Attenuation of teratoma formation by p27 overexpression in induced pluripotent stem cells. Stem Cell Res Ther 7: 30, 2016. doi: 10.1186/s13287-016-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Mácia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 28: 1568–1570, 2010. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol 167: 663–671, 2005. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev 85: 635–678, 2005. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 77.Araujo AB, Salton GD, Furlan JM, Schneider N, Angeli MH, Laureano ÁM, Silla L, Passos EP, Paz AH. Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy 19: 577–585, 2017. doi: 10.1016/j.jcyt.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells 6: 195–202, 2014. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steigman SA, Fauza DO. Isolation of mesenchymal stem cells from amniotic fluid and placenta. Curr Protoc Stem Cell Biol 1: 1E.2.1–1E.2.12, 2007. doi: 10.1002/9780470151808.sc01e02s1. [DOI] [PubMed] [Google Scholar]
- 80.Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Ayame H, Iwasaki K, Taki A, Oshima N. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther 8: 219, 2017. doi: 10.1186/s13287-017-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun 438: 410–419, 2013. doi: 10.1016/j.bbrc.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 82.Sagar R, Walther-Jallow L, David AL, Götherström C, Westgren M. Fetal mesenchymal stromal cells: an opportunity for prenatal cellular therapy. Curr Stem Cell Rep 4: 61–68, 2018. doi: 10.1007/s40778-018-0118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shang F, Yu Y, Liu S, Ming L, Zhang Y, Zhou Z, Zhao J, Jin Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater 6: 666–683, 2021. doi: 10.1016/j.bioactmat.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsiko A, Levingstone TJ, O'Brien FJ. Advanced strategies for articular cartilage defect repair. Materials (Basel) 6: 637–668, 2013. doi: 10.3390/ma6020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther 8: 45, 2017. doi: 10.1186/s13287-017-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J 18: e264–e277, 2018. doi: 10.18295/squmj.2018.18.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 163: 399–408, 2014. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 88.Li C-y, Wu X-y, Tong J-B, Yang X-X, Zhao J-L, Zheng Q-F, Zhao G-B, Ma Z-J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther 6: 55, 2015. doi: 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, Feng G, Cai Y, Xia C, Liu H, Shen W, Hu X, Ouyang H. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med 47: 1722–1733, 2019. doi: 10.1177/0363546519848678. [DOI] [PubMed] [Google Scholar]
- 90.Hladik D, Höfig I, Oestreicher U, Beckers J, Matjanovski M, Bao X, Scherthan H, Atkinson MJ, Rosemann M. Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Res Ther 10: 218, 2019. doi: 10.1186/s13287-019-1334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noguchi H, Miyagi-Shiohira C, Nakashima Y. Induced tissue-specific stem cells and epigenetic memory in induced pluripotent stem cells. Int J Mol Sci 19: 930, 2018. doi: 10.3390/ijms19040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flahou C, Morishima T, Takizawa H, Sugimoto N. Fit-for-all iPSC-derived cell therapies and their evaluation in humanized mice with nk cell immunity. Front Immunol 12: 662360, 2021. doi: 10.3389/fimmu.2021.662360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu M, Xue R, Wang P, Wang X, Tian X, Liu Y, Wang S, Cui A, Xie J, Le L, Zhao M, Quan J, Li N, Meng D, Wang X, Sun N, Chen AF, Xiang M, Chen S. Induced pluripotent stem cells attenuate chronic allogeneic vasculopathy in an integrin β-1-dependent manner. Am J Transplant 20: 2755–2767, 2020. doi: 10.1111/ajt.15900. [DOI] [PubMed] [Google Scholar]
- 94.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 14: 47–59, 2011. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC, Putnam AJ. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316: 813–825, 2010. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel J-C, Cugno C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int 2020: 4356359, 2020. 2020. doi: 10.1155/2020/4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y, Anderson JD, Rahnama LMA, Gu SV, Knowlton AA. Exosomes in disease and regeneration: biological functions, diagnostics, and beneficial effects. Am J Physiol Heart Circ Physiol 319: H1162–H1180, 2020. doi: 10.1152/ajpheart.00075.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 99.Vittorio O, Jacchetti E, Pacini S, Cecchini M. Endothelial differentiation of mesenchymal stromal cells: when traditional biology meets mechanotransduction. Integr Biol (Camb) 5: 291–299, 2013. doi: 10.1039/c2ib20152f. [DOI] [PubMed] [Google Scholar]
- 100.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 94: 230–238, 2004. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 101.Kikuchi-Taura A, Okinaka Y, Saino O, Takeuchi Y, Ogawa Y, Kimura T, Gul S, Claussen C, Boltze J, Taguchi A. Gap junction-mediated cell-cell interaction between transplanted mesenchymal stem cells and vascular endothelium in stroke. Stem Cells 39: 904–912, 2021. doi: 10.1002/stem.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kikuchi-Taura A, Okinaka Y, Takeuchi Y, Ogawa Y, Maeda M, Kataoka Y, Yasui T, Kimura T, Gul S, Claussen C, Boltze J, Taguchi A. Bone marrow mononuclear cells activate angiogenesis via gap junction-mediated cell-cell interaction. Stroke 51: 1279–1289, 2020. doi: 10.1161/STROKEAHA.119.028072. [DOI] [PubMed] [Google Scholar]
- 103.Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg 30: 353–361, 2006. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 104.Wang X, Bai L, Liu X, Shen W, Tian H, Liu W, Yu B. Cardiac microvascular functions improved by MSC-derived exosomes attenuate cardiac fibrosis after ischemia-reperfusion via PDGFR-β modulation. Int J Cardiol 344: 13–24, 2021. doi: 10.1016/j.ijcard.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 105.Liu K, Guo L, Zhou Z, Pan M, Yan C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res 123: 74–80, 2019. doi: 10.1016/j.mvr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y, Zhao S, Luo L, Wang J, Zhu Z, Xiang Q, Deng Y, Zhao Z. Mesenchymal stem cell-derived exosomes ameliorate erection by reducing oxidative stress damage of corpus cavernosum in a rat model of artery injury. J Cell Mol Med 23: 7462–7473, 2019. doi: 10.1111/jcmm.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mikami S, Nakashima A, Nakagawa K, Maruhashi T, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, Ochi M, Nishimura M, Tsuji K, Kato Y, Goto C, Higashi Y. Autologous bone-marrow mesenchymal stem cell implantation and endothelial function in a rabbit ischemic limb model. PLoS One 8: e67739, 2013. doi: 10.1371/journal.pone.0067739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Florea V, Landin AM, Castellanos AM, Natsumeda M, Tompkins BA, Premer C, Difede D, Balkan W, Schulman IH, Hare J. The role of gender on mesenchymal stem cell therapy in non-ischemic dilated cardiomyopathy: a subanalysis of the POSEIDON-DCM trial. Am Coll Cardiol 69: 863, 2017. doi: 10.1016/S0735-1097(17)34252-3. [DOI] [Google Scholar]
- 109.Sharma S, Pandey NN, Sinha M, Kumar S, Jagia P, Gulati GS, Gond K, Mohanty S, Bhargava B. Randomized, double-blind, placebo-controlled trial to evaluate safety and therapeutic efficacy of angiogenesis induced by intraarterial autologous bone marrow-derived stem cells in patients with severe peripheral arterial disease. J Vasc Interv Radiol 32: 157–163, 2021. doi: 10.1016/j.jvir.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Mathiasen AB, Haack-Sørensen M, Jørgensen E, Kastrup J. Autotransplantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina—final 3-year follow-up. Int J Cardiol 170: 246–251, 2013. doi: 10.1016/j.ijcard.2013.10.079. [DOI] [PubMed] [Google Scholar]
- 111.Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, Zhao S, Yuan H, Yang X, Shi J, Zhao H. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol 318: C848–C856, 2020. doi: 10.1152/ajpcell.00041.2020. [DOI] [PubMed] [Google Scholar]
- 112.Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem 121: 2089–2102, 2020. doi: 10.1002/jcb.27399. [DOI] [PubMed] [Google Scholar]
- 113.Zhu X-Y, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 31: 117–125, 2013. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnson TK, Zhao L, Zhu D, Wang Y, Xiao Y, Oguljahan B, Zhao X, Kirlin WG, Yin L, Chilian WM, Liu D. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am J Physiol Heart Circ Physiol 317: H765–H776, 2019. doi: 10.1152/ajpheart.00247.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang K, Chen X, Li H, Feng G, Nie Y, Wei Y, Li N, Han Z, Han ZC, Kong D, Guo Z, Zhao Q, Li Z. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Acta Biomater 113: 289–304, 2020. doi: 10.1016/j.actbio.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 116.Motawea SM, Noreldin RI, Naguib YM. Potential therapeutic effects of endothelial cells trans-differentiated from Wharton's Jelly-derived mesenchymal stem cells on altered vascular functions in aged diabetic rat model. Diabetol Metab Syndr 12: 40, 2020. doi: 10.1186/s13098-020-00546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gorjipour F, Hosseini-Gohari L, Alizadeh Ghavidel A, Hajimiresmaiel SJ, Naderi N, Darbandi Azar A, Pazoki-Toroudi H. Mesenchymal stem cells from human amniotic membrane differentiate into cardiomyocytes and endothelial-like cells without improving cardiac function after surgical administration in rat model of chronic heart failure. J Cardiovasc Thorac Res 11: 35–42, 2019. doi: 10.15171/jcvtr.2019.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei Y, Wu Y, Zhao R, Zhang K, Midgley AC, Kong D, Li Z, Zhao Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials 204: 13–24, 2019. doi: 10.1016/j.biomaterials.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 119.Klein D, Steens J, Wiesemann A, Schulz F, Kaschani F, Röck K, Yamaguchi M, Wirsdörfer F, Kaiser M, Fischer JW, Stuschke M, Jendrossek V. Mesenchymal stem cell therapy protects lungs from radiation-induced endothelial cell loss by restoring superoxide dismutase 1 expression. Antioxid Redox Signal 26: 563–582, 2017. doi: 10.1089/ars.2016.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen J, Park H-C, Addabbo F, Ni J, Pelger E, Li H, Plotkin M, Goligorsky MS. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int 74: 879–889, 2008. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting pericytes for angiogenic therapies. Microcirculation 21: 345–357, 2014. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Azimi MS, Motherwell JM, Dutreil M, Fishel RL, Nice M, Hodges NA, Bunnell BA, Katz A, Murfee WL. A novel tissue culture model for evaluating the effect of aging on stem cell fate in adult microvascular networks. Geroscience 42: 515–526, 2020. doi: 10.1007/s11357-020-00178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barker TH. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials 32: 4211–4214, 2011. doi: 10.1016/j.biomaterials.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 124.Wu C-C, Chao Y-C, Chen C-N, Chien S, Chen Y-C, Chien C-C, Chiu J-J, Linju Yen B. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech 41: 813–821, 2008. doi: 10.1016/j.jbiomech.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Nassiri SM, Rahbarghazi R. Interactions of mesenchymal stem cells with endothelial cells. Stem Cells Dev 23: 319–332, 2014. doi: 10.1089/scd.2013.0419. [DOI] [PubMed] [Google Scholar]
- 126.Lee KW, Kim DH, Lee JH, Youn YN. The effect of pulsatile flow on bMSC-derived endothelial-like cells in a small-sized artificial vessel made by 3-dimensional bioprinting. Stem Cells Int 2018: 7823830, 2018. doi: 10.1155/2018/7823830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohtani K, Vlachojannis GJ, Koyanagi M, Boeckel J-N, Urbich C, Farcas R, Bonig H, Marquez VE, Zeiher AM, Dimmeler S. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res 109: 1219–1229, 2011. doi: 10.1161/CIRCRESAHA.111.247304. [DOI] [PubMed] [Google Scholar]
- 128.Gomes SA, Rangel EB, Premer C, Dulce RA, Cao Y, Florea V, Balkan W, Rodrigues CO, Schally AV, Hare JM. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci USA 110: 2834–2839, 2013. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rytlewski JA, Alejandra Aldon M, Lewis EW, Suggs LJ. Mechanisms of tubulogenesis and endothelial phenotype expression by MSCs. Microvasc Res 99: 26–35, 2015. doi: 10.1016/j.mvr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Portalska KJ, Teixeira LM, Leijten JCH, Jin R, van Blitterswijk C, de Boer J, Karperien M. Boosting angiogenesis and functional vascularization in injectable dextran-hyaluronic acid hydrogels by endothelial-like mesenchymal stromal cells. Tissue Eng Part A 20: 819–829, 2014. doi: 10.1089/ten.TEA.2013.0280. [DOI] [PubMed] [Google Scholar]
- 131.Zhang C, Lin Y, Liu Q, He J, Xiang P, Wang D, Hu X, Chen J, Zhu W, Yu H. Growth differentiation factor 11 promotes differentiation of MSCs into endothelial-like cells for angiogenesis. J Cell Mol Med 24: 8703–8717, 2020. doi: 10.1111/jcmm.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jang E, Kim J-H, Lee J, Kim D-H, Youn Y-N. Enhanced biocompatibility of multi-layered, 3D bio-printed artificial vessels composed of autologous mesenchymal stem cells. Polymers (Basel) 12: 538, 2020. doi: 10.3390/polym12030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rahbarghazi R, Nassiri SM, Khazraiinia P, Kajbafzadeh A-M, Ahmadi SH, Mohammadi E, Molazem M, Zamani-Ahmadmahmudi M. Juxtacrine and paracrine interactions of rat marrow-derived mesenchymal stem cells, muscle-derived satellite cells, and neonatal cardiomyocytes with endothelial cells in angiogenesis dynamics. Stem Cells Dev 22: 855–865, 2013. doi: 10.1089/scd.2012.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fan X, Teng Y, Ye Z, Zhou Y, Tan WS. The effect of gap junction-mediated transfer of miR-200b on osteogenesis and angiogenesis in a co-culture of MSCs and HUVECs. J Cell Sci 131: jcs216135, 2018. doi: 10.1242/jcs.216135. [DOI] [PubMed] [Google Scholar]
- 135.Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res 92: 10–18, 2014. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 136.Ge Q, Zhang H, Hou J, Wan L, Cheng W, Wang X, Dong D, Chen C, Xia J, Guo J, Chen X, Wu X. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol Med Rep 17: 1667–1675, 2018. doi: 10.3892/mmr.2017.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang B, Yang S, Zhang Y, Sun Z, Xu W, Ye S. Co-culture of mesenchymal stem cells with umbilical vein endothelial cells under hypoxic condition. J Huazhong Univ Sci Technol Med Sci 32: 173–180, 2012. doi: 10.1007/s11596-012-0031-9. [DOI] [PubMed] [Google Scholar]
- 138.Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles 7: 1522236, 2018. doi: 10.1080/20013078.2018.1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark 4: 7, 2015. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology 16: 81, 2018. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krawczenko A, Bielawska-Pohl A, Paprocka M, Kraskiewicz H, Szyposzynska A, Wojdat E, Klimczak A. Microvesicles from human immortalized cell lines of endothelial progenitor cells and mesenchymal stem/stromal cells of adipose tissue origin as carriers of bioactive factors facilitating angiogenesis. Stem Cells Int 2020: 1289380, 2020. doi: 10.1155/2020/1289380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park S-R, Kim J-W, Jun H-S, Roh JY, Lee H-Y, Hong I-S. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther 26: 606–617, 2018. doi: 10.1016/j.ymthe.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chatterjee V, Yang X, Ma Y, Wu MH, Yuan SY. Extracellular vesicles: new players in regulating vascular barrier function. Am J Physiol Heart Circ Physiol 319: H1181–H1196, 2020. doi: 10.1152/ajpheart.00579.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yu Q, Wang D, Wen X, Tang X, Qi D, He J, Zhao Y, Deng W, Zhu T. Adipose-derived exosomes protect the pulmonary endothelial barrier in ventilator-induced lung injury by inhibiting the TRPV4/Ca(2+) signaling pathway. Am J Physiol Lung Cell Mol Physiol 318: L723–L741, 2020. doi: 10.1152/ajplung.00255.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6: 287–296, 2016. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang XC, Gao JQ, Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm 521: 167–175, 2017. doi: 10.1016/j.ijpharm.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 147.Lee JH, Ha DH, Go H-K, Youn J, Kim H-K, Jin RC, Miller RB, Kim D-H, Cho BS, Yi YW. Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int J Mol Sci 21: 4774, 2020. doi: 10.3390/ijms21134774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 21: 581–592, 2019. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 149.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol 3: 399–425, 2008. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luo R, Li L, Liu X, Yuan Y, Zhu W, Li L, Liu J, Lu Y, Cheng J, Chen Y. Mesenchymal stem cells alleviate palmitic acid-induced endothelial-to-mesenchymal transition by suppressing endoplasmic reticulum stress. Am J Physiol Endocrinol Metab 319: E961–E980, 2020. doi: 10.1152/ajpendo.00155.2020. [DOI] [PubMed] [Google Scholar]
- 151.Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med 9: 985–1006, 2020. doi: 10.1002/sctm.19-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther 25: 465–479, 2017. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19: 1885–1893, 2010. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 154.Gorbunov NV, Garrison BR, McDaniel DP, Zhai M, Liao P-J, Nurmemet D, Kiang JG. Adaptive redox response of mesenchymal stromal cells to stimulation with lipopolysaccharide inflammagen: mechanisms of remodeling of tissue barriers in sepsis. Oxid Med Cell Longev 2013: 186795, 2013. doi: 10.1155/2013/186795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu T, Ma X, Ouyang T, Chen H, Lin J, Liu J, Xiao Y, Yu J, Huang Y. SIRT1 reverses senescence via enhancing autophagy and attenuates oxidative stress-induced apoptosis through promoting p53 degradation. Int J Biol Macromol 117: 225–234, 2018. doi: 10.1016/j.ijbiomac.2018.05.174. [DOI] [PubMed] [Google Scholar]
- 156.Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Tang F, Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res 26: 190–205, 2016. doi: 10.1038/cr.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu S-H, Huang J-P, Lee RK-K, Huang M-C, Wu Y-H, Chen C-Y, Chen C-P. Paracrine factors from human placental multipotent mesenchymal stromal cells protect endothelium from oxidative injury via STAT3 and manganese superoxide dismutase activation. Biol Reprod 82: 905–913, 2010. doi: 10.1095/biolreprod.109.081828. [DOI] [PubMed] [Google Scholar]
- 158.Shen Y, Jiang X, Meng L, Xia C, Zhang L, Xin Y. Transplantation of bone marrow mesenchymal stem cells prevents radiation-induced artery injury by suppressing oxidative stress and inflammation. Oxid Med Cell Longev 2018: 5942916, 2018. doi: 10.1155/2018/5942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10: 301–312, 2013. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 160.Hogan SE, Rodriguez Salazar MP, Cheadle J, Glenn R, Medrano C, Petersen TH, Ilagan RM. Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 316: L723–L737, 2019. doi: 10.1152/ajplung.00058.2018. [DOI] [PubMed] [Google Scholar]
- 161.Du S, Ling H, Guo Z, Cao Q, Song C. Roles of exosomal miRNA in vascular aging. Pharmacol Res 165: 105278, 2021. doi: 10.1016/j.phrs.2020.105278. [DOI] [PubMed] [Google Scholar]
- 162.Zheng S, Shi A, Hill S, Grant C, Kokkinos MI, Murthi P, Georgiou HM, Brennecke SP, Kalionis B. Decidual mesenchymal stem/stromal cell-derived extracellular vesicles ameliorate endothelial cell proliferation, inflammation, and oxidative stress in a cell culture model of preeclampsia. Pregnancy Hypertens 22: 37–46, 2020. doi: 10.1016/j.preghy.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 163.Xie C, Jin J, Lv X, Tao J, Wang R, Miao D. Anti-aging effect of transplanted amniotic membrane mesenchymal stem cells in a premature aging model of BMI-1 deficiency . Sci Rep 5: 13975, 2015. doi: 10.1038/srep13975. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164.Zhao M, Liu S, Wang C, Wang Y, Wan M, Liu F, Gong M, Yuan Y, Chen Y, Cheng J, Lu Y, Liu J. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano 15: 1519–1538, 2021. [Erratum in ACS Nano15: 20692, 2021]. doi: 10.1021/acsnano.0c08947. [DOI] [PubMed] [Google Scholar]
- 165.Dutra Silva J, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, O'Kane C, Krasnodembskaya AD. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J 58: 2002978, 2021. doi: 10.1183/13993003.02978-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu W, Yuan Y, Liao G, Li L, Liu J, Chen Y, Zhang J, Cheng J, Lu Y. Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis 9: 837, 2018. doi: 10.1038/s41419-018-0861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yuan Y, Shi M, Li L, Liu J, Chen B, Chen Y, An X, Liu S, Luo R, Long D, Zhang W, Newsholme P, Cheng J, Lu Y. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1α pathway. Clin Sci (Lond) 130: 2181–2198, 2016. doi: 10.1042/CS20160235. [DOI] [PubMed] [Google Scholar]
- 168.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18: 759–765, 2012. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li C, Cheung MKH, Han S, Zhang Z, Chen L, Chen J, Zeng H, Qiu J. Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep 39: BSR20182417, 2019. doi: 10.1042/BSR20182417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vallabhaneni KC, Haller H, Dumler I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev 21: 3104–3113, 2012. doi: 10.1089/scd.2011.0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mahrouf-Yorgov M, Augeul L, Da Silva CC, Jourdan M, Rigolet M, Manin S, Ferrera R, Ovize M, Henry A, Guguin A, Meningaud JP, Dubois-Randé JL, Motterlini R, Foresti R, Rodriguez AM. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ 24: 1224–1238, 2017. doi: 10.1038/cdd.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park SH, Kwon OS, Park S-Y, Weavil JC, Hydren JR, Reese V, Andtbacka RHI, Hyngstrom JR, Richardson RS. Vasodilatory and vascular mitochondrial respiratory function with advancing age: evidence of a free radically mediated link in the human vasculature. Am J Physiol Regul Integr Comp Physiol 318: R701–R711, 2020. doi: 10.1152/ajpregu.00268.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Te Winkel J, John QE, Hosfield BD, Drucker NA, Das A, Olson KR, Markel TA. Mesenchymal stem cells promote mesenteric vasodilation through hydrogen sulfide and endothelial nitric oxide. Am J Physiol Gastrointest Liver Physiol 317: G441–G446, 2019. doi: 10.1152/ajpgi.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Korkmaz-Icöz S, Zhou P, Guo Y, Loganathan S, Brlecic P, Radovits T, Sayour AA, Ruppert M, Veres G, Karck M, Szabó G. Mesenchymal stem cell-derived conditioned medium protects vascular grafts of brain-dead rats against in vitro ischemia/reperfusion injury. Stem Cell Res Ther 12: 144, 2021. doi: 10.1186/s13287-021-02166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bhayadia R, Schmidt BMW, Melk A, Hömme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontol A Biol Sci Med Sci 71: 161–169, 2016. doi: 10.1093/gerona/glv008. [DOI] [PubMed] [Google Scholar]
- 176.Shi HZ, Zeng JC, Shi SH, Giannakopoulos H, Zhang QZ, Le AD. Extracellular vesicles of GMSCs alleviate aging-related cell senescence. J Dent Res 100: 283–292, 2021. doi: 10.1177/0022034520962463. [DOI] [PubMed] [Google Scholar]
- 177.Ding Q, Sun R, Wang P, Zhang H, Xiang M, Meng D, Sun N, Chen AF, Chen S. Protective effects of human induced pluripotent stem cell-derived exosomes on high glucose-induced injury in human endothelial cells. Exp Ther Med 15: 4791–4797, 2018. doi: 10.3892/etm.2018.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lei X, He N, Zhu L, Zhou M, Zhang K, Wang C, Huang H, Chen S, Li Y, Liu Q, Han Z, Guo Z, Han Z, Li Z. Mesenchymal stem cell-derived extracellular vesicles attenuate radiation-induced lung injury via miRNA-214-3p. Antioxid Redox Signal 35: 849–862, 2020. doi: 10.1089/ars.2019.7965. [DOI] [PubMed] [Google Scholar]
- 179.Wang S, Tong M, Hu S, Chen X. The bioactive substance secreted by MSC retards mouse aortic vascular smooth muscle cells calcification. Biomed Res Int 2018: 6053567, 2018. doi: 10.1155/2018/6053567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang Y, Ma W-Q, Zhu Y, Han X-Q, Liu N. Exosomes derived from mesenchymal stromal cells pretreated with advanced glycation end product-bovine serum albumin inhibit calcification of vascular smooth muscle cells. Front Endocrinol (Lausanne) 9: 524, 2018. doi: 10.3389/fendo.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang D, Gao B, Yue J, Liu F, Liu Y, Fu W, Si Y. Exosomes from mesenchymal stem cells expressing miR-125b inhibit neointimal hyperplasia via myosin IE. J Cell Mol Med 23: 1528–1540, 2019. doi: 10.1111/jcmm.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gopinath B, Chiha J, Plant AJH, Thiagalingam A, Burlutsky G, Kovoor P, Liew G, Mitchell P. Associations between retinal microvascular structure and the severity and extent of coronary artery disease. Atherosclerosis 236: 25–30, 2014. doi: 10.1016/j.atherosclerosis.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 183.Siasos G, Sara JD, Zaromytidou M, Park KH, Coskun AU, Lerman LO, Oikonomou E, Maynard CC, Fotiadis D, Stefanou K, Papafaklis M, Michalis L, Feldman C, Lerman A, Stone PH. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J Am Coll Cardiol 71: 2092–2102, 2018. doi: 10.1016/j.jacc.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 184.Katunaric B, Cohen KE, Beyer AM, Gutterman DD, Freed JK. Sweat the small stuff: the human microvasculature and heart disease. Microcirculation 28: e12658, 2021.. doi: 10.1111/micc.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Câmara NO, Bergamaschi CT, Campos RR, Boim MA. Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One 8: e78464, 2013. doi: 10.1371/journal.pone.0078464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oliveira-Sales EB, Boim MA. Mesenchymal stem cells and chronic renal artery stenosis. Am J Physiol Renal Physiol 310: F6–F9, 2016. doi: 10.1152/ajprenal.00341.2015. [DOI] [PubMed] [Google Scholar]
- 187.Pietrosi G, Fernández-Iglesias A, Pampalone M, Ortega-Ribera M, Lozano JJ, García-Calderó H, Abad-Jordà L, Conaldi PG, Parolini O, Vizzini G, Luca A, Bosch J, Gracia-Sancho J. Human amniotic stem cells improve hepatic microvascular dysfunction and portal hypertension in cirrhotic rats. Liver Int 40: 2500–2514, 2020. doi: 10.1111/liv.14610. [DOI] [PubMed] [Google Scholar]
- 188.Scheiner ZS, Talib S, Feigal EG. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J Biol Chem 289: 4571–4577, 2014. doi: 10.1074/jbc.R113.509588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khorraminejad-Shirazi M, Dorvash M, Estedlal A, Hoveidaei AH, Mazloomrezaei M, Mosaddeghi P. Aging: a cell source limiting factor in tissue engineering. World J Stem Cells 11: 787–802, 2019. doi: 10.4252/wjsc.v11.i10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sanchez-Diaz M, Quiñones-Vico MI, Sanabria de la Torre R, Montero-Vílchez T, Sierra-Sánchez A, Molina-Leyva A, Arias-Santiago S. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: a systematic review. J Clin Med 10: 2925, 2021. doi: 10.3390/jcm10132925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li M, Zhang HP, Wang XY, Chen ZG, Lin XF, Zhu W. Mesenchymal stem cell-derived exosomes ameliorate dermal fibrosis in a murine model of bleomycin-induced scleroderma. Stem Cells Dev 30: 981–990, 2021. doi: 10.1089/scd.2021.0112. [DOI] [PubMed] [Google Scholar]
- 192.Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med 9: 17–27, 2020. doi: 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-γ-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood 107: 2570–2577, 2006. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 194.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 195.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42–48, 2002. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 196.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 197.Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, Ueda T, Gee P, Nishikawa M, Nomura M, Kitaoka F, Takahashi T, Okita K, Yoshida Y, Kaneko S, Hotta A. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell 24: 566–578.e7, 2019. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 198.Merola J, Reschke M, Pierce RW, Qin L, Spindler S, Baltazar T, Manes TD, Lopez-Giraldez F, Li G, Bracaglia LG, Xie C, Kirkiles-Smith N, Saltzman WM, Tietjen GT, Tellides G, Pober JS. Progenitor-derived human endothelial cells evade alloimmunity by CRISPR/Cas9-mediated complete ablation of MHC expression. JCI Insight 4: e129739, 2019. doi: 10.1172/jci.insight.129739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cottrill A, Samadi Y, Marra K. Adipose stem cells and donor demographics: impact of anatomic location, donor sex, race, BMI, and health. In: Scientific Principles of Adipose Stem Cells, edited by Kokai L, Marra K, Rubin P.. Academic Press, 2022, p. 149–163. [Google Scholar]
- 200.Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 201.Frazier T, Lee S, Bowles A, Semon J, Bunnell B, Wu X, Gimble J. Gender and age-related cell compositional differences in C57BL/6 murine adipose tissue stromal vascular fraction. Adipocyte 7: 183–189, 2018. doi: 10.1080/21623945.2018.1460009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lim S, Kim I-K, Choi J-W, Seo H-H, Lim KH, Lee S, Lee HB, Kim SW, Hwang KC. Gender-dimorphic effects of adipose-derived stromal vascular fractions on HUVECs exposed to oxidative stress. Int J Med Sci 14: 911–919, 2017. doi: 10.7150/ijms.19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Redondo J, Sarkar P, Kemp K, Virgo PF, Pawade J, Norton A, Emery DC, Guttridge MG, Marks DI, Wilkins A, Scolding NJ, Rice CM. Reduced cellularity of bone marrow in multiple sclerosis with decreased MSC expansion potential and premature ageing in vitro. Mult Scler 24: 919–931, 2018. doi: 10.1177/1352458517711276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Isik B, Thaler R, Goksu BB, Conley SM, Al-Khafaji H, Mohan A, Afarideh M, Abumoawad AM, Zhu XY, Krier JD, Saadiq IM, Tang H, Eirin A, Hickson LJ, van Wijnen AJ, Textor SC, Lerman LO, Herrmann SM. Hypoxic preconditioning induces epigenetic changes and modifies swine mesenchymal stem cell angiogenesis and senescence in experimental atherosclerotic renal artery stenosis. Stem Cell Res Ther 12: 240, 2021. doi: 10.1186/s13287-021-02310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Karina K, Rosliana I, Sobariah S, Rosadi I, Afini I, Widyastuti T, Remelia M, Sukmawati D, Adiwinata Pawitan J. Diabetes mellitus type 2 reduces the viability, proliferation, and angiogenic marker of adipose-derived stem cells cultured in low-glucose anti-oxidant-serum supplemented medium. Biomed Res Ther 6: 3073–3082, 2019. doi: 10.15419/bmrat.v6i3.530. [DOI] [Google Scholar]
- 206.Aird AL, Nevitt CD, Christian K, Williams SK, Hoying JB, LeBlanc AJ. Adipose-derived stromal vascular fraction cells isolated from old animals exhibit reduced capacity to support the formation of microvascular networks. Exp Gerontol 63: 18–26, 2015. doi: 10.1016/j.exger.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dos-Anjos Vilaboa S, Navarro-Palou M, Llull R. Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy 16: 1092–1097, 2014. doi: 10.1016/j.jcyt.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 208.Andjelkov K, Conde-Green A, Mosahebi A. Smoking and physical activity significantly influence stromal vascular fraction cell yield and viability. Aesthetic Plast Surg 45: 315–321, 2021. doi: 10.1007/s00266-020-02008-2. [DOI] [PubMed] [Google Scholar]
- 209.Barwinska D. Damaging Effects of Cigarette Smoke on Organs and Stem/Progenitor Cells and the Restorative Potential of Cell Therapy (PhD thesis). Bloomington: Indiana University, 2017, p. 158. [Google Scholar]
- 210.Frazier TP, Gimble JM, Devay JW, Tucker HA, Chiu ES, Rowan BG. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol 14: 34, 2013. doi: 10.1186/1471-2121-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Denu RA, Hematti P. Optimization of oxidative stress for mesenchymal stromal/stem cell engraftment, function and longevity. Free Radic Biol Med 167: 193–200, 2021. doi: 10.1016/j.freeradbiomed.2021.02.042. [DOI] [PubMed] [Google Scholar]
- 212.Garrido-Pascual P, Alonso-Varona A, Castro B, Burón M, Palomares T. H2O2− preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Res Ther 11: 335, 2020. doi: 10.1186/s13287-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bai Y, Han Y-D, Yan X-L, Ren J, Zeng Q, Li X-D, Pei XT, Han Y. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem Biophys Res Commun 500: 310–317, 2018. doi: 10.1016/j.bbrc.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 214.Guo L, Du J, Yuan D-F, Zhang Y, Zhang S, Zhang H-C, Mi JW, Ning YL, Chen MJ, Wen DL, Sun JH, Liu D, Zeng L, Zhang A, Jiang J, Huang H. Optimal H2O2 preconditioning to improve bone marrow mesenchymal stem cells' engraftment in wound healing. Stem Cell Res Ther 11: 434, 2020. doi: 10.1186/s13287-020-01910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hao D, He C, Ma B, Lankford L, Reynaga L, Farmer DL, Guo F, Wang A. Hypoxic preconditioning enhances survival and proangiogenic capacity of human first trimester chorionic villus-derived mesenchymal stem cells for fetal tissue engineering. Stem Cells Int 2019: 9695239, 2019. doi: 10.1155/2019/9695239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol 299: C1562–C1570, 2010. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kubo M, Li T-S, Suzuki R, Shirasawa B, Morikage N, Ohshima M, Qin SL, Hamano K. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am J Physiol Heart Circ Physiol 294: H590–H595, 2008. doi: 10.1152/ajpheart.00856.2007. [DOI] [PubMed] [Google Scholar]
- 218.Mathew SA, Chandravanshi B, Bhonde R. Hypoxia primed placental mesenchymal stem cells for wound healing. Life Sci 182: 85–92, 2017. doi: 10.1016/j.lfs.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 219.Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic properties of mesenchymal stromal/stem cells: the need of cell priming for cell-free therapies in regenerative medicine. Int J Mol Sci 22: 763, 2021. doi: 10.3390/ijms22020763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Costa V, Raimondi L, Conigliaro A, Salamanna F, Carina V, De Luca A, Bellavia D, Alessandro R, Fini M, Giavaresi G. Hypoxia-inducible factor 1Alpha may regulate the commitment of mesenchymal stromal cells toward angio-osteogenesis by miRNA-675-5P. Cytotherapy 19: 1412–1425, 2017. doi: 10.1016/j.jcyt.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 221.Wang XY, Liu C, Li SD, Xu Y, Chen P, Liu Y, Ding Q, Wahafu W, Hong B, Yang M. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One 10: e0118951, 2015. doi: 10.1371/journal.pone.0118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Muzakkir AF, Suryawan I, Yusrizal T. Hypoxic preconditioning effects of bone marrow-derived culture mesenchymal stem cells on CD31+ expression, vascular endothelial growth factors-a (VEGF-A) and stromal-derived sactors-1 α (SDF-1α). IOP Conf Series: Earth Environ Sci 441: 012161, 2020. doi: 10.1088/1755-1315/441/1/012161. [DOI] [Google Scholar]
- 223.Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J, Sadoine J, Beckouche N, Berndt S, Novais A, Lesage M, Hosten B, Vercellino L, Merlet P, Le-Denmat D, Marchiol C, Letourneur D, Nicoletti A, Vital SO, Poliard A, Salmon B, Muller L, Chaussain C, Germain S. Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem Cells Transl Med 5: 392–404, 2016. doi: 10.5966/sctm.2015-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leroux L, Descamps B, Tojais NF, Séguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamazière JM, Dufourcq P, Couffinhal T, Duplàa C. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 18: 1545–1552, 2010. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee JH, Yoon YM, Lee SH. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt axis. Int J Mol Sci 18: 1320, 2017. doi: 10.3390/ijms18061320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Han Y-S, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis 7: e2395, 2016. doi: 10.1038/cddis.2016.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhou P, Tan Y-Z, Wang H-J, Wang G-D. Hypoxic preconditioning-induced autophagy enhances survival of engrafted endothelial progenitor cells in ischaemic limb. J Cell Mol Med 21: 2452–2464, 2017. doi: 10.1111/jcmm.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gorgun C, Ceresa D, Lesage R, Villa F, Reverberi D, Balbi C, Santamaria S, Cortese K, Malatesta P, Geris L, Quarto R, Tasso R. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 269: 120633, 2021. doi: 10.1016/j.biomaterials.2020.120633. [DOI] [PubMed] [Google Scholar]
- 229.Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol 7: 292, 2019. doi: 10.3389/fbioe.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Zhou Z, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater 103: 196–212, 2020.doi: 10.1016/j.actbio.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 231.Gao Y, Wu D, Jia D, Guo Q, Wang M, Yang R, Zhang X, Chen M, Zhang D. Hypoxic stem cell-derived extracellular vesicles for cardiac repair in preclinical animal models of myocardial infarction: a meta-analysis. Stem Cells Dev 30: 891–907, 2021. doi: 10.1089/scd.2021.0084. [DOI] [PubMed] [Google Scholar]
- 232.Kuss MA, Harms R, Wu S, Wang Y, Untrauer JB, Carlson MA, Duan B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv 7: 29312–29320, 2017. doi: 10.1039/c7ra04372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Taylor CJ, Church JE, Williams MD, Gerrand Y-W, Keramidaris E, Palmer JA, Galea LA, Penington AJ, Morrison WA, Mitchell GM. Hypoxic preconditioning of myoblasts implanted in a tissue engineering chamber significantly increases local angiogenesis via upregulation of myoblast vascular endothelial growth factor-A expression and downregulation of miRNA-1, miRNA-206 and angiopoietin-1. J Tissue Eng Regen Med 12: e408–e421, 2018. doi: 10.1002/term.2440. [DOI] [PubMed] [Google Scholar]
- 234.Zubkova ES, Beloglazova IB, Makarevich PI, Boldyreva MA, Sukhareva OY, Shestakova MV, Dergilev KV, Parfyonova YV, Menshikov MY. Regulation of adipose tissue stem cells angiogenic potential by tumor necrosis factor-α. J Cell Biochem 117: 180–196, 2016. doi: 10.1002/jcb.25263. [DOI] [PubMed] [Google Scholar]
- 235.Sun J, Chen J, Cao J, Li T, Zhuang S, Jiang X. IL-1β-stimulated β-catenin up-regulation promotes angiogenesis in human lung-derived mesenchymal stromal cells through a NF-κB-dependent microRNA-433 induction. Oncotarget 7: 59429–59440, 2016. doi: 10.18632/oncotarget.10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 29: 1727–1737, 2011. doi: 10.1002/stem.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Beegle JR, Magner NL, Kalomoiris S, Harding A, Zhou P, Nacey C, White JL, Pepper K, Gruenloh W, Annett G, Nolta JA, Fierro FA. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol Ther Methods Clin Dev 3: 16053, 2016. doi: 10.1038/mtm.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fierro FA, Magner N, Beegle J, Dahlenburg H, Logan White J, Zhou P, Pepper K, Fury B, Coleal-Bergum DP, Bauer G, Gruenloh W, Annett G, Pifer C, Nolta JA. Mesenchymal stem/stromal cells genetically engineered to produce vascular endothelial growth factor for revascularization in wound healing and ischemic conditions. Transfusion 59: 893–897, 2019. doi: 10.1111/trf.14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 33: 1818–1828, 2015. doi: 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Madonna R, Taylor DA, Geng Y-J, De Caterina R, Shelat H, Perin EC, Willerson JT. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ Res 113: 902–914, 2013. doi: 10.1161/CIRCRESAHA.113.301690. [DOI] [PubMed] [Google Scholar]
- 241.Sonoda S, Murata S, Nishida K, Kato H, Uehara N, Kyumoto YN, Yamaza H, Takahashi I, Kukita T, Yamaza T. Extracellular vesicles from deciduous pulp stem cells recover bone loss by regulating telomerase activity in an osteoporosis mouse model. Stem Cell Res Ther 11: 296, 2020. doi: 10.1186/s13287-020-01818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, Zhang Y, Zhang Y, Xie W, Yan B, Liu F, Yip HK, Yu XY, Li Y. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-κB/TNF-α signaling pathway. Oxid Med Cell Longev 2019: 9739258, 2019. doi: 10.1155/2019/9739258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gutkin A, Uziel O, Beery E, Nordenberg J, Pinchasi M, Goldvaser H, Henick S, Goldberg M, Lahav M. Tumor cells derived exosomes contain hTERT mRNA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget 7: 59173–59188, 2016. doi: 10.18632/oncotarget.10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vellingiri V, Mehta D. TERTing the hyperoxic lung. Am J Physiol Heart Circ Physiol 321: H1103–H1105, 2021. doi: 10.1152/ajpheart.00580.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tracy E, Rowe G, Toro L, Beyer A, Leblanc A. Telomerase reverse transcriptase mediates restoration of functional vasodilation in isolated coronary microvessels of aged female rats. FASEB J 34: 1, 2020. doi: 10.1096/fasebj.2020.34.s1.07603. [DOI] [Google Scholar]
- 246.Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther 11: 373, 2020. doi: 10.1186/s13287-020-01881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 35: 1747–1759, 2017. doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 248.Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X, Liu L. miRNA-126-3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo. Stem Cell Res Ther 11: 464, 2020. doi: 10.1186/s13287-020-01978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Houri K, Mori T, Onodera Y, Tsujimoto T, Takehara T, Nakao S, Teramura T, Fukuda K. miR-142 induces accumulation of reactive oxygen species (ROS) by inhibiting pexophagy in aged bone marrow mesenchymal stem cells. Sci Rep 10: 3735, 2020. doi: 10.1038/s41598-020-60346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hua P, Liu L-B, Liu J-L, Wang M, Jiang H-Q, Zeng K, Yang YQ, Yang SR. Inhibition of apoptosis by knockdown of caspase-3 with siRNA in rat bone marrow mesenchymal stem cells. Exp Biol Med (Maywood) 238: 991–998, 2013. doi: 10.1177/1535370213497320. [DOI] [PubMed] [Google Scholar]
- 251.Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc 1: e002238, 2012. doi: 10.1161/JAHA.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sanchez-Sanchez R, Gómez-Ferrer M, Reinal I, Buigues M, Villanueva-Bádenas E, Ontoria-Oviedo I, Hernándiz A, González-King H, Peiró-Molina E, Dorronsoro A, Sepúlveda P. miR-4732-3p in extracellular vesicles from mesenchymal stromal cells is cardioprotective during myocardial ischemia. Front Cell Dev Biol 9: 734143, 2021. doi: 10.3389/fcell.2021.734143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shi Z, Wang S, Deng J, Gong Z. PGC-1α attenuates the oxidative stress-induced impaired osteogenesis and angiogenesis regulation effects of mesenchymal stem cells in the presence of diabetic serum. Biochem Biophys Rep 27: 101070, 2021. doi: 10.1016/j.bbrep.2021.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yan P, Li Q, Wang L, Lu P, Suzuki K, Liu Z, Lei J, Li W, He X, Wang S, Ding J, Chan P, Zhang W, Song M, Izpisua Belmonte JC, Qu J, Tang F, Liu GH. FOXO3-engineered human ESC-derived vascular cells promote vascular protection and regeneration. Cell Stem Cell 24: 447–461.e8, 2019. doi: 10.1016/j.stem.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 255.Zhu Q, Hao H, Xu H, Fichman Y, Cui Y, Yang C, Wang M, Mittler R, Hill MA, Cowan PJ, Zhang G, He X, Zhou S, Liu Z. Combination of antioxidant enzyme overexpression and n-acetylcysteine treatment enhances the survival of bone marrow mesenchymal stromal cells in ischemic limb in mice with type 2 diabetes. J Am Heart Assoc 10: e023491, 2021. doi: 10.1161/JAHA.121.023491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu X, Hu D, Zeng Z, Zhu W, Zhang N, Yu H, Chen H, Wang K, Wang Y, Wang L, Zhao J, Zhang L, Wu R, Hu X, Wang J. SRT1720 promotes survival of aged human mesenchymal stem cells via FAIM: a pharmacological strategy to improve stem cell-based therapy for rat myocardial infarction. Cell Death Dis 8: e2731, 2017. doi: 10.1038/cddis.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yuen DA, Zhang Y, Thai K, Spring C, Chan L, Guo X, Advani A, Sivak JM, Gilbert RE. Angiogenic dysfunction in bone marrow-derived early outgrowth cells from diabetic animals is attenuated by SIRT1 activation. Stem Cells Transl Med 1: 921–926, 2012. doi: 10.5966/sctm.2012-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kornicka K, Śmieszek A, Węgrzyn AS, Röcken M, Marycz K. Immunomodulatory properties of adipose-derived stem cells treated with 5-azacytydine and resveratrol on peripheral blood mononuclear cells and macrophages in metabolic syndrome animals. J Clin Med 7: 383, 2018. doi: 10.3390/jcm7110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kornicka K, Szłapka-Kosarzewska J, Śmieszek A, Marycz K. 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J Cell Mol Med 23: 237–259, 2019. doi: 10.1111/jcmm.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kornicka-Garbowska K, Pędziwiatr R, Woźniak P, Kucharczyk K, Marycz K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse—a case report. Stem Cell Res Ther 10: 394, 2019. doi: 10.1186/s13287-019-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Noronha N. D C, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther 10: 131, 2019. [Erratum in Stem Cell Res Ther 10: 132, 2019]. doi: 10.1186/s13287-019-1224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xu R, Chen W, Zhang Z, Qiu Y, Wang Y, Zhang B, Lu W. Integrated data analysis identifies potential inducers and pathways during the endothelial differentiation of bone-marrow stromal cells by DNA methyltransferase inhibitor, 5-aza-2'-deoxycytidine. Gene 657: 9–18, 2018. doi: 10.1016/j.gene.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 263.Khan I, Ali A, Akhter MA, Naeem N, Chotani MA, Mustafa T, Salim A. Preconditioning of mesenchymal stem cells with 2,4-dinitrophenol improves cardiac function in infarcted rats. Life Sci 162: 60–69, 2016. doi: 10.1016/j.lfs.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 264.Hu X, Yang J, Wang Y, Zhang Y, Ii M, Shen Z, Hui J. Mesenchymal stem cells preconditioned with trimetazidine promote neovascularization of hearts under hypoxia/reoxygenation injury. Int J Clin Exp Med 8: 16991–17005, 2015. [PMC free article] [PubMed] [Google Scholar]
- 265.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther 329: 543–550, 2009. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Oses C, Olivares B, Ezquer M, Acosta C, Bosch P, Donoso M, Léniz P, Ezquer F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: potential application in the treatment of diabetic neuropathy. PLoS One 12: e0178011, 2017. doi: 10.1371/journal.pone.0178011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Holden P, Nair LS. Deferoxamine: an angiogenic and antioxidant molecule for tissue regeneration. Tissue Eng Part B Rev 25: 461–470, 2019. doi: 10.1089/ten.TEB.2019.0111. [DOI] [PubMed] [Google Scholar]
- 268.Lian K, Wang Q, Zhao S, Yang M, Chen G, Chen Y, Li C, Gao H, Li C. Pretreatment of diabetic adipose-derived stem cells with mitoTEMPO reverses their defective proangiogenic function in diabetic mice with critical limb ischemia. Cell Transplant 28: 1652–1663, 2019. doi: 10.1177/0963689719885076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Touani FK, Borie M, Azzi F, Trudel D, Noiseux N, Der Sarkissian S, Lerouge S. Pharmacological preconditioning improves the viability and proangiogenic paracrine function of hydrogel-encapsulated mesenchymal stromal cells. Stem Cells Int 2021: 6663467, 2021. doi: 10.1155/2021/6663467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Palikuqi B, Nguyen D-HT, Li G, Schreiner R, Pellegata AF, Liu Y, Redmond D, Geng F, Lin Y, Gómez-Salinero JM, Yokoyama M, Zumbo P, Zhang T, Kunar B, Witherspoon M, Han T, Tedeschi AM, Scottoni F, Lipkin SM, Dow L, Elemento O, Xiang JZ, Shido K, Spence JR, Zhou QJ, Schwartz RE, De Coppi P, Rabbany SY, Rafii S. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 585: 426–432, 2020. doi: 10.1038/s41586-020-2712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Xing Z, Zhao C, Wu S, Zhang C, Liu H, Fan Y. Hydrogel-based therapeutic angiogenesis: an alternative treatment strategy for critical limb ischemia. Biomaterials 274: 120872, 2021. doi: 10.1016/j.biomaterials.2021.120872. [DOI] [PubMed] [Google Scholar]
- 272.Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L, Tian Z, Yan Y, Li Q, Zhong W, Xing M, Zhang L, Zhang L. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep 5: 18104, 2015. doi: 10.1038/srep18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, Longaker MT, Gurtner GC. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 33: 80–90, 2012. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Amani S, Shahrooz R, Hobbenaghi R, Mohammadi R, Baradar Khoshfetrat A, Karimi A, Bakhtiari Z, Adcock IM, Mortaz E. Angiogenic effects of cell therapy within a biomaterial scaffold in a rat hind limb ischemia model. Sci Rep 11: 20545, 2021. doi: 10.1038/s41598-021-99579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces 13: 18472–18487, 2021. doi: 10.1021/acsami.0c22671. [DOI] [PubMed] [Google Scholar]
- 276.Zhang B, Huang J, Liu J, Lin F, Ding Z, Xu J. Injectable composite hydrogel promotes osteogenesis and angiogenesis in spinal fusion by optimizing the bone marrow mesenchymal stem cell microenvironment and exosomes secretion. Mater Sci Eng C Mater Biol Appl 123: 111782, 2021. doi: 10.1016/j.msec.2020.111782. [DOI] [PubMed] [Google Scholar]
- 277.Wang K, Dong R, Tang J, Li H, Dang J, Zhang Z, Yu Z, Guo B, Yi C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via redulating macrophage polarization to accelerate angiogenesis. Chem Eng J 430: 132664, 2022. doi: 10.1016/j.cej.2021.132664. [DOI] [Google Scholar]
- 278.Jeong G-J, Song SY, Kang M, Go S, Sohn HS, Kim B-S. An injectable decellularized matrix that improves mesenchymal stem cell engraftment for therapeutic angiogenesis. ACS Biomater Sci Eng 4: 2571–2581, 2018. doi: 10.1021/acsbiomaterials.8b00617. [DOI] [PubMed] [Google Scholar]
- 279.Tan SH, Loo SJ, Gao Y, Tao ZH, Su LP, Wang CX, Zhang SL, Mu YH, Cui YH, Abdurrachim D, Wang WH, Lalic J, Lim KC, Bu J, Tan RS, Lee TH, Zhang J, Ye L. Thymosin β4 increases cardiac cell proliferation, cell engraftment, and the reparative potency of human induced-pluripotent stem cell-derived cardiomyocytes in a porcine model of acute myocardial infarction. Theranostics 11: 7879–7895, 2021. doi: 10.7150/thno.56757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ezquerra S, Zuleta A, Arancibia R, Estay J, Aulestia F, Carrion F. Functional properties of human-derived mesenchymal stem cell spheroids: a meta-analysis and systematic review. Stem Cells Int 2021: 8825332, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Costa MHG, Serra J, McDevitt TC, Cabral JMS, da Silva CL, Ferreira FC. Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3D spheroids. Biotechnol J 16: e2000389, 2021. doi: 10.1002/biot.202000389. [DOI] [PubMed] [Google Scholar]
- 282.Lee J-H, Parthiban P, Jin G-Z, Knowles JC, Kim H-W. Materials roles for promoting angiogenesis in tissue regeneration. Prog Mater Sci 117: 100732, 2021. doi: 10.1016/j.pmatsci.2020.100732. [DOI] [Google Scholar]
- 283.Spater T, Tobias AL, Menger MM, Nickels RM, Menger MD, Laschke MW. Biological coating with platelet-rich plasma and adipose tissue-derived microvascular fragments improves the vascularization, biocompatibility and tissue incorporation of porous polyethylene. Acta Biomater 108: 194–206, 2020. doi: 10.1016/j.actbio.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 284.Pilia M, McDaniel JS, Guda T, Chen XK, Rhoads RP, Allen RE, Corona BT, Rathbone CR. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur Cell Mater 28: 11–23, 2014. doi: 10.22203/eCM.v028a02. [DOI] [PubMed] [Google Scholar]
- 285.Ergün S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal 15: 981–995, 2011. doi: 10.1089/ars.2010.3507. [DOI] [PubMed] [Google Scholar]
- 286.Altalhi W, Hatkar R, Hoying JB, Aghazadeh Y, Nunes SS. Type I diabetes delays perfusion and engraftment of 3D constructs by impinging on angiogenesis; which can be rescued by hepatocyte growth factor supplementation. Cell Mol Bioeng 12: 443–454, 2019. [Erratum in Cell Mol Bioeng 12: 541–542, 2019]. doi: 10.1007/s12195-019-00574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Laschke MW, Spater T, Menger MD. Microvascular fragments: more than just natural vascularization units. Trends Biotechnol 39: 24–33, 2021. doi: 10.1016/j.tibtech.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 288.Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng 13: 2871–2879, 2007. doi: 10.1089/ten.2007.0025. [DOI] [PubMed] [Google Scholar]
- 289.Spater T, Menger MM, Nickels RM, Menger MD, Laschke MW. Macrophages promote network formation and maturation of transplanted adipose tissue-derived microvascular fragments. J Tissue Eng 11: 2041731420911816, 2020. doi: 10.1177/2041731420911816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One 6: e15846, 2011. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.McDaniel JS, Pilia M, Ward CL, Pollot BE, Rathbone CR. Characterization and multilineage potential of cells derived from isolated microvascular fragments. J Surg Res 192: 214–222, 2014. doi: 10.1016/j.jss.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 292.Nunes SS, Maijub JG, Krishnan L, Ramakrishnan VM, Clayton LR, Williams SK, Hoying JB, Boyd NL. Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures. Sci Rep 3: 2141, 2013. doi: 10.1038/srep02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Spater T, Frueh FS, Nickels RM, Menger MD, Laschke MW. Prevascularization of collagen-glycosaminoglycan scaffolds: stromal vascular fraction versus adipose tissue-derived microvascular fragments. J Biol Eng 12: 24, 2018. doi: 10.1186/s13036-018-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sun X, Wu J, Qiang B, Romagnuolo R, Gagliardi M, Keller G, Laflamme MA, Li R-K, Nunes SS. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci Transl Med 12: eaax2992, 2020. doi: 10.1126/scitranslmed.aax2992. [DOI] [PubMed] [Google Scholar]
- 295.Vasconcelos SN, Sun X, Wu J, Li RK. Ready-made microvessels robustly integrate into the infarcted coronary vasculature promoting graft perfusion, enhancing cardiac remuscularization and function. Circ Res 125: A418, 2019. doi: 10.1161/res.125.suppl_1.418. [DOI] [Google Scholar]
- 296.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A 14: 433–440, 2008. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 297.Nunes SS, Krishnan L, Gerard CS, Dale JR, Maddie MA, Benton RL, Hoying JB. Angiogenic potential of microvessel fragments is independent of the tissue of origin and can be influenced by the cellular composition of the implants. Microcirculation 17: 557–567, 2010. doi: 10.1111/j.1549-8719.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Laschke MW, Karschnia P, Scheuer C, Heß A, Metzger W, Menger MD. Effects of cryopreservation on adipose tissue-derived microvascular fragments. J Tissue Eng Regen Med 12: 1020–1030, 2018. doi: 10.1002/term.2591. [DOI] [PubMed] [Google Scholar]
- 299.Karschnia P, Scheuer C, Heß A, Später T, Menger MD, Laschke MW. Erythropoietin promotes network formation of transplanted adipose tissue-derived microvascular fragments. Eur Cell Mater 35: 268–280, 2018. doi: 10.22203/eCM.v035a19. [DOI] [PubMed] [Google Scholar]
- 300.Laschke MW, Kontaxi E, Scheuer C, Heß A, Karschnia P, Menger MD. Insulin-like growth factor 1 stimulates the angiogenic activity of adipose tissue-derived microvascular fragments. J Tissue Eng 10: 2041731419879837, 2019. doi: 10.1177/2041731419879837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Laschke MW, Seifert MS, Scheuer C, Kontaxi E, Metzger W, Menger MD. High glucose exposure promotes proliferation and in vivo network formation of adipose-tissue-derived microvascular fragments. Eur Cell Mater 38: 188–200, 2019. doi: 10.22203/eCM.v038a13. [DOI] [PubMed] [Google Scholar]
- 302.Laschke MW, Grässer C, Kleer S, Scheuer C, Eglin D, Alini M, Menger MD. Adipose tissue-derived microvascular fragments from aged donors exhibit an impaired vascularisation capacity. Eur Cell Mater 28: 287–298, 2014. doi: 10.22203/ecm.v028a20. [DOI] [PubMed] [Google Scholar]
- 303.Aly RM. Current state of stem cell-based therapies: an overview. Stem Cell Investig 7: 8, 2020. doi: 10.21037/sci-2020-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moyé L, Simari RD, Wolf A, Hare JM; Cardiovascular Cell Therapy Research Network. Concise review: Review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med 5: 186–191, 2016. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hamamoto H, Gorman JH, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg 87: 794–801, 2009. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J 29: 251–259, 2008. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 307.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J 30: 2722–2732, 2009. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ma J, Ge J, Zhang S, Sun A, Shen J, Chen L, Wang K, Zou Y. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol 100: 217–223, 2005. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]
- 309.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 287: H2670–H2676, 2004. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 310.Wysoczynski M, Khan A, Bolli R. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res 123: 138–158, 2018. doi: 10.1161/CIRCRESAHA.118.313251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hong KU, Li Q-H, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 108: 346, 2013. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 112: 18, 2017. doi: 10.1007/s00395-017-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ammar HI, Shamseldeen AM, Shoukry HS, Ashour H, Kamar SS, Rashed LA, Fadel M, Srivastava A, Dhingra S. Metformin impairs homing ability and efficacy of mesenchymal stem cells for cardiac repair in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol Heart Circ Physiol 320: H1290–H1302, 2021. doi: 10.1152/ajpheart.00317.2020. [DOI] [PubMed] [Google Scholar]
- 314.Halkos ME, Zhao Z-Q, Kerendi F, Wang N-P, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol 103: 525–536, 2008. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 315.Jun Hong S, Rogers PI, Kihlken J, Warfel J, Bull C, Deuter-Reinhard M, Feng D, Xie J, Kyle A, Merfeld-Clauss S, Johnstone BH, Traktuev DO, Chen PS, Lindner JR, March KL. Intravenous xenogeneic transplantation of human adipose-derived stem cells improves left ventricular function and microvascular integrity in swine myocardial infarction model. Catheter Cardiovasc Interv 86: E38–E48, 2015. doi: 10.1002/ccd.25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol 122: 17–28, 2007. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 317.Wang W, Jiang Q, Zhang H, Jin P, Yuan X, Wei Y, Hu S. Intravenous administration of bone marrow mesenchymal stromal cells is safe for the lung in a chronic myocardial infarction model. Regen Med 6: 179–190, 2011. doi: 10.2217/rme.10.104. [DOI] [PubMed] [Google Scholar]
- 318.Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, Waksman R, Epstein SE. Intravenously delivered mesenchymal stem cells: systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ Res 120: 1598–1613, 2017. doi: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 319.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 38: 187–197, 2017. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 321.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang X-H, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol 106: 1299–1310, 2011. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 322.Guo J, Lin G-S, Bao C-y, Hu Z-M, Hu M-Y. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation 30: 97–104, 2007. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 323.Liao YY, Chen ZY, Wang YX, Lin Y, Yang F, Zhou QL. New progress in angiogenesis therapy of cardiovascular disease by ultrasound targeted microbubble destruction. Biomed Res Int 2014: 872984, 2014. doi: 10.1155/2014/872984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Imada T, Tatsumi T, Mori Y, Nishiue T, Yoshida M, Masaki H, Okigaki M, Kojima H, Nozawa Y, Nishiwaki Y, Nitta N, Iwasaka T, Matsubara H. Targeted delivery of bone marrow mononuclear cells by ultrasound destruction of microbubbles induces both angiogenesis and arteriogenesis response. Arterioscler Thromb Vasc Biol 25: 2128–2134, 2005. doi: 10.1161/01.ATV.0000179768.06206.cb. [DOI] [PubMed] [Google Scholar]
- 325.Song X, Zhu H, Jin L, Wang J, Yang Q, Jin P, Li X. Ultrasound-mediated microbubble destruction enhances the efficacy of bone marrow mesenchymal stem cell transplantation and cardiac function. Clin Exp Pharmacol Physiol 36: 267–271, 2009. doi: 10.1111/j.1440-1681.2008.05049.x. [DOI] [PubMed] [Google Scholar]
- 326.Garcia-Belda P, Prima-García H, Aliena-Valero A, Castelló-Ruiz M, Ulloa-Navas MJ, Ten-Esteve A, Martí-Bonmatí L, Salom JB, García-Verdugo JM, Gil-Perotín S. Intravenous SPION-labeled adipocyte-derived stem cells targeted to the brain by magnetic attraction in a rat stroke model: an ultrastructural insight into cell fate within the brain. Nanomedicine 39: 102464, 2021. doi: 10.1016/j.nano.2021.102464. [DOI] [PubMed] [Google Scholar]
- 327.Soto PA, Vence M, Piñero GM, Coral DF, Usach V, Muraca D, Cueto A, Roig A, van Raap MBF, Setton-Avruj CP. Sciatic nerve regeneration after traumatic injury using magnetic targeted adipose-derived mesenchymal stem cells. Acta Biomater 130: 234–247, 2021. doi: 10.1016/j.actbio.2021.05.050. [DOI] [PubMed] [Google Scholar]
- 328.Copcu HE. Three states of stromal cells-solid, liquid, and aerosol-and innovative delivery methods not previously reported. Arch Plast Surg 48: 549–552, 2021. doi: 10.5999/aps.2021.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Baris R, Kankaya Y, Ozer K, Kocer RG, Bektas CI, Karatas A, Kocer U, Koca G, Astarci HM. The effect of microneedling with a roller device on the viability of random skin flaps in rats. Plast Reconstr Surg 131: 1024–1034, 2013. doi: 10.1097/PRS.0b013e3182879edf. [DOI] [PubMed] [Google Scholar]
- 330.Lee HJ, Lee EG, Kang S, Sung J-H, Chung H-M, Kim DH. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: a randomized, controlled, blinded split-face study. Ann Dermatol 26: 584–591, 2014. doi: 10.5021/ad.2014.26.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Aguilera Y, Mellado-Damas N, Olmedo-Moreno L, López V, Panadero-Morón C, Benito M, Guerrero-Cázares H, Márquez-Vega C, Martín-Montalvo A, Capilla-González V. Preclinical safety evaluation of intranasally delivered human mesenchymal stem cells in juvenile mice. Cancers (Basel) 13: 1169, 2021. doi: 10.3390/cancers13051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Simorgh S, Alizadeh R, Shabani R, Karimzadeh F, Seidkhani E, Majidpoor J, Moradi F, Kasbiyan H. Olfactory mucosa stem cells delivery via nasal route: a simple way for the treatment of Parkinson disease. Neurotox Res 39: 598–608, 2021. doi: 10.1007/s12640-020-00290-1. [DOI] [PubMed] [Google Scholar]
- 333.Alizadeh R, Boroujeni ME, Kamrava SK, Tehrani AM, Bagher Z, Heidari F, Bluyssen HAR, Farhadi M. From transcriptome to behavior: intranasal injection of late passage human olfactory stem cells displays potential in a rat model of Parkinson's disease. ACS Chem Neurosci 12: 2209–2217, 2021. doi: 10.1021/acschemneuro.1c00225. [DOI] [PubMed] [Google Scholar]
- 334.Lu MH, Ji WL, Chen H, Sun YY, Zhao XY, Wang F, Shi Y, Hu YN, Liu BX, Wu JW, Xu DE, Zheng JW, Liu CF, Ma QH. Intranasal transplantation of human neural stem cells ameliorates Alzheimer's disease-like pathology in a mouse model. Front Aging Neurosci 13: 650103, 2021. doi: 10.3389/fnagi.2021.650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sullivan S, Stacey GN, Akazawa C, Aoyama N, Baptista R, Bedford P, Bennaceur Griscelli A, Chandra A, Elwood N, Girard M, Kawamata S, Hanatani T, Latsis T, Lin S, Ludwig TE, Malygina T, Mack A, Mountford JC, Noggle S, Pereira LV, Price J, Sheldon M, Srivastava A, Stachelscheid H, Velayudhan SR, Ward NJ, Turner ML, Barry J, Song J. Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med 13: 859–866, 2018. doi: 10.2217/rme-2018-0095. [DOI] [PubMed] [Google Scholar]
- 336.Cai Y, Liu W, Lian L, Xu Y, Bai X, Xu S, Zhang J. Stroke treatment: is exosome therapy superior to stem cell therapy? Biochimie 179: 190–204, 2020.doi: 10.1016/j.biochi.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 337.You D, Jang MJ, Kim BH, Song G, Lee C, Suh N, Jeong IG, Ahn TY, Kim CS. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med 4: 351–358, 2015. doi: 10.5966/sctm.2014-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Roman S, Mangir N, Hearnden V. Angiogenic potential of adipose derived stem cells compared to the stromal vascular fraction. Eur Cells Mater 32: 40, 2016. doi: 10.1007/s11626-017-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zhang HZ, Chae DS, Kim SW. ASC and SVF cells synergistically induce neovascularization in ischemic hindlimb following cotransplantation. Int J Mol Sci 23: 185, 2021. doi: 10.3390/ijms23010185. [DOI] [PMC free article] [PubMed] [Google Scholar]