Summary
Under natural environmental settings or in the human body, the majority of microorganisms exist in complex polymicrobial biofilms adhered to abiotic and biotic surfaces. These microorganisms exhibit symbiotic, mutualistic, synergistic, or antagonistic relationships with other species during biofilm colonization and development. These polymicrobial interactions are heterogeneous, complex, and hard to control, thereby often yielding worse outcomes than monospecies infections.
Concerning fungi, Candida spp., in particular, Candida albicans is often detected with various bacterial species in oral biofilms. These Candida-bacterial interactions may induce the transition of C. albicans from commensal to pathobiont or dysbiotic organism. Consequently, Candida–bacterial interactions are largely associated with various oral diseases, including denture stomatitis, dental caries, periodontitis, peri-implantitis, endodontic infections, and oral cancer. Given the severity of oral diseases caused by cross-kingdom consortia that develop hard-to-remove and highly drug-resistant biofilms, fundamental research is warranted to strategically develop cost-effective and safe therapies to prevent and treat cross-kingdom interactions and subsequent biofilm development. While studies have shed some light, targeting fungal-involved polymicrobial biofilms has been limited. This mini-review outlines the key features of Candida-bacterial interactions and their impact on various oral diseases. In addition, current knowledge on therapeutic strategies to target Candida-bacterial polymicrobial biofilms is discussed.
Keywords: Candida albicans, bacteria, cross-kingdom biofilm, oral diseases, therapeutics
Introduction
In a wide variety of environments from natural settings to the human body, the majority of microorganisms exist in complex polymicrobial biofilms adhered to abiotic and biotic surfaces. During biofilm colonization and development, microbes exhibit symbiotic, mutualistic, synergistic, or antagonistic relationships with other species. Those polymicrobial biofilms are often detrimental, causing food spoilage, industrial pipe fouling and corrosion, as well as human infectious diseases. Specifically, polymicrobial biofilms can cause various infections in a wide range of the human body, from the oral cavity (Kolenbrander 2000, Schaudinn et al. 2009) to lung (Stressmann et al. 2012, Zhao et al. 2012, Filkins et al. 2015) to urinary tract (Ronald 2002, Kline et al. 2016) to chronic wounds (Gjødsbøl et al. 2006, Dowd et al. 2008). These polymicrobial biofilms tend to be challenging to treat and often yield worse outcomes than monospecies infections by altering the sensitivity to antimicrobial agents (Orazi et al. 2019).
The gastrointestinal tract and the oral cavity are the representative human body parts that harbor a complex and diverse multitude of microorganisms, where they serve an essential role in local and systemic health. In particular, the oral cavity is a unique ecosystem that contains 600 to 1,000 bacterial species as well as more than 100 fungal species, colonizing soft and hard tissues either permanently or transiently (Aas et al. 2005, Manson et al. 2008, Peters et al. 2012a, Brown et al. 2019). In health, commensal microbiota inhibits pathogen colonization while supplying the host with essential nutrients, maintaining a stable micro-ecosystem (Martín et al. 2013, Negrini et al. 2021). However, disruptions of such commensal microbial communities from steady-state composition may result in the imbalance of host-microbiome interaction and illness (Negrini et al. 2021). Although clinical evidence indicates that the coexistence of bacteria and fungus in the oral cavity may accelerate susceptibility to host infection, previous oral biofilm studies have largely focused on the development of bacterial biofilms (mostly monospecies), and the aspect of cross-kingdom interactions have been underexplored. However, recent mechanistic studies exhibit the role and importance of bacterial-fungal interactions during biofilm formation and development as well as their implication in oral health and disease states.
Concerning fungi, Candida spp. are the most commonly detected fungal species in the oral cavity (Ghannoum et al. 2010, Dupuy et al. 2014, Witherden et al. 2017, Delaney et al. 2019). Particularly, C. albicans is often found with various bacterial species in oral polymicrobial biofilms which may induce the transition of C. albicans from commensal to pathobiont or dysbiotic organism (O’Donnell et al. 2015, Janus et al. 2016, Delaney et al. 2018, Xiao et al. 2018, de Cássia Negrini et al. 2019). In this cross-kingdom interaction, the cell wall of C. albicans, a critical structure for maintaining the cell shape and immunogenicity (Hall et al. 2013), plays an important role as the major point of contact between the fungus and bacteria (Buurman et al. 1998, Hoyer 2001). For example, hypha-specific adhesins, ALS (agglutinin-like sequence) group of cell wall glycoproteins (e.g., Als1 and Als3), are shown to mediate the cross-kingdom interaction of C. albicans with various bacteria commonly found in the oral cavity, such as Streptoccus gordonii (Silverman et al. 2010, Bamford et al. 2015), Streptococcus oralis (Xu et al. 2017), Porphyromonas gingivalis (Sztukowska et al. 2018), Staphylococcus aureus (Peters et al. 2012b), and Staphylococcus epidermidis (Beaussart et al. 2013). These Candida–bacterial interactions have been found to be associated with various oral diseases including dental caries, denture stomatitis, periodontitis, peri-implantitis, and oral cancer. Unfortunately, drug susceptibility studies revealed that it is challenging to eradicate those Candida-bacterial polymicrobial biofilm-induced diseases due to alterations of the efficacy of antibiotics by either fungal cells or bacteria (Jenkinson et al. 2002) and lack of targeting polymicrobial interactions (Kim et al. 2021).
This mini-review aims to present the characteristics of Candida-bacterial interactions and their impact on polymicrobial biofilm formations in the context of various oral diseases. In addition, diverse therapeutic strategies to target Candida-bacterial polymicrobial biofilms are introduced.
Candida-bacterial biofilm-associated oral diseases
Bacterial colonization and biofilm formation on tooth surfaces or oral soft tissues are modulated by the type of species that initially bind and subsequent colonizers interacting with those. Candida-bacterial cross-kingdom interactions may also participate in those processes and affect biofilm development, thus contributing to the severity of biofilm-associated oral diseases. There are numerous pieces of evidence showing that the association of C. albicans and various bacteria is implicated in diverse aspects of oral diseases (Figure 1), which are summarized in the following subsections.
Figure 1.
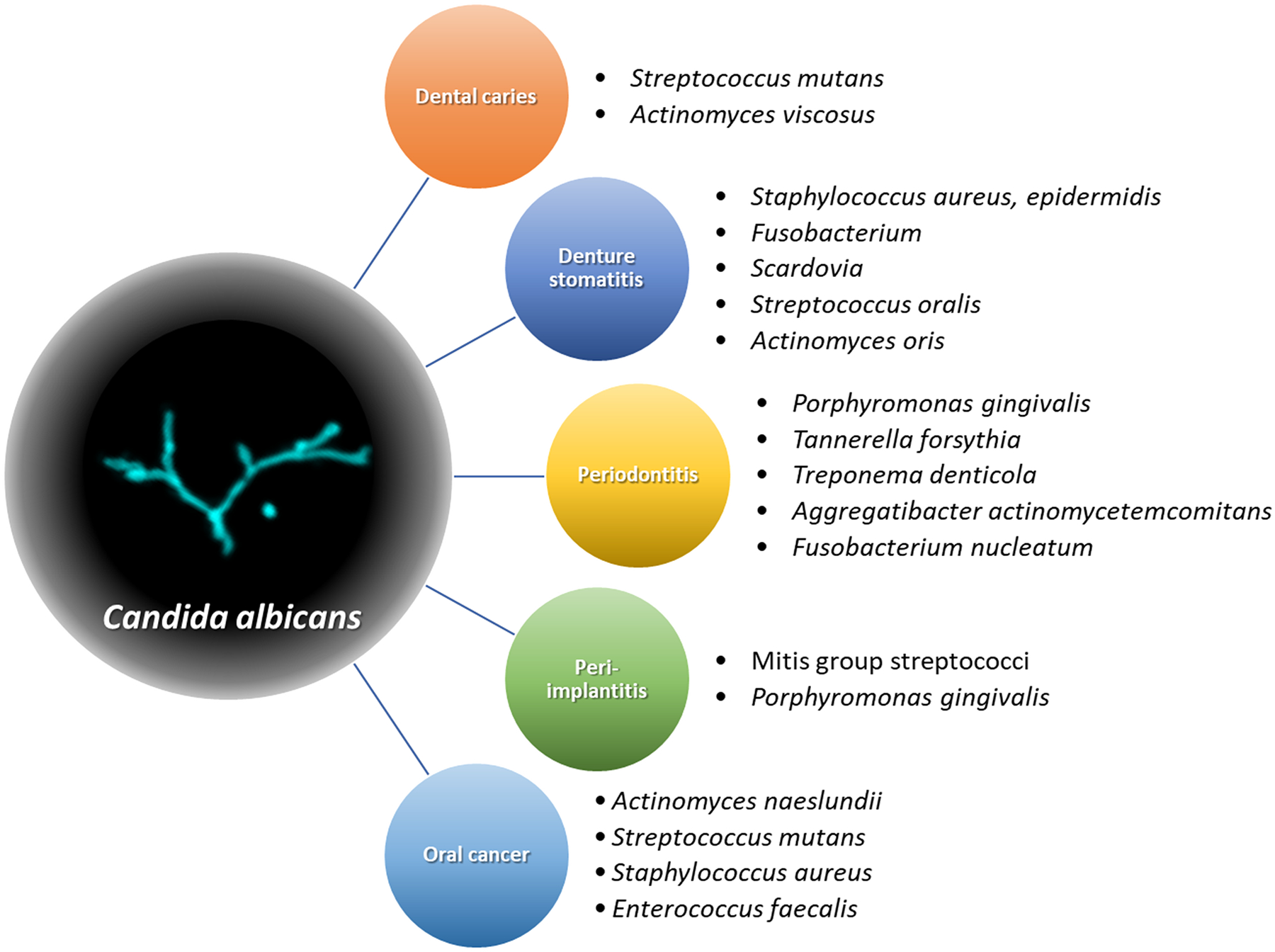
Association of Candida albicans and various bacteria in oral diseases. A variety of gram-positive and -negative oral bacteria interact with C. albicans, contributing to virulences of diverse oral diseases ranging from dental caries to oral cancer.
Dental caries
Dental caries, also known as tooth decay, is a representative biofilm- and diet-dependent oral disease (Sheiham et al. 2015, Bowen et al. 2018). Among various fermentable sugars, sucrose is considered the most cariogenic (Leme et al. 2006) due to its contribution to biofilm formation and development by serving as a substrate for the production of extracellular polysaccharides (EPS) (Bowen et al. 2018). While bacteria have been traditionally considered as a major component of the etiology of dental caries (Thomas et al. 2012, Wolff et al. 2013, Simón-Soro et al. 2015), many recent studies revealed that C. albicans are often detected from plaque biofilms, particularly in children with severe early childhood caries (ECC) (Hajishengallis et al. 2017, Jean et al. 2018, Xiao et al. 2018, Garcia et al. 2021). Specifically, synergistic interaction between C. albicans and cariogenic bacterium Streptococcus mutans is heavily studied in vitro and in vivo in the context of dental caries (Falsetta et al. 2014, Ellepola et al. 2017, He et al. 2017, Hwang et al. 2017, Kim et al. 2017). The consensus is that EPS produced by S. mutans plays an important role in mediating C. albicans-S. mutans cross-kingdom interaction, generating a virtuous cycle whereby it enhances C. albicans growth and metabolic activity, in turn, accelerating S. mutans growth and EPS production as well. This enhanced EPS production also facilitated the surface coating of C. albicans with EPS, established the alliance between C. albicans and S. mutans at an early stage of biofilm development, thereby outperforming S. gordonii in a 3-species mixed biofilm model (Figure 2) (Wan et al. 2021). Interestingly, a more recent study showed that only C. albicans-S. mutans cross-kingdom biofilm matured and created an acidic microenvironment when cultured in human saliva, while S. mutans alone were not successful (Kim et al. 2020). In addition, a variety of other factors that involved in C. albicans-S. mutans interaction has been discussed. For example, one study revealed that the removal of extracellular DNA disrupted the initial stage of cross-kingdom biofilm formation (Guo et al. 2021). Other studies suggested that S. mutans antigen I/II (Yang et al. 2018), S. mutans collagen-binding proteins (Garcia et al. 2021), deletion of the S. mutans delta subunit of RNA polymerase (RpoE) (Xue et al. 2011), C. albicans-derived polysaccharide biofilm matrix (Khoury et al. 2020), or the presence of alkaloid nicotine (Liu et al. 2017) can boost the C. albicans-S. mutans cross-kingdom biofilm formation. Furthermore, the new critical role of C. albicans in inducing oral microbial dysbiosis that exacerbates the pathogenesis of root caries has been reported (Du et al. 2021) and the new cross-feeding mechanism between S. mutans and C. albicans has been suggested with the aid of multi-omics analyses (Ellepola et al. 2019). Other than C. albicans-S. mutans biofilms, cross-kingdom interactions of C. albicans with Actinomyces viscosus also significantly increased the cariogenic virulence of biofilm (Deng et al. 2019a). Although some antagonistic interactions between C. albicans and S. mutans regarding inhibition of C. albicans hyphal formation have been reported (Jarosz et al. 2009, Vílchez et al. 2010), it appears that most cross-kingdom interactions facilitate biofilm accumulation while increasing the acidogenicity of biofilm, amplifying the virulence of biofilms.
Figure 2.
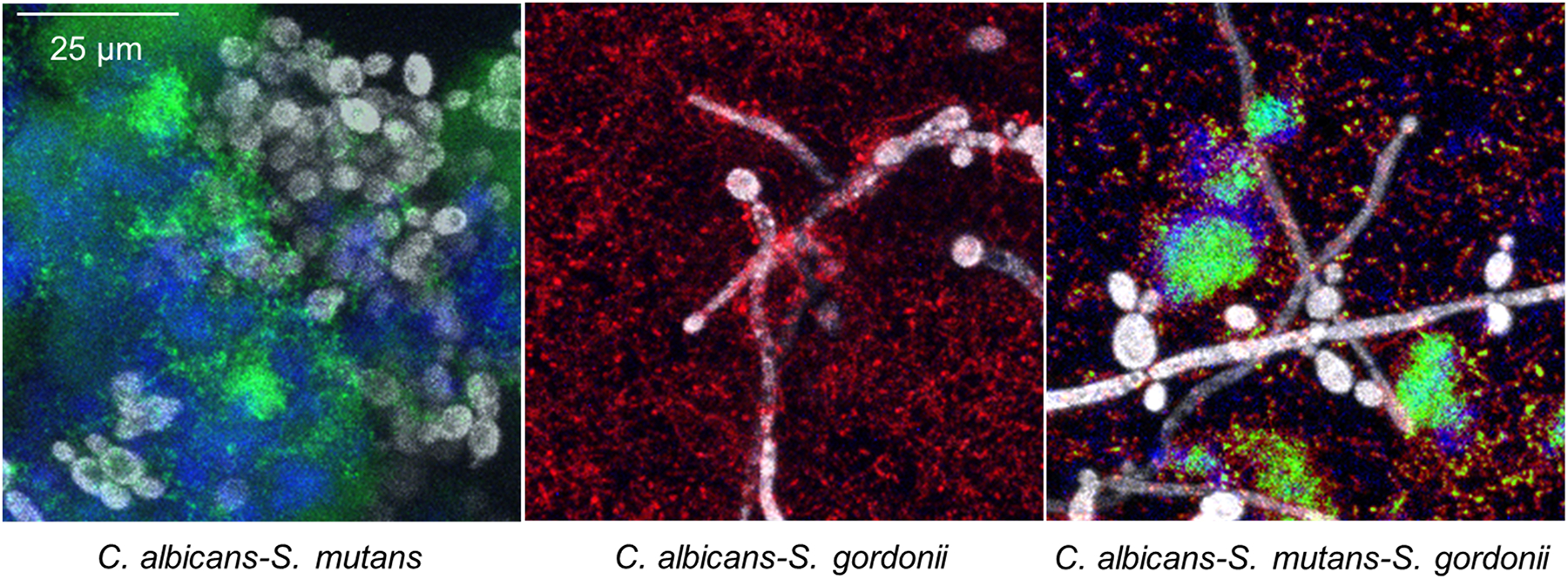
Representative confocal images of C. albicans-S. mutans, C. albicans-S. gordonii, and C. albicans-S. mutans–S. gordonii biofilms cultured in media supplemented with 1% sucrose. Gray, green, red, and blue colors indicate C. albicans, S. mutans, S. gordonii, and extracellular polysaccharides (EPS)-matrix, respectively. Adapted with permission from Wan et al. (2021) Cross-Kingdom Cell-to-Cell Interactions in Cariogenic Biofilm Initiation. Journal of Dental Research, Vol. 100(1) 74–81. Copyright © International & American Associations for Dental Research 2020
Denture stomatitis
Denture stomatitis is an inflamed condition of the oral mucosa that is directly in contact with dentures. Although C. albicans has been extensively studied as the sole main etiological factor of denture stomatitis, recent studies revealed that cross-kingdom interactions between C. albicans and bacteria often prosper in denture biofilms. For example, several studies reported frequent isolation of C. albicans with S. aureus or S. epidermidis from the oral mucous of patients wearing dental prostheses (Tawara et al. 1996, Baena-Monroy et al. 2005, Peters et al. 2010, Pereira et al. 2013). In another study, Fusobacterium nucleatum as well as F. nucleatum subsp. animalis and vincentii were exclusively detected in high numbers with C. albicans from denture stomatitis patients (Shi et al. 2016). A recent profiling study demonstrated that a gram-positive anaerobe Scardovia showed a positive correlation with C. albicans from plaque formed inside of a denture, while three anaerobes (Leptotrichia, Lachnoanaerobaculum, and Moryella) showed a negative correlation (Fujinami et al. 2021). In addition, cooperative physical and metabolic processes among C. albicans, S. oralis, and Actinomyces oris were found to contribute to early biofilm formation on denture material from in vitro model (Cavalcanti et al. 2016a). Similar to the findings from the dental caries study (Liu et al. 2017), the effect of nicotine is also appeared to increase the coaggregation of C. albicans and S. mutans in denture biofilm (Ashkanane et al. 2019).
Periodontitis
Periodontitis is caused by an imbalance between the microbiota and immune defense that results in the loss of soft-tissue seal around teeth, formation of periodontal pockets, and subsequent bone destruction (Buduneli et al. 2011, Lamont et al. 2018, Jabri et al. 2021). While various microorganisms have been known to be associated with the initiation and progression of the periodontitis, red complex, P. gingivalis, Tannerella forsythia, and Treponema denticola, as well as Aggregatibacter actinomycetemcomitans have been considered the most pathogenic bacteria involved in periodontitis (Teles et al. 2013). Lately, the role of yeast and its cross-kingdom interaction with various bacteria in periodontitis pathogenesis were discussed. Investigation of the associations of Candida and periodontopathic bacteria from seniors (≥ 60 years old) demonstrated that the surface area of inflamed periodontal tissue was significantly greater when Tannerella forsythia and Treponema denticola were detected together with C. albicans from patients (Shigeishi et al. 2021). Also, several studies revealed various interaction mechanisms between C. albicans and P. gingivalis. For example, fungal cell adhesins Als3 and Mp65, aspartic proteases Sap6 and Sap9, and protein enolase appeared to mediate the direct physical contact with P. gingivalis (Bartnicka et al. 2019). Particularly, C. albicans Als3 directly interacted with P. gingivalis InlJ, acting as an adhesin-receptor system for C. albicans-P. gingivalis association (Sztukowska et al. 2018). Similarly, C. albicans surface mannoprotein Flo9 and F. nucleatum outer membrane protein RadD were involved in interspecies co-adherence (Wu et al. 2015). In other studies, it showed that virulence factors of P. gingivalis such as cysteine proteases and peptidylarginine deiminase enzymes played a crucial role in C. albicans-P. gingivalis association (Karkowska-Kuleta et al. 2018, Karkowska-Kuleta et al. 2020). As an environmental factor affecting C. albicans-P. gingivalis association, heme, an important iron source for both species, was shown to enhance the pathogenic potential of P. gingivalis while interacting with C. albicans (Guo et al. 2020). Such C. albicans-P. gingivalis association facilitated the invasion and infection of gingival tissue cells (Tamai et al. 2011, Bartnicka et al. 2020), and hampered wound closure (Haverman et al. 2017).
Peri-implantitis
Osseointegrated dental implants have become a clinical standard for replacing missing teeth (Nickenig et al. 2008, Johannsen et al. 2012, Park et al. 2020). The inflammatory response of the gingival tissue around implants represents a growing challenge as many studies demonstrated a high incidence of peri-implant diseases after implantation (Atieh et al. 2013, Gomes et al. 2015, Papathanasiou et al. 2016, Gurgel et al. 2017, Lee et al. 2017). Such peri-implant diseases could lead to destructive failures, resulting in discomfort, painful and costly surgical replacement of failed implants, and the potential breakdown of overall oral health (Charalampakis et al. 2012, Sakka et al. 2012, Rosen et al. 2013). The microbiota linked to dental implant failure has been shown to be associated with a higher prevalence of periodontal pathogens, such as P. gingivalis, Prevotella intermedia, Fusobacterium spp, as well as gram-negative cocci, together with Candida spp (Alcoforado et al. 1991, Leonhardt et al. 1999, Hultin et al. 2002, Canullo et al. 2015). While the mechanistic investigation of cross-kingdom interaction between Candida and bacteria on peri-implantitis has been limited, there are some studies describing their implications. For example, one study demonstrated the mutualistic relationship between C. albicans and mitis group streptococci (i.e., Streptococcus mitis, Streptococcus sanguinis, S. oralis, and S. gordonii) promoted biofilm formation on titanium surfaces, resulting in increased tissue damage (Souza et al. 2020). Another study demonstrated similar findings that mutualistic C. albicans-S. gordonii cross-kingdom interactions enhanced biofilm formation and fostered a high level of resistance to combination therapy with antifungal and antibacterial drugs (Montelongo-Jauregui et al. 2018). In addition, there was a study aimed at evaluating the interaction between C. albicans and Streptococcus salivarius biofilms developed on titanium surfaces, under reduced oxygen levels (Martorano-Fernandes et al. 2020). Unlike their antagonistic relationship observed in oral candidiasis models (Ishijima et al. 2012, James et al. 2016), the presence of S. salivarius did not affect fungus growth or C. albicans virulence in the context of peri-implant disease (Martorano-Fernandes et al. 2020). Interestingly, virulence factors of C. albicans expressed in biofilms formed on titanium (i.e., expression of genes associated with adhesins and hydrolytic enzymes) significantly varied depending on associated bacterial species (e.g., S. sanguinis, S. mutans, and P. gingivalis) (Cavalcanti et al. 2016b).
Oral cancer
Oral cancer is one of the most prevalent cancers which mainly occurs in the squamous cells (Pushalkar et al. 2011, Arzmi et al. 2019). While various risk factors for oral cancer are known, including tobacco use, heavy alcohol consumption, and human papillomavirus infection, microbial infections also can contribute to its pathogenesis (Arzmi et al. 2019). In particular, C. albicans is considered one of the major microorganisms contributing to oral cancer development, potentially promoting carcinogenesis via several mechanisms (Kaźmierczak-Siedlecka et al. 2020). For instance, cross-kingdom interactions of C. albicans with oral bacteria A. naeslundii and S. mutans enhanced invasion of oral squamous cell carcinoma and increased the expression of cancerous inflammatory cytokines, which promoted oral carcinogenesis (Arzmi et al. 2018). Also, metabolites from C. albicans-S. aureus cross-kingdom biofilm promoted changes in proto-oncogenes and cell cycle gene expression in normal and neoplastic oral epithelial cell lines (Amaya Arbeláez et al. 2021). It is worth noting that those cross-kingdom interactions are not only involved in the pathogenesis of oral cancer but also cause catastrophic complication. During cytotoxic chemotherapy, the dysbiotic state is often promoted, elevating the risk of oral candidiasis, which results in infectious complications that are a common cause of morbidity and mortality in cancer patients (Bertolini et al. 2019). This was proposed whereby the mutualistic relationships between C. albicans and Enterococcus faecalis facilitate their overgrowth, which augments mucosal barrier breach by releasing proteolytic enzymes and enhancing virulence gene expression by C. albicans (Bertolini et al. 2019).
Combined, cross-kingdom interactions between C. albicans and oral bacteria are widely associated with the virulence of various oral diseases. Thus, their mechanism of action should be further understood to successfully manage Candida-involved complex biofilm-associated oral diseases. Further investigations using clinically relevant ecological biofilm models combined with powerful analytical tools may progress our knowledge to the next level.
Therapeutic approaches for Candida-bacterial biofilm-associated oral diseases
Given the aggressive damage caused by cross-kingdom consortia that develop hard-to-remove and highly drug-resistant biofilms, there is a great need to strategically develop cost-effective and safe therapies to prevent cross-kingdom interactions and subsequent biofilm development. While there have been endeavors to develop therapeutic strategies to treat pathogenic bacterial biofilms, targeting fungal-involved polymicrobial biofilms has been limited. Since fungal-bacterial biofilms exhibit dynamic inter-kingdom interactions and diverse drug resistance patterns (Orazi et al. 2019, Khan et al. 2021), efficacies of antibiofilm agents are often limited. Here, the use of naturally derived bioactive molecules, chemically synthesized compounds, nano-formulated drugs, alternative biofilm treatment strategies as well as antibiofilm surfaces aimed at targeting Candida-bacterial biofilms are summarized (also illustrated in Figure 3).
Figure 3.
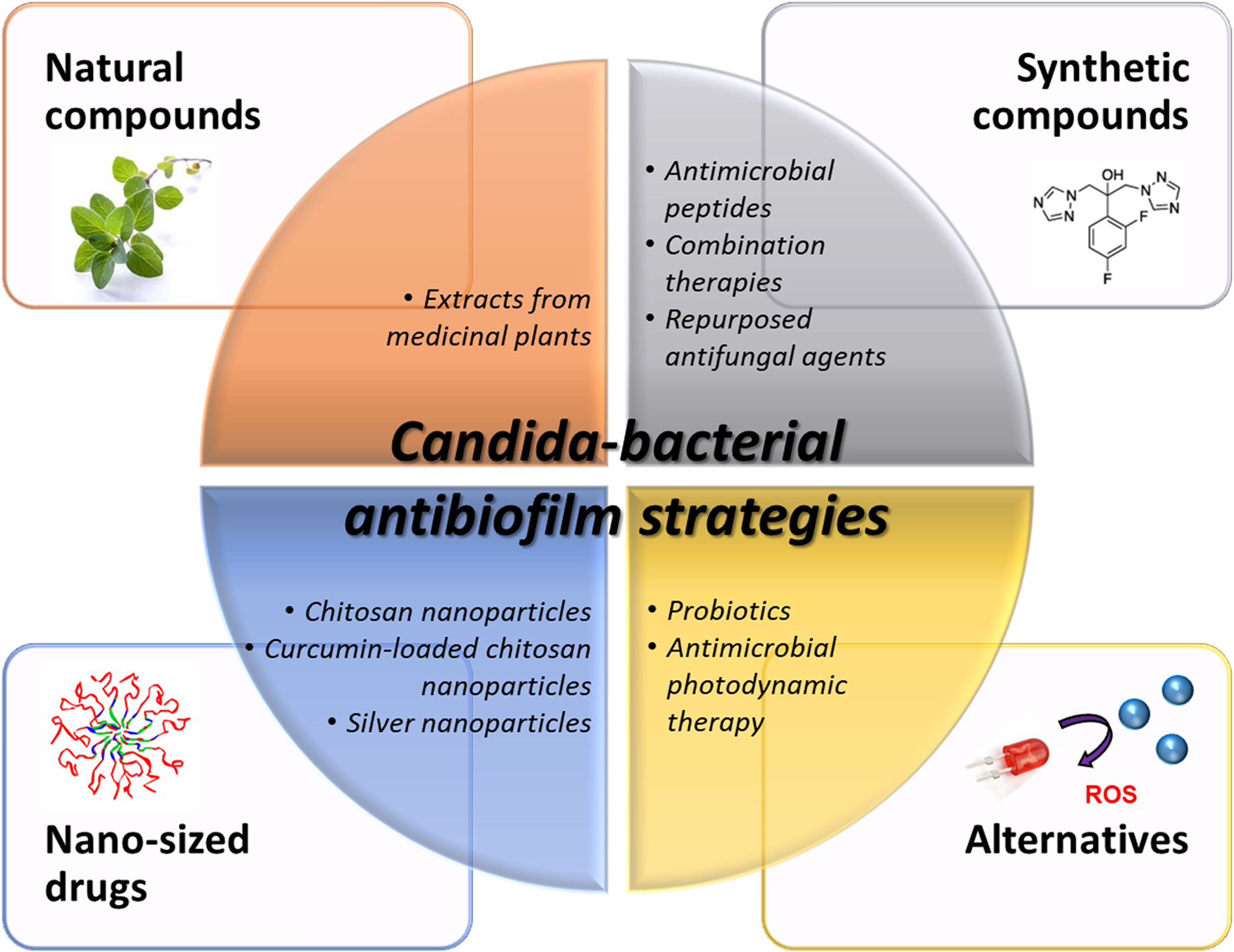
Various antibiofilm strategies to eradicate Candida-bacterial cross-kingdom biofilm. It includes but is not limited to naturally derived bioactive molecules, chemically synthesized compounds, nano-formulated drugs, and alternative biofilm treatment strategies. ROS denotes reactive oxygen species.
Natural antibiofilm products
A variety of natural antibiofilm agents derived from medicinal plants have been introduced due to their unique characteristics such as low toxicity, high biocompatibility, and low manufacturing cost. Among them, a portion of bioactive molecules exhibits antibiofilm activities that can target different stages of cross-kingdom biofilm development. For example, cajuputs candy was able to inhibit C. albicans hyphal transformation and suppress insoluble glucan formation by S. mutans (Septiana et al. 2019). Similarly, the use of curcumin concomitantly downregulated glucosyltransferase and quorum sensing-related gene expression of S. mutans as well as the ALS family of C. albicans (Li et al. 2019). Other natural compounds extracted from Cranberry (Philip et al. 2019), green tea (Farkash et al. 2019), Rhamnus prinoides (Campbell et al. 2020), Camellia japonica and Thuja orientalis (Choi et al. 2017), olive oil (Arias et al. 2016) as well as Casearia sylvestris (Ribeiro et al. 2019) have been also reported to exert antibiofilm activity against C. albicans-S. mutans cross-kingdom biofilms. In regards to other Candida-bacterial biofilms, gymnemic acids, isolated from Gymnema sylvestre, prevented the development of C. albicans-S. gordonii biofilm by inhibiting S. gordonii binding to C. albicans hyphae (Veerapandian et al. 2019).
Synthetic antibiofilm products
To improve the efficacy and equip diverse functions, extensive efforts have been made to develop chemically synthesized antibiofilm agents. Synthetic antimicrobial peptide (AMP) is one of the widely applied chemically synthesized antibiofilm agents, mainly used to target monospecies biofilm. Recently, however, cyclic dipeptides have been shown to inhibit S. mutans and C. albicans adhesion to a hydroxyapatite disc, thereby preventing their cross-kingdom biofilm formation (Simon et al. 2019). In addition, cholic acid-peptide conjugates (Gupta et al. 2019), ceragenins (Hacioglu et al. 2019), and guanylated polymethacrylates (Qu et al. 2016), which are synthetic compounds mimicking AMP, have shown to be effective in disrupting C. albicans-S. aureus biofilms. Ceragenins were also tested against C. albicans cross-kingdom biofilms with Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, or Klebsiella pneumoniae, exhibiting its superior efficacy to naturally occurring AMPs (Hacioglu et al. 2020). Other synthetic compounds include nitrochalcone (Bombarda et al. 2019) and peroxynitric acid solution (Iwaki et al. 2020) that were effective against C. albicans-S. mutans biofilm, which outperformed broad-spectrum antimicrobial agent chlorohexidine with less cytotoxicity. There also have been attempts to combine synthetic compounds and a classic anticaries agent such as fluoride. Sodium trimetaphosphate accompanied with fluoride elevated pH of C. albicans-S. mutans biofilm close to the neutral values, which may reduce the tooth demineralization (Cavazana et al. 2020). The combinations of tt-farnesol and 4’-hydroxychalcone were effective against preformed C. albicans-S. mutans biofilm when combined with fluoride (Lobo et al. 2021). Other various combination therapies involving multiple synthetic and natural compounds have also demonstrated enhanced antibiofilm efficacy against cross-kingdom biofilms. Povidone-iodine and fluconazole combination treatment inhibited the production of α-glucan by S. mutans and enhanced fluconazole efficacy against C. albicans (Kim et al. 2018). Eugenol with fluconazole or azithromycin also exhibited enhanced antibiofilm activity against C. albicans-S. mutans biofilm (Jafri et al. 2020). In addition, 2-aminobenzimidazole with curcumin (Tan et al. 2019), berberine and amphotericin B (Gao et al. 2021), anidulafungin and tigecycline (Rogiers et al. 2018), and fluconazole and minocycline (Li et al. 2015) were effective in deterring C. albicans-S. aureus biofilm. Interestingly, some antifungal agents are repurposed to treat C. albicans involved cross-kingdom biofilms. For example, voriconazole, a second-generation broad-spectrum triazole antifungal drug, was found to regulate the ergosterol pathway that is critical for the interaction of C. albicans and A. viscosus (Deng et al. 2019b).
Nanotechnology-based antibiofilm products
While several natural and synthetic biofilm agents exhibit potent antibiofilm activity, their efficacy is often hampered by the limited penetration of these drugs into dense biofilms. To address this issue, recently nanotechnology-based biofilm eradication strategies have been extensively applied to enhance drug penetration and delivery (Besinis et al. 2015, Koo et al. 2017, Benoit et al. 2019, Liu et al. 2019). However, these were mainly tested against monospecies biofilms, and utilization of bioactive nanoparticles to deter cross-kingdom biofilms has been very limited. Encouragingly, one study reported that 20–30 nm-sized chitosan nanoparticles reduced the viability of C. albicans and S. mutans as well as biomass of the cross-kingdom biofilm (Ikono et al. 2019). More recent studies also demonstrated that loaded curcumin on chitosan nanoparticles (Ma et al. 2020) and positively-charged silver nanoparticles (Lara et al. 2020) exhibited excellent antibiofilm activity against polymicrobial biofilms of C. albicans and S. aureus. Another study using nanoemulsion containing a quaternary ammonium salt also revealed that it effectively inhibited adherence of C. albicans, S. mutans, Lactobacillus casei, and A. viscosus to glass surfaces and subsequent biofilm formation by combinations thereof (Ramalingam et al. 2012).
Alternative antibiofilm strategies
In spite of the advent of such diverse pioneering antibiofilm approaches, antimicrobial resistance and off-target effect due to broad-spectrum antimicrobials demand searching for alternative biofilm treatment strategies. As consequence, the use of probiotic bacteria to suppress pathogenic strains has been suggested as an alternative strategy for controlling oral biofilms (Söderling et al. 2011, Saha et al. 2014). Among them, the effect of Lactobacillus genus against C. albicans-S. mutans biofilms has been mainly investigated. Interestingly, Lactobacillus plantarum 108 supernatant (Srivastava et al. 2020) and Lactobacillus salivarius (Krzyściak et al. 2017) exhibited capabilities of disrupting C. albicans-S. mutans biofilm formation and development while inhibiting the hyphal transformation of C. albicans. Furthermore, the use of Lactobacillus plantarum CCFM8724 outperformed chlorhexidine in treating and preventing dental caries induced by C. albicans-S. mutans biofilm in vivo (Zhang et al. 2020). Similarly, antagonizing Streptococcus parasanguinis disrupted C. albicans-S. mutans biofilm by altering sugar metabolism and glucosyltransferase activity of S. mutans (Huffines et al. 2020), which is critical for EPS-matrix development and GtfB-mediated cross-kingdom interaction (Hwang et al. 2017, Kim et al. 2021). Another alternative approach for biofilm eradication is antimicrobial photodynamic therapy (aPDT). aPDT requires a photosensitizer that generates short-lived cytotoxic reactive oxygen species (ROS) only under stimulating light, enabling localized and time-controlled therapy. A study showed that aPDT therapy using erythrosine as the photosensitizer exhibited a significant antimicrobial effect against biofilm composed of C. albicans, S. mutans, and Lactobacillus casei (Gong et al. 2019). Also, photodithazine- (Quishida et al. 2015a, Quishida et al. 2015b) and curcumin-based aPDT (Quishida et al. 2016) significantly reduced the metabolic activity and total biomass of multispecies biofilms of C. albicans, S. mutans, and Candida glabrata.
Antibiofilm surfaces
Finally, the sharp increase in using dental implants and devices causes a new class of microbially induced infectious diseases via biofilm accumulation on these surfaces (Arciola et al. 2018, Dhall et al. 2021). By recognizing the importance of fungal-bacterial interaction in pathogenic biofilm development, it is crucial to invent infection-resistant biomaterials that can confront polymicrobial biofilms to mitigate the prevalence of microbially induced medical device infections. In this regard, a fluoride-releasing dental prosthesis copolymer was developed that can interrupt polymicrobial biofilm interactions of C. albicans, L. casei, and S. mutans (Yassin et al. 2016). Also, a self-adhesive sealant modified with di-n-butyldimethacrylate-tin showed strong anti-biofilm efficacy against C. albicans-S. mutans biofilm neither affects the mechanical properties of the sealant nor causes cytotoxicities (Cocco et al. 2020). While current reports describing the surface that can deal with cross-kingdom biofilms are extremely limited, diverse active biomaterials targeting various mechanisms of action are desired.
Concluding Remarks
As summarized here, polymicrobial biofilms containing C. albicans and various pathogenic bacteria together are ubiquitously associated with a variety of oral diseases and have the potential to exacerbate diseases. Therefore, it iterates the importance of expanding biofilm investigations from single-species systems to complex cross-kingdom relationships. Furthermore, it turned out that HIV-infected children are highly susceptible to C. albicans associated oral lesions, candidiasis (Charone et al. 2013, Rosa Oliveira et al. 2016, Charone et al. 2017), which is an important marker of immune suppression that may progress to more severe infections with other pathogens. Thus, more vigorous research efforts should be made to develop innovative antibiofilm therapeutics against fungal-associated polymicrobial biofilms. Although several approaches have been introduced to deal with cross-kingdom biofilms, the vast majority is relying on broad-spectrum antimicrobial activity that can kill both fungus and bacterium but lack targeting polymicrobial interactions. A recent binding mechanism-based non-microbicidal approach that intervenes in symbiotic C. albicans-S. mutans biofilm interaction suggests a new paradigm in treating cross-kingdom biofilms (Kim et al. 2021). Furthermore, it is worth noting that most of the current therapeutics has been mainly tested using in vitro model. A thorough assessment of the efficacy and safety of new therapeutics using an appropriate in vivo model should be accompanied for developing successful applications in the prevention and treatment of polymicrobial infections.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes for Dental and Craniofacial Research (NIDCR) grants DE027970.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I and Dewhirst FE (2005). Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43(11): 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoforado GA, Rams TE, Feik D and Slots J (1991). Microbial aspects of failing osseointegrated dental implants in humans. J Parodontol 10(1): 11–18. [PubMed] [Google Scholar]
- Amaya Arbeláez MI, de Paula ESACA, Navegante G, Valente V, Barbugli PA and Vergani CE (2021). Proto-Oncogenes and Cell Cycle Gene Expression in Normal and Neoplastic Oral Epithelial Cells Stimulated With Soluble Factors From Single and Dual Biofilms of Candida albicans and Staphylococcus aureus. Front Cell Infect Microbiol 11: 627043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciola CR, Campoccia D and Montanaro L (2018). Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 16(7): 397–409. [DOI] [PubMed] [Google Scholar]
- Arias LS, Delbem ACB, Fernandes RA, Barbosa DB and Monteiro DR (2016). Activity of tyrosol against single and mixed-species oral biofilms. J Appl Microbiol 120(5): 1240–1249. [DOI] [PubMed] [Google Scholar]
- Arzmi MH, Cirillo N, Lenzo JC, Catmull DV, O’Brien-Simpson N, Reynolds EC, Dashper S and McCullough M (2018). Monospecies and polymicrobial biofilms differentially regulate the phenotype of genotype-specific oral cancer cells. Carcinogenesis 40(1): 184–193. [DOI] [PubMed] [Google Scholar]
- Arzmi MH, Dashper S and McCullough M (2019). Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J Oral Pathol Med 48(7): 546–551. [DOI] [PubMed] [Google Scholar]
- Ashkanane A, Gomez GF, Levon J, Windsor LJ, Eckert GJ and Gregory RL (2019). Nicotine Upregulates Coaggregation of Candida albicans and Streptococcus mutans. J Prosthodont 28(7): 790–796. [DOI] [PubMed] [Google Scholar]
- Atieh MA, Alsabeeha NHM, Faggion CM and Duncan WJ (2013). The frequency of peri‐implant diseases: a systematic review and meta‐analysis. J Periodontol 84(11): 1586–1598. [DOI] [PubMed] [Google Scholar]
- Baena-Monroy T, Moreno-Maldonado V, Franco-Martínez F, Aldape-Barrios B, Quindós G and Sánchez-Vargas LO (2005). Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal 10 Suppl 1: E27–39. [PubMed] [Google Scholar]
- Bamford CV, Nobbs AH, Barbour ME, Lamont RJ and Jenkinson HF (2015). Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii. Microbiology (Reading) 161(Pt 1): 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicka D, Gonzalez-Gonzalez M, Sykut J, Koziel J, Ciaston I, Adamowicz K, Bras G, Zawrotniak M, Karkowska-Kuleta J, Satala D, Kozik A, Zyla E, Gawron K, Lazarz-Bartyzel K, Chomyszyn-Gajewska M and Rapala-Kozik M (2020). Candida albicans Shields the Periodontal Killer Porphyromonas gingivalis from Recognition by the Host Immune System and Supports the Bacterial Infection of Gingival Tissue. Int J Mol Sci 21(6): 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicka D, Karkowska-Kuleta J, Zawrotniak M, Satała D, Michalik K, Zielinska G, Bochenska O, Kozik A, Ciaston I, Koziel J, Dutton LC, Nobbs AH, Potempa B, Baster Z, Rajfur Z, Potempa J and Rapala-Kozik M (2019). Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci Rep 9(1): 4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaussart A, Herman P, El-Kirat-Chatel S, Lipke PN, Kucharíková S, Van Dijck P and Dufrêne YF (2013). Single-cell force spectroscopy of the medically important Staphylococcus epidermidis-Candida albicans interaction. Nanoscale 5(22): 10894–10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit DSW, Sims KR Jr. and Fraser D (2019). Nanoparticles for Oral Biofilm Treatments. ACS Nano 13(5): 4869–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M, Ranjan A, Thompson A, Diaz PI, Sobue T, Maas K and Dongari-Bagtzoglou A (2019). Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog 15(4): e1007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besinis A, De Peralta T, Tredwin CJ and Handy RD (2015). Review of nanomaterials in dentistry: interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano 9(3): 2255–2289. [DOI] [PubMed] [Google Scholar]
- Bombarda GF, Rosalen PL, Paganini ER, Garcia MA, Silva DR, Lazarini JG, Freires IA, Regasini LO and Sardi JC (2019). Bioactive molecule optimized for biofilm reduction related to childhood caries. Future Microbiol 14(14): 1207–1220. [DOI] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H and Koo H (2018). Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol 26(3): 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Johnston W, Delaney C, Short B, Butcher MC, Young T, Butcher J, Riggio M, Culshaw S and Ramage G (2019). Polymicrobial oral biofilm models: simplifying the complex. J Med Microbiol 68(11): 1573–1584. [DOI] [PubMed] [Google Scholar]
- Buduneli N and Kinane DF (2011). Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol 38(s11): 85–105. [DOI] [PubMed] [Google Scholar]
- Buurman ET, Westwater C, Hube B, Brown AJ, Odds FC and Gow NA (1998). Molecular analysis of CaMnt1p, a mannosyl transferase important for adhesion and virulence of Candida albicans. Proc Natl Acad Sci USA 95(13): 7670–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Fathi R, Cheng SY, Ho A and Gilbert ES (2020). Rhamnus prinoides (gesho) stem extract prevents co-culture biofilm formation by Streptococcus mutans and Candida albicans. Lett Appl Microbiol 71(3): 294–302. [DOI] [PubMed] [Google Scholar]
- Canullo L, Peñarrocha-Oltra D, Covani U and Rossetti PH (2015). Microbiologic and Clinical Findings of Implants in Healthy Condition and with Peri-Implantitis. Int J Oral Maxillofac Implants 30(4): 834–842. [DOI] [PubMed] [Google Scholar]
- Cavalcanti IM, Nobbs AH, Ricomini-Filho AP, Jenkinson HF and Del Bel Cury AA (2016a). Interkingdom cooperation between Candida albicans, Streptococcus oralis and Actinomyces oris modulates early biofilm development on denture material. Pathog Dis 74(3). [DOI] [PubMed] [Google Scholar]
- Cavalcanti YW, Wilson M, Lewis M, Del-Bel-Cury AA, da Silva WJ and Williams DW (2016b). Modulation of Candida albicans virulence by bacterial biofilms on titanium surfaces. Biofouling 32(2): 123–134. [DOI] [PubMed] [Google Scholar]
- Cavazana TP, Pessan JP, Hosida TY, Sampaio C, Amarante VOZ, Monteiro DR and Delbem ACB (2020). Effects of Sodium Trimetaphosphate, Associated or Not with Fluoride, on the Composition and pH of Mixed Biofilms, before and after Exposure to Sucrose. Caries Res 54(4): 358–368. [DOI] [PubMed] [Google Scholar]
- Charalampakis G, Leonhardt Å, Rabe P and Dahlén G (2012). Clinical and microbiological characteristics of peri‐implantitis cases: a retrospective multicentre study. Clin Oral Implants Res 23(9): 1045–1054. [DOI] [PubMed] [Google Scholar]
- Charone S, Portela MB, das Chagas MS, de Araújo Soares RM and de Araújo Castro GF (2013). Biofilm of Candida albicans from oral cavity of an HIV-infected child: challenge on enamel microhardness. Oral Surg Oral Med Oral Pathol Oral Radiol 115(4): 500–504. [DOI] [PubMed] [Google Scholar]
- Charone S, Portela MB, Martins KO, Soares RM and Castro GF (2017). Role of Candida species from HIV infected children in enamel caries lesions: an in vitro study. J Appl Oral Sci 25(1): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HA, Cheong DE, Lim HD, Kim WH, Ham MH, Oh MH, Wu Y, Shin HJ and Kim GJ (2017). Antimicrobial and Anti-Biofilm Activities of the Methanol Extracts of Medicinal Plants against Dental Pathogens Streptococcus mutans and Candida albicans. J Microbiol Biotechnol 27(7): 1242–1248. [DOI] [PubMed] [Google Scholar]
- Cocco AR, Cuevas-Suárez CE, Liu Y, Lund RG, Piva E and Hwang G (2020). Anti-biofilm activity of a novel pit and fissure self-adhesive sealant modified with metallic monomers. Biofouling 36(3): 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cássia Negrini T, Koo H and Arthur RA (2019). Candida–bacterial biofilms and host–microbe interactions in oral diseases. Oral Mucosal Immunity and Microbiome: 119–141. [DOI] [PubMed] [Google Scholar]
- Delaney C, Kean R, Short B, Tumelty M, McLean W, Nile CJ and Ramage G (2018). Fungi at the scene of the crime: innocent bystanders or accomplices in oral infections? Curr Clin Microbiol Rep 5(3): 190–200. [Google Scholar]
- Delaney C, O’Donnell LE, Kean R, Sherry L, Brown JL, Calvert G, Nile CJ, Cross L, Bradshaw DJ and Brandt BW (2019). Interkingdom interactions on the denture surface: Implications for oral hygiene. Biofilm 1: 100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Li W, He Y, Wu J, Ren B and Zou L (2019a). Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch Oral Biol 100: 106–112. [DOI] [PubMed] [Google Scholar]
- Deng L, Zou L, Wu J, Liu H, Luo T, Zhou X, Li W and Ren B (2019b). Voriconazole inhibits cross-kingdom interactions between Candida albicans and Actinomyces viscosus through the ergosterol pathway. Int J Antimicrob Agents 53(6): 805–813. [DOI] [PubMed] [Google Scholar]
- Dhall A, Islam S, Park M, Zhang Y, Kim A and Hwang G (2021). Bimodal Nanocomposite Platform with Antibiofilm and Self-Powering Functionalities for Biomedical Applications. ACS Appl Mater Interfaces 13(34): 40379–40391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA and Wolcott RD (2008). Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 8(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q, Ren B, He J, Peng X, Guo Q, Zheng L, Li J, Dai H, Chen V, Zhang L, Zhou X and Xu X (2021). Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J 15(3): 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy AK, David MS, Li L, Heider TN, Peterson JD, Montano EA, Dongari-Bagtzoglou A, Diaz PI and Strausbaugh LD (2014). Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: discovery of Malassezia as a prominent commensal. PLoS One 9(3): e90899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellepola K, Liu Y, Cao T, Koo H and Seneviratne CJ (2017). Bacterial GtfB Augments Candida albicans Accumulation in Cross-Kingdom Biofilms. J Dent Res 96(10): 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellepola K, Truong T, Liu Y, Lin Q, Lim T, Lee Y, Cao T, Koo H and Seneviratne C (2019). Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans-Candida albicans mixed-species biofilms. Infect Immun 87(10): e00339–00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai C-H, Gonzalez-Begne M, Watson G, Krysan DJ and Bowen WH (2014). Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infec Immun 82(5): 1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkash Y, Feldman M, Ginsburg I, Steinberg D and Shalish M (2019). Polyphenols Inhibit Candida albicans and Streptococcus mutans Biofilm Formation. Dent J (Basel) 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins LM and O’Toole GA (2015). Cystic fibrosis lung infections: polymicrobial, complex, and hard to treat. PLoS Pathog 11(12): e1005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami W, Nishikawa K, Ozawa S, Hasegawa Y and Takebe J (2021). Correlation between the relative abundance of oral bacteria and Candida albicans in denture and dental plaques. J Oral Biosci 63(2): 175–183. [DOI] [PubMed] [Google Scholar]
- Gao S, Zhang S and Zhang S (2021). Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J Appl Microbiol 130(4): 1154–1172. [DOI] [PubMed] [Google Scholar]
- Garcia BA, Acosta NC, Tomar SL, Roesch LFW, Lemos JA, Mugayar LRF and Abranches J (2021). Association of Candida albicans and Cbp(+) Streptococcus mutans with early childhood caries recurrence. Sci Rep 11(1): 10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A and Gillevet PM (2010). Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 6(1): e1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM and Krogfelt KA (2006). Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3(3): 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes SC, Corvello P, Romagna R, Müller LH, Angst P and Oppermann RV (2015). How do peri-implant mucositis and gingivitis respond to supragingival biofilm control-an intra-individual longitudinal cohort study. Eur J Oral Implantol 8(1): 65–73. [PubMed] [Google Scholar]
- Gong J, Park H, Lee J, Seo H and Lee S (2019). Effect of Photodynamic Therapy on Multispecies Biofilms, Including Streptococcus mutans, Lactobacillus casei, and Candida albicans. Photobiomodul Photomed Laser Surg 37(5): 282–287. [DOI] [PubMed] [Google Scholar]
- Guo H, Chen Y, Guo W and Chen J (2021). Effects of extracellular DNA on dual-species biofilm formed by Streptococcus mutans and Candida albicans. Microb Pathog 154: 104838. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Wang Y, Jin Y and Wang C (2020). Heme Competition Triggers an Increase in the Pathogenic Potential of Porphyromonas gingivalis in Porphyromonas gingivalis-Candida albicans Mixed Biofilm. Front Microbiol 11: 596459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Thakur J, Pal S, Gupta R, Mishra D, Kumar S, Yadav K, Saini A, Yavvari PS, Vedantham M, Singh A, Srivastava A, Prasad R and Bajaj A (2019). Cholic Acid-Peptide Conjugates as Potent Antimicrobials against Interkingdom Polymicrobial Biofilms. Antimicrob Agents Chemother 63(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel B. C. d. V., Montenegro SCL, Dantas PMC, Pascoal A. L. d. B., Lima KC and Calderon P. d. S. (2017). Frequency of peri‐implant diseases and associated factors. Clin Oral Implants Res 28(10): 1211–1217. [DOI] [PubMed] [Google Scholar]
- Hacioglu M, Haciosmanoglu E, Birteksoz-Tan AS, Bozkurt-Guzel C and Savage PB (2019). Effects of ceragenins and conventional antimicrobials on Candida albicans and Staphylococcus aureus mono and multispecies biofilms. Diagn Microbiol Infect Dis 95(3): 114863. [DOI] [PubMed] [Google Scholar]
- Hacioglu M, Oyardi O, Bozkurt-Guzel C and Savage PB (2020). Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J Antibiot 73(7): 455–462. [DOI] [PubMed] [Google Scholar]
- Hajishengallis E, Parsaei Y, Klein MI and Koo H (2017). Advances in the microbial etiology and pathogenesis of early childhood caries. Mol Oral Microbiol 32(1): 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA and Gow NA (2013). Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol 90(6): 1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverman TM, Laheij A, de Soet JJ, de Lange J and Rozema FR (2017). Candida and Porphyromonas gingivalis: the effect on wound closure in vitro. J Oral Microbiol 9(1): 1328266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Kim D, Zhou X, Ahn SJ, Burne RA, Richards VP and Koo H (2017). RNA-Seq Reveals Enhanced Sugar Metabolism in Streptococcus mutans Co-cultured with Candida albicans within Mixed-Species Biofilms. Front Microbiol 8: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL (2001). The ALS gene family of Candida albicans. Trends Microbiol 9(4): 176–180. [DOI] [PubMed] [Google Scholar]
- Huffines JT and Scoffield JA (2020). Disruption of Streptococcus mutans and Candida albicans synergy by a commensal streptococcus. Sci Rep 10(1): 19661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultin M, Gustafsson A, Hallström H, Johansson LA, Ekfeldt A and Klinge B (2002). Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res 13(4): 349–358. [DOI] [PubMed] [Google Scholar]
- Hwang G, Liu Y, Kim D, Li Y, Krysan DJ and Koo H (2017). Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. Plos Pathog 13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikono R, Vibriani A, Wibowo I, Saputro KE, Muliawan W, Bachtiar BM, Mardliyati E, Bachtiar EW, Rochman NT, Kagami H, Xianqi L, Nagamura-Inoue T and Tojo A (2019). Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res Notes 12(1): 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima SA, Hayama K, Burton JP, Reid G, Okada M, Matsushita Y and Abe S (2012). Effect of Streptococcus salivarius K12 on the in vitro growth of Candida albicans and its protective effect in an oral candidiasis model. Appl Environ Microbiol 78(7): 2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T, Ohshima T, Tasaki T, Momoi Y, Ikawa S, Kitano K and Yamamoto T (2020). High microbicidal effect of peroxynitric acid on biofilm-infected dentin in a root carious tooth model and verification of tissue safety. J Oral Biosci 62(2): 189–194. [DOI] [PubMed] [Google Scholar]
- Jabri B, Iken M, Achmit M, Rida S and Ennibi OK (2021). Occurrence of Candida albicans in Periodontitis. Int J Dent 2021: 5589664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri H, Banerjee G, Khan MSA, Ahmad I, Abulreesh HH and Althubiani AS (2020). Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 10(1): 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KM, MacDonald KW, Chanyi RM, Cadieux PA and Burton JP (2016). Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J Med Microbiol 65(4): 328–336. [DOI] [PubMed] [Google Scholar]
- Janus MM, Willems HM and Krom BP (2016). Candida albicans in multispecies oral communities; a keystone commensal? Fungal Biofilms and related infections: 13–20. [DOI] [PubMed] [Google Scholar]
- Jarosz LM, Deng DM, van der Mei HC, Crielaard W and Krom BP (2009). Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell 8(11): 1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J, Goldberg S, Khare R, Bailey LC, Forrest CB, Hajishengallis E and Koo H (2018). Retrospective analysis of Candida-related conditions in infancy and early childhood caries. Pediatr Dent 40(2): 131–135. [PMC free article] [PubMed] [Google Scholar]
- Jenkinson H and Douglas L (2002). Candida interactions with bacterial biofilms. Polymicrobial diseases: 357–373. [Google Scholar]
- Johannsen A, Westergren A and Johannsen G (2012). Dental implants from the patients perspective: transition from tooth loss, through amputation to implants–negative and positive trajectories. J Clin Periodontol 39(7): 681–687. [DOI] [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Bartnicka D, Zawrotniak M, Zielinska G, Kieronska A, Bochenska O, Ciaston I, Koziel J, Potempa J, Baster Z, Rajfur Z and Rapala-Kozik M (2018). The activity of bacterial peptidylarginine deiminase is important during formation of dual-species biofilm by periodontal pathogen Porphyromonas gingivalis and opportunistic fungus Candida albicans. Pathog Dis 76(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkowska-Kuleta J, Surowiec M, Gogol M, Koziel J, Potempa B, Potempa J, Kozik A and Rapala-Kozik M (2020). Peptidylarginine Deiminase of Porphyromonas gingivalis Modulates the Interactions between Candida albicans Biofilm and Human Plasminogen and High-Molecular-Mass Kininogen. Int J Mol Sci 21(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaźmierczak-Siedlecka K, Dvořák A, Folwarski M, Daca A, Przewłócka K and Makarewicz W (2020). Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers (Basel) 12(5): 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, Bamunuarachchi NI, Pham DTN, Tabassum N, Khan MSA and Kim YM (2021). Mixed biofilms of pathogenic Candida-bacteria: regulation mechanisms and treatment strategies. Crit Rev Microbiol 47(6): 699–727. [DOI] [PubMed] [Google Scholar]
- Khoury ZH, Vila T, Puthran TR, Sultan AS, Montelongo-Jauregui D, Melo MAS and Jabra-Rizk MA (2020). The Role of Candida albicans Secreted Polysaccharides in Augmenting Streptococcus mutans Adherence and Mixed Biofilm Formation: In vitro and in vivo Studies. Front Microbiol 11: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Liu Y, Benhamou RI, Sanchez H, Simón-Soro Á, Li Y, Hwang G, Fridman M, Andes DR and Koo H (2018). Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J 12(6): 1427–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Sengupta A, Niepa TH, Lee B-H, Weljie A, Freitas-Blanco VS, Murata RM, Stebe KJ, Lee D and Koo H (2017). Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep 7(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-E, Liu Y, Dhall A, Bawazir M, Koo H and Hwang G (2020). Synergism of Streptococcus mutans and Candida albicans reinforces biofilm maturation and acidogenicity in saliva: an in vitro study. Front Cell Infect Microbiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Dhall A, Liu Y, Bawazir M, Koo H and Hwang G (2021). Intervening in Symbiotic Cross-Kingdom Biofilm Interactions: a Binding Mechanism-Based Nonmicrobicidal Approach. Mbio 12(3): e00651–00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline KA and Lewis AL (2016). Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr 4(2): 4.2. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE (2000). Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 54(1): 413–437. [DOI] [PubMed] [Google Scholar]
- Koo H, Allan RN, Howlin RP, Stoodley P and Hall-Stoodley L (2017). Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15(12): 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyściak W, Kościelniak D, Papież M, Vyhouskaya P, Zagórska-Świeży K, Kołodziej I, Bystrowska B and Jurczak A (2017). Effect of a Lactobacillus Salivarius Probiotic on a Double-Species Streptococcus Mutans and Candida Albicans Caries Biofilm. Nutrients 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Koo H and Hajishengallis G (2018). The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16(12): 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara HH and Lopez-Ribot JL (2020). Inhibition of Mixed Biofilms of Candida albicans and Methicillin-Resistant Staphylococcus aureus by Positively Charged Silver Nanoparticles and Functionalized Silicone Elastomers. Pathogens 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-T, Huang Y-W, Zhu L and Weltman R (2017). Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent 62: 1–12. [DOI] [PubMed] [Google Scholar]
- Leme AP, Koo H, Bellato C, Bedi G and Cury J (2006). The role of sucrose in cariogenic dental biofilm formation—new insight. J Dent Res 85(10): 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt A, Renvert S and Dahlén G (1999). Microbial findings at failing implants. Clin Oral Implants Res 10(5): 339–345. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang C, Liu P, Liu W, Gao Y and Sun S (2015). In vitro interactions between fluconazole and minocycline against mixed cultures of Candida albicans and Staphylococcus aureus. J Microbiol Immunol Infect 48(6): 655–661. [DOI] [PubMed] [Google Scholar]
- Li X, Yin L, Ramage G, Li B, Tao Y, Zhi Q, Lin H and Zhou Y (2019). Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Microbiologyopen 8(12): e937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Qiu W, Zhang K, Zhou X, Ren B, He J, Xu X, Cheng L and Li M (2017). Nicotine Enhances Interspecies Relationship between Streptococcus mutans and Candida albicans. Biomed Res Int 2017: 7953920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shi L, Su L, van der Mei HC, Jutte PC, Ren Y and Busscher HJ (2019). Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev 48(2): 428–446. [DOI] [PubMed] [Google Scholar]
- Lobo CIV, Lopes A. C. U. d. A. and Klein MI (2021). Compounds with Distinct Targets Present Diverse Antimicrobial and Antibiofilm Efficacy against Candida albicans and Streptococcus mutans, and Combinations of Compounds Potentiate Their Effect. J Fungi 7(5): 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Moser D, Han F, Leonhard M, Schneider-Stickler B and Tan Y (2020). Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr Polym 241: 116254. [DOI] [PubMed] [Google Scholar]
- Manson JM, Rauch M and Gilmore MS (2008). The commensal microbiology of the gastrointestinal tract. GI microbiota and regulation of the immune system: 15–28. [DOI] [PubMed] [Google Scholar]
- Martín R, Miquel S, Ulmer J, Kechaou N, Langella P and Bermúdez-Humarán LG (2013). Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact 12: 71–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorano-Fernandes L, Rodrigues NC, de Souza Borges MH, Cavalcanti YW and de Almeida LFD (2020). Interkingdom interaction between C. albicans and S. salivarius on titanium surfaces. BMC Oral Health 20(1): 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelongo-Jauregui D, Srinivasan A, Ramasubramanian AK and Lopez-Ribot JL (2018). An In Vitro Model for Candida albicans⁻Streptococcus gordonii Biofilms on Titanium Surfaces. J Fungi 4(2): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini T. d. C., Carlos IZ, Duque C, Caiaffa KS and Arthur RA (2021). Interplay Among the Oral Microbiome, Oral Cavity Conditions, the Host Immune Response, Diabetes Mellitus, and Its Associated-Risk Factors—An Overview. Front Oral Health 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig H-J, Wichmann M, Andreas SK and Eitner S (2008). Oral health–related quality of life in partially edentulous patients: Assessments before and after implant therapy. J Cranio-Maxillofac Surg 36(8): 477–480. [DOI] [PubMed] [Google Scholar]
- O’Donnell LE, Millhouse E, Sherry L, Kean R, Malcolm J, Nile CJ and Ramage G (2015). Polymicrobial Candida biofilms: friends and foe in the oral cavity. FEMS Yeast Res 15(7). [DOI] [PubMed] [Google Scholar]
- Orazi G and O’Toole GA (2019). “It takes a village”: mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J Bact 202(1): e00530–00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanasiou E, Finkelman M, Hanley J and Parashis AO (2016). Prevalence, etiology and treatment of peri‐implant mucositis and peri‐implantitis: A survey of periodontists in the United States. J Periodontol 87(5): 493–501. [DOI] [PubMed] [Google Scholar]
- Park M, Islam S, Kim HE, Korostoff J, Blatz MB, Hwang G and Kim A (2020). Human Oral Motion‐Powered Smart Dental Implant (SDI) for In Situ Ambulatory Photo‐biomodulation Therapy. Adv Healthc Mater 9(16): 2000658. [DOI] [PubMed] [Google Scholar]
- Pereira CA, Toledo BC, Santos CT, Pereira Costa ACB, Back-Brito GN, Kaminagakura E and Jorge AOC (2013). Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn Microbiol Infect Dis 76(4): 419–424. [DOI] [PubMed] [Google Scholar]
- Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW and Shirtliff ME (2012a). Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 25(1): 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW and Shirtliff ME (2010). Microbial interactions and differential protein expression in Staphylococcus aureus -Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol 59(3): 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BM, Ovchinnikova ES, Krom BP, Schlecht LM, Zhou H, Hoyer LL, Busscher HJ, van der Mei HC, Jabra-Rizk MA and Shirtliff ME (2012b). Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 158(12): 2975–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, Leishman SJ, Bandara H and Walsh LJ (2019). Polyphenol-Rich Cranberry Extracts Modulate Virulence of Streptococcus mutans-Candida albicans Biofilms Implicated in the Pathogenesis of Early Childhood Caries. Pediatr Dent 41(1): 56–62. [PubMed] [Google Scholar]
- Pushalkar S, Mane SP, Ji X, Li Y, Evans C, Crasta OR, Morse D, Meagher R, Singh A and Saxena D (2011). Microbial diversity in saliva of oral squamous cell carcinoma. FEMS Immunol Med Microbiol 61(3): 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Locock K, Verma-Gaur J, Hay ID, Meagher L and Traven A (2016). Searching for new strategies against polymicrobial biofilm infections: guanylated polymethacrylates kill mixed fungal/bacterial biofilms. J. Antimicrob. Chemother 71(2): 413–421. [DOI] [PubMed] [Google Scholar]
- Quishida CC, Carmello JC, Mima EG, Bagnato VS, Machado AL and Pavarina AC (2015a). Susceptibility of multispecies biofilm to photodynamic therapy using Photodithazine®. Lasers Med Sci 30(2): 685–694. [DOI] [PubMed] [Google Scholar]
- Quishida CC, De Oliveira Mima EG, Jorge JH, Vergani CE, Bagnato VS and Pavarina AC (2016). Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers Med Sci 31(5): 997–1009. [DOI] [PubMed] [Google Scholar]
- Quishida CC, Mima EG, Dovigo LN, Jorge JH, Bagnato VS and Pavarina AC (2015b). Photodynamic inactivation of a multispecies biofilm using Photodithazine(®) and LED light after one and three successive applications. Lasers Med Sci 30(9): 2303–2312. [DOI] [PubMed] [Google Scholar]
- Ramalingam K, Amaechi BT, Ralph RH and Lee VA (2012). Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Arch Oral Biol 57(1): 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SM, Fratucelli É DO, Bueno PCP, de Castro MKV, Francisco AA, Cavalheiro AJ and Klein MI (2019). Antimicrobial and antibiofilm activities of Casearia sylvestris extracts from distinct Brazilian biomes against Streptococcus mutans and Candida albicans. BMC Complement Altern Med 19(1): 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogiers O, Holtappels M, Siala W, Lamkanfi M, Van Bambeke F, Lagrou K, Van Dijck P and Kucharíková S (2018). Anidulafungin increases the antibacterial activity of tigecycline in polymicrobial Candida albicans/Staphylococcus aureus biofilms on intraperitoneally implanted foreign bodies. J Antimicrob Chemother 73(10): 2806–2814. [DOI] [PubMed] [Google Scholar]
- Ronald A (2002). The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med 113(1): 14–19. [DOI] [PubMed] [Google Scholar]
- Rosa Oliveira CA, Charone S, de Araújo Soares RM, Portela MB and de Aráujo Castro GF (2016). Association of Candida Species Isolated From the Dental Plaque of HIV-infected Children and Prevalence of Early Carious Lesions. J Dent Child (Chic) 83(3): 139–145. [PubMed] [Google Scholar]
- Rosen P, Clem D, Cochran DL, Froum S, McAllister B, Renvert S and Wang HL (2013). Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol 84(4): 436–443. [DOI] [PubMed] [Google Scholar]
- Saha S, Tomaro-Duchesneau C, Rodes L, Malhotra M, Tabrizian M and Prakash S (2014). Investigation of probiotic bacteria as dental caries and periodontal disease biotherapeutics. Benef Microbes 5(4): 447–460. [DOI] [PubMed] [Google Scholar]
- Sakka S, Baroudi K and Nassani MZ (2012). Factors associated with early and late failure of dental implants. J Investig Clin Dent 3(4): 258–261. [DOI] [PubMed] [Google Scholar]
- Schaudinn C, Gorur A, Keller D, Sedghizadeh PP and Costerton JW (2009). Periodontitis: an archetypical biofilm disease. J Am Dent Assoc 140(8): 978–986. [DOI] [PubMed] [Google Scholar]
- Septiana S, Bachtiar BM, Yuliana ND and Wijaya CH (2019). Cajuputs candy impairs Candida albicans and Streptococcus mutans mixed biofilm formation in vitro. F1000 Res 8: 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiham A and James W (2015). Diet and dental caries: the pivotal role of free sugars reemphasized. J Dent Res 94(10): 1341–1347. [DOI] [PubMed] [Google Scholar]
- Shi B, Wu T, McLean J, Edlund A, Young Y, He X, Lv H, Zhou X, Shi W, Li H and Lux R (2016). The Denture-Associated Oral Microbiome in Health and Stomatitis. mSphere 1(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeishi H, Nakamura M, Oka I, Su CY, Yano K, Ishikawa M, Kaneyasu Y, Sugiyama M and Ohta K (2021). The Associations of Periodontopathic Bacteria and Oral Candida with Periodontal Inflamed Surface Area in Older Adults Receiving Supportive Periodontal Therapy. Diagnostics (Basel) 11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME and Jenkinson HF (2010). Interaction of Candida albicans Cell Wall Als3 Protein with Streptococcus gordonii SspB Adhesin Promotes Development of Mixed-Species Communities. Infect Immun 78(11): 4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Soro A and Mira A (2015). Solving the etiology of dental caries. Trends Microbiol 23(2): 76–82. [DOI] [PubMed] [Google Scholar]
- Simon G, Bérubé C, Voyer N and Grenier D (2019). Anti-biofilm and anti-adherence properties of novel cyclic dipeptides against oral pathogens. Bioorg Med Chem 27(12): 2323–2331. [DOI] [PubMed] [Google Scholar]
- Söderling EM, Marttinen AM and Haukioja AL (2011). Probiotic Lactobacilli Interfere with Streptococcus mutans Biofilm Formation In Vitro. Curr Microbiol 62(2): 618–622. [DOI] [PubMed] [Google Scholar]
- Souza JGS, Bertolini M, Thompson A, Barão VAR and Dongari-Bagtzoglou A (2020). Biofilm Interactions of Candida albicans and Mitis Group Streptococci in a Titanium-Mucosal Interface Model. Appl Environ Microbiol 86(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N, Ellepola K, Venkiteswaran N, Chai LYA, Ohshima T and Seneviratne CJ (2020). Lactobacillus Plantarum 108 Inhibits Streptococcus mutans and Candida albicans Mixed-Species Biofilm Formation. Antibiotics (Basel) 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stressmann FA, Rogers GB, Van Der Gast CJ, Marsh P, Vermeer LS, Carroll MP, Hoffman L, Daniels TW, Patel N and Forbes B (2012). Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax 67(10): 867–873. [DOI] [PubMed] [Google Scholar]
- Sztukowska MN, Dutton LC, Delaney C, Ramsdale M, Ramage G, Jenkinson HF, Nobbs AH and Lamont RJ (2018). Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als3. mBio 9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai R, Sugamata M and Kiyoura Y (2011). Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb Pathog 51(4): 250–254. [DOI] [PubMed] [Google Scholar]
- Tan Y, Leonhard M, Moser D, Ma S and Schneider-Stickler B (2019). Antibiofilm efficacy of curcumin in combination with 2-aminobenzimidazole against single- and mixed-species biofilms of Candida albicans and Staphylococcus aureus. Colloids Surf B Biointerfaces 174: 28–34. [DOI] [PubMed] [Google Scholar]
- Tawara Y, Honma K and Naito Y (1996). Methicillin-resistant Staphylococcus aureus and Candida albicans on denture surfaces. Bull Tokyo Dent Coll 37(3): 119–128. [PubMed] [Google Scholar]
- Teles R, Teles F, Frias‐Lopez J, Paster B and Haffajee A (2013). Lessons learned and unlearned in periodontal microbiology. Periodontology 2000 62(1): 95–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Zijnge V, Cicek A, De Soet J, Harmsen H and Huysmans M (2012). Shifts in the microbial population in relation to in situ caries progression. Caries Res 46(5): 427–431. [DOI] [PubMed] [Google Scholar]
- Veerapandian R and Vediyappan G (2019). Gymnemic Acids Inhibit Adhesive Nanofibrillar Mediated Streptococcus gordonii-Candida albicans Mono-Species and Dual-Species Biofilms. Front Microbiol 10: 2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vílchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H and Wagner-Döbler I (2010). Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 11(11): 1552–1562. [DOI] [PubMed] [Google Scholar]
- Wan SX, Tian J, Liu Y, Dhall A, Koo H and Hwang G (2021). Cross-Kingdom Cell-to-Cell Interactions in Cariogenic Biofilm Initiation. J Dent Res 100(1): 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherden EA, Shoaie S, Hall RA and Moyes DL (2017). The human mucosal mycobiome and fungal community interactions. J Fungi 3(4): 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff D, Frese C, Maier-Kraus T, Krueger T and Wolff B (2013). Bacterial biofilm composition in caries and caries-free subjects. Caries Res 47(1): 69–77. [DOI] [PubMed] [Google Scholar]
- Wu T, Cen L, Kaplan C, Zhou X, Lux R, Shi W and He X (2015). Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum. J Dent Res 94(10): 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, Castillo DA, Campbell F, Davis J and Herzog K (2018). Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res 52(1–2): 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sobue T, Bertolini M, Thompson A, Vickerman M, Nobile CJ and Dongari-Bagtzoglou A (2017). S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 8(8): 1602–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Sztajer H, Buddruhs N, Petersen J, Rohde M, Talay SR and Wagner-Döbler I (2011). Lack of the delta subunit of RNA polymerase increases virulence related traits of Streptococcus mutans. PLoS One 6(5): e20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Scoffield J, Wu R, Deivanayagam C, Zou J and Wu H (2018). Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol Oral Microbiol 33(4): 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin SA, German MJ, Rolland SL, Rickard AH and Jakubovics NS (2016). Inhibition of multispecies biofilms by a fluoride-releasing dental prosthesis copolymer. J Dent 48: 62–70. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Qin S, Xu X, Zhao J, Zhang H, Liu Z and Chen W (2020). Inhibitory Effect of Lactobacillus plantarum CCFM8724 towards Streptococcus mutans- and Candida albicans-Induced Caries in Rats. Oxid Med Cell Longev 2020: 4345804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S and Li JZ (2012). Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA 109(15): 5809–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]


