Abstract
Background
Colletotrichum gloeosporioides, a soil-borne fungal pathogen, causes significant yield losses in many plants, including cultivated strawberry (Fragaria × ananassa, 2n = 8x = 56). Thaumatin-like proteins (TLPs) are a large and complex family of proteins that play a vital role in plant host defense and other physiological processes.
Methods
To enhance our understanding of the antifungal activity of F. × ananassa TLPs (FaTLP), we investigated the genome-wide identification of FaTLP gene families and their expression patterns in F. × ananassa plants upon pathogen infection. Moreover, we used RNA sequencing (RNA-seq) to detect the differences in the expression patterns of TLP genes between different resistant strawberry cultivars in response to C. gloeosporioides infection.
Results
In total, 76 TLP genes were identified from the octoploid cultivated strawberry genome with a mean length of 1,439 bp. They were distributed on 24 F. × ananassa chromosomes. The FaTLP family was then divided into ten groups (Group I–X) according to the comparative phylogenetic results. Group VIII contained the highest number of TLP family genes. qRT-PCR analysis results indicated that FaTLP40, FaTLP41, FaTLP43, FaTLP68, and FaTLP75 were upregulated following C. gloeosporioides infection in the resistant octoploid strawberry.
Conclusions
The data showed some differences in TLP gene expression patterns across different resistant strawberry cultivars, as well as faster TLP defense responses to pathogenic fungi in resistant cultivars. This study will aid in the characterization of TLP gene family members found in octoploid strawberries and their potential biological functions in plants’ defenses against pathogenic fungi.
Keywords: Fragaria × ananassa, Thaumatin like-proteins, Colletotrichum gloeosporioides, Gene family, Expression analysis, Antifungal activity
Introduction
Thaumatin-like proteins (TLPs) are important members of the highly complex gene family pathogenesis-related protein group 5 (PR5). They are highly homologous with sweet-tasting thaumatin protein produced by Thaumatococcus daniellii fruit (Wel & Loeve, 1972; Velazhahan, Datta & Muthukrishnan, 1999; Christensen et al., 2002; Loon, Rep & Pieterse, 2006). TLPs are abundant and highly diversified in plants (Loon, Rep & Pieterse, 2006; O’Leary, Poulis & von Aderkas, 2007; Liu, Zamani & Ekramoddoullah, 2010). According to a survey conducted in the UniProt database (https://www.uniprot.org/uniprot/?query=Thaumatin-like+proteins&sort=score), 1,816 TLPs (1,720 angiosperms, 82 gymnosperms, seven bryophytes, and seven algae species) from 187 different species have been identified (Faillace et al., 2019; Jesús-Pires et al., 2020). A great number of isoforms activated by biotic or abiotic stresses and multiple rounds of distinct gene duplication events were observed across the TLP gene family’s expansion and evolution in plants, suggesting that this superfamily has a complex pattern of molecular evolution (Liu, Zamani & Ekramoddoullah, 2010; Cao et al., 2016). Previous studies have indicated that plant TLPs consist of approximately 200 amino acids, have a molecular weight of 21–26 kD (Velazhahan, Datta & Muthukrishnan, 1999), and contain 10 to 16 conserved motifs (Hulo et al., 2008). These proteins exhibit a series of responses to biotic and abiotic stress factors in plants, such as pathogen invasion, drought, wounding, freezing, and salinity (Petre et al., 2011). They also play roles in a variety of physiological and developmental processes, including organ formation, fruit ripening, and seed germination (Salzman et al., 1998; Seo et al., 2008).
The octoploid cultivated strawberry (Fragaria × ananassa, 2n = 8x = 56) is an economically important perennial horticultural crop (FAO, IFAD, UNICEF, WFP, WHO, 2017) that is widely cultivated in China, but the main cultivar is ‘Benihoppe’ from Japan. ‘Benihoppe’ is susceptible to a range of diseases including anthracnose, which is one of the most destructive fungal diseases that results in considerable losses in strawberry production (Hammerschlag et al., 2006; Dean et al., 2012), especially at the seedling stage and early stages following transplanting. On the east coast of China, anthracnose in strawberry is mainly caused by the fungus Colletotrichum gloeosporioides (Zhang et al., 2017), which can infect all aerial plant parts, and the most severe symptoms are dwarf-stem and foliar lesions.
Pathogenesis-related (PR) proteins, including TLPs, exhibit significant antifungal activity in plants (Velazhahan, Datta & Muthukrishnan, 1999). TLP gene expression is responsive to infection by a variety of fungal pathogens, such as Colletotrichum, Podosphaera, Phytophthora, Fusarium, and Neurospora (Woloshuk et al., 1991; Narasimhan et al., 2003; Liu, Zamani & Ekramoddoullah, 2010; Rather et al., 2015). TLPs play multiple roles in inhibiting hyphal growth and spore germination of various pathogenic fungi (Roberts & Selitrennikoff, 1990; Woloshuk et al., 1991; De Freitas et al., 2011), binding β-1,3-glucans to destroy the stability of fungal membranes (Grenier, Potvin & Asselin, 2000; Osmond et al., 2001; Zareie, Melanson & Murphy, 2002), and stimulating plant defensive responses against pathogenic fungi to prevent fungal damage or reduce disease symptoms. Because of their effective antifungal activity, TLP genes are promising candidates for improving plant disease resistance. Overexpression of defense-related TLP genes in transgenic Arabidopsis (Rout, Nanda & Joshi, 2016), rice (Datta et al., 1999), tobacco (Singh et al., 2013), grape (He et al., 2017), wheat (Mackintosh et al., 2007), and potato (Acharya et al., 2013) significantly enhanced plant resistance to fungal diseases.
Resistance to a variety of pathogens has been a key breeding purpose in the development of commercial strawberry cultivars. Research on plant defensive responses against fungal pathogens indicate that TLPs are promising candidates for developing better anthracnose resistance in strawberry. Although the TLP gene has been studied in a variety of plants, the composition of the TLP gene family members in octoploid strawberry and their potential defense response to the invasion of pathogenic fungi, particularly C. gloeosporioides, remain largely unexplored. Additionally, many studies on strawberry anthracnose have focused on fungicides used for disease control, but not the molecular resistance mechanisms and engineered plant disease resistance of the octoploid strawberry.
This study aimed to identify the TLP gene family members in octoploid cultivated strawberry (F. × ananassa), and examine their transcription and expression characteristics during pathogenic fungi infection. The purpose of this study was to expand our understanding of the function of strawberry TLP genes in defense responses against C. gloeosporioides.
Materials and Methods
Identification and characterization of TLPs in F. × ananassa
A hidden Markov model (Eddy, 1998) was constructed using HMMER software (version 3.0) based on the TLP sequences of Arabidopsis (https://www.arabidopsis.org) and rice (https://rapdb.dna.affrc.go.jp). It was then used as query sequences to identify homologs in F. × ananassa (https://www.rosaceae.org/organism/24345). We used BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to retrieve TLP sequences for F. × ananassa with an expectation value of e−10. After filtering out the redundant sequences, the aligned sequences were considered candidate sequences for further analysis. The SMART (http://smart.embl-heidelberg.de/) and Pfam (http://xfam.org/) databases were used to verify our identification results, and only the sequence containing the TLP domain (PF00314) was considered the final FaTLP sequence (Ivica, Tobias & Peer, 2012; El-Gebali et al., 2018). We used the ExPASy ProtParam tool (http://web.expasy.org/protparam/) to predict the biophysical properties of the final FaTLP proteins (Gasteiger et al., 2003), including their relative molecular weight (kDa) and theoretical isoelectric point (pI).
Phylogenetic analysis of TLP genes
We used the amino acid sequences of the TLP proteins identified in F. × ananassa, Fragaria vesca, Arabidopsis, and rice in the phylogenetic analysis. One representative sequence was selected from each clade of Arabidopsis and rice TLPs according to the phylogeny of these two species (Shatters et al., 2006; Zhao & Su, 2010), which we then used as reference sequences in our phylogenetic analysis. The F. vesca sequences were obtained from the Genome Database for Rosaceae (GDR; https://www.rosaceae.org/species/fragaria_vesca/genome_v4.0.a1), and the Arabidopsis and Oryza sequences were obtained from The Arabidopsis Information Resource (TAIR) (http://arabidopsis.org) and The Institute for Genomic Research (TIGR) (http://rice.plantbiology.msu.edu/blast.shtml) database respectively. ClustalW (http://www.clustal.org/) (Larkin et al., 2007) was used for multiple sequence alignment. MEGA 6.0 (http://www.megasoftware.net/) (Tamura et al., 2013) was used to perform a phylogenetic analysis of the aligned protein sequences using the neighbor-joining method with 1,000 bootstrap replicates.
Protein motif analysis
The conserved motifs of the TLP proteins in F. × ananassa were predicted using MEME software (http://meme.nbcr.net/meme/, v4.11.0) and the following criteria: minimum motif width of 6, maximum motif width of 200, and maximum number of motifs set at 20.
Chromosome distribution analysis
To determine the physical locations of FaTLP genes, we established the starting positions of all FaTLP genes identified from the F. × ananassa genome database. A diagram of the chromosome locations of FaTLP genes was generated using MG2C (http://mg2c.iask.in/mg2c_v2.0/).
Plant materials
‘Benihoppe’ (susceptible) and ‘Kaorino’ (resistant) strawberry seedlings (Mangandi, Peres & Whitaker, 2015; Han et al., 2019) were cultivated at the Zhejiang Academy of Agricultural Sciences. Experimental plants of the two strawberry cultivars were propagated from runners, rooted in 10-cm diameter pots, and grown in a dedicated room with a 16 h photoperiod. The average daytime temperature was 25.0 ± 1.5 °C while the nighttime temperature was around 18.0 ± 1.5 °C with 75% relative humidity. No fungicides were applied and fertilizer was applied proportionally as needed.
C. gloeosporioides infection
The pathogenic fungus C. gloeosporioides was cultured and provided by the Institute of Plant Protection and Microbiology, Zhejiang Academy of Agricultural Sciences. For the propagation of C. gloeosporioides, we used oatmeal agar (OA) medium and the following steps: we weighed 30 g of oatmeal (Solarbio, https://www.solarbio.com/), boiled it for 1 h, and then strained it using four layers of gauze. We added 17 g of agar to the filtrate, adjusted the volume to 1 L using sterile water, autoclaved it at 121 °C for 15 min, and then divided it into petri dishes following sterilization. After cooling down, the pathogenic fungus was propagated on OA medium and stored for 7–10 days at 28 °C. The propagated spores were washed and collected with sterile water, and the concentration was adjusted to 4 × 106 spores/mL with sterile distilled water. The prepared C. gloeosporioides fungal suspension was used for inoculation. Three hundred plants were divided into three subgroups (control, susceptible, and resistant) with 100 plants each. Sixty-day-old strawberry seedlings were used for fungal inoculation. The strawberry leaves in the susceptible and resistant groups were sprayed with the spore suspension (4 × 106 spores/mL) using an atomizer until dripping. Control plants were similarly inoculated with sterile water. Twenty randomly-selected leaves from each group were sampled at 2, 6, 12, 24, and 48 h, and at 3, 4, 5, 6, and 7 d after inoculation. The samples were immediately frozen in liquid nitrogen and stored at −80 °C for further processing. Three replicates were sampled at each time point.
Transcriptome analysis
To determine the transcriptome profile of different resistant strawberry cultivars in response to C. gloeosporioides, 12 samples were used for RNA-seq analysis with three replicates per treatment: ‘Kaorino’-infected (24 h post-inoculation, hpi), ‘Kaorino’-uninfected, ‘Benihoppe’-infected (24 hpi), and ‘Benihoppe’-uninfected. The RNA-seq transcriptome library was prepared using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA). We used HisAT2 (v2.1.0) (Kim et al., 2019) for sequence alignment and an annotated genome (Fragaria × ananassa Camarosa Genome Assembly v1.0.a1), available from the GDR (https://www.rosaceae.org/organism/24345), as a reference. The fragments per kilobase million (FPKM) value (Malone & Oliver, 2011) was used to identify differentially expressed genes (DEGs) between the two different samples. DESeq2 software (Anders & Huber, 2010) was used for differential expression analysis.
RNA isolation and quantitative RT-PCR (qRT-PCR) analysis
Total RNA was isolated using the modified CTAB method (Chang et al., 2007). The integrity of the RNA samples was examined using a U-0080D Protein nucleic acid spectrophotometer (Hitachi, Tokyo, Japan). cDNA was synthesized from 2 μg of total RNA using TranScript® II One-Step gDNA Removal and cDNA Synthesis SuperMix (TransScript®, Beijing, China). qRT-PCR was then carried out in a LightCycler® 96 real-time PCR system (Roche, Basel, Switzerland) with a DNA Green Master (Roche, Basel, Switzerland). The primers used for the validation of DEGs are shown in Table S5. The Actin gene was used as the reference gene. Each sample was repeated in triplicate.
Statistical analysis
Statistical analysis was carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). The significance level was p < 0.05.
Results
Genome-wide identification of TLP genes in F. × ananassa
To understand the potential roles of TLPs in strawberry, we used cultivated strawberry (F. × ananassa) for genome-wide identification and characterization of TLP genes. We identified a total of 76 TLP gene members from F. × ananassa (designated as ‘FaTLP’), which was a greater amount than the number of TLP genes in many other plant species (De Jesus-Pires et al., 2020). Among the 76 FaTLPs identified, FaTLP68 was the longest (over 4,370 bp), FaTLP5 was the shortest (384 bp), and the mean length was approximately 1,439 bp. The molecular weights of these TLP genes ranged from 13.36 to 138.40 kD, with pI values between 4.27 and 8.77. Most of these genes were 200–400 aa in length with one to two introns and two to three exons, although several genes had over 10 introns/exons. Detailed information on the TLP genes, including their names, coding sequence (CDS) lengths, molecular weights, and pI values, can be found in Table 1.
Table 1. Physical and molecular characteristics of TLP genes in F. × ananassa.
| Gene name | Scaffold location (bp) | Chromosome position |
Subgroup | Length (bp) |
Size (aa) | MW (Da) |
pI | Extron | Intron | |
|---|---|---|---|---|---|---|---|---|---|---|
| FaTLP1 | 7,737,020 | 7,738,607 | Chr1-2 | VII | 1,344 | 310 | 31,952.79 | 4.66 | 3 | 2 |
| FaTLP2 | 24,918,873 | 24,919,901 | Chr2-1 | VI | 1,029 | 342 | 35,319.38 | 4.41 | 1 | 0 |
| FaTLP3 | 6,717,318 | 6,718,064 | Chr3-4 | III | 747 | 248 | 26,625.50 | 8.58 | 1 | 0 |
| FaTLP4 | 1,053,888 | 1,055,785 | Chr5-1 | V | 1,218 | 405 | 43,590.76 | 5.43 | 2 | 1 |
| FaTLP5 | 9,55,336 | 9,55,816 | Chr5-4 | V | 384 | 127 | 13,364.01 | 6.79 | 2 | 1 |
| FaTLP6 | 18,563,346 | 18,565,549 | Chr6-1 | VIII | 1,208 | 332 | 35,850.27 | 8.61 | 2 | 1 |
| FaTLP7 | 33,108,008 | 33,109,182 | Chr6-1 | VIII | 1,033 | 250 | 26,542.12 | 8.27 | 2 | 1 |
| FaTLP8 | 30,176,529 | 30,177,597 | Chr7-1 | VIII | 858 | 285 | 30,512.75 | 7.8 | 3 | 2 |
| FaTLP9 | 31,754,141 | 31,755,199 | Chr7-1 | VIII | 780 | 259 | 27,642.45 | 7.36 | 4 | 3 |
| FaTLP10 | 24,146,688 | 24,154,818 | Chr7-2 | V | 3,289 | 848 | 92,352.63 | 5.06 | 10 | 9 |
| FaTLP11 | 4,809,914 | 4,815,227 | Chr7-4 | V | 3,069 | 792 | 87,475.76 | 5.44 | 9 | 8 |
| FaTLP12 | 21,872,342 | 21,874,277 | Chr1-1 | VI | 1,493 | 295 | 30,430.31 | 4.77 | 3 | 2 |
| FaTLP13 | 21,880,819 | 21,883,234 | Chr1-1 | VII | 1,540 | 409 | 42,778.42 | 6.31 | 5 | 4 |
| FaTLP14 | 7,745,129 | 7,747,037 | Chr1-2 | VI | 1,468 | 295 | 30,413.37 | 4.88 | 3 | 2 |
| FaTLP15 | 6,305,276 | 6,306,911 | Chr1-3 | VII | 1,409 | 314 | 32,383.19 | 4.53 | 3 | 2 |
| FaTLP16 | 6,313,153 | 6,315,086 | Chr1-3 | VI | 1,480 | 295 | 30,476.41 | 5.05 | 3 | 2 |
| FaTLP17 | 5,551,432 | 5,554,101 | Chr1-4 | VII | 1,469 | 393 | 41,477.24 | 5.96 | 5 | 4 |
| FaTLP18 | 5,559,900 | 5,561,737 | Chr1-4 | VI | 1,385 | 296 | 30,473.33 | 4.79 | 3 | 2 |
| FaTLP19 | 17,180,554 | 17,182,275 | Chr2-1 | X | 1,219 | 286 | 30,586.86 | 8.05 | 3 | 2 |
| FaTLP20 | 25,893,167 | 25,894,288 | Chr2-1 | IX | 1,039 | 228 | 23,591.69 | 4.56 | 2 | 1 |
| FaTLP21 | 3,82,116 | 3,84,430 | Chr2-1 | VII | 1,714 | 335 | 34,244.90 | 4.29 | 3 | 2 |
| FaTLP22 | 3,89,384 | 3,92,027 | Chr2-1 | VI | 1,443 | 276 | 29,395.70 | 8.11 | 3 | 2 |
| FaTLP23 | 24,923,609 | 24,925,890 | Chr2-1 | VI | 1,596 | 311 | 32,878.54 | 5.33 | 4 | 3 |
| FaTLP24 | 22,298,735 | 22,301,183 | Chr2-2 | VI | 1,323 | 253 | 26,818.66 | 7.83 | 2 | 1 |
| FaTLP25 | 22,313,583 | 22,315,865 | Chr2-2 | VII | 1,694 | 335 | 34,269.89 | 4.29 | 3 | 2 |
| FaTLP26 | 4,205,420 | 4,209,653 | Chr2-2 | X | 1,452 | 388 | 41,391.17 | 8.57 | 7 | 6 |
| FaTLP27 | 1,581,587 | 1,583,723 | Chr2-3 | X | 1,633 | 286 | 30,503.79 | 8.03 | 3 | 2 |
| FaTLP28 | 22,764,585 | 22,767,122 | Chr2-3 | VI | 1,401 | 276 | 29,459.78 | 7.83 | 3 | 2 |
| FaTLP29 | 22,784,341 | 22,786,568 | Chr2-3 | VII | 1,628 | 340 | 34,734.50 | 4.27 | 3 | 2 |
| FaTLP30 | 2,891,948 | 2,893,222 | Chr2-3 | IX | 1,187 | 255 | 26,322.57 | 4.29 | 2 | 1 |
| FaTLP31 | 4,012,565 | 4,015,252 | Chr2-3 | VI | 2,015 | 372 | 39,197.11 | 4.95 | 3 | 2 |
| FaTLP32 | 25,284,514 | 25,286,825 | Chr2-4 | VI | 1,707 | 373 | 39,232.11 | 4.96 | 3 | 2 |
| FaTLP33 | 25,287,065 | 25,288,842 | Chr2-4 | VI | 819 | 272 | 28,126.24 | 4.28 | 2 | 1 |
| FaTLP34 | 19,300,457 | 19,310,292 | Chr2-4 | X | 1,620 | 429 | 45,971.35 | 8.76 | 9 | 8 |
| FaTLP35 | 5,05,512 | 5,18,409 | Chr2-4 | VI | 2,885 | 319 | 34,141.17 | 8.26 | 5 | 4 |
| FaTLP36 | 10,599,943 | 10,601,571 | Chr5-1 | I | 1,484 | 253 | 27,092.00 | 8.77 | 2 | 1 |
| FaTLP37 | 9,829,098 | 9,830,623 | Chr5-2 | I | 1,400 | 253 | 27,084.96 | 8.77 | 2 | 1 |
| FaTLP38 | 18,257,006 | 18,258,448 | Chr5-3 | I | 1,317 | 253 | 27,088.95 | 8.77 | 2 | 1 |
| FaTLP39 | 24,102,586 | 24,103,807 | Chr5-3 | VIII | 1,076 | 247 | 26,010.34 | 5.16 | 2 | 1 |
| FaTLP40 | 26,365,436 | 26,371,141 | Chr5-3 | V | 2,208 | 735 | 79,611.59 | 6.51 | 3 | 2 |
| FaTLP41 | 9,73,897 | 9,76,302 | Chr5-4 | V | 1,278 | 425 | 46,444.21 | 6.74 | 2 | 1 |
| FaTLP42 | 18,566,292 | 18,567,674 | Chr6-1 | VIII | 1,266 | 237 | 25,411.68 | 5.78 | 2 | 1 |
| FaTLP43 | 24,059,925 | 24,061,244 | Chr6-1 | VIII | 1,016 | 244 | 25,726.88 | 4.46 | 2 | 1 |
| FaTLP44 | 27,284,404 | 27,285,539 | Chr6-1 | II | 1,050 | 244 | 25,699.44 | 7.39 | 2 | 1 |
| FaTLP45 | 3,114,373 | 3,115,524 | Chr6-1 | IV | 1,055 | 259 | 28,086.23 | 8.26 | 2 | 1 |
| FaTLP46 | 32,540,333 | 32,541,588 | Chr6-1 | VIII | 1,114 | 250 | 26,542.12 | 8.27 | 2 | 1 |
| FaTLP47 | 30,036,263 | 30,037,271 | Chr6-2 | IV | 910 | 259 | 28,148.17 | 7.38 | 2 | 1 |
| FaTLP48 | 33,609,425 | 33,610,486 | Chr6-3 | II | 976 | 244 | 25,706.48 | 7.39 | 2 | 1 |
| FaTLP49 | 2,574,069 | 2,574,953 | Chr6-3 | IV | 740 | 244 | 26,497.36 | 7.91 | 3 | 2 |
| FaTLP50 | 63,531 | 64,471 | Chr6-4 | IV | 842 | 262 | 28,599.78 | 8.27 | 2 | 1 |
| FaTLP51 | 11,952,207 | 11,953,479 | Chr6-4 | VIII | 991 | 244 | 25,783.89 | 4.35 | 2 | 1 |
| FaTLP52 | 18,037,077 | 18,040,378 | Chr6-4 | VIII | 1,481 | 247 | 26,227.58 | 4.75 | 2 | 1 |
| FaTLP53 | 9,212,045 | 9,213,306 | Chr6-4 | II | 1,176 | 244 | 25,651.40 | 7.39 | 2 | 1 |
| FaTLP54 | 25,239,035 | 25,240,789 | Chr7-1 | II | 1,053 | 290 | 30,926.20 | 8.03 | 3 | 2 |
| FaTLP55 | 25,879,973 | 25,884,142 | Chr7-1 | V | 2,994 | 658 | 72,264.64 | 5.11 | 8 | 7 |
| FaTLP56 | 25,867,595 | 25,875,423 | Chr7-1 | V | 2,577 | 858 | 94,842.94 | 5.84 | 13 | 12 |
| FaTLP57 | 23,075,906 | 23,077,305 | Chr7-2 | II | 873 | 290 | 30,932.29 | 8.21 | 3 | 2 |
| FaTLP58 | 23,519,637 | 23,522,646 | Chr7-2 | V | 2,045 | 651 | 71,453.60 | 5.25 | 7 | 6 |
| FaTLP59 | 24,128,411 | 24,131,354 | Chr7-2 | V | 1,941 | 646 | 71,288.52 | 5.42 | 8 | 7 |
| FaTLP60 | 29,017,016 | 29,018,415 | Chr7-2 | II | 873 | 290 | 30,886.26 | 8.21 | 3 | 2 |
| FaTLP61 | 1,564,471 | 1,566,282 | Chr7-3 | VIII | 1,621 | 332 | 35,466.06 | 5.00 | 3 | 2 |
| FaTLP62 | 6,391,832 | 6,394,818 | Chr7-3 | V | 2,031 | 661 | 72,913.56 | 5.38 | 8 | 7 |
| FaTLP63 | 7,013,691 | 7,015,141 | Chr7-3 | II | 1,156 | 362 | 39,230.87 | 8.62 | 4 | 3 |
| FaTLP64 | 6,395,823 | 6,402,472 | Chr7-3 | V | 2,787 | 844 | 92,124.41 | 5.85 | 9 | 8 |
| FaTLP65 | 4,87,772 | 4,89,436 | Chr7-4 | VIII | 1,474 | 332 | 35,497.10 | 5.00 | 3 | 2 |
| FaTLP66 | 5,545,850 | 5,547,249 | Chr7-4 | II | 1,089 | 362 | 39,081.68 | 8.42 | 3 | 2 |
| FaTLP67 | 6,225,650 | 6,227,172 | Chr7-4 | II | 996 | 331 | 35,782.12 | 7.45 | 3 | 2 |
| FaTLP68 | 4,792,251 | 4,809,304 | Chr7-4 | V | 4,370 | 1,255 | 1,38,401.93 | 6.19 | 18 | 17 |
| FaTLP69 | 4,018,164 | 4,020,305 | Chr2-3 | VI | 1,134 | 377 | 39,155.99 | 4.62 | 3 | 2 |
| FaTLP70 | 24,864,686 | 24,865,432 | Chr3-2 | III | 747 | 248 | 26,635.50 | 8.58 | 1 | 0 |
| FaTLP71 | 22,604,600 | 22,605,641 | Chr4-2 | VIII | 726 | 241 | 24,694.60 | 4.62 | 2 | 1 |
| FaTLP72 | 29,107,013 | 29,108,091 | Chr4-3 | VIII | 726 | 241 | 24,674.58 | 4.48 | 2 | 1 |
| FaTLP73 | 1,058,206 | 1,058,952 | Chr5-1 | V | 747 | 248 | 26,881.21 | 5.55 | 1 | 0 |
| FaTLP74 | 24,100,394 | 24,101,427 | Chr5-3 | VIII | 921 | 247 | 26,326.74 | 4.75 | 2 | 1 |
| FaTLP75 | 16,618,205 | 16,619,717 | Chr6-2 | VIII | 1,127 | 297 | 32,080.80 | 4.28 | 3 | 2 |
| FaTLP76 | 24,122,820 | 24,126,239 | Chr7-2 | V | 2,392 | 230 | 24,400.62 | 6.70 | 2 | 1 |
Note:
Detailed information on the FaTLP genes, including their names, chromosome position, CDS lengths, molecular weights, and pI values.
Phylogenetic relationships of TLPs in major plant species
To investigate the evolutionary relationship of the TLP gene families, 10 representative Arabidopsis thaliana and Oryza sativa TLPs sequences were used as reference sequences together with F. × ananassa and F. vesca TLPs in the phylogenetic analysis. Similar to the phylogenic results of Arabidopsis and Oryza TLPs (Shatters et al., 2006), the 76 FaTLPs were also classified into 10 phylogenetic groups, Groups I to X (Fig. 1A). A maximum number (16) of FaTLPs were clustered in Group VIII, followed by Group V (15), and these two groups accounted for almost half of the FaTLPs. Groups III and IX contained the least amount of TLP family genes with two genes each. Similarly, most of the F. vesca TLPs were concentrated in Groups VIII and V, and their distribution was more uneven than the FaTLPs.
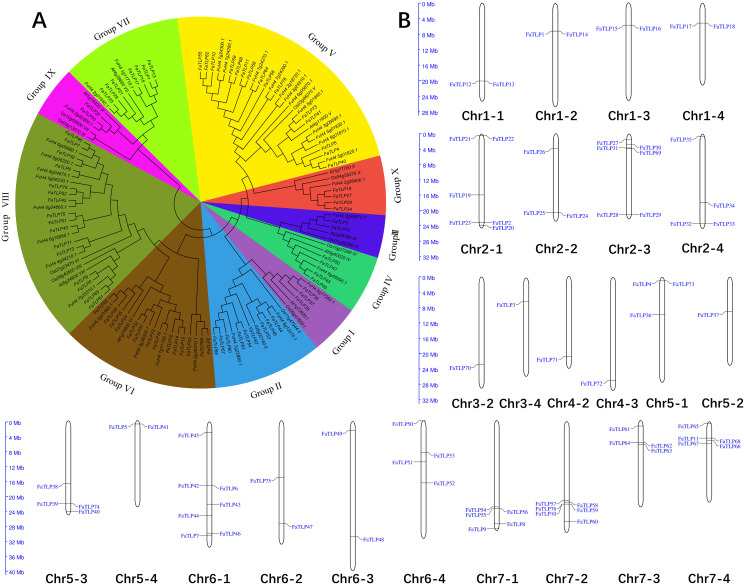
Figure 1. Phylogenetic analysis and chromosome distribution analyses of the TLP gene family.
(A) A phylogenetic tree was constructed for F. × ananassa, F. vesca, Arabidopsis thaliana, and Oryza sativa using MEGA 6.0 with bootstrap values of 1,000. All of the tested TLP genes were divided into 10 groups (Groups I to X), represented by different colors. (B) The distribution of FaTLP on 24 different chromosomes.
Chromosomal distribution of TLP family genes in F. × ananassa
To explore the distribution of TLP family genes on the chromosomes of F. × ananassa, we performed a chromosomal localization analysis. The results showed that the FaTLP members were distributed on 24 (42.9% of 56) F. × ananassa chromosomes (Fig. 1B). The number of FaTLP genes for each chromosome varied significantly. The largest number of genes (seven) was detected on Chr6-1; followed by six genes on Chr2-1, Chr2-3, and Chr7-2; five genes on Chr7-1 and Chr7-4; and the fewest were found on Chr3-2, Chr3-4, Chr4-2, Chr4-3, and Chr5-2 (one per chromosome). Similar chromosomal distribution has been shown in other species, Arabidopsis TLP genes were distributed on seven (29.2% of 24) chromosomes, and there were 25 (56.8% of 44) in rice and 28 (57.1% of 49) in poplar TLP genes (Cao et al., 2016). Additionally, the same paraphyletic group of genes were not distributed on a certain chromosome; that is, all seven genes in Group VII were distributed on seven chromosomes (Chr1-1, Chr1-2, Chr1-3, Chr1-4, Chr2-1, Chr2-2, and Chr2-3), and all nine genes in Group II were distributed on seven chromosomes (Chr6-1, Chr6-3, Chr6-4, Chr7-1, Chr7-2, Chr7-3, and Chr7-4). Segmental duplications might be a major factor that contributed to these distributional properties (Cannon et al., 2004). However, there are a few exceptions. For example, five genes from Group VIII were distributed on the same chromosome (Chr6-1), suggesting that they may come from the tandem duplications (Cao et al., 2016; Faillace et al., 2019). Our results indicated that the expansion and diversification of the TLP gene family in F. × ananassa might be caused by tandem and segmental duplication events. Moreover, the genes were not evenly distributed on a certain chromosome and more genes were distributed at both ends of the chromosome. For example, Chr1-1, Chr2-2, Chr2-3, Chr3-2, Chr4-2, Chr4-3, Chr5-4, Chr6-3, Chr7-1, and Chr7-2 were closer to the ends, while Chr5-2 and Chr6-4 were near the centromere. This character may be due to the distribution of more repeat sequences in the centromere (Wang et al., 2020).
Conserved motifs of TLP genes
The diversity of the motif compositions of TLP genes in F. × ananassa was assessed using MEME software, and a total of 15 conserved motifs were obtained. The distribution of these 15 motifs in the TLPs are shown in Fig. 2. FaTLP10 and FaTLP68 contained all 15 conserved motifs. In addition, four genes (FaTLP55, FaTLP56, FaTLP59, and FaTLP62) contained 14 motifs but did not include motif11. Motif1, 2, 3, 4, 5, 6, 7, 10, 11, and 15 were found in most of the FaTLP genes. Furthermore, conserved motif6 was widely distributed across all 76 FaTLP genes. Motif2 and motif7 were absent only in one gene (FaTLP5), and motif3 was not found in two genes (FaTLP9 and FaTLP33). These 10 conserved motifs were also common across all groups (Group I to X). Moreover, some members of Group V (FaTLP10, FaTLP11, FaTLP55, FaTLP56, FaTLP58, FaTLP59, FaTLP62, FaTLP64, and FaTLP68) shared several unique motifs, namely motif8, 9, 12, 13, and 14. These results suggested that the TLP genes in each group shared several unique motifs and may have certain functional similarities. Moreover, these motifs were relatively conserved, which is why they may be used as markers for the identification of TLP genes and important functional components of the TLP gene family.
Figure 2. The conserved motif analyses of the TLP proteins of F. × ananassa.
(A) The motif compositions were predicted using MEME software, and the 15 conserved motifs are represented by different colors. (B) The sequence logos of all 15 motifs of the FaTLP.
Transcriptome changes in different resistant strawberry cultivars in response to C. gloeosporioides infection
To better understand the transcriptome profile of different resistant strawberry cultivars in response to C. gloeosporioides, RNA-seq analysis was used on 12 samples (‘Kaorino’-infected, ‘Kaorino’-uninfected, ‘Benihoppe’-infected, and ‘Benihoppe’-uninfected) with three replicates per treatment. Approximately 576 million raw reads were obtained, and the clean reads were mapped to the F. × ananassa genome (Table S1). Based on p-adjust < 0.05, and log2FC ≥ 1, a total of 10,462 and 13,682 DEGs were detected in the ‘Kaorino’-infected/uninfected (resistant (R) group) and ‘Benihoppe’-infected/uninfected (susceptible (S) group) leaves, respectively. Additionally, 5,490 (40.12%) genes were downregulated in the S-group compared to 4,765 (45.55%) in the R-group (log2FC ≤ −1) (Fig. S1, Table S2). More genes were upregulated in the S-group (8,192, 59.87%) than in the R-group (5,697, 54.45%) (log2FC ≥ 1) (Fig. S1, Table S3). Based on sequence homology, the DEGs were classified into 48 functional groups belonging to three main GO ontologies: cellular components, molecular functions, and biological processes. Among these DEGs, 2,993 (28.61%) genes in R-group and 3,564 (26.05%) genes in S-group were involved in the GO categories “response to stimulus” (Fig. S2), which contained three subgroups associated with fungal resistance: GO:0050832, GO:0009817, and GO:0009620 (Fig. S3). Six DEGs annotated as TLPs were categorized into these functional subgroups, indicating that these DEGs varied greatly in response to C. gloeosporioides (Table S4). According to our identification of the TLP gene family in octoploid strawberry, the six differentially expressed TLP genes were identified as FaTLP40, FaTLP41, FaTLP43, FaTLP62, FaTLP68, and FaTLP75. The RNA-seq results showed that at 24 hpi, FaTLP40 and FaTLP41 were upregulated and FaTLP75 was downregulated in the resistant group, while FaTLP62 was upregulated in the susceptible group, and FaTLP43 was downregulated and FaTLP68 was upregulated in both groups.
qRT-PCR analysis of TLP genes in response to C. gloeosporioides infection
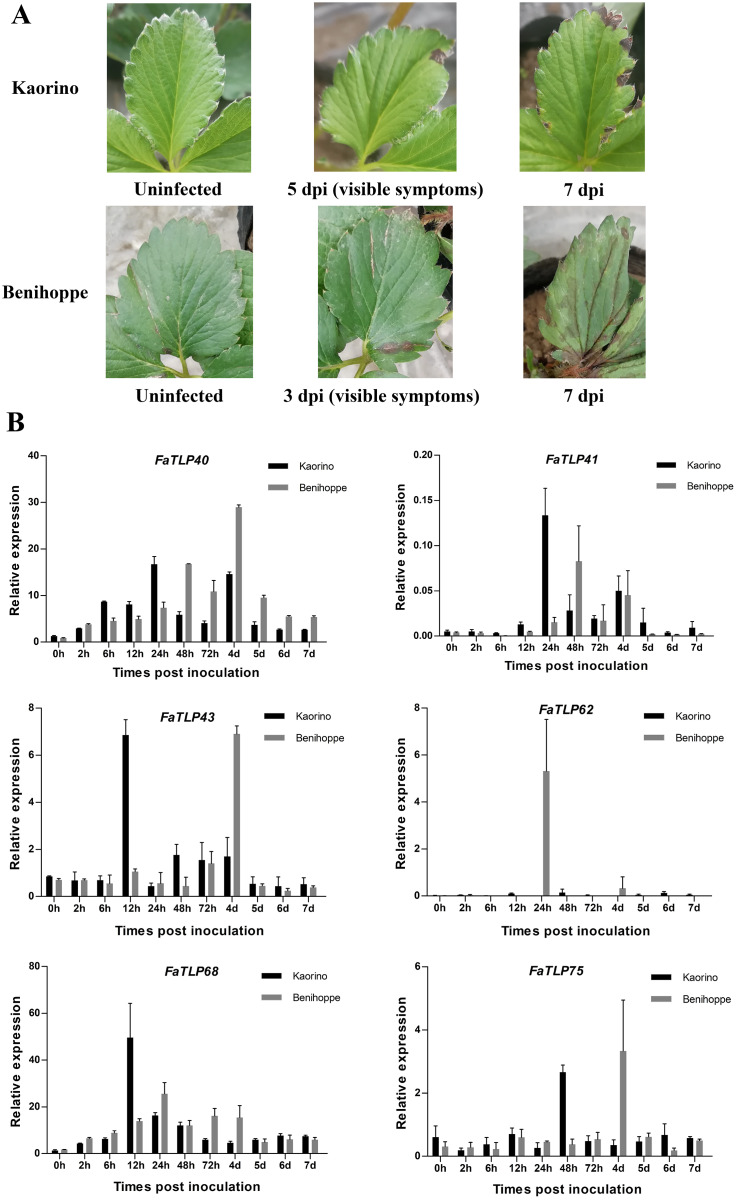
To further understand the roles of TLP genes in strawberry, we investigated the expression profiles of FaTLP genes in F. × ananassa. The qRT-PCR results of these TLP genes in different resistant strawberry cultivars after infection with the fungal pathogen showed a wide range of expression responses (Fig. 3B, File S1). The induction expression of FaTLP68 was significant in the resistant cultivar (‘Kaorino’) at 12 hpi, although it decreased over time, whereas the upregulation of FaTLP68 was detected at 24 hpi in the susceptible cultivar (‘Benihoppe’), and its expression peak appeared later than that of the resistant cultivar. FaTLP40 and FaTLP41 were also highly induced in both strawberry cultivars but there were two expression peaks. This induction increased over time and reached its first peak at 24 hpi and the second peak at 4 d post-inoculation (dpi) in the resistant cultivar, whereas the susceptible cultivar had its first expression peaks of FaTLP40 and FaTLP41 at 48 hpi. The expression of FaTLP62 was considerably upregulated at 24 hpi only in the susceptible cultivar. These results showed a trend similar to the one observed using RNA-seq. Nevertheless, a gradual downregulation was observed for FaTLP43 and FaTLP75 in ‘Kaorino’ after pathogen infection, which then significantly increased at 12 hpi and 48 hpi, respectively, followed by a decrease until 7 dpi. This was not entirely consistent with the results of the transcriptome analysis, indicating that FaTLP43 and FaTLP75 were downregulated at 24 hpi. However, in the susceptible cultivar, the expression patterns of FaTLP43 and FaTLP75 were similar during the initial stage after inoculation but the maximum peak occurred at 4 dpi (1 day after disease symptoms were visible to the naked eye; Fig. 3A). Overall, the qRT-PCR analysis indicated a clear upregulation of the six abovementioned TLP genes following C. gloeosporioides infection in the octoploid strawberry.
Figure 3. Time course of different resistant strawberry leaves and the qRT-PCR results of TLP gene responses to C. gloeosporioides infection.
(A) The progression of symptoms between ‘Kaorino’ and ‘Benihoppe’ after C. gloeosporioides inoculation. Visible symptoms appeared earlier and progressed faster in ‘Benihoppe’. (B) The expression analysis of FaTLP (FaTLP40, FaTLP41, FaTLP43, FaTLP62, FaTLP68, and FaTLP75) in different resistant strawberry cultivars after infection. The x-axis represents the different times post-inoculation. The y-axis represents relative expression. Black and gray sections represent ‘Kaorino’ and ‘Benihoppe’, respectively. The results were normalized against the reference gene and shown as means of three replicates ± SDs.
Discussion
TLPs are a PR protein family that plays a key role in plant defense. In this study, 76 TLP gene members were identified in F. × ananassa, and their characteristics, phylogenetic relationships, motif organization, and chromosomal location were investigated. Using reference sequences from Arabidopsis and Oryza, F. × ananassa TLP genes were clustered into 10 paraphyletic groups, although the distribution of the TLP genes in each group was not uniform. Phylogenetic studies using Arabidopsis, Oryza (Shatters et al., 2006), Populus (Zhao & Su, 2010) and barley (Iqbal et al., 2020) species found that TLPs were also unevenly distributed across the groups, whereas the largest groups contained 11 (36.67%), 5 (23.81), 25 (45.45%), and 7 (36.84%) TLPs, respectively. Previous studies hypothesized that those clades containing the largest gene members would be more active against pathogens or have other exogenous stimuli and more rapid adaptive evolution (Zhao & Su, 2010). In this study, Groups V and VIII were the largest gene clades in our paraphyletic results. Our reference TLP sequence (AT4G11650) from Arabidopsis, annotated as an osmotin-like gene ATOSM34, was found in Group V. Three other PR TLP genes from Arabidopsis (At5g24620) and Oryza (Os07g23470 and Os08g40600) involved in defense response to pathogens (Ascencio-Ibáñez et al., 2008; Liu, Zamani & Ekramoddoullah, 2010) were also clustered in Group V and Group VIII, although their exact functions have not been verified. Osmotin is a TLP belonging to the PR5 family that was originally regarded as a salt-induced protein (Singh et al., 1989), and its antifungal mechanism of action has been studied in detail (Xu et al., 1994; Ibeas et al., 2001; Salzman et al., 2004). ATOSM34 has been reported as a key Arabidopsis gene involved in the defense response to biotic and abiotic stress (Capelli et al., 1997; Mukherjee et al., 2010; Vibhuti et al., 2016; Park & Kim, 2021). We used transcriptome data to identify the six genes encoding TLP proteins associated with plant responses to C. gloeosporioides infection, FaTLP40, FaTLP41, FaTLP43, FaTLP62, FaTLP68, and FaTLP75, which were also clustered in Group V (FaTLP40, FaTLP41, FaTLP62, and FaTLP68) and Group VIII (FaTLP43 and FaTLP75), respectively. These FaTLP genes clustered in the same clade as the reference genes, which have anti-fungal functions, indicating that the TLP genes belonging to the same phylogenetic group may have certain functional similarities.
Further qRT-PCR analysis verified that five of the TLP genes were up-regulated in resistant strawberry cultivars upon C. gloeosporioides infection (Fig. 3). Upregulation or overexpression of TLP genes often results in enhanced antifungal activity against several different pathogenic fungi (Datta et al., 1999; Fagoaga et al., 2001; Kalpana et al., 2006). For example, overexpression of barley TLP-1 in transgenic wheat lines improved pathogen resistance to Fusarium graminearum (Mackintosh et al., 2007). Similarly, increased TLP gene expression in transgenic tobacco plants enhanced resistance to Pythium aphanidermatum and Rhizoctonia solani (Rajam et al., 2007), while overexpression of the TLP gene VaTLP improved downy mildew resistance in Vitis vinifera (He et al., 2017). In turn, significant upregulation of FaPR5-1 and FaPR5-2 was observed in the salicylic acid-primed defense response of octoploid strawberry to Podosphaera aphanis (Feng et al., 2020). Ultimately, these results suggest that FaTLP40, FaTLP41, FaTLP43, FaTLP68, and FaTLP75 may have potential functions in plant resistance responses to C. gloeosporioides.
Our findings demonstrated an initiation of transcriptional responses in strawberry leaves during C. gloeosporioides invasion for some FaTLP genes. Additionally, the expression patterns of these genes revealed that the two different resistant strawberry cultivars differed in their temporal defense responses. FaTLP43 and FaTLP75 activated defense responses much faster in ‘Kaorino’ (<48 hpi) than in the susceptible cultivar ‘Benihoppe’ (4 dpi). Therefore, we hypothesized that during C. gloeosporioides inoculation in strawberry leaves, the spread of the fungus was suppressed because of a early defense response in the resistant cultivar while the pathogen spread out of control in the susceptible cultivar due to the delayed defense response. Similar results were also reported in the response of strawberries to Verticillium dahliae (Besbes, Habegger & Schwab, 2019) and Podosphaera aphanis (Feng et al., 2020) infection, which supports our hypothesis. The early activated defense mechanism of these TLP genes in resistant cultivar such as ‘Kaorino’ needs to be further studied. Our results provide a new strategy for developing anthracnose-resistant strawberry cultivars in the future by promoting early response TLP gene expression with antifungal activity in susceptible cultivars.
Conclusion
In this study, we performed genome-wide identification and characterization of TLPs in octoploid strawberry. A total of 76 TLP genes (FaTLP1–76) were identified using genome-wide screening. Comparative phylogenetic analysis divided the TLPs into 10 groups and the functions of TLPs in F. × ananassa were analyzed. Our qRT-PCR analysis indicated a clear upregulation of five TLP genes in resistant strawberry leaves infected with C. gloeosporioides. Furthermore, our results showed differences in TLP gene expression patterns between two different resistant strawberry cultivars. We concluded that the TLPs’ early activated defense to pathogenic fungi might be a reason why the resistant strawberry cultivar ‘Kaorino’ showed greater anthracnose resistance than the susceptible cultivar ‘Benihoppe’. This study lays the foundation for further exploration of the antifungal function of TLP genes in F. × ananassa, and provides new strategies for improving strawberry resistance to anthracnose through genetic engineering.
Supplemental Information
Based on p-adjust < 0.05, and |log2FC| > 1.
Based on p-adjust < 0.05, and |log2FC| > 1.
Based on p-adjust < 0.05, and |log2FC| > 1.
The Actin gene was used as a reference gene. Each sample was repeated in triplicate.
The x-axis represents the different treatments. The y-axis indicates the number of DEGs. Black and gray sections represent upregulated and downregulated genes, respectively.
The x-axis represents enriched GO processes, and different colors represent different GO processes. The y-axis indicates the total number of genes annotated to each GO process.
The x-axis represents the number of DEGs. The y-axis indicates the three subgroups associated with fungal resistance. Black and gray sections represent ‘Kaorino’-infected/uninfected and ‘Benihoppe’-infected/uninfected, respectively.
Acknowledgments
We thank Dr. Li Fang and Dr. Yunye Xie of the Institute of Plant Protection and Microbiology, Zhejiang Academy of Agricultural Sciences for providing the pathogenic fungus C. gloeosporioides used in this study.
Funding Statement
This work was supported by Science and Technology Program of Zhejiang Province (No. LY20C150003, No. 2021C02066-7, No. 2021XTTGSC02-2, No. 2021SNLF009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Yuchao Zhang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Lixiang Miao performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Xiaofang Yang performed the experiments, prepared figures and/or tables, and approved the final draft.
Guihua Jiang conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data of the RNA-seq experiment is available at NCBI Sequence Read Archive: PRJNA754844.
References
- Acharya et al. (2013).Acharya K, Pal AK, Gulati A, Kumar S, Singh AK, Ahuja PS. Overexpression of Camellia sinensis thaumatin-like protein, CsTLP in potato confers enhanced resistance to Macrophomina phaseolina and Phytophthora infestans infection. Molecular Biotechnology. 2013;54(2):609–622. doi: 10.1007/s12033-012-9603-y. [DOI] [PubMed] [Google Scholar]
- Anders & Huber (2010).Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11(10):106–117. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascencio-Ibáñez et al. (2008).Ascencio-Ibáñez JT, Sozzani R, Lee TJ, Chu TM, Wolfinger RD, Cella R, Hanley-Bowdoin L. Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiology. 2008;148(1):436–454. doi: 10.1104/pp.108.121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besbes, Habegger & Schwab (2019).Besbes F, Habegger R, Schwab W. Induction of PR-10 genes and metabolites in strawberry plants in response to Verticillium dahliae infection. BMC Plant Biology. 2019;19(1):128–144. doi: 10.1186/s12870-019-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon et al. (2004).Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biology. 2004;4(1):10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2016).Cao J, Lv Y, Hou Z, Li X, Ding L. Expansion and evolution of thaumatin-like protein (TLP) gene family in six plants. Plant Growth Regulation. 2016;79(3):299–307. doi: 10.1007/s10725-015-0134-y. [DOI] [Google Scholar]
- Capelli et al. (1997).Capelli N, Diogon N, Greppin H, Simon P. Isolation and characterization of a cDNA clone encoding an osmotin-like protein from Arabidopsis thaliana. Gene. 1997;191(1):51–56. doi: 10.1016/S0378-1119(97)00029-2. [DOI] [PubMed] [Google Scholar]
- Chang et al. (2007).Chang L, Zhang Z, Yang H, Li H, Dai H. Detection of strawberry RNA and DNA viruses by RT-PCR using total nucleic acid as a template. Journal of Phytopathology. 2007;155(7–8):431–436. doi: 10.1111/j.1439-0434.2007.01254.x. [DOI] [Google Scholar]
- Christensen et al. (2002).Christensen AB, Cho BH, Næsby M, Gregersen PL, Brandt J, Madriz-Ordeñana K, Collinge DB, Thordal-Christensen H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Molecular Plant Pathology. 2002;3(3):135–144. doi: 10.1046/j.1364-3703.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- Datta et al. (1999).Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK. Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theoretical and Applied Genetics. 1999;98(6–7):1138–1145. doi: 10.1007/s001220051178. [DOI] [Google Scholar]
- De Freitas et al. (2011).De Freitas CD, Lopes JL, Beltramini LM, De Oliveira RS, Oliveira JT, Ramos MV. Osmotin from Calotropis procera latex: new insights into structure and antifungal properties. Biochimica et Biophysica Acta. 2011;1808(10):2501–2507. doi: 10.1016/j.bbamem.2011.07.014. [DOI] [PubMed] [Google Scholar]
- De Jesus-Pires et al. (2020).De Jesus-Pires C, Ferreira-Neto JRC, Bezerra-Neto JP, Kido EA, de Oliveira Silva RL, Pandolfi V, Wanderley-Nogueira AC, Binneck E, da Costa AF, Pio-Ribeiro G, Pereira-Andrade G, Sittolin IM, Freire-Filho F, Benko-Iseppon AM. Plant thaumatin-like proteins: function, evolution and biotechnological applications. Current Protein and Peptide Science. 2020;21(1):36–51. doi: 10.2174/1389203720666190318164905. [DOI] [PubMed] [Google Scholar]
- Dean et al. (2012).Dean R, Kan JALV, Pretorius ZA, Hammond-Kosack KE, Pietro AD, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology. 2012;13(4):414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy (1998).Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14(9):755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- El-Gebali et al. (2018).El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD. The Pfam protein families database in 2019. Nucleic Acids Research. 2018;47(D1):427–432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagoaga et al. (2001).Fagoaga C, Rodrigo I, Conejero V, Hinarejos C, Tuset JJ, Arnau J, Pina JA, Navarro L, Peña L. Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Molecular Breeding. 2001;7(2):175–185. doi: 10.1023/A:1011358005054. [DOI] [Google Scholar]
- Faillace et al. (2019).Faillace GR, Turchetto-Zolet AC, Guzman FL, Oliveira-Busatto LA, Bodanese-Zanettini MH. Genome-wide analysis and evolution of plant thaumatin-like proteins: a focus on the origin and diversifcation of osmotins. Molecular Genetics and Genomics. 2019;294(5):1137–1157. doi: 10.1007/s00438-019-01554-y. [DOI] [PubMed] [Google Scholar]
- FAO, IFAD, UNICEF, WFP, WHO (2017).FAO, IFAD, UNICEF, WFP, WHO . The state of food security and nutrition in the world 2017. Building resilience for peace and food security. Rome: FAO; 2017. [Google Scholar]
- Feng et al. (2020).Feng J, Zhang M, Yang KN, Zheng CX. Salicylic acid-primed defence response in octoploid strawberry ‘Benihoppe’ leaves induces resistance against Podosphaera aphanis through enhanced accumulation of proanthocyanidins and upregulation of pathogenesis-related genes. BMC Plant Biology. 2020;20(1):149–166. doi: 10.1186/s12870-020-02353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger et al. (2003).Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 2003;31(13):3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier, Potvin & Asselin (2000).Grenier J, Potvin C, Asselin A. Some fungi express b-1, 3-glucanases similar to thaumatin-like proteins. Mycologia. 2000;92(5):841–848. doi: 10.2307/3761579. [DOI] [Google Scholar]
- Hammerschlag et al. (2006).Hammerschlag F, Garcés S, Koch-Dean M, Ray S, Lewers K, Maas J, Smith BJ. In vitro response of strawberry cultivars and regenerants to Colletotrichum acutatum. Plant Cell, Tissue and Organ Culture. 2006;84(3):255–261. doi: 10.1007/s11240-005-9027-5. [DOI] [Google Scholar]
- Han et al. (2019).Han YC, Zeng XG, Xiang FY, Guo C, Zhang QH, Chen FY, Guan W. In vitro evaluation of strawberry germplasm resources for resistance to anthracnose. Scientia Agricultura Sinica. 2019;52(20):3585–3594. doi: 10.3864/j.issn.0578-1752.2019.20.009. (In Chinese) [DOI] [Google Scholar]
- He et al. (2017).He RR, Wu J, Zhang YL, Agüero CB, Li XL, Liu SL, Wang CX, Walker MA, Lu J. Overexpression of a thaumatin-like protein gene from Vitis amurensis improves downy mildew resistance in Vitis vinifera grapevine. Protoplasma. 2017;254(4):1579–1589. doi: 10.1007/s00709-016-1047-y. [DOI] [PubMed] [Google Scholar]
- Hulo et al. (2008).Hulo N, Bairoch A, Bulliard V, Cerutti L, Cuche BA, de Castro E, Lachaize C, Langendijk-Genevaux PS, Sigrist CJA. The 20 years of PROSITE. Nucleic Acids Research. 2008;36(Database):245–249. doi: 10.1093/nar/gkm977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibeas et al. (2001).Ibeas JI, Yun DJ, Damsz B, Narasimhan ML, Uesono Y, Ribas JC, Lee H, Hasegawa PM, Bressan RA, Pardo JM. Resistance to the plant PR-5 protein osmotin in the model fungus Saccharomyces cerevisiae is mediated by the regulatory effects of SSD1 on cell wall composition. Plant Journal. 2001;2(3):271–280. doi: 10.1046/j.1365-313x.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Iqbal et al. (2020).Iqbal I, Tripathi RK, Wilkins O, Singh J. Thaumatin-like protein (TLP) gene family in barley: genome-wide exploration and expression analysis during germination. Genes. 2020;11(9):1080–1093. doi: 10.3390/genes11091080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivica, Tobias & Peer (2012).Ivica L, Tobias D, Peer B. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Research. 2012;40(Database issue):302–305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesús-Pires et al. (2020).Jesús-Pires C, Ferreira-Neto JRC, Bezerra-Neto JP, Kido EA, Oliveira Silva RL, Pandolfi V, Wanderley-Nogueira AC, Binneck E, da Costa AF, Pio-Ribeiro G, Pereira-Andrade G, Sittolin IM, Freire-Filho F, Benko-Iseppon AM. Plant thaumatin-like proteins: function, evolution and biotechnological applications. Current Protein & Peptide Science. 2020;21(1):36–51. doi: 10.2174/1389203720666190318164905. [DOI] [PubMed] [Google Scholar]
- Kalpana et al. (2006).Kalpana K, Maruthasalama S, Rajesha T, Poovannana K, Kumara KK, Kokiladevia E, Raja JAJ, Sudhakar D, Velazhahan R, Samiyappan R, Balasubramanian P. Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Science. 2006;170(2):203–215. doi: 10.1016/j.plantsci.2005.08.002. [DOI] [Google Scholar]
- Kim et al. (2019).Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nature Biotechnology. 2019;37(8):907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin et al. (2007).Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Liu, Zamani & Ekramoddoullah (2010).Liu JJ, Zamani A, Ekramoddoullah AKM. Expression profiling of a complex thaumatin-like protein family in western white pine. Planta. 2010;231:637–651. doi: 10.1007/s00425-009-1068-2. [DOI] [PubMed] [Google Scholar]
- Loon, Rep & Pieterse (2006).Loon LCV, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology. 2006;44(1):135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Mackintosh et al. (2007).Mackintosh CA, Lewis J, Radmer LE, Shin S, Heinen SJ, Smith LA, Wyckoff MN, Dill-Macky R, Evans CK, Kravchenko S, Baldridge GD, Zeyen RJ, Muehlbauer GJ. Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Reports. 2007;26(4):479–488. doi: 10.1007/s00299-006-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone & Oliver (2011).Malone JH, Oliver B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biology. 2011;9(1):34–42. doi: 10.1186/1741-7007-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangandi, Peres & Whitaker (2015).Mangandi J, Peres NA, Whitaker VM. Identifying resistance to crown rot caused by Colletotrichum gloeosporioides in strawberry. Plant Disease. 2015;99(7):954–961. doi: 10.1094/pdis-09-14-0907-re. [DOI] [PubMed] [Google Scholar]
- Mukherjee et al. (2010).Mukherjee AK, Carp MJ, Zuchman R, Ziv T, Horwitz BA, Gepstein S. Proteomics of the response of Arabidopsis thaliana to infection with Alternaria brassicicola. Journal of Proteomics. 2010;73(4):709–720. doi: 10.1016/j.jprot.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Narasimhan et al. (2003).Narasimhan ML, Lee H, Damsz B, Singh NK, Ibeas JI, Matsumoto TK, Woloshuk CP, Bressan RA. Overexpression of a cell wall glycoprotein in Fusarium oxysporum increases virulence and resistance to a plant PR-5 protein. Plant Journal. 2003;36(3):390–400. doi: 10.1046/j.1365-313X.2003.01886.x. [DOI] [PubMed] [Google Scholar]
- Osmond et al. (2001).Osmond RIW, Hrmova M, Fontaine F, Imberty A, Fincher GB. Binding interactions between barley thaumatin-like proteins and (1, 3)-ß-D-glucans. Kinetics, specificity, structural analysis and biological implications. European Journal of Biochemistry. 2001;268(15):4190–4199. doi: 10.1046/j.1432-1327.2001.02331.x/abs. [DOI] [PubMed] [Google Scholar]
- O’Leary, Poulis & von Aderkas (2007).O’Leary SJB, Poulis BAD, von Aderkas P. Identification of two thaumatin-like proteins (TLPs) in the pollination drop of hybrid yew that may play a role in pathogen defence during pollen collection. Tree Physiology. 2007;27(12):1649–1659. doi: 10.1093/treephys/27.12.1649. [DOI] [PubMed] [Google Scholar]
- Park & Kim (2021).Park EJ, Kim TH. Arabidopsis OSMOTIN 34 functions in the ABA signaling pathway and is regulated by proteolysis. International Journal of Molecular Medicine. 2021;22(15):7915–7924. doi: 10.3390/ijms22157915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre et al. (2011).Petre B, Major I, Rouhier N, Duplessis S. Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biology. 2011;11(1):33–48. doi: 10.1186/1471-2229-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajam et al. (2007).Rajam MV, Chandola N, Goud PS, Singh D, Kashyap V, Choudhary ML, Sihachakr D. Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biologia Plantarum. 2007;51(1):135–141. doi: 10.1007/s10535-007-0026-8. [DOI] [Google Scholar]
- Rather et al. (2015).Rather IA, Awasthi P, Mahajan V, Bedi YS, Vishwakarma RA, Gandhi SG. Molecular cloning and functional characterization of an antifungal PR-5 protein from Ocimum basilicum. Gene. 2015;558(1):143–151. doi: 10.1016/j.gene.2014.12.055. [DOI] [PubMed] [Google Scholar]
- Roberts & Selitrennikoff (1990).Roberts WK, Selitrennikoff CP. Zeamatin, an antifungal protein from maize with membrane-permeabilizing activity. Journal of General Microbiology. 1990;136(9):1771–1778. doi: 10.1099/00221287-136-9-1771. [DOI] [Google Scholar]
- Rout, Nanda & Joshi (2016).Rout E, Nanda S, Joshi RK. Molecular characterization and heterologous expression of a pathogen induced PR5 gene from garlic (Allium sativum L.) conferring enhanced resistance to necrotrophic fungi. European Journal of Plant Pathology. 2016;144(2):345–360. doi: 10.1007/s10658-015-0772-y. [DOI] [Google Scholar]
- Salzman et al. (2004).Salzman RA, Koiwa H, Ibeas JI, Pardo JM, Hasegawa PM, Bressan RA. Inorganic cations mediate plant PR5 protein antifungal activity through fungal Mnn1- and Mnn4-regulated cell surface glycans. Molecular Plant-Microbe Interactions. 2004;17(7):780–788. doi: 10.1094/MPMI.2004.17.7.780. [DOI] [PubMed] [Google Scholar]
- Salzman et al. (1998).Salzman RA, Tikhonova I, Bordelon BP, Hasegawa PM, Bressan RA. Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiology. 1998;117(2):465–472. doi: 10.1104/pp.117.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo et al. (2008).Seo PJ, Lee AK, Xiang F, Park CM. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant and Cell Physiology. 2008;49(3):334–344. doi: 10.1093/pcp/pcn011. [DOI] [PubMed] [Google Scholar]
- Shatters et al. (2006).Shatters RG, Boykin LM, Lapointe SL, Hunter WB, Weathersbee AA. Phylogenetic and structural relationships of the PR5 gene family reveal an ancient multigene family conserved in plants and select animal taxa. Journal of Molecular Evolution. 2006;63(1):12–29. doi: 10.1007/s00239-005-0053-z. [DOI] [PubMed] [Google Scholar]
- Singh et al. (2013).Singh NK, Kumar KRR, Kumar D, Shukla P, Kirti PB. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachisdiogoi, a wild peanut. PLOS ONE. 2013;8(12):e83963. doi: 10.1371/journal.pone.0083963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh et al. (1989).Singh NK, Nelson DE, Kuhn D, Hasegawa PM, Bressan PA. Molecular cloning of osmotin and regulation of its expression by ABA and adaptation to low water potential. Plant Physiology. 1989;90:1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2013).Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazhahan, Datta & Muthukrishnan (1999).Velazhahan R, Datta SK, Muthukrishnan S. Pathogenesis-Related Proteins in Plants. Boca Raton, FL, USA: CRC Press; 1999. The PR-5 family: thaumatin-like proteins; pp. 107–129. [Google Scholar]
- Vibhuti et al. (2016).Vibhuti M, Kumar A, Sheoran N, Nadakkakath AV, Eapen SJ. Molecular basis of endophytic Bacillus megaterium-induced growth promotion in Arabidopsis thaliana: Revelationby microarray-based gene expression analysis. Journal of Plant Growth Regulation. 2016;36(1):118–130. doi: 10.1007/s00344-016-9624-z. [DOI] [Google Scholar]
- Wang et al. (2020).Wang T, Hu JJ, Ma X, Li CJ, Yang QH, Feng SY, Li MM, Li N, Song XM. Identification, evolution and expression analyses of whole genome-wide TLP gene family in Brassica napus. BMC Genomics. 2020;21(1):264–277. doi: 10.1186/s12864-020-6678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wel & Loeve (1972).Wel H, Loeve K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. European Journal of Biochemistry. 1972;31(2):221–225. doi: 10.1111/j.1432-1033.1972.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Woloshuk et al. (1991).Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, Van den Elzen PJ, Cornelissen BJ. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991;3(6):619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (1994).Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6(8):1077–1085. doi: 10.2307/3869886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie, Melanson & Murphy (2002).Zareie R, Melanson DL, Murphy PJ. Isolation of fungal cell wall degrading proteins from barley (Hordeum vulgare L.) leaves infected with Rhynchosporium secalis. Molecular Plant-Microbe Interactions. 2002;15(10):1031–1039. doi: 10.1094/MPMI.2002.15.10.1031. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang Y, Wang G, Dong JQ, Zhong C, Chang L, Zhang H. The current progress in strawberry breeding in China. VIII International Strawberry Symposium; 2017. pp. 7–12. [Google Scholar]
- Zhao & Su (2010).Zhao JP, Su XH. Patterns of molecular evolution and predicted function in thaumatin-like proteins of Populus trichocarpa. Planta. 2010;232(4):949–962. doi: 10.1007/s00425-010-1218-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Based on p-adjust < 0.05, and |log2FC| > 1.
Based on p-adjust < 0.05, and |log2FC| > 1.
Based on p-adjust < 0.05, and |log2FC| > 1.
The Actin gene was used as a reference gene. Each sample was repeated in triplicate.
The x-axis represents the different treatments. The y-axis indicates the number of DEGs. Black and gray sections represent upregulated and downregulated genes, respectively.
The x-axis represents enriched GO processes, and different colors represent different GO processes. The y-axis indicates the total number of genes annotated to each GO process.
The x-axis represents the number of DEGs. The y-axis indicates the three subgroups associated with fungal resistance. Black and gray sections represent ‘Kaorino’-infected/uninfected and ‘Benihoppe’-infected/uninfected, respectively.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data of the RNA-seq experiment is available at NCBI Sequence Read Archive: PRJNA754844.