Abstract
Background: Renovascular hypertension elicits cardiac damage and remodeling. Two-kidney, one-clip (2K1C) is an experimental model used to study hypertension pathophysiology. In this model, the renin-angiotensin-system (RAS) is overactive due to renal artery stenosis, leading to cardiac remodeling. Redox mechanisms underlying RAS activation mediate hypertension-induced cardiovascular damage. Preclinical studies and clinical trials demonstrated resveratrol’s protective effects in cardiovascular diseases, mainly attributed to its antioxidant properties. We hypothesized resveratrol alone or in combination with an angiotensin-converting enzyme (ACE) inhibitor would be beneficial against cardiac damage caused by renovascular hypertension. Objective: We investigated the benefits of resveratrol against cardiac remodeling in 2K1C rats compared with captopril. Methods: Male Wistar rats underwent unilateral renal stenosis – 2K1C Goldblatt model of hypertension. Systolic Blood Pressure (SBP) was measured before and 6 weeks after surgery. Hypertensive 2K1C rats presented SBP≥160 mmHg. From the 6th week after the surgery, the animals received oral resveratrol (20 mg/kg), captopril (12 mg/kg), or their combination for 3 times per week for 3 weeks. Whole heart hypertrophy was evaluated. Histological assays assessed left ventricle hypertrophy and fibrosis. Results: Renovascular hypertension caused cardiac hypertrophy, accompanied by increased myocyte diameter and collagen deposition. Resveratrol reduced 2K1C rats’ SBP and whole heart hypertrophy, independently of captopril. Resveratrol caused a higher reduction in ventricular hypertrophy than captopril. Collagen deposition was greater reduced by 2K1C treated only with resveratrol than with captopril alone or combined with resveratrol. Conclusion: Independent of captopril, resveratrol prompts cardioprotective effects on cardiomyocyte remodeling and fibrosis resulting from renovascular hypertension in 2K1C rats.
Keywords: Resveratrol, Oxidative Stress, Cardiac Remodeling and Fibrosis, Renovascular Hypertension, Renin-Angiotensin System (RAS), Captopril
Introduction
Cardiac remodeling results from genome expression, molecular, cellular, and interstitial modification with increased collagen deposition and also manifests after heart injuries due to underlying diseases, such as hypertension [1,2]. Approximately 30% of the global population are expected to have hypertension by 2025 [3] and may consequently be at risk for the development of cardiomyocyte hypertrophy [1,2].
Hypertension-induced cardiac remodeling is an initial adaptive/compensatory response to an increased left ventricle wall stress [2,4-7]. This can be caused by augmented hemodynamic load and neurohormonal activation, such as the renin-angiotensin system (RAS) [8].
RAS can be activated under reduced kidney blood flow and regulates systemic blood pressure, fluid, and electrolyte balance [9,10]. Overactivation of RAS leads to inflammation, endothelial dysfunction, microvascular damage, high blood pressure, and heart remodeling/hypertrophy [6,9,10]. Renovascular disease accounts for 1 to 5% of all hypertension cases in the general population [11,12]. Renal artery stenosis is the primary cause of hypertension, and it is associated with increased systemic levels of Angiotensin II (AngII). AngII, produced by the action of the angiotensin-converting enzyme (ACE), is the primary effector molecule of the RAS [9-12].
The Goldblatt two kidney, one-clip (2K1C) is an experimental model of hypertension [13], resulting from reduced renal blood perfusion. 2K1C hypertensive rats are widely used to investigate aspects of persistent increased systemic blood pressure affecting patients with renovascular hypertension [14,15], including the consequences of overactivation of the RAS pathways [16]. The heart from 2K1C animals presents augmented left ventricular mass [13,14,17].
In the RAS-dependent phase of the 2K1C model of hypertension [17], reactive oxygen species (ROS) are massively produced, contributing to the genesis and progression of cardiac remodeling [18,19]. ACE inhibitors effectively prevent cardiac remodeling and/or fibrosis/hypertrophy in rats with renovascular hypertension [20-22]. Other works have shown cardioprotective effects of captopril in different animal models developing heart disease [23,24].
AngII-mediated cardiovascular inflammatory and hypertrophic responses seem to depend on increased ROS production during hypertension progression [25-27]. Clinical and experimental studies establish significant benefits from the pharmacological inhibition of RAS [16,28]. ACE inhibitors are part of the arsenal to treat cardiovascular diseases (CVD) and their underlying conditions [28]. Although ACE inhibitors are among the first choices to treat hypertension and heart failure, this approach has limitations in clinical practice [28], particularly in specific patient groups, such as the Black population [29] and patients with reduced renal function [30].
Oxidative stress is an essential pathomechanism of cardiovascular risk factors and heart disease, so alternative approaches that counteract the consequences of oxidative stress are of therapeutic interest [31]. The most prominent ROS involved in the pathogenesis of renal disease are superoxide (O2̇−), hydroxyl radical (·OH), H2O2, nitric oxide (·NO), and peroxynitrite (ONOO−) in the kidney [32]. In renovascular hypertension, O2̇− is the more common oxidative agent [33,34].
Resveratrol antioxidant mechanisms have been extensively demonstrated as related with those ROS involved in the pathogenesis of renal disease [35-38]. Its pharmacological effects are widely reported in clinical and animal experimental studies [39,40]. Resveratrol produced antihypertensive effects in rats with partial nephrectomy-induced cardiac hypertrophy [41]. The protective benefits of resveratrol are shown to be mediated by different pathways, including the lowering of oxidative stress and inflammation [42].
Our group had shown the benefits of resveratrol (orally administered) on reducing systolic blood pressure (SBP) levels in 2K1C rats and reducing ROS from their aorta [43]. We demonstrated that in synergy with captopril, resveratrol promoted protective effects against vascular remodeling caused by 2K1C hypertension [44]. The cardiac benefits of resveratrol against damage caused by renal hypertension have not been reported.
We hypothesized that resveratrol would protect the heart from renovascular hypertensive rats against cardiac fibrosis and remodeling. In this sense, we aimed to evaluate the impact of resveratrol on fibrosis and cardiac remodeling in 2K1C rats compared to the effects of an ACE inhibitor (captopril).
Materials and Methods
Animals
Male Wistar rats (180-220g or ~10-12 weeks of age) were used. All experimental procedures were approved by the Institutional Ethics Committee in Research (protocol no. 007/2010) that follows the national [45] and international [46] regulations and guidelines for ethical conduct in the care and use of animals in research. The animals were kept under a 12h light: 12h darkness-cycle and fed with regular chow and water ad libitum.
Surgical Procedures to Obtain Hypertensive (2K-1C) and Normotensive (2K) Rats
The animals were anesthetized with 0.1 mL (100 µL)/100 g of body weight, via i.p., with the mixture 0.2 mL ketamine + 0.1 mL xylazine and underwent a surgical procedure to induce renovascular hypertension according to the 2K1C Goldblatt model as previously described [13,43,44]. Briefly, for unilateral renal stenosis, the rats underwent a laparotomy. Their left kidney was exposed through a small flank incision and externalized. The left renal artery was individualized over a short segment by blunt dissection. A silver clip (0.2 mm of internal diameter) was placed around it. The normotensive (two-kidney, 2K) group comprised sham-operated rats that underwent laparotomy to manipulate the left renal artery without clipping it. At the end of the surgery, both groups received a single dose of the antibiotic oxytetracycline (0.2 g/Kg, im).
Blood Pressure Measurements and Treatments
Animals (N=59) had their systolic blood pressure (SBP) measured by indirect tail-cuff plethysmography as a baseline before the surgical procedure. Six weeks after the surgery, SBP was measured. 2K1C rats were considered hypertensive when their SBP was higher than 160 mmHg [43,44]. Then 2K1C (N=30) and 2K (N=29) rats were randomly assigned to receive the active compounds (treated group) or vehicle (control group) 3 times a week for 3 weeks. The hypertensive disease resultant from 2K1C model comprises three distinct phases [17]. Because we aim to study aspects associated with the RAS pathways, the treatments were initiated on the 6th week after the surgery, representing the end of the first stage of the RAS overactivation.
Treated groups were administered by gavage [43,44], resveratrol 20 mg/Kg (RESV, 2K1C: N=8; 2K: N=7), captopril 12 mg/Kg (CAP, 2K1C: N=8; 2K: N=5), or captopril 12 mg/Kg combined with resveratrol 20 mg/Kg (CAP+RESV, 2K1C: N=9; 2K: N=8). Control groups 2K1C: N=8; 2K: N=6) received, by gavage, one mL of the resveratrol’s vehicle (deionized water + 0.05% Tween-80) [43,44].
After 3 weeks of treatments, which was at the end of the 9th week after surgery, SBP was measured again.
Heart Isolation
At the end of the treatments (9th week), rats were administrated, intraperitoneally, 0.15 mL per 100 g of body weight of a mixture of 0.2 mL ketamine + 0.1 mL xylazine (2:1). The effectiveness of deep anesthesia was confirmed by a lack of paw pinch and eye-blink reflexes. Death was assured by decapitation and exsanguination. The animals (N=59) from all groups underwent the same procedure. All protocols were approved by the Institutional Animal Care and Use Committee and follow the “Guide for the Care and Use of Laboratory Animals,” 8th edition (2011) and ARRIVE guidelines [45,46]. The whole hearts were harvested, washed out, and weighed, as previously described [44], to perform the subsequent protocols.
Cardiac Hypertrophy Measurements
Cardiac hypertrophy was assessed based upon the ratio between whole heart weight (HW) and body weight (BW) expressed in mg/g.
Morphology and Morphometry of Cardiomyocytes
After weighing, the hearts were cut transversely and fixed in phosphate-buffered 10% formalin for 24 hours. The hearts were embedded into paraffin for histological processing, and each block was serially cut with 5 mm thickness. The sections were stained with Hematoxylin and Eosin (H&E) and picrosirius red. For morphometric analysis, the minor diameter of myocytes of the left ventricle was measured in the H&E stained tissue sections. The diameter was estimated as the distance between the cytoplasm, crossing the nucleus perpendicularly, only in the fibers sectioned longitudinally. The collagen deposition was measured as the percentage of picrosirius red. The diameter of each fiber and the collagen deposition were blindly assessed as the percentage of left ventricle Sirius red labeling (red) in 30 high-power randomized fields (HPF; x40 magnification). The mean value was obtained from each heart, 30 fields per region per rat randomly selected using Image J software (Image J, 1.33u, NIH, USA). Thus, collagen surface % is expressed as high-power fields (HPF).
Drugs
Resveratrol (3,4′,5-Trihydroxy-trans-stilbene), captopril, and Tween-80 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Resveratrol was dissolved in deionized water with 0.05% Tween-80. All of the drugs and vehicles were prepared on the day of the respective treatment. Ketamine chloride was purchased from Agener (Sao Paulo – SP, Brazil) and Xylazine hydrochloride was purchased from Hertape Calier (Juatuba – MG, Brazil). Isoflurane was purchased from Baxter Healthcare Co. Oxytetracycline was purchased from Pfizer (Guarulhos – SP, Brazil).
Statistical Analysis
Data, described in the bars from the respective figures, are expressed as means±SEM and were analyzed by the One-way analysis of variance (ANOVA) followed by Newman-Keuls post-hoc test or unpaired Student t-test. The values are reported in the bars in the respective figures. The significance level considered in all tests was P<0.05 (IC95). Graphical design and statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
Results
Surgical Procedure to Establish Renovascular (2K1C) Hypertension was Effective
The results depicted at the Figure 1A (indicated as means±SEM of mmHg) confirmed the effectiveness of surgical procedures in obtaining 2K (normotensive) and 2K1C (hypertensive) groups. Before the surgical procedure, naïve rats presented normal SBP. No differences were observed between SBP from naïve vs 2K rats. Six weeks after the surgery, before initiating the treatments, rats that underwent unilateral renal stenosis had highest (P<0.0001) SBP (2K1C: 186+12.0mmHg, N=29) compared to sham-operated (2K: 124+2.0mmHg, N=69) and naïve rats (125+4.0mmHg, N=30).
Figure 1.
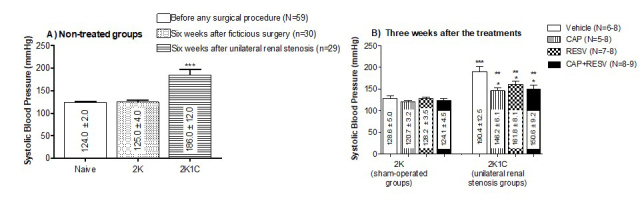
Systolic blood pressure (SBP, mmHg) measured by indirect tail-cuff plethysmography. Bars represent means±SEM for the number (N) of animals indicated in the parentheses. Data were analyzed through One-way ANOVA, Newman-Keuls post hoc. A: SBP (mmHg) measured in non-treated animals before any surgical procedures (naïve group) and 6 weeks after fictitious surgery (2K group) or after the left renal artery stenosis (2K1C group). *** Difference (P<0.0001) between 2K1C rats and the other groups (naïve and 2K). B: SBP measured 3 weeks after treatments. *** Difference (P<0.0001) between 2K1C vehicle-treated group vs all the 2K groups of treatment. ** Difference (P<0.01) between 2K1C groups treated with CAP, RESV, or CAP+RESV vs 2K1C vehicle-treated group. * Difference (P<0.05) between 2K1C group treated with CAP, RESV, or CAP+RESV vs all the 2K groups.
2K1C Rats had their SBP Reduced by Resveratrol Independently of Co-Treatment with Captopril
The treatments were initiated in the 6th week after the surgery, and 3 weeks later, the rats had their SBP measured (indicated as means±SEM of mmHg). None of the treatments affected the SBP in 2K rats (vehicle:128.6+5.0mmHg; CAP:120.7+3.2mmHg; RESV: 128.2+3.5mmHg; CAP+RESV: 124.1+4.5mmHg, P>0.05, N=6-8; Figure 1B).
It is noteworthy that there is no difference between the SBP of the 2K1C rats treated for 3 weeks with the vehicle (190+12.5mmHg; Figure 1B) and the 2K1C animals before initiating any of the treatments, (186+12.0mmHg; Figure 1A).
Three weeks after treatments, 2K1C rats treated with vehicle exhibited SBP (190+12.5mmHg; Figure 1B) significantly higher than all the 2K groups (P<0.0001).
Although the treatments did not normalize the high SBP in 2K1C rats to levels of the normotensive control – 2K groups (P<0.05; Figure 1B), the treatment of the hypertensive rats with CAP (146.4+6.1mmHg) RESV (161.8+8.1mmHg) as well as with the combination (CAP+RES: 150.6+9.2mmHg) significantly reduced the SBP in comparison with 2K1C rats treated with vehicle (P<0.01, N=8-9; Figure 1B).
Resveratrol Reduces Cardiac Hypertrophy Independently of Captopril in 2K1C Rats
The ratios between whole heart weight (HW) and body weight (BW) were assessed to investigate the effect of CAP, RESV, or the combined treatments on the cardiac hypertrophy triggered by renovascular hypertension (values are expressed as heart weight (mg)/ body weight(mg) as means±SEM; Figure 2). 2K1C rats treated with vehicle had significantly more cardiac hypertrophy (3.41±0.1) when compared with all the other groups, either 2K (vehicle:2.57±0.1; CAP:2.49±0.1; RESV:2.31±0.1; CAP+RESV:2.58±0.1) or 2K1C treated with drugs (CAP: 2.68±0.1; RESV: 2.44±0.1; CAP+RESV: 2K1C:2.41±0.1) (N=6-9, P<0.0001; Figure 2).
Figure 2.
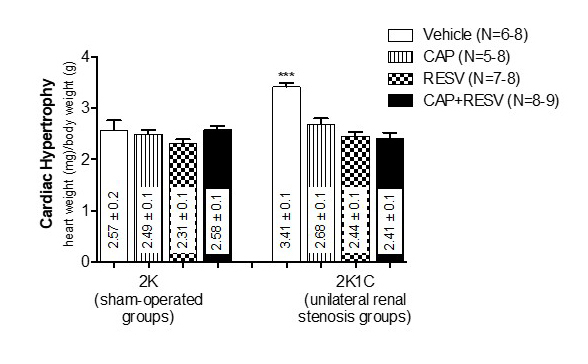
Cardiac hypertrophy expressed by the ratio between the whole heart weight (HW, mg) and body weight (BW, g). Bars represent means±SEM for the number (N) of animals indicated in the parentheses. Data were analyzed through One-way ANOVA, Newman-Keuls post hoc. *** Difference (P<0.0001) between vehicle-treated 2K1C rats and the rats from all the 2K groups or the 2K1C rats treated with CAP, RESV, or CAP+RESV.
Treatment with CAP, RESV, or CAP+RESV similarly (P<0.05) blunted the cardiac hypertrophy in 2K1C rats. There were no differences among 2K animals (P>0.05) treated with vehicle, CAP, RESV, and CAP+RESV.
Resveratrol Produces a More Significant Reduction in Cardiomyocyte Diameter from 2K1C Rats than Captopril
Ventricular hypertrophy measurements were performed in H&E stained left ventricular myocyte tissue sections to evaluate cardiac remodeling (myocyte diameters are indicated as means±SEM µm; Figure 3).
Figure 3.
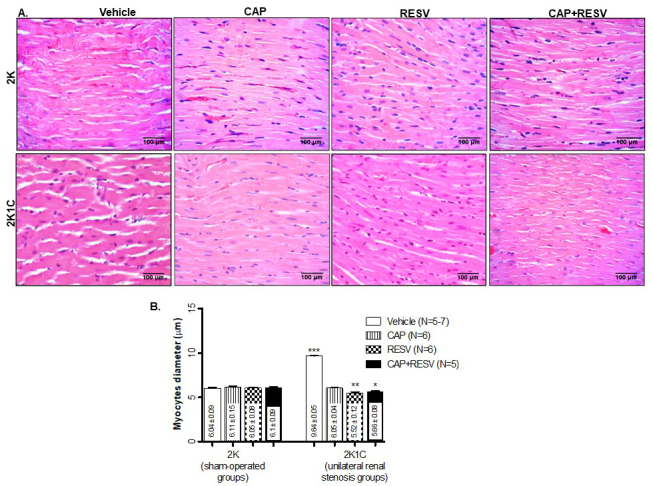
Morphometric analysis of the left ventricular myocyte’s diameters from 2K and 2K1C treated with vehicle, RESV (20 mg/Kg), CAP (12 mg/Kg), and CAP (12 mg/Kg) + RESV (20 mg/Kg). A: Representative images of H&E-stained sections of left ventricle heart tissues. B: Quantitative values are expressed as µm of thickness; bars represent means±SEM for the number (N) of animals indicated in the parentheses. Data were analyzed through One-way ANOVA, Newman-Keuls post hoc. *** Difference (P<0.0001) between 2K1C vehicle-treated group vs all the 2K groups or vs the 2K1C groups treated with CAP, RESV, or CAP+RESV. ** Difference (P<0.01) between 2K1C treated with RESV vs the 2K1C CAP-treated group. * Difference (P<0.05) between 2K1C treated with CAP+RESV vs 2K1C treated with CAP.
Myocyte thickness in all the 2K groups (N=5-6) was not different regardless of treatment (vehicle:6.04±0.09; CAP:6.11±0.15; RESV:6.05±0.08; CAP+RESV:6.1±0.09). The treatments with CAP and RESV combined (5.66±0.08), or isolated (CAP:6.05±0.04; RESV: 5.52±0.12) prevented myocyte thickening in 2K1C groups (N=5-7) because their results are similar (P>0.05) to all the sham (2K) groups. Without any drug treatments (CAP, RESV, or the combination), the hypertensive animals (2K1C vehicle) presented with the highest increase of the myocytes thickness (9.64±0.06, P<0.0001; Figure 3).
Interestingly, a significantly higher reduction in ventricular hypertrophy was observed in myocytes from 2K1C rats treated with RESV (5.52±0.12, P<0.01; Figure 3) or CAP+RESV (5.66±0.08, P<0.05; Figure 3) compared to the hypertensive rats treated only with CAP (6.05±0.04).
Resveratrol Causes the Highest Reduction in the Collagen Deposition in Ventricular Cardiomyocytes from 2K1C Rats
Left ventricular cardiomyocyte tissue sections were stained with picrosirius red to study the collagen deposition and, consequently, fibrosis. Results are indicated in Figure 4, panels A and B depict images of Picrosirius-Red-stained sections under direct microscopy and polarized light, respectively. Panel C: bar graphs express the means±SEM %HPF.
Figure 4.
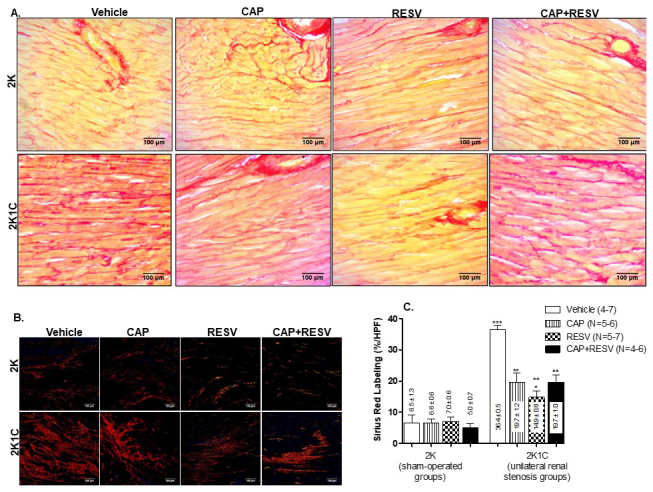
Percentage of collagen deposition of the left ventricle from 2K and 2K1C treated with vehicle, RESV (20 mg/Kg), CAP (12 mg/Kg) and CAP (12 mg/Kg) + RESV (20 mg/Kg). A: Representative images of Picrosirius-Red-stained sections of left ventricular heart tissue under direct microscopy and B: Polarized light. C: Quantification of left ventricle picrosirius red labeling expressed as collagen surface (% high-power fields- HPF); bars represent means±SEM for the number (N) of animals indicated in the parentheses. Data were analyzed through One-way ANOVA, Newman-Keuls post hoc. *** Difference (P<0.0001) between 2K1C vehicle-treated group and all the other groups. ** Difference (P<0.01) between 2K1C groups treated with RESV, CAP, or CAP+RESV vs and all the 2K groups. * Difference (P<0.05) between 2K1C groups treated with RESV vs 2K1C groups treated with CAP or CAP+ RESV.
The picrosirius red labeling was not different among any of the 2K groups, indicating that the treatments did not affect collagen deposition in normotensive rats (Figure 4C). Nonetheless, renovascular hypertension significantly increased (P<0.001; Figure 4C) collagen deposition in the left ventricle from 2K1C rats treated with vehicle (36.4±0.5) compared to all the treated 2K groups (vehicle:6.5±1.35; CAP:6.6±0.6; RESV:7.0±0.6; CAP+RESV:5.0±0.7).
Compared with 2K1C rats treated with vehicle (36.4±0.5), treatment of the 2K1C groups with CAP (19.7±1.2), RESV (14.9±0.8) or CAP+RESV (19.7±1.0) significantly reduced collagen deposition (P<0.0001, N=6-7; Figure 4C).
In comparison with 2K1C rats treated with vehicle, RESV or CAP isolated, as well as CAP+RESV, effectively reduced the picrosirius red labeling induced by renal artery stenosis (P<0.0001; Figure 4C) without normalizing it to the level of 2K groups (P<0.01; Figure 4C). There was no difference between the picrosirius red labeling in cardiomyocytes from 2K1C rats treated with CAP (19.7±1.2) vs. 2K1C rats treated with CAP+RESV (19.7±1.0). Nevertheless, it is noticeable that the 2K1C rats treated only with RESV (14.9±0.8) had a more substantial reduction of cardiac collagen deposition than the 2K1C groups treated with CAP alone or CAP+RESV (P<0.05; Figure 4C).
Discussion
This study’s most significant new finding is that in an experimental model, resveratrol promoted more efficient protection from renovascular hypertension-induced cardiac remodeling than captopril.
To our knowledge, no published studies have evaluated the effects of resveratrol or its combination with ACE inhibitors on the cardiac damage triggered by renovascular hypertension.
Three consecutive and distinct phases characterize the progression of hypertensive disease subsequent to the renal stenosis in the 2K1C model [17]. The first stage lasts for 6 weeks and involves the ACE/AngII axis of RAS. The second phase persists for 2 weeks, in which RAS-associated high blood pressure is mainly due to salt and water retention [17]. In the third and ultimate stage reached from the 9th week following renal ischemia, a steady high BP does not depend on RAS [17]. Hypertension developed by 2K1C in its first phase of RAS overactivation is characterized by an acute increase in circulating levels of AngII, and the subsequent substantial increase in plasma renin activity [17]. We sought to investigate the benefits of resveratrol with or without the ACE inhibitor against the cardiomyocyte damage progression under hypertensive status. Thus, the treatments started on the 6th week, coinciding with the end of the first phase.
There was no difference in the SBP measures from both naïve and 2K (sham) control groups, considered normotensives, showing the surgical procedure was effective. These preliminary results were essential to the internal consistency of the overall findings.
Except for the hypertensive rats treated only with the vehicle, all the treatment designs (resveratrol plus captopril, captopril, or resveratrol alone) similarly reduced the SBP. We formerly published that only when combined with captopril, resveratrol produced SBP significant reduction [44]. In that work, although the animals submitted to renovascular occlusion developed high SBP, it was not as high as reported in the present article [44]. Taking together previous and current results indicate the higher the hypertensive status, the higher the susceptibility to resveratrol’s antihypertensive effects in 2K1C.
Oxidative stress biomarkers positively correlate with increased blood pressure in 2K1C renovascular hypertension and cardiac oxidative damage caused by leading to left ventricle hypertrophy [47]. We have previously shown that high SBP in the RAS-activation phase of 2K1C hypertension is associated with increased ROS production susceptible to resveratrol’s vascular protective effects [43]. Through Live Data Mode acquisition, ROS were directly measured in fresh isolated vascular cells from 2K1C and 2K [43] using DHE. Renovascular hypertensive rats treated with resveratrol had a significant ROS reduction in the aorta [43].
Oxidative stress mediates AngII-induced cardiac hypertrophy, apoptosis, fibrosis inflammation, and cardiomyocyte contractile dysfunction [48]. Massive ROS generation results from AngII-induced NAD(P)H oxidase machinery in the vessels [49] and heart [50]. Based on our previous work demonstrating that resveratrol reduces ROS in vessels from 2K1C rats [33], we conclude resveratrol’s heart tissue benefits may also be associated with lowering ROS-mediated mechanisms that damage cardiomyocytes.
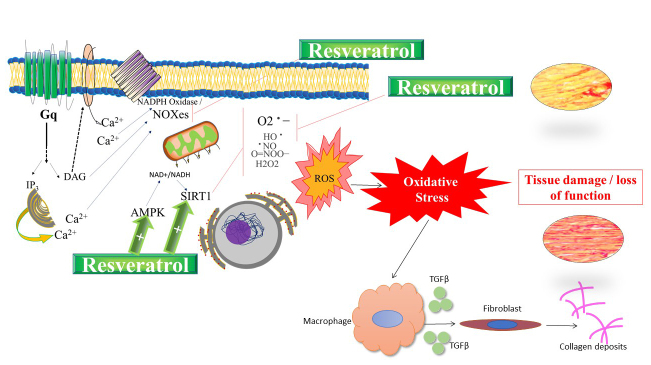
Hypothetical cellular/molecular mechanisms of tissue protection developed by resveratrol are summarized as a graphical representation (Figure 5). We propose that, at least partially, resveratrol produces benefits against tissue damage caused by oxidative pathways triggered by the activation of receptors coupled to Gq protein, such as AT1R mediated by AngII-agonism [49]. We have shown that, through a mechanism involving IP3-receptor, Protein Kinase-C and NADPH oxidase, resveratrol promotes anti-contractile effects in non-vascular smooth muscle under stimulation of α1-adrenergic, Gq protein-coupled, receptor [51]. In the present work, we propose that cardioprotective actions would be due to direct antioxidant mechanisms, interfering with NOXes and/or by enhancing protection led by AMP-activated protein kinase (AMPK) [52] and/or SIRT [53,54] pathways, which are remarkable components of antioxidant intracellular mechanisms. The foundation for our theoretical proposal is based on evidence reporting a negative correlation between the RAS and AMPK [55]. Inhibition of RAS up-regulates the activity of AMPK [55]. Activation of AMPK ameliorated pathological damage induced by AngII in rat cardiomyocytes. Moreover, overexpression of a cardiac-specific SIRT protected against AngII-induced hypertrophy by activating liver kinase B1 (LKB1), which ultimately resulted in maintenance of AMPK levels in hypertrophy [56]. Cao et al. [57] demonstrated that resveratrol prevents AngII-induced hypertension via AMPK activation in mice.
Figure 5.
Graphical abstract of hypothetical cellular/molecular cardioprotective mechanisms developed by resveratrol. Resveratrol produces benefits against tissue damage caused by oxidative pathways triggered by the activation of AT1R interfering with NOXes, AMP-activated protein kinase (AMPK) and/or sirtuins (SIRT) pathways. An additional protective mechanism of resveratrol would be associated with heart fibrosis/hypertrophy is the involvement of MMP and cytokines.
It is known that activation of AMPK is an essential cellular energy sensor and develops protective actions in several diseases, including cardiovascular, metabolic, and renal diseases (for reviews, see: [55,58,59]). For example, AMPK activation inhibits cardiac hypertrophy and cardiomyopathy [58]. Resveratrol was reported to mitigate ventricular remodeling by activating AMPK to induce autophagy that recycles cardiomyocytes in a failing heart [52].
Daily chronic oral administration of resveratrol alleviated hypertension in the experimental model of AngII-induced hypertensive mice, and these effects were accompanied by the activation of AMPK. This is another piece of evidence supporting our mechanistic hypotheses involving AMPK to explain the overactivation of RAS in 2K1C with the molecule associated with resveratrol.
Whether resveratrol acts as a direct activator of SIRT1 remains under debate [60], yet it is well known its actions stimulate SIRT [61] and AMPK [52,62]. Resveratrol developed protective effects in dysfunctional cardiomyocytes isolated from animal models with heart failure by enhancing the expression of AMPK and improving cardiac function through the activation of SIRT1 [54].
An additional protective mechanism of resveratrol associated with heart fibrosis/hypertrophy is the involvement of matrix metalloproteinases (MMP) and cytokines. Rizzi et al. [47] demonstrated that 2K1C hypertension induced-LV hypertrophy is associated with increased cardiac MMP-2 activation due to ROS formation. Antioxidants agents mitigate 2K1C-induced cardiac collagen deposition particularly via MMP inhibition. In addition, our group has shown that resveratrol attenuates cardiac fibrosis and reduces matrix metalloproteinase-2 activity in an experimental model of heart failure [63]. Thus, antioxidant effects of resveratrol could inhibit MMP-2 and decrease fibrosis, such as we found in the present study. Direct or indirect relationship between resveratrol’s mechanisms involving MMPs with SIRT/AMPK pathways remain to be elucidated (for review, see [64]).
In this study, the hypertensive rats treated only with the vehicle presented with whole cardiac hypertrophy, ventricular hypertrophy, and increased collagen deposition. These findings are consistent with other studies showing the 2K1C hypertensive status leads to left ventricle fibrosis and remodeling [19,47]. None of the treatments caused ventricle fibrosis and remodeling in our normotensive control groups (2K).
Based on the current results and on what is acknowledged regarding the resveratrol’s mechanisms on the cardiovascular system [41,42,65], our data suggests that resveratrol’s benefits in decreasing end-organ damage may be related to its antihypertensive action.
Although captoril treatment blunted the markers of cardiac tissue damage during the establishment of renovascular hypertension, resveratrol, with or without captopril, reduced the 2K1C rats’ whole heart hypertrophy. Resveratrol supplanted the ACE inhibitor benefits regarding left ventricle cardiomyocyte diameters and fibrosis, evidencing a greater reduction in cardiac remodeling.
ACE inhibitors are broadly used to treat high blood pressure and its comorbidities in renovascular hypertension during the RAS-dependent phases [28]. In experimental studies, ACE inhibitors prevented vascular remodeling and fibrosis in hypertensive 2K1C rats [20-22]. In patients with CVD, ACE inhibitors also modify the process of cardiac remodeling and other clinically relevant benefits, reducing morbidity and mortality [31]. We had predicted that inhibition of the ACE and consequent diminished AngII production would result in higher positive benefits on the histological markers than treatment with resveratrol alone. However, in the current study, treatment with resveratrol resulted in greater cardiac tissue benefits than with captopril. This finding implicates the cardioprotective effects of resveratrol are beyond reducing the damaging effects mediated by AngII.
The ACE inhibitors class of drugs is among the top tier of agents to treat hypertension. However, there are concerns about the safety and efficacy of ACE inhibitors in the Black population [29]. Also, ACE inhibitors are known to decrease renal perfusion pressure and cause acute renal failure during renal stenosis [30]. In this sense, these drugs may be a risk for patients with reduced renal function due to renal stenosis – particularly the elderly, who may develop renal ischemia and secondary renovascular hypertension – accelerating preexisting hypertensive conditions progressing to chronic kidney disease or acute kidney injury [14,15,28].
Data from studies with humans show resveratrol is very well tolerated after oral administration, with satisfactory bioavailability and safety [66]. Concerns with the use of resveratrol are related to drug interactions [36] and its multiple targets [67]. Of note, since resveratrol reduces platelet aggregation, it is contraindicated for patients taking anticoagulants, antiplatelets, or non-steroidal anti-inflammatory drugs (NSAIDs) [68-70]. However, several studies (including the current one) are being conducted to use these additive interactions as an advantage to decrease the dose of medication when used in combination with resveratrol [30,67]. Several human and animal studies focused on resveratrol’s safety have been reported [35] and demonstrating that its overall low toxicity in humans encourages further translational research [71-72].
Although further investigation on pharmacokinetics profile resveratrol in 2K1C hypertension rats is needed, our control group validates the present results. Additionally, evidence of acceptable pharmacokinetic parameters for resveratrol have been reported in a variety of different experimental models and in human [36,66,67,73,74].
Clinical trials report myriad positive outcomes after oral use of resveratrol [36,42,73,75-79]. The favorable impact of resveratrol on blood pressure in patients was reported through systematic review and meta-analysis of randomized clinical trials [80].
As a clinical implication from the current results, the cardioprotective effects of resveratrol make it a suitable therapeutic agent to optimize the reduction of hypertensive heart disease progression and its consequences. This includes reducing risks for comorbidities associated with cardiac hypertrophy, such as renal artery stenosis and hypertension.
Our data encourage further investigation to evaluate whether chronic treatment with resveratrol promotes cardioprotective effects in patients with end-organ injury, specifically cardiac hypertrophy, with and without hypertension, mainly secondary to kidney function impairment.
Limitations
The present study aimed to investigate resveratrol’s effects on the SBP, cardiac remodeling, and fibrosis, but not its mechanisms. Approaches examining its intrinsic mechanisms are needed, particularly questions not directly linked to ACE pathways and actions of ACE inhibitors.
Conclusions
In summary, we demonstrated that resveratrol reduces SBP similar to ACE inhibition in the 2K1C renovascular model of hypertension. The 2K1C hypertensive status leads to cardiomyocyte fibrosis and remodeling. This damage is counteracted by oral resveratrol oral, which surpassed captopril’s cardioprotective effects. Further investigations are needed to target cardioprotective actions of resveratrol in renovascular hypertension, to lower the risks associated with kidney function impairment.
Key-Message
• Resveratrol reduces systolic blood pressure similarly to captopril in 2K1C rats.
• Independently of captopril, resveratrol normalized cardiac fibrosis in 2K1C rats.
• Resveratrol decreased cardiac remodeling in 2K1C rats, independently of captopril.
• Resveratrol may reduce end-organ cardiac damage triggered by renovascular hypertension.
Acknowledgments
The authors would like to thank Ms. Carla R. K. Antonietto and Mr. Carlos Adriano da Cruz Alves for their technical assistance. The authors are grateful for the thorough revision of the text and insightful comments from Ms. Suzanne Guimond Wilson, MSN, RN-Senior Academic Specialist, MSU College of Osteopathic Medicine. The authors also thank the College of Osteopathic Medicine-MSU and Pharmacology & Toxicology Dept, MSU, and UNAERP, RP, Brazil, for the academic support.
Glossary
- 2K
Two-kidney
- 2K1C
Two-kidney, one-clip
- ACE
angiotensin-converting enzyme
- AngII
Angiotensin II
- BW
body weight
- CAP
captopril
- CVD
cardiovascular disease(s)
- H&E
Hematoxylin and Eosin
- HPF
high-power fields
- HW
heart weight
- RAS
renin-angiotensin-system
- RESV
resveratrol
- ROS
reactive oxygen species
- SBP
Systolic Blood Pressure
Author Contributions
CBAR: conceived the project, designed the protocols, acquisition, analysis, interpretation of data, and wrote the manuscript. CBAR, AFEG, HMN, MFAC, VFN, and ER: performed renal stenosis surgical procedures and carried out animal treatments, blood pressure measurements, and organs isolation for histology processing. LNZR: performed all the histological sample preparation and worked on analyzing and interpreting the images. CBAR, AFEG, HMN, MFAC, VFN, ER, and LNZR: critically revised and approved the manuscript’s final version. CBAR, AFEG, HMN, MFAC, VFN, ER, and LNZR: responsible for the intellectual content of the final version to be published.
Source of funding for all authors
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number 121957/2013-6 (2014/1)] (Brazil)
References
- Schirone L, Forte M, Palmerio S, Yee D, Nocella C, Angelini F, et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid Med Cell Longev. 2017;2017:3920195. 10.1155/2017/3920195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000. Mar;35(3):569–82. 10.1016/s0735-1097(99)00630-0 [DOI] [PubMed] [Google Scholar]
- Perkovic V, Huxley R, Wu Y, Prabhakaran D, MacMahon S. The burden of blood pressure-related disease: a neglected priority for global health. Hypertension. 2007. Dec;50(6):991–7. 10.1161/HYPERTENSIONAHA.107.095497 [DOI] [PubMed] [Google Scholar]
- Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004. Jun;94(12):1543–53. 10.1161/01.RES.0000130526.20854.fa [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999. Jan;79(1):215–62. 10.1152/physrev.1999.79.1.215 [DOI] [PubMed] [Google Scholar]
- Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013. Jul;128(4):388–400. 10.1161/CIRCULATIONAHA.113.001878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006. Jan;367(9507):356–67. 10.1016/S0140-6736(06)68074-4 [DOI] [PubMed] [Google Scholar]
- Ferrario CM. Cardiac remodelling and RAS inhibition. Ther Adv Cardiovasc Dis. 2016. Jun;10(3):162–71. 10.1177/1753944716642677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977. Apr;57(2):313–70. 10.1152/physrev.1977.57.2.313 [DOI] [PubMed] [Google Scholar]
- Bernstein KE. Two ACEs and a heart. Nature. 2002. Jun;417(6891):799–802. 10.1038/417799a [DOI] [PubMed] [Google Scholar]
- Herrmann SM, Textor SC. Renovascular Hypertension. Endocrinol Metab Clin North Am. 2019. Dec;48(4):765–78. 10.1016/j.ecl.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Wang SY, Sun YJ, Ren JH, Guo FJ. [Diagnostic value of contrast-enhanced ultrasound for accessory renal artery among patients suspected of renal artery stenosis]. Zhonghua Yi Xue Za Zhi. 2019. Mar;99(11):838–40. 10.3760/cma.j.issn.0376-2491.2019.11.008 [DOI] [PubMed] [Google Scholar]
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934. Feb;59(3):347–79. 10.1084/jem.59.3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar LG, Zou L, Von Thun A, Tarng Wang C, Imig JD, Mitchell KD. Unraveling the Mystery of Goldblatt Hypertension. News Physiol Sci. 1998. Aug;13(4):170–6. 10.1152/physiologyonline.1998.13.4.170 [DOI] [PubMed] [Google Scholar]
- Elliott WJ. Renovascular hypertension: an update. J Clin Hypertens (Greenwich). 2008. Jul;10(7):522–33. 10.1111/j.1751-7176.2008.07788.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düsing R. Mega clinical trials which have shaped the RAS intervention clinical practice. Ther Adv Cardiovasc Dis. 2016. Jun;10(3):133–50. 10.1177/1753944716644131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991. May;17(5):707–19. 10.1161/01.hyp.17.5.707 [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, et al. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med. 2009. May;46(9):1298–307. 10.1016/j.freeradbiomed.2009.02.011 [DOI] [PubMed] [Google Scholar]
- Ceron CS, Rizzi E, Guimarães DA, Martins-Oliveira A, Gerlach RF, Tanus-Santos JE. Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-β and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic Biol Med. 2013. Dec;65:47–56. 10.1016/j.freeradbiomed.2013.06.033 [DOI] [PubMed] [Google Scholar]
- Jalil JE, Janicki JS, Pick R, Weber KT. Coronary vascular remodeling and myocardial fibrosis in the rat with renovascular hypertension. Response to captopril. Am J Hypertens. 1991. Jan;4(1 Pt 1):51–5. 10.1093/ajh/4.1.51 [DOI] [PubMed] [Google Scholar]
- Sheng H, Zhu J, Wu X, Yang D, Zhang J. Angiotensin-converting enzyme inhibitor suppresses activation of calcineurin in renovascular hypertensive rats. Hypertens Res. 2007. Dec;30(12):1247–54. 10.1291/hypres.30.1247 [DOI] [PubMed] [Google Scholar]
- Akbar Nekooeian A, Rasti Pour A, Dehghani F, Mashghoolozekr E, Esmaeilpour T. Effects of Captopril and Losartan on Cardiac Stereology in Rats with Renovascular Hypertension. Iran J Med Sci. 2021. May;46(3):169–79. 10.30476/ijms.2020.81948.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Peres LC. Effect of captopril on the prevention and regression of myocardial cell hypertrophy and interstitial fibrosis in pressure overload cardiac hypertrophy. Am Heart J. 1992. Sep;124(3):700–9. 10.1016/0002-8703(92)90281-y [DOI] [PubMed] [Google Scholar]
- Rials SJ, Wu Y, Xu X, Filart RA, Marinchak RA, Kowey PR. Regression of left ventricular hypertrophy with captopril restores normal ventricular action potential duration, dispersion of refractoriness, and vulnerability to inducible ventricular fibrillation. Circulation. 1997. Aug;96(4):1330–6. 10.1161/01.CIR.96.4.1330 [DOI] [PubMed] [Google Scholar]
- Sriramula S, Francis J. Tumor necrosis factor-alpha is essential for angiotensin II-induced ventricular remodeling: role for oxidative stress. PLoS One. 2015. Sep;10(9):e0138372. 10.1371/journal.pone.0138372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006. Feb;69(3):666–76. 10.1016/j.cardiores.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010. Apr;12(2):135–42. 10.1007/s11906-010-0100-z [DOI] [PubMed] [Google Scholar]
- Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020. Jun;75(6):1334–57. 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and β-adrenergic blockers? A systematic review. BMC Med. 2013. May;11(1):141. 10.1186/1741-7015-11-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber BR, Dieter RS. Renal artery stenosis: epidemiology and treatment. Int J Nephrol Renovasc Dis. 2014. May;7:169–81. 10.2147/IJNRD.S40175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Horke S, Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. 2013. Jun;34(6):313–9. 10.1016/j.tips.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Ishimoto Y, Tanaka T, Yoshida Y, Inagi R. Physiological and pathophysiological role of reactive oxygen species and reactive nitrogen species in the kidney. Clin Exp Pharmacol Physiol. 2018. Nov;45(11):1097–105. 10.1111/1440-1681.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002. Jun;346(25):1954–62. 10.1056/NEJMoa013591 [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am J Physiol Regul Integr Comp Physiol. 2002. Feb;282(2):R335–42. 10.1152/ajpregu.00605.2001 [DOI] [PubMed] [Google Scholar]
- Den Hartogh DJ, Tsiani E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients. 2019. Jul;11(7):1624. 10.3390/nu11071624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, et al. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines. 2018. Sep;6(3):91. 10.3390/biomedicines6030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004. Apr;489(1-2):39–48. 10.1016/j.ejphar.2004.02.031 [DOI] [PubMed] [Google Scholar]
- Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006. Jan;53(1):6–15. 10.1016/j.phrs.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009. Nov-Dec;2(5):270–8. 10.4161/oxim.2.5.9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011. Jan;1215(1):161–9. 10.1111/j.1749-6632.2010.05853.x [DOI] [PubMed] [Google Scholar]
- Liu Z, Song Y, Zhang X, Liu Z, Zhang W, Mao W, et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin Exp Pharmacol Physiol. 2005. Dec;32(12):1049–54. 10.1111/j.1440-1681.2005.04303.x [DOI] [PubMed] [Google Scholar]
- Breuss JM, Atanasov AG, Uhrin P. Resveratrol and Its Effects on the Vascular System. Int J Mol Sci. 2019. Mar;20(7):1523. 10.3390/ijms20071523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JC, Antonietto CR, Scalabrini AC, Marinho TS, Pernomian L, Corrêa JW, et al. Antioxidant protective effects of the resveratrol on the cardiac and vascular tissues from renal hypertensive rats. Open J Med Chem. 2012;2(3):61–71. 10.4236/ojmc.2012.23008 [DOI] [Google Scholar]
- Natalin HM, Garcia AF, Ramalho LN, Restini CB. Resveratrol improves vasoprotective effects of captopril on aortic remodeling and fibrosis triggered by renovascular hypertension. Cardiovasc Pathol. 2016. Mar-Apr;25(2):116–9. 10.1016/j.carpath.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Conselho Nacional de Controle de Experimentação Animal. Diretriz Brasileira para o cuidado e a utilização de animais para fins científicos e didáticos. Brasília 2013: Ministério da Ciência, Tecnologia e Inovação. Available: online(http://www.mct.gov.br/index.php/content/view/364015/Diretriz_Brasileira_para_o_Cuidado_e_a_Utilizacao_de_Animais_em_Atividades_de_Ensino_ou_de_Pesquisa_Cientifica___DBCA.html)
- National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington 2011: National Academy Press. Available online (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf)
- Rizzi E, Ceron CS, Guimaraes DA, Prado CM, Rossi MA, Gerlach RF, et al. Temporal changes in cardiac matrix metalloproteinase activity, oxidative stress, and TGF-β in renovascular hypertension-induced cardiac hypertrophy. Exp Mol Pathol. 2013. Feb;94(1):1–9. 10.1016/j.yexmp.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Wen H, Gwathmey JK, Xie LH. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J Hypertens. 2012. Aug;2(4):34–44. 10.5494/wjh.v2.i4.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda). 2006. Aug;21(4):269–80. 10.1152/physiol.00004.2006 [DOI] [PubMed] [Google Scholar]
- Chapter 4. In: Restini CB, Garcia AF, Natalin HM, Natalin GM, Rizzi E. Signaling Pathways of Cardiac Remodeling Related to Angiotensin II. Book: Renin-Angiotensin System - Past. Present and Future; 2016. pp. 51–68. 10.5772/66076 [DOI] [Google Scholar]
- Restini CB, Vieira DF, Oliveira DA, Barnett B, Marcos JM. Anti-Contractile Mechanism of Resveratrol in Non-Vascular Smooth Muscle Under α1-Adrenoceptor Stimulation involves IP3-Receptor, Protein Kinase-C and NADPH Oxidase. Open Acc J of Toxicol. 2019;4(1):555627. 10.19080/OAJT.2019.04.555627 [DOI] [Google Scholar]
- Takemura G, Kanamori H, Okada H, Miyazaki N, Watanabe T, Tsujimoto A, et al. Anti-apoptosis in nonmyocytes and pro-autophagy in cardiomyocytes: two strategies against postinfarction heart failure through regulation of cell death/degeneration. Heart Fail Rev. 2018. Sep;23(5):759–72. 10.1007/s10741-018-9708-x [DOI] [PubMed] [Google Scholar]
- Kane AE, Sinclair DA. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ Res. 2018. Sep;123(7):868–85. 10.1161/CIRCRESAHA.118.312498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XS, Wang ZB, Ye Z, Lei JP, Li L, Su DF, et al. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet Mol Res. 2014. Jan;13(1):323–35. 10.4238/2014.January.17.17 [DOI] [PubMed] [Google Scholar]
- Liu J, Li X, Lu Q, Ren D, Sun X, Rousselle T, et al. AMPK: a balancer of the renin-angiotensin system. Biosci Rep. 2019. Sep;39(9):BSR20181994. 10.1042/BSR20181994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Chen XF, Wang NY, Wang XM, Liang ST, Zheng W, et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation. 2017. Nov;136(21):2051–67. 10.1161/CIRCULATIONAHA.117.028728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Luo T, Luo X, Tang Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens Res. 2014. Sep;37(9):803–10. 10.1038/hr.2014.90 [DOI] [PubMed] [Google Scholar]
- Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond). 2009. Apr;116(8):607–20. 10.1042/CS20080066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng RS, Pei ZH, Yin R, Zhang CX, Chen BL, Zhang Y, et al. Adenosine monophosphate-activated protein kinase inhibits cardiac hypertrophy through reactivating peroxisome proliferator-activated receptor-alpha signaling pathway. Eur J Pharmacol. 2009. Oct;620(1-3):63–70. 10.1016/j.ejphar.2009.08.024 [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010. Mar;285(11):8340–51. 10.1074/jbc.M109.088682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Wang M, Qiu X, Liu D, Jiang H, Yang N, et al. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015. Jun;29(12):1316–25. 10.1101/gad.265462.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan D, Zhou Y, Wang K, Hou Y, Hou R, Ye X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res Bull. 2016. Mar;121:255–62. 10.1016/j.brainresbull.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Durand MT, Orlandin CB, Bonácio GF, Rizzi E, Durão MP, Lataro RM, et al. A Silva CA, Amaral JH Restini CB, Salgado HC. Antioxidant Resveratrol Attenuates Cardiac Fibrosis and Reduces Matrix Metalloproteinase-2 Activity in Heart Failure Rats. Hypertension. 2019;74:AP2005. Available from: https://www.ahajournals.org/doi/10.1161/hyp.74.suppl_1.P2005 10.1161/hyp.74.suppl_1.P2005 [DOI] [Google Scholar]
- Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta. 2015. Jun;1852(6):1071–113. 10.1016/j.bbadis.2015.01.014 [DOI] [PubMed] [Google Scholar]
- Li H, Xia N, Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012. Feb;26(2):102–10. 10.1016/j.niox.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Sergides C, Chirilă M, Silvestro L, Pitta D, Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp Ther Med. 2016. Jan;11(1):164–70. 10.3892/etm.2015.2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzuto JM. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol Ther. 2019;27(1):1-14. DOI: 10.4062/biomolther.2018.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17(1):1–3. [PubMed] [Google Scholar]
- Shen MY, Hsiao G, Liu CL, Fong TH, Lin KH, Chou DS, et al. Inhibitory mechanisms of resveratrol in platelet activation: pivotal roles of p38 MAPK and NO/cyclic GMP. Br J Haematol. 2007. Nov;139(3):475–85. 10.1111/j.1365-2141.2007.06788.x [DOI] [PubMed] [Google Scholar]
- Huang TY, Yu CP, Hsieh YW, Lin SP, Hou YC. Resveratrol stereoselectively affected (±)warfarin pharmacokinetics and enhanced the anticoagulation effect. Sci Rep. 2020. Sep;10(1):15910. 10.1038/s41598-020-72694-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen S, Weiskirchen R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv Nutr. 2016. Jul;7(4):706–18. 10.3945/an.115.011627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. 10.1371/journal.pone.0019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010. Nov;70(22):9003–11. 10.1158/0008-5472.CAN-10-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006. Jun;5(6):493–506. 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018. Apr;55(4):341–53. 10.1007/s00592-017-1098-3 [DOI] [PubMed] [Google Scholar]
- Huang H, Chen G, Liao D, Zhu Y, Pu R, Xue X. The effects of resveratrol intervention on risk markers of cardiovascular health in overweight and obese subjects: a pooled analysis of randomized controlled trials. Obes Rev. 2016. Dec;17(12):1329–40. 10.1111/obr.12458 [DOI] [PubMed] [Google Scholar]
- Tomé-Carneiro J, Larrosa M, Yáñez-Gascón MJ, Dávalos A, Gil-Zamorano J, Gonzálvez M, et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013. Jun;72:69–82. 10.1016/j.phrs.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010. Jun;91(6):1590–7. 10.3945/ajcn.2009.28641 [DOI] [PubMed] [Google Scholar]
- Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer’s disease. Ann N Y Acad Sci. 2017. Sep;1403(1):142–9. 10.1111/nyas.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogacci F, Tocci G, Presta V, Fratter A, Borghi C, Cicero AF. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit Rev Food Sci Nutr. 2019;59(10):1605–18. 10.1080/10408398.2017.1422480 [DOI] [PubMed] [Google Scholar]