Abstract
Background and Objectives
Although flavonoids have the potential to exert neuroprotective benefits, evidence of their role in improving survival rates among individuals with Parkinson disease (PD) remains lacking. We aimed to prospectively study the association between prediagnosis and postdiagnosis flavonoid intakes and risk of mortality among individuals with PD identified from 2 large ongoing cohorts of US men and women.
Methods
Included in the current analysis were 599 women from the Nurses' Health Study and 652 men from the Health Professionals Follow-Up Study who were newly diagnosed with PD during follow-up. Dietary intakes of total flavonoid and its subclasses, together with major flavonoid-rich foods (tea, apples, berries, orange and orange juice, and red wine), were repeatedly assessed with a validated food frequency questionnaire every 4 years. Mortality was ascertained via the National Death Index and state vital statistics records.
Results
We documented 944 deaths during 32 to 34 years of follow-up. A higher total flavonoid intake before PD diagnosis was associated with a lower future risk for all-cause mortality in men (hazard ratio [HR] comparing 2 extreme quartiles 0.53, 95% confidence interval [CI] 0.39, 0.71; p for trend < 0.001) but not in women (HR 0.93, 95% CI 0.68, 1.28; p for trend = 0.69) after adjustment for age, smoking status, total energy intake, and other covariates. The pooled HR comparing the extreme quartiles was 0.70 (95% CI 0.40, 1.22; p for trend = 0.25) with significant heterogeneity (p = 0.01). For flavonoid subclasses, the highest quartile of anthocyanins, flavones, and flavan-3-ols intakes before diagnosis had a lower mortality risk compared to the lowest quartile (pooled HR 0.66, 0.78, and 0.69, respectively; p < 0.05 for all); for berries and red wine, participants consuming ≥3 servings per week had a lower risk (pooled HR 0.77, 95% CI 0.58, 1.02; and pooled HR 0.68, 95% CI 0.51, 0.91, respectively) compared to <1 serving per month. After PD diagnosis, greater consumptions of total flavonoid, subclasses including flavonols, anthocyanins, flavan-3-ols, and polymers, and berries and red wine were associated with lower mortality risk (p < 0.05 for all).
Discussion
Among individuals with PD, higher consumption of flavonoids, especially anthocyanins and flavan-3-ols, and flavonoid-rich food such as berries and red wine was likely to be associated with a lower risk of mortality.
Parkinson disease (PD) substantially reduces quality of life and increases the risk of morbidity and mortality.1 Mortality risk among individuals with PD was greater than that in the general population2,3 and could be even greater than the risk of individuals with other major chronic diseases such as colorectal cancer, stroke, ischemic heart disease, or chronic obstructive pulmonary disease.4 However, few studies have examined the potential role of modifiable risk factors such as dietary factors in survival among individuals with PD.5 Several dietary components such as flavonoids and flavonoid-rich foods have previously been shown to be associated with a lower risk of developing PD.6
Flavonoids are plant-derived polyphenolic molecules present naturally in a variety of fruits, vegetables, and several beverages (e.g., tea and red wine).7 After ingestion, they are rapidly metabolized, and these metabolites can cross the blood-brain barrier, resulting in diverse functions such as the alleviation of oxidative stress, inflammation, and atherosclerosis.7 Evidence suggests that they can reduce injury caused by nitric oxide synthase and xanthine oxidase activity in the brain and thus are valued for their neuroprotective potential.8-10 Despite several recent animal studies on PD to examine the potential therapeutic effects of flavonoid compounds,11 evidence on the association between flavonoids and PD prognosis from human studies is lacking. Therefore, we prospectively studied the association between habitual dietary flavonoid intakes, flavonoid-rich food intakes, and risk of all-cause mortality among individuals who were diagnosed with PD from 2 large US cohorts.
Individuals with PD generally have difficulty in swallowing, jaw rigidity, and impaired cutlery and food handling (i.e., dysphagia symptoms),12-14 which could lead to low consumption of flavonoid-rich foods (e.g., fruit/vegetables) and poor disease prognosis.15 To minimize the potential for reverse causality due to dysphagia and other relevant motor disorders after PD motor symptom onset and to provide evidence for the role of diet across the disease progression of PD since the preclinical stage, we used flavonoid intakes collected both before and after PD diagnosis as the primary exposures in this study.
Methods
Study Population
The current analyses were based on 2 ongoing cohorts: the Nurses' Health Study (NHS), which started in 1976 with 121,700 female registered nurses 30 to 55 years of age, and the Health Professionals Follow-Up Study (HPFS), which started in 1986 with 51,529 male health professionals 40 to 75 years of age. Questionnaires were mailed to the participants at baseline and biennially thereafter to collect and update information on personal demographics, lifestyles, medical history, and chronic disease occurrence, as detailed elsewhere.6
We included 599 women and 652 men who were newly diagnosed with PD during follow-up until 2012 and had dietary data available. PD cases were first identified by self-reported questionnaires and were confirmed by a review of the medical records by a neurologist specialized in movement disorder, as detailed elsewhere.6
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required.
Dietary Flavonoid Intake Assessment
Diet was assessed with a validated semi-quantitative food frequency questionnaire (FFQ) since 1980 in the NHS and since 1986 in the HPFS and updated every 2 to 4 years thereafter. Because the 1980 NHS FFQ included only half the number of food items in all following FFQs and did not include several major food items that contribute to habitual flavonoid intake, we used 1984 as the baseline for NHS.6
The updated and expanded US Department of Agriculture flavonoid content of foods and the proanthocyanidin databases were used to quantify flavonoid levels from the FFQ, as previously described.16 Intakes of individual compounds were calculated as the sum of the intake frequency multiplied by the content for the specified portion size of each food. We included intakes of the 6 main flavonoid subclasses commonly consumed in the US diet, specifically flavonols (quercetin, kaempferol, myricetin, and isorhamnetin), anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, petunidin, and peonidin), flavones (luteolin and apigenin), flavanones (eriodictyol, hesperetin, and naringenin), flavan-3-ols (catechins and epicatechins), and flavonoid polymers (proanthocyanidins, theaflavins, and thearubigins).16 Total flavonoid intakes were calculated as the sum of the 6 component subclasses. The raw intakes were then calibrated by total energy intake. In the validation study of the FFQs compared to the weighed 7-day dietary records, the energy-adjusted correlations for total flavonoid were 0.65 for men and 0.63 for women.17 The correlations for flavonoid subclasses ranged from 0.19 (flavones) to 0.76 (anthocyanins). The validity estimates for all major food contributors to flavonoid intake were also moderate to high: in men, the correlations were 0.78 for apples, 0.77 for blueberries, 0.74 for strawberries, 0.71 for tea, 0.81 for oranges and orange juice, and 0.88 for red wines; in women, the correlations were similar.17
Mortality Ascertainment
All-cause mortality was the primary outcome of this study. Deaths were identified from state vital statistics records, the National Death Index, reports from next of kin, or the postal authorities. More than 97% of deaths were identified for both cohorts; records matched by the National Death Index had a sensitivity of 97% and specificity of 100%.18,19 The cause of death was confirmed by physicians reviewing death certificates or medical records and coded according to the ICD-8 for the NHS and ICD-9 for the HPFS.20 Participants who did not have death records by June 2018 were censored.
Covariates Assessment
Information on age, smoking status, height, body weight, disease history, and the use of nonsteroidal anti-inflammatory drugs and postmenopausal hormone use in women was collected through biennial questionnaires.21,22 Body mass index was calculated as weight in kilograms divided by height in meters squared. Intakes of total energy, caffeine, alcohol, lactose, vitamin C, vitamin E, and beta carotene were also calculated from the FFQs, as detailed previously.21,22
Statistical Analysis
To limit the potential for reverse causality due to PD severity and relevant symptoms, we separately examined the prediagnosis and postdiagnosis flavonoid intakes. For prediagnosis analyses, the person-time of follow-up for each participant was calculated from the date of returning the baseline FFQ until the first instance of death or the end of follow-up (June 2018). For postdiagnosis analyses, person-time was calculated from the date of returning the first FFQ after PD diagnosis until the first instance of death or the end of follow-up.
For prediagnosis analyses, cumulative average intakes of flavonoids, which represent the long-term average intake, were calculated by taking the mean intake from all available FFQs before PD onset. For example, if an individual had PD onset in January 2000, the cumulative average intakes were calculated from all available dietary intake data until 1998 (the last dietary assessment before PD onset). For postdiagnosis analyses, cumulative averages were calculated by taking the mean intake from all available FFQs after PD onset up to the end of follow-up. Cohort-specific quartiles were calculated for the cumulative average of energy-adjusted total and individual flavonoids before or after diagnosis separately.
We also examined foods that are among the top contributors of dietary flavonoids in the US diet, specifically tea, apples, berries (strawberries and blueberries), orange and orange juice, and red wine.6 Flavonoid-rich foods were categorized into 4 groups that were based on the frequency of consumption: ≤1 serving per month (reference group), 1 to 3 servings per month, 1 to 2 servings per week, or ≥3 servings per week; for orange and orange juice intake, because the overall intake frequency was higher than the other food, it was categorized into quartiles.
Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) with the lowest intake category as the reference. The median intake of each category was assigned to all participants in that category and entered as a continuous exposure to test for linear trends. Multivariable models were adjusted for age (year), smoking status (never smoker or <10, 10–24, 25–44, or ≥45 pack-years), body mass index status (<21, 21–24.9, 25–29.9, or ≥30 kg/m2), physical activity (metabolic-equivalent hours, in quartiles), use of nonsteroidal anti-inflammatory drugs (yes/no), hypertension (yes/no), type 2 diabetes (yes/no), hypercholesterolemia (yes/no), and intakes of total energy (kilocalories per day, in quartiles), alcohol (0, 1–4.9, 5–9.9, 10–14.9, or ≥15 g/d for women; 0, 1–9.9, 10–19.9, 20–29.9, or ≥30 g/d for men), caffeine (milligrams per day, in quartiles), lactose (milligrams per day, in quartiles), and postmenopausal hormone use in women (premenopausal/never used, current user, or past user). The primary outcome was all-cause mortality, and the secondary outcome was mortality from the top causes. Pooled HRs were obtained by pooling the estimates from both cohorts with the use of random-effects models.
Several sensitivity analyses were performed. To exclude potential confounding from other antioxidants that are rich in plant-derived foods, we further adjusted for energy-calibrated vitamin C, vitamin E, and beta carotene intake (milligrams per day, in quartiles) in the sensitivity analysis. To examine sex heterogeneity in flavonoid intake, we applied the same quartile cutoff values generated in the HPFS to the NHS and estimated the risks. To minimize the potential for reverse causality due to prodromal symptoms (for prediagnosis intake) and disease severity (for postdiagnosis intake), we performed 4-year lagged analyses in which we stopped updating the dietary assessments by 4 years before PD diagnosis for prediagnosis analysis and by 4 years before death or end of follow-up for postdiagnosis analysis. We tested the interaction between flavonoid intake and smoking status, alcohol intake, and caffeine intake.
To evaluate the effect of unmeasured residual confounding, we calculated E values using the HRs and CIs derived from the main models to estimate the minimum risk ratio that an unmeasured confounder outside the covariates in the adjusted model would need to have with both the exposure (flavonoid intake) and the outcome (mortality) to fully explain away the observed association.23,24 A large E value implies a robust model because considerable unmeasured confounding effect would be needed to explain the independent effect of the exposure.
All statistical tests were 2 sided with a value of p < 0.05 as the significance level and performed with SAS version 9.2 for UNIX (SAS Institute, Cary, NC).
Data Availability
Further information, including the procedures to obtain and access data from the NHS and HPFS, is available from the corresponding author on reasonable request.
Results
Mean ages at PD diagnosis were 72.8 (SD 8.3) years for men and 71.9 (SD 7.6) years for women. Participant characteristics at the last FFQ return before diagnosis are listed in Table 1. The mean time between the last prediagnosis dietary assessment and the time of PD diagnosis was 33.3 months in men and 31.5 months in women. In both cohorts, individuals who consumed more flavonoids were more likely to never smoke and to have higher physical activity levels and higher intakes of vitamin C, vitamin E, and beta carotene. We documented 528 deaths in men and 416 deaths in women during 32 to 34 years of follow-up.
Table 1.
Characteristics at the Last Diet Assessment Before Diagnosis of Parkinson Disease
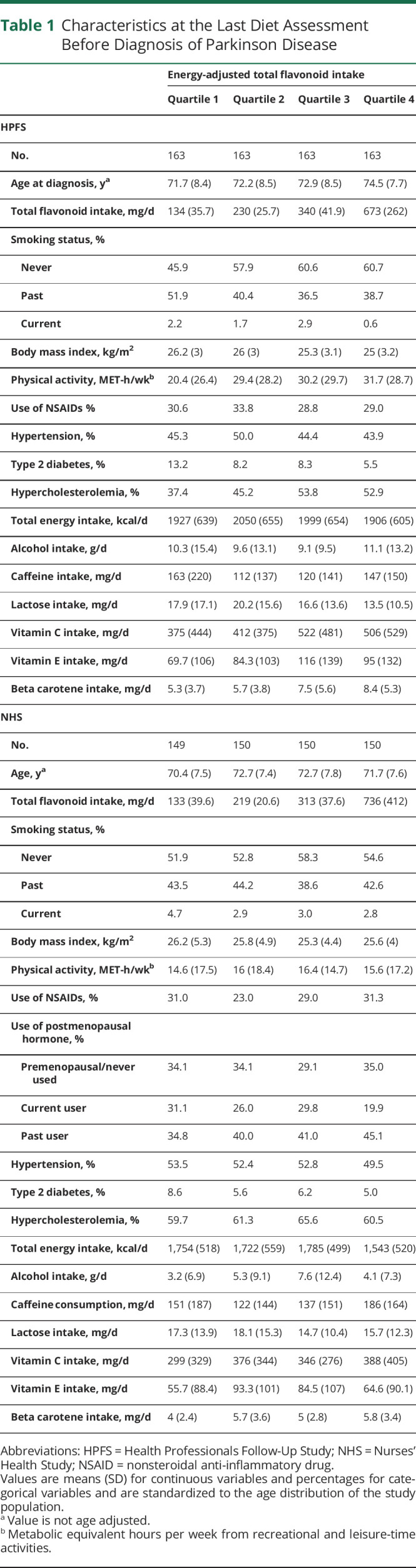
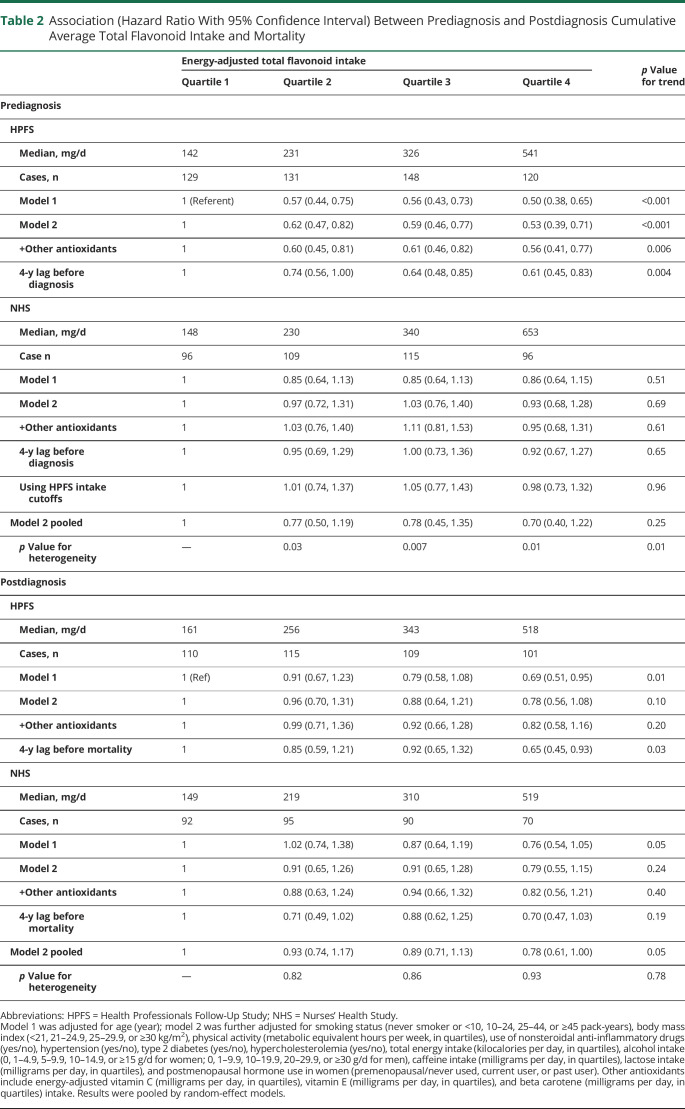
In the multivariable adjusted models, a higher total flavonoid intake before PD diagnosis was associated with a lower future risk for all-cause mortality after PD diagnosis in men (p for trend < 0.001) but not in women (p for trend = 0.69; p for heterogeneity = 0.01; Table 2). Per 100 mg/d higher total flavonoid intake, the pooled adjusted HR was 0.93 (95% CI 0.83, 1.05), and the E value was 1.28. The HR comparing the highest and the lowest quartile was 0.53 (95% CI 0.39, 0.71) in men and 0.93 (95% CI 0.68, 1.28) in women; the pooled HR was 0.70 (95% CI 0.40, 1.22; p for trend = 0.25; p for heterogeneity = 0.01). The E values comparing the extreme quartiles were 2.47 in men and 1.28 in women, also suggesting that the association was more robust in men and that it is more unlikely to be explained away by an unmeasured confounder.
Table 2.
Association (Hazard Ratio With 95% Confidence Interval) Between Prediagnosis and Postdiagnosis Cumulative Average Total Flavonoid Intake and Mortality
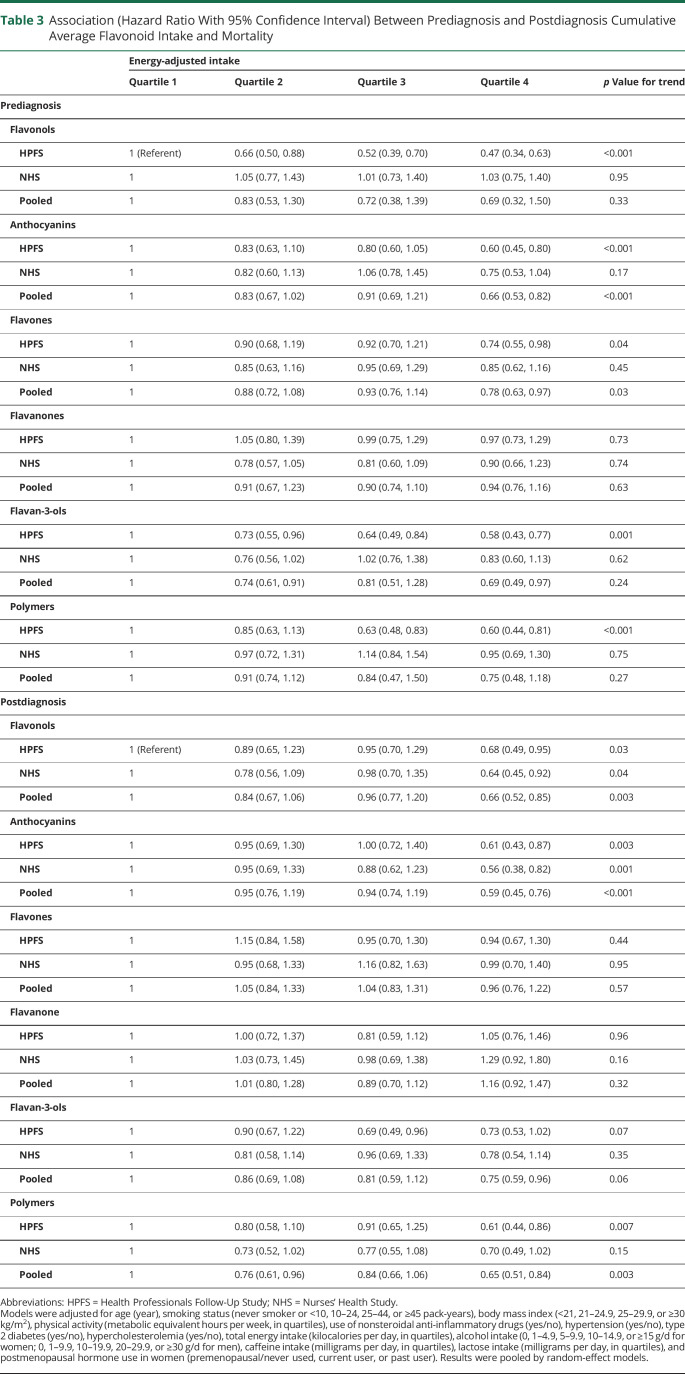
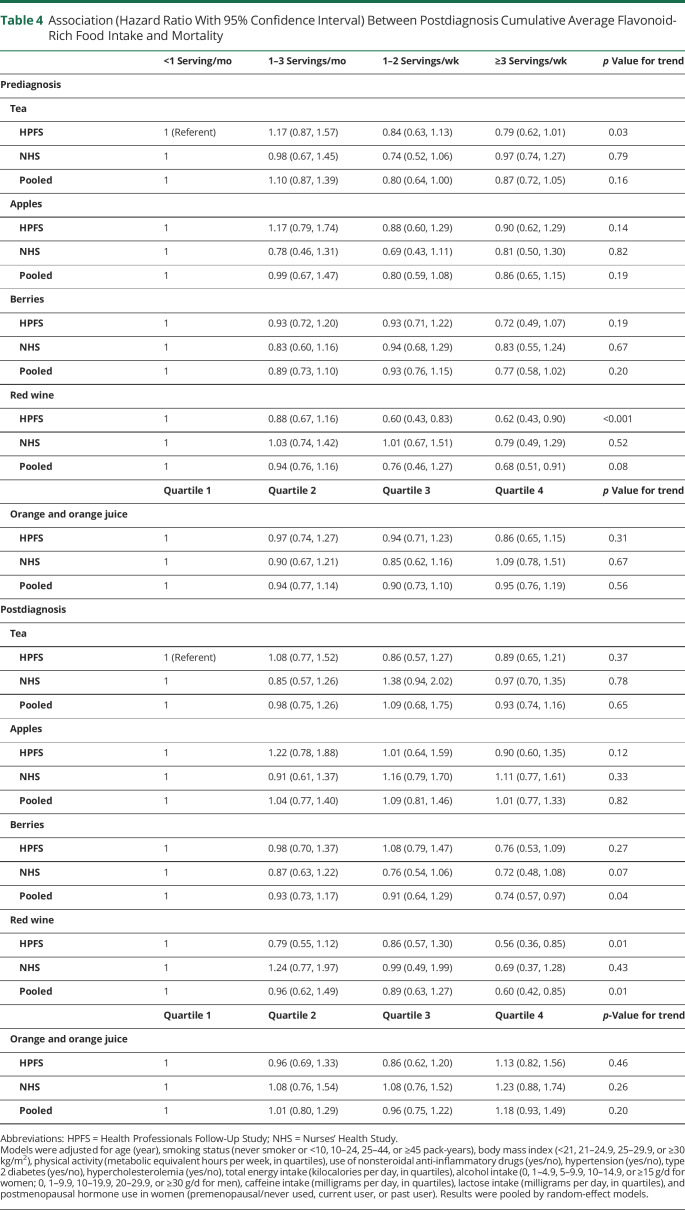
In the analyses of flavonoid subclasses, we found significant associations between a higher anthocyanins, flavones, and flavan-3-ols intake and lower mortality risk in the pooled analyses (Table 3). The pooled HR comparing the highest vs the lowest intake quartiles was 0.66 (95% CI 0.53, 0.82; p for trend < 0.001, p for heterogeneity = 0.34) for anthocyanin, 0.78 (95% CI 0.63, 0.97; p for trend = 0.03; p for heterogeneity = 0.52) for flavones, and 0.69 (95% CI 0.49, 0.97; p for trend = 0.24; p for heterogeneity = 0.10) for flavan-3-ols. In the analyses of flavonoid-rich foods, a higher intake of red wine, but not other foods, was associated with lower mortality risk (pooled HR comparing ≥3 servings per week and <1 serving per month 0.68, 95% CI 0.51, 0.91; p for heterogeneity = 0.44; Table 4).
Table 3.
Association (Hazard Ratio With 95% Confidence Interval) Between Prediagnosis and Postdiagnosis Cumulative Average Flavonoid Intake and Mortality
Table 4.
Association (Hazard Ratio With 95% Confidence Interval) Between Postdiagnosis Cumulative Average Flavonoid-Rich Food Intake and Mortality
Compared to prediagnosis intake, the postdiagnosis cumulative average flavonoid intake increased slightly by 9.79 mg/d (standard error [SE] 4.07, p = 0.02) in the HPFS during 9.78 years but decreased by −38.4 mg/d (SE 4.78, p < 0.001) in the NHS during 10.6 years. When we examined the association between intakes of flavonoids and flavonoid-rich foods after PD diagnosis, similar patterns were observed (Tables 2–4). Higher intakes of total flavonoid and several subclasses, including flavonols, anthocyanins, flavan-3-ols, and polymers, were associated with lower mortality risk (pooled adjusted HR comparing the extreme quartiles 0.78, 0.66, 0.59, 0.75, and 0.65, respectively; p < 0.05 for all). For flavonoid-rich foods, a higher intake of berries and red wine was associated with lower mortality risk (pooled HR comparing ≥3 servings per week to <1 serving per month 0.74 and 0.60, respectively; p < 0.05 for both).
In both the prediagnosis and postdiagnosis analyses, further adjustment for vitamin C, vitamin E, and beta carotene intake did not materially change the results (Table 2). The 4-year lagged analyses showed similar results (Table 2). In the NHS, using the same cutoff values as in the HPFS for grouping did not materially change the results (Table 2). We did not find any significant interaction between total flavonoid intake and age, smoking, caffeine, and alcohol intake (p for interaction > 0.05 for all).
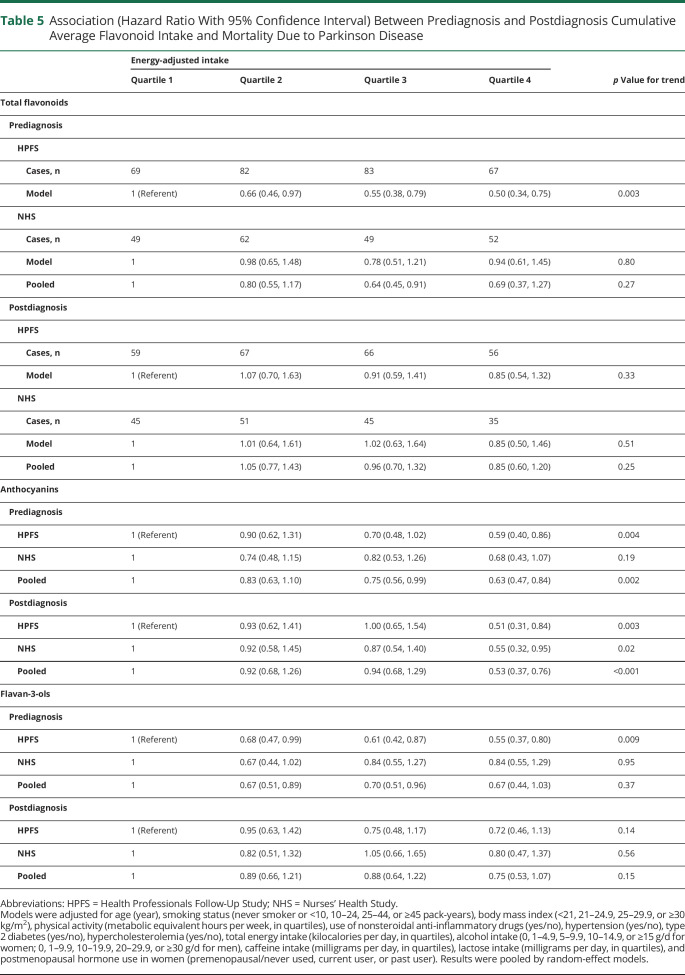
Among the 944 deaths in total, we identified 513 deaths resulting from PD, 112 deaths resulting from cardiovascular diseases, and 69 deaths resulting from cancers. Because of the relatively small case numbers, only the results for mortality due to PD are presented in Table 5. Prediagnosis total flavonoid intake was inversely associated with PD-specific mortality in men (adjusted HR comparing the extreme quartiles 0.50, 95% CI 0.34, 0.75) but not in women (HR 0.94, 95% CI 0.61, 1.45). When we examined the flavonoid subclasses that showed significant associations with all-cause mortality, significant inverse associations were found for anthocyanins intake both before and after diagnosis (pooled adjusted HR comparing the extreme quartiles 0.63 and 0.53, respectively; p < 0.05 for both).
Table 5.
Association (Hazard Ratio With 95% Confidence Interval) Between Prediagnosis and Postdiagnosis Cumulative Average Flavonoid Intake and Mortality Due to Parkinson Disease
Discussion
In this prospective cohort study, we found that individuals with PD who habitually consumed more flavonoids and certain subclasses of flavonoids (e.g., anthocyanin and flavan-3-ols) were likely to have a lower risk of all-cause mortality relative to those with lower intake. Consistently, greater consumption of berries and red wine, which are rich in anthocyanins, was also associated with lower mortality. The observed associations between flavonoid consumption before PD onset and future risk of mortality were more pronounced in men than in women.
This study examined the risk of mortality among individuals with PD in relation to the habitual diet. Although direct comparison with other studies cannot be made, our results are in line with those from previous studies on related topics, for example, an association between flavonoid intake and risk of developing PD and between flavonoid intake and mortality in general populations. In our previous study using the data from the same cohorts, higher flavonoid intakes were associated with lower incident PD risk in men (HR comparing 2 extreme quintiles 0.60, 95% CI 0.43, 0.83) but not in women (HR comparing 2 extreme quintiles 1.01, 95% CI 0.70, 1.44). Consistently, in subclass analyses, higher intakes of anthocyanins and anthocyanin-rich foods such as berries were associated with lower PD risk.6 A case-control study reported that individuals with PD (n = 81) consumed fewer blueberries and strawberries compared to their sibling controls (odds ratio 0.62 for blueberries and 0.42 for strawberries), although the difference did not reach statistical significance.25 Two recent meta-analyses found that consumption of total flavonoids, flavones, and anthocyanins was inversely associated with the risk of all-cause mortality and cardiovascular disease–attributed mortality.26,27 However, these studies did not examine mortality attributed to other reasons such as PD or neurodegenerative diseases. Our results add to the current evidence of the beneficial effect of increasing dietary flavonoid intake but additionally are focused on a group with special disease etiology and high mortality risk.
The potential biological explanations for the observed protective effect of dietary flavonoids on mortality in individuals with PD could be complex because subtle differences in structure can have marked effects on the bioavailability and bioactivity of flavonoid metabolites. We proposed the following potential mechanisms underlying the association. One main neuroprotective pathway could be through direct radical scavenging, which inhibits low-density lipoprotein oxidation and lowers chronic neuroinflammation levels.7 Flavonoids can also alter enzyme activities and thus regulate oxidative activities. This includes inhibiting nitric oxide synthase to reduce nitric oxide injury and subsequent cell membrane damage,28 increasing intracellular glutathione,29 and preventing the influx of Ca2+ despite high levels of reactive oxygen species. Studies on other plant-based bioactives, including vitamin E, beta carotene, or other carotenoids, and PD risk found mixed results,30,31 suggesting the importance of studies on flavonoids against neuroinflammation. Another explanation could be the potential protective effect of flavonoids on other major chronic diseases such as hypertension,16 cardiovascular disease,32 stroke,33 and cancers.34 Moreover, studies have reported that flavonoids protect against cognitive decline35 and depression,36 which are associated with higher mortality risk. Due to the limited number of cases in this study, we were unable to explore the association for deaths caused by other diseases except for PD. Future studies with enough power to examine cause-specific mortality are needed.
We found that higher intakes of anthocyanins and flavan-3-ols both before and after diagnosis of PD were associated with lower mortality risk. Consistently, top food contributors to dietary anthocyanins and flavan-3-ols are berries and red wines,37,38 which tended to be associated with lower mortality risk. Besides the aforementioned anti-inflammatory properties for the flavonoids family,39 anthocyanins have been shown to exert antiapoptosis effects and to protect cognition and motor functions.37 Rats fed grape juice showed an increase in dopamine release and motor performances that rely on balance, coordination, and strength.40 Moreover, anthocyanins and flavan-3-ols may improve cerebral blood flow.37,41 Anthocyanins were distributed in several regions of the brain, including the striatum.42 In animal models, the concentration of anthocyanins in the brain rises very shortly after introducing to the stomach.43 These observations further support the brain as an important site for anthocyanin functioning. Flavan-3-ols and related metabolites could act as scavengers of reactive oxygen and reactive nitrogen species44 and interact with cell signaling to downregulate cell apoptosis.38 In the Framingham Heart Study Offspring Cohort, intakes of flavonoids, especially flavan-3-ols, were reported to be inversely associated with brain MRI measures that are markers for Alzheimer disease and related dementia (p for trend = 0.01).45
The prediagnosis associations seemed to be more robust in men than in women. Using the same cutoff values as in HPFS for grouping in the NHS did not result in a significant association, suggesting that the difference is not due to sex-specific flavonoid intake level. This was consistent with findings from previous studies in which sex differences have been reported for other risk factors of PD, for example, flavonoid intake,6 caffeine intake,46 dairy intake,47 and plasma urate.48 However, because of the lack of results on flavonoids and PD prognosis from other cohorts, we cannot exclude the possibility of a chance finding. Genetic, hormonal, and inflammatory factors could interact with each other and possibly contribute to the sex difference in the potential neuroprotective effects of flavonoids at the preclinical and prodromal stages.49 Studies suggested that the clinical motor symptom could be different between men and women, and this could imply heterogeneity in the underlying causes or domain of neurodegeneration.50 Unfortunately, information on the clinical phenotypes, for example, tremor- or non–tremor-dominant PD, was not available for both NHS and HPFS participants; we thus could not examine the sex differences.
We found that postdiagnosis flavonoid intake slightly increased in the HPFS but decreased in the NHS, although the magnitudes of change (9.79 and −38.4 mg/d) were relatively small compared to the quartile median differences (range 82–313 mg/d). Due to the high risk of mortality in PD, effective prevention and treatment strategies are urgently needed to reduce disease burden and mortality. Dietary flavonoids have been suggested and tested in animal models as novel therapies for PD.9 A recent review summarized the preclinical pharmacologic studies in animals that used flavonoid compounds in neurodegenerative disease treatment.11 However, these preliminary works focused mostly on traditional Eastern medicine. Most of these animal studies suffered from poor quality, for example, lack of sample size calculation, randomization, and blinding of investigators.11 The hypotheses should be tested with well-designed drugs and rigorous study designs.
Our study fills a significant literature gap in examining the role of dietary habits on mortality among individuals with PD. This was made plausible by repeated dietary assessments over 30 years in both cohorts. We were able to examine prediagnosis and postdiagnosis flavonoid intake separately. We calculated cumulative average intake to minimize the random measurement and at the same time to capture the change in dietary intake. Several limitations also need to be considered. First, although we adjusted for major confounders, we do not have detailed information on disease severity, which may confound the association. For example, the severity of the disease, especially the severity of motor symptoms such as difficulty with chewing, was not available but could confound the observed association. We thus used prediagnosis intake as the primary exposure when motor symptoms should be less severe in general. For postdiagnosis intake, the mean time between the last dietary assessment and death/end of follow-up was 5.4 years in men and 6.4 years in women, and we further performed 4-year lagged analysis to limit the risk of reverse causality. We also calculated E values to estimate the effect an unmeasured confounder needed to fully explain away the exposure-outcome association. The E value for the highest vs the lowest quartile of flavonoid intake in men was 2.47, meaning that the observed association could be explained away only by an unmeasured confounder that was associated with both dietary flavonoid intake and all-cause mortality by a risk ratio of 2.47-fold each, above and beyond the measured confounders. Although the E value needs to be interpreted with caution by carefully considering the study design and possible bias, it is a promising statistical instrument for observational studies to measure the magnitude of residual confounding. Second, mortality was the only prognostic outcome in this study. Data on PD progression would be useful to better illustrate the neuropathologic effect of flavonoids. Meanwhile, although the NHS and HPFS cohorts had a high-follow-up rate of >97% mortality cases, there could be cases lost to the follow-up or misclassified outcomes due to the limitation of data access. Cause-specific mortality among the included participants with PD was not confirmed by neurologists, which may lead to misclassification. Third, the NHS and HPFS include predominantly White health care professionals, which limits the generalizability to individuals of other races/ethnicities and socioeconomic backgrounds. Last, future studies could examine this association among individuals without PD, which would allow a better assessment of the specificity of this finding for PD. On the basis of these limitations, caution needs to be taken in the interpretation of the findings of this study.
In this prospective cohort study, individuals with PD who consumed more flavonoids, especially anthocyanins and flavan-3-ols, had a lower risk of all-cause mortality. Consistently, higher intake of flavonoid-rich food, especially berries and red wine, was associated with a lower mortality risk. Results based on prediagnosis flavonoid intakes were more pronounced in men than in women. These findings suggest the importance of dietary flavonoids as a potentially modifiable factor to improve prognostic and life expectancy among individuals with PD.
Acknowledgment
The authors thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina; Tennessee; Texas, Virginia, Washington, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Glossary
- CI
confidence interval
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-Up Study
- HR
hazard ratio
- ICD
International Classification of Disease
- NHS
Nurses' Health Study
- PD
Parkinson disease
- SE
standard error
Appendix. Authors

Study Funding
The NHS is supported by the NIH through grant UM1 CA186107, and the HPFS is supported by the NIH through grant U01 CA167552. This study is funded by the National Institute of Neurological Disorders and Stroke through grant 1R01NS102735-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Centers for Disease Control and Prevention. QuickStats: Age-Adjusted Death Rates for Parkinson Disease Among Adults Aged ≥65 Years—National Vital Statistics System, United States, 1999–2017. MMWR Morb Mortal Wkly Rep. 2019;68:773. Accessed January 2021. 10.15585/mmwr.mm6835a6. [DOI] [PubMed] [Google Scholar]
- 2.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70(16 Pt 2):1423-1430. [DOI] [PubMed] [Google Scholar]
- 3.Herlofson K, Lie SA, Arsland D, Larsen JP. Mortality and Parkinson disease: a community based study. Neurology. 2004;62(6):937-942. [DOI] [PubMed] [Google Scholar]
- 4.Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol. 2012;69(5):601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascherio A, Weisskopf MG, O'Reilly EJ, et al. Coffee consumption, gender, and Parkinson's disease mortality in the Cancer Prevention Study II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977-984. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology. 2012;78(15):1138-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418-425. [DOI] [PubMed] [Google Scholar]
- 8.Jones QR, Warford J, Rupasinghe HP, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol Sci. 2012;33(11):602-610. [DOI] [PubMed] [Google Scholar]
- 9.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules (Basel, Switzerland). 2010;15:7792-7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascherio A, Schwarzschild MA. Dietary antioxidants and Parkinson's disease. Mov Disord. 2017;32(11):1501-1503. [DOI] [PubMed] [Google Scholar]
- 11.de Andrade Teles RB, Diniz TC, Costa Pinto TC, et al. Flavonoids as therapeutic agents in Alzheimer's and Parkinson's diseases: a systematic review of preclinical evidences. Oxid Med Cell Longev. 2018;2018:7043213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson's disease. Dysphagia. 1996;11(1):14-22. [DOI] [PubMed] [Google Scholar]
- 13.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson's disease. Age Ageing. 2006;35(6):614-618. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa C, Gemmell E, Kenworthy J, Speyer R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson's disease, Alzheimer's disease, head injury, and pneumonia. Dysphagia. 2016;31(3):434-441. [DOI] [PubMed] [Google Scholar]
- 15.Clarke CE, Gullaksen E, Macdonald S, Lowe F. Referral criteria for speech and language therapy assessment of dysphagia caused by idiopathic Parkinson's disease. Acta Neurol Scand. 1998;97(1):27-35. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy A, O'Reilly EJ, Kay C, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93(2):338-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue Y, Petimar J, Willett WC, et al. Dietary flavonoids and flavonoid-rich foods: validity and reproducibility of FFQ-derived intake estimates. Public Health Nutr. 2020;23(18):3295-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am J Epidemiol. 1994;140:1016-1019. [DOI] [PubMed] [Google Scholar]
- 19.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the national death index. Am J Epidemiol. 1984;119(5):837-839. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Li Y, Satija A, et al. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365:l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858-867. [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790-796. [DOI] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. [DOI] [PubMed] [Google Scholar]
- 24.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602-603. [DOI] [PubMed] [Google Scholar]
- 25.Golbe LI, Farrell TM, Davis PH. Case-control study of early life dietary factors in Parkinson's disease. Arch Neurol. 1988;45(12):1350-1353. [DOI] [PubMed] [Google Scholar]
- 26.Grosso G, Micek A, Godos J, et al. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: systematic review and dose-response meta-analysis. Am J Epidemiol. 2017;185(12):1304-1316. [DOI] [PubMed] [Google Scholar]
- 27.Liu XM, Liu YJ, Huang Y, et al. Dietary total flavonoids intake and risk of mortality from all causes and cardiovascular disease in the general population: a systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. 2017;61:1601003. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y-C, Huang Y-T, Tsai S-H, Lin-Shiau S-Y, Chen C-F, Lin J-K. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20(10):1945-1952. [DOI] [PubMed] [Google Scholar]
- 29.Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30(4):433-446. [DOI] [PubMed] [Google Scholar]
- 30.Hughes KC, Gao X, Kim IY, et al. Intake of antioxidant vitamins and risk of Parkinson's disease. Mov Disord. 2016;31(12):1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hantikainen E, Trolle Lagerros Y, Ye W, et al. Dietary antioxidants and the risk of Parkinson disease: the Swedish National March Cohort. Neurology. 2021;96(6):e895-e903. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11. [DOI] [PubMed] [Google Scholar]
- 33.Wang ZM, Zhao D, Nie ZL, et al. Flavonol intake and stroke risk: a meta-analysis of cohort studies. Nutrition. 2014;30(5):518-523. [DOI] [PubMed] [Google Scholar]
- 34.Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary flavonoids and the risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Nutrients. 2018;10:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh T-S, Yuan C, Ascherio A, Rosner BA, Willett WC, Blacker D. Long-term dietary flavonoid intake and subjective cognitive decline in US men and women. Neurology. 2021;97(10):e1041-e1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godos J, Castellano S, Ray S, Grosso G, Galvano F. Dietary polyphenol intake and depression: results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) study. Molecules. 2018;23:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf. 2013;12(5):483-508. [DOI] [PubMed] [Google Scholar]
- 38.Spencer JP, Schroeter H, Rechner AR, Rice-Evans C. Bioavailability of flavan-3-ols and procyanidins: gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid Redox Signal. 2001;3(6):1023-1039. [DOI] [PubMed] [Google Scholar]
- 39.Mena P, Dominguez-Perles R, Girones-Vilaplana A, Baenas N, Garcia-Viguera C, Villano D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life. 2014;66(11):745-758. [DOI] [PubMed] [Google Scholar]
- 40.Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295-302. [DOI] [PubMed] [Google Scholar]
- 41.Decroix L, Tonoli C, Soares DD, Tagougui S, Heyman E, Meeusen R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl Physiol Nutr Metab. 2016;41(12):1225-1232. [DOI] [PubMed] [Google Scholar]
- 42.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111-120. [DOI] [PubMed] [Google Scholar]
- 43.Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agric Food Chem. 2005;53(18):7029-7034. [DOI] [PubMed] [Google Scholar]
- 44.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res. 2008;52(1):79-104. [DOI] [PubMed] [Google Scholar]
- 45.Shishtar E, Rogers GT, Blumberg JB, Au R, DeCarli C, Jacques PF. Flavonoid intake and MRI markers of brain health in the Framingham Offspring Cohort. J Nutr. 2020;150(6):1545-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50(1):56-63. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, O'Reilly E, McCullough ML, et al. Consumption of dairy products and risk of Parkinson's disease. Am J Epidemiol. 2007;165(9):998-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao X, O'Reilly EJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. 2016;86(6):520-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerri S, Mus L, Blandini F. Parkinson's disease in women and men: what's the difference?. J Parkinsons Dis. 2019;9:501-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaidya B, Dhamija K, Guru P, Sharma SS. Parkinson's disease in women: mechanisms underlying sex differences. Eur J Pharmacol. 2021;895:173862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further information, including the procedures to obtain and access data from the NHS and HPFS, is available from the corresponding author on reasonable request.