Abstract
Running is among the most popular sporting hobbies and often chosen specifically for intrinsic psychological benefits. However, up to 40% of runners may experience exercise-induced dyspnoea as a result of cascading physiological phenomena, possibly causing negative psychological states or barriers to participation. Breathing techniques such as slow, deep breathing have proven benefits at rest, but it is unclear if they can be used during exercise to address respiratory limitations or improve performance. While direct experimental evidence is limited, diverse findings from exercise physiology and sports science combined with anecdotal knowledge from Yoga, meditation, and breathwork suggest that many aspects of breathing could be improved via purposeful strategies. Hence, we sought to synthesize these disparate sources to create a new theoretical framework called “Breath Tools” proposing breathing strategies for use during running to improve tolerance, performance, and lower barriers to long-term enjoyment.
Keywords: breathing pattern, coupling, running, techniques, strategies, respiration, ventilation
Introduction
Breathing is natural and automatic, sustaining life by the simple movement of air. Despite the apparent simplicity of this process, the understanding of breathing has recently been advanced extensively through investigations in medicine, sports science, and psychophysiology. The recent SARS-COVID-19 global epidemic has reminded many of the significance of breathing and the consequences of respiratory distress.
Several recent studies have brought renewed attention to the anthropological roots of breathing and its effect upon overall well-being. Yogic techniques have for millenia utilized breath awareness and exercises to cultivate “prana” (meaning both “breath” and “life force” in Sanskrit), while meditation, breathwork practices, and freediving also take advantage of breathing techniques for calm, focus, and performance. Resonant frequency breathing performed in heart-rate variability (HRV) biofeedback has significant positive effects upon HRV itself, overall autonomic nervous system regulation, and related emotional states such as anxiety and depression (Lehrer et al., 2020). Performing these breathing techniques at rest has additive effects upon cognitive function, decision-making, and concentration in sport (Jimenez Morgan and Molina Mora, 2017; De Couck et al., 2019). These effects are extremely valuable in sports contexts where both mental and physical performance affect positive psychological states such as perceived efficacy and enjoyment (Ogles et al., 1995). Although slow breathing is demonstrably efficacious at rest, the utility of slow breathing during exercise is understudied.
Recent reviews have explored the various mechanisms that may cause breathing to limit physical performance during exercise (Amann, 2012; Dempsey et al., 2020), but little work has attempted to address these mechanisms or improve breathing directly during exercise. Although running is both one of the most popular (Statista, 2018) and well-studied physical activities, very few studies have directly investigated the use of breathing techniques during running as done during Yoga, meditation, and cycling (Vickery, 2008; Saoji et al., 2019). Running deserves special attention not only for its immense global popularity but also because runners are driven by a complex mix of psychological and emotional motives (Ogles et al., 1995; Pereira et al., 2021). Since breathing can heavily affect the psychological perception of exercise (Laviolette and Laveneziana, 2014), improving breathing during running may influence tolerance or psychological state during activity. Considering the popularity of running and the diverse benefits of breathing strategies in other contexts, we sought to synthesize the available evidence to demonstrate how breathing could be used to ease respiratory distress and improve running performance and psychological states.
This narrative synthesis has three main goals:
Provide an updated overview of exercise breathing pattern, and identify respiratory limitations to running
Define and describe breathing strategies that provide physical and mental benefits for runners
Discuss practical applications and recommendations for future studies of breathing techniques during running.
Respiration During Exercise
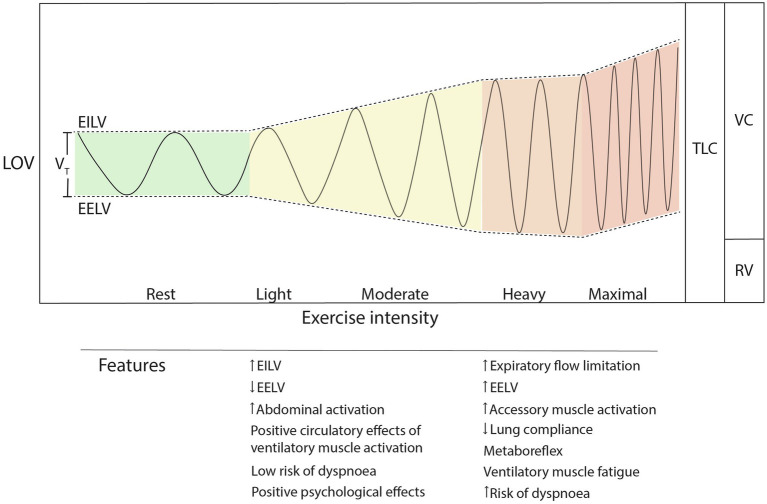
The onset of exercise stimulates rapid, characteristic changes in ventilation (VE) over 20 times greater than that at rest (hyperpnoea). The increase from an average 6 L/min up to 150 L/min occurs in response to various metabolic, homeostatic, and peripheral stimuli (Figure 1). While not completely understood, humans’ precise control of exercise hyperpnoea is driven by multiple redundant control mechanisms, such as biochemical feedback loops [especially by the partial pressure of carbon dioxide (pCO2) and blood pH], central command (neural feed-forward), and peripheral afferent feedback from the working limbs (Forster et al., 2012). During steady-state exercise, the healthy respiratory system precisely tunes VE to match metabolic rate and maintain equal O2 and CO2 balance at every level of the system (Ferretti et al., 2017). Above the respiratory compensation point (RCP; a.k.a., second ventilatory threshold), VE increases nonlinearly beyond the increase in CO2 consumption (VCO2). Ventilatory change points are trait- and state-dependent, continuously adjusting to factors like anaerobic energy utilization, blood buffering, and metabolic acidosis. Indeed, the respiratory system is remarkable in responding “just right” to exercise in most scenarios, efficiently managing VE proportional to CO2 production (VCO2). Exercise VE increases linearly (r2 = 0.99) with inspiratory drive (VT/TI; measured as mean inspiratory flow), reflecting increased neural drive to the inspiratory musculature (Naranjo et al., 2005). This is achieved via patterns of breathing rate (BR), depth, and coordinated muscle activity that maximize O2 perfusion and minimize the metabolic work of breathing (WOB; Dempsey et al., 2020). Nevertheless, there is considerable individual and situational variance in breathing pattern (BP) response to VE demands (Naranjo et al., 2005; Gravier et al., 2013).
Figure 1.
Exercise breathing pattern changes during increasing exercise intensity. Note the nonlinear increase in breathing rate and unequal partitioning of EILV and EELV as intensity increases. TLC, total lung capacity; LOV, lung operating volume; VC, vital capacity; RV, residual volume; EILV, end-inspiratory lung volume; EELV, end-expiratory lung volume; and VT, tidal volume.
Exercise Breathing Pattern
During exercise, it is thought that individuals intuitively select the BP that minimizes the metabolic cost of VE (Mead, 1960; Benchetrit, 2000; Welch et al., 2019). This is termed the “principle of minimal effort.” The popular definition of BP includes an inhale, an exhale, and a pause (Table 1). It is primarily determined by four principal variables: inspiratory flow profile (rate of airflow during inhale), inspiratory duration (TI), expiratory flow profile, and expiratory duration (TE; Tipton et al., 2017). These variables determine the typical BP descriptors of BR (60*TB−1, where TB = TE + TI) and depth (tidal volume; VT). Duty cycle (dc or breath ratio; measured as TI/TB) constrains flow to determine BP timing, depth, and airway (nose vs. mouth; Naranjo et al., 2005). These components are regulated by multiple overlapping control mechanisms, leading to a variety of coordinated patterns to achieve respiratory homeostasis. Unlike other physiological processes, BP can be consciously altered, for example to be faster or slower. Although sometime conscious, it is largely unconscious, with bidirectionality between physiological and psychological mechanisms; this qualifies BP as a “psychophysiological” construct. While VE is ultimately the product of BR and VT (Equation 1), these determinants adjust differently to various regulatory mechanisms, as do related subcomponents of BP such as timing, coordination, and coupling, discussed below (Table 1).
Table 1.
List of breathing pattern components and common abbreviations.
| Abbreviation | Variable (units) | Definition |
|---|---|---|
| BP | Breathing pattern | Differential trait and state-dependent control of breathing rhythm and mechanics |
| BR | Breathing rate (bpm) | Respiratory frequency; number of breaths taken per minute |
| Dc | Duty cycle, breath ratio (%) | Breath timing; relative percentage of inhale time to the complete breath cycle (TI/TB in %) |
| EELV | End-expiratory lung volume | Volume of the lungs at the end of an expiration |
| EID | Exercise-induced dyspnoea | Excessive perceived breathlessness during activity |
| EILV | End-inspiratory lung volume | Volume of the lungs at the end of an inspiration |
| FR | Flow reversal | Instant of breath switching; e.g., from inhale to exhale or exhale to inhale |
| LOV | Lung operating volume (%) | Mean diaphragm position at a given tidal volume (mean of EELV + EILV as % of TLC) |
| LRC | Locomotor-respiratory coupling (steps:breath) | Synchronization between flow reversal and movement; e.g., running footstrike |
| RV | Reserve volume (l) | Amount of air that remaining in airway and lungs after maximal expiration |
| TB | Breath cycle time (s) | Total breath time from inspiration to next inspiration (TI + TE) |
| TE | Exhale time (s) | Exhale duration during one breath cycle |
| TI | Inhale time (s) | Inhale duration during one breath cycle |
| TLC | Total lung capacity (l) | Total amount of air present in lungs after maximal inspiration |
| TLD | Thoraco-lumbar depth (%) | Ratio of thorax to abdominal expansion contributing to total tidal volume |
| VC | Vital capacity (l) | Total amount of air exhaled after maximal inspiration (TLC-RV) |
| VT | Tidal volume (l), depth | Breathing depth; total amount of air inspired during one breath cycle |
| VD | Ventilatory dead space (l) | Sum of airway volumes which do not contribute to gas exchange |
| VE | Minute ventilation (L/min) | Quantity of air breathed per minute |
| VO2 | Oxygen consumption (L/min) | Oxygen consumption; difference between oxygen inspired and oxygen expired in a unit of time |
| WOB | Work of breathing | Metabolic energy demand of ventilation |
| - | Thoraco-lumbar coordination (s) | Breathing coordination; time lag between thoracic and abdominal flow reversal |
| - | Ventilatory drive (l/s) | Total output of ventilatory pump; mean inspiratory flow rate (VT/TI) |
| - | Ventilatory efficiency | Ventilatory pump response to increasing demands, frequently measured as VE/VCO2 slope |
Exercise BP is modulated by central and peripheral neural mechanisms, chemoreflex stimulation, attention, and emotions, and biomechanical rhythms, among other factors. While VE increase necessitates elevated breathing rate (BR, a.k.a. respiratory frequency) and/or VT, their increases are independently regulated. Recent work indicates that during exercise BR is more “behavioral” and primarily driven by central command (activity in motor and premotor areas of the brain) and muscle afferents (Amann et al., 2010; Nicolo et al., 2016, 2017a, 2018). BR and effort are closely correlated across many different exercise intensities and experimental conditions (Nicolò et al., 2020b) because perceived exertion is likely signaled by the magnitude of central command outflow (Marcora, 2009). At submaximal intensities, BR is also affected by cognitive load, emotions, environmental stress, and exercise rhythm (more on this below; Homma and Masaoka, 2008; Grassmann et al., 2016; Tipton et al., 2017). BR is acutely responsive, adjusting almost immediately to abrupt changes in exercise intensity and stress, such as anticipatory anxiety, pain, and cold exposure (Masaoka and Homma, 2001; Tipton et al., 2017). At high relative intensities above the RCP, continued increases in VE are accomplished primarily via BR (tachypnoea; fast BR above ~80% peak BR); this point is termed the “tachypnoeic shift” (Sheel and Romer, 2011). The correlation between BR and perceived effort is particularly strong at these intensities as BR adjusts independent of absolute workload, metabolism, or muscular activation (Nicolò et al., 2018; Cochrane-Snyman et al., 2019). This may be due to increased levels of central command activity compared with low or moderate-intensity exercise (Nicolò et al., 2020a). At maximal exertion, peak BR varies substantially between individuals from 35 to 70 breaths per minute (bpm; Blackie et al., 1991; Naranjo et al., 2005).
While BR responds quickly to “fast” inputs, evidence suggests that during exercise VT adjusts slowly to optimally match alveolvar VE to VCO2 (Nicolò et al., 2017a, 2018). In their “unbalanced interdependence” model, Nicolo and Sacchetti (2019) propose that VT is secondarily regulated on the basis of BR to maintain biochemical homeostasis. This differential control likely extends across most exercise intensities (Nicolo et al., 2020). Studies report diverse responses of VT to increasing exercise intensity; in untrained exercisers, VT tends to increase until either the first or second ventilatory threshold, after which it either plateaus or declines (Gravier et al., 2013). While this plateau generally coincides with the tachypnoeic shift, some elite athletes appear to increase VT beyond the RCP and up to total exhaustion (Lucía et al., 1999). Generally, VT peaks around 50%–60% (1.9–2.7 L) of vital capacity (VC; total amount of air exhaled after maximal inspiration; Blackie et al., 1991), although in some untrained persons as low as 35% (Gravier et al., 2013) and elite athletes as high as 70% VC (Lucía et al., 1999).
The tachypnoeic shift typical of the RCP cannot entirely explain VT plateau during normal exercise conditions, since the plateau occurs before the RCP in some individuals (Gravier et al., 2013), and not at all in others (Lucía et al., 1999). Lung mechanoreceptor feedback may explain some of these disparities. VT limits are likely governed by the principle of minimal effort, as vagally-mediated afferent feedback from pulmonary stretch receptors regulates lung operating volumes [LOV; relative to end-inspiratory (EILV) and end-expiratory volume (EELV)] to minimize the WOB (Breuer, 1868; Hering, 1868; Clark and von Euler, 1972; Sheel and Romer, 2011). These mechanical limitations may interact with pCO2, which is known to suppress pulmonary stretch receptor outflow (Schelegle and Green, 2001). Clark et al. (1980) observed this phenomenon with progressive levels of hypercapnia during incremental exercise increasing VT peak. Nevertheless, despite higher relative VT peak, CO2 levels are similar or reduced in elite athletes vs. untrained persons at equivalent absolute work rates (Lucía et al., 1999). Hence, some of the mechanisms that affect the VT plateau and tachypnoeic shift during exercise are not yet entirely clear. Despite large inter-individual differences in relative VT peak, it is unclear if this is a fixed characteristic of BP; fitness level and training appear to have no effect on the VT plateau or the VE/VCO2 relationship (Salazar-Martinez et al., 2016, 2018). It is believed that the attainment of VT peak is the only circumstance at which VT substantially affects BR (Sheel and Romer, 2011; Nicolò et al., 2018).
While the tachypnoeic shift is an adaptive, essential response to maintain respiratory homeostasis at high relative exercise intensities, it coincides with increased WOB, decreased ventilatory efficiency, and peripheral fatigue (Naranjo et al., 2005; Ward, 2007; Gravier et al., 2013). Although exercise below the RCP triggers near-universal positive affect, there are homogenously negative psychological changes above the RCP (Ekkekakis et al., 2011). This may be explained by the close correlation (r = 0.71) between tachypnoea and dyspnoea during incremental exercise (Tsukada et al., 2017). While the mechanisms causing dyspnoea are complex and varied (Sheel et al., 2011), recent studies suggest that the psychological “unpleasantness” dimension of dypsnoea at its onset may contribute substantially to the near-simultaneous presentation of tachypnoea (Izumizaki et al., 2011; Tsukada et al., 2017).
Humans generally switch airway from the nose to mouth as VE increases above 40 L/min (Saibene et al., 1978). Duty cycle (dc; TI/TB) increases from resting values from about 40% (slightly longer exhale than inhale) to 50% (equal inhale to exhale) or greater at maximal intensity (Naranjo et al., 2005; Kift and Williams, 2007; Salazar-Martinez et al., 2018). Shorter TE vs. TI implies that mean expiratory flow rate must exceed mean inspiratory flow rate (rate of airflow during breath phase) in order to maintain constant LOV.
Exercise-induced VE and drive increases trigger altered ventilatory pump musculature activity and coordination. From rest to 70% max workload, diaphragmatic pressure increases more than twofold, accompanied by an increased velocity of shortening, which contributes 70%–80% of the total inspiratory force (Wallden, 2017). As exercise intensity increases, active exhales (expiratory muscle activation) lower the inspiratory WOB by reducing end-expiratory lung volume, modulating lung compliance, and storing elastic energy in the ventilatory pump musculature (Aliverti, 2016). The primary expiratory muscles are the internal obliques, which may reach 50% maximum voluntary contraction at maximal intensity (Ito et al., 2016). The intercostals, parasternals, scalenes, and neck muscles contribute to ventilation at high intensities by moderating EILV and airway caliber (e.g., dilation and inflammation). Altogether, the diaphragm and associated ventilatory pump musculature are remarkably efficient [~3%–5% total O2 consumption (VO2)] and fatigue-resistant at submaximal intensities (Welch et al., 2019; Sheel et al., 2020).
Locomotor-Respiratory Coupling
Humans are among a large proportion of animals that entrain BR to movement. The synchronization of locomotion to breath is termed “locomotor-respiratory coupling” (LRC), and involves a dual-synchronization not only of frequency [e.g., BR = step rate (SR)] but also event phase (e.g., footstrike synchronized with breath onset; O’Halloran et al., 2012). While most quadrupedal mammals utilize a 1:1 phase-locked locomotion-to-breath ratio while running due to mechanical constraints of the thorax, humans’ upright gait permits BR adjustment independent of locomotion (Bramble and Carrier, 1983). Although humans lack this mechnical constraint on breathing, they have been observed performing LRC during several rhythmic activities, such as walking, running, cycling, rowing, cross-country skiing, and even finger-tapping (Bechbache and Duffin, 1977; Persegol et al., 1991; Fabre et al., 2007; Bjorklund et al., 2015; Mathias et al., 2020). Swimming is a prime example of phase-locked breathing, as swimmers inhale during specific phases of the stroke when the face is not underwater.
Despite an apparent freedom from quadrupedal thorax constraints on breathing, LRC in humans is likely affected by various biomechanical phenomena specific to running. The “visceral piston” (three-dimensional displacement of the abdominal mass during locomotion) affects diaphragmatic contraction via direct ligamentous connections (Daley et al., 2013). Axial-appendicular dynamics have the potential to positively or negatively affect VT depending on the phasic relationship to inhale and exhale (Bramble, 1989). The effect of footstrike timing and impact forces upon VT is termed “step-driven flows,” and may affect VE up to 10%–12% (Daley et al., 2013). This could be detrimental when the timing of footstrike is out of phase (unsynchronized) with breath onset (flow reversal; FR) but additive when in-phase (synchronized). When the inhale is synchronized with peak visceral downward velocity, it pulls on the diaphragm, increasing the velocity of shortening. Daley et al. (2013) found that runners naturally prefer LRC with phase synchronziation at additive (flow-enhancing) phases, and that ventilatory transitions (change from inspiration to expiration) were quicker in these conditions of LRC. They concluded that the visceral piston and rhythmic arm movement substantially affect step-driven flows and LRC has a physiologically significant mechanical effect on breathing dynamics. These findings suggest that LRC is a result of the “minimal effort” hypothesis of breathing. If LRC reduces the WOB, it may contribute to a delayed onset of ventilatory muscle fatigue, especially at high exercise intensities, long exercise durations, or in special populations predisposed to respiratory distress (discussed below; Daley et al., 2013).
Locomotor-respiratory coupling is likely modulated by an interaction of mechanical, neurological, and metabolic interactions during running. Recent work indicates that LRC in humans is probably neurophysiological in origin, as there is a direct neurological link in humans between the respiratory and locomotor central pattern generators in the spinal cord (Le Gal et al., 2014; Del Negro et al., 2018). Group III and IV afferent feedback from the working limbs appears to affect LRC, since activities with higher-frequency limb movement produce higher levels of entrainment (Bechbache and Duffin, 1977; Caterini et al., 2016). However, close associations between limb movement, BR, and pCO2 suggest that chemoreflexive feedback affects the strength of entrainment (Forster et al., 2012). Cyclical, high-frequency activities such as running are more likely to induce entrainment vs., for example, walking, and LRC is most likely to occur at higher intensities near VO2max (maximal oxygen uptake; Bechbache and Duffin, 1977; Bernasconi and Kohl, 1993). Notably, these studies reported that increases in velocity of movement affected the strength of LRC more than intensity increase via load or gradient. There appears to be an influence of training history upon entrainment, where task preference and experience are positively associated with LRC onset and strength (Kohl et al., 1981; Bramble and Carrier, 1983; Stickford et al., 2020). These relationships were independent of overall fitness, so sport-specific experience may coincide with LRC as a learned skill (perhaps unconsciously). Finally, studies utilizing metronomes to instruct movement appeared to quickly and strongly influence LRC (Bechbache and Duffin, 1977; Bernasconi et al., 1995). Entrainment is likely to occur spontaneously and consistently in the presence of some or all of the above conditions.
Respiration as a Limiting Factor
The respiratory system in healthy individuals is considered to be generally well-adapted for the demands of exercise (Amann, 2012; Dempsey et al., 2020). Nevertheless, accumulating evidence strongly suggests that the respiratory system is “underbuilt” for the demands of intense exercise. At exercise around or above 80%–85% VO2max, three primary mechanisms cause the respiratory system to limit performance: exercise-induced arterial oxyhemoglobin desaturation, excessive ventilatory muscle work, and intrathoracic pressure effects on cardiac output (Amann, 2012). Specific scenarios (e.g., hypoxia and cold/dry climates) expose respiratory system vulnerabilities at submaximal intensities, and certain populations (e.g., elite athletes, females, and elderly) are especially susceptible; these phenomena have been recently detailed in extensive reviews (Dempsey et al., 2020; Archiza et al., 2021). While the exact limiting mechanisms differ (structural or functional), these situations and individuals bring the respiratory system close to its physiological limits. However, physiological limits do not fully explain the prevalence of exercise-induced breathlessness (a.k.a dyspnoea; EID).
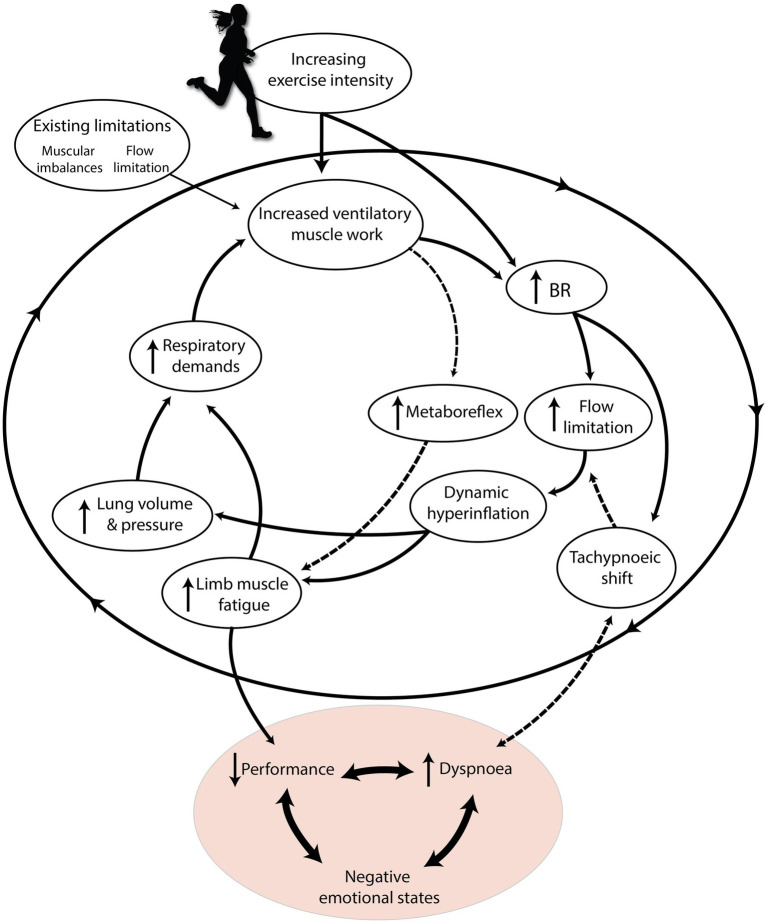
An estimated 20%–40% of otherwise healthy runners experience EID even at low absolute exercise intensities (Johansson et al., 2015; Smoliga et al., 2016; Ersson et al., 2020). This could be because unfit or deconditioned individuals may approach high levels of exertion and experience limb fatigue at low absolute workloads (Abu-Hasan et al., 2005). It could also be related to mouth breathing, since mouth-only breathing at submaximal intensities causes airway irritation, and possibly subsequent exercise-induced laryngeal obstruction (EILO; Mangla and Menon, 1981; Johansson et al., 2015). While the majority of EID prevalence may be explained by physiological limitations and deconditioning, the other most likely cause is dysfunctional breathing (Depiazzi and Everard, 2016). Distinct from pathology, dysfunctional breathing (DB; suboptimal BP) can cause otherwise healthy runners to experience premature onset of fatigue and subsequent EID (Boulding et al., 2016). Depiazzi and Everard (2016) submit that any BP deviating from slow, coordinated, diaphragmatic breathing has the potential to be “dysfunctional.” Chronic stress (internal or external) or negative emotional states could cause habitual DB during exercise (Homma and Masaoka, 2008; Tipton et al., 2017). Whether caused by physiological or psychological limits, fatigue and EID could contribute to cessation of exercise, increased rating of perceived exertion (RPE), or negative emotional states (Figure 2; Bradley and Clifton-Smith, 2009; Weinberger and Abu-Hasan, 2009). Hence, here we aim to identify three important shared phenomena that lead to respiration limiting exercise performance, tolerance, and enjoyment: dynamic hyperinflation, blood stealing, and hyperventilation.
Figure 2.
The “respiratory limiting cycle” cascade of phenomena leading to respiration limiting exercise performance and enjoyment. Increasing exercise intensity interacts with pre-existing individual constraints, causing an accumulation of respiratory phenomena that ultimately harm performance and cause dyspnoea. Dashed arrows indicate mechanisms specific to high relative exercise intensities. Adapted with permission from BradCliff® and Bradley and Clifton-Smith (2009).
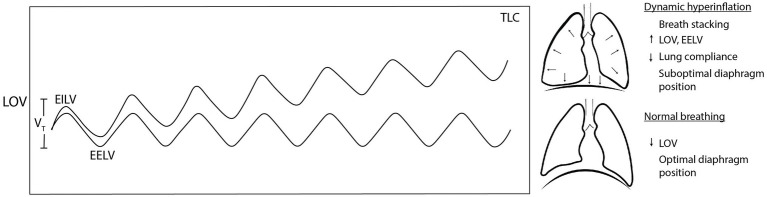
Exercise BP may fail to provide the “just right” response in the presence of flow limitation. During high intensity exercise, large increases in ventilatory flow may cause narrowing of the airway related to the Venturi effect and Bernoulli principle, among other constraints. This is termed “exercise-induced largyngeal obstruction,” and it is particularly common in elite athletes who generate large VE at high intensities (Smoliga et al., 2016). Up to 20% of elite athletes, females, adolescents, and overweight individuals may experience this during low-intensity activity (Smith et al., 2017; Dempsey et al., 2020; Ersson et al., 2020). DB phenotypes including upper-thoracic-dominant breathing and core muscle hypertonicity (such as in low back pain compensation) are also risk factors (Chaitow et al., 2014). Flow limitation could lead to “breath stacking,” a negative consequence when subsequent breaths have slightly larger inspiratory than expiratory flow (Ward, 2007). Breath stacking causes EILV and EELV to progressively increase, leading to dynamic hyperinflation (Figure 3; Sheel et al., 2020). At these higher LOV, the lungs are stiffer, less compliant, and require more muscle work to expand (Sheel et al., 2020). Unfortunately, dynamic hyperinflation places the diaphragm in a suboptimal length for expanding the lungs and managing intrathoracic pressures, further fatiguing the ventilatory musculature (Aliverti, 2016).
Figure 3.
Dynamic hyperinflation occurs when accumulated breath stacking progressively increases lung operating volume. When lung operating volume approaches total lung capacity, lung stiffness, and suboptimal diaphragm position increase the work of breathing (WOB) and dyspnoea. TLC, total lung capacity; LOV, lung operating volume; EILV, end-inspiratory lung volume; EELV, end-expiratory lung volume; and VT, tidal volume.
Diaphragmatic breathing positively affects blood shifting between the trunk and the extremities during exercise (Aliverti et al., 2010). However, during heavy exercise above ~80% peak work rate, increasing intra-thoracic pressure acts like a Valsalva maneuver, decreasing stroke volume and cardiac output (Aliverti, 2016). Furthermore, at sustained high intensities the diaphragm fatigues; it demands up to 14%–20% of cardiac output and 10%–16% of VO2 on top of concurrent accessory and expiratory muscle fatigue (Welch et al., 2019). Ventilatory muscle fatigue at high intensities triggers the metaboreflex, which ensures that the ventilatory pump maintains adequate perfusion by shunting blood from the working muscles via sympathetically-mediated vasocontriction. This competition for oxygen-rich blood is termed “blood stealing”; a detailed review is available elsewhere (Sheel et al., 2018). Although its negative haemodynamic effects generally only occur above 85% VO2max, its relationship to BP is unclear. Since the tachypnoeic shift and dynamic inflation associated with heavy exercise also elevate the WOB, it is likely that they contribute to blood stealing (Amann, 2012).
Exercise hyperpnoea is usually a “just right” respiratory response to maintaining biochemical homeostasis with increasing intensity (Dempsey et al., 2020). However, high BR may be psychologically disadvantageous, since the tachypnoeic shift onset is closely associated with EID (Izumizaki et al., 2011; Tsukada et al., 2017). Some runners may experience tachypnoea and associated dyspnoea prematurely. Healthy adult females, for instance, have hormonally-determined lung and airway limitations that predispose them to higher average BR, lower VT, and increased risk of EID (Itoh et al., 2007; Dempsey et al., 2020). During running, VT is constrained more than in other activities, and the tachypnoeic shift occurs relatively earlier (Elliott and Grace, 2010; Marko, 2020). This limitation is partially attributed to competing demands for postural and ventilatory function upon the diaphragm as well as step-driven flows (Chaitow et al., 2014; Stickford and Stickford, 2014). Another factor could be surface inclination; Bernardi et al. (2017) demonstrated that gradients above 20%–30% decrease thoraco-lumbar coordination (r = 0.99) and subsequent ventilatory efficiency (r = −0.265). Subsequently, this harmed BP (lower VT, increased BR), oxygen saturation, and performance. Since these effects were independent of absolute altitude and fatigue, they concluded that this was due to trunk inclination limiting ribcage expansion.
If the tachypnoeic response is early, or inappropriately dramatic, such as in hyperventilation DB, hypocapnia could reduce peripheral muscle perfusion via the Bohr effect (Depiazzi and Everard, 2016). This may accelerate blood stealing and limb muscle fatigue. Additionally, lower pCO2 is associated with earlier VT peak (Clark et al., 1980), which could lead to accelerated increases in BR to increase VE. Hyperventilation is accompanied by increased flow rates, which could lead to airway narrowing and flow limitation (Dempsey et al., 2020). Hyperventilation, hyperinflation, and blood stealing might together form a negative feedback loop if unchecked. If this cycle is not addressed, it could lead to EID, impaired performance, or negative emotional affect (Bradley and Clifton-Smith, 2009; Chaitow et al., 2014). If these phenomena could be avoided, we theorize that individuals could benefit from enhanced performance, reduced perception of fatigue, or prevention of negative psychological states (Figure 2).
A related, but intensity-independent aspect of respiratory limitations almost unique to running is exercise-related transient abdominal pain (ETAP), also known as “side stitch.” First mentioned by Pliny the Elder, there is still no consensus on the exact etiology of this unpleasant phenomenon (Morton and Callister, 2015). Unfortunately, this unpleasant, painful experience affects up to 70% of runners per year, which is at best frustrating and at worst a reason for exercise cessation. Some experts believe that phrenic nerve irritation related to repeated right footstrike and exhalation synchronization might be the cause (Coates and Kowalchik, 2013). Specifically, irritation of the parietal peritoneum is the most likely cause of ETAP, especially during running and in the right lower quadrant (Morton and Callister, 2015). It could be that LRC at even ratios (such as 2:1 strides per breath), leading to ipsilateral footstrike on expiration, is actually a risk factor for developing side stitch in runners.
Breathing Patterns Can Be Modified and Improved
While breathing usually provides the “just right” response to the physiological demands of exercise, respiratory limitations can lead to negative performance or psychological outcomes. If BP can be “improved” to prevent or delay the onset of dyspnoea, or to increase ventilatory efficiency, then it can benefit not only exercise performance but also the psychological effects of exercise. Although acute BP modification and longer-term breathing “retraining” have well-established benefits for human health (Zaccaro et al., 2018; Lehrer et al., 2020), this field has only recently gained attention in exercise science. A recent review addressed this disparity by exploring the utility of breath retraining for respiratory-limited athletes (Allado et al., 2021); they found that several targeted techniques (e.g., Olin EILOBI breathing) can improve symptoms of flow limitation. Several studies have utilized principles of breath retraining at rest (e.g., slow diaphragmatic breathing) to demonstrate increased exercise performance (Jimenez Morgan and Molina Mora, 2017; Bahensky et al., 2021), but it is unclear if these can be implemented during exercise, and what psychological benefits result. Whether modifying BP is possible without compromising the “minimal effort” homeostasis of the respiratory system requires discussion and more direct study. In fact, one study examining the effects of internal attentional focus upon breathing reported no overall benefit for movement economy, despite positive effects upon VE, respiratory quotient, and heart rate (Schucker et al., 2014). Nonetheless, it is known that breathing techniques improve positive emotion (Zaccaro et al., 2018), and that positive emotions can increase running economy (Brick et al., 2018), so some accommodation might unlock such performance benefits. In fact, doing so during running might be the most specific application to maximize adaptations. Despite a lack of direct evidence, theoretical and experimental findings from fields, such as cycling, respiratory medicine, and Yoga indicate various limiting mechanisms that can be addressed with breathing techniques. We hypothesize that breathing strategies employed during running could improve performance, attenuate EID, or enhance psychological states.
Breath Tools
Renewed attention to breathing techniques has inspired substantial scientific scrutiny and interest among practioners, but to our knowledge, no one has yet attempted to summarize breathing strategies for exercise in an evidence-based, organized manner. Thus, the following section is a description of techniques and “breath tools” with potential benefits to the runner. Each tool is described with an acknowledgement of some historical and anecdotal perspectives as well as a synthesis of its benefits for running biomechanics, biochemistry, and psychophysiology. The “advanced” tools are slightly different, as they increase respiratory stress to catalyze positive adaptations via training. We summarize these strategies in roughly ascending order of benefit, complexity, and risk.
Rate
Humans have long known the value of slower BR. While religious ceremonies, Yoga, and meditation rituals have explored the practice for thousands of years, recent work has confirmed the value of slow breathing for biochemical and psychophysiological benefits at rest (Russo et al., 2017; Zaccaro et al., 2018). Although evidence suggests that sustained high BR during activity could lead to respiratory limitations (see “Respiration as a Limiting Factor”), reduced BR has been understudied as a standalone breathing strategy during running.
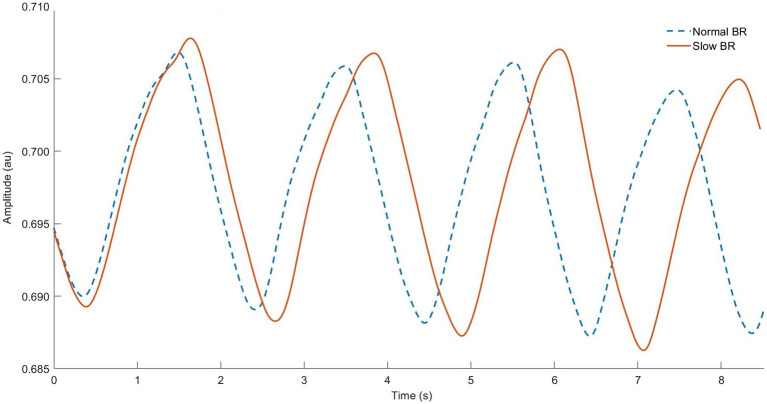
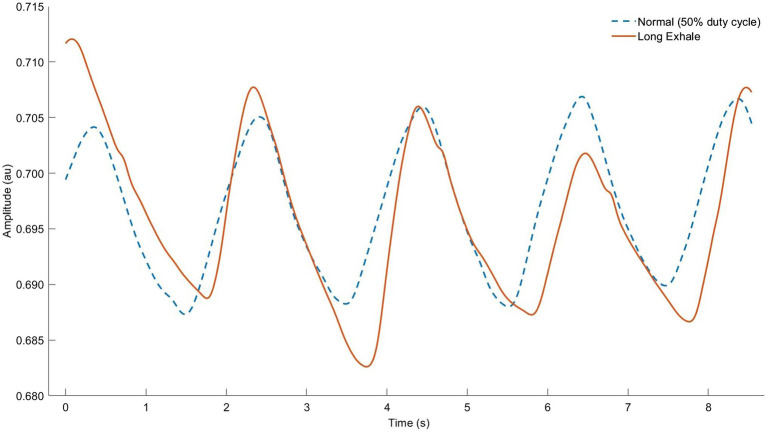
Slow BR may reduce the work of accessory respiratory muscles and subsequent WOB during exercise (Chaitow et al., 2014; Welch et al., 2019). Perhaps, the most simple advantage is improved gas exchange. Since BR is inversely proportional to VT at a given VE (Equation 1), slower breathing implicity induces greater depth of breathing (Figure 4). Since there is a fixed anatomic dead space (VD; average 150 ml of airway segments that do not participate in gas exchange), increases in VE via VT (instead of BR) beneficially manipulate relative VD (VD:VT ratio). For example, in conditions of isoventilation (Equation 1), a BR increase from 20 to 40 bpm at 10 L/min VE causes an increase of 30% VD relative to VT, and a reduction of 300 ml in alveolar ventilation (Table 2). In contrast, increasing VE to 20 L/min by only increasing VT (to 1.0 L) has the effect of halved relative VD (15%) and a 21% increase in alveolar ventilation. Simplistically, increasing VE via VT (instead of BR) allows for more oxygen-rich breaths and greater alveolar ventilation.
Figure 4.
Respiratory inductance plethysmography data from our lab showing normal breathing (dashed line) vs. “rate” breathing strategy (solid line). Note longer breath duration (horizontal) and related larger tidal volume (vertical) for each breath cycle.
Table 2.
Effect of breathing pattern on alveolar ventilation and dead space in three scenarios. Adapted from Braun (1990).
| Breathing pattern | Minute ventilation (VE, L/min) | Breathing rate (BR, bpm) | Tidal volume (VT, L) | Dead space volume (VD, ml) | Alveolar ventilation rate (L/min) | Relative VD (VD/VT, %) |
|---|---|---|---|---|---|---|
| Normal | 10 | 20 | 0.50 | 150 | 7.0 | 30% |
| Slow, deep | 10 | 10 | 1.0 | 150 | 8.5 | 15% |
| Fast, shallow | 10 | 40 | 0.25 | 150 | 4.0 | 60% |
Several studies have demonstrated remarkable plasticity of VT in healthy individuals at submaximal intensities (Vickery, 2008; Bahensky et al., 2019; Cleary, 2019; Bahensky et al., 2020). These findings may stand in contrast to the “minimal effort” hypothesis. We suspect that since an already-small percentage of VC is used for normal VT during exercise, an incrementally larger VT does not undermine the lung volume/pressure relationship, and the BR/VT relationship may be more “flexible” than previously thought. This is likely not the case above the RCP, where BR increases are driven substantially by central command (Nicolò et al., 2018) and most exercisers reach VT peak (Blackie et al., 1991). We suspect that this strategy is most appropriate at low relative exercise intensities, and perhaps not helpful or even harmful at high exercise intensities; future studies should investigate this difference.
Given the close association between BR and RPE (Nicolò et al., 2018; Cochrane-Snyman et al., 2019), we speculate that slower BR may decrease perceived feelings of effort at a given exercise intensity. Hypothetically, slower BR may “trick” the brain into feeling exercise to be easier. Hence, lower perceived effort might be reflected in improved performance or positive psychological states (Noakes, 2012). Moreover, since BR reflects the physiological response to cognitive and environmental stress at rest (Grassmann et al., 2016; Tipton et al., 2017), slowing BR during exercise may improve mental performance and calmness. As slow BR is known to positively impact autonomic nervous system balance and vagal tone at rest (Lehrer et al., 2020), it is possible that there is a similarly “optimal” BR during running that enhances the pleasant feelings of exercise (Homma and Masaoka, 2008). One study that manipulated BR during cycling found lower RPE, suppressed sympathetic and increased parasympathetic activity when breathing at very low BR of 10 bpm vs. unconstrained BR (Matsumoto et al., 2011). More studies are needed to evaluate such findings in running.
Another potential application of the “rate” strategy is to regulate exercise intensity. As BR is closely correlated with physical effort, we speculate that constant BR may limit physical output. Since mechanical limitations partially determine the comfortable limit of VT, constant “paced” BR therefore has a theoretical upper limit of VE. Paced BR therefore deterministically limits overexertion since VE cannot easily increase. For example, given a typical VC of 4 L and assumed VT peak of 60% VC (Naranjo et al., 2005), running with paced BR at 20 bpm would limit comfortable VT to 2.4 L and VE to 48 L/m. If the runner speeds up, increasing metabolic demands but not VE, there might be a dissociation of the VCO2/VE relationship. Increased pCO2 could trigger dyspnoea and air hunger (Sheel et al., 2011); this is a strong cue to “slow down.” In this way, breathing could be used to deliberately impose a limit on exercise intensity, potentially aiding in sustainable pacing of exercise. This could be especially helpful for unfit beginner runners to prevent overexertion. Considering the complex “minimal effort” regulation of BP, paced BR during exercise could cause adverse effects such as respiratory discomfort or EID; this requires more study. Nonetheless, elite athletes express lower levels of BP variability during exercise vs. healthy sedentary individuals (Castro et al., 2017), suggesting that decreased BR variability could be advantageous. Experimental investigations could address this topic by including subjective assessment of dyspnoea intensity and discomfort on top of objective measurement of physiological performance (Lewthwaite and Jensen, 2021).
Practical application of paced breathing during running invites scientific exploration. In biofeedback studies, visual feedback has been used to successfully fix BR at specific rates (Davis et al., 1999; Blum et al., 2019). Auditive feedback may be especially appropriate for field running, since over 60% of runners listen to audio, on average, during their run (Nolan, 2016). We have demonstrated in our lab that runners can easily follow continuous and periodic auditive BR instruction during running (van Rheden et al., 2021). The specific parameters for the “rate” strategy need further definition: there is likely not an absolute “best” BR for all runners, but rather a relative decrease that optimizes the benefits outlined above. Nicolò et al. (2017b) suggest monitoring BR as a percentage of an individual’s peak BR (BR/BRPeak), and this could be used similarly for the “Rate” strategy. A decrease of 10%–20% is perhaps prudent as used in previous studies with breathing retraining (Bahensky et al., 2021).
Deep
Given the interdependence of BR and VT, depth of breathing is largely dependent upon the former. However, equivalent VT can be achieved with more or less diaphragmatic engagement, and at variable LOV. Pranayama Yoga, Zen, and Transcedental meditation practices include conscious diaphragmatic breathing exercises shown to be effective for improving exercise capacity, stress reduction, and reducing symptoms of respiratory disease (Hamasaki, 2020). Thus, breathing depth ought to be considered distinctly modifiable.
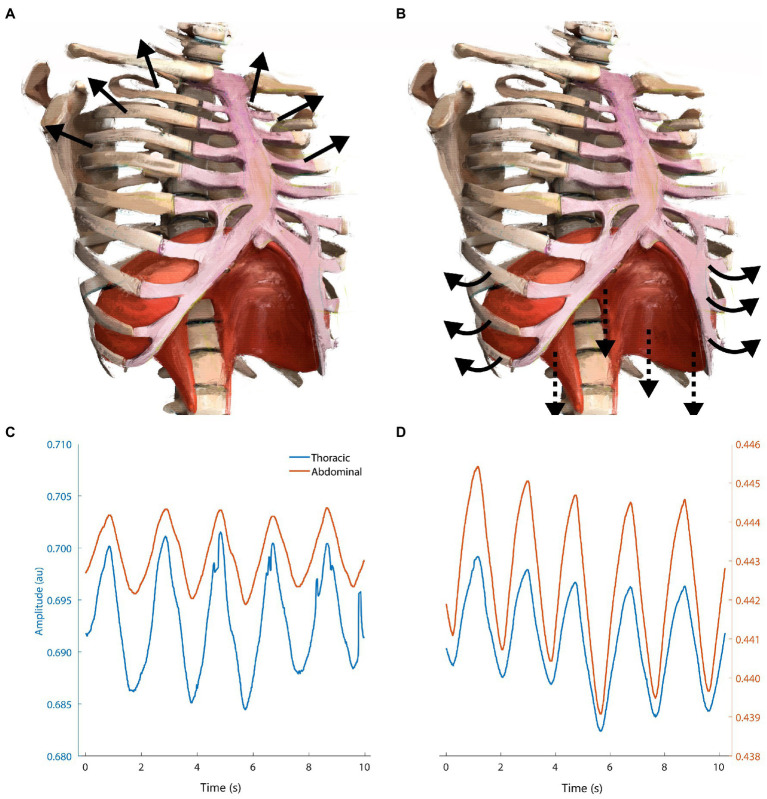
Although VT must adjust proportionally with BR to match VE demands, it can perhaps be altered independently. Elite athletes have demonstrated ventilatory compensation strategies favoring VT increases relatively greater than non-elites, especially in acute hypoxia (Lucía et al., 2001; Tipton et al., 2017). This may be an adaptive mechanism to aid in the elevated VE demands of high performance, as lung structure is remarkably intractable even with training (Dempsey et al., 2020). Not only is increasing VE via VT preferable and possible (section “rate”), but this “depth” should come from the abdominal ribcage (Figure 5).
Figure 5.
Schematic showing the difference between upper-thoracic dominant breathing (A,C) vs. “deep” diaphragmatic breathing (B,D). (A) Upper-thoracic breathing elevates and expands the upper ribcage, visible in (C) respiratory inductance plethysmography measurements from our lab showing increased amplitude in thoracic vs. abdominal bands. (B) Deep breathing flattens the diaphragm against the inferior abdominal viscera, expanding the abdominal ribcage via pump- and bucket-handle mechanisms. Adapted from Isometric angle of diaphragm and ribcage by Chest Heart & Stroke Scotland and Stuart Brett, The University of Edinburgh 2018 CC BY-NC-SA; arrows added for emphasis.
Upper-thoracic dominant breathing is associated with increased flow limitation, WOB, hyperventilation, and postural instability (Nelson, 2012; Chaitow et al., 2014; Depiazzi and Everard, 2016; Wallden, 2017). Conversely, diaphragmatic breathing is correlated with various positive health benefits, including reduced resting heart rate, post-exercise oxidative stress, increased postural control, and baroreflex sensitivity (Hazlett-Stevens and Craske, 2009; Martarelli et al., 2011; Nelson, 2012; Hamasaki, 2020). The effect of diaphragmatic breathing during exercise on these parameters is unknown. We suspect that this is due to methodological difficulties in measuring diaphragmatic contribution to breathing noninvasively. Nonetheless, diaphragmatic deep breathing might help to attenuate respiratory limitations in vulnerable individuals and situations. We suspect that this could result in reduced risk for EID and negative psychophysiological consequences.
Greater depth of breathing can be achieved through exercises to improve diaphragmatic function, and thoraco-lumbar coordination. Several publications have provided summaries of such exercises, which include paced breathing, biofeedback, and manual therapy (Hazlett-Stevens and Craske, 2009; Saoji et al., 2019; Hamasaki, 2020). On the other hand, examples of manipulating breathing depth during exercise are limited. One simple method is implementing the “rate” strategy to force VT to increase in response to slow BR (section “rate”). However, this does not necessarily cue diaphragmatic breathing as we have described. Some products such as the Buteyko belt® (Buteyko Clinic International, Galway, Ireland) may bring awareness to the diagphragm and abdominal ribcage via external tactile stimulation, but it is unclear if these are suitable during exercise. Sensors such as the Hexoskin garments (Carre Technologies, Canada) are capable of measuring abdominal ribcage expansion, but they experience significant signal contamination as a result of soft tissue artifact when used during running (Harbour et al., 2021). Visual and auditive methods from the field of biofeedback could be particularly suitable for cueing this strategy during running.
Nose
The nose is the primary point of entry and exit of the airway during healthy breathing at rest. It is functionally equipped to humidify, warm, and filter inspired air (Walker et al., 2016). It also increases nitric oxide production in the airway, and has positive effects upon pulmonary perfusion (Sanchez Crespo et al., 2010), head posture (Sabatucci et al., 2015), and cognitive function (Zelano et al., 2016). Mouth breathing is more common during exercise and for those with nasal breathing difficulties (Niinimaa, 1983). However, unlike nose breathing, habitual mouth breathing is linked with DB and numerous pathologies, including upper respiratory tract infections, rhinitis, and asthma (Chaitow et al., 2014; Walker et al., 2016). Despite these concrete advantages at rest, nasal breathing during exercise has seen mixed attention and enthusiam as a standalone breathing strategy. Nose breathing is encouraged during Pranayama Yoga exercises, and also popularized by authors/bloggers Brian McKenzie (Sh//ft.®) and Patrick McKeown (Oxygen Advantage®) as a psychophysiological state modulator. Humans usually switch to mouth breathing at VE = 40 L/min, leading to the assumption that mouth breathing is a requirement during exercise (Saibene et al., 1978). Despite this assumption, studies have demonstrated that humans have surprising flexibility in airway choice during exercise (Morton et al., 1995; Dallam and Kies, 2020).
Thomas et al. (2009) reported that subjects were able to maintain nasal breathing up to 85% VO2max during exercise when instructed with a familiarization but no other accommodation. With an adaptation period, nasal breathing during exercise may cause reduced BR, reduced hypocapnia, and increased nitric oxide production (Dallam et al., 2018). Nitric oxide production is itself beneficial as a vasodilator and bronchodilator (Sanchez Crespo et al., 2010; Thornadtsson et al., 2017), perhaps reducing the risk for flow limitation. While nasal breathing utilizes a smaller airway, which is a limitation at higher exercise intensities, it appears to increase diaphragmatic function (Trevisan et al., 2015), which could be a long-term advantage (see section “Deep”). Some studies have reported favorable performance effects, such as decreased respiratory exchange ratio, VO2, and increased running economy and time to exhaustion (Morton et al., 1995; Recinto et al., 2017). We estimate that these effects might benefically decrease RPE or dyspnoeic sensations, although direct study is required. Conversely, nasal breathing during heavy exercise leads to higher exercise HR and no difference in power output or anaerobic performance, perhaps as a result of greater inspiratory muscle load (Recinto et al., 2017).
The filtration and humidification functions of the nose may help at any exercise intensity to prevent EID and pathogen or particulate inhalation (Mangla and Menon, 1981; Aydın et al., 2014). The risk for Rhinitis and upper respiratory tract infections is substantially reduced with nasal breathing during exercise (Walker et al., 2016). Airway choice also impacts head posture and glossopharyngeal mechanics (Okuro et al., 2011; Sabatucci et al., 2015), suggesting that nasal breathing could be a long-term strategy to prevent EILO. Although there is limited evidence on the psychophysiological correlates of nasal breathing during exercise, studies suggest that nasal breathing at rest leads to improved cognitive function, emotional appraisal, memory, and lower perception of fear (Zelano et al., 2016). Hence, we suggest that nasal breathing is beneficial for its positive effects on performance, airway quality, and cognitive function during low-intensity exercise.
Implementing nasal breathing during exercise requires awareness and accommodation. Anecdotal evidence suggests that 10–12 weeks are required for meaningful changes in nasal breathing comfort and relief of airway restriction to occur, while intervention studies have examined learning periods of up to 6 months (Dallam et al., 2018). Conversely, several reports indicate that nasally-restricted breathing causes nasal airway resistance to drop in days and even minutes as a result of nitric oxide production and shifting nasal mucosa (Shturman-Ellstein et al., 1978; McCaffrey and Kern, 1979; Mertz et al., 1984). Rather, nasal breathing is self-manifesting: performing it encourages subsequent ease. In fact, nasal airway resistance falls during exercise, regardless of the airway used (Olson and Strohl, 1987). This is why the “nose” strategy is indeed an accessible choice for most runners; barriers to uptake are most likely related to habituation alone. Nasal breathing requires some adaptation, but the ideal protocols and individual differences need more investigation. An understudied aspect is whether diaphragm fatigue is improved or harmed with nose breathing, given its active resistance as a smaller airway. Finally, we recommend exercising caution when performing studies on nasal breathing in an exercise physiology setting specifically related to spiroergometry masks and their adverse effects on BP (Gilbert et al., 1972; Laveneziana et al., 2019). Future studies should explore nasal breathing in natural running settings with minimally invasive equipment, and also the details of nasal breathing accommodation.
Active Exhale
The benefits of long, slow exhales have been long promoted in Yoga and meditation fields to enhance health and well-being (Saoji et al., 2019). Some running coaches and experts have also touted this strategy, suggesting it enhances breathing depth and aerobic endurance (Jackson, 2002; Coates and Kowalchik, 2013). Although there are few studies directly examining manipulation of the exhale phase during exercise, combined evidence from other domains supports several advantages of “active” exhales.
Longer exhales may exploit respiratory sinus arrythmia to improve HRV and subjective well-being at rest (Matsumoto et al., 2008; Van Diest et al., 2014). While inspiration enhances sympathetic and suppresses parasympathetic activation, during expiration, the opposite occurs, triggering vagal afferents (Seals et al., 1993; Hayano et al., 1994). This phenomenon has been observed during incremental exercise (Blain et al., 2005). Indeed, breathing may be the main mechanism responsible for short-term HR fluctuations, especially at higher intensities (Bernardi et al., 1990; Prigent et al., 2021). Matsumoto et al. (2011) tested this effect during exercise: longer exhales (33 vs. 50% dc) caused improved HRV, ventilatory efficiency (VE/VCO2 19.1 ± 2.9 vs. 22.1 ± 4.4), and VO2 during incremental cycling. These profound results have yet to be replicated in the literature.
A separate but related approach to exhale manipulation is conscious recruitment of the expiratory musculature. Although expiration becomes active by default during exercise, additional contraction of the abdominals may confer additional benefits. In other words, stronger, forced exhales in combination with a lower duty cycle make the “active exhale.” Active exhales cause a fuller upward excursion of the diaphragm, which generates passive elastic forces, lowers diaphragmatic work, and assists in postural stabilization (Wuthrich et al., 2014; Wallden, 2017). Greater abdominal recruitment might help to partition the WOB and delay the onset of ventilatory muscle fatigue at high intensities, but this aspect is apparently unstudied. While active exhales are automatic in most situations, the respiratory limitations outlined above can cause this pattern to become dysregulated. Hence, in the presence of these limitations, it is possible that purposeful active exhales could assist in maintaining optimal LOV (Figure 6). Lower duty cycle limits inspiratory flow, and allows more time for expiratory flow; this may reduce expiratory flow limitation. In addition, the assymetric flow profile accompanying long exhales implies that peak negative inspiratory pressure exceeds negative expiratory pressure. This could have net positive effects on intrathoracic pressure as a limiter to cardiac output during high-intensity exercise (Amann, 2012). No studies have explored this aspect yet in the literature.
Figure 6.
Respiratory inductance plethysmography (RIP) data from our lab showing normal breathing (dashed line) vs. “active exhale” breathing strategy (solid line). Note that raw RIP data depict inductance, where signal increases (upward slope) correspond to the exhale phase. Observe the identical breath cycle time, but shorter relative inhale and longer exhale (smaller breath ratio) as well as lower average lung operating volume throughout the breath cycle (higher signal units indicating decreased sensor stretch).
The mountaineering community has long-touted a version of active exhales (“rescue breath” or “pressure breath”) for managing respiratory distress at altitude (Expeditions, 2014). Ian Jackson’s Breathplay technique highlights this strategy, claiming a number of benefits that were examined by Wojta et al. (1987) after a 3-day training period. The study found a delayed onset of fatigue, lower HR (1.9%), and longer time to exhaustion (7.2%) during an incremental cycling test to exhaustion. In addition, they reported a substantial delay in the onset of peak CO2 (40%), presentation of RQ = 1 (60 s later), and anaerobic threshold (120 s). Replication studies are needed to confirm these distinct results. Positive expiratory pressure may contribute to these ergogenic effects; Rupp et al. (2019) reported that forced exhales benefit peripheral and central circulation and oxygenation, especially in hypoxia, that is probably caused by increased alveolar pressure and resultant hyperperfusion. When used intermittently, this may allow marginal increases in pCO2, which enhance the Bohr Effect. This may be especially relevant for individuals and situations predisposed to hypocapnia, such as intense exercise and hyperventilation DB.
A valuable addition to the active exhale is phonation. For example, the yogic technique Bhramari Pranayama (humming during the exhale) may be effective in cueing active exhales since it not only adds additional airway resistance on the out-breath, but it profoundly increases free nitric oxide (up to 15-fold at rest; Weitzberg and Lundberg, 2002; Pramanik et al., 2009). This may enable nasal breathing at higher intensities, or ease flow limitation. This unconventional aspect has the additional benefit of amusement for the runner (or those around them). Future work should clarify whether this technique can reduce respiratory limitations or if it might adversely irritate laryngeal structures, especially whether there is an ideal frequency to perform it.
Performing active exhales during running probably requires attention, instruction, and habituation. Visual modes of biofeedback may be especially effective if displaying real-time LOV. A valuable cue may be to “squeeze all the air out” (Jackson, 2002) or fully “empty” the lungs before the inhale (Johnston et al., 2018). Other techniques such as pursed lips breathing could be combined to exploit the ergogenic effects of positive expiratory pressure, which is particularly relevant when exercising at high intensity or altitude (Rupp et al., 2019). A major limitation to studying or performing the active exhale is its deviation from the “minimal effort” BP; duty cycle is remarkably constant in most healthy exercisers (Naranjo et al., 2005), and it may require substantial cognitive focus to maintain this technique for long periods of time.
Sync
Locomotor-respiratory coupling, once penned “rhythmic breathing” by medical doctor Irwin Hance in 1919, has been the object of much scientific investigation for at least 50 years, and has wide cultural influence (Hey et al., 1966; Coates and Kowalchik, 2013). While bipedalism gives humans flexibility to perform it or not during locomotion, LRC has been observed at many ratios during running (commonly reported 4:1, 6:1, 8:1, 5:1, and 3:1 steps per breath; Bramble and Lieberman, 2004; Stickford and Stickford, 2014).
The passive assistance of step-driven flows may assist VE increases without elevating the WOB (Daley et al., 2013; Stickford and Stickford, 2014). Several studies report that LRC decreases VO2, increases running economy, and reduces dyspnoea (Garlando et al., 1985; Bernasconi et al., 1995; Takano and Deguchi, 1997; Hoffmann et al., 2012). Some have speculated that active exhales may further enhance the exhale phase in combination with LRC, as concentric contraction of the abdominal and pelvic floor musculature may optimize visceral compressive forces when synchronized with step-driven flows (Daley et al., 2013; Wallden, 2017). The “free” work granted by step-driven flows may realize some of the benefits of other strategies, since greater VT enables slower BR and reduced flow velocity at a given VE. If this eases flow limitation or EID, then it can also lead to associated positive psychological outcomes.
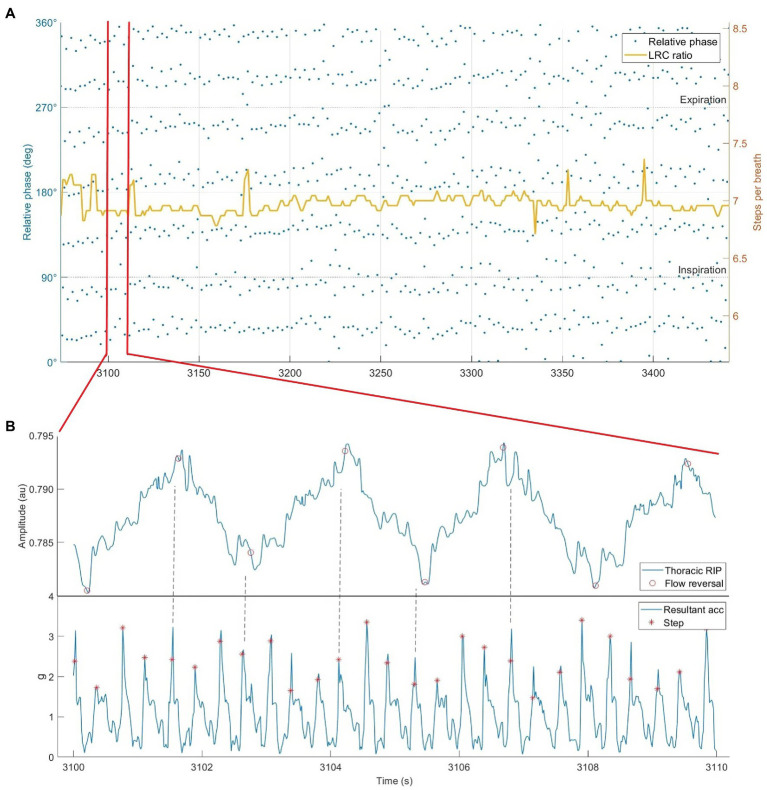
As noted in section “respiration as a limiting factor,” LRC at even ratios (e.g., 4:1 or 6:1) could be a risk factor for side stitch. However, it might also be used to prevent it. Some experts recommend exhalation on alternate steps specifically to avoid side stitch (Jackson, 2002; Coates and Kowalchik, 2013). Using an odd-numbered LRC ratio (e.g., 5:1 or 7:1; Figure 7) causes exhales to occur on opposite footstrikes, potentially limiting parietal peritoneum irritation. This might avoid such unpleasant pain and discomfort during running.
Figure 7.
Respiratory inductance plethysmography data from our lab showing locomotor-respiratory coupling (LRC). (A) Phase synchrogram and LRC ratio plotted during 8 min of running at an instructed LRC ratio 3:4 (steps per inhale:steps per exhale). Note the quantity of steps synchronized with inspiration vs. expiration. All relative phase shifted 90° for visibility. (B) Subsection of 10 s of raw RIP and hip-mounted accelerometer data while running at 3:4 LRC. Dotted lines added to emphasize step & flow reversal synchronization.
Locomotor-respiratory coupling may be energetically advantageous not only for its synergistic respiratory advantages, but also as a mediator of BR and running pace. As described in the “rate” strategy, given the close correlation between BR and RPE, and also the known stability SR during running (Van Oeveren et al., 2019), LRC may be an effective way to stabilize running pace. LRC ratios of 4:1 and 5:1 for a runner with a preferred SR around 180 (right and left, steps per minute) would thus correlate to BR of 45 and 36, respectively. Different LRC ratios could thus be utilized as a “gears” system corresponding to different perceptual and physiological levels of effort.
Since a primary mechanism of LRC during running appears to be neurological, purposeful performance of LRC may have additive psychological benefits. The immersive psychophysiological experience of flow is suggested to occur with the presence of three conditions: an activity with clear goals and progress, immediate feedback, and balance between perceived challenges and competence (Snyder et al., 2020). Entrainment, such as that of breath to step during LRC, is likely to enhance flow experience in runners (Bood et al., 2013; Nijs et al., 2020). Indeed, LRC during running satisfies the primary conditions for inducing a trance state: physical exertion, rhythm, and concentration (Damm et al., 2020). Such rhythmicity is comforting, sedating, and hypnotic, and rhythmic stability may lower stress on the nervous system by reducing cognitive fatigue (Ross et al., 2013). Future studies should explore LRC and flow phenomenology especially as it pertains to runners.
The practical application of LRC is not trivial. It is possible that the additional concentration required to execute LRC during running negates any physiological benefit. Nevertheless, past studies that found no benefit for LRC examined it in untrained individuals or during unfamiliar tasks (Yonge, 1983; Maclennan et al., 1994). Perhaps some learning and accommodation is required to realize ergogenic advantages. Although many elite runners perform it unconsciously (Bonsignore et al., 1998; McDermott et al., 2003), limited evidence is available regarding learning this as a deliberate breathing strategy, especially in sub-elite runners. A notable exception instructed LRC via haptic feedback (vibration) timed with footstrikes on either the exhale or inhale (Valsted et al., 2017). They found comparable success when the feedback was periodic (1 min of instruction followed by 2 min or no instruction) or self-selected vs. continuous. Nonetheless, some runners found LRC difficult or the instruction annoying. Attention is needed in this field to develop intelligent systems for LRC instruction and feedback, with auditive modes being understudied. Runners should probably synchronize breath to step, instead of step to breath, since deviating from individually preferred SR might be energetically disadvantageous or increase injury risk (Adams et al., 2018; De Ruiter et al., 2019). Smart feedback systems should consider adapting breath instruction to the current SR to avoid such effects and to maximize entrainment (Bood et al., 2013; Van Dyck et al., 2015). Practically, runners should use an odd ratio (such as 5:1 or 7:1) to capture the benefits of longer exhales and side stitch prevention.
Advanced Breath Tools
These breathing strategies are labeled “advanced” because they require either special equipment or are especially difficult to perform. They also carry some risk, which should be considered in context vs. the potential benefits and population of interest. Nevertheless, they are included here because they have demonstrated ergogenic benefits and are suitable for application during running.
Strength
Respiratory muscle training (RMT) has been extensively studied as an alternative strategy to improve breathing during exercise. The use of resistive breathing devices such as the Training Mask® and POWERbreathe® stress the respiratory system, resulting in positive changes in ventilatory efficiency, muscle recruitment patterns, oxygen delivery, and reduced WOB and dyspnoea (Karsten et al., 2018; Shei, 2018; Lorca-Santiago et al., 2020). Readers are directed to these three recent reviews for a detailed explanation of these mechanisms. While the majority of studies leverage these methods at rest, several studies have examined the effects of concurrent resistive breathing during exercise (Hellyer et al., 2015; Porcari et al., 2016; Barbieri et al., 2020). Experts in this field have suggested that concurrent RMT is underexplored and may, in fact, be the most effective means of transferring the benefits of RMT to sport performance (Karsten et al., 2019). Unfortunately, high-quality studies examining these scenarios are lacking.
The Olin EILOBI techniques were developed by J. Tod Olin and colleagues as a variant of inspiratory resistance breathing specifically to address EILO (Johnston et al., 2018). They were conceived to be used specifically during exercise when EILO occurs to maximize specificity. Although primarily developed for clinical applications, the self-resisted nature of this technique may qualify as RMT and be suitable for other settings. Johnston et al. (2018) report alleviation of EILO symptoms in 66% of their participants, and we suspect that this could be valuable for other runners to prevent flow limitation. While this technique is complex to learn, some components (emptying, abdominal ribcage focus) may be helpful for improving breathing mechanics.
We found exactly one study that specifically tested RMT methods during running. Granados et al. (2016) reported that wearing the Training Mask® (Training Mask LLC; Cadillac, MI, United States) during running at 60% VO2max induced hypoxaemia without substantial increases in RPE or anxiety. They concluded that incorporation of RMT methods part-time in a training routine is a convenient, time-efficient approach to benefit from RMT. Nevertheless, more studies are needed to explore long-term use of such methods. If the muscle recruitment pattern triggered by resisted breathing is not deep & diaphragmatic, it may not accumulate adequate stimulus to induce diaphragmatic hypertrophy, or it may habituate DB (Karsten et al., 2018, 2019). While many respiratory-limited individuals could benefit substantially from RMT’s subsequent reduction in EID (Bernardi et al., 2015), females appear less receptive to its benefits (Schaer et al., 2019). Finally, while it could be dangerous to induce additional respiratory distress during running, the potential ergogenic and psychological benefits suggest that careful protocol development is a key to making this a viable breathing “strategy” among runners.
Hold
Breath holding (BH, also known as hypoventilation, CO2 tolerance, or air hunger training) garnered recent popularity due to large performance benefits reported in swimming and techniques popularized by freediving (Holfelder and Becker, 2019). The various effects of hypoxia and hypercapnia have been rigorously studied (Millet et al., 2016; Girard et al., 2020), and BH is an accessible method for runners to replicate such benefits. In short, BH is a strong metabolic stressor similar to hypoxic training that causes accelerated muscle deoxygenation, hypercapnia, and increased muscle activity during exercise (Kume et al., 2016; Toubekis et al., 2017). BH protocols lasting 3–5 weeks reported performance gains of 3%–4% related to two acute mechanisms: increased stroke volume (up to 30%) and haemoglobin concentration (up to 10%; Woorons et al., 2016; Lapointe et al., 2020; Woorons et al., 2020). These ergogenic benefits are likely due to increased left ventricular stroke volume (Woorons et al., 2021b) and post-BH spleen contraction (Inoue et al., 2013). Only one study was found that examined the acute effects of BH during running (Woorons et al., 2021a). They reported dramatic central and peripheral deoxygenation when performing maximal end-expiratory BH at 60%–100% of maximal aerobic velocity, which could provide adequate stimulus for the aforementioned training effects if performed systematically.
Characteristics of elite free-divers suggest that long-term adaptations to BH include reduced CO2 chemosensitivity and increased lung volume (Bain et al., 2018; Elia et al., 2019). Repeated hypercapnia (Bloch-Salisbury et al., 1996) and endurance training (Katayama et al., 1999) cause long-term adaptations to lower chemosensitivity (measured as the ventilatory response to a given absolute workload). Moreover, reduced chemosensitivity during exercise is a characteristic of trained athletes vs. healthy sedentary individuals (McConnell and Semple, 1996). While increased pCO2 is responsible for the sensation of “air hunger” (Banzett et al., 1990), it also allows for enhanced O2 transport via the Bohr Effect. Decreased CO2 sensitivity, therefore, may allow for enhanced ventilatory efficiency and reduced BR. No studies could be found directly investigating this mechanism in exercise.
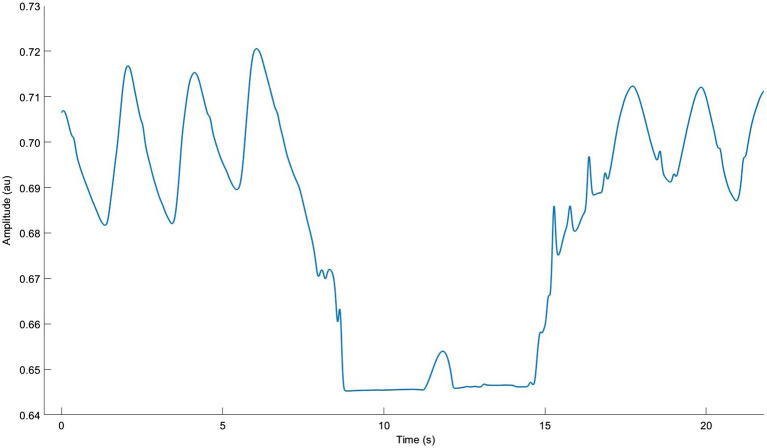
Performing BH during running is best in a safe, supervised environment with prior familiarization with BH techniques at rest. The cited studies suggest a work:rest ratio of 1:1.5 or 1:2 (e.g., 10 s hold followed by 20 s running) for 10–12 repetitions. Notably, participant instructions often include counting cycles per breath to “pace” BH duration; this is could facilitate use of the “hold” tool in the field. Most protocols recommend end-expiratory BH since it accelerates hypoxaemia and hypercapnia; this is done by performing a long exhale, and then another, down to residual volume (Figure 8). End-inspiratory BH and very slow BR trigger similar levels of hypercapnia, but not of hypoxaemia required to maximize training effects (Yamamoto et al., 1988; Holfelder and Becker, 2019). Adverse effects include hypercapnia-induced headaches, lung injury, syncope, and neurological harm if performed too often or aggressively (Matsuo et al., 2014). “Hold” is likely to be intensely difficult and psychologically unpleasant since it induces large feelings of air hunger. This may increase the risk for anxiety and emotional distress in many individuals (von Leupoldt et al., 2008). Conversely, if it can be tolerated long enough to realize its dramatic performance benefits, it may cause beneficial reductions in EID, especially at high intensities. Important work is being done to assist the execution and safety of BH with wearable devices (Vinetti et al., 2020), but this has not extended to BH during running.
Figure 8.
Respiratory inductance plethysmography data from our lab showing normal breathing with one approximate 10 s end-expiratory breath hold. Note the very long double exhale and brief diaphragm twitch.
Practical Applications
Breathing is Quantifiable
The control and expression of breathing pattern is tractibly understood and every step can be reliably and specifically measured (Del Negro et al., 2018). We suggest measuring and reporting dyspnoea intensity and discomfort in exercise studies since these data points assist substantially in identifying respiratory limitations (Lewthwaite and Jensen, 2021). If a runner has access to qualified exercise physiology personnel, cardiopulmonary exercise testing procedures can be especially useful; we recommend measuring ventilatory thresholds, tachypnoeic shift onset, VO2max, and approximate representations of ventilatory efficiency (including, but not limited to: running economy, VE/VCO2 slope, and VT/BR quotient). Voluntary spirometry measures such as forced expiratory volume and maximum voluntary ventilation may provide additional insights. For a detailed description of these laboratory-based procedures, the reader is directed to recent reviews (Janssens et al., 2013; Barnes and Kilding, 2015; Laveneziana et al., 2019; Segizbaeva and Aleksandrova, 2019; Ionescu et al., 2020).
Recent developments in wearable sensors have expanded the capabilities of respiratory system monitoring outside of the lab. Non-contact methods such as respiratory inductance plethysmography and capacitive sensors can provide estimations of BR, thoraco-lumbar coordination, and the ratio of thoracic-to-abdominal ribcage breathing (thoraco-lumbar depth) and VT in the field (Bernardi et al., 2017; Leutheuser et al., 2017; Massaroni et al., 2019). Combined FR and step detection in garments such as the Hexoskin® (Carre Technologies, Canada) could enable LRC estimation in the field (Harbour et al., 2021), although such applications are scarce. When viewed in combination with performance measures, monitoring BP may therefore reveal deep individual constraints and context-specific insights that could be modified or improved with these proposed breath tools. Alternatively, these BP measurement methods and protocols could also be used to scientifically evaluate the effectiveness of these strategies during breath retraining interventions.
Using Breath Tools
Although the value and quantity of resources related to breath retraining at rest is substantial, less is known regarding implementing breathing strategies during running. While we have provided some specific recommendations for each strategy, some general recommendations can be made. Simple awareness of BP encourages slow BR and greater depth during running (Schucker et al., 2014; Schucker and Parrington, 2019). Breathing exercises at rest can develop awareness of thoraco-lumbar coordination that carries over into exercise performance (Hagman et al., 2011; Kiesel et al., 2020). Runners can easily access such exercises in many Yoga and meditation practices (Ma et al., 2017; Saoji et al., 2019).
A unified theory of breathing strategy prescription would carefully choose the techniques above specific to the needs of the runner and scenario (Table 3). We propose the “Sync” tool as a near-universal practical recommendation, since it can be leveraged to manipulate BR, depth, and timing. However, there is limited knowledge available on how to learn this skill. Simply counting steps per breath is likely only suitable for skilled runners with experience in rhythm (e.g., musicians or dancers). Coates and Kowalchik (2013) describe a multi-step learning process that may be suitable for coaches and athletes in field running. This could be combined with the “gears” system suggested by McKenzie (2020) to adjust BP to running intensity, or the postural and verbal cues of Jackson (2002) to maintain active exhales and proper abdominal engagement. Preliminary data from our lab suggest that even novice runners can perform this skill within one session given step-synchronous audio guidance, although with variable cognitive load.
Table 3.
Overview of breath tools strategies description and application.
| Breath tool | Description | Primary mechanisms | Advantages | Disadvantages | Applications |
|---|---|---|---|---|---|
| Rate | ↓ and/or paced BR | ↓ relative VD; ANS regulation | ↑ perfusion; ↓ dyspnoea; and pacing assistance | ↑ VT at less-compliant lung volumes, initial air hunger | Novice runners; low-intensity exercise |
| Deep | ↑ VT via diaphragmatic engagement | ↓ BR; ↑ abdominal ribcage contribution to VE | ↓ WOB, LOV; ↑ postural control | Difficult to cue | Biofeedback; thoracic-dominant breathers |
| Nose | Constant or intermittent nasal breathing | ↑ NO; ↑ air humidification, warming, and filtration | ↓ airway constriction; ↑ diaphragmatic activation | Difficult at high intensities; time required for habituation | Low intensity exercise; extreme climates |
| Active exhale | Longer, forceful exhale phase with/without phonation | ↓ expiratory flow velocity: ↑ abdominal engagement, expiratory pressure, and NO | ↓ flow limitation, LOV; ↑ perfusion; and ANS regulation | ↓ relative TI; difficult to cue | Constant for calming effects; intermittent during high intensity or at altitude |
| Sync | Step & breath synchronization at whole-integer ratios | Step-driven flows; rhythmic entrainment | ↓ WOB; pacing assistance; hypnotic | Difficult to learn; even ratios ↑ side stitch | Odd ratios for ↓ side stitch; ↑ breath awareness |
| Strength | Respiratory muscle resistance training | ↑ ventilatory muscle activation, metabolic stress | ↓ WOB, dyspnoea; ↑ diaphragmatic activation | Special equipment needed; unclear protocols | Low intensity exercise; training for competition |
| Hold | Intermittent brief end-expiratory breath holds | ↑ biochemical stress, spleen contraction | ↓ chemosensitivity; cardiovascular performance | Risk of syncope, intense air hunger unpleasant | Pre-competition; elite sport |
Mechanisms, advantages, and disadvantages are based on a mixture of theoretical and empirical evidence as described in the main text. Applications are based on preliminary subjective findings of the authors, and do not constitute absolute recommendations. ANS, autonomic nervous system; BR, breathing rate; LOV, lung operating volume; NO, nitric oxide; TI, inhale time; WOB, work of breathing; VT, tidal volume; and VD, dead space.
The field of Human-Computer Interaction shows immense promise in learning breathing strategies during running, with demonstrators such as Strive (Valsted et al., 2017) and Counterpace® (Constantini et al., 2018) exploiting step-synchronized feedback modes. Core principles such as multi-sensory experience, user-centered design, and embodied interaction can guide the design of future systems to teach runners how to breathe during running (Wiehr et al., 2017; Mencarini et al., 2019; van Rheden et al., 2020). We propose auditive and haptic-based feedback systems that are field-ready with real-time learning possibilities regarding the runner’s current BP and adherence to the desired strategy.
Evidence suggests that breathing strategies such as LRC might be more effective at relative intensities lesser or greater than self-selected speeds (Stickford and Stickford, 2014). Variations away from preferred gait speed tend to increase the energy cost of transport (Hunter and Smith, 2007); thus, at metabolically “suboptimal” speeds, breathing strategies have a greater theoretical benefit. At lower intensities, we hypothesize that most runners could benefit from slower, deeper, nose breathing, which reduces the risk for respiratory limitations. These benefits may be especially helpful in extreme environments (hypoxic, dry, and cold) when the risk for EID is higher (Weiler et al., 2016). At higher intensities, strategies such as the active exhale and sync might be more helpful, as they might improve gas exchange, optimize trunk kinematics, lower the WOB, and maintain sustaintable BR. We speculate that the greatest benefits would be realized in respiratory-limited runners; this requires more study.
Limitations
Although breathing during running is measurable, modifiable, and improvable, several limitations must be addressed before further study and application. Our review includes a mix of experimental and theoretical evidence which requires more direct investigation. Notably, there is a strong hypothesis that BP manipulation is likely to be ineffective or even harmful when initially employed. If indeed healthy human ventilatory response is “just right,” and lung structure is not plastic (Dempsey et al., 2020), then perhaps changing BP will not result in improved respiratory performance. Ventilatory efficiency and overall BP are considered the result of complexity in a well-adjusted system (Benchetrit, 2000); any pertubations could be not only cognitively demanding, but also energetically costly. On the other hand, studies have shown that positive BP changes can be habituated over time periods spanning 2–6 months (Vickery, 2008; Dallam et al., 2018; Bahensky et al., 2019, 2021). It is unknown when exactly BP changes occur and under what conditions. Some studies have questioned the benefit of internal focus during running, suggesting it leads to technique breakdown and performance disruption (Beilock et al., 2002; Hill et al., 2020). Thus, there may be a switching point when the mental effort to change BP decreases, perhaps unlocking resultant benefits. Nevertheless, changing BP requires a suppression of natural reflexes and ingrained habits. More work is needed to clarify if, how, and when BP changes and which conditions facilitate this.
Conclusion
We have synthesized the evidence to demonstrate how purposeful breathing strategies might improve running via specific biochemical, biomechanical, and, ultimately, psychophysiological mechanisms. Breathing strategies have the potential to significantly improve ventilatory efficiency and exercise performance but estimates of effect size are scarce and variable. It is likely that breathing strategies do not acutely improve exercise performance but have the potential to increase it 1%–5% over a longer learning period. Respiratory-limited individuals have the most to gain by using these techniques. We theorize that the greatest benefits are psychological; increased exercise tolerance or positive psychological states might increase runners’ exercise habits and long-term training adherence. Intervention studies are needed to study these likely transformative benefits in vivo, especially over longer durations and with populations predisposed to respiratory limitations.
Author Contributions
EH and TF: conceptualization. EH: writing—original draft preparation and visualization. EH, TF, and TS: writing—review and editing. TF and HS: supervision and funding acquisition. HS: project administration. All authors contributed to the article and approved the submitted version.
Funding
This work was partly funded by the Austrian Federal Ministry for Transport, Innovation and Technology, the Austrian Federal Ministry for Digital and Economic Affairs, and the federal state of Salzburg under the research program COMET—Competence Centers for Excellent Technologies—in the project Digital Motion in Sports, Fitness and Well-being (DiMo).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abu-Hasan M., Tannous B., Weinberger M. (2005). Exercise-induced dyspnea in children and adolescents: if not asthma then what? Ann. Allergy Asthma Immunol. 94, 366–371. doi: 10.1016/S1081-1206(10)60989-1 PMID: [DOI] [PubMed] [Google Scholar]
- Adams D., Pozzi F., Willy R. W., Carrol A., Zeni J. (2018). Altering cadence or vertical oscillation during running: effects on running related injury factors. Int. J. Sports Phys. Ther. 13, 633–642. doi: 10.26603/ijspt20180633 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliverti A. (2016). The respiratory muscles during exercise. Breathe 12, 165–168. doi: 10.1183/20734735.008116 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliverti A., Uva B., Laviola M., Bovio D., Mauro A. L., Tarperi C., et al. (2010). Concomitant ventilatory and circulatory functions of the diaphragm and abdominal muscles. J. Appl. Physiol. 109, 1432–1440. doi: 10.1152/japplphysiol.00576.2010 PMID: [DOI] [PubMed] [Google Scholar]
- Allado E., Poussel M., Hily O., Chenuel B. (2021). The interest of rehabilitation of respiratory disorders in athletes: myth or reality? Ann. Phys. Rehabil. Med. 65:101461. doi: 10.1016/j.rehab.2020.101461 PMID: [DOI] [PubMed] [Google Scholar]
- Amann M. (2012). Pulmonary system limitations to endurance exercise performance in humans. Exp. Physiol. 97, 311–318. doi: 10.1113/expphysiol.2011.058800 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M., Blain G. M., Proctor L. T., Sebranek J. J., Pegelow D. F., Dempsey J. A. (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 109, 966–976. doi: 10.1152/japplphysiol.00462.2010 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archiza B., Leahy M. G., Kipp S., Sheel A. W. (2021). An integrative approach to the pulmonary physiology of exercise: when does biological sex matter? Eur. J. Appl. Physiol. 121, 2377–2391. doi: 10.1007/s00421-021-04690-9 PMID: [DOI] [PubMed] [Google Scholar]
- Aydın S., Cingi C., San T., Ulusoy S., Orhan I. (2014). The effects of air pollutants on nasal functions of outdoor runners. Eur. Arch. Otorhinolaryngol. 271, 713–717. doi: 10.1007/s00405-013-2610-1 PMID: [DOI] [PubMed] [Google Scholar]
- Bahensky P., Bunc V., Malatova R., Marko D., Grosicki G. J., Schuster J. (2021). Impact of a breathing intervention on engagement of abdominal, thoracic, and subclavian musculature during exercise, a randomized trial. J. Clin. Med. 10:3514. doi: 10.3390/jcm10163514 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahensky P., Bunc V., Marko D., Malatova R. (2020). Dynamics of ventilation parameters at different load intensities and the options to influence it by a breathing exercise. J. Sports Med. Phys. Fitness 60, 1101–1109. doi: 10.23736/S0022-4707.20.10793-X PMID: [DOI] [PubMed] [Google Scholar]
- Bahensky P., Malatova R., Bunc V. (2019). Changed dynamic ventilation parameters as a result of a breathing exercise intervention program. J. Sports Med. Phys. Fitness 59, 1369–1375. doi: 10.23736/S0022-4707.19.09483-0 PMID: [DOI] [PubMed] [Google Scholar]
- Bain A. R., Drvis I., Dujic Z., Macleod D. B., Ainslie P. N. (2018). Physiology of static breath holding in elite apneists. Exp. Physiol. 103, 635–651. doi: 10.1113/EP086269 PMID: [DOI] [PubMed] [Google Scholar]
- Banzett R. B., Lansing R. W., Brown R., Topulos G. P., Yager D., Steele S. M., et al. (1990). ‘Air hunger’ from increased PCO2 persists after complete neuromuscular block in humans. Respir. Physiol. 81, 1–17. doi: 10.1016/0034-5687(90)90065-7 PMID: [DOI] [PubMed] [Google Scholar]
- Barbieri J. F., Gaspari A. F., Teodoro C. L., Motta L., Castano L. A. A., Bertuzzi R., et al. (2020). The effect of an airflow restriction mask (ARM) on metabolic, ventilatory, and electromyographic responses to continuous cycling exercise. PLoS One 15:e0237010. doi: 10.1371/journal.pone.0237010 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K. R., Kilding A. E. (2015). Running economy: measurement, norms, and determining factors. Sports Med. Open 1:8. doi: 10.1186/s40798-015-0007-y PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechbache R., Duffin J. (1977). The entrainment of breathing frequency by exercise rhythm. Phys. J. 272, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilock S. L., Carr T. H., Macmahon C., Starkes J. L. (2002). When paying attention becomes counterproductive: impact of divided versus skill-focused attention on novice and experienced performance of sensorimotor skills. J. Exp. Psychol. Appl. 8:6. doi: 10.1037/1076-898X.8.1.6 PMID: [DOI] [PubMed] [Google Scholar]
- Benchetrit G. (2000). Breathing pattern in humans: diversity and individuality. Respir. Physiol. 122, 123–129. doi: 10.1016/S0034-5687(00)00154-7 PMID: [DOI] [PubMed] [Google Scholar]
- Bernardi E., Pomidori L., Bassal F., Contoli M., Cogo A. (2015). Respiratory muscle training with normocapnic hyperpnea improves ventilatory pattern and thoracoabdominal coordination, and reduces oxygen desaturation during endurance exercise testing in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 1899–1906. doi: 10.2147/COPD.S88609 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi E., Pratali L., Mandolesi G., Spiridonova M., Roi G. S., Cogo A. (2017). Thoraco-abdominal coordination and performance during uphill running at altitude. PLoS One 12:e0174927. doi: 10.1371/journal.pone.0174927 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L., Salvucci F., Suardi R., Soldá P. L., Calciati A., Perlini S., et al. (1990). Evidence for an intrinsic mechanism regulating heart rate variability in the transplanted and the intact heart during submaximal dynamic exercise? Cardiovasc. Res. 24, 969–981. doi: 10.1093/cvr/24.12.969 PMID: [DOI] [PubMed] [Google Scholar]
- Bernasconi P., Bürki P., Bührer A., Koller E., Kohl J. (1995). Running training and co-ordination between breathing and running rhythms during aerobic and anaerobic conditions in humans. Eur. J. Appl. Physiol. Occup. Physiol. 70, 387–393. doi: 10.1007/BF00618488 PMID: [DOI] [PubMed] [Google Scholar]
- Bernasconi P., Kohl J. (1993). Analysis of co-ordination between breathing and exercise rhythms in man. Phys. J. 471, 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund G., Holmberg H. C., Stoggl T. (2015). The effects of prior high intensity double poling on subsequent diagonal stride skiing characteristics. Springerplus 4:40. doi: 10.1186/s40064-015-0796-y PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackie S. P., Fairbarn M. S., Mcelvaney N. G., Wilcox P. G., Morrison N. J., Pardy R. L. (1991). Normal values and ranges for ventilation and breathing pattern at maximal exercise. Chest 100, 136–142. doi: 10.1378/chest.100.1.136 PMID: [DOI] [PubMed] [Google Scholar]
- Blain G., Meste O., Bermon S. (2005). Influences of breathing patterns on respiratory sinus arrhythmia in humans during exercise. Am. J. Physiol. Heart Circ. Physiol. 288, H887–H895. doi: 10.1152/ajpheart.00767.2004 PMID: [DOI] [PubMed] [Google Scholar]
- Bloch-Salisbury E., Shea S. A., Brown R., Evans K., Banzett R. B. (1996). Air hunger induced by acute increase in PCO2 adapts to chronic elevation of PCO2 in ventilated humans. J. Appl. Physiol. 81, 949–956. [DOI] [PubMed] [Google Scholar]
- Blum J., Rockstroh C., Goritz A. S. (2019). Heart rate variability biofeedback based on slow-paced breathing with immersive virtual reality nature scenery. Front. Psychol. 10:2172. doi: 10.3389/fpsyg.2019.02172 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore M. R., Morici G., Abate P., Romano S., Bonsignore G. (1998). Ventilation and entrainment of breathing during cycling and running in triathletes. Med. Sci. Sports Exerc. 30, 239–245. doi: 10.1097/00005768-199802000-00011 PMID: [DOI] [PubMed] [Google Scholar]
- Bood R. J., Nijssen M., Van Der Kamp J., Roerdink M. (2013). The power of auditory-motor synchronization in sports: enhancing running performance by coupling cadence with the right beats. PLoS One 8:e70758. doi: 10.1371/journal.pone.0070758 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulding R., Stacey R., Niven R., Fowler S. J. (2016). Dysfunctional breathing: a review of the literature and proposal for classification. Eur. Respir. Rev. 25, 287–294. doi: 10.1183/16000617.0088-2015 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Clifton-Smith T. (2009). BradCliff® Manual. Auckland, New Zealand: Writers Inc. [Google Scholar]
- Bramble D. M. (1989). Axial-appendicular dynamics and the integration of breathing and gait in mammal. Am. Zool. 29, 171–186. doi: 10.1093/icb/29.1.171 [DOI] [Google Scholar]
- Bramble D. M., Carrier D. R. (1983). Running and breathing in mammals. Science 219, 251–256. doi: 10.1126/science.6849136 PMID: [DOI] [PubMed] [Google Scholar]
- Bramble D. M., Lieberman D. E. (2004). Endurance running and the evolution of homo. Nature 432, 345–352. doi: 10.1038/nature03052 PMID: [DOI] [PubMed] [Google Scholar]
- Braun S. R. (1990). Respiratory Rate and Pattern. Stoneham, MA, USA: Butterworth Publishers, 226–230 [PubMed] [Google Scholar]
- Breuer J. (1868). Die Selbststeuerung der Athmung durch den Nervus vagus. Sitzungsberichte der kaiserlichen Akademie der Wissenschaften. Mathematisch–naturwissenschaftliche Classe, Wien, 1868, 58 Band, II. Abtheilung, 909–937.
- Brick N. E., Mcelhinney M. J., Metcalfe R. S. (2018). The effects of facial expression and relaxation cues on movement economy, physiological, and perceptual responses during running. Psychol. Sport Exerc. 34, 20–28. doi: 10.1016/j.psychsport.2017.09.009 [DOI] [Google Scholar]
- Castro R. R. T., Lima S. P., Sales A. R. K., Nobrega A. (2017). Minute-ventilation variability during cardiopulmonary exercise test is higher in sedentary men than in athletes. Arq. Bras. Cardiol. 109, 185–190. doi: 10.5935/abc.20170104 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterini J. E., Duffin J., Wells G. D. (2016). Limb movement frequency is a significant modulator of the ventilatory response during submaximal cycling exercise in humans. Respir. Physiol. Neurobiol. 220, 10–16. doi: 10.1016/j.resp.2015.09.004 PMID: [DOI] [PubMed] [Google Scholar]
- Chaitow L., Bradley D., Gilbert C. (2014). Recognizing and Treating Breathing Disorders. London: Elsevier Health Sciences. [Google Scholar]
- Clark J. M., Sinclair R. D., Lenox J. B. (1980). Chemical and nonchemical components of ventilation during hypercapnic exercise in man. J. Appl. Physiol. 48, 1065–1076. doi: 10.1152/jappl.1980.48.6.1065 PMID: [DOI] [PubMed] [Google Scholar]
- Clark F., Von Euler C. V. (1972). On the regulation of depth and rate of breathing. Phys. J. 222, 267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary S. (2019). The Effects of Deep Breathing on Exercise Performance in Humans. PhD thesis. Dublin City University.
- Coates B., Kowalchik C. (2013). Runner’s World Running on Air: The Revolutionary Way to Run Better by Breathing Smarter. New York: Rodale Books. [Google Scholar]
- Cochrane-Snyman K. C., Housh T. J., Smith C. M., Hill E. C., Jenkins N. D. (2019). Treadmill running using an RPE-clamp model: mediators of perception and implications for exercise prescription. Eur. J. Appl. Physiol. 119, 2083–2094. doi: 10.1007/s00421-019-04197-4 PMID: [DOI] [PubMed] [Google Scholar]
- Constantini K., Stickford A. S. L., Bleich J. L., Mannheimer P. D., Levine B. D., Chapman R. F. (2018). Synchronizing gait with cardiac cycle phase alters heart rate response during running. Med. Sci. Sports Exerc. 50, 1046–1053. doi: 10.1249/MSS.0000000000001515 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley M. A., Bramble D. M., Carrier D. R. (2013). Impact loading and locomotor-respiratory coordination significantly influence breathing dynamics in running humans. PLoS One 8:e70752. doi: 10.1371/journal.pone.0070752 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallam G., Kies B. (2020). The effect of nasal breathing versus oral and oronasal breathing during exercise: a review. J. Sports Res. 7, 1–10. doi: 10.18488/journal.90.2020.71.1.10 [DOI] [Google Scholar]
- Dallam G., Mcclaran S., Cox D., Foust C. (2018). Effect of nasal versus oral breathing on vo2max and physiological economy in recreational runners following an extended period spent using nasally restricted breathing. Int. J. Kinesiol. Sports Sci. 6:22. doi: 10.7575/aiac.ijkss.v.6n.2p.22 [DOI] [Google Scholar]
- Damm L., Varoqui D., De Cock V. C., Dalla Bella S., Bardy B. (2020). Why do we move to the beat? A multi-scale approach, from physical principles to brain dynamics. Neurosci. Biobehav. Rev. 112, 553–584. doi: 10.1016/j.neubiorev.2019.12.024 PMID: [DOI] [PubMed] [Google Scholar]
- Davis A. M., Ottenweller J. E., Lamanca J., Reisman S. S., Findley T. W., Natelson B. H. (1999). A simple biofeedback digital data collection instrument to control ventilation during autonomic investigations. J. Auton. Nerv. Syst. 77, 55–59. doi: 10.1016/S0165-1838(99)00035-1 PMID: [DOI] [PubMed] [Google Scholar]
- De Couck M., Caers R., Musch L., Fliegauf J., Giangreco A., Gidron Y. (2019). How breathing can help you make better decisions: two studies on the effects of breathing patterns on heart rate variability and decision-making in business cases. Int. J. Psychophysiol. 139, 1–9. doi: 10.1016/j.ijpsycho.2019.02.011 PMID: [DOI] [PubMed] [Google Scholar]
- De Ruiter C. J., Van Daal S., Van Dieen J. H. (2019). Individual optimal step frequency during outdoor running. Eur. J. Sport Sci. 20, 1–9. doi: 10.1080/17461391.2019.1626911 [DOI] [PubMed] [Google Scholar]
- Del Negro C. A., Funk G. D., Feldman J. L. (2018). Breathing matters. Nat. Rev. Neurosci. 19, 351–367. doi: 10.1038/s41583-018-0003-6 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey J. A., La Gerche A., Hull J. H. (2020). Is the healthy respiratory system built just right, overbuilt, or underbuilt to meet the demands imposed by exercise? J. Appl. Physiol. 129, 1235–1256. doi: 10.1152/japplphysiol.00444.2020 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depiazzi J., Everard M. L. (2016). Dysfunctional breathing and reaching one’s physiological limit as causes of exercise-induced dyspnoea. Breathe 12, 120–129. doi: 10.1183/20734735.007216 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekkekakis P., Parfitt G., Petruzzello S. J. (2011). The pleasure and displeasure people feel when they exercise at different intensities. Sports Med. 41, 641–671. doi: 10.2165/11590680-000000000-00000 PMID: [DOI] [PubMed] [Google Scholar]
- Elia A., Wilson O. J., Lees M., Parker P. J., Barlow M. J., Cocks M., et al. (2019). Skeletal muscle, haematological and splenic volume characteristics of elite breath-hold divers. Eur. J. Appl. Physiol. 119, 2499–2511. doi: 10.1007/s00421-019-04230-6 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. D., Grace F. (2010). An examination of exercise mode on ventilatory patterns during incremental exercise. Eur. J. Appl. Physiol. 110, 557–562. doi: 10.1007/s00421-010-1541-4 PMID: [DOI] [PubMed] [Google Scholar]
- Ersson K., Mallmin E., Malinovschi A., Norlander K., Johansson H., Nordang L. (2020). Prevalence of exercise-induced bronchoconstriction and laryngeal obstruction in adolescent athletes. Pediatr. Pulmonol. 55, 3509–3516. doi: 10.1002/ppul.25104 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expeditions (2014). Moving Air: Breathing For Performance. Mountaineering Training [Online]. Available at: https://www.rmiguides.com/blog/2014/07/07/mountaineering_training_moving_air_breathing_for_performance (Accessed October 15, 2021).
- Fabre N., Perrey S., Arbez L., Rouillon J.-D. (2007). Neuro-mechanical and chemical influences on locomotor respiratory coupling in humans. Respir. Physiol. Neurobiol. 155, 128–136. doi: 10.1016/j.resp.2006.04.015 PMID: [DOI] [PubMed] [Google Scholar]
- Ferretti G., Fagoni N., Taboni A., Bruseghini P., Vinetti G. (2017). The physiology of submaximal exercise: The steady state concept. Respir. Physiol. Neurobiol. 246, 76–85. doi: 10.1016/j.resp.2017.08.005 PMID: [DOI] [PubMed] [Google Scholar]
- Forster H. V., Haouzi P., Dempsey J. A. (2012). Control of breathing during exercise. Compr. Physiol. 2, 743–777. doi: 10.1002/cphy.c100045 PMID: [DOI] [PubMed] [Google Scholar]
- Garlando F., Kohl J., Koller E., Pietsch P. (1985). Effect of coupling the breathing-and cycling rhythms on oxygen uptake during bicycle ergometry. Eur. J. Appl. Physiol. Occup. Physiol. 54, 497–501. doi: 10.1007/BF00422959 PMID: [DOI] [PubMed] [Google Scholar]
- Gilbert R., Auchincloss J., Jr., Brodsky J., Boden W. A. (1972). Changes in tidal volume, frequency, and ventilation induced by their measurement. J. Appl. Physiol. 33, 252–254. doi: 10.1152/jappl.1972.33.2.252 PMID: [DOI] [PubMed] [Google Scholar]
- Girard O., Brocherie F., Goods P. S. R., Millet G. P. (2020). An updated panorama of “living low-training high” altitude/hypoxic methods. Front. Sports Act. Living 2:26. doi: 10.3389/fspor.2020.00026 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados J., Gillum T. L., Castillo W., Christmas K. M., Kuennen M. R. (2016). “Functional” respiratory muscle training during endurance exercise causes modest hypoxemia but overall is well tolerated. J. Strength Cond. Res. 30, 755–762. doi: 10.1519/JSC.0000000000001151 PMID: [DOI] [PubMed] [Google Scholar]
- Grassmann M., Vlemincx E., Von Leupoldt A., Mittelstadt J. M., Van Den Bergh O. (2016). Respiratory changes in response to cognitive load: a systematic review. Neural Plast. 2016:8146809. doi: 10.1155/2016/8146809 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravier G., Delliaux S., Delpierre S., Guieu R., Jammes Y. (2013). Inter-individual differences in breathing pattern at high levels of incremental cycling exercise in healthy subjects. Respir. Physiol. Neurobiol. 189, 59–66. doi: 10.1016/j.resp.2013.06.027 PMID: [DOI] [PubMed] [Google Scholar]
- Hagman C., Janson C., Emtner M. (2011). Breathing retraining—a five-year follow-up of patients with dysfunctional breathing. Respir. Med. 105, 1153–1159. doi: 10.1016/j.rmed.2011.03.006 PMID: [DOI] [PubMed] [Google Scholar]
- Hamasaki H. (2020). Effects of diaphragmatic breathing on health: a narrative review. Medicine 7:65. doi: 10.3390/medicines7100065 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour E., Lasshofer M., Genitrini M., Schwameder H. (2021). Enhanced breathing pattern detection during running using wearable sensors. Sensors 21:5606. doi: 10.3390/s21165606 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano J., Mukai S., Sakakibara M., Okada A., Takata K., Fujinami T. (1994). Effects of respiratory interval on vagal modulation of heart rate. Am. J. Physiol. Heart Circ. Physiol. 267, H33–H40. doi: 10.1152/ajpheart.1994.267.1.H33 PMID: [DOI] [PubMed] [Google Scholar]
- Hazlett-Stevens H., Craske M. G. (2009). “Breathing retraining and diaphragmatic breathing techniques,” in Cognitive Behavior Therapy: Applying Empirically Supported Techniques in your Practice. eds. O’donohue W. T., Fisher J. E., Hayes S. C. (Hoboken, New Jersey: John Wiley & Sons; ), 68–73. [Google Scholar]
- Hellyer N. J., Folsom I. A., Gaz D. V., Kakuk A. C., Mack J. L., Ver Mulm J. A. (2015). Respiratory muscle activity during simultaneous stationary cycling and inspiratory muscle training. J. Strength Cond. Res. 29, 3517–3522. doi: 10.1097/JSC.0000000000000238 PMID: [DOI] [PubMed] [Google Scholar]
- Hering K. E. K. (1868). Die Selbststeuerung der Athmung durch den Nervus vagus. Sitzungsberichte der kaiserlichen Akademie der Wissenschaften. Mathematisch–naturwissenschaftliche Classe, Wien, 1868, 57 Band, II. Abtheilung, 672–677.
- Hey E., Lloyd B., Cunningham D., Jukes M., Bolton D. (1966). Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir. Physiol. 1, 193–205. doi: 10.1016/0034-5687(66)90016-8 PMID: [DOI] [PubMed] [Google Scholar]
- Hill A., Schücker L., Wiese M., Hagemann N., Strauß B. (2020). The influence of mindfulness training on running economy and perceived flow under different attentional focus conditions–an intervention study. Int. J. Sport Exerc. Psychol. 19, 1–20. doi: 10.1080/1612197X.2020.1739110 [DOI] [Google Scholar]
- Hoffmann C. P., Torregrosa G., Bardy B. G. (2012). Sound stabilizes locomotor-respiratory coupling and reduces energy cost. PLoS One 7:e45206. doi: 10.1371/journal.pone.0045206 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holfelder B., Becker F. (2019). Hypoventilation training: a systematic review. Sports Exerc. Med. Switzerland. 66. doi: 10.34045/SSEM/2018/23 [DOI] [Google Scholar]
- Homma I., Masaoka Y. (2008). Breathing rhythms and emotions. Exp. Physiol. 93, 1011–1021. doi: 10.1113/expphysiol.2008.042424 PMID: [DOI] [PubMed] [Google Scholar]
- Hunter I., Smith G. A. (2007). Preferred and optimal stride frequency, stiffness and economy: changes with fatigue during a 1-h high-intensity run. Eur. J. Appl. Physiol. 100, 653–661. doi: 10.1007/s00421-007-0456-1 PMID: [DOI] [PubMed] [Google Scholar]
- Inoue Y., Nakajima A., Mizukami S., Hata H. (2013). Effect of breath holding on spleen volume measured by magnetic resonance imaging. PLoS One 8:e68670. doi: 10.1371/journal.pone.0068670 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu M. F., Mani-Babu S., Degani-Costa L. H., Johnson M., Paramasivan C., Sylvester K., et al. (2020). Cardiopulmonary exercise testing in the assessment of dysfunctional breathing. Front. Physiol. 11:620955. doi: 10.3389/fphys.2020.620955 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Nonaka K., Ogaya S., Ogi A., Matsunaka C., Horie J. (2016). Surface electromyography activity of the rectus abdominis, internal oblique, and external oblique muscles during forced expiration in healthy adults. J. Electromyogr. Kinesiol. 28, 76–81. doi: 10.1016/j.jelekin.2016.03.007 PMID: [DOI] [PubMed] [Google Scholar]
- Itoh M., Ueoka H., Aoki T., Hotta N., Kaneko Y., Takita C., et al. (2007). Exercise hyperpnea and hypercapnic ventilatory responses in women. Respir. Med. 101, 446–452. doi: 10.1016/j.rmed.2006.07.011 PMID: [DOI] [PubMed] [Google Scholar]
- Izumizaki M., Masaoka Y., Homma I. (2011). Coupling of dyspnea perception and tachypneic breathing during hypercapnia. Respir. Physiol. Neurobiol. 179, 276–286. doi: 10.1016/j.resp.2011.09.007 PMID: [DOI] [PubMed] [Google Scholar]
- Jackson I. (2002). The benefit of breathing out and in, instead of in and out. Healing Breath 4:2. [Google Scholar]
- Janssens L., Brumagne S., Mcconnell A. K., Raymaekers J., Goossens N., Gayan-Ramirez G., et al. (2013). The assessment of inspiratory muscle fatigue in healthy individuals: a systematic review. Respir. Med. 107, 331–346. doi: 10.1016/j.rmed.2012.11.019 PMID: [DOI] [PubMed] [Google Scholar]
- Jimenez Morgan S., Molina Mora J. A. (2017). Effect of heart rate variability biofeedback on sport performance, a systematic review. Appl. Psychophysiol. Biofeedback 42, 235–245. doi: 10.1007/s10484-017-9364-2 PMID: [DOI] [PubMed] [Google Scholar]
- Johansson H., Norlander K., Berglund L., Janson C., Malinovschi A., Nordvall L., et al. (2015). Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 70, 57–63. doi: 10.1136/thoraxjnl-2014-205738 PMID: [DOI] [PubMed] [Google Scholar]
- Johnston K. L., Bradford H., Hodges H., Moore C. M., Nauman E., Olin J. T. (2018). The Olin EILOBI breathing techniques: description and initial case series of novel respiratory retraining strategies for athletes with exercise-induced laryngeal obstruction. J. Voice 32, 698–704. doi: 10.1016/j.jvoice.2017.08.020 PMID: [DOI] [PubMed] [Google Scholar]
- Karsten M., Ribeiro G. S., Esquivel M. S., Matte D. L. (2018). The effects of inspiratory muscle training with linear workload devices on the sports performance and cardiopulmonary function of athletes: A systematic review and meta-analysis. Phys. Ther. Sport 34, 92–104. doi: 10.1016/j.ptsp.2018.09.004 PMID: [DOI] [PubMed] [Google Scholar]
- Karsten M., Ribeiro G. S., Esquivel M. S., Matte D. L. (2019). Maximizing the effectiveness of inspiratory muscle training in sports performance: A current challenge. Phys. Ther. Sport 36, 68–69. doi: 10.1016/j.ptsp.2019.01.004 PMID: [DOI] [PubMed] [Google Scholar]
- Katayama K., Sato Y., Morotome Y., Shima N., Ishida K., Mori S., et al. (1999). Ventilatory chemosensitive adaptations to intermittent hypoxic exposure with endurance training and detraining. J. Appl. Physiol. 86, 1805–1811. doi: 10.1152/jappl.1999.86.6.1805 PMID: [DOI] [PubMed] [Google Scholar]
- Kiesel K., Burklow M., Garner M. B., Hayden J., Hermann A. J., Kingshott E., et al. (2020). Exercise intervention for individuals with dysfunctional breathing: a matched controlled trial. Int. J. Sports Phys. Ther. 15, 114–125. doi: 10.26603/ijspt20200114 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kift J., Williams E. M. (2007). The respiratory time and flow profile at volitional exercise termination. J. Sports Sci. 25, 1599–1606. doi: 10.1080/02640410701275201 PMID: [DOI] [PubMed] [Google Scholar]
- Kohl J., Koller E., Jäger M. (1981). Relation between pedalling-and breathing rhythm. Eur. J. Appl. Physiol. Occup. Physiol. 47, 223–237. doi: 10.1007/BF00422468 PMID: [DOI] [PubMed] [Google Scholar]
- Kume D., Akahoshi S., Yamagata T., Wakimoto T., Nagao N. (2016). Does voluntary hypoventilation during exercise impact EMG activity? Springerplus 5:149. doi: 10.1186/s40064-016-1845-x PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J., Paradis-Deschenes P., Woorons X., Lemaitre F., Billaut F. (2020). Impact of hypoventilation training on muscle oxygenation, myoelectrical changes, systemic [k(+)], and repeated-sprint ability in basketball players. Front. Sports Act. Living 2:29. doi: 10.3389/fspor.2020.00029 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laveneziana P., Albuquerque A., Aliverti A., Babb T., Barreiro E., Dres M., et al. (2019). ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 53:1801214. doi: 10.1183/13993003.01214-2018 PMID: [DOI] [PubMed] [Google Scholar]
- Laviolette L., Laveneziana P. (2014). Dyspnoea: a multidimensional and multidisciplinary approach. Eur. Respir. J. 43, 1750–1762. doi: 10.1183/09031936.00092613 PMID: [DOI] [PubMed] [Google Scholar]
- Le Gal J. P., Juvin L., Cardoit L., Thoby-Brisson M., Morin D. (2014). Remote control of respiratory neural network by spinal locomotor generators. PLoS One 9:e89670. doi: 10.1371/journal.pone.0089683 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P., Kaur K., Sharma A., Shah K., Huseby R., Bhavsar J., et al. (2020). Heart rate variability biofeedback improves emotional and physical health and performance: a systematic review and meta analysis. Appl. Psychophysiol. Biofeedback 45, 109–129. doi: 10.1007/s10484-020-09466-z PMID: [DOI] [PubMed] [Google Scholar]
- Leutheuser H., Heyde C., Roecker K., Gollhofer A., Eskofier B. M. (2017). Reference-free adjustment of respiratory inductance plethysmography for measurements during physical exercise. IEEE Trans. Biomed. Eng. 64, 2836–2846. doi: 10.1109/TBME.2017.2675941 PMID: [DOI] [PubMed] [Google Scholar]
- Lewthwaite H., Jensen D. (2021). Multidimensional breathlessness assessment during cardiopulmonary exercise testing in healthy adults. Eur. J. Appl. Physiol. 121, 499–511. doi: 10.1007/s00421-020-04537-9 PMID: [DOI] [PubMed] [Google Scholar]
- Lorca-Santiago J., Jimenez S. L., Pareja-Galeano H., Lorenzo A. (2020). Inspiratory muscle training in intermittent sports modalities: a systematic review. Int. J. Environ. Res. Public Health 17:4448. doi: 10.3390/ijerph17124448 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucía A., Carvajal A., Calderón F. J., Alfonso A., Chicharro J. L. (1999). Breathing pattern in highly competitive cyclists during incremental exercise. Eur. J. Appl. Physiol. Occup. Physiol. 79, 512–521. [DOI] [PubMed] [Google Scholar]
- Lucía A., Hoyos J., Pardo J., Chicharro J. L. (2001). Effects of endurance training on the breathing pattern of professional cyclists. Jpn. J. Physiol. 51, 133–141. doi: 10.2170/jjphysiol.51.133 PMID: [DOI] [PubMed] [Google Scholar]
- Ma X., Yue Z. Q., Gong Z. Q., Zhang H., Duan N. Y., Shi Y. T., et al. (2017). The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front. Psychol. 8:874. doi: 10.3389/fpsyg.2017.00874 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan S. E., Silvestri G. A., Ward J., Mahler D. A. (1994). Does entrained breathing improve the economy of rowing? Med. Sci. Sports Exerc. 26, 610–614. PMID: [PubMed] [Google Scholar]
- Mangla P., Menon M. (1981). Effect of nasal and oral breathing on exercise-induced asthma. Clin. Exp. Allergy 11, 433–439. doi: 10.1111/j.1365-2222.1981.tb01616.x PMID: [DOI] [PubMed] [Google Scholar]
- Marcora S. (2009). Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J. Appl. Physiol. 106, 2060–2062. doi: 10.1152/japplphysiol.90378.2008 PMID: [DOI] [PubMed] [Google Scholar]
- Marko D. (2020). “Comparison of results of spiroergometry on running and bicycle ergometer of athletes with running and cycling specialization.” in Proceedings of the 12th International Conference on Kinanthropology. November 7-9, 2019.
- Martarelli D., Cocchioni M., Scuri S., Pompei P. (2011). Diaphragmatic breathing reduces exercise-induced oxidative stress. Evid. Based Complement. Alternat. Med. 2011:932430. doi: 10.1093/ecam/nep169 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka Y., Homma I. (2001). The effect of anticipatory anxiety on breathing and metabolism in humans. Respir. Physiol. 128, 171–177. doi: 10.1016/S0034-5687(01)00278-X PMID: [DOI] [PubMed] [Google Scholar]
- Massaroni C., Nicolò A., Lo Presti D., Sacchetti M., Silvestri S., Schena E. (2019). Contact-based methods for measuring respiratory rate. Sensors 19:908. doi: 10.3390/s19040908 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias B., Zamm A., Gianferrara P. G., Ross B., Palmer C. (2020). Rhythm complexity modulates behavioral and neural dynamics during auditory–motor synchronization. J. Cogn. Neurosci. 32, 1864–1880. doi: 10.1162/jocn_a_01601 PMID: [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Masuda T., Hotta K., Shimizu R., Ishii A., Kutsuna T., et al. (2011). Effects of prolonged expiration breathing on cardiopulmonary responses during incremental exercise. Respir. Physiol. Neurobiol. 178, 275–282. doi: 10.1016/j.resp.2011.06.025 PMID: [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Matsunaga A., Hara M., Saitoh M., Yonezawa R., Ishii A., et al. (2008). Effects of the breathing mode characterized by prolonged expiration on respiratory and cardiovascular responses and autonomic nervous activity during the exercise. Jpn. J. Phys. Fit. 57, 315–326. doi: 10.7600/jspfsm.57.315 [DOI] [Google Scholar]
- Matsuo R., Kamouchi M., Arakawa S., Furuta Y., Kanazawa Y., Kitazono T. (2014). Magnetic resonance imaging in breath-hold divers with cerebral decompression sickness. Case Rep. Neurol. 6, 23–27. doi: 10.1159/000357169 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccaffrey T. V., Kern E. B. (1979). Response of nasal airway resistance to hypercapnia and hypoxia in man. Ann. Otol. Rhinol. Laryngol. 88, 247–252. doi: 10.1177/000348947908800217 PMID: [DOI] [PubMed] [Google Scholar]
- Mcconnell A. K., Semple E. (1996). Ventilatory sensitivity to carbon dioxide: the influence of exercise and athleticism. Med. Sci. Sports Exerc. 28, 685–691. doi: 10.1097/00005768-199606000-00007 PMID: [DOI] [PubMed] [Google Scholar]
- Mcdermott W. J., Van Emmerik R. E., Hamill J. (2003). Running training and adaptive strategies of locomotor-respiratory coordination. Eur. J. Appl. Physiol. 89, 435–444. doi: 10.1007/s00421-003-0831-5 PMID: [DOI] [PubMed] [Google Scholar]
- Mckenzie B. (2020). “The Gears,” (ed.) Sh//Ft. (Vimeo).
- Mead J. (1960). Control of respiratory frequency. J. Appl. Physiol. 15, 325–336. doi: 10.1152/jappl.1960.15.3.325 PMID: 35099342 [DOI] [Google Scholar]
- Mencarini E., Rapp A., Tirabeni L., Zancanaro M. (2019). Designing wearable systems for sports: a review of trends and opportunities in human–computer interaction. IEEE Trans. Hum. Mach. Syst. 49, 314–325. doi: 10.1109/THMS.2019.2919702 [DOI] [Google Scholar]
- Mertz J. S., Mccaffrey T. V., Kern E. B. (1984). Role of the nasal airway in regulation of airway resistance during hypercapnia and exercise. Otolaryngol. Head Neck Surg. 92, 302–307. doi: 10.1177/019459988409200311 PMID: [DOI] [PubMed] [Google Scholar]
- Millet G. P., Debevec T., Brocherie F., Malatesta D., Girard O. (2016). Therapeutic use of exercising in hypoxia: promises and limitations. Front. Physiol. 7:224. doi: 10.3389/fphys.2016.00224 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D., Callister R. (2015). Exercise-related transient abdominal pain (ETAP). Sports Med. 45, 23–35. doi: 10.1007/s40279-014-0245-z PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton A., King K., Papalia S., Goodman C., Turley K., Wilmore J. (1995). Comparison of maximal oxygen consumption with oral and nasal breathing. Aust. J. Sci. Med. Sport 27, 51–55. PMID: [PubMed] [Google Scholar]
- Naranjo J., Centeno R. A., Galiano D., Beaus M. (2005). A nomogram for assessment of breathing patterns during treadmill exercise. Br. J. Sports Med. 39, 80–83. doi: 10.1136/bjsm.2003.009316 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. (2012). Diaphragmatic breathing: the foundation of core stability. Strength Cond. J. 34, 34–40. doi: 10.1519/SSC.0b013e31826ddc07 [DOI] [Google Scholar]
- Nicolò A., Girardi M., Bazzucchi I., Felici F., Sacchetti M. (2018). Respiratory frequency and tidal volume during exercise: differential control and unbalanced interdependence. Phys. Rep. 6:e13908. doi: 10.14814/phy2.13908 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolò A., Girardi M., Sacchetti M. (2017a). Control of the depth and rate of breathing: metabolic vs. non-metabolic inputs. J. Physiol. 595, 6363–6364. doi: 10.1113/JP275013 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo A., Marcora S. M., Sacchetti M. (2016). Respiratory frequency is strongly associated with perceived exertion during time trials of different duration. J. Sports Sci. 34, 1199–1206. doi: 10.1080/02640414.2015.1102315 PMID: [DOI] [PubMed] [Google Scholar]
- Nicolo A., Marcora S. M., Sacchetti M. (2020). Last word on viewpoint: time to reconsider how ventilation is regulated above the respiratory compensation point during incremental exercise. J. Appl. Physiol. 128:1456. doi: 10.1152/japplphysiol.00285.2020 PMID: [DOI] [PubMed] [Google Scholar]
- Nicolò A., Marcora S. M., Sacchetti M. (2020a). Time to reconsider how ventilation is regulated above the respiratory compensation point during incremental exercise. J. Appl. Physiol. 128, 1447–1449. doi: 10.1152/japplphysiol.00814.2019 PMID: [DOI] [PubMed] [Google Scholar]
- Nicolò A., Massaroni C., Passfield L. (2017b). Respiratory frequency during exercise: the neglected physiological measure. Front. Physiol. 8:922. doi: 10.3389/fphys.2017.00922 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolò A., Massaroni C., Schena E., Sacchetti M. (2020b). The importance of respiratory rate monitoring: from healthcare to sport and exercise. Sensors 20:6396. doi: 10.3390/s20216396 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo A., Sacchetti M. (2019). A new model of ventilatory control during exercise. Exp. Physiol. 104, 1331–1332. doi: 10.1113/EP087937 PMID: [DOI] [PubMed] [Google Scholar]
- Niinimaa V. (1983). Oronasal airway choice during running. Respir. Physiol. 53, 129–133. doi: 10.1016/0034-5687(83)90021-X PMID: [DOI] [PubMed] [Google Scholar]
- Nijs A., Roerdink M., Beek P. J. (2020). Cadence modulation in walking and running: pacing steps or strides? Brain Sci. 10:273. doi: 10.3390/brainsci10050273 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes T. D. (2012). Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front. Physiol. 3:82. doi: 10.3389/fphys.2012.00082 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan A. (2016). The State of the American Runner [Online]. Runner’s World. Available at: https://www.runnersworld.com/runners-stories/a20825842/the-state-of-the-american-runner-2016/ (Accessed 11 October 2021).
- O’halloran J., Hamill J., Mcdermott W. J., Remelius J. G., Van Emmerik R. E. (2012). Locomotor-respiratory coupling patterns and oxygen consumption during walking above and below preferred stride frequency. Eur. J. Appl. Physiol. 112, 929–940. doi: 10.1007/s00421-011-2040-y PMID: [DOI] [PubMed] [Google Scholar]
- Ogles B., Masters K., Richardson S. (1995). Obligatory running and gender: an analysis of participative motives and training habits. Int. J. Sport Psychol. 26, 233–248. [Google Scholar]
- Okuro R. T., Morcillo A. M., Sakano E., Schivinski C. I. S., Ribeiro M. Â. G. O., Ribeiro J. D. (2011). Exercise capacity, respiratory mechanics and posture in mouth breathers. Braz. J. Otorhinolaryngol. 77, 656–662. doi: 10.1590/S1808-86942011000500020 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. G., Strohl K. P. (1987). The response of the nasal airway to exercise. Am. Rev. Respir. Dis. 135, 356–359. doi: 10.1164/arrd.1987.135.2.356 PMID: [DOI] [PubMed] [Google Scholar]
- Pereira H. V., Palmeira A. L., Encantado J., Marques M. M., Santos I., Carraa E. V., et al. (2021). Systematic review of psychological and behavioral correlates of recreational running. Front. Psychol. 12:624783. doi: 10.3389/fpsyg.2021.624783 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persegol L., Jordan M., Viala D. (1991). Evidence for the entrainment of breathing by locomotor pattern in human. J. Physiol. 85, 38–43. PMID: [PubMed] [Google Scholar]
- Porcari J. P., Probst L., Forrester K., Doberstein S., Foster C., Cress M. L., et al. (2016). Effect of wearing the elevation training mask on aerobic capacity, lung function, and hematological variables. J. Sports Sci. Med. 15, 379–386. PMID: [PMC free article] [PubMed] [Google Scholar]
- Pramanik T., Sharma H. O., Mishra S., Mishra A., Prajapati R., Singh S. (2009). Immediate effect of slow pace bhastrika pranayama on blood pressure and heart rate. J. Altern. Complement. Med. 15, 293–295. doi: 10.1089/acm.2008.0440 PMID: [DOI] [PubMed] [Google Scholar]
- Prigent G., Aminian K., Rodrigues T., Vesin J. M., Millet G. P., Falbriard M., et al. (2021). Indirect estimation of breathing rate from heart rate monitoring system during running. Sensors 21:5651. doi: 10.3390/s21165651 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinto C., Efthemeou T., Boffelli P. T., Navalta J. W. (2017). Effects of nasal or oral breathing on anaerobic power output and metabolic responses. Int. J. Exerc. Sci. 10, 506–514. PMID: [PMC free article] [PubMed] [Google Scholar]
- Ross C. F., Blob R. W., Carrier D. R., Daley M. A., Deban S. M., Demes B., et al. (2013). The evolution of locomotor rhythmicity in tetrapods. Evolution 67, 1209–1217. doi: 10.1111/evo.12015 PMID: [DOI] [PubMed] [Google Scholar]
- Rupp T., Saugy J. J., Bourdillon N., Verges S., Millet G. P. (2019). Positive expiratory pressure improves arterial and cerebral oxygenation in acute normobaric and hypobaric hypoxia. Am. J. Phys. Regul. Integr. Comp. Phys. 317, R754–R762. doi: 10.1152/ajpregu.00025.2019 PMID: [DOI] [PubMed] [Google Scholar]
- Russo M. A., Santarelli D. M., O’rourke D. (2017). The physiological effects of slow breathing in the healthy human. Breathe 13, 298–309. doi: 10.1183/20734735.009817 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatucci A., Raffaeli F., Mastrovincenzo M., Luchetta A., Giannone A., Ciavarella D. (2015). Breathing pattern and head posture: changes in craniocervical angles. Minerva Stomatol. 64, 59–74. PMID: [PubMed] [Google Scholar]
- Saibene F., Mognoni P., Lafortuna C. L., Mostardi R. (1978). Oronasal breathing during exercise. Pflugers Arch. 378, 65–69. doi: 10.1007/BF00581959 PMID: [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E., De Matos T. R., Arrans P., Santalla A., Orellana J. N. (2018). Ventilatory efficiency response is unaffected by fitness level, ergometer type, age or body mass index in male athletes. Biol. Sport 35, 393–398. doi: 10.5114/biolsport.2018.78060 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Martinez E., Terrados N., Burtscher M., Santalla A., Naranjo Orellana J. (2016). Ventilatory efficiency and breathing pattern in world-class cyclists: A three-year observational study. Respir. Physiol. Neurobiol. 229, 17–23. doi: 10.1016/j.resp.2016.04.001 PMID: [DOI] [PubMed] [Google Scholar]
- Sanchez Crespo A., Hallberg J., Lundberg J. O., Lindahl S. G., Jacobsson H., Weitzberg E., et al. (2010). Nasal nitric oxide and regulation of human pulmonary blood flow in the upright position. J. Appl. Physiol. 108, 181–188. doi: 10.1152/japplphysiol.00285.2009 PMID: [DOI] [PubMed] [Google Scholar]
- Saoji A. A., Raghavendra B. R., Manjunath N. K. (2019). Effects of yogic breath regulation: a narrative review of scientific evidence. J. Ayurveda Integr. Med. 10, 50–58. doi: 10.1016/j.jaim.2017.07.008 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer C. E., Wuthrich T. U., Beltrami F. G., Spengler C. M. (2019). Effects of sprint-interval and endurance respiratory muscle training regimens. Med. Sci. Sports Exerc. 51, 361–371. doi: 10.1249/MSS.0000000000001782 PMID: [DOI] [PubMed] [Google Scholar]
- Schelegle E. S., Green J. F. (2001). An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir. Physiol. 125, 17–31. doi: 10.1016/S0034-5687(00)00202-4 PMID: [DOI] [PubMed] [Google Scholar]
- Schucker L., Knopf C., Strauss B., Hagemann N. (2014). An internal focus of attention is not always as bad as its reputation: how specific aspects of internally focused attention do not hinder running efficiency. J. Sport Exerc. Psychol. 36, 233–243. doi: 10.1123/jsep.2013-0200 PMID: [DOI] [PubMed] [Google Scholar]
- Schucker L., Parrington L. (2019). Thinking about your running movement makes you less efficient: attentional focus effects on running economy and kinematics. J. Sports Sci. 37, 638–646. doi: 10.1080/02640414.2018.1522697 PMID: [DOI] [PubMed] [Google Scholar]
- Seals D. R., Suwarno N. O., Joyner M. J., Iber C., Copeland J. G., Dempsey J. A. (1993). Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ. Res. 72, 440–454. doi: 10.1161/01.RES.72.2.440 PMID: [DOI] [PubMed] [Google Scholar]
- Segizbaeva M. O., Aleksandrova N. P. (2019). Assessment of the functional state of respiratory muscles: methodological aspects and data interpretation. Hum. Physiol. 45, 213–224. doi: 10.1134/S0362119719010110 [DOI] [Google Scholar]
- Sheel A. W., Boushel R., Dempsey J. A. (2018). Competition for blood flow distribution between respiratory and locomotor muscles: implications for muscle fatigue. J. Appl. Physiol. 125, 820–831. doi: 10.1152/japplphysiol.00189.2018 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel A. W., Foster G. E., Romer L. M. (2011). Exercise and its impact on dyspnea. Curr. Opin. Pharmacol. 11, 195–203. doi: 10.1016/j.coph.2011.04.004 PMID: [DOI] [PubMed] [Google Scholar]
- Sheel A. W., Romer L. M. (2011). Ventilation and respiratory mechanics. Compr. Physiol. 2, 1093–1142. doi: 10.1002/cphy.c100046 PMID: [DOI] [PubMed] [Google Scholar]
- Sheel A. W., Taylor J. L., Katayama K. (2020). The hyperpnoea of exercise in health: respiratory influences on neurovascular control. Exp. Physiol. 105, 1984–1989. doi: 10.1113/EP088103 PMID: [DOI] [PubMed] [Google Scholar]
- Shei R. J. (2018). Recent advancements in our understanding of the ergogenic effect of respiratory muscle training in healthy humans: a systematic review. J. Strength Cond. Res. 32, 2665–2676. doi: 10.1519/JSC.0000000000002730 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shturman-Ellstein R., Zeballos R., Buckley J., Souhrada J. (1978). The beneficial effect of nasal breathing on exercise-induced bronchoconstriction. Am. Rev. Respir. Dis. 118, 65–73. doi: 10.1164/arrd.1978.118.1.65 PMID: [DOI] [PubMed] [Google Scholar]
- Smith J. R., Kurti S. P., Meskimen K., Harms C. A. (2017). Expiratory flow limitation and operating lung volumes during exercise in older and younger adults. Respir. Physiol. Neurobiol. 240, 26–31. doi: 10.1016/j.resp.2016.12.016 PMID: [DOI] [PubMed] [Google Scholar]
- Smoliga J. M., Mohseni Z. S., Berwager J. D., Hegedus E. J. (2016). Common causes of dyspnoea in athletes: a practical approach for diagnosis and management. Breathe 12, e22–e37. doi: 10.1183/20734735.006416 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder C. R., Lopez S. J., Edwards L. M., Marques S. C. (2020). The Oxford Handbook of Positive Psychology. New York: Oxford university press. [Google Scholar]
- Statista (2018). “Number of participants in running/jogging and trail running in the U.S. from 2006 to 2017 (in millions).” (Statista: Outdoor Foundation).
- Stickford A. S., Stickford J. L. (2014). Ventilation and locomotion in humans: mechanisms, implications, and perturbations to the coupling of these two rhythms. Springer Sci. Rev. 2, 95–118. doi: 10.1007/s40362-014-0020-4 [DOI] [Google Scholar]
- Stickford A. S. L., Stickford J. L., Fulton T. J., Lovci T. L., Chapman R. F. (2020). Attentional focus does not impact locomotor-respiratory coupling in trained runners. Eur. J. Appl. Physiol. 120, 2477–2486. doi: 10.1007/s00421-020-04475-6 PMID: [DOI] [PubMed] [Google Scholar]
- Takano N., Deguchi H. (1997). Sensation of breathlessness and respiratory oxygen cost during cycle exercise with and without conscious entrainment of the breathing rhythm. Eur. J. Appl. Physiol. Occup. Physiol. 76, 209–213. doi: 10.1007/s004210050238 PMID: [DOI] [PubMed] [Google Scholar]
- Thomas S.A., Phillips V., Mock C., Lock M., Cox G., Baxter J. (2009). “The effects of nasal breathing on exercise tolerance,” in Chartered Society of Physiotherapy Annual Congress. October 17, 2009.
- Thornadtsson A., Drca N., Ricciardolo F., Hogman M. (2017). Increased levels of alveolar and airway exhaled nitric oxide in runners. Ups. J. Med. Sci. 122, 85–91. doi: 10.1080/03009734.2017.1317886 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton M. J., Harper A., Paton J. F., Costello J. T. (2017). The human ventilatory response to stress: rate or depth? Phys. J. 595, 5729–5752. doi: 10.1113/JP274596 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubekis A. G., Beidaris N., Botonis P. G., Koskolou M. (2017). Severe hypoxemia induced by prolonged expiration and reduced frequency breathing during submaximal swimming. J. Sports Sci. 35, 1025–1033. doi: 10.1080/02640414.2016.1209304 PMID: [DOI] [PubMed] [Google Scholar]
- Trevisan M. E., Boufleur J., Soares J. C., Haygert C. J., Ries L. G., Correa E. C. (2015). Diaphragmatic amplitude and accessory inspiratory muscle activity in nasal and mouth-breathing adults: a cross-sectional study. J. Electromyogr. Kinesiol. 25, 463–468. doi: 10.1016/j.jelekin.2015.03.006 PMID: [DOI] [PubMed] [Google Scholar]
- Tsukada S., Masaoka Y., Yoshikawa A., Okamoto K., Homma I., Izumizaki M. (2017). Coupling of dyspnea perception and occurrence of tachypnea during exercise. J. Physiol. Sci. 67, 173–180. doi: 10.1007/s12576-016-0452-5 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsted F.M., Nielsen C.V., Jensen J.Q., Sonne T., Jensen M.M. (2017). “Strive: exploring assistive haptic feedback on the run.” in Proceedings of the 29th Australian Conference on Computer-Human Interaction: ACM. November 28 - December 01, 2017; 275–284.
- Van Diest I., Verstappen K., Aubert A. E., Widjaja D., Vansteenwegen D., Vlemincx E. (2014). Inhalation/exhalation ratio modulates the effect of slow breathing on heart rate variability and relaxation. Appl. Psychophysiol. Biofeedback 39, 171–180. doi: 10.1007/s10484-014-9253-x PMID: [DOI] [PubMed] [Google Scholar]
- Van Dyck E., Moens B., Buhmann J., Demey M., Coorevits E., Dalla Bella S., et al. (2015). Spontaneous entrainment of running cadence to music tempo. Sports Med. Int. Open 1:15. doi: 10.1186/s40798-015-0025-9 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oeveren B. T., De Ruiter C. J., Hoozemans M. J. M., Beek P. J., Van Dieen H. (2019). Inter-individual differences in stride frequencies during running obtained from wearable data. J. Sports Sci. 37, 1996–2006. doi: 10.1080/02640414.2019.1614137 PMID: [DOI] [PubMed] [Google Scholar]
- Van Rheden V., Grah T., Meschtscherjakov A. (2020). “Sonification approaches in sports in the past decade.” in Proceedings of the 15th International Conference on Audio Mostly. September 16, 2020.
- Van Rheden V., Harbour E., Finkenzeller T., Burr L.A., Meschtscherjakov A., Tscheligi M. (2021). “Run, beep, breathe: exploring the effects on adherence and user experience of 5 breathing instruction sounds while running,” in Audio Mostly 2021. October 15, 2021.
- Vickery R.L. (2008). The effect of breathing pattern retraining on performance in competitive cyclists. dissertation/thesis. Auckland University of Technology.
- Vinetti G., Lopomo N. F., Taboni A., Fagoni N., Ferretti G. (2020). The current use of wearable sensors to enhance safety and performance in breath-hold diving: a systematic review. Diving Hyperb. Med. 50, 54–65. doi: 10.28920/dhm50.1.54-65 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Leupoldt A., Sommer T., Kegat S., Baumann H. J., Klose H., Dahme B., et al. (2008). The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am. J. Respir. Crit. Care Med. 177, 1026–1032. doi: 10.1164/rccm.200712-1821OC PMID: [DOI] [PubMed] [Google Scholar]
- Walker A., Surda P., Rossiter M., Little S. (2016). Nasal function and dysfunction in exercise. J. Laryngol. Otol. 130, 431–434. doi: 10.1017/S0022215116000128 PMID: [DOI] [PubMed] [Google Scholar]
- Wallden M. (2017). The diaphragm–more than an inspired design. J. Bodyw. Mov. Ther. 21, 342–349. doi: 10.1016/j.jbmt.2017.03.013 PMID: [DOI] [PubMed] [Google Scholar]
- Ward S. A. (2007). Ventilatory control in humans: constraints and limitations. Exp. Physiol. 92, 357–366. doi: 10.1113/expphysiol.2006.034371 PMID: [DOI] [PubMed] [Google Scholar]
- Weiler J. M., Brannan J. D., Randolph C. C., Hallstrand T. S., Parsons J., Silvers W., et al. (2016). Exercise-induced bronchoconstriction update-2016. J. Allergy Clin. Immunol. 138, 1292–1295.e36. doi: 10.1016/j.jaci.2016.05.029 PMID: [DOI] [PubMed] [Google Scholar]
- Weinberger M., Abu-Hasan M. (2009). Perceptions and pathophysiology of dyspnea and exercise intolerance. Pediatr. Clin. N. Am. 56, 33–48, ix. doi: 10.1016/j.pcl.2008.10.015 PMID: [DOI] [PubMed] [Google Scholar]
- Weitzberg E., Lundberg J. O. (2002). Humming greatly increases nasal nitric oxide. Am. J. Respir. Crit. Care Med. 166, 144–145. doi: 10.1164/rccm.200202-138BC PMID: [DOI] [PubMed] [Google Scholar]
- Welch J. F., Kipp S., Sheel A. W. (2019). Respiratory muscles during exercise: mechanics, energetics, and fatigue. Curr. Opin. Physiol. 10, 102–109. doi: 10.1016/j.cophys.2019.04.023 PMID: 16677807 [DOI] [Google Scholar]
- Wiehr F., Kosmalla F., Daiber F., Krüger A. (2017). “FootStriker: an EMS-based assistance system for real-time running style correction.” in Proceedings of the 19th International Conference on Human-Computer Interaction with Mobile Devices and Services. September 04, 2017; 1–6.
- Wojta D., Flores X., Andres F. (1987). Effect of “breathplay” on the physiological performance of trained cyclists. Med. Sci. Sports Exerc. 19:S85. doi: 10.1249/00005768-198704001-00509 [DOI] [Google Scholar]
- Woorons X., Billaut F., Lamberto C. (2021a). Running exercise with end-expiratory breath holding up to the breaking point induces large and early fall in muscle oxygenation. Eur. J. Appl. Physiol. 121, 3515–3525. doi: 10.1007/s00421-021-04813-2 PMID: [DOI] [PubMed] [Google Scholar]
- Woorons X., Billaut F., Vandewalle H. (2020). Transferable benefits of cycle hypoventilation training for run-based performance in team-sport athletes. Int. J. Sports Physiol. Perform. 1–6. doi: 10.1123/ijspp.2019-0583 [Epub ahead of print]. [DOI] [PubMed]
- Woorons X., Lemaitre F., Claessen G., Woorons C., Vandewalle H. (2021b). Exercise with end-expiratory breath holding induces large increase in stroke volume. Int. J. Sports Med. 42, 56–65. doi: 10.1055/a-1179-6093 PMID: [DOI] [PubMed] [Google Scholar]
- Woorons X., Mucci P., Richalet J. P., Pichon A. (2016). Hypoventilation training at supramaximal intensity improves swimming performance. Med. Sci. Sports Exerc. 48, 1119–1128. doi: 10.1249/MSS.0000000000000863 PMID: [DOI] [PubMed] [Google Scholar]
- Wuthrich T. U., Eberle E. C., Spengler C. M. (2014). Locomotor and diaphragm muscle fatigue in endurance athletes performing time-trials of different durations. Eur. J. Appl. Physiol. 114, 1619–1633. doi: 10.1007/s00421-014-2889-7 PMID: [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Takei Y., Mutoh Y., Miyashita M. (1988). Delayed appearance of blood lactate with reduced frequency breathing during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 57, 462–466. doi: 10.1007/BF00417994 PMID: [DOI] [PubMed] [Google Scholar]
- Yonge R. (1983). Entrainment of breathing in rhythmic exercise. Model. Control Breath. 197–204. [Google Scholar]
- Zaccaro A., Piarulli A., Laurino M., Garbella E., Menicucci D., Neri B., et al. (2018). How breath-control can change your life: a systematic review on psycho-physiological correlates of slow breathing. Front. Hum. Neurosci. 12:353. doi: 10.3389/fnhum.2018.00353 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C., Jiang H., Zhou G., Arora N., Schuele S., Rosenow J., et al. (2016). Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 36, 12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]