Abstract
Background
Patients with kidney failure require vascular access to receive maintenance haemodialysis (HD), which can be achieved by an arteriovenous fistula or a central venous catheter (CVC). CVC use is related to frequent complications such as venous stenosis and infection. Venous stenosis occurs mainly due to trauma caused by the entrance of the catheter into the venous lumen and repeated contact with the vein wall. A biofilm, a colony of irreversible adherent and self‐sufficient micro‐organisms embedded in a self‐produced matrix of exopolysaccharides, is associated with the development of infections in patients with indwelling catheters. Despite its clinical relevance, the treatment of catheter‐related bloodstream infections (CRBSIs) in patients receiving maintenance HD remains controversial, especially regarding catheter management. Antibiotic lock solutions may sterilise the catheter, treat the infection and prevent unnecessary catheter procedures. However, such treatment may also lead to antibiotic resistance or even clinical worsening in certain more virulent pathogens. Catheter removal and delayed replacement may remove the source of infection, improving infectious outcomes, but this approach may also increase vascular access stenosis, thrombosis or both, or even central vein access failure. Catheter guidewire exchange attempts to remove the source of infection while maintaining access to the same vein and, therefore, may improve clinical outcomes and preserve central veins for future access.
Objectives
To assess the benefits and harms of different interventions for CRBSI treatment in patients receiving maintenance HD through a permanent CVC, such as systemic antibiotics alone or systemic antibiotics combined with either lock solutions or catheter guidewire exchange or catheter replacement.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 21 December 2021 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register were identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal, and ClinicalTrials.gov.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs evaluating the management of CRBSI in permanent CVCs in people receiving maintenance HD.
Data collection and analysis
Two authors independently selected studies for inclusion, assessed their risk of bias, and performed data extraction. Results were expressed as risk ratios (RR) or hazard ratios (HR) for dichotomous outcomes and mean difference (MD) for continuous outcomes, with their 95% confidence intervals (CI). The certainty of the evidence was assessed using GRADE.
Main results
We identified two RCTs and one quasi‐RCT that enrolled 760 participants addressing the treatment of CRBSIs in people (children and adults) receiving maintenance HD through CVC. No two studies compared the same interventions. The quasi‐RCT compared two different lock solutions (tissue plasminogen activator (TPA) and heparin) with concurrent systemic antibiotics. One RCT compared systemic antibiotics alone and in association with an ethanol lock solution, and the other compared systemic antibiotics with different catheter management strategies (guidewire exchange versus removal and replacement). The overall certainty of the evidence was downgraded due to the small number of participants, high risk of bias in many domains, especially randomisation, allocation, and other sources of bias, and missing outcome data. It is uncertain whether an ethanol lock solution used with concurrent systemic antibiotics improved CRBSI eradication compared to systemic antibiotics alone (RR 1.61, 95% CI 1.16 to 2.23) because the certainty of this evidence is very low. There were no reported differences between the effects of TPA and heparin lock solutions on cure rates (RR 0.92, 95% CI 0.74 to 1.15) or between catheter guidewire exchange versus catheter removal with delayed replacement, expressed as catheter infection‐free survival (HR 0.88, 95% CI 0.43 to 1.79). To date, no results are available comparing other interventions.
Outcomes such as venous stenosis and/or thrombosis, antibiotic resistance, death, and adverse events were not reported.
Authors' conclusions
Currently, there is no available high certainty evidence to support one treatment over another for CRBSIs. The benefit of using ethanol lock treatment in combination with systemic antibiotics compared to systemic antibiotics alone for CRBSIs in patients receiving maintenance HD remains uncertain due to the very low certainty of the evidence. Hence, further RCTs to identify the benefits and harms of CRBSI treatment options are needed. Future studies should unify CRBSI and cure definitions and improve methodological design.
Plain language summary
Interventions for treating catheter‐related bloodstream infections in people receiving maintenance haemodialysis
What is the issue?
Patients with kidney failure require kidney replacement therapy (KRT) to sustain life. Among KRT options, haemodialysis (HD) is the primary method of dialysis. Patients receiving HD via an indwelling catheter have a higher risk of developing bloodstream infections. There are several options for treating these bloodstream infections. These include lock solutions (the infusion of high doses of antibiotic inside each of the catheter ports between the dialysis sessions), removal of the catheter followed by a new insertion after initial clinical improvement, exchange of the catheter for a new one by a guidewire (inserted through one of the catheter's ports into the same vein, allowing the preservation of the venous site), and the use of systemic antibiotics (used in isolated or combined with other treatments). Each treatment has its own inherent risks.
What did we do?
We searched Cochrane Kidney and Transplant’s Specialised Register up to 21 December 2021 and performed a systematic review of studies investigating treatment options for catheter‐related bloodstream infection in patients undergoing HD.
What did we find?
We found three studies enrolling 760 participants that compared various treatments for catheter‐related bloodstream infections. No studies compared similar treatment strategies or had similar outcomes, and therefore we were unable to combine data in our meta‐analyses. The comparisons included systemic antibiotics with two different lock solutions, systemic antibiotics alone versus systemic antibiotics plus an ethanol lock solution, and systemic antibiotics plus catheter removal versus systemic antibiotics plus catheter exchange.
One study reported a higher success rate for clearing infection with systemic antibiotics plus ethanol locking treatment than systemic antibiotics alone. The other studies reported no difference between their two treatment arms. Outcomes such as venous stenosis and/or thrombosis, antibiotic resistance, death, and adverse events were not reported.
Conclusions
Further randomised studies to identify the benefits and harms of catheter‐related bloodstream infection treatments are needed.
Summary of findings
Summary of findings 1. Systemic antibiotics plus antibiotic lock solutions plus tissue plasminogen activator versus systemic antibiotics plus antibiotic lock solutions plus heparin.
| Systemic antibiotics plus antibiotic lock solutions plus TPA versus systemic antibiotics plus antibiotic lock solutions plus heparin | |||||
|
Patient or population: patients on maintenance HD with CRBSI Settings: inpatient Intervention: systemic antibiotics plus antibiotic lock solution plus TPA Comparison: systemic antibiotics plus antibiotic lock solution plus heparin | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | |
| Risk with heparin | Risk with TPA | ||||
| Cure: short‐term success Follow‐up: 2 weeks |
1000 per 1000 | 920 per 1000 (740 to 1150) |
RR 0.92 (0.74 to 1.15) | 18; 24 catheters (1 quasi‐RCT) | ⊕⊝⊝⊝ very low 1,2 |
| Cure: long‐term success Follow‐up: 6 weeks |
917 per 1000 | 587 per 1000 (348 to 972) |
RR 064 (0.38 to 1.06) | 18; 24 catheters (1 quasi‐RCT) | ⊕⊝⊝⊝ very low 1,2 |
| Cure: infection‐free survival Follow‐up: between the final dose of antibiotics and the first positive blood culture obtained from the catheter |
Infection‐free survival was 27.7 days less with TPA (88.68 days less to 33.28 days more) than with heparin | ‐ | 18; 24 catheters (1 quasi‐RCT) | ⊕⊝⊝⊝ very low 1,2 |
|
| Stenosis or thrombosis of vascular access site after catheter removal | Not reported | Not reported | ‐ | ‐ | ‐ |
| Death | Not reported | Not reported | ‐ | ‐ | ‐ |
| Development of antibiotic resistance | Not reported | Not reported | ‐ | ‐ | ‐ |
| Adverse effects | Not reported | Not reported | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CRBSI: Catheter‐related bloodstream infection; HD: Haemodialysis; RR: Risk Ratio; TPA: Tissue plasminogen activator | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1High risk of bias in random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), inconsistencies regarding the description of the data analysis and the tables, protocol break and also conflicting information between the author's reply and the article (other bias); downgraded by 2 levels (methodological limitation)
2Small number of participants and studies (doubt about the reproducibility of the data); downgraded by 1 level (imprecision)
Summary of findings 2. Systemic antibiotics alone versus systematic antibiotics plus antibiotic lock solution.
| Systemic antibiotics alone versus systematic antibiotics plus antibiotic lock solution | |||||
|
Patient or population: patients on maintenance HD with CRBSI Settings: inpatient Intervention: systemic antibiotics alone Comparison: systemic antibiotics and antibiotic lock solution (ethanol) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with systemic antibiotics alone | Risk with systemic antibiotic plus ethanol antibiotic lock solution | ||||
| Cure: successful eradication of the infection Follow‐up: 2 days |
563 per 1000 |
906 per 1000 (653 to 1000) |
RR 1.61 (1.16 to 2.23) |
64 (1) | ⊕⊝⊝⊝ 1,2,3 very low |
| Stenosis or thrombosis of vascular access site after catheter removal | Not reported | Not reported | ‐ | ‐ | ‐ |
| Death | Not reported | Not reported | ‐ | ‐ | ‐ |
| Development of antibiotic resistance | Not reported | Not reported | ‐ | ‐ | ‐ |
| Adverse effects | Not reported | Not reported | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CRBSI: Catheter‐related bloodstream infection; HD: Haemodialysis; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1High risk of bias in selective reporting because of retrospective trial registration (reporting bias) and other bias once 45% of the included population have negative blood cultures; downgraded by 1 level (methodological limitation).
2Small number of participants and studies (doubt about the reproducibility of the data); downgraded by 1 level (imprecision).
3Outcome cure defined only by clinical improvement in signs and symptoms, without laboratory confirmation; downgraded by 1 level (indirectness).
Summary of findings 3. Catheter guidewire exchange versus catheter removal and replacement.
| Catheter guidewire exchange versus catheter removal and replacement | |||||
|
Patient or population: patients on maintenance HD with CRBSI Settings: inpatient Intervention: systemic antibiotics and catheter guidewire exchange Comparison: systemic antibiotics and catheter removal and replacement | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with systemic catheter removal | Risk with guidewire exchange | ||||
| Cure: infection‐free survival Follow‐up: 45 days |
720 per 1000 | 749 per 1000 (555 to 868) |
HR 0.881 (0.43 to 1.79) |
678 (1) | ⊕⊝⊝⊝ 2,3,4 very low |
| Stenosis or thrombosis of vascular access site after catheter removal | Not reported | Not reported | ‐ | ‐ | ‐ |
| Death | Not reported |
Not reported | ‐ | ‐ | ‐ |
| Development of antibiotic resistance | Not reported | Not reported | ‐ | ‐ | ‐ |
| Adverse effects | Not reported | Not reported | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CRBSI: Catheter‐related bloodstream infection; HD: Haemodialysis; HR: Hazard Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Outcome cure was measured as a time‐to‐event outcome. Comparator group risk of event‐free survival at 90 days. Baseline risk line of 0.72 comes from the study's Kaplan‐Meier survival curves at 90 days time point, which represents the middle of the follow‐up
2High risk of bias in blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias), selective reporting because of retrospective trial registration (reporting bias) and other bias; downgraded by 1 level (methodological limitation).
3Small number of participants and studies (doubt about the reproducibility of the data); downgraded by 1 level (imprecision)
4Use of catheter infection‐free survival as an indication of cure; downgraded by 1 level (indirectness)
Background
Description of the condition
Patients with kidney failure require kidney replacement therapy (KRT) to sustain life. Among KRT options, haemodialysis (HD) is the primary method of dialysis used in 79% of countries with a frequency of 80% (Saran 2019). The creation and maintenance of a point of access into the patient’s vascular system are crucial for achieving safe and effective HD. In their latest guidelines, the National Kidney Foundation’s Dialysis Outcomes Quality Initiative (NKF‐DOQI) and the European Society of Vascular Surgeons (ESVS) recommend the use of an arteriovenous fistula (AVF) as the primary option for achieving vascular access for HD. However, there are situations in which the use of an AVF is not feasible, either because of the patient’s limited life expectancy or their comorbidities (e.g. congestive heart failure or atherosclerosis with ischaemia), AVF maturation time, the absence of venous outflow or reduced venous diameter. In such cases, the use of a tunnelled and cuffed central venous catheter (CVC) for vascular access is acceptable. Additionally, compared to AVF, tunnelled and cuffed CVC has benefits such as needle‐free connection to the dialysis circuit, the possibility of immediate use, the elimination of cannulation‐related complications, and the simplicity of device implantation.
However, patients receiving maintenance HD through CVC frequently present with clinically relevant complications, such as venous thrombosis, central venous stenosis, and especially infection (Liangos 2006). Sepsis in these patients is related to a higher occurrence of cardiovascular events and lead to a higher death rate (Foley 2004). Catheter‐related infections (CRI) (also termed bacteraemia or catheter‐related bloodstream infection (CRBSI)) may occur at the exit site, at the subcutaneous tunnel, or in the bloodstream.
The clinical features of CRBSI might not always be present. The most sensitive. although nonspecific manifestations. are fever and chills, while others, such as haemodynamic instability, catheter dysfunction, hypothermia, nausea and vomiting, and generalized malaise may also occur. Especially for surveillance purposes, any patient with a HD catheter and signs and symptoms of bloodstream infection without an alternate source of infection is suspected to have CRBSI (CDC 2018 Dialysis Event Surveillance Protocol). Although there is no consensus related to CRBSI definition, most guidelines and associations require laboratory confirmation (Mermel 2009; O'Grady 2011; Vascular Access 2006 Work Group 2006). In this systematic review, definite CRBSI is defined as suspected CRBSI with concurrent positive blood cultures of the same organism from the catheter and a peripheral vein. The blood from a peripheral source can be obtained from a vein or the dialysis circuit. The colony count is considered positive if there are > 15 colony forming units (CFU)/catheter segment (in the hub or tip) in semi‐quantitative culture or > 100 CFU/catheter segment (hub or tip) in quantitative culture. In addition, the differential time to positivity of two hours, which is the differential period of catheter culture versus peripheral blood culture, is considered positive. If available, simultaneous quantitative cultures of blood samples with a ratio of ≥ 3:1 (catheter hub/tip versus peripheral dialysis circuit/vein) would be supportive.
Description of the intervention
Patients receiving maintenance HD through CVC have increased susceptibility to infections as a result of the acquired immunodeficiency status, a characteristic of kidney failure (due to uraemia, advanced age and associated comorbidities such as diabetes mellitus), the high bacterial virulence (related to biofilm formation), and the frequent and repeated exposure to risk factors inherent in the typical HD process (Sarnak 2000).
Several pathogens can cause CRBSI. Coagulase‐negative staphylococci and Staphylococcus aureus are the most frequent causes and account for 40% to 80% of cases (Allon 2004). Non‐staphylococcal infections are mostly due to enterococci and gram‐negative bacteria. Up to 12% to 38% of CRBSIs are caused by methicillin‐resistant S. aureus (MRSA) (Lok 2011). Although less common, fungal infection can also occur and, in 7% to 21% of cases, multiple organisms are responsible, making treatment even more challenging.
The severity of the infection is closely linked to the type of organism present. Serious metastatic infections, including osteomyelitis, thrombophlebitis, endocarditis, septic arthritis, epidural abscess, and large atrial thrombi occur more frequently due to S. aureus. S. aureus is also commonly associated with significant morbidity and death rates, with some series showing a three‐fold higher risk of infectious complications and a 20% higher death rate compared to all other pathogens (Allon 2004). MRSA, however, has been associated with three to five times higher death rates compared with methicillin‐sensitive strains (Beigi 2010).
The isolation of the pathogen also indicates the treatment required. Although the use of systemic empirical antibiotic therapy is similar at the beginning of treatment, tailoring might be necessary following the microbiological results. In addition, some guidelines suggest different catheter management depending on each aetiological agent (Mermel 2009; Vascular Access 2006 Work Group 2006).
Since the use of the Dacron cuff leads to a local inflammatory response and is thought to create a mechanical barrier avoiding bacterial migration from the skin, biofilm formation is the presumed source of CRI (Valliant 2014). As such, CRBSI treatment should also address catheter sterilisation, which can be achieved through using antibiotic lock solutions or by the removal of the CVC, either over a guidewire in the same vein or with replacement in a new vein. Although there is evidence of inferior rates of cure through the use of systemic antibiotics alone, it remains an intervention option in many centres (Aslam 2014).
Antibiotic lock solutions are high concentrations (approximately 100‐fold higher than those used systemically) of antibiotic or antimicrobial solutions in small volumes with or without an anticoagulant. This intervention is adjunctive to systemic antibiotic therapy and consists of catheter salvage with the instillation of a solution into each catheter lumen at the end of each dialysis session for the same duration as the systemic therapy. Common antibiotic lock solutions contain gentamicin, cefazolin, cefotaxime, vancomycin, linezolid, vancomycin plus gentamicin, cefazolin plus gentamicin, trimethoprim plus sulfamethoxazole, or minocycline. Antimicrobial lock solutions may contain taurolidine, 30% citrate, ethanol, EDTA, or methylene blue. The anticoagulants typically used in collaboration with lock treatment are heparin, citrate or tissue plasminogen activator (TPA) (Justo 2014).
CVC removal with CVC exchange over a guidewire at the same site is a single procedure performed by inserting a guidewire through the infected catheter lumen to the accessed vein, followed by CVC removal and placement of a new CVC over the guidewire, therefore, maintaining the same access site (i.e. the same vein) (Duszak 1998).
CVC removal with a new CVC placed at a new site involves the removal of the infected catheter and the placement of a new HD catheter, which can be done in one procedure by positioning a new CVC for HD, or in two separate procedures: one to place a CVC that is neither tunnelled nor cuffed and a second to exchange it for a new tunnelled and cuffed CVC. Additionally, it may or may not include a CVC‐free duration (i.e. a period when there is no CVC in situ) (Lok 2019).
How the intervention might work
Biofilm is associated with the development of infections in patients with indwelling catheters. Within 24 hours of catheter insertion, all vascular devices show micro‐organism colonization. The process of biofilm formation is of irreversible adhesion of micro‐organisms to a surface such as medical devices (e.g. venous and urinary catheters); the micro‐organisms can multiply slowly and create a self‐sufficient flora protected by a self‐produced matrix of exopolysaccharides. Unlike planktonic micro‐organisms, biofilm flora have a lower sensitivity to antibiotics, requiring high concentrations for its eradication (Donlan 2001).
The systematic review by Arechabala 2018 showed that the use of antibiotics and combined antimicrobial lock solutions reduced the risk of CRI compared to the use of control lock solutions (usually containing only an anticoagulant). Benefits were also observed in tunnelled HD CVC. Thus, the use of antibiotic lock solutions to treat CRI represents a logical strategy.
Central venous stenosis is a frequent complication among patients receiving maintenance HD (MacRae 2005). The trauma caused by the entrance of the catheter into the venous lumen, the torque of the CVC over the distal third of the subclavian vein at the first rib topography, and its repeated contact with the vein wall, aggravated by the cardiac cycle, are contributing factors to the development of venous stenosis (Liangos 2006).
Patients with venous stenosis requiring CVC removal may develop thrombosis and occlusion of the venous access. In addition to the risk of developing superior vena cava stenosis and superior vena cava syndrome (facial oedema and dyspnoea), central venous occlusion reduces the chances of posterior vascular access due to failure of AVF maturation, inadequate flow or the impossibility of new CVC (MacRae 2005).
Therefore, the treatment of CRI should include the caveat of trying to preserve venous sites. The passage of a guidewire through the CVC ensures access to the vein lumen, allowing the passage of a new device in the same place without thrombosis and removing the infection and its source altogether.
Why it is important to do this review
Infection is a major cause of CVC‐related morbidity and death, with an incidence of one to six episodes of CRI/1000 catheter‐days (Lok 2011; Ravani 2013; Saad 1999; Tanriover 2000). Infection is the second highest cause of death among patients receiving HD, behind only cardiovascular causes (Perl 2011; Saran 2018). Moreover, the occurrence of sepsis in patients with kidney failure results in higher death than in the general population (Sarnak 2000) and can be considered a harbinger of cardiovascular events (Foley 2004; Ishani 2005). Additionally, CVC carries a 1.5‐ to three‐fold increased risk of death from infectious causes and a two‐ to three‐fold greater risk of death from all causes (Polkinghorne 2004).
By the end of 2016, 63.1% of patients with prevalent kidney failure in the USA underwent HD. Four out of five of these patients started HD through CVC, and up to three months later, more than two‐thirds of them had maintained the CVC as vascular access. Approximately 39% of the AVFs created between June 2014 and May 2016 in the USA had maturation failure (Saran 2018). And so, despite the risks of catheter use and the policies to encourage fistulae creation, the use of tunnelled and cuffed CVC has increased in many countries (Rayner 2010).
The ideal CRBSI treatment should be able to resolve the potentially life‐threatening infection, without leading to antibiotic resistance or the development of venous stenosis or occlusion. This systematic review aimed to evaluate the current state of the evidence on CRBSI management, as this information is essential for vascular surgeons, nephrologists and interventional radiologists who can treat CRBSI and patients with kidney failure for whom the presence of a viable access site is a lifeline.
Objectives
This review aimed to look at the benefits and harms of the different interventions proposed for bloodstream infections in permanent CVC for patients on maintenance HD, such as systemic antibiotics alone or systemic antibiotics combined with either lock solutions or catheter guidewire exchange or catheter replacement.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the management of CRBSI in permanent CVCs in people receiving maintenance HD.
Types of participants
Inclusion criteria
Patients with kidney failure on maintenance HD by tunnelled and cuffed CVC with CRBSI.
Exclusion criteria
Patients with kidney failure on maintenance HD using subcutaneously tunnelled and cuffed CVC with exit site infections or tunnel infections without associated CRBSI
Patients with kidney failure on maintenance HD using subcutaneously tunnelled and cuffed CVC with other sources of infection
Patients with kidney failure on other types of KRT
Patients on HD with CVC infection but with acute kidney injury
Patients with kidney failure on maintenance HD with short‐term CVC use.
Types of interventions
All types of interventions compared included systemic antibiotic use, either used alone or in combination with a catheter management option.
Planned comparisons
Systemic antibiotic alone (any substance, dose or administration) versus catheter guidewire exchange
Systemic antibiotic alone (any substance, dose or administration) versus catheter removal and replacement
Systemic antibiotic alone (any substance, dose or administration) versus lock treatment (any substance, preparation, dose or administration)
Catheter guidewire exchange versus catheter removal and replacement
Catheter guidewire exchange versus catheter retainment and lock treatment (any substance, preparation, dose or administration)
Catheter removal replacement versus catheter retainment and lock treatment (any substance, preparation, dose or administration)
Catheter removal and replacement, single procedure (new tunnelled and cuffed CVC) versus catheter removal and replacement, two different procedures (non tunnelled CVC followed by tunnelled and cuffed CVC)
Catheter retainment and lock treatment (any substance, preparation, dose or administration) versus catheter retainment and other lock treatment (different substance, preparation, dose or administration).
Types of outcome measures
This review did not exclude studies based on the non‐reporting of outcomes of interest.
The outcomes selected included the relevant SONG core outcome sets as specified by the Standardized Outcomes in Nephrology initiative (SONG 2017).
Primary outcomes
Cure: clinical resolution associated with negative blood cultures at least 72 hours after completion of systemic antibiotic treatment
Stenosis or thrombosis of vascular access site after catheter removal: narrowing or occlusion of a central vein used for tunnelled and cuffed CVC insertion (e.g. internal jugular vein, subclavian vein, femoral vein).
Secondary outcomes
Death
Catheter malfunction (failure to attain and maintain an extracorporeal blood flow ≥ 300 mL/min in an adult patient to perform HD without significantly lengthening the HD treatment) (Vascular Access 2006 Work Group 2006)
Duration of hospitalisation
Development of antibiotic resistance
Fatigue
Cardiovascular disease
Adverse outcomes, such as bleeding (major or minor), allergic reactions, urticaria, anaphylaxis, or other adverse effects defined by the study authors.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 21 December 2021 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Contacting relevant individuals/organizations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations, and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were not searched.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain the titles and abstracts of studies potentially relevant to the review. The titles and abstracts were screened independently by two authors (BMA, DGC) and discarded when not applicable; studies and reviews that could include relevant data were initially retained. We independently assessed the retrieved abstracts and, when necessary, the full text of these studies to determine which ones satisfied our inclusion criteria. Disagreements were resolved in consultation with a third author (DHM). If a study did not meet our inclusion criteria, it was excluded and the reasons documented.
Data extraction and management
Two authors (BMA, DGC) independently carried out data extraction using standard data extraction forms. Data extraction included characteristics of the population, the intervention described, the outcomes assessed, and the duration of the follow‐up. Disagreements were resolved in consultation with a third author (DHM). Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of a study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancies between published versions were to be highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors (BMA, DGC) using the risk of bias assessment tool (Higgins 2020) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
We used the risk ratio (RR) for dichotomous outcomes (e.g. death, cure, stenosis or thrombosis of vascular access site after catheter removal, catheter malfunction outcomes), with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. duration of hospitalisation, fatigue, cardiovascular disease, development of antibiotic resistance), we planned to use the mean difference (MD); and if different scales were used, the standardised mean difference (SMD).
We planned to tabulate and assess the adverse effects with descriptive techniques.
Unit of analysis issues
Outcomes were analysed at the individual patient level. If the unit of randomisation was not the same as the level of analysis, that is, the patient, adjustments were made to address the potential impact of clustering on the outcome.
Dealing with missing data
We included all available data and requested further information twice from the original authors by written correspondence (emailing corresponding author/s) and obtained a response from two. The relevant information obtained was included in the review. Evaluation of important numerical data such as screened, randomised patients, as well as intention‐to‐treat, as‐treated, and per‐protocol population, were carefully performed. Attrition rates such as drop‐outs, losses to follow‐up, and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2020).
Assessment of heterogeneity
The heterogeneity was first assessed by visual inspection of the forest plot, and after quantified by statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was used as follows.
0% to 30%: might not be important
30% to 50%: may represent moderate heterogeneity
50% to 75%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of the evidence for heterogeneity (e.g. P‐value from the Chi² test, or a 95% CI for I²) (Higgins 2020).
Assessment of reporting biases
We planned to use funnel plots to assess the potential existence of publication bias and other bias reports if sufficient studies (more than 10) were identified for inclusion in the meta‐analysis (Higgins 2020).
Data synthesis
Data were intended to be pooled using the random‐effects model, but the fixed‐effect model would also have been used to ensure the robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we had intended to perform subgroup analysis to explore possible sources of heterogeneity, which were to include the following.
First versus multiple treatments and catheter salvage attempts
Initial catheter access site: either jugular, subclavian, or femoral
Presence of other CRI in association with CRBSI, such as exit‐site infection, infection of the tunnel track or CRBSI alone
Inpatient versus outpatient
Previous use or not of prophylactic antibiotic lock solutions
Aetiological agent of the infection (such as gram positive, gram negative, anaerobes, fungi).
Heterogeneity among participants could be related to age and renal pathology (gender, ethnicity, diabetics versus non‐diabetics, smoking, vascular disease, body mass index, age category, dialysis vintage and duration of catheter use before development of CRI). Heterogeneity in treatments could be related to a patient’s access site conditions (patients with femoral tunnelled and cuffed CVC usually have poor vascular access prognosis), to the placement or not of a not tunnelled CVC prior to a tunnelled and cuffed CVC at CVC removal, prior agent(s) used, and the agent, dose and duration of therapy (such as the lock solution substance, dose, preparation, and administration). Statistical heterogeneity in study design and risk of bias was evaluated.
Sensitivity analysis
We had planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis considering the risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Summary of findings and assessment of the certainty of the evidence
We have presented the main results of the review in the 'Summary of findings' tables. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2020a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the certainty of a body of evidence as to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. This approach will be assessed by two authors. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2020b). The following outcomes were intended to be presented in the 'Summary of findings' tables.
Cure
Stenosis or thrombosis of vascular access site after catheter removal
Death
Development of antibiotic resistance
Adverse effects.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification
Results of the search
After searching the Specialised Register, a total of 25 records were identified. After screening the titles and abstracts and then full‐text review, three studies (five reports) were included and 13 studies (19 reports) were excluded. One study (Khosroshahi 2006b) is as awaiting assessment (Figure 1).
1.
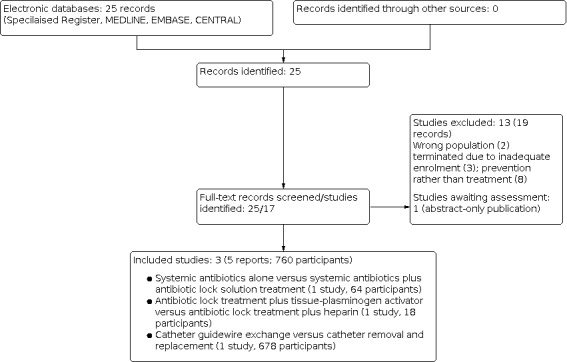
Study flow diagram.
Included studies
We identified two RCTs (Khosroshahi 2015; Saleh 2017) and one quasi‐RCT (Onder 2008) (760 participants). See Characteristics of included studies.
Khosroshahi 2015 enrolled 64 patients with kidney failure on maintenance HD with suspected CRBSI. This single‐centre RCT compared an ethanol lock solution plus concomitant systemic antibiotics with systemic antibiotics alone to treat CRBSIs. The outcome measured was the successful eradication of the infection.
Onder 2008 enrolled 18 children with kidney failure on maintenance HD (a total of 24 catheters) with suspected CRBSI. This study compared two different anticoagulants (TPA and heparin) used with an antibiotic lock solution with concomitant systemic antibiotics to treat CRBSIs. The investigation was a single‐centre quasi‐RCT that evaluated successful eradication of infection, recurrence of infection, and infection‐free catheter survival as outcomes.
Saleh 2017 compared two different catheter management strategies (catheter exchange over a guidewire to catheter removal and replacement) with concomitant systemic antibiotics to treat CRBSIs. This multicentre RCT analysed 678 patients with kidney failure and suspected CRBSI for cure, treatment failure, and indeterminate outcomes.
No two studies compared the same interventions, so a meta‐analysis could not be undertaken. Additionally, the studies applied different definitions for confirmed CRBSIs and for outcomes such as cure or eradication of infection.
Excluded studies
Thirteen studies were excluded.
Eight studies investigated the prevention of CRBSI rather than its treatment (Bosma 2010; ELVIS 2015; Harris 1997; Hymes 2017; Levin 1989; Oliver 2007; Rosenblum 2014; Zwiech 2016).
Abdel Azim 2018 only included patients with kidney failure and non‐cuffed and non‐tunnelled CVC.
Three studies were abandoned early due to inadequate enrolment (NCT01483872; NCT02040818), one of which also did not evaluate the interventions of this review (NCT00108433)
Dahlberg 1986 included the wrong population and compared the incidence of CRBSI and not its treatment.
For individual details of the excluded studies, see Characteristics of excluded studies.
Studies awaiting classification
Khosroshahi 2006b was published as an abstract over 10 years ago and both its full‐text and raw data are not available according to the author.
Risk of bias in included studies
The 'Risk of bias' assessments for all included studies are shown in Figure 2 and Figure 3. Overall, most studies were classified as having a high risk of bias due to methodological limitations and poorly reported results.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Two studies were judged to be at low risk of bias. Khosroshahi 2015 used a computer‐generated list, and Saleh 2017 used a sealed envelope service to provide the randomised sequence. Onder 2008 was judged to be at high risk because although it used sealed envelopes for the first 18 patients, six recurrences were further included as a non‐planned cross‐over and were not randomised.
Allocation concealment
Two studies were judged to be at low risk of bias. Khosroshahi 2015 responded by correspondence that the randomised list was provided to the HD centre nurse, and the randomisation service used by Saleh 2017 also provided allocation concealment. Onder 2008 was judged to be at high risk of bias. The envelopes were not described as being opaque, and the allocation remains unclear because the information provided by the article conflicted with the author's response.
Blinding
Khosroshahi 2015 reported that the practitioner who evaluated the outcome was unaware of the patient’s treatment, but the researchers failed to report how and if the participants were blinded. We thus classified the study as having an unclear risk for performance bias and a low risk of detection bias.
Onder 2008 described the study as randomised, prospective and non‐blinded. We contacted the author and received only one reply with conflicting data. The masking was said to have included the patient, the practitioner, and the observer, but while describing the allocation, it was said that the sealed envelope was given to the patient’s care nurse – which disabled blinding of providers. Due to unreliable information, we classified the performance bias as high risk. Because the outcomes were direct and objective, although there were the mentioned discrepancies, we considered that the assessment was not affected and thus classified the detection bias as low risk.
Saleh 2017 compared two different surgical techniques for which it was impossible to mask the personnel and the patient. Therefore, the performance bias was classified as high risk. The article did not describe the detection bias, but the clinical study’s retrospective registration points out that there was single masking for the care provider, which probably concerns the outcome assessment observer. Consequently, we classified it as having a low risk for detection bias.
Incomplete outcome data
In Saleh 2017, participants consisted of those with suspected CRBSIs and positive blood cultures from both the catheter and a peripheral vein. The authors described that negative catheter tips were an exclusion criterion. There is no report of how many patients initially diagnosed with CRBSI were included, randomised, but had further negative catheter tips and were then excluded from the study. There is also no mention of withdrawals or deaths, or descriptions of which groups these patients belonged to. These facts led to a high risk for attrition bias in this study.
Onder 2008 did not describe if there were losses during the follow‐up. For this reason, we classified the study as having an unclear risk for this domain.
Khosroshahi 2015 replied all participants completed the treatment and the follow‐up. Therefore, the study was classified as having a low risk for attrition bias.
Selective reporting
Onder 2008 had an unclear risk of bias for selective reporting because although the authors reported the planned outcomes, there was no published trial protocol.
Both Khosroshahi 2015 and Saleh 2017 had retrospective trial registration, and neither study thoroughly reported the outcomes. Therefore, we classified both studies as having a high risk of reporting bias.
Other potential sources of bias
There were differences regarding the description of the data analysis and the tables, protocol breaks, and conflicting information between the author's reply and the published article by Onder 2008. Therefore we classified this study as having a high risk of other potential sources of bias.
Khosroshahi 2015 was judged to be at high risk of bias. The study considered all suspected CRBSIs as their included population, without the need for laboratory confirmation, which resulted in 45% of unconfirmed cases (negative blood cultures). Although the authors did not exclude patients with negative blood cultures, they failed to describe or compare which groups these patients belonged to, which could yield misleading conclusions.
Saleh 2017 reported that 65% of the incident and 41% of the prevalent patients on maintenance HD use catheters as vascular access in their centres and that this is a substantially higher rate than those observed in America and Europe. Although these peculiarities influence the study's external validity, they do not alter its risk of bias. However, patients whose catheter was not replaced within 10 days because of death were among the exclusion criteria. This exclusion criterion could be concealing a critical adverse event for this intervention. Hence, this study was classified as high risk for other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Antibiotic lock treatment plus tissue plasminogen activator versus antibiotic lock treatment plus heparin
Onder 2008 compared different antibiotic lock treatments in association with systemic antibiotics. Eighteen children with confirmed infection of tunnelled and cuffed HD catheters were included. The patients were all treated with systemic antibiotics (initially empiric and tailored afterwards if needed) and randomised into two groups based on the type of antibiotic lock solution used, which was a mixture of an antibiotic (tobramycin or vancomycin, depending on the blood culture) with either TPA or heparin. Thirty‐five patients with suspected catheter‐related bacteraemia entered the study with only 24 completions due to laboratory confirmations from positive blood cultures (69%). Additionally, a significant proportion of the participants were not randomised (6/18 patients had recurrences and underwent an unplanned cross‐over treatment). The unit of analysis was the episode of catheter‐related bacteraemia. The primary outcomes were short‐ and long‐term success, defined as clearance of infection at the end of two weeks and infection‐free status at the end of six weeks, respectively. Blood cultures were collected during the two weeks of maintained systemic antibiotic treatment, and no further surveillance cultures were obtained in asymptomatic children. Thus, these outcomes cannot be included as the cure definition for this review. The study’s authors reported that 23 catheters were cleared of infection after two weeks of treatment and that one was cleared after four weeks (because of antifungal treatment) (Analysis 1.1 (24 catheters): RR 0.92, 95% CI 0.74 to 1.15); the long‐term success was 58% (7 catheters) for the TPA group and 91% (11 catheters) for the heparin group (Analysis 1.2).
1.1. Analysis.

Comparison 1: Tissue plasminogen activator‐antibiotic lock treatment versus heparin‐antibiotic lock treatment, Outcome 1: Short‐term success
1.2. Analysis.

Comparison 1: Tissue plasminogen activator‐antibiotic lock treatment versus heparin‐antibiotic lock treatment, Outcome 2: Long‐term success
The secondary outcomes of this study were infection‐free catheter survival (defined as the time period between the final dose of antibiotics), the first subsequent positive blood culture obtained from the catheter, and overall catheter survival, with no reported definition. The mean infection‐free survival was 126.8 ± 81.6 days for the TPA group and 154.5 ± 70.4 days for the heparin group (Analysis 1.3). Overall, Onder 2008 did not demonstrate a difference in short‐ or long‐term success or infection‐free survival between TPA and heparin.
1.3. Analysis.

Comparison 1: Tissue plasminogen activator‐antibiotic lock treatment versus heparin‐antibiotic lock treatment, Outcome 3: Infection‐free survival
Systemic antibiotics alone versus systemic antibiotics plus antibiotic lock solution treatment
Khosroshahi 2015 compared systemic antibiotics alone versus systemic antibiotics with concurrent antibiotic lock solution, randomising 64 patients with kidney failure and suspected infections of tunnelled and cuffed HD catheters. All participants were initially treated with empiric antibiotics (vancomycin and third‐generation cephalosporin) further modified by blood culture results. Participants were followed for three weeks. Laboratory confirmation of CRBSI was obtained thereafter, leading to 45% of the included patients presenting negative blood cultures, and, therefore, outside this review’s inclusion criteria. The primary outcome measured was successful eradication of infection, defined as clinical improvement in signs and symptoms within 48 hours of initiating treatment, which does not comply with this review’s definition of cure. Overall, the study reported a success rate for clearing infection of 90.6% (29 patients) in the systemic antibiotic and ethanol lock group and 56.2% (18 patients) in the systemic antibiotics alone group (P = 0.002) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Systemic antibiotic alone versus systemic antibiotic plus antibiotic lock solution, Outcome 1: Successful eradication of infection
Catheter guidewire exchange versus catheter removal and replacement
Saleh 2017 compared over‐the‐wire catheter exchange with catheter removal and late replacement (3 to 7 days later), randomising 678 with kidney failure and suspected infection of tunnelled and cuffed HD catheters. Concurrent positive blood cultures from the catheter and a peripheral vein were obtained for laboratory confirmation of CRBSI, and catheter tips were also sent for culture. The number of negative blood cultures (including patients with no laboratory CRBSI confirmation) was not reported. Negative catheter tip cultures were considered an exclusion criterion, and the number of these excluded patients was also not reported. The primary outcome was catheter infection‐free survival, without a clear definition. The study’s authors presented definitions for cure, treatment failure, and indeterminate results, but these outcomes were not reported. Although it was not a planned outcome, the researchers also described infection recurrence rates, with no reported differences between the interventions. Overall, Saleh 2017 did not report a difference in catheter infection‐free survival between catheter guidewire exchange and catheter removal with late replacement (Analysis 3.2 (678 participants): Hazard Ratio 0.88, 95% CI 0.43 to 1.79).
3.2. Analysis.

Comparison 3: Catheter guidewire exchange versus catheter removal and replacement, Outcome 2: Infection‐free survival
Primary outcomes
Cure (clinical resolution associated with negative blood cultures at least 72 hours after completion of systemic antibiotic treatment) was not correctly defined and evaluated by any of the included studies. Onder 2008 performed blood cultures, but these tests were performed during the use of antibiotics. Khosroshahi 2015 considered cure only as clinical improvement, without laboratory confirmation. The definition of cure considered by Saleh 2017 was in accordance with our review; however, the study did not report results for this outcome.
Stenosis and thrombosis of vascular access after catheter removal were not reported by any of the included studies.
Secondary outcomes
Death, catheter malfunction, duration of hospitalisation, development of antibiotic resistance, fatigue, cardiovascular disease, and adverse outcomes (e.g., bleeding or anaphylaxis) were not reported by any of the included studies.
Subgroup and sensitivity analysis
Subgroup and sensitivity analyses were not performed due to the limited available data.
Discussion
Summary of main results
The findings of this review suggest that it remains uncertain if systemic antibiotic in association with an ethanol lock solution is superior to systemic antibiotics alone for the treatment of CRBSIs in patients with kidney failure on maintenance HD. This uncertainty is mainly because of imprecision, directness, and a high risk of bias for many domains. This evidence was restricted to one single‐centre study (64 participants), with 45% of the considered CRBSIs presenting negative blood cultures and with cure defined only by a clinical improvement in signs and symptoms, without laboratory confirmation. Additionally, the safety of this treatment is also uncertain, as the study did not report other outcomes, such as adverse events, death, antibiotic resistance, or venous stenosis or thrombosis after catheter removal.
No differences were reported for catheter infection‐free survival when comparing catheter guidewire exchange to catheter removal and late replacement or when comparing two different locking treatments (which differed from anticoagulant or thrombolytic agent use only).
Overall completeness and applicability of evidence
This review aimed to assess the different treatments for bloodstream infections in patients with permanent CVCs receiving maintenance HD. Only three studies met our inclusion criteria, and each study compared different interventions: Onder 2008 compared the use of anticoagulants and thrombolytic for antibiotic lock solutions, Khosroshahi 2015 compared the inclusion of an ethanol lock solution with the use of systemic antibiotics alone, and Saleh 2017 compared different catheter removal manoeuvres. We found no other studies evaluating other comparisons. The secondary outcomes of this review, as well as the presence of venous stenosis or thrombosis, were not assessed by the included studies. Therefore, the data on the treatment of CRBSI remains incomplete, and other relevant questions regarding antibiotic resistance after antibiotic locking and the development of venous stenosis and thrombosis after catheter removal remain unanswered.
A critical feature observed in this review was the different definitions of cure used by each study. Lok 2019 highlighted the lack of consensus among associations for CRBSI definition impairs accurate reports and reliable comparisons between studies. Accordingly, none of the included studies’ definitions of cure fit our primary outcome. Therefore, we were unable to perform a combined analysis of this crucial outcome.
It is also important to note the significant clinical heterogeneity between the participants: one study only included children with kidney failure (Onder 2008), and two studies only included adults with kidney failure (Khosroshahi 2015; Saleh 2017). The mean age of the included participants was 15.1 ± 6.8 years for children and 57.5 ± 15.6 years for adults, and the primary aetiology of kidney failure differed significantly between these groups. In addition, although Saleh 2017 was a multicentre study, it included only Middle Eastern institutions, and the authors reported a substantially higher rate of CVC use for patients on prevalent HD in their centres than the corresponding rate observed in America and Europe.
The methodological limitations, small samples, and mostly single‐centre settings of the included studies also lead to concerns regarding external validity.
Quality of the evidence
We classified the quality of the evidence as very low. We downgraded the quality of evidence due to serious limitations in the design (high risk of bias in critical domains such as randomisation, allocation, and other sources of bias), indirectness (lack of clear outcome definitions and use of catheter infection‐free survival as an indication of cure) and because of imprecision (small number of participants, only 760 in total, and just three included studies).
Potential biases in the review process
One study is awaiting classification (Khosroshahi 2006b). It was published as an abstract, the full report and results were not available, and doubts regarding participants' use of short‐ or long‐term catheters remained even after correspondence. We attempted to communicate with the authors responsible for the included studies and obtained two replies. The author of Khosroshahi 2006b and Khosroshahi 2015 clarified the allocation process of his last study but unfortunately did not eliminate many of our doubts once the raw data from both studies were unavailable. Onder 2008’s response presented conflicting information when compared to the article’s description, and further questions were not clarified. No response was obtained from Saleh 2017.
Agreements and disagreements with other studies or reviews
We identified other previous reviews that examined the treatments of CRBSIs (Allon 2004; Aslam 2014; Lok 2011).
Allon 2004 focused on observational, non‐randomised studies. The researchers reported clinical cure in only 22% to 37% of CRBSIs treated with systemic antibiotics alone and considered this method a suboptimal approach. The authors compared this finding with that derived from three other catheter strategies (including removal and delayed replacement, guidewire exchange, and maintenance with lock treatment) and reported better clinical cure with similar infection‐free survival rates between them.
Lok 2011 was a descriptive review of the literature. It addressed both CRBSI prevention and treatment and compared the use of systemic antibiotics alone to its association with different catheter approaches (lock treatment, catheter exchange over guidewire, and catheter removal with late replacement). Of the 12 included studies, only one was a quasi‐randomised study (Onder 2008), and the rest were either prospective, uncontrolled or observational studies. Overall, it concluded that catheter salvage by any means is associated more with failure rates (above 65%), and it should thus be avoided.
In the more recent of these reviews, Aslam 2014 conducted a systematic review and meta‐analysis that included 28 publications ranging from observational cohorts to chart reviews. Although the study described a thorough protocol, its findings were jeopardized by conducting a meta‐analysis with different study designs and different outcome definitions. The study focused on the role of antibiotic lock solutions and catheter guidewire exchange compared to systemic antibiotics alone. Decisions regarding whether the patient would undergo a catheter salvage attempt were made at the physician's discretion in many of the included studies, leading to an important selection bias. As in our review, the researchers reported poor cure proportions with the use of systemic antibiotics alone compared to antibiotic lock solution. They also reported a significant improvement in cure proportions when comparing systemic antibiotics alone with catheter guidewire exchange – which could not be evaluated in our review due to the lack of RCTs. Aslam 2014 found no significant differences in cure outcomes between antibiotic lock solution and catheter guidewire exchange. The authors also pooled the microbiological data and reported that infections by coagulase‐negative staphylococci exhibited higher cure proportions, followed by gram‐negative and S. aureus infections. In addition, they found that S. aureus CRBSI showed better resolutions when treated by catheter guidewire exchange than by antibiotic lock solution. Our review planned to analyse the outcomes by pathogen subgroup; however, we did not have enough data.
Authors' conclusions
Implications for practice.
It is uncertain whether the use of antibiotic lock solutions in addition to systemic antibiotics yields better cure outcomes for CRBSI treatment than the use of systemic antibiotics alone because the certainty of this evidence is very low. There is currently no evidence supporting catheter removal and delayed replacement in addition to systemic antibiotics over catheter guidewire exchange in conjunction with systemic antibiotics. Additionally, there is no evidence to support the use of one specific antibiotic lock solution over another. For these comparisons, the uncertainty of the currently available evidence prevents recommendations for clinical practice.
No eligible randomised or controlled clinical trials addressed the other comparisons we planned to analyse. Therefore, high‐quality studies are needed to enable a comparison between lock treatment versus catheter guidewire exchange, lock treatment versus catheter removal and replacement, catheter guidewire exchange versus systemic antibiotic treatment alone and catheter removal and replacement versus systemic antibiotic treatment alone.
Of note, limitations were that no studies reported outcomes including adverse events and death, leading to uncertainty about these interventions' safety.
Implications for research.
General
Patients with kidney failure should receive individualised care to plan their means of achieving vascular access when HD is needed. Although several studies highlight the increased risk of infectious complications related to the use of CVCs over fistulae (Foley 2004; Liangos 2006; Sarnak 2000), patients’ clinical conditions could make AVF creation impractical. Hence, there will always be a subset of patients requiring CVCs and therefore exposed to CRBSIs.
Because CRBSI can present severe complications in HD patients, it is crucial to establish a fast and precise diagnosis, which requires a clear definition. However, there is no consensus on the CRBSI definition among the associations and guidelines (Lok 2019). Strict definitions can enhance the specificity but might reduce the sensitivity and thus miss some cases of true CRBSI. This could lead to clinical worsening by delaying the diagnosis and, therefore, the treatment. By contrast, the least rigorous definition may over‐diagnose CRBSI and result in patients without true CRBSIs undergoing unnecessary procedures and, in the end, jeopardizing future vascular access.
Thus, it is imperative to unify a definition for CRBSI to allow proper surveillance and permit reliable studies comparing its treatments.
Design
High‐quality RCTs that compare different treatments for CRBSIs in people receiving maintenance HD are needed. We suggest that future trials investigating CRBSI treatment should incorporate the following characteristics.
Clearly describe the methods of randomisation and concealment of allocation
Use clearly described criteria to define CRBSI (such as the KDOQI 2020 definition – Lok 2019)
Include a greater number of participants, since CRBSI is a frequent complication in patients on HD through CVC
Assess cure utilizing a clear and unified definition
Evaluate outcomes other than cure, such as the development of venous stenosis and/or thrombosis, antibiotic resistance and adverse events, including death
Compare interventions, such as catheter removal and delayed replacement versus antibiotic lock solutions and versus systemic antibiotics alone
Compare interventions, such as catheter exchange over a guidewire versus antibiotic lock solutions and versus systemic antibiotics alone
Investigate and report adverse events.
Measurement
The most important outcomes to be measured are cure, development of stenosis and/or thrombosis of vascular access, antibiotic resistance, death, and adverse events. However, a minority of the studies reported outcomes other than cure.
Although the majority of the studies that addressed CRBSI measured cure, each one did so by adopting a different definition (Aslam 2014). Some analysed infection‐free rates, others evaluated successful eradication of infection, but most of them relied only on clinical improvement rather than on laboratory confirmation, the latter requiring negative blood culture after the completion of the treatment. It is essential to measure outcomes by the same criteria in order to compare results from different studies properly.
In addition to selected outcomes from this review, a cost analysis should be considered as an outcome in future studies.
History
Protocol first published: Issue 3, 2020
Acknowledgements
The Methods section of this review is based on a standard template used by Cochrane Kidney and Transplant.
We would like to thank Cochrane Vascular, Cochrane Brazil and the Division of Vascular and Endovascular Surgery of Federal University of São Paulo, SP, Brazil, for their methodological support.
The authors are grateful to the following peer reviewers for their time and comments: Takeshi Hasegawa, MD, MPH, PhD (Showa University, Tokyo, Japan); Mohamed Elrggal, MD (Kidney and Urology Center, Alexandria, Egypt); Eugene Kovalik MD, CM, FRCP(C), FACP, FASN (Professor of Medicine at Duke University Medical Center, USA); Deirdre Hahn (The Children’s Hospital at Westmead, Westmead, Australia).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Tissue plasminogen activator‐antibiotic lock treatment versus heparin‐antibiotic lock treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Short‐term success | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.15] |
| 1.2 Long‐term success | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.06] |
| 1.3 Infection‐free survival | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐27.70 [‐88.68, 33.28] |
Comparison 2. Systemic antibiotic alone versus systemic antibiotic plus antibiotic lock solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Successful eradication of infection | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.16, 2.23] |
Comparison 3. Catheter guidewire exchange versus catheter removal and replacement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Infection recurrence | 1 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.43, 5.27] |
| 3.2 Infection‐free survival | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.88 [0.43, 1.79] |
3.1. Analysis.

Comparison 3: Catheter guidewire exchange versus catheter removal and replacement, Outcome 1: Infection recurrence
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Khosroshahi 2015.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly divided into two groups using the www.randomization.org website |
| Allocation concealment (selection bias) | Low risk | The randomisation list was provided to the HD centre nurses (data supplied via correspondence) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Practitioners were blinded, but there was no mention of the blinding of patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The practitioner who evaluated the outcome was not aware of the patient groups and type of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study, and there were no losses during the follow‐up |
| Selective reporting (reporting bias) | High risk | Trial registration (https://www.irct.ir/trial/2728) was completed retrospectively. Fever and chills, considered as primary consequences (observed at the beginning of the study and during each HD session) and culture results and catheter removal, considered secondary consequences, were not reported as planned |
| Other bias | High risk | The study population considered suspected CRBSI, which resulted of 45% unconfirmed cases (negative blood cultures). There is no mention of which group these negative cultures belonged to, which could significantly influence the results |
Onder 2008.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | TPA group
Heparin group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sealed envelopes used with 18 patients. The 6 catheter‐related infection recurrences "were placed on the other arm for the second CRB without randomization". |
| Allocation concealment (selection bias) | High risk | A sealed envelope was picked by a blinded observer and given to the nurse caring for the patient who met the inclusion criteria. The envelopes were not described as being opaque, and the allocation remains uncertain because the information provided by the article conflicted with the author's response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was randomised, prospective and non‐blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although the author's reply points out "blinded observer" and the article reports a "non‐blinded study", this did not affect the assessment of the outcomes, as they are direct and objective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not describe or report missing data |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not published |
| Other bias | High risk | Differences in the description, analysis and tables. There was a protocol break ‐ catheters were removed if symptoms remained after 48 hours of treatment. However, the study reported the clearance of 83% of symptoms at 48 hours but that the catheters were maintained until 72 hours. There is conflicting information between the author's reply and the article |
Saleh 2017.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions | Catheter exchange group
Catheter removal group
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prospective randomisation was conducted by sealed envelope randomisation service |
| Allocation concealment (selection bias) | Low risk | The randomisation service also provided allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was impossible to blind the personnel and patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The published trial protocol reported single masking (care provider) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary outcomes were not fully reported |
| Selective reporting (reporting bias) | High risk | The trial protocol (NCT03054714) was published retrospectively. Additionally, did not mention excluded or withdrawn patients, deaths, treatment failures and absolute numbers for cure |
| Other bias | High risk | Among the exclusion criteria were patients in whom the catheter was not replaced within 10 days because of death. This exclusion criterion could be concealing an important adverse event for this specific intervention |
AVF ‐ arteriovenous fistula; CRB ‐ catheter‐related bacteraemia; CRBSI ‐ catheter‐related bloodstream infection; HD ‐ haemodialysis; RCT ‐ randomised controlled trial; SD ‐ standard deviation; TPA ‐ tissue plasminogen activator
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel Azim 2018 | Wrong population: includes only patients on HD by non‐tunnelled catheters |
| Bosma 2010 | Wrong intervention: biofilm prevention rather than treatment of catheter infection |
| Dahlberg 1986 | Wrong population, methods and intervention: study involved 3 different groups with randomised and non‐randomised patients and compared incidence of CRBSI in long‐term versus short‐term dialysis catheters rather than the intervention options for its treatment |
| ELVIS 2015 | Wrong intervention: prevention rather than treatment of catheter infection, also compared dysfunction |
| Harris 1997 | Wrong intervention: study assessed the prevention of infection and dysfunction for a specific catheter rather than treatment of catheter infection |
| Hymes 2017 | Wrong intervention: prevention rather than treatment of catheter infection |
| Levin 1989 | Wrong intervention: prevention rather than treatment of catheter infection |
| NCT00108433 | Study abandoned and not published with the wrong population and intervention: includes short‐term catheters and compared two different systemic antibiotics alone without catheter management |
| NCT01483872 | Study abandoned early due to inadequate enrolment |
| NCT02040818 | Study abandoned early due to inadequate enrolment |
| Oliver 2007 | Wrong intervention: use of fibrin sheath disruption for prevention rather than treatment of catheter infection |
| Rosenblum 2014 | Wrong intervention: prevention rather than treatment of catheter infection |
| Zwiech 2016 | Wrong intervention: use of taurolidine‐citrate‐heparin lock solution for prevention rather than treatment of catheter infection |
CRBSI ‐ catheter‐related bloodstream infection; HD ‐ haemodialysis
Characteristics of studies awaiting classification [ordered by study ID]
Khosroshahi 2006b.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Both groups
|
| Outcomes |
|
| Notes |
|
CRBSI ‐ catheter‐related bloodstream infection; CVC ‐ central venous catheter; HD ‐ haemodialysis; M/F ‐ male/female; RCT ‐ randomised controlled trial; SD ‐ standard deviation
Differences between protocol and review
The objectives were updated to not only compare catheter guidewire exchange to other treatments, but to analyse all possible treatments for catheter‐related bloodstream infection.
Contributions of authors
Draft the protocol: BMA, DC
Study selection: BMA, DC
Extract data from studies: BMA, DC
Enter data into RevMan: BMA
Carry out the analysis: BMA, DC
Interpret the analysis: BMA, DC
Draft and comment on the final review: BMA, DC, VV, DHM
Disagreement resolution: DHM
Update the review: BMA
Sources of support
Internal sources
Brazilian Cochrane Center, Brazil
Postgraduate Program in Evidence Based Medicine, UNIFESP, Brazil
External sources
No sources of support provided
Declarations of interest
Beatriz M Almeida: no known conflicts of interest
Daniel H Moreno: no known conflicts of interest
Vladimir Vasconcelos: no known conflicts of interest
Daniel G Cacione: no known conflicts of interest
New
References
References to studies included in this review
Khosroshahi 2015 {published data only}
- Khosroshahi HT, Mahdipur H, Parkhideh S, Basmenji S, Khalilzadeh M, Tozihi M.The effectiveness of systemic antibiotic therapy with and without ethanol-locked solution in the treatment of hemodialysis-related catheter infection. Saudi Journal of Kidney Diseases & Transplantation 2015;26(3):477-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Onder 2008 {published data only}
- Onder AM, Chandar J, Simon N, Diaz R, Nwobi O, Abitbol CL, et al.Comparison of tissue plasminogen activator-antibiotic locks with heparin-antibiotic locks in children with catheter-related bacteraemia. Nephrology Dialysis Transplantation 2008;23(8):2604-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saleh 2017 {published data only}
- Saleh HM, Abouellail H.Prospective, randomized study of tunneled cuffed hemodialysis catheter removal and delayed insertion versus guidewire exchange to treat catheter related blood stream infection. Interim results of first 100 cases. Life Science Journal 2017;14:45-9. [DOI] [PubMed] [Google Scholar]
- Saleh HM, Tawfik MM, Abouellail H.Prospective, randomized study of long-term hemodialysis catheter removal versus guidewire exchange to treat catheter-related bloodstream infection. Journal of Vascular Surgery 2017;66(5):1427-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saleh HM, Tawfik MM, Abouellail H.Prospective, randomized study of long-term hemodialysis catheter removal versus guidewire exchange to treat catheter-related bloodstream infection [abstract]. European Journal of Vascular & Endovascular Surgery 2017;54(5):671. [EMBASE: 619106979] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel Azim 2018 {published data only}
- Abdel Azim AB, ElSaid TW, El Said HW, Hemida W, Zaghlool S, Ramadan A, et al.A randomized controlled clinical trial of 4% sodium citrate versus heparin as locking solution for temporary dialysis catheters among hemodialysis patients. Clinical Nephrology 2018;90(5):341-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bosma 2010 {published data only}
- Bosma JW, Siegert CE, Peerbooms PG, Weijmer MC.Reduction of biofilm formation with trisodium citrate in haemodialysis catheters: a randomized controlled trial. Nephrology Dialysis Transplantation 2010;25(4):1213-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bosma JW, Weijmer MC, Peerbooms PG, Siegert CE.Reduction of biofilm formation with trisodium citrate in haemodialysis catheters: a randomized controlled trial [abstract]. Journal of Vascular Access 2009;10(2):83. [DOI] [PubMed] [Google Scholar]
Dahlberg 1986 {published data only}
- Dahlberg PJ, Yutuc WR, Newcomer KL.Subclavian hemodialysis catheter infections. American Journal of Kidney Diseases 1986;7(5):421-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ELVIS 2015 {published data only}
- Coupez E, Timsit JF, Boyer A, Bouadma L, Canet E, Klouche K, et al.Guidewire exchange vs new-site placement for temporary dialysis catheters insertion in ICU patients: is there a greater risk of colonization or dysfunction? [abstract]. Intensive Care Medicine Experimental 2015;3(Suppl 1):A463. [EMBASE: 611852790] [Google Scholar]
- Coupez E, Timsit JF, Ruckly S, Schwebel C, Gruson D, Canet E, et al.Guidewire exchange vs new site placement for temporary dialysis catheter insertion in ICU patients: is there a greater risk of colonization or dysfunction? Critical Care 2016;20(1):230. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souweine B, Lautrette A, Gruson D, Canet E, Klouche K, Argaud L, et al.Ethanol lock and risk of hemodialysis catheter infection in critically ill patients. A randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 2015;191(9):1024-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 1997 {published data only}
- Harris FE, Nelson SR.Preliminary experience of methacryloylphosphoryl-choline laurylmethacrylate coated vascular access catheters [abstract no: P1399]. Nephrology 1997;3(Suppl 1):S422. [Google Scholar]
Hymes 2017 {published data only}
- Hymes JL, Mooney A, Van Zandt CR, Lynch L, Ziebol R, Killion D.A novel device for reducing catheter related bloodstream, infections: a multi-center, controlled, randomized clinical trials of the Clearguard HD antimicrobial barrier cap [abstract no: SA-PO1054]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):879A. [CENTRAL: CN-01657423] [Google Scholar]
- Hymes JL, Mooney A, Van Zandt CR, Lynch L, Ziebol R, Killion D.Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. American Journal of Kidney Diseases 2017;69(2):220-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levin 1989 {published data only}
- Levin A, Mason AJ, Fong IW, Jindal KK, Goldstein MB.Effectiveness of topical betadine ointment (B) against hemodialysis (HD), subclavian line (SCL) related sepsis [abstract]. Kidney International 1989;35(1):253. [CENTRAL: CN-01658130] [Google Scholar]
- Levin A, Mason AJ, Fong IW, Jindal KK, Goldstein MB.Effectiveness of topical betadine ointment (B) against hemodialysis (HD), subclavian line (SCL) sepsis [abstract]. Kidney International 1990;37(1):307. [CENTRAL: CN-01658129] [Google Scholar]
NCT00108433 {published data only}
- Linezolid in the treatment of hemodialysis patients with catheter-related gram-positive bloodstream infections [Linezolid vs vancomycin/cefazolin in the treatment of hemodialysis patients with catheter-related gram-positive bloodstream infections]. clinicaltrials.gov/show/NCT00108433 (first received 18 April 2005).
NCT01483872 {published data only}
- Aslam S.Use of a novel catheter lock solution for treatment of hemodialysis catheter infections [Phase II trial of a novel catheter lock solution for adjunctive treatment of hemodialysis catheter-associated bacteremia]. clinicaltrials.gov/show/NCT01483872 (first received 2 December 2011).
NCT02040818 {published data only}
- Aslam S.Treatment of hemodialysis catheter-related bacteremia [RCT for the treatment of hemodialysis catheter-related bacteremia]. clinicaltrials.gov/show/NCT02040818 (first received 20 January 2014).
Oliver 2007 {published data only}
- Oliver MJ, Mendelssohn DC, Quinn RR, Richardson EP, Rajan DK, Pugash RA, et al.Catheter patency and function after catheter sheath disruption: a pilot study. Clinical Journal of the American Society of Nephrology: CJASN 2007;2(6):1201-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosenblum 2014 {published data only}
- Rosenblum A, Wang W, Ball LK, Latham C, Maddux FW, Lacson EJ.Hemodialysis catheter care strategies: a cluster-randomized quality improvement initiative. American Journal of Kidney Diseases 2014;63(2):259-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenblum AJ, Hall JA, Wang W, Maddux FW, Lacson EK.Comparative effectiveness of hemodialysis catheter care protocols to prevent bacteremia [abstract no: TH-PO256]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):152A. [Google Scholar]
Zwiech 2016 {published data only}
- Zwiech R, Adelt M, Chrul S.A taurolidine-citrate-heparin lock solution effectively eradicates pathogens from the catheter biofilm in hemodialysis patients. American Journal of Therapeutics 2016;23(2):e363-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Khosroshahi 2006b {published data only}
- Khosroshahi HT, Aghazadeh MM, Oskuii SF, Soja MM, Khalilzadeh M.Comparisonal management of hemodialysis catheter related infection with and without an adjunctive antibiotic lock solution [abstract no: SP657]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv237. [CENTRAL: CN-01657227] [Google Scholar]
Additional references
Allon 2004
- Allon M.Dialysis catheter-related bacteremia: treatment and prophylaxis. American Journal of Kidney Diseases 2004;44(5):779-91. [MEDLINE: ] [PubMed] [Google Scholar]
Arechabala 2018
- Arechabala MC, Catoni MI, Claro JC, Rojas NP, Rubio ME, Calvo MA, et al.Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD010597. [DOI: 10.1002/14651858.CD010597.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aslam 2014
- Aslam S, Vaida F, Ritter M, Mehta RL.Systematic review and meta-analysis on management of hemodialysis catheter-related bacteremia. Journal of the American Society of Nephrology 2014;25(12):2927-41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beigi 2010
- Beigi AA, Khansoltani M, Masoudpour H, Atapour AA, Eshaghian A, Khademi EF.Influence of intralumenal and antibiotic-lock of vancomycin on the rate of catheter removal in the patients with permanent hemodialysis catheters. Saudi Journal of Kidney Diseases & Transplantation 2010;21(1):54-8. [MEDLINE: ] [PubMed] [Google Scholar]
Donlan 2001
- Donlan RM.Biofilm formation: a clinically relevant microbiological process. Clinical Infectious Diseases 2001;33(8):1387-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duszak 1998
- Duszak R Jr, Haskal ZJ, Thomas-Hawkins C, Soulen MC, Baum RA, Shlansky-Goldberg RD, et al.Replacement of failing tunneled hemodialysis catheters through pre-existing subcutaneous tunnels: a comparison of catheter function and infection rates for de novo placements and over-the-wire exchanges. Journal of Vascular & Interventional Radiology 1998;9(2):321-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 2004
- Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ.Septicemia in the United States dialysis population, 1991 to 1999. Journal of the American Society of Nephrology 2004;15(4):1038-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al.GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al.Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Ishani 2005
- Ishani A, Collins AJ, Herzog CA, Foley RN.Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney International 2005;68(1):311-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Justo 2014
- Justo JA, Bookstaver PB.Antibiotic lock therapy: review of technique and logistical challenges. Infection & Drug Resistance 2014;7:343-63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liangos 2006
- Liangos O, Gul A, Madias NE, Jaber BL.Long-term management of the tunneled venous catheter. Seminars in Dialysis 2006;19(2):158-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lok 2011
- Lok CE, Mokrzycki MH.Prevention and management of catheter-related infection in hemodialysis patients. Kidney International 2011;79(6):587-98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lok 2019
MacRae 2005
- MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M.Central vein stenosis: a common problem in patients on hemodialysis. ASAIO Journal 2005;51(1):77-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mermel 2009
- Mermel LA, Allon M, Bouza E, Craven E, Flynn P, O'Grady NP, et al.Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America [Erratum in: Clin Infect Dis. 2010 Apr 1;50(7):1079 Note: Dosage error in article text] [Erratum in: Clin Infect Dis. 2010 Feb 1;50(3):457]. Clinical Infectious Diseases 2009;49(1):1-45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al.Guidelines for the prevention of intravascular catheter-related infections. Clinical Infectious Diseases 2011;52(9):e162-93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perl 2011
- Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, et al.Hemodialysis vascular access modifies the association between dialysis modality and survival. Journal of the American Society of Nephrology 2011;22(6):1113-21. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polkinghorne 2004
- Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG.Vascular access and all-cause mortality: a propensity score analysis. Journal of the American Society of Nephrology 2004;15(2):477-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ravani 2013
- Ravani P, Gillespie BW, Quinn RR, MacRae J, Manns B, Mendelssohn D, et al.Temporal risk profile for infectious and noninfectious complications of hemodialysis access. Journal of the American Society of Nephrology 2013;24(10):1668-77. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayner 2010
- Rayner HC, Pisoni RL.The increasing use of hemodialysis catheters: evidence from the DOPPS on its significance and ways to reverse it. Seminars in Dialysis 2010;23(1):6-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saad 1999
- Saad TF.Bacteremia associated with tunneled, cuffed hemodialysis catheters. American Journal of Kidney Diseases 1999;34(6):1114-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saran 2018
- Saran R, Robinson B, Abbott KC, Agodoa LY, Bhave N, Bragg-Gresham J, et al.US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States [Erratum in: Am J Kidney Dis. 2018 Apr;71(4):501; PMID: 29579418]. American Journal of Kidney Diseases 2018;71(3 Suppl 1):A7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saran 2019
- Saran R, Robinson B, Abbott KC, Agoda LY, Braff-Gresham J, et al.US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. American Journal of Kidney Diseases 2019;73(3 Suppl 1):A7-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarnak 2000
- Sarnak MJ, Jaber BL.Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney International 2000;58(4):1758-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2020a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
Schunemann 2020b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al.Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
SONG 2017
- SONG Initiative.The SONG Handbook Version 1.0, 2017. www.songinitiative.org/reports-and-publications/ (last accessed 3 March 2020).
Tanriover 2000
- Tanriover B, Carlton D, Saddekni S, Hamrick K, Oser R, Westfall AO, et al.Bacteremia associated with tunneled dialysis catheters: Comparison of two treatment strategies. Kidney International 2000;57(5):2151-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valliant 2014
- Valliant AM, Chaudhry MK, Yevzlin A, Astor B, Chan MR.Tunneled dialysis catheter exchange with fibrin sheath disruption is not associated with increased rate of bacteremia. Journal of Vascular Access 2014;16(1):52-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vascular Access 2006 Work Group 2006
- Vascular Access 2006 Work Group.Clinical practice guidelines for vascular access. American Journal of Kidney Diseases 2006;48 Suppl 1:S176-247. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Almeida 2020
- Almeida BM, Moreno DH, Vasconcelos V, Cacione DG.Interventions for treating catheter‐related bloodstream infections in people receiving maintenance haemodialysis. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013554. [DOI: 10.1002/14651858.CD013554] [DOI] [PMC free article] [PubMed] [Google Scholar]


