Abstract
We performed a systematic review, meta‐analysis, and meta‐regression to determine if increasing daily protein ingestion contributes to gaining lean body mass (LBM), muscle strength, and physical/functional test performance in healthy subjects. A protocol for the present study was registered (PROSPERO, CRD42020159001), and a systematic search of Medline, Embase, CINAHL, and Web of Sciences databases was undertaken. Only randomized controlled trials (RCT) where participants increased their daily protein intake and were healthy and non‐obese adults were included. Research questions focused on the main effects on the outcomes of interest and subgroup analysis, splitting the studies by participation in a resistance exercise (RE), age (<65 or ≥65 years old), and levels of daily protein ingestion. Three‐level random‐effects meta‐analyses and meta‐regressions were conducted on data from 74 RCT. Most of the selected studies tested the effects of additional protein ingestion during RE training. The evidence suggests that increasing daily protein ingestion may enhance gains in LBM in studies enrolling subjects in RE (SMD [standardized mean difference] = 0.22, 95% CI [95% confidence interval] 0.14:0.30, P < 0.01, 62 studies, moderate level of evidence). The effect on LBM was significant in subjects ≥65 years old ingesting 1.2–1.59 g of protein/kg/day and for younger subjects (<65 years old) ingesting ≥1.6 g of protein/kg/day submitted to RE. Lower‐body strength gain was slightly higher by additional protein ingestion at ≥1.6 g of protein/kg/day during RE training (SMD = 0.40, 95% CI 0.09:0.35, P < 0.01, 19 studies, low level of evidence). Bench press strength is slightly increased by ingesting more protein in <65 years old subjects during RE training (SMD = 0.18, 95% CI 0.03:0.33, P = 0.01, 32 studies, low level of evidence). The effects of ingesting more protein are unclear when assessing handgrip strength and only marginal for performance in physical function tests. In conclusion, increasing daily protein ingestion results in small additional gains in LBM and lower body muscle strength gains in healthy adults enrolled in resistance exercise training. There is a slight effect on bench press strength and minimal effect performance in physical function tests. The effect on handgrip strength is unclear.
Keywords: Muscle mass, Muscle strength, Protein quantity, Physical function
Introduction
Skeletal muscle is the main component of lean body mass (LBM), and beyond locomotion, muscle has several health‐related roles. 1 Reduced skeletal muscle mass and function in adults have been linked to chronic diseases, poor quality of life, sarcopenia, physical disability, increased risk of fractures, and risk for frailty. 2 , 3 , 4 , 5 , 6 , 7 , 8 Protein ingestion and resistance exercise (RE) are the main non‐pharmacologic factors driving anabolic signals to increase or maintain skeletal muscle mass. 9 Nonetheless, many questions still arise when constructing dietary or physical activity guidelines focusing on skeletal muscle health. 10 Particularly, the optimal daily protein intake level required to optimize skeletal muscle mass gain or maintenance in healthy adults, which is still largely unclear. 10 , 11 , 12 , 13 It is also unclear whether additional protein ingestion can preserve lean body mass and muscle function in healthy adults who do not engage in RE. 14
It appears that ingesting sufficient protein is required to maintain muscle mass. 11 , 15 , 16 Recommended protein intakes for healthy adults slightly vary worldwide but are generally in the range of 0.8–0.9 g protein/kg body weight (BW)/day; an intake proposed to maintain nitrogen balance in ~98% of individuals. For example, the current Recommended Dietary Allowance in the USA and Canada is 0.8 g protein/kg BW/day, 17 the FAO of the UN/WHO recommendation is 0.83 g/kg BW/day, 18 and the European Food Safety Authority also established its population reference intake for protein as 0.83 g/kg BW/day for all adults. These dietary protein intake recommendations have traditionally been the same for adults (>18 years old), regardless of age or sex. Nevertheless, a higher daily protein intake (1.2–1.6 g/kg BW/day) has been suggested to improve lean body mass gain or maintain muscle mass in young and old healthy adults. 5 , 10 , 19
Previous meta‐analyses have been conducted to ascertain whether additional protein ingestion is linked to increases in LBM (i.e. muscle mass), muscle strength, or physical function in adults. 13 , 14 , 20 , 21 , 22 , 23 However, the effects of protein ingestion independent of RE are not commonly explored. 13 , 22 Furthermore, the population inclusion criteria in previous meta‐analyses have, we propose, led to confusion in the interpretation of the results. Meta‐analyses of studies testing weight loss protocols, including obese subjects or subjects with chronic illnesses, sarcopenia, frailty, chronic diseases, or multigradient supplements, make it challenging to translate the findings. 13 , 20 , 21 , 22
We performed a systematic review, meta‐analysis, and meta‐regression to determine if providing additional dietary protein (protein to exceed participants' habitual protein intake) contributes to increasing LBM (i.e. muscle mass, fat‐free mass, lean soft‐tissue mass, bone and fat‐free mass), strength, and physical/functional test performance in healthy adult subjects who were free from conditions that have been shown to affect skeletal muscle protein turnover and muscle function. Also, we sought to know if additional protein ingestion affects the proposed outcomes independent of RE and age. We hypothesized that additional protein ingestion would improve all outcomes, independent of age or the performance of RE; however, there may be a dose–response effect.
Methods
This systematic review, meta‐analysis, and meta‐regression followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions 24 and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) report. 25 The procedures for identification, screening, data extraction, and analysis were agreed upon in advance by the team of researchers involved in the study. Details of the intended methods were documented in advance in a protocol registered at the International Prospective Register of Systematic Reviews (PROSPERO, CRD CRD42020159001) before literature search, data extraction, and analysis.
Eligibility criteria
The research questions were established using the PICOS (population, intervention, comparison, outcome, and setting) criteria (Table 1). Participants were untrained or trained healthy men or women 18 or older. Studies including weight loss diet protocols, obese, and subjects with any diagnosed or self‐reported disease were not included. Studies including obese subjects were excluded since it has been shown that obesity negatively affects postprandial myofibrillar protein synthetic response to nutrition and exercise. 26 Also, interventions were carefully screened for the presence of any potential active ingredient that might impact lean body mass gain other than protein (i.e. creatine, phosphatidic acid, omega‐3 fatty acids, anabolic steroids, and beta‐hydroxy‐beta‐methylbutyrate [HMB]). If a particular study testing potential active supplements had intervention groups fitting our inclusion criteria, the study was included and assessed to extract the respective data of protein and control/placebo interventions only. No restrictions were imposed regarding additional protein dose or the duration of the intervention protocol.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Adult participants (healthy) aged 18 years or older | Subjects with decreased mobility, frailty, obesity, or any chronic or infectious diseases were not included |
| Intervention | Additional protein ingestion with or without the addition of resistance exercise (increasing protein ingestion as the primary intervention using nutritional supplements or diet at any dose or level) | Intervention aiming to cause weight loss (i.e. negative energy balance) or with the presence of other potential active ingredients in the intervention to change lean body mass (e.g. creatine, phosphatidic acid, omega‐3 fatty acids, anabolic steroids, beta‐hydroxy‐beta‐methylbutyrate [HMB]) |
| Comparator | Placebo or no intervention (control) | No control or placebo groups |
| Outcomes | Lean body mass (or a similar measure), muscle strength (lower body, bench press, and handgrip strength) and performance in physical tests | Not assessing at least one of the target outcomes |
| Study Design | Randomized controlled trials (RCT) | Not a RCT |
| Research questions |
Main question:
Sub‐questions:
|
Systematic search strategy
A literature search for randomized controlled trials (RCT) investigating the effect of ingesting additional protein on lean body mass, muscle strength, and physical test performance in healthy adults was conducted up to September 2020 by electronic searching of relevant literature databases. The literature search was conducted on Medical Literature Analysis and Retrieval System Online (Medline), Excerpta Medica (Embase), Cumulative Index of Nursing and Allied Health Literature (CINHAL), and Web of Science core collection. Two distinct search strategies were used to assess studies using protein ingestion only or protein ingestion in parallel to a RE training protocol as an intervention. Results from both searches were combined and screened according to our inclusion and exclusion criteria (see Supplementary files ‐ Search strategies). Limits were applied to the electronic search, restricting studies to adults and humans, English only, and excluding diseases (e.g. cancer and diabetes). The systematic search team (E. A. N., L. C. S., S. R. M., T. Y., and S. M. P.) conducted additional filtering to exclude specific studies based on inclusion and exclusion criteria, visually screening titles, abstracts, and full‐texts when necessary. Lists of references from database searches were imported to the software Endnote X9.3.3 for title screening and additional filtering using semi‐automated tools. The remaining references selected during title screening were uploaded to Rayyan—a web and mobile app for systematic reviews. 27 Using Ryaan, three reviewers (E. A. N., S. M. P., and T. Y.) screened titles and abstracts independently. Conflicts were solved by reassessing the respective references during group discussion after unblinding the results. Abstracts and conference proceedings were not included.
Data extraction and outcome measures
Studies were reviewed and screened for the study design, protein supplementation or increased protein prescription intervention, subject characteristics, placebo/control information, body composition, resistance training protocols, and strength or physical testing outcomes. Data were extracted not independently by three investigators (E. A. N., S. M. P., and T. Y.) and checked for consistency after extraction. First, each member extracted data from an equal number of different studies. Then, the extracted data were checked for accuracy and reviewed by a second member. Body composition outcomes were extracted as changes in any variable targeting ‘muscle’ mass (i.e. muscle mass, whole‐body lean mass, lean body mass, fat‐free mass, and bone and fat‐free lean soft tissue mass). Methods applied to measure body composition included dual‐energy X‐ray absorptiometry (DXA), hydrodensitometry, bioimpedance (BIA), skinfolds, and/or whole‐body air plethysmography (BodPod®). Daily protein ingestion, additional protein given during the intervention, protein source (e.g. animal‐based, plant‐based or blended protein sources) data were also extracted. Strength testing outcomes were repetition‐maximum (isotonic) strength (measured by 1‐3RM strength tests) or any isometric testing for strength. Upper body strength was obtained from bench/chest press exercises testing data. For lower body strength, leg press, squat, leg extension, leg curls, or similar exercises were used for data extraction. Physical testing included timed up and go (TUG), chair‐based testing, sit‐to‐stand tests, gait speed tests, balance tests, short physical performance battery tests, stair climb tests, time or distance‐limited walking tests, and tests involving activities of daily life. Authors were not contacted for missing data. If not available in tables or the text, data were extracted from figures using the online tool WebPlotDigitizer. 28
Risk of bias, heterogeneity, quality of the evidence, and sensitivity analysis
The risk of bias was assessed according to the Cochrane Collaboration risk‐of‐bias tool using RevMan5 by two team members (E. A. N. and S. M. P.). 24 , 29 Studies were carefully reviewed for details, including randomization methods, participant allocation, and blinding of the subjects and researchers directly involved with the subjects or data analysis. Studies not reporting randomization or blinding procedures were considered high risk in the domain allocation concealment and blinding of participants and personnel. Also, attrition, incomplete outcome data, selective reporting, and other sources of bias were assessed. Cochrane's Q was employed to detect statistical heterogeneity and I 2 statistic to quantify the magnitude of statistical heterogeneity between studies where I 2 30% to 60% represents moderate and I 2 60% to 90% represents substantial heterogeneity across studies. The quality of the evidence was assessed by two team members (E. A. N. and S. M. P.) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system of rating uploading the list of studies on GRADEpro platform (https://gdt.gradepro.org/) and performing the grading manually. 30 Funnel plots were generated for visual assessment for asymmetry and potential publication bias 31 (see Figures S20–S29). Studies identified as potential outliers during the visual analysis of funnel plots and assessed with three (3) or more domains judged as potentially high risk of bias were submitted to sensitivity analyses. These analyses were conducted for all outcomes by the ‘remove 1’ technique to assess whether individual studies had a disproportionate effect on the meta‐analyses results 32 (Tables S6–S10).
Statistical analysis
The analysis was conducted using change from baseline to immediate post‐treatment data (means, standard deviations) for both intervention and control/placebo groups to generate the summary measures of effect in the form of standardized mean difference (SMD). Means and standard deviation (SD) for changes were calculated or imputed from the available data in the paper. Correlation coefficients were estimated from the data and used to impute missing SD for change for some studies according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions. 24 Calculated correlation coefficients were in the range of 0.7–0.9 for all outcomes. Therefore, a median point of 0.8 was applied as the correlation coefficient for any necessary SD imputation.
The SMD was used as a summary statistic since studies in this systematic review often assessed the same outcome measured in various ways (i.e. muscle mass, lean body mass, bone and fat‐free mass and lower body strength measured by leg press, squat, or leg extension). In this situation, it was necessary to standardize the results of the studies before they could be compared across studies or combined in the quantitative synthesis. SMDs were estimated using Hedge's g approach (also known as bias‐corrected effect size). The SMDs of 0.2–0.5, 0.5–0.8, and >0.8 were considered small, medium, and large effects, respectively. To analyse physical performance measures, we standardized the direction of effect to ensure consistency of desirable outcome responses (i.e. a reduction measured in seconds to cover a given distance reflects a faster gait speed and thus a better outcome, whereas an increase in gait speed measured in m/s reflects a positive outcome). Similarly, a reduction in the sit‐to‐stand test (s), five chair repetition test (s), and timed up‐and‐go test (s) is desirable. When available, multiple data were extracted from the same study for lower‐body strength or performance in physical tests and included in the analysis.
We used a random‐effects three‐level meta‐analytic approach to account for dependency between effect sizes (i.e. the correlation between effect sizes due to multiple measures or sub‐measures of the same outcome within a study or the comparison of multiple interventions to a single control group). In such cases, multiples measures and comparisons from the same study are nested within studies first, and variance in observed effect sizes is decomposed into sampling variance, with‐in study variance, and between‐study variance to account for intracluster (or intraclass) correlation in the true effects. In addition, we submitted the data to three‐level meta‐regression analyses based on the use of exercise/resistance training (‘yes’ or ‘no’), age (<65 vs. ≥65 years old), and the level of protein intake (continuous as g/kg/day or categorical—‘<1.2 g/kg/day’, ‘1.2–1.59 g/kg/day’ and ‘≥1.6 g/kg/day’) when possible. All analyses and figures were made with RStudio v.1.4.1717 (metafor R package).
Results
Literature search and study selection
The results of the literature search are presented in Figure 1. Four databases were searched, applying search strategies for augmented protein intake alone or in addition to resistance exercise interventions resulting in 23 757 records. After screening for duplicates and study characteristics, 164 studies were selected for full‐text screening and eligibility. Finally, 74 RCT were obtained at the end of our screening process.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow chart shows the number of studies involved in each systematic search and screening step. Medline: Medical Literature Analysis and Retrieval System Online.
Study characteristics for randomized controlled trials
Table S1 shows summary information from all RCT studies included in the meta‐analysis. In the studies assessing protein ingestion, daily total protein ingestion varied from 1 to 4.4 g/kg/day in the intervention groups (33% of the studies 1.2–1.59 g/kg/day and 54% of the studies ≥1.6 g/kg/day) and from 0.8 to 2.3 g/kg/day in the placebo/control groups. However, it is noteworthy that in ~80% of the studies, baseline protein ingestion was at least 1.2 g of protein/kg/day. The participants' mean age ranged from 19 to 85 years, and study protocols lasted from 6 to 108 weeks (76% of the studies between 8 and 12 weeks). Studies varied vastly regarding the quantity of additional protein provided to research participants. Dietary or supplemented protein ranged from 5 to 100 g/day, depending on the study (56% of the studies between 10 and 30 g/day and 28% between 31 and 50 g/day). Six studies had intervention groups ingesting a blend of proteins (supplements or food), 33 , 34 , 35 , 36 , 37 , 38 , 39 and nine used plant‐based (primarily soy) protein supplements. 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 In some cases, the same study tested more than one protein source or supplement. 40 , 42 , 43 , 45 , 47 , 48 , 49
Sixty‐six out of 74 studies were included in the lean body mass change analysis, utilizing 2665 subjects. 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 Six studies presented intervention groups not using RE training 41 , 46 , 94 , 97 , 98 , 99 ; of these, four studies tested protein ingestion exclusively, 41 , 97 , 98 , 99 and two studies tested protein ingestion in groups without and with resistance exercise. 46 , 94 Changes in strength data resulting from the additional protein intervention were extracted from 50 studies testing 2283 subjects for lower‐body strength 33 , 36 , 37 , 38 , 39 , 43 , 44 , 47 , 48 , 49 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 61 , 62 , 63 , 64 , 65 , 67 , 68 , 69 , 70 , 72 , 74 , 75 , 76 , 77 , 78 , 82 , 84 , 86 , 88 , 89 , 90 , 91 , 92 , 94 , 96 , 100 , 101 , 102 , 103 , 104 and only three studies with intervention groups without RE. 41 , 99 , 105 Thirty‐four studies tested bench‐press strength 33 , 36 , 37 , 38 , 43 , 47 , 48 , 49 , 53 , 54 , 55 , 62 , 63 , 64 , 65 , 67 , 68 , 70 , 72 , 74 , 75 , 77 , 78 , 82 , 84 , 86 , 87 , 88 , 90 , 91 , 93 , 95 , 96 , 99 with 1049 subjects. The duration of the studies was, on average, 12 weeks for both bench‐press and lower body strength. However, one study testing lower‐body strength was 108 weeks long. 105 Only one study testing bench‐press strength did not use RE in the protocol. 99 Handgrip strength data were extracted from 10 studies in total (612 subjects), 41 , 50 , 58 , 76 , 81 , 83 , 97 , 99 , 104 , 105 four studies using RE training 41 , 97 , 99 , 105 and two studies testing only young participants. 41 , 81 The approximate duration of the studies testing the effects of protein ingestion on handgrip strength was 12 weeks, except for one study lasting 108 weeks. 105 Data regarding the effects of additional protein ingestion on physical or functional tests were extracted from 15 studies enrolling 1173 subjects 6 , 39 , 50 , 52 , 56 , 58 , 72 , 75 , 76 , 94 , 97 , 99 , 100 , 104 , 105 and an approximate duration of 12 weeks, 6 , 39 , 50 , 52 , 56 , 58 , 72 , 75 , 76 , 94 , 97 , 99 , 100 , 104 except for one study lasting 108 weeks. 105 Eleven studies tested the effect of protein intervention on physical function in parallel to RE training. 39 , 50 , 52 , 56 , 58 , 72 , 75 , 76 , 94 , 100 , 104
Risk of bias and heterogeneity of randomized controlled trials
Risk of bias analysis showed that six studies had a potential unclear or high risk of selection bias due to missing information regarding randomization or allocation procedures. Seventeen studies out of 74 presented a potentially high risk of performance bias for blinding research participants or staff. Nine studies reporting the use of single‐blind protocols were scored as unclear risk of performance bias. Fourteen studies presented a potentially high risk of detection bias since the research staff was aware of which individuals received the intervention. Eleven studies were scored an unclear risk of detection bias since it was not described whether the research team knew which treatment the participants were assigned to during the intervention and testing. In 21 studies, there was a potential unclear risk of attrition bias. Eleven studies were scored as unclear risk of reporting bias. A summary of the risk of bias analysis is presented in Figure S1 . A supplementary figure shows the per‐study risk of bias analysis (Figure S2).
Heterogeneity for overall main effects in most of the analysis regarding changes in lean body mass was low (I 2 ≤ 25%) (Table 2). Overall heterogeneity was moderate for the main effect of ingesting protein on bench press strength (I 2 = 43%, Table 3). Subgroup analysis of studies by age (<65 and ≥65 years old) returned moderate heterogeneity for the overall effect (I 2 = 39.4%, Table 3). However, heterogeneity was even higher in the subgroup <65 years old (I 2 = 55%, Table 3). When analysed by the level of protein ingestion, overall, and in each protein level subgroup, heterogeneity for bench press strength was small to moderate (I 2 = 23.3–54.7%, Table 3). Heterogeneity in low body strength data was moderate (I 2 = 52.8%, Table 4). Subgroups of studies by the level of protein intake, resistance exercise presence, and age showed small to high heterogeneity for subgroups (I 2 = 26.1–51.6%, Table 4). Handgrip strength data had low heterogeneity (I 2 = 0%, Table 5). Heterogeneity in studies reporting physical and functional testing outcomes was moderate (I 2 = 46.4–58%, Table 5).
Table 2.
Effects of protein supplementation on changes in lean body mass
| Groups/subgroups | SMD | 95% CI | Number of trials/intervention groups | P‐value | I 2 (%) |
|---|---|---|---|---|---|
| All RCT | 0.22 | 0.15:0.29 | 66/93 | <0.01 | 7 |
| RCT without resistance exercise | 0.21 | ‐0.15:0.58 | 6/6 | 0.38 | 25 |
| RCT with resistance exercise (RE) | 0.22 | 0.14:0.30 | 62/87 | <0.01 | 6.2 |
| <65 years old | 0.25 | 0.16:0.35 | 48/70 | <0.01 | 8.1 |
| ≥65 years old | 0.13 | −0.00:0.28 | 14/17 | 0.06 | 6.2 |
| RCT with RE reporting protein ingestion | 0.19 | 0.11:0.28 | 51/72 | <0.01 | 6.9 |
| RCT with RE ingesting <1.2 g/kg/day | −0.14 | −0.56:0.27 | 4/4 | 0.35 | 0 |
| RCT with RE ingesting 1.2–1.59 g/kg/day | 0.17 | 0.06:0.28 | 24/34 | <0.01 | 0 |
| <65 years old | 0.15 | −0.02:0.31 | 15/23 | 0.07 | 2.8 |
| ≥65 years old | 0.20 | 0.02:0.37 | 9/11 | 0.03 | 0 |
| RCT with RE ingesting ≥1.6 g/kg/day | 0.30 | 0.17:0.43 | 23/34 | <0.01 | 0 |
| <65 years old | 0.30 | 0.17:0.43 | 23/34 | <0.01 | 0 |
| ≥65 years old a | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in all RCT reporting protein ingestion |
0.13 | −0.00:0.26 | 55/77 | 0.06 | NA |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in studies using RE | 0.14 | 0.00:0.27 | 51/72 | 0.04 | NA |
BW, body weight; CI, confidence intervals; NA, not applicable; RCT, randomized clinical trials; RE, resistance exercise; SMD, standardized mean deviation.
No studies in the dataset.
Table 3.
Effects of protein supplementation on changes in bench press strength
| Groups/subgroups | SMD | 95% CI | Number of trials/intervention groups | P‐value | I 2 (%) |
|---|---|---|---|---|---|
| All RCT – bench press strength | 0.20 | 0.06:0.34 | 34/50 | <0.01 | 42.8 |
| RCT without resistance exercise | 0.89 | −0.07:1.82 | 1/1 | NA | 0 |
| RCT with resistance exercise (RE) | 0.18 | 0.04:0.32 | 33/49 | 0.01 | 39.4 |
| <65 years old | 0.18 | 0.03:0.33 | 32/48 | 0.01 | 55 |
| ≥65 years old | 0.28 | −0.51:1.07 | 1/1 | NA | 0 |
| RCT with RE testing bench press and reporting protein ingestion | 0.15 | 0.02:0.28 | 31/46 | 0.03 | 27 |
| RCT with RE ingesting <1.2 g/kg/day | −0.16 | −1.09:0.77 | 1/1 | NA | 0 |
| RCT with RE ingesting 1.2–1.59 g/kg/day | 0.17 | −0.01:0.35 | 14/21 | 0.07 | 23.3 |
| RCT with RE ingesting ≥1.6 g/kg/day | 0.13 | −0.15:0.41 | 16/24 | 0.33 | 54.7 |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in all RCT reporting protein ingestion | −0.00 | −0.22:0.22 | 32/48 | 0.999 | NA |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in studies using RE | 0.01 | −0.20:0.23 | 31/47 | 0.869 | NA |
BW, body weight; CI, confidence intervals; NA, not applicable; RCT, randomized clinical trials; RE, resistance exercise; SMD, standardized mean deviation.
Table 4.
Effects of protein supplementation on changes in lower‐body strength
| Groups/subgroups | SMD | 95% CI | Number of trials/intervention groups | P‐value | I 2 (%) |
|---|---|---|---|---|---|
| All RCT reporting lower‐body strength | 0.20 | 0.08:0.33 | 50/70 | <0.01 | 52.8 |
| RCT without resistance exercise | 0.14 | −0.36:0.64 | 4/4 | 0.44 | 20.4 |
| RCT with resistance exercise (RE) | 0.21 | 0.08:0.34 | 47/66 | <0.01 | 54.5 |
| <65 years old | 0.19 | 0.03:0.36 | 35/52 | 0.02 | 52.8 |
| ≥65 years old | 0.25 | 0.01:0.48 | 12/14 | 0.04 | 60.6 |
| RCT with RE reporting protein ingestion | 0.21 | 0.08:0.34 | 41/56 | <0.01 | 49.5 |
| Ingesting <1.2 g/kg/day | −0.01 | −1.85:1.83 | 2/2 | 0.95 | 0 |
| Ingesting 1.2–1.59 g/kg/day | 0.08 | −0.10:0.27 | 20/28 | 0.37 | 51.6 |
| Ingesting ≥1.6 g/kg/day | 0.40 | 0.23:0.57 | 19/26 | <0.01 | 26.1 |
| <65 years old | 0.38 | 0.19:0.56 | 17/24 | <0.01 | 62 |
| ≥65 years old | 0.55 | 0.04:1.06 | 2/2 | 0.03 | 0 |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in all RCT reporting protein ingestion | 0.25 | 0.05:0.45 | 44/60 | 0.016 | NA |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in studies using RE | 0.26 | 0.05:0.47 | 41/56 | 0.014 | NA |
BW, body weight; CI, confidence intervals; NA, not applicable; RCT, randomized clinical trials; RE, resistance exercise; SMD, standardized mean deviation.
Table 5.
Effects of protein supplementation on the change of handgrip strength and functional or physical test performance
| Groups/subgroups | SMD | 95% CI | Number of trials/intervention groups | P‐value | I 2 (%) |
|---|---|---|---|---|---|
| Handgrip strength – All RCT | 0.15 | −0.03:0.32 | 10/11 | 0.10 | 0 |
| RCT without resistance exercise | 0.20 | −0.17:0.57 | 4/4 | 0.18 | 0 |
| RCT with resistance exercise | 0.10 | −0.18:0.37 | 6/7 | 0.43 | 0 |
| Meta‐regression considering protein ingestion as a continuous variable (g/kg BW/day) a | −0.09 | −1.09:0.91 | 8/8 | 0.84 | ‐ |
| Functional and physical performance tests – All RCT b | 0.15 | 0.00:0.29 | 15/19 | 0.04 | 46.4 |
| RCT without Resistance Exercise | 0.09 | −0.08:0.25 | 5/6 | 0.28 | 0 |
| RCT with Resistance Exercise | 0.17 | −0.03:0.37 | 11/13 | 0.10 | 58 |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in all RCT reporting protein ingestion | −0.23 | −0.99:0.52 | 13/16 | 0.54 | ‐ |
| Meta regression – protein ingestion as a continuous variable (g/kg BW/day) in studies using RE | −0.26 | −1.30:0.77 | 9/10 | 0.61 | ‐ |
BW, body weight; CI, confidence intervals; NA, not applicable; RCT, randomized clinical trials; RE, resistance exercise; SMD, standardized mean deviation.
50% of the RCT accessing handgrip strength reporting protein ingestion were conducted in subjects also submitted to RE.
One study was conducted in subjects <65 years old.
Meta‐analysis and meta‐regression
Effect of additional protein intake on lean body mass
A summary of the effects of additional protein ingestion on LBM is presented in Table 2. Additional protein ingestion probably leads to a small increase in lean body mass (SMD = 0.22, 95% CI 0.15:0.30, P < 0.01, n = 66 studies, moderate certainty of evidence) (Figure S3). The change represents approximately 1.3–1.4 kg lean mass gain during the intervention compared with an average of ~0.8 kg gain in the placebo/control group (~0.5–0.7 kg difference between groups). We found the same small significant positive main effect on lean body mass gain when isolating studies with resistance exercise (RE) (SMD = 0.22, 95% CI 0.14:0.30, n = 62 studies with RE, moderate certainty of the evidence). Only six studies presented intervention groups assessing LBM when providing additional protein without RE in healthy subjects (Figure S3). Our analysis showed a small, non‐significant intervention effect when only increased protein ingestion was applied (SMD = 0.21, 95% CI −0.15:0.58, n = 6 studies with intervention groups not using RE, low certainty of the evidence; Figure S3). Following this result, we conducted further subgroup analyses only in studies submitting subjects to RE.
Ingesting additional protein increased LBM in studies with younger subjects and older subjects submitted to RE (SMD = 0.25, 95% CI 0.16:0.35, n = 48 studies vs. SMD = 0.13, 95% CI −0.00:0.28, n = 14 studies, low certainty of the evidence; Table 2). The effect of protein in LBM was more pronounced in young subjects since the main effect in older subjects was marginal and not significant. Still, there was not a significant difference when performing the analysis by subgroups of age (P > 0.05) (Figure S4). Considering only studies using RE and reporting daily protein ingestion, additional protein still likely has a significant effect on lean body mass (SMD = 0.19, 95% CI 0.11:0.28, P < 0.01, n = 51 studies, moderate certainty of the evidence; Table 2; Figure 2). Subgroup analysis by daily protein ingestion showed that ingesting more protein may increase LBM gain in older subjects at 1.2–1.59 g/kg/day (SMD = 0.20, 95% CI 0.02:0.37, n = 9 studies, low certainty of the evidence; Table 2) and younger subjects at 1.6 g/kg/day or higher (SMD = 0.30, 95% CI 0.17:0.43, n = 23 studies, moderate certainty of the evidence; Table 2). A post hoc sensitivity analysis revealed that excluding Nakayama et al. 76 from the subgroup of studies testing older subjects at 1.2–1.59 g/kg/day changed the main effect to a non‐significant value (SMD = 0.12, 95% CI −0.08:0.32, Table S6). Our systematic search resulted in no studies testing the effect of additional protein ingestion on LBM and RE using intakes ≥1.6 g of protein/kg/day in older subjects (Table 2).
Figure 2.
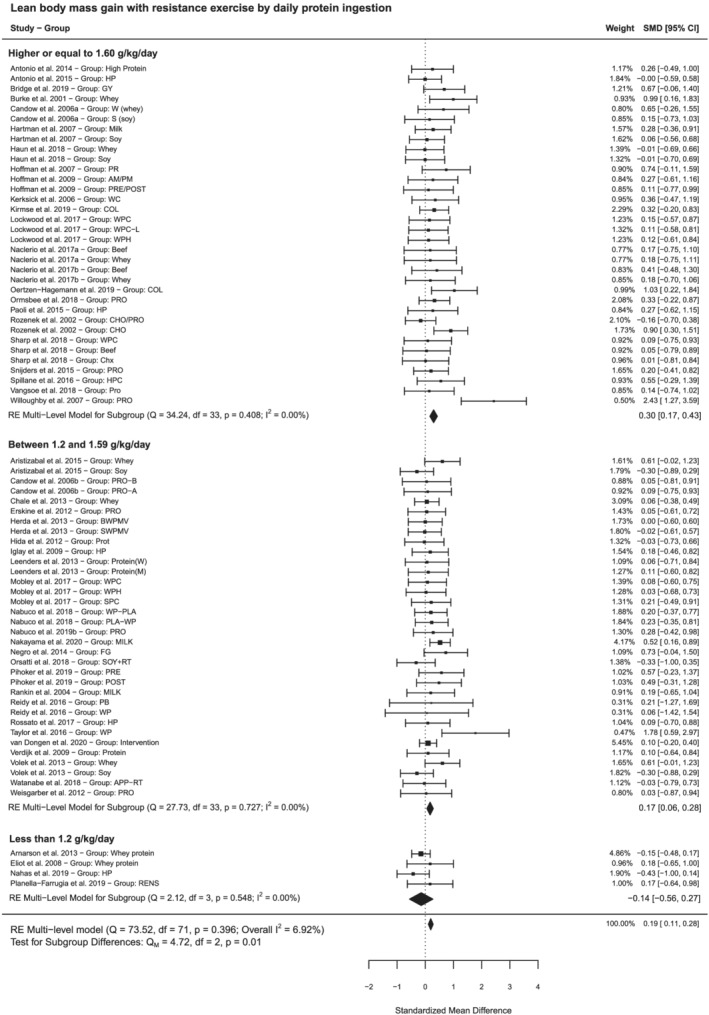
Forest plot showing effects of additional protein ingestion on changes in lean body mass by daily protein ingestion in subjects submitted to resistance exercise training.
A three‐level meta‐regression considering protein ingestion as a continuous variable in studies using significant but marginal main effect on lean body mass (SMD = 0.14, 95% CI 0.00:0.27, P < 0.04, n = 51 studies and 72 intervention groups). The same analysis considering all RCT independent of RE returned no significant results (Table 2). Bubble plots showing regression curves are in the supplementary files (Figures S11 and S12).
Effect of dietary protein intake on muscle strength
Effect of dietary protein intake on bench press strength
Bench press strength gains may be significantly higher in subjects supplemented with protein (SMD = 0.20, 95% CI 0.06:0.34, n = 34 studies, low certainty of the evidence) (Table 3). Thirty‐three out of 34 studies testing the effect of additional protein on bench press strength enrolled subjects in resistance exercise programs (Figure S5). When subgrouping studies by the age of subjects, a small positive main effect of additional protein ingestion on bench press strength was detected in <65 years old subjects (SMD = 0.18, 95% CI 0.03:0.33, n = 32 studies, low certainty of the evidence). Noteworthy, only one study was conducted on older subjects. The effect was also small on bench press strength considering studies reporting protein ingestion (SMD = 0.15, 95% CI 0.02:0.28, n = 31 studies, low certainty of the evidence). Still, no significant effects were found when searching for the effects of different daily protein ingestion levels. However, the sensitivity analysis showed the effect size for the extract of >1.6 g of protein/kg/day changed to significant after excluding Vangsoe et al. 91 (Table S7). Three‐level meta‐regression analysis using daily protein ingestion as a continuous variable was not significant for the effect of additional protein in bench press strength (Table 3). Bubble plots showing regression curves are in the supporting files (Figures S13 and S14).
Effect of dietary protein intake on lower‐body strength
Effects of additional protein ingestion on lower‐body strength are presented in Table 4. Lower‐body strength was slightly higher in subjects ingesting more protein (SMD = 0.20, 95% CI 0.08:0.33, n = 50 studies, low certainty of the evidence). Four studies measuring lower‐body strength did not enrol subjects in RE training, and the effect of ingesting additional protein was not significant (Figure S6). However, ingesting more protein produced a small significant effect on lower‐body strength in subjects submitted to RE (SMD = 0.21, 95% CI 0.08:0.34, n = 47 studies, low certainty of the evidence) (Figure S6). This small effect may be independent of the age in subjects submitted to RE (Table 4) (Figure S7). However, after excluding Burke et al. 2001 54 during sensitivity analysis, the significant main effect for older subjects was not present anymore (SMD = 0.16, 95% CI −0.00:0.32, Table S8). Noteworthy, only levels >1.6 g of protein/kg/day may significantly increased lower‐body strength (SMD = 0.40, 95% CI 0.23:0.57, n = 20 studies, low certainty of the evidence; Figure S8) and mainly in young subjects (Table 4). Meta‐regression using protein ingestion as a continuous variable in studies using RE showed a small significant effect of protein on lower‐body strength for all RCT (SMD = 0.25, 95% CI 0.05:0.45, n = 45 studies and 60 intervention groups) or studies using RE (SMD = 0.26, 95% CI 0.05:0.47, n = 41 studies and 56 intervention groups; Table 3). Bubble plots showing regression curves are in the supporting files (Figures S15 and S16).
Effect of dietary protein intake on handgrip strength and physical performance functional tests
The evidence shows that ingesting more protein produced a slightly positive but not significant main effect in handgrip strength (SMD = 0.15, 95% CI −0.03:0.32, n = 10 studies, very low certainty of the evidence; Figure S9). Meta‐regression of handgrip strength data vs. protein ingestion as a continuous variable was not significant (Table 5 and Figure S17).
The evidence was very uncertain about the effect of ingesting more protein and performance on physical and functional performance tests (SMD = 0.15, 95% CI 0.00:0.29, n = 15 studies, very low certainty of the evidence). Five studies did not use RE training protocol (Figure S10). Meta‐regression of performance data in physical and functional tests using daily protein ingestion as a continuous variable was not significant (Table 5). Bubble plots showing regression curves are in the supporting files (Figures S18 and S19).
Discussion
This systematic review and meta‐analysis aimed to investigate the efficacy of increasing dietary protein ingestion to improve lean body mass gain, skeletal muscle strength, and physical function in healthy subjects. To our knowledge, this is the first systematic review and meta‐analysis investigating such outcomes restricting the literature search to studies with healthy, not obese adults (i.e. no minor illnesses, not frail, and not sarcopenic), and including no weight‐loss study protocols. Furthermore, the literature search was restricted to studies testing protein interventions only (i.e. no additional supplement ingredients). Finally, when considering physical activity intervention, only resistance exercise was in our inclusion criteria. The main findings of the present meta‐analysis were that additional protein ingestion together with RE leads to small additional lean body mass and lower body strength. This effect seems to be more prominent in younger subjects ingesting ≥1.6 g/kg/day when enrolled in RE. The number of studies with healthy older subjects to conduct a proper analysis is relatively low what levels down the certainty of the evidence. Effects on bench press strength, handgrip strength, and improved performance in physical tests in healthy adults seem to be trivial. For most outcomes, the evidence is unclear due to the low number of studies or increased heterogeneity. A critical finding of our systematic review is that more RCT testing increasing protein ingestion as a solo intervention in healthy, not obese, adults are needed.
Most meta‐analyses have reported consistently positive results regarding the effect of additional protein ingestion on RE training‐induced increases in LBM. 13 , 14 , 21 , 23 Cermak et al. 23 showed a significant main effect for protein supplementation in muscle mass in young and old subjects during resistance exercise‐like training. Tagawa et al. 13 found significant effects of additional protein ingestion on LBM in adults (19–81 years old) independent of resistance exercise (≥2 weeks). Wirth et al. 21 also found a significant effect of additional protein ingestion on LBM in adults (18–55 years old or ≥55 years old). Conversely, Haaf et al. 22 found no effect of additional protein supplementation in LBM in non‐frail community‐dwelling older adults (>50 years old), even when combined with resistance exercise (≥4 weeks). Noteworthy, is the fact that divergent inclusion criteria are an important source of variability when comparing different meta‐analyses. The insertion of clinical trials testing multi‐ingredient supplements, 106 including energy‐restricted weight‐loss diets, 13 or using different cut‐off points for age sub‐groups likely explain the differences in main effects and conclusions when comparing studies. 14 , 21 , 22 , 23 Still, a meta‐analysis conducted by our group showed that protein ingestion could significantly increase the RE training‐induced gains in lean mass in young (<45 years old) and old (≥45 years old) healthy subjects. 14 One of the present meta‐analysis objectives was to expand our previous findings to studies that have included protein supplements but not having subjects enrolled in RE training. However, after a systematic review, we identified only six studies matching our criteria, which restricts the possibility of a proper analysis.
Our data show a small increment in LBM caused by ingesting additional protein and RE. Older subjects would likely respond differently since anabolic resistance develops with ageing, and higher per‐meal protein doses are postulated to be necessary to stimulate muscle protein synthesis in this population. 107 Present protein ingestion recommendations for healthy young and old subjects range from 0.67 to 0.8 g/kg BW/day. 17 , 18 This meta‐analysis also found that LBM was slightly increased by protein and RE in older subjects in studies testing daily protein ingestion at 1.2–1.59 g/kg/day. However, it is relevant to highlight that the study of Nakayama et al., 76 was the main contributor to this result according to our sensitivity analysis. Probably, because the study sample in Nakayama et al. 76 is relatively large (n = 122) when compared with other studies in the subgroup analysis. Therefore, because the effect of protein supplementation is significant only when Nakayama et al. 76 data are included in the analysis, it is possible that resistance exercise per se is the main contributor to lean body mass gains in studies with older participants. Curiously, our study showed a significant effect of ingesting more protein and RE in younger subjects only when ingesting ≥1.6 g of protein/kg/day. Our current findings in some way support the hypothesis that higher daily protein ingestion may be needed to increase LBM in young 108 and maybe older healthy subjects. 109 Noteworthy, as highlighted in our results, most of the studies included in our analysis (~80%) reported baseline daily protein ingestion of at least 1.2 g/kg BW. This is 50% higher than current protein ingestion recommendations for healthy adults. 17 , 18 Such observation might explain the small effect of the intervention on the different outcomes. A relevant question is how much of LBM is muscle mass? 110 This question is relevant as protein supplementation rarely substantially affects strength outcomes, 14 , 21 , 22 , 23 which highlights that the extra LBM gain stimulated by protein supplementation may not be muscle, 111 or at least not sufficient muscle that is contributing to increases in strength.
According to previous meta‐analyses from our group and others, the effects of increasing daily protein ingestion on muscle strength are highly variable. 14 , 21 , 22 , 23 Previous data from our group 14 and Cermak et al. 23 showed very small but significant effect of additional protein ingestion on strength, mainly lower body strength data when selecting RE studies. In contrast, Wirth et al. 21 and Haaf et al. 22 found no effect of additional protein ingestion and exercise on lower‐body strength. However, some particularities in the inclusion criteria in these two meta‐analyses (i.e. aerobic exercise training or the cut‐off point during age subgroup analysis) might cause such contrast compared with our findings. Our current data support a small effect of ingesting more protein on lower‐body strength. Still, a high daily protein ingestion (≥1.6 g/kg BW/day) might be necessary to increase strength in the lower body. Such a level of protein ingestion represents twice the current RDA for protein for healthy adults. 17 This observation reinforces the idea that optimal skeletal muscle increases in strength during RE, while small, might require greater protein ingestion. 14
Handgrip strength has been positively linked to several relevant variables related to the quality of life and physical function, especially for older subjects. 112 Also, growing evidence shows that handgrip strength is associated with total strength, bone mineral density, fractures, falls, cognitive impairment, depression levels, and overall diet quality. 112 , 113 However, because few studies investigated the effect of protein ingestion on handgrip strength in healthy adults, it is unclear if additional protein ingestion would improve this outcome. The search strategy used in the present meta‐analysis selected 10 studies investigating handgrip strength changes due to additional protein ingestion. Nevertheless, only five studies did not enrol participants in a RE training protocol.
As mentioned, handgrip strength seems to be considered a potential marker related to several aspects of functional capacity and quality of life. 112 , 113 However, we intended to explore the effects of additional protein ingestion in functional tests directly. We found a small marginal effect of protein ingestion on performance in physical function tests. Our results are in line with a previous meta‐analysis 22 showing no significant effect of protein ingestion added to RE on gait speed or chair‐rise time in healthy subjects. In contrast, Liao et al. 106 found additive significant main effects for additional protein (but included numerous other supplement ingredients) ingestion and RE in the performance of physical function tests in older overweight or obese subjects. Of note, Liao et al. 106 compared the effect of additional protein ingestion and RE with no intervention as a control group; therefore, it is reasonable to suggest that the RE was the primary intervention leading to the main findings.
There are several strengths of this review. We restricted our search to studies with healthy non‐obese adults. We think this is essential to reduce the influence of minor illnesses such as diagnosed sarcopenia, frailty, arthritis, and even obesity, which have all been shown to perturb muscle protein turnover. 26 Our inclusion criteria excluded studies using multi‐ingredient supplements or combining other added nutrients or compounds in the intervention group to isolate the effect of protein. Also, we restricted our systematic search and inclusion criteria to research including resistance exercise only if a study included physical activity. Altogether, these criteria are essential to narrow our findings to the effect of additional protein in healthy adults. Finally, we applied GRADE to qualify our level of evidence. Using GRADE, we show that despite being statistically significant, some of our findings were downgraded in terms of certainty, highlighting that study design issues hamper making further conclusions. The main reasons for downgrading the certainty of evidence were increased risk of bias, mainly in the blinding domains, moderate to high heterogeneity, and, for some subgroup analysis, the low number of the subject in each respective group. In some way, this highlights that future studies testing additional protein as a primary intervention and examining outcomes relevant to strength and lean body mass need to focus on trial planning and control of variables known to affect study quality. We are aware that blinding ingestion in studies testing dietary interventions can be challenging. However, overcoming such challenges might be necessary to increase the quality of the evidence if one is applying the current tools available to grade the evidence in meta‐analysis.
We also have some notable limitations that we must acknowledge. In general, study protocols were highly variable, which is probably the cause of the distinct heterogeneity in response to the intervention. Most of the selected studies in this meta‐analysis (65 out of 74) provided animal protein to their subjects. Therefore, our findings reflect mainly the effect of animal‐based protein sources. 11 Approximately a quarter of the selected studies showed an increased risk of bias due to poor blinding during the study or the data analysis (Figures S1 and S2). The relevance of such increased risk of bias escalates when subgrouping studies by age or levels of daily protein intake. Consequently, some conclusions presented in the current meta‐analysis might change in the future in the case of the addition of studies with improved blinding procedures.
In conclusion, our systematic review showed few studies testing protein intervention in healthy non‐obese subjects and assessing LMB, strength, or physical function outcomes in the absence of a parallel RE training program. Therefore, more studies are needed to conduct a proper meta‐analysis and answer our research question regarding the use of dietary protein intervention solely in healthy subjects. Alternatively, the evidence in this meta‐analysis supports the hypothesis that additional protein ingestion (1.6 g of protein/kg/day or higher) leads to small increments in lean body mass in studies enrolling young subjects in RE training. The results on older subjects seem marginal or influenced by individual studies. Lower body muscle strength was also marginally increased by additional protein ingestion in studies with RE training. Bench press strength, handgrip strength, and performance in physical or functional tests were slightly or not affected by ingestion of additional protein. Noteworthy, 80% of the studies reported subjects ingesting at least 1.2 g of protein/kg/day in their habitual diets. Such baseline protein ingestion is a potential contributor for minor or the absence of additional effects of a protein intervention in combination with RE. Still, the downgrading of the evidence for some outcomes in the current meta‐analysis highlights the necessity of more studies testing protein interventions in healthy subjects with improved planning of RCTs, fulfilling important aspects as proper blinding of research participants and staff.
Conflict of interest
Stuart M. Phillips reports that he is an inventor on a patent (WO/2018/157258) held by Exerkine Corporation. Stuart M. Phillips is an unpaid member of the scientific advisory board of Enhanced Recovery™ (https://ersportsdrink.com/). Stuart M. Phillips has received, in the last 5 years, honoraria and travel expenses from the US National Dairy Council, the National Cattlemen's Beef Association, and the US Dairy Export Council. Philip J. Atherton received research funding and/or honoraria for protein nutrition research from Abbott Nutrition, Fresenius‐Kabi, and Ajinomoto Co., Inc. Francesco Landi received financial support from Abbott Nutrition and Nutricia. Maria Camprubi Robles, Michelle Braun, and Sandra Naranjo‐Modad are employees of Abbott Nutrition, International Flavors & Fragrances, and Givaudan, respectively. Everson A. Nunes, Lauren Colenso‐Semple, Sean R. McKellar, Thomas Yau, Muhammad Usman Ali, Donna Fitzpatrick‐Lewis, Diana Sherifali, Claire Gaudichon, and Daniel Tomé declare that they have no conflict of interest.
Funding
This research received a grant from the International Life Science Institute (Europe) to Stuart M. Phillips. Everson A Nunes is a Tier 2 Research Productivity Fellow supported by the Brazilian National Council for Scientific and Technological Development (CNPq)—grant number 308584/2019‐8. Experts are not paid for the time spent on this work; however, the non‐industry members within the expert group were offered support for travel and accommodation costs from the Health Benefits Assessment of Food Task Force to attend meetings to discuss the manuscript and a small compensatory sum (honorarium) with the option to decline.
Supporting information
Table S1 ‐ Characteristics of the studies
Table S2 – GRADE evidence profile rating for lean body mass changes in studies testing additional dietary protein intervention in healthy subjects.
Table S3 – GRADE evidence profile rating for muscle strength changes in studies testing additional dietary protein intervention in healthy subjects.
Table S4 – GRADE evidence profile rating for handgrip strength changes in studies testing additional dietary protein intervention in healthy subjects.
Table S5 – GRADE evidence profile rating for physical testing performance changes in studies providing additional dietary protein to healthy subjects.
Table S6 ‐ Sensitivity analysis lean body mass
Table S7 – Sensitivity analysis bench press strength
Table S8 – Sensitivity analysis lower body strength
Table S9 – Sensitivity analysis handgrip strength
Table S10 – Sensitivity analysis physical function
Figure S1 – Risk of bias analysis of all studies included in the meta‐analysis. (+) – Circles filled in green = Low risk of bias; (?) – Circles filled in yellow = Unclear risk of bias; (−) – Circles filled in red = High risk of bias.
Figure S2 Summary of risk of bias analysis showing the percentage of studies with potential unclear or high risk of selection, performance, detection, attrition, reporting, or other bias.
Figure S3 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' lean body or muscle mass, including or not a resistance exercise program.
Figure S4 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' lean body or muscle mass of subjects categorized by age < 65 or ≥ 65 years old in studies including a resistance exercise program.
Figure S5 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein and resistance exercise on adults' bench press strength.
Figure S6 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' lower‐body strength by the presence or not of resistance exercise training in a study research protocol.
Figure S7 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies reporting daily protein ingestion and testing the effects of ingesting additional protein and resistance exercise on adults' lower body strength by age < 65 or ≥ 65 years old.
Figure S8 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies reporting daily protein ingestion and testing the effects of ingesting additional protein on adults' lower body strength by the level of protein ingestion.
Figure S9 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' handgrip strength by the presence or not of resistance exercise training in a study research protocol.
Figure S10 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' physical testing performance by the presence or not of resistance exercise training in a study research protocol.
Figure S11 – Bubble plot of three‐level meta‐regression analysis of the main effect on lean body mass vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S12 – Bubble plot of three‐level meta‐regression analysis of the main effect on lean body mass vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S13 – Bubble plot of three‐level meta‐regression analysis of the main effect on bench press strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S14 – Bubble plot of three‐level meta‐regression analysis of the main effect on bench press strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S15 – Bubble plot of three‐level meta‐regression analysis of the main effect on lower‐body strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S16 – Bubble plot of three‐level meta‐regression analysis of the main effect on lower‐body strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S17 – Bubble plot of three‐level meta‐regression analysis of the main effect on handgrip strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S18 – Bubble plot of three‐level meta‐regression analysis of the main effect on performance in physical and functional tests vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S19 – Bubble plot of three‐level meta‐regression analysis of the main effect on performance in physical and functional tests vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S20 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lean body or muscle mass including or not a resistance exercise program.
Figure S21 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lean body or muscle mass only in studies including a resistance exercise program.
Figure S22 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lean body or muscle mass only in studies including a resistance exercise program reporting protein ingestion.
Figure S23 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' bench press strength in studies including or not a resistance exercise program.
Figure S24 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' bench press strength only in studies including a resistance exercise program reporting protein ingestion.
Figure S25 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lower‐body strength in studies including or not a resistance exercise program.
Figure S26 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lower‐body strength only in studies including a resistance exercise program.
Figure S27 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lower‐body strength only in studies including a resistance exercise program reporting protein ingestion.
Figure S28 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' handgrip strength in studies including or not a resistance exercise program.
Figure S29 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' performance in physical and functional tests in studies including or not a resistance exercise program.
Search_strategies
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 114 This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. The research question addressed in this publication and potential contributing experts in the field were identified by the Health Benefits Assessment of Food Task Force. Industry members of this task force are listed on the ILSI Europe website at http://ilsi.eu/wp‐content/uploads/sites/3/2020/09/HBA_TFonepager_sep2020.pdf. According to ILSI Europe policies, the EG is composed by at least 50% of external non‐industry members. Once the expert group was formed, the research project was handed over to them to independently refine the research question. Consequently, the expert group carried out the work, that is, collecting/analysing data/information and writing the scientific paper independently of other activities of the task force. The research reported is the result of a scientific evaluation in line with ILSI Europe's framework to provide a pre‐competitive setting for public‐private partnership. ILSI Europe (Ms. Naomi Venlet and former ILSI Europe staff members Dr. Michela Miani and Dr. Kirsi Forsberg) facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call +3227710014.
Nunes E. A., Colenso‐Semple L., McKellar S. R., Yau T., Ali M. U., Fitzpatrick‐Lewis D., Sherifali D., Gaudichon C., Tomé D., Atherton P. J., Robles M. C., Naranjo‐Modad S., Braun M., Landi F., and Phillips S. M. (2022) Systematic review and meta‐analysis of protein intake to support muscle mass and function in healthy adults, Journal of Cachexia, Sarcopenia and Muscle, 13, 795–810, 10.1002/jcsm.12922
References
- 1. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–482. [DOI] [PubMed] [Google Scholar]
- 2. Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Dietary protein is associated with musculoskeletal health independently of dietary pattern: the Framingham Third Generation Study. Am J Clin Nutr 2017;105:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coll PP, Phu S, Hajjar SH, Kirk B, Duque G, Taxel P. The prevention of osteoporosis and sarcopenia in older adults. J Am Geriatr Soc 2021;69:1388–1398. [DOI] [PubMed] [Google Scholar]
- 4. Marcos‐Pardo PJ, Gonzalez‐Galvez N, Lopez‐Vivancos A, Espeso‐Garcia A, Martinez‐Aranda LM, Gea‐Garcia GM, et al. Sarcopenia, diet, physical activity and obesity in European middle‐aged and older adults: The LifeAge Study. Nutrients 2020;13. 10.3390/nu13010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phillips SM, Paddon‐Jones D, Layman DK. Optimizing adult protein intake during catabolic health conditions. Adv Nutr 2020;11:S1058–S1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim D, Park Y. Amount of Protein Required to Improve Muscle Mass in Older Adults. Nutrients 2020;12:1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SH, Jeong JB, Kang J, Ahn DW, Kim JW, Kim BG, et al. Association between sarcopenia level and metabolic syndrome. PLoS One 2021;16:e0248856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song P, Han P, Zhao Y, Zhang Y, Wang L, Tao Z, et al. Muscle mass rather than muscle strength or physical performance is associated with metabolic syndrome in community‐dwelling older Chinese adults. BMC Geriatr 2021;21:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 2012;590:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morton RW, Phillips SM. Does protein supplementation really augment hypertrophy in older persons with resistance exercise training? Am J Clin Nutr 2018;107:1054–1056. [DOI] [PubMed] [Google Scholar]
- 11. Nunes E, Currier B, Lim C, Phillips S. Nutrient‐dense protein as a primary dietary strategy in healthy ageing: Please sir, may we have more? Proc Nutr Soc 2020;80:1–14. [DOI] [PubMed] [Google Scholar]
- 12. Santarpia L, Contaldo F, Pasanisi F. Dietary protein content for an optimal diet: a clinical view. J Cachexia Sarcopenia Muscle 2017;8:345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tagawa R, Watanabe D, Ito K, Ueda K, Nakayama K, Sanbongi C, et al. Dose‐response relationship between protein intake and muscle mass increase: a systematic review and meta‐analysis of randomized controlled trials. Nutr Rev 2020;79:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morton RW, Murphy KT, McKellar SR Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta‐analysis and meta‐regression of the effect of protein supplementation on resistance training‐induced gains in muscle mass and strength in healthy adults. Br J Sports Med 2018;52:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JE, O'Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta‐analysis. Nutr Rev 2016;74:210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community‐dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–155. [DOI] [PubMed] [Google Scholar]
- 17. Institute of Medicine . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC, USA: The National Academies Press; 2005. 10.17226/10490 [DOI] [Google Scholar]
- 18. World Health Organization (WHO) . Protein and amino acid requirements in human nutrition: report of a Joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: World Health Organisation (WHO); 2007. https://apps.who.int/iris/handle/10665/43411 [Google Scholar]
- 19. Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the 'anabolic resistance' of ageing. Nutr Metab (Lond) 2011;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin‐Cantero A, Reijnierse EM, Gill BMT, Maier AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta‐analysis. Nutr Rev 2021;79:315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wirth J, Hillesheim E, Brennan L. The role of protein intake and its timing on body composition and muscle function in healthy adults: a systematic review and meta‐analysis of randomized controlled trials. J Nutr 2020;150:1443–1460. [DOI] [PubMed] [Google Scholar]
- 22. Ten Haaf DSM, Nuijten MAH, Maessen MFH, Horstman AMH, Eijsvogels TMH, Hopman MTE. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community‐dwelling older adults: a systematic review and meta‐analysis. Am J Clin Nutr 2018;108:1043–1059. [DOI] [PubMed] [Google Scholar]
- 23. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance‐type exercise training: a meta‐analysis. Am J Clin Nutr 2012;96:1454–1464. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ & Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.: 2020. 10.1002/9781119536604 [DOI]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097, 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beals JW, Burd NA, Moore DR, van Vliet S. Obesity alters the muscle protein synthetic response to nutrition and exercise. Front Nutr 2019;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rohatgi A. WebPlotDigitizer version:4.5 Pacifica, California, USA. 2021.
- 29. (RevMan) RM . [Computer program]. Version 5.4 The Cochrane Collaboration. 2020.
- 30. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 32. Schünemann H, Brozek J, Guyatt G & Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. https://gdt.gradepro.org/app/handbook/handbook.html: 2013.
- 33. Antonio J, Ellerbroek A, Silver T, Orris S, Scheiner M, Gonzalez A, et al. A high protein diet (3.4 g/kg/d) combined with a heavy resistance training program improves body composition in healthy trained men and women—a follow‐up investigation. J Int Soc Sports Nutr 2015;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deibert P, Solleder F, Konig D, Vitolins MZ, Dickhuth HH, Gollhofer A, et al. Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males. Aging Male 2011;14:273–279. [DOI] [PubMed] [Google Scholar]
- 35. Kim HH, Kim YJ, Lee SY, Jeong DW, Lee JG, Yi YH, et al. Interactive effects of an isocaloric high‐protein diet and resistance exercise on body composition, ghrelin, and metabolic and hormonal parameters in untrained young men: a randomized clinical trial. J Diab Investig 2014;5:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orsatti FL, Maesta N, de Oliveira EP, Nahas Neto J, Burini RC, Nunes PRP, et al. Adding soy protein to milk enhances the effect of resistance training on muscle strength in postmenopausal women. J Diet Suppl 2018;15:140–152. [DOI] [PubMed] [Google Scholar]
- 37. Reidy PT, Borack MS, Markofski MM, Dickinson JM, Deer RR, Husaini SH, et al. Protein supplementation has minimal effects on muscle adaptations during resistance exercise training in young men: a double‐blind randomized clinical trial. J Nutr 2016;146:1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossato LT, Nahas PC, de Branco FMS, Martins FM, Souza AP, Carneiro MAS, et al. Higher protein intake does not improve lean mass gain when compared with RDA recommendation in postmenopausal women following resistance exercise protocol: a randomized clinical trial. Nutrients 2017;9. 10.3390/nu9091007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Dongen EJI, Haveman‐Nies A, Doets EL, Dorhout BG, de Groot L. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in practice. J Am Med Directors Assoc 2020;21:1065−+. doi: 8 1072.e3 [DOI] [PubMed] [Google Scholar]
- 40. Aristizabal JC, Freidenreich DJ, Volk BM, Kupchak BR, Saenz C, Maresh CM, et al. Effect of resistance training on resting metabolic rate and its estimation by a dual‐energy X‐ray absorptiometry metabolic map. Eur J Clin Nutr 2015;69:831–836. [DOI] [PubMed] [Google Scholar]
- 41. Bartholomae E, Incollingo A, Vizcaino M, Wharton C, Johnston CS. Mung bean protein supplement improves muscular strength in healthy, underactive vegetarian adults. Nutrients 2019;11. 10.3390/nu11102423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown EC, DiSilvestro RA, Babaknia A, Devor ST. Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status. Nutr J 2004;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Candow DG, Burke NC, Smith‐Palmer T, Burke DG. Effect of whey and soy protein supplementation combined with resistance training in young adults. Int J Sport Nutr Exerc Metab 2006;16:233–244. [DOI] [PubMed] [Google Scholar]
- 44. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat‐free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86:373–381. [DOI] [PubMed] [Google Scholar]
- 45. Haun CT, Mobley CB, Vann CG, Romero MA, Roberson PA, Mumford PW, et al. Soy protein supplementation is not androgenic or estrogenic in college‐aged men when combined with resistance exercise training (vol 8, pg 11151, 2018). Sci Rep 2018;8. 10.1038/s41598-018-29591-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maesta N, Nahas EAP, Nahas‐Neto J, Orsatti FL, Fernandes CE, Traiman P, et al. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas. 2007;56(4):350–358. 10.1016/j.maturitas.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 47. Mobley CB, Haun CT, Roberson PA, Mumford PW, Romero MA, Kephart WC, et al. Effects of whey, soy or leucine supplementation with 12 weeks of resistance training on strength, body composition, and skeletal muscle and adipose tissue histological attributes in college‐aged males. Nutrients 2017;9. 10.3390/nu9090972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Volek JS, Volk BM, Gomez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr 2013;32:122–135. [DOI] [PubMed] [Google Scholar]
- 49. Obradović J, Vukadinović Jurišić M, Rakonjac D. The effects of leucine and whey protein supplementation with eight weeks of resistance training on strength and body composition. J Sports Med Phys Fitness. 2020;60(6):864–869. 10.23736/s0022-4707.20.09742-x [DOI] [PubMed] [Google Scholar]
- 50. Amasene M, Besga A, Echeverria I, Urquiza M, Ruiz JR, Rodriguez‐Larrad A, et al. Effects of leucine‐enriched whey protein supplementation on physical function in post‐hospitalized older adults participating in 12‐weeks of resistance training program: a randomized controlled trial. Nutrients 2019;11. 10.3390/nu11102337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antonio J, Peacock CA, Ellerbroek A, Fromhoff B, Silver T. The effects of consuming a high protein diet (4.4 g/kg/d) on body composition in resistance‐trained individuals. J Int Soc Sports Nutr 2014;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arnarson A, Geirsdottir OG, Ramel A, Briem K, Jonsson PV, Thorsdottir I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: double blind, randomised controlled trial. Eur J Clin Nutr 2013;67:821–826. [DOI] [PubMed] [Google Scholar]
- 53. Bridge A, Brown J, Snider H, Nasato M, Ward WE, Roy BD, et al. Greek yogurt and 12 weeks of exercise training on strength, muscle thickness and body composition in lean, untrained, university‐aged males. Front Nutr 2019;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burke DG, Chilibeck PD, Davidson KS, Ow DG, Farthing J, Smith‐Palmer T. The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Int J Sport Nutr Exerc Metab 2001;11:349–364. [DOI] [PubMed] [Google Scholar]
- 55. Candow DG, Chilibeck PD, Facci M, Abeysekara S, Zello GA. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol 2006;97:548–556. [DOI] [PubMed] [Google Scholar]
- 56. Chalé A, Cloutier G, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise–induced changes in lean mass, muscle strength, and physical function in mobility‐limited older adults. J Gerontol: Series A. 2013;68(6):682–690. 10.1093/gerona/gls221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coburn JW, Housh DJ, Housh TJ, Malek MH, Beck TW, Cramer JT, et al. Effects of leucine and whey protein supplementation during eight weeks of unilateral resistance training. J Strength Cond Res 2006;20:284–291. [DOI] [PubMed] [Google Scholar]
- 58. Dulac MC, Pion CH, Lemieux FC, Pinheiro Carvalho L, El Hajj Boutros G, Bélanger M, et al. Effects of slow‐ v. fast‐digested protein supplementation combined with mixed power training on muscle function and functional capacities in older men. Br J Nutr 2021;125(9):1017–1033. 10.1017/s0007114520001932 [DOI] [PubMed] [Google Scholar]
- 59. Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging. 2008;12(3):208–212. 10.1007/bf02982622 [DOI] [PubMed] [Google Scholar]
- 60. Erskine RM, Fletcher G, Hanson B, Foll PJ. Whey protein does not enhance the adaptations to elbow flexor resistance training. Med Sci Sports Exerc 2012;44:1791–1800. [DOI] [PubMed] [Google Scholar]
- 61. Gryson C, Ratel S, Rance M, Pen, o S , Bonhomme C, Ruyet PL, et al. Four‐month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc 2014;15:958 e1–e9. 12 [DOI] [PubMed] [Google Scholar]
- 62. Herda AA, Herda TJ, Costa PB, Ryan ED, Stout JR, Cramer JT. Muscle performance, size, and safety responses after eight weeks of resistance training and protein supplementation: a randomized, double‐blinded, placebo‐controlled clinical trial. J Strength Cond Res 2013;27:3091–3100. [DOI] [PubMed] [Google Scholar]
- 63. Hida A, Hasegawa Y, Mekata Y, Usuda M, Masuda Y, Kawano H, et al. Effects of egg white protein supplementation on muscle strength and serum free amino acid concentrations. Nutrients 2012;4:1504–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoffman JR, Ratamess NA, Kang J, Falvo MJ, Faigenbaum AD. Effects of protein supplementation on muscular performance and resting hormonal changes in college football players. J Sports Sci Med 2007;6:85–92. [PMC free article] [PubMed] [Google Scholar]
- 65. Hoffman JR, Ratamess NA, Tranchina CP, Rashti SL, Kang J, Faigenbaum AD. Effect of protein‐supplement timing on strength, power, and body‐composition changes in resistance‐trained men. Int J Sport Nutr Exerc Metab 2009;19:172–185. [DOI] [PubMed] [Google Scholar]
- 66. Iglay HB, Apolzan JW, Gerrard DE, Eash JK, Anderson JC, Campbell WW. Moderately increased protein intake predominately from egg sources does not influence whole body, regional, or muscle composition responses to resistance training in older people. J Nutr Health Aging 2009;13:108–114. [DOI] [PubMed] [Google Scholar]
- 67. Kerksick CM, Rasmussen CJ, Lancaster SL, Magu B, Smith P, Melton C, et al. The effects of protein and amino acid supplementation on performance and training adaptations during ten weeks of resistance training. J Strength Cond Res 2006;20:643–653. [PubMed] [Google Scholar]
- 68. Kirmse M, Oertzen‐Hagemann V, de Marees M, Bloch W, Platen P. Prolonged collagen peptide supplementation and resistance exercise training affects body composition in recreationally active men. Nutrients 2019;11:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leenders M, Verdijk LB, Van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig W, et al. Protein supplementation during resistance‐type exercise training in the elderly. Med Sci Sports Exerc 2013;45:542–552. [DOI] [PubMed] [Google Scholar]
- 70. Lockwood CM, Roberts MD, Dalbo VJ, Smith‐Ryan AE, Kendall KL, Moon JR, et al. Effects of hydrolyzed whey versus other whey protein supplements on the physiological response to 8 weeks of resistance exercise in college‐aged males. J Am Coll Nutr 2017;36:16–27. [DOI] [PubMed] [Google Scholar]
- 71. Nabuco HCG, Tomeleri CM, Fernandes RR, Sugihara P, Cavalcante EF, Venturini D, et al. Effects of protein intake beyond habitual intakes associated with resistance training on metabolic syndrome‐related parameters, isokinetic strength, and body composition in older women. J Aging Phys Activity 2019;27:545–552. [DOI] [PubMed] [Google Scholar]
- 72. Nabuco HCG, Tomeleri CM, Sugihara P, Fernandes RR, Cavalcante EF, Antunes M, et al. Effects of whey protein supplementation pre‐ or post‐resistance training on muscle mass, muscular strength, and functional capacity in pre‐conditioned older women: a randomized clinical trial. Nutrients 2018;10. 10.3390/nu10050563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Naclerio F, Larumbe‐Zabala E, Ashrafi N, Seijo M, Nielsen B, Allgrove J, et al. Effects of protein‐carbohydrate supplementation on immunity and resistance training outcomes: a double‐blind, randomized, controlled clinical trial. Eur J Appl Physiol 2017;117:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Naclerio F, Seijo‐Bujia M, Larumbe‐Zabala E, Earnest CP. Carbohydrates alone or mixing with beef or whey protein promote similar training outcomes in resistance training males: a double‐blind, randomized controlled clinical trial. Int J Sport Nutr Exerc Metab 2017;27:408–420. [DOI] [PubMed] [Google Scholar]
- 75. Nahas PC, Rossato LT, Martins FM, Souza AP, de Branco FMS, Carneiro MAS, et al. Moderate increase in protein intake promotes a small additional improvement in functional capacity, but not in muscle strength and lean mass quality, in postmenopausal women following resistance exercise: a randomized clinical trial. Nutrients 2019;11. 10.3390/nu11061323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakayama K, Saito Y, Sanbongi C, Murata K, Urashima T. Effects of low‐dose milk protein supplementation following low‐to‐moderate intensity exercise training on muscle mass in healthy older adults: a randomized placebo‐controlled trial. Eur J Nutr 2020;60:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Negro M, oni M , Ottobrini S, Codrons E, Correale L, Buonocore D, et al. Protein supplementation with low fat meat after resistance training: effects on body composition and strength. Nutrients 2014;6:3040–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oertzen‐Hagemann V, Kirmse M, Eggers B, Pfeiffer K, Marcus K, de Marees M, et al. Effects of 12 weeks of hypertrophy resistance exercise training combined with collagen peptide supplementation on the skeletal muscle proteome in recreationally active men. Nutrients 2019;11. 10.3390/nu11051072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ormsbee MJ, Willingham BD, Marchant T, Binkley TL, Specker BL, Vukovich MD. Protein supplementation during a 6‐month concurrent training program: effect on body composition and muscular strength in sedentary individuals. Int J Sport Nutr Exerc Metab 2018;28:619–628. [DOI] [PubMed] [Google Scholar]
- 80. Ozan M, Buzdagli Y, Siktar E, Ucan I. The effect of protein and carbohydrate consumption during 10‐week strength training on maximal strength and body composition. Int J Appl Exerc Physiol 2020;9:161–171. [Google Scholar]
- 81. Paoli A, Pacelli QF, Neri M, Toniolo L, Cancellara P, Canato M, et al. Protein supplementation increases postexercise plasma myostatin concentration after 8 weeks of resistance training in young physically active subjects. J Med Food 2015;18:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pihoker AA, Peterjohn AM, Trexler ET, Hirsch KR, Blue MNM, Anderson KC, et al. The effects of nutrient timing on training adaptations in resistance‐trained females. J Sci Med Sport 2019;22:472–477. [DOI] [PubMed] [Google Scholar]
- 83. Planella‐Farrugia C, Comas F, Sabater‐Masdeu M, Moreno M, Moreno‐Navarrete JM, Rovira O, et al. Circulating irisin and myostatin as markers of muscle strength and physical condition in elderly subjects. Front Physiol 2019;10. 10.3389/fphys.2019.00871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rankin JW, Goldman LP, Puglisi MJ, Nickols‐Richardson SM, Earthman CP, Gwazdauskas FC. Effect of post‐exercise supplement consumption on adaptations to resistance training. J Am Coll Nutr 2004;23:322–330. [DOI] [PubMed] [Google Scholar]
- 85. Reidy PT, Fry CS, Igbinigie S, Deer RR, Jennings K, Cope MB, et al. Protein supplementation does not affect myogenic adaptations to resistance training. Med Sci Sports Exerc 2017;49:1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rozenek R, Ward P, Long S, Garhammer J. Effects of high‐calorie supplements on body composition and muscular strength following resistance training. J Sports Med Phys Fitness 2002;42:340–347. [PubMed] [Google Scholar]
- 87. Sharp MH, Lowery RP, Shields KA, Lane JR, Gray JL, Partl JM, et al. The effects of beef, chicken, or whey protein after workout on body composition and muscle performance. J Strength Cond Res 2018;32:2233–2242. [DOI] [PubMed] [Google Scholar]
- 88. Snijders T, Res PT, Smeets JSJ, van Vliet S, van Kranenburg J, Maase K, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance‐type exercise training in healthy young men. J Nutr 2015;145:1178–1184. [DOI] [PubMed] [Google Scholar]
- 89. Spillane M, Willoughby DS. Daily overfeeding from protein and/or carbohydrate supplementation for eight weeks in conjunction with resistance training does not improve body composition and muscle strength or increase markers indicative of muscle protein synthesis and myogenesis in resistance‐trained males. J Sports Sci Med 2016;15:17–25. [PMC free article] [PubMed] [Google Scholar]
- 90. Taylor LW, Wilborn C, Roberts MD, White A, Dugan K. Eight weeks of pre‐ and postexercise whey protein supplementation increases lean body mass and improves performance in Division III collegiate female basketball players. Appl Physiol Nutr Metab 2016;41:249–254. [DOI] [PubMed] [Google Scholar]
- 91. Vangsoe MT, Joergensen MS, Heckmann LHL, Hansen M. Effects of insect protein supplementation during resistance training on changes in muscle mass and strength in young men. Nutrients 2018;10. 10.3390/nu10030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Verdijk LB, Jonkers RAM, Gleeson BG, Beelen M, Meijer K, Savelberg H, et al. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 2009;89:608–616. [DOI] [PubMed] [Google Scholar]
- 93. Walker TB, Smith J, Herrera M, Lebegue B, Pinchak A, Fischer J. The influence of 8 weeks of whey‐protein and leucine supplementation on physical and cognitive performance. Int J Sport Nutr Exerc Metab 2010;20:409–417. [DOI] [PubMed] [Google Scholar]
- 94. Watanabe K, Holobar A, Mita Y, Kouzaki M, Ogawa M, Akima H, et al. Effect of resistance training and fish protein intake on motor unit firing pattern and motor function of elderly. Front Physiol 2018;9:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weisgarber KD, Candow DG, Vogt ESM. Whey protein before and during resistance exercise has no effect on muscle mass and strength in untrained young adults. Int J Sport Nutr Exerc Metab 2012;22:463–469. [DOI] [PubMed] [Google Scholar]
- 96. Willoughby DS, Stout JR, Wilborn CD. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2007;32:467–477. [DOI] [PubMed] [Google Scholar]
- 97. Aleman‐Mateo H, Gallegos Aguilar A, Ramirez Carreon V, Macias L, Astiazaran Garcia H, Ramos Enriquez JR. Nutrient‐rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: a single‐blind randomized clinical trial. Clin Interv Aging. 2014;1517–1525. 10.2147/cia.s67449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Norton C, Toomey C, McCormack WG, Francis P, Saunders J, Kerin E, et al. Protein supplementation at breakfast and lunch for 24 weeks beyond habitual intakes increases whole‐body lean tissue mass in healthy older adults. J Nutr 2016;146:65–69. [DOI] [PubMed] [Google Scholar]
- 99. Ottestad I, Lovstad AT, Gjevestad GO, Hamarsl H, Benth JS, Andersen LF, et al. Intake of a protein‐enriched milk and effects on muscle mass and strength. A 12‐week randomized placebo controlled trial among community‐dwelling older adults. J Nutr Health Aging 2017;21(10):1160–1169. [DOI] [PubMed] [Google Scholar]
- 100. Aas SN, Seynnes O, Benestad HB, Raastad T. Strength training and protein supplementation improve muscle mass, strength, and function in mobility‐limited older adults: a randomized controlled trial. Aging Clin Exp Res 2020;32:605–616. [DOI] [PubMed] [Google Scholar]
- 101. Babault N, Deley G, Le Ruyet P, Morgan F, Allaert FA. Effects of soluble milk protein or casein supplementation on muscle fatigue following resistance training program: a randomized, double‐blind, and placebo‐controlled study. J Int Soc Sports Nutr 2014;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Campbell B, Aguilar D, Conlin L, Vargas A, Schoenfeld BJ, Corson A, et al. Effects of high versus low protein intake on body composition and maximal strength in aspiring female physique athletes engaging in an 8‐week resistance training program. Int J Sport Nutr Exerc Metab 2018;28:580–585. [DOI] [PubMed] [Google Scholar]
- 103. Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, et al. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports 2014;24:788–798. [DOI] [PubMed] [Google Scholar]
- 104. Mori H, Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: a randomized controlled trial. Geriatr Gerontol Int 2018;18:1398–1404. [DOI] [PubMed] [Google Scholar]
- 105. Zhu K, Kerr DA, Meng XQ, Devine A, Solah V, Binns CW, et al. Two‐year whey protein supplementation did not enhance muscle mass and physical function in well‐nourished healthy older postmenopausal women. J Nutr 2015;145:2520–2526. 11 [DOI] [PubMed] [Google Scholar]
- 106. Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta‐analysis. Am J Clin Nutr 2017;106:1078–1091. [DOI] [PubMed] [Google Scholar]
- 107. Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BB, Verdijk LB, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10:e0140903, 10.1371/journal.pone.0140903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mazzulla M, Sawan SA, Williamson E, Hannaian SJ, Volterman KA, West DWD, et al. Protein intake to maximize whole‐body anabolism during postexercise recovery in resistance‐trained men with high habitual intakes is severalfold greater than the current recommended dietary allowance. J Nutr 2020;150:505–511. [DOI] [PubMed] [Google Scholar]
- 109. Churchward‐Venne TA, Holwerda AM, Phillips SM, van Loon LJ. What is the optimal amount of protein to support post‐exercise skeletal muscle reconditioning in the older adult? Sports Med 2016;46:1205–1212. [DOI] [PubMed] [Google Scholar]
- 110. Orwoll ES, Peters KE, Hellerstein M, Cummings SR, Evans WJ, Cawthon PM. The importance of muscle versus fat mass in sarcopenic obesity: a re‐evaluation using D3‐creatine muscle mass versus DXA lean mass measurements. J Gerontol A Biol Sci Med Sci 2020;75:1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reidy PT. Muscle or nothing! Where is the excess protein going in men with high protein intakes engaged in strength training? J Nutr 2020;150:421–422. [DOI] [PubMed] [Google Scholar]
- 112. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging 2019;14:1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fanelli Kuczmarski M, Pohlig RT, Stave Shupe E, Zonderman AB, Evans MK. Dietary protein intake and overall diet quality are associated with handgrip strength in African American and White adults. J Nutr Health Aging 2018;22:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ‐ Characteristics of the studies
Table S2 – GRADE evidence profile rating for lean body mass changes in studies testing additional dietary protein intervention in healthy subjects.
Table S3 – GRADE evidence profile rating for muscle strength changes in studies testing additional dietary protein intervention in healthy subjects.
Table S4 – GRADE evidence profile rating for handgrip strength changes in studies testing additional dietary protein intervention in healthy subjects.
Table S5 – GRADE evidence profile rating for physical testing performance changes in studies providing additional dietary protein to healthy subjects.
Table S6 ‐ Sensitivity analysis lean body mass
Table S7 – Sensitivity analysis bench press strength
Table S8 – Sensitivity analysis lower body strength
Table S9 – Sensitivity analysis handgrip strength
Table S10 – Sensitivity analysis physical function
Figure S1 – Risk of bias analysis of all studies included in the meta‐analysis. (+) – Circles filled in green = Low risk of bias; (?) – Circles filled in yellow = Unclear risk of bias; (−) – Circles filled in red = High risk of bias.
Figure S2 Summary of risk of bias analysis showing the percentage of studies with potential unclear or high risk of selection, performance, detection, attrition, reporting, or other bias.
Figure S3 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' lean body or muscle mass, including or not a resistance exercise program.
Figure S4 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' lean body or muscle mass of subjects categorized by age < 65 or ≥ 65 years old in studies including a resistance exercise program.
Figure S5 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein and resistance exercise on adults' bench press strength.
Figure S6 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' lower‐body strength by the presence or not of resistance exercise training in a study research protocol.
Figure S7 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies reporting daily protein ingestion and testing the effects of ingesting additional protein and resistance exercise on adults' lower body strength by age < 65 or ≥ 65 years old.
Figure S8 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies reporting daily protein ingestion and testing the effects of ingesting additional protein on adults' lower body strength by the level of protein ingestion.
Figure S9 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' handgrip strength by the presence or not of resistance exercise training in a study research protocol.
Figure S10 – Forest plot showing results of the three‐level random‐effects meta‐analysis of studies testing the effects of ingesting additional protein on adults' physical testing performance by the presence or not of resistance exercise training in a study research protocol.
Figure S11 – Bubble plot of three‐level meta‐regression analysis of the main effect on lean body mass vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S12 – Bubble plot of three‐level meta‐regression analysis of the main effect on lean body mass vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S13 – Bubble plot of three‐level meta‐regression analysis of the main effect on bench press strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S14 – Bubble plot of three‐level meta‐regression analysis of the main effect on bench press strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S15 – Bubble plot of three‐level meta‐regression analysis of the main effect on lower‐body strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S16 – Bubble plot of three‐level meta‐regression analysis of the main effect on lower‐body strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S17 – Bubble plot of three‐level meta‐regression analysis of the main effect on handgrip strength vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S18 – Bubble plot of three‐level meta‐regression analysis of the main effect on performance in physical and functional tests vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT reporting protein ingestion.
Figure S19 – Bubble plot of three‐level meta‐regression analysis of the main effect on performance in physical and functional tests vs. total daily protein ingestion (g/kg/day) in studies testing interventions to increase protein ingestion in all included RCT using resistance exercise training and reporting protein ingestion.
Figure S20 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lean body or muscle mass including or not a resistance exercise program.
Figure S21 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lean body or muscle mass only in studies including a resistance exercise program.
Figure S22 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lean body or muscle mass only in studies including a resistance exercise program reporting protein ingestion.
Figure S23 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' bench press strength in studies including or not a resistance exercise program.
Figure S24 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' bench press strength only in studies including a resistance exercise program reporting protein ingestion.
Figure S25 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lower‐body strength in studies including or not a resistance exercise program.
Figure S26 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lower‐body strength only in studies including a resistance exercise program.
Figure S27 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' lower‐body strength only in studies including a resistance exercise program reporting protein ingestion.
Figure S28 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' handgrip strength in studies including or not a resistance exercise program.
Figure S29 – Funnel plot showing results studies testing the effects of ingesting additional protein on adults' performance in physical and functional tests in studies including or not a resistance exercise program.
Search_strategies


