Abstract
Purpose
We aimed to evaluate the efficacy and safety of penile girth enhancement (PGE) using hyaluronic acid (HA) filler with different physical properties from previous studies. Additionally, we evaluated the clinical impact on ejaculation after PGE.
Materials and Methods
This was a prospective, patient/evaluator-blinded, randomized, active-controlled, multicenter trial. Patients recruited between December 2017 and March 2018 were randomly assigned to the HA filler or control group (polylactic acid [PLA] filler). Penile girth, satisfaction level, Premature Ejaculation Profile (PEP), and self-estimated intravaginal ejaculation latency time (IELT) were assessed at baseline and at 24 weeks post-injection.
Results
Sixty-four subjects (32 in each group) completed the trial. The mean increase in girth was 22.74±12.60 mm and 20.23±8.73 mm in the HA and control groups, respectively. Satisfaction level regarding penile appearance and sexual life significantly increased in both groups. There was no statistically significant difference between the groups in terms of increase in penile girth or change in satisfaction level. Both groups showed significant improvements in PEP index scores. Self-estimated IELT also significantly increased in the HA group (from 5.36±3.51 to 7.86±4.73 minutes, p=0.0001) and control group (from 5.23±3.55 to 6.43±4.22 minutes, p=0.021). No serious adverse events (AEs) were reported.
Conclusions
PGE with HA and PLA fillers resulted in significant enhancement of girth without serious AEs with no significant differences. Furthermore, PGE using filler improved clinical symptoms related to ejaculation.
Keywords: Ejaculation, Filler, Girth enhancement, Hyaluronic acid, Penis
INTRODUCTION
Penile augmentation (PA), including penile girth enhancement (PGE), has been primarily performed in men with sexual dysfunction caused by anatomical abnormalities, such as in Peyronie's disease [1,2]. PGE for cosmetic purposes requires clarification on various aspects, such as standardized methods and performing the procedure in patients with psychiatric distress [3]. Meanwhile, the demand for surgical treatment to enlarge genital circumference has steadily increased, and various procedures are available, including PGE using dermal acellular graft [4], autologous fat injection [5], and other scaffolds [6]. Although these surgical techniques do not cause serious complications, such as observed after the use of mineral oils [7] and paraffin injections [8], complications such as palpable nodules, asymmetry, curvatures resulting from fibrosis, penile shortening or deformity, seroma, infection, and common necrosis occur [9].
Since hyaluronic acid (HA) filler was approved by the U.S. Food and Drug Administration (FDA) in 2003, it has become the filler of choice in the dermal filler market and clinical practice [10]. A major advantage of HA filler over temporary fillers such as fat and collagen is its low complication rate [11]. Additionally, the slow degradation of the HA filler through cross-linkage increases its longevity by a hundred times compared to that of natural polymer implants, without a decrease in biocompatibility [11].
Based on the properties of HA fillers, in 2011, a group of researchers reported the feasibility and safety of PGE using HA fillers [12]. Recently, several studies have demonstrated the efficacy and safety of PGE using HA fillers [13,14,15]. Consequently, the Korean Ministry of Food and Drug Safety has approved several HA fillers for PGE. Although previous approval studies have evaluated subjective satisfaction regarding sexual performance, detailed information regarding ejaculation after PGE using injectable fillers is still unknown. Furthermore, it is necessary to determine whether HA fillers with different properties (HA concentration and cross-linking degree) show similar effects and safety to those of previously studied HA fillers. Therefore, in this randomized controlled trial, we aimed to evaluate the efficacy and safety of PGE using HA filler and its clinical impact on ejaculation.
MATERIALS AND METHODS
1. Subjects and study design
This was designed as a prospective, patient/evaluator-blinded, randomized, active-controlled, multicenter trial to evaluate the efficacy, safety, and clinical impact of ejaculation following PGE using injectable filler 24 weeks after the injection.
Patients were recruited through advertisements at Korea University Guro Hospital, Korea University Anam Hospital, and Seoul St. Mary's Hospital and screened between December 2017 and March 2018. Among voluntary applicants for the clinical trial, healthy men aged between 20 and 65 years seeking cosmetic PGE and having a heterosexual and stable sexual relationship were included. The exclusion criteria were as follows: any congenital or acquired penile malformation including micropenis, concealed penis, severe phimosis, and Peyronie's disease; previous penile surgery including PGE and insertion of a penile prosthesis; psychiatric or psychological disorder including body dysmorphic disorder after psychological counseling; and any chronic major systemic disease including coagulopathy or taking anticoagulant medication.
During the screening period, we obtained the subjects' demographics and medical history, and all subjects underwent a physical examination.
After the screening period, the enrolled patients were randomly assigned in a 1:1 ratio to the HA filler group or control group using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) by an independent statistician. The control group was injected with polylactic acid (PLA) filler, previously approved by the KMFDS for PGE. Although the composition of the PLA filler is different from that of the HA filler, the application methods and expected effects are identical. Thus, PLA filler was selected as a control to evaluate the effect and safety of HA filler injection for PGE in this study.
The subjects' penile girth was measured at baseline (before the procedure) and at 4, 12, and 24 weeks after the procedure. Questionnaires were distributed to document the participants' responses regarding satisfaction with penile appearance and sexual life and ejaculation function, including the Premature Ejaculation Diagnostic Tool (PEDT) and Premature Ejaculation Profile (PEP), at baseline and after 12 and 24 weeks. Satisfaction was rated on a scale of 1–5 (1=dissatisfied; 5=satisfied). Additionally, the patients' self-estimated intravaginal ejaculation latency time (IELT) was documented at baseline and 24 weeks after the procedure. During the study period, assessments before and after the procedure were performed by a single physician, independent of the filler injection at each institution (evaluator-blinded).
2. Injected filler product
The injected HA filler (Doublofill; Humedix, Co., Ltd., Anyang, Korea) is a newly developed PGE product consisting of 23 mg of cross-linked HA in 2 mL of saline. It is a biphasic HA product with a particle size of approximately 700 μm.
The PLA filler (PowerFill; REGEN Biotech, Inc., Seoul, Korea) is a currently commercially available powder consisting of 10 g of 50-μm PLA microparticles suspended in 3 mL of methylcellulose and carboxy-methylcellulose that is reconstituted with 8 mL of saline immediately before injection.
3. Injection technique
The injection technique for PGE has been described in detail earlier [12]. Briefly, under local anesthesia with the application of EMLA cream (EMLA 5% cream; AstraZeneca Korea, Co., Ltd., Korea) or penile dorsal nerve block, patients were placed in the supine position, and a medical curtain was placed at the umbilical level for a patient-blinded procedure. Entry sites were made using 21-gauge or 18-gauge needles for the HA or PLA filler, respectively. Four entry sites were made on the penile base and distal penile shaft at the 2 o'clock and 10 o'clock positions, respectively. Subsequently, using a 22-gauge or 20-gauge cannula, HA or PLA filler was injected in the respective group of patients, between the Buck's fascia and the dartos fascia, by combining the back-and-forth and fanning techniques. Depending on the penile size, 15–22 mL of HA or PLA filler was injected. After the procedure, a mild compressive dressing was applied to the penis and left in place overnight. Patients were advised to abstain from sexual intercourse for 4 weeks after the procedure.
4. Outcome measures
The primary outcome was the difference in the change in mean penile girth in the HA and PLA groups at each follow-up visit. As the PGE procedure was expected to increase the entire penile circumference, the mean penile girth was defined as the mean of the proximal, mid, and distal circumference measurements of the penis.
Secondary outcomes were as follows: 1) difference in satisfaction regarding penile appearance and sexual life at 12 and 24 weeks after the procedure compared to baseline, 2) change in each domain score and index score (the mean of all four measures) of PEP at 24 weeks after the procedure compared to baseline, and 3) change in the self-estimated IELT between baseline and 24 weeks after the procedure.
5. Safety assessments
Patients were observed for 30 minutes after the PGE procedure to monitor injection site complications such as hemorrhage, pain, induration, swelling, and redness. During the study period, treatment-related AEs were assessed through vital signs, physical examination, and medical history obtained at each visit. Laboratory tests were performed 24 weeks post-injection.
6. Statistical analysis
All statistical analyses were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). Demographic data for all subjects were expressed as the mean±standard deviation (SD), and comparison of the differences between groups was analyzed using Student's t-test and Fisher's exact test. Data including penile girth; 5-scale satisfaction regarding penile appearance and sexual life; and self-estimated IELT, PEDT, and PEP measurements at baseline and at 12 and 24 weeks for all subjects were expressed as mean±SD. Between-group comparisons, including differences in the change in mean penile girth, satisfaction level, PEP score, and self-estimated IELT, were analyzed by analysis of covariance using the injected volume of filler as a covariate. Within-group comparisons were analyzed using the paired t-test. A p-value <0.05 was considered statistically significant.
7. Ethics statement
The present study protocol was reviewed and approved by the Korean Ministry of Food and Drug Safety (file no.782-2017) and by each institutional review board (nos 2017GR0824 at Korea University Guro Hospital, 2017AN0305 at Korea University Anam Hospital, and KC17DDDE0545 at Catholic University Seoul Saint Mary's Hospital). All subjects were informed and instructed about the objectives and procedures of the current clinical trial. They were asked to sign the informed consent after a detailed explanation of possible adverse events (AEs) related to the filler and filler injection procedure.
RESULTS
1. Subjects
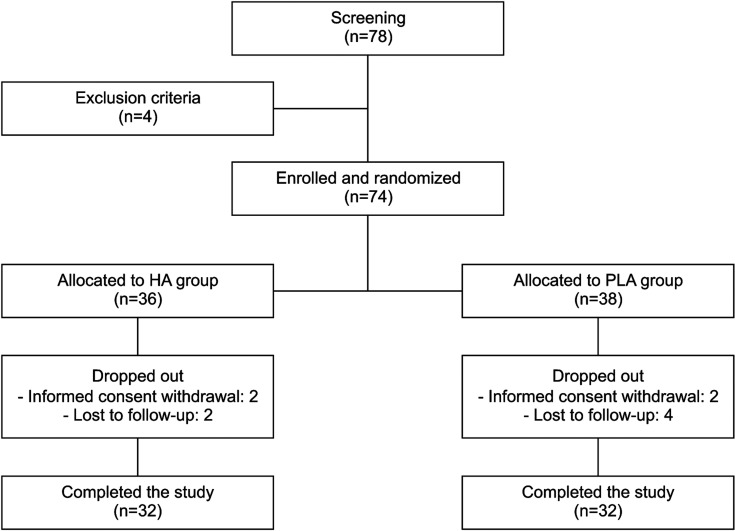
Of the 78 subjects who were screened, 4 who met the exclusion criteria were excluded; thus, 74 subjects were enrolled in the study and randomly assigned to the HA or control group. Ten patients dropped out (four withdrew informed consent and six were lost to follow-up), leaving 64 subjects (32 each in the HA and control groups) who completed the trial (Fig. 1). Baseline demographic and clinical characteristics were not significantly different between the groups (Table 1). Only 1 enrolled patient had a medication history of phosphodiesterase-5 inhibitor for erectile dysfunction and there was no patient who had a medication history for other sexual dysfunctions.
Fig. 1. Flow diagram for study subjects. HA: hyaluronic acid, PLA: polylactic acid.
Table 1. Baseline characteristics.
| Variable | HA group (n=32) |
Control group (n=32) |
p-value |
|---|---|---|---|
| Age (y) | 40.47±12.12 | 43.31±11.47 | 0.279a |
| BMI (kg/m2) | 24.94±3.45 | 25.28±3.70 | 0.701a |
| Hypertension | 3 (9.4) | 1 (3.1) | 0.302b |
| Diabetes mellitus | 0 | 1 (3.1) | 0.313b |
| Erectile dysfunction | 1 (3.1) | 0 | 0.313b |
| Premature ejaculation | 8 (25.0) | 10 (31.3) | 0.578b |
Values are presented as mean±standard deviation or number (%).
BMI: body mass index, HA: hyaluronic acid.
aStudent's t-test; bFisher's exact test.
2. Penile girth
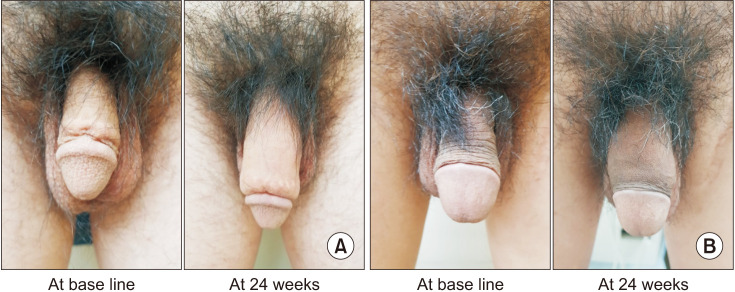
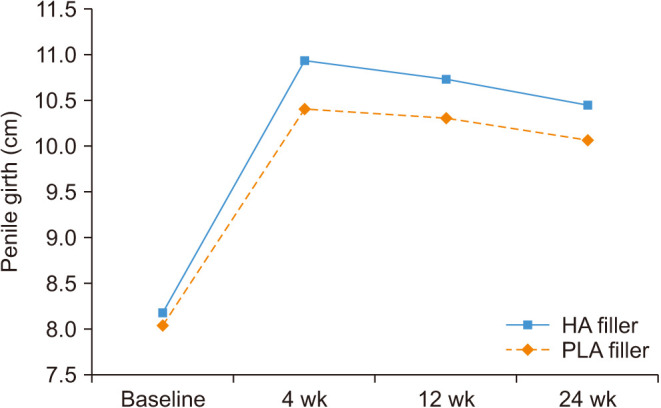
The mean penile girth at baseline was 81.75±9.86 mm in the HA group and 80.37±6.79 mm in the control group (no significant difference). The mean injected volume of the filler was 19.69±0.74 and 20.00±0.00 mL in the HA and control group, respectively (p=0.022). On gross examination, injected fillers were well distributed throughout the penile shaft, enhancing penile girth, which was maintained for up to 24 weeks (Fig. 2). The mean penile girth at 4, 12, and 24 weeks increased significantly in both groups compared to that at baseline (p<0.001 for each time point). The mean increase in girth at 24 weeks was 22.74±12.60 mm and 20.23±8.73 mm in the HA and control groups, respectively. There was no statistically significant difference between the groups at each time point in the increase in penile girth compared to that at baseline (Fig. 3).
Fig. 2. Representative photos of penis before and 24 weeks after filler injections. (A) HA filler, (B) PLA filler. HA: hyaluronic acid, PLA: polylactic acid. Informed consent was obtained from the patients for using photographs.
Fig. 3. Changes in penile girth at week 4, 12, and 24 were significantly increased in both HA and PLA groups compared to the baseline (all p<0.0001). There was no significant difference between groups in increase of penile girth compared to baseline at all time points (at 4 weeks, p=0.073; at 12 weeks, p=0.089; at 24 weeks, p=0.092). HA: hyaluronic acid, PLA: polylactic acid.
3. Satisfaction level
The satisfaction levels regarding penile appearance and sexual life during the study period are summarized in Table 2. The satisfaction level of the subjects on penile appearance and sexual life at 12 and 24 weeks post-injection significantly increased in both groups compared to that at baseline. There was no statistically significant difference between the groups with respect to change in the subjects' satisfaction with penile appearance compared to the baseline value at 12 and 24 weeks post-injection. The change in satisfaction with sexual life from baseline to 12 weeks was significantly greater in the HA group than in the control group (p=0.0143); however, there was no difference between the two groups at 24 weeks post-injection (p=0.3608).
Table 2. Changes in the satisfaction of subjects with penile appearance and sexual life during the study period.
| Variable | Satisfaction with penile appearance | Satisfaction with sexual life | |||
|---|---|---|---|---|---|
| HA group (n=32) | Control group (n=32) | HA group (n=32) | Control group (n=32) | ||
| Baseline | 2.13±0.55 | 2.06±0.80 | 2.47±0.67 | 2.47±0.88 | |
| Week 12 | 3.45±1.03 | 2.71±0.97 | 3.65±0.80 | 2.87±0.99 | |
| Change from baseline at week 12 | 1.32±1.19 | 0.65±1.14 | 1.16±1.07 | 0.42±0.89 | |
| p-valuea | <0.0001 | 0.0037 | <0.0001 | 0.0132 | |
| Treatment difference | 0.62 [-0.00, 1.25] | 0.66 [0.14, 1.18] | |||
| p-valueb | 0.0504 | 0.0143 | |||
| Week 24 | 3.25±1.11 | 2.88±1.07 | 3.47±0.92 | 3.09±0.96 | |
| Change from baseline at week 24 | 1.13±1.21 | 0.81±1.20 | 1.00±1.11 | 0.63±1.10 | |
| p-valuea | <0.0001 | 0.0006 | <0.0001 | 0.003 | |
| Treatment difference | 0.22 [-0.41, 0.86] | 0.26 [-0.31, 0.84] | |||
| p-valueb | 0.4797 | 0.3608 | |||
Values are presented as mean±standard deviation or least squares mean difference [95% confidence interval].
HA: hyaluronic acid.
aPaired t-test; bAnalysis of covariance test (injected volume of filler as a covariate).
4. Clinical impact on ejaculation
The ejaculation assessment was completed by 44 subjects (22 in the HA group and 22 in the control group). At baseline, 20 subjects (10 each in the HA and control groups) reported a PEDT score ≥9. Both groups showed significantly improved PEP index scores at 24 weeks post-injection; no significant differences were observed between the groups (Table 3). Within PEP, the score for control over ejaculation improved significantly in both groups. The scores for other domains within the PEP are summarized in Table 3.
Table 3. Changes in PEP index score (the mean of all four measures), each PEP domain score, and self-estimated IELT between baseline and 24 weeks.
| Variable | HA group (n=22) | Control group (n=22) | p-valueb
(HA vs. control) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | p-valuea | Baseline | Week 24 | p-valuea | |||
| PEP index score | 2.09±0.68 | 2.55±0.74 | 0.007 | 2.02±0.83 | 2.38±0.89 | 0.017 | 0.617 | |
| PEP domain | ||||||||
| Perceived control over ejaculation | 1.77±0.53 | 2.32±0.72 | 0.001 | 1.68±1.00 | 2.09±0.92 | 0.047 | 0.574 | |
| Satisfaction with sexual intercourse | 1.91±0.68 | 2.27±0.88 | 0.057 | 1.91±0.75 | 2.23±0.87 | 0.090 | 0.859 | |
| Distress related to ejaculation | 2.23±0.97 | 2.55±1.01 | 0.129 | 2.05±1.00 | 2.23±1.15 | 0.257 | 0.596 | |
| Interpersonal difficulty | 2.45±1.10 | 3.05±1.05 | 0.016 | 2.45±1.01 | 2.95±1.13 | 0.380 | 0.777 | |
| Self-estimated IELT (min) | 5.36±3.51 | 7.86±4.73 | 0.001 | 5.23±3.55 | 6.43±4.22 | 0.021 | 0.063 | |
Values are presented as mean±standard deviation.
PEP: premature ejaculation profile, IELT: intravaginal ejaculation latency time, HA: hyaluronic acid.
aPaired t-test; bStudent's t-test.
At baseline, 18 subjects (8 in the HA group and 10 in the control group) reported a self-estimated IELT of <3 minutes. Self-estimated IELT at 24 weeks post-injection was significantly increased compared to that at baseline in both the HA (from 5.36±3.51 minutes to 7.86±4.73 minutes, p=0.001) and control (from 5.23±3.55 minutes to 6.43±4.22 minutes, p=0.021) group. Additionally, among 18 subjects reporting <3 minutes of self-estimated IELT, 12 (5 in the HA group and 7 in the control group) reported improvement from baseline IELT at 24 weeks post-injection.
5. Safety
Filler injection-related AEs in the HA and control groups were reported in two (6.3%) and three (9.4%) subjects, respectively. Two subjects in each group reported inflammation at the site of injection that was mild and improved with conservative treatment. Pain at the injection site was reported in one subject in the control group, and it was transient and self-limited. No serious AEs were reported during the study period.
DISCUSSION
Esthetic PGE in patients with small penis syndrome who wish to have a penis enlargement despite a normal penis size requires a careful approach. These patients might suffer from a penile dysmorphophobic disorder, which might lead to poor satisfaction with penile enlargement treatment due to their unrealistic expectations of penile size [9]. Therefore, esthetic PGE should not be performed in patients with psychiatric disorders, and a multidisciplinary approach, including psychological counseling, is necessary. In this study, patients with psychiatric disorders were excluded, and procedures were performed after careful advice on the potential results of treatment and potential complications during the screening period. As a result, we achieved a high satisfaction rate for penile appearance during the post-injection follow-up period. This is consistent with other recent studies on PGE using HA and PLA fillers [13,14,15].
Another limitation of conventional PA is that the procedure is invasive [16]. In particular, a major concern is that PGE is mainly performed for cosmetic purposes, unlike penile lengthening [9]. However, since 2000, various soft fillers for minimally invasive procedures have been approved by the FDA, leading to several trials of dermal fillers that offer a less invasive alternative to the traditional surgical interventions for PGE. HA, calcium hydroxylapatite, polymethylmethacrylate microspheres, and PLA fillers have been examined for use in PGEs [12,17,18]. Although an ideal filler for PGE has not yet been established, among reversible fillers, HA has become dominant and popular [19]. The natural-state HA filler, which is considered the most ideal filler, has a short half-life, which is a major drawback; however, a cross-linking process is used during manufacturing to stabilize the HA, leading to increased longevity [20]. By making minimal alterations to the material, manufacturers have created HA products that are well tolerated by the immune system and exhibit favorable properties of longevity and nonreactivity [21]. The PLA filler used for the control group in this study was also a nonpermanent filler and has been approved for PA in many countries [14]. The injected PLA absorbs water and causes tissue swelling after injection, and the micro-particles of the injected PLA filler are surrounded by connective tissue capsules that are gradually degraded by hydrolysis; thus, the injected site is bulky because of the fibrous tissue reaction with collagen deposition [22]. Although the mechanisms of tissue expansion of the two fillers are different, there was no significant difference in the effects and stability of the two fillers in this study, consistent with the results of previous studies [13,14,15].
Recent studies on PGE used HA fillers from different manufacturers, differing in HA concentration and degree of cross-linking, which determine the physical properties of HA fillers [13,14,15]. The HA filler used in this study had a higher degree of cross-linking and HA concentration than the HA fillers used in previous studies, thus increasing the viscosity and cohesivity of the filler, which in turn increases the longevity and tissue volume of the injection site [11]. However, this can cause a foreign body reaction, which leads to discomfort, swelling, and even granuloma formation [11]. Interestingly, the increase in mean penile girth by 22.7 mm in this study exceeded the results of previous studies that reported changes from 19.1.–21.0 mm. This was considered to be due to the difference in the properties of the HA filler used in this study and in previous studies. In fact, the HA concentration of the filler in this study was 23 mg/mL, which was higher than the 20 mg/mL HA concentration in previous studies, and the cross-linking degree was 12%, which was higher than the 6%–10% reported in previous studies. Nevertheless, there were no differences in the incidence of inflammatory AEs of concern compared to previous studies, and granuloma formation was not observed. The properties of HA fillers and the results of previous studies and the current study are summarized in Table 4.
Table 4. Summary of studies addressing the outcome of penile girth enhancement using HA fillers.
| Trade name (manufacturer, country) |
Study design | Sample size | Mean penile girth increase at 24 weeks, mm | Complications | Injection dosage, mL (mean±SD) | HA concentration, mg/mL | Particle size, µm | Cross-linking, degree |
|---|---|---|---|---|---|---|---|---|
| Potenfill (Medytox, Korea) [14] |
Prospective, patient/evaluator-blinded, randomized, multicenter trial | 65 (HA: 33, PLA: 32) | 21 | Injection site deformation: 1; injection site inflammation: 1 | 20.8±1.5 | 20 | ~700 | BDDE, 10% |
| HyaFilia Impact (CHA Meditech, Korea) [15] | Prospective, patient/ evaluator-blinded, randomized, multicenter trial | 62 (HA: 30, PLA: 32) |
19.1 | Injection site inflammation: 1; injection site pain: 2 | 16.4±2.7 | 20 | ~500 | BDDE, 6% |
| THE CHAEUM; Shape 10 (Hugel Bio, Korea) [13] |
Prospective, patient/ evaluator-blinded, randomized, multicenter trial | 59 (HA: 30, PLA: 29) |
20.6 | Injection site induration: 1 | 19.1±1.4 | 20 | ~600 | BDDE, no record |
| Doublofill (Humedix, Korea)a |
Prospective, patient/ evaluator-blinded, randomized, multicenter trial | 64 (HA: 32, PLA: 32) |
22.7 | Injection site inflammation: 2 | 19.7±0.7 | 23 | ~700 | BDDE, 12% |
HA: hyaluronic acid, PLA: polylactic acid, BDDE: 1,4-butanediol diglycidyl ether.
aCurrent study.
Another interesting aspect of this study is that PGE improved clinical symptoms related to ejaculation in several patients. Several studies have demonstrated the therapeutic efficacy of glans penis augmentation using HA filler for premature ejaculation (PE) [3,23]; however, the effect on ejaculation of PGE using HA filler without injection into the glans is unknown. Although the reason for this has not been clearly demonstrated, it is considered to be the same mechanism as that of glans penis augmentation using HA filler for the treatment of PE [24]. It is well known that one of the two axons of the dorsal nerve is located along the dorsal midline and terminates in the glans, while the other group radiates from the main trunk over the lateral and ventral aspects of the penile shaft [25]. Therefore, the filler injected between the Buck's fascia and the dartos fascia is speculated to act as a barrier between the tactile stimuli and the dorsal nerve ending receptor in the penile shaft, which reduces the sensation threshold. Indeed, in the initial feasibility study of PGE using HA filler, most patients experienced decreased tactile sensation of the shaft [12]. In addition, in this study, we found that the effect on ejaculation did not negatively affect satisfaction with sexual life.
This study had several limitations. First, the study duration limits drawing a strong conclusion regarding the long-term efficacy of PGE using HA filler. Second, it is possible to criticize the study design, which uses an active control rather than a placebo control. Although a placebo-controlled study allows better evaluation of the clinical effectiveness of a new treatment, there is a higher risk that nontreatment would be deemed ethically unacceptable by an ethics commission. Additionally, even if nontreatment does not harm the patients, it is difficult to conduct a blinded study using saline injection as a placebo control because volume expansion disappears soon in saline-injected patents. Therefore, the current study used active treatment in the control group, as in previous studies. Third, there was a limitation in evaluating ejaculation. Although the PEDT questionnaire was developed mainly as a diagnostic tool for PE, reliance on the PEDT is flawed according to the current International Society for Sexual Medicine definition of PE. The PEP questionnaire has yet to be validated as a sensitive tool for evaluating PE. Additionally, among the subjects who completed the ejaculation assessment, less than half showed clinical PE. As the present study was not specifically designed to evaluate PGE using injectable fillers for the treatment of PE, further studies are needed to demonstrate the therapeutic effect of PGE on PE.
Despite these limitations, the present study showed the efficacy and safety of PGE using HA fillers with properties different from those of previous studies. We believe that our study will provide useful information for clinicians to choose HA fillers among various available products. Additionally, our study is the first to report the impact on ejaculation of PGE using HA filler and is expected to serve as a catalyst for future research on PGE for the treatment of PE.
CONCLUSIONS
Although an ideal penile filler has not yet been established, PGE with HA and PLA filler resulted in significant PGE for 24 weeks post-injection and appears to be safe and stable. In particular, the current study showed safety using HA filler with a higher HA concentration and cross-linking degree than the HA filler in previous studies. Despite some concerns about PGE using injectable filler in terms of patient satisfaction, a high satisfaction level with penile appearance and sexual life through a careful approach to men looking for penile enhancement was achieved in the current study. Furthermore, it was demonstrated that PGE using fillers improved clinical symptoms related to ejaculation. However, further studies are recommended regarding the efficacy and safety of PGE using fillers in patients with severe PE.
ACKNOWLEDGEMENTS
This work was supported by Humedix, Co., Ltd., Anyang, Korea.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: DGM.
- Formal analysis: STA, JSS.
- Funding Acquisition: DGM.
- Investigation: STA, JSS, WJB, SWK, JJK, DGM.
- Methodology: SWK, JJK, DGM.
- Visualization: STA.
- Supervision: SWK.
- Writing — Original Draft: STA.
- Writing — Review & Editing: STA, SWK, DGM.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time due to legal and ethical reasons.
References
- 1.Chung E. Penile reconstructive surgery in Peyronie disease: challenges in restoring normal penis size, shape, and function. World J Mens Health. 2020;38:1–8. doi: 10.5534/wjmh.170056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokolakis I, Schönbauer P, Mykoniatis I, Kübler H, Gschwend J, Lahme S, et al. Long-term results after surgical treatment of congenital penile curvature using a modified Nesbit technique. World J Mens Health. 2020;38:564–572. doi: 10.5534/wjmh.190092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon du G, Kwak TI, Kim JJ. Glans penis augmentation using hyaluronic acid gel as an injectable filler. World J Mens Health. 2015;33:50–61. doi: 10.5534/wjmh.2015.33.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alei G, Letizia P, Ricottilli F, Simone P, Alei L, Massoni F, et al. Original technique for penile girth augmentation through porcine dermal acellular grafts: results in a 69-patient series. J Sex Med. 2012;9:1945–1953. doi: 10.1111/j.1743-6109.2012.02744.x. [DOI] [PubMed] [Google Scholar]
- 5.Kang DH, Chung JH, Kim YJ, Lee HN, Cho SH, Chang TH, et al. Efficacy and safety of penile girth enhancement by autologous fat injection for patients with thin penises. Aesthetic Plast Surg. 2012;36:813–818. doi: 10.1007/s00266-012-9891-4. [DOI] [PubMed] [Google Scholar]
- 6.Solomon MP, Komlo C, Defrain M. Allograft materials in phalloplasty: a comparative analysis. Ann Plast Surg. 2013;71:297–299. doi: 10.1097/SAP.0b013e318281aece. [DOI] [PubMed] [Google Scholar]
- 7.Francis J, Poh Choo Choo A, Wansaicheong Khin-Lin G. Ultrasound and MRI features of penile augmentation by “Jamaica oil” injection. A case series. Med Ultrason. 2014;16:372–376. doi: 10.11152/mu.201.3.2066.164.apchoo. [DOI] [PubMed] [Google Scholar]
- 8.Chon W, Koo JY, Park MJ, Choi KU, Park HJ, Park NC. Paraffin granuloma associated with buried glans penis-induced sexual and voiding dysfunction. World J Mens Health. 2017;35:129–132. doi: 10.5534/wjmh.2017.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hehemann MC, Towe M, Huynh LM, El-Khatib FM, Yafi FA. Penile girth enlargement strategies: what's the evidence? Sex Med Rev. 2019;7:535–547. doi: 10.1016/j.sxmr.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 10.American Society of Plastic Surgeons. 2019 Plastic surgery statistics report [Internet] Arlington Heights (IL): American Society of Plastic Surgeons; c2019. [cited 2021 Jan 5]. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf. [Google Scholar]
- 11.Herrmann JL, Hoffmann RK, Ward CE, Schulman JM, Grekin RC. Biochemistry, physiology, and tissue interactions of contemporary biodegradable injectable dermal fillers. Dermatol Surg. 2018;44(Suppl 1):S19–S31. doi: 10.1097/DSS.0000000000001582. [DOI] [PubMed] [Google Scholar]
- 12.Kwak TI, Oh M, Kim JJ, Moon du G. The effects of penile girth enhancement using injectable hyaluronic acid gel, a filler. J Sex Med. 2011;8:3407–3413. doi: 10.1111/j.1743-6109.2010.01748.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang DY, Ko K, Lee SH, Lee WK. A comparison of the efficacy and safety between hyaluronic acid and polylactic acid filler injection in penile augmentation: a multicenter, patient/evaluator-blinded, randomized trial. J Sex Med. 2019;16:577–585. doi: 10.1016/j.jsxm.2019.01.310. [DOI] [PubMed] [Google Scholar]
- 14.Yang DY, Jeong HC, Ahn ST, Bae WJ, Moon DG, Kim SW, et al. A comparison between hyaluronic acid and polylactic acid filler injections for temporary penile augmentation in patients with small penis syndrome: a multicenter, patient/evaluator-blind, comparative, randomized trial. J Sex Med. 2020;17:133–141. doi: 10.1016/j.jsxm.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Yang DY, Jeong HC, Ko K, Lee SH, Lee YG, Lee WK. Comparison of clinical outcomes between hyaluronic and polylactic acid filler injections for penile augmentation in men reporting a small penis: a multicenter, patient-blinded/evaluator-blinded, non-inferiority, randomized comparative trial with 18 months of follow-up. J Clin Med. 2020;9:1024. doi: 10.3390/jcm9041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Otero J, Manfredi C, Ralph D, Osmonov D, Verze P, Castiglione F, et al. Non-invasive and surgical penile enhancement interventions for aesthetic or therapeutic purposes: a systematic review. BJU Int. 2021;127:269–291. doi: 10.1111/bju.15145. [DOI] [PubMed] [Google Scholar]
- 17.Yang DY, Ko K, Lee SH, Moon DG, Kim JW, Lee WK. Efficacy and safety of a newly developed polylactic acid microsphere as an injectable bulking agent for penile augmentation: 18-months follow-up. Int J Impot Res. 2017;29:136–141. doi: 10.1038/ijir.2017.10. [DOI] [PubMed] [Google Scholar]
- 18.Kim MT, Ko K, Lee WK, Kim SC, Yang DY. Long-term safety and longevity of a mixture of polymethyl methacrylate and cross-linked dextran (Lipen-10®) after penile augmentation: extension study from six to 18 months of follow-up. World J Mens Health. 2015;33:202–208. doi: 10.5534/wjmh.2015.33.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WK, Yang DY. In: Penile augmentation. Park NC, Kim SW, Moon DG, editors. Berlin/Heidelberg: Springer; 2016. Classification of soft tissue filler; pp. 71–82. [Google Scholar]
- 20.Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302–312. doi: 10.1111/j.1524-4725.2008.01046.x. [DOI] [PubMed] [Google Scholar]
- 21.De Boulle K, Glogau R, Kono T, Nathan M, Tezel A, Roca-Martinez JX, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39:1758–1766. doi: 10.1111/dsu.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzat WH, Keller GS. The use of poly-L-lactic acid filler in facial aesthetics. Facial Plast Surg. 2011;27:503–509. doi: 10.1055/s-0031-1298782. [DOI] [PubMed] [Google Scholar]
- 23.Alahwany A, Ragab MW, Zaghloul A, Abdallah H, Mostafa T. Hyaluronic acid injection in glans penis for treatment of premature ejaculation: a randomized controlled cross-over study. Int J Impot Res. 2019;31:348–355. doi: 10.1038/s41443-018-0104-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Kwak TI, Jeon BG, Cheon J, Moon DG. Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation. Int J Impot Res. 2004;16:547–551. doi: 10.1038/sj.ijir.3901226. [DOI] [PubMed] [Google Scholar]
- 25.Yang CC, Bradley WE. Neuroanatomy of the penile portion of the human dorsal nerve of the penis. Br J Urol. 1998;82:109–113. doi: 10.1046/j.1464-410x.1998.00669.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time due to legal and ethical reasons.