Keywords: inflammation, osteoarthritis, rehabilitation, skeletal muscle
Abstract
Many individuals with end-stage osteoarthritis (OA) undergo elective total hip/knee arthroplasty (THA/TKA) to relieve pain, improve mobility and quality of life. However, ∼30% suffer long-term mobility impairment following surgery. This may be in part due to muscle inflammation susceptibility (MuIS+), an overt proinflammatory pathology localized to skeletal muscle surrounding the diseased joint, present in some patients with TKA/THA. We interrogated the hypothesis that MuIS+ status results in a perturbed perioperative gene expression profile and decreases skeletal muscle integrity in patients with end-stage OA. Samples were leveraged from the two-site, randomized, controlled trial R01HD084124, NCT02628795. Participants were dichotomized based on surgical (SX) muscle gene expression of TNFRSF1A (TNF-αR). MuIS+/− samples were probed for gene expression and fibrosis. Paired and independent two-tailed t tests were used to determine differences between contralateral (CTRL) and surgical (SX) limbs and between-subject comparisons, respectively. Significance was declared at P < 0.05. Seventy participants (26M/44F; mean age 62.41 ± 8.86 yr; mean body mass index 31.10 ± 4.91 kg/m2) undergoing THA/TKA were clustered as MuIS+ (n = 24) or MuIS− (n = 46). Lower skeletal muscle integrity (greater fibrosis) exists on the SX versus CTRL limb (P < 0.001). Furthermore, MuIS+ versus MuIS− muscle exhibited higher proinflammatory (IL-6R and TNF-α) and catabolic (TRIM63) gene expression (P < 0.001, P = 0.004, and 0.024 respectively), with a trend for greater fibrosis (P = 0.087). Patients with MuIS+ exhibit more inflammation and catabolic gene expression in skeletal muscle of the SX limb, accompanied by decreased skeletal muscle integrity (Trend). This highlights the impact of MuIS+ status emphasizing the potential value of perioperative MuIS assessment to inform optimal postsurgical care.
NEW & NOTEWORTHY This study assessed the skeletal muscle molecular characteristics associated with end-stage osteoarthritis and refined an important phenotype, in some patients, termed muscle inflammation susceptibility (MuIS+) that may be an important consideration following surgery. Furthermore, we provide evidence of differential inflammatory and catabolic gene expression between the contralateral and surgical limbs along with differences between the skeletal muscle surrounding the diseased hip versus knee joints.
INTRODUCTION
Osteoarthritis (OA) is the most predominant form of arthritis (1), with knee and hip OA ranked 11th among chronic conditions that lead to disability worldwide (2). Although commonly described as a degenerative joint disease, OA presents as an inflammatory condition pervasive in the joint along with periarticular structures (3). When OA progresses to end-stage, patients often pursue total knee or hip arthroplasty (TKA/THA), which is an effective means to improve quality of life in many patients (4, 5). However, ∼30% are unable to overcome the physiological burden of OA and the additive trauma of surgery resulting in long-term mobility impairments postsurgery, possibly stemming from musculoskeletal deconditioning at the time of surgery (6, 7). Currently, the field lacks mechanistic insights to comprehensively describe factors driving poor post-arthroplasty outcomes, which limits effective postsurgical rehabilitation.
Although the mechanisms behind the heterogeneity in recovery following joint arthroplasty have not fully been elucidated, our laboratory has previously demonstrated that, at the time of hip arthroplasty, some patients exhibit an overt proinflammatory state within the skeletal muscle surrounding the diseased joint (8). We have termed this hyperinflammatory state, “muscle inflammation susceptibility” (MuIS+) because it is distinct from systemic inflammation. MuIS+ may limit muscle recovery potential through aberrant signaling via proinflammatory receptors such as tumor necrosis factor α (TNF-α) receptor (aka TNF-αR), interleukin-6 receptor (IL-6R), and fibroblast growth factor-inducible 14 (Fn14) (8, 9). Specifically, our previous cohort of patients with THA dichotomized as MuIS+ based on skeletal muscle Fn14 gene expression presented with approximately threefold and sixfold greater gene expression of TNF-αR and IL-6R respectively, in the muscle surrounding the diseased joint compared with the MuIS− counterparts (8). Excessive inflammation, often characterized by robust expression of TNF-α/TNF-αR, IL-6/IL-6R, and/or TNF-like weak inducer of apoptosis (TWEAK)/Fn14 (8–11), is associated with skeletal muscle atrophy (12–16) and impairments in physiological functioning (9, 17, 18). Our laboratory has illustrated heightened muscle inflammation in atrophic conditions including burn injury (19, 20), spinal cord injury (21), and attenuation of myogenesis in aging (9). In addition, recent work has shown greater skeletal muscle fibrosis as OA progresses (22). Fibrosis results from excessive production of extracellular matrix, which provides support to skeletal muscle under normal conditions, but in excess, minimizes skeletal muscle integrity and regenerative capacity (23).
The negative effect of muscle inflammation appears multifaceted, as anabolic signaling decreases (20) and atrogenes increase (9). The inflammatory microenvironment within skeletal muscle involves a coordinated series of signaling cascades that may contribute to poor rehabilitative outcomes in MuIS+ individuals. For instance, the transcription factor nuclear factor κ-light-chain enhancer of activated B cells (NFκB) is activated by a number of cytokine and receptors binding such as TWEAK/Fn14 and TNF-α/TNF-αR (10, 24–26). Specifically, TNF-α binding to TNF-αR degrades cytoplasmic IκB enabling nuclear translocation of the NFκB heterodimer (24, 25). NFκB is associated with muscle fibrosis (27–29) and promotes ubiquitin-proteasome activity via the E3 ligases FBXO32 (muscle atrophy F-box, MAFbx) and TRIM63 (muscle ring finger-1, MuRF1) (15, 29–32) exacerbating loss of muscle integrity.
This present work focused on MuIS+ status aims to advance the understanding of its impact on gene expression, muscle integrity, and function in end-stage osteoarthritis at the time of joint arthroplasty surgery. We hypothesize that patients dichotomized into the MuIS+ group will exhibit higher localized inflammatory gene expression and lower skeletal muscle integrity compared with their MuIS− counterparts. These data provide novel insight and highlight potential pathways for focused interventions.
METHODS
Human Participants
Seventy adults between the ages of 40 and 80 yr were recruited to the University of Alabama at Birmingham (UAB) or University of Arkansas for Medical Sciences (UAMS) to participate in the parent clinical trial (NCT02628795). Eligible participants were individuals undergoing their first knee or hip replacement on the given joint. Participants were excluded if they were undergoing bilateral joint replacement, had a history of alcoholism or liver disease, coagulation disorders, pregnant or lactating, unable to participate in exercise training, any uncontrolled cardiometabolic disease, currently using androgen or anabolic agents, allergic to lidocaine, history of resistance training in the past year, or any other condition or event deemed exclusionary by the principal investigators or physician co-investigators. Participants provided written informed consent for all biospecimens to be used in future studies. The parent clinical trial was reviewed and approved by the UAB and UAMS Institutional Review Boards and operated in accordance with the Declaration of Helsinki.
Skeletal Muscle Biopsy and Tissue Preparation
All tissues were collected in the fasting state. Tissue collection time of day was determined by the scheduling of the joint replacement surgery. For the nonsurgical limb (contralateral, CTRL), skeletal muscle biopsy samples were obtained from the vastus lateralis using a 5-mm Bergström needle with suction, as previously described (33) immediately before the onset of the first surgical incision. Biopsy samples from the surgical limb (SX) were obtained using a scalpel from the skeletal muscle surrounding the diseased hip (gluteus maximus or tensor fascia latae) for THA (n = 22) or vastus medialis for TKA (n = 48) during joint replacement surgery. Biopsies were immediately processed to remove excess connective tissue, fat, and blood. A portion of skeletal muscle intended for gene expression analyses (∼20 mg) was immediately snap frozen in liquid nitrogen (LN2). A portion of the muscle biopsy (∼50 mg) was prepared for immunohistochemistry by aligning the fiber bundles cross sectionally in a mounting medium comprising tragacanth gum mixed with Tissue Tek O.C.T. compound (Sakura Finetek, Torrance, CA), and frozen in isopentane cooled in LN2. All samples were stored at −80°C until downstream analyses.
Targeted Skeletal Muscle Gene Expression
Skeletal muscle (∼20 mg) was homogenized, twice for 20 s at 4.2 m/s, in a Bead Ruptor Elite bead mill homogenizer (Omni International, Kennesaw, GA). Total RNA was extracted using miRNeasy Mini kits (Qiagen, Valencia, CA). RNA was quantified using spectrophotometry (NanoDrop ND-1000, Thermo Scientific, Rockford, IL), and quality was assessed for a subset of samples via an Agilent 2100 Bioanalyzer (Santa Clara, CA) (n = 54; CTRL RIN = 7.60 ± 1.33, SX RIN = 7.28 ± 1.50). Using concentrations from the spectrophotometer, cDNA was synthesized with 1 μg of RNA using the SuperScript VILO cDNA synthesis kit (Invitrogen, Carlsbad, CA). Within skeletal muscle, eight genes were assessed for expression using TaqMan PCR: TNF-α (Hs00174128_m1), TNF receptor 1A (TNF-αR, Hs00533560_m1), TNF-like weak inducer of apoptosis (TWEAK, Hs00356411_m1), TWEAK receptor (Fn14, Hs0017993_m1), interleukin-6 (IL-6, Hs00985639_m1), IL-6 receptor (IL-6R, Hs00794121_m1), FBXO32 (MAFbx, Hs01041408_m1), and TRIM63 (MuRF1, Hs00822397_m1). The arithmetic mean of glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Hs02758991_g1) and chromosome-1 open reading frame 43 (CH1ORF43, Hs01555103_ g1) served as internal controls as represented in Table 1 for all comparisons. All samples were run in triplicate using the Quantstudio 3 (Applied Biosystems, StepOne software v. 2.2.2). Fold differences within the participant’s CTRL and SX limbs were calculated using the 2−DDCT method (34) with the expression of each gene on the CTRL limb set to a value of 1 (graphically represented as a dotted line in accompanying figure). All between-subject comparisons (TKA vs. THA, MuIS+ vs. MuIS−, and male vs. female) gene expression was determined using the arbitrary units 2−DCT method (35).
Table 1.
Average housekeeping gene expression for each transcript and comparison
| Transcripts (CT) | ||||||||
|---|---|---|---|---|---|---|---|---|
| TNF-αR | Fn14 | IL-6R | TNF-α | TWEAK | IL-6 | TRIM63 | FBXO32 | |
| CTRL | 22.46 ± (0.68) | 22.40 ± (0.63) | 22.42 ± (0.64) | 22.45 ± (0.67) | 22.19 ± (0.67) | 22.43 ± (0.74) | 22.03 ± (0.64) | 22.11 ± (0.66) |
| SX | 22.55 ± (0.83) | 22.49 ± (0.84) | 22.53 ± (0.88) | 22.49 ± (0.86) | 22.25 ± (0.92) | 22.46 ± (0.89) | 22.13 ± (0.84) | 22.18 ± (0.84) |
| SX Transcripts (CT) | TKA vs. THA | MuIS− vs. MuIS+ | Female vs. Male |
|---|---|---|---|
| TNF-αR | 22.47 ± (0.59); 22.72 ± (2.21) | 22.47 ± (0.91); 22.70 ± (0.64) | 22.54 ± (0.57); 22.57 ± (1.17) |
| Fn14 | 22.37 ± (0.62); 22.75 ± (1.17) | 22.37 ± (0.87); 22.72 ± (0.73) | 22.47 ± (0.57); 22.52 ± (1.18) |
| IL-6R | 22.41 ± (0.58); 22.78 ± (1.30) | 22.32 ± (0.90); 22.91 ± (0.71) | 22.52 ± (0.57); 22.53 ± (1.26) |
| TNF-α | 22.36 ± (0.63); 22.78 ± (1.18) | 22.35 ± (0.89); 22.76 ± (0.74) | 22.52 ± (0.62); 22.44 ± (1.17) |
| TWEAK | 22.05 ± (0.73); 22.69 ± (1.13) | 22.00 ± (0.90); 22.72 ± (0.76) | 22.26 ± (0.76); 22.24 ± (1.15) |
| IL-6 | 22.29 ± (0.64); 22.83 ± (1.21) | 22.31 ± (0.89); 22.74 ± (0.84) | 22.43 ± (0.65); 22.49 ± (1.21) |
| TRIM63 | 22.05 ± (0.57); 22.31 ± (1.24) | 22.12 ± (0.91); 22.16 ± (0.71) | 22.11 ± (0.57); 22.17 ± (1.18) |
| FBXO32 | 22.08 ± (0.57); 22.39 ± (1.23) | 22.18 ± (0.90); 22.16 ± (0.71) | 22.17 ± (0.56); 22.18 ± (1.18) |
Values are means ± (SD). Housekeeping genes = GAPDH and C1orf43. Sample size: contralateral (CTRL)/surgical (SX) limbs = 70; total hip arthroplasty (THA) = 22; total knee arthroplasty (TKA) = 48; muscle inflammation susceptibility (MuIS)− = 46; MuIS+ = 24; female = 44; male = 26. FBXO32, muscle atrophy F-box (MAFbx); Fn14, fibroblast growth factor-inducible 14; IL-6, interleukin-6; IL-6R, interleukin-6 receptor; TNF-α, tumor necrosis factor alpha; TNF-αR, tumor necrosis factor alpha receptor; TRIM63, muscle ring finger-1 (MuRF1); TWEAK, TNF-like weak inducer of apoptosis.
MuIS Determination
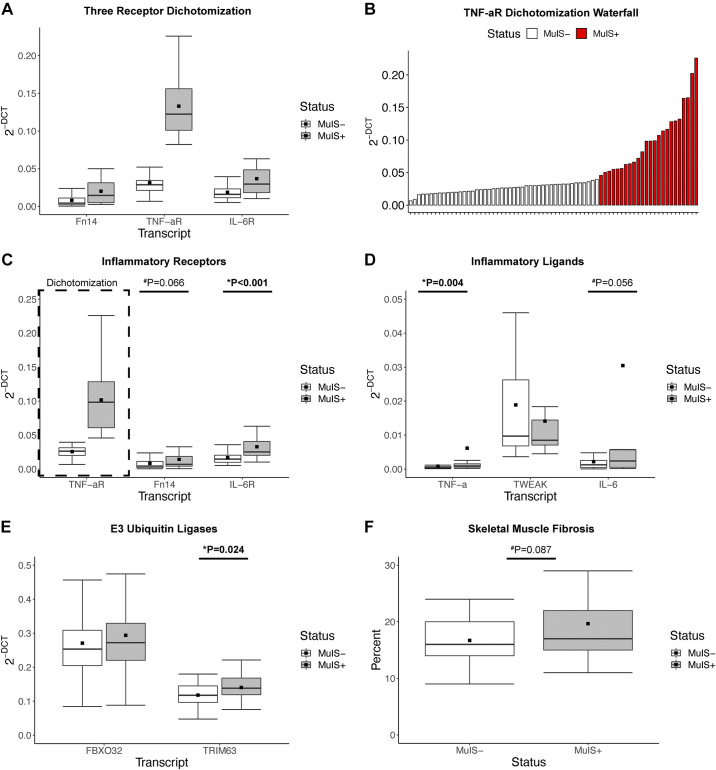
In this cohort of patients with TKA or THA, we initially dichotomized on three inflammatory receptor transcripts vital to skeletal muscle signaling (10, 12, 26) (TNF-αR, Fn14, and IL-6R) using an unbiased clustering algorithm (K-means). TNF-αR expression was the dominant contributor to cluster assignment (Fig. 3A). Therefore, we defined MuIS status on TNF-αR expression as opposed to Fn14 used in our previous work (8). Briefly, MuIS status (either positive: MuIS+, or negative: MuIS−) was determined based on TNF-αR gene expression in skeletal muscle of the SX limb. Participants were assigned to groups using K-means clustering, as previously performed by our laboratory for grouping of complex physiological phenomena (36). NbClust (R v. 4.0.3, package v. 3.0) was used to determine the most appropriate number of centers based on the distribution of the data. Based on the results of the NbClust algorithm, K-means was run with a seed set at 100 and the number of centers at 4. The cluster with the lowest level of TNF-αR expression was deemed MuIS−, whereas the remaining participants with higher expression were classified as MuIS+ (Fig. 3B).
Systemic Inflammation
Circulating cytokines (IFN-γ, IL-10, IL-6, IL-8, TNF-α, IL-1β, and IL-12P70) were assessed in 64 participants using the MesoScale Discovery Human Proinflammatory Panel I Kits (Rockville, MD) on serum collected at the time of surgery using standard procedures. The cytokines IL-1β and IL-12P70 were excluded from the analyses because the majority of samples failed to meet detection thresholds (IL-1β n = 60, IL-12P70 n = 37). The remaining cytokines were run in duplicate with an intra-assay coefficient of variation (CV)% of 2.46 (IFN-γ), 3.31 (IL-10), 6.60 (IL-6), 3.29 (IL-8), and 4.53 (TNF-α).
Skeletal Muscle Fibrosis
Skeletal muscle fibrosis as a proxy for muscle integrity was determined in histological specimens using wheat germ agglutinin (WGA) conjugated to Texas Red (Thermo Fisher, W21405) as we have previously described (21, 37). Briefly, 6-µm cryosections were place in a humidity chamber for 10 min at room temperature, fixed for 3 min in 1:2 acetone and methanol, washed three times for 5 min in 1× PBS, incubated in Texas Red conjugated WGA for 1 h, washed again with 1× PBS three times for 5 min, coated with mounting media, and secured with a coverslip. Slides were protected from light and stored at −20°C for no more than three days before imaging. Images were captured at ×20 using an Olympus BX51 fluorescent microscope with an Olympus XM10 camera (Olympus) and CellSens Dimensions software (Olympus). Images were analyzed for percent positive red pixel area using Image Pro Premier software (v. 9.1).
Thigh Muscle Mass
Surgical (SX) limb muscle mass was assessed by dual energy X-ray absorptiometry (DXA, Lunar Prodigy Model No. 8743, GE Lunar Corporation, Madison, WI and Hologic Discovery model W) before surgery following our validated protocol (38). Thigh muscle mass was normalized to participant’s height (cm) and analyzed using enCORE/APEX software (v. 18/13.3) according to manufacturer’s instructions.
Leg Extension Power
Knee extension power was conducted unilaterally on the surgical leg before surgery. We have previously utilized knee extension power as a valuable index of muscle performance (39). Briefly, participants completed five full unilateral knee extension repetitions, with the concentric action being completed as explosively as possible against a resistance equal to 40% maximum voluntary isometric contraction (MVIC) force (Biodex System 4 Pro, Shirley, NY, and Cybex Isokinetic System, Ronkonkoma, NY).
Leg Extension Isometric Strength
Peak unilateral knee extension MVIC strength was determined for the surgical limb before surgery. MVIC was assessed by fixing the participant’s leg at 60° of knee flexion then following two familiarization repetitions, three maximum efforts to extend the shank against a fixed dynamometer (Biodex System 4 Pro, Shirley, NY, and Cybex Isokinetic System, Ronkonkoma, NY) were completed.
Statistical Analyses
Statistical analyses were performed using R version 4.0.3 and RStudio version 1.2.5033. All data are shown as median, 25th and 75th quartiles ± 1.5 the interquartile range unless otherwise noted. The central limit theorem (40) was invoked for the CTRL versus SX comparisons and paired two-tailed t tests were used to determine differences between limbs. Normality was assessed for variables with n < 30 using the Shapiro–Wilk test and Q-Q plots (40). Variables that were non-normally distributed were Log10 transformed; if they remained non-normally distributed, the Wilcoxon rank-sum test was performed to determine statistical differences between groups. For all TKA/THA, MuIS+/MuIS−, and female/male comparisons, homogeneity of variance was tested using Levene’s tests. If the Levene’s test was significant or nonsignificant, between-subject comparisons were performed using the Welch two-tailed t test or the two-tailed Student’s t test, respectively. Pearson correlations were performed to assess associations among transcript expression, muscle integrity, and functional outcomes. For all analyses significance was declared at P < 0.05 and trends (P value between 0.05 and 0.10) are discussed. The sample size for each analysis is provided in results.
RESULTS
Participant Characteristics and Impact of End-Stage Osteoarthritis
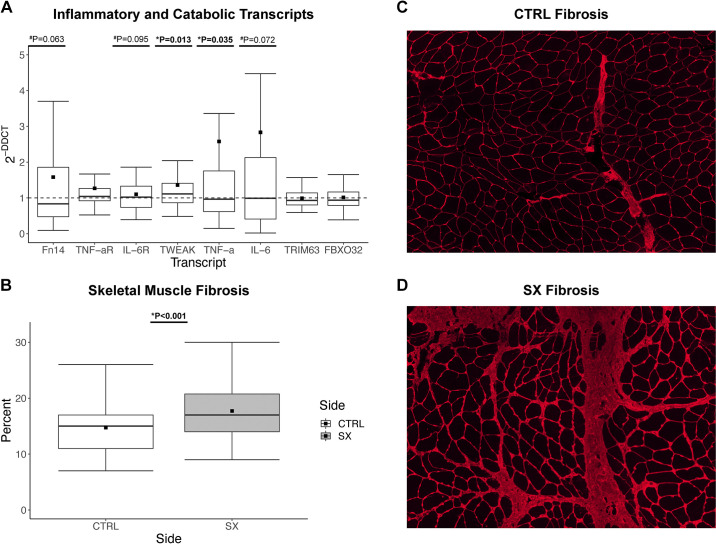
Participant characteristics are presented in Table 2. The 70 participants were predominantly female (44/70) and the majority underwent TKA (n = 48) versus THA (n = 22). On average, participants were obese in accordance with body mass index (BMI) criteria. The SX limb exhibited a greater inflammatory gene expression profile compared with the CTRL limb (dotted line) with significantly greater TWEAK and TNF-α ligand expression (P = 0.013 and P = 0.035, respectively; Fig. 1A). Fn14, IL-6R, and IL-6 trended greater in the SX limb (P = 0.063, P = 0.095, and P = 0.072 respectively; Fig. 1A). In addition, fibrosis (n = 62) was 18% greater in the SX limb (P < 0.001) (Fig. 1B; representative image of CTRL and SX fibrosis respectively Fig. 1, C and D). No other genes were significantly different between limbs. However, TWEAK (P = 0.003) and Fn14 (P < 0.001) gene expression was significantly greater and IL-6 (P = 0.07) trended greater for females compared with males (data not shown).
Table 2.
Descriptive characteristics
| Variable | n = 70 |
|---|---|
| Surgery | |
| THA | 22 |
| TKA | 48 |
| Sex | |
| F | 44 |
| M | 26 |
| Age, yr | 62.41 ± (8.86) |
| Height, m | 1.70 ± (0.11) |
| Weight, kg | 89.57 ± (16.69) |
| BMI | 31.10 ± (4.91) |
Values are means ± (SD). BMI, body mass index; THA, total hip arthroplasty; TKA, total knee arthroplasty.
Figure 1.
The influence of end-stage osteoarthritis on gene expression and skeletal muscle fibrosis. A: fold difference in expression of proinflammatory and catabolic skeletal muscle genes between contralateral (CTRL) and surgical (SX) limbs; boxplots represent SX gene expression relative to CTRL, while the –– line represents CTRL gene expression (n = 70). B: percent skeletal muscle fibrosis between the CTRL and SX limbs (n = 62). C: representative image of CTRL skeletal muscle fibrosis. D: representative image of SX skeletal muscle fibrosis. Boxplots = median (solid line), mean (square), box-ends = 1st and 3rd quartile, whiskers = ±1.5 interquartile range. *Significant P < 0.05. #Trend P < 0.1.
Influence of Knee or Hip Osteoarthritis on Skeletal Muscle Gene Expression
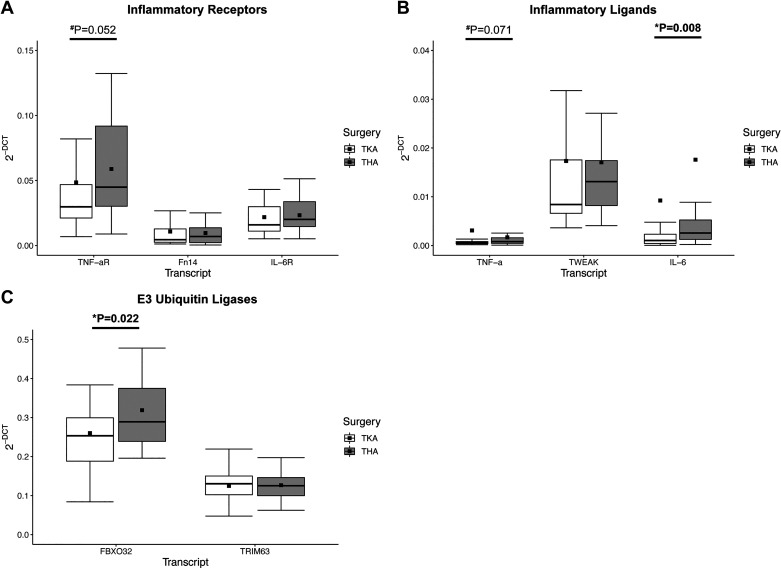
Comparing SX muscles in TKA versus THA, IL-6 ligand and FBXO32 gene expression were greater in the THA than vastus medialis TKA (1.90-fold, P = 0.008 and 1.23-fold, P = 0.022 respectively; Fig. 2, B and C). In addition, TNF-αR trended toward higher gene expression in THA versus TKA (P = 0.052; Fig. 2A), whereas TNF-α trended greater in the vastus medialis (P = 0.071; Fig. 2B). None of the remaining genes nor fibrosis were significantly different across surgery location.
Figure 2.
The influence of knee or hip osteoarthritis on inflammatory and catabolic gene expression. A: expression of proinflammatory receptors comparing TKA and THA. B: proinflammatory ligands comparing total knee arthroplasty (TKA) and total hip arthroplasty (THA). C: markers of skeletal muscle catabolism; E3 ubiquitin ligases comparing TKA and THA. Boxplots = median (solid line), mean (square), box-ends = 1st and 3rd quartile, whiskers = ±1.5 interquartile range. *Significant P < 0.05. #Trend P < 0.1. Data are representing n = 22 THA and n = 48 TKA participants.
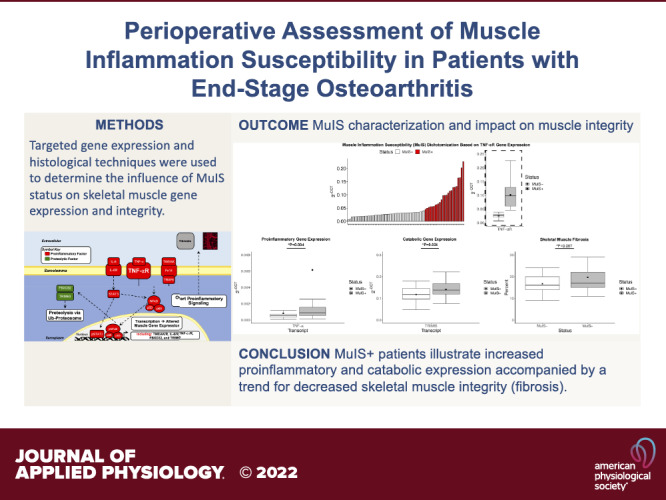
MuIS Characterization and Influence on Skeletal Muscle Integrity
Using K-means clustering on TNF-αR, 46 participants were determined to be MuIS− and 24 participants were MuIS+ (Table 3). The K-means clustering approach resulted in a fourfold difference in TNF-αR gene expression among MuIS+ versus MuIS− (Fig. 3C). Furthermore, IL-6R, TNF-α, and TRIM63 were higher in the MuIS+ group (1.96-fold P < 0.001, 7.41-fold P = 0.004, and 1.19-fold P = 0.024; Fig. 3, C–E, respectively). Strong trends (MuIS+ vs. MuIS−) were noted for the expression of Fn14 (P = 0.066, Fig. 3C) and IL-6 (P = 0.056, Fig. 3D), as well as the histological measure of skeletal muscle fibrosis (P = 0.087, MuIS− = 41 and MuIS+ = 21, Fig. 3F). As hypothesized, markers of systemic (serum) inflammation (42 MuIS− and 22 MuIS+) were not different between groups (Table 3).
Table 3.
Descriptive characteristics by MuIS status
| Variable | MuIS−, n = 46 | MuIS+, n = 24 | P value* |
|---|---|---|---|
| Surgery | |||
| THA | 11 | 11 | |
| TKA | 35 | 13 | |
| Sex | |||
| F | 28 | 16 | |
| M | 18 | 8 | |
| Age, yr | 62.61 ± (9.17) | 62.04 ± (8.43) | |
| Height, m | 1.70 ± (0.13) | 1.70 ± (0.09) | |
| Weight, kg | 88.83 ± (17.96) | 90.98 ± (14.20) | |
| BMI | 30.84 ± (5.11) | 31.60 ± (4.58) | |
| IFN-γ, pg/mL | 15.34 ± (40.64) | 8.84 ± (4.73) | 0.074 |
| IL-10, pg/mL | 0.70 ± (1.30) | 0.37 ± (0.23) | 0.461 |
| IL-6, pg/mL | 4.11 ± (12.11) | 2.47 ± (2.64) | 0.689 |
| IL-8, pg/mL | 17.89 ± (32.48) | 11.82 ± (3.21) | 0.208 |
| TNF-α, pg/mL | 3.21 ± (1.21) | 3.33 ± (1.01) | 0.721 |
Values are means ± (SD). IFN-γ, IL-10, IL-6, IL-8, and TNF-α (n = 42 MuIS−, 22 MuIS+). BMI, body mass index; MuIS, muscle inflammation susceptibility; THA, total hip arthroplasty; TKA, total knee arthroplasty.
Wilcoxon rank-sum test.
Figure 3.
The influence of muscle inflammation susceptibility (MuIS) status on gene expression and muscle integrity. A: inflammatory transcript receptor expression following initial dichotomization combining tumor necrosis factor alpha receptor (TNF-αR), fibroblast growth factor-inducible 14 (Fn14), and interleukin-6 receptor (IL-6R) that resulted in n = 56 MuIS− and n = 14 MuIS+. B: waterfall plot illustrating the inflection point between MuIS+ and MuIS− status using TNF-αR expression to cluster resulting in n = 46 MuIS− and n = 24 MuIS+ participants. C: expression of proinflammatory receptors comparing MuIS+ and MuIS− status, as dichotomized based on TNF-αR expression. D: proinflammatory ligands comparing MuIS+ and MuIS− status. E: markers of skeletal muscle catabolism; E3 ubiquitin ligases comparing MuIS+ and MuIS− status. F: percent skeletal muscle fibrosis across MuIS+ and MuIS− status. Boxplots = median (solid line), mean (square), box-ends = 1st and 3rd quartile, whiskers = ±1.5 interquartile range. *Significant P < 0.05. #Trend P < 0.1.
Association of Proinflammatory Receptor Gene Expression with Muscle Integrity and Function
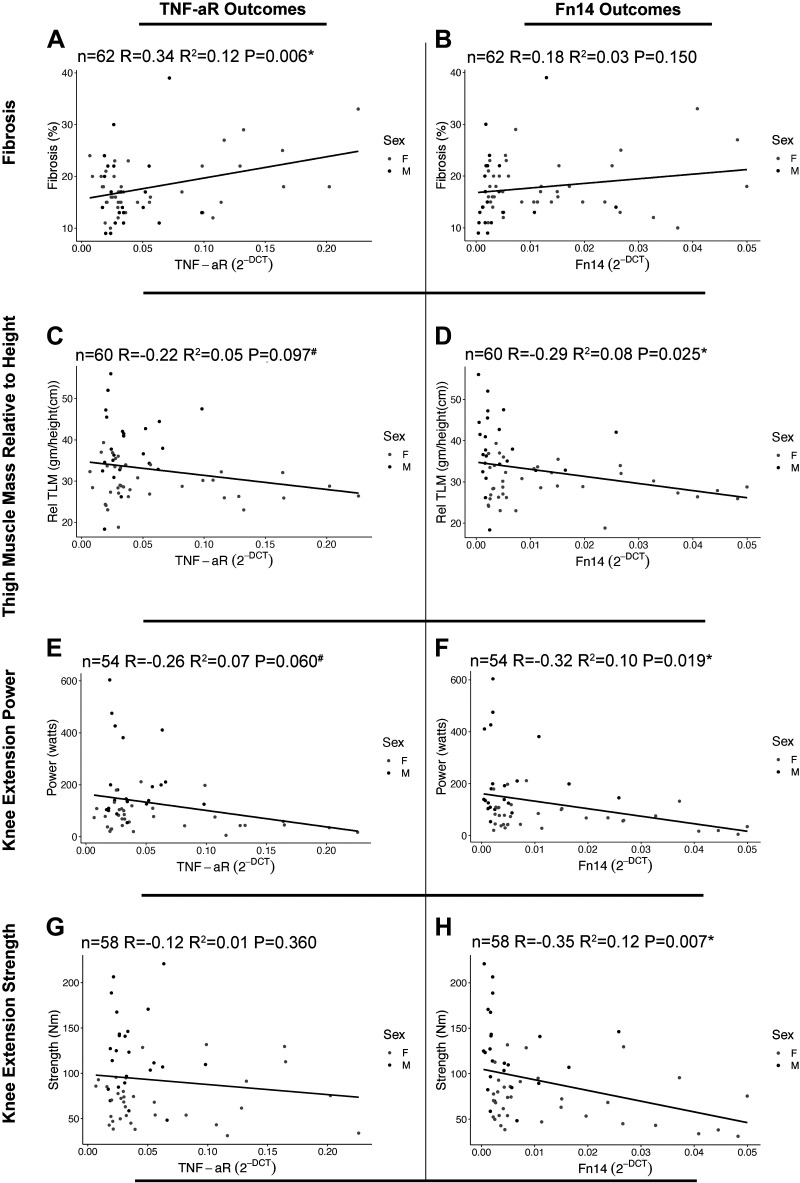
TNF-αR gene expression possessed a significant positive association with skeletal muscle fibrosis (R = 0.34, P = 0.006; Fig. 4A). For TNF-αR, negative associations trended with relative thigh muscle mass (R = −0.22, P = 0.097, Fig. 4C) and knee extension power (R = −0.26, P = 0.060, Fig. 4E), but not knee extension strength (R = −0.12, P = 0.360, Fig. 4G). Contrarily, Fn14 gene expression was significantly associated with relative thigh muscle mass (R = −0.29, P = 0.025, Fig. 4D), knee extension power (R = −0.32, P = 0.019, Fig. 4F), and knee extension strength (R = −0.35, P = 0.007, Fig. 4H), but not skeletal muscle fibrosis (R = 0.18, P = 0.150, Fig. 4B).
Figure 4.
Association of proinflammatory receptor gene expression with skeletal muscle integrity and function. A: surgical (SX) tumor necrosis factor alpha receptor (TNF-αR) gene expression associated with fibrosis. B: SX fibroblast growth factor-inducible 14 (Fn14) gene expression associated with fibrosis. C: SX TNF-αR gene expression associated with thigh muscle mass relative to height. D: SX Fn14 gene expression associated with thigh muscle mass relative to height. E: SX TNF-αR gene expression associated with knee extension power. F: SX Fn14 gene expression associated with knee extension power. G: SX TNF-αR gene expression associated with knee extension strength. H: SX Fn14 gene expression associated with knee extension strength. *Significant P < 0.05. #Trend P < 0.1. Data are representing n = 62 (A and B), n = 60 (C and D), n = 54 (E and F), n = 58 (G and H).
DISCUSSION
Through application of targeted transcript analysis, histological fibrosis assessment, and serum cytokine profiling, we interrogated differences between surgical and contralateral limbs, site of osteoarthritis, and refined MuIS+ dichotomization to advance our understanding of MuIS+ status (8). Furthermore, we tested the potential effects of biological sex, and also evaluated potential associations between transcript receptor expression and muscle integrity/functional outcomes perioperatively. These novel findings set the stage for follow-up analyses which we are currently undertaking to determine if MuIS+ status attenuates recovery postsurgery and highlight the need for targeted interventions to overcome this overt proinflammatory state.
Influence of Disease on Skeletal Muscle Inflammation and Integrity
The within-subjects model used in this study is powerful, yet uncommon in human molecular skeletal muscle OA research. We therefore must describe our findings in relation to other studies comparing OA to healthy controls. The importance of inflammation in OA progression has been described (41), and previous work has shown patients with OA possess greater inflammation, fibrotic signaling, and histologically assessed fibrosis as OA increases in severity compared with healthy controls (22, 42). Our findings corroborate these data as TWEAK and TNF-α ligands are significantly upregulated in muscle surrounding the OA joint that is accompanied by more fibrosis compared with the contralateral limb. With the growing number of patients electing to undergo TKA/THA (5), and the preponderance of individuals continuing to have lasting movement dysfunction postsurgery (6), these data suggest poor recovery may be in-part due to inflammation and fibrosis. However, an acknowledged limitation is that patients will often pursue a subsequent joint replacement on the CTRL (43) limb; meaning, we cannot eliminate the possible influence of OA pathology on the CTRL limb in our comparisons.
Influence of Knee or Hip Osteoarthritis on Inflammatory and Catabolic Gene Expression
With regional differences associated with knee or hip OA, and the surrounding muscles differing in both size and function, we explored potential differences in gene expression between the affected periarticular muscles. During TKA a tourniquet may be used to minimize blood loss, but this approach has been shown to impact catabolic signaling via hypoxic stress (44, 45). Specifically, both FBXO32 and TRIM63 are significantly upregulated from presurgery to the height of ischemia in the vastus lateralis (44). However, the muscle specimens collected during TKA, from this present work, were either isolated without a tourniquet or within 30 s of tourniquet application precluding deleterious hypoxic signaling. In addition, our data illustrate significantly greater IL-6 and FBXO32 expression in the patients undergoing THA, a surgery that does not use tourniquets, and therefore should not have ischemia-associated atrophy signaling (46, 47). Alternatively, these data may be due to differential loading at the hip versus knee during daily living before surgery. In healthy conditions, the vasti musculature endures greater loading than the musculature surrounding the hip during walking (48). In addition, in older adults performing tasks of daily living such as walking or descending stairs, the compressive forces observed at the knee versus the hip were greater (49). Therefore, the knee joint may be under greater load than the hip while performing activities such as walking during end-stage OA. In conjunction with inflammation, unloading appears to influence muscle atrophy in OA (50), suggesting the greater FBXO32 expression may reflect a relatively lower loading profile surrounding the hip.
Influence of MuIS Status on Gene Expression and Muscle Integrity
Our laboratory’s previous work interrogating the impact of MuIS highlighted the potential impact of inflammation on the regenerative capacity of skeletal muscle (9). Specifically, MuIS+ status in patients with end-stage hip OA was associated with higher inflammatory and catabolic gene expression in the skeletal muscle surrounding the diseased joint leading to lower rates of protein synthesis (8). The current results expand upon those data verifying that in a more robust sample size, MuIS+ status does in fact promote greater inflammatory and catabolic gene expression in the periarticular skeletal muscle. Our hypothesis that the MuIS+ phenotype is a localized inflammatory phenotype was confirmed through the lack of differences observed in the serum cytokine panel across groups. Furthermore, although not significant, a strong trend toward greater fibrosis was noted in MuIS+ compared with MuIS−. As a whole, these data suggest that MuIS+ status may be an important consideration at the time of surgery, but we acknowledge limitations including focusing on a limited inflammatory profile, and factors such as disease duration being unknown. It has been suggested that to better understand the breadth of perturbed signaling surrounding a diseased joint, carefully designed untargeted “omics” studies are necessary (51). This is a valuable future pursuit, and the present study findings provide a foundation for continuing to develop an understanding of the molecular environment associated with MuIS+ status and how it affects postsurgical rehabilitation prognosis.
Association of Proinflammatory Receptor Gene Expression with Skeletal Muscle Integrity and Function
Correlations were performed to illustrate the potential impact of inflammatory gene receptors TNF-αR and Fn14 on markers of muscle integrity and function. Although only TNF-αR was significantly associated with fibrosis and Fn14 with thigh muscle mass, knee extension power, and knee extension strength, the trend lines depict similar directionality with differing magnitudes. The similarity in these data are insightful, yet expected as TNF-αR and Fn14 both act on NFκB (10, 24–26) that promotes fibrosis and reduces muscle integrity (15, 28–31, 52). Ultimately, it appears that while in this cohort TNF-αR expression is the most robust, other inflammatory receptors such as Fn14 may cooperatively signal to drive these observed phenotypes.
Conclusions
This study provides novel molecular and histological phenotyping of skeletal muscle from surgical versus contralateral limbs in people with end-stage OA. Our findings highlight the divergence in skeletal muscle gene expression between the surgical and contralateral limbs and between sites of OA (knee vs. hip). In addition, these data expand upon our work emphasizing that some patients with end-stage OA possess greater dysregulated skeletal muscle surrounding the diseased joint (8). Overall, the findings provide new insight into the MuIS+ phenotype. We suggest patients deemed MuIS+ will likely require a robust rehabilitation protocol to overcome the resultant MuIS+ sequelae.
GRANTS
This study was supported by HHS|NIH|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under Grants R01HD084124, P2CHD086851, and T32HD071866 via the National Center for Medical Rehabilitation Research (NCMRR).
DISCLOSURES
J. A. Singh has received consultant fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two laboratories Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health and the American College of Rheumatology. J. A. Singh owns stock options in TPT Global Tech, Vaxart pharmaceuticals, and Charlotte’s Web Holdings, Inc. J. A. Singh previously owned stock options in Amarin, Viking, and Moderna pharmaceuticals. J. A. Singh is on the speaker’s bureau of Simply Speaking. J. A. Singh is a member of the executive of Outcomes Measures in Rheumatology (OMERACT), an organization that develops outcome measures in rheumatology and receives arms-length funding from eight companies. J. A. Singh serves on the Food and Drug Administration Arthritis Advisory Committee. J. A. Singh is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. J. A. Singh is the editor and the Director of the University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. J. A. Singh previously served as a member of the following committees: member, the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee and the co-chair of the ACR Criteria and Response Criteria subcommittee. None of the other authors have any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
D.J.D., I.A., J.A.S., A.A.F., S.L.B., and M.M.B. conceived and designed research; D.J.D., R.S., D.W., G.T., S.Y., C.K., S.C.T., B.P., H.S., E.G., S.S., and C.L.B. performed experiments; D.J.D., J.S.M., and K.M.L. analyzed data; D.J.D., J.S.M., K.M.L., D.W., and M.M.B. interpreted results of experiments; D.J.D. and K.M.L. prepared figures; D.J.D. drafted manuscript; D.J.D., J.S.M., R.S., I.A., K.M.L., D.W., G.T., S.Y., C.K., S.C.T., B.P., H.S., E.G., J.A.S., S.S., C.L.B., A.A.F., S.L.B., and M.M.B. edited and revised manuscript; D.J.D., J.S.M., R.S., I.A., K.M.L., D.W., G.T., S.Y., C.K., S.C.T., B.P., H.S., E.G., J.A.S., S.S., C.L.B., A.A.F., S.L.B., and M.M.B. approved final version of manuscript.
REFERENCES
- 1.Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med 59: 333–339, 2016. doi: 10.1016/j.rehab.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Mobasheri A, Saarakkala S, Finnilä M, Karsdal MA, Bay-Jensen A-C, van Spil WE. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 8: 2091, 2019. doi: 10.12688/f1000research.20575.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamman MM, Wick TM, Carmona-Moran CA, Bridges SL Jr.. Exercise medicine for osteoarthritis: research strategies to maximize effectiveness. Arthritis Care Res (Hoboken) 68: 288–291, 2016. doi: 10.1002/acr.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster J-Y. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 86: 963–974, 2004. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020–2040 using the National Inpatient Sample. J Rheumatol 46: 1134–1140, 2019. doi: 10.3899/jrheum.170990. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA, Lewallen DG. Predictors of activity limitation and dependence on walking aids after primary total hip arthroplasty. J Am Geriatr Soc 58: 2387–2393, 2010. doi: 10.1111/j.1532-5415.2010.03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilton ME, Gioe T, Noorbaloochi S, Singh JA. Increasing comorbidity is associated with worsening physical function and pain after primary total knee arthroplasty. BMC Musculoskelet Disord 17: 421, 2016. doi: 10.1186/s12891-016-1261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamman MM, Ferrando AA, Evans RP, Stec MJ, Kelly NA, Gruenwald JM, Corrick KL, Trump JR, Singh JA. Muscle inflammation susceptibility: a prognostic index of recovery potential after hip arthroplasty? Am J Physiol Endocrinol Physiol 308: E670–E679, 2015. doi: 10.1152/ajpendo.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Tuggle SC, Kosek DJ, Kim J, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Liu B, Liang C, Li Y, Song Y-H. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol Metab 27: 335–347, 2016. doi: 10.1016/j.tem.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Llovera M, García-Martínez C, López-Soriano J, Agell N, López-Soriano FJ, Garcia I, Argilés JM. Protein turnover in skeletal muscle of tumour-bearing transgenic mice overexpressing the soluble TNF receptor-1. Cancer Lett 130: 19–27, 1998. doi: 10.1016/S0304-3835(98)00137-2. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98: 911–917, 2005. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Bhatnagar S, Paul PK. TWEAK and TRAF6 regulate skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 15: 233–239, 2012. doi: 10.1097/MCO.0b013e328351c3fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham ZA, Lavin KM, O'Bryan SM, Thalacker-Mercer AE, Buford TW, Ford KM, Broderick TJ, Bamman MM. Mechanisms of exercise as a preventative measure to muscle wasting. Am J Physiol Cell Physiol 321: C40–C57, 2021. doi: 10.1152/ajpcell.00056.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J 21: 1857–1869, 2007. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y-P, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factorα. FASEB J 12: 871–880, 1998. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Ogura Y, Mishra V, Shin J, Bhatnagar S, Hill BG, Kumar A. TWEAK promotes exercise intolerance by decreasing skeletal muscle oxidative phosphorylation capacity. Skelet Muscle 3: 18, 2013. doi: 10.1186/2044-5040-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato S, Ogura Y, Kumar A. TWEAK/Fn14 signaling axis mediates skeletal muscle atrophy and metabolic dysfunction. Front Immunol 5: 18, 2014. doi: 10.3389/fimmu.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merritt EK, Cross JM, Bamman MM. Inflammatory and protein metabolism signaling responses in human skeletal muscle following burn injury. J Burn Care Res 33: 291–297, 2012. doi: 10.1097/BCR.0b013e3182331e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrick KL, Stec MJ, Merritt EK, Windham ST, Thomas SJ, Cross JM, Bamman MM. Serum from human burn victims impairs myogenesis and protein synthesis in primary myoblasts. Front Physiol 6: 184, 2015. doi: 10.3389/fphys.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarar-Fisher C, Bickel CS, Kelly NA, Stec MJ, Windham ST, McLain AB, Oster RA, Bamman MM. Heightened TWEAK-NF-κB signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am J Physiol Endocrinol Physiol 310: E754–E761, 2016. doi: 10.1152/ajpendo.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noehren B, Kosmac K, Walton RG, Murach KA, Lyles MF, Loeser RF, Peterson CA, Messier SP. Alterations in quadriceps muscle cellular and molecular properties in adults with moderate knee osteoarthritis. Osteoarthritis Cartilage 26: 1359–1368, 2018. doi: 10.1016/j.joca.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahdy MAA. Skeletal muscle fibrosis: an overview. Cell Tissue Res 375: 575–588, 2019. doi: 10.1007/s00441-018-2955-2. [DOI] [PubMed] [Google Scholar]
- 24.Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, McDermott MF. Tumour necrosis factor signalling in health and disease. F1000Res 8: 111, 2019. doi: 10.12688/f1000research.17023.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol 26: 253–266, 2014. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatnagar S, Kumar A. The TWEAK-Fn14 system: breaking the silence of cytokine-induced skeletal muscle wasting. Curr Mol Med 12: 3–13, 2012. doi: 10.2174/156652412798376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Reay DP, Salay MN, Mi MY, Clemens PR, Guttridge DC, Robbins PD, Huard J, Wang B. Inhibition of the IKK/NF-κB pathway by AAV gene transfer improves muscle regeneration in older mdx mice. Gene Ther 17: 1476–1483, 2010. doi: 10.1038/gt.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourkioti F, Kratsios P, Luedde T, Song Y-H, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest 116: 2945–2954, 2006. [Erratum in J Clin Invest 117: 277, 2007]. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 86: 1113–1126, 2008. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Physiol 307: E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peris-Moreno D, Taillandier D, Polge C. MuRF1/TRIM63, master regulator of muscle mass. Int J Mol Sci 21: 6663, 2020. doi: 10.3390/ijms21186663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu C-L, Cornwell EW, Jackman RW, Kandarian SC. NF-κB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am J Physiol Cell Physiol 306: C762–C767, 2014. doi: 10.1152/ajpcell.00361.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stec MJ, Thalacker-Mercer A, Mayhew DL, Kelly NA, Tuggle SC, Merritt EK, Brown CJ, Windham ST, Dell’Italia LJ, Bickel CS, Roberts BM, Vaughn KM, Isakova-Donahue I, Many GM, Bamman MM. Randomized, four-arm, dose-response clinical trial to optimize resistance exercise training for older adults with age-related muscle atrophy. Exp Gerontol 99: 98–109, 2017. doi: 10.1016/j.exger.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 128: 87–99, 2020. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamman MM, Petrella JK, Kim J, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 102: 2232–2239, 2007. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 37.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 116: 582–592, 2014. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bamman MM, Ragan RC, Kim J-S, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol (1985) 97: 1329–1337, 2004. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- 39.Petrella JK, Kim J, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol (1985) 98: 211–220, 2005. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- 40.Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10: 486–489, 2012. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12: 580–592, 2016. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, McKenna MJ, Cameron-Smith D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum 63: 1343–1348, 2011. doi: 10.1002/art.30287. [DOI] [PubMed] [Google Scholar]
- 43.Sanders TL, Maradit Kremers H, Schleck CD, Larson DR, Berry DJ. Subsequent total joint arthroplasty after primary total knee or hip arthroplasty. J Bone Joint Surg 99: 396–401, 2017. doi: 10.2106/JBJS.16.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey AN, Hocker AD, Vermillion BR, Smolkowski K, Shah SN, Jewett BA, Dreyer HC. MAFbx, MuRF1, and the stress-activated protein kinases are upregulated in muscle cells during total knee arthroplasty. Am J Physiol Regul Integr Comp Physiol 303: R376–R386, 2012. doi: 10.1152/ajpregu.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreyer HC. Tourniquet use during knee replacement surgery may contribute to muscle atrophy in older adults. Exerc Sport Sci Rev 44: 61–70, 2016. doi: 10.1249/JES.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Han SB. Patient blood management in hip replacement arthroplasty. Hip Pelvis 27: 201–208, 2015. doi: 10.5371/hp.2015.27.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petis S, Howard JL, Lanting BL, Vasarhelyi EM. Surgical approach in primary total hip arthroplasty: anatomy, technique and clinical outcomes. Can J Surg 58: 128–139, 2015. doi: 10.1503/cjs.007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki K, Neptune RR. Individual muscle contributions to the axial knee joint contact force during normal walking. J Biomech 43: 2780–2784, 2010. doi: 10.1016/j.jbiomech.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luepongsak N, Amin S, Krebs DE, McGibbon CA, Felson D. The contribution of type of daily activity to loading across the hip and knee joints in the elderly. Osteoarthritis Cartilage 10: 353–359, 2002. doi: 10.1053/joca.2000.0511. [DOI] [PubMed] [Google Scholar]
- 50.Shorter E, Sannicandro AJ, Poulet B, Goljanek-Whysall K. Skeletal muscle wasting and its relationship with osteoarthritis: a mini-review of mechanisms and current interventions. Curr Rheumatol Rep 21: 40, 2019. doi: 10.1007/s11926-019-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henrotin Y, Sanchez C, Bay-Jensen AC, Mobasheri A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: current status and future perspectives. Ann Phys Rehabil Med 59: 145–148, 2016. doi: 10.1016/j.rehab.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Peterson JM, Wang DJ, Shettigar V, Roof SR, Canan BD, Bakkar N, Shintaku J, Gu J-M, Little SC, Ratnam NM, Londhe P, Lu L, Gaw CE, Petrosino JM, Liyanarachchi S, Wang H, Janssen PML, Davis JP, Ziolo MT, Sharma SM, Guttridge DC. NF-κB inhibition rescues cardiac function by remodeling calcium genes in a Duchenne muscular dystrophy model. Nat Commun 9: 1–14, 2018. doi: 10.1038/s41467-018-05910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]