Abstract
In addition to producing profound subjective effects following acute administration, psychedelic compounds can induce beneficial behavioral changes relevant to the treatment of neuropsychiatric disorders that last long after the compounds have been cleared from the body. One hypothesis with the potential to explain the remarkable enduring effects of psychedelics is related to their abilities to promote structural and functional neuroplasticity in the prefrontal cortex (PFC). A hallmark of many stress-related neuropsychiatric diseases—including depression, post-traumatic stress disorder (PTSD), and addiction—is the atrophy of neurons in the PFC. Psychedelics appear to be particularly effective catalysts for the growth of these key neurons, ultimately leading to restoration of synaptic connectivity in this critical brain region. Furthermore, evidence suggests that the hallucinogenic effects of psychedelics are not directly linked to their ability to promote structural and functional neuroplasticity. If we are to develop improved alternatives to psychedelics for treating neuropsychiatric diseases, we must fully characterize the molecular mechanisms that give rise to psychedelic-induced neuroplasticity. Here, I review our current understanding of the biochemical signaling pathways activated by psychedelics and related neuroplasticity-promoting molecules, with an emphasis on key unanswered questions.
Keywords: Psychedelic, psilocybin, LSD, DMT, neuroplasticity, spinogenesis, synaptogenesis, dendritogenesis, mTOR, TrkB, BDNF, psychLight, TBG
Graphical Abstract
Increasing preclinical1,2,3,4,5,6,7 and clinical8,9,10,11,12,13 evidence suggests that psychedelics produce therapeutic effects relevant to treating neuropsychiatric diseases like depression, PTSD, and substance use disorder (SUD). 14,15,16,17,18,19,20 Moreover, these effects exhibit rapid onset (within 24 h), occur after only a single or a few doses, and last long after the compounds have been cleared from the body. The sustained behavioral effects of psychedelics are truly remarkable and differentiate these compounds from traditional neurotherapeutics that must be administered daily. Currently, it is unclear exactly how psychedelics produce such long-lasting effects. One hypothesis is that psychedelics induce mystical-type experiences that can facilitate interactions with therapists, enable patients to gain insight into their disorders, and perhaps even enhance the placebo effect.21,22,23,24 Another non-mutually exclusive explanation involves the ability of psychedelics to promote structural and functional neuroplasticity in the prefrontal cortex (PFC) enabling pathological circuits controlling mood, fear, and reward to be repaired.25,26,27,28
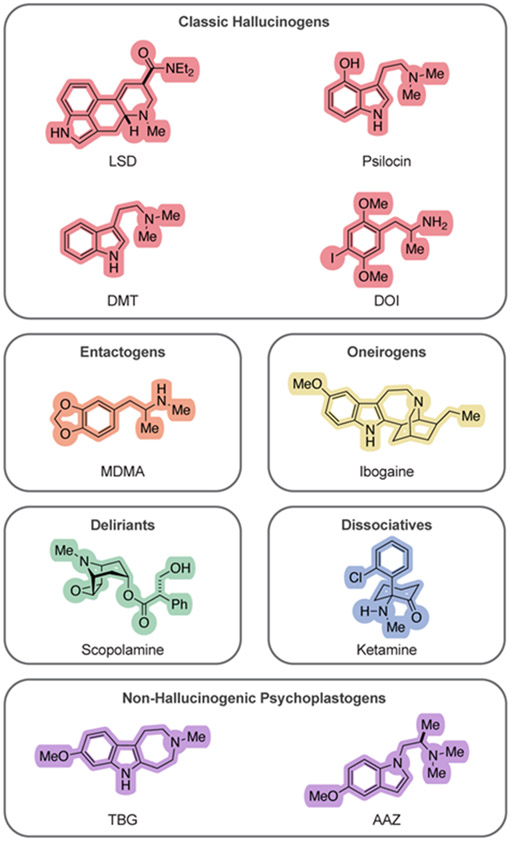
Cortical atrophy and dysfunction underlie many stress-related neuropsychiatric diseases including depression, PTSD, and SUD.29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44 Thus, compounds capable of rapidly and robustly re-growing atrophied neurons in the PFC have broad therapeutic potential. Our group has hypothesized that compound-induced cortical neuron growth might explain why psychedelics produce therapeutic effects across several distinct neuropsychiatric diseases,45 giving them the semblance of panaceas. Psychedelics belong to a broader class of compounds known as psychoplastogens (Figure 1),46 and unlike other small molecules capable of promoting induced plasticity (iPlasticity)47 such as fluoxetine, psychoplastogens produce robust, and lasting changes in cortical neuron growth following a single administration. The list of known psychoplastogens continues to grow and includes classic serotonergic hallucinogens such as lysergic acid diethylamide (LSD), psilocin, N,N-dimethyltryptmaine (DMT), and 2,5-dimethoxy-4-iodoamphetamine (DOI),6,7,48 entactogens like 3,4-methylenedioxymethamphetamine (MDMA),48 oneirogens like ibogaine,49 deliriants like scopolamine,50 and dissociatives like ketamine.51,52,53 Moreover, several non-hallucinogenic psychoplastogens, such as tabernanthalog (TBG) have recently been identified,49,54,55 suggesting that it may be possible to decouple the hallucinogenic effects of psychedelics from their sustained beneficial effects on behavior,56,57,58 though this hypothesis requires further testing in humans.
Figure 1.
Chemical structures of psychoplastogens from various pharmacological classes
Penzes and co-workers were the first to demonstrate that serotonergic psychedelics can impact structural neuroplasticity.59 Using cultured cortical neurons, they demonstrated that DOI transiently increased dendritic spine size 30 mins after treatment, but that spine size returned to baseline after an hour. Muma and co-workers later demonstrated that DOI-induced changes in spine morphology involve 5-HT2A/5-HT2C-mediated activation of transglutaminase, Rac1, and Cdc42.60 In addition to promoting changes in spine morphology, Shiga and co-workers demonstrated that DOI increases spine density in embryonic rat cortical cultures treated for 24 h.61 They also showed that activation of 5-HT2 receptors by DOI increases the size of cortical neuron dendritic growth cones in vitro.62
All of the early work studying the effects of psychedelics on structural plasticity had been performed with DOI, leaving open the possibility that the effects of DOI on neuronal structure could be an inherent property of the amphetamine scaffold rather than a general attribute of psychedelics. To address this question, our group directly compared the psychoplastogenic effects of psychedelics from the amphetamine, tryptamine, and ergoline families.48 We found that psychedelic compounds across diverse chemical space could all robustly promote neuritogenesis, spinogenesis, and synaptogenesis in rat embryonic cortical cultures,48 and that these changes can be induced by only transient stimulation (~1 h).63 Interestingly, unlike DOI, D-amphetamine was unable to promote neuritogenesis,48 demonstrating that the psychoplastogenic effects of DOI were due to its pharmacological properties rather than its core chemical structure. In vivo, D-amphetamine has been shown to promote growth in the medial PFC, though it decreases spine density in the orbital PFC and has no effect on neuronal growth in the parietal and occipital cortices.64,65,66 Moreover, these effects were observed after chronic dosing, which might yield different results than acute treatment. Like D-amphetamine, we found that serotonin did not promote the growth of cultured cortical neurons,48 suggesting that psychedelics have a unique ability to promote structural neuroplasticity.
In addition to producing psychoplastogenic effects in vitro, psychedelics also impact neuronal structure in vivo and across species (i.e, rodents and Drosophila).48 We found that a single administration of DMT to rats led to increased dendritic spine density measured in the PFC long after the compound had been cleared from the body. Moreover, this change in structural plasticity was accompanied by functional changes as well, including sustained increases in the amplitude and frequency of spontaneous excitatory postsynaptic currents (sEPSCs).48 In collaboration with Yi Zuo and co-workers, we performed two-photon imaging in live mice to demonstrate that both hallucinogenic (i.e., DOI) and non-hallucinogenic (i.e., TBG) psychoplastogens increase the rate of spine formation, but not elimination, over the course of 24 h.49 Furthermore, a single dose of TBG partially rescued dendritic spine loss induced by unpredictable mild stress and completely normalized the activity of cortical neurons.67 Following these studies, Kwan and co-workers reported that a single administration of psilocybin increases cortical spine density for at least a month in mice, with females responding more robustly than males.6 Using a recently developed PET ligand, Knudsen and co-workers demonstrated the psilocybin increases cortical density of the presynaptic marker synaptic vesicle glycoprotein 2A (SV2A).68 Taken together, these long-lasting changes in neuronal structure and function could potentially explain why psychoplastogens produce sustained behavioral effects after a single dose.
Like psychedelics, several non-serotonergic psychoplastogens, including ketamine and scopolamine, increase dendritic spine density in the PFC50,51,52 and promote dendritogenesis in cortical cultures.48,63 Recently, an elegant study by Liston and co-workers demonstrated a causal relationship between ketamine-induced spine growth in the PFC and the long-lasting antidepressant-like behavioral effects of the drug.53 While it is reasonable to hypothesize that spine growth in the PFC also underlies the long-lasting antidepressant-like effects of psychedelics in rodents, an experiment testing this hypothesis has not yet been performed. Interestingly, the effects of ketamine on spine density and antidepressant-like behavior last for approximately one week,69 while the effects of psilocybin appear to be significantly more enduring.2,6 Though all psychoplastogens appear to engage similar downstream biochemical signaling pathways leading to neuronal growth, their primary molecular targets can be distinct.26,70 For example, ketamine and scopolamine target NMDA and muscarinic receptors, respectively, while serotonergic psychedelics exert their primary effects through activation of 5-HT2A receptors.
Serotonergic psychedelics exhibit complex polypharmacology71 with many of these compounds targeting several GPCRs implicated in structural neuroplasticity including 5-HT6 and 5-HT7 receptors.72,73,74,75,76 In fact, the unique polypharmacology of psychedelics might contribute to their psychoplastogenic and/or therapeutic effects.77 However, the one commonality shared by all classic serotonergic psychedelics is high affinity for 5-HT2 receptors.78,79 There are three 5-HT2 receptor subtypes—5-HT2A, 5-HT2B, and 5-HT2C—with 5-HT2A and 5-HT2C receptors being highly expressed in the brain. The exact contributions of 5-HT2A and 5-HT2C receptors to the effects of psychedelics have yet to be fully elucidated, though increasing evidence suggest that 5-HT2A receptor activation plays a critical role in both the hallucinogenic and psychoplastogenic effects of these compounds.
Glennon and co-workers found that the affinities of psychedelics for 5-HT2 receptors correlate very well with their human hallucinogenic potencies,80 and that 5-HT2A/5-HT2C antagonists can block the discriminative stimulus properties of psychedelics in rodents, suggesting that 5-HT2B activation does not play a role in their subjective effects.81 Blocking 5-HT2 receptors in humans with the antagonist ketanserin eliminates hallucinations produced by both psilocybin82 and LSD,83,84 and the intensity of the hallucinogenic experience correlates with 5-HT2 receptor occupancy.85
Given the high sequence homology between 5-HT2A and 5-HT2C receptors, it has been challenging to identify selective pharmacological tools to disentangle their respective contributions to psychedelic-induced effects, though some evidence suggests that the 5-HT2A receptor affinities of antagonists correlate better with their abilities to block the discriminative stimulus properties of psychedelics than do their 5-HT2C receptor affinities.86 Given the selectivity issues associated with pharmacological probes, genetic tools have proven extremely valuable. Genetic knockout (KO) of 5-HT2A receptors completely abolishes psychedelic-induced head-twitch response (HTR) behavior in mice,87 while 5-HT2C receptor KO only leads to a ~50% reduction.88 Potency in the HTR assay correlates exceptionally well with human hallucinogenic potency across a wide range of psychedelic compounds.89,90
Like their hallucinogenic effects, the psychoplastogenic effects of psychedelics appear to be mediated by 5-HT2A receptors. While ketanserin completely blocks the ability of psychedelics to promote dendritogenesis, spinogenesis, and synaptogenesis in cortical cultures,48 ketanserin pretreatment only leads to a partial block of psilocybin-induced structural plasticity in vivo.6 The inability of ketanserin to completely block the effects of psilocybin in vivo is likely due to pharmacokinetic considerations, as ketanserin is known to exhibit poor brain penetration91 and only occupies ~30% of cortical 5-HT2A receptors when administered to rats at 1 mg/kg.92 Our group has found that the passive diffusion of ketanserin across non-polar membranes is surprisingly poor, as measured via a PAMPA assay (unpublished results). To avoid the issues associated with 5-HT2 antagonists, González-Maeso and co-workers recently used 5-HT2A receptor KO mice to demonstrate that these receptors are critical for the increases in spine density observed following DOI administration.7 While evidence strongly suggests that 5-HT2A receptors mediate the psychoplastogenic effects of psychedelics, it is still unclear why serotonin cannot produce similar effects on structural plasticity.48
Though the sequences of 5-HT2A receptors are very similar across species, there are several key differences between the human and rodent receptors that lead to functional differences. In humans, residue 242 is a serine, while it is an alanine in rodents. In the human receptor, this particular serine can form a hydrogen bond with certain ligands, drastically impacting their binding potencies and kinetics.93,94,95 Additionally, the rat and human 5-HT2A receptors exhibit differences in recycling and internalization, which have been linked to their divergent C-terminal sequences.96 These important differences should be taken into consideration when evaluating psychoplastogenic effects across species.
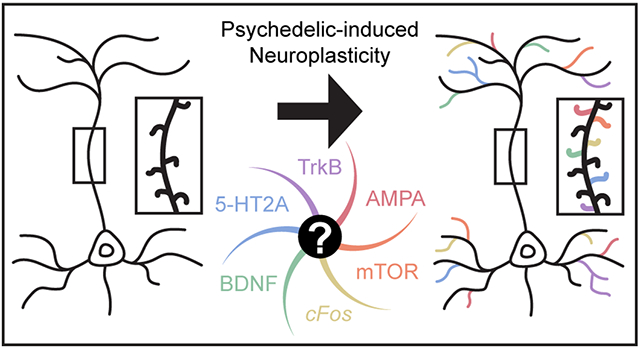
Exactly how 5-HT2A receptor stimulation leads to structural plasticity remains a mystery, though several clues have emerged. Like ketamine and scopolamine,50,51 psychedelics seem to require TrkB, AMPA receptor, and mTOR signaling to produce psychoplastogenic effects48,63,97 with mTOR being a critical downstream kinase responsible for producing plasticity-related proteins.98 Using shotgun proteomics, Rehen and co-workers found that 5-MeO-DMT modulated levels of proteins associated with structural neuroplasticity in cerebral organoids.99
The prevailing hypothesis is that both ketamine and psychedelics induce a glutamate burst100,101,102,103 leading to AMPA receptor activation and subsequent secretion of brain-derived neurotrophic factor (BDNF).104,105 Secreted BDNF then binds to TrkB resulting in mTOR activation. As mTOR activation is known to increase the production of BDNF,106 and BDNF can facilitate nonexocytotic glutamate release,107 the pathway can stay activated for some time though this autoregulatory feedback loop.63
While psychoplastogens appear to catalyze neuronal growth processes involving AMPA receptors, TrkB, and mTOR, several questions remain. Activation of AMPA receptors seems to be necessary for psychoplastogen-induced neuronal growth, but it is unclear if a large glutamate burst is essential. Psychedelic- and ketamine-induced glutamate release in the cortex has been hypothesized to result in hallucinogenic effects through increased cortical excitation.108 Given that non-hallucinogenic analogs of psychedelics can produce similar psychoplastogenic effects,49,54,55 it is unclear if a large glutamate burst is critical to turn on biochemical pathways leading to sustained neuronal growth. Moreover, several alternative mechanisms do not invoke a glutamate burst to explain the effects of ketamine on pyramidal neuron structure and function. Monteggia and co-workers have hypothesized that ketamine might promote neuronal growth through homeostatic synaptic upscaling,109,110 while Kwan and co-workers suggest that ketamine might increase pyramidal neuron excitability by blocking NMDA receptors on GABAergic neurons within cortical microcircuits.26
Several studies have demonstrated that BDNF plays a critical role in mediating the effects of ketamine and scopolamine. The antidepressant effects of ketamine are absent when the drug is administered to inducible BDNF KO mice111 or Val66Met mutant mice.112 Similarly, infusion of an anti-BDNF antibody into the PFC can block the antidepressant-like effects of scopolamine.113 While it is largely assumed that BDNF is essential to the psychoplastogenic effects of serotonergic psychedelics, similar mechanistic studies have not yet been performed.
Though a causal link between BDNF and psychedelic-induced neuroplasticity has not yet been definitively established, psychedelics do increase BDNF gene expression in the cortex, and this effect is blocked by pretreatment with a 5-HT2A receptor antagonist.114 Psychedelics also increase the expression of immediate early genes (IEGs) associated with neuroplasticity such as c-Fos, arc, egr-1, and egr-2, among others, and these increases in expression are abolished by 5-HT2A receptor antagonists or in 5-HT2A receptor KO mice.87, 115,116,117,118,119,120,121,122,123,124,125,126 Using selective inhibitors, Vaidya and co-workers found that psychedelic-induced expression of plasticity-related genes required activation of both CaMKII and MAPK pathways.127 Even though psychedelics produce profound, long-lasting changes in behavior, they induce differential expression of relatively few genes.7,128,129 Interestingly, a recent study suggests that a single administration of DOI leads to sustained epigenomic changes in the frontal cortex of mice, and that these changes were primarily found at enhancer regions of genes implicated in neuroplasticity.7 Given that the antidepressant-like effects of serotonergic psychedelics appear to be more sustained than those of ketamine,2 it would be interesting to directly compare the long-lasting epigenomic profiles of these classes of psychoplastogens.
Canonical G protein signaling pathways are believed to be responsible for some, but not all, of the gene expression changes observed after treatment with psychedelics.87,120,127 The 5-HT2A receptor typically couples to Gq,130 and thus, stimulation of 5-HT2A receptors can lead to activation of phospholipase C (PLC), the production of inositol triphosphate (IP3), and an increase in intracellular calcium.131,132,133 Psychedelics such as LSD, DOI, and 5-MeO-DMT act as partial agonists of this pathway, as do several non-hallucinogenic 5-HT2A ligands such as lisuride, 6-F-DET, and TBG.49,134,135,136,137,138 Increased c-Fos expression following treatment with either hallucinogenic or non-hallucinogenic agonists of the 5-HT2A receptor is abolished in 5-HT2A receptor KO neurons or by pretreatment with a PLC inhibitor.87,120,127 However, the contribution of Gq signaling to the behavioral effects of psychedelics is unclear given that non-hallucinogenic 5-HT2A receptor ligands can activate Gq, and DOI still produces a robust HTR in Gq KO mice.139 Moreover, it is currently unknown what role, if any, canonical Gq activation plays in the psychoplastogenic effects of psychedelics. Full agonists like serotonin do not necessarily promote plasticity, and partial agonists like LSD can induce large increases in structural plasticity.48
In addition to activating PLC, psychedelics have also been shown to increase arachidonic acid release through activation of phospholipase A2 (PLA2),140,141 While this pathway is quite opaque compared to the pathway leading to PLC activation, it appears that it may require Gi/o, Gβγ, and G12/13 in NIH3T3–5HT2A cells.142 Cellular context seems to be critical for determining which signaling pathways psychedelics can activate, as Roth and co-workers recently used TRUPATH143 to demonstrate that LSD selectively activates Gq, G11, and G15 in HEK293T cells while Gonzalez-Maeso, Meana, and co-workers have shown that psychedelics can activate Gi in neurons.87,144 Given that non-hallucinogenic 5-HT2A agonists do not appear to be capable of activating Gi, yet they can promote neuroplasticity, it is unclear what role Gi signaling plays in the psychoplastogenic effects of psychedelics.
Stimulation of 5-HT2A receptors can activate a variety of other downstream effectors known to be involved in cell growth including, but not limited to, ERK,142,145 JAK2,146 and GSK3β,147 though no studies to date have assessed the roles of these key proteins in the psychoplastogenic effects of psychedelics. Similarly, β-arrestin activation can play important roles in the downstream effects of 5-HT2A ligands,148,149,150,151,152 but we currently do not know if β-arrestin is involved in psychedelic-induced structural neuroplasticity.
Given that the potencies and efficacies of 5-HT2A ligands for activating various 5-HT2A-dependent signaling cascades do not correlate well with either their hallucinogenic or psychoplastogenic effects, we were interested in developing a direct fluorescence readout of 5-HT2A receptor conformation. To achieve this goal, we fused a circularly permuted green fluorescent protein to the third intracellular loop of the 5-HT2A receptor.55 Activation and inactivation of the sensor increases and decreases fluorescence intensity, respectively. Interestingly, when the sensor is expressed in HEK293T cells, its activation correlates very well with human hallucinogenic potency. Moreover, non-hallucinogenic agonists of the PLC pathway like lisuride, TBG, and 6-F-DET act as inverse agonists of this sensor. Given its ability to predict hallucinogenic potential across a wide range of structurally diverse compounds, we started calling this sensor psychLight.55 While psychLight is quite good at predicting hallucinogenicity, the current version of the sensor cannot predict psychoplastogenicity.
Ultimately, the integration of various 5-HT2A receptor signaling pathways can lead to compound-specific changes in the phosphoproteome and/or transcriptome. Several efforts have attempted to distinguish between hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists by comparing their phosphoproteomic145,153 or transcriptomic signatures.87,120 While these initial results are promising, the effects of many more compounds from diverse chemical classes need to be assessed before any claims can be made about a particular phosphorylation or gene expression pattern being a hallmark of one group of compounds over another. Similar efforts should be undertaken to compare proteomic, phosphoproteomic, and transcriptomic signatures of psychoplastogens against their structurally related non-psychoplastogenic congeners.
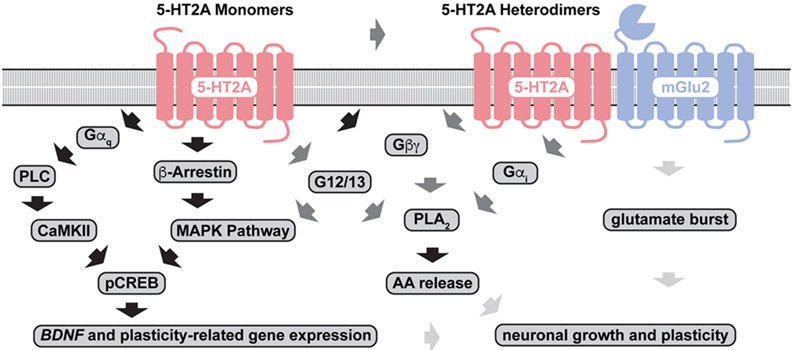
Though we know relatively little about how 5-HT2A receptor signaling converges on activation of TrkB, AMPA receptors, and mTOR to promote neuronal growth, it is clear that ligands for this receptor can exhibit a high degree of functional selectivity or biased agonism.87,154,155,148,156,157 The 5-HT2A receptor interacts with a number of scaffolding proteins158 and forms heterodimeric complexes with metabotropic glutamate,155 dopamine,159 cannabinoid,160 and serotonin161 receptors that can alter its signaling profile, though the in vivo functional relevance of these heterodimers is highly debated.162 The 5-HT2A-mGlu2 heterodimer155,163,164,165 has received a lot of attention given that it seems to be selectively activated by hallucinogens.155 It is interesting to note that DOI-induced BDNF expression in the cortex can be modulated by mGlu2 receptor ligands.166 Thus, it is possible that psychedelics induce glutamate release through a presynaptic mechanism167,168 involving a putative 5-HT2A-mGlu2 heterodimer. In theory, this glutamate burst could activate AMPA receptors leading to upregulation of BDNF/TrkB signaling. However, the role of a 5-HT2A-mGlu2 heterodimer in the psychoplastogenic effects of psychedelics is still unclear given that non-hallucinogenic ligands do not appear to activate this heterodimer and yet several non-hallucinogenic psychoplastogens have recently been discovered.
Because it is still unknown which 5-HT2A receptor signaling pathways are most critical to promoting neuronal growth (Figure 2), we focused our medicinal chemistry efforts on using phenotypic screening in neuronal cultures to identify non-hallucinogenic psychoplastogens.49,54,55 These compounds are structural analogs of psychedelics that do not induce a HTR, but are still capable of producing robust psychoplastogenic effects and sustained therapeutic behavioral responses after a single administration. Currently, tabernanthalog (TBG) is the most studied non-hallucinogenic psychoplastogen having demonstrated the ability to repair neural circuitry damaged by chronic stress67 and to produce long-lasting behavioral effects relevant to treating both depression and addiction.49,169 Efforts to design new non-hallucinogenic psychoplastogens have relied heavily on structure-activity relationship studies, but with the advent of high resolution structures of the 5-HT2A receptor in both active and inactive states,95,170 rational design of improved psychedelic-related therapeutics might be possible in the near future.
Figure 2.
Biochemical pathways activated by psychedelics. Pathways with with strong, moderate, and weak supporting evidence are indicated with black, dark grey, and light grey arrows, respectively.
CONCLUSION
Biochemical signaling resulting from 5-HT2A receptor activation is complex and depends on both the nature of the ligand and the cellular environment. In order to understand which pathways lead to psychedelic-induced neuroplasticity, we need to use a variety of pharmacological ad genetic tools to block these pathways in neurons. Additionally, the development of high-throughput assays to assess psychoplastogenic effects will be essential for correlating psychoplastogenic potencies and efficacies with those of more traditional assays relevant to 5-HT2A receptor signaling. While we know that 5-HT2 receptors appear to be essential for the psychoplastogenic effects of psychedelics, several key questions remain. Does the genetic localization of 5-HT2 receptors impart a level of cell-type selectivity in the psychoplastogenic effects of psychedelics? Do psychedelics induce growth of non-neuronal cells expressing 5-HT2 receptors? These are some of the many questions that need to be answered if we are to engineer better neuroplasticity-promoting therapeutics based on psychedelics. For other perspectives on psychedelic-induced neuroplasticity and the molecular mechanisms of psychedelics, please see several excellent recent reviews.25,71,171,172
Acknowledgement
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997), the Camille and Henry Dreyfus Foundation, a Dr. Mohsen Najafi Research Award in Medicinal Chemistry, and the Boone Family Foundation.
Footnotes
Disclosure
David E. Olson is a co-founder and the chief innovation officer of Delix Therapeutics, Inc.
REFERENCES
- 1.Catlow BJ; Song S; Paredes DA; Kirstein CL; Sanchez-Ramos J Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res, 2013, 228, 481–491. [DOI] [PubMed] [Google Scholar]
- 2.Hibicke M; Landry AN; Kramer HM; Talman ZK; Nichols CD Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci 2020, 11, 864–871. [DOI] [PubMed] [Google Scholar]
- 3.Cameron LP; Benson CJ; Dunlap LE; Olson DE Effects of N,N-dimethyltryptamine (DMT) on rat behaviors relevant to anxiety and depression. ACS Chem. Neurosci 2018, 9, 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron LP; Benson CJ; DeFelice BC; Fiehn O; Olson DE Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci 2019, 10, 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesselgrave N; Troppoli TA; Wulff AB; Cole AB; Thompson SM Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A. 2021, 118, e2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao LX; Liao C; Gregg I; Davoudian PA; Savalia NK; Delagarza K; Kwan AC Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021, 109, 2535–2544.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Fuente Revenga M, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, Toneatti R, Sierra S, Wolstenholme JT, Beardsley PM, Huntley GW, Lu C, Gonzelez-Maeso J. Prolonged epigenetic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep., 2021, 37, 109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JM; Bogenschutz M; Lilienstein A et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021. 27, 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis AK; Barrett FS; May DG; Cosimano MP; Sepeda ND; Johnson MW; Finan PH; Griffiths RR Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2021, 78, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carhart-Harris R; Giribaldi B; Watts R et al. Trial of Psilocybin versus Escitalopram for Depression. The New England Journal of Medicine. 2021, 384, 1402–1411. [DOI] [PubMed] [Google Scholar]
- 11.Carhart-Harris RL; Bolstridge M; Rucker J; Day CMJ; Erritzoe D; Kaelen M; Bloomfield M; Rickard JA; Forbes B; Feilding A; Taylor D; Pilling S; Curran VH; Nutt DJ Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry, 2016, 3, 619–627. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths RR; Johnson MW; Carducci MA; Umbricht A; Richards WA; Richards BD; Cosimano MP; Klinedinst MA Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol., 2016, 30, 1181–1197. doi: 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carhart-Harris RL; Bolstridge M; Day CMJ; Rucker J; Watts R; Erritzoe DE; Kaelen M; Giribaldi B; Bloomfield M; Pilling S; Rickard JA Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl)., 2018, 235, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mithoefer MC; Grob CS; Brewerton TD Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry. 2016, 3, 481–488. [DOI] [PubMed] [Google Scholar]
- 15.Carhart-Harris RL; Goodwin GM The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology. 2017, 42, 2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols DE; Johnson MW; Nichols CD Psychedelics as medicines: an emerging new paradigm. Clin. Pharmacol. Ther 2017, 101, 209–219. [DOI] [PubMed] [Google Scholar]
- 17.Vollenweider F, Kometer M The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010, 11, 642–651. [DOI] [PubMed] [Google Scholar]
- 18.Dos Santos RG; Osório FL; Crippa JA; Riba J; Zuardi AW; Hallak JE Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther. Adv. Psychopharmacol 2016, 6, 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyzar EJ; Nichols CD; Gainetdinov RR; Nichols DE; Kalueff AV Psychedelic drugs in biomedicine. Trends Pharmacol. Sci 2017, 38, 992–1005. [DOI] [PubMed] [Google Scholar]
- 20.Vollenweider FX; Kometer M The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci, 2010, 11, 642–651. [DOI] [PubMed] [Google Scholar]
- 21.Yaden DB; Griffiths RR The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci. 2020, 4, 568–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartogsohn I The meaning-enhancing properties of psychedelics and their mediator role in psychedelic therapy, spirituality, and creativity. Front Neurosci., 2018, 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Set Hartogsohn I. and setting, psychedelics and the placebo response: an extra-pharmacological perspective on psychopharmacology. J Psychopharmacol., 2016, 30, 1259–1267. [DOI] [PubMed] [Google Scholar]
- 24.Olson JA; Suissa-Rocheleau L; Lifshitz M; Raz A; Veissière SPL. Tripping on nothing: placebo psychedelics and contextual factors. Psychopharmacology., 2020, 237, 1371–1382. [DOI] [PubMed] [Google Scholar]
- 25.Aleksandrova LR; Phillips AG Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci., 2021, S0165–6147(21)00157-7. [DOI] [PubMed] [Google Scholar]
- 26.Savalia NK; Shao LX; Kwan AC A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci., 2021, 44, 260–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks MI; Zahid Z; Jones NT; Sultan ZW; Wenthur CJ Catalysts for change: the cellular neurobiology of psychedelics. Mol Biol Cell., 2021, 32, 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artin H; Zisook S; Ramanathan D How do serotonergic psychedelics treat depression: The potential role of neuroplasticity. World J Psychiatry. 2021, 11, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eero Castrén E; Monteggia LM Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol Psychiatry, 2021, 90, 128–136. [DOI] [PubMed] [Google Scholar]
- 30.Duman RS; Monteggia LM A neurotrophic model for stress-related mood disorders. Biol Psychiatry., 2006, 59, 1116–1127. [DOI] [PubMed] [Google Scholar]
- 31.Björkholm C; Monteggia LM BDNF - a key transducer of antidepressant effects. Neuropharmacology, 2016, 102, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castrén E; Kojima M Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis., 2017, 97, 119–126. [DOI] [PubMed] [Google Scholar]
- 33.Duman RS; Deyama S; Fogaça MV Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci., 2021, 53, 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T; Nie Z; Shu H; Kuang Y; Chen X; Cheng J; Yu S; Liu H The Role of BDNF on Neural Plasticity in Depression. Front Cell Neurosci., 2020, 14, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duman RS; Aghajanian GK; Sanacora G; Krystal JH Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med., 2016, 22, 238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein RZ; Volkow ND Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci., 2011, 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein RZ; Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry., 2002, 159, 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkow ND; Fowler JS; Wang GJ; Goldstein RZ Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem., 2002, 78, 610–624. [DOI] [PubMed] [Google Scholar]
- 39.Pahng AR; McGinn MA; Paulsen RI; Edwards S The Prefrontal Cortex as a Critical Gate of Negative Affect and Motivation in Alcohol Use Disorder. Curr Opin Behav Sci., 2017, 13, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnsten AF; Raskind MA; Taylor FB; Connor DF The Effects of Stress Exposure on Prefrontal Cortex: Translating Basic Research into Successful Treatments for Post-Traumatic Stress Disorder. Neurobiol Stress., 2015, 1, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenigs M; Grafman J Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist., 2009, 15, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hare BD; Duman RS Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry, 2020, 25, 2742–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kühn S; Gallinat J Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry, 2013, 73, 70–74. [DOI] [PubMed] [Google Scholar]
- 44.Asensio S; Morales JL; Senabre I; Romero MJ; Beltran MA; Flores-Bellver M; Barcia JM; Romero FJ Magnetic resonance imaging structural alterations in brain of alcohol abusers and its association with impulsivity. Addict Biol., 2016, 21, 962–971. [DOI] [PubMed] [Google Scholar]
- 45.Vargas MV; Meyer R; Avanes AA; Rus M; Olson DE Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry, 2021, 12, 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson DE Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. of Exp. Neurosci 2018, 12, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castrén E; Antila H Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry, 2017, 8, 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ly C; Greb AC; Cameron LP; Wong JM; Barragan EV; Wilson PC; Burbach KF; Soltanzadeh Zarandi S; Sood A; Paddy MR; Duim WC; Dennis MY; McAllister AK; Ori-McKenney KM; Gray JA; Olson DE Psychedelics promote structural and functional neural plasticity. Cell Rep, 2018, 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron LP; Tombari RJ; Lu J; Pell AJ; Hurley ZQ; Ehinger Y; Vargas MV; McCarroll MN; Taylor JC; Myers-Turnbull D; Liu T; Yaghoobi B; Laskowski LJ; Anderson EI; Zhang G; Viswanathan J; Brown BM; Tjia M; Dunlap LE; Rabow ZT; Fiehn O; Wulff H; McCorvy JD; Lein PJ; Kokel D; Ron D; Peters J; Zuo Y; Olson DE A Non-Hallucinogenic Psychedelic Analogue with Therapeutic Potential. Nature, 2021, 589, 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voleti B; Navarria A; Liu RJ; Banasr M; Li N; Terwilliger R; Sanacora G; Eid T; Aghajanian G; Duman RS Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry, 2013, 74, 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li N; Lee B; Liu RJ; Banasr M; Dwyer JM; Iwata M; Li XY; Aghajanian G; Duman RS mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 2010, 329, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Browne CA; Lucki I Antidepressant effects of ketamine: Mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol, 2013, 4, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moda-Sava RN; Murdock MH; Parekh PK; Fetcho RN; Huang BS; Huynh TN; Witztum J; Shaver DC; Rosenthal DL; Always EJ; Lopez K; Meng Y; Nellissen L; Grosenick L; Milner TA; Deisseroth K; Bito H; Kasai H; Liston C Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science, 2019, 364, pii: eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunlap LE; Azinfar A; Ly C; Cameron LP; Viswanathan J; Tombari RJ; Myers-Turnbull D; Taylor JC; Grodzki AC; Lein PJ; Kokel D Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogues through Structure-Activity Relationship Studies. J Med Chem., 2020, 63, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong C; Ly C; Dunlap LE; Vargas MV; Sun J; Hwang I-W; Azinfar A; Oh WC; Wetsel WC; Olson DE; Tian L Psychedelic-Inspired Drug Discovery Using an Engineered Biosensor. Cell, 2021, 10, 2779–2792.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olson DE The Subjective Effects of Psychedelics May Not Be Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol Transl Sci., 2020, 4, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cameron LP; Olson DE The Evolution of the Psychedelic Revolution. Neuropsychopharmacology, 2021, 47, 413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters J; Olson DE Engineering Safer Psychedelics for Treating Addiction. Neuroscience Insights, 2021, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones KA; Srivastava DP; Allen JA; Strachan RT; Roth BL; Penzes P Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A, 2009, 106, 19575–19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mi Z; Si T; Kapadia K; Li Q; Muma NA Receptor-stimulated transamidation induces activation of Rac1 and Cdc42 and the regulation of dendritic spines. Neuropharmacology. 2017, 117, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida H; Kanamaru C; Ohtani A; Li F; Senzaki K; Shiga T Subtype specific roles of serotonin receptors in the spine formation of cortical neurons in vitro. Neurosci Res., 2011, 71, 311–314. [DOI] [PubMed] [Google Scholar]
- 62.Ohtani A; Kozono N; Senzaki K; Shiga T Serotonin 2A receptor regulates microtubule assembly and induces dynamics of dendritic growth cones in rat cortical neurons in vitro. Neurosci Res., 2014, 81-82, 11–20. [DOI] [PubMed] [Google Scholar]
- 63.Ly C; Greb CA; Vargas MV; Duim WC; Grodzki ACG; Lein PJ; Olson DE Transient Stimulation with Psychoplastogens is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci, 2020, 4, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson TE; Kolb B Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology, 2004, 47, 33–46. [DOI] [PubMed] [Google Scholar]
- 65.Robinson TE; Kolb B Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. Journal of Neuroscience, 1997, 17, 8491–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crombag HS; Gorny G; Li Y; Kolb B; Robinson TE Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebral Cortex, 2005, 15, 341–348. [DOI] [PubMed] [Google Scholar]
- 67.Lu J; Tjia M; Mullen B; Cao B; Lukasiewicz K; Shah-Morales S; Weiser S; Cameron LP; Olson DE; Chen L; Zuo Y An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol Psychiatry, 2021, doi: 10.1038/s41380-021-01159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raval NR; Johansen A; Donovan LL; Ros NF; Ozenne B; Hansen HD; Knudsen GM Single dose of psilocybin increases synaptic density and decreases 5-HT2A receptor density in the pig brain. Int J Mol Sci., 2021, 22, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phoumthipphavong V; Barthas F; Hassett S; Kwan AC Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro, 2016, 3, ENEURO.0133–15.2016. doi: 10.1523/ENEURO.0133-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kadriu B; Greenwald M; Henter ID; Gilbert JR; Kraus C; Park LT, Zarate CA, Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of rapid-acting antidepressants. Int. J. Neuropsychopharmacol, 2021, 24, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inserra A; De Gregorio D; Gobbi G Psychedelics in psychiatry: neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacological Reviews, 2021, 73, 202–277. [DOI] [PubMed] [Google Scholar]
- 72.Quiedeville A; Boulouard M; Da Silva Costa-Aze V; Dauphin F; Bouet V; Freret T 5-HT6 receptor antagonists as treatment for age-related cognitive decline. Rev Neurosci., 2014, 25, 417–427. [DOI] [PubMed] [Google Scholar]
- 73.Upton N; Chuang TT; Hunter AJ; Virley DJ 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease. Neurotherapeutics. 2008, 5, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meneses A Memory formation and memory alterations: 5-HT6 and 5-HT7 receptors, novel alternative. Rev Neurosci., 2014, 25, 325–356. [DOI] [PubMed] [Google Scholar]
- 75.Speranza L; Labus J; Volpicelli F; Guseva D; Lacivita E; Leopoldo M; Bellenchi GC; di Porzio U Bijata M; Perrone-Capano C; Ponimaskin E. Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J Neurochem., 2017, 141, 647–661. [DOI] [PubMed] [Google Scholar]
- 76.Crispino M; Volpicelli F; Perrone-Capano C Role of the Serotonin Receptor 7 in Brain Plasticity: From Development to Disease. International journal of molecular sciences, 2020, 21, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oña G; Bouso JC Therapeutic Potential of Natural Psychoactive Drugs for Central Nervous System Disorders: A Perspective from Polypharmacology. Curr Med Chem., 2021, 28, 53–68. [DOI] [PubMed] [Google Scholar]
- 78.Nichols DE Psychedelics. Pharmacol Rev., 2016, 68, 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nichols DE Hallucinogens. Pharmacol. Ther, 2004, 101, 131–181. [DOI] [PubMed] [Google Scholar]
- 80.Glennon RA; Titeler M; McKenney JD Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci., 1984, 35, 2505–2511. [DOI] [PubMed] [Google Scholar]
- 81.Nelson DL; Lucaites VL; Wainscott DB; Glennon RA Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn-Schmiedeberg's archives of pharmacology, 1999, 359, 1–6. [DOI] [PubMed] [Google Scholar]
- 82.Vollenweider FX; Vollenweider-Scherpenhuyzen MF; Bäbler A; Vogel H; Hell D Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport, 1998, 9, 3897–3902. [DOI] [PubMed] [Google Scholar]
- 83.Preller KH; Burt JB; Ji JL; Schleifer CH; Adkinson BD; Stämpfli P; Seifritz E; Repovs G; Krystal JH; Murray JD; Vollenweider FX Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018, 7, e35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holze F; Vizeli P; Ley L; Müller F; Dolder P; Stocker M; Duthaler U; Varghese N; Eckert A; Borgwardt S; Liechti ME Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacol. 2021, 46, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madsen MK; Fisher PM; Burmester D; Dyssegaard A; Stenbæk DS; Kristiansen S; Johansen SS; Lehel S; Linnet K; Svarer C; Erritzoe D, Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology, 2019, 44, 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiorella D; Rabin RA; Winter JC The role of the 5-HT 2A and 5-HT 2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology, 1995, 121, 347–356. [DOI] [PubMed] [Google Scholar]
- 87.González-Maeso J; Weisstaub NV; Zhou M; Chan P; Ivic L; Ang R; Lira A; Bradley-Moore M; Ge Y; Zhou Q; Sealfon SC; Gingrich JA Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007, 53, 439–452. [DOI] [PubMed] [Google Scholar]
- 88.Canal CE; Da Silva UBO; Gresch PJ; Watt EE; Sanders-Bush E; Airey DC The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology, 2010, 209, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halberstadt AL; Chatha M; Klein AK; Wallach J; Brandt SD Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020, 167, 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanks JB; González-Maeso J Animal models of serotonergic psychedelics. ACS Chem. Neurosci 2013, 4, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Michiels M; Monbaliu J; Meuldermans W; Hendriks R; Geerts R; Woestenborghs R; Heykants J; Pharmacokinetics and tissue distribution of ketanserin in rat, rabbit and dog. Arzneimittel-forschung, 1988, 38, 775–784. [PubMed] [Google Scholar]
- 92.Smith RL; Barrett RJ; Sanders-Bush E Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor. Journal of Pharmacology and Experimental Therapeutics, 1995, 275, 1050–1057. [PubMed] [Google Scholar]
- 93.Johnson MP; Loncharich RJ; Baez M; Nelson DL Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure-activity relationship of certain ergolines and tryptamines. Molecular Pharmacology, 1994, 45, 277–286. [PubMed] [Google Scholar]
- 94.Canal CE; Cordova-Sintjago T; Liu Y; Kim MS; Morgan D; Booth RG Molecular pharmacology and ligand docking studies reveal a single amino acid difference between mouse and human serotonin 5-HT2A receptors that impacts behavioral translation of novel 4-phenyl-2-dimethylaminotetralin ligands. Journal of Pharmacology and Experimental Therapeutics, 2013, 347, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim K; Che T; Panova O; DiBerto JF; Lyu J; Krumm BE; Wacker D; Robertson MJ; Seven AB; Nichols DE; Shoichet BK Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell, 2020, 182, 1574–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhattacharya A; Sankar S; Panicker MM Differences in the C-terminus contribute to variations in trafficking between rat and human 5-HT2A receptor isoforms: identification of a primate-specific tripeptide ASK motif that confers GRK-2 and β arrestin-2 interactions. Journal of Neurochemistry, 2010. 112, 723–732. [DOI] [PubMed] [Google Scholar]
- 97.De Gregorio D; Popic J; Enns JP; Inserra A; Skalecka A; Markopoulos A; Posa L; Lopez-Canul M; Qianzi H; Lafferty CK; Britt JP; Comai S; Aguilar-Valles A; Sonenberg N; Gobbi G. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proceedings of the National Academy of Sciences. 2021, 118 e2020705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Switon K; Kotulska K; Janusz-Kaminska A; Zmorzynska J; Jaworski J Molecular Neurobiology of mTOR. Neuroscience. 2017, 341, 112–153. [DOI] [PubMed] [Google Scholar]
- 99.Dakic V; Nascimento JM; Sartore RC; Maciel RM; de Araujo DB; Ribeiro S, et al. Short term changes in the proteome of human cerebral organoids induced by 5-methoxy-N,N-dimethyltryptamine. Sci Rep., 2017, 7, 12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moghaddam B; Adams B; Verma A; Daly D Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal of Neuroscience, 1997, 17, 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stone JM; Dietrich C; Edden R; Mehta MA; De Simoni S; Reed LJ; Krystal JH; Nutt D; Barker GJ Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Molecular psychiatry, 2012, 17, 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muschamp JW; Regina MJ; Hull EM; Winter JC; Rabin RA, Lysergic acid diethylamide and [−]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Research. 2004, 1023, 134–140. [DOI] [PubMed] [Google Scholar]
- 103.Scruggs JL; Patel S; Bubser M; Deutch AY DOI-induced activation of the cortex: Dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. The Journal of Neuroscience, 2000, 20, 8846–8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jourdi H; Hsu YT; Zhou M; Qin Q; Bi X; Baudry M Positive AMPA Receptor Modulation Rapidly Stimulates BDNF Release and Increases Dendritic mRNA Translation. J. Neurosci, 2009, 29, 8688–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takei N; Inamura N; Kawamura M; Namba H; Hara K, Yonezawa K; Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci 2004, 24, 9760–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeon MT; Nam JH; Shin WH; Leem E; Jeong KH; Jung UJ; Bae YS; Jin YH; Kholodilov N; Burke RE; Lee SG In vivo AAV1 transduction with hRheb (S16H) protects hippocampal neurons by BDNF production. Molecular Therapy, 2015, 23, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takei N; Numakawa T; Kozaki S; Sakai N; Endo Y; Takahashi M; Hatanaka H Brain-derived neurotrophic factor induces rapid and transient release of glutamate through the non-exocytotic pathway from cortical neurons. J. Biol. Chem 1998, 273, 27620–27624. [DOI] [PubMed] [Google Scholar]
- 108.Vollenweider FX; Kometer M The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nature Reviews Neuroscience, 2010, 11, 642–651. [DOI] [PubMed] [Google Scholar]
- 109.Kavalali ET; Monteggia LM Targeting homeostatic synaptic plasticity for treatment of mood disorders. Neuron, 2020, 106, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suzuki K; Kim JW; Nosyreva E; Kavalali ET; Monteggia LM Convergence of distinct signaling pathways on synaptic scaling to trigger rapid antidepressant action. Cell Reports, 2021, 37, 109918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Autry AE; Adachi M; Nosyreva E; Na ES; Los MF; Cheng PF; Kavalali ET; Monteggia LM Receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature, 2011, 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu RJ; Lee FS; Li XY; Bambico F; Duman RS; Aghajanian GK Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry, 2012, 71, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghosal S; Bang E; Yue W; Hare BD; Lepack AE; Girgenti MJ; Duman RS Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biological psychiatry, 2018, 83, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vaidya VA; Marek GJ; Aghajanian GK; Duman RS 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997, 17, 2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin DA; Nichols CD The Effects of Hallucinogens on Gene Expression. Curr Top Behav Neurosci., 2018, 36, 137–158. [DOI] [PubMed] [Google Scholar]
- 116.Leslie RA; Moorman JM; Coulson A; Grahame-Smith DG Serotonin 2/1 C receptor activation causes a localized expression of the immediate-early gene c-fos in rat brain: evidence for involvement of dorsal raphe nucleus projection fibres. Neuroscience. 1993, 53, 457–463. [DOI] [PubMed] [Google Scholar]
- 117.Frankel PS; Cunningham KA The hallucinogen d-lysergic acid diethylamide (d-LSD) induces the immediate-early gene c-Fos in rat forebrain. Brain Res. 2002, 958, 251–260. [DOI] [PubMed] [Google Scholar]
- 118.Erdtmann-Vourliotis M; Mayer P; Riechert U; Hollt V Acute injection of drugs with low addictive potential (delta(9)-tetrahydrocannabinol, 3,4-methylenedioxymethamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than highly addicting drugs (cocaine and morphine). Brain Res Mol Brain Res. 1999, 71, 313–324. [DOI] [PubMed] [Google Scholar]
- 119.Gresch PJ; Strickland LV; Sanders-Bush E Lysergic acid diethylamide-induced Fos expression in rat brain: role of serotonin-2A receptors. Neuroscience. 2002, 114, 707–713. [DOI] [PubMed] [Google Scholar]
- 120.Gonzalez-Maeso J; Yuen T; Ebersole BJ; et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003, 23, 8836–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin DA; Nichols CD. Psychedelics recruit multiple cellular types and produce complex transcriptional responses within the brain. EBioMedicine. 2016, 11, 262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pei Q; Lewis L; Sprakes ME; Jones EJ; Grahame-Smith DG; Zetterstrom TS Serotonergic regulation of mRNA expression of Arc, an immediate early gene selectively localized at neuronal dendrites. Neuropharmacology. 2000, 39, 463–470. [DOI] [PubMed] [Google Scholar]
- 123.Pei Q; Tordera R; Sprakes M; Sharp T Glutamate receptor activation is involved in 5-HT2 agonist-induced Arc gene expression in the rat cortex. Neuropharmacology. 2004, 46, 331–339. [DOI] [PubMed] [Google Scholar]
- 124.Nichols CD; Sanders-Bush E A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002, 26, 634–642. [DOI] [PubMed] [Google Scholar]
- 125.Nichols CD; Garcia EE; Sanders-Bush E Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Brain Res Mol Brain Res. 2003, 111, 182–188. [DOI] [PubMed] [Google Scholar]
- 126.Nichols CD; Sanders-Bush E Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase-1, C/EBP-beta and ILAD-1, a novel gene with homology to arrestins. J Neurochem. 2004, 90, 576–584. [DOI] [PubMed] [Google Scholar]
- 127.Desouza LA; Benekareddy M; Fanibunda SE; Mohammad F; Janakiraman B; Ghai U; Gur T; Blendy JA; Vaidya VA The Hallucinogenic Serotonin2A Receptor Agonist, 2, 5-Dimethoxy-4-Iodoamphetamine, Promotes cAMP Response Element Binding Protein-Dependent Gene Expression of Specific Plasticity-Associated Genes in the Rodent Neocortex. Frontiers in Molecular Neuroscience, 2021, 14, 790213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nichols CD; Sanders-Bush E A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology, 2002, 26, 634–642. [DOI] [PubMed] [Google Scholar]
- 129.Donovan LL; Johansen JV; Ros NF; Jaberi E; Linnet K; Johansen SS; Ozenne B; Issazadeh-Navikas S; Hansen HD; Knudsen GM Effects of a single dose of psilocybin on behaviour, brain 5-HT2A receptor occupancy and gene expression in the pig. European Neuropsychopharmacology, 2021, 42, 1–11. [DOI] [PubMed] [Google Scholar]
- 130.Nichols DE; Nichols CD Serotonin receptors. Chem Rev, 2008, 108, 1614–1641. [DOI] [PubMed] [Google Scholar]
- 131.Roth B; Nakaki T; Chuang D; Costa E Aortic Recognition Sites For Serotonin (5HT) are Coupled to Phospholipase-C and Modulate Phosphatidylinositol Turnover. Neuropharmacology, 1984, 23, 1223–1225. [DOI] [PubMed] [Google Scholar]
- 132.Conn PJ; Sanders-Bush E Selective 5HT-2 antagonists inhibit serotonin stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology, 1984, 23, 993–996. [DOI] [PubMed] [Google Scholar]
- 133.Bhatnagar A; Sheffler DJ; Kroeze WK; Compton-Toth B; Roth BL Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. The Journal of Biological Chemistry, 2004, 279, 34614–34623. [DOI] [PubMed] [Google Scholar]
- 134.Marona-Lewicka D; Kurrasch-Orbaugh DM; Selken JR; Cumbay MG; Lisnicchia JG; Nichols DE Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine 1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology, 2002, 164, 93–107. [DOI] [PubMed] [Google Scholar]
- 135.Porter RH; Benwell KR; Lamb H; Malcolm CS; Allen NH; Revell DF; Adams DR; Sheardown MJ Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol, 1999, 128, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Egan CT; Herrick-Davis K; Miller K; Glennon RA; Teitler M Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology, 1998, 136, 409–414. [DOI] [PubMed] [Google Scholar]
- 137.Cussac D; Boutet-Robinet E; Ailhaud MC; Newman-Tancredi A; Martel JC; Danty N; Rauly-Lestienne I Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur. J. Pharmacol, 2008, 594, 32–38. [DOI] [PubMed] [Google Scholar]
- 138.Rabin RA; Regina M; Doat M; Winter JC 5-HT2A receptor- stimulated phosphoinositide hydrolysis in the stimulus effects of hallucino- gens. Pharmacol. Biochem. Behav, 2002, 72, 29–37. [DOI] [PubMed] [Google Scholar]
- 139.Garcia EE; Smith RL; Sanders-Bush E Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology, 2007, 52, 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kurrasch-Orbaugh DM; Watts VJ; Barker EL; Nichols DE Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther, 2003, 304, 229–237. [DOI] [PubMed] [Google Scholar]
- 141.Berg KA; Maayani S; Goldfarb J; Scaramellini C; Leff P; Clarke WP Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol, 1998, 54, 94–104. [PubMed] [Google Scholar]
- 142.Kurrasch-Orbaugh DM; Parrish JC; Watts VJ; Nichols DE A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem, 2003, 86, 980–991. [DOI] [PubMed] [Google Scholar]
- 143.Olsen RHJ; DiBerto JF; English JG; Glaudin AM; Krumm BE; Slocum ST; Che T; Gavin AC; McCorvy JD; Roth BL; Strachan RT TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol, 2020, 16, 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.García-Bea A; Miranda-Azpiazu P; Muguruza C; Marmolejo-Martinez-Artesero S; Diez-Alarcia R; Gabilondo AM; Callado LF; Morentin B; González-Maeso J; Meana JJ; Serotonin 5-HT2A receptor expression and functionality in postmortem frontal cortex of subjects with schizophrenia: selective biased agonism via Gαi1-proteins. European Neuropsychopharmacology, 2019, 29, 1453–1463. [DOI] [PubMed] [Google Scholar]
- 145.Karaki S; Becamel C; Murat S; La Cour CM; Millan MJ; Prézeau L; Bockaert J; Marin P; Vandermoere F Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Molecular & Cellular Proteomics, 2014, 13, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oufkir T; Arseneault M; Sanderson JT; Vaillancourt C; The 5-HT2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK–ERK1/2 and JAK2–STAT3 signalling pathways in human choriocarcinoma cell lines. Placenta, 2010, 31, 439–447. [DOI] [PubMed] [Google Scholar]
- 147.Abbas A; Yadav P; Yao W; Arbuckle M; Grant S; Caron M; Roth B PSD-95 Is Essential for Hallucinogen and Atypical Antipsychotic Drug Actions at Serotonin Receptors. Journal of Neuroscience, 2009, 29, 7124–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmid CL; Raehal KM; Bohn LM Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schmid CL; Bohn LM Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. Journal of Neuroscience, 2010, 30, 13513–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gray JA; Bhatnagar A; Gurevich VV; Roth BL The interaction of a constitutively active arrestin with the arrestin-insensitive 5-HT2A receptor induces agonist-independent internalization. Molecular pharmacology, 2003, 63, 961–972. [DOI] [PubMed] [Google Scholar]
- 151.Wacker D; Wang C; Katritch V; Han GW; Huang XP; Vardy E; McCorvy JD; Jiang Y; Chu M; Siu FY; Liu W Structural features for functional selectivity at serotonin receptors. Science, 2013, 340, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rodriguiz RM; Nadkarni V; Means CR; Pogorelov VM; Chiu YT; Roth BL; Wetsel WC LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1. Scientific reports, 2021, 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Banerjee AA; Vaidya VA; Differential signaling signatures evoked by DOI versus lisuride stimulation of the 5-HT2A receptor. Biochemical and Biophysical Research Communications, 2020, 531, 609–614. [DOI] [PubMed] [Google Scholar]
- 154.López-Giménez JF; González-Maeso J Hallucinogens and serotonin 5-HT 2A receptor-mediated signaling pathways. Behavioral Neurobiology of Psychedelic Drugs, 2017, 45–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.González-Maeso J; Ang RL; Yuen T; Chan P; Weisstaub NV; López-Giménez JF; Zhou M; Okawa Y; Callado LF; Milligan G; Gingrich JA Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008, 452, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Berg KA; Maayani S; Goldfarb J; Scaramellini C; Leff P; Clarke WP Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol 1998, 54, 94–104. [PubMed] [Google Scholar]
- 157.Fribourg M; Moreno JL; Holloway T; Provasi D; Baki L; Mahajan R; Park G; Adney SK; Hatcher C; Eltit JM; Ruta JD; Albizu L; Li Z; Umali A; Shim J; Fabiato A; MacKerell AD Jr; Brezina V; Sealfon SC; Filizola M; González-Maeso J, Logothetis DE Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell. 2011, 147, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Slocum ST; DiBerto JF; Roth BL Molecular insights into psychedelic drug action. Journal of neurochemistry. 2021, doi: 10.1111/jnc.15540. [DOI] [PubMed] [Google Scholar]
- 159.Lukasiewicz S; Polit A; Kędracka-Krok S; Wędzony K; Maćkowiak M; Dziedzicka-Wasylewska M Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta, 2010, 1803, 1347–1358. [DOI] [PubMed] [Google Scholar]
- 160.Viñals X; Moreno E; Lanfumey L; Cordomí A; Pastor A; de La Torre R; Gasperini P; Navarro G; Howell LA, Pardo L; Lluís C; Canela E; McCormick PJ; Maldonado R; Robledo P; Cognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A Receptors. PLoS Biol., 2015, 13, e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moutkine I; Quentin E; Guiard BP; Maroteaux L; Doly S Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. Journal of Biological Chemistry, 2017, 292, 6352–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Delille HK; Mezler M; Marek GJ The two faces of the pharmacological interaction of mGlu2 and 5-HT2A–relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology, 2013, 70, 296–305. [DOI] [PubMed] [Google Scholar]
- 163.Toneatti R; Shin JM; Shah UH; Mayer CR; Saunders JM; Fribourg M; Arsenovic PT; Janssen WG; Sealfon SC; López-Giménez JF; Benson DL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shah UH; Toneatti R; Gaitonde SA; Shin JM; González-Maeso J Site-specific incorporation of genetically encoded photo-crosslinkers locates the heteromeric interface of a GPCR complex in living cells. Cell Chemical Biology, 2020, 27, 1308–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moreno JL; Holloway T; Albizu L; Sealfon SC; González-Maeso J Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neuroscience letters, 2011, 493, 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gewirtz JC; Chen AC, Terwilliger R; Duman RC; Marek GJ Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacology Biochemistry and Behavior, 2002, 73, 317–326. [DOI] [PubMed] [Google Scholar]
- 167.Aghajanian GK; Marek GJ. Serotonin induces excitatory post-synaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997, 36, 589–599. [DOI] [PubMed] [Google Scholar]
- 168.Marek GJ; Aghajanian GK 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999, 367, 197–206. [DOI] [PubMed] [Google Scholar]
- 169.Peters J; Olson DE Engineering Safer Psychedelics for Treating Addiction. Neuroscience Insights, 2021, 16, 10.1177/26331055211033847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kimura KT; Asada H; Inoue A; Kadji FMN; Im D; Mori C; Arakawa T; Hirata K; Nomura Y; Nomura N; Aoki J Structures of the 5-HT 2A receptor in complex with the antipsychotics risperidone and zotepine. Nature structural & molecular biology, 2019, 26, 121–128. [DOI] [PubMed] [Google Scholar]
- 171.Lukasiewicz K; Baker JJ; Zuo Y; Lu J Serotonergic Psychedelics in Neural Plasticity. Frontiers in Molecular Neuroscience, 2021, 14, 748359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jaster AM; de la Fuente Revenga M; González-Maeso J. Molecular targets of psychedelic-induced plasticity. Journal of neurochemistry, 2021, doi: 10.1111/jnc.15536. [DOI] [PMC free article] [PubMed] [Google Scholar]