Abstract
Over the course of the COVID-19 pandemic, numerous studies have aimed to address the challenges faced by patients with kidney disease and their caregivers. These studies addressed areas of concern such as the high infection and mortality risk of patients on in-centre haemodialysis and transplant recipients. However, the ability to draw meaningful conclusions from these studies has in some instances been challenging, owing to barriers in aspects of usual care, data limitations and problematic methodological practices. In many settings, access to SARS-CoV-2 testing differed substantially between patient groups, whereas the incidence of SARS-CoV-2 infection varied over time and place because of differences in viral prevalence, targeted public health policies and vaccination rates. The absence of baseline kidney function data posed problems in the classification of chronic kidney disease and acute kidney injury in some studies, potentially compromising the generalizability of findings. Study findings also require attentive appraisal in terms of the effects of confounding, collider bias and chance. As this pandemic continues and in the future, the implementation of sustainable and integrated research infrastructure is needed in settings across the world to minimize infection transmission and both prevent and plan for the short-term and long-term complications of infectious diseases. Registries can support the real-world evaluation of vaccines and therapies in patients with advanced kidney disease while enabling monitoring of rare complications.
Subject terms: Nephrology, Epidemiology
Patients with kidney disease are at particular risk of the adverse outcomes of COVID-19. Throughout the pandemic, epidemiological studies have been performed to inform clinical care; however, these studies have faced a number of methodological challenges. This Review discusses current understanding of the effects of COVID-19 on patients with kidney disease and some of the major obstacles encountered when conducting epidemiological research in a pandemic setting.
Key points
Patients who are receiving in-centre dialysis have a higher risk of exposure to SARS-CoV-2 than members of the general population, owing to their limited ability to isolate.
Studies have found a dose–response of increasing risk of mortality from COVID-19 with decreasing kidney function, with particularly high mortality seen in people with kidney failure and those on kidney replacement therapy.
Evidence of how infection risk can be mitigated in patients with chronic kidney disease is often of poor quality due to the many challenges of conducting epidemiological research in fragmented health-care settings.
Observational studies of associations between risk factors, such as chronic kidney disease or transplantation, and outcomes, such as COVID-19-related mortality, can be distorted as a result of collider bias, and thus care must be taken in study design and evaluation.
Recognizing the challenges that affect epidemiological studies in pandemic settings together with information on local health contexts enables rigorous assessment of study quality; adequate reporting of aspects relevant to local care availability enables readers to identify studies that contribute robust findings to the literature.
To confront the challenges wrought by future pandemics, a sustainable and integrated global infrastructure is needed to identify evidence-based approaches to minimize infection transmission and adverse outcomes.
Introduction
The abrupt arrival of the COVID-19 pandemic in early 2020 posed unforeseen challenges for patients with kidney disease and their care providers. Day-to-day priorities shifted towards the rapid reconfiguration of services to protect patients on in-centre haemodialysis who were unable to strictly adhere to social distancing policies due to their need to attend treatment. Concerns also existed that transplantation might place new recipients at a heightened risk of postoperative death. There were instances during the pandemic in which some critical care units became overwhelmed with an unprecedented demand for acute kidney replacement therapy (KRT). Despite the rapid development of vaccines and identification of effective treatments for severe disease, many of these challenges persist with the continuing emergence of novel SARS-CoV-2 variants. Lessons from learned experiences and the published literature must, therefore, be rapidly applied to better cope with ongoing challenges and similar crises that may arise in the future.
To best inform clinical care, epidemiological studies — ranging from small single-centre case series to large registry and population-wide cohorts — have been conducted across a range of settings. However, the COVID-19 pandemic has presented unique challenges, and many of these studies have encountered methodological difficulties arising from barriers in aspects of usual care, limitations in data collection and challenges in study design (Table 1; Supplementary Table 1). For example, such studies should ideally investigate and account for variations in health-care delivery, temporal trends and geographical factors that arose as a consequence of the pandemic. These methodological challenges have also hindered comparisons between epidemiological studies. Meta-analyses that use aggregated outcomes from studies that have not investigated and/or accounted for these variables may be limited in their conclusions — a fact that has been well-acknowledged1.
Table 1.
Biases that can occur in epidemiological studies of kidney disease populations as a result of barriers to health care
| Type of bias | Barriers to accessing health care pre-pandemic | Barriers to SARS-CoV-2 testing | Barriers to accessing health care during the COVID-19 pandemic |
|---|---|---|---|
| Information bias (misclassification of the study exposure or outcome) |
Misclassification of CKD as non-CKD (absence of testing or coding for CKD) Misclassification of subclinical CKD as AKI and vice versa |
Misclassification of infection as non-infection | Misclassification of cause of hospital admission |
| Confounding (when a measured or unmeasured variable, not on the causal pathway, influences both the study exposure and the study outcome) |
Reasons for getting a particular type of care will confound results Applies to all kidney disease populations |
Reasons for getting a test will confound results Applies to all kidney disease populations |
Reasons for getting a particular type of care will confound results Applies to all kidney disease populations |
| Collider bias (when the study sample collected is conditional on a variable on the causal pathway between study exposure and study outcome) | When a retrospective health record study only includes those with baseline and repeat creatinine test results (in a conventional cohort study people with missing test results would be logged as ‘lost to follow-up’ and appropriately censored/analysed) | Access to SARS-CoV-2 testing may depend on COVID-19 severity and varies between kidney disease populations; comparisons of outcomes amongst those tested may be biased | Access to hospital/ICU care depends on COVID-19 severity and underlying chances of survival/comorbidity, which varies between kidney disease populations; therefore, analyses of hospitalized/ICU patient populations may not always provide information on pathobiology |
| Selection bias (for a cohort study, selection bias is a systematic bias in the ability to capture study outcomes dependent on the (unobserved) study outcome; for a case–control study, selection bias occurs when cases are drawn from a different source population than the controls) |
Not applicable for studies where COVID-19 is an outcome For studies that consider kidney outcomes after COVID-19, barriers to kidney care/dialysis/transplantation can introduce selection bias |
Especially for transplant, home dialysis, CKD and AKI populations where there was no systematic screening for COVID-19 |
Applies to all kidney disease populations Some populations may have had fewer barriers than others (e.g. in-centre haemodialysis or transplant recipients) Some populations potentially may have had less access to ICU care |
AKI, acute kidney injury; CKD, chronic kidney disease; ICU, intensive care unit.
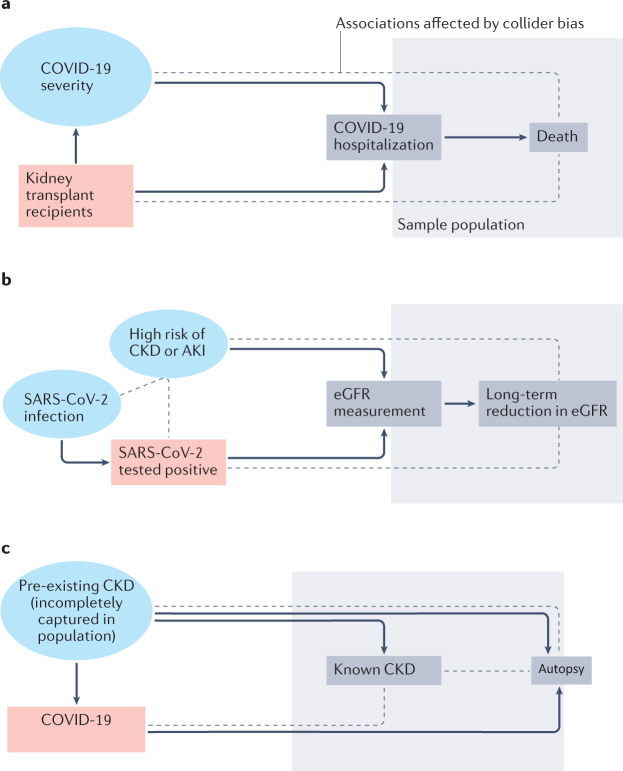
Collider bias is an important problem encountered in COVID-19 epidemiology2 (Fig. 1, Table 1; Supplementary Table 1). Collider bias occurs when both the risk factor or exposure of interest (for example, kidney transplantation) and the factors on the pathway to the outcome of interest (for example, disease severity on the pathway to death) influence the mechanisms behind selection into a study sample population. Causal inference based on analyses of such a selected study sample cannot then be generalized to a wider population of interest and, depending on the circumstances, may not be internally valid due to selection bias. Epidemiological studies therefore require well-defined study populations in which outcome events (for example, SARS-CoV-2 infection and its consequences) are determined as accurately as possible for every study participant. Although individuals on long-term KRT are generally included in national registries, a lack of baseline kidney function data makes it difficult to define populations with chronic kidney disease (CKD) and acute kidney injury (AKI) for prospective outcome assessment.
Fig. 1. The effect of collider bias on COVID-19 epidemiology studies.
Collider bias occurs when both the risk factor or exposure of interest and the factors on the pathway to the outcome of interest influence the mechanisms behind selection into a study sample population. This can result in biased associations between the exposure and outcome. a | Collider bias can occur in studies of the association between kidney transplantation (the risk factor, red box) and death (the outcome) in people hospitalized with COVID-19 (the sample population). Hospitalization is related to unmeasured COVID-19 severity (blue circle). By restricting the sample population to those who are hospitalized (grey box), collider bias may alter associations between kidney transplantation and death that cannot be generalized to the wider population (dotted lines), because the indications for hospitalization may differ between transplant recipients and other patient groups. Similar problems arise when investigating associations in populations admitted to intensive care. b | Collider bias can also occur when investigations of long-term reductions in estimated glomerular filtration rate (eGFR) following SARS-CoV-2 infection are restricted to those with available eGFR measurements and SARS-CoV-2 test results (grey box). In such instances, infection is only partially observed as a consequence of limited access to testing (in most cases early in the pandemic, based on disease severity). Serum creatinine testing is also more likely in those who are at risk of declining kidney function (for example, people with diabetes or cardiovascular disease, or those on certain drugs), or in those at risk of, or suspected to have acute kidney injury (AKI). Collider bias can induce and/or alter associations between the variables (indicated by dotted lines). c | Autopsy studies in patients who have died with COVID-19 are also at risk of collider bias. As only people who died after developing COVID-19 are selected (grey box), and because pre-existing chronic kidney disease (CKD) is a risk factor for severe COVID-19 and death more generally, collider bias can alter associations between COVID-19 and histological features of CKD at autopsy (dotted lines).
In this Review, we discuss a range of epidemiological challenges posed by the COVID-19 pandemic — a unique situation in which timely, reliable research was needed in the face of an unprecedented public health challenge. We discuss some of the major obstacles encountered when conducting epidemiological research in populations with kidney disease in a pandemic setting (Table 1; Supplementary Table 1), including challenges in ascertaining COVID-19-related outcomes in the dialysis and transplant populations, confounding factors such as barriers to health care, and the challenges in identifying and defining people with CKD or AKI (the ‘denominators’). We focus particularly on studies that have relevance for public health and their ability to inform future research endeavours and study design.
COVID-19 in patients on dialysis
Estimating the incidence of COVID-19 among patients on in-centre haemodialysis
Data on the incidence of COVID-19 among patients receiving in-centre haemodialysis is generally of high quality. The first cases of COVID-19 among patients receiving in-centre haemodialysis were from Wuhan, China3, with additional reports emerging as the pandemic spread to other parts of the world4–7.Throughout the pandemic, patients on in-centre haemodialysis were more likely than members of the general population to be exposed to SARS-CoV-2, as a consequence of the requirement to attend dialysis centres three times weekly, even during lockdowns. Often, attendance would involve shared patient transportation to and from home and interactions with other patients and dialysis staff. Despite the implementation of infection control procedures, these interactions increased exposure risk, especially in the early phases of the pandemic when asymptomatic screening was unavailable8 and personal protective equipment was lacking in some settings9. It is therefore unsurprising that the incidence of COVID-19 is higher in patients on in-centre haemodialysis than in the general population (Box 1). In the Flanders region of Belgium, the age-standardized estimate of the incidence of SARS-CoV-2 infection among patients on maintenance haemodialysis was 2.5% in May 2020 — more than fourfold greater than in the general population10. By the end of August 2020 in England, 11.2% of patients on in-centre haemodialysis tested positive for SARS-CoV-2 compared to only 2.9% of patients on home dialysis11. These findings highlight the greater exposure risk for those requiring in-centre treatment.
However, a diagnosis of SARS-CoV-2 infection is dependent on the availability of testing; the greater access to testing for patients on in-centre haemodialysis as part of infection control processes compared with that in the general population or patients on home dialysis may also account for the higher incidence of infection among this patient group.
Estimates of SARS-CoV-2 incidence are also heavily influenced by the sampling frame, both temporally and geographically, owing to differences in population viral prevalence, targeted public health policies, the infectivity of virus variants and vaccination rates. In early 2020, for example, local efforts to detect infection often led to the rapid implementation of local mitigation strategies, resulting in regional differences in viral prevalence. Thus, regional estimates cannot be reliably extrapolated to entire countries, as demonstrated in France where in early May 2020, the nationwide incidence of SARS-CoV-2 infection in patients on maintenance dialysis was 3.3%, but as high as 10% in some regions12.
Seroprevalence studies may improve insight into the past burden of SARS-CoV-2 infection among patients on dialysis. One study in patients on in-centre haemodialysis in London, UK, identified SARS-CoV-2 antibodies in 44 of 235 patients (18.7%) who were asymptomatic, and had therefore not been tested by PCR. Of the 42 symptomatic patients who had been tested but were PCR-negative, eight (19.0%) had SARS-CoV-2 antibodies8. A survey of samples from >28,000 patients on in-centre haemodialysis from across the USA in July 2020 found a seropositivity rate of 9.3% after standardization for age, sex and region, ranging from 0% in seven states to 34% in New York State13. However, these values are likely to be an underestimate of the burden of COVID-19 among the dialysis population, as they do not include patients who died from COVID-19, patients who did not seroconvert, or those whose antibody levels diminished over time. This study found that the likelihood of past infection was higher among individuals of Black, Hispanic or undisclosed ethnicity as recorded on their electronic health record (EHR), as well as those living in majority Black and Hispanic neighbourhoods, and correlated with neighbourhood poverty and population density13, after adjustment for age and sex, consistent with the reported ethnic and socioeconomic disparities in SARS-CoV-2 incidence in England14.
Box 1 The effects of COVID-19 on patients on dialysis and kidney transplant recipients.
A study of primary care electronic health records from England, UK, up to early May 2020, found that patients on dialysis and recipients of a solid organ transplant had a >3.5-fold increased risk of death from COVID-19 than individuals without a transplant or on dialysis60.
Approximately 20% of patients on dialysis died within 1 month of SARS-CoV-2 infection — substantially higher than the risk of death associated with other infections or many cardiovascular events1,15,16.
Excess mortality among patients on maintenance dialysis was nearly 30% higher relative to preceding 5-year trends during the initial height of the pandemic in the USA and the UK14,17.
Meta-analyses have shown that more than one in five kidney transplant recipients die after infection with SARS-CoV-2. The risk of death varied by time since transplantation: nearly one in three patients transplanted in the 15 months before infection died, compared with roughly one in five who received a transplant 16–60 months previously65,66.
Approximately nine in ten patients on maintenance dialysis demonstrated evidence of seroconversion after immunization with the BNT162b2 Pfizer and mRNA-1273 Moderna vaccines, but titres rapidly decreased in the ensuing months, suggesting the need for additional doses135.
Kidney and other transplant recipients have more persistent viral shedding than patients who did not receive a transplant, which might result in higher PCR testing sensitivity relative to the general population29–32.
Mortality in patients on maintenance dialysis
Although early reports from Wuhan, China, suggested that the effects of COVID-19 among patients on dialysis may be mild3, contradictory case series from the Lombardy region of Italy warned of a high mortality risk in this patient population4,5. These and other studies found that approximately 20–25% of patients on dialysis died within 1 month of a COVID-19 diagnosis1,15,16. During this early stage of the pandemic, excess mortality (compared with all-cause mortality in the same period in previous years) was 30% higher among patients on dialysis in the USA and the UK than historical trends14,17. However, not all of these deaths were formally attributed to COVID-19. As outlined by the WHO, International Classification of Disease (ICD) codes were inconsistently applied in the early stages of the pandemic18; in the USA, for example, COVID-19 received a dedicated ICD code only on 1 April 2020. Furthermore, the understandable focus of most health systems on the provision of acute care during the early phases of the pandemic hinders our ability to retrospectively establish how much of the excess mortality was driven by a lack of access to timely diagnosis and care for chronic, non-COVID-19 conditions.
The proportion of patients who died in the weeks and months following SARS-CoV-2 infection is even more striking when considered in the context of other acute illnesses that are common among patients on dialysis. Prior to the emergence of COVID-19, approximately 8.5% of patients on dialysis hospitalized for cardiovascular disease died during hospitalization or within 30 days of discharge19, and 13% of patients hospitalized for infection died during the index hospitalization or within 30 days of discharge20.
The European Renal Association COVID-19 Database (ERACODA), a prospective voluntary registry of 98 centres, found that age, frailty, vascular cause of kidney disease, obesity and heart failure were associated with higher COVID-19-related mortality in patients on dialysis21. Notably, patients on dialysis who were undergoing assessment or on a waiting list for transplantation had an 81% lower mortality than those not on a waiting list because of the known selection of healthier patients on to kidney transplant waiting lists. Registry data from England and Wales up to June 2020 also show that Asian ethnicity and time on dialysis (>5 years) are associated with COVID-19-related mortality among patients on in-centre haemodialysis15.
Practice-related and care delivery-related factors
Concerns about the potential increased exposure of patients on dialysis to SARS-CoV-2 and their increased mortality risk prompted dialysis services worldwide to rapidly implement infection control measures. These policies were largely implemented in the absence of established evidence specifically relating to their effects on SARS-CoV-2 transmission, and outside the setting of prospective trials. Thus, while observational analyses of these policies can identify trends and associations, ideally, trials are needed to derive definitive causal conclusions as to which of the implemented processes are most effective in preventing SARS-CoV-2 transmission.
One audit of >5,700 patients undergoing haemodialysis in centres in London, UK, found that the wearing of facemasks by asymptomatic patients was associated with a 36% decreased hazard of hospital admission for COVID-19 (HR 0.64, 95% CI 0.44–0.93)22. Patients admitted to larger centres or centres with fewer side rooms to separate those with suspected or confirmed disease tended to show worse outcomes. By contrast, isolation strategies (which varied by unit) were not associated with admission for COVID-19, although statistical power was likely to have been too low to detect minor effects of these strategies. Some studies also identified high rates of infection among nursing staff, which would probably contribute to transmission to patients and limit the ability of the centres to deliver services safely6,9. Before the availability of vaccines, some centres reduced the weekly frequency of in-centre dialysis (for example, from three to two sessions per week) during outbreaks, to limit patients’ risk of exposure at treatment centres and during transportation23. Whether such measures were beneficial is unknown; missed dialysis sessions were more common in low-income and in lower-income to middle-income countries24. The lack of dedicated transport for patients attending in-centre haemodialysis with confirmed or suspected SARS-CoV-2 infection also led to hospitalization to prevent co-transportation with other patients23; these occurrences will affect the conclusions of studies that assume hospitalization as a measure of COVID-19 severity.
COVID-19 testing is now more readily available than it was during the early stages of the pandemic. Many dialysis units now use regular asymptomatic surveillance testing to diagnose and isolate infected patients and minimize transmission to other patients. Thus, a greater number of patients identified as infected may be asymptomatic than in the early phases of the pandemic, when for the most-part, only symptomatic patients were tested.
COVID-19 and kidney transplantation
Estimating the incidence of COVID-19 in kidney transplant recipients
As for epidemiological studies in any other population, estimates of the incidence of SARS-CoV-2 infection in kidney transplant recipients depend on the availability of SARS-CoV-2 PCR testing. In early stages of the pandemic, such testing was often limited to hospitalized patients25–27. Nevertheless, a number of studies found a higher incidence of SARS-CoV-2 infection among kidney transplant recipients than in the general population10,28. The reasons for this higher incidence are unclear, but may have been due to high numbers of unavoidable health and social care interactions, susceptibility to infection as a consequence of immunosuppression, or greater access to testing owing to the higher risk of severe disease or as a result of established relationships with health-care providers10,28. Analyses also suggest that viral shedding may persist for longer in transplant recipients following infection, which might yield higher sensitivity from PCR testing than in the general population29–32 (Box 1).
Despite the higher incidence of SARS-CoV-2 infection in transplant recipients than in the general population, regional and national registry studies from regions such as Colombia, England, Flanders, France and Wales have consistently shown that the incidence of SARS-CoV-2 infection among transplant recipients is lower than in patients on dialysis or those on a waiting list for kidney transplantation10,25,33–35. This finding may potentially reflect the inability of many patients on dialysis to isolate given their need to attend thrice-weekly in-centre appointments and/or the outcomes of rigorous SARS-CoV-2 testing, which was implemented in many dialysis centres for infection control.
As for patients on dialysis, seroprevalence studies performed before the availability of COVID-19 vaccines provided insights into the prevalence of SARS-CoV-2 infection among kidney transplant recipients. In a study of kidney transplant recipients in London, UK, from July 2020, only 3.9% of recipients tested positive by PCR; however, 10.4% tested positive for SARS-CoV-2 antibodies by serological screening36. Of note, serological surveys may actually underestimate the incidence of infection among transplant recipients since patients on immunosuppression may be less likely to seroconvert or experience more rapid waning of antibodies. A study from New York City, USA, in late July 2020 found that 20.3% of transplant recipients who had tested positive by PCR did not have detectable antibodies a median of 44 days after diagnosis; seropositivity without PCR-positivity was associated with younger age, an absence of diabetes mellitus and better graft function26. By combining the results of PCR and serology testing, the researchers found an overall prevalence of SARS-CoV-2 infection among transplant recipients of 23.4% — notably lower than the 33% seroprevalence estimated for the local general population in a governmental survey26. As described above, this difference may have been due to lower rates of seroconversion in asymptomatic or mildly symptomatic transplant recipients, more rapid waning of antibodies or a consequence of better adherence to social distancing and/or isolation guidance. It should be noted that serological surveys of transplant recipients may not be representative if some groups of patients were less likely to attend a clinic for testing than others (for example, those more cautious about the risk of transmission). It is also noteworthy that the available seroconversion studies have included few, if any, non-hospitalized kidney transplant recipients37–42.
Mortality in kidney transplant recipients
Studies from early in the pandemic that generally underestimated the incidence of SARS-CoV-2 as a consequence of limited testing in patients with mild or asymptomatic disease may have conversely overestimated the rates of adverse outcomes, such as death. For example, a single-centre study of transplant recipients found that COVID-19-related mortality reduced from 32% to 15% by including cases that were identified through serology testing36. However, as seroconversion rates in non-hospitalized recipients are unknown, this rate may still be an overestimate37–42.
Barriers in access to testing and care, combined with our incomplete understanding of seroconversion rates, means that analyses of risk factors for death in transplant recipients must be scrutinized in terms of the population(s) represented by datasets (Table 1; Supplementary Table 1). The most consistently identified pre-existing risk factors for death in transplant recipients are age, diabetes, cardiovascular disease and receipt of a deceased donor organ16,26,27,36,43–47. Ethnic and socioeconomic inequalities in outcomes have also been found in some settings47. Some studies have found an association between reduced levels of immunosuppression therapy and mortality, but this association is likely to have been a result of confounding by indication (that is, that patients with more severe illness are likely to have their immunosuppression reduced).
As the pandemic progressed, studies investigated whether mortality among transplant recipients is comparable to that in other populations after accounting for age and comorbidities. However, such studies may be susceptible to collider bias (Fig. 1a). As non-kidney solid organ transplants are relatively rare, some studies have investigated overall mortality outcomes across all solid organ recipients combined. Thus, despite the fact that kidney transplantation is overwhelmingly more common than transplantation of other solid organs, reports of mortality outcomes may be biased by better outcomes in liver recipients (the next most transplanted organ), potentially because liver transplant recipients typically have fewer cardiovascular comorbidities and less immunosuppression than kidney recipients28,35.
Outcomes among transplant recipients requiring intensive care are poor. However, a propensity-score matched comparison that matched kidney transplant recipients with non-transplant patients by age, comorbidities and medication profile across 68 intensive care units (ICUs) in the USA found a similar mortality of 40%48. This finding is in line with those of several observational studies that compared outcomes of kidney or solid organ transplant recipients with those of matched, non-transplant patients49–55. However, these findings should be interpreted with caution given the considerable risks of collider bias and residual confounding associated with restricting the study populations to those who received intensive care (Table 1). Some of these studies were also small, which limits the effectiveness of matching on covariables51,53. Diagnosis, clinical presentation, hospitalization and treatment thresholds may also be different for transplant recipients compared to the general population. For instance, some studies found that more transplant recipients than non-transplant patients had no oxygen requirements at admission52,56. This observation may in part reflect the fact that many transplant recipients were admitted for predominantly gastrointestinal symptoms and/or graft impairment as a result of COVID-19, which were associated with a more favourable prognosis57,58. One study found that 21% of patients in the general comparator group of patients with COVID-19 had a ‘do not resuscitate’ order, compared with 9% of transplant recipients with COVID-19, suggesting fundamental differences in the populations included in the study56. The comparable mortality outcomes in transplant recipients and in the general population may also, in part, reflect the beneficial effects of immunomodulatory therapies. In some studies, for example, transplant recipients were more likely than patients in the comparator group to receive tocilizumab52,53,56, which has since demonstrated benefits in reducing mortality in severe COVID-19 (ref.59). Similarly, many transplant recipients would have received prednisolone as part of their immunosuppression regimen; corticosteroids are now routinely used in patients hospitalized with COVID-19.
In contrast to the abovementioned studies that sampled hospitalized groups of patients, however, large studies in unselected populations have demonstrated poorer outcomes among transplant recipients than in the general population. A study of primary care EHRs linked to death registry data from England from the beginning of the pandemic until early May 2020 found that organ recipient status was associated a 3.5-fold higher risk of death from COVID-19 compared with non-transplant status, after accounting for recorded comorbidities60. This finding is in line with registry data from Flanders, Italy and Sweden10,28,61. Similarly, a study of EHR data from the USA reported that transplant recipients had a nearly twofold higher risk of death than non-transplanted patients, after accounting for comorbidities62.
Using mortality data to guide kidney transplantation during the pandemic
Of particular interest to clinicians and policymakers is whether transplant recipients are at a greater risk of death than those on transplant waiting lists, to inform whether transplantation activity should be resumed or continued, particularly given the higher absolute long-term risks of infection and COVID-19-related mortality among patients on in-centre haemodialysis11.
Again, it is essential to consider differential access to testing and care when making comparisons. A study from a hospital network in New York City, USA, found that patients on the transplant waiting list had a 3.6-fold greater odds of death than transplant recipients. Patients the transplant waiting list were also less likely to require oxygen during hospitalization, suggesting less-severe respiratory disease43. However, contradictory findings were reported by the ERACODA collaboration, which reported mortality of 5% among patients on the waiting list compared with 21% in transplant recipients and 25% in all patients on dialysis irrespective of waiting list status21. Similarly, a cohort study in England found mortality of 26% in transplant recipients compared with 10% in those on the waiting list35.
One important consideration when deciding whether to continue transplant programmes is the initial increased infection risk in the acute phase (weeks and months) after surgery. Thus, time since transplantation is an important factor that is often ignored by simple comparisons between patients on the waiting list and transplanted patients. Several studies found mortality of >30% among transplant recipients in the acute phase following transplantation. This high mortality may be a consequence of aggressive immunosuppression and/or more frequent health-care interactions in the early post-transplantation period21,44,57,63. In one large centre in London, a mortality of 24% was found among kidney recipients — including seropositive patients — in their first year after transplantation compared with just 7% among those on the waiting list45. By contrast, a study of several transplant centres across India found that only 15% of recipients transplanted during 2020 who developed COVID-19, died, although the mean age of the transplanted population was 39 years and 95% of transplants were from living donors64. A study from Italy found a negligible effect of time after transplantation with 24% mortality among solid organ transplant recipients within 4 months of transplantation and 27% among transplant recipients overall28. A meta-analysis showed mortality of 30% <15 months after transplantation and 20% for the period 16–60 months after transplantation among kidney transplant recipients during the COVID-19 pandemic65. In view of the generally high mortality associated with SARS-CoV-2 infection, local decision making as to whether transplantation programmes can proceed should be guided by high-quality local data on infection risks in the hospital and dialysis units. Transplant programmes may choose to continue, provided, for example, that transplant recipients can shield themselves in the community, that transplant recipients develop and maintain effective antibody responses after vaccination (despite induction immunosuppression), that effective antiviral treatment is available, and that absolute numbers of hospital-acquired infections can be minimized. However, these requirements will become increasingly difficult to ensure as restrictions are lifted, especially if more virulent strains of SARS-CoV-2 emerge.
Critical illness in transplant recipients
Meta-analyses have shown that 25–29% of kidney transplant recipients with COVID-19 were admitted to an ICU65,66. However, access to critical care facilities varies between centres and regions, and some studies have therefore analysed composite outcomes of death or mechanical ventilation. One of these studies found no difference in the composite outcome of death or mechanical ventilation between solid organ transplant recipients and matched non-transplant patients50, whereas another reported a trend towards increased rates of the composite outcome in transplant recipients55. Studies from both the USA and the UK have found similar rates of mechanical ventilation between critically ill patients on the transplant waiting list and transplant recipients following SARS-CoV-2 infection43,63.
Kidney graft-related outcomes
Kidney transplant recipients have baseline serum creatinine measurements available from routine consultations, and thus AKI can generally be detected from changes in serum creatinine level following admission with COVID-19. Available evidence suggests that AKI is more common in transplant recipients than in other patients hospitalized with COVID-19, with estimates as high as 75–83%30,67,68. One study from the USA found that solid organ transplant recipients hospitalized with COVID-19 were 3.5-times more likely to require dialysis than non-transplant patients55.
Early multicentre studies found varying rates of graft loss, from 4% in a French registry analysis46 to 12% in centres in London, UK63. Registries that are linked to COVID-19 testing data may be able to provide updated reports in the context of more widespread testing but may not have sufficient detail about disease severity and management to enable investigation of possible pathophysiological processes or determine whether outcomes in transplant recipients are different from those in non-transplanted patients.
Although the mechanisms underlying graft loss following SARS-CoV-2 infection are unclear, potential mechanisms include the exacerbation of COVID-19-related microthrombotic complications by calcineurin inhibitors. Alternatively, reduction in immunosuppression following infection might lead to allosensitization and acute rejection. A large single-centre study from São Paulo, Brazil, found that 19% of transplant recipients who were alive 28 days after the onset of COVID-19 symptoms had persistent graft impairment; of the 30% who underwent biopsy, acute rejection was seen in 35% and tubular injury was present in all30. A single-centre study from New York City of 18 kidney transplant recipients with SARS-CoV-2 infection who underwent graft biopsy before May 2021 found vascular rejection in 33% in the first month after SARS-CoV-2 positivity69. Single-centre reports also exist of cytomegalovirus and BK polyomavirus activation after COVID-19 (refs70,71), and of patients developing new donor-specific antibodies72. Of note, observational, single-centre studies are susceptible to selection bias from variation in clinical decision making and the availability and timing of investigations such as biopsies and anti-HLA testing, and must therefore be interpreted with caution.
COVID-19 and chronic kidney disease
Estimating the incidence of COVID-19 among patients with CKD
Estimating the incidence of COVID-19 in patients with CKD who are not on KRT is particularly difficult (Table 1; Supplementary Table 1). Throughout the pandemic, access to SARS-CoV-2 testing often depended on temporal and geographical factors, disease severity and/or perceived risk. Patients in some settings — for example, those in nursing homes — may have had access to universal screening, whereas testing in others may have been more limited73. In addition, the ‘denominator population’ — that is, the total number of individuals with CKD within a given population — is typically unknown since CKD is often under-reported as a consequence of incomplete coding and under-diagnosed in groups perceived to be at lower risk (for example, younger individuals and those without diabetes or cardiovascular disease), and albuminuria is infrequently measured in the general practice setting74. Patients with diagnosed CKD may have been more likely than those with undiagnosed CKD to be tested for SARS-CoV-2 infection due to their more frequent interactions with health services, resulting in collider bias.
Estimates of COVID-19-related mortality in patients with CKD
Validation studies from the UK have demonstrated that the prevalence of estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 can be reliably estimated from UK primary care EHRs75. An analysis of such data from over 17 million adults in England up to early May 2020 found that individuals with eGFR in the range 30–60 ml/min/1.73 m2 had a 33% higher risk of COVID-19-related death than those with no documented eGFR <60 ml/min/1.73 m2, even after taking into account all recorded comorbidities, whereas the risk of COVID-19-related death in those with eGFR <30 ml/min/1.73 m2 was more than double that in individuals with normal kidney function60 (Box 2). This higher risk of COVID-19-related death among patients with CKD may in part reflect the unprecedented demand for critical care resources during the early peak of the pandemic in England, which resulted in reduced access for patients who were considered to be at highest risk of poor outcomes76.
A separate study of health data from >54 million individuals in the National Health Service Digital Trusted Research Environment database in England — currently published in preprint form — identified over 46,000 excess deaths between March 2020 and March 2021 among the >2.3 million individuals identified with CKD (mostly CKD stages 3–5). Of note, although some of these deaths will have been driven by COVID-19, this high mortality also reflects the multi-morbidity and poor outcomes associated with CKD itself77.
Box 2 The effects of COVID-19 on CKD and AKI.
In individuals diagnosed with COVID-19, the risk of death among those with CKD stage 4–5 was 2.5-fold greater than that of individuals with normal kidney function or with mild CKD (stage 1–2)60.
In a large prospective cohort study from the UK, 31.5% of hospitalized patients developed AKI. AKI risk was associated with pre-existing CKD, black ethnicity and tachypnoea at presentation. Mortality correlated with AKI severity and 2.6% of hospitalized patients required KRT79.
The initial weeks of the pandemic in early 2020 were associated with a decrease in the total number of individuals registered with new onset chronic kidney failure requiring KRT. In April 2020 in the USA, there was a 25% decrease in the incidence of kidney failure relative to historical projections; by 1 year after the start of the pandemic, there were about 3.5% fewer KRT patients in the USA than would have been projected90,91. Possible explanations include high mortality of patients with pre-dialysis CKD from COVID-19.
Among military veterans in the USA, 30-day survivors of COVID-19 had a 1.6-fold higher risk of a 50% decline in eGFR and a nearly threefold increased risk of developing kidney failure than individuals who were not infected101.
AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy.
Critical illness in patients with CKD
The Global Burden of Disease collaboration identified CKD as the most prevalent risk factor for severe COVID-19 requiring critical care78. The authors calculated that removing CKD would decrease the proportion of the global population at increased risk of severe COVID-19 from 22% to 17%78. This calculation assumed that CKD occurs in isolation from other conditions; however, in high-income settings, the presence of CKD is an indicator condition of multi-morbidity77. Therefore, this estimate may be an underestimate, since the availability of critical care services for multi-morbid patients varies between clinical settings and over time, particularly when health services are stretched.
Kidney complications in patients with CKD
AKI is readily detectable in hospitalized patients for whom baseline serum creatinine measurements from before admission are available. A large, prospective cohort study by the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) in patients admitted with COVID-19 to >250 hospitals in the UK up to early December 2020 found that patients with underlying CKD were 66% more likely to develop AKI and had a greater than threefold higher risk of requiring acute KRT79 (Box 2). To our knowledge, no studies have evaluated the risk of requiring chronic KRT in patients with CKD after surviving COVID-19.
Arterial and venous thromboembolic complications in patients with CKD
COVID-19 can induce a prothrombotic state, which can increase the risk of venous and arterial thromboembolic events80. Conflicting findings exist as to whether individuals with CKD are at greater risk of thromboembolic events after SARS-CoV-2 infection than those with normal kidney function. The interpretation of available studies is complicated by the fact that many studies do not adequately define or document CKD at baseline, or lack systematic follow-up. A prospective, multicentre registry of over 4,900 hospitalized patients with COVID-19 from New York City, USA, found that CKD was associated with a greater than twofold higher risk of a composite outcome of venous or arterial thromboembolic events and all-cause mortality within 90 days of hospital discharge81. However, that study did not provide a definition of CKD. Another study that used well-accepted definitions of CKD to identify patients with pre-existing CKD before critical care admission found that the occurrence of thromboembolic events among critically ill patients with COVID-19 was similar regardless of CKD status82.
COVID-19 and kidney outcomes in the general population
Acute kidney injury
The development of AKI in patients with COVID-19 is strongly associated with an increased risk of adverse short-term outcomes, such as in-hospital death79. Variations in rates of AKI reported may reflect different national and regional policies regarding criteria for hospital admission, which in turn may complicate comparisons between different settings. For example, reports indicate that patients in China were hospitalized with fewer comorbidities and less-severe disease than patients in other regions. This lower threshold for hospital admission may underlie the very low incidence of AKI found in early studies from China83. In contrast, an analysis of >40,000 patients who were hospitalized with COVID-19 in the UK ISARIC study found that 31.5% developed AKI. Development of AKI was associated with pre-existing CKD, black ethnicity and tachypnoea at presentation, and AKI severity was correlated with higher mortality79. Whether prevention or treatment of AKI can reduce the risk of adverse outcomes, such as in-hospital mortality or long-term kidney complications among patients with COVID-19, is unknown. Until such data are available, AKI could be considered a ‘prognostic factor’ that reflects COVID-19 disease severity, underlying frailty, or reduced renal reserve.
Many published studies have not provided clear definitions or staging of AKI, nor provided information on renal recovery or follow-up. The distinction between AKI in patients with normal baseline kidney function and AKI superimposed on pre-existing CKD is also rarely made. Urine output is reported infrequently outside critical care settings, which may contribute to an underestimate of AKI incidence. A paucity of baseline serum creatinine measurements prior to hospital admission also impedes the identification of patients with undiagnosed, pre-existing CKD. This lack of information complicates the reliable diagnosis and staging of AKI. The assignment of a presumed eGFR of 75 ml/min/1.73 m2 for patients without baseline serum creatinine measurements is problematic, in that it may lead to overestimates of AKI incidence among some individuals (for example, older patients who are likely to have baseline eGFR <75 ml/min/1.73 m2)84. Similarly, the use of the lowest serum creatinine value during hospitalization as the baseline with which to guide retrospective AKI diagnosis at admission may underestimate AKI incidence. Such inaccuracies will consequently distort our understanding of the association between AKI and adverse outcomes, such as in-hospital mortality and long-term kidney failure. Sensitivity analyses can be used to examine whether different definitions and assumptions change study conclusions.
Impacts of AKI on health-care systems
As levels of COVID-19 surged, health systems in various regions faced an increased demand for KRT as a consequence of the high numbers of patients developing AKI. ISARIC reported that 2.6% of patients hospitalized with COVID-19 in the UK required KRT79 whereas an analysis of registry data up to August 2020 found that KRT was required in up to 27% of patients admitted to ICUs in the UK85. In some settings, this high incidence of AKI led to unforeseen shortages of dialysis machines and/or consumables86. Supply chains were in some instances compromised as a consequence of lockdowns or workforce challenges, further threatening local shortages87. More recent studies have found a reduced need for acute KRT among hospitalized patients with COVID-19, which may be due to improvements in fluid management or the use of drugs such as dexamethasone85.
In some resource-limited settings, the reported high mortality of ventilated patients with severe AKI76 led local physicians to consider initiation of KRT almost futile23,88. It has been suggested that the use of a wider variety of modalities (for example, continuous venovenous haemofiltration, prolonged intermittent KRT, sustained low-efficiency dialysis and acute peritoneal dialysis) may enable a greater number of patients to receive KRT89. In addition, strategies such as moderating treatment intensity to conserve fluids, lowering blood flow rate to reduce citrate consumption, or using higher clearance rates to treat more patients per machine could form part of a local response87. However, in the absence of clinical trials or meticulous data capture in large, multicentre registries, it is difficult to evaluate the outcomes of these strategies.
In some settings, the number of patients in whom chronic dialysis was initiated decreased as infection rates increased. This inverse relationship may reflect mortality as a competing outcome, whereby many patients with CKD who, without COVID-19, would have progressed to chronic dialysis, instead died. There were also reduced rates of transplantation, which contributed to delayed haemodialysis initiation in some settings due to a lack of capacity90,91.
De novo immune-mediated kidney disease
A number of case reports suggest that COVID-19 may induce de novo immune-mediated kidney diseases such as IgA nephropathy92, vasculitis93, membranous nephropathy94, minimal change disease95 and collapsing focal segmental glomerulosclerosis96,97. Larger studies are needed to quantify the extent of these associations by comparing, for example, the incidence of specific kidney diseases before and after the pandemic within histopathology registries98. However, patients may be less likely to undergo biopsy during the COVID-19 pandemic and therefore data may be unrepresentative. Another approach would be to compare individuals with and without COVID-19 for the development of de novo immune-mediated kidney disease within a comparative cohort study with protocolized follow-up. Routine data will be problematic because patients with COVID-19 may be more likely to receive follow-up investigations (for example, for serum creatinine, urinary abnormalities and blood pressure) and, therefore, more likely to be diagnosed than those without COVID-19, leading to overestimation of the association.
Long-term kidney outcomes
A growing number of studies are now focusing on post-COVID-19 complications, including kidney diseases99,100. The investigation of rare outcomes, such as kidney failure requires very large cohorts; however, analyses of data from well-established health-care systems that fund KRT for all patients who need it can use initiation of KRT as an outcome proxy defining kidney failure. Of note, however, in many patients with CKD stage 5 (eGFR <15 ml/min/1.73 m2), dialysis may not be initiated until kidney function worsens substantially and/or symptoms appear.
One US cohort study of >89,000 military veterans who had survived COVID-19 and >1.6 million non-infected military veteran controls found that individuals who had survived COVID-19 exhibited an increased risk of all studied outcomes (incidence of AKI, eGFR decline, kidney failure and major adverse kidney events (defined as a decline in eGFR of ≥50%, kidney failure or all-cause mortality)), irrespective of whether they had been hospitalized or admitted to the ICU101. Overall, the rate of kidney failure was almost threefold higher in survivors than in individuals without known infection. Assessment of eGFR decline can be affected by collider bias (Fig. 1b), since only those with an available eGFR assessment are included in such a study. Serum creatinine testing is most likely to be offered to individuals who are at risk of CKD (for example, those with diabetes or cardiovascular disease), thereby distorting the strength of any association between SARS-CoV-2 infection and kidney outcomes.
In a separate cohort study from Hamburg, Germany, median eGFR was found to be similar among 443 adults who had survived SARS-CoV-2 infection (over 90% of whom had not been hospitalized) and in matched population-based controls recruited before the pandemic (median eGFR 108.9 versus 109.1 ml/min/1.73 m2, respectively)102. However, the findings may also have been affected by selection bias, as some participants were recruited through public announcements. Outcomes such as decline in kidney function (for example, a decrease in eGFR below a valid threshold value such as <60 ml/min/1.73 m2 or <30 ml/min/1.73 m2)103, time taken to reach percentage decline in eGFR101,104, and/or longitudinal eGFR decline using linear mixed models105 are likely to be more informative than median residual eGFR in evaluating kidney outcomes following infection. Of note, serum creatinine-based GFR estimates may overestimate true GFR in survivors of severe COVID-19, particularly in those with a prolonged, severe or complex course of illness owing to changes in body composition; thus the true long-term impact of COVID-19 on kidney function loss in such individuals may in turn be underestimated.
The pathophysiological processes by which COVID-19 might lead to a decline in kidney function remain unknown. Autopsy studies of patients who have died with COVID-19 suggest that SARS-CoV-2 may directly infect the kidney, causing upregulation of profibrotic cell signalling pathways, although large immunohistochemistry series of kidney biopsies have not found evidence of SARS-CoV-2 expression98,106. However, these findings may be affected by collider bias, since pre-existing CKD is a risk factor for death from severe COVID-19, which in turn may distort associations between COVID-19 and the histological features of CKD at autopsy. Moreover, it is difficult to establish temporality — that is, whether histological or molecular changes seen on autopsy are present because people with CKD are more likely to die from COVID-19 (that is, that the histological changes preceded COVID-19) or whether the changes were caused by COVID-19 (Fig. 1c).
COVID-19 pharmacoepidemiology in kidney disease
Safety of existing drugs in COVID-19
Early in the pandemic, potential safety concerns were raised about the use of angiotensin-converting enzyme (ACE) inhibitors, which are considered a standard of care for many patients with hypertension, CKD, ischaemic heart disease and heart failure. These concerns arose from the finding that SARS-CoV-2 enters cells via the functional receptor, ACE2 (ref.107), and some suggestions that ACE2 expression might be upregulated by ACE inhibitors108. However, observational studies from Lombardy, Italy109, New York City, USA110, and other regions111–113, have consistently suggested that no association exists between ACE inhibitor use and the incidence and/or progression of COVID-19. This finding has since been confirmed by two randomized controlled trials that demonstrated no difference in COVID-19 outcomes, such as COVID-19 progression and death, among hospitalized patients, regardless of whether they continued or discontinued ACE inhibitor treatment114,115.
Clinical trials are imperfect vehicles for detecting rare outcomes. Trial data can be complemented by analyses of administrative databases, which can provide rapid insights into persistent safety concerns about commonly used drugs, such as ACE inhibitors109–113 and non-steroidal anti-inflammatory drugs116,117, in a real-world setting. However, caution is needed in such analyses to appropriately address confounding by indication. For example, patients with and without an ACE inhibitor prescription are likely to be fundamentally different in terms of the existence of comorbidities, such as hypertension, CKD and heart failure. To reduce the influence of confounding by indication, an active comparator study design (for example, one that compares patients with hypertension on ACE inhibitors with similar patients on other antihypertensives) may be a more rigorous analytical approach.
Anti-COVID-19 therapies
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial demonstrated benefits of dexamethasone, tocilizumab and baricitinib in reducing mortality in hospitalized patients with COVID-19 (refs59,118,119). The study also found no benefit of other therapies that were used widely in patients with kidney disease early in the pandemic120, including lopinavir–ritonavir, hydroxychloroquine and azithromycin121–123. Dexamethasone reduced the requirement for KRT by 39%118, and tocilizumab by 28%59. The RECOVERY trial is ongoing and will continue to investigate the effects of repurposed therapies. To date it has recruited >45,000 participants, but although the trial includes individuals with pre-existing kidney diseases, subgroup analyses have not been reported and would probably be underpowered. Of note, participants can be considered unsuitable for randomization to specific therapies, which may affect generalizability. For example, 28% of recruited participants with eGFR <30 ml/min/1.73 m2 and a third of people with diabetes were considered unsuitable for randomization to dexamethasone118. Although the RECOVERY trial can serve as a blueprint for an approach to rapidly determine effective therapies in future pandemics and other clinical settings, real-world analyses of inpatient prescription data are also required to support safety and efficacy in subgroups, such as patients with kidney disease. Such pharmacoepidemiology requires specific methodological considerations to generate valid and reliable evidence, such as active comparator study designs (to minimize confounding by indication) and valid definitions of outcomes124.
An accumulating body of evidence showing impaired vaccine responses among patients on dialysis and kidney transplant recipients highlights the urgent need for studies to evaluate the ability of novel antibody and antiviral therapies to reduce COVID-19 disease severity in these high-risk groups125. The SARS-CoV-2 monoclonal antibody sotrovimab markedly reduced hospitalization and death among high-risk, non-hospitalized patients with COVID-19. Although the trial included CKD in its definition of ‘high-risk’, patients with eGFR <60 ml/min/1.73 m2 comprised <1% of participants126. The RECOVERY trial found that combination casirivimab–imdevimab monoclonal antibody therapy reduced mortality in seronegative patients hospitalized with COVID-19; participants included those on dialysis and kidney transplant recipients127. A living systematic review and network meta-analysis (including 47 randomized controlled trials published up to 21 July 2021) concluded that casirivimab–imdevimab and some other antibody therapies may reduce hospitalization in patients with non-severe COVID-19, whereas convalescent plasma, intravenous immunoglobulin and other antibody and cellular therapies are unlikely to provide meaningful benefit, although most of the studies included in the analysis did not seem to include patients with kidney diseases128.
COVID-19 vaccines
A number of studies have examined the efficacy and safety of COVID-19 vaccines in patients with kidney diseases — including those on dialysis and kidney transplant recipients129,130. The Renal Patients COVID-19 Vaccination Immune Response (RECOVAC IR) study, which includes patients with CKD not on dialysis, is ongoing131. The available evidence indicates that kidney transplant recipients and patients on dialysis have an impaired response to vaccines compared with that in the general population125,132,133. A prospective study found that patients on dialysis exhibited higher antibody development rates than kidney transplant recipients (>95% versus 42%)134. In a study of over 9,000 patients on dialysis, 87% and 96% of patients had developed a seroresponse to the BNT162b2 Pfizer and mRNA-1273 Moderna mRNA vaccines, respectively, but only 37% had developed a seroresponse to Ad26.COV2.S Janssen adenoviral vector vaccine 14–74 days after completion of the vaccination schedule135. However, the longer-term follow-up demonstrated that antibody responses to the mRNA vaccines declined within 6 months136. These findings and others prompted policy changes to increase the routine vaccination series to include three primary doses in these patients.
A more recent study found that a third dose of BNT162b2 Pfizer vaccine improved humoral immune responses in kidney transplant recipients137; however, the effectiveness of this approach on clinical outcomes such as death and hospitalization remains unknown. An analysis of national transplant registry data from England found that while fewer deaths occurred among transplant recipients vaccinated with the ChAdOx1-S Oxford–AstraZeneca vaccine than among unvaccinated transplant recipients following SARS-CoV-2 infection, there was no survival benefit associated with the BNT162b2 Pfizer vaccine138. However, this apparent lack of benefit from the BNT162b2 Pfizer vaccine might have been due to the result of residual confounding due to comorbidities or age (which was classified, somewhat crudely, as 16–49 or >50 years) if those considered higher risk were more likely to receive BNT162b2 Pfizer in the earliest stages of the vaccine rollout.
Some case reports suggest that COVID-19 vaccines may also induce de novo or reactivation of intrinsic kidney diseases139. Large studies comparing patients with and without vaccination are needed to confirm these signals at the population level. However, in countries with high rates of vaccine uptake — particularly in those in which populations were prioritized for vaccination based on clinical risk — the pool from which to draw from in the comparison group may be very small, and patients with and without vaccination may be systematically different in terms of their health status and behaviours.
Conclusions and future directions
The global science community should be praised for the speed at which epidemiological studies contributed to clinical decision making for the benefit of patients with kidney disease; however, the pandemic also provides an opportunity to further improve how epidemiological research can serve the public health good. We have highlighted how barriers to health care during the pandemic can contribute to biased epidemiological data. These biases can be addressed by adequate study design, but careful planning and thought as to the most important research questions in the context of a fast-moving pandemic are needed in order to have study designs and data collection tools at the ready to meet future challenges.
Despite many advances in our understanding of COVID-19 and its interactions with comorbidities such as kidney disease, a number of questions remain unanswered (Box 3; Table 2). Quantifying the long-term impacts of the effects of COVID-19 on the kidney on the development and progression of CKD is a public health priority, as is the identification of approaches to improve outcomes in patients with kidney disease. EHRs have great potential to identify changes in disease epidemiology as the pandemic progresses, and to enable the rapid evaluation of the safety and efficacy of therapies in real-world settings. However, for EHR systems to be useful, nephrology researchers must understand the extent to which their patients’ data are ‘captured’ in local and national databases. Kidney disease registries must be readily integrated with EHR systems and be able to receive real-time data to monitor outbreaks and help plan local infection control processes.
Table 2.
Addressing challenges in conducting epidemiological research in populations with kidney disease
| Challenges | Potential solutions |
|---|---|
| Unrepresentative denominator populations |
Representative registries of patients with CKD, AKI and on KRT. In settings where legislation prevents analysis of data without consent, ensure prospective consent of patients for inclusion in follow-up studies and trials |
| Incomplete capture of outcomes | Work with health systems to prospectively capture routine clinical care and outcomes in registry populations |
| Health policy changes without sufficient evidence | Create trial protocols for protective measures taken and/or treatments that can be implemented at short notice |
| Small samples sizes | Share protocols for case definitions across international registries to enable adequately powered and more representative studies in rare disease populations |
| Data not generalizable to low-resource settings with different at-risk population profiles | To aid local policy makers, registries should be built globally and not just in high-income settings |
AKI, acute kidney injury; CKD, chronic kidney disease; KRT, kidney replacement therapy.
Unfortunately, COVID-19 will not be the last infectious disease pandemic. Nephrology and public health communities need to work together to establish protocols for future pandemics, ideally relying on findings from representative patient populations. The UK ISARIC study is a good example of a secondary care prospective study that was rapidly implemented to accumulate data from hospitals across the UK, having been designed several years earlier in anticipation of a pandemic79.
Collider bias — a constant threat to public health reporting efforts — can be avoided only by breaking down barriers to care and the associated documentation of health needs. For example, using defined cohorts of pre-consented and engaged patients, there is no reason why technological advancements cannot be developed to enable gathering of symptoms data in real time, particularly in patients who are self-isolating.
Protocols should be in place, including randomized components, to evaluate the efficacy of centre-level interventions where clinical equipoise exists. Such randomized trials are the gold standard with which to assess the causality of interventions; for example, whether a temporary reduction to twice-weekly dialysis or cessation of transplantation programmes reduces exposure at infection epicentres and whether these interventions are associated with overall benefit.
In patients with relatively rare conditions who are typically excluded from clinical trials, such as patients on KRT or those with rare immune-mediated kidney diseases, global trial protocols should be in place to allow participating disease registries to rapidly implement adequately powered treatment and vaccination trials when called upon. Registries with systematic, real-time capture of incident and relapsed intrinsic kidney disease, with linkages to other records in defined-catchment populations, are required to keep track of rare complications of infections and treatments.
A stark imbalance is apparent between economically advanced regions and those with fewer resources both in their capacity to conduct studies using comprehensive EHR sources and in their universal provision of care for kidney diseases140. The pandemic has highlighted the importance of economic empowerment and of targeted approaches to deliver equitable and sustainable global solutions, and this principle should extend to the infrastructure needed to conduct large-scale studies at speed. Lack of access to appropriate tools for local data collection, surveillance, planning and development may lead to unexpected and costly consequences when it comes to the provision of care and may lead to premature loss of life. Future pandemics will doubtless occur, and may arise in low-income settings due to factors such as ongoing biodiversity loss and the increasing urbanization that characterizes even the poorest countries. Timely high-quality epidemiological research holds the potential to save countless lives worldwide when future pandemics arise.
Box 3 Possible directions for future research.
Quantifying the long-term impacts of COVID-19 on the development and progression of CKD should be a public health priority.
Pharmacological and non-pharmacological clinical trials are required in patients who developed AKI following SARS-CoV-2 infection (and as a result of other causes) to identify approaches to minimize the risk of adverse outcomes, including the development of CKD, kidney failure, heart failure and thrombotic events.
The risk of SARS-CoV-2 reinfection, and in particular the role of vaccination (including additional and booster doses) in reducing the risk of severe illness in patients with kidney disease, requires greater understanding.
The effects of ‘long COVID’ in patients on dialysis and kidney transplant recipients should be studied, particularly in terms of whether this phenomenon represents an additional source of morbidity in a population with an already high comorbidity burden.
The safety and efficacy of emerging drugs for COVID-19 (for example, antibody therapies) should be evaluated specifically in patients with kidney disease. Patients with kidney disease (for example, transplant recipients) may potentially be amongst those to benefit most from early intervention to reduce infection severity, given that vaccine responses may be impaired and mortality high in this population.
Research is needed to establish whether drugs such as SGLT2 inhibitors, for example, are acutely renoprotective in the setting of COVID-19-related AKI.
Systematic multicentre registries of patients with intrinsic kidney diseases within well-defined catchment populations with capacity for linkage to data on infection and vaccination should be established to enable rapid assessment of the ability of infections and vaccines to induce de novo or reactivation of kidney disease.
AKI, acute kidney injury; CKD, chronic kidney disease
Supplementary information
Glossary
- Viral shedding
The release of viral particles from the respiratory tract, which may lead to infection transmission and/or viral detection by PCR.
- Sensitivity analyses
Analyses exploring varying statistical assumptions and data definitions (if unsure which is best) to confirm the robustness of study findings. If analysis results change drastically under different analysis assumptions, biases may contribute to study findings as opposed to there being an association in truth.
- Linear mixed models
Statistical methodology that takes account within-person correlations of repeated continuous measurements taken in the same patient.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Nephrology thanks Ziyad Al-Aly, Annette Bruchfeld and Priya Vart for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41581-022-00570-3.
References
- 1.Chung EYM, et al. Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am. J. Kidney Dis. 2021;78:804–815. doi: 10.1053/j.ajkd.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith GJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Y, et al. Epidemiological, clinical, and immunological features of a cluster of COVID-19-contracted hemodialysis patients. Kidney Int. Rep. 2020;5:1333–1341. doi: 10.1016/j.ekir.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberici F, et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int. Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Milia V, et al. COVID-19 outbreak in a large hemodialysis center in Lombardy, Italy. Kidney Int. Rep. 2020;5:1095–1099. doi: 10.1016/j.ekir.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett RW, et al. Epidemiology of COVID-19 in an urban dialysis center. J. Am. Soc. Nephrol. 2020;31:1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goicoechea M, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke C, et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J. Am. Soc. Nephrol. 2020;31:1969–1975. doi: 10.1681/ASN.2020060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugawara Y, et al. Infection prevention measures for patients undergoing hemodialysis during the COVID-19 pandemic in Japan: a nationwide questionnaire survey. Ren. Replace. Ther. 2021;7:27. doi: 10.1186/s41100-021-00350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Meester J, et al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J. Am. Soc. Nephrol. 2021;32:385–396. doi: 10.1681/ASN.2020060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Kidney Association. COVID-19 surveillance report. https://ukkidney.org/audit-research/publications-presentations/report/covid-19-surveillance-reports (2022).
- 12.Couchoud C, et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020;98:1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand S, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396:1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manuela Savino SS, et al. Outcomes of patients with COVID-19 on kidney replacement therapy: a comparison among modalities in England. Clin. Kidney J. 2021;14:5273–2581. doi: 10.1093/ckj/sfab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savino M, et al. Sociodemographic features and mortality of individuals on haemodialysis treatment who test positive for SARS-CoV-2: a UK Renal Registry data analysis. PLoS ONE. 2020;15:e0241263. doi: 10.1371/journal.pone.0241263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jager KJ, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinhandl ED, et al. Initial effects of COVID-19 on patients with ESKD. J. Am. Soc. Nephrol. 2021;32:1444–1453. doi: 10.1681/ASN.2021010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Emergency use ICD codes for COVID-19 disease outbreak. https://www.who.int/standards/classifications/classification-of-diseases/emergency-use-icd-codes-for-covid-19-disease-outbreak (2022).
- 19.Wetmore JB, et al. Readmissions following a hospitalization for cardiovascular events in dialysis patients: a retrospective cohort study. J. Am. Heart Assoc. 2018 doi: 10.1161/jaha.117.007231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalrymple LS, et al. Outcomes of infection-related hospitalization in Medicare beneficiaries receiving in-center hemodialysis. Am. J. Kidney Dis. 2015;65:754–762. doi: 10.1053/j.ajkd.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilbrands LB, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol. Dial. Transpl. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplin B, et al. Risk of COVID-19 disease, dialysis unit attributes, and infection control strategy among London in-center hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2021;16:1237–1246. doi: 10.2215/CJN.03180321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones ESW, et al. COVID-19 and the kidney: a South African state healthcare experience. Clin. Nephrol. 2021;95:171–181. doi: 10.5414/CN110390. [DOI] [PubMed] [Google Scholar]
- 24.Tannor EK, et al. The COVID-19 pandemic identifies significant global inequities in hemodialysis care in low and lower middle-income countries–an ISN/DOPPS survey. Kidney Int. Rep. 2022 doi: 10.1016/j.ekir.2022.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias-Murillo YR, et al. SARS-CoV2/COVID-19 infection in transplant recipients and in patients on the organ transplant waiting list in Colombia. Transpl. Proc. 2021;53:1237–1244. doi: 10.1016/j.transproceed.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzi Y, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98:1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favà A, et al. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: a multicentric cohort study. Am. J. Transpl. 2020;20:3030–3041. doi: 10.1111/ajt.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapani S, et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am. J. Transpl. 2021;21:2509–2521. doi: 10.1111/ajt.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaston DC, et al. Clinical implications of SARS-CoV-2 cycle threshold values in solid organ transplant recipients. Am. J. Transpl. 2021;21:1304–1311. doi: 10.1111/ajt.16357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cristelli MP, et al. The full spectrum of COVID-19 development and recovery among kidney transplant recipients. Transplantation. 2021;105:1433–1444. doi: 10.1097/TP.0000000000003751. [DOI] [PubMed] [Google Scholar]
- 31.Benotmane I, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am. J. Transpl. 2020;20:3162–3172. doi: 10.1111/ajt.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nahi SL, Shetty AA, Tanna SD, Leventhal JR. Renal allograft function in kidney transplant recipients infected with SARS-CoV 2: an academic single center experience. PLoS ONE. 2021;16:e0252979. doi: 10.1371/journal.pone.0252979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thaunat O, et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) Kidney Int. 2020;98:1568–1577. doi: 10.1016/j.kint.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalid U, Ilham MA, Nagaraja P, Elker D, Asderakis A. SARS-CoV-2 in kidney transplant and waitlisted patients during the first peak: the Welsh experience. Transpl. Proc. 2021;53:1154–1159. doi: 10.1016/j.transproceed.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravanan R, et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am. J. Transpl. 2020;20:3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willicombe M, et al. Identification of patient characteristics associated with SARS-CoV-2 infection and outcome in kidney transplant patients using serological screening. Transplantation. 2021;105:151–157. doi: 10.1097/TP.0000000000003526. [DOI] [PubMed] [Google Scholar]
- 37.Bajpai D, et al. Development and longevity of antibodies against SARS-CoV-2 in kidney transplant recipients after symptomatic COVID-19. Transpl. Infect. Dis. 2021;23:e13646. doi: 10.1111/tid.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benotmane I, et al. Persistence of SARS-CoV-2 antibodies in kidney transplant recipients. Am. J. Transpl. 2021;21:2307–2310. doi: 10.1111/ajt.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyarsky BJ, et al. Early development and durability of SARS-CoV-2 antibodies among solid organ transplant recipients: a pilot study. Transplantation. 2021;105:e52–e53. doi: 10.1097/TP.0000000000003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruno PF, et al. COVID-19 infection: viral clearance and antibody response in dialysis patients and renal transplant recipients. Nephron. 2021;145:363–370. doi: 10.1159/000515128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavarot N, et al. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int. 2021;99:486–488. doi: 10.1016/j.kint.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zervou FN, Ali NM, Neumann HJ, Madan RP, Mehta SA. SARS-CoV-2 antibody responses in solid organ transplant recipients. Transpl. Infect. Dis. 2021;23:e13728. doi: 10.1111/tid.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craig-Schapiro R, et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am. J. Transpl. 2021;21:1576–1585. doi: 10.1111/ajt.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salto-Alejandre S, et al. Risk factors for unfavorable outcome and impact of early post-transplant infection in solid organ recipients with COVID-19: a prospective multicenter cohort study. PLoS ONE. 2021;16:e0250796. doi: 10.1371/journal.pone.0250796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke C, et al. Informing the risk of kidney transplantation versus remaining on the waitlist in the coronavirus disease 2019 era. Kidney Int. Rep. 2021;6:46–55. doi: 10.1016/j.ekir.2020.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caillard S, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elias M, et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J. Am. Soc. Nephrol. 2020;31:2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molnar MZ, et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am. J. Transpl. 2020;20:3061–3071. doi: 10.1111/ajt.16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chavarot N, et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am. J. Transpl. 2021;21:1285–1294. doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linares L, et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS ONE. 2021;16:e0247251. doi: 10.1371/journal.pone.0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pereira MR, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl. Infect. Dis. 2021;23:e13637. doi: 10.1111/tid.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma P, et al. COVID-19 outcomes among solid organ transplant recipients: a case-control study. Transplantation. 2021;105:128–137. doi: 10.1097/TP.0000000000003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hugo C, Vehreschild J, Stecher M. Response to Invited Commentary “Undoubtedly, kidney transplant recipients have a higher mortality due to COVID-19 disease compared to the general population”. Transpl. Int. 2021;34:771–773. doi: 10.1111/tri.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nair V, et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am. J. Transpl. 2021;21:2522–2531. doi: 10.1111/ajt.16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avery RK, et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: a retrospective cohort. Am. J. Transpl. 2021;21:2498–2508. doi: 10.1111/ajt.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crespo M, et al. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104:2225–2233. doi: 10.1097/TP.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 58.Villanego F, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish registry. Am. J. Transpl. 2021;21:2573–2582. doi: 10.1111/ajt.16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Søfteland JM, et al. COVID-19 in solid organ transplant recipients: a national cohort study from Sweden. Am. J. Transpl. 2021;21:2762–2773. doi: 10.1111/ajt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher AM, et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin. Transpl. 2021;35:e14216. doi: 10.1111/ctr.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mamode N, et al. Mortality rates in transplant recipients and transplantation candidates in a high-prevalence COVID-19 environment. Transplantation. 2021;105:212–215. doi: 10.1097/TP.0000000000003533. [DOI] [PubMed] [Google Scholar]
- 64.Jha PK, et al. A retrospective multi-center experience of renal transplants from India during COVID-19 pandemic. Clin. Transpl. 2021;35:e14423. doi: 10.1111/ctr.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kremer D, et al. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: lessons to be learned. Am. J. Transpl. 2021;21:3936–3945. doi: 10.1111/ajt.16742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raja MA, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transpl. Rev. 2021;35:100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajpai D, et al. Recovery of kidney function after AKI because of COVID-19 in kidney transplant recipients. Transpl. Int. 2021;34:1074–1082. doi: 10.1111/tri.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benotmane I, et al. Biomarkers of cytokine release syndrome predict disease severity and mortality from COVID-19 in kidney transplant recipients. Transplantation. 2021;105:158–169. doi: 10.1097/TP.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 69.Daniel E, et al. Kidney allograft biopsy findings after COVID-19. Am. J. Transpl. 2021;21:4032–4042. doi: 10.1111/ajt.16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meshram HS, Kute VB, Chauhan S. BK polyomavirus infection following COVID-19 infection in renal transplant recipients: a single-center experience. Kidney Res. Clin. Pract. 2021;40:496–500. doi: 10.23876/j.krcp.21.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larrosa-Garcia M, et al. Long-term effects of COVID-19 in solid organ transplantation recipients. Transpl. Infect. Dis. 2021;23:e13677. doi: 10.1111/tid.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kute VB, et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021;105:851–860. doi: 10.1097/TP.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dumyati G, Gaur S, Nace DA, Jump RLP. Does universal testing for COVID-19 work for everyone? J. Am. Med. Dir. Assoc. 2020;21:1525–1532. doi: 10.1016/j.jamda.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonald HI, et al. Methodological challenges when carrying out research on CKD and AKI using routine electronic health records. Kidney Int. 2016;90:943–949. doi: 10.1016/j.kint.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Iwagami M, et al. Validity of estimated prevalence of decreased kidney function and renal replacement therapy from primary care electronic health records compared with national survey and registry data in the United Kingdom. Nephrol. Dial. Transpl. 2017;32:ii142–ii150. doi: 10.1093/ndt/gfw318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doidge JC, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am. J. Respir. Crit. Care Med. 2021;203:565–574. doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dashtban, M. et al. Predicting and validating risk of pre-pandemic and excess mortality in individuals with chronic kidney disease. Preprint at Lancethttps://papers.ssrn.com/sol3/papers.cfm?abstract_id=3970707 (2021).
- 78.Clark A, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob. Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan MK, et al. Acute kidney injury in patients hospitalised with COVID-19 from the ISARIC WHO CCP-UK study: a prospective, multicentre cohort study. Nephrol. Dial. Transpl. 2021 doi: 10.1093/ndt/gfab303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bikdeli B, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giannis D, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flythe JE, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am. J. Kidney Dis. 2021;77:190–203.e1. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guthrie G, et al. Developing an AKI consensus definition for database research: findings from a scoping review and expert opinion using a Delphi process. Am. J. Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 85.Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland 23 October 2020. https://www.icnarc.org/DataServices/Attachments/Download/342c4f9c-5115-eb11-912b-00505601089b (2020).
- 86.Chua HR, et al. Ensuring sustainability of continuous kidney replacement therapy in the face of extraordinary demand: lessons from the COVID-19 pandemic. Am. J. Kidney Dis. 2020;76:392–400. doi: 10.1053/j.ajkd.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burgner A, Ikizler TA, Dwyer JP. COVID-19 and the inpatient dialysis unit: managing resources during contingency planning pre-crisis. Clin. J. Am. Soc. Nephrol. 2020;15:720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Critical Care Society of Southern Africa. Allocation of scarce critical care resources during the COVID-19 public health emergency in South Africa. https://criticalcare.org.za/wp-content/uploads/2020/06/V3-2020-May-05-Allocation-of-Scarce-Critical-Care-Resources-During-the-COVID-19-Public-Health-Emergency-in-South-Africa-FINAL-.pdf (2020). [PubMed]
- 89.Nadim MK, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020;16:747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wetmore JB, et al. Changes in treatment of patients with incident ESKD during the novel coronavirus disease 2019 pandemic. J. Am. Soc. Nephrol. 2021;32:2948–2957. doi: 10.1681/ASN.2021040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinhandl ED, Gilbertson DT, Wetmore JB, Johansen KL. COVID-19-associated decline in the size of the end-stage kidney disease population in the United States. Kidney Int. Rep. 2021;6:2698–2701. doi: 10.1016/j.ekir.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farooq H, et al. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: a systematic review. J. Taibah Univ. Med. Sci. 2021 doi: 10.1016/j.jtumed.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3:e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miao J, Fidler ME, Nasr SH, Larsen CP, Zoghby ZM. Membranous nephropathy in a patient with coronavirus disease 2019 (COVID-19): a case report. Clin. Nephrol. Case Stud. 2021;9:11–18. doi: 10.5414/CNCS110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gupta RK, Bhargava R, Shaukat AA, Albert E, Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21:326. doi: 10.1186/s12882-020-01970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magoon S, et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2:488–492. doi: 10.1016/j.xkme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma Y, et al. COVID-19-associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2:493–497. doi: 10.1016/j.xkme.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.May RM, et al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in coronavirus 2019 disease (COVID-19) Kidney Int. 2021;100:1303–1315. doi: 10.1016/j.kint.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu T, et al. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ayoubkhani D, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J. Am. Soc. Nephrol. 2021;32:2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petersen EL, et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: the Hamburg City Health Study COVID programme. Eur. Heart J. 2022 doi: 10.1093/eurheartj/ehab914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Novak M, et al. Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in patients with diabetes with comorbid depression. Diabetes Care. 2016;39:1940–1947. doi: 10.2337/dc16-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klatte DCF, et al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153:702–710. doi: 10.1053/j.gastro.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 105.Janmaat CJ, et al. Lower serum calcium is independently associated with CKD progression. Sci. Rep. 2018;8:5148. doi: 10.1038/s41598-018-23500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jansen J, et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022;29:217–231.e8. doi: 10.1016/j.stem.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferrario CM, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 109.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reynolds HR, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morales DR, et al. Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit. Health. 2021;3:e98–e114. doi: 10.1016/S2589-7500(20)30289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Savarese G, Benson L, Sundström J, Lund LH. Association between renin-angiotensin-aldosterone system inhibitor use and COVID-19 hospitalization and death: a 1.4 million patient nationwide registry analysis. Eur. J. Heart Fail. 2021;23:476–485. doi: 10.1002/ejhf.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jung SY, Choi JC, You SH, Kim WY. Association of renin-angiotensin-aldosterone system inhibitors with coronavirus disease 2019 (COVID-19)-related outcomes in Korea: a nationwide population-based cohort study. Clin. Infect. Dis. 2020;71:2121–2128. doi: 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopes RD, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cohen JB, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir. Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lund LC, et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-CoV-2: a Danish nationwide cohort study. PLoS Med. 2020;17:e1003308. doi: 10.1371/journal.pmed.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeong HE, et al. Association between nonsteroidal antiinflammatory drug use and adverse clinical outcomes among adults hospitalized with coronavirus 2019 in South Korea: a nationwide study. Clin. Infect. Dis. 2021;73:e4179–e4188. doi: 10.1093/cid/ciaa1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Horby P, et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Preprint at medRxiv10.1101/2022.03.02.22271623 (2022). [DOI] [PMC free article] [PubMed]
- 120.Mahalingasivam V, et al. A systematic review of COVID-19 and kidney transplantation. Kidney Int. Rep. 2021;6:24–45. doi: 10.1016/j.ekir.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Group RC. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Horby P, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Group RC. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pottegård A, Kurz X, Moore N, Christiansen CF, Klungel O. Considerations for pharmacoepidemiological analyses in the SARS-CoV-2 pandemic. Pharmacoepidemiol. Drug Saf. 2020;29:825–831. doi: 10.1002/pds.5029. [DOI] [PubMed] [Google Scholar]
- 125.The OpenSAFELY Collaborative. Describing the population experiencing COVID-19 vaccine breakthrough following second vaccination in England: a cohort study from OpenSAFELY. Preprint at medRxiv10.1101/2021.11.08.21265380 (2021). [DOI] [PMC free article] [PubMed]
- 126.Gupta A, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 127.RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siemieniuk RA, et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ. 2021;374:n2231. doi: 10.1136/bmj.n2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glenn DA, et al. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int. Rep. 2021;6:1407–1410. doi: 10.1016/j.ekir.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Windpessl M, et al. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021;17:291–293. doi: 10.1038/s41581-021-00406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kho MML, et al. The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis or living with a kidney transplant. Nephrol. Dial. Transpl. 2021;36:1761–1764. doi: 10.1093/ndt/gfab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ravanan R, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021;105:e263–e264. doi: 10.1097/TP.0000000000003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qin CX, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105:e265–e266. doi: 10.1097/TP.0000000000003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stumpf J, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021;9:100178. doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu CM, et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients. Am. J. Kidney Dis. 2022;79:307–310. doi: 10.1053/j.ajkd.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hsu CM, et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients over 6 months. Clin. J. Am. Soc. Nephrol. 2022;17:403–413. doi: 10.2215/CJN.12250921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Masset C, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100:1132–1135. doi: 10.1016/j.kint.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Callaghan CJ, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106:436–446. doi: 10.1097/TP.0000000000004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. 2021;100:959–965. doi: 10.1016/j.kint.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.See EJ, et al. Global coverage of health information systems for kidney disease: availability, challenges, and opportunities for development. Kidney Int. Suppl. 2018;8:74–81. doi: 10.1016/j.kisu.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.