Abstract
Metformin can suppress gluconeogenesis and reduce blood sugar by activating adenosine monophosphate-activated protein kinase (AMPK) and inducing small heterodimer partner (SHP) expression in the liver cells. The main mechanism of metformin’s action is related to its activation of the AMPK enzyme and regulation of the energy balance. AMPK is a heterothermic serine/threonine kinase made of a catalytic alpha subunit and two subunits of beta and a gamma regulator. This enzyme can measure the intracellular ratio of AMP/ATP. If this ratio is high, the amino acid threonine 172 available in its alpha chain would be activated by the phosphorylated liver kinase B1 (LKB1), leading to AMPK activation. Several studies have indicated that apart from its significant role in the reduction of blood glucose level, metformin activates the AMPK enzyme that in turn has various efficient impacts on the regulation of various processes, including controlling inflammatory conditions, altering the differentiation pathway of immune and non-immune cell pathways, and the amelioration of various cancers, liver diseases, inflammatory bowel disease (IBD), kidney diseases, neurological disorders, etc. Metformin’s activation of AMPK enables it to control inflammatory conditions, improve oxidative status, regulate the differentiation pathways of various cells, change the pathological process in various diseases, and finally have positive therapeutic effects on them. Due to the activation of AMPK and its role in regulating several subcellular signalling pathways, metformin can be effective in altering the cells’ proliferation and differentiation pathways and eventually in the prevention and treatment of certain diseases.
Graphical abstract
Keywords: AMPK, Metformin, Diseases
Introduction
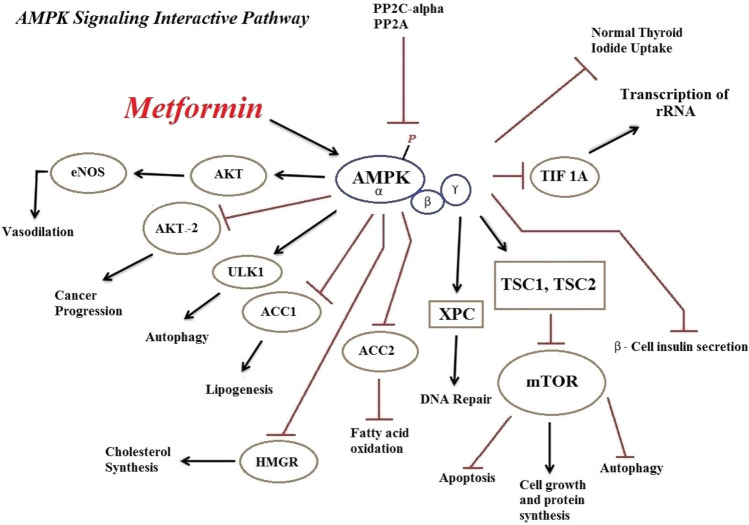
Metformin is known as one of the oldest, and most widely used oral medications in the treatment of type two diabetes or diabetes mellitus (Beladi Mousavi 2012; Taleb et al. 2014). As a result of metformin’s activity, the mitochondrial respiratory chain complex (MRCC) (Baur and Birnbaum 2014) is blocked, the rate of glucose production from glycogen in liver cells is reduced, and sensitivity to insulin and glucose uptake through the activation of the adenosine monophosphate-activated protein kinase (AMPK) increases in the liver and peripheral tissues (Ismaiel et al. 2016; Mummidi et al. 2016; Wang et al. 2016). Metformin can suppress gluconeogenesis and reduce blood sugar by activating AMPK and inducing small heterodimer partner expression in the liver (Kim et al. 2008; Chanda et al. 2009). AMPK is a heterothermic serine/threonine kinase made of a catalytic alpha subunit and two subunits of beta and a gamma regulator (Oakhill et al. 2009; Hasanvand et al. 2016). This enzyme can measure the intracellular ratio of AMP/ATP. If this ratio is high, the amino acid threonine 172 available in its alpha chain would be activated by the phosphorylated liver kinase B1 (LKB1), leading to AMPK activation (Young and Zaha 2012; Hardie 2015). In other words, since it is an intracellular energy sensor, AMPK interferes with the regulation of glucose, cellular as well as whole‐body energy homeostasis, and fatty acid metabolism (Shaw et al. 2005; Bright et al. 2009; Oakhill et al. 2009). Moreover, the muscle activity, physiological stress and oxidative factors are also able to activate the AMPK (Kim, Yang et al. 2016).
AMPK, metabolic and non-metabolic pathways
Several studies have examined the role of AMPK signalling pathway in various metabolic processes. This enzyme inhibits anabolic pathways (ATP consumer), and activates catabolic pathways to re-supply the cellular energy sources. The results of a study conducted by Suet Ching Chen in 2017 revealed that metformin can suppress adipogenesis through processes that are either dependent or independent of AMPK (Chen et al. 2017). Moreover, the effect of metformin independent of AMPK depends on the cell type and its evolutionary stage. Metformin in AMPK-mediated mechanisms leads to reduced liver glucose production and an increase in its consumption. AMPK is, indeed, considered as the main regulator of sugar and fat metabolisms. Ampk activation results in a diminish in the production of lipogenic enzymes, the induction of fatty acids oxidation, and a reduction in the activity of Acetyl-CoA carboxylase enzyme (Zhou et al. 2001; An and He 2016). Furthermore, the chronic activation of AMPK will induce the expression of hexokinase and glucose carrier (Zhou et al. 2001). In a research conducted on experimental model, it was revealed that the intra-duodenum injection of metformin into these rats could reduce glucose production from liver cells via the activation of AMPK of duodenum mucosa (Duca et al. 2015). Moreover, several studies have confirmed the role of the AMPK pathway in the reduction of lipid mass of the body and improvement of NAFLD by inducing liver fatty acid oxidation (Zang et al. 2004a, b; Smith et al. 2016). It has recently been found that AMPK acts as an inhibitor of Farnesoid X receptor (FXR). FXR regulates lipid and glucose metabolism, and is involved in controlling the production and circulation of bile acids in the liver. The cultivation of liver and intestinal cells revealed that AMPK activity would inhibit FXR transcription (Brandmaier et al. 2015). It has been indicated in some studies that occurrence of intermediate fibrosis in the omental fat storage of obese people, which is associated with AMPK deactivation, results in TGF-β/SMAD3 signalling pathway induction, activation of myofibroblasts and apoptosis of adipocytes. Metformin is capable of preventing other complications and development of fibrosis via activating AMPK and suppressing the TGF-β/SMAD3 pathway (Luo et al. 2016).
AMPK is able to participate in various non-metabolic pathways, including nitric oxide synthesis and anti-inflammatory processes (Reihill et al. 2007; Salminen et al. 2011; Salt and Palmer 2012). Metformin is involved in controlling oxidative stress by controlling complex I of the mitochondrial electron transfer chain (Kinaan et al. 2015; Wiernsperger 2015) and can be effective in reducing kidney damage induced by gentamicin (Darabi and Hasanvand 2018; Hasanvand 2018). Many studies have shown that the anti-inflammatory and antioxidative stress effects of metformin in various diseases, such as rheumatoid arthritis, neuropathic pain, renal disorders and Ankylosing spondylitis (Son et al. 2014, Afshari et al. 2018, Driver et al. 2018, Qin et al. 2018a, b, Rajaei, Haybar et al. 2018). This effect of metformin is achieved via activating multiple signalling pathways, including AMPK and Pi3K/AKT. Accordingly, it can attenuated the levels of inflammatory cytokines, including TNF-α, Nrf2, IL-6 and etc. (Yan, Zhou et al. 2015, Ci, Zhou et al. 2017). Furthermore, metformin reduces the levels of inflammatory cytokines by inducing stimuli caused by lipopolysaccharide, activation of AMPK, and inhibition of phosphorylation of JNK1 in macrophages (Woo, Xu et al. 2014). AMPK chemical activators such as metformin reduce the transcription of the NF-β factor and MDR1 expression in MCF-7/adr cells (Kim et al. 2011). Continuous activity of AMPK signalling inhibits this factor and its consequent inflammation (Cacicedo et al. 2004; Yang et al. 2010). Using AMPK activators, Chunfen Mo et al. indicated in a research that this enzyme has a role in stimulating the expression of the Nrf2 transcription factor. This transcription activating factor is one of the factors that are effective in antioxidant responses (Mo et al. 2014). Nrf2 targets several genes, including NQO-1, HO-1 and glutathione S-transferase (GST), hence plays a role in regulating the antioxidant system (Tkachev et al. 2011).
Different factors can activate macrophages and cause inflammatory conditions in diabetic patients. One of the most important of these factors is the AGEs (Qin et al. 2012; Jin et al. 2015). These factors have a receptor of RAGE/NF-β on the surface of the macrophages. Various investigations have revealed that RAGE signalling plays a pivotal role in inflammation caused by the activation of macrophages by AGE (Salminen et al. 2011; Huang et al. 2015). By activating AMPK, metformin is able to reduce the expression of RAGE and suppress NF-βB activity, and accordingly, reduce the expression of macrophage inflammatory cytokines, such as IL-1β, IL-6 and TGF-β. Moreover, metformin increases the expression of IL-10 anti-inflammatory cytokines (Cai et al. 2015, Zhou et al. 2016). Various investigations suggest the relationship between the formation of AGE and development of neurological disorders and inflammatory responses in diabetic patients. Ming-Min Chung indicated that the cultivation of human neural stem cells in the presence of AGE decreases the survival of these cells and increases the production of inflammatory cytokines and oxidative enzymes. Treatment with metformin results in reducing the expression of inflammatory transcription factors, such as NF-κB and IKK, and also decreasing the production of AGE (Chung et al. 2017). By phosphorylating and stimulating AMPK, metformin can increase the rate of expression of telomerase reverse transcriptase (hTERT) enzyme and postpone the aging process of endothelial cells (Karnewar et al. 2018).
The results showed that metformin could reduce the activity of MRCC-1 and eventually reduce oxygen consumption (El-Mir et al. 2000). This inhibition of MRCC-1 by metformin affects the AMP/ATP ratio and the NAD + /NADH ratio, which prevents gluconeogenesis (Apostolova et al. 2020).
Regulation of transcription of hepatic gluconeogenesis induced by metformin has various mechanisms, such as inhibition of CREB-mediated transcription of gluconeogenic genes through breducing cyclic AMP accumulation (Miller et al. 2013; Johanns et al. 2016). In addition, activation of AMPK by metformin could reduce the expression of gluconeogenic gene. It has been suggested that decreased expression of G6pc and Pck1 may be due to separation of the CREB transcription set and mediated by AMPK activity (He et al. 2009).
AMPK and cellular differentiation
In a recent research conducted by Wei et al. the role of metformin in increasing the differentiation of dental pulp cells into odontoblast was examined. In this research, metformin could induce differentiation and mineralization of dental pulp cells and be effective in the reconstruction of palpation ulcers via regulating AMPK activity. Moreover, the inhibition of AMPK reduced the activity of alkaline phosphatase (Qin et al. 2018a, b). Metformin also significantly activated AMPK in bone marrow progenitor cells (BMPCs). Prescription of metformin in a time-dependent manner stimulates the differentiation of BMPCs into osteoblast by activating specific osteoblast transcription factors, such as Runx2/Cbfa1 and activating AMPK (Molinuevo et al. 2010). As the activation of AMPK was intensified by metformin, phosphorylation of the STAT3 transcription factor was reduced. This factor is central to the stimulation of conversion monocyte to macrophage by regulating the signalling of pro-inflammatory events and creating inflammatory micro-environment. Hence, as its phosphorylation is reduced by AMPK, this differentiation pathway is disrupted and inflammation will be more likely to be reduced (Vasamsetti et al. 2015). Moreover, the oral prescription of metformin in the systemic lupus erythematosus disease prevents the differentiation of B cells into antibody-producing plasma cells by inhibiting the mTOR/STAT3 signalling pathway (Lee et al. 2017). It has been found that metformin increases the proliferation of regulatory T cells and raises the expression of its specific transcription factor (FOXP3) by reducing the expression of STAT3 and increasing the expression of STAT5 (Passerini et al. 2008; Goodman et al. 2011; Maddur et al. 2012). This effect is simultaneously observed with reduced power of proliferation of TH17 cells. Metformin can alter the pathway of polarity of cell differentiation from the inflammatory phenotype of TH17 to the Treg inhibitory phenotype by regulating this pathway (Lee, Lee et al. 2015a, b).
Through its effect on multiple factors, the AMPK activity can alter the differentiation pathway of macrophages towards anti-inflammatory phenotype. In fact, if macrophages are stimulated by anti-inflammatory factors, AMPK phosphorylation occurs and the macrophage phenotype would be of anti-inflammatory type. Moreover, AMPK dephosphorylation occurs if macrophage is exposed to inflammatory cytokines (Sag et al. 2008). However, it was revealed, in tumor conditions, that metformin might be involved in inhibiting the spread of tumors and suppressing cancer development by altering the pathway of tumor-associated macrophages (TAMs) from phenotypes M2 to M1. Classic macrophages or M1 have pre-inflammatory activity and the alternative type or M2 has an inhibitory effect on immune responses, particularly on anti-tumor responses (Mukhtar et al. 2011; Ding et al. 2015). Chi-Fu Chiang et al. indicated that metformin-treated cancer cells increase the production of cytokines such as IL-12 and TNF-α, which act as macrophage inhibitors to the inflammatory phenotype or M1 by activating the signalling pathway of AMPK/NF-β. Moreover, the activation of this pathway reduces the production of inhibitory cytokines, such as IL-10, IL-4, IL-13 and TGF-β, and suppresses the differentiation of macrophages towards the anti-inflammatory phenotype M2. As a result of these processes, the microenvironment surrounding the cancer cells or the tumor is likely to suppress the tumor and destroy cancer cells (Chiang et al. 2017).
Activation of AMPK–mTOR via metformin inhibits the complex activity of the MRCC, which ultimately leads to cell death or inhibition of cell proliferation by growth factors (Cai et al. 2016). Akt kinase is an important kinase in human kinoma, which has three subtypes such as AKT1, AKT2, and AKT3. AKT2 subtype has been shown to play an important role in breast cancer and its proliferation and cell survival (Santi and Lee 2011). However, the increasing of expression of miR-200c and its effect on the activity pathway of AKT2, c-Myc and Bcl-2 thourth by metformin therapy and its antitumor effects may indicate the anti-cancer potency of metformin and AMPK (Pulito et al. 2014; Zhang et al. 2017).
Metformin and therapeutic goals
Therapeutic actions of metformin in COVID-19
With the advent of coronavirus in 2019, many studies have been conducted with regard to its treatment. However, the ACE2 receptor has been shown to facilitate infection at the surface of coronary target cells (Guan et al. 2020, Hoffmann et al. 2020). It has been indicated that metformin activates the AMPK pathway that can ultimately prevent the virus binding to the receptor by phosphorylating the ACE2 receptor and altering its structure (Sharma, Ray et al. 2020). Studies have shown that two proteins in humans that are regulated by the mTOR signalling pathway interact with coronavirus proteins (Sharma, Ray et al. 2020). Moreover, it has been indicated that the AMPK signalling pathway can inhibit the mTOR pathway (Ramaiah 2020). Meanwhile, functional disorders of vital organs of the body, which are among the most important coronary complications, including endothelia, cardiovascular, hematological, and brain disorders, etc., are affected by oxidative stress and inflammation processes. Activation of the AMPK pathway and subsequently inhibition of mTOR, suppression of oxidative stress and inflammation, and inhibition of the increase in genes encoding proinflammatory cytokines, enables metformin to reduce mortality in patients with confirmed COVID-19 (Kamyshnyi et al. 2021). Decreased endosome cell pH has been shown to be associated with increased maturation of coronary virion, and metformin inhibits the endocytic cycle and maturation of virions by increasing the pH level. On the other hand, it can be effective in reducing the mortality of these patients by preventing the disruption of the normal intestinal flora, (Varghese, Samuel et al. 2021). Studies have shown that metformin can have a reduced risk of death in COVID-19 patients. This effect was mediated by inhibits mTORC1 and active LKB1 by AMPK phosphorylation (Kamyshnyi et al. 2021). Recent studies have shown that the use of metformin by AMPK activation can phosphorylate ACE2 and increase the stability of ACE2 and then decrease the host cell acceptance level for SARS-Cov-2 (Bangi et al. 2020, Sharma, Ray et al. 2020, Shen et al. 2020). Induction of the AMPK signalling pathway by metformin effectively reduces and modifies neutrophil extracellular trap activity and suppresses the inflammatory response in SARS-CoV-2 (Sharma, Chang et al. 2020, Kamyshnyi et al. 2021). In addition, Esam Z. et al. Also showed that the use of metformin could reduce the risk of lung fibrosis associated with SARS-Cov-2 (Esam 2020). There are numerous other studies in this area, all of which suggest that metformin may be a useful adjunctive therapy for COVID-19 patients (Bhutta et al. 2021, Bielka et al. 2021, Ibrahim et al. 2021, Varghese, Samuel et al. 2021, Zangiabadian et al. 2021).
Therapeutic actions of metformin as an anti-cancer agent
The relationship between the spread of tumors and cell metabolism processes, particularly the role of AMPK in these processes, has been investigated in various researches over a long period of time, and their use in developing new anti-cancer strategies are being examined (Cairns et al. 2011). The mTOR signalling pathway has a significant role in controlling the translation and construction of protein, spread of lymphocyte population, tumor genesis and drug resistance (Weichhart and Saemann 2009; Witzig and Gupta 2010; Perez-Galan et al. 2011; Zoncu et al. 2011). Epidemiological investigations have shown that using metformin is associated with reduced incidence of various types of cancers including pancreatic (Li et al. 2009), colon (Currie et al. 2009), breast (Bodmer et al. 2010), and prostate cancers (Wright and Stanford 2009). The results of different investigations have emphasized that metformin is highly likely to inhibit mTOR and perform its anti-tumor role by activating AMPK. Moreover, the AMPK Alpha subunit interacts with mitotic and cytokine regulators. This process is also associated with the suppressing role of AMPK in combating various kinds of cancers (Vazquez-Martin et al. 2009a, b; Green et al. 2010).
Moreover, another study conducted by Tao Lu et al. stated that exposure to metformin thourth regulating AMPK–CEBPB–PDL1 signalling pathway can a significant reduction in the risk of non-small cell lung cancer (Lu et al. 2021). Also, Zhuang Luo et al. showed that metformin can induces apoptotic cytotoxicity and finally degradation thourth AMPK/PKA/GSK-3β-mediated in non-small cell lung cancer (Luo et al. 2019). Various research have shown that the combination therapy of metformin with anticancer drugs mades synergic anticancer effects, such as radiation (Storozhuk et al. 2013), gefitinib (Morgillo et al. 2013; Li et al. 2017), erlotinib (Wang et al. 2017), sorafenib (Groenendijk et al. 2015), car-boplatin (Liu et al. 2017), cisplatin (Lin et al. 2013), and TRAIL (Nazim et al. 2016).
Park et al. reported that metformin regulates β‑catenin to reduce cell proliferation by activating AMPK in colon carcinoma (Park et al. 2019) and other study showed that in colorectal cancer cells, inhibits non-canonical Ser552 phosphorylation in β-catenin through an AMPK/PI3K/Akt activation with metformin (Amable et al. 2019). The role of AMPK and its downstream pathway in repression of protein prenylation through MVA pathway and LKB1/AMPK pathway is linked to inhibition of tumor growth has also been reported in several study (Carretero et al. 2007; Seo et al. 2020). In another study demonstrated that activating ampk by metformin attenuated tight junction assembly in intestinal epithelium and promotes expression of colonic epithelial Caco2 cells (Chen, Wang et al. 2018). Several studies have shown that activation of cells (Vazquez-Martin et al. 2009a, b; Yi et al. 2017). Metformin inhibits the progress of cells in cancer by inducing AMPK and then LKB1 levels and ultimately inhibits translation. Also, metformin reduces the phosphorylation of S6Ks and prevents mTOR activity (Saraei et al. 2019). Metformin effectively decrease the risk of proliferation and metastasis of pancreatic cancer (Oliveria et al. 2008; Ruiter et al. 2012). In addition, evidence suggests that a reduced risk of pancreatic cancer suggests that significant inhibition of mTOR may be TSC2-independent or dependent but associated with phosphorylated AMPK (Dowling et al. 2007; Gwinn et al. 2008; Mohammed et al. 2013).
Therapeutic actions of metformin as a nephroprotective agent
It has also been indicated that metformin can have a nephroprotective effect by activating the AMPK signalling pathway (Hasanvand et al. 2018). Another research revealed that AMPK activation by metformin was associated with reduced TGF-β-induced collagen production in kidney fibroblasts in mice. This factor, which is considered as one of the important fibrogenic cytokines, plays a significant role in the pathogenesis of kidney diseases (Hills and Squires 2011; Lu et al. 2015; Hasanvand and Saberi 2018). Moreover, the positive effect of metformin in reducing the incidence of nephropathies in diabetic patients has been confirmed (Rafieian-Kopaei 2013; Dissanayake et al. 2017). This drug prevents the formation of kidney stones with its antioxidant activity (Yang et al. 2016). It has been shown that treatment with metformin, as an AMPK agonist, can well moderate mitophagia in epithelial cells of the proximal tubule of the kidney (Zhao and Sun 2020). In addition, Ya-chun Han et al. Also showed that the use of metformin could decrease the risk of fibrosis in the renal interstitial tube associated with mitophage activation via the AMPK-Pink1-Parkin pathway (Han et al. 2021). Another study showed that the use of metformin in renal nephrectomy models following renal disease could halt the progression of chronic renal disease, including renal fibrosis, and that activation of AMPK may contribute to the protective effect of metformin nephroprotechnics (Borges et al. 2020). Based on several clinical trials, metformin has shown beneficial therapeutic effects on the survival of CKD patients as well as the survival of a transplanted kidney (Stephen et al. 2014; De Broe et al. 2018).
Therapeutic actions of metformin as a neuroprotective agent
A study has shown that stimulation of AMPK is a major molecular mechanism of “feeding behaviour” in the hypothalamus of the brain (Blanco Martinez de Morentin, Gonzalez et al. 2011). In vitro studies have shown that stimulation of AMPK reverses neuropathic allodynia. Moreover, metformin could diminish chemotherapy-induced neuropathic pain in animals (Taylor et al. 2013). In rat models, metformin has been shown to have antinociceptive effects on the alleviation of pain in nerves damaged by diabetes (Ma et al. 2015). Metformin administration could up-regulate the expression of intrinsic factors linked to nerve regeneration such as apolipoprotein E (ApoE) after nerve damage (Melemedjian et al. 2013). It was indicated in animal models of focal cerebral ischemia studies conducted by Harada et al. that neuroprotective effects of AMPK signalling activated by metformin diminishes the glucose intolerance. Moreover, they have reported a decrease in variation in the mnemonic tests (Harada et al. 2010). Activation of AMPK could be considered as a novel therapeutic purpose for the tentative treatment of neuropathic pain (Yerra et al. 2018). It was reported that activation of TRPA1 could induce pain-related behaviors in mice (Miura et al. 2013). However, treatment with AMPK activators could attenuate these behavioral and molecular changes in the pathophysiological profile of metabolic dysfunction (Wang et al. 2018). Various studies have shown that treatment with metformin is effective in neurological diseases, including high MPTP and increased BDNF (Patil et al. 2014, Lu et al. 2016), Parkinson’s disease (Choi et al. 2010; Arbeláez-Quintero and Palacios 2017; Lu et al. 2020, Paudel et al. 2020), epilepsy (H S, Paudel et al. 2019, Demaré et al. 2021; Sanz et al. 2021, Salvati et al. 2022), traumatic brain injury (Tao et al. 2018; Taheri et al. 2019; Fan et al. 2020; Rahimi et al. 2020), neuroprotection of the heart (Zhu et al. 2018, Benjanuwattra et al. 2020, Leech et al. 2020), and preconditioning in ischemic brain injury (Wang et al. 2021a, b, c). Various studies have shown that BDNF can affect the structure of the anal sphincter (Singh and Rattan 2021, Singh, Singh et al. 2021).
Therapeutic actions of metformin as a cardioprotective agent
Consumption of metformin in diabetic patients is associated with a significant reduction in cardiac infarction and atherosclerosis (Matsumoto et al. 2004). With its effect on reducing the recruitment of monocytes to the vascular wall and their differentiation into inflammatory macrophages, metformin reduces the formation of atherosclerotic plaques and decreases the levels of inflammatory cytokines (Vasamsetti et al. 2015). The effects of metformin are mediated through an increase in p-AMPK and by up-regulating p-eNOS. Moreover, it improves cardiac function. The cardioprotective effects of metformin are independent of its anti-hyperglycemic effects. Moreover, an improved myocardial remodelling after an ischemic insult with metformin use was indicated in a research (Varjabedian et al. 2018). A study in patients showed that the progression of the medial thickness of the carotid artery was reduced in metformin-treated diabetic patients. The median thickness of the carotid artery is a known indicator of atherosclerotic progression. It has also been indicated that activation of AMPK by metformin can have a pleiotropic effect (Zang et al. 2004a, b; Vasamsetti et al. 2015). Additionally, various studies have shown that treatment with metformin is effective in heart diseases, including cardiotoxicity (Kuburas et al. 2022, O’Neill et al. 2022, Park, Park et al. 2022), heart failure (Benes et al. 2022, Buczyńska et al. 2022, Hendawy et al. 2022), atrial fibrillation (Bai et al. 2019; Liu et al. 2020, Zhou et al. 2022), coronary heart disease (Hua et al. 2018; Luo et al. 2020).
Therapeutic actions of metformin in reproductive system diseases
In a research conducted by HyeRan Gwak et al. in 2017, the mechanism of action of metformin on ovarian cancer was examined. The results of this research confirmed that metformin can inhibit AKt and P70S6K by activating AMPK. Following this process, the GSK3β protein is activated, and finally during the ubiquitin/ proteasome process, the value of cyclin D1 decreases. In fact, metformin decreases the amount of cyclin D1 without affecting its transcription levels (Gwak et al. 2017). Cyclin D1 is an essential regulator of the cell cycle in phase G1. Investigations have indicated that high expression of this factor is associated with resistance to treatment and prognosis of ovarian cancer (Bali et al. 2004; Hashimoto et al. 2011). Activation of AMPK by metformin in ovarian syndrome or metabolic syndrome in vitro and in vivo results in the inhibition of metabolism, cessation of the cell cycle, and finally apoptosis of cells (Ben Sahra et al. 2008; Pierotti et al. 2013). Research studies have shown that using metformin is associated with improved semen parameters and testicular weight, reduced apoptosis in testicular cell, and finally restoration of hormonal homeostasis (Yan, Mu et al. 2015a, b). Indeed, metformin administration in STZ-diabetic rats resulted in improved testosterone, LH and FSH hormones levels (Nasrolahi et al. 2013). Various studies have shown that treatment with metformin is effective in reproductive system diseases, including female and male reproduction in endocrine pathologies (Lee et al. 2019; Shpakov 2021, ul haq Shah, Shrivastava et al. 2022), endometriosis (Zhao et al. 2018, Mu et al. 2020, Stochino-Loi et al. 2021, Kimber-Trojnar et al. 2022), polycystic ovary syndrome (Chen et al. 2019; Fornes et al. 2022, Xu et al. 2022) and prostate (Sun et al. 2018, Chen, Wang et al. 2021a, b, c, Aydın et al. 2022, Morale et al. 2022).
Therapeutic actions of metformin in the bone structure
Available evidence suggests the occurrence of fibroblasts asphyxiation and blockage of AMPK activity in the ankylosing spondylitis disease. Moreover, laboratory investigations conducted on metformin have indicated its anti-osteogenic effects and also its agonist property concerning AMPK. Findings of the research carried out by Xiong Qin et al. in 2018 showed that osteogenic markers and inhibition of ossification are reduced significantly by metformin prescription to fibroblasts extracted from patients with ankylosing spondylitis (Qin et al. 2018a, b). Metformin is able to suppress the differentiation of osteoblasts and inhibit the signalling pathway of OPG/RANKL/RANK (Shao et al. 2014). In another study carried out in 2010, the mechanism of metformin’s activity on the differentiation of osteoblasts was examined. This research revealed that metformin could increase the expression of osteogenic genes such as bone sialoprotein, alkaline phosphatase, Runx2, osteocalcin, and SHP. Metformin induces the physical interaction and the formation of a complex between SHP and Runx2 in the osteocalcin promoter. The research findings showed that metformin might stimulate osteoblasts differentiation via changing the activation of Runx2 by upstream stimulatory factor-1 AMPK/USF-1/SHP (Jang et al. 2011). Min-Ji Ahn and Goang-Won Cho indicated, in a research conducted in 2017, that activation of AMPK by metformin could have a stimulatory role in human bone marrow mesenchymal stem cells towards neural differentiation (Ahn and Cho 2017). Various studies have shown that treatment with metformin is effective in bone structure, including osteoarthritis (Feng et al. 2020; Li et al. 2020a, b; Li et al. 2020a, b), osteoporosis (Blümel et al. 2020, Guo et al. 2022, Song et al. 2022), bone regeneration (Ren et al. 2021; Fang et al. 2022; Sun et al. 2022) and osteosarcoma (Paiva-Oliveira et al. 2018; Zhao et al. 2019, Lu et al. 2021).
Therapeutic actions of metformin in digestive system diseases
Min-Jie Lin et al. showed that activation of the metformin / AMPK pathway could improve the non-alcoholic fatty liver disease in obese mice (Lin et al. 2017a, b). Stimulation of this pathway has a positive effect on the activity of the LXRα transcription factor. This factor results in the decreasing regulation of expression of apolipoprotein (Jakel et al. 2004; Shu et al. 2007; Shu et al. 2010; Gao et al. 2012). Hence, due to the influence on the reduction of its expression in the aforementioned process, metformin ameliorates this complication (Lin et al. 2017a, b). Another study revealed that metformin could play an inhibitory role in the cell proliferation of Esophageal squamous cell carcinomas by positive regulation of AMPK, P53, P21 and P27 (Cai et al. 2015). Metformin significantly reduces the severity of inflammatory bowel disease (IBD) via suppressing the signalling pathway of STAT3. As the expression of STAT3 transcription factor is increased in this disease, the TH17 phenotype and the production of inflammatory cytokines, particularly IL-17, IL-6 and TNF-α increase. Metformin can reduce the expression of STAT3 and increase the expression of P53 through the AMPK pathway, which is in the upstream of the mTOR transcription factor. Regulation of these conditions improves the clinical status of IBD patients by reducing inflammation (Shackelford and Shaw 2009, Micic et al. 2011, Gálvez 2014, Lee, Lee et al. 2015a, b). Furthermore, another investigation revealed that inhibiting the activation of the two pathways of STAT3 and NF-βB by AMPK has been effective in inhibiting the growth of pancreatic tumours (Tan et al. 2015). In addition, various studies have shown that treatment with metformin is effective in digestive system diseases, including colitis (El-Mahdy et al. 2021, Liu, Liao et al. 2021, El-Ghannam et al. 2022), Pancreatitis (He et al. 2021, Wang et al. 2021a, b, c, Wang, Yu et al. 2021), Liver Disease (Saeedi Saravi et al. 2016, Pinyopornpanish et al. 2021, Xie, Wang et al. 2021a, b, c) and Gut Microbiota (Lee, Chae et al. 2021b, a, Lee, Kim et al. 2021, Liu, Liao et al. 2021).
Conclusions
Many studies have investigated the role of metformin in the treatment of various diseases, including inflammatory diseases, autoimmune diseases and cancer. A considerable number of these investigations have revealed that the capacity of metformin to activate AMPK and also the consequent activation or inhibition of different factors by metformin enable it to alter the pathological pathways of the disease, direct cell differentiation, and moderate the inflammatory conditions. Since its therapeutic use, even at high doses, does not lead to severe side effects, metformin has a significant role in either treating various diseases or reducing their symptoms.
Funding
The author have not disclosed any funding.
Data availability
Enquiries about data availability should be directed to the authors.
Declarations
Conflict of interest
The author have not disclosed any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afshari K, Dehdashtian A, Haddadi NS, Haj-Mirzaian A, Iranmehr A, Ebrahimi MA, Tavangar SM, Faghir-Ghanesefat H, Mohammadi F, Rahimi N, Javidan AN, Dehpour AR. Anti-inflammatory effects of metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord. 2018;56(11):1032–1041. doi: 10.1038/s41393-018-0168-x. [DOI] [PubMed] [Google Scholar]
- Ahn MJ, Cho GW. Metformin promotes neuronal differentiation and neurite outgrowth through AMPK activation in human bone marrow-mesenchymal stem cells. Biotechnol Appl Biochem. 2017;64(6):836–842. doi: 10.1002/bab.1584. [DOI] [PubMed] [Google Scholar]
- Amable G, Martínez-León E, Picco ME, Di Siervi N, Davio C, Rozengurt E, Rey O. Metformin inhibits β-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int J Biochem Cell Biol. 2019;112:88–94. doi: 10.1016/j.biocel.2019.05.004. [DOI] [PubMed] [Google Scholar]
- An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol. 2016;228(3):R97–106. doi: 10.1530/JOE-15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020;34:101517. doi: 10.1016/j.redox.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeláez-Quintero I, Palacios M. To use or not to use metformin in cerebral ischemia: a review of the application of metformin in stroke rodents. Stroke Res Treat. 2017;2017:9756429. doi: 10.1155/2017/9756429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydın PK, Karabulut-Bulan O, Bugan I, Turkyilmaz IB, Altun S, Yanardag R. The protective effect of metformin against testicular damage in diabetes and prostate cancer model. Cell Biochem Funct. 2022;40(1):60–70. doi: 10.1002/cbf.3674. [DOI] [PubMed] [Google Scholar]
- Bai F, Liu Y, Tu T, Li B, Xiao Y, Ma Y, Qin F, Xie J, Zhou S, Liu Q. Metformin regulates lipid metabolism in a canine model of atrial fibrillation through AMPK/PPAR-α/VLCAD pathway. Lipids Health Dis. 2019;18(1):1–9. doi: 10.1186/s12944-019-1059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali A, O'Brien PM, Edwards LS, Sutherland RL, Hacker NF, Henshall SM. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004;10(15):5168–5177. doi: 10.1158/1078-0432.CCR-03-0751. [DOI] [PubMed] [Google Scholar]
- Bangi S, Barve R, Qamar A. Protective effects of CVD and DM Medications in SARS-CoV-2 Infection. SN Compr Clin Med. 2020;2(9):1296–1298. doi: 10.1007/s42399-020-00452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Birnbaum MJ. Control of gluconeogenesis by metformin: does redox trump energy charge? Cell Metab. 2014;20(2):197–199. doi: 10.1016/j.cmet.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beladi Mousavi SS. Metformin improves diabetic kidney disease. J Nephropharmacology. 2012;1(1):1–2. [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27(25):3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- Benes J, Kotrc M, Kroupova K, Wohlfahrt P, Kovar J, Franekova J, Hegarova M, L Hoskova, E Hoskova and T Pelikanova (2022) Metformin in the management of patients with diabetes and advanced heart failure (HFrEF): a propensity score-matched analysis [DOI] [PMC free article] [PubMed]
- Benjanuwattra J, Apaijai N, Chunchai T, Kerdphoo S, Jaiwongkam T, Arunsak B, Wongsuchai S, Chattipakorn N, Chattipakorn SC. Metformin preferentially provides neuroprotection following cardiac ischemia/reperfusion in non-diabetic rats. Biochim et Biophys Acta (BBA) - Mol Basis Dis. 2020;1866(10):165893. doi: 10.1016/j.bbadis.2020.165893. [DOI] [PubMed] [Google Scholar]
- Bhutta MS, Gallo ES, Borenstein R. Multifaceted role of AMPK in viral infections. Cells. 2021;10(5):1118. doi: 10.3390/cells10051118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielka W, Przezak A, Pawlik A. Therapy of type 2 diabetes in patients with SARS-CoV-2 infection. Int J Mol Sci. 2021;22(14):7605. doi: 10.3390/ijms22147605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümel JE, Arteaga E, Aedo S, Arriola-Montenegro J, López M, Martino M, Miranda C, Miranda O, Mostajo D, Ñañez M, Ojeda E, Pilnik S, Rojas J, Salinas C, Sosa L, Spritzer PM, Tserotas K, Vallejo MS, Belardo A, Fighera TM, Chedraui P. Metformin use is associated with a lower risk of osteoporosis in adult women independent of type 2 diabetes mellitus and obesity. REDLINC IX study. Gynecol Endocrinol. 2020;36(5):421–425. doi: 10.1080/09513590.2020.1718092. [DOI] [PubMed] [Google Scholar]
- Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33(6):1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges C M, Fujihara C K, Malheiros D M A C, F V, de Ávila G, Formigari P, José B, de Faria L. Metformin arrests the progression of established kidney disease in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol-Renal Physiol. 2020;318(5):F1229–F1236. doi: 10.1152/ajprenal.00539.2019. [DOI] [PubMed] [Google Scholar]
- Brandmaier S, Xu T, Illig T, Suhre K, Adamski J, Wang-Sattler R. Response to comment on Xu et al. effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. diabetes care 2015;38:1858–1867. Diabetes Care. 2015;38(12):e216–e217. doi: 10.2337/dci15-0022. [DOI] [PubMed] [Google Scholar]
- Bright NJ, Thornton C, Carling D. The regulation and function of mammalian AMPK-related kinases. Acta Physiol (oxf) 2009;196(1):15–26. doi: 10.1111/j.1748-1716.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- Buczyńska A, Sidorkiewicz I, Krętowski AJ, Zbucka-Krętowska M, Adamska A. Metformin intervention—A panacea for cancer treatment? Cancers. 2022;14(5):1336. doi: 10.3390/cancers14051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324(4):1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen X, Guan Y, Chen C, Jing X. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal squamous cell carcinomas in vitro and in vivo. PLoS One. 2015;10(7):e0133349. doi: 10.1371/journal.pone.0133349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Zhang Y, Han TK, Everett RS, Thakker DR. Cation-selective transporters are critical to the AMPK-mediated antiproliferative effects of metformin in human breast cancer cells. Int J Cancer. 2016;138(9):2281–2292. doi: 10.1002/ijc.29965. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Carretero J, Medina PP, Blanco R, Smit L, Tang M, Roncador G, Maestre L, Conde E, Lopez-Rios F, Clevers HC, Sanchez-Cespedes M. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26(11):1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- Chanda D, Li T, Song KH, Kim YH, Sim J, Lee CH, Chiang JY, Choi HS. Hepatocyte growth factor family negatively regulates hepatic gluconeogenesis via induction of orphan nuclear receptor small heterodimer partner in primary hepatocytes. J Biol Chem. 2009;284(42):28510–28521. doi: 10.1074/jbc.M109.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Brooks R, Houskeeper J, Bremner SK, Dunlop J, Viollet B, Logan PJ, Salt IP, Ahmed SF, Yarwood SJ. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2017;440:57–68. doi: 10.1016/j.mce.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wei H, Zhao X, Xin X, Peng L, Ning Y, Wang Y, Lan Y, Zhang Q. Metformin treatment alleviates polycystic ovary syndrome by decreasing the expression of MMP-2 and MMP-9 via H19/miR-29b-3p and AKT/mTOR/autophagy signaling pathways. J Cell Physiol. 2019;234(11):19964–19976. doi: 10.1002/jcp.28594. [DOI] [PubMed] [Google Scholar]
- Chiang CF, Chao TT, Su YF, Hsu CC, Chien CY, Chiu KC, Shiah SG, Lee CH, Liu SY, Shieh YS. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-kappaB signaling. Oncotarget. 2017;8(13):20706–20718. doi: 10.18632/oncotarget.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Park C, Jeong JW. AMP-activated protein kinase is activated in Parkinson's disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem Biophys Res Commun. 2010;391(1):147–151. doi: 10.1016/j.bbrc.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Chung MM, Nicol CJ, Cheng YC, Lin KH, Chen YL, Pei D, Lin CH, Shih YN, Yen CH, Chen SJ, Huang RN, Chiang MC. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res. 2017;352(1):75–83. doi: 10.1016/j.yexcr.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- Darabi S, Hasanvand A. Protective effect of metformin on diabetes mellitus, diabetic kidney disease and hepatocytes. Ann Res Antioxid. 2018;3(1):e03. [Google Scholar]
- De Broe ME, Kajbaf F, Lalau JD. Renoprotective effects of metformin. Nephron. 2018;138(4):261–274. doi: 10.1159/000481951. [DOI] [PubMed] [Google Scholar]
- Demaré S, Kothari A, Calcutt NA, Fernyhough P. Metformin as a potential therapeutic for neurological disease: mobilizing AMPK to repair the nervous system. Expert Rev Neurother. 2021;21(1):45–63. doi: 10.1080/14737175.2021.1847645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H, Zhou Y, Wu H, Yang B, He Q. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6(34):36441–36455. doi: 10.18632/oncotarget.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake AM, Wheldon MC, Ahmed J, Hood CJ. Extending metformin use in diabetic kidney disease: a pharmacokinetic study in stage 4 diabetic nephropathy. Kidney Int Rep. 2017;2(4):705–712. doi: 10.1016/j.ekir.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- Driver C, Hayangah JA, Nyane NA, Owira PMO. Metformin with insulin relieves oxidative stress and confers renoprotection in type 1 diabetes in vivo. J Nephropathol. 2018;7(3):171–181. doi: 10.15171/jnp.2018.37. [DOI] [Google Scholar]
- Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21(5):506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghannam MS, Saad MA, Nassar NN, El-Yamany MF, El-Bahy AA. Linagliptin ameliorates acetic acid-induced colitis via modulating AMPK/SIRT1/PGC-1α and JAK2/STAT3 signaling pathway in rats. Toxicol Appl Pharmacol. 2022;438:115906. doi: 10.1016/j.taap.2022.115906. [DOI] [PubMed] [Google Scholar]
- El-Mahdy NA, El-Sayad ME-S, El-Kadem AH, Abu-Risha SE-S. Metformin alleviates inflammation in oxazolone induced ulcerative colitis in rats: plausible role of sphingosine kinase 1/sphingosine 1 phosphate signaling pathway. Immunopharmacol Immunotoxicol. 2021;43(2):192–202. doi: 10.1080/08923973.2021.1878214. [DOI] [PubMed] [Google Scholar]
- El-Mir M-Y, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Esam Z. A proposed mechanism for the possible therapeutic potential of Metformin in COVID-19. Diabetes Res Clin Pract. 2020;167:108282. doi: 10.1016/j.diabres.2020.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y-Y, Wang Y-J, Guo J, Wu M-N, Zhang M-S, Niu B-L, Li Y, Zhao J, Yang C-H, Li Y. Delayed metformin treatment improves functional recovery following traumatic brain injury via central AMPK-dependent brain tissue repair. Brain Res Bull. 2020;164:146–156. doi: 10.1016/j.brainresbull.2020.08.021. [DOI] [PubMed] [Google Scholar]
- Fang C-H, Sun C-K, Lin Y-W, Hung M-C, Lin H-Y, Li C-H, Lin I-P, Chang H-C, Sun J-S, Chang JZ-C. Metformin-incorporated gelatin/nano-hydroxyapatite scaffolds promotes bone regeneration in critical size rat alveolar bone defect model. Int J Mol Sci. 2022;23(1):558. doi: 10.3390/ijms23010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Pan J, Li J, Zeng C, Qi W, Shao Y, Liu X, Liu L, Xiao G, Zhang H. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging (albany NY) 2020;12(2):1087. doi: 10.18632/aging.102635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornes R, Simin J, Nguyen MH, Cruz G, Crisosto N, van der Schaaf M, Engstrand L, Brusselaers N. Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: a nationwide population-based study. Reprod Biol Endocrinol. 2022;20(1):1–12. doi: 10.1186/s12958-022-00905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez J (2014) Role of Th17 cells in the pathogenesis of human IBD. Int Sch Res Notices [DOI] [PMC free article] [PubMed]
- Gao X, Forte TM, Ryan RO. Influence of apolipoprotein A-V on hepatocyte lipid droplet formation. Biochem Biophys Res Commun. 2012;427(2):361–365. doi: 10.1016/j.bbrc.2012.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Young AB, McCormick TS, Cooper KD, Levine AD. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J Immunol. 2011;186(6):3336–3345. doi: 10.4049/jimmunol.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, Boyer O, Bardet V, Park S, Foretz M, Viollet B, Ifrah N, Dreyfus F, Hermine O, Moura IC, Lacombe C, Mayeux P, Bouscary D, Tamburini J. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood. 2010;116(20):4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- Groenendijk FH, Mellema WW, van der Burg E, Schut E, Hauptmann M, Horlings HM, Willems SM, van den Heuvel MM, Jonkers J, Smit EF, Bernards R. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int J Cancer. 2015;136(6):1434–1444. doi: 10.1002/ijc.29113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W-J, Liang W-H, Zhao Y, Liang H-R, Chen Z-S, Li Y-M, Liu X-Q, Chen R-C, C-l. Tang and T. Wang, Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yang R, Zong S, Wang Z, Zhao J, Chen C, Wang C, Wang S (2022) Metformin combined with alendronate ameliorates osteoarthritis by attenuating RANKL-induced bone resorption and protecting cartilage against degradation
- Gwak H, Kim Y, An H, Dhanasekaran DN, Song YS. Metformin induces degradation of cyclin D1 via AMPK/GSK3beta axis in ovarian cancer. Mol Carcinog. 2017;56(2):349–358. doi: 10.1002/mc.22498. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y-C, Tang S-Q, Liu Y-T, Li A-M, Zhan M, Yang M, Song N, Zhang W, Wu X-Q, Peng C-H, Zhang H, Yang S. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021;12(10):925. doi: 10.1038/s41419-021-04184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S, Fujita-Hamabe W, Tokuyama S. The importance of regulation of blood glucose levels through activation of peripheral 5′-AMP-activated protein kinase on ischemic neuronal damage. Brain Res. 2010;1351:254–263. doi: 10.1016/j.brainres.2010.06.052. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Hasanvand A. Antioxidative and anti-inflammatory effects of metformin; a new look to an old drug. J Renal Endocrinol. 2018;4(1):2–2. [Google Scholar]
- Hasanvand A, Saberi S. Renin angiotensin system and different mediators induce renal fibrosis. J Renal Endocrinol. 2018;4(1):9–9. [Google Scholar]
- Hasanvand A, Amini-Khoei H, Hadian MR, Abdollahi A, Tavangar SM, Dehpour AR, Semiei E, Mehr SE. Anti-inflammatory effect of AMPK signaling pathway in rat model of diabetic neuropathy. Inflammopharmacology. 2016;24(5):207–219. doi: 10.1007/s10787-016-0275-2. [DOI] [PubMed] [Google Scholar]
- Hasanvand A, Amini-Khoei H, Jahanabadi S, Hadian M-R, Abdollahi A, Tavangar SM, Jtemaei Mehr S, Dehpour AR. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through activation of AMPK signaling pathway. J Nephropathol. 2018;7(1):37–42. doi: 10.15171/jnp.2018.11. [DOI] [Google Scholar]
- Hashimoto T, Yanaihara N, Okamoto A, Nikaido T, Saito M, Takakura S, Yasuda M, Sasaki H, Ochiai K, Tanaka T. Cyclin D1 predicts the prognosis of advanced serous ovarian cancer. Exp Ther Med. 2011;2(2):213–219. doi: 10.3892/etm.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137(4):635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Gao F, Hou J, Li T, Tan J, Wang C, Liu X, Wang M, Liu H, Chen Y. Metformin inhibits MAPK signaling and rescues pancreatic aquaporin 7 expression to induce insulin secretion in type 2 diabetes mellitus. J Biol Chem. 2021;297(2):101002. doi: 10.1016/j.jbc.2021.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendawy M, Ramy A and Mohie I. (2022). "Autophagy promotion and fibrosis inhibition by combination of GLP1 analogue and metformin decreasing the progression of type II diabetic cardiomyopathy of albino rats: immunohistochemical study." Bull Egypt Soc Physiol Sci: 202–212
- Hills CE, Squires PE. The role of TGF-beta and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011;22(3):131–139. doi: 10.1016/j.cytogfr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Liu Z, Liu Z, An D, Lai W, Zhan Q, Zeng Q, Ren H, Xu D. Metformin increases cardiac rupture after myocardial infarction via the AMPK-MTOR/PGC-1α signaling pathway in rats with acute myocardial infarction. Med Sci Monit: Int Med J Exp Clin Res. 2018;24:6989. doi: 10.12659/MSM.910930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BP, Lin CH, Chen HM, Lin JT, Cheng YF, Kao SH. AMPK activation inhibits expression of proinflammatory mediators through downregulation of PI3K/p38 MAPK and NF-kappaB signaling in murine macrophages. DNA Cell Biol. 2015;34(2):133–141. doi: 10.1089/dna.2014.2630. [DOI] [PubMed] [Google Scholar]
- Ibrahim S, Lowe JR, Bramante CT, Shah S, Klatt NR, Sherwood N, Aronne L, Puskarich M, Tamariz L, Palacio A, Bomberg E, Usher M, King S, Benson B, Vojta D, Tignanelli C, Ingraham N. Metformin and Covid-19: focused review of mechanisms and current literature suggesting benefit. Front Endocrinol (Lausanne) 2021;12:587801. doi: 10.3389/fendo.2021.587801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaiel AA, Espinosa-Oliva AM, Santiago M, Garcia-Quintanilla A, Oliva-Martin MJ, Herrera AJ, Venero JL, de Pablos RM. Metformin, besides exhibiting strong in vivo anti-inflammatory properties, increases mptp-induced damage to the nigrostriatal dopaminergic system. Toxicol Appl Pharmacol. 2016;298:19–30. doi: 10.1016/j.taap.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Jakel H, Nowak M, Moitrot E, Dehondt H, Hum DW, Pennacchio LA, Fruchart-Najib J, Fruchart JC. The liver X receptor ligand T0901317 down-regulates APOA5 gene expression through activation of SREBP-1c. J Biol Chem. 2004;279(44):45462–45469. doi: 10.1074/jbc.M404744200. [DOI] [PubMed] [Google Scholar]
- Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS, Koh JT. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48(4):885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Jin X, Yao T, Zhou Z, Zhu XJ, Zhang S, Hu W, Shen C. Advanced glycation end products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κB pathway. BioMed Res Int. 2015;2015:12. doi: 10.1155/2015/732450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanns M, Lai Y-C, Hsu M-F, Jacobs R, Vertommen D, Van Sande J, Dumont JE, Woods A, Carling D, Hue L. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat Commun. 2016;7(1):1–12. doi: 10.1038/ncomms10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamyshnyi O, Matskevych V, Lenchuk T, Strilbytska O, Storey K, Lushchak O. Metformin to decrease COVID-19 severity and mortality: Molecular mechanisms and therapeutic potential. Biomed Pharmacother. 2021;144:112230. doi: 10.1016/j.biopha.2021.112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnewar S, Neeli PK, Panuganti D, Kotagiri S, Mallappa S, Jain N, Jerald MK, Kotamraju S. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: relevance in age-associated vascular dysfunction. Biochim Biophys Acta. 2018;1864((4.Pt A)):1115–1128. doi: 10.1016/j.bbadis.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, Lee CH, Choi HS. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes. 2008;57(2):306–314. doi: 10.2337/db07-0381. [DOI] [PubMed] [Google Scholar]
- Kim HG, Hien TT, Han EH, Hwang YP, Choi JH, Kang KW, Kwon K, Kim BH, Kim SK, Song GY, Jeong TC, Jeong HG. Metformin inhibits P-glycoprotein expression via the NF-κB pathway and CRE transcriptional activity through AMPK activation. Br J Pharmacol. 2011;162(5):1096–1108. doi: 10.1111/j.1476-5381.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber-Trojnar Ż, Dłuski DF, Wierzchowska-Opoka M, Ruszała M, Leszczyńska-Gorzelak B. Metformin as a potential treatment option for endometriosis. Cancers. 2022;14(3):577. doi: 10.3390/cancers14030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinaan M, Ding H, Triggle CR. Metformin: an old drug for the treatment of diabetes but a new drug for the protection of the endothelium. Med Princ Pract. 2015;24(5):401–415. doi: 10.1159/000381643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuburas R, Gharanei M, Haussmann I, Maddock H, Sandhu H (2022) Metformin protects against sunitinib-induced cardiotoxicity: investigating the role of AMPK: Investigating the role of AMPK. Authorea Preprints [DOI] [PubMed]
- Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PLoS One. 2015;10(9):e0135858. doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PLoS One. 2015;10(9):e0135858. doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Moon SJ, Kim EK, Seo HB, Yang EJ, Son HJ, Kim JK, Min JK, Park SH, Cho ML. Metformin suppresses systemic autoimmunity in roquin(san/san) Mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J Immunol. 2017;198(7):2661–2670. doi: 10.4049/jimmunol.1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Shin Y-J, Kim H, Kim H, Kim J, Min S-A, Kim P, Do Yu S, Park K. Metformin-induced endocrine disruption and oxidative stress of Oryzias latipes on two-generational condition. J Hazard Mater. 2019;367:171–181. doi: 10.1016/j.jhazmat.2018.12.084. [DOI] [PubMed] [Google Scholar]
- Lee CB, Chae SU, Jo SJ, Jerng UM, Bae SK. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int J Mol Sci. 2021;22(7):3566. doi: 10.3390/ijms22073566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim AH, Kim E, Lee S, Yu K-S, Jang I-J, Chung J-Y, Cho J-Y. Changes in the gut microbiome influence the hypoglycemic effect of metformin through the altered metabolism of branched-chain and nonessential amino acids. Diabetes Res Clin Pract. 2021;178:108985. doi: 10.1016/j.diabres.2021.108985. [DOI] [PubMed] [Google Scholar]
- Leech T, Apaijai N, Palee S, Higgins LA, Maneechote C, Chattipakorn N, Chattipakorn SC. Acute administration of metformin prior to cardiac ischemia/reperfusion injury protects brain injury. Eur J Pharmacol. 2020;885:173418. doi: 10.1016/j.ejphar.2020.173418. [DOI] [PubMed] [Google Scholar]
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KL, Li L, Zhang P, Kang J, Wang YB, Chen HY, He Y. A multicenter double-blind phase II study of metformin with Gefitinib as first-line therapy of locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2017;18(3):340–343. doi: 10.1016/j.cllc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Li H, Ding X, Terkeltaub R, Lin H, Zhang Y, Zhou B, He K, Li K, Liu Z, Wei J. Exploration of metformin as novel therapy for osteoarthritis: preventing cartilage degeneration and reducing pain behavior. Arthritis Res Ther. 2020;22(1):1–11. doi: 10.1186/s13075-020-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang B, Liu W-X, Lu K, Pan H, Wang T, Yi D, Huang J, Zhao L, Ning G. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79(5):635–645. doi: 10.1136/annrheumdis-2019-216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Yeh HH, Huang WL, Yan JJ, Lai WW, Su WP, Chen HH, Su WC. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am J Respir Cell Mol Biol. 2013;49(2):241–250. doi: 10.1165/rcmb.2012-0244OC. [DOI] [PubMed] [Google Scholar]
- Lin MJ, Dai W, Scott MJ, Li R, Zhang YQ, Yang Y, Chen LZ, Huang XS. Metformin improves nonalcoholic fatty liver disease in obese mice via down-regulation of apolipoprotein A5 as part of the AMPK/LXRalpha signaling pathway. Oncotarget. 2017;8(65):108802–108809. doi: 10.18632/oncotarget.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MJ, Dai W, Scott MJ, Li R, Zhang YQ, Yang Y, Chen LZ, Huang XS. Metformin improves nonalcoholic fatty liver disease in obese mice via down-regulation of apolipoprotein A5 as part of the AMPK/LXRα signaling pathway. Oncotarget. 2017;8(65):108802–108809. doi: 10.18632/oncotarget.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He C, Huang X. Metformin partially reverses the carboplatin-resistance in NSCLC by inhibiting glucose metabolism. Oncotarget. 2017;8(43):75206–75216. doi: 10.18632/oncotarget.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bai F, Liu N, Zhang B, Qin F, Tu T, Li B, Li J, Ma Y, Ouyang F. Metformin improves lipid metabolism and reverses the Warburg effect in a canine model of chronic atrial fibrillation. BMC Cardiovasc Disord. 2020;20(1):1–9. doi: 10.1186/s12872-020-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Shi J, Li M, Gui B, Fu R, Yao G, Duan Z, Lv Z, Yang Y, Chen Z, Jia L, Tian L. Activation of AMPK by metformin inhibits TGF-beta-induced collagen production in mouse renal fibroblasts. Life Sci. 2015;127:59–65. doi: 10.1016/j.lfs.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Lu M, Su C, Qiao C, Bian Y, Ding J, Hu G. Metformin prevents dopaminergic neuron death in MPTP/P-induced mouse model of Parkinson's disease via autophagy and mitochondrial ROS clearance. Int J Neuropsychopharmacol. 2016;19(9):pyw047. doi: 10.1093/ijnp/pyw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Chen H, Nie F, Wei X, Tao Z, Ma J. The potential role of metformin in the treatment of Parkinson’s disease. J Bio-X Res. 2020;3(01):27–35. [Google Scholar]
- Lu G, Wu Z, Shang J, Xie Z, Chen C, C. zhang, The effects of metformin on autophagy. Biomed Pharmacother. 2021;137:111286. doi: 10.1016/j.biopha.2021.111286. [DOI] [PubMed] [Google Scholar]
- Luo T, Nocon A, Fry J, Sherban A, Rui X, Jiang B, Xu XJ, Han J, Yan Y, Yang Q, Li Q, Zang M. AMPK activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes. 2016;65(8):2295–2310. doi: 10.2337/db15-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Zhu T, Luo W, Lv Y, Zhang L, Wang C, Li M, Wu W, Shi S. Metformin induces apoptotic cytotoxicity depending on AMPK/PKA/GSK-3β-mediated c-FLIP(L) degradation in non-small cell lung cancer. Cancer Manag Res. 2019;11:681–689. doi: 10.2147/CMAR.S178688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Schooling CM, Wong ICK, Au Yeung SL. Evaluating the impact of AMPK activation, a target of metformin, on risk of cardiovascular diseases and cancer in the UK Biobank: a Mendelian randomisation study. Diabetologia. 2020;63(11):2349–2358. doi: 10.1007/s00125-020-05243-z. [DOI] [PubMed] [Google Scholar]
- Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. Metformin attenuates hyperalgesia and allodynia in rats with painful diabetic neuropathy induced by streptozotocin. Eur J Pharmacol. 2015;764:599–606. doi: 10.1016/j.ejphar.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181(1):8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Sera Y, Abe Y, Tominaga T, Yeki Y, Miyake S. Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabetes Res Clin Pract. 2004;64(3):225–228. doi: 10.1016/j.diabres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Melemedjian OK, Yassine HN, Shy A, Price TJ. Proteomic and functional annotation analysis of injured peripheral nerves reveals ApoE as a protein upregulated by injury that is modulated by metformin treatment. Mol Pain. 2013;9:14. doi: 10.1186/1744-8069-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micic D, Cvijovic G, Trajkovic V, Duntas LH, Polovina S. Metformin: its emerging role in oncology. Hormones (athens) 2011;10(1):5–15. doi: 10.14310/horm.2002.1288. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Takahashi K, Imagawa T, Uchida K, Saito S, Tominaga M, Ohta T. Involvement of TRPA1 activation in acute pain induced by cadmium in mice. Mol Pain. 2013;9:7. doi: 10.1186/1744-8069-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, Xiao H. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20(4):574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Transl Oncol. 2013;6(6):649–659. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo MS, Schurman L, McCarthy AD, Cortizo AM, Tolosa MJ, Gangoiti MV, Arnol V, Sedlinsky C. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010;25(2):211–221. doi: 10.1359/jbmr.090732. [DOI] [PubMed] [Google Scholar]
- Morale MG, Tamura RE, Rubio IGS. Metformin and cancer hallmarks: molecular mechanisms in thyroid, prostate and head and neck cancer models. Biomolecules. 2022;12(3):357. doi: 10.3390/biom12030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgillo F, Sasso FC, Della Corte CM, Vitagliano D, D'Aiuto E, Troiani T, Martinelli E, De Vita F, Orditura M, De Palma R, Ciardiello F. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res. 2013;19(13):3508–3519. doi: 10.1158/1078-0432.CCR-12-2777. [DOI] [PubMed] [Google Scholar]
- Mu N, Xu T, Gao M, Dong M, Tang Q, Hao L, Wang G, Li Z, Wang W, Yang Y. Therapeutic effect of metformin in the treatment of endometrial cancer. Oncol Lett. 2020;20(5):1–1. doi: 10.3892/ol.2020.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar RA, Nseyo O, Campbell MJ, Esserman LJ. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn. 2011;11(1):91–100. doi: 10.1586/erm.10.97. [DOI] [PubMed] [Google Scholar]
- Mummidi S, Das NA, Carpenter AJ, Kandikattu H, Krenz M, Siebenlist U, Valente AJ, Chandrasekar B. Metformin inhibits aldosterone-induced cardiac fibroblast activation, migration and proliferation in vitro, and reverses aldosterone+salt-induced cardiac fibrosis in vivo. J Mol Cell Cardiol. 2016;98:95–102. doi: 10.1016/j.yjmcc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- N HS, P YN, K KL. Envisioning the neuroprotective effect of metformin in experimental epilepsy: a portrait of molecular crosstalk. Life Sci. 2019;233:116686. doi: 10.1016/j.lfs.2019.116686. [DOI] [PubMed] [Google Scholar]
- Nasrolahi O, Khaneshi F, Rahmani F, Razi M. Honey and metformin ameliorated diabetes-induced damages in testes of rat; correlation with hormonal changes. Iran J Reprod Med. 2013;11(12):1013–1020. [PMC free article] [PubMed] [Google Scholar]
- Nazim UM, Moon JH, Lee JH, Lee YJ, Seol JW, Eo SK, Lee JH, Park SY. Activation of autophagy flux by metformin downregulates cellular FLICE-like inhibitory protein and enhances TRAIL- induced apoptosis. Oncotarget. 2016;7(17):23468–23481. doi: 10.18632/oncotarget.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill EJ, Moore J, Song J, Tsiani EL. Inhibition of non-small cell lung cancer proliferation and survival by rosemary extract is associated with activation of ERK and AMPK. Life. 2022;12(1):52. doi: 10.3390/life12010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JS, Scott JW, Kemp BE. Structure and function of AMP-activated protein kinase. Acta Physiol (oxf) 2009;196(1):3–14. doi: 10.1111/j.1748-1716.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- Oliveria SA, Koro CE, Yood MU, Sowell M. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes Metab Syndr. 2008;2(1):47–57. doi: 10.1016/j.dsx.2007.11.002. [DOI] [Google Scholar]
- Paiva-Oliveira DI, Martins-Neves SR, Abrunhosa AJ, Fontes-Ribeiro C, Gomes CM. Therapeutic potential of the metabolic modulator metformin on osteosarcoma cancer stem-like cells. Cancer Chemother Pharmacol. 2018;81(1):49–63. doi: 10.1007/s00280-017-3467-6. [DOI] [PubMed] [Google Scholar]
- Park SY, Kim D, Kee SH. Metformin-activated AMPK regulates β-catenin to reduce cell proliferation in colon carcinoma RKO cells. Oncol Lett. 2019;17(3):2695–2702. doi: 10.3892/ol.2019.9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-W, Park J-E, Kim S-R, Sim M-K, Kang C-M, Kim KS (2022) Metformin alleviates ionizing radiation-induced senescence by restoring BARD1-mediated DNA repair in human aortic endothelial cells. Exp Gerontol 111706 [DOI] [PubMed]
- Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25- effector T cells. Int Immunol. 2008;20(3):421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson's disease in mice. Neuroscience. 2014;277:747–754. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Paudel YN, Angelopoulou E, Piperi C, Shaikh MF, Othman I. Emerging neuroprotective effect of metformin in Parkinson’s disease: a molecular crosstalk. Pharmacol Res. 2020;152:104593. doi: 10.1016/j.phrs.2019.104593. [DOI] [PubMed] [Google Scholar]
- Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti MA, Berrino F, Gariboldi M, Melani C, Mogavero A, Negri T, Pasanisi P, Pilotti S. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene. 2013;32(12):1475–1487. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- Pinyopornpanish K, Leerapun A, Pinyopornpanish K, Chattipakorn N. Effects of metformin on hepatic steatosis in adults with nonalcoholic fatty liver disease and diabetes: insights from the cellular to patient levels. Gut and Liver. 2021;15(6):827. doi: 10.5009/gnl20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulito C, Donzelli S, Muti P, Puzzo L, Strano S, Blandino G. microRNAs and cancer metabolism reprogramming: the paradigm of metformin. Ann Transl Med. 2014;2(6):58. doi: 10.3978/j.issn.2305-5839.2014.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Niu J, Wang Z, Xu W, Qiao Z, Gu Y. Astragalus membranaceus inhibits inflammation via Phospho-P38 mitogen-activated protein kinase (MAPK) and nuclear Factor (NF)-κB pathways in advanced glycation end product-stimulated macrophages. Int J Mol Sci. 2012;13(7):8379–8387. doi: 10.3390/ijms13078379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Gao X, Ma T, Weir MD, Zou J, Song B, Lin Z, Schneider A, Xu HHK. Metformin enhances the differentiation of dental pulp cells into odontoblasts by activating AMPK signaling. J Endod. 2018;44(4):576–584. doi: 10.1016/j.joen.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Jiang T, Liu S, Tan J, Wu H, Zheng L, Zhao J. Effect of metformin on ossification and inflammation of fibroblasts in ankylosing spondylitis: An in vitro study. J Cell Biochem. 2018;119(1):1074–1082. doi: 10.1002/jcb.26275. [DOI] [PubMed] [Google Scholar]
- Rafieian-Kopaei M. Combination of metformin with other antioxidants may increase its renoprotective efficacy. J Renal Inj Prev. 2013;2(2):35–36. doi: 10.12861/jrip.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi S, Ferdowsi A, Siahposht-Khachaki A. Neuroprotective effects of metformin on traumatic brain injury in rats is associated with the AMP-activated protein kinase signaling pathway. Metab Brain Dis. 2020;35(7):1135–1144. doi: 10.1007/s11011-020-00594-3. [DOI] [PubMed] [Google Scholar]
- Rajaei E, Haybar H, Mowla K, Zayeri ZD. Metformin one in a million efficient medicines for rheumatoid arthritis complications: inflammation, osteoblastogenesis, cardiovascular disease, malignancies. Curr Rheumatol Rev. 2018;15:116–122. doi: 10.2174/1573397114666180717145745. [DOI] [PubMed] [Google Scholar]
- Ramaiah MJ. mTOR inhibition and p53 activation, microRNAs: The possible therapy against pandemic COVID-19. Gene Rep. 2020;20:100765. doi: 10.1016/j.genrep.2020.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007;354(4):1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Hao X, Wang L, Hu Y, Meng L, Zheng S, Ren F, Bu W, Wang H, Li D. Metformin Carbon Dots for promoting periodontal bone regeneration via activation of ERK/AMPK pathway. Adv Healthcare Mater. 2021;10(12):2100196. doi: 10.1002/adhm.202100196. [DOI] [PubMed] [Google Scholar]
- Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, Straus SM, Herings RM, Stricker BH. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35(1):119–124. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi Saravi SS, Hasanvand A, Shahkarami K, Dehpour AR. The protective potential of metformin against acetaminophen-induced hepatotoxicity in BALB/C mice. Pharm Biol. 2016;54(12):2830–2837. doi: 10.1080/13880209.2016.1185633. [DOI] [PubMed] [Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181(12):8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (berl) 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt IP, Palmer TM. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin Investig Drugs. 2012;21(8):1155–1167. doi: 10.1517/13543784.2012.696609. [DOI] [PubMed] [Google Scholar]
- Salvati KA, Ritger ML, Davoudian PA, O’Dell F, Wyskiel DR, Souza GMPR, Lu AC, Perez-Reyes E, Drake JC, Yan Z, Beenhakker MP. AMPK-mediated potentiation of GABAergic signalling drives hypoglycaemia-provoked spike-wave seizures. Brain. 2022 doi: 10.1093/brain/awac037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi SA, Lee H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One. 2011;6(1):e14614. doi: 10.1371/journal.pone.0014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz P, Serratosa JM, Sánchez MP. Beneficial effects of metformin on the central nervous system, with a focus on epilepsy and Lafora disease. Int J Mol Sci. 2021;22(10):5351. doi: 10.3390/ijms22105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res. 2019;11:3295–3313. doi: 10.2147/CMAR.S200059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Kim J, Park SJ, Park JJ, Cheon JH, Kim WH, Kim TI. Metformin suppresses cancer stem cells through AMPK activation and inhibition of protein prenylation of the mevalonate pathway in colorectal cancer. Cancers. 2020;12(9):2554. doi: 10.3390/cancers12092554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Cao X, Song G, Zhao Y, Shi B. Metformin rescues the MG63 osteoblasts against the effect of high glucose on proliferation. J Diabetes Res. 2014;2014:453940. doi: 10.1155/2014/453940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhang J, Wang C, Jain PP, Xiong M, Shi X, Lei Y, Chen S, Yin Q, Thistlethwaite PA, Wang J, Gong K, Yuan ZY, Yuan JX, Shyy JY. MDM2-mediated ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension. Circulation. 2020;142(12):1190–1204. doi: 10.1161/CIRCULATIONAHA.120.048191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpakov AO. Improvement effect of metformin on female and male reproduction in endocrine pathologies and its mechanisms. Pharmaceuticals. 2021;14(1):42. doi: 10.3390/ph14010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Chan J, Ryan RO, Forte TM. Apolipoprotein A-V association with intracellular lipid droplets. J Lipid Res. 2007;48(7):1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- Shu X, Nelbach L, Ryan RO, Forte TM. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim Biophys Acta. 2010;1801(5):605–608. doi: 10.1016/j.bbalip.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Singh J and Rattan S (2021) Evidence for the presence and release of BDNF in the neuronal and non-neuronal structures of the internal anal sphincter. Neurogastroenterol Motil 34(4):e14099 [DOI] [PMC free article] [PubMed]
- Singh A, Rattan S. BDNF rescues aging-associated internal anal sphincter dysfunction. Am J Physiol Gastrointest Liver Physiol. 2021;321(1):G87–g97. doi: 10.1152/ajpgi.00090.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311(4):E730–e740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- Son H-J, Lee J, Lee S-Y, Kim E-K, Park M-J, Kim K-W, Park S-H, Cho M-L. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:13. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wu Z, Zhao P (2022) The function of metformin in aging-related musculoskeletal disorders. Front Pharmacol 13:865524. 10.3389/fphar.2022.865524 [DOI] [PMC free article] [PubMed]
- Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol. 2014;40(6):546–553. doi: 10.1159/000370034. [DOI] [PubMed] [Google Scholar]
- Stochino-Loi E, Major AL, Gillon TER, Ayoubi JM, Feki A, Bouquet de Joliniere J. Metformin, the rise of a new medical therapy for endometriosis? a systematic review of the literature. Front Med (Lausanne) 2021;8:581311. doi: 10.3389/fmed.2021.581311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021–2032. doi: 10.1038/bjc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Gong F, Liu P, Miao Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene. 2018;664:50–57. doi: 10.1016/j.gene.2018.04.045. [DOI] [PubMed] [Google Scholar]
- Sun C-K, Weng P-W, Chang JZ-C, Lin Y-W, Tsuang F-Y, Lin F-H, Tsai T-H, Sun J-S. Metformin-incorporated gelatin/hydroxyapatite nanofiber scaffold for bone regeneration. Tissue Eng Part A. 2022;28(1–2):1–12. doi: 10.1089/ten.tea.2021.0038. [DOI] [PubMed] [Google Scholar]
- Taheri A, Emami M, Asadipour E, Kasirzadeh S, Rouini M-R, Najafi A, Heshmat R, Abdollahi M, Mojtahedzadeh M. A randomized controlled trial on the efficacy, safety, and pharmacokinetics of metformin in severe traumatic brain injury. J Neurol. 2019;266(8):1988–1997. doi: 10.1007/s00415-019-09366-1. [DOI] [PubMed] [Google Scholar]
- Taleb S, Moghaddas P, Rahimi Balaei M, Taleb S, Rahimpour S, Abbasi A, Ejtemaei-Mehr S, Dehpour AR. Metformin improves skin flap survival through nitric oxide system. J Surg Res. 2014;192(2):686–691. doi: 10.1016/j.jss.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Tan XL, Bhattacharyya KK, Dutta SK, Bamlet WR, Rabe KG, Wang E, Smyrk TC, Oberg AL, Petersen GM, Mukhopadhyay D. Metformin suppresses pancreatic tumor growth with inhibition of NFkappaB/STAT3 inflammatory signaling. Pancreas. 2015;44(4):636–647. doi: 10.1097/MPA.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y, Gao R, Chen G. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res Bull. 2018;140:154–161. doi: 10.1016/j.brainresbull.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Taylor A, Westveld AH, Szkudlinska M, Guruguri P, Annabi E, Patwardhan A, Price TJ, Yassine HN. The use of metformin is associated with decreased lumbar radiculopathy pain. J Pain Res. 2013;6:755–763. doi: 10.2147/JPR.S52205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev VO, Menshchikova EB, Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (mosc) 2011;76(4):407–422. doi: 10.1134/S0006297911040031. [DOI] [PubMed] [Google Scholar]
- ul haq Shah MZ, Shrivastava V, Mir MA (2022) Metformin treatment ameliorates endocrine-metabolic disturbances in letrozole-induced PCOS mice model by modulating adiponectin status. Obesity Med 31:100392
- Varjabedian L, Bourji M, Pourafkari L, Nader ND. Cardioprotection by metformin: beneficial effects beyond glucose reduction. Am J Cardiovasc Drugs. 2018;18(3):181–193. doi: 10.1007/s40256-018-0266-3. [DOI] [PubMed] [Google Scholar]
- Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64(6):2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Menendez JA. AMPK: evidence for an energy-sensing cytokinetic tumor suppressor. Cell Cycle. 2009;8(22):3679–3683. doi: 10.4161/cc.8.22.9905. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8(1):88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, Guo Y, Li Z, Yao T, Mei X. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. 2016;477(4):534–540. doi: 10.1016/j.bbrc.2016.05.148. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen K, Yu Y, Xiang Y, Kim JH, Gong W, Huang J, Shi G, Li Q, Zhou M, Sayers T, Tewary P, Gao B, Wang JM. Metformin sensitizes lung cancer cells to treatment by the tyrosine kinase inhibitor erlotinib. Oncotarget. 2017;8(65):109068–109078. doi: 10.18632/oncotarget.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes. 2018;67(1):98–109. doi: 10.2337/db17-0503. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang T, Liao Q, Dai M, Guo J, Yang X, Tan W, Lin D, Wu C, Zhao Y. Metformin inhibits pancreatic cancer metastasis caused by SMAD4 deficiency and consequent HNF4G upregulation. Protein Cell. 2021;12(2):128–144. doi: 10.1007/s13238-020-00760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang A, Guo H, Zhang Z, Wang S, Pei T, Liu Z, Yang D, Liu Y, Ruan C. Neuroprotective effects of long-term metformin preconditioning on rats with ischemic brain injuries. Eur Neurol. 2021;84(3):212–218. doi: 10.1159/000514431. [DOI] [PubMed] [Google Scholar]
- Wang X-D, Yu W-L, Sun Y. Activation of AMPK restored impaired autophagy and inhibited inflammation reaction by up-regulating SIRT1 in acute pancreatitis. Life Sci. 2021;277:119435. doi: 10.1016/j.lfs.2021.119435. [DOI] [PubMed] [Google Scholar]
- Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30(5):218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wiernsperger N. Metformin as a cellular protector; a synoptic view of modern evidences. J Nephropharmacol. 2015;4(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Witzig TE, Gupta M. Signal transduction inhibitor therapy for lymphoma. Hematology Am Soc Hematol Educ Program. 2010;2010:265–270. doi: 10.1182/asheducation-2010.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, Guo X, Guo T, Botchlett R, Qi T, Pei Y, Zheng J, Xu Y, An X, Chen L, Chen L, Li Q, Xiao X, Huo Y, Wu C. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014;9(3):e91111. doi: 10.1371/journal.pone.0091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20(9):1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, B, Dai W, Liu L, Han H, Zhang J, Du X, Pei X and Fu X (2022) "Metformin ameliorates polycystic ovary syndrome in a rat model by decreasing excessive autophagy in ovarian granulosa cells via the PI3K/AKT/mTOR pathway" Endocrine J EJ21–0480. [DOI] [PubMed]
- Yan H, Zhou H, Hu Y, Pham CTN. Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J Rheum Dis Treat. 2015;1(1):5. doi: 10.23937/2469-5726/1510005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Mu Y, Yu N, Yi T, Zhang Y, Pang X, Cheng D, Yang J. Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J Assist Reprod Genet. 2015;32(7):1097–1104. doi: 10.1007/s10815-015-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285(25):19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ding H, Qin Z, Zhang C, Qi S, Zhang H, Yang T, He Z, Yang K, Du E, Liu C, Xu Y, Zhang Z. Metformin prevents renal stone formation through an antioxidant mechanism in vitro and in vivo. Oxid Med Cell Longev. 2016;2016:10. doi: 10.1155/2016/4156075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerra VG, Kalvala AK, Sherkhane B, Areti A, Kumar A. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology. 2018;131:256–270. doi: 10.1016/j.neuropharm.2017.12.029. [DOI] [PubMed] [Google Scholar]
- Yi Y, Chen D, Ao J, Sun S, Wu M, Li X, Bergholz J, Zhang Y, Xiao Z-X. Metformin promotes AMP-activated protein kinase-independent suppression of ΔNp63α protein expression and inhibits cancer cell viability. J Biol Chem. 2017;292(13):5253–5261. doi: 10.1074/jbc.M116.769141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LH, Zaha VG. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res. 2012;111(6):800–814. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279(46):47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- Zangiabadian M, Nejadghaderi SA, Zahmatkesh MM, Hajikhani B, Mirsaeidi M, Nasiri MJ. The efficacy and potential mechanisms of metformin in the treatment of COVID-19 in the diabetics: a systematic review. Front Endocrinol (Lausanne) 2021;12:645194. doi: 10.3389/fendo.2021.645194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li G, Chen Y, Fang L, Guan C, Bai F, Ma M, Lyu J, Meng QH. Metformin inhibits tumorigenesis and tumor growth of breast cancer cells by Upregulating miR-200c but downregulating AKT2 expression. J Cancer. 2017;8(10):1849–1864. doi: 10.7150/jca.19858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sun M. Metformin rescues Parkin protein expression and mitophagy in high glucose-challenged human renal epithelial cells by inhibiting NF-κB via PP2A activation. Life Sci. 2020;246:117382. doi: 10.1016/j.lfs.2020.117382. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun H, Feng M, Zhao J, Zhao X, Wan Q, Cai D. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol Endocrinol. 2018;34(5):428–432. doi: 10.1080/09513590.2017.1409714. [DOI] [PubMed] [Google Scholar]
- Zhao B, Luo J, Wang Y, Zhou L, Che J, Wang F, Peng S, Zhang G, Shang P. Metformin suppresses self-renewal ability and tumorigenicity of osteosarcoma stem cells via reactive oxygen species-mediated apoptosis and autophagy. Oxid Med Cell Longev. 2019;2019:9290728. doi: 10.1155/2019/9290728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Tang Y, Jin X, Chen C, Lu Y, Liu L, Shen C. Metformin inhibits advanced glycation end products-induced inflammatory response in murine macrophages partly through AMPK activation and RAGE/NFkappaB pathway suppression. J Diabetes Res. 2016;2016:4847812. doi: 10.1155/2016/4847812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang G, Chang C, Chou OHI, Lee S, Leung KSK, Wong WT, Liu T, Wai AKC, Cheng SH (2022) Metformin versus sulphonylureas for new onset atrial fibrillation and stroke in type 2 diabetes mellitus: a population-based study. Acta Diabetol 1–13 [DOI] [PubMed]
- Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, Ji Z. Metformin improves neurologic outcome via amp-activated protein kinase–mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc. 2018;7(12):e008389. doi: 10.1161/JAHA.117.008389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Enquiries about data availability should be directed to the authors.