Abstract
Despite the use of pneumococcal conjugate vaccines for pediatric immunization, North American Indigenous populations continue to experience high burden of pneumococcal infections. Naturally acquired antibodies, which can protect unvaccinated adults against pneumococcal infections, have not previously been studied in Canadian Indigenous people. We analysed concentrations of natural serum IgG, IgM and IgA antibodies specific to 7 serotype-specific capsular polysaccharides (3, 6B, 9V, 14, 19A, 19F and 23F) in 141 healthy individuals (age between 18 and 80 years), including Indigenous adults living in 2 geographical different areas of Ontario, Canada, and non-Indigenous residing in northwestern Ontario. Regardless of the geographical area, concentrations of IgG specific to serotypes 6B, 9V, and 14, IgM specific to 9V, and all serotype-specific IgA were significantly higher in Indigenous study participants as compared to non-Indigenous. The differences are likely attributed to an increased exposure of Indigenous individuals to Streptococcus pneumoniae and/or cross-reactive antigens of other microorganisms or plants present in the environment. Although in non-Indigenous adults concentrations of IgM specific to 9V, 19A, 19F, and 23F significantly decreased with age, this was not observed in Indigenous individuals suggesting that Indigenous people may experience continuous exposure to pneumococci and cross-reactive antigens over the life span. Women had generally higher concentrations of natural IgG and IgM concentrations than men, with more striking differences found in Indigenous adults, potentially associated with larger exposure of women to young children, the major reservoir of pneumococci in communities. Our data suggest that increased rates of pneumococcal infections among Indigenous people are unlikely related to deficiency of naturally acquired antibodies, at least those specific to 7 common serotypes. Determining serological correlates of protection for adults will be essential to identify the groups in need of adult pneumococcal immunizations that may prevent excessive burden of the disease among North American Indigenous people.
Introduction
Streptococcus pneumoniae (pneumococcus) is Gram-positive, encapsulated, extracellular bacterium, which asymptomatically colonizes mucosal surfaces of the upper respiratory tract and spreads through respiratory droplets [1,2]. When pneumococci evade the host defenses, they can cause non-invasive (otitis media, sinusitis, pneumonia) and invasive (meningitis, septicaemia, pericarditis) infections, if the bacteria invade normally sterile body sites [3,4]. Children under 5 years of age, the elderly, and immunocompromised adults have the highest incidence of invasive pneumococcal disease (IPD) and pneumococcal pneumonia [2,3]. Over 90 pneumococcal serotypes have been identified through antigenic differences in their polysaccharide capsules [5]. The capsule is a key virulence factor in the pathogenesis of pneumococcal disease; it facilitates colonization of mucosal surfaces, inhibits the classic and alternative complement pathways, and reduces opsonization by impeding interactions between complement fragments or antibodies with their receptors on phagocytes [1,6].
Since the introduction of pneumococcal conjugate vaccines (PCV), which stimulate T-cell dependent production of antibodies to pneumococcal capsular polysaccharides, into pediatric immunization programs two decades ago, incidence rates of IPD caused by vaccine serotypes decreased among both immunized children and unimmunized adults. The indirect effect of pediatric immunization is the result of decreased transmission of S. pneumoniae from immunized children to adults due to reduced nasopharyngeal colonization [7]. In the post-PCV era, the colonization rates of vaccine serotypes have also decreased in adults [8,9]. In Canada, PCV containing 7 serotype-specific pneumococcal capsular polysaccharides, i.e. 4, 6B, 9V, 14, 18C, 19F, and 23F (PCV7) introduced into publicly-funded universal pediatric immunization program in 2005, was replaced by PCV13, which includes 6 additional serotypes (1, 3, 5, 6A, 7F, 19A), in 2010–2011 [10]. The 23-valent pneumococcal polysaccharide vaccine (PPV23) containing purified capsular polysaccharides of serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F is currently used for immunization of adults ≥65 years of age as well as groups of risk for IPD >2 years of age [11]. However, pneumococcal vaccine uptake in Canadian adults is low; amongst the adult population with chronic conditions predisposing to IPD, only 17.3% of 18–64 year-old and 36.5% of ≥65 year-old individuals received PPV23 in 2014 [12]. Similar to other countries, in Canada, over the last years, the incidence of IPD caused by PCV13 serotypes has decreased in both young children and older adults although the overall rates did not decrease mainly due to the increasing incidence of PPV23 unique and non-vaccine serotypes [13]. While the numbers of hospitalized adult cases of community-acquired pneumococcal pneumonia decreased between 2011 and 2014, they increased in 2015, with a particular growth in cases caused by serotype 3 [10].
The province of Ontario is a large region of over one million square kilometers characterized by diverse climate conditions, ranging from subarctic in the north to moderate humid continental climate in the south. Northwestern Ontario has the largest proportion of Indigenous people, i.e. 25.4% of the population compared to 2.83% in the whole province [14,15]. In North America, the incidence of IPD remains higher in Indigenous people compared to the general population despite the use of pneumococcal vaccines [16–19]. Eton et al. (2017) investigated the cases of IPD admitted to a hospital serving Indigenous communities in northwestern Ontario. The incidence of IPD over a period of 2010–2015 was more than double the 2013 rate for all of Canada (23.1 vs. 9.0/100,000/year) [20]. Among IPD cases admitted to the major hospital serving northwestern Ontario between 2006 and 2015, 29.1% of adult patients were Indigenous, and 72% of cases were caused by non-PCV13 serotypes [21].
We recently studied immune response to PCV13 in adults with severe chronic kidney disease in northwestern Ontario and unexpectedly found higher concentrations of naturally acquired 6B, 9V, 14, 19F, and 23F serotype-specific IgG antibodies in Indigenous individuals as compared to their non-Indigenous counterpart [22]. As no studies have previously addressed natural pneumococcal antibodies in Canadian Indigenous people, we extended the analysis to include healthy adults residing in different parts of the province to account for potential effect of distinct environmental conditions. The analysis involved serum IgG, IgM and IgA antibodies to 7 serotype-specific capsular polysaccharides included into PCV13 and PPV23 (3, 6B, 9V, 14, 19A, 19F and 23F). We found that regardless of the geographical area, concentrations of several serotype-specific IgG and IgM, and all serotype-specific IgA were higher in Indigenous study participants as compared to non-Indigenous individuals residing in northwestern Ontario.
Materials and methods
Ethics statement
The study has been conducted according to the World Medical Association Declaration of Helsinki. We adhered to the principles of Ownership, Control, Access, and Possession (OCAP) as defined by the National Aboriginal Health Organization [23], and the guidelines of Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2), specifically those outlined in Chapter 9: Research Involving First Nations, Inuit and Métis Peoples of Canada [24]. The study was approved by the research ethics board of Lakehead University (REB #117 11-12/Romeo #1462496, Thunder Bay, Ontario).
Study participants
The study included 141 healthy adults (Table 1). Indigenous adults were recruited from two Ojibwa First Nations communities, one in southern Ontario (Group 1) and the other in northwestern Ontario (Group 2). Non-Indigenous adults were recruited from two cities in northwestern Ontario, Thunder Bay (Group 3) and Kenora (Group 4). Participants were considered eligible if they were over the age of 18 years, self-declared generally healthy, did not have a history of taking immunosuppressive medications, and declared that they did not receive a pneumococcal vaccine. Ethnicity was determined based on self-declaration. Serum samples from both Indigenous communities, Kenora, and a part of the Thunder Bay samples were collected during January—November 2015, as we previously described [25]. The remaining Thunder Bay samples were collected between November 2016 and November 2017. The samples were collected under informed written consent and stored at -80°C prior to analysis.
Table 1. Participant demographics.
| Age (years) | |||||
|---|---|---|---|---|---|
| Group | Number | Mean ± SEM | Median | Range | % Female |
| Indigenous a | 77 | 41 (1.67) | 39 | 18–80 | 49.4 |
| non-Indigenous b | 64 | 45 (2.11) | 52 | 20–72 | 66.6* |
| Group 1 c# | 30 | 46 (2.75) ** | 51 | 20–80 | 50 |
| Group 2 d | 47 | 37 (1.94) | 34 | 18–67 | 48.9 |
| Group 3 e | 45 | 46 (2.48) ** | 52 | 22–72 | 62.2 |
| Group 4 f& | 19 | 43 (4.04) | 48 | 20–68 | 77.8 |
a Indigenous: Indigenous First Nations adults residing in Ontario (combination of groups 1 and 2).
b non-Indigenous: Non-Indigenous adults residing in Ontario (combination of groups 3 and 4).
c Group 1: Indigenous adults residing in a southern Ontario First Nations Community.
d Group 2: Indigenous adults residing in a northwestern Ontario First Nations Community.
e Group 3: Non-Indigenous adults residing in Thunder Bay, Ontario.
f Group 4: Non-Indigenous adults residing in Kenora, Ontario.
#Age of one participant is unknown.
&Sex of one participant is unknown.
* p < 0.05, Indigenous females vs. non-Indigenous females (Fisher’s exact test).
** p < 0.01, in comparison to group 2 (Mann-Whitney U test).
SEM, Standard error of the mean.
No significant differences in the proportions of females between groups 1–4 (Fisher’s exact test).
Antibody analysis
Serum concentrations of IgG, IgM and IgA specific to the pneumococcal serotypes 3, 6B, 9V, 14, 19A, 19F and 23F were determined by enzyme-linked immunosorbent assay (ELISA) according to the WHO protocol [26].
Statistical analysis
The serotype-specific antibodies were reported as geometric mean concentrations (GMC) with two-sided 95% confidence intervals (CI). Groups were compared using Mann-Whitney U test, or Kruskal-Wallis test with Dunn’s ad hoc tests depending on the number of groups compared; Fishers’ exact test was conducted to compare categorical variables. To study the association of antibody concentrations with age, linear regression and Spearman correlation analyses were performed. A p value of < 0.05 was reported as statistically significant. Statistical analysis was performed using Graph-Pad Prism 9 (GraphPad Prism Software Inc., San Diego, CA).
Results
Demographics of 141 study participants are presented in Table 1. The age range was between 18 and 80 years, without significant differences between Indigenous and non-Indigenous individuals. The non-Indigenous participants (Group 3 and Group 4 combined) had a higher proportion of females compared to the Indigenous (Group 1 and Group 2 combined). Group 2 was significantly younger compared to Groups 1 or 3 and it had the lowest proportion of women while Group 4 had the highest (Table 1).
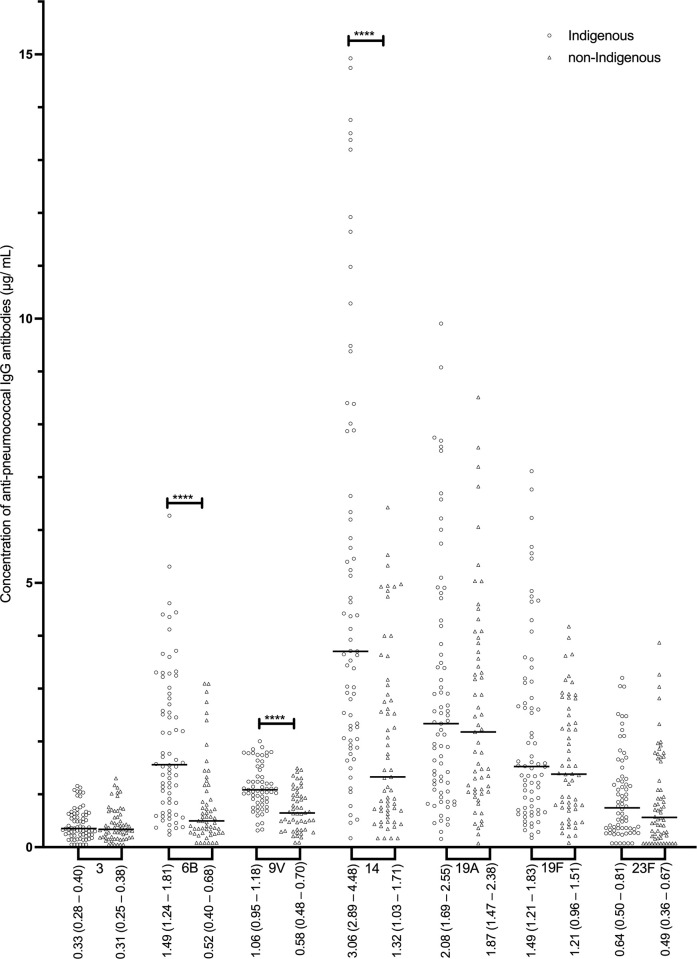
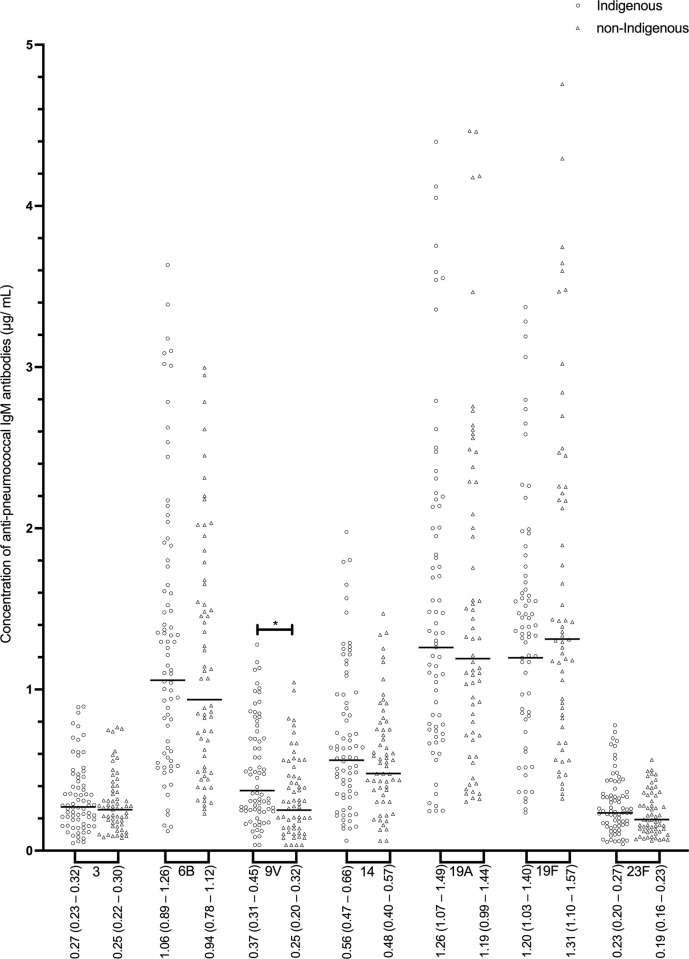
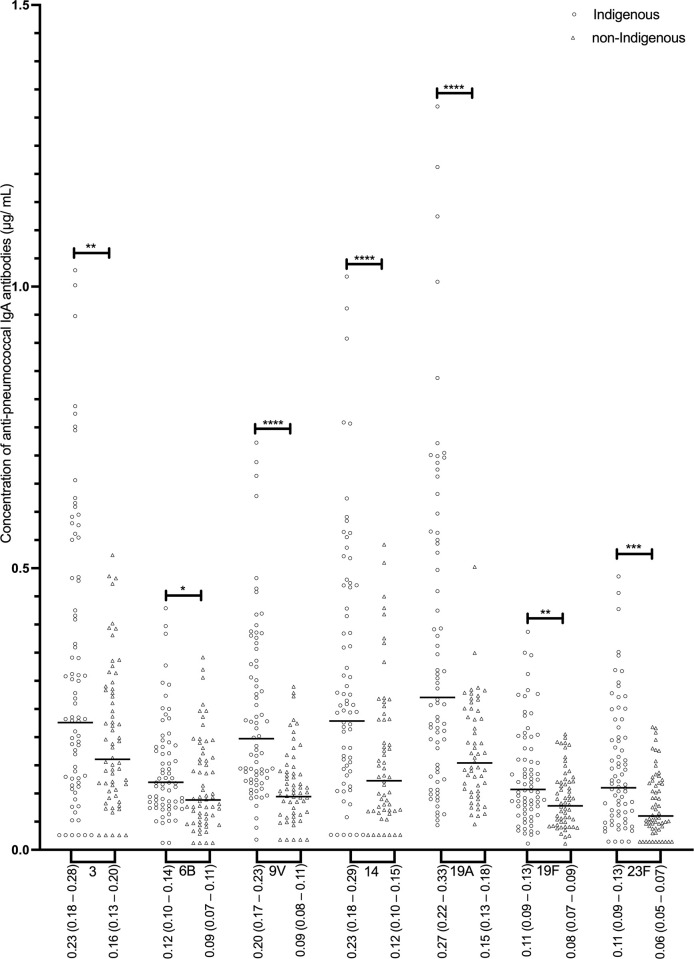
Indigenous individuals (Group 1 and Group 2 combined) had significantly higher concentrations of 3 out of 7 serotype-specific IgG (anti-6B, -9V, and -14) compared to non-Indigenous (Fig 1). In addition, proportions of Indigenous adults who had concentrations of IgG specific to serotypes 6B, 9V, and 14 above 1.0 μg/mL and above 1.3 μg/mL were significantly higher than the corresponding proportions of non-Indigenous individuals (S1 Table). Among Indigenous individuals, GMC for IgG specific to serotypes 19A and 19F were significantly higher in Group 1 than Group 2; no differences in GMC between non-Indigenous individuals residing in different communities (Group 3 vs. Group 4) were present (Table 2A). Concentrations of serotype-specific IgM were generally similar among all the groups (Table 2B) although Indigenous individuals (Group 1 and Group 2 combined) had higher IgM specific to 9V compared to non-Indigenous (Group 3 and Group 4 combined), as demonstrated in Fig 2. In contrast, concentrations of all 7 serotype-specific IgA were significantly higher in Indigenous than non-Indigenous study participants (Fig 3). Although no differences were present between Indigenous (between Groups 1 and 2) or non-Indigenous individuals (between Groups 3 and 4) residing in different communities, GMC of specific IgA in Indigenous people living in southern Ontario (Group 1) were significantly higher for serotypes 9V, 14, and 23F as compared to Group 3, and for serotypes 9V, 14, and 19A compared to Group 4. Similarly, Indigenous people living in northwestern Ontario (Group 2) had higher GMC of IgA specific to 9V, 19A, and 23F as compared to Group 3, and to 9V and 19A compared to Group 4 (Table 2C).
Fig 1. Concentrations of serotype-specific pneumococcal IgG antibodies for Indigenous (n = 77) and non-Indigenous groups (n = 64).
Geometric mean antibody concentrations (GMC) are displayed (μg/ mL) and the 95% confidence intervals of the GMC. Statistical significance was determined using a Mann-Whitney U test for all serotypes. **** p < 0.0001.
Table 2A. The geometric mean concentrations of anti-pneumococcal IgG antibodies (μg/ mL, 95% confidence intervals of the geometric mean).
| Serotype | Group 1 | Group 2 | Group 3 | Group 4 | p value (Kruskal-Wallis test) |
| 3 | 0.39 (0.29–0.53) | 0.29 (0.23–0.37) | 0.25 (0.19–0.33) | 0.45 (0.35–0.57) | 0.019 |
| 6B | 1.59 (1.13–2.23) | 1.47 (1.17–1.86) $ $ $ $ | 0.46 (0.34–0.61) #### | 0.68 (0.38–1.22) ^ & | <0.0001 |
| 9V | 1.02 (0.86–1.21) | 1.09 (0.94–1.25) $ $ $ $ | 0.53 (0.41–0.67) ## | 0.73 (0.55–0.95) & | <0.0001 |
| 14 | 4.50 (3.02–6.72) | 3.18 (2.44–4.15) $ $ $ | 1.28 (0.93–1.77) #### | 1.43 (0.94–2.18) ^^ & | <0.0001 |
| 19A | 2.98 (2.03–4.39) * | 1.60 (1.28–1.99) | 1.65 (1.20–2.27) | 2.48 (1.77–3.49) | 0.0088 |
| 19F | 2.33 (1.57–3.45) ** | 1.20 (0.95–1.51) | 1.08 (0.80–1.46) ## | 1.53 (1.10–2.12) | 0.0043 |
| 23F | 0.88 (0.59–1.32) | 0.54 (0.40–0.72) | 0.38 (0.26–0.56) # | 0.84 (0.59–1.19) | 0.013 |
| Group 1: Indigenous adults residing in a southern Ontario First Nations Community. | |||||
| Group 2: Indigenous adults residing in a northwestern Ontario First Nations Community. | |||||
| Group 3: Non-Indigenous adults residing in Thunder Bay, Ontario. | |||||
| Group 4: Non-Indigenous adults residing in Kenora, Ontario. | |||||
| Significance determined by Dunn’s multiple comparisons test. | |||||
| Group 1 vs. Group 2 | |||||
| * p < 0.05 | |||||
| ** p < 0.01. | |||||
| Group 1 vs. Group 3 | |||||
| # p < 0.05 | |||||
| ## p < 0.01 | |||||
| #### p < 0.0001. | |||||
| Group 1 vs. Group 4 | |||||
| ^ p < 0.05 | |||||
| ^^ p < 0.01. | |||||
| Group 2 vs. Group 3 | |||||
| $ $ $ p < 0.001 | |||||
| $ $ $ $ p < 0.0001. | |||||
| Group 2 vs. Group 4 | |||||
| & < 0.05. | |||||
| Group 3 vs. Group 4: No significant differences for any serotypes. | |||||
| Table 2B The geometric mean concentrations of anti-pneumococcal IgM antibodies (μg/ mL, 95% confidence intervals of the geometric mean). | |||||
| Serotype | Group 1 | Group 2 | Group 3 | Group 4 | p value (Kruskal-Wallis test) |
| 3 | 0.28 (0.21–0.37) | 0.27 (0.22–0.34) | 0.28 (0.23–0.34) | 0.20 (0.16–0.26) | > 0.05 |
| 6B | 0.98 (0.72–1.34) | 1.11 (0.89–1.37) | 1.01 (0.81–1.27) | 0.84 (0.61–1.17) | > 0.05 |
| 9V | 0.34 (0.24–0.49) | 0.41 (0.33–0.50) | 0.26 (0.20–0.34) | 0.23 (0.15–0.37) | > 0.05 |
| 14 | 0.48 (0.35–0.66) | 0.61 (0.51–0.75) | 0.55 (0.44–0.68) | 0.38 (0.28–0.53) | > 0.05 |
| 19A | 1.31 (0.97–1.76) | 1.27 (1.03–1.57) | 1.27 (1.01–1.60) | 1.02 (0.73–1.43) | > 0.05 |
| 19F | 1.27 (0.93–1.73) | 1.20 (1.01–1.43) | 1.39 (1.11–1.74) | 1.24 (0.91–1.69) | > 0.05 |
| 23F | 0.22 (0.16–0.28) | 0.24 (0.19–0.29) | 0.21 (0.17–0.25) | 0.16 (0.12–0.22) | > 0.05 |
| Group 1: Indigenous adults residing in a southern Ontario First Nations Community. | |||||
| Group 2: Indigenous adults residing in a northwestern Ontario First Nations Community. | |||||
| Group 3: Non-Indigenous adults residing in Thunder Bay, Ontario. | |||||
| Group 4: Non-Indigenous adults residing in Kenora, Ontario. | |||||
| Dunn’s multiple comparisons test did not detect any statistically significant differences in IgM concentrations between groups for any serotype. | |||||
| Table 2C The geometric mean concentrations of anti-pneumococcal IgA antibodies (μg/ mL, 95% confidence intervals of the geometric mean). | |||||
| Serotype | Group 1 | Group 2 | Group 3 | Group 4 | p value (Kruskal-Wallis test) |
| 3 | 0.20 (0.14–0.30) | 0.24 (0.18–0.32) | 0.16 (0.13–0.21) | 0.17 (0.11–0.25) | > 0.05 |
| 6B | 0.12 (0.09–0.17) | 0.13 (0.10–0.15) | 0.09 (0.07–0.11) | 0.09 (0.06–0.13) | > 0.05 |
| 9V | 0.17 (0.13–0.24) | 0.21 (0.18–0.26) $ $ $ $ | 0.10 (0.08–0.12) ## | 0.07 (0.05–0.11) ^^ &&&& | <0.0001 |
| 14 | 0.32 (0.22–0.46) | 0.21 (0.16–0.28) | 0.14 (0.11–0.18) ## | 0.12 (0.07–0.21) ^^ | 0.0008 |
| 19A | 0.26 (0.19–0.37) | 0.27 (0.21–0.34) $ | 0.16 (0.14–0.19) | 0.14 (0.10–0.18) ^ & | 0.0013 |
| 19F | 0.11 (0.08–0.16) | 0.10 (0.08–0.12) | 0.08 (0.07–0.10) | 0.07 (0.06–0.10) | > 0.05 |
| 23F | 0.11 (0.07–0.16) | 0.11 (0.09–0.14) $ $ | 0.06 (0.04–0.07) # | 0.07 (0.04–0.10) | 0.0017 |
| Group 1: Indigenous adults residing in a southern Ontario First Nations Community. | |||||
| Group 2: Indigenous adults residing in a northwestern Ontario First Nations Community. | |||||
| Group 3: Non-Indigenous adults residing in Thunder Bay, Ontario. | |||||
| Group 4: Non-Indigenous adults residing in Kenora, Ontario. | |||||
| Significance determined by Dunn’s multiple comparisons test. | |||||
| Group 1 vs. Group 2: No significant differences for any serotypes. | |||||
| Group 1 vs. Group 3 | |||||
| # p < 0.05 | |||||
| ## p < 0.01. | |||||
| Group 1 vs. Group 4 | |||||
| ^ p < 0.05 | |||||
| ^^ p < 0.01. | |||||
| Group 2 vs. Group 3 | |||||
| $ p < 0.05 | |||||
| $ $ p < 0.01 | |||||
| $ $ $ $ p < 0.0001. | |||||
| Group 2 vs. Group 4 | |||||
| & p < 0.05 | |||||
| &&&& p < 0.0001. | |||||
| Group 3 vs. Group 4: No significant differences for any serotypes. | |||||
Fig 2. Concentrations of serotype-specific pneumococcal IgM antibodies for Indigenous (n = 77) and non-Indigenous groups (n = 64).
Geometric mean antibody concentrations (GMC) are displayed (μg/ mL) and the 95% confidence intervals of the GMC. Statistical significance was determined using a Mann-Whitney U test for all serotypes. * p < 0.05.
Fig 3. Concentrations of serotype-specific pneumococcal IgA antibodies for Indigenous (n = 77) and non-Indigenous groups (n = 64).
Geometric mean antibody concentrations are displayed (μg/ mL) and the 95% confidence intervals of the GMC. Statistical significance was determined using a Mann-Whitney U test for all serotypes. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
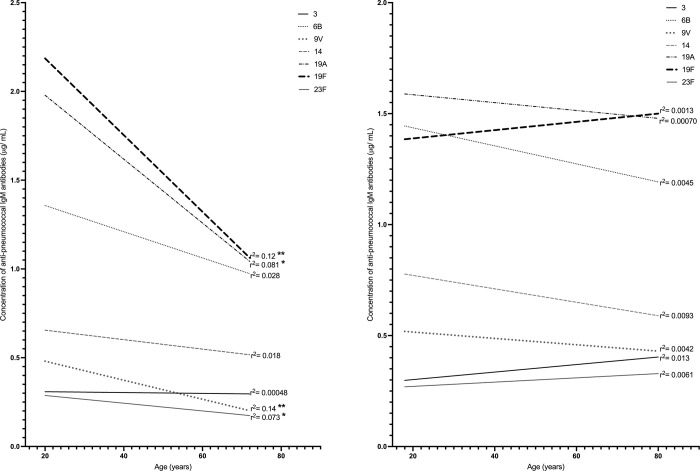
To determine if concentrations of natural pneumococcal antibodies were dependent on age, linear regression analyses were performed for Indigenous and non-Indigenous groups for each of the seven serotype-specific IgG, IgM and IgA. No consistent or clear association of most IgG (S2 Table) or IgA (S3 Table) concentrations with age for either group was observed. However, concentrations of IgM specific to serotypes 9V, 19A, 19F, and 23F significantly decreased with age in non-Indigenous (Fig 4A), but not in Indigenous individuals (Fig 4B). Spearman correlation analysis indicated statistically significant, but weak negative correlation between age of non-Indigenous adults and concentrations of IgM specific to serotypes 9V (r = −0.28, p < 0.05) and 19F (r = −0.25, p < 0.05). No significant correlation between age and the rest of serotype-specific IgM concentrations was found (S4 Table).
Fig 4.
Linear regression analysis of association of serotype-specific IgM antibodies with age for (A) non-Indigenous groups (n = 64) and (B) Indigenous (n = 76). The goodness of the fit is reported by the r2 value; the p value determines if the slope is significantly non-zero. * p < 0.05, ** p < 0.01.
As differences in antibody concentrations between men and women could potentially account for the above observations, we compared GMC of serotype-specific IgG, IgM, and IgA between male and female individuals of the same ethnic group. No significant differences in mean ages between males and females of the same ethnicity were present (S5 Table). As shown in S6 Table, concentrations of IgG and IgM antibodies were generally higher in Indigenous women than men, with statistically significant differences identified for 14-, 19A-, and 19F-specific IgG, and for all but one serotype-specific IgM (except for serotype 14). In non-Indigenous individuals, higher female IgG antibody concentrations were found only in case of serotype 23F; all but one serotype-specific IgM (except for serotype 6B) were significantly higher in female than male individuals. Concentrations of IgA antibodies did not differ between male and female individuals of either ethnicity (S6 Table).
Discussion
In this study, we quantified natural antibodies specific to seven pneumococcal serotypes (3, 6B, 9V, 14, 19A, 19F and 23F), which are all included in currently used pneumococcal vaccines (PPV23 and PCV13) owing to their epidemiological importance. These serotypes have distinct biological characteristics and different prevalence in various forms of pneumococcal infections [27].
The principal rationale for specifically selecting these 7 pneumococcal serotypes was based on their distinct immunogenic characteristics recognized from studies of antibody response to multi-component pneumococcal vaccines. It is known that serological thresholds for protection against infections caused by these serotypes substantially differ. A large multi-national study by Voysey et al. (2018) demonstrated that antibody concentrations associated with protection against carriage greatly varied between 10 different serotypes (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F). The highest protective threshold was found for serotypes 14 and 19F, and the lowest for serotypes 6B and 23F; the threshold for serotype 9V was intermediate [28]. Including analysis of antibody for serotype 3 was of special interest for our study. Pneumococci of this serotype are heavily encapsulated are highly resistant to phagocytosis. As serotype 3 is typically associated with invasive disease rather than with nasopharyngeal carriage, this may result in poor development of natural antibody [29]. We were interested in antibodies to serotype 14 because its capsule is structurally similar to the capsular polysaccharide of Group B Streptococcus type III that could influence the development of natural immunity; immunological cross-reactivity between these bacteria was reported [30]. Considering that pneumococci of the serogroup 19 widely cross-react with other common bacteria including Klebsiella and streptococci, we were interested in the analysis of naturally-acquired antibodies against serotypes 19A and 19F [31]. In addition, antibiotic-resistant serotype 19A emerged as replacement disease following the introduction of the first-generation pneumococcal conjugate vaccine (PCV7) in early 2000s, and became highly significant in the epidemiology of pneumococcal infections until immunization with PCV13 greatly reduced its prevalence [32].
Prior to the introduction of pneumococcal conjugate vaccines, all these 7 serotypes were highly significant in epidemiology of IPD as well as commonly associated with nasopharyngeal carriage in young children [33,34]. In Canada, the rates of disease caused by PCV13 vaccine serotypes (except for serotype 3), including IPD in all age groups, and pneumococcal pneumonia in adults, have greatly decreased since the implementation of PCV13 [13]. According to the most recent data by the National Laboratory Surveillance of Invasive Streptococcal Disease (2017), among all IPD cases, the prevalence of disease caused by serotypes 6B, 9V, 14 or 23F was less than 1%, 2.5% for 19F and 4.8% for 19A, while serotype 3 accounted for 9.7% [35]. We observed a decrease in the prevalence of PCV13 serotypes in the etiology of adult IPD in northwestern Ontario indicating an indirect effect of pediatric immunization in a region with a large population of Indigenous people [21].
Historically, North American Indigenous people experienced the highest IPD incidence rates in the world [36]. Pre-PCV-era population-based studies conducted in the White Mountain Apache reported the highest global incidence rates of IPD, i.e. 2396 cases per 100,000 population of children between 1 and 2 years of age [37]. In Alaska, annual incidence of IPD among Indigenous children < 2 years of age was 624 per 100,000, with meningitis or pneumonia rates being 8–10 times higher than elsewhere in the U.S. [38]. Although the rates of IPD greatly decreased in Indigenous children following the introduction of PCV, they remained over 5-fold higher in White Mountain Apache children compared to the same age group in the general U.S. population [39]. Epidemiological studies identified several modifiable risk factors for IPD in Indigenous adults, including renal failure, alcoholism, and unemployment that could potentially explain a large difference in incidence rates between the Indigenous and the general U.S. population [40]. While the introduction of PCV led to elimination of IPD due to vaccine serotypes, incidence rates of disease caused by non-vaccine serotypes increased in Alaska [41]. That study identified the lack of in-home piped water as an essential factor associated with high incidence rates of IPD in Alaska. Similarly, increased incidence of IPD in Indigenous people as compared to the general population has been reported from Canada including our region of interest, northwestern Ontario [18–21]. Nevertheless, despite a decreasing prevalence of PCV13 serotypes in the etiology of IPD in northwestern Ontario, the proportion of PCV or non-vaccine serotypes did not differ between Indigenous and non-Indigenous adults with IPD [21].
What can be the reason/s for the differences in concentrations of naturally acquired pneumococcal antibodies between Indigenous and non-Indigenous study participants? Indeed, healthy adult Indigenous residents of both Ojibwe communities, one in northwestern, another in southern Ontario, had significantly higher concentrations of three serotype-specific IgG, one serotype-specific IgM and all seven serotype-specific IgA as compared to their non-Indigenous counterparts in northwestern Ontario. Of note, the two communities have considerable differences in geographical location, climate, environment, distance to large urban centers, and access to services. One in the north is remote and exposed to harsh weather during winters. The southern one has a short-distance access to a large city, more developed services on-site, and more favourable climate with milder winters and warm summers. Regardless of these differences, people in both Indigenous communities have stronger natural pneumococcal immunity as compared to non-Indigenous healthy adults living in a smaller town of Kenora (15,000 habitants), which is only 80 km away from the northwestern Indigenous community, or in a larger northwestern city of Thunder Bay (110,000 habitants).
One possibility could be higher carriage rates in Indigenous compared to non-Indigenous communities, as more frequent exposure to pneumococci would contribute to higher concentrations of natural pneumococcal antibodies. The nasopharyngeal colonization and repeated exposure to pneumococci are important for immune defenses in adults; these are immunizing events that result in the production of natural antibodies and maintain the memory B cell pool [42]. Even in the setting of high PCV immunization rates, substantial burden of pneumococcal colonization persisted in children and adults in North American Indigenous communities, with children being the major drivers of intra-household transmission [43,44]. According to our data, women had generally higher concentrations of natural IgG and IgM antibodies than men, with more striking differences found in Indigenous groups, that is in line with earlier observations in a different population [45]. Among 30–64 year old Finnish people, concentrations of naturally acquired pneumococcal IgG and IgM antibodies were higher in women than men, with significant differences identified for 4/6 serotype-specific IgG (3, 6B, 14, and 23F) and all serotype-specific IgM (3, 4, 6B, 9V, 14, and 23F) that could be related to larger exposure of women to children [45]. Indeed, studies conducted in various populations in pre- and post-PCV time demonstrated that pneumococcal carriage in adults is directly associated with exposure to young children [8,46].
It is plausible to suggest that acquisition of natural pneumococcal antibodies by adults may be caused by persistent carriage of S. pneumoniae in children within Indigenous communities. Although the latter may potentially be related to insufficient pediatric immunization coverage in the region, no data to support or rule out this idea are available. No publicly accessible data are available on immunization coverage in specific communities of Ontario, while the overall PCV13 vaccination coverage of children by two years of age in Canada is 81.4% [47]. Based on official data by Public Health Ontario, the average rates of immunization of school pupils in Ontario with pneumococcal conjugate vaccine during the time of our study were the following: 2015–2016: 79.0%, 2016–2017: 79.7%, 2017–2018: 74.1%; however, immunization coverage reports in this province do not include any information of the race or ethnicity [48]. For comparison, in the remote Nordic region of Nunavik (province of Québec), with 90% of the population being Indigenous, the uptake rates of PCV7 in 2005 were similar to those in the whole province of Québec, and higher than the average Canadian data [49]. However, despite high immunization rates, pneumococcal disease burden in Nunavik Indigenous children remained higher that elsewhere in Canada [50]. Overall, Indigenous people in Canada continue to experience poorer health and higher mortality rates compared to the general population, attributed to the consequences of colonialism and unfavourable social determinants of health such as poverty, overcrowded housing, and inadequate access to clean water [51]. In addition to high rates of IPD, significant burden of other invasive bacterial diseases, including H. influenzae, group A and B streptococcal infections, and meningococcal disease has been reported in Indigenous communities [52].
However, persistent carriage of pneumococci in children is not exclusive in Indigenous people and may depend on specific characteristics of individual serotypes and their interactions with other bacteria sharing the nasopharyngeal microbiome. Different immunogenicity of pneumococcal serotypes can influence their abilities to colonize the nasopharynx; notably, capsular polysaccharides of serotypes 6B, 14, and 19F are poorly immunogenic [53,54]. Salt et al. (2007) suggested that the low immunogenicity of serotype 6B explained its propensity to frequently colonize the nasopharynx of young children [55]. In addition to dissimilar immunogenic properties, pneumococcal serotypes have different virulence; some are more commonly associated with invasive disease rather than carriage [29]. One example is serotype 3, which has a thick polysaccharide capsule and is highly resistant to phagocytosis resulting in the most severe forms of pneumococcal infection [29]. In our study, both Indigenous and non-indigenous individuals had the lowest concentrations of antibody against serotype 3 among all the studied serotype-specific IgG, suggesting poor natural immunity against this serotype. Notably, current pediatric PCV13 immunization decreased the prevalence of all vaccine serotypes in etiology of pneumococcal infections, with exception of IPD caused by serotype 3 [10]. Although the serological correlates of protection against adult IPD are unknown, studies in children indicated higher immunological threshold for serotype 3 than for other PCV13 serotypes [28,56].
Natural antibodies to pneumococcal capsular polysaccharides can be induced by the exposure to cross-reactive microbial or environmental antigens. In particular, common oral non-pathogenic streptococci, i.e. S. mitis, S. orali, and S. infantis are able to carry capsular polysaccharides cross-reactive to S. pneumoniae and may then contribute to acquisition of natural pneumococcal antibodies [57]. As prevalence, distribution, and exposure to cross-reactive antigens depends on the environment, lifestyle, and hygienic practices, their role in the development of natural immunity may be more prominent in Indigenous than non-Indigenous communities. Earlier studies demonstrated serological cross-reactivity between capsular polysaccharides of pneumococcal serotype 6B and Haemophilus influenzae serotype a (Hia) [58]. Considering that Hia is a pathogen which is more prevalent among North American Indigenous people as compared to the general population [59], and invasive Hia disease has consistently been present in northwestern Ontario over the last 2 decades [60,61], cross-reactive antibody induced by exposure to Hia may contribute to the development of natural antibody specific to serotype 6B among Indigenous adults. Indigenous people living in rural communities may also be frequently exposed to cross-reactive antigens of Klebsiella pneumoniae, Pseudomonas aeruginosa, or certain fungi common in the natural environment [62], as well as some derived from plant polysaccharides traditionally consumed by Indigenous people for nutrition or medicine, such as fruit pectin or Althaea officinalis [63,64].
Published data on natural pneumococcal antibody in North American Indigenous adults are limited by 2 studies of several serotype-specific IgG conducted in a different population, elderly Alaska Native people, precluding direct comparison to our data [65,66]. It is even more difficult to compare our data to published seroprevalence data collected in other adult populations (U.S., UK, Finland, The Netherlands, Australia) that used different assay methods and reported a wide range of antibody concentrations. Nevertheless, the concentrations of specific IgG in our sample are similar to the previously reported data (S7 Table). Published studies mostly addressed IgG antibodies with only data available on concentrations of naturally acquired serotype-specific IgM in a Finnish population [45]. Although IgG is a dominant immunoglobulin, which plays essential role in opsonisation of pneumococci, IgM is characterized by superior abilities to activate complement, and IgA can trigger phagocytosis of pneumococci by neutrophils [67,68]. Despite the differences in populations, our data show similarities with data by Simell et al. 2008, i.e. higher concentrations of several serotype-specific IgM in women than men, and a decrease in concentrations of natural IgM antibody with age [45]. However, we observed significant age-dependent decrease in IgM specific to serotypes 9V, 19A, 19F, and 23F in non-Indigenous, rather than Indigenous study participants. These observations imply that older Indigenous people may experience continuous exposure to cross-reactive antigens over their life span. To the best of our knowledge, no previous studies of natural pneumococcal IgA antibodies have been conducted. Although concentrations of serum IgA antibodies were inferior to IgG or IgM, the former were significantly higher in Indigenous than non-Indigenous adults that may potentially reflect differences in antigenic stimulation through the mucosal surfaces. While the role of secretory IgA in mucosal defences against bacterial infections has been well established, the function of serum IgA is less known. Earlier studies have demonstrated that capsular polysaccharide-specific serum IgA antibodies are able to opsonize pneumococci for phagocytosis by neutrophils via the process involving the alternative complement pathway and interaction with IgA Fc receptor (FcαRI) [68,69]. Recent data indicated that IgA was more efficient, than IgG, in the formation of neutrophil extracellular traps, suggesting yet another mechanism of anti-bacterial action of serum IgA [70,71]. Considering that serum concentrations of natural IgA antibodies are low, a question to what degree serum IgA may contribute to natural pneumococcal immunity deserves further study.
Considering higher burden of IPD in Indigenous than non-Indigenous people, our findings of higher concentrations of several serotype-specific antibodies among Indigenous adults may sound paradoxical. For interpretation of our data it is important to bear in mind that we assessed natural immunity in generally healthy adults. However, high burden of chronic diseases and immunocompromising conditions, along with unfavourable socioeconomic conditions, are important factors predisposing Indigenous people to invasive bacterial disease [40,72]. As Indigenous Canadians have high burden of obesity, diabetes, circulatory diseases, chronic kidney disease, and cancer, this suggests that an increased IPD rate in Indigenous adults is due to the increased prevalence of risk factors rather than lack of anti-capsular antibodies [73,74]. Indeed, according to our recent data, among northwestern Ontario adults with IPD, prevalence of immunocompromising conditions was much higher in Indigenous than in non-Indigenous patients (66% vs. 29.5%, p < 0.001) [21]. However, for interpretation of serological data, it is important to consider that anti-capsular antibodies detected by ELISA may have different functional abilities because of dissimilar avidity [75]. On the other hand, protection induced by the natural exposure to S. pneumoniae may depend on antibodies to non-capsular antigens, such as antibody to the pneumococcal cell wall polysaccharide or pneumococcal proteins [76,77].
Although serological correlates of protection against pneumococcal disease in adults have not been established, certain concentrations of specific IgG have been suggested for the interpretation of antibody responses to pneumococcal immunization. In the literature, the concentration of 1.3 μg/mL has been used as a cut-off for protection, and as an indicator of the adequate response to pneumococcal immunization in adults [78]; the concentration of 1 μg/mL was suggested as a threshold of protection against pneumococcal pneumonia in the elderly [79], although the protective threshold is serotype-dependent and may be different for naturally acquired as compared to post-vaccination antibodies. In our study, Indigenous individuals more frequently than non-Indigenous ones, had serotype-specific IgG above these thresholds, with statistically significant differences found for 6B, 9V, and 14, reflecting the differences in geometric mean antibody concentrations between the groups.
Limitations and future directions
We analyzed natural antibodies specific to 7 select pneumococcal serotypes chosen for their diverse biological characteristics, significance in epidemiology, and inclusion in currently used vaccines. However, we did not study natural antibody specific to non-vaccine serotypes that would be important considering the dynamic changes in epidemiology of pneumococcal infections and emergence of new serotypes in the post-conjugate vaccine era. In this study, we have reported only concentrations of serotype-specific IgG, IgA, and IgM antibodies. Although recognizing that analysis of the functional antibody abilities could provide better understanding of the protective capacities of natural antibodies, conducting the opsonophagocytic assays in our lab was not feasible.
There is always a potential for inaccurate information in any study when demographic data are collected by self-declaration. However, we made efforts to avoid recruiting participants with co-morbidities that could potentially impact their immunity, via asking them to complete a detailed questionnaire on their health status, with the list of common chronic conditions, co-morbidities, and history of taking corticosteroids or other immunosuppressive medications. As adult pneumococcal vaccination in Canada is not very common, especially among Indigenous populations, it would be very unlikely that they provided an incorrect record of pneumococcal vaccination.
A sample size was insufficient to complete analysis by age-stratified groups; non-Indigenous study participants from southern Ontario could not been recruited because of logistical reasons. Immunological correlates of protection against pneumococcal disease in adults have not been determined that presented challenges for clinical interpretation of collected data. The lack of data on pneumococcal carriage in the communities of interest precluded us from making direct conclusions on the effect of different carriage rates on the amounts of natural antibody in respective population groups. Collecting accurate data on carriage is problematic mainly due to its transitory manner that requires longitudinal studies and as it was previously recognized, “even with monthly collection of swabs, episodes of carriage are likely to be missed” [42]. For interpretation of our findings, we considered previously published data on pneumococcal carriage in different populations of various ages, while recognizing an inherent deficiency of such an approach. Finally, we recognize that Canadian Indigenous peoples represent a highly diverse group with different history, genetic backgrounds, cultural traditions, etc. While we made attempt to assess two different populations living in geographically diverse environments, it is impossible to generalize our findings towards other Indigenous groups.
Conclusions
Comparison between healthy Indigenous and non-Indigenous adults suggests that increased rates of IPD and other forms of pneumococcal infections among Indigenous people are unlikely related to deficiency of naturally acquired antibodies, at least those specific to 7 common S. pneumoniae serotypes. Adverse social determinants of health, high burden of non-communicable chronic diseases, and increased prevalence of immunocompromising conditions most likely predispose Indigenous people to pneumococcal infections. One plausible solution to this problem would be the improvement of immunization coverage of adult groups at risk. Determining serological correlates of protection for adults will be essential to identify the groups in need of pneumococcal immunizations that may prevent an excessive burden of the disease among North American Indigenous people.
Supporting information
Fisher’s exact test compared proportions of Indigenous and non-Indigenous participants with IgG concentrations > 1.3 μg/ mL and > 1.0 μg/ mL for each serotype.
(DOCX)
The goodness of the fit is reported by the r2 value; the p value determines if the slope is significantly non-zero.
(DOCX)
The goodness of the fit is reported by the r2 value; the p value determines if the slope is significantly non-zero.
(DOCX)
(DOCX)
Mann-Whitney U tests compared ages of males and females of the same ethnicity, there were no significant differences between groups. a One participant with an unknown age.
(DOCX)
Concentrations of antibodies were compared between males and females of the same ethnicity using a Mann-Whitney U test.
(DOCX)
(DOCX)
Indigenous adults southern Ontario (Group 1), Indigenous adults northwestern Ontario (Group 2), non-Indigenous adults Thunder Bay (Group 3), and non-Indigenous adults Kenora (Group 4). Participant group, ID number, age and ethnicity are displayed.
(DOCX)
Indigenous adults southern Ontario (Group 1), Indigenous adults northwestern Ontario (Group 2), non-Indigenous adults Thunder Bay (Group 3), and non-Indigenous adults Kenora (Group 4). All data displayed are the original values. For our statistical analyses, the lower limit of detection was determined for each serotype according to the WHO pneumococcal ELISA protocol. The lower limits of detection are serotype 3 (0.100 μg/ mL), serotype 6B (0.167 μg/ mL), 9V (0.175 μg/ mL), 14 (0.339 μg/ mL), 19A (0.144 μg/ mL), 19F (0.166 μg/ mL) and 23F (0.144 μg/ mL). All values below the lower limits of detection were reported as half the value for statistical purposes.
(DOCX)
Indigenous adults southern Ontario (Group 1), Indigenous adults northwestern Ontario (Group 2), non-Indigenous adults Thunder Bay (Group 3), and non-Indigenous adults Kenora (Group 4). All data displayed are the original values. For our statistical analyses, the lower limit of detection was determined for each serotype according to the WHO pneumococcal ELISA protocol. The lower limits of detection are serotype 3 (0.035 μg/ mL), serotype 6B (0.088 μg/ mL), 9V (0.070 μg/ mL), 14 (0.124 μg/ mL), 19A (0.078 μg/ mL), 19F (0.130 μg/ mL) and 23F (0.031 μg/ mL). All values below the lower limits of detection were reported as half the value for statistical purposes.
(DOCX)
Indigenous adults southern Ontario (Group 1), Indigenous adults northwestern Ontario (Group 2), non-Indigenous adults Thunder Bay (Group 3), and non-Indigenous adults Kenora (Group 4). All data displayed are the original values. For our statistical analyses, the lower limit of detection was determined for each serotype according to the WHO pneumococcal ELISA protocol. The lower limits of detection are serotype 3 (0.052 μg/ mL), serotype 6B (0.025 μg/ mL), 9V (0.036 μg/ mL), 14 (0.053 μg/ mL), 19A (0.025 μg/ mL), 19F (0.022 μg/ mL) and 23F (0.029 μg/ mL). All values below the lower limits of detection were reported as half the value for statistical purposes.
(DOCX)
(DOCX)
Acknowledgments
We would like to thank all the participants who donated their serum for the study. We are indebted to our collaborators, especially the health care providers at Indigenous Health Access Centers and First Nations community members who kindly helped us with collecting samples, with our special thanks to Annette Schroeter, Connee Badiuk, Anita Cameron, Cynthia Price, Wayne Hyacinthe, Karen Fobister, Rekha Netto, Sherisse McLaughlin, Trudy Jacobs, and Lori Sinclair. We also thank Hanan Alsarmi for collecting the healthy Thunder Bay participant samples as well as Twyla Biluk (Thunder Bay 55 Plus Centre) for assistance with recruitment of healthy Thunder Bay participants. We thank Angele Desbiens-Forget, Brenda Huska (Northern Ontario School of Medicine) and Amanda Bakke for technical help.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work received support from: Mitacs Accelerate with contribution by Bruce Power; IT05441; www.mitacs.ca/en/programs/accelerate to MU, DB; Northern Ontario Academic Medicine Association (AHSC AFP Innovation Fund); www.noama.ca; A-12-06 to WM, MU; Pfizer (Investigator Initiated Research Project, Grant 53232197) to M.U. https://www.cybergrants.com/pfizer/Research; and NOSM Summer Medical Student Research Awards to EBN and JT, https://www.nosm.ca/research/student-research-at-nosm-2/deans-summer-medical-student-research-awards/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6(4):288–301. doi: 10.1038/nrmicro1871 . [DOI] [PubMed] [Google Scholar]
- 2.Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3(7). Epub 2013/07/03. doi: 10.1101/cshperspect.a010215 ; PubMed Central PMCID: PMC3685878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20 Suppl 5:45–51. doi: 10.1111/1469-0691.12461 . [DOI] [PubMed] [Google Scholar]
- 4.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O’Brien KL, et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–55. doi: 10.1586/erv.12.53 . [DOI] [PubMed] [Google Scholar]
- 5.Jauneikaite E, Tocheva AS, Jefferies JM, Gladstone RA, Faust SN, Christodoulides M, et al. Current methods for capsular typing of Streptococcus pneumoniae. J Microbiol Methods. 2015;113:41–9. Epub 2015/03/31. doi: 10.1016/j.mimet.2015.03.006 . [DOI] [PubMed] [Google Scholar]
- 6.Paton JC, Trappetti C. Streptococcus pneumoniae Capsular Polysaccharide. Microbiol Spectr. 2019;7(2). Epub 2019/04/13. doi: 10.1128/microbiolspec.GPP3-0019-2018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayhty H, Auranen K, Nohynek H, Dagan R, Makela H. Nasopharyngeal colonization: a target for pneumococcal vaccination. Expert Rev Vaccines. 2006;5(5):651–67. Epub 2006/12/22. doi: 10.1586/14760584.5.5.651 . [DOI] [PubMed] [Google Scholar]
- 8.Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, Reasonover A, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006;193(11):1487–94. Epub 2006/05/03. doi: 10.1086/503805 . [DOI] [PubMed] [Google Scholar]
- 9.Millar EV, Watt JP, Bronsdon MA, Dallas J, Reid R, Santosham M, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47(8):989–96. Epub 2008/09/11. doi: 10.1086/591966 . [DOI] [PubMed] [Google Scholar]
- 10.LeBlanc JJ, ElSherif M, Ye L, MacKinnon-Cameron D, Ambrose A, Hatchette TF, et al. Streptococcus pneumoniae serotype 3 is masking PCV13-mediated herd immunity in Canadian adults hospitalized with community acquired pneumonia: A study from the Serious Outcomes Surveillance (SOS) Network of the Canadian immunization research Network (CIRN). Vaccine. 2019;37(36):5466–73. Epub 2019/07/28. doi: 10.1016/j.vaccine.2019.05.003 . [DOI] [PubMed] [Google Scholar]
- 11.Government of Canada PHC. Page 16: Canadian Immunization Guide: Part 4- Active Vaccines 2016. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-16-pneumococcal-vaccine.html. [Google Scholar]
- 12.Government of Canada PHAoC. Vaccine uptake in Canadian adults: results from the 2014 adult National Immunization Coverage Survey 2014. [cited 2019]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-uptake-canadian-adults-results-2014-adult-national-immunization-coverage-survey.html. [Google Scholar]
- 13.Wijayasri S, Hillier K, Lim GH, Harris TM, Wilson SE, Deeks SL. The shifting epidemiology and serotype distribution of invasive pneumococcal disease in Ontario, Canada, 2007–2017. PLoS One. 2019;14(12):e0226353. Epub 2019/12/14. doi: 10.1371/journal.pone.0226353 ; PubMed Central PMCID: PMC6910703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Census Profile, 2016 Census, Northwest Ontario and Ontario [Internet]. 2016. [cited January 21, 2019]. Available from: http://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=ER&Code1=3595&Geo2=PR&Code2=35&Data=Count&SearchText=Northwest&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=3595&TABID=1. [Google Scholar]
- 15.Government of Canada PHAoC. Aboriginal Peoples Highlight Tables, 2016 Census 2016. [cited 2021]. Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/abo-aut/Table.cfm?Lang=Eng&T=101&S=99&O=A. [Google Scholar]
- 16.Said MA, O’Brien KL, Nuorti JP, Singleton R, Whitney CG, Hennessy TW. The epidemiologic evidence underlying recommendations for use of pneumococcal polysaccharide vaccine among American Indian and Alaska Native populations. Vaccine. 2011;29(33):5355–62. Epub 2011/06/15. doi: 10.1016/j.vaccine.2011.05.086 . [DOI] [PubMed] [Google Scholar]
- 17.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–92. Epub 2007/04/26. doi: 10.1001/jama.297.16.1784 . [DOI] [PubMed] [Google Scholar]
- 18.Vanderkooi OG, Kellner JD. The challenge of reducing invasive pneumococcal disease in indigenous Indian populations. Clin Infect Dis. 2010;50(9):1247–8. Epub 2010/04/07. doi: 10.1086/651681 . [DOI] [PubMed] [Google Scholar]
- 19.Helferty M, Rotondo JL, Martin I, Desai S. The epidemiology of invasive pneumococcal disease in the Canadian North from 1999 to 2010. Int J Circumpolar Health. 2013;72. Epub 2013/08/24. doi: 10.3402/ijch.v72i0.21606 ; PubMed Central PMCID: PMC3749849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eton V, Schroeter A, Kelly L, Kirlew M, Tsang RSW, Ulanova M. Epidemiology of invasive pneumococcal and Haemophilus influenzae diseases in Northwestern Ontario, Canada, 2010–2015. Int J Infect Dis. 2017;65:27–33. doi: 10.1016/j.ijid.2017.09.016 . [DOI] [PubMed] [Google Scholar]
- 21.Dalcin D, Sieswerda L, Dubois S, Ulanova M. Epidemiology of invasive pneumococcal disease in indigenous and non-indigenous adults in northwestern Ontario, Canada, 2006–2015. BMC Infect Dis. 2018;18(1):621. Epub 2018/12/06. doi: 10.1186/s12879-018-3531-9 ; PubMed Central PMCID: PMC6280531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulanova M, Huska B, Desbiens A, Gaultier GN, Domonkos V, McCready WG. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in 23-valent pneumococcal polysaccharide vaccine-naive and previously immunized adult patients with severe chronic kidney disease. Vaccine. 2021;39(4):699–710. Epub 2020/12/29. doi: 10.1016/j.vaccine.2020.12.035 . [DOI] [PubMed] [Google Scholar]
- 23.Ownership Schnarch B., control, access, and possession (OCAP) or self-determination applied to research: A critical analysis of contemporary First Nations research and some options for First Nations communities. International Journal of Indigenous Health 2004. 1(1):80–95. [Google Scholar]
- 24.Canadian Institutes of Health Research NSaERCoC, and Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. December 2014. [Google Scholar]
- 25.Nix EB, Choi J, Anthes C, Gaultier GN, Thorgrimson J, Cox AD, et al. Characterization of natural bactericidal antibody against Haemophilus influenzae type a in Canadian First Nations: A Canadian Immunization Research Network (CIRN) Clinical Trials Network (CTN) study. PLoS One. 2018;13(8):e0201282. doi: 10.1371/journal.pone.0201282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laboratories WHOPSR. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn Ps ELISA). Available from: https://www.vaccine.uab.edu/uploads/mdocs/ELISAProtocol(007sp).pdf. [Google Scholar]
- 27.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, et al. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev. 2015;28(3):871–99. Epub 2015/06/19. doi: 10.1128/CMR.00024-15 ; PubMed Central PMCID: PMC4475641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voysey M, Fanshawe TR, Kelly DF, O’Brien KL, Kandasamy R, Shrestha S, et al. Serotype-Specific Correlates of Protection for Pneumococcal Carriage: An Analysis of Immunity in 19 Countries. Clin Infect Dis. 2018;66(6):913–20. Epub 2017/10/27. doi: 10.1093/cid/cix895 . [DOI] [PubMed] [Google Scholar]
- 29.Weinberger DM, Trzcinski K, Lu YJ, Bogaert D, Brandes A, Galagan J, et al. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009;5(6):e1000476. Epub 2009/06/13. doi: 10.1371/journal.ppat.1000476 ; PubMed Central PMCID: PMC2689349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crumrine MH, Fischer GW, Balk MW. Immunochemical cross-reactions between type III group B Streptococcus and type 14 Streptococcus pneumoniae. Infect Immun. 1979;25(3):960–3. Epub 1979/09/01. doi: 10.1128/iai.25.3.960-963.1979 PubMed Central PMCID: PMC414541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CJ, Wang Z. Induction of increased antibody responses to pneumococcal type 19F polysaccharide by Klebsiella polysaccharide. J Infect Dis. 1985;151(4):658–64. Epub 1985/04/01. doi: 10.1093/infdis/151.4.658 [DOI] [PubMed] [Google Scholar]
- 32.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73. Epub 2011/04/16. doi: 10.1016/S0140-6736(10)62225-8 ; PubMed Central PMCID: PMC3256741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18 Suppl A:35–45. Epub 1986/07/01. doi: 10.1093/jac/18.supplement_a.35 . [DOI] [PubMed] [Google Scholar]
- 34.Scott JA, Hall AJ, Dagan R, Dixon JM, Eykyn SJ, Fenoll A, et al. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996;22(6):973–81. Epub 1996/06/01. doi: 10.1093/clinids/22.6.973 . [DOI] [PubMed] [Google Scholar]
- 35.National Laboratory Surveillance of Invasive Streptococcal Disease in Canada—Annual Summary 2017: Government of Canada, Public Health Agency of Canada; 2019. Available from: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-laboratory-surveillance-invasive-streptococcal-disease-annual-summary-2017.html. [Google Scholar]
- 36.Watt JP, O’Brien KL, Benin AL, Whitney CG, Robinson K, Parkinson AJ, et al. Invasive pneumococcal disease among Navajo adults, 1989–1998. Clin Infect Dis. 2004;38(4):496–501. Epub 2004/02/07. doi: 10.1086/381198 . [DOI] [PubMed] [Google Scholar]
- 37.Cortese MM, Wolff M, Almeido-Hill J, Reid R, Ketcham J, Santosham M. High incidence rates of invasive pneumococcal disease in the White Mountain Apache population. Arch Intern Med. 1992;152(11):2277–82. Epub 1992/11/01. . [PubMed] [Google Scholar]
- 38.Davidson M, Parkinson AJ, Bulkow LR, Fitzgerald MA, Peters HV, Parks DJ. The epidemiology of invasive pneumococcal disease in Alaska, 1986-1990—ethnic differences and opportunities for prevention. J Infect Dis. 1994;170(2):368–76. Epub 1994/08/01. doi: 10.1093/infdis/170.2.368 . [DOI] [PubMed] [Google Scholar]
- 39.Lacapa R, Bliss SJ, Larzelere-Hinton F, Eagle KJ, McGinty DJ, Parkinson AJ, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008;47(4):476–84. Epub 2008/07/17. doi: 10.1086/590001 . [DOI] [PubMed] [Google Scholar]
- 40.Watt JP, O’Brien KL, Benin AL, McCoy SI, Donaldson CM, Reid R, et al. Risk factors for invasive pneumococcal disease among Navajo adults. Am J Epidemiol. 2007;166(9):1080–7. Epub 2007/08/19. doi: 10.1093/aje/kwm178 . [DOI] [PubMed] [Google Scholar]
- 41.Wenger JD, Zulz T, Bruden D, Singleton R, Bruce MG, Bulkow L, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J. 2010;29(3):251–6. Epub 2009/12/03. doi: 10.1097/INF.0b013e3181bdbed5 . [DOI] [PubMed] [Google Scholar]
- 42.Goldblatt D, Hussain M, Andrews N, Ashton L, Virta C, Melegaro A, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192(3):387–93. Epub 2005/07/05. doi: 10.1086/431524 . [DOI] [PubMed] [Google Scholar]
- 43.Mosser JF, Grant LR, Millar EV, Weatherholtz RC, Jackson DM, Beall B, et al. Nasopharyngeal carriage and transmission of Streptococcus pneumoniae in American Indian households after a decade of pneumococcal conjugate vaccine use. PLoS One. 2014;9(1):e79578. Epub 2014/01/28. doi: 10.1371/journal.pone.0079578 ; PubMed Central PMCID: PMC3894936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott JR, Millar EV, Lipsitch M, Moulton LH, Weatherholtz R, Perilla MJ, et al. Impact of more than a decade of pneumococcal conjugate vaccine use on carriage and invasive potential in Native American communities. J Infect Dis. 2012;205(2):280–8. doi: 10.1093/infdis/jir730 ; PubMed Central PMCID: PMC3244367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simell B, Lahdenkari M, Reunanen A, Kayhty H, Vakevainen M. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin Vaccine Immunol. 2008;15(9):1391–7. Epub 2008/07/04. doi: 10.1128/CVI.00110-08 ; PubMed Central PMCID: PMC2546667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miellet WR, van Veldhuizen J, Nicolaie MA, Mariman R, Bootsma HJ, Bosch T, et al. Influenza-like Illness Exacerbates Pneumococcal Carriage in Older Adults. Clin Infect Dis. 2020. Epub 2020/10/31. doi: 10.1093/cid/ciaa1551 . [DOI] [PubMed] [Google Scholar]
- 47.Canada TPHAo. Estimated national vaccination coverage of routine childhood vaccines by two years of age, by sex-childhood National Immunization Coverage Survey, Canada, 2017. Two-year-old vaccination coverage, % (95% CI) 2020. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/2017-vaccine-uptake-canadian-children-survey.html. [Google Scholar]
- 48.Ontario PH. Immunization Coverage Report for School Pupils in Ontario 2015–2018 [cited 2022]. Available from: https://www.publichealthontario.ca/en/health-topics/immunization/vaccine-coverage. [Google Scholar]
- 49.Cleophat JE, Le Meur JB, Proulx JF, De Wals P. Uptake of pneumococcal vaccines in the Nordic region of Nunavik, province of Quebec, Canada. Can J Public Health. 2014;105(4):e268–72. Epub 2014/08/29. doi: 10.17269/cjph.105.4315 ; PubMed Central PMCID: PMC6972132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Wals P, Zhou Z, LeMeur JB, Proulx JF. Burden of respiratory infections and otitis media in the Inuit population of Nunavik, Quebec, Canada. Int J Circumpolar Health. 2020;79(1):1799688. Epub 2020/07/31. doi: 10.1080/22423982.2020.1799688 ; PubMed Central PMCID: PMC7480588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitrou F, Cooke M, Lawrence D, Povah D, Mobilia E, Guimond E, et al. Gaps in Indigenous disadvantage not closing: a census cohort study of social determinants of health in Australia, Canada, and New Zealand from 1981–2006. BMC Public Health. 2014;14:201. Epub 2014/02/27. doi: 10.1186/1471-2458-14-201 ; PubMed Central PMCID: PMC3937433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bocking N, Matsumoto CL, Loewen K, Teatero S, Marchand-Austin A, Gordon J, et al. High Incidence of Invasive Group A Streptococcal Infections in Remote Indigenous Communities in Northwestern Ontario, Canada. Open Forum Infect Dis. 2017;4(1):ofw243. Epub 2017/05/10. doi: 10.1093/ofid/ofw243 ; PubMed Central PMCID: PMC5414009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez ME, van den Dobbelsteen GP, Oomen LA, de Weers O, van Buren L, Beurret M, et al. Immunogenicity of Streptococcus pneumoniae type 6B and 14 polysaccharide-tetanus toxoid conjugates and the effect of uncoupled polysaccharide on the antigen-specific immune response. Vaccine. 1998;16(20):1941–9. Epub 1998/10/31. doi: 10.1016/s0264-410x(98)00129-7 . [DOI] [PubMed] [Google Scholar]
- 54.van Dam JE, Fleer A, Snippe H. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Van Leeuwenhoek. 1990;58(1):1–47. Epub 1990/06/01. doi: 10.1007/BF02388078 . [DOI] [PubMed] [Google Scholar]
- 55.Salt P, Banner C, Oh S, Yu LM, Lewis S, Pan D, et al. Social mixing with other children during infancy enhances antibody response to a pneumococcal conjugate vaccine in early childhood. Clin Vaccine Immunol. 2007;14(5):593–9. Epub 2007/03/09. doi: 10.1128/CVI.00344-06 ; PubMed Central PMCID: PMC1865629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46. Epub 2014/07/22. doi: 10.1016/S1473-3099(14)70822-9 . [DOI] [PubMed] [Google Scholar]
- 57.Gertz RE Jr., Pimenta FC, Chochua S, Larson S, Venero AK, Bigogo G, et al. Nonpneumococcal Strains Recently Recovered from Carriage Specimens and Expressing Capsular Serotypes Highly Related or Identical to Pneumococcal Serotypes 2, 4, 9A, 13, and 23A. mBio. 2021;12(3). Epub 2021/05/20. doi: 10.1128/mBio.01037-21 ; PubMed Central PMCID: PMC8262907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagergard T, Branefors P. Nature of cross-reactivity between Haemophilus influenzae types a and b and Streptococcus pneumoniae types 6A and 6B. Acta Pathol Microbiol Immunol Scand C. 1983;91(6):371–6. Epub 1983/12/01. [PubMed] [Google Scholar]
- 59.Soeters HM, Oliver SE, Plumb ID, Blain AE, Zulz T, Simons BC, et al. Epidemiology of Invasive Haemophilus influenzae Serotype a Disease-United States, 2008–2017. Clin Infect Dis. 2021;73(2):e371–e9. Epub 2020/06/27. doi: 10.1093/cid/ciaa875 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown VM, Madden S, Kelly L, Jamieson FB, Tsang RS, Ulanova M. Invasive Haemophilus influenzae disease caused by non-type b strains in Northwestern Ontario, Canada, 2002–2008. Clin Infect Dis. 2009;49(8):1240–3. doi: 10.1086/605671 . [DOI] [PubMed] [Google Scholar]
- 61.Cerqueira A, Byce S, Tsang RSW, Jamieson FB, Kus JV, Ulanova M. Continuing surveillance of invasive Haemophilus influenzae disease in northwestern Ontario emphasizes the importance of serotype a and non-typeable strains as causes of serious disease: a Canadian Immunization Research Network (CIRN) Study. Can J Microbiol. 2019;65(11):805–13. Epub 2019/06/27. doi: 10.1139/cjm-2019-0210 . [DOI] [PubMed] [Google Scholar]
- 62.Heidelberger M, Bernheimer AW. Cross-reactions of polysaccharides of fungi, molds, and yeasts in anti-pneumococcal and other antisera. Proc Natl Acad Sci U S A. 1984;81(16):5247–9. Epub 1984/08/01. doi: 10.1073/pnas.81.16.5247 ; PubMed Central PMCID: PMC391675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heidelberger M. Cross-reactions of plant polysaccharides in antipneumococcal and other antisera, an update. Fortschr Chem Org Naturst. 1982;42:287–96. Epub 1982/01/01. doi: 10.1007/978-3-7091-8677-0_2 . [DOI] [PubMed] [Google Scholar]
- 64.Nahm MH, Yu J, Vlach J, Bar-Peled M. A Common Food Glycan, Pectin, Shares an Antigen with Streptococcus pneumoniae Capsule. mSphere. 2020;5(2). Epub 2020/04/10. doi: 10.1128/mSphere.00074-20 ; PubMed Central PMCID: PMC7142292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55–74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine. 2011;29(12):2287–95. Epub 2011/01/25. doi: 10.1016/j.vaccine.2011.01.029 . [DOI] [PubMed] [Google Scholar]
- 66.Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, et al. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in alaska native adults 55–70 years of age. Clin Infect Dis. 2009;49(2):241–8. Epub 2009/06/16. doi: 10.1086/599824 . [DOI] [PubMed] [Google Scholar]
- 67.Simell B, Nurkka A, Ekstrom N, Givon-Lavi N, Kayhty H, Dagan R. Serum IgM antibodies contribute to high levels of opsonophagocytic activities in toddlers immunized with a single dose of the 9-valent pneumococcal conjugate vaccine. Clin Vaccine Immunol. 2012;19(10):1618–23. Epub 2012/08/10. doi: 10.1128/CVI.00248-12 ; PubMed Central PMCID: PMC3485875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Pol W, Vidarsson G, Vile HA, van de Winkel JG, Rodriguez ME. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via FcalphaRI (CD89). J Infect Dis. 2000;182(4):1139–45. Epub 2000/09/09. doi: 10.1086/315825 . [DOI] [PubMed] [Google Scholar]
- 69.Janoff EN, Fasching C, Orenstein JM, Rubins JB, Opstad NL, Dalmasso AP. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Invest. 1999;104(8):1139–47. Epub 1999/10/19. doi: 10.1172/JCI6310 ; PubMed Central PMCID: PMC408571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Storisteanu DM, Pocock JM, Cowburn AS, Juss JK, Nadesalingam A, Nizet V, et al. Evasion of Neutrophil Extracellular Traps by Respiratory Pathogens. Am J Respir Cell Mol Biol. 2017;56(4):423–31. Epub 2016/11/18. doi: 10.1165/rcmb.2016-0193PS ; PubMed Central PMCID: PMC5449512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gimpel AK, Maccataio A, Unterweger H, Sokolova MV, Schett G, Steffen U. IgA Complexes Induce Neutrophil Extracellular Trap Formation More Potently Than IgG Complexes. Front Immunol. 2021;12:761816. Epub 2022/02/01. doi: 10.3389/fimmu.2021.761816 ; PubMed Central PMCID: PMC8792984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewnard JA, Hanage WP. Making sense of differences in pneumococcal serotype replacement. Lancet Infect Dis. 2019;19(6):e213–e20. Epub 2019/02/03. doi: 10.1016/S1473-3099(18)30660-1 . [DOI] [PubMed] [Google Scholar]
- 73.King M. Chronic diseases and mortality in Canadian Aboriginal peoples: learning from the knowledge. Chronic Dis Can. 2010;31(1):2–3. Epub 2010/12/24. . [PubMed] [Google Scholar]
- 74.Kelly L, Matsumoto CL, Schreiber Y, Gordon J, Willms H, Olivier C, et al. Prevalence of chronic kidney disease and cardiovascular comorbidities in adults in First Nations communities in northwest Ontario: a retrospective observational study. CMAJ Open. 2019;7(3):E568–E72. Epub 2019/09/11. doi: 10.9778/cmajo.20190040 ; PubMed Central PMCID: PMC6768774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29(2):281–8. Epub 1999/09/07. doi: 10.1086/520200 . [DOI] [PubMed] [Google Scholar]
- 76.Musher DM, Johnson B Jr., Watson DA. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect Immun. 1990;58(12):3871–6. Epub 1990/12/01. doi: 10.1128/iai.58.12.3871-3876.1990 ; PubMed Central PMCID: PMC313748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferreira DM, Neill DR, Bangert M, Gritzfeld JF, Green N, Wright AK, et al. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med. 2013;187(8):855–64. Epub 2013/02/02. doi: 10.1164/rccm.201212-2277OC ; PubMed Central PMCID: PMC3707375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park MA, Jenkins SM, Smith CY, Pyle RC, Sacco KA, Ryu E, et al. Pneumococcal serotype-specific cut-offs based on antibody responses to pneumococcal polysaccharide vaccination in healthy adults. Vaccine. 2021;39(21):2850–6. Epub 2021/04/27. doi: 10.1016/j.vaccine.2021.04.015 . [DOI] [PubMed] [Google Scholar]
- 79.Ortqvist A, Henckaerts I, Hedlund J, Poolman J. Non-response to specific serotypes likely cause for failure to 23-valent pneumococcal polysaccharide vaccine in the elderly. Vaccine. 2007;25(13):2445–50. Epub 2006/10/21. doi: 10.1016/j.vaccine.2006.09.018 . [DOI] [PubMed] [Google Scholar]