Abstract
Introduction
Obesity in women is often associated with hyperandrogenism, but the role of adipose tissue (AT) in androgen synthesis remains unclear. Therefore, we studied whether AT could be a source of androgens promoting hyperandrogenism.
Methods
Subcutaneous and visceral (visc) AT was collected from lean and obese women. Androgen levels were evaluated in serum, AT, and cell-culture supernatant. Gene and protein expression of steroidogenic enzymes were determined.
Results
Obese subjects had elevated serum androgen levels, which reduced after weight loss. Androgens were measurable in AT and in cell-culture supernatants of adipocytes. Steroids were higher in AT from obese women, with the highest difference for testosterone in visc AT (+7.9-fold, p = 0.032). Steroidogenic enzymes were expressed in human AT with depot-specific differences. Obese women showed a significantly higher expression of genes of the backdoor pathway and of CYP19 in visc AT.
Conclusion
The whole steroidogenic machinery of the classical and backdoor pathways of steroidogenesis, and the capacity for androgen biosynthesis, were found in both AT depots and cultured adipocytes. Therefore, we hypothesize that AT is a de novo site of androgen production and the backdoor pathway of steroidogenesis might be a new pathomechanism for hyperandrogenism in women with obesity.
Keywords: Hyperandrogenism in women, Androgens, Obesity, Adipocytes, Steroidogenic capacity
Introduction
Obesity in women is often associated with hyperandrogenism. High testosterone levels can be associated with obesity, hypertension, amenorrhea, and ovulatory dysfunction, which can lead to infertility. A great number of hyperandrogenic women are diagnosed with the polycystic ovarian syndrome (PCOS). The prevalence is 5–15% of premenopausal women worldwide [1, 2]. PCOS is often associated with insulin resistance and cardiometabolic risk factors [3]. An excess of androgen production is considered to be an important pathomechanism of PCOS [4, 5, 6]. Insulin resistance and hyperinsulinism were identified to increase ovarian and adrenal androgen secretion in obese women [6, 7]. This creates a vicious circle as hyperandrogenism and associated clinical features such as hirsutism, acne, alopecia, type 2 diabetes, central obesity, the metabolic syndrome, and reproductive disorders often predict the metabolic phenotype of these women [8, 9, 10].
Other research groups have suggested that besides the adrenal glands and ovaries, other sites including adipose tissue (AT), hair follicles, and genital skin could contribute to androgen biosynthesis and circulating androgens [11, 12]. They found expression of certain steroid-genic genes on RNA level including 3β-HSD2, 17β-HSD3, and 5α-reductase 2 [12, 13]. AT is capable of converting circulating plasma DHEA-S, androstenedione, and testosterone to more potent androgens [14]. Until today, as many as 15 steroidogenic and steroid-inactivating enzymes have been detected in human AT [15, 16]. Therefore, the presence of the steroidogenic machinery suggests that locally produced steroids may modulate AT activity and function [12]. Recent studies also suggested the presence of StAR and CYP11A1, which are essential players for de novo steroidogenesis from cholesterol in AT [14]. Indran et al. [11] have estimated that 25% of testosterone in women is produced by ovaries, 25% by adrenals, and 50% by peripheral tissues including AT. This highlights the importance of investigating the role of AT in steroid production and storage to better understand the mechanisms of hyperandrogenism.
Steroidogenesis is regulated by the transcription and posttranslational modification of steroidogenic enzymes and co-factors. Most steroidogenic enzymes are either hydroxysteroid dehydrogenases or cytochrome P450 enzymes, which activities are modulated by posttranslational modifications and co-factors, especially electron-donating redox partners. The frontdoor pathway proceeds from cholesterol via pregnenolone, 17OH-Preg and DHEA to androstenedione or androstenediol and then to testosterone. The backdoor pathway proceeds mainly from 17OH-Preg to 17OH-Prog, 17OH-DHP, 17OH-Allo, androsterone, androstanediol and then to DHT. The alternative pathway is characterized by the presence of 5α-reductase type 1, reductive 3α-HSD activity catalyzed by AKR1C2 and AKR1C4 and oxidative 3α-HSD activity, which is catalyzed by 17β-HSD6 (RoDH) (24, 25). The backdoor pathway functions in steroidogenic tissues that express these enzymes in physiological and pathological conditions.
To study the role of AT in androgen production in females, we used a rodent model with lean and obese rats (25). Substantial amounts of testosterone were measurable in AT and in the supernatant of cultured preadipocytes and adipocytes from periovarian and inguinal AT. Most steroidogenic enzymes were expressed in AT and cultured cells of both depots. With thin-layer chromatography, we were able to show that pre- and adipocytes were able to convert pregnenolone to testosterone. Higher levels of all steroidogenic enzymes were found in obese compared with lean rats [17]. We concluded that the whole steroidogenic machinery and capacity for testosterone biosynthesis were found in periovarian and inguinal AT of female rats. These findings support the hypothesis that AT may contribute substantially to hyperandrogenism in human female obesity. To find support for this hypothesis, we analyzed in detail the steroidogenic machinery and capacity for testosterone biosynthesis on RNA and protein levels in human paired AT samples (subcutaneous (sc) and visceral (visc)) of women that had a normal body weight compared to obesity.
Materials and Methods
Cohorts and Patient Characteristics
Our study included two different cohorts to investigate androgen production and steroidogenesis in AT of lean (n = 10) and obese (n = 10) women. In the first cohort, we investigated serum androgens, steroidogenic gene expression, and androgens in sc and visc AT. In the second cohort (n = 10), we explored the changes of androgens in serum and AT in women with weight loss following bariatric surgery.
Subjects fulfilled the following inclusion criteria: (1) absence of any acute inflammatory disease (normal leukocyte count, CRP, no signs of infection); (2) no malignant disease; (3) no alcohol, smoking, or drug abuse; (4) no pregnancy and no use of oral contraceptives. Baseline characteristics of each cohort are given in Table 1. Patients have been consecutively recruited by the Department of Medicine, the Department of Surgical intervention, and the Obesity Outpatients Clinic at the University Hospital of Leipzig. AT samples were collected during elective laparoscopies or Sleeve Gastrectomy or Roux-en-Y Gastric Bypass Surgery. Prior to bariatric surgery, all obese patients attended an interdisciplinary weight loss program for at least 6 months, including frequent physical exercise, behavioral education, and diet counseling. However, only 40% of the participants lost weight before bariatric surgery (−3.1 kg), whereas 60% gained 2.9 kg on average during the intervention program. Percentage of body fat was measured by dual X-ray absorptiometry and calculated as described previously [18]. All blood samples were collected between 8:00 a.m. and 10:00 a.m. after overnight fasting.
Table 1.
Anthropometric cohort characteristics between the different groups
| Parameter | Lean | Obese | p values lean/obese | Bariatric surgery | p values lean/bs |
|---|---|---|---|---|---|
| Age, years | 65.3±11.9 | 43.7±13.5 | <0.001 | 47.9±6.9 | <0.001 |
| Body weight, kg | 57.3±6.1 | 136.5±14.2 | <0.001 | 132.6±61 | <0.001 |
| BMI, kg/m2 | 22.3±2.3 | 50.5±7.7 | <0.001 | 49.6±21 | <0.001 |
| Body fat, % | 17.9±3.3 | 59.4±3.1 | <0.001 | 47.5±18 | 0.016 |
| HbA1c, % | 5.1±0.3 | 6.5±1.3 | <0.001 | 6.61±3.2 | <0.001 |
| Fasting glucose, mmol/L | 5.20±0.73 | 5.80±1.72 | 0.68 | 5.77±1.18 | 0.90 |
| CRP, mg/L | 3.5±3.2 | 10.4±8.7 | 0.035 | 8.58±8 | 0.038 |
p values are given comparing the lean and obese cohort and the lean and the bariatric surgery group. Results are expressed as means ± SD from 10 samples per group.
Isolation of Preadipocytes
AT from five obese female patients was used to isolate preadipocytes as described before [19]. The stromal vascular fraction (SVF) was separated by collagenase digestion (1 mg/mL) for 45 min at 37°C. Cells were centrifuged for 5 min at 400 × g, and the supernatant was removed to separate adipocytes and SVF. This process was repeated. The SVF was washed twice in PBS and filtered (30µm-mesh). Cells were centrifuged for 5 min at 400 × g and erythrocytes lysed with lysis buffer. Cells were washed twice in PBS and counted. SVF cells were seeded at 20,000 cells/cm2 on a 96-well plate and incubated in culture medium (DMEM/F-12, 10% FBS, 100 units penicillin, 0.1 mg/mL streptomycin) at 37°C and 5% CO2. After reaching 90% confluence, differentiation was induced with insulin, dexamethasone, and rosiglitazone. Each time before collecting the supernatant, cells were washed 3x and starved with serum-free medium for 24 h (DMEM/F-12, 100 units penicillin, 0.1 mg/mL streptomycin). 200 µL cell-culture supernatant was always frozen for further analysis. In wells without cells but just media, no androgens were measurable and therefore always three wells were used as a negative control.
Blood Parameters
Blood parameters were assessed by a certified laboratory (Institute of Laboratory Medicine, University Hospital Leipzig). HbA1c was measured with an enzyme immunometric assay for the IMMULITE automated analyzer (Diagnostic Products, Los Angeles, CA, USA). Serum CRP was measured by an ELISA kit (Bio Vendor, Czech Republic).
Laboratory Hormone Measurements
Serum estradiol, progesterone, testosterone, DHEA-S, SHBG, LH, and FSH levels were measured using an elektrochemilumineszenz immunoassay (Cobas 801; Roche Diagnostics, Mannheim, Germany). Serum androstenedione was detected using a solid-phase, competitive chemiluminescent enzyme immunoassay (IMMULITE 2000; Siemens Healthcare Diagnostics, Munich, Germany).
Mass-Spectrometry
Progesterone, androstenedione, testosterone, DHEA-S, and DHT were measured in cell-culture supernatants and isolated AT, according to protocols established for human plasma analysis. Steroids were determined by LC-MS/MS, as previously described [20, 21]. Briefly, samples, calibrators, and controls were combined with the internal standard mixture to monitor recovery. All samples were extracted using Oasis MAX SPE system plates (Waters, Milford, MA, USA). Chromatographic separation was carried out using an UPLC system, connected to a Quattro Premier/XE triple Quad mass spectrometer (Waters, Milford, MA, USA). A Waters Acquity UPLC BEH C18 column (1.7 μm, 100 × 2.1) was used at a flow rate of 0.4 mL/min at 50°C. Water and acetonitrile with 0.01% formic acid were used as the mobile phase. Two mass transitions were monitored for each hormone. Data were acquired using MassLynx 4.1 software and quantified with QuanLynx software (Waters). During all analyses, the ambient temperature was maintained at 21°C by air conditioning. The FBS-free medium was also measured and no androgens were detectable.
Determination of Androgens in AT
Steroids were extracted from AT as described previously [22]. In brief, the tissue was weighed and homogenized by sonication (2 × 20 sec) in a sodium phosphate buffer solution (PBS). Homogenates were centrifuged at 10,000g for 10 min. Testosterone was extracted with ethyl acetate and evaporated overnight. The pellet was resuspended in PBS and testosterone concentrations were determined employing LC-MS/MS. The steroid concentration in nmol/L was divided by the concentration used (150 µL), and the amount of AT used (in mg) to get the amount of steroid in pmol/mg AT.
Isolation of RNA and cDNA Production
Total RNA was extracted from different depots of AT using RNeasy Lipid Tissue kit (Qiagen, Hilden, Germany), according to the manufacturer's protocol. The RNA was pretreated with DNAse (RNase-free DNase Set, Qiagen) according to the manufacturer's instructions. The amount of RNA was measured by Nanodrop (Thermo Fisher Scientific, NanoDrop2000). The RNA was kept at −80°C until experiments were performed. Total RNA was processed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's protocol.
Gene Expression Analysis by qPCR
The samples for qPCR were prepared using iQ SYBR Green Supermix (Bio-Rad Laboratories), and the PCR cycles were run at 95°C for 10 s, 56–63°C for 45 s (depending on the melting curve for each primer pair), 95°C for 60 s, and 55°C for 60 s followed by a melting curve from 55 to 95°C in steps of 0.5°C and then held at 4°C (iCycler iQ, Bio-Rad Laboratories, Hercules, CA, USA) after having estimated the best reaction conditions by running a temperature gradient for each primer pair. All results were normalized to RPLP0, 36B4, and 18 s as housekeeping genes. To control the effectivity of the process, a negative control was always added to each qPCR assay. The 2−ΔΔCt method was used to calculate the fold changes in gene expression. Primer sequences are available in online supplementary Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000521571.
Automated Western-Blot Analysis
Automated Western blotting of steroidogenic enzymes was performed as described previously [23]. Briefly, the tissue was lysed in CelLytic cell reagent (Sigma Chemical Co., St. Louis, MO, USA) and the lysates diluted with sample buffer to a protein concentration of 0.3 μg in 4 μL were mixed with the 5× Master Mix (DTT, fluorescence-labeled marker, SDS) in a ratio 5:1 and then incubated at 95°C for 5 min. The samples, protein ladder, blocking reagent, primary antibodies, HRP-conjugated secondary antibodies, chemiluminescent substrate, and stacking matrices were loaded into individual wells of the sample plate. Antibodies were diluted with antibody diluent buffer. After plate loading, the separation electrophoresis and immunodetection steps took place in the capillary system and were fully automated in the Wes instrument. The 30 min incubation with primary antibodies against StAR, CYP17A1, CYP11A1, AKR1C2, and GAPDH and 60 min incubation with the 3β-HSD2 antibody were followed by incubation with HRP-conjugated goat anti-rabbit secondary antibody for 30 min. Luminol and peroxide (ProteinSimple) were then added to generate chemiluminescence. The digital images obtained were analyzed with the Compass software (ProteinSimple). Band densities were normalized against GAPDH and the expression of steroidogenic enzymes was expressed as percentages of the corresponding values of visc AT in obese women (online suppl. Table 1).
Statistical Analyses
The differences between values were analyzed for statistical significance by Student's t test, and paired t test using the SigmaPlot (v. 11) program (Systat Software, Inc., San Jose, CA, USA). In case that the normality test (Shapiro-Wilk) failed, a Mann-Whitney Rank Sum Test was used. *p < 0.05 was considered statistically significant; **p < 0.01 was considered highly significant.
Results
Cohort Characteristics
The lean cohort had an average body weight of 57.3+/−6.1 kg (BMI 22.3+/−2.3 kg/m2), the obese cohort of 136.4+/−14.2 kg (BMI 50.5+/− 7.7 kg/m2) and the bariatric cohort of 132.6+/− 61 kg (BMI of 49.6+/−21 kg/m2) (Table 1). The patients lost on average 34 kg during the 12 months after surgery and reduced their BMI to 36.8 kg/m2 (Table 2).
Table 2.
Anthropometric characteristics before and 12 months after bariatric surgery
| Parameter | Before bsc | After bsc | Δ% | Significance |
|---|---|---|---|---|
| Body weight, kg | 132.6±61 | 98.6±26 | −26% | p = 0.006 |
| BMI, kg/m2 | 49.6±21 | 36.8±8 | −26% | p = 0.004 |
| Body fat, % | 47.5±18 | 39.8±8 | −16% | p = 0.040 |
| Fat mass, kg | 63.4±42 | 40.2±17 | −37% | p < 0.001 |
| HbA1c, % | 6.61±3.2 | 5.28±0.9 | −20% | p = 0.003 |
| CRP, mg/L | 8.58±8 | 2.06±2.1 | −76% | p = 0.014 |
| Androgens | ||||
| Androstendione, µg/L | 1.3±0.6 | 0.9±0.5 | −27% | p = 0.008 |
| Testosterone, µg/L | 0.35±0.2 | 0.22±0.1 | −35% | p = 0.003 |
| Free androgen index, % | 6.9±11 | 2.2±1.8 | −68% | p = 0.002 |
| DHEAs, mg/L | 1.7±1.2 | 1.3±11 | −23% | p = 0.032 |
Results are expressed as means ± SD, from 10 samples per group. Δ% depicts the difference before and after bariatric surgery (bsc). p values are given in the table.
Obese Women Showed Elevated Serum Androgen Levels Compared to Lean Women
We found elevated androgen levels in the obese cohort compared to the lean cohort, with testosterone levels of 0.22 +/−0.2 μg/L in the lean compared to significantly higher (0.63 +/− 0.5 μg/L (p < 0.001)) in the obese cohort. The following androgens were higher in women with obesity: free androgen index (FAI) was 3.4+/−6.4% in the lean and 11.7+/−23.3% (p = 0.001) in the obese, DHEA-S 0.62+/−1.3 mg/L and 1.9+/−1.9 mg/L (p = 0.004), and androstendione 1.1+/−1.1 μg/L and 1.4+/−1.6 μg/L (not significant) (Table 3).
Table 3.
Serum androgen concentrations in lean and obese women. Results are expressed as means ± SD from 10 samples per group
| Androgens in serum | Lean women | Obese women | p values |
|---|---|---|---|
| Androstendione, µg/L | 1.1±1.4 | 1.4±1.6 | ns |
| Testosterone, µg/L | 0.2±0.2 | 0.6±0.5 | <0.001 |
| Free androgen index, % | 3.5±6.4 | 11.7±23.3 | 0.001 |
| DHEA-s, mg/L | 0.6±1.3 | 1.9±1.9 | 0.004 |
p values are given in the table.
Serum Androgen Levels Declined after Weight Loss due to Bariatric Surgery
Serum androgens decreased in all patients with weight loss 12 months after bariatric surgery: testosterone by 35% (p = 0.003), FAI by 68% (p = 0.002), androstendione by 27% (p = 0.008), and DHEA-S by 23% (p = 0.023) (Table 2).
Androgen Levels Are Depot Specific and Serum Concentrations Are Higher in Patients with Obesity
Steroids were isolated from both AT depots. In general, we found high androgen levels in obese patients. Due to strong interindividual variation, most androgen levels were not significantly different in the two cohorts due to the small sample size. We found higher androstendione levels by 2-folds in the sc AT of lean compared to obese and by 2.2-folds in visc AT (p = 0.085 and p = 0.19 respectively). The difference between lean and obese women in dihydrotestosterone (DHT) levels was 4.5-fold in the sc depot and 4.7-fold in the visc depot (p = 0.31 and p = 0.26, respectively). The highest difference was measured for testosterone with an increase of 6.6-fold in the sc (p = 0.69) and even 7.9-fold in the visc AT of obese women which was significant (p = 0.032*).
If the amount of total body fat is taken into consideration, lean women would have average testosterone levels of 0.039 nmol/kg body weight, whereas obese women would have around 3.38 nmol/kg body weight. For DHT, lean women would have on average 0.159 nmol/kg and obese women 5.82 nmol/kg body weight. For androstendione, we estimated 7.13 nmol/kg in lean and 164.35 nmol/kg in obese women.
Androgens Were Detectable in the Cell-Culture Supernatant during Adipocyte Differentiation
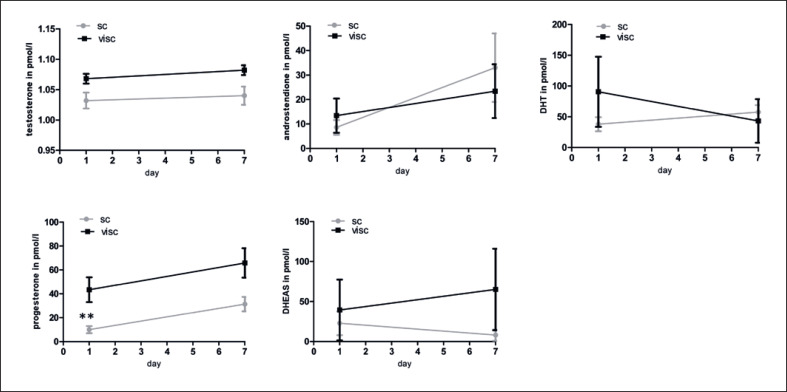
We measured androgen levels in the cell-culture supernatant of preadipocytes at day 1 and of adipocytes during differentiation at day 7 with LC-MS/MS. Lipid droplets adequately formed during differentiation and the proportion of cells with lipid droplets per well was evaluated and counted with a microscope. As expected adipocytes isolated from sc AT differentiated better (80–90% lipid droplets in sc AT), as compared to adipocytes that were isolated from visc AT (25–40% in visc AT). We found slightly higher levels of testosterone, progesterone, and DHEA-S in the supernatant of adipocytes isolated from visc compared to sc AT, although the adipocytes from visc AT differentiated less well than adipocytes from sc AT (Fig. 1).
Fig. 1.
Production of testosterone, progesterone, androstendione, dihydrotestosterone and DHEA-S during adipocyte differentiation at day 1 and day 7 in pmol/L (measured with Mass-Spectrometry). Results are expressed as means ± SEM from 5 samples per group. The different degrees of significance was indicated as follows in the graphs *p < 0.05;**p< 0.01.
Steroidogenic Enzymes Were Expressed in Sc and Visc at from Lean and Obese Women and Showed Depot-Specific Differences
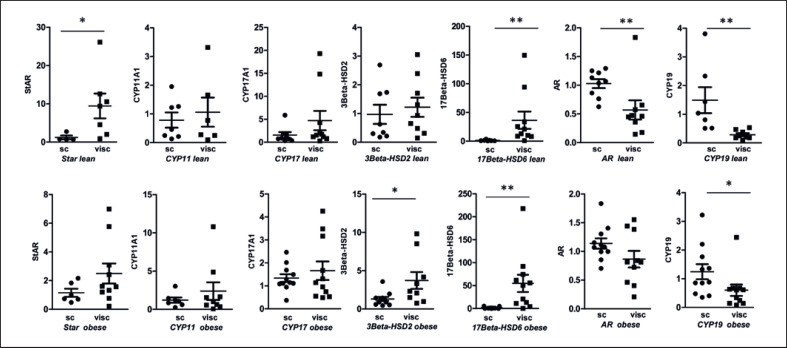
We measured the mRNA expression of steoroidogenic enzymes required for androgen production. All genes that were expressed before PCR cycle 35 were included in the data evaluation. Expression of StAR, CYP11A1, 3β-HSD2, and 17β-HSD3 was not detectable in all biopsies, whereas the following genes were found in all visc AT samples of obese patients: CYP17A1, 17β-HSD6, 5α-Reductase 1/2, CYP19/Aromatase, AR, LH-r, AKR1C2, and AKR1C3. In 9 out of 10 patients with obesity, we found significant expression levels for StAR, CYP17A1, 3β-HSD2, and 17β-HSD3. For CYP11A1; we only found expression in 6 out of 10 samples from women with obesity. Furthermore, we investigated depot-specific differences. In lean women, we found significant differences in gene expression between sc and visc AT. We found a significant upregulation of StAR by 7.7-fold (p = 0.024) and 17β-HSD6 by 28.3-fold (p = 0.002) in visc AT. A lower expression was found for AR by 1.5-fold (p = 0.008) and CYP19 by 1.8-fold (p = 0.001) in visc compared to sc AT (Fig. 2). In women with obesity, we found a significantly higher expression in visc AT of the following genes: 3β-HSD2 by 2.8-fold (p = 0.023) and 17β-HSD6 by 32.1-fold (p = 0.002). Again, CYP19 expression was lower by 1.5-fold (p = 0.014). Thus, we found similar depot-specific differences in lean and obese women (Fig. 2).
Fig. 2.
Depot-specific differences of relative gene expression of steroidogenic enzymes. RPLP0 was used as a reference gene. First row represents depot-specific differences in lean women and second row in obese women. Sc and visc AT are compared and results are expressed as dot blots plus mean ± SEM. N = 6–10 samples in each group. The different degrees of significance was indicated as follows in the graphs *p < 0.05; **p < 0.01.
In addition, steroidogenic enzyme protein levels were measured by automated Western blot analysis. We did not find significant amounts of StAR and CYP17A1, but found high levels of CYP11A1 and could prove protein expression of 3-BetaHSD2 and AKR1C2 in all samples. In lean women, the expression of CYP11A1 (<0.001) and 3Beta-HSD2 (p = 0.029) was significantly higher in visc than sc AT, whereas in obese samples we found a significantly higher protein expression for CYP11A1 (p = 0.029) and 3Beta-HSD2 (p = 0.029) in the sc depot compared to the visc (online suppl. Fig. 2).
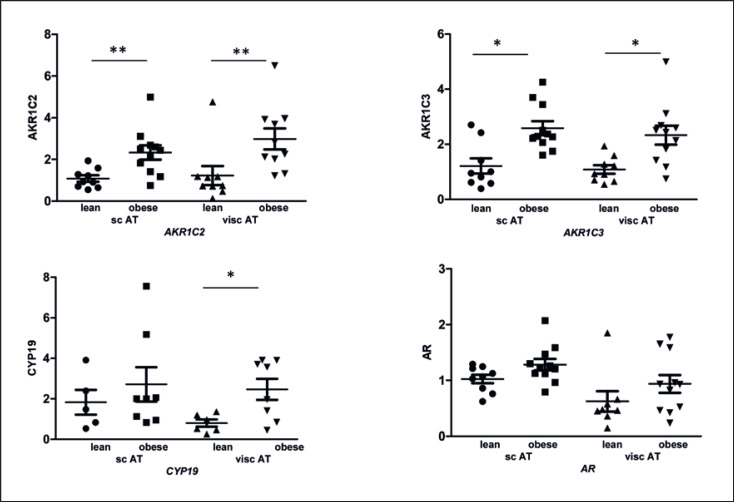
Aromatase and AKR1C2 and 3 Were Upregulated in Obese Patients
Interestingly, we found significant differences in CYP19/aromatase expression (Fig. 3) between lean and obese women. There was an increase of expression in visc AT of obese compared to lean patients by 4.1-fold (p = 0.012). Furthermore, we measured AKR1C2 and AKR1C3, which are genes of the steroidogenic backdoor pathway. AKR1C2 was significantly upregulated in sc AT of obese women by 2.5-fold (p = 0.007) and in visc AT by 2.4-fold (p = 0.004) compared to lean women. In addition, AKR1C3 was significantly upregulated in sc AT of obese women by 2.1-fold (p = 0.01) and in visc AT by 2.4-fold (p = 0.018) (Fig. 3).
Fig. 3.
Relative gene expression level of steroidogenic enzymes always compared to AT of sc depots of lean women. Differences of expression of lean and obese females and from both depots are depicted. RPLP0 was used as a reference gene. Results are expressed as dot blots plus mean ± SEM from 10 samples per group. The different degrees of significance was indicated as follows in the graphs *p < 0.05; **p < 0.01.
Discussion
Our data provide the first evidence that the whole steroidogenic machinery of the classical and backdoor pathway of steroidogenesis and the capacity for androgen biosynthesis are present in human female AT. Serum androgen levels were significantly higher in obese women than in lean. Most studies support our hypothesis, but there is also conflicting data on this topic [24, 25, 26], as some women with obesity do present with a nonhyperandrogenic state. The reason for this remains unclear [27]. After a considerable weight loss following bariatric surgery serum androgen levels decreased. This is in line with other published data: weight loss, which was achieved by intervention programs or bariatric surgery, resulted in a decrease of androgens [28, 29]. With that experiment, we measured the androgen content in AT, but in addition, we wanted to evaluate if preadipocytes and adipocytes might have the capacity to de novo produce androgens when cultured in vitro. Substantial amounts of androgens were measurable in the cell-culture supernatant. Data from our previous rat study revealed that pre- and adipocytes were able to convert pregnenolone to testosterone and 3 alpha-diol, confirming the functional activity of steroidogenic enzymes in adipocytes [17, 30]. To test our hypothesis that also human AT is a site of de novo production of steroids, we measured the expression of steroidogenic enzymes. We detected all selected steroidogenic genes that are necessary for steroid production through the frontdoor pathway, in both AT depots, but not in all 20 samples that were analyzed. These observations are also in line with recent findings by others [11, 12, 13]. Active steroid hormones are synthesized from cholesterol, which is initiated by CYP11A1 or through the conversion of steroid precursors [12, 31]. We and others found StAR expression in AT of female rats [17] [13, 32, 33] and in human AT [34, 35], but we and others failed to show protein expression by Western blot [36, 37]. The detection methods might not be sensitive enough to prove protein expression. Other steroidogenic tissues lack StAR expression [38], e.g., the brain and the placenta, and are still defined as steroidogenic organs. The first and rate limiting reaction is the conversion of cholesterol to pregnenolone, which is catalyzed by CYP11A1 [12, 39]. CYP11A1 was expressed in most of our AT samples on RNA level and in all samples on protein level. CYP11A1 expression was shown in 8 women after caesarean section on RNA level [13] and in sc AT of women with PCOS [35]. We found CYP17A1 expression in all obese and most of the lean AT samples on RNA level but not on protein level. Some groups were not able to find the expression of CYP17A1 in human AT [13, 40], whereas others confirmed the expression on mRNA level [35] and with LC-MS/MS showed strong enzyme activity. Others have also shown that most steroid-converting enzymes downstream of pregnenolone are present in human AT [14, 31]. 3β-HSD1 and -2 were detected in AT [13, 41] and breast tissue [42] and obese women with PCOS showed elevated gene expression levels in sc AT [12].
We found a significantly higher expression of StAR, 17β-HSD6 in visc compared to sc AT in lean women. This fits with higher androgens in visc than in sc AT and in the supernatant.
Furthermore, we evaluated the differences of steroidogenic gene expression between lean women and women with obesity. In contrast to our rat study, we did not find significant differences for most of the steroidogenic enzymes in human AT on RNA level [17]. Most probably, the steroidogenic gene expression depends on many factors: e.g., circadian rhythm, weight loss before surgery, chronic disease, and hormonal factors. Interestingly, we found a higher CYP19 expression in visc AT of obese women. Therefore, we speculate that AT of obese women and especially visc AT converts higher amounts of testosterone to estradiol, or androstendione to estron, trying to lower high androgen levels.
The ovary is considered to be the main source of androgen excess in PCOS [10, 43, 44, 45, 46]. Theca cells from PCOS patients showed increased CYP17A1 and 3β-HSD2 enzyme activity [11, 44] and androgen production. Interestingly, we also found a strong upregulation of CYP17A1 and 3β-HSD2 enzymes in our obese rats. Since LH receptors are also expressed in AT [47], raised LH levels, as in PCOS patients, could stimulate the steroidogenic activity in the ovaries and AT and therefore add to high androgens. Increased intra-abdominal fat deposition in PCOS patients could promote metabolic dysfunction [48]. Additionally, we detected AKR1C2 and AKR1C3, which are genes of the “alternative” or “backdoor pathway.” Those genes were significantly upregulated in both depots of obese women. The “backdoor” pathway produces DHT without testosterone intermediacy [49]. AKR1C2 converts androstanediol to DHT and AKR1C3 converts androstenedione to testosterone, androsterone to androstanediol and androstanedione to DHT. Hence, to the more potent forms of androgens, Quinkler et al. [50] also proved AKR1C3 expression in human AT from women with PCOS. The expression in the sc tissue correlated with the BMI of the patients and the expression level of AKR1C3 decreased with weight [50]. Recently, Ostinelli et al. [51] found AKR1C2 and 3 expressions in AT samples which were positively associated with percentage of trunk fat mass in women. They were able to confirm a link between AKR1C2, adipogenic differentiation and AT distribution. We showed that all necessary genes for the backdoor pathway are expressed in human AT and therefore, we speculate that the “front-” and “backdoor pathway” are functional in AT defining the AT as a steroidogenic organ.
In addition, cell-culture experiments of preadipocytes and differentiating adipocytes revealed that the function of the steroidogenic enzymes is strong enough to produce significant amounts of androgens that were measurable in cell-culture supernatants during differentiation. In a follow-up study, we would like to analyze the capacity of converting steroid precursors and steroids to androgens and to test steroid inhibitors.
Our study has some limitations. First, the sample size is rather small. As steroid measurements are depending on many influencing factors, e.g., circadian rhythm, menstrual cycles, etc. we found strong interindividual variation. A greater sample size would have been beneficial to possibly identify if there are any significant differences. Second, the average age of our lean cohort was 21.6 years older than our obese cohort. Therefore, we had more postmenopausal patients in our lean and more menstruating women in our obese cohort. This factor might unfortunately strongly influence our data. Circulating androgens slightly decrease with increasing age. But several studies were able to show that in postmenopausal women, the ovaries produce significant amounts of androgens for many years after the menopause [43, 52, 53, 54, 55]. Davinson et al. [54] reported that serum androgen levels mostly decline in the early reproductive years and do not vary because a consequence of natural menopause and that the postmenopausal ovary appears to be an ongoing site of testosterone production. Burger et al. [52] concluded that SHBG and the FAI levels slightly change at the time of the menopause, at least partially due to the decline in estradiol but testosterone remains unchanged during the menopausal years. SHBG and DHEAS show a high degree of stability within an individual over time [52]. But of course we cannot exclude that the older age and the greater number of postmenopausal women in the lean cohort also influenced testosterone, androstendione, and DHT levels and need to consider this aspect for data evaluation. Furthermore, we were not able to consider the phase of the menstrual cycle of the patient, if the menstrual cycle was regular or if and when menopause started. Furthermore, we did not have the chance to measure enzyme activity ex vivo. Hence, it would be of great importance to take those aspects into consideration when planning a new study. Patients that underwent bariatric surgery completed an intervention program prior to surgery. Therefore, some individuals could already have improved their metabolic profile and as we have seen reduced testosterone levels and the FAI. This may also have influenced the expression levels of steroidogenic enzymes. Furthermore, changes in AT morphology before versus after the weight loss and between the different depots could have affected the measured steroid concentrations. With our experiments, we were unfortunately not able to differentiate if differences are coming from less lipid content or fewer or smaller adipocytes, and the steroid concentration was normalized for the amount of the adipose tissue in mg.
As our obese cohort had 30–40% more AT compared to our lean cohort, the higher amount of AT would already be sufficient for higher androgen production. Testosterone levels were 564% higher in sc and 687% higher in visc AT of obese women. Androstendione was elevated in AT of obese women by 103% in sc and by 124% in visc AT and DHT by 355% in sc and by 368% in visc AT compared to lean women. That strong differences in androgens in AT could of course be a factor that might partly explain hyperandrogenism and the disruption of the menstrual cycle in obese women. Thus, we suggest that AT is a significant source of de novo androgen production and may contribute to androgen-associated disturbances in women with obesity. The “backdoor pathway” of steroidogenesis might be an additional pathomechanism which could result in increased androgen production in AT of women with obesity (online suppl. Fig. 1). We would therefore support the hypothesis of Indran et al. [11] that significant amounts of testosterone, of androstendione, and of DHEA-S might be produced by peripheral tissues. Taken together, our data strongly suggest a pathophysiological role of AT in women with hyperandrogenism. These findings need to be taken into consideration in the development of novel therapeutic concepts for hyperandrogenism, such as blockage of steroidogenic pathways in AT.
Conclusion
In conclusion, we provide a model that the whole steroidogenic machinery of the classical and backdoor pathways of steroidogenesis, and the capacity for androgen biosynthesis are active in both AT depots as well as in cultured adipocytes. Therefore, we hypothesize that AT is a de novo site of androgen production and hence might add to hyperandrogenism in women with obesity.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Subjects have given their written informed consent to participate in the study, and the study protocol was approved by the Ethics Committee of the University of Leipzig (096-11-07032011).
Conflict of Interest Statement
We declare not having any conflict of interest and not any competing financial interest in relation to the work described.
Funding Sources
This work was supported by grants from the European Union's Horizon 2020 research and innovation programme (grant agreement No 634880), the Swedish Research Council, the Frimurare Barnhuset Foundation, Kronprinsessan Lovisas Foundation, the “Sällskapet Barnavård,” and the “Stiftelsen Samariten.” I.V.W. was supported by the ESPE Research Fellowship (European Society for Pediatric Endocrinology) and the IFB Adiposity Diseases. Animal experiments were supported by SFB1052/2 (B04 to NK) and IFB Adiposity Diseases (FKZ 01E01501 to NK).
Author Contributions
I.V.W., I.S., L.S., A.K., N.K., A.D., P.M.H., J.D., M.B., and O.S. performed experiments, collected samples and included patients, acquired, analyzed and/or interpreted data, revised the manuscript critically for important intellectual content, and approved the manuscript. I.V.W. wrote the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
Many thanks to all the patients that took part in our study and all medical doctors that helped in recruiting the patients. We like to thank Viola Döbel for the cell culture studies and Daniela Kern and Susan Kralisch-Jäcklein for their technical support.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89((6)):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27((10)):3067–3073. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 3.Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33((5)):812–841. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wickenheisser JK, Nelson-Degrave VL, McAllister JM. Dysregulation of cytochrome P450 17alpha-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90((3)):1720–1727. doi: 10.1210/jc.2004-1860. [DOI] [PubMed] [Google Scholar]
- 5.Medeiros SF, Barbosa JS, Yamamoto MM. Comparison of steroidogenic pathways among normoandrogenic and hyperandrogenic polycystic ovary syndrome patients and normal cycling women. J Obstet Gynaecol Res. 2015;41((2)):254–263. doi: 10.1111/jog.12524. [DOI] [PubMed] [Google Scholar]
- 6.Alpanes M, Luque-Ramirez M, Martinez-Garcia MA, Fernandez-Duran E, Alvarez-Blasco F, Escobar-Morreale HF. Influence of adrenal hyperandrogenism on the clinical and metabolic phenotype of women with polycystic ovary syndrome. Fertil Steril. 2015;103((3)):795–801 e2. doi: 10.1016/j.fertnstert.2014.12.105. [DOI] [PubMed] [Google Scholar]
- 7.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33((6)):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghetti P, Tosi F, Bonin C, Di Sarra D, Fiers T, Kaufman JM, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98((4)):E628–37. doi: 10.1210/jc.2012-3908. [DOI] [PubMed] [Google Scholar]
- 9.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18((6)):618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 10.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352((12)):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 11.Indran IR, Lee BH, Yong EL. Cellular and animal studies: insights into pathophysiology and therapy of PCOS. Best Pract Res Clin Obstet Gynaecol. 2016;37:12–24. doi: 10.1016/j.bpobgyn.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Papadopoulos V, Vihma V. Steroid biosynthesis in adipose tissue. Steroids. 2015;103:89–104. doi: 10.1016/j.steroids.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie SM, Huda SS, Sattar N, Fraser R, Connell JM, Davies E. Depot-specific steroidogenic gene transcription in human adipose tissue. Clin Endocrinol. 2008;69((6)):848–854. doi: 10.1111/j.1365-2265.2008.03262.x. [DOI] [PubMed] [Google Scholar]
- 14.Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34((11–12)):737–745. doi: 10.1055/s-2002-38265. [DOI] [PubMed] [Google Scholar]
- 15.Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126((2)):1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- 16.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301((1–2)):97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Wagner IV, Sahlin L, Savchuk I, Klöting N, Svechnikov K, Söder O. Adipose tissue is a potential source of hyperandrogenism in obese female rats. Obesity. 2018;26((7)):1161–1167. doi: 10.1002/oby.22198. [DOI] [PubMed] [Google Scholar]
- 18.Loffler D, Muller U, Scheuermann K, Friebe D, Gesing J, Bielitz J, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. 2015;100((4)):1289–1299. doi: 10.1210/jc.2014-2932. [DOI] [PubMed] [Google Scholar]
- 19.Landgraf K, Rockstroh D, Wagner IV, Weise S, Tauscher R, Schwartze JT, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2015;64((4)):1249–1261. doi: 10.2337/db14-0744. [DOI] [PubMed] [Google Scholar]
- 20.Kulle AE, Welzel M, Holterhus PM, Riepe FG. Implementation of a liquid chromatography tandem mass spectrometry assay for eight adrenal C-21 steroids and pediatric reference data. Horm Res Paediatr. 2013;79((1)):22–31. doi: 10.1159/000346406. [DOI] [PubMed] [Google Scholar]
- 21.Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. J Clin Endocrinol Metab. 2010;95((5)):2399–409. doi: 10.1210/jc.2009-1670. [DOI] [PubMed] [Google Scholar]
- 22.Robertson KM, Schuster GU, Steffensen KR, Hovatta O, Meaney S, Hultenby K, et al. The liver X receptor-{beta} is essential for maintaining cholesterol homeostasis in the testis. Endocrinology. 2005;146((6)):2519–2530. doi: 10.1210/en.2004-1413. [DOI] [PubMed] [Google Scholar]
- 23.Savchuk I, Morvan ML, Antignac JP, Gemzell-Danielsson K, Le Bizec B, Soder O, et al. Androgenic potential of human fetal adrenals at the end of the first trimester. Endocr Connect. 2017;6((6)):348–359. doi: 10.1530/EC-17-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85((5)):1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali R, Vicennati V, Gambineri A, Pagotto U. Sex-dependent role of glucocorticoids and androgens in the pathophysiology of human obesity. Int J Obes. 2008;32((12)):1764–1779. doi: 10.1038/ijo.2008.129. [DOI] [PubMed] [Google Scholar]
- 26.McCartney CR, Burt Solorzano CM, Patrie JT, Marshall JC, Haisenleder DJ. Estimating testosterone concentrations in adolescent girls: comparison of two direct immunoassays to liquid chromatography-tandem mass spectrometry. Steroids. 2018;140:62–9. doi: 10.1016/j.steroids.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchernof A, Brochu D, Maltais-Payette I, Mansour MF, Marchand GB, Carreau AM, et al. Androgens and the regulation of adiposity and body fat distribution in humans. Compr Physiol. 2018;8((4)):1253–1290. doi: 10.1002/cphy.c170009. [DOI] [PubMed] [Google Scholar]
- 28.Ennour-Idrissi K, Maunsell E, Diorio C. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015;17((1)):139. doi: 10.1186/s13058-015-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst B, Wilms B, Thurnheer M, Schultes B. Reduced circulating androgen levels after gastric bypass surgery in severely obese women. Obes Surg. 2013;23((5)):602–7. doi: 10.1007/s11695-012-0823-9. [DOI] [PubMed] [Google Scholar]
- 30.Rey R, Campo S, Ayuso S, Nagle C, Chemes H. Testicular steroidogenesis in the Cebus monkey throughout postnatal development. Biol Reprod. 1995;52((5)):997–1002. doi: 10.1095/biolreprod52.5.997. [DOI] [PubMed] [Google Scholar]
- 31.Tchernof A, Mansour MF, Pelletier M, Boulet MM, Nadeau M, Luu-The V. Updated survey of the steroid-converting enzymes in human adipose tissues. J Steroid Biochem Mol Biol. 2015;147:56–69. doi: 10.1016/j.jsbmb.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Atzmon G, Yang XM, Muzumdar R, Ma XH, Gabriely I, Barzilai N. Differential gene expression between visceral and subcutaneous fat depots. Horm Metab Res. 2002;34((11–12)):622–8. doi: 10.1055/s-2002-38250. [DOI] [PubMed] [Google Scholar]
- 33.Campion J, Milagro FI, Fernandez D, Martinez JA. Vitamin C supplementation influences body fat mass and steroidogenesis-related genes when fed a high-fat diet. Int J Vitam Nutr Res. 2008;78((2)):87–95. doi: 10.1024/0300-9831.78.2.87. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Morante JJ, Gomez-Santos C, Milagro F, Campion J, Martinez JA, Zamora S, et al. Expression of cortisol metabolism-related genes shows circadian rhythmic patterns in human adipose tissue. Int J Obesity. 2009;33((4)):473–480. doi: 10.1038/ijo.2009.4. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Li S, Zhao A, Tao T, Mao X, Zhang P, et al. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J Steroid Biochem Mol Biol. 2012;132((1–2)):120–6. doi: 10.1016/j.jsbmb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Daly E, Campioli E, Wabitsch M, Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J Biol Chem. 2014;289((2)):747–764. doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anuka E, Gal M, Stocco DM, Orly J. Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Mol Cell Endocrinol. 2013;371((1–2)):47–61. doi: 10.1016/j.mce.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52((12)):2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalla Valle L, Toffolo V, Nardi A, Fiore C, Bernante P, Di Liddo R, et al. Tissue-specific transcriptional initiation and activity of steroid sulfatase complementing dehydroepiandrosterone sulfate uptake and intracrine steroid activations in human adipose tissue. J Endocrinol. 2006;190((1)):129–139. doi: 10.1677/joe.1.06811. [DOI] [PubMed] [Google Scholar]
- 41.Blouin K, Nadeau M, Mailloux J, Daris M, Lebel S, Luu-The V, et al. Pathways of adipose tissue androgen metabolism in women: depot differences and modulation by adipogenesis. Am J Physiol Endocrinol Metabol. 2009;296((2)):E244–55. doi: 10.1152/ajpendo.00039.2008. [DOI] [PubMed] [Google Scholar]
- 42.Killinger DW, Strutt BJ, Roncari DA, Khalil MW. Estrone formation from dehydroepiandrosterone in cultured human breast adipose stromal cells. J Steroid Biochem Mol Biol. 1995;52((2)):195–201. doi: 10.1016/0960-0760(94)00164-h. [DOI] [PubMed] [Google Scholar]
- 43.Burger HG. Androgen production in women. Fertil Steril. 2002;77((Suppl 4)):3–5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 44.Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86((12)):5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 45.Kempna P, Marti N, Udhane S, Fluck CE. Regulation of androgen biosynthesis: a short review and preliminary results from the hyperandrogenic starvation NCI-H295R cell model. Mol Cell Endocrinol. 2015;408:124–132. doi: 10.1016/j.mce.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13((6)):946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 47.Schaffler A, Binart N, Scholmerich J, Buchler C. Hypothesis paper brain talks with fat: evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides. 2005;39((4)):363–7. doi: 10.1016/j.npep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab. 2016;101((11)):4178–4188. doi: 10.1210/jc.2016-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Dev Dyn. 2013;242((4)):320–9. doi: 10.1002/dvdy.23892. [DOI] [PubMed] [Google Scholar]
- 50.Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity: a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183((2)):331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 51.Ostinelli G, Vijay J, Vohl MC, Grundberg E, Tchernof A. AKR1C2 and AKR1C3 expression in adipose tissue: Association with body fat distribution and regulatory variants. Mol Cell Endocrinol. 2021;527:111220. doi: 10.1016/j.mce.2021.111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85((8)):2832–2838. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 53.Davey DA. Androgens in women before and after the menopause and post bilateral oophorectomy: clinical effects and indications for testosterone therapy. Womens Health. 2012;8((4)):437–446. doi: 10.2217/whe.12.27. [DOI] [PubMed] [Google Scholar]
- 54.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90((7)):3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 55.Pasquali R, Oriolo C. Obesity and androgens in women. Front Horm Res. 2019;53:120–134. doi: 10.1159/000494908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.