Key Points
Question
How can insomnia disorder be treated effectively in Black women over the internet, and how can intervention engagement be increased?
Findings
In this randomized clinical trial including 333 Black women, participants receiving both a standard internet-delivered cognitive behavioral intervention for insomnia and a version tailored for Black women reported significantly greater insomnia improvement compared with those receiving sleep education alone. Participants were more likely to complete the full intervention if they received the tailored program, with intervention completion associated with greater insomnia improvement.
Meaning
In this study, known sleep health disparities facing Black women in the US were addressed with an evidence-based treatment, and engagement was increased using a culturally adapted intervention.
This randomized clinical trial compares the efficacy of a standard version of an internet-delivered cognitive behavioral therapy for insomnia program, a culturally tailored version, and a sleep education control at improving insomnia symptoms among Black women with insomnia disorder.
Abstract
Importance
Black women are at risk for insomnia disorder. Despite interest in addressing sleep health disparities, there is limited research investigating the efficacy of criterion-standard treatment (cognitive behavioral therapy for insomnia [CBT-I]) among this racial minority population.
Objective
To compare the efficacy of a standard version of an internet-delivered CBT-I program, a culturally tailored version, and a sleep education control at improving insomnia symptoms.
Design, Setting, and Participants
In this single-blind, 3-arm randomized clinical trial, participants in a national, longitudinal cohort (Black Women’s Health Study [BWHS]) were recruited between October 2019 and June 2020. BWHS participants with elevated insomnia symptoms were enrolled and randomized in the current study.
Interventions
Participants were randomized to receive (1) an automated internet-delivered treatment called Sleep Healthy Using the Internet (SHUTi); (2) a stakeholder-informed, tailored version of SHUTi for Black women (SHUTi-BWHS); or (3) patient education (PE) about sleep.
Main Outcomes and Measures
The primary outcome was insomnia severity (Insomnia Severity Index [ISI]). Index score ranged from 0 to 28 points, with those scoring less than 8 points considered to not have clinically significant insomnia symptoms and a score of 15 points or higher suggesting insomnia disorder. An ISI score reduction of more than 7 points was considered a clinically significant improvement in insomnia symptoms. The SHUTi-BWHS program was hypothesized to be more effective at significantly decreasing insomnia severity compared with the SHUTi program and PE.
Results
A total of 333 Black women were included in this trial, and their mean (SD) age was 59.5 (8.0) years. Those randomized to receive either SHUTi or SHUTi-BWHS reported significantly greater reductions in ISI score at 6-month follow-up (SHUTi: −10.0 points; 95% CI, −11.2 to −8.7; SHUTi-BWHS: −9.3 points; 95% CI, −10.4 to −8.2) than those randomized to receive PE (−3.6 points; 95% CI, −4.5 to −2.1) (P < .001). Significantly more participants randomized to SHUTi-BWHS completed the intervention compared with those randomized to SHUTi (86 of 110 [78.2%] vs 70 of 108 [64.8%]; P = .008). Participants who completed either intervention showed greater reductions in insomnia severity compared with noncompleters (−10.4 points [95% CI, −11.4 to −9.4] vs −6.2 points [95% CI, −8.6 to −3.7]).
Conclusions and Relevance
In this randomized clinical trial, both the SHUTi and SHUTi-BWHS programs decreased insomnia severity and improved sleep outcomes more than PE. The culturally tailored SHUTi-BWHS program was more effective at engaging participants with the program, as a greater proportion completed the full intervention. Program completion was associated with greater improvements in sleep.
Trial Registration
ClinicalTrials.gov Identifier: NCT03613519
Introduction
Insomnia is associated more frequently with psychiatric disorders than any other medical illness,1 contributes to an increased risk of cardiovascular disease,2 obesity,3 and home, motor vehicle, and workplace unintentional injuries,4 and results in substantially poorer quality of life.5 Women are up to 40% more likely to have insomnia disorder during their lifetime than men and are more likely to experience multiple insomnia symptoms.6,7 In one national survey, 67% of women reported at least a few nights of sleep difficulty in the past month.8
Poor sleep has also been shown to disproportionately affect Black individuals.9,10 For example, Black adults in the US have shorter total sleep duration, experience lighter and more fragmented sleep,10,11,12,13,14,15,16 and report worse sleep quality compared with other racial groups.14,17,18,19 Consistent with this literature, we demonstrated that insomnia is a major problem for participants in the Black Women’s Health Study (BWHS), a longitudinal cohort of Black women from across the US.20 Among 26 139 BWHS participants, approximately 45% had insomnia symptoms, and 15% had symptoms consistent with insomnia disorder.20
The American Academy of Sleep Medicine (AASM)21 and American College of Physicians22 recommend cognitive behavioral therapy for insomnia (CBT-I) as criterion-standard treatment for insomnia. CBT-I is a multicomponent intervention that addresses the maladaptive sleep behaviors and beliefs maintaining insomnia disorder, incorporating sleep restriction (limiting time in bed to reflect sleep duration), stimulus control (avoidance of nonsleep activities in bed), sleep hygiene (implementing positive sleep behaviors and improving social/environmental factors), cognitive therapy (reframing negative thoughts surrounding sleep and its daytime impact), and relaxation exercises.23 Because there are few CBT-I–trained clinicians and state licensure regulations often prohibit cross-border practice, treatment availability is a challenge.24,25 Internet-delivered CBT-I programs (eg, Sleep Healthy Using the Internet [SHUTi]) can increase access. The SHUTi program is an automated intervention that has been studied across multiple patient populations around the world, with consistent data demonstrating its effectiveness.26,27,28,29,30 While internet-delivered interventions offer better access to evidence-based care, patient adherence with automated programs that are designed to change health behaviors can be an issue.31,32 In a prior SHUTi trial, Black women were less likely than women of other races and ethnicities to initiate and continue with treatment. This difference is critical, as SHUTi participant engagement was associated with how much insomnia symptoms improved.33
The development of a culturally tailored intervention may be the key to better engaging racial and ethnic minority patients with proven insomnia treatment.34,35 Despite increasing awareness of the importance of the sociocultural context in understanding racial and ethnic sleep health disparities,36 to our knowledge, there has not previously been a cultural adaptation of CBT-I for any racial or ethnic minority group.37 This limitation is reflected in a recent American Academy of Sleep Medicine systematic review and meta-analysis, which concluded that racial and ethnic minority groups have been largely overlooked in CBT-I trials.38 Other meta-analyses do not discuss the effect of race and ethnicity on intervention efficacy,39,40 with participant data on race and ethnicity often not even reported.41 The failure to learn how to engage underserved racial and ethnic minority populations with CBT-I and establish the effectiveness of sleep treatments in these populations is a health inequity requiring attention.9,42
This report describes, to our knowledge, the first published trial of an insomnia program designed for Black women. We collaborated with a stakeholder group to develop a SHUTi adaptation including elements hypothesized to better engage Black women while retaining the core CBT-I components which make SHUTi effective. We then conducted a randomized clinical trial among BWHS participants comparing the efficacy of SHUTi, a version of SHUTi adapted specifically for Black women, and patient education (PE) about sleep. We hypothesized that the SHUTi-BWHS program would be more effective at decreasing insomnia symptom severity compared with the SHUTi program and compared with PE. We also evaluated the extent to which the tailored SHUTi-BWHS program affected user engagement.
Methods
Study Design
We conducted a single-blind (participants), parallel randomized clinical trial among Black women with insomnia disorder, using an equal allocation ratio. Participants were recruited from the BWHS, a national cohort study that enrolled 59 000 Black women from across the US in 1995.43
The study was approved by the Boston University Medical Campus Institutional Review Board as well as the institutional review boards of the participating institutions. Oral informed consent was obtained from all participants. The trial protocol can be found in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
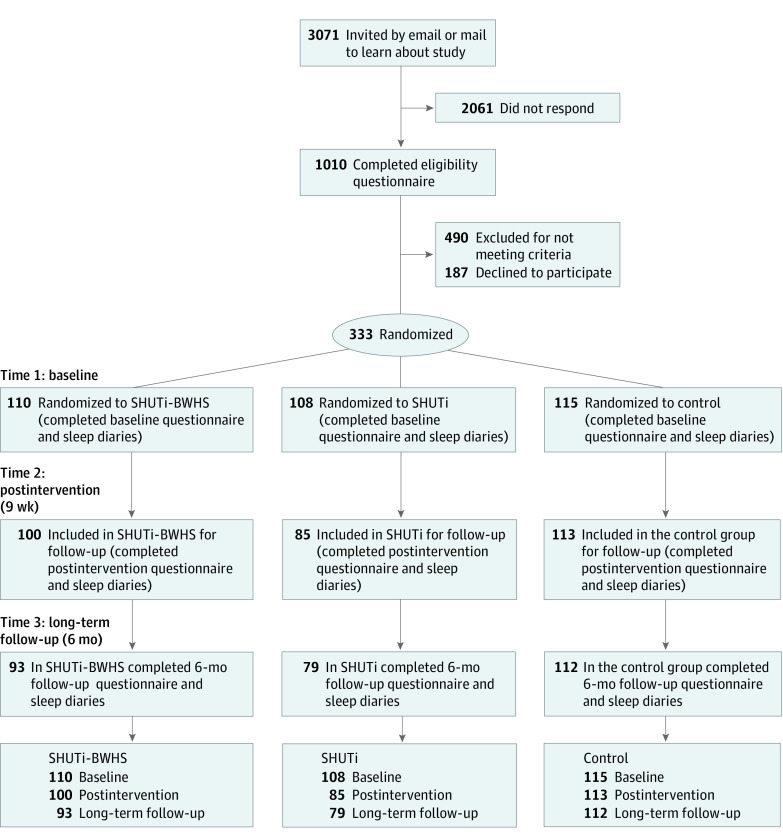
Between October 2019 and May 2020, invitation letters were sent to BWHS participants selected at random from those with an ISI score of 15 points or greater (reflecting clinical insomnia44) in the 2015 BWHS questionnaire and who had completed the most recent (2019) questionnaire. Of 3071 women to whom invitations were sent, 1010 completed an online eligibility questionnaire. Based on responses, 490 were excluded (Figure). As some women reported more than 1 reason, the total sums to more than the total number of women. Among those eligible, 333 women enrolled and were randomized. The randomized participants differed from the 677 who completed the eligibility questionnaire but were not enrolled on level of education (significantly more enrolled participants had completed postgraduate education [147 of 333 vs 213 of 667; P < .001]) but did not differ with respect to age or insomnia symptom severity. Participants were randomized individually using a table populated with numbers from 1 to 3 that was generated using the randomization feature of an SQL server. Enrollment occurred from October 2019 to June 2020, with all data collected by March 2021.
Figure. Study Enrollment Flow.
BWHS indicates Black Women’s Health Study; SHUTi, Sleep Healthy Using the Internet.
Study Interventions
The SHUTi program is a 6-session program (45 to 60 minutes per session) delivered over 6 to 9 weeks. SHUTi incorporates all core elements of CBT-I, tailoring content based on each participant’s reported baseline sleep function, treatment adherence, and sleep progress. The version of SHUTi used in this trial was evaluated among adults 55 years and older.45
The adapted version of SHUTi (SHUTi-BWHS) was developed over 1 year of collaboration with stakeholders, which included 3 Black women (a BWHS participant, a Black woman with a history of insomnia, and a Black woman with participatory research experience), a sleep physician from a medical center where more than 70% of the patient population are from racial and ethnic minority groups, a sleep researcher serving in a leadership role at a national health organization, and study investigators. In addition, 2 Black women who served on the project team were intimately involved in the adaptation process. All stakeholders were tasked to individually complete the standard SHUTi program with the goal of identifying content that could improve the engagement of Black women with the intervention. After completion of each session, interviewers conducted a semistructured interview with each stakeholder asking their views of the strengths and weaknesses of that session as it related to program engagement for a Black woman. Interviews were recorded and transcripts were analyzed and summarized by a doctoral-level qualitative researcher according to standard comprehensive, thematic qualitative analysis methods. A summary of the identified themes was then presented to the stakeholder group, followed by a discussion among group members about the adaptations that needed to be made. First, stakeholders advised the overhaul of all visual content to include only Black men and women. To address this, we replaced still photographs and filmed new videos with Black actors for the patient vignettes. In addition, we filmed videos of Black female sleep physicians who served as the sleep experts in the program content. Second, stakeholders recommended the introduction of didactic content that spoke to the cultural and social contexts in which insomnia occurs for Black women. Examples of didactic content modifications included a discussion of how to successfully implement stimulus control in a crowded living environment and ways to address neighborhood noise levels.
PE material about sleep tends to reflect usual care and served as the active control condition. The PE website focused on symptoms of insomnia, the impact, prevalence, and causes of insomnia, considerations on when to see a doctor, and basic lifestyle, environmental, and behavioral strategies to improve sleep; it has been used extensively in prior SHUTi trials.27
Study Measures
Study assessments were conducted at time 1 (baseline), time 2 (postintervention; approximately 9 weeks after time 1), and time 3 (long-term follow-up; approximately 6 months after time 2). Participants were compensated $25 for completion of questionnaires and diaries at time 1, $50 at time 2, and $75 at time 3.
Sleep-Related Outcomes
The primary study outcome was insomnia severity, assessed with the ISI,44 a 7-item questionnaire well validated in insomnia research in multiple patient populations, including via online administration and in Black women.46,47,48,49 Total scores range from 0 to 28 points, with those scoring less than 8 points considered to not have clinically significant insomnia symptoms and a score of 15 points or higher suggesting insomnia disorder. An ISI score reduction of more than 7 points was considered a clinically significant improvement in insomnia symptoms.50 Secondary sleep outcomes were collected via online sleep diaries, which include time taken to fall asleep (sleep onset latency), wake after sleep onset, total sleep, total time in bed, and sleep efficiency (total sleep divided by total time in bed, multiplied by 100). Additionally, the Pittsburgh Sleep Quality Index (PSQI),51 a 19-item survey assessing overall sleep quality, was a secondary sleep outcome. The PSQI has been validated extensively, including among Black women.52
Covariates
Variables used to describe the study sample (Table 1) were obtained from several sources. Age, marital status, employment status, bed partners, sleep apnea diagnosis, and alcohol consumption were collected at time 1. Highest educational level achieved was obtained from the 2003 BWHS questionnaire, with all other covariate data obtained from the 2015 BWHS questionnaire.
Table 1. Characteristics of Women Randomized in the Triala.
| Characteristic | No. (%) | ||||
|---|---|---|---|---|---|
| Total (N = 333) | Treatment group | ||||
| SHUTi-BWHS (n = 110) | SHUTi (n = 108) | PE (n = 115) | P valueb | ||
| Age, mean (SD), yc | 59.5 (8.0) | 59.6 (8.4) | 59.9 (8.0) | 59.2 (7.7) | .83 |
| Education leveld | .44 | ||||
| ≤12 y | 23 (6.9) | 6 (5.5) | 10 (9.3) | 7 (6.1) | |
| Some college | 73 (21.9) | 22 (20.0) | 26 (24.1) | 25 (21.7) | |
| College degree | 92 (27.6) | 28 (25.5) | 25 (23.2) | 39 (33.9) | |
| ≥Graduate degree | 145 (43.5) | 54 (49.1) | 47 (43.5) | 44 (38.3) | |
| Marital statuse | .55 | ||||
| Married or living as married | 128 (38.4) | 46 (41.8) | 42 (38.9) | 40 (34.8) | |
| Single, separated, divorced, or widowed | 205 (61.6) | 64 (58.2) | 66 (61.1) | 75 (65.2) | |
| Living arrangement | .38 | ||||
| Live alone | 125 (37.5) | 39 (35.4) | 37 (34.3) | 49 (42.6) | |
| Live with others | 208 (62.5) | 71 (64.6) | 71 (65.7) | 66 (57.4) | |
| Bed partner | .42 | ||||
| No bed partner | 216 (64.9) | 66 (60.0) | 72 (66.7) | 78 (67.8) | |
| Bed partner | 117 (35.1) | 44 (40.0) | 36 (33.3) | 37 (32.2) | |
| Geographic region | .29 | ||||
| Northeast | 62 (18.6) | 24 (18.6) | 23 (21.3) | 15 (13.0) | |
| South | 142 (42.6) | 44 (40.0) | 45 (41.7) | 53 (46.1) | |
| Midwest | 56 (16.8) | 14 (12.7) | 17 (15.7) | 25 (21.7) | |
| West | 73 (21.9) | 28 (25.5) | 23 (21.3) | 22 (19.1) | |
| Employment statuse | .55 | ||||
| Currently employed | 203 (61.0) | 63 (57.3) | 66 (61.1) | 74 (64.4) | |
| Not currently employed | 130 (39.0) | 47 (42.7) | 42 (38.9) | 41 (35.7) | |
| Parityf | .97 | ||||
| Nulliparous | 85 (25.5) | 29 (26.4) | 27 (25.0) | 29 (25.2) | |
| Parous | 248 (74.5) | 81 (73.6) | 81 (75.0) | 86 (74.8) | |
| Menopause status | .82 | ||||
| Premenopausal | 128 (38.4) | 43 (39.1) | 39 (36.1) | 46 (40.0) | |
| Postmenopausal | 205 (61.6) | 67 (60.9) | 69 (63.9) | 69 (60.0) | |
| Alcoholic drinks per week, mean (SD)e | 1.4 (2.2) | 1.1 (1.9) | 1.8 (2.6) | 1.2 (2.1) | .03 |
| Smoking history | .14 | ||||
| Current | 20 (6.0) | 5 (4.6) | 10 (9.3) | 5 (4.4) | |
| Past | 78 (23.4) | 32 (29.1) | 18 (16.7) | 28 (24.4) | |
| Never | 235 (70.6) | 73 (66.4) | 80 (74.1) | 82 (71.3) | |
| Body mass indexg | .21 | ||||
| <25 | 76 (22.8) | 33 (30.0) | 25 (23.2) | 18 (15.7) | |
| 25-29 | 84 (25.2) | 25 (22.7) | 28 (25.9) | 31 (27.0) | |
| ≥30 | 154 (46.2) | 44 (40.0) | 51 (47.2) | 59 (51.3) | |
| Unknown | 19 (5.7) | 8 (7.3) | 4 (3.7) | 7 (6.1) | |
| Hypertension history | .85 | ||||
| No | 190 (57.1) | 62 (56.4) | 64 (59.3) | 64 (55.7) | |
| Yes | 143 (42.9) | 48 (43.6) | 44 (40.7) | 51 (44.3) | |
| Type II diabetes | .85 | ||||
| No | 278 (83.5) | 90 (81.8) | 91 (84.3) | 97 (84.4) | |
| Yes | 55 (16.5) | 20 (18.2) | 17 (15.7) | 18 (15.7) | |
| Ever medication-treated depression | .06 | ||||
| No | 190 (57.1) | 53 (48.2) | 64 (59.3) | 73 (63.5) | |
| Yes | 143 (42.9) | 57 (51.8) | 44 (40.7) | 42 (36.5) | |
| Current medication-treated depression | .62 | ||||
| No | 306 (91.9) | 100 (90.9) | 98 (90.7) | 108 (93.9) | |
| Yes | 27 (8.1) | 10 (9.1) | 10 (9.3) | 7 (6.1) | |
| Sleep apnea (actively treated)e | .06 | ||||
| No | 316 (94.9) | 102 (92.7) | 107 (99.1) | 107 (93.0) | |
| Yes | 17 (5.1) | 8 (7.3) | 1 (0.9) | 8 (7.0) | |
Abbreviations: BWHS, Black Women’s Health Study; PE, patient education; SHUTi, Sleep Healthy Using the Internet.
Values are from the 2015 BWHS health questionnaire unless otherwise indicated.
P values of continuous variables calculated using analyis of variance and of categorical variables using χ2 test.
Age in 2020.
Value last collected from BWHS in 2003.
Value from eligibility questionnaire.
Value last collected from BWHS in 2013.
Calculated as weight in kilograms divided by height in meters squared.
Statistical Analysis
Study groups are described on baseline characteristics by means and SDs for continuous characteristics and counts and percentages for categorical characteristics. For ISI (primary study outcome), factorial 3 (groups) × 3 (times) mixed-effects linear model analyses compared improvement in the treatment groups to the usual care group and to one another. Use of a linear-mixed model analysis where we treated time as a categorical factor accounted for the within-participant correlation of ISI values over time through a random effect and allowed us to include available data from participants who did not complete assessments at the second or third time points. Differences in changes in ISI scores over time between intervention groups were represented through group × time interactions, and model contrasts were used to make specific pairwise comparisons between the 3 interventions. We corrected for multiple testing of pairwise comparisons across the 3 study groups by using the Hochberg sequentially acceptive step-up procedure.53,54 Analyses of secondary sleep outcomes paralleled the primary analyses and examined the percentage of participants who responded to treatment (a decrease of 8 or more points on the ISI from time 1 to time 3) or whose insomnia remitted (ISI score of 7 or less at time 3).50 Percentages were compared between the SHUTi-BWHS and SHUTi arms through χ2 tests. To examine the association between treatment completion and treatment effect, secondary analyses examined changes in sleep parameters from time 1 to time 3 for participants in the SHUTi-BWHS and SHUTi programs who completed all 6 sessions vs those who completed fewer through the 2-sample t test.
Primary analyses were based on all available data; however, some participants did not respond to the time 2 or time 3 assessments and the level of missing data varied across treatment group (Figure). Time 2 or time 3 assessment completers did not significantly differ from those who failed to complete that assessment on demographic (age, education), health (body mass index; calculated as weight in kilograms divided by height in meters squared), or disease-specific (baseline ISI score) variables. The 2 groups did differ on the number of intervention sessions completed, with assessment completers more likely to have finished more intervention sessions of either SHUTi-BWHS or SHUTi (mean [SD] of 5.4 [1.5] sessions) compared with noncompleters (mean [SD] of 2.2 [2.1] sessions). As this suggests that data may not be missing at random, we conducted sensitivity analyses conservatively assigning baseline values to the study’s primary outcome (ISI score) when participants were missing data. These analyses did not change the direction or significance of any of our findings.
Power calculations were conducted using Proc Power in SAS version 9.4 (SAS Institute) based on data collected from a prior SHUTi intervention trial that included a subset of Black women.27 This study showed a reduction in mean (SD) Insomnia Severity Index (ISI)44 scores from baseline to 6 months of 17.0 (4.0) points to 8.4 (5.7) points in the SHUTi group compared with 18.0 (4.0) points to 12.2 (5.6) points in a patient education (PE) group, corresponding to a Cohen d of 0.71 and a 32% difference in mean ISI score at 6 months. Powering our study at 80% to detect this moderate to large difference between any 2 of our study arms via a 2-samples t test of the mean change scores, at a pairwise P < .01 to account for multiple testing, a minimum analysis sample of 72 in each study group is needed. Conservatively estimating 28% study attrition based on previous SHUTi trials, a recruitment sample of 101 for each randomized group (303 overall) was targeted. The study was not powered to detect smaller differences between the SHUTi and SHUTi-BWHS intervention arms.
Results
A total of 333 Black women were included in this trial, and their mean (SD) age was 59.5 (8.0) years (Table 1). Most were employed, parous, and postmenopausal. A total of 154 participants (46.2%) were obese (body mass index of 30 or greater), 143 (42.9%) had hypertension, 55 (16.5%) had type 2 diabetes, and 17 (5.1%) reported sleep apnea that was actively treated. Some (143 [42.9%]) had received drug treatment for depression at some point in their lives.
Changes to Sleep During the Study Period
Significant improvements from time 1 to time 3 were seen in the ISI, PSQI, and sleep diary outcomes (sleep onset latency, wake after sleep onset, sleep efficiency) for participants receiving SHUTi-BWHS and SHUTi but not for participants receiving PE (Table 2; eFigure in Supplement 2). The declines from time 1 to time 3 for the ISI total score for SHUTi-BWHS, SHUTi, and PE were 9.3, 10.0, and 3.6, respectively, with a bigger decrease indicating greater reduction in insomnia severity. Sleep quality (PSQI) decreased from time 1 to time 3 by 4.8, 5.3, and 2.0, respectively, with lower scores indicating better sleep quality. Sleep efficiency improved from time 1 to time 3 by 11.4% among SHUTi-BWHS participants, 12.2% in SHUTi participants, and 4.3% in PE participants. The changes in ISI, PSQI, and sleep diary outcomes from time 1 to time 3 were significantly greater in each of the SHUTi groups than in the PE group, while the changes in the SHUTi-BWHS and SHUTi groups did not differ from each other (Table 2).
Table 2. Changes in Sleep Parameters Over Time.
| Measure | Mean sleep parameter (SE)a | Change from time 1 to time 2 | Change from time 1 to time 3 | Change from time 2 to time 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time 1 (baseline) | Time 2 (9 wk after time 1) | Time 3 (6 mo after time 2) | Change in scoreb | P valuec | P value vs PEd | P value vs SHUTie | Change in score | P valuec | P value vs PEd | P value vs SHUTie | Change in score | P valuec | P value vs PEd | P value vs SHUTie | |
| Insomnia Severity Index (P < .001)f | |||||||||||||||
| All participants | 18.8 (0.2) | 12.6 (0.3) | 11.1 (0.3) | −6.2 | <.001 | NA | NA | −7.6 | <.001 | NA | NA | −1.4 | <.001 | NA | NA |
| SHUTi-BWHS | 18.7 (0.4) | 11 (0.5) | 9.4 (0.6) | −7.8 | <.001 | <.001 | .48 | −9.3 | <.001 | <.001 | .44 | −1.5 | .002 | .85 | .85 |
| SHUTi | 18.3 (0.4) | 10 (0.6) | 8.4 (0.6) | −8.3 | <.001 | <.001 | NA | −10.0 | <.001 | <.001 | NA | −1.7 | .002 | .85 | NA |
| PE | 19.2 (0.3) | 16.7 (0.5) | 15.6 (0.5) | −2.5 | <.001 | NA | NA | −3.6 | <.001 | NA | NA | −1.1 | .01 | NA | NA |
| Sleep onset latency, min (P = .001) | |||||||||||||||
| All participants | 42.3 (1.6) | 27.5 (1.2) | 27.4 (1.3) | −14.8 | <.001 | NA | NA | −15.0 | <.001 | NA | NA | −0.2 | .89 | NA | NA |
| SHUTi-BWHS | 38.0 (2.7) | 21.9 (2.1) | 20.3 (2.2) | −16.1 | <.001 | .02 | .14 | −17.7 | <.001 | .01 | .54 | −1.6 | .47 | .66 | .66 |
| SHUTi | 47.5 (2.7) | 25.9 (2.3) | 27.3 (2.4) | −21.6 | <.001 | <.001 | NA | −20.2 | <.001 | .00 | NA | 1.4 | .57 | .66 | NA |
| PE | 41.6 (2.6) | 34.8 (2.0) | 34.5 (2.1) | −6.7 | .01 | NA | NA | −7.0 | .01 | NA | NA | −0.3 | .89 | NA | NA |
| Wake after sleep onset, min (P = .03) | |||||||||||||||
| All participants | 39.7 (1.8) | 26.4 (1.6) | 24.9 (1.7) | −13.3 | <.001 | NA | NA | −14.8 | <.001 | NA | NA | −1.6 | .25 | NA | NA |
| SHUTi-BWHS | 41.2 (3.2) | 25.4 (2.7) | 21.5 (2.9) | −15.8 | <.001 | .08 | .80 | −19.7 | <.001 | .01 | .64 | −3.9 | .10 | .70 | .70 |
| SHUTi | 37.4 (3.2) | 20.4 (2.9) | 19.8 (3.1) | −17.0 | <.001 | .07 | NA | −17.6 | <.001 | .04 | NA | −0.6 | .81 | .89 | NA |
| PE | 40.5 (3.1) | 33.5 (2.6) | 33.3 (2.7) | −7.1 | .02 | NA | NA | −7.2 | .01 | NA | NA | −0.2 | .94 | NA | NA |
| Total sleep time, h (P = .02) | |||||||||||||||
| All participants | 5.8 (0.1) | 6.1 (0.1) | 6.3 (0.1) | 0.3 | <.001 | NA | NA | 0.5 | <.001 | NA | NA | 0.2 | <.001 | NA | NA |
| SHUTi-BWHS | 6.0 (0.1) | 6.0 (0.1) | 6.3 (0.1) | 0.1 | .47 | .04 | .07 | 0.3 | .003 | .24 | .03 | 0.3 | .003 | .34 | .45 |
| SHUTi | 5.7 (0.1) | 6.1 (0.1) | 6.4 (0.1) | 0.4 | <.001 | .81 | NA | 0.8 | <.001 | .24 | NA | 0.4 | <.001 | .11 | NA |
| PE | 5.7 (0.1) | 6.2 (0.1) | 6.3 (0.1) | 0.4 | <.001 | NA | NA | 0.5 | <.001 | NA | NA | 0.1 | .24 | NA | NA |
| Time in bed, h (P < .001)f | |||||||||||||||
| All participants | 8.0 (0.1) | 7.5 (0.1) | 7.8 (0.1) | −0.5 | <.001 | NA | NA | −0.2 | <.001 | NA | NA | 0.3 | <.001 | NA | NA |
| SHUTi-BWHS | 8.2 (0.1) | 7.3 (0.1) | 7.5 (0.1) | −0.9 | <.001 | <.001 | .22 | −0.7 | <.001 | <.001 | .01 | 0.2 | .02 | .51 | .12 |
| SHUTi | 8.0 (0.1) | 7.3 (0.1) | 7.8 (0.1) | −0.7 | <.001 | <.001 | NA | −0.3 | .03 | .002 | NA | 0.5 | <.001 | .03 | NA |
| PE | 7.9 (0.1) | 8.0 (0.1) | 8.2 (0.1) | 0.1 | .20 | NA | NA | 0.3 | .01 | NA | NA | 0.1 | .12 | NA | NA |
| Sleep efficiency, % (P < .001) g | |||||||||||||||
| All participants | 72.2 (0.7) | 81.2 (0.6) | 81.5 (0.6) | 9.0 | <.001 | NA | NA | 9.3 | <.001 | NA | NA | 0.3 | .51 | NA | NA |
| SHUTi-BWHS | 73.1 (1.1) | 83.5 (1.0) | 84.5 (1.1) | 10.4 | <.001 | <.001 | .27 | 11.4 | <.001 | <.001 | .61 | 1.0 | .26 | .93 | .93 |
| SHUTi | 70.9 (1.1) | 83.1 (1.1) | 83.1 (1.1) | 12.2 | <.001 | <.001 | NA | 12.3 | <.001 | <.001 | NA | 0.0 | .97 | .98 | NA |
| PE | 72.7 (1.1) | 77.0 (1.0) | 77.0 (1.0) | 4.3 | <.001 | NA | NA | 4.3 | <.001 | NA | NA | 0.0 | .99 | NA | NA |
| Pittsburgh Sleep Quality Index (P < .001) | |||||||||||||||
| All participants | 12.7 (0.2) | 9.2 (0.2) | 8.7 (0.2) | −3.6 | <.001 | NA | NA | −4.0 | <.001 | NA | NA | −0.5 | .02 | NA | NA |
| SHUTi-BWHS | 12.3 (0.3) | 8.1 (0.3) | 7.5 (0.4) | −4.2 | <.001 | <.001 | .43 | −4.8 | <.001 | <.001 | .29 | −0.6 | .07 | .86 | .86 |
| SHUTi | 12.7 (0.3) | 8 .0 (0.4) | 7.5 (0.4) | −4.8 | <.001 | <.001 | NA | −5.3 | <.001 | <.001 | NA | −0.5 | .16 | .86 | NA |
| PE | 13.1 (0.3) | 11.4 (0.3) | 11.1 (0.4) | −1.7 | <.001 | NA | NA | −2.0 | <.001 | NA | NA | −0.3 | .35 | NA | NA |
Abbreviations: BWHS, Black Women’s Health Study; NA, not applicable; PE, patient education; SHUTi, Sleep Healthy Using the Internet.
Means presented are least squares means from mixed-effects linear regression model.
Estimated within-group change from mixed-effects linear regression model.
P value for within-group change.
P value for change between indicated group and PE group from mixed-effects linear regression model with Hochberg correction for multiple comparisons.
P value for change between indicated group and SHUTi group from mixed-effects linear regression model with Hochberg correction for multiple comparisons.
P value for comparing the 3 study groups on change over time, from the group × time interaction in mixed-effects linear regression model.
Calculated as total time spent asleep divided by total time spent in bed, multiplied by 100.
There were no statistically significant differences in the change in ISI scores from time 1 to time 3 between the SHUTi-BWHS and SHUTi participants (Table 3). Among participants receiving SHUTi-BWHS, 52 of 110 (47.3%) had more than a 7-point decrease in ISI score (treatment responder), and 41 of 110 (37.3%) reported an ISI score of less than 8 points by time 3 (insomnia fully resolved). Among SHUTi participants, 50 of 108 (46.3%) had an ISI decrease of more than 7 points, and 41 of 108 (38.0%) had an ISI score of less than 8 points by Time 3. Few SHUTi-BWHS and SHUTi participants (5 of 110 [4.5%] and 6 of 108 [5.6%], respectively) increased in ISI score during the study period.
Table 3. Treatment Response and Insomnia Remission After Time 3 (6-Month Assessment).
| Outcome | Treatment arm, No. (%) | P valuea | ||
|---|---|---|---|---|
| SHUTi-BWHS (n = 110) | SHUTi (n = 108) | PE (n = 115) | ||
| Treatment response (ISI score decreased by >7 points) | 52 (47.3) | 50 (46.3) | 17 (14.8) | .89 |
| Insomnia remitted (final ISI score <8) | 41 (37.3) | 41 (38.0) | 7 (6.1) | .92 |
Abbreviations: BWHS, Black Women’s Health Study; ISI, Insomnia Severity Index; PE, patient education; SHUTi, Sleep Healthy Using the Internet.
P value of difference in proportions between SHUTi-BWHS and SHUTi treatment arms.
Intervention Engagement
Significantly more participants randomized to the SHUTi-BWHS program completed the full intervention compared with those randomized to the SHUTi program. The completion rate for all 6 sessions among SHUTi-BWHS participants was 78.2% (86 of 110) compared with 64.8% (70 of 108) among SHUTi participants (P < .001). Full completers of either program had significantly greater improvements in their insomnia symptoms and in other sleep parameters than noncompleters (Table 4). Differences between time 1 and time 3 among completers vs noncompleters were ISI score (10.4-point decrease vs 6.2-point decrease, respectively), sleep onset latency (20.6-minute decrease vs 10.0-minute decrease), wake after sleep onset (21.4-minute decrease vs 3.5-minute decrease) sleep efficiency (13.3% increase vs 3.3% increase), and sleep quality (measured by PSQI; 5.7-point decrease vs 1.8-point decrease). We note that full completers of either program did not differ from noncompleters with respect to baseline insomnia severity.
Table 4. Changes in Sleep Parameters From Time 1 to Time 3 Comparing Participants Who Fully Completed Treatment in Either Intervention vs Those Who Did Not Fully Complete Treatment.
| Outcome | Treatment completion, mean (SD)a | P value | |
|---|---|---|---|
| Yes | No | ||
| Insomnia Severity Index | −10.39 (6.2) | −6.16 (6.0) | .002 |
| Sleep onset latency, min | −20.56 (28.4) | −10.04 (36.7) | .10 |
| Wake after sleep onset, min | −21.37 (33.8) | −3.54 (25.7) | .01 |
| Total sleep time, h | 0.56 (1.2) | 0.28 (1.2) | .26 |
| Time in bed, h | −0.60 (1.2) | 0.02 (0.8) | .01 |
| Sleep efficiency, %b | 13.31 (12.1) | 3.27 (9.5) | <.001 |
| Pittsburgh Sleep Quality Index | −5.66 (4.1) | −1.83 (4.2) | <.001 |
Treatment completion defined as completion of all 6 sessions of their assigned treatment by participants in Sleep Healthy Using the Internet or Sleep Healthy Using the Internet–Black Women’s Health Study.
Calculated as total time spent asleep divided by total time spent in bed, multiplied by 100.
Discussion
CBT-I is the criterion-standard treatment for insomnia disorder. As access to CBT-I is limited,24 automated CBT-I programs have been developed. The advantage of providing evidence-based insomnia treatment at scale is limited by adherence challenges,55 especially when patients are asked to make major behavioral changes, such as during CBT-I.56 Exacerbating this limitation, racial and ethnic minority populations are consistently less likely to initiate, continue, or complete behavioral health treatments.57 Little is known about why this is the case or what can been done to address this challenge, with some hypothesizing that it may be because psychosocial intervention content is typically developed for and tested primarily in non-Hispanic White men and women.58,59 Thus, we evaluated whether tailoring an automated CBT-I intervention for Black women would better engage participants. We compared a standard version of an internet-delivered CBT-I program (SHUTi) with a stakeholder-informed version tailored specifically for Black women (SHUTi-BWHS), assessing both treatment efficacy and program engagement.
Our findings indicate that both the SHUTi and SHUTi-BWHS programs significantly improved sleep health outcomes compared with PE about sleep; the improvements did not differ significantly between the SHUTi and SHUTi-BWHS programs. Comparable efficacy of the programs is not surprising, as core CBT-I principles were identical in both interventions. Our results add to SHUTi’s considerable body of evidence. At 6 months postintervention, insomnia improvements among intervention recipients were dramatic. Almost 40% of SHUTi or SHUTi-BWHS recipients experienced insomnia remission, and close to 50% reported clinically significant insomnia symptom improvements. Further, intervention participants saw their sleep efficiency increase by 10% to 12% through the 6-month follow-up. These improvements are comparable with those seen in prior SHUTi trials with samples that were comprised predominantly of non–racial and ethnic minority individuals26,27 and when CBT-I is delivered in-person.60 To put this degree of sleep efficiency improvement in context, a 10% improvement in sleep efficiency for women with breast cancer who were poor sleepers was associated with a 32% increase in survival.61
While it is important to demonstrate that SHUTi can improve the sleep of Black women, the significant differences in terms of treatment engagement are noteworthy: more Black women fully completed the version of the intervention that was tailored specifically for them. Similar to prior SHUTi trials, there was a direct association between the participant’s level of intervention engagement and their improvement in sleep.33 The differences observed in our trial were not merely statistically significant but clinically meaningful as well. These results showing that it is possible to adapt a proven intervention to significantly increase treatment engagement for a racial minority group are compelling. Improving participation with a treatment as challenging as CBT-I is no small feat as it requires people to overhaul their sleep patterns, behaviors, and thoughts. While there has been past research showing that culturally tailored mental health programming is associated with improved treatment outcomes,62 there have been limited efforts for patients with insomnia disorder.63,64 At the community level, the scale of public health improvement that is possible with a 13% increase in treatment engagement is immense, especially in a racial group that faces multiple barriers to treatment.65,66 As it appears that tailoring intervention content may be important to maximizing the impact of CBT-I, future research in how to successfully adapt this treatment approach in other racial and ethnic minority groups is needed.
Limitations
This study has limitations. While the study sample was comprised entirely of a racial minority group (Black women), they were of a higher socioeconomic background than US Black women as a whole. This can limit the generalizability of our findings to Black women for whom health disparities are most salient. On the other hand, the women lived in a wide range of settings across the country, encountering a variety of circumstances that might impair sleep.67 Medical mistrust exists for many marginalized groups, which can lead to poorer management of health conditions.68 This issue was not directly addressed in the present study and is deserving of future attention. Only women participated in our study. There are known sex differences, with women more likely to report insomnia to health care professionals, seek treatment, and implement intervention strategies.69,70 The study group was comprised of middle-aged and older women, which may limit the generalizability of study findings to other age groups. However, prior literature has demonstrated the efficacy of the SHUTi program and CBT-I across the life span. Study recruitment and data collection occurred before and during the COVID-19 pandemic. We did not systematically study this potential confounder. However, data suggests that treatment outcomes are likely to be similar for the pre– and post–COVID-19 group.71 Next, while our study was powered to detect moderate to large differences on the ISI as expected between a SHUTi intervention vs PE, it was not powered to detect smaller differences between 2 SHUTi intervention versions. Thus, our finding that participants given SHUTi and SHUTi-BWHS did not differ on the ISI should be taken with some caution. Additionally, this trial was conducted within an established cohort with a long history of engagement with members of the research team. This may explain why intervention completion rates even for the standard version of SHUTi were higher than among Black men and women in a recent trial of another online CBT-I program.72 Importantly, this further underscores the importance of working to connect with the community that programs are designed to reach.
Conclusions
Black individuals in the US are among the most likely to experience disparities in access to health care and, ultimately, poor health outcomes. There is a critical need to develop and evaluate evidence-based interventions to address these persistent racial gaps. The lack of culturally adapted interventions to improve sleep health among racial and ethnic minority populations persists, and to our knowledge, this is the first CBT-I intervention adapted for Black women. Results from the present trial provide intriguing data to encourage further studies in the effort to address health disparities in the context of insomnia disorder.
Trial protocol.
eFigure. Participant changes in insomnia severity and sleep efficiency across study time points.
Data sharing statement.
References
- 1.Benca RM. Consequences of insomnia and its therapies. J Clin Psychiatry. 2001;62(suppl 10):33-38. [PubMed] [Google Scholar]
- 2.Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435-444. doi: 10.1016/j.chest.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan WS, Levsen MP, McCrae CS. A meta-analysis of associations between obesity and insomnia diagnosis and symptoms. Sleep Med Rev. 2018;40:170-182. doi: 10.1016/j.smrv.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 4.Léger D, Bayon V, Ohayon MM, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143-152. doi: 10.1111/jsr.12104 [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, Wall M, Liu SM, Morin CM, Blanco C. Insomnia and impaired quality of life in the United States. J Clin Psychiatry. 2018;79(5):17m12020. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85-93. doi: 10.1093/sleep/29.1.85 [DOI] [PubMed] [Google Scholar]
- 7.Jaussent I, Dauvilliers Y, Ancelin ML, et al. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19(1):88-97. doi: 10.1097/JGP.0b013e3181e049b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasens ER, Twerski SR, Yang K, Umlauf MG. Sleepiness and health in midlife women: results of the National Sleep Foundation’s 2007 Sleep in America poll. Behav Sleep Med. 2010;8(3):157-171. doi: 10.1080/15402002.2010.487462 [DOI] [PubMed] [Google Scholar]
- 9.Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behav Sleep Med. 2006;4(1):29-44. doi: 10.1207/s15402010bsm0401_3 [DOI] [PubMed] [Google Scholar]
- 10.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: a meta-analysis. Sleep Med. 2011;12(3):209-214. doi: 10.1016/j.sleep.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Williams NJ, Grandner MA, Wallace DM, et al. Social and behavioral predictors of insufficient sleep among African Americans and Caucasians. Sleep Med. 2016;18:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: role of racial/ethnic differences. Sleep Med Rev. 2013;17(4):255-262. doi: 10.1016/j.smrv.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol. 2002;14(3):321-326. doi: 10.1002/ajhb.10032 [DOI] [PubMed] [Google Scholar]
- 14.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70(4):410-416. doi: 10.1097/PSY.0b013e31816fdf21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurina LM, Thisted RA, Chen J-H, McClintock MK, Waite LJ, Lauderdale DS. Actigraphic sleep characteristics among older Americans. Sleep Health. 2015;1(4):285-292. doi: 10.1016/j.sleh.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biol Psychiatry. 2000;47(10):921-927. doi: 10.1016/S0006-3223(99)00169-9 [DOI] [PubMed] [Google Scholar]
- 17.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73-82. [PMC free article] [PubMed] [Google Scholar]
- 18.Karacan I, Thornby JI, Anch M, et al. Prevalence of sleep disturbance in a primarily urban Florida County. Soc Sci Med. 1976;10(5):239-244. doi: 10.1016/0037-7856(76)90006-8 [DOI] [PubMed] [Google Scholar]
- 19.Pigeon WR, Heffner K, Duberstein P, Fiscella K, Moynihan J, Chapman BP. Elevated sleep disturbance among blacks in an urban family medicine practice. J Am Board Fam Med. 2011;24(2):161-168. doi: 10.3122/jabfm.2011.02.100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethea TN, Zhou ES, Schernhammer ES, Castro-Webb N, Cozier YC, Rosenberg L. Perceived racial discrimination and risk of insomnia among middle-aged and elderly Black women. Sleep. 2020;43(1):zsz208. doi: 10.1093/sleep/zsz208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262. doi: 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133. doi: 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 23.Morin CM. Cognitive-behavioral therapy of insomnia. Sleep Med Clin. 2006;1(3):375-386. doi: 10.1016/j.jsmc.2006.06.008 [DOI] [Google Scholar]
- 24.Thomas A, Grandner M, Nowakowski S, Nesom G, Corbitt C, Perlis ML. Where are the behavioral sleep medicine providers and where are they needed? a geographic assessment. Behav Sleep Med. 2016;14(6):687-698. doi: 10.1080/15402002.2016.1173551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb C, Orwig J. Expanding our reach: telehealth and licensure implications for psychologists. J Clin Psychol Med Settings. 2015;22(4):243-250. doi: 10.1007/s10880-015-9440-9 [DOI] [PubMed] [Google Scholar]
- 26.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66(7):692-698. doi: 10.1001/archgenpsychiatry.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinical trial. JAMA Psychiatry. 2017;74(1):68-75. doi: 10.1001/jamapsychiatry.2016.3249 [DOI] [PubMed] [Google Scholar]
- 28.Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333-341. doi: 10.1016/S2215-0366(15)00536-2 [DOI] [PubMed] [Google Scholar]
- 29.Zhou ES, Recklitis CJ. Internet-delivered insomnia intervention improves sleep and quality of life for adolescent and young adult cancer survivors. Pediatr Blood Cancer. 2020;67(9):e28506. doi: 10.1002/pbc.28506 [DOI] [PubMed] [Google Scholar]
- 30.Hagatun S, Vedaa Ø, Nordgreen T, et al. The short-term efficacy of an unguided internet-based cognitive-behavioral therapy for insomnia: a randomized controlled trial with a six-month nonrandomized follow-up. Behav Sleep Med. 2019;17(2):137-155. doi: 10.1080/15402002.2017.1301941 [DOI] [PubMed] [Google Scholar]
- 31.Vigerland S, Lenhard F, Bonnert M, et al. Internet-delivered cognitive behavior therapy for children and adolescents: a systematic review and meta-analysis. Clin Psychol Rev. 2016;50:1-10. doi: 10.1016/j.cpr.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 32.Josephine K, Josefine L, Philipp D, David E, Harald B. Internet- and mobile-based depression interventions for people with diagnosed depression: a systematic review and meta-analysis. J Affect Disord. 2017;223:28-40. doi: 10.1016/j.jad.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 33.Batterham PJ, Christensen H, Mackinnon AJ, et al. Trajectories of change and long-term outcomes in a randomised controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2017;3(5):228-235. doi: 10.1192/bjpo.bp.117.005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrera M Jr, Castro FG, Strycker LA, Toobert DJ. Cultural adaptations of behavioral health interventions: a progress report. J Consult Clin Psychol. 2013;81(2):196-205. doi: 10.1037/a0027085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jean-Louis G, Grandner M. Importance of recognizing sleep health disparities and implementing innovative interventions to reduce these disparities. Sleep Med. 2016;18:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams NJ, Grandne MA, Snipes A, et al. Racial/ethnic disparities in sleep health and health care: importance of the sociocultural context. Sleep Health. 2015;1(1):28-35. doi: 10.1016/j.sleh.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcántara C, Giorgio Cosenzo L, McCullough E, Vogt T, Falzon AL, Perez Ibarra I. Cultural adaptations of psychological interventions for prevalent sleep disorders and sleep disturbances: a systematic review of randomized controlled trials in the United States. Sleep Med Rev. 2021;56:101455. doi: 10.1016/j.smrv.2021.101455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(2):263-298. doi: 10.5664/jcsm.8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204. doi: 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- 40.Ho FY, Chung KF, Yeung WF, et al. Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;19:17-28. doi: 10.1016/j.smrv.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 41.Polo AJ, Makol BA, Castro AS, Colón-Quintana N, Wagstaff AE, Guo S. Diversity in randomized clinical trials of depression: a 36-year review. Clin Psychol Rev. 2019;67:22-35. doi: 10.1016/j.cpr.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 42.Airhihenbuwa CO, Iwelunmor JI, Ezepue CJ, Williams NJ, Jean-Louis G. I sleep, because we sleep: a synthesis on the role of culture in sleep behavior research. Sleep Med. 2016;18:67-73. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972). 1995;50(2):56-58. [PubMed] [Google Scholar]
- 44.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 45.Sleep Healthy Using The Internet for Older Adult Sufferers of Insomnia and Sleeplessness (SHUTiOASIS). ClinicalTrials.gov identifier: NCT03213132. Updated April 29, 2021. Accessed January 16, 2022. https://clinicaltrials.gov/ct2/show/NCT03213132
- 46.Gagnon C, Bélanger L, Ivers H, Morin CM. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26(6):701-710. doi: 10.3122/jabfm.2013.06.130064 [DOI] [PubMed] [Google Scholar]
- 47.Michaud AL, Zhou ES, Chang G, Recklitis CJ. Validation of the Insomnia Severity Index (ISI) for identifying insomnia in young adult cancer survivors: comparison with a Structured Clinical Diagnostic Interview of the DSM-5 (SCID-5). Sleep Med. 2021;81:80-85. doi: 10.1016/j.sleep.2021.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorndike FP, Ritterband LM, Saylor DK, Magee JC, Gonder-Frederick LA, Morin CM. Validation of the Insomnia Severity Index as a web-based measure. Behav Sleep Med. 2011;9(4):216-223. doi: 10.1080/15402002.2011.606766 [DOI] [PubMed] [Google Scholar]
- 49.Yusufov M, Recklitis C, Zhou ES, Bethea TN, Rosenberg L. A population-based psychometric analysis of the Insomnia Severity Index in Black women with and without a history of cancer. J Sleep Res. 2022;31(1):e13421. doi: 10.1111/jsr.13421 [DOI] [PubMed] [Google Scholar]
- 50.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601-608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 52.Beaudreau SA, Spira AP, Stewart A, et al. ; Study of Osteoporotic Fractures . Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older Black and White women. Sleep Med. 2012;13(1):36-42. doi: 10.1016/j.sleep.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800-802. doi: 10.1093/biomet/75.4.800 [DOI] [Google Scholar]
- 54.Keselman H, Lix LM. Improved repeated measures stepwise multiple comparison procedures. J Educ Behav Stat. 1995;20(1):83-99. doi: 10.3102/10769986020001083 [DOI] [Google Scholar]
- 55.Christensen H, Griffiths KM, Farrer L. Adherence in internet interventions for anxiety and depression. J Med Internet Res. 2009;11(2):e13. doi: 10.2196/jmir.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horsch C, Lancee J, Beun RJ, Neerincx MA, Brinkman WP. Adherence to technology-mediated insomnia treatment: a meta-analysis, interviews, and focus groups. J Med Internet Res. 2015;17(9):e214. doi: 10.2196/jmir.4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alegria M, Falgas-Bague I, Fong HF. Engagement of ethnic minorities in mental health care. World Psychiatry. 2020;19(1):35-36. doi: 10.1002/wps.20695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miranda J, Bernal G, Lau A, Kohn L, Hwang WC, LaFromboise T. State of the science on psychosocial interventions for ethnic minorities. Annu Rev Clin Psychol. 2005;1:113-142. doi: 10.1146/annurev.clinpsy.1.102803.143822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maura J, Weisman de Mamani A. Mental health disparities, treatment engagement, and attrition among racial/ethnic minorities with severe mental illness: a review. J Clin Psychol Med Settings. 2017;24(3-4):187-210. doi: 10.1007/s10880-017-9510-2 [DOI] [PubMed] [Google Scholar]
- 60.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204. doi: 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- 61.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837-842. doi: 10.5665/sleep.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huey SJ Jr, Tilley JL. Effects of mental health interventions with Asian Americans: a review and meta-analysis. J Consult Clin Psychol. 2018;86(11):915-930. doi: 10.1037/ccp0000346 [DOI] [PubMed] [Google Scholar]
- 63.Spadola CE, Rottapel RE, Zhou ES, et al. A sleep hygiene and yoga intervention conducted in affordable housing communities: pilot study results and lessons for a future trial. Complement Ther Clin Pract. 2020;39:101121. doi: 10.1016/j.ctcp.2020.101121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rottapel RE, Zhou ES, Spadola CE, et al. Adapting sleep hygiene for community interventions: a qualitative investigation of sleep hygiene behaviors among racially/ethnically diverse, low-income adults. Sleep Health. 2020;6(2):205-213. doi: 10.1016/j.sleh.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie Z, St Clair P, Goldman DP, Joyce G. Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS One. 2019;14(2):e0212117. doi: 10.1371/journal.pone.0212117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy EJ, Kassem L, Chemerinski A, Rush AJ, Laje G, McMahon FJ. Retention and attrition among African Americans in the STAR*D study: what causes research volunteers to stay or stray? Depress Anxiety. 2013;30(11):1137-1144. doi: 10.1002/da.22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cozier YC, Palmer JR, Horton NJ, Fredman L, Wise LA, Rosenberg L. Relation between neighborhood median housing value and hypertension risk among Black women in the United States. Am J Public Health. 2007;97(4):718-724. doi: 10.2105/AJPH.2005.074740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaiswal J, Halkitis PN. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav Med. 2019;45(2):79-85. doi: 10.1080/08964289.2019.1619511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Zhang J, Lam SP, et al. Help-seeking behaviors for insomnia in Hong Kong Chinese: a community-based study. Sleep Med. 2016;21:106-113. doi: 10.1016/j.sleep.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 70.Sidani S, Guruge S, Fox M, Collins L. Gender differences in perpetuating factors, experience and management of chronic insomnia. J Gend Stud. 2019;28(4):402-413. doi: 10.1080/09589236.2018.1491394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahoney A, Li I, Grierson A, Millard M, Haskelberg H, Mason E. Internet-based cognitive behaviour therapy for insomnia before and during the COVID-19 pandemic. Aust Psychol. 2021;57(1):65-76. doi: 10.1080/00050067.2021.1979884 [DOI] [Google Scholar]
- 72.Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49(3):491-500. doi: 10.1017/S0033291718001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eFigure. Participant changes in insomnia severity and sleep efficiency across study time points.
Data sharing statement.