Abstract
For a century, since the pioneering work of Otto Warburg, the interwoven relationship between metabolism and cancer has been appreciated. More recently, with obesity rates rising in the U.S. and worldwide, epidemiologic evidence has supported a link between obesity and cancer. A substantial body of work seeks to mechanistically unpack the association between obesity, altered metabolism, and cancer. Without question, these relationships are multifactorial and cannot be distilled to a single obesity- and metabolism-altering hormone, substrate, or factor. However, it is important to understand the hormone-specific associations between metabolism and cancer. Here, we review the links between obesity, metabolic dysregulation, insulin, and cancer, with an emphasis on current investigational metabolic adjuncts to standard-of-care cancer treatment.
Keywords: cancer metabolism, diabetes, immunometabolism
Introduction: obesity and cancer epidemiology
Currently, the Centers for Disease Control have identified thirteen tumor types of which overweight and obesity increase risk [1–27] (Table 1). In most of these, excess weight also worsens prognosis. It should be noted that there may be a sampling bias in the link between obesity and cancer, though it is complex: a meta-analysis by Fagan et al. [28] demonstrated that obesity was associated with a decreased likelihood of screening for cervical and colorectal cancer, an increased likelihood of screening for prostate cancer, and no difference in rates of screening for breast cancer. Interestingly, there are other tumor types in which overweight and/or obesity may confer an improved response to treatment: in both lung cancer [29–30] and melanoma [31–33], as well as in pooled analyses of patients with any tumor treated with immune checkpoint inhibitors [34,35], outcomes were improved in overweight/obese subjects. There appear to be opposing continua of both tumor immunogenicity and association with obesity: in general, more immunogenic tumors appear to be less positively associated with overweight and obesity, and vice versa. In this review, we concentrate on tumors positively correlated with obesity, and the role of insulin in driving tumor progression, while recognizing that no monolith regarding the relationship between metabolism and cancer exists.
Table 1. Cancers associated with obesity in humans (adapted from [1]).
| Postmenopausal breast |
| Colorectal |
| Endometrial/uterine |
| Esophageal adenocarcinoma |
| Gallbladder |
| Gastric |
| Hepatocellular |
| Meningioma |
| Multiple myeloma |
| Ovarian |
| Pancreatic |
| Renal |
| Thyroid |
Obesity's link to insulin resistance and hyperinsulinemia
Obesity linked to high insulin
A critical consequence of excess adiposity is insulin resistance, which has been thoroughly reviewed elsewhere [36–38]. There exists a wide spectrum of insulin resistance, where an insulin sensitive individual will have low basal and postprandial insulin concentrations, an insulin resistant individual will have hyperinsulinemia in both settings, and an individual with overt type 2 diabetes, whose pancreatic beta cells cannot properly secrete insulin in response to elevated glucose, presents with hyperglycemia without hyperinsulinemia [39]. A mildly insulin resistant individual will have obesity with or without hyperglycemia, but elevated insulin concentrations in the basal and postprandial state. Therefore, insulin resistant individuals have a decreased capacity to store plasma glucose as muscle and liver glycogen and suppress hepatic gluconeogenesis in response to insulin, commonly resulting in simultaneous hyperinsulinemia and hyperglycemia [38].
The molecular mechanism of insulin resistance, and thus elevated insulin levels, is downstream of the insulin receptor (IR) in at least muscle, liver, and adipose tissue, and may be the result of the accumulation of ectopic lipids, including ceramides and/or diacylglycerols, in these tissues. Several alternative mechanisms for the induction of insulin resistance in obesity are also supported by the literature. Elevated non-esterified fatty acids, branch-chained amino acids, and glucose have all been reported in the setting of obesity, each of which can contribute to nutrient-induced insulin resistance through various purported mechanisms [36]. Systemic and tissue-specific inflammation has also been implicated as a mechanistic link between obesity and insulin resistance, with interleukin-6 and c-Jun N-terminal kinase (JNK) signaling as key mediators [40–42]. Thus, it has become apparent that both the local and systemic environment can play substantial roles in the context of insulin resistance.
Nuanced definition of obesity (body weight versus adiposity)
Though obesity is often defined epidemiologically by excess weight (typically BMI > 30 kg/m2), there are shortfalls to this approach in metabolic disease and cancer. BMI appears to relatively underestimate body fat percentage in certain populations [43,44], and overestimate in others [45]. Nonetheless, at large scale, risk of morbidity and mortality has been well described with BMI representing a proxy for excess adiposity. However, other surrogates for adiposity, such as waist circumference, waist-hip ratio [46], skin-fold measurements, medical imaging such as dual-energy x-ray absorptiometry (DXA) [47], computed tomography (CT) [48,49], or magnetic resonance imaging (MRI) provide much more accurate measures of body fat. In addition, medical imaging allows for the distinction between visceral and subcutaneous adiposity. Though visceral fat is only ∼5% and 3% of total adipose tissue for men and women, respectively [50], it confers a greater deleterious consequences for metabolic disease and cancer than excess weight alone [51]. In addition, visceral fat content is one of the strongest independent predictors of insulin resistance and hyperinsulinemia [52].
Few mechanisms have been explored in humans that interrogate how visceral adiposity modulates tumor biology [51,53,54]. As alluded to earlier in this review, obesity appears to be protective for survival in lung cancer. Recent work from our group tested the hypothesis that obesity as defined by BMI would uncover different immunometabolic characteristics of tumors than using visceral adiposity as a readout of metabolic health [48]. We demonstrated that when tumor gene expression analyses were performed on high versus low BMI patients, there were more differentially expressed genes with beneficial prognosis, including CBX6, TOX3, and TMPRSS2 in patients with high BMI, consistent with BMI having a protective effect. However, high visceral adiposity versus low visceral adiposity analyses demonstrated an opposite effect on prognosis: expression of detrimentally prognostic genes (as determined from the PRECOG database [55]) including KRT6A, FEM1B, and S100A2, reveal that visceral adiposity, the more deleterious component of excess body mass, is associated with vastly different transcriptional profiles within the tumors. The mechanistic links of visceral adiposity to these transcriptomic profiles remain to be uncovered. In addition to altered transcriptomics between BMI and visceral adiposity comparisons, increased glucose uptake within lung tumors was positively correlated with visceral fat content, but not BMI, providing support for more nuanced relationships between body composition, metabolism, and prognosis than simply relying upon BMI. Other research has implicated adipose-derived inflammatory mediators (including IL-6, and IL-1β, but not MCP1) [53], altered amino acid metabolism (including serine/glycine and tryptophan metabolism) [56], or reactive oxygen species [57] as potential mediators of the link between body composition and cancer progression. These findings among others have led some to interrogate the concept of metabolically healthy obesity, and metabolically unhealthy leanness in metabolic disease and cancer [58–61].
Nuanced definition of obesity (metabolically healthy obesity)
Though there is no clear definition of metabolically healthy obesity, the concept is that a person with a BMI greater than 30 kg/m2 can have normal blood glucose, triglycerides, cholesterol, and blood pressure, and that these individuals, though obese, may not have elevated risk for disease [62]. However, large epidemiological studies have shown that metabolically healthy obese individuals have greater all-cause mortality than metabolically healthy lean individuals [63], and that metabolically healthy obese individuals have greater odds of developing cancer than metabolically healthy lean individuals [64]. Conversely, there exist individuals with a BMI between 18.5 and 25 kg/m2 who nevertheless exhibit elevated cardiometabolic risk factors with decreased skeletal muscle mass and elevated visceral fat mass, referred to as metabolically unhealthy normal-weight individuals [65], who have higher risk for diabetes [66], a three-folder high risk of all-cause mortality/cardiovascular disease [67], and an increased risk of cancer [58].
Nuanced definition of obesity (sarcopenic obesity and cachexia)
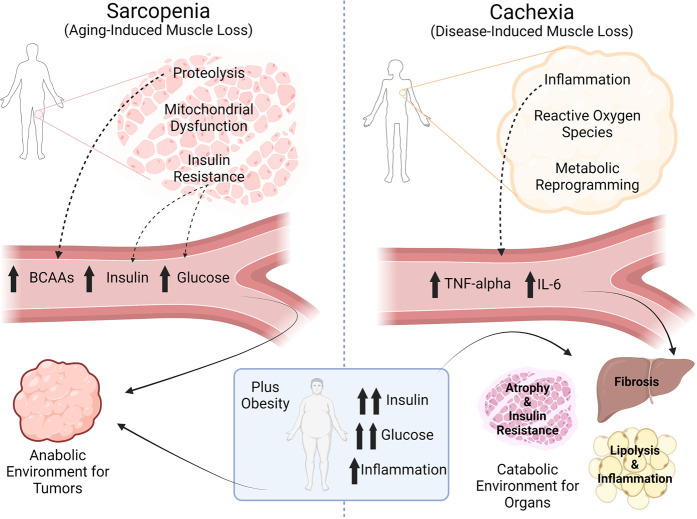
Sarcopenic (loss of muscle mass, often associated with ageing) obesity is the concept that an individual can be meet clinical definitions of obesity (BMI > 30 kg/m2) and simultaneously exhibit skeletal muscle wasting [68,69]. Sarcopenic skeletal muscles are known to be insulin resistant even in the setting of low whole-body fat stores [70], and considering that skeletal muscle is a primary site of both insulin action and glucose uptake/storage [71–75], sarcopenia contributes significantly to systemic metabolic syndrome [76]. Numerous studies have shown detrimental epidemiological consequences of sarcopenic obesity on cancer incidence, progression, and survival, with the largest influence on cancer incidence [53,54,69,77–81]. Likely mechanisms of sarcopenia-associated insulin resistance include reduced mitochondrial function (i.e. the ability to oxidize metabolites) [82,83], reduced skeletal muscle mass and thus reduced skeletal muscle glucose disposal [53,84], as well as protein wasting that involves the release of deleterious metabolites [85] (Figure 1). For example, elevated branched-chain amino acid (BCAA) concentrations are an independent predictor of type 2 diabetes risk and incidence [86–89], and considering that BCAAs are essential amino acids that cannot be synthesized de novo, BCAA concentrations in plasma must reflect either dietary intake and/or an imbalance between skeletal muscle protein anabolism and skeletal muscle protein catabolism. Sarcopenia tips the balance towards the net release of BCAAs into the plasma, and when combined with obesity-associated hyperinsulinemia, could provide a tumor-promoting hormonal and metabolic milieu in the plasma. It should be mentioned that cancer cachexia, in the presence or absence of obesity, likely shares similar metabolic derangements induced by sarcopenia [53,90].
Figure 1.
Sarcopenia creates an anabolic environment for tumors, while cancer cachexia creates a catabolic environment for organs. Made in BioRender.com.
The definition of cachexia differs from sarcopenia based on the underlying cause. Cachexia is wasting of lean mass due to underlying illness, while sarcopenia is lean mass wasting often associated with natural ageing [90]. Cancer-associated cachexia illustrates another concept: cancer per se may cause systemic metabolic perturbations in skeletal muscle and other tissues. Tumor-derived inflammatory mediators, including IL-6 [91–93] and TNFα [94–98] have causal roles in tissue-specific insulin resistance, including liver, adipose tissue, and skeletal muscle. In addition, once a tumor grows to a certain size, it is likely that it can compete for nutrients to a similar degree compared with that of other organs: 18F-FDG PET/CT data comparing tissue-specific glucose uptake shows that maximal glucose uptake capacity in breast, head and neck squamous cell, soft tissue sarcoma, and non-small cell lung tumors is 3–10 times greater than other organs including skeletal muscle, adipose tissue, and spleen [99]. Thus, in considering the tumor as another fractional contributor to the consumption of circulating metabolites, it is clear that relative sizes of each compartment (vital organs, muscle, adipose tissue, and tumors) can drive relatively large changes in systemic nutrient partitioning.
In sum, there are manifold mechanisms related to excess adiposity, location of adipose tissue, and metabolic derangements independent of body mass that confer risk for metabolic disease, cancer, and all-cause mortality.
Canonical insulin signaling
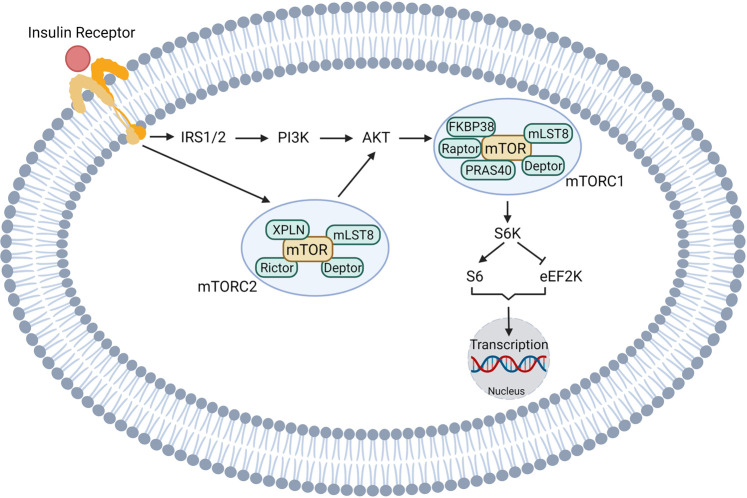
Insulin is secreted by beta cells in the pancreatic islet and is recognized by IRs, which are expressed in all cell types in the body, including tumor cells. Canonical insulin signaling pathways in tumor cells are depicted in Figure 2. In support of a critical role for insulin in cancer progression, high IR expression is a poor prognostic factor in lung cancer [100], breast cancer [101], and colon cancer [102]. After ligand binding, IR activates its tyrosine kinase and initiates downstream signaling including the PI3K–AKT [103–105], mTOR [106–108], and RAS–MAPK pathways [105,109,110]. Insulin receptor substrates (IRSs), which are the adaptor proteins of the IR, recruit multiple signaling complexes [111–114]. In particular, growth factor receptor-bound protein 2 (Grb2) is recruited to the binding motif on IRS [115,116], which in turn forms a complex with guanine nucleotide exchange factor Son of Sevenless and phosphorylates RAS. Activated RAS (RAS-GTP) activates the mitogen-activated protein kinase (MAPK) signaling cascade, including extracellular signal-regulated kinase 1/2 (ERK1/2) [117,118]. Rac1, a member of the superfamily of small guanosine triphosphatases [119], is another important signaling pathway downstream of the IR. Rac1 functions as a key regulator of insulin-induced glucose uptake [120,121] and glucose-induced insulin secretion [122]. More importantly, up-regulation of Rac1 is closely related to tumor development in multiple cancer types by promoting cell proliferation and migration as well as angiogenesis [119,123–126]. Taken together, there is no doubt that these kinases promote gene expression in pathways related to cell survival and proliferation [127].
Figure 2. Insulin signaling promotes cell division in tumors. Made in BioRender.com.
Insulin can also bind to the insulin-like growth factor 1 (IGF1) receptor [116,128,129], which consequently activates the mitogenic signaling pathways that promotes cellular growth and proliferation. Although there is significant redundancy in the intracellular insulin and IGF signaling pathways, some studies imply that they may have distinct roles in malignancies. For instance, Gallagher et al. [130] showed in vivo that IR phosphorylation, but not IGF-IR or hybrid receptor phosphorylation, promotes mammary tumor growth in mice with skeletal muscle insulin resistance. These authors also showed that AspB10, an insulin analog that binds specifically to the IR, has a similar effect to increase tumor growth independently of IGF signaling.
However, the IGF signaling pathway also supports the formation and maintenance of cancer stem cells [131–133], which play an important role in the epithelial-to-mesenchymal transition [134,135] and consequent tumor metastasis in both liver cancer [135] and leukemia [136]. Recently, Shahbazi et al. [137] demonstrated that insulin acts as a key stimulator of the mRNA transcriptome, seeding, proliferation, and phosphorylation in human induced pluripotent stem cells (hiPSC). These data highlight a new mechanism by which insulin may promote tumor progression by inducing and enhancing cancer stem cells, leading to tumor growth and metastasis.
Insulin and tumor cell energetics
Compared with healthy cells, tumor cells have tremendous energy requirements to support proliferation and invasion. Therefore, tumor cells tend to modify their metabolic pattern, exemplified by the transition of the primary glucose utilization pathway from oxidative phosphorylation to glycolysis, i.e. the Warburg effect [138–140]. This metabolic shift not only allows tumor cells to convert nutrients into energy in an oxygen-deprived microenvironment, but also provides building blocks for biosynthesis and cellular proliferation. For instance, glucose-6-phosphate (a glycolytic intermediate) will enter the pentose phosphate pathway to generate ribulose-5-phosphate, a precursor used for DNA and NADPH generation, as well as lipid synthesis [141]. Besides the proliferative and survival effect described previously, insulin also controls whole-body as well as intracellular metabolism by substrate (glucose) partitioning [142]. Aberrant PI3K–mTOR signaling is common in tumor cells. For instance, a hyperactivated mutation in eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1), a downstream effector of mTOR that forms its transcription complex, is commonly observed in the head and neck squamous cell carcinomas [143]. mTOR also alters glucose availability in tumor cells by regulating glucose uptake [144] and glycogenolysis [107]. Glycogen synthase kinase (GSK)-3 can inhibit mTOR signaling via phosphorylation of tuberous sclerosis complex subunit 2 (TSC2). Buller and colleagues showed that impeding activity of the tumor suppressor gene TSC2 resulted in a substantial increase in GLUT1-mediated glucose uptake. This phenotype depended on mTOR activity, suggesting a role for mTOR in modulating cellular glucose metabolism which may translate to tumor cells [145], likely with a varying impact on cell division in different tumor types. Considering that GLUT1 is the primary glucose transporter expressed in most tumor types, including breast [146], lung [147], renal cell [148], colorectal [149], and melanoma [150], and high expression correlates with poorer prognosis of most tumor types found in the Human Protein Atlas (available from www.proteinatlas.org) [151] including breast, cervical, endometrial, ovarian, head and neck, liver, lung, pancreatic, renal, urothelial, and glioma, it is likely that part of mTOR's effect on cancer progression can be attributed to its modulation of glucose uptake and, consequently, metabolism.
However, many of these studies measure only enzymatic activities and nutrient/metabolite concentrations, lacking the gold-standard steady-state isotopic tracer analysis of metabolic fluxes. Without tracers, it is difficult to distinguish between the effects of oncogenic signaling to reprogram tumor metabolism, from the nutrient-dependent, cancer driver-independent, direct effects of metabolic reprogramming on cancer cell division. The use of isotope tracers to assess tumor metabolism will identify metabolic targets of interest within the complex interactions between oncogenic signaling pathways and pathways regulating substrate metabolism. The importance of the interactions between metabolic and oncogenic signaling pathways is highlighted by the fact that, because insulin signaling pathways are not specific to tumor cells, interventions directly targeting IR signaling pathways result in deleterious effects on liver cells, muscle cells, and other tissues. Specifically, treatment with PI3K–AKT–mTOR inhibitors can cause hyperglycemia and, in rare cases, diabetic ketoacidosis due to the effect of these drugs to interfere with systemic insulin signaling [152–160], and hyperinsulinemia resulting from the β-cells’ attempts to normalize blood glucose can limit the efficacy of these agents [104]. However, use of glucose-wasting sodium-glucose cotransporter-2 (SGLT2) inhibitors or low-carbohydrate ketogenic diets can minimize the deleterious effects of PI3K–AKT–mTOR inhibitors on both systemic glucose homeostasis and on tumor growth, at least in rodent models [104]. Several case reports have suggested that the efficacy of SGLT2 inhibitors and low-carbohydrate diets to prevent PI3K inhibitor-induced hyperglycemia may translate to humans [161,162], and a search of the U.S. ClinicalTrials.gov registry on November 21, 2021 revealed three ongoing trials examining the efficacy of adding SGLT2 inhibitors and/or low-carbohydrate diets in patients treated with PI3K inhibitors. Other experimental strategies to indirectly target insulin signaling in combination with other cancer treatments, such as chemotherapy [163] and immunotherapy [32], are also being actively pursued in the clinic, and will be discussed later in this review.
Epidemiology: hyperinsulinemia and cancer
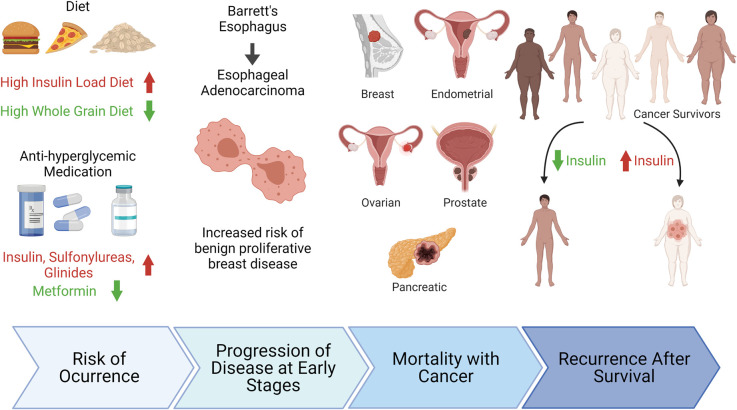
Hyperinsulinemia is associated with increased risk of breast, endometrial, ovarian [164–166], and prostate cancer [167,168]; increased pancreatic [169] and breast cancer mortality [170]; and increased any cancer mortality [171]. This association holds true in both obese and normal-weight individuals [172], regardless of diabetes, visceral adiposity, or metabolic syndrome status [173]. Hyperinsulinemic dietary patterns are associated with poorer survival and also with increased risk of recurrence in colorectal cancer patients [174–177] and with increased all-cause mortality [178], while high whole-grain and dietary fiber intake lowers the risk of bladder cancer [179] (Figure 3). Other evidence suggests that the influence of insulin on tumor formation has effects in the early stages of cancer, as hyperinsulinemia is independently associated with benign proliferative breast disease [180], and insulin resistance is a risk factor for progressing from Barrett's esophagus to esophageal adenocarcinoma [181]. Evidence for a direct effect of hyperinsulinemia of tumor progression has been suggested by the significantly higher presence of the IR on malignant than benign prostate epithelial cells from human biopsies [182].
Figure 3. Plasma Insulin is an independent tumor-promoting factor through all stages of cancer progression. Made in BioRender.com.
In combination with other risk factors including inflammatory markers, sex hormones, and elevated glucose levels, insulin appears to confer independent and perhaps synergistic effects on tumor progression and cancer outcomes.
Epidemiology: diabetes and cancer
Both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) result in hyperglycemia, though their etiologies are strikingly different. T1DM is caused by an autoimmune-mediated destruction of the insulin-producing pancreatic beta cells leading to hyperglycemia through insulinopenia. The lack of endogenous insulin production in T1DM is treated by subcutaneous administration of exogenous insulin. Oftentimes supraphysiologic doses of exogenous insulin are required to suppress endogenous hepatic glucose production and to allow for metabolism of exogenous carbohydrate intake [183]. Endogenous insulin, produced by the pancreas of healthy individuals, can directly enter the portal vein to regulate hepatic glucose metabolism, but this is lacking in those with T1DM. Therefore, to maintain glucose homeostasis as effectively as endogenous insulin does, oftentimes supraphysiologic doses of exogenous insulin are needed. T2DM, on the other hand, is caused by insulin resistance and thus impaired glucose clearance. In an attempt to overcome the inherent insulin resistance, the pancreas increases its insulin production, leading to hyperinsulinemia. The resultant circulating insulin levels in both T1DM, treated with exogenous insulin, and T2DM are substantially higher than those produced by the pancreas of healthy controls. Concomitant hyperinsulinemia in both types of diabetes has now become one of the major proposed mechanisms by which diabetes might promote cancer development, regardless of its origin (i.e. endogenous or exogenous).
In recent years, epidemiologic studies have shown evidence for a higher incidence of various site-specific cancers in people with diabetes mellitus, especially T2DM and to a lesser extent T1DM, compared with the general population. More than a twofold relative risk has been reported for endometrial, hepatic, and pancreatic cancer and an up to 1.5-fold relative risk for bladder, breast and colorectal cancer in T2DM [184–188]. In addition to the higher risk for developing the aforementioned cancers, patients with diabetes reportedly suffer from higher age adjusted short- and long-term mortality rates when diagnosed with cancer [184,189]. The epidemiological association between diabetes mellitus and cancer has led to the investigation of possible mechanistic links between the two as well as between the potential role of diabetes therapeutics in the development of cancer.
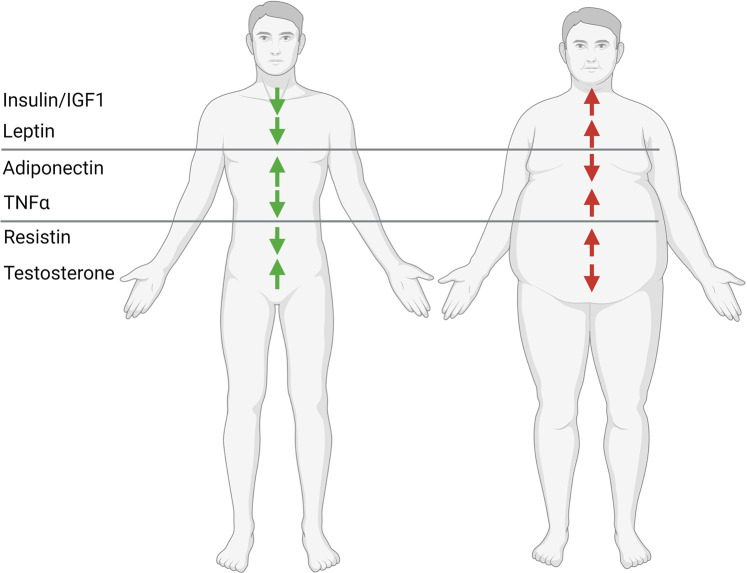
One of the major proposed mechanisms by which diabetes might promote cancer development is hyperinsulinemia, regardless of its cause (endogenous or exogenous). The link between insulin and cancer is the topic of this review, but we recognize the high likelihood that insulin is not the only link between obesity, diabetes, and cancer. Additional proposed cancer-promoting factors, especially in the conjunction with concomitant obesity, are hyperglycemia, hyperlipidemia as well as increasing circulating levels of leptin, estrogen, resistin, and inflammatory cytokines along with reduced concentrations of IGF binding proteins and adiponectin levels [190] which are proposed to play a permissive role in tumor cell proliferation, dissemination, and oncogene expression [191].
In addition to diabetes, anti-diabetes therapy has also been implicated in the development of cancer [5]. The list of agents includes incretin analogs, such as GLP-1 receptor agonists, incretin enhancers, such as dipeptidyl peptidase-4 inhibitors, insulins like glargine, along with pioglitazone, and sulfonylureas. All of which have been associated with cancer pathogenesis due to their enhancement of circulating insulin levels. However, many of the studies performed were flawed by inadequate methodology, in vitro/in vivo conditions that were not concordant/congruent with actual physiology and/or significant bias in study design and data interpretation, such as prevalent-user bias, immortal time bias, and time-lag bias/confounding by indication. Furthermore, many studies did not account and adjust for many covariates, such as disease duration, and severity, amongst others.
Despite the highly suggestive association between diabetes and cancer [184–188,192–201], the underlying molecular and mechanistic links still remain fairly obscure. In addition, it is possible that there is no linear, direct causality between diabetes and cancer, but the link might rather be as multifactorial as the pathology of diabetes itself. For instance, mutuality between diabetes and cancer might be attributable to their common predisposing factors, such as unhealthy lifestyles, including physical inactivity and excess caloric intake, higher adipose mass and decreased lean muscle mass as well as ageing itself. Therefore, further research is needed to identify exact underlying mechanistic causality and identify novel therapeutic and interventional targets.
Hyperinsulinemia as a therapeutic target in animals with cancer
Despite mechanistic uncertainties, considering the strong epidemiologic evidence in support of a pathogenic link between insulin and cancer, numerous in vivo preclinical studies have explored this possibility interventionally. Both endogenous hyperinsulinemia [202] and exogenous insulin injection [203] promote colorectal cancer growth in rats. Similarly, insulin injection also promotes the progression of pancreatic cancer in Syrian hamsters [204], as well as the development and metastasis of breast [205–207] and colon cancer in mice [208]. To be noted, insulin did not significantly alter body composition in these studies, consistent with a direct impact of insulin to accelerate tumor growth. Preclinical in vivo rodent studies have demonstrated that hyperinsulinemia can activate not only its cognate IR but can also bind to and hence stimulate the insulin-like growth factor 1 receptor (IGF1R). Additionally, hyperinsulinemia also promoted increased production of IGF-1 by the liver which in turn further amplified IGF1R signaling through the PI3K–AKT–mTOR and RAS–MAPK pathways, which stimulate expression of the MYC proto-oncogene, cell proliferation, anti-apoptotic, and anabolic effects in tumor cells [104,190,209–212]. Of course, hyperinsulinemia is not the only mechanistic link between obesity and cancer. Although discussion of these alternative mechanisms is beyond the scope of this review, we acknowledge that insulin-independent mechanisms — for example, lipid peroxidation and metabolism [213], fibroblast growth factor receptor-1 [214], creatine [215], leptin [216], inflammatory cytokines [217], and many others which space limitations do not permit us to discuss in any detail (Figure 4) — play a key role in the progression of obesity-associated cancers as well.
Figure 4. Proposed mechanisms by which obesity may promote the progression of certain tumors. Made in BioRender.com.
Hyperinsulinemia as a therapeutic target in patients with cancer
Several epidemiologic studies have correlated antihyperglycemic medication use with risk or outcomes of cancer, and have generally concluded that patients with type 2 diabetes treated with insulin and with sulfonylureas, which stimulate insulin secretion, have higher cancer incidence and mortality than those treated with metformin [218–223]. However, these results do not uniformly point to a direct link between insulin and cancer: despite strong preclinical evidence [208,224,225], the addition of metformin to chemotherapy for non-small cell lung cancer reduced hyperinsulinemia, but did not provide survival benefit [226]. In this same study, however, patients with high 18F-Fluorodeoxyglucose uptake on their PET scans received a mortality benefit from metformin, suggesting certain glucose-dependent but insulin-independent effects in the tumor microenvironment. In addition, metformin reduced the risk of cancer in a type 2 diabetic population, with no differences in fasting insulin or the homeostatic model assessment for insulin resistance (HOMA-IR), but the metformin group had less exogenous insulin use [227]. Another study found that use of insulin, glinides, and sulfonylureas increased the risk for gastrointestinal and lung cancers [220]. Clearly, more evidence is required in humans to determine whether and how the reversal of hyperinsulinemia can slow tumor growth and improve clinical outcomes.
Likely, it is of particular importance to develop and apply strategies to enhance systemic metabolism during cancer treatment because of the effects of standard-of-care therapies to induce metabolic dysfunction. In addition to the effect of immune checkpoint inhibitors to cause autoimmune diabetes, which will be discussed later in this review, chemotherapy commonly causes weight gain and insulin resistance [228]. Moreover, insulin resistance is a predictor of poor outcomes in those treated with chemotherapy: a recent study found the probability of a pathological complete response to treatment for breast cancer to be close to five times lower in those with insulin resistance, as compared with those without [229], with other studies generating similar conclusions regarding the detrimental effect of chemotherapy on metabolic health in multiple tumor types [230–234]. Although less commonly studied, androgen deprivation therapy [235], radiotherapy [236–238], and stem cell transplant [239], as well as chemotherapy in combination with radiotherapy [240], also appear to pose an increased risk of excess weight and impaired insulin sensitivity in cancer survivors. However, surprisingly little work has focused on the mechanisms by which these standard-of-care cancer treatments may lead to metabolic dysfunction. Historically, chemotherapy was commonly administered in 5% glucose water; although normal saline is increasingly preferred, the epidemiologic data may recommend a more robust examination of this common practice, considering the extra carbohydrate load presented by glucose diluent and its potential impact on systemic metabolism during chemotherapy treatment.
Exercise and cancer
Exercise is a well-established insulin-sensitizing intervention. Studies dating back at least 100 years [241] have demonstrated that both acutely and chronically, aerobic exercise has the capacity to reduce plasma glycemia and enhance insulin action in skeletal muscle, in both an intensity- and duration-dependent manner [242–262]. Exercise research has been fundamental in understanding glucose transport, and exercise was used as a model to illustrate insulin-independent (GLUT4-dependent) skeletal muscle glucose uptake, making exercise prescription in patients with insulin resistance an appealing therapeutic modality [263,264]. As the links between insulin resistance and cancer have emerged over the past several decades, exercise quickly became a standard adjuvant for cancer therapy [265–273].
Basic and translational studies have consistently shown immense effects of exercise (most commonly voluntary wheel running in rodents) to slow tumor growth [274–282] and reduce metastases [283–286] in tumor-bearing animals. Multiple mechanisms have been suggested to mediate exercise's anti-cancer effects: enhanced angiogenesis and thus increased immune cell infiltration [280,284], forced-swimming-induced catecholamine induction has been suggested to enhance natural killer cell infiltration into tumors [282,287], and exercise training induced improvements in insulin resistance [288,289], and thus reductions in tumor anabolism, among others, have been suggested.
As would be predicted by the epidemiologic and clinical data, exercise exerts a modest but significant effect to reduce cancer risk and slow tumor progression [290–294]; however, whether and to what extent the beneficial effect of exercise is mediated by reversal of hyperinsulinemia per se and to what extent this effect is reliant on alterations in tumor and/or immunometabolism is an open question. The PreHAB study, where obese women with breast cancer were randomized to a combination of aerobic and resistance exercise training program, compared with a mindfulness control group, for 4 weeks prior to surgical excision, demonstrated that exercise reduced circulating insulin, IGF1, and leptin, though only leptin reductions were significantly different from control patients [295]. Future clinical trials on the impact of exercise on tumor biology should continue to collect biomarker data to provide further insight into the mechanistic basis of exercise and cancer interactions. In addition, the frequency, intensity, type, and duration of exercise necessary to induce beneficial metabolic and anti-tumor effects in patients is unknown and largely unexplored.
A topic of recent interest involves how exercise may alter immune function through metabolic reprogramming. Work primarily derived from RNA sequencing and metabolomics has suggested that different substrates play key regulatory roles in immune action. Conventional wisdom holds that glucose and glutamine metabolism may be crucial to promote differentiation, activation, and clonal expansion [296–306], while fatty acid metabolism may also play a key role in immune cell longevity, including preventing exhaustion in T cells and dendritic cells [307,308] and promoting regulatory and memory T cell formation and survival [303,307,309–314]. These data would predict that approaches to transiently increase systemic glucose metabolism in a cyclic pattern, while chronically increasing fatty acid metabolism, would be most beneficial in improving anti-cancer immunity.
Aerobic exercise is a classic means of inducing just these changes. Acutely upon initiation of intense exercise, there is a shift to glucose metabolism [315–317]; however, during recovery from exercise and during exercise training, a shift in whole-body metabolism to increase fatty acid oxidation has been repeatedly observed [318–320]. As discussed in the next section, it is possible — though not yet proven — that both alterations may yield anti-cancer benefits by their actions on immune cells.
Cancer immunology, nutrients, and insulin
With rising recognition of the interactions between insulin, substrates, and tumor progression, dietary modifications represent an area of burgeoning interest in cancer therapeutics. The impact of diet on tumor metabolism and prognosis is likely nuanced: while a low-carbohydrate, ketogenic diet reduces tumor glucose uptake in human patients [321–323], a high fat, low-carbohydrate diet — seemingly paradoxically — increases tumor glucose uptake in rodents [207,208,324]. In addition to potential species differences, studies in this vein are plagued by a critical confounder: there is wide variance in both adherence to any diet, and in the total caloric load ingested on most diets, leading to discrepancies in dietary intervention studies in terms of whether participants experience a positive, negative, or neutral energy balance. These differences in diet-induced alterations in energy balance across various studies may lead to differences in the effect of the diet on insulin responsiveness.
Additionally, recent research has highlighted the possibility that various diets may affect cancer outcomes in a tumor cell-autonomous manner: by affecting the immune response to cancer. Insulin has been implicated in the modulation of different immune phenotypes and responses [325], as evidenced by the expression of insulin receptors (IRs) on T, B cells and macrophages after activation [326,327]. Furthermore, conditional knockdown of these IRs has been found to reduce aerobic glycolysis, which is evidenced in the decreased expression of GLUT 3,4 and the reduction in lactate production [328], all of which are hallmarks of the Warburg effect of cancer. The immunomodulatory role of insulin has been mechanistically linked to the PI3K/Akt/mTOR signaling pathway; binding of insulin to an IR results in its dimerization and autophosphorylation, which results in the activation of IRSs [329,330]. These activated IRSs in-turn stimulates PI3K resulting in the phosphorylation of AKT at tyrosine-308 by PDK1 and at Serine-473 by mTORC2. Interestingly, PI3K/Akt/mTOR signaling pathway is also often dysregulated in many cancer pathologies. Furthermore, IR-deficient T cells have been found to have decreased expression of Myc, which is a transcription factor that is downstream of the PI3K/Akt/mTOR signaling pathway and it is involved in glycolytic metabolism [329,331]. Myc is also an oncogene, whose dysregulation results in the propagation of many cancer pathologies [332].
Whether insulin-dependent or -independent, it is clear that substrate metabolism also plays a role in immune function and longevity. It has been suggested that metabolic competition in the TME is a key mechanism by which immune cells limit tumor growth [296,297,333]. While this possibility could be dismissed offhand by considering the much greater biomass of tumor cells versus immune cells in a typical tumor, it is important to consider the primary glucose transporters expressed by each cell type. While as mentioned earlier GLUT1 is the primary glucose transporter expressed in most tumor types, the higher-affinity GLUT3 is the primary glucose transporter expressed by T cells, and data from the open-access Immunological Proteome Resource (ImmPRes) show that its expression is markedly increased in activated T cells. As the Km of GLUT1 is 7–26 mM [334,335] while the Km of GLUT3 is less than 2 mM [335], GLUT3 is better able to facilitate glucose uptake at the low glucose concentrations characteristic of the TME, suggesting that tumor-T cell competition for glucose may be relevant in determining cancer prognosis.
In addition, exercise is another well-studied modulator of systemic and tissue-specific nutrient partitioning. A single bout of exercise stimulates whole-body glucose metabolism by increasing both insulin-mediated and insulin-independent glucose uptake in tissues [245,247,249,253,336–343]. During acute exercise, the exercising muscle and, therefore, whole organism rely first on breakdown of glycogen, the short-term storage form of glucose. Once endurance exercise is sustained for longer durations (beyond ∼10 min), de novo glucose synthesis (gluconeogenesis) increases to enable systemic increases in rates of aerobic and non-aerobic glucose metabolism [344,345]. This rapid increase in systemic reliance upon glucose is made possible by a 2–3-fold induction of hepatic, and possibly renal, glucose production [249,340–342]. As glucose is considered the primary driver of T cell activation [306,346–350], exercise-induced increases in glucose metabolism could mediate the effect of exercise to promote cytotoxic T cell function in mice with cancer.
However, chronically, exercise rehabilitation and training reduce the whole-organism respiratory exchange ratio, reflecting a shift in systemic metabolism from oxidation of glucose to oxidation of fatty acids [351–356]. This increased reliance on fatty acids has important implications for all-cause mortality: a lower respiratory exchange ratio is associated with a lower incidence of postoperative complications [357–359] and improved survival in patients with sepsis [360,361] and heart failure [362–364] as well as in ageing mice [365], highlighting the intriguing possibility that chronically increased systemic fatty acid metabolism may improve outcomes in other conditions in which the immune system is important, including cancer.
While glucose metabolism has received most of the attention paid to cancer immunometabolism, increasing evidence suggests that fatty acid metabolism should not be ignored. Metabolic flexibility appears to be critical in promoting cytotoxic effector function while also preserving long-term immune cell health and longevity, with glucose and glutamine metabolism promoting effector function [296,297,366–370] and fatty acid metabolism predominantly fueling naïve T cell metabolism [298,371–373], Treg formation [313], memory T cell formation and survival [307,312,314,374–377], and natural killer cell [378] and dendritic cell maturation and function [379]. Systemic metabolic inflexibility — that is, a constant and exclusive reliance upon either glucose or fatty acid metabolism would, then, be predicted to worsen outcomes: excessive reliance on glucose may acutely promote effector function but chronically promote exhaustion and worsen memory cell formation, whereas excessive reliance on fatty acids may enhance longevity but worsen effector function.
In considering possible targets to mimic the effect of exercise on anti-cancer immune function, carnitine palmitoyltransferase I (CPT1) represents an attractive target. CPT1 is considered the gatekeeper for mitochondrial fatty acid oxidation, as it catalyzes the formation of acylcarnitines for transport from the cytosol into the mitochondria. Chronic exercise increases CPT1 expression in skeletal muscles and peripheral blood mononuclear cells of rodents [380–386] and humans [387–390]; however, future studies will be required to determine the functional relevance of this increase in CPT1 expression on anti-cancer immune function per se.
Given these links between insulin, immune function and cancer, including the exhaustive evidence provided for the connections between obesity, inflammation, insulin-dependent diabetes, and cancer, this serves the logical thinking that there are links between cancer immunology and insulin. Though this field is relatively understudied, there are certain lines of evidence that could invigorate future research work. One line of such evidence described in 2015, follows the use of anti-programmed cell death-1 (PD-1) therapies such as pembrolizumab in a patient with BRAF wild-type cutaneous melanoma that subsequently developed autoimmune diabetes [391]. Pembrolizumab is an immune checkpoint inhibitor and an IgG4 monoclonal antibody that targets PD-1 [391]. Interestingly, immune checkpoint inhibitors such as pembrolizumab seem to modulate the same nodal networks that are involved insulin signaling [331]. Though the ability of immune checkpoint inhibitors to induce autoimmune diabetes has been well described, this serious adverse effect of immune checkpoint inhibitors is extremely rare at ∼1% of those treated with ICIs for cancer [392].
Though a clear mechanistic relationship between immune checkpoint inhibitors and the subsequent presentation of autoimmune diabetes is yet to be described, some inferences can be made. CD28 is a co-activator for T cell function and similar to the IR activation of the PI3K/Akt/mTOR signaling pathway described above, tyrosine phosphorylation of the cytoplasmic tail of CD28 up-regulates the activity PI3K/Akt signaling in T cells [393]. Given that activation of PD-1 directly and CTLA-4 indirectly antagonizes the up-regulation of PI3K/Akt signaling, it is quite possible that efficacy of immune checkpoint inhibitors is tied to insulin signaling.
Concluding thoughts
Although many studies, including from our group, have attempted to draw linear relationships between obesity, diabetes, hyperinsulinemia, and cancer, and have assessed the links between these devastating conditions in isolation, it is likely that the relationships between these conditions are more complex than that. While scientific rigor requires one to choose a target of interest and probe it as independently as possible, in vivo there is undoubtedly interplay between insulin and many other tumor-promoting or -limiting factors. However, this does not undercut the potential for insulin-targeting therapies to serve as a useful adjunct to standard-of-care therapies in cancer. What is unarguably clear at this time is that there is a link between altered systemic metabolism and cancer. Future studies to mechanistically understand this link are of critical importance as we stand on a precipice of continuing increases in rates of both obesity, diabetes, and cancer in the U.S. and worldwide.
Acknowledgements
The authors declare no relevant conflicts of interest.
Abbreviations
- BCAA
branched-chain amino acid
- CPT1
carnitine palmitoyltransferase I
- CT
computed tomography
- IGF1
insulin-like growth factor 1
- IGF1R
insulin-like growth factor 1 receptor
- ImmPRes
immunological proteome resource
- IR
insulin receptor
- IRSs
insulin receptor substrates
- MAPK
mitogen-activated protein kinase
- SGLT2
sodium-glucose cotransporter-2
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1. https://www.cdc.gov/cancer/obesity/index.htm (2021, March 10) Obesity and Cancer | CDC.
- 2.Zhang, D., Chen, J., Wang, J., Gong, S., Jin, H., Sheng, P.et al. (2016) Body mass index and risk of brain tumors: a systematic review and dose-response meta-analysis. Eur. J. Clin. Nutr. 70, 757–765 10.1038/ejcn.2016.4 [DOI] [PubMed] [Google Scholar]
- 3.Niedermaier, T., Behrens, G., Schmid, D., Schlecht, I., Fischer, B. and Leitzmann, M.F. (2015) Body mass index, physical activity, and risk of adult meningioma and glioma: a meta-analysis. Neurology 85, 1342–1350 10.1212/WNL.0000000000002020 [DOI] [PubMed] [Google Scholar]
- 4.Chen, Q., Zhuang, H. and Liu, Y. (2012) The association between obesity factor and esophageal cancer. J. Gastrointest. Oncol. 3, 226–231 10.3978/j.issn.2078-6891.2012.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott, J.A. and Reynolds, J.V. (2021) Visceral obesity, metabolic syndrome, and esophageal adenocarcinoma. Front. Oncol. 11, 692 10.3389/fonc.2021.627270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman, G.D. and Herrinton, L.J. (1994) Obesity and multiple myeloma. Cancer Causes Control 5, 479–483 10.1007/BF01694762 [DOI] [PubMed] [Google Scholar]
- 7.Teras, L.R., Kitahara, C.M., Birmann, B.M., Hartge, P.A., Wang, S.S., Robien, K., et al. (2014) Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br. J. Haematol. 166, 667–676 10.1111/bjh.12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson, K.M. and Cho, E. (2016) Obesity and kidney cancer. Recent Results Cancer Res. 208, 81–93 10.1007/978-3-319-42542-9_5 [DOI] [PubMed] [Google Scholar]
- 9.Liu, X., Sun, Q., Hou, H., Zhu, K., Wang, Q., Liu, H.et al. (2018) The association between BMI and kidney cancer risk. Medicine (Baltimore) 97, e12860 10.1097/MD.0000000000012860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onstad, M.A., Schmandt, R.E. and Lu, K.H. (2016) Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 34, 4225–4230 10.1200/JCO.2016.69.4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitson, S.J. and Crosbie, E.J. (2019) Endometrial cancer and obesity. Obstet. Gynaecol. 21, 237–245 10.1111/tog.12601 [DOI] [Google Scholar]
- 12.Foong, K.W. and Bolton, H. (2017) Obesity and ovarian cancer risk: a systematic review. Post Reprod. Health 23, 183–198 10.1177/2053369117709225 [DOI] [PubMed] [Google Scholar]
- 13.Nagle, C.M., Dixon, S.C., Jensen, A., Kjaer, S.K., Modugno, F., deFazio, A., et al. (2015) Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br. J. Cancer 113, 817–826 10.1038/bjc.2015.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardou, M., Barkun, A.N. and Martel, M. (2013) Obesity and colorectal cancer. Gut 62, 933–947 10.1136/gutjnl-2013-304701 [DOI] [PubMed] [Google Scholar]
- 15.Liu, P.-H., Wu, K., Ng, K., Zauber, A.G., Nguyen, L.H., Song, M., et al. (2019) Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 5, 37–44 10.1001/jamaoncol.2018.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao, Z.G., Guo, X.G., Ba, C.X., Wang, W., Yang, Y.Y., Wang, J.et al. (2012) Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J. Int. Med. Res. 40, 2041–2050 10.1177/030006051204000601 [DOI] [PubMed] [Google Scholar]
- 17.Xu, L., Port, M., Landi, S., Gemignani, F., Cipollini, M., Elisei, R., et al. (2014) Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case–control studies. Thyroid 24, 966–974 10.1089/thy.2013.0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engin, A. (2017) Obesity-associated breast cancer: analysis of risk factors. Adv. Exp. Med. Biol. 960, 571–606 10.1007/978-3-319-48382-5_25 [DOI] [PubMed] [Google Scholar]
- 19.Lohmann, A.E., Soldera, S.V., Pimentel, I., Ribnikar, D., Ennis, M., Amir, E.et al. (2021) Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta-analysis. J. Natl Cancer Inst. 113, 1465–1475 10.1093/jnci/djab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn, W., Lee, H.W., Lee, S., Lim, J.H., Lee, M.W., Park, C.H.et al. (2021) Obesity and the risk of primary liver cancer: a systematic review and meta-analysis. Clin. Mol. Hepatol. 27, 157–174 10.3350/cmh.2020.0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitta, C., Pollicino, T. and Raimondo, G. (2019) Obesity and liver cancer. Ann. Hepatol. 18, 810–815 10.1016/j.aohep.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 22.Larsson, S.C. and Wolk, A. (2007) Obesity and the risk of gallbladder cancer: a meta-analysis. Br. J. Cancer 96, 1457–1461 10.1038/sj.bjc.6603703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, L., Gan, Y., Li, W., Wu, C. and Lu, Z. (2016) Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: a meta-analysis of observational studies. Obesity 24, 1786–1802 10.1002/oby.21505 [DOI] [PubMed] [Google Scholar]
- 24.Yang, P., Zhou, Y., Chen, B., Wan, H.-W., Jia, G.-Q., Bai, H.-L.et al. (2009) Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur. J. Cancer 45, 2867–2873 10.1016/j.ejca.2009.04.019 [DOI] [PubMed] [Google Scholar]
- 25.Lin, X.-J., Wang, C.-P., Liu, X.-D., Yan, K.-K., Li, S., Bao, H.-H.et al. (2014) Body mass index and risk of gastric cancer: a meta-analysis. Jpn. J. Clin. Oncol. 44, 783–791 10.1093/jjco/hyu082 [DOI] [PubMed] [Google Scholar]
- 26.Berrington de Gonzalez, A., Sweetland, S. and and Spencer, E. (2003) A meta-analysis of obesity and the risk of pancreatic cancer. Br. J. Cancer 89, 519–523 10.1038/sj.bjc.6601140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, D., Morris, J.S., Liu, J., Hassan, M.M., Day, R.S., Bondy, M.L.et al. (2009) Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 301, 2553–2562 10.1001/jama.2009.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagan, H.B., Wender, R., Myers, R.E. and Petrelli, N. (2011) Obesity and cancer screening according to race and gender. J. Obes. 2011, e218250 10.1155/2011/218250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kichenadasse, G., Miners, J.O., Mangoni, A.A., Rowland, A., Hopkins, A.M. and Sorich, M.J. (2019) Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 6, 512–518 10.1001/jamaoncol.2019.5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortellini, A., Ricciuti, B., Tiseo, M., Bria, E., Banna, G.L., Aerts, J.G., et al. (2020) Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J. Immunother. Cancer 8, e001403 10.1136/jitc-2020-001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly, D., Bajaj, S., Yu, J., Hsu, M., Balar, A., Pavlick, A.et al. (2019) The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 7, 222 10.1186/s40425-019-0699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuade, J.L., Daniel, C.R., Hess, K.R., Mak, C., Wang, D.Y., Rai, R.R., et al. (2018) Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19, 310–322 10.1016/S1470-2045(18)30078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naik, G.S., Waikar, S.S., Johnson, A.E.W., Buchbinder, E.I., Haq, R., Hodi, F.S.et al. (2019) Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J. Immunother. Cancer 7, 89 10.1186/s40425-019-0512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortellini, A., Bersanelli, M., Buti, S., Cannita, K., Santini, D., Perrone, F., et al. (2019) A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J. Immunother Cancer 7, 57 10.1186/s40425-019-0527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An, Y., Wu, Z., Wang, N., Yang, Z., Li, Y., Xu, B.et al. (2020) Association between body mass index and survival outcomes for cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J. Transl. Med. 18, 235 10.1186/s12967-020-02404-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gancheva, S., Jelenik, T., Álvarez-Hernández, E. and Roden, M. (2018) Interorgan metabolic crosstalk in human insulin resistance. Physiol. Rev. 98, 1371–1415 10.1152/physrev.00015.2017 [DOI] [PubMed] [Google Scholar]
- 37.Petersen, M.C. and Shulman, G.I. (2018) Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 10.1152/physrev.00063.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel, V.T. and Shulman, G.I. (2016) The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126, 12–22 10.1172/JCI77812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roden, M. and Shulman, G.I. (2019) The integrative biology of type 2 diabetes. Nature 576, 51–60 10.1038/s41586-019-1797-8 [DOI] [PubMed] [Google Scholar]
- 40.Hirosumi, J., Tuncman, G., Chang, L., Görgün, C.Z., Uysal, K.T., Maeda, K.et al. (2002) A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 10.1038/nature01137 [DOI] [PubMed] [Google Scholar]
- 41.Han, M.S., Jung, D.Y., Morel, C., Lakhani, S.A., Kim, J.K., Flavell, R.A.et al. (2013) JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. science. Am. Assoc. Adv. Sci. 339, 218–222 10.1126/science.1227568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry, R.J., Camporez, J.-P.G., Kursawe, R., Titchenell, P.M., Zhang, D., Perry, C.J., et al. (2015) Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 10.1016/j.cell.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranasinghe, C., Gamage, P., Katulanda, P., Andraweera, N., Thilakarathne, S. and Tharanga, P. (2013) Relationship between body mass index (BMI) and body fat percentage, estimated by bioelectrical impedance, in a group of Sri Lankan adults: a cross sectional study. BMC Public Health 13, 797 10.1186/1471-2458-13-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hudzik, B., Nowak, J., Szkodzinski, J., Danikiewicz, A., Korzonek-Szlacheta, I. and Zubelewicz-Szkodzińska, B. (2021) Discordance between body-mass index and body adiposity index in the classification of weight status of elderly patients with stable coronary artery disease. J. Clin. Med. 10, 943 10.3390/jcm10050943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell, L., Bel-Serrat, S., Heinen, M., Mehegan, J., Murrin, C., O'Brien, S.et al. (2021) Waist circumference-to-height ratio and body mass index for obesity classification in Irish children. Acta Paediatr. 110, 1541–1547 10.1111/apa.15724 [DOI] [PubMed] [Google Scholar]
- 46.Borugian, M.J., Sheps, S.B., Kim-Sing, C., Olivotto, I.A., Van Patten, C., Dunn, B.P.et al. (2003) Waist-to-hip ratio and breast cancer mortality. Am. J. Epidemiol. 158, 963–968 10.1093/aje/kwg236 [DOI] [PubMed] [Google Scholar]
- 47.Shah, N.R. and Braverman, E.R. (2012) Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One 7, e33308 10.1371/journal.pone.0033308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leitner, B.P., Ospanova, S., Beisenbayeva, A., Givechian, K.B., Politi, K. and Perry, R.J. (2021) Multimodal analysis reveals differential immuno-metabolic features in lung squamous cell carcinoma and adenocarcinoma. npj Precis. Oncol. 6, 8 10.1038/s41698-021-00248-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goncalves, M.D., Taylor, S., Halpenny, D.F., Schwitzer, E., Gandelman, S., Jackson, J., et al. (2018) Imaging skeletal muscle volume, density, and FDG uptake before and after induction therapy for non-small cell lung cancer. Clin. Radiol. 73, 505.e1–505.e8 10.1016/j.crad.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasai, H., Brychta, R.J., Wood, R.P., Rothney, M.P., Zhao, X., Skarulis, M.C.et al. (2015) Does visceral fat estimated by dual-energy X-ray absorptiometry independently predict cardiometabolic risks in adults? J. Diabetes Sci. Technol. 9, 917–924 10.1177/1932296815577424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbi, J., Patnaik, S.K., Pabla, S., Zollo, R., Smith, R.J., Sass, S.N., et al. (2021) Visceral obesity promotes lung cancer progression—toward resolution of the obesity paradox in lung cancer. J. Thorac. Oncol. 6, 1333–1348 10.1016/j.jtho.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lalia, A.Z., Dasari, S., Johnson, M.L., Robinson, M.M., Konopka, A.R., Distelmaier, K., et al. (2016) Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. J. Clin. Endocrinol. Metab. 101, 626–634 10.1210/jc.2015-2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson, L.J., Lee, J., Anderson, B., Lee, B., Migula, D., Sauer, A., et al. (2022) Whole-body and adipose tissue metabolic phenotype in cancer patients. J. Cachexia Sarcopenia Muscle 10.1002/jcsm.12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Runkel, M., Diallo, T.D., Lang, S.A., Bamberg, F., Benndorf, M. and Fichtner-Feigl, S. (2021) The role of visceral obesity, sarcopenia and sarcopenic obesity on surgical outcomes after liver resections for colorectal metastases. World J. Surg. 45, 2218–2226 10.1007/s00268-021-06073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gentles, A.J., Newman, A.M., Liu, C.L., Bratman, S.V., Feng, W., Kim, D., et al. (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catanese, S., Beuchel, C.F., Sawall, T., Lordick, F., Brauer, R., Scholz, M.et al. (2021) Biomarkers related to fatty acid oxidative capacity are predictive for continued weight loss in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 12, 2101–2110 10.1002/jcsm.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burlaka, A.P., Virko, S.V., Burlaka, A.A. and Krupnyk, K.L. (2021) Redox dependent features of tumors, adipose tissue, neutrophiles and platelets in patients with metastatic colorectal cancer. Exp. Oncol. 43, 261–265 10.32471/exp-oncology.2312-8852.vol-43-no-3.16571 [DOI] [PubMed] [Google Scholar]
- 58.Park, Y.-M.M., White, A.J., Nichols, H.B., O'Brien, K.M., Weinberg, C.R. and Sandler, D.P. (2017) The association between metabolic health, obesity phenotype and the risk of breast cancer. Int. J. Cancer 140, 2657–2666 10.1002/ijc.30684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donini, L., Merola, G., Poggiogalle, E., Lubrano, C., Gnessi, L., Mariani, S., et al. (2016) Disability, physical inactivity, and impaired health-related quality of life are not different in metabolically healthy vs. unhealthy obese subjects. Nutrients 8, 759 10.3390/nu8120759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blüher, M. (2010) The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr. Opin. Lipidol. 21, 38 10.1097/MOL.0b013e3283346ccc [DOI] [PubMed] [Google Scholar]
- 61.Moore, L.L., Chadid, S., Singer, M.R., Kreger, B.E. and Denis, G.V. (2014) Metabolic health reduces risk of obesity-related cancer in framingham study adults. Cancer Epidemiol. Biomark. Prev. 23, 2057–2065 10.1158/1055-9965.EPI-14-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blüher, M. (2020) Metabolically healthy obesity. Endocr. Rev. 41, 405–420 10.1210/endrev/bnaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kramer, C.K., Zinman, B. and Retnakaran, R. (2013) Are metabolically healthy overweight and obesity benign conditions? Ann. Intern. Med. 159, 758–769 10.7326/0003-4819-159-11-201312030-00008 [DOI] [PubMed] [Google Scholar]
- 64.Lin, C.-J., Chang, Y.-C., Cheng, T.-Y., Lo, K., Liu, S.-J. and Yeh, T.L. (2020) The association between metabolically healthy obesity and risk of cancer: a systematic review and meta-analysis of prospective cohort studies. Obes. Rev. 21, e13049 10.1111/obr.13049 [DOI] [PubMed] [Google Scholar]
- 65.Stefan, N. (2020) Metabolically healthy and unhealthy normal weight and obesity. Endocrinol. Metab. (Seoul) 35, 487–493 10.3803/EnM.2020.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckel, N., Mühlenbruch, K., Meidtner, K., Boeing, H., Stefan, N. and Schulze, M.B. (2015) Characterization of metabolically unhealthy normal-weight individuals: risk factors and their associations with type 2 diabetes. Metabolism 64, 862–871 10.1016/j.metabol.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 67.Stefan, N., Schick, F. and Häring, H.-U. (2017) Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 26, 292–300 10.1016/j.cmet.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 68.Bilski, J., Pierzchalski, P., Szczepanik, M., Bonior, J. and Zoladz, J.A. (2022) Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. Cells 11, 160 10.3390/cells11010160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fehrenbach, U., Wuensch, T., Gabriel, P., Segger, L., Yamaguchi, T., Auer, T.A., et al. (2021) CT body composition of sarcopenia and sarcopenic obesity: predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers 13, 2921 10.3390/cancers13122921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armandi, A., Rosso, C., Caviglia, G.P., Ribaldone, D.G. and Bugianesi, E. (2021) The impact of dysmetabolic sarcopenia among insulin sensitive tissues: a narrative review. Front. Endocrinol. 12 10.3389/fendo.2021.716533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newsholme, E. and Randle, P. (1961) Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, Adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem. J. 80, 655–662 10.1042/bj0800655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cline, G.W., Petersen, K.F., Krssak, M., Shen, J., Hundal, R.S., Trajanoski, Z.et al. (1999) Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341, 240–246 10.1056/NEJM199907223410404 [DOI] [PubMed] [Google Scholar]
- 73.Halse, R., Bonavaud, S.M., Armstrong, J.L., McCormack, J.G. and Yeaman, S.J. (2001) Control of glycogen synthesis by glucose, glycogen, and insulin in cultured human muscle cells. Diabetes 50, 720–726 10.2337/diabetes.50.4.720 [DOI] [PubMed] [Google Scholar]
- 74.Randle, P.J., Newsholme, E.A. and Garland, P.B. (1964) Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem. J. 93, 652–665 10.1042/bj0930652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klip, A. and Pâquet, M.R. (1990) Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care 13, 228–243 10.2337/diacare.13.3.228 [DOI] [PubMed] [Google Scholar]
- 76.Nishikawa, H., Asai, A., Fukunishi, S., Nishiguchi, S. and Higuchi, K. (2021) Metabolic syndrome and sarcopenia. Nutrients 13, 3519 10.3390/nu13103519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almarzouq, A., Kool, R., Al Bulushi, Y., Marcq, G., Souhami, L., Cury, F.L.et al. (2021) Impact of sarcopenia on outcomes of patients treated with trimodal therapy for muscle invasive bladder cancer. Urol. Oncol. 10.1016/j.urolonc.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 78.Icard, P., Schussler, O., Loi, M., Bobbio, A., Mansuet Lupo, A., Wislez, M.et al. (2020) Pre-disease and pre-surgery BMI, weight loss and sarcopenia impact survival of resected lung cancer independently of tumor stage. Cancers 12, 266 10.3390/cancers12020266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrmann, T., Mione, C., Montoriol, P.-F., Molnar, I., Ginzac, A., Durando, X.et al. (2022) Body mass index, sarcopenia, and their variations in predicting outcomes for patients treated with nivolumab for metastatic renal cell carcinoma. Oncology 100, 114–123 10.1159/000520833 [DOI] [PubMed] [Google Scholar]
- 80.Peng, Y.-C., Wu, C.-H., Tien, Y.-W., Lu, T.-P., Wang, Y.-H. and Chen, B.-B. (2021) Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur. Radiol. 31, 2472–2481 10.1007/s00330-020-07294-7 [DOI] [PubMed] [Google Scholar]
- 81.Ligibel, J.A., Schmitz, K.H. and Berger, N.A. (2020) Sarcopenia in aging, obesity, and cancer. Transl. Cancer Res. 9, 5760 10.21037/tcr-2019-eaoc-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim, K.W., Baek, M.-O., Yoon, M.-S. and Son, K.H. (2021) Deterioration of mitochondrial function in the human intercostal muscles differs among individuals with sarcopenia, obesity, and sarcopenic obesity. Clin. Nutr. 40, 2697–2706 10.1016/j.clnu.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 83.Kemp, P.R., Paul, R., Hinken, A.C., Neil, D., Russell, A. and Griffiths, M.J. (2020) Metabolic profiling shows pre-existing mitochondrial dysfunction contributes to muscle loss in a model of ICU-acquired weakness. J. Cachexia Sarcopenia Muscle 11, 1321–1335 10.1002/jcsm.12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gould, D.W., Lahart, I., Carmichael, A.R., Koutedakis, Y. and Metsios, G.S. (2013) Cancer cachexia prevention via physical exercise: molecular mechanisms. J. Cachexia Sarcopenia Muscle 4, 111–124 10.1007/s13539-012-0096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alldritt, I., Greenhaff, P.L. and Wilkinson, D.J. (2021) Metabolomics as an important tool for determining the mechanisms of human skeletal muscle deconditioning. Int. J. Mol. Sci. 22, 13575 10.3390/ijms222413575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lynch, C.J. and Adams, S.H. (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736 10.1038/nrendo.2014.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mann, G., Mora, S., Madu, G. and Adegoke, O.A.J. (2021) Branched-chain amino acids: catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front. Physiol. 12 10.3389/fphys.2021.702826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neinast, M., Murashige, D. and Arany, Z. (2019) Branched chain amino acids. Annu. Rev. Physiol. 81, 139–164 10.1146/annurev-physiol-020518-114455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Supruniuk, E., Żebrowska, E. and Chabowski, A. (2021) Branched chain amino acids—friend or foe in the control of energy substrate turnover and insulin sensitivity? Crit. Rev. Food Sci. Nutr. 1–39 10.1080/10408398.2021.1977910 [DOI] [PubMed] [Google Scholar]
- 90.Ali, S. and Garcia, J.M. (2014) Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology 60, 294–305 10.1159/000356760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kristiansen, O.P. and Mandrup-Poulsen, T. (2005) Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54, S114–S124 10.2337/diabetes.54.suppl_2.S114 [DOI] [PubMed] [Google Scholar]
- 92.Grunfeld, C., Adi, S., Soued, M., Moser, A., Fiers, W. and Feingold, K.R. (1991) Search for mediators of the lipogenic effects of tumor necrosis factor: potential role for interleukin 6. Cancer Res. 50, 4233–4238 https://pubmed.ncbi.nlm.nih.gov/2032220/ [PubMed] [Google Scholar]
- 93.Kim, J., Bachmann, R. A. and Chen, J. (2009) Chapter 21 interleukin-6 and insulin resistance. In Vitamins & Hormones (Litwack, G., ed.), pp. 613–633, Academic Press, Cambridge, MA, USA; [DOI] [PubMed] [Google Scholar]
- 94.del Aguila, L.F., Claffey, K.P. and Kirwan, J.P. (1999) TNF-α impairs insulin signaling and insulin stimulation of glucose uptake in C2C12muscle cells. Am. J. Physiol. 276, E849–E855 10.1152/ajpendo.1999.276.5.E849 [DOI] [PubMed] [Google Scholar]
- 95.Hotamisligil, G.S., Shargill, N.S. and Spiegelman, B.M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 96.Wellen, K.E. and Hotamisligil, G.S. (2005) Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 10.1172/JCI25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kern, L., Mittenbühler, M.J., Vesting, A.J., Ostermann, A.L., Wunderlich, C.M. and Wunderlich, F.T. (2018) Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation—driven liver and colorectal cancers. Cancers (Basel) 11 10.3390/cancers11010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park, E.J., Lee, J.H., Yu, G.-Y., He, G., Ali, S.R., Holzer, R.G.et al. (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 10.1016/j.cell.2009.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leitner, B.P. and Perry, R.J. (2020) The impact of obesity on tumor glucose uptake in breast and lung cancer. JNCI Cancer Spectr. 4 10.1093/jncics/pkaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim, J.-S., Kim, E.S., Liu, D., Lee, J.J., Solis, L., Behrens, C.et al. (2012) Prognostic impact of insulin receptor expression on survival of patients with nonsmall cell lung cancer. Cancer 118, 2454–2465 10.1002/cncr.26492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gallagher, E.J., Fei, K., Feldman, S.M., Port, E., Friedman, N.B., Boolbol, S.K., et al. (2020) Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Res. 22, 40 10.1186/s13058-020-01281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heckl, S.M., Pellinghaus, M., Krüger, S., Bosselmann, C., Wilhelm, F., Behrens, H.-M.et al. (2018) Epithelial insulin receptor expression-prognostic relevance in colorectal cancer. Oncotarget 9, 37497–37508 10.18632/oncotarget.26490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hopkins, B.D., Goncalves, M.D. and Cantley, L.C. (2020) Insulin-PI3 K signalling: an evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 16, 276–283 10.1038/s41574-020-0329-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hopkins, B.D., Pauli, C., Du, X., Wang, D.G., Li, X., Wu, D., et al. (2018) Suppression of insulin feedback enhances the efficacy of PI3 K inhibitors. Nature 560, 499–503 10.1038/s41586-018-0343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Molinaro, A., Becattini, B., Mazzoli, A., Bleve, A., Radici, L., Maxvall, I.et al. (2019) Insulin-driven PI3K-AKT signaling in the hepatocyte is mediated by redundant PI3Kα and PI3Kβ activities and Is promoted by RAS. Cell Metab. 29, 1400–1409.e5 10.1016/j.cmet.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 106.Yoon, M.-S. (2017) The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 9, E1176 10.3390/nu9111176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nemazanyy, I., Espeillac, C., Pende, M. and Panasyuk, G. (2013) Role of PI3 K, mTOR and Akt2 signalling in hepatic tumorigenesis via the control of PKM2 expression. Biochem. Soc. Trans. 41, 917–922 10.1042/BST20130034 [DOI] [PubMed] [Google Scholar]
- 108.Sarbassov, D.D., Ali, S.M. and Sabatini, D.M. (2005) Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 10.1016/j.ceb.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 109.Di Camillo, B., Carlon, A., Eduati, F. and Toffolo, G.M. (2016) A rule-based model of insulin signalling pathway. BMC Syst. Biol. 10, 38 10.1186/s12918-016-0281-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yonezawa, K., Ando, A., Kaburagi, Y., Yamamoto-Honda, R., Kitamura, T., Hara, K.et al. (1994) Signal transduction pathways from insulin receptors to Ras. Analysis by mutant insulin receptors. J. Biol. Chem. 269, 4634–4640 10.1016/S0021-9258(17)41823-0 [DOI] [PubMed] [Google Scholar]
- 111.Mardilovich, K., Pankratz, S.L. and Shaw, L.M. (2009) Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun. Signal. 7, 14 10.1186/1478-811X-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belfiore, A., Frasca, F., Pandini, G., Sciacca, L. and Vigneri, R. (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 30, 586–623 10.1210/er.2008-0047 [DOI] [PubMed] [Google Scholar]
- 113.Kido, Y., Nakae, J. and Accili, D. (2001) The insulin receptor and its cellular targets. J. Clin. Endocrinol. Metab. 86, 972–979 10.1210/jcem.86.3.7306 [DOI] [PubMed] [Google Scholar]
- 114.Gutmann, T., Schäfer, I.B., Poojari, C., Brankatschk, B., Vattulainen, I., Strauss, M. et al. et al. (2020) Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. J. Cell Biol. 219, e201907210 10.1083/jcb.201907210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myers, M.G., Wang, L.M., Sun, X.J., Zhang, Y., Yenush, L., Schlessinger, J.et al. (1994) Role of IRS-1-GRB-2 complexes in insulin signaling. Mol. Cell. Biol. 14, 3577–3587 10.1128/mcb.14.6.3577-3587.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagao, H., Cai, W., Wewer Albrechtsen, N.J., Steger, M., Batista, T.M., Pan, H.et al. (2021) Distinct signaling by insulin and IGF-1 receptors and their extra- and intracellular domains. Proc. Natl Acad. Sci. U.S.A. 118, e2019474118 10.1073/pnas.2019474118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang, S., Weinheimer, C., Courtois, M., Kovacs, A., Zhang, C.E., Cheng, A.M.et al. (2003) The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J. Clin. Invest. 111, 833–841 10.1172/JCI16290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang, W. and Liu, H.T. (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 12, 9–18 10.1038/sj.cr.7290105 [DOI] [PubMed] [Google Scholar]
- 119.Schnelzer, A., Prechtel, D., Knaus, U., Dehne, K., Gerhard, M., Graeff, H.et al. (2000) Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19, 3013–3020 10.1038/sj.onc.1203621 [DOI] [PubMed] [Google Scholar]
- 120.Audzeyenka, I., Rogacka, D., Rachubik, P., Typiak, M., Rychłowski, M., Angielski, S.et al. (2021) The PKGIα–Rac1 pathway is a novel regulator of insulin-dependent glucose uptake in cultured rat podocytes. J. Cell Physiol. 236, 4655–4668 10.1002/jcp.30188 [DOI] [PubMed] [Google Scholar]
- 121.Takenaka, N., Nakao, M., Hasegawa, K., Chan, M.P. and Satoh, T. (2020) The guanine nucleotide exchange factor FLJ00068 activates Rac1 in adipocyte insulin signaling. FEBS Lett. 594, 4370–4380 10.1002/1873-3468.13939 [DOI] [PubMed] [Google Scholar]
- 122.Thamilselvan, V. and Kowluru, A. (2021) Paradoxical regulation of glucose-induced Rac1 activation and insulin secretion by RhoGDIβ in pancreatic β-cells. Small GTPases 12, 114–121 10.1080/21541248.2019.1635403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bid, H.K., Roberts, R.D., Manchanda, P.K. and Houghton, P.J. (2013) RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol. Cancer Ther. 12, 1925–1934 10.1158/1535-7163.MCT-13-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zou, T., Mao, X., Yin, J., Li, X., Chen, J., Zhu, T.et al. (2017) Emerging roles of RAC1 in treating lung cancer patients: emerging roles of RAC1 in treating lung cancer patients. Clin. Genet. 91, 520–528 10.1111/cge.12908 [DOI] [PubMed] [Google Scholar]
- 125.Yang, W.-H., Lan, H.-Y., Huang, C.-H., Tai, S.-K., Tzeng, C.-H., Kao, S.-Y.et al. (2012) RAC1 activation mediates Twist1-induced cancer cell migration. Nat. Cell Biol. 14, 366–374 10.1038/ncb2455 [DOI] [PubMed] [Google Scholar]
- 126.Heid, I., Lubeseder–Martellato, C., Sipos, B., Mazur, P.K., Lesina, M., Schmid, R.M.et al. (2011) Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology 141, 719–730.e7 10.1053/j.gastro.2011.04.043 [DOI] [PubMed] [Google Scholar]
- 127.Avruch, J. (2007) MAP kinase pathways: the first twenty years. Biochim. Biophys. Acta 1773, 1150–1160 10.1016/j.bbamcr.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Werner, H., Weinstein, D. and Bentov, I. (2008) Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Arch. Physiol. Biochem. 114, 17–22 10.1080/13813450801900694 [DOI] [PubMed] [Google Scholar]
- 129.Cao, J. and Yee, D. (2021) Disrupting insulin and IGF receptor function in cancer. Int. J. Mol. Sci. 22, 555 10.3390/ijms22020555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gallagher, E.J., Alikhani, N., Tobin-Hess, A., Blank, J., Buffin, N.J., Zelenko, Z.et al. (2013) Insulin receptor phosphorylation by endogenous insulin or the insulin analog AspB10 promotes mammary tumor growth independent of the IGF-I receptor. Diabetes 62, 3553–3560 10.2337/db13-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bendall, S.C., Stewart, M.H., Menendez, P., George, D., Vijayaragavan, K., Werbowetski-Ogilvie, T., et al. (2007) IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448, 1015–1021 10.1038/nature06027 [DOI] [PubMed] [Google Scholar]
- 132.Ngo, M.-H.T., Jeng, H.-Y., Kuo, Y.-C., Diony Nanda, J., Brahmadhi, A., Ling, T.-Y.et al. (2021) The role of IGF/IGF-1R signaling in hepatocellular carcinomas: stemness-related properties and drug resistance. IJMS 22, 1931 10.3390/ijms22041931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramakrishnan, V., Xu, B., Akers, J., Nguyen, T., Ma, J., Dhawan, S., et al. (2020) Radiation-induced extracellular vesicle (EV) release of miR-603 promotes IGF1-mediated stem cell state in glioblastomas. EBioMedicine 55, 102736 10.1016/j.ebiom.2020.102736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wicha, M.S., Liu, S. and Dontu, G. (2006) Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 66, 1883–1890. discussion 1895–1896 10.1158/0008-5472.CAN-05-3153 [DOI] [PubMed] [Google Scholar]
- 135.Shan, J., Shen, J., Liu, L., Xia, F., Xu, C., Duan, G., et al. (2012) Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 56, 1004–1014 10.1002/hep.25745 [DOI] [PubMed] [Google Scholar]
- 136.Medyouf, H., Gusscott, S., Wang, H., Tseng, J.-C., Wai, C., Nemirovsky, O., et al. (2011) High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J. Exp. Med. 208, 1809–1822 10.1084/jem.20110121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shahbazi, M., Cundiff, P., Zhou, W., Lee, P., Patel, A., D'Souza, S.L.et al. (2019) The role of insulin as a key regulator of seeding, proliferation, and mRNA transcription of human pluripotent stem cells. Stem Cell Res. Ther. 10, 228 10.1186/s13287-019-1319-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heiden, V., Cantley, M.G., and Thompson, L.C. and B, C. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Warburg, O. (1925) The metabolism of carcinoma cells. J. Cancer Res. 9, 148–163 10.1158/jcr.1925.148 [DOI] [Google Scholar]
- 140.Warburg, O., Wind, F. and Negelein, E. (1927) The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin, L. and Zhou, Y. (2019) Crucial role of the pentose phosphate pathway in malignant tumors. Oncol. Lett. 17, 4213–4221 10.3892/ol.2019.10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Magkos, F., Wang, X. and Mittendorfer, B. (2010) Metabolic actions of insulin in men and women. Nutrition 26, 686–693 10.1016/j.nut.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang, Z., Feng, X., Molinolo, A.A., Martin, D., Vitale-Cross, L., Nohata, N., et al. (2019) 4E-BP1 is a tumor suppressor protein reactivated by mTOR inhibition in head and neck cancer. Cancer Res. 79, 1438–1450 10.1158/0008-5472.CAN-18-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nejad, E., Najafgholian, A., Rostami, S., Sistani, A., Shojaeifar, A., Esparvarinha, S., (2021) The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 21, 62 10.1186/s12935-020-01719-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buller, C.L., Loberg, R.D., Fan, M.-H., Zhu, Q., Park, J.L., Vesely, E.et al. (2008) A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am. J. Physiol. Cell Physiol. 295, C836–C843 10.1152/ajpcell.00554.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brown, R.S. and Wahl, R.L. (1993) Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer 72, 2979–2985 [DOI] [PubMed] [Google Scholar]
- 147.Zhao, H., Sun, J., Shao, J., Zou, Z., Qiu, X., Wang, E.et al. (2019) Glucose transporter 1 promotes the malignant phenotype of non-small cell lung cancer through integrin β1/Src/FAK signaling. J. Cancer 10, 4989–4997 10.7150/jca.30772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ambrosetti, D., Dufies, M., Dadone, B., Durand, M., Borchiellini, D., Amiel, J., et al. (2018) The two glycolytic markers GLUT1 and MCT1 correlate with tumor grade and survival in clear-cell renal cell carcinoma. PLoS One 13, e0193477 10.1371/journal.pone.0193477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Haber, R.S., Rathan, A., Weiser, K.R., Pritsker, A., Itzkowitz, S.H., Bodian, C.et al. (1998) GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer 83, 34–40 [DOI] [PubMed] [Google Scholar]
- 150.Koch, A., Lang, S.A., Wild, P.J., Gantner, S., Mahli, A., Spanier, G.et al. (2015) Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget 6, 32748–32760 10.18632/oncotarget.4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uhlen, M., Zhang, C., Lee, S., Sjöstedt, E., Fagerberg, L., Bidkhori, G., et al. (2017) A pathology atlas of the human cancer transcriptome. Science 357, eaan2507 10.1126/science.aan2507 [DOI] [PubMed] [Google Scholar]
- 152.Geuna, E., Roda, D., Rafii, S., Jimenez, B., Capelan, M., Rihawi, K., et al. (2015) Complications of hyperglycaemia with PI3K–AKT–mTOR inhibitors in patients with advanced solid tumours on phase I clinical trials. Br. J. Cancer 113, 1541–1547 10.1038/bjc.2015.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Busaidy, N.L., Farooki, A., Dowlati, A., Perentesis, J.P., Dancey, J.E., Doyle, L.A.et al. (2012) Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J. Clin. Oncol. 30, 2919–2928 10.1200/JCO.2011.39.7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sivendran, S., Agarwal, N., Gartrell, B., Ying, J., Boucher, K.M., Choueiri, T.K.et al. (2014) Metabolic complications with the use of mTOR inhibitors for cancer therapy. Cancer Treat Rev. 40, 190–196 10.1016/j.ctrv.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hudes, G., Carducci, M., Tomczak, P., Dutcher, J., Figlin, R., Kapoor, A., et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 156.Motzer, R.J., Escudier, B., Oudard, S., Hutson, T.E., Porta, C., Bracarda, S., et al. (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma. Cancer 116, 4256–4265 10.1002/cncr.25219 [DOI] [PubMed] [Google Scholar]
- 157.Yao, J.C., Shah, M.H., Ito, T., Bohas, C.L., Wolin, E.M., Van Cutsem, E., et al. (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baselga, J., Campone, M., Piccart, M., Burris, H.A., Rugo, H.S., Sahmoud, T., et al. (2012) Everolimus in postmenopausal hormone-receptor–Ppsitive advanced breast cancer. N. Engl. J. Med. 366, 520–529 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baselga, J., van Dam, P., Greil, R., Gardner, H., Bandaru, R., Molloy, B.et al. (2008) Improved clinical and cell cycle response with an mTOR inhibitor, daily oral RAD001 (everolimus) plus letrozole versus placebo plus letrozole in a randomized phase II neoadjuvant trial in ER plus breast cancer. J. Clin. Oncol. 26, 1582–1584 10.1200/JCO.2007.15.3700 [DOI] [Google Scholar]
- 160.Khan, K.H., Wong, M., Rihawi, K., Bodla, S., Morganstein, D., Banerji, U.et al. (2016) Hyperglycemia and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3 K/AKT/mTOR) inhibitors in phase I trials: incidence, predictive factors, and management. Oncologist 21, 855–860 10.1634/theoncologist.2015-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sahakian, N., Cattieuw, L., Ramillon-Cury, C., Corroller, A.B.-L., Silvestre-Aillaud, P., Béliard, S.et al. (2021) SGLT2 inhibitors as potentially helpful drugs in PI3 K inhibitor-induced diabetes: a case report. Clin. Diabetes Endocrinol. 7, 17 10.1186/s40842-021-00125-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blow, T., Hyde, P.N., Falcone, J.N., Neinstein, A., Vasan, N., Chitkara, R., et al. (2021) Treating alpelisib-induced hyperglycemia with very low carbohydrate diets and sodium-glucose co-transporter 2 inhibitors: a case series. Integr. Cancer Ther. 20, 15347354211032284 10.1177/15347354211032283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zeiss, K., Parhofer, K.G., Heinemann, V., Haas, M., Laubender, R.P., Holdenrieder, S.et al. (2013) Glucose and lipid metabolism in patients with advanced pancreatic cancer receiving palliative chemotherapy. Anticancer Res. 33, 287–292 https://ar.iiarjournals.org/content/33/1/287 [PubMed] [Google Scholar]
- 164.Kabat, G.C., Kim, M.Y., Lane, D.S., Zaslavsky, O., Ho, G.Y.F., Luo, J., et al. (2018) Serum glucose and insulin and risk of cancers of the breast, endometrium, and ovary in postmenopausal women. Eur. J. Cancer Prev. 27, 261–268 10.1097/CEJ.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 165.Gunter, M.J., Hoover, D.R., Yu, H., Wassertheil-Smoller, S., Manson, J.E., Li, J., et al. (2008) A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol. Biomark. Prev. 17, 921–929 10.1158/1055-9965.EPI-07-2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Del Giudice, M.E., Fantus, I.G., Ezzat, S., McKeown-Eyssen, G., Page, D. and Goodwin, P.J. (1998) Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res. Treat. 47, 111–120 10.1023/A:1005831013718 [DOI] [PubMed] [Google Scholar]
- 167.Pandeya, D.R., Mittal, A., Sathian, B. and Bhatta, B. (2014) Role of hyperinsulinemia in increased risk of prostate cancer: a case control study from Kathmandu valley. Asian Pac. J. Cancer Prev. 15, 1031–1033 10.7314/APJCP.2014.15.2.1031 [DOI] [PubMed] [Google Scholar]
- 168.Nandeesha, H., Koner, B.C. and Dorairajan, L.N. (2008) Altered insulin sensitivity, insulin secretion and lipid profile in non-diabetic prostate carcinoma. Acta Physiol. Hung. 95, 97–105 10.1556/APhysiol.95.2008.1.7 [DOI] [PubMed] [Google Scholar]
- 169.Kim, N.H., Chang, Y., Lee, S.R., Ryu, S. and Kim, H.J. (2020) Glycemic status, insulin resistance, and risk of pancreatic cancer mortality in individuals with and without diabetes. Am. J. Gastroenterol. 115, 1840–1848 10.14309/ajg.0000000000000956 [DOI] [PubMed] [Google Scholar]
- 170.Pan, K., Chlebowski, R.T., Mortimer, J.E., Gunter, M.J., Rohan, T., Vitolins, M.Z.et al. (2020) Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women's Health Initiative. Cancer 126, 3638–3647 10.1002/cncr.33002 [DOI] [PubMed] [Google Scholar]
- 171.Wargny, M., Balkau, B., Lange, C., Charles, M.-A., Giral, P. and Simon, D. (2018) Association of fasting serum insulin and cancer mortality in a healthy population – 28-year follow-up of the French TELECOM study. Diabetes Metab. 44, 30–37 10.1016/j.diabet.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 172.Tsujimoto, T., Kajio, H. and Sugiyama, T. (2017) Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: a population-based observational study. Int. J. Cancer 141, 102–111 10.1002/ijc.30729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perseghin, G., Calori, G., Lattuada, G., Ragogna, F., Dugnani, E., Garancini, M.P., et al. (2012) Insulin resistance/hyperinsulinemia and cancer mortality: the Cremona study at the 15th year of follow-up. Acta Diabetol. 49, 421–428 10.1007/s00592-011-0361-2 [DOI] [PubMed] [Google Scholar]
- 174.Tabung, F.K., Noonan, A., Lee, D.H., Song, M., Clinton, S.K., Spakowicz, D., et al. (2020) Post-diagnosis dietary insulinemic potential and survival outcomes among colorectal cancer patients. BMC Cancer 20, 817 10.1186/s12885-020-07288-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morales-Oyarvide, V., Yuan, C., Babic, A., Zhang, S., Niedzwiecki, D., Brand-Miller, J.C., et al. (2019) Dietary insulin load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803 (Alliance). J. Natl Cancer Inst. 111, 170–179 10.1093/jnci/djy098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tabung, F.K., Wang, W., Fung, T.T., Smith-Warner, S.A., Keum, N., Wu, K.et al. (2018) Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am. J. Clin. Nutr. 108, 363–370 10.1093/ajcn/nqy093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yuan, C., Bao, Y., Sato, K., Nimptsch, K., Song, M., Brand-Miller, J.C., et al. (2017) Influence of dietary insulin scores on survival in colorectal cancer patients. Br. J. Cancer 117, 1079–1087 10.1038/bjc.2017.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mazidi, M., Katsiki, N., Mikhailidis, D. P. and Banach, M., and International Lipid Expert Panel (ILEP). (2020) Effect of dietary insulinemia on all-cause and cause-specific mortality: results from a cohort study. J. Am. Coll. Nutr. 39, 407–413. 10.1080/07315724.2019.1646167 [DOI] [PubMed] [Google Scholar]
- 179.Yu, E.Y.W., Wesselius, A., Mehrkanoon, S., Brinkman, M., van den Brandt, P., White, E., et al. (2020) Grain and dietary fiber intake and bladder cancer risk: a pooled analysis of prospective cohort studies. Am. J. Clin. Nutr. 112, 1252–1266 10.1093/ajcn/nqaa215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Catsburg, C., Gunter, M.J., Chen, C., Cote, M.L., Kabat, G.C., Nassir, R.et al. (2014) Insulin, estrogen, inflammatory markers, and risk of benign proliferative breast disease. Cancer Res. 74, 3248–3258 10.1158/0008-5472.CAN-13-3514 [DOI] [PubMed] [Google Scholar]
- 181.Duggan, C., Onstad, L., Hardikar, S., Blount, P.L., Reid, B.J. and Vaughan, T.L. (2013) Association between markers of obesity and progression from Barrett's esophagus to esophageal adenocarcinoma. Clin. Gastroenterol. Hepatol. 11, 934–943 10.1016/j.cgh.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cox, M.E., Gleave, M.E., Zakikhani, M., Bell, R.H., Piura, E., Vickers, E.et al. (2009) Insulin receptor expression by human prostate cancers. Prostate 69, 33–40 10.1002/pros.20852 [DOI] [PubMed] [Google Scholar]
- 183.Gregory, J.M., Smith, T.J., Slaughter, J.C., Mason, H.R., Hughey, C.C., Smith, M.S., et al. (2019) Iatrogenic hyperinsulinemia, not hyperglycemia, drives insulin resistance in type 1 diabetes as revealed by comparison with GCK-MODY (MODY2). Diabetes 68, 1565–1576 10.2337/db19-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barone, B.B., Yeh, H.-C., Snyder, C.F., Peairs, K.S., Stein, K.B., Derr, R.L.et al. (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. J. Am. Med. Assoc. 300, 2754–2764 10.1001/jama.2008.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lipscombe, L.L., Goodwin, P.J., Zinman, B., McLaughlin, J.R. and Hux, J.E. (2008) The impact of diabetes on survival following breast cancer. Breast Cancer Res. Treat. 109, 389–395 10.1007/s10549-007-9654-0 [DOI] [PubMed] [Google Scholar]
- 186.Ma, J., Li, H., Giovannucci, E., Mucci, L., Qiu, W., Nguyen, P.L.et al. (2008) Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 9, 1039–1047 10.1016/S1470-2045(08)70235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wolpin, B.M., Meyerhardt, J.A., Chan, A.T., Ng, K., Chan, J.A., Wu, K.et al. (2009) Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J. Clin. Oncol. 27, 176–185 10.1200/JCO.2008.17.9945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.García-Jiménez, C., Gutiérrez-Salmerón, M., Chocarro-Calvo, A., García-Martinez, J.M., Castaño, A. and De la Vieja, A. (2016) From obesity to diabetes and cancer: epidemiological links and role of therapies. Br. J. Cancer 114, 716–722 10.1038/bjc.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Currie, C.J., Poole, C.D., Jenkins-Jones, S., Gale, E.A.M., Johnson, J.A. and Morgan, C.L. (2012) Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 35, 299–304 10.2337/dc11-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Perry, R.J. and Shulman, G.I. (2020) Mechanistic links between obesity, insulin, and cancer. Trends Cancer 6, 75–78 10.1016/j.trecan.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rahman, I., Athar, M.T. and Islam, M. (2020) Type 2 diabetes, obesity, and cancer share some common and critical pathways. Front. Oncol. 10, 600824 10.3389/fonc.2020.600824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Boakye, D., Günther, K., Niedermaier, T., Haug, U., Ahrens, W. and Nagrani, R. (2021) Associations between comorbidities and advanced stage diagnosis of lung, breast, colorectal, and prostate cancer: a systematic review and meta-analysis. Cancer Epidemiol. 75, 102054 10.1016/j.canep.2021.102054 [DOI] [PubMed] [Google Scholar]
- 193.Wang, L., Zhong, L., Xu, B., Chen, M. and Huang, H. (2020) Diabetes mellitus and the risk of ovarian cancer: a systematic review and meta-analysis of cohort and case-control studies. BMJ Open 10, e040137 10.1136/bmjopen-2020-040137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramos-Garcia, P., Roca-Rodriguez, M.D.M., Aguilar-Diosdado, M. and Gonzalez-Moles, M.A. (2021) Diabetes mellitus and oral cancer/oral potentially malignant disorders: a systematic review and meta-analysis. Oral Dis. 27, 404–421 10.1111/odi.13289 [DOI] [PubMed] [Google Scholar]
- 195.Yi, Z.-H., Luther, Y., Xiong, G.-H., Ni, Y.-L., Yun, F., Chen, J.et al. (2020) Association between diabetes mellitus and lung cancer: meta-analysis. Eur. J. Clin. Invest. 50, e13332 10.1111/eci.13332 [DOI] [PubMed] [Google Scholar]
- 196.Ling, S., Brown, K., Miksza, J.K., Howells, L., Morrison, A., Issa, E.et al. (2020) Association of type 2 diabetes with cancer: a meta-analysis With bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care 43, 2313–2322 10.2337/dc20-0204 [DOI] [PubMed] [Google Scholar]
- 197.Raffone, A., Travaglino, A., Saccone, G., D'Alessandro, P., Arduino, B., Mascolo, M.et al. (2020) Diabetes mellitus is associated with occult cancer in endometrial hyperplasia. Pathol. Oncol. Res. 26, 1377–1384 10.1007/s12253-019-00684-3 [DOI] [PubMed] [Google Scholar]
- 198.Zhang, J.-J., Jia, J.-P., Shao, Q. and Wang, Y.-K. (2019) Diabetes mellitus and risk of pancreatic cancer in China: a meta-analysis based on 26 case-control studies. Prim Care Diabetes 13, 276–282 10.1016/j.pcd.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 199.Saed, L., Varse, F., Baradaran, H.R., Moradi, Y., Khateri, S., Friberg, E., et al. (2019) The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer 19, 527 10.1186/s12885-019-5748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li, H. and Qian, J. (2017) Association of diabetes mellitus with thyroid cancer risk: a meta-analysis of cohort studies. Medicine (Baltimore) 96, e8230 10.1097/MD.0000000000008230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu, Y., Huo, R., Chen, X. and Yu, X. (2017) Diabetes mellitus and the risk of bladder cancer: a PRISMA-compliant meta-analysis of cohort studies. Medicine (Baltimore) 96, e8588 10.1097/MD.0000000000008588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tran, T.T., Medline, A. and Bruce, W.R. (1996) Insulin promotion of colon tumors in rats. Cancer Epidemiol. Biomarkers Prev. 5, 1013–1015 https://pubmed.ncbi.nlm.nih.gov/8959325/ [PubMed] [Google Scholar]
- 203.Corpet, D.E., Jacquinet, C., Peiffer, G. and Taché, S. (1997) Insulin injections promote the growth of aberrant crypt foci in the colon of rats. Nutr. Cancer 27, 316–320 10.1080/01635589709514543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pour, P.M., Kazakoff, K. and Carlson, K. (1990) Inhibition of streptozotocin-induced islet cell tumors and N-nitrosobis(2-oxopropyl)amine-induced pancreatic exocrine tumors in Syrian hamsters by exogenous insulin. Cancer Res. 50, 1634–1639 https://pubmed.ncbi.nlm.nih.gov/2154330/ [PubMed] [Google Scholar]
- 205.Novosyadlyy, R., Lann, D.E., Vijayakumar, A., Rowzee, A., Lazzarino, D.A., Fierz, Y., et al. (2010) Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 70, 741–751 10.1158/0008-5472.CAN-09-2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pan, F. and Hong, L.-Q. (2014) Insulin promotes proliferation and migration of breast cancer cells through the extracellular regulated kinase pathway. Asian Pac. J. Cancer Prev. 15, 6349–6352 10.7314/APJCP.2014.15.15.6349 [DOI] [PubMed] [Google Scholar]
- 207.Nasiri, A.R., Rodrigues, M.R., Li, Z., Leitner, B.P. and Perry, R.J. (2019) SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 7, 10 10.1186/s40170-019-0203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang, Y., Nasiri, A.R., Damsky, W.E., Perry, C.J., Zhang, X.-M., Rabin-Court, A.et al. (2018) Uncoupling hepatic oxidative phosphorylation reduces tumor growth in Two murine models of colon cancer. Cell Rep. 24, 47–55 10.1016/j.celrep.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Boucher, J., Kleinridders, A. and Kahn, C.R. (2014) Insulin receptor signaling in normal and insulin-resistant states. Cold Spring. Harb. Perspect. Biol. 6, a009191 10.1101/cshperspect.a009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gallagher, E.J. and LeRoith, D. (2020) Hyperinsulinaemia in cancer. Nat. Rev. Cancer 20, 629–644 10.1038/s41568-020-0295-5 [DOI] [PubMed] [Google Scholar]
- 211.Haeusler, R.A., McGraw, T.E. and Accili, D. (2018) Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 19, 31–44 10.1038/nrm.2017.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guertin, D.A. and Sabatini, D.M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 10.1016/j.ccr.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 213.Marino, N., German, R., Rao, X., Simpson, E., Liu, S., Wan, J., et al. (2020) Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. npj Breast Cancer 6, 1–13 10.1038/s41523-020-00191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wellberg, E.A., Kabos, P., Gillen, A.E., Jacobsen, B.M., Brechbuhl, H.M., Johnson, S.J., et al. (2018) FGFR1 underlies obesity-associated progression of estrogen receptor-positive breast cancer after estrogen deprivation. JCI Insight 3, 120594 10.1172/jci.insight.120594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Maguire, O.A., Ackerman, S.E., Szwed, S.K., Maganti, A.V., Marchildon, F., Huang, X., et al. (2021) Creatine-mediated crosstalk between adipocytes and cancer cells regulates obesity-driven breast cancer. Cell Metab. 33, 499–512.e6 10.1016/j.cmet.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen, Y.-C., Chien, C.-Y., Hsu, C.-C., Lee, C.-H., Chou, Y.-T., Shiah, S.-G., et al. (2021) Obesity-associated leptin promotes chemoresistance in colorectal cancer through YAP-dependent AXL upregulation. Am. J. Cancer Res. 11, 4220–4240 https://pubmed.ncbi.nlm.nih.gov/34659884/ [PMC free article] [PubMed] [Google Scholar]
- 217.Padmanabhan, S., Gaire, B., Zou, Y., Uddin, M.M., DeLeon, D. and Vancurova, I. (2021) IFNγ induces JAK1/STAT1/p65 NFκB-dependent interleukin-8 expression in ovarian cancer cells, resulting in their increased migration. Int. J. Biochem. Cell Biol. 141, 106093 10.1016/j.biocel.2021.106093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bowker, S.L., Majumdar, S.R., Veugelers, P. and Johnson, J.A. (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29, 254–258 10.2337/diacare.29.02.06.dc05-1558 [DOI] [PubMed] [Google Scholar]
- 219.Haring, A., Murtola, T.J., Talala, K., Taari, K., Tammela, T.L.J. and Auvinen, A. (2017) Antidiabetic drug use and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Scand J. Urol. 51, 5–12 10.1080/21681805.2016.1271353 [DOI] [PubMed] [Google Scholar]
- 220.Chang, C.-H., Lin, J.-W., Wu, L.-C., Lai, M.-S. and Chuang, L.-M. (2012) Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 97, E1170–E1175 10.1210/jc.2012-1162 [DOI] [PubMed] [Google Scholar]
- 221.Hsieh, M.-C., Lee, T.-C., Cheng, S.-M., Tu, S.-T., Yen, M.-H. and Tseng, C.-H. (2012) The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp. Diabetes Res. 2012, 413782 10.1155/2012/413782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kawaguchi, T., Taniguchi, E., Morita, Y., Shirachi, M., Tateishi, I., Nagata, E.et al. (2010) Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 30, 479–486 10.1111/j.1478-3231.2009.02191.x [DOI] [PubMed] [Google Scholar]
- 223.Liu, X.-L., Wu, H., Zhao, L.-G., Xu, H.-L., Zhang, W. and Xiang, Y.-B. (2018) Association between insulin therapy and risk of liver cancer among diabetics: a meta-analysis of epidemiological studies. Eur. J. Gastroenterol. Hepatol. 30, 1–8 10.1097/MEG.0000000000001001 [DOI] [PubMed] [Google Scholar]
- 224.Alsheikh, H.A.M., Metge, B.J., Ha, C.-M., Hinshaw, D.C., Mota, M.S.V., Kammerud, S.C., et al. (2021) Normalizing glucose levels reconfigures the mammary tumor immune and metabolic microenvironment and decreases metastatic seeding. Cancer Lett. 517, 24–34 10.1016/j.canlet.2021.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Farahi, A., Abedini, M.R., Javdani, H., Arzi, L., Chamani, E., Farhoudi, R.et al. (2021) Crocin and metformin suppress metastatic breast cancer progression via VEGF and MMP9 downregulations: in vitro and in vivo studies. Mol. Cell Biochem. 476, 3341–3351 10.1007/s11010-020-04043-8 [DOI] [PubMed] [Google Scholar]
- 226.Lee, Y., Joo, J., Lee, Y.J., Lee, E.K., Park, S., Kim, T.-S., et al. (2021) Randomized phase II study of platinum-based chemotherapy plus controlled diet with or without metformin in patients with advanced non-small cell lung cancer. Lung Cancer 151, 8–15 10.1016/j.lungcan.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 227.Kim, H.J., Lee, S., Chun, K.H., Jeon, J.Y., Han, S.J., Kim, D.J., et al. (2018) Metformin reduces the risk of cancer in patients with type 2 diabetes: an analysis based on the Korean national diabetes program cohort. Medicine 97, e0036 10.1097/MD.0000000000010036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Makari-Judson, G., Katz, D., Barham, R. and Mertens, W. (2009) Deleterious effect of chemotherapy on measures of insulin resistance in patients with newly-diagnosed invasive breast cancer. Cancer Res. 69, 1054–1054 10.1158/0008-5472.SABCS-09-1054 [DOI] [Google Scholar]
- 229.Alan, O., Akin Telli, T., Aktas, B., Koca, S., Ökten, I.N., Hasanov, R., et al. (2020) Is insulin resistance a predictor for complete response in breast cancer patients who underwent neoadjuvant treatment? World J. Surg. Oncol. 18, 242 10.1186/s12957-020-02019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Willemse, P.M., Burggraaf, J., Hamdy, N.A.T., Weijl, N.I., Vossen, C.Y., van Wulften, L.et al. (2013) Prevalence of the metabolic syndrome and cardiovascular disease risk in chemotherapy-treated testicular germ cell tumour survivors. Br. J. Cancer 109, 60–67 10.1038/bjc.2013.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Haugnes, H.S., Aass, N., Fosså, S.D., Dahl, O., Klepp, O., Wist, E.A.et al. (2007) Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann. Oncol. 18, 241–248 10.1093/annonc/mdl372 [DOI] [PubMed] [Google Scholar]
- 232.Demark-Wahnefried, W., Winer, E.P. and Rimer, B.K. (1993) Why women gain weight with adjuvant chemotherapy for breast cancer. J. Clin Oncol. 11, 1418–1429 10.1200/JCO.1993.11.7.1418 [DOI] [PubMed] [Google Scholar]
- 233.Nuver, J., Smit, A.J., Sleijfer, D.T., van Gessel, A.I., van Roon, A.M., van der Meer, J., et al. (2004) Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur. J. Cancer 40, 701–706 10.1016/j.ejca.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 234.Meinardi, M.T., Gietema, J.A., van der Graaf, W.T.A., van Veldhuisen, D.J., Runne, M.A., Sluiter, W.J., et al. (2000) Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J. Clin. Oncol. 18, 1725–1732 10.1200/JCO.2000.18.8.1725 [DOI] [PubMed] [Google Scholar]
- 235.Braga-Basaria, M., Dobs, A.S., Muller, D.C., Carducci, M.A., John, M., Egan, J.et al. (2006) Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J. Clin. Oncol. 24, 3979–3983 10.1200/JCO.2006.05.9741 [DOI] [PubMed] [Google Scholar]
- 236.Oymak, Y., Uysal, B., Hilkay Karapinar, T., Ay, Y., Ince, D., Demirag, B., et al. (2014) Obesity and insulin resistance after chemotherapy in patients with acute lymphoblastic leukemia. Blood 124, 5250 10.1182/blood.V124.21.5250.5250 [DOI] [Google Scholar]
- 237.Meacham, L.R., Chow, E.J., Ness, K.K., Kamdar, K.Y., Chen, Y., Yasui, Y.et al. (2010) Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer Epidemiol. Biomarkers Prev. 19, 170–181 10.1158/1055-9965.EPI-09-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nottage, K.A., Ness, K.K., Li, C., Srivastava, D., Robison, L.L. and Hudson, M.M. (2014) Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia - from the St. Jude Lifetime Cohort. Br. J. Haematol. 165, 364–374 10.1111/bjh.12754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li, C., Liu, P., Liu, L., Zhang, X., Yang, P., Sheng, H.et al. (2014) Metabolic syndrome in hematologic malignancies survivors: a meta-analysis. Med. Oncol. 32, 422 10.1007/s12032-014-0422-9 [DOI] [PubMed] [Google Scholar]
- 240.Talvensaari, K.K., Lanning, M., Tapanainen, P. and Knip, M. (1996) Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J. Clin. Endocrinol. Metab. 81, 3051–3055 10.1210/jcem.81.8.8768873 [DOI] [PubMed] [Google Scholar]
- 241.Lawrence, R.D. (1926) The effect of exercise on insulin action in diabetes. Br. Med. J. 1, 648–650 10.1136/bmj.1.3406.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Richter, E.A., Garetto, L.P., Goodman, M.N. and Ruderman, N.B. (1982) Muscle glucose metabolism following exercise in the Rat: INCREASED SENSITIVITY TO INSULIN. J. Clin. Invest. 69, 785–793 10.1172/JCI110517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ren, J.M., Semenkovich, C.F., Gulve, E.A., Gao, J. and Holloszy, J.O. (1994) Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J. Biol. Chem. 269, 14396–14401 10.1016/S0021-9258(17)36636-X [DOI] [PubMed] [Google Scholar]
- 244.Pruett, E.D.R. (1971) Plasma insulin levels during prolonged exercise. In Muscle Metabolism During Exercise: Proceedings of a Karolinska Institutet Symposium held in Stockholm, Sweden, September 6–9, 1970 Honorary guest: E Hohwü Christensen (Pernow, B. and Saltin, B., eds.), pp. 165–175, Springer US, Boston, MA [Google Scholar]
- 245.Knudsen, J.R., Steenberg, D.E., Hingst, J.R., Hodgson, L.R., Henriquez-Olguin, C., Li, Z., et al. (2020) Prior exercise in humans redistributes intramuscular GLUT4 and enhances insulin-stimulated sarcolemmal and endosomal GLUT4 translocation. Mol. Metab. 39, 100998 10.1016/j.molmet.2020.100998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sonne, B., Mikines, K.J. and Galbo, H. (1987) Glucose turnover in 48-hour-fasted running rats. Am. J. Physiol. 252, R587–R593 10.1152/ajpregu.1987.252.3.R587 [DOI] [PubMed] [Google Scholar]
- 247.Koyama, Y., Galassetti, P., Coker, R.H., Pencek, R.R., Lacy, D.B., Davis, S.N.et al. (2002) Prior exercise and the response to insulin-induced hypoglycemia in the dog. Am. J. Physiol. Endocrinol. Metab. 282, E1128–E1138 10.1152/ajpendo.00370.2001 [DOI] [PubMed] [Google Scholar]
- 248.Abbott, M.J. and Turcotte, L.P. (2014) AMPK-α2 is involved in exercise training-induced adaptations in insulin-stimulated metabolism in skeletal muscle following high-fat diet. J. Appl. Physiol. 117, 869–879 10.1152/japplphysiol.01380.2013 [DOI] [PubMed] [Google Scholar]
- 249.Justice, T.D., Hammer, G.L., Davey, R.J., Paramalingam, N., Guelfi, K.J., Lewis, L.et al. (2015) Effect of antecedent moderate-intensity exercise on the glycemia-increasing effect of a 30-sec maximal sprint: a sex comparison. Physiol. Rep. 3, e12386 10.14814/phy2.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Santiworakul, A., Chuaychoo, B., Kriengsinyos, W., Saengsirisuwan, V. and Jalayondeja, W. (2014) Substrate utilization during and after high intensity exercise in healthy lean and obese men. J. Med. Assoc. Thail. 97, S50–S54 https://pubmed.ncbi.nlm.nih.gov/25141527/ [PubMed] [Google Scholar]
- 251.Havel, R.J. (1971) Influence of intensity and duration of exercise on supply and use of fuels. In Muscle Metabolism During Exercise: Proceedings of a Karolinska Institutet Symposium held in Stockholm, Sweden, September 6–9, 1970 Honorary guest: E Hohwü Christensen (Pernow, B., and Saltin, B., eds.), pp. 315–325, Springer US, Boston, MA [Google Scholar]
- 252.Romijn, J.A., Coyle, E.F., Sidossis, L.S., Gastaldelli, A., Horowitz, J.F., Endert, E.et al. (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 265, E380–E391 10.1152/ajpendo.1993.265.3.E380 [DOI] [PubMed] [Google Scholar]
- 253.Nguyen, T.-T.P., Jacobs, P.G., Castle, J.R., Wilson, L.M., Kuehl, K., Branigan, D., et al. (2021) Separating insulin-mediated and non-insulin-mediated glucose uptake during and after aerobic exercise in type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 320, E425–E437 10.1152/ajpendo.00534.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Arciero, P.J., Vukovich, M.D., Holloszy, J.O., Racette, S.B. and Kohrt, W.M. (1999) Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J. Appl. Physiol. 86, 1930–1935 10.1152/jappl.1999.86.6.1930 [DOI] [PubMed] [Google Scholar]
- 255.Cartee, G.D. (2015) Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am. J. Physiol. 309, E949–E959 10.1152/ajpendo.00416.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Douen, A.G., Ramlal, T., Rastogi, S., Bilan, P.J., Cartee, G.D., Vranic, M.et al. (1990) Exercise induces recruitment of the ‘insulin-responsive glucose transporter’. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J. Biol. Chem. 265, 13427–13430 10.1016/S0021-9258(18)77362-6 [DOI] [PubMed] [Google Scholar]
- 257.Cartee, G.D., Young, D.A., Sleeper, M.D., Zierath, J., Wallberg-Henriksson, H. and Holloszy, J.O. (1989) Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am. J. Physiol. 256, E494–E499 10.1152/ajpendo.1989.256.4.E494 [DOI] [PubMed] [Google Scholar]
- 258.Wojtaszewski, J.F., Hansen, B.F., Gade, K., Markuns, B., Goodyear, J.F., and Richter, L.J.et al. (2000) Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49, 325–331 10.2337/diabetes.49.3.325 [DOI] [PubMed] [Google Scholar]
- 259.Sylow, L., Kleinert, M., Richter, E.A. and Jensen, T.E. (2017) Exercise-stimulated glucose uptake — regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 13, 133–148 10.1038/nrendo.2016.162 [DOI] [PubMed] [Google Scholar]
- 260.Hamada, T., Arias, E.B. and Cartee, G.D. (2006) Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J. Appl. Physiol. 101, 1368–1376 10.1152/japplphysiol.00416.2006 [DOI] [PubMed] [Google Scholar]
- 261.Henríquez-Olguin, C., Knudsen, J.R., Raun, S.H., Li, Z., Dalbram, E., Treebak, J.T., et al. (2019) Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 10 10.1038/s41467-019-12523-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Richter, E.A. and Hargreaves, M. (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93, 993–1017 10.1152/physrev.00038.2012 [DOI] [PubMed] [Google Scholar]
- 263.Stefani, L. and Galanti, G. (2017) Physical exercise prescription in metabolic chronic disease. In Translational Informatics in Smart Healthcare (Shen, B., ed.), pp. 123–141, Springer Singapore, Singapore: [DOI] [PubMed] [Google Scholar]
- 264.Hansen, D., Niebauer, J., Cornelissen, V., Barna, O., Neunhäuserer, D., Stettler, C., et al. (2018) Exercise prescription in patients with different combinations of cardiovascular disease risk factors: a consensus statement from the EXPERT working group. Sports Med. 48, 1781–1797 10.1007/s40279-018-0930-4 [DOI] [PubMed] [Google Scholar]
- 265.Adeline, F., Hugo, P.-R., René, M., Tàmàs, F., Eléonor, R. and Michel, P. (2021) Effects of a mixed exercise program on cancer related-fatigue and health-related quality of life in oncogeriatric patients: a feasibility study. J. Geriatr. Oncol. 12, 915–921 10.1016/j.jgo.2021.02.025 [DOI] [PubMed] [Google Scholar]
- 266.Adams, S.C., Iyengar, N.M., Scott, J.M. and Jones, L.W. (2018) Exercise implementation in oncology: one size does not fit all. J. Clin. Oncol. 36, 925–926 10.1200/JCO.2017.76.2906 [DOI] [PubMed] [Google Scholar]
- 267.D'Ascenzi, F., Anselmi, F., Fiorentini, C., Mannucci, R., Bonifazi, M. and Mondillo, S. (2019) The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur. J. Prev. Cardiol. 10.1177/2047487319874900 [DOI] [PubMed] [Google Scholar]
- 268.Fairman, C.M., Zourdos, M.C., Helms, E.R. and Focht, B.C. (2017) A scientific rationale to improve resistance training prescription in exercise oncology. Sports Med. 47, 1457–1465 10.1007/s40279-017-0673-7 [DOI] [PubMed] [Google Scholar]
- 269.Sasso, J.P., Eves, N.D., Christensen, J.F., Koelwyn, G.J., Scott, J. and Jones, L.W. (2015) A framework for prescription in exercise-oncology research. J. Cachexia Sarcopenia Muscle 6, 115–124 10.1002/jcsm.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jones, L.W. and Alfano, C.M. (2013) Exercise-oncology research: past, present, and future. Acta Oncol. 52, 195–215 10.3109/0284186X.2012.742564 [DOI] [PubMed] [Google Scholar]
- 271.Iyengar, N.M. and Jones, L.W. (2019) Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 5, 1620–1627 10.1001/jamaoncol.2019.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Baumann, F.T., Bloch, W. and Beulertz, J. (2013) Clinical exercise interventions in pediatric oncology: a systematic review. Pediatr. Res. 74, 366–374 10.1038/pr.2013.123 [DOI] [PubMed] [Google Scholar]
- 273.Balakrishnan, V.S. (2016) Physical exercise might affect breast cancer outcomes. Lancet Oncol. 17, e380 10.1016/S1470-2045(16)30389-8 [DOI] [PubMed] [Google Scholar]
- 274.de Lima, C., Alves, L.E., Iagher, F., Machado, A.F., Bonatto, S.J., Kuczera, D., et al. (2008) Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur. J. Appl. Physiol. 104, 957 10.1007/s00421-008-0849-9 [DOI] [PubMed] [Google Scholar]
- 275.de Lima, C., Alves, L., Iagher, F., Machado, A.F., Kryczyk, M., Yamazaki, R.K.et al. (2011) Tumor growth reduction in Walker 256 tumor-bearing rats performing anaerobic exercise: participation of Bcl-2, Bax, apoptosis, and peroxidation. Appl. Physiol. Nutr. Metab. 36, 533–538 10.1139/h11-047 [DOI] [PubMed] [Google Scholar]
- 276.Hoffman, S.A., Paschkis, K.E., DeBias, D.A., Cantarow, A. and Williams, T.L. (1962) The influence of exercise on the growth of transplanted Rat tumors. Cancer Res. 22, 597–599 https://pubmed.ncbi.nlm.nih.gov/14042313/ [PubMed] [Google Scholar]
- 277.Lu, M., Sanderson, S.M., Zessin, A., Ashcraft, K.A., Jones, L.W., Dewhirst, M.W.et al. (2018) Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab. 6, 14 10.1186/s40170-018-0190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Padilha, C.S., Testa, M.T., Marinello, P.C., Cella, P.S., Voltarelli, F.A., Frajacomo, F.T.et al. (2019) Resistance exercise counteracts tumor growth in two carcinoma rodent models. Med. Sci. Sports Exerc. 51, 2003 10.1249/MSS.0000000000002009 [DOI] [PubMed] [Google Scholar]
- 279.Rundqvist, H., Veliça, P., Barbieri, L., Gameiro, P.A., Bargiela, D., Gojkovic, M., et al. (2020) Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 9, e59996 10.7554/eLife.59996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zheng, X., Cui, X.-X., Huang, M.-T., Liu, Y., Shih, W.J., Lin, Y.et al. (2008) Inhibitory effect of voluntary running wheel exercise on the growth of human pancreatic Panc-1 and prostate PC-3 xenograft tumors in immunodeficient mice. Oncol. Rep. 19, 1583–1588 https://pubmed.ncbi.nlm.nih.gov/18497969/ [PMC free article] [PubMed] [Google Scholar]
- 281.Dethlefsen, C., Hansen, L.S., Lillelund, C., Andersen, C., Gehl, J., Christensen, J.F.et al. (2017) Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 77, 4894–4904 10.1158/0008-5472.CAN-16-3125 [DOI] [PubMed] [Google Scholar]
- 282.Pedersen, L., Idorn, M., Olofsson, G.H., Lauenborg, B., Nookaew, I., Hansen, R.H., et al. (2016) Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 23, 554–562 10.1016/j.cmet.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 283.Alvarado, A., da Costa, R.M.G., Faustino-Rocha, A.I., Ferreira, R., Lopes, C., Oliveira, P.A.et al. (2017) Effects of exercise training on breast cancer metastasis in a rat model. Int. J. Exp. Pathol. 98, 40–46 10.1111/iep.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ashcraft, K.A., Peace, R.M., Betof, A.S., Dewhirst, M.W. and Jones, L.W. (2016) Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 76, 4032–4050 10.1158/0008-5472.CAN-16-0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hoffman-Goetz, L. (1994) Exercise, natural immunity, and tumor metastasis. Med. Sci. Sports Exerc. 26, 157–163 10.1249/00005768-199402000-00005 [DOI] [PubMed] [Google Scholar]
- 286.Rincón-Castanedo, C., Morales, J.S., Martín-Ruiz, A., Valenzuela, P.L., Ramírez, M., Santos-Lozano, A.et al. (2020) Physical exercise effects on metastasis: a systematic review and meta-analysis in animal cancer models. Cancer Metastasis Rev. 39, 91–114 10.1007/s10555-020-09851-4 [DOI] [PubMed] [Google Scholar]
- 287.Idorn, M. and Hojman, P. (2016) Exercise-dependent regulation of NK cells in cancer protection. Trends Mol. Med. 22, 565–577 10.1016/j.molmed.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 288.Irwin, M.L., Varma, K., Alvarez-Reeves, M., Cadmus, L., Wiley, A., Chung, G.G.et al. (2009) Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale exercise and survivorship study. Cancer Epidemiol. Biomarkers Prev. 18, 306–313 10.1158/1055-9965.EPI-08-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lee, M.K., Kim, J.-Y., Kim, D.-I., Kang, D.-W., Park, J., Ahn, K.-Y., et al. (2017) Effect of home-based exercise intervention on fasting insulin and adipocytokines in colorectal cancer survivors: a randomized controlled trial. Metabolism 76, 23–31 10.1016/j.metabol.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 290.Niehoff, N.M., Nichols, H.B., Zhao, S., White, A.J. and Sandler, D.P. (2019) Adult physical activity and breast cancer risk in women with a family history of breast cancer. Cancer Epidemiol. Biomarkers Prev. 28, 51–58 10.1158/1055-9965.EPI-18-0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Steiner, J.L., Davis, J.M., McClellan, J.L., Enos, R.T. and Murphy, E.A. (2013) Effects of voluntary exercise on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Int. J. Oncol. 42, 1466–1472 10.3892/ijo.2013.1827 [DOI] [PubMed] [Google Scholar]
- 292.Murphy, E.A., Davis, J.M., Barrilleaux, T.L., McClellan, J.L., Steiner, J.L., Carmichael, M.D.et al. (2011) Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine 55, 274–279 10.1016/j.cyto.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Moore, S.C., Lee, I.-M., Weiderpass, E., Campbell, P.T., Sampson, J.N., Kitahara, C.M., (2016) Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 176, 816–825 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Brown, J.C., Winters-Stone, K., Lee, A. and Schmitz, K.H. (2012) Cancer, physical activity, and exercise. Compr. Physiol. 2, 2775–2809 10.1002/cphy.c120005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ligibel, J.A., Dillon, D., Giobbie-Hurder, A., McTiernan, A., Frank, E., Cornwell, M., et al. (2019) Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: results from the pre-operative health and body (PreHAB) study. Clin. Cancer Res. 25, 5398–5406 10.1158/1078-0432.CCR-18-3143 [DOI] [PubMed] [Google Scholar]
- 296.Ho, P.-C., Bihuniak, J.D., Macintyre, A.N., Staron, M., Liu, X., Amezquita, R., et al. (2015) Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 10.1016/j.cell.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chang, C.-H., Qiu, J., O'Sullivan, D., Buck, M.D., Noguchi, T., Curtis, J.D., et al. (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 10.1016/j.cell.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang, R., Dillon, C.P., Shi, L.Z., Milasta, S., Carter, R., Finkelstein, D., et al. (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 10.1016/j.immuni.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sbarra, A.J. and Karnovsky, M.L. (1959) The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 234, 1355–1362 10.1016/S0021-9258(18)70011-2 [DOI] [PubMed] [Google Scholar]
- 300.Borregaard, N. and Herlin, T. (1982) Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest. 70, 550–557 10.1172/JCI110647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Borregaard, N. and Kragballe, K. (1982) The oxygen-dependent antibody-dependent cell-mediated cytotoxicity of human monocytes and neutrophils. Adv. Exp. Med. Biol. 141, 71–84 10.1007/978-1-4684-8088-7_8 [DOI] [PubMed] [Google Scholar]
- 302.Cramer, T., Yamanishi, Y., Clausen, B.E., Förster, I., Pawlinski, R., Mackman, N., et al. (2003) HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 10.1016/S0092-8674(03)00154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shi, L.Z., Wang, R., Huang, G., Vogel, P., Neale, G., Green, D.R.et al. (2011) HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cham, C.M. and Gajewski, T.F. (2005) Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 174, 4670–4677 10.4049/jimmunol.174.8.4670 [DOI] [PubMed] [Google Scholar]
- 305.Cham, C.M., Driessens, G., O'Keefe, J.P. and Gajewski, T.F. (2008) Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 38, 2438–2450 10.1002/eji.200838289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jacobs, S.R., Herman, C.E., MacIver, N.J., Wofford, J.A., Wieman, H.L., Hammen, J.J.et al. (2008) Glucose uptake Is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180, 4476–4486 10.4049/jimmunol.180.7.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Loschinski, R., Böttcher, M., Stoll, A., Bruns, H., Mackensen, A. and Mougiakakos, D. (2018) IL-21 modulates memory and exhaustion phenotype of T-cells in a fatty acid oxidation-dependent manner. Oncotarget 9, 13125–13138 10.18632/oncotarget.24442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lavoie, S., Chun, E., Bae, S., Brennan, C.A., Gallini Comeau, C.A., Lang, J.K., et al. (2020) Expression of free fatty acid receptor 2 by dendritic cells prevents their expression of interleukin 27 and is required for maintenance of mucosal barrier and immune response against colorectal tumors in mice. Gastroenterology 158, 1359–1372.e9 10.1053/j.gastro.2019.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Liu, F., Liu, W., Zhou, S., Yang, C., Tian, M., Jia, G., et al. (2020) Identification of FABP5 as an immunometabolic marker in human hepatocellular carcinoma. J. Immunother. Cancer 8 10.1136/jitc-2019-000501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Patsoukis, N., Bardhan, K., Chatterjee, P., Sari, D., Liu, B., Bell, L.N., et al. (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6, 6692 10.1038/ncomms7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Charlton, J.J., Chatzidakis, I., Tsoukatou, D., Boumpas, D.T., Garinis, G.A. and Mamalaki, C. (2013) Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J. Immunol. 190, 6104–6114 10.4049/jimmunol.1201617 [DOI] [PubMed] [Google Scholar]
- 312.Pearce, E.L., Walsh, M.C., Cejas, P.J., Harms, G.M., Shen, H., Wang, L.-S.et al. (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 10.1038/nature08097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Michalek, R.D., Gerriets, V.A., Jacobs, S.R., Macintyre, A.N., MacIver, N.J., Mason, E.F.et al. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Araki, K., Turner, A.P., Shaffer, V.O., Gangappa, S., Keller, S.A., Bachmann, M.F.et al. (2009) mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 10.1038/nature08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Roepstorff, C., Steffensen, C.H., Madsen, M., Stallknecht, B., Kanstrup, I.-L., Richter, E.A.et al. (2002) Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am. J. Physiol. 282, E435–E447 10.1152/ajpendo.00266.2001 [DOI] [PubMed] [Google Scholar]
- 316.Benthem, L., Bolhuis, J.W., van der Leest, J., Steffens, A.B., Zock, J.P. and Zijlstra, W.G. (1994) Methods for measurement of energy expenditure and substrate concentrations in swimming rats. Physiol. Behav. 56, 151–159 10.1016/0031-9384(94)90273-9 [DOI] [PubMed] [Google Scholar]
- 317.Goedecke, J.H., Gibson, A.S.C., Grobler, L., Collins, M., Noakes, T.D. and Lambert, E.V. (2000) Determinants of the variability in respiratory exchange ratio at rest and during exercise in trained athletes. Am. J. Physiol. 279, E1325–E1334 10.1152/ajpendo.2000.279.6.E1325 [DOI] [PubMed] [Google Scholar]
- 318.Bresnahan, J.J., Farkas, G.J., Clasey, J.L., Yates, J.W. and Gater, D.R. (2019) Arm crank ergometry improves cardiovascular disease risk factors and community mobility independent of body composition in high motor complete spinal cord injury. J. Spinal Cord Med. 42, 272–280 10.1080/10790268.2017.1412562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hallsworth, K., Fattakhova, G., Hollingsworth, K.G., Thoma, C., Moore, S., Taylor, R.et al. (2011) Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60, 1278–1283 10.1136/gut.2011.242073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.P, F., Castinheiras Neto, A.G. and Amorim, P.R.S. (2016) Oxygen consumption and substrate utilization during and after resistance exercises performed with different muscle mass. Int. J. Exerc. Sci. 9, 77–88 https://pubmed.ncbi.nlm.nih.gov/27293507/ [PMC free article] [PubMed] [Google Scholar]
- 321.Moasses-Ghafari, B., Fallahi, B., Esfehani, A.F., Eftekhari, M., Rahmani, K., Eftekhari, A.et al. (2021) Effect of diet on physiologic bowel 18F-FDG uptake. J. Nucl. Med. Technol. 49, 241–245 10.2967/jnmt.120.257857 [DOI] [PubMed] [Google Scholar]
- 322.Nebeling, L.C., Miraldi, F., Shurin, S.B. and Lerner, E. (1995) Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J. Am. Coll. Nutr. 14, 202–208 10.1080/07315724.1995.10718495 [DOI] [PubMed] [Google Scholar]
- 323.Fine, E.J., Segal-Isaacson, C.J., Feinman, R.D., Herszkopf, S., Romano, M.C., Tomuta, N.et al. (2012) Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition 28, 1028–1035 10.1016/j.nut.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 324.Mashhedi, H., Blouin, M.-J., Zakikhani, M., David, S., Zhao, Y., Bazile, M., et al. (2011) Metformin abolishes increased tumor 18 F-2-fluoro-2-deoxy-D-glucose uptake associated with a high energy diet. Cell Cycle 10, 2770–2778 10.4161/cc.10.16.16219 [DOI] [PubMed] [Google Scholar]
- 325.van Niekerk, G., Christowitz, C., Conradie, D. and Engelbrecht, A.-M. (2020) Insulin as an immunomodulatory hormone. Cytokine Growth Factor Rev. 52, 34–44 10.1016/j.cytogfr.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 326.Helderman, J.H. and Strom, T.B. (1978) Specific insulin binding site on T and B lymphocytes as a marker of cell activation. Nature 274, 62–63 10.1038/274062a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Bar, R.S., Gorden, P., Roth, J., Kahn, C.R. and De Meyts, P. (1976) Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J. Clin. Invest. 58, 1123–1135 10.1172/JCI108565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Fischer, H.J., Sie, C., Schumann, E., Witte, A.-K., Dressel, R. and Reichardt, H.M.) The insulin receptor plays a critical role in T cell function and adaptive immunity. J. Immunol. 12, 1910–1920 10.4049/jimmunol.1601011 [DOI] [PubMed] [Google Scholar]
- 329.Tsai, S., Clemente-Casares, X., Zhou, A.C., Lei, H., Ahn, J.J., Chan, Y.T., et al. (2018) Insulin receptor-Mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 28, 922–934.e4 10.1016/j.cmet.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 330.Taniguchi, C.M., Emanuelli, B. and Kahn, C.R. (2006) Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 10.1038/nrm1837 [DOI] [PubMed] [Google Scholar]
- 331.van Niekerk, G., Dalgleish, A.G., Joubert, F., Joubert, A. and Engelbrecht, A.-M. (2021) The immuno-oncological implications of insulin. Life Sci. 264, 118716 10.1016/j.lfs.2020.118716 [DOI] [PubMed] [Google Scholar]
- 332.Dang, C.V. (2012) MYC on the path to cancer. Cell 149, 22–35 10.1016/j.cell.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Edwards, D.N., Ngwa, V.M., Raybuck, A.L., Wang, S., Hwang, Y., Kim, L.C., et al. (2021) Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Invest. 131 10.1172/JCI140100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nishimura, H., Pallardo, F.V., Seidner, G.A., Vannucci, S., Simpson, I.A. and Birnbaum, M.J. (1993) Kinetics of GLUT1 and GLUT4 glucose transporters expressed in Xenopus oocytes. J. Biol. Chem. 268, 8514–8520 10.1016/S0021-9258(18)52905-7 [DOI] [PubMed] [Google Scholar]
- 335.Burant, C.F. and Bell, G.I. (1992) Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry 31, 10414–10420 10.1021/bi00157a032 [DOI] [PubMed] [Google Scholar]
- 336.Kreisman, S.H., Halter, J.B., Vranic, M. and Marliss, E.B. (2003) Combined infusion of epinephrine and norepinephrine during moderate exercise reproduces the glucoregulatory response of intense exercise. Diabetes 52, 1347–1354 10.2337/diabetes.52.6.1347 [DOI] [PubMed] [Google Scholar]
- 337.Angus, D.J., Febbraio, M.A. and Hargreaves, M. (2002) Plasma glucose kinetics during prolonged exercise in trained humans when fed carbohydrate. Am. J. Physiol. Endocrinol. Metab. 283, E573–E577 10.1152/ajpendo.00443.2001 [DOI] [PubMed] [Google Scholar]
- 338.Mora-Rodriguez, R., Hodgkinson, B.J., Byerley, L.O. and Coyle, E.F. (2001) Effects of β-adrenergic receptor stimulation and blockade on substrate metabolism during submaximal exercise. Am. J. Physiol. 280, E752–E760 10.1152/ajpendo.2001.280.5.E752 [DOI] [PubMed] [Google Scholar]
- 339.Morrison, D.J., Kowalski, G.M., Grespan, E., Mari, A., Bruce, C.R. and Wadley, G.D. (2018) Measurement of postprandial glucose fluxes in response to acute and chronic endurance exercise in healthy humans. Am. J. Physiol. Endocrinol. Metab. 314, E503–E511 10.1152/ajpendo.00316.2017 [DOI] [PubMed] [Google Scholar]
- 340.Choi, K. and Weber, J.-M. (2015) Coping with an exogenous glucose overload: glucose kinetics of rainbow trout during graded swimming. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 310, R493–R501 10.1152/ajpregu.00330.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hansen, M., Palsøe, M.K., Helge, J.W. and Dela, F. (2015) The effect of metformin on glucose homeostasis during moderate exercise. Diabetes Care 38, 293–301 10.2337/dc14-1480 [DOI] [PubMed] [Google Scholar]
- 342.Knudsen, S.H., Karstoft, K., Pedersen, B.K., van Hall, G. and Solomon, T.P.J. (2014) The immediate effects of a single bout of aerobic exercise on oral glucose tolerance across the glucose tolerance continuum. Physiol. Rep. 2 10.14814/phy2.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wang, X., Patterson, B.W., Smith, G.I., Kampelman, J., Reeds, D.N., Sullivan, S.A.et al. (2013) A ∼60-min brisk walk increases insulin-stimulated glucose disposal but has no effect on hepatic and adipose tissue insulin sensitivity in older women. J. Appl. Physiol. (1985) 114, 1563–1568 10.1152/japplphysiol.01364.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Howell, L.B. (1971) The Liver as an Energy Source in Man During Exercise. In Muscle Metabolism During Exercise: Proceedings of a Karolinska Institutet Symposium held in Stockholm, Sweden, September 6–9, 1970 Honorary guest: E Hohwü Christensen (Pernow, B., and Saltin, B., eds.), pp. 127–141, Springer US, Boston, MA [Google Scholar]
- 345.Knudsen, J.G., Biensø, R.S., Hassing, H.A., Jakobsen, A.H. and Pilegaard, H. (2015) Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver. Mol. Cell Biochem. 403, 209–217 10.1007/s11010-015-2351-0 [DOI] [PubMed] [Google Scholar]
- 346.Shyer, J.A., Flavell, R.A. and Bailis, W. (2020) Metabolic signaling in T cells. Cell Res. 30, 649–659 10.1038/s41422-020-0379-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Palmer, C.S., Ostrowski, M., Balderson, B., Christian, N. and Crowe, S.M. (2015) Glucose metabolism regulates T cell activation, differentiation, and functions. Front. Immunol. 6, 1 10.3389/fimmu.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Chang, C.-H., Curtis, J.D., Maggi, L.B., Faubert, B., Villarino, A.V., O'Sullivan, D., et al. (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Menk, A.V., Scharping, N.E., Moreci, R.S., Zeng, X., Guy, C., Salvatore, S., et al. (2018) Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 10.1016/j.celrep.2018.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Bantug, G.R., Galluzzi, L., Kroemer, G. and Hess, C. (2018) The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 19–34 10.1038/nri.2017.99 [DOI] [PubMed] [Google Scholar]
- 351.Bergman, B.C. and Brooks, G.A. (1999) Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. (1985) 86, 479–487 10.1152/jappl.1999.86.2.479 [DOI] [PubMed] [Google Scholar]
- 352.Bergman, B.C., Wolfel, E.E., Butterfield, G.E., Lopaschuk, G.D., Casazza, G.A., Horning, M.A.et al. (1999) Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 87, 1684–1696 10.1152/jappl.1999.87.5.1684 [DOI] [PubMed] [Google Scholar]
- 353.von Korn, P., Keating, S., Mueller, S., Haller, B., Kraenkel, N., Dinges, S., et al. (2021) The effect of exercise intensity and volume on metabolic phenotype in patients with metabolic syndrome: a randomized controlled trial. Metab. Syndr. Relat. Disord. 19, 107–114 10.1089/met.2020.0105 [DOI] [PubMed] [Google Scholar]
- 354.Wills, J.A., Drain, J., Fuller, J.T. and Doyle, T.L.A. (2020) Physiological responses of female load carriage improves after 10 weeks of training. Med. Sci. Sports Exerc. 52, 1763–1769 10.1249/MSS.0000000000002321 [DOI] [PubMed] [Google Scholar]
- 355.McGregor, S.J., Busa, M.A., Yaggie, J.A. and Bollt, E.M. (2009) High resolution MEMS accelerometers to estimate VO2 and compare running mechanics between highly trained inter-collegiate and untrained runners. PLoS One 4, e7355 10.1371/journal.pone.0007355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chen, L. and Tang, L. (2020) Effects of interval training versus continuous training on coronary artery disease: an updated meta-analysis of randomized controlled trials. Physiother. Theory Pract. 37, 1–10 10.1080/09593985.2019.1706213 [DOI] [PubMed] [Google Scholar]
- 357.Bar, S., Grenez, C., Nguyen, M., de Broca, B., Bernard, E., Abou-Arab, O.et al. (2020) Predicting postoperative complications with the respiratory exchange ratio after high-risk noncardiac surgery: a prospective cohort study. Eur. J. Anaesthesiol. 37, 1050–1057 10.1097/EJA.0000000000001111 [DOI] [PubMed] [Google Scholar]
- 358.Bar, S., Santarelli, D., de Broca, B., Abou Arab, O., Leviel, F., Miclo, M.et al. (2020) Predictive value of the respiratory exchange ratio for the occurrence of postoperative complications in laparoscopic surgery: a prospective and observational study. J. Clin. Monit. Comput. 35, 849–858 10.1007/s10877-020-00544-5 [DOI] [PubMed] [Google Scholar]
- 359.Colson, M., Baglin, J., Bolsin, S. and Grocott, M.P.W. (2012) Cardiopulmonary exercise testing predicts 5 yr survival after major surgery. Br. J. Anaesth. 109, 735–741 10.1093/bja/aes263 [DOI] [PubMed] [Google Scholar]
- 360.Mesquida, J., Saludes, P., Pérez-Madrigal, A., Proença, L., Cortes, E., Enseñat, L.et al. (2018) Respiratory quotient estimations as additional prognostic tools in early septic shock. J. Clin. Monit. Comput. 32, 1065–1072 10.1007/s10877-018-0113-8 [DOI] [PubMed] [Google Scholar]
- 361.Wang, M., Liu, T., Niu, Z., Zuo, J. and Qi, D. (2021) Utility of venous-to-arterial carbon dioxide changes to arteriovenous oxygen content ratios in the prognosis of severe sepsis and septic shock: a systematic review and meta-analysis. Hong Kong J. Emerg. Med. 28, 241–253 10.1177/1024907921994970 [DOI] [Google Scholar]
- 362.Kakutani, N., Fukushima, A., Yokota, T., Katayama, T., Nambu, H., Shirakawa, R., et al. (2018) Impact of high respiratory exchange ratio during submaximal exercise on adverse clinical outcome in heart failure. Circ. J. 82, 2753–2760 10.1253/circj.CJ-18-0103 [DOI] [PubMed] [Google Scholar]
- 363.Mezzani, A., Corrà, U., Bosimini, E., Giordano, A. and Giannuzzi, P. (2003) Contribution of peak respiratory exchange ratio to peak VO2 prognostic reliability in patients with chronic heart failure and severely reduced exercise capacity. Am. Heart J. 145, 1102–1107 10.1016/S0002-8703(03)00100-5 [DOI] [PubMed] [Google Scholar]
- 364.Woods, P.R., Bailey, K.R., Wood, C.M. and Johnson, B.D. (2011) Submaximal exercise gas exchange is an important prognostic tool to predict adverse outcomes in heart failure. Eur. J. Heart Fail. 13, 303–310 10.1093/eurjhf/hfq187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Icyuz, M., Zhang, F., Fitch, M.P., Joyner, M.R., Challa, A.K. and Sun, L.Y. (2021) Physiological and metabolic characteristics of novel double-mutant female mice with targeted disruption of both growth hormone-releasing hormone and growth hormone receptor. Aging Cell 20, e13339 10.1111/acel.13339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Momcilovic, M., Bailey, S.T., Lee, J.T., Fishbein, M.C., Braas, D., Go, J., et al. (2018) The GSK3 signaling axis regulates adaptive glutamine metabolism in lung squamous cell carcinoma. Cancer Cell 33, 905–921.e5 10.1016/j.ccell.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Lee, Y.-M., Lee, G., Oh, T.-I., Kim, B.M., Shim, D.-W., Lee, K.-H.et al. (2016) Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 48, 399–408 10.3892/ijo.2015.3243 [DOI] [PubMed] [Google Scholar]
- 368.Sun, L., Yin, Y., Clark, L.H., Sun, W., Sullivan, S.A., Tran, A.-Q., et al. (2017) Dual inhibition of glycolysis and glutaminolysis as a therapeutic strategy in the treatment of ovarian cancer. Oncotarget 8, 63551–63561 10.18632/oncotarget.18854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Briceño, P., Rivas-Yañez, E., Rosemblatt, M.V., Parra-Tello, B., Farías, P., Vargas, L., et al. (2021) CD73 ectonucleotidase restrains CD8+ T cell metabolic fitness and anti-tumoral activity. Front. Cell Dev. Biol. 9, 638037 10.3389/fcell.2021.638037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kvacskay, P., Yao, N., Schnotz, J.-H., Scarpone, R., de Carvalho, R.A., Klika, K.D.et al. (2021) Increase of aerobic glycolysis mediated by activated T helper cells drives synovial fibroblasts towards an inflammatory phenotype: new targets for therapy? Arthritis Res. Ther. 23, 56 10.1186/s13075-021-02437-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Verma, V., Jafarzadeh, N., Boi, S., Kundu, S., Jiang, Z., Fan, Y., et al. (2021) MEK inhibition reprograms CD8+ T lymphocytes into memory stem cells with potent antitumor effects. Nat. Immunol. 22, 53–66 10.1038/s41590-020-00818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Huang, X., Yi, S., Hu, J., Du, Z., Wang, Q., Ye, Z., et al. (2020) Analysis of the role of palmitoleic acid in acute anterior uveitis. Int. Immunopharmacol. 84, 106552 10.1016/j.intimp.2020.106552 [DOI] [PubMed] [Google Scholar]
- 373.Nicoli, F., Papagno, L., Frere, J.J., Cabral-Piccin, M.P., Clave, E., Gostick, E.et al. (2018) Naïve CD8+ T-cells engage a versatile metabolic program upon activation in humans and differ energetically from memory CD8+ T-cells. Front. Immunol. 9, 2736 10.3389/fimmu.2018.02736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.O'Sullivan, D., van der Windt, G.J.W., Huang, S.C.-C., Curtis, J.D., Chang, C.-H., Buck, M.D.et al. (2014) Memory CD8+ T cells Use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 10.1016/j.immuni.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Pearce, E.L., Poffenberger, M.C., Chang, C.-H. and Jones, R.G. (2013) Fueling immunity: insights into metabolism and lymphocyte function. Science 342 10.1126/science.1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.van der Windt, G.J.W., Everts, B., Chang, C.-H., Curtis, J.D., Freitas, T.C., Amiel, E.et al. (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 10.1016/j.immuni.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.van der Windt, G.J.W., O'Sullivan, D., Everts, B., Huang, S.C.-C., Buck, M.D., Curtis, J.D., et al. (2013) CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl Acad. Sci. U.S.A. 110, 14336–14341 10.1073/pnas.1221740110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Marçais, A., Cherfils-Vicini, J., Viant, C., Degouve, S., Viel, S., Fenis, A., et al. (2014) The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 15, 749–757 10.1038/ni.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Hoppenbrouwers, T., Fogliano, V., Garssen, J., Pellegrini, N., Willemsen, L.E.M. and Wichers, H.J. (2020) Specific polyunsaturated fatty acids Can modulate in vitro human moDC2s and subsequent Th2 cytokine release. Front. Immunol. 11, 748 10.3389/fimmu.2020.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kostić, M., Korićanac, G., Tepavčević, S., Stanišić, J., Romić, S., Ćulafić, T.et al. (2021) Low-intensity exercise diverts cardiac fatty acid metabolism from triacylglycerol synthesis to beta oxidation in fructose-fed rats. Arch. Physiol. Biochem. 1–11 10.1080/13813455.2021.1886118 [DOI] [PubMed] [Google Scholar]
- 381.Kim, S., Park, J., Shin, J., You, Y., Kim, O.-K., Lee, J.et al. (2020) Ethanolic extract of vaccinium corymbosum alleviates muscle fatigue in mice. J. Med. Food 23, 1225–1229 10.1089/jmf.2020.4753 [DOI] [PubMed] [Google Scholar]
- 382.Khalafi, M., Mohebbi, H., Symonds, M.E., Karimi, P., Akbari, A., Tabari, E.et al. (2020) The impact of moderate-intensity continuous or high-intensity interval training on adipogenesis and browning of subcutaneous adipose tissue in obese male rats. Nutrients 12 10.3390/nu12040925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Baati, N., Feillet-Coudray, C., Fouret, G., Vernus, B., Goustard, B., Jollet, M., et al. (2019) New evidence of exercise training benefits in myostatin-deficient mice: effect on lipidomic abnormalities. Biochem. Biophys. Res. Commun. 516, 89–95 10.1016/j.bbrc.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 384.Ok, D.-P., Ko, K. and Bae, J.Y. (2018) Exercise without dietary changes alleviates nonalcoholic fatty liver disease without weight loss benefits. Lipids Health Dis. 17, 207 10.1186/s12944-018-0852-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Cho, J.-K., Kim, S.-U., Hong, H.-R., Yoon, J.-H. and Kang, H.-S. (2015) Exercise training improves whole body insulin resistance via adiponectin receptor 1. Int. J. Sports Med. 36, e24–e30 10.1055/s-0035-1559715 [DOI] [PubMed] [Google Scholar]
- 386.Shen, Y., Xu, X., Yue, K. and Xu, G. (2015) Effect of different exercise protocols on metabolic profiles and fatty acid metabolism in skeletal muscle in high-fat diet-fed rats. Obesity (Silver Spring) 23, 1000–1006 10.1002/oby.21056 [DOI] [PubMed] [Google Scholar]
- 387.De Carvalho, F.G., Brandao, C.F.C., Batitucci, G., de Souza, A.O., Ferrari, G.D., Alberici, L.C., et al. (2021) Taurine supplementation associated with exercise increases mitochondrial activity and fatty acid oxidation gene expression in the subcutaneous white adipose tissue of obese women. Clin. Nutr. 40, 2180–2187 10.1016/j.clnu.2020.09.044 [DOI] [PubMed] [Google Scholar]
- 388.Hammond, K.M., Sale, C., Fraser, W., Tang, J., Shepherd, S.O., Strauss, J.A., et al. (2019) Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: implications for training adaptation. J. Physiol. 597, 4779–4796 10.1113/JP278209 [DOI] [PubMed] [Google Scholar]
- 389.Lefai, E., Blanc, S., Momken, I., Antoun, E., Chery, I., Zahariev, A.et al. (2017) Exercise training improves fat metabolism independent of total energy expenditure in sedentary overweight men, but does not restore lean metabolic phenotype. Int. J. Obes. (Lond.) 41, 1728–1736 10.1038/ijo.2017.151 [DOI] [PubMed] [Google Scholar]
- 390.Molé, P.A., Oscai, L.B. and Holloszy, J.O. (1971) Adaptation of muscle to exercise: increase in levels of palmityl CoA synthetase, carnitine palmityltransferase, and palmityl CoA dehydrogenase, and in the capacity to oxidize fatty acids. J. Clin. Invest. 50, 2323–2330 10.1172/JCI106730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Martin-Liberal, J., Furness, A.J., Joshi, K., Peggs, K.S., Quezada, S.A. and Larkin, J. (2015) Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol. Immunother. 64, 765–767 10.1007/s00262-015-1689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Stamatouli, A.M., Quandt, Z., Perdigoto, A.L., Clark, P.L., Kluger, H., Weiss, S.A., et al. (2018) Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 67, 1471–1480 10.2337/dbi18-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Frauwirth, K.A., Riley, J.L., Harris, M.H., Parry, R.V., Rathmell, J.C., Plas, D.R.et al. (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]