Abstract
Intestinal alkaline phosphatase (IALP) has recently assumed a special relevance, being the subject of study in the prevention and treatment of certain diseases related to leaky gut. This brush border enzyme (ecto-enzyme) plays an important role in the maintenance of intestinal microbial homeostasis and intestinal barrier function through its ability to dephosphorylate lipopolysaccharide (LPS). This review addresses how IALP and intestinal barrier dysfunction may be implicated in the pathophysiology of specific diseases such as inflammatory bowel disease, necrotizing enterocolitis, and metabolic syndrome. The use of IALP as a possible biomarker to assess intestinal barrier function and strategies to modulate IALP activity are also discussed.
Keywords: inflammatory bowel disease, intestinal alkaline phosphatase, intestinal barrier function, low-grade chronic inflammation, metabolic dysfunction, necrotizing enterocolitis, obesity
1. Introduction
Alkaline phosphatase (ALP), first described in 1907 by Suzuki et al. [1], is a glycoprotein bound to plasma membranes [2,3] that hydrolyzes several monophosphate esters optimally at an alkaline pH with the release of inorganic phosphates [4,5,6,7,8].
Although structurally and functionally distinct from protein phosphatases, some studies have shown that ALP is also capable of dephosphorylating proteins [9,10,11]. Protein phosphorylation/dephosphorylation balance plays an important role in the regulation of several cellular functions, such as proliferation and differentiation. Thus, it is possible that ALP may also be involved in the regulation of those functions as well [10,12,13].
Human ALP is divided into four isoenzymes depending on the tissue where it is expressed: placental ALP; germ cell ALP; liver, bone, or kidney ALP (tissue non-specific ALP); and intestinal ALP (IALP) [3].
IALP is an ectoenzyme usually expressed in intestinal epithelial cells (enterocytes), and their levels vary along the longitudinal axis of the intestine [10,14,15]. This brush border enzyme is involved in fatty acid absorption and plays an important role in the maintenance of intestinal microbial homeostasis and intestinal barrier function through its ability to dephosphorylate lipopolysaccharide (LPS). Tissue-non-specific ALP may also be present in the intestine, having the same functions as IALP [16]. IALP is also involved in the regulation of the intestinal surface pH and in the control of the composition, function, and anatomical location of intestinal microbiota constituents [8].
This review focuses on intestinal alkaline phosphatase (IALP) and on its role in the maintenance of the intestinal barrier function. In addition, this review addresses how IALP and intestinal barrier dysfunction may be implicated in the pathophysiology of some specific diseases such as inflammatory bowel disease, necrotizing enterocolitis, and metabolic syndrome. Gut microbiota dysbiosis and increased intestinal permeability have been recently pointed out as mechanisms involved in the etiology of these diseases, and in this context, IALP may assume a special relevance not only as a biomarker but also as a preventive and therapeutic strategy. The use of IALP as a possible biomarker to assess intestinal barrier function and strategies to modulate IALP activity is also discussed.
2. IALP and Intestinal Barrier Function
Trillions of generally harmless bacteria inhabit the surface of the host’s intestinal epithelium [17]. However, these bacteria can cause infection and septic shock and can be a threat if a protective intestinal barrier does not exist [17]. The intestinal barrier is what protects the host from an invasion of microorganisms, maintaining homeostasis and preventing infections [17,18]. The first lines of defense essential to maintaining the intestinal barrier are the mucus layer and antimicrobial peptides (AMPs) [18]. Tight junctions between intestinal epithelial cells and the lamina propria, where innate immune cells reside, are also essential for the integrity of the intestinal barrier [17,18].
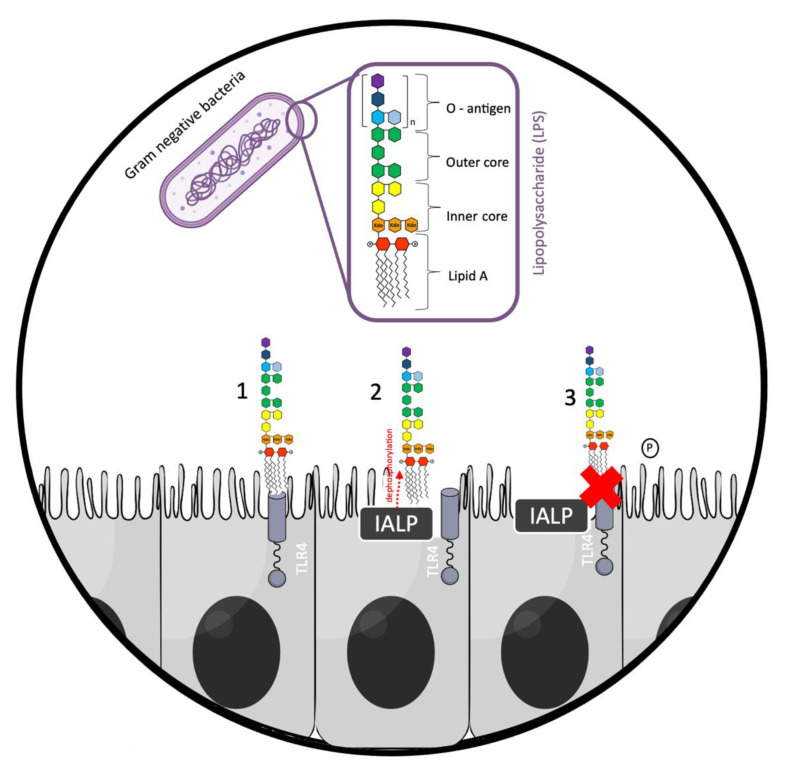
Present in the membrane of intestinal cells, pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), are innate immune receptors that can detect highly conserved bacterial patterns, such as LPS [17]. LPS present on the outer membrane of Gram-negative bacteria is an endotoxin that can cause septic shock in animals [8,19]. LPS acts as a toxin by over-stimulating the innate immune signaling of the Toll-like receptor 4 (TLR4), which induces an exacerbated inflammatory response [8,19]. Nevertheless, IALP is able to detoxify bacterial LPS by dephosphorylation [16]. Lipid A is responsible for the toxicity of LPS and contains two phosphate groups coupled to glucosamines, allowing LPS to bind TLR4, triggering an inflammatory response (e.g., the release of pro-inflammatory cytokines) (Figure 1) [16]. However, the removal of one of the phosphate groups from lipid A by IALP generates a monophosphoryl lipid A, which is 100 times less toxic than unmodified lipid A [20,21,22]. Thus, IALP manages to prevent inflammatory processes that would be triggered if LPS were not dephosphorylated. The prevention of this inflammatory process may contribute to the maintenance of intestinal barrier integrity.
Figure 1.
Detoxification of Gram-negative bacterial lipopolysaccharide (LPS) by intestinal alkaline phosphatase (IALP). 1—Absence of intestinal alkaline phosphatase: LPS binds to Toll-like receptor 4 (TLR4); 2—presence of IALP: lipid A phosphate group from LPS is dephosphorylated; 3—LPS dephosphorylation: no binding of LPS to TLR4 receptor, preventing the triggering of an inflammatory cascade. The red cross in the figure means no binding of LPS to TLR4.
Several studies have been conducted with the intention of verifying the role of IALP in the defense of the intestinal mucosa and its interaction with the intestinal microbiota. The authors found that IALP is involved in the maintenance of normal gut microbial homeostasis and may have a therapeutic potential in the prevention/treatment of dysbiosis and infections caused by pathogenic bacteria [23].
In a study conducted in rats, Buchet et al. found that oral IALP supplementation favors the growth of commensal bacteria, increasing the restoration of the intestinal microbiota lost due to antibiotic treatment and inhibiting the growth of a pathogenic bacterium (Salmonella typhimurium) [16]. This is due to the ability of IALP to dephosphorylate other luminal phosphates such as adenosine triphosphate (ATP), increasing the secretion of bicarbonate ions that are important to control luminal pH and, consequently, inhibiting pathogens growth [8,24,25,26]. Thus, oral administration of IALP may also have therapeutic potential against dysbiosis and pathogenic infections [8,16]. In addition, an in vitro study by Shin et al. showed that IALP alleviated the toxicity of LPS in intestinal epithelial cells (IECs), inhibited the activity of nuclear factor kappa B (NF-κB), and blocked the invasion and displacement of bacterial pathogens into the IECs, assisting in the preservation of the intestinal barrier integrity [27].
Moreover, the role of endogenous IALP in the maintenance of intestinal barrier function has also been associated with the regulation of tight junction proteins (TJP) expression. In mouse embryonic fibroblasts (MEFs) generated from IALP-knockout mice, the protein expression levels of zolunin-1 (ZO-1), zonulin-2 (ZO-2), and occludin were significantly lower compared to the levels of these proteins in wild-type control cells [28]. In addition, overexpression of IALP in human colorectal adenocarcinoma Caco-2 and T84 cells markedly enhanced ZO-1 and ZO-2 expression [28]. These authors have further demonstrated that exogenous IALP pretreatment of Caco-2 cells is able to prevent the LPS-induced alteration in the location and assembly of TJP and ameliorate its effect on intestinal permeability [28].
Lastly, Larrick et al. reported decreased IALP activity with aging, along with decreased integrity of the intestinal epithelial barrier [29]. In addition, the leaky-gut-associated inflammation was prevented by supplementation with IALP, suggesting that IALP can be a major regulator of intestinal permeability. Therefore, it can be concluded that IALP plays a fundamental role in maintaining the intestinal barrier function and that it could be involved in different intestinal and metabolic diseases in which intestinal permeability is increased.
3. IALP and Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) comprises a group of disorders that involves chronic inflammation, including Crohn’s disease (CD) and ulcerative colitis (UC) [30]. Dysbiosis is a common feature in patients with IBD [31]. In addition, it has been shown that patients with CD or UC have reduced IALP mRNA expression and activity in intestinal biopsies compared to healthy individuals [32,33]. Moreover, Tuin et al. have shown that in these patients, IALP mRNA expression is reduced in inflamed intestinal tissue compared to non-inflamed intestinal tissue [32].
In animal models of experimental colitis triggered by dextran sulfate sodium (DSS) or piroxicam, oral or intrarectal administration of IALP has been shown to significantly reduce intestinal inflammation [32,34,35,36]. Results from these studies demonstrated that, in experimental colitis, a lower endogenous IALP activity is associated with higher severity of the intestinal injury, whereas intestinal inflammation can be reversed by supplementation with exogenous IALP.
Danielak et al. studied the effect of IALP combined with moderate physical activity (voluntary wheel running) on experimental colitis induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) in mice fed a standard diet (SD) or high-fat diet (HFD) [37]. The authors found that in sedentary SD-fed mice, macroscopic and microscopic colitis was accompanied by a significant decrease in colonic blood flow and a significant increase in the colonic expression of tumor necrosis factor-alpha (TNF-α), IL-6, IL-1β, and leptin [37]. The same effects were exacerbated in sedentary HFD mice but reduced in active (exercising) animals, potentiated by IALP treatment, namely in obese mice [37]. They concluded that the combination of voluntary exercise and oral treatment with IALP synergistically favored healing of intestinal inflammation, strengthened antioxidant defense, and improved the course of experimental colitis [37]. In a subsequent study, the authors evaluated whether these effects were mediated by the intestinal microbiota [38]. They found that TNBS-induced colitis was worsened in obese sedentary mice and that IALP supplementation in combination with moderate physical activity attenuated the severity of murine colitis through a mechanism that involved the negative regulation of the intestinal cytokine/chemokine network and oxidative stress, an improvement of muscle strength and the modulation of the intestinal microbiota [38]. In particular, the authors proposed that the increase in the Ruminococcus genus, a butyrate-producing genus that has been found to exert anti-inflammatory properties, restoring and maintaining normal gastrointestinal tract function and integrity, may explain the effects observed [38].
In a study carried out on patients with UC, IALP was administered daily for 7 days via a duodenal catheter [39]. The authors found a short-term improvement in disease activity scores, accompanied by reductions in C-reactive protein and fecal calprotectin, within 21 days. Treatment with exogenous IALP was well tolerated and nonimmunogenic [39].
Since IALP can dephosphorylate and detoxify LPS, it is conceivable that IALP may exert these protective and anti-inflammatory effects via LPS detoxification. Thus, IALP may play an important role in IBD management, controlling LPS dephosphorylation and intestinal inflammation, and may be therapeutically effective, without the harmful risks associated with currently accepted therapies [40]. Furthermore, there is also evidence that intestinal permeability can play a fundamental role in the pathophysiology of IBD [41,42,43,44]. Thus, in addition to reducing inflammation, IALP may also contribute to IBD management by decreasing intestinal permeability, as explained in Section 2.
4. IALP and Necrotizing Enterocolitis (NEC)
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in premature infants, having an associated high mortality rate [45,46] and long-term associated morbidities, such as short bowel syndrome, nutritional deficiency, and delayed neurological development [46,47,48].
Newborn infants, especially those with very low birth weight, are more susceptible to sepsis due to prolonged hospitalizations, invasive instrumentation, underdeveloped innate immunity, and altered immune responses. These last two physiological states, together with an immature intestinal barrier, may contribute to NEC development [49,50].
Interestingly, IALP activity measured in fecal samples is significantly lower in infants with severe NEC compared to infants without NEC [46]. Since there is a decrease in IALP activity, further studies are needed to evaluate whether IALP supplementation could be effective in NEC prevention and treatment.
In 2014, a group of researchers investigated whether IALP supplementation could be protective of the premature intestine [51]. They evaluated premature newborn rats that were formula-fed with or without IALP supplementation. The authors showed that although there were no differences between groups regarding IALP activity, the animals supplemented with IALP had decreased mRNA expression of inflammatory cytokines in the terminal ileum [51]. IALP supplementation also decreased intestinal permeability (evaluated ex vivo) and the expression of inflammatory cytokines after exposure to LPS when compared to control animals fed with formula only [51]. These results support that IALP is beneficial for the premature intestine, reducing intestinal damage and inflammation caused by LPS [51].
Recent findings indicate that NEC is also preceded and accompanied by changes in the intestinal microbiota composition, which are associated with the host’s immune pathways responsible for activating intestinal inflammation [50,52]. By neutralizing LPS, IALP may prevent a cascade of pro-inflammatory signals in the intestine and contribute to the beneficial maturation of the microbiota [46]. This could constitute another mechanism by which IALP may be protective against NEC.
Nevertheless, IALP supplementation is not the only way to prevent intestinal damage and inflammation that characterizes NEC. Preterm infants who receive human milk instead of formula have a lower incidence of NEC [53]. Additionally, as recently demonstrated by our group, premature infants who received human milk had their endogenous IALP activity increased [53]. In our recent work, very premature infants fed with mothers’ own milk (MOM) or donor human milk (DHM) had increased fecal ALP activity on the 26th day of life compared to formula-fed infants [53]. MOM and DHM concomitantly stimulated the growth of Bifidobacterium, although the mechanisms by which human milk consumption increase IALP activity remain to be further elucidated.
Lastly, as proposed by other authors, as the activity of the IALP precedes the beginning of the signaling cascades that trigger inflammation, the abundance and enzymatic activity of the IALP eliminated in the feces could be used as a specific biomarker in establishing the diagnosis of severe NEC, monitoring disease progression and surveilling high-risk infant groups [46].
5. IALP and Metabolic Dysfunction
Metabolic syndrome is a set of several interrelated factors such as central obesity, hypertension, insulin resistance, and dyslipidemia that increase the risk of cardiovascular disease and type 2 diabetes [54,55].
Obesity is characterized by a state of low-grade chronic inflammation that could be a result of LPS and other microbial metabolites being absorbed along with dietary fats [56]. Evidence shows that high-fat diets decrease gut microbiota diversity (causing dysbiosis) and increase plasma concentration of LPS which can, in turn, trigger weight gain, inflammation, and insulin resistance [56,57].
Narisawa et al. have shown that IALP-deficient mice maintained on a high-fat diet showed faster body weight gain than wild-type animals [58]. Histological examination revealed accelerated transport of fat droplets through the intestinal epithelium and elevated serum triacylglyceride levels in IALP-deficient mice compared to wild-type mice, enlightening that IALP participates in the rate-limiting step that regulates fat absorption [58,59]. Nevertheless, although IALP-deficient mice might have gained more weight due to higher dietary fat absorption, the contribution of LPS circulating levels cannot be ruled out. In fact, these mice may have increased intestinal permeability (due to the lack of IALP) and, consequently, increased LPS levels (metabolic endotoxemia), which could have contributed to the increased weight gain [56].
In this regard, Kaliannan et al. have later confirmed that IALP knockout mice have increased gut permeability and suffer from metabolic endotoxemia, presenting obesity and associated metabolic disorders [60]. In this study, the authors also found that both endogenous and exogenous IALP inhibit LPS absorption and that oral IALP supplementation is capable of preventing and reversing metabolic syndrome [60].
Furthermore, a study published in 2015 evaluated the levels of IALP activity in fecal samples of diabetic (with or without overweight) and healthy non-diabetic patients [61]. This study related fecal IALP deficiency to T2D and showed that a high level of IALP is protective against diabetes, regardless of obesity [61].
Early childhood exposure to antibiotics has been implicated in the pathogenesis of metabolic syndrome later in adulthood [62]. In this context, Economopoulos et al. have shown that the use of antibiotics concomitantly with IALP supplementation early in life may have a preventive role against metabolic syndrome [62]. In this study, mice were treated for three intermittent 7-day cycles with azithromycin and supplemented with oral IALP. At the end of the last cycle, mice were treated with a regular diet for five weeks and then with a high-fat diet for another five weeks [62]. Co-administration of IALP with azithromycin prevented susceptibility to metabolic syndrome by decreasing total body weight, serum lipids, glucose levels, and liver lipids to the levels of control mice [62]. This effect may be explained through the prevention of antibiotic-induced changes in the microbiota by IALP.
In recent years, artificial sweeteners have been used as a substitute for sugar and as a weight control strategy [63]. However, several studies suggest that this exchange does not improve weight loss and may even contribute to the development of metabolic syndrome [64,65,66]. Recent evidence points out that artificial sweeteners, specifically aspartame (ASP), can have a direct effect on the intestinal microbiota that could explain the metabolic changes that occur after the consumption of high doses of artificial sweeteners [67,68,69]. In addition, a recently published cross-sectional study showed significant differences in the human gut microbiota when comparing the consumption of a diet rich in artificial sweeteners to that of a regular diet [70]. Furthermore, Frankenfeld et al. demonstrated a different bacterial composition in patients (healthy adults) after 4 days of consuming ASP, also showing that the consumption of low doses of ASP alters the intestinal microbiota [70].
ASP is the most used artificial sweetener used and is also known to be metabolized by intestinal esterases and peptidases to L-aspartic acid, L-phenylalanine (PHE), and methanol [71]. Since the amino acid PHE is a well-known IALP inhibitor [72], it may hinder the IALP-mediated detoxification of LPS [71]. As verified by Gul et al. in an isolated intestinal loop model, IALP activity was significantly reduced by the presence of ASP [71]. These results confirm that ASP can reduce the endogenous IALP activity at the intestinal brush border and lumen [71].
Thus, as already demonstrated in mice, the ingestion of 60 mg of ASP per liter of water altered the microbiota composition, verifying an increase in the number of bacteria of the Enterobacteriaceae and Clostridium leptum family (strain associated with the microbiota of obese individuals) [67]. Thus, by altering the composition of the intestinal microbiota and compromising the action of IALP, interfering with the neutralization of bacterial toxins such as LPS, ASP can contribute to the development of metabolic syndrome.
6. IALP—How Can It Be Modulated?
Diet appears to have an important impact on the modulation of IALP [73]. Fasting dramatically decreases IALP activity, while feeding retrieves it [8,74].
From a nutritional perspective, strategies that lead to a healthy lifestyle should be adopted, namely, the adoption of a Mediterranean dietary pattern [75]. As demonstrated by Ismael et al., the activity of fecal IALP positively correlates with gut microbiota diversity promoted by a Mediterranean diet [76].
The Mediterranean diet is a diet rich in fiber, which can explain its effects on IALP activity. Fiber stimulates the growth and/or activity of bacteria in the colon, promoting the production of short-chain fatty acids that increase IALP activity [77,78].
As verified by the study of Gibson et al. carried out in rats, different food sources of fiber (uncooked potato starch; cooked potato starch; precooked corn flour; whole bran; guar gum; wheat bran; methylcellulose; oat bran) increased IALP activity in the colon. Intestinal mechanical stimulation induced by fiber intake increases the proliferation of colonic epithelium and, consequently, increases the activity of IALP [73,79].
On the other hand, the Mediterranean diet is low in trans fatty acids and privileges the consumption of food sources rich in polyunsaturated and monounsaturated fats. A diet supplemented with fish oil or corn oil increases IALP activity [80]. Triglycerides increase IALP expression and activity in the intestinal lumen; that is, the amount of fat in the diet determines the amount of IALP secretion [81,82,83,84]. On the other hand, the Mediterranean diet, which is rich in unsaturated fatty acids, increases IALP activity [81,85,86]. Different types of unsaturated fatty acids can influence IALP activity [85]. For example, sunflower oil decreases IALP activity compared to olive oil due to changes in phospholipid composition in the apical membrane of intestinal epithelial cells [85].
Trans fatty acids decrease IALP activity at the intestinal brush border membranes when the amount of linoleic acid in the diet is low, with no effect in the presence of greater amounts of linoleic acid [87].
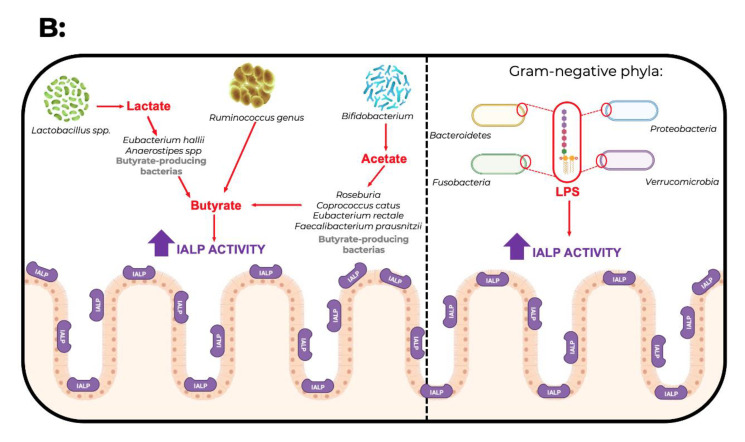
The activity of IALP in the intestine can also be differently modulated by the microbiota [73]. Okazaki et al. showed a significant positive correlation between colon IALP activity, Bifidobacterium, mucins, and butyrate [88]. Some studies indicate that the consumption of fermentable oligosaccharides such as fructooligosaccharides and galactooligosaccharides (raffinose), as well as lactulose, modulate the gut microbiota by increasing both Bifidobacterium and butyrate [88,89,90,91,92]. Interestingly, butyrate is known to increase IALP activity [53]. However, further studies are needed to confirm the relationship between the non-digestible oligosaccharides-induced increase in colonic ALP and colonic barrier function [88].
In the human intestine, the main butyrate-producing bacteria belong to the Firmicutes phylum, in particular, Faecalibacterium prausnitzii and Clostridium leptum of the Ruminococcaceae family and Eubacterium rectale and Roseburia spp. of the family Lachnospiraceae [93]. There are also bacteria that use sugar and/or lactate to produce butyrate, such as Eubacterium hallii and Anaerostipes spp. [93,94]. Nevertheless, butyrate-producing bacteria may be present in higher numbers in the human intestine because members of Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria, Spirochaetes, and Thermotogae are potential butyrate producers according to the genes they express, including those encoding enzymes that synthesize butyrate, such as butyryl—CoA dehydrogenase, butyryl-CoA transferase, and/or butyrate kinase [93].
In addition to butyrate, the production of other SCFAs is mediated by bacteria such as Bifidobacterium species (belonging to the Phylum Actinobacteria), which produce acetate and lactate during carbohydrate fermentation [93,94]. In addition, the mucin-degrading bacterium Akkermansia muciniphila (Phylum Verrucomicrobia) produces propionate and acetate [93,94]. In turn, acetate and lactate may be used as substrates by butyrate-producing bacteria (Figure 2B).
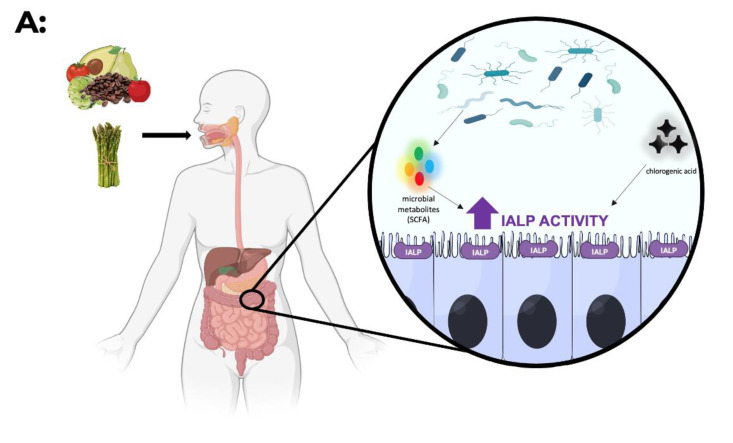
Figure 2.
(A) Diet has an impact on the modulation of IALP (intestinal alkaline phosphatase) activity. Foods rich in fermentable fiber (e.g., artichoke, asparagus) are used as a substrate for the microbiota. This fermentation results in the production of short-chain fatty acids (e.g., butyrate) that increase IALP activity. On the other hand, consumption of foods rich in chlorogenic acid (coffee, artichoke, apple, pear, tomato, and avocado) also increases the activity/expression of IALP. (B) The gut microbiota may modulate the expression/activity of IALP. On the one hand, butyrate-producing bacteria (Gram-positive bacteria) may increase IALP due to the increase in butyrate. On the other hand, Gram-negative bacteria may also increase IALP due to the presence of LPS (the presence of LPS as a substrate may enhance enzyme activity).
A study carried out with pregnant women by Selma-Royo et al. did not observe a direct relationship between high-fat diets and IALP expression [95]. However, they found that ingesting fat (saturated (SFA) and monounsaturated (MUFA) fatty acids) increased Firmicutes, a strain associated with higher concentrations of IALP [95]. Furthermore, a lower abundance of the phylum Proteobacteria was associated with higher IALP activity [95].
Polyphenols seem to have an impact on the modulation of alkaline phosphatase activity; however, further work on this topic is needed since conclusions are scarce [96,97].
As verified by Zhou et al., chlorogenic acid showed a protective effect in the intestine of endotoxin-infused female Sprague-Dawley rats. With intragastric administration of 60 mg/kg of body weight of chlorogenic acid twice daily for 28 days, there was an improvement in endotoxin-induced intestinal damage and an increase in alkaline phosphatase activity. Thus, chlorogenic acid present in coffee and in a variety of vegetables and fruits may have an impact against increased intestinal permeability (Figure 2A) [97].
When used as a drug, IALP is usually administered by intravenous injections so that degradation and digestion in the stomach and upper intestinal tract does not occur [8]. Nevertheless, enteral administration of exogenous ALP has two main effects: detoxifying PAMPs and stimulating the production of endogenous IALP by the enterocyte. The mechanism by which exogenous IALP stimulates the production of endogenous IALP by the enterocyte is unknown, but it may be indirect, resulting from the reduction of local inflammation [98,99]. IALP administered intraperitoneally or intravenously contributes to the detoxification of circulating PAMPs without a positive feedback effect on IALP in the small intestine [98,99].
Regarding rectal enemas that allow the direct entry of IALP into the large intestine and exogenous IALP in the form of enteric-coated or delayed-release capsules, their effects have not yet been reported in human studies.
There are some published studies on the administration of IALP in an animal model (Table 1) and in humans (Table 2); however, the conclusions are still scarce. Therefore, it is necessary to study and understand the effect of IALP supplementation and IALP modulation through the diet and microbiota in the prevention and treatment of diseases where its activity can be compromised, such as inflammatory bowel disease, necrotizing enterocolitis, and metabolic syndrome.
Table 1.
Summary of the most recent studies on the administration of IALP in animal models.
| Aim of Study | Route of Administration | Treatment Effect | Animal Model | Ref. |
|---|---|---|---|---|
| Examine whether co-administration of IALP with antibiotics early in life have a preventive role against metabolic syndrome | Oral IALP (100 units/mL drinking water) supplementation ad libitumfor three intermittent 7-day cycles | Co-administration of IALP with AZT early in life prevents mice from susceptibility to the later development of HFD-induced obesity and MetS | C57BL/6 mice | Economopoulos et al. (2016) [62] |
| Investigate whether oral IALP supplementation protects against alcohol-induced liver disease | Oral IALP supplementation (200 U/mL) for 10 days | IALP treatment protected mice from alcohol-induced hepatotoxicity and steatosis | Female C57BL/6 mice | Hamarneh et al. (2017) [100] |
| Evaluate whether the protective effect of IALP on DSS-induced colitis is mediated by the Toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB) pathway | IALP (300 IU/day) via oral gavage for 7 days | Oral gavage administration of IALP significantly attenuated the severity of colitis via the TLR4/NF-κB pathway | WT C57BL/6 mice and TLR4-/- mice | Hwang et al. (2018) [101] |
| Determine if bovine intestinal ALP (BiAP) infusion prevents AKI | BiAP was administered by continuous infusion (25 U/kg/hr) via a femoral central venous catheter | BiAP infusion corrects serum and tissue ALP deficiency and may prevent AKI | Porcine model of early infant CPB/DHCA -induced AKI | Davidson et al. (2019) [102] |
| Evaluate the effect of IALP combined with moderate physical activity (voluntary wheel running) on the experimental colitis | IALP (200 U/day) was administered intragastrical for 12 weeks | Oral IALP treatment synergistically favored healing of intestinal inflammation, strengthened the antioxidant defense, and ameliorated the course of experimental colitis | SD- and HFD-fed C57BL/6 mice with experimental colitis induced by TNBS | Danielak et al. (2021) [37] |
| Evaluate the effect of IALP combined with moderate physical activity (voluntary wheel running) on experimental colitis | Oral IALP supplementation (200 U/day) in drinking water for 2 weeks | Administration of IALP combined with moderate physical activity significantly reduced gross and microscopic inflammatory response and oxidative stress markers | HFD female C57BL/6J mice with experimental colitis induced by TNBS | Wojcik-Grzybek Dagmara et al. (2022) [38] |
BiALP—bovine intestinal alkaline phosphatase; IALP—intestinal alkaline phosphatase; AZT—azithromycin; DSS—dextran sulfate sodium; CPB—cardiopulmonary bypass; DHCA—deep hypothermic circulatory arrest); SD—standard diet; MetS—metabolic syndrome; TLR4—Toll-like receptor 4; NF-κB—nuclear factor-kappa B; HFD—high-fat diet; SW—spinning wheel; TNBS—2,4,6-trinitrobenzenesulfonic acid; AKI—acute kidney injury.
Table 2.
Studies on administration of alkaline phosphatase in humans.
| Aim of Study | Study Design | Route of Administration | Treatment Effect | Sample Size/ Estimated Enrollment |
Ref. |
|---|---|---|---|---|---|
| Evaluate the safety and preliminary efficacy of exogenous ALP administered to patients with UC | Interventionalallocation: N/A; intervention model: single group assignment; masking: none (open label); primary purpose: treatment | bIAP bolus 30,000 U/24 h for 7 consecutive days via a duodenal catheter | AP enzyme treatment was well tolerated and nonimmunogenic | 21 | M. Lukas et al. [39] (2010) |
| Evaluate the safety, pharmacokinetics, and pharmacodynamics of IV administration of exogenous ALP | Randomized; double-blind; placebo-controlled sequential protocols | Administered exogenous, 10 min IV infusions (three ascending doses) or 24–72 h continuous (132.5–200 U kg−1 24 h−1) IV | Exogenous AP administration in severe sepsis patients may play a renal protective role | 103 | P. Pickkers et al. [103] (2009) |
| Evaluate whether alkaline phosphatase injections can reduce acute inflammation in patients with rheumatoid arthritis | Interventional (clinical trial); non-randomized | s.c. injections of bovine intestinal Alkaline Phosphatase daily subcutaneous treatment with two injections of 2000 IU bIAP for three days | Ongoing study | 6 | NCT01416493 * |
| Evaluate the efficacy and safety of bovine intestinal alkaline phosphatase (bIAP) in reducing the pro-inflammatory post-surgical responses | Allocation: randomized; intervention model: parallel assignment; masking: quadruple (participant, care provider, investigator, outcomes assessor); primary purpose: prevention | bIAP bolus and 8 h infusion intravenous as a bolus of bIAP (1000 IU) just prior to surgery followed by a 40 IU/kg bIAP infusion during the first 8 h post-surgery | Ongoing study | 53 | NCT01144611 * |
* Clinical studies without published final results obtained from clinictrials.gov; bIAP—bovine intestinal alkaline phosphatase; s.c. —subcutaneous; UC—ulcerative colitis; IV—intravenous.
7. Concluding Remarks and Future Perspectives
To assess intestinal permeability in a research setting, the lactulose/mannitol test is usually used as a gold standard [104]. This test can aid in the non-invasive diagnosis of barrier dysfunction in patients with diarrheal diseases, malnutrition, surgical stress, Crohn’s disease, active inflammatory bowel disease, and irritable bowel syndrome, among other pathologies [105,106,107,108]. Nevertheless, it may not be feasible to implement routinely in clinical practice since urine is collected from 5 to 6 h after the ingestion of the solution containing lactulose and mannitol, making patients wait a long time for the results [104].
Other potential biomarkers such as fecal calprotectin and zonulin, as well as plasma intestinal fatty acid-binding protein (I-FABP), lipopolysaccharide-binding protein (LBP), and zonulin, have been proposed to assess intestinal permeability [109]. Nevertheless, fecal IALP activity would constitute a more promising alternative since it can be performed routinely by any clinical analysis laboratory, using a non-invasive single fecal sample collection, which translates into lower costs and fast results. Even so, further studies are necessary to validate the use of fecal IALP as a marker of intestinal permeability.
Author Contributions
Conceptualization, G.M.S. and C.M.; research methodology, G.M.S.; validation, C.M.; preparation of draft writing, G.M.S.; review and editing, S.I., J.M., J.R.A., A.F., C.C. and C.M.; supervision, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by ERDF through the operation POCI-01-0145-ERDF-007746 funded by Programa Operacional Competitividade e Internacionalização—COMPETE2020 and by National Funds through FCT—Fundação para a Ciência e a Tecnologia within CINTESIS, R&D Unit (reference UID/IC/4255/2013) and (PTDC/BAA-AGR/7419/2020).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suzuki U.Y.K., Takashi M. Uber ein enzyme–phytase‖ das anhydro-oxy-methylen-diphosphorsaure spaltet. Bull. Coll. Agric. Tokyo Imp. Univ. 1907;7:503–512. [Google Scholar]
- 2.Tsai L.C., Hung M.W., Chen Y.H., Su W.C., Chang G.G., Chang T.C. Expression and regulation of alkaline phosphatases in human breast cancer MCF-7 cells. Eur. J. Biochem. 2000;267:1330–1339. doi: 10.1046/j.1432-1327.2000.01100.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharma U., Pal D., Prasad R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014;29:269–278. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benham F., Cottell D.C., Franks M., Wilson P.D. Alkaline phosphatase activity in human bladder tumor cell lines. J. Histochem. Cytochem. 1997;25:266–274. doi: 10.1177/25.4.870558. [DOI] [PubMed] [Google Scholar]
- 5.Mornet E., Stura E., Lia-Baldini A.S., Stigbrand T., Menez A., Le Du M.H. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J. Biol. Chem. 2001;276:31171–31178. doi: 10.1074/jbc.M102788200. [DOI] [PubMed] [Google Scholar]
- 6.Weiss M.J., Henthorn P.S., Lafferty M.A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. USA. 1986;83:7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma U., Singh S.K., Pal D., Khajuria R., Mandal A.K., Prasad R. Implication of BBM lipid composition and fluidity in mitigated alkaline phosphatase activity in renal cell carcinoma. Mol. Cell. Biochem. 2012;369:287–293. doi: 10.1007/s11010-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 8.Estaki M., DeCoffe D., Gibson D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014;20:15650–15656. doi: 10.3748/wjg.v20.i42.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J.R., Stinson R.A. Dephosphorylation of phosphoproteins of human liver plasma membranes by endogenous and purified liver alkaline phosphatases. J. Biol. Chem. 1986;261:7635–7639. doi: 10.1016/S0021-9258(19)57445-2. [DOI] [PubMed] [Google Scholar]
- 10.Anagnostou F., Plas C., Forest N. Ecto-alkaline phosphatase considered as levamisole-sensitive phosphohydrolase at physiological pH range during mineralization in cultured fetal calvaria cells. J. Cell. Biochem. 1996;60:484–494. doi: 10.1002/(SICI)1097-4644(19960315)60:4<484::AID-JCB5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Scheibe R.J., Kuehl H., Krautwald S., Meissner J.D., Mueller W.H. Ecto-alkaline phosphatase activity identified at physiological pH range on intact P19 and HL-60 cells is induced by retinoic acid. J. Cell. Biochem. 2000;76:420–436. doi: 10.1002/(SICI)1097-4644(20000301)76:3<420::AID-JCB10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Nonoyama S., Karakida T., Chiba-Ohkuma R., Yamamoto R., Ujiie Y., Nagano T., Yamakoshi Y., Gomi K. Development and Characterization of Alkaline Phosphatase-Positive Human Umbilical Cord Perivascular Cells. Cells. 2021;10:3011. doi: 10.3390/cells10113011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki A., Palmer G., Bonjour J.P., Caverzasio J. Regulation of Alkaline Phosphatase Activity by p38 MAP Kinase in Response to Activation of Gi Protein-Coupled Receptors by Epinephrine in Osteoblast-Like Cells. Endocrinology. 1999;140:3177–3182. doi: 10.1210/endo.140.7.6857. [DOI] [PubMed] [Google Scholar]
- 14.Calhau C., Martel F., Hipólito-Reis C., Azevedo I. Differences between duodenal and jejunal rat alkaline phosphatase. Clin. Biochem. 2000;33:571–577. doi: 10.1016/S0009-9120(00)00171-5. [DOI] [PubMed] [Google Scholar]
- 15.Nakano T., Inoue I., Alpers D.H., Akiba Y., Katayama S., Shinozaki R., Kaunitz J.D., Ohshima S., Akita M., Takahashi S., et al. Role of lysophosphatidylcholine in brush-border intestinal alkaline phosphatase release and restoration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G207–G214. doi: 10.1152/ajpgi.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchet R., Millan J.L., Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol. Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 17.Geddes K., Philpott D.J. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology. 2008;135:8–12. doi: 10.1053/j.gastro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Wells J.M., Brummer R.J., Derrien M., MacDonald T.T., Troost F., Cani P.D., Theodorou V., Dekker J., Meheust A., de Vos W.M., et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates J.M., Akerlund J., Mittge E., Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutler B., Rietschel E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. 2003;169:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 21.Schromm A.B., Brandenburg K., Loppnow H., Zähringer U., Rietschel E.T., Carroll S.F., Koch M.H., Kusumoto S., Seydel U. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J. Immunol. 1998;161:5464–5471. [PubMed] [Google Scholar]
- 22.Galanos C., Lüderitz O., Rietschel E.T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H., et al. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 23.Malo M.S., Alam S.N., Mostafa G., Zeller S.J., Johnson P.V., Mohammad N., Chen K.T., Moss A.K., Ramasamy S., Faruqui A., et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59:1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 24.Malo M.S., Moaven O., Muhammad N., Biswas B., Alam S.N., Economopoulos K.P., Gul S.S., Hamarneh S.R., Malo N.S., Teshager A., et al. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G826–G838. doi: 10.1152/ajpgi.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam S.N., Yammine H., Moaven O., Ahmed R., Moss A.K., Biswas B., Muhammad N., Biswas R., Raychowdhury A., Kaliannan K., et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 2014;259:715–722. doi: 10.1097/SLA.0b013e31828fae14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiba Y., Mizumori M., Guth P.H., Engel E., Kaunitz J.D. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1223–G1233. doi: 10.1152/ajpgi.00313.2007. [DOI] [PubMed] [Google Scholar]
- 27.Shin J., Carr A., Corner G.A., Togel L., Davalos-Salas M., Tran H., Chueh A.C., Al-Obaidi S., Chionh F., Ahmed N., et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like factor 5 (KLF5)-dependent manner. J. Biol. Chem. 2014;289:25306–25316. doi: 10.1074/jbc.M114.557546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Hu D., Huo H., Zhang W., Adiliaghdam F., Morrison S., Ramirez J.M., Gul S.S., Hamarneh S.R., Hodin R.A. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J. Am. Coll. Surg. 2016;222:1009–1017. doi: 10.1016/j.jamcollsurg.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larrick J.W., Mendelsohn A.R. Supplementation with Brush Border Enzyme Alkaline Phosphatase Slows Aging. Rejuvenation Res. 2020;23:171–175. doi: 10.1089/rej.2020.2335. [DOI] [PubMed] [Google Scholar]
- 30.Abraham C., Cho J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manichanh C., Borruel N., Casellas F., Guarner F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 32.Tuin A., Poelstra K., de Jager-Krikken A., Bok L., Raaben W., Velders M.P., Dijkstra G. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58:379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 33.Torres M.I., Lorite P., Lopez-Casado M.A., Rios A. A new approach using tissue alkaline phosphatase histochemistry to identify Crohn’s disease. Pathol. Res. Pract. 2007;203:485–487. doi: 10.1016/j.prp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Ramasamy S., Nguyen D.D., Eston M.A., Alam S.N., Moss A.K., Ebrahimi F., Biswas B., Mostafa G., Chen K.T., Kaliannan K., et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm. Bowel Dis. 2011;17:532–542. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C., Chun J., Hwang S.W., Kang S.J., Im J.P., Kim J.S. The effect of intestinal alkaline phosphatase on intestinal epithelial cells, macrophages and chronic colitis in mice. Life Sci. 2014;100:118–124. doi: 10.1016/j.lfs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Moya P., Ortega-Gonzalez M., Gonzalez R., Anzola A., Ocon B., Hernandez-Chirlaque C., Lopez-Posadas R., Suarez M.D., Zarzuelo A., Martinez-Augustin O., et al. Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacol. Res. 2012;66:144–153. doi: 10.1016/j.phrs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Danielak A., Wojcik D., Mazur-Bialy A., Surmiak M., Bilski J., Targosz A., Magierowski M., Chmura A., Strzalka M., Krzysiek-Maczka G., et al. Intestinal Alkaline Phosphatase Combined with Voluntary Physical Activity Alleviates Experimental Colitis in Obese Mice. Involvement of Oxidative Stress, Myokines, Adipokines and Proinflammatory Biomarkers. Antioxidants. 2021;10:240. doi: 10.3390/antiox10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wojcik-Grzybek D., Hubalewska-Mazgaj M., Surmiak M., Sliwowski Z., Dobrut A., Mlodzinska A., Wojcik A., Kwiecien S., Magierowski M., Mazur-Bialy A., et al. The Combination of Intestinal Alkaline Phosphatase Treatment with Moderate Physical Activity Alleviates the Severity of Experimental Colitis in Obese Mice via Modulation of Gut Microbiota, Attenuation of Proinflammatory Cytokines, Oxidative Stress Biomarkers and DNA Oxidative Damage in Colonic Mucosa. Int. J. Mol. Sci. 2022;23:2964. doi: 10.3390/ijms23062964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukas M., Drastich P., Konecny M., Gionchetti P., Urban O., Cantoni F., Bortlik M., Duricova D., Bulitta M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel Dis. 2010;16:1180–1186. doi: 10.1002/ibd.21161. [DOI] [PubMed] [Google Scholar]
- 40.Bilski J., Mazur-Bialy A., Wojcik D., Zahradnik-Bilska J., Brzozowski B., Magierowski M., Mach T., Magierowska K., Brzozowski T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017;2017:9074601. doi: 10.1155/2017/9074601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mankertz J., Amasheh M., Krug S.M., Fromm A., Amasheh S., Hillenbrand B., Tavalali S., Fromm M., Schulzke J.D. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- 42.Jager S., Stange E.F., Wehkamp J. Inflammatory bowel disease: An impaired barrier disease. Langenbecks Arch. Surg. 2013;398:1–12. doi: 10.1007/s00423-012-1030-9. [DOI] [PubMed] [Google Scholar]
- 43.Michielan A., D’Inca R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015;2015:628157. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoni L., Nuding S., Wehkamp J., Stange E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014;20:1165–1179. doi: 10.3748/wjg.v20.i5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin P.W., Stoll B.J. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 46.Heath M., Buckley R., Gerber Z., Davis P., Linneman L., Gong Q., Barkemeyer B., Fang Z., Good M., Penn D., et al. Association of Intestinal Alkaline Phosphatase With Necrotizing Enterocolitis Among Premature Infants. JAMA Netw. Open. 2019;2:e1914996. doi: 10.1001/jamanetworkopen.2019.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yee W.H., Soraisham A.S., Shah V.S., Aziz K., Yoon W., Lee S.K., Canadian Neonatal N. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–e304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 48.Young C., Sharma R., Handfield M., Mai V., Neu J. Biomarkers for infants at risk for necrotizing enterocolitis: Clues to prevention? Pediatr. Res. 2009;65:91–97. doi: 10.1203/PDR.0b013e31819dba7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neu J., Walker W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanthakumar N., Meng D., Goldstein A.M., Zhu W., Lu L., Uauy R., Llanos A., Claud E.C., Walker W.A. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE. 2011;6:e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinzerling N.P., Liedel J.L., Welak S.R., Fredrich K., Biesterveld B.E., Pritchard K.A., Jr., Gourlay D.M. Intestinal alkaline phosphatase is protective to the preterm rat pup intestine. J. Pediatr. Surg. 2014;49:954–960. doi: 10.1016/j.jpedsurg.2014.01.031. discussion 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mai V., Young C.M., Ukhanova M., Wang X., Sun Y., Casella G., Theriaque D., Li N., Sharma R., Hudak M., et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morais J., Marques C., Faria A., Teixeira D., Barreiros-Mota I., Durao C., Araujo J., Ismael S., Brito S., Cardoso M., et al. Influence of Human Milk on Very Preterms’ Gut Microbiota and Alkaline Phosphatase Activity. Nutrients. 2021;13:1564. doi: 10.3390/nu13051564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundy S.M., Brewer H.B., Jr., Cleeman J.I., Smith S.C., Jr., Lenfant C., American Heart A., National Heart L., Blood I. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 55.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 56.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 57.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 58.Narisawa S., Huang L., Iwasaki A., Hasegawa H., Alpers D.H., Millan J.L. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol. Cell. Biol. 2003;23:7525–7530. doi: 10.1128/MCB.23.21.7525-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z., Shi C., Yang J., Zhang P., Ma Y., Wang F., Qin H. Molecular regulation of the intestinal epithelial barrier: Implication in human diseases. Front. Biosci. 2011;16:2903–2909. doi: 10.2741/3888. [DOI] [PubMed] [Google Scholar]
- 60.Kaliannan K., Hamarneh S.R., Economopoulos K.P., Nasrin Alam S., Moaven O., Patel P., Malo N.S., Ray M., Abtahi S.M., Muhammad N., et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA. 2013;110:7003–7008. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malo M.S. A High Level of Intestinal Alkaline Phosphatase Is Protective Against Type 2 Diabetes Mellitus Irrespective of Obesity. EBioMedicine. 2015;2:2016–2023. doi: 10.1016/j.ebiom.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Economopoulos K.P., Ward N.L., Phillips C.D., Teshager A., Patel P., Mohamed M.M., Hakimian S., Cox S.B., Ahmed R., Moaven O., et al. Prevention of antibiotic-associated metabolic syndrome in mice by intestinal alkaline phosphatase. Diabetes Obes. Metab. 2016;18:519–527. doi: 10.1111/dom.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anton S.D., Martin C.K., Han H., Coulon S., Cefalu W.T., Geiselman P., Williamson D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55:37–43. doi: 10.1016/j.appet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardener H., Rundek T., Markert M., Wright C.B., Elkind M.S., Sacco R.L. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J. Gen. Intern. Med. 2012;27:1120–1126. doi: 10.1007/s11606-011-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swithers S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013;24:431–441. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imamura F., O’Connor L., Ye Z., Mursu J., Hayashino Y., Bhupathiraju S.N., Forouhi N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmnas M.S., Cowan T.E., Bomhof M.R., Su J., Reimer R.A., Vogel H.J., Hittel D.S., Shearer J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE. 2014;9:e109841. doi: 10.1371/journal.pone.0109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 69.Burke M.V., Small D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015;152:381–388. doi: 10.1016/j.physbeh.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frankenfeld C.L., Sikaroodi M., Lamb E., Shoemaker S., Gillevet P.M. High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann. Epidemiol. 2015;25:736–742.e734. doi: 10.1016/j.annepidem.2015.06.083. [DOI] [PubMed] [Google Scholar]
- 71.Gul S.S., Hamilton A.R., Munoz A.R., Phupitakphol T., Liu W., Hyoju S.K., Economopoulos K.P., Morrison S., Hu D., Zhang W., et al. Inhibition of the gut enzyme intestinal alkaline phosphatase may explain how aspartame promotes glucose intolerance and obesity in mice. Appl. Physiol. Nutr. Metab. 2017;42:77–83. doi: 10.1139/apnm-2016-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh N.K., Fishman W.H. On the Mechanism of Inhibition of Intestinal Alkaline Phosphatase by l-Phenylalanine. J. Biol. Chem. 1966;241:2516–2522. doi: 10.1016/S0021-9258(18)96570-1. [DOI] [PubMed] [Google Scholar]
- 73.Lalles J.P. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 74.Bamba T., Vaja S., Murphy G.M., Dowling R.H. Effect of fasting and feeding on polyamines and related enzymes along the villus: Crypt axis. Digestion. 1990;46((Suppl. S2)):424–429. doi: 10.1159/000200417. [DOI] [PubMed] [Google Scholar]
- 75.Antoniazzi L., Arroyo-Olivares R., Bittencourt M.S., Tada M.T., Lima I., Jannes C.E., Krieger J.E., Pereira A.C., Quintana-Navarro G., Muniz-Grijalvo O., et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021;31:2014–2022. doi: 10.1016/j.numecd.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Ismael S., Silvestre M.P., Vasques M., Araujo J.R., Morais J., Duarte M.I., Pestana D., Faria A., Pereira-Leal J.B., Vaz J., et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients. 2021;13:1228. doi: 10.3390/nu13041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitehead R.H., Young G.P., Bhathal P.S. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215) Gut. 1986;27:1457–1463. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibson P.R., Nov R., Fielding M., Mcintyre A., Finch C.F., Rosella O., Mariadason J.M., Barkla D.H., Young G.P. Relationship of hydrolase activities to epithelial cell turnover in distal colonic mucosa of normal rats. J. Gastroenterol. Hepatol. 1999;14:866–872. doi: 10.1046/j.1440-1746.1999.01973.x. [DOI] [PubMed] [Google Scholar]
- 80.Stenson W.F., Seetharam B., Talkad V., Pickett W., Dudeja P., Brasitus T.A. Effects of dietary fish oil supplementation on membrane fluidity and enzyme activity in rat small intestine. Biochem. J. 1989;263:41–45. doi: 10.1042/bj2630041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudley M.A., Wang H., Hachey D.L., Shulman R.J., Perkinson J.S., Rosenberger J., Mersmann H.J. Mersmann. Jejunal Brush Border Hydrolase Activity Is Higher in Tallow-Fed Pigs than in Corn Oil-Fed Pigs. J. Nutr. 1994;124:1996–2005. doi: 10.1093/jn/124.10.1996. [DOI] [PubMed] [Google Scholar]
- 82.Takase S., Goda T. Effects of medium-chain triglycerides on brush border membrane-bound enzyme activity in rat small intestine. J. Nutr. 1990;120:969–976. doi: 10.1093/jn/120.9.969. [DOI] [PubMed] [Google Scholar]
- 83.Kaur M., Kaur J., Ojha S., Mahmood A. Dietary fat effects on brush border membrane composition and enzyme activities in rat intestine. Ann. Nutr. Metab. 1996;40:269–276. doi: 10.1159/000177967. [DOI] [PubMed] [Google Scholar]
- 84.Kaur J., Madan S., Hamid A., Singla A., Mahmood A. Intestinal alkaline phosphatase secretion in oil-fed rats. Dig. Dis. Sci. 2007;52:665–670. doi: 10.1007/s10620-006-9384-x. [DOI] [PubMed] [Google Scholar]
- 85.Vázquez C.M., Zanetti R., Santa-María C., Ruíz-Gutiérrez V. Effects of two highly monounsaturated oils on lipid composition and enzyme activities in rat jejunum. Biosci. Rep. 2000;20:355–368. doi: 10.1023/A:1010377900745. [DOI] [PubMed] [Google Scholar]
- 86.Wahnon R., Cogan U., Mokady S. Dietary fish oil modulates the alkaline phosphatase activity and not the fluidity of rat intestinal microvillus membrane. J. Nutr. 1992;122:1077–1084. doi: 10.1093/jn/122.5.1077. [DOI] [PubMed] [Google Scholar]
- 87.Ghafoorunissa S.A.I. Influence of dietary partially hydrogenated fat high in trans fatty acids on lipid composition and function of intestinal brush border membrane in rats. J. Nutr. Biochem. 2001;12:116–120. doi: 10.1016/S0955-2863(00)00150-9. [DOI] [PubMed] [Google Scholar]
- 88.Okazaki Y., Katayama T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br. J. Nutr. 2019;121:146–154. doi: 10.1017/S0007114518003082. [DOI] [PubMed] [Google Scholar]
- 89.Bornet F.R., Brouns F. Immune-stimulating and gut health-promoting properties of short-chain fructo-oligosaccharides. Pt 1Nutr. Rev. 2002;60:326–334. doi: 10.1301/002966402320583442. [DOI] [PubMed] [Google Scholar]
- 90.Watzl B., Girrbach S., Roller M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005;93((Suppl. S1)):S49–S55. doi: 10.1079/BJN20041357. [DOI] [PubMed] [Google Scholar]
- 91.Tanabe H., Ito H., Sugiyama K., Kiriyama S., Morita T. Dietary indigestible components exert different regional effects on luminal mucin secretion through their bulk-forming property and fermentability. Biosci. Biotechnol. Biochem. 2006;70:1188–1194. doi: 10.1271/bbb.70.1188. [DOI] [PubMed] [Google Scholar]
- 92.Komura M., Fukuta T., Genda T., Hino S., Aoe S., Kawagishi H., Morita T. A short-term ingestion of fructo-oligosaccharides increases immunoglobulin A and mucin concentrations in the rat cecum, but the effects are attenuated with the prolonged ingestion. Biosci. Biotechnol. Biochem. 2014;78:1592–1602. doi: 10.1080/09168451.2014.925782. [DOI] [PubMed] [Google Scholar]
- 93.Parada Venegas D., De la Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 95.Selma-Royo M., Garcia-Mantrana I., Calatayud M., Parra-Llorca A., Martinez-Costa C., Collado M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021;60:1429–1442. doi: 10.1007/s00394-020-02337-7. [DOI] [PubMed] [Google Scholar]
- 96.Negrão M.R., Keating E., Faria A., Azevedo I., Martins M.J. Acute effect of tea, wine, beer, and polyphenols on ecto-alkaline phosphatase activity in human vascular smooth muscle cells. J. Agric. Food Chem. 2006;54:4982–4988. doi: 10.1021/jf060505u. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Y., Ruan Z., Zhou L., Yang Y., Mi S., Deng Z., Yin Y. Chlorogenic acid decreased intestinal permeability and ameliorated intestinal injury in rats via amelioration of mitochondrial respiratory chain dysfunction. Food Sci. Biotechnol. 2016;25:253–260. doi: 10.1007/s10068-016-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lalles J.P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014;72:82–94. doi: 10.1111/nure.12082. [DOI] [PubMed] [Google Scholar]
- 99.Lalles J.P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019;77:710–724. doi: 10.1093/nutrit/nuz015. [DOI] [PubMed] [Google Scholar]
- 100.Hamarneh S.R., Kim B.M., Kaliannan K., Morrison S.A., Tantillo T.J., Tao Q., Mohamed M.M.R., Ramirez J.M., Karas A., Liu W., et al. Intestinal Alkaline Phosphatase Attenuates Alcohol-Induced Hepatosteatosis in Mice. Dig. Dis. Sci. 2017;62:2021–2034. doi: 10.1007/s10620-017-4576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hwang S.W., Kim J.H., Lee C., Im J.P., Kim J.S. Intestinal alkaline phosphatase ameliorates experimental colitis via toll-like receptor 4-dependent pathway. Eur. J. Pharmacol. 2018;820:156–166. doi: 10.1016/j.ejphar.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 102.Davidson J.A., Khailova L., Treece A., Robison J., Soranno D.E., Jaggers J., Ing R.J., Lawson S., Lujan S.O. Alkaline Phosphatase Treatment of Acute Kidney Injury in an Infant Piglet Model of Cardiopulmonary Bypass with Deep Hypothermic Circulatory Arrest. Sci. Rep. 2019;9:14175. doi: 10.1038/s41598-019-50481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pickkers P., Snellen F., Rogiers P., Bakker J., Jorens P., Meulenbelt J., Spapen H., Tulleken J.E., Lins R., Ramael S., et al. Clinical pharmacology of exogenously administered alkaline phosphatase. Eur. J. Clin. Pharmacol. 2009;65:393–402. doi: 10.1007/s00228-008-0591-6. [DOI] [PubMed] [Google Scholar]
- 104.Musa M.A., Kabir M., Hossain M.I., Ahmed E., Siddique A., Rashid H., Mahfuz M., Mondal D., Ahmed T., Petri W.A., et al. Measurement of intestinal permeability using lactulose and mannitol with conventional five hours and shortened two hours urine collection by two different methods: HPAE-PAD and LC-MSMS. PLoS ONE. 2019;14:e0220397. doi: 10.1371/journal.pone.0220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barboza M.S., Jr., Silva T.M.J., Guerrant R.L., Lima A.A. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz. J. Med. Biol. Res. 1999;32:1499–1504. doi: 10.1590/S0100-879X1999001200008. [DOI] [PubMed] [Google Scholar]
- 106.Hossain M.I., Nahar B., Hamadani J.D., Ahmed T., Roy A.K., Brown K.H. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J. Pediatr. Gastroenterol. Nutr. 2010;51:638–644. doi: 10.1097/MPG.0b013e3181eb3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andre F., Andre C., Emery Y., Forichon J., Descos L., Minaire Y. Assessment of the lactulose-mannitol test in Crohn’s disease. Gut. 1988;29:511–515. doi: 10.1136/gut.29.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marshall J.K., Thabane M., Garg A.X., Clark W., Meddings J., Collins S.M., Investigators W.E.L. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment. Pharmacol. Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 109.Seethaler B., Basrai M., Neyrinck A.M., Nazare J.A., Walter J., Delzenne N.M., Bischoff S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021;321:G11–G17. doi: 10.1152/ajpgi.00113.2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.