Abstract
The rising prevalence of diabetes mellitus (DM) leads on to an increase in chronic diabetic complications. Diabetic peripheral neuropathies (DPNs) are common chronic complications of diabetes. Distal symmetric polyneuropathy is the most prevalent form. Most patients with DPN will remain pain-free; however, painful DPN (PDPN) occurs in 6–34% of all DM patients and is associated with reduced health-related-quality-of-life and substantial economic burden. Symptomatic treatment of PDPN and diabetic autonomic neuropathy is the key treatment goals. Using certain patient related characteristics, subjects with PDPN can be stratified and assigned targeted therapies to produce better pain outcomes. The aim of this review is to discuss the various pathogenetic mechanisms of DPN with special reference to the mechanisms leading to PDPN and the various pharmacological and non-pharmacological therapies available for its management. Recommended pharmacological therapies include anticonvulsants, antidepressants, opioid analgesics, and topical medications.
Keywords: Diabetes mellitus, Diabetic peripheral neuropathy, Gabapentinoids, Opioids, Painful diabetic peripheral neuropathy
The current global prevalence of diabetes mellitus (DM) among adults (aged 20–70 years) is 537 million (one in every ten adults) that is expected to raise to 643 million by the year 2030, and 783 million by the year 2045.[1] The increasing prevalence of DM has led to a rise in chronic diabetic complications. Diabetic peripheral neuropathies (DPNs) are the most common chronic complications of diabetes.[2] Among these, the distal symmetric polyneuropathy (DSPN) is the most prevalent form which may affect up to 50% of patients with type 2 DM (T2DM) after 10 years of the disease, and at least 20% with type 1 DM (T1DM) after 20 years of the disease.[3] Furthermore, nearly 10–15% of newly diagnosed T2DM may have DSPN.
Although a vast majority of patients with DPN may remain pain-free, painful DPN (PDPN) is estimated to affect 6–34% of all patients with DM.[4] The disease burden from PDPN is directly proportional to the severity of neuropathic pain, which is associated with reduced health-related-quality-of-life (HRQoL) because of the effect of pain on the day-to-day functioning, quality/quantity of sleep, and anxiety/depression levels. It is also associated with impaired work productivity, increased healthcare expenditure and substantial economic burden. In the United States (U.S.), among T1DM and T2DM patients, nearly 27% of the total annual costs on diabetes care and nearly 9% of overall healthcare costs incurred are attributable to DPN and its complications.[5]
The diabetic microvascular complications including diabetic retinopathy and nephropathy can be diagnosed relatively early, and both these diseases have reasonably effective disease-modifying therapies. On the other hand, with the currently used crude diagnostic tests, the diabetic neuropathy is often diagnosed quite late in the disease course after the sensory loss has already established, with severe irreversible nerve damage.[3] The early stages of DPN with predominantly small fiber neuropathy can be reversed or at least prevented from progression, whereas the late stages of DPN with loss of protective sensation in the feet cannot be reversed.[6] Hence, early diagnosis and timely intervention are the keys to prevent the development and progression of DPN. Due to the limited understanding of the pathogenic mechanisms leading to the development of PDPN, highly effective disease-modifying therapies have not yet been developed.[7] Apart from modification of multiple cardiovascular risk factors including raised triglycerides, body mass index, hypertension, and smoking that are associated with incident DPN, symptomatic treatment of PDPN, and diabetic autonomic neuropathy (DAN) remains the mainstays in the management.[8]
The U.S. Food and Drug Administration (FDA) has approved Pregabalin and Duloxetine nearly 25 years ago and Tapentadol nearly 6 years ago for the management of pain in patients with PDPN. However, these agents are often inadequate for symptom relief in PDPN patients, given their relatively modest effects on pain control with common troublesome side effects.[7] A novel agent with superior efficacy and safety profile (mirogabalin) has recently received approval in Japan for the management of PDPN, and is undergoing further clinical trials.[9] It is recently proposed that using certain patient related characteristics including the clinical profile, quantitative sensory testing, genetics, and cerebral imaging, PDPN patients can be stratified and assigned targeted therapies to produce better pain outcomes.[10] In this review article, we discuss the various pathogenetic mechanisms of DPN with special reference to the mechanisms leading to PDPN, and the various pharmacological and non-pharmacological therapies available in its management.
Classification of Diabetic Neuropathy
The classification of diabetic neuropathies based on American Diabetes Association (ADA) is given in Table 1.
Table 1.
Classification of diabetic neuropathies based on American Diabetes Association.[3]
| Diabetic Neuropathies | |||
|---|---|---|---|
| A | Diffuse neuropathy | ||
| DSPN (Distal Symmetric Polyneuropathy) | Predominantly small-fiber | ||
| Predominantly large-fiber | |||
| Mixed small and large fiber | |||
| Autonomic | Cardiovascular, gastrointestinal, urogenital, sudomotor, hypoglycemia unawareness, or abnormal pupillary function | ||
| B | Mononeuropathy | ||
| Isolated cranial/peripheral nerve | 3rd cranial, ulnar, median, femoral, peroneal | ||
| Mononeuritis multiplex | If confluent may resemble polyneuropathy | ||
| C | Radiculopathy | ||
| Radiculoplexus neuropathy | Asymmetric proximal motor neuropathy | ||
| Thoracic radiculopathy | |||
| Nondiabetic neuropathies common in diabetes | |||
| Chronic inflammatory demyelinating polyneuropathy, radiculoplexus neuropathy, acute painful small-fiber neuropathy (treatment induced), and pressure palsy | |||
Clinical Features of Diabetic Neuropathy
Patients with PDPN present with neuropathic pain that has distinct presentations as burning, sharp, aching, electric shock like, and evoked pains.[3] The pain can be mild or intractable; sporadic or constant; transient (disappear completely after some time) or chronic. Regardless of the presence of pain, these patients may also develop numbness, tingling, and pins and needle sensations. The DPN symptoms begin with asymmetrical involvement of distal lower limbs, and progress to involve proximal lower limbs, before finally involving the upper limbs. Physical signs in early DPN include impairment of light touch, pinprick, and temperature sensation. The signs in advanced DPN include loss of vibration, proprioception, 10-g monofilament sensation, ankle reflexes, and motor involvement (muscle weakness, muscle wasting, and clawed toes).[11]
Although the presenting symptoms between painful and painless DPN patients are markedly different, the physical signs are usually indistinct between the two subtypes. Thus, most of the PDPN patients exhibits sensory loss on physical examination. But a minority of PDPN patients (irritable nociceptor phenotype) show evidence of “gain of function” or “positive sensory” signs including allodynia, where a normally non-noxious stimulus evokes pain, and hyperalgesia.[12] Moreover, some patients with PDPN may have a pure small fiber neuropathy characterized by loss of small fiber function (pinprick and temperature) with intact large fiber function (vibration and proprioception).[13]
Pathogenesis of DPN
The Schwann cells provide energy substrates and cytoskeletal support to the axons of nerves. These cells are essential for preserving the structure, function, and survival of axons, by sheathing the unmyelinated axons, myelinating the myelinated axons, and secreting the neurotrophic factors.[14] Uncontrolled hyperglycemia and dyslipidemia contribute to the pathogenesis of DPN (Fig. 1). While hyperglycemia is the main driver of DPN in T1DM, dyslipidemia is the main driver of DPN in T2DM.[15] DM is associated with high substrate load to Schwann cells (glucose and free fatty acids). In hyperglycemic states, glucose enters the Schwann cells through glucose transporter 3 in surplus amounts, and undergoes glycolysis. Thus, the amount of pyruvate exceeds the metabolic handling capacity of the tricarboxylic acid (TCA) cycle, resulting in a shift to anaerobic metabolism and lactate accumulation. The lactate is subsequently shuttled from Schwann cells into axons, resulting in mitochondrial dysfunction and axonal degeneration.[16]
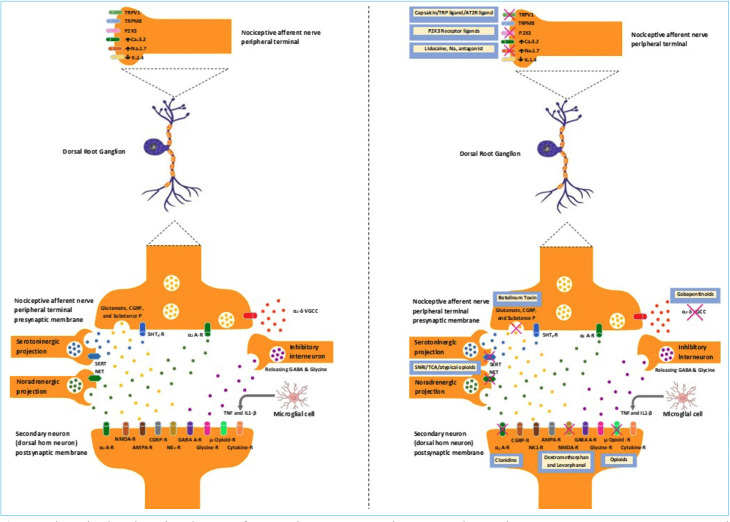
Figure 1.
The pathophysiological mechanisms of PDPN and agents acting on the corresponding mechanisms. TRPV1: Transient receptor potential vanilloid 1, TRPM8: Transient receptor potential melastatin 8, P2X3: Purinoceptor 3, α2 A-R: α2-AdrenoReceptor, 5HT3-R: 5-Hydroxytryptamine receptor, SERT: Serotonin transporter, NET: Norepinephrine transporter, CGRP-R: Calcitonin gene-related peptide receptor, NK1-R: Neurokinin 1 receptor (NK1-R acts as receptor for substance P), NMDA-R: N-methyl-D-aspartate receptor, AMPA-R: α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (NAMDA-R and AMPA-R acts as receptor for glutamate), GABA A-R: Gamma aminobutyric acid A receptor, Glycine-R: Glycine receptor, TNF: Tumor necrosis factor, IL1-β: Interleukin 1 beta, Cytokine-R: Cytokine receptor (acts as receptor for TNF and IL1-β).
Hyperglycemia-driven excessive activation of the electron transport chain leads to mitochondrial dysfunction, reactive oxygen species (ROS) generation, oxidative stress, deoxyribonucleic acid damage, and activation of polyadenosine diphosphate-ribose polymerase (PARP). PARP activation inhibits the enzyme glyceraldehyde-3-phosphate dehydrogenase resulting in accumulation of glycolytic metabolites, upregulation of polyol, hexosamine, diacylglycerol and protein kinase C pathways, as well as generation of Advanced Glycation End-products (AGEs).[17-23] The AGE-receptors for AGE (RAGE) interactions, oxidative stress, endoplasmic reticulum (ER) stress and upregulated non-glycolytic pathways result in endothelial dysfunction and microvascular damage. The microvascular damage decreases the neuronal blood flow resulting in hypoxia, demyelination, axonal loss, decreased myelinated fiber density, and reduced nerve conduction velocity.[23]
The mechanisms leading to excessive ROS generation and oxidative stress in patients with hyperglycemia include excessive activation of the electron transport chain, AGE-RAGE interaction, pro-inflammatory cytokines, upregulated non-glycolytic pathways, and high protein folding load.[17,22] The AGE accumulation and hexosamine/polyol pathway upregulation are associated with a rise in misfolded or unfolded proteins and, therefore, ER stress. The oxidative stress would increase the misfolding of proteins with worsening ER stress. Similarly, misfolded proteins result in adenosine triphosphate (ATP) depletion, increasing the oxidative stress.[17,22] Therefore, there exists a crosstalk between oxidative and ER stress.[17]
Dyslipidemia-driven increased substrate load of long chain saturated fatty acids (LCSFAs; palmitate and stearate) is associated with increased β-oxidation to form acetyl CoA. When the β-oxidation capacity of the TCA cycle is exceeded, toxic acylcarnitine accumulates inside Schwann cells, which is then shuttled into axons, resulting in mitochondrial dysfunction and axonal degeneration.[15] Additionally, oxidation of cholesterol into oxysterols in neuronal cells results in neuronal injury and apoptosis.[24,25] Moreover, neurotoxic deoxysphingolipids generated by altered sphingolipid metabolism results in neuronal cell damage.[26,27]
Pathogenesis of PDPN
Several theories have been proposed for the pathogenesis of neuropathic pain related to the diabetic neuropathy, as outlined in Table 2 and Figure 1. The pharmacological agents acting on the corresponding mechanisms are also depicted in the figure. Some individuals with DSPN develop severe neuropathic pain, whereas others with similar extent of neuropathy remain entirely asymptomatic. Although it was hypothesized that the PDPN might be associated with increasing severity of DSPN, experimental studies do not support this hypothesis. PDPN is a heterogenous condition caused by a complex interaction of various environmental factors (female gender, cultural, and psycho-social), genetic predisposition (gain of function mutation of Nav1.7 gene is associated with severe PDPN), metabolic disturbances (hyperglycemia, hyperlipidemia, and metabolic syndrome), and vascular dysfunction leading on to downstream alterations in the peripheral and central nervous system.[30]
Table 2.
Pathophysiological mechanisms of painful diabetic peripheral neuropathy
| Peripheral mechanisms |
|---|
| Small fiber neuropathy involving thinly myelinated Aδ and unmyelinated C fibers[28] |
| Autonomic neuropathy → microvascular dysfunction → hypoxic nerve injury[29] |
| Nerve injury → neuronal inflammation → neuronal hyperexcitability[30] |
| Nerve injury → altered expression and function of noxious transducers including TRPV1, TRPA1, TRPM8 and P2X3 → neuronal hyperexcitability[31,32] |
| Gain of function of ion channels (“channels sprouting”) → neuronal hyperexcitability |
| Nerve injury → persistent nociceptive input → enhanced pre-synaptic neurotransmitter release (glutamate and substance P) and enhanced post-synaptic signaling to spinal cord (via AMPA and NMDA receptor activation)[39] |
| Nerve injury→ microglial activation→ synthesis of cytokines, chemokines, and cytotoxic substances (BDNF, nitric oxide, and free radicals) → pro-inflammatory milieu in DRG[40] |
| Central mechanisms (altered central nervous system pain processing) |
| Enhanced glutamate release from primary afferents in spinal cord and enhanced spinal NMDA expression, and reduced expression of GABAB receptors → hyperexcitability of spinal neurons (central sensitization)[41] |
| Enhanced spontaneous neuronal activity associated with enhanced blood flow of ventral posterolateral neurons of the thalamus → generation and amplification of pain response[42] |
| Individuals with painful DPN have preserved thalamic N-acetyl aspartate (NAA) and GABA levels, whereas those with painless DPN have reduced NAA and GABA levels, indicating that these neurotransmitters are essential for transmission and amplification of pain[42] |
| Disruption of connectivity between thalamus and somatosensory cortical areas involved in behavioral/cognitive/emotional pain processing (anterior cingulate and insular cortex) → reduced thalamic feedback → aberrant pain processing and mood/sleep disturbances[43] |
| Impaired descending inhibition through periaqueductal gray and rostroventromedial medulla (PAG and RVM) → reduced pain inhibition and enhanced pain amplification[44] |
| Enhanced descending facilitation and enhanced ascending pain messages[44] |
DRG: Dorsal root ganglia, TRPV1: Transient receptor potential vanilloid 1, TRPA1: Transient receptor potential ankyrin 1, TRPM8: Transient receptor potential melastatin 8, P2X3: Purinoceptor 3, AMPA: A-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, NMDA: N-methyl-D-aspartate, BDNF: Brain derived neurotrophic factor, GABA: Gamma aminobutyric acid, NAA: N-acetyl aspartate, PAG: Periaqueductal gray, RVM: Rostroventromedial medulla, PDPN: Painful diabetic peripheral neuropathy
Management of PDPN
Currently Available Pharmacological Management
GABA analogs (Gabapentinoids) including pregabalin and gabapentin are considered as the first line pharmacological agents in the management of PDPN by various international agencies. The National Institute for Health and Care Excellence (NICE, 2013) guidelines consider gabapentin, pregabalin, amitriptyline, or duloxetine as the first line drugs and tramadol as the second line drug.[45] The guideline recommends capsaicin for localized neuropathic pain, and for those who cannot tolerate, has contraindications, or wish to avoid oral pharmacological agents.
The French recommendations for the management of neuropathic pain (2020) recommends duloxetine 60–120 mg/day, venlafaxine 150–225 mg/day, gabapentin 1200–3600 mg/day, or tricyclic antidepressants (TCAs) 10–150 mg/day as the first line for the central or non-focal peripheral neuropathic pain.[46] The second line agents for the group include pregabalin 150–600 mg/day, tramadol 100–400 mg/day, or antidepressants-gabapentinoids combination. For the focal peripheral neuropathic pain, the guideline recommends lidocaine plasters (1–3 plasters, 12 h/day) or transcutaneous electrical neural stimulation (TENS) (≥30 min/day) as the first line; and capsaicin 8% patch (1–4 patches every 3 months) or Botulinum toxin A (BTX-A) (50–300 units every 3 months) as the second line. The third line agents for either group include repetitive transcranial magnetic stimulation, spinal cord stimulation, or strong opioids.
The (ADA, 2017) considers pregabalin and duloxetine as the first line; gabapentin and amitriptyline as the second line; and opioids such as tramadol and tapentadol as the third line.[3] The American Academy of Neurology (AAN, 2011) guidelines consider pregabalin as the first line, and the drugs including gabapentin, sodium valproate, amitriptyline, duloxetine, venlafaxine, opioids, capsaicin patch, lidocaine patch, and isosorbide dinitrate spray as the second line.[47] They suggests not to consider the following drugs in the management of PDPN: anticonvulsant drugs such as oxcarbazepine, lamotrigine, and lacosamide; antidepressant drugs such as imipramine, desipramine, fluoxetine, nortriptyline and fluphenazine; miscellaneous drugs such as clonidine, pentoxifylline, and mexiletine.[47]
Various pharmacological agents and their effectiveness in the management of PDPN with emphasis on the number needed to treat (when data are available) for an additional harmful outcome number needed to harm (NNTH) and the number needed to treat for an additional beneficial outcome number needed to treat benefit (NNTB) are mentioned in the Table 3.
Table 3.
Pharmacological agents available in the management of PDPN and their effectiveness (NNTB and NNTH)[48-68]
| Name of the drug | Mechanism of action | Starting dose/day | Maximum dose/day |
NNTB (95% confidence interval)
|
NNTH (95% confidence interval)
|
||||
|---|---|---|---|---|---|---|---|---|---|
|
NNTB 30%
|
NNTB 50%
|
NNTB PGIC
|
Adverse events (AEs)
and AE-withdrawals |
||||||
| Pregabalin[48] | α2δ Ligand | 150 mg | Somnolence | Dizziness | Withdrawal | ||||
| 300 mg | 22 (12–200) | 14 (9.7–26) | 4.9 (3.8–6.9) | 13 (11–17) | 10 (8.6–13) | 35 (22–82) | |||
| 600 mg | 9.6 (5.5–41) | 6.1 (4.7–8.8) | 3.7 (2.8–5.3) | 9.6 (7.5–13) | 5.6 (4.8–6.7) | 12 (9.2–19) | |||
| Gabapentin[49] | α2δ Ligand | 900 mg | 3600 mg | Any AEs | Serious AEs | Withdrawal | |||
| 6.6 (4.9–9.9) | 6.6 (5.0–9.7) | 4.9 (3.6–7.6) | 7.5 (6.1–9.6) | No data | 30 (20–66) | ||||
| Duloxetine[50,51] | SNRI | 30–60 mg | 60–120 mg | 5.0 (3.0–8.0) | 5.0 (4.0–7.0) | No data | No data | No data | 17 (12–50) |
| Venlafaxine[52,53] | SNRI | 37.5–75 mg | 150–225 mg | 5.2 (2.7–5.9) | No data | No data | 9.6 (3.5–13) | No data | 16.2 (8–436) |
| Amitriptyline | TCA[53-55] | 10–25 mg | 150 mg | No data | 4.6 (3.6–6.6) | No data | 5.2 (3.6–9.1) | No data | 28 (17.6–69) |
| Nortriptyline[56] | TCA | 10–25 mg | 150 mg | No data | No data | No data | No data | No data | No data |
| Imipramine[57] | TCA | 10–25 mg | 75 mg | No data | No data | No data | No data | No data | No data |
| Desipramine[58] | TCA | 10–25 mg | 150 mg | No data | No data | No data | No data | No data | No data |
| Tramadol[59] | μ agonist | 50 mg bd | 400 mg | No data | 4.4 (2.9–8.8) | No data | 4.2 (2.8–8.3) | No data | 8.2 (5.8–14) |
| Tapentadol[60-62] | μ agonist | 50 mg bd | 500 mg | No data | No data | No data | No data | No data | No data |
| Oxycodone[63] | μ agonist | 10 mg bd | 60–120 mg | 5.7 (4.0–9.9) | No data | No data | 4.3 (3.1–7.0) | No data | No data |
| Morphine[64] | μ agonist | Adjust dose | 120 mg | 3.7 (2.6–6.5) | No data | No data | No data | No data | No data |
| Lidocaine[65-67] | NaV blocker | 5% Topical | 1–3 patches | No data | No data | No data | No data | No data | No data |
| Capsaicin[68] | TRPV ligand | 8% Topical | 1–4 patches | 10.6 (7.4–19) | No data | No data | No data | No data | No data |
| BTX-A SC[68] | Botulinum | 50–200 units | Q 3 months | 1.9 (1.5–2.4) | No data | No data | No data | No data | No data |
NNTB: Number needed to treat benefit, NNTH: Number needed to harm, PDPN: Painful diabetic peripheral neuropathy, TCA: Tricarboxylic acid, BTX-A: Botulinum toxin-A, SNRI: Serotonin-norepinephrine reuptake inhibitors.
A meta-analysis of 229 double-blind randomized controlled trials (RCTs) on oral and topical agents for the neuropathic pain calculated the NNTBs for 50% neuropathic pain relief.[68] The study observed that NNTBs were 3.6 (95% CI: 3.0–4.4) for the TCAs, 6.4 (95% CI: 5.2–8.4) for the serotonin-norepinephrine reuptake inhibitors (SNRIs) - duloxetine-venlafaxine combined, 7.7 (95% CI: 6.5–9.4) for pregabalin, 6.3 (95% CI: 5.0–8.3) for gabapentin immediate release or IR, 8.3 (95% CI: 6.2–13) for gabapentin extended release or ER, 7.2 (95% CI: 5.9–9.2) for gabapentin combined, 4.73 (95% CI: 3.6–6.7) for tramadol, 4.26 (95% CI: 3.4–5.8) for strong opioids, 10.64 (95% CI: 7.4–19) for capsaicin, and 1.85 (95% CI: 1.5–2.4) for BTX-A.
The study also observed that the NNTHs were 13.4 (95% CI: 9.3–24.4) for the TCAs, 11.8 (95% CI: 9.5–15.2) for combined duloxetine and venlafaxine, 13.9 (95% CI: 11.6–17.4) for pregabalin, 25.6 (95% CI: 15.3–78.6) for gabapentin IR, 31.9 (95% CI: 17.1–230) for gabapentin ER, 12.6 (95% CI: 8.4–25.3) for tramadol, and 11.7 (95% CI: 8.4–19.3) for strong opioids.[68] Based on this, the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain (NeuPSIG-IASP, 2015) recommended the TCAs, the SNRIs, pregabalin, and gabapentin as the first line (strong recommendation); lidocaine patches, capsaicin high-strength patches, and tramadol as the second line (weak recommendation); and strong opioids and BTX-A as the third line (weak recommendation) therapeutic agents in the management of neuropathic pain.
Pregabalin and Gabapentin
Gabapentinoids are anticonvulsant drugs that blocks the presynaptic alpha-2-delta (α2-δ) voltage-gated calcium channels (VGCC) in the dorsal root ganglia. These agents have high affinity for the α2δ-1 and α2δ-2 subunits of the VGCC. Pregabalin and Gabapentin are non-selective ligands of α2δ-1 and α2δ-2, wherein the drug inhibits the cellular calcium influx; reduces the number of synaptic vesicles fusing with the presynaptic membrane; decreases the release of various neurotransmitters including γ-aminobutyric acid (GABA), glutamate, norepinephrine, substance P, and calcitonin gene-related peptide (CGRP) into the synapse; and suppresses the activity of excitatory primary afferent fibers that carry nociceptive impulses to the dorsal horn.[69] In comparison to gabapentin, pregabalin has a predictable linear pharmacokinetics, exhibiting a dose-proportional absorption and response.
A superior therapeutic response was noted with pregabalin 300 mg compared to pregabalin 600 mg. In comparison to gabapentin, pregabalin exhibits a rapid onset of action, requires lesser dose titration, and has convenience of twice daily administration.[70] The lower doses of gabapentinoids (pregabalin <300 mg and gabapentin <1200 mg) are less likely to be beneficial for PDPN.[68] The maximal approved doses for PDPN include 600 mg/day for pregabalin and 3600 mg/day for gabapentin. Compared to placebo, both drugs can be associated with adverse effects in nearly 3%, including drowsiness, dizziness, edema, gait disturbances, and ataxia.[68] When taken at therapeutic doses, pregabalin is associated with lower healthcare and non-healthcare costs than gabapentin in patients with PDPN.[71] Pregabalin has got FDA approval for the management of neuropathic pain, whereas gabapentin has not.
A systematic review of 83 RCTs have observed that amitriptyline, duloxetine, gabapentin, pregabalin and venlafaxine have the best available evidence as monotherapy, and oxycodone has the best available evidence as add-on therapy for the management of PDPN.[72] Tramadol is effective when used as monotherapy as well as add-on therapy. A network meta-analysis that assessed the efficacy and safety of six drugs used in the management of PDPN including amitriptyline, duloxetine, gabapentin, pregabalin, valproate, and venlafaxine, observed that gabapentin has the highest efficacy and amitriptyline has the lowest safety.[73] They concluded that gabapentin exhibited the most favorable balance between efficacy and safety.
A recently published meta-analysis of 43 RCTs observed that, on a pairwise meta-analysis gabapentin exhibited superiority over placebo in achieving 50% pain reduction in comparison to desvenlafaxine, lacosamide, lamotrigine, oxcarbazepine, and tapentadol.[74] The study also observed that carbamazepine and venlafaxine exhibited inferiority in comparison to pregabalin in achieving 50% pain reduction, whereas mirogabalin, duloxetine, and duloxetine-gabapentin combination exhibited non-inferiority (statistically non-significant superiority) compared to pregabalin in achieving 50% pain reduction.
There is contrasting evidence regarding efficacy of gabapentin in some meta-analyses. A network meta-analysis of 57 RCTs observed that SNRIs, capsaicin, TCAs, pregabalin, and oxcarbazepine were better than placebo for short-term pain control. However, this study observed that gabapentin was not superior to placebo in the management of PDPN.[75] A similar observation was made in yet another systematic review of 106 RCTs that observed that SNRIs (duloxetine and venlafaxine), pregabalin (but not gabapentin), oxcarbazepine, TCAs, atypical opioids, and BTX are superior to placebo in the management of PDPN.[76] In comparison to other anticonvulsant drugs, the gabapentinoids do not have significant drug-drug interactions as they lack hepatic metabolism and cytochrome P450 induction.
Mirogabalin is a new α2-δ ligand that has received approval in Japan (2019) for the treatment of neuropathic pain. While pregabalin and gabapentin are non-selective ligands of α2-δ1 and α2-δ2 subunit of the VGCC, mirogabalin is selective ligand of α2-δ1 and α2-δ2. The binding of mirogabalin with α2-δ1 subunit is more potent.[77] Mirogabalin binds to α2-δ1 and α2-δ2 subunits for a longer time (longer dissociation half-life) in comparison to pregabalin, especially longer dissociation half from α2-δ1 subunit. This feature confers improved efficacy and safety to this molecule in comparison to pregabalin and gabapentin. In the phase 3 trial, mirogabalin 30 mg/day is found to be superior to placebo.[78] Although the adverse events (AEs) are like that of other α2-δ ligands (somnolence, dizziness, peripheral edema, and weight gain), they are only mild or moderate in severity.[79] In patients with PDPN, switching from pregabalin to mirogabalin improves the pain intensity and is well tolerated.[80]
SNRIS
SNRIs including duloxetine and venlafaxine amplify the activity of noradrenergic and serotonergic neurons in the descending inhibitory pathways of the dorsal horn, suppressing the excessive nociceptive impulses from reaching the brain.[81] SNRIs also exerts an effect on neuropathic pain through inhibition of neuroimmune mechanisms accompanying nerve injury.[82] Duloxetine has received FDA approval for the management of neuropathic pain, whereas venlafaxine has not. Duloxetine is effective in the management of PDPN at doses of 60–120 mg/day,[83] but is ineffective at doses <60 mg/day.[51] Furthermore, an increase in duloxetine dose from 60 mg/day to 120 mg/day did not achieve any significant extra pain reduction.[84] The recommendation is to start with duloxetine 30 mg/day in the morning and increase the dose to 60 mg or up to 120 mg as a single morning dose after 7–14 days.[85]
Apart from PDPN, duloxetine is approved as a first-line agent in major depressive disorder (MDD), generalized anxiety disorder (GAD), fibromyalgia syndrome, and stress urinary incontinence. A recent systematic review of 85 studies that analyzed the efficacy, safety, and tolerability of duloxetine in these indications observed that the drug is effective in over 80% of cases.[86] The common treatment emergent AEs (TEAEs) were dry mouth, somnolence, nausea, constipation, or hyperhidrosis. These TEAEs tend to decrease over time and disappear with continuation of duloxetine therapy. Among the observed cardiovascular AEs, such as hypertension, increase in heart rate, and myocardial ischemia, only the tachycardia was statistically significant, although not clinically relevant. Overall, duloxetine is a safe and well-tolerated agent even in elderly patients and those with cardiovascular disease.[86]
Duloxetine monotherapy is associated with <20% treatment discontinuation due to TEAEs.[87] Although the discontinuation rate due to TEAEs is higher in older population, duloxetine monotherapy is well tolerated and efficacious regardless of the age.[88] Although an increase in glucose, glycosylated hemoglobin (HbA1c), total cholesterol, and bodyweight is noted on long-term monotherapy with duloxetine, none of these were clinically relevant.[89] A randomized controlled trial that compared the efficacy of pregabalin, amitriptyline, and duloxetine, and their effect on sleep, daytime functioning, and quality of life in patients with PDPN observed no significant difference in analgesic efficacy between these agents when used as monotherapy.[90] However, in regard to the sleep-wake cycle, pregabalin improved the sleep duration (p<0.001), whereas duloxetine increased the wake duration and reduced the total sleep duration (p<0.01 and <0.001). Duloxetine monotherapy was associated with enhanced performance of sensory-motor task, whereas pregabalin was associated with higher AEs.
Another randomized controlled trial compared the efficacy and safety of duloxetine monotherapy, pregabalin monotherapy, or duloxetine-gabapentin combination therapy in the management of PDPN in those with inadequate treatment response to gabapentin ≥900 mg/day.[91] The study observed that duloxetine is noninferior to pregabalin for the treatment of PDPN. However, there was difference in the AEs. Nausea, insomnia, hyperhidrosis, and loss of appetite were higher with duloxetine compared to pregabalin. On the other hand, peripheral edema was more frequent with pregabalin compared to duloxetine. Insomnia is less frequent with duloxetine-gabapentin combination therapy compared to duloxetine monotherapy, whereas nausea, vomiting, hyperhidrosis, and loss of appetite are more frequent with duloxetine-gabapentin combination therapy compared to pregabalin.
Venlafaxine extended-release formulation has received FDA approval for MDD, GAD, social anxiety disorder, and panic disorder.[92] It is effective in the management of PDPN at doses of 150–225 mg/day.[93,94] Its efficacy is equal or superior to carbamazepine, equal to that of imipramine, but inferior to that of pregabalin.[95-97] A comparative effectiveness network meta-analysis observed that venlafaxine is marginally superior over duloxetine in the management of PDPN.[75] However, there is lack of support for this observation from larger comprehensive trials. Mechanism of action of venlafaxine in the management of PDPN include activation of descending inhibitory pathways, reduction in spinal hyperexcitability through inhibition of the central 5-HT1A serotoninergic receptors, and a reduction in tumor necrosis factor α production through α2-adrenoreceptor mediated as well as β2-adrenoreceptor (within the dorsal root ganglia) mediated mechanisms.[98] The common AEs include nausea, somnolence, and ECG changes. Venlafaxine should be weaned off slowly to reduce the adverse effects. Desvenlafaxine at doses of 200 and 400 mg/day was tried in the management of PDPD and was found to be effective in pain reduction and well tolerated.[99]
TCAS
Antidepressants achieved pain relief in patients with or without depression, and the analgesic effect occurred at much lower doses than that is required to improve depression.[100] Moreover, the analgesic effect of antidepressants occurred within few days to 1 week, whereas the antidepressant effect occurred only in 2–4 weeks.[101] TCAs inhibit the presynaptic reuptake of serotonin and norepinephrine, which activate the descending inhibitory pathways of the dorsal horn, suppressing the excessive nociceptive impulses from reaching the brain. Apart from this, the TCAs block the sodium channels, calcium channels, histamine (H1) receptors, α1-adrenergic receptors, and N-methyl-D-aspartate (NMDA) receptors. Moreover, the TCAs activate the potassium channels, opioid receptors, and GABA-B receptors. All these mechanisms are associated with reduction in neuropathic pain.[102]
Based on the pharmacological structure, the TCAs are subdivided into tertiary amines and secondary amines.[103] Tertiary amines are amitriptyline, imipramine, and clomipramine, whereas secondary amines are nortriptyline, desipramine, and desmethylclomipramine.[104] The secondary amines are the metabolites of the tertiary amines formed by demethylation of tertiary amines.[105] The secondary amines selectively block the reuptake of norepinephrine. On the other hand, the tertiary amines block the reuptake of both serotonin as well as norepinephrine; the reuptake of serotonin is blocked by the tertiary amine itself, whereas the reuptake of norepinephrine is blocked by the metabolite. In view of the above, the tertiary amines are also known as dual-type TCAs. The NNTB for 50% neuropathic pain relief for TCAs in general is approximately 2–3, for tertiary amines is 2.1, and for secondary amines is 2.5.[68]
The common AEs associated with TCA therapy include dry mouth, blurred vision, postural hypotension, QTc prolongation, cardiac arrhythmias, myocardial ischemia, drowsiness, cognitive impairment, excessive sleepiness, excessive weight gain, constipation, and urinary retention. With regard to safety, the secondary amines are better tolerated compared to tertiary amines, as the latter are associated with increased rates of drowsiness (resulting from increased histaminergic and α1-adrenergic blocking), anticholinergic side-effects, and cardiac side-effects including hypotension (resulting from increased α1-adrenergic blocking). Hence, ADA suggests that secondary amines are preferred over tertiary amines in those who are prone for these side effects and in elderly.[3] Overall, the TCAs should be used with caution in PDPN patients with known or suspected cardiovascular disease.
With regard to efficacy, the tertiary amine - amitriptyline - is considered as the most efficient agent among all TCAs by AAN.[47] It is as effective as gabapentin[106] and duloxetine.[105-107] Its secondary amine counterpart - nortriptyline - also is equally effective to gabapentin in the management of neuropathic pain, though the combination of nortriptyline and gabapentin was more effective than either drug given alone.[108] In general, the effective therapeutic dose ranges from 25 to 150 mg/day, with the most or all the doses taken at night-time. The “Start-Low, Go-Slow” approach seems to be the best approach in elderly, with the dose up-titrated over a period of 6–8 weeks at weekly increments of 10–25 mg. Thus, the pain relief would be noted only after several weeks, which would compromise on the compliance.[45]
Tapentadol and Tramadol
The opioids can be classified into strong opioids which include molecules such as morphine, oxycodone, hydromorphone, buprenorphine, and fentanyl; and weak opioids which include molecules such as codeine, dihydrocodeine, tramadol, and tapentadol.[79] Opioids are μ-receptor agonists that act by blocking the ascending pain signals in the spinal cord. The term atypical opioid is coined for those μ-receptor agonists that have one additional non-μ-receptor mediated mechanism of action. Tapentadol, tramadol, buprenorphine, levorphanol, and methadone are atypical opioids.[109] Tapentadol exhibits additional norepinephrine reuptake inhibition, whereas tramadol exhibits additional serotonin and norepinephrine reuptake inhibition. These additional mechanisms activate the descending inhibitory pathways, complement, and potentiate the μ-opioid receptor activation, contributing to an opioid-sparing effect. This opioid-sparing effect result in reduced incidence of opioid induced gastrointestinal AEs including nausea as well as constipation. Tapentadol ER with a half-life of 4.4–5.9 h should be initiated at 50 mg twice daily. The dose should be increased every 3 days in increments of no more than 50 mg twice daily to the effective therapeutic dose of 100–250 mg twice daily.
According to the neuropathic pain special interest group-international association for the study of pain (NeuPSIG-IASP) recommendations, the weak opioids are considered as second line agents and the strong opioids are considered as third line agents in the management of PDPN.[68] The strong opioids are associated with high abuse potential, and with resultant high mortality from overdosage.[110] Hence, the weak opioid tapentadol ER is the only agent from this group that has received FDA approval in the management of PDPN. Tapentadol ER offers pain relief at 100–250 mg doses twice daily for up to a period of 1 year. Moreover, it offers better compliance in comparison to oxycodone-controlled release tablets.[111] It is effective across different patient subgroups regardless of age, gender, ethnicity, opioid experience, or intensity of pain.[60] It has a better safety profile, is well tolerated, and is associated with improved HRQoL in patients with PDPN. The TEAEs were nausea in 21.1% and vomiting in 12.7%.[61]
A 6-month open extension trial following a 6-week randomization observed that tramadol, in doses of 50–400 mg/day, is effective in achieving long-term pain relief in patients with diabetic neuropathy.[112] The patients treated with tramadol experienced superior physical and social functioning, though the commonest TEAEs were headache, excessive sleepiness, nausea, and constipation.[113] However, a Cochrane systematic review observed that the NNTB for 50% pain relief from neuropathic pain is 4.4 (95% CI 2.9–8.8) with tramadol, whereas the NNTH is 4.2 (2.8–8.3).[59] However, the evidence showing benefit from tramadol was of low or even very low quality. Moreover, due to the additional serotonin reuptake blocking effect, there is a risk of serotonin syndrome characterized by labile blood pressure, confusion, seizures, or coma, especially when the tramadol is administered for the management of breakthrough pain in combination with TCAs or SNRIs.[114]
A systematic review that compared the efficacy and safety of tapentadol and tramadol in adults observed that the use of tramadol in neuropathic pain and tapentadol in PDPN has only low level of supportive evidence.[115] Tapentadol is associated with fewer serotoninergic side effects (nausea, vomiting, and hypoglycemia), higher opioid side effects (constipation, respiratory suppression, and abuse potential), and lesser drug-to-drug interactions in comparison to tramadol. Even though tapentadol has been proved to be generally safe among the elderly population, there is lack of sufficient evidence regarding its safety in vulnerable elderly patients, and in those with severe liver or kidney impairment.
Another recently published systematic review observed that various opioids including morphine, hydromorphone, oxycodone, tramadol, and buprenorphine are effective in relieving the neuropathic pain by ≥50% and in reducing the disability associated with neuropathic pain without any clinically relevant treatment related AEs.[116] The authors also observed that tapentadol is effective in relieving the neuropathic pain associated with PDPN by ≥50% and in reducing the disability without any clinically relevant treatment related AEs. In a retrospective cohort study, the abuse liability of tapentadol active pharmaceutical ingredient is compared with that of morphine, hydromorphone, oxymorphone, hydrocodone, oxycodone, and tramadol. The study observed that the population-level abuse rate is significantly lower than other opioids, but when adjustments are made for availability of drug, the abuse rates were lower, but not the lowest.[117]
Buprenorphine is an atypical opioid: A partial agonist at the μ-opioid receptor, agonist at the δ-opioid and opioid receptor-like 1 (ORL-1) receptor and a weak antagonist at the κ-opioid receptor.[109] Levorphanol is another atypical opioid: Agonist at the μ-opioid, the δ-opioid, and the κ-opioid receptors; antagonist at the NMDA receptors and having an SNRI like effect. A recent systematic review that evaluated the effects of atypical opioids including tapentadol, buprenorphine and levorphanol in patients with neuropathic pain observed that the evidence is strong for tapentadol, whereas it is weak for buprenorphine and levorphanol.[116] The transdermal buprenorphine does not achieve a clinically significant pain relief due to high withdrawal rates. The oral levorphanol is as effective as first line and second line agents in the management of neuropathic pain. The study concluded that atypical opioids with lesser abuse potential can be considered as an alternate option in those with neuropathic pain that is refractory to first- and second-line agents, but only after further research. A Cochrane review that evaluated the effect of atypical opioid methadone in the management of neuropathic pain observed that definite conclusions cannot be made due to very limited and very low-quality evidence available.[118]
Topical Capsaicin
The transient receptor potential vanilloid 1 (TRPV1) receptors are expressed on the primary nociceptive afferents which are the small Aδ and C nerve fibers.[119] Capsaicin is a natural alkaloid present in red chili peppers and is a selective agonist of TRPV1 receptors. The downstream signals from TRPV1 receptors release substance P and other neurotransmitters. The repeated exposure to topical capsaicin results in neurotransmitter depletion and a reduction in pain signals reaching to central nervous system from peripheral nervous system. Capsaicin at low concentrations achieves analgesia lasting for hours secondary to only short-term reversible loss of function of nociceptive afferents.[120] On the other hand, capsaicin at high concentration achieves analgesia lasting for several months secondary to long-term loss of function of TRPV1 expression in nociceptive afferent terminals. The long-term analgesic effects of capsaicin are mediated by mitochondrial dysfunction, microtubule disorganization, and the calcium-calpain-mediated degeneration of the afferent axons.[121-123]
Capsaicin is recommended as the second-line agent in the management of neuropathic pain by AAN and as the third-line agent by the NICE. It can be used as capsaicin topical cream or as an 8% capsaicin patch. The capsaicin topical creams (0.025% and 0.075%) have compliance issues, as they need to be applied four times daily. On the other hand, a single 30-min application of capsaicin 8% patch can have long-lasting pain relief lasting up to 3 months.[124] Up to four capsaicin 8% patches can be applied simultaneously. The capsaicin 8% patch has received European Medicines Agency (2015) and FDA (2020) approval for the management of PDPN.[125,126] The initial stimulation of the nociceptive afferent neurons results in a burning pain at the site of application associated with redness, itching, swelling etc. These “application site reactions” are transient, and they diminish with repeated use - “capsaicin desensitization.” However, during this desensitization phase the neurons become unresponsive to thermal nociception with a possible high risk for diabetic foot ulceration. The capsaicin patch is tolerated well. The transient application site reactions do not improve with lidocaine plaster pre-treatment.[127] Moreover, the lidocaine plaster does not improve the efficacy, tolerability, and compliance to capsaicin patch therapy.
A Cochrane review observed that there is insufficient data regarding efficacy and tolerability of low-concentration capsaicin (<1%) in patients with neuropathic pain.[128] Another Cochrane review showed that high-concentration capsaicin improves the neuropathic pain in a small proportion of people.[129] Those with positive response in neuropathic pain also get better sleep, and improvement of depression, energy levels, and quality of life. Because of the high cost associated with capsaicin patch therapy; this treatment should be reserved only for adults with neuropathic pain who are refractory to all other available therapies. Moreover, repeated applications should only be done in cases exhibiting significant objective pain relief.
Topical Lidocaine
Lidocaine blocks the voltage gated sodium channels (Nav1.7 and Nav1.8) on the primary nociceptors Aδ and C afferent neurons, and this reduces the ectopic discharges.[130] Similarly, the drug regulates T cell activity and suppresses the nitric oxide generation and exhibits an anti-inflammatory effect. The lidocaine also directly activates the TRPV1 and TRPA1 channels in the nociceptive afferents, produces membrane depolarization, and reduces the electrical activity in the afferent neurons. The drug is available as 5% lidocaine-medicated plasters and it is applied once daily for 12 h. Up to three plasters can be applied simultaneously. It is well tolerated, though transient “application site reactions” may occur that resolve with removal.
When used as monotherapy, 5% lidocaine-medicated plasters are associated with similar degree of pain relief, but with significantly fewer TEAEs when compared to pregabalin.[131] When these patients who were initially on monotherapy with 5% lidocaine medicated plaster or pregabalin were treated with combination therapy, they achieved additional pain relief with improved patient satisfaction. Moreover, the combination therapy was safe and was well tolerated.[132] Similar improvement in neuropathic pain and quality of life was noted with a combination of lidocaine medicated plaster and gabapentin.[133] A systematic review and network meta-analysis of 23 studies observed that the lidocaine medicated plaster has a comparable efficacy to amitriptyline, gabapentin, pregabalin, and capsaicin, with fewer and less severe AEs.[134] A recent study has noted that a combination of capsaicin and lidocaine patch is safe and effective in patients with PDPN.[135]
Topical GTN and Topical Clonidine
Glyceryl Trinitrate (GTN) patch is used as an off-label treatment to alleviate the neuropathic pain associated with PDPN.[136-138] The GTN is proposed to act via alteration in neuronal nitric oxide synthase in the dorsal root ganglion cells and the spinal cord contributing to neuronal plasticity.[139] Topical clonidine gel, an α2-adrenergic agonist, has been shown to decrease the pain in patients with PDPN.[140] In a Cochrane review regarding the use of topical clonidine in PDPN, the NNTB for 30% neuropathic pain relief is 8.33 (95% CI: 4.3–50). The authors concluded that topical clonidine can be considered when no better treatment options are available due to adverse effects, contraindications, or lack of efficacy.[141]
Intravenous Lidocaine
Intravenous infusion of lidocaine administered, at a dose of 3–5 mg/kg, over an hour, offered short-term pain relief without any improvement in quality of life.[142,143]
BTX-A
BTX is a neurotoxin produced by Clostridium botulinum and it blocks the acetylcholine release at neuromuscular junctions causing muscle relaxation.[144] The analgesic effect of BTX is due to inhibition of release of neurotransmitters including substance P, glutamate, and CGRP from the afferent nerves. Moreover, the BTX reduces the activity of TRPV1. The BTX administered every 3 months subcutaneously at doses of 50–300 mg into the painful area reduces the neuropathic pain intensity, improves the sleep quality and quality of life as observed in three RCTs.[145-147] However, the RCT evidence is weak due to small sample sizes.[148] According to NeuPSIG-IASP recommendations, BTX is considered as the third line agent in the management of neuropathic pain.[68]
Novel Pharmacological Treatment Options
Sodium Channel Antagonists
Genes encoding the voltage gated sodium channels have a significant role in the pathogenesis of small fiber neuropathies. Gain of function mutations of genes encoding Nav1.7, Nav1.8, and Nav1.9 are associated with PDPN.[149-152] As Nav1.7, Nav1.8, and Nav1.9 are preferentially expressed by the peripheral nociceptors, their antagonism would improve pain without causing central and cardiac TEAEs.[153] Vixotrigine is an oral Nav1.7 antagonist. In a phase 2 RCT, vixotrigine 200 mg administered twice daily achieved statistically significant reduction in average daily pain score at 12th week in diabetes-associated small fiber neuropathy, but not in idiopathic small fibre neuropathy.[154] The drug was well tolerated with the common TEAEs (incidence ≥2.5%) include dizziness, headache, vertigo, and nausea. VX-150 is an oral Nav1.8 antagonist. In a phase 2 RCT, VX-150 1250 mg administered once daily achieved significant pain relief in patients with small fiber neuropathy and was well tolerated.[155]
Cibinetide (Innate Repair Receptor [IRR] Ligand)
Cibinetide (previously ARA290) is a novel peptide analogous to erythropoietin, and it acts by selectively interacting with the IRR.[156] Cibinetide improves the neuropathic pain via anti-inflammatory effects and through stimulation of the regrowth of the nerve fibers from the damaged axons, both mediated by IRR. In a phase 2 study, 4 mg Cibinetide, administered subcutaneously resulted in improvement in HbA1c, lipid levels, neuropathic symptoms and small fiber neuropathy. Another potential use of cibinetide is to use with low-dose tacrolimus in improving the survival after pancreatic islet transplantation.[157] The islet protection could be mediated by reduction in the Instant Blood Mediated Inflammatory Reaction and a delay in the alloreactivity. The drug may also improve the engraftment after pancreatic islet transplantation.[158]
Transient Receptor Potential (TRP) Agonists
TRP agonists are an attractive option as novel analgesics for the management of neuropathic pain.[159] Few members from this group have reached the phase 2 stage: TRPV1 agonist (AZD1386),[160] TRPA1 agonist (GRC-17536),[161] and TRPV3 agonist (SAR292833).[162] The phase 2 trials for AZD1386 for the neuropathic pain and osteoarthritis were terminated due to various reasons while GRC-17536 has successfully completed phase 2 trials.[163]
P2X3 Receptor Ligands
P2X3 or purinoceptor-3 is a ligand-gated ion channel in the peripheral nervous system with which the ATP signaling results in neuronal sensitization and neuropathic pain. The selective antagonist that blocks the P2X3 receptor (eliapixant) and the nonselective antagonist that blocks both P2X3 and P2X2/3Rs (Gefapixant) have recently completed Phase 3 clinical trials for refractory chronic cough.[164] However, these agents are potential drug targets for the management of neuropathic pain.[165]
Vitamin D
Vitamin D deficiency is associated with greater severity of neuropathic pain in patients with diabetic neuropathy.[166] Vitamin D treatment is associated with improvement in pain severity, pain-related disability, and neuropathy-specific quality of life in patients with PDPN.[167] Vitamin D is an endogenous partial agonist of TRPV1, and this could possibly explain the analgesic effect of Vitamin D.[168]
Angiotensin II Receptor 2 (AT2R) Antagonist
Upregulation of AT2R and TRPV1 is considered to contribute to neuropathic pain. Olodanrigan antagonizes AT2R and inhibits the direct phosphorylation of TRPV1.[169] In 2 phase 2b trials, the drug administered as 100 mg twice daily achieved clinically significant pain relief in patients with post herpetic neuralgia and PDPN. However, these two RCTs were prematurely terminated due to potential for liver toxicity on long-term administration observed in preclinical studies, this was not observed in the phase 2b trials.[169]
Trazadone and Gabapentinoid Combination
Trazadone is a second-generation antidepressant with a sedative effect. It has α1-adrenergic, H1 histaminergic, and 5HT2 receptor blocking action in the post-synaptic membrane at the lower doses, and it has serotonin reuptake inhibitory action in the presynaptic membrane at higher doses.[170] Hence, its mechanism of action is often referred as Serotonin Antagonist - Reuptake Inhibition. A pilot RCT showed a clinically significant improvement in the neuropathic pain symptoms with trazodone-gabapentin combination.[171] A phase 2 trial comparing trazodone-gabapentin fixed dose combination with gabapentin alone or placebo in the management of PDPN is in progress.[172]
Dextromethorphan and Quinidine Combination
Dextromethorphan is an NMDA receptor antagonist. In addition, it is a sigma-1 receptor (σ1) agonist, N-type calcium channel antagonist, and a serotonin reuptake inhibitor. It has a rapid and extensive metabolism by hepatic cytochrome P450 2D6. Quinidine, a potent cytochrome P450 2D6 inhibitor, can help in maintaining a bioavailability of dextromethorphan so that it can exert a better therapeutic effect. At doses of 45/30 mg, or 30/30 mg the combination has shown its efficacy and safety for neuropathic pain.[173]
A summary of available pharmacological therapies for the management of neuropathic pain as per recommendations from various guidelines is summarized in Table 4.
Table 4.
Available pharmacological therapies for the management of neuropathic pain as per various guidelines
| AAN[47] | NICE[45] | IASP[68] | ADA[3] | IDF[174] | Canada[175] | French[46] | German[86] | |
|---|---|---|---|---|---|---|---|---|
| 2011 | 2013 | 2015 | 2017 | 2017 | 2018 | 2020 | 2020 | |
| Pregabalin | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Gabapentin | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 |
| Duloxetine | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| Venlafaxine | 2 | No mention | 1 | No mention | No mention | 2 | 1 | No mention |
| Amitriptyline | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 |
| Tramadol | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 3 |
| Tapentadol | No mention | No mention | 2 | 3 | No mention | 3 | 3 | 3 |
| Oxycodone ER | 2 | No mention | 3 | No mention | No mention | 3 | 3 | 3 |
| Other Opioids | 2 | No mention | 3 | No mention | 2 | No mention | 3 | 2 |
| Dextromethorphan | 2 | No mention | No mention | No mention | No mention | 3 | No mention | No mention |
| Lidocaine patch | May be used | No mention | 2 | No mention | No mention | No mention | 1 (focal) | 2 |
| Capsaicin patch | 2 | No mention | 2 | No mention | No mention | No mention | 2 (focal) | 2 |
| BTX-A | No mention | No mention | 3 | No mention | No mention | No mention | 2 (focal) | 3 |
| Sodium Valproate | 2 | No mention | No mention | No mention | No mention | 2 | No mention | No mention |
| Neuromodulation | No mention | No mention | No mention | No mention | No mention | No mention | 3 | No mention |
BTX-A: Botulinum toxin-A.
Non-Pharmacological Management
The various available neuromodulation techniques used in the management of neuropathic pain are given in Table 5. The Repetitive Transcranial Magnetic Stimulation and transcranial direct current stimulation are the commonly used central non-invasive neuromodulation (NINM) techniques, whereas TENS is the most common peripheral NINM technique.[177] The NINM techniques induce changes in neuronal membrane polarity, thereby modulating the brain function and pain perception. They also regulate the endogenous inhibitory pain pathways and reverse the maladaptive synaptic plasticity. A recent meta-analysis of various NINM techniques observed that the central NINM techniques and the peripheral electrical NINM techniques reduce the neuropathic pain in patients with PDPN.[178] The high frequency (10 kHz) spinal cord stimulation provides clinically significant pain relief and improvement in HRQoL in patients with PDPN refractory to conventional therapy are.[179] As the purpose our review is to critically appraise the use of pharmacotherapy for PDPN, we are not elaborating these modalities of treatment in this review.
Table 5.
| Non-invasive neuromodulation (NINM) techniques | ||
| Peripheral NINM | ||
| Electrical | TENS | Transcutaneous Electrical Neural Stimulation |
| PENS | Percutaneous Electrical Nerve Stimulation | |
| Electromagnetic | PEMF | Pulsed Electromagnetic Field |
| FREMS | Frequency-Modulated Electromagnetic Neural Stimulation | |
| Central NINM | ||
| Electromagnetic | rTMS | Repetitive Transcranial Magnetic Stimulation |
| Electrical | tDCS | Transcranial Direct Current Stimulation |
| MDM | Mesodiencephalic Modulation | |
| Invasive neuromodulation techniques | ||
| ITT | Intrathecal Therapy with Ziconotide | |
| SCS | Spinal Cord Stimulation | |
| Conventional SCS | ||
| Burst SCS | ||
| High Frequency (10 kHz) SCS | ||
PDPN: Painful diabetic peripheral neuropathy.
Summary and Conclusions
Vast majority of patients with DPN remain pain-free, but PDPN is estimated to affect 6–34% of all DM and is associated with reduced HRQoL and substantial economic burden. Early diagnosis and timely intervention are essential to prevent the development and progression of DPN. Delayed diagnosis of DPN after established sensory loss results in severe irreversible nerve damage, which could have been prevented if diagnosed earlier. Symptomatic treatment of PDPN and DAN remains the mainstays of management. Using certain patient related characteristics, PDPN patients can be stratified and assigned targeted therapies to produce better pain outcomes. PDPN is a heterogenous condition caused by a complex interaction of various environmental factors, genetic predisposition, metabolic disturbances, and vascular dysfunction leading on to downstream alterations in the peripheral and central nervous system. Understanding the pathogenesis of PDPN helps in the development of new treatments targets for the management of neuropathic pain.
Management of DPN can be divided into pharmacological and non-pharmacological agents. Currently available pharmacological agents include GABA analogues such as pregabalin and gabapentin either of which are considered as the first line pharmacological agents in the management of PDPN by various international agencies. The lower doses of GABA analogues (pregabalin <300 mg and gabapentin <1200 mg) are less likely to be beneficial for the management of PDPN. SNRIs and TCAs have also been used as first line treatments. Amitriptyline is considered as the most efficient agent among all TCAs by AAN. Regarding opioids, the weaker ones are considered as second line agents and the stronger ones are considered as third line agents in the management of PDPN. Capsaicin is recommended as the second-line agent in the management of neuropathic pain by AAN and as the third-line agent by the NICE. Topical lidocaine plasters can be used as monotherapy or as combination therapy with pregabalin with good outcome. BTX is considered as the third line agent in the management of neuropathic pain.
GTN patch can be considered as an off-label treatment to alleviate the neuropathic pain associated with PDPN and topical clonidine can be considered when no better treatment options are available due to adverse effects, contraindications, or lack of efficacy. Vitamin D treatment is also associated with improvement in pain severity, pain-related disability, and neuropathy-specific quality of life in patients with PDPN. A few novel pharmacological treatment options that are promising but are still in experimental stage include sodium channel antagonists, IRR ligands, P2X3 receptor ligands, trazadone-gabapentinoid combination, and dextromethorphan-quinidine combination.
Disclosure
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept – C.J.F., J.M.P.; Design – C.J.F., J.M.P., S.T.; Supervision – J.M.P., S.T.; Materials – C.J.F., K.K., D.V.G.; Data collection & Processing – C.J.F., J.M.P.; Analysis & interpretation – C.J.F., J.M.P., S.T.; Literature search – C.J.F., K.K., D.V.G.; Writing – C.J.F., D.V.G., K.K.; Critical review & revision – C.F.J., S.T., K.K., D.V.G., J.M.P.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Available at: https://www.diabetesatlas.org/en/. Accessed Dec 25, 2020.
- 2.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817–24. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 3.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alleman CJ, Westerhout KY, Hensen M, Chambers C, Stoker M, Long S, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res Clin Pract. 2015;109:215–25. doi: 10.1016/j.diabres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol (Lausanne) 2021;12:671257. doi: 10.3389/fendo.2021.671257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azmi S, Alam U, Burgess J, Malik RA. State-of-the-art pharmacotherapy for diabetic neuropathy. Expert Opin Pharmacother. 2021;22:55–68. doi: 10.1080/14656566.2020.1812578. [DOI] [PubMed] [Google Scholar]
- 8.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. EURODIAB Prospective Complications Study Group Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–50. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 9.Deeks ED. Mirogabalin: first global approval. Drugs. 2019;79:463–8. doi: 10.1007/s40265-019-01070-8. [DOI] [PubMed] [Google Scholar]
- 10.Themistocleous AC, Crombez G, Baskozos G, Bennett DL. Using stratified medicine to understand, diagnose, and treat neuropathic pain. Pain. 2018;159(Suppl 1):S31–42. doi: 10.1097/j.pain.0000000000001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–8. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 12.Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157:1132–45. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naruse K. Schwann cells as crucial players in diabetic neuropathy. Adv Exp Med Biol. 2019;1190:345–56. doi: 10.1007/978-981-32-9636-7_22. [DOI] [PubMed] [Google Scholar]
- 15.Stino AM, Rumora AE, Kim B, Feldman EL. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J Peripher Nerv Syst. 2020;25:76–84. doi: 10.1111/jns.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maamoun H, Benameur T, Pintus G, Munusamy S, Agouni A. Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1. Front Physiol. 2019;10:70. doi: 10.3389/fphys.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farias VX, Uchoa PN, Aquino CP, Britto LRG, Fonteles MC, Leal-Cardoso JH, et al. Expression of myo-inositol cotransporters in the sciatic nerve and dorsal root ganglia in experimental diabetes. Braz J Med Biol Res. 2019;52:e8589. doi: 10.1590/1414-431X20198589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizukami H, Osonoi S, Takaku S, Yamagishi SI, Ogasawara S, Sango K, et al. Role of glucosamine in development of diabetic neuropathy independent of the aldose reductase pathway. Brain Commun. 2020;2:fcaa168. doi: 10.1093/braincomms/fcaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizukami H, Osonoi S. Pathogenesis and molecular treatment strategies of diabetic neuropathy collateral glucose-utilizing pathways in diabetic polyneuropathy. Int J Mol Sci. 2020;22:94. doi: 10.3390/ijms22010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–34. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, et al. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177–209. doi: 10.1016/bs.irn.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Sloan G, Ye Y, Wang S, Duan B, Tesfaye S, et al. New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol (Lausanne) 2020;10:929. doi: 10.3389/fendo.2019.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 25.Jang ER, Lee CS. 7-ketocholesterol induces apoptosis in differentiated PC12 cells via reactive oxygen species-dependent activation of NF-κB and Akt pathways. Neurochem Int. 2011;58:52–9. doi: 10.1016/j.neuint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Othman A, Bianchi R, Alecu I, Wei Y, Porretta-Serapiglia C, Lombardi R, et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes. 2015;64:1035–45. doi: 10.2337/db14-1325. [DOI] [PubMed] [Google Scholar]
- 27.Callaghan BC, Gallagher G, Fridman V, Feldman EL. Diabetic neuropathy: what does the future hold? Diabetologia. 2020;63:891–7. doi: 10.1007/s00125-020-05085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36:2456–65. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes. 2015;6:432–44. doi: 10.4239/wjd.v6.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep. 2019;19:32. doi: 10.1007/s11892-019-1150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pabbidi MR, Premkumar LS. Role of transient receptor potential channels Trpv1 and Trpm8 in diabetic peripheral neuropathy. J Diabetes Treat. 2017;2017:029. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, et al. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 2018;14:359–69. doi: 10.1007/s11302-018-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zemel BM, Ritter DM, Covarrubias M, Muqeem T. A-type KV channels in dorsal root ganglion neurons: diversity, function, and dysfunction. Front Mol Neurosci. 2018;11:253. doi: 10.3389/fnmol.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodwin G, McMahon SB. The physiological function of different voltage-gated sodium channels in pain. Nat Rev Neurosci. 2021;22:263–74. doi: 10.1038/s41583-021-00444-w. [DOI] [PubMed] [Google Scholar]
- 35.Blesneac I, Themistocleous AC, Fratter C, Conrad LJ, Ramirez JD, Cox JJ, et al. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain. 2018;159:469–80. doi: 10.1097/j.pain.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18:926–33. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 37.Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem. 2010;114:1460–75. doi: 10.1111/j.1471-4159.2010.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joksimovic SL, Evans JG, McIntire WE, Orestes P, Barrett PQ, Jevtovic-Todorovic V, et al. Glycosylation of CaV3.2 channels contributes to the hyperalgesia in peripheral neuropathy of type 1 diabetes. Front Cell Neurosci. 2020;14:605312. doi: 10.3389/fncel.2020.605312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17:400–20. doi: 10.1038/s41574-021-00496-z. [DOI] [PubMed] [Google Scholar]
- 40.Rajchgot T, Thomas SC, Wang JC, Ahmadi M, Balood M, Crosson T, et al. Neurons and microglia; a sickly-sweet duo in diabetic pain neuropathy. Front Neurosci. 2019;13:25. doi: 10.3389/fnins.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer TZ, Waxman SG. Neuropathic pain in diabetes--evidence for a central mechanism. Nat Rev Neurol. 2010;6:462–6. doi: 10.1038/nrneurol.2010.90. [DOI] [PubMed] [Google Scholar]
- 42.Selvarajah D, Wilkinson ID, Gandhi R, Griffiths PD, Tesfaye S. Microvascular perfusion abnormalities of the Thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care. 2011;34:718–20. doi: 10.2337/dc10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe K, Hirano S, Kojima K, Nagashima K, Mukai H, Sato T, et al. Altered cerebral blood flow in the anterior cingulate cortex is associated with neuropathic pain. J Neurol Neurosurg Psychiatry. 2018;89:1082–7. doi: 10.1136/jnnp-2017-316601. [DOI] [PubMed] [Google Scholar]
- 44.Segerdahl AR, Themistocleous AC, Fido D, Bennett DL, Tracey I. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain. 2018;141:357–64. doi: 10.1093/brain/awx337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centre for Clinical Practice at NICE (UK). London:: National Institute for Health and Care Excellence, (UK); 2013. Nov, Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings [Internet]. [PubMed] [Google Scholar]
- 46.Moisset X, Bouhassira D, Avez Couturier J, Alchaar H, Conradi S, Delmotte MH, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev Neurol (Paris) 2020;176:325–52. doi: 10.1016/j.neurol.2020.01.361. [DOI] [PubMed] [Google Scholar]
- 47.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1:CD007076. doi: 10.1002/14651858.CD007076.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD007938. doi: 10.1002/14651858.CD007938.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev. 2009:CD007115. doi: 10.1002/14651858.CD007115.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014:CD007115. doi: 10.1002/14651858.CD007115.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallagher HC, Gallagher RM, Butler M, Buggy DJ, Henman MC. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;2015:CD011091. doi: 10.1002/14651858.CD011091.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007:CD005454. doi: 10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;12:CD008242. doi: 10.1002/14651858.CD008242.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;2015:CD008242. doi: 10.1002/14651858.CD008242.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;1:CD011209. doi: 10.1002/14651858.CD011209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hearn L, Derry S, Phillips T, Moore RA, Wiffen PJ. Imipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;2014:CD010769. doi: 10.1002/14651858.CD010769.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;2014:CD011003. doi: 10.1002/14651858.CD011003.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD003726. doi: 10.1002/14651858.CD003726.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forget P, Vermeersch M. To what extent are we confident that tapentadol induces less constipation and other side effects than the other opioids in chronic pain patients? a confidence evaluation in network meta-analysis. Br J Pain. 2021;15:380–7. doi: 10.1177/2049463720945289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz S, Etropolski MS, Shapiro DY, Rauschkolb C, Vinik AI, Lange B, et al. A pooled analysis evaluating the efficacy and tolerability of tapentadol extended release for chronic, painful diabetic peripheral neuropathy. Clin Drug Investig. 2015;35:95–108. doi: 10.1007/s40261-014-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vinik AI, Shapiro DY, Rauschkolb C, Lange B, Karcher K, Pennett D, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37:2302–9. doi: 10.2337/dc13-2291. [DOI] [PubMed] [Google Scholar]
- 63.Gaskell H, Derry S, Stannard C, Moore RA. Oxycodone for neuropathic pain in adults. Cochrane Database Syst Rev. 2016;7:CD010692. doi: 10.1002/14651858.CD010692.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper TE, Chen J, Wiffen PJ, Derry S, Carr DB, Aldington D, et al. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;5:CD011669. doi: 10.1002/14651858.CD011669.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meier T, Wasner G, Faust M, Kuntzer T, Ochsner F, Hueppe M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106:151–8. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 66.Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev. 2014;2014:CD010958. doi: 10.1002/14651858.CD010958.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demant DT, Lund K, Finnerup NB, Vollert J, Maier C, Segerdahl MS, et al. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. Pain. 2015;156:2234–44. doi: 10.1097/j.pain.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 68.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alles SRA, Cain SM, Snutch TP. Pregabalin as a pain therapeutic: beyond calcium channels. Front Cell Neurosci. 2020;14:83. doi: 10.3389/fncel.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(Suppl 6):13–8. doi: 10.1111/j.0013-9580.2004.455003.x. [DOI] [PubMed] [Google Scholar]
- 71.Sicras-Mainar A, Rejas-Gutiérrez J, Perez-Paramo M, Navarro-Artieda R. Cost of treating peripheral neuropathic pain with pregabalin or gabapentin at therapeutic doses in routine practice. J Comp Eff Res. 2018;7:615–25. doi: 10.2217/cer-2018-0008. [DOI] [PubMed] [Google Scholar]
- 72.Liampas A, Rekatsina M, Vadalouca A, Paladini A, Varrassi G, Zis P. Pharmacological management of painful peripheral neuropathies: a systematic review. Pain Ther. 2021;10:55–68. doi: 10.1007/s40122-020-00210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudroju N, Bansal D, Talakokkula ST, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16:E705–14. [PubMed] [Google Scholar]
- 74.Asrar MM, Kumari S, Sekhar BC, Bhansali A, Bansal D. Relative efficacy and safety of pharmacotherapeutic interventions for diabetic peripheral neuropathy: a systematic review and bayesian network meta-analysis. Pain Physician. 2021;24:E1–14. [PubMed] [Google Scholar]
- 75.Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161:639–49. doi: 10.7326/M14-0511. [DOI] [PubMed] [Google Scholar]
- 76.Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology. 2017;88:1958–67. doi: 10.1212/WNL.0000000000003882. [DOI] [PubMed] [Google Scholar]
- 77.Zajączkowska R, Mika J, Leppert W, Kocot-Kępska M, Malec-Milewska M, Wordliczek J. Mirogabalin-a novel selective ligand for the α2δ calcium channel subunit. Pharmaceuticals (Basel) 2021;14:112. doi: 10.3390/ph14020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baba M, Matsui N, Kuroha M, Wasaki Y, Ohwada S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019;10:1299–306. doi: 10.1111/jdi.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tetsunaga T, Tetsunaga T, Nishida K, Misawa H, Takigawa T, Yamane K, et al. Short-term outcomes of mirogabalin in patients with peripheral neuropathic pain: a retrospective study. J Orthop Surg Res. 2020;15:191. doi: 10.1186/s13018-020-01709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura Y, Yamaguchi S, Suzuki T, Kato J, Chiba S, Hirakawa N, et al. Switching from pregabalin to mirogabalin in patients with peripheral neuropathic pain: a multi-center, prospective, single-arm, open-label study (MIROP Study). Pain Ther. 2021;10:711–27. doi: 10.1007/s40122-021-00255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience. 2016;338:183–206. doi: 10.1016/j.neuroscience.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 82.Kremer M, Yalcin I, Goumon Y, Wurtz X, Nexon L, Daniel D, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci. 2018;38:9934–54. doi: 10.1523/JNEUROSCI.1004-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67:1411–20. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 84.Kajdasz DK, Iyengar S, Desaiah D, Backonja MM, Farrar JT, Fishbain DA, et al. Duloxetine for the management of diabetic peripheral neuropathic pain: evidence-based findings from post hoc analysis of three multicenter, randomized, double-blind, placebo-controlled, parallel-group studies. Clin Ther. 2007;29 Suppl:2536–46. doi: 10.1016/j.clinthera.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Schlereth T. Guideline "diagnosis and non interventional therapy of neuropathic pain" of the German Society of Neurology (deutsche Gesellschaft für Neurologie). Neurol Res Pract. 2020;2:16. doi: 10.1186/s42466-020-00063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodrigues-Amorim D, Olivares JM, Spuch C, Rivera-Baltanás T. A systematic review of efficacy, safety, and tolerability of duloxetine. Front Psychiatry. 2020;11:554899. doi: 10.3389/fpsyt.2020.554899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–18. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 88.Wasan AD, Ossanna MJ, Raskin J, Wernicke JF, Robinson MJ, Hall JA, et al. Safety and efficacy of duloxetine in the treatment of diabetic peripheral neuropathic pain in older patients. Curr Drug Saf. 2009;4:22–9. doi: 10.2174/157488609787354404. [DOI] [PubMed] [Google Scholar]
- 89.Yasuda H, Hotta N, Kasuga M, Kashiwagi A, Kawamori R, Yamada T, et al. Efficacy and safety of 40 mg or 60 mg duloxetine in Japanese adults with diabetic neuropathic pain: Results from a randomized, 52-week, open-label study. J Diabetes Investig. 2016;7:100–8. doi: 10.1111/jdi.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boyle J, Eriksson ME, Gribble L, Gouni R, Johnsen S, Coppini DV, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35:2451–8. doi: 10.2337/dc12-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanenberg RJ, Irving GA, Risser RC, Ahl J, Robinson MJ, Skljarevski V, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc. 2011;86:6152–6. doi: 10.4065/mcp.2010.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venlafaxine Dosage. 2021. Available at: https://www.drugs.com/dosage/venlafaxine.html. Accessed Feb 02, 2022.
- 93.Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110:697–706. doi: 10.1016/j.pain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 94.Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18:1999–2012. doi: 10.1093/pm/pnw261. [DOI] [PubMed] [Google Scholar]
- 95.Hai-Yan J, Qifu L, Dian-Ping S, Zhen-Mei A, Yu-Ping L, Xing-Wu R, et al. Effects of venlafaxine and carbamazepine for painful peripheral diabetic neuropathy: a randomized, double-blind and double-dummy, controlled multi-center trial. Chin J Evid Based Med. 2006;6:74414617. [Google Scholar]
- 96.Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60:1284–9. doi: 10.1212/01.wnl.0000058749.49264.bd. [DOI] [PubMed] [Google Scholar]
- 97.Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy. A randomized, double-blind trial. Neurosciences (Riyadh) 2014;19:192–8. [PMC free article] [PubMed] [Google Scholar]
- 98.Trouvin AP, Perrot S, Lloret-Linares C. Efficacy of venlafaxine in neuropathic pain: a narrative review of optimized treatment. Clin Ther. 2017;39:1104–22. doi: 10.1016/j.clinthera.2017.05.347. [DOI] [PubMed] [Google Scholar]
- 99.Allen R, Sharma U, Barlas S. Clinical experience with desvenlafaxine in treatment of pain associated with diabetic peripheral neuropathy. J Pain Res. 2014;7:339–51. doi: 10.2147/JPR.S55682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hameroff SR, Weiss JL, Lerman JC, Cork RC, Watts KS, Crago BR, et al. Doxepin's effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45:47–53. [PubMed] [Google Scholar]
- 101.Onghena P, Van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: a meta-analysis of 39 placebo-controlled studies. Pain. 1992;49:205–19. doi: 10.1016/0304-3959(92)90144-Z. [DOI] [PubMed] [Google Scholar]
- 102.Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther. 2017;6:25–33. doi: 10.1007/s40122-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–48. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takano A, Nag S, Gulyás B, Halldin C, Farde L. NET occupancy by clomipramine and its active metabolite, desmethylclomipramine, in non-human primates in vivo. Psychopharmacology (Berl) 2011;216:279–86. doi: 10.1007/s00213-011-2212-9. [DOI] [PubMed] [Google Scholar]
- 105.Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18:2483. doi: 10.3390/ijms18112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chou R, Carson S, Chan BK. Gabapentin versus tricyclic antidepressants for diabetic neuropathy and post-herpetic neuralgia: discrepancies between direct and indirect meta-analyses of randomized controlled trials. J Gen Intern Med. 2009;24:178–88. doi: 10.1007/s11606-008-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care. 2011;34:818–22. doi: 10.2337/dc10-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374:1252–61. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 109.Erosa SC, Haffey PR, Mehta N, Gulati A. Tapentadol, buprenorphine, and levorphanol for the treatment of neuropathic pain: a systematic review. Curr Pain Headache Rep. 2021;25:18. doi: 10.1007/s11916-020-00934-z. [DOI] [PubMed] [Google Scholar]
- 110.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Afilalo M, Morlion B. Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician. 2013;16:27–40. [PubMed] [Google Scholar]
- 112.Harati Y, Gooch C, Swenson M, Edelman SV, Greene D, Raskin P, et al. Maintenance of the long-term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. J Diabetes Complications. 2000;14:65–70. doi: 10.1016/s1056-8727(00)00060-x. [DOI] [PubMed] [Google Scholar]
- 113.Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–6. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 114.Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40:828–49. doi: 10.1016/j.clinthera.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Roulet L, Rollason V, Desmeules J, Piguet V. Tapentadol versus tramadol: a narrative and comparative review of their pharmacological, efficacy and safety profiles in adult patients. Drugs. 2021;81:1257–72. doi: 10.1007/s40265-021-01515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sommer C, Klose P, Welsch P, Petzke F, Häuser W. Opioids for chronic non-cancer neuropathic pain. An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Eur J Pain. 2020;24:3–18. doi: 10.1002/ejp.1494. [DOI] [PubMed] [Google Scholar]
- 117.Vosburg SK, Severtson SG, Dart RC, Cicero TJ, Kurtz SP, Parrino MW, et al. Assessment of tapentadol API abuse liability with the researched abuse, diversion and addiction-related surveillance system. J Pain. 2018;19:439–53. doi: 10.1016/j.jpain.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 118.McNicol ED, Ferguson MC, Schumann R. Methadone for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;5:CD012499. doi: 10.1002/14651858.CD012499.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma K, Jiang W, Wang YX, Wang L, Lv Y, Liu JF, et al. Expert consensus of the Chinese Association for the Study of Pain on pain treatment with the transdermal patch. World J Clin Cases. 2021;9:2110–22. doi: 10.12998/wjcc.v9.i9.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arora V, Campbell JN, Chung MK. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol Ther. 2021;220:107743. doi: 10.1016/j.pharmthera.2020.107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiang H, Ohno N, Hsieh YL, Mahad DJ, Kikuchi S, Komuro H, et al. Mitochondrial fission augments capsaicin-induced axonal degeneration. Acta Neuropathol. 2015;129:81–96. doi: 10.1007/s00401-014-1354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goswami C, Dreger M, Otto H, Schwappach B, Hucho F. Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J Neurochem. 2006;96:254–66. doi: 10.1111/j.1471-4159.2005.03551.x. [DOI] [PubMed] [Google Scholar]
- 123.Wang S, Wang S, Asgar J, Joseph J, Ro JY, Wei F, et al. Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J Biol Chem. 2017;292:8291–303. doi: 10.1074/jbc.M117.778290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wagner T, Roth-Daniek A, Sell A, England J, Kern KU. Capsaicin 8% patch for peripheral neuropathic pain: review of treatment best practice from 'real-world' clinical experience. Pain Manag. 2012;2:239–50. doi: 10.2217/pmt.12.13. [DOI] [PubMed] [Google Scholar]
- 125.European Medicines Agency. Qutenza: Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/qutenza-epar-product-information_en.pdf. Accessed Sep 20, 2021.
- 126.Averitas pharma announces FDA approval of Qutenza for the treatment of neuropathic pain associated with diabetic peripheral neuropathy of the feet. Available at: https://www.drugs.com/newdrugs/averitas-pharma-announces-fda-approval-qutenza-neuropathic-pain-associated-diabetic-peripheral-5301.html. Accessed Sep 20, 2021.
- 127.Kern KU, Nowack W, Poole C. Treatment of neuropathic pain with the capsaicin 8% patch: is pretreatment with lidocaine necessary? Pain Pract. 2014;14:E42–50. doi: 10.1111/papr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012;2012:CD010111. doi: 10.1002/14651858.CD010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derry S, Sven-Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013:CD007393. doi: 10.1002/14651858.CD007393.pub3. [DOI] [PubMed] [Google Scholar]
- 130.Baron R, Allegri M, Correa-Illanes G, Hans G, Serpell M, Mick G, et al. The 5% lidocaine-medicated plaster: its inclusion in international treatment guidelines for treating localized neuropathic pain, and clinical evidence supporting its use. Pain Ther. 2016;5:149–69. doi: 10.1007/s40122-016-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig. 2009;29:231–41. doi: 10.2165/00044011-200929040-00002. [DOI] [PubMed] [Google Scholar]
- 132.Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of combination therapy with 5% lidocaine medicated plaster and pregabalin in post-herpetic neuralgia and diabetic polyneuropathy. Curr Med Res Opin. 2009;25:1677–87. doi: 10.1185/03007990903048078. [DOI] [PubMed] [Google Scholar]
- 133.White WT, Patel N, Drass M, Nalamachu S. Lidocaine patch 5% with systemic analgesics such as gabapentin: a rational polypharmacy approach for the treatment of chronic pain. Pain Med. 2003;4:321–30. doi: 10.1111/j.1526-4637.2003.03045.x. [DOI] [PubMed] [Google Scholar]
- 134.Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Med Wkly. 2010;140:297–306. doi: 10.4414/smw.2010.12995. [DOI] [PubMed] [Google Scholar]
- 135.Hussain N, Said ASA, Javaid FA, Al Haddad AHI, Anwar M, Khan Z, et al. The efficacy and safety profile of capsaicin 8% patch versus 5% Lidocaine patch in patients with diabetic peripheral neuropathic pain: a randomized, placebo-controlled study of south Asian male patients. J Diabetes Metab Disord. 2021;20:271–8. doi: 10.1007/s40200-021-00741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuen KC, Baker NR, Rayman G. Treatment of chronic painful diabetic neuropathy with isosorbide dinitrate spray: a double-blind placebo-controlled cross-over study. Diabetes Care. 2002;25:1699–703. doi: 10.2337/diacare.25.10.1699. [DOI] [PubMed] [Google Scholar]
- 137.Agrawal RP, Choudhary R, Sharma P, Sharma S, Beniwal R, Kaswan K, et al. Glyceryl trinitrate spray in the management of painful diabetic neuropathy: a randomized double blind placebo controlled cross-over study. Diabetes Res Clin Pract. 2007;77:161–7. doi: 10.1016/j.diabres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Taheri A, Farbood A, Heshmat R, Samadi A, Khashayar P, Qorbani M, et al. The effect of transdermal nitroglycerin on pain control in diabetic patients with peripheral neuropathy. J Diabetes Metab Disord. 2015;14:86. doi: 10.1186/s40200-015-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cízková D, Lukácová N, Marsala M, Marsala J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res Bull. 2002;58:161–71. doi: 10.1016/s0361-9230(02)00761-x. [DOI] [PubMed] [Google Scholar]
- 140.Campbell CM, Kipnes MS, Stouch BC, Brady KL, Kelly M, Schmidt WK, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815–23. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wrzosek A, Woron J, Dobrogowski J, Jakowicka-Wordliczek J, Wordliczek J. Topical clonidine for neuropathic pain. Cochrane Database Syst Rev. 2015;8:CD010967. doi: 10.1002/14651858.CD010967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim YC, Castañeda AM, Lee CS, Jin HS, Park KS, Moon JY. Efficacy and safety of lidocaine ınfusion treatment for neuropathic pain: a randomized, double-blind, and placebo-controlled study. Reg Anesth Pain Med. 2018;43:415–24. doi: 10.1097/AAP.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 143.Moulin DE, Morley-Forster PK, Pirani Z, Rohfritsch C, Stitt L. Intravenous lidocaine in the management of chronic peripheral neuropathic pain: a randomized-controlled trial. Can J Anaesth. 2019;66:820–7. doi: 10.1007/s12630-019-01395-8. [DOI] [PubMed] [Google Scholar]
- 144.Egeo G, Fofi L, Barbanti P. Botulinum neurotoxin for the treatment of neuropathic pain. Front Neurol. 2020;11:716. doi: 10.3389/fneur.2020.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Salehi H, Moussaei M, Kamiab Z, Vakilian A. The effects of botulinum toxin type A injection on pain symptoms, quality of life, and sleep quality of patients with diabetic neuropathy: A randomized double-blind clinical trial. Iran J Neurol. 2019;18:99–107. [PMC free article] [PubMed] [Google Scholar]
- 146.Ghasemi M, Ansari M, Basiri K, Shaigannejad V. The effects of intradermal botulinum toxin type a injections on pain symptoms of patients with diabetic neuropathy. J Res Med Sci. 2014;19:106–11. [PMC free article] [PubMed] [Google Scholar]
- 147.Yuan RY, Sheu JJ, Yu JM, Chen WT, Tseng IJ, Chang HH, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72:1473–8. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]
- 148.Lakhan SE, Velasco DN, Tepper D. Botulinum toxin-a for painful diabetic neuropathy: a meta-analysis. Pain Med. 2015;16:1773–80. doi: 10.1111/pme.12728. [DOI] [PubMed] [Google Scholar]
- 149.Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, et al. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 150.Alsaloum M, Estacion M, Almomani R, Gerrits MM, Bönhof GJ, Ziegler D, et al. Propane Study Group A gain-of-function sodium channel β2-subunit mutation in painful diabetic neuropathy. Mol Pain. 2019;15:1744806919849802. doi: 10.1177/1744806919849802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Faber CG, Lauria G, Merkies IS, Cheng X, Han C, Ahn HS, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A. 2012 Nov 20;109:19444–9. doi: 10.1073/pnas.1216080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers JG, Gerrits MM, et al. PROPANE Study Group Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain. 2014;137:1627–42. doi: 10.1093/brain/awu079. [DOI] [PubMed] [Google Scholar]
- 153.Alsaloum M, Higerd GP, Effraim PR, Waxman SG. Status of peripheral sodium channel blockers for non-addictive pain treatment. Nat Rev Neurol. 2020;16:689–705. doi: 10.1038/s41582-020-00415-2. [DOI] [PubMed] [Google Scholar]
- 154.Biogen announces positive topline results from phase 2 CONVEY study in small fiber neuropathy. 2021 Sep; Available at: https://investors.biogen.com/news-releases/news-release-details/biogen-announces-positive-topline-results-phase-2-convey-study. Accessed Nov 2, 2020. [Google Scholar]
- 155.Vertex announces positive phase 2 data in third proof-of-concept study with the NaV1.8 inhibitor VX-150. Available at: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-positive-phase-2-data-third-proof-concept-study. 2018 Dec; Accessed Nov 2, 2020. [Google Scholar]
- 156.Brines M, Dunne AN, van Velzen M, Proto PL, Ostenson CG, Kirk RI, et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. 2015;20:658–66. doi: 10.2119/molmed.2014.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao M, Watanabe M, Sun S, Tokodai K, Cerami A, Brines M, et al. Improvement of islet allograft function using cibinetide, an innate repair receptor ligand. Transplantation. 2020;104:2048–58. doi: 10.1097/TP.0000000000003284. [DOI] [PubMed] [Google Scholar]
- 158.Yao M, Domogatskaya A, Ågren N, Watanabe M, Tokodai K, Brines M, et al. Cibinetide protects isolated human islets in a stressful environment and improves engraftment in the perspective of intra portal islet transplantation. Cell Transplant. 2021;30:9636897211039739. doi: 10.1177/09636897211039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carrasco C, Naziroğlu M, Rodríguez AB, Pariente JA. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol. 2018;9:95. doi: 10.3389/fphys.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.AstraZeneca. Study to evaluate the efficacy, safety, tolerability, and pharmacokinetics of AZD1386 in patients with osteoarthritis (OA) of the knee (OA19). Available at: https://clinicaltrials.gov/show/NCT00878501. Accessed Sep 21, 2020.
- 161.A clinical trial to study the effects of GRC 17536 in patients with painful diabetic peripheral neuropathy (painful extremities due to peripheral nerve damage in diabetic patients). Available at: https://clinicaltrials.gov/ct2/show/NCT01726413?term=GRC-17536. Accessed Sep 21, 2020.
- 162.Sanofi. Efficacy and safety of SAR292833 administration for 4 weeks in patients with chronic peripheral neuropathic pain (Alchemilla). Available at: https://clinicaltrials.gov/ct2/show/NCT01463397?term=NCT01463397. Accessed Sep 21, 2020.
- 163.Koivisto AP, Belvisi MG, Gaudet R, Szallasi A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov. 2022;21:41–59. doi: 10.1038/s41573-021-00268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spinaci A, Buccioni M, Dal Ben D, Marucci G, Volpini R, Lambertucci C. P2X3 receptor ligands: structural features and potential therapeutic applications. Front Pharmacol. 2021;12:653561. doi: 10.3389/fphar.2021.653561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krajewski JL. P2X3-containing receptors as targets for the treatment of chronic pain. Neurotherapeutics. 2020;17:826–38. doi: 10.1007/s13311-020-00934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shillo P, Selvarajah D, Greig M, Gandhi R, Rao G, Wilkinson ID, et al. Reduced vitamin D levels in painful diabetic peripheral neuropathy. Diabet Med. 2019;36:44–51. doi: 10.1111/dme.13798. [DOI] [PubMed] [Google Scholar]
- 167.Davoudi M, Allame Z, Niya RT, Taheri AA, Ahmadi SM. The synergistic effect of vitamin D supplement and mindfulness training on pain severity, pain-related disability and neuropathy-specific quality of life dimensions in painful diabetic neuropathy: a randomized clinical trial with placebo-controlled. J Diabetes Metab Disord. 2021;20:49–58. doi: 10.1007/s40200-020-00700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Long W, Fatehi M, Soni S, Panigrahi R, Philippaert K, Yu Y, et al. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J Physiol. 2020;598:4321–38. doi: 10.1113/JP279961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rice ASC, Dworkin RH, Finnerup NB, Attal N, Anand P, Freeman R, et al. Efficacy and safety of EMA401 in peripheral neuropathic pain: results of 2 randomised, double-blind, phase 2 studies in patients with postherpetic neuralgia and painful diabetic neuropathy. Pain. 2021;162:2578–89. doi: 10.1097/j.pain.0000000000002252. [DOI] [PubMed] [Google Scholar]
- 170.Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14:536–46. doi: 10.1017/s1092852900024020. [DOI] [PubMed] [Google Scholar]
- 171.Lipone P, Ehler E, Nastaj M, Palka-Kisielowska I, Cruccu G, Truini A, et al. Efficacy and safety of low doses of trazodone in patients affected by painful diabetic neuropathy and treated with gabapentin: a randomized controlled pilot study. CNS Drugs. 2020;34:1177–89. doi: 10.1007/s40263-020-00760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aziende Chimiche Riunite Angelini Francesco S.p.A. Trazodone/gabapentin fixed dose combination products in painful diabetic neuropathy. Available at: https://clinicaltrials.gov/ct2/show/NCT03749642. Accessed Sep 21, 2020.
- 173.Shaibani AI, Pope LE, Thisted R, Hepner A. Efficacy and safety of dextromethorphan/quinidine at two dosage levels for diabetic neuropathic pain: a double-blind, placebo-controlled, multicenter study. Pain Med. 2012;13:243–54. doi: 10.1111/j.1526-4637.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- 174.Ibrahim A. IDF Clinical Practice Recommendation on the Diabetic Foot: A guide for healthcare professionals. Diabetes Res Clin Pract. 2017;127:285–7. doi: 10.1016/j.diabres.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 175.Diabetes Canada Clinical Practice Guidelines Expert Committee. Bril V, Breiner A, Perkins BA, Zochodne D. Neuropathy. Can J Diabetes. 2018;42(Suppl 1):S217–21. doi: 10.1016/j.jcjd.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 176.Zeng H, Pacheco-Barrios K, Cao Y, Li Y, Zhang J, Yang C, et al. Non-invasive neuromodulation effects on painful diabetic peripheral neuropathy: a systematic review and meta-analysis. Sci Rep. 2020;10:19184. doi: 10.1038/s41598-020-75922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gupta M, Knezevic NN, Abd-Elsayed A, Ray M, Patel K, Chowdhury B. Treatment of painful diabetic neuropathy-a narrative review of pharmacological and interventional approaches. Biomedicines. 2021;9:573. doi: 10.3390/biomedicines9050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, White JL, Sills SM, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. 2021;78:687–98. doi: 10.1001/jamaneurol.2021.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]